User login
How gynecologic procedures and pharmacologic treatments can affect the uterus
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
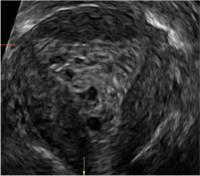
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
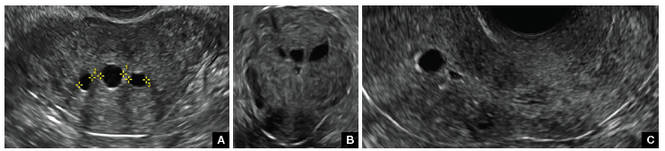
| FIGURE 1 Asherman syndrome | ||||
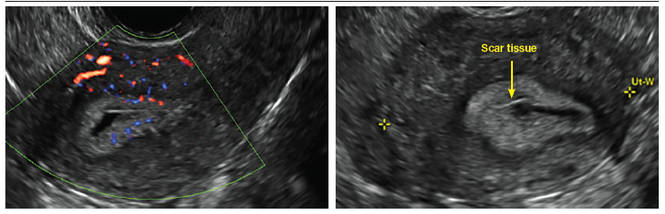
|
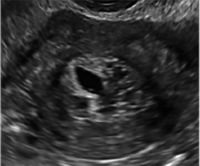
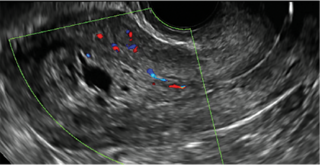
Postablation endometrial destruction
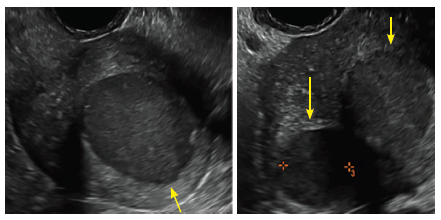
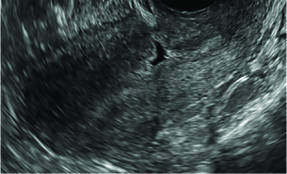
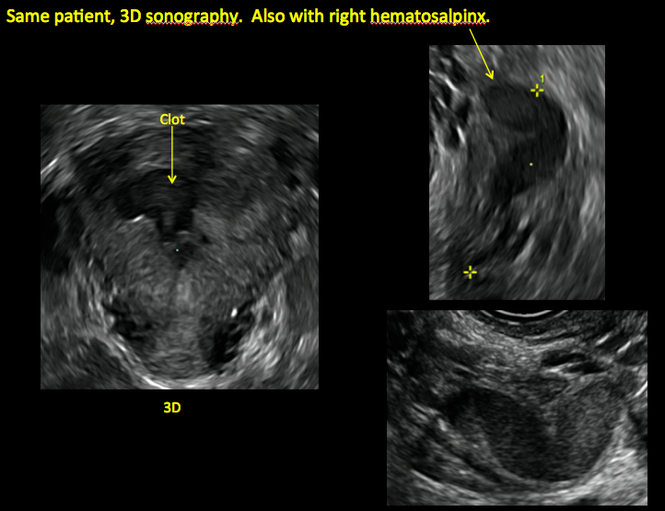
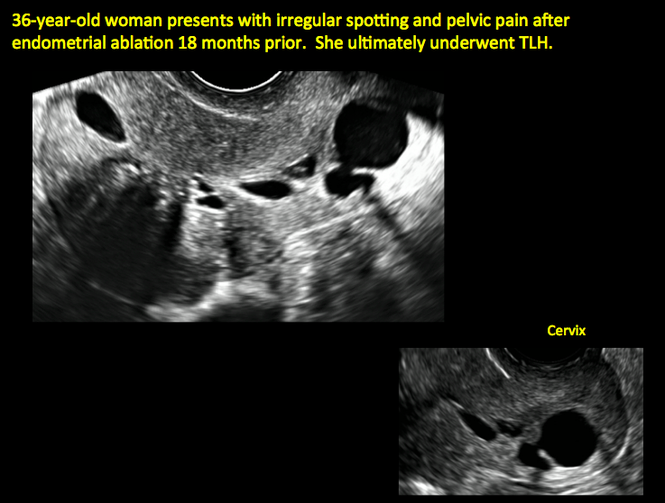
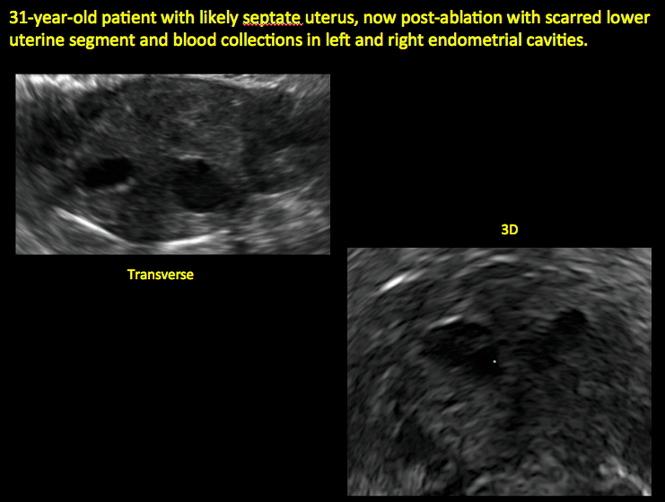
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
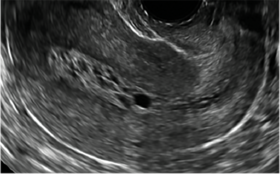
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
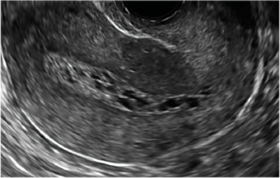
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
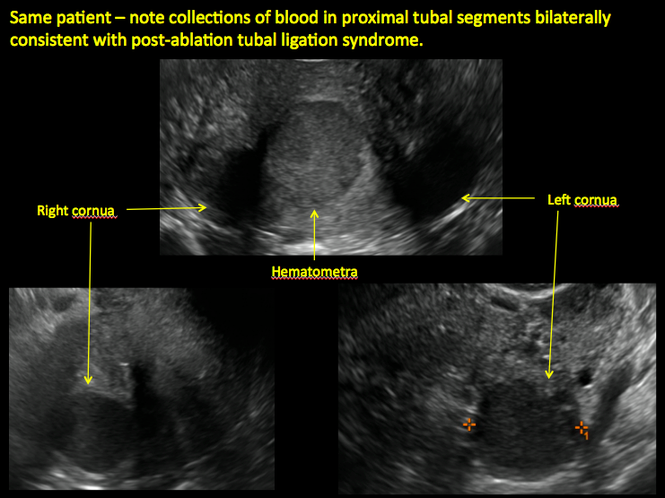
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
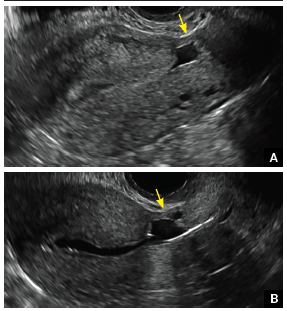
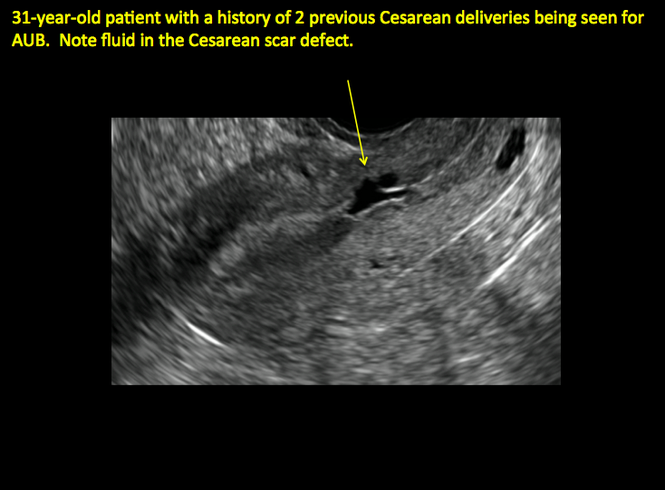
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
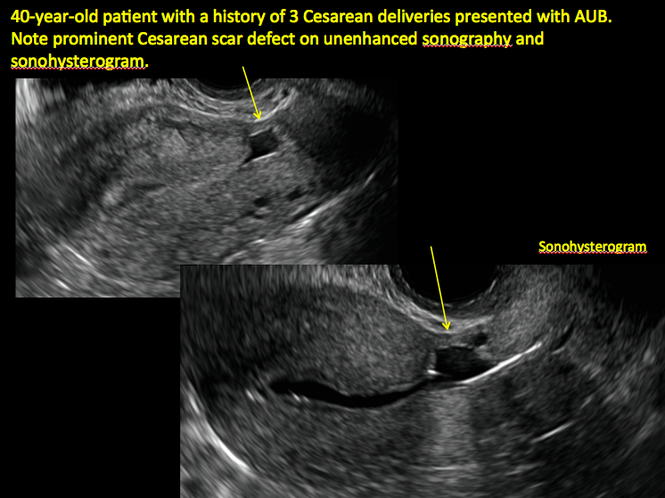
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
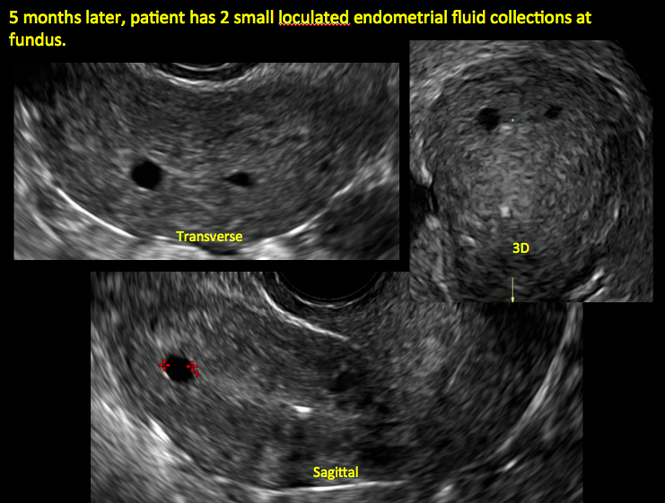
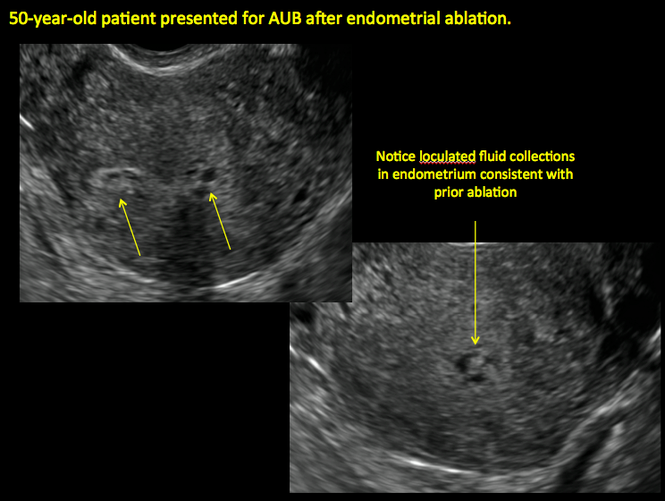
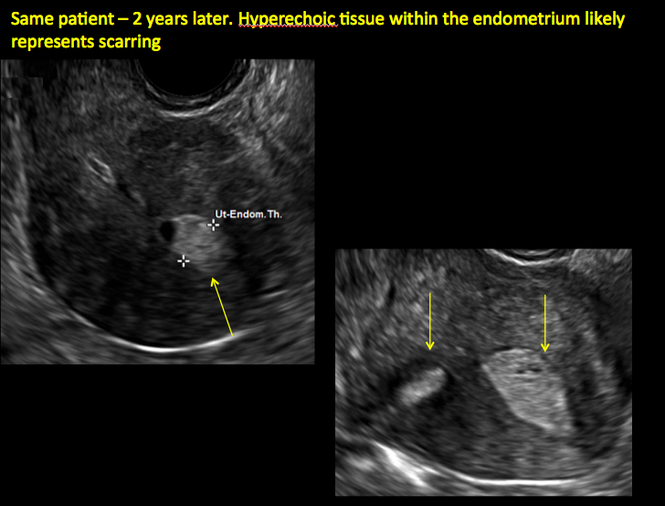
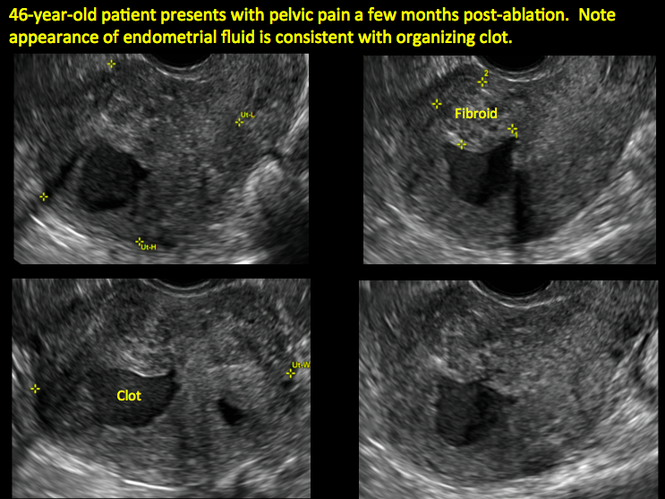
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
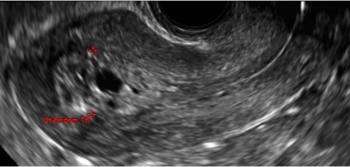
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
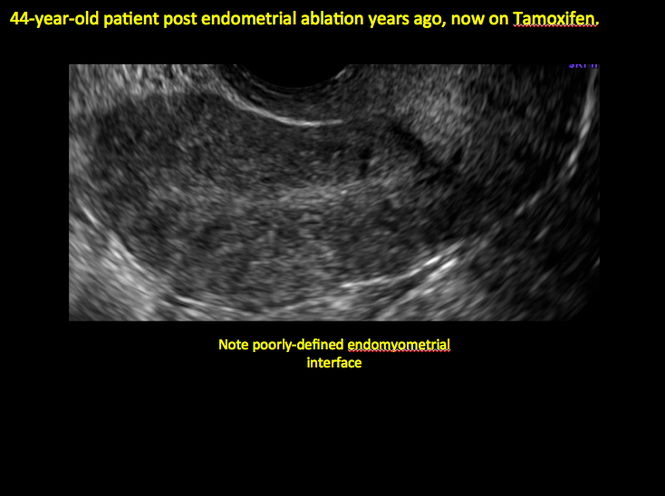
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||
| 
| 
| ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||
| 
| 
| ||
ADDITIONAL CASES - Postablation

ADDITIONAL CASES - Cesarean scar defect
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
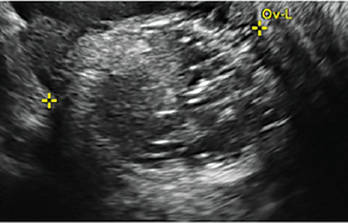
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||

|
Postablation endometrial destruction
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||

| 
| 
| ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||

| 
| 
| ||
ADDITIONAL CASES - Postablation










ADDITIONAL CASES - Cesarean scar defect


Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||

|
Postablation endometrial destruction
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||

| 
| 
| ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||

| 
| 
| ||
ADDITIONAL CASES - Postablation










ADDITIONAL CASES - Cesarean scar defect


Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
In this Article
- Foreword by Steven R. Goldstein, MD
- Uterine changes postablation
- Endometrial changes with tamoxifen use
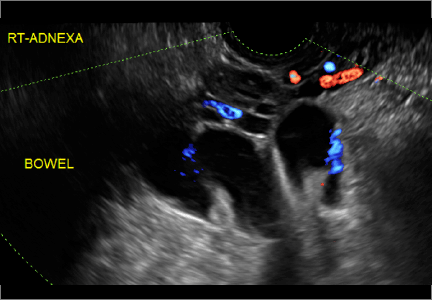
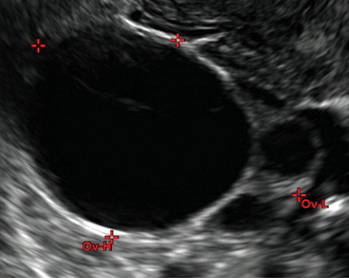
Imaging the suspected ovarian malignancy: 14 cases
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts given its ability to characterize such cysts with high resolution and accuracy. Most cystic adnexal masses have characteristic findings that can guide counseling and management decisions. For instance, mature cystic teratomas have hyperechoic lines/dots and acoustic shadowing; hydrosalpinx are tubular or s shaped and show a “waist sign.”
In parts 1 through 3 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma)
- hydrosalpinx and pelvic inclusion cysts (Part 3: “Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts)
In this conclusion to the series, we detail imaging for ovarian neoplasias (including cystadenoma and cystadenocarcinoma).
OVARIAN NEOPLASIA
A woman’s lifetime risk of undergoing surgery for suspected ovarian malignancy is 5% to 10% in the United States, and only about 13% to 21% of those undergoing surgery will actually be diagnosed with ovarian cancer.1 Therefore, the goal of diagnostic evaluation is to exclude malignancy.
Diagnostic evaluation includes:
- imaging
- lab work
- history
- physical findings.
The preferred imaging modality for a pelvic mass in asymptomatic premenopausal and postmenopausal women is transvaginal ultrasonography according to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin, which was reaffirmed in 2013.1 “No alternative imaging modality has demonstrated sufficient superiority to transvaginal ultrasonography to justify its routine use.”1
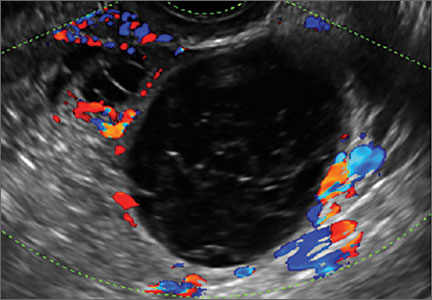
Transvaginal ultrasonography with color Doppler interrogation has demonstrated a sensitivity of 0.86% and a specificity of 0.91% for discriminating between malignant and benign ovarian masses.
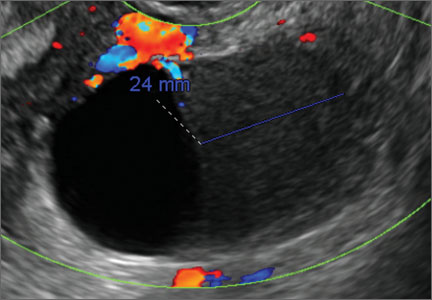
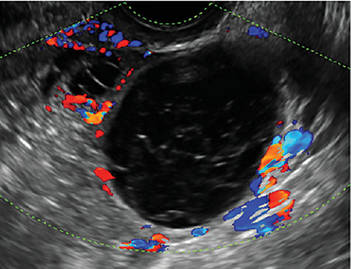
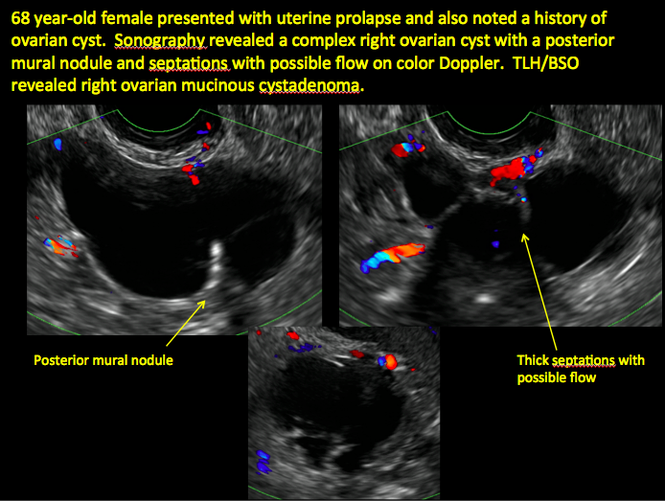
Sonographic features that are worrisome for malignancy include:
- Multiple thin septations (if indeterminate, the mass may possibly be benign)
- Thick (> 3 mm), irregular septations
- Focal areas of wall thickening (> 3 mm)
- Mural nodules or papillary projections
- Levine and colleagues note that a cyst with a mural nodule with internal blood flow on color Doppler has the highest likelihood of being malignant2
- Moderate or large amount of ascitic fluid in pelvis (in conjunction with ovarian mass showing the above characteristics)
Various morphology indices have been developed that combine these criteria with ovarian mass volume to determine the preoperative predictive value for malignancy.
In the images that follow, we present 14 cases that demonstrate cystadenoma, low malignant potential tumors, and ovarian neoplasia.
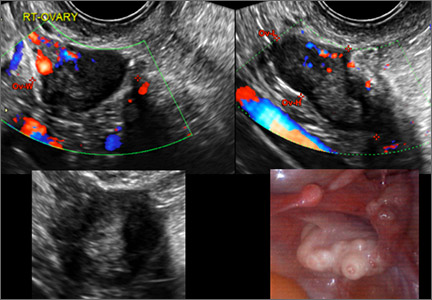
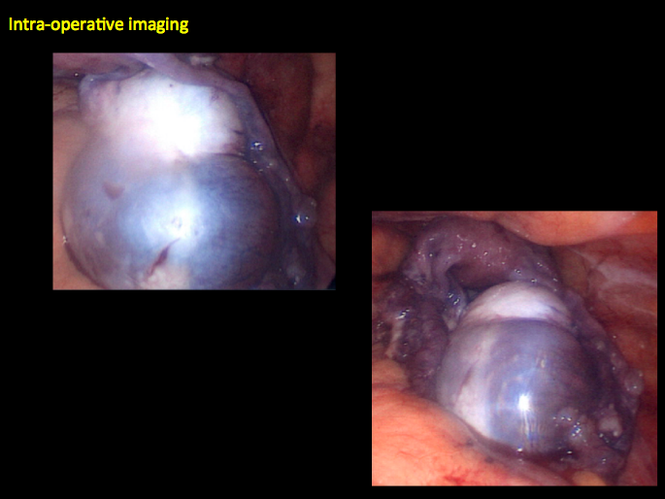
CASE 1. Right ovarian mucinous cystadenoma in 68-year-old woman with uterine prolapse and history of ovarian cyst
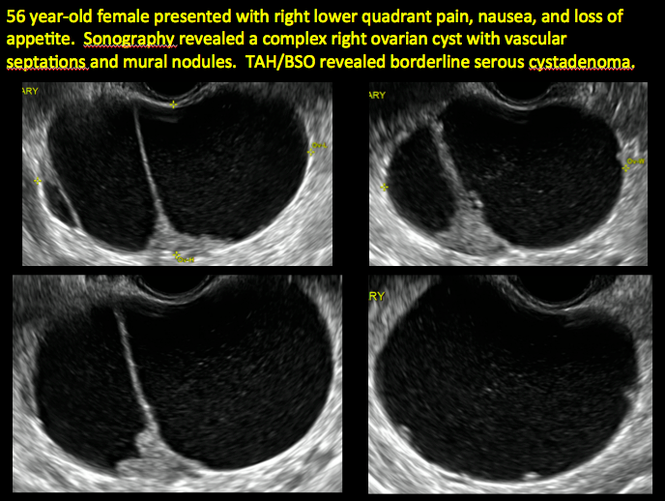
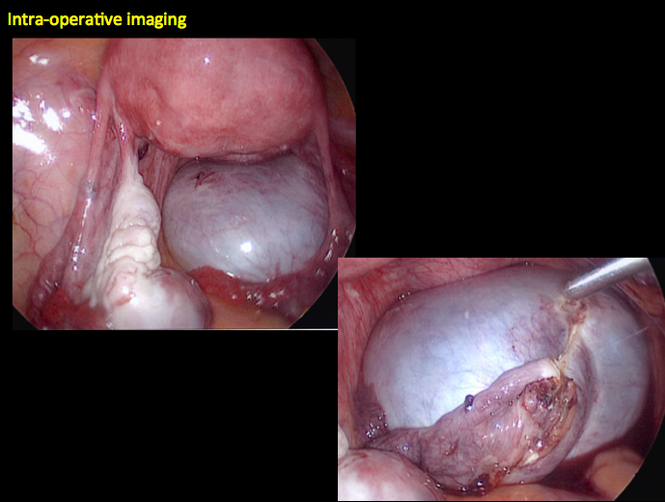
CASE 2. Borderline serous cystadenoma in 56-year-old woman with right lower quadrant pain, nausea, and loss of appetite

CASE 3. Mucinous cystadenoma in 38-year-old woman undergoing sonography for spontaneous abortion

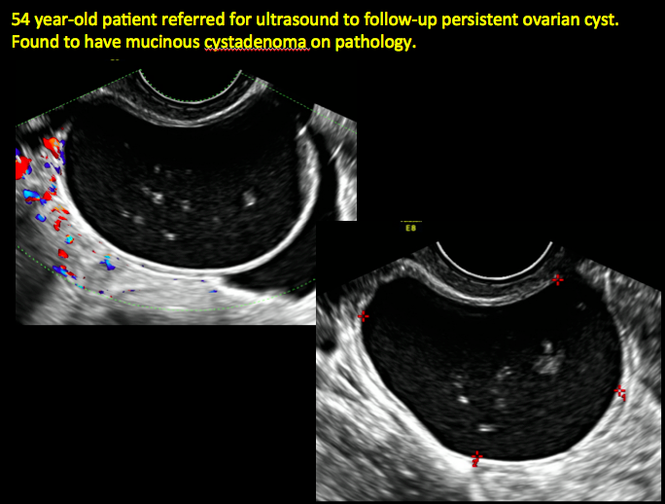
CASE 4. Mucinous cystadenoma in 54-year-old woman undergoing follow-up ultrasound for persistent ovarian cyst
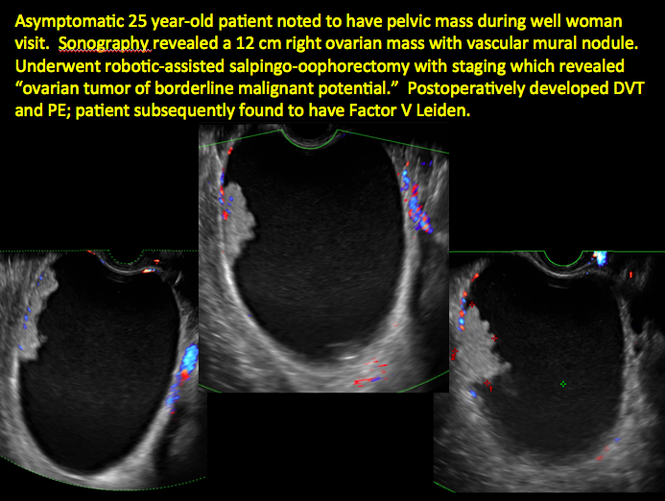
CASE 7. Mature cystic teratoma in 31-year-old woman with progressively heavier bleeding and pelvic pain

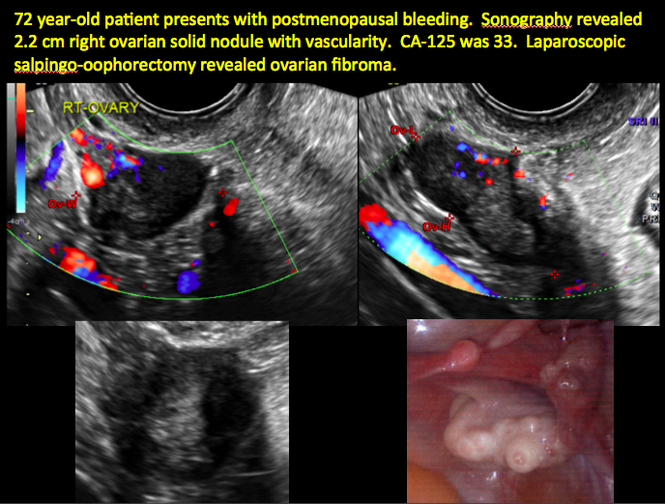
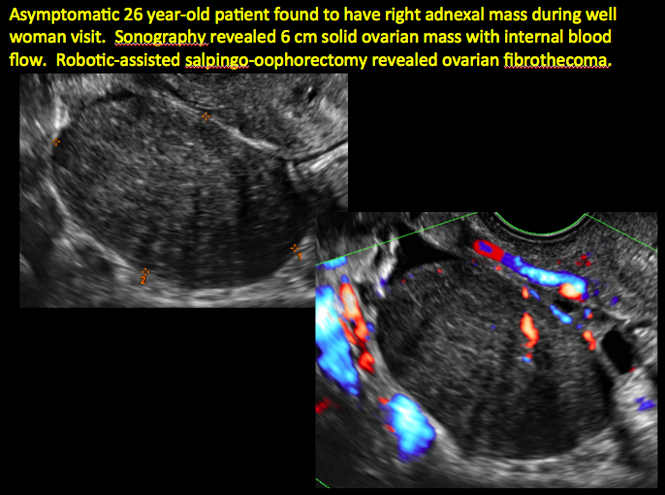
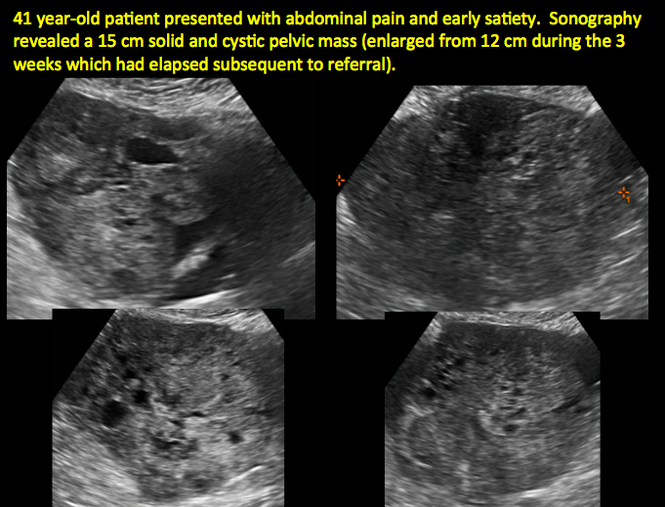
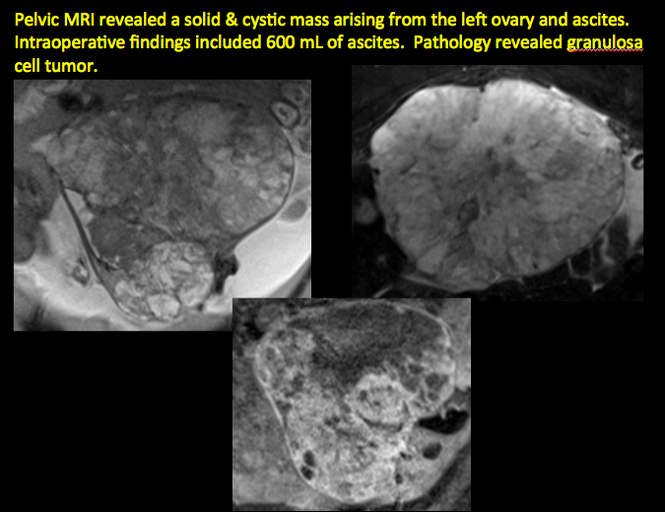
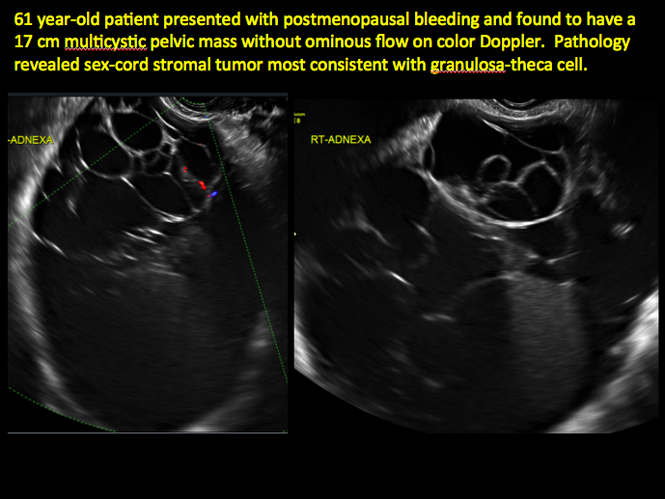
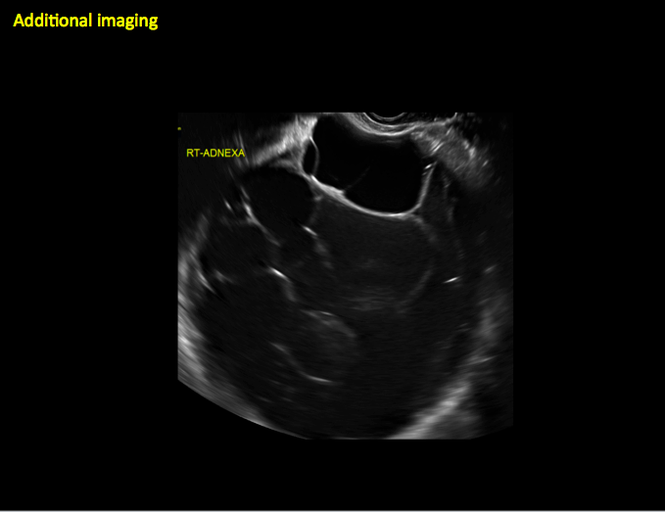
CASE 11. Sex-cord stromal tumor in 61-year-old woman with postmenopausal bleeding
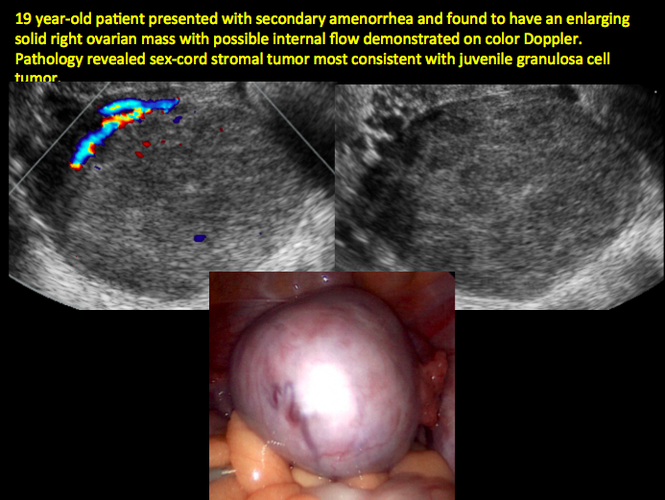
CASE 12. Juvenile granulosa cell tumor in 19-year-old patient with secondary amenorrhea
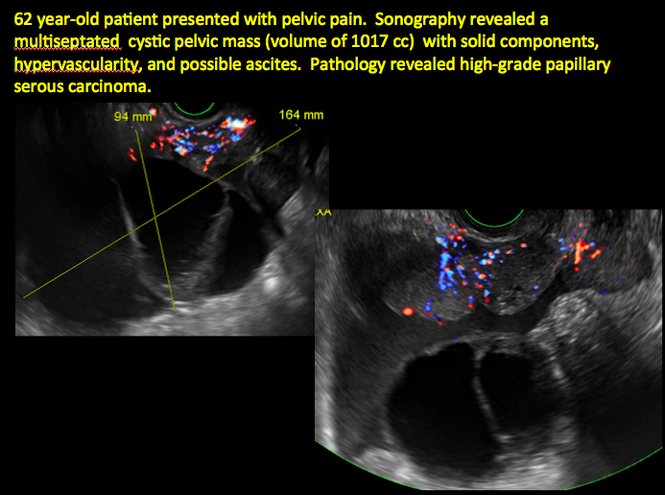
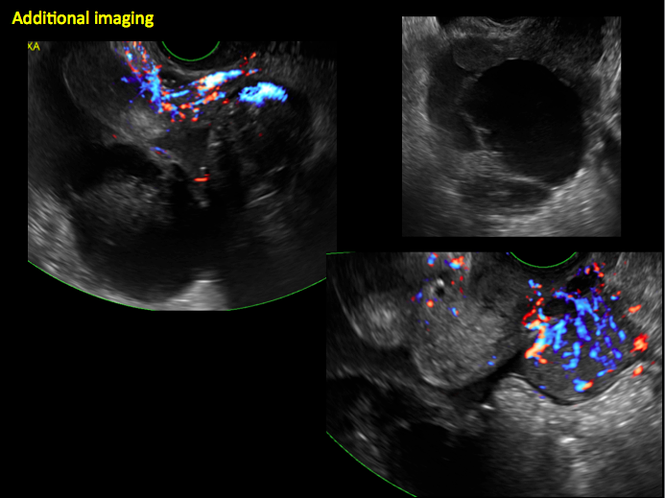
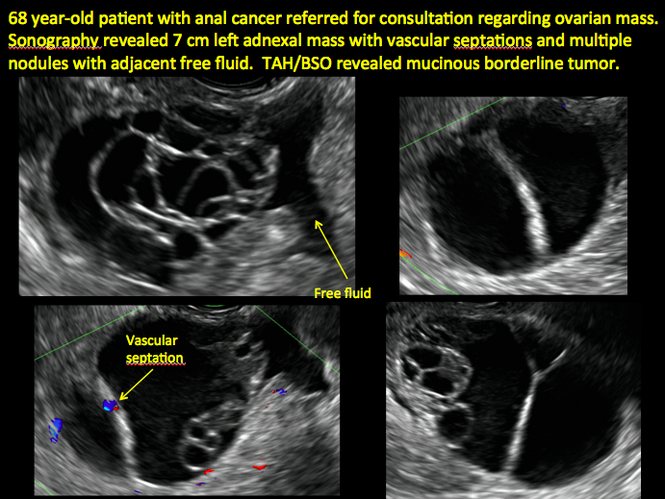
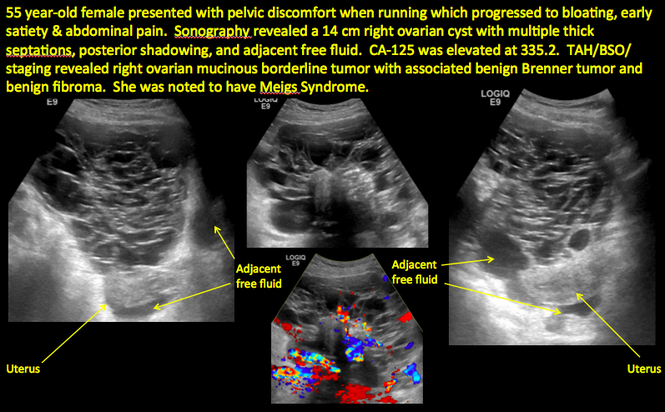
CASE 14. Mucinous borderline tumor in 55-year-old woman with pelvic discomfort
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts given its ability to characterize such cysts with high resolution and accuracy. Most cystic adnexal masses have characteristic findings that can guide counseling and management decisions. For instance, mature cystic teratomas have hyperechoic lines/dots and acoustic shadowing; hydrosalpinx are tubular or s shaped and show a “waist sign.”
In parts 1 through 3 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma)
- hydrosalpinx and pelvic inclusion cysts (Part 3: “Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts)
In this conclusion to the series, we detail imaging for ovarian neoplasias (including cystadenoma and cystadenocarcinoma).
OVARIAN NEOPLASIA
A woman’s lifetime risk of undergoing surgery for suspected ovarian malignancy is 5% to 10% in the United States, and only about 13% to 21% of those undergoing surgery will actually be diagnosed with ovarian cancer.1 Therefore, the goal of diagnostic evaluation is to exclude malignancy.
Diagnostic evaluation includes:
- imaging
- lab work
- history
- physical findings.
The preferred imaging modality for a pelvic mass in asymptomatic premenopausal and postmenopausal women is transvaginal ultrasonography according to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin, which was reaffirmed in 2013.1 “No alternative imaging modality has demonstrated sufficient superiority to transvaginal ultrasonography to justify its routine use.”1
Transvaginal ultrasonography with color Doppler interrogation has demonstrated a sensitivity of 0.86% and a specificity of 0.91% for discriminating between malignant and benign ovarian masses.
Sonographic features that are worrisome for malignancy include:
- Multiple thin septations (if indeterminate, the mass may possibly be benign)
- Thick (> 3 mm), irregular septations
- Focal areas of wall thickening (> 3 mm)
- Mural nodules or papillary projections
- Levine and colleagues note that a cyst with a mural nodule with internal blood flow on color Doppler has the highest likelihood of being malignant2
- Moderate or large amount of ascitic fluid in pelvis (in conjunction with ovarian mass showing the above characteristics)
Various morphology indices have been developed that combine these criteria with ovarian mass volume to determine the preoperative predictive value for malignancy.
In the images that follow, we present 14 cases that demonstrate cystadenoma, low malignant potential tumors, and ovarian neoplasia.
CASE 1. Right ovarian mucinous cystadenoma in 68-year-old woman with uterine prolapse and history of ovarian cyst


CASE 2. Borderline serous cystadenoma in 56-year-old woman with right lower quadrant pain, nausea, and loss of appetite


CASE 3. Mucinous cystadenoma in 38-year-old woman undergoing sonography for spontaneous abortion


CASE 4. Mucinous cystadenoma in 54-year-old woman undergoing follow-up ultrasound for persistent ovarian cyst



CASE 7. Mature cystic teratoma in 31-year-old woman with progressively heavier bleeding and pelvic pain





CASE 11. Sex-cord stromal tumor in 61-year-old woman with postmenopausal bleeding


CASE 12. Juvenile granulosa cell tumor in 19-year-old patient with secondary amenorrhea



CASE 14. Mucinous borderline tumor in 55-year-old woman with pelvic discomfort

Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts given its ability to characterize such cysts with high resolution and accuracy. Most cystic adnexal masses have characteristic findings that can guide counseling and management decisions. For instance, mature cystic teratomas have hyperechoic lines/dots and acoustic shadowing; hydrosalpinx are tubular or s shaped and show a “waist sign.”
In parts 1 through 3 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma)
- hydrosalpinx and pelvic inclusion cysts (Part 3: “Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts)
In this conclusion to the series, we detail imaging for ovarian neoplasias (including cystadenoma and cystadenocarcinoma).
OVARIAN NEOPLASIA
A woman’s lifetime risk of undergoing surgery for suspected ovarian malignancy is 5% to 10% in the United States, and only about 13% to 21% of those undergoing surgery will actually be diagnosed with ovarian cancer.1 Therefore, the goal of diagnostic evaluation is to exclude malignancy.
Diagnostic evaluation includes:
- imaging
- lab work
- history
- physical findings.
The preferred imaging modality for a pelvic mass in asymptomatic premenopausal and postmenopausal women is transvaginal ultrasonography according to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin, which was reaffirmed in 2013.1 “No alternative imaging modality has demonstrated sufficient superiority to transvaginal ultrasonography to justify its routine use.”1
Transvaginal ultrasonography with color Doppler interrogation has demonstrated a sensitivity of 0.86% and a specificity of 0.91% for discriminating between malignant and benign ovarian masses.
Sonographic features that are worrisome for malignancy include:
- Multiple thin septations (if indeterminate, the mass may possibly be benign)
- Thick (> 3 mm), irregular septations
- Focal areas of wall thickening (> 3 mm)
- Mural nodules or papillary projections
- Levine and colleagues note that a cyst with a mural nodule with internal blood flow on color Doppler has the highest likelihood of being malignant2
- Moderate or large amount of ascitic fluid in pelvis (in conjunction with ovarian mass showing the above characteristics)
Various morphology indices have been developed that combine these criteria with ovarian mass volume to determine the preoperative predictive value for malignancy.
In the images that follow, we present 14 cases that demonstrate cystadenoma, low malignant potential tumors, and ovarian neoplasia.
CASE 1. Right ovarian mucinous cystadenoma in 68-year-old woman with uterine prolapse and history of ovarian cyst


CASE 2. Borderline serous cystadenoma in 56-year-old woman with right lower quadrant pain, nausea, and loss of appetite


CASE 3. Mucinous cystadenoma in 38-year-old woman undergoing sonography for spontaneous abortion


CASE 4. Mucinous cystadenoma in 54-year-old woman undergoing follow-up ultrasound for persistent ovarian cyst



CASE 7. Mature cystic teratoma in 31-year-old woman with progressively heavier bleeding and pelvic pain





CASE 11. Sex-cord stromal tumor in 61-year-old woman with postmenopausal bleeding


CASE 12. Juvenile granulosa cell tumor in 19-year-old patient with secondary amenorrhea



CASE 14. Mucinous borderline tumor in 55-year-old woman with pelvic discomfort

“Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts
Ultrasonography is the preferred imaging method to evaluate most adnexal cysts. Most types of pelvic cyst pathology have characteristic findings that, when identified, can guide counseling and management decisions. For instance, simple cysts have thin walls, are uniformly hypoechoic, and show no blood flow on color Doppler. Endometriomas, on the other hand, demonstrate diffuse, low-level internal echoes on ultrasonography.
In parts 1 and 2 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- and mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma).
In this part 3, we detail imaging for hydrosalpinx and pelvic inclusion cysts. In part 4 we will consider cystadenomas and ovarian neoplasias.
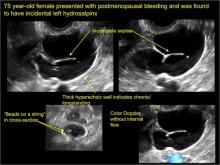
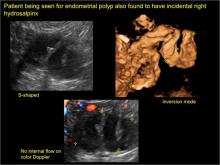
hydrosalpinx
These cysts are caused by fimbrial obstruction and result in tubal distention with serous fluid. A hydrosalpinx may occur following an episode of salpingitis or pelvic surgery.
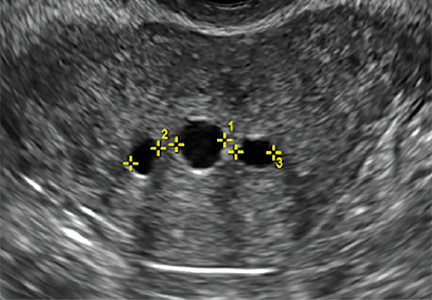
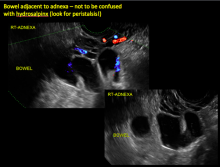
Sonographic features diagnostic for hydrosalpinx include a tubular or S-shaped cystic mass separate from the ovary, with:
- “beads on a string” or “cogwheel” appearance (small round nodules less than 3 mm in size that represent endosalpingeal folds when viewed in cross section)
- “waist sign” (indentations on opposite sides)
- incomplete septations, which result from segments of distended tube folding over/adhering to other tubal segments
Levine and colleagues noted that 3-dimensional imaging may be helpful when the diagnosis is uncertain.1
When a mass is noted that has features classic for hydrosalpinx, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommends1:
- no further imaging is necessary to establish the diagnosis
- frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
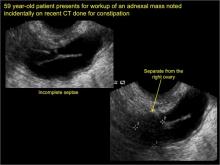
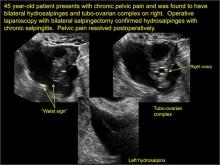
In FIGURES 1 through 6 below (slides of image collections), we present 5 cases, including one of a 45-year-old patient presenting with chronic pelvic pain who was found to have bilateral hydrosalginges and right-sided tubo-ovarian complex.
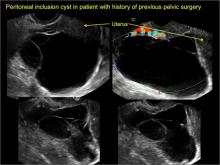
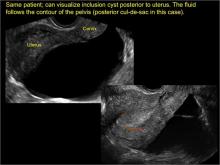
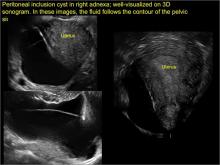
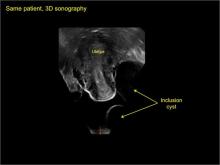
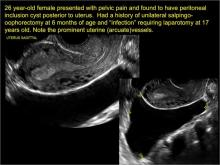
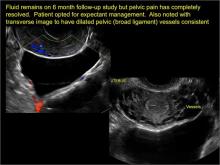
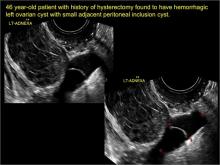
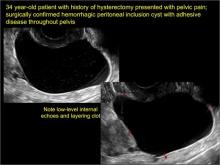
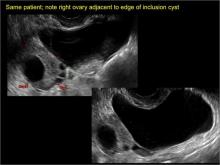
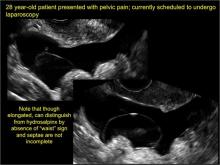
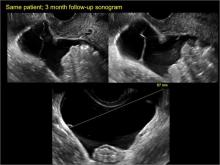
pelvic inclusion cysts
Pelvic/peritoneal inclusion cysts, or peritoneal pseudocysts, are typically associated with factors that increase the risk for pelvic adhesive disease (including endometriosis, pelvic inflammatory disease, or prior pelvic surgery).
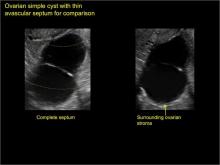
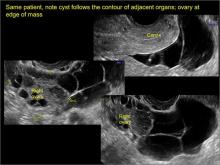
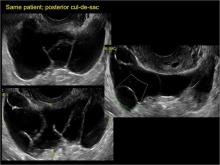
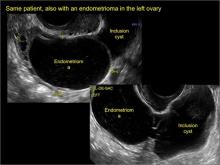
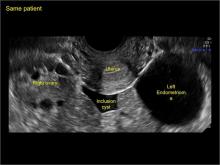
Classic sonographic features of pelvic inclusion cysts are:
- cystic mass, usually with septations/loculations
- the mass follows the contour of adjacent organs
- ovary at edge of the mass or sometimes suspended within it
- with or without flow in septation on color Doppler
When a mass is noted that has features classic for a peritoneal inclusion cyst, the US Society of Radiologists in Ultrasound recommends that1:
- no further imaging is necessary to establish the diagnosis (although further imaging may be needed if the diagnosis is uncertain)
- the frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
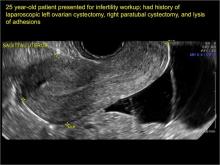
In FIGURES 7 through 22 below (slides of image collections), we present several cases that demonstrate pelvic inclusion cysts on imaging. One case involves a 25-year-old patient presenting for 2- and 3-dimensional pelvic imaging due to infertility. She had a history of laparoscopic left ovarian cystectomy, right paratubal cystectomy, and lysis of adhesions. She was found to have a pelvic inclusion cyst and an endometrioma in the left ovary.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12
Figure 13
Figure 14
Figure 15
Figure 16
Figure 17
Figure 18
Figure 19
Figure 20
Figure 21
Figure 22
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Ultrasound Q. 2010;26(3):121−131.
Ultrasonography is the preferred imaging method to evaluate most adnexal cysts. Most types of pelvic cyst pathology have characteristic findings that, when identified, can guide counseling and management decisions. For instance, simple cysts have thin walls, are uniformly hypoechoic, and show no blood flow on color Doppler. Endometriomas, on the other hand, demonstrate diffuse, low-level internal echoes on ultrasonography.
In parts 1 and 2 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- and mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma).
In this part 3, we detail imaging for hydrosalpinx and pelvic inclusion cysts. In part 4 we will consider cystadenomas and ovarian neoplasias.
hydrosalpinx
These cysts are caused by fimbrial obstruction and result in tubal distention with serous fluid. A hydrosalpinx may occur following an episode of salpingitis or pelvic surgery.
Sonographic features diagnostic for hydrosalpinx include a tubular or S-shaped cystic mass separate from the ovary, with:
- “beads on a string” or “cogwheel” appearance (small round nodules less than 3 mm in size that represent endosalpingeal folds when viewed in cross section)
- “waist sign” (indentations on opposite sides)
- incomplete septations, which result from segments of distended tube folding over/adhering to other tubal segments
Levine and colleagues noted that 3-dimensional imaging may be helpful when the diagnosis is uncertain.1
When a mass is noted that has features classic for hydrosalpinx, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommends1:
- no further imaging is necessary to establish the diagnosis
- frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 1 through 6 below (slides of image collections), we present 5 cases, including one of a 45-year-old patient presenting with chronic pelvic pain who was found to have bilateral hydrosalginges and right-sided tubo-ovarian complex.
pelvic inclusion cysts
Pelvic/peritoneal inclusion cysts, or peritoneal pseudocysts, are typically associated with factors that increase the risk for pelvic adhesive disease (including endometriosis, pelvic inflammatory disease, or prior pelvic surgery).
Classic sonographic features of pelvic inclusion cysts are:
- cystic mass, usually with septations/loculations
- the mass follows the contour of adjacent organs
- ovary at edge of the mass or sometimes suspended within it
- with or without flow in septation on color Doppler
When a mass is noted that has features classic for a peritoneal inclusion cyst, the US Society of Radiologists in Ultrasound recommends that1:
- no further imaging is necessary to establish the diagnosis (although further imaging may be needed if the diagnosis is uncertain)
- the frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 7 through 22 below (slides of image collections), we present several cases that demonstrate pelvic inclusion cysts on imaging. One case involves a 25-year-old patient presenting for 2- and 3-dimensional pelvic imaging due to infertility. She had a history of laparoscopic left ovarian cystectomy, right paratubal cystectomy, and lysis of adhesions. She was found to have a pelvic inclusion cyst and an endometrioma in the left ovary.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12
Figure 13
Figure 14
Figure 15
Figure 16
Figure 17
Figure 18
Figure 19
Figure 20
Figure 21
Figure 22
Ultrasonography is the preferred imaging method to evaluate most adnexal cysts. Most types of pelvic cyst pathology have characteristic findings that, when identified, can guide counseling and management decisions. For instance, simple cysts have thin walls, are uniformly hypoechoic, and show no blood flow on color Doppler. Endometriomas, on the other hand, demonstrate diffuse, low-level internal echoes on ultrasonography.
In parts 1 and 2 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- and mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma).
In this part 3, we detail imaging for hydrosalpinx and pelvic inclusion cysts. In part 4 we will consider cystadenomas and ovarian neoplasias.
hydrosalpinx
These cysts are caused by fimbrial obstruction and result in tubal distention with serous fluid. A hydrosalpinx may occur following an episode of salpingitis or pelvic surgery.
Sonographic features diagnostic for hydrosalpinx include a tubular or S-shaped cystic mass separate from the ovary, with:
- “beads on a string” or “cogwheel” appearance (small round nodules less than 3 mm in size that represent endosalpingeal folds when viewed in cross section)
- “waist sign” (indentations on opposite sides)
- incomplete septations, which result from segments of distended tube folding over/adhering to other tubal segments
Levine and colleagues noted that 3-dimensional imaging may be helpful when the diagnosis is uncertain.1
When a mass is noted that has features classic for hydrosalpinx, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommends1:
- no further imaging is necessary to establish the diagnosis
- frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 1 through 6 below (slides of image collections), we present 5 cases, including one of a 45-year-old patient presenting with chronic pelvic pain who was found to have bilateral hydrosalginges and right-sided tubo-ovarian complex.
pelvic inclusion cysts
Pelvic/peritoneal inclusion cysts, or peritoneal pseudocysts, are typically associated with factors that increase the risk for pelvic adhesive disease (including endometriosis, pelvic inflammatory disease, or prior pelvic surgery).
Classic sonographic features of pelvic inclusion cysts are:
- cystic mass, usually with septations/loculations
- the mass follows the contour of adjacent organs
- ovary at edge of the mass or sometimes suspended within it
- with or without flow in septation on color Doppler
When a mass is noted that has features classic for a peritoneal inclusion cyst, the US Society of Radiologists in Ultrasound recommends that1:
- no further imaging is necessary to establish the diagnosis (although further imaging may be needed if the diagnosis is uncertain)
- the frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 7 through 22 below (slides of image collections), we present several cases that demonstrate pelvic inclusion cysts on imaging. One case involves a 25-year-old patient presenting for 2- and 3-dimensional pelvic imaging due to infertility. She had a history of laparoscopic left ovarian cystectomy, right paratubal cystectomy, and lysis of adhesions. She was found to have a pelvic inclusion cyst and an endometrioma in the left ovary.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12
Figure 13
Figure 14
Figure 15
Figure 16
Figure 17
Figure 18
Figure 19
Figure 20
Figure 21
Figure 22
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Ultrasound Q. 2010;26(3):121−131.
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Ultrasound Q. 2010;26(3):121−131.
Imaging the endometrioma and mature cystic teratoma
The preferred imaging method to evaluate the majority of adnexal cysts is ultrasonography, which can help characterize the cyst type. Common benign adnexal cyst types include simple, hemorrhagic, endometrioma, and mature teratoma (dermoid cyst). In this part 2 of a 4-part series on cystic adnexal pathology, we focus on imaging signs for, and follow-up of, endometriomas and mature teratomas.
Endometriomas
Endometriomas are common, typically benign, cysts that produce homogenous, low-level internal echoes and a “ground glass” appearance on ultrasonography. No internal flow is apparent on color Doppler. The presence of tiny echogenic wall foci can distinguish an endometrioma from a hemorrhagic cyst.
Rarely, endometriomas may undergo malignant transformation. Usually this occurs with cysts greater than 9 cm and in patients aged 45 years or older. A malignancy often exhibits rapid growth or the development of a solid nodule with flow on color Doppler.
Management
Although surgery remains the first-line management for women with symptomatic or enlarging endometriomas, there appears to be a role for sonographic observation, with continuous progestational treatment, in women with small (< 5 cm) asymptomatic endometriomas.
The Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommended1:
- Short-interval follow-up (6 to 12 weeks) in reproductive-aged women to ensure acute hemorrhagic cysts are not mistaken for endometriomas
- If not removed surgically, sonographic follow-up is recommended, with frequency of follow up based on patient age and symptoms and cyst size and characteristics.
In FIGURES 1 through 11 (slides of image collections), we present several cases, including one of a 25-year-old patient presenting with pelvic pain and dyspareunia who was later found to have bilateral endometriomas.
Mature teratomas
Mature cystic teratomas display several telltale signs on imaging, including:
- hyperechoic lines/dots (“dermoid mesh”) corresponding to hair/skeletal components
- “Rokitanski nodule” – a peripherally placed mass of sebum, bones, and hair
- posterior acoustic shadowing
- cystic or floating spherical structures
- no internal flow on color Doppler
Rarely, dermoid cysts may undergo malignant transformation. Usually this occurs in cysts greater than 10 cm and in patients aged 50 years or older. Internal flow on color Doppler, branching, or invasion into adjacent structures can indicate malignancy.
Management
The traditional treatment for dermoid cysts is surgical. However, given the ability for accurate diagnosis with vaginal ultrasonography, there appears to be a role for sonographic observation in asymptomatic women with small dermoids.2
If the cyst is not surgically removed, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommended initial sonographic follow up at no more than 6 months to 1 year to ensure no change in size or internal architecture.1
In FIGURES 12 through 24 below (slides of image collections), we offer imaging from the case presentation and follow-up of a 19-year-old patient with pelvic pain who has a history of ovarian cystectomy for dermoid cyst, as well as 6 additional case illustrations.
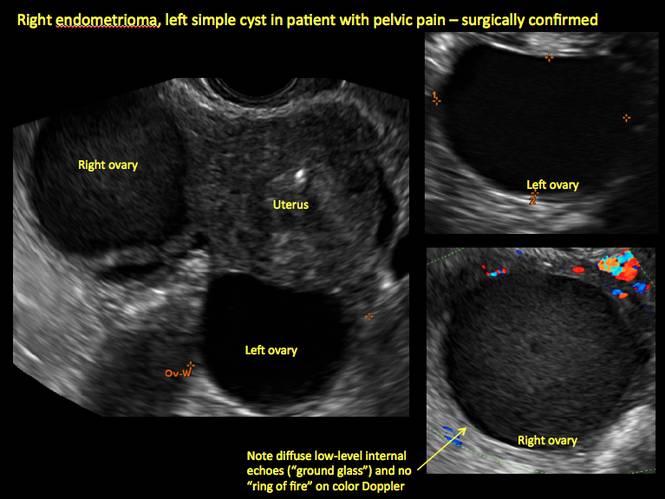
Figure 1
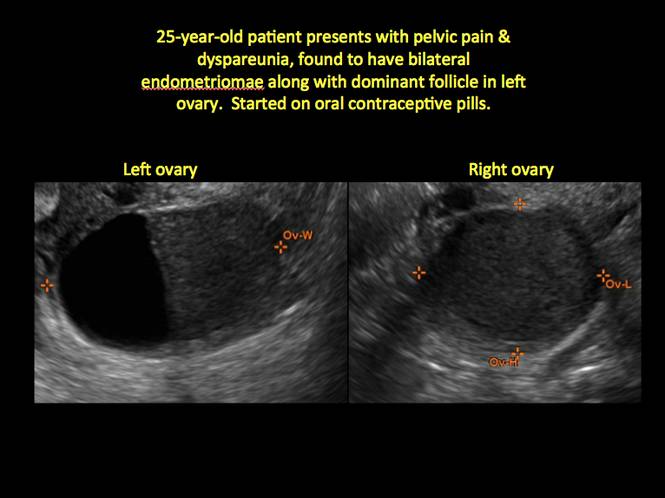
Figure 2
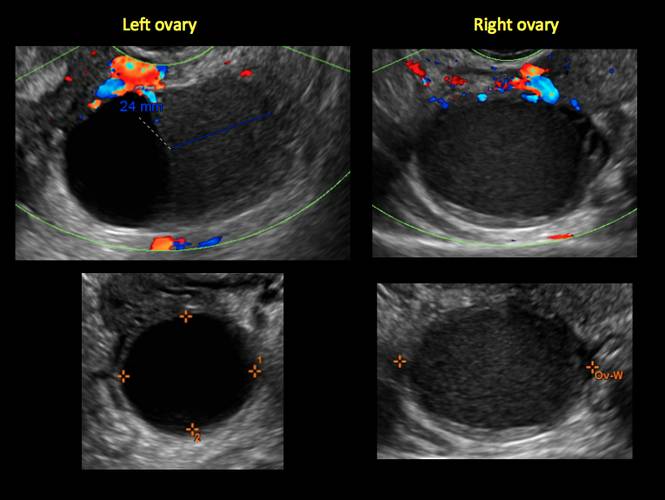
Figure 3
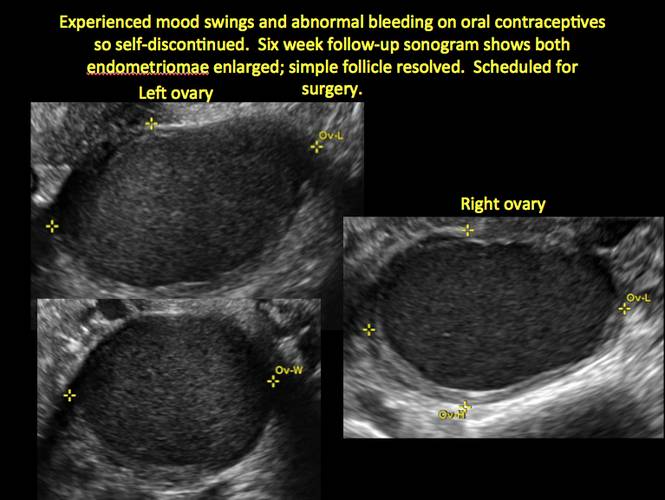
Figure 4
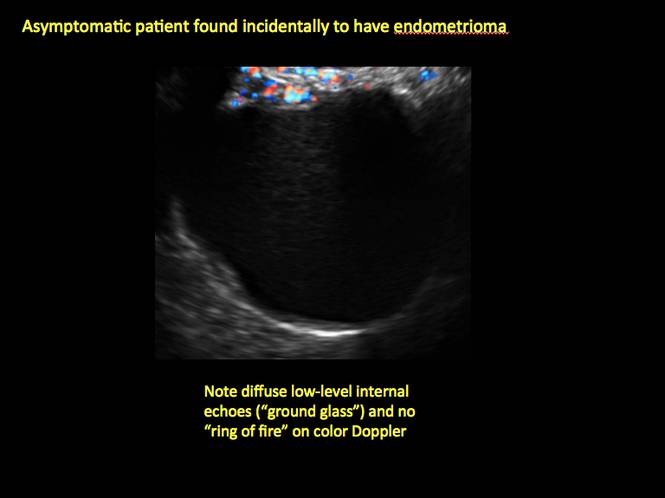
Figure 5
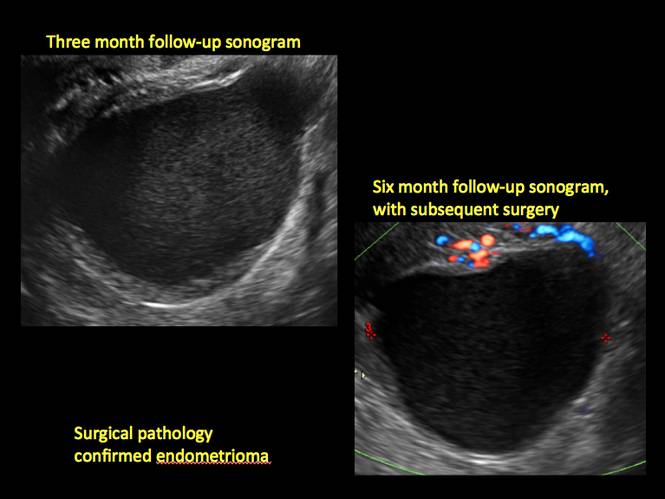
Figure 6
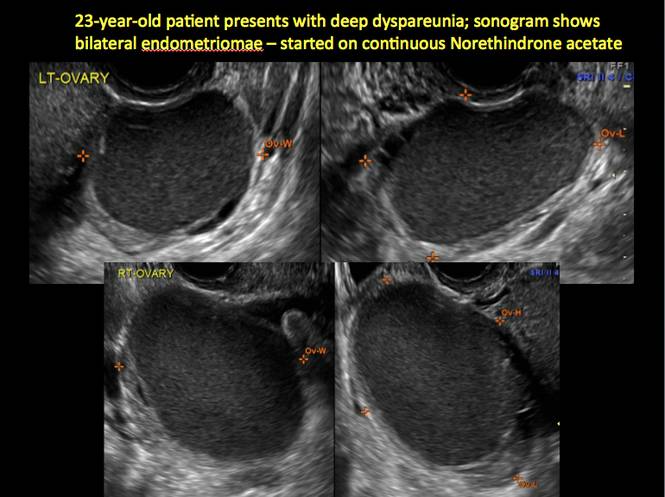
Figure 7
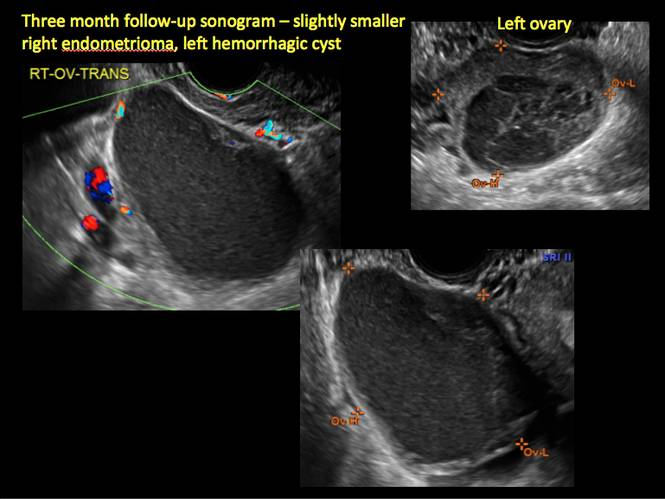
Figure 8
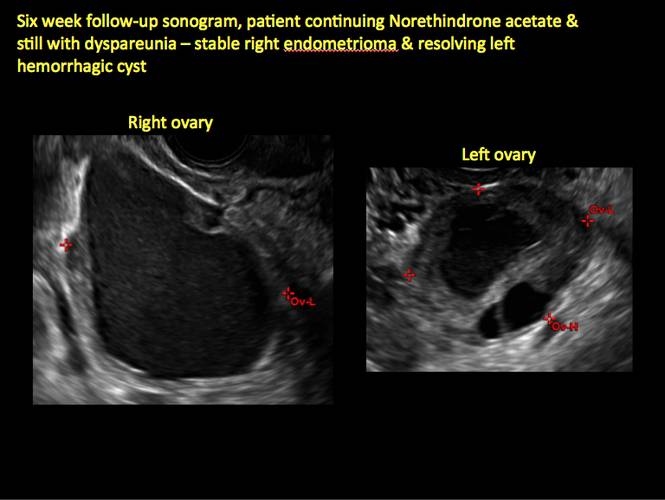
Figure 9
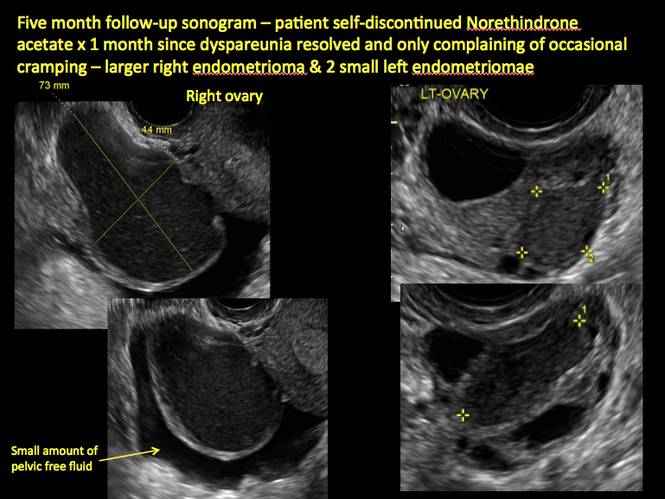
Figure 10
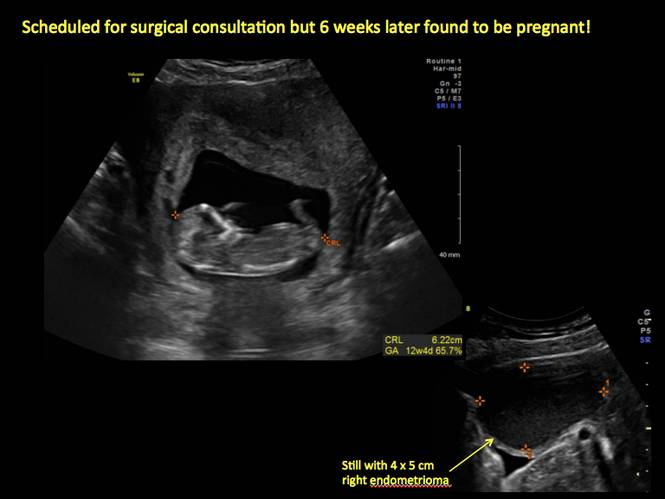
Figure 11
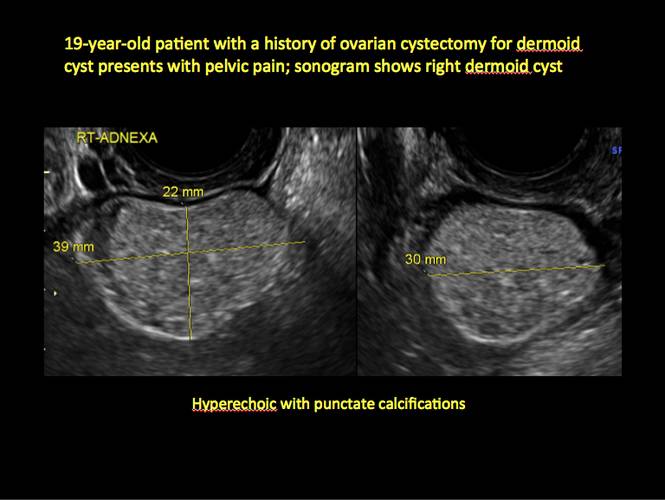
Figure 12
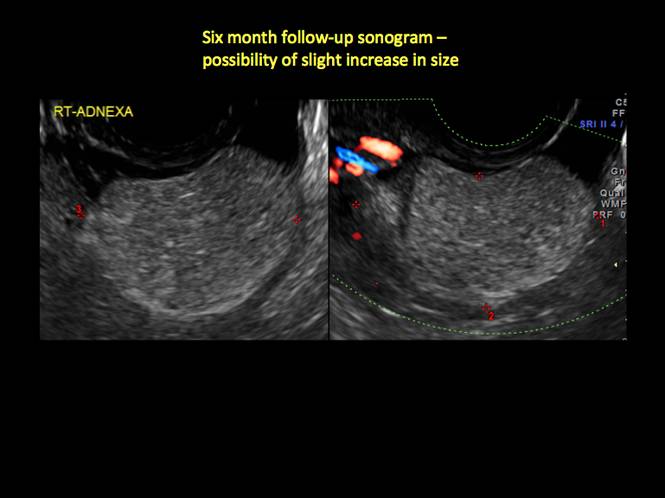
Figure 13
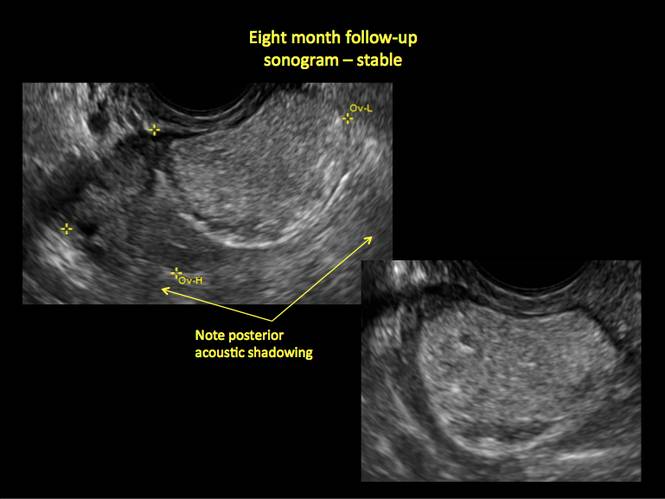
Figure 14
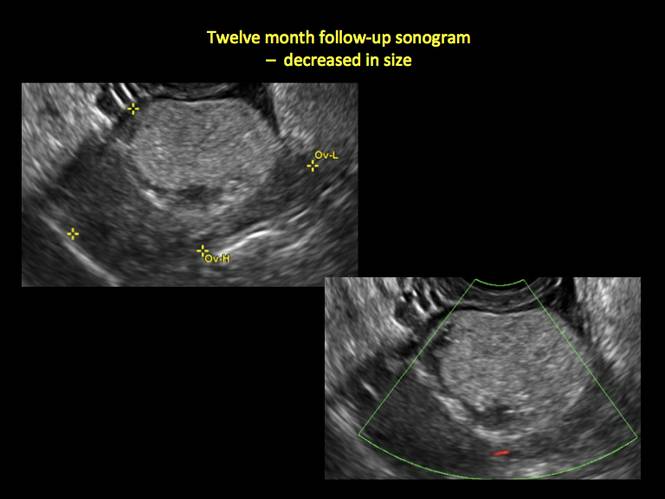
Figure 15
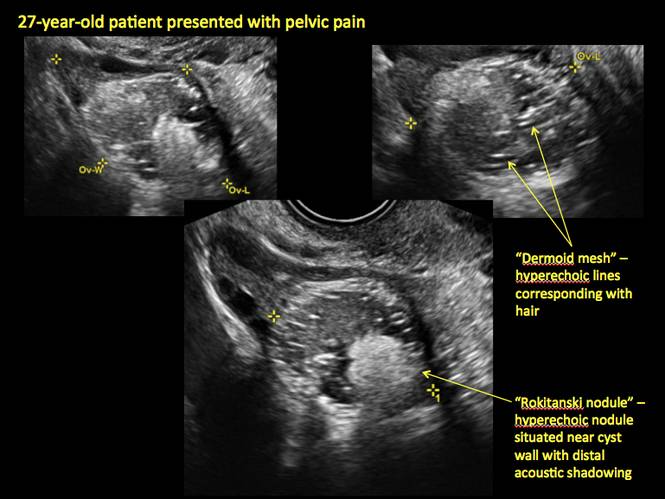
Figure 16
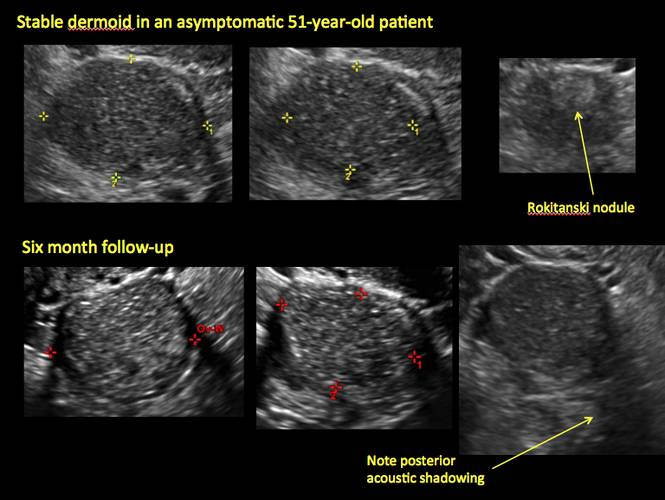
Figure 17
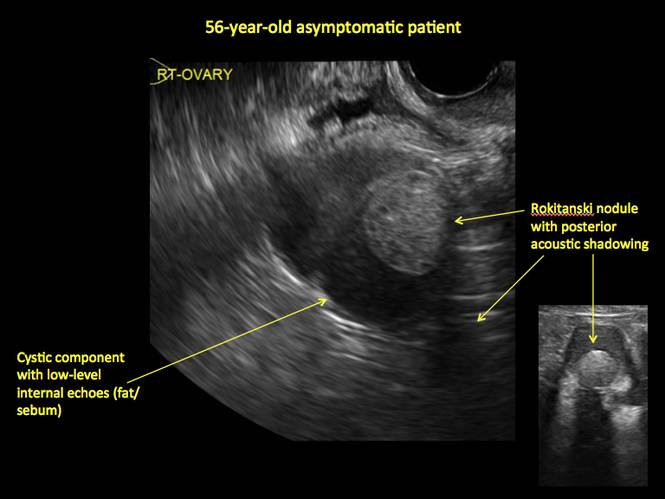
Figure 18
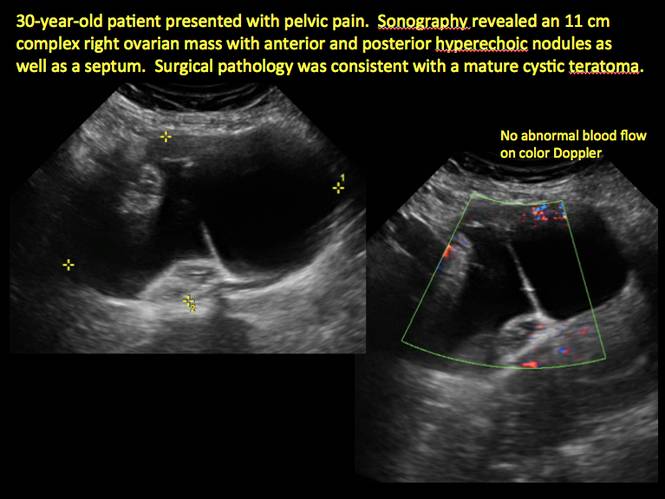
Figure 19
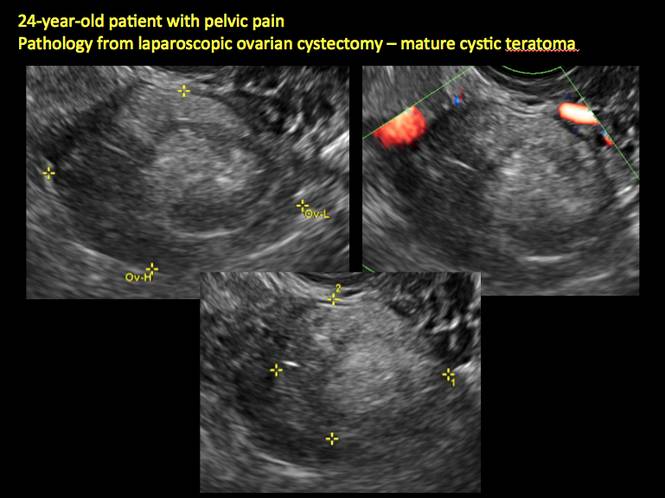
Figure 20
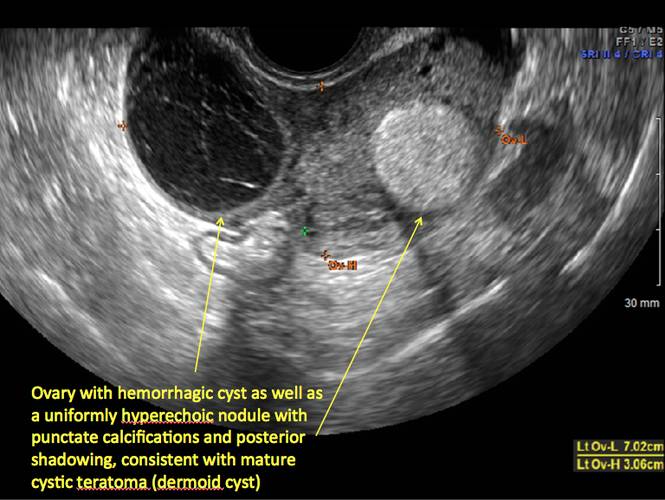
Figure 21
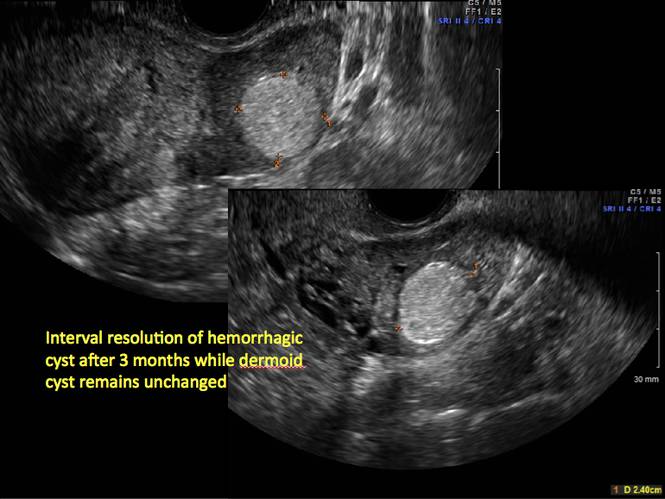
Figure 22
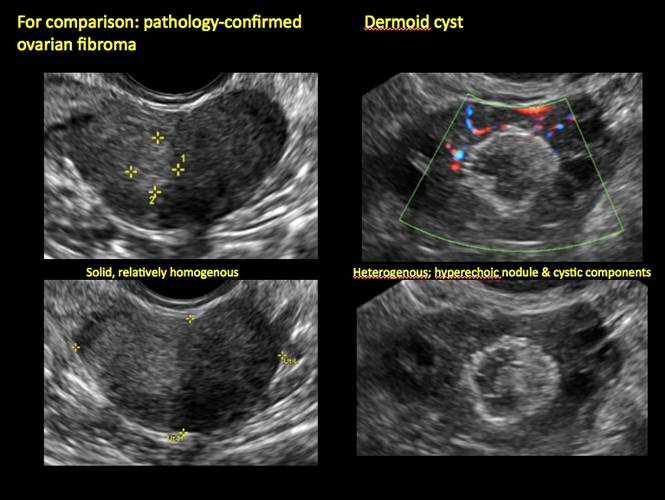
Figure 23
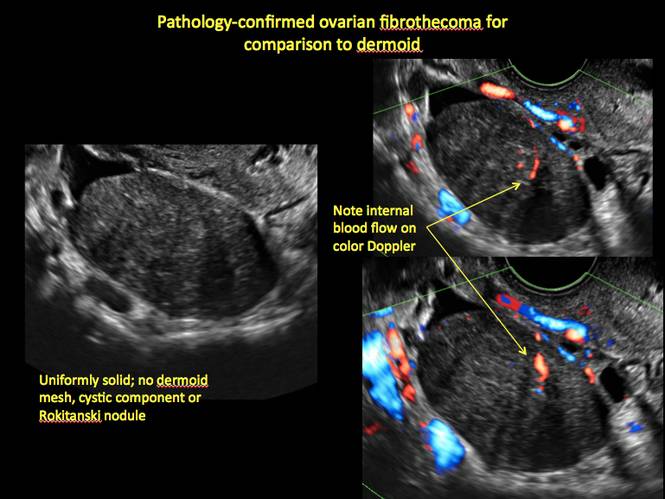
Figure 24
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943–954.
2. Hoo WL, Yazbek WL, Holland T, Mavrelos D, Tong EN, Jurkovic D. Expectant management of ultrasonically diagnosed ovarian dermoid cysts: is it possible to predict outcome? Ultrasound Obstet Gynecol. 2010;36(2): 235–240.
The preferred imaging method to evaluate the majority of adnexal cysts is ultrasonography, which can help characterize the cyst type. Common benign adnexal cyst types include simple, hemorrhagic, endometrioma, and mature teratoma (dermoid cyst). In this part 2 of a 4-part series on cystic adnexal pathology, we focus on imaging signs for, and follow-up of, endometriomas and mature teratomas.
Endometriomas
Endometriomas are common, typically benign, cysts that produce homogenous, low-level internal echoes and a “ground glass” appearance on ultrasonography. No internal flow is apparent on color Doppler. The presence of tiny echogenic wall foci can distinguish an endometrioma from a hemorrhagic cyst.
Rarely, endometriomas may undergo malignant transformation. Usually this occurs with cysts greater than 9 cm and in patients aged 45 years or older. A malignancy often exhibits rapid growth or the development of a solid nodule with flow on color Doppler.
Management
Although surgery remains the first-line management for women with symptomatic or enlarging endometriomas, there appears to be a role for sonographic observation, with continuous progestational treatment, in women with small (< 5 cm) asymptomatic endometriomas.
The Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommended1:
- Short-interval follow-up (6 to 12 weeks) in reproductive-aged women to ensure acute hemorrhagic cysts are not mistaken for endometriomas
- If not removed surgically, sonographic follow-up is recommended, with frequency of follow up based on patient age and symptoms and cyst size and characteristics.
In FIGURES 1 through 11 (slides of image collections), we present several cases, including one of a 25-year-old patient presenting with pelvic pain and dyspareunia who was later found to have bilateral endometriomas.
Mature teratomas
Mature cystic teratomas display several telltale signs on imaging, including:
- hyperechoic lines/dots (“dermoid mesh”) corresponding to hair/skeletal components
- “Rokitanski nodule” – a peripherally placed mass of sebum, bones, and hair
- posterior acoustic shadowing
- cystic or floating spherical structures
- no internal flow on color Doppler
Rarely, dermoid cysts may undergo malignant transformation. Usually this occurs in cysts greater than 10 cm and in patients aged 50 years or older. Internal flow on color Doppler, branching, or invasion into adjacent structures can indicate malignancy.
Management
The traditional treatment for dermoid cysts is surgical. However, given the ability for accurate diagnosis with vaginal ultrasonography, there appears to be a role for sonographic observation in asymptomatic women with small dermoids.2
If the cyst is not surgically removed, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommended initial sonographic follow up at no more than 6 months to 1 year to ensure no change in size or internal architecture.1
In FIGURES 12 through 24 below (slides of image collections), we offer imaging from the case presentation and follow-up of a 19-year-old patient with pelvic pain who has a history of ovarian cystectomy for dermoid cyst, as well as 6 additional case illustrations.

Figure 1

Figure 2

Figure 3

Figure 4

Figure 5

Figure 6

Figure 7

Figure 8

Figure 9

Figure 10

Figure 11

Figure 12

Figure 13

Figure 14

Figure 15

Figure 16

Figure 17

Figure 18

Figure 19

Figure 20

Figure 21

Figure 22

Figure 23

Figure 24
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The preferred imaging method to evaluate the majority of adnexal cysts is ultrasonography, which can help characterize the cyst type. Common benign adnexal cyst types include simple, hemorrhagic, endometrioma, and mature teratoma (dermoid cyst). In this part 2 of a 4-part series on cystic adnexal pathology, we focus on imaging signs for, and follow-up of, endometriomas and mature teratomas.
Endometriomas
Endometriomas are common, typically benign, cysts that produce homogenous, low-level internal echoes and a “ground glass” appearance on ultrasonography. No internal flow is apparent on color Doppler. The presence of tiny echogenic wall foci can distinguish an endometrioma from a hemorrhagic cyst.
Rarely, endometriomas may undergo malignant transformation. Usually this occurs with cysts greater than 9 cm and in patients aged 45 years or older. A malignancy often exhibits rapid growth or the development of a solid nodule with flow on color Doppler.
Management
Although surgery remains the first-line management for women with symptomatic or enlarging endometriomas, there appears to be a role for sonographic observation, with continuous progestational treatment, in women with small (< 5 cm) asymptomatic endometriomas.
The Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommended1:
- Short-interval follow-up (6 to 12 weeks) in reproductive-aged women to ensure acute hemorrhagic cysts are not mistaken for endometriomas
- If not removed surgically, sonographic follow-up is recommended, with frequency of follow up based on patient age and symptoms and cyst size and characteristics.
In FIGURES 1 through 11 (slides of image collections), we present several cases, including one of a 25-year-old patient presenting with pelvic pain and dyspareunia who was later found to have bilateral endometriomas.
Mature teratomas
Mature cystic teratomas display several telltale signs on imaging, including:
- hyperechoic lines/dots (“dermoid mesh”) corresponding to hair/skeletal components
- “Rokitanski nodule” – a peripherally placed mass of sebum, bones, and hair
- posterior acoustic shadowing
- cystic or floating spherical structures
- no internal flow on color Doppler
Rarely, dermoid cysts may undergo malignant transformation. Usually this occurs in cysts greater than 10 cm and in patients aged 50 years or older. Internal flow on color Doppler, branching, or invasion into adjacent structures can indicate malignancy.
Management
The traditional treatment for dermoid cysts is surgical. However, given the ability for accurate diagnosis with vaginal ultrasonography, there appears to be a role for sonographic observation in asymptomatic women with small dermoids.2
If the cyst is not surgically removed, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommended initial sonographic follow up at no more than 6 months to 1 year to ensure no change in size or internal architecture.1
In FIGURES 12 through 24 below (slides of image collections), we offer imaging from the case presentation and follow-up of a 19-year-old patient with pelvic pain who has a history of ovarian cystectomy for dermoid cyst, as well as 6 additional case illustrations.

Figure 1

Figure 2

Figure 3

Figure 4

Figure 5

Figure 6

Figure 7

Figure 8

Figure 9

Figure 10

Figure 11

Figure 12

Figure 13

Figure 14

Figure 15

Figure 16

Figure 17

Figure 18

Figure 19

Figure 20

Figure 21

Figure 22

Figure 23

Figure 24
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943–954.
2. Hoo WL, Yazbek WL, Holland T, Mavrelos D, Tong EN, Jurkovic D. Expectant management of ultrasonically diagnosed ovarian dermoid cysts: is it possible to predict outcome? Ultrasound Obstet Gynecol. 2010;36(2): 235–240.
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943–954.
2. Hoo WL, Yazbek WL, Holland T, Mavrelos D, Tong EN, Jurkovic D. Expectant management of ultrasonically diagnosed ovarian dermoid cysts: is it possible to predict outcome? Ultrasound Obstet Gynecol. 2010;36(2): 235–240.
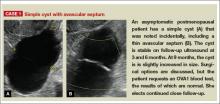
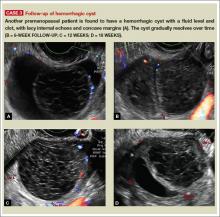
Telltale sonographic features of simple and hemorrhagic cysts
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts, given its ability to accurately characterize their various aspects:
- Simple cysts are uniformly hypoechoic, with thin walls and no blood flow on color Doppler (FIGURE 1).
- Hemorrhagic cysts produce lacy/reticular echoes and clot with concave margins
(FIGURE 2). - Mature cystic teratomas produce hyperechoic lines and dots, sometimes known as “dermoid mesh,” acoustic shadowing, and a hyperechoic nodule (FIGURE 3).
- Endometriomas produce diffuse, low-level internal echoes and a “ground glass” appearance (FIGURE 4).
In the first of this 4-part series on the sonographic features of cystic adnexal pathology, we focus on simple and hemorrhagic cysts. In the following parts we will highlight:
- mature cystic teratomas and endometriomas (Part 2)
- hydrosalpinx and pelvic inclusion cysts (Part 3)
- cystadenoma and ovarian neoplasia (Part 4).
An earlier installment of this series entitled “Hemorrhagic ovarian cysts: one entity with many appearances” (May 2014) also focused on cystic pathology.
Figure 1: Simple cyst
A simple cyst in a 32-year-old patient. Figure 2: Hemorrhagic cyst
Note the lacy/reticular internal echoes and lack of internal blood flow on color Doppler. Figure 3: Cystic teratoma
This cyst exhibits the “dermoid mesh” and hyperechoic lines that correspond with hair. Figure 4: Endometrioma
Note diffuse low-level internal echoes (“ground glass”) and no “ring of fire” on color Doppler. |
Characteristics of simple cysts
A simple cyst typically is round or oval, anechoic, and has smooth, thin walls. It contains no solid component or septation (with rare exceptions), and no internal flow is visible on color Doppler imaging.
Levine and colleagues observed that simple adnexal cysts as large as 10 cm carry a risk of malignancy of less than 1%, regardless of the age of the patient. In its 2010 Consensus Conference Statement,1 the Society of Radiologists in Ultrasound recommended the following management strategies for women with simple cysts:
Reproductive-aged women
- Cyst <3 cm: No action necessary; the cyst is a normal physiologic finding and should be referred to as a follicle.
- 3–5 cm: No follow-up necessary; the cyst is almost certainly benign.
- 5–7 cm: Yearly imaging; the cyst is highly likely to be benign.
- >7 cm: Additional imaging is recommended.
Postmenopausal women
- <1 cm: No follow-up necessary; the cyst is almost certainly benign.
- 1–7 cm: Yearly imaging; the cyst is likely to be benign.
- >7 cm: Additional imaging is recommended.
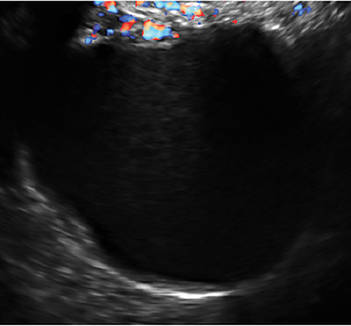
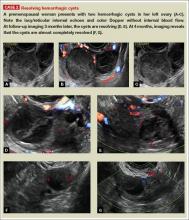
Characteristics of hemorrhagic cysts
These cysts can be quite variable in appearance. Among their sonographic features:
- reticular (lacy, cobweb, or fishnet) internal echoes, due to fibrin strands
- solid-appearing areas with concave margins
- on color Doppler, there may be circumferential peripheral flow (“ring of fire”) and no internal flow.
In its 2010 Consensus Conference Statement, the Society of Radiologists in Ultrasound recommended the following management strategies1:
Premenopausal women
- ≤5 cm: No follow-up imaging unless the diagnosis is uncertain.
- >5 cm: Short-interval follow-up ultrasound (6–12 weeks).
Recently menopausal women
- Any size: Follow-up ultrasound in 6–12 weeks to ensure resolution.
Later postmenopausal women
- Any size: Consider surgical removal, as the cyst may be neoplastic.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943–954.
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts, given its ability to accurately characterize their various aspects:
- Simple cysts are uniformly hypoechoic, with thin walls and no blood flow on color Doppler (FIGURE 1).
- Hemorrhagic cysts produce lacy/reticular echoes and clot with concave margins
(FIGURE 2). - Mature cystic teratomas produce hyperechoic lines and dots, sometimes known as “dermoid mesh,” acoustic shadowing, and a hyperechoic nodule (FIGURE 3).
- Endometriomas produce diffuse, low-level internal echoes and a “ground glass” appearance (FIGURE 4).
In the first of this 4-part series on the sonographic features of cystic adnexal pathology, we focus on simple and hemorrhagic cysts. In the following parts we will highlight:
- mature cystic teratomas and endometriomas (Part 2)
- hydrosalpinx and pelvic inclusion cysts (Part 3)
- cystadenoma and ovarian neoplasia (Part 4).
An earlier installment of this series entitled “Hemorrhagic ovarian cysts: one entity with many appearances” (May 2014) also focused on cystic pathology.
Figure 1: Simple cyst
A simple cyst in a 32-year-old patient. Figure 2: Hemorrhagic cyst
Note the lacy/reticular internal echoes and lack of internal blood flow on color Doppler. Figure 3: Cystic teratoma
This cyst exhibits the “dermoid mesh” and hyperechoic lines that correspond with hair. Figure 4: Endometrioma
Note diffuse low-level internal echoes (“ground glass”) and no “ring of fire” on color Doppler. |
Characteristics of simple cysts
A simple cyst typically is round or oval, anechoic, and has smooth, thin walls. It contains no solid component or septation (with rare exceptions), and no internal flow is visible on color Doppler imaging.
Levine and colleagues observed that simple adnexal cysts as large as 10 cm carry a risk of malignancy of less than 1%, regardless of the age of the patient. In its 2010 Consensus Conference Statement,1 the Society of Radiologists in Ultrasound recommended the following management strategies for women with simple cysts:
Reproductive-aged women
- Cyst <3 cm: No action necessary; the cyst is a normal physiologic finding and should be referred to as a follicle.
- 3–5 cm: No follow-up necessary; the cyst is almost certainly benign.
- 5–7 cm: Yearly imaging; the cyst is highly likely to be benign.
- >7 cm: Additional imaging is recommended.
Postmenopausal women
- <1 cm: No follow-up necessary; the cyst is almost certainly benign.
- 1–7 cm: Yearly imaging; the cyst is likely to be benign.
- >7 cm: Additional imaging is recommended.
Characteristics of hemorrhagic cysts
These cysts can be quite variable in appearance. Among their sonographic features:
- reticular (lacy, cobweb, or fishnet) internal echoes, due to fibrin strands
- solid-appearing areas with concave margins
- on color Doppler, there may be circumferential peripheral flow (“ring of fire”) and no internal flow.
In its 2010 Consensus Conference Statement, the Society of Radiologists in Ultrasound recommended the following management strategies1:
Premenopausal women
- ≤5 cm: No follow-up imaging unless the diagnosis is uncertain.
- >5 cm: Short-interval follow-up ultrasound (6–12 weeks).
Recently menopausal women
- Any size: Follow-up ultrasound in 6–12 weeks to ensure resolution.
Later postmenopausal women
- Any size: Consider surgical removal, as the cyst may be neoplastic.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts, given its ability to accurately characterize their various aspects:
- Simple cysts are uniformly hypoechoic, with thin walls and no blood flow on color Doppler (FIGURE 1).
- Hemorrhagic cysts produce lacy/reticular echoes and clot with concave margins
(FIGURE 2). - Mature cystic teratomas produce hyperechoic lines and dots, sometimes known as “dermoid mesh,” acoustic shadowing, and a hyperechoic nodule (FIGURE 3).
- Endometriomas produce diffuse, low-level internal echoes and a “ground glass” appearance (FIGURE 4).
In the first of this 4-part series on the sonographic features of cystic adnexal pathology, we focus on simple and hemorrhagic cysts. In the following parts we will highlight:
- mature cystic teratomas and endometriomas (Part 2)
- hydrosalpinx and pelvic inclusion cysts (Part 3)
- cystadenoma and ovarian neoplasia (Part 4).
An earlier installment of this series entitled “Hemorrhagic ovarian cysts: one entity with many appearances” (May 2014) also focused on cystic pathology.
Figure 1: Simple cyst
A simple cyst in a 32-year-old patient. Figure 2: Hemorrhagic cyst
Note the lacy/reticular internal echoes and lack of internal blood flow on color Doppler. Figure 3: Cystic teratoma
This cyst exhibits the “dermoid mesh” and hyperechoic lines that correspond with hair. Figure 4: Endometrioma
Note diffuse low-level internal echoes (“ground glass”) and no “ring of fire” on color Doppler. |
Characteristics of simple cysts
A simple cyst typically is round or oval, anechoic, and has smooth, thin walls. It contains no solid component or septation (with rare exceptions), and no internal flow is visible on color Doppler imaging.
Levine and colleagues observed that simple adnexal cysts as large as 10 cm carry a risk of malignancy of less than 1%, regardless of the age of the patient. In its 2010 Consensus Conference Statement,1 the Society of Radiologists in Ultrasound recommended the following management strategies for women with simple cysts:
Reproductive-aged women
- Cyst <3 cm: No action necessary; the cyst is a normal physiologic finding and should be referred to as a follicle.
- 3–5 cm: No follow-up necessary; the cyst is almost certainly benign.
- 5–7 cm: Yearly imaging; the cyst is highly likely to be benign.
- >7 cm: Additional imaging is recommended.
Postmenopausal women
- <1 cm: No follow-up necessary; the cyst is almost certainly benign.
- 1–7 cm: Yearly imaging; the cyst is likely to be benign.
- >7 cm: Additional imaging is recommended.
Characteristics of hemorrhagic cysts
These cysts can be quite variable in appearance. Among their sonographic features:
- reticular (lacy, cobweb, or fishnet) internal echoes, due to fibrin strands
- solid-appearing areas with concave margins
- on color Doppler, there may be circumferential peripheral flow (“ring of fire”) and no internal flow.
In its 2010 Consensus Conference Statement, the Society of Radiologists in Ultrasound recommended the following management strategies1:
Premenopausal women
- ≤5 cm: No follow-up imaging unless the diagnosis is uncertain.
- >5 cm: Short-interval follow-up ultrasound (6–12 weeks).
Recently menopausal women
- Any size: Follow-up ultrasound in 6–12 weeks to ensure resolution.
Later postmenopausal women
- Any size: Consider surgical removal, as the cyst may be neoplastic.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943–954.
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943–954.
Congenital uterine anomalies: A resource of diagnostic images, Part 2
As detailed in Part 1 of this installment on uterine anomalies, a uterus that has developed abnormally can appear to be normal on 2D sonography and on unenhanced sonohysterography (Figure). Without the application of 3D coronal ultrasonography, accurate identification of the fundal contour, and ultimately the type and classification of the uterine anomaly, is not possible.1-3 Fortunately, the lowered cost (compared with magnetic resonance imaging) and the noninvasive nature of this more detailed imaging modality makes its use convenient to both the physician and the patient.
In part 1 of this 2-part installment of our imaging series, we discussed the frequency with which uterine anomalies occur and their types and classifications, as well as offered an imaging library showing the normal endometrial cavity, arcuate uterus, incomplete (partial) uterine septum, and complete uterine septum. Here, we provide two cases demonstrating 3D sonography of the unicornuate, bicornuate, didelphic, and DES-exposed uterus.
A. | B. 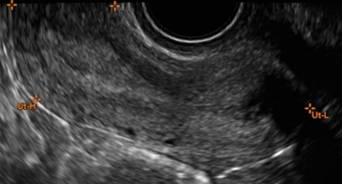
|
C. 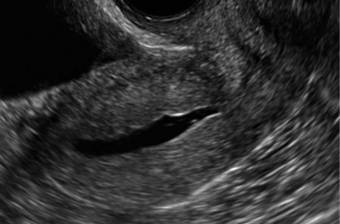
| D. 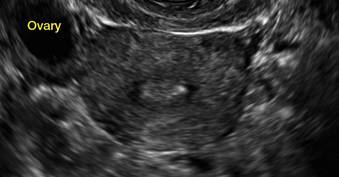
|
E. 
| F. 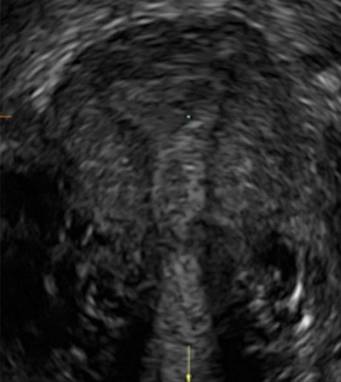
|
G. 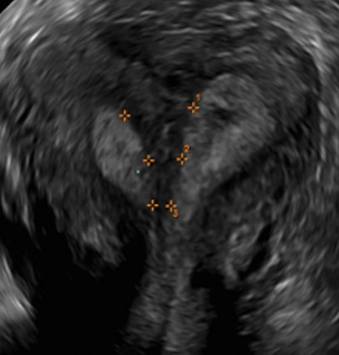
In sagittal view, a uterus with a congenital anomaly can appear normal. Sagittal views of a normal uterus (A) and didelphic uterus (B) and sonohysterogram of a unicornuate uterus (C). Transverse views of a normal (D) and didelphic uterus (E). 3D coronal views of a normal (F) and didelphic uterus (G).
Case 1: Unicornuate uterus
Transverse view of Mirena IUD in right horn and noncommunicating rudimentary left horn.
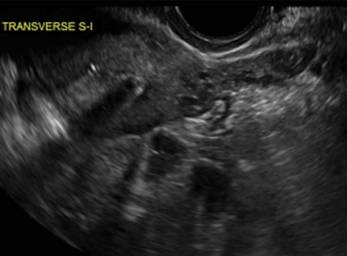
Case 2: Bicornuate uterus, with concave contour
A patient reporting pelvic pain is examined by 2D sonography, which reveals a bicornuate uterus (A). Note the concave fundal contour (arrow), indicating bicornuate uterus, both horns communicating. 3D imaging (B) revealing fundal “dimple” (concave contour, >1 cm), which is indicative of bicornuate uterus. Complete separation of cavities (C).
A. 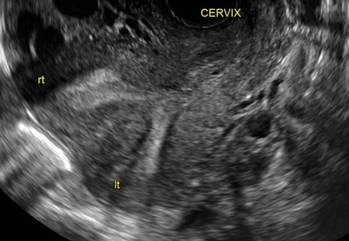
B. 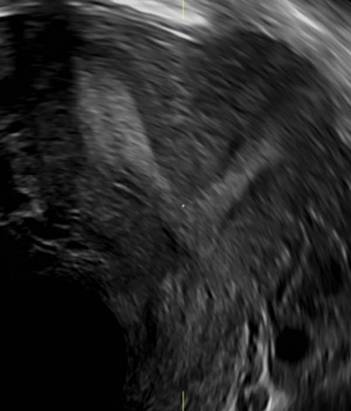
C. 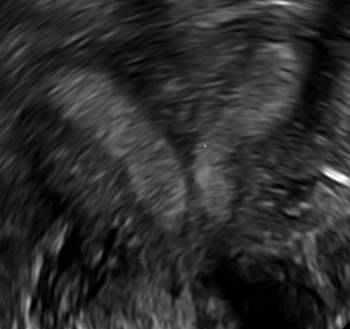
Case 3: Didelphic uterus
A patient presenting with primary infertility is found to have a didelphic uterus on 2D and 3D imaging. Note complete separation of uterine cavities on transverse, 2D views (A and B). The left horn sagittal, 2D view shows a normal appearing uterus (C). 3D imaging (D).
A. 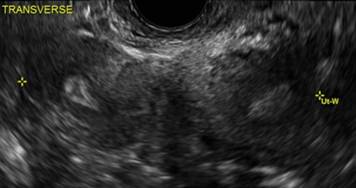
B. 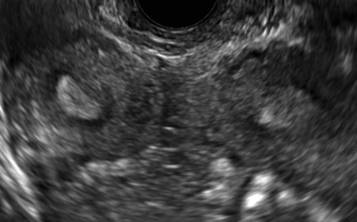
C. 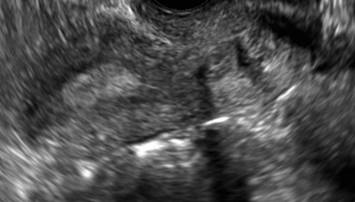
D. 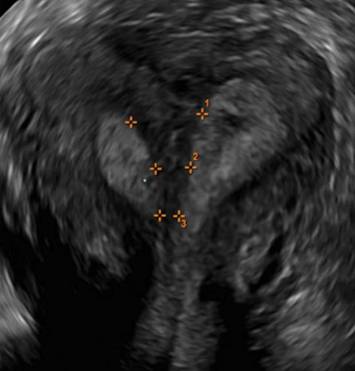
Additional images
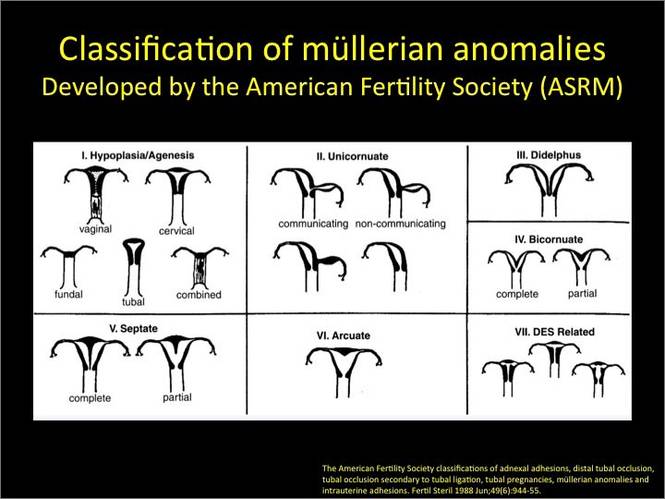
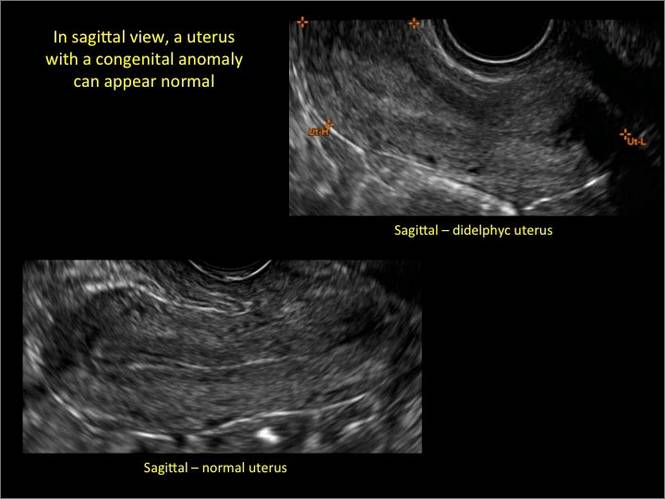
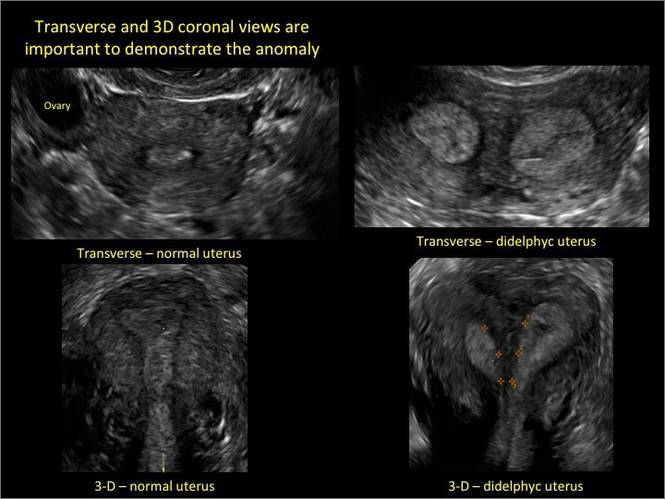
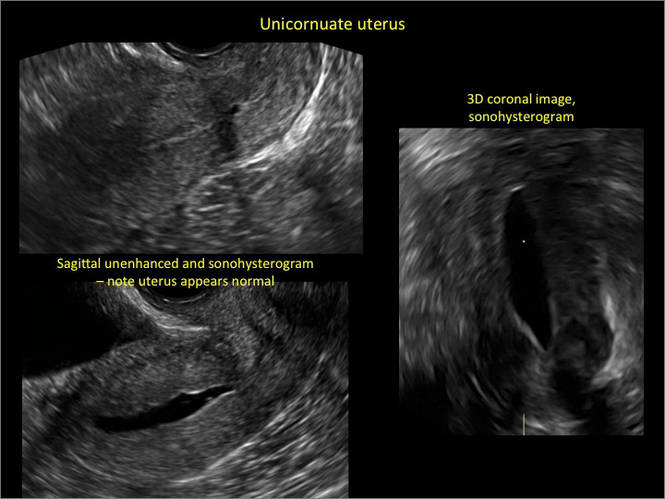
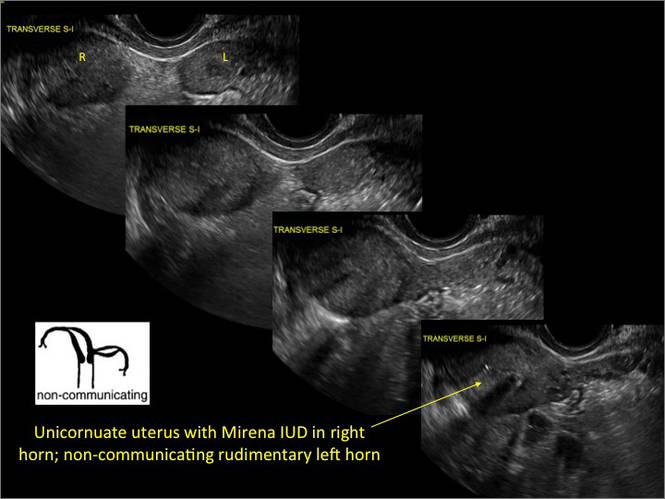
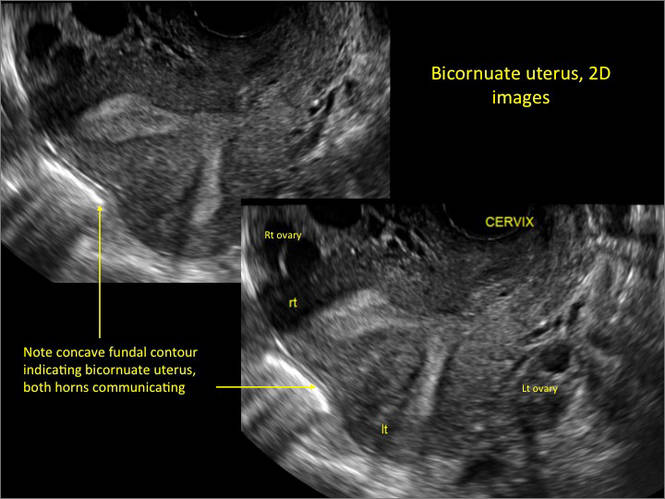
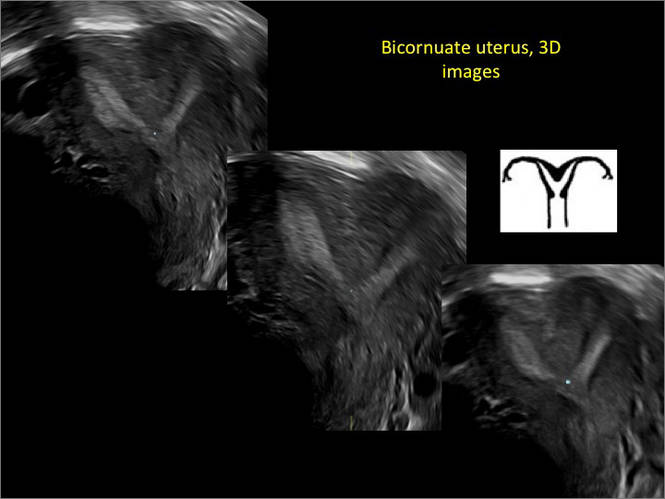
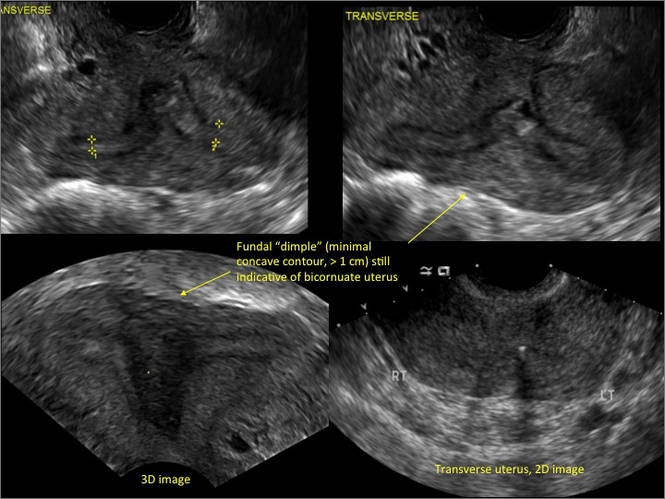
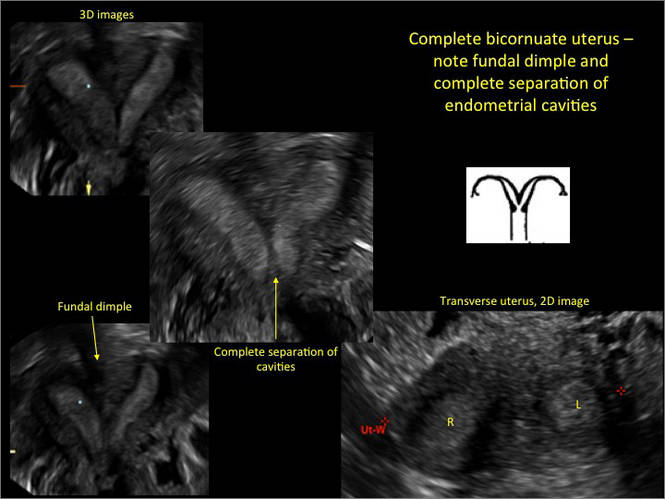
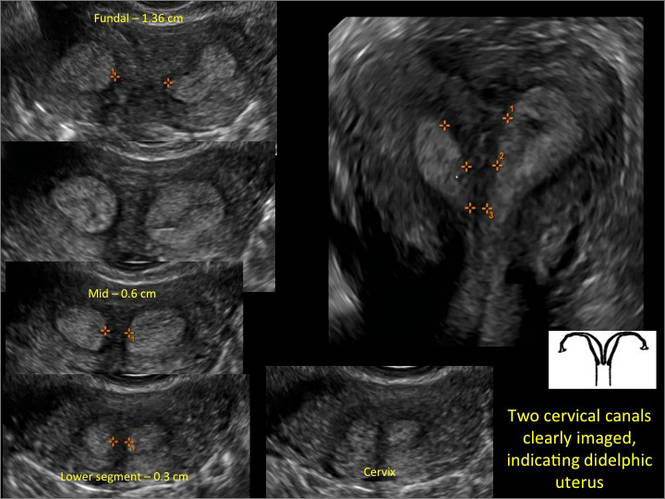

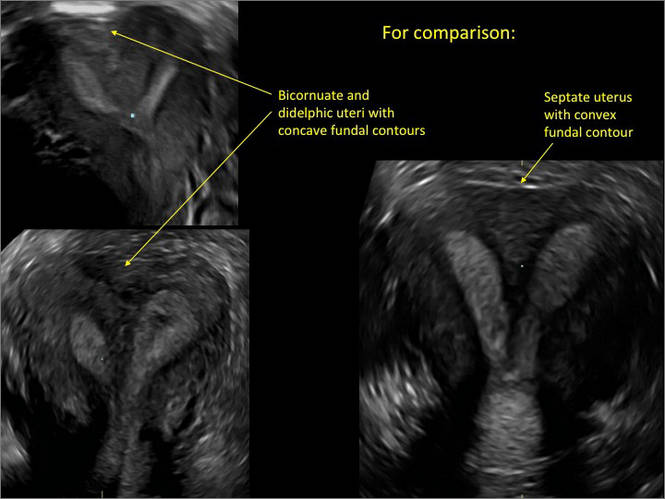
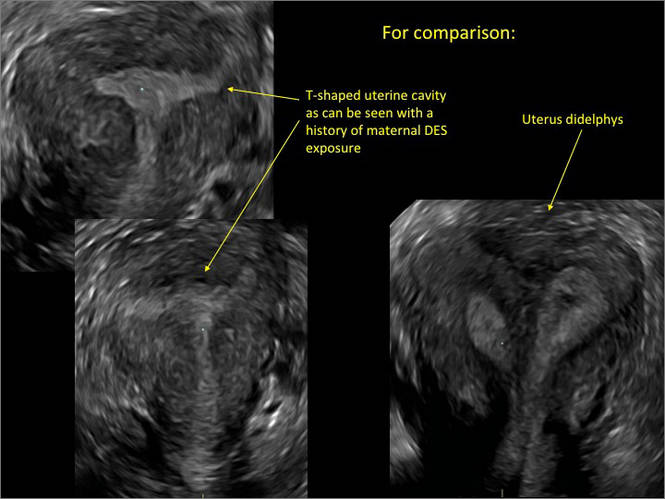
- Deutch T, Bocca S, Oehninger S, et al. Magnetic resonance imaging versus three-dimensional transvaginal ultrasound for the diagnosis of müllerian anomalies [abstract]. Fertil Steril 2006; 86(suppl):S308.15.
- Wu MH, Hsu CC, Huang KE. Detection of congenital müllerian duct anomalies using three-dimensional ultrasound. J Clin Ultrasound 1997; 25:487–492.
- Deutch TD, Abuhamad AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of müllerian duct anomalies: a review of the literature. J Ultrasound Med 2008; 27:413–423.
As detailed in Part 1 of this installment on uterine anomalies, a uterus that has developed abnormally can appear to be normal on 2D sonography and on unenhanced sonohysterography (Figure). Without the application of 3D coronal ultrasonography, accurate identification of the fundal contour, and ultimately the type and classification of the uterine anomaly, is not possible.1-3 Fortunately, the lowered cost (compared with magnetic resonance imaging) and the noninvasive nature of this more detailed imaging modality makes its use convenient to both the physician and the patient.
In part 1 of this 2-part installment of our imaging series, we discussed the frequency with which uterine anomalies occur and their types and classifications, as well as offered an imaging library showing the normal endometrial cavity, arcuate uterus, incomplete (partial) uterine septum, and complete uterine septum. Here, we provide two cases demonstrating 3D sonography of the unicornuate, bicornuate, didelphic, and DES-exposed uterus.
A. | B. 
|
C. 
| D. 
|
E. 
| F. 
|
G. 
In sagittal view, a uterus with a congenital anomaly can appear normal. Sagittal views of a normal uterus (A) and didelphic uterus (B) and sonohysterogram of a unicornuate uterus (C). Transverse views of a normal (D) and didelphic uterus (E). 3D coronal views of a normal (F) and didelphic uterus (G).
Case 1: Unicornuate uterus
Transverse view of Mirena IUD in right horn and noncommunicating rudimentary left horn.

Case 2: Bicornuate uterus, with concave contour
A patient reporting pelvic pain is examined by 2D sonography, which reveals a bicornuate uterus (A). Note the concave fundal contour (arrow), indicating bicornuate uterus, both horns communicating. 3D imaging (B) revealing fundal “dimple” (concave contour, >1 cm), which is indicative of bicornuate uterus. Complete separation of cavities (C).
A. 
B. 
C. 
Case 3: Didelphic uterus
A patient presenting with primary infertility is found to have a didelphic uterus on 2D and 3D imaging. Note complete separation of uterine cavities on transverse, 2D views (A and B). The left horn sagittal, 2D view shows a normal appearing uterus (C). 3D imaging (D).
A. 
B. 
C. 
D. 
Additional images













As detailed in Part 1 of this installment on uterine anomalies, a uterus that has developed abnormally can appear to be normal on 2D sonography and on unenhanced sonohysterography (Figure). Without the application of 3D coronal ultrasonography, accurate identification of the fundal contour, and ultimately the type and classification of the uterine anomaly, is not possible.1-3 Fortunately, the lowered cost (compared with magnetic resonance imaging) and the noninvasive nature of this more detailed imaging modality makes its use convenient to both the physician and the patient.
In part 1 of this 2-part installment of our imaging series, we discussed the frequency with which uterine anomalies occur and their types and classifications, as well as offered an imaging library showing the normal endometrial cavity, arcuate uterus, incomplete (partial) uterine septum, and complete uterine septum. Here, we provide two cases demonstrating 3D sonography of the unicornuate, bicornuate, didelphic, and DES-exposed uterus.
A. | B. 
|
C. 
| D. 
|
E. 
| F. 
|
G. 
In sagittal view, a uterus with a congenital anomaly can appear normal. Sagittal views of a normal uterus (A) and didelphic uterus (B) and sonohysterogram of a unicornuate uterus (C). Transverse views of a normal (D) and didelphic uterus (E). 3D coronal views of a normal (F) and didelphic uterus (G).
Case 1: Unicornuate uterus
Transverse view of Mirena IUD in right horn and noncommunicating rudimentary left horn.

Case 2: Bicornuate uterus, with concave contour
A patient reporting pelvic pain is examined by 2D sonography, which reveals a bicornuate uterus (A). Note the concave fundal contour (arrow), indicating bicornuate uterus, both horns communicating. 3D imaging (B) revealing fundal “dimple” (concave contour, >1 cm), which is indicative of bicornuate uterus. Complete separation of cavities (C).
A. 
B. 
C. 
Case 3: Didelphic uterus
A patient presenting with primary infertility is found to have a didelphic uterus on 2D and 3D imaging. Note complete separation of uterine cavities on transverse, 2D views (A and B). The left horn sagittal, 2D view shows a normal appearing uterus (C). 3D imaging (D).
A. 
B. 
C. 
D. 
Additional images













- Deutch T, Bocca S, Oehninger S, et al. Magnetic resonance imaging versus three-dimensional transvaginal ultrasound for the diagnosis of müllerian anomalies [abstract]. Fertil Steril 2006; 86(suppl):S308.15.
- Wu MH, Hsu CC, Huang KE. Detection of congenital müllerian duct anomalies using three-dimensional ultrasound. J Clin Ultrasound 1997; 25:487–492.
- Deutch TD, Abuhamad AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of müllerian duct anomalies: a review of the literature. J Ultrasound Med 2008; 27:413–423.
- Deutch T, Bocca S, Oehninger S, et al. Magnetic resonance imaging versus three-dimensional transvaginal ultrasound for the diagnosis of müllerian anomalies [abstract]. Fertil Steril 2006; 86(suppl):S308.15.
- Wu MH, Hsu CC, Huang KE. Detection of congenital müllerian duct anomalies using three-dimensional ultrasound. J Clin Ultrasound 1997; 25:487–492.
- Deutch TD, Abuhamad AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of müllerian duct anomalies: a review of the literature. J Ultrasound Med 2008; 27:413–423.