User login
64-year-old woman • hot flashes, facial flushing, excessive sweating, and palpitations • daily headaches • history of hypertension • Dx?
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.
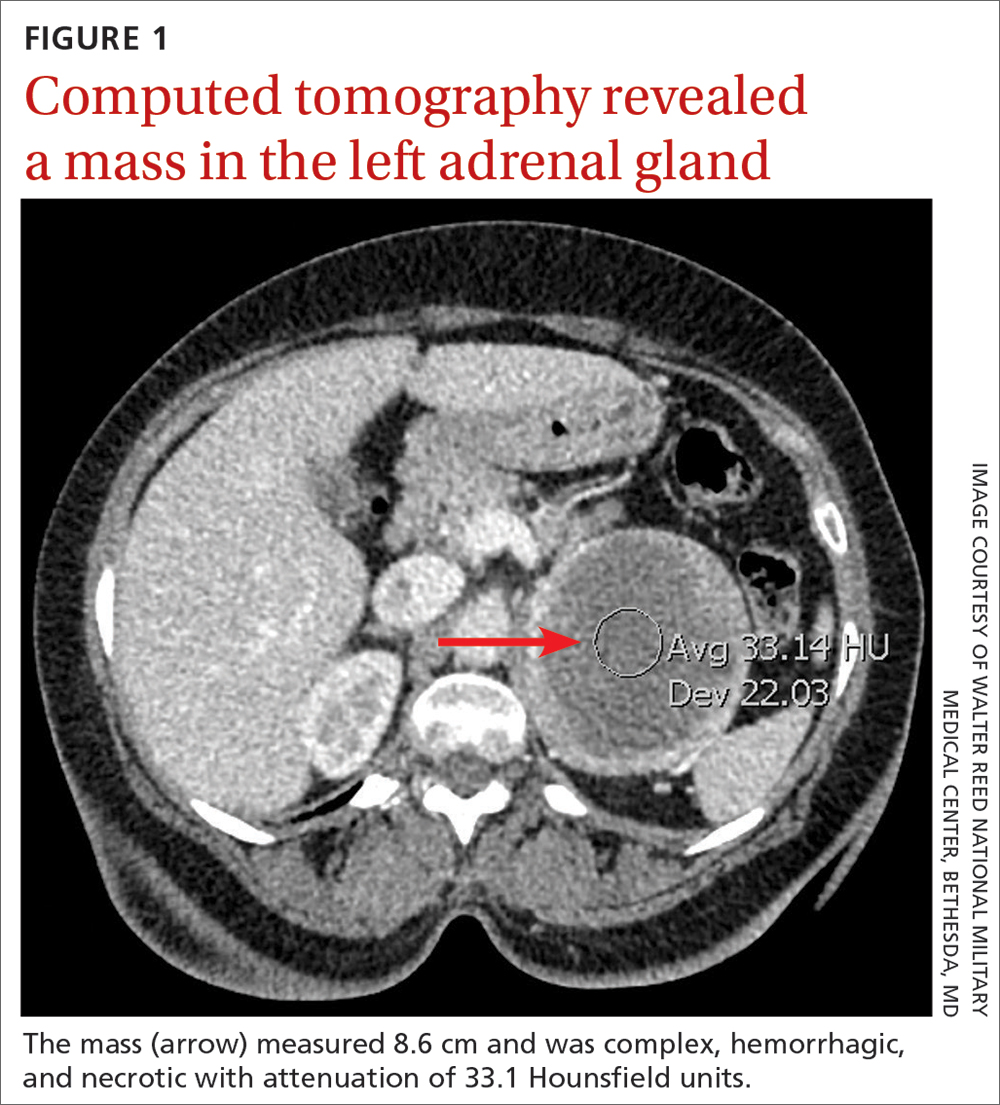
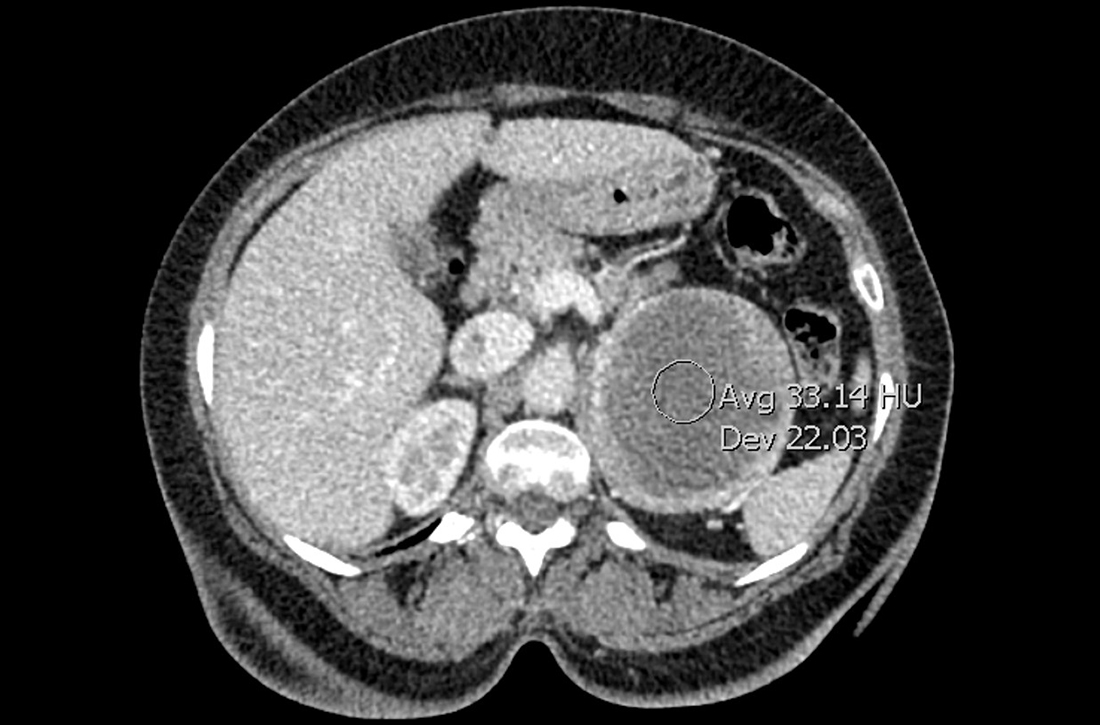
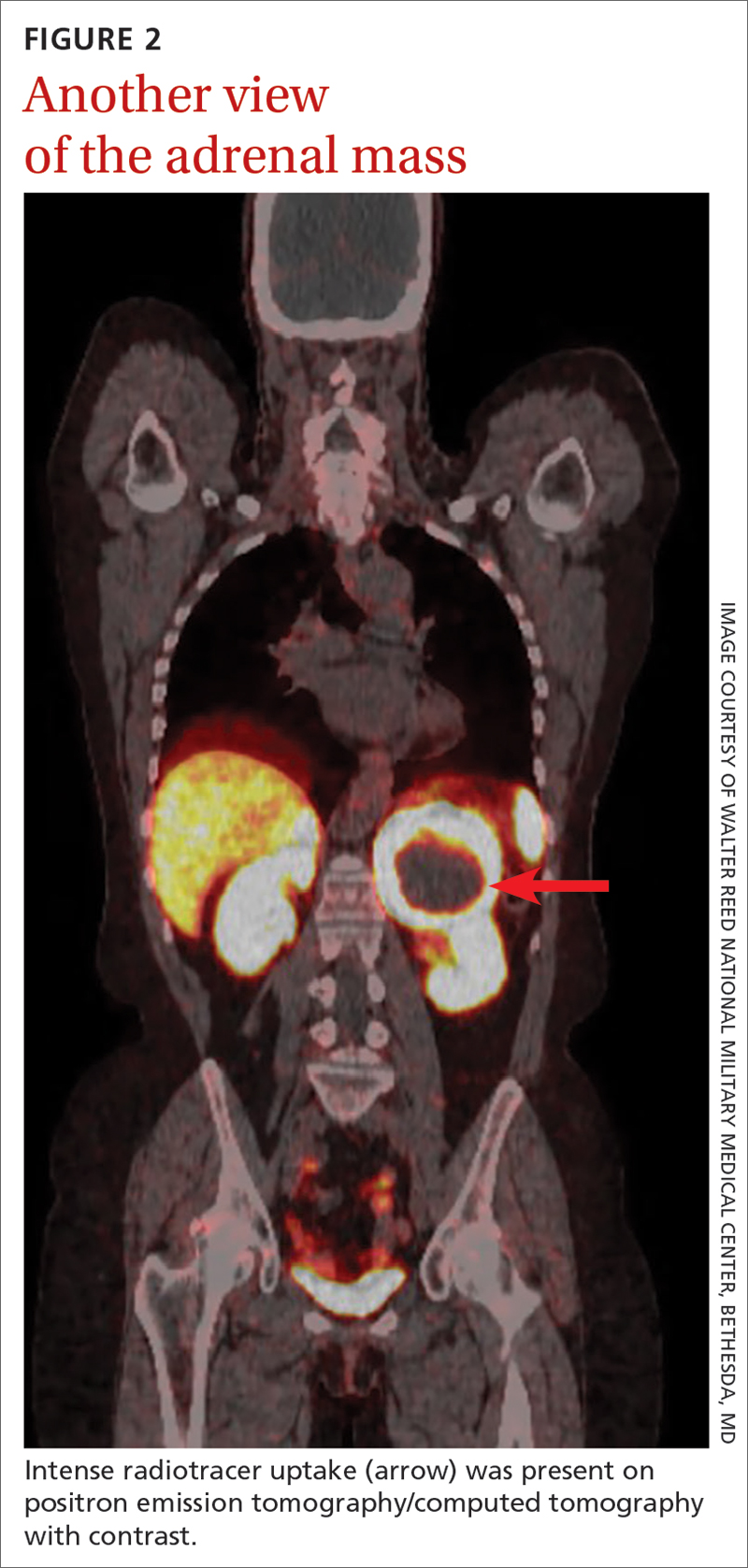
Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).
THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.

Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).

THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.

Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).

THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
► Hot flashes, facial flushing, excessive sweating, and palpitations
► Daily headaches
► History of hypertension
Strategies for improved management of hypothyroidism
The hormones thyroxine (T4) and triiodothyronine (T3), produced by the thyroid gland, are crucial for maintaining metabolism. A deficit of thyroid hormone production—hypothyroidism—is a common endocrine disorder seen in primary care.
Although the diagnosis and management of hypothyroidism are considered straightforward, many patients with hypothyroidism do not achieve optimal treatment goals or see an improvement in their quality of life. In this article, we address the questionable utility of screening; outline the diagnostic approach, including the central role of laboratory testing; and explain why treatment requires a precise approach to address the range of patient types.
Epidemiology and classification
Estimates are that almost 5% of Americans 12 years or older have hypothyroidism; older people and women are more likely to develop the condition. 1 In the US National Health and Nutrition Examination Survey (NHANES III) of 13,344 people without known thyroid disease or a family history, hypothyroidism was found in 4.6% (overt [clinical] in 0.3% and subclinical in 4.3%); 11% had a high serum thyroid peroxidase antibody level, which increases their risk of hypothyroidism, and is treated the same as hypothyroidism of other causes; and, overall, lower serum thyroid-stimulating hormone (TSH) levels were seen in Blacks, compared to Whites and Mexican Americans.1

Primary hypothyroidism accounts for > 95% of cases of hypothyroidism, representing a failure of the thyroid gland to produce sufficient hormone. It has been shown that, in iodine-replete countries such as the United States, the prevalence of spontaneous hypothyroidism is 1% to 2%, and it is 10 times more common in women.2,3
Central hypothyroidism is caused by insufficient stimulation of the thyroid gland by TSH, due to pituitary (secondary hypothyroidism) or hypothalamic (tertiary hypothyroidism) disease and is estimated to occur in 1 in every 20,000 to 80,000 people in the general population.4
How does hypothyroidism manifest?
Signs and symptoms. Manifestations of hypothyroidism range from life-threatening to minimal or no clinical signs and symptoms (TABLE W1). Signs and symptoms of low thyroid function vary by the degree of hypothyroidism at presentation.
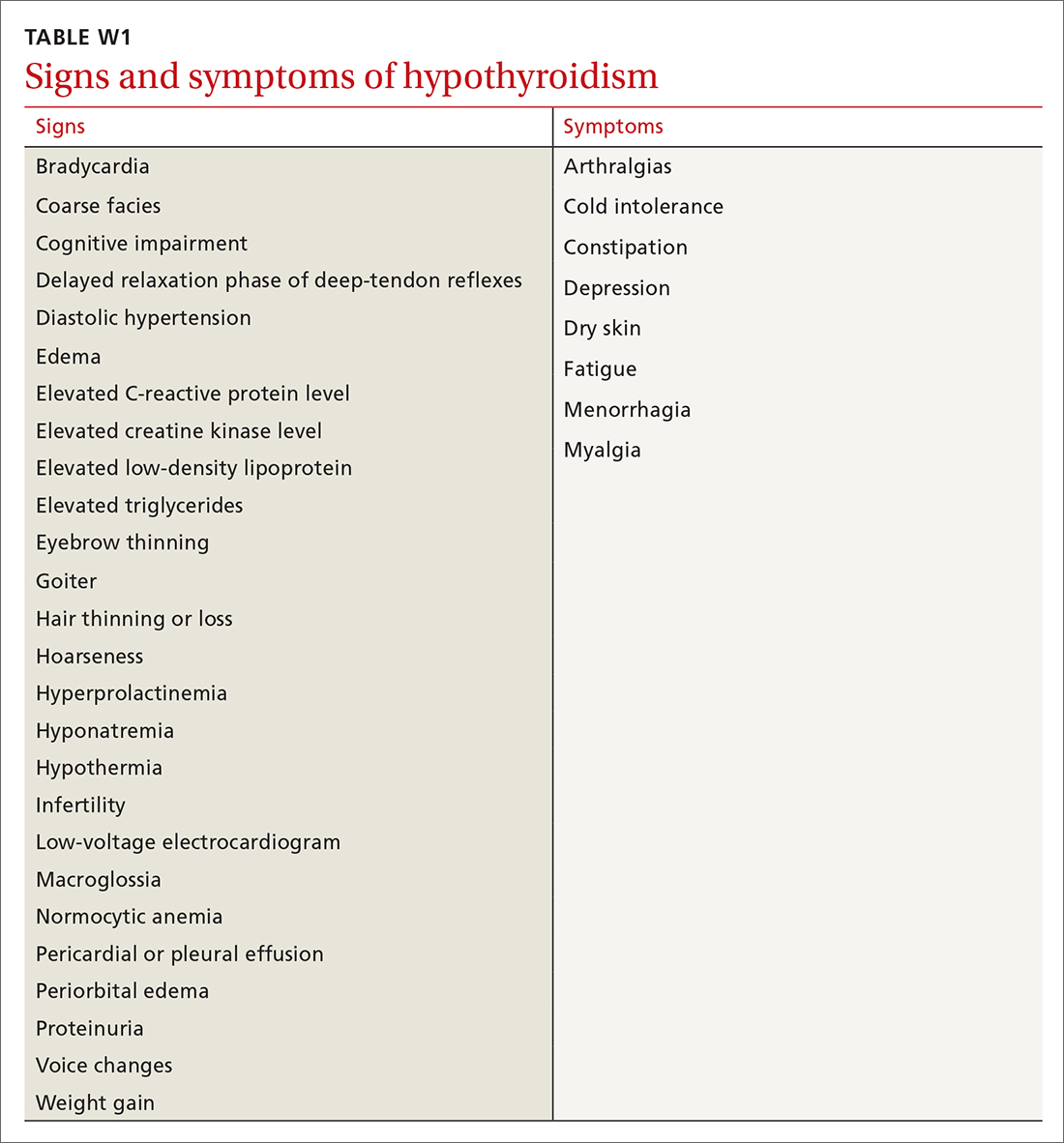
Common signs and symptoms of low thyroid function include fatigue, weight gain, dry skin, brittle hair, hair loss, morning stiffness, muscle aches, joint pain, cold intolerance, diffuse headache, constipation, difficulty concentrating, low libido, depression, and menstrual irregularities. On physical examination, a patient might present with bradycardia, hypotension, hypothermia with slow speech or movement, coarse facial appearance, goiter, diffuse hair loss, cold hands and feet, and a prolonged Achilles tendon reflex.5 Skin findings, such as keratosis pilaris, palmoplantar keratoderma (thickening of the skin), and pityriasis rubra pilar, can be associated with autoimmune hypothyroidism.6,7
Continue to: Carpal tunnel syndrome...
Carpal tunnel syndrome, plantar fasciitis, infertility or miscarriage, dyspepsia, and small intestinal bacterial overgrowth can be associated with hypothyroidism; thyroid function should therefore be assessed in patients who have any of these conditions, along with other signs and symptoms of low thyroid function.8,9 A patient with severe hypothyroidism might present with hemodynamic instability, pericardial or pleural effusion, and myxedema coma.10
Clues in the history and from the lab. A history of radiation to the head, neck, or chest area and a history or family history of autoimmune disorders are risk factors for autoimmune thyroid disease.11,12 Laboratory findings can include markers of oxidative stress, such as elevated levels of low-density lipoprotein cholesterol and serum malondialdehyde.13-15
Screening and diagnosis
Screening. The US Preventive Services Task Force has asserted that evidence is insufficient by which to evaluate the benefits and risks of routine screening for thyroid dysfunction in nonpregnant, asymptomatic adults.16 According to the American Thyroid Association and the American Association of Clinical Endocrinologists, screening should be considered in high-risk patients, including those who take medication that affects thyroid function or the results of thyroid hormone assays (TABLE W2, available at mdedge.com/familymedicine).17-20
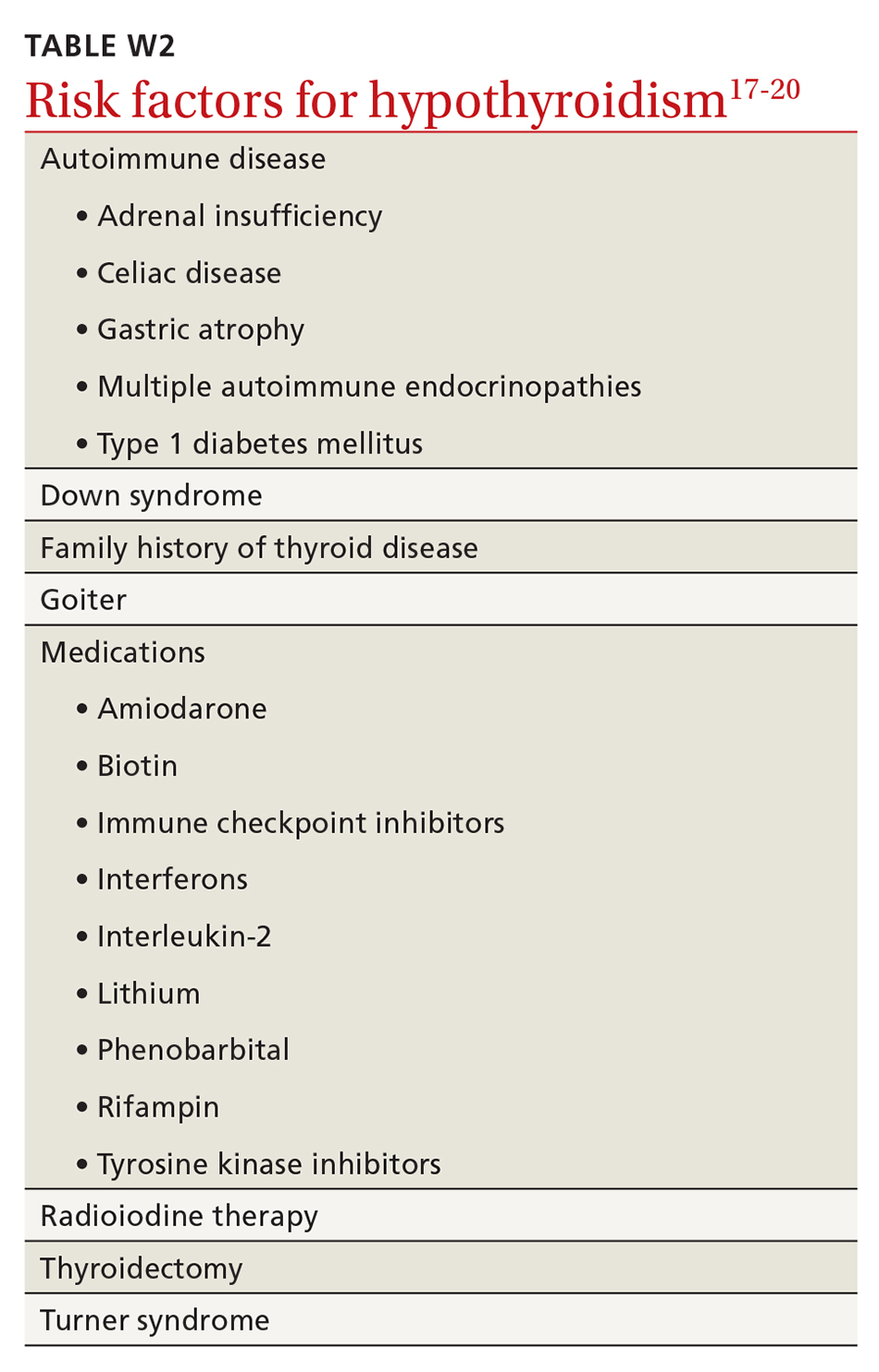
Screening inpatients is challenging and usually not recommended unless thyroid disease is strongly suspected. This is because changes in the levels of thyroid hormones, binding proteins, and the TSH concentration can occur in severe nonthyroidal illness; in addition, assay interference by antibodies and other substances can affect thyroid hormone measurement.21
Testing strategy. Generally, screening and diagnosis of hypothyroidism are based primarily on laboratory testing, because signs and symptoms are nonspecific (FIGURE 15). A serum TSH level is usually the initial test when screening for thyroid dysfunction. A normal serum TSH value ranges from 0.5-5.0 mIU/L.
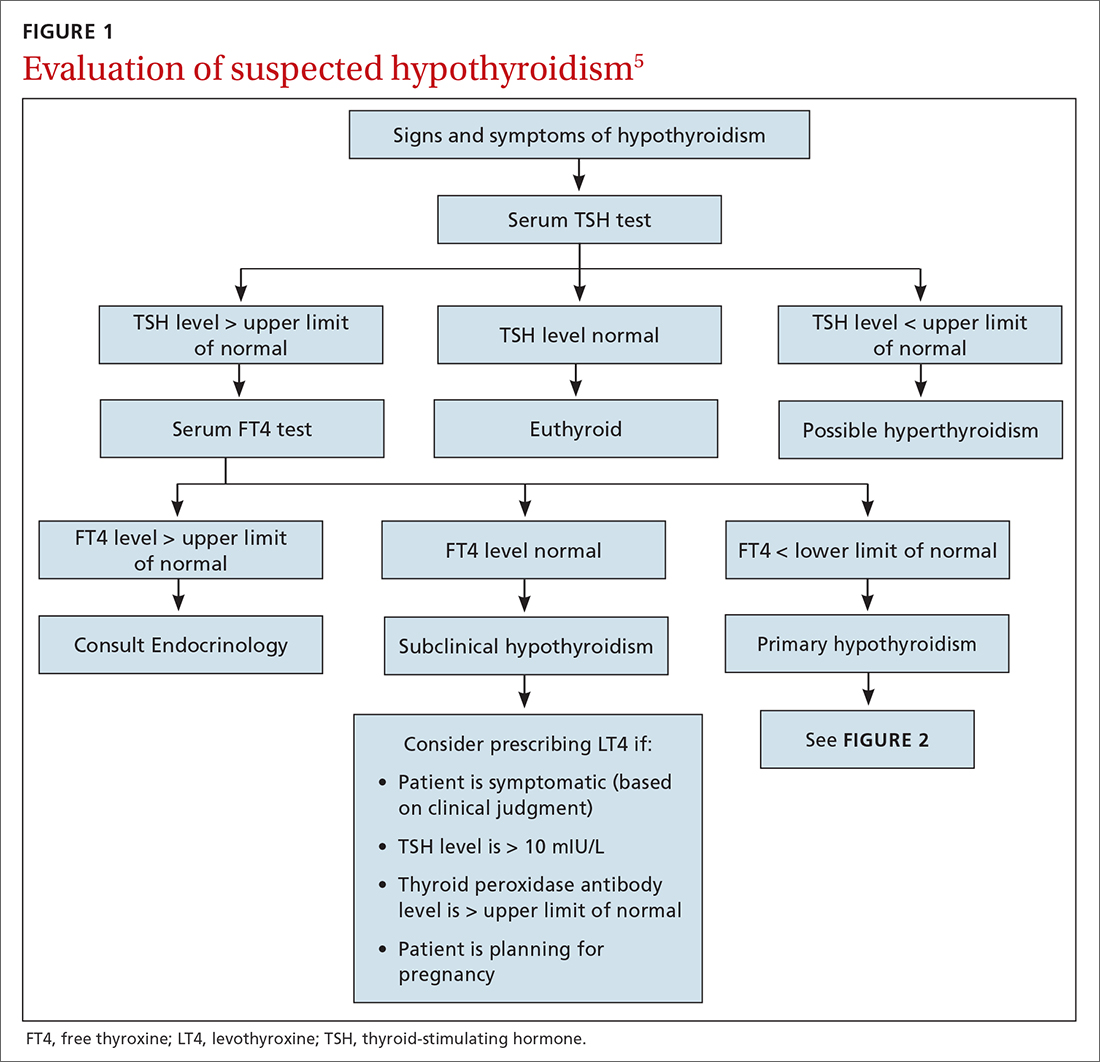
When an abnormal serum TSH level is found, further tests can be performed to investigate, including a serum free thyroxine (FT4) test. (Our preference is to order TSH and FT4 assays simultaneously to facilitate and confirm the diagnosis.) An FT4 test measures the amount of unattached, or free, thyroxine in blood by immunoassay. A normal FT4 value usually ranges from 0.7-1.9 ng/dL.
The combination of a high TSH level and a low FT4 level could be an indication of an underactive thyroid gland (ie, clinical or overt hypothyroidism). Milder, subclinical hypothyroidism is characterized by a higher-than-normal TSH level but a normal FT4 level.22 Central (secondary) hypothyroidism is characterized by a low serum FT4 level and a serum TSH level that can be below the reference range, low normal, or even slightly high.4
Continue to: These measurements...
These measurements must be interpreted within the context of the laboratory-specific normal range for each test. Third-generation serum TSH assays are more sensitive and specific than serum FT4 measurements for hypothyroidism. FT4 is usually measured by automated analogue immunoassay, which generally provides reliable results; abnormal binding proteins or other interferences occur in some patients, however, resulting in reporting of a falsely high, or falsely low, FT4 level. In such cases, FT4 by direct dialysis, or total T4, can be measured for further evaluation. In primary care, you are most likely to encounter primary hypothyroidism; secondary (central) hypothyroidism is much rarer (< 5% of cases).4
The ins and outs of treatment
For most patients, hypothyroidism is a permanent disorder requiring lifelong thyroid hormone replacement therapy—unless the disease is transient (ie, painless or subacute thyroiditis); reversible, because it is caused by medication; or responsive to medical intervention that addresses the underlying autoimmune condition.19 Goals of treatment (Figure 25,23) are to:
- normalize the TSH level to 0.5-5.0 mIU/L (the main goal), with an age-related shift toward a higher TSH goal in older patients (and an upper limit of normal of 7.5 mIU/L in patients who are ≥ 80 years of age)20
- restore the euthyroid state
- relieve symptoms
- reduce any goiter
- avoid overtreatment (iatrogenic thyrotoxicosis).
Desiccated thyroid extract (DTE), developed in the late 1880s and made from the dried thyroid gland of pigs, sheep, or cows, was the earliest treatment for hypothyroidism. The use of DTE has declined since the introduction of synthetic thyroxine (T4, or levothyroxine [here, referred to as LT4]), which is now the standard treatment.20-22 LT4 is deiodinated in peripheral tissues to form T3, the active thyroid hormone; this process accounts for 80% of total T3 production daily.24
LT4 formulations. LT4 is commercially available in tablet, soft-gel, and liquid preparations. Most patients are treated with the tablet; the soft-gel capsule or liquid is an option for patients who absorb the tablet poorly (because of atrophic gastritis, celiac disease, or gluten sensitivity or because they are post bariatric surgery). Increasing the dosage of the tablet form of LT4, with ongoing TSH monitoring, is more cost effective than moving to an alternative preparation.
If a switch of LT4 formulation is made (ie, from one manufacturer to another), test the serum TSH level to ensure that the therapeutic goal is being reached. Also, in our experience, it is best to prescribe a brand-name preparation of levothyroxine, not a generic, whenever possible, due to the variability in generic formulations and the potential presence of other (inert) ingredients.25
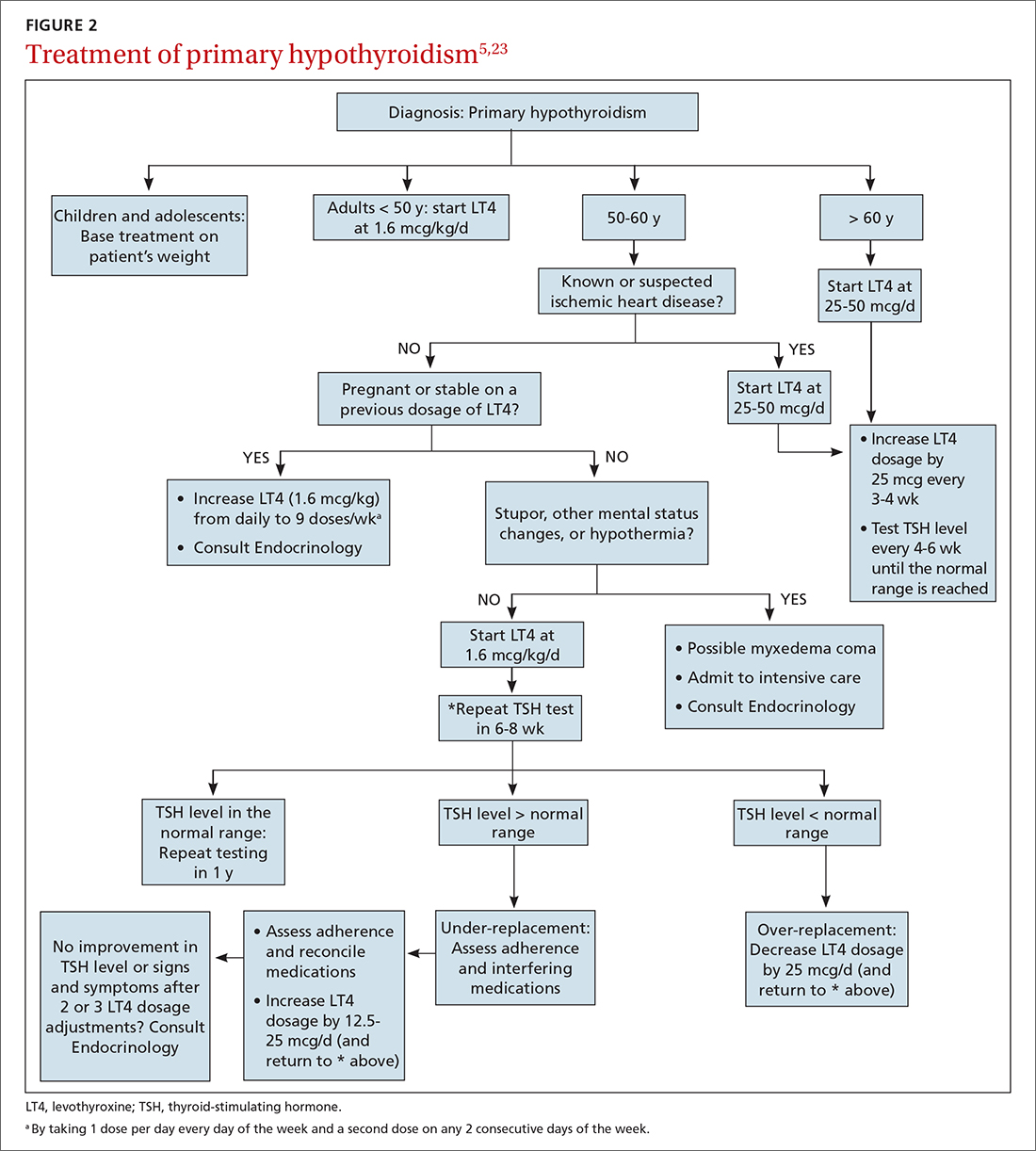
Dosing (TABLE 320,23). The average full replacement dosage of LT4 for a young, healthy adult is approximately 1.6 mcg/kg/d. Older patients (> 65 years) or those with coronary artery disease (CAD) should be started on a lower dosage (25-50 mcg/d) and titrated to goal accordingly.
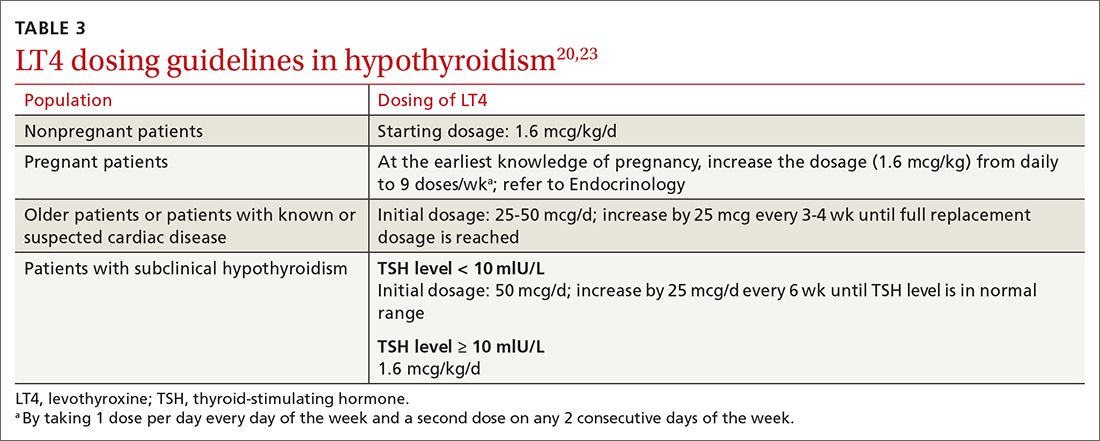
LT4 (tablets, soft-gel capsules, or liquid) should be administered on an empty stomach, with water only, 30 to 60 minutes before breakfast. Medications that interfere with LT4 absorption (eg, bile acid resins, calcium carbonate, ferrous sulfate) should be taken several hours after LT4. For patients who cannot take LT4 in the morning, taking it at bedtime (≥ 2-3 hours after the last meal) is acceptable.
Continue to: Monitoring and titrating
Monitoring and titrating. Hypothyroid symptoms usually improve after 2 or 3 weeks of LT4 treatment; in severe hypothyroidism, complete recovery might take months. Approximately 6 weeks after LT4 therapy is initiated, serum TSH should be measured. After assessing whether administration of LT4 at the starting dosage is appropriate, that dosage can be increased, or decreased, every 4 to 6 weeks until the TSH goal is reached. Once the patient is maintained at a given dosage, measure serum TSH once a year—more often if there is an abnormal result or a change in the patient’s health status.23
Adverse effects of LT4 therapy are rare, unless over-replacement occurs. Rarely, patients have an allergy to the dye or an excipient (filler) in the tablet.26-28 The white, 50-mcg tablets can be given safely to patients with dye sensitivity. For those who have an allergy to an excipient (except gelatin) or gluten intolerance, the LT4 soft-gel capsule or liquid preparation (Tirosint) can be prescribed.
Pure LT4, in a capsule made from vegetable sources, can be ordered through a compounding pharmacy for patients who are allergic to animal products.
Anemia, especially iron-deficiency anemia, can cause intolerance to LT4 therapy; in such patients, lowering the starting dosage and treating anemia are indicated.29
Persistent symptoms (despite a normal TSH level). Because many hypothyroid symptoms are nonspecific, patients might come to think that their LT4 dosage is inadequate if they feel tired or gain weight. Persistent hypothyroid symptoms despite a normal serum TSH level might be due to (1) the inability of LT4 therapy to restore tissue thyroid hormone levels to normal or (2) other variables unrelated to hypothyroidism, including disorders associated with inflammation or autoimmune disease, certain medications, diet, lifestyle, and environmental toxins.
These patients might benefit from a detailed history to identify other causes and a switch to either LT4 + liothyronine (LT3; synthetic T3) combination therapy or DTE26,30-33 (TABLE 434), although a beneficial effect of LT4 + LT3 therapy was not seen in several studies.35,36 Over-replacement with LT4 should be discouraged, due to concerns about thyrotoxicosis and its complications (eg, atrial fibrillation, accelerated bone loss).
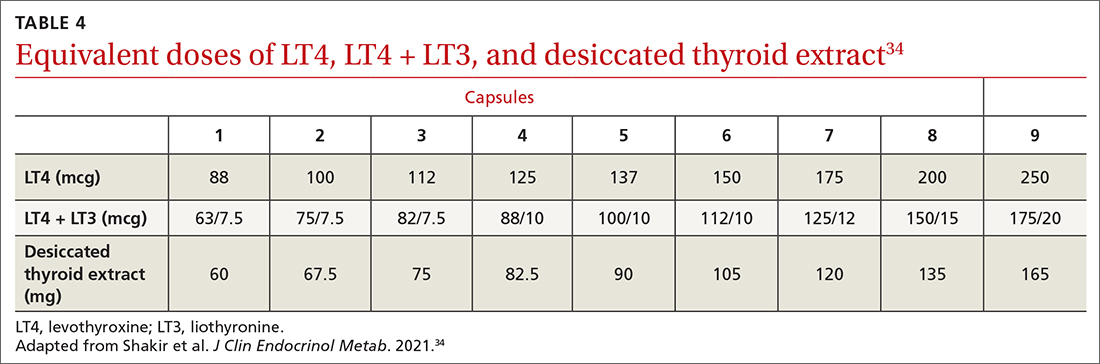
DTE and LT4 + LT3. Use of DTE has decreased since the 1970s, when LT4 became the therapy of choice. Subsequently, anecdotal evidence emerged that some patients did not feel well on LT4 and preferred to return to DTE.32,33
Continue to: Several clinical trials...
Several clinical trials addressed the question of whether residual symptoms could be resolved through LT4 + LT3 combination therapy31-39 (TABLE 434), but evidence of any consistent superiority of combination therapy was not demonstrated.35-39 In selected cases, patients might prefer the combination approach.31,33,39 The quality of life of hypothyroid patients was found to be similarly improved with LT4 or DTE, but the latter was associated with modest weight loss (approximately 4 lbs); nearly 50% of study patients preferred treatment with DTE over LT4.33 A follow-up study did not confirm weight loss with DTE, however.34
When LT4 monotherapy and LT4 + LT3 combination therapy were compared, results were mixed31-39; responsiveness to therapy containing LT3 might therefore depend on multiple variables, including genetic background, nutritional and lifestyle factors, stress, presence of comorbidities and autoimmune disorders, and other unidentified or poorly defined variables.40-48
Although combination therapy and DTE are not generally recommended over LT4 monotherapy, they might offer better options for patients who are still symptomatic when being treated with LT4 only: In a randomized, double-blind, crossover study that compared LT4 with DTE and with LT4 + LT3, one-third of the most highly symptomatic patients who had low scores on mood, cognitive, and quality-of-life assessments improved significantly after they were switched to combination therapy or DTE.34
The 2014 American Thyroid Association guidelines24 do not support routine use of LT4 + LT3 in hypothyroid patients who have residual symptoms after LT4 monotherapy; however, a therapeutic trial of LT4 + LT3, while maintaining a normal serum TSH, is reasonable in selected patients. Candidates for DTE or LT4 + LT3 might include patients who do not feel well on LT4 monotherapy, are post thyroidectomy or post radioiodine therapy, or have a low serum T3 level. DTE and combination therapy are discouraged in older patients, patients who have underlying CAD, and pregnant patients.
Special treatment circumstances
A number of patient variables have the potential to alter management strategies for hypothyroidism.18,20,23,40,49-53
Age, comorbidity. Older patients (> 65 years) and patients with cardiopulmonary disease or CAD should be treated with LT4, 25 to 50 mcg/d, initially; that dosage can be titrated upward by 12.5 to 25 mcg/d every 4 to 6 weeks until the TSH goal is reached—preferably, in the range of 4 to 8 mIU/L. An increase in the dosage of LT4 might be required in the presence of malabsorption (eg, gastrointestinal disorders, celiac disease) and in nephrotic syndrome.18,20,23
Body weight. A decrease in the dosage of LT4 might be indicated in the setting of significant weight loss (> 10% body weight).23
Continue to: Co-pharmacy
Co-pharmacy. An increase in the dosage of LT4 might be required when other drugs (eg, phenytoin, phenobarbital, rifampin, and carbamazepine) have led to an increased rate of thyroid hormone metabolism. A decrease in the dosage of LT4 might be necessary after initiation of androgen therapy.23
Pregnancy. Women with pre-existing hypothyroidism require an increase of 25% to 50% in their LT4 dosage during pregnancy to maintain a TSH level in the recommended pregnancy reference range. Thyroid function should be monitored every 4 to 6 weeks to ensure that the TSH target for each trimester is reached (first trimester, 0.1-4 mIU/L; second trimester, 0.2-4 mIU/L; third trimester, 0.3-4 mIU/L). Postpartum, LT4 can be reduced to the prepartum dosage; TSH should be checked every 4 to 6 weeks to maintain the TSH goal.23
Estrogen therapy. Hypothyroid women who are receiving estrogen therapy might require an increase in their LT4 dosage because serum thyroxine-binding globulin levels are increased by estrogens or through other mechanisms that have not been identified.23
Surgical candidacy. Observational studies show few adverse outcomes in surgical patients with mild (subclinical) hypothyroidism or moderate hypothyroidism; however, the risk of adverse surgical outcome might be increased in patients with severe hypothyroidism. For patients in whom surgery is planned and who have:
- subclinical hypothyroidism (elevated TSH and normal FT4), we recommend that surgery—urgent or elective—not be posptoned but proceed.
- moderate (overt) hypothyroidism who require urgent surgery, we recommend not postponing surgery, even though minor perioperative complications might develop. Such patients should be treated with LT4 as soon as the diagnosis for which surgery is required has been made. Alternatively, when moderate hypothyroidism is discovered in a patient who is being evaluated for elective surgery, we recommend postponing surgery until the euthyroid state is restored.
- severe hypothyroidism (myxedema coma [discussed in a bit]; severe clinical symptoms of chronic hypothyroidism, such as altered mental status, pericardial effusion, or heart failure; or a very low level of T4), surgery should be delayed until hypothyroidism has been treated. When emergency surgery is required for a severely hypothyroid patient, they should be treated with LT4 as soon as the diagnosis for which surgery is indicated has been made. When emergency surgery must be performed in a patient with myxedema coma, we recommend treatment with LT4 + LT3, rather than LT4 alone, often administered intravenously because LT4 is poorly absorbed in these patients.
Nonadherence. For patients who do not take LT4 regularly or do not respond to efforts to improve adherence, LT4 can be given weekly, instead of daily, although this interval is not ideal. Weekly dosing should not be used in older patients with CAD.23
Thyroid cancer. Patients who are post total thyroidectomy for thyroid cancer need to take LT4 to treat hypothyroidism and to prevent recurrence of thyroid cancer. The goal TSH level should be based on the cancer stage and risk of recurrence and should be monitored by an endocrinologist.
Myxedema coma. This medical emergency has high mortality. Myxedema coma occurs when severe hypothyroidism leads to any, or a combination, of the following: diminished mentation; hypothermia; bradycardia; hyponatremia; hypotension; cardiovascular, respiratory, and gastrointestinal dysfunction; and renal insufficiency. LT4, LT3, and glucocorticoids should be administered intravenously and the patient monitored closely—preferably in consultation with an endocrinologist.
Continue to: When to seek consultation
When to seek consultation
A patient with hypothyroidism should be referred to Endocrinology if they are < 18 years of age, pregnant, unresponsive to therapy, or have cardiac disease, coexisting endocrine disease, suspected myxedema coma, goiter or thyroid nodules, or a structural thyroid abnormality.
What we know about nutrition and hypothyroidism
Although it is commonly recognized that iodine is essential for production of thyroid hormone, other nutritional factors might contribute to proper production of thyroid hormones, including:
- adequate intake of iron, tyrosine, selenium, zinc, and vitamins E, B2, B3, B6, C, and D44,45
- selenium and zinc, which increase conversion of T4 to T3 and might be important in the management of hypothyroid patients40,46
- vitamin A, zinc, and regular exercise, which have been shown to improve cellular sensitivity to thyroid hormones.
Low iron stores can contribute to persistent symptoms and poor quality of life in patients with hypothyroidism, despite their being treated according to guidelines.29,47
Despite what is known about these nutritional connections, there is insufficient evidence that improving nutrition can reverse hypothyroidism.
Prevention
Prevention of hypothyroidism should take into account variables that affect or inhibit thyroid function, such as stress, infection (eg, Epstein-Barr virus), excessive fluoride intake, toxins (eg, pesticides, solvents, mercury, cadmium, and lead), autoimmune disease (eg, celiac disease), and food sensitivity.54,55 Oxidative stress can also cause thyroid impairment.40-48,54-58
Otherwise, there are, at present, no effective strategies for preventing thyroid disorders.
Subclinical hypothyroidism: Elusive management target
Subclinical hypothyroidism is defined as a normal serum FT4 level in the presence of an elevated serum TSH level. The prevalence of subclinical hypothyroidism varies from 3% to 15%, depending on the population studied; a higher incidence has been noted in women and older people.59 In the NHANES III,1 which excluded people with previously diagnosed thyroid disease, the incidence of subclinical hypothyroidism was 4.3%.
Continue to: Causes of subclinical...
Causes of subclinical hypothyroidism are the same as those of overt hypothyroidism, and include Hashimoto disease. The combination of an elevated TSH level and a normal FT4 level is associated with disorders characterized by protein-binding variations (eg, pregnancy, genetic disorders, drugs), TSH-secreting pituitary adenoma, class II and III obesity (respectively, body mass index, ≥ 35 but < 40 and ≥ 40), and assay variability.49,51
Lab diagnosis: Fraught with difficulty
The serum TSH level and either the total T4 level or the FT4 level should be measured to make a diagnosis of subclinical hypothyroidism. Most laboratories use a 1-step analogue immunoassay to determine free thyroid hormones; protein-binding variations can thus affect measurement of FT4.
Several scenarios that can result in inaccurate measurement of FT4 by radioimmunoassay include genetic disorders that affect binding proteins; pregnancy; use of certain drugs, including heparin, furosemide, antiepileptic agents, salicylate, ferrous sulfate, and cholesterol-binding resins; and some medical conditions, including cardiac surgery, critical illness, and renal failure. Variables that inhibit proper production of thyroid hormones—stress, infection, fluoride (an iodine antagonist), toxins (pesticides, mercury, cadmium, lead) and autoimmune conditions, such as celiac disease—should be considered when attempting to determine the cause of subclinical hypothyroidism.
Liquid chromatography–mass spectrometry measurement of thyroid hormones might be more accurate than immunoassay.53 Measuring serum total T4 and FT4 by dialysis, free from interfering proteins, might also be useful when measurement of FT4 by immunoassay is affected by binding-protein variations.
Features of subclinical hypothyroidism
Most patients who have subclinical hypothyroidism and a serum TSH level < 10 mIU/L are asymptomatic. Some might have nonspecific symptoms of hypothyroidism, however, such as reduced quality of life, poor cognitive function, and poor memory—symptoms that do not typically correlate with the serum TSH level.
It has been suggested that some elderly people normally have a higher level of serum TSH, and that they might have even a prolonged lifespan.51 Additionally, it has been shown that, in nonpregnant adult patients with subclinical hypothyroidism and a serum TSH level of 4.5 to 10 mIU/L, treatment with LT4 was not associated with improvement in thyroid-related symptoms or general quality of life.52
Treat, or don't treat, subclinical hypothyroidism?
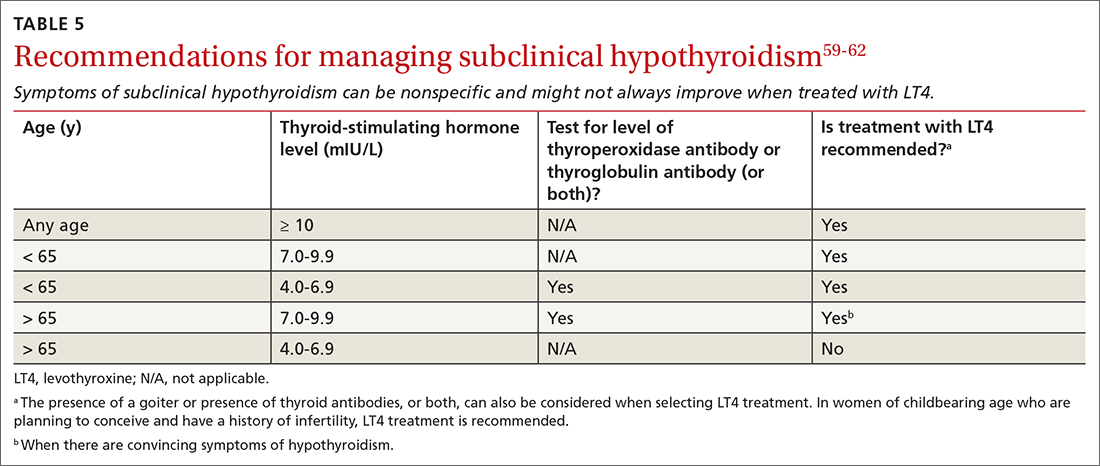
It is well accepted that the goal of therapy in hypothyroid patients is to normalize the serum TSH level; however, the American Thyroid Association and the American Association of Clinical Endocrinology recommend starting LT4 in patients with a serum TSH level ≥ 10 mIU/L (TABLE 5).59-62 The principal reason for not treating subclinical hypothyroidism is the lack of benefit in reducing the risk of cardiovascular morbidity and mortality when the TSH level is between 7.5 and 10 mIU/L.62
Continue to: Routine treatment
Routine treatment of patients with a serum TSH level of 4.5 to 10 mIU/L remains controversial. When TSH is 7.0 to 9.9 mIU/L, treatment is recommended for (1) patients < 65 years and (2) for older patients (> 65 years) only when there are convincing hypothyroid symptoms because of concern about unintended overtreatment.
When the TSH level is anywhere above the upper limit of normal to 6.9 mIU/L, treatment is recommended for patients < 65 years old, patients who have a high titer of thyroid peroxidase antibodies, and patients with goiter—but not for patients > 65 years (and, especially, not for octogenarians) because their upper limit of normal could be as high as 6 to 8 mIU/L, especially if they are otherwise healthy.
Treatment should be considered for women with subclinical hypothyroidism who are trying to conceive or experiencing an infertility problem.
For patients with subclinical hypothyroidism who are not being treated, monitor thyroid function every 6 to 12 months by testing TSH and FT4.
CORRESPONDENCE
Thanh D. Hoang, DO, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499. doi: 10.1210/jcem.87.2.8182
2. Vanderpump MPJ. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39-51. doi: 10.1093/bmb/ldr030
3. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
4. Persani L. Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab. 2012;97:3068-3078. doi: 10.1210/jc.2012-1616
5. Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am. 2012;96:203-221. doi: 10.1016/j.mcna.2012.01.005
6. Ai J, Leonhardt JM, Heymann WR. Autoimmune thyroid diseases: etiology, pathogenesis and dermatologic manifestations. J Am Acad Dermatol. 2003;48:641-659. doi: 10.1067/mjd.2003.257
7. Franzotti AM, Avelar JCD, Cardoso TA, et al. Pityriasis rubra pilar and hypothyroidism. An Bras Dermatol. 2014;89:497-500. doi: 10.1590/abd1806-4841.20142994
8. Yaylali O, Kirac S, Yilmaz M, et al. Does hypothyroidism affect gastrointestinal motility? Gastroenterol Res Pract. 2009;2009:529802. doi: 10.1155/2009/529802
9. Patil AD. Link between hypothyroidism and small intestinal bacterial overgrowth. Indian J Endocrinol Metab. 2014;18:307-309.
10. Ono Y, Ono S, Yasunaga H, et al. Clinical characteristics and outcomes of myxedema coma: analysis of a national inpatient database in Japan. J Epidemiol. 2017;27:117-122. doi: 10.1016/j.je.2016.04.002
11. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5. doi: 10.1016/j.radonc.2011.03.002
12. Boelaert K, Newby PR, Simmonds MJ, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123:183.e1-e9. doi: 10.1016/j.amjmed.2009.06.030
13. Cheserek MJ, Wu G-R, Ntazinda A, et al. Association between thyroid hormones, lipids and oxidative stress markers in subclinical hypothyroidism. J Med Biochem. 2015;34:323-331. doi: 10.2478/jomb-2014-0044
14. Zha K, Zuo C, Wang A, et al. LDL in patients with subclinical hypothyroidism shows increased lipid peroxidation. Lipids Health Dis. 2015;14:95. doi: 10.1186/s12944-015-0092-4
15. Tejovathi B, Suchitra MM, Suresh V, et al. Association of lipid oxidation with endothelial dysfunction in patients with overt hypothyroidism. Exp Clin Endocrinol Diabetes. 2013;121:306-309. doi: 10.1055/s-0032-1333298
16. LeFevre ML; U.S. Preventive Services Task Force. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
17. Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet. 2017;390:1550-1562. doi: 10.1016/S0140-6736(17)30703-1
18. Vaidya B, Pearce SHS. Management of hypothyroidism in adults. BMJ. 2008;337:a801. doi: 10.1136/bmj.a801
19. Iyer PC, Cabanillas ME, Waguespack SG, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. 2018;28:1243-1251. doi: 10.1089/thy.2018.0116
20. Garber JR, Cobin RH, Gharib H, et al; American Association Of Clinical Endocrinologists And American Thyroid Association Taskforce On Hypothyroidism In Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Maiden MJ, Torpy DJ. Thyroid hormones in critical illness. Crit Care Clin. 2019;35:375-388. doi: 10.1016/j.ccc.2018.11.012
22. Peeters RP. Subclinical hypothyroidism. N Engl J Med. 2017;376:2556-2565. doi: 10.1056/NEJMcp1611144
23. Benvenga S, Carlé A. Levothyroxine formulations: pharmacological and clinical implications of generic substitution. Adv Ther. 2019;36(suppl 2):59-71. doi: 10.1007/s12325-019-01079-1
24. Jonklaas J, Bianco AC, Bauer AJ, et al; . Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
25. Engler D, Burger AG. The deiodination of the iodothyronines and of their derivatives in man. Endocr Rev. 1984;5:151-184. doi: 10.1210/edrv-5-2-151
26. Ettleson MD, Bianco AC. Individualized therapy for hypothyroidism: is T4 enough for everyone? J Clin Endocrinol Metab. 2020;105:e3090-e3104. doi: 10.1210/clinem/dgaa430
27. Slayden TA, Shakir MKM, Hoang TD. A bull in a pill shop: alpha-gal allergy complicating treatment options for postprocedural hypothyroidism. AACE Clin Case Rep. 2020;6:e101-e104. doi: 10.4158/ACCR-2019-0495
28. Chamorro-Pareja N, Carrillo-Martin I, Haehn DA, et al. Self-reported allergy to thyroid replacement therapy: a multicenter retrospective chart review. Endocr Pract. 2020;26:761-767. doi: 10.4158/EP-2019-0488
29. Shakir MKM, Turton D, Aprill BS, et al. Anemia: a cause of intolerance to thyroxine sodium. Mayo Clin Proc. 2000;75:189-192.
30. Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid. 2021;31:156-182. doi: 10.1089/thy.2020.0720
31. Appelhof BC, Fliers E, Wekking EM, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab. 2005;90:2666-2674. doi: 10.1210/jc.2004-2111
32. Escobar-Morreale HF, Botella-Carretero JI, M, et al. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med. 2005;142:412-424. doi: 10.7326/0003-4819-142-6-200503150-00007
33. Hoang TD, Olsen CH, Mai VQ, et al. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98:1982-1990. doi: 10.1210/jc.2012-4107
34. Shakir MKM, Brooks DI, McAninch EA, et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine+liothyronine in hypothyroidism. J Clin Endocrinol Metab. 2021;106:e4400-e4413. doi: 10.1210/clinem/dgab478
35. Valizadeh M, Seyyed-Majidi MR, Hajibeigloo H, et al. Efficacy of combined levothyroxine and liothyronine as compared with levothyroxine monotherapy in primary hypothyroidism: a randomized controlled trial. Endocr Res. 2009;34:80-89. doi: 10.1080/07435800903156340
36. Walsh JP, Shiels L, Lim EM, et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab. 2003;88:4543-4550. doi: 10.1210/jc.2003-030249
37. Rodriguez T, Lavis VR, Meininger JC, et al. Substitution of liothyronine at a 1:5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocr Pract. 2005;11:223-233. doi: 10.4158/EP.11.4.223
38. Sawka AM, Gerstein HC, Marriott MJ, et al. Does a combination regimen of thyroxine (T4) and 3,5,3’-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. 2003;88:4551-4555. doi: 10.1210/jc.2003-030139
39. Clyde PW, Harari AE, Getka EJ, et al. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA. 2003;290:2952-2958. doi: 10.1001/jama.290.22.2952
40. Duntas LH. Selenium and the thyroid: a close-knit connection. J Clin Endocrinol Metab. 2010;95:5180-5188. doi: 10.1210/jc.2010-0191
41. Winther KH, Wichman JEM, Bonnema SJ, et al. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine. 2017;55:376-385. doi: 10.1007/s12020-016-1098-z
42. Parva NR, Tadepalli S, Singh P, et al. Prevalence of vitamin D deficiency and associated risk factors in the US population (2011-2012). Cureus. 2018;10:e2741. doi: 10.7759/cureus.2741
43. Wang J, Lv S, Chen G, et al. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. 2015,7:2485-2498. doi: 10.3390/nu7042485
44. Wilson MM, Reedy J, Krebs-Smith SM. American diet quality: where it is, where it is heading, and what it could be. J Acad Nutr Diet. 2016;116:302-310.e1. doi: 10.1016/j.jand.2015.09.020
45. Babiker A, Alawi A, Al Atawi M, et al. The role of micronutrients in thyroid dysfunction. Sudan J Paediatr. 2020;20:13-19. doi: 10.24911/SJP.106-1587138942
46. Knezevic J, Starchl C, Tmava Berisha A, et al. Thyroid-gut-axis: How does the microbiota influence thyroid function? Nutrients. 2020;12:1769. doi: 10.3390/nu12061769
47. Rayman MP. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc Nutr Soc. 2019;78:34-44. doi: 10.1017/S0029665118001192
48. Chakrabarti SK, Ghosh S, Banerjee S, et al. Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian J Endocrinol Metab. 2016;20:674-678. doi: 10.4103/2230-8210.190555
49. Tseng F-Y, Lin W-Y, Lin C-C, et al. Subclinical hypothyroidism is associated with increased risk for all-cause and cardiovascular mortality in adults. J Am Coll Cardiol. 2012;60:730-737. doi: 10.1016/j.jacc.2012.03.047
50. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Intern Med. 2006;145:573-581. doi: 10.7326/0003-4819-145-8-200610170-00006
51. Gussekloo J, van Exel E, de Craen AJM, et al. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591-2599. doi: 10.1001/jama.292.21.2591
52. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
53. Monzani F, Dardano A, Caraccio N. Does treating subclinical hypothyroidism improve markers of cardiovascular risk? Treat Endocrinol. 2006;5:65-81. doi: 10.2165/00024677-200605020-00001
54. Duntas LH. Does celiac disease trigger autoimmune thyroiditis? Nat Rev Endocrinol. 2009;5:190-191. doi: 10.1038/nrendo.2009.46
55. Lerner A, Jeremias P, Matthias T. Gut-thyroid axis and celiac disease. Endocr Connect. 2017;6:R52-R58. doi: 10.1530/EC-17-0021
56. Janegova A, Janega P, Rychly B, et al. The role of Epstein-Barr virus infection in the development of autoimmune thyroid diseases. Endokrynol Pol. 2015;66:132-136. doi: 10.5603/EP.2015.0020
57. Brent GA. Environmental exposures and autoimmune thyroid disease. Thyroid. 2010;20:755-761. doi: 10.1089/thy.2010.1636
58. Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84. doi: 10.1016/j.biocel.2006.07.001
59. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028. doi: 10.4158/EP12280.GL
60. Welsh KJ, Soldin SJ. Diagnosis of endocrine disease: How reliable are free thyroid and total T3 hormone assays? Eur J Endocrinol. 2016;175:R255-R263. doi: 10.1530/EJE-16-0193
61. Grossman A, Feldhamer I, Meyerovitch J. Treatment with levothyroxin in subclinical hypothyroidism is associated with increased mortality in the elderly. Eur J Intern Med. 2018;50:65-68. doi: 10.1016/j.ejim.2017.11.010
62. Pearce SHS, Brabant G, Duntas LH, et al. 2013 ETA Guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
The hormones thyroxine (T4) and triiodothyronine (T3), produced by the thyroid gland, are crucial for maintaining metabolism. A deficit of thyroid hormone production—hypothyroidism—is a common endocrine disorder seen in primary care.
Although the diagnosis and management of hypothyroidism are considered straightforward, many patients with hypothyroidism do not achieve optimal treatment goals or see an improvement in their quality of life. In this article, we address the questionable utility of screening; outline the diagnostic approach, including the central role of laboratory testing; and explain why treatment requires a precise approach to address the range of patient types.
Epidemiology and classification
Estimates are that almost 5% of Americans 12 years or older have hypothyroidism; older people and women are more likely to develop the condition. 1 In the US National Health and Nutrition Examination Survey (NHANES III) of 13,344 people without known thyroid disease or a family history, hypothyroidism was found in 4.6% (overt [clinical] in 0.3% and subclinical in 4.3%); 11% had a high serum thyroid peroxidase antibody level, which increases their risk of hypothyroidism, and is treated the same as hypothyroidism of other causes; and, overall, lower serum thyroid-stimulating hormone (TSH) levels were seen in Blacks, compared to Whites and Mexican Americans.1

Primary hypothyroidism accounts for > 95% of cases of hypothyroidism, representing a failure of the thyroid gland to produce sufficient hormone. It has been shown that, in iodine-replete countries such as the United States, the prevalence of spontaneous hypothyroidism is 1% to 2%, and it is 10 times more common in women.2,3
Central hypothyroidism is caused by insufficient stimulation of the thyroid gland by TSH, due to pituitary (secondary hypothyroidism) or hypothalamic (tertiary hypothyroidism) disease and is estimated to occur in 1 in every 20,000 to 80,000 people in the general population.4
How does hypothyroidism manifest?
Signs and symptoms. Manifestations of hypothyroidism range from life-threatening to minimal or no clinical signs and symptoms (TABLE W1). Signs and symptoms of low thyroid function vary by the degree of hypothyroidism at presentation.

Common signs and symptoms of low thyroid function include fatigue, weight gain, dry skin, brittle hair, hair loss, morning stiffness, muscle aches, joint pain, cold intolerance, diffuse headache, constipation, difficulty concentrating, low libido, depression, and menstrual irregularities. On physical examination, a patient might present with bradycardia, hypotension, hypothermia with slow speech or movement, coarse facial appearance, goiter, diffuse hair loss, cold hands and feet, and a prolonged Achilles tendon reflex.5 Skin findings, such as keratosis pilaris, palmoplantar keratoderma (thickening of the skin), and pityriasis rubra pilar, can be associated with autoimmune hypothyroidism.6,7
Continue to: Carpal tunnel syndrome...
Carpal tunnel syndrome, plantar fasciitis, infertility or miscarriage, dyspepsia, and small intestinal bacterial overgrowth can be associated with hypothyroidism; thyroid function should therefore be assessed in patients who have any of these conditions, along with other signs and symptoms of low thyroid function.8,9 A patient with severe hypothyroidism might present with hemodynamic instability, pericardial or pleural effusion, and myxedema coma.10
Clues in the history and from the lab. A history of radiation to the head, neck, or chest area and a history or family history of autoimmune disorders are risk factors for autoimmune thyroid disease.11,12 Laboratory findings can include markers of oxidative stress, such as elevated levels of low-density lipoprotein cholesterol and serum malondialdehyde.13-15
Screening and diagnosis
Screening. The US Preventive Services Task Force has asserted that evidence is insufficient by which to evaluate the benefits and risks of routine screening for thyroid dysfunction in nonpregnant, asymptomatic adults.16 According to the American Thyroid Association and the American Association of Clinical Endocrinologists, screening should be considered in high-risk patients, including those who take medication that affects thyroid function or the results of thyroid hormone assays (TABLE W2, available at mdedge.com/familymedicine).17-20

Screening inpatients is challenging and usually not recommended unless thyroid disease is strongly suspected. This is because changes in the levels of thyroid hormones, binding proteins, and the TSH concentration can occur in severe nonthyroidal illness; in addition, assay interference by antibodies and other substances can affect thyroid hormone measurement.21
Testing strategy. Generally, screening and diagnosis of hypothyroidism are based primarily on laboratory testing, because signs and symptoms are nonspecific (FIGURE 15). A serum TSH level is usually the initial test when screening for thyroid dysfunction. A normal serum TSH value ranges from 0.5-5.0 mIU/L.

When an abnormal serum TSH level is found, further tests can be performed to investigate, including a serum free thyroxine (FT4) test. (Our preference is to order TSH and FT4 assays simultaneously to facilitate and confirm the diagnosis.) An FT4 test measures the amount of unattached, or free, thyroxine in blood by immunoassay. A normal FT4 value usually ranges from 0.7-1.9 ng/dL.
The combination of a high TSH level and a low FT4 level could be an indication of an underactive thyroid gland (ie, clinical or overt hypothyroidism). Milder, subclinical hypothyroidism is characterized by a higher-than-normal TSH level but a normal FT4 level.22 Central (secondary) hypothyroidism is characterized by a low serum FT4 level and a serum TSH level that can be below the reference range, low normal, or even slightly high.4
Continue to: These measurements...
These measurements must be interpreted within the context of the laboratory-specific normal range for each test. Third-generation serum TSH assays are more sensitive and specific than serum FT4 measurements for hypothyroidism. FT4 is usually measured by automated analogue immunoassay, which generally provides reliable results; abnormal binding proteins or other interferences occur in some patients, however, resulting in reporting of a falsely high, or falsely low, FT4 level. In such cases, FT4 by direct dialysis, or total T4, can be measured for further evaluation. In primary care, you are most likely to encounter primary hypothyroidism; secondary (central) hypothyroidism is much rarer (< 5% of cases).4
The ins and outs of treatment
For most patients, hypothyroidism is a permanent disorder requiring lifelong thyroid hormone replacement therapy—unless the disease is transient (ie, painless or subacute thyroiditis); reversible, because it is caused by medication; or responsive to medical intervention that addresses the underlying autoimmune condition.19 Goals of treatment (Figure 25,23) are to:
- normalize the TSH level to 0.5-5.0 mIU/L (the main goal), with an age-related shift toward a higher TSH goal in older patients (and an upper limit of normal of 7.5 mIU/L in patients who are ≥ 80 years of age)20
- restore the euthyroid state
- relieve symptoms
- reduce any goiter
- avoid overtreatment (iatrogenic thyrotoxicosis).

Desiccated thyroid extract (DTE), developed in the late 1880s and made from the dried thyroid gland of pigs, sheep, or cows, was the earliest treatment for hypothyroidism. The use of DTE has declined since the introduction of synthetic thyroxine (T4, or levothyroxine [here, referred to as LT4]), which is now the standard treatment.20-22 LT4 is deiodinated in peripheral tissues to form T3, the active thyroid hormone; this process accounts for 80% of total T3 production daily.24
LT4 formulations. LT4 is commercially available in tablet, soft-gel, and liquid preparations. Most patients are treated with the tablet; the soft-gel capsule or liquid is an option for patients who absorb the tablet poorly (because of atrophic gastritis, celiac disease, or gluten sensitivity or because they are post bariatric surgery). Increasing the dosage of the tablet form of LT4, with ongoing TSH monitoring, is more cost effective than moving to an alternative preparation.
If a switch of LT4 formulation is made (ie, from one manufacturer to another), test the serum TSH level to ensure that the therapeutic goal is being reached. Also, in our experience, it is best to prescribe a brand-name preparation of levothyroxine, not a generic, whenever possible, due to the variability in generic formulations and the potential presence of other (inert) ingredients.25
Dosing (TABLE 320,23). The average full replacement dosage of LT4 for a young, healthy adult is approximately 1.6 mcg/kg/d. Older patients (> 65 years) or those with coronary artery disease (CAD) should be started on a lower dosage (25-50 mcg/d) and titrated to goal accordingly.

LT4 (tablets, soft-gel capsules, or liquid) should be administered on an empty stomach, with water only, 30 to 60 minutes before breakfast. Medications that interfere with LT4 absorption (eg, bile acid resins, calcium carbonate, ferrous sulfate) should be taken several hours after LT4. For patients who cannot take LT4 in the morning, taking it at bedtime (≥ 2-3 hours after the last meal) is acceptable.
Continue to: Monitoring and titrating
Monitoring and titrating. Hypothyroid symptoms usually improve after 2 or 3 weeks of LT4 treatment; in severe hypothyroidism, complete recovery might take months. Approximately 6 weeks after LT4 therapy is initiated, serum TSH should be measured. After assessing whether administration of LT4 at the starting dosage is appropriate, that dosage can be increased, or decreased, every 4 to 6 weeks until the TSH goal is reached. Once the patient is maintained at a given dosage, measure serum TSH once a year—more often if there is an abnormal result or a change in the patient’s health status.23
Adverse effects of LT4 therapy are rare, unless over-replacement occurs. Rarely, patients have an allergy to the dye or an excipient (filler) in the tablet.26-28 The white, 50-mcg tablets can be given safely to patients with dye sensitivity. For those who have an allergy to an excipient (except gelatin) or gluten intolerance, the LT4 soft-gel capsule or liquid preparation (Tirosint) can be prescribed.
Pure LT4, in a capsule made from vegetable sources, can be ordered through a compounding pharmacy for patients who are allergic to animal products.
Anemia, especially iron-deficiency anemia, can cause intolerance to LT4 therapy; in such patients, lowering the starting dosage and treating anemia are indicated.29
Persistent symptoms (despite a normal TSH level). Because many hypothyroid symptoms are nonspecific, patients might come to think that their LT4 dosage is inadequate if they feel tired or gain weight. Persistent hypothyroid symptoms despite a normal serum TSH level might be due to (1) the inability of LT4 therapy to restore tissue thyroid hormone levels to normal or (2) other variables unrelated to hypothyroidism, including disorders associated with inflammation or autoimmune disease, certain medications, diet, lifestyle, and environmental toxins.
These patients might benefit from a detailed history to identify other causes and a switch to either LT4 + liothyronine (LT3; synthetic T3) combination therapy or DTE26,30-33 (TABLE 434), although a beneficial effect of LT4 + LT3 therapy was not seen in several studies.35,36 Over-replacement with LT4 should be discouraged, due to concerns about thyrotoxicosis and its complications (eg, atrial fibrillation, accelerated bone loss).

DTE and LT4 + LT3. Use of DTE has decreased since the 1970s, when LT4 became the therapy of choice. Subsequently, anecdotal evidence emerged that some patients did not feel well on LT4 and preferred to return to DTE.32,33
Continue to: Several clinical trials...
Several clinical trials addressed the question of whether residual symptoms could be resolved through LT4 + LT3 combination therapy31-39 (TABLE 434), but evidence of any consistent superiority of combination therapy was not demonstrated.35-39 In selected cases, patients might prefer the combination approach.31,33,39 The quality of life of hypothyroid patients was found to be similarly improved with LT4 or DTE, but the latter was associated with modest weight loss (approximately 4 lbs); nearly 50% of study patients preferred treatment with DTE over LT4.33 A follow-up study did not confirm weight loss with DTE, however.34
When LT4 monotherapy and LT4 + LT3 combination therapy were compared, results were mixed31-39; responsiveness to therapy containing LT3 might therefore depend on multiple variables, including genetic background, nutritional and lifestyle factors, stress, presence of comorbidities and autoimmune disorders, and other unidentified or poorly defined variables.40-48
Although combination therapy and DTE are not generally recommended over LT4 monotherapy, they might offer better options for patients who are still symptomatic when being treated with LT4 only: In a randomized, double-blind, crossover study that compared LT4 with DTE and with LT4 + LT3, one-third of the most highly symptomatic patients who had low scores on mood, cognitive, and quality-of-life assessments improved significantly after they were switched to combination therapy or DTE.34
The 2014 American Thyroid Association guidelines24 do not support routine use of LT4 + LT3 in hypothyroid patients who have residual symptoms after LT4 monotherapy; however, a therapeutic trial of LT4 + LT3, while maintaining a normal serum TSH, is reasonable in selected patients. Candidates for DTE or LT4 + LT3 might include patients who do not feel well on LT4 monotherapy, are post thyroidectomy or post radioiodine therapy, or have a low serum T3 level. DTE and combination therapy are discouraged in older patients, patients who have underlying CAD, and pregnant patients.
Special treatment circumstances
A number of patient variables have the potential to alter management strategies for hypothyroidism.18,20,23,40,49-53
Age, comorbidity. Older patients (> 65 years) and patients with cardiopulmonary disease or CAD should be treated with LT4, 25 to 50 mcg/d, initially; that dosage can be titrated upward by 12.5 to 25 mcg/d every 4 to 6 weeks until the TSH goal is reached—preferably, in the range of 4 to 8 mIU/L. An increase in the dosage of LT4 might be required in the presence of malabsorption (eg, gastrointestinal disorders, celiac disease) and in nephrotic syndrome.18,20,23
Body weight. A decrease in the dosage of LT4 might be indicated in the setting of significant weight loss (> 10% body weight).23
Continue to: Co-pharmacy
Co-pharmacy. An increase in the dosage of LT4 might be required when other drugs (eg, phenytoin, phenobarbital, rifampin, and carbamazepine) have led to an increased rate of thyroid hormone metabolism. A decrease in the dosage of LT4 might be necessary after initiation of androgen therapy.23
Pregnancy. Women with pre-existing hypothyroidism require an increase of 25% to 50% in their LT4 dosage during pregnancy to maintain a TSH level in the recommended pregnancy reference range. Thyroid function should be monitored every 4 to 6 weeks to ensure that the TSH target for each trimester is reached (first trimester, 0.1-4 mIU/L; second trimester, 0.2-4 mIU/L; third trimester, 0.3-4 mIU/L). Postpartum, LT4 can be reduced to the prepartum dosage; TSH should be checked every 4 to 6 weeks to maintain the TSH goal.23
Estrogen therapy. Hypothyroid women who are receiving estrogen therapy might require an increase in their LT4 dosage because serum thyroxine-binding globulin levels are increased by estrogens or through other mechanisms that have not been identified.23
Surgical candidacy. Observational studies show few adverse outcomes in surgical patients with mild (subclinical) hypothyroidism or moderate hypothyroidism; however, the risk of adverse surgical outcome might be increased in patients with severe hypothyroidism. For patients in whom surgery is planned and who have:
- subclinical hypothyroidism (elevated TSH and normal FT4), we recommend that surgery—urgent or elective—not be posptoned but proceed.
- moderate (overt) hypothyroidism who require urgent surgery, we recommend not postponing surgery, even though minor perioperative complications might develop. Such patients should be treated with LT4 as soon as the diagnosis for which surgery is required has been made. Alternatively, when moderate hypothyroidism is discovered in a patient who is being evaluated for elective surgery, we recommend postponing surgery until the euthyroid state is restored.
- severe hypothyroidism (myxedema coma [discussed in a bit]; severe clinical symptoms of chronic hypothyroidism, such as altered mental status, pericardial effusion, or heart failure; or a very low level of T4), surgery should be delayed until hypothyroidism has been treated. When emergency surgery is required for a severely hypothyroid patient, they should be treated with LT4 as soon as the diagnosis for which surgery is indicated has been made. When emergency surgery must be performed in a patient with myxedema coma, we recommend treatment with LT4 + LT3, rather than LT4 alone, often administered intravenously because LT4 is poorly absorbed in these patients.
Nonadherence. For patients who do not take LT4 regularly or do not respond to efforts to improve adherence, LT4 can be given weekly, instead of daily, although this interval is not ideal. Weekly dosing should not be used in older patients with CAD.23
Thyroid cancer. Patients who are post total thyroidectomy for thyroid cancer need to take LT4 to treat hypothyroidism and to prevent recurrence of thyroid cancer. The goal TSH level should be based on the cancer stage and risk of recurrence and should be monitored by an endocrinologist.
Myxedema coma. This medical emergency has high mortality. Myxedema coma occurs when severe hypothyroidism leads to any, or a combination, of the following: diminished mentation; hypothermia; bradycardia; hyponatremia; hypotension; cardiovascular, respiratory, and gastrointestinal dysfunction; and renal insufficiency. LT4, LT3, and glucocorticoids should be administered intravenously and the patient monitored closely—preferably in consultation with an endocrinologist.
Continue to: When to seek consultation
When to seek consultation
A patient with hypothyroidism should be referred to Endocrinology if they are < 18 years of age, pregnant, unresponsive to therapy, or have cardiac disease, coexisting endocrine disease, suspected myxedema coma, goiter or thyroid nodules, or a structural thyroid abnormality.
What we know about nutrition and hypothyroidism
Although it is commonly recognized that iodine is essential for production of thyroid hormone, other nutritional factors might contribute to proper production of thyroid hormones, including:
- adequate intake of iron, tyrosine, selenium, zinc, and vitamins E, B2, B3, B6, C, and D44,45
- selenium and zinc, which increase conversion of T4 to T3 and might be important in the management of hypothyroid patients40,46
- vitamin A, zinc, and regular exercise, which have been shown to improve cellular sensitivity to thyroid hormones.
Low iron stores can contribute to persistent symptoms and poor quality of life in patients with hypothyroidism, despite their being treated according to guidelines.29,47
Despite what is known about these nutritional connections, there is insufficient evidence that improving nutrition can reverse hypothyroidism.
Prevention
Prevention of hypothyroidism should take into account variables that affect or inhibit thyroid function, such as stress, infection (eg, Epstein-Barr virus), excessive fluoride intake, toxins (eg, pesticides, solvents, mercury, cadmium, and lead), autoimmune disease (eg, celiac disease), and food sensitivity.54,55 Oxidative stress can also cause thyroid impairment.40-48,54-58
Otherwise, there are, at present, no effective strategies for preventing thyroid disorders.
Subclinical hypothyroidism: Elusive management target
Subclinical hypothyroidism is defined as a normal serum FT4 level in the presence of an elevated serum TSH level. The prevalence of subclinical hypothyroidism varies from 3% to 15%, depending on the population studied; a higher incidence has been noted in women and older people.59 In the NHANES III,1 which excluded people with previously diagnosed thyroid disease, the incidence of subclinical hypothyroidism was 4.3%.
Continue to: Causes of subclinical...
Causes of subclinical hypothyroidism are the same as those of overt hypothyroidism, and include Hashimoto disease. The combination of an elevated TSH level and a normal FT4 level is associated with disorders characterized by protein-binding variations (eg, pregnancy, genetic disorders, drugs), TSH-secreting pituitary adenoma, class II and III obesity (respectively, body mass index, ≥ 35 but < 40 and ≥ 40), and assay variability.49,51
Lab diagnosis: Fraught with difficulty
The serum TSH level and either the total T4 level or the FT4 level should be measured to make a diagnosis of subclinical hypothyroidism. Most laboratories use a 1-step analogue immunoassay to determine free thyroid hormones; protein-binding variations can thus affect measurement of FT4.
Several scenarios that can result in inaccurate measurement of FT4 by radioimmunoassay include genetic disorders that affect binding proteins; pregnancy; use of certain drugs, including heparin, furosemide, antiepileptic agents, salicylate, ferrous sulfate, and cholesterol-binding resins; and some medical conditions, including cardiac surgery, critical illness, and renal failure. Variables that inhibit proper production of thyroid hormones—stress, infection, fluoride (an iodine antagonist), toxins (pesticides, mercury, cadmium, lead) and autoimmune conditions, such as celiac disease—should be considered when attempting to determine the cause of subclinical hypothyroidism.
Liquid chromatography–mass spectrometry measurement of thyroid hormones might be more accurate than immunoassay.53 Measuring serum total T4 and FT4 by dialysis, free from interfering proteins, might also be useful when measurement of FT4 by immunoassay is affected by binding-protein variations.
Features of subclinical hypothyroidism
Most patients who have subclinical hypothyroidism and a serum TSH level < 10 mIU/L are asymptomatic. Some might have nonspecific symptoms of hypothyroidism, however, such as reduced quality of life, poor cognitive function, and poor memory—symptoms that do not typically correlate with the serum TSH level.
It has been suggested that some elderly people normally have a higher level of serum TSH, and that they might have even a prolonged lifespan.51 Additionally, it has been shown that, in nonpregnant adult patients with subclinical hypothyroidism and a serum TSH level of 4.5 to 10 mIU/L, treatment with LT4 was not associated with improvement in thyroid-related symptoms or general quality of life.52
Treat, or don't treat, subclinical hypothyroidism?
It is well accepted that the goal of therapy in hypothyroid patients is to normalize the serum TSH level; however, the American Thyroid Association and the American Association of Clinical Endocrinology recommend starting LT4 in patients with a serum TSH level ≥ 10 mIU/L (TABLE 5).59-62 The principal reason for not treating subclinical hypothyroidism is the lack of benefit in reducing the risk of cardiovascular morbidity and mortality when the TSH level is between 7.5 and 10 mIU/L.62

Continue to: Routine treatment
Routine treatment of patients with a serum TSH level of 4.5 to 10 mIU/L remains controversial. When TSH is 7.0 to 9.9 mIU/L, treatment is recommended for (1) patients < 65 years and (2) for older patients (> 65 years) only when there are convincing hypothyroid symptoms because of concern about unintended overtreatment.
When the TSH level is anywhere above the upper limit of normal to 6.9 mIU/L, treatment is recommended for patients < 65 years old, patients who have a high titer of thyroid peroxidase antibodies, and patients with goiter—but not for patients > 65 years (and, especially, not for octogenarians) because their upper limit of normal could be as high as 6 to 8 mIU/L, especially if they are otherwise healthy.
Treatment should be considered for women with subclinical hypothyroidism who are trying to conceive or experiencing an infertility problem.
For patients with subclinical hypothyroidism who are not being treated, monitor thyroid function every 6 to 12 months by testing TSH and FT4.
CORRESPONDENCE
Thanh D. Hoang, DO, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
The hormones thyroxine (T4) and triiodothyronine (T3), produced by the thyroid gland, are crucial for maintaining metabolism. A deficit of thyroid hormone production—hypothyroidism—is a common endocrine disorder seen in primary care.
Although the diagnosis and management of hypothyroidism are considered straightforward, many patients with hypothyroidism do not achieve optimal treatment goals or see an improvement in their quality of life. In this article, we address the questionable utility of screening; outline the diagnostic approach, including the central role of laboratory testing; and explain why treatment requires a precise approach to address the range of patient types.
Epidemiology and classification
Estimates are that almost 5% of Americans 12 years or older have hypothyroidism; older people and women are more likely to develop the condition. 1 In the US National Health and Nutrition Examination Survey (NHANES III) of 13,344 people without known thyroid disease or a family history, hypothyroidism was found in 4.6% (overt [clinical] in 0.3% and subclinical in 4.3%); 11% had a high serum thyroid peroxidase antibody level, which increases their risk of hypothyroidism, and is treated the same as hypothyroidism of other causes; and, overall, lower serum thyroid-stimulating hormone (TSH) levels were seen in Blacks, compared to Whites and Mexican Americans.1

Primary hypothyroidism accounts for > 95% of cases of hypothyroidism, representing a failure of the thyroid gland to produce sufficient hormone. It has been shown that, in iodine-replete countries such as the United States, the prevalence of spontaneous hypothyroidism is 1% to 2%, and it is 10 times more common in women.2,3
Central hypothyroidism is caused by insufficient stimulation of the thyroid gland by TSH, due to pituitary (secondary hypothyroidism) or hypothalamic (tertiary hypothyroidism) disease and is estimated to occur in 1 in every 20,000 to 80,000 people in the general population.4
How does hypothyroidism manifest?
Signs and symptoms. Manifestations of hypothyroidism range from life-threatening to minimal or no clinical signs and symptoms (TABLE W1). Signs and symptoms of low thyroid function vary by the degree of hypothyroidism at presentation.

Common signs and symptoms of low thyroid function include fatigue, weight gain, dry skin, brittle hair, hair loss, morning stiffness, muscle aches, joint pain, cold intolerance, diffuse headache, constipation, difficulty concentrating, low libido, depression, and menstrual irregularities. On physical examination, a patient might present with bradycardia, hypotension, hypothermia with slow speech or movement, coarse facial appearance, goiter, diffuse hair loss, cold hands and feet, and a prolonged Achilles tendon reflex.5 Skin findings, such as keratosis pilaris, palmoplantar keratoderma (thickening of the skin), and pityriasis rubra pilar, can be associated with autoimmune hypothyroidism.6,7
Continue to: Carpal tunnel syndrome...
Carpal tunnel syndrome, plantar fasciitis, infertility or miscarriage, dyspepsia, and small intestinal bacterial overgrowth can be associated with hypothyroidism; thyroid function should therefore be assessed in patients who have any of these conditions, along with other signs and symptoms of low thyroid function.8,9 A patient with severe hypothyroidism might present with hemodynamic instability, pericardial or pleural effusion, and myxedema coma.10
Clues in the history and from the lab. A history of radiation to the head, neck, or chest area and a history or family history of autoimmune disorders are risk factors for autoimmune thyroid disease.11,12 Laboratory findings can include markers of oxidative stress, such as elevated levels of low-density lipoprotein cholesterol and serum malondialdehyde.13-15
Screening and diagnosis
Screening. The US Preventive Services Task Force has asserted that evidence is insufficient by which to evaluate the benefits and risks of routine screening for thyroid dysfunction in nonpregnant, asymptomatic adults.16 According to the American Thyroid Association and the American Association of Clinical Endocrinologists, screening should be considered in high-risk patients, including those who take medication that affects thyroid function or the results of thyroid hormone assays (TABLE W2, available at mdedge.com/familymedicine).17-20

Screening inpatients is challenging and usually not recommended unless thyroid disease is strongly suspected. This is because changes in the levels of thyroid hormones, binding proteins, and the TSH concentration can occur in severe nonthyroidal illness; in addition, assay interference by antibodies and other substances can affect thyroid hormone measurement.21
Testing strategy. Generally, screening and diagnosis of hypothyroidism are based primarily on laboratory testing, because signs and symptoms are nonspecific (FIGURE 15). A serum TSH level is usually the initial test when screening for thyroid dysfunction. A normal serum TSH value ranges from 0.5-5.0 mIU/L.

When an abnormal serum TSH level is found, further tests can be performed to investigate, including a serum free thyroxine (FT4) test. (Our preference is to order TSH and FT4 assays simultaneously to facilitate and confirm the diagnosis.) An FT4 test measures the amount of unattached, or free, thyroxine in blood by immunoassay. A normal FT4 value usually ranges from 0.7-1.9 ng/dL.
The combination of a high TSH level and a low FT4 level could be an indication of an underactive thyroid gland (ie, clinical or overt hypothyroidism). Milder, subclinical hypothyroidism is characterized by a higher-than-normal TSH level but a normal FT4 level.22 Central (secondary) hypothyroidism is characterized by a low serum FT4 level and a serum TSH level that can be below the reference range, low normal, or even slightly high.4
Continue to: These measurements...
These measurements must be interpreted within the context of the laboratory-specific normal range for each test. Third-generation serum TSH assays are more sensitive and specific than serum FT4 measurements for hypothyroidism. FT4 is usually measured by automated analogue immunoassay, which generally provides reliable results; abnormal binding proteins or other interferences occur in some patients, however, resulting in reporting of a falsely high, or falsely low, FT4 level. In such cases, FT4 by direct dialysis, or total T4, can be measured for further evaluation. In primary care, you are most likely to encounter primary hypothyroidism; secondary (central) hypothyroidism is much rarer (< 5% of cases).4
The ins and outs of treatment
For most patients, hypothyroidism is a permanent disorder requiring lifelong thyroid hormone replacement therapy—unless the disease is transient (ie, painless or subacute thyroiditis); reversible, because it is caused by medication; or responsive to medical intervention that addresses the underlying autoimmune condition.19 Goals of treatment (Figure 25,23) are to:
- normalize the TSH level to 0.5-5.0 mIU/L (the main goal), with an age-related shift toward a higher TSH goal in older patients (and an upper limit of normal of 7.5 mIU/L in patients who are ≥ 80 years of age)20
- restore the euthyroid state
- relieve symptoms
- reduce any goiter
- avoid overtreatment (iatrogenic thyrotoxicosis).

Desiccated thyroid extract (DTE), developed in the late 1880s and made from the dried thyroid gland of pigs, sheep, or cows, was the earliest treatment for hypothyroidism. The use of DTE has declined since the introduction of synthetic thyroxine (T4, or levothyroxine [here, referred to as LT4]), which is now the standard treatment.20-22 LT4 is deiodinated in peripheral tissues to form T3, the active thyroid hormone; this process accounts for 80% of total T3 production daily.24
LT4 formulations. LT4 is commercially available in tablet, soft-gel, and liquid preparations. Most patients are treated with the tablet; the soft-gel capsule or liquid is an option for patients who absorb the tablet poorly (because of atrophic gastritis, celiac disease, or gluten sensitivity or because they are post bariatric surgery). Increasing the dosage of the tablet form of LT4, with ongoing TSH monitoring, is more cost effective than moving to an alternative preparation.
If a switch of LT4 formulation is made (ie, from one manufacturer to another), test the serum TSH level to ensure that the therapeutic goal is being reached. Also, in our experience, it is best to prescribe a brand-name preparation of levothyroxine, not a generic, whenever possible, due to the variability in generic formulations and the potential presence of other (inert) ingredients.25
Dosing (TABLE 320,23). The average full replacement dosage of LT4 for a young, healthy adult is approximately 1.6 mcg/kg/d. Older patients (> 65 years) or those with coronary artery disease (CAD) should be started on a lower dosage (25-50 mcg/d) and titrated to goal accordingly.

LT4 (tablets, soft-gel capsules, or liquid) should be administered on an empty stomach, with water only, 30 to 60 minutes before breakfast. Medications that interfere with LT4 absorption (eg, bile acid resins, calcium carbonate, ferrous sulfate) should be taken several hours after LT4. For patients who cannot take LT4 in the morning, taking it at bedtime (≥ 2-3 hours after the last meal) is acceptable.
Continue to: Monitoring and titrating
Monitoring and titrating. Hypothyroid symptoms usually improve after 2 or 3 weeks of LT4 treatment; in severe hypothyroidism, complete recovery might take months. Approximately 6 weeks after LT4 therapy is initiated, serum TSH should be measured. After assessing whether administration of LT4 at the starting dosage is appropriate, that dosage can be increased, or decreased, every 4 to 6 weeks until the TSH goal is reached. Once the patient is maintained at a given dosage, measure serum TSH once a year—more often if there is an abnormal result or a change in the patient’s health status.23
Adverse effects of LT4 therapy are rare, unless over-replacement occurs. Rarely, patients have an allergy to the dye or an excipient (filler) in the tablet.26-28 The white, 50-mcg tablets can be given safely to patients with dye sensitivity. For those who have an allergy to an excipient (except gelatin) or gluten intolerance, the LT4 soft-gel capsule or liquid preparation (Tirosint) can be prescribed.
Pure LT4, in a capsule made from vegetable sources, can be ordered through a compounding pharmacy for patients who are allergic to animal products.
Anemia, especially iron-deficiency anemia, can cause intolerance to LT4 therapy; in such patients, lowering the starting dosage and treating anemia are indicated.29
Persistent symptoms (despite a normal TSH level). Because many hypothyroid symptoms are nonspecific, patients might come to think that their LT4 dosage is inadequate if they feel tired or gain weight. Persistent hypothyroid symptoms despite a normal serum TSH level might be due to (1) the inability of LT4 therapy to restore tissue thyroid hormone levels to normal or (2) other variables unrelated to hypothyroidism, including disorders associated with inflammation or autoimmune disease, certain medications, diet, lifestyle, and environmental toxins.
These patients might benefit from a detailed history to identify other causes and a switch to either LT4 + liothyronine (LT3; synthetic T3) combination therapy or DTE26,30-33 (TABLE 434), although a beneficial effect of LT4 + LT3 therapy was not seen in several studies.35,36 Over-replacement with LT4 should be discouraged, due to concerns about thyrotoxicosis and its complications (eg, atrial fibrillation, accelerated bone loss).

DTE and LT4 + LT3. Use of DTE has decreased since the 1970s, when LT4 became the therapy of choice. Subsequently, anecdotal evidence emerged that some patients did not feel well on LT4 and preferred to return to DTE.32,33
Continue to: Several clinical trials...
Several clinical trials addressed the question of whether residual symptoms could be resolved through LT4 + LT3 combination therapy31-39 (TABLE 434), but evidence of any consistent superiority of combination therapy was not demonstrated.35-39 In selected cases, patients might prefer the combination approach.31,33,39 The quality of life of hypothyroid patients was found to be similarly improved with LT4 or DTE, but the latter was associated with modest weight loss (approximately 4 lbs); nearly 50% of study patients preferred treatment with DTE over LT4.33 A follow-up study did not confirm weight loss with DTE, however.34
When LT4 monotherapy and LT4 + LT3 combination therapy were compared, results were mixed31-39; responsiveness to therapy containing LT3 might therefore depend on multiple variables, including genetic background, nutritional and lifestyle factors, stress, presence of comorbidities and autoimmune disorders, and other unidentified or poorly defined variables.40-48
Although combination therapy and DTE are not generally recommended over LT4 monotherapy, they might offer better options for patients who are still symptomatic when being treated with LT4 only: In a randomized, double-blind, crossover study that compared LT4 with DTE and with LT4 + LT3, one-third of the most highly symptomatic patients who had low scores on mood, cognitive, and quality-of-life assessments improved significantly after they were switched to combination therapy or DTE.34
The 2014 American Thyroid Association guidelines24 do not support routine use of LT4 + LT3 in hypothyroid patients who have residual symptoms after LT4 monotherapy; however, a therapeutic trial of LT4 + LT3, while maintaining a normal serum TSH, is reasonable in selected patients. Candidates for DTE or LT4 + LT3 might include patients who do not feel well on LT4 monotherapy, are post thyroidectomy or post radioiodine therapy, or have a low serum T3 level. DTE and combination therapy are discouraged in older patients, patients who have underlying CAD, and pregnant patients.
Special treatment circumstances
A number of patient variables have the potential to alter management strategies for hypothyroidism.18,20,23,40,49-53
Age, comorbidity. Older patients (> 65 years) and patients with cardiopulmonary disease or CAD should be treated with LT4, 25 to 50 mcg/d, initially; that dosage can be titrated upward by 12.5 to 25 mcg/d every 4 to 6 weeks until the TSH goal is reached—preferably, in the range of 4 to 8 mIU/L. An increase in the dosage of LT4 might be required in the presence of malabsorption (eg, gastrointestinal disorders, celiac disease) and in nephrotic syndrome.18,20,23
Body weight. A decrease in the dosage of LT4 might be indicated in the setting of significant weight loss (> 10% body weight).23
Continue to: Co-pharmacy
Co-pharmacy. An increase in the dosage of LT4 might be required when other drugs (eg, phenytoin, phenobarbital, rifampin, and carbamazepine) have led to an increased rate of thyroid hormone metabolism. A decrease in the dosage of LT4 might be necessary after initiation of androgen therapy.23
Pregnancy. Women with pre-existing hypothyroidism require an increase of 25% to 50% in their LT4 dosage during pregnancy to maintain a TSH level in the recommended pregnancy reference range. Thyroid function should be monitored every 4 to 6 weeks to ensure that the TSH target for each trimester is reached (first trimester, 0.1-4 mIU/L; second trimester, 0.2-4 mIU/L; third trimester, 0.3-4 mIU/L). Postpartum, LT4 can be reduced to the prepartum dosage; TSH should be checked every 4 to 6 weeks to maintain the TSH goal.23
Estrogen therapy. Hypothyroid women who are receiving estrogen therapy might require an increase in their LT4 dosage because serum thyroxine-binding globulin levels are increased by estrogens or through other mechanisms that have not been identified.23
Surgical candidacy. Observational studies show few adverse outcomes in surgical patients with mild (subclinical) hypothyroidism or moderate hypothyroidism; however, the risk of adverse surgical outcome might be increased in patients with severe hypothyroidism. For patients in whom surgery is planned and who have:
- subclinical hypothyroidism (elevated TSH and normal FT4), we recommend that surgery—urgent or elective—not be posptoned but proceed.
- moderate (overt) hypothyroidism who require urgent surgery, we recommend not postponing surgery, even though minor perioperative complications might develop. Such patients should be treated with LT4 as soon as the diagnosis for which surgery is required has been made. Alternatively, when moderate hypothyroidism is discovered in a patient who is being evaluated for elective surgery, we recommend postponing surgery until the euthyroid state is restored.
- severe hypothyroidism (myxedema coma [discussed in a bit]; severe clinical symptoms of chronic hypothyroidism, such as altered mental status, pericardial effusion, or heart failure; or a very low level of T4), surgery should be delayed until hypothyroidism has been treated. When emergency surgery is required for a severely hypothyroid patient, they should be treated with LT4 as soon as the diagnosis for which surgery is indicated has been made. When emergency surgery must be performed in a patient with myxedema coma, we recommend treatment with LT4 + LT3, rather than LT4 alone, often administered intravenously because LT4 is poorly absorbed in these patients.
Nonadherence. For patients who do not take LT4 regularly or do not respond to efforts to improve adherence, LT4 can be given weekly, instead of daily, although this interval is not ideal. Weekly dosing should not be used in older patients with CAD.23
Thyroid cancer. Patients who are post total thyroidectomy for thyroid cancer need to take LT4 to treat hypothyroidism and to prevent recurrence of thyroid cancer. The goal TSH level should be based on the cancer stage and risk of recurrence and should be monitored by an endocrinologist.
Myxedema coma. This medical emergency has high mortality. Myxedema coma occurs when severe hypothyroidism leads to any, or a combination, of the following: diminished mentation; hypothermia; bradycardia; hyponatremia; hypotension; cardiovascular, respiratory, and gastrointestinal dysfunction; and renal insufficiency. LT4, LT3, and glucocorticoids should be administered intravenously and the patient monitored closely—preferably in consultation with an endocrinologist.
Continue to: When to seek consultation
When to seek consultation
A patient with hypothyroidism should be referred to Endocrinology if they are < 18 years of age, pregnant, unresponsive to therapy, or have cardiac disease, coexisting endocrine disease, suspected myxedema coma, goiter or thyroid nodules, or a structural thyroid abnormality.
What we know about nutrition and hypothyroidism
Although it is commonly recognized that iodine is essential for production of thyroid hormone, other nutritional factors might contribute to proper production of thyroid hormones, including:
- adequate intake of iron, tyrosine, selenium, zinc, and vitamins E, B2, B3, B6, C, and D44,45
- selenium and zinc, which increase conversion of T4 to T3 and might be important in the management of hypothyroid patients40,46
- vitamin A, zinc, and regular exercise, which have been shown to improve cellular sensitivity to thyroid hormones.
Low iron stores can contribute to persistent symptoms and poor quality of life in patients with hypothyroidism, despite their being treated according to guidelines.29,47
Despite what is known about these nutritional connections, there is insufficient evidence that improving nutrition can reverse hypothyroidism.
Prevention
Prevention of hypothyroidism should take into account variables that affect or inhibit thyroid function, such as stress, infection (eg, Epstein-Barr virus), excessive fluoride intake, toxins (eg, pesticides, solvents, mercury, cadmium, and lead), autoimmune disease (eg, celiac disease), and food sensitivity.54,55 Oxidative stress can also cause thyroid impairment.40-48,54-58
Otherwise, there are, at present, no effective strategies for preventing thyroid disorders.
Subclinical hypothyroidism: Elusive management target
Subclinical hypothyroidism is defined as a normal serum FT4 level in the presence of an elevated serum TSH level. The prevalence of subclinical hypothyroidism varies from 3% to 15%, depending on the population studied; a higher incidence has been noted in women and older people.59 In the NHANES III,1 which excluded people with previously diagnosed thyroid disease, the incidence of subclinical hypothyroidism was 4.3%.
Continue to: Causes of subclinical...
Causes of subclinical hypothyroidism are the same as those of overt hypothyroidism, and include Hashimoto disease. The combination of an elevated TSH level and a normal FT4 level is associated with disorders characterized by protein-binding variations (eg, pregnancy, genetic disorders, drugs), TSH-secreting pituitary adenoma, class II and III obesity (respectively, body mass index, ≥ 35 but < 40 and ≥ 40), and assay variability.49,51
Lab diagnosis: Fraught with difficulty
The serum TSH level and either the total T4 level or the FT4 level should be measured to make a diagnosis of subclinical hypothyroidism. Most laboratories use a 1-step analogue immunoassay to determine free thyroid hormones; protein-binding variations can thus affect measurement of FT4.
Several scenarios that can result in inaccurate measurement of FT4 by radioimmunoassay include genetic disorders that affect binding proteins; pregnancy; use of certain drugs, including heparin, furosemide, antiepileptic agents, salicylate, ferrous sulfate, and cholesterol-binding resins; and some medical conditions, including cardiac surgery, critical illness, and renal failure. Variables that inhibit proper production of thyroid hormones—stress, infection, fluoride (an iodine antagonist), toxins (pesticides, mercury, cadmium, lead) and autoimmune conditions, such as celiac disease—should be considered when attempting to determine the cause of subclinical hypothyroidism.
Liquid chromatography–mass spectrometry measurement of thyroid hormones might be more accurate than immunoassay.53 Measuring serum total T4 and FT4 by dialysis, free from interfering proteins, might also be useful when measurement of FT4 by immunoassay is affected by binding-protein variations.
Features of subclinical hypothyroidism
Most patients who have subclinical hypothyroidism and a serum TSH level < 10 mIU/L are asymptomatic. Some might have nonspecific symptoms of hypothyroidism, however, such as reduced quality of life, poor cognitive function, and poor memory—symptoms that do not typically correlate with the serum TSH level.
It has been suggested that some elderly people normally have a higher level of serum TSH, and that they might have even a prolonged lifespan.51 Additionally, it has been shown that, in nonpregnant adult patients with subclinical hypothyroidism and a serum TSH level of 4.5 to 10 mIU/L, treatment with LT4 was not associated with improvement in thyroid-related symptoms or general quality of life.52
Treat, or don't treat, subclinical hypothyroidism?
It is well accepted that the goal of therapy in hypothyroid patients is to normalize the serum TSH level; however, the American Thyroid Association and the American Association of Clinical Endocrinology recommend starting LT4 in patients with a serum TSH level ≥ 10 mIU/L (TABLE 5).59-62 The principal reason for not treating subclinical hypothyroidism is the lack of benefit in reducing the risk of cardiovascular morbidity and mortality when the TSH level is between 7.5 and 10 mIU/L.62

Continue to: Routine treatment
Routine treatment of patients with a serum TSH level of 4.5 to 10 mIU/L remains controversial. When TSH is 7.0 to 9.9 mIU/L, treatment is recommended for (1) patients < 65 years and (2) for older patients (> 65 years) only when there are convincing hypothyroid symptoms because of concern about unintended overtreatment.
When the TSH level is anywhere above the upper limit of normal to 6.9 mIU/L, treatment is recommended for patients < 65 years old, patients who have a high titer of thyroid peroxidase antibodies, and patients with goiter—but not for patients > 65 years (and, especially, not for octogenarians) because their upper limit of normal could be as high as 6 to 8 mIU/L, especially if they are otherwise healthy.
Treatment should be considered for women with subclinical hypothyroidism who are trying to conceive or experiencing an infertility problem.
For patients with subclinical hypothyroidism who are not being treated, monitor thyroid function every 6 to 12 months by testing TSH and FT4.
CORRESPONDENCE
Thanh D. Hoang, DO, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499. doi: 10.1210/jcem.87.2.8182
2. Vanderpump MPJ. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39-51. doi: 10.1093/bmb/ldr030
3. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
4. Persani L. Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab. 2012;97:3068-3078. doi: 10.1210/jc.2012-1616
5. Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am. 2012;96:203-221. doi: 10.1016/j.mcna.2012.01.005
6. Ai J, Leonhardt JM, Heymann WR. Autoimmune thyroid diseases: etiology, pathogenesis and dermatologic manifestations. J Am Acad Dermatol. 2003;48:641-659. doi: 10.1067/mjd.2003.257
7. Franzotti AM, Avelar JCD, Cardoso TA, et al. Pityriasis rubra pilar and hypothyroidism. An Bras Dermatol. 2014;89:497-500. doi: 10.1590/abd1806-4841.20142994
8. Yaylali O, Kirac S, Yilmaz M, et al. Does hypothyroidism affect gastrointestinal motility? Gastroenterol Res Pract. 2009;2009:529802. doi: 10.1155/2009/529802
9. Patil AD. Link between hypothyroidism and small intestinal bacterial overgrowth. Indian J Endocrinol Metab. 2014;18:307-309.
10. Ono Y, Ono S, Yasunaga H, et al. Clinical characteristics and outcomes of myxedema coma: analysis of a national inpatient database in Japan. J Epidemiol. 2017;27:117-122. doi: 10.1016/j.je.2016.04.002
11. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5. doi: 10.1016/j.radonc.2011.03.002
12. Boelaert K, Newby PR, Simmonds MJ, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123:183.e1-e9. doi: 10.1016/j.amjmed.2009.06.030
13. Cheserek MJ, Wu G-R, Ntazinda A, et al. Association between thyroid hormones, lipids and oxidative stress markers in subclinical hypothyroidism. J Med Biochem. 2015;34:323-331. doi: 10.2478/jomb-2014-0044
14. Zha K, Zuo C, Wang A, et al. LDL in patients with subclinical hypothyroidism shows increased lipid peroxidation. Lipids Health Dis. 2015;14:95. doi: 10.1186/s12944-015-0092-4
15. Tejovathi B, Suchitra MM, Suresh V, et al. Association of lipid oxidation with endothelial dysfunction in patients with overt hypothyroidism. Exp Clin Endocrinol Diabetes. 2013;121:306-309. doi: 10.1055/s-0032-1333298
16. LeFevre ML; U.S. Preventive Services Task Force. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
17. Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet. 2017;390:1550-1562. doi: 10.1016/S0140-6736(17)30703-1
18. Vaidya B, Pearce SHS. Management of hypothyroidism in adults. BMJ. 2008;337:a801. doi: 10.1136/bmj.a801
19. Iyer PC, Cabanillas ME, Waguespack SG, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. 2018;28:1243-1251. doi: 10.1089/thy.2018.0116
20. Garber JR, Cobin RH, Gharib H, et al; American Association Of Clinical Endocrinologists And American Thyroid Association Taskforce On Hypothyroidism In Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Maiden MJ, Torpy DJ. Thyroid hormones in critical illness. Crit Care Clin. 2019;35:375-388. doi: 10.1016/j.ccc.2018.11.012
22. Peeters RP. Subclinical hypothyroidism. N Engl J Med. 2017;376:2556-2565. doi: 10.1056/NEJMcp1611144
23. Benvenga S, Carlé A. Levothyroxine formulations: pharmacological and clinical implications of generic substitution. Adv Ther. 2019;36(suppl 2):59-71. doi: 10.1007/s12325-019-01079-1
24. Jonklaas J, Bianco AC, Bauer AJ, et al; . Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
25. Engler D, Burger AG. The deiodination of the iodothyronines and of their derivatives in man. Endocr Rev. 1984;5:151-184. doi: 10.1210/edrv-5-2-151
26. Ettleson MD, Bianco AC. Individualized therapy for hypothyroidism: is T4 enough for everyone? J Clin Endocrinol Metab. 2020;105:e3090-e3104. doi: 10.1210/clinem/dgaa430
27. Slayden TA, Shakir MKM, Hoang TD. A bull in a pill shop: alpha-gal allergy complicating treatment options for postprocedural hypothyroidism. AACE Clin Case Rep. 2020;6:e101-e104. doi: 10.4158/ACCR-2019-0495
28. Chamorro-Pareja N, Carrillo-Martin I, Haehn DA, et al. Self-reported allergy to thyroid replacement therapy: a multicenter retrospective chart review. Endocr Pract. 2020;26:761-767. doi: 10.4158/EP-2019-0488
29. Shakir MKM, Turton D, Aprill BS, et al. Anemia: a cause of intolerance to thyroxine sodium. Mayo Clin Proc. 2000;75:189-192.
30. Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid. 2021;31:156-182. doi: 10.1089/thy.2020.0720
31. Appelhof BC, Fliers E, Wekking EM, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab. 2005;90:2666-2674. doi: 10.1210/jc.2004-2111
32. Escobar-Morreale HF, Botella-Carretero JI, M, et al. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med. 2005;142:412-424. doi: 10.7326/0003-4819-142-6-200503150-00007
33. Hoang TD, Olsen CH, Mai VQ, et al. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98:1982-1990. doi: 10.1210/jc.2012-4107
34. Shakir MKM, Brooks DI, McAninch EA, et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine+liothyronine in hypothyroidism. J Clin Endocrinol Metab. 2021;106:e4400-e4413. doi: 10.1210/clinem/dgab478
35. Valizadeh M, Seyyed-Majidi MR, Hajibeigloo H, et al. Efficacy of combined levothyroxine and liothyronine as compared with levothyroxine monotherapy in primary hypothyroidism: a randomized controlled trial. Endocr Res. 2009;34:80-89. doi: 10.1080/07435800903156340
36. Walsh JP, Shiels L, Lim EM, et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab. 2003;88:4543-4550. doi: 10.1210/jc.2003-030249
37. Rodriguez T, Lavis VR, Meininger JC, et al. Substitution of liothyronine at a 1:5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocr Pract. 2005;11:223-233. doi: 10.4158/EP.11.4.223
38. Sawka AM, Gerstein HC, Marriott MJ, et al. Does a combination regimen of thyroxine (T4) and 3,5,3’-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. 2003;88:4551-4555. doi: 10.1210/jc.2003-030139
39. Clyde PW, Harari AE, Getka EJ, et al. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA. 2003;290:2952-2958. doi: 10.1001/jama.290.22.2952
40. Duntas LH. Selenium and the thyroid: a close-knit connection. J Clin Endocrinol Metab. 2010;95:5180-5188. doi: 10.1210/jc.2010-0191
41. Winther KH, Wichman JEM, Bonnema SJ, et al. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine. 2017;55:376-385. doi: 10.1007/s12020-016-1098-z
42. Parva NR, Tadepalli S, Singh P, et al. Prevalence of vitamin D deficiency and associated risk factors in the US population (2011-2012). Cureus. 2018;10:e2741. doi: 10.7759/cureus.2741
43. Wang J, Lv S, Chen G, et al. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. 2015,7:2485-2498. doi: 10.3390/nu7042485
44. Wilson MM, Reedy J, Krebs-Smith SM. American diet quality: where it is, where it is heading, and what it could be. J Acad Nutr Diet. 2016;116:302-310.e1. doi: 10.1016/j.jand.2015.09.020
45. Babiker A, Alawi A, Al Atawi M, et al. The role of micronutrients in thyroid dysfunction. Sudan J Paediatr. 2020;20:13-19. doi: 10.24911/SJP.106-1587138942
46. Knezevic J, Starchl C, Tmava Berisha A, et al. Thyroid-gut-axis: How does the microbiota influence thyroid function? Nutrients. 2020;12:1769. doi: 10.3390/nu12061769
47. Rayman MP. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc Nutr Soc. 2019;78:34-44. doi: 10.1017/S0029665118001192
48. Chakrabarti SK, Ghosh S, Banerjee S, et al. Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian J Endocrinol Metab. 2016;20:674-678. doi: 10.4103/2230-8210.190555
49. Tseng F-Y, Lin W-Y, Lin C-C, et al. Subclinical hypothyroidism is associated with increased risk for all-cause and cardiovascular mortality in adults. J Am Coll Cardiol. 2012;60:730-737. doi: 10.1016/j.jacc.2012.03.047
50. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Intern Med. 2006;145:573-581. doi: 10.7326/0003-4819-145-8-200610170-00006
51. Gussekloo J, van Exel E, de Craen AJM, et al. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591-2599. doi: 10.1001/jama.292.21.2591
52. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
53. Monzani F, Dardano A, Caraccio N. Does treating subclinical hypothyroidism improve markers of cardiovascular risk? Treat Endocrinol. 2006;5:65-81. doi: 10.2165/00024677-200605020-00001
54. Duntas LH. Does celiac disease trigger autoimmune thyroiditis? Nat Rev Endocrinol. 2009;5:190-191. doi: 10.1038/nrendo.2009.46
55. Lerner A, Jeremias P, Matthias T. Gut-thyroid axis and celiac disease. Endocr Connect. 2017;6:R52-R58. doi: 10.1530/EC-17-0021
56. Janegova A, Janega P, Rychly B, et al. The role of Epstein-Barr virus infection in the development of autoimmune thyroid diseases. Endokrynol Pol. 2015;66:132-136. doi: 10.5603/EP.2015.0020
57. Brent GA. Environmental exposures and autoimmune thyroid disease. Thyroid. 2010;20:755-761. doi: 10.1089/thy.2010.1636
58. Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84. doi: 10.1016/j.biocel.2006.07.001
59. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028. doi: 10.4158/EP12280.GL
60. Welsh KJ, Soldin SJ. Diagnosis of endocrine disease: How reliable are free thyroid and total T3 hormone assays? Eur J Endocrinol. 2016;175:R255-R263. doi: 10.1530/EJE-16-0193
61. Grossman A, Feldhamer I, Meyerovitch J. Treatment with levothyroxin in subclinical hypothyroidism is associated with increased mortality in the elderly. Eur J Intern Med. 2018;50:65-68. doi: 10.1016/j.ejim.2017.11.010
62. Pearce SHS, Brabant G, Duntas LH, et al. 2013 ETA Guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499. doi: 10.1210/jcem.87.2.8182
2. Vanderpump MPJ. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39-51. doi: 10.1093/bmb/ldr030
3. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
4. Persani L. Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab. 2012;97:3068-3078. doi: 10.1210/jc.2012-1616
5. Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am. 2012;96:203-221. doi: 10.1016/j.mcna.2012.01.005
6. Ai J, Leonhardt JM, Heymann WR. Autoimmune thyroid diseases: etiology, pathogenesis and dermatologic manifestations. J Am Acad Dermatol. 2003;48:641-659. doi: 10.1067/mjd.2003.257
7. Franzotti AM, Avelar JCD, Cardoso TA, et al. Pityriasis rubra pilar and hypothyroidism. An Bras Dermatol. 2014;89:497-500. doi: 10.1590/abd1806-4841.20142994
8. Yaylali O, Kirac S, Yilmaz M, et al. Does hypothyroidism affect gastrointestinal motility? Gastroenterol Res Pract. 2009;2009:529802. doi: 10.1155/2009/529802
9. Patil AD. Link between hypothyroidism and small intestinal bacterial overgrowth. Indian J Endocrinol Metab. 2014;18:307-309.
10. Ono Y, Ono S, Yasunaga H, et al. Clinical characteristics and outcomes of myxedema coma: analysis of a national inpatient database in Japan. J Epidemiol. 2017;27:117-122. doi: 10.1016/j.je.2016.04.002
11. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5. doi: 10.1016/j.radonc.2011.03.002
12. Boelaert K, Newby PR, Simmonds MJ, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123:183.e1-e9. doi: 10.1016/j.amjmed.2009.06.030
13. Cheserek MJ, Wu G-R, Ntazinda A, et al. Association between thyroid hormones, lipids and oxidative stress markers in subclinical hypothyroidism. J Med Biochem. 2015;34:323-331. doi: 10.2478/jomb-2014-0044
14. Zha K, Zuo C, Wang A, et al. LDL in patients with subclinical hypothyroidism shows increased lipid peroxidation. Lipids Health Dis. 2015;14:95. doi: 10.1186/s12944-015-0092-4
15. Tejovathi B, Suchitra MM, Suresh V, et al. Association of lipid oxidation with endothelial dysfunction in patients with overt hypothyroidism. Exp Clin Endocrinol Diabetes. 2013;121:306-309. doi: 10.1055/s-0032-1333298
16. LeFevre ML; U.S. Preventive Services Task Force. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
17. Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet. 2017;390:1550-1562. doi: 10.1016/S0140-6736(17)30703-1
18. Vaidya B, Pearce SHS. Management of hypothyroidism in adults. BMJ. 2008;337:a801. doi: 10.1136/bmj.a801
19. Iyer PC, Cabanillas ME, Waguespack SG, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. 2018;28:1243-1251. doi: 10.1089/thy.2018.0116
20. Garber JR, Cobin RH, Gharib H, et al; American Association Of Clinical Endocrinologists And American Thyroid Association Taskforce On Hypothyroidism In Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Maiden MJ, Torpy DJ. Thyroid hormones in critical illness. Crit Care Clin. 2019;35:375-388. doi: 10.1016/j.ccc.2018.11.012
22. Peeters RP. Subclinical hypothyroidism. N Engl J Med. 2017;376:2556-2565. doi: 10.1056/NEJMcp1611144
23. Benvenga S, Carlé A. Levothyroxine formulations: pharmacological and clinical implications of generic substitution. Adv Ther. 2019;36(suppl 2):59-71. doi: 10.1007/s12325-019-01079-1
24. Jonklaas J, Bianco AC, Bauer AJ, et al; . Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
25. Engler D, Burger AG. The deiodination of the iodothyronines and of their derivatives in man. Endocr Rev. 1984;5:151-184. doi: 10.1210/edrv-5-2-151
26. Ettleson MD, Bianco AC. Individualized therapy for hypothyroidism: is T4 enough for everyone? J Clin Endocrinol Metab. 2020;105:e3090-e3104. doi: 10.1210/clinem/dgaa430
27. Slayden TA, Shakir MKM, Hoang TD. A bull in a pill shop: alpha-gal allergy complicating treatment options for postprocedural hypothyroidism. AACE Clin Case Rep. 2020;6:e101-e104. doi: 10.4158/ACCR-2019-0495
28. Chamorro-Pareja N, Carrillo-Martin I, Haehn DA, et al. Self-reported allergy to thyroid replacement therapy: a multicenter retrospective chart review. Endocr Pract. 2020;26:761-767. doi: 10.4158/EP-2019-0488
29. Shakir MKM, Turton D, Aprill BS, et al. Anemia: a cause of intolerance to thyroxine sodium. Mayo Clin Proc. 2000;75:189-192.
30. Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid. 2021;31:156-182. doi: 10.1089/thy.2020.0720
31. Appelhof BC, Fliers E, Wekking EM, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab. 2005;90:2666-2674. doi: 10.1210/jc.2004-2111
32. Escobar-Morreale HF, Botella-Carretero JI, M, et al. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med. 2005;142:412-424. doi: 10.7326/0003-4819-142-6-200503150-00007
33. Hoang TD, Olsen CH, Mai VQ, et al. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98:1982-1990. doi: 10.1210/jc.2012-4107
34. Shakir MKM, Brooks DI, McAninch EA, et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine+liothyronine in hypothyroidism. J Clin Endocrinol Metab. 2021;106:e4400-e4413. doi: 10.1210/clinem/dgab478
35. Valizadeh M, Seyyed-Majidi MR, Hajibeigloo H, et al. Efficacy of combined levothyroxine and liothyronine as compared with levothyroxine monotherapy in primary hypothyroidism: a randomized controlled trial. Endocr Res. 2009;34:80-89. doi: 10.1080/07435800903156340
36. Walsh JP, Shiels L, Lim EM, et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab. 2003;88:4543-4550. doi: 10.1210/jc.2003-030249
37. Rodriguez T, Lavis VR, Meininger JC, et al. Substitution of liothyronine at a 1:5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocr Pract. 2005;11:223-233. doi: 10.4158/EP.11.4.223
38. Sawka AM, Gerstein HC, Marriott MJ, et al. Does a combination regimen of thyroxine (T4) and 3,5,3’-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. 2003;88:4551-4555. doi: 10.1210/jc.2003-030139
39. Clyde PW, Harari AE, Getka EJ, et al. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA. 2003;290:2952-2958. doi: 10.1001/jama.290.22.2952
40. Duntas LH. Selenium and the thyroid: a close-knit connection. J Clin Endocrinol Metab. 2010;95:5180-5188. doi: 10.1210/jc.2010-0191
41. Winther KH, Wichman JEM, Bonnema SJ, et al. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine. 2017;55:376-385. doi: 10.1007/s12020-016-1098-z
42. Parva NR, Tadepalli S, Singh P, et al. Prevalence of vitamin D deficiency and associated risk factors in the US population (2011-2012). Cureus. 2018;10:e2741. doi: 10.7759/cureus.2741
43. Wang J, Lv S, Chen G, et al. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. 2015,7:2485-2498. doi: 10.3390/nu7042485
44. Wilson MM, Reedy J, Krebs-Smith SM. American diet quality: where it is, where it is heading, and what it could be. J Acad Nutr Diet. 2016;116:302-310.e1. doi: 10.1016/j.jand.2015.09.020
45. Babiker A, Alawi A, Al Atawi M, et al. The role of micronutrients in thyroid dysfunction. Sudan J Paediatr. 2020;20:13-19. doi: 10.24911/SJP.106-1587138942
46. Knezevic J, Starchl C, Tmava Berisha A, et al. Thyroid-gut-axis: How does the microbiota influence thyroid function? Nutrients. 2020;12:1769. doi: 10.3390/nu12061769
47. Rayman MP. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc Nutr Soc. 2019;78:34-44. doi: 10.1017/S0029665118001192
48. Chakrabarti SK, Ghosh S, Banerjee S, et al. Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian J Endocrinol Metab. 2016;20:674-678. doi: 10.4103/2230-8210.190555
49. Tseng F-Y, Lin W-Y, Lin C-C, et al. Subclinical hypothyroidism is associated with increased risk for all-cause and cardiovascular mortality in adults. J Am Coll Cardiol. 2012;60:730-737. doi: 10.1016/j.jacc.2012.03.047
50. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Intern Med. 2006;145:573-581. doi: 10.7326/0003-4819-145-8-200610170-00006
51. Gussekloo J, van Exel E, de Craen AJM, et al. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591-2599. doi: 10.1001/jama.292.21.2591
52. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
53. Monzani F, Dardano A, Caraccio N. Does treating subclinical hypothyroidism improve markers of cardiovascular risk? Treat Endocrinol. 2006;5:65-81. doi: 10.2165/00024677-200605020-00001
54. Duntas LH. Does celiac disease trigger autoimmune thyroiditis? Nat Rev Endocrinol. 2009;5:190-191. doi: 10.1038/nrendo.2009.46
55. Lerner A, Jeremias P, Matthias T. Gut-thyroid axis and celiac disease. Endocr Connect. 2017;6:R52-R58. doi: 10.1530/EC-17-0021
56. Janegova A, Janega P, Rychly B, et al. The role of Epstein-Barr virus infection in the development of autoimmune thyroid diseases. Endokrynol Pol. 2015;66:132-136. doi: 10.5603/EP.2015.0020
57. Brent GA. Environmental exposures and autoimmune thyroid disease. Thyroid. 2010;20:755-761. doi: 10.1089/thy.2010.1636
58. Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84. doi: 10.1016/j.biocel.2006.07.001
59. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028. doi: 10.4158/EP12280.GL
60. Welsh KJ, Soldin SJ. Diagnosis of endocrine disease: How reliable are free thyroid and total T3 hormone assays? Eur J Endocrinol. 2016;175:R255-R263. doi: 10.1530/EJE-16-0193
61. Grossman A, Feldhamer I, Meyerovitch J. Treatment with levothyroxin in subclinical hypothyroidism is associated with increased mortality in the elderly. Eur J Intern Med. 2018;50:65-68. doi: 10.1016/j.ejim.2017.11.010
62. Pearce SHS, Brabant G, Duntas LH, et al. 2013 ETA Guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
PRACTICE RECOMMENDATIONS
› Prescribe levothyroxine (LT4) to maintain thyroid-stimulating hormone (TSH) at 4 to 7 mIU/L in select patients with primary hypothyroidism for whom that range of the serum TSH level can be considered appropriate (ie, those older than 65 years and those who have underlying coronary artery disease or another debilitating chronic disorder). A
› Counsel all women of childbearing age with primary hypothyroidism that they need to have their dosage of LT4 increased as soon as pregnancy is suspected. A
› Keep in mind that treating hypothyroidism is not always necessary in older patients who have subclinical disease and a serum TSH level < 10 mIU/L. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series