User login
Bimekizumab for Hidradenitis Suppurativa: Pathophysiology and Promising Interventions
Bimekizumab for Hidradenitis Suppurativa: Pathophysiology and Promising Interventions
Hidradenitis suppurativa (HS) is a debilitating dermatologic condition characterized by recurrent episodes of neutrophilic inflammation affecting the apocrine and pilosebaceous units that most commonly affects individuals aged 20 to 40 years. Originating from the hair follicles, inflammation initiates the formation of painful nodules and abscesses that can progress to sinus tracts or fistulas accompanied by the development of extensive scarring, exquisite pain, and malodorous drainage.1 The lesions most commonly occur in intertriginous zones as well as areas rich in apocrine glands. The distinctive and sometimes irreversible clinical features of HS profoundly influence patients’ well-being and have lasting social, personal, and emotional impacts on their lives.2
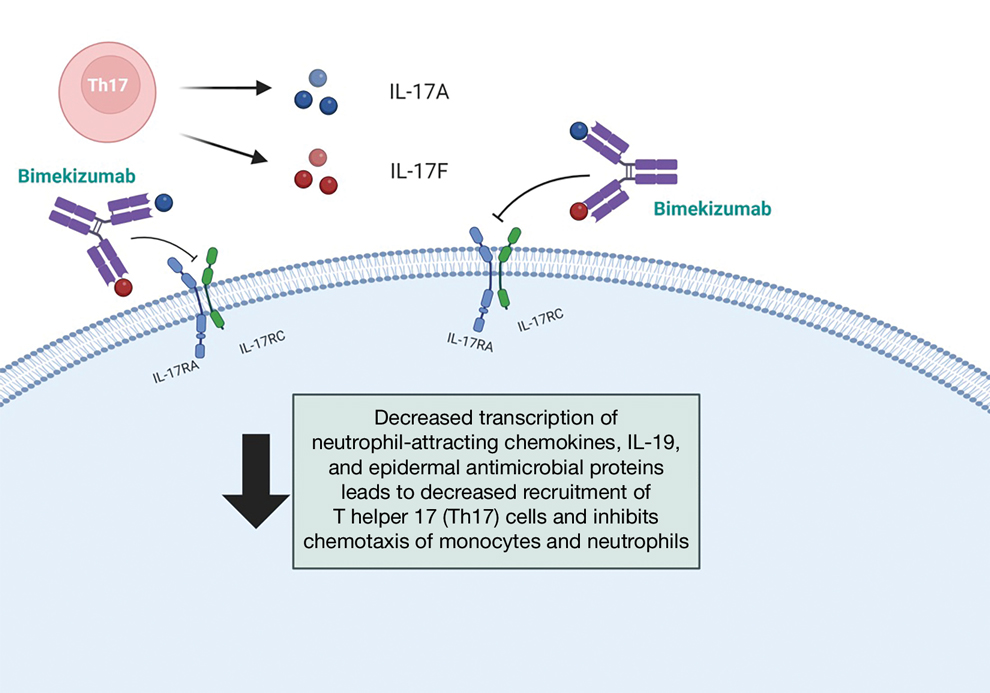
Bimekizumab is a monoclonal antibody that specifically targets IL-17A and IL-17F, aiming to inhibit the downstream effects responsible for the chronic inflammation and tissue damage characteristic of HS.3 In HS lesions, IL-17 cytokines produced by T helper 17 (Th17) cells stimulate the production of chemokines (such as CC motif chemokine ligand 20) and neutrophil-attracting chemokines (including C-X-C motif chemokine ligands 1 and 8), cytokines (such as granulocyte colony-stimulating factor and IL-19), and epidermal antimicrobial proteins.1,2 This cascade results in the chemotaxis of monocytes and neutrophils in the skin, recruiting additional Th17 and myeloid cells and further amplifying IL-17 production.1
Bimekizumab’s mechanism of action strategically disrupts this feed-forward inflammatory loop, decreasing the transcription of neutrophil-attracting chemokines, IL-19, and epidermal antimicrobial proteins (Figure).1,2 This leads to diminished recruitment of Th17 cells and inhibits the chemotaxis of monocytes and neutrophils in the skin, effectively addressing the chronic inflammation and tissue damage characteristic of HS.
We present a comprehensive review of the current standards of care, the underlying molecular pathophysiology of HS, and evaluation of the efficacy and safety of bimekizumab.
Evaluating HS Severity
The Hurley staging system provides a valuable framework for evaluating the severity of HS based on lesion characteristics. Stage I is characterized by abscess formation without tracts or scars. Stage II is characterized by recurrent abscesses with sinus tracts and scarring. Stage III is characterized by diffuse involvement, multiple interconnected sinus tracts, and abscesses across an entire area, leaving little to no uninvolved skin.4
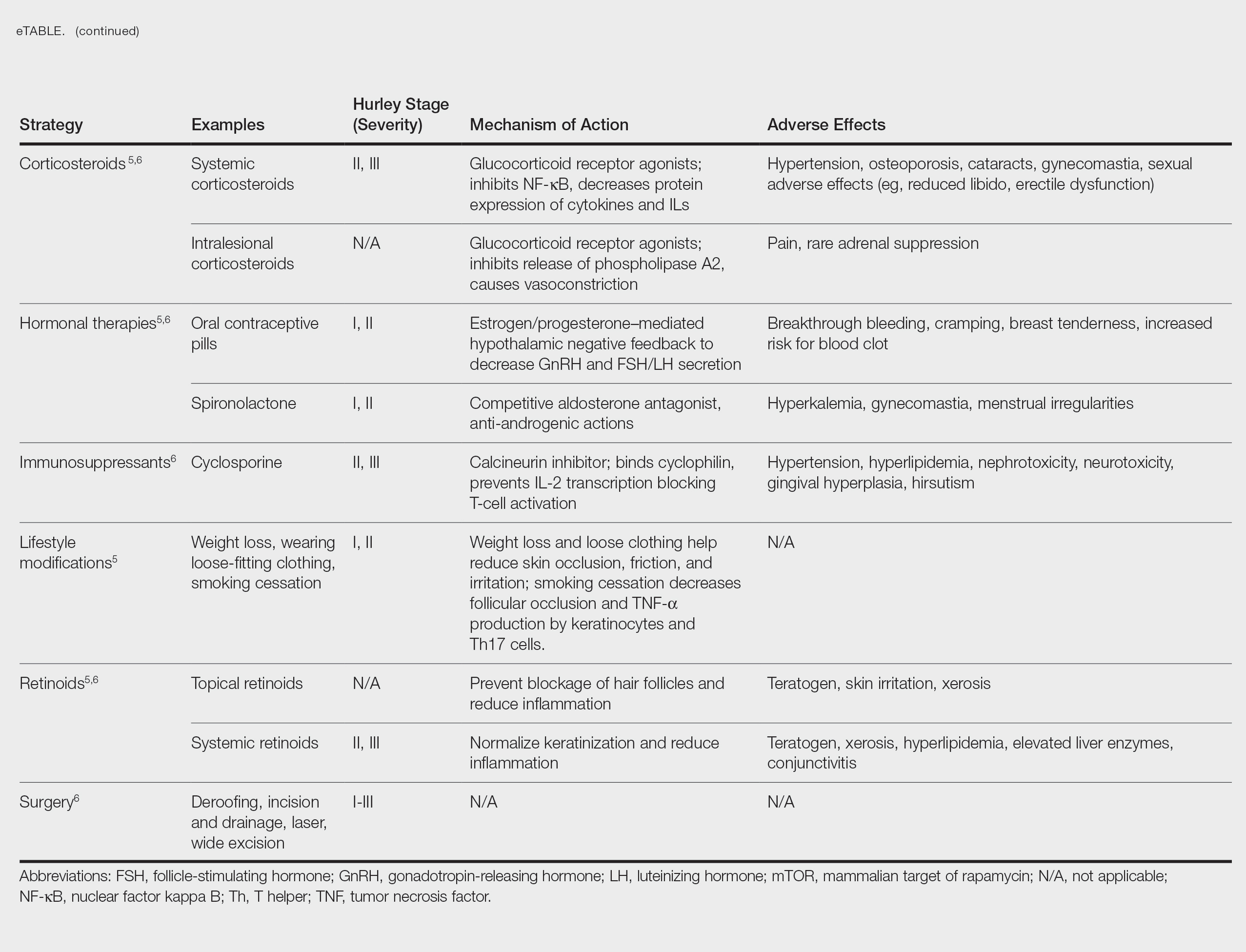
Treatment strategies for HS vary based on Hurley staging (eTable).5-11 For mild cases (stage I), topical and intralesional therapies are common, while moderate to severe cases (stages II and III) may require extensive surgical approaches or systemic drugs such as antibiotics, hormonal therapies, retinoids, or immunosuppressive/biologic agents.2
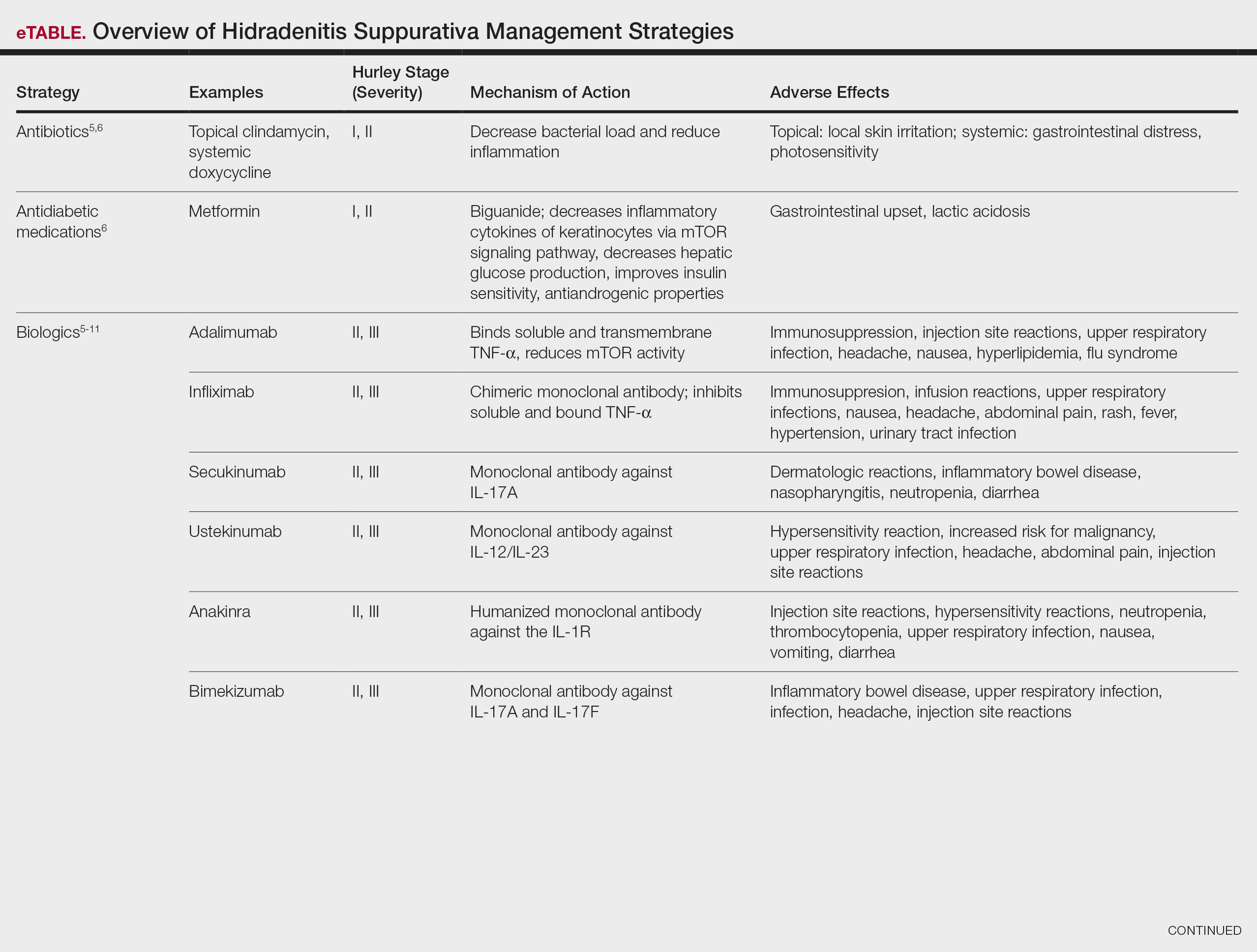
Adalimumab, an anti–tumor necrosis factor (TNF) α monoclonal antibody, was the first US Food and Drug Administration (FDA)–approved biologic for HS. Secukinumab, a monoclonal antibody against IL-17A, subsequently was approved by the FDA for moderate to severe HS.12 Off-label use of biologics including infliximab and ustekinumab expands the available treatment options for HS. In one Phase II randomized clinical trial (RCT), infliximab showed efficacy in reducing Hidradenitis Suppurativa Severity Index scores, with 26.7% (4/15) of patients achieving a 50% or greater reduction compared to placebo, although this was not statistically significant. Similarly, ustekinumab demonstrated promising results, with 47.1% (8/17) of patients achieving Hidradenitis Suppurativa Clinical Response (HiSCR) at week 40.2 This multifaceted approach aims to address the varying degrees of severity and optimize outcomes for individuals with HS.
Molecular Pathophysiology of HS
The pathogenesis of HS is multifactorial, involving a complex interplay of genetic, environmental, and behavioral factors.2 Approximately 33% to 40% of patients with HS worldwide report a first-degree relative with the condition, indicating a hereditary element with an autosomal-dominant transmission pattern and highlighting the global relevance of genetic factors in HS.4 Hidradenitis suppurativa is highly prevalent in individuals with obesity, likely due to increased intertriginous surface area, skin friction, sweat production, and hormonal changes in these patients. Smoking also commonly is associated with HS, with nicotine potentially contributing to increased follicular plugging.1 Hormonal influences also play a role, as evidenced by a greater prevalence of HS in females, disease onset typically occurring between puberty and menopause, and symptomatic fluctuations correlating with menstrual cycles and exogenous hormones.4
Altered infundibular keratinization with subsequent hyperkeratosis/occlusion and innate immune pathway activation are key events leading to development of HS.1 These events are mediated by release of pathogen- and danger-associated molecular patterns, leading to inflammasome-mediated IL-1α release, followed by downstream cytokine release.2 Elevated levels of TNF-α, IL-1Β, IL-10, IL-17, and particularly IL-17A have been detected in HS lesional skin. The IL-17 family comprises multiple members, namely IL-17A, IL-17C, IL-17E, and IL17F. IL-17A and IL-17F often are co-expressed and secreted predominantly by a subset of CD4+ T helper cells, namely Th17 cells.2 IL-17 cytokines exert pro-inflammatory effects, influencing immune cell activity and contributing to skin inflammation, particularly in HS.
Given the pivotal role of IL-17 in the pathogenesis of HS, the exploration of IL-17–targeted agents has become a focal point in clinical research. Bimekizumab, a novel IL-17 inhibitor, has emerged as a promising candidate, offering a potential breakthrough in the treatment landscape for individuals affected by HS.
Bimekizumab for HS Management
A phase II, double-blind, placebo-controlled RCT included 90 patients with moderate to severe HS (age range, 18-70 years) who were randomly assigned in a 2:1:1 ratio to receive either bimekizumab 320 mg every 2 weeks (with a 640-mg loading dose at baseline)(n=46), placebo (n=21), or adalimumab 40 mg once weekly from week 4 onward (following an initial 160-mg loading dose at baseline and 80-mg dose at week 2)(n=21). The study included a 12-week treatment period followed by a 20-week safety follow-up period. The primary endpoint was the achievement of HiSCR50—defined as a reduction of at least 50% nodules, coupled with no increase in the number of abscesses or draining fistulas relative to baseline—at week 12. Additionally, the study assessed the number of patients who achieved a modified HiSCR with 75% reduction (HiSCR75) of combined abscess and inflammatory nodule count or a modified HiSCR with 90% reduction (HiSCR90). At week 12, the modeled response rates were estimated using a Bayesian logistic regression model. For HiSCR50, the modeled rate for bimekizumab was 57.3%, with an observed rate of 62.5% (25/40), compared to a modeled rate of 26.1% for placebo (observed rate, 27.8% [5/18]). The posterior probability of superiority for bimekizumab over placebo was 0.998. By week 12, bimekizumab-treated patients achieved modeled HiSCR75 and HiSCR90 rates of 46.0% and 32.0%, respectively, with observed rates of 50.0% (20/40) for HiSCR75 and 35.0% (14/40) for HiSCR90. In comparison, placebo-treated patients achieved modeled HiSCR75 and HiSCR90 rates of 10.0% and 0%, respectively, with observed rates of 11.1% (2/18) for HiSCR75 and 0% (0/18) for HiSCR90. Adalimumab-treated participants demonstrated intermediate results, achieving modeled HiSCR75 and HiSCR90 rates of 35.0% and 15.0%, respectively, with observed rates of 38.88% (7/18) for HiSCR75 and 16.66% (3/18) for HiSCR90.7
Bimekizumab was effective in the treatment of moderate to severe HS with comparable results to adalimumab.7 The incidence of treatment-emergent adverse events was similar across treatment arms (bimekizumab, 69.6% [32/46]; placebo, 61.9% [13/21]; adalimumab, 71.4% [15/21]). The most common treatment-emergent adverse events in the biologic treatment arms were infections (43.5% [20/46] in the bimekizumab group and 42.9% [9/21] in the adalimumab group), skin and subcutaneous tissue disorders (28.3% [13/46] in the bimekizumab group and 42.9% [9/21] in the adalimumab group), and general disorders/administration site conditions (21.7% [10/46] in the bimekizumab group and 23.8% [5/21] in the adalimumab group). Serious adverse events occurred in 4.3% (2/46) of patients in the bimekizumab group, 9.5% (2/21) of patients in the placebo group, and 4.8% (1/21) of patients in the adalimumab group. Serious adverse events that required hospitalization were due to anemia and empyema in the bimekizumab group; worsening HS in the adalimumab group; and myocardial infarction, hypoesthesia, headache, and dizziness in the placebo group. No deaths occurred in this study. Overall, bimekizumab was well tolerated, and discontinuation rates were low across all arms. The primary reason for discontinuation was withdrawal of consent (not due to an adverse event) or loss to follow-up.7
Two completed 48-week phase III RCTs, BE HEARD I and BE HEARD II, evaluated the efficacy and safety of bimekizumab in patients with moderate to severe HS.13 In both trials, 2 bimekizumab dosing regimens (320 mg every 2 weeks and 320 mg every 4 weeks) were compared with placebo during the 16-week initial and 32-week maintenance treatment periods. The primary endpoint of week 16 was achieved by 47.8% (138/289) and 51.9% (151/291) of patients receiving bimekizumab every 2 weeks in BE HEARD I (n=505) and BE HEARD II (n=509), respectively, compared with 29.2% (21/72) and 32.4% (24/74) of the placebo group. The bimekizumab 320 mg every 4 weeks dosing regimen met the primary endpoint only in BE HEARD II, with 53.5% (77/144) of patients achieving HiSCR50 compared to 32.4% (24/74) with placebo (P=0.0038).13 Both trials met the key secondary endpoint of HiSCR75 at week 16 for bimekizumab 320 mg every 2 weeks vs placebo. In BE HEARD I, 33.6% (97/289) of patients receiving bimekizumab achieved HiSCR75 versus 18.1% (13/72) taking placebo. In BE HEARD II, 35.7% (104/291) of patients receiving bimekizumab achieved HiSCR75 vs 16.2% (12/74) taking placebo. Responses were maintained or increased through week 48 in both trials. The most common treatment-emergent adverse events through week 48 were worsening HS, COVID-19 infection, diarrhea, oral candidiasis, and headache.13
A smaller scale case series investigated the use of bimekizumab in 4 female patients aged 20 to 62 years with moderate to severe HS and concomitant plaque or inverse psoriasis.8 A monthly loading dose of 320 mg was given during weeks 0 to 12 followed by a maintenance dose of 320 mg administered every 8 weeks. The International Hidradenitis Suppurativa Score System, visual analogue scale, and Dermatology Life Quality Index were used to assess the effectiveness of therapy by comparing scores before and after 4 and 16 weeks of treatment. A reduction of pain and improvement of HS lesions was observed in 3 (75.0%) patients after the first dosage of bimekizumab, with completed remission of HS by week 16. The fourth patient (25.0%) experienced substantial improvement in all measures, although not complete remission. All 4 patients remained on bimekizumab, and no adverse effects were reported.8
A meta-analysis evaluated 16 RCTs of 9 biologics and 3 small-molecule inhibitors in 2076 patients with HS.10 Secukinumab was not included in this meta-analysis. Only adalimumab (risk ratio, 1.77; 95% CI, 1.44-2.17) and bimekizumab (risk ratio, 2.25; 95% CI, 1.03-4.92) were superior to placebo in achieving HiSCR response at weeks 12 to 16 in 5 RCTs and 1 RCT, respectively; however, no statistically significant differences were noted between adalimumab and bimekizumab (P=.56). This analysis concluded that adalimumab and bimekizumab are the only 2 biologics efficacious in reaching HiSCR and consistently improved both disease severity and quality of life in patients with HS with an acceptable safety profile.10 Furthermore, these biologics had no increase in serious adverse events when compared to placebo.10
A network meta-analysis of 10 clinical trials involving more than 900 total participants evaluated nonsurgical therapies for HS. The analysis used Surface Under the Cumulative Ranking curve (SUCRA) values to estimate the efficacy of treatments in achieving clinical response according to HiSCR criteria. These values range from 0% to 100%, with 100% representing the best possible ranking for efficacy. Bimekizumab showed the highest estimated efficacy with a SUCRA value of 67%, followed by adalimumab (64%), anakinra (49%), and placebo (19%). These SUCRA values indicate the relative ranking of treatments, with higher values suggesting greater likelihood of achieving clinical response, rather than representing the actual percentage of patients achieving HiSCR. Bimekizumab was found to be more efficacious than placebo (P<.05).14
Building on the initial evidence of bimekizumab’s efficacy, BE HEARD I and BE HEARD II addressed some limitations of prior studies, including small sample sizes and insufficient stratification.13 Notably, stratification by baseline Hurley stage severity (ie, the most severe stage of disease assigned at baseline) and baseline systemic antibiotic use helped mitigate bias and ensured a more robust assessment of treatment efficacy; however, certain limitations persist. While the trials demonstrated rapid and clinically meaningful responses maintained up to 48 weeks, longer-term data beyond this period are limited, leaving gaps in understanding the durability of treatment effects over years. Additionally, despite appropriate stratification, the generalizability of the findings to broader patient populations remains unclear, as trial participants may not fully represent the diversity of patients seen in clinical practice.13
Future research is needed to address these limitations. The use of validated HS biomarkers as endpoints could enhance the ability to evaluate biologic efficacy and identify predictors of response. Comparative studies with other biologics also are warranted to establish the relative efficacy of bimekizumab within the growing therapeutic landscape for HS. Finally, real-world evidence from larger and more diverse populations will be critical to confirm the trial findings and assess long-term safety and effectiveness in routine clinical practice.13
Conclusion
The existing literature and recent phase III RCTs, BE HEARD I and BE HEARD II, demonstrate that bimekizumab is an effective treatment for moderate to severe HS, with robust efficacy according to HiSCR scores and sustained responses through 48 weeks. These trials addressed some prior limitations, including small sample sizes and insufficient stratification, providing a more comprehensive evaluation of bimekizumab’s clinical impact. The safety profile of bimekizumab remains favorable, with low discontinuation rates and manageable adverse events, such as infection, gastrointestinal upset, headache, and injection-site reactions. Long-term efficacy and safety data beyond 48 weeks still are needed to fully establish its durability and impact in diverse populations. The recent FDA approval of bimekizumab for moderate to severe HS provides patients with a new treatment option, offering a more positive clinical outlook.
- Malvaso D, Calabrese L, Chiricozzi A, et al. IL-17 inhibition: a valid therapeutic strategy in the management of hidradenitis suppurativa. Pharmaceutics. 2023;15:2450. doi:10.3390 /pharmaceutics15102450
- Markota C¡agalj A, Marinovic´ B, Bukvic´ Mokos Z. New and emerging targeted therapies for hidradenitis suppurativa. Int J Mol Sci. 2022;23:3753. doi:10.3390/ijms23073753
- Zouboulis CC, Frew JW, Giamarellos-Bourboulis EJ, et al. Target molecules for future hidradenitis suppurativa treatment. Exp Dermatol. 2021;30 suppl 1:8-17. doi:10.1111/exd.14338
- Ballard K, Shuman VL. Hidradenitis suppurativa. StatPearls [Internet]. Updated May 6, 2024. Accessed December 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534867/
- Rathod U, Prasad PN, Patel BM, et al. Hidradenitis suppurativa: a literature review comparing current therapeutic modalities. Cureus. 2023;15:E43695. doi:10.7759/cureus.43695
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: current and emerging treatments. J Am Acad Dermatol. 2020;82:1061-1082. doi:10.1016/j.jaad.2019.08.089
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Molinelli E, Gambini D, Maurizi A, et al. Bimekizumab in hidradenitis suppurativa: a valid and effective emerging treatment. Clin Exp Dermatol. 2023;48:1272-1274. doi:10.1093/ced/llad229
- Martora F, Megna M, Battista T, et al. Adalimumab, ustekinumab, and secukinumab in the management of hidradenitis suppurativa: a review of the real-life experience. Clin Cosmet Investig Dermatol. 2023;16:135-148. doi:10.2147/CCID.S391356
- Huang CH, Huang IH, Tai CC, et al. Biologics and small molecule inhibitors for treating hidradenitis suppurativa: a systematic review and meta-analysis. Biomedicines. 2022;10:1303. doi:10.3390 /biomedicines10061303
- Ojeda Gómez A, Madero Velázquez L, Buendía Sanchez L, et al. Inflammatory bowel disease new-onset during secukinumab therapy: real-world data from a tertiary center. Rev Esp Enferm Dig. 2021;113: 858-859. doi:10.17235/reed.2021.8397/2021
- Martora F, Marasca C, Cacciapuoti S, et al. Secukinumab in hidradenitis suppurativa patients who failed adalimumab: a 52-week real-life study. Clin Cosmet Investig Dermatol. 2024;17:159-166. doi:10.2147 /CCID.S449367
- Kimball AB, Jemec GBE, Sayed CJ, et al. Efficacy and safety of bimekizumab in patients with moderate-to-severe hidradenitis suppurativa (BE HEARD I and BE HEARD II): two 48-week, randomised, double-blind, placebo-controlled, multicentre phase 3 trials. Lancet. 2024;403:2504-2519. doi:10.1016 /S0140-6736(24)00101-6
- Gupta AK, Shear NH, Piguet V, et al. Efficacy of non-surgical monotherapies for hidradenitis suppurativa: a systematic review and network meta-analyses of randomized trials. J Dermatolog Treat. 2022;33:2149-2160. doi:10.1080/09546634.2021.1927949
Hidradenitis suppurativa (HS) is a debilitating dermatologic condition characterized by recurrent episodes of neutrophilic inflammation affecting the apocrine and pilosebaceous units that most commonly affects individuals aged 20 to 40 years. Originating from the hair follicles, inflammation initiates the formation of painful nodules and abscesses that can progress to sinus tracts or fistulas accompanied by the development of extensive scarring, exquisite pain, and malodorous drainage.1 The lesions most commonly occur in intertriginous zones as well as areas rich in apocrine glands. The distinctive and sometimes irreversible clinical features of HS profoundly influence patients’ well-being and have lasting social, personal, and emotional impacts on their lives.2
Bimekizumab is a monoclonal antibody that specifically targets IL-17A and IL-17F, aiming to inhibit the downstream effects responsible for the chronic inflammation and tissue damage characteristic of HS.3 In HS lesions, IL-17 cytokines produced by T helper 17 (Th17) cells stimulate the production of chemokines (such as CC motif chemokine ligand 20) and neutrophil-attracting chemokines (including C-X-C motif chemokine ligands 1 and 8), cytokines (such as granulocyte colony-stimulating factor and IL-19), and epidermal antimicrobial proteins.1,2 This cascade results in the chemotaxis of monocytes and neutrophils in the skin, recruiting additional Th17 and myeloid cells and further amplifying IL-17 production.1
Bimekizumab’s mechanism of action strategically disrupts this feed-forward inflammatory loop, decreasing the transcription of neutrophil-attracting chemokines, IL-19, and epidermal antimicrobial proteins (Figure).1,2 This leads to diminished recruitment of Th17 cells and inhibits the chemotaxis of monocytes and neutrophils in the skin, effectively addressing the chronic inflammation and tissue damage characteristic of HS.

We present a comprehensive review of the current standards of care, the underlying molecular pathophysiology of HS, and evaluation of the efficacy and safety of bimekizumab.
Evaluating HS Severity
The Hurley staging system provides a valuable framework for evaluating the severity of HS based on lesion characteristics. Stage I is characterized by abscess formation without tracts or scars. Stage II is characterized by recurrent abscesses with sinus tracts and scarring. Stage III is characterized by diffuse involvement, multiple interconnected sinus tracts, and abscesses across an entire area, leaving little to no uninvolved skin.4
Treatment strategies for HS vary based on Hurley staging (eTable).5-11 For mild cases (stage I), topical and intralesional therapies are common, while moderate to severe cases (stages II and III) may require extensive surgical approaches or systemic drugs such as antibiotics, hormonal therapies, retinoids, or immunosuppressive/biologic agents.2


Adalimumab, an anti–tumor necrosis factor (TNF) α monoclonal antibody, was the first US Food and Drug Administration (FDA)–approved biologic for HS. Secukinumab, a monoclonal antibody against IL-17A, subsequently was approved by the FDA for moderate to severe HS.12 Off-label use of biologics including infliximab and ustekinumab expands the available treatment options for HS. In one Phase II randomized clinical trial (RCT), infliximab showed efficacy in reducing Hidradenitis Suppurativa Severity Index scores, with 26.7% (4/15) of patients achieving a 50% or greater reduction compared to placebo, although this was not statistically significant. Similarly, ustekinumab demonstrated promising results, with 47.1% (8/17) of patients achieving Hidradenitis Suppurativa Clinical Response (HiSCR) at week 40.2 This multifaceted approach aims to address the varying degrees of severity and optimize outcomes for individuals with HS.
Molecular Pathophysiology of HS
The pathogenesis of HS is multifactorial, involving a complex interplay of genetic, environmental, and behavioral factors.2 Approximately 33% to 40% of patients with HS worldwide report a first-degree relative with the condition, indicating a hereditary element with an autosomal-dominant transmission pattern and highlighting the global relevance of genetic factors in HS.4 Hidradenitis suppurativa is highly prevalent in individuals with obesity, likely due to increased intertriginous surface area, skin friction, sweat production, and hormonal changes in these patients. Smoking also commonly is associated with HS, with nicotine potentially contributing to increased follicular plugging.1 Hormonal influences also play a role, as evidenced by a greater prevalence of HS in females, disease onset typically occurring between puberty and menopause, and symptomatic fluctuations correlating with menstrual cycles and exogenous hormones.4
Altered infundibular keratinization with subsequent hyperkeratosis/occlusion and innate immune pathway activation are key events leading to development of HS.1 These events are mediated by release of pathogen- and danger-associated molecular patterns, leading to inflammasome-mediated IL-1α release, followed by downstream cytokine release.2 Elevated levels of TNF-α, IL-1Β, IL-10, IL-17, and particularly IL-17A have been detected in HS lesional skin. The IL-17 family comprises multiple members, namely IL-17A, IL-17C, IL-17E, and IL17F. IL-17A and IL-17F often are co-expressed and secreted predominantly by a subset of CD4+ T helper cells, namely Th17 cells.2 IL-17 cytokines exert pro-inflammatory effects, influencing immune cell activity and contributing to skin inflammation, particularly in HS.
Given the pivotal role of IL-17 in the pathogenesis of HS, the exploration of IL-17–targeted agents has become a focal point in clinical research. Bimekizumab, a novel IL-17 inhibitor, has emerged as a promising candidate, offering a potential breakthrough in the treatment landscape for individuals affected by HS.
Bimekizumab for HS Management
A phase II, double-blind, placebo-controlled RCT included 90 patients with moderate to severe HS (age range, 18-70 years) who were randomly assigned in a 2:1:1 ratio to receive either bimekizumab 320 mg every 2 weeks (with a 640-mg loading dose at baseline)(n=46), placebo (n=21), or adalimumab 40 mg once weekly from week 4 onward (following an initial 160-mg loading dose at baseline and 80-mg dose at week 2)(n=21). The study included a 12-week treatment period followed by a 20-week safety follow-up period. The primary endpoint was the achievement of HiSCR50—defined as a reduction of at least 50% nodules, coupled with no increase in the number of abscesses or draining fistulas relative to baseline—at week 12. Additionally, the study assessed the number of patients who achieved a modified HiSCR with 75% reduction (HiSCR75) of combined abscess and inflammatory nodule count or a modified HiSCR with 90% reduction (HiSCR90). At week 12, the modeled response rates were estimated using a Bayesian logistic regression model. For HiSCR50, the modeled rate for bimekizumab was 57.3%, with an observed rate of 62.5% (25/40), compared to a modeled rate of 26.1% for placebo (observed rate, 27.8% [5/18]). The posterior probability of superiority for bimekizumab over placebo was 0.998. By week 12, bimekizumab-treated patients achieved modeled HiSCR75 and HiSCR90 rates of 46.0% and 32.0%, respectively, with observed rates of 50.0% (20/40) for HiSCR75 and 35.0% (14/40) for HiSCR90. In comparison, placebo-treated patients achieved modeled HiSCR75 and HiSCR90 rates of 10.0% and 0%, respectively, with observed rates of 11.1% (2/18) for HiSCR75 and 0% (0/18) for HiSCR90. Adalimumab-treated participants demonstrated intermediate results, achieving modeled HiSCR75 and HiSCR90 rates of 35.0% and 15.0%, respectively, with observed rates of 38.88% (7/18) for HiSCR75 and 16.66% (3/18) for HiSCR90.7
Bimekizumab was effective in the treatment of moderate to severe HS with comparable results to adalimumab.7 The incidence of treatment-emergent adverse events was similar across treatment arms (bimekizumab, 69.6% [32/46]; placebo, 61.9% [13/21]; adalimumab, 71.4% [15/21]). The most common treatment-emergent adverse events in the biologic treatment arms were infections (43.5% [20/46] in the bimekizumab group and 42.9% [9/21] in the adalimumab group), skin and subcutaneous tissue disorders (28.3% [13/46] in the bimekizumab group and 42.9% [9/21] in the adalimumab group), and general disorders/administration site conditions (21.7% [10/46] in the bimekizumab group and 23.8% [5/21] in the adalimumab group). Serious adverse events occurred in 4.3% (2/46) of patients in the bimekizumab group, 9.5% (2/21) of patients in the placebo group, and 4.8% (1/21) of patients in the adalimumab group. Serious adverse events that required hospitalization were due to anemia and empyema in the bimekizumab group; worsening HS in the adalimumab group; and myocardial infarction, hypoesthesia, headache, and dizziness in the placebo group. No deaths occurred in this study. Overall, bimekizumab was well tolerated, and discontinuation rates were low across all arms. The primary reason for discontinuation was withdrawal of consent (not due to an adverse event) or loss to follow-up.7
Two completed 48-week phase III RCTs, BE HEARD I and BE HEARD II, evaluated the efficacy and safety of bimekizumab in patients with moderate to severe HS.13 In both trials, 2 bimekizumab dosing regimens (320 mg every 2 weeks and 320 mg every 4 weeks) were compared with placebo during the 16-week initial and 32-week maintenance treatment periods. The primary endpoint of week 16 was achieved by 47.8% (138/289) and 51.9% (151/291) of patients receiving bimekizumab every 2 weeks in BE HEARD I (n=505) and BE HEARD II (n=509), respectively, compared with 29.2% (21/72) and 32.4% (24/74) of the placebo group. The bimekizumab 320 mg every 4 weeks dosing regimen met the primary endpoint only in BE HEARD II, with 53.5% (77/144) of patients achieving HiSCR50 compared to 32.4% (24/74) with placebo (P=0.0038).13 Both trials met the key secondary endpoint of HiSCR75 at week 16 for bimekizumab 320 mg every 2 weeks vs placebo. In BE HEARD I, 33.6% (97/289) of patients receiving bimekizumab achieved HiSCR75 versus 18.1% (13/72) taking placebo. In BE HEARD II, 35.7% (104/291) of patients receiving bimekizumab achieved HiSCR75 vs 16.2% (12/74) taking placebo. Responses were maintained or increased through week 48 in both trials. The most common treatment-emergent adverse events through week 48 were worsening HS, COVID-19 infection, diarrhea, oral candidiasis, and headache.13
A smaller scale case series investigated the use of bimekizumab in 4 female patients aged 20 to 62 years with moderate to severe HS and concomitant plaque or inverse psoriasis.8 A monthly loading dose of 320 mg was given during weeks 0 to 12 followed by a maintenance dose of 320 mg administered every 8 weeks. The International Hidradenitis Suppurativa Score System, visual analogue scale, and Dermatology Life Quality Index were used to assess the effectiveness of therapy by comparing scores before and after 4 and 16 weeks of treatment. A reduction of pain and improvement of HS lesions was observed in 3 (75.0%) patients after the first dosage of bimekizumab, with completed remission of HS by week 16. The fourth patient (25.0%) experienced substantial improvement in all measures, although not complete remission. All 4 patients remained on bimekizumab, and no adverse effects were reported.8
A meta-analysis evaluated 16 RCTs of 9 biologics and 3 small-molecule inhibitors in 2076 patients with HS.10 Secukinumab was not included in this meta-analysis. Only adalimumab (risk ratio, 1.77; 95% CI, 1.44-2.17) and bimekizumab (risk ratio, 2.25; 95% CI, 1.03-4.92) were superior to placebo in achieving HiSCR response at weeks 12 to 16 in 5 RCTs and 1 RCT, respectively; however, no statistically significant differences were noted between adalimumab and bimekizumab (P=.56). This analysis concluded that adalimumab and bimekizumab are the only 2 biologics efficacious in reaching HiSCR and consistently improved both disease severity and quality of life in patients with HS with an acceptable safety profile.10 Furthermore, these biologics had no increase in serious adverse events when compared to placebo.10
A network meta-analysis of 10 clinical trials involving more than 900 total participants evaluated nonsurgical therapies for HS. The analysis used Surface Under the Cumulative Ranking curve (SUCRA) values to estimate the efficacy of treatments in achieving clinical response according to HiSCR criteria. These values range from 0% to 100%, with 100% representing the best possible ranking for efficacy. Bimekizumab showed the highest estimated efficacy with a SUCRA value of 67%, followed by adalimumab (64%), anakinra (49%), and placebo (19%). These SUCRA values indicate the relative ranking of treatments, with higher values suggesting greater likelihood of achieving clinical response, rather than representing the actual percentage of patients achieving HiSCR. Bimekizumab was found to be more efficacious than placebo (P<.05).14
Building on the initial evidence of bimekizumab’s efficacy, BE HEARD I and BE HEARD II addressed some limitations of prior studies, including small sample sizes and insufficient stratification.13 Notably, stratification by baseline Hurley stage severity (ie, the most severe stage of disease assigned at baseline) and baseline systemic antibiotic use helped mitigate bias and ensured a more robust assessment of treatment efficacy; however, certain limitations persist. While the trials demonstrated rapid and clinically meaningful responses maintained up to 48 weeks, longer-term data beyond this period are limited, leaving gaps in understanding the durability of treatment effects over years. Additionally, despite appropriate stratification, the generalizability of the findings to broader patient populations remains unclear, as trial participants may not fully represent the diversity of patients seen in clinical practice.13
Future research is needed to address these limitations. The use of validated HS biomarkers as endpoints could enhance the ability to evaluate biologic efficacy and identify predictors of response. Comparative studies with other biologics also are warranted to establish the relative efficacy of bimekizumab within the growing therapeutic landscape for HS. Finally, real-world evidence from larger and more diverse populations will be critical to confirm the trial findings and assess long-term safety and effectiveness in routine clinical practice.13
Conclusion
The existing literature and recent phase III RCTs, BE HEARD I and BE HEARD II, demonstrate that bimekizumab is an effective treatment for moderate to severe HS, with robust efficacy according to HiSCR scores and sustained responses through 48 weeks. These trials addressed some prior limitations, including small sample sizes and insufficient stratification, providing a more comprehensive evaluation of bimekizumab’s clinical impact. The safety profile of bimekizumab remains favorable, with low discontinuation rates and manageable adverse events, such as infection, gastrointestinal upset, headache, and injection-site reactions. Long-term efficacy and safety data beyond 48 weeks still are needed to fully establish its durability and impact in diverse populations. The recent FDA approval of bimekizumab for moderate to severe HS provides patients with a new treatment option, offering a more positive clinical outlook.
Hidradenitis suppurativa (HS) is a debilitating dermatologic condition characterized by recurrent episodes of neutrophilic inflammation affecting the apocrine and pilosebaceous units that most commonly affects individuals aged 20 to 40 years. Originating from the hair follicles, inflammation initiates the formation of painful nodules and abscesses that can progress to sinus tracts or fistulas accompanied by the development of extensive scarring, exquisite pain, and malodorous drainage.1 The lesions most commonly occur in intertriginous zones as well as areas rich in apocrine glands. The distinctive and sometimes irreversible clinical features of HS profoundly influence patients’ well-being and have lasting social, personal, and emotional impacts on their lives.2
Bimekizumab is a monoclonal antibody that specifically targets IL-17A and IL-17F, aiming to inhibit the downstream effects responsible for the chronic inflammation and tissue damage characteristic of HS.3 In HS lesions, IL-17 cytokines produced by T helper 17 (Th17) cells stimulate the production of chemokines (such as CC motif chemokine ligand 20) and neutrophil-attracting chemokines (including C-X-C motif chemokine ligands 1 and 8), cytokines (such as granulocyte colony-stimulating factor and IL-19), and epidermal antimicrobial proteins.1,2 This cascade results in the chemotaxis of monocytes and neutrophils in the skin, recruiting additional Th17 and myeloid cells and further amplifying IL-17 production.1
Bimekizumab’s mechanism of action strategically disrupts this feed-forward inflammatory loop, decreasing the transcription of neutrophil-attracting chemokines, IL-19, and epidermal antimicrobial proteins (Figure).1,2 This leads to diminished recruitment of Th17 cells and inhibits the chemotaxis of monocytes and neutrophils in the skin, effectively addressing the chronic inflammation and tissue damage characteristic of HS.

We present a comprehensive review of the current standards of care, the underlying molecular pathophysiology of HS, and evaluation of the efficacy and safety of bimekizumab.
Evaluating HS Severity
The Hurley staging system provides a valuable framework for evaluating the severity of HS based on lesion characteristics. Stage I is characterized by abscess formation without tracts or scars. Stage II is characterized by recurrent abscesses with sinus tracts and scarring. Stage III is characterized by diffuse involvement, multiple interconnected sinus tracts, and abscesses across an entire area, leaving little to no uninvolved skin.4
Treatment strategies for HS vary based on Hurley staging (eTable).5-11 For mild cases (stage I), topical and intralesional therapies are common, while moderate to severe cases (stages II and III) may require extensive surgical approaches or systemic drugs such as antibiotics, hormonal therapies, retinoids, or immunosuppressive/biologic agents.2


Adalimumab, an anti–tumor necrosis factor (TNF) α monoclonal antibody, was the first US Food and Drug Administration (FDA)–approved biologic for HS. Secukinumab, a monoclonal antibody against IL-17A, subsequently was approved by the FDA for moderate to severe HS.12 Off-label use of biologics including infliximab and ustekinumab expands the available treatment options for HS. In one Phase II randomized clinical trial (RCT), infliximab showed efficacy in reducing Hidradenitis Suppurativa Severity Index scores, with 26.7% (4/15) of patients achieving a 50% or greater reduction compared to placebo, although this was not statistically significant. Similarly, ustekinumab demonstrated promising results, with 47.1% (8/17) of patients achieving Hidradenitis Suppurativa Clinical Response (HiSCR) at week 40.2 This multifaceted approach aims to address the varying degrees of severity and optimize outcomes for individuals with HS.
Molecular Pathophysiology of HS
The pathogenesis of HS is multifactorial, involving a complex interplay of genetic, environmental, and behavioral factors.2 Approximately 33% to 40% of patients with HS worldwide report a first-degree relative with the condition, indicating a hereditary element with an autosomal-dominant transmission pattern and highlighting the global relevance of genetic factors in HS.4 Hidradenitis suppurativa is highly prevalent in individuals with obesity, likely due to increased intertriginous surface area, skin friction, sweat production, and hormonal changes in these patients. Smoking also commonly is associated with HS, with nicotine potentially contributing to increased follicular plugging.1 Hormonal influences also play a role, as evidenced by a greater prevalence of HS in females, disease onset typically occurring between puberty and menopause, and symptomatic fluctuations correlating with menstrual cycles and exogenous hormones.4
Altered infundibular keratinization with subsequent hyperkeratosis/occlusion and innate immune pathway activation are key events leading to development of HS.1 These events are mediated by release of pathogen- and danger-associated molecular patterns, leading to inflammasome-mediated IL-1α release, followed by downstream cytokine release.2 Elevated levels of TNF-α, IL-1Β, IL-10, IL-17, and particularly IL-17A have been detected in HS lesional skin. The IL-17 family comprises multiple members, namely IL-17A, IL-17C, IL-17E, and IL17F. IL-17A and IL-17F often are co-expressed and secreted predominantly by a subset of CD4+ T helper cells, namely Th17 cells.2 IL-17 cytokines exert pro-inflammatory effects, influencing immune cell activity and contributing to skin inflammation, particularly in HS.
Given the pivotal role of IL-17 in the pathogenesis of HS, the exploration of IL-17–targeted agents has become a focal point in clinical research. Bimekizumab, a novel IL-17 inhibitor, has emerged as a promising candidate, offering a potential breakthrough in the treatment landscape for individuals affected by HS.
Bimekizumab for HS Management
A phase II, double-blind, placebo-controlled RCT included 90 patients with moderate to severe HS (age range, 18-70 years) who were randomly assigned in a 2:1:1 ratio to receive either bimekizumab 320 mg every 2 weeks (with a 640-mg loading dose at baseline)(n=46), placebo (n=21), or adalimumab 40 mg once weekly from week 4 onward (following an initial 160-mg loading dose at baseline and 80-mg dose at week 2)(n=21). The study included a 12-week treatment period followed by a 20-week safety follow-up period. The primary endpoint was the achievement of HiSCR50—defined as a reduction of at least 50% nodules, coupled with no increase in the number of abscesses or draining fistulas relative to baseline—at week 12. Additionally, the study assessed the number of patients who achieved a modified HiSCR with 75% reduction (HiSCR75) of combined abscess and inflammatory nodule count or a modified HiSCR with 90% reduction (HiSCR90). At week 12, the modeled response rates were estimated using a Bayesian logistic regression model. For HiSCR50, the modeled rate for bimekizumab was 57.3%, with an observed rate of 62.5% (25/40), compared to a modeled rate of 26.1% for placebo (observed rate, 27.8% [5/18]). The posterior probability of superiority for bimekizumab over placebo was 0.998. By week 12, bimekizumab-treated patients achieved modeled HiSCR75 and HiSCR90 rates of 46.0% and 32.0%, respectively, with observed rates of 50.0% (20/40) for HiSCR75 and 35.0% (14/40) for HiSCR90. In comparison, placebo-treated patients achieved modeled HiSCR75 and HiSCR90 rates of 10.0% and 0%, respectively, with observed rates of 11.1% (2/18) for HiSCR75 and 0% (0/18) for HiSCR90. Adalimumab-treated participants demonstrated intermediate results, achieving modeled HiSCR75 and HiSCR90 rates of 35.0% and 15.0%, respectively, with observed rates of 38.88% (7/18) for HiSCR75 and 16.66% (3/18) for HiSCR90.7
Bimekizumab was effective in the treatment of moderate to severe HS with comparable results to adalimumab.7 The incidence of treatment-emergent adverse events was similar across treatment arms (bimekizumab, 69.6% [32/46]; placebo, 61.9% [13/21]; adalimumab, 71.4% [15/21]). The most common treatment-emergent adverse events in the biologic treatment arms were infections (43.5% [20/46] in the bimekizumab group and 42.9% [9/21] in the adalimumab group), skin and subcutaneous tissue disorders (28.3% [13/46] in the bimekizumab group and 42.9% [9/21] in the adalimumab group), and general disorders/administration site conditions (21.7% [10/46] in the bimekizumab group and 23.8% [5/21] in the adalimumab group). Serious adverse events occurred in 4.3% (2/46) of patients in the bimekizumab group, 9.5% (2/21) of patients in the placebo group, and 4.8% (1/21) of patients in the adalimumab group. Serious adverse events that required hospitalization were due to anemia and empyema in the bimekizumab group; worsening HS in the adalimumab group; and myocardial infarction, hypoesthesia, headache, and dizziness in the placebo group. No deaths occurred in this study. Overall, bimekizumab was well tolerated, and discontinuation rates were low across all arms. The primary reason for discontinuation was withdrawal of consent (not due to an adverse event) or loss to follow-up.7
Two completed 48-week phase III RCTs, BE HEARD I and BE HEARD II, evaluated the efficacy and safety of bimekizumab in patients with moderate to severe HS.13 In both trials, 2 bimekizumab dosing regimens (320 mg every 2 weeks and 320 mg every 4 weeks) were compared with placebo during the 16-week initial and 32-week maintenance treatment periods. The primary endpoint of week 16 was achieved by 47.8% (138/289) and 51.9% (151/291) of patients receiving bimekizumab every 2 weeks in BE HEARD I (n=505) and BE HEARD II (n=509), respectively, compared with 29.2% (21/72) and 32.4% (24/74) of the placebo group. The bimekizumab 320 mg every 4 weeks dosing regimen met the primary endpoint only in BE HEARD II, with 53.5% (77/144) of patients achieving HiSCR50 compared to 32.4% (24/74) with placebo (P=0.0038).13 Both trials met the key secondary endpoint of HiSCR75 at week 16 for bimekizumab 320 mg every 2 weeks vs placebo. In BE HEARD I, 33.6% (97/289) of patients receiving bimekizumab achieved HiSCR75 versus 18.1% (13/72) taking placebo. In BE HEARD II, 35.7% (104/291) of patients receiving bimekizumab achieved HiSCR75 vs 16.2% (12/74) taking placebo. Responses were maintained or increased through week 48 in both trials. The most common treatment-emergent adverse events through week 48 were worsening HS, COVID-19 infection, diarrhea, oral candidiasis, and headache.13
A smaller scale case series investigated the use of bimekizumab in 4 female patients aged 20 to 62 years with moderate to severe HS and concomitant plaque or inverse psoriasis.8 A monthly loading dose of 320 mg was given during weeks 0 to 12 followed by a maintenance dose of 320 mg administered every 8 weeks. The International Hidradenitis Suppurativa Score System, visual analogue scale, and Dermatology Life Quality Index were used to assess the effectiveness of therapy by comparing scores before and after 4 and 16 weeks of treatment. A reduction of pain and improvement of HS lesions was observed in 3 (75.0%) patients after the first dosage of bimekizumab, with completed remission of HS by week 16. The fourth patient (25.0%) experienced substantial improvement in all measures, although not complete remission. All 4 patients remained on bimekizumab, and no adverse effects were reported.8
A meta-analysis evaluated 16 RCTs of 9 biologics and 3 small-molecule inhibitors in 2076 patients with HS.10 Secukinumab was not included in this meta-analysis. Only adalimumab (risk ratio, 1.77; 95% CI, 1.44-2.17) and bimekizumab (risk ratio, 2.25; 95% CI, 1.03-4.92) were superior to placebo in achieving HiSCR response at weeks 12 to 16 in 5 RCTs and 1 RCT, respectively; however, no statistically significant differences were noted between adalimumab and bimekizumab (P=.56). This analysis concluded that adalimumab and bimekizumab are the only 2 biologics efficacious in reaching HiSCR and consistently improved both disease severity and quality of life in patients with HS with an acceptable safety profile.10 Furthermore, these biologics had no increase in serious adverse events when compared to placebo.10
A network meta-analysis of 10 clinical trials involving more than 900 total participants evaluated nonsurgical therapies for HS. The analysis used Surface Under the Cumulative Ranking curve (SUCRA) values to estimate the efficacy of treatments in achieving clinical response according to HiSCR criteria. These values range from 0% to 100%, with 100% representing the best possible ranking for efficacy. Bimekizumab showed the highest estimated efficacy with a SUCRA value of 67%, followed by adalimumab (64%), anakinra (49%), and placebo (19%). These SUCRA values indicate the relative ranking of treatments, with higher values suggesting greater likelihood of achieving clinical response, rather than representing the actual percentage of patients achieving HiSCR. Bimekizumab was found to be more efficacious than placebo (P<.05).14
Building on the initial evidence of bimekizumab’s efficacy, BE HEARD I and BE HEARD II addressed some limitations of prior studies, including small sample sizes and insufficient stratification.13 Notably, stratification by baseline Hurley stage severity (ie, the most severe stage of disease assigned at baseline) and baseline systemic antibiotic use helped mitigate bias and ensured a more robust assessment of treatment efficacy; however, certain limitations persist. While the trials demonstrated rapid and clinically meaningful responses maintained up to 48 weeks, longer-term data beyond this period are limited, leaving gaps in understanding the durability of treatment effects over years. Additionally, despite appropriate stratification, the generalizability of the findings to broader patient populations remains unclear, as trial participants may not fully represent the diversity of patients seen in clinical practice.13
Future research is needed to address these limitations. The use of validated HS biomarkers as endpoints could enhance the ability to evaluate biologic efficacy and identify predictors of response. Comparative studies with other biologics also are warranted to establish the relative efficacy of bimekizumab within the growing therapeutic landscape for HS. Finally, real-world evidence from larger and more diverse populations will be critical to confirm the trial findings and assess long-term safety and effectiveness in routine clinical practice.13
Conclusion
The existing literature and recent phase III RCTs, BE HEARD I and BE HEARD II, demonstrate that bimekizumab is an effective treatment for moderate to severe HS, with robust efficacy according to HiSCR scores and sustained responses through 48 weeks. These trials addressed some prior limitations, including small sample sizes and insufficient stratification, providing a more comprehensive evaluation of bimekizumab’s clinical impact. The safety profile of bimekizumab remains favorable, with low discontinuation rates and manageable adverse events, such as infection, gastrointestinal upset, headache, and injection-site reactions. Long-term efficacy and safety data beyond 48 weeks still are needed to fully establish its durability and impact in diverse populations. The recent FDA approval of bimekizumab for moderate to severe HS provides patients with a new treatment option, offering a more positive clinical outlook.
- Malvaso D, Calabrese L, Chiricozzi A, et al. IL-17 inhibition: a valid therapeutic strategy in the management of hidradenitis suppurativa. Pharmaceutics. 2023;15:2450. doi:10.3390 /pharmaceutics15102450
- Markota C¡agalj A, Marinovic´ B, Bukvic´ Mokos Z. New and emerging targeted therapies for hidradenitis suppurativa. Int J Mol Sci. 2022;23:3753. doi:10.3390/ijms23073753
- Zouboulis CC, Frew JW, Giamarellos-Bourboulis EJ, et al. Target molecules for future hidradenitis suppurativa treatment. Exp Dermatol. 2021;30 suppl 1:8-17. doi:10.1111/exd.14338
- Ballard K, Shuman VL. Hidradenitis suppurativa. StatPearls [Internet]. Updated May 6, 2024. Accessed December 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534867/
- Rathod U, Prasad PN, Patel BM, et al. Hidradenitis suppurativa: a literature review comparing current therapeutic modalities. Cureus. 2023;15:E43695. doi:10.7759/cureus.43695
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: current and emerging treatments. J Am Acad Dermatol. 2020;82:1061-1082. doi:10.1016/j.jaad.2019.08.089
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Molinelli E, Gambini D, Maurizi A, et al. Bimekizumab in hidradenitis suppurativa: a valid and effective emerging treatment. Clin Exp Dermatol. 2023;48:1272-1274. doi:10.1093/ced/llad229
- Martora F, Megna M, Battista T, et al. Adalimumab, ustekinumab, and secukinumab in the management of hidradenitis suppurativa: a review of the real-life experience. Clin Cosmet Investig Dermatol. 2023;16:135-148. doi:10.2147/CCID.S391356
- Huang CH, Huang IH, Tai CC, et al. Biologics and small molecule inhibitors for treating hidradenitis suppurativa: a systematic review and meta-analysis. Biomedicines. 2022;10:1303. doi:10.3390 /biomedicines10061303
- Ojeda Gómez A, Madero Velázquez L, Buendía Sanchez L, et al. Inflammatory bowel disease new-onset during secukinumab therapy: real-world data from a tertiary center. Rev Esp Enferm Dig. 2021;113: 858-859. doi:10.17235/reed.2021.8397/2021
- Martora F, Marasca C, Cacciapuoti S, et al. Secukinumab in hidradenitis suppurativa patients who failed adalimumab: a 52-week real-life study. Clin Cosmet Investig Dermatol. 2024;17:159-166. doi:10.2147 /CCID.S449367
- Kimball AB, Jemec GBE, Sayed CJ, et al. Efficacy and safety of bimekizumab in patients with moderate-to-severe hidradenitis suppurativa (BE HEARD I and BE HEARD II): two 48-week, randomised, double-blind, placebo-controlled, multicentre phase 3 trials. Lancet. 2024;403:2504-2519. doi:10.1016 /S0140-6736(24)00101-6
- Gupta AK, Shear NH, Piguet V, et al. Efficacy of non-surgical monotherapies for hidradenitis suppurativa: a systematic review and network meta-analyses of randomized trials. J Dermatolog Treat. 2022;33:2149-2160. doi:10.1080/09546634.2021.1927949
- Malvaso D, Calabrese L, Chiricozzi A, et al. IL-17 inhibition: a valid therapeutic strategy in the management of hidradenitis suppurativa. Pharmaceutics. 2023;15:2450. doi:10.3390 /pharmaceutics15102450
- Markota C¡agalj A, Marinovic´ B, Bukvic´ Mokos Z. New and emerging targeted therapies for hidradenitis suppurativa. Int J Mol Sci. 2022;23:3753. doi:10.3390/ijms23073753
- Zouboulis CC, Frew JW, Giamarellos-Bourboulis EJ, et al. Target molecules for future hidradenitis suppurativa treatment. Exp Dermatol. 2021;30 suppl 1:8-17. doi:10.1111/exd.14338
- Ballard K, Shuman VL. Hidradenitis suppurativa. StatPearls [Internet]. Updated May 6, 2024. Accessed December 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534867/
- Rathod U, Prasad PN, Patel BM, et al. Hidradenitis suppurativa: a literature review comparing current therapeutic modalities. Cureus. 2023;15:E43695. doi:10.7759/cureus.43695
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: current and emerging treatments. J Am Acad Dermatol. 2020;82:1061-1082. doi:10.1016/j.jaad.2019.08.089
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Molinelli E, Gambini D, Maurizi A, et al. Bimekizumab in hidradenitis suppurativa: a valid and effective emerging treatment. Clin Exp Dermatol. 2023;48:1272-1274. doi:10.1093/ced/llad229
- Martora F, Megna M, Battista T, et al. Adalimumab, ustekinumab, and secukinumab in the management of hidradenitis suppurativa: a review of the real-life experience. Clin Cosmet Investig Dermatol. 2023;16:135-148. doi:10.2147/CCID.S391356
- Huang CH, Huang IH, Tai CC, et al. Biologics and small molecule inhibitors for treating hidradenitis suppurativa: a systematic review and meta-analysis. Biomedicines. 2022;10:1303. doi:10.3390 /biomedicines10061303
- Ojeda Gómez A, Madero Velázquez L, Buendía Sanchez L, et al. Inflammatory bowel disease new-onset during secukinumab therapy: real-world data from a tertiary center. Rev Esp Enferm Dig. 2021;113: 858-859. doi:10.17235/reed.2021.8397/2021
- Martora F, Marasca C, Cacciapuoti S, et al. Secukinumab in hidradenitis suppurativa patients who failed adalimumab: a 52-week real-life study. Clin Cosmet Investig Dermatol. 2024;17:159-166. doi:10.2147 /CCID.S449367
- Kimball AB, Jemec GBE, Sayed CJ, et al. Efficacy and safety of bimekizumab in patients with moderate-to-severe hidradenitis suppurativa (BE HEARD I and BE HEARD II): two 48-week, randomised, double-blind, placebo-controlled, multicentre phase 3 trials. Lancet. 2024;403:2504-2519. doi:10.1016 /S0140-6736(24)00101-6
- Gupta AK, Shear NH, Piguet V, et al. Efficacy of non-surgical monotherapies for hidradenitis suppurativa: a systematic review and network meta-analyses of randomized trials. J Dermatolog Treat. 2022;33:2149-2160. doi:10.1080/09546634.2021.1927949
Bimekizumab for Hidradenitis Suppurativa: Pathophysiology and Promising Interventions
Bimekizumab for Hidradenitis Suppurativa: Pathophysiology and Promising Interventions
PRACTICE POINTS
- Management of hidradenitis suppurativa (HS) includes lifestyle modifications as well as topical and systemic antibiotics, intralesional and systemic corticosteroids, retinoids, hormonal therapies, immunosuppressants, biologic agents, and minor to invasive surgical procedures.
- Adalimumab, secukinumab, and more recently bimekizumab are biologics that are approved by the US Food and Drug Administration for the treatment of moderate to severe HS.
- Bimekizumab is a monoclonal antibody targeting IL-17A and IL-17F that has demonstrated strong clinical efficacy in generating a sustained clinical response in moderate to severe HS-related clinical features.