User login
Nonatherosclerotic limb ischemia: Prompt evaluation and diagnosis
Timely diagnosis of limb ischemia is critical to limb health and limb salvage. The cause in most cases is related to atherosclerosis, and patients with limb ischemia are usually older and have risk factors for atherosclerosis, such as smoking, diabetes, hypertension, hyperlipidemia, and coronary artery disease. When younger patients develop limb ischemia, the diagnosis is often delayed since the index of suspicion is quite low in the absence of the usual risk factors.
Here, we discuss several nonatherosclerotic causes of limb ischemia: popliteal artery entrapment syndrome, popliteal artery aneurysm, cystic adventitial disease, persistent sciatic artery, phlegmasia cerulea dolens, Buerger disease, Takayasu arteritis, arterial thoracic outlet syndrome, and external iliac endofibrosis (Table 1). Our goal is to help clinicians make a timely diagnosis and ultimately save the patient’s limb.
POPLITEAL ARTERY ENTRAPMENT SYNDROME
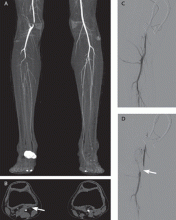
Popliteal artery entrapment syndrome occurs when the popliteal artery becomes compressed in the popliteal fossa, particularly during exercise.1,2 The underlying problem may be that the popliteal artery has an aberrant course lateral to the medial head of the gastrocnemius muscle, or the medial head of the gastrocnemius may have an abnormal insertion, or there may be fibrous bands in the popliteal fossa, or a combination of these (Figure 1).1–3 Functional popliteal artery entrapment syndrome occurs when there is compression of the artery without an anatomic cause.1–3
The classic clinical presentation is a young athletic patient with calf or foot claudication (crampy pain with exercise, relieved with rest), but other symptoms can include coldness, paresthesias, and numbness. Pain at rest and tissue loss are rare on presentation but may develop if the diagnosis and treatment are delayed.3
Continued compression and microtrauma to the artery may lead to an intramural hematoma, thrombus formation, aneurysmal degeneration, dissection, or even acute thrombosis.2 If the diagnosis is delayed, the patient’s condition may progress from intermittent arterial compression with plantar flexion to complete arterial thrombosis and critical limb ischemia, putting the patient at risk of limb loss.
Diagnosing popliteal artery entrapment syndrome
The diagnostic workup includes a detailed history with a focus on the cause of pain (usually exercise), a comprehensive physical examination that includes looking for wounds, and a thorough pulse examination.
The workup should start with noninvasive imaging such as duplex arterial ultrasonography with and without provocative measures (plantar flexion), the ankle-brachial index with and without provocative measures, and exercise treadmill testing with ankle-brachial index measurement.1,2 Plantar flexion may be necessary to elicit arterial compression that is usually absent at rest.
Magnetic resonance imaging (MRI) and computed tomography (CT) of the lower extremity are useful to identify an arterial abnormality and aberrant muscle anatomy1,3; MRI is currently the gold standard for delineating the muscles of the popliteal fossa.4 If these studies do not shed light on the diagnosis, arterial angiography with and without provocative maneuvers is useful in identifying compression of the popliteal artery.1–3
Treating popliteal artery entrapment syndrome
Treatment depends on the level of arterial injury.
For patients with symptoms but no evidence of arterial injury, the most common procedure offered is popliteal fossa decompression.1–3 This involves surgical release of the medial head of the gastrocnemius muscle and other muscles compressing the popliteal artery.
For patients with evidence of arterial injury such as stenosis, dissection, or aneurysm, bypass grafting may be required.
For patients who present with acute limb ischemia, both surgical thrombectomy with possible bypass and intraarterial lysis have been described.1,2,5
POPLITEAL ARTERY ANEURYSM
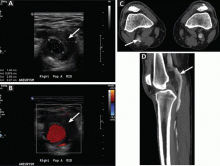
Popliteal artery aneurysm (Figure 2) is the most common type of aneurysm of the peripheral arteries of the lower extremity and is present in about 1% of men over age 65. Fifty percent are bilateral, and 50% are associated with an abdominal aortic aneurysm.6,7 While up to 80% patients with this type of aneurysm have no symptoms at the time of diagnosis, symptoms develop at a rate of 14% per year, with acute limb ischemia occurring in up to one-third of cases.6,7
When popliteal artery aneurysm progresses to acute limb ischemia, the consequences are often deleterious, as the tibial arteries distal to the popliteal artery are often occluded, limiting treatment options.
Popliteal artery aneurysm is defined as a local dilation of the artery of 2 cm or greater or an increase in the diameter to 1.5 times normal.6
Acute thrombosis of the aneurysm with limb ischemia is the most common presenting symptom and occurs in 50% of symptomatic cases of popliteal artery aneurysm.7 Almost 25% of patients present with intermittent claudication secondary to thrombosis, partial thrombosis with distal embolization, or combined aneurysmal and atherosclerotic disease. Compression of the popliteal vein by the popliteal artery aneurysm can cause leg swelling with or without deep vein thrombosis in up to 5% of patients.6 Rupture is very rare, with a rate of 2% to 4%.6,7
Diagnosing popliteal artery aneurysm
The diagnosis can be made with arterial duplex ultrasonography, which is also useful for follow-up surveillance.6–8 In the acute setting, computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is useful not only to identify the popliteal aneurysm, but also to define the distal tibial outflow vessels.6,7
Treating popliteal artery aneurysm
Management of an acutely thrombosed popliteal artery aneurysm starts with systemic anticoagulation with intravenous heparin, followed initially by arterial angiography and lysis.8–11 This approach has been shown to be safe and effective even in the absence of arterial runoff distal to the thrombosed popliteal aneurysm. Conversion to open thrombectomy and bypass can be done if initial lytic therapy fails, if the patient develops complications of lytic therapy, or if the patient needs emergency revascularization because of motor and neurologic deficits in the affected extremity.8,10,11
How to manage the asymptomatic patient depends on the size of the aneurysm. Most studies recommend 2 cm or larger as the criterion for repair,6–8,12 while others suggest treating even smaller aneurysms if thrombus is detected.9 Preoperative imaging before elective treatment of an asymptomatic popliteal artery aneurysm includes either CTA or MRA,8,10 which allows the surgeon to visualize the full extent of the aneurysm to best plan the surgical approach. Diagnostic angiography can help determine the most suitable bypass target and can better characterize tibial outflow.
Asymptomatic popliteal artery aneurysm has traditionally been treated with surgical bypass with exclusion of the aneurysm,6–8,12 but more recently, endovascular approaches using self-expanding stent grafts have been described. Further study is needed to determine the long-term efficacy of the endovascular approach.8,10
CYSTIC ADVENTITIAL DISEASE
Cystic adventitial disease is a rare condition in which a blood vessel is narrowed due to mucin-containing cysts in the adventitia. More than 80% of cases occur in the popliteal artery, but it has been described in other peripheral arteries and veins.13,14 It is more common in men than in women and typically occurs in the 4th or 5th decade of life. Most patients present with the sudden onset of calf claudication without the usual risk factors for peripheral vascular disease.13
Diagnosing cystic adventitial disease
Noninvasive arterial or venous duplex ultrasonography can be a good screening tool, as the cysts appear hypoechoic, but results are operator-dependent. CTA and MRA are the imaging tests of choice, as they can detect the cystic lesions and define vessel anatomy for intervention. Diagnostic angiography does not show the cysts themselves but instead reveals a classic “hourglass” and “scimitar” pattern of arterial narrowing that suggests the underlying pathology.13,14
Treating cystic adventitial disease
Usual treatment is complete cyst resection and vessel reconstruction by surgical bypass. Other therapies include open surgical cyst evacuation and removal of the cyst wall, open surgical cyst aspiration, aspiration guided by ultrasonography or CT, and percutaneous angioplasty. However, these nonsurgical treatments have not been shown to be as effective and long-lasting as cyst excision and bypass.13,14
PERSISTENT SCIATIC ARTERY
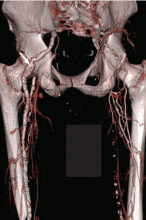
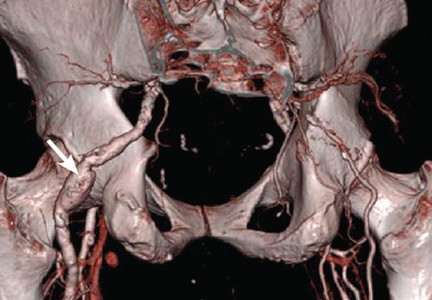
Persistent sciatic artery is a rare developmental abnormality.15–17 Normally, as the femoral artery develops in the embryo, the sciatic artery involutes to form the inferior gluteal artery. But if the femoral system fails to mature, the sciatic artery, which is adjacent to the sciatic nerve posteriorly as it goes through the sciatic foramen, persists and functions as the major artery supplying the lower extremity, continuing to the posterior thigh and joining the popliteal artery (Figure 3).15,17
Persistent sciatic artery has an incidence of 2.5 to 4 per 10,000 per year15 and is bilateral in almost half of cases.16 Up to 40% of patients have no symptoms, but symptoms may develop by age 40 to 50. Because of repeated trauma to the vessel as it passes through the sciatic foramen,18 the persistent sciatic artery typically sustains accelerated atherosclerotic changes that make it susceptible to aneurysm formation,15 and up to 46% of patients present with aneurysmal degeneration.17
Classically, patients present with lower extremity ischemia from atherosclerotic changes in the persistent sciatic artery or aneurysmal degeneration and thromboembolism.15 Rarely, these aneurysms rupture.15,17 Other signs and symptoms include a pulsatile mass in the buttock, lower extremity numbness, motor weakness, and radicular pain along the sciatic nerve distribution from nerve compression.15–17
Physical findings vary but are distinguished by the lack of femoral pulses in the presence of pedal pulses. A pulsatile buttock mass with evidence of lower extremity nerve compression or limb ischemia or both is pathognomonic of a persistent sciatic artery aneurysm.16,18
Diagnosing persistent sciatic artery
Diagnostic angiography is the gold standard imaging test,15,19 although CTA is starting to replace it.16,18
Treating persistent sciatic artery
Persistent sciatic artery that is asymptomatic and is found incidentally does not require repair; however, it should be followed with duplex ultrasonography to look for evidence of aneurysm degeneration. Degeneration requires repair in most cases.15,16,18,19 When the persistent sciatic artery is the only blood supply to the distal extremity, open aneurysm excision and bypass is the treatment of choice.15,16,19 If collateral flow is adequate, endovascular coil embolization is an option.15 Endovascular stent graft placement has also been described.16,19
PHLEGMASIA CERULEA DOLENS
Phlegmasia cerulea dolens is a rare syndrome caused by extensive acute thrombosis of the ileofemoral vein.20–23 It is defined as total or near-total occlusion of the venous outflow of an extremity, causing massive swelling and congestion that impedes arterial inflow.20,22
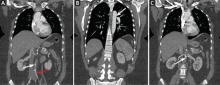
Phlegmasia cerulea dolens is associated with four cardinal signs: edema, violaceous discoloration, pain, and severe venous outflow obstruction (Figure 4).22 Patients present with sudden onset of lower extremity pain, swelling, cyanosis, and arterial ischemia with or without loss of distal pulses.20,22
This syndrome can progress to gangrene and massive fluid sequestration leading to shock and death.21–23 From 25% to 40% of patients die, and of those who survive, 20% to 50% require amputation of the limb.20,23
Risk factors include malignancy, immobility, heart failure, heparin-induced thrombocytopenia, antiphospholipid syndrome, pregnancy, venous catheterization (eg, to insert an inferior vena cava filter), and surgery.20–22
Diagnosing phlegmasia cerulea dolens
The diagnosis is made on clinical suspicion with evidence of iliofemoral deep vein thrombosis. Most experts suggest venous duplex ultrasonography to identify the deep vein thrombosis,23 although CT or MR venography can be used to better delineate the proximal extent of the thrombus.20,23
Treating phlegmasia cerulea dolens
Initial management is aggressive fluid resuscitation, elevation of the affected limb, strict bed rest, and anticoagulation with intravenous heparin.20,23 Interventions are aimed at urgently restoring venous outflow to prevent progression to venous gangrene and limb loss.
Although conservative therapy can succeed by itself,23 if the condition does not improve or has already progressed to an advanced stage, the two mainstays of treatment are open venous thrombectomy and endovascular treatment.21–23 Endovascular treatment includes catheter-directed thrombolytic therapy (with or without percutaneous mechanical or pharmacomechanical thrombectomy) and stenting.20,23 The success rate for endovascular therapy can be as high as 90% with near-complete resolution of thrombosis.20 A disadvantage is that, compared with open surgical thrombectomy, more time is needed to achieve venous outflow.20,22
If endovascular therapy is ineffective, if lytic therapy is contraindicated, or if the disease has progressed to gangrene, open surgical thrombectomy with possible fasciotomy is the preferred option.20,21,23 Open surgery has the advantage of restoring venous outflow faster, but disadvantages include the inability to open the smaller veins of the extremity, blood loss, and risks associated with general anesthesia.20–22
BUERGER DISEASE
Buerger disease (thromboangiitis obliterans) is a nonatherosclerotic segmental inflammatory disease involving the small and medium-sized vessels of the arms and legs.24–27 It is differentiated from other vasculitides by its marked male predominance, its close association with smoking, the rarity of systemic signs and symptoms, and the absence of elevated inflammatory markers.26
The rate of major amputation is reported to be 11% at 5 years and 23% at 20 years.24
The classic patient is a young male smoker with symptoms of arterial disease before age 45.24,26 Patients can present with migratory thrombophlebitis or signs of arterial insufficiency in the upper or lower extremities. Two or more limbs are commonly involved. Arterial insufficiency can range from claudication and exertional discomfort of the extremity to ischemic pain at rest leading to ulceration of the distal fingers and toes. Physical findings are similar to those seen in peripheral vascular disease and arterial insufficiency, with decreased arterial brachial index, cool extremities, and wounds.
Diagnosing Buerger disease
- The Shionoya diagnostic criteria for Buerger disease are the following five clinical features24,27:
- History of smoking
- Onset before age 50
- Infrapopliteal arterial occlusive disease
- Upper-limb involvement or phlebitis migrans
- Absence of atherosclerotic risk factors other than heavy smoking.
Various other major and minor criteria have been described to make the diagnosis as well.24
There is no specific laboratory test to confirm the diagnosis of Buerger disease. A full panel of laboratory tests should be sent to rule out other causes of arterial insufficiency and vasculitides; these tests should include C-reactive protein, rheumatoid factor, erythrocyte sedimentation rate, antinuclear antibodies, antiphospholipid antibodies, anti-Scl-70 antibodies, anticentromere antibodies, complement level measurement, and hypercoagulability workup.
Imaging studies include arterial duplex ultrasonography with ankle-brachial indices and segmental pressures and CTA or MRA.26 Angiography can show a “corkscrew” pattern of occlusive disease and collateral formation, which is highly associated with Buerger disease.24
Treating Buerger disease
The only treatment shown to reduce the risk of amputation is complete abstention from tobacco and nicotine (smoking, secondhand smoke, and nicotine patches and gum).24,26
Symptoms of claudication can be managed with aspirin, clopidogrel, vasodilators, pentoxifylline, and cilostazol.26
Surgical bypass is rarely an option, as Buerger disease typically affects the distal blood vessels, thus precluding bypass, and the 5-year patency rate is only 49%.26 Other treatments including arterial thrombolysis, sympathectomy, stem cell injection, spinal cord stimulators, omental grafting, and immunomodulation have been described, but there are only limited data to offer guidance in choosing the appropriate one.24
TAKAYASU ARTERITIS
Takayasu arteritis is a form of vasculitis involving the aorta and its main branches (Figure 5).28 Although seen around the world, it has a higher incidence in young Asian women. Patients can present with systemic symptoms such as fever, fatigue, vague pain, and cardinal signs of limb ischemia associated with Takayasu arteritis, such as weak or absent pulses, differences between the arms in pulses and blood pressures, unobtainable blood pressure measurement in one or both arms, limb fatigability, and pain.28
Diagnosing Takayasu arteritis
Multiple diagnostic criteria have been proposed to define Takayasu arteritis.28 CTA, MRA, and positron emission tomography have replaced invasive angiography as the diagnostic imaging tests of choice.29
Treating Takayasu arteritis
Takayasu arteritis has an acute and chronic course. Interventions are typically reserved for severe cases, with indications that include uncontrollable hypertension from renal artery stenosis, severe coronary or cerebrovascular disease, severe aortic regurgitation or coarctation, stenotic or occlusive lesions resulting in critical limb ischemia, and aneurysm at risk of rupture.28–30
THORACIC OUTLET SYNDROME
Thoracic outlet syndrome is compression of the brachial plexus, subclavian vein, or subclavian artery as it exits the thoracic outlet through an area known as the scalene triangle, which is bordered by the anterior scalene, first rib, and clavicle.31 Presenting symptoms depend on the structure compressed.
By far the most common presentation32 is neurogenic thoracic outlet syndrome, accounting for more than 90% of cases, followed by venous thoracic outlet syndrome. Arterial thoracic outlet syndrome is the least frequent at less than 1%, but carries the greatest morbidity with potential for limb loss.31–33
The subclavian artery exits the thoracic outlet between the anterior and middle scalene muscles, and then travels over the first rib and underneath the clavicle.31 Repeated trauma from compression of the artery results in intimal injury leading to compression, stenosis, occlusion, or aneurysm formation.31,32
Symptoms of arterial thoracic outlet syndrome can start out as effort fatigue of the upper extremity secondary to compression. These symptoms are usually vague and difficult to define,31 as these patients typically are young and do not have atherosclerotic risk factors that would prompt suspicion of a vascular cause.
The most common presentation of arterial thoracic outlet syndrome is upper extremity embolization from a partially thrombosed aneurysm or area of stenosis with ischemia.32 Symptoms can range from ischemia of the fingers due to microembolization to acute limb ischemia due to complete thrombosis of the subclavian artery.31,32 Arterial thoracic outlet syndrome is most commonly associated with a bony abnormality (ie, cervical rib or anomalous first rib),31–33 and on physical examination the bony abnormality may be palpated in the supraclavicular fossa.31
Other physical findings include a bruit over the subclavian artery, a blood pressure difference of 20 mm Hg or more between the affected and unaffected arms, loss of brachial, radial, or ulnar pulses with arm abduction, and loss of the radial pulse with the head rotated to the affected side as the patient takes a deep breath (the Adson maneuver).31 While postural changes in the pulse examination hint at arterial thoracic outlet syndrome, extremity pulses may be reduced or even absent in up to 60% of normal patients.32
Diagnosing thoracic outlet syndrome
The workup should start with noninvasive imaging with pulse volume recording and wrist and finger systolic pressures, followed by arterial duplex ultrasonography.
Chest radiography may be able to identify bony abnormalities, and MRA or CTA with the patient in two positions—ie, arms down at the sides, and arms held above the head—can help identify arterial compression from bony or muscular structures in the thoracic outlet. Upper extremity angiography provides high-resolution imaging of the digital arteries and can help identify a subclavian artery aneurysm, which may be a subtle finding.31
It is important to have objective evidence of arterial or venous mechanical obstruction before deciding to remove the first rib.
Treating thoracic outlet syndrome
Treatment is determined by the severity and acuity of symptoms. If the patient presents with acute limb ischemia, prompt treatment with either open surgery or endovascular treatment is required.31,32,34 Once the acute phase has resolved or if the patient presents with chronic disease, open surgical repair is needed to remove the compression of the artery. If an arterial abnormality is identified (aneurysm or significant stenosis), an arterial reconstruction with bypass may be required.31
The standard treatment for thoracic outlet syndrome is resection of the first rib (and removal of the cervical rib if present).31,34 This can be by a transaxillary approach unless arterial reconstruction is needed, in which case a supraclavicular approach is used.31,34 When a patient without symptoms is found to have evidence of arterial compression, most experts would recommend resection of the first rib if there is evidence of an arterial abnormality, or follow-up with duplex imaging for patients with only subtle findings.31
EXTERNAL ILIAC ENDOFIBROSIS
External iliac endofibrosis is a rare cause of intermittent claudication, typically in high-performance athletes, resulting from thickening of the intima in the external iliac artery causing luminal narrowing and resultant ischemia.35–37 The estimated incidence is as high as 20% in elite competitive cyclists, and the condition has been described in other sports as well.37
External iliac endofibrosis typically presents as unilateral leg pain or cramping at near-maximal exercise with an associated feeling of swelling and numbness on the affected side.35,37 It is bilateral in up to 15% of cases.35 While claudication of the thigh is the predominant presenting symptom, dissection and thrombosis of the external iliac artery have been described, presenting with acute limb ischemia in up to 4% of patients.35,36
The condition has been attributed to factors such as physical position, psoas hypertrophy, tethering of the external iliac artery to the psoas muscle, kinking and tortuosity of the vessel, and high-flow states secondary to increased cardiac output and adaptive systolic hypertension.36,37
Diagnosing external iliac endofibrosis
The diagnosis is difficult, as symptoms typically manifest only during maximal exercise. Delays of 12 to 41 months between the onset of symptoms and diagnosis have been reported.37 Physical findings are nonspecific, and pulses and ankle-brachial indices are typically normal at rest. A careful history with a focus on location and duration of symptoms and a high index of suspicion have been shown to increase the sensitivity of diagnosis.36
Noninvasive vascular imaging with arterial duplex ultrasonography with physiologic studies (the ankle-brachial index) at rest and at maximal exertion should be obtained first.35,37 If findings on ultrasonography are positive, CTA or MRA can be used to identify a suspected stenosis.
Diagnostic angiography is still the gold standard for imaging, as real-time images of the artery with different leg positions can be obtained and pressure gradients can be measured with or without the use of a vasodilator to determine the hemodynamic significance of a lesion.35–37
Treating external iliac endofibrosis
Treatment should initially be conservative. Recreational athletes should consider changing to a sport that does not require hip flexion, and cyclists should be advised to reduce the amount of time spent cycling and to raise the handlebars or bring the saddle position forward to minimize hip flexion.37
Definitive treatment is open surgical repair. Surgical options include arterial release of the tethered artery, endofibrosectomy and vessel shortening, endofibrosectomy and patch angioplasty, and interposition bypass grafting.35–37
- Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg 2012; 55:252–262.e30.
- Gokkus K, Sagtas E, Bakalim T, Taskaya E, Aydin AT. Popliteal entrapment syndrome. A systematic review of the literature and case presentation. Muscles Ligaments Tendons J 2014; 4:141–148.
- Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg 2008; 48(suppl 6):61S–65S.
- Liu Y, Sun Y, He X, et al. Imaging diagnosis and surgical treatment of popliteal artery entrapment syndrome: a single-center experience. Ann Vasc Surg 2014; 28:330–337.
- Kim SY, Min SK, Ahn S, Min SI, Ha J, Kim SJ. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg 2012; 55:90–97.
- Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl 2007; 89:466–471.
- Dawson J, Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene—is rupture really a danger? Prog Cardiovasc Dis 2013; 56:26–35.
- Stone PA, Jagannath P, Thompson SN, et al. Evolving treatment of popliteal artery aneurysms. J Vasc Surg 2013; 57:1306–1310.
- Eslami MH, Rybin D, Doros G, Farber A. Open repair of asymptomatic popliteal artery aneurysm is associated with better outcomes than endovascular repair. J Vasc Surg 2015; 61:663–669.
- Serrano Hernando FJ, Martínez López I, Hernández Mateo MM, et al. Comparison of popliteal artery aneurysm therapies. J Vasc Surg 2015; 61:655–661.
- Marty B, Wicky S, Ris HB, et al. Success of thrombolysis as a predictor of outcome in acute thrombosis of popliteal aneurysms. J Vasc Surg 2002; 35:487–493.
- Hall HA, Minc S, Babrowski T. Peripheral artery aneurysm. Surg Clin North Am 2013; 93:911–923.
- Veraldi GF, Scudo G, Scorsone L, Mezzetto L, Castellani RL. Cystic adventitial disease of the popliteal artery: report of two cases and review of the literature. G Chir 2014; 35:229–234.
- Desy NM, Spinner RJ. The etiology and management of cystic adventitial disease. J Vasc Surg 2014; 60:235–245.e1–e11.
- Patel MV, Patel NH, Schneider JR, Kim S, Verta MJ. Persistent sciatic artery presenting with limb ischemia. J Vasc Surg 2013; 57:225–229.
- Kesri G, Mangtani J, Kumar G, Dangayach KK. Persistent sciatic artery aneurysm with lower limb ischemia. Case Rep Vasc Med 2014; 2014:183969.
- Nuño-Escobar C, Pérez-Durán MA, Ramos-López R, et al. Persistent sciatic artery aneurysm. Ann Vasc Surg 2013; 27:1182.e13–e16.
- Vaz C, Machado R, Rego D, Matos A, Almeida R. Hybrid approach in a case of persistent sciatic artery aneurysm. Ann Vasc Surg 2014; 28:1313.e5–e7.
- Abularrage CJ, Crawford RS, Patel VI, Conrad MF. Diagnostic strategies for the persistent sciatic artery. Vasc Endovascular Surg 2009; 43:485–489.
- Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010; 22:223–230.
- Vysetti S, Shinde S, Chaudhry S, Subramoney K. Phlegmasia cerulea dolens—a rare, life-threatening condition. ScientificWorldJournal 2009; 9:1105–1106.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Dargon PT, Landry GJ. Buerger’s disease. Ann Vasc Surg 2012; 26:871–880.
- Faizer R, Forbes TL. Buerger’s disease. J Vasc Surg 2007; 46:812.
- Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—current practices. Int J Inflam 2013; 2013:156905.
- Ohta T, Ishibashi H, Sugimoto I, et al. The clinical course of Buerger’s disease. Ann Vasc Dis 2008; 1:85–90.
- de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 2014; 48–49:79–83.
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med 2013; 2013:618910.
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014; 53:793–801.
- Sanders RJ, Annest SJ. Thoracic outlet and pectoralis minor syndromes. Semin Vasc Surg 2014; 27:86–117.
- Criado E, Berguer R, Greenfield L. The spectrum of arterial compression at the thoracic outlet. J Vasc Surg 2010; 52:406–411.
- Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2014; 11:CD007218.
- Orlando MS, Likes KC, Mirza S, et al. A decade of excellent outcomes after surgical intervention in 538 patients with thoracic outlet syndrome. J Am Coll Surg 2015; 220:934–939.
- Bucci F, Ottaviani N, Plagnol P. Acute thrombosis of external iliac artery secondary to endofibrosis. Ann Vasc Surg 2011; 25:698.e5–e7.
- Willson TD, Revesz E, Podbielski FJ, Blecha MJ. External iliac artery dissection secondary to endofibrosis in a cyclist. J Vasc Surg 2010; 52:219–221.
- Peach G, Schep G, Palfreeman R, Beard JD, Thompson MM, Hinchliffe RJ. Endofibrosis and kinking of the Iliac arteries in athletes: a systematic review. Eur J Vasc Endovasc Surg 2012; 43:208–217.
Timely diagnosis of limb ischemia is critical to limb health and limb salvage. The cause in most cases is related to atherosclerosis, and patients with limb ischemia are usually older and have risk factors for atherosclerosis, such as smoking, diabetes, hypertension, hyperlipidemia, and coronary artery disease. When younger patients develop limb ischemia, the diagnosis is often delayed since the index of suspicion is quite low in the absence of the usual risk factors.
Here, we discuss several nonatherosclerotic causes of limb ischemia: popliteal artery entrapment syndrome, popliteal artery aneurysm, cystic adventitial disease, persistent sciatic artery, phlegmasia cerulea dolens, Buerger disease, Takayasu arteritis, arterial thoracic outlet syndrome, and external iliac endofibrosis (Table 1). Our goal is to help clinicians make a timely diagnosis and ultimately save the patient’s limb.
POPLITEAL ARTERY ENTRAPMENT SYNDROME
Popliteal artery entrapment syndrome occurs when the popliteal artery becomes compressed in the popliteal fossa, particularly during exercise.1,2 The underlying problem may be that the popliteal artery has an aberrant course lateral to the medial head of the gastrocnemius muscle, or the medial head of the gastrocnemius may have an abnormal insertion, or there may be fibrous bands in the popliteal fossa, or a combination of these (Figure 1).1–3 Functional popliteal artery entrapment syndrome occurs when there is compression of the artery without an anatomic cause.1–3
The classic clinical presentation is a young athletic patient with calf or foot claudication (crampy pain with exercise, relieved with rest), but other symptoms can include coldness, paresthesias, and numbness. Pain at rest and tissue loss are rare on presentation but may develop if the diagnosis and treatment are delayed.3
Continued compression and microtrauma to the artery may lead to an intramural hematoma, thrombus formation, aneurysmal degeneration, dissection, or even acute thrombosis.2 If the diagnosis is delayed, the patient’s condition may progress from intermittent arterial compression with plantar flexion to complete arterial thrombosis and critical limb ischemia, putting the patient at risk of limb loss.
Diagnosing popliteal artery entrapment syndrome
The diagnostic workup includes a detailed history with a focus on the cause of pain (usually exercise), a comprehensive physical examination that includes looking for wounds, and a thorough pulse examination.
The workup should start with noninvasive imaging such as duplex arterial ultrasonography with and without provocative measures (plantar flexion), the ankle-brachial index with and without provocative measures, and exercise treadmill testing with ankle-brachial index measurement.1,2 Plantar flexion may be necessary to elicit arterial compression that is usually absent at rest.
Magnetic resonance imaging (MRI) and computed tomography (CT) of the lower extremity are useful to identify an arterial abnormality and aberrant muscle anatomy1,3; MRI is currently the gold standard for delineating the muscles of the popliteal fossa.4 If these studies do not shed light on the diagnosis, arterial angiography with and without provocative maneuvers is useful in identifying compression of the popliteal artery.1–3
Treating popliteal artery entrapment syndrome
Treatment depends on the level of arterial injury.
For patients with symptoms but no evidence of arterial injury, the most common procedure offered is popliteal fossa decompression.1–3 This involves surgical release of the medial head of the gastrocnemius muscle and other muscles compressing the popliteal artery.
For patients with evidence of arterial injury such as stenosis, dissection, or aneurysm, bypass grafting may be required.
For patients who present with acute limb ischemia, both surgical thrombectomy with possible bypass and intraarterial lysis have been described.1,2,5
POPLITEAL ARTERY ANEURYSM
Popliteal artery aneurysm (Figure 2) is the most common type of aneurysm of the peripheral arteries of the lower extremity and is present in about 1% of men over age 65. Fifty percent are bilateral, and 50% are associated with an abdominal aortic aneurysm.6,7 While up to 80% patients with this type of aneurysm have no symptoms at the time of diagnosis, symptoms develop at a rate of 14% per year, with acute limb ischemia occurring in up to one-third of cases.6,7
When popliteal artery aneurysm progresses to acute limb ischemia, the consequences are often deleterious, as the tibial arteries distal to the popliteal artery are often occluded, limiting treatment options.
Popliteal artery aneurysm is defined as a local dilation of the artery of 2 cm or greater or an increase in the diameter to 1.5 times normal.6
Acute thrombosis of the aneurysm with limb ischemia is the most common presenting symptom and occurs in 50% of symptomatic cases of popliteal artery aneurysm.7 Almost 25% of patients present with intermittent claudication secondary to thrombosis, partial thrombosis with distal embolization, or combined aneurysmal and atherosclerotic disease. Compression of the popliteal vein by the popliteal artery aneurysm can cause leg swelling with or without deep vein thrombosis in up to 5% of patients.6 Rupture is very rare, with a rate of 2% to 4%.6,7
Diagnosing popliteal artery aneurysm
The diagnosis can be made with arterial duplex ultrasonography, which is also useful for follow-up surveillance.6–8 In the acute setting, computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is useful not only to identify the popliteal aneurysm, but also to define the distal tibial outflow vessels.6,7
Treating popliteal artery aneurysm
Management of an acutely thrombosed popliteal artery aneurysm starts with systemic anticoagulation with intravenous heparin, followed initially by arterial angiography and lysis.8–11 This approach has been shown to be safe and effective even in the absence of arterial runoff distal to the thrombosed popliteal aneurysm. Conversion to open thrombectomy and bypass can be done if initial lytic therapy fails, if the patient develops complications of lytic therapy, or if the patient needs emergency revascularization because of motor and neurologic deficits in the affected extremity.8,10,11
How to manage the asymptomatic patient depends on the size of the aneurysm. Most studies recommend 2 cm or larger as the criterion for repair,6–8,12 while others suggest treating even smaller aneurysms if thrombus is detected.9 Preoperative imaging before elective treatment of an asymptomatic popliteal artery aneurysm includes either CTA or MRA,8,10 which allows the surgeon to visualize the full extent of the aneurysm to best plan the surgical approach. Diagnostic angiography can help determine the most suitable bypass target and can better characterize tibial outflow.
Asymptomatic popliteal artery aneurysm has traditionally been treated with surgical bypass with exclusion of the aneurysm,6–8,12 but more recently, endovascular approaches using self-expanding stent grafts have been described. Further study is needed to determine the long-term efficacy of the endovascular approach.8,10
CYSTIC ADVENTITIAL DISEASE
Cystic adventitial disease is a rare condition in which a blood vessel is narrowed due to mucin-containing cysts in the adventitia. More than 80% of cases occur in the popliteal artery, but it has been described in other peripheral arteries and veins.13,14 It is more common in men than in women and typically occurs in the 4th or 5th decade of life. Most patients present with the sudden onset of calf claudication without the usual risk factors for peripheral vascular disease.13
Diagnosing cystic adventitial disease
Noninvasive arterial or venous duplex ultrasonography can be a good screening tool, as the cysts appear hypoechoic, but results are operator-dependent. CTA and MRA are the imaging tests of choice, as they can detect the cystic lesions and define vessel anatomy for intervention. Diagnostic angiography does not show the cysts themselves but instead reveals a classic “hourglass” and “scimitar” pattern of arterial narrowing that suggests the underlying pathology.13,14
Treating cystic adventitial disease
Usual treatment is complete cyst resection and vessel reconstruction by surgical bypass. Other therapies include open surgical cyst evacuation and removal of the cyst wall, open surgical cyst aspiration, aspiration guided by ultrasonography or CT, and percutaneous angioplasty. However, these nonsurgical treatments have not been shown to be as effective and long-lasting as cyst excision and bypass.13,14
PERSISTENT SCIATIC ARTERY
Persistent sciatic artery is a rare developmental abnormality.15–17 Normally, as the femoral artery develops in the embryo, the sciatic artery involutes to form the inferior gluteal artery. But if the femoral system fails to mature, the sciatic artery, which is adjacent to the sciatic nerve posteriorly as it goes through the sciatic foramen, persists and functions as the major artery supplying the lower extremity, continuing to the posterior thigh and joining the popliteal artery (Figure 3).15,17
Persistent sciatic artery has an incidence of 2.5 to 4 per 10,000 per year15 and is bilateral in almost half of cases.16 Up to 40% of patients have no symptoms, but symptoms may develop by age 40 to 50. Because of repeated trauma to the vessel as it passes through the sciatic foramen,18 the persistent sciatic artery typically sustains accelerated atherosclerotic changes that make it susceptible to aneurysm formation,15 and up to 46% of patients present with aneurysmal degeneration.17
Classically, patients present with lower extremity ischemia from atherosclerotic changes in the persistent sciatic artery or aneurysmal degeneration and thromboembolism.15 Rarely, these aneurysms rupture.15,17 Other signs and symptoms include a pulsatile mass in the buttock, lower extremity numbness, motor weakness, and radicular pain along the sciatic nerve distribution from nerve compression.15–17
Physical findings vary but are distinguished by the lack of femoral pulses in the presence of pedal pulses. A pulsatile buttock mass with evidence of lower extremity nerve compression or limb ischemia or both is pathognomonic of a persistent sciatic artery aneurysm.16,18
Diagnosing persistent sciatic artery
Diagnostic angiography is the gold standard imaging test,15,19 although CTA is starting to replace it.16,18
Treating persistent sciatic artery
Persistent sciatic artery that is asymptomatic and is found incidentally does not require repair; however, it should be followed with duplex ultrasonography to look for evidence of aneurysm degeneration. Degeneration requires repair in most cases.15,16,18,19 When the persistent sciatic artery is the only blood supply to the distal extremity, open aneurysm excision and bypass is the treatment of choice.15,16,19 If collateral flow is adequate, endovascular coil embolization is an option.15 Endovascular stent graft placement has also been described.16,19
PHLEGMASIA CERULEA DOLENS
Phlegmasia cerulea dolens is a rare syndrome caused by extensive acute thrombosis of the ileofemoral vein.20–23 It is defined as total or near-total occlusion of the venous outflow of an extremity, causing massive swelling and congestion that impedes arterial inflow.20,22
Phlegmasia cerulea dolens is associated with four cardinal signs: edema, violaceous discoloration, pain, and severe venous outflow obstruction (Figure 4).22 Patients present with sudden onset of lower extremity pain, swelling, cyanosis, and arterial ischemia with or without loss of distal pulses.20,22
This syndrome can progress to gangrene and massive fluid sequestration leading to shock and death.21–23 From 25% to 40% of patients die, and of those who survive, 20% to 50% require amputation of the limb.20,23
Risk factors include malignancy, immobility, heart failure, heparin-induced thrombocytopenia, antiphospholipid syndrome, pregnancy, venous catheterization (eg, to insert an inferior vena cava filter), and surgery.20–22
Diagnosing phlegmasia cerulea dolens
The diagnosis is made on clinical suspicion with evidence of iliofemoral deep vein thrombosis. Most experts suggest venous duplex ultrasonography to identify the deep vein thrombosis,23 although CT or MR venography can be used to better delineate the proximal extent of the thrombus.20,23
Treating phlegmasia cerulea dolens
Initial management is aggressive fluid resuscitation, elevation of the affected limb, strict bed rest, and anticoagulation with intravenous heparin.20,23 Interventions are aimed at urgently restoring venous outflow to prevent progression to venous gangrene and limb loss.
Although conservative therapy can succeed by itself,23 if the condition does not improve or has already progressed to an advanced stage, the two mainstays of treatment are open venous thrombectomy and endovascular treatment.21–23 Endovascular treatment includes catheter-directed thrombolytic therapy (with or without percutaneous mechanical or pharmacomechanical thrombectomy) and stenting.20,23 The success rate for endovascular therapy can be as high as 90% with near-complete resolution of thrombosis.20 A disadvantage is that, compared with open surgical thrombectomy, more time is needed to achieve venous outflow.20,22
If endovascular therapy is ineffective, if lytic therapy is contraindicated, or if the disease has progressed to gangrene, open surgical thrombectomy with possible fasciotomy is the preferred option.20,21,23 Open surgery has the advantage of restoring venous outflow faster, but disadvantages include the inability to open the smaller veins of the extremity, blood loss, and risks associated with general anesthesia.20–22
BUERGER DISEASE
Buerger disease (thromboangiitis obliterans) is a nonatherosclerotic segmental inflammatory disease involving the small and medium-sized vessels of the arms and legs.24–27 It is differentiated from other vasculitides by its marked male predominance, its close association with smoking, the rarity of systemic signs and symptoms, and the absence of elevated inflammatory markers.26
The rate of major amputation is reported to be 11% at 5 years and 23% at 20 years.24
The classic patient is a young male smoker with symptoms of arterial disease before age 45.24,26 Patients can present with migratory thrombophlebitis or signs of arterial insufficiency in the upper or lower extremities. Two or more limbs are commonly involved. Arterial insufficiency can range from claudication and exertional discomfort of the extremity to ischemic pain at rest leading to ulceration of the distal fingers and toes. Physical findings are similar to those seen in peripheral vascular disease and arterial insufficiency, with decreased arterial brachial index, cool extremities, and wounds.
Diagnosing Buerger disease
- The Shionoya diagnostic criteria for Buerger disease are the following five clinical features24,27:
- History of smoking
- Onset before age 50
- Infrapopliteal arterial occlusive disease
- Upper-limb involvement or phlebitis migrans
- Absence of atherosclerotic risk factors other than heavy smoking.
Various other major and minor criteria have been described to make the diagnosis as well.24
There is no specific laboratory test to confirm the diagnosis of Buerger disease. A full panel of laboratory tests should be sent to rule out other causes of arterial insufficiency and vasculitides; these tests should include C-reactive protein, rheumatoid factor, erythrocyte sedimentation rate, antinuclear antibodies, antiphospholipid antibodies, anti-Scl-70 antibodies, anticentromere antibodies, complement level measurement, and hypercoagulability workup.
Imaging studies include arterial duplex ultrasonography with ankle-brachial indices and segmental pressures and CTA or MRA.26 Angiography can show a “corkscrew” pattern of occlusive disease and collateral formation, which is highly associated with Buerger disease.24
Treating Buerger disease
The only treatment shown to reduce the risk of amputation is complete abstention from tobacco and nicotine (smoking, secondhand smoke, and nicotine patches and gum).24,26
Symptoms of claudication can be managed with aspirin, clopidogrel, vasodilators, pentoxifylline, and cilostazol.26
Surgical bypass is rarely an option, as Buerger disease typically affects the distal blood vessels, thus precluding bypass, and the 5-year patency rate is only 49%.26 Other treatments including arterial thrombolysis, sympathectomy, stem cell injection, spinal cord stimulators, omental grafting, and immunomodulation have been described, but there are only limited data to offer guidance in choosing the appropriate one.24
TAKAYASU ARTERITIS
Takayasu arteritis is a form of vasculitis involving the aorta and its main branches (Figure 5).28 Although seen around the world, it has a higher incidence in young Asian women. Patients can present with systemic symptoms such as fever, fatigue, vague pain, and cardinal signs of limb ischemia associated with Takayasu arteritis, such as weak or absent pulses, differences between the arms in pulses and blood pressures, unobtainable blood pressure measurement in one or both arms, limb fatigability, and pain.28
Diagnosing Takayasu arteritis
Multiple diagnostic criteria have been proposed to define Takayasu arteritis.28 CTA, MRA, and positron emission tomography have replaced invasive angiography as the diagnostic imaging tests of choice.29
Treating Takayasu arteritis
Takayasu arteritis has an acute and chronic course. Interventions are typically reserved for severe cases, with indications that include uncontrollable hypertension from renal artery stenosis, severe coronary or cerebrovascular disease, severe aortic regurgitation or coarctation, stenotic or occlusive lesions resulting in critical limb ischemia, and aneurysm at risk of rupture.28–30
THORACIC OUTLET SYNDROME
Thoracic outlet syndrome is compression of the brachial plexus, subclavian vein, or subclavian artery as it exits the thoracic outlet through an area known as the scalene triangle, which is bordered by the anterior scalene, first rib, and clavicle.31 Presenting symptoms depend on the structure compressed.
By far the most common presentation32 is neurogenic thoracic outlet syndrome, accounting for more than 90% of cases, followed by venous thoracic outlet syndrome. Arterial thoracic outlet syndrome is the least frequent at less than 1%, but carries the greatest morbidity with potential for limb loss.31–33
The subclavian artery exits the thoracic outlet between the anterior and middle scalene muscles, and then travels over the first rib and underneath the clavicle.31 Repeated trauma from compression of the artery results in intimal injury leading to compression, stenosis, occlusion, or aneurysm formation.31,32
Symptoms of arterial thoracic outlet syndrome can start out as effort fatigue of the upper extremity secondary to compression. These symptoms are usually vague and difficult to define,31 as these patients typically are young and do not have atherosclerotic risk factors that would prompt suspicion of a vascular cause.
The most common presentation of arterial thoracic outlet syndrome is upper extremity embolization from a partially thrombosed aneurysm or area of stenosis with ischemia.32 Symptoms can range from ischemia of the fingers due to microembolization to acute limb ischemia due to complete thrombosis of the subclavian artery.31,32 Arterial thoracic outlet syndrome is most commonly associated with a bony abnormality (ie, cervical rib or anomalous first rib),31–33 and on physical examination the bony abnormality may be palpated in the supraclavicular fossa.31
Other physical findings include a bruit over the subclavian artery, a blood pressure difference of 20 mm Hg or more between the affected and unaffected arms, loss of brachial, radial, or ulnar pulses with arm abduction, and loss of the radial pulse with the head rotated to the affected side as the patient takes a deep breath (the Adson maneuver).31 While postural changes in the pulse examination hint at arterial thoracic outlet syndrome, extremity pulses may be reduced or even absent in up to 60% of normal patients.32
Diagnosing thoracic outlet syndrome
The workup should start with noninvasive imaging with pulse volume recording and wrist and finger systolic pressures, followed by arterial duplex ultrasonography.
Chest radiography may be able to identify bony abnormalities, and MRA or CTA with the patient in two positions—ie, arms down at the sides, and arms held above the head—can help identify arterial compression from bony or muscular structures in the thoracic outlet. Upper extremity angiography provides high-resolution imaging of the digital arteries and can help identify a subclavian artery aneurysm, which may be a subtle finding.31
It is important to have objective evidence of arterial or venous mechanical obstruction before deciding to remove the first rib.
Treating thoracic outlet syndrome
Treatment is determined by the severity and acuity of symptoms. If the patient presents with acute limb ischemia, prompt treatment with either open surgery or endovascular treatment is required.31,32,34 Once the acute phase has resolved or if the patient presents with chronic disease, open surgical repair is needed to remove the compression of the artery. If an arterial abnormality is identified (aneurysm or significant stenosis), an arterial reconstruction with bypass may be required.31
The standard treatment for thoracic outlet syndrome is resection of the first rib (and removal of the cervical rib if present).31,34 This can be by a transaxillary approach unless arterial reconstruction is needed, in which case a supraclavicular approach is used.31,34 When a patient without symptoms is found to have evidence of arterial compression, most experts would recommend resection of the first rib if there is evidence of an arterial abnormality, or follow-up with duplex imaging for patients with only subtle findings.31
EXTERNAL ILIAC ENDOFIBROSIS
External iliac endofibrosis is a rare cause of intermittent claudication, typically in high-performance athletes, resulting from thickening of the intima in the external iliac artery causing luminal narrowing and resultant ischemia.35–37 The estimated incidence is as high as 20% in elite competitive cyclists, and the condition has been described in other sports as well.37
External iliac endofibrosis typically presents as unilateral leg pain or cramping at near-maximal exercise with an associated feeling of swelling and numbness on the affected side.35,37 It is bilateral in up to 15% of cases.35 While claudication of the thigh is the predominant presenting symptom, dissection and thrombosis of the external iliac artery have been described, presenting with acute limb ischemia in up to 4% of patients.35,36
The condition has been attributed to factors such as physical position, psoas hypertrophy, tethering of the external iliac artery to the psoas muscle, kinking and tortuosity of the vessel, and high-flow states secondary to increased cardiac output and adaptive systolic hypertension.36,37
Diagnosing external iliac endofibrosis
The diagnosis is difficult, as symptoms typically manifest only during maximal exercise. Delays of 12 to 41 months between the onset of symptoms and diagnosis have been reported.37 Physical findings are nonspecific, and pulses and ankle-brachial indices are typically normal at rest. A careful history with a focus on location and duration of symptoms and a high index of suspicion have been shown to increase the sensitivity of diagnosis.36
Noninvasive vascular imaging with arterial duplex ultrasonography with physiologic studies (the ankle-brachial index) at rest and at maximal exertion should be obtained first.35,37 If findings on ultrasonography are positive, CTA or MRA can be used to identify a suspected stenosis.
Diagnostic angiography is still the gold standard for imaging, as real-time images of the artery with different leg positions can be obtained and pressure gradients can be measured with or without the use of a vasodilator to determine the hemodynamic significance of a lesion.35–37
Treating external iliac endofibrosis
Treatment should initially be conservative. Recreational athletes should consider changing to a sport that does not require hip flexion, and cyclists should be advised to reduce the amount of time spent cycling and to raise the handlebars or bring the saddle position forward to minimize hip flexion.37
Definitive treatment is open surgical repair. Surgical options include arterial release of the tethered artery, endofibrosectomy and vessel shortening, endofibrosectomy and patch angioplasty, and interposition bypass grafting.35–37
Timely diagnosis of limb ischemia is critical to limb health and limb salvage. The cause in most cases is related to atherosclerosis, and patients with limb ischemia are usually older and have risk factors for atherosclerosis, such as smoking, diabetes, hypertension, hyperlipidemia, and coronary artery disease. When younger patients develop limb ischemia, the diagnosis is often delayed since the index of suspicion is quite low in the absence of the usual risk factors.
Here, we discuss several nonatherosclerotic causes of limb ischemia: popliteal artery entrapment syndrome, popliteal artery aneurysm, cystic adventitial disease, persistent sciatic artery, phlegmasia cerulea dolens, Buerger disease, Takayasu arteritis, arterial thoracic outlet syndrome, and external iliac endofibrosis (Table 1). Our goal is to help clinicians make a timely diagnosis and ultimately save the patient’s limb.
POPLITEAL ARTERY ENTRAPMENT SYNDROME
Popliteal artery entrapment syndrome occurs when the popliteal artery becomes compressed in the popliteal fossa, particularly during exercise.1,2 The underlying problem may be that the popliteal artery has an aberrant course lateral to the medial head of the gastrocnemius muscle, or the medial head of the gastrocnemius may have an abnormal insertion, or there may be fibrous bands in the popliteal fossa, or a combination of these (Figure 1).1–3 Functional popliteal artery entrapment syndrome occurs when there is compression of the artery without an anatomic cause.1–3
The classic clinical presentation is a young athletic patient with calf or foot claudication (crampy pain with exercise, relieved with rest), but other symptoms can include coldness, paresthesias, and numbness. Pain at rest and tissue loss are rare on presentation but may develop if the diagnosis and treatment are delayed.3
Continued compression and microtrauma to the artery may lead to an intramural hematoma, thrombus formation, aneurysmal degeneration, dissection, or even acute thrombosis.2 If the diagnosis is delayed, the patient’s condition may progress from intermittent arterial compression with plantar flexion to complete arterial thrombosis and critical limb ischemia, putting the patient at risk of limb loss.
Diagnosing popliteal artery entrapment syndrome
The diagnostic workup includes a detailed history with a focus on the cause of pain (usually exercise), a comprehensive physical examination that includes looking for wounds, and a thorough pulse examination.
The workup should start with noninvasive imaging such as duplex arterial ultrasonography with and without provocative measures (plantar flexion), the ankle-brachial index with and without provocative measures, and exercise treadmill testing with ankle-brachial index measurement.1,2 Plantar flexion may be necessary to elicit arterial compression that is usually absent at rest.
Magnetic resonance imaging (MRI) and computed tomography (CT) of the lower extremity are useful to identify an arterial abnormality and aberrant muscle anatomy1,3; MRI is currently the gold standard for delineating the muscles of the popliteal fossa.4 If these studies do not shed light on the diagnosis, arterial angiography with and without provocative maneuvers is useful in identifying compression of the popliteal artery.1–3
Treating popliteal artery entrapment syndrome
Treatment depends on the level of arterial injury.
For patients with symptoms but no evidence of arterial injury, the most common procedure offered is popliteal fossa decompression.1–3 This involves surgical release of the medial head of the gastrocnemius muscle and other muscles compressing the popliteal artery.
For patients with evidence of arterial injury such as stenosis, dissection, or aneurysm, bypass grafting may be required.
For patients who present with acute limb ischemia, both surgical thrombectomy with possible bypass and intraarterial lysis have been described.1,2,5
POPLITEAL ARTERY ANEURYSM
Popliteal artery aneurysm (Figure 2) is the most common type of aneurysm of the peripheral arteries of the lower extremity and is present in about 1% of men over age 65. Fifty percent are bilateral, and 50% are associated with an abdominal aortic aneurysm.6,7 While up to 80% patients with this type of aneurysm have no symptoms at the time of diagnosis, symptoms develop at a rate of 14% per year, with acute limb ischemia occurring in up to one-third of cases.6,7
When popliteal artery aneurysm progresses to acute limb ischemia, the consequences are often deleterious, as the tibial arteries distal to the popliteal artery are often occluded, limiting treatment options.
Popliteal artery aneurysm is defined as a local dilation of the artery of 2 cm or greater or an increase in the diameter to 1.5 times normal.6
Acute thrombosis of the aneurysm with limb ischemia is the most common presenting symptom and occurs in 50% of symptomatic cases of popliteal artery aneurysm.7 Almost 25% of patients present with intermittent claudication secondary to thrombosis, partial thrombosis with distal embolization, or combined aneurysmal and atherosclerotic disease. Compression of the popliteal vein by the popliteal artery aneurysm can cause leg swelling with or without deep vein thrombosis in up to 5% of patients.6 Rupture is very rare, with a rate of 2% to 4%.6,7
Diagnosing popliteal artery aneurysm
The diagnosis can be made with arterial duplex ultrasonography, which is also useful for follow-up surveillance.6–8 In the acute setting, computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is useful not only to identify the popliteal aneurysm, but also to define the distal tibial outflow vessels.6,7
Treating popliteal artery aneurysm
Management of an acutely thrombosed popliteal artery aneurysm starts with systemic anticoagulation with intravenous heparin, followed initially by arterial angiography and lysis.8–11 This approach has been shown to be safe and effective even in the absence of arterial runoff distal to the thrombosed popliteal aneurysm. Conversion to open thrombectomy and bypass can be done if initial lytic therapy fails, if the patient develops complications of lytic therapy, or if the patient needs emergency revascularization because of motor and neurologic deficits in the affected extremity.8,10,11
How to manage the asymptomatic patient depends on the size of the aneurysm. Most studies recommend 2 cm or larger as the criterion for repair,6–8,12 while others suggest treating even smaller aneurysms if thrombus is detected.9 Preoperative imaging before elective treatment of an asymptomatic popliteal artery aneurysm includes either CTA or MRA,8,10 which allows the surgeon to visualize the full extent of the aneurysm to best plan the surgical approach. Diagnostic angiography can help determine the most suitable bypass target and can better characterize tibial outflow.
Asymptomatic popliteal artery aneurysm has traditionally been treated with surgical bypass with exclusion of the aneurysm,6–8,12 but more recently, endovascular approaches using self-expanding stent grafts have been described. Further study is needed to determine the long-term efficacy of the endovascular approach.8,10
CYSTIC ADVENTITIAL DISEASE
Cystic adventitial disease is a rare condition in which a blood vessel is narrowed due to mucin-containing cysts in the adventitia. More than 80% of cases occur in the popliteal artery, but it has been described in other peripheral arteries and veins.13,14 It is more common in men than in women and typically occurs in the 4th or 5th decade of life. Most patients present with the sudden onset of calf claudication without the usual risk factors for peripheral vascular disease.13
Diagnosing cystic adventitial disease
Noninvasive arterial or venous duplex ultrasonography can be a good screening tool, as the cysts appear hypoechoic, but results are operator-dependent. CTA and MRA are the imaging tests of choice, as they can detect the cystic lesions and define vessel anatomy for intervention. Diagnostic angiography does not show the cysts themselves but instead reveals a classic “hourglass” and “scimitar” pattern of arterial narrowing that suggests the underlying pathology.13,14
Treating cystic adventitial disease
Usual treatment is complete cyst resection and vessel reconstruction by surgical bypass. Other therapies include open surgical cyst evacuation and removal of the cyst wall, open surgical cyst aspiration, aspiration guided by ultrasonography or CT, and percutaneous angioplasty. However, these nonsurgical treatments have not been shown to be as effective and long-lasting as cyst excision and bypass.13,14
PERSISTENT SCIATIC ARTERY
Persistent sciatic artery is a rare developmental abnormality.15–17 Normally, as the femoral artery develops in the embryo, the sciatic artery involutes to form the inferior gluteal artery. But if the femoral system fails to mature, the sciatic artery, which is adjacent to the sciatic nerve posteriorly as it goes through the sciatic foramen, persists and functions as the major artery supplying the lower extremity, continuing to the posterior thigh and joining the popliteal artery (Figure 3).15,17
Persistent sciatic artery has an incidence of 2.5 to 4 per 10,000 per year15 and is bilateral in almost half of cases.16 Up to 40% of patients have no symptoms, but symptoms may develop by age 40 to 50. Because of repeated trauma to the vessel as it passes through the sciatic foramen,18 the persistent sciatic artery typically sustains accelerated atherosclerotic changes that make it susceptible to aneurysm formation,15 and up to 46% of patients present with aneurysmal degeneration.17
Classically, patients present with lower extremity ischemia from atherosclerotic changes in the persistent sciatic artery or aneurysmal degeneration and thromboembolism.15 Rarely, these aneurysms rupture.15,17 Other signs and symptoms include a pulsatile mass in the buttock, lower extremity numbness, motor weakness, and radicular pain along the sciatic nerve distribution from nerve compression.15–17
Physical findings vary but are distinguished by the lack of femoral pulses in the presence of pedal pulses. A pulsatile buttock mass with evidence of lower extremity nerve compression or limb ischemia or both is pathognomonic of a persistent sciatic artery aneurysm.16,18
Diagnosing persistent sciatic artery
Diagnostic angiography is the gold standard imaging test,15,19 although CTA is starting to replace it.16,18
Treating persistent sciatic artery
Persistent sciatic artery that is asymptomatic and is found incidentally does not require repair; however, it should be followed with duplex ultrasonography to look for evidence of aneurysm degeneration. Degeneration requires repair in most cases.15,16,18,19 When the persistent sciatic artery is the only blood supply to the distal extremity, open aneurysm excision and bypass is the treatment of choice.15,16,19 If collateral flow is adequate, endovascular coil embolization is an option.15 Endovascular stent graft placement has also been described.16,19
PHLEGMASIA CERULEA DOLENS
Phlegmasia cerulea dolens is a rare syndrome caused by extensive acute thrombosis of the ileofemoral vein.20–23 It is defined as total or near-total occlusion of the venous outflow of an extremity, causing massive swelling and congestion that impedes arterial inflow.20,22
Phlegmasia cerulea dolens is associated with four cardinal signs: edema, violaceous discoloration, pain, and severe venous outflow obstruction (Figure 4).22 Patients present with sudden onset of lower extremity pain, swelling, cyanosis, and arterial ischemia with or without loss of distal pulses.20,22
This syndrome can progress to gangrene and massive fluid sequestration leading to shock and death.21–23 From 25% to 40% of patients die, and of those who survive, 20% to 50% require amputation of the limb.20,23
Risk factors include malignancy, immobility, heart failure, heparin-induced thrombocytopenia, antiphospholipid syndrome, pregnancy, venous catheterization (eg, to insert an inferior vena cava filter), and surgery.20–22
Diagnosing phlegmasia cerulea dolens
The diagnosis is made on clinical suspicion with evidence of iliofemoral deep vein thrombosis. Most experts suggest venous duplex ultrasonography to identify the deep vein thrombosis,23 although CT or MR venography can be used to better delineate the proximal extent of the thrombus.20,23
Treating phlegmasia cerulea dolens
Initial management is aggressive fluid resuscitation, elevation of the affected limb, strict bed rest, and anticoagulation with intravenous heparin.20,23 Interventions are aimed at urgently restoring venous outflow to prevent progression to venous gangrene and limb loss.
Although conservative therapy can succeed by itself,23 if the condition does not improve or has already progressed to an advanced stage, the two mainstays of treatment are open venous thrombectomy and endovascular treatment.21–23 Endovascular treatment includes catheter-directed thrombolytic therapy (with or without percutaneous mechanical or pharmacomechanical thrombectomy) and stenting.20,23 The success rate for endovascular therapy can be as high as 90% with near-complete resolution of thrombosis.20 A disadvantage is that, compared with open surgical thrombectomy, more time is needed to achieve venous outflow.20,22
If endovascular therapy is ineffective, if lytic therapy is contraindicated, or if the disease has progressed to gangrene, open surgical thrombectomy with possible fasciotomy is the preferred option.20,21,23 Open surgery has the advantage of restoring venous outflow faster, but disadvantages include the inability to open the smaller veins of the extremity, blood loss, and risks associated with general anesthesia.20–22
BUERGER DISEASE
Buerger disease (thromboangiitis obliterans) is a nonatherosclerotic segmental inflammatory disease involving the small and medium-sized vessels of the arms and legs.24–27 It is differentiated from other vasculitides by its marked male predominance, its close association with smoking, the rarity of systemic signs and symptoms, and the absence of elevated inflammatory markers.26
The rate of major amputation is reported to be 11% at 5 years and 23% at 20 years.24
The classic patient is a young male smoker with symptoms of arterial disease before age 45.24,26 Patients can present with migratory thrombophlebitis or signs of arterial insufficiency in the upper or lower extremities. Two or more limbs are commonly involved. Arterial insufficiency can range from claudication and exertional discomfort of the extremity to ischemic pain at rest leading to ulceration of the distal fingers and toes. Physical findings are similar to those seen in peripheral vascular disease and arterial insufficiency, with decreased arterial brachial index, cool extremities, and wounds.
Diagnosing Buerger disease
- The Shionoya diagnostic criteria for Buerger disease are the following five clinical features24,27:
- History of smoking
- Onset before age 50
- Infrapopliteal arterial occlusive disease
- Upper-limb involvement or phlebitis migrans
- Absence of atherosclerotic risk factors other than heavy smoking.
Various other major and minor criteria have been described to make the diagnosis as well.24
There is no specific laboratory test to confirm the diagnosis of Buerger disease. A full panel of laboratory tests should be sent to rule out other causes of arterial insufficiency and vasculitides; these tests should include C-reactive protein, rheumatoid factor, erythrocyte sedimentation rate, antinuclear antibodies, antiphospholipid antibodies, anti-Scl-70 antibodies, anticentromere antibodies, complement level measurement, and hypercoagulability workup.
Imaging studies include arterial duplex ultrasonography with ankle-brachial indices and segmental pressures and CTA or MRA.26 Angiography can show a “corkscrew” pattern of occlusive disease and collateral formation, which is highly associated with Buerger disease.24
Treating Buerger disease
The only treatment shown to reduce the risk of amputation is complete abstention from tobacco and nicotine (smoking, secondhand smoke, and nicotine patches and gum).24,26
Symptoms of claudication can be managed with aspirin, clopidogrel, vasodilators, pentoxifylline, and cilostazol.26
Surgical bypass is rarely an option, as Buerger disease typically affects the distal blood vessels, thus precluding bypass, and the 5-year patency rate is only 49%.26 Other treatments including arterial thrombolysis, sympathectomy, stem cell injection, spinal cord stimulators, omental grafting, and immunomodulation have been described, but there are only limited data to offer guidance in choosing the appropriate one.24
TAKAYASU ARTERITIS
Takayasu arteritis is a form of vasculitis involving the aorta and its main branches (Figure 5).28 Although seen around the world, it has a higher incidence in young Asian women. Patients can present with systemic symptoms such as fever, fatigue, vague pain, and cardinal signs of limb ischemia associated with Takayasu arteritis, such as weak or absent pulses, differences between the arms in pulses and blood pressures, unobtainable blood pressure measurement in one or both arms, limb fatigability, and pain.28
Diagnosing Takayasu arteritis
Multiple diagnostic criteria have been proposed to define Takayasu arteritis.28 CTA, MRA, and positron emission tomography have replaced invasive angiography as the diagnostic imaging tests of choice.29
Treating Takayasu arteritis
Takayasu arteritis has an acute and chronic course. Interventions are typically reserved for severe cases, with indications that include uncontrollable hypertension from renal artery stenosis, severe coronary or cerebrovascular disease, severe aortic regurgitation or coarctation, stenotic or occlusive lesions resulting in critical limb ischemia, and aneurysm at risk of rupture.28–30
THORACIC OUTLET SYNDROME
Thoracic outlet syndrome is compression of the brachial plexus, subclavian vein, or subclavian artery as it exits the thoracic outlet through an area known as the scalene triangle, which is bordered by the anterior scalene, first rib, and clavicle.31 Presenting symptoms depend on the structure compressed.
By far the most common presentation32 is neurogenic thoracic outlet syndrome, accounting for more than 90% of cases, followed by venous thoracic outlet syndrome. Arterial thoracic outlet syndrome is the least frequent at less than 1%, but carries the greatest morbidity with potential for limb loss.31–33
The subclavian artery exits the thoracic outlet between the anterior and middle scalene muscles, and then travels over the first rib and underneath the clavicle.31 Repeated trauma from compression of the artery results in intimal injury leading to compression, stenosis, occlusion, or aneurysm formation.31,32
Symptoms of arterial thoracic outlet syndrome can start out as effort fatigue of the upper extremity secondary to compression. These symptoms are usually vague and difficult to define,31 as these patients typically are young and do not have atherosclerotic risk factors that would prompt suspicion of a vascular cause.
The most common presentation of arterial thoracic outlet syndrome is upper extremity embolization from a partially thrombosed aneurysm or area of stenosis with ischemia.32 Symptoms can range from ischemia of the fingers due to microembolization to acute limb ischemia due to complete thrombosis of the subclavian artery.31,32 Arterial thoracic outlet syndrome is most commonly associated with a bony abnormality (ie, cervical rib or anomalous first rib),31–33 and on physical examination the bony abnormality may be palpated in the supraclavicular fossa.31
Other physical findings include a bruit over the subclavian artery, a blood pressure difference of 20 mm Hg or more between the affected and unaffected arms, loss of brachial, radial, or ulnar pulses with arm abduction, and loss of the radial pulse with the head rotated to the affected side as the patient takes a deep breath (the Adson maneuver).31 While postural changes in the pulse examination hint at arterial thoracic outlet syndrome, extremity pulses may be reduced or even absent in up to 60% of normal patients.32
Diagnosing thoracic outlet syndrome
The workup should start with noninvasive imaging with pulse volume recording and wrist and finger systolic pressures, followed by arterial duplex ultrasonography.
Chest radiography may be able to identify bony abnormalities, and MRA or CTA with the patient in two positions—ie, arms down at the sides, and arms held above the head—can help identify arterial compression from bony or muscular structures in the thoracic outlet. Upper extremity angiography provides high-resolution imaging of the digital arteries and can help identify a subclavian artery aneurysm, which may be a subtle finding.31
It is important to have objective evidence of arterial or venous mechanical obstruction before deciding to remove the first rib.
Treating thoracic outlet syndrome
Treatment is determined by the severity and acuity of symptoms. If the patient presents with acute limb ischemia, prompt treatment with either open surgery or endovascular treatment is required.31,32,34 Once the acute phase has resolved or if the patient presents with chronic disease, open surgical repair is needed to remove the compression of the artery. If an arterial abnormality is identified (aneurysm or significant stenosis), an arterial reconstruction with bypass may be required.31
The standard treatment for thoracic outlet syndrome is resection of the first rib (and removal of the cervical rib if present).31,34 This can be by a transaxillary approach unless arterial reconstruction is needed, in which case a supraclavicular approach is used.31,34 When a patient without symptoms is found to have evidence of arterial compression, most experts would recommend resection of the first rib if there is evidence of an arterial abnormality, or follow-up with duplex imaging for patients with only subtle findings.31
EXTERNAL ILIAC ENDOFIBROSIS
External iliac endofibrosis is a rare cause of intermittent claudication, typically in high-performance athletes, resulting from thickening of the intima in the external iliac artery causing luminal narrowing and resultant ischemia.35–37 The estimated incidence is as high as 20% in elite competitive cyclists, and the condition has been described in other sports as well.37
External iliac endofibrosis typically presents as unilateral leg pain or cramping at near-maximal exercise with an associated feeling of swelling and numbness on the affected side.35,37 It is bilateral in up to 15% of cases.35 While claudication of the thigh is the predominant presenting symptom, dissection and thrombosis of the external iliac artery have been described, presenting with acute limb ischemia in up to 4% of patients.35,36
The condition has been attributed to factors such as physical position, psoas hypertrophy, tethering of the external iliac artery to the psoas muscle, kinking and tortuosity of the vessel, and high-flow states secondary to increased cardiac output and adaptive systolic hypertension.36,37
Diagnosing external iliac endofibrosis
The diagnosis is difficult, as symptoms typically manifest only during maximal exercise. Delays of 12 to 41 months between the onset of symptoms and diagnosis have been reported.37 Physical findings are nonspecific, and pulses and ankle-brachial indices are typically normal at rest. A careful history with a focus on location and duration of symptoms and a high index of suspicion have been shown to increase the sensitivity of diagnosis.36
Noninvasive vascular imaging with arterial duplex ultrasonography with physiologic studies (the ankle-brachial index) at rest and at maximal exertion should be obtained first.35,37 If findings on ultrasonography are positive, CTA or MRA can be used to identify a suspected stenosis.
Diagnostic angiography is still the gold standard for imaging, as real-time images of the artery with different leg positions can be obtained and pressure gradients can be measured with or without the use of a vasodilator to determine the hemodynamic significance of a lesion.35–37
Treating external iliac endofibrosis
Treatment should initially be conservative. Recreational athletes should consider changing to a sport that does not require hip flexion, and cyclists should be advised to reduce the amount of time spent cycling and to raise the handlebars or bring the saddle position forward to minimize hip flexion.37
Definitive treatment is open surgical repair. Surgical options include arterial release of the tethered artery, endofibrosectomy and vessel shortening, endofibrosectomy and patch angioplasty, and interposition bypass grafting.35–37
- Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg 2012; 55:252–262.e30.
- Gokkus K, Sagtas E, Bakalim T, Taskaya E, Aydin AT. Popliteal entrapment syndrome. A systematic review of the literature and case presentation. Muscles Ligaments Tendons J 2014; 4:141–148.
- Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg 2008; 48(suppl 6):61S–65S.
- Liu Y, Sun Y, He X, et al. Imaging diagnosis and surgical treatment of popliteal artery entrapment syndrome: a single-center experience. Ann Vasc Surg 2014; 28:330–337.
- Kim SY, Min SK, Ahn S, Min SI, Ha J, Kim SJ. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg 2012; 55:90–97.
- Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl 2007; 89:466–471.
- Dawson J, Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene—is rupture really a danger? Prog Cardiovasc Dis 2013; 56:26–35.
- Stone PA, Jagannath P, Thompson SN, et al. Evolving treatment of popliteal artery aneurysms. J Vasc Surg 2013; 57:1306–1310.
- Eslami MH, Rybin D, Doros G, Farber A. Open repair of asymptomatic popliteal artery aneurysm is associated with better outcomes than endovascular repair. J Vasc Surg 2015; 61:663–669.
- Serrano Hernando FJ, Martínez López I, Hernández Mateo MM, et al. Comparison of popliteal artery aneurysm therapies. J Vasc Surg 2015; 61:655–661.
- Marty B, Wicky S, Ris HB, et al. Success of thrombolysis as a predictor of outcome in acute thrombosis of popliteal aneurysms. J Vasc Surg 2002; 35:487–493.
- Hall HA, Minc S, Babrowski T. Peripheral artery aneurysm. Surg Clin North Am 2013; 93:911–923.
- Veraldi GF, Scudo G, Scorsone L, Mezzetto L, Castellani RL. Cystic adventitial disease of the popliteal artery: report of two cases and review of the literature. G Chir 2014; 35:229–234.
- Desy NM, Spinner RJ. The etiology and management of cystic adventitial disease. J Vasc Surg 2014; 60:235–245.e1–e11.
- Patel MV, Patel NH, Schneider JR, Kim S, Verta MJ. Persistent sciatic artery presenting with limb ischemia. J Vasc Surg 2013; 57:225–229.
- Kesri G, Mangtani J, Kumar G, Dangayach KK. Persistent sciatic artery aneurysm with lower limb ischemia. Case Rep Vasc Med 2014; 2014:183969.
- Nuño-Escobar C, Pérez-Durán MA, Ramos-López R, et al. Persistent sciatic artery aneurysm. Ann Vasc Surg 2013; 27:1182.e13–e16.
- Vaz C, Machado R, Rego D, Matos A, Almeida R. Hybrid approach in a case of persistent sciatic artery aneurysm. Ann Vasc Surg 2014; 28:1313.e5–e7.
- Abularrage CJ, Crawford RS, Patel VI, Conrad MF. Diagnostic strategies for the persistent sciatic artery. Vasc Endovascular Surg 2009; 43:485–489.
- Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010; 22:223–230.
- Vysetti S, Shinde S, Chaudhry S, Subramoney K. Phlegmasia cerulea dolens—a rare, life-threatening condition. ScientificWorldJournal 2009; 9:1105–1106.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Dargon PT, Landry GJ. Buerger’s disease. Ann Vasc Surg 2012; 26:871–880.
- Faizer R, Forbes TL. Buerger’s disease. J Vasc Surg 2007; 46:812.
- Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—current practices. Int J Inflam 2013; 2013:156905.
- Ohta T, Ishibashi H, Sugimoto I, et al. The clinical course of Buerger’s disease. Ann Vasc Dis 2008; 1:85–90.
- de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 2014; 48–49:79–83.
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med 2013; 2013:618910.
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014; 53:793–801.
- Sanders RJ, Annest SJ. Thoracic outlet and pectoralis minor syndromes. Semin Vasc Surg 2014; 27:86–117.
- Criado E, Berguer R, Greenfield L. The spectrum of arterial compression at the thoracic outlet. J Vasc Surg 2010; 52:406–411.
- Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2014; 11:CD007218.
- Orlando MS, Likes KC, Mirza S, et al. A decade of excellent outcomes after surgical intervention in 538 patients with thoracic outlet syndrome. J Am Coll Surg 2015; 220:934–939.
- Bucci F, Ottaviani N, Plagnol P. Acute thrombosis of external iliac artery secondary to endofibrosis. Ann Vasc Surg 2011; 25:698.e5–e7.
- Willson TD, Revesz E, Podbielski FJ, Blecha MJ. External iliac artery dissection secondary to endofibrosis in a cyclist. J Vasc Surg 2010; 52:219–221.
- Peach G, Schep G, Palfreeman R, Beard JD, Thompson MM, Hinchliffe RJ. Endofibrosis and kinking of the Iliac arteries in athletes: a systematic review. Eur J Vasc Endovasc Surg 2012; 43:208–217.
- Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg 2012; 55:252–262.e30.
- Gokkus K, Sagtas E, Bakalim T, Taskaya E, Aydin AT. Popliteal entrapment syndrome. A systematic review of the literature and case presentation. Muscles Ligaments Tendons J 2014; 4:141–148.
- Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg 2008; 48(suppl 6):61S–65S.
- Liu Y, Sun Y, He X, et al. Imaging diagnosis and surgical treatment of popliteal artery entrapment syndrome: a single-center experience. Ann Vasc Surg 2014; 28:330–337.
- Kim SY, Min SK, Ahn S, Min SI, Ha J, Kim SJ. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg 2012; 55:90–97.
- Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl 2007; 89:466–471.
- Dawson J, Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene—is rupture really a danger? Prog Cardiovasc Dis 2013; 56:26–35.
- Stone PA, Jagannath P, Thompson SN, et al. Evolving treatment of popliteal artery aneurysms. J Vasc Surg 2013; 57:1306–1310.
- Eslami MH, Rybin D, Doros G, Farber A. Open repair of asymptomatic popliteal artery aneurysm is associated with better outcomes than endovascular repair. J Vasc Surg 2015; 61:663–669.
- Serrano Hernando FJ, Martínez López I, Hernández Mateo MM, et al. Comparison of popliteal artery aneurysm therapies. J Vasc Surg 2015; 61:655–661.
- Marty B, Wicky S, Ris HB, et al. Success of thrombolysis as a predictor of outcome in acute thrombosis of popliteal aneurysms. J Vasc Surg 2002; 35:487–493.
- Hall HA, Minc S, Babrowski T. Peripheral artery aneurysm. Surg Clin North Am 2013; 93:911–923.
- Veraldi GF, Scudo G, Scorsone L, Mezzetto L, Castellani RL. Cystic adventitial disease of the popliteal artery: report of two cases and review of the literature. G Chir 2014; 35:229–234.
- Desy NM, Spinner RJ. The etiology and management of cystic adventitial disease. J Vasc Surg 2014; 60:235–245.e1–e11.
- Patel MV, Patel NH, Schneider JR, Kim S, Verta MJ. Persistent sciatic artery presenting with limb ischemia. J Vasc Surg 2013; 57:225–229.
- Kesri G, Mangtani J, Kumar G, Dangayach KK. Persistent sciatic artery aneurysm with lower limb ischemia. Case Rep Vasc Med 2014; 2014:183969.
- Nuño-Escobar C, Pérez-Durán MA, Ramos-López R, et al. Persistent sciatic artery aneurysm. Ann Vasc Surg 2013; 27:1182.e13–e16.
- Vaz C, Machado R, Rego D, Matos A, Almeida R. Hybrid approach in a case of persistent sciatic artery aneurysm. Ann Vasc Surg 2014; 28:1313.e5–e7.
- Abularrage CJ, Crawford RS, Patel VI, Conrad MF. Diagnostic strategies for the persistent sciatic artery. Vasc Endovascular Surg 2009; 43:485–489.
- Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010; 22:223–230.
- Vysetti S, Shinde S, Chaudhry S, Subramoney K. Phlegmasia cerulea dolens—a rare, life-threatening condition. ScientificWorldJournal 2009; 9:1105–1106.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Dargon PT, Landry GJ. Buerger’s disease. Ann Vasc Surg 2012; 26:871–880.
- Faizer R, Forbes TL. Buerger’s disease. J Vasc Surg 2007; 46:812.
- Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—current practices. Int J Inflam 2013; 2013:156905.
- Ohta T, Ishibashi H, Sugimoto I, et al. The clinical course of Buerger’s disease. Ann Vasc Dis 2008; 1:85–90.
- de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 2014; 48–49:79–83.
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med 2013; 2013:618910.
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014; 53:793–801.
- Sanders RJ, Annest SJ. Thoracic outlet and pectoralis minor syndromes. Semin Vasc Surg 2014; 27:86–117.
- Criado E, Berguer R, Greenfield L. The spectrum of arterial compression at the thoracic outlet. J Vasc Surg 2010; 52:406–411.
- Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2014; 11:CD007218.
- Orlando MS, Likes KC, Mirza S, et al. A decade of excellent outcomes after surgical intervention in 538 patients with thoracic outlet syndrome. J Am Coll Surg 2015; 220:934–939.
- Bucci F, Ottaviani N, Plagnol P. Acute thrombosis of external iliac artery secondary to endofibrosis. Ann Vasc Surg 2011; 25:698.e5–e7.
- Willson TD, Revesz E, Podbielski FJ, Blecha MJ. External iliac artery dissection secondary to endofibrosis in a cyclist. J Vasc Surg 2010; 52:219–221.
- Peach G, Schep G, Palfreeman R, Beard JD, Thompson MM, Hinchliffe RJ. Endofibrosis and kinking of the Iliac arteries in athletes: a systematic review. Eur J Vasc Endovasc Surg 2012; 43:208–217.
KEY POINTS
- A high index of suspicion should be maintained to recognize symptoms consistent with limb ischemia in a younger patient in the absence of the usual atherosclerosis risk factors.
- A workup for most conditions includes noninvasive vascular ultrasonography to detect and quantify limb ischemia.
- Prompt referral for surgical or endovascular treatment is necessary for optimal limb salvage.