User login
Guiding Resuscitation in the Emergency Department
Resuscitation of critically ill patients in shock from cardiogenic, hypovolemic, obstructive, distributive, or neurogenic etiology is a cornerstone of the care delivered by emergency physicians (EPs).1 Regardless of the etiology, it is essential that the treating EP initiate resuscitative measures in a timely manner and closely trend the patient’s response to these interventions.
The early goal-directed therapy (EGDT) initially proposed by Rivers et al2 in 2001 demonstrated a bundled approach to fluid resuscitation by targeting end points for volume resuscitation, mean arterial blood pressure (MAP), oxygen (O2) delivery/extraction (mixed venous O2 saturation, [SvO2]), hemoglobin (Hgb) concentration, and cardiac contractility. Since then, advancements in laboratory testing and hemodynamic monitoring (HDM) devices further aid and guide resuscitative efforts, and are applicable to any etiology of shock.
In addition to these advancements, the growing evidence of the potential harm from improper fluid resuscitation, such as the administration of excessive intravascular fluid (IVF),3 underscores the importance of a precise, targeted, and individualized approach to care. This article reviews the background, benefits, and limitations of some of the common and readily available tools in the ED that the EP can employ to guide fluid resuscitation in critically ill patients.
Physical Examination
Background
The rapid recognition and treatment of septic shock in the ED is associated with lower rates of in-hospital morbidity and mortality.4 The physical examination by the EP begins immediately upon examining the patient. The acquisition of vital signs and recognition of physical examination findings suggestive of intravascular volume depletion allows the EP to initiate treatment immediately.
In this discussion, hypotension is defined as systolic blood pressure (SBP) of less than 95 mm Hg, MAP of less than 65 mm Hg, or a decrease in SBP of more than 40 mm Hg from baseline measurements. Subsequently, shock is defined as hypotension with evidence of tissue hypoperfusion-induced dysfunction.5,6 Although the use of findings from the physical examination to guide resuscitation allows for rapid patient assessment and treatment, the predictive value of the physical examination to assess hemodynamic status is limited.
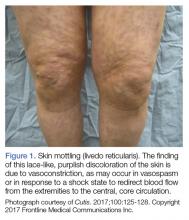
Visual inspection of the patient’s skin and mucous membranes can serve as an indicator of volume status. The patient’s tongue should appear moist with engorged sublingual veins; a dry tongue and diminished veins may suggest the need for volume resuscitation. On examination of the skin, delayed capillary refill of the digits and cool, clammy extremities suggest the shunting of blood by systemic circulation from the skin to central circulation. Patients who progress to more severe peripheral vasoconstriction develop skin mottling, referred to as livedo reticularis (Figure 1).
Benefits
The major benefit of the physical examination as a tool to evaluate hemodynamic status is its ease and rapid acquisition. The patient’s vital signs and physical examination can be obtained in the matter of moments upon presentation, without the need to wait on results of laboratory evaluation or additional equipment. Additionally, serial examinations by the same physician can be helpful to monitor a patient’s response to resuscitative efforts. The negative predictive value (NPV) of the physical examination in evaluating for hypovolemia may be helpful, but only when it is taken in the appropriate clinical context and is used in conjunction with other diagnostic tools. The physical examination can exclude hypovolemic volume status with an NPV of approximately 70%.7
A constellation of findings from the physical examination may include altered mentation, hypotension, tachycardia, and decreased urinary output by 30% to 40% intravascular volume loss.8,9Findings from the physical examination to assess fluid status should be used with caution as interobserver reliability has proven to be poor and the prognostic value is limited.
Limitations
The literature shows the limited prognostic value of the physical examination in determining a patient’s volume status and whether fluid resuscitation is indicated. For example, in one meta-analysis,10 supine hypotension and tachycardia were frequently absent on examination—even in patients who underwent large volume phlebotomy.8 This study also showed postural dizziness to be of no prognostic value.
Another study by Saugel et al7 that compared the physical examination (skin assessment, lung auscultation, and percussion) to transpulmonary thermodilution measurements of the cardiac index, global end-diastolic volume index, and extravascular lung water index, found poor interobserver correlation and agreement among physicians.
The physical examination is also associated with weak predictive capabilities for the estimation of volume status compared to the device measurements. Another contemporary study by Saugel et al9 evaluated the predictive value of the physical examination to accurately identify volume responsiveness replicated these results, and reported poor interobserver correlation (κ coefficient 0.01; 95% caval index [CI] -0.39-0.42) among physical examination findings, with a sensitivity of only 71%, specificity of 23.5%, positive predictive value of 27.8%, and negative predictive value of 66.7%.9
Serum Lactate Levels
Background
In the 1843 book titled, Investigations of Pathological Substances Obtained During the Epidemic of Puerperal Fever, Johann Joseph Scherer described the cases of seven young peripartum female patients who died from a clinical picture of what is now understood to be septic shock.11 In his study of these cases, Scherer demonstrated the presence of lactic acid in patients with pathological conditions. Prior to this discovery, lactic acid had never been isolated in a healthy individual. These results were recreated in 1851 by Scherer and Virchow,11 who demonstrated the presence of lactic acid in the blood of a patient who died from leukemia. The inference based on Scherer and Virchow’s work correlated the presence of excessive lactic acid with bodily deterioration and severe disease. Since this finding, there has been a great deal of interest in measuring serum lactic acid as a means to identify and manage critical illness.
In a 2001 groundbreaking study of EGDT for severe sepsis and septic shock, Rivers et al2 studied lactic acid levels as a marker for severe disease. Likewise, years later, the 2014 Protocol-Based Care for Early Septic Shock (PROCESS), Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE), and Australasian Resuscitation in Sepsis Evaluation (ARISE) trials used lactate levels in a similar manner to identify patients appropriate for randomization.12-14 While the purpose of measuring lactic acid was only employed in these studies to identify patients at risk for critical illness, the 2012 Surviving Sepsis Campaign Guidelines recommended serial measurement of lactate, based on the assumption that improved lactate levels signified better tissue perfusion.15
Although much of the studies on lactate levels appear to be based on the treatment and management of septic patients, findings can be applied to any etiology of shock. For example, a serum lactate level greater than 2 mmol/L is considered abnormal, and a serum lactate greater than 4 mmol/L indicates a significantly increased risk for in-hospital mortality.16
Benefits
It is now a widely accepted belief that the rapid identification, triage, and treatment of critically ill patients has a dramatic effect on morbidity and mortality.4 As previously noted, lactate has been extensively studied and identified as a marker of severe illness.17,18 A serum lactate level, which can be rapidly processed in the ED, can be easily obtained from a minimally invasive venous, arterial, or capillary blood draw.18 The only risk associated with serum lactate testing is that of any routine venipuncture; the test causes minimal, if any, patient discomfort.
Thanks to advances in point-of-care (POC) technology, the result of serum lactate assessment can be available within 10 minutes from blood draw. This technology is inexpensive and can be easily deployed in the prehospital setting or during the initial triage assessment of patients arriving at the ED.19 These POC instruments have been well correlated with whole blood measurements and permit for the rapid identification and treatment of at risk patients.
Limitations
The presence of elevated serum lactate levels is believed to represent the presence of cellular anaerobic metabolism due to impaired O2 delivery in the shock state. Abnormal measurements therefore prompt aggressive interventions aimed at maximizing O2 delivery to the tissues, such as intravenous fluid boluses, vasopressor therapy, or even blood product administration.
A return to a normalized serum lactate level is assumed to represent a transition back to aerobic metabolism. Lactate elevations, however, are not solely an indication of anaerobic metabolism and may only represent a small degree of lactate production.20 While the specific cellular mechanics are out of the scope of this article, it has been postulated that the increase in plasma lactate concentration is primarily driven by β-2 receptor stimulation from increased circulating catecholamines leading to increased aerobic glycolysis. Increased lactate levels could therefore be an adaptive mechanism of energy production—aggressive treatment and rapid clearance may, in fact, be harmful. Type A lactic acidosis is categorized as elevated serum levels due to tissue hypoperfusion.21
However, lactate elevations do not exclusively occur in severe illness. The use of β-2 receptor agonists such as continuous albuterol treatments or epinephrine may cause abnormal lactate levels.22 Other medications have also been associated with elevated serum lactate levels, including, but not limited to linezolid, metformin, and propofol.23-25 Additionally, lactate levels may be elevated after strenuous exercise, seizure activity, or in liver and kidney disease.26 These “secondary” causes of lactic acidosis that are not due to tissue hypoperfusion are referred to as type B lactic acidosis. Given these multiple etiologies and lack of specificity for this serum measurement, a failure to understand these limitations may result in over aggressive or unnecessary medical treatments.
Central Venous Pressure
Background
Central venous pressure (CVP) measurements can be obtained through a catheter, the distal tip of which transduces pressure of the superior vena cava at the entrance of the right atrium (RA). Thus, CVP is often used as a representation of RA pressure (RAP) and therefore an estimate of right ventricular (RV) preload. While CVP is used to diagnose and determine the etiology of shock, evidence and controversy regarding the use of CVP as a marker for resuscitation comes largely from sepsis-focused literature.5 Central venous pressure is meant to represent preload, which is essential for stroke volume as described by the Frank-Starling mechanism; however, its use as a target in distributive shock, a state in which it is difficult to determine a patient’s volume status, has been popularized by EGDT since 2001.2
Since the publication of the 2004 Surviving Sepsis Guidelines, CVP monitoring has been in the spotlight of sepsis resuscitation, albeit with some controversy.27 Included as the result of two studies, this recommendation has been removed in the most recent guidelines after 12 years of further study and scrutiny.2,27,28
Hypovolemic and hemorrhagic shock are usually diagnosed clinically and while a low CVP can be helpful in the diagnosis, the guidelines do not support CVP as a resuscitation endpoint. Obstructive and cardiogenic shock will both result in elevated CVP; however, treatment of obstructive shock is generally targeted at the underlying cause. While cardiogenic shock can be preload responsive, the mainstay of therapy in the ED is identification of patients for revascularization and inotropic support.29
Benefits
The CVP has been used as a surrogate for RV preload volume. If a patient’s preload volume is low, the treating physician can administer fluids to improve stroke volume and cardiac output (CO). Clinically, CVP measurements are easy to obtain provided a central venous line has been placed with the distal tip at the entrance to the RA. Central venous pressure is measured by transducing the pressure via manometry and connecting it to the patient’s bedside monitor. This provides an advantage of being able to provide serial or even continuous measurements. The “normal” RAP should be a low value (1-5 mm Hg, mean of 3 mm Hg), as this aids in the pressure gradient to drive blood from the higher pressures of the left ventricle (LV) and aorta through the circulation back to the low-pressure of the RA.30 The value of the CVP is meant to correspond to the physical examination findings of jugular venous distension.31,32 Thus, a low CVP may be “normal” and seen in patients with hypovolemic shock, whereas an elevated CVP can suggest volume overload or obstructive shock. However, this is of questionable value in distributive shock cases.
Aside from the two early studies on CVP monitoring during treatment of septic patients, there are few data to support the use of CVP measurement in the early resuscitation of patients with shock.2,28 More recent trials (PROMISE, ARISE, PROCESS) that compared protocolized sepsis care to standard care showed no benefit to bundles including CVP measurements.12-14 However, a subsequent, large observational trial spanning 7.5 years demonstrated improvements in sepsis-related mortality in patients who received a central venous catheter (CVC) and CVP-targeted therapy.33 Thus, it is possible that protocols including CVP are still beneficial in combination with other therapies even though CVP in isolation is not.
Limitations
The traditional two assumptions in CVP monitoring are CVP value represents the overall volume status of the patient, and the LV is able to utilize additional preload volume. The latter assumption, however, may be hampered by the presence of sepsis-induced myocardial dysfunction, which may be present in up to 40% of critically ill patients.34 The former assumption does not always hold true due to processes that change filling pressures independent of intravascular volume—eg, acute or chronic pulmonary hypertension, cardiac tamponade, intra-abdominal hypertension, or LV failure. Even before the landmark EGDT study, available data suggested that CVP was not a reliable marker for resuscitation management.35 A recent systematic review by Gottlieb and Hunter36 showed that the area under the receiver-operator curve for low, mid-range, or high CVPs was equivocal at best. In addition to its unreliability and lack of specificity, another significant drawback to using CVP to guide resuscitation therapy in the ED is that it necessitates placement of a CVC, which can be time-consuming and, if not otherwise indicated, lead to complications of infection, pneumothorax, and/or thrombosis.37
Mixed Venous Oxygen
Background
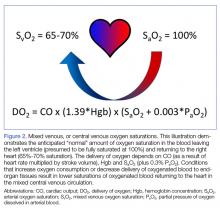
Most EPs are familiar with the use of ScvO2 in EGDT protocols to guide volume resuscitation of septic patients.2 A patient’s ScvO2 represents the O2 saturation of venous blood obtained via a CVC at the confluence of the superior vena cava and the RA, and thus it reflects tissue O2 consumption as a surrogate for tissue perfusion. The measurement parallels the SvO2 obtained from the pulmonary artery. In a healthy patient, SvO2 is around 65% to 70% and includes blood returning from both the superior and inferior vena cava (IVC). As such, ScvO2 values are typically 3% to 5% lower than SvO2 owing to the lower O2 extracted by tissues draining into the IVC compared to the mixed venous blood sampled from the pulmonary artery.38
Though a debate over the benefit of EGDT in treating sepsis continues, understanding the physiology of ScvO2 measurements is another potential tool the EP can use to guide the resuscitation of critically ill patients.39 A patient’s SvO2 and, by extension, ScvO2 represents the residual O2 saturation after the tissues have extracted the amount of O2 necessary to meet metabolic demands (Figure 2).
Conversely, cellular dysfunction, which can occur in certain toxicities or in severe forms of sepsis, can lead to decreased tissue O2 consumption with a concomitant rise in ScvO2 to supernormal values.38 The EP should take care, however, to consider whether ScvO2 values exceeding 80% represent successful therapeutic intervention or impaired tissue O2 extraction and utilization. There are data from ED patients suggesting an increased risk of mortality with both extremely low and extremely high values of ScvO2.40
Benefits
A critically ill patient’s ScvO2 can potentially provide EPs with insight into the patient’s global tissue perfusion and the source of any mismatch between O2 delivery and consumption. Using additional tools and measurements (physical examination, serum Hgb levels, and pulse oximetry) in conjunction with an ScvO2 measurement, assists EPs in identifying targets for therapeutic intervention. The effectiveness of this intervention can then be assessed using serial ScvO2 measurements, as described in Rivers et al2 EGDT protocol. Importantly, EPs should take care to measure serial ScvO2 values to maximize its utility.38 Similar to a CVP measurement, ScvO2is easily obtained from blood samples for serial laboratory measurements, assuming the patient already has a CVC with the distal tip at the entrance to the RA (ScvO2) or a pulmonary artery catheter (PAC) (SvO2).
Limitations
Serial measurements provide the most reliable information, which may be more useful in patients who spend extended periods of their resuscitation in the ED. In comparison to other measures of global tissue hypoxia, work by Jones et al41 suggests non-inferiority of peripherally sampled, serial lactate measurements as an alternative to ScvO2. This, in conjunction with the requirement for an internal jugular CVC, subclavian CVC, or PAC with their associated risks, may make ScvO2 a less attractive guide for the resuscitation of critically ill patients in the ED.
Monitoring Devices
Background
As noted throughout this review, it is important not only to identify and rapidly treat shock, but to also correctly identify the type of shock, such that treatment can be appropriately directed at its underlying cause. However, prior work suggests that EPs are unable to grossly estimate CO or systemic vascular resistance when compared to objective measurements of these parameters.42 This is in agreement with the overall poor performance of physical examination and clinical evaluation as a means of predicting volume responsiveness or guiding resuscitation, as discussed previously. Fortunately, a wide variety of devices to objectively monitor hemodynamics are now available to the EP.
In 1970, Swan et al43 published their initial experience with pulmonary artery catheterization at the bedside, using a balloon-tipped, flow-guided PAC in lieu of fluoroscopy, which had been mandated by earlier techniques. The ability to measure CO, right heart pressures, pulmonary arterial pressures, and estimate LV end diastolic pressure ushered in an era of widespread PAC use, despite an absence of evidence for causation of improved patient outcomes. The utilization of PACs has fallen, as the literature suggests that the empiric placement of PACs in critically ill patients does not improve mortality, length of stay, or cost, and significant complication rates have been reported in large trials.44,45Subsequently, a number of non-invasive or less-invasive HDM devices have been developed. Amongst the more commonly encountered modern devices, the techniques utilized for providing hemodynamic assessments include thermodilution and pulse contour analysis (PiCCOTM), pulse contour analysis (FloTrac/VigileoTM), and lithium chemodilution with pulse power analysis (LiDCOplusTM).46 The primary utility of these devices for the EP lies in the ability to quantify CO, stroke volume, and stroke volume or pulse pressure variation (PPV) to predict or assess response to resuscitative interventions (volume administration, vasopressors, inotropes, etc).
Benefits
Many of these devices require placement of an arterial catheter. Some require the addition of a CVC. Both of these procedures are well within the clinical scope of the EP, and are performed with fair frequency on critically ill patients. This is a distinct advantage when compared to pulmonary artery catheterization, a higher risk procedure that is rarely performed outside of the intensive care unit or cardiac catheterization laboratory. In addition, all of the devices below present hemodynamic data in a graphical, easy-to-read format, in real time. All of the devices discussed report stroke volume variation (SVV) or PPV continuously.
Limitations
Though these measures have validated threshold values that predict volume responsiveness, they require the patient to be intubated with a set tidal volume of greater than or equal to 8 mL/kg without spontaneous respirations and cardiac arrhythmias, in order to accurately do so. All of the HDM devices that rely on pulse contour analysis as the primary means of CO measurement cannot be used in the presence of significant cardiac arrhythmias (ie, atrial fibrillation), or mechanical circulatory assistance devices (ie, intra-aortic balloon counterpulsation). None of these devices are capable of monitoring microcirculatory changes, felt to be of increasing clinical importance in the critically ill.
The use of HDM devices to monitor CO with a reasonable degree of accuracy, trend CO, and assess for volume responsiveness using a number of previously validated parameters such as SVV is now in little doubt. However, these devices are still invasive, if less so than a pulmonary artery. The crux of the discussion of HDM devices for use in ED resuscitation revolves around whether or not the use of such devices to drive previously validated, protocolized care results in better outcomes for patients. The EP can now have continuous knowledge of a large number of hemodynamic parameters at their fingertips with relatively minimal additional efforts. At the time of this writing, though, this is both untested and unproven, with respect to the ED population.
Point-of-Care Ultrasound
Background
Over the past two decades, ultrasound (US) has become an integral part of the practice of emergency medicine (EM), and is now included in all United States Accreditation Council for Graduate Medical Education Emergency Medicine Residency Programs.47,48 It has emerged as a very important bedside tool performed by the clinician to identify type of shock and guide resuscitation, and has been endorsed by both EM and critical care societies.49-51 This section reviews the utility of US as a modality in identifying shock and guiding resuscitation, in addition to the pitfalls and limitations of this important tool.
In 2010, Perera et al47 described in their landmark article the Rapid Ultrasound in SHock (RUSH) examination, which describes a stepwise (the pump, tank, pipes) approach to identify the type of shock (cardiogenic, hypovolemic, obstructive, or distributive) in the crashing, hypotensive ED patient. We do not describe the full RUSH examination in this review, but discuss key elements of it as examples of how POCUS can assist the EP to make a rapid diagnosis and aid in the management of patients in shock. The “pump” is the heart, which is assessed in four different views to identify a pericardial effusion and possible tamponade, assess contractility or ejection fraction of the LV (severely decreased, decreased, normal, or hyperdynamic), and right heart strain which is identified by an RV that is larger than the LV, indicative of a potential pulmonary embolus.
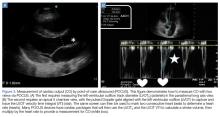
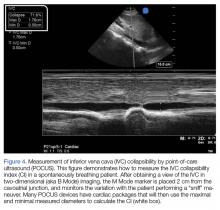
The “tank” is then assessed by visualizing the IVC in the subxiphoid plane, and is evaluated for respiratory collapsibility (CI) and maximum size. This has been quite the debated topic over the last two decades. In 1988, Simonson and Schiller52 were the first to describe a correlation in spontaneously breathing patients between IVC caliber (measured 2 cm from the cavoatrial junction) and variation and RAP, where a larger IVC diameter and less respiratory variation correlated with a high RAP. Kircher et al53 later went on to describe that a CI greater than 50% correlated with an RAP of less than 10 mm Hg and vice versa in spontaneously breathing patients. Since then there have been more studies attempting to verify these findings in both spontaneously breathing and mechanically ventilated patients.54-56 The purpose of performing these measurements is not to estimate CVP, but to assess fluid responsiveness (ie, a blood pressure response to a fluid challenge). It can be assumed in states of shock that a small IVC, or one with a high CI, in the presence of a hyperdynamic heart is indicative of an underfilled ventricle and fluid responsiveness, especially if the IVC size increases with fluid.55,57 However, there are several caveats to this. First, in mechanically ventilated patients, the IVC is already plethoric due to positive pressure ventilation, and increases in diameter with inspiration and decreases with expiration as compared to spontaneously breathing patients. Second, the CI value to predict volume responsiveness in ventilated patients is set at 15% instead of 50%.55 Third, it is important to always take the clinical scenario in context; a dilated IVC with small CI is not necessarily only due to volume overload and congestive heart failure, but can be due to elevated RAP from obstructive shock due to cardiac tamponade or massive pulmonary embolus, which is why it is important to assess the “pump” first.47,58 It is also crucial to not forget to assess the abdominal and thoracic cavities, as intraperitoneal or pleural fluid with a collapsed IVC can potentially make a diagnosis of hemorrhagic or hypovolemic shock depending on the clinical scenario.47 The final part of the RUSH protocol is to evaluate the “pipes,” inclusive of the lower extremity deep venous system for evaluation of potential thrombosis that could increase suspicion for a pulmonary embolism causing obstructive shock, and the aorta with the common iliac arteries if there is concern for aortic dissection or aneurysmal rupture.
Benefits
Some of the most significant advantages to the use of POCUS to guide resuscitation is that it is quick, non-invasive, does not use ionizing radiation, and can be easily repeated. As noted above, it is a requirement for EM residencies to teach its use, so that contemporary graduates are entering the specialty competent in applying it to the care of their patients. Furthermore, POCUS is done at the bedside, limiting the need to potentially transport unstable patients.
In the most basic applications, POCUS provides direct visualization of a patient’s cardiac function, presence or absence of lung sliding to suggest a pneumothorax, presence of pulmonary edema, assessment of CVP pressures or potential for fluid responsiveness, as well as identification of potential thoracic, peritoneal, or pelvic cavity fluid accumulation that may suggest hemorrhage. There is literature to support that these assessments performed by the EP have been shown to be comparable to those of cardiologists.59,60 With continued practice and additional training, it is possible for EPs to even perform more “advanced” hemodynamic assessments to both diagnose and guide therapy to patients in shock (Figures 3 and 4).61
Limitations
Although POCUS has been shown as a remarkable tool to help assist the EP in making rapid decisions regarding resuscitation, it is always important to remember its limitations. Most of the studies regarding its use are of very small sample sizes, and further prospective studies have to be performed in order for this modality to be fully relied on.62Compared to some of the previously mentioned HDM devices that may provide continuous data, POCUS needs to be performed by the treating physician, thereby occurring intermittently. Emergency physicians need to be aware of their own experience and limitations with this modality, as errors in misdiagnosis can lead to unnecessary procedures, with resulting significant morbidity and mortality. Blanco and Volpicelli63 describe several common errors that include misdiagnosing the stomach as a peritoneal effusion, assuming adequate volume resuscitation when the IVC is seen to be plethoric in the setting of cardiac tamponade, or mistaking IVC movement as indicative of collapsibility, amongst other described misinterpretations. Several other studies have shown that, despite adequate performance of EPs in POCUS, diagnostic sensitivities remained higher when performed by radiologists.64-67 Thus it remains important for the EPs to be vigilant and not anchor on a diagnosis when in doubt, and to consult early with radiology, particularly if there is any question, to avoid potential adverse patient outcomes.
Summary
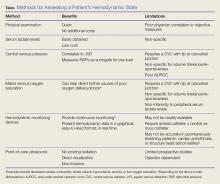
There are several ways to diagnose and track resuscitation in the ED, which include physical examination, assessment of serum laboratory values, monitoring of hemodynamic status, and use of POCUS. Unfortunately, none of these methods provides a perfect assessment, and no method has been proven superior and effective over the others. Therefore, it is important for EPs treating patients in shock to be aware of the strengths and limitations of each assessment method (Table).
1. Richards JB, Wilcox SR. Diagnosis and management of shock in the emergency department. Emerg Med Pract. 2014;16(3):1-22; quiz 22-23.
2. Rivers E, Nguyen B, Havstad S, et al; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377.
3. Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259-265. doi:10.1097/CCM.0b013e3181feeb15.
4. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235-2244. doi:10.1056/NEJMoa1703058.
5. Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(12):1795-1815. doi:10.1007/s00134-014-3525-z.
6. Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726-1734. doi:10.1056/NEJMra1208943.
7. Saugel B, Ringmaier S, Holzapfel K, et al. Physical examination, central venous pressure, and chest radiography for the prediction of transpulmonary thermodilution-derived hemodynamic parameters in critically ill patients: a prospective trial. J Crit Care. 2011;26(4):402-410. doi:10.1016/j.jcrc.2010.11.001.
8. American College of Surgeons. Committee on Trauma. Shock. In: American College of Surgeons. Committee on Trauma, ed. Advanced Trauma Life Support: Student Course Manual. 9th ed. Chicago, IL: American College of Surgeons; 2012:69.
9. Saugel B, Kirsche SV, Hapfelmeier A, et al. Prediction of fluid responsiveness in patients admitted to the medical intensive care unit. J Crit Care. 2013:28(4):537.e1-e9. doi:10.1016/j.jcrc.2012.10.008.
10. McGee S, Abernethy WB 3rd, Simel DV. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281(11):1022-1029.
11. Kompanje EJ, Jansen TC, van der Hoven B, Bakker J. The first demonstration of lactic acid in human blood in shock by Johann Joseph Scherer (1814-1869) in January 1843. Intensive Care Med. 2007;33(11):1967-1971. doi:10.1007/s00134-007-0788-7.
12. The ProCESS Investigators. A Randomized Trial of Protocol-Based Care for Early Septic Shock. N Engl J Med. 2014; 370:1683-1693. doi:10.1056/NEJMoa1401602.
13. Mouncey PR, Osborn TM, Power GS, et al. Protocolised Management In Sepsis (ProMISe): a multicentre randomised controlled trial of the clinical effectiveness and cost-effectiveness of early, goal-directed, protocolised resuscitation for emerging septic shock. Health Technol Assess. 2015;19(97):i-xxv, 1-150. doi:10.3310/hta19970.
14. ARISE Investigators; ANZICS Clinical Trials Group; Peake SL, Delaney A, Bailey M, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496-1506. doi:10.1056/NEJMoa1404380.
15. Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Group. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228. doi:10.1007/s00134-012-2769-8.
16. Casserly B, Phillips GS, Schorr C, et al: Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med. 2015;43(3):567-573. doi:10.1097/CCM.0000000000000742.
17. Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care. 2013;3(1):12. doi:10.1186/2110-5820-3-12.
18. Kruse O, Grunnet N, Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scand J Trauma Resusc Emerg Med. 2011;19:74. doi:10.1186/1757-7241-19-74.
19. Gaieski DF, Drumheller BC, Goyal M, Fuchs BD, Shofer FS, Zogby K. Accuracy of handheld point-of-care fingertip lactate measurement in the emergency department. West J Emerg Med. 2013;14(1):58-62. doi:10.5811/westjem.2011.5.6706.
20. Marik PE, Bellomo R. Lactate clearance as a target of therapy in sepsis: a flawed paradigm. OA Critical Care. 2013;1(1):3.
21. Kreisberg RA. Lactate homeostasis and lactic acidosis. Ann Intern Med. 1980;92(2 Pt 1):227-237.
22. Dodda VR, Spiro P. Can albuterol be blamed for lactic acidosis? Respir Care. 2012; 57(12):2115-2118. doi:10.4187/respcare.01810.
23. Scale T, Harvey JN. Diabetes, metformin and lactic acidosis. Clin Endocrinol (Oxf). 2011;74(2):191-196. doi:10.1111/j.1365-2265.2010.03891.x.
24. Velez JC, Janech MG. A case of lactic acidosis induced by linezolid. Nat Rev Nephrol. 2010;6(4):236-242. doi:10.1038/nrneph.2010.20.
25. Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia. 2007;62(7):690-701.
26. Griffith FR Jr, Lockwood JE, Emery FE. Adrenalin lactacidemia: proportionality with dose. Am J Physiol. 1939;127(3):415-421.
27. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6.
28. Early Goal-Directed Therapy Collaborative Group of Zhejiang Province. The effect of early goal-directed therapy on treatment of critical patients with severe sepsis/ septic shock: a multi-center, prospective, randomised, controlled study. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2010;22(6):331-334.
29. Thiele H, Ohman EM, Desch S, Eitel I, de Waha S. Management of cardiogenic shock. Eur Heart J. 2015;36(20):1223-1230. doi:10.1093/eurheartj/ehv051.
30. Lee M, Curley GF, Mustard M, Mazer CD. The Swan-Ganz catheter remains a critically important component of monitoring in cardiovascular critical care. Can J Cardiol. 2017;33(1):142-147. doi:10.1016/j.cjca.2016.10.026.
31. Morgan BC, Abel FL, Mullins GL, Guntheroth WG. Flow patterns in cavae, pulmonary artery, pulmonary vein, and aorta in intact dogs. Am J Physiol. 1966;210(4):903-909. doi:10.1152/ajplegacy.1966.210.4.903.
32. Brecher GA, Hubay CA. Pulmonary blood flow and venous return during spontaneous respiration. Circ Res. 1955;3(2):210-214.

33. Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med. 2014;40(11):1623-1633. doi:10.1007/s00134-014-3496-0.
34. Fernandes CJ Jr, Akamine N, Knobel E. Cardiac troponin: a new serum marker of myocardial injury in sepsis. Intensive Care Med. 1999;25(10):1165-1168. doi:10.1007/s001340051030.
35. Rady MY, Rivers EP, Nowak RM. Resuscitation of the critically III in the ED: responses of blood pressure, heart rate, shock index, central venous oxygen saturation, and lactate. Am J Emerg Med. 1996;14(2):218-225. doi:10.1016/s0735-6757(96)90136-9.
36. Gottlieb M, Hunter B. Utility of central venous pressure as a predictor of fluid responsiveness. Ann Emerg Med. 2016;68(1):114-116. doi:10.1016/j.annemergmed.2016.02.009.
37. Kornbau C, Lee KC, Hughes GD, Firstenberg MS. Central line complications. Int J Critical Illn Inj Sci. 2015;5(3):170-178. doi:10.4103/2229-5151.164940.
38. Walley KR. Use of central venous oxygen saturation to guide therapy. Am J Respir Crit Care Med. 2011;184(5):514-520. doi:10.1164/rccm.201010-1584CI.
39. PRISM Investigators, Rowan KM, Angus DC, et al. Early, goal-directed therapy for septic shock - a patient-level meta-analysis. N Engl J Med. 2017;376(23):2223-2234. doi:10.1056/NEJMoa1701380.
40. Pope JV, Jones AE, Gaieski DF, Arnold RC, Trzeciak S, Shapiro NI; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Multicenter study of central venous oxygen saturation (ScvO(2)) as a predictor of mortality in patients with sepsis. Ann Emerg Med. 2010;55(1):40-46.e1. doi:10.1016/j.annemergmed.2009.08.014.
41. Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303(8):739-746. doi:10.1001/jama.2010.158.
42. Nowak RM, Sen A, Garcia, AJ, et al. The inability of emergency physicians to adequately clinically estimate the underlying hemodynamic profiles of acutely ill patients. Am J Emerg Med. 2012;30(6):954-960. doi:10.1016/j.ajem.2011.05.021.
43. Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med. 1970;283(9):447-451. doi:10.1056/NEJM197008272830902.
44. Hadian M, Pinsky MR. Evidence-based review of the use of the pulmonary artery catheter: impact data and complications. Crit Care. 2006;10 Suppl 3:S8.
45. Rajaram SS, Desai, NK, Kalra A, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev. 2013;(2):CD003408. doi:10.1002/14651858.CD003408.pub3.
46. Laher AE, Watermeyer MJ, Buchanan SK, et al. A review of hemodynamic monitoring techniques, methods and devices for the emergency physician. Am J Emerg Med. 2017;35(9):1335-1347. doi:10.1016/j.ajem.2017.03.036.
47. Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid Ultrasound in SHock in the evaluation of the critically lll. Emerg Med Clin North Am. 2010;28(1):29-56, vii. doi:10.1016/j.emc.2009.09.010.
48. Heller MB, Mandavia D, Tayal VS, et al. Residency training in emergency ultrasound: fulfilling the mandate. Acad Emerg Med. 2002;9(8):835-839.
49. Ultrasound guidelines: emergency, point-of-care and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2016;69(5):e27-e54. doi:10.1016/j.annemergmed.2016.08.457.
50. Expert Round Table on Ultrasound in ICU. International expert statement on training standards for critical care ultrasonography. Intensive Care Med. 2011;37(7):1077-1083. doi:10.1007/s00134-011-2246-9.
51. Neri L, Storti E, Lichtenstein D. Toward an ultrasound curriculum for critical care medicine. Crit Care Med. 2007;35(5 Suppl):S290-S304.
52. Simonson JS, Schiller NB. Sonospirometry: a new method for noninvasive estimation of mean right atrial pressure based on two-dimensional echographic measurements of the inferior vena cava during measured inspiration. J Am Coll Cardiol. 1988;11(3):557-564.
53. Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66(4):493-496.
54. Nagdev AD, Merchant RC, Tirado-Gonzalez A, Sisson CA, Murphy MC. Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55(3):290-295. doi:10.1016/j.annemergmed.2009.04.021.
55. Barbier C, Loubières Y, Schmit C, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30(9):1740-1746.
56. Corl KA, George NR, Romanoff J, et al. Inferior vena cava collapsibility detects fluid responsiveness among spontaneously breathing critically-ill patients. J Crit Care. 2017;41:130-137. doi:10.1016/j.jcrc.2017.05.008.
57. Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30(9):1834-1837.
58. Blehar DJ, Dickman E, Gaspari R. Identification of congestive heart failure via respiratory variation of inferior vena cava diameter. Am J Emerg Med. 2009;27(1):71-75. doi:10.1016/j.ajem.2008.01.002.
59. Moore CL, Rose GA, Tayal VS, Sullivan DM, Arrowood JA, Kline JA. Determination of left ventricular function by emergency physician echocardiography of hypotensive patients. Acad Emerg Med. 2002;9(3):186-193.
60. Mandavia DP, Hoffner RJ, Mahaney K, Henderson SO. Bedside echocardiography by emergency physicians. Ann Emerg Med. 2001;38(4):377-382.
61. Mosier JM, Martin J, Andrus P, et al. Advanced hemodynamic and cardiopulmonary ultrasound for critically ill patients in the emergency department. Emerg Med. 2018;50(1):17-34. doi:10.12788/emed.2018.0078.
62. Agarwal S, Swanson S, Murphy A, Yaeger K, Sharek P, Halamek LP. Comparing the utility of a standard pediatric resuscitation cart with a pediatric resuscitation cart based on the Broselow tape: a randomized, controlled, crossover trial involving simulated resuscitation scenarios. Pediatrics. 2005;116(3):e326-e333.
63. Blanco P, Volpicelli G. Common pitfalls in point-of-care ultrasound: a practical guide for emergency and critical care physicians. Crit Ultrasound J. 2016;8(1):15.
64. Tajoddini S, Shams Vahdati S. Ultrasonographic diagnosis of abdominal free fluid: accuracy comparison of emergency physicians and radiologists. Eur J Trauma Emerg Surg. 2013;39(1):9-13. doi:10.1007/s00068-012-0219-5.

65 Abbasi S, Bolverdi E, Zare MA, et al. Comparison of diagnostic value of conventional ultrasonography by emergency physicians with Doppler ultrasonography by radiology physicians for diagnosis of deep vein thrombosis. J Pak Med Assoc. 2012;62(5):461-465.
66. Arhami Dolatabadi A, Amini A, Hatamabadi H, et al. Comparison of the accuracy and reproducibility of focused abdominal sonography for trauma performed by emergency medicine and radiology residents. Ultrasound Med Biol. 2014;40(7):1476-1482. doi:10.1016/j.ultrasmedbio.2014.01.017.
67. Karimi E, Aminianfar M, Zarafshani K, Safaie A. The accuracy of emergency physicians in ultrasonographic screening of acute appendicitis; a cross sectional study. Emerg (Tehran). 2017;5(1):e22.
Resuscitation of critically ill patients in shock from cardiogenic, hypovolemic, obstructive, distributive, or neurogenic etiology is a cornerstone of the care delivered by emergency physicians (EPs).1 Regardless of the etiology, it is essential that the treating EP initiate resuscitative measures in a timely manner and closely trend the patient’s response to these interventions.
The early goal-directed therapy (EGDT) initially proposed by Rivers et al2 in 2001 demonstrated a bundled approach to fluid resuscitation by targeting end points for volume resuscitation, mean arterial blood pressure (MAP), oxygen (O2) delivery/extraction (mixed venous O2 saturation, [SvO2]), hemoglobin (Hgb) concentration, and cardiac contractility. Since then, advancements in laboratory testing and hemodynamic monitoring (HDM) devices further aid and guide resuscitative efforts, and are applicable to any etiology of shock.
In addition to these advancements, the growing evidence of the potential harm from improper fluid resuscitation, such as the administration of excessive intravascular fluid (IVF),3 underscores the importance of a precise, targeted, and individualized approach to care. This article reviews the background, benefits, and limitations of some of the common and readily available tools in the ED that the EP can employ to guide fluid resuscitation in critically ill patients.
Physical Examination
Background
The rapid recognition and treatment of septic shock in the ED is associated with lower rates of in-hospital morbidity and mortality.4 The physical examination by the EP begins immediately upon examining the patient. The acquisition of vital signs and recognition of physical examination findings suggestive of intravascular volume depletion allows the EP to initiate treatment immediately.
In this discussion, hypotension is defined as systolic blood pressure (SBP) of less than 95 mm Hg, MAP of less than 65 mm Hg, or a decrease in SBP of more than 40 mm Hg from baseline measurements. Subsequently, shock is defined as hypotension with evidence of tissue hypoperfusion-induced dysfunction.5,6 Although the use of findings from the physical examination to guide resuscitation allows for rapid patient assessment and treatment, the predictive value of the physical examination to assess hemodynamic status is limited.
Visual inspection of the patient’s skin and mucous membranes can serve as an indicator of volume status. The patient’s tongue should appear moist with engorged sublingual veins; a dry tongue and diminished veins may suggest the need for volume resuscitation. On examination of the skin, delayed capillary refill of the digits and cool, clammy extremities suggest the shunting of blood by systemic circulation from the skin to central circulation. Patients who progress to more severe peripheral vasoconstriction develop skin mottling, referred to as livedo reticularis (Figure 1).
Benefits
The major benefit of the physical examination as a tool to evaluate hemodynamic status is its ease and rapid acquisition. The patient’s vital signs and physical examination can be obtained in the matter of moments upon presentation, without the need to wait on results of laboratory evaluation or additional equipment. Additionally, serial examinations by the same physician can be helpful to monitor a patient’s response to resuscitative efforts. The negative predictive value (NPV) of the physical examination in evaluating for hypovolemia may be helpful, but only when it is taken in the appropriate clinical context and is used in conjunction with other diagnostic tools. The physical examination can exclude hypovolemic volume status with an NPV of approximately 70%.7
A constellation of findings from the physical examination may include altered mentation, hypotension, tachycardia, and decreased urinary output by 30% to 40% intravascular volume loss.8,9Findings from the physical examination to assess fluid status should be used with caution as interobserver reliability has proven to be poor and the prognostic value is limited.
Limitations
The literature shows the limited prognostic value of the physical examination in determining a patient’s volume status and whether fluid resuscitation is indicated. For example, in one meta-analysis,10 supine hypotension and tachycardia were frequently absent on examination—even in patients who underwent large volume phlebotomy.8 This study also showed postural dizziness to be of no prognostic value.
Another study by Saugel et al7 that compared the physical examination (skin assessment, lung auscultation, and percussion) to transpulmonary thermodilution measurements of the cardiac index, global end-diastolic volume index, and extravascular lung water index, found poor interobserver correlation and agreement among physicians.
The physical examination is also associated with weak predictive capabilities for the estimation of volume status compared to the device measurements. Another contemporary study by Saugel et al9 evaluated the predictive value of the physical examination to accurately identify volume responsiveness replicated these results, and reported poor interobserver correlation (κ coefficient 0.01; 95% caval index [CI] -0.39-0.42) among physical examination findings, with a sensitivity of only 71%, specificity of 23.5%, positive predictive value of 27.8%, and negative predictive value of 66.7%.9
Serum Lactate Levels
Background
In the 1843 book titled, Investigations of Pathological Substances Obtained During the Epidemic of Puerperal Fever, Johann Joseph Scherer described the cases of seven young peripartum female patients who died from a clinical picture of what is now understood to be septic shock.11 In his study of these cases, Scherer demonstrated the presence of lactic acid in patients with pathological conditions. Prior to this discovery, lactic acid had never been isolated in a healthy individual. These results were recreated in 1851 by Scherer and Virchow,11 who demonstrated the presence of lactic acid in the blood of a patient who died from leukemia. The inference based on Scherer and Virchow’s work correlated the presence of excessive lactic acid with bodily deterioration and severe disease. Since this finding, there has been a great deal of interest in measuring serum lactic acid as a means to identify and manage critical illness.
In a 2001 groundbreaking study of EGDT for severe sepsis and septic shock, Rivers et al2 studied lactic acid levels as a marker for severe disease. Likewise, years later, the 2014 Protocol-Based Care for Early Septic Shock (PROCESS), Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE), and Australasian Resuscitation in Sepsis Evaluation (ARISE) trials used lactate levels in a similar manner to identify patients appropriate for randomization.12-14 While the purpose of measuring lactic acid was only employed in these studies to identify patients at risk for critical illness, the 2012 Surviving Sepsis Campaign Guidelines recommended serial measurement of lactate, based on the assumption that improved lactate levels signified better tissue perfusion.15
Although much of the studies on lactate levels appear to be based on the treatment and management of septic patients, findings can be applied to any etiology of shock. For example, a serum lactate level greater than 2 mmol/L is considered abnormal, and a serum lactate greater than 4 mmol/L indicates a significantly increased risk for in-hospital mortality.16
Benefits
It is now a widely accepted belief that the rapid identification, triage, and treatment of critically ill patients has a dramatic effect on morbidity and mortality.4 As previously noted, lactate has been extensively studied and identified as a marker of severe illness.17,18 A serum lactate level, which can be rapidly processed in the ED, can be easily obtained from a minimally invasive venous, arterial, or capillary blood draw.18 The only risk associated with serum lactate testing is that of any routine venipuncture; the test causes minimal, if any, patient discomfort.
Thanks to advances in point-of-care (POC) technology, the result of serum lactate assessment can be available within 10 minutes from blood draw. This technology is inexpensive and can be easily deployed in the prehospital setting or during the initial triage assessment of patients arriving at the ED.19 These POC instruments have been well correlated with whole blood measurements and permit for the rapid identification and treatment of at risk patients.
Limitations
The presence of elevated serum lactate levels is believed to represent the presence of cellular anaerobic metabolism due to impaired O2 delivery in the shock state. Abnormal measurements therefore prompt aggressive interventions aimed at maximizing O2 delivery to the tissues, such as intravenous fluid boluses, vasopressor therapy, or even blood product administration.
A return to a normalized serum lactate level is assumed to represent a transition back to aerobic metabolism. Lactate elevations, however, are not solely an indication of anaerobic metabolism and may only represent a small degree of lactate production.20 While the specific cellular mechanics are out of the scope of this article, it has been postulated that the increase in plasma lactate concentration is primarily driven by β-2 receptor stimulation from increased circulating catecholamines leading to increased aerobic glycolysis. Increased lactate levels could therefore be an adaptive mechanism of energy production—aggressive treatment and rapid clearance may, in fact, be harmful. Type A lactic acidosis is categorized as elevated serum levels due to tissue hypoperfusion.21
However, lactate elevations do not exclusively occur in severe illness. The use of β-2 receptor agonists such as continuous albuterol treatments or epinephrine may cause abnormal lactate levels.22 Other medications have also been associated with elevated serum lactate levels, including, but not limited to linezolid, metformin, and propofol.23-25 Additionally, lactate levels may be elevated after strenuous exercise, seizure activity, or in liver and kidney disease.26 These “secondary” causes of lactic acidosis that are not due to tissue hypoperfusion are referred to as type B lactic acidosis. Given these multiple etiologies and lack of specificity for this serum measurement, a failure to understand these limitations may result in over aggressive or unnecessary medical treatments.
Central Venous Pressure
Background
Central venous pressure (CVP) measurements can be obtained through a catheter, the distal tip of which transduces pressure of the superior vena cava at the entrance of the right atrium (RA). Thus, CVP is often used as a representation of RA pressure (RAP) and therefore an estimate of right ventricular (RV) preload. While CVP is used to diagnose and determine the etiology of shock, evidence and controversy regarding the use of CVP as a marker for resuscitation comes largely from sepsis-focused literature.5 Central venous pressure is meant to represent preload, which is essential for stroke volume as described by the Frank-Starling mechanism; however, its use as a target in distributive shock, a state in which it is difficult to determine a patient’s volume status, has been popularized by EGDT since 2001.2
Since the publication of the 2004 Surviving Sepsis Guidelines, CVP monitoring has been in the spotlight of sepsis resuscitation, albeit with some controversy.27 Included as the result of two studies, this recommendation has been removed in the most recent guidelines after 12 years of further study and scrutiny.2,27,28
Hypovolemic and hemorrhagic shock are usually diagnosed clinically and while a low CVP can be helpful in the diagnosis, the guidelines do not support CVP as a resuscitation endpoint. Obstructive and cardiogenic shock will both result in elevated CVP; however, treatment of obstructive shock is generally targeted at the underlying cause. While cardiogenic shock can be preload responsive, the mainstay of therapy in the ED is identification of patients for revascularization and inotropic support.29
Benefits
The CVP has been used as a surrogate for RV preload volume. If a patient’s preload volume is low, the treating physician can administer fluids to improve stroke volume and cardiac output (CO). Clinically, CVP measurements are easy to obtain provided a central venous line has been placed with the distal tip at the entrance to the RA. Central venous pressure is measured by transducing the pressure via manometry and connecting it to the patient’s bedside monitor. This provides an advantage of being able to provide serial or even continuous measurements. The “normal” RAP should be a low value (1-5 mm Hg, mean of 3 mm Hg), as this aids in the pressure gradient to drive blood from the higher pressures of the left ventricle (LV) and aorta through the circulation back to the low-pressure of the RA.30 The value of the CVP is meant to correspond to the physical examination findings of jugular venous distension.31,32 Thus, a low CVP may be “normal” and seen in patients with hypovolemic shock, whereas an elevated CVP can suggest volume overload or obstructive shock. However, this is of questionable value in distributive shock cases.
Aside from the two early studies on CVP monitoring during treatment of septic patients, there are few data to support the use of CVP measurement in the early resuscitation of patients with shock.2,28 More recent trials (PROMISE, ARISE, PROCESS) that compared protocolized sepsis care to standard care showed no benefit to bundles including CVP measurements.12-14 However, a subsequent, large observational trial spanning 7.5 years demonstrated improvements in sepsis-related mortality in patients who received a central venous catheter (CVC) and CVP-targeted therapy.33 Thus, it is possible that protocols including CVP are still beneficial in combination with other therapies even though CVP in isolation is not.
Limitations
The traditional two assumptions in CVP monitoring are CVP value represents the overall volume status of the patient, and the LV is able to utilize additional preload volume. The latter assumption, however, may be hampered by the presence of sepsis-induced myocardial dysfunction, which may be present in up to 40% of critically ill patients.34 The former assumption does not always hold true due to processes that change filling pressures independent of intravascular volume—eg, acute or chronic pulmonary hypertension, cardiac tamponade, intra-abdominal hypertension, or LV failure. Even before the landmark EGDT study, available data suggested that CVP was not a reliable marker for resuscitation management.35 A recent systematic review by Gottlieb and Hunter36 showed that the area under the receiver-operator curve for low, mid-range, or high CVPs was equivocal at best. In addition to its unreliability and lack of specificity, another significant drawback to using CVP to guide resuscitation therapy in the ED is that it necessitates placement of a CVC, which can be time-consuming and, if not otherwise indicated, lead to complications of infection, pneumothorax, and/or thrombosis.37
Mixed Venous Oxygen
Background
Most EPs are familiar with the use of ScvO2 in EGDT protocols to guide volume resuscitation of septic patients.2 A patient’s ScvO2 represents the O2 saturation of venous blood obtained via a CVC at the confluence of the superior vena cava and the RA, and thus it reflects tissue O2 consumption as a surrogate for tissue perfusion. The measurement parallels the SvO2 obtained from the pulmonary artery. In a healthy patient, SvO2 is around 65% to 70% and includes blood returning from both the superior and inferior vena cava (IVC). As such, ScvO2 values are typically 3% to 5% lower than SvO2 owing to the lower O2 extracted by tissues draining into the IVC compared to the mixed venous blood sampled from the pulmonary artery.38
Though a debate over the benefit of EGDT in treating sepsis continues, understanding the physiology of ScvO2 measurements is another potential tool the EP can use to guide the resuscitation of critically ill patients.39 A patient’s SvO2 and, by extension, ScvO2 represents the residual O2 saturation after the tissues have extracted the amount of O2 necessary to meet metabolic demands (Figure 2).
Conversely, cellular dysfunction, which can occur in certain toxicities or in severe forms of sepsis, can lead to decreased tissue O2 consumption with a concomitant rise in ScvO2 to supernormal values.38 The EP should take care, however, to consider whether ScvO2 values exceeding 80% represent successful therapeutic intervention or impaired tissue O2 extraction and utilization. There are data from ED patients suggesting an increased risk of mortality with both extremely low and extremely high values of ScvO2.40
Benefits
A critically ill patient’s ScvO2 can potentially provide EPs with insight into the patient’s global tissue perfusion and the source of any mismatch between O2 delivery and consumption. Using additional tools and measurements (physical examination, serum Hgb levels, and pulse oximetry) in conjunction with an ScvO2 measurement, assists EPs in identifying targets for therapeutic intervention. The effectiveness of this intervention can then be assessed using serial ScvO2 measurements, as described in Rivers et al2 EGDT protocol. Importantly, EPs should take care to measure serial ScvO2 values to maximize its utility.38 Similar to a CVP measurement, ScvO2is easily obtained from blood samples for serial laboratory measurements, assuming the patient already has a CVC with the distal tip at the entrance to the RA (ScvO2) or a pulmonary artery catheter (PAC) (SvO2).
Limitations
Serial measurements provide the most reliable information, which may be more useful in patients who spend extended periods of their resuscitation in the ED. In comparison to other measures of global tissue hypoxia, work by Jones et al41 suggests non-inferiority of peripherally sampled, serial lactate measurements as an alternative to ScvO2. This, in conjunction with the requirement for an internal jugular CVC, subclavian CVC, or PAC with their associated risks, may make ScvO2 a less attractive guide for the resuscitation of critically ill patients in the ED.
Monitoring Devices
Background
As noted throughout this review, it is important not only to identify and rapidly treat shock, but to also correctly identify the type of shock, such that treatment can be appropriately directed at its underlying cause. However, prior work suggests that EPs are unable to grossly estimate CO or systemic vascular resistance when compared to objective measurements of these parameters.42 This is in agreement with the overall poor performance of physical examination and clinical evaluation as a means of predicting volume responsiveness or guiding resuscitation, as discussed previously. Fortunately, a wide variety of devices to objectively monitor hemodynamics are now available to the EP.
In 1970, Swan et al43 published their initial experience with pulmonary artery catheterization at the bedside, using a balloon-tipped, flow-guided PAC in lieu of fluoroscopy, which had been mandated by earlier techniques. The ability to measure CO, right heart pressures, pulmonary arterial pressures, and estimate LV end diastolic pressure ushered in an era of widespread PAC use, despite an absence of evidence for causation of improved patient outcomes. The utilization of PACs has fallen, as the literature suggests that the empiric placement of PACs in critically ill patients does not improve mortality, length of stay, or cost, and significant complication rates have been reported in large trials.44,45Subsequently, a number of non-invasive or less-invasive HDM devices have been developed. Amongst the more commonly encountered modern devices, the techniques utilized for providing hemodynamic assessments include thermodilution and pulse contour analysis (PiCCOTM), pulse contour analysis (FloTrac/VigileoTM), and lithium chemodilution with pulse power analysis (LiDCOplusTM).46 The primary utility of these devices for the EP lies in the ability to quantify CO, stroke volume, and stroke volume or pulse pressure variation (PPV) to predict or assess response to resuscitative interventions (volume administration, vasopressors, inotropes, etc).
Benefits
Many of these devices require placement of an arterial catheter. Some require the addition of a CVC. Both of these procedures are well within the clinical scope of the EP, and are performed with fair frequency on critically ill patients. This is a distinct advantage when compared to pulmonary artery catheterization, a higher risk procedure that is rarely performed outside of the intensive care unit or cardiac catheterization laboratory. In addition, all of the devices below present hemodynamic data in a graphical, easy-to-read format, in real time. All of the devices discussed report stroke volume variation (SVV) or PPV continuously.
Limitations
Though these measures have validated threshold values that predict volume responsiveness, they require the patient to be intubated with a set tidal volume of greater than or equal to 8 mL/kg without spontaneous respirations and cardiac arrhythmias, in order to accurately do so. All of the HDM devices that rely on pulse contour analysis as the primary means of CO measurement cannot be used in the presence of significant cardiac arrhythmias (ie, atrial fibrillation), or mechanical circulatory assistance devices (ie, intra-aortic balloon counterpulsation). None of these devices are capable of monitoring microcirculatory changes, felt to be of increasing clinical importance in the critically ill.
The use of HDM devices to monitor CO with a reasonable degree of accuracy, trend CO, and assess for volume responsiveness using a number of previously validated parameters such as SVV is now in little doubt. However, these devices are still invasive, if less so than a pulmonary artery. The crux of the discussion of HDM devices for use in ED resuscitation revolves around whether or not the use of such devices to drive previously validated, protocolized care results in better outcomes for patients. The EP can now have continuous knowledge of a large number of hemodynamic parameters at their fingertips with relatively minimal additional efforts. At the time of this writing, though, this is both untested and unproven, with respect to the ED population.
Point-of-Care Ultrasound
Background
Over the past two decades, ultrasound (US) has become an integral part of the practice of emergency medicine (EM), and is now included in all United States Accreditation Council for Graduate Medical Education Emergency Medicine Residency Programs.47,48 It has emerged as a very important bedside tool performed by the clinician to identify type of shock and guide resuscitation, and has been endorsed by both EM and critical care societies.49-51 This section reviews the utility of US as a modality in identifying shock and guiding resuscitation, in addition to the pitfalls and limitations of this important tool.
In 2010, Perera et al47 described in their landmark article the Rapid Ultrasound in SHock (RUSH) examination, which describes a stepwise (the pump, tank, pipes) approach to identify the type of shock (cardiogenic, hypovolemic, obstructive, or distributive) in the crashing, hypotensive ED patient. We do not describe the full RUSH examination in this review, but discuss key elements of it as examples of how POCUS can assist the EP to make a rapid diagnosis and aid in the management of patients in shock. The “pump” is the heart, which is assessed in four different views to identify a pericardial effusion and possible tamponade, assess contractility or ejection fraction of the LV (severely decreased, decreased, normal, or hyperdynamic), and right heart strain which is identified by an RV that is larger than the LV, indicative of a potential pulmonary embolus.
The “tank” is then assessed by visualizing the IVC in the subxiphoid plane, and is evaluated for respiratory collapsibility (CI) and maximum size. This has been quite the debated topic over the last two decades. In 1988, Simonson and Schiller52 were the first to describe a correlation in spontaneously breathing patients between IVC caliber (measured 2 cm from the cavoatrial junction) and variation and RAP, where a larger IVC diameter and less respiratory variation correlated with a high RAP. Kircher et al53 later went on to describe that a CI greater than 50% correlated with an RAP of less than 10 mm Hg and vice versa in spontaneously breathing patients. Since then there have been more studies attempting to verify these findings in both spontaneously breathing and mechanically ventilated patients.54-56 The purpose of performing these measurements is not to estimate CVP, but to assess fluid responsiveness (ie, a blood pressure response to a fluid challenge). It can be assumed in states of shock that a small IVC, or one with a high CI, in the presence of a hyperdynamic heart is indicative of an underfilled ventricle and fluid responsiveness, especially if the IVC size increases with fluid.55,57 However, there are several caveats to this. First, in mechanically ventilated patients, the IVC is already plethoric due to positive pressure ventilation, and increases in diameter with inspiration and decreases with expiration as compared to spontaneously breathing patients. Second, the CI value to predict volume responsiveness in ventilated patients is set at 15% instead of 50%.55 Third, it is important to always take the clinical scenario in context; a dilated IVC with small CI is not necessarily only due to volume overload and congestive heart failure, but can be due to elevated RAP from obstructive shock due to cardiac tamponade or massive pulmonary embolus, which is why it is important to assess the “pump” first.47,58 It is also crucial to not forget to assess the abdominal and thoracic cavities, as intraperitoneal or pleural fluid with a collapsed IVC can potentially make a diagnosis of hemorrhagic or hypovolemic shock depending on the clinical scenario.47 The final part of the RUSH protocol is to evaluate the “pipes,” inclusive of the lower extremity deep venous system for evaluation of potential thrombosis that could increase suspicion for a pulmonary embolism causing obstructive shock, and the aorta with the common iliac arteries if there is concern for aortic dissection or aneurysmal rupture.
Benefits
Some of the most significant advantages to the use of POCUS to guide resuscitation is that it is quick, non-invasive, does not use ionizing radiation, and can be easily repeated. As noted above, it is a requirement for EM residencies to teach its use, so that contemporary graduates are entering the specialty competent in applying it to the care of their patients. Furthermore, POCUS is done at the bedside, limiting the need to potentially transport unstable patients.
In the most basic applications, POCUS provides direct visualization of a patient’s cardiac function, presence or absence of lung sliding to suggest a pneumothorax, presence of pulmonary edema, assessment of CVP pressures or potential for fluid responsiveness, as well as identification of potential thoracic, peritoneal, or pelvic cavity fluid accumulation that may suggest hemorrhage. There is literature to support that these assessments performed by the EP have been shown to be comparable to those of cardiologists.59,60 With continued practice and additional training, it is possible for EPs to even perform more “advanced” hemodynamic assessments to both diagnose and guide therapy to patients in shock (Figures 3 and 4).61
Limitations
Although POCUS has been shown as a remarkable tool to help assist the EP in making rapid decisions regarding resuscitation, it is always important to remember its limitations. Most of the studies regarding its use are of very small sample sizes, and further prospective studies have to be performed in order for this modality to be fully relied on.62Compared to some of the previously mentioned HDM devices that may provide continuous data, POCUS needs to be performed by the treating physician, thereby occurring intermittently. Emergency physicians need to be aware of their own experience and limitations with this modality, as errors in misdiagnosis can lead to unnecessary procedures, with resulting significant morbidity and mortality. Blanco and Volpicelli63 describe several common errors that include misdiagnosing the stomach as a peritoneal effusion, assuming adequate volume resuscitation when the IVC is seen to be plethoric in the setting of cardiac tamponade, or mistaking IVC movement as indicative of collapsibility, amongst other described misinterpretations. Several other studies have shown that, despite adequate performance of EPs in POCUS, diagnostic sensitivities remained higher when performed by radiologists.64-67 Thus it remains important for the EPs to be vigilant and not anchor on a diagnosis when in doubt, and to consult early with radiology, particularly if there is any question, to avoid potential adverse patient outcomes.
Summary
There are several ways to diagnose and track resuscitation in the ED, which include physical examination, assessment of serum laboratory values, monitoring of hemodynamic status, and use of POCUS. Unfortunately, none of these methods provides a perfect assessment, and no method has been proven superior and effective over the others. Therefore, it is important for EPs treating patients in shock to be aware of the strengths and limitations of each assessment method (Table).
Resuscitation of critically ill patients in shock from cardiogenic, hypovolemic, obstructive, distributive, or neurogenic etiology is a cornerstone of the care delivered by emergency physicians (EPs).1 Regardless of the etiology, it is essential that the treating EP initiate resuscitative measures in a timely manner and closely trend the patient’s response to these interventions.
The early goal-directed therapy (EGDT) initially proposed by Rivers et al2 in 2001 demonstrated a bundled approach to fluid resuscitation by targeting end points for volume resuscitation, mean arterial blood pressure (MAP), oxygen (O2) delivery/extraction (mixed venous O2 saturation, [SvO2]), hemoglobin (Hgb) concentration, and cardiac contractility. Since then, advancements in laboratory testing and hemodynamic monitoring (HDM) devices further aid and guide resuscitative efforts, and are applicable to any etiology of shock.
In addition to these advancements, the growing evidence of the potential harm from improper fluid resuscitation, such as the administration of excessive intravascular fluid (IVF),3 underscores the importance of a precise, targeted, and individualized approach to care. This article reviews the background, benefits, and limitations of some of the common and readily available tools in the ED that the EP can employ to guide fluid resuscitation in critically ill patients.
Physical Examination
Background
The rapid recognition and treatment of septic shock in the ED is associated with lower rates of in-hospital morbidity and mortality.4 The physical examination by the EP begins immediately upon examining the patient. The acquisition of vital signs and recognition of physical examination findings suggestive of intravascular volume depletion allows the EP to initiate treatment immediately.
In this discussion, hypotension is defined as systolic blood pressure (SBP) of less than 95 mm Hg, MAP of less than 65 mm Hg, or a decrease in SBP of more than 40 mm Hg from baseline measurements. Subsequently, shock is defined as hypotension with evidence of tissue hypoperfusion-induced dysfunction.5,6 Although the use of findings from the physical examination to guide resuscitation allows for rapid patient assessment and treatment, the predictive value of the physical examination to assess hemodynamic status is limited.
Visual inspection of the patient’s skin and mucous membranes can serve as an indicator of volume status. The patient’s tongue should appear moist with engorged sublingual veins; a dry tongue and diminished veins may suggest the need for volume resuscitation. On examination of the skin, delayed capillary refill of the digits and cool, clammy extremities suggest the shunting of blood by systemic circulation from the skin to central circulation. Patients who progress to more severe peripheral vasoconstriction develop skin mottling, referred to as livedo reticularis (Figure 1).
Benefits
The major benefit of the physical examination as a tool to evaluate hemodynamic status is its ease and rapid acquisition. The patient’s vital signs and physical examination can be obtained in the matter of moments upon presentation, without the need to wait on results of laboratory evaluation or additional equipment. Additionally, serial examinations by the same physician can be helpful to monitor a patient’s response to resuscitative efforts. The negative predictive value (NPV) of the physical examination in evaluating for hypovolemia may be helpful, but only when it is taken in the appropriate clinical context and is used in conjunction with other diagnostic tools. The physical examination can exclude hypovolemic volume status with an NPV of approximately 70%.7
A constellation of findings from the physical examination may include altered mentation, hypotension, tachycardia, and decreased urinary output by 30% to 40% intravascular volume loss.8,9Findings from the physical examination to assess fluid status should be used with caution as interobserver reliability has proven to be poor and the prognostic value is limited.
Limitations
The literature shows the limited prognostic value of the physical examination in determining a patient’s volume status and whether fluid resuscitation is indicated. For example, in one meta-analysis,10 supine hypotension and tachycardia were frequently absent on examination—even in patients who underwent large volume phlebotomy.8 This study also showed postural dizziness to be of no prognostic value.
Another study by Saugel et al7 that compared the physical examination (skin assessment, lung auscultation, and percussion) to transpulmonary thermodilution measurements of the cardiac index, global end-diastolic volume index, and extravascular lung water index, found poor interobserver correlation and agreement among physicians.
The physical examination is also associated with weak predictive capabilities for the estimation of volume status compared to the device measurements. Another contemporary study by Saugel et al9 evaluated the predictive value of the physical examination to accurately identify volume responsiveness replicated these results, and reported poor interobserver correlation (κ coefficient 0.01; 95% caval index [CI] -0.39-0.42) among physical examination findings, with a sensitivity of only 71%, specificity of 23.5%, positive predictive value of 27.8%, and negative predictive value of 66.7%.9
Serum Lactate Levels
Background
In the 1843 book titled, Investigations of Pathological Substances Obtained During the Epidemic of Puerperal Fever, Johann Joseph Scherer described the cases of seven young peripartum female patients who died from a clinical picture of what is now understood to be septic shock.11 In his study of these cases, Scherer demonstrated the presence of lactic acid in patients with pathological conditions. Prior to this discovery, lactic acid had never been isolated in a healthy individual. These results were recreated in 1851 by Scherer and Virchow,11 who demonstrated the presence of lactic acid in the blood of a patient who died from leukemia. The inference based on Scherer and Virchow’s work correlated the presence of excessive lactic acid with bodily deterioration and severe disease. Since this finding, there has been a great deal of interest in measuring serum lactic acid as a means to identify and manage critical illness.
In a 2001 groundbreaking study of EGDT for severe sepsis and septic shock, Rivers et al2 studied lactic acid levels as a marker for severe disease. Likewise, years later, the 2014 Protocol-Based Care for Early Septic Shock (PROCESS), Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE), and Australasian Resuscitation in Sepsis Evaluation (ARISE) trials used lactate levels in a similar manner to identify patients appropriate for randomization.12-14 While the purpose of measuring lactic acid was only employed in these studies to identify patients at risk for critical illness, the 2012 Surviving Sepsis Campaign Guidelines recommended serial measurement of lactate, based on the assumption that improved lactate levels signified better tissue perfusion.15
Although much of the studies on lactate levels appear to be based on the treatment and management of septic patients, findings can be applied to any etiology of shock. For example, a serum lactate level greater than 2 mmol/L is considered abnormal, and a serum lactate greater than 4 mmol/L indicates a significantly increased risk for in-hospital mortality.16
Benefits
It is now a widely accepted belief that the rapid identification, triage, and treatment of critically ill patients has a dramatic effect on morbidity and mortality.4 As previously noted, lactate has been extensively studied and identified as a marker of severe illness.17,18 A serum lactate level, which can be rapidly processed in the ED, can be easily obtained from a minimally invasive venous, arterial, or capillary blood draw.18 The only risk associated with serum lactate testing is that of any routine venipuncture; the test causes minimal, if any, patient discomfort.
Thanks to advances in point-of-care (POC) technology, the result of serum lactate assessment can be available within 10 minutes from blood draw. This technology is inexpensive and can be easily deployed in the prehospital setting or during the initial triage assessment of patients arriving at the ED.19 These POC instruments have been well correlated with whole blood measurements and permit for the rapid identification and treatment of at risk patients.
Limitations
The presence of elevated serum lactate levels is believed to represent the presence of cellular anaerobic metabolism due to impaired O2 delivery in the shock state. Abnormal measurements therefore prompt aggressive interventions aimed at maximizing O2 delivery to the tissues, such as intravenous fluid boluses, vasopressor therapy, or even blood product administration.
A return to a normalized serum lactate level is assumed to represent a transition back to aerobic metabolism. Lactate elevations, however, are not solely an indication of anaerobic metabolism and may only represent a small degree of lactate production.20 While the specific cellular mechanics are out of the scope of this article, it has been postulated that the increase in plasma lactate concentration is primarily driven by β-2 receptor stimulation from increased circulating catecholamines leading to increased aerobic glycolysis. Increased lactate levels could therefore be an adaptive mechanism of energy production—aggressive treatment and rapid clearance may, in fact, be harmful. Type A lactic acidosis is categorized as elevated serum levels due to tissue hypoperfusion.21
However, lactate elevations do not exclusively occur in severe illness. The use of β-2 receptor agonists such as continuous albuterol treatments or epinephrine may cause abnormal lactate levels.22 Other medications have also been associated with elevated serum lactate levels, including, but not limited to linezolid, metformin, and propofol.23-25 Additionally, lactate levels may be elevated after strenuous exercise, seizure activity, or in liver and kidney disease.26 These “secondary” causes of lactic acidosis that are not due to tissue hypoperfusion are referred to as type B lactic acidosis. Given these multiple etiologies and lack of specificity for this serum measurement, a failure to understand these limitations may result in over aggressive or unnecessary medical treatments.
Central Venous Pressure
Background
Central venous pressure (CVP) measurements can be obtained through a catheter, the distal tip of which transduces pressure of the superior vena cava at the entrance of the right atrium (RA). Thus, CVP is often used as a representation of RA pressure (RAP) and therefore an estimate of right ventricular (RV) preload. While CVP is used to diagnose and determine the etiology of shock, evidence and controversy regarding the use of CVP as a marker for resuscitation comes largely from sepsis-focused literature.5 Central venous pressure is meant to represent preload, which is essential for stroke volume as described by the Frank-Starling mechanism; however, its use as a target in distributive shock, a state in which it is difficult to determine a patient’s volume status, has been popularized by EGDT since 2001.2
Since the publication of the 2004 Surviving Sepsis Guidelines, CVP monitoring has been in the spotlight of sepsis resuscitation, albeit with some controversy.27 Included as the result of two studies, this recommendation has been removed in the most recent guidelines after 12 years of further study and scrutiny.2,27,28
Hypovolemic and hemorrhagic shock are usually diagnosed clinically and while a low CVP can be helpful in the diagnosis, the guidelines do not support CVP as a resuscitation endpoint. Obstructive and cardiogenic shock will both result in elevated CVP; however, treatment of obstructive shock is generally targeted at the underlying cause. While cardiogenic shock can be preload responsive, the mainstay of therapy in the ED is identification of patients for revascularization and inotropic support.29
Benefits
The CVP has been used as a surrogate for RV preload volume. If a patient’s preload volume is low, the treating physician can administer fluids to improve stroke volume and cardiac output (CO). Clinically, CVP measurements are easy to obtain provided a central venous line has been placed with the distal tip at the entrance to the RA. Central venous pressure is measured by transducing the pressure via manometry and connecting it to the patient’s bedside monitor. This provides an advantage of being able to provide serial or even continuous measurements. The “normal” RAP should be a low value (1-5 mm Hg, mean of 3 mm Hg), as this aids in the pressure gradient to drive blood from the higher pressures of the left ventricle (LV) and aorta through the circulation back to the low-pressure of the RA.30 The value of the CVP is meant to correspond to the physical examination findings of jugular venous distension.31,32 Thus, a low CVP may be “normal” and seen in patients with hypovolemic shock, whereas an elevated CVP can suggest volume overload or obstructive shock. However, this is of questionable value in distributive shock cases.
Aside from the two early studies on CVP monitoring during treatment of septic patients, there are few data to support the use of CVP measurement in the early resuscitation of patients with shock.2,28 More recent trials (PROMISE, ARISE, PROCESS) that compared protocolized sepsis care to standard care showed no benefit to bundles including CVP measurements.12-14 However, a subsequent, large observational trial spanning 7.5 years demonstrated improvements in sepsis-related mortality in patients who received a central venous catheter (CVC) and CVP-targeted therapy.33 Thus, it is possible that protocols including CVP are still beneficial in combination with other therapies even though CVP in isolation is not.
Limitations
The traditional two assumptions in CVP monitoring are CVP value represents the overall volume status of the patient, and the LV is able to utilize additional preload volume. The latter assumption, however, may be hampered by the presence of sepsis-induced myocardial dysfunction, which may be present in up to 40% of critically ill patients.34 The former assumption does not always hold true due to processes that change filling pressures independent of intravascular volume—eg, acute or chronic pulmonary hypertension, cardiac tamponade, intra-abdominal hypertension, or LV failure. Even before the landmark EGDT study, available data suggested that CVP was not a reliable marker for resuscitation management.35 A recent systematic review by Gottlieb and Hunter36 showed that the area under the receiver-operator curve for low, mid-range, or high CVPs was equivocal at best. In addition to its unreliability and lack of specificity, another significant drawback to using CVP to guide resuscitation therapy in the ED is that it necessitates placement of a CVC, which can be time-consuming and, if not otherwise indicated, lead to complications of infection, pneumothorax, and/or thrombosis.37
Mixed Venous Oxygen
Background
Most EPs are familiar with the use of ScvO2 in EGDT protocols to guide volume resuscitation of septic patients.2 A patient’s ScvO2 represents the O2 saturation of venous blood obtained via a CVC at the confluence of the superior vena cava and the RA, and thus it reflects tissue O2 consumption as a surrogate for tissue perfusion. The measurement parallels the SvO2 obtained from the pulmonary artery. In a healthy patient, SvO2 is around 65% to 70% and includes blood returning from both the superior and inferior vena cava (IVC). As such, ScvO2 values are typically 3% to 5% lower than SvO2 owing to the lower O2 extracted by tissues draining into the IVC compared to the mixed venous blood sampled from the pulmonary artery.38
Though a debate over the benefit of EGDT in treating sepsis continues, understanding the physiology of ScvO2 measurements is another potential tool the EP can use to guide the resuscitation of critically ill patients.39 A patient’s SvO2 and, by extension, ScvO2 represents the residual O2 saturation after the tissues have extracted the amount of O2 necessary to meet metabolic demands (Figure 2).
Conversely, cellular dysfunction, which can occur in certain toxicities or in severe forms of sepsis, can lead to decreased tissue O2 consumption with a concomitant rise in ScvO2 to supernormal values.38 The EP should take care, however, to consider whether ScvO2 values exceeding 80% represent successful therapeutic intervention or impaired tissue O2 extraction and utilization. There are data from ED patients suggesting an increased risk of mortality with both extremely low and extremely high values of ScvO2.40
Benefits
A critically ill patient’s ScvO2 can potentially provide EPs with insight into the patient’s global tissue perfusion and the source of any mismatch between O2 delivery and consumption. Using additional tools and measurements (physical examination, serum Hgb levels, and pulse oximetry) in conjunction with an ScvO2 measurement, assists EPs in identifying targets for therapeutic intervention. The effectiveness of this intervention can then be assessed using serial ScvO2 measurements, as described in Rivers et al2 EGDT protocol. Importantly, EPs should take care to measure serial ScvO2 values to maximize its utility.38 Similar to a CVP measurement, ScvO2is easily obtained from blood samples for serial laboratory measurements, assuming the patient already has a CVC with the distal tip at the entrance to the RA (ScvO2) or a pulmonary artery catheter (PAC) (SvO2).
Limitations
Serial measurements provide the most reliable information, which may be more useful in patients who spend extended periods of their resuscitation in the ED. In comparison to other measures of global tissue hypoxia, work by Jones et al41 suggests non-inferiority of peripherally sampled, serial lactate measurements as an alternative to ScvO2. This, in conjunction with the requirement for an internal jugular CVC, subclavian CVC, or PAC with their associated risks, may make ScvO2 a less attractive guide for the resuscitation of critically ill patients in the ED.
Monitoring Devices
Background
As noted throughout this review, it is important not only to identify and rapidly treat shock, but to also correctly identify the type of shock, such that treatment can be appropriately directed at its underlying cause. However, prior work suggests that EPs are unable to grossly estimate CO or systemic vascular resistance when compared to objective measurements of these parameters.42 This is in agreement with the overall poor performance of physical examination and clinical evaluation as a means of predicting volume responsiveness or guiding resuscitation, as discussed previously. Fortunately, a wide variety of devices to objectively monitor hemodynamics are now available to the EP.
In 1970, Swan et al43 published their initial experience with pulmonary artery catheterization at the bedside, using a balloon-tipped, flow-guided PAC in lieu of fluoroscopy, which had been mandated by earlier techniques. The ability to measure CO, right heart pressures, pulmonary arterial pressures, and estimate LV end diastolic pressure ushered in an era of widespread PAC use, despite an absence of evidence for causation of improved patient outcomes. The utilization of PACs has fallen, as the literature suggests that the empiric placement of PACs in critically ill patients does not improve mortality, length of stay, or cost, and significant complication rates have been reported in large trials.44,45Subsequently, a number of non-invasive or less-invasive HDM devices have been developed. Amongst the more commonly encountered modern devices, the techniques utilized for providing hemodynamic assessments include thermodilution and pulse contour analysis (PiCCOTM), pulse contour analysis (FloTrac/VigileoTM), and lithium chemodilution with pulse power analysis (LiDCOplusTM).46 The primary utility of these devices for the EP lies in the ability to quantify CO, stroke volume, and stroke volume or pulse pressure variation (PPV) to predict or assess response to resuscitative interventions (volume administration, vasopressors, inotropes, etc).
Benefits
Many of these devices require placement of an arterial catheter. Some require the addition of a CVC. Both of these procedures are well within the clinical scope of the EP, and are performed with fair frequency on critically ill patients. This is a distinct advantage when compared to pulmonary artery catheterization, a higher risk procedure that is rarely performed outside of the intensive care unit or cardiac catheterization laboratory. In addition, all of the devices below present hemodynamic data in a graphical, easy-to-read format, in real time. All of the devices discussed report stroke volume variation (SVV) or PPV continuously.
Limitations
Though these measures have validated threshold values that predict volume responsiveness, they require the patient to be intubated with a set tidal volume of greater than or equal to 8 mL/kg without spontaneous respirations and cardiac arrhythmias, in order to accurately do so. All of the HDM devices that rely on pulse contour analysis as the primary means of CO measurement cannot be used in the presence of significant cardiac arrhythmias (ie, atrial fibrillation), or mechanical circulatory assistance devices (ie, intra-aortic balloon counterpulsation). None of these devices are capable of monitoring microcirculatory changes, felt to be of increasing clinical importance in the critically ill.
The use of HDM devices to monitor CO with a reasonable degree of accuracy, trend CO, and assess for volume responsiveness using a number of previously validated parameters such as SVV is now in little doubt. However, these devices are still invasive, if less so than a pulmonary artery. The crux of the discussion of HDM devices for use in ED resuscitation revolves around whether or not the use of such devices to drive previously validated, protocolized care results in better outcomes for patients. The EP can now have continuous knowledge of a large number of hemodynamic parameters at their fingertips with relatively minimal additional efforts. At the time of this writing, though, this is both untested and unproven, with respect to the ED population.
Point-of-Care Ultrasound
Background
Over the past two decades, ultrasound (US) has become an integral part of the practice of emergency medicine (EM), and is now included in all United States Accreditation Council for Graduate Medical Education Emergency Medicine Residency Programs.47,48 It has emerged as a very important bedside tool performed by the clinician to identify type of shock and guide resuscitation, and has been endorsed by both EM and critical care societies.49-51 This section reviews the utility of US as a modality in identifying shock and guiding resuscitation, in addition to the pitfalls and limitations of this important tool.
In 2010, Perera et al47 described in their landmark article the Rapid Ultrasound in SHock (RUSH) examination, which describes a stepwise (the pump, tank, pipes) approach to identify the type of shock (cardiogenic, hypovolemic, obstructive, or distributive) in the crashing, hypotensive ED patient. We do not describe the full RUSH examination in this review, but discuss key elements of it as examples of how POCUS can assist the EP to make a rapid diagnosis and aid in the management of patients in shock. The “pump” is the heart, which is assessed in four different views to identify a pericardial effusion and possible tamponade, assess contractility or ejection fraction of the LV (severely decreased, decreased, normal, or hyperdynamic), and right heart strain which is identified by an RV that is larger than the LV, indicative of a potential pulmonary embolus.
The “tank” is then assessed by visualizing the IVC in the subxiphoid plane, and is evaluated for respiratory collapsibility (CI) and maximum size. This has been quite the debated topic over the last two decades. In 1988, Simonson and Schiller52 were the first to describe a correlation in spontaneously breathing patients between IVC caliber (measured 2 cm from the cavoatrial junction) and variation and RAP, where a larger IVC diameter and less respiratory variation correlated with a high RAP. Kircher et al53 later went on to describe that a CI greater than 50% correlated with an RAP of less than 10 mm Hg and vice versa in spontaneously breathing patients. Since then there have been more studies attempting to verify these findings in both spontaneously breathing and mechanically ventilated patients.54-56 The purpose of performing these measurements is not to estimate CVP, but to assess fluid responsiveness (ie, a blood pressure response to a fluid challenge). It can be assumed in states of shock that a small IVC, or one with a high CI, in the presence of a hyperdynamic heart is indicative of an underfilled ventricle and fluid responsiveness, especially if the IVC size increases with fluid.55,57 However, there are several caveats to this. First, in mechanically ventilated patients, the IVC is already plethoric due to positive pressure ventilation, and increases in diameter with inspiration and decreases with expiration as compared to spontaneously breathing patients. Second, the CI value to predict volume responsiveness in ventilated patients is set at 15% instead of 50%.55 Third, it is important to always take the clinical scenario in context; a dilated IVC with small CI is not necessarily only due to volume overload and congestive heart failure, but can be due to elevated RAP from obstructive shock due to cardiac tamponade or massive pulmonary embolus, which is why it is important to assess the “pump” first.47,58 It is also crucial to not forget to assess the abdominal and thoracic cavities, as intraperitoneal or pleural fluid with a collapsed IVC can potentially make a diagnosis of hemorrhagic or hypovolemic shock depending on the clinical scenario.47 The final part of the RUSH protocol is to evaluate the “pipes,” inclusive of the lower extremity deep venous system for evaluation of potential thrombosis that could increase suspicion for a pulmonary embolism causing obstructive shock, and the aorta with the common iliac arteries if there is concern for aortic dissection or aneurysmal rupture.
Benefits
Some of the most significant advantages to the use of POCUS to guide resuscitation is that it is quick, non-invasive, does not use ionizing radiation, and can be easily repeated. As noted above, it is a requirement for EM residencies to teach its use, so that contemporary graduates are entering the specialty competent in applying it to the care of their patients. Furthermore, POCUS is done at the bedside, limiting the need to potentially transport unstable patients.
In the most basic applications, POCUS provides direct visualization of a patient’s cardiac function, presence or absence of lung sliding to suggest a pneumothorax, presence of pulmonary edema, assessment of CVP pressures or potential for fluid responsiveness, as well as identification of potential thoracic, peritoneal, or pelvic cavity fluid accumulation that may suggest hemorrhage. There is literature to support that these assessments performed by the EP have been shown to be comparable to those of cardiologists.59,60 With continued practice and additional training, it is possible for EPs to even perform more “advanced” hemodynamic assessments to both diagnose and guide therapy to patients in shock (Figures 3 and 4).61
Limitations
Although POCUS has been shown as a remarkable tool to help assist the EP in making rapid decisions regarding resuscitation, it is always important to remember its limitations. Most of the studies regarding its use are of very small sample sizes, and further prospective studies have to be performed in order for this modality to be fully relied on.62Compared to some of the previously mentioned HDM devices that may provide continuous data, POCUS needs to be performed by the treating physician, thereby occurring intermittently. Emergency physicians need to be aware of their own experience and limitations with this modality, as errors in misdiagnosis can lead to unnecessary procedures, with resulting significant morbidity and mortality. Blanco and Volpicelli63 describe several common errors that include misdiagnosing the stomach as a peritoneal effusion, assuming adequate volume resuscitation when the IVC is seen to be plethoric in the setting of cardiac tamponade, or mistaking IVC movement as indicative of collapsibility, amongst other described misinterpretations. Several other studies have shown that, despite adequate performance of EPs in POCUS, diagnostic sensitivities remained higher when performed by radiologists.64-67 Thus it remains important for the EPs to be vigilant and not anchor on a diagnosis when in doubt, and to consult early with radiology, particularly if there is any question, to avoid potential adverse patient outcomes.
Summary
There are several ways to diagnose and track resuscitation in the ED, which include physical examination, assessment of serum laboratory values, monitoring of hemodynamic status, and use of POCUS. Unfortunately, none of these methods provides a perfect assessment, and no method has been proven superior and effective over the others. Therefore, it is important for EPs treating patients in shock to be aware of the strengths and limitations of each assessment method (Table).
1. Richards JB, Wilcox SR. Diagnosis and management of shock in the emergency department. Emerg Med Pract. 2014;16(3):1-22; quiz 22-23.
2. Rivers E, Nguyen B, Havstad S, et al; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377.
3. Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259-265. doi:10.1097/CCM.0b013e3181feeb15.
4. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235-2244. doi:10.1056/NEJMoa1703058.
5. Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(12):1795-1815. doi:10.1007/s00134-014-3525-z.
6. Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726-1734. doi:10.1056/NEJMra1208943.
7. Saugel B, Ringmaier S, Holzapfel K, et al. Physical examination, central venous pressure, and chest radiography for the prediction of transpulmonary thermodilution-derived hemodynamic parameters in critically ill patients: a prospective trial. J Crit Care. 2011;26(4):402-410. doi:10.1016/j.jcrc.2010.11.001.
8. American College of Surgeons. Committee on Trauma. Shock. In: American College of Surgeons. Committee on Trauma, ed. Advanced Trauma Life Support: Student Course Manual. 9th ed. Chicago, IL: American College of Surgeons; 2012:69.
9. Saugel B, Kirsche SV, Hapfelmeier A, et al. Prediction of fluid responsiveness in patients admitted to the medical intensive care unit. J Crit Care. 2013:28(4):537.e1-e9. doi:10.1016/j.jcrc.2012.10.008.
10. McGee S, Abernethy WB 3rd, Simel DV. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281(11):1022-1029.
11. Kompanje EJ, Jansen TC, van der Hoven B, Bakker J. The first demonstration of lactic acid in human blood in shock by Johann Joseph Scherer (1814-1869) in January 1843. Intensive Care Med. 2007;33(11):1967-1971. doi:10.1007/s00134-007-0788-7.
12. The ProCESS Investigators. A Randomized Trial of Protocol-Based Care for Early Septic Shock. N Engl J Med. 2014; 370:1683-1693. doi:10.1056/NEJMoa1401602.
13. Mouncey PR, Osborn TM, Power GS, et al. Protocolised Management In Sepsis (ProMISe): a multicentre randomised controlled trial of the clinical effectiveness and cost-effectiveness of early, goal-directed, protocolised resuscitation for emerging septic shock. Health Technol Assess. 2015;19(97):i-xxv, 1-150. doi:10.3310/hta19970.
14. ARISE Investigators; ANZICS Clinical Trials Group; Peake SL, Delaney A, Bailey M, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496-1506. doi:10.1056/NEJMoa1404380.
15. Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Group. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228. doi:10.1007/s00134-012-2769-8.
16. Casserly B, Phillips GS, Schorr C, et al: Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med. 2015;43(3):567-573. doi:10.1097/CCM.0000000000000742.
17. Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care. 2013;3(1):12. doi:10.1186/2110-5820-3-12.
18. Kruse O, Grunnet N, Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scand J Trauma Resusc Emerg Med. 2011;19:74. doi:10.1186/1757-7241-19-74.
19. Gaieski DF, Drumheller BC, Goyal M, Fuchs BD, Shofer FS, Zogby K. Accuracy of handheld point-of-care fingertip lactate measurement in the emergency department. West J Emerg Med. 2013;14(1):58-62. doi:10.5811/westjem.2011.5.6706.
20. Marik PE, Bellomo R. Lactate clearance as a target of therapy in sepsis: a flawed paradigm. OA Critical Care. 2013;1(1):3.
21. Kreisberg RA. Lactate homeostasis and lactic acidosis. Ann Intern Med. 1980;92(2 Pt 1):227-237.
22. Dodda VR, Spiro P. Can albuterol be blamed for lactic acidosis? Respir Care. 2012; 57(12):2115-2118. doi:10.4187/respcare.01810.
23. Scale T, Harvey JN. Diabetes, metformin and lactic acidosis. Clin Endocrinol (Oxf). 2011;74(2):191-196. doi:10.1111/j.1365-2265.2010.03891.x.
24. Velez JC, Janech MG. A case of lactic acidosis induced by linezolid. Nat Rev Nephrol. 2010;6(4):236-242. doi:10.1038/nrneph.2010.20.
25. Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia. 2007;62(7):690-701.
26. Griffith FR Jr, Lockwood JE, Emery FE. Adrenalin lactacidemia: proportionality with dose. Am J Physiol. 1939;127(3):415-421.
27. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6.
28. Early Goal-Directed Therapy Collaborative Group of Zhejiang Province. The effect of early goal-directed therapy on treatment of critical patients with severe sepsis/ septic shock: a multi-center, prospective, randomised, controlled study. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2010;22(6):331-334.
29. Thiele H, Ohman EM, Desch S, Eitel I, de Waha S. Management of cardiogenic shock. Eur Heart J. 2015;36(20):1223-1230. doi:10.1093/eurheartj/ehv051.
30. Lee M, Curley GF, Mustard M, Mazer CD. The Swan-Ganz catheter remains a critically important component of monitoring in cardiovascular critical care. Can J Cardiol. 2017;33(1):142-147. doi:10.1016/j.cjca.2016.10.026.
31. Morgan BC, Abel FL, Mullins GL, Guntheroth WG. Flow patterns in cavae, pulmonary artery, pulmonary vein, and aorta in intact dogs. Am J Physiol. 1966;210(4):903-909. doi:10.1152/ajplegacy.1966.210.4.903.
32. Brecher GA, Hubay CA. Pulmonary blood flow and venous return during spontaneous respiration. Circ Res. 1955;3(2):210-214.

33. Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med. 2014;40(11):1623-1633. doi:10.1007/s00134-014-3496-0.
34. Fernandes CJ Jr, Akamine N, Knobel E. Cardiac troponin: a new serum marker of myocardial injury in sepsis. Intensive Care Med. 1999;25(10):1165-1168. doi:10.1007/s001340051030.
35. Rady MY, Rivers EP, Nowak RM. Resuscitation of the critically III in the ED: responses of blood pressure, heart rate, shock index, central venous oxygen saturation, and lactate. Am J Emerg Med. 1996;14(2):218-225. doi:10.1016/s0735-6757(96)90136-9.
36. Gottlieb M, Hunter B. Utility of central venous pressure as a predictor of fluid responsiveness. Ann Emerg Med. 2016;68(1):114-116. doi:10.1016/j.annemergmed.2016.02.009.
37. Kornbau C, Lee KC, Hughes GD, Firstenberg MS. Central line complications. Int J Critical Illn Inj Sci. 2015;5(3):170-178. doi:10.4103/2229-5151.164940.
38. Walley KR. Use of central venous oxygen saturation to guide therapy. Am J Respir Crit Care Med. 2011;184(5):514-520. doi:10.1164/rccm.201010-1584CI.
39. PRISM Investigators, Rowan KM, Angus DC, et al. Early, goal-directed therapy for septic shock - a patient-level meta-analysis. N Engl J Med. 2017;376(23):2223-2234. doi:10.1056/NEJMoa1701380.
40. Pope JV, Jones AE, Gaieski DF, Arnold RC, Trzeciak S, Shapiro NI; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Multicenter study of central venous oxygen saturation (ScvO(2)) as a predictor of mortality in patients with sepsis. Ann Emerg Med. 2010;55(1):40-46.e1. doi:10.1016/j.annemergmed.2009.08.014.
41. Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303(8):739-746. doi:10.1001/jama.2010.158.
42. Nowak RM, Sen A, Garcia, AJ, et al. The inability of emergency physicians to adequately clinically estimate the underlying hemodynamic profiles of acutely ill patients. Am J Emerg Med. 2012;30(6):954-960. doi:10.1016/j.ajem.2011.05.021.
43. Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med. 1970;283(9):447-451. doi:10.1056/NEJM197008272830902.
44. Hadian M, Pinsky MR. Evidence-based review of the use of the pulmonary artery catheter: impact data and complications. Crit Care. 2006;10 Suppl 3:S8.
45. Rajaram SS, Desai, NK, Kalra A, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev. 2013;(2):CD003408. doi:10.1002/14651858.CD003408.pub3.
46. Laher AE, Watermeyer MJ, Buchanan SK, et al. A review of hemodynamic monitoring techniques, methods and devices for the emergency physician. Am J Emerg Med. 2017;35(9):1335-1347. doi:10.1016/j.ajem.2017.03.036.
47. Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid Ultrasound in SHock in the evaluation of the critically lll. Emerg Med Clin North Am. 2010;28(1):29-56, vii. doi:10.1016/j.emc.2009.09.010.
48. Heller MB, Mandavia D, Tayal VS, et al. Residency training in emergency ultrasound: fulfilling the mandate. Acad Emerg Med. 2002;9(8):835-839.
49. Ultrasound guidelines: emergency, point-of-care and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2016;69(5):e27-e54. doi:10.1016/j.annemergmed.2016.08.457.
50. Expert Round Table on Ultrasound in ICU. International expert statement on training standards for critical care ultrasonography. Intensive Care Med. 2011;37(7):1077-1083. doi:10.1007/s00134-011-2246-9.
51. Neri L, Storti E, Lichtenstein D. Toward an ultrasound curriculum for critical care medicine. Crit Care Med. 2007;35(5 Suppl):S290-S304.
52. Simonson JS, Schiller NB. Sonospirometry: a new method for noninvasive estimation of mean right atrial pressure based on two-dimensional echographic measurements of the inferior vena cava during measured inspiration. J Am Coll Cardiol. 1988;11(3):557-564.
53. Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66(4):493-496.
54. Nagdev AD, Merchant RC, Tirado-Gonzalez A, Sisson CA, Murphy MC. Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55(3):290-295. doi:10.1016/j.annemergmed.2009.04.021.
55. Barbier C, Loubières Y, Schmit C, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30(9):1740-1746.
56. Corl KA, George NR, Romanoff J, et al. Inferior vena cava collapsibility detects fluid responsiveness among spontaneously breathing critically-ill patients. J Crit Care. 2017;41:130-137. doi:10.1016/j.jcrc.2017.05.008.
57. Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30(9):1834-1837.
58. Blehar DJ, Dickman E, Gaspari R. Identification of congestive heart failure via respiratory variation of inferior vena cava diameter. Am J Emerg Med. 2009;27(1):71-75. doi:10.1016/j.ajem.2008.01.002.
59. Moore CL, Rose GA, Tayal VS, Sullivan DM, Arrowood JA, Kline JA. Determination of left ventricular function by emergency physician echocardiography of hypotensive patients. Acad Emerg Med. 2002;9(3):186-193.
60. Mandavia DP, Hoffner RJ, Mahaney K, Henderson SO. Bedside echocardiography by emergency physicians. Ann Emerg Med. 2001;38(4):377-382.
61. Mosier JM, Martin J, Andrus P, et al. Advanced hemodynamic and cardiopulmonary ultrasound for critically ill patients in the emergency department. Emerg Med. 2018;50(1):17-34. doi:10.12788/emed.2018.0078.
62. Agarwal S, Swanson S, Murphy A, Yaeger K, Sharek P, Halamek LP. Comparing the utility of a standard pediatric resuscitation cart with a pediatric resuscitation cart based on the Broselow tape: a randomized, controlled, crossover trial involving simulated resuscitation scenarios. Pediatrics. 2005;116(3):e326-e333.
63. Blanco P, Volpicelli G. Common pitfalls in point-of-care ultrasound: a practical guide for emergency and critical care physicians. Crit Ultrasound J. 2016;8(1):15.
64. Tajoddini S, Shams Vahdati S. Ultrasonographic diagnosis of abdominal free fluid: accuracy comparison of emergency physicians and radiologists. Eur J Trauma Emerg Surg. 2013;39(1):9-13. doi:10.1007/s00068-012-0219-5.

65 Abbasi S, Bolverdi E, Zare MA, et al. Comparison of diagnostic value of conventional ultrasonography by emergency physicians with Doppler ultrasonography by radiology physicians for diagnosis of deep vein thrombosis. J Pak Med Assoc. 2012;62(5):461-465.
66. Arhami Dolatabadi A, Amini A, Hatamabadi H, et al. Comparison of the accuracy and reproducibility of focused abdominal sonography for trauma performed by emergency medicine and radiology residents. Ultrasound Med Biol. 2014;40(7):1476-1482. doi:10.1016/j.ultrasmedbio.2014.01.017.
67. Karimi E, Aminianfar M, Zarafshani K, Safaie A. The accuracy of emergency physicians in ultrasonographic screening of acute appendicitis; a cross sectional study. Emerg (Tehran). 2017;5(1):e22.
1. Richards JB, Wilcox SR. Diagnosis and management of shock in the emergency department. Emerg Med Pract. 2014;16(3):1-22; quiz 22-23.
2. Rivers E, Nguyen B, Havstad S, et al; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377.
3. Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259-265. doi:10.1097/CCM.0b013e3181feeb15.
4. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235-2244. doi:10.1056/NEJMoa1703058.
5. Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(12):1795-1815. doi:10.1007/s00134-014-3525-z.
6. Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726-1734. doi:10.1056/NEJMra1208943.
7. Saugel B, Ringmaier S, Holzapfel K, et al. Physical examination, central venous pressure, and chest radiography for the prediction of transpulmonary thermodilution-derived hemodynamic parameters in critically ill patients: a prospective trial. J Crit Care. 2011;26(4):402-410. doi:10.1016/j.jcrc.2010.11.001.
8. American College of Surgeons. Committee on Trauma. Shock. In: American College of Surgeons. Committee on Trauma, ed. Advanced Trauma Life Support: Student Course Manual. 9th ed. Chicago, IL: American College of Surgeons; 2012:69.
9. Saugel B, Kirsche SV, Hapfelmeier A, et al. Prediction of fluid responsiveness in patients admitted to the medical intensive care unit. J Crit Care. 2013:28(4):537.e1-e9. doi:10.1016/j.jcrc.2012.10.008.
10. McGee S, Abernethy WB 3rd, Simel DV. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281(11):1022-1029.
11. Kompanje EJ, Jansen TC, van der Hoven B, Bakker J. The first demonstration of lactic acid in human blood in shock by Johann Joseph Scherer (1814-1869) in January 1843. Intensive Care Med. 2007;33(11):1967-1971. doi:10.1007/s00134-007-0788-7.
12. The ProCESS Investigators. A Randomized Trial of Protocol-Based Care for Early Septic Shock. N Engl J Med. 2014; 370:1683-1693. doi:10.1056/NEJMoa1401602.
13. Mouncey PR, Osborn TM, Power GS, et al. Protocolised Management In Sepsis (ProMISe): a multicentre randomised controlled trial of the clinical effectiveness and cost-effectiveness of early, goal-directed, protocolised resuscitation for emerging septic shock. Health Technol Assess. 2015;19(97):i-xxv, 1-150. doi:10.3310/hta19970.
14. ARISE Investigators; ANZICS Clinical Trials Group; Peake SL, Delaney A, Bailey M, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496-1506. doi:10.1056/NEJMoa1404380.
15. Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Group. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228. doi:10.1007/s00134-012-2769-8.
16. Casserly B, Phillips GS, Schorr C, et al: Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med. 2015;43(3):567-573. doi:10.1097/CCM.0000000000000742.
17. Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care. 2013;3(1):12. doi:10.1186/2110-5820-3-12.
18. Kruse O, Grunnet N, Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scand J Trauma Resusc Emerg Med. 2011;19:74. doi:10.1186/1757-7241-19-74.
19. Gaieski DF, Drumheller BC, Goyal M, Fuchs BD, Shofer FS, Zogby K. Accuracy of handheld point-of-care fingertip lactate measurement in the emergency department. West J Emerg Med. 2013;14(1):58-62. doi:10.5811/westjem.2011.5.6706.
20. Marik PE, Bellomo R. Lactate clearance as a target of therapy in sepsis: a flawed paradigm. OA Critical Care. 2013;1(1):3.
21. Kreisberg RA. Lactate homeostasis and lactic acidosis. Ann Intern Med. 1980;92(2 Pt 1):227-237.
22. Dodda VR, Spiro P. Can albuterol be blamed for lactic acidosis? Respir Care. 2012; 57(12):2115-2118. doi:10.4187/respcare.01810.
23. Scale T, Harvey JN. Diabetes, metformin and lactic acidosis. Clin Endocrinol (Oxf). 2011;74(2):191-196. doi:10.1111/j.1365-2265.2010.03891.x.
24. Velez JC, Janech MG. A case of lactic acidosis induced by linezolid. Nat Rev Nephrol. 2010;6(4):236-242. doi:10.1038/nrneph.2010.20.
25. Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia. 2007;62(7):690-701.
26. Griffith FR Jr, Lockwood JE, Emery FE. Adrenalin lactacidemia: proportionality with dose. Am J Physiol. 1939;127(3):415-421.
27. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6.
28. Early Goal-Directed Therapy Collaborative Group of Zhejiang Province. The effect of early goal-directed therapy on treatment of critical patients with severe sepsis/ septic shock: a multi-center, prospective, randomised, controlled study. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2010;22(6):331-334.
29. Thiele H, Ohman EM, Desch S, Eitel I, de Waha S. Management of cardiogenic shock. Eur Heart J. 2015;36(20):1223-1230. doi:10.1093/eurheartj/ehv051.
30. Lee M, Curley GF, Mustard M, Mazer CD. The Swan-Ganz catheter remains a critically important component of monitoring in cardiovascular critical care. Can J Cardiol. 2017;33(1):142-147. doi:10.1016/j.cjca.2016.10.026.
31. Morgan BC, Abel FL, Mullins GL, Guntheroth WG. Flow patterns in cavae, pulmonary artery, pulmonary vein, and aorta in intact dogs. Am J Physiol. 1966;210(4):903-909. doi:10.1152/ajplegacy.1966.210.4.903.
32. Brecher GA, Hubay CA. Pulmonary blood flow and venous return during spontaneous respiration. Circ Res. 1955;3(2):210-214.

33. Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med. 2014;40(11):1623-1633. doi:10.1007/s00134-014-3496-0.
34. Fernandes CJ Jr, Akamine N, Knobel E. Cardiac troponin: a new serum marker of myocardial injury in sepsis. Intensive Care Med. 1999;25(10):1165-1168. doi:10.1007/s001340051030.
35. Rady MY, Rivers EP, Nowak RM. Resuscitation of the critically III in the ED: responses of blood pressure, heart rate, shock index, central venous oxygen saturation, and lactate. Am J Emerg Med. 1996;14(2):218-225. doi:10.1016/s0735-6757(96)90136-9.
36. Gottlieb M, Hunter B. Utility of central venous pressure as a predictor of fluid responsiveness. Ann Emerg Med. 2016;68(1):114-116. doi:10.1016/j.annemergmed.2016.02.009.
37. Kornbau C, Lee KC, Hughes GD, Firstenberg MS. Central line complications. Int J Critical Illn Inj Sci. 2015;5(3):170-178. doi:10.4103/2229-5151.164940.
38. Walley KR. Use of central venous oxygen saturation to guide therapy. Am J Respir Crit Care Med. 2011;184(5):514-520. doi:10.1164/rccm.201010-1584CI.
39. PRISM Investigators, Rowan KM, Angus DC, et al. Early, goal-directed therapy for septic shock - a patient-level meta-analysis. N Engl J Med. 2017;376(23):2223-2234. doi:10.1056/NEJMoa1701380.
40. Pope JV, Jones AE, Gaieski DF, Arnold RC, Trzeciak S, Shapiro NI; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Multicenter study of central venous oxygen saturation (ScvO(2)) as a predictor of mortality in patients with sepsis. Ann Emerg Med. 2010;55(1):40-46.e1. doi:10.1016/j.annemergmed.2009.08.014.
41. Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303(8):739-746. doi:10.1001/jama.2010.158.
42. Nowak RM, Sen A, Garcia, AJ, et al. The inability of emergency physicians to adequately clinically estimate the underlying hemodynamic profiles of acutely ill patients. Am J Emerg Med. 2012;30(6):954-960. doi:10.1016/j.ajem.2011.05.021.
43. Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med. 1970;283(9):447-451. doi:10.1056/NEJM197008272830902.
44. Hadian M, Pinsky MR. Evidence-based review of the use of the pulmonary artery catheter: impact data and complications. Crit Care. 2006;10 Suppl 3:S8.
45. Rajaram SS, Desai, NK, Kalra A, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev. 2013;(2):CD003408. doi:10.1002/14651858.CD003408.pub3.
46. Laher AE, Watermeyer MJ, Buchanan SK, et al. A review of hemodynamic monitoring techniques, methods and devices for the emergency physician. Am J Emerg Med. 2017;35(9):1335-1347. doi:10.1016/j.ajem.2017.03.036.
47. Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid Ultrasound in SHock in the evaluation of the critically lll. Emerg Med Clin North Am. 2010;28(1):29-56, vii. doi:10.1016/j.emc.2009.09.010.
48. Heller MB, Mandavia D, Tayal VS, et al. Residency training in emergency ultrasound: fulfilling the mandate. Acad Emerg Med. 2002;9(8):835-839.
49. Ultrasound guidelines: emergency, point-of-care and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2016;69(5):e27-e54. doi:10.1016/j.annemergmed.2016.08.457.
50. Expert Round Table on Ultrasound in ICU. International expert statement on training standards for critical care ultrasonography. Intensive Care Med. 2011;37(7):1077-1083. doi:10.1007/s00134-011-2246-9.
51. Neri L, Storti E, Lichtenstein D. Toward an ultrasound curriculum for critical care medicine. Crit Care Med. 2007;35(5 Suppl):S290-S304.
52. Simonson JS, Schiller NB. Sonospirometry: a new method for noninvasive estimation of mean right atrial pressure based on two-dimensional echographic measurements of the inferior vena cava during measured inspiration. J Am Coll Cardiol. 1988;11(3):557-564.
53. Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66(4):493-496.
54. Nagdev AD, Merchant RC, Tirado-Gonzalez A, Sisson CA, Murphy MC. Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55(3):290-295. doi:10.1016/j.annemergmed.2009.04.021.
55. Barbier C, Loubières Y, Schmit C, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30(9):1740-1746.
56. Corl KA, George NR, Romanoff J, et al. Inferior vena cava collapsibility detects fluid responsiveness among spontaneously breathing critically-ill patients. J Crit Care. 2017;41:130-137. doi:10.1016/j.jcrc.2017.05.008.
57. Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30(9):1834-1837.
58. Blehar DJ, Dickman E, Gaspari R. Identification of congestive heart failure via respiratory variation of inferior vena cava diameter. Am J Emerg Med. 2009;27(1):71-75. doi:10.1016/j.ajem.2008.01.002.
59. Moore CL, Rose GA, Tayal VS, Sullivan DM, Arrowood JA, Kline JA. Determination of left ventricular function by emergency physician echocardiography of hypotensive patients. Acad Emerg Med. 2002;9(3):186-193.
60. Mandavia DP, Hoffner RJ, Mahaney K, Henderson SO. Bedside echocardiography by emergency physicians. Ann Emerg Med. 2001;38(4):377-382.
61. Mosier JM, Martin J, Andrus P, et al. Advanced hemodynamic and cardiopulmonary ultrasound for critically ill patients in the emergency department. Emerg Med. 2018;50(1):17-34. doi:10.12788/emed.2018.0078.
62. Agarwal S, Swanson S, Murphy A, Yaeger K, Sharek P, Halamek LP. Comparing the utility of a standard pediatric resuscitation cart with a pediatric resuscitation cart based on the Broselow tape: a randomized, controlled, crossover trial involving simulated resuscitation scenarios. Pediatrics. 2005;116(3):e326-e333.
63. Blanco P, Volpicelli G. Common pitfalls in point-of-care ultrasound: a practical guide for emergency and critical care physicians. Crit Ultrasound J. 2016;8(1):15.
64. Tajoddini S, Shams Vahdati S. Ultrasonographic diagnosis of abdominal free fluid: accuracy comparison of emergency physicians and radiologists. Eur J Trauma Emerg Surg. 2013;39(1):9-13. doi:10.1007/s00068-012-0219-5.

65 Abbasi S, Bolverdi E, Zare MA, et al. Comparison of diagnostic value of conventional ultrasonography by emergency physicians with Doppler ultrasonography by radiology physicians for diagnosis of deep vein thrombosis. J Pak Med Assoc. 2012;62(5):461-465.
66. Arhami Dolatabadi A, Amini A, Hatamabadi H, et al. Comparison of the accuracy and reproducibility of focused abdominal sonography for trauma performed by emergency medicine and radiology residents. Ultrasound Med Biol. 2014;40(7):1476-1482. doi:10.1016/j.ultrasmedbio.2014.01.017.
67. Karimi E, Aminianfar M, Zarafshani K, Safaie A. The accuracy of emergency physicians in ultrasonographic screening of acute appendicitis; a cross sectional study. Emerg (Tehran). 2017;5(1):e22.
Critical Care in the ED: Mechanical Ventilation, Sepsis, Neurological Hypertensive Emergencies, and Pressors in Shock
Emergency medicine and critical care medicine share a responsibility for the care of acutely ill patients with life-threatening pathologies. The skills required of both emergency physicians (EPs) and critical care specialists to recognize, diagnose, and resuscitate such patients have resulted in many shared guidelines, recommendations, and publications. When critically ill patients enter the hospital through the ED, the care provided by EPs greatly impacts both the early and long-term outcomes. It is not uncommon for critically ill patients to spend several hours under the care of an EP while awaiting an available inpatient bed in the intensive care unit (ICU) or “step down” monitored unit.
This article provides a summary review of current guidelines, evidence-based medicine recommendations, and the results of recent trials involving ventilator management, treatment of sepsis, management of hypertension accompanying neurological emergencies, and the selection of pressors for the treatment of different shock states.
Ventilator Management
Mechanical ventilation is frequently undertaken in the ED for patients with respiratory failure—the origin of which is not always immediately clear. Data from the National Heart, Lung, and Blood Institute’s (NHLBI) acute respiratory distress syndrome (ARDS) clinical network (http://www.ardsnet.org) and other clinical trials have established the benefit of low tidal-volume, “lung-protective” ventilation in the patient with ARDS.1,2 Numerous studies have also shown the benefit of low-tidal-volume (TV), ventilation in patients without ARDS, and its use is now the standard of care for a large range of respiratory conditions causing compromise.3
The prompt initiation of lung-protective ventilation has a significant impact on reducing ICU mortality.4 A recent retrospective review of 3.5 million ED visits showed the median length of stay for patients started on mechanical ventilation in the ED to be greater than 3 hours.5 Such a length of time on mechanical ventilation in this setting can have significant effects on the course of illness; however, it is not clear whether mechanical ventilation performed in the ED typically conforms to evidence-based standards. In one study performed in an academic center, less than one-third of patients with sepsis and respiratory failure received low-volume ventilation in the ED.6 Another study suggested that emergency medicine residents may not receive as much dedicated education on the initial management of ventilators as needed—despite the potentially unforgiving physiologic process of positive-pressure mechanical ventilation.7
The fundamental principles required to safely manage most patients in respiratory failure are not difficult to master. There are several simple evidence-based ventilator strategies for managing patients with respiratory failure. The three primary principles of initiating and providing effective mechanical ventilation are: (1) avoiding traumatic ventilation; (2) maintaining normoxia; and (3) maintaining appropriate acid-base balance. Each of these principles can be achieved in a stepwise fashion.
Step I: Establishing Lung-Protective Settings on the Ventilator
Three central parameters must be selected at the initiation of assist-control mechanical ventilation: TV, respiratory rate (RR), and positive end-expiratory pressure (PEEP). These parameters have been extensively studied, and there is excellent evidence to guide the EP in choosing the correct settings.
Tidal Volume. Although the normal human lung can accommodate about 6 L of air, in cases of respiratory failure, the surface area available for gas exchange is significantly reduced due to a pathologic process undermining entire regions of the air-blood interface. Consequently, a person whose normal lungs are suddenly required to perform the life-sustaining gas exchanges in critical illness with the much smaller lung surface is at a significant disadvantage.
The widely accepted lung-protective volumes range from 6 to 8 mL/kg of predicted body weight (PBW), a height-based calculation.8 For example, in a 6-foot tall man, 6 mL/kg of PBW amounts to a TV of 466 mL; in a 5-foot tall woman, the same amount of PBW amounts to a TV of 273 mL. Volumes may be referenced using PBW tables from the NHLBI ARDS network or by employing the following equations:
Adult men: PBW (kg) = 50 + 2.3 (height [in] – 60)
Adult women: PBW (kg) = 45.5 + 2.3 (height [in] – 60).9
Respiratory Rate. The RR should be set somewhat higher than normal because the TV per breath has been slightly reduced, and also because sick patients in a catabolic state may have larger minute ventilations than they would when healthy. As previously described, since the TV is restricted, RR is the most mobile parameter in maintaining appropriate minute ventilation. Minute ventilation (MV) is the product of RR multiplied by TV (MV = RR x TV), and this should be calculated to approximate the patient’s own efforts, which are dependent upon the clinical circumstances. For example, patients whose bodies are trying to compensate for an acidosis will require much higher rates than those who are simply obtunded and intubated for airway protection. In other words, in order to remove carbon dioxide (CO2) in an acidemic patient, a higher RR rate may be required, whereas a lower rate may be selected to compensate for alkalemia while maintaining appropriate oxygen (O2) levels in both cases.10
Positive End-Expiratory Pressure. Previous recommendations for ventilation in respiratory failure called for large TVs (ie, 10 to 15 mL/kg), partly out of concern that smaller volumes would promote distal airway collapse, thereby increasing the amount of lung that received blood but not air, consequently worsening overall oxygenation.11 Although administering such large volumes has clearly proved harmful, the valid concern about distal airway collapse can be addressed in part by adjustments to PEEP, which acts to “stent” open airways after most of the tidal breath has left the airways.
Positive end-expiratory pressure, however, is not without risks.12 Blood from the rest of the body will encounter resistance returning to a thoracic cavity persistently inflated by positive pressure, and this decrease in preload may contribute to hypotension. Similarly, a weak right ventricle may struggle to push blood into the compressed pulmonary vasculature, increasing the cardiac workload and further compromising hemodynamics.13 In general, PEEP should be set as low as the maintenance of adequate oxygenation permits. The NHLBI ARDS guidelines provide a table on balancing PEEP and the fraction of inspired O2 (FiO2), as well as hypotension, in refractory hypoxemic patients—with the limitation on PEEP set by the patients’ pulmonary compliance (plateau pressures, discussed next).8
After making these selections, several parameters must be monitored closely. Those most relevant to lung-protective ventilation are the peak airway pressure and, most importantly, the plateau pressure. Numerous animal studies now demonstrate serious lung injury in both healthy and diseased lungs from high peak pressures (defined as a plateau pressure >30 cm water [H2O]).14,15 A high-pressure alarm sounding on the ventilator must be promptly addressed by an evaluation for easily reversible causes, such as tube obstruction, pneumothorax, breath stacking, pulmonary edema, or pleural effusions. A full discussion of the causes of elevated peak and plateau pressures is beyond the scope of this review, but if the plateau pressures remain consistently high, a reduction in TV may be necessary.
Step II: Maintaining Normoxia
As a severely hypoxic patient will rapidly decompensate with progression to death, a host of monitoring devices are used to alert the nurse or physician that O2 levels have fallen below the normal range. Strategies to manage refractory hypoxia in the ventilated patient are complex. For most patients, 100% FiO2 is initiated immediately after intubation to increase the safety of the procedure, but there is animal evidence that high O2 levels promote inflammatory responses, and human data suggest hyperoxia can be deleterious to long-term outcomes, particularly following cardiac arrest and stroke.16,17 A persistent O2 saturation of 100% on pulse oximetry or a supraphysiologic partial pressure of O2 (PaO2) on an arterial blood gas (defined as >200 mm Hg) may actually cause the patient more harm than good. Therefore, the fraction of inspired O2 should be titrated to maintain normoxia. The ARDS protocol, for example, targets an O2 saturation of 88% to 95% and a PaO2 of 55 to 80 mm Hg.8
Step III: Maintaining Acid-Base Balance
The basic principles of acid-base physiology should be familiar to EPs. When a patient is sedated and the airway secured, the primary means by which blood pH is maintained is now in the hands of the intubating physician. Patients with respiratory failure may have compensated for a preexisting derangement in their blood pH. If the preexisting condition is not recognized and ventilator settings are not maintained appropriately, they may be vulnerable to developing another derangement. Even on settings that allow the patient to breathe over a set rate, the sedation required to tolerate an endotracheal tube may cause significant respiratory depression, making it impossible for the patient to auto-regulate the respiratory component of acid-base homeostasis (ie, by hyperventilation).
As in the discussion of RR, TVs are “fixed” based on low-TV lung-protective ventilation. Therefore, changing the patient’s set RR is the easiest method to adjust the partial pressure of CO2 (PaCO2), and consequently address any respiratory acidosis. An increase in the RR will increase the patient’s minute ventilation, leading to a decrease in serum PaCO2 levels, whereas a decrease in the RR will have the converse effect. It is important to obtain an arterial blood-gas reading shortly after intubation and to continue to monitor the impact of any ventilator titrations on the patient’s acid-base status.
Studies of “permissive hypercapnia” in ARDS patients have shown that prioritizing lung-protective ventilator settings, even at the expense of a normal CO2, reduce mortality.1,18 Even in situations where it is not necessary to maintain hypercapnia for lung-protective settings, the hypercapnia appears to have beneficial effects.19-21 No upper limits on hypercapnia have been established, and even extreme levels have been associated with successful patient outcomes.22 However, a study by Hickling et al23 demonstrated that an initial trial of lung-protective ventilation demonstrated benefit from unbuffered hypercarbia and acidosis, reporting an average CO2 level of 66 and a pH of 7. These guidelines should be appropriate for use in the ED.
In summary, assuming control of a patient’s respiratory system—with its nuanced and responsive role in acid-base, oxygenation, and cardiopulmonary hemodynamics—is one of the most difficult situations routinely encountered by an EP. While the procedure itself may be life-saving, the next several hours can have significant impact on the patient’s long-term outcome.
Treating Sepsis and Surviving Sepsis: Recommendations Versus the ARISE/ProCESS Trials
Sepsis and Septic Shock
Sepsis is defined as an infection plus systemic inflammatory response syndrome (SIRS). Severe sepsis is sepsis plus sepsis-induced organ dysfunction or tissue hypoperfusion resulting in or caused by lactic acidosis, acute lung injury, altered mental status, or coagulation abnormalities. Septic shock refers to persistent sepsis-induced hypotension despite adequate fluid resuscitation.24 The ambiguity of these definitions may invariably lead to a practitioner’s underappreciation or misconception of the importance of sepsis.
Sepsis is one of the most common, yet least-recognized, entities. In the United States, it is estimated that 3 in 1,000 people annually are affected by sepsis, and every few seconds, a person dies of sepsis.25 Both numbers underestimate the effects on the elderly. Clinical manifestations of sepsis vary, and the condition may originate from both community-acquired and healthcare-associated sources.
In 2001, a landmark study demonstrated that early goal-directed therapy (EGDT) reduced mortality and improved patient outcomes in patients presenting to the ED in severe sepsis.26 The estimated 12% to 16% reduction in mortality reported in this trial began an initiative to broaden the scope and awareness of sepsis.
Current Literature and Evidence-Based Guidelines
The most recent guidelines for the management of septic shock from the Surviving Sepsis Campaign are summarized in Table 1. With its last revision, the Surviving Sepsis guidelines of 2012 has two main management foci—initiating treatment within the first 3 and the first 6 hours after recognition within the first 3 hours and those within the first 6 hours after sepsis recognition (Table 2). Within the first 3 hours, the treatment team should draw a serum lactate level; obtain cultures prior to the administration of antibiotics; initiate broad-spectrum antibiotics as early as possible; and administer 20 to 30 mL/kg of crystalloid fluids in patients with hypotension or a lactate level greater than 4 mmol/L. Within the first 6 hours, the clinician should administer intravenous (IV) vasopressors, preferentially norepinephrine, for persistent hypotension after a fluid challenge to maintain a mean arterial pressure >65 mm Hg; place a supra-diaphragmatic central venous catheter to measure a serum mixed venous O2 saturation (ScvO2) and central venous pressure (CVP); and measure serial serum lactate levels if they were initially elevated (lactate ≥4 mmol/L [36 mg/dL]).24,30,31 The targets for ScvO2 and CVP are ≥70%, and >8 mm Hg, respectively.
Summary
Sepsis is a prevalent ED presentation associated with mortality that can present in a complex fashion. Early recognition and management is essential and can be condensed into a few key recommendations. Becoming familiar with and incorporating these recommendations into daily practice will enable EPs to deliver quality care to every patient presenting with sepsis, and will also reduce mortality.
Blood Pressure Management for Select Neurological Emergencies
Patients with ischemic stroke, spontaneous intracerebral hemorrhage (ICH), and aneurysmal subarachnoid hemorrhage (SAH) often present with elevated blood pressures (BPs).34-36 In caring for these patients, EPs face the question of how, or even if, the patient’s BP should be managed. What are the appropriate BP targets for each of the aforementioned pathologies? Does aggressive BP management benefit or harm the patient?
Background
The relationship between hypertension and stroke is different for each stroke type. Retrospective data show a U-shaped relationship between BP and mortality in ischemic stroke, with the highest mortality observed at both extremes of the BP curve.34 Data also suggest increased mortality when ICH is accompanied by hypertension.35 Hypertension may also be associated with a higher risk of rebleeding in patients with SAH due to aneurysms.36 Because of the variable relationship between stroke and hypertension, therapeutic recommendations for each type of stroke can be confusing.
Current Literature and Evidence-Based Guidelines
Firm evidence to make therapeutic recommendations remains elusive. The recent American Heart Association (AHA) guidelines covering ischemic stroke, ICH, and SAH were published between late 2010 and early 2013, and several trials investigating the role of BP control in ischemic and hemorrhagic stroke have subsequently been published.37-41
When the Cochrane Collaboration updated its systematic review on vasoactive medications in stroke in 2014 to include recent evidence,42 it ultimately concluded that lowering BP does not improve mortality, neurological deterioration, or quality of life regardless of stroke type, and suggest that further investigations should be undertaken.9 However, the Cochrane authors noted that two recent trials showed a statistically significant association between improved quality of life and BP reduction within 6 hours of stroke onset.38-39 Although the data were compiled from just 2,835 patients of the 15,432 included in the entire Cochrane review, it suggested that interventions initiated in the ED may contribute to any potential beneficial outcomes from intensive BP control.
Ischemic Stroke
The China Antihypertensive Trial in Acute Ischemic Stroke (CATIS) investigated the initiation of BP-control measures within 48 hours of onset of ischemic stroke in approximately 4,000 patients and found no significant difference in death or disability between the group that received BP-control interventions and the group that did not.37 The Rapid Intervention With Glyceryl Trinitrate in Hypertensive Stroke Trial (RIGHT) included patients with both ischemic and hemorrhagic strokes. Though it studied only 41 patients, this trial suggests that early BP control is safe and may be associated with lower disability.38 These findings are bolstered by the more recent Efficacy of Nitric Oxide in Stroke (ENOS) trial showing a similar safety profile for BP control in both ischemic and hemorrhagic strokes, though the mean difference in systolic BP after therapy was a mere 7 mm Hg.40 The combined data from the RIGHT and ENOS trials offer little to clarify the question of appropriate BP control.
For now, the EP is left with the AHA/American Stroke Association (ASA) guideline’s recommendation “not to lower the BP during the initial 24 hours of acute ischemic stroke unless the BP is greater than 220/120 mm Hg.”34 The recommendation differs in cases when a patient receives thrombolytics and hemorrhagic transformation is a risk. There have been no new data to change the AHA/ASA’s recommendations for patients receiving thrombolytics. In such cases, the EP should ensure the patient’s BP is below 185/110 mm Hg prior to thrombolytic administration and below 180/105 mm Hg during therapy.34 A variety of agents is available to lower BP in this situation, and includes IV labetalol, nicardipine, esmolol, and others.
Intracerebral Hemorrhage
The recent literature on blood pressure control in ICH has also increased since the most recent AHA/ASA recommendations. The Second Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT-2 included over 2,800 patients randomized to intensive early therapy to reduce BP to less than 140 mm Hg or less than 180 mm Hg and found no significant difference in mortality or safety between the two groups, though intensive therapy was associated with less disability.39 The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial (ICH-ADAPT) trial further clarified the issue of safety during intensive BP control by showing no decrease in perihematomal cerebral blood flow in patients whose BP control was targeted to less than 150 mm Hg compared to those whose BP was less than 180 mm Hg, suggesting that aggressive BP reduction does not cause iatrogenic ischemic stroke.41
These combined data suggest that intensive BP management is safe for patients with ICH, providing reassurance for the AHA/ASA guideline recommendation that “in patients…with a systolic BP of 150 to 220 mm Hg, acute lowering of systolic BP to 140 mm Hg is probably safe.”35 Whether this improves patient outcomes remains unclear. Again, multiple agents are available for BP control, including IV labetalol or nicardipine, with no agent identified as superior in producing better patient outcomes. A continuous infusion is recommended if several boluses are ineffective in achieving and maintaining the target BP, as BP variability has been associated with poorer outcomes.43
Subarachnoid Hemorrhage
There are no large recent studies in the literature on antihypertensive therapy in SAH. The AHA/ASA guidelines updated in 2012 reflect the consensus that elevated BPs are associated with increased risk of aneurysmal rebleeding and thus poorer patient-oriented outcomes. The consensus remains to use a titratable agent to target a systolic BP less than 160 mm Hg until definitive neurosurgical therapy, such as aneurysmal coiling or clipping.36 Given the variability of the sodium nitroprusside dose-response relationship, IV labetalol, and nicardipine, are recommended agents for continuous control, though data showing differences in mortality and/or disability are lacking.36 Again, retrospective data suggest that BP variability negatively impacts mortality and disability, so consider early initiation of continuous infusions to achieve and maintain consistency in the chosen target.44
Summary
Pressors in the Management of Hypotension
Norepinephrine
Norepinephrine is one of the most commonly used agents for shock in the ED, with indications spanning multiple etiologies. It is an endogenous neurotransmitter that works predominantly on a1 receptors as well as exerting some modest effects on b1 and b2 receptors, for combined vasopressor and improved cardiac contractility effect.46-47 Norepinephrine is currently the recommended initial agent for sepsis-induced tissue hypoperfusion.24 However, a recent Cochrane systematic review and meta-analysis supports evidence of limited differences among various pressors.48 Several comparative randomized control trials show norepinephrine is as effective as other agents, but with fewer side effects.24,49 With the ease and familiarity of its use by most EPs, and a wide therapeutic index for targeted effect versus arrhythmias, norepinephrine is a reasonable choice as the initial pressor in managing a wide variety of shock syndromes.
Vasopressin
Another commonly used agent in the treatment of shock, vasopressin is an analogue of the antidiuretic hormone secreted from the posterior pituitary gland, exerting its CV effects primarily as a vasoconstrictor by increasing intracellular calcium.50 Vasopressin doses are 0.03 or 0.04 U/min IV without titration.24,50 Early studies of septic patients demonstrated a relative deficiency of serum vasopressin levels, leading clinicians to utilize it in the treatment of sepsis-induced shock. However, the Vasopressin and Septic Shock Trial (VASST) trial demonstrated that the addition of vasopressin to norepinephrine did not produce any improvements in morbidity or mortality compared with norepinephrine alone.51 Despite these findings, vasopressin is still commonly used as a secondary agent to correct continued hypotension. Vasopressin may be appropriate for patients who specifically require peripheral vasoconstriction in the setting of good cardiac output and volume status, ability to tolerate increases in afterload, or in patients at risk for dysrhythmias.
Dopamine
Dopamine had been previously recommended as the initial choice of pharmacologic support for the management of shock.24,52-54 Dopamine is an adrenergic agonist agent that works via a1 and b1 receptors as well as a precursor to the synthesis of norepinephrine and epinephrine.55 There are dose-dependent effects on various receptors from escalating amounts administered,55-56 but the literature does not support the concept of “renal-dose” dopamine.57-59 A study by DeBaker et al49 suggested no difference in efficacy between dopamine and norepinephrine, but demonstrated a greater tendency toward cardiac dysrhythmias with dopamine. For these reasons noted above, norepinephrine may be the initial agent for pharmaceutical support of shock, particularly in septic syndromes, with dopamine as a secondary or adjunct agent in patients at low risk for tachyarrhythmia or a relative bradycardia. 24, 56
Dobutamine
Dobutamine is another adrenergic agonist that is similar to dopamine but with a greater effect on inotropic cardiac contractility due to a preferential action at b1 receptors.60 It can potentially induce peripheral vasodilatation due to its effect on arterial b1 receptors. Given this balance, dobutamine is an agent that should be utilized for cardiogenic shock when increased contractility is needed. These effects are particularly useful in patients with “wet and cold” heart failure who have a low cardiac output and volume-overloaded status.61, 62 However, it may be necessary to add another agent to provide additional peripheral vasoconstriction should the use of dobutamine affect lead to excessive vasodilatation.
Epinephrine
One of the most powerful vasoactive agents, epinephrine has a high affinity for all b1, b2 and a1 receptors.63 These combined effects lead to increased cardiac output and improved BP by increasing cardiac contractility and peripheral vasoconstriction. The effect of epinephrine in limiting mast cell release of histamine makes it the preferred choice for the treatment of anaphylaxis.64 However, side effects of epinephrine include hypertension, tachydysrhythmias, tissue ischemia from vigorous vasoconstriction, and induced lactic acidosis.63
Phenylephrine
Phenylephrine is an a-adrenergic agonist that activates a1 receptors on arteriole smooth muscle, resulting in vasoconstriction.65 It is currently recommended only for hypotension related to procedural sedation.47 Phenylephrine is not recommended for treating patients with septic shock, except when there are concerns about tachydysrhythmias; persistent hypotension with a high cardiac output after treatment with other vasoconstrictor and inotropic drugs; or when a “pure” vasoconstrictor may be preferred.24,56,65
Summary
Although there are many other vasoactive agents that can be used, the selected agents discussed above represent those most commonly used in the ED. All demonstrate significant crossover effects and receptor activation, as well as impact on cardiac contractility and vasoconstriction. The suggested specific indications for each agent are based on current evidenced-based medicine, clinical guidelines, and theoretical benefits on clinical scenarios. But, as always, clinical decisions should be individualized for critically ill patients.
Conclusion
The resuscitation and initiation of care for critically ill patients must typically be immediately upon their arrival in the ED. While general guidelines or recommendations exist for commonly encountered pathologies, treatment should always be patient-centered, based on the needs and nuances unique to each patient in this vulnerable population. The initiation of mechanical ventilation, treatment of sepsis, management of hypertensive neurosurgical emergencies, and use of pressors in shock states are among the most critically important tasks an EP is called upon to perform. This review of current evidence-based guidelines and recommendations will help EPs provide the appropriate and unique care each patient requires.
Dr Brubaker is a resident in the department of emergency medicine at the University of Pittsburgh, Pennsylvania. Dr Yu is a fellow of adult critical care medicine – emergency medicine in the department of critical care medicine, University of Pittsburgh Medical Center, Pennsylvania. Dr Goodmanson is a resident in the department of emergency medicine at the University of Pittsburgh, Pennsylvania. Dr Schott is an assistant professor, department of emergency medicine and critical care medicine; assistant director of ultrasonography; director, critical care elective student rotation; and director, point of care ultrasound elective student rotation, at the University of Pittsburgh, Pennsylvania
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308.
- Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32(9):1817-1824.
- Brower RG, Rubenfeld GD. Lung-protective ventilation strategies in acute lung injury. Crit Care Med. 2003;31(4):S312-S316.
- Needham DM, Yang T, Dinglas VD. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. A prospective cohort study. Am J Respir Crit Care Med. 2015;191(2):177-185.
- Easter BD, Fischer C, Fisher J. The use of mechanical ventilation in the ED. Am J Emerg Med. 2012;30(7):1183-1188.
- Fuller BM, Mohr NM, Dettmer M, et al. Mechanical ventilation and acute lung injury in emergency department patients with severe sepsis and septic shock: an observational study. Acad Emerg Med. 2013;20(7):659-669.
- Wilcox SR, Seigel TA, Strout TD, et al. Emergency medicine residents’ knowledge of mechanical ventilation. J Emerg Med. 2014. doi:10.1016/j.jemermed.2014.09.059. [Epub ahead of print]
- National Institutes of Health’s National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Clinical Network Mechanical Ventilator Protocol Summary. http://www.ardsnet.org/system/files/Ventilator%20Protocol%20Card.pdf. Accessed March 5, 2015.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (NHLBI ARDS) Network. Predicted body weight calculator. http://www.ardsnet.org/node/77460. Accessed March 5, 2015.
- Boron WF. Acid-base physiology. In: Boron WF, Boulpaep EL, eds. Medical Physiology: A Cellular And Molecular Approach. Philadelphia, PA: Saunders/Elsevier; 2009:647-649.
- Orebaugh, SL. Initiation of mechanical ventilation in the emergency department. Am J Emerg Med. 1996;14(1):59-69.
- Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149(5):1327-1334.
- Marino PL, Sutin KM. Principles of mechanical ventilation. In: Marino PL, ed. The ICU Book. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:457-472.
- Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection with positive end-expiratory pressures. Am Rev Resp Dis. 1974;110(5):556-565.
- Parker JC, Hernandez LA, Peevy KJ. Mechanisms of ventilator-induced lung injury. Crit Care Med. 1993;21(1):131-143.
- Bhandari V. Molecular mechanisms of hyperoxia-induced acute lung injury. Front Biosci. 2008;13:6653-6661.
- Kilgannon JH, Jones AE, Shapiro NI, et al; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165-2171.
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347-354.
- Laffey JG, Tanaka M, Engelberts D, et al. Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. Am J Respir Crit Care Med. 2000;162(6):2287-2294.
- Costello J, Higgins B, Contreras M, et al. Hypercapnic acidosis attenuates shock and lung injury in early and prolonged systemic sepsis. Crit Care Med. 2009;37(8):2412-2420.
- Ijland MM, Heunks LM, van der Hoeven JG. Bench-to-bedside review: hypercapnic acidosis in lung injury—from ‘permissive’ to ‘therapeutic.’ Crit Care. 2010;14(6):237.
- Garg SK. Permissive hypercapnia: Is there any upper limit? Indian J Crit Care Med. 2014;18(9):612-614.
- Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med. 1994;22(10):1568-1578.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup Surviving Sepsis Campaign. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228.
- Peake SL, Delaney A, Bailey M, et al; ARISE Investigators; ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496-1506.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;8;345(19):1368-1377.
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(21):2063.
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303-1310.
- Rivers EP, Ahrens T. Improving outcomes for severe sepsis and septic shock: tools for early identification of at-risk patients and treatment protocol implementation. Crit Care Clin. 2008;24(3 Suppl):S1-S47.
- Nguyen HB, Rivers EP, Abrahamian FM, et al; Emergency Department Sepsis Education Program and Strategies to Improve Survival (ED-SEPSIS) Working Group. Severe sepsis and septic shock: review of the literature and emergency department management guidelines. Ann Emerg Med. 2006;48(1):28-54.
- Jones AE, Puskarich MA. The Surviving Sepsis Campaign guidelines 2012: update for emergency physicians. Ann Emerg Med. 2014;63(1):35-47.
- Yealy DM, Kellum JA, Huang DT, et al.;ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-1693.
- Huang DT, Angus DC, Barnato A, et al; ProCESS/ARISE/ProMISe Methodology Writing Committee. Harmonizing international trials of early goal-directed resuscitation for severe sepsis and septic shock: methodology of ProCESS, ARISE, and ProMISe. Intensive Care Med. 2013;39(10):1760-1775.
- Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
- Morgenstern LB, Hemphill JC 3rd, Anderson C, et al; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108-2129.
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43(6):1711-1737.
- He J, Zhang Y, Xu T, et al; The China Antihypertensive Trial in Acute Ischemic Stroke (CATIS) Investigators. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA. 2014;311(5):479-489.
- Ankolekar S, Fuller M, Cross I, et al. Feasibility of an ambulance-based stroke trial, and safety of glyceryl trinitrate in ultra-acute stroke: The Rapid Intervention With Glyceryl Trinitrate in Hypertensive Stroke Trial (RIGHT, ISRCTN66434824). Stroke. 2013;44(11):3120-3128.
- Anderson CS, Heeley E, Huang Y, et al; The Second Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT-2) Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368(25):2355-2365.
- Bath PM, Woodhouse L, Scutt P, et al; ENOS Trial Investigators. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet. 2014;385(9968):617-628
- Butcher KS, Jeerakathil T, Hill M, et al; ICH ADAPT Investigators. The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial. Stroke. 2013;44(3):620-626.
- Bath PM, Krishnan K. Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database Syst Rev. 2014;10:CD000039.
- Tanaka E, Koga M, Kobayashi J, et al. Blood pressure variability on antihypertensive therapy in acute intracerebral hemorrhage: the Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-intracerebral hemorrhage study. Stroke. 2014;45(8):2275-2279.
- Beseoglu K, Unfrau K, Steiger HJ, Hänggi D. Influence of blood pressure variability on short-term outcome in patients with subarachnoid hemorrhage. Cent Eur Neurosurg. 2010;71(2):69-74.
- Hinshaw LB, Cox BG, eds. The fundamental mechanisms of shock. Proceedings of a Symposium Held in Oklahoma City, Oklahoma, October 1-2, 1971. In: Advances in Experimental Medicine and Biology, Vol 23. New York, NY: Plenum Press; 1972.
- Norepinephrine. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=norepinephrine&x=0&y=0. March 5, 2015.
- Overgaard CB, Dzavik V. Inotropes and vasopressors: review of physiology and clinical use in cardiovascular disease. Circulation. 2008;118(10):1047-1056.
- Havel C, Arrich J, Losert H, et al. Vasopressors for hypotensive shock. Cochrane Database Syst Rev. 2011;(5):CD003709.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010;362(9):779-789.
- Vasopressin. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=vasopressin&x=0&y=0. March 5, 2015.
- Russell JA, Walley KR, Singer J, et al; VASST Investigators. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877-887.
- Martin C, Papazian L, Perrin G, Saux P, Gouin F. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest. 1993;103(6):1826-1831.
- De Backer D, Creteur J, Silva E, Vincent JL. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: which is best? Crit Care Med. 2003;31(6)1659-1667.
- Day NP, Phu NH, Bethell DP, et al. The effects of dopamine and adrenaline infusions on acid-base balance and systemic haemodynamics in severe infection. Lancet. 1996;348(9022):219-223.
- Dopamine. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=dopamine&x=0&y=0. March 5, 2015.
- Manaker S. Use of vasopressors and inotropes. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/use-of-vasopressors-and-inotropes?source=search_result&search=Use+of+vasopressors+and+isotopes&selectedTitle=1%7E150. Accessed March 5, 2015.
- Lauschke A, Teichgräber UK, Frei U, Eckardt KU. ‘Low-dose’ dopamine worsens renal perfusion in patients with acute renal failure. Kidney Int. 2006;69(9):1669-1674.
- Bellomo R, Chapman M, Finfer S, Hickling K, Myburgh J. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356(9248):2139-2143.
- Kellum JA, M Decker J. Use of dopamine in acute renal failure: a meta-analysis. Crit Care Med. 2001;29(8):1526-1531.
- Dobutamine. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=Dobutamine&x=0&y=0. Accessed March 5, 2015.
- Nohria A, Mielniczuk LM, Stevenson LW. Evaluation and monitoring of patients with acute heart failure syndromes. Am J Cardiol. 2005;96(6A):32G-40G.
- Joseph SM, Cedars AM, Ewald GA, Geltman EM, Mann DL. Acute decompensated heart failure: contemporary medical management. Tex Heart Inst J. 2009;36(6):510-520.
- Epinephrine. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=epinephrine. Accessed March 5, 2015.
- Vadas P, Perelman B. Effect of epinephrine on platelet-activating factor-stimulated human vascular smooth muscle cells. J Allergy Clin Immunol. 2012;129(5):1329-1333.
- Phenylephrine. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=65.%09Phenylephrine&x=10&y=10. Accessed March 5, 2015.
Emergency medicine and critical care medicine share a responsibility for the care of acutely ill patients with life-threatening pathologies. The skills required of both emergency physicians (EPs) and critical care specialists to recognize, diagnose, and resuscitate such patients have resulted in many shared guidelines, recommendations, and publications. When critically ill patients enter the hospital through the ED, the care provided by EPs greatly impacts both the early and long-term outcomes. It is not uncommon for critically ill patients to spend several hours under the care of an EP while awaiting an available inpatient bed in the intensive care unit (ICU) or “step down” monitored unit.
This article provides a summary review of current guidelines, evidence-based medicine recommendations, and the results of recent trials involving ventilator management, treatment of sepsis, management of hypertension accompanying neurological emergencies, and the selection of pressors for the treatment of different shock states.
Ventilator Management
Mechanical ventilation is frequently undertaken in the ED for patients with respiratory failure—the origin of which is not always immediately clear. Data from the National Heart, Lung, and Blood Institute’s (NHLBI) acute respiratory distress syndrome (ARDS) clinical network (http://www.ardsnet.org) and other clinical trials have established the benefit of low tidal-volume, “lung-protective” ventilation in the patient with ARDS.1,2 Numerous studies have also shown the benefit of low-tidal-volume (TV), ventilation in patients without ARDS, and its use is now the standard of care for a large range of respiratory conditions causing compromise.3
The prompt initiation of lung-protective ventilation has a significant impact on reducing ICU mortality.4 A recent retrospective review of 3.5 million ED visits showed the median length of stay for patients started on mechanical ventilation in the ED to be greater than 3 hours.5 Such a length of time on mechanical ventilation in this setting can have significant effects on the course of illness; however, it is not clear whether mechanical ventilation performed in the ED typically conforms to evidence-based standards. In one study performed in an academic center, less than one-third of patients with sepsis and respiratory failure received low-volume ventilation in the ED.6 Another study suggested that emergency medicine residents may not receive as much dedicated education on the initial management of ventilators as needed—despite the potentially unforgiving physiologic process of positive-pressure mechanical ventilation.7
The fundamental principles required to safely manage most patients in respiratory failure are not difficult to master. There are several simple evidence-based ventilator strategies for managing patients with respiratory failure. The three primary principles of initiating and providing effective mechanical ventilation are: (1) avoiding traumatic ventilation; (2) maintaining normoxia; and (3) maintaining appropriate acid-base balance. Each of these principles can be achieved in a stepwise fashion.
Step I: Establishing Lung-Protective Settings on the Ventilator
Three central parameters must be selected at the initiation of assist-control mechanical ventilation: TV, respiratory rate (RR), and positive end-expiratory pressure (PEEP). These parameters have been extensively studied, and there is excellent evidence to guide the EP in choosing the correct settings.
Tidal Volume. Although the normal human lung can accommodate about 6 L of air, in cases of respiratory failure, the surface area available for gas exchange is significantly reduced due to a pathologic process undermining entire regions of the air-blood interface. Consequently, a person whose normal lungs are suddenly required to perform the life-sustaining gas exchanges in critical illness with the much smaller lung surface is at a significant disadvantage.
The widely accepted lung-protective volumes range from 6 to 8 mL/kg of predicted body weight (PBW), a height-based calculation.8 For example, in a 6-foot tall man, 6 mL/kg of PBW amounts to a TV of 466 mL; in a 5-foot tall woman, the same amount of PBW amounts to a TV of 273 mL. Volumes may be referenced using PBW tables from the NHLBI ARDS network or by employing the following equations:
Adult men: PBW (kg) = 50 + 2.3 (height [in] – 60)
Adult women: PBW (kg) = 45.5 + 2.3 (height [in] – 60).9
Respiratory Rate. The RR should be set somewhat higher than normal because the TV per breath has been slightly reduced, and also because sick patients in a catabolic state may have larger minute ventilations than they would when healthy. As previously described, since the TV is restricted, RR is the most mobile parameter in maintaining appropriate minute ventilation. Minute ventilation (MV) is the product of RR multiplied by TV (MV = RR x TV), and this should be calculated to approximate the patient’s own efforts, which are dependent upon the clinical circumstances. For example, patients whose bodies are trying to compensate for an acidosis will require much higher rates than those who are simply obtunded and intubated for airway protection. In other words, in order to remove carbon dioxide (CO2) in an acidemic patient, a higher RR rate may be required, whereas a lower rate may be selected to compensate for alkalemia while maintaining appropriate oxygen (O2) levels in both cases.10
Positive End-Expiratory Pressure. Previous recommendations for ventilation in respiratory failure called for large TVs (ie, 10 to 15 mL/kg), partly out of concern that smaller volumes would promote distal airway collapse, thereby increasing the amount of lung that received blood but not air, consequently worsening overall oxygenation.11 Although administering such large volumes has clearly proved harmful, the valid concern about distal airway collapse can be addressed in part by adjustments to PEEP, which acts to “stent” open airways after most of the tidal breath has left the airways.
Positive end-expiratory pressure, however, is not without risks.12 Blood from the rest of the body will encounter resistance returning to a thoracic cavity persistently inflated by positive pressure, and this decrease in preload may contribute to hypotension. Similarly, a weak right ventricle may struggle to push blood into the compressed pulmonary vasculature, increasing the cardiac workload and further compromising hemodynamics.13 In general, PEEP should be set as low as the maintenance of adequate oxygenation permits. The NHLBI ARDS guidelines provide a table on balancing PEEP and the fraction of inspired O2 (FiO2), as well as hypotension, in refractory hypoxemic patients—with the limitation on PEEP set by the patients’ pulmonary compliance (plateau pressures, discussed next).8
After making these selections, several parameters must be monitored closely. Those most relevant to lung-protective ventilation are the peak airway pressure and, most importantly, the plateau pressure. Numerous animal studies now demonstrate serious lung injury in both healthy and diseased lungs from high peak pressures (defined as a plateau pressure >30 cm water [H2O]).14,15 A high-pressure alarm sounding on the ventilator must be promptly addressed by an evaluation for easily reversible causes, such as tube obstruction, pneumothorax, breath stacking, pulmonary edema, or pleural effusions. A full discussion of the causes of elevated peak and plateau pressures is beyond the scope of this review, but if the plateau pressures remain consistently high, a reduction in TV may be necessary.
Step II: Maintaining Normoxia
As a severely hypoxic patient will rapidly decompensate with progression to death, a host of monitoring devices are used to alert the nurse or physician that O2 levels have fallen below the normal range. Strategies to manage refractory hypoxia in the ventilated patient are complex. For most patients, 100% FiO2 is initiated immediately after intubation to increase the safety of the procedure, but there is animal evidence that high O2 levels promote inflammatory responses, and human data suggest hyperoxia can be deleterious to long-term outcomes, particularly following cardiac arrest and stroke.16,17 A persistent O2 saturation of 100% on pulse oximetry or a supraphysiologic partial pressure of O2 (PaO2) on an arterial blood gas (defined as >200 mm Hg) may actually cause the patient more harm than good. Therefore, the fraction of inspired O2 should be titrated to maintain normoxia. The ARDS protocol, for example, targets an O2 saturation of 88% to 95% and a PaO2 of 55 to 80 mm Hg.8
Step III: Maintaining Acid-Base Balance
The basic principles of acid-base physiology should be familiar to EPs. When a patient is sedated and the airway secured, the primary means by which blood pH is maintained is now in the hands of the intubating physician. Patients with respiratory failure may have compensated for a preexisting derangement in their blood pH. If the preexisting condition is not recognized and ventilator settings are not maintained appropriately, they may be vulnerable to developing another derangement. Even on settings that allow the patient to breathe over a set rate, the sedation required to tolerate an endotracheal tube may cause significant respiratory depression, making it impossible for the patient to auto-regulate the respiratory component of acid-base homeostasis (ie, by hyperventilation).
As in the discussion of RR, TVs are “fixed” based on low-TV lung-protective ventilation. Therefore, changing the patient’s set RR is the easiest method to adjust the partial pressure of CO2 (PaCO2), and consequently address any respiratory acidosis. An increase in the RR will increase the patient’s minute ventilation, leading to a decrease in serum PaCO2 levels, whereas a decrease in the RR will have the converse effect. It is important to obtain an arterial blood-gas reading shortly after intubation and to continue to monitor the impact of any ventilator titrations on the patient’s acid-base status.
Studies of “permissive hypercapnia” in ARDS patients have shown that prioritizing lung-protective ventilator settings, even at the expense of a normal CO2, reduce mortality.1,18 Even in situations where it is not necessary to maintain hypercapnia for lung-protective settings, the hypercapnia appears to have beneficial effects.19-21 No upper limits on hypercapnia have been established, and even extreme levels have been associated with successful patient outcomes.22 However, a study by Hickling et al23 demonstrated that an initial trial of lung-protective ventilation demonstrated benefit from unbuffered hypercarbia and acidosis, reporting an average CO2 level of 66 and a pH of 7. These guidelines should be appropriate for use in the ED.
In summary, assuming control of a patient’s respiratory system—with its nuanced and responsive role in acid-base, oxygenation, and cardiopulmonary hemodynamics—is one of the most difficult situations routinely encountered by an EP. While the procedure itself may be life-saving, the next several hours can have significant impact on the patient’s long-term outcome.
Treating Sepsis and Surviving Sepsis: Recommendations Versus the ARISE/ProCESS Trials
Sepsis and Septic Shock
Sepsis is defined as an infection plus systemic inflammatory response syndrome (SIRS). Severe sepsis is sepsis plus sepsis-induced organ dysfunction or tissue hypoperfusion resulting in or caused by lactic acidosis, acute lung injury, altered mental status, or coagulation abnormalities. Septic shock refers to persistent sepsis-induced hypotension despite adequate fluid resuscitation.24 The ambiguity of these definitions may invariably lead to a practitioner’s underappreciation or misconception of the importance of sepsis.
Sepsis is one of the most common, yet least-recognized, entities. In the United States, it is estimated that 3 in 1,000 people annually are affected by sepsis, and every few seconds, a person dies of sepsis.25 Both numbers underestimate the effects on the elderly. Clinical manifestations of sepsis vary, and the condition may originate from both community-acquired and healthcare-associated sources.
In 2001, a landmark study demonstrated that early goal-directed therapy (EGDT) reduced mortality and improved patient outcomes in patients presenting to the ED in severe sepsis.26 The estimated 12% to 16% reduction in mortality reported in this trial began an initiative to broaden the scope and awareness of sepsis.
Current Literature and Evidence-Based Guidelines
The most recent guidelines for the management of septic shock from the Surviving Sepsis Campaign are summarized in Table 1. With its last revision, the Surviving Sepsis guidelines of 2012 has two main management foci—initiating treatment within the first 3 and the first 6 hours after recognition within the first 3 hours and those within the first 6 hours after sepsis recognition (Table 2). Within the first 3 hours, the treatment team should draw a serum lactate level; obtain cultures prior to the administration of antibiotics; initiate broad-spectrum antibiotics as early as possible; and administer 20 to 30 mL/kg of crystalloid fluids in patients with hypotension or a lactate level greater than 4 mmol/L. Within the first 6 hours, the clinician should administer intravenous (IV) vasopressors, preferentially norepinephrine, for persistent hypotension after a fluid challenge to maintain a mean arterial pressure >65 mm Hg; place a supra-diaphragmatic central venous catheter to measure a serum mixed venous O2 saturation (ScvO2) and central venous pressure (CVP); and measure serial serum lactate levels if they were initially elevated (lactate ≥4 mmol/L [36 mg/dL]).24,30,31 The targets for ScvO2 and CVP are ≥70%, and >8 mm Hg, respectively.
Summary
Sepsis is a prevalent ED presentation associated with mortality that can present in a complex fashion. Early recognition and management is essential and can be condensed into a few key recommendations. Becoming familiar with and incorporating these recommendations into daily practice will enable EPs to deliver quality care to every patient presenting with sepsis, and will also reduce mortality.
Blood Pressure Management for Select Neurological Emergencies
Patients with ischemic stroke, spontaneous intracerebral hemorrhage (ICH), and aneurysmal subarachnoid hemorrhage (SAH) often present with elevated blood pressures (BPs).34-36 In caring for these patients, EPs face the question of how, or even if, the patient’s BP should be managed. What are the appropriate BP targets for each of the aforementioned pathologies? Does aggressive BP management benefit or harm the patient?
Background
The relationship between hypertension and stroke is different for each stroke type. Retrospective data show a U-shaped relationship between BP and mortality in ischemic stroke, with the highest mortality observed at both extremes of the BP curve.34 Data also suggest increased mortality when ICH is accompanied by hypertension.35 Hypertension may also be associated with a higher risk of rebleeding in patients with SAH due to aneurysms.36 Because of the variable relationship between stroke and hypertension, therapeutic recommendations for each type of stroke can be confusing.
Current Literature and Evidence-Based Guidelines
Firm evidence to make therapeutic recommendations remains elusive. The recent American Heart Association (AHA) guidelines covering ischemic stroke, ICH, and SAH were published between late 2010 and early 2013, and several trials investigating the role of BP control in ischemic and hemorrhagic stroke have subsequently been published.37-41
When the Cochrane Collaboration updated its systematic review on vasoactive medications in stroke in 2014 to include recent evidence,42 it ultimately concluded that lowering BP does not improve mortality, neurological deterioration, or quality of life regardless of stroke type, and suggest that further investigations should be undertaken.9 However, the Cochrane authors noted that two recent trials showed a statistically significant association between improved quality of life and BP reduction within 6 hours of stroke onset.38-39 Although the data were compiled from just 2,835 patients of the 15,432 included in the entire Cochrane review, it suggested that interventions initiated in the ED may contribute to any potential beneficial outcomes from intensive BP control.
Ischemic Stroke
The China Antihypertensive Trial in Acute Ischemic Stroke (CATIS) investigated the initiation of BP-control measures within 48 hours of onset of ischemic stroke in approximately 4,000 patients and found no significant difference in death or disability between the group that received BP-control interventions and the group that did not.37 The Rapid Intervention With Glyceryl Trinitrate in Hypertensive Stroke Trial (RIGHT) included patients with both ischemic and hemorrhagic strokes. Though it studied only 41 patients, this trial suggests that early BP control is safe and may be associated with lower disability.38 These findings are bolstered by the more recent Efficacy of Nitric Oxide in Stroke (ENOS) trial showing a similar safety profile for BP control in both ischemic and hemorrhagic strokes, though the mean difference in systolic BP after therapy was a mere 7 mm Hg.40 The combined data from the RIGHT and ENOS trials offer little to clarify the question of appropriate BP control.
For now, the EP is left with the AHA/American Stroke Association (ASA) guideline’s recommendation “not to lower the BP during the initial 24 hours of acute ischemic stroke unless the BP is greater than 220/120 mm Hg.”34 The recommendation differs in cases when a patient receives thrombolytics and hemorrhagic transformation is a risk. There have been no new data to change the AHA/ASA’s recommendations for patients receiving thrombolytics. In such cases, the EP should ensure the patient’s BP is below 185/110 mm Hg prior to thrombolytic administration and below 180/105 mm Hg during therapy.34 A variety of agents is available to lower BP in this situation, and includes IV labetalol, nicardipine, esmolol, and others.
Intracerebral Hemorrhage
The recent literature on blood pressure control in ICH has also increased since the most recent AHA/ASA recommendations. The Second Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT-2 included over 2,800 patients randomized to intensive early therapy to reduce BP to less than 140 mm Hg or less than 180 mm Hg and found no significant difference in mortality or safety between the two groups, though intensive therapy was associated with less disability.39 The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial (ICH-ADAPT) trial further clarified the issue of safety during intensive BP control by showing no decrease in perihematomal cerebral blood flow in patients whose BP control was targeted to less than 150 mm Hg compared to those whose BP was less than 180 mm Hg, suggesting that aggressive BP reduction does not cause iatrogenic ischemic stroke.41
These combined data suggest that intensive BP management is safe for patients with ICH, providing reassurance for the AHA/ASA guideline recommendation that “in patients…with a systolic BP of 150 to 220 mm Hg, acute lowering of systolic BP to 140 mm Hg is probably safe.”35 Whether this improves patient outcomes remains unclear. Again, multiple agents are available for BP control, including IV labetalol or nicardipine, with no agent identified as superior in producing better patient outcomes. A continuous infusion is recommended if several boluses are ineffective in achieving and maintaining the target BP, as BP variability has been associated with poorer outcomes.43
Subarachnoid Hemorrhage
There are no large recent studies in the literature on antihypertensive therapy in SAH. The AHA/ASA guidelines updated in 2012 reflect the consensus that elevated BPs are associated with increased risk of aneurysmal rebleeding and thus poorer patient-oriented outcomes. The consensus remains to use a titratable agent to target a systolic BP less than 160 mm Hg until definitive neurosurgical therapy, such as aneurysmal coiling or clipping.36 Given the variability of the sodium nitroprusside dose-response relationship, IV labetalol, and nicardipine, are recommended agents for continuous control, though data showing differences in mortality and/or disability are lacking.36 Again, retrospective data suggest that BP variability negatively impacts mortality and disability, so consider early initiation of continuous infusions to achieve and maintain consistency in the chosen target.44
Summary
Pressors in the Management of Hypotension
Norepinephrine
Norepinephrine is one of the most commonly used agents for shock in the ED, with indications spanning multiple etiologies. It is an endogenous neurotransmitter that works predominantly on a1 receptors as well as exerting some modest effects on b1 and b2 receptors, for combined vasopressor and improved cardiac contractility effect.46-47 Norepinephrine is currently the recommended initial agent for sepsis-induced tissue hypoperfusion.24 However, a recent Cochrane systematic review and meta-analysis supports evidence of limited differences among various pressors.48 Several comparative randomized control trials show norepinephrine is as effective as other agents, but with fewer side effects.24,49 With the ease and familiarity of its use by most EPs, and a wide therapeutic index for targeted effect versus arrhythmias, norepinephrine is a reasonable choice as the initial pressor in managing a wide variety of shock syndromes.
Vasopressin
Another commonly used agent in the treatment of shock, vasopressin is an analogue of the antidiuretic hormone secreted from the posterior pituitary gland, exerting its CV effects primarily as a vasoconstrictor by increasing intracellular calcium.50 Vasopressin doses are 0.03 or 0.04 U/min IV without titration.24,50 Early studies of septic patients demonstrated a relative deficiency of serum vasopressin levels, leading clinicians to utilize it in the treatment of sepsis-induced shock. However, the Vasopressin and Septic Shock Trial (VASST) trial demonstrated that the addition of vasopressin to norepinephrine did not produce any improvements in morbidity or mortality compared with norepinephrine alone.51 Despite these findings, vasopressin is still commonly used as a secondary agent to correct continued hypotension. Vasopressin may be appropriate for patients who specifically require peripheral vasoconstriction in the setting of good cardiac output and volume status, ability to tolerate increases in afterload, or in patients at risk for dysrhythmias.
Dopamine
Dopamine had been previously recommended as the initial choice of pharmacologic support for the management of shock.24,52-54 Dopamine is an adrenergic agonist agent that works via a1 and b1 receptors as well as a precursor to the synthesis of norepinephrine and epinephrine.55 There are dose-dependent effects on various receptors from escalating amounts administered,55-56 but the literature does not support the concept of “renal-dose” dopamine.57-59 A study by DeBaker et al49 suggested no difference in efficacy between dopamine and norepinephrine, but demonstrated a greater tendency toward cardiac dysrhythmias with dopamine. For these reasons noted above, norepinephrine may be the initial agent for pharmaceutical support of shock, particularly in septic syndromes, with dopamine as a secondary or adjunct agent in patients at low risk for tachyarrhythmia or a relative bradycardia. 24, 56
Dobutamine
Dobutamine is another adrenergic agonist that is similar to dopamine but with a greater effect on inotropic cardiac contractility due to a preferential action at b1 receptors.60 It can potentially induce peripheral vasodilatation due to its effect on arterial b1 receptors. Given this balance, dobutamine is an agent that should be utilized for cardiogenic shock when increased contractility is needed. These effects are particularly useful in patients with “wet and cold” heart failure who have a low cardiac output and volume-overloaded status.61, 62 However, it may be necessary to add another agent to provide additional peripheral vasoconstriction should the use of dobutamine affect lead to excessive vasodilatation.
Epinephrine
One of the most powerful vasoactive agents, epinephrine has a high affinity for all b1, b2 and a1 receptors.63 These combined effects lead to increased cardiac output and improved BP by increasing cardiac contractility and peripheral vasoconstriction. The effect of epinephrine in limiting mast cell release of histamine makes it the preferred choice for the treatment of anaphylaxis.64 However, side effects of epinephrine include hypertension, tachydysrhythmias, tissue ischemia from vigorous vasoconstriction, and induced lactic acidosis.63
Phenylephrine
Phenylephrine is an a-adrenergic agonist that activates a1 receptors on arteriole smooth muscle, resulting in vasoconstriction.65 It is currently recommended only for hypotension related to procedural sedation.47 Phenylephrine is not recommended for treating patients with septic shock, except when there are concerns about tachydysrhythmias; persistent hypotension with a high cardiac output after treatment with other vasoconstrictor and inotropic drugs; or when a “pure” vasoconstrictor may be preferred.24,56,65
Summary
Although there are many other vasoactive agents that can be used, the selected agents discussed above represent those most commonly used in the ED. All demonstrate significant crossover effects and receptor activation, as well as impact on cardiac contractility and vasoconstriction. The suggested specific indications for each agent are based on current evidenced-based medicine, clinical guidelines, and theoretical benefits on clinical scenarios. But, as always, clinical decisions should be individualized for critically ill patients.
Conclusion
The resuscitation and initiation of care for critically ill patients must typically be immediately upon their arrival in the ED. While general guidelines or recommendations exist for commonly encountered pathologies, treatment should always be patient-centered, based on the needs and nuances unique to each patient in this vulnerable population. The initiation of mechanical ventilation, treatment of sepsis, management of hypertensive neurosurgical emergencies, and use of pressors in shock states are among the most critically important tasks an EP is called upon to perform. This review of current evidence-based guidelines and recommendations will help EPs provide the appropriate and unique care each patient requires.
Dr Brubaker is a resident in the department of emergency medicine at the University of Pittsburgh, Pennsylvania. Dr Yu is a fellow of adult critical care medicine – emergency medicine in the department of critical care medicine, University of Pittsburgh Medical Center, Pennsylvania. Dr Goodmanson is a resident in the department of emergency medicine at the University of Pittsburgh, Pennsylvania. Dr Schott is an assistant professor, department of emergency medicine and critical care medicine; assistant director of ultrasonography; director, critical care elective student rotation; and director, point of care ultrasound elective student rotation, at the University of Pittsburgh, Pennsylvania
Emergency medicine and critical care medicine share a responsibility for the care of acutely ill patients with life-threatening pathologies. The skills required of both emergency physicians (EPs) and critical care specialists to recognize, diagnose, and resuscitate such patients have resulted in many shared guidelines, recommendations, and publications. When critically ill patients enter the hospital through the ED, the care provided by EPs greatly impacts both the early and long-term outcomes. It is not uncommon for critically ill patients to spend several hours under the care of an EP while awaiting an available inpatient bed in the intensive care unit (ICU) or “step down” monitored unit.
This article provides a summary review of current guidelines, evidence-based medicine recommendations, and the results of recent trials involving ventilator management, treatment of sepsis, management of hypertension accompanying neurological emergencies, and the selection of pressors for the treatment of different shock states.
Ventilator Management
Mechanical ventilation is frequently undertaken in the ED for patients with respiratory failure—the origin of which is not always immediately clear. Data from the National Heart, Lung, and Blood Institute’s (NHLBI) acute respiratory distress syndrome (ARDS) clinical network (http://www.ardsnet.org) and other clinical trials have established the benefit of low tidal-volume, “lung-protective” ventilation in the patient with ARDS.1,2 Numerous studies have also shown the benefit of low-tidal-volume (TV), ventilation in patients without ARDS, and its use is now the standard of care for a large range of respiratory conditions causing compromise.3
The prompt initiation of lung-protective ventilation has a significant impact on reducing ICU mortality.4 A recent retrospective review of 3.5 million ED visits showed the median length of stay for patients started on mechanical ventilation in the ED to be greater than 3 hours.5 Such a length of time on mechanical ventilation in this setting can have significant effects on the course of illness; however, it is not clear whether mechanical ventilation performed in the ED typically conforms to evidence-based standards. In one study performed in an academic center, less than one-third of patients with sepsis and respiratory failure received low-volume ventilation in the ED.6 Another study suggested that emergency medicine residents may not receive as much dedicated education on the initial management of ventilators as needed—despite the potentially unforgiving physiologic process of positive-pressure mechanical ventilation.7
The fundamental principles required to safely manage most patients in respiratory failure are not difficult to master. There are several simple evidence-based ventilator strategies for managing patients with respiratory failure. The three primary principles of initiating and providing effective mechanical ventilation are: (1) avoiding traumatic ventilation; (2) maintaining normoxia; and (3) maintaining appropriate acid-base balance. Each of these principles can be achieved in a stepwise fashion.
Step I: Establishing Lung-Protective Settings on the Ventilator
Three central parameters must be selected at the initiation of assist-control mechanical ventilation: TV, respiratory rate (RR), and positive end-expiratory pressure (PEEP). These parameters have been extensively studied, and there is excellent evidence to guide the EP in choosing the correct settings.
Tidal Volume. Although the normal human lung can accommodate about 6 L of air, in cases of respiratory failure, the surface area available for gas exchange is significantly reduced due to a pathologic process undermining entire regions of the air-blood interface. Consequently, a person whose normal lungs are suddenly required to perform the life-sustaining gas exchanges in critical illness with the much smaller lung surface is at a significant disadvantage.
The widely accepted lung-protective volumes range from 6 to 8 mL/kg of predicted body weight (PBW), a height-based calculation.8 For example, in a 6-foot tall man, 6 mL/kg of PBW amounts to a TV of 466 mL; in a 5-foot tall woman, the same amount of PBW amounts to a TV of 273 mL. Volumes may be referenced using PBW tables from the NHLBI ARDS network or by employing the following equations:
Adult men: PBW (kg) = 50 + 2.3 (height [in] – 60)
Adult women: PBW (kg) = 45.5 + 2.3 (height [in] – 60).9
Respiratory Rate. The RR should be set somewhat higher than normal because the TV per breath has been slightly reduced, and also because sick patients in a catabolic state may have larger minute ventilations than they would when healthy. As previously described, since the TV is restricted, RR is the most mobile parameter in maintaining appropriate minute ventilation. Minute ventilation (MV) is the product of RR multiplied by TV (MV = RR x TV), and this should be calculated to approximate the patient’s own efforts, which are dependent upon the clinical circumstances. For example, patients whose bodies are trying to compensate for an acidosis will require much higher rates than those who are simply obtunded and intubated for airway protection. In other words, in order to remove carbon dioxide (CO2) in an acidemic patient, a higher RR rate may be required, whereas a lower rate may be selected to compensate for alkalemia while maintaining appropriate oxygen (O2) levels in both cases.10
Positive End-Expiratory Pressure. Previous recommendations for ventilation in respiratory failure called for large TVs (ie, 10 to 15 mL/kg), partly out of concern that smaller volumes would promote distal airway collapse, thereby increasing the amount of lung that received blood but not air, consequently worsening overall oxygenation.11 Although administering such large volumes has clearly proved harmful, the valid concern about distal airway collapse can be addressed in part by adjustments to PEEP, which acts to “stent” open airways after most of the tidal breath has left the airways.
Positive end-expiratory pressure, however, is not without risks.12 Blood from the rest of the body will encounter resistance returning to a thoracic cavity persistently inflated by positive pressure, and this decrease in preload may contribute to hypotension. Similarly, a weak right ventricle may struggle to push blood into the compressed pulmonary vasculature, increasing the cardiac workload and further compromising hemodynamics.13 In general, PEEP should be set as low as the maintenance of adequate oxygenation permits. The NHLBI ARDS guidelines provide a table on balancing PEEP and the fraction of inspired O2 (FiO2), as well as hypotension, in refractory hypoxemic patients—with the limitation on PEEP set by the patients’ pulmonary compliance (plateau pressures, discussed next).8
After making these selections, several parameters must be monitored closely. Those most relevant to lung-protective ventilation are the peak airway pressure and, most importantly, the plateau pressure. Numerous animal studies now demonstrate serious lung injury in both healthy and diseased lungs from high peak pressures (defined as a plateau pressure >30 cm water [H2O]).14,15 A high-pressure alarm sounding on the ventilator must be promptly addressed by an evaluation for easily reversible causes, such as tube obstruction, pneumothorax, breath stacking, pulmonary edema, or pleural effusions. A full discussion of the causes of elevated peak and plateau pressures is beyond the scope of this review, but if the plateau pressures remain consistently high, a reduction in TV may be necessary.
Step II: Maintaining Normoxia
As a severely hypoxic patient will rapidly decompensate with progression to death, a host of monitoring devices are used to alert the nurse or physician that O2 levels have fallen below the normal range. Strategies to manage refractory hypoxia in the ventilated patient are complex. For most patients, 100% FiO2 is initiated immediately after intubation to increase the safety of the procedure, but there is animal evidence that high O2 levels promote inflammatory responses, and human data suggest hyperoxia can be deleterious to long-term outcomes, particularly following cardiac arrest and stroke.16,17 A persistent O2 saturation of 100% on pulse oximetry or a supraphysiologic partial pressure of O2 (PaO2) on an arterial blood gas (defined as >200 mm Hg) may actually cause the patient more harm than good. Therefore, the fraction of inspired O2 should be titrated to maintain normoxia. The ARDS protocol, for example, targets an O2 saturation of 88% to 95% and a PaO2 of 55 to 80 mm Hg.8
Step III: Maintaining Acid-Base Balance
The basic principles of acid-base physiology should be familiar to EPs. When a patient is sedated and the airway secured, the primary means by which blood pH is maintained is now in the hands of the intubating physician. Patients with respiratory failure may have compensated for a preexisting derangement in their blood pH. If the preexisting condition is not recognized and ventilator settings are not maintained appropriately, they may be vulnerable to developing another derangement. Even on settings that allow the patient to breathe over a set rate, the sedation required to tolerate an endotracheal tube may cause significant respiratory depression, making it impossible for the patient to auto-regulate the respiratory component of acid-base homeostasis (ie, by hyperventilation).
As in the discussion of RR, TVs are “fixed” based on low-TV lung-protective ventilation. Therefore, changing the patient’s set RR is the easiest method to adjust the partial pressure of CO2 (PaCO2), and consequently address any respiratory acidosis. An increase in the RR will increase the patient’s minute ventilation, leading to a decrease in serum PaCO2 levels, whereas a decrease in the RR will have the converse effect. It is important to obtain an arterial blood-gas reading shortly after intubation and to continue to monitor the impact of any ventilator titrations on the patient’s acid-base status.
Studies of “permissive hypercapnia” in ARDS patients have shown that prioritizing lung-protective ventilator settings, even at the expense of a normal CO2, reduce mortality.1,18 Even in situations where it is not necessary to maintain hypercapnia for lung-protective settings, the hypercapnia appears to have beneficial effects.19-21 No upper limits on hypercapnia have been established, and even extreme levels have been associated with successful patient outcomes.22 However, a study by Hickling et al23 demonstrated that an initial trial of lung-protective ventilation demonstrated benefit from unbuffered hypercarbia and acidosis, reporting an average CO2 level of 66 and a pH of 7. These guidelines should be appropriate for use in the ED.
In summary, assuming control of a patient’s respiratory system—with its nuanced and responsive role in acid-base, oxygenation, and cardiopulmonary hemodynamics—is one of the most difficult situations routinely encountered by an EP. While the procedure itself may be life-saving, the next several hours can have significant impact on the patient’s long-term outcome.
Treating Sepsis and Surviving Sepsis: Recommendations Versus the ARISE/ProCESS Trials
Sepsis and Septic Shock
Sepsis is defined as an infection plus systemic inflammatory response syndrome (SIRS). Severe sepsis is sepsis plus sepsis-induced organ dysfunction or tissue hypoperfusion resulting in or caused by lactic acidosis, acute lung injury, altered mental status, or coagulation abnormalities. Septic shock refers to persistent sepsis-induced hypotension despite adequate fluid resuscitation.24 The ambiguity of these definitions may invariably lead to a practitioner’s underappreciation or misconception of the importance of sepsis.
Sepsis is one of the most common, yet least-recognized, entities. In the United States, it is estimated that 3 in 1,000 people annually are affected by sepsis, and every few seconds, a person dies of sepsis.25 Both numbers underestimate the effects on the elderly. Clinical manifestations of sepsis vary, and the condition may originate from both community-acquired and healthcare-associated sources.
In 2001, a landmark study demonstrated that early goal-directed therapy (EGDT) reduced mortality and improved patient outcomes in patients presenting to the ED in severe sepsis.26 The estimated 12% to 16% reduction in mortality reported in this trial began an initiative to broaden the scope and awareness of sepsis.
Current Literature and Evidence-Based Guidelines
The most recent guidelines for the management of septic shock from the Surviving Sepsis Campaign are summarized in Table 1. With its last revision, the Surviving Sepsis guidelines of 2012 has two main management foci—initiating treatment within the first 3 and the first 6 hours after recognition within the first 3 hours and those within the first 6 hours after sepsis recognition (Table 2). Within the first 3 hours, the treatment team should draw a serum lactate level; obtain cultures prior to the administration of antibiotics; initiate broad-spectrum antibiotics as early as possible; and administer 20 to 30 mL/kg of crystalloid fluids in patients with hypotension or a lactate level greater than 4 mmol/L. Within the first 6 hours, the clinician should administer intravenous (IV) vasopressors, preferentially norepinephrine, for persistent hypotension after a fluid challenge to maintain a mean arterial pressure >65 mm Hg; place a supra-diaphragmatic central venous catheter to measure a serum mixed venous O2 saturation (ScvO2) and central venous pressure (CVP); and measure serial serum lactate levels if they were initially elevated (lactate ≥4 mmol/L [36 mg/dL]).24,30,31 The targets for ScvO2 and CVP are ≥70%, and >8 mm Hg, respectively.
Summary
Sepsis is a prevalent ED presentation associated with mortality that can present in a complex fashion. Early recognition and management is essential and can be condensed into a few key recommendations. Becoming familiar with and incorporating these recommendations into daily practice will enable EPs to deliver quality care to every patient presenting with sepsis, and will also reduce mortality.
Blood Pressure Management for Select Neurological Emergencies
Patients with ischemic stroke, spontaneous intracerebral hemorrhage (ICH), and aneurysmal subarachnoid hemorrhage (SAH) often present with elevated blood pressures (BPs).34-36 In caring for these patients, EPs face the question of how, or even if, the patient’s BP should be managed. What are the appropriate BP targets for each of the aforementioned pathologies? Does aggressive BP management benefit or harm the patient?
Background
The relationship between hypertension and stroke is different for each stroke type. Retrospective data show a U-shaped relationship between BP and mortality in ischemic stroke, with the highest mortality observed at both extremes of the BP curve.34 Data also suggest increased mortality when ICH is accompanied by hypertension.35 Hypertension may also be associated with a higher risk of rebleeding in patients with SAH due to aneurysms.36 Because of the variable relationship between stroke and hypertension, therapeutic recommendations for each type of stroke can be confusing.
Current Literature and Evidence-Based Guidelines
Firm evidence to make therapeutic recommendations remains elusive. The recent American Heart Association (AHA) guidelines covering ischemic stroke, ICH, and SAH were published between late 2010 and early 2013, and several trials investigating the role of BP control in ischemic and hemorrhagic stroke have subsequently been published.37-41
When the Cochrane Collaboration updated its systematic review on vasoactive medications in stroke in 2014 to include recent evidence,42 it ultimately concluded that lowering BP does not improve mortality, neurological deterioration, or quality of life regardless of stroke type, and suggest that further investigations should be undertaken.9 However, the Cochrane authors noted that two recent trials showed a statistically significant association between improved quality of life and BP reduction within 6 hours of stroke onset.38-39 Although the data were compiled from just 2,835 patients of the 15,432 included in the entire Cochrane review, it suggested that interventions initiated in the ED may contribute to any potential beneficial outcomes from intensive BP control.
Ischemic Stroke
The China Antihypertensive Trial in Acute Ischemic Stroke (CATIS) investigated the initiation of BP-control measures within 48 hours of onset of ischemic stroke in approximately 4,000 patients and found no significant difference in death or disability between the group that received BP-control interventions and the group that did not.37 The Rapid Intervention With Glyceryl Trinitrate in Hypertensive Stroke Trial (RIGHT) included patients with both ischemic and hemorrhagic strokes. Though it studied only 41 patients, this trial suggests that early BP control is safe and may be associated with lower disability.38 These findings are bolstered by the more recent Efficacy of Nitric Oxide in Stroke (ENOS) trial showing a similar safety profile for BP control in both ischemic and hemorrhagic strokes, though the mean difference in systolic BP after therapy was a mere 7 mm Hg.40 The combined data from the RIGHT and ENOS trials offer little to clarify the question of appropriate BP control.
For now, the EP is left with the AHA/American Stroke Association (ASA) guideline’s recommendation “not to lower the BP during the initial 24 hours of acute ischemic stroke unless the BP is greater than 220/120 mm Hg.”34 The recommendation differs in cases when a patient receives thrombolytics and hemorrhagic transformation is a risk. There have been no new data to change the AHA/ASA’s recommendations for patients receiving thrombolytics. In such cases, the EP should ensure the patient’s BP is below 185/110 mm Hg prior to thrombolytic administration and below 180/105 mm Hg during therapy.34 A variety of agents is available to lower BP in this situation, and includes IV labetalol, nicardipine, esmolol, and others.
Intracerebral Hemorrhage
The recent literature on blood pressure control in ICH has also increased since the most recent AHA/ASA recommendations. The Second Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT-2 included over 2,800 patients randomized to intensive early therapy to reduce BP to less than 140 mm Hg or less than 180 mm Hg and found no significant difference in mortality or safety between the two groups, though intensive therapy was associated with less disability.39 The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial (ICH-ADAPT) trial further clarified the issue of safety during intensive BP control by showing no decrease in perihematomal cerebral blood flow in patients whose BP control was targeted to less than 150 mm Hg compared to those whose BP was less than 180 mm Hg, suggesting that aggressive BP reduction does not cause iatrogenic ischemic stroke.41
These combined data suggest that intensive BP management is safe for patients with ICH, providing reassurance for the AHA/ASA guideline recommendation that “in patients…with a systolic BP of 150 to 220 mm Hg, acute lowering of systolic BP to 140 mm Hg is probably safe.”35 Whether this improves patient outcomes remains unclear. Again, multiple agents are available for BP control, including IV labetalol or nicardipine, with no agent identified as superior in producing better patient outcomes. A continuous infusion is recommended if several boluses are ineffective in achieving and maintaining the target BP, as BP variability has been associated with poorer outcomes.43
Subarachnoid Hemorrhage
There are no large recent studies in the literature on antihypertensive therapy in SAH. The AHA/ASA guidelines updated in 2012 reflect the consensus that elevated BPs are associated with increased risk of aneurysmal rebleeding and thus poorer patient-oriented outcomes. The consensus remains to use a titratable agent to target a systolic BP less than 160 mm Hg until definitive neurosurgical therapy, such as aneurysmal coiling or clipping.36 Given the variability of the sodium nitroprusside dose-response relationship, IV labetalol, and nicardipine, are recommended agents for continuous control, though data showing differences in mortality and/or disability are lacking.36 Again, retrospective data suggest that BP variability negatively impacts mortality and disability, so consider early initiation of continuous infusions to achieve and maintain consistency in the chosen target.44
Summary
Pressors in the Management of Hypotension
Norepinephrine
Norepinephrine is one of the most commonly used agents for shock in the ED, with indications spanning multiple etiologies. It is an endogenous neurotransmitter that works predominantly on a1 receptors as well as exerting some modest effects on b1 and b2 receptors, for combined vasopressor and improved cardiac contractility effect.46-47 Norepinephrine is currently the recommended initial agent for sepsis-induced tissue hypoperfusion.24 However, a recent Cochrane systematic review and meta-analysis supports evidence of limited differences among various pressors.48 Several comparative randomized control trials show norepinephrine is as effective as other agents, but with fewer side effects.24,49 With the ease and familiarity of its use by most EPs, and a wide therapeutic index for targeted effect versus arrhythmias, norepinephrine is a reasonable choice as the initial pressor in managing a wide variety of shock syndromes.
Vasopressin
Another commonly used agent in the treatment of shock, vasopressin is an analogue of the antidiuretic hormone secreted from the posterior pituitary gland, exerting its CV effects primarily as a vasoconstrictor by increasing intracellular calcium.50 Vasopressin doses are 0.03 or 0.04 U/min IV without titration.24,50 Early studies of septic patients demonstrated a relative deficiency of serum vasopressin levels, leading clinicians to utilize it in the treatment of sepsis-induced shock. However, the Vasopressin and Septic Shock Trial (VASST) trial demonstrated that the addition of vasopressin to norepinephrine did not produce any improvements in morbidity or mortality compared with norepinephrine alone.51 Despite these findings, vasopressin is still commonly used as a secondary agent to correct continued hypotension. Vasopressin may be appropriate for patients who specifically require peripheral vasoconstriction in the setting of good cardiac output and volume status, ability to tolerate increases in afterload, or in patients at risk for dysrhythmias.
Dopamine
Dopamine had been previously recommended as the initial choice of pharmacologic support for the management of shock.24,52-54 Dopamine is an adrenergic agonist agent that works via a1 and b1 receptors as well as a precursor to the synthesis of norepinephrine and epinephrine.55 There are dose-dependent effects on various receptors from escalating amounts administered,55-56 but the literature does not support the concept of “renal-dose” dopamine.57-59 A study by DeBaker et al49 suggested no difference in efficacy between dopamine and norepinephrine, but demonstrated a greater tendency toward cardiac dysrhythmias with dopamine. For these reasons noted above, norepinephrine may be the initial agent for pharmaceutical support of shock, particularly in septic syndromes, with dopamine as a secondary or adjunct agent in patients at low risk for tachyarrhythmia or a relative bradycardia. 24, 56
Dobutamine
Dobutamine is another adrenergic agonist that is similar to dopamine but with a greater effect on inotropic cardiac contractility due to a preferential action at b1 receptors.60 It can potentially induce peripheral vasodilatation due to its effect on arterial b1 receptors. Given this balance, dobutamine is an agent that should be utilized for cardiogenic shock when increased contractility is needed. These effects are particularly useful in patients with “wet and cold” heart failure who have a low cardiac output and volume-overloaded status.61, 62 However, it may be necessary to add another agent to provide additional peripheral vasoconstriction should the use of dobutamine affect lead to excessive vasodilatation.
Epinephrine
One of the most powerful vasoactive agents, epinephrine has a high affinity for all b1, b2 and a1 receptors.63 These combined effects lead to increased cardiac output and improved BP by increasing cardiac contractility and peripheral vasoconstriction. The effect of epinephrine in limiting mast cell release of histamine makes it the preferred choice for the treatment of anaphylaxis.64 However, side effects of epinephrine include hypertension, tachydysrhythmias, tissue ischemia from vigorous vasoconstriction, and induced lactic acidosis.63
Phenylephrine
Phenylephrine is an a-adrenergic agonist that activates a1 receptors on arteriole smooth muscle, resulting in vasoconstriction.65 It is currently recommended only for hypotension related to procedural sedation.47 Phenylephrine is not recommended for treating patients with septic shock, except when there are concerns about tachydysrhythmias; persistent hypotension with a high cardiac output after treatment with other vasoconstrictor and inotropic drugs; or when a “pure” vasoconstrictor may be preferred.24,56,65
Summary
Although there are many other vasoactive agents that can be used, the selected agents discussed above represent those most commonly used in the ED. All demonstrate significant crossover effects and receptor activation, as well as impact on cardiac contractility and vasoconstriction. The suggested specific indications for each agent are based on current evidenced-based medicine, clinical guidelines, and theoretical benefits on clinical scenarios. But, as always, clinical decisions should be individualized for critically ill patients.
Conclusion
The resuscitation and initiation of care for critically ill patients must typically be immediately upon their arrival in the ED. While general guidelines or recommendations exist for commonly encountered pathologies, treatment should always be patient-centered, based on the needs and nuances unique to each patient in this vulnerable population. The initiation of mechanical ventilation, treatment of sepsis, management of hypertensive neurosurgical emergencies, and use of pressors in shock states are among the most critically important tasks an EP is called upon to perform. This review of current evidence-based guidelines and recommendations will help EPs provide the appropriate and unique care each patient requires.
Dr Brubaker is a resident in the department of emergency medicine at the University of Pittsburgh, Pennsylvania. Dr Yu is a fellow of adult critical care medicine – emergency medicine in the department of critical care medicine, University of Pittsburgh Medical Center, Pennsylvania. Dr Goodmanson is a resident in the department of emergency medicine at the University of Pittsburgh, Pennsylvania. Dr Schott is an assistant professor, department of emergency medicine and critical care medicine; assistant director of ultrasonography; director, critical care elective student rotation; and director, point of care ultrasound elective student rotation, at the University of Pittsburgh, Pennsylvania
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308.
- Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32(9):1817-1824.
- Brower RG, Rubenfeld GD. Lung-protective ventilation strategies in acute lung injury. Crit Care Med. 2003;31(4):S312-S316.
- Needham DM, Yang T, Dinglas VD. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. A prospective cohort study. Am J Respir Crit Care Med. 2015;191(2):177-185.
- Easter BD, Fischer C, Fisher J. The use of mechanical ventilation in the ED. Am J Emerg Med. 2012;30(7):1183-1188.
- Fuller BM, Mohr NM, Dettmer M, et al. Mechanical ventilation and acute lung injury in emergency department patients with severe sepsis and septic shock: an observational study. Acad Emerg Med. 2013;20(7):659-669.
- Wilcox SR, Seigel TA, Strout TD, et al. Emergency medicine residents’ knowledge of mechanical ventilation. J Emerg Med. 2014. doi:10.1016/j.jemermed.2014.09.059. [Epub ahead of print]
- National Institutes of Health’s National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Clinical Network Mechanical Ventilator Protocol Summary. http://www.ardsnet.org/system/files/Ventilator%20Protocol%20Card.pdf. Accessed March 5, 2015.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (NHLBI ARDS) Network. Predicted body weight calculator. http://www.ardsnet.org/node/77460. Accessed March 5, 2015.
- Boron WF. Acid-base physiology. In: Boron WF, Boulpaep EL, eds. Medical Physiology: A Cellular And Molecular Approach. Philadelphia, PA: Saunders/Elsevier; 2009:647-649.
- Orebaugh, SL. Initiation of mechanical ventilation in the emergency department. Am J Emerg Med. 1996;14(1):59-69.
- Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149(5):1327-1334.
- Marino PL, Sutin KM. Principles of mechanical ventilation. In: Marino PL, ed. The ICU Book. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:457-472.
- Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection with positive end-expiratory pressures. Am Rev Resp Dis. 1974;110(5):556-565.
- Parker JC, Hernandez LA, Peevy KJ. Mechanisms of ventilator-induced lung injury. Crit Care Med. 1993;21(1):131-143.
- Bhandari V. Molecular mechanisms of hyperoxia-induced acute lung injury. Front Biosci. 2008;13:6653-6661.
- Kilgannon JH, Jones AE, Shapiro NI, et al; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165-2171.
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347-354.
- Laffey JG, Tanaka M, Engelberts D, et al. Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. Am J Respir Crit Care Med. 2000;162(6):2287-2294.
- Costello J, Higgins B, Contreras M, et al. Hypercapnic acidosis attenuates shock and lung injury in early and prolonged systemic sepsis. Crit Care Med. 2009;37(8):2412-2420.
- Ijland MM, Heunks LM, van der Hoeven JG. Bench-to-bedside review: hypercapnic acidosis in lung injury—from ‘permissive’ to ‘therapeutic.’ Crit Care. 2010;14(6):237.
- Garg SK. Permissive hypercapnia: Is there any upper limit? Indian J Crit Care Med. 2014;18(9):612-614.
- Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med. 1994;22(10):1568-1578.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup Surviving Sepsis Campaign. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228.
- Peake SL, Delaney A, Bailey M, et al; ARISE Investigators; ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496-1506.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;8;345(19):1368-1377.
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(21):2063.
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303-1310.
- Rivers EP, Ahrens T. Improving outcomes for severe sepsis and septic shock: tools for early identification of at-risk patients and treatment protocol implementation. Crit Care Clin. 2008;24(3 Suppl):S1-S47.
- Nguyen HB, Rivers EP, Abrahamian FM, et al; Emergency Department Sepsis Education Program and Strategies to Improve Survival (ED-SEPSIS) Working Group. Severe sepsis and septic shock: review of the literature and emergency department management guidelines. Ann Emerg Med. 2006;48(1):28-54.
- Jones AE, Puskarich MA. The Surviving Sepsis Campaign guidelines 2012: update for emergency physicians. Ann Emerg Med. 2014;63(1):35-47.
- Yealy DM, Kellum JA, Huang DT, et al.;ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-1693.
- Huang DT, Angus DC, Barnato A, et al; ProCESS/ARISE/ProMISe Methodology Writing Committee. Harmonizing international trials of early goal-directed resuscitation for severe sepsis and septic shock: methodology of ProCESS, ARISE, and ProMISe. Intensive Care Med. 2013;39(10):1760-1775.
- Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
- Morgenstern LB, Hemphill JC 3rd, Anderson C, et al; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108-2129.
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43(6):1711-1737.
- He J, Zhang Y, Xu T, et al; The China Antihypertensive Trial in Acute Ischemic Stroke (CATIS) Investigators. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA. 2014;311(5):479-489.
- Ankolekar S, Fuller M, Cross I, et al. Feasibility of an ambulance-based stroke trial, and safety of glyceryl trinitrate in ultra-acute stroke: The Rapid Intervention With Glyceryl Trinitrate in Hypertensive Stroke Trial (RIGHT, ISRCTN66434824). Stroke. 2013;44(11):3120-3128.
- Anderson CS, Heeley E, Huang Y, et al; The Second Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT-2) Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368(25):2355-2365.
- Bath PM, Woodhouse L, Scutt P, et al; ENOS Trial Investigators. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet. 2014;385(9968):617-628
- Butcher KS, Jeerakathil T, Hill M, et al; ICH ADAPT Investigators. The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial. Stroke. 2013;44(3):620-626.
- Bath PM, Krishnan K. Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database Syst Rev. 2014;10:CD000039.
- Tanaka E, Koga M, Kobayashi J, et al. Blood pressure variability on antihypertensive therapy in acute intracerebral hemorrhage: the Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-intracerebral hemorrhage study. Stroke. 2014;45(8):2275-2279.
- Beseoglu K, Unfrau K, Steiger HJ, Hänggi D. Influence of blood pressure variability on short-term outcome in patients with subarachnoid hemorrhage. Cent Eur Neurosurg. 2010;71(2):69-74.
- Hinshaw LB, Cox BG, eds. The fundamental mechanisms of shock. Proceedings of a Symposium Held in Oklahoma City, Oklahoma, October 1-2, 1971. In: Advances in Experimental Medicine and Biology, Vol 23. New York, NY: Plenum Press; 1972.
- Norepinephrine. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=norepinephrine&x=0&y=0. March 5, 2015.
- Overgaard CB, Dzavik V. Inotropes and vasopressors: review of physiology and clinical use in cardiovascular disease. Circulation. 2008;118(10):1047-1056.
- Havel C, Arrich J, Losert H, et al. Vasopressors for hypotensive shock. Cochrane Database Syst Rev. 2011;(5):CD003709.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010;362(9):779-789.
- Vasopressin. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=vasopressin&x=0&y=0. March 5, 2015.
- Russell JA, Walley KR, Singer J, et al; VASST Investigators. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877-887.
- Martin C, Papazian L, Perrin G, Saux P, Gouin F. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest. 1993;103(6):1826-1831.
- De Backer D, Creteur J, Silva E, Vincent JL. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: which is best? Crit Care Med. 2003;31(6)1659-1667.
- Day NP, Phu NH, Bethell DP, et al. The effects of dopamine and adrenaline infusions on acid-base balance and systemic haemodynamics in severe infection. Lancet. 1996;348(9022):219-223.
- Dopamine. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=dopamine&x=0&y=0. March 5, 2015.
- Manaker S. Use of vasopressors and inotropes. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/use-of-vasopressors-and-inotropes?source=search_result&search=Use+of+vasopressors+and+isotopes&selectedTitle=1%7E150. Accessed March 5, 2015.
- Lauschke A, Teichgräber UK, Frei U, Eckardt KU. ‘Low-dose’ dopamine worsens renal perfusion in patients with acute renal failure. Kidney Int. 2006;69(9):1669-1674.
- Bellomo R, Chapman M, Finfer S, Hickling K, Myburgh J. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356(9248):2139-2143.
- Kellum JA, M Decker J. Use of dopamine in acute renal failure: a meta-analysis. Crit Care Med. 2001;29(8):1526-1531.
- Dobutamine. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=Dobutamine&x=0&y=0. Accessed March 5, 2015.
- Nohria A, Mielniczuk LM, Stevenson LW. Evaluation and monitoring of patients with acute heart failure syndromes. Am J Cardiol. 2005;96(6A):32G-40G.
- Joseph SM, Cedars AM, Ewald GA, Geltman EM, Mann DL. Acute decompensated heart failure: contemporary medical management. Tex Heart Inst J. 2009;36(6):510-520.
- Epinephrine. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=epinephrine. Accessed March 5, 2015.
- Vadas P, Perelman B. Effect of epinephrine on platelet-activating factor-stimulated human vascular smooth muscle cells. J Allergy Clin Immunol. 2012;129(5):1329-1333.
- Phenylephrine. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=65.%09Phenylephrine&x=10&y=10. Accessed March 5, 2015.
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308.
- Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32(9):1817-1824.
- Brower RG, Rubenfeld GD. Lung-protective ventilation strategies in acute lung injury. Crit Care Med. 2003;31(4):S312-S316.
- Needham DM, Yang T, Dinglas VD. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. A prospective cohort study. Am J Respir Crit Care Med. 2015;191(2):177-185.
- Easter BD, Fischer C, Fisher J. The use of mechanical ventilation in the ED. Am J Emerg Med. 2012;30(7):1183-1188.
- Fuller BM, Mohr NM, Dettmer M, et al. Mechanical ventilation and acute lung injury in emergency department patients with severe sepsis and septic shock: an observational study. Acad Emerg Med. 2013;20(7):659-669.
- Wilcox SR, Seigel TA, Strout TD, et al. Emergency medicine residents’ knowledge of mechanical ventilation. J Emerg Med. 2014. doi:10.1016/j.jemermed.2014.09.059. [Epub ahead of print]
- National Institutes of Health’s National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Clinical Network Mechanical Ventilator Protocol Summary. http://www.ardsnet.org/system/files/Ventilator%20Protocol%20Card.pdf. Accessed March 5, 2015.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (NHLBI ARDS) Network. Predicted body weight calculator. http://www.ardsnet.org/node/77460. Accessed March 5, 2015.
- Boron WF. Acid-base physiology. In: Boron WF, Boulpaep EL, eds. Medical Physiology: A Cellular And Molecular Approach. Philadelphia, PA: Saunders/Elsevier; 2009:647-649.
- Orebaugh, SL. Initiation of mechanical ventilation in the emergency department. Am J Emerg Med. 1996;14(1):59-69.
- Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149(5):1327-1334.
- Marino PL, Sutin KM. Principles of mechanical ventilation. In: Marino PL, ed. The ICU Book. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:457-472.
- Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection with positive end-expiratory pressures. Am Rev Resp Dis. 1974;110(5):556-565.
- Parker JC, Hernandez LA, Peevy KJ. Mechanisms of ventilator-induced lung injury. Crit Care Med. 1993;21(1):131-143.
- Bhandari V. Molecular mechanisms of hyperoxia-induced acute lung injury. Front Biosci. 2008;13:6653-6661.
- Kilgannon JH, Jones AE, Shapiro NI, et al; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165-2171.
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347-354.
- Laffey JG, Tanaka M, Engelberts D, et al. Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. Am J Respir Crit Care Med. 2000;162(6):2287-2294.
- Costello J, Higgins B, Contreras M, et al. Hypercapnic acidosis attenuates shock and lung injury in early and prolonged systemic sepsis. Crit Care Med. 2009;37(8):2412-2420.
- Ijland MM, Heunks LM, van der Hoeven JG. Bench-to-bedside review: hypercapnic acidosis in lung injury—from ‘permissive’ to ‘therapeutic.’ Crit Care. 2010;14(6):237.
- Garg SK. Permissive hypercapnia: Is there any upper limit? Indian J Crit Care Med. 2014;18(9):612-614.
- Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med. 1994;22(10):1568-1578.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup Surviving Sepsis Campaign. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228.
- Peake SL, Delaney A, Bailey M, et al; ARISE Investigators; ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496-1506.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;8;345(19):1368-1377.
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(21):2063.
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303-1310.
- Rivers EP, Ahrens T. Improving outcomes for severe sepsis and septic shock: tools for early identification of at-risk patients and treatment protocol implementation. Crit Care Clin. 2008;24(3 Suppl):S1-S47.
- Nguyen HB, Rivers EP, Abrahamian FM, et al; Emergency Department Sepsis Education Program and Strategies to Improve Survival (ED-SEPSIS) Working Group. Severe sepsis and septic shock: review of the literature and emergency department management guidelines. Ann Emerg Med. 2006;48(1):28-54.
- Jones AE, Puskarich MA. The Surviving Sepsis Campaign guidelines 2012: update for emergency physicians. Ann Emerg Med. 2014;63(1):35-47.
- Yealy DM, Kellum JA, Huang DT, et al.;ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-1693.
- Huang DT, Angus DC, Barnato A, et al; ProCESS/ARISE/ProMISe Methodology Writing Committee. Harmonizing international trials of early goal-directed resuscitation for severe sepsis and septic shock: methodology of ProCESS, ARISE, and ProMISe. Intensive Care Med. 2013;39(10):1760-1775.
- Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
- Morgenstern LB, Hemphill JC 3rd, Anderson C, et al; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108-2129.
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43(6):1711-1737.
- He J, Zhang Y, Xu T, et al; The China Antihypertensive Trial in Acute Ischemic Stroke (CATIS) Investigators. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA. 2014;311(5):479-489.
- Ankolekar S, Fuller M, Cross I, et al. Feasibility of an ambulance-based stroke trial, and safety of glyceryl trinitrate in ultra-acute stroke: The Rapid Intervention With Glyceryl Trinitrate in Hypertensive Stroke Trial (RIGHT, ISRCTN66434824). Stroke. 2013;44(11):3120-3128.
- Anderson CS, Heeley E, Huang Y, et al; The Second Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT-2) Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368(25):2355-2365.
- Bath PM, Woodhouse L, Scutt P, et al; ENOS Trial Investigators. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet. 2014;385(9968):617-628
- Butcher KS, Jeerakathil T, Hill M, et al; ICH ADAPT Investigators. The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial. Stroke. 2013;44(3):620-626.
- Bath PM, Krishnan K. Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database Syst Rev. 2014;10:CD000039.
- Tanaka E, Koga M, Kobayashi J, et al. Blood pressure variability on antihypertensive therapy in acute intracerebral hemorrhage: the Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-intracerebral hemorrhage study. Stroke. 2014;45(8):2275-2279.
- Beseoglu K, Unfrau K, Steiger HJ, Hänggi D. Influence of blood pressure variability on short-term outcome in patients with subarachnoid hemorrhage. Cent Eur Neurosurg. 2010;71(2):69-74.
- Hinshaw LB, Cox BG, eds. The fundamental mechanisms of shock. Proceedings of a Symposium Held in Oklahoma City, Oklahoma, October 1-2, 1971. In: Advances in Experimental Medicine and Biology, Vol 23. New York, NY: Plenum Press; 1972.
- Norepinephrine. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=norepinephrine&x=0&y=0. March 5, 2015.
- Overgaard CB, Dzavik V. Inotropes and vasopressors: review of physiology and clinical use in cardiovascular disease. Circulation. 2008;118(10):1047-1056.
- Havel C, Arrich J, Losert H, et al. Vasopressors for hypotensive shock. Cochrane Database Syst Rev. 2011;(5):CD003709.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010;362(9):779-789.
- Vasopressin. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=vasopressin&x=0&y=0. March 5, 2015.
- Russell JA, Walley KR, Singer J, et al; VASST Investigators. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877-887.
- Martin C, Papazian L, Perrin G, Saux P, Gouin F. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest. 1993;103(6):1826-1831.
- De Backer D, Creteur J, Silva E, Vincent JL. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: which is best? Crit Care Med. 2003;31(6)1659-1667.
- Day NP, Phu NH, Bethell DP, et al. The effects of dopamine and adrenaline infusions on acid-base balance and systemic haemodynamics in severe infection. Lancet. 1996;348(9022):219-223.
- Dopamine. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=dopamine&x=0&y=0. March 5, 2015.
- Manaker S. Use of vasopressors and inotropes. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/use-of-vasopressors-and-inotropes?source=search_result&search=Use+of+vasopressors+and+isotopes&selectedTitle=1%7E150. Accessed March 5, 2015.
- Lauschke A, Teichgräber UK, Frei U, Eckardt KU. ‘Low-dose’ dopamine worsens renal perfusion in patients with acute renal failure. Kidney Int. 2006;69(9):1669-1674.
- Bellomo R, Chapman M, Finfer S, Hickling K, Myburgh J. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356(9248):2139-2143.
- Kellum JA, M Decker J. Use of dopamine in acute renal failure: a meta-analysis. Crit Care Med. 2001;29(8):1526-1531.
- Dobutamine. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=Dobutamine&x=0&y=0. Accessed March 5, 2015.
- Nohria A, Mielniczuk LM, Stevenson LW. Evaluation and monitoring of patients with acute heart failure syndromes. Am J Cardiol. 2005;96(6A):32G-40G.
- Joseph SM, Cedars AM, Ewald GA, Geltman EM, Mann DL. Acute decompensated heart failure: contemporary medical management. Tex Heart Inst J. 2009;36(6):510-520.
- Epinephrine. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=epinephrine. Accessed March 5, 2015.
- Vadas P, Perelman B. Effect of epinephrine on platelet-activating factor-stimulated human vascular smooth muscle cells. J Allergy Clin Immunol. 2012;129(5):1329-1333.
- Phenylephrine. UpToDate Web site. Post TW, ed. UpToDate, Waltham, MA. http://www.uptodate.com/contents/search?search=65.%09Phenylephrine&x=10&y=10. Accessed March 5, 2015.