User login
Utility of Blood Cultures in Pneumonia
Blood cultures (BCs) have long been a mainstay of the diagnostic evaluation of patients hospitalized with community‐acquired pneumonia (CAP). They have been strongly recommended by professional societies13 and are often expected by admitting physicians. A large retrospective study of Medicare patients with pneumonia found that obtaining BCs is associated with lower mortality.4 In 2002, when the National Hospital Quality Measures (NHQM) were introduced, BCs were included as a quality measure for pneumonia.5, 6
However, there is uncertainty about the actual utility of BCs in CAP. In large studies they are true‐positive in only 7 to 11% of cases and false‐positive in 5%,2, 7 and whether they affect clinical management has been strongly questioned.810 Their impact may be limited by slow results, low frequency of bacterial resistance to the empiric antibiotic regimen, and reluctance of physicians to narrow antibiotic coverage.9, 11 Recent updates to professional society guidelines no longer recommend BCs in all admitted CAP patients.12
To evaluate the clinical utility of BCs and the appropriateness of pnemonia quality measures based on BCs, we performed a systematic review of the literature to determine the effect of BCs on the management of adults with CAP requiring hospitalization.
PATIENTS AND METHODS
Data Sources and Searches
We searched the English‐language literature via MEDLINE (1966 through September 2007), MEDLINE‐In Process, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, and ACP Journal Club. Within each of these databases we used keywords and exploded Medical Subject Headings (MeSH) to produce the following search strategy: blood culture(s) (keyword), bacteriological techniques (MeSH), blood [microbiology] (MeSH), bacteremia [microbiology or drug therapy] (MeSH), or diagnostic tests, routine (MeSH) combined with pneumonia (keyword), pneumonia (MeSH), lower respiratory tract infection(s) (keyword), or community‐acquired infections (MeSH). To maximize capture of BC or bacteremia studies with subgroups of CAP patients we added the following search strategy: explode microbiological techniques [utilization] (MeSH), explode blood specimen collection [utilization] (MeSH), or focus bacteremia [drug therapy] (MeSH). We reviewed the reference lists of all included studies as well as those of important background articles. Finally, we asked experts to evaluate the completeness of our list.
Study Selection
We included studies in which: (1) subjects were adults hospitalized with CAP; (2) BCs were obtained at or near hospital admission; and (3) the effects of BCs on management (change in antibiotic therapy or other effects such as duration of parenteral therapy, length of hospitalization, or level of care) were reported. The first 2 requirements could be satisfied by a subgroup.
From retrieved citations, relevant abstracts were reviewed, and studies with any potential to meet inclusion criteria were chosen for full‐text review. Two authors (N.A., R.S.) independently analyzed each full‐text article to determine inclusion for data analysis. A third author (J.T.) analyzed all included and narrowly excluded articles to confirm the final list of included studies. Disagreements were resolved by discussion.
Data Extraction
For the included studies, 2 authors (N.A., K.A.) independently abstracted the following data using a standardized collection instrument: study design and setting, inclusion and exclusion criteria, number of hospitalized CAP patients in whom BCs were obtained, empiric antibiotic regimens, number of true‐positive and false‐positive BCs, bacteria isolated in true‐positive BCs, BC‐directed antibiotic narrowing, BC‐directed antibiotic broadening ultimately associated with a resistant organism, and any other management effects reported. Narrowing refers to coverage of fewer organisms, while broadening refers to coverage of a larger or different spectrum of organisms.
If a study included patients not meeting our selection criteria, our analysis was limited to the subset of patients meeting criteria. We also analyzed each study to determine whether a subgroup of severely ill patients was reported separately and whether such a group benefited from BCs. The 2 authors independently repeated all data abstraction to confirm accuracy. We attempted to contact authors for clarification when needed.
Data Synthesis
Data were synthesized by compilation of characteristic summary tables. In the primary analysis, the proportion of positive BCs (both true and false) and the frequency of BC‐directed changes in antimicrobial therapy (narrowing, or broadening ultimately associated with a resistant organism) were determined and reported for each study and then described as an aggregate range. This compilation required studies to provide a particular denominatorthe number of patients in whom BCs were performed. If a study did not do so, it was described separately in the secondary analysis, where we also assessed the cost of BCs as well as the impact of BCs in critically ill patients and on outcomes other than antibiotic change. Heterogeneity of subject inclusion and exclusion criteria and empiric antibiotic use were summarized qualitatively. Two authors (N.A., R.S.) assessed each study's quality.
DATA SYNTHESIS
Search Results
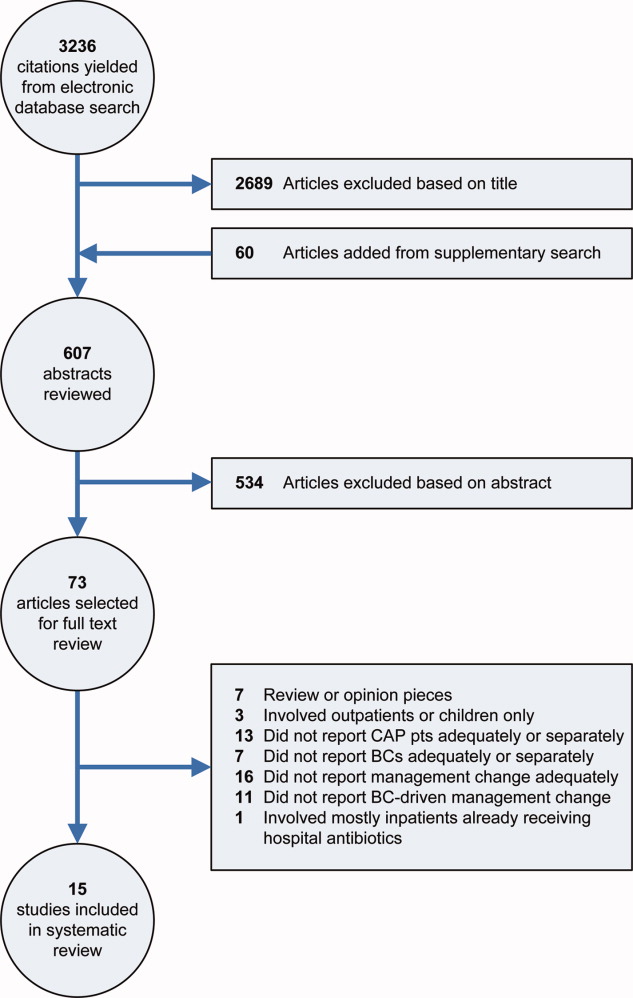
Our electronic database search yielded 3236 citations. From this list and the supplementary search of references, we reviewed 607 abstracts; of these, we selected 73 articles for full‐text review, and 15 were included in the final analysis (Figure 1). One study was narrowly excluded because it largely included CAP patients that had already been admitted to the hospital and failed an empiric antibiotic trial before BCs were obtained.13
Study Characteristics
Fifteen studies with a total of 3898 patients evaluated BC‐directed management changes in adults admitted with CAP.11, 1427 However, 2 of these, involving only patients with bacteremic pneumococcal CAP, by design could not report the number of patients that had BCs done; thus they were not included in the primary analysis.16, 25
The 13 studies amenable to the primary analysis (Table 1) all had an observational cohort design; 6 were prospective11, 18, 20, 24, 26, 27 and 7 were retrospective.14, 15, 17, 19, 2123 Sample size varied from 52 to 760 patients. Settings included university and community hospitals in the U.S. and 4 other countries, with patient enrollment spanning the years 19882003 (publication dates 19912007).
| Study Author, Year, Design, Setting | Inclusion Criteria | Exclusion Criteria | CAP Patients with BCs, n*; True‐Positive BCs, n (%); False‐Positive BCs, n (%) | BCs Directed Antibiotic Narrowing, n (%) | BCs Directed Antibiotic Broadening and Organism was Resistant, n (%) | Comments |
|---|---|---|---|---|---|---|
| ||||||
| Benenson et al.,14 2007; retrospective, U.S. suburban | ED ICD‐9 dx and discharge dx of PNA | None | n = 684; 23 (3.4); 54 (7.9) | 3 (0.4) | 0 (0) | 11% of pts with ED dx of PNA not eligible due to different dx at discharge; 25% from nursing homes, 18% recently hospitalized, 14% immunocompromised; Abxs narrowed in 3/21 eligible pts |
| Ramanujamand Rathlev,22 2006; retrospective; U.S. urban | ED, ICD‐9, and discharge dx of PNA, and ED BCs before abxs | IC, active cancer, chronic renal failure, hospitalized last 1 week, nursing home resident, aspiration | n = 289; 13 (4.5%); 13 (4.5%) | 1 (0.3%) | 0 (0%) | 532 pts screened; 3% not eligible due to different dx at discharge; of eligible pts, 9% excluded due to HCAP and 31% excluded due to other risk factors; Abxs were narrowed in 1/10 eligible pts; Cost: $8,000 for the 1 pt with abx change |
| Mountain et al.,21 2006; retrospective, Australian suburban | All pts who had BCs done in the ED during a 2‐month period (PNA pts were a subgroup) | None | n = 52; Not reported; Not reported | 1 (1.9) | 0 (0) | 52/218 study pts had clinical PNA. Overall BCs true‐positive in 6.4%, false‐positive in 7.3%; frequencies for PNA pts not reported separately; Reason for abx change (ceftriaxone to ciprofloxacin) not reported, but thought not to be associated with resistant organism (personal communication); Cost: $1,950 (U.S.) per BC that altered treatment |
| Kennedy et al.,20 2005; prospective, U.S urban | Clinical and radiographic PNA and BCs in ED or within 3 hours of admission | None | n = 385; 27 (7.0); 6.0% | 11 (2.9) | 4 (1.0) | 23% of pts from nursing homes, 22% admitted to ICU; 3/4 pts whose abxs were broadened due to a resistant organism came from nursing homes; Abxs were narrowed in 11/19 eligible pts; BCs were false‐positive in 25/414 (6%) pts, including 29 pts discharged from the ED |
| Corbo et al.,17 2004; retrospective, U.S. urban | Primary diagnosis of CAP, positive CXR, and ED BCs before abxs | IC, cancer, recent hospitalization, nursing home resident | n = 355; 33 (9.3); 37 (10.4) | 7 (2.0) | 0 (0) | 821 pts admitted with CAP; 24% not eligible due to non‐confirmatory CXR; of eligible pts, 22% excluded due to HCAP, 23% excluded due to other risk factors; 6 pts with false‐positive BCs had abx change due to BCs ‐ authors suggest hospitalization prolonged in these cases; Physicians reluctant to narrow abxs per authors |
| Campbell et al.,11 2003; prospective, Canadian multiple (19) hospitals | Two signs or sxms of PNA and positive CXR | IC, shock, direct ICU admission, chronic kidney disease, pregnant or nursing, alcoholism | n = 760; 43 (5.7); Not reported | 12 (1.6) | 2 (0.3) | 38% of pts screened with suspected CAP either ineligible or excluded due to risk factors; Abxs were narrowed in 12/35 eligible pts; In one case, BCs grew MRSA resistant to empiric abxs, but abxs had been changed before BC results available; Cost: $1550 (U.S.) per BC leading to abx change |
| Waterer and Wunderink,26 2001; prospective; U.S. urban | Signs and sxms of PNA, positive CXR, and BCs before abxs | IC, hospitalized last 30 days, nursing home residents (if non‐ambulatory) | n = 209; 29 (13.9); 9 (4.3) | 5 (2.4) | 1 (0.5) | BCs only changed management in pts in PSI class 4 and 5 |
| Theerthakarai et al.,24 2001; prospective, U.S. suburban | Acute febrile illness with respiratory sxms and a positive CXR | IC, cancer, age >65, alcoholism, IVDU, COPD, IDDM, neurologic disease, renal failure, recent abx, severe or complicated PNA | n = 74; 0 (0); 0 (0) | 0 (0%) | 0 (0%) | Very strict exclusion criteria: 62% of eligible pts excluded due to risk factors; Authors reported that 28% of included pts could have been treated as outpatients per ATS guidelines |
| Sanyal et al.,23 1999; retrospective, U.S. urban | Acute lower respiratory tract infection and positive CXR | IC, cancer, hospitalized last 12 weeks, IVDU, bronchiectasis, splenectomy, not treated per ATS guidelines | n = 174; 19 (10.9); Not reported | Not reported | 1 (0.6%) | BC‐directed antibiotic changes only reported for pts who did not respond to initial abxs, so BC‐directed narrowing could not be determined; The pt whose abxs were broadened was a nursing home resident with severe pneumonia (by ATS criteria) |
| Glerant et al.,18 1999; prospective, French suburban | Acute septic episode with respiratory sxms and positive CXR | IC, ICU admission, hospitalized last 2 weeks, aspiration | n = 53; 5 (9.4); 2 (3.8) | 0 (0) | 0 (0) | BCs done during first 48 hours so not clear how many BCs sent after hospital abxs started; 23 pts were on abxs before admission; Cost: $6006 (U.S.), no abx changes |
| Kelly,19 1998; retrospective, Australian suburban | All pts who had BCs done in the ED over a 9‐ month period (PNA pts were a subgroup) | None | n = 260; 5%; Not reported | 1% | 1% | 260/1062 study pts had PNA; 14% of all pts discharged; for CAP pts percentage not reported; False‐positive rate 3.8% for all pts, but not reported separately for PNA pts; 1% of PNA pts had abx change due to BCs; type of change not reported, hence reporting of 1% in outcome columns; Cost: $4800 (U.S.) per abx change |
| Chalasani et al.,15 1995; retrospective; U.S. urban | Dx of PNA, respiratory sxms, positive CXR, and 2 sets of BCs before abxs | IC, cancer, hospitalized last 2 weeks, nursing home resident | n = 517; 34 (6.6); 25 (4.8) | 7 (1.4) | 0 (0) | 1250 pts screened with discharge dx of PNA; 59% either ineligible or excluded due to risk factors (authors did not report number ineligible due to the BC requirement); In one case, BCs grew H. influenzae resistant to empiric abxs, but sputum cultures drove the abx change; Cost: $4875 per abx change |
| Woodhead et al.,27 1991; prospective, British urban (2 hospitals) | Clinical features of CAP and positive CXR | IC, cancer, admitted to geriatric or communicable disease ward | n = 86; 9 (10.5%); Not reported | 2 (2.3) | 1 (1.2) | 8% of pts meeting inclusion and exclusion criteria were later excluded due to different dx at discharge |
Included patients were usually required to have clinical features of pneumonia and a confirmatory chest x‐ray. Treating physicians were required to obtain BCs (either by study or hospital protocol) in only 3 studies14, 22, 24 and in a subgroup of another study;11 otherwise the performance of BCs was left to physician discretion.
Nine studies excluded patients who were immunocompromised,11, 15, 17, 18, 2224, 26, 27 a label that was often incompletely defined. Otherwise, exclusion criteria were variable. Notably, only 3 studies excluded patients admitted to the intensive care unit (ICU),11, 18, 24 while 6 excluded patients with cancer15, 17, 2224, 27 and 6 excluded either nursing home residents15, 17, 22, 26 or the elderly (de facto exclusion of most nursing home residents).24, 27
Empiric antibiotic regimens, where reported, were predominantly cephalosporin plus macrolide combinations in 4 studies,17, 2224 fluoroquinolones in 3 studies,11, 14, 26 and penicillin or 1 of its derivatives in 1 study.27
Concerning the 2 studies not included in the primary analysis, the one by Waterer et al.25 was a retrospective review of all cases of pneumococcal bacteremia (n = 74) associated with an admission diagnosis of CAP (N = 1805) in a US urban hospital over a 3‐year period. The one by Chang et al.16 was a retrospective case‐control study of 288 randomly‐selected, immunocompetent Medicare patients with bacteremic pneumococcal CAP who survived to discharge. They were matched 1:1 with blood and sputum culture‐negative controls to study the rate of fluoroquinolone use at discharge in the 2 groups.
Study Findings
Primary Analysis
As shown in Table 1, BCs were positive for a true pathogen in 0% to 14% of cases. Details of microbiology and empiric antibiotic selection are reported in Table 2. S. pneumoniae was by far the most common pathogen: of the 9 studies that had positive BCs and reported the organisms, S. pneumoniae represented 50% to 91% of the pathogens, with penicillin‐resistance found in 0% to 20%.11, 14, 15, 17, 18, 20, 22, 23, 26 S. aureus was next most common, occurring in 6 studies and growing in 3% to 23% of positive BCs;11, 14, 17, 20, 23, 26 its sensitivity to methicillin was reported in 3 studies, with methicillin‐resistant S. aureus (MRSA) representing 0/3, 3/7, and 1/1 of cases.14, 20, 23 E. coli represented 3% to 11% of pathogens in 6 studies,11, 14, 15, 20, 23, 26 while H. influenzae represented 2% to 15% of pathogens in 7 studies.11, 14, 15, 18, 22, 23, 26
| Study: Author, Year | Empiric Antibiotics Given: Frequency, Agent | Bacteria Isolated in True‐Positive BCs: n, Organism | Organisms in BCs Resistant to Empiric Antibiotics |
|---|---|---|---|
| |||
| Benenson et al.,14 2007 | Mild to moderate PNA: levofloxacin; If ICU admission: levofloxacin + azithromycin; If HCAP: levofloxacin + clindamycin; If risk for MRSA: added vancomycin; If structural lung disease: added tobramycin | 14 S. pneumoniae; 3 S. aureus (all MSSA); 2 Group B Strep; 2 H. influenzae; 1 E. coli; 1 Group A Strep | None |
| Ramanujam and Rathlev,22 2006 | Ceftriaxone + oral azithromycin | 11 S. pneumoniae (1 PCN interm res); 2 H. influenzae | None |
| Mountain et al.,21 2006 | Not reported | Not reported completely | None |
| Kennedy et al.,20 2005 | Not reported | 15 S. pneumoniae (3 PCN res); 7 S. aureus (3 MRSA); 3 E. coli; 1 Coagulase‐negative Staph; 1 Pseudomonas; 1 Proteus; 1 Moraxella; 1 E. faecalis | 2 MRSA; 1 MSSA (res to levofloxacin, clindamycin); 1 E. coli (res to levofloxacin) |
| Corbo et al.,17 2004 | 48% ceftriaxone + macrolide; 21% cephalosporin only; 6% quinolone only | 30 S. pneumoniae; 2 S. aureus (# MRSA not reported); 1 Staph haemolyticus | None |
| Campbell et al.,11 2003 | 55% levofloxacin; 45% antibiotic not reported | 30 S. pneumoniae (1 PCN res); 5 S. aureus (total # MRSA not reported); 5 E. coli; 1 H. influenzae; 1 E. faecalis; 1 K. pneumoniae; 1 Enterobacter | 1 MRSA (antibiotic changed before BC results available); 1 MSSA (res not reported); 1 S. pneumoniae (PCN res) |
| Waterer and Wunderink,26 2001 | 60% quinolone only; 25% quinolone + other antibiotic(s) | 20 S. pneumoniae (3 PCN res); 3 S. viridans; 1 H. influenzae; 1 S. aureus (# MRSA not reported); 1 Enterobacter; 1 E. coli; 1 Group B Strep; 1 Group D Strep; 1 Group G Strep; 1 Acinetobacter | 1 Group D Strep (res to levofloxacin) |
| Theerthakarai et al.,24 2001 | Cephalosporin + macrolide | None | None |
| Sanyal et al.,23 1999 | Severe CAP: erythromycin + ceftazidime or ticarcillin/clavulanate; Nonsevere CAP: 76% cefuroxime + erythromycin, 18% cefuroxime only | 14 S. pneumoniae (0 PCN res); 2 H. influenzae; 1 S. aureus (MRSA); 1 K. pneumoniae; 1 E. coli | 1 MRSA |
| Glerant et al.,18 1999 | Not reported | 4 S. pneumoniae (0 PCN res); 1 H. influenzae | None |
| Kelly,19 1998 | Not reported | Not reported | Cannot determine |
| Chalasani et al.,15 1995 | Not reported | 29 S. pneumoniae (0 PCN res); 3 H. influenzae; 1 S. pyogenes; 1 E. coli | H. influenzae (sputum culture drove the antibiotic change) |
| Woodhead et al.,27 1991 | 78% included penicillin, aminopenicillin, or amoxicillin/clavulanate; 33% included erythromycin; 21% ‐lactam + erythromycin | Not reported separately for BCs | E. coli (res to erythromycin) |
| Chang et al.,16 2005 | BC+/Controls: 34%/21%/Quinolones; 86%/88%/ ‐lactam; 1%/1%/Amox/PCN; 38%/37%/ Macrolide | 288 S. pneumoniae (only organism, by design) | Not reported |
| Waterer et al.,25 1999 | 38% Cephalosporin + macrolide other; 27% Quinolone other | 74 S. pneumoniae (only organism, by design); 11 PCN interm res; 4 PCN res | 2 S. pneumoniae (both resistant; degree of resistance not specified) |
In the 8 studies that reported false‐positive BCs, the false‐positive rate was 0% to 10%,14, 15, 17, 18, 20, 22, 24, 26 with 5 studies finding comparable false‐positive and true‐positive BC rates15, 17, 20, 22, 24 and 1 study finding a substantially higher frequency of false‐positive than true‐positive BCs (Table 1).14
BCs led to narrowing of antibiotic coverage in 0% to 3% of cases (Table 1). Four studies reported that physicians narrowed antibiotics when BCs indicated that it was possible to do so, but only in 10%, 14%, 34%, and 58% of eligible cases.11, 14, 20, 22
BCs led to antibiotic broadening ultimately associated with a resistant organism in 0% to 1% of cases (Table 1). The pathogens were MRSA (3), methicillin‐sensitive S. aureus (2), E. coli (2), S. pneumoniae (1), and Group D Streptococcus (1). Details about these patients' medical histories and demographics were absent or sparse in all but 1 study.20 For several of the above cases it was not explicitly stated that BCs directed the antibiotic changes, though it was usually implied; thus we assumed causation.
Secondary Analyses
In the pneumococcal bacteremia study by Waterer et al.,25 BCs altered management in 31 of the 74 cases of pneumococcemia, but in only 2 patients was this associated with antibiotic resistance. Most of the other 29 cases involved narrowing of antibiotics, though switching to penicillin or dropping atypical coverage occurred in only 22% and 37% of eligible patients, respectively. In the study by Chang et al.,16 there was no significant difference in fluoroquinolone use at discharge between the pneumococcemic and culture‐negative groups (the primary endpoint), though there was significantly higher ‐lactam use and lower macrolide use in the pneumococcemic patients at discharge. From the data provided it was not possible to determine how often antibiotic broadening occurred.
Only 2 of the 15 studies stratified management effects based on severity of illness, and neither specified the proportion of severely ill patients admitted to the ICU. Waterer and Wunderink26 prospectively hypothesized that sicker patients were more likely to benefit from BCs. They found that the 30 patients in pneumonia severity index class 5 were most likely to have a BC‐driven antibiotic change, though in at most 1 of these patients was associated with a resistant organism. Sanyal et al.23 stratified patients by severity based on expert guidelines. They found that 19 of 174patients had severe CAP that did not respond to the initial antibiotic regimen, with 1 having a BC‐driven antibiotic change; this was due to resistance.
Only 1 study reported an outcome other than antibiotic change, which in this case was duration of parenteral therapy. In the study, 5 of 43 patients with true‐positive BCs remained on intravenous antibiotics for the full course of treatment probably due to bacteremia alone.11
The direct cost of BCs per BC‐directed antibiotic change (or total cost of BCs if there was no antibiotic change) was reported in 6 studies and, not adjusted for inflation, ranged from $1550 to $8000 (U.S.).11, 15, 18, 19, 21, 22
Quality of the Studies
A detailed listing of the strengths and weaknesses of each study is provided in the Appendix. Briefly, all 15 studies included in this review were observational. Most did not prospectively require BCs in all patients admitted with CAP. This could have biased the results in favor of BC utility as physicians presumably order BCs in patients with a higher probability of bacteremia. Conversely, several studies did not explicitly require two sets of BCs or that BCs be done prior to antibiotics, so they may not have revealed the maximum utility of BCs. The 2 studies limited to pneumococcal bacteremia and described in the secondary analysis were inherently biased against BC utility, as pneumococcus is more likely to be antibiotic‐sensitive than other CAP pathogens.
Eligibility was based only on an emergency department (ED)/admission diagnosis of CAP, a criteria that approximates real world practice, in 3 studies.19, 21, 25 The other studies required either a confirmatory radiograph or a hospital discharge diagnosis of pneumonia. Consequent ED/admission misdiagnosis rates were 3%, 8%, 11%, 24% in the 4 studies that reported them;14, 17, 22, 27 the final diagnoses, when reported, were nearly all noninfections or proximal respiratory tract infections.22, 27
Five studies included all eligible patients.14, 1921, 25 However, 3 studies excluded 23%, 31%, and 62% of eligible patients based on risk factors for bacteremia or resistant pathogens,17, 22, 24 and the rest did not report the number excluded.
DISCUSSION
Summary of Findings
Our systematic review of the literature finds that BCs rarely alter empiric antibiotic therapy in adults hospitalized with community‐acquired pneumonia. Even when there is a change in treatment it usually is not of the type most likely to impact patient outcome, which is antibiotic broadening ultimately associated with a resistant organism. In the 13 studies that could quantify this effect, it occurred in only 0% to 1% of cases in which BCs were obtained. Antibiotic narrowing occurred in 0% to 3% of cases, with physicians often choosing not to narrow antibiotics when BC results suggested that they could do so.
Limits on BC Utility
‐Lactam‐Resistant Pneumococcus
In the studies reviewed here 50%‐90% of positive BCs grew pneumococcus, consistent with the 60% to 67% rate reported elsewhere.2, 28, 29 Pneumococci that invade the bloodstream have disproportionately low rates of ‐lactam resistance,30, 31 inherently limiting the utility of BCs for detecting inadequate empiric antibiotic therapy. Though pneumococcal resistance to ‐lactams has risen over the last 2 decades, third‐generation cephalosporins, preferred agents for CAP, are still extremely effective. Even when the organism is by historical standards moderately resistant to them, these cephalosporins at standard doses maintain bactericidal efficacy in the lung,32, 33 and their use in the setting of such resistance is not associated with higher mortality.3437 By newer laboratory standards 97% and 96% of S. pneumoniae isolates in mid‐2003 were sensitive to ceftriaxone and cefotaxime, respectively.38 Thus a major potential benefit of BCsdetecting cephalosporin‐resistant pneumococcusremains a rare occurrence.
Polymicrobial Infection
If positive BCs in CAP mostly reveal antibiotic‐sensitive pathogens, one may infer that at least they lead to narrowing of therapy. However, the studies reviewed here reveal that this usually does not happen.
One explanation for this reluctance to narrow antibiotics is that CAP is often a polymicrobial disease. When rigorous serologic testing is done, multiple pathogens are found in up to 40% of cases.39 The occult copathogen is frequently an intracellular one and thus cannot be detected by BCs. Though the evidence for empirically treating these atypical organisms is mixed,40, 41 expert guidelines recommend doing so,12 and guideline‐concordant antibiotic therapy in CAP is associated with lower mortality.42 Even in bacteremic pneumococcal CAP, monotherapy is associated with higher mortality.4346 Thus, stopping antibiotic coverage of atypical pathogens in response to BCs alone might not always be appropriate.
Prognosis
Another rationale given for ordering BCs is that bacteremic pneumonia is a morbid disease so positive BCs may demand prolonged parenteral therapy or extended hospitalization. Although mortality for bacteremic pneumococcal pneumonia (the predominantly studied variety of bacteremic pneumonia) has historically been high at 20%,47, 48 studies that have examined pneumococcal bacteremia as an independent risk factor for death in CAP have yielded mixed results.2 Moreover, it appears that patients with bacteremic pneumococcal pneumonia who reach clinical stability may be safely switched to oral antibiotics.49
It is not clear that positive BCs in pneumonia (at least in the case of S. pneumoniae) should alter the duration of parenteral therapy or hospitalization, though whether or not such effects occur in clinical practice was largely unaddressed by the studies reviewed here.
Epidemiology
One theoretical benefit of BCs is their epidemiologic value. When true‐positive in pneumonia, perhaps more than any other test they identify with great specificity at least 1 of the causative agents. Unfortunately, as discussed above, BCs alone provide an incomplete and skewed picture of the microbiology of CAP. They underestimate atypical organisms, overestimate pneumococcus, and, because bacteremic pneumococcus is more likely to be antibiotic‐susceptible, they underestimate antibiotic resistance.11 Tracking pathogens in bacteremic pneumonia may be useful nonetheless, but perhaps a more accurate method for determining etiologic trends is periodic comprehensive microbiological investigation, including BCs, sputum/bronchial cultures, and serology.
Costs
In the studies reviewed here, based on reported costs of $15 to $65 per set of BCs or per patient, BCs cost $1550 to $8000 (U.S.) per BC‐directed antibiotic change. Considering that very few of these antibiotic changes involved broadening associated with a resistant organism, the cost/benefit ratio was quite high. Today BCs may be even more expensive, as U.S. hospitals now often charge over $150 per set of BCs.50, 51
The cost of false‐positive BCs must also be taken into account. The false‐positive rate in the studies reviewed here was 0% to 10%, similar to that reported elsewhere.7 False‐positive BCs increase hospital length of stay by 3 to 5 days and hospital charges by $4400 to $8800.51, 52
Limitations of the Review
Our search strategy was designed to be sensitive and included backup methods such as searching article references and querying experts. Nevertheless, we may have missed studies, especially if there were small eligible subgroups or if determining management effects was not a primary purpose. We chose not to measure instances of antibiotic broadening that were not associated with a resistant organism, though in unusual cases (eg, Pseudomonas bacteremia) this effect of BCs may be useful.
The methodologies of the included studies were adequate to measure the key outcomes with reasonable validity. Biases were evident, though they occurred both for and against BC utility.
Eligibility varied across studies, and most investigations excluded immunocompromised or other high‐risk patient groups, which could have biased results against BC utility. However, results of these studies were consistent with those that included all patients with CAP, suggesting the degree of bias was probably small. Still, given this concern, it would be prudent not to generalize the findings of this review to immunocompromised patients. Moreover, although the critically ill and those who today would be classified as having healthcare‐associated pneumonia (HCAP)nursing home residents, the recently hospitalized, and hemodialysis patientswere included in most studies, their numbers were small, and these groups were not analyzed separately; thus, the results might not be generalizable to these populations either. Finally, the reported studies, which enrolled patients through 2003, do not reflect more recent increases in the prevalence of resistant pathogens, such as MRSA, in the community.
BCs as a Quality Measure
The adoption of BCs as a quality measure was largely predicated on the widely‐cited study by Meehan et al.,4 which showed an association between BC obtainment and reduced mortality. This study, which associated processes of care with hard outcomes such as mortality, was limited by uncontrolled confounders, including variation in hospital quality.53 A more recent study of pneumonia processes of care found no association between BC collection and mortality.54 Another study often cited to support BC use, by Arbo and Snydman,55 showed that positive BCs were associated with changes in antibiotic therapy, but it included very few pneumonia patients and did not describe results for them separately.
The inclusion of BC acquisition in 2 quality measures in the NHQM guidelines for pneumonia impacts the clinical practice of hospitals and physicians, which may be rated and reimbursed differentially based on their compliance with such measures. One of the quality measures requires BCs in patients admitted to the ICU. The other requires that ED BCs for pneumonia, if obtained, be drawn before antibiotics are given.6
The studies we reviewed are not specific to these quality measures, but are relevant to them. With regard to the first measure, all but 3 studies included patients admitted to the ICU and found BCs to be of minimal benefit overall. Our subgroup analysis of severely ill patients was unrevealing. The ICU measure is tentative in its validity, but it is not unreasonable given that these patients have a life‐threatening infection and may be at risk for bacteremia with resistant pathogens.12
The second measure, though perhaps simply seeking to maximize the potential for BCs to turn positive, depends for its validity on BCs being useful in a large proportion of patients with CAP. Though we cannot exclude the possibility that BCs benefit certain subsets of patients, such as those who are immunocompromised or have HCAP, our findings do not support obtaining BCs in all or even most adults hospitalized with CAP. This conclusion is reflected in the 2007 Infectious Diseases Society of America/American Thoracic Society management guidelines for CAP, which state than BCs are optional except for patients with severe pneumonia, some immunocompromised states, and particular radiographic abnormalities.12
With such data and guidelines in mind, a physician seeking to minimize treatment delays in a patient with pneumonia may give antibiotics early in the ED course (the basis of another quality measure) without obtaining BCs. If she later determines that the patient is particularly high‐risk for bacteremia or a resistant pathogen, should she be discouraged from ordering BCs? Experts specifically state that BCs, even after antibiotics, are warranted for such a patient.12
With the scope of medical practice captured in quality measures being so narrow, having 2 measures based on a test with such limited benefit is itself questionable.
- ,,,,,.Practice guidelines for the management of community‐acquired pneumonia in adults. Infectious diseases society of America.Clin Infect Dis.2000;31:347–382.
- ,,,,.Canadian guidelines for the initial management of community‐acquired pneumonia: an evidence‐based update by the Canadian infectious diseases society and the Canadian thoracic society. The Canadian community‐acquired pneumonia working group.Clin Infect Dis.2000;31:383–421.
- ,,, et al.Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,, et al.Quality of care, process, and outcomes in elderly patients with pneumonia.JAMA.1997;278:2080–2084.
- Hospital quality initiative, overview, centers for Medicare and Medicaid services. Available at: http://www.cms.hhs.gov/HospitalQualityInits. Accessed September2007.
- Specifications manual for national hospital quality measures, version 2.3b. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Current+NHQM+Manual.htm. Accessed October2007.
- ,,,.Predicting bacteremia in patients with community‐acquired pneumonia.Am J Respir Crit Care Med.2004;169:342–347.
- ,.The joint commission on accreditation of healthcare organizations and center for Medicare and Medicaid services community‐acquired pneumonia initiative: what went wrong?Ann Emerg Med.2005;46:409–411.
- .Blood cultures in community‐acquired pneumonia: Are we ready to quit?Chest.2003;123:977–978.
- .Blood cultures for community‐acquired pneumonia: piecing together a mosaic for doing less.Am J Respir Crit Care Med.2004;169:327–328.
- ,,,,.The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community‐acquired pneumonia: a prospective observational study.Chest.2003;123:1142–1150.
- ,,, et al.Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults.Clin Infect Dis.2007;44 (Suppl 2):S27–S72.
- ,,,,,.Value of routine microbial investigation in community‐acquired pneumonia treated in a tertiary care center.Respiration.1996;63:164–169.
- ,,,.Selective use of blood cultures in emergency department pneumonia patients.J Emerg Med.2007;33:1–8.
- ,,,,.Clinical utility of blood cultures in adult patients with community‐acquired pneumonia without defined underlying risks.Chest.1995;108:932–936.
- ,,,,,.Blood culture and susceptibility results and allergy history do not influence fluoroquinolone use in the treatment of community‐acquired pneumonia.Pharmacotherapy.2005;25:59–66.
- ,,,.Limited usefulness of initial blood cultures in community acquired pneumonia.Emerg Med J.2004;21:446–448.
- ,,,,.Utility of blood cultures in community‐acquired pneumonia requiring hospitalization: Influence of antibiotic treatment before admission.Respir Med.1999;93:208–212.
- .Clinical impact of blood cultures taken in the emergency department.J Accid Emerg Med.1998;15:254–256.
- ,,,,,.Do emergency department blood cultures change practice in patients with pneumonia?Ann Emerg Med.2005;46:393–400.
- ,,,.Blood cultures ordered in the adult emergency department are rarely useful.Eur J Emerg Med.2006;13:76–79.
- ,.Blood cultures do not change management in hospitalized patients with community‐acquired pneumonia.Acad Emerg Med.2006;13:740–745.
- ,,,,,.Initial microbiologic studies did not affect outcome in adults hospitalized with community‐acquired pneumonia.Am J Respir Crit Care Med.1999;160:346–348.
- ,,,,.Nonvalue of the initial microbiological studies in the management of nonsevere community‐acquired pneumonia.Chest.2001;119:181–184.
- ,,.The impact of blood cultures on antibiotic therapy in pneumococcal pneumonia.Chest.1999;116:1278–1281.
- ,.The influence of the severity of community‐acquired pneumonia on the usefulness of blood cultures.Respir Med.2001;95:78–82.
- ,,,,.The value of routine microbial investigation in community‐acquired pneumonia.Respir Med.1991;85:313–317.
- ,,, et al.Study of community acquired pneumonia aetiology (scapa) in adults admitted to hospital: implications for management guidelines.Thorax.2001;56:296–301.
- ,.Etiology of community‐acquired pneumonia.Clin Chest Med.2005;26:47–55.
- ,,,,.Early predictors of mortality in pneumococcal bacteraemia.Ann Acad Med Singapore.2005;34:426–431.
- ,,,.Penicillin‐nonsusceptible Streptococcus pneumoniae at San Francisco general hospital.Clin Infect Dis.1999;29:580–585.
- .Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men.Clin Infect Dis.1998;26:1–10; quiz 11–12.
- .The significance of serum vs tissue levels of antibiotics in the treatment of penicillin‐resistant Streptococcus pneumoniae and community‐acquired pneumonia: are we looking in the wrong place?Chest.1999;116:535–538.
- ,,, et al.Pneumonia acquired in the community through drug‐resistant Streptococcus pneumoniae.Am J Respir Crit Care Med.1999;159:1835–1842.
- ,,, et al.The effect of cephalosporin resistance on mortality in adult patients with nonmeningeal systemic pneumococcal infections.Am J Med.2002;113:120–126.
- ,,, et al.Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain.N Engl J Med.1995;333:474–480.
- ,,, et al.An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome.Clin Infect Dis.2003;37:230–237.
- ,,,,,.Tracking the implementation of NCCLS m100‐s12 expanded‐spectrum cephalosporin MIC breakpoints for non‐meningeal isolates of Streptococcus pneumoniae by clinical laboratories in the united states during 2002 and 2003.Ann Clin Microbiol Antimicrob.2004;3:1.
- ,,, et al.Multiple pathogens in adult patients admitted with community‐acquired pneumonia: a one year prospective study of 346 consecutive patients.Thorax.1996;51:179–184.
- ,,,,.How good is the evidence for the recommended empirical antimicrobial treatment of patients hospitalized because of community‐acquired pneumonia? A systematic review.J Antimicrob Chemother.2003;52:555–563.
- ,,,.Empirical atypical coverage for inpatients with community‐acquired pneumonia: systematic review of randomized controlled trials.Arch Intern Med.2005;165:1992–2000.
- ,,,.Impact of guideline‐concordant empiric antibiotic therapy in community‐acquired pneumonia.Am J Med.2006;119:865–871.
- ,,, et al.Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia [see comment].Am J Respir Crit Care Med.2004;170:440–444.
- ,,, et al.Addition of a macrolide to a beta‐lactam‐based empirical antibiotic regimen is associated with lower in‐hospital mortality for patients with bacteremic pneumococcal pneumonia. [see comment].Clin Infect Dis.2003;36:389–395.
- ,,.Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia.Arch Intern Med.2001;161:1837–1842.
- ,,, et al.Clinical characteristics at initial presentation and impact of dual therapy on the outcome of bacteremic Streptococcus pneumoniae pneumonia in adults.Can Respir J.2004;11:589–593.
- ,.Pneumococcal bacteremia with special reference to bacteremic pneumococcal pneumonia.Arch Intern Med.1964;60:759–776.
- ,,, et al.Prognosis and outcomes of patients with community‐acquired pneumonia. A meta‐analysis.JAMA.1996;275:134–141.
- ,.Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community‐acquired Streptococcus pneumoniae pneumonia.Arch Intern Med.2001;161:848–850.
- Cleveland Clinic patient price information list. Available at:http://cms.clevelandclinic.org/documents/CCMain_HB197_2007.pdf. Accessed January2008.
- ,.Analysis of strategies to improve cost effectiveness of blood cultures.J Hosp Med.2006;1:272–276.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,,,.Patient and hospital characteristics associated with recommended processes of care for elderly patients hospitalized with pneumonia: results from the Medicare quality indicator system pneumonia module.Arch Intern Med.2002;162:827–833.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,.Influence of blood culture results on antibiotic choice in the treatment of bacteremia.Arch Intern Med.1994;154:2641–2645.
Blood cultures (BCs) have long been a mainstay of the diagnostic evaluation of patients hospitalized with community‐acquired pneumonia (CAP). They have been strongly recommended by professional societies13 and are often expected by admitting physicians. A large retrospective study of Medicare patients with pneumonia found that obtaining BCs is associated with lower mortality.4 In 2002, when the National Hospital Quality Measures (NHQM) were introduced, BCs were included as a quality measure for pneumonia.5, 6
However, there is uncertainty about the actual utility of BCs in CAP. In large studies they are true‐positive in only 7 to 11% of cases and false‐positive in 5%,2, 7 and whether they affect clinical management has been strongly questioned.810 Their impact may be limited by slow results, low frequency of bacterial resistance to the empiric antibiotic regimen, and reluctance of physicians to narrow antibiotic coverage.9, 11 Recent updates to professional society guidelines no longer recommend BCs in all admitted CAP patients.12
To evaluate the clinical utility of BCs and the appropriateness of pnemonia quality measures based on BCs, we performed a systematic review of the literature to determine the effect of BCs on the management of adults with CAP requiring hospitalization.
PATIENTS AND METHODS
Data Sources and Searches
We searched the English‐language literature via MEDLINE (1966 through September 2007), MEDLINE‐In Process, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, and ACP Journal Club. Within each of these databases we used keywords and exploded Medical Subject Headings (MeSH) to produce the following search strategy: blood culture(s) (keyword), bacteriological techniques (MeSH), blood [microbiology] (MeSH), bacteremia [microbiology or drug therapy] (MeSH), or diagnostic tests, routine (MeSH) combined with pneumonia (keyword), pneumonia (MeSH), lower respiratory tract infection(s) (keyword), or community‐acquired infections (MeSH). To maximize capture of BC or bacteremia studies with subgroups of CAP patients we added the following search strategy: explode microbiological techniques [utilization] (MeSH), explode blood specimen collection [utilization] (MeSH), or focus bacteremia [drug therapy] (MeSH). We reviewed the reference lists of all included studies as well as those of important background articles. Finally, we asked experts to evaluate the completeness of our list.
Study Selection
We included studies in which: (1) subjects were adults hospitalized with CAP; (2) BCs were obtained at or near hospital admission; and (3) the effects of BCs on management (change in antibiotic therapy or other effects such as duration of parenteral therapy, length of hospitalization, or level of care) were reported. The first 2 requirements could be satisfied by a subgroup.
From retrieved citations, relevant abstracts were reviewed, and studies with any potential to meet inclusion criteria were chosen for full‐text review. Two authors (N.A., R.S.) independently analyzed each full‐text article to determine inclusion for data analysis. A third author (J.T.) analyzed all included and narrowly excluded articles to confirm the final list of included studies. Disagreements were resolved by discussion.
Data Extraction
For the included studies, 2 authors (N.A., K.A.) independently abstracted the following data using a standardized collection instrument: study design and setting, inclusion and exclusion criteria, number of hospitalized CAP patients in whom BCs were obtained, empiric antibiotic regimens, number of true‐positive and false‐positive BCs, bacteria isolated in true‐positive BCs, BC‐directed antibiotic narrowing, BC‐directed antibiotic broadening ultimately associated with a resistant organism, and any other management effects reported. Narrowing refers to coverage of fewer organisms, while broadening refers to coverage of a larger or different spectrum of organisms.
If a study included patients not meeting our selection criteria, our analysis was limited to the subset of patients meeting criteria. We also analyzed each study to determine whether a subgroup of severely ill patients was reported separately and whether such a group benefited from BCs. The 2 authors independently repeated all data abstraction to confirm accuracy. We attempted to contact authors for clarification when needed.
Data Synthesis
Data were synthesized by compilation of characteristic summary tables. In the primary analysis, the proportion of positive BCs (both true and false) and the frequency of BC‐directed changes in antimicrobial therapy (narrowing, or broadening ultimately associated with a resistant organism) were determined and reported for each study and then described as an aggregate range. This compilation required studies to provide a particular denominatorthe number of patients in whom BCs were performed. If a study did not do so, it was described separately in the secondary analysis, where we also assessed the cost of BCs as well as the impact of BCs in critically ill patients and on outcomes other than antibiotic change. Heterogeneity of subject inclusion and exclusion criteria and empiric antibiotic use were summarized qualitatively. Two authors (N.A., R.S.) assessed each study's quality.
DATA SYNTHESIS
Search Results
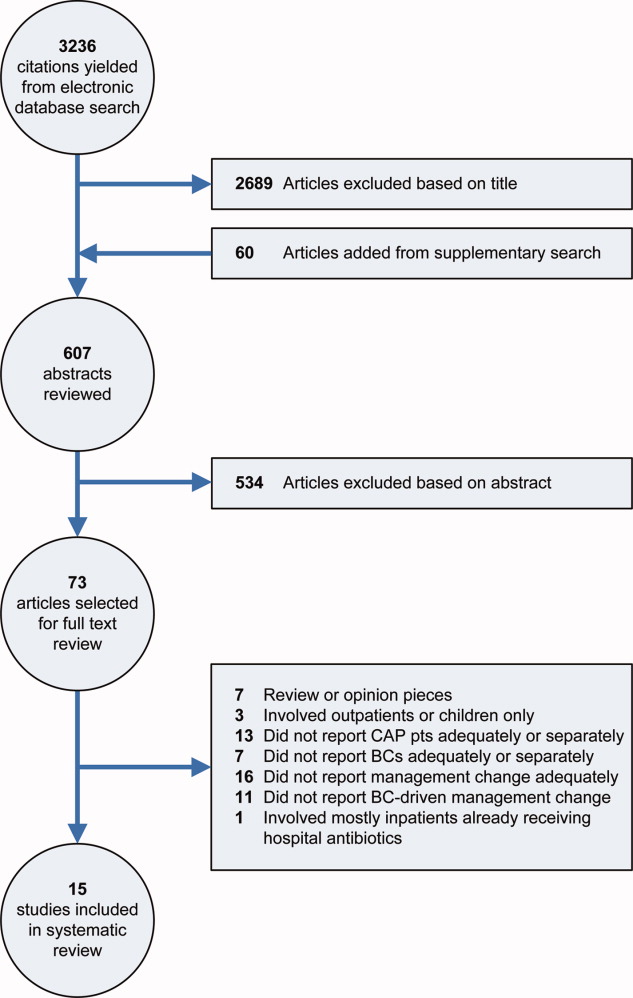
Our electronic database search yielded 3236 citations. From this list and the supplementary search of references, we reviewed 607 abstracts; of these, we selected 73 articles for full‐text review, and 15 were included in the final analysis (Figure 1). One study was narrowly excluded because it largely included CAP patients that had already been admitted to the hospital and failed an empiric antibiotic trial before BCs were obtained.13

Study Characteristics
Fifteen studies with a total of 3898 patients evaluated BC‐directed management changes in adults admitted with CAP.11, 1427 However, 2 of these, involving only patients with bacteremic pneumococcal CAP, by design could not report the number of patients that had BCs done; thus they were not included in the primary analysis.16, 25
The 13 studies amenable to the primary analysis (Table 1) all had an observational cohort design; 6 were prospective11, 18, 20, 24, 26, 27 and 7 were retrospective.14, 15, 17, 19, 2123 Sample size varied from 52 to 760 patients. Settings included university and community hospitals in the U.S. and 4 other countries, with patient enrollment spanning the years 19882003 (publication dates 19912007).
| Study Author, Year, Design, Setting | Inclusion Criteria | Exclusion Criteria | CAP Patients with BCs, n*; True‐Positive BCs, n (%); False‐Positive BCs, n (%) | BCs Directed Antibiotic Narrowing, n (%) | BCs Directed Antibiotic Broadening and Organism was Resistant, n (%) | Comments |
|---|---|---|---|---|---|---|
| ||||||
| Benenson et al.,14 2007; retrospective, U.S. suburban | ED ICD‐9 dx and discharge dx of PNA | None | n = 684; 23 (3.4); 54 (7.9) | 3 (0.4) | 0 (0) | 11% of pts with ED dx of PNA not eligible due to different dx at discharge; 25% from nursing homes, 18% recently hospitalized, 14% immunocompromised; Abxs narrowed in 3/21 eligible pts |
| Ramanujamand Rathlev,22 2006; retrospective; U.S. urban | ED, ICD‐9, and discharge dx of PNA, and ED BCs before abxs | IC, active cancer, chronic renal failure, hospitalized last 1 week, nursing home resident, aspiration | n = 289; 13 (4.5%); 13 (4.5%) | 1 (0.3%) | 0 (0%) | 532 pts screened; 3% not eligible due to different dx at discharge; of eligible pts, 9% excluded due to HCAP and 31% excluded due to other risk factors; Abxs were narrowed in 1/10 eligible pts; Cost: $8,000 for the 1 pt with abx change |
| Mountain et al.,21 2006; retrospective, Australian suburban | All pts who had BCs done in the ED during a 2‐month period (PNA pts were a subgroup) | None | n = 52; Not reported; Not reported | 1 (1.9) | 0 (0) | 52/218 study pts had clinical PNA. Overall BCs true‐positive in 6.4%, false‐positive in 7.3%; frequencies for PNA pts not reported separately; Reason for abx change (ceftriaxone to ciprofloxacin) not reported, but thought not to be associated with resistant organism (personal communication); Cost: $1,950 (U.S.) per BC that altered treatment |
| Kennedy et al.,20 2005; prospective, U.S urban | Clinical and radiographic PNA and BCs in ED or within 3 hours of admission | None | n = 385; 27 (7.0); 6.0% | 11 (2.9) | 4 (1.0) | 23% of pts from nursing homes, 22% admitted to ICU; 3/4 pts whose abxs were broadened due to a resistant organism came from nursing homes; Abxs were narrowed in 11/19 eligible pts; BCs were false‐positive in 25/414 (6%) pts, including 29 pts discharged from the ED |
| Corbo et al.,17 2004; retrospective, U.S. urban | Primary diagnosis of CAP, positive CXR, and ED BCs before abxs | IC, cancer, recent hospitalization, nursing home resident | n = 355; 33 (9.3); 37 (10.4) | 7 (2.0) | 0 (0) | 821 pts admitted with CAP; 24% not eligible due to non‐confirmatory CXR; of eligible pts, 22% excluded due to HCAP, 23% excluded due to other risk factors; 6 pts with false‐positive BCs had abx change due to BCs ‐ authors suggest hospitalization prolonged in these cases; Physicians reluctant to narrow abxs per authors |
| Campbell et al.,11 2003; prospective, Canadian multiple (19) hospitals | Two signs or sxms of PNA and positive CXR | IC, shock, direct ICU admission, chronic kidney disease, pregnant or nursing, alcoholism | n = 760; 43 (5.7); Not reported | 12 (1.6) | 2 (0.3) | 38% of pts screened with suspected CAP either ineligible or excluded due to risk factors; Abxs were narrowed in 12/35 eligible pts; In one case, BCs grew MRSA resistant to empiric abxs, but abxs had been changed before BC results available; Cost: $1550 (U.S.) per BC leading to abx change |
| Waterer and Wunderink,26 2001; prospective; U.S. urban | Signs and sxms of PNA, positive CXR, and BCs before abxs | IC, hospitalized last 30 days, nursing home residents (if non‐ambulatory) | n = 209; 29 (13.9); 9 (4.3) | 5 (2.4) | 1 (0.5) | BCs only changed management in pts in PSI class 4 and 5 |
| Theerthakarai et al.,24 2001; prospective, U.S. suburban | Acute febrile illness with respiratory sxms and a positive CXR | IC, cancer, age >65, alcoholism, IVDU, COPD, IDDM, neurologic disease, renal failure, recent abx, severe or complicated PNA | n = 74; 0 (0); 0 (0) | 0 (0%) | 0 (0%) | Very strict exclusion criteria: 62% of eligible pts excluded due to risk factors; Authors reported that 28% of included pts could have been treated as outpatients per ATS guidelines |
| Sanyal et al.,23 1999; retrospective, U.S. urban | Acute lower respiratory tract infection and positive CXR | IC, cancer, hospitalized last 12 weeks, IVDU, bronchiectasis, splenectomy, not treated per ATS guidelines | n = 174; 19 (10.9); Not reported | Not reported | 1 (0.6%) | BC‐directed antibiotic changes only reported for pts who did not respond to initial abxs, so BC‐directed narrowing could not be determined; The pt whose abxs were broadened was a nursing home resident with severe pneumonia (by ATS criteria) |
| Glerant et al.,18 1999; prospective, French suburban | Acute septic episode with respiratory sxms and positive CXR | IC, ICU admission, hospitalized last 2 weeks, aspiration | n = 53; 5 (9.4); 2 (3.8) | 0 (0) | 0 (0) | BCs done during first 48 hours so not clear how many BCs sent after hospital abxs started; 23 pts were on abxs before admission; Cost: $6006 (U.S.), no abx changes |
| Kelly,19 1998; retrospective, Australian suburban | All pts who had BCs done in the ED over a 9‐ month period (PNA pts were a subgroup) | None | n = 260; 5%; Not reported | 1% | 1% | 260/1062 study pts had PNA; 14% of all pts discharged; for CAP pts percentage not reported; False‐positive rate 3.8% for all pts, but not reported separately for PNA pts; 1% of PNA pts had abx change due to BCs; type of change not reported, hence reporting of 1% in outcome columns; Cost: $4800 (U.S.) per abx change |
| Chalasani et al.,15 1995; retrospective; U.S. urban | Dx of PNA, respiratory sxms, positive CXR, and 2 sets of BCs before abxs | IC, cancer, hospitalized last 2 weeks, nursing home resident | n = 517; 34 (6.6); 25 (4.8) | 7 (1.4) | 0 (0) | 1250 pts screened with discharge dx of PNA; 59% either ineligible or excluded due to risk factors (authors did not report number ineligible due to the BC requirement); In one case, BCs grew H. influenzae resistant to empiric abxs, but sputum cultures drove the abx change; Cost: $4875 per abx change |
| Woodhead et al.,27 1991; prospective, British urban (2 hospitals) | Clinical features of CAP and positive CXR | IC, cancer, admitted to geriatric or communicable disease ward | n = 86; 9 (10.5%); Not reported | 2 (2.3) | 1 (1.2) | 8% of pts meeting inclusion and exclusion criteria were later excluded due to different dx at discharge |
Included patients were usually required to have clinical features of pneumonia and a confirmatory chest x‐ray. Treating physicians were required to obtain BCs (either by study or hospital protocol) in only 3 studies14, 22, 24 and in a subgroup of another study;11 otherwise the performance of BCs was left to physician discretion.
Nine studies excluded patients who were immunocompromised,11, 15, 17, 18, 2224, 26, 27 a label that was often incompletely defined. Otherwise, exclusion criteria were variable. Notably, only 3 studies excluded patients admitted to the intensive care unit (ICU),11, 18, 24 while 6 excluded patients with cancer15, 17, 2224, 27 and 6 excluded either nursing home residents15, 17, 22, 26 or the elderly (de facto exclusion of most nursing home residents).24, 27
Empiric antibiotic regimens, where reported, were predominantly cephalosporin plus macrolide combinations in 4 studies,17, 2224 fluoroquinolones in 3 studies,11, 14, 26 and penicillin or 1 of its derivatives in 1 study.27
Concerning the 2 studies not included in the primary analysis, the one by Waterer et al.25 was a retrospective review of all cases of pneumococcal bacteremia (n = 74) associated with an admission diagnosis of CAP (N = 1805) in a US urban hospital over a 3‐year period. The one by Chang et al.16 was a retrospective case‐control study of 288 randomly‐selected, immunocompetent Medicare patients with bacteremic pneumococcal CAP who survived to discharge. They were matched 1:1 with blood and sputum culture‐negative controls to study the rate of fluoroquinolone use at discharge in the 2 groups.
Study Findings
Primary Analysis
As shown in Table 1, BCs were positive for a true pathogen in 0% to 14% of cases. Details of microbiology and empiric antibiotic selection are reported in Table 2. S. pneumoniae was by far the most common pathogen: of the 9 studies that had positive BCs and reported the organisms, S. pneumoniae represented 50% to 91% of the pathogens, with penicillin‐resistance found in 0% to 20%.11, 14, 15, 17, 18, 20, 22, 23, 26 S. aureus was next most common, occurring in 6 studies and growing in 3% to 23% of positive BCs;11, 14, 17, 20, 23, 26 its sensitivity to methicillin was reported in 3 studies, with methicillin‐resistant S. aureus (MRSA) representing 0/3, 3/7, and 1/1 of cases.14, 20, 23 E. coli represented 3% to 11% of pathogens in 6 studies,11, 14, 15, 20, 23, 26 while H. influenzae represented 2% to 15% of pathogens in 7 studies.11, 14, 15, 18, 22, 23, 26
| Study: Author, Year | Empiric Antibiotics Given: Frequency, Agent | Bacteria Isolated in True‐Positive BCs: n, Organism | Organisms in BCs Resistant to Empiric Antibiotics |
|---|---|---|---|
| |||
| Benenson et al.,14 2007 | Mild to moderate PNA: levofloxacin; If ICU admission: levofloxacin + azithromycin; If HCAP: levofloxacin + clindamycin; If risk for MRSA: added vancomycin; If structural lung disease: added tobramycin | 14 S. pneumoniae; 3 S. aureus (all MSSA); 2 Group B Strep; 2 H. influenzae; 1 E. coli; 1 Group A Strep | None |
| Ramanujam and Rathlev,22 2006 | Ceftriaxone + oral azithromycin | 11 S. pneumoniae (1 PCN interm res); 2 H. influenzae | None |
| Mountain et al.,21 2006 | Not reported | Not reported completely | None |
| Kennedy et al.,20 2005 | Not reported | 15 S. pneumoniae (3 PCN res); 7 S. aureus (3 MRSA); 3 E. coli; 1 Coagulase‐negative Staph; 1 Pseudomonas; 1 Proteus; 1 Moraxella; 1 E. faecalis | 2 MRSA; 1 MSSA (res to levofloxacin, clindamycin); 1 E. coli (res to levofloxacin) |
| Corbo et al.,17 2004 | 48% ceftriaxone + macrolide; 21% cephalosporin only; 6% quinolone only | 30 S. pneumoniae; 2 S. aureus (# MRSA not reported); 1 Staph haemolyticus | None |
| Campbell et al.,11 2003 | 55% levofloxacin; 45% antibiotic not reported | 30 S. pneumoniae (1 PCN res); 5 S. aureus (total # MRSA not reported); 5 E. coli; 1 H. influenzae; 1 E. faecalis; 1 K. pneumoniae; 1 Enterobacter | 1 MRSA (antibiotic changed before BC results available); 1 MSSA (res not reported); 1 S. pneumoniae (PCN res) |
| Waterer and Wunderink,26 2001 | 60% quinolone only; 25% quinolone + other antibiotic(s) | 20 S. pneumoniae (3 PCN res); 3 S. viridans; 1 H. influenzae; 1 S. aureus (# MRSA not reported); 1 Enterobacter; 1 E. coli; 1 Group B Strep; 1 Group D Strep; 1 Group G Strep; 1 Acinetobacter | 1 Group D Strep (res to levofloxacin) |
| Theerthakarai et al.,24 2001 | Cephalosporin + macrolide | None | None |
| Sanyal et al.,23 1999 | Severe CAP: erythromycin + ceftazidime or ticarcillin/clavulanate; Nonsevere CAP: 76% cefuroxime + erythromycin, 18% cefuroxime only | 14 S. pneumoniae (0 PCN res); 2 H. influenzae; 1 S. aureus (MRSA); 1 K. pneumoniae; 1 E. coli | 1 MRSA |
| Glerant et al.,18 1999 | Not reported | 4 S. pneumoniae (0 PCN res); 1 H. influenzae | None |
| Kelly,19 1998 | Not reported | Not reported | Cannot determine |
| Chalasani et al.,15 1995 | Not reported | 29 S. pneumoniae (0 PCN res); 3 H. influenzae; 1 S. pyogenes; 1 E. coli | H. influenzae (sputum culture drove the antibiotic change) |
| Woodhead et al.,27 1991 | 78% included penicillin, aminopenicillin, or amoxicillin/clavulanate; 33% included erythromycin; 21% ‐lactam + erythromycin | Not reported separately for BCs | E. coli (res to erythromycin) |
| Chang et al.,16 2005 | BC+/Controls: 34%/21%/Quinolones; 86%/88%/ ‐lactam; 1%/1%/Amox/PCN; 38%/37%/ Macrolide | 288 S. pneumoniae (only organism, by design) | Not reported |
| Waterer et al.,25 1999 | 38% Cephalosporin + macrolide other; 27% Quinolone other | 74 S. pneumoniae (only organism, by design); 11 PCN interm res; 4 PCN res | 2 S. pneumoniae (both resistant; degree of resistance not specified) |
In the 8 studies that reported false‐positive BCs, the false‐positive rate was 0% to 10%,14, 15, 17, 18, 20, 22, 24, 26 with 5 studies finding comparable false‐positive and true‐positive BC rates15, 17, 20, 22, 24 and 1 study finding a substantially higher frequency of false‐positive than true‐positive BCs (Table 1).14
BCs led to narrowing of antibiotic coverage in 0% to 3% of cases (Table 1). Four studies reported that physicians narrowed antibiotics when BCs indicated that it was possible to do so, but only in 10%, 14%, 34%, and 58% of eligible cases.11, 14, 20, 22
BCs led to antibiotic broadening ultimately associated with a resistant organism in 0% to 1% of cases (Table 1). The pathogens were MRSA (3), methicillin‐sensitive S. aureus (2), E. coli (2), S. pneumoniae (1), and Group D Streptococcus (1). Details about these patients' medical histories and demographics were absent or sparse in all but 1 study.20 For several of the above cases it was not explicitly stated that BCs directed the antibiotic changes, though it was usually implied; thus we assumed causation.
Secondary Analyses
In the pneumococcal bacteremia study by Waterer et al.,25 BCs altered management in 31 of the 74 cases of pneumococcemia, but in only 2 patients was this associated with antibiotic resistance. Most of the other 29 cases involved narrowing of antibiotics, though switching to penicillin or dropping atypical coverage occurred in only 22% and 37% of eligible patients, respectively. In the study by Chang et al.,16 there was no significant difference in fluoroquinolone use at discharge between the pneumococcemic and culture‐negative groups (the primary endpoint), though there was significantly higher ‐lactam use and lower macrolide use in the pneumococcemic patients at discharge. From the data provided it was not possible to determine how often antibiotic broadening occurred.
Only 2 of the 15 studies stratified management effects based on severity of illness, and neither specified the proportion of severely ill patients admitted to the ICU. Waterer and Wunderink26 prospectively hypothesized that sicker patients were more likely to benefit from BCs. They found that the 30 patients in pneumonia severity index class 5 were most likely to have a BC‐driven antibiotic change, though in at most 1 of these patients was associated with a resistant organism. Sanyal et al.23 stratified patients by severity based on expert guidelines. They found that 19 of 174patients had severe CAP that did not respond to the initial antibiotic regimen, with 1 having a BC‐driven antibiotic change; this was due to resistance.
Only 1 study reported an outcome other than antibiotic change, which in this case was duration of parenteral therapy. In the study, 5 of 43 patients with true‐positive BCs remained on intravenous antibiotics for the full course of treatment probably due to bacteremia alone.11
The direct cost of BCs per BC‐directed antibiotic change (or total cost of BCs if there was no antibiotic change) was reported in 6 studies and, not adjusted for inflation, ranged from $1550 to $8000 (U.S.).11, 15, 18, 19, 21, 22
Quality of the Studies
A detailed listing of the strengths and weaknesses of each study is provided in the Appendix. Briefly, all 15 studies included in this review were observational. Most did not prospectively require BCs in all patients admitted with CAP. This could have biased the results in favor of BC utility as physicians presumably order BCs in patients with a higher probability of bacteremia. Conversely, several studies did not explicitly require two sets of BCs or that BCs be done prior to antibiotics, so they may not have revealed the maximum utility of BCs. The 2 studies limited to pneumococcal bacteremia and described in the secondary analysis were inherently biased against BC utility, as pneumococcus is more likely to be antibiotic‐sensitive than other CAP pathogens.
Eligibility was based only on an emergency department (ED)/admission diagnosis of CAP, a criteria that approximates real world practice, in 3 studies.19, 21, 25 The other studies required either a confirmatory radiograph or a hospital discharge diagnosis of pneumonia. Consequent ED/admission misdiagnosis rates were 3%, 8%, 11%, 24% in the 4 studies that reported them;14, 17, 22, 27 the final diagnoses, when reported, were nearly all noninfections or proximal respiratory tract infections.22, 27
Five studies included all eligible patients.14, 1921, 25 However, 3 studies excluded 23%, 31%, and 62% of eligible patients based on risk factors for bacteremia or resistant pathogens,17, 22, 24 and the rest did not report the number excluded.
DISCUSSION
Summary of Findings
Our systematic review of the literature finds that BCs rarely alter empiric antibiotic therapy in adults hospitalized with community‐acquired pneumonia. Even when there is a change in treatment it usually is not of the type most likely to impact patient outcome, which is antibiotic broadening ultimately associated with a resistant organism. In the 13 studies that could quantify this effect, it occurred in only 0% to 1% of cases in which BCs were obtained. Antibiotic narrowing occurred in 0% to 3% of cases, with physicians often choosing not to narrow antibiotics when BC results suggested that they could do so.
Limits on BC Utility
‐Lactam‐Resistant Pneumococcus
In the studies reviewed here 50%‐90% of positive BCs grew pneumococcus, consistent with the 60% to 67% rate reported elsewhere.2, 28, 29 Pneumococci that invade the bloodstream have disproportionately low rates of ‐lactam resistance,30, 31 inherently limiting the utility of BCs for detecting inadequate empiric antibiotic therapy. Though pneumococcal resistance to ‐lactams has risen over the last 2 decades, third‐generation cephalosporins, preferred agents for CAP, are still extremely effective. Even when the organism is by historical standards moderately resistant to them, these cephalosporins at standard doses maintain bactericidal efficacy in the lung,32, 33 and their use in the setting of such resistance is not associated with higher mortality.3437 By newer laboratory standards 97% and 96% of S. pneumoniae isolates in mid‐2003 were sensitive to ceftriaxone and cefotaxime, respectively.38 Thus a major potential benefit of BCsdetecting cephalosporin‐resistant pneumococcusremains a rare occurrence.
Polymicrobial Infection
If positive BCs in CAP mostly reveal antibiotic‐sensitive pathogens, one may infer that at least they lead to narrowing of therapy. However, the studies reviewed here reveal that this usually does not happen.
One explanation for this reluctance to narrow antibiotics is that CAP is often a polymicrobial disease. When rigorous serologic testing is done, multiple pathogens are found in up to 40% of cases.39 The occult copathogen is frequently an intracellular one and thus cannot be detected by BCs. Though the evidence for empirically treating these atypical organisms is mixed,40, 41 expert guidelines recommend doing so,12 and guideline‐concordant antibiotic therapy in CAP is associated with lower mortality.42 Even in bacteremic pneumococcal CAP, monotherapy is associated with higher mortality.4346 Thus, stopping antibiotic coverage of atypical pathogens in response to BCs alone might not always be appropriate.
Prognosis
Another rationale given for ordering BCs is that bacteremic pneumonia is a morbid disease so positive BCs may demand prolonged parenteral therapy or extended hospitalization. Although mortality for bacteremic pneumococcal pneumonia (the predominantly studied variety of bacteremic pneumonia) has historically been high at 20%,47, 48 studies that have examined pneumococcal bacteremia as an independent risk factor for death in CAP have yielded mixed results.2 Moreover, it appears that patients with bacteremic pneumococcal pneumonia who reach clinical stability may be safely switched to oral antibiotics.49
It is not clear that positive BCs in pneumonia (at least in the case of S. pneumoniae) should alter the duration of parenteral therapy or hospitalization, though whether or not such effects occur in clinical practice was largely unaddressed by the studies reviewed here.
Epidemiology
One theoretical benefit of BCs is their epidemiologic value. When true‐positive in pneumonia, perhaps more than any other test they identify with great specificity at least 1 of the causative agents. Unfortunately, as discussed above, BCs alone provide an incomplete and skewed picture of the microbiology of CAP. They underestimate atypical organisms, overestimate pneumococcus, and, because bacteremic pneumococcus is more likely to be antibiotic‐susceptible, they underestimate antibiotic resistance.11 Tracking pathogens in bacteremic pneumonia may be useful nonetheless, but perhaps a more accurate method for determining etiologic trends is periodic comprehensive microbiological investigation, including BCs, sputum/bronchial cultures, and serology.
Costs
In the studies reviewed here, based on reported costs of $15 to $65 per set of BCs or per patient, BCs cost $1550 to $8000 (U.S.) per BC‐directed antibiotic change. Considering that very few of these antibiotic changes involved broadening associated with a resistant organism, the cost/benefit ratio was quite high. Today BCs may be even more expensive, as U.S. hospitals now often charge over $150 per set of BCs.50, 51
The cost of false‐positive BCs must also be taken into account. The false‐positive rate in the studies reviewed here was 0% to 10%, similar to that reported elsewhere.7 False‐positive BCs increase hospital length of stay by 3 to 5 days and hospital charges by $4400 to $8800.51, 52
Limitations of the Review
Our search strategy was designed to be sensitive and included backup methods such as searching article references and querying experts. Nevertheless, we may have missed studies, especially if there were small eligible subgroups or if determining management effects was not a primary purpose. We chose not to measure instances of antibiotic broadening that were not associated with a resistant organism, though in unusual cases (eg, Pseudomonas bacteremia) this effect of BCs may be useful.
The methodologies of the included studies were adequate to measure the key outcomes with reasonable validity. Biases were evident, though they occurred both for and against BC utility.
Eligibility varied across studies, and most investigations excluded immunocompromised or other high‐risk patient groups, which could have biased results against BC utility. However, results of these studies were consistent with those that included all patients with CAP, suggesting the degree of bias was probably small. Still, given this concern, it would be prudent not to generalize the findings of this review to immunocompromised patients. Moreover, although the critically ill and those who today would be classified as having healthcare‐associated pneumonia (HCAP)nursing home residents, the recently hospitalized, and hemodialysis patientswere included in most studies, their numbers were small, and these groups were not analyzed separately; thus, the results might not be generalizable to these populations either. Finally, the reported studies, which enrolled patients through 2003, do not reflect more recent increases in the prevalence of resistant pathogens, such as MRSA, in the community.
BCs as a Quality Measure
The adoption of BCs as a quality measure was largely predicated on the widely‐cited study by Meehan et al.,4 which showed an association between BC obtainment and reduced mortality. This study, which associated processes of care with hard outcomes such as mortality, was limited by uncontrolled confounders, including variation in hospital quality.53 A more recent study of pneumonia processes of care found no association between BC collection and mortality.54 Another study often cited to support BC use, by Arbo and Snydman,55 showed that positive BCs were associated with changes in antibiotic therapy, but it included very few pneumonia patients and did not describe results for them separately.
The inclusion of BC acquisition in 2 quality measures in the NHQM guidelines for pneumonia impacts the clinical practice of hospitals and physicians, which may be rated and reimbursed differentially based on their compliance with such measures. One of the quality measures requires BCs in patients admitted to the ICU. The other requires that ED BCs for pneumonia, if obtained, be drawn before antibiotics are given.6
The studies we reviewed are not specific to these quality measures, but are relevant to them. With regard to the first measure, all but 3 studies included patients admitted to the ICU and found BCs to be of minimal benefit overall. Our subgroup analysis of severely ill patients was unrevealing. The ICU measure is tentative in its validity, but it is not unreasonable given that these patients have a life‐threatening infection and may be at risk for bacteremia with resistant pathogens.12
The second measure, though perhaps simply seeking to maximize the potential for BCs to turn positive, depends for its validity on BCs being useful in a large proportion of patients with CAP. Though we cannot exclude the possibility that BCs benefit certain subsets of patients, such as those who are immunocompromised or have HCAP, our findings do not support obtaining BCs in all or even most adults hospitalized with CAP. This conclusion is reflected in the 2007 Infectious Diseases Society of America/American Thoracic Society management guidelines for CAP, which state than BCs are optional except for patients with severe pneumonia, some immunocompromised states, and particular radiographic abnormalities.12
With such data and guidelines in mind, a physician seeking to minimize treatment delays in a patient with pneumonia may give antibiotics early in the ED course (the basis of another quality measure) without obtaining BCs. If she later determines that the patient is particularly high‐risk for bacteremia or a resistant pathogen, should she be discouraged from ordering BCs? Experts specifically state that BCs, even after antibiotics, are warranted for such a patient.12
With the scope of medical practice captured in quality measures being so narrow, having 2 measures based on a test with such limited benefit is itself questionable.
Blood cultures (BCs) have long been a mainstay of the diagnostic evaluation of patients hospitalized with community‐acquired pneumonia (CAP). They have been strongly recommended by professional societies13 and are often expected by admitting physicians. A large retrospective study of Medicare patients with pneumonia found that obtaining BCs is associated with lower mortality.4 In 2002, when the National Hospital Quality Measures (NHQM) were introduced, BCs were included as a quality measure for pneumonia.5, 6
However, there is uncertainty about the actual utility of BCs in CAP. In large studies they are true‐positive in only 7 to 11% of cases and false‐positive in 5%,2, 7 and whether they affect clinical management has been strongly questioned.810 Their impact may be limited by slow results, low frequency of bacterial resistance to the empiric antibiotic regimen, and reluctance of physicians to narrow antibiotic coverage.9, 11 Recent updates to professional society guidelines no longer recommend BCs in all admitted CAP patients.12
To evaluate the clinical utility of BCs and the appropriateness of pnemonia quality measures based on BCs, we performed a systematic review of the literature to determine the effect of BCs on the management of adults with CAP requiring hospitalization.
PATIENTS AND METHODS
Data Sources and Searches
We searched the English‐language literature via MEDLINE (1966 through September 2007), MEDLINE‐In Process, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, and ACP Journal Club. Within each of these databases we used keywords and exploded Medical Subject Headings (MeSH) to produce the following search strategy: blood culture(s) (keyword), bacteriological techniques (MeSH), blood [microbiology] (MeSH), bacteremia [microbiology or drug therapy] (MeSH), or diagnostic tests, routine (MeSH) combined with pneumonia (keyword), pneumonia (MeSH), lower respiratory tract infection(s) (keyword), or community‐acquired infections (MeSH). To maximize capture of BC or bacteremia studies with subgroups of CAP patients we added the following search strategy: explode microbiological techniques [utilization] (MeSH), explode blood specimen collection [utilization] (MeSH), or focus bacteremia [drug therapy] (MeSH). We reviewed the reference lists of all included studies as well as those of important background articles. Finally, we asked experts to evaluate the completeness of our list.
Study Selection
We included studies in which: (1) subjects were adults hospitalized with CAP; (2) BCs were obtained at or near hospital admission; and (3) the effects of BCs on management (change in antibiotic therapy or other effects such as duration of parenteral therapy, length of hospitalization, or level of care) were reported. The first 2 requirements could be satisfied by a subgroup.
From retrieved citations, relevant abstracts were reviewed, and studies with any potential to meet inclusion criteria were chosen for full‐text review. Two authors (N.A., R.S.) independently analyzed each full‐text article to determine inclusion for data analysis. A third author (J.T.) analyzed all included and narrowly excluded articles to confirm the final list of included studies. Disagreements were resolved by discussion.
Data Extraction
For the included studies, 2 authors (N.A., K.A.) independently abstracted the following data using a standardized collection instrument: study design and setting, inclusion and exclusion criteria, number of hospitalized CAP patients in whom BCs were obtained, empiric antibiotic regimens, number of true‐positive and false‐positive BCs, bacteria isolated in true‐positive BCs, BC‐directed antibiotic narrowing, BC‐directed antibiotic broadening ultimately associated with a resistant organism, and any other management effects reported. Narrowing refers to coverage of fewer organisms, while broadening refers to coverage of a larger or different spectrum of organisms.
If a study included patients not meeting our selection criteria, our analysis was limited to the subset of patients meeting criteria. We also analyzed each study to determine whether a subgroup of severely ill patients was reported separately and whether such a group benefited from BCs. The 2 authors independently repeated all data abstraction to confirm accuracy. We attempted to contact authors for clarification when needed.
Data Synthesis
Data were synthesized by compilation of characteristic summary tables. In the primary analysis, the proportion of positive BCs (both true and false) and the frequency of BC‐directed changes in antimicrobial therapy (narrowing, or broadening ultimately associated with a resistant organism) were determined and reported for each study and then described as an aggregate range. This compilation required studies to provide a particular denominatorthe number of patients in whom BCs were performed. If a study did not do so, it was described separately in the secondary analysis, where we also assessed the cost of BCs as well as the impact of BCs in critically ill patients and on outcomes other than antibiotic change. Heterogeneity of subject inclusion and exclusion criteria and empiric antibiotic use were summarized qualitatively. Two authors (N.A., R.S.) assessed each study's quality.
DATA SYNTHESIS
Search Results
Our electronic database search yielded 3236 citations. From this list and the supplementary search of references, we reviewed 607 abstracts; of these, we selected 73 articles for full‐text review, and 15 were included in the final analysis (Figure 1). One study was narrowly excluded because it largely included CAP patients that had already been admitted to the hospital and failed an empiric antibiotic trial before BCs were obtained.13

Study Characteristics
Fifteen studies with a total of 3898 patients evaluated BC‐directed management changes in adults admitted with CAP.11, 1427 However, 2 of these, involving only patients with bacteremic pneumococcal CAP, by design could not report the number of patients that had BCs done; thus they were not included in the primary analysis.16, 25
The 13 studies amenable to the primary analysis (Table 1) all had an observational cohort design; 6 were prospective11, 18, 20, 24, 26, 27 and 7 were retrospective.14, 15, 17, 19, 2123 Sample size varied from 52 to 760 patients. Settings included university and community hospitals in the U.S. and 4 other countries, with patient enrollment spanning the years 19882003 (publication dates 19912007).
| Study Author, Year, Design, Setting | Inclusion Criteria | Exclusion Criteria | CAP Patients with BCs, n*; True‐Positive BCs, n (%); False‐Positive BCs, n (%) | BCs Directed Antibiotic Narrowing, n (%) | BCs Directed Antibiotic Broadening and Organism was Resistant, n (%) | Comments |
|---|---|---|---|---|---|---|
| ||||||
| Benenson et al.,14 2007; retrospective, U.S. suburban | ED ICD‐9 dx and discharge dx of PNA | None | n = 684; 23 (3.4); 54 (7.9) | 3 (0.4) | 0 (0) | 11% of pts with ED dx of PNA not eligible due to different dx at discharge; 25% from nursing homes, 18% recently hospitalized, 14% immunocompromised; Abxs narrowed in 3/21 eligible pts |
| Ramanujamand Rathlev,22 2006; retrospective; U.S. urban | ED, ICD‐9, and discharge dx of PNA, and ED BCs before abxs | IC, active cancer, chronic renal failure, hospitalized last 1 week, nursing home resident, aspiration | n = 289; 13 (4.5%); 13 (4.5%) | 1 (0.3%) | 0 (0%) | 532 pts screened; 3% not eligible due to different dx at discharge; of eligible pts, 9% excluded due to HCAP and 31% excluded due to other risk factors; Abxs were narrowed in 1/10 eligible pts; Cost: $8,000 for the 1 pt with abx change |
| Mountain et al.,21 2006; retrospective, Australian suburban | All pts who had BCs done in the ED during a 2‐month period (PNA pts were a subgroup) | None | n = 52; Not reported; Not reported | 1 (1.9) | 0 (0) | 52/218 study pts had clinical PNA. Overall BCs true‐positive in 6.4%, false‐positive in 7.3%; frequencies for PNA pts not reported separately; Reason for abx change (ceftriaxone to ciprofloxacin) not reported, but thought not to be associated with resistant organism (personal communication); Cost: $1,950 (U.S.) per BC that altered treatment |
| Kennedy et al.,20 2005; prospective, U.S urban | Clinical and radiographic PNA and BCs in ED or within 3 hours of admission | None | n = 385; 27 (7.0); 6.0% | 11 (2.9) | 4 (1.0) | 23% of pts from nursing homes, 22% admitted to ICU; 3/4 pts whose abxs were broadened due to a resistant organism came from nursing homes; Abxs were narrowed in 11/19 eligible pts; BCs were false‐positive in 25/414 (6%) pts, including 29 pts discharged from the ED |
| Corbo et al.,17 2004; retrospective, U.S. urban | Primary diagnosis of CAP, positive CXR, and ED BCs before abxs | IC, cancer, recent hospitalization, nursing home resident | n = 355; 33 (9.3); 37 (10.4) | 7 (2.0) | 0 (0) | 821 pts admitted with CAP; 24% not eligible due to non‐confirmatory CXR; of eligible pts, 22% excluded due to HCAP, 23% excluded due to other risk factors; 6 pts with false‐positive BCs had abx change due to BCs ‐ authors suggest hospitalization prolonged in these cases; Physicians reluctant to narrow abxs per authors |
| Campbell et al.,11 2003; prospective, Canadian multiple (19) hospitals | Two signs or sxms of PNA and positive CXR | IC, shock, direct ICU admission, chronic kidney disease, pregnant or nursing, alcoholism | n = 760; 43 (5.7); Not reported | 12 (1.6) | 2 (0.3) | 38% of pts screened with suspected CAP either ineligible or excluded due to risk factors; Abxs were narrowed in 12/35 eligible pts; In one case, BCs grew MRSA resistant to empiric abxs, but abxs had been changed before BC results available; Cost: $1550 (U.S.) per BC leading to abx change |
| Waterer and Wunderink,26 2001; prospective; U.S. urban | Signs and sxms of PNA, positive CXR, and BCs before abxs | IC, hospitalized last 30 days, nursing home residents (if non‐ambulatory) | n = 209; 29 (13.9); 9 (4.3) | 5 (2.4) | 1 (0.5) | BCs only changed management in pts in PSI class 4 and 5 |
| Theerthakarai et al.,24 2001; prospective, U.S. suburban | Acute febrile illness with respiratory sxms and a positive CXR | IC, cancer, age >65, alcoholism, IVDU, COPD, IDDM, neurologic disease, renal failure, recent abx, severe or complicated PNA | n = 74; 0 (0); 0 (0) | 0 (0%) | 0 (0%) | Very strict exclusion criteria: 62% of eligible pts excluded due to risk factors; Authors reported that 28% of included pts could have been treated as outpatients per ATS guidelines |
| Sanyal et al.,23 1999; retrospective, U.S. urban | Acute lower respiratory tract infection and positive CXR | IC, cancer, hospitalized last 12 weeks, IVDU, bronchiectasis, splenectomy, not treated per ATS guidelines | n = 174; 19 (10.9); Not reported | Not reported | 1 (0.6%) | BC‐directed antibiotic changes only reported for pts who did not respond to initial abxs, so BC‐directed narrowing could not be determined; The pt whose abxs were broadened was a nursing home resident with severe pneumonia (by ATS criteria) |
| Glerant et al.,18 1999; prospective, French suburban | Acute septic episode with respiratory sxms and positive CXR | IC, ICU admission, hospitalized last 2 weeks, aspiration | n = 53; 5 (9.4); 2 (3.8) | 0 (0) | 0 (0) | BCs done during first 48 hours so not clear how many BCs sent after hospital abxs started; 23 pts were on abxs before admission; Cost: $6006 (U.S.), no abx changes |
| Kelly,19 1998; retrospective, Australian suburban | All pts who had BCs done in the ED over a 9‐ month period (PNA pts were a subgroup) | None | n = 260; 5%; Not reported | 1% | 1% | 260/1062 study pts had PNA; 14% of all pts discharged; for CAP pts percentage not reported; False‐positive rate 3.8% for all pts, but not reported separately for PNA pts; 1% of PNA pts had abx change due to BCs; type of change not reported, hence reporting of 1% in outcome columns; Cost: $4800 (U.S.) per abx change |
| Chalasani et al.,15 1995; retrospective; U.S. urban | Dx of PNA, respiratory sxms, positive CXR, and 2 sets of BCs before abxs | IC, cancer, hospitalized last 2 weeks, nursing home resident | n = 517; 34 (6.6); 25 (4.8) | 7 (1.4) | 0 (0) | 1250 pts screened with discharge dx of PNA; 59% either ineligible or excluded due to risk factors (authors did not report number ineligible due to the BC requirement); In one case, BCs grew H. influenzae resistant to empiric abxs, but sputum cultures drove the abx change; Cost: $4875 per abx change |
| Woodhead et al.,27 1991; prospective, British urban (2 hospitals) | Clinical features of CAP and positive CXR | IC, cancer, admitted to geriatric or communicable disease ward | n = 86; 9 (10.5%); Not reported | 2 (2.3) | 1 (1.2) | 8% of pts meeting inclusion and exclusion criteria were later excluded due to different dx at discharge |
Included patients were usually required to have clinical features of pneumonia and a confirmatory chest x‐ray. Treating physicians were required to obtain BCs (either by study or hospital protocol) in only 3 studies14, 22, 24 and in a subgroup of another study;11 otherwise the performance of BCs was left to physician discretion.
Nine studies excluded patients who were immunocompromised,11, 15, 17, 18, 2224, 26, 27 a label that was often incompletely defined. Otherwise, exclusion criteria were variable. Notably, only 3 studies excluded patients admitted to the intensive care unit (ICU),11, 18, 24 while 6 excluded patients with cancer15, 17, 2224, 27 and 6 excluded either nursing home residents15, 17, 22, 26 or the elderly (de facto exclusion of most nursing home residents).24, 27
Empiric antibiotic regimens, where reported, were predominantly cephalosporin plus macrolide combinations in 4 studies,17, 2224 fluoroquinolones in 3 studies,11, 14, 26 and penicillin or 1 of its derivatives in 1 study.27
Concerning the 2 studies not included in the primary analysis, the one by Waterer et al.25 was a retrospective review of all cases of pneumococcal bacteremia (n = 74) associated with an admission diagnosis of CAP (N = 1805) in a US urban hospital over a 3‐year period. The one by Chang et al.16 was a retrospective case‐control study of 288 randomly‐selected, immunocompetent Medicare patients with bacteremic pneumococcal CAP who survived to discharge. They were matched 1:1 with blood and sputum culture‐negative controls to study the rate of fluoroquinolone use at discharge in the 2 groups.
Study Findings
Primary Analysis
As shown in Table 1, BCs were positive for a true pathogen in 0% to 14% of cases. Details of microbiology and empiric antibiotic selection are reported in Table 2. S. pneumoniae was by far the most common pathogen: of the 9 studies that had positive BCs and reported the organisms, S. pneumoniae represented 50% to 91% of the pathogens, with penicillin‐resistance found in 0% to 20%.11, 14, 15, 17, 18, 20, 22, 23, 26 S. aureus was next most common, occurring in 6 studies and growing in 3% to 23% of positive BCs;11, 14, 17, 20, 23, 26 its sensitivity to methicillin was reported in 3 studies, with methicillin‐resistant S. aureus (MRSA) representing 0/3, 3/7, and 1/1 of cases.14, 20, 23 E. coli represented 3% to 11% of pathogens in 6 studies,11, 14, 15, 20, 23, 26 while H. influenzae represented 2% to 15% of pathogens in 7 studies.11, 14, 15, 18, 22, 23, 26
| Study: Author, Year | Empiric Antibiotics Given: Frequency, Agent | Bacteria Isolated in True‐Positive BCs: n, Organism | Organisms in BCs Resistant to Empiric Antibiotics |
|---|---|---|---|
| |||
| Benenson et al.,14 2007 | Mild to moderate PNA: levofloxacin; If ICU admission: levofloxacin + azithromycin; If HCAP: levofloxacin + clindamycin; If risk for MRSA: added vancomycin; If structural lung disease: added tobramycin | 14 S. pneumoniae; 3 S. aureus (all MSSA); 2 Group B Strep; 2 H. influenzae; 1 E. coli; 1 Group A Strep | None |
| Ramanujam and Rathlev,22 2006 | Ceftriaxone + oral azithromycin | 11 S. pneumoniae (1 PCN interm res); 2 H. influenzae | None |
| Mountain et al.,21 2006 | Not reported | Not reported completely | None |
| Kennedy et al.,20 2005 | Not reported | 15 S. pneumoniae (3 PCN res); 7 S. aureus (3 MRSA); 3 E. coli; 1 Coagulase‐negative Staph; 1 Pseudomonas; 1 Proteus; 1 Moraxella; 1 E. faecalis | 2 MRSA; 1 MSSA (res to levofloxacin, clindamycin); 1 E. coli (res to levofloxacin) |
| Corbo et al.,17 2004 | 48% ceftriaxone + macrolide; 21% cephalosporin only; 6% quinolone only | 30 S. pneumoniae; 2 S. aureus (# MRSA not reported); 1 Staph haemolyticus | None |
| Campbell et al.,11 2003 | 55% levofloxacin; 45% antibiotic not reported | 30 S. pneumoniae (1 PCN res); 5 S. aureus (total # MRSA not reported); 5 E. coli; 1 H. influenzae; 1 E. faecalis; 1 K. pneumoniae; 1 Enterobacter | 1 MRSA (antibiotic changed before BC results available); 1 MSSA (res not reported); 1 S. pneumoniae (PCN res) |
| Waterer and Wunderink,26 2001 | 60% quinolone only; 25% quinolone + other antibiotic(s) | 20 S. pneumoniae (3 PCN res); 3 S. viridans; 1 H. influenzae; 1 S. aureus (# MRSA not reported); 1 Enterobacter; 1 E. coli; 1 Group B Strep; 1 Group D Strep; 1 Group G Strep; 1 Acinetobacter | 1 Group D Strep (res to levofloxacin) |
| Theerthakarai et al.,24 2001 | Cephalosporin + macrolide | None | None |
| Sanyal et al.,23 1999 | Severe CAP: erythromycin + ceftazidime or ticarcillin/clavulanate; Nonsevere CAP: 76% cefuroxime + erythromycin, 18% cefuroxime only | 14 S. pneumoniae (0 PCN res); 2 H. influenzae; 1 S. aureus (MRSA); 1 K. pneumoniae; 1 E. coli | 1 MRSA |
| Glerant et al.,18 1999 | Not reported | 4 S. pneumoniae (0 PCN res); 1 H. influenzae | None |
| Kelly,19 1998 | Not reported | Not reported | Cannot determine |
| Chalasani et al.,15 1995 | Not reported | 29 S. pneumoniae (0 PCN res); 3 H. influenzae; 1 S. pyogenes; 1 E. coli | H. influenzae (sputum culture drove the antibiotic change) |
| Woodhead et al.,27 1991 | 78% included penicillin, aminopenicillin, or amoxicillin/clavulanate; 33% included erythromycin; 21% ‐lactam + erythromycin | Not reported separately for BCs | E. coli (res to erythromycin) |
| Chang et al.,16 2005 | BC+/Controls: 34%/21%/Quinolones; 86%/88%/ ‐lactam; 1%/1%/Amox/PCN; 38%/37%/ Macrolide | 288 S. pneumoniae (only organism, by design) | Not reported |
| Waterer et al.,25 1999 | 38% Cephalosporin + macrolide other; 27% Quinolone other | 74 S. pneumoniae (only organism, by design); 11 PCN interm res; 4 PCN res | 2 S. pneumoniae (both resistant; degree of resistance not specified) |
In the 8 studies that reported false‐positive BCs, the false‐positive rate was 0% to 10%,14, 15, 17, 18, 20, 22, 24, 26 with 5 studies finding comparable false‐positive and true‐positive BC rates15, 17, 20, 22, 24 and 1 study finding a substantially higher frequency of false‐positive than true‐positive BCs (Table 1).14
BCs led to narrowing of antibiotic coverage in 0% to 3% of cases (Table 1). Four studies reported that physicians narrowed antibiotics when BCs indicated that it was possible to do so, but only in 10%, 14%, 34%, and 58% of eligible cases.11, 14, 20, 22
BCs led to antibiotic broadening ultimately associated with a resistant organism in 0% to 1% of cases (Table 1). The pathogens were MRSA (3), methicillin‐sensitive S. aureus (2), E. coli (2), S. pneumoniae (1), and Group D Streptococcus (1). Details about these patients' medical histories and demographics were absent or sparse in all but 1 study.20 For several of the above cases it was not explicitly stated that BCs directed the antibiotic changes, though it was usually implied; thus we assumed causation.
Secondary Analyses
In the pneumococcal bacteremia study by Waterer et al.,25 BCs altered management in 31 of the 74 cases of pneumococcemia, but in only 2 patients was this associated with antibiotic resistance. Most of the other 29 cases involved narrowing of antibiotics, though switching to penicillin or dropping atypical coverage occurred in only 22% and 37% of eligible patients, respectively. In the study by Chang et al.,16 there was no significant difference in fluoroquinolone use at discharge between the pneumococcemic and culture‐negative groups (the primary endpoint), though there was significantly higher ‐lactam use and lower macrolide use in the pneumococcemic patients at discharge. From the data provided it was not possible to determine how often antibiotic broadening occurred.
Only 2 of the 15 studies stratified management effects based on severity of illness, and neither specified the proportion of severely ill patients admitted to the ICU. Waterer and Wunderink26 prospectively hypothesized that sicker patients were more likely to benefit from BCs. They found that the 30 patients in pneumonia severity index class 5 were most likely to have a BC‐driven antibiotic change, though in at most 1 of these patients was associated with a resistant organism. Sanyal et al.23 stratified patients by severity based on expert guidelines. They found that 19 of 174patients had severe CAP that did not respond to the initial antibiotic regimen, with 1 having a BC‐driven antibiotic change; this was due to resistance.
Only 1 study reported an outcome other than antibiotic change, which in this case was duration of parenteral therapy. In the study, 5 of 43 patients with true‐positive BCs remained on intravenous antibiotics for the full course of treatment probably due to bacteremia alone.11
The direct cost of BCs per BC‐directed antibiotic change (or total cost of BCs if there was no antibiotic change) was reported in 6 studies and, not adjusted for inflation, ranged from $1550 to $8000 (U.S.).11, 15, 18, 19, 21, 22
Quality of the Studies
A detailed listing of the strengths and weaknesses of each study is provided in the Appendix. Briefly, all 15 studies included in this review were observational. Most did not prospectively require BCs in all patients admitted with CAP. This could have biased the results in favor of BC utility as physicians presumably order BCs in patients with a higher probability of bacteremia. Conversely, several studies did not explicitly require two sets of BCs or that BCs be done prior to antibiotics, so they may not have revealed the maximum utility of BCs. The 2 studies limited to pneumococcal bacteremia and described in the secondary analysis were inherently biased against BC utility, as pneumococcus is more likely to be antibiotic‐sensitive than other CAP pathogens.
Eligibility was based only on an emergency department (ED)/admission diagnosis of CAP, a criteria that approximates real world practice, in 3 studies.19, 21, 25 The other studies required either a confirmatory radiograph or a hospital discharge diagnosis of pneumonia. Consequent ED/admission misdiagnosis rates were 3%, 8%, 11%, 24% in the 4 studies that reported them;14, 17, 22, 27 the final diagnoses, when reported, were nearly all noninfections or proximal respiratory tract infections.22, 27
Five studies included all eligible patients.14, 1921, 25 However, 3 studies excluded 23%, 31%, and 62% of eligible patients based on risk factors for bacteremia or resistant pathogens,17, 22, 24 and the rest did not report the number excluded.
DISCUSSION
Summary of Findings
Our systematic review of the literature finds that BCs rarely alter empiric antibiotic therapy in adults hospitalized with community‐acquired pneumonia. Even when there is a change in treatment it usually is not of the type most likely to impact patient outcome, which is antibiotic broadening ultimately associated with a resistant organism. In the 13 studies that could quantify this effect, it occurred in only 0% to 1% of cases in which BCs were obtained. Antibiotic narrowing occurred in 0% to 3% of cases, with physicians often choosing not to narrow antibiotics when BC results suggested that they could do so.
Limits on BC Utility
‐Lactam‐Resistant Pneumococcus
In the studies reviewed here 50%‐90% of positive BCs grew pneumococcus, consistent with the 60% to 67% rate reported elsewhere.2, 28, 29 Pneumococci that invade the bloodstream have disproportionately low rates of ‐lactam resistance,30, 31 inherently limiting the utility of BCs for detecting inadequate empiric antibiotic therapy. Though pneumococcal resistance to ‐lactams has risen over the last 2 decades, third‐generation cephalosporins, preferred agents for CAP, are still extremely effective. Even when the organism is by historical standards moderately resistant to them, these cephalosporins at standard doses maintain bactericidal efficacy in the lung,32, 33 and their use in the setting of such resistance is not associated with higher mortality.3437 By newer laboratory standards 97% and 96% of S. pneumoniae isolates in mid‐2003 were sensitive to ceftriaxone and cefotaxime, respectively.38 Thus a major potential benefit of BCsdetecting cephalosporin‐resistant pneumococcusremains a rare occurrence.
Polymicrobial Infection
If positive BCs in CAP mostly reveal antibiotic‐sensitive pathogens, one may infer that at least they lead to narrowing of therapy. However, the studies reviewed here reveal that this usually does not happen.
One explanation for this reluctance to narrow antibiotics is that CAP is often a polymicrobial disease. When rigorous serologic testing is done, multiple pathogens are found in up to 40% of cases.39 The occult copathogen is frequently an intracellular one and thus cannot be detected by BCs. Though the evidence for empirically treating these atypical organisms is mixed,40, 41 expert guidelines recommend doing so,12 and guideline‐concordant antibiotic therapy in CAP is associated with lower mortality.42 Even in bacteremic pneumococcal CAP, monotherapy is associated with higher mortality.4346 Thus, stopping antibiotic coverage of atypical pathogens in response to BCs alone might not always be appropriate.
Prognosis
Another rationale given for ordering BCs is that bacteremic pneumonia is a morbid disease so positive BCs may demand prolonged parenteral therapy or extended hospitalization. Although mortality for bacteremic pneumococcal pneumonia (the predominantly studied variety of bacteremic pneumonia) has historically been high at 20%,47, 48 studies that have examined pneumococcal bacteremia as an independent risk factor for death in CAP have yielded mixed results.2 Moreover, it appears that patients with bacteremic pneumococcal pneumonia who reach clinical stability may be safely switched to oral antibiotics.49
It is not clear that positive BCs in pneumonia (at least in the case of S. pneumoniae) should alter the duration of parenteral therapy or hospitalization, though whether or not such effects occur in clinical practice was largely unaddressed by the studies reviewed here.
Epidemiology
One theoretical benefit of BCs is their epidemiologic value. When true‐positive in pneumonia, perhaps more than any other test they identify with great specificity at least 1 of the causative agents. Unfortunately, as discussed above, BCs alone provide an incomplete and skewed picture of the microbiology of CAP. They underestimate atypical organisms, overestimate pneumococcus, and, because bacteremic pneumococcus is more likely to be antibiotic‐susceptible, they underestimate antibiotic resistance.11 Tracking pathogens in bacteremic pneumonia may be useful nonetheless, but perhaps a more accurate method for determining etiologic trends is periodic comprehensive microbiological investigation, including BCs, sputum/bronchial cultures, and serology.
Costs
In the studies reviewed here, based on reported costs of $15 to $65 per set of BCs or per patient, BCs cost $1550 to $8000 (U.S.) per BC‐directed antibiotic change. Considering that very few of these antibiotic changes involved broadening associated with a resistant organism, the cost/benefit ratio was quite high. Today BCs may be even more expensive, as U.S. hospitals now often charge over $150 per set of BCs.50, 51
The cost of false‐positive BCs must also be taken into account. The false‐positive rate in the studies reviewed here was 0% to 10%, similar to that reported elsewhere.7 False‐positive BCs increase hospital length of stay by 3 to 5 days and hospital charges by $4400 to $8800.51, 52
Limitations of the Review
Our search strategy was designed to be sensitive and included backup methods such as searching article references and querying experts. Nevertheless, we may have missed studies, especially if there were small eligible subgroups or if determining management effects was not a primary purpose. We chose not to measure instances of antibiotic broadening that were not associated with a resistant organism, though in unusual cases (eg, Pseudomonas bacteremia) this effect of BCs may be useful.
The methodologies of the included studies were adequate to measure the key outcomes with reasonable validity. Biases were evident, though they occurred both for and against BC utility.
Eligibility varied across studies, and most investigations excluded immunocompromised or other high‐risk patient groups, which could have biased results against BC utility. However, results of these studies were consistent with those that included all patients with CAP, suggesting the degree of bias was probably small. Still, given this concern, it would be prudent not to generalize the findings of this review to immunocompromised patients. Moreover, although the critically ill and those who today would be classified as having healthcare‐associated pneumonia (HCAP)nursing home residents, the recently hospitalized, and hemodialysis patientswere included in most studies, their numbers were small, and these groups were not analyzed separately; thus, the results might not be generalizable to these populations either. Finally, the reported studies, which enrolled patients through 2003, do not reflect more recent increases in the prevalence of resistant pathogens, such as MRSA, in the community.
BCs as a Quality Measure
The adoption of BCs as a quality measure was largely predicated on the widely‐cited study by Meehan et al.,4 which showed an association between BC obtainment and reduced mortality. This study, which associated processes of care with hard outcomes such as mortality, was limited by uncontrolled confounders, including variation in hospital quality.53 A more recent study of pneumonia processes of care found no association between BC collection and mortality.54 Another study often cited to support BC use, by Arbo and Snydman,55 showed that positive BCs were associated with changes in antibiotic therapy, but it included very few pneumonia patients and did not describe results for them separately.
The inclusion of BC acquisition in 2 quality measures in the NHQM guidelines for pneumonia impacts the clinical practice of hospitals and physicians, which may be rated and reimbursed differentially based on their compliance with such measures. One of the quality measures requires BCs in patients admitted to the ICU. The other requires that ED BCs for pneumonia, if obtained, be drawn before antibiotics are given.6
The studies we reviewed are not specific to these quality measures, but are relevant to them. With regard to the first measure, all but 3 studies included patients admitted to the ICU and found BCs to be of minimal benefit overall. Our subgroup analysis of severely ill patients was unrevealing. The ICU measure is tentative in its validity, but it is not unreasonable given that these patients have a life‐threatening infection and may be at risk for bacteremia with resistant pathogens.12
The second measure, though perhaps simply seeking to maximize the potential for BCs to turn positive, depends for its validity on BCs being useful in a large proportion of patients with CAP. Though we cannot exclude the possibility that BCs benefit certain subsets of patients, such as those who are immunocompromised or have HCAP, our findings do not support obtaining BCs in all or even most adults hospitalized with CAP. This conclusion is reflected in the 2007 Infectious Diseases Society of America/American Thoracic Society management guidelines for CAP, which state than BCs are optional except for patients with severe pneumonia, some immunocompromised states, and particular radiographic abnormalities.12
With such data and guidelines in mind, a physician seeking to minimize treatment delays in a patient with pneumonia may give antibiotics early in the ED course (the basis of another quality measure) without obtaining BCs. If she later determines that the patient is particularly high‐risk for bacteremia or a resistant pathogen, should she be discouraged from ordering BCs? Experts specifically state that BCs, even after antibiotics, are warranted for such a patient.12
With the scope of medical practice captured in quality measures being so narrow, having 2 measures based on a test with such limited benefit is itself questionable.
- ,,,,,.Practice guidelines for the management of community‐acquired pneumonia in adults. Infectious diseases society of America.Clin Infect Dis.2000;31:347–382.
- ,,,,.Canadian guidelines for the initial management of community‐acquired pneumonia: an evidence‐based update by the Canadian infectious diseases society and the Canadian thoracic society. The Canadian community‐acquired pneumonia working group.Clin Infect Dis.2000;31:383–421.
- ,,, et al.Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,, et al.Quality of care, process, and outcomes in elderly patients with pneumonia.JAMA.1997;278:2080–2084.
- Hospital quality initiative, overview, centers for Medicare and Medicaid services. Available at: http://www.cms.hhs.gov/HospitalQualityInits. Accessed September2007.
- Specifications manual for national hospital quality measures, version 2.3b. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Current+NHQM+Manual.htm. Accessed October2007.
- ,,,.Predicting bacteremia in patients with community‐acquired pneumonia.Am J Respir Crit Care Med.2004;169:342–347.
- ,.The joint commission on accreditation of healthcare organizations and center for Medicare and Medicaid services community‐acquired pneumonia initiative: what went wrong?Ann Emerg Med.2005;46:409–411.
- .Blood cultures in community‐acquired pneumonia: Are we ready to quit?Chest.2003;123:977–978.
- .Blood cultures for community‐acquired pneumonia: piecing together a mosaic for doing less.Am J Respir Crit Care Med.2004;169:327–328.
- ,,,,.The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community‐acquired pneumonia: a prospective observational study.Chest.2003;123:1142–1150.
- ,,, et al.Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults.Clin Infect Dis.2007;44 (Suppl 2):S27–S72.
- ,,,,,.Value of routine microbial investigation in community‐acquired pneumonia treated in a tertiary care center.Respiration.1996;63:164–169.
- ,,,.Selective use of blood cultures in emergency department pneumonia patients.J Emerg Med.2007;33:1–8.
- ,,,,.Clinical utility of blood cultures in adult patients with community‐acquired pneumonia without defined underlying risks.Chest.1995;108:932–936.
- ,,,,,.Blood culture and susceptibility results and allergy history do not influence fluoroquinolone use in the treatment of community‐acquired pneumonia.Pharmacotherapy.2005;25:59–66.
- ,,,.Limited usefulness of initial blood cultures in community acquired pneumonia.Emerg Med J.2004;21:446–448.
- ,,,,.Utility of blood cultures in community‐acquired pneumonia requiring hospitalization: Influence of antibiotic treatment before admission.Respir Med.1999;93:208–212.
- .Clinical impact of blood cultures taken in the emergency department.J Accid Emerg Med.1998;15:254–256.
- ,,,,,.Do emergency department blood cultures change practice in patients with pneumonia?Ann Emerg Med.2005;46:393–400.
- ,,,.Blood cultures ordered in the adult emergency department are rarely useful.Eur J Emerg Med.2006;13:76–79.
- ,.Blood cultures do not change management in hospitalized patients with community‐acquired pneumonia.Acad Emerg Med.2006;13:740–745.
- ,,,,,.Initial microbiologic studies did not affect outcome in adults hospitalized with community‐acquired pneumonia.Am J Respir Crit Care Med.1999;160:346–348.
- ,,,,.Nonvalue of the initial microbiological studies in the management of nonsevere community‐acquired pneumonia.Chest.2001;119:181–184.
- ,,.The impact of blood cultures on antibiotic therapy in pneumococcal pneumonia.Chest.1999;116:1278–1281.
- ,.The influence of the severity of community‐acquired pneumonia on the usefulness of blood cultures.Respir Med.2001;95:78–82.
- ,,,,.The value of routine microbial investigation in community‐acquired pneumonia.Respir Med.1991;85:313–317.
- ,,, et al.Study of community acquired pneumonia aetiology (scapa) in adults admitted to hospital: implications for management guidelines.Thorax.2001;56:296–301.
- ,.Etiology of community‐acquired pneumonia.Clin Chest Med.2005;26:47–55.
- ,,,,.Early predictors of mortality in pneumococcal bacteraemia.Ann Acad Med Singapore.2005;34:426–431.
- ,,,.Penicillin‐nonsusceptible Streptococcus pneumoniae at San Francisco general hospital.Clin Infect Dis.1999;29:580–585.
- .Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men.Clin Infect Dis.1998;26:1–10; quiz 11–12.
- .The significance of serum vs tissue levels of antibiotics in the treatment of penicillin‐resistant Streptococcus pneumoniae and community‐acquired pneumonia: are we looking in the wrong place?Chest.1999;116:535–538.
- ,,, et al.Pneumonia acquired in the community through drug‐resistant Streptococcus pneumoniae.Am J Respir Crit Care Med.1999;159:1835–1842.
- ,,, et al.The effect of cephalosporin resistance on mortality in adult patients with nonmeningeal systemic pneumococcal infections.Am J Med.2002;113:120–126.
- ,,, et al.Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain.N Engl J Med.1995;333:474–480.
- ,,, et al.An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome.Clin Infect Dis.2003;37:230–237.
- ,,,,,.Tracking the implementation of NCCLS m100‐s12 expanded‐spectrum cephalosporin MIC breakpoints for non‐meningeal isolates of Streptococcus pneumoniae by clinical laboratories in the united states during 2002 and 2003.Ann Clin Microbiol Antimicrob.2004;3:1.
- ,,, et al.Multiple pathogens in adult patients admitted with community‐acquired pneumonia: a one year prospective study of 346 consecutive patients.Thorax.1996;51:179–184.
- ,,,,.How good is the evidence for the recommended empirical antimicrobial treatment of patients hospitalized because of community‐acquired pneumonia? A systematic review.J Antimicrob Chemother.2003;52:555–563.
- ,,,.Empirical atypical coverage for inpatients with community‐acquired pneumonia: systematic review of randomized controlled trials.Arch Intern Med.2005;165:1992–2000.
- ,,,.Impact of guideline‐concordant empiric antibiotic therapy in community‐acquired pneumonia.Am J Med.2006;119:865–871.
- ,,, et al.Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia [see comment].Am J Respir Crit Care Med.2004;170:440–444.
- ,,, et al.Addition of a macrolide to a beta‐lactam‐based empirical antibiotic regimen is associated with lower in‐hospital mortality for patients with bacteremic pneumococcal pneumonia. [see comment].Clin Infect Dis.2003;36:389–395.
- ,,.Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia.Arch Intern Med.2001;161:1837–1842.
- ,,, et al.Clinical characteristics at initial presentation and impact of dual therapy on the outcome of bacteremic Streptococcus pneumoniae pneumonia in adults.Can Respir J.2004;11:589–593.
- ,.Pneumococcal bacteremia with special reference to bacteremic pneumococcal pneumonia.Arch Intern Med.1964;60:759–776.
- ,,, et al.Prognosis and outcomes of patients with community‐acquired pneumonia. A meta‐analysis.JAMA.1996;275:134–141.
- ,.Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community‐acquired Streptococcus pneumoniae pneumonia.Arch Intern Med.2001;161:848–850.
- Cleveland Clinic patient price information list. Available at:http://cms.clevelandclinic.org/documents/CCMain_HB197_2007.pdf. Accessed January2008.
- ,.Analysis of strategies to improve cost effectiveness of blood cultures.J Hosp Med.2006;1:272–276.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,,,.Patient and hospital characteristics associated with recommended processes of care for elderly patients hospitalized with pneumonia: results from the Medicare quality indicator system pneumonia module.Arch Intern Med.2002;162:827–833.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,.Influence of blood culture results on antibiotic choice in the treatment of bacteremia.Arch Intern Med.1994;154:2641–2645.
- ,,,,,.Practice guidelines for the management of community‐acquired pneumonia in adults. Infectious diseases society of America.Clin Infect Dis.2000;31:347–382.
- ,,,,.Canadian guidelines for the initial management of community‐acquired pneumonia: an evidence‐based update by the Canadian infectious diseases society and the Canadian thoracic society. The Canadian community‐acquired pneumonia working group.Clin Infect Dis.2000;31:383–421.
- ,,, et al.Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,, et al.Quality of care, process, and outcomes in elderly patients with pneumonia.JAMA.1997;278:2080–2084.
- Hospital quality initiative, overview, centers for Medicare and Medicaid services. Available at: http://www.cms.hhs.gov/HospitalQualityInits. Accessed September2007.
- Specifications manual for national hospital quality measures, version 2.3b. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Current+NHQM+Manual.htm. Accessed October2007.
- ,,,.Predicting bacteremia in patients with community‐acquired pneumonia.Am J Respir Crit Care Med.2004;169:342–347.
- ,.The joint commission on accreditation of healthcare organizations and center for Medicare and Medicaid services community‐acquired pneumonia initiative: what went wrong?Ann Emerg Med.2005;46:409–411.
- .Blood cultures in community‐acquired pneumonia: Are we ready to quit?Chest.2003;123:977–978.
- .Blood cultures for community‐acquired pneumonia: piecing together a mosaic for doing less.Am J Respir Crit Care Med.2004;169:327–328.
- ,,,,.The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community‐acquired pneumonia: a prospective observational study.Chest.2003;123:1142–1150.
- ,,, et al.Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults.Clin Infect Dis.2007;44 (Suppl 2):S27–S72.
- ,,,,,.Value of routine microbial investigation in community‐acquired pneumonia treated in a tertiary care center.Respiration.1996;63:164–169.
- ,,,.Selective use of blood cultures in emergency department pneumonia patients.J Emerg Med.2007;33:1–8.
- ,,,,.Clinical utility of blood cultures in adult patients with community‐acquired pneumonia without defined underlying risks.Chest.1995;108:932–936.
- ,,,,,.Blood culture and susceptibility results and allergy history do not influence fluoroquinolone use in the treatment of community‐acquired pneumonia.Pharmacotherapy.2005;25:59–66.
- ,,,.Limited usefulness of initial blood cultures in community acquired pneumonia.Emerg Med J.2004;21:446–448.
- ,,,,.Utility of blood cultures in community‐acquired pneumonia requiring hospitalization: Influence of antibiotic treatment before admission.Respir Med.1999;93:208–212.
- .Clinical impact of blood cultures taken in the emergency department.J Accid Emerg Med.1998;15:254–256.
- ,,,,,.Do emergency department blood cultures change practice in patients with pneumonia?Ann Emerg Med.2005;46:393–400.
- ,,,.Blood cultures ordered in the adult emergency department are rarely useful.Eur J Emerg Med.2006;13:76–79.
- ,.Blood cultures do not change management in hospitalized patients with community‐acquired pneumonia.Acad Emerg Med.2006;13:740–745.
- ,,,,,.Initial microbiologic studies did not affect outcome in adults hospitalized with community‐acquired pneumonia.Am J Respir Crit Care Med.1999;160:346–348.
- ,,,,.Nonvalue of the initial microbiological studies in the management of nonsevere community‐acquired pneumonia.Chest.2001;119:181–184.
- ,,.The impact of blood cultures on antibiotic therapy in pneumococcal pneumonia.Chest.1999;116:1278–1281.
- ,.The influence of the severity of community‐acquired pneumonia on the usefulness of blood cultures.Respir Med.2001;95:78–82.
- ,,,,.The value of routine microbial investigation in community‐acquired pneumonia.Respir Med.1991;85:313–317.
- ,,, et al.Study of community acquired pneumonia aetiology (scapa) in adults admitted to hospital: implications for management guidelines.Thorax.2001;56:296–301.
- ,.Etiology of community‐acquired pneumonia.Clin Chest Med.2005;26:47–55.
- ,,,,.Early predictors of mortality in pneumococcal bacteraemia.Ann Acad Med Singapore.2005;34:426–431.
- ,,,.Penicillin‐nonsusceptible Streptococcus pneumoniae at San Francisco general hospital.Clin Infect Dis.1999;29:580–585.
- .Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men.Clin Infect Dis.1998;26:1–10; quiz 11–12.
- .The significance of serum vs tissue levels of antibiotics in the treatment of penicillin‐resistant Streptococcus pneumoniae and community‐acquired pneumonia: are we looking in the wrong place?Chest.1999;116:535–538.
- ,,, et al.Pneumonia acquired in the community through drug‐resistant Streptococcus pneumoniae.Am J Respir Crit Care Med.1999;159:1835–1842.
- ,,, et al.The effect of cephalosporin resistance on mortality in adult patients with nonmeningeal systemic pneumococcal infections.Am J Med.2002;113:120–126.
- ,,, et al.Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain.N Engl J Med.1995;333:474–480.
- ,,, et al.An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome.Clin Infect Dis.2003;37:230–237.
- ,,,,,.Tracking the implementation of NCCLS m100‐s12 expanded‐spectrum cephalosporin MIC breakpoints for non‐meningeal isolates of Streptococcus pneumoniae by clinical laboratories in the united states during 2002 and 2003.Ann Clin Microbiol Antimicrob.2004;3:1.
- ,,, et al.Multiple pathogens in adult patients admitted with community‐acquired pneumonia: a one year prospective study of 346 consecutive patients.Thorax.1996;51:179–184.
- ,,,,.How good is the evidence for the recommended empirical antimicrobial treatment of patients hospitalized because of community‐acquired pneumonia? A systematic review.J Antimicrob Chemother.2003;52:555–563.
- ,,,.Empirical atypical coverage for inpatients with community‐acquired pneumonia: systematic review of randomized controlled trials.Arch Intern Med.2005;165:1992–2000.
- ,,,.Impact of guideline‐concordant empiric antibiotic therapy in community‐acquired pneumonia.Am J Med.2006;119:865–871.
- ,,, et al.Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia [see comment].Am J Respir Crit Care Med.2004;170:440–444.
- ,,, et al.Addition of a macrolide to a beta‐lactam‐based empirical antibiotic regimen is associated with lower in‐hospital mortality for patients with bacteremic pneumococcal pneumonia. [see comment].Clin Infect Dis.2003;36:389–395.
- ,,.Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia.Arch Intern Med.2001;161:1837–1842.
- ,,, et al.Clinical characteristics at initial presentation and impact of dual therapy on the outcome of bacteremic Streptococcus pneumoniae pneumonia in adults.Can Respir J.2004;11:589–593.
- ,.Pneumococcal bacteremia with special reference to bacteremic pneumococcal pneumonia.Arch Intern Med.1964;60:759–776.
- ,,, et al.Prognosis and outcomes of patients with community‐acquired pneumonia. A meta‐analysis.JAMA.1996;275:134–141.
- ,.Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community‐acquired Streptococcus pneumoniae pneumonia.Arch Intern Med.2001;161:848–850.
- Cleveland Clinic patient price information list. Available at:http://cms.clevelandclinic.org/documents/CCMain_HB197_2007.pdf. Accessed January2008.
- ,.Analysis of strategies to improve cost effectiveness of blood cultures.J Hosp Med.2006;1:272–276.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,,,.Patient and hospital characteristics associated with recommended processes of care for elderly patients hospitalized with pneumonia: results from the Medicare quality indicator system pneumonia module.Arch Intern Med.2002;162:827–833.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,.Influence of blood culture results on antibiotic choice in the treatment of bacteremia.Arch Intern Med.1994;154:2641–2645.
One Hundred Years Later
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 36 year‐old male physician was admitted to a Baltimore hospital in April 1907 with weight loss, weakness, arthralgias, and abdominal distension that had progressed over 5 years.
In 1907, major causes of unexplained weight loss included tuberculosis, hyperthyroidism, cancer, and diabetes. Arthralgias and weakness are not specific. The insidious progression over 5 years narrows the infectious possibilities; tuberculosis and syphilis are important considerations. Since surgical removal was the main treatment for malignancy in 1907, a history of prior surgery might point to a previously diagnosed malignancy that is now progressing.
Five years earlier, while visiting Turkey as a medical missionary, he first noted the onset of arthralgias that lasted 6 to 8 hours and occurred 3 to 4 times per week. Over time, these attacks lasted up to 24 hours and became associated with warmth, swelling, and tenderness of both small and large joints. He gradually lost weight and strength. One year prior to arrival in the hospital, he developed a cough productive of yellow sputum. Seven months prior, he returned from Turkey to Atlanta and noticed an increase in his cough, along with fevers of 100 Fahrenheit and night sweats.
The primacy of the arthralgias in this illness lead me to consider primary rheumatic diseases, and multisystem diseases (including infections) with a predominant skeletal component. In 1907, tests for lupus and the rheumatoid factor were not available. Neither skeletal remains nor works of art provide evidence that rheumatoid arthritis existed until the 19th century, whereas ankylosing spondylitis, gout, and rickets were present by then.
As a medical missionary, he might have acquired a disease endemic to the areas he visited, or the travel history may be a red herring. Familial Mediterranean fever, though prevalent in Turkey and a cause of arthralgias accompanied by recurrent attacks of abdominal pain and fever, is not an acquired disease. Behcet's disease, also known as Silk Trader's Route disease, is found in descendents of the countries that comprised the ancient Silk Route from Japan to the Middle East and may cause arthritis along with oral ulcers, genital lesions, pathergy or uveitis. I would inquire about his ancestry and fevers before dismissing these possibilities.
Although 5 years would be unusually long for tuberculosis to go unrecognized, a physician in the first half of the 20th century would place tuberculosis near the top of possible diagnoses. In 1930, a time when the population of the United States was considerably less, there were over 300,000 cases of tuberculosis. Physicians, and in particular pathologists since autopsies were more commonly performed, often died from tuberculosis since streptomycin, the first antituberculous medication, did not arrive until 1944. At the turn of the 20th century, the ability to detect tubercle bacilli was quite good. Thus, I would include tuberculous peritonitis as a cause of the progressive abdominal symptoms in this physician. In approximately one‐third of patients with tuberculous peritonitis there is evidence of pulmonary disease, and I would try to culture tuberculosis in samples of sputum, a test then so common it probably rivaled our frequent complete blood counts in popularity.
Six months prior, examinations of sputum were negative for tubercle bacilli. Four months prior to arrival, the patient moved to New Mexico. His cough improved but he continued to lose weight and had diarrhea consisting of 3 to 4 loose or semiformed bowel movements per day. Three months prior to admission, he noted an increase in abdominal girth along with right lower quadrant fullness. One month prior, he noted painful swelling and warmth in both ankles as well as dyspnea with exertion.
The increased abdominal girth in the context of chronic illness might be due to ascites, adenopathy, visceromegaly, or mass lesions such as a neoplasm or abscess. If ascites is the cause, one would need to consider primary hepatic disorders, as well as extrahepatic diseases that could progress over years. Infection with hepatitis A virus does not cause chronic liver disease. Hepatitis B, in those days, was referred to as serum hepatitis, and a serum marker for the B virusthe Australia antigenwas not identified until 1967. Cardiac causes of ascites include congestive heart failure and constrictive pericarditis, the latter an important consideration because it is potentially curable. Also, constrictive pericarditis can present as an indolent weight‐losing disease because of chronic visceral congestion. Other considerations include nephrotic syndrome, infection, and neoplasm, including mesothelioma.
Abdominal distention might also be seen with a smoldering abscess. In addition to an appendiceal process, the travel and right lower quadrant localization reminds us to consider ameboma. This patient surely was in an area where amebiasis was endemic, and amebomaa chronic inflammatory form of infection with E. histolytica not associated with diarrhea or liver cystsmay mimic cecal carcinoma. Exertional dyspnea suggests at least the possibility of cardiac disease. Despite the negative sputum cultures, tuberculosis remains high on the list as a cause of constrictive pericarditis or peritonitis, either of which may occur in the absence of active pulmonary disease.
Past medical history included measles and whooping cough as a child, mild pleurisy at age 14, mild influenza 7 years previously. The patient had a tonsillectomy as a child and had a portion of his inferior turbinate bone removed in an attempt to relieve a nasal condition.
On physical exam, the patient was thin and the skin over his face and hands was deep brown. His temperature was 101.5 Fahrenheit, the heart rate was 100, and the respiratory rate was 24. Small lymph nodes were palpable in the axillary and epitrochlear areas. His thorax moved asymmetrically, with less movement on the left apex and slight dullness to percussion in that area. The pulmonic component of the second heart sound was mildly accentuated. The abdomen displayed fullness and tympany, most pronounced in the right lower quadrant without hepatosplenomegaly. The left ankle was swollen, and the overlying skin was tense, shiny, and hot. On both lower legs, areas of discoloration and slight induration were observed, felt to be consistent with faded erythema nodosum.
Though pleurisy has numerous causes, its presence raises the specter of tuberculosis again. The nasal condition triggers thoughts of Wegener's granulomatosis or lethal midline granuloma, both unlikely diagnoses here. The pulmonary exam suggests an apical process, such as tuberculosis, and the accentuated pulmonic heart sound implies pulmonary hypertension, which could be due to a number of chronic pulmonary diseases. The epitrochlear nodes are of interest since lymphoma and Hodgkin's disease rarely involve this area; syphilis and human immunodeficiency virus (HIV) are a few of the chronic diseases that may involve this lymph node region. More helpful is the absence of hepatosplenomegaly, since many indolent malignancies and infections would be expected to enlarge these organs by this point.
Monoarticular arthritis is often due to infection, and less likely due to rheumatoid disease. When rheumatoid arthritis flares, the entire skeleton flares, not single joints. Given the indolence and this single joint involvement, tuberculosis again comes to mind.
I would next want to obtain a plain chest radiograph, looking for evidence of tuberculosis. As with any test, one should ask how this will change management. In 1907, antituberculous medications were not available, so therapy was directed at lowering oxygen tension in the primary site of infection; for example, pulmonary disease was addressed via pneumothorax. If the chest radiograph provides little hint of tuberculosis, then consideration must be given to exploratory surgery of the abdomen given the focal abnormality in the right lower quadrant.
A peripheral blood smear revealed a hypochromic microcytic anemia. The total red blood cell count was 4.468 million/mm3 (normal range for men is 4.52‐5.90 million/mm3), white blood cell count was 8180/mm3, including 80% granulocytes and 9% eosinophils. On gross inspection, the stool was clay‐colored, and stool microscopy demonstrated large numbers of neutral fat droplets, but no ova, parasites, or tubercle bacilli. Urinalysis revealed no albumin or casts, and the bones were normal on ankle radiographs. Another sample of sputum revealed no tubercle bacilli, and intradermal placement of tuberculin provoked no reaction.
His negative tuberculin skin reaction is unusual for that era, because of the prevalence of tuberculosis. Most likely, he is anergic because of his severe underlying illness, and the absent reaction is thus not all that helpful a clue. Multiple negative sputum examinations lower the possibility of pulmonary, but not extrapulmonary, tuberculosis. The absence of bony destruction on ankle radiographs lowers my suspicion for tuberculous arthritis.
The excess stool fat implies steatogenic diarrhea from malabsorption, and 2 categories here are pancreatic and luminal diseases. Of these 2, pancreatic etiologies produce more severe malabsorption. We do not hear mention of jaundice, however, and I cannot see how to link the pancreas to the arthritis. A chronic infection which may produce malabsorption and eosinophilia is strongyloidiasis, endemic in the southeastern United States. However, this patient did not manifest the most common finding of chronic strongyloidiasis, namely asthma. Adrenal insufficiency, as might result from disseminated tuberculosis, is associated with increased skin pigmentation, diarrhea, and eosinophilia. However, the diarrhea of adrenal insufficiency is not malabsorptive, and serum electrolytes and cortisol tests were not available then to confirm this diagnosis antemortem.
In an attempt to identify a unifying cause of chronic arthritis, malabsorption, and increased skin pigmentation, I must consider Whipple's disease first and foremost. Physicians then were strapped and observation was often the default mode of the day. Given the abdominal findings, an exploratory laparotomy would be warranted if his condition deteriorated.
Despite forced oral feedings, the patient continued to lose weight, from his normal of 175 pounds to a nadir of 145 pounds. Because of worsening abdominal distention, the patient underwent exploratory abdominal surgery on the twenty‐first hospital day. Intraoperatively, no ascites was seen, but his mesenteric lymph nodes were hard and markedly enlarged. The abdomen was closed without further intervention. Two days after the surgery, the patient abruptly developed dyspnea. His respirations were 40 per minute, heart rate was 120, and he had minimal rales at the lung bases without findings of consolidation. He died 2 hours later, on the twenty‐third hospital day, and an autopsy was performed.
The final event may have been a pulmonary embolism. As for the adenopathy, lymphoma and tuberculosis are possible. Heavy chain disease, an unusual lymphoproliferative disorder found in persons from the old Silk Trader's Route from the Middle East to the Orient, is a remote prospect. However, 5 years is just too indolent for most cancers and would be very unusual for tuberculosis. I think the findings support Whipple's disease, and I wonder if this was the first reported case.
On postmortem examination, the abdominal adenopathy was striking. The small intestine contained enlarged villi with thickened submucosa, and the mesenteric nodes were enlarged with fat deposits and abnormal foamy cells. Within these foamy cells, microscopy revealed numerous rod‐shaped organisms. All studies were negative for tuberculosis, and although the pathologist, Dr. George Hoyt Whipple, suspected an infectious etiology, he offered the name intestinal lipodystrophy to emphasize the striking small intestinal changes he witnessed at autopsy, and which are the hallmarks of the disease that now bears his name. Whipple also shared the 1934 Nobel Prize in Physiology or Medicine with Minot and Murphy for their discovery that a nutritional substance in liver, now known as vitamin B12, was beneficial in treating pernicious anemia.
COMMENTARY
This is the index case of Whipple's disease, summarized from the original 1907 description.1 George Hoyt Whipple, then a pathologist at Johns Hopkins, highlights the value of keen observation and a well‐done case report in describing a new disease entity. One of the roles of case reports is to detail the features of an unknown disease. In this capacity, Whipple's summary is exemplary. His achievement was having the openness of mind to realize he was witnessing something novel, and to take the first step on the road to discovery. Although Whipple suspected he was staring at a unique disease, he could not pinpoint the culprit bacteria and he had trouble squaring the extraintestinal findings with the marked intestinal anomalies. It was left to decades of input from others to confirm the association of arthralgias, eosinophilia, skin hyperpigmentation, and cardiac valve abnormalities with intestinal malabsorption, and to culture the infectious agent.
In his discussion, Whipple recognized he was confronted with a novel clinical entity. Prior to surgery, pulmonary and mesenteric tuberculosis were suspected, based on the fevers, weight loss, cough, fat malabsorption, and lymphadenopathy. However, he felt the left apical exam was more representative of retraction from prior disease than active infection. He was also bothered by the negative skin reaction and sputum tests. At surgery, the pronounced adenopathy suggested sarcoma or Hodgkin's disease but postmortem examination eliminated these possibilities. At autopsy, the abdominal findings were most striking. The small intestine demonstrated enlarged villi with thickened submucosa and markedly enlarged mesenteric lymph glands containing large fat deposits and distinctly abnormal foamy cells. These foamy macrophages contained great numbers of rod‐shaped organisms resembling the tubercle bacillus. However, all tests were negative for tuberculosis, and the lungs contained no active disease. Though he suspected an infectious etiology, Whipple offered the name intestinal lipodystrophy to emphasize the striking small intestinal pathology.
Although Whipple had surmised a novel infectious agent in 1907, it took almost a century to isolate the causative microbe. Granules within foamy macrophages of the small intestine were detected on periodic acid‐Schiff (PAS) staining in 1949.2 Similar PAS‐positive granules were soon discovered in other tissues and fluid, providing a plausible explanation for the systemic features of the disease.3 Electron microscopy confirmed the presence of infectious bacilli in 1961,4 ushering in the era of antimicrobial treatment for this disease. More recently, using polymerase chain reaction (PCR), a unique bacterial 16S ribosomal RNA gene was isolated in patients with Whipple's disease.5, 6 Phylogenetically classified with the actinobacteria, Tropheryma whipplei (fom the Greek trophe, nourishment, and eryma, barrier) was ultimately subcultured in 2000,7 and immunodetection testing became possible. Using this technique, the archived pathology specimens from the 1907 index case demonstrated numerous intracellular bacteria in the lamina propria, closing the loop started by Whipple nearly a century earlier.8
Whipple's index case report described most of the manifestations of the disease we are familiar with today. As in the original description, arthralgias are the most common initial symptom and may precede diagnosis by a mean of 8 years. Other cardinal features include weight loss, abdominal pain and steatorrhea due to small intestinal involvement. Table 1 summarizes the important signs and symptoms of Whipple's disease.9, 10 One notable manifestation missing in Whipple's report is central nervous system involvement. Central nervous system (CNS) disease ranges from cognitive deficits to encephalitis and focal defects, and may occur years after treatment and without concomitant intestinal symptoms.
| Clinical Feature | Comment |
|---|---|
| |
| Cardinal features (present in 60% to 90%) | |
| Arthropathy | Most common initial symptom, preceding diagnosis by a mean of 8 years. Migratory, nonerosive, mainly in the peripheral joints. |
| Weight loss | |
| Diarrhea | Usually steatorrhea, may be associated with pain or occult blood in the stool |
| Other common features (present in 20% to 45%) | |
| Fever | |
| Lymphadenopathy | May present as a palpable mass |
| Increased skin pigmentation | Mechanism unknown (evidence of adrenal insufficiency has not been found in Whipple's) |
| Cardiac disease | Culture‐negative endocarditis |
| Hypotension | |
| Peripheral edema | |
| Uncommon clinical features | |
| Central nervous system involvement | May be global (dementia, personality change, sleep disturbance) or focal (cranial neuropathy, nystagmus) |
| Eye disease | Uveitis, retinitis |
| Hepatosplenomegaly | |
| Polyserositis | |
| Ascites | |
A remaining mystery is why this pathogen results only rarely in clinical disease. Caucasians comprise the majority of infected patients, and men are affected 8 times more often than women. An overrepresentation of HLA‐B27 suggests a genetic predisposition, though its role in pathogenesis is unclear. T. whipplei has been identified by PCR methods in asymptomatic individuals, implying additional abnormalities must be present in susceptible hosts for symptoms to occur following colonization.11 The exact immune defects are speculative, and immunodeficiency states (such as HIV) have not been consistently identified in patients with Whipple's disease.
The cornerstone of diagnosing Whipple's disease is upper endoscopy with duodenal biopsy. Flattening of the villi and markedly increased PAS‐positive staining of lamina propria macrophages are strongly suggestive of the diagnosis. PAS‐positive staining is not unique to T. whipplei, however. In patients with profound immunodeficiency, Mycobacterium avium intracellulare may stain positive with PAS. Since Whipple's disease is only rarely associated with HIV, a negative HIV test would favor a diagnosis of Whipple's disease. Electron microscopy may distinguish T. whipplei from its mimickers by morphology. For extraintestinal disease, PCR testing on samples from infected tissue has been found to be a reliable diagnostic aid.9
Given the rarity of the disease, controlled clinical trials addressing optimal treatment are lacking. Current recommendations include initial therapy for 14 days with an agent that crosses the blood‐brain barrier (eg, ceftriaxone) to reduce the incidence of CNS disease. This is then followed by a year or more of oral antimicrobial therapy with trimethoprim‐sulfamethoxazole or a tetracycline.9 While most patients respond within 2 to 3 weeks, relapse may occur in as many as one‐third of patients.
Historical case reports reinforce the case‐based learning paradigm. As the discussant remarks, observation was all too often the only recourse for physicians a century ago. In recounting the 7‐year progression of disease in 1 individual, Whipple provides a unique window into the natural evolution of the key features of this systemic disease. Viewed through the prism of Whipple's eyes, we can recall the striking lymphoid hyperplasia and unusual organisms in the small intestine, cementing our understanding of the pathogenesis of this disorder. Revisiting past cases allows us to learn of and learn from the past.
Teaching Points
-
Whipple's disease should be considered in patients with unexplained arthralgias accompanied by weight loss, malabsorption, and abdominal pain.
-
For suspected intestinal Whipple's disease, diagnosis is best made by duodenal biopsy demonstrating PAS‐positive staining in lamina propria macrophages.
-
Systemic manifestations of Whipple's disease include culture‐negative endocarditis and CNS disease. PCR testing of involved sites for T. whipplei is recommended to confirm extraintestinal disease.
- .A hitherto undescribed disease characterized anatomically by deposits of fat and fatty acids in the intestinal and mesenteric lymphatic tissues.Bull Johns Hopkins Hosp.1907;18:382–391.
- .The tinctoral demonstration of a glycoprotein in Whipple's disease.Proc Soc Exp Biol Med.1949;72:225–227.
- ,,.Whipple's disease: clinical, biochemical, and histopathologic features and assessment of treatment in 29 patients.Mayo Clin Proc.1988;63:539–551.
- ,.Combined electron and light microscopy in Whipple's disease: demonstration of “bacillary bodies” in the intestine.Bull Johns Hopkins Hosp.1961;109:80–98.
- ,,,.Phylogeny of the Whipple's disease‐associated bacterium.Lancet.1991;338:474–475.
- ,,,.Identification of the uncultured bacillus of Whipple's disease.N Engl J Med.1992;327:293–301.
- ,,, et al.Cultivation of the bacillus of Whipple's disease.N Engl J Med.2000;342:620–625.
- ,,,.Immunodetection of Tropheryma whipplei in intestinal tissue from Dr. Whipple's 1907 patient.N Engl J Med.2003;348:1411– 1412.
- ,.Whipple's disease.Lancet.2003;361:239–246.
- ,,, et al.Diagnostic guidelines in central nervous system Whipple's disease.Ann Neurol.1996;40:561–568.
- ,,, et al.PCR‐positive tests for Tropheryma whippleii in patients without Whipple's disease.Lancet.1999;353:2214.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 36 year‐old male physician was admitted to a Baltimore hospital in April 1907 with weight loss, weakness, arthralgias, and abdominal distension that had progressed over 5 years.
In 1907, major causes of unexplained weight loss included tuberculosis, hyperthyroidism, cancer, and diabetes. Arthralgias and weakness are not specific. The insidious progression over 5 years narrows the infectious possibilities; tuberculosis and syphilis are important considerations. Since surgical removal was the main treatment for malignancy in 1907, a history of prior surgery might point to a previously diagnosed malignancy that is now progressing.
Five years earlier, while visiting Turkey as a medical missionary, he first noted the onset of arthralgias that lasted 6 to 8 hours and occurred 3 to 4 times per week. Over time, these attacks lasted up to 24 hours and became associated with warmth, swelling, and tenderness of both small and large joints. He gradually lost weight and strength. One year prior to arrival in the hospital, he developed a cough productive of yellow sputum. Seven months prior, he returned from Turkey to Atlanta and noticed an increase in his cough, along with fevers of 100 Fahrenheit and night sweats.
The primacy of the arthralgias in this illness lead me to consider primary rheumatic diseases, and multisystem diseases (including infections) with a predominant skeletal component. In 1907, tests for lupus and the rheumatoid factor were not available. Neither skeletal remains nor works of art provide evidence that rheumatoid arthritis existed until the 19th century, whereas ankylosing spondylitis, gout, and rickets were present by then.
As a medical missionary, he might have acquired a disease endemic to the areas he visited, or the travel history may be a red herring. Familial Mediterranean fever, though prevalent in Turkey and a cause of arthralgias accompanied by recurrent attacks of abdominal pain and fever, is not an acquired disease. Behcet's disease, also known as Silk Trader's Route disease, is found in descendents of the countries that comprised the ancient Silk Route from Japan to the Middle East and may cause arthritis along with oral ulcers, genital lesions, pathergy or uveitis. I would inquire about his ancestry and fevers before dismissing these possibilities.
Although 5 years would be unusually long for tuberculosis to go unrecognized, a physician in the first half of the 20th century would place tuberculosis near the top of possible diagnoses. In 1930, a time when the population of the United States was considerably less, there were over 300,000 cases of tuberculosis. Physicians, and in particular pathologists since autopsies were more commonly performed, often died from tuberculosis since streptomycin, the first antituberculous medication, did not arrive until 1944. At the turn of the 20th century, the ability to detect tubercle bacilli was quite good. Thus, I would include tuberculous peritonitis as a cause of the progressive abdominal symptoms in this physician. In approximately one‐third of patients with tuberculous peritonitis there is evidence of pulmonary disease, and I would try to culture tuberculosis in samples of sputum, a test then so common it probably rivaled our frequent complete blood counts in popularity.
Six months prior, examinations of sputum were negative for tubercle bacilli. Four months prior to arrival, the patient moved to New Mexico. His cough improved but he continued to lose weight and had diarrhea consisting of 3 to 4 loose or semiformed bowel movements per day. Three months prior to admission, he noted an increase in abdominal girth along with right lower quadrant fullness. One month prior, he noted painful swelling and warmth in both ankles as well as dyspnea with exertion.
The increased abdominal girth in the context of chronic illness might be due to ascites, adenopathy, visceromegaly, or mass lesions such as a neoplasm or abscess. If ascites is the cause, one would need to consider primary hepatic disorders, as well as extrahepatic diseases that could progress over years. Infection with hepatitis A virus does not cause chronic liver disease. Hepatitis B, in those days, was referred to as serum hepatitis, and a serum marker for the B virusthe Australia antigenwas not identified until 1967. Cardiac causes of ascites include congestive heart failure and constrictive pericarditis, the latter an important consideration because it is potentially curable. Also, constrictive pericarditis can present as an indolent weight‐losing disease because of chronic visceral congestion. Other considerations include nephrotic syndrome, infection, and neoplasm, including mesothelioma.
Abdominal distention might also be seen with a smoldering abscess. In addition to an appendiceal process, the travel and right lower quadrant localization reminds us to consider ameboma. This patient surely was in an area where amebiasis was endemic, and amebomaa chronic inflammatory form of infection with E. histolytica not associated with diarrhea or liver cystsmay mimic cecal carcinoma. Exertional dyspnea suggests at least the possibility of cardiac disease. Despite the negative sputum cultures, tuberculosis remains high on the list as a cause of constrictive pericarditis or peritonitis, either of which may occur in the absence of active pulmonary disease.
Past medical history included measles and whooping cough as a child, mild pleurisy at age 14, mild influenza 7 years previously. The patient had a tonsillectomy as a child and had a portion of his inferior turbinate bone removed in an attempt to relieve a nasal condition.
On physical exam, the patient was thin and the skin over his face and hands was deep brown. His temperature was 101.5 Fahrenheit, the heart rate was 100, and the respiratory rate was 24. Small lymph nodes were palpable in the axillary and epitrochlear areas. His thorax moved asymmetrically, with less movement on the left apex and slight dullness to percussion in that area. The pulmonic component of the second heart sound was mildly accentuated. The abdomen displayed fullness and tympany, most pronounced in the right lower quadrant without hepatosplenomegaly. The left ankle was swollen, and the overlying skin was tense, shiny, and hot. On both lower legs, areas of discoloration and slight induration were observed, felt to be consistent with faded erythema nodosum.
Though pleurisy has numerous causes, its presence raises the specter of tuberculosis again. The nasal condition triggers thoughts of Wegener's granulomatosis or lethal midline granuloma, both unlikely diagnoses here. The pulmonary exam suggests an apical process, such as tuberculosis, and the accentuated pulmonic heart sound implies pulmonary hypertension, which could be due to a number of chronic pulmonary diseases. The epitrochlear nodes are of interest since lymphoma and Hodgkin's disease rarely involve this area; syphilis and human immunodeficiency virus (HIV) are a few of the chronic diseases that may involve this lymph node region. More helpful is the absence of hepatosplenomegaly, since many indolent malignancies and infections would be expected to enlarge these organs by this point.
Monoarticular arthritis is often due to infection, and less likely due to rheumatoid disease. When rheumatoid arthritis flares, the entire skeleton flares, not single joints. Given the indolence and this single joint involvement, tuberculosis again comes to mind.
I would next want to obtain a plain chest radiograph, looking for evidence of tuberculosis. As with any test, one should ask how this will change management. In 1907, antituberculous medications were not available, so therapy was directed at lowering oxygen tension in the primary site of infection; for example, pulmonary disease was addressed via pneumothorax. If the chest radiograph provides little hint of tuberculosis, then consideration must be given to exploratory surgery of the abdomen given the focal abnormality in the right lower quadrant.
A peripheral blood smear revealed a hypochromic microcytic anemia. The total red blood cell count was 4.468 million/mm3 (normal range for men is 4.52‐5.90 million/mm3), white blood cell count was 8180/mm3, including 80% granulocytes and 9% eosinophils. On gross inspection, the stool was clay‐colored, and stool microscopy demonstrated large numbers of neutral fat droplets, but no ova, parasites, or tubercle bacilli. Urinalysis revealed no albumin or casts, and the bones were normal on ankle radiographs. Another sample of sputum revealed no tubercle bacilli, and intradermal placement of tuberculin provoked no reaction.
His negative tuberculin skin reaction is unusual for that era, because of the prevalence of tuberculosis. Most likely, he is anergic because of his severe underlying illness, and the absent reaction is thus not all that helpful a clue. Multiple negative sputum examinations lower the possibility of pulmonary, but not extrapulmonary, tuberculosis. The absence of bony destruction on ankle radiographs lowers my suspicion for tuberculous arthritis.
The excess stool fat implies steatogenic diarrhea from malabsorption, and 2 categories here are pancreatic and luminal diseases. Of these 2, pancreatic etiologies produce more severe malabsorption. We do not hear mention of jaundice, however, and I cannot see how to link the pancreas to the arthritis. A chronic infection which may produce malabsorption and eosinophilia is strongyloidiasis, endemic in the southeastern United States. However, this patient did not manifest the most common finding of chronic strongyloidiasis, namely asthma. Adrenal insufficiency, as might result from disseminated tuberculosis, is associated with increased skin pigmentation, diarrhea, and eosinophilia. However, the diarrhea of adrenal insufficiency is not malabsorptive, and serum electrolytes and cortisol tests were not available then to confirm this diagnosis antemortem.
In an attempt to identify a unifying cause of chronic arthritis, malabsorption, and increased skin pigmentation, I must consider Whipple's disease first and foremost. Physicians then were strapped and observation was often the default mode of the day. Given the abdominal findings, an exploratory laparotomy would be warranted if his condition deteriorated.
Despite forced oral feedings, the patient continued to lose weight, from his normal of 175 pounds to a nadir of 145 pounds. Because of worsening abdominal distention, the patient underwent exploratory abdominal surgery on the twenty‐first hospital day. Intraoperatively, no ascites was seen, but his mesenteric lymph nodes were hard and markedly enlarged. The abdomen was closed without further intervention. Two days after the surgery, the patient abruptly developed dyspnea. His respirations were 40 per minute, heart rate was 120, and he had minimal rales at the lung bases without findings of consolidation. He died 2 hours later, on the twenty‐third hospital day, and an autopsy was performed.
The final event may have been a pulmonary embolism. As for the adenopathy, lymphoma and tuberculosis are possible. Heavy chain disease, an unusual lymphoproliferative disorder found in persons from the old Silk Trader's Route from the Middle East to the Orient, is a remote prospect. However, 5 years is just too indolent for most cancers and would be very unusual for tuberculosis. I think the findings support Whipple's disease, and I wonder if this was the first reported case.
On postmortem examination, the abdominal adenopathy was striking. The small intestine contained enlarged villi with thickened submucosa, and the mesenteric nodes were enlarged with fat deposits and abnormal foamy cells. Within these foamy cells, microscopy revealed numerous rod‐shaped organisms. All studies were negative for tuberculosis, and although the pathologist, Dr. George Hoyt Whipple, suspected an infectious etiology, he offered the name intestinal lipodystrophy to emphasize the striking small intestinal changes he witnessed at autopsy, and which are the hallmarks of the disease that now bears his name. Whipple also shared the 1934 Nobel Prize in Physiology or Medicine with Minot and Murphy for their discovery that a nutritional substance in liver, now known as vitamin B12, was beneficial in treating pernicious anemia.
COMMENTARY
This is the index case of Whipple's disease, summarized from the original 1907 description.1 George Hoyt Whipple, then a pathologist at Johns Hopkins, highlights the value of keen observation and a well‐done case report in describing a new disease entity. One of the roles of case reports is to detail the features of an unknown disease. In this capacity, Whipple's summary is exemplary. His achievement was having the openness of mind to realize he was witnessing something novel, and to take the first step on the road to discovery. Although Whipple suspected he was staring at a unique disease, he could not pinpoint the culprit bacteria and he had trouble squaring the extraintestinal findings with the marked intestinal anomalies. It was left to decades of input from others to confirm the association of arthralgias, eosinophilia, skin hyperpigmentation, and cardiac valve abnormalities with intestinal malabsorption, and to culture the infectious agent.
In his discussion, Whipple recognized he was confronted with a novel clinical entity. Prior to surgery, pulmonary and mesenteric tuberculosis were suspected, based on the fevers, weight loss, cough, fat malabsorption, and lymphadenopathy. However, he felt the left apical exam was more representative of retraction from prior disease than active infection. He was also bothered by the negative skin reaction and sputum tests. At surgery, the pronounced adenopathy suggested sarcoma or Hodgkin's disease but postmortem examination eliminated these possibilities. At autopsy, the abdominal findings were most striking. The small intestine demonstrated enlarged villi with thickened submucosa and markedly enlarged mesenteric lymph glands containing large fat deposits and distinctly abnormal foamy cells. These foamy macrophages contained great numbers of rod‐shaped organisms resembling the tubercle bacillus. However, all tests were negative for tuberculosis, and the lungs contained no active disease. Though he suspected an infectious etiology, Whipple offered the name intestinal lipodystrophy to emphasize the striking small intestinal pathology.
Although Whipple had surmised a novel infectious agent in 1907, it took almost a century to isolate the causative microbe. Granules within foamy macrophages of the small intestine were detected on periodic acid‐Schiff (PAS) staining in 1949.2 Similar PAS‐positive granules were soon discovered in other tissues and fluid, providing a plausible explanation for the systemic features of the disease.3 Electron microscopy confirmed the presence of infectious bacilli in 1961,4 ushering in the era of antimicrobial treatment for this disease. More recently, using polymerase chain reaction (PCR), a unique bacterial 16S ribosomal RNA gene was isolated in patients with Whipple's disease.5, 6 Phylogenetically classified with the actinobacteria, Tropheryma whipplei (fom the Greek trophe, nourishment, and eryma, barrier) was ultimately subcultured in 2000,7 and immunodetection testing became possible. Using this technique, the archived pathology specimens from the 1907 index case demonstrated numerous intracellular bacteria in the lamina propria, closing the loop started by Whipple nearly a century earlier.8
Whipple's index case report described most of the manifestations of the disease we are familiar with today. As in the original description, arthralgias are the most common initial symptom and may precede diagnosis by a mean of 8 years. Other cardinal features include weight loss, abdominal pain and steatorrhea due to small intestinal involvement. Table 1 summarizes the important signs and symptoms of Whipple's disease.9, 10 One notable manifestation missing in Whipple's report is central nervous system involvement. Central nervous system (CNS) disease ranges from cognitive deficits to encephalitis and focal defects, and may occur years after treatment and without concomitant intestinal symptoms.
| Clinical Feature | Comment |
|---|---|
| |
| Cardinal features (present in 60% to 90%) | |
| Arthropathy | Most common initial symptom, preceding diagnosis by a mean of 8 years. Migratory, nonerosive, mainly in the peripheral joints. |
| Weight loss | |
| Diarrhea | Usually steatorrhea, may be associated with pain or occult blood in the stool |
| Other common features (present in 20% to 45%) | |
| Fever | |
| Lymphadenopathy | May present as a palpable mass |
| Increased skin pigmentation | Mechanism unknown (evidence of adrenal insufficiency has not been found in Whipple's) |
| Cardiac disease | Culture‐negative endocarditis |
| Hypotension | |
| Peripheral edema | |
| Uncommon clinical features | |
| Central nervous system involvement | May be global (dementia, personality change, sleep disturbance) or focal (cranial neuropathy, nystagmus) |
| Eye disease | Uveitis, retinitis |
| Hepatosplenomegaly | |
| Polyserositis | |
| Ascites | |
A remaining mystery is why this pathogen results only rarely in clinical disease. Caucasians comprise the majority of infected patients, and men are affected 8 times more often than women. An overrepresentation of HLA‐B27 suggests a genetic predisposition, though its role in pathogenesis is unclear. T. whipplei has been identified by PCR methods in asymptomatic individuals, implying additional abnormalities must be present in susceptible hosts for symptoms to occur following colonization.11 The exact immune defects are speculative, and immunodeficiency states (such as HIV) have not been consistently identified in patients with Whipple's disease.
The cornerstone of diagnosing Whipple's disease is upper endoscopy with duodenal biopsy. Flattening of the villi and markedly increased PAS‐positive staining of lamina propria macrophages are strongly suggestive of the diagnosis. PAS‐positive staining is not unique to T. whipplei, however. In patients with profound immunodeficiency, Mycobacterium avium intracellulare may stain positive with PAS. Since Whipple's disease is only rarely associated with HIV, a negative HIV test would favor a diagnosis of Whipple's disease. Electron microscopy may distinguish T. whipplei from its mimickers by morphology. For extraintestinal disease, PCR testing on samples from infected tissue has been found to be a reliable diagnostic aid.9
Given the rarity of the disease, controlled clinical trials addressing optimal treatment are lacking. Current recommendations include initial therapy for 14 days with an agent that crosses the blood‐brain barrier (eg, ceftriaxone) to reduce the incidence of CNS disease. This is then followed by a year or more of oral antimicrobial therapy with trimethoprim‐sulfamethoxazole or a tetracycline.9 While most patients respond within 2 to 3 weeks, relapse may occur in as many as one‐third of patients.
Historical case reports reinforce the case‐based learning paradigm. As the discussant remarks, observation was all too often the only recourse for physicians a century ago. In recounting the 7‐year progression of disease in 1 individual, Whipple provides a unique window into the natural evolution of the key features of this systemic disease. Viewed through the prism of Whipple's eyes, we can recall the striking lymphoid hyperplasia and unusual organisms in the small intestine, cementing our understanding of the pathogenesis of this disorder. Revisiting past cases allows us to learn of and learn from the past.
Teaching Points
-
Whipple's disease should be considered in patients with unexplained arthralgias accompanied by weight loss, malabsorption, and abdominal pain.
-
For suspected intestinal Whipple's disease, diagnosis is best made by duodenal biopsy demonstrating PAS‐positive staining in lamina propria macrophages.
-
Systemic manifestations of Whipple's disease include culture‐negative endocarditis and CNS disease. PCR testing of involved sites for T. whipplei is recommended to confirm extraintestinal disease.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 36 year‐old male physician was admitted to a Baltimore hospital in April 1907 with weight loss, weakness, arthralgias, and abdominal distension that had progressed over 5 years.
In 1907, major causes of unexplained weight loss included tuberculosis, hyperthyroidism, cancer, and diabetes. Arthralgias and weakness are not specific. The insidious progression over 5 years narrows the infectious possibilities; tuberculosis and syphilis are important considerations. Since surgical removal was the main treatment for malignancy in 1907, a history of prior surgery might point to a previously diagnosed malignancy that is now progressing.
Five years earlier, while visiting Turkey as a medical missionary, he first noted the onset of arthralgias that lasted 6 to 8 hours and occurred 3 to 4 times per week. Over time, these attacks lasted up to 24 hours and became associated with warmth, swelling, and tenderness of both small and large joints. He gradually lost weight and strength. One year prior to arrival in the hospital, he developed a cough productive of yellow sputum. Seven months prior, he returned from Turkey to Atlanta and noticed an increase in his cough, along with fevers of 100 Fahrenheit and night sweats.
The primacy of the arthralgias in this illness lead me to consider primary rheumatic diseases, and multisystem diseases (including infections) with a predominant skeletal component. In 1907, tests for lupus and the rheumatoid factor were not available. Neither skeletal remains nor works of art provide evidence that rheumatoid arthritis existed until the 19th century, whereas ankylosing spondylitis, gout, and rickets were present by then.
As a medical missionary, he might have acquired a disease endemic to the areas he visited, or the travel history may be a red herring. Familial Mediterranean fever, though prevalent in Turkey and a cause of arthralgias accompanied by recurrent attacks of abdominal pain and fever, is not an acquired disease. Behcet's disease, also known as Silk Trader's Route disease, is found in descendents of the countries that comprised the ancient Silk Route from Japan to the Middle East and may cause arthritis along with oral ulcers, genital lesions, pathergy or uveitis. I would inquire about his ancestry and fevers before dismissing these possibilities.
Although 5 years would be unusually long for tuberculosis to go unrecognized, a physician in the first half of the 20th century would place tuberculosis near the top of possible diagnoses. In 1930, a time when the population of the United States was considerably less, there were over 300,000 cases of tuberculosis. Physicians, and in particular pathologists since autopsies were more commonly performed, often died from tuberculosis since streptomycin, the first antituberculous medication, did not arrive until 1944. At the turn of the 20th century, the ability to detect tubercle bacilli was quite good. Thus, I would include tuberculous peritonitis as a cause of the progressive abdominal symptoms in this physician. In approximately one‐third of patients with tuberculous peritonitis there is evidence of pulmonary disease, and I would try to culture tuberculosis in samples of sputum, a test then so common it probably rivaled our frequent complete blood counts in popularity.
Six months prior, examinations of sputum were negative for tubercle bacilli. Four months prior to arrival, the patient moved to New Mexico. His cough improved but he continued to lose weight and had diarrhea consisting of 3 to 4 loose or semiformed bowel movements per day. Three months prior to admission, he noted an increase in abdominal girth along with right lower quadrant fullness. One month prior, he noted painful swelling and warmth in both ankles as well as dyspnea with exertion.
The increased abdominal girth in the context of chronic illness might be due to ascites, adenopathy, visceromegaly, or mass lesions such as a neoplasm or abscess. If ascites is the cause, one would need to consider primary hepatic disorders, as well as extrahepatic diseases that could progress over years. Infection with hepatitis A virus does not cause chronic liver disease. Hepatitis B, in those days, was referred to as serum hepatitis, and a serum marker for the B virusthe Australia antigenwas not identified until 1967. Cardiac causes of ascites include congestive heart failure and constrictive pericarditis, the latter an important consideration because it is potentially curable. Also, constrictive pericarditis can present as an indolent weight‐losing disease because of chronic visceral congestion. Other considerations include nephrotic syndrome, infection, and neoplasm, including mesothelioma.
Abdominal distention might also be seen with a smoldering abscess. In addition to an appendiceal process, the travel and right lower quadrant localization reminds us to consider ameboma. This patient surely was in an area where amebiasis was endemic, and amebomaa chronic inflammatory form of infection with E. histolytica not associated with diarrhea or liver cystsmay mimic cecal carcinoma. Exertional dyspnea suggests at least the possibility of cardiac disease. Despite the negative sputum cultures, tuberculosis remains high on the list as a cause of constrictive pericarditis or peritonitis, either of which may occur in the absence of active pulmonary disease.
Past medical history included measles and whooping cough as a child, mild pleurisy at age 14, mild influenza 7 years previously. The patient had a tonsillectomy as a child and had a portion of his inferior turbinate bone removed in an attempt to relieve a nasal condition.
On physical exam, the patient was thin and the skin over his face and hands was deep brown. His temperature was 101.5 Fahrenheit, the heart rate was 100, and the respiratory rate was 24. Small lymph nodes were palpable in the axillary and epitrochlear areas. His thorax moved asymmetrically, with less movement on the left apex and slight dullness to percussion in that area. The pulmonic component of the second heart sound was mildly accentuated. The abdomen displayed fullness and tympany, most pronounced in the right lower quadrant without hepatosplenomegaly. The left ankle was swollen, and the overlying skin was tense, shiny, and hot. On both lower legs, areas of discoloration and slight induration were observed, felt to be consistent with faded erythema nodosum.
Though pleurisy has numerous causes, its presence raises the specter of tuberculosis again. The nasal condition triggers thoughts of Wegener's granulomatosis or lethal midline granuloma, both unlikely diagnoses here. The pulmonary exam suggests an apical process, such as tuberculosis, and the accentuated pulmonic heart sound implies pulmonary hypertension, which could be due to a number of chronic pulmonary diseases. The epitrochlear nodes are of interest since lymphoma and Hodgkin's disease rarely involve this area; syphilis and human immunodeficiency virus (HIV) are a few of the chronic diseases that may involve this lymph node region. More helpful is the absence of hepatosplenomegaly, since many indolent malignancies and infections would be expected to enlarge these organs by this point.
Monoarticular arthritis is often due to infection, and less likely due to rheumatoid disease. When rheumatoid arthritis flares, the entire skeleton flares, not single joints. Given the indolence and this single joint involvement, tuberculosis again comes to mind.
I would next want to obtain a plain chest radiograph, looking for evidence of tuberculosis. As with any test, one should ask how this will change management. In 1907, antituberculous medications were not available, so therapy was directed at lowering oxygen tension in the primary site of infection; for example, pulmonary disease was addressed via pneumothorax. If the chest radiograph provides little hint of tuberculosis, then consideration must be given to exploratory surgery of the abdomen given the focal abnormality in the right lower quadrant.
A peripheral blood smear revealed a hypochromic microcytic anemia. The total red blood cell count was 4.468 million/mm3 (normal range for men is 4.52‐5.90 million/mm3), white blood cell count was 8180/mm3, including 80% granulocytes and 9% eosinophils. On gross inspection, the stool was clay‐colored, and stool microscopy demonstrated large numbers of neutral fat droplets, but no ova, parasites, or tubercle bacilli. Urinalysis revealed no albumin or casts, and the bones were normal on ankle radiographs. Another sample of sputum revealed no tubercle bacilli, and intradermal placement of tuberculin provoked no reaction.
His negative tuberculin skin reaction is unusual for that era, because of the prevalence of tuberculosis. Most likely, he is anergic because of his severe underlying illness, and the absent reaction is thus not all that helpful a clue. Multiple negative sputum examinations lower the possibility of pulmonary, but not extrapulmonary, tuberculosis. The absence of bony destruction on ankle radiographs lowers my suspicion for tuberculous arthritis.
The excess stool fat implies steatogenic diarrhea from malabsorption, and 2 categories here are pancreatic and luminal diseases. Of these 2, pancreatic etiologies produce more severe malabsorption. We do not hear mention of jaundice, however, and I cannot see how to link the pancreas to the arthritis. A chronic infection which may produce malabsorption and eosinophilia is strongyloidiasis, endemic in the southeastern United States. However, this patient did not manifest the most common finding of chronic strongyloidiasis, namely asthma. Adrenal insufficiency, as might result from disseminated tuberculosis, is associated with increased skin pigmentation, diarrhea, and eosinophilia. However, the diarrhea of adrenal insufficiency is not malabsorptive, and serum electrolytes and cortisol tests were not available then to confirm this diagnosis antemortem.
In an attempt to identify a unifying cause of chronic arthritis, malabsorption, and increased skin pigmentation, I must consider Whipple's disease first and foremost. Physicians then were strapped and observation was often the default mode of the day. Given the abdominal findings, an exploratory laparotomy would be warranted if his condition deteriorated.
Despite forced oral feedings, the patient continued to lose weight, from his normal of 175 pounds to a nadir of 145 pounds. Because of worsening abdominal distention, the patient underwent exploratory abdominal surgery on the twenty‐first hospital day. Intraoperatively, no ascites was seen, but his mesenteric lymph nodes were hard and markedly enlarged. The abdomen was closed without further intervention. Two days after the surgery, the patient abruptly developed dyspnea. His respirations were 40 per minute, heart rate was 120, and he had minimal rales at the lung bases without findings of consolidation. He died 2 hours later, on the twenty‐third hospital day, and an autopsy was performed.
The final event may have been a pulmonary embolism. As for the adenopathy, lymphoma and tuberculosis are possible. Heavy chain disease, an unusual lymphoproliferative disorder found in persons from the old Silk Trader's Route from the Middle East to the Orient, is a remote prospect. However, 5 years is just too indolent for most cancers and would be very unusual for tuberculosis. I think the findings support Whipple's disease, and I wonder if this was the first reported case.
On postmortem examination, the abdominal adenopathy was striking. The small intestine contained enlarged villi with thickened submucosa, and the mesenteric nodes were enlarged with fat deposits and abnormal foamy cells. Within these foamy cells, microscopy revealed numerous rod‐shaped organisms. All studies were negative for tuberculosis, and although the pathologist, Dr. George Hoyt Whipple, suspected an infectious etiology, he offered the name intestinal lipodystrophy to emphasize the striking small intestinal changes he witnessed at autopsy, and which are the hallmarks of the disease that now bears his name. Whipple also shared the 1934 Nobel Prize in Physiology or Medicine with Minot and Murphy for their discovery that a nutritional substance in liver, now known as vitamin B12, was beneficial in treating pernicious anemia.
COMMENTARY
This is the index case of Whipple's disease, summarized from the original 1907 description.1 George Hoyt Whipple, then a pathologist at Johns Hopkins, highlights the value of keen observation and a well‐done case report in describing a new disease entity. One of the roles of case reports is to detail the features of an unknown disease. In this capacity, Whipple's summary is exemplary. His achievement was having the openness of mind to realize he was witnessing something novel, and to take the first step on the road to discovery. Although Whipple suspected he was staring at a unique disease, he could not pinpoint the culprit bacteria and he had trouble squaring the extraintestinal findings with the marked intestinal anomalies. It was left to decades of input from others to confirm the association of arthralgias, eosinophilia, skin hyperpigmentation, and cardiac valve abnormalities with intestinal malabsorption, and to culture the infectious agent.
In his discussion, Whipple recognized he was confronted with a novel clinical entity. Prior to surgery, pulmonary and mesenteric tuberculosis were suspected, based on the fevers, weight loss, cough, fat malabsorption, and lymphadenopathy. However, he felt the left apical exam was more representative of retraction from prior disease than active infection. He was also bothered by the negative skin reaction and sputum tests. At surgery, the pronounced adenopathy suggested sarcoma or Hodgkin's disease but postmortem examination eliminated these possibilities. At autopsy, the abdominal findings were most striking. The small intestine demonstrated enlarged villi with thickened submucosa and markedly enlarged mesenteric lymph glands containing large fat deposits and distinctly abnormal foamy cells. These foamy macrophages contained great numbers of rod‐shaped organisms resembling the tubercle bacillus. However, all tests were negative for tuberculosis, and the lungs contained no active disease. Though he suspected an infectious etiology, Whipple offered the name intestinal lipodystrophy to emphasize the striking small intestinal pathology.
Although Whipple had surmised a novel infectious agent in 1907, it took almost a century to isolate the causative microbe. Granules within foamy macrophages of the small intestine were detected on periodic acid‐Schiff (PAS) staining in 1949.2 Similar PAS‐positive granules were soon discovered in other tissues and fluid, providing a plausible explanation for the systemic features of the disease.3 Electron microscopy confirmed the presence of infectious bacilli in 1961,4 ushering in the era of antimicrobial treatment for this disease. More recently, using polymerase chain reaction (PCR), a unique bacterial 16S ribosomal RNA gene was isolated in patients with Whipple's disease.5, 6 Phylogenetically classified with the actinobacteria, Tropheryma whipplei (fom the Greek trophe, nourishment, and eryma, barrier) was ultimately subcultured in 2000,7 and immunodetection testing became possible. Using this technique, the archived pathology specimens from the 1907 index case demonstrated numerous intracellular bacteria in the lamina propria, closing the loop started by Whipple nearly a century earlier.8
Whipple's index case report described most of the manifestations of the disease we are familiar with today. As in the original description, arthralgias are the most common initial symptom and may precede diagnosis by a mean of 8 years. Other cardinal features include weight loss, abdominal pain and steatorrhea due to small intestinal involvement. Table 1 summarizes the important signs and symptoms of Whipple's disease.9, 10 One notable manifestation missing in Whipple's report is central nervous system involvement. Central nervous system (CNS) disease ranges from cognitive deficits to encephalitis and focal defects, and may occur years after treatment and without concomitant intestinal symptoms.
| Clinical Feature | Comment |
|---|---|
| |
| Cardinal features (present in 60% to 90%) | |
| Arthropathy | Most common initial symptom, preceding diagnosis by a mean of 8 years. Migratory, nonerosive, mainly in the peripheral joints. |
| Weight loss | |
| Diarrhea | Usually steatorrhea, may be associated with pain or occult blood in the stool |
| Other common features (present in 20% to 45%) | |
| Fever | |
| Lymphadenopathy | May present as a palpable mass |
| Increased skin pigmentation | Mechanism unknown (evidence of adrenal insufficiency has not been found in Whipple's) |
| Cardiac disease | Culture‐negative endocarditis |
| Hypotension | |
| Peripheral edema | |
| Uncommon clinical features | |
| Central nervous system involvement | May be global (dementia, personality change, sleep disturbance) or focal (cranial neuropathy, nystagmus) |
| Eye disease | Uveitis, retinitis |
| Hepatosplenomegaly | |
| Polyserositis | |
| Ascites | |
A remaining mystery is why this pathogen results only rarely in clinical disease. Caucasians comprise the majority of infected patients, and men are affected 8 times more often than women. An overrepresentation of HLA‐B27 suggests a genetic predisposition, though its role in pathogenesis is unclear. T. whipplei has been identified by PCR methods in asymptomatic individuals, implying additional abnormalities must be present in susceptible hosts for symptoms to occur following colonization.11 The exact immune defects are speculative, and immunodeficiency states (such as HIV) have not been consistently identified in patients with Whipple's disease.
The cornerstone of diagnosing Whipple's disease is upper endoscopy with duodenal biopsy. Flattening of the villi and markedly increased PAS‐positive staining of lamina propria macrophages are strongly suggestive of the diagnosis. PAS‐positive staining is not unique to T. whipplei, however. In patients with profound immunodeficiency, Mycobacterium avium intracellulare may stain positive with PAS. Since Whipple's disease is only rarely associated with HIV, a negative HIV test would favor a diagnosis of Whipple's disease. Electron microscopy may distinguish T. whipplei from its mimickers by morphology. For extraintestinal disease, PCR testing on samples from infected tissue has been found to be a reliable diagnostic aid.9
Given the rarity of the disease, controlled clinical trials addressing optimal treatment are lacking. Current recommendations include initial therapy for 14 days with an agent that crosses the blood‐brain barrier (eg, ceftriaxone) to reduce the incidence of CNS disease. This is then followed by a year or more of oral antimicrobial therapy with trimethoprim‐sulfamethoxazole or a tetracycline.9 While most patients respond within 2 to 3 weeks, relapse may occur in as many as one‐third of patients.
Historical case reports reinforce the case‐based learning paradigm. As the discussant remarks, observation was all too often the only recourse for physicians a century ago. In recounting the 7‐year progression of disease in 1 individual, Whipple provides a unique window into the natural evolution of the key features of this systemic disease. Viewed through the prism of Whipple's eyes, we can recall the striking lymphoid hyperplasia and unusual organisms in the small intestine, cementing our understanding of the pathogenesis of this disorder. Revisiting past cases allows us to learn of and learn from the past.
Teaching Points
-
Whipple's disease should be considered in patients with unexplained arthralgias accompanied by weight loss, malabsorption, and abdominal pain.
-
For suspected intestinal Whipple's disease, diagnosis is best made by duodenal biopsy demonstrating PAS‐positive staining in lamina propria macrophages.
-
Systemic manifestations of Whipple's disease include culture‐negative endocarditis and CNS disease. PCR testing of involved sites for T. whipplei is recommended to confirm extraintestinal disease.
- .A hitherto undescribed disease characterized anatomically by deposits of fat and fatty acids in the intestinal and mesenteric lymphatic tissues.Bull Johns Hopkins Hosp.1907;18:382–391.
- .The tinctoral demonstration of a glycoprotein in Whipple's disease.Proc Soc Exp Biol Med.1949;72:225–227.
- ,,.Whipple's disease: clinical, biochemical, and histopathologic features and assessment of treatment in 29 patients.Mayo Clin Proc.1988;63:539–551.
- ,.Combined electron and light microscopy in Whipple's disease: demonstration of “bacillary bodies” in the intestine.Bull Johns Hopkins Hosp.1961;109:80–98.
- ,,,.Phylogeny of the Whipple's disease‐associated bacterium.Lancet.1991;338:474–475.
- ,,,.Identification of the uncultured bacillus of Whipple's disease.N Engl J Med.1992;327:293–301.
- ,,, et al.Cultivation of the bacillus of Whipple's disease.N Engl J Med.2000;342:620–625.
- ,,,.Immunodetection of Tropheryma whipplei in intestinal tissue from Dr. Whipple's 1907 patient.N Engl J Med.2003;348:1411– 1412.
- ,.Whipple's disease.Lancet.2003;361:239–246.
- ,,, et al.Diagnostic guidelines in central nervous system Whipple's disease.Ann Neurol.1996;40:561–568.
- ,,, et al.PCR‐positive tests for Tropheryma whippleii in patients without Whipple's disease.Lancet.1999;353:2214.
- .A hitherto undescribed disease characterized anatomically by deposits of fat and fatty acids in the intestinal and mesenteric lymphatic tissues.Bull Johns Hopkins Hosp.1907;18:382–391.
- .The tinctoral demonstration of a glycoprotein in Whipple's disease.Proc Soc Exp Biol Med.1949;72:225–227.
- ,,.Whipple's disease: clinical, biochemical, and histopathologic features and assessment of treatment in 29 patients.Mayo Clin Proc.1988;63:539–551.
- ,.Combined electron and light microscopy in Whipple's disease: demonstration of “bacillary bodies” in the intestine.Bull Johns Hopkins Hosp.1961;109:80–98.
- ,,,.Phylogeny of the Whipple's disease‐associated bacterium.Lancet.1991;338:474–475.
- ,,,.Identification of the uncultured bacillus of Whipple's disease.N Engl J Med.1992;327:293–301.
- ,,, et al.Cultivation of the bacillus of Whipple's disease.N Engl J Med.2000;342:620–625.
- ,,,.Immunodetection of Tropheryma whipplei in intestinal tissue from Dr. Whipple's 1907 patient.N Engl J Med.2003;348:1411– 1412.
- ,.Whipple's disease.Lancet.2003;361:239–246.
- ,,, et al.Diagnostic guidelines in central nervous system Whipple's disease.Ann Neurol.1996;40:561–568.
- ,,, et al.PCR‐positive tests for Tropheryma whippleii in patients without Whipple's disease.Lancet.1999;353:2214.
In the Literature
Literature at a Glance
A guide to this month’s studies.
- Carotid stenting is equivalent to endarterectomy in carotid artery stenosis.
- Idiopathic ventricular fibrillation is associated with early repolarization.
- Aggressive LDL and blood pressure management may benefit diabetic patients.
- Treatment of hypertension in patients older than age 80 improves outcomes.
- Length of stay predicts mortality in pulmonary embolism patients.
- Poor symptom control and lack of family support during end-of-life hospital stays.
- Potentially inappropriate medication use in hospitalized elders is common.
- Steroids may not help in pediatric meningitis.
- PCI and CABG may be equivalent in the treatment of left main disease.
Is Stenting or Endarterectomy Best for Carotid Artery Stenosis?
Background: Patients with moderate to severe symptomatic carotid artery stenosis and those with severe asymptomatic carotid stenosis benefit from carotid endarterectomy. Carotid stenting may provide an alternative therapy, but the long-term protection against stroke compared with endarterectomy is unclear.
Study Design: Prospective randomized trial.
Setting: 29 centers in the United States.
Synopsis: This article reports the long-term (three years) follow-up of the SAPPHIRE trial, published in 2004, which compared carotid stenting to endarterectomy in patients at high surgical risk. In that trial, 334 patients randomized to either stenting or endarterectomy had similar outcomes at one year. Patients were followed for three years with death and major cardiovascular events as endpoints.
Rates of stroke at three years were approximately 10% with an overall death rate of approximately 20%. There was no difference between carotid stenting and endarterectomy with regards to death, stroke, or other cardiovascular outcome.
Notably, follow-up was not complete (78%), a specific type of stenting procedure was used, and the patient population was at high risk for surgical complications. Therefore, results may not be applicable in other centers or in other patient populations. Yet, this trial provides follow-up, long-term evidence that carotid stenting may be a viable alternative to endarterectomy in patients with carotid artery stenosis.
Bottom line: Carotid stenting and endarterectomy had similar outcomes at three years in high-risk patients with carotid artery stenosis.
Citation: Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572-1579.
Is Early Repolarization on EKG Associated with Sudden Cardiac Arrest?
Background: Electrocardiographic early repolarization, defined as elevation of the QRS-ST junction of at least 0.1mV from baseline in the inferior or lateral leads (manifested as slurring or notching), occurs in 1% to 5% of patients. It is considered benign, but experimental studies have suggested it may be arrhythmogenic.
Study Design: Prospective case-control.
Setting: 22 international tertiary care centers.
Synopsis: Case subjects were less than 60 years of age and were resuscitated after ventricular fibrillation (VF) arrest ultimately deemed idiopathic. All had normal echocardiograms, no evidence of coronary artery disease, and no repolarization abnormalities (including Brugada and long-QT). Of 206 patients, 31% had early repolarization on EKG, versus only 5% in controls without heart disease. In case subjects with prior EKGs, early repolarization was proven to be pre-existing.
The mean magnitude of J-point elevation was 2 mm in cases versus 1.2 mm in controls, and in cases this magnitude increased during later episodes of arrhythmia. Electrophysiologic mapping showed that ectopy originated at sites concordant with the location of abnormal repolarization. During five years of follow-up, arrhythmic recurrence was twice as common in cases with early repolarization.
Although long-term observational studies of persons with early repolarization have shown a benign natural course, this study may change our approach to those with syncope or a family history of sudden death.
Bottom line: Early repolarization on EKG is associated with idiopathic ventricular fibrillation.
Citation: Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358(19):2016-2023.
Does Aggressive Blood Pressure and LDL Treatment in Diabetics Affect Development of Subclinical Atherosclerosis?
Background: There is evidence to suggest more aggressive treatment of LDL cholesterol in patients with known coronary artery disease is beneficial and more aggressive blood pressure control can improve outcomes in some patient populations. However, it is unclear if patients with diabetes without cardiovascular disease would benefit from more aggressive LDL and systolic blood pressure (SBP) treatment.
Study Design: Randomized, open-label, blinded-to-end point trial.
Setting: Four centers in Oklahoma, Arizona, and South Dakota.
Synopsis: Investigators studied 499 type 2 diabetic American Indian men with no history of cardiovascular disease. Patients were randomized to receive treatment to achieve aggressive (70 mg/dL and 115 mmHg) or standard (100 mg/dL and 130 mmHg) targets for their LDL cholesterol and SBP, respectively.
At three years, the aggressive group showed decreased carotid intima-media thickness (IMT) and decreased left ventricular mass, whereas both IMT and left ventricular volume increased in the standard group. There were no differences in clinical cardiovascular events between the aggressive and standard group and both groups had lower-than-expected clinical events.
This study included no women and was limited to an American Indian population. Of note, there was an increase in adverse events related to blood pressure medications in the aggressive group. It also is unclear how the surrogates of cardiovascular disease or subclinical atherosclerosis relate to significant clinical outcomes.
Bottom line: More aggressive LDL and SBP treatment in diabetics without coronary disease decreased subclinical atherosclerosis but did not impact clinical outcomes.
Citation: Howard B, Roman M, Devereux R, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes. JAMA. 2008;299(14):1678-1689.
Should We Treat Hypertension in Patients Older Than 80?
Background: There is debate about whether treatment of hypertension in the elderly is beneficial. Numerous studies suggest blood pressure control does less to prevent strokes in patients older than 80 years than for younger patients. Moreover, other evidence shows controlling blood pressure in elderly patients may result in an increase in mortality even if there was a decreased risk of stroke.
Study Design: Randomized, double-blind, placebo-controlled trial.
Setting: 195 centers in 13 countries in Europe, China, Australasia, and North Africa.
Synopsis: This study evaluated 3,845 patients, age 80 or older, with a sustained systolic blood pressure (SBP) of 160 mmHg and randomized them to receive indapamide (sustained release) or placebo. Perindopril, or placebo, was added if necessary to achieve a target blood pressure of 150/80 mmHg. Patients who received the indapamide with or without the perindopril had lower blood pressure, lower rate of stroke, lower rate of heart failure, lower rate of death from a cardiovascular cause, and a 21% reduction in all-cause mortality (all statistically significant). There were very few adverse drug events and fewer adverse events overall in the treatment group.
Of note, exclusion criteria included a history of heart failure requiring anti-hypertensive medication, dementia, need for nursing care, an inability to stand or walk, and a creatinine more than 1.7 mg/dL. As well, the “target” SBP of 150 mmHg (which only half of the treatment group achieved) is still considered hypertensive according to the JNC 7 guidelines.
Bottom line: In some patients older than 80, treatment of hypertension may reduce the incidence of stroke, death from stroke, heart failure, and all-cause mortality.
Citation: Beckett N, Peters R, Fletcher A, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
What Is the Optimal Hospital LOS for Patients with PE?
Background: Though there are clear trends toward shorter hospital stays after pulmonary embolism (PE), especially with the introduction of low molecular weight heparin, the optimal timing of discharge and the effect of decreased length of stay (LOS) on post-discharge mortality are unknown. Furthermore, there is no risk stratification strategy used to identify low-risk patients with PE who can safely be discharged early or treated in the outpatient setting.
Study Design: Retrospective cohort study.
Setting: 186 acute care hospitals in Pennsylvania from January 2000 to November 2002.
Synopsis: Using a statewide database of 15,531 patients discharged with pulmonary embolism (PE), the authors sought to identify patient and hospital factors associated with LOS and assess whether LOS was associated with post-discharge mortality.
Findings indicate there is considerable variation in LOS for PE between and within hospitals in Pennsylvania. The median LOS for patients with PE was six days; patients with a LOS of four or fewer days had significantly higher post-discharge mortality than patients hospitalized five to eight days. More than half the patients discharged at four or fewer days were classified as high-risk, with Pulmonary Embolism Severity Index (PESI) scores of III-V (3.1% to 24.5% risk of mortality at 30 days).
Although we cannot infer causation (i.e., early discharge=death), clinicians should be aware of the results and consider severity of illness (using PESI or other criteria) in the discharge decision in patients with PE. Future prognostic models and evidence-based criteria would be helpful to identify patients with PE who can be safely discharged early.
Bottom line: Physicians may inappropriately select patients with PE for early discharge who are at increased risk of complications.
Citation: Aujesky D, Stone RA, Kim S, et al. Length of hospital stay and post-discharge mortality in patients with pulmonary embolism. Arch Intern Med. 2008;168(7):706-712.
Do Patients Have a “Good Death” in the Hospital?
Background: Despite an increasing focus on providing appropriate end-of-life care, the majority of patients in developed countries die in the hospital. The circumstances and quality of care provided at the time of death are poorly described.
Study Design: Cross-sectional survey.
Setting: 613 departments in 200 French hospitals.
Synopsis: For 3,793 in-hospital deaths, the investigators surveyed the bedside nurses about the circumstances and details of the death. Twenty-three percent of the patients were admitted for end-of-life care, 29% had a malignancy, and 50% of patients were identified as terminally ill for three days prior to their death.
A family member or relative was present in only 25% of all deaths; 20% of patients were alone at the time of death. In the last few hours of life, up to 70% of patients had symptoms of respiratory distress, while only 44% received opiate analgesia. Only 35% of nurses were satisfied with the quality of death. Satisfaction increased with presence of family members and having written protocols for care at the end of life.
This large, multicenter study has limitations but provides a concerning snapshot of death in the hospital. Hospitalists should be aggressive about symptom control at the end of life as well as attempt to ensure patients are not alone at the time of death.
Bottom line: Many patients die in the hospital in some degree of respiratory distress and without family or friends at the bedside.
Citation: Ferrand E, Jabre P, Vincent-Genod C, et al. Circumstances of death in hospitalized patients and nurses’ perceptions. Arch Intern Med. 2008;168(8):867-875.
How Common Is Potentially Inappropriate Medication Use in the Hospital?
Background: Use of potentially inappropriate medications (PIM) in the elderly based on the Beers’ List is common in nursing homes, the emergency department (ED), and outpatient settings and is associated with adverse outcomes and hospitalization. Frequency of PIM use the inpatient setting has not been well studied.
Study Design: A retrospective cohort study.
Setting: 384 U.S. hospitals.
Synopsis: In this retrospective cohort study of 493,971 inpatients (older than 65) admitted with medical diagnoses to non-surgeons, PIM prescription was evaluated. Forty nine percent of all patients were prescribed at least one PIM, while 6% were prescribed three or more. In a multivariable model, physician specialty was associated with variation in high severity PIM (HSPIM) prescription. In comparison with internal medicine physicians, cardiologists (odds ratio [OR] 1.32) and pulmonologists (OR 1.10) were more likely to prescribe HSPIMs, while hospitalists (OR 0.90) and geriatricians (OR 0.60) were less likely. In addition, patient age older than 85 was associated with decreased HSPIM prescription (OR 0.59) compared with those younger than 85.
Compared with patients in the Midwest, patients in the South (OR 1.63) and West (OR 1.43) were more likely to prescribe HSPIMs, while those in the Northeast (OR 0.85) were less likely. Hospitals with geriatric services had less PIM use. The study couldn’t account for continuation of chronic medications and did not evaluate adverse outcomes from PIM prescribing.
Bottom line: PIM prescription to hospitalized geriatric patients is common and associated with provider and hospital characteristics.
Citation: Rothberg MB, Pekow PS, Liu F, et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3:91-102:91-102.
Is There a Benefit to Corticosteroids When Treating Bacterial Meningitis in Children?
Background: The benefit of adjuvant corticosteroids in the treatment of bacterial meningitis in children in the developed world remains unclear; recent expert guidelines reflect this uncertainty.
Study Design: Retrospective cohort study.
Setting: Twenty-seven tertiary care hospitals in the United States.
Synopsis: Researchers examined 2,780 children with a primary diagnosis of bacterial meningitis discharged from 27 tertiary care centers in the U.S. from 2001-2006. Using a propensity analysis (to control for severity of illness), the study compared those who had received adjunctive corticosteroids with those who had not, with mortality and length of study (LOS) as primary outcomes.
The median age was nine months, 8.9% of children received corticosteroids, and the overall mortality rate was 4.2%. Adjuvent corticosteroids did not reduce mortality or LOS. The outcomes were unchanged in subgroup analyses.
Although limited by its retrospective design and lack of other outcome measures (e.g., hearing loss, neurological deficits), this study provides reasonable evidence that corticosteroid use in bacterial meningitis in children may not save lives or shorten LOS. Pediatric hospitalists may not want to routinely give steroids in this setting pending large randomized-controlled trials.
Bottom line: Adjunctive corticosteroids therapy in children with bacterial meningitis may not save lives or reduce LOS.
Citation: Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055.
Should Unprotected Left Main Disease Be Treated With PCI or CABG?
Background: The current standard of care for the treatment of left main coronary artery disease is coronary-artery bypass grafting (CABG). With the advent of drug-eluting stents, there is growing interest in the use of percutaneous coronary intervention (PCI) to treat left main disease.
Study Design: Prospective observational study.
Setting: Twelve Korean cardiac centers.
Synopsis: From 2000 to 2006, patients with left main disease were treated with PCI or CABG at the discretion of the physician. Nearly 1,100 patients in each cohort were compared and evaluated for death and a composite outcome of death, myocardial infarction, or stroke. Propensity-matching was employed to control for confounders.
In the overall cohort matched by propensity score, there was no significant difference in death or the composite outcome between the PCI and CABG groups after three years. Type of stent (bare metal vs. drug-eluting) did not affect the outcome. Rates of target-vessel revascularization were significantly higher in the group that received stents.
The results are limited by the observational nature and the need for propensity analysis and yet provide an intriguing result. The standard of care for treatment of left main disease remains CABG, but clinicians may be more comfortable treating with stents while we await randomized-controlled trials.
Bottom line: In this observational study, PCI and CABG had similar outcomes in patients with left main disease.
Citation: Seung KB, Park D, Kim Y, Lee S. Stents versus coronary-artery bypass grafting for left main coronary artery disease. N Engl J Med. 2008;358:1781-1792.
Literature at a Glance
A guide to this month’s studies.
- Carotid stenting is equivalent to endarterectomy in carotid artery stenosis.
- Idiopathic ventricular fibrillation is associated with early repolarization.
- Aggressive LDL and blood pressure management may benefit diabetic patients.
- Treatment of hypertension in patients older than age 80 improves outcomes.
- Length of stay predicts mortality in pulmonary embolism patients.
- Poor symptom control and lack of family support during end-of-life hospital stays.
- Potentially inappropriate medication use in hospitalized elders is common.
- Steroids may not help in pediatric meningitis.
- PCI and CABG may be equivalent in the treatment of left main disease.
Is Stenting or Endarterectomy Best for Carotid Artery Stenosis?
Background: Patients with moderate to severe symptomatic carotid artery stenosis and those with severe asymptomatic carotid stenosis benefit from carotid endarterectomy. Carotid stenting may provide an alternative therapy, but the long-term protection against stroke compared with endarterectomy is unclear.
Study Design: Prospective randomized trial.
Setting: 29 centers in the United States.
Synopsis: This article reports the long-term (three years) follow-up of the SAPPHIRE trial, published in 2004, which compared carotid stenting to endarterectomy in patients at high surgical risk. In that trial, 334 patients randomized to either stenting or endarterectomy had similar outcomes at one year. Patients were followed for three years with death and major cardiovascular events as endpoints.
Rates of stroke at three years were approximately 10% with an overall death rate of approximately 20%. There was no difference between carotid stenting and endarterectomy with regards to death, stroke, or other cardiovascular outcome.
Notably, follow-up was not complete (78%), a specific type of stenting procedure was used, and the patient population was at high risk for surgical complications. Therefore, results may not be applicable in other centers or in other patient populations. Yet, this trial provides follow-up, long-term evidence that carotid stenting may be a viable alternative to endarterectomy in patients with carotid artery stenosis.
Bottom line: Carotid stenting and endarterectomy had similar outcomes at three years in high-risk patients with carotid artery stenosis.
Citation: Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572-1579.
Is Early Repolarization on EKG Associated with Sudden Cardiac Arrest?
Background: Electrocardiographic early repolarization, defined as elevation of the QRS-ST junction of at least 0.1mV from baseline in the inferior or lateral leads (manifested as slurring or notching), occurs in 1% to 5% of patients. It is considered benign, but experimental studies have suggested it may be arrhythmogenic.
Study Design: Prospective case-control.
Setting: 22 international tertiary care centers.
Synopsis: Case subjects were less than 60 years of age and were resuscitated after ventricular fibrillation (VF) arrest ultimately deemed idiopathic. All had normal echocardiograms, no evidence of coronary artery disease, and no repolarization abnormalities (including Brugada and long-QT). Of 206 patients, 31% had early repolarization on EKG, versus only 5% in controls without heart disease. In case subjects with prior EKGs, early repolarization was proven to be pre-existing.
The mean magnitude of J-point elevation was 2 mm in cases versus 1.2 mm in controls, and in cases this magnitude increased during later episodes of arrhythmia. Electrophysiologic mapping showed that ectopy originated at sites concordant with the location of abnormal repolarization. During five years of follow-up, arrhythmic recurrence was twice as common in cases with early repolarization.
Although long-term observational studies of persons with early repolarization have shown a benign natural course, this study may change our approach to those with syncope or a family history of sudden death.
Bottom line: Early repolarization on EKG is associated with idiopathic ventricular fibrillation.
Citation: Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358(19):2016-2023.
Does Aggressive Blood Pressure and LDL Treatment in Diabetics Affect Development of Subclinical Atherosclerosis?
Background: There is evidence to suggest more aggressive treatment of LDL cholesterol in patients with known coronary artery disease is beneficial and more aggressive blood pressure control can improve outcomes in some patient populations. However, it is unclear if patients with diabetes without cardiovascular disease would benefit from more aggressive LDL and systolic blood pressure (SBP) treatment.
Study Design: Randomized, open-label, blinded-to-end point trial.
Setting: Four centers in Oklahoma, Arizona, and South Dakota.
Synopsis: Investigators studied 499 type 2 diabetic American Indian men with no history of cardiovascular disease. Patients were randomized to receive treatment to achieve aggressive (70 mg/dL and 115 mmHg) or standard (100 mg/dL and 130 mmHg) targets for their LDL cholesterol and SBP, respectively.
At three years, the aggressive group showed decreased carotid intima-media thickness (IMT) and decreased left ventricular mass, whereas both IMT and left ventricular volume increased in the standard group. There were no differences in clinical cardiovascular events between the aggressive and standard group and both groups had lower-than-expected clinical events.
This study included no women and was limited to an American Indian population. Of note, there was an increase in adverse events related to blood pressure medications in the aggressive group. It also is unclear how the surrogates of cardiovascular disease or subclinical atherosclerosis relate to significant clinical outcomes.
Bottom line: More aggressive LDL and SBP treatment in diabetics without coronary disease decreased subclinical atherosclerosis but did not impact clinical outcomes.
Citation: Howard B, Roman M, Devereux R, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes. JAMA. 2008;299(14):1678-1689.
Should We Treat Hypertension in Patients Older Than 80?
Background: There is debate about whether treatment of hypertension in the elderly is beneficial. Numerous studies suggest blood pressure control does less to prevent strokes in patients older than 80 years than for younger patients. Moreover, other evidence shows controlling blood pressure in elderly patients may result in an increase in mortality even if there was a decreased risk of stroke.
Study Design: Randomized, double-blind, placebo-controlled trial.
Setting: 195 centers in 13 countries in Europe, China, Australasia, and North Africa.
Synopsis: This study evaluated 3,845 patients, age 80 or older, with a sustained systolic blood pressure (SBP) of 160 mmHg and randomized them to receive indapamide (sustained release) or placebo. Perindopril, or placebo, was added if necessary to achieve a target blood pressure of 150/80 mmHg. Patients who received the indapamide with or without the perindopril had lower blood pressure, lower rate of stroke, lower rate of heart failure, lower rate of death from a cardiovascular cause, and a 21% reduction in all-cause mortality (all statistically significant). There were very few adverse drug events and fewer adverse events overall in the treatment group.
Of note, exclusion criteria included a history of heart failure requiring anti-hypertensive medication, dementia, need for nursing care, an inability to stand or walk, and a creatinine more than 1.7 mg/dL. As well, the “target” SBP of 150 mmHg (which only half of the treatment group achieved) is still considered hypertensive according to the JNC 7 guidelines.
Bottom line: In some patients older than 80, treatment of hypertension may reduce the incidence of stroke, death from stroke, heart failure, and all-cause mortality.
Citation: Beckett N, Peters R, Fletcher A, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
What Is the Optimal Hospital LOS for Patients with PE?
Background: Though there are clear trends toward shorter hospital stays after pulmonary embolism (PE), especially with the introduction of low molecular weight heparin, the optimal timing of discharge and the effect of decreased length of stay (LOS) on post-discharge mortality are unknown. Furthermore, there is no risk stratification strategy used to identify low-risk patients with PE who can safely be discharged early or treated in the outpatient setting.
Study Design: Retrospective cohort study.
Setting: 186 acute care hospitals in Pennsylvania from January 2000 to November 2002.
Synopsis: Using a statewide database of 15,531 patients discharged with pulmonary embolism (PE), the authors sought to identify patient and hospital factors associated with LOS and assess whether LOS was associated with post-discharge mortality.
Findings indicate there is considerable variation in LOS for PE between and within hospitals in Pennsylvania. The median LOS for patients with PE was six days; patients with a LOS of four or fewer days had significantly higher post-discharge mortality than patients hospitalized five to eight days. More than half the patients discharged at four or fewer days were classified as high-risk, with Pulmonary Embolism Severity Index (PESI) scores of III-V (3.1% to 24.5% risk of mortality at 30 days).
Although we cannot infer causation (i.e., early discharge=death), clinicians should be aware of the results and consider severity of illness (using PESI or other criteria) in the discharge decision in patients with PE. Future prognostic models and evidence-based criteria would be helpful to identify patients with PE who can be safely discharged early.
Bottom line: Physicians may inappropriately select patients with PE for early discharge who are at increased risk of complications.
Citation: Aujesky D, Stone RA, Kim S, et al. Length of hospital stay and post-discharge mortality in patients with pulmonary embolism. Arch Intern Med. 2008;168(7):706-712.
Do Patients Have a “Good Death” in the Hospital?
Background: Despite an increasing focus on providing appropriate end-of-life care, the majority of patients in developed countries die in the hospital. The circumstances and quality of care provided at the time of death are poorly described.
Study Design: Cross-sectional survey.
Setting: 613 departments in 200 French hospitals.
Synopsis: For 3,793 in-hospital deaths, the investigators surveyed the bedside nurses about the circumstances and details of the death. Twenty-three percent of the patients were admitted for end-of-life care, 29% had a malignancy, and 50% of patients were identified as terminally ill for three days prior to their death.
A family member or relative was present in only 25% of all deaths; 20% of patients were alone at the time of death. In the last few hours of life, up to 70% of patients had symptoms of respiratory distress, while only 44% received opiate analgesia. Only 35% of nurses were satisfied with the quality of death. Satisfaction increased with presence of family members and having written protocols for care at the end of life.
This large, multicenter study has limitations but provides a concerning snapshot of death in the hospital. Hospitalists should be aggressive about symptom control at the end of life as well as attempt to ensure patients are not alone at the time of death.
Bottom line: Many patients die in the hospital in some degree of respiratory distress and without family or friends at the bedside.
Citation: Ferrand E, Jabre P, Vincent-Genod C, et al. Circumstances of death in hospitalized patients and nurses’ perceptions. Arch Intern Med. 2008;168(8):867-875.
How Common Is Potentially Inappropriate Medication Use in the Hospital?
Background: Use of potentially inappropriate medications (PIM) in the elderly based on the Beers’ List is common in nursing homes, the emergency department (ED), and outpatient settings and is associated with adverse outcomes and hospitalization. Frequency of PIM use the inpatient setting has not been well studied.
Study Design: A retrospective cohort study.
Setting: 384 U.S. hospitals.
Synopsis: In this retrospective cohort study of 493,971 inpatients (older than 65) admitted with medical diagnoses to non-surgeons, PIM prescription was evaluated. Forty nine percent of all patients were prescribed at least one PIM, while 6% were prescribed three or more. In a multivariable model, physician specialty was associated with variation in high severity PIM (HSPIM) prescription. In comparison with internal medicine physicians, cardiologists (odds ratio [OR] 1.32) and pulmonologists (OR 1.10) were more likely to prescribe HSPIMs, while hospitalists (OR 0.90) and geriatricians (OR 0.60) were less likely. In addition, patient age older than 85 was associated with decreased HSPIM prescription (OR 0.59) compared with those younger than 85.
Compared with patients in the Midwest, patients in the South (OR 1.63) and West (OR 1.43) were more likely to prescribe HSPIMs, while those in the Northeast (OR 0.85) were less likely. Hospitals with geriatric services had less PIM use. The study couldn’t account for continuation of chronic medications and did not evaluate adverse outcomes from PIM prescribing.
Bottom line: PIM prescription to hospitalized geriatric patients is common and associated with provider and hospital characteristics.
Citation: Rothberg MB, Pekow PS, Liu F, et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3:91-102:91-102.
Is There a Benefit to Corticosteroids When Treating Bacterial Meningitis in Children?
Background: The benefit of adjuvant corticosteroids in the treatment of bacterial meningitis in children in the developed world remains unclear; recent expert guidelines reflect this uncertainty.
Study Design: Retrospective cohort study.
Setting: Twenty-seven tertiary care hospitals in the United States.
Synopsis: Researchers examined 2,780 children with a primary diagnosis of bacterial meningitis discharged from 27 tertiary care centers in the U.S. from 2001-2006. Using a propensity analysis (to control for severity of illness), the study compared those who had received adjunctive corticosteroids with those who had not, with mortality and length of study (LOS) as primary outcomes.
The median age was nine months, 8.9% of children received corticosteroids, and the overall mortality rate was 4.2%. Adjuvent corticosteroids did not reduce mortality or LOS. The outcomes were unchanged in subgroup analyses.
Although limited by its retrospective design and lack of other outcome measures (e.g., hearing loss, neurological deficits), this study provides reasonable evidence that corticosteroid use in bacterial meningitis in children may not save lives or shorten LOS. Pediatric hospitalists may not want to routinely give steroids in this setting pending large randomized-controlled trials.
Bottom line: Adjunctive corticosteroids therapy in children with bacterial meningitis may not save lives or reduce LOS.
Citation: Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055.
Should Unprotected Left Main Disease Be Treated With PCI or CABG?
Background: The current standard of care for the treatment of left main coronary artery disease is coronary-artery bypass grafting (CABG). With the advent of drug-eluting stents, there is growing interest in the use of percutaneous coronary intervention (PCI) to treat left main disease.
Study Design: Prospective observational study.
Setting: Twelve Korean cardiac centers.
Synopsis: From 2000 to 2006, patients with left main disease were treated with PCI or CABG at the discretion of the physician. Nearly 1,100 patients in each cohort were compared and evaluated for death and a composite outcome of death, myocardial infarction, or stroke. Propensity-matching was employed to control for confounders.
In the overall cohort matched by propensity score, there was no significant difference in death or the composite outcome between the PCI and CABG groups after three years. Type of stent (bare metal vs. drug-eluting) did not affect the outcome. Rates of target-vessel revascularization were significantly higher in the group that received stents.
The results are limited by the observational nature and the need for propensity analysis and yet provide an intriguing result. The standard of care for treatment of left main disease remains CABG, but clinicians may be more comfortable treating with stents while we await randomized-controlled trials.
Bottom line: In this observational study, PCI and CABG had similar outcomes in patients with left main disease.
Citation: Seung KB, Park D, Kim Y, Lee S. Stents versus coronary-artery bypass grafting for left main coronary artery disease. N Engl J Med. 2008;358:1781-1792.
Literature at a Glance
A guide to this month’s studies.
- Carotid stenting is equivalent to endarterectomy in carotid artery stenosis.
- Idiopathic ventricular fibrillation is associated with early repolarization.
- Aggressive LDL and blood pressure management may benefit diabetic patients.
- Treatment of hypertension in patients older than age 80 improves outcomes.
- Length of stay predicts mortality in pulmonary embolism patients.
- Poor symptom control and lack of family support during end-of-life hospital stays.
- Potentially inappropriate medication use in hospitalized elders is common.
- Steroids may not help in pediatric meningitis.
- PCI and CABG may be equivalent in the treatment of left main disease.
Is Stenting or Endarterectomy Best for Carotid Artery Stenosis?
Background: Patients with moderate to severe symptomatic carotid artery stenosis and those with severe asymptomatic carotid stenosis benefit from carotid endarterectomy. Carotid stenting may provide an alternative therapy, but the long-term protection against stroke compared with endarterectomy is unclear.
Study Design: Prospective randomized trial.
Setting: 29 centers in the United States.
Synopsis: This article reports the long-term (three years) follow-up of the SAPPHIRE trial, published in 2004, which compared carotid stenting to endarterectomy in patients at high surgical risk. In that trial, 334 patients randomized to either stenting or endarterectomy had similar outcomes at one year. Patients were followed for three years with death and major cardiovascular events as endpoints.
Rates of stroke at three years were approximately 10% with an overall death rate of approximately 20%. There was no difference between carotid stenting and endarterectomy with regards to death, stroke, or other cardiovascular outcome.
Notably, follow-up was not complete (78%), a specific type of stenting procedure was used, and the patient population was at high risk for surgical complications. Therefore, results may not be applicable in other centers or in other patient populations. Yet, this trial provides follow-up, long-term evidence that carotid stenting may be a viable alternative to endarterectomy in patients with carotid artery stenosis.
Bottom line: Carotid stenting and endarterectomy had similar outcomes at three years in high-risk patients with carotid artery stenosis.
Citation: Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572-1579.
Is Early Repolarization on EKG Associated with Sudden Cardiac Arrest?
Background: Electrocardiographic early repolarization, defined as elevation of the QRS-ST junction of at least 0.1mV from baseline in the inferior or lateral leads (manifested as slurring or notching), occurs in 1% to 5% of patients. It is considered benign, but experimental studies have suggested it may be arrhythmogenic.
Study Design: Prospective case-control.
Setting: 22 international tertiary care centers.
Synopsis: Case subjects were less than 60 years of age and were resuscitated after ventricular fibrillation (VF) arrest ultimately deemed idiopathic. All had normal echocardiograms, no evidence of coronary artery disease, and no repolarization abnormalities (including Brugada and long-QT). Of 206 patients, 31% had early repolarization on EKG, versus only 5% in controls without heart disease. In case subjects with prior EKGs, early repolarization was proven to be pre-existing.
The mean magnitude of J-point elevation was 2 mm in cases versus 1.2 mm in controls, and in cases this magnitude increased during later episodes of arrhythmia. Electrophysiologic mapping showed that ectopy originated at sites concordant with the location of abnormal repolarization. During five years of follow-up, arrhythmic recurrence was twice as common in cases with early repolarization.
Although long-term observational studies of persons with early repolarization have shown a benign natural course, this study may change our approach to those with syncope or a family history of sudden death.
Bottom line: Early repolarization on EKG is associated with idiopathic ventricular fibrillation.
Citation: Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358(19):2016-2023.
Does Aggressive Blood Pressure and LDL Treatment in Diabetics Affect Development of Subclinical Atherosclerosis?
Background: There is evidence to suggest more aggressive treatment of LDL cholesterol in patients with known coronary artery disease is beneficial and more aggressive blood pressure control can improve outcomes in some patient populations. However, it is unclear if patients with diabetes without cardiovascular disease would benefit from more aggressive LDL and systolic blood pressure (SBP) treatment.
Study Design: Randomized, open-label, blinded-to-end point trial.
Setting: Four centers in Oklahoma, Arizona, and South Dakota.
Synopsis: Investigators studied 499 type 2 diabetic American Indian men with no history of cardiovascular disease. Patients were randomized to receive treatment to achieve aggressive (70 mg/dL and 115 mmHg) or standard (100 mg/dL and 130 mmHg) targets for their LDL cholesterol and SBP, respectively.
At three years, the aggressive group showed decreased carotid intima-media thickness (IMT) and decreased left ventricular mass, whereas both IMT and left ventricular volume increased in the standard group. There were no differences in clinical cardiovascular events between the aggressive and standard group and both groups had lower-than-expected clinical events.
This study included no women and was limited to an American Indian population. Of note, there was an increase in adverse events related to blood pressure medications in the aggressive group. It also is unclear how the surrogates of cardiovascular disease or subclinical atherosclerosis relate to significant clinical outcomes.
Bottom line: More aggressive LDL and SBP treatment in diabetics without coronary disease decreased subclinical atherosclerosis but did not impact clinical outcomes.
Citation: Howard B, Roman M, Devereux R, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes. JAMA. 2008;299(14):1678-1689.
Should We Treat Hypertension in Patients Older Than 80?
Background: There is debate about whether treatment of hypertension in the elderly is beneficial. Numerous studies suggest blood pressure control does less to prevent strokes in patients older than 80 years than for younger patients. Moreover, other evidence shows controlling blood pressure in elderly patients may result in an increase in mortality even if there was a decreased risk of stroke.
Study Design: Randomized, double-blind, placebo-controlled trial.
Setting: 195 centers in 13 countries in Europe, China, Australasia, and North Africa.
Synopsis: This study evaluated 3,845 patients, age 80 or older, with a sustained systolic blood pressure (SBP) of 160 mmHg and randomized them to receive indapamide (sustained release) or placebo. Perindopril, or placebo, was added if necessary to achieve a target blood pressure of 150/80 mmHg. Patients who received the indapamide with or without the perindopril had lower blood pressure, lower rate of stroke, lower rate of heart failure, lower rate of death from a cardiovascular cause, and a 21% reduction in all-cause mortality (all statistically significant). There were very few adverse drug events and fewer adverse events overall in the treatment group.
Of note, exclusion criteria included a history of heart failure requiring anti-hypertensive medication, dementia, need for nursing care, an inability to stand or walk, and a creatinine more than 1.7 mg/dL. As well, the “target” SBP of 150 mmHg (which only half of the treatment group achieved) is still considered hypertensive according to the JNC 7 guidelines.
Bottom line: In some patients older than 80, treatment of hypertension may reduce the incidence of stroke, death from stroke, heart failure, and all-cause mortality.
Citation: Beckett N, Peters R, Fletcher A, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
What Is the Optimal Hospital LOS for Patients with PE?
Background: Though there are clear trends toward shorter hospital stays after pulmonary embolism (PE), especially with the introduction of low molecular weight heparin, the optimal timing of discharge and the effect of decreased length of stay (LOS) on post-discharge mortality are unknown. Furthermore, there is no risk stratification strategy used to identify low-risk patients with PE who can safely be discharged early or treated in the outpatient setting.
Study Design: Retrospective cohort study.
Setting: 186 acute care hospitals in Pennsylvania from January 2000 to November 2002.
Synopsis: Using a statewide database of 15,531 patients discharged with pulmonary embolism (PE), the authors sought to identify patient and hospital factors associated with LOS and assess whether LOS was associated with post-discharge mortality.
Findings indicate there is considerable variation in LOS for PE between and within hospitals in Pennsylvania. The median LOS for patients with PE was six days; patients with a LOS of four or fewer days had significantly higher post-discharge mortality than patients hospitalized five to eight days. More than half the patients discharged at four or fewer days were classified as high-risk, with Pulmonary Embolism Severity Index (PESI) scores of III-V (3.1% to 24.5% risk of mortality at 30 days).
Although we cannot infer causation (i.e., early discharge=death), clinicians should be aware of the results and consider severity of illness (using PESI or other criteria) in the discharge decision in patients with PE. Future prognostic models and evidence-based criteria would be helpful to identify patients with PE who can be safely discharged early.
Bottom line: Physicians may inappropriately select patients with PE for early discharge who are at increased risk of complications.
Citation: Aujesky D, Stone RA, Kim S, et al. Length of hospital stay and post-discharge mortality in patients with pulmonary embolism. Arch Intern Med. 2008;168(7):706-712.
Do Patients Have a “Good Death” in the Hospital?
Background: Despite an increasing focus on providing appropriate end-of-life care, the majority of patients in developed countries die in the hospital. The circumstances and quality of care provided at the time of death are poorly described.
Study Design: Cross-sectional survey.
Setting: 613 departments in 200 French hospitals.
Synopsis: For 3,793 in-hospital deaths, the investigators surveyed the bedside nurses about the circumstances and details of the death. Twenty-three percent of the patients were admitted for end-of-life care, 29% had a malignancy, and 50% of patients were identified as terminally ill for three days prior to their death.
A family member or relative was present in only 25% of all deaths; 20% of patients were alone at the time of death. In the last few hours of life, up to 70% of patients had symptoms of respiratory distress, while only 44% received opiate analgesia. Only 35% of nurses were satisfied with the quality of death. Satisfaction increased with presence of family members and having written protocols for care at the end of life.
This large, multicenter study has limitations but provides a concerning snapshot of death in the hospital. Hospitalists should be aggressive about symptom control at the end of life as well as attempt to ensure patients are not alone at the time of death.
Bottom line: Many patients die in the hospital in some degree of respiratory distress and without family or friends at the bedside.
Citation: Ferrand E, Jabre P, Vincent-Genod C, et al. Circumstances of death in hospitalized patients and nurses’ perceptions. Arch Intern Med. 2008;168(8):867-875.
How Common Is Potentially Inappropriate Medication Use in the Hospital?
Background: Use of potentially inappropriate medications (PIM) in the elderly based on the Beers’ List is common in nursing homes, the emergency department (ED), and outpatient settings and is associated with adverse outcomes and hospitalization. Frequency of PIM use the inpatient setting has not been well studied.
Study Design: A retrospective cohort study.
Setting: 384 U.S. hospitals.
Synopsis: In this retrospective cohort study of 493,971 inpatients (older than 65) admitted with medical diagnoses to non-surgeons, PIM prescription was evaluated. Forty nine percent of all patients were prescribed at least one PIM, while 6% were prescribed three or more. In a multivariable model, physician specialty was associated with variation in high severity PIM (HSPIM) prescription. In comparison with internal medicine physicians, cardiologists (odds ratio [OR] 1.32) and pulmonologists (OR 1.10) were more likely to prescribe HSPIMs, while hospitalists (OR 0.90) and geriatricians (OR 0.60) were less likely. In addition, patient age older than 85 was associated with decreased HSPIM prescription (OR 0.59) compared with those younger than 85.
Compared with patients in the Midwest, patients in the South (OR 1.63) and West (OR 1.43) were more likely to prescribe HSPIMs, while those in the Northeast (OR 0.85) were less likely. Hospitals with geriatric services had less PIM use. The study couldn’t account for continuation of chronic medications and did not evaluate adverse outcomes from PIM prescribing.
Bottom line: PIM prescription to hospitalized geriatric patients is common and associated with provider and hospital characteristics.
Citation: Rothberg MB, Pekow PS, Liu F, et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3:91-102:91-102.
Is There a Benefit to Corticosteroids When Treating Bacterial Meningitis in Children?
Background: The benefit of adjuvant corticosteroids in the treatment of bacterial meningitis in children in the developed world remains unclear; recent expert guidelines reflect this uncertainty.
Study Design: Retrospective cohort study.
Setting: Twenty-seven tertiary care hospitals in the United States.
Synopsis: Researchers examined 2,780 children with a primary diagnosis of bacterial meningitis discharged from 27 tertiary care centers in the U.S. from 2001-2006. Using a propensity analysis (to control for severity of illness), the study compared those who had received adjunctive corticosteroids with those who had not, with mortality and length of study (LOS) as primary outcomes.
The median age was nine months, 8.9% of children received corticosteroids, and the overall mortality rate was 4.2%. Adjuvent corticosteroids did not reduce mortality or LOS. The outcomes were unchanged in subgroup analyses.
Although limited by its retrospective design and lack of other outcome measures (e.g., hearing loss, neurological deficits), this study provides reasonable evidence that corticosteroid use in bacterial meningitis in children may not save lives or shorten LOS. Pediatric hospitalists may not want to routinely give steroids in this setting pending large randomized-controlled trials.
Bottom line: Adjunctive corticosteroids therapy in children with bacterial meningitis may not save lives or reduce LOS.
Citation: Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055.
Should Unprotected Left Main Disease Be Treated With PCI or CABG?
Background: The current standard of care for the treatment of left main coronary artery disease is coronary-artery bypass grafting (CABG). With the advent of drug-eluting stents, there is growing interest in the use of percutaneous coronary intervention (PCI) to treat left main disease.
Study Design: Prospective observational study.
Setting: Twelve Korean cardiac centers.
Synopsis: From 2000 to 2006, patients with left main disease were treated with PCI or CABG at the discretion of the physician. Nearly 1,100 patients in each cohort were compared and evaluated for death and a composite outcome of death, myocardial infarction, or stroke. Propensity-matching was employed to control for confounders.
In the overall cohort matched by propensity score, there was no significant difference in death or the composite outcome between the PCI and CABG groups after three years. Type of stent (bare metal vs. drug-eluting) did not affect the outcome. Rates of target-vessel revascularization were significantly higher in the group that received stents.
The results are limited by the observational nature and the need for propensity analysis and yet provide an intriguing result. The standard of care for treatment of left main disease remains CABG, but clinicians may be more comfortable treating with stents while we await randomized-controlled trials.
Bottom line: In this observational study, PCI and CABG had similar outcomes in patients with left main disease.
Citation: Seung KB, Park D, Kim Y, Lee S. Stents versus coronary-artery bypass grafting for left main coronary artery disease. N Engl J Med. 2008;358:1781-1792.