User login
Tissue Isn’t the Issue
A 43-year-old man with a history of asplenia, hepatitis C, and nephrolithiasis reported right-flank pain. He described severe, sharp pain that came in waves and radiated to the right groin, associated with nausea and nonbloody emesis. He noted “pink urine” but no dysuria. He had 4prior similar episodes during which he had passed kidney stones, although stone analysis had never been performed. He denied having fevers or chills.
The patient had been involved in a remote motor vehicle accident complicated by splenic laceration, for which he underwent splenectomy. He was appropriately immunized. The patient also suffered from bipolar affective disorder and untreated chronic hepatitis C infection with no evidence of cirrhosis. He smoked one pack of tobacco per day for the last 10 years and reported distant alcohol and methamphetamine use.
Right-flank pain can arise from conditions affecting the lower thorax (effusion, pneumonia, pulmonary embolism), abdomen (hepatobiliary or intestinal disease), retroperitoneum (hemorrhage or infection), musculoskeletal system, peripheral nerves (herpes zoster), or the genitourinary system (pyelonephritis). Pain radiating to the groin, discolored urine (suggesting hematuria), and history of kidney stones increase the likelihood of renal colic from nephrolithiasis.
Less commonly, flank pain and hematuria may present as initial symptoms of renal cell carcinoma, renal infarction, or aortic dissection. The patient’s immunosuppression from asplenia and active injection drug use could predispose him to septic emboli to his kidneys. Prior trauma causing aortic injury could predispose himto subsequent dissection.
The patient appeared well with a heart rate of 100 beats per minute, blood pressure 122/76 mmHg, temperature 36.8°C, respiratory rate 16 breaths per minute, and oxygen saturation 96% on room air. His cardiopulmonary and abdominal examinations were normal, and he had no costovertebral angle tenderness. His skin was warm and dry without rashes. His white blood cell (WBC) count was 26,000/μL; absolute neutrophil count was 22,000/μL. Serum chemistries were normal, including creatinine 0.63 mg/dL, calcium 8.8 mg/dL, and phosphorus 3.1 mg/dL. Lactate was 0.8 mmol/L (reference range: 0-2.0 mmol/L). Urinalysis revealed large ketones, >50 red blood cells (RBC) per high power field (HPF), <5 WBC per HPF, 1+ calcium oxalate crystals and pH 6.0. A bedside ultrasound showed mild right hydronephrosis. Computed tomography (CT) with intravenous contrast of his abdomen and pelvis demonstrated diffuse, mildly prominent subcentimeter mesenteric lymphadenopathy and no kidney stones. He was treated with intravenous fluids and pain control, and was discharged with a presumptive diagnosis of a passed kidney stone.
A passed stone would not explain this degree of leukocytosis. The CT results reduce the likelihood of a renal neoplasm, renal infarction, or pyelonephritis. Mesenteric lymphadenopathy is nonspecific, but it may signal underlying infection or malignancy with spread to lymph nodes, or it may be part of a systemic disorder causing generalized lymphadenopathy. Malignant causes of mesenteric lymphadenopathy (with no apparent primary tumor) include testicular cancer, lymphoma, and primary urogenital neoplasms.
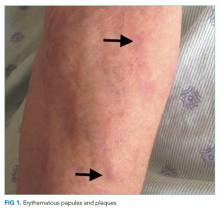
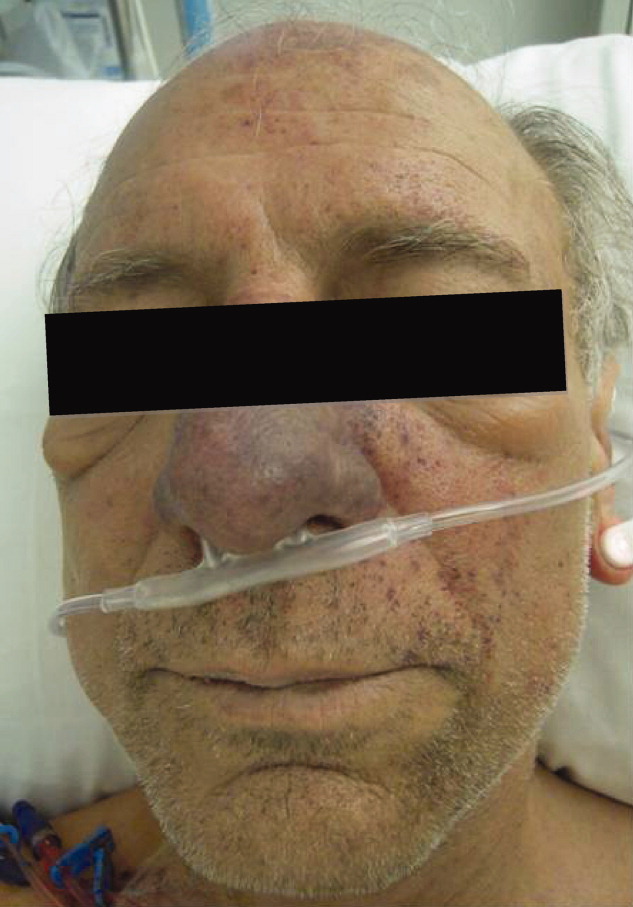
The lower extremity nodules are consistent with erythema nodosum, which may be observed in numerous infectious and noninfectious illnesses. The rapid tempo of this febrile illness mandates early consideration of infection. Splenectomized patients are at risk for overwhelming post-splenectomy infection from encapsulated organisms, although this risk is significantly mitigated with appropriate immunization. The patient is at risk of bacterial endocarditis, which could explain his fevers and polyarthritis, although plaques, pustules, and oral ulcers would be unusual. Disseminated gonococcal infection causes fevers, oral lesions, polyarthritis and pustular skin lesions, but plaques are uncommon. Disseminated mycobacterial and fungal infections may cause oral ulcers, but affected patients tend to be severely ill and have profound immunosuppression. Secondary syphilis may account for many of the findings; however, oral ulcers would be unusual, and the rash tends to be more widespread, with a predilection for the palms and soles. Human immunodeficiency virus (HIV) can cause oral ulcers and is the chief viral etiology to consider.
Noninfectious illnesses to consider include neoplasms and connective tissue diseases. Malignancy would be unlikely to manifest this abruptly or produce a paraneoplastic disorder with these features.
The patient described severe fatigue and drenching night sweats for two months prior to admission. He denied dyspnea or cough. He was born in the southwestern United States and had lived in California for almost a decade. He had been incarcerated for a few years and released three years prior. He had intermittently lived in homeless shelters, but currently lived alone in downtown San Francisco. He had traveled remotely to the Caribbean, and more recently traveled frequently to the Central Valley in California. The patient formerly worked as a pipe-fitter and welder. He denied animal exposure or recent sick contacts. He was sexually active with women, and intermittently used barrier protection.
His years in the southwestern United States may have exposed the patient to blastomycosis or histoplasmosis; both can mimic mycobacterial disease. Blastomycosis demonstrates a slightly stronger predilection for spreading to the bones, genitourinary tract, and central nervous system, whereas histoplasmosis is a more frequent cause of polyarthrtitis and mesenteric adenopathy. The patient’s travel to the Central Valley, California raises the possibility of coccidioidomycosis, which typically starts with pulmonary disease prior to dissemination to bones, skin, and other less common sites. Pipe-fitters are predisposed to asbestos-related illnesses, including lung cancer and mesothelioma, which would not explain this patient’s presentation. Incarceration and high-risk sexual practices increase his risk for tuberculosis, HIV, and syphilis. Widespread skin involvement is more characteristic of syphilis or primary HIV infection than of disseminated fungal or mycobacterial infection.
WBC measured 29,000/uL with a neutrophilic predominance. His peripheral blood smear was unremarkable. A comprehensive metabolic panel was normal. Lactate dehydrogenase (LDH) was 317 U/L (reference range 140-280 U/L). Erythrocyte sedimentation rate (ESR) was 39 mm/hr (reference range < 20 mm/hr) and C-reactive protein (CRP) was 66 mg/L (reference range <6.3 mg/L). Blood, urine, and throat cultures were sent. Chest radiograph showed clear lungs without adenopathy. Ankle and knee radiographs identified small effusions bilaterally without bony abnormalities. CT of his brain showed a small, hypodense lesion in the right lacrimal gland. A lumbar puncture with cerebrospinal fluid (CSF) analysis showed absence of RBCs; WBC, 2/µL; protein, 35 mg/dL; glucose, 62 mg/dL; negative gram stain. CSF bacterial and fungal cultures, venereal disease research laboratory (VDRL), herpes simplex virus polymerase chain reaction (HSV PCR), and cryptococcal antigen were sent for laboratory analysis. The patient was started on vancomycin and aztreonam.
Lesions of the lacrimal gland feature multiple causes, including autoimmune diseases (Sjögren’s, Behçet’s disease), granulomatous diseases (sarcoidosis, granulomatosis with polyangiitis), neoplasms (salivary gland tumors, lymphoma), and infections. Initiating broad-spectrum antibiotics is reasonable while awaiting additional information from blood and urine cultures, serologies for HIV and syphilis, and purified protein derivative or interferon-gamma release assay (IGRA).
If these tests fail to reveal a diagnosis, the search for atypical infections and noninfectious possibilities should expand.
The patient continued to have intermittent fevers, sweats, and malaise over the next 3 days. All bacterial and fungal cultures remained negative, and antibiotics were discontinued. Rheumatoid factor, anticyclic citrullinated peptide, antinuclear antibody, and cryoglobulins were negative. Serum C3, C4, and angiotensin-converting enzyme (ACE) levels were normal. A rapid plasma reagin (RPR), HIV antibody, IGRA, and serum antibodies for Coccidioides, histoplasmosis, and West Nile virus were negative. Urine nucleic acid amplification testing for gonorrhea and chlamydia was negative. CSF VDRL, HSV PCR and cryptococcal antigen were negative. HSV culture from an oral ulcer showed no growth. The patient had a reactive hepatitis C antibody with a viral load of 3 million virus equivalents/mL.
The additional test results lower
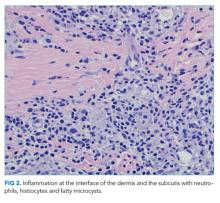
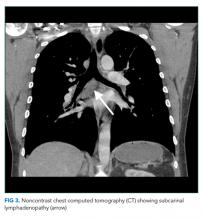
The most likely diagnosis is Löfgren’s syndrome, a variant of sarcoidosis characterized by erythema nodosum, bilateral hilar lymphadenopathy, and polyarthralgias or polyarthritis. Löfgren’s syndrome may include fevers, uveitis, widespread skin lesions and other systemic manifestations. Sarcoidosis could explain the lacrimal gland lesion, and could manifest with recurrent kidney stones. Oral lesions may occur in sarcoidosis. A normal serum ACE level may be observed in up to half of patients. The lack of visualized granulomas on the submental node FNA may reflect sampling error, lower likelihood of visualizing granulomas on FNA (compared with excisional biopsy), or biopsy location (hilar nodes are more likely to demonstrate sarcoid granulomas).
Although Löfgren’s syndrome is often self-limited, treatment can ameliorate symptoms. Nonsteroidal anti-inflammatory medication can be tried first, with prednisone reserved for refractory cases.
The constellation of bilateral hilar adenopathy, arthritis, and erythema nodosum was consistent with Löfgren’s syndrome, further supported by granulomatous infiltrates on biopsy. The patient’s symptoms resolved with naproxen. He was scheduled for follow-up in dermatology and rheumatology clinics and was referred to hepatology for management of hepatitis C.
COMMENTARY
Sarcoidosis is a multisystem granulomatous disease of unclear etiology. The disease derives its name from Boeck’s 1899 report describing benign cutaneous lesions that resembled sarcomas.1 Sarcoidosis most commonly manifests as bilateral hilar adenopathy and pulmonary infiltrates, but may impact any tissue or organ, including the eyes, nonhilar lymph nodes, liver, spleen, joints, mucous membranes, and skin. Nephrolithiasis may result from hypercalcemia and/or hypercalciuria (related to granulomatous production of 1,25 vitamin D) and can be the presenting feature of sarcoidosis.2 Less common presentations include neurologic sarcoidosis (which can present with seizures, aseptic meningitis, encephalopathy, neuroendocrine dysfunction, myelopathy and peripheral neuropathies), cardiac sarcoidosis (which may present with arrhythmias, valvular dysfunction, heart failure, ischemia, or pericardial disease), and Heerfordt syndrome (the constellation of parotid gland enlargement, facial palsy, anterior uveitis, and fever). Sarcoidosis may mimic other diseases, including malignancy, idiopathic pulmonary fibrosis, and infiltrative tuberculosis.3 Sarcoidosis-like reactions have occurred in response to malignancy and medications.4
The patient’s rash demonstrated a predilection for areas of prior scarring, which has a limited differential diagnosis. Keloids and hypertrophic scars occur at sites of former surgical wounds, lacerations, or areas of inflammation. Pruritic urticarial papules and plaques of pregnancy (PUPPP) is a benign inflammatory condition where papules cluster in areas of prior striae. Cutaneous lesions of Behçet’s syndrome display pathergy, where pustular response is observed at sites of injury. Granulomatous infiltration in sarcoidosis may demonstrate a predilection for scars and tattoos (ie, scar or tattoo sarcoidosis).5 Sarcoidosis can have other cutaneous manifestations, including psoriaform, ulcerative, or erythrodermic lesions; subcutaneous nodules; scarring or nonscarring alopecia; and lupus pernio – violaceous, nodular and plaque-like lesions on the nose, earlobes, cheeks, and digits.5
Löfgren’s syndrome is a distinct variant of sarcoidosis.In 1952, Dr. Löfgren described a case series of patients with bilateral hilar lymphadenopathy and coexisting erythema nodosum and polyarthralgia.6 The epidemiology favors young women.7 Patients with Löfgren’s syndrome present acutely (as in this case), which differs from the typical subacute course observed with sarcoidosis. In addition to the classic presentation described above, patients with Löfgren’s syndrome may demonstrate additional manifestations of sarcoidosis, including fevers, peripheral adenopathy, arthritis, and granulomatous skin lesions. Painful symptoms may require short-term anti-inflammatory treatments. Most patients do not require systemic immunosuppression. Symptoms usually decrease over several months, and the majority of patients experience complete remission within years. Rare recurrences have been described up to several years.8
In confirming the diagnosis of sarcoidosis, current guidelines recommend exclusion of other diseases that present similarly, a work-up that generally includes compatible laboratory tests and imaging, and histologic demonstration of noncaseating granulomas.9 However, Löfgren’s syndrome is a notable exception. The constellation of fever, bilateral hilar adenopathy, polyarthralgia, and erythema nodosum suffices to diagnose Löfgren’s syndrome as long as the disease remits rapidly and spontaneously.9 Thus, in this case, although granulomatous infiltrates were confirmed on biopsy, the diagnosis of Löfgren’s syndrome could have been based on clinical and radiologic features alone.
KEY LEARNING POINTS
- Sarcoidosis is a multisystem granulomatous disease that most commonly presents with bilateral hilar adenopathy and pulmonary infiltrates but can also present atypically, including with nephrolithiasis from hypercalcemia, neurologic syndromes, and cardiac involvement.
- Löfgren’s syndrome, a variant of sarcoidosis, is characterized by relatively acute onset of fevers, erythema nodosum, bilateral hilar adenopathy, and polyarthralgia or polyarthritis. Most patients recover and manifest complete remission.
- A limited differential exists for rashes with a predilection for areas of tattoos and prior scarring, including keloids, PUPPP, Behçet’s disease, and granulomatous infiltration.
Disclosure
There are no conflicts of interest or financial disclosures to report.
1. Multiple Benign Sarcoids of the Skin. JAMA. 1899;XXXIII(26):1620-1621.
2. Rizzato G, Fraioli P, Montemurro L. Nephrolithiasis as a presenting feature of chronic sarcoidosis. Thorax. 1995;50(5):555-559. PubMed
3. Romanov V. Atypical variants of clinical course of sarcoidosis. Eur Respir J. 2014;44(58):3782. PubMed
4. Arish N, Kuint R, Sapir E, et al. Characteristics of Sarcoidosis in Patients with Previous Malignancy: Causality or Coincidence? Respiration. 2017;93(4):247-252. PubMed
5. Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25(3):295-302. PubMed
6. Löfgren S. The Bilateral Hilar Lymphoma Syndrome. Acta Med Scand. 1952;142(4):265-273. PubMed
7. Mañá J, Gómez-Vaquero C, Montero A et al. Löfgren’s syndrome revisited: a study of 186 patients. Am J Med. 1999;107(3):240-245. PubMed
8. Gran J, Bohmer E. Acute Sarcoid Arthritis: A Favourable Outcome? Scand J Rheumatol. 1996;25(2):70-73. PubMed
9. American Thoracic Society. Statement on Sarcoidosis. Am J Respir Crit Care Med. 1999;160:736-755.Otate voluptiatia qui aut iur, utendi quiae incipis m PubMed
A 43-year-old man with a history of asplenia, hepatitis C, and nephrolithiasis reported right-flank pain. He described severe, sharp pain that came in waves and radiated to the right groin, associated with nausea and nonbloody emesis. He noted “pink urine” but no dysuria. He had 4prior similar episodes during which he had passed kidney stones, although stone analysis had never been performed. He denied having fevers or chills.
The patient had been involved in a remote motor vehicle accident complicated by splenic laceration, for which he underwent splenectomy. He was appropriately immunized. The patient also suffered from bipolar affective disorder and untreated chronic hepatitis C infection with no evidence of cirrhosis. He smoked one pack of tobacco per day for the last 10 years and reported distant alcohol and methamphetamine use.
Right-flank pain can arise from conditions affecting the lower thorax (effusion, pneumonia, pulmonary embolism), abdomen (hepatobiliary or intestinal disease), retroperitoneum (hemorrhage or infection), musculoskeletal system, peripheral nerves (herpes zoster), or the genitourinary system (pyelonephritis). Pain radiating to the groin, discolored urine (suggesting hematuria), and history of kidney stones increase the likelihood of renal colic from nephrolithiasis.
Less commonly, flank pain and hematuria may present as initial symptoms of renal cell carcinoma, renal infarction, or aortic dissection. The patient’s immunosuppression from asplenia and active injection drug use could predispose him to septic emboli to his kidneys. Prior trauma causing aortic injury could predispose himto subsequent dissection.
The patient appeared well with a heart rate of 100 beats per minute, blood pressure 122/76 mmHg, temperature 36.8°C, respiratory rate 16 breaths per minute, and oxygen saturation 96% on room air. His cardiopulmonary and abdominal examinations were normal, and he had no costovertebral angle tenderness. His skin was warm and dry without rashes. His white blood cell (WBC) count was 26,000/μL; absolute neutrophil count was 22,000/μL. Serum chemistries were normal, including creatinine 0.63 mg/dL, calcium 8.8 mg/dL, and phosphorus 3.1 mg/dL. Lactate was 0.8 mmol/L (reference range: 0-2.0 mmol/L). Urinalysis revealed large ketones, >50 red blood cells (RBC) per high power field (HPF), <5 WBC per HPF, 1+ calcium oxalate crystals and pH 6.0. A bedside ultrasound showed mild right hydronephrosis. Computed tomography (CT) with intravenous contrast of his abdomen and pelvis demonstrated diffuse, mildly prominent subcentimeter mesenteric lymphadenopathy and no kidney stones. He was treated with intravenous fluids and pain control, and was discharged with a presumptive diagnosis of a passed kidney stone.
A passed stone would not explain this degree of leukocytosis. The CT results reduce the likelihood of a renal neoplasm, renal infarction, or pyelonephritis. Mesenteric lymphadenopathy is nonspecific, but it may signal underlying infection or malignancy with spread to lymph nodes, or it may be part of a systemic disorder causing generalized lymphadenopathy. Malignant causes of mesenteric lymphadenopathy (with no apparent primary tumor) include testicular cancer, lymphoma, and primary urogenital neoplasms.
The lower extremity nodules are consistent with erythema nodosum, which may be observed in numerous infectious and noninfectious illnesses. The rapid tempo of this febrile illness mandates early consideration of infection. Splenectomized patients are at risk for overwhelming post-splenectomy infection from encapsulated organisms, although this risk is significantly mitigated with appropriate immunization. The patient is at risk of bacterial endocarditis, which could explain his fevers and polyarthritis, although plaques, pustules, and oral ulcers would be unusual. Disseminated gonococcal infection causes fevers, oral lesions, polyarthritis and pustular skin lesions, but plaques are uncommon. Disseminated mycobacterial and fungal infections may cause oral ulcers, but affected patients tend to be severely ill and have profound immunosuppression. Secondary syphilis may account for many of the findings; however, oral ulcers would be unusual, and the rash tends to be more widespread, with a predilection for the palms and soles. Human immunodeficiency virus (HIV) can cause oral ulcers and is the chief viral etiology to consider.
Noninfectious illnesses to consider include neoplasms and connective tissue diseases. Malignancy would be unlikely to manifest this abruptly or produce a paraneoplastic disorder with these features.
The patient described severe fatigue and drenching night sweats for two months prior to admission. He denied dyspnea or cough. He was born in the southwestern United States and had lived in California for almost a decade. He had been incarcerated for a few years and released three years prior. He had intermittently lived in homeless shelters, but currently lived alone in downtown San Francisco. He had traveled remotely to the Caribbean, and more recently traveled frequently to the Central Valley in California. The patient formerly worked as a pipe-fitter and welder. He denied animal exposure or recent sick contacts. He was sexually active with women, and intermittently used barrier protection.
His years in the southwestern United States may have exposed the patient to blastomycosis or histoplasmosis; both can mimic mycobacterial disease. Blastomycosis demonstrates a slightly stronger predilection for spreading to the bones, genitourinary tract, and central nervous system, whereas histoplasmosis is a more frequent cause of polyarthrtitis and mesenteric adenopathy. The patient’s travel to the Central Valley, California raises the possibility of coccidioidomycosis, which typically starts with pulmonary disease prior to dissemination to bones, skin, and other less common sites. Pipe-fitters are predisposed to asbestos-related illnesses, including lung cancer and mesothelioma, which would not explain this patient’s presentation. Incarceration and high-risk sexual practices increase his risk for tuberculosis, HIV, and syphilis. Widespread skin involvement is more characteristic of syphilis or primary HIV infection than of disseminated fungal or mycobacterial infection.
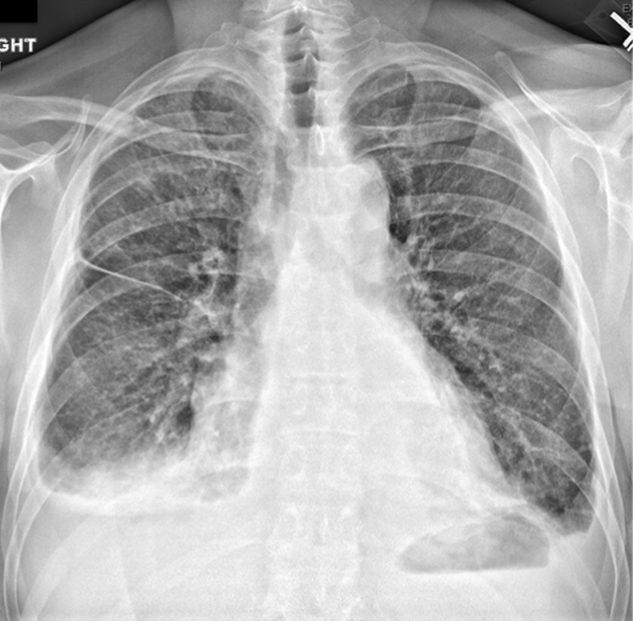
WBC measured 29,000/uL with a neutrophilic predominance. His peripheral blood smear was unremarkable. A comprehensive metabolic panel was normal. Lactate dehydrogenase (LDH) was 317 U/L (reference range 140-280 U/L). Erythrocyte sedimentation rate (ESR) was 39 mm/hr (reference range < 20 mm/hr) and C-reactive protein (CRP) was 66 mg/L (reference range <6.3 mg/L). Blood, urine, and throat cultures were sent. Chest radiograph showed clear lungs without adenopathy. Ankle and knee radiographs identified small effusions bilaterally without bony abnormalities. CT of his brain showed a small, hypodense lesion in the right lacrimal gland. A lumbar puncture with cerebrospinal fluid (CSF) analysis showed absence of RBCs; WBC, 2/µL; protein, 35 mg/dL; glucose, 62 mg/dL; negative gram stain. CSF bacterial and fungal cultures, venereal disease research laboratory (VDRL), herpes simplex virus polymerase chain reaction (HSV PCR), and cryptococcal antigen were sent for laboratory analysis. The patient was started on vancomycin and aztreonam.
Lesions of the lacrimal gland feature multiple causes, including autoimmune diseases (Sjögren’s, Behçet’s disease), granulomatous diseases (sarcoidosis, granulomatosis with polyangiitis), neoplasms (salivary gland tumors, lymphoma), and infections. Initiating broad-spectrum antibiotics is reasonable while awaiting additional information from blood and urine cultures, serologies for HIV and syphilis, and purified protein derivative or interferon-gamma release assay (IGRA).
If these tests fail to reveal a diagnosis, the search for atypical infections and noninfectious possibilities should expand.
The patient continued to have intermittent fevers, sweats, and malaise over the next 3 days. All bacterial and fungal cultures remained negative, and antibiotics were discontinued. Rheumatoid factor, anticyclic citrullinated peptide, antinuclear antibody, and cryoglobulins were negative. Serum C3, C4, and angiotensin-converting enzyme (ACE) levels were normal. A rapid plasma reagin (RPR), HIV antibody, IGRA, and serum antibodies for Coccidioides, histoplasmosis, and West Nile virus were negative. Urine nucleic acid amplification testing for gonorrhea and chlamydia was negative. CSF VDRL, HSV PCR and cryptococcal antigen were negative. HSV culture from an oral ulcer showed no growth. The patient had a reactive hepatitis C antibody with a viral load of 3 million virus equivalents/mL.
The additional test results lower
The most likely diagnosis is Löfgren’s syndrome, a variant of sarcoidosis characterized by erythema nodosum, bilateral hilar lymphadenopathy, and polyarthralgias or polyarthritis. Löfgren’s syndrome may include fevers, uveitis, widespread skin lesions and other systemic manifestations. Sarcoidosis could explain the lacrimal gland lesion, and could manifest with recurrent kidney stones. Oral lesions may occur in sarcoidosis. A normal serum ACE level may be observed in up to half of patients. The lack of visualized granulomas on the submental node FNA may reflect sampling error, lower likelihood of visualizing granulomas on FNA (compared with excisional biopsy), or biopsy location (hilar nodes are more likely to demonstrate sarcoid granulomas).
Although Löfgren’s syndrome is often self-limited, treatment can ameliorate symptoms. Nonsteroidal anti-inflammatory medication can be tried first, with prednisone reserved for refractory cases.
The constellation of bilateral hilar adenopathy, arthritis, and erythema nodosum was consistent with Löfgren’s syndrome, further supported by granulomatous infiltrates on biopsy. The patient’s symptoms resolved with naproxen. He was scheduled for follow-up in dermatology and rheumatology clinics and was referred to hepatology for management of hepatitis C.
COMMENTARY
Sarcoidosis is a multisystem granulomatous disease of unclear etiology. The disease derives its name from Boeck’s 1899 report describing benign cutaneous lesions that resembled sarcomas.1 Sarcoidosis most commonly manifests as bilateral hilar adenopathy and pulmonary infiltrates, but may impact any tissue or organ, including the eyes, nonhilar lymph nodes, liver, spleen, joints, mucous membranes, and skin. Nephrolithiasis may result from hypercalcemia and/or hypercalciuria (related to granulomatous production of 1,25 vitamin D) and can be the presenting feature of sarcoidosis.2 Less common presentations include neurologic sarcoidosis (which can present with seizures, aseptic meningitis, encephalopathy, neuroendocrine dysfunction, myelopathy and peripheral neuropathies), cardiac sarcoidosis (which may present with arrhythmias, valvular dysfunction, heart failure, ischemia, or pericardial disease), and Heerfordt syndrome (the constellation of parotid gland enlargement, facial palsy, anterior uveitis, and fever). Sarcoidosis may mimic other diseases, including malignancy, idiopathic pulmonary fibrosis, and infiltrative tuberculosis.3 Sarcoidosis-like reactions have occurred in response to malignancy and medications.4
The patient’s rash demonstrated a predilection for areas of prior scarring, which has a limited differential diagnosis. Keloids and hypertrophic scars occur at sites of former surgical wounds, lacerations, or areas of inflammation. Pruritic urticarial papules and plaques of pregnancy (PUPPP) is a benign inflammatory condition where papules cluster in areas of prior striae. Cutaneous lesions of Behçet’s syndrome display pathergy, where pustular response is observed at sites of injury. Granulomatous infiltration in sarcoidosis may demonstrate a predilection for scars and tattoos (ie, scar or tattoo sarcoidosis).5 Sarcoidosis can have other cutaneous manifestations, including psoriaform, ulcerative, or erythrodermic lesions; subcutaneous nodules; scarring or nonscarring alopecia; and lupus pernio – violaceous, nodular and plaque-like lesions on the nose, earlobes, cheeks, and digits.5
Löfgren’s syndrome is a distinct variant of sarcoidosis.In 1952, Dr. Löfgren described a case series of patients with bilateral hilar lymphadenopathy and coexisting erythema nodosum and polyarthralgia.6 The epidemiology favors young women.7 Patients with Löfgren’s syndrome present acutely (as in this case), which differs from the typical subacute course observed with sarcoidosis. In addition to the classic presentation described above, patients with Löfgren’s syndrome may demonstrate additional manifestations of sarcoidosis, including fevers, peripheral adenopathy, arthritis, and granulomatous skin lesions. Painful symptoms may require short-term anti-inflammatory treatments. Most patients do not require systemic immunosuppression. Symptoms usually decrease over several months, and the majority of patients experience complete remission within years. Rare recurrences have been described up to several years.8
In confirming the diagnosis of sarcoidosis, current guidelines recommend exclusion of other diseases that present similarly, a work-up that generally includes compatible laboratory tests and imaging, and histologic demonstration of noncaseating granulomas.9 However, Löfgren’s syndrome is a notable exception. The constellation of fever, bilateral hilar adenopathy, polyarthralgia, and erythema nodosum suffices to diagnose Löfgren’s syndrome as long as the disease remits rapidly and spontaneously.9 Thus, in this case, although granulomatous infiltrates were confirmed on biopsy, the diagnosis of Löfgren’s syndrome could have been based on clinical and radiologic features alone.
KEY LEARNING POINTS
- Sarcoidosis is a multisystem granulomatous disease that most commonly presents with bilateral hilar adenopathy and pulmonary infiltrates but can also present atypically, including with nephrolithiasis from hypercalcemia, neurologic syndromes, and cardiac involvement.
- Löfgren’s syndrome, a variant of sarcoidosis, is characterized by relatively acute onset of fevers, erythema nodosum, bilateral hilar adenopathy, and polyarthralgia or polyarthritis. Most patients recover and manifest complete remission.
- A limited differential exists for rashes with a predilection for areas of tattoos and prior scarring, including keloids, PUPPP, Behçet’s disease, and granulomatous infiltration.
Disclosure
There are no conflicts of interest or financial disclosures to report.
A 43-year-old man with a history of asplenia, hepatitis C, and nephrolithiasis reported right-flank pain. He described severe, sharp pain that came in waves and radiated to the right groin, associated with nausea and nonbloody emesis. He noted “pink urine” but no dysuria. He had 4prior similar episodes during which he had passed kidney stones, although stone analysis had never been performed. He denied having fevers or chills.
The patient had been involved in a remote motor vehicle accident complicated by splenic laceration, for which he underwent splenectomy. He was appropriately immunized. The patient also suffered from bipolar affective disorder and untreated chronic hepatitis C infection with no evidence of cirrhosis. He smoked one pack of tobacco per day for the last 10 years and reported distant alcohol and methamphetamine use.
Right-flank pain can arise from conditions affecting the lower thorax (effusion, pneumonia, pulmonary embolism), abdomen (hepatobiliary or intestinal disease), retroperitoneum (hemorrhage or infection), musculoskeletal system, peripheral nerves (herpes zoster), or the genitourinary system (pyelonephritis). Pain radiating to the groin, discolored urine (suggesting hematuria), and history of kidney stones increase the likelihood of renal colic from nephrolithiasis.
Less commonly, flank pain and hematuria may present as initial symptoms of renal cell carcinoma, renal infarction, or aortic dissection. The patient’s immunosuppression from asplenia and active injection drug use could predispose him to septic emboli to his kidneys. Prior trauma causing aortic injury could predispose himto subsequent dissection.
The patient appeared well with a heart rate of 100 beats per minute, blood pressure 122/76 mmHg, temperature 36.8°C, respiratory rate 16 breaths per minute, and oxygen saturation 96% on room air. His cardiopulmonary and abdominal examinations were normal, and he had no costovertebral angle tenderness. His skin was warm and dry without rashes. His white blood cell (WBC) count was 26,000/μL; absolute neutrophil count was 22,000/μL. Serum chemistries were normal, including creatinine 0.63 mg/dL, calcium 8.8 mg/dL, and phosphorus 3.1 mg/dL. Lactate was 0.8 mmol/L (reference range: 0-2.0 mmol/L). Urinalysis revealed large ketones, >50 red blood cells (RBC) per high power field (HPF), <5 WBC per HPF, 1+ calcium oxalate crystals and pH 6.0. A bedside ultrasound showed mild right hydronephrosis. Computed tomography (CT) with intravenous contrast of his abdomen and pelvis demonstrated diffuse, mildly prominent subcentimeter mesenteric lymphadenopathy and no kidney stones. He was treated with intravenous fluids and pain control, and was discharged with a presumptive diagnosis of a passed kidney stone.
A passed stone would not explain this degree of leukocytosis. The CT results reduce the likelihood of a renal neoplasm, renal infarction, or pyelonephritis. Mesenteric lymphadenopathy is nonspecific, but it may signal underlying infection or malignancy with spread to lymph nodes, or it may be part of a systemic disorder causing generalized lymphadenopathy. Malignant causes of mesenteric lymphadenopathy (with no apparent primary tumor) include testicular cancer, lymphoma, and primary urogenital neoplasms.
The lower extremity nodules are consistent with erythema nodosum, which may be observed in numerous infectious and noninfectious illnesses. The rapid tempo of this febrile illness mandates early consideration of infection. Splenectomized patients are at risk for overwhelming post-splenectomy infection from encapsulated organisms, although this risk is significantly mitigated with appropriate immunization. The patient is at risk of bacterial endocarditis, which could explain his fevers and polyarthritis, although plaques, pustules, and oral ulcers would be unusual. Disseminated gonococcal infection causes fevers, oral lesions, polyarthritis and pustular skin lesions, but plaques are uncommon. Disseminated mycobacterial and fungal infections may cause oral ulcers, but affected patients tend to be severely ill and have profound immunosuppression. Secondary syphilis may account for many of the findings; however, oral ulcers would be unusual, and the rash tends to be more widespread, with a predilection for the palms and soles. Human immunodeficiency virus (HIV) can cause oral ulcers and is the chief viral etiology to consider.
Noninfectious illnesses to consider include neoplasms and connective tissue diseases. Malignancy would be unlikely to manifest this abruptly or produce a paraneoplastic disorder with these features.
The patient described severe fatigue and drenching night sweats for two months prior to admission. He denied dyspnea or cough. He was born in the southwestern United States and had lived in California for almost a decade. He had been incarcerated for a few years and released three years prior. He had intermittently lived in homeless shelters, but currently lived alone in downtown San Francisco. He had traveled remotely to the Caribbean, and more recently traveled frequently to the Central Valley in California. The patient formerly worked as a pipe-fitter and welder. He denied animal exposure or recent sick contacts. He was sexually active with women, and intermittently used barrier protection.
His years in the southwestern United States may have exposed the patient to blastomycosis or histoplasmosis; both can mimic mycobacterial disease. Blastomycosis demonstrates a slightly stronger predilection for spreading to the bones, genitourinary tract, and central nervous system, whereas histoplasmosis is a more frequent cause of polyarthrtitis and mesenteric adenopathy. The patient’s travel to the Central Valley, California raises the possibility of coccidioidomycosis, which typically starts with pulmonary disease prior to dissemination to bones, skin, and other less common sites. Pipe-fitters are predisposed to asbestos-related illnesses, including lung cancer and mesothelioma, which would not explain this patient’s presentation. Incarceration and high-risk sexual practices increase his risk for tuberculosis, HIV, and syphilis. Widespread skin involvement is more characteristic of syphilis or primary HIV infection than of disseminated fungal or mycobacterial infection.
WBC measured 29,000/uL with a neutrophilic predominance. His peripheral blood smear was unremarkable. A comprehensive metabolic panel was normal. Lactate dehydrogenase (LDH) was 317 U/L (reference range 140-280 U/L). Erythrocyte sedimentation rate (ESR) was 39 mm/hr (reference range < 20 mm/hr) and C-reactive protein (CRP) was 66 mg/L (reference range <6.3 mg/L). Blood, urine, and throat cultures were sent. Chest radiograph showed clear lungs without adenopathy. Ankle and knee radiographs identified small effusions bilaterally without bony abnormalities. CT of his brain showed a small, hypodense lesion in the right lacrimal gland. A lumbar puncture with cerebrospinal fluid (CSF) analysis showed absence of RBCs; WBC, 2/µL; protein, 35 mg/dL; glucose, 62 mg/dL; negative gram stain. CSF bacterial and fungal cultures, venereal disease research laboratory (VDRL), herpes simplex virus polymerase chain reaction (HSV PCR), and cryptococcal antigen were sent for laboratory analysis. The patient was started on vancomycin and aztreonam.
Lesions of the lacrimal gland feature multiple causes, including autoimmune diseases (Sjögren’s, Behçet’s disease), granulomatous diseases (sarcoidosis, granulomatosis with polyangiitis), neoplasms (salivary gland tumors, lymphoma), and infections. Initiating broad-spectrum antibiotics is reasonable while awaiting additional information from blood and urine cultures, serologies for HIV and syphilis, and purified protein derivative or interferon-gamma release assay (IGRA).
If these tests fail to reveal a diagnosis, the search for atypical infections and noninfectious possibilities should expand.
The patient continued to have intermittent fevers, sweats, and malaise over the next 3 days. All bacterial and fungal cultures remained negative, and antibiotics were discontinued. Rheumatoid factor, anticyclic citrullinated peptide, antinuclear antibody, and cryoglobulins were negative. Serum C3, C4, and angiotensin-converting enzyme (ACE) levels were normal. A rapid plasma reagin (RPR), HIV antibody, IGRA, and serum antibodies for Coccidioides, histoplasmosis, and West Nile virus were negative. Urine nucleic acid amplification testing for gonorrhea and chlamydia was negative. CSF VDRL, HSV PCR and cryptococcal antigen were negative. HSV culture from an oral ulcer showed no growth. The patient had a reactive hepatitis C antibody with a viral load of 3 million virus equivalents/mL.
The additional test results lower
The most likely diagnosis is Löfgren’s syndrome, a variant of sarcoidosis characterized by erythema nodosum, bilateral hilar lymphadenopathy, and polyarthralgias or polyarthritis. Löfgren’s syndrome may include fevers, uveitis, widespread skin lesions and other systemic manifestations. Sarcoidosis could explain the lacrimal gland lesion, and could manifest with recurrent kidney stones. Oral lesions may occur in sarcoidosis. A normal serum ACE level may be observed in up to half of patients. The lack of visualized granulomas on the submental node FNA may reflect sampling error, lower likelihood of visualizing granulomas on FNA (compared with excisional biopsy), or biopsy location (hilar nodes are more likely to demonstrate sarcoid granulomas).
Although Löfgren’s syndrome is often self-limited, treatment can ameliorate symptoms. Nonsteroidal anti-inflammatory medication can be tried first, with prednisone reserved for refractory cases.
The constellation of bilateral hilar adenopathy, arthritis, and erythema nodosum was consistent with Löfgren’s syndrome, further supported by granulomatous infiltrates on biopsy. The patient’s symptoms resolved with naproxen. He was scheduled for follow-up in dermatology and rheumatology clinics and was referred to hepatology for management of hepatitis C.
COMMENTARY
Sarcoidosis is a multisystem granulomatous disease of unclear etiology. The disease derives its name from Boeck’s 1899 report describing benign cutaneous lesions that resembled sarcomas.1 Sarcoidosis most commonly manifests as bilateral hilar adenopathy and pulmonary infiltrates, but may impact any tissue or organ, including the eyes, nonhilar lymph nodes, liver, spleen, joints, mucous membranes, and skin. Nephrolithiasis may result from hypercalcemia and/or hypercalciuria (related to granulomatous production of 1,25 vitamin D) and can be the presenting feature of sarcoidosis.2 Less common presentations include neurologic sarcoidosis (which can present with seizures, aseptic meningitis, encephalopathy, neuroendocrine dysfunction, myelopathy and peripheral neuropathies), cardiac sarcoidosis (which may present with arrhythmias, valvular dysfunction, heart failure, ischemia, or pericardial disease), and Heerfordt syndrome (the constellation of parotid gland enlargement, facial palsy, anterior uveitis, and fever). Sarcoidosis may mimic other diseases, including malignancy, idiopathic pulmonary fibrosis, and infiltrative tuberculosis.3 Sarcoidosis-like reactions have occurred in response to malignancy and medications.4
The patient’s rash demonstrated a predilection for areas of prior scarring, which has a limited differential diagnosis. Keloids and hypertrophic scars occur at sites of former surgical wounds, lacerations, or areas of inflammation. Pruritic urticarial papules and plaques of pregnancy (PUPPP) is a benign inflammatory condition where papules cluster in areas of prior striae. Cutaneous lesions of Behçet’s syndrome display pathergy, where pustular response is observed at sites of injury. Granulomatous infiltration in sarcoidosis may demonstrate a predilection for scars and tattoos (ie, scar or tattoo sarcoidosis).5 Sarcoidosis can have other cutaneous manifestations, including psoriaform, ulcerative, or erythrodermic lesions; subcutaneous nodules; scarring or nonscarring alopecia; and lupus pernio – violaceous, nodular and plaque-like lesions on the nose, earlobes, cheeks, and digits.5
Löfgren’s syndrome is a distinct variant of sarcoidosis.In 1952, Dr. Löfgren described a case series of patients with bilateral hilar lymphadenopathy and coexisting erythema nodosum and polyarthralgia.6 The epidemiology favors young women.7 Patients with Löfgren’s syndrome present acutely (as in this case), which differs from the typical subacute course observed with sarcoidosis. In addition to the classic presentation described above, patients with Löfgren’s syndrome may demonstrate additional manifestations of sarcoidosis, including fevers, peripheral adenopathy, arthritis, and granulomatous skin lesions. Painful symptoms may require short-term anti-inflammatory treatments. Most patients do not require systemic immunosuppression. Symptoms usually decrease over several months, and the majority of patients experience complete remission within years. Rare recurrences have been described up to several years.8
In confirming the diagnosis of sarcoidosis, current guidelines recommend exclusion of other diseases that present similarly, a work-up that generally includes compatible laboratory tests and imaging, and histologic demonstration of noncaseating granulomas.9 However, Löfgren’s syndrome is a notable exception. The constellation of fever, bilateral hilar adenopathy, polyarthralgia, and erythema nodosum suffices to diagnose Löfgren’s syndrome as long as the disease remits rapidly and spontaneously.9 Thus, in this case, although granulomatous infiltrates were confirmed on biopsy, the diagnosis of Löfgren’s syndrome could have been based on clinical and radiologic features alone.
KEY LEARNING POINTS
- Sarcoidosis is a multisystem granulomatous disease that most commonly presents with bilateral hilar adenopathy and pulmonary infiltrates but can also present atypically, including with nephrolithiasis from hypercalcemia, neurologic syndromes, and cardiac involvement.
- Löfgren’s syndrome, a variant of sarcoidosis, is characterized by relatively acute onset of fevers, erythema nodosum, bilateral hilar adenopathy, and polyarthralgia or polyarthritis. Most patients recover and manifest complete remission.
- A limited differential exists for rashes with a predilection for areas of tattoos and prior scarring, including keloids, PUPPP, Behçet’s disease, and granulomatous infiltration.
Disclosure
There are no conflicts of interest or financial disclosures to report.
1. Multiple Benign Sarcoids of the Skin. JAMA. 1899;XXXIII(26):1620-1621.
2. Rizzato G, Fraioli P, Montemurro L. Nephrolithiasis as a presenting feature of chronic sarcoidosis. Thorax. 1995;50(5):555-559. PubMed
3. Romanov V. Atypical variants of clinical course of sarcoidosis. Eur Respir J. 2014;44(58):3782. PubMed
4. Arish N, Kuint R, Sapir E, et al. Characteristics of Sarcoidosis in Patients with Previous Malignancy: Causality or Coincidence? Respiration. 2017;93(4):247-252. PubMed
5. Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25(3):295-302. PubMed
6. Löfgren S. The Bilateral Hilar Lymphoma Syndrome. Acta Med Scand. 1952;142(4):265-273. PubMed
7. Mañá J, Gómez-Vaquero C, Montero A et al. Löfgren’s syndrome revisited: a study of 186 patients. Am J Med. 1999;107(3):240-245. PubMed
8. Gran J, Bohmer E. Acute Sarcoid Arthritis: A Favourable Outcome? Scand J Rheumatol. 1996;25(2):70-73. PubMed
9. American Thoracic Society. Statement on Sarcoidosis. Am J Respir Crit Care Med. 1999;160:736-755.Otate voluptiatia qui aut iur, utendi quiae incipis m PubMed
1. Multiple Benign Sarcoids of the Skin. JAMA. 1899;XXXIII(26):1620-1621.
2. Rizzato G, Fraioli P, Montemurro L. Nephrolithiasis as a presenting feature of chronic sarcoidosis. Thorax. 1995;50(5):555-559. PubMed
3. Romanov V. Atypical variants of clinical course of sarcoidosis. Eur Respir J. 2014;44(58):3782. PubMed
4. Arish N, Kuint R, Sapir E, et al. Characteristics of Sarcoidosis in Patients with Previous Malignancy: Causality or Coincidence? Respiration. 2017;93(4):247-252. PubMed
5. Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25(3):295-302. PubMed
6. Löfgren S. The Bilateral Hilar Lymphoma Syndrome. Acta Med Scand. 1952;142(4):265-273. PubMed
7. Mañá J, Gómez-Vaquero C, Montero A et al. Löfgren’s syndrome revisited: a study of 186 patients. Am J Med. 1999;107(3):240-245. PubMed
8. Gran J, Bohmer E. Acute Sarcoid Arthritis: A Favourable Outcome? Scand J Rheumatol. 1996;25(2):70-73. PubMed
9. American Thoracic Society. Statement on Sarcoidosis. Am J Respir Crit Care Med. 1999;160:736-755.Otate voluptiatia qui aut iur, utendi quiae incipis m PubMed
© 2018 Society of Hospital Medicine
A Prescription for Note Bloat: An Effective Progress Note Template
The widespread adoption of electronic health records (EHRs) has led to significant progress in the modernization of healthcare delivery. Ease of access has improved clinical efficiency, and digital data have allowed for point-of-care decision support tools ranging from predicting the 30-day risk of readmission to providing up-to-date guidelines for the care of various diseases.1,2 Documentation tools such as copy-forward and autopopulation increase the speed of documentation, and typed notes improve legibility and ease of note transmission.3,4
However, all of these benefits come with a potential for harm, particularly with respect to accurate and concise documentation. Many experts have described the perpetuation of false information leading to errors, copying-forward of inconsistent and outdated information, and the phenomenon of “note bloat” — physician notes that contain multiple pages of nonessential information, often leaving key aspects buried or lost.5-7 Providers seem to recognize the hazards of copy-and-paste functionality yet persist in utilizing it. In 1 survey, more than 70% of attendings and residents felt that copy and paste led to inaccurate and outdated information, yet 80% stated they would still use it.8
There is little evidence to guide institutions on ways to improve EHR documentation practices. Recent studies have shown that operative note templates improved documentation and decreased the number of missing components.9,10 In the nonoperative setting, 1 small pilot study of pediatric interns demonstrated that a bundled intervention composed of a note template and classroom teaching resulted in improvement in overall note quality and a decrease in “note clutter.”11 In a larger study of pediatric residents, a standardized and simplified note template resulted in a shorter note, although notes were completed later in the day.12 The present study seeks to build upon these efforts by investigating the effect of didactic teaching and an electronic progress note template on note quality, length, and timeliness across 4 academic internal medicine residency programs.
METHODS
Study Design
This prospective quality improvement study took place across 4 academic institutions: University of California Los Angeles (UCLA), University of California San Francisco (UCSF), University of California San Diego (UCSD), and University of Iowa, all of which use Epic EHR (Epic Corp., Madison, WI). The intervention combined brief educational conferences directed at housestaff and attendings with the implementation of an electronic progress note template. Guided by resident input, a note-writing task force at UCSF and UCLA developed a set of best practice guidelines and an aligned note template for progress notes (supplementary Appendix 1). UCSD and the University of Iowa adopted them at their respective institutions. The template’s design minimized autopopulation while encouraging providers to enter relevant data via free text fields (eg, physical exam), prompts (eg, “I have reviewed all the labs from today. Pertinent labs include…”), and drop-down menus (eg, deep vein thrombosis [DVT] prophylaxis: enoxaparin, heparin subcutaneously, etc; supplementary Appendix 2). Additionally, an inpatient checklist was included at the end of the note to serve as a reminder for key inpatient concerns and quality measures, such as Foley catheter days, discharge planning, and code status. Lectures that focused on issues with documentation in the EHR, the best practice guidelines, and a review of the note template with instructions on how to access it were presented to the housestaff. Each institution tailored the lecture to suit their culture. Housestaff were encouraged but not required to use the note template.
Selection and Grading of Progress Notes
Progress notes were eligible for the study if they were written by an intern on an internal medicine teaching service, from a patient with a hospitalization length of at least 3 days with a progress note selected from hospital day 2 or 3, and written while the patient was on the general medicine wards. The preintervention notes were authored from September 2013 to December 2013 and the postintervention notes from April 2014 to June 2014. One note was selected per patient and no more than 3 notes were selected per intern. Each institution selected the first 50 notes chronologically that met these criteria for both the preintervention and the postintervention periods, for a total of 400 notes. The note-grading tool consisted of the following 3 sections to analyze note quality: (1) a general impression of the note (eg, below average, average, above average); (2) the validated Physician Documentation Quality Instrument, 9-item version (PDQI-9) that evaluates notes on 9 domains (up to date, accurate, thorough, useful, organized, comprehensible, succinct, synthesized, internally consistent) on a Likert scale from 1 (not at all) to 5 (extremely); and (3) a note competency questionnaire based on the Accreditation Council for Graduate Medical Education competency note checklist that asked yes or no questions about best practice elements (eg, is there a relevant and focused physical exam).12
Graders were internal medicine teaching faculty involved in the study and were assigned to review notes from their respective sites by directly utilizing the EHR. Although this introduces potential for bias, it was felt that many of the grading elements required the grader to know details of the patient that would not be captured if the note was removed from the context of the EHR. Additionally, graders documented note length (number of lines of text), the time signed by the housestaff, and whether the template was used. Three different graders independently evaluated each note and submitted ratings by using Research Electronic Data Capture.13
Statistical Analysis
Means for each item on the grading tool were computed across raters for each progress note. These were summarized by institution as well as by pre- and postintervention. Cumulative logit mixed effects models were used to compare item responses between study conditions. The number of lines per note before and after the note template intervention was compared by using a mixed effects negative binomial regression model. The timestamp on each note, representing the time of day the note was signed, was compared pre- and postintervention by using a linear mixed effects model. All models included random note and rater effects, and fixed institution and intervention period effects, as well as their interaction. Inter-rater reliability of the grading tool was assessed by calculating the intraclass correlation coefficient (ICC) using the estimated variance components. Data obtained from the PDQI-9 portion were analyzed by individual components as well as by sum score combining each component. The sum score was used to generate odds ratios to assess the likelihood that postintervention notes that used the template compared to those that did not would increase PDQI-9 sum scores. Both cumulative and site-specific data were analyzed. P values < .05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
The mean general impression score significantly improved from 2.0 to 2.3 (on a 1-3 scale in which 2 is average) after the intervention (P < .001). Additionally, note quality significantly improved across each domain of the PDQI-9 (P < .001 for all domains, Table 1). The ICC was 0.245 for the general impression score and 0.143 for the PDQI-9 sum score.
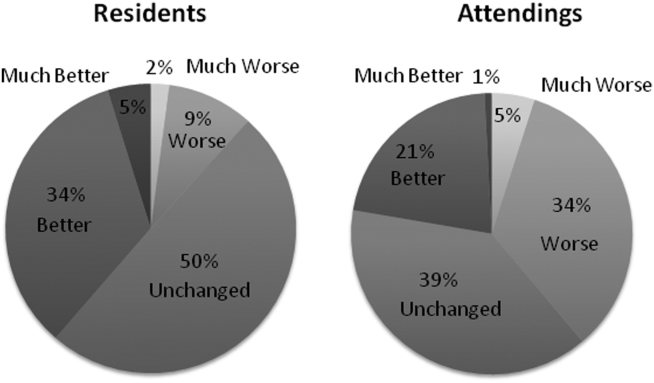
Three of 4 institutions documented the number of lines per note and the time the note was signed by the intern. Mean number of lines per note decreased by 25% (361 lines preintervention, 265 lines postintervention, P < .001). Mean time signed was approximately 1 hour and 15 minutes earlier in the day (3:27
Site-specific data revealed variation between sites. Template use was 92% at UCSF, 90% at UCLA, 79% at Iowa, and 21% at UCSD. The mean general impression score significantly improved at UCSF, UCLA, and UCSD, but not at Iowa. The PDQI-9 score improved across all domains at UCSF and UCLA, 2 domains at UCSD, and 0 domains at Iowa. Documentation of pertinent labs and studies significantly improved at UCSF, UCLA, and Iowa, but not UCSD. Note length decreased at UCSF and UCLA, but not at UCSD. Notes were signed earlier at UCLA and UCSD, but not at UCSF.
When comparing postintervention notes based on template use, notes that used the template were significantly more likely to receive a higher mean impression score (odds ratio [OR] 11.95, P < .001), higher PDQI-9 sum score (OR 3.05, P < .001), be approximately 25% shorter (326 lines vs 239 lines, P < .001), and be completed approximately 1 hour and 20 minutes earlier (3:07
DISCUSSION
A bundled intervention consisting of educational lectures and a best practice progress note template significantly improved the quality, decreased the length, and resulted in earlier completion of inpatient progress notes. These findings are consistent with a prior study that demonstrated that a bundled note template intervention improved total note score and reduced note clutter.11 We saw a broad improvement in progress notes across all 9 domains of the PDQI-9, which corresponded with an improved general impression score. We also found statistically significant improvements in 7 of the 13 categories of the competency questionnaire.
Arguably the greatest impact of the intervention was shortening the documentation of labs and studies. Autopopulation can lead to the appearance of a comprehensive note; however, key data are often lost in a sea of numbers and imaging reports.6,14 Using simple prompts followed by free text such as, “I have reviewed all the labs from today. Pertinent labs include…” reduced autopopulation and reminded housestaff to identify only the key information that affected patient care for that day, resulting in a more streamlined, clear, and high-yield note.
The time spent documenting care is an important consideration for physician workflow and for uptake of any note intervention.14-18 One study from 2016 revealed that internal medicine housestaff spend more than half of an average shift using the computer, with 52% of that time spent on documentation.17 Although functions such as autopopulation and copy-forward were created as efficiency tools, we hypothesize that they may actually prolong note writing time by leading to disorganized, distended notes that are difficult to use the following day. There was concern that limiting these “efficiency functions” might discourage housestaff from using the progress note template. It was encouraging to find that postintervention notes were signed 1.3 hours earlier in the day. This study did not measure the impact of shorter notes and earlier completion time, but in theory, this could allow interns to spend more time in direct patient care and to be at lower risk of duty hour violations.19 Furthermore, while the clinical impact of this is unknown, it is possible that timely note completion may improve patient care by making notes available earlier for consultants and other members of the care team.
We found that adding an “inpatient checklist” to the progress note template facilitated a review of key inpatient concerns and quality measures. Although we did not specifically compare before-and-after documentation of all of the components of the checklist, there appeared to be improvement in the domains measured. Notably, there was a 31% increase (P < .001) in the percentage of notes documenting the “discharge plan, goals of hospitalization, or estimated length of stay.” In the surgical literature, studies have demonstrated that incorporating checklists improves patient safety, the delivery of care, and potentially shortens the length of stay.20-22 Future studies should explore the impact of adding a checklist to the daily progress note, as there may be potential to improve both process and outcome measures.
Institution-specific data provided insightful results. UCSD encountered low template use among their interns; however, they still had evidence of improvement in note quality, though not at the same level of UCLA and UCSF. Some barriers to uptake identified were as follows: (1) interns were accustomed to import labs and studies into their note to use as their rounding report, and (2) the intervention took place late in the year when interns had developed a functional writing system that they were reluctant to change. The University of Iowa did not show significant improvement in their note quality despite a relatively high template uptake. Both of these outcomes raise the possibility that in addition to the template, there were other factors at play. Perhaps because UCSF and UCLA created the best practice guidelines and template, it was a better fit for their culture and they had more institutional buy-in. Or because the educational lectures were similar, but not standardized across institutions, some lectures may have been more effective than others. However, when evaluating the postintervention notes at UCSD and Iowa, templated notes were found to be much more likely to score higher on the PDQI-9 than nontemplated notes, which serves as evidence of the efficacy of the note template.
Some of the strengths of this study include the relatively large sample size spanning 4 institutions and the use of 3 different assessment tools for grading progress note quality (general impression score, PDQI-9, and competency note questionnaire). An additional strength is our unique finding suggesting that note writing may be more efficient by removing, rather than adding, “efficiency functions.” There were several limitations of this study. Pre- and postintervention notes were examined at different points in the same academic year, thus certain domains may have improved as interns progressed in clinical skill and comfort with documentation, independent of our intervention.21 However, our analysis of postintervention notes across the same time period revealed that use of the template was strongly associated with higher quality, shorter notes and earlier completion time arguing that the effect seen was not merely intern experience. The poor interrater reliability is also a limitation. Although the PDQI-9 was previously validated, future use of the grading tool may require more rater training for calibration or more objective wording.23 The study was not blinded, and thus, bias may have falsely elevated postintervention scores; however, we attempted to minimize bias by incorporating a more objective yes/no competency questionnaire and by having each note scored by 3 graders. Other studies have attempted to address this form of bias by printing out notes and blinding the graders. This design, however, isolates the note from all other data in the medical record, making it difficult to assess domains such as accuracy and completeness. Our inclusion of objective outcomes such as note length and time of note completion help to mitigate some of the bias.
Future research can expand on the results of this study by introducing similar progress note interventions at other institutions and/or in nonacademic environments to validate the results and expand generalizability. Longer term follow-up would be useful to determine if these effects are transient or long lasting. Similarly, it would be interesting to determine if such results are sustained even after new interns start suggesting that institutional culture can be changed. Investigators could focus on similar projects to improve other notes that are particularly at a high risk for propagating false information, such as the History and Physical or Discharge Summary. Future research should also focus on outcomes data, including whether a more efficient note can allow housestaff to spend more time with patients, decrease patient length of stay, reduce clinical errors, and improve educational time for trainees. Lastly, we should determine if interventions such as this can mitigate the widespread frustrations with electronic documentation that are associated with physician and provider burnout.15,24 One would hope that the technology could be harnessed to improve provider productivity and be effectively integrated into comprehensive patient care.
Our research makes progress toward recommendations made by the American College of Physicians “to improve accuracy of information recorded and the value of information,” and develop automated tools that “enhance documentation quality without facilitating improper behaviors.”19 Institutions should consider developing internal best practices for clinical documentation and building structured note templates.19 Our research would suggest that, combined with a small educational intervention, such templates can make progress notes more accurate and succinct, make note writing more efficient, and be harnessed to improve quality metrics.
ACKNOWLEDGMENTS
The authors thank Michael Pfeffer, MD, and Sitaram Vangala, MS, for their contributions to and support of this research study and manuscript.
Disclosure: The authors declare no conflicts of interest.
1. Herzig SJ, Guess JR, Feinbloom DB, et al. Improving appropriateness of acid-suppressive medication use via computerized clinical decision support. J Hosp Med. 2015;10(1):41-45. PubMed
2. Nguyen OK, Makam AN, Clark C, et al. Predicting all-cause readmissions using electronic health record data from the entire hospitalization: Model development and comparison. J Hosp Med. 2016;11(7):473-480. PubMed
3. Donati A, Gabbanelli V, Pantanetti S, et al. The impact of a clinical information system in an intensive care unit. J Clin Monit Comput. 2008;22(1):31-36. PubMed
4. Schiff GD, Bates DW. Can electronic clinical documentation help prevent diagnostic errors? N Engl J Med. 2010;362(12):1066-1069. PubMed
5. Hartzband P, Groopman J. Off the record--avoiding the pitfalls of going electronic. N Engl J Med. 2008;358(16):1656-1658. PubMed
6. Hirschtick RE. A piece of my mind. Copy-and-paste. JAMA. 2006;295(20):2335-2336. PubMed
7. Hirschtick RE. A piece of my mind. John Lennon’s elbow. JAMA. 2012;308(5):463-464. PubMed
8. O’Donnell HC, Kaushal R, Barrón Y, Callahan MA, Adelman RD, Siegler EL. Physicians’ attitudes towards copy and pasting in electronic note writing. J Gen Intern Med. 2009;24(1):63-68. PubMed
9. Mahapatra P, Ieong E. Improving Documentation and Communication Using Operative Note Proformas. BMJ Qual Improv Rep. 2016;5(1):u209122.w3712. PubMed
10. Thomson DR, Baldwin MJ, Bellini MI, Silva MA. Improving the quality of operative notes for laparoscopic cholecystectomy: Assessing the impact of a standardized operation note proforma. Int J Surg. 2016;27:17-20. PubMed
11. Dean SM, Eickhoff JC, Bakel LA. The effectiveness of a bundled intervention to improve resident progress notes in an electronic health record. J Hosp Med. 2015;10(2):104-107. PubMed
12. Aylor M, Campbell EM, Winter C, Phillipi CA. Resident Notes in an Electronic Health Record: A Mixed-Methods Study Using a Standardized Intervention With Qualitative Analysis. Clin Pediatr (Phila). 2016;6(3):257-262.
13. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. PubMed
14. Chi J, Kugler J, Chu IM, et al. Medical students and the electronic health record: ‘an epic use of time’. Am J Med. 2014;127(9):891-895. PubMed
15. Martin SA, Sinsky CA. The map is not the territory: medical records and 21st century practice. Lancet. 2016;388(10055):2053-2056. PubMed
16. Oxentenko AS, Manohar CU, McCoy CP, et al. Internal medicine residents’ computer use in the inpatient setting. J Grad Med Educ. 2012;4(4):529-532. PubMed
17. Mamykina L, Vawdrey DK, Hripcsak G. How Do Residents Spend Their Shift Time? A Time and Motion Study With a Particular Focus on the Use of Computers. Acad Med. 2016;91(6):827-832. PubMed
18. Chen L, Guo U, Illipparambil LC, et al. Racing Against the Clock: Internal Medicine Residents’ Time Spent On Electronic Health Records. J Grad Med Educ. 2016;8(1):39-44. PubMed
19. Kuhn T, Basch P, Barr M, Yackel T, Physicians MICotACo. Clinical documentation in the 21st century: executive summary of a policy position paper from the American College of Physicians. Ann Intern Med. 2015;162(4):301-303. PubMed
20. Treadwell JR, Lucas S, Tsou AY. Surgical checklists: a systematic review of impacts and implementation. BMJ Qual Saf. 2014;23(4):299-318. PubMed
21. Ko HC, Turner TJ, Finnigan MA. Systematic review of safety checklists for use by medical care teams in acute hospital settings--limited evidence of effectiveness. BMC Health Serv Res. 2011;11:211. PubMed
22. Diaz-Montes TP, Cobb L, Ibeanu OA, Njoku P, Gerardi MA. Introduction of checklists at daily progress notes improves patient care among the gynecological oncology service. J Patient Saf. 2012;8(4):189-193. PubMed
23. Stetson PD, Bakken S, Wrenn JO, Siegler EL. Assessing Electronic Note Quality Using the Physician Documentation Quality Instrument (PDQI-9). Appl Clin Inform. 2012;3(2):164-174. PubMed
24. Friedberg MW, Chen PG, Van Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. Santa Monica, CA: RAND Corporation; 2013. PubMed
The widespread adoption of electronic health records (EHRs) has led to significant progress in the modernization of healthcare delivery. Ease of access has improved clinical efficiency, and digital data have allowed for point-of-care decision support tools ranging from predicting the 30-day risk of readmission to providing up-to-date guidelines for the care of various diseases.1,2 Documentation tools such as copy-forward and autopopulation increase the speed of documentation, and typed notes improve legibility and ease of note transmission.3,4
However, all of these benefits come with a potential for harm, particularly with respect to accurate and concise documentation. Many experts have described the perpetuation of false information leading to errors, copying-forward of inconsistent and outdated information, and the phenomenon of “note bloat” — physician notes that contain multiple pages of nonessential information, often leaving key aspects buried or lost.5-7 Providers seem to recognize the hazards of copy-and-paste functionality yet persist in utilizing it. In 1 survey, more than 70% of attendings and residents felt that copy and paste led to inaccurate and outdated information, yet 80% stated they would still use it.8
There is little evidence to guide institutions on ways to improve EHR documentation practices. Recent studies have shown that operative note templates improved documentation and decreased the number of missing components.9,10 In the nonoperative setting, 1 small pilot study of pediatric interns demonstrated that a bundled intervention composed of a note template and classroom teaching resulted in improvement in overall note quality and a decrease in “note clutter.”11 In a larger study of pediatric residents, a standardized and simplified note template resulted in a shorter note, although notes were completed later in the day.12 The present study seeks to build upon these efforts by investigating the effect of didactic teaching and an electronic progress note template on note quality, length, and timeliness across 4 academic internal medicine residency programs.
METHODS
Study Design
This prospective quality improvement study took place across 4 academic institutions: University of California Los Angeles (UCLA), University of California San Francisco (UCSF), University of California San Diego (UCSD), and University of Iowa, all of which use Epic EHR (Epic Corp., Madison, WI). The intervention combined brief educational conferences directed at housestaff and attendings with the implementation of an electronic progress note template. Guided by resident input, a note-writing task force at UCSF and UCLA developed a set of best practice guidelines and an aligned note template for progress notes (supplementary Appendix 1). UCSD and the University of Iowa adopted them at their respective institutions. The template’s design minimized autopopulation while encouraging providers to enter relevant data via free text fields (eg, physical exam), prompts (eg, “I have reviewed all the labs from today. Pertinent labs include…”), and drop-down menus (eg, deep vein thrombosis [DVT] prophylaxis: enoxaparin, heparin subcutaneously, etc; supplementary Appendix 2). Additionally, an inpatient checklist was included at the end of the note to serve as a reminder for key inpatient concerns and quality measures, such as Foley catheter days, discharge planning, and code status. Lectures that focused on issues with documentation in the EHR, the best practice guidelines, and a review of the note template with instructions on how to access it were presented to the housestaff. Each institution tailored the lecture to suit their culture. Housestaff were encouraged but not required to use the note template.
Selection and Grading of Progress Notes
Progress notes were eligible for the study if they were written by an intern on an internal medicine teaching service, from a patient with a hospitalization length of at least 3 days with a progress note selected from hospital day 2 or 3, and written while the patient was on the general medicine wards. The preintervention notes were authored from September 2013 to December 2013 and the postintervention notes from April 2014 to June 2014. One note was selected per patient and no more than 3 notes were selected per intern. Each institution selected the first 50 notes chronologically that met these criteria for both the preintervention and the postintervention periods, for a total of 400 notes. The note-grading tool consisted of the following 3 sections to analyze note quality: (1) a general impression of the note (eg, below average, average, above average); (2) the validated Physician Documentation Quality Instrument, 9-item version (PDQI-9) that evaluates notes on 9 domains (up to date, accurate, thorough, useful, organized, comprehensible, succinct, synthesized, internally consistent) on a Likert scale from 1 (not at all) to 5 (extremely); and (3) a note competency questionnaire based on the Accreditation Council for Graduate Medical Education competency note checklist that asked yes or no questions about best practice elements (eg, is there a relevant and focused physical exam).12
Graders were internal medicine teaching faculty involved in the study and were assigned to review notes from their respective sites by directly utilizing the EHR. Although this introduces potential for bias, it was felt that many of the grading elements required the grader to know details of the patient that would not be captured if the note was removed from the context of the EHR. Additionally, graders documented note length (number of lines of text), the time signed by the housestaff, and whether the template was used. Three different graders independently evaluated each note and submitted ratings by using Research Electronic Data Capture.13
Statistical Analysis
Means for each item on the grading tool were computed across raters for each progress note. These were summarized by institution as well as by pre- and postintervention. Cumulative logit mixed effects models were used to compare item responses between study conditions. The number of lines per note before and after the note template intervention was compared by using a mixed effects negative binomial regression model. The timestamp on each note, representing the time of day the note was signed, was compared pre- and postintervention by using a linear mixed effects model. All models included random note and rater effects, and fixed institution and intervention period effects, as well as their interaction. Inter-rater reliability of the grading tool was assessed by calculating the intraclass correlation coefficient (ICC) using the estimated variance components. Data obtained from the PDQI-9 portion were analyzed by individual components as well as by sum score combining each component. The sum score was used to generate odds ratios to assess the likelihood that postintervention notes that used the template compared to those that did not would increase PDQI-9 sum scores. Both cumulative and site-specific data were analyzed. P values < .05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
The mean general impression score significantly improved from 2.0 to 2.3 (on a 1-3 scale in which 2 is average) after the intervention (P < .001). Additionally, note quality significantly improved across each domain of the PDQI-9 (P < .001 for all domains, Table 1). The ICC was 0.245 for the general impression score and 0.143 for the PDQI-9 sum score.
Three of 4 institutions documented the number of lines per note and the time the note was signed by the intern. Mean number of lines per note decreased by 25% (361 lines preintervention, 265 lines postintervention, P < .001). Mean time signed was approximately 1 hour and 15 minutes earlier in the day (3:27
Site-specific data revealed variation between sites. Template use was 92% at UCSF, 90% at UCLA, 79% at Iowa, and 21% at UCSD. The mean general impression score significantly improved at UCSF, UCLA, and UCSD, but not at Iowa. The PDQI-9 score improved across all domains at UCSF and UCLA, 2 domains at UCSD, and 0 domains at Iowa. Documentation of pertinent labs and studies significantly improved at UCSF, UCLA, and Iowa, but not UCSD. Note length decreased at UCSF and UCLA, but not at UCSD. Notes were signed earlier at UCLA and UCSD, but not at UCSF.
When comparing postintervention notes based on template use, notes that used the template were significantly more likely to receive a higher mean impression score (odds ratio [OR] 11.95, P < .001), higher PDQI-9 sum score (OR 3.05, P < .001), be approximately 25% shorter (326 lines vs 239 lines, P < .001), and be completed approximately 1 hour and 20 minutes earlier (3:07
DISCUSSION
A bundled intervention consisting of educational lectures and a best practice progress note template significantly improved the quality, decreased the length, and resulted in earlier completion of inpatient progress notes. These findings are consistent with a prior study that demonstrated that a bundled note template intervention improved total note score and reduced note clutter.11 We saw a broad improvement in progress notes across all 9 domains of the PDQI-9, which corresponded with an improved general impression score. We also found statistically significant improvements in 7 of the 13 categories of the competency questionnaire.
Arguably the greatest impact of the intervention was shortening the documentation of labs and studies. Autopopulation can lead to the appearance of a comprehensive note; however, key data are often lost in a sea of numbers and imaging reports.6,14 Using simple prompts followed by free text such as, “I have reviewed all the labs from today. Pertinent labs include…” reduced autopopulation and reminded housestaff to identify only the key information that affected patient care for that day, resulting in a more streamlined, clear, and high-yield note.
The time spent documenting care is an important consideration for physician workflow and for uptake of any note intervention.14-18 One study from 2016 revealed that internal medicine housestaff spend more than half of an average shift using the computer, with 52% of that time spent on documentation.17 Although functions such as autopopulation and copy-forward were created as efficiency tools, we hypothesize that they may actually prolong note writing time by leading to disorganized, distended notes that are difficult to use the following day. There was concern that limiting these “efficiency functions” might discourage housestaff from using the progress note template. It was encouraging to find that postintervention notes were signed 1.3 hours earlier in the day. This study did not measure the impact of shorter notes and earlier completion time, but in theory, this could allow interns to spend more time in direct patient care and to be at lower risk of duty hour violations.19 Furthermore, while the clinical impact of this is unknown, it is possible that timely note completion may improve patient care by making notes available earlier for consultants and other members of the care team.
We found that adding an “inpatient checklist” to the progress note template facilitated a review of key inpatient concerns and quality measures. Although we did not specifically compare before-and-after documentation of all of the components of the checklist, there appeared to be improvement in the domains measured. Notably, there was a 31% increase (P < .001) in the percentage of notes documenting the “discharge plan, goals of hospitalization, or estimated length of stay.” In the surgical literature, studies have demonstrated that incorporating checklists improves patient safety, the delivery of care, and potentially shortens the length of stay.20-22 Future studies should explore the impact of adding a checklist to the daily progress note, as there may be potential to improve both process and outcome measures.
Institution-specific data provided insightful results. UCSD encountered low template use among their interns; however, they still had evidence of improvement in note quality, though not at the same level of UCLA and UCSF. Some barriers to uptake identified were as follows: (1) interns were accustomed to import labs and studies into their note to use as their rounding report, and (2) the intervention took place late in the year when interns had developed a functional writing system that they were reluctant to change. The University of Iowa did not show significant improvement in their note quality despite a relatively high template uptake. Both of these outcomes raise the possibility that in addition to the template, there were other factors at play. Perhaps because UCSF and UCLA created the best practice guidelines and template, it was a better fit for their culture and they had more institutional buy-in. Or because the educational lectures were similar, but not standardized across institutions, some lectures may have been more effective than others. However, when evaluating the postintervention notes at UCSD and Iowa, templated notes were found to be much more likely to score higher on the PDQI-9 than nontemplated notes, which serves as evidence of the efficacy of the note template.
Some of the strengths of this study include the relatively large sample size spanning 4 institutions and the use of 3 different assessment tools for grading progress note quality (general impression score, PDQI-9, and competency note questionnaire). An additional strength is our unique finding suggesting that note writing may be more efficient by removing, rather than adding, “efficiency functions.” There were several limitations of this study. Pre- and postintervention notes were examined at different points in the same academic year, thus certain domains may have improved as interns progressed in clinical skill and comfort with documentation, independent of our intervention.21 However, our analysis of postintervention notes across the same time period revealed that use of the template was strongly associated with higher quality, shorter notes and earlier completion time arguing that the effect seen was not merely intern experience. The poor interrater reliability is also a limitation. Although the PDQI-9 was previously validated, future use of the grading tool may require more rater training for calibration or more objective wording.23 The study was not blinded, and thus, bias may have falsely elevated postintervention scores; however, we attempted to minimize bias by incorporating a more objective yes/no competency questionnaire and by having each note scored by 3 graders. Other studies have attempted to address this form of bias by printing out notes and blinding the graders. This design, however, isolates the note from all other data in the medical record, making it difficult to assess domains such as accuracy and completeness. Our inclusion of objective outcomes such as note length and time of note completion help to mitigate some of the bias.
Future research can expand on the results of this study by introducing similar progress note interventions at other institutions and/or in nonacademic environments to validate the results and expand generalizability. Longer term follow-up would be useful to determine if these effects are transient or long lasting. Similarly, it would be interesting to determine if such results are sustained even after new interns start suggesting that institutional culture can be changed. Investigators could focus on similar projects to improve other notes that are particularly at a high risk for propagating false information, such as the History and Physical or Discharge Summary. Future research should also focus on outcomes data, including whether a more efficient note can allow housestaff to spend more time with patients, decrease patient length of stay, reduce clinical errors, and improve educational time for trainees. Lastly, we should determine if interventions such as this can mitigate the widespread frustrations with electronic documentation that are associated with physician and provider burnout.15,24 One would hope that the technology could be harnessed to improve provider productivity and be effectively integrated into comprehensive patient care.
Our research makes progress toward recommendations made by the American College of Physicians “to improve accuracy of information recorded and the value of information,” and develop automated tools that “enhance documentation quality without facilitating improper behaviors.”19 Institutions should consider developing internal best practices for clinical documentation and building structured note templates.19 Our research would suggest that, combined with a small educational intervention, such templates can make progress notes more accurate and succinct, make note writing more efficient, and be harnessed to improve quality metrics.
ACKNOWLEDGMENTS
The authors thank Michael Pfeffer, MD, and Sitaram Vangala, MS, for their contributions to and support of this research study and manuscript.
Disclosure: The authors declare no conflicts of interest.
The widespread adoption of electronic health records (EHRs) has led to significant progress in the modernization of healthcare delivery. Ease of access has improved clinical efficiency, and digital data have allowed for point-of-care decision support tools ranging from predicting the 30-day risk of readmission to providing up-to-date guidelines for the care of various diseases.1,2 Documentation tools such as copy-forward and autopopulation increase the speed of documentation, and typed notes improve legibility and ease of note transmission.3,4
However, all of these benefits come with a potential for harm, particularly with respect to accurate and concise documentation. Many experts have described the perpetuation of false information leading to errors, copying-forward of inconsistent and outdated information, and the phenomenon of “note bloat” — physician notes that contain multiple pages of nonessential information, often leaving key aspects buried or lost.5-7 Providers seem to recognize the hazards of copy-and-paste functionality yet persist in utilizing it. In 1 survey, more than 70% of attendings and residents felt that copy and paste led to inaccurate and outdated information, yet 80% stated they would still use it.8
There is little evidence to guide institutions on ways to improve EHR documentation practices. Recent studies have shown that operative note templates improved documentation and decreased the number of missing components.9,10 In the nonoperative setting, 1 small pilot study of pediatric interns demonstrated that a bundled intervention composed of a note template and classroom teaching resulted in improvement in overall note quality and a decrease in “note clutter.”11 In a larger study of pediatric residents, a standardized and simplified note template resulted in a shorter note, although notes were completed later in the day.12 The present study seeks to build upon these efforts by investigating the effect of didactic teaching and an electronic progress note template on note quality, length, and timeliness across 4 academic internal medicine residency programs.
METHODS
Study Design
This prospective quality improvement study took place across 4 academic institutions: University of California Los Angeles (UCLA), University of California San Francisco (UCSF), University of California San Diego (UCSD), and University of Iowa, all of which use Epic EHR (Epic Corp., Madison, WI). The intervention combined brief educational conferences directed at housestaff and attendings with the implementation of an electronic progress note template. Guided by resident input, a note-writing task force at UCSF and UCLA developed a set of best practice guidelines and an aligned note template for progress notes (supplementary Appendix 1). UCSD and the University of Iowa adopted them at their respective institutions. The template’s design minimized autopopulation while encouraging providers to enter relevant data via free text fields (eg, physical exam), prompts (eg, “I have reviewed all the labs from today. Pertinent labs include…”), and drop-down menus (eg, deep vein thrombosis [DVT] prophylaxis: enoxaparin, heparin subcutaneously, etc; supplementary Appendix 2). Additionally, an inpatient checklist was included at the end of the note to serve as a reminder for key inpatient concerns and quality measures, such as Foley catheter days, discharge planning, and code status. Lectures that focused on issues with documentation in the EHR, the best practice guidelines, and a review of the note template with instructions on how to access it were presented to the housestaff. Each institution tailored the lecture to suit their culture. Housestaff were encouraged but not required to use the note template.
Selection and Grading of Progress Notes
Progress notes were eligible for the study if they were written by an intern on an internal medicine teaching service, from a patient with a hospitalization length of at least 3 days with a progress note selected from hospital day 2 or 3, and written while the patient was on the general medicine wards. The preintervention notes were authored from September 2013 to December 2013 and the postintervention notes from April 2014 to June 2014. One note was selected per patient and no more than 3 notes were selected per intern. Each institution selected the first 50 notes chronologically that met these criteria for both the preintervention and the postintervention periods, for a total of 400 notes. The note-grading tool consisted of the following 3 sections to analyze note quality: (1) a general impression of the note (eg, below average, average, above average); (2) the validated Physician Documentation Quality Instrument, 9-item version (PDQI-9) that evaluates notes on 9 domains (up to date, accurate, thorough, useful, organized, comprehensible, succinct, synthesized, internally consistent) on a Likert scale from 1 (not at all) to 5 (extremely); and (3) a note competency questionnaire based on the Accreditation Council for Graduate Medical Education competency note checklist that asked yes or no questions about best practice elements (eg, is there a relevant and focused physical exam).12
Graders were internal medicine teaching faculty involved in the study and were assigned to review notes from their respective sites by directly utilizing the EHR. Although this introduces potential for bias, it was felt that many of the grading elements required the grader to know details of the patient that would not be captured if the note was removed from the context of the EHR. Additionally, graders documented note length (number of lines of text), the time signed by the housestaff, and whether the template was used. Three different graders independently evaluated each note and submitted ratings by using Research Electronic Data Capture.13
Statistical Analysis
Means for each item on the grading tool were computed across raters for each progress note. These were summarized by institution as well as by pre- and postintervention. Cumulative logit mixed effects models were used to compare item responses between study conditions. The number of lines per note before and after the note template intervention was compared by using a mixed effects negative binomial regression model. The timestamp on each note, representing the time of day the note was signed, was compared pre- and postintervention by using a linear mixed effects model. All models included random note and rater effects, and fixed institution and intervention period effects, as well as their interaction. Inter-rater reliability of the grading tool was assessed by calculating the intraclass correlation coefficient (ICC) using the estimated variance components. Data obtained from the PDQI-9 portion were analyzed by individual components as well as by sum score combining each component. The sum score was used to generate odds ratios to assess the likelihood that postintervention notes that used the template compared to those that did not would increase PDQI-9 sum scores. Both cumulative and site-specific data were analyzed. P values < .05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
The mean general impression score significantly improved from 2.0 to 2.3 (on a 1-3 scale in which 2 is average) after the intervention (P < .001). Additionally, note quality significantly improved across each domain of the PDQI-9 (P < .001 for all domains, Table 1). The ICC was 0.245 for the general impression score and 0.143 for the PDQI-9 sum score.
Three of 4 institutions documented the number of lines per note and the time the note was signed by the intern. Mean number of lines per note decreased by 25% (361 lines preintervention, 265 lines postintervention, P < .001). Mean time signed was approximately 1 hour and 15 minutes earlier in the day (3:27
Site-specific data revealed variation between sites. Template use was 92% at UCSF, 90% at UCLA, 79% at Iowa, and 21% at UCSD. The mean general impression score significantly improved at UCSF, UCLA, and UCSD, but not at Iowa. The PDQI-9 score improved across all domains at UCSF and UCLA, 2 domains at UCSD, and 0 domains at Iowa. Documentation of pertinent labs and studies significantly improved at UCSF, UCLA, and Iowa, but not UCSD. Note length decreased at UCSF and UCLA, but not at UCSD. Notes were signed earlier at UCLA and UCSD, but not at UCSF.
When comparing postintervention notes based on template use, notes that used the template were significantly more likely to receive a higher mean impression score (odds ratio [OR] 11.95, P < .001), higher PDQI-9 sum score (OR 3.05, P < .001), be approximately 25% shorter (326 lines vs 239 lines, P < .001), and be completed approximately 1 hour and 20 minutes earlier (3:07
DISCUSSION
A bundled intervention consisting of educational lectures and a best practice progress note template significantly improved the quality, decreased the length, and resulted in earlier completion of inpatient progress notes. These findings are consistent with a prior study that demonstrated that a bundled note template intervention improved total note score and reduced note clutter.11 We saw a broad improvement in progress notes across all 9 domains of the PDQI-9, which corresponded with an improved general impression score. We also found statistically significant improvements in 7 of the 13 categories of the competency questionnaire.
Arguably the greatest impact of the intervention was shortening the documentation of labs and studies. Autopopulation can lead to the appearance of a comprehensive note; however, key data are often lost in a sea of numbers and imaging reports.6,14 Using simple prompts followed by free text such as, “I have reviewed all the labs from today. Pertinent labs include…” reduced autopopulation and reminded housestaff to identify only the key information that affected patient care for that day, resulting in a more streamlined, clear, and high-yield note.
The time spent documenting care is an important consideration for physician workflow and for uptake of any note intervention.14-18 One study from 2016 revealed that internal medicine housestaff spend more than half of an average shift using the computer, with 52% of that time spent on documentation.17 Although functions such as autopopulation and copy-forward were created as efficiency tools, we hypothesize that they may actually prolong note writing time by leading to disorganized, distended notes that are difficult to use the following day. There was concern that limiting these “efficiency functions” might discourage housestaff from using the progress note template. It was encouraging to find that postintervention notes were signed 1.3 hours earlier in the day. This study did not measure the impact of shorter notes and earlier completion time, but in theory, this could allow interns to spend more time in direct patient care and to be at lower risk of duty hour violations.19 Furthermore, while the clinical impact of this is unknown, it is possible that timely note completion may improve patient care by making notes available earlier for consultants and other members of the care team.
We found that adding an “inpatient checklist” to the progress note template facilitated a review of key inpatient concerns and quality measures. Although we did not specifically compare before-and-after documentation of all of the components of the checklist, there appeared to be improvement in the domains measured. Notably, there was a 31% increase (P < .001) in the percentage of notes documenting the “discharge plan, goals of hospitalization, or estimated length of stay.” In the surgical literature, studies have demonstrated that incorporating checklists improves patient safety, the delivery of care, and potentially shortens the length of stay.20-22 Future studies should explore the impact of adding a checklist to the daily progress note, as there may be potential to improve both process and outcome measures.
Institution-specific data provided insightful results. UCSD encountered low template use among their interns; however, they still had evidence of improvement in note quality, though not at the same level of UCLA and UCSF. Some barriers to uptake identified were as follows: (1) interns were accustomed to import labs and studies into their note to use as their rounding report, and (2) the intervention took place late in the year when interns had developed a functional writing system that they were reluctant to change. The University of Iowa did not show significant improvement in their note quality despite a relatively high template uptake. Both of these outcomes raise the possibility that in addition to the template, there were other factors at play. Perhaps because UCSF and UCLA created the best practice guidelines and template, it was a better fit for their culture and they had more institutional buy-in. Or because the educational lectures were similar, but not standardized across institutions, some lectures may have been more effective than others. However, when evaluating the postintervention notes at UCSD and Iowa, templated notes were found to be much more likely to score higher on the PDQI-9 than nontemplated notes, which serves as evidence of the efficacy of the note template.
Some of the strengths of this study include the relatively large sample size spanning 4 institutions and the use of 3 different assessment tools for grading progress note quality (general impression score, PDQI-9, and competency note questionnaire). An additional strength is our unique finding suggesting that note writing may be more efficient by removing, rather than adding, “efficiency functions.” There were several limitations of this study. Pre- and postintervention notes were examined at different points in the same academic year, thus certain domains may have improved as interns progressed in clinical skill and comfort with documentation, independent of our intervention.21 However, our analysis of postintervention notes across the same time period revealed that use of the template was strongly associated with higher quality, shorter notes and earlier completion time arguing that the effect seen was not merely intern experience. The poor interrater reliability is also a limitation. Although the PDQI-9 was previously validated, future use of the grading tool may require more rater training for calibration or more objective wording.23 The study was not blinded, and thus, bias may have falsely elevated postintervention scores; however, we attempted to minimize bias by incorporating a more objective yes/no competency questionnaire and by having each note scored by 3 graders. Other studies have attempted to address this form of bias by printing out notes and blinding the graders. This design, however, isolates the note from all other data in the medical record, making it difficult to assess domains such as accuracy and completeness. Our inclusion of objective outcomes such as note length and time of note completion help to mitigate some of the bias.
Future research can expand on the results of this study by introducing similar progress note interventions at other institutions and/or in nonacademic environments to validate the results and expand generalizability. Longer term follow-up would be useful to determine if these effects are transient or long lasting. Similarly, it would be interesting to determine if such results are sustained even after new interns start suggesting that institutional culture can be changed. Investigators could focus on similar projects to improve other notes that are particularly at a high risk for propagating false information, such as the History and Physical or Discharge Summary. Future research should also focus on outcomes data, including whether a more efficient note can allow housestaff to spend more time with patients, decrease patient length of stay, reduce clinical errors, and improve educational time for trainees. Lastly, we should determine if interventions such as this can mitigate the widespread frustrations with electronic documentation that are associated with physician and provider burnout.15,24 One would hope that the technology could be harnessed to improve provider productivity and be effectively integrated into comprehensive patient care.
Our research makes progress toward recommendations made by the American College of Physicians “to improve accuracy of information recorded and the value of information,” and develop automated tools that “enhance documentation quality without facilitating improper behaviors.”19 Institutions should consider developing internal best practices for clinical documentation and building structured note templates.19 Our research would suggest that, combined with a small educational intervention, such templates can make progress notes more accurate and succinct, make note writing more efficient, and be harnessed to improve quality metrics.
ACKNOWLEDGMENTS
The authors thank Michael Pfeffer, MD, and Sitaram Vangala, MS, for their contributions to and support of this research study and manuscript.
Disclosure: The authors declare no conflicts of interest.
1. Herzig SJ, Guess JR, Feinbloom DB, et al. Improving appropriateness of acid-suppressive medication use via computerized clinical decision support. J Hosp Med. 2015;10(1):41-45. PubMed
2. Nguyen OK, Makam AN, Clark C, et al. Predicting all-cause readmissions using electronic health record data from the entire hospitalization: Model development and comparison. J Hosp Med. 2016;11(7):473-480. PubMed
3. Donati A, Gabbanelli V, Pantanetti S, et al. The impact of a clinical information system in an intensive care unit. J Clin Monit Comput. 2008;22(1):31-36. PubMed
4. Schiff GD, Bates DW. Can electronic clinical documentation help prevent diagnostic errors? N Engl J Med. 2010;362(12):1066-1069. PubMed
5. Hartzband P, Groopman J. Off the record--avoiding the pitfalls of going electronic. N Engl J Med. 2008;358(16):1656-1658. PubMed
6. Hirschtick RE. A piece of my mind. Copy-and-paste. JAMA. 2006;295(20):2335-2336. PubMed
7. Hirschtick RE. A piece of my mind. John Lennon’s elbow. JAMA. 2012;308(5):463-464. PubMed
8. O’Donnell HC, Kaushal R, Barrón Y, Callahan MA, Adelman RD, Siegler EL. Physicians’ attitudes towards copy and pasting in electronic note writing. J Gen Intern Med. 2009;24(1):63-68. PubMed
9. Mahapatra P, Ieong E. Improving Documentation and Communication Using Operative Note Proformas. BMJ Qual Improv Rep. 2016;5(1):u209122.w3712. PubMed
10. Thomson DR, Baldwin MJ, Bellini MI, Silva MA. Improving the quality of operative notes for laparoscopic cholecystectomy: Assessing the impact of a standardized operation note proforma. Int J Surg. 2016;27:17-20. PubMed
11. Dean SM, Eickhoff JC, Bakel LA. The effectiveness of a bundled intervention to improve resident progress notes in an electronic health record. J Hosp Med. 2015;10(2):104-107. PubMed
12. Aylor M, Campbell EM, Winter C, Phillipi CA. Resident Notes in an Electronic Health Record: A Mixed-Methods Study Using a Standardized Intervention With Qualitative Analysis. Clin Pediatr (Phila). 2016;6(3):257-262.
13. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. PubMed
14. Chi J, Kugler J, Chu IM, et al. Medical students and the electronic health record: ‘an epic use of time’. Am J Med. 2014;127(9):891-895. PubMed
15. Martin SA, Sinsky CA. The map is not the territory: medical records and 21st century practice. Lancet. 2016;388(10055):2053-2056. PubMed
16. Oxentenko AS, Manohar CU, McCoy CP, et al. Internal medicine residents’ computer use in the inpatient setting. J Grad Med Educ. 2012;4(4):529-532. PubMed
17. Mamykina L, Vawdrey DK, Hripcsak G. How Do Residents Spend Their Shift Time? A Time and Motion Study With a Particular Focus on the Use of Computers. Acad Med. 2016;91(6):827-832. PubMed
18. Chen L, Guo U, Illipparambil LC, et al. Racing Against the Clock: Internal Medicine Residents’ Time Spent On Electronic Health Records. J Grad Med Educ. 2016;8(1):39-44. PubMed
19. Kuhn T, Basch P, Barr M, Yackel T, Physicians MICotACo. Clinical documentation in the 21st century: executive summary of a policy position paper from the American College of Physicians. Ann Intern Med. 2015;162(4):301-303. PubMed
20. Treadwell JR, Lucas S, Tsou AY. Surgical checklists: a systematic review of impacts and implementation. BMJ Qual Saf. 2014;23(4):299-318. PubMed
21. Ko HC, Turner TJ, Finnigan MA. Systematic review of safety checklists for use by medical care teams in acute hospital settings--limited evidence of effectiveness. BMC Health Serv Res. 2011;11:211. PubMed
22. Diaz-Montes TP, Cobb L, Ibeanu OA, Njoku P, Gerardi MA. Introduction of checklists at daily progress notes improves patient care among the gynecological oncology service. J Patient Saf. 2012;8(4):189-193. PubMed
23. Stetson PD, Bakken S, Wrenn JO, Siegler EL. Assessing Electronic Note Quality Using the Physician Documentation Quality Instrument (PDQI-9). Appl Clin Inform. 2012;3(2):164-174. PubMed
24. Friedberg MW, Chen PG, Van Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. Santa Monica, CA: RAND Corporation; 2013. PubMed
1. Herzig SJ, Guess JR, Feinbloom DB, et al. Improving appropriateness of acid-suppressive medication use via computerized clinical decision support. J Hosp Med. 2015;10(1):41-45. PubMed
2. Nguyen OK, Makam AN, Clark C, et al. Predicting all-cause readmissions using electronic health record data from the entire hospitalization: Model development and comparison. J Hosp Med. 2016;11(7):473-480. PubMed
3. Donati A, Gabbanelli V, Pantanetti S, et al. The impact of a clinical information system in an intensive care unit. J Clin Monit Comput. 2008;22(1):31-36. PubMed
4. Schiff GD, Bates DW. Can electronic clinical documentation help prevent diagnostic errors? N Engl J Med. 2010;362(12):1066-1069. PubMed
5. Hartzband P, Groopman J. Off the record--avoiding the pitfalls of going electronic. N Engl J Med. 2008;358(16):1656-1658. PubMed
6. Hirschtick RE. A piece of my mind. Copy-and-paste. JAMA. 2006;295(20):2335-2336. PubMed
7. Hirschtick RE. A piece of my mind. John Lennon’s elbow. JAMA. 2012;308(5):463-464. PubMed
8. O’Donnell HC, Kaushal R, Barrón Y, Callahan MA, Adelman RD, Siegler EL. Physicians’ attitudes towards copy and pasting in electronic note writing. J Gen Intern Med. 2009;24(1):63-68. PubMed
9. Mahapatra P, Ieong E. Improving Documentation and Communication Using Operative Note Proformas. BMJ Qual Improv Rep. 2016;5(1):u209122.w3712. PubMed
10. Thomson DR, Baldwin MJ, Bellini MI, Silva MA. Improving the quality of operative notes for laparoscopic cholecystectomy: Assessing the impact of a standardized operation note proforma. Int J Surg. 2016;27:17-20. PubMed
11. Dean SM, Eickhoff JC, Bakel LA. The effectiveness of a bundled intervention to improve resident progress notes in an electronic health record. J Hosp Med. 2015;10(2):104-107. PubMed
12. Aylor M, Campbell EM, Winter C, Phillipi CA. Resident Notes in an Electronic Health Record: A Mixed-Methods Study Using a Standardized Intervention With Qualitative Analysis. Clin Pediatr (Phila). 2016;6(3):257-262.
13. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. PubMed
14. Chi J, Kugler J, Chu IM, et al. Medical students and the electronic health record: ‘an epic use of time’. Am J Med. 2014;127(9):891-895. PubMed
15. Martin SA, Sinsky CA. The map is not the territory: medical records and 21st century practice. Lancet. 2016;388(10055):2053-2056. PubMed
16. Oxentenko AS, Manohar CU, McCoy CP, et al. Internal medicine residents’ computer use in the inpatient setting. J Grad Med Educ. 2012;4(4):529-532. PubMed
17. Mamykina L, Vawdrey DK, Hripcsak G. How Do Residents Spend Their Shift Time? A Time and Motion Study With a Particular Focus on the Use of Computers. Acad Med. 2016;91(6):827-832. PubMed
18. Chen L, Guo U, Illipparambil LC, et al. Racing Against the Clock: Internal Medicine Residents’ Time Spent On Electronic Health Records. J Grad Med Educ. 2016;8(1):39-44. PubMed
19. Kuhn T, Basch P, Barr M, Yackel T, Physicians MICotACo. Clinical documentation in the 21st century: executive summary of a policy position paper from the American College of Physicians. Ann Intern Med. 2015;162(4):301-303. PubMed
20. Treadwell JR, Lucas S, Tsou AY. Surgical checklists: a systematic review of impacts and implementation. BMJ Qual Saf. 2014;23(4):299-318. PubMed
21. Ko HC, Turner TJ, Finnigan MA. Systematic review of safety checklists for use by medical care teams in acute hospital settings--limited evidence of effectiveness. BMC Health Serv Res. 2011;11:211. PubMed
22. Diaz-Montes TP, Cobb L, Ibeanu OA, Njoku P, Gerardi MA. Introduction of checklists at daily progress notes improves patient care among the gynecological oncology service. J Patient Saf. 2012;8(4):189-193. PubMed
23. Stetson PD, Bakken S, Wrenn JO, Siegler EL. Assessing Electronic Note Quality Using the Physician Documentation Quality Instrument (PDQI-9). Appl Clin Inform. 2012;3(2):164-174. PubMed
24. Friedberg MW, Chen PG, Van Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. Santa Monica, CA: RAND Corporation; 2013. PubMed
© 2018 Society of Hospital Medicine
Forging ahead
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 45-year-old woman presented to the emergency department with 2 days of generalized, progressive weakness. Her ability to walk and perform daily chores was increasingly limited. On the morning of her presentation, she was unable to stand up without falling.
A complaint of weakness must be classified as either functional weakness related to a systemic process or true neurologic weakness from dysfunction of the central nervous system (eg, brain, spinal cord) or peripheral nervous system (eg, anterior horn cell, nerve, neuromuscular junction, or muscle). More information on her clinical course and a detailed neurologic exam will help clarify this key branch point.
She was 2 weeks status-post laparoscopic Roux-en-Y gastric bypass and gastric band removal performed in Europe. Immediately following surgery, she experienced abdominal discomfort and nausea with occasional nonbloody, nonbilious emesis, attributed to expected postoperative anatomical changes. She developed a postoperative pneumonia treated with amoxicillin-clavulanate. She tolerated her flight back to the United States, but her abdominal discomfort persisted and she had minimal oral intake due to her nausea.
Functional weakness may stem from hypovolemia from insufficient oral intake, anemia related to the recent surgery, electrolyte abnormalities, chronic nutritional issues associated with obesity and weight-reduction surgery, and pneumonia. Prolonged air travel, obesity, and recent surgery place her at risk for venous thromboembolism, which may manifest as reduced exercise tolerance. Nausea, vomiting, and abdominal pain persisting for 2 weeks after a Roux-en-Y gastric bypass surgery raises several concerns, including gastric remnant distension (although hiccups are often prominent); stomal stenosis, which typically presents several weeks after surgery; marginal ulceration; or infection at the surgical site or from an anastomotic leak. She may also have a surgery- or medication-related myopathy.
The patient had a history of obesity, hypertension, hyperlipidemia, migraine headaches, and nonalcoholic steatohepatitis. Four years previously, she had undergone gastric banding complicated by band migration and ulceration at the banding site. Her medications were amlodipine, losartan, ranitidine, acetaminophen, and nadroparin for venous thromboembolism prophylaxis during her flight. She denied alcohol, tobacco, or illicit drug use. On further questioning, she reported diaphoresis, mild dyspnea, loose stools, and a sensation of numbness and “heaviness” in her arms. Her abdominal pain was limited to the surgical incision and was controlled with acetaminophen. She denied fevers, cough, chest pain, diplopia, or dysphagia.
Heaviness in both arms could result from an acutely presenting myopathic or neuropathic process, while the coexistence of numbness suggests a sensorimotor polyneuropathy. Obesity and gastric bypass surgery increase her nutritional risk, and thiamine deficiency may present as an acute axonal polyneuropathy (ie, beriberi). Unlike vitamin B12 deficiency, which may take years to develop, thiamine deficiency can present within 4 weeks of gastric bypass surgery. Her dyspnea may be a manifestation of diaphragmatic weakness, although her ostensibly treated pneumonia or as of yet unproven postoperative anemia may be contributing. Chemoprophylaxis mitigates her risk of venous thromboembolism, which is, nonetheless, unlikely to account for the gastrointestinal symptoms and upper extremity weakness. If she is continuing to take amlodipine and losartan but has become volume-depleted, hypotension may be contributing to the generalized weakness.
Physical examination revealed an obese, pale and diaphoretic woman. Her temperature was 36.9°C, heart rate 77 beats per minute, blood pressure 158/90 mm Hg, respiratory rate 28 breaths per minute, and O2 saturation 99% on ambient air. She had no cervical lymphadenopathy and a normal thyroid exam. There were no murmurs on cardiac examination, and jugular venous pressure was estimated at 10 cm of water. Her lung sounds were clear. Her abdomen was soft, nondistended, with localized tenderness and fluctuance around the midline surgical incision with a small amount of purulent drainage. She was alert and oriented to name, date, place, and situation. Cranial nerves II through XII were grossly intact. Strength was 4/5 in bilateral biceps, triceps and distal hand and finger extensors, 3/5 in bilateral deltoids. Strength in hip flexors was 4/5 and it was 5/5 in distal lower extremities. Sensation was intact to pinprick in upper and lower extremities. Biceps reflexes were absent; patellar and ankle reflexes were 1+ and symmetric. The remainder of the physical exam was unremarkable.
The patient has symmetric proximal muscle weakness with upper extremity predominance and preserved strength in her distal lower extremities. A myopathy could explain this pattern of weakness, further substantiated by absent reflexes and reportedly intact sensation. Subacute causes of myopathy include hypokalemia, hyperkalemia, toxic myopathies from medications, or infection-induced rhabdomyolysis. However, she does not report muscle pain, and the loss of reflexes is faster than would be expected with a myopathy. A more thorough sensory examination would inform the assessment of potential neuropathic processes. Guillain-Barré syndrome (GBS) is possible; it most commonly presents as an ascending, distally predominant acute inflammatory demyelinating polyneuropathy (AIDP), although her upper extremity weakness predominates and there are no clear sensory changes. It remains to be determined how her wound infection might relate to her overall presentation.
Her white blood cell count was 12,600/μL (reference range: 3,400-10,000/μL), hemoglobin was 10.2 g/dL, and platelet count was 698,000/μL. Mean corpuscular volume was 86 fL. Serum chemistries were: sodium 138 mEq/L, potassium 3.8 mEq/L, chloride 106 mmol/L, bicarbonate 15 mmol/L, blood urea nitrogen 5 mg/dL, creatinine 0.65 mg/dL, glucose 125 mg/dL, calcium 8.3 mg/dL, magnesium 1.9 mg/dL, phosphorous 2.4 mg/dL, and lactate 1.8 mmol/L (normal: < 2.0 mmol/L). Creatinine kinase (CK), liver function tests, and coagulation panel were normal. Total protein was 6.4 g/dL, and albumin was 2.7 g/dL. Venous blood gas was: pH 7.39 and PCO2 25 mmHg. Urinalysis revealed ketones. Blood and wound cultures were sent for evaluation. A chest x-ray was unremarkable. An electrocardiogram showed normal sinus rhythm. Computed tomography (CT) of the abdomen and pelvis revealed a multiloculated rim-enhancing fluid collection in the anterior abdominal wall (Figure 1).
She does not have any notable electrolyte derangements that would account for her weakness, and the normal creatinine kinase lowers the probability of a myopathy and excludes rhabdomyolysis. Progression of weakness from proximal to distal muscles in a symmetric fashion is consistent with botulism, and she has an intra-abdominal wound infection that could be harboring Clostridium botulinum. Nonetheless, the normal cranial nerve exam and the rarity of botulism occurring with surgical wounds argue against this diagnosis. She should receive intravenous (IV) thiamine for the possibility of beriberi. A lumbar puncture should be performed to assess for albuminocytologic dissociation, which can be seen in patients with GBS.
The patient received high-dose IV thiamine, IV vancomycin, IV piperacillin-tazobactam, and acetaminophen. Over the subsequent 4 hours, her anion gap acidosis worsened. She declined arterial puncture. Repeat venous blood gas was: pH 7.22, PCO2 28 mmHg, and bicarbonate 11 mmol/L. Lactate and glucose were normal. Serum osmolarity was 292 mmol/kg (reference range: 283-301 mmol/kg). She was started on an IV sodium bicarbonate infusion without improvement in her acidemia.
An acute anion gap metabolic acidosis suggests a limited differential diagnosis that includes lactic acidosis, D-lactic acidosis, severe starvation ketoacidosis, acute renal failure, salicylate, or other drug or poison ingestion. Starvation ketoacidosis may be contributing, but a bicarbonate value this low would be unusual. There is no history of alcohol use or other ingestions, and the normal serum osmolality and low osmolal gap (less than 10 mOsm/kg) argue against a poisoning with ethanol, ethylene glycol, or methanol. The initial combined anion gap metabolic acidosis and respiratory alkalosis is consistent with salicylate toxicity, but she does not report aspirin ingestion. Acetaminophen use in the setting of malnutrition or starvation physiology raises the possibility of 5-oxoproline accumulation.
Routine serum lactate does not detect D-lactate, which is produced by colonic bacteria and has been reported in short bowel syndrome and following intestinal bypass surgery. This may occur weeks to months after intestinal procedures, following ingestion of a heavy carbohydrate load, and almost invariably presents with altered mental status and increased anion gap metabolic acidosis, although generalized weakness has been reported.
A surgical consultant drained her wound infection. Fluid Gram stain was negative. D-lactate, salicylate and acetaminophen levels were undetectable. Thiamine pyrophosphate level was 229 nmol/L (reference range: 78-185 nmol/L). Acetaminophen was discontinued and N-acetylcysteine infusion was started for possible 5-oxoprolinemia. Her anion gap acidosis rapidly improved. Twelve hours after admission, she reported sudden onset of blurry vision. Her vital signs were: temperature 37oC, heart rate 110 beats per minute, respiratory rate 40 breaths per minute, blood pressure 168/90, and oxygen saturation 100% on ambient air. Telemetry showed ventricular bigeminy. On examination, she was unable to abduct her right eye; muscle strength was 1/5 in all extremities; biceps, ankle, and patellar reflexes were absent.
Her neurological deficits have progressed over hours to near complete paralysis, asymmetric cranial nerve paresis, and areflexia. Although botulism can cause blurred vision and absent deep tendon reflexes, patients almost always have symmetrical bulbar findings followed by descending paralysis. Should the “numbness” in her arms reported earlier represent undetected sensory deficits, this, too would be inconsistent with botulism.
A diagnosis of GBS ties together several aspects of her presentation and clinical course. Several variants show different patterns of weakness and may involve cranial nerves. Her tachypnea and dyspnea are concerning signs of potential impending respiratory failure. The ventricular bigeminy and mild hypertension could represent autonomic dysfunction that is seen in many cases of GBS.
She was intubated for airway protection. Computed tomography angiography and magnetic resonance imaging of her brain were normal. Cerebral spinal fluid analysis obtained through lumbar puncture showed the following: white blood cell count 3/μL, red blood cell count 11/μL, protein 63 mg/dL (reference range: 15-60mg/dL), and glucose 128 mg/dL (reference range: 40-80mg/dL).
The lumbar puncture is consistent with GBS given the slightly elevated protein and cell count well below 50/μL. Given the severity of her symptoms, treatment with IV immunoglobulin or plasmapheresis should be initiated. Nerve conduction studies (NCS) and electromyography (EMG) are indicated for diagnostic confirmation.
EMG and NCS revealed a severe sensorimotor polyneuropathy with demyelinating features including a conduction block at a noncompressible site, consistent with AIDP. Left sural nerve biopsy confirmed acute demyelinating and mild axonal neuropathy (Figure 2). On hospital day 2, treatment with IV immunoglobulins (IVIG) was initiated; however, she developed anaphylaxis following her second administration and subsequently received plasmapheresis. A tracheostomy was performed for respiratory muscle weakness, and she was discharged to a nursing facility. C. botulinum cultures from the wound eventually returned negative. Following her hospitalization, a serum 5-oxoproline level sent 10 hours after admission returned as elevated, confirming the additional diagnosis of 5-oxoprolinemia. On follow-up, she can sit up and feed herself without assistance, and her gait continues to improve with physical therapy.
DISCUSSION
This patient presented with rapidly progressive weakness that developed in the 2 weeks following bariatric surgery. In the postsurgical setting, patient complaints of weakness are commonly encountered and can pose a diagnostic challenge. Asthenia (ie, general loss of strength or energy) is frequently reported in the immediate postoperative period, and may result from the stress of surgery, pain, deconditioning, or infection. This must be distinguished from true neurologic weakness, which results from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, or muscle. The initial history can help elucidate the inciting events such as preceding surgery, infections or ingestions, and can also categorize the pattern of weakness. The neurologic examination can localize the pathology within the neuraxis. EMG and NCS can distinguish neuropathy from radiculopathy, and categorize the process as axonal, demyelinating, or mixed. In this case, the oculomotor weakness, sensory abnormalities and areflexia signaled a severe sensorimotor polyneuropathy, and EMG/NCS confirmed a demyelinating process consistent with GBS.
Guillain-Barré syndrome is an acute, immune-mediated polyneuropathy. Patients with GBS often present with a preceding respiratory or diarrheal illness; however, the stress of a recent surgery can serve as an inciting event. The syndrome, acute postgastric reduction surgery (APGARS) neuropathy, was introduced in the literature in 2002, describing 3 patients who presented with progressive vomiting, weakness, and hyporeflexia following bariatric surgery.1 The term has been used to describe bariatric surgery patients who developed postoperative quadriparesis, cranial nerve deficits, and respiratory compromise.2 Given the clinical heterogeneity in the literature with relation to APGARS, it is probable that the cases described could result from multiple etiologies. While GBS is purely immune-mediated and can be precipitated by the stress of surgery itself, postbariatric surgery patients are susceptible to many nutritional deficiencies that can lead to similar presentations.3 For example, thiamine (vitamin B1) and cobalamin (vitamin B12) deficiencies cause distinct postbariatric surgery neuropathies.4 Thiamine deficiency may manifest weeks to months after surgery and can rapidly progress, whereas cobalamin deficiency generally develops over 3 to 5 years. Both of these syndromes demonstrate an axonal pattern of nerve injury on EMG/NCS, in contrast to the demyelinating pattern typically seen in GBS. In addition, bariatric surgery patients are at higher risk for copper deficiency, which usually presents as a myeloneuropathy with subacute gait decline and upper motor neuron signs including spasticity.
Although GBS classically presents with symmetric ascending weakness and sensory abnormalities, it may manifest in myriad ways. Factors influencing the presentation include the types of nerve fibers involved (motor, sensory, cranial or autonomic), the predominant mode of injury (axonal vs demyelinating), and the presence or absence of alteration in consciousness.5 The most common form of GBS is AIDP. The classic presentation involves paresthesias in the fingertips and toes followed by lower extremity weakness that ascends over hours to days to involve the arms and potentially the muscles of respiration. A minority of patients with GBS first experience weakness in the upper extremities or facial muscles, and oculomotor involvement is rare.5 Pain is common and often severe.6 Dysautonomia affects most patients with GBS and may manifest as labile blood pressure or arrhythmias.5 Several variant GBS presentation patterns have been described, including acute motor axonal neuropathy, a pure motor form of GBS; ophthalmoplegia, ataxia, and areflexia in Miller Fisher syndrome; and alteration in consciousness, hyperreflexia, ataxia, and ophthalmoparesis in Bickerstaff’s brain stem encephalitis.5
Patients with GBS can progress rapidly to respiratory failure. Serial neurologic exams may signal the diagnosis and inform triage to the appropriate level of care. Measurement of bedside pulmonary function, including mean inspiratory force and functional vital capacity, help to determine if there is weakness of diaphragmatic muscles. Patients with signs or symptoms of diaphragmatic weakness require monitoring in an intensive care unit and potentially early intubation. Treatment with IVIG or plasmapheresis has been found to hasten recovery from GBS, including earlier improvement in muscle strength and a reduced need for mechanical ventilation.7 Treatment selection is based on available resources as both modalities are felt to be equivalent.The majority of patients with GBS make a full recovery over a period of weeks to months, although many have persistent motor weakness. Despite immunotherapy, up to 20% of patients remain severely disabled and approximately 5% die.8 Advanced age, rapid progression of weakness over a period of less than 72 hours, need for mechanical ventilation, and absent compound muscle action potentials on NCS are all associated with prolonged and incomplete recovery.9
This patient developed respiratory failure within 12 hours of hospitalization, prior to being diagnosed with GBS. Even in that short time, the treating clinicians encountered a series of clinical diversions. The initial proximal pattern of muscle weakness suggested a possible myopathic process; the wound infection introduced the possibility of botulism; obesity and recent bariatric surgery triggered concern for thiamine deficiency; and the anion gap acidosis from 5-oxoprolinemia created yet another clinical detour. While the path from presentation to diagnosis is seldom a straight line, when faced with rapidly progressive weakness, it is paramount to forge ahead with an efficient diagnostic evaluation and timely therapeutic intervention.
KEY TEACHING POINTS
- A complaint of general weakness requires distinction between asthenia (ie, general loss of strength or energy) and true neuromuscular weakness from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, and/or muscle.
- Guillain-Barré syndrome may present in a variety of atypical fashions not limited to ascending, distally predominant weakness.
- Acute postgastric reduction surgery neuropathy should be considered in patients presenting with weakness, vomiting, or hyporeflexia after bariatric surgery.
- Acute inflammatory demyelinating polyneuropathy may rapidly progress to respiratory failure, and warrants serial neurologic examinations, monitoring of pulmonary function, and an expedited diagnostic evaluation.
Disclosure
Nothing to report.
1. Akhtar M, Collins MP, Kissel JT. Acute postgastric reduction surgery (APGARS) Neuropathy: A polynutritional, multisystem disorder. Neurology. 2002;58:A68. PubMed
2. Chang CG, Adams-Huet B, Provost DA. Acute post-gastric reduction surgery (APGARS) neuropathy. Obes Surg. 2004;14(2):182-189. PubMed
3. Chang CG, Helling TS, Black WE, Rymer MM. Weakness after gastric bypass. Obes Surg. 2002;12(4):592-597. PubMed
4. Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26(11-12):1031-1037. PubMed
5. Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31(2):491-510. PubMed
6. Ruts L, Drenthen J, Jongen JL, et al. Pain in Guillain-Barré syndrome: a long-term follow-up study. Neurology. 2010;75(16):1439-1447. PubMed
7. Hughes RAC, Wijdicks EFM, Barohn R, et al: Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;61:736-740. PubMed
8. Hughes RA, Swan AV, Raphaël JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barré syndrome: a systematic review. Brain. 2007;130(Pt 9):2245-2257. PubMed
9. Rajabally YA, Uncini A. Outcome and predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 2012;83(7):711-718. PubMed
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 45-year-old woman presented to the emergency department with 2 days of generalized, progressive weakness. Her ability to walk and perform daily chores was increasingly limited. On the morning of her presentation, she was unable to stand up without falling.
A complaint of weakness must be classified as either functional weakness related to a systemic process or true neurologic weakness from dysfunction of the central nervous system (eg, brain, spinal cord) or peripheral nervous system (eg, anterior horn cell, nerve, neuromuscular junction, or muscle). More information on her clinical course and a detailed neurologic exam will help clarify this key branch point.
She was 2 weeks status-post laparoscopic Roux-en-Y gastric bypass and gastric band removal performed in Europe. Immediately following surgery, she experienced abdominal discomfort and nausea with occasional nonbloody, nonbilious emesis, attributed to expected postoperative anatomical changes. She developed a postoperative pneumonia treated with amoxicillin-clavulanate. She tolerated her flight back to the United States, but her abdominal discomfort persisted and she had minimal oral intake due to her nausea.
Functional weakness may stem from hypovolemia from insufficient oral intake, anemia related to the recent surgery, electrolyte abnormalities, chronic nutritional issues associated with obesity and weight-reduction surgery, and pneumonia. Prolonged air travel, obesity, and recent surgery place her at risk for venous thromboembolism, which may manifest as reduced exercise tolerance. Nausea, vomiting, and abdominal pain persisting for 2 weeks after a Roux-en-Y gastric bypass surgery raises several concerns, including gastric remnant distension (although hiccups are often prominent); stomal stenosis, which typically presents several weeks after surgery; marginal ulceration; or infection at the surgical site or from an anastomotic leak. She may also have a surgery- or medication-related myopathy.
The patient had a history of obesity, hypertension, hyperlipidemia, migraine headaches, and nonalcoholic steatohepatitis. Four years previously, she had undergone gastric banding complicated by band migration and ulceration at the banding site. Her medications were amlodipine, losartan, ranitidine, acetaminophen, and nadroparin for venous thromboembolism prophylaxis during her flight. She denied alcohol, tobacco, or illicit drug use. On further questioning, she reported diaphoresis, mild dyspnea, loose stools, and a sensation of numbness and “heaviness” in her arms. Her abdominal pain was limited to the surgical incision and was controlled with acetaminophen. She denied fevers, cough, chest pain, diplopia, or dysphagia.
Heaviness in both arms could result from an acutely presenting myopathic or neuropathic process, while the coexistence of numbness suggests a sensorimotor polyneuropathy. Obesity and gastric bypass surgery increase her nutritional risk, and thiamine deficiency may present as an acute axonal polyneuropathy (ie, beriberi). Unlike vitamin B12 deficiency, which may take years to develop, thiamine deficiency can present within 4 weeks of gastric bypass surgery. Her dyspnea may be a manifestation of diaphragmatic weakness, although her ostensibly treated pneumonia or as of yet unproven postoperative anemia may be contributing. Chemoprophylaxis mitigates her risk of venous thromboembolism, which is, nonetheless, unlikely to account for the gastrointestinal symptoms and upper extremity weakness. If she is continuing to take amlodipine and losartan but has become volume-depleted, hypotension may be contributing to the generalized weakness.
Physical examination revealed an obese, pale and diaphoretic woman. Her temperature was 36.9°C, heart rate 77 beats per minute, blood pressure 158/90 mm Hg, respiratory rate 28 breaths per minute, and O2 saturation 99% on ambient air. She had no cervical lymphadenopathy and a normal thyroid exam. There were no murmurs on cardiac examination, and jugular venous pressure was estimated at 10 cm of water. Her lung sounds were clear. Her abdomen was soft, nondistended, with localized tenderness and fluctuance around the midline surgical incision with a small amount of purulent drainage. She was alert and oriented to name, date, place, and situation. Cranial nerves II through XII were grossly intact. Strength was 4/5 in bilateral biceps, triceps and distal hand and finger extensors, 3/5 in bilateral deltoids. Strength in hip flexors was 4/5 and it was 5/5 in distal lower extremities. Sensation was intact to pinprick in upper and lower extremities. Biceps reflexes were absent; patellar and ankle reflexes were 1+ and symmetric. The remainder of the physical exam was unremarkable.
The patient has symmetric proximal muscle weakness with upper extremity predominance and preserved strength in her distal lower extremities. A myopathy could explain this pattern of weakness, further substantiated by absent reflexes and reportedly intact sensation. Subacute causes of myopathy include hypokalemia, hyperkalemia, toxic myopathies from medications, or infection-induced rhabdomyolysis. However, she does not report muscle pain, and the loss of reflexes is faster than would be expected with a myopathy. A more thorough sensory examination would inform the assessment of potential neuropathic processes. Guillain-Barré syndrome (GBS) is possible; it most commonly presents as an ascending, distally predominant acute inflammatory demyelinating polyneuropathy (AIDP), although her upper extremity weakness predominates and there are no clear sensory changes. It remains to be determined how her wound infection might relate to her overall presentation.
Her white blood cell count was 12,600/μL (reference range: 3,400-10,000/μL), hemoglobin was 10.2 g/dL, and platelet count was 698,000/μL. Mean corpuscular volume was 86 fL. Serum chemistries were: sodium 138 mEq/L, potassium 3.8 mEq/L, chloride 106 mmol/L, bicarbonate 15 mmol/L, blood urea nitrogen 5 mg/dL, creatinine 0.65 mg/dL, glucose 125 mg/dL, calcium 8.3 mg/dL, magnesium 1.9 mg/dL, phosphorous 2.4 mg/dL, and lactate 1.8 mmol/L (normal: < 2.0 mmol/L). Creatinine kinase (CK), liver function tests, and coagulation panel were normal. Total protein was 6.4 g/dL, and albumin was 2.7 g/dL. Venous blood gas was: pH 7.39 and PCO2 25 mmHg. Urinalysis revealed ketones. Blood and wound cultures were sent for evaluation. A chest x-ray was unremarkable. An electrocardiogram showed normal sinus rhythm. Computed tomography (CT) of the abdomen and pelvis revealed a multiloculated rim-enhancing fluid collection in the anterior abdominal wall (Figure 1).
She does not have any notable electrolyte derangements that would account for her weakness, and the normal creatinine kinase lowers the probability of a myopathy and excludes rhabdomyolysis. Progression of weakness from proximal to distal muscles in a symmetric fashion is consistent with botulism, and she has an intra-abdominal wound infection that could be harboring Clostridium botulinum. Nonetheless, the normal cranial nerve exam and the rarity of botulism occurring with surgical wounds argue against this diagnosis. She should receive intravenous (IV) thiamine for the possibility of beriberi. A lumbar puncture should be performed to assess for albuminocytologic dissociation, which can be seen in patients with GBS.
The patient received high-dose IV thiamine, IV vancomycin, IV piperacillin-tazobactam, and acetaminophen. Over the subsequent 4 hours, her anion gap acidosis worsened. She declined arterial puncture. Repeat venous blood gas was: pH 7.22, PCO2 28 mmHg, and bicarbonate 11 mmol/L. Lactate and glucose were normal. Serum osmolarity was 292 mmol/kg (reference range: 283-301 mmol/kg). She was started on an IV sodium bicarbonate infusion without improvement in her acidemia.
An acute anion gap metabolic acidosis suggests a limited differential diagnosis that includes lactic acidosis, D-lactic acidosis, severe starvation ketoacidosis, acute renal failure, salicylate, or other drug or poison ingestion. Starvation ketoacidosis may be contributing, but a bicarbonate value this low would be unusual. There is no history of alcohol use or other ingestions, and the normal serum osmolality and low osmolal gap (less than 10 mOsm/kg) argue against a poisoning with ethanol, ethylene glycol, or methanol. The initial combined anion gap metabolic acidosis and respiratory alkalosis is consistent with salicylate toxicity, but she does not report aspirin ingestion. Acetaminophen use in the setting of malnutrition or starvation physiology raises the possibility of 5-oxoproline accumulation.
Routine serum lactate does not detect D-lactate, which is produced by colonic bacteria and has been reported in short bowel syndrome and following intestinal bypass surgery. This may occur weeks to months after intestinal procedures, following ingestion of a heavy carbohydrate load, and almost invariably presents with altered mental status and increased anion gap metabolic acidosis, although generalized weakness has been reported.
A surgical consultant drained her wound infection. Fluid Gram stain was negative. D-lactate, salicylate and acetaminophen levels were undetectable. Thiamine pyrophosphate level was 229 nmol/L (reference range: 78-185 nmol/L). Acetaminophen was discontinued and N-acetylcysteine infusion was started for possible 5-oxoprolinemia. Her anion gap acidosis rapidly improved. Twelve hours after admission, she reported sudden onset of blurry vision. Her vital signs were: temperature 37oC, heart rate 110 beats per minute, respiratory rate 40 breaths per minute, blood pressure 168/90, and oxygen saturation 100% on ambient air. Telemetry showed ventricular bigeminy. On examination, she was unable to abduct her right eye; muscle strength was 1/5 in all extremities; biceps, ankle, and patellar reflexes were absent.
Her neurological deficits have progressed over hours to near complete paralysis, asymmetric cranial nerve paresis, and areflexia. Although botulism can cause blurred vision and absent deep tendon reflexes, patients almost always have symmetrical bulbar findings followed by descending paralysis. Should the “numbness” in her arms reported earlier represent undetected sensory deficits, this, too would be inconsistent with botulism.
A diagnosis of GBS ties together several aspects of her presentation and clinical course. Several variants show different patterns of weakness and may involve cranial nerves. Her tachypnea and dyspnea are concerning signs of potential impending respiratory failure. The ventricular bigeminy and mild hypertension could represent autonomic dysfunction that is seen in many cases of GBS.
She was intubated for airway protection. Computed tomography angiography and magnetic resonance imaging of her brain were normal. Cerebral spinal fluid analysis obtained through lumbar puncture showed the following: white blood cell count 3/μL, red blood cell count 11/μL, protein 63 mg/dL (reference range: 15-60mg/dL), and glucose 128 mg/dL (reference range: 40-80mg/dL).
The lumbar puncture is consistent with GBS given the slightly elevated protein and cell count well below 50/μL. Given the severity of her symptoms, treatment with IV immunoglobulin or plasmapheresis should be initiated. Nerve conduction studies (NCS) and electromyography (EMG) are indicated for diagnostic confirmation.
EMG and NCS revealed a severe sensorimotor polyneuropathy with demyelinating features including a conduction block at a noncompressible site, consistent with AIDP. Left sural nerve biopsy confirmed acute demyelinating and mild axonal neuropathy (Figure 2). On hospital day 2, treatment with IV immunoglobulins (IVIG) was initiated; however, she developed anaphylaxis following her second administration and subsequently received plasmapheresis. A tracheostomy was performed for respiratory muscle weakness, and she was discharged to a nursing facility. C. botulinum cultures from the wound eventually returned negative. Following her hospitalization, a serum 5-oxoproline level sent 10 hours after admission returned as elevated, confirming the additional diagnosis of 5-oxoprolinemia. On follow-up, she can sit up and feed herself without assistance, and her gait continues to improve with physical therapy.
DISCUSSION
This patient presented with rapidly progressive weakness that developed in the 2 weeks following bariatric surgery. In the postsurgical setting, patient complaints of weakness are commonly encountered and can pose a diagnostic challenge. Asthenia (ie, general loss of strength or energy) is frequently reported in the immediate postoperative period, and may result from the stress of surgery, pain, deconditioning, or infection. This must be distinguished from true neurologic weakness, which results from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, or muscle. The initial history can help elucidate the inciting events such as preceding surgery, infections or ingestions, and can also categorize the pattern of weakness. The neurologic examination can localize the pathology within the neuraxis. EMG and NCS can distinguish neuropathy from radiculopathy, and categorize the process as axonal, demyelinating, or mixed. In this case, the oculomotor weakness, sensory abnormalities and areflexia signaled a severe sensorimotor polyneuropathy, and EMG/NCS confirmed a demyelinating process consistent with GBS.
Guillain-Barré syndrome is an acute, immune-mediated polyneuropathy. Patients with GBS often present with a preceding respiratory or diarrheal illness; however, the stress of a recent surgery can serve as an inciting event. The syndrome, acute postgastric reduction surgery (APGARS) neuropathy, was introduced in the literature in 2002, describing 3 patients who presented with progressive vomiting, weakness, and hyporeflexia following bariatric surgery.1 The term has been used to describe bariatric surgery patients who developed postoperative quadriparesis, cranial nerve deficits, and respiratory compromise.2 Given the clinical heterogeneity in the literature with relation to APGARS, it is probable that the cases described could result from multiple etiologies. While GBS is purely immune-mediated and can be precipitated by the stress of surgery itself, postbariatric surgery patients are susceptible to many nutritional deficiencies that can lead to similar presentations.3 For example, thiamine (vitamin B1) and cobalamin (vitamin B12) deficiencies cause distinct postbariatric surgery neuropathies.4 Thiamine deficiency may manifest weeks to months after surgery and can rapidly progress, whereas cobalamin deficiency generally develops over 3 to 5 years. Both of these syndromes demonstrate an axonal pattern of nerve injury on EMG/NCS, in contrast to the demyelinating pattern typically seen in GBS. In addition, bariatric surgery patients are at higher risk for copper deficiency, which usually presents as a myeloneuropathy with subacute gait decline and upper motor neuron signs including spasticity.
Although GBS classically presents with symmetric ascending weakness and sensory abnormalities, it may manifest in myriad ways. Factors influencing the presentation include the types of nerve fibers involved (motor, sensory, cranial or autonomic), the predominant mode of injury (axonal vs demyelinating), and the presence or absence of alteration in consciousness.5 The most common form of GBS is AIDP. The classic presentation involves paresthesias in the fingertips and toes followed by lower extremity weakness that ascends over hours to days to involve the arms and potentially the muscles of respiration. A minority of patients with GBS first experience weakness in the upper extremities or facial muscles, and oculomotor involvement is rare.5 Pain is common and often severe.6 Dysautonomia affects most patients with GBS and may manifest as labile blood pressure or arrhythmias.5 Several variant GBS presentation patterns have been described, including acute motor axonal neuropathy, a pure motor form of GBS; ophthalmoplegia, ataxia, and areflexia in Miller Fisher syndrome; and alteration in consciousness, hyperreflexia, ataxia, and ophthalmoparesis in Bickerstaff’s brain stem encephalitis.5
Patients with GBS can progress rapidly to respiratory failure. Serial neurologic exams may signal the diagnosis and inform triage to the appropriate level of care. Measurement of bedside pulmonary function, including mean inspiratory force and functional vital capacity, help to determine if there is weakness of diaphragmatic muscles. Patients with signs or symptoms of diaphragmatic weakness require monitoring in an intensive care unit and potentially early intubation. Treatment with IVIG or plasmapheresis has been found to hasten recovery from GBS, including earlier improvement in muscle strength and a reduced need for mechanical ventilation.7 Treatment selection is based on available resources as both modalities are felt to be equivalent.The majority of patients with GBS make a full recovery over a period of weeks to months, although many have persistent motor weakness. Despite immunotherapy, up to 20% of patients remain severely disabled and approximately 5% die.8 Advanced age, rapid progression of weakness over a period of less than 72 hours, need for mechanical ventilation, and absent compound muscle action potentials on NCS are all associated with prolonged and incomplete recovery.9
This patient developed respiratory failure within 12 hours of hospitalization, prior to being diagnosed with GBS. Even in that short time, the treating clinicians encountered a series of clinical diversions. The initial proximal pattern of muscle weakness suggested a possible myopathic process; the wound infection introduced the possibility of botulism; obesity and recent bariatric surgery triggered concern for thiamine deficiency; and the anion gap acidosis from 5-oxoprolinemia created yet another clinical detour. While the path from presentation to diagnosis is seldom a straight line, when faced with rapidly progressive weakness, it is paramount to forge ahead with an efficient diagnostic evaluation and timely therapeutic intervention.
KEY TEACHING POINTS
- A complaint of general weakness requires distinction between asthenia (ie, general loss of strength or energy) and true neuromuscular weakness from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, and/or muscle.
- Guillain-Barré syndrome may present in a variety of atypical fashions not limited to ascending, distally predominant weakness.
- Acute postgastric reduction surgery neuropathy should be considered in patients presenting with weakness, vomiting, or hyporeflexia after bariatric surgery.
- Acute inflammatory demyelinating polyneuropathy may rapidly progress to respiratory failure, and warrants serial neurologic examinations, monitoring of pulmonary function, and an expedited diagnostic evaluation.
Disclosure
Nothing to report.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 45-year-old woman presented to the emergency department with 2 days of generalized, progressive weakness. Her ability to walk and perform daily chores was increasingly limited. On the morning of her presentation, she was unable to stand up without falling.
A complaint of weakness must be classified as either functional weakness related to a systemic process or true neurologic weakness from dysfunction of the central nervous system (eg, brain, spinal cord) or peripheral nervous system (eg, anterior horn cell, nerve, neuromuscular junction, or muscle). More information on her clinical course and a detailed neurologic exam will help clarify this key branch point.
She was 2 weeks status-post laparoscopic Roux-en-Y gastric bypass and gastric band removal performed in Europe. Immediately following surgery, she experienced abdominal discomfort and nausea with occasional nonbloody, nonbilious emesis, attributed to expected postoperative anatomical changes. She developed a postoperative pneumonia treated with amoxicillin-clavulanate. She tolerated her flight back to the United States, but her abdominal discomfort persisted and she had minimal oral intake due to her nausea.
Functional weakness may stem from hypovolemia from insufficient oral intake, anemia related to the recent surgery, electrolyte abnormalities, chronic nutritional issues associated with obesity and weight-reduction surgery, and pneumonia. Prolonged air travel, obesity, and recent surgery place her at risk for venous thromboembolism, which may manifest as reduced exercise tolerance. Nausea, vomiting, and abdominal pain persisting for 2 weeks after a Roux-en-Y gastric bypass surgery raises several concerns, including gastric remnant distension (although hiccups are often prominent); stomal stenosis, which typically presents several weeks after surgery; marginal ulceration; or infection at the surgical site or from an anastomotic leak. She may also have a surgery- or medication-related myopathy.
The patient had a history of obesity, hypertension, hyperlipidemia, migraine headaches, and nonalcoholic steatohepatitis. Four years previously, she had undergone gastric banding complicated by band migration and ulceration at the banding site. Her medications were amlodipine, losartan, ranitidine, acetaminophen, and nadroparin for venous thromboembolism prophylaxis during her flight. She denied alcohol, tobacco, or illicit drug use. On further questioning, she reported diaphoresis, mild dyspnea, loose stools, and a sensation of numbness and “heaviness” in her arms. Her abdominal pain was limited to the surgical incision and was controlled with acetaminophen. She denied fevers, cough, chest pain, diplopia, or dysphagia.
Heaviness in both arms could result from an acutely presenting myopathic or neuropathic process, while the coexistence of numbness suggests a sensorimotor polyneuropathy. Obesity and gastric bypass surgery increase her nutritional risk, and thiamine deficiency may present as an acute axonal polyneuropathy (ie, beriberi). Unlike vitamin B12 deficiency, which may take years to develop, thiamine deficiency can present within 4 weeks of gastric bypass surgery. Her dyspnea may be a manifestation of diaphragmatic weakness, although her ostensibly treated pneumonia or as of yet unproven postoperative anemia may be contributing. Chemoprophylaxis mitigates her risk of venous thromboembolism, which is, nonetheless, unlikely to account for the gastrointestinal symptoms and upper extremity weakness. If she is continuing to take amlodipine and losartan but has become volume-depleted, hypotension may be contributing to the generalized weakness.
Physical examination revealed an obese, pale and diaphoretic woman. Her temperature was 36.9°C, heart rate 77 beats per minute, blood pressure 158/90 mm Hg, respiratory rate 28 breaths per minute, and O2 saturation 99% on ambient air. She had no cervical lymphadenopathy and a normal thyroid exam. There were no murmurs on cardiac examination, and jugular venous pressure was estimated at 10 cm of water. Her lung sounds were clear. Her abdomen was soft, nondistended, with localized tenderness and fluctuance around the midline surgical incision with a small amount of purulent drainage. She was alert and oriented to name, date, place, and situation. Cranial nerves II through XII were grossly intact. Strength was 4/5 in bilateral biceps, triceps and distal hand and finger extensors, 3/5 in bilateral deltoids. Strength in hip flexors was 4/5 and it was 5/5 in distal lower extremities. Sensation was intact to pinprick in upper and lower extremities. Biceps reflexes were absent; patellar and ankle reflexes were 1+ and symmetric. The remainder of the physical exam was unremarkable.
The patient has symmetric proximal muscle weakness with upper extremity predominance and preserved strength in her distal lower extremities. A myopathy could explain this pattern of weakness, further substantiated by absent reflexes and reportedly intact sensation. Subacute causes of myopathy include hypokalemia, hyperkalemia, toxic myopathies from medications, or infection-induced rhabdomyolysis. However, she does not report muscle pain, and the loss of reflexes is faster than would be expected with a myopathy. A more thorough sensory examination would inform the assessment of potential neuropathic processes. Guillain-Barré syndrome (GBS) is possible; it most commonly presents as an ascending, distally predominant acute inflammatory demyelinating polyneuropathy (AIDP), although her upper extremity weakness predominates and there are no clear sensory changes. It remains to be determined how her wound infection might relate to her overall presentation.
Her white blood cell count was 12,600/μL (reference range: 3,400-10,000/μL), hemoglobin was 10.2 g/dL, and platelet count was 698,000/μL. Mean corpuscular volume was 86 fL. Serum chemistries were: sodium 138 mEq/L, potassium 3.8 mEq/L, chloride 106 mmol/L, bicarbonate 15 mmol/L, blood urea nitrogen 5 mg/dL, creatinine 0.65 mg/dL, glucose 125 mg/dL, calcium 8.3 mg/dL, magnesium 1.9 mg/dL, phosphorous 2.4 mg/dL, and lactate 1.8 mmol/L (normal: < 2.0 mmol/L). Creatinine kinase (CK), liver function tests, and coagulation panel were normal. Total protein was 6.4 g/dL, and albumin was 2.7 g/dL. Venous blood gas was: pH 7.39 and PCO2 25 mmHg. Urinalysis revealed ketones. Blood and wound cultures were sent for evaluation. A chest x-ray was unremarkable. An electrocardiogram showed normal sinus rhythm. Computed tomography (CT) of the abdomen and pelvis revealed a multiloculated rim-enhancing fluid collection in the anterior abdominal wall (Figure 1).
She does not have any notable electrolyte derangements that would account for her weakness, and the normal creatinine kinase lowers the probability of a myopathy and excludes rhabdomyolysis. Progression of weakness from proximal to distal muscles in a symmetric fashion is consistent with botulism, and she has an intra-abdominal wound infection that could be harboring Clostridium botulinum. Nonetheless, the normal cranial nerve exam and the rarity of botulism occurring with surgical wounds argue against this diagnosis. She should receive intravenous (IV) thiamine for the possibility of beriberi. A lumbar puncture should be performed to assess for albuminocytologic dissociation, which can be seen in patients with GBS.
The patient received high-dose IV thiamine, IV vancomycin, IV piperacillin-tazobactam, and acetaminophen. Over the subsequent 4 hours, her anion gap acidosis worsened. She declined arterial puncture. Repeat venous blood gas was: pH 7.22, PCO2 28 mmHg, and bicarbonate 11 mmol/L. Lactate and glucose were normal. Serum osmolarity was 292 mmol/kg (reference range: 283-301 mmol/kg). She was started on an IV sodium bicarbonate infusion without improvement in her acidemia.
An acute anion gap metabolic acidosis suggests a limited differential diagnosis that includes lactic acidosis, D-lactic acidosis, severe starvation ketoacidosis, acute renal failure, salicylate, or other drug or poison ingestion. Starvation ketoacidosis may be contributing, but a bicarbonate value this low would be unusual. There is no history of alcohol use or other ingestions, and the normal serum osmolality and low osmolal gap (less than 10 mOsm/kg) argue against a poisoning with ethanol, ethylene glycol, or methanol. The initial combined anion gap metabolic acidosis and respiratory alkalosis is consistent with salicylate toxicity, but she does not report aspirin ingestion. Acetaminophen use in the setting of malnutrition or starvation physiology raises the possibility of 5-oxoproline accumulation.
Routine serum lactate does not detect D-lactate, which is produced by colonic bacteria and has been reported in short bowel syndrome and following intestinal bypass surgery. This may occur weeks to months after intestinal procedures, following ingestion of a heavy carbohydrate load, and almost invariably presents with altered mental status and increased anion gap metabolic acidosis, although generalized weakness has been reported.
A surgical consultant drained her wound infection. Fluid Gram stain was negative. D-lactate, salicylate and acetaminophen levels were undetectable. Thiamine pyrophosphate level was 229 nmol/L (reference range: 78-185 nmol/L). Acetaminophen was discontinued and N-acetylcysteine infusion was started for possible 5-oxoprolinemia. Her anion gap acidosis rapidly improved. Twelve hours after admission, she reported sudden onset of blurry vision. Her vital signs were: temperature 37oC, heart rate 110 beats per minute, respiratory rate 40 breaths per minute, blood pressure 168/90, and oxygen saturation 100% on ambient air. Telemetry showed ventricular bigeminy. On examination, she was unable to abduct her right eye; muscle strength was 1/5 in all extremities; biceps, ankle, and patellar reflexes were absent.
Her neurological deficits have progressed over hours to near complete paralysis, asymmetric cranial nerve paresis, and areflexia. Although botulism can cause blurred vision and absent deep tendon reflexes, patients almost always have symmetrical bulbar findings followed by descending paralysis. Should the “numbness” in her arms reported earlier represent undetected sensory deficits, this, too would be inconsistent with botulism.
A diagnosis of GBS ties together several aspects of her presentation and clinical course. Several variants show different patterns of weakness and may involve cranial nerves. Her tachypnea and dyspnea are concerning signs of potential impending respiratory failure. The ventricular bigeminy and mild hypertension could represent autonomic dysfunction that is seen in many cases of GBS.
She was intubated for airway protection. Computed tomography angiography and magnetic resonance imaging of her brain were normal. Cerebral spinal fluid analysis obtained through lumbar puncture showed the following: white blood cell count 3/μL, red blood cell count 11/μL, protein 63 mg/dL (reference range: 15-60mg/dL), and glucose 128 mg/dL (reference range: 40-80mg/dL).
The lumbar puncture is consistent with GBS given the slightly elevated protein and cell count well below 50/μL. Given the severity of her symptoms, treatment with IV immunoglobulin or plasmapheresis should be initiated. Nerve conduction studies (NCS) and electromyography (EMG) are indicated for diagnostic confirmation.
EMG and NCS revealed a severe sensorimotor polyneuropathy with demyelinating features including a conduction block at a noncompressible site, consistent with AIDP. Left sural nerve biopsy confirmed acute demyelinating and mild axonal neuropathy (Figure 2). On hospital day 2, treatment with IV immunoglobulins (IVIG) was initiated; however, she developed anaphylaxis following her second administration and subsequently received plasmapheresis. A tracheostomy was performed for respiratory muscle weakness, and she was discharged to a nursing facility. C. botulinum cultures from the wound eventually returned negative. Following her hospitalization, a serum 5-oxoproline level sent 10 hours after admission returned as elevated, confirming the additional diagnosis of 5-oxoprolinemia. On follow-up, she can sit up and feed herself without assistance, and her gait continues to improve with physical therapy.
DISCUSSION
This patient presented with rapidly progressive weakness that developed in the 2 weeks following bariatric surgery. In the postsurgical setting, patient complaints of weakness are commonly encountered and can pose a diagnostic challenge. Asthenia (ie, general loss of strength or energy) is frequently reported in the immediate postoperative period, and may result from the stress of surgery, pain, deconditioning, or infection. This must be distinguished from true neurologic weakness, which results from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, or muscle. The initial history can help elucidate the inciting events such as preceding surgery, infections or ingestions, and can also categorize the pattern of weakness. The neurologic examination can localize the pathology within the neuraxis. EMG and NCS can distinguish neuropathy from radiculopathy, and categorize the process as axonal, demyelinating, or mixed. In this case, the oculomotor weakness, sensory abnormalities and areflexia signaled a severe sensorimotor polyneuropathy, and EMG/NCS confirmed a demyelinating process consistent with GBS.
Guillain-Barré syndrome is an acute, immune-mediated polyneuropathy. Patients with GBS often present with a preceding respiratory or diarrheal illness; however, the stress of a recent surgery can serve as an inciting event. The syndrome, acute postgastric reduction surgery (APGARS) neuropathy, was introduced in the literature in 2002, describing 3 patients who presented with progressive vomiting, weakness, and hyporeflexia following bariatric surgery.1 The term has been used to describe bariatric surgery patients who developed postoperative quadriparesis, cranial nerve deficits, and respiratory compromise.2 Given the clinical heterogeneity in the literature with relation to APGARS, it is probable that the cases described could result from multiple etiologies. While GBS is purely immune-mediated and can be precipitated by the stress of surgery itself, postbariatric surgery patients are susceptible to many nutritional deficiencies that can lead to similar presentations.3 For example, thiamine (vitamin B1) and cobalamin (vitamin B12) deficiencies cause distinct postbariatric surgery neuropathies.4 Thiamine deficiency may manifest weeks to months after surgery and can rapidly progress, whereas cobalamin deficiency generally develops over 3 to 5 years. Both of these syndromes demonstrate an axonal pattern of nerve injury on EMG/NCS, in contrast to the demyelinating pattern typically seen in GBS. In addition, bariatric surgery patients are at higher risk for copper deficiency, which usually presents as a myeloneuropathy with subacute gait decline and upper motor neuron signs including spasticity.
Although GBS classically presents with symmetric ascending weakness and sensory abnormalities, it may manifest in myriad ways. Factors influencing the presentation include the types of nerve fibers involved (motor, sensory, cranial or autonomic), the predominant mode of injury (axonal vs demyelinating), and the presence or absence of alteration in consciousness.5 The most common form of GBS is AIDP. The classic presentation involves paresthesias in the fingertips and toes followed by lower extremity weakness that ascends over hours to days to involve the arms and potentially the muscles of respiration. A minority of patients with GBS first experience weakness in the upper extremities or facial muscles, and oculomotor involvement is rare.5 Pain is common and often severe.6 Dysautonomia affects most patients with GBS and may manifest as labile blood pressure or arrhythmias.5 Several variant GBS presentation patterns have been described, including acute motor axonal neuropathy, a pure motor form of GBS; ophthalmoplegia, ataxia, and areflexia in Miller Fisher syndrome; and alteration in consciousness, hyperreflexia, ataxia, and ophthalmoparesis in Bickerstaff’s brain stem encephalitis.5
Patients with GBS can progress rapidly to respiratory failure. Serial neurologic exams may signal the diagnosis and inform triage to the appropriate level of care. Measurement of bedside pulmonary function, including mean inspiratory force and functional vital capacity, help to determine if there is weakness of diaphragmatic muscles. Patients with signs or symptoms of diaphragmatic weakness require monitoring in an intensive care unit and potentially early intubation. Treatment with IVIG or plasmapheresis has been found to hasten recovery from GBS, including earlier improvement in muscle strength and a reduced need for mechanical ventilation.7 Treatment selection is based on available resources as both modalities are felt to be equivalent.The majority of patients with GBS make a full recovery over a period of weeks to months, although many have persistent motor weakness. Despite immunotherapy, up to 20% of patients remain severely disabled and approximately 5% die.8 Advanced age, rapid progression of weakness over a period of less than 72 hours, need for mechanical ventilation, and absent compound muscle action potentials on NCS are all associated with prolonged and incomplete recovery.9
This patient developed respiratory failure within 12 hours of hospitalization, prior to being diagnosed with GBS. Even in that short time, the treating clinicians encountered a series of clinical diversions. The initial proximal pattern of muscle weakness suggested a possible myopathic process; the wound infection introduced the possibility of botulism; obesity and recent bariatric surgery triggered concern for thiamine deficiency; and the anion gap acidosis from 5-oxoprolinemia created yet another clinical detour. While the path from presentation to diagnosis is seldom a straight line, when faced with rapidly progressive weakness, it is paramount to forge ahead with an efficient diagnostic evaluation and timely therapeutic intervention.
KEY TEACHING POINTS
- A complaint of general weakness requires distinction between asthenia (ie, general loss of strength or energy) and true neuromuscular weakness from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, and/or muscle.
- Guillain-Barré syndrome may present in a variety of atypical fashions not limited to ascending, distally predominant weakness.
- Acute postgastric reduction surgery neuropathy should be considered in patients presenting with weakness, vomiting, or hyporeflexia after bariatric surgery.
- Acute inflammatory demyelinating polyneuropathy may rapidly progress to respiratory failure, and warrants serial neurologic examinations, monitoring of pulmonary function, and an expedited diagnostic evaluation.
Disclosure
Nothing to report.
1. Akhtar M, Collins MP, Kissel JT. Acute postgastric reduction surgery (APGARS) Neuropathy: A polynutritional, multisystem disorder. Neurology. 2002;58:A68. PubMed
2. Chang CG, Adams-Huet B, Provost DA. Acute post-gastric reduction surgery (APGARS) neuropathy. Obes Surg. 2004;14(2):182-189. PubMed
3. Chang CG, Helling TS, Black WE, Rymer MM. Weakness after gastric bypass. Obes Surg. 2002;12(4):592-597. PubMed
4. Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26(11-12):1031-1037. PubMed
5. Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31(2):491-510. PubMed
6. Ruts L, Drenthen J, Jongen JL, et al. Pain in Guillain-Barré syndrome: a long-term follow-up study. Neurology. 2010;75(16):1439-1447. PubMed
7. Hughes RAC, Wijdicks EFM, Barohn R, et al: Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;61:736-740. PubMed
8. Hughes RA, Swan AV, Raphaël JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barré syndrome: a systematic review. Brain. 2007;130(Pt 9):2245-2257. PubMed
9. Rajabally YA, Uncini A. Outcome and predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 2012;83(7):711-718. PubMed
1. Akhtar M, Collins MP, Kissel JT. Acute postgastric reduction surgery (APGARS) Neuropathy: A polynutritional, multisystem disorder. Neurology. 2002;58:A68. PubMed
2. Chang CG, Adams-Huet B, Provost DA. Acute post-gastric reduction surgery (APGARS) neuropathy. Obes Surg. 2004;14(2):182-189. PubMed
3. Chang CG, Helling TS, Black WE, Rymer MM. Weakness after gastric bypass. Obes Surg. 2002;12(4):592-597. PubMed
4. Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26(11-12):1031-1037. PubMed
5. Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31(2):491-510. PubMed
6. Ruts L, Drenthen J, Jongen JL, et al. Pain in Guillain-Barré syndrome: a long-term follow-up study. Neurology. 2010;75(16):1439-1447. PubMed
7. Hughes RAC, Wijdicks EFM, Barohn R, et al: Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;61:736-740. PubMed
8. Hughes RA, Swan AV, Raphaël JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barré syndrome: a systematic review. Brain. 2007;130(Pt 9):2245-2257. PubMed
9. Rajabally YA, Uncini A. Outcome and predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 2012;83(7):711-718. PubMed
© 2017 Society of Hospital Medicine
Breakdown
The patient's tachycardia and leukocytosis suggest sepsis. Potential sources include soft tissue infection or osteomyelitis from his sacral ulcers, Clostridium difficile, or a urinary tract infection. Impaired visceral sensation from his spinal cord injury may dampen his response to an intra‐abdominal process, such as mesenteric ischemia or toxic megacolon. Records from other hospitals should be reviewed to assess the acuity of change in his WBC count, hemoglobin, and creatinine. His anemia may be from chronic inflammation (eg, osteomyelitis), renal insufficiency, hemolysis, or occult blood loss, including retroperitoneal and gastrointestinal sources. His kidney injury may be from tubular necrosis in the setting of sepsis or obstructive uropathy related to a neurogenic bladder.
Potential contributors to his PEA and cardiovascular collapse are drug use (cocaine), alcohol withdrawal, infection, hypovolemia, myocardial ischemia, or heart failure. Severe hemorrhage, hyperkalemia, or acidosis from acute kidney injury and sepsis could also account for his cardiac arrest. His paraplegia and hospitalization raise the risk of venous thromboembolism, which can lead to PEA from pulmonary embolus and prolonged hypoxia.
His profound anemia is the likely cause of his PEA arrest and severe lactic acidosis. Massive hemolysis is most likely given no overt evidence of bleeding to account for the precipitous fall in hematocrit. Hemolysis can result from disorders intrinsic or extrinsic to the red blood cell (RBC). Intrinsic defects are usually congenital and involve the membrane, hemoglobin, or metabolic enzymes within the RBC. Extrinsic hemolysis arises from processes that injure the RBC from the outside: antibodies, infections, and mechanical shearing.
A rapidly declining platelet count is seen in microangiopathic hemolytic conditions such as disseminated intravascular coagulation (DIC) or thrombotic thrombocytopenic purpura (TTP), where platelets are consumed along with RBCs; sepsis makes DIC more likely. Autoimmune hemolytic anemia (AIHA) is sometimes accompanied by immune thrombocytopenia. AIHA arises from antibodies that are idiopathic or produced in response to infection, autoimmune conditions (eg, systemic lupus erythematosus), lymphoproliferative disease, or drugs (eg, ‐lactam antibiotics). The antiphospholipid syndrome can lead to thrombocytopenia, hemolysis, and kidney injury. Devitalized tissue in his sacral ulcers may predispose the patient to infection with Clostridium perfringens, which can elaborate enzymes that trigger massive hemolysis.
Because automated hemoglobin measurement is performed by spectrophotometry (light absorption and scatter), high concentrations of poorly soluble autoantibodies can increase the turbidity of the sample and preclude the measurement of hemoglobin concentration. This could lead to the report of interfering substances.
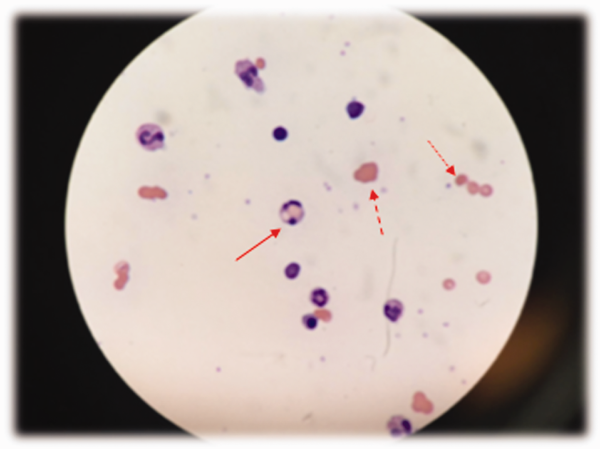
Low haptoglobin, elevated LDH, and hyperbilirubinemia confirm hemolysis. A more robust reticulocytosis is expected in the face of profound anemia, but the patient may also suffer from a concomitant hypoproliferative state (eg, nutritional deficiency). More likely, the rapidity of his decline outpaced the marrow's response, which can be delayed by days.
The most common cause of a combined elevation of the INR/PT and aPTT in a critically ill patient is DIC. Although no schistocytes were detected on the peripheral smear, they can be absent in up to 50% of DIC cases. TTP is associated with hemolytic anemia, kidney injury, and thrombocytopenia, but it generally does not cause coagulopathy.
The combination of red cell agglutination and hemophagocytosis suggests that the RBCs are coated with autoantibodies that cross‐link the cells and make them targets for phagocytosis by neutrophils in the circulation. This is distinct from the hemophagocytic syndrome, a rare immune activation syndrome characterized by macrophage phagocytosis of RBCs in the reticuloendothelial system. The blood smear also shows microspherocytes, which are seen in AIHA and hereditary spherocytosis.
Acute tubular necrosis could result from sepsis, ischemic injury from DIC, hypotension during cardiac arrest, or heme pigment toxicity. Urine sediment should be reviewed for dysmorphic RBCs or RBC casts that would indicate glomerulonephritis (eg, from an underlying autoimmune process associated with AIHA).
Urine hemoglobin that is disproportionate to the degree of hematuria suggests hemoglobinuria, which in turn defines the hemolysis as intravascular. Processes that directly lyse RBCs in circulation via mechanical shearing, activation of complement, infection of the RBC, or enzymatic or oxidative destruction of the membrane cause intravascular hemolysis. Leading considerations include microangiopathy (eg, DIC, TTP), clostridial sepsis, and AIHA.
AIHA can be broadly classified as warm or cold. Warm AIHA is caused by immunoglobulin IgG antibodies that bind most avidly at body temperature. Because warm AIHA does not activate complement, patients present with evidence of extravascular hemolysis that is typically chronic and mild to moderate in severity. It does not typically cause the acute, fulminant, intravascular hemolytic condition seen here.
Cold AIHA is characterized by autoantibodies that bind at lower temperatures and comes in 2 forms: cold agglutinin disease and (rarely) paroxysmal cold hemoglobinuria (PCH). Cold agglutinins are most often IgM antibodies produced in response to infection (Mycoplasma pneumoniae, infectious mononucleosis), drugs, or a hematologic malignancy. These IgM antibodies bind RBCs, causing them to agglutinate, and fix complement (including C3) to the surface of RBCs when blood circulates to cooler parts of the body. This results in complement activation, formation of the membrane attack complex, and intravascular hemolysis when bound and activated complement is present in large numbers. Acute infection can increase the complement available for binding to the surface of RBCs. Through a slightly different mechanism, PCH causes intravascular hemolysis through direct IgG activation of complement fixed to the surface of RBCs. During a hemolytic episode the direct antibody test (DAT) is positive using anti‐C3 and negative for IgG.
Based on the patient's clinical evidence of intravascular hemolysis and a suspected autoimmune etiology, the leading diagnosis at this time is cold AIHA.
The DAT detects IgG or complement adherent to RBCs. This patient has tested positive for both IgG and C3, though much more strongly for IgG, suggesting an unusual ability of the patient's IgG to activate complement. The phenomenon of mixed AIHA, in which the patient has both warm‐ and cold‐reacting antibodies, is rare.
Regarding infections associated with AIHA, there is no cough or rash to suggest M pneumoniae, and there is no sore throat, fever, lymphadenopathy, splenomegaly, or atypical lymphocytosis to suggest infectious mononucleosis. He should be tested for human immunodeficiency virus, which is also associated with AIHA. His leukocytosis may raise suspicion for an underlying hematologic malignancy, but he does not have blasts, dysplastic leukocytes, or lymphocytosis on his peripheral blood smear. Systemic lupus erythematosus can be associated with AIHA, thrombocytopenia, and renal failure, but he lacks the more common clinical manifestations of rash, arthralgias, and fever.
Drug‐induced immune hemolytic anemia (DIIHA) can cause both the clinical and serologic profile of an AIHA, as seen here. DIIHA can be distinguished from mixed AIHA if hemolysis abates with discontinuation of an offending drug. His deterioration is temporally associated with drug administration at the time of admission. Cephalosporins and ‐lactams (e.g., piperacillin) are the most common causes of DIIHA, and ‐lactamases such as tazobactam have also been implicated. By exclusion of other causes, DIIHA secondary to piperacillin is most likely responsible for his massive intravascular hemolysis.
COMMENTARY
This case illustrates a dramatic presentation of fulminant intravascular hemolysis secondary to piperacillin. The incidence of DIIHA is estimated to be 1 in 1 million.[1] Historically, methyldopa and high‐dose penicillin have been responsible for the majority of cases,[2] but in recent years complex penicillins, including piperacillin, and second‐ and third‐generation cephalosporins have been implicated.[3, 4] Cases of DIIHA are often underdiagnosed or misdiagnosed, as smoldering or less severe cases may not be recognized or are attributed to other causes.
A positive DAT, suggesting immunoglobulin and/or complement binding to RBCs, is the most reliable laboratory finding in DIIHA.[5] However, a positive DAT does not identify the source of the antigen and may result in misattribution of the immune hemolysis to autoimmunity rather than to a drug. Repeated or continued administration of the offending drug (as in this case) may perpetuate or worsen the hemolysis. Drug‐specific antibody tests may help to confirm the diagnosis, but these tests are complex and take significant time for specialized laboratories to run.
Severe hemolysis should be considered when a patient has a sudden and dramatic drop in his hemoglobin level in the absence of bleeding. Because DIIHA can be rapidly progressive, discontinuing a suspected culprit drug is the most important diagnostic and therapeutic measure. Typically, when an offending drug is stopped, the hemolysis stops as well. The time course over which this occurs depends on the rapidity of drug clearance.[4] Hemodialysis or plasmapheresis may be required in cases where the medication is renally excreted, particularly in cases of concomitant kidney injury. Evidence supporting corticosteroid use in DIIHA is limited, as the offending agent is usually discontinued by the time corticosteroids are initiated.[4]
This patient's DAT confirmed both IgG and complement activation, consistent with DIIHA caused by an immune complexlike reaction. This mechanism involves the antibody binding to a mixed epitope of the drug and a RBC membrane glycoprotein.[6] The offending drug was stopped only when review of his medical records established a clear temporal association between antibiotic administration and prior hemolysis.
The 2009 Health Information Technology for Economic and Clinical Health Act created an electronic health record (EHR) incentive program (meaningful use criteria).[7] By 2012, only 6% of hospitals met all of the stage 2 criteria, which include EHR interoperability across health systems.[8] The patient's preceding hemolytic event was described in records faxed by the outside hospitals, but without EHR interoperability, the treating clinicians did not have timely access to this information. Instead, the familiar manual process of obtaining outside records involving signed forms, phone calls, fax machines, and reams of paper progressed at its usual pace. Real‐time access to health records might have guided providers to select an alternative antibiotic regimen. Instead, a communication breakdown contributed to a catastrophic drug reaction and to this tragic patient outcome.
KEY TEACHING POINTS
- In a patient presenting with acute hemolysis and a positive DAT, consider DIIHA.
- Both piperacillin and tazobactam can cause a severe, complement‐mediated immune hemolytic anemia (DIIHA).
- Drug‐induced antibodies are detected by direct antiglobulin testing, but a complete medication history is the key to diagnosis.
- Management of drug‐induced hemolytic anemia involves immediate discontinuation of the culprit medication, supportive care, and potentially corticosteroids, plasmapheresis, and/or hemodialysis to expedite removal of the offending agent.
- EHR interoperability may provide timely access to important health information across different hospitals, expedite health information exchange, and reduce adverse patient outcomes that stem from communication delays.
This case was submitted anonymously to AHRQ WebM&M on July 18, 2014, and was accepted on August 7, 2014. The case and WebM&M commentary were published online on October 26, 2015.[9] This separate commentary on the same case was later submitted to the Journal of Hospital Medicine on September 2, 2015, accepted on November 24, 2015, and published on January 22, 2016. The 2 publications are written by different authors, and although they reference the same case, they make different but valuable points.
Disclosure
Nothing to report.
The patient's tachycardia and leukocytosis suggest sepsis. Potential sources include soft tissue infection or osteomyelitis from his sacral ulcers, Clostridium difficile, or a urinary tract infection. Impaired visceral sensation from his spinal cord injury may dampen his response to an intra‐abdominal process, such as mesenteric ischemia or toxic megacolon. Records from other hospitals should be reviewed to assess the acuity of change in his WBC count, hemoglobin, and creatinine. His anemia may be from chronic inflammation (eg, osteomyelitis), renal insufficiency, hemolysis, or occult blood loss, including retroperitoneal and gastrointestinal sources. His kidney injury may be from tubular necrosis in the setting of sepsis or obstructive uropathy related to a neurogenic bladder.
Potential contributors to his PEA and cardiovascular collapse are drug use (cocaine), alcohol withdrawal, infection, hypovolemia, myocardial ischemia, or heart failure. Severe hemorrhage, hyperkalemia, or acidosis from acute kidney injury and sepsis could also account for his cardiac arrest. His paraplegia and hospitalization raise the risk of venous thromboembolism, which can lead to PEA from pulmonary embolus and prolonged hypoxia.
His profound anemia is the likely cause of his PEA arrest and severe lactic acidosis. Massive hemolysis is most likely given no overt evidence of bleeding to account for the precipitous fall in hematocrit. Hemolysis can result from disorders intrinsic or extrinsic to the red blood cell (RBC). Intrinsic defects are usually congenital and involve the membrane, hemoglobin, or metabolic enzymes within the RBC. Extrinsic hemolysis arises from processes that injure the RBC from the outside: antibodies, infections, and mechanical shearing.
A rapidly declining platelet count is seen in microangiopathic hemolytic conditions such as disseminated intravascular coagulation (DIC) or thrombotic thrombocytopenic purpura (TTP), where platelets are consumed along with RBCs; sepsis makes DIC more likely. Autoimmune hemolytic anemia (AIHA) is sometimes accompanied by immune thrombocytopenia. AIHA arises from antibodies that are idiopathic or produced in response to infection, autoimmune conditions (eg, systemic lupus erythematosus), lymphoproliferative disease, or drugs (eg, ‐lactam antibiotics). The antiphospholipid syndrome can lead to thrombocytopenia, hemolysis, and kidney injury. Devitalized tissue in his sacral ulcers may predispose the patient to infection with Clostridium perfringens, which can elaborate enzymes that trigger massive hemolysis.
Because automated hemoglobin measurement is performed by spectrophotometry (light absorption and scatter), high concentrations of poorly soluble autoantibodies can increase the turbidity of the sample and preclude the measurement of hemoglobin concentration. This could lead to the report of interfering substances.

Low haptoglobin, elevated LDH, and hyperbilirubinemia confirm hemolysis. A more robust reticulocytosis is expected in the face of profound anemia, but the patient may also suffer from a concomitant hypoproliferative state (eg, nutritional deficiency). More likely, the rapidity of his decline outpaced the marrow's response, which can be delayed by days.
The most common cause of a combined elevation of the INR/PT and aPTT in a critically ill patient is DIC. Although no schistocytes were detected on the peripheral smear, they can be absent in up to 50% of DIC cases. TTP is associated with hemolytic anemia, kidney injury, and thrombocytopenia, but it generally does not cause coagulopathy.
The combination of red cell agglutination and hemophagocytosis suggests that the RBCs are coated with autoantibodies that cross‐link the cells and make them targets for phagocytosis by neutrophils in the circulation. This is distinct from the hemophagocytic syndrome, a rare immune activation syndrome characterized by macrophage phagocytosis of RBCs in the reticuloendothelial system. The blood smear also shows microspherocytes, which are seen in AIHA and hereditary spherocytosis.
Acute tubular necrosis could result from sepsis, ischemic injury from DIC, hypotension during cardiac arrest, or heme pigment toxicity. Urine sediment should be reviewed for dysmorphic RBCs or RBC casts that would indicate glomerulonephritis (eg, from an underlying autoimmune process associated with AIHA).
Urine hemoglobin that is disproportionate to the degree of hematuria suggests hemoglobinuria, which in turn defines the hemolysis as intravascular. Processes that directly lyse RBCs in circulation via mechanical shearing, activation of complement, infection of the RBC, or enzymatic or oxidative destruction of the membrane cause intravascular hemolysis. Leading considerations include microangiopathy (eg, DIC, TTP), clostridial sepsis, and AIHA.
AIHA can be broadly classified as warm or cold. Warm AIHA is caused by immunoglobulin IgG antibodies that bind most avidly at body temperature. Because warm AIHA does not activate complement, patients present with evidence of extravascular hemolysis that is typically chronic and mild to moderate in severity. It does not typically cause the acute, fulminant, intravascular hemolytic condition seen here.
Cold AIHA is characterized by autoantibodies that bind at lower temperatures and comes in 2 forms: cold agglutinin disease and (rarely) paroxysmal cold hemoglobinuria (PCH). Cold agglutinins are most often IgM antibodies produced in response to infection (Mycoplasma pneumoniae, infectious mononucleosis), drugs, or a hematologic malignancy. These IgM antibodies bind RBCs, causing them to agglutinate, and fix complement (including C3) to the surface of RBCs when blood circulates to cooler parts of the body. This results in complement activation, formation of the membrane attack complex, and intravascular hemolysis when bound and activated complement is present in large numbers. Acute infection can increase the complement available for binding to the surface of RBCs. Through a slightly different mechanism, PCH causes intravascular hemolysis through direct IgG activation of complement fixed to the surface of RBCs. During a hemolytic episode the direct antibody test (DAT) is positive using anti‐C3 and negative for IgG.
Based on the patient's clinical evidence of intravascular hemolysis and a suspected autoimmune etiology, the leading diagnosis at this time is cold AIHA.
The DAT detects IgG or complement adherent to RBCs. This patient has tested positive for both IgG and C3, though much more strongly for IgG, suggesting an unusual ability of the patient's IgG to activate complement. The phenomenon of mixed AIHA, in which the patient has both warm‐ and cold‐reacting antibodies, is rare.
Regarding infections associated with AIHA, there is no cough or rash to suggest M pneumoniae, and there is no sore throat, fever, lymphadenopathy, splenomegaly, or atypical lymphocytosis to suggest infectious mononucleosis. He should be tested for human immunodeficiency virus, which is also associated with AIHA. His leukocytosis may raise suspicion for an underlying hematologic malignancy, but he does not have blasts, dysplastic leukocytes, or lymphocytosis on his peripheral blood smear. Systemic lupus erythematosus can be associated with AIHA, thrombocytopenia, and renal failure, but he lacks the more common clinical manifestations of rash, arthralgias, and fever.
Drug‐induced immune hemolytic anemia (DIIHA) can cause both the clinical and serologic profile of an AIHA, as seen here. DIIHA can be distinguished from mixed AIHA if hemolysis abates with discontinuation of an offending drug. His deterioration is temporally associated with drug administration at the time of admission. Cephalosporins and ‐lactams (e.g., piperacillin) are the most common causes of DIIHA, and ‐lactamases such as tazobactam have also been implicated. By exclusion of other causes, DIIHA secondary to piperacillin is most likely responsible for his massive intravascular hemolysis.
COMMENTARY
This case illustrates a dramatic presentation of fulminant intravascular hemolysis secondary to piperacillin. The incidence of DIIHA is estimated to be 1 in 1 million.[1] Historically, methyldopa and high‐dose penicillin have been responsible for the majority of cases,[2] but in recent years complex penicillins, including piperacillin, and second‐ and third‐generation cephalosporins have been implicated.[3, 4] Cases of DIIHA are often underdiagnosed or misdiagnosed, as smoldering or less severe cases may not be recognized or are attributed to other causes.
A positive DAT, suggesting immunoglobulin and/or complement binding to RBCs, is the most reliable laboratory finding in DIIHA.[5] However, a positive DAT does not identify the source of the antigen and may result in misattribution of the immune hemolysis to autoimmunity rather than to a drug. Repeated or continued administration of the offending drug (as in this case) may perpetuate or worsen the hemolysis. Drug‐specific antibody tests may help to confirm the diagnosis, but these tests are complex and take significant time for specialized laboratories to run.
Severe hemolysis should be considered when a patient has a sudden and dramatic drop in his hemoglobin level in the absence of bleeding. Because DIIHA can be rapidly progressive, discontinuing a suspected culprit drug is the most important diagnostic and therapeutic measure. Typically, when an offending drug is stopped, the hemolysis stops as well. The time course over which this occurs depends on the rapidity of drug clearance.[4] Hemodialysis or plasmapheresis may be required in cases where the medication is renally excreted, particularly in cases of concomitant kidney injury. Evidence supporting corticosteroid use in DIIHA is limited, as the offending agent is usually discontinued by the time corticosteroids are initiated.[4]
This patient's DAT confirmed both IgG and complement activation, consistent with DIIHA caused by an immune complexlike reaction. This mechanism involves the antibody binding to a mixed epitope of the drug and a RBC membrane glycoprotein.[6] The offending drug was stopped only when review of his medical records established a clear temporal association between antibiotic administration and prior hemolysis.
The 2009 Health Information Technology for Economic and Clinical Health Act created an electronic health record (EHR) incentive program (meaningful use criteria).[7] By 2012, only 6% of hospitals met all of the stage 2 criteria, which include EHR interoperability across health systems.[8] The patient's preceding hemolytic event was described in records faxed by the outside hospitals, but without EHR interoperability, the treating clinicians did not have timely access to this information. Instead, the familiar manual process of obtaining outside records involving signed forms, phone calls, fax machines, and reams of paper progressed at its usual pace. Real‐time access to health records might have guided providers to select an alternative antibiotic regimen. Instead, a communication breakdown contributed to a catastrophic drug reaction and to this tragic patient outcome.
KEY TEACHING POINTS
- In a patient presenting with acute hemolysis and a positive DAT, consider DIIHA.
- Both piperacillin and tazobactam can cause a severe, complement‐mediated immune hemolytic anemia (DIIHA).
- Drug‐induced antibodies are detected by direct antiglobulin testing, but a complete medication history is the key to diagnosis.
- Management of drug‐induced hemolytic anemia involves immediate discontinuation of the culprit medication, supportive care, and potentially corticosteroids, plasmapheresis, and/or hemodialysis to expedite removal of the offending agent.
- EHR interoperability may provide timely access to important health information across different hospitals, expedite health information exchange, and reduce adverse patient outcomes that stem from communication delays.
This case was submitted anonymously to AHRQ WebM&M on July 18, 2014, and was accepted on August 7, 2014. The case and WebM&M commentary were published online on October 26, 2015.[9] This separate commentary on the same case was later submitted to the Journal of Hospital Medicine on September 2, 2015, accepted on November 24, 2015, and published on January 22, 2016. The 2 publications are written by different authors, and although they reference the same case, they make different but valuable points.
Disclosure
Nothing to report.
The patient's tachycardia and leukocytosis suggest sepsis. Potential sources include soft tissue infection or osteomyelitis from his sacral ulcers, Clostridium difficile, or a urinary tract infection. Impaired visceral sensation from his spinal cord injury may dampen his response to an intra‐abdominal process, such as mesenteric ischemia or toxic megacolon. Records from other hospitals should be reviewed to assess the acuity of change in his WBC count, hemoglobin, and creatinine. His anemia may be from chronic inflammation (eg, osteomyelitis), renal insufficiency, hemolysis, or occult blood loss, including retroperitoneal and gastrointestinal sources. His kidney injury may be from tubular necrosis in the setting of sepsis or obstructive uropathy related to a neurogenic bladder.
Potential contributors to his PEA and cardiovascular collapse are drug use (cocaine), alcohol withdrawal, infection, hypovolemia, myocardial ischemia, or heart failure. Severe hemorrhage, hyperkalemia, or acidosis from acute kidney injury and sepsis could also account for his cardiac arrest. His paraplegia and hospitalization raise the risk of venous thromboembolism, which can lead to PEA from pulmonary embolus and prolonged hypoxia.
His profound anemia is the likely cause of his PEA arrest and severe lactic acidosis. Massive hemolysis is most likely given no overt evidence of bleeding to account for the precipitous fall in hematocrit. Hemolysis can result from disorders intrinsic or extrinsic to the red blood cell (RBC). Intrinsic defects are usually congenital and involve the membrane, hemoglobin, or metabolic enzymes within the RBC. Extrinsic hemolysis arises from processes that injure the RBC from the outside: antibodies, infections, and mechanical shearing.
A rapidly declining platelet count is seen in microangiopathic hemolytic conditions such as disseminated intravascular coagulation (DIC) or thrombotic thrombocytopenic purpura (TTP), where platelets are consumed along with RBCs; sepsis makes DIC more likely. Autoimmune hemolytic anemia (AIHA) is sometimes accompanied by immune thrombocytopenia. AIHA arises from antibodies that are idiopathic or produced in response to infection, autoimmune conditions (eg, systemic lupus erythematosus), lymphoproliferative disease, or drugs (eg, ‐lactam antibiotics). The antiphospholipid syndrome can lead to thrombocytopenia, hemolysis, and kidney injury. Devitalized tissue in his sacral ulcers may predispose the patient to infection with Clostridium perfringens, which can elaborate enzymes that trigger massive hemolysis.
Because automated hemoglobin measurement is performed by spectrophotometry (light absorption and scatter), high concentrations of poorly soluble autoantibodies can increase the turbidity of the sample and preclude the measurement of hemoglobin concentration. This could lead to the report of interfering substances.

Low haptoglobin, elevated LDH, and hyperbilirubinemia confirm hemolysis. A more robust reticulocytosis is expected in the face of profound anemia, but the patient may also suffer from a concomitant hypoproliferative state (eg, nutritional deficiency). More likely, the rapidity of his decline outpaced the marrow's response, which can be delayed by days.
The most common cause of a combined elevation of the INR/PT and aPTT in a critically ill patient is DIC. Although no schistocytes were detected on the peripheral smear, they can be absent in up to 50% of DIC cases. TTP is associated with hemolytic anemia, kidney injury, and thrombocytopenia, but it generally does not cause coagulopathy.
The combination of red cell agglutination and hemophagocytosis suggests that the RBCs are coated with autoantibodies that cross‐link the cells and make them targets for phagocytosis by neutrophils in the circulation. This is distinct from the hemophagocytic syndrome, a rare immune activation syndrome characterized by macrophage phagocytosis of RBCs in the reticuloendothelial system. The blood smear also shows microspherocytes, which are seen in AIHA and hereditary spherocytosis.
Acute tubular necrosis could result from sepsis, ischemic injury from DIC, hypotension during cardiac arrest, or heme pigment toxicity. Urine sediment should be reviewed for dysmorphic RBCs or RBC casts that would indicate glomerulonephritis (eg, from an underlying autoimmune process associated with AIHA).
Urine hemoglobin that is disproportionate to the degree of hematuria suggests hemoglobinuria, which in turn defines the hemolysis as intravascular. Processes that directly lyse RBCs in circulation via mechanical shearing, activation of complement, infection of the RBC, or enzymatic or oxidative destruction of the membrane cause intravascular hemolysis. Leading considerations include microangiopathy (eg, DIC, TTP), clostridial sepsis, and AIHA.
AIHA can be broadly classified as warm or cold. Warm AIHA is caused by immunoglobulin IgG antibodies that bind most avidly at body temperature. Because warm AIHA does not activate complement, patients present with evidence of extravascular hemolysis that is typically chronic and mild to moderate in severity. It does not typically cause the acute, fulminant, intravascular hemolytic condition seen here.
Cold AIHA is characterized by autoantibodies that bind at lower temperatures and comes in 2 forms: cold agglutinin disease and (rarely) paroxysmal cold hemoglobinuria (PCH). Cold agglutinins are most often IgM antibodies produced in response to infection (Mycoplasma pneumoniae, infectious mononucleosis), drugs, or a hematologic malignancy. These IgM antibodies bind RBCs, causing them to agglutinate, and fix complement (including C3) to the surface of RBCs when blood circulates to cooler parts of the body. This results in complement activation, formation of the membrane attack complex, and intravascular hemolysis when bound and activated complement is present in large numbers. Acute infection can increase the complement available for binding to the surface of RBCs. Through a slightly different mechanism, PCH causes intravascular hemolysis through direct IgG activation of complement fixed to the surface of RBCs. During a hemolytic episode the direct antibody test (DAT) is positive using anti‐C3 and negative for IgG.
Based on the patient's clinical evidence of intravascular hemolysis and a suspected autoimmune etiology, the leading diagnosis at this time is cold AIHA.
The DAT detects IgG or complement adherent to RBCs. This patient has tested positive for both IgG and C3, though much more strongly for IgG, suggesting an unusual ability of the patient's IgG to activate complement. The phenomenon of mixed AIHA, in which the patient has both warm‐ and cold‐reacting antibodies, is rare.
Regarding infections associated with AIHA, there is no cough or rash to suggest M pneumoniae, and there is no sore throat, fever, lymphadenopathy, splenomegaly, or atypical lymphocytosis to suggest infectious mononucleosis. He should be tested for human immunodeficiency virus, which is also associated with AIHA. His leukocytosis may raise suspicion for an underlying hematologic malignancy, but he does not have blasts, dysplastic leukocytes, or lymphocytosis on his peripheral blood smear. Systemic lupus erythematosus can be associated with AIHA, thrombocytopenia, and renal failure, but he lacks the more common clinical manifestations of rash, arthralgias, and fever.
Drug‐induced immune hemolytic anemia (DIIHA) can cause both the clinical and serologic profile of an AIHA, as seen here. DIIHA can be distinguished from mixed AIHA if hemolysis abates with discontinuation of an offending drug. His deterioration is temporally associated with drug administration at the time of admission. Cephalosporins and ‐lactams (e.g., piperacillin) are the most common causes of DIIHA, and ‐lactamases such as tazobactam have also been implicated. By exclusion of other causes, DIIHA secondary to piperacillin is most likely responsible for his massive intravascular hemolysis.
COMMENTARY
This case illustrates a dramatic presentation of fulminant intravascular hemolysis secondary to piperacillin. The incidence of DIIHA is estimated to be 1 in 1 million.[1] Historically, methyldopa and high‐dose penicillin have been responsible for the majority of cases,[2] but in recent years complex penicillins, including piperacillin, and second‐ and third‐generation cephalosporins have been implicated.[3, 4] Cases of DIIHA are often underdiagnosed or misdiagnosed, as smoldering or less severe cases may not be recognized or are attributed to other causes.
A positive DAT, suggesting immunoglobulin and/or complement binding to RBCs, is the most reliable laboratory finding in DIIHA.[5] However, a positive DAT does not identify the source of the antigen and may result in misattribution of the immune hemolysis to autoimmunity rather than to a drug. Repeated or continued administration of the offending drug (as in this case) may perpetuate or worsen the hemolysis. Drug‐specific antibody tests may help to confirm the diagnosis, but these tests are complex and take significant time for specialized laboratories to run.
Severe hemolysis should be considered when a patient has a sudden and dramatic drop in his hemoglobin level in the absence of bleeding. Because DIIHA can be rapidly progressive, discontinuing a suspected culprit drug is the most important diagnostic and therapeutic measure. Typically, when an offending drug is stopped, the hemolysis stops as well. The time course over which this occurs depends on the rapidity of drug clearance.[4] Hemodialysis or plasmapheresis may be required in cases where the medication is renally excreted, particularly in cases of concomitant kidney injury. Evidence supporting corticosteroid use in DIIHA is limited, as the offending agent is usually discontinued by the time corticosteroids are initiated.[4]
This patient's DAT confirmed both IgG and complement activation, consistent with DIIHA caused by an immune complexlike reaction. This mechanism involves the antibody binding to a mixed epitope of the drug and a RBC membrane glycoprotein.[6] The offending drug was stopped only when review of his medical records established a clear temporal association between antibiotic administration and prior hemolysis.
The 2009 Health Information Technology for Economic and Clinical Health Act created an electronic health record (EHR) incentive program (meaningful use criteria).[7] By 2012, only 6% of hospitals met all of the stage 2 criteria, which include EHR interoperability across health systems.[8] The patient's preceding hemolytic event was described in records faxed by the outside hospitals, but without EHR interoperability, the treating clinicians did not have timely access to this information. Instead, the familiar manual process of obtaining outside records involving signed forms, phone calls, fax machines, and reams of paper progressed at its usual pace. Real‐time access to health records might have guided providers to select an alternative antibiotic regimen. Instead, a communication breakdown contributed to a catastrophic drug reaction and to this tragic patient outcome.
KEY TEACHING POINTS
- In a patient presenting with acute hemolysis and a positive DAT, consider DIIHA.
- Both piperacillin and tazobactam can cause a severe, complement‐mediated immune hemolytic anemia (DIIHA).
- Drug‐induced antibodies are detected by direct antiglobulin testing, but a complete medication history is the key to diagnosis.
- Management of drug‐induced hemolytic anemia involves immediate discontinuation of the culprit medication, supportive care, and potentially corticosteroids, plasmapheresis, and/or hemodialysis to expedite removal of the offending agent.
- EHR interoperability may provide timely access to important health information across different hospitals, expedite health information exchange, and reduce adverse patient outcomes that stem from communication delays.
This case was submitted anonymously to AHRQ WebM&M on July 18, 2014, and was accepted on August 7, 2014. The case and WebM&M commentary were published online on October 26, 2015.[9] This separate commentary on the same case was later submitted to the Journal of Hospital Medicine on September 2, 2015, accepted on November 24, 2015, and published on January 22, 2016. The 2 publications are written by different authors, and although they reference the same case, they make different but valuable points.
Disclosure
Nothing to report.
Perceptions of Current Note Quality
The electronic health record (EHR) has revolutionized the practice of medicine. As part of the economic stimulus package in 2009, Congress enacted the Health Information Technology for Economic and Clinical Health Act, which included incentives for physicians and hospitals to adopt an EHR by 2015. In the setting of more limited duty hours and demands for increased clinical productivity, EHRs have functions that may improve the quality and efficiency of clinical documentation.[1, 2, 3, 4, 5]
The process of note writing and the use of notes for clinical care have changed substantially with EHR implementation. Use of efficiency tools (ie, copy forward functions and autopopulation of data) may increase the speed of documentation.[5] Notes in an EHR are more legible and accessible and may be able to organize data to improve clinical care.[6]
Yet, many have commented on the negative consequences of documentation in an EHR. In a New England Journal of Medicine Perspective article, Drs. Hartzband and Groopman wrote, we have observed the electronic medical record become a powerful vehicle for perpetuating erroneous information, leading to diagnostic errors that gain momentum when passed on electronically.[7] As a result, the copy forward and autopopulation functions have come under significant scrutiny.[8, 9, 10] A survey conducted at 2 academic institutions found that 71% of residents and attendings believed that the copy forward function led to inconsistencies and outdated information.[11] Autopopulation has been criticized for creating lengthy notes full of trivial or redundant data, a phenomenon termed note bloat. Bloated notes may be less effective as a communication tool.[12] Additionally, the process of composing a note often stimulates critical thinking and may lead to changes in care. The act of copying forward a previous note and autopopulating data bypasses that process and in effect may suppress critical thinking.[13] Previous studies have raised numerous concerns regarding copy forward and autopopulation functionality in the EHR. Many have described the duplication of outdated data and the possibility of the introduction and perpetuation of errors.[14, 15, 16] The Veterans Affairs (VA) Puget Sound Health system evaluated 6322 copy events and found that 1 in 10 electronic patient charts contained an instance of high‐risk copying.[17] In a survey of faculty and residents at a single academic medical center, the majority of users of copy and paste functionality recognized the hazards; they responded that their notes may contain more outdated (66%) and more inconsistent information (69%). Yet, most felt copy forwarding improved the documentation of the entire hospital course (87%), overall physician documentation (69%), and should definitely be continued (91%).[11] Others have complained about the impact of copy forward on the expression of clinical reasoning.[7, 9, 18]
Previous discussions on the topic of overall note quality following EHR implementation have been limited to perspectives or opinion pieces of individual attending providers.[18] We conducted a survey across 4 academic institutions to analyze both housestaff and attendings perceptions of the quality of notes since the implementation of an EHR to better inform the discussion of the impact of an EHR on note quality.
METHODS
Participants
Surveys were administered via email to interns, residents (second‐, third‐, or fourth‐year residents, hereafter referred to as residents) and attendings at 4 academic hospitals that use the Epic EHR (Epic Corp., Madison, WI). The 4 institutions each adopted the Epic EHR, with mandatory faculty and resident training, between 1 and 5 years prior to the survey. Three of the institutions previously used systems with electronic notes, whereas the fourth institution previously used a system with handwritten notes. The study participation emails included a link to an online survey in REDCap.[19] We included interns and residents from the following types of residency programs: internal medicine categorical or primary care, medicine‐pediatrics, or medicine‐psychiatry. For housestaff (the combination of both interns and residents), exclusion criteria included preliminary or transitional year interns, or any interns or residents from other specialties who rotate on the medicine service. For attendings, participants included hospitalists, general internal medicine attendings, chief residents, and subspecialty medicine attendings, each of whom had worked for any amount of time on the inpatient medicine teaching service in the prior 12 months.
Design
We developed 3 unique surveys for interns, residents, and attendings to assess their perception of inpatient progress notes (see Supporting Information, Appendix, in the online version of this article). The surveys incorporated questions from 2 previously published sources, the 9‐item Physician Documentation Quality Instrument (PDQI‐9) (see online Appendix), a validated note‐scoring tool, and the Accreditation Council for Graduate Medical Education note‐writing competency checklists.[20] Additionally, faculty at the participating institutions developed questions to address practices and attitudes toward autopopulation, copy forward, and the purposes of a progress note. Responses were based on a 5‐point Likert scale. The intern and resident surveys asked for self‐evaluation of their own progress notes and those of their peers, whereas the attending surveys asked for assessment of housestaff notes.
The survey was left open for a total of 55 days and participants were sent reminder emails. The study received a waiver from the institutional review board at all 4 institutions.
Data Analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of California, San Francisco (UCSF).[19] The survey data were analyzed and the figures were created using Microsoft Excel 2008 (Microsoft Corp., Redmond, WA). Mean values for each survey question were calculated. Differences between the means among the groups were assessed using 2‐sample t tests. P values <0.05 were considered statistically significant.
RESULTS
Demographics
We received 99 completed surveys from interns, 155 completed surveys from residents, and 153 completed surveys from attendings across the 4 institutions. The overall response rate for interns was 68%, ranging from 59% at the University of California, San Diego (UCSD) to 74% at the University of Iowa. The overall response rate for residents was 49%, ranging from 38% at UCSF to 66% at the University of California, Los Angeles. The overall response rate for attendings was 70%, ranging from 53% at UCSD to 74% at UCSF.
A total of 78% of interns and 72% of residents had used an EHR at a prior institution. Of the residents, 90 were second‐year residents, 64 were third‐year residents, and 2 were fourth‐year residents. A total of 76% of attendings self‐identified as hospitalists.
Overall Assessment of Note Quality
Participants were asked to rate the quality of progress notes on a 5‐point scale (poor, fair, good, very good, excellent). Half of interns and residents rated their own progress notes as very good or excellent. A total of 44% percent of interns and 24% of residents rated their peers notes as very good or excellent, whereas only 15% of attending physicians rated housestaff notes as very good or excellent.
When asked to rate the change in progress note quality since their hospital had adopted the EHR, the majority of residents answered unchanged or better, and the majority of attendings answered unchanged or worse (Figure 1).
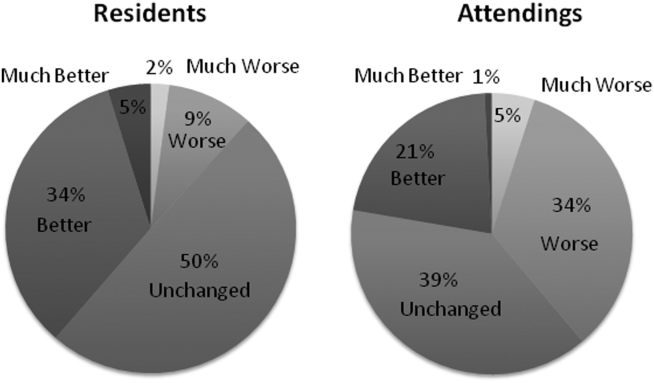
PDQI‐9 Framework
Participants answered each PDQI‐9 question on a 5‐point Likert scale ranging from not at all (1) to extremely (5). In 8 of the 9 PDQI‐9 domains, there were no significant differences between interns and residents. Across each domain, attending perceptions of housestaff notes were significantly lower than housestaff perceptions of their own notes (P<0.001) (Figure 2). Both housestaff and attendings gave the highest ratings to thorough, up to date, and synthesized and the lowest rating to succinct.
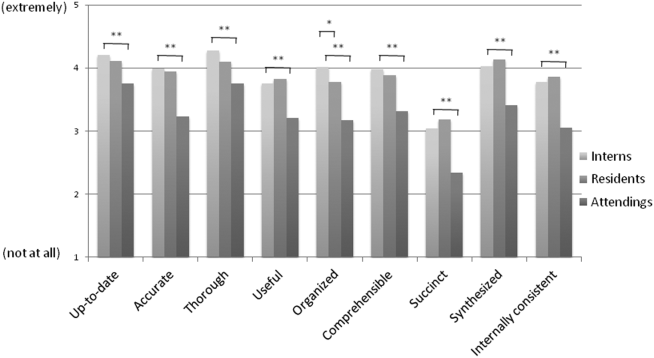
Copy Forward and Autopopulation
Overall, the effect of copy forward and autopopulation on critical thinking, note accuracy, and prioritizing the problem list was thought to be neutral or somewhat positive by interns, neutral by residents, and neutral or somewhat negative by attendings (P<0.001) (Figure 3). In all, 16% of interns, 22% of residents, and 55% of attendings reported that copy forward had a somewhat negative or very negative impact on critical thinking (P<0.001). In all, 16% of interns, 29% of residents and 39% of attendings thought that autopopulation had a somewhat negative or very negative impact on critical thinking (P<0.001).
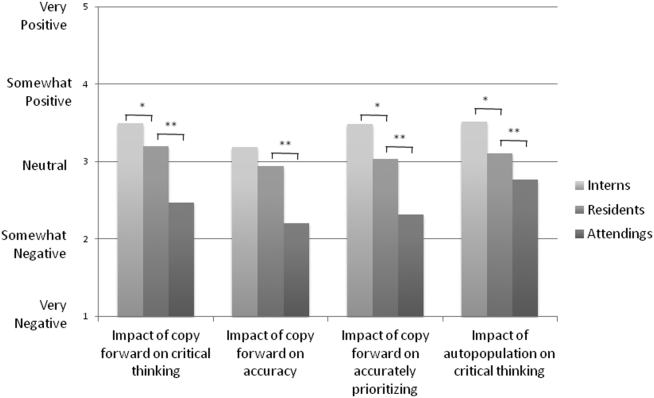
Purpose of Progress Notes
Participants were provided with 7 possible purposes of a progress note and asked to rate the importance of each stated purpose. There was nearly perfect agreement between interns, residents, and attendings in the rank order of the importance of each purpose of a progress note (Table 1). Attendings and housestaff ranked communication with other providers and documenting important events and the plan for the day as the 2 most important purposes of a progress note, and billing and quality improvement as less important.
| Interns | Residents | Attendings | |
|---|---|---|---|
| Communication with other providers | 1 | 1 | 2 |
| Documenting important events and the plan for the day | 2 | 2 | 1 |
| Prioritizing issues going forward in the patient's care | 3 | 3 | 3 |
| Medicolegal | 4 | 4 | 4 |
| Stimulate critical thinking | 5 | 5 | 5 |
| Billing | 6 | 6 | 6 |
| Quality improvement | 7 | 7 | 7 |
DISCUSSION
This is the first large multicenter analysis of both attendings and housestaff perceptions of note quality in the EHR era. The findings provide insight into important differences and similarities in the perceptions of the 2 groups. Most striking is the difference in opinion of overall note quality, with only a small minority of faculty rating current housestaff notes as very good or excellent, whereas a much larger proportion of housestaff rated their own notes and those of their peers to be of high quality. Though participants were not specifically asked why note quality in general was suboptimal, housestaff and faculty rankings of specific domains from the PDQI‐9 may yield an important clue. Specifically, all groups expressed that the weakest attribute of current progress notes is succinct. This finding is consistent with the note bloat phenomenon, which has been maligned as a consequence of EHR implementation.[7, 14, 18, 21, 22]
One interesting finding was that only 5% of interns rated the notes of other housestaff as fair or poor. One possible explanation for this may be the tendency for an individual to enhance or augment the status or performance of the group to which he or she belongs as a mechanism to increase self‐image, known as the social identity theory.[23] Thus, housestaff may not criticize their peers to allow for identification with a group that is not deficient in note writing.
The more positive assessment of overall note quality among housestaff could be related to the different roles of housestaff and attendings on a teaching service. On a teaching service, housestaff are typically the writer, whereas attendings are almost exclusively the reader of progress notes. Housestaff may reap benefits, including efficiency, beyond the finished product. A perception of higher quality may reflect the process of note writing, data gathering, and critical thinking required to build an assessment and plan. The scores on the PDQI‐9 support this notion, as housestaff rated all 9 domains significantly higher than attendings.
Housestaff and attendings held greater differences of opinion with respect to the EHR's impact on note quality. Generally, housestaff perceived the EHR to have improved progress note quality, whereas attendings perceived the opposite. One explanation could be that these results reflect changing stages of development of physicians well described through the RIME framework (reporter, interpreter, manager, educator). Attendings may expect notes to reflect synthesis and analysis, whereas trainees may be satisfied with the data gathering that an EHR facilitates. In our survey, the trend of answers from intern to resident to attending suggests an evolving process of attitudes toward note quality.
The above reasons may also explain why housestaff were generally more positive than attendings about the effect of copy forward and autopopulation functions on critical thinking. Perhaps, as these functions can potentially increase efficiency and decrease time spent at the computer, although data are mixed on this finding, housestaff may have more time to spend with patients or develop a thorough plan and thus rate these functions positively.
Notably, housestaff and attendings had excellent agreement on the purposes of a progress note. They agreed that the 2 most important purposes were communication with other providers and documenting important events and the plan for the day. These are the 2 listed purposes that are most directly related to patient care. If future interventions to improve note quality require housestaff and attendings to significantly change their behavior, a focus on the impact on patient care might yield the best results.
There were several limitations in our study. Any study based on self‐assessment is subject to bias. A previous meta‐analysis and review described poor to moderate correlations between self‐assessed and external measures of performance.[24, 25] The survey data were aggregated from 4 institutions despite somewhat different, though relatively high, response rates between the institutions. There could be a response bias; those who did not respond may have systematically different perceptions of note quality. It should be noted that the general demographics of the respondents reflected those of the housestaff and attendings at 4 academic centers. All 4 of the participating institutions adopted the Epic EHR within the last several years of the survey being administered, and perceptions of note quality may be biased depending on the prior system used (ie, change from handwritten to electronic vs electronic to other electronic system). In addition, the survey results reflect experience with only 1 EHR, and our results may not apply to other EHR vendors or institutions like the VA, which have a long‐standing system in place. Last, we did not explore the impact of perceived note quality on the measured or perceived quality of care. One previous study found no direct correlation between note quality and clinical quality.[26]
There are several future directions for research based on our findings. First, potential differences between housestaff and attending perceptions of note quality could be further teased apart by studying the perceptions of attendings on a nonteaching service who write their own daily progress notes. Second, housestaff perceptions on why copy forward and autopopulation may increase critical thinking could be explored further with more direct questioning. Finally, although our study captured only perceptions of note quality, validated tools could be used to objectively measure note quality; these measurements could then be compared to perception of note quality as well as clinical outcomes.
Given the prevalence and the apparent belief that the benefits of an EHR outweigh the hazards, institutions should embrace these innovations but take steps to mitigate the potential errors and problems associated with copy forward and autopopulation. The results of our study should help inform future interventions.
Acknowledgements
The authors acknowledge the contributions of Russell Leslie from the University of Iowa.
Disclosure: Nothing to report.
- , , , et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144(10):742–752.
- , , , , . Clinical information technologies and inpatient outcomes: a multiple hospital study. Arch Intern Med. 2009;169(2):108–114.
- , , , et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280(15):1311–1316.
- , , , . Electronic health records and quality of diabetes care. N Engl J Med. 2011;365(9):825–833.
- , , , et al. The impact of a clinical information system in an intensive care unit. J Clin Monit Comput. 2008;22(1):31–36.
- , . Can electronic clinical documentation help prevent diagnostic errors? N Engl J Med. 2010;362(12):1066–1069.
- , . Off the record—avoiding the pitfalls of going electronic. N Eng J Med. 2008;358(16):1656–1658.
- , , . Copying and pasting of examinations within the electronic medical record. Int J Med Inform. 2007;76(suppl 1):S122–S128.
- , . Copy and paste: a remediable hazard of electronic health records. Am J Med. 2009;122(6):495–496.
- , , . The role of copy‐and‐paste in the hospital electronic health record. JAMA Intern Med. 2014;174(8):1217–1218.
- , , , , , . Physicians’ attitudes towards copy and pasting in electronic note writing. J Gen Intern Med. 2009;24(1):63–68.
- , , , , . Medical education in the electronic medical record (EMR) era: benefits, challenges, and future directions. Acad Med. 2013;88(6):748–752.
- , . Educational impact of the electronic medical record. J Surg Educ. 2012;69(1):105–112.
- , , , , , . Direct text entry in electronic progress notes. An evaluation of input errors. Methods Inf Med. 2003;42(1):61–67.
- . The clinical record: a 200‐year‐old 21st‐century challenge. Ann Intern Med. 2010;153(10):682–683.
- . Sloppy and paste. Morbidity and Mortality Rounds on the Web. Available at: http://www.webmm.ahrq.gov/case.aspx?caseID=274. Published July 2012. Accessed September 26, 2014.
- , , , . Are electronic medical records trustworthy? Observations on copying, pasting and duplication. AMIA Annu Symp Proc. 2003:269–273.
- . A piece of my mind. John Lennon's elbow. JAMA. 2012;308(5):463–464.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- , , . ACGME competency note checklist. Available at: http://www.im.org/p/cm/ld/fid=831. Accessed August 8, 2013.
- , , , . Assessing electronic note quality using the Physician Documentation Quality Instrument (PDQI‐9). Appl Clin Inform. 2012;3(2):164–174.
- , , , . Quantifying clinical narrative redundancy in an electronic health record. J Am Med Inform Assoc. 2010;17(1):49–53.
- , . The social identity theory of intergroup behavior. In: Psychology of Intergroup Relations. 2nd ed. Chicago, IL: Nelson‐Hall Publishers; 1986:7–24.
- , . Student self‐assessment in higher education: a meta‐analysis. Rev Educ Res. 1989;59:395–430.
- . A review of the validity and accuracy of self‐assessments in health professions training. Acad Med. 1991;66:762–769.
- , , , , . Association of note quality and quality of care: a cross‐sectional study. BMJ Qual Saf. 2014;23(5):406–413.
The electronic health record (EHR) has revolutionized the practice of medicine. As part of the economic stimulus package in 2009, Congress enacted the Health Information Technology for Economic and Clinical Health Act, which included incentives for physicians and hospitals to adopt an EHR by 2015. In the setting of more limited duty hours and demands for increased clinical productivity, EHRs have functions that may improve the quality and efficiency of clinical documentation.[1, 2, 3, 4, 5]
The process of note writing and the use of notes for clinical care have changed substantially with EHR implementation. Use of efficiency tools (ie, copy forward functions and autopopulation of data) may increase the speed of documentation.[5] Notes in an EHR are more legible and accessible and may be able to organize data to improve clinical care.[6]
Yet, many have commented on the negative consequences of documentation in an EHR. In a New England Journal of Medicine Perspective article, Drs. Hartzband and Groopman wrote, we have observed the electronic medical record become a powerful vehicle for perpetuating erroneous information, leading to diagnostic errors that gain momentum when passed on electronically.[7] As a result, the copy forward and autopopulation functions have come under significant scrutiny.[8, 9, 10] A survey conducted at 2 academic institutions found that 71% of residents and attendings believed that the copy forward function led to inconsistencies and outdated information.[11] Autopopulation has been criticized for creating lengthy notes full of trivial or redundant data, a phenomenon termed note bloat. Bloated notes may be less effective as a communication tool.[12] Additionally, the process of composing a note often stimulates critical thinking and may lead to changes in care. The act of copying forward a previous note and autopopulating data bypasses that process and in effect may suppress critical thinking.[13] Previous studies have raised numerous concerns regarding copy forward and autopopulation functionality in the EHR. Many have described the duplication of outdated data and the possibility of the introduction and perpetuation of errors.[14, 15, 16] The Veterans Affairs (VA) Puget Sound Health system evaluated 6322 copy events and found that 1 in 10 electronic patient charts contained an instance of high‐risk copying.[17] In a survey of faculty and residents at a single academic medical center, the majority of users of copy and paste functionality recognized the hazards; they responded that their notes may contain more outdated (66%) and more inconsistent information (69%). Yet, most felt copy forwarding improved the documentation of the entire hospital course (87%), overall physician documentation (69%), and should definitely be continued (91%).[11] Others have complained about the impact of copy forward on the expression of clinical reasoning.[7, 9, 18]
Previous discussions on the topic of overall note quality following EHR implementation have been limited to perspectives or opinion pieces of individual attending providers.[18] We conducted a survey across 4 academic institutions to analyze both housestaff and attendings perceptions of the quality of notes since the implementation of an EHR to better inform the discussion of the impact of an EHR on note quality.
METHODS
Participants
Surveys were administered via email to interns, residents (second‐, third‐, or fourth‐year residents, hereafter referred to as residents) and attendings at 4 academic hospitals that use the Epic EHR (Epic Corp., Madison, WI). The 4 institutions each adopted the Epic EHR, with mandatory faculty and resident training, between 1 and 5 years prior to the survey. Three of the institutions previously used systems with electronic notes, whereas the fourth institution previously used a system with handwritten notes. The study participation emails included a link to an online survey in REDCap.[19] We included interns and residents from the following types of residency programs: internal medicine categorical or primary care, medicine‐pediatrics, or medicine‐psychiatry. For housestaff (the combination of both interns and residents), exclusion criteria included preliminary or transitional year interns, or any interns or residents from other specialties who rotate on the medicine service. For attendings, participants included hospitalists, general internal medicine attendings, chief residents, and subspecialty medicine attendings, each of whom had worked for any amount of time on the inpatient medicine teaching service in the prior 12 months.
Design
We developed 3 unique surveys for interns, residents, and attendings to assess their perception of inpatient progress notes (see Supporting Information, Appendix, in the online version of this article). The surveys incorporated questions from 2 previously published sources, the 9‐item Physician Documentation Quality Instrument (PDQI‐9) (see online Appendix), a validated note‐scoring tool, and the Accreditation Council for Graduate Medical Education note‐writing competency checklists.[20] Additionally, faculty at the participating institutions developed questions to address practices and attitudes toward autopopulation, copy forward, and the purposes of a progress note. Responses were based on a 5‐point Likert scale. The intern and resident surveys asked for self‐evaluation of their own progress notes and those of their peers, whereas the attending surveys asked for assessment of housestaff notes.
The survey was left open for a total of 55 days and participants were sent reminder emails. The study received a waiver from the institutional review board at all 4 institutions.
Data Analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of California, San Francisco (UCSF).[19] The survey data were analyzed and the figures were created using Microsoft Excel 2008 (Microsoft Corp., Redmond, WA). Mean values for each survey question were calculated. Differences between the means among the groups were assessed using 2‐sample t tests. P values <0.05 were considered statistically significant.
RESULTS
Demographics
We received 99 completed surveys from interns, 155 completed surveys from residents, and 153 completed surveys from attendings across the 4 institutions. The overall response rate for interns was 68%, ranging from 59% at the University of California, San Diego (UCSD) to 74% at the University of Iowa. The overall response rate for residents was 49%, ranging from 38% at UCSF to 66% at the University of California, Los Angeles. The overall response rate for attendings was 70%, ranging from 53% at UCSD to 74% at UCSF.
A total of 78% of interns and 72% of residents had used an EHR at a prior institution. Of the residents, 90 were second‐year residents, 64 were third‐year residents, and 2 were fourth‐year residents. A total of 76% of attendings self‐identified as hospitalists.
Overall Assessment of Note Quality
Participants were asked to rate the quality of progress notes on a 5‐point scale (poor, fair, good, very good, excellent). Half of interns and residents rated their own progress notes as very good or excellent. A total of 44% percent of interns and 24% of residents rated their peers notes as very good or excellent, whereas only 15% of attending physicians rated housestaff notes as very good or excellent.
When asked to rate the change in progress note quality since their hospital had adopted the EHR, the majority of residents answered unchanged or better, and the majority of attendings answered unchanged or worse (Figure 1).

PDQI‐9 Framework
Participants answered each PDQI‐9 question on a 5‐point Likert scale ranging from not at all (1) to extremely (5). In 8 of the 9 PDQI‐9 domains, there were no significant differences between interns and residents. Across each domain, attending perceptions of housestaff notes were significantly lower than housestaff perceptions of their own notes (P<0.001) (Figure 2). Both housestaff and attendings gave the highest ratings to thorough, up to date, and synthesized and the lowest rating to succinct.

Copy Forward and Autopopulation
Overall, the effect of copy forward and autopopulation on critical thinking, note accuracy, and prioritizing the problem list was thought to be neutral or somewhat positive by interns, neutral by residents, and neutral or somewhat negative by attendings (P<0.001) (Figure 3). In all, 16% of interns, 22% of residents, and 55% of attendings reported that copy forward had a somewhat negative or very negative impact on critical thinking (P<0.001). In all, 16% of interns, 29% of residents and 39% of attendings thought that autopopulation had a somewhat negative or very negative impact on critical thinking (P<0.001).

Purpose of Progress Notes
Participants were provided with 7 possible purposes of a progress note and asked to rate the importance of each stated purpose. There was nearly perfect agreement between interns, residents, and attendings in the rank order of the importance of each purpose of a progress note (Table 1). Attendings and housestaff ranked communication with other providers and documenting important events and the plan for the day as the 2 most important purposes of a progress note, and billing and quality improvement as less important.
| Interns | Residents | Attendings | |
|---|---|---|---|
| Communication with other providers | 1 | 1 | 2 |
| Documenting important events and the plan for the day | 2 | 2 | 1 |
| Prioritizing issues going forward in the patient's care | 3 | 3 | 3 |
| Medicolegal | 4 | 4 | 4 |
| Stimulate critical thinking | 5 | 5 | 5 |
| Billing | 6 | 6 | 6 |
| Quality improvement | 7 | 7 | 7 |
DISCUSSION
This is the first large multicenter analysis of both attendings and housestaff perceptions of note quality in the EHR era. The findings provide insight into important differences and similarities in the perceptions of the 2 groups. Most striking is the difference in opinion of overall note quality, with only a small minority of faculty rating current housestaff notes as very good or excellent, whereas a much larger proportion of housestaff rated their own notes and those of their peers to be of high quality. Though participants were not specifically asked why note quality in general was suboptimal, housestaff and faculty rankings of specific domains from the PDQI‐9 may yield an important clue. Specifically, all groups expressed that the weakest attribute of current progress notes is succinct. This finding is consistent with the note bloat phenomenon, which has been maligned as a consequence of EHR implementation.[7, 14, 18, 21, 22]
One interesting finding was that only 5% of interns rated the notes of other housestaff as fair or poor. One possible explanation for this may be the tendency for an individual to enhance or augment the status or performance of the group to which he or she belongs as a mechanism to increase self‐image, known as the social identity theory.[23] Thus, housestaff may not criticize their peers to allow for identification with a group that is not deficient in note writing.
The more positive assessment of overall note quality among housestaff could be related to the different roles of housestaff and attendings on a teaching service. On a teaching service, housestaff are typically the writer, whereas attendings are almost exclusively the reader of progress notes. Housestaff may reap benefits, including efficiency, beyond the finished product. A perception of higher quality may reflect the process of note writing, data gathering, and critical thinking required to build an assessment and plan. The scores on the PDQI‐9 support this notion, as housestaff rated all 9 domains significantly higher than attendings.
Housestaff and attendings held greater differences of opinion with respect to the EHR's impact on note quality. Generally, housestaff perceived the EHR to have improved progress note quality, whereas attendings perceived the opposite. One explanation could be that these results reflect changing stages of development of physicians well described through the RIME framework (reporter, interpreter, manager, educator). Attendings may expect notes to reflect synthesis and analysis, whereas trainees may be satisfied with the data gathering that an EHR facilitates. In our survey, the trend of answers from intern to resident to attending suggests an evolving process of attitudes toward note quality.
The above reasons may also explain why housestaff were generally more positive than attendings about the effect of copy forward and autopopulation functions on critical thinking. Perhaps, as these functions can potentially increase efficiency and decrease time spent at the computer, although data are mixed on this finding, housestaff may have more time to spend with patients or develop a thorough plan and thus rate these functions positively.
Notably, housestaff and attendings had excellent agreement on the purposes of a progress note. They agreed that the 2 most important purposes were communication with other providers and documenting important events and the plan for the day. These are the 2 listed purposes that are most directly related to patient care. If future interventions to improve note quality require housestaff and attendings to significantly change their behavior, a focus on the impact on patient care might yield the best results.
There were several limitations in our study. Any study based on self‐assessment is subject to bias. A previous meta‐analysis and review described poor to moderate correlations between self‐assessed and external measures of performance.[24, 25] The survey data were aggregated from 4 institutions despite somewhat different, though relatively high, response rates between the institutions. There could be a response bias; those who did not respond may have systematically different perceptions of note quality. It should be noted that the general demographics of the respondents reflected those of the housestaff and attendings at 4 academic centers. All 4 of the participating institutions adopted the Epic EHR within the last several years of the survey being administered, and perceptions of note quality may be biased depending on the prior system used (ie, change from handwritten to electronic vs electronic to other electronic system). In addition, the survey results reflect experience with only 1 EHR, and our results may not apply to other EHR vendors or institutions like the VA, which have a long‐standing system in place. Last, we did not explore the impact of perceived note quality on the measured or perceived quality of care. One previous study found no direct correlation between note quality and clinical quality.[26]
There are several future directions for research based on our findings. First, potential differences between housestaff and attending perceptions of note quality could be further teased apart by studying the perceptions of attendings on a nonteaching service who write their own daily progress notes. Second, housestaff perceptions on why copy forward and autopopulation may increase critical thinking could be explored further with more direct questioning. Finally, although our study captured only perceptions of note quality, validated tools could be used to objectively measure note quality; these measurements could then be compared to perception of note quality as well as clinical outcomes.
Given the prevalence and the apparent belief that the benefits of an EHR outweigh the hazards, institutions should embrace these innovations but take steps to mitigate the potential errors and problems associated with copy forward and autopopulation. The results of our study should help inform future interventions.
Acknowledgements
The authors acknowledge the contributions of Russell Leslie from the University of Iowa.
Disclosure: Nothing to report.
The electronic health record (EHR) has revolutionized the practice of medicine. As part of the economic stimulus package in 2009, Congress enacted the Health Information Technology for Economic and Clinical Health Act, which included incentives for physicians and hospitals to adopt an EHR by 2015. In the setting of more limited duty hours and demands for increased clinical productivity, EHRs have functions that may improve the quality and efficiency of clinical documentation.[1, 2, 3, 4, 5]
The process of note writing and the use of notes for clinical care have changed substantially with EHR implementation. Use of efficiency tools (ie, copy forward functions and autopopulation of data) may increase the speed of documentation.[5] Notes in an EHR are more legible and accessible and may be able to organize data to improve clinical care.[6]
Yet, many have commented on the negative consequences of documentation in an EHR. In a New England Journal of Medicine Perspective article, Drs. Hartzband and Groopman wrote, we have observed the electronic medical record become a powerful vehicle for perpetuating erroneous information, leading to diagnostic errors that gain momentum when passed on electronically.[7] As a result, the copy forward and autopopulation functions have come under significant scrutiny.[8, 9, 10] A survey conducted at 2 academic institutions found that 71% of residents and attendings believed that the copy forward function led to inconsistencies and outdated information.[11] Autopopulation has been criticized for creating lengthy notes full of trivial or redundant data, a phenomenon termed note bloat. Bloated notes may be less effective as a communication tool.[12] Additionally, the process of composing a note often stimulates critical thinking and may lead to changes in care. The act of copying forward a previous note and autopopulating data bypasses that process and in effect may suppress critical thinking.[13] Previous studies have raised numerous concerns regarding copy forward and autopopulation functionality in the EHR. Many have described the duplication of outdated data and the possibility of the introduction and perpetuation of errors.[14, 15, 16] The Veterans Affairs (VA) Puget Sound Health system evaluated 6322 copy events and found that 1 in 10 electronic patient charts contained an instance of high‐risk copying.[17] In a survey of faculty and residents at a single academic medical center, the majority of users of copy and paste functionality recognized the hazards; they responded that their notes may contain more outdated (66%) and more inconsistent information (69%). Yet, most felt copy forwarding improved the documentation of the entire hospital course (87%), overall physician documentation (69%), and should definitely be continued (91%).[11] Others have complained about the impact of copy forward on the expression of clinical reasoning.[7, 9, 18]
Previous discussions on the topic of overall note quality following EHR implementation have been limited to perspectives or opinion pieces of individual attending providers.[18] We conducted a survey across 4 academic institutions to analyze both housestaff and attendings perceptions of the quality of notes since the implementation of an EHR to better inform the discussion of the impact of an EHR on note quality.
METHODS
Participants
Surveys were administered via email to interns, residents (second‐, third‐, or fourth‐year residents, hereafter referred to as residents) and attendings at 4 academic hospitals that use the Epic EHR (Epic Corp., Madison, WI). The 4 institutions each adopted the Epic EHR, with mandatory faculty and resident training, between 1 and 5 years prior to the survey. Three of the institutions previously used systems with electronic notes, whereas the fourth institution previously used a system with handwritten notes. The study participation emails included a link to an online survey in REDCap.[19] We included interns and residents from the following types of residency programs: internal medicine categorical or primary care, medicine‐pediatrics, or medicine‐psychiatry. For housestaff (the combination of both interns and residents), exclusion criteria included preliminary or transitional year interns, or any interns or residents from other specialties who rotate on the medicine service. For attendings, participants included hospitalists, general internal medicine attendings, chief residents, and subspecialty medicine attendings, each of whom had worked for any amount of time on the inpatient medicine teaching service in the prior 12 months.
Design
We developed 3 unique surveys for interns, residents, and attendings to assess their perception of inpatient progress notes (see Supporting Information, Appendix, in the online version of this article). The surveys incorporated questions from 2 previously published sources, the 9‐item Physician Documentation Quality Instrument (PDQI‐9) (see online Appendix), a validated note‐scoring tool, and the Accreditation Council for Graduate Medical Education note‐writing competency checklists.[20] Additionally, faculty at the participating institutions developed questions to address practices and attitudes toward autopopulation, copy forward, and the purposes of a progress note. Responses were based on a 5‐point Likert scale. The intern and resident surveys asked for self‐evaluation of their own progress notes and those of their peers, whereas the attending surveys asked for assessment of housestaff notes.
The survey was left open for a total of 55 days and participants were sent reminder emails. The study received a waiver from the institutional review board at all 4 institutions.
Data Analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of California, San Francisco (UCSF).[19] The survey data were analyzed and the figures were created using Microsoft Excel 2008 (Microsoft Corp., Redmond, WA). Mean values for each survey question were calculated. Differences between the means among the groups were assessed using 2‐sample t tests. P values <0.05 were considered statistically significant.
RESULTS
Demographics
We received 99 completed surveys from interns, 155 completed surveys from residents, and 153 completed surveys from attendings across the 4 institutions. The overall response rate for interns was 68%, ranging from 59% at the University of California, San Diego (UCSD) to 74% at the University of Iowa. The overall response rate for residents was 49%, ranging from 38% at UCSF to 66% at the University of California, Los Angeles. The overall response rate for attendings was 70%, ranging from 53% at UCSD to 74% at UCSF.
A total of 78% of interns and 72% of residents had used an EHR at a prior institution. Of the residents, 90 were second‐year residents, 64 were third‐year residents, and 2 were fourth‐year residents. A total of 76% of attendings self‐identified as hospitalists.
Overall Assessment of Note Quality
Participants were asked to rate the quality of progress notes on a 5‐point scale (poor, fair, good, very good, excellent). Half of interns and residents rated their own progress notes as very good or excellent. A total of 44% percent of interns and 24% of residents rated their peers notes as very good or excellent, whereas only 15% of attending physicians rated housestaff notes as very good or excellent.
When asked to rate the change in progress note quality since their hospital had adopted the EHR, the majority of residents answered unchanged or better, and the majority of attendings answered unchanged or worse (Figure 1).

PDQI‐9 Framework
Participants answered each PDQI‐9 question on a 5‐point Likert scale ranging from not at all (1) to extremely (5). In 8 of the 9 PDQI‐9 domains, there were no significant differences between interns and residents. Across each domain, attending perceptions of housestaff notes were significantly lower than housestaff perceptions of their own notes (P<0.001) (Figure 2). Both housestaff and attendings gave the highest ratings to thorough, up to date, and synthesized and the lowest rating to succinct.

Copy Forward and Autopopulation
Overall, the effect of copy forward and autopopulation on critical thinking, note accuracy, and prioritizing the problem list was thought to be neutral or somewhat positive by interns, neutral by residents, and neutral or somewhat negative by attendings (P<0.001) (Figure 3). In all, 16% of interns, 22% of residents, and 55% of attendings reported that copy forward had a somewhat negative or very negative impact on critical thinking (P<0.001). In all, 16% of interns, 29% of residents and 39% of attendings thought that autopopulation had a somewhat negative or very negative impact on critical thinking (P<0.001).

Purpose of Progress Notes
Participants were provided with 7 possible purposes of a progress note and asked to rate the importance of each stated purpose. There was nearly perfect agreement between interns, residents, and attendings in the rank order of the importance of each purpose of a progress note (Table 1). Attendings and housestaff ranked communication with other providers and documenting important events and the plan for the day as the 2 most important purposes of a progress note, and billing and quality improvement as less important.
| Interns | Residents | Attendings | |
|---|---|---|---|
| Communication with other providers | 1 | 1 | 2 |
| Documenting important events and the plan for the day | 2 | 2 | 1 |
| Prioritizing issues going forward in the patient's care | 3 | 3 | 3 |
| Medicolegal | 4 | 4 | 4 |
| Stimulate critical thinking | 5 | 5 | 5 |
| Billing | 6 | 6 | 6 |
| Quality improvement | 7 | 7 | 7 |
DISCUSSION
This is the first large multicenter analysis of both attendings and housestaff perceptions of note quality in the EHR era. The findings provide insight into important differences and similarities in the perceptions of the 2 groups. Most striking is the difference in opinion of overall note quality, with only a small minority of faculty rating current housestaff notes as very good or excellent, whereas a much larger proportion of housestaff rated their own notes and those of their peers to be of high quality. Though participants were not specifically asked why note quality in general was suboptimal, housestaff and faculty rankings of specific domains from the PDQI‐9 may yield an important clue. Specifically, all groups expressed that the weakest attribute of current progress notes is succinct. This finding is consistent with the note bloat phenomenon, which has been maligned as a consequence of EHR implementation.[7, 14, 18, 21, 22]
One interesting finding was that only 5% of interns rated the notes of other housestaff as fair or poor. One possible explanation for this may be the tendency for an individual to enhance or augment the status or performance of the group to which he or she belongs as a mechanism to increase self‐image, known as the social identity theory.[23] Thus, housestaff may not criticize their peers to allow for identification with a group that is not deficient in note writing.
The more positive assessment of overall note quality among housestaff could be related to the different roles of housestaff and attendings on a teaching service. On a teaching service, housestaff are typically the writer, whereas attendings are almost exclusively the reader of progress notes. Housestaff may reap benefits, including efficiency, beyond the finished product. A perception of higher quality may reflect the process of note writing, data gathering, and critical thinking required to build an assessment and plan. The scores on the PDQI‐9 support this notion, as housestaff rated all 9 domains significantly higher than attendings.
Housestaff and attendings held greater differences of opinion with respect to the EHR's impact on note quality. Generally, housestaff perceived the EHR to have improved progress note quality, whereas attendings perceived the opposite. One explanation could be that these results reflect changing stages of development of physicians well described through the RIME framework (reporter, interpreter, manager, educator). Attendings may expect notes to reflect synthesis and analysis, whereas trainees may be satisfied with the data gathering that an EHR facilitates. In our survey, the trend of answers from intern to resident to attending suggests an evolving process of attitudes toward note quality.
The above reasons may also explain why housestaff were generally more positive than attendings about the effect of copy forward and autopopulation functions on critical thinking. Perhaps, as these functions can potentially increase efficiency and decrease time spent at the computer, although data are mixed on this finding, housestaff may have more time to spend with patients or develop a thorough plan and thus rate these functions positively.
Notably, housestaff and attendings had excellent agreement on the purposes of a progress note. They agreed that the 2 most important purposes were communication with other providers and documenting important events and the plan for the day. These are the 2 listed purposes that are most directly related to patient care. If future interventions to improve note quality require housestaff and attendings to significantly change their behavior, a focus on the impact on patient care might yield the best results.
There were several limitations in our study. Any study based on self‐assessment is subject to bias. A previous meta‐analysis and review described poor to moderate correlations between self‐assessed and external measures of performance.[24, 25] The survey data were aggregated from 4 institutions despite somewhat different, though relatively high, response rates between the institutions. There could be a response bias; those who did not respond may have systematically different perceptions of note quality. It should be noted that the general demographics of the respondents reflected those of the housestaff and attendings at 4 academic centers. All 4 of the participating institutions adopted the Epic EHR within the last several years of the survey being administered, and perceptions of note quality may be biased depending on the prior system used (ie, change from handwritten to electronic vs electronic to other electronic system). In addition, the survey results reflect experience with only 1 EHR, and our results may not apply to other EHR vendors or institutions like the VA, which have a long‐standing system in place. Last, we did not explore the impact of perceived note quality on the measured or perceived quality of care. One previous study found no direct correlation between note quality and clinical quality.[26]
There are several future directions for research based on our findings. First, potential differences between housestaff and attending perceptions of note quality could be further teased apart by studying the perceptions of attendings on a nonteaching service who write their own daily progress notes. Second, housestaff perceptions on why copy forward and autopopulation may increase critical thinking could be explored further with more direct questioning. Finally, although our study captured only perceptions of note quality, validated tools could be used to objectively measure note quality; these measurements could then be compared to perception of note quality as well as clinical outcomes.
Given the prevalence and the apparent belief that the benefits of an EHR outweigh the hazards, institutions should embrace these innovations but take steps to mitigate the potential errors and problems associated with copy forward and autopopulation. The results of our study should help inform future interventions.
Acknowledgements
The authors acknowledge the contributions of Russell Leslie from the University of Iowa.
Disclosure: Nothing to report.
- , , , et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144(10):742–752.
- , , , , . Clinical information technologies and inpatient outcomes: a multiple hospital study. Arch Intern Med. 2009;169(2):108–114.
- , , , et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280(15):1311–1316.
- , , , . Electronic health records and quality of diabetes care. N Engl J Med. 2011;365(9):825–833.
- , , , et al. The impact of a clinical information system in an intensive care unit. J Clin Monit Comput. 2008;22(1):31–36.
- , . Can electronic clinical documentation help prevent diagnostic errors? N Engl J Med. 2010;362(12):1066–1069.
- , . Off the record—avoiding the pitfalls of going electronic. N Eng J Med. 2008;358(16):1656–1658.
- , , . Copying and pasting of examinations within the electronic medical record. Int J Med Inform. 2007;76(suppl 1):S122–S128.
- , . Copy and paste: a remediable hazard of electronic health records. Am J Med. 2009;122(6):495–496.
- , , . The role of copy‐and‐paste in the hospital electronic health record. JAMA Intern Med. 2014;174(8):1217–1218.
- , , , , , . Physicians’ attitudes towards copy and pasting in electronic note writing. J Gen Intern Med. 2009;24(1):63–68.
- , , , , . Medical education in the electronic medical record (EMR) era: benefits, challenges, and future directions. Acad Med. 2013;88(6):748–752.
- , . Educational impact of the electronic medical record. J Surg Educ. 2012;69(1):105–112.
- , , , , , . Direct text entry in electronic progress notes. An evaluation of input errors. Methods Inf Med. 2003;42(1):61–67.
- . The clinical record: a 200‐year‐old 21st‐century challenge. Ann Intern Med. 2010;153(10):682–683.
- . Sloppy and paste. Morbidity and Mortality Rounds on the Web. Available at: http://www.webmm.ahrq.gov/case.aspx?caseID=274. Published July 2012. Accessed September 26, 2014.
- , , , . Are electronic medical records trustworthy? Observations on copying, pasting and duplication. AMIA Annu Symp Proc. 2003:269–273.
- . A piece of my mind. John Lennon's elbow. JAMA. 2012;308(5):463–464.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- , , . ACGME competency note checklist. Available at: http://www.im.org/p/cm/ld/fid=831. Accessed August 8, 2013.
- , , , . Assessing electronic note quality using the Physician Documentation Quality Instrument (PDQI‐9). Appl Clin Inform. 2012;3(2):164–174.
- , , , . Quantifying clinical narrative redundancy in an electronic health record. J Am Med Inform Assoc. 2010;17(1):49–53.
- , . The social identity theory of intergroup behavior. In: Psychology of Intergroup Relations. 2nd ed. Chicago, IL: Nelson‐Hall Publishers; 1986:7–24.
- , . Student self‐assessment in higher education: a meta‐analysis. Rev Educ Res. 1989;59:395–430.
- . A review of the validity and accuracy of self‐assessments in health professions training. Acad Med. 1991;66:762–769.
- , , , , . Association of note quality and quality of care: a cross‐sectional study. BMJ Qual Saf. 2014;23(5):406–413.
- , , , et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144(10):742–752.
- , , , , . Clinical information technologies and inpatient outcomes: a multiple hospital study. Arch Intern Med. 2009;169(2):108–114.
- , , , et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280(15):1311–1316.
- , , , . Electronic health records and quality of diabetes care. N Engl J Med. 2011;365(9):825–833.
- , , , et al. The impact of a clinical information system in an intensive care unit. J Clin Monit Comput. 2008;22(1):31–36.
- , . Can electronic clinical documentation help prevent diagnostic errors? N Engl J Med. 2010;362(12):1066–1069.
- , . Off the record—avoiding the pitfalls of going electronic. N Eng J Med. 2008;358(16):1656–1658.
- , , . Copying and pasting of examinations within the electronic medical record. Int J Med Inform. 2007;76(suppl 1):S122–S128.
- , . Copy and paste: a remediable hazard of electronic health records. Am J Med. 2009;122(6):495–496.
- , , . The role of copy‐and‐paste in the hospital electronic health record. JAMA Intern Med. 2014;174(8):1217–1218.
- , , , , , . Physicians’ attitudes towards copy and pasting in electronic note writing. J Gen Intern Med. 2009;24(1):63–68.
- , , , , . Medical education in the electronic medical record (EMR) era: benefits, challenges, and future directions. Acad Med. 2013;88(6):748–752.
- , . Educational impact of the electronic medical record. J Surg Educ. 2012;69(1):105–112.
- , , , , , . Direct text entry in electronic progress notes. An evaluation of input errors. Methods Inf Med. 2003;42(1):61–67.
- . The clinical record: a 200‐year‐old 21st‐century challenge. Ann Intern Med. 2010;153(10):682–683.
- . Sloppy and paste. Morbidity and Mortality Rounds on the Web. Available at: http://www.webmm.ahrq.gov/case.aspx?caseID=274. Published July 2012. Accessed September 26, 2014.
- , , , . Are electronic medical records trustworthy? Observations on copying, pasting and duplication. AMIA Annu Symp Proc. 2003:269–273.
- . A piece of my mind. John Lennon's elbow. JAMA. 2012;308(5):463–464.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- , , . ACGME competency note checklist. Available at: http://www.im.org/p/cm/ld/fid=831. Accessed August 8, 2013.
- , , , . Assessing electronic note quality using the Physician Documentation Quality Instrument (PDQI‐9). Appl Clin Inform. 2012;3(2):164–174.
- , , , . Quantifying clinical narrative redundancy in an electronic health record. J Am Med Inform Assoc. 2010;17(1):49–53.
- , . The social identity theory of intergroup behavior. In: Psychology of Intergroup Relations. 2nd ed. Chicago, IL: Nelson‐Hall Publishers; 1986:7–24.
- , . Student self‐assessment in higher education: a meta‐analysis. Rev Educ Res. 1989;59:395–430.
- . A review of the validity and accuracy of self‐assessments in health professions training. Acad Med. 1991;66:762–769.
- , , , , . Association of note quality and quality of care: a cross‐sectional study. BMJ Qual Saf. 2014;23(5):406–413.
© 2015 Society of Hospital Medicine
A coat with a clue
A 59‐year‐old man with a history of hypertension was admitted with 6 months of shortness of breath, night sweats, and debilitating fatigue. His symptoms were initially mild and would persist for weeks at a time, after which he would feel better for several days. Over the 2 weeks prior to admission his symptoms had progressed, and he had become dyspneic with minimal exertion.
Progressive dyspnea has a broad differential that includes diseases of the heart (eg, congestive heart failure, aortic stenosis, constrictive pericarditis), lung (eg, chronic obstructive pulmonary disease, interstitial lung disease, pulmonary hypertension, pleural effusion), and blood (eg, anemia).
Night sweats suggest an inflammatory condition, but do not help prioritize infection, malignancy, or autoimmunity. Any of those conditions can be relapsing and remitting, at least in their early phases, but the return to normalcy raises the possibility of hypersensitivity pneumonitis from a periodic exposure.
The 6‐month duration makes typical bacterial and viral infections less likely and suggests indolent infections such as mycobacteria, fungi, or human immunodeficiency virus. Lymphoma or chronic leukemia could cause dyspnea through pleural or pulmonary involvement or from anemia. Autoimmune conditions such as systemic lupus erythematosus or adult Still's disease could also present with this course.
On admission, he described progressive orthopnea, lower extremity edema, and a 15‐lb weight gain. He denied chest pain or palpitations. His symptoms did not correlate with environmental or occupational exposures. He had been diagnosed with essential hypertension a few years earlier but was not taking any medications. He worked as an editor for a newspaper and had traveled throughout California. He never used tobacco and drank alcohol in moderation. He previously smoked marijuana. His father died of Alzheimer's disease, and his mother and 2 siblings were healthy.
Orthopnea, lower extremity edema, and weight gain suggest volume overload, which can result from heart failure, cirrhosis, renal failure, or nephrotic syndrome. The untreated hypertension is a principal risk factor for heart failure. Subacute bacterial endocarditis is an important consideration in a patient with suspected heart failure and night sweats. Travel through the central valley of California may have exposed him to coccidiodomycosis, which can cause chronic pulmonary and extrapulmonary infection.
Physical examination revealed a chronically ill‐appearing man in mild respiratory distress. His temperature was 37.2C, heart rate was 83 bpm, and blood pressure was 168/81 mm Hg. His oxygen saturation was 97% with a respiratory rate of 17 while breathing ambient air. Bilateral chemosis was present. He had crackles at the lung bases. There was a 2/6 systolic murmur loudest at the left lower sternal border with apical radiation. His jugular venous pressure was 2 cm above the sternal angle at 45. He had mild pitting edema of both lower extremities. His abdomen was soft and nondistended. He demonstrated full range of motion of all extremities and had no rashes. He was alert and oriented to person, place, and time. There were no cranial nerve deficits. His strength, sensation and coordination were intact, and he had a normal gait.
Chemosis (conjunctival edema) usually represents conjunctival irritation from an allergic, infectious, or toxic process. It can also be seen in cases of increased ophthalmic venous pressure such as hyperthyroid ophthalmopathy, superior vena cava syndrome, or carotid‐cavernous sinus fistula. The crackles, weight gain, borderline jugular venous distention, and edema suggest some systemic volume overload, but not enough to produce chemosis.
The location and timing of the murmur suggests regurgitation through the mitral or tricuspid valve, a ventricular septal defect, or hypertrophic cardiomyopathy. Tricuspid regurgitation may indicate pulmonary hypertension with right ventricular failure. Despite the absence of fever, subacute bacterial endocarditis remains a concern.
Laboratory evaluation revealed a white blood cell count of 9600/L, hemoglobin of 8.7 g/dL, and platelet count of 522,000/L. Mean corpuscular volume was 88 fL. Serum chemistries were normal; serum creatinine was 1.2 mg/dL. Serum albumin was 2.6 g/dL. A urinalysis was normal. An electrocardiogram demonstrated normal sinus rhythm and left ventricular hypertrophy (LVH). A chest x‐ray revealed interstitial edema and small bilateral pleural effusions. A transthoracic echocardiogram demonstrated normal left ventricular systolic function, an ejection fraction of 65%, mild LVH, and mild diastolic dysfunction. Mild mitral regurgitation, a mildly dilated left atrium, and a minimal pericardial effusion were also noted. A renal ultrasound revealed an atrophic left kidney without arterial flow. He was treated with diuretics for presumed heart failure related to diastolic dysfunction. His dyspnea partially improved, and he was discharged.
Heart failure with preserved ejection fraction may be contributing to his dyspnea but is unlikely to be entirely explanatory given the laboratory abnormalities. The absence of valvular vegetations on transthoracic echocardiogram lowers the probability of bacterial endocarditis. The interstitial pulmonary markings may represent pulmonary edema but alternatively could reflect interstitial lung disease, lymphangitic spread of cancer, infection (eg, Pneumocystis jiroveci), or diffuse alveolar hemorrhage.
Anemia may also be contributing to his dyspnea. There is no evidence of bleeding on history, examination, or imaging. Hemolysis is unlikely given the absence of jaundice, splenomegaly, or a known predisposing condition. The normocytic anemia may also arise from chronic inflammation. Severe anemia can cause high output heart failure, but usually the hemoglobin level is much lower and the echocardiogram would have suggestive findings. Thrombocytosis suggests inflammation, a primary myeloproliferative disorder, or severe iron deficiency (not suspected here). His hypoalbuminemia is further evidence of chronic inflammation especially in the absence of nephropathy, hepatopathy, or a protein‐losing enteropathy.
An atrophic kidney may be congenital or result from long‐standing unilateral renal ischemia, infection, or obstruction. Diminished arterial flow in a middle‐aged man with hypertension may simply reflect atherosclerotic renal artery stenosis, but mass effect within the left renal artery from thrombus, infection, or cancer cannot be ruled out.
Four weeks later he was readmitted for progressive dyspnea and persistent night sweats. He was afebrile, fatigued, and in marked respiratory distress. The remainder of his physical examination was unchanged. Laboratory evaluation revealed a white blood cell count of 20,000/L with neutrophilic predominance, hemoglobin of 11 g/dL, and platelet count of 614,000/L. Creatinine was 1.4 mg/dL. Erythrocyte sedimentation rate (ESR) was greater than 100 mm/h, and C‐reactive protein (CRP) was 44 mg/L. Blood cultures were negative. Chest x‐ray (Figure 1) revealed persistent interstitial edema and increased bilateral pleural effusions.
Although clinical and radiologic features continue to suggest heart failure, the marked respiratory distress and persistent chest x‐ray abnormalities imply that a superimposed process is affecting the lungs. The night sweats, neutrophilia, and elevated ESR and CRP strongly suggest an inflammatory state from infection, malignancy, or autoimmunity.
A computed tomography (CT) scan of the lungs would help assess for interstitial lung disease, lymphangitic carcinomatosis and septic emboli. Blood cultures should be repeated to definitively exclude subacute endocarditis. A peripheral blood smear is needed to evaluate for hematologic malignancy. Finally, human immunodeficiency virus antibody testing is indicated.
CT of the abdomen and pelvis demonstrated left renal artery stenosis, an atrophic left kidney, right kidney edema with mild perinephric stranding, and mild‐to‐moderate right hydroureter without an obstructing mass or calculus. There was mild splenomegaly and mesenteric lymphadenopathy up to 3 cm in diameter. The distal thoracic and suprarenal abdominal aorta had crescentic high‐density wall thickening. There were small sclerotic densities of the proximal femora, pelvic girdle, and thoracolumbar spine (Figure 2). Contrast chest CT demonstrated severe wall thickening of his entire thoracic aorta. There was also cardiomegaly, mild interlobular septal thickening, small bilateral pleural effusions, a 3.2‐cm right upper lobe paratracheal lymph node, and nodular pleural thickening (Figure 3).
Diffuse aortopathy is caused by inflammatory, infectious, or infiltrative processes. Large vessel vasculitides such as Behet's disease, giant cell arteritis, and Takayasu's arteritis are unlikely, as the patient lacks the associated clinical findings or epidemiology. Imaging does not reveal preexisting aortic pathology, such as an aneurysm or atherosclerotic plaque, which could predispose him to bacterial endovascular infection.
Urinary system dilation without an obvious obstruction could be explained by retroperitoneal fibrosis. Generalized lymphadenopathy, (suspected) retroperitoneal fibrosis, sclerotic bone lesions, and cardiopulmonary disease collectively suggest a widespread infiltrative process. Lymphoma may lead to lymphadenopathy and bone lesions but would not explain the aortopathy. He lacks risk factors for infections like tuberculosis or tertiary syphilis, a well‐known cause of aortopathy in the past.
Widespread multisystem involvement invites consideration of nonmalignant, noninfectious infiltrative disorders such as immunoglobulin G4‐related disease (IgG4‐RD), histiocytoses such as Erdheim‐Chester disease (ECD), systemic mastocytosis (SM), and sarcoidosis. ECD is a disorder of non‐Langerhans histiocytes that infiltrate the aorta, bones, retroperitoneum, lungs, myocardium, and periorbital structures. Perinephric stranding is sometimes seen in this condition. The lymphoplasmacytes in IgG4‐RD and noncaseating granulomas of sarcoidosis infiltrate many of the same organs. Common sites infiltrated by mast cells in SM include the bone and lymph nodes. Among these diseases, ECD and IgG4‐RD more commonly manifest with aortic and retroperitoneal infiltration and thus are prioritized on this differential diagnosis.
A positron emission tomography (PET) scan revealed abnormal fluordeoxyglucose uptake involving the thoracic aorta, right apical pleural surface, perinephric soft tissue, and various marrow spaces. Core needle biopsy of a sclerotic lesion in the right ischium demonstrated focal marrow replacement by a fibrohistiocytic process. No malignant cells or pathogenic organisms were identified. Biopsy of the right kidney revealed chronic interstitial nephritis with features of megalocytic interstitial nephritis (histiocytic inflammation) and arteriolar nephrosclerosis. A transbronchial biopsy demonstrated alveolar tissue with focal intra‐alveolar hemorrhage and organization, but no malignancy, atypia, or pathogenic organisms.
The biopsy results do not support infection, lymphoma, or carcinoma. The absence of noncaseating granulomas and mastocytes on multiple biopsies essentially rules out sarcoidosis and SM, respectively. None of the characteristic pathologic features of IgG4‐RDlymphoplasmacytic infiltrate, obliterative phlebitis, and fibrosiswere observed. The pulmonary pathology points to injury, but not the underlying cause. The bone and kidney tissue samples reveal histiocytic infiltration.
The abnormalities of the aorta, bone, lung, kidney, and retroperitoneum can be explained by the diffuse histiocytic involvement seen in ECD. The perinephric stranding detected on CT and perinephric inflammation on the PET scan may reflect the hairy kidney of ECD, which is a result of histiocytic infiltration. It is possible that the chemosis relates to exophthalmos from histiocytic orbital infiltrates. Sensitivity for detecting orbital pathology on a PET scan is limited because of the high signal from the adjacent brain.
ECD should be distinguished from Langerhans cell histiocytosis (LCH) by immunohistologic staining and microscopic characteristics of the histiocytes. LCH usually does not involve the aorta, and it more commonly involves the skin.
Serum IgG4 was within normal limits, and immunohistochemical staining of pathology specimens for IgG4 was negative. The BRAF V600E mutation, which is present in the majority of patients with ECD, was detected in a subsequent right perinephric biopsy specimen. The patient was diagnosed with ECD.
Prednisone and pegylated interferon‐ led to a rapid improvement in his symptoms. As the prednisone was tapered, he developed bilateral periorbital swelling. Magnetic resonance imaging (MRI) revealed well‐circumscribed, intraorbital soft tissue masses with partial encasement of his optic nerves and superior ophthalmic veins, as well as infiltration of his transverse sinuses, consistent with intracranial manifestations of ECD. There was no evidence of pituitary, hypothalamic, or other brain parenchymal infiltration. His dyspnea, night sweats, and hypertension improved; however, 3 months into therapy he developed an extensive rash. Interferon was discontinued. Vemurafinib, a serine kinase inhibitor that targets the BRAF mutation, was prescribed with subsequent resolution of the rash.
COMMENTARY
This patient suffered from a chronic, progressive, inflammatory illness. Although the disease initially appeared to be confined to the heart and lungs, laboratory testing signaled a more systemic condition, and subsequent imaging demonstrated involvement of a disparate group of organs. Subacute disease processes with elevated markers of inflammation and diffuse organ involvement often fall into 1 of 3 categories: infectious, autoimmune, or neoplastic. The histiocytoses inhabit a fourth and easily overlooked category that can be described as infiltrative. Infiltrative diseases are a heterogeneous group of conditions that cause illness when cells or substances not normally found in tissues lead to organ dysfunction.
Although traditional teaching has focused on sarcoidosis, amyloidosis, and hemochromatosis as the primary representatives of this category, the medical literature describes a number of other infiltrative disease processes. IgG4‐RD is a fibroinflammatory disorder characterized by space‐occupying lesions, a lymphoplasmacytic infiltrate of IgG4‐positive plasma cells, and storiform (matted and irregularly whorled microscopic pattern) fibrosis.[1] IgG4‐RD, like sarcoidosis, blurs the categorical line between infiltrative and autoimmune diseases. Other infiltrative cellular disorders, such as histiocytosis and mastocytosis, exist on a spectrum between monoclonal proliferation and neoplastic invasion.
The histiocytoses represent a diverse group of disorders with an evolving nomenclature, characterized by localized or diffuse infiltration of macrophages, monocytes, and dendritic cells (Table 1). ECD is a rare, non‐Langerhans histiocytosis characterized by excessive recruitment and activation of histiocytes through kinase signaling pathways.[2, 3] Immunohistochemical staining for CD68, CD163, and Factor XIIIa, with lack of staining for CD1a, S100, and CD207, supports the diagnosis.[3] Mutations in the BRAF V600E gene (a protein kinase involved in cell proliferation) represent the most likely etiology of this overactivation. An estimated 38% to 100% of patients with ECD harbor this mutation, with detection rates influenced by the sensitivity of testing techniques.[3] The serine kinase inhibitor vemurafinib targets this mutation, and early experience with this agent in ECD demonstrates encouraging results.[4]
| Dendritic cell disorders |
| Langerhans cell histiocytosis |
| Secondary dendritic cell processes |
| Juvenile xanthogranuloma and related disorders (including Erdheim‐Chester disease) |
| Solitary histiocytomas with a dendritic phenotype |
| Macrophage‐related disorders |
| Primary hemophagocytic lymphohistiocytosis (familial and sporadic) |
| Secondary hemophagocytic syndromes |
| Sinus histiocytosis with massive lymphadenopathy (Rosai‐Dorfman disease) |
| Solitary histiocytoma with a macrophage phenotype |
| Malignant histiocytic disorders |
| Monocyte‐related leukemias |
| Extramedullary monocytic tumor or sarcoma |
| Dendritic cell‐related histiocytic sarcoma |
| Macrophage‐related histiocytic sarcoma |
ECD presents heterogeneously, occurring most commonly between the ages of 40 and 70 years. Nonspecific symptoms include weakness, fatigue, fever, chills, weight loss, and night sweats. Typical sites of involvement include the bone, central nervous system, cardiovascular system, lungs, and retroperitoneum. Bone involvement is nearly universal, and bone pain is the most common presenting symptom. Symmetric diaphyseal and metaphyseal osteosclerotic lesions may be seen on x‐rays, bone scan, PET, CT, and MRI.[3] Approximately 50% of patients have extraskeletal involvement at diagnosis.[5] Neurologic manifestations may result from invasion of histiocytes into the facial bones, orbits, meninges, and intracranial vessels, as eventually developed in this patient. Diabetes insipidus is the most common neurologic manifestation of ECD, followed by exophthalmos, cerebellar ataxia, panhypopituitarism, and papilledema.[6, 7] Approximately 75% of patients eventually suffer from cardiovascular disease, including hypertension, congestive heart failure, acute myocardial infarction, valvular dysfunction, pericardial infiltration, and cardiac tamponade.[8] Vascular involvement includes perivascular infiltration and periaortic fibrosis, resulting in the coated aorta seen in 20% of patients with ECD.[3] Pulmonary manifestations of ECD include interstitial, pleural, and consolidative lung disease. A review of high‐resolution chest CTs of patients with ECD demonstrated that greater than half had evidence of parenchymal lung disease, with interlobular septal thickening being the most common finding.[9] Infiltration and fibrosis of retroperitoneal structures is common. Infiltration of perinephric fat creates irregular renal borders, appearing radiographically as hairy kidneys.
Arriving at the diagnosis in this case proved to be challenging because the early presentation was consistent with congestive heart failure. As the patient's conditioned deteriorated, imaging suggested multisystem involvement. It was the extensive aortopathy in particularnot the less specific bone, kidney, lymph node, or pulmonary findingsthat allowed the clinicians to hone the extensive differential diagnosis. The coated aorta is a finding that has been strongly associated with ECD; few other conditions coat the aorta in a similar fashion.[10] In most mysteries, the perpetrator's coat conceals his identity; however, in this story the coat gave it away.
KEY LEARNING POINTS
- Subacute, inflammatory, multiorgan disease is usually explained by 3 categoriesinfection, autoimmunity, and neoplasiabut a fourth category, infiltrative disorders, sometimes warrants consideration.
- ECD presents heterogeneously, ranging from localized disease to widespread organ infiltration. The classic presentation includes bone pain, diabetes insipidus, and exophthalmos.
- Characteristic radiological findings that suggest ECD include long bone osteosclerosis, a coated aorta from periaortic infiltration, and hairy kidneys from perinephric infiltration.
Disclosure
Nothing to report
- , , IgG4‐related disease. N Engl J Med. 2012;366(6):539–551.
- , , , Diagnosing Erdheim‐Chester disease. Ann Rheum Dis. 2013;72(7):e19.
- , , , et al. Consensus guidelines for the diagnosis and clinical management of Erdheim‐Chester disease. Blood. 2014;124(4):483–492.
- , , , et al. The efficacy of vemurafenib in Erdheim‐Chester Disease and Langerhans Cell Histiocytosis: preliminary results from VE‐Basket Study. Blood. 2014;124(21):635.
- , , , et al. Erdheim‐Chester disease. Clinical and radiologic characteristics of 59 cases. Medicine (Baltimore). 1996;75(3):157–169.
- , , , et al. Neurological manifestations and neuroradiological presentation of Erdheim‐Chester disease: report of 6 cases and systematic review of the literature. J Neurol. 2006;253(10):1267–1277.
- , , , et al. Cerebral, facial, and orbital involvement in Erdheim‐Chester disease: CT and MR imaging findings. Radiology. 2010;255(2):586–594.
- , , , et al. Images in cardiovascular medicine. Cardiac involvement in Erdheim‐Chester disease: magnetic resonance and computed tomographic scan imaging in a monocentric series of 37 patients. Circulation. 2009;119(25):e597–e598.
- , , , et al. Pulmonary involvement in Erdheim‐Chester disease: a single‐center study of thirty‐four patients and a review of the literature. Arthritis Rheum. 2010;62(11):3504–3512.
- , , , et al. “Coated aorta”: a new sign of Erdheim‐Chester disease. J Rheumatol. 2000;27(6):1550–1553.
A 59‐year‐old man with a history of hypertension was admitted with 6 months of shortness of breath, night sweats, and debilitating fatigue. His symptoms were initially mild and would persist for weeks at a time, after which he would feel better for several days. Over the 2 weeks prior to admission his symptoms had progressed, and he had become dyspneic with minimal exertion.
Progressive dyspnea has a broad differential that includes diseases of the heart (eg, congestive heart failure, aortic stenosis, constrictive pericarditis), lung (eg, chronic obstructive pulmonary disease, interstitial lung disease, pulmonary hypertension, pleural effusion), and blood (eg, anemia).
Night sweats suggest an inflammatory condition, but do not help prioritize infection, malignancy, or autoimmunity. Any of those conditions can be relapsing and remitting, at least in their early phases, but the return to normalcy raises the possibility of hypersensitivity pneumonitis from a periodic exposure.
The 6‐month duration makes typical bacterial and viral infections less likely and suggests indolent infections such as mycobacteria, fungi, or human immunodeficiency virus. Lymphoma or chronic leukemia could cause dyspnea through pleural or pulmonary involvement or from anemia. Autoimmune conditions such as systemic lupus erythematosus or adult Still's disease could also present with this course.
On admission, he described progressive orthopnea, lower extremity edema, and a 15‐lb weight gain. He denied chest pain or palpitations. His symptoms did not correlate with environmental or occupational exposures. He had been diagnosed with essential hypertension a few years earlier but was not taking any medications. He worked as an editor for a newspaper and had traveled throughout California. He never used tobacco and drank alcohol in moderation. He previously smoked marijuana. His father died of Alzheimer's disease, and his mother and 2 siblings were healthy.
Orthopnea, lower extremity edema, and weight gain suggest volume overload, which can result from heart failure, cirrhosis, renal failure, or nephrotic syndrome. The untreated hypertension is a principal risk factor for heart failure. Subacute bacterial endocarditis is an important consideration in a patient with suspected heart failure and night sweats. Travel through the central valley of California may have exposed him to coccidiodomycosis, which can cause chronic pulmonary and extrapulmonary infection.
Physical examination revealed a chronically ill‐appearing man in mild respiratory distress. His temperature was 37.2C, heart rate was 83 bpm, and blood pressure was 168/81 mm Hg. His oxygen saturation was 97% with a respiratory rate of 17 while breathing ambient air. Bilateral chemosis was present. He had crackles at the lung bases. There was a 2/6 systolic murmur loudest at the left lower sternal border with apical radiation. His jugular venous pressure was 2 cm above the sternal angle at 45. He had mild pitting edema of both lower extremities. His abdomen was soft and nondistended. He demonstrated full range of motion of all extremities and had no rashes. He was alert and oriented to person, place, and time. There were no cranial nerve deficits. His strength, sensation and coordination were intact, and he had a normal gait.
Chemosis (conjunctival edema) usually represents conjunctival irritation from an allergic, infectious, or toxic process. It can also be seen in cases of increased ophthalmic venous pressure such as hyperthyroid ophthalmopathy, superior vena cava syndrome, or carotid‐cavernous sinus fistula. The crackles, weight gain, borderline jugular venous distention, and edema suggest some systemic volume overload, but not enough to produce chemosis.
The location and timing of the murmur suggests regurgitation through the mitral or tricuspid valve, a ventricular septal defect, or hypertrophic cardiomyopathy. Tricuspid regurgitation may indicate pulmonary hypertension with right ventricular failure. Despite the absence of fever, subacute bacterial endocarditis remains a concern.
Laboratory evaluation revealed a white blood cell count of 9600/L, hemoglobin of 8.7 g/dL, and platelet count of 522,000/L. Mean corpuscular volume was 88 fL. Serum chemistries were normal; serum creatinine was 1.2 mg/dL. Serum albumin was 2.6 g/dL. A urinalysis was normal. An electrocardiogram demonstrated normal sinus rhythm and left ventricular hypertrophy (LVH). A chest x‐ray revealed interstitial edema and small bilateral pleural effusions. A transthoracic echocardiogram demonstrated normal left ventricular systolic function, an ejection fraction of 65%, mild LVH, and mild diastolic dysfunction. Mild mitral regurgitation, a mildly dilated left atrium, and a minimal pericardial effusion were also noted. A renal ultrasound revealed an atrophic left kidney without arterial flow. He was treated with diuretics for presumed heart failure related to diastolic dysfunction. His dyspnea partially improved, and he was discharged.
Heart failure with preserved ejection fraction may be contributing to his dyspnea but is unlikely to be entirely explanatory given the laboratory abnormalities. The absence of valvular vegetations on transthoracic echocardiogram lowers the probability of bacterial endocarditis. The interstitial pulmonary markings may represent pulmonary edema but alternatively could reflect interstitial lung disease, lymphangitic spread of cancer, infection (eg, Pneumocystis jiroveci), or diffuse alveolar hemorrhage.
Anemia may also be contributing to his dyspnea. There is no evidence of bleeding on history, examination, or imaging. Hemolysis is unlikely given the absence of jaundice, splenomegaly, or a known predisposing condition. The normocytic anemia may also arise from chronic inflammation. Severe anemia can cause high output heart failure, but usually the hemoglobin level is much lower and the echocardiogram would have suggestive findings. Thrombocytosis suggests inflammation, a primary myeloproliferative disorder, or severe iron deficiency (not suspected here). His hypoalbuminemia is further evidence of chronic inflammation especially in the absence of nephropathy, hepatopathy, or a protein‐losing enteropathy.
An atrophic kidney may be congenital or result from long‐standing unilateral renal ischemia, infection, or obstruction. Diminished arterial flow in a middle‐aged man with hypertension may simply reflect atherosclerotic renal artery stenosis, but mass effect within the left renal artery from thrombus, infection, or cancer cannot be ruled out.
Four weeks later he was readmitted for progressive dyspnea and persistent night sweats. He was afebrile, fatigued, and in marked respiratory distress. The remainder of his physical examination was unchanged. Laboratory evaluation revealed a white blood cell count of 20,000/L with neutrophilic predominance, hemoglobin of 11 g/dL, and platelet count of 614,000/L. Creatinine was 1.4 mg/dL. Erythrocyte sedimentation rate (ESR) was greater than 100 mm/h, and C‐reactive protein (CRP) was 44 mg/L. Blood cultures were negative. Chest x‐ray (Figure 1) revealed persistent interstitial edema and increased bilateral pleural effusions.

Although clinical and radiologic features continue to suggest heart failure, the marked respiratory distress and persistent chest x‐ray abnormalities imply that a superimposed process is affecting the lungs. The night sweats, neutrophilia, and elevated ESR and CRP strongly suggest an inflammatory state from infection, malignancy, or autoimmunity.
A computed tomography (CT) scan of the lungs would help assess for interstitial lung disease, lymphangitic carcinomatosis and septic emboli. Blood cultures should be repeated to definitively exclude subacute endocarditis. A peripheral blood smear is needed to evaluate for hematologic malignancy. Finally, human immunodeficiency virus antibody testing is indicated.
CT of the abdomen and pelvis demonstrated left renal artery stenosis, an atrophic left kidney, right kidney edema with mild perinephric stranding, and mild‐to‐moderate right hydroureter without an obstructing mass or calculus. There was mild splenomegaly and mesenteric lymphadenopathy up to 3 cm in diameter. The distal thoracic and suprarenal abdominal aorta had crescentic high‐density wall thickening. There were small sclerotic densities of the proximal femora, pelvic girdle, and thoracolumbar spine (Figure 2). Contrast chest CT demonstrated severe wall thickening of his entire thoracic aorta. There was also cardiomegaly, mild interlobular septal thickening, small bilateral pleural effusions, a 3.2‐cm right upper lobe paratracheal lymph node, and nodular pleural thickening (Figure 3).


Diffuse aortopathy is caused by inflammatory, infectious, or infiltrative processes. Large vessel vasculitides such as Behet's disease, giant cell arteritis, and Takayasu's arteritis are unlikely, as the patient lacks the associated clinical findings or epidemiology. Imaging does not reveal preexisting aortic pathology, such as an aneurysm or atherosclerotic plaque, which could predispose him to bacterial endovascular infection.
Urinary system dilation without an obvious obstruction could be explained by retroperitoneal fibrosis. Generalized lymphadenopathy, (suspected) retroperitoneal fibrosis, sclerotic bone lesions, and cardiopulmonary disease collectively suggest a widespread infiltrative process. Lymphoma may lead to lymphadenopathy and bone lesions but would not explain the aortopathy. He lacks risk factors for infections like tuberculosis or tertiary syphilis, a well‐known cause of aortopathy in the past.
Widespread multisystem involvement invites consideration of nonmalignant, noninfectious infiltrative disorders such as immunoglobulin G4‐related disease (IgG4‐RD), histiocytoses such as Erdheim‐Chester disease (ECD), systemic mastocytosis (SM), and sarcoidosis. ECD is a disorder of non‐Langerhans histiocytes that infiltrate the aorta, bones, retroperitoneum, lungs, myocardium, and periorbital structures. Perinephric stranding is sometimes seen in this condition. The lymphoplasmacytes in IgG4‐RD and noncaseating granulomas of sarcoidosis infiltrate many of the same organs. Common sites infiltrated by mast cells in SM include the bone and lymph nodes. Among these diseases, ECD and IgG4‐RD more commonly manifest with aortic and retroperitoneal infiltration and thus are prioritized on this differential diagnosis.
A positron emission tomography (PET) scan revealed abnormal fluordeoxyglucose uptake involving the thoracic aorta, right apical pleural surface, perinephric soft tissue, and various marrow spaces. Core needle biopsy of a sclerotic lesion in the right ischium demonstrated focal marrow replacement by a fibrohistiocytic process. No malignant cells or pathogenic organisms were identified. Biopsy of the right kidney revealed chronic interstitial nephritis with features of megalocytic interstitial nephritis (histiocytic inflammation) and arteriolar nephrosclerosis. A transbronchial biopsy demonstrated alveolar tissue with focal intra‐alveolar hemorrhage and organization, but no malignancy, atypia, or pathogenic organisms.
The biopsy results do not support infection, lymphoma, or carcinoma. The absence of noncaseating granulomas and mastocytes on multiple biopsies essentially rules out sarcoidosis and SM, respectively. None of the characteristic pathologic features of IgG4‐RDlymphoplasmacytic infiltrate, obliterative phlebitis, and fibrosiswere observed. The pulmonary pathology points to injury, but not the underlying cause. The bone and kidney tissue samples reveal histiocytic infiltration.
The abnormalities of the aorta, bone, lung, kidney, and retroperitoneum can be explained by the diffuse histiocytic involvement seen in ECD. The perinephric stranding detected on CT and perinephric inflammation on the PET scan may reflect the hairy kidney of ECD, which is a result of histiocytic infiltration. It is possible that the chemosis relates to exophthalmos from histiocytic orbital infiltrates. Sensitivity for detecting orbital pathology on a PET scan is limited because of the high signal from the adjacent brain.
ECD should be distinguished from Langerhans cell histiocytosis (LCH) by immunohistologic staining and microscopic characteristics of the histiocytes. LCH usually does not involve the aorta, and it more commonly involves the skin.
Serum IgG4 was within normal limits, and immunohistochemical staining of pathology specimens for IgG4 was negative. The BRAF V600E mutation, which is present in the majority of patients with ECD, was detected in a subsequent right perinephric biopsy specimen. The patient was diagnosed with ECD.
Prednisone and pegylated interferon‐ led to a rapid improvement in his symptoms. As the prednisone was tapered, he developed bilateral periorbital swelling. Magnetic resonance imaging (MRI) revealed well‐circumscribed, intraorbital soft tissue masses with partial encasement of his optic nerves and superior ophthalmic veins, as well as infiltration of his transverse sinuses, consistent with intracranial manifestations of ECD. There was no evidence of pituitary, hypothalamic, or other brain parenchymal infiltration. His dyspnea, night sweats, and hypertension improved; however, 3 months into therapy he developed an extensive rash. Interferon was discontinued. Vemurafinib, a serine kinase inhibitor that targets the BRAF mutation, was prescribed with subsequent resolution of the rash.
COMMENTARY
This patient suffered from a chronic, progressive, inflammatory illness. Although the disease initially appeared to be confined to the heart and lungs, laboratory testing signaled a more systemic condition, and subsequent imaging demonstrated involvement of a disparate group of organs. Subacute disease processes with elevated markers of inflammation and diffuse organ involvement often fall into 1 of 3 categories: infectious, autoimmune, or neoplastic. The histiocytoses inhabit a fourth and easily overlooked category that can be described as infiltrative. Infiltrative diseases are a heterogeneous group of conditions that cause illness when cells or substances not normally found in tissues lead to organ dysfunction.
Although traditional teaching has focused on sarcoidosis, amyloidosis, and hemochromatosis as the primary representatives of this category, the medical literature describes a number of other infiltrative disease processes. IgG4‐RD is a fibroinflammatory disorder characterized by space‐occupying lesions, a lymphoplasmacytic infiltrate of IgG4‐positive plasma cells, and storiform (matted and irregularly whorled microscopic pattern) fibrosis.[1] IgG4‐RD, like sarcoidosis, blurs the categorical line between infiltrative and autoimmune diseases. Other infiltrative cellular disorders, such as histiocytosis and mastocytosis, exist on a spectrum between monoclonal proliferation and neoplastic invasion.
The histiocytoses represent a diverse group of disorders with an evolving nomenclature, characterized by localized or diffuse infiltration of macrophages, monocytes, and dendritic cells (Table 1). ECD is a rare, non‐Langerhans histiocytosis characterized by excessive recruitment and activation of histiocytes through kinase signaling pathways.[2, 3] Immunohistochemical staining for CD68, CD163, and Factor XIIIa, with lack of staining for CD1a, S100, and CD207, supports the diagnosis.[3] Mutations in the BRAF V600E gene (a protein kinase involved in cell proliferation) represent the most likely etiology of this overactivation. An estimated 38% to 100% of patients with ECD harbor this mutation, with detection rates influenced by the sensitivity of testing techniques.[3] The serine kinase inhibitor vemurafinib targets this mutation, and early experience with this agent in ECD demonstrates encouraging results.[4]
| Dendritic cell disorders |
| Langerhans cell histiocytosis |
| Secondary dendritic cell processes |
| Juvenile xanthogranuloma and related disorders (including Erdheim‐Chester disease) |
| Solitary histiocytomas with a dendritic phenotype |
| Macrophage‐related disorders |
| Primary hemophagocytic lymphohistiocytosis (familial and sporadic) |
| Secondary hemophagocytic syndromes |
| Sinus histiocytosis with massive lymphadenopathy (Rosai‐Dorfman disease) |
| Solitary histiocytoma with a macrophage phenotype |
| Malignant histiocytic disorders |
| Monocyte‐related leukemias |
| Extramedullary monocytic tumor or sarcoma |
| Dendritic cell‐related histiocytic sarcoma |
| Macrophage‐related histiocytic sarcoma |
ECD presents heterogeneously, occurring most commonly between the ages of 40 and 70 years. Nonspecific symptoms include weakness, fatigue, fever, chills, weight loss, and night sweats. Typical sites of involvement include the bone, central nervous system, cardiovascular system, lungs, and retroperitoneum. Bone involvement is nearly universal, and bone pain is the most common presenting symptom. Symmetric diaphyseal and metaphyseal osteosclerotic lesions may be seen on x‐rays, bone scan, PET, CT, and MRI.[3] Approximately 50% of patients have extraskeletal involvement at diagnosis.[5] Neurologic manifestations may result from invasion of histiocytes into the facial bones, orbits, meninges, and intracranial vessels, as eventually developed in this patient. Diabetes insipidus is the most common neurologic manifestation of ECD, followed by exophthalmos, cerebellar ataxia, panhypopituitarism, and papilledema.[6, 7] Approximately 75% of patients eventually suffer from cardiovascular disease, including hypertension, congestive heart failure, acute myocardial infarction, valvular dysfunction, pericardial infiltration, and cardiac tamponade.[8] Vascular involvement includes perivascular infiltration and periaortic fibrosis, resulting in the coated aorta seen in 20% of patients with ECD.[3] Pulmonary manifestations of ECD include interstitial, pleural, and consolidative lung disease. A review of high‐resolution chest CTs of patients with ECD demonstrated that greater than half had evidence of parenchymal lung disease, with interlobular septal thickening being the most common finding.[9] Infiltration and fibrosis of retroperitoneal structures is common. Infiltration of perinephric fat creates irregular renal borders, appearing radiographically as hairy kidneys.
Arriving at the diagnosis in this case proved to be challenging because the early presentation was consistent with congestive heart failure. As the patient's conditioned deteriorated, imaging suggested multisystem involvement. It was the extensive aortopathy in particularnot the less specific bone, kidney, lymph node, or pulmonary findingsthat allowed the clinicians to hone the extensive differential diagnosis. The coated aorta is a finding that has been strongly associated with ECD; few other conditions coat the aorta in a similar fashion.[10] In most mysteries, the perpetrator's coat conceals his identity; however, in this story the coat gave it away.
KEY LEARNING POINTS
- Subacute, inflammatory, multiorgan disease is usually explained by 3 categoriesinfection, autoimmunity, and neoplasiabut a fourth category, infiltrative disorders, sometimes warrants consideration.
- ECD presents heterogeneously, ranging from localized disease to widespread organ infiltration. The classic presentation includes bone pain, diabetes insipidus, and exophthalmos.
- Characteristic radiological findings that suggest ECD include long bone osteosclerosis, a coated aorta from periaortic infiltration, and hairy kidneys from perinephric infiltration.
Disclosure
Nothing to report
A 59‐year‐old man with a history of hypertension was admitted with 6 months of shortness of breath, night sweats, and debilitating fatigue. His symptoms were initially mild and would persist for weeks at a time, after which he would feel better for several days. Over the 2 weeks prior to admission his symptoms had progressed, and he had become dyspneic with minimal exertion.
Progressive dyspnea has a broad differential that includes diseases of the heart (eg, congestive heart failure, aortic stenosis, constrictive pericarditis), lung (eg, chronic obstructive pulmonary disease, interstitial lung disease, pulmonary hypertension, pleural effusion), and blood (eg, anemia).
Night sweats suggest an inflammatory condition, but do not help prioritize infection, malignancy, or autoimmunity. Any of those conditions can be relapsing and remitting, at least in their early phases, but the return to normalcy raises the possibility of hypersensitivity pneumonitis from a periodic exposure.
The 6‐month duration makes typical bacterial and viral infections less likely and suggests indolent infections such as mycobacteria, fungi, or human immunodeficiency virus. Lymphoma or chronic leukemia could cause dyspnea through pleural or pulmonary involvement or from anemia. Autoimmune conditions such as systemic lupus erythematosus or adult Still's disease could also present with this course.
On admission, he described progressive orthopnea, lower extremity edema, and a 15‐lb weight gain. He denied chest pain or palpitations. His symptoms did not correlate with environmental or occupational exposures. He had been diagnosed with essential hypertension a few years earlier but was not taking any medications. He worked as an editor for a newspaper and had traveled throughout California. He never used tobacco and drank alcohol in moderation. He previously smoked marijuana. His father died of Alzheimer's disease, and his mother and 2 siblings were healthy.
Orthopnea, lower extremity edema, and weight gain suggest volume overload, which can result from heart failure, cirrhosis, renal failure, or nephrotic syndrome. The untreated hypertension is a principal risk factor for heart failure. Subacute bacterial endocarditis is an important consideration in a patient with suspected heart failure and night sweats. Travel through the central valley of California may have exposed him to coccidiodomycosis, which can cause chronic pulmonary and extrapulmonary infection.
Physical examination revealed a chronically ill‐appearing man in mild respiratory distress. His temperature was 37.2C, heart rate was 83 bpm, and blood pressure was 168/81 mm Hg. His oxygen saturation was 97% with a respiratory rate of 17 while breathing ambient air. Bilateral chemosis was present. He had crackles at the lung bases. There was a 2/6 systolic murmur loudest at the left lower sternal border with apical radiation. His jugular venous pressure was 2 cm above the sternal angle at 45. He had mild pitting edema of both lower extremities. His abdomen was soft and nondistended. He demonstrated full range of motion of all extremities and had no rashes. He was alert and oriented to person, place, and time. There were no cranial nerve deficits. His strength, sensation and coordination were intact, and he had a normal gait.
Chemosis (conjunctival edema) usually represents conjunctival irritation from an allergic, infectious, or toxic process. It can also be seen in cases of increased ophthalmic venous pressure such as hyperthyroid ophthalmopathy, superior vena cava syndrome, or carotid‐cavernous sinus fistula. The crackles, weight gain, borderline jugular venous distention, and edema suggest some systemic volume overload, but not enough to produce chemosis.
The location and timing of the murmur suggests regurgitation through the mitral or tricuspid valve, a ventricular septal defect, or hypertrophic cardiomyopathy. Tricuspid regurgitation may indicate pulmonary hypertension with right ventricular failure. Despite the absence of fever, subacute bacterial endocarditis remains a concern.
Laboratory evaluation revealed a white blood cell count of 9600/L, hemoglobin of 8.7 g/dL, and platelet count of 522,000/L. Mean corpuscular volume was 88 fL. Serum chemistries were normal; serum creatinine was 1.2 mg/dL. Serum albumin was 2.6 g/dL. A urinalysis was normal. An electrocardiogram demonstrated normal sinus rhythm and left ventricular hypertrophy (LVH). A chest x‐ray revealed interstitial edema and small bilateral pleural effusions. A transthoracic echocardiogram demonstrated normal left ventricular systolic function, an ejection fraction of 65%, mild LVH, and mild diastolic dysfunction. Mild mitral regurgitation, a mildly dilated left atrium, and a minimal pericardial effusion were also noted. A renal ultrasound revealed an atrophic left kidney without arterial flow. He was treated with diuretics for presumed heart failure related to diastolic dysfunction. His dyspnea partially improved, and he was discharged.
Heart failure with preserved ejection fraction may be contributing to his dyspnea but is unlikely to be entirely explanatory given the laboratory abnormalities. The absence of valvular vegetations on transthoracic echocardiogram lowers the probability of bacterial endocarditis. The interstitial pulmonary markings may represent pulmonary edema but alternatively could reflect interstitial lung disease, lymphangitic spread of cancer, infection (eg, Pneumocystis jiroveci), or diffuse alveolar hemorrhage.
Anemia may also be contributing to his dyspnea. There is no evidence of bleeding on history, examination, or imaging. Hemolysis is unlikely given the absence of jaundice, splenomegaly, or a known predisposing condition. The normocytic anemia may also arise from chronic inflammation. Severe anemia can cause high output heart failure, but usually the hemoglobin level is much lower and the echocardiogram would have suggestive findings. Thrombocytosis suggests inflammation, a primary myeloproliferative disorder, or severe iron deficiency (not suspected here). His hypoalbuminemia is further evidence of chronic inflammation especially in the absence of nephropathy, hepatopathy, or a protein‐losing enteropathy.
An atrophic kidney may be congenital or result from long‐standing unilateral renal ischemia, infection, or obstruction. Diminished arterial flow in a middle‐aged man with hypertension may simply reflect atherosclerotic renal artery stenosis, but mass effect within the left renal artery from thrombus, infection, or cancer cannot be ruled out.
Four weeks later he was readmitted for progressive dyspnea and persistent night sweats. He was afebrile, fatigued, and in marked respiratory distress. The remainder of his physical examination was unchanged. Laboratory evaluation revealed a white blood cell count of 20,000/L with neutrophilic predominance, hemoglobin of 11 g/dL, and platelet count of 614,000/L. Creatinine was 1.4 mg/dL. Erythrocyte sedimentation rate (ESR) was greater than 100 mm/h, and C‐reactive protein (CRP) was 44 mg/L. Blood cultures were negative. Chest x‐ray (Figure 1) revealed persistent interstitial edema and increased bilateral pleural effusions.

Although clinical and radiologic features continue to suggest heart failure, the marked respiratory distress and persistent chest x‐ray abnormalities imply that a superimposed process is affecting the lungs. The night sweats, neutrophilia, and elevated ESR and CRP strongly suggest an inflammatory state from infection, malignancy, or autoimmunity.
A computed tomography (CT) scan of the lungs would help assess for interstitial lung disease, lymphangitic carcinomatosis and septic emboli. Blood cultures should be repeated to definitively exclude subacute endocarditis. A peripheral blood smear is needed to evaluate for hematologic malignancy. Finally, human immunodeficiency virus antibody testing is indicated.
CT of the abdomen and pelvis demonstrated left renal artery stenosis, an atrophic left kidney, right kidney edema with mild perinephric stranding, and mild‐to‐moderate right hydroureter without an obstructing mass or calculus. There was mild splenomegaly and mesenteric lymphadenopathy up to 3 cm in diameter. The distal thoracic and suprarenal abdominal aorta had crescentic high‐density wall thickening. There were small sclerotic densities of the proximal femora, pelvic girdle, and thoracolumbar spine (Figure 2). Contrast chest CT demonstrated severe wall thickening of his entire thoracic aorta. There was also cardiomegaly, mild interlobular septal thickening, small bilateral pleural effusions, a 3.2‐cm right upper lobe paratracheal lymph node, and nodular pleural thickening (Figure 3).


Diffuse aortopathy is caused by inflammatory, infectious, or infiltrative processes. Large vessel vasculitides such as Behet's disease, giant cell arteritis, and Takayasu's arteritis are unlikely, as the patient lacks the associated clinical findings or epidemiology. Imaging does not reveal preexisting aortic pathology, such as an aneurysm or atherosclerotic plaque, which could predispose him to bacterial endovascular infection.
Urinary system dilation without an obvious obstruction could be explained by retroperitoneal fibrosis. Generalized lymphadenopathy, (suspected) retroperitoneal fibrosis, sclerotic bone lesions, and cardiopulmonary disease collectively suggest a widespread infiltrative process. Lymphoma may lead to lymphadenopathy and bone lesions but would not explain the aortopathy. He lacks risk factors for infections like tuberculosis or tertiary syphilis, a well‐known cause of aortopathy in the past.
Widespread multisystem involvement invites consideration of nonmalignant, noninfectious infiltrative disorders such as immunoglobulin G4‐related disease (IgG4‐RD), histiocytoses such as Erdheim‐Chester disease (ECD), systemic mastocytosis (SM), and sarcoidosis. ECD is a disorder of non‐Langerhans histiocytes that infiltrate the aorta, bones, retroperitoneum, lungs, myocardium, and periorbital structures. Perinephric stranding is sometimes seen in this condition. The lymphoplasmacytes in IgG4‐RD and noncaseating granulomas of sarcoidosis infiltrate many of the same organs. Common sites infiltrated by mast cells in SM include the bone and lymph nodes. Among these diseases, ECD and IgG4‐RD more commonly manifest with aortic and retroperitoneal infiltration and thus are prioritized on this differential diagnosis.
A positron emission tomography (PET) scan revealed abnormal fluordeoxyglucose uptake involving the thoracic aorta, right apical pleural surface, perinephric soft tissue, and various marrow spaces. Core needle biopsy of a sclerotic lesion in the right ischium demonstrated focal marrow replacement by a fibrohistiocytic process. No malignant cells or pathogenic organisms were identified. Biopsy of the right kidney revealed chronic interstitial nephritis with features of megalocytic interstitial nephritis (histiocytic inflammation) and arteriolar nephrosclerosis. A transbronchial biopsy demonstrated alveolar tissue with focal intra‐alveolar hemorrhage and organization, but no malignancy, atypia, or pathogenic organisms.
The biopsy results do not support infection, lymphoma, or carcinoma. The absence of noncaseating granulomas and mastocytes on multiple biopsies essentially rules out sarcoidosis and SM, respectively. None of the characteristic pathologic features of IgG4‐RDlymphoplasmacytic infiltrate, obliterative phlebitis, and fibrosiswere observed. The pulmonary pathology points to injury, but not the underlying cause. The bone and kidney tissue samples reveal histiocytic infiltration.
The abnormalities of the aorta, bone, lung, kidney, and retroperitoneum can be explained by the diffuse histiocytic involvement seen in ECD. The perinephric stranding detected on CT and perinephric inflammation on the PET scan may reflect the hairy kidney of ECD, which is a result of histiocytic infiltration. It is possible that the chemosis relates to exophthalmos from histiocytic orbital infiltrates. Sensitivity for detecting orbital pathology on a PET scan is limited because of the high signal from the adjacent brain.
ECD should be distinguished from Langerhans cell histiocytosis (LCH) by immunohistologic staining and microscopic characteristics of the histiocytes. LCH usually does not involve the aorta, and it more commonly involves the skin.
Serum IgG4 was within normal limits, and immunohistochemical staining of pathology specimens for IgG4 was negative. The BRAF V600E mutation, which is present in the majority of patients with ECD, was detected in a subsequent right perinephric biopsy specimen. The patient was diagnosed with ECD.
Prednisone and pegylated interferon‐ led to a rapid improvement in his symptoms. As the prednisone was tapered, he developed bilateral periorbital swelling. Magnetic resonance imaging (MRI) revealed well‐circumscribed, intraorbital soft tissue masses with partial encasement of his optic nerves and superior ophthalmic veins, as well as infiltration of his transverse sinuses, consistent with intracranial manifestations of ECD. There was no evidence of pituitary, hypothalamic, or other brain parenchymal infiltration. His dyspnea, night sweats, and hypertension improved; however, 3 months into therapy he developed an extensive rash. Interferon was discontinued. Vemurafinib, a serine kinase inhibitor that targets the BRAF mutation, was prescribed with subsequent resolution of the rash.
COMMENTARY
This patient suffered from a chronic, progressive, inflammatory illness. Although the disease initially appeared to be confined to the heart and lungs, laboratory testing signaled a more systemic condition, and subsequent imaging demonstrated involvement of a disparate group of organs. Subacute disease processes with elevated markers of inflammation and diffuse organ involvement often fall into 1 of 3 categories: infectious, autoimmune, or neoplastic. The histiocytoses inhabit a fourth and easily overlooked category that can be described as infiltrative. Infiltrative diseases are a heterogeneous group of conditions that cause illness when cells or substances not normally found in tissues lead to organ dysfunction.
Although traditional teaching has focused on sarcoidosis, amyloidosis, and hemochromatosis as the primary representatives of this category, the medical literature describes a number of other infiltrative disease processes. IgG4‐RD is a fibroinflammatory disorder characterized by space‐occupying lesions, a lymphoplasmacytic infiltrate of IgG4‐positive plasma cells, and storiform (matted and irregularly whorled microscopic pattern) fibrosis.[1] IgG4‐RD, like sarcoidosis, blurs the categorical line between infiltrative and autoimmune diseases. Other infiltrative cellular disorders, such as histiocytosis and mastocytosis, exist on a spectrum between monoclonal proliferation and neoplastic invasion.
The histiocytoses represent a diverse group of disorders with an evolving nomenclature, characterized by localized or diffuse infiltration of macrophages, monocytes, and dendritic cells (Table 1). ECD is a rare, non‐Langerhans histiocytosis characterized by excessive recruitment and activation of histiocytes through kinase signaling pathways.[2, 3] Immunohistochemical staining for CD68, CD163, and Factor XIIIa, with lack of staining for CD1a, S100, and CD207, supports the diagnosis.[3] Mutations in the BRAF V600E gene (a protein kinase involved in cell proliferation) represent the most likely etiology of this overactivation. An estimated 38% to 100% of patients with ECD harbor this mutation, with detection rates influenced by the sensitivity of testing techniques.[3] The serine kinase inhibitor vemurafinib targets this mutation, and early experience with this agent in ECD demonstrates encouraging results.[4]
| Dendritic cell disorders |
| Langerhans cell histiocytosis |
| Secondary dendritic cell processes |
| Juvenile xanthogranuloma and related disorders (including Erdheim‐Chester disease) |
| Solitary histiocytomas with a dendritic phenotype |
| Macrophage‐related disorders |
| Primary hemophagocytic lymphohistiocytosis (familial and sporadic) |
| Secondary hemophagocytic syndromes |
| Sinus histiocytosis with massive lymphadenopathy (Rosai‐Dorfman disease) |
| Solitary histiocytoma with a macrophage phenotype |
| Malignant histiocytic disorders |
| Monocyte‐related leukemias |
| Extramedullary monocytic tumor or sarcoma |
| Dendritic cell‐related histiocytic sarcoma |
| Macrophage‐related histiocytic sarcoma |
ECD presents heterogeneously, occurring most commonly between the ages of 40 and 70 years. Nonspecific symptoms include weakness, fatigue, fever, chills, weight loss, and night sweats. Typical sites of involvement include the bone, central nervous system, cardiovascular system, lungs, and retroperitoneum. Bone involvement is nearly universal, and bone pain is the most common presenting symptom. Symmetric diaphyseal and metaphyseal osteosclerotic lesions may be seen on x‐rays, bone scan, PET, CT, and MRI.[3] Approximately 50% of patients have extraskeletal involvement at diagnosis.[5] Neurologic manifestations may result from invasion of histiocytes into the facial bones, orbits, meninges, and intracranial vessels, as eventually developed in this patient. Diabetes insipidus is the most common neurologic manifestation of ECD, followed by exophthalmos, cerebellar ataxia, panhypopituitarism, and papilledema.[6, 7] Approximately 75% of patients eventually suffer from cardiovascular disease, including hypertension, congestive heart failure, acute myocardial infarction, valvular dysfunction, pericardial infiltration, and cardiac tamponade.[8] Vascular involvement includes perivascular infiltration and periaortic fibrosis, resulting in the coated aorta seen in 20% of patients with ECD.[3] Pulmonary manifestations of ECD include interstitial, pleural, and consolidative lung disease. A review of high‐resolution chest CTs of patients with ECD demonstrated that greater than half had evidence of parenchymal lung disease, with interlobular septal thickening being the most common finding.[9] Infiltration and fibrosis of retroperitoneal structures is common. Infiltration of perinephric fat creates irregular renal borders, appearing radiographically as hairy kidneys.
Arriving at the diagnosis in this case proved to be challenging because the early presentation was consistent with congestive heart failure. As the patient's conditioned deteriorated, imaging suggested multisystem involvement. It was the extensive aortopathy in particularnot the less specific bone, kidney, lymph node, or pulmonary findingsthat allowed the clinicians to hone the extensive differential diagnosis. The coated aorta is a finding that has been strongly associated with ECD; few other conditions coat the aorta in a similar fashion.[10] In most mysteries, the perpetrator's coat conceals his identity; however, in this story the coat gave it away.
KEY LEARNING POINTS
- Subacute, inflammatory, multiorgan disease is usually explained by 3 categoriesinfection, autoimmunity, and neoplasiabut a fourth category, infiltrative disorders, sometimes warrants consideration.
- ECD presents heterogeneously, ranging from localized disease to widespread organ infiltration. The classic presentation includes bone pain, diabetes insipidus, and exophthalmos.
- Characteristic radiological findings that suggest ECD include long bone osteosclerosis, a coated aorta from periaortic infiltration, and hairy kidneys from perinephric infiltration.
Disclosure
Nothing to report
- , , IgG4‐related disease. N Engl J Med. 2012;366(6):539–551.
- , , , Diagnosing Erdheim‐Chester disease. Ann Rheum Dis. 2013;72(7):e19.
- , , , et al. Consensus guidelines for the diagnosis and clinical management of Erdheim‐Chester disease. Blood. 2014;124(4):483–492.
- , , , et al. The efficacy of vemurafenib in Erdheim‐Chester Disease and Langerhans Cell Histiocytosis: preliminary results from VE‐Basket Study. Blood. 2014;124(21):635.
- , , , et al. Erdheim‐Chester disease. Clinical and radiologic characteristics of 59 cases. Medicine (Baltimore). 1996;75(3):157–169.
- , , , et al. Neurological manifestations and neuroradiological presentation of Erdheim‐Chester disease: report of 6 cases and systematic review of the literature. J Neurol. 2006;253(10):1267–1277.
- , , , et al. Cerebral, facial, and orbital involvement in Erdheim‐Chester disease: CT and MR imaging findings. Radiology. 2010;255(2):586–594.
- , , , et al. Images in cardiovascular medicine. Cardiac involvement in Erdheim‐Chester disease: magnetic resonance and computed tomographic scan imaging in a monocentric series of 37 patients. Circulation. 2009;119(25):e597–e598.
- , , , et al. Pulmonary involvement in Erdheim‐Chester disease: a single‐center study of thirty‐four patients and a review of the literature. Arthritis Rheum. 2010;62(11):3504–3512.
- , , , et al. “Coated aorta”: a new sign of Erdheim‐Chester disease. J Rheumatol. 2000;27(6):1550–1553.
- , , IgG4‐related disease. N Engl J Med. 2012;366(6):539–551.
- , , , Diagnosing Erdheim‐Chester disease. Ann Rheum Dis. 2013;72(7):e19.
- , , , et al. Consensus guidelines for the diagnosis and clinical management of Erdheim‐Chester disease. Blood. 2014;124(4):483–492.
- , , , et al. The efficacy of vemurafenib in Erdheim‐Chester Disease and Langerhans Cell Histiocytosis: preliminary results from VE‐Basket Study. Blood. 2014;124(21):635.
- , , , et al. Erdheim‐Chester disease. Clinical and radiologic characteristics of 59 cases. Medicine (Baltimore). 1996;75(3):157–169.
- , , , et al. Neurological manifestations and neuroradiological presentation of Erdheim‐Chester disease: report of 6 cases and systematic review of the literature. J Neurol. 2006;253(10):1267–1277.
- , , , et al. Cerebral, facial, and orbital involvement in Erdheim‐Chester disease: CT and MR imaging findings. Radiology. 2010;255(2):586–594.
- , , , et al. Images in cardiovascular medicine. Cardiac involvement in Erdheim‐Chester disease: magnetic resonance and computed tomographic scan imaging in a monocentric series of 37 patients. Circulation. 2009;119(25):e597–e598.
- , , , et al. Pulmonary involvement in Erdheim‐Chester disease: a single‐center study of thirty‐four patients and a review of the literature. Arthritis Rheum. 2010;62(11):3504–3512.
- , , , et al. “Coated aorta”: a new sign of Erdheim‐Chester disease. J Rheumatol. 2000;27(6):1550–1553.
The Hand That Feeds You
A 66‐year‐old man presented to the Emergency Department (ED) with rash and malaise in early April. He was in his usual state of good health until the morning of presentation, when he awoke feeling lethargic. Over the course of the day, his hands and feet grew cold and numb, his nose became dark red, and he developed a diffuse, net‐like red rash over his legs, hands, buttocks, and trunk. He had multiple maroon bowel movements. His wife noted that he became incoherent and brought him to the ED.
This apparently previously healthy man presented with an acute episode of fatigue and altered mental status accompanied by a prominent cutaneous eruption. The differential diagnosis will ultimately be guided by the morphology of the rash. At this stage, infectious diseases, drug or toxin exposure, and allergic processes including anaphylaxis must all be considered in this patient with rash and acute illness. The maroon bowel movements likely represent a gastrointestinal bleed that may be part of a unifying diagnosisa hematologic disorder, a vasculitis, or liver disease.
In the ED, the patient was reportedly febrile (exact temperature not recorded) with a blood pressure of 96/54 mmHg. He had pulse oximetry of 88% on room air and a diffuse purpuric rash. The patient was noted to have a leukocytosis, thrombocytopenia, coagulopathy, and an elevation of his creatinine and cardiac enzymes. He was given fluids, fresh frozen plasma, and broad‐spectrum antibiotics, and transferred directly to the intensive care unit of a tertiary medical center for further management.
Upon arrival to the intensive care unit, he complained of fatigue, progression of his nonpruritic, nonpainful rash, and worsening numbness and tingling of his extremities. He denied headache, nuchal rigidity, photophobia, vision or hearing changes, chest pain, cough, abdominal pain, myalgias, or arthralgias. While being interviewed, he had dark brown emesis and a bloody bowel movement.
The patient's past medical history included bacterial pericarditis as a teenager and remote hepatitis of unclear etiology. He rarely saw a physician, took no medications, and had no known medication allergies.
The patient worked as president of a software company and lived with his wife. He had smoked 1 to 2 packs of cigarettes a day for the past 30 years. He endorsed 2 of 4 CAGE criteria (need to Cut down, Annoyed when asked about alcohol, feel Guilty about drinking, need for an Eye opener), and his wife and had never been tested for human immunodeficiency virus (HIV). Family history was unremarkable.
The patient's presentation is concerning for a life‐threatening disease process with a rapid course. In the setting of the laboratory abnormalities demonstrating multi‐organ dysfunction, aggressive volume resuscitation and prompt initiation of broad‐spectrum antibiotics are indicated. The history does not reveal an obvious source of infection or exposure to a new drug, toxin, or allergen. His apparent gastrointestinal bleed could be explained by complications of liver disease from chronic alcohol use. For example, he could have variceal bleeding or gastropathy from portal hypertension. Alternatively, he may have bleeding secondary to a coagulopathy from decreased synthetic function of clotting factors. Other possibilities include a perforated viscus (eg, peptic ulcer) leading to bleeding and peritonitis or mesenteric ischemia, though the absence of abdominal pain makes these unlikely.
At this point, the overall presentation is most concerning for infection, especially given his chronic alcohol use and the vague history of hepatitis. The acute onset and severity of the illness are consistent with an aggressive, suppurative bacterial infection. The most likely causative organisms include gram‐negative bacteria, especially Neisseria meningitidis (with or without meningitis), as well as Staphylococcus aureus, Streptococcus pyogenes, and Rickettsia rickettsii (Rocky Mountain spotted fever).
Several months prior to presentation, he had traveled to Mexico. Two months prior to presentation, he made a trip to North Carolina and Ohio to visit his brother, who subsequently died of pneumonia. One month prior to presentation, he had traveled to urban China for work.
Because the presentation is so acute and the patient's travel took place over 1 month ago, this is unlikely to be a travel‐associated illness. Furthermore, the course is too acute to be consistent with endemic diseases of Central America and the midwestern United States, such as tuberculosis, brucellosis, and histoplasmosis.
He had a temperature of 38.7C. His heart rate was 110 beats per minute. His blood pressure was 115/78 mmHg, respiratory rate was 24 breaths per minute, and oxygen saturation was 99% on 6 liters via nasal cannula. The patient was a well‐nourished, middle‐aged man who appeared uncomfortable. He was in mild respiratory distress, though able to speak in full sentences. He was alert, coherent, and oriented to self, place, date, and time.
Skin examination revealed nonblanching purpuric papules coalescing into stellate plaques on his scalp, forehead, nose, cheeks, bilateral ears, hands, and feet (Figure 1). Acral surfaces, including hands and feet, were cyanotic without evidence of gangrene. He had nonblanching retiform purpuric plaques on his right flank, lower abdomen, low back, buttock, penis, scrotum, thighs, and legs (Figure 2). His right dorsal hand had 3 healing erosions of 3 to 10 mm in size without associated edema, erythema, or drainage.
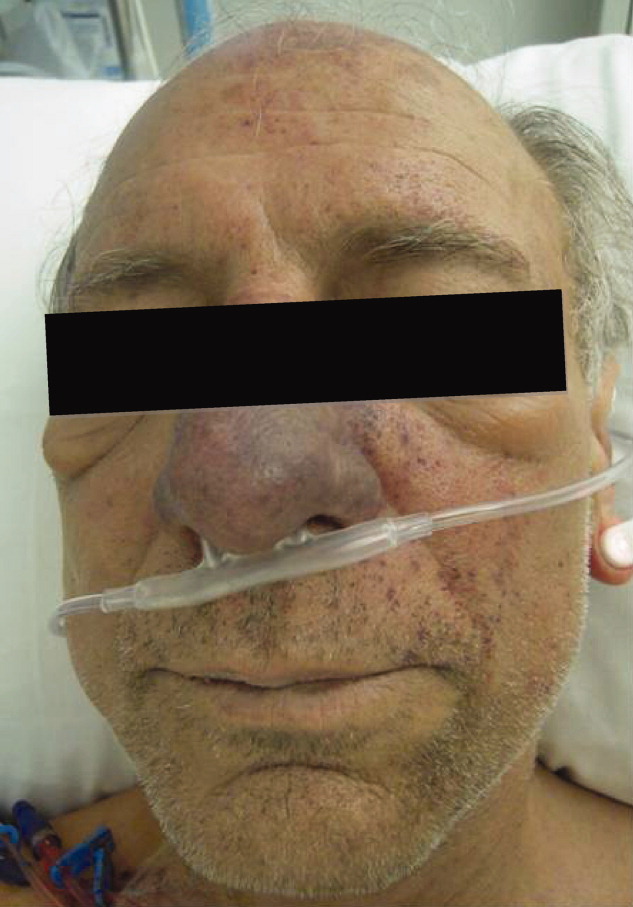
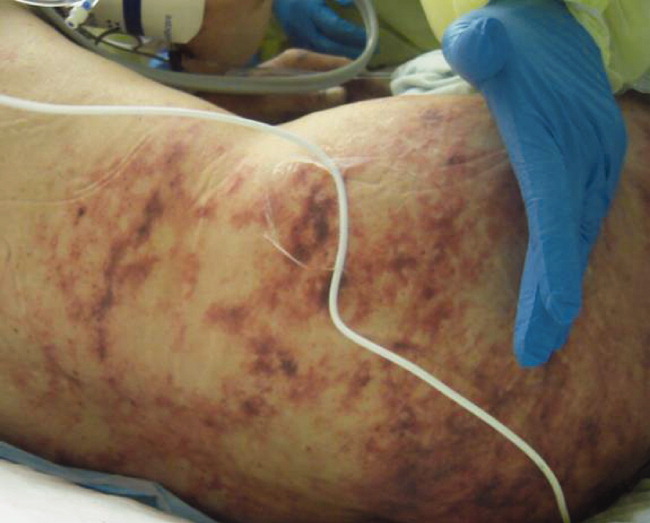
Mucous membranes were dry without lesions. Cardiac examination demonstrated tachycardia without appreciable murmur. He was mildly tachypneic and his lungs were clear to auscultation without adventitious breath sounds. His abdominal examination was unremarkable. His hands and feet were cool with decreased sensation to touch. He had full range of motion and intact muscle strength, but mild bilateral dysmetria with finger‐nose‐finger testing. His radial and dorsalis pedis pulses were symmetric and brisk. Rectal exam revealed guaiac‐positive stool.
The patient's vital signs are compatible with the systemic inflammatory response syndrome. The presence of retiform purpura raises concerns for a systemic vasculitis with destruction of the vessel wall, or intravascular occlusion with thrombosis or emboli. Absence of murmur does not rule out endocarditis but makes it less likely. He has no risk factors for vasculitis, so the purpura, in conjunction with both bleeding and thrombosis, is much more suggestive of disseminated intravascular coagulation (DIC). This clotting disorder can result from a noninfectious trigger, such as acute pancreatitis or malignancy, but his presentation is more worrisome for a severe infection leading to DIC and complicated by purpura fulminans. He does not show signs of hepatic encephalopathy or cirrhosis, making decompensated liver disease a less likely inciting factor of his presentation.
Further exposure history was obtained: The patient often spent time outdoors near his rural home and used a weed‐whacker in his yard the day before admission. He owned 3 horses which he fed and often rode. He had 3 healthy dogs and had been bitten in attempts to break up fights among them, most recently 3 days prior to admission. He lived in mountain lion territory but had no direct exposure to lions. He had no known insect bites. He regularly drank well water, and consumed medium‐rare hamburgers 4 days prior to admission. One week prior to admission, a child with possible streptococcal pharyngitis visited his home.
With this history, the patient was treated with aggressive intravenous fluids and meningeal doses of ceftriaxone, vancomycin, and metronidazole.
In the summer, outdoor exposure to brush confers a risk of tick‐borne infections, including rickettsial diseases, ehrlichiosis, and spirochetal relapsing fever. However, this patient presented in the spring, and apart from rickettsial spotted fever, these illnesses tend to be indolent. It is conceivable, though unlikely, that the weed‐cutting device may have aerosolized fulminant zoonotic pathogens such as Francisella tularensis or plague that can be found in mountain lion territory.
Well water exposure suggests leptospirosis, which can present in a fulminant fashion with multi‐organ dysfunction, but is more often a subacute illness (developing over many days to a week or two). His ingestion of potentially undercooked meat raises the possibility of enterohemorrhagic infection complicated by the hemolytic uremic syndrome (HUS). However, while the purpuric rash and renal failure are compatible with HUS, the pace of illness and accompanying hypotension once again favor alternative infectious diagnoses.
The incubation period and presentation is concerning overwhelming bacterial infection related to the dog bite. Microbiological considerations include streptococcal species, Staphylococcus aureus, and gram‐negative organisms including Pasteurella species and Capnocytophaga canimorsus. The latter 2 organisms are of particular interest since they tend to cause severe sepsis in patients with alcoholism.
The antibiotic selection in this case is not straightforward. In general, empiric therapy for infections related to dog bites should include treatment for beta‐lactamaseproducing bacteria and anaerobes (eg, piperacillin/tazobactam). Yet, given the clinical presentation, severity of illness, and possible DIC, it is appropriate to be concerned about meningococcemia. Unfortunately, the tazobactam in piperacillin/tazobactam has poor central nervous system penetration so would be suboptimal treatment for meningitis. At this point, ceftriaxone, vancomycin, and metronidazole is a reasonable regimen.
Laboratory results were notable for blood urea nitrogen 50 mg/dL, creatinine 3.47 mg/dL, white cell count 21,800/L, with an absolute neutrophil count of 20,690/L, hematocrit 35.9%, platelet count 34,000/L, International Normalized Ratio 1.5, and partial thromboplastin time 44.0 seconds. His alanine aminotransferase was 356 U/L (1641 U/L), aspartate aminotransferase 959 U/L (1259 U/L), alkaline phosphatase 50 U/L (29111 U/L), and total bilirubin 1.7 mg/dL (0.31.3 mg/dL). Fibrinogen was 283 g/L (202430 g/L), lactate dehydrogenase was 1883 U/L (91185 IU/L), and uric acid was 10.5 mg/dL (3.77.7 mg/dL). His troponin I was 1.18 ng/mL (0.05 ng/ml), and his electrocardiogram showed sinus tachycardia but no evidence of myocardial ischemia. Chest x‐ray showed no infiltrate or evidence of volume overload. Lumbar puncture was deferred out of concern for ongoing disseminated intravascular coagulation.
Transthoracic echocardiogram revealed global hypokinesis and reduced left ventricular systolic function with ejection fraction of 35%. There was no evidence of vegetations or thrombus.
The patient's thrombocytopenia and prolonged coagulation parameters further support the presence of DIC. A peripheral blood smear should be examined. If microangiopathic changes are found, other diagnoses such as thrombotic thrombocytopenic purpura might be considered, although the rapid pace of illness and presence of hypotension still make sepsis with DIC more likely.
While septic shock often causes multi‐organ system failure secondary to hypoperfusion, the presumed rapid onset of hepatic and renal abnormalities suggests that microvascular thrombosis is playing a larger role in his organ system dysfunction. Microvascular thrombosis could also contribute to his myocardial injury, though globally depressed ejection fraction and elevated troponin might also be explained by infectious myocarditis. A third possibility is that his severe sepsis caused his myocardial dysfunction. Regardless of its etiology, the patient has no clinical evidence of congestive heart failure, so no specific therapy is required at this time. However, his cardiopulmonary exam should be monitored closely, and if he survives, he should have repeat echocardiography to monitor for resolution of the global hypokinesis.
Further evaluation revealed creatine kinase of 45,000 ng/ml (55380 ng/ml) and repeat troponin of >22 ng/ml. Protein C level was low at 30%. Testing for HIV was negative. Blood smear from time of transfer had few schistocytes. Urinalysis showed muddy brown casts but no dysmorphic red blood cells or red cell casts. The patient was placed on continuous veno‐venous hemofiltration (CVVH) for worsening renal failure and oliguria from presumed acute tubular necrosis in the setting of rhabdomyolysis and sepsis.
The patient has severe rhabdomyolysis that cannot fully be explained by his initial hypoperfusion and is more likely related to the overwhelming infection and microthrombosis. Rhabdomyolysis probably contributed to his acute tubular necrosis and renal failure.
Dermatology consultation identified the rash as likely purpura fulminans. They recommended a skin biopsy to rule out vasculitis. Three skin biopsies revealed micro‐vascular thrombosis; direct immunofluorescence test was negative for vasculitis; his skin tissue culture was negative for bacterial, mycobacterial, and fungal organisms.
Input from the dermatology service was key in identifying the rash. Purpura fulminans has a limited differential that includes severe infection from gram‐negative organisms and protein C and S deficiency. Since the biopsy results made vasculitis unlikely, the team was able to focus greater attention on potential pathogens such as Pasteurella species and C. canimorsus.
The biopsy also confirms the clinical suspicion that microvascular thrombosis is causing the patient's acute kidney injury, rhabdomyolysis, and myocardial ischemia. The presence of microvascular thrombosis prompts consideration of antithrombotic therapy such as heparin, but benefits of this therapy must be weighed against contraindications including bleeding and thrombocytopenia.
Ultimately out of concerns for recurrent gastrointestinal bleeding, the primary team decided not to treat with heparin or other antithrombotic therapy.
After several days of supportive care with antibiotics and renal replacement therapy, the patient showed gradual improvement of his retiform purpura, sensory neuropathy, laboratory data, and other markers of end‐organ dysfunction. Purpura of his fingertips, feet, and toes progressed to dry gangrene (Figure 3), which was monitored for potential need for amputation. He remained dependent on intermittent hemodialysis.
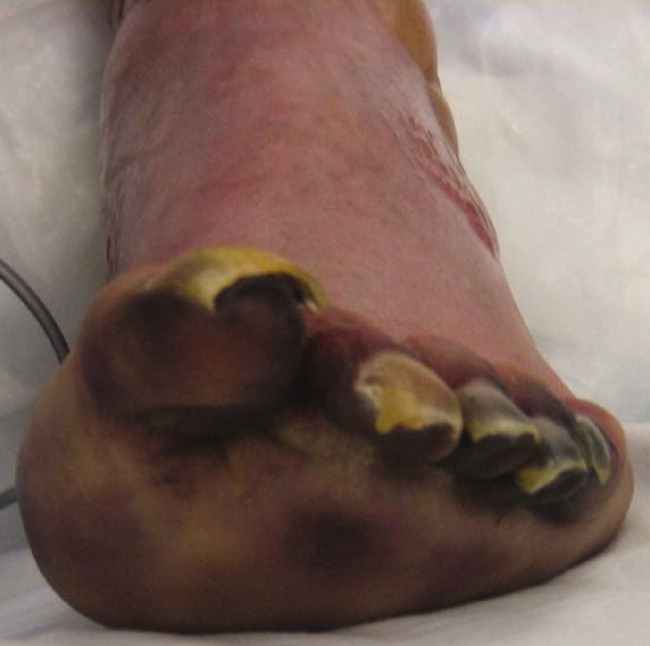
His initial antibiotic regimen was narrowed to ceftriaxone monotherapy. Five days after initial presentation, blood cultures drawn from the outside emergency department grew a gram‐negative rod in the anaerobic broth. Ten days later, this gram‐negative rod was identified as Capnocytophaga canimorsus. He was ultimately discharged to a skilled nursing facility.
Generally growth of an organism in broth only suggests either a very low inoculum or that the isolate is a contaminant. In this case, it was because the causative organism, C. canimorsus, is an obligate anaerobe and quite fastidious, so unlikely to grow easily. The identification of C. canimorsus from the initial blood culture is not surprising in this patient who presented with severe sepsis, DIC, and purpura fulminans after a recent dog bite. While the patient's chronic alcohol use may explain his fulminant infection from an atypical organism, one should always consider occult underlying malignancy as a predisposing factor, particularly in patients of this age group.
With the appropriate course of antibiotics, C. canimorsus infection should be completely cured. However, recovery of kidney and cardiac function could take weeks to months, and his dry gangrene may or may not resolve.
COMMENTARY
Capnocytophaga canimorsis sepsis is a rare and potentially deadly complication of dog bites that can present with rash, cellulitis, purpura fulminans, arthritis, meningitis, and endocarditis. The discussant considered a broad differential for the presentation of fever, rash, and acute illness. While the travel history was intriguing, the severity and pace of illness allowed him to focus attention on more recent infectious exposures. The ultimate key to the diagnosis was the patient's history of dog bite, an important but underrecognized source of serious infection in the United States.
According to the Centers for Disease Control and Prevention, there are approximately 4 million dog bites in the country each year. Of these, 300,000 bite victims seek care in the emergency department, resulting in 13,000 hospitalizations and 20 deaths annually.1 Infected dog bite wounds often grow polymicrobial flora. Pasteurella species are the most frequently found organisms in both dog and cat bite wounds. However, other aerobes such as streptococci, staphylococci, Moraxella, and Neisseria, as well as anaerobes including Fusobacterium and Bacteroides species, are also common.2
C. canimorsis is a facultative, fastidious gram‐negative bacillus found in the mouth flora of not only dogs but also cats and humans. It is often mistaken for other gram‐negative rod species.3 As with the patient described in this report, systemic infection from C. canimorsis can follow even superficial or well‐healed bite wounds.
Since this bacterium was first described in the literature 30 years ago, more than 100 cases of C. canimorsus infection have been described, with a mortality rate of nearly 30%.4 C. canimorsus occurs more frequently in males and in patients 50 to 70 years of age. Traditional risk factors include alcohol abuse, asplenia, immunosuppression, and corticosteroid treatment. However, in a case series of 56 isolates in California, only 10% of patients with Capnocytophaga sepsis were asplenic and none had alcohol abuse reported in their medical charts. In this series, median time from dog bite to the onset of symptoms was 3 days. Eighty‐five percent of patients presented with fever, while 32% had sepsis and 13% had DIC or septic shock.3
While C. canimorsus was once susceptible to a range of antibiotics, several reports from Canada and Europe document rising rates of beta‐lactamaseproducing strains that have caused clinically significant disease.5, 6 Individual susceptibility data take days to obtain, so it is important to start with empiric therapy. In general, empiric therapy for all serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes, for example, with amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam. If the patient is allergic to penicillin, clindamycin plus a fluoroquinolone can be used instead.
There are previous reports of purpura fulminans and symmetric peripheral gangrene following Capnocytophaga infection from dog bites.7, 8 Purpura fulminans is defined as rapidly progressive skin necrosis due to dermal vascular thrombosis, often in the setting of DIC. Early involvement occurs at acral sites, such as the nose, ears, fingers, and toes. Purpuric lesions often progress to skin necrosis or dry gangrene within 24 to 48 hours. In a review of 12 patients with purpura fulminans, only 9 survived. Eight of the 9 survivors required amputation of at least 1 limb, and 4 of them required 4‐limb amputation.7
In this patient who presented with fever and rash, the discussant recognized early on an underlying infectious etiology. Although the patient's exposure history led the discussant to consider a host of possibilities, the recognition of purpura fulminans allowed him to narrow his differential. Ultimately, the dog's bite clinched the diagnosis.
KEY TEACHING POINTS
-
Sepsis caused by C. canimorsus is often characterized by rash, cellulitis, arthritis, meningitis, and endocarditis. In some instances, infection can progress to purpura fulminans.
-
In cases where fastidious organisms are suspected as an infectious source, microbiology labs should be notified of suspected organisms so they can extend incubation periods or use special media to maximize culture yield and the likelihood of accurate identification.
-
Empiric therapy for serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes. Consider using amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
Acknowledgements
The authors thank Snigdha Vallabhaneni, MD, from the UCSF Division of Infectious Diseases, for her contributions to the discussion on C. canimorsus. They also thank Kanade Shinkai, MD, PhD, from the UCSF Department of Dermatology, and Heather Nye, MD, PhD, from the UCSF Division of Hospital Medicine, for their review of the manuscript.
Disclosure: Nothing to report.
- ,,.Incidence of dog bite injuries treated in emergency departments.JAMA.1998;279:51–53.
- ,,,,.Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group.N Engl J Med.1999;340:85–92.
- ,,,.Diagnosing Capnocytophaga canimorsus infections.Emerg Infect Dis.2006;12:340–342.
- ,,.Capnocytophaga canimorsus infections in human: review of the literature and cases report.Eur J Epidemiol.1996;12:521–533.
- ,,,,.Antimicrobial susceptibilities and beta‐lactamase characterization of Capnocytophaga species.Antimicrob Agents Chemother.1992;36:2197–2200.
- ,,,,,.Bacteremia due to Capnocytophaga species in patients with neutropenia: high frequency of beta‐lactamase‐producing strains.Clin Infect Dis.1999;28:1172–1174.
- ,,.Presentation and outcome of purpura fulminans associated with peripheral gangrene in 12 patients at Mayo Clinic.J Am Acad Dermatol.2007;57:944–956.
- ,,,.Capnocytophaga canimorsus sepsis with purpura fulminans and symmetrical gangrene following a dog bite in a shelter employee.Am J Med Sci.2004:327:369–372.
A 66‐year‐old man presented to the Emergency Department (ED) with rash and malaise in early April. He was in his usual state of good health until the morning of presentation, when he awoke feeling lethargic. Over the course of the day, his hands and feet grew cold and numb, his nose became dark red, and he developed a diffuse, net‐like red rash over his legs, hands, buttocks, and trunk. He had multiple maroon bowel movements. His wife noted that he became incoherent and brought him to the ED.
This apparently previously healthy man presented with an acute episode of fatigue and altered mental status accompanied by a prominent cutaneous eruption. The differential diagnosis will ultimately be guided by the morphology of the rash. At this stage, infectious diseases, drug or toxin exposure, and allergic processes including anaphylaxis must all be considered in this patient with rash and acute illness. The maroon bowel movements likely represent a gastrointestinal bleed that may be part of a unifying diagnosisa hematologic disorder, a vasculitis, or liver disease.
In the ED, the patient was reportedly febrile (exact temperature not recorded) with a blood pressure of 96/54 mmHg. He had pulse oximetry of 88% on room air and a diffuse purpuric rash. The patient was noted to have a leukocytosis, thrombocytopenia, coagulopathy, and an elevation of his creatinine and cardiac enzymes. He was given fluids, fresh frozen plasma, and broad‐spectrum antibiotics, and transferred directly to the intensive care unit of a tertiary medical center for further management.
Upon arrival to the intensive care unit, he complained of fatigue, progression of his nonpruritic, nonpainful rash, and worsening numbness and tingling of his extremities. He denied headache, nuchal rigidity, photophobia, vision or hearing changes, chest pain, cough, abdominal pain, myalgias, or arthralgias. While being interviewed, he had dark brown emesis and a bloody bowel movement.
The patient's past medical history included bacterial pericarditis as a teenager and remote hepatitis of unclear etiology. He rarely saw a physician, took no medications, and had no known medication allergies.
The patient worked as president of a software company and lived with his wife. He had smoked 1 to 2 packs of cigarettes a day for the past 30 years. He endorsed 2 of 4 CAGE criteria (need to Cut down, Annoyed when asked about alcohol, feel Guilty about drinking, need for an Eye opener), and his wife and had never been tested for human immunodeficiency virus (HIV). Family history was unremarkable.
The patient's presentation is concerning for a life‐threatening disease process with a rapid course. In the setting of the laboratory abnormalities demonstrating multi‐organ dysfunction, aggressive volume resuscitation and prompt initiation of broad‐spectrum antibiotics are indicated. The history does not reveal an obvious source of infection or exposure to a new drug, toxin, or allergen. His apparent gastrointestinal bleed could be explained by complications of liver disease from chronic alcohol use. For example, he could have variceal bleeding or gastropathy from portal hypertension. Alternatively, he may have bleeding secondary to a coagulopathy from decreased synthetic function of clotting factors. Other possibilities include a perforated viscus (eg, peptic ulcer) leading to bleeding and peritonitis or mesenteric ischemia, though the absence of abdominal pain makes these unlikely.
At this point, the overall presentation is most concerning for infection, especially given his chronic alcohol use and the vague history of hepatitis. The acute onset and severity of the illness are consistent with an aggressive, suppurative bacterial infection. The most likely causative organisms include gram‐negative bacteria, especially Neisseria meningitidis (with or without meningitis), as well as Staphylococcus aureus, Streptococcus pyogenes, and Rickettsia rickettsii (Rocky Mountain spotted fever).
Several months prior to presentation, he had traveled to Mexico. Two months prior to presentation, he made a trip to North Carolina and Ohio to visit his brother, who subsequently died of pneumonia. One month prior to presentation, he had traveled to urban China for work.
Because the presentation is so acute and the patient's travel took place over 1 month ago, this is unlikely to be a travel‐associated illness. Furthermore, the course is too acute to be consistent with endemic diseases of Central America and the midwestern United States, such as tuberculosis, brucellosis, and histoplasmosis.
He had a temperature of 38.7C. His heart rate was 110 beats per minute. His blood pressure was 115/78 mmHg, respiratory rate was 24 breaths per minute, and oxygen saturation was 99% on 6 liters via nasal cannula. The patient was a well‐nourished, middle‐aged man who appeared uncomfortable. He was in mild respiratory distress, though able to speak in full sentences. He was alert, coherent, and oriented to self, place, date, and time.
Skin examination revealed nonblanching purpuric papules coalescing into stellate plaques on his scalp, forehead, nose, cheeks, bilateral ears, hands, and feet (Figure 1). Acral surfaces, including hands and feet, were cyanotic without evidence of gangrene. He had nonblanching retiform purpuric plaques on his right flank, lower abdomen, low back, buttock, penis, scrotum, thighs, and legs (Figure 2). His right dorsal hand had 3 healing erosions of 3 to 10 mm in size without associated edema, erythema, or drainage.


Mucous membranes were dry without lesions. Cardiac examination demonstrated tachycardia without appreciable murmur. He was mildly tachypneic and his lungs were clear to auscultation without adventitious breath sounds. His abdominal examination was unremarkable. His hands and feet were cool with decreased sensation to touch. He had full range of motion and intact muscle strength, but mild bilateral dysmetria with finger‐nose‐finger testing. His radial and dorsalis pedis pulses were symmetric and brisk. Rectal exam revealed guaiac‐positive stool.
The patient's vital signs are compatible with the systemic inflammatory response syndrome. The presence of retiform purpura raises concerns for a systemic vasculitis with destruction of the vessel wall, or intravascular occlusion with thrombosis or emboli. Absence of murmur does not rule out endocarditis but makes it less likely. He has no risk factors for vasculitis, so the purpura, in conjunction with both bleeding and thrombosis, is much more suggestive of disseminated intravascular coagulation (DIC). This clotting disorder can result from a noninfectious trigger, such as acute pancreatitis or malignancy, but his presentation is more worrisome for a severe infection leading to DIC and complicated by purpura fulminans. He does not show signs of hepatic encephalopathy or cirrhosis, making decompensated liver disease a less likely inciting factor of his presentation.
Further exposure history was obtained: The patient often spent time outdoors near his rural home and used a weed‐whacker in his yard the day before admission. He owned 3 horses which he fed and often rode. He had 3 healthy dogs and had been bitten in attempts to break up fights among them, most recently 3 days prior to admission. He lived in mountain lion territory but had no direct exposure to lions. He had no known insect bites. He regularly drank well water, and consumed medium‐rare hamburgers 4 days prior to admission. One week prior to admission, a child with possible streptococcal pharyngitis visited his home.
With this history, the patient was treated with aggressive intravenous fluids and meningeal doses of ceftriaxone, vancomycin, and metronidazole.
In the summer, outdoor exposure to brush confers a risk of tick‐borne infections, including rickettsial diseases, ehrlichiosis, and spirochetal relapsing fever. However, this patient presented in the spring, and apart from rickettsial spotted fever, these illnesses tend to be indolent. It is conceivable, though unlikely, that the weed‐cutting device may have aerosolized fulminant zoonotic pathogens such as Francisella tularensis or plague that can be found in mountain lion territory.
Well water exposure suggests leptospirosis, which can present in a fulminant fashion with multi‐organ dysfunction, but is more often a subacute illness (developing over many days to a week or two). His ingestion of potentially undercooked meat raises the possibility of enterohemorrhagic infection complicated by the hemolytic uremic syndrome (HUS). However, while the purpuric rash and renal failure are compatible with HUS, the pace of illness and accompanying hypotension once again favor alternative infectious diagnoses.
The incubation period and presentation is concerning overwhelming bacterial infection related to the dog bite. Microbiological considerations include streptococcal species, Staphylococcus aureus, and gram‐negative organisms including Pasteurella species and Capnocytophaga canimorsus. The latter 2 organisms are of particular interest since they tend to cause severe sepsis in patients with alcoholism.
The antibiotic selection in this case is not straightforward. In general, empiric therapy for infections related to dog bites should include treatment for beta‐lactamaseproducing bacteria and anaerobes (eg, piperacillin/tazobactam). Yet, given the clinical presentation, severity of illness, and possible DIC, it is appropriate to be concerned about meningococcemia. Unfortunately, the tazobactam in piperacillin/tazobactam has poor central nervous system penetration so would be suboptimal treatment for meningitis. At this point, ceftriaxone, vancomycin, and metronidazole is a reasonable regimen.
Laboratory results were notable for blood urea nitrogen 50 mg/dL, creatinine 3.47 mg/dL, white cell count 21,800/L, with an absolute neutrophil count of 20,690/L, hematocrit 35.9%, platelet count 34,000/L, International Normalized Ratio 1.5, and partial thromboplastin time 44.0 seconds. His alanine aminotransferase was 356 U/L (1641 U/L), aspartate aminotransferase 959 U/L (1259 U/L), alkaline phosphatase 50 U/L (29111 U/L), and total bilirubin 1.7 mg/dL (0.31.3 mg/dL). Fibrinogen was 283 g/L (202430 g/L), lactate dehydrogenase was 1883 U/L (91185 IU/L), and uric acid was 10.5 mg/dL (3.77.7 mg/dL). His troponin I was 1.18 ng/mL (0.05 ng/ml), and his electrocardiogram showed sinus tachycardia but no evidence of myocardial ischemia. Chest x‐ray showed no infiltrate or evidence of volume overload. Lumbar puncture was deferred out of concern for ongoing disseminated intravascular coagulation.
Transthoracic echocardiogram revealed global hypokinesis and reduced left ventricular systolic function with ejection fraction of 35%. There was no evidence of vegetations or thrombus.
The patient's thrombocytopenia and prolonged coagulation parameters further support the presence of DIC. A peripheral blood smear should be examined. If microangiopathic changes are found, other diagnoses such as thrombotic thrombocytopenic purpura might be considered, although the rapid pace of illness and presence of hypotension still make sepsis with DIC more likely.
While septic shock often causes multi‐organ system failure secondary to hypoperfusion, the presumed rapid onset of hepatic and renal abnormalities suggests that microvascular thrombosis is playing a larger role in his organ system dysfunction. Microvascular thrombosis could also contribute to his myocardial injury, though globally depressed ejection fraction and elevated troponin might also be explained by infectious myocarditis. A third possibility is that his severe sepsis caused his myocardial dysfunction. Regardless of its etiology, the patient has no clinical evidence of congestive heart failure, so no specific therapy is required at this time. However, his cardiopulmonary exam should be monitored closely, and if he survives, he should have repeat echocardiography to monitor for resolution of the global hypokinesis.
Further evaluation revealed creatine kinase of 45,000 ng/ml (55380 ng/ml) and repeat troponin of >22 ng/ml. Protein C level was low at 30%. Testing for HIV was negative. Blood smear from time of transfer had few schistocytes. Urinalysis showed muddy brown casts but no dysmorphic red blood cells or red cell casts. The patient was placed on continuous veno‐venous hemofiltration (CVVH) for worsening renal failure and oliguria from presumed acute tubular necrosis in the setting of rhabdomyolysis and sepsis.
The patient has severe rhabdomyolysis that cannot fully be explained by his initial hypoperfusion and is more likely related to the overwhelming infection and microthrombosis. Rhabdomyolysis probably contributed to his acute tubular necrosis and renal failure.
Dermatology consultation identified the rash as likely purpura fulminans. They recommended a skin biopsy to rule out vasculitis. Three skin biopsies revealed micro‐vascular thrombosis; direct immunofluorescence test was negative for vasculitis; his skin tissue culture was negative for bacterial, mycobacterial, and fungal organisms.
Input from the dermatology service was key in identifying the rash. Purpura fulminans has a limited differential that includes severe infection from gram‐negative organisms and protein C and S deficiency. Since the biopsy results made vasculitis unlikely, the team was able to focus greater attention on potential pathogens such as Pasteurella species and C. canimorsus.
The biopsy also confirms the clinical suspicion that microvascular thrombosis is causing the patient's acute kidney injury, rhabdomyolysis, and myocardial ischemia. The presence of microvascular thrombosis prompts consideration of antithrombotic therapy such as heparin, but benefits of this therapy must be weighed against contraindications including bleeding and thrombocytopenia.
Ultimately out of concerns for recurrent gastrointestinal bleeding, the primary team decided not to treat with heparin or other antithrombotic therapy.
After several days of supportive care with antibiotics and renal replacement therapy, the patient showed gradual improvement of his retiform purpura, sensory neuropathy, laboratory data, and other markers of end‐organ dysfunction. Purpura of his fingertips, feet, and toes progressed to dry gangrene (Figure 3), which was monitored for potential need for amputation. He remained dependent on intermittent hemodialysis.

His initial antibiotic regimen was narrowed to ceftriaxone monotherapy. Five days after initial presentation, blood cultures drawn from the outside emergency department grew a gram‐negative rod in the anaerobic broth. Ten days later, this gram‐negative rod was identified as Capnocytophaga canimorsus. He was ultimately discharged to a skilled nursing facility.
Generally growth of an organism in broth only suggests either a very low inoculum or that the isolate is a contaminant. In this case, it was because the causative organism, C. canimorsus, is an obligate anaerobe and quite fastidious, so unlikely to grow easily. The identification of C. canimorsus from the initial blood culture is not surprising in this patient who presented with severe sepsis, DIC, and purpura fulminans after a recent dog bite. While the patient's chronic alcohol use may explain his fulminant infection from an atypical organism, one should always consider occult underlying malignancy as a predisposing factor, particularly in patients of this age group.
With the appropriate course of antibiotics, C. canimorsus infection should be completely cured. However, recovery of kidney and cardiac function could take weeks to months, and his dry gangrene may or may not resolve.
COMMENTARY
Capnocytophaga canimorsis sepsis is a rare and potentially deadly complication of dog bites that can present with rash, cellulitis, purpura fulminans, arthritis, meningitis, and endocarditis. The discussant considered a broad differential for the presentation of fever, rash, and acute illness. While the travel history was intriguing, the severity and pace of illness allowed him to focus attention on more recent infectious exposures. The ultimate key to the diagnosis was the patient's history of dog bite, an important but underrecognized source of serious infection in the United States.
According to the Centers for Disease Control and Prevention, there are approximately 4 million dog bites in the country each year. Of these, 300,000 bite victims seek care in the emergency department, resulting in 13,000 hospitalizations and 20 deaths annually.1 Infected dog bite wounds often grow polymicrobial flora. Pasteurella species are the most frequently found organisms in both dog and cat bite wounds. However, other aerobes such as streptococci, staphylococci, Moraxella, and Neisseria, as well as anaerobes including Fusobacterium and Bacteroides species, are also common.2
C. canimorsis is a facultative, fastidious gram‐negative bacillus found in the mouth flora of not only dogs but also cats and humans. It is often mistaken for other gram‐negative rod species.3 As with the patient described in this report, systemic infection from C. canimorsis can follow even superficial or well‐healed bite wounds.
Since this bacterium was first described in the literature 30 years ago, more than 100 cases of C. canimorsus infection have been described, with a mortality rate of nearly 30%.4 C. canimorsus occurs more frequently in males and in patients 50 to 70 years of age. Traditional risk factors include alcohol abuse, asplenia, immunosuppression, and corticosteroid treatment. However, in a case series of 56 isolates in California, only 10% of patients with Capnocytophaga sepsis were asplenic and none had alcohol abuse reported in their medical charts. In this series, median time from dog bite to the onset of symptoms was 3 days. Eighty‐five percent of patients presented with fever, while 32% had sepsis and 13% had DIC or septic shock.3
While C. canimorsus was once susceptible to a range of antibiotics, several reports from Canada and Europe document rising rates of beta‐lactamaseproducing strains that have caused clinically significant disease.5, 6 Individual susceptibility data take days to obtain, so it is important to start with empiric therapy. In general, empiric therapy for all serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes, for example, with amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam. If the patient is allergic to penicillin, clindamycin plus a fluoroquinolone can be used instead.
There are previous reports of purpura fulminans and symmetric peripheral gangrene following Capnocytophaga infection from dog bites.7, 8 Purpura fulminans is defined as rapidly progressive skin necrosis due to dermal vascular thrombosis, often in the setting of DIC. Early involvement occurs at acral sites, such as the nose, ears, fingers, and toes. Purpuric lesions often progress to skin necrosis or dry gangrene within 24 to 48 hours. In a review of 12 patients with purpura fulminans, only 9 survived. Eight of the 9 survivors required amputation of at least 1 limb, and 4 of them required 4‐limb amputation.7
In this patient who presented with fever and rash, the discussant recognized early on an underlying infectious etiology. Although the patient's exposure history led the discussant to consider a host of possibilities, the recognition of purpura fulminans allowed him to narrow his differential. Ultimately, the dog's bite clinched the diagnosis.
KEY TEACHING POINTS
-
Sepsis caused by C. canimorsus is often characterized by rash, cellulitis, arthritis, meningitis, and endocarditis. In some instances, infection can progress to purpura fulminans.
-
In cases where fastidious organisms are suspected as an infectious source, microbiology labs should be notified of suspected organisms so they can extend incubation periods or use special media to maximize culture yield and the likelihood of accurate identification.
-
Empiric therapy for serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes. Consider using amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
Acknowledgements
The authors thank Snigdha Vallabhaneni, MD, from the UCSF Division of Infectious Diseases, for her contributions to the discussion on C. canimorsus. They also thank Kanade Shinkai, MD, PhD, from the UCSF Department of Dermatology, and Heather Nye, MD, PhD, from the UCSF Division of Hospital Medicine, for their review of the manuscript.
Disclosure: Nothing to report.
A 66‐year‐old man presented to the Emergency Department (ED) with rash and malaise in early April. He was in his usual state of good health until the morning of presentation, when he awoke feeling lethargic. Over the course of the day, his hands and feet grew cold and numb, his nose became dark red, and he developed a diffuse, net‐like red rash over his legs, hands, buttocks, and trunk. He had multiple maroon bowel movements. His wife noted that he became incoherent and brought him to the ED.
This apparently previously healthy man presented with an acute episode of fatigue and altered mental status accompanied by a prominent cutaneous eruption. The differential diagnosis will ultimately be guided by the morphology of the rash. At this stage, infectious diseases, drug or toxin exposure, and allergic processes including anaphylaxis must all be considered in this patient with rash and acute illness. The maroon bowel movements likely represent a gastrointestinal bleed that may be part of a unifying diagnosisa hematologic disorder, a vasculitis, or liver disease.
In the ED, the patient was reportedly febrile (exact temperature not recorded) with a blood pressure of 96/54 mmHg. He had pulse oximetry of 88% on room air and a diffuse purpuric rash. The patient was noted to have a leukocytosis, thrombocytopenia, coagulopathy, and an elevation of his creatinine and cardiac enzymes. He was given fluids, fresh frozen plasma, and broad‐spectrum antibiotics, and transferred directly to the intensive care unit of a tertiary medical center for further management.
Upon arrival to the intensive care unit, he complained of fatigue, progression of his nonpruritic, nonpainful rash, and worsening numbness and tingling of his extremities. He denied headache, nuchal rigidity, photophobia, vision or hearing changes, chest pain, cough, abdominal pain, myalgias, or arthralgias. While being interviewed, he had dark brown emesis and a bloody bowel movement.
The patient's past medical history included bacterial pericarditis as a teenager and remote hepatitis of unclear etiology. He rarely saw a physician, took no medications, and had no known medication allergies.
The patient worked as president of a software company and lived with his wife. He had smoked 1 to 2 packs of cigarettes a day for the past 30 years. He endorsed 2 of 4 CAGE criteria (need to Cut down, Annoyed when asked about alcohol, feel Guilty about drinking, need for an Eye opener), and his wife and had never been tested for human immunodeficiency virus (HIV). Family history was unremarkable.
The patient's presentation is concerning for a life‐threatening disease process with a rapid course. In the setting of the laboratory abnormalities demonstrating multi‐organ dysfunction, aggressive volume resuscitation and prompt initiation of broad‐spectrum antibiotics are indicated. The history does not reveal an obvious source of infection or exposure to a new drug, toxin, or allergen. His apparent gastrointestinal bleed could be explained by complications of liver disease from chronic alcohol use. For example, he could have variceal bleeding or gastropathy from portal hypertension. Alternatively, he may have bleeding secondary to a coagulopathy from decreased synthetic function of clotting factors. Other possibilities include a perforated viscus (eg, peptic ulcer) leading to bleeding and peritonitis or mesenteric ischemia, though the absence of abdominal pain makes these unlikely.
At this point, the overall presentation is most concerning for infection, especially given his chronic alcohol use and the vague history of hepatitis. The acute onset and severity of the illness are consistent with an aggressive, suppurative bacterial infection. The most likely causative organisms include gram‐negative bacteria, especially Neisseria meningitidis (with or without meningitis), as well as Staphylococcus aureus, Streptococcus pyogenes, and Rickettsia rickettsii (Rocky Mountain spotted fever).
Several months prior to presentation, he had traveled to Mexico. Two months prior to presentation, he made a trip to North Carolina and Ohio to visit his brother, who subsequently died of pneumonia. One month prior to presentation, he had traveled to urban China for work.
Because the presentation is so acute and the patient's travel took place over 1 month ago, this is unlikely to be a travel‐associated illness. Furthermore, the course is too acute to be consistent with endemic diseases of Central America and the midwestern United States, such as tuberculosis, brucellosis, and histoplasmosis.
He had a temperature of 38.7C. His heart rate was 110 beats per minute. His blood pressure was 115/78 mmHg, respiratory rate was 24 breaths per minute, and oxygen saturation was 99% on 6 liters via nasal cannula. The patient was a well‐nourished, middle‐aged man who appeared uncomfortable. He was in mild respiratory distress, though able to speak in full sentences. He was alert, coherent, and oriented to self, place, date, and time.
Skin examination revealed nonblanching purpuric papules coalescing into stellate plaques on his scalp, forehead, nose, cheeks, bilateral ears, hands, and feet (Figure 1). Acral surfaces, including hands and feet, were cyanotic without evidence of gangrene. He had nonblanching retiform purpuric plaques on his right flank, lower abdomen, low back, buttock, penis, scrotum, thighs, and legs (Figure 2). His right dorsal hand had 3 healing erosions of 3 to 10 mm in size without associated edema, erythema, or drainage.


Mucous membranes were dry without lesions. Cardiac examination demonstrated tachycardia without appreciable murmur. He was mildly tachypneic and his lungs were clear to auscultation without adventitious breath sounds. His abdominal examination was unremarkable. His hands and feet were cool with decreased sensation to touch. He had full range of motion and intact muscle strength, but mild bilateral dysmetria with finger‐nose‐finger testing. His radial and dorsalis pedis pulses were symmetric and brisk. Rectal exam revealed guaiac‐positive stool.
The patient's vital signs are compatible with the systemic inflammatory response syndrome. The presence of retiform purpura raises concerns for a systemic vasculitis with destruction of the vessel wall, or intravascular occlusion with thrombosis or emboli. Absence of murmur does not rule out endocarditis but makes it less likely. He has no risk factors for vasculitis, so the purpura, in conjunction with both bleeding and thrombosis, is much more suggestive of disseminated intravascular coagulation (DIC). This clotting disorder can result from a noninfectious trigger, such as acute pancreatitis or malignancy, but his presentation is more worrisome for a severe infection leading to DIC and complicated by purpura fulminans. He does not show signs of hepatic encephalopathy or cirrhosis, making decompensated liver disease a less likely inciting factor of his presentation.
Further exposure history was obtained: The patient often spent time outdoors near his rural home and used a weed‐whacker in his yard the day before admission. He owned 3 horses which he fed and often rode. He had 3 healthy dogs and had been bitten in attempts to break up fights among them, most recently 3 days prior to admission. He lived in mountain lion territory but had no direct exposure to lions. He had no known insect bites. He regularly drank well water, and consumed medium‐rare hamburgers 4 days prior to admission. One week prior to admission, a child with possible streptococcal pharyngitis visited his home.
With this history, the patient was treated with aggressive intravenous fluids and meningeal doses of ceftriaxone, vancomycin, and metronidazole.
In the summer, outdoor exposure to brush confers a risk of tick‐borne infections, including rickettsial diseases, ehrlichiosis, and spirochetal relapsing fever. However, this patient presented in the spring, and apart from rickettsial spotted fever, these illnesses tend to be indolent. It is conceivable, though unlikely, that the weed‐cutting device may have aerosolized fulminant zoonotic pathogens such as Francisella tularensis or plague that can be found in mountain lion territory.
Well water exposure suggests leptospirosis, which can present in a fulminant fashion with multi‐organ dysfunction, but is more often a subacute illness (developing over many days to a week or two). His ingestion of potentially undercooked meat raises the possibility of enterohemorrhagic infection complicated by the hemolytic uremic syndrome (HUS). However, while the purpuric rash and renal failure are compatible with HUS, the pace of illness and accompanying hypotension once again favor alternative infectious diagnoses.
The incubation period and presentation is concerning overwhelming bacterial infection related to the dog bite. Microbiological considerations include streptococcal species, Staphylococcus aureus, and gram‐negative organisms including Pasteurella species and Capnocytophaga canimorsus. The latter 2 organisms are of particular interest since they tend to cause severe sepsis in patients with alcoholism.
The antibiotic selection in this case is not straightforward. In general, empiric therapy for infections related to dog bites should include treatment for beta‐lactamaseproducing bacteria and anaerobes (eg, piperacillin/tazobactam). Yet, given the clinical presentation, severity of illness, and possible DIC, it is appropriate to be concerned about meningococcemia. Unfortunately, the tazobactam in piperacillin/tazobactam has poor central nervous system penetration so would be suboptimal treatment for meningitis. At this point, ceftriaxone, vancomycin, and metronidazole is a reasonable regimen.
Laboratory results were notable for blood urea nitrogen 50 mg/dL, creatinine 3.47 mg/dL, white cell count 21,800/L, with an absolute neutrophil count of 20,690/L, hematocrit 35.9%, platelet count 34,000/L, International Normalized Ratio 1.5, and partial thromboplastin time 44.0 seconds. His alanine aminotransferase was 356 U/L (1641 U/L), aspartate aminotransferase 959 U/L (1259 U/L), alkaline phosphatase 50 U/L (29111 U/L), and total bilirubin 1.7 mg/dL (0.31.3 mg/dL). Fibrinogen was 283 g/L (202430 g/L), lactate dehydrogenase was 1883 U/L (91185 IU/L), and uric acid was 10.5 mg/dL (3.77.7 mg/dL). His troponin I was 1.18 ng/mL (0.05 ng/ml), and his electrocardiogram showed sinus tachycardia but no evidence of myocardial ischemia. Chest x‐ray showed no infiltrate or evidence of volume overload. Lumbar puncture was deferred out of concern for ongoing disseminated intravascular coagulation.
Transthoracic echocardiogram revealed global hypokinesis and reduced left ventricular systolic function with ejection fraction of 35%. There was no evidence of vegetations or thrombus.
The patient's thrombocytopenia and prolonged coagulation parameters further support the presence of DIC. A peripheral blood smear should be examined. If microangiopathic changes are found, other diagnoses such as thrombotic thrombocytopenic purpura might be considered, although the rapid pace of illness and presence of hypotension still make sepsis with DIC more likely.
While septic shock often causes multi‐organ system failure secondary to hypoperfusion, the presumed rapid onset of hepatic and renal abnormalities suggests that microvascular thrombosis is playing a larger role in his organ system dysfunction. Microvascular thrombosis could also contribute to his myocardial injury, though globally depressed ejection fraction and elevated troponin might also be explained by infectious myocarditis. A third possibility is that his severe sepsis caused his myocardial dysfunction. Regardless of its etiology, the patient has no clinical evidence of congestive heart failure, so no specific therapy is required at this time. However, his cardiopulmonary exam should be monitored closely, and if he survives, he should have repeat echocardiography to monitor for resolution of the global hypokinesis.
Further evaluation revealed creatine kinase of 45,000 ng/ml (55380 ng/ml) and repeat troponin of >22 ng/ml. Protein C level was low at 30%. Testing for HIV was negative. Blood smear from time of transfer had few schistocytes. Urinalysis showed muddy brown casts but no dysmorphic red blood cells or red cell casts. The patient was placed on continuous veno‐venous hemofiltration (CVVH) for worsening renal failure and oliguria from presumed acute tubular necrosis in the setting of rhabdomyolysis and sepsis.
The patient has severe rhabdomyolysis that cannot fully be explained by his initial hypoperfusion and is more likely related to the overwhelming infection and microthrombosis. Rhabdomyolysis probably contributed to his acute tubular necrosis and renal failure.
Dermatology consultation identified the rash as likely purpura fulminans. They recommended a skin biopsy to rule out vasculitis. Three skin biopsies revealed micro‐vascular thrombosis; direct immunofluorescence test was negative for vasculitis; his skin tissue culture was negative for bacterial, mycobacterial, and fungal organisms.
Input from the dermatology service was key in identifying the rash. Purpura fulminans has a limited differential that includes severe infection from gram‐negative organisms and protein C and S deficiency. Since the biopsy results made vasculitis unlikely, the team was able to focus greater attention on potential pathogens such as Pasteurella species and C. canimorsus.
The biopsy also confirms the clinical suspicion that microvascular thrombosis is causing the patient's acute kidney injury, rhabdomyolysis, and myocardial ischemia. The presence of microvascular thrombosis prompts consideration of antithrombotic therapy such as heparin, but benefits of this therapy must be weighed against contraindications including bleeding and thrombocytopenia.
Ultimately out of concerns for recurrent gastrointestinal bleeding, the primary team decided not to treat with heparin or other antithrombotic therapy.
After several days of supportive care with antibiotics and renal replacement therapy, the patient showed gradual improvement of his retiform purpura, sensory neuropathy, laboratory data, and other markers of end‐organ dysfunction. Purpura of his fingertips, feet, and toes progressed to dry gangrene (Figure 3), which was monitored for potential need for amputation. He remained dependent on intermittent hemodialysis.

His initial antibiotic regimen was narrowed to ceftriaxone monotherapy. Five days after initial presentation, blood cultures drawn from the outside emergency department grew a gram‐negative rod in the anaerobic broth. Ten days later, this gram‐negative rod was identified as Capnocytophaga canimorsus. He was ultimately discharged to a skilled nursing facility.
Generally growth of an organism in broth only suggests either a very low inoculum or that the isolate is a contaminant. In this case, it was because the causative organism, C. canimorsus, is an obligate anaerobe and quite fastidious, so unlikely to grow easily. The identification of C. canimorsus from the initial blood culture is not surprising in this patient who presented with severe sepsis, DIC, and purpura fulminans after a recent dog bite. While the patient's chronic alcohol use may explain his fulminant infection from an atypical organism, one should always consider occult underlying malignancy as a predisposing factor, particularly in patients of this age group.
With the appropriate course of antibiotics, C. canimorsus infection should be completely cured. However, recovery of kidney and cardiac function could take weeks to months, and his dry gangrene may or may not resolve.
COMMENTARY
Capnocytophaga canimorsis sepsis is a rare and potentially deadly complication of dog bites that can present with rash, cellulitis, purpura fulminans, arthritis, meningitis, and endocarditis. The discussant considered a broad differential for the presentation of fever, rash, and acute illness. While the travel history was intriguing, the severity and pace of illness allowed him to focus attention on more recent infectious exposures. The ultimate key to the diagnosis was the patient's history of dog bite, an important but underrecognized source of serious infection in the United States.
According to the Centers for Disease Control and Prevention, there are approximately 4 million dog bites in the country each year. Of these, 300,000 bite victims seek care in the emergency department, resulting in 13,000 hospitalizations and 20 deaths annually.1 Infected dog bite wounds often grow polymicrobial flora. Pasteurella species are the most frequently found organisms in both dog and cat bite wounds. However, other aerobes such as streptococci, staphylococci, Moraxella, and Neisseria, as well as anaerobes including Fusobacterium and Bacteroides species, are also common.2
C. canimorsis is a facultative, fastidious gram‐negative bacillus found in the mouth flora of not only dogs but also cats and humans. It is often mistaken for other gram‐negative rod species.3 As with the patient described in this report, systemic infection from C. canimorsis can follow even superficial or well‐healed bite wounds.
Since this bacterium was first described in the literature 30 years ago, more than 100 cases of C. canimorsus infection have been described, with a mortality rate of nearly 30%.4 C. canimorsus occurs more frequently in males and in patients 50 to 70 years of age. Traditional risk factors include alcohol abuse, asplenia, immunosuppression, and corticosteroid treatment. However, in a case series of 56 isolates in California, only 10% of patients with Capnocytophaga sepsis were asplenic and none had alcohol abuse reported in their medical charts. In this series, median time from dog bite to the onset of symptoms was 3 days. Eighty‐five percent of patients presented with fever, while 32% had sepsis and 13% had DIC or septic shock.3
While C. canimorsus was once susceptible to a range of antibiotics, several reports from Canada and Europe document rising rates of beta‐lactamaseproducing strains that have caused clinically significant disease.5, 6 Individual susceptibility data take days to obtain, so it is important to start with empiric therapy. In general, empiric therapy for all serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes, for example, with amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam. If the patient is allergic to penicillin, clindamycin plus a fluoroquinolone can be used instead.
There are previous reports of purpura fulminans and symmetric peripheral gangrene following Capnocytophaga infection from dog bites.7, 8 Purpura fulminans is defined as rapidly progressive skin necrosis due to dermal vascular thrombosis, often in the setting of DIC. Early involvement occurs at acral sites, such as the nose, ears, fingers, and toes. Purpuric lesions often progress to skin necrosis or dry gangrene within 24 to 48 hours. In a review of 12 patients with purpura fulminans, only 9 survived. Eight of the 9 survivors required amputation of at least 1 limb, and 4 of them required 4‐limb amputation.7
In this patient who presented with fever and rash, the discussant recognized early on an underlying infectious etiology. Although the patient's exposure history led the discussant to consider a host of possibilities, the recognition of purpura fulminans allowed him to narrow his differential. Ultimately, the dog's bite clinched the diagnosis.
KEY TEACHING POINTS
-
Sepsis caused by C. canimorsus is often characterized by rash, cellulitis, arthritis, meningitis, and endocarditis. In some instances, infection can progress to purpura fulminans.
-
In cases where fastidious organisms are suspected as an infectious source, microbiology labs should be notified of suspected organisms so they can extend incubation periods or use special media to maximize culture yield and the likelihood of accurate identification.
-
Empiric therapy for serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes. Consider using amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
Acknowledgements
The authors thank Snigdha Vallabhaneni, MD, from the UCSF Division of Infectious Diseases, for her contributions to the discussion on C. canimorsus. They also thank Kanade Shinkai, MD, PhD, from the UCSF Department of Dermatology, and Heather Nye, MD, PhD, from the UCSF Division of Hospital Medicine, for their review of the manuscript.
Disclosure: Nothing to report.
- ,,.Incidence of dog bite injuries treated in emergency departments.JAMA.1998;279:51–53.
- ,,,,.Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group.N Engl J Med.1999;340:85–92.
- ,,,.Diagnosing Capnocytophaga canimorsus infections.Emerg Infect Dis.2006;12:340–342.
- ,,.Capnocytophaga canimorsus infections in human: review of the literature and cases report.Eur J Epidemiol.1996;12:521–533.
- ,,,,.Antimicrobial susceptibilities and beta‐lactamase characterization of Capnocytophaga species.Antimicrob Agents Chemother.1992;36:2197–2200.
- ,,,,,.Bacteremia due to Capnocytophaga species in patients with neutropenia: high frequency of beta‐lactamase‐producing strains.Clin Infect Dis.1999;28:1172–1174.
- ,,.Presentation and outcome of purpura fulminans associated with peripheral gangrene in 12 patients at Mayo Clinic.J Am Acad Dermatol.2007;57:944–956.
- ,,,.Capnocytophaga canimorsus sepsis with purpura fulminans and symmetrical gangrene following a dog bite in a shelter employee.Am J Med Sci.2004:327:369–372.
- ,,.Incidence of dog bite injuries treated in emergency departments.JAMA.1998;279:51–53.
- ,,,,.Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group.N Engl J Med.1999;340:85–92.
- ,,,.Diagnosing Capnocytophaga canimorsus infections.Emerg Infect Dis.2006;12:340–342.
- ,,.Capnocytophaga canimorsus infections in human: review of the literature and cases report.Eur J Epidemiol.1996;12:521–533.
- ,,,,.Antimicrobial susceptibilities and beta‐lactamase characterization of Capnocytophaga species.Antimicrob Agents Chemother.1992;36:2197–2200.
- ,,,,,.Bacteremia due to Capnocytophaga species in patients with neutropenia: high frequency of beta‐lactamase‐producing strains.Clin Infect Dis.1999;28:1172–1174.
- ,,.Presentation and outcome of purpura fulminans associated with peripheral gangrene in 12 patients at Mayo Clinic.J Am Acad Dermatol.2007;57:944–956.
- ,,,.Capnocytophaga canimorsus sepsis with purpura fulminans and symmetrical gangrene following a dog bite in a shelter employee.Am J Med Sci.2004:327:369–372.
Successfully Promoted Academic Hospitalists
The growth of academic hospital medicine has been driven by multiple factors including expanding clinical needs, housestaff duty hours' limitations, and an increasing focus on quality and patient safety.1 Hospitalists at academic medical centers frequently assume roles that differ substantially from traditional faculty positions. Academic hospitalists may have predominantly clinical positions, and may be involved in quality improvement and patient safety projects.24 Because of these commitments, many academic hospitalists spend less time on research or educational efforts.1, 5 Many have raised concerns that these unique job descriptions might lead to less time to devote to scholarship and academic pursuits, and consequently greater challenges in the promotions process.2, 5
There are little published data on promotion and tenure in academics, and even less specifically focused on the promotion of hospitalists. Theoretically, promotion should recognize an individual's contribution to his or her institution and field. However, each institution has unique criteria though which faculty achieve promotion. Previous articles addressing specific groups, such as part‐time,6 clinical faculty,79 or clinician‐educators10 may be relevant to hospitalists, as hospitalists may be more likely to fall into these categories. These reports suggest general agreement that promotion committees should consider and weigh clinical and educational work (in addition to scholarly publications) in the promotions process, but assessment methods vary across institutions and the contribution of activities, such as quality improvement, remain unclear. The educator's portfolio has gained momentum as a way to document valued teaching in many institutions,11, 12 but academic hospitalist participation in education may be limited.13
Literature related to the development of Divisions of General Internal Medicine is relevant insofar as similar concerns for promotion were expressed with the growth of their faculty.14, 15 However, its applicability may be limited by differences between roles of hospitalists and more traditional general medicine faculty.
To better understand the factors influencing promotion for academic hospitalists, the Society of General Internal Medicine (SGIM) Academic Hospitalist Task Force (AHTF) undertook a survey of promoted hospitalists who had successfully reached the rank of Associate Professor or higher.
Methods
Development of the Survey
The AHTF is a group of 18 academic hospitalists representing 15 institutions. Draft survey questions were developed by the group and sent to its members for refinement based on group consensus. Three cycles of refinement were performed, and the final survey (Appendix) was converted into an electronic format distributed through SurveyMonkey (SurveyMonkey.com, Portland, OR).
Identification of Survey Recipients
We identified a convenience sample of hospitalists who had been promoted to Associate or Full Professor of Medicine by querying members of the AHTF, the Society of Hospital Medicine (SHM) Academic Committee, and colleagues of academic medical centers with established hospitalist programs. We identified 33 promoted hospitalists.
Each recipient received an email from the AHTF cochairs in January 2009 asking them to complete the survey. If a response was not received in three weeks, a second email was sent. If a response was again not received, an AHTF task‐force member who knew the recipient asked him or her to complete the survey. All responses were received by March 2009.
Data Analysis
We examined responses using descriptive measures. Responses were analyzed across all respondents, as well as between these two subgroups. Statistical analysis with Fisher's exact test was performed using Stata 9.0 (StataCorp, College Station, TX).
Results
Of the 33 hospitalists who received the survey, 26 responded (response rate of 79%). Of these, 25 completed the survey in its entirely and were included in our analysis; 1 did not submit details regarding specific promotion‐related activities. General information regarding the respondents and their programs at the time of their promotion is contained in Table 1.
| |
| No. of institutions represented | 20 |
| Program age | 5.7 years (range 110) |
| Size of hospitalist program at the time of promotion | 10 (range 128) |
| Size of hospitalist program currently | 25 (range 745) |
| Programs that were separate divisions at the time of respondent promotion | 4 (20%) |
| Programs that are now separate divisions | 8 (40%) |
| Programs with 1‐track* promotion system | 2 (10%) |
| Programs with 2‐track promotion system | 8 (40%) |
| Programs with 3‐track promotion system | 9 (45%) |
| Other type of promotion system | 1 (5%) |
| Tenure track* | 8 (32%) |
| Institutions with tenure and promotion criteria that explicitly recognized hospitalist work | 8 (40%) |
The seven nonrespondents were from seven different institutions; however two of these institutions were represented by respondents. One nonrespondent had achieved a rank of Professor (through general medicine); the rest had been promoted to Associate Professor. One nonrespondent is known by the authors to hold a research position.
Ten respondents identified themselves as clinician‐educators (40%), ten as clinician‐administrators (40%), and five as clinician‐researchers (20%). Seventeen (68%) of the promoted hospitalists were not on a tenure track (as defined by them); they were more likely to have administrative or educational roles than a research appointment. Though the majority of self‐identified researchers were among the earliest to have been promoted, there were no statistically significant differences in self‐defined job description between more and less recently promoted hospitalists.
Promoted hospitalists were involved in a diverse range of activities which supported their promotion, including service (eg, institutional committees), education, research, and quality improvement. Nearly all hospitalists surveyed listed teaching and educational activities, and almost all had disseminated scholarly output and some degree of grant funding. Table 2 lists the specific activities in which respondents reported being engaged in each of these domains.
| Activity | Percent of Respondents Engaged in Activity |
|---|---|
| Service | |
| School of Medicine | 56 |
| Department of Medicine | 84 |
| Hospital | 80 |
| Professional societies | 92 |
| Administration | 67 |
| Education | |
| Medical student | 72 |
| Housestaff lectures | 84 |
| Ward/consult attending | 96 |
| Clinic precepting | 40 |
| Course director/curriculum development | 80 |
| Program director (or associate) | 36 |
| Research | |
| Peer‐reviewed publications | 92 |
| Abstract/poster presentations | 80 |
| Invited speaker | 96 |
| Reviewer/editor | 80 |
| Study section | 24 |
| Federal grants | 32 |
| Nonfederal grants (internal and external) | 72 |
| Quality improvement/patient safety | |
| Project member | 36 |
| Project leader | 52 |
| Institutional leadership | 32 |
| Curriculum development | 32 |
A range of individuals assisted the respondents in the promotion process. Twenty‐three (92%) respondents identified the individuals who supported their promotion, and all listed more than one person. Respondents most commonly credited their Section or Division Chief (43%) with facilitating their promotion, followed by Departmental Chairs or Vice/Associate Chairs (22%). Mentors (13%) or peers (8%) were also named. Four respondents (17%) named themselves as the person providing most guidance through the promotions process.
No consistent themes regarding obstacles emerged from free‐text responses to questions about the promotions process. One respondent felt that high clinical expectations made participation in other academic activities a challenge. The only other barriers noted were not being on the radar screen of the Division Chief of GIM, and difficulty identifying external, senior hospitalists to write letters in support of promotion.
When asked about the most important activities supporting their promotion, 24 respondents listed one to two key activities, detailed in Table 3. The most common response was peer‐reviewed publications (33%). Activities related to education and/or teaching were the next most common response (29%), specifically teaching, educational activities, curriculum design, or program director. Research or research funding represented 26% of responses. Valued activities outside of the respondent's institution included national reputation (21%) and service in professional societies (16%). Service or administrative responsibilities were mentioned by 25% of respondents.
| Category of Activity | Frequency of Response* (%) |
|---|---|
| |
| Research | 14 (58) |
| Peer‐reviewed publications | 8 (33) |
| Research | 4 (16) |
| Research funding | 2 (8) |
| Activities outside institution | 8 (33) |
| National reputation | 5 (21) |
| Professional society membership | 3 (13) |
| Education | 7 (29) |
| Teaching | 3 (13) |
| Educational activities | 2 (8) |
| Residency Director | 1 (4) |
| Curriculum development | 1 (4) |
| Service | 6 (25) |
| Service | 3 (13) |
| Administration/leadership of group | 3 (13) |
Discussion
We conducted a unique and comprehensive survey of academic hospitalists who have been promoted since 1995. We identified the most common and important activities contributing to promotion. Contrary to our expectations, survey respondents generally did not report being a hospitalist was a barrier in the promotions process.
Respondents were engaged in a diverse range of activities, including service, education, and research. Interestingly, no one identified him or herself primarily as a clinician. Teaching appeared to be a core component for all surveyed, regardless of academic appointment. Only one felt that her clinical workload as a hospitalist was an obstacle that prevented her from being engaged in other activities important for promotion. With more programs potentially evolving to separate divisions, the issue of being on the radar screen of a General Internal Medicine Division Chief may become less common over time. We hope that as programs mature and the numbers of associate and full professors increase, there will not be difficulty obtaining outside letters.
Although only 23% self‐identified as clinician‐researchers, nearly all had peer‐reviewed publications and other evidence of disseminated scholarly work. Grant funding, both federal and nonfederal, was also common among this group. This finding is consistent with self‐reported activities of a cohort of junior internal medicine faculty followed over three years who were eventually promoted, though the majority of those participants were classified as having either traditional clinician‐educator or clinician‐researcher positions.16
Despite outlining a seemingly clear pathway to promotion for hospitalists, concerns remain. Most importantly, those surveyed seem to have achieved promotion through relatively traditional academic job descriptions. Obtaining or maintaining these types of positions may be difficult as clinical needs at academic centers increase. According to a recent survey of hospitalist faculty,13 over one‐third spend more than 60% of their time on nonteaching clinical services. In that survey, over half of respondents had little or no protected time for scholarly activities. The contrast between this survey's findings and ours raises the question of whether our promoted sample had positions similar to those of most academic hospitalists. Given that the majority of our respondents noted peer‐reviewed publications and grant funding to be among the most important activities for promotion, there may be a dangerous disconnect for junior academic hospitalists who spend the majority of their time in direct patient care. Moreover, the promoted hospitalists in our survey reported relatively less participation in quality improvement/patient safety activities, in contrast to both anecdotal and survey reports that these activities are a major component of many academic hospitalist positions.5, 17 Most academic medical centers do not yet consider achievements in this area in their promotions criteria, potentially creating a barrier for the ranks of clinician quality improvers.1 Thus, significant obstacles to promotion of academic hospitalists may exist.
Leaders in academic hospital medicine are recognizing these potential barriers. A diverse group from major professional societies recently published a summary of the challenges and opportunities for the field of academic hospital medicine.1 Several needs and areas for intervention were identified, including enhanced faculty development and improved documentation of quality improvement activities. The SGIM, the SHM, and the Association of Chiefs and Leaders of General Internal Medicine (ACLGIM) recently cosponsored an intensive four‐day faculty development course for junior faculty to promote skills necessary for academic hospitalist success. Early reports indicate that this was a success.1820
In addition, the AHTF has developed a Quality Portfolio, paralleling the Educator's Portfolio, that can be used as a tool for documenting quality improvement and patient safety activities in a way that can be useful for career development and promotion.4 Lastly, the Society of Hospital Medicine has hosted the inaugural Academic Hospital Medicine Leadership Summit as part of the national meeting to provide mentorship and professional development opportunities for junior faculty. Our hope is that these opportunities, coupled with the growth of mid‐level and senior leaders in hospital medicine, will provide greater infrastructure for the development and promotion of junior faculty.
Our results may have relevance beyond hospitalist groups. With anticipated further limits on housestaff duty hours, more academic physicians may be asked to fill predominantly clinical roles. In addition, a growing emphasis on quality and patient safety may lead to a more general expansion of academicians who focus on these areas.15
Our survey and methodology have limitations. By including only promoted individuals, we did not survey hospitalists with the most difficulties in the promotions processthose who were not promoted. Thus, we are unable to directly compare successful versus unsuccessful strategies. Identifying nonpromoted academic hospitalists to understand the reasons they were not (or have not yet been) promoted could be a next step in this line of inquiry. Additionally, understanding the attitudes of promotions committees regarding hospitalists, and the clinical and quality improvement roles in which they are engaged, could enhance our current results. Finally, we surveyed a convenience sample of a limited numbers of hospitalists and institutions, and were unable to systematically account for variations in promotions criteria across institutions. However, to our knowledge, this is the most comprehensive study of promotions among academic hospitalists to date. Given the common themes that emerged in terms of activities that supported promotion, mentors, and advice, we believe that our sample was sufficient to identify important themes and advance our understanding of this nascent specialty.
In conclusion, our survey of promoted hospital medicine faculty provides valuable information for junior faculty and hospitalist leaders. Success was found through engaging in a diverse set of activities in the traditional areas of education, service, and scholarship, frequently in conjunction with developing recognition outside of their institutions. While all respondents were clinically active, none described themselves as having purely clinical roles. As academic hospitalist roles evolve, academic leaders will need to provide adequate mentorship, create time for scholarly pursuits, and promote documentation and recognition of nontraditional activities that may nonetheless be worthy of promotion.
- ,,,,,.Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit.J Gen Intern Med.2009;24(5):636–641.
- ,,.Hospitalist educators: future of inpatient internal medicine training.Mt Sinai J Med.2008;75(5):436–451.
- ,,,.Hospitalists as emerging leaders in patient safety: lessons learned and future directions.J Patient Saf.2009;5(1):3–8.
- Quality Portfolio—Template and Instructions. Available at: http://www.sgim.org/userfiles/file/AHTF%20QP%20WEB%20TEMPLATE%20INS TRUCTIONS.pdf. Accessed on April 24,2010.
- .An innovative approach to support hospitalist physicians toward academic success.J Hosp Med.2008;3:314–318.
- ,,,.Institutional policies of U.S. medical schools regarding tenure, promotion, and benefits for part‐time faculty.Acad Med.2000;75(8):846–849.
- ,,,,,.Revising appointment, promotion, and tenure procedures to incorporate an expanded definition of scholarship: the University of Kentucky College of Medicine experience.Acad Med.2000;75(9)913–924.
- ,,,.Attitudes of clinical faculty about career progress, career success and recognition, and commitment to academic medicine. Results of a survey.Arch Intern Med.2000;160(17):2625–2629.
- ,,, et al.Promotion criteria for clinician‐educators.J Gen Intern Med.2003;18(9):711–716.
- ,,,.Documentation systems for educators seeking academic promotion in U.S. medical schools.Acad Med.2004;79(8):783–790.
- ,,,.Faculty development: academic opportunities for emergency medicine faculty on education career tracks.Acad Emerg Med.2003;10(10):1113–1117.
- ,,,.Burnout and internal medicine resident work hours restrictions.Arch Intern Med.2005;165(22):2595–2600.
- ,.Development of a Division of General Medicine in a Department of Internal Medicine.J Med Ed.1981;56:390–396.
- .The evolution of departments of medicine.N Engl J Med.1980;303(9):489–496.
- ,.Clinicians in quality improvement: a new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- ,,.A time to be promoted. The prospective study of promotion in academia.J Gen Intern Med.2006;21(2):123–129.
- Academic Hospitalist Academy. Available at: http://www.sgim.org/index. cfm?pageId=815. Accessed on April 24,2010.
- .Reflections on the Academic Hospitalist Academy.SGIM Forum.2010;33(1):5.
- .The Academic Hospitalist Academy: Get anchored, equipped, and energized.SGIM Forum.2010;33(1):5–6.
- .Four formative days in the life of an academic hospitalist: the Academic Hospitalist Academy.SGIM Forum.2010;33(1):6.
The growth of academic hospital medicine has been driven by multiple factors including expanding clinical needs, housestaff duty hours' limitations, and an increasing focus on quality and patient safety.1 Hospitalists at academic medical centers frequently assume roles that differ substantially from traditional faculty positions. Academic hospitalists may have predominantly clinical positions, and may be involved in quality improvement and patient safety projects.24 Because of these commitments, many academic hospitalists spend less time on research or educational efforts.1, 5 Many have raised concerns that these unique job descriptions might lead to less time to devote to scholarship and academic pursuits, and consequently greater challenges in the promotions process.2, 5
There are little published data on promotion and tenure in academics, and even less specifically focused on the promotion of hospitalists. Theoretically, promotion should recognize an individual's contribution to his or her institution and field. However, each institution has unique criteria though which faculty achieve promotion. Previous articles addressing specific groups, such as part‐time,6 clinical faculty,79 or clinician‐educators10 may be relevant to hospitalists, as hospitalists may be more likely to fall into these categories. These reports suggest general agreement that promotion committees should consider and weigh clinical and educational work (in addition to scholarly publications) in the promotions process, but assessment methods vary across institutions and the contribution of activities, such as quality improvement, remain unclear. The educator's portfolio has gained momentum as a way to document valued teaching in many institutions,11, 12 but academic hospitalist participation in education may be limited.13
Literature related to the development of Divisions of General Internal Medicine is relevant insofar as similar concerns for promotion were expressed with the growth of their faculty.14, 15 However, its applicability may be limited by differences between roles of hospitalists and more traditional general medicine faculty.
To better understand the factors influencing promotion for academic hospitalists, the Society of General Internal Medicine (SGIM) Academic Hospitalist Task Force (AHTF) undertook a survey of promoted hospitalists who had successfully reached the rank of Associate Professor or higher.
Methods
Development of the Survey
The AHTF is a group of 18 academic hospitalists representing 15 institutions. Draft survey questions were developed by the group and sent to its members for refinement based on group consensus. Three cycles of refinement were performed, and the final survey (Appendix) was converted into an electronic format distributed through SurveyMonkey (SurveyMonkey.com, Portland, OR).
Identification of Survey Recipients
We identified a convenience sample of hospitalists who had been promoted to Associate or Full Professor of Medicine by querying members of the AHTF, the Society of Hospital Medicine (SHM) Academic Committee, and colleagues of academic medical centers with established hospitalist programs. We identified 33 promoted hospitalists.
Each recipient received an email from the AHTF cochairs in January 2009 asking them to complete the survey. If a response was not received in three weeks, a second email was sent. If a response was again not received, an AHTF task‐force member who knew the recipient asked him or her to complete the survey. All responses were received by March 2009.
Data Analysis
We examined responses using descriptive measures. Responses were analyzed across all respondents, as well as between these two subgroups. Statistical analysis with Fisher's exact test was performed using Stata 9.0 (StataCorp, College Station, TX).
Results
Of the 33 hospitalists who received the survey, 26 responded (response rate of 79%). Of these, 25 completed the survey in its entirely and were included in our analysis; 1 did not submit details regarding specific promotion‐related activities. General information regarding the respondents and their programs at the time of their promotion is contained in Table 1.
| |
| No. of institutions represented | 20 |
| Program age | 5.7 years (range 110) |
| Size of hospitalist program at the time of promotion | 10 (range 128) |
| Size of hospitalist program currently | 25 (range 745) |
| Programs that were separate divisions at the time of respondent promotion | 4 (20%) |
| Programs that are now separate divisions | 8 (40%) |
| Programs with 1‐track* promotion system | 2 (10%) |
| Programs with 2‐track promotion system | 8 (40%) |
| Programs with 3‐track promotion system | 9 (45%) |
| Other type of promotion system | 1 (5%) |
| Tenure track* | 8 (32%) |
| Institutions with tenure and promotion criteria that explicitly recognized hospitalist work | 8 (40%) |
The seven nonrespondents were from seven different institutions; however two of these institutions were represented by respondents. One nonrespondent had achieved a rank of Professor (through general medicine); the rest had been promoted to Associate Professor. One nonrespondent is known by the authors to hold a research position.
Ten respondents identified themselves as clinician‐educators (40%), ten as clinician‐administrators (40%), and five as clinician‐researchers (20%). Seventeen (68%) of the promoted hospitalists were not on a tenure track (as defined by them); they were more likely to have administrative or educational roles than a research appointment. Though the majority of self‐identified researchers were among the earliest to have been promoted, there were no statistically significant differences in self‐defined job description between more and less recently promoted hospitalists.
Promoted hospitalists were involved in a diverse range of activities which supported their promotion, including service (eg, institutional committees), education, research, and quality improvement. Nearly all hospitalists surveyed listed teaching and educational activities, and almost all had disseminated scholarly output and some degree of grant funding. Table 2 lists the specific activities in which respondents reported being engaged in each of these domains.
| Activity | Percent of Respondents Engaged in Activity |
|---|---|
| Service | |
| School of Medicine | 56 |
| Department of Medicine | 84 |
| Hospital | 80 |
| Professional societies | 92 |
| Administration | 67 |
| Education | |
| Medical student | 72 |
| Housestaff lectures | 84 |
| Ward/consult attending | 96 |
| Clinic precepting | 40 |
| Course director/curriculum development | 80 |
| Program director (or associate) | 36 |
| Research | |
| Peer‐reviewed publications | 92 |
| Abstract/poster presentations | 80 |
| Invited speaker | 96 |
| Reviewer/editor | 80 |
| Study section | 24 |
| Federal grants | 32 |
| Nonfederal grants (internal and external) | 72 |
| Quality improvement/patient safety | |
| Project member | 36 |
| Project leader | 52 |
| Institutional leadership | 32 |
| Curriculum development | 32 |
A range of individuals assisted the respondents in the promotion process. Twenty‐three (92%) respondents identified the individuals who supported their promotion, and all listed more than one person. Respondents most commonly credited their Section or Division Chief (43%) with facilitating their promotion, followed by Departmental Chairs or Vice/Associate Chairs (22%). Mentors (13%) or peers (8%) were also named. Four respondents (17%) named themselves as the person providing most guidance through the promotions process.
No consistent themes regarding obstacles emerged from free‐text responses to questions about the promotions process. One respondent felt that high clinical expectations made participation in other academic activities a challenge. The only other barriers noted were not being on the radar screen of the Division Chief of GIM, and difficulty identifying external, senior hospitalists to write letters in support of promotion.
When asked about the most important activities supporting their promotion, 24 respondents listed one to two key activities, detailed in Table 3. The most common response was peer‐reviewed publications (33%). Activities related to education and/or teaching were the next most common response (29%), specifically teaching, educational activities, curriculum design, or program director. Research or research funding represented 26% of responses. Valued activities outside of the respondent's institution included national reputation (21%) and service in professional societies (16%). Service or administrative responsibilities were mentioned by 25% of respondents.
| Category of Activity | Frequency of Response* (%) |
|---|---|
| |
| Research | 14 (58) |
| Peer‐reviewed publications | 8 (33) |
| Research | 4 (16) |
| Research funding | 2 (8) |
| Activities outside institution | 8 (33) |
| National reputation | 5 (21) |
| Professional society membership | 3 (13) |
| Education | 7 (29) |
| Teaching | 3 (13) |
| Educational activities | 2 (8) |
| Residency Director | 1 (4) |
| Curriculum development | 1 (4) |
| Service | 6 (25) |
| Service | 3 (13) |
| Administration/leadership of group | 3 (13) |
Discussion
We conducted a unique and comprehensive survey of academic hospitalists who have been promoted since 1995. We identified the most common and important activities contributing to promotion. Contrary to our expectations, survey respondents generally did not report being a hospitalist was a barrier in the promotions process.
Respondents were engaged in a diverse range of activities, including service, education, and research. Interestingly, no one identified him or herself primarily as a clinician. Teaching appeared to be a core component for all surveyed, regardless of academic appointment. Only one felt that her clinical workload as a hospitalist was an obstacle that prevented her from being engaged in other activities important for promotion. With more programs potentially evolving to separate divisions, the issue of being on the radar screen of a General Internal Medicine Division Chief may become less common over time. We hope that as programs mature and the numbers of associate and full professors increase, there will not be difficulty obtaining outside letters.
Although only 23% self‐identified as clinician‐researchers, nearly all had peer‐reviewed publications and other evidence of disseminated scholarly work. Grant funding, both federal and nonfederal, was also common among this group. This finding is consistent with self‐reported activities of a cohort of junior internal medicine faculty followed over three years who were eventually promoted, though the majority of those participants were classified as having either traditional clinician‐educator or clinician‐researcher positions.16
Despite outlining a seemingly clear pathway to promotion for hospitalists, concerns remain. Most importantly, those surveyed seem to have achieved promotion through relatively traditional academic job descriptions. Obtaining or maintaining these types of positions may be difficult as clinical needs at academic centers increase. According to a recent survey of hospitalist faculty,13 over one‐third spend more than 60% of their time on nonteaching clinical services. In that survey, over half of respondents had little or no protected time for scholarly activities. The contrast between this survey's findings and ours raises the question of whether our promoted sample had positions similar to those of most academic hospitalists. Given that the majority of our respondents noted peer‐reviewed publications and grant funding to be among the most important activities for promotion, there may be a dangerous disconnect for junior academic hospitalists who spend the majority of their time in direct patient care. Moreover, the promoted hospitalists in our survey reported relatively less participation in quality improvement/patient safety activities, in contrast to both anecdotal and survey reports that these activities are a major component of many academic hospitalist positions.5, 17 Most academic medical centers do not yet consider achievements in this area in their promotions criteria, potentially creating a barrier for the ranks of clinician quality improvers.1 Thus, significant obstacles to promotion of academic hospitalists may exist.
Leaders in academic hospital medicine are recognizing these potential barriers. A diverse group from major professional societies recently published a summary of the challenges and opportunities for the field of academic hospital medicine.1 Several needs and areas for intervention were identified, including enhanced faculty development and improved documentation of quality improvement activities. The SGIM, the SHM, and the Association of Chiefs and Leaders of General Internal Medicine (ACLGIM) recently cosponsored an intensive four‐day faculty development course for junior faculty to promote skills necessary for academic hospitalist success. Early reports indicate that this was a success.1820
In addition, the AHTF has developed a Quality Portfolio, paralleling the Educator's Portfolio, that can be used as a tool for documenting quality improvement and patient safety activities in a way that can be useful for career development and promotion.4 Lastly, the Society of Hospital Medicine has hosted the inaugural Academic Hospital Medicine Leadership Summit as part of the national meeting to provide mentorship and professional development opportunities for junior faculty. Our hope is that these opportunities, coupled with the growth of mid‐level and senior leaders in hospital medicine, will provide greater infrastructure for the development and promotion of junior faculty.
Our results may have relevance beyond hospitalist groups. With anticipated further limits on housestaff duty hours, more academic physicians may be asked to fill predominantly clinical roles. In addition, a growing emphasis on quality and patient safety may lead to a more general expansion of academicians who focus on these areas.15
Our survey and methodology have limitations. By including only promoted individuals, we did not survey hospitalists with the most difficulties in the promotions processthose who were not promoted. Thus, we are unable to directly compare successful versus unsuccessful strategies. Identifying nonpromoted academic hospitalists to understand the reasons they were not (or have not yet been) promoted could be a next step in this line of inquiry. Additionally, understanding the attitudes of promotions committees regarding hospitalists, and the clinical and quality improvement roles in which they are engaged, could enhance our current results. Finally, we surveyed a convenience sample of a limited numbers of hospitalists and institutions, and were unable to systematically account for variations in promotions criteria across institutions. However, to our knowledge, this is the most comprehensive study of promotions among academic hospitalists to date. Given the common themes that emerged in terms of activities that supported promotion, mentors, and advice, we believe that our sample was sufficient to identify important themes and advance our understanding of this nascent specialty.
In conclusion, our survey of promoted hospital medicine faculty provides valuable information for junior faculty and hospitalist leaders. Success was found through engaging in a diverse set of activities in the traditional areas of education, service, and scholarship, frequently in conjunction with developing recognition outside of their institutions. While all respondents were clinically active, none described themselves as having purely clinical roles. As academic hospitalist roles evolve, academic leaders will need to provide adequate mentorship, create time for scholarly pursuits, and promote documentation and recognition of nontraditional activities that may nonetheless be worthy of promotion.
The growth of academic hospital medicine has been driven by multiple factors including expanding clinical needs, housestaff duty hours' limitations, and an increasing focus on quality and patient safety.1 Hospitalists at academic medical centers frequently assume roles that differ substantially from traditional faculty positions. Academic hospitalists may have predominantly clinical positions, and may be involved in quality improvement and patient safety projects.24 Because of these commitments, many academic hospitalists spend less time on research or educational efforts.1, 5 Many have raised concerns that these unique job descriptions might lead to less time to devote to scholarship and academic pursuits, and consequently greater challenges in the promotions process.2, 5
There are little published data on promotion and tenure in academics, and even less specifically focused on the promotion of hospitalists. Theoretically, promotion should recognize an individual's contribution to his or her institution and field. However, each institution has unique criteria though which faculty achieve promotion. Previous articles addressing specific groups, such as part‐time,6 clinical faculty,79 or clinician‐educators10 may be relevant to hospitalists, as hospitalists may be more likely to fall into these categories. These reports suggest general agreement that promotion committees should consider and weigh clinical and educational work (in addition to scholarly publications) in the promotions process, but assessment methods vary across institutions and the contribution of activities, such as quality improvement, remain unclear. The educator's portfolio has gained momentum as a way to document valued teaching in many institutions,11, 12 but academic hospitalist participation in education may be limited.13
Literature related to the development of Divisions of General Internal Medicine is relevant insofar as similar concerns for promotion were expressed with the growth of their faculty.14, 15 However, its applicability may be limited by differences between roles of hospitalists and more traditional general medicine faculty.
To better understand the factors influencing promotion for academic hospitalists, the Society of General Internal Medicine (SGIM) Academic Hospitalist Task Force (AHTF) undertook a survey of promoted hospitalists who had successfully reached the rank of Associate Professor or higher.
Methods
Development of the Survey
The AHTF is a group of 18 academic hospitalists representing 15 institutions. Draft survey questions were developed by the group and sent to its members for refinement based on group consensus. Three cycles of refinement were performed, and the final survey (Appendix) was converted into an electronic format distributed through SurveyMonkey (SurveyMonkey.com, Portland, OR).
Identification of Survey Recipients
We identified a convenience sample of hospitalists who had been promoted to Associate or Full Professor of Medicine by querying members of the AHTF, the Society of Hospital Medicine (SHM) Academic Committee, and colleagues of academic medical centers with established hospitalist programs. We identified 33 promoted hospitalists.
Each recipient received an email from the AHTF cochairs in January 2009 asking them to complete the survey. If a response was not received in three weeks, a second email was sent. If a response was again not received, an AHTF task‐force member who knew the recipient asked him or her to complete the survey. All responses were received by March 2009.
Data Analysis
We examined responses using descriptive measures. Responses were analyzed across all respondents, as well as between these two subgroups. Statistical analysis with Fisher's exact test was performed using Stata 9.0 (StataCorp, College Station, TX).
Results
Of the 33 hospitalists who received the survey, 26 responded (response rate of 79%). Of these, 25 completed the survey in its entirely and were included in our analysis; 1 did not submit details regarding specific promotion‐related activities. General information regarding the respondents and their programs at the time of their promotion is contained in Table 1.
| |
| No. of institutions represented | 20 |
| Program age | 5.7 years (range 110) |
| Size of hospitalist program at the time of promotion | 10 (range 128) |
| Size of hospitalist program currently | 25 (range 745) |
| Programs that were separate divisions at the time of respondent promotion | 4 (20%) |
| Programs that are now separate divisions | 8 (40%) |
| Programs with 1‐track* promotion system | 2 (10%) |
| Programs with 2‐track promotion system | 8 (40%) |
| Programs with 3‐track promotion system | 9 (45%) |
| Other type of promotion system | 1 (5%) |
| Tenure track* | 8 (32%) |
| Institutions with tenure and promotion criteria that explicitly recognized hospitalist work | 8 (40%) |
The seven nonrespondents were from seven different institutions; however two of these institutions were represented by respondents. One nonrespondent had achieved a rank of Professor (through general medicine); the rest had been promoted to Associate Professor. One nonrespondent is known by the authors to hold a research position.
Ten respondents identified themselves as clinician‐educators (40%), ten as clinician‐administrators (40%), and five as clinician‐researchers (20%). Seventeen (68%) of the promoted hospitalists were not on a tenure track (as defined by them); they were more likely to have administrative or educational roles than a research appointment. Though the majority of self‐identified researchers were among the earliest to have been promoted, there were no statistically significant differences in self‐defined job description between more and less recently promoted hospitalists.
Promoted hospitalists were involved in a diverse range of activities which supported their promotion, including service (eg, institutional committees), education, research, and quality improvement. Nearly all hospitalists surveyed listed teaching and educational activities, and almost all had disseminated scholarly output and some degree of grant funding. Table 2 lists the specific activities in which respondents reported being engaged in each of these domains.
| Activity | Percent of Respondents Engaged in Activity |
|---|---|
| Service | |
| School of Medicine | 56 |
| Department of Medicine | 84 |
| Hospital | 80 |
| Professional societies | 92 |
| Administration | 67 |
| Education | |
| Medical student | 72 |
| Housestaff lectures | 84 |
| Ward/consult attending | 96 |
| Clinic precepting | 40 |
| Course director/curriculum development | 80 |
| Program director (or associate) | 36 |
| Research | |
| Peer‐reviewed publications | 92 |
| Abstract/poster presentations | 80 |
| Invited speaker | 96 |
| Reviewer/editor | 80 |
| Study section | 24 |
| Federal grants | 32 |
| Nonfederal grants (internal and external) | 72 |
| Quality improvement/patient safety | |
| Project member | 36 |
| Project leader | 52 |
| Institutional leadership | 32 |
| Curriculum development | 32 |
A range of individuals assisted the respondents in the promotion process. Twenty‐three (92%) respondents identified the individuals who supported their promotion, and all listed more than one person. Respondents most commonly credited their Section or Division Chief (43%) with facilitating their promotion, followed by Departmental Chairs or Vice/Associate Chairs (22%). Mentors (13%) or peers (8%) were also named. Four respondents (17%) named themselves as the person providing most guidance through the promotions process.
No consistent themes regarding obstacles emerged from free‐text responses to questions about the promotions process. One respondent felt that high clinical expectations made participation in other academic activities a challenge. The only other barriers noted were not being on the radar screen of the Division Chief of GIM, and difficulty identifying external, senior hospitalists to write letters in support of promotion.
When asked about the most important activities supporting their promotion, 24 respondents listed one to two key activities, detailed in Table 3. The most common response was peer‐reviewed publications (33%). Activities related to education and/or teaching were the next most common response (29%), specifically teaching, educational activities, curriculum design, or program director. Research or research funding represented 26% of responses. Valued activities outside of the respondent's institution included national reputation (21%) and service in professional societies (16%). Service or administrative responsibilities were mentioned by 25% of respondents.
| Category of Activity | Frequency of Response* (%) |
|---|---|
| |
| Research | 14 (58) |
| Peer‐reviewed publications | 8 (33) |
| Research | 4 (16) |
| Research funding | 2 (8) |
| Activities outside institution | 8 (33) |
| National reputation | 5 (21) |
| Professional society membership | 3 (13) |
| Education | 7 (29) |
| Teaching | 3 (13) |
| Educational activities | 2 (8) |
| Residency Director | 1 (4) |
| Curriculum development | 1 (4) |
| Service | 6 (25) |
| Service | 3 (13) |
| Administration/leadership of group | 3 (13) |
Discussion
We conducted a unique and comprehensive survey of academic hospitalists who have been promoted since 1995. We identified the most common and important activities contributing to promotion. Contrary to our expectations, survey respondents generally did not report being a hospitalist was a barrier in the promotions process.
Respondents were engaged in a diverse range of activities, including service, education, and research. Interestingly, no one identified him or herself primarily as a clinician. Teaching appeared to be a core component for all surveyed, regardless of academic appointment. Only one felt that her clinical workload as a hospitalist was an obstacle that prevented her from being engaged in other activities important for promotion. With more programs potentially evolving to separate divisions, the issue of being on the radar screen of a General Internal Medicine Division Chief may become less common over time. We hope that as programs mature and the numbers of associate and full professors increase, there will not be difficulty obtaining outside letters.
Although only 23% self‐identified as clinician‐researchers, nearly all had peer‐reviewed publications and other evidence of disseminated scholarly work. Grant funding, both federal and nonfederal, was also common among this group. This finding is consistent with self‐reported activities of a cohort of junior internal medicine faculty followed over three years who were eventually promoted, though the majority of those participants were classified as having either traditional clinician‐educator or clinician‐researcher positions.16
Despite outlining a seemingly clear pathway to promotion for hospitalists, concerns remain. Most importantly, those surveyed seem to have achieved promotion through relatively traditional academic job descriptions. Obtaining or maintaining these types of positions may be difficult as clinical needs at academic centers increase. According to a recent survey of hospitalist faculty,13 over one‐third spend more than 60% of their time on nonteaching clinical services. In that survey, over half of respondents had little or no protected time for scholarly activities. The contrast between this survey's findings and ours raises the question of whether our promoted sample had positions similar to those of most academic hospitalists. Given that the majority of our respondents noted peer‐reviewed publications and grant funding to be among the most important activities for promotion, there may be a dangerous disconnect for junior academic hospitalists who spend the majority of their time in direct patient care. Moreover, the promoted hospitalists in our survey reported relatively less participation in quality improvement/patient safety activities, in contrast to both anecdotal and survey reports that these activities are a major component of many academic hospitalist positions.5, 17 Most academic medical centers do not yet consider achievements in this area in their promotions criteria, potentially creating a barrier for the ranks of clinician quality improvers.1 Thus, significant obstacles to promotion of academic hospitalists may exist.
Leaders in academic hospital medicine are recognizing these potential barriers. A diverse group from major professional societies recently published a summary of the challenges and opportunities for the field of academic hospital medicine.1 Several needs and areas for intervention were identified, including enhanced faculty development and improved documentation of quality improvement activities. The SGIM, the SHM, and the Association of Chiefs and Leaders of General Internal Medicine (ACLGIM) recently cosponsored an intensive four‐day faculty development course for junior faculty to promote skills necessary for academic hospitalist success. Early reports indicate that this was a success.1820
In addition, the AHTF has developed a Quality Portfolio, paralleling the Educator's Portfolio, that can be used as a tool for documenting quality improvement and patient safety activities in a way that can be useful for career development and promotion.4 Lastly, the Society of Hospital Medicine has hosted the inaugural Academic Hospital Medicine Leadership Summit as part of the national meeting to provide mentorship and professional development opportunities for junior faculty. Our hope is that these opportunities, coupled with the growth of mid‐level and senior leaders in hospital medicine, will provide greater infrastructure for the development and promotion of junior faculty.
Our results may have relevance beyond hospitalist groups. With anticipated further limits on housestaff duty hours, more academic physicians may be asked to fill predominantly clinical roles. In addition, a growing emphasis on quality and patient safety may lead to a more general expansion of academicians who focus on these areas.15
Our survey and methodology have limitations. By including only promoted individuals, we did not survey hospitalists with the most difficulties in the promotions processthose who were not promoted. Thus, we are unable to directly compare successful versus unsuccessful strategies. Identifying nonpromoted academic hospitalists to understand the reasons they were not (or have not yet been) promoted could be a next step in this line of inquiry. Additionally, understanding the attitudes of promotions committees regarding hospitalists, and the clinical and quality improvement roles in which they are engaged, could enhance our current results. Finally, we surveyed a convenience sample of a limited numbers of hospitalists and institutions, and were unable to systematically account for variations in promotions criteria across institutions. However, to our knowledge, this is the most comprehensive study of promotions among academic hospitalists to date. Given the common themes that emerged in terms of activities that supported promotion, mentors, and advice, we believe that our sample was sufficient to identify important themes and advance our understanding of this nascent specialty.
In conclusion, our survey of promoted hospital medicine faculty provides valuable information for junior faculty and hospitalist leaders. Success was found through engaging in a diverse set of activities in the traditional areas of education, service, and scholarship, frequently in conjunction with developing recognition outside of their institutions. While all respondents were clinically active, none described themselves as having purely clinical roles. As academic hospitalist roles evolve, academic leaders will need to provide adequate mentorship, create time for scholarly pursuits, and promote documentation and recognition of nontraditional activities that may nonetheless be worthy of promotion.
- ,,,,,.Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit.J Gen Intern Med.2009;24(5):636–641.
- ,,.Hospitalist educators: future of inpatient internal medicine training.Mt Sinai J Med.2008;75(5):436–451.
- ,,,.Hospitalists as emerging leaders in patient safety: lessons learned and future directions.J Patient Saf.2009;5(1):3–8.
- Quality Portfolio—Template and Instructions. Available at: http://www.sgim.org/userfiles/file/AHTF%20QP%20WEB%20TEMPLATE%20INS TRUCTIONS.pdf. Accessed on April 24,2010.
- .An innovative approach to support hospitalist physicians toward academic success.J Hosp Med.2008;3:314–318.
- ,,,.Institutional policies of U.S. medical schools regarding tenure, promotion, and benefits for part‐time faculty.Acad Med.2000;75(8):846–849.
- ,,,,,.Revising appointment, promotion, and tenure procedures to incorporate an expanded definition of scholarship: the University of Kentucky College of Medicine experience.Acad Med.2000;75(9)913–924.
- ,,,.Attitudes of clinical faculty about career progress, career success and recognition, and commitment to academic medicine. Results of a survey.Arch Intern Med.2000;160(17):2625–2629.
- ,,, et al.Promotion criteria for clinician‐educators.J Gen Intern Med.2003;18(9):711–716.
- ,,,.Documentation systems for educators seeking academic promotion in U.S. medical schools.Acad Med.2004;79(8):783–790.
- ,,,.Faculty development: academic opportunities for emergency medicine faculty on education career tracks.Acad Emerg Med.2003;10(10):1113–1117.
- ,,,.Burnout and internal medicine resident work hours restrictions.Arch Intern Med.2005;165(22):2595–2600.
- ,.Development of a Division of General Medicine in a Department of Internal Medicine.J Med Ed.1981;56:390–396.
- .The evolution of departments of medicine.N Engl J Med.1980;303(9):489–496.
- ,.Clinicians in quality improvement: a new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- ,,.A time to be promoted. The prospective study of promotion in academia.J Gen Intern Med.2006;21(2):123–129.
- Academic Hospitalist Academy. Available at: http://www.sgim.org/index. cfm?pageId=815. Accessed on April 24,2010.
- .Reflections on the Academic Hospitalist Academy.SGIM Forum.2010;33(1):5.
- .The Academic Hospitalist Academy: Get anchored, equipped, and energized.SGIM Forum.2010;33(1):5–6.
- .Four formative days in the life of an academic hospitalist: the Academic Hospitalist Academy.SGIM Forum.2010;33(1):6.
- ,,,,,.Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit.J Gen Intern Med.2009;24(5):636–641.
- ,,.Hospitalist educators: future of inpatient internal medicine training.Mt Sinai J Med.2008;75(5):436–451.
- ,,,.Hospitalists as emerging leaders in patient safety: lessons learned and future directions.J Patient Saf.2009;5(1):3–8.
- Quality Portfolio—Template and Instructions. Available at: http://www.sgim.org/userfiles/file/AHTF%20QP%20WEB%20TEMPLATE%20INS TRUCTIONS.pdf. Accessed on April 24,2010.
- .An innovative approach to support hospitalist physicians toward academic success.J Hosp Med.2008;3:314–318.
- ,,,.Institutional policies of U.S. medical schools regarding tenure, promotion, and benefits for part‐time faculty.Acad Med.2000;75(8):846–849.
- ,,,,,.Revising appointment, promotion, and tenure procedures to incorporate an expanded definition of scholarship: the University of Kentucky College of Medicine experience.Acad Med.2000;75(9)913–924.
- ,,,.Attitudes of clinical faculty about career progress, career success and recognition, and commitment to academic medicine. Results of a survey.Arch Intern Med.2000;160(17):2625–2629.
- ,,, et al.Promotion criteria for clinician‐educators.J Gen Intern Med.2003;18(9):711–716.
- ,,,.Documentation systems for educators seeking academic promotion in U.S. medical schools.Acad Med.2004;79(8):783–790.
- ,,,.Faculty development: academic opportunities for emergency medicine faculty on education career tracks.Acad Emerg Med.2003;10(10):1113–1117.
- ,,,.Burnout and internal medicine resident work hours restrictions.Arch Intern Med.2005;165(22):2595–2600.
- ,.Development of a Division of General Medicine in a Department of Internal Medicine.J Med Ed.1981;56:390–396.
- .The evolution of departments of medicine.N Engl J Med.1980;303(9):489–496.
- ,.Clinicians in quality improvement: a new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- ,,.A time to be promoted. The prospective study of promotion in academia.J Gen Intern Med.2006;21(2):123–129.
- Academic Hospitalist Academy. Available at: http://www.sgim.org/index. cfm?pageId=815. Accessed on April 24,2010.
- .Reflections on the Academic Hospitalist Academy.SGIM Forum.2010;33(1):5.
- .The Academic Hospitalist Academy: Get anchored, equipped, and energized.SGIM Forum.2010;33(1):5–6.
- .Four formative days in the life of an academic hospitalist: the Academic Hospitalist Academy.SGIM Forum.2010;33(1):6.
Copyright © 2011 Society of Hospital Medicine
In the Literature
Literature at a Glance
A guide to this month’s studies.
- Carotid stenting is equivalent to endarterectomy in carotid artery stenosis.
- Idiopathic ventricular fibrillation is associated with early repolarization.
- Aggressive LDL and blood pressure management may benefit diabetic patients.
- Treatment of hypertension in patients older than age 80 improves outcomes.
- Length of stay predicts mortality in pulmonary embolism patients.
- Poor symptom control and lack of family support during end-of-life hospital stays.
- Potentially inappropriate medication use in hospitalized elders is common.
- Steroids may not help in pediatric meningitis.
- PCI and CABG may be equivalent in the treatment of left main disease.
Is Stenting or Endarterectomy Best for Carotid Artery Stenosis?
Background: Patients with moderate to severe symptomatic carotid artery stenosis and those with severe asymptomatic carotid stenosis benefit from carotid endarterectomy. Carotid stenting may provide an alternative therapy, but the long-term protection against stroke compared with endarterectomy is unclear.
Study Design: Prospective randomized trial.
Setting: 29 centers in the United States.
Synopsis: This article reports the long-term (three years) follow-up of the SAPPHIRE trial, published in 2004, which compared carotid stenting to endarterectomy in patients at high surgical risk. In that trial, 334 patients randomized to either stenting or endarterectomy had similar outcomes at one year. Patients were followed for three years with death and major cardiovascular events as endpoints.
Rates of stroke at three years were approximately 10% with an overall death rate of approximately 20%. There was no difference between carotid stenting and endarterectomy with regards to death, stroke, or other cardiovascular outcome.
Notably, follow-up was not complete (78%), a specific type of stenting procedure was used, and the patient population was at high risk for surgical complications. Therefore, results may not be applicable in other centers or in other patient populations. Yet, this trial provides follow-up, long-term evidence that carotid stenting may be a viable alternative to endarterectomy in patients with carotid artery stenosis.
Bottom line: Carotid stenting and endarterectomy had similar outcomes at three years in high-risk patients with carotid artery stenosis.
Citation: Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572-1579.
Is Early Repolarization on EKG Associated with Sudden Cardiac Arrest?
Background: Electrocardiographic early repolarization, defined as elevation of the QRS-ST junction of at least 0.1mV from baseline in the inferior or lateral leads (manifested as slurring or notching), occurs in 1% to 5% of patients. It is considered benign, but experimental studies have suggested it may be arrhythmogenic.
Study Design: Prospective case-control.
Setting: 22 international tertiary care centers.
Synopsis: Case subjects were less than 60 years of age and were resuscitated after ventricular fibrillation (VF) arrest ultimately deemed idiopathic. All had normal echocardiograms, no evidence of coronary artery disease, and no repolarization abnormalities (including Brugada and long-QT). Of 206 patients, 31% had early repolarization on EKG, versus only 5% in controls without heart disease. In case subjects with prior EKGs, early repolarization was proven to be pre-existing.
The mean magnitude of J-point elevation was 2 mm in cases versus 1.2 mm in controls, and in cases this magnitude increased during later episodes of arrhythmia. Electrophysiologic mapping showed that ectopy originated at sites concordant with the location of abnormal repolarization. During five years of follow-up, arrhythmic recurrence was twice as common in cases with early repolarization.
Although long-term observational studies of persons with early repolarization have shown a benign natural course, this study may change our approach to those with syncope or a family history of sudden death.
Bottom line: Early repolarization on EKG is associated with idiopathic ventricular fibrillation.
Citation: Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358(19):2016-2023.
Does Aggressive Blood Pressure and LDL Treatment in Diabetics Affect Development of Subclinical Atherosclerosis?
Background: There is evidence to suggest more aggressive treatment of LDL cholesterol in patients with known coronary artery disease is beneficial and more aggressive blood pressure control can improve outcomes in some patient populations. However, it is unclear if patients with diabetes without cardiovascular disease would benefit from more aggressive LDL and systolic blood pressure (SBP) treatment.
Study Design: Randomized, open-label, blinded-to-end point trial.
Setting: Four centers in Oklahoma, Arizona, and South Dakota.
Synopsis: Investigators studied 499 type 2 diabetic American Indian men with no history of cardiovascular disease. Patients were randomized to receive treatment to achieve aggressive (70 mg/dL and 115 mmHg) or standard (100 mg/dL and 130 mmHg) targets for their LDL cholesterol and SBP, respectively.
At three years, the aggressive group showed decreased carotid intima-media thickness (IMT) and decreased left ventricular mass, whereas both IMT and left ventricular volume increased in the standard group. There were no differences in clinical cardiovascular events between the aggressive and standard group and both groups had lower-than-expected clinical events.
This study included no women and was limited to an American Indian population. Of note, there was an increase in adverse events related to blood pressure medications in the aggressive group. It also is unclear how the surrogates of cardiovascular disease or subclinical atherosclerosis relate to significant clinical outcomes.
Bottom line: More aggressive LDL and SBP treatment in diabetics without coronary disease decreased subclinical atherosclerosis but did not impact clinical outcomes.
Citation: Howard B, Roman M, Devereux R, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes. JAMA. 2008;299(14):1678-1689.
Should We Treat Hypertension in Patients Older Than 80?
Background: There is debate about whether treatment of hypertension in the elderly is beneficial. Numerous studies suggest blood pressure control does less to prevent strokes in patients older than 80 years than for younger patients. Moreover, other evidence shows controlling blood pressure in elderly patients may result in an increase in mortality even if there was a decreased risk of stroke.
Study Design: Randomized, double-blind, placebo-controlled trial.
Setting: 195 centers in 13 countries in Europe, China, Australasia, and North Africa.
Synopsis: This study evaluated 3,845 patients, age 80 or older, with a sustained systolic blood pressure (SBP) of 160 mmHg and randomized them to receive indapamide (sustained release) or placebo. Perindopril, or placebo, was added if necessary to achieve a target blood pressure of 150/80 mmHg. Patients who received the indapamide with or without the perindopril had lower blood pressure, lower rate of stroke, lower rate of heart failure, lower rate of death from a cardiovascular cause, and a 21% reduction in all-cause mortality (all statistically significant). There were very few adverse drug events and fewer adverse events overall in the treatment group.
Of note, exclusion criteria included a history of heart failure requiring anti-hypertensive medication, dementia, need for nursing care, an inability to stand or walk, and a creatinine more than 1.7 mg/dL. As well, the “target” SBP of 150 mmHg (which only half of the treatment group achieved) is still considered hypertensive according to the JNC 7 guidelines.
Bottom line: In some patients older than 80, treatment of hypertension may reduce the incidence of stroke, death from stroke, heart failure, and all-cause mortality.
Citation: Beckett N, Peters R, Fletcher A, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
What Is the Optimal Hospital LOS for Patients with PE?
Background: Though there are clear trends toward shorter hospital stays after pulmonary embolism (PE), especially with the introduction of low molecular weight heparin, the optimal timing of discharge and the effect of decreased length of stay (LOS) on post-discharge mortality are unknown. Furthermore, there is no risk stratification strategy used to identify low-risk patients with PE who can safely be discharged early or treated in the outpatient setting.
Study Design: Retrospective cohort study.
Setting: 186 acute care hospitals in Pennsylvania from January 2000 to November 2002.
Synopsis: Using a statewide database of 15,531 patients discharged with pulmonary embolism (PE), the authors sought to identify patient and hospital factors associated with LOS and assess whether LOS was associated with post-discharge mortality.
Findings indicate there is considerable variation in LOS for PE between and within hospitals in Pennsylvania. The median LOS for patients with PE was six days; patients with a LOS of four or fewer days had significantly higher post-discharge mortality than patients hospitalized five to eight days. More than half the patients discharged at four or fewer days were classified as high-risk, with Pulmonary Embolism Severity Index (PESI) scores of III-V (3.1% to 24.5% risk of mortality at 30 days).
Although we cannot infer causation (i.e., early discharge=death), clinicians should be aware of the results and consider severity of illness (using PESI or other criteria) in the discharge decision in patients with PE. Future prognostic models and evidence-based criteria would be helpful to identify patients with PE who can be safely discharged early.
Bottom line: Physicians may inappropriately select patients with PE for early discharge who are at increased risk of complications.
Citation: Aujesky D, Stone RA, Kim S, et al. Length of hospital stay and post-discharge mortality in patients with pulmonary embolism. Arch Intern Med. 2008;168(7):706-712.
Do Patients Have a “Good Death” in the Hospital?
Background: Despite an increasing focus on providing appropriate end-of-life care, the majority of patients in developed countries die in the hospital. The circumstances and quality of care provided at the time of death are poorly described.
Study Design: Cross-sectional survey.
Setting: 613 departments in 200 French hospitals.
Synopsis: For 3,793 in-hospital deaths, the investigators surveyed the bedside nurses about the circumstances and details of the death. Twenty-three percent of the patients were admitted for end-of-life care, 29% had a malignancy, and 50% of patients were identified as terminally ill for three days prior to their death.
A family member or relative was present in only 25% of all deaths; 20% of patients were alone at the time of death. In the last few hours of life, up to 70% of patients had symptoms of respiratory distress, while only 44% received opiate analgesia. Only 35% of nurses were satisfied with the quality of death. Satisfaction increased with presence of family members and having written protocols for care at the end of life.
This large, multicenter study has limitations but provides a concerning snapshot of death in the hospital. Hospitalists should be aggressive about symptom control at the end of life as well as attempt to ensure patients are not alone at the time of death.
Bottom line: Many patients die in the hospital in some degree of respiratory distress and without family or friends at the bedside.
Citation: Ferrand E, Jabre P, Vincent-Genod C, et al. Circumstances of death in hospitalized patients and nurses’ perceptions. Arch Intern Med. 2008;168(8):867-875.
How Common Is Potentially Inappropriate Medication Use in the Hospital?
Background: Use of potentially inappropriate medications (PIM) in the elderly based on the Beers’ List is common in nursing homes, the emergency department (ED), and outpatient settings and is associated with adverse outcomes and hospitalization. Frequency of PIM use the inpatient setting has not been well studied.
Study Design: A retrospective cohort study.
Setting: 384 U.S. hospitals.
Synopsis: In this retrospective cohort study of 493,971 inpatients (older than 65) admitted with medical diagnoses to non-surgeons, PIM prescription was evaluated. Forty nine percent of all patients were prescribed at least one PIM, while 6% were prescribed three or more. In a multivariable model, physician specialty was associated with variation in high severity PIM (HSPIM) prescription. In comparison with internal medicine physicians, cardiologists (odds ratio [OR] 1.32) and pulmonologists (OR 1.10) were more likely to prescribe HSPIMs, while hospitalists (OR 0.90) and geriatricians (OR 0.60) were less likely. In addition, patient age older than 85 was associated with decreased HSPIM prescription (OR 0.59) compared with those younger than 85.
Compared with patients in the Midwest, patients in the South (OR 1.63) and West (OR 1.43) were more likely to prescribe HSPIMs, while those in the Northeast (OR 0.85) were less likely. Hospitals with geriatric services had less PIM use. The study couldn’t account for continuation of chronic medications and did not evaluate adverse outcomes from PIM prescribing.
Bottom line: PIM prescription to hospitalized geriatric patients is common and associated with provider and hospital characteristics.
Citation: Rothberg MB, Pekow PS, Liu F, et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3:91-102:91-102.
Is There a Benefit to Corticosteroids When Treating Bacterial Meningitis in Children?
Background: The benefit of adjuvant corticosteroids in the treatment of bacterial meningitis in children in the developed world remains unclear; recent expert guidelines reflect this uncertainty.
Study Design: Retrospective cohort study.
Setting: Twenty-seven tertiary care hospitals in the United States.
Synopsis: Researchers examined 2,780 children with a primary diagnosis of bacterial meningitis discharged from 27 tertiary care centers in the U.S. from 2001-2006. Using a propensity analysis (to control for severity of illness), the study compared those who had received adjunctive corticosteroids with those who had not, with mortality and length of study (LOS) as primary outcomes.
The median age was nine months, 8.9% of children received corticosteroids, and the overall mortality rate was 4.2%. Adjuvent corticosteroids did not reduce mortality or LOS. The outcomes were unchanged in subgroup analyses.
Although limited by its retrospective design and lack of other outcome measures (e.g., hearing loss, neurological deficits), this study provides reasonable evidence that corticosteroid use in bacterial meningitis in children may not save lives or shorten LOS. Pediatric hospitalists may not want to routinely give steroids in this setting pending large randomized-controlled trials.
Bottom line: Adjunctive corticosteroids therapy in children with bacterial meningitis may not save lives or reduce LOS.
Citation: Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055.
Should Unprotected Left Main Disease Be Treated With PCI or CABG?
Background: The current standard of care for the treatment of left main coronary artery disease is coronary-artery bypass grafting (CABG). With the advent of drug-eluting stents, there is growing interest in the use of percutaneous coronary intervention (PCI) to treat left main disease.
Study Design: Prospective observational study.
Setting: Twelve Korean cardiac centers.
Synopsis: From 2000 to 2006, patients with left main disease were treated with PCI or CABG at the discretion of the physician. Nearly 1,100 patients in each cohort were compared and evaluated for death and a composite outcome of death, myocardial infarction, or stroke. Propensity-matching was employed to control for confounders.
In the overall cohort matched by propensity score, there was no significant difference in death or the composite outcome between the PCI and CABG groups after three years. Type of stent (bare metal vs. drug-eluting) did not affect the outcome. Rates of target-vessel revascularization were significantly higher in the group that received stents.
The results are limited by the observational nature and the need for propensity analysis and yet provide an intriguing result. The standard of care for treatment of left main disease remains CABG, but clinicians may be more comfortable treating with stents while we await randomized-controlled trials.
Bottom line: In this observational study, PCI and CABG had similar outcomes in patients with left main disease.
Citation: Seung KB, Park D, Kim Y, Lee S. Stents versus coronary-artery bypass grafting for left main coronary artery disease. N Engl J Med. 2008;358:1781-1792.
Literature at a Glance
A guide to this month’s studies.
- Carotid stenting is equivalent to endarterectomy in carotid artery stenosis.
- Idiopathic ventricular fibrillation is associated with early repolarization.
- Aggressive LDL and blood pressure management may benefit diabetic patients.
- Treatment of hypertension in patients older than age 80 improves outcomes.
- Length of stay predicts mortality in pulmonary embolism patients.
- Poor symptom control and lack of family support during end-of-life hospital stays.
- Potentially inappropriate medication use in hospitalized elders is common.
- Steroids may not help in pediatric meningitis.
- PCI and CABG may be equivalent in the treatment of left main disease.
Is Stenting or Endarterectomy Best for Carotid Artery Stenosis?
Background: Patients with moderate to severe symptomatic carotid artery stenosis and those with severe asymptomatic carotid stenosis benefit from carotid endarterectomy. Carotid stenting may provide an alternative therapy, but the long-term protection against stroke compared with endarterectomy is unclear.
Study Design: Prospective randomized trial.
Setting: 29 centers in the United States.
Synopsis: This article reports the long-term (three years) follow-up of the SAPPHIRE trial, published in 2004, which compared carotid stenting to endarterectomy in patients at high surgical risk. In that trial, 334 patients randomized to either stenting or endarterectomy had similar outcomes at one year. Patients were followed for three years with death and major cardiovascular events as endpoints.
Rates of stroke at three years were approximately 10% with an overall death rate of approximately 20%. There was no difference between carotid stenting and endarterectomy with regards to death, stroke, or other cardiovascular outcome.
Notably, follow-up was not complete (78%), a specific type of stenting procedure was used, and the patient population was at high risk for surgical complications. Therefore, results may not be applicable in other centers or in other patient populations. Yet, this trial provides follow-up, long-term evidence that carotid stenting may be a viable alternative to endarterectomy in patients with carotid artery stenosis.
Bottom line: Carotid stenting and endarterectomy had similar outcomes at three years in high-risk patients with carotid artery stenosis.
Citation: Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572-1579.
Is Early Repolarization on EKG Associated with Sudden Cardiac Arrest?
Background: Electrocardiographic early repolarization, defined as elevation of the QRS-ST junction of at least 0.1mV from baseline in the inferior or lateral leads (manifested as slurring or notching), occurs in 1% to 5% of patients. It is considered benign, but experimental studies have suggested it may be arrhythmogenic.
Study Design: Prospective case-control.
Setting: 22 international tertiary care centers.
Synopsis: Case subjects were less than 60 years of age and were resuscitated after ventricular fibrillation (VF) arrest ultimately deemed idiopathic. All had normal echocardiograms, no evidence of coronary artery disease, and no repolarization abnormalities (including Brugada and long-QT). Of 206 patients, 31% had early repolarization on EKG, versus only 5% in controls without heart disease. In case subjects with prior EKGs, early repolarization was proven to be pre-existing.
The mean magnitude of J-point elevation was 2 mm in cases versus 1.2 mm in controls, and in cases this magnitude increased during later episodes of arrhythmia. Electrophysiologic mapping showed that ectopy originated at sites concordant with the location of abnormal repolarization. During five years of follow-up, arrhythmic recurrence was twice as common in cases with early repolarization.
Although long-term observational studies of persons with early repolarization have shown a benign natural course, this study may change our approach to those with syncope or a family history of sudden death.
Bottom line: Early repolarization on EKG is associated with idiopathic ventricular fibrillation.
Citation: Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358(19):2016-2023.
Does Aggressive Blood Pressure and LDL Treatment in Diabetics Affect Development of Subclinical Atherosclerosis?
Background: There is evidence to suggest more aggressive treatment of LDL cholesterol in patients with known coronary artery disease is beneficial and more aggressive blood pressure control can improve outcomes in some patient populations. However, it is unclear if patients with diabetes without cardiovascular disease would benefit from more aggressive LDL and systolic blood pressure (SBP) treatment.
Study Design: Randomized, open-label, blinded-to-end point trial.
Setting: Four centers in Oklahoma, Arizona, and South Dakota.
Synopsis: Investigators studied 499 type 2 diabetic American Indian men with no history of cardiovascular disease. Patients were randomized to receive treatment to achieve aggressive (70 mg/dL and 115 mmHg) or standard (100 mg/dL and 130 mmHg) targets for their LDL cholesterol and SBP, respectively.
At three years, the aggressive group showed decreased carotid intima-media thickness (IMT) and decreased left ventricular mass, whereas both IMT and left ventricular volume increased in the standard group. There were no differences in clinical cardiovascular events between the aggressive and standard group and both groups had lower-than-expected clinical events.
This study included no women and was limited to an American Indian population. Of note, there was an increase in adverse events related to blood pressure medications in the aggressive group. It also is unclear how the surrogates of cardiovascular disease or subclinical atherosclerosis relate to significant clinical outcomes.
Bottom line: More aggressive LDL and SBP treatment in diabetics without coronary disease decreased subclinical atherosclerosis but did not impact clinical outcomes.
Citation: Howard B, Roman M, Devereux R, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes. JAMA. 2008;299(14):1678-1689.
Should We Treat Hypertension in Patients Older Than 80?
Background: There is debate about whether treatment of hypertension in the elderly is beneficial. Numerous studies suggest blood pressure control does less to prevent strokes in patients older than 80 years than for younger patients. Moreover, other evidence shows controlling blood pressure in elderly patients may result in an increase in mortality even if there was a decreased risk of stroke.
Study Design: Randomized, double-blind, placebo-controlled trial.
Setting: 195 centers in 13 countries in Europe, China, Australasia, and North Africa.
Synopsis: This study evaluated 3,845 patients, age 80 or older, with a sustained systolic blood pressure (SBP) of 160 mmHg and randomized them to receive indapamide (sustained release) or placebo. Perindopril, or placebo, was added if necessary to achieve a target blood pressure of 150/80 mmHg. Patients who received the indapamide with or without the perindopril had lower blood pressure, lower rate of stroke, lower rate of heart failure, lower rate of death from a cardiovascular cause, and a 21% reduction in all-cause mortality (all statistically significant). There were very few adverse drug events and fewer adverse events overall in the treatment group.
Of note, exclusion criteria included a history of heart failure requiring anti-hypertensive medication, dementia, need for nursing care, an inability to stand or walk, and a creatinine more than 1.7 mg/dL. As well, the “target” SBP of 150 mmHg (which only half of the treatment group achieved) is still considered hypertensive according to the JNC 7 guidelines.
Bottom line: In some patients older than 80, treatment of hypertension may reduce the incidence of stroke, death from stroke, heart failure, and all-cause mortality.
Citation: Beckett N, Peters R, Fletcher A, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
What Is the Optimal Hospital LOS for Patients with PE?
Background: Though there are clear trends toward shorter hospital stays after pulmonary embolism (PE), especially with the introduction of low molecular weight heparin, the optimal timing of discharge and the effect of decreased length of stay (LOS) on post-discharge mortality are unknown. Furthermore, there is no risk stratification strategy used to identify low-risk patients with PE who can safely be discharged early or treated in the outpatient setting.
Study Design: Retrospective cohort study.
Setting: 186 acute care hospitals in Pennsylvania from January 2000 to November 2002.
Synopsis: Using a statewide database of 15,531 patients discharged with pulmonary embolism (PE), the authors sought to identify patient and hospital factors associated with LOS and assess whether LOS was associated with post-discharge mortality.
Findings indicate there is considerable variation in LOS for PE between and within hospitals in Pennsylvania. The median LOS for patients with PE was six days; patients with a LOS of four or fewer days had significantly higher post-discharge mortality than patients hospitalized five to eight days. More than half the patients discharged at four or fewer days were classified as high-risk, with Pulmonary Embolism Severity Index (PESI) scores of III-V (3.1% to 24.5% risk of mortality at 30 days).
Although we cannot infer causation (i.e., early discharge=death), clinicians should be aware of the results and consider severity of illness (using PESI or other criteria) in the discharge decision in patients with PE. Future prognostic models and evidence-based criteria would be helpful to identify patients with PE who can be safely discharged early.
Bottom line: Physicians may inappropriately select patients with PE for early discharge who are at increased risk of complications.
Citation: Aujesky D, Stone RA, Kim S, et al. Length of hospital stay and post-discharge mortality in patients with pulmonary embolism. Arch Intern Med. 2008;168(7):706-712.
Do Patients Have a “Good Death” in the Hospital?
Background: Despite an increasing focus on providing appropriate end-of-life care, the majority of patients in developed countries die in the hospital. The circumstances and quality of care provided at the time of death are poorly described.
Study Design: Cross-sectional survey.
Setting: 613 departments in 200 French hospitals.
Synopsis: For 3,793 in-hospital deaths, the investigators surveyed the bedside nurses about the circumstances and details of the death. Twenty-three percent of the patients were admitted for end-of-life care, 29% had a malignancy, and 50% of patients were identified as terminally ill for three days prior to their death.
A family member or relative was present in only 25% of all deaths; 20% of patients were alone at the time of death. In the last few hours of life, up to 70% of patients had symptoms of respiratory distress, while only 44% received opiate analgesia. Only 35% of nurses were satisfied with the quality of death. Satisfaction increased with presence of family members and having written protocols for care at the end of life.
This large, multicenter study has limitations but provides a concerning snapshot of death in the hospital. Hospitalists should be aggressive about symptom control at the end of life as well as attempt to ensure patients are not alone at the time of death.
Bottom line: Many patients die in the hospital in some degree of respiratory distress and without family or friends at the bedside.
Citation: Ferrand E, Jabre P, Vincent-Genod C, et al. Circumstances of death in hospitalized patients and nurses’ perceptions. Arch Intern Med. 2008;168(8):867-875.
How Common Is Potentially Inappropriate Medication Use in the Hospital?
Background: Use of potentially inappropriate medications (PIM) in the elderly based on the Beers’ List is common in nursing homes, the emergency department (ED), and outpatient settings and is associated with adverse outcomes and hospitalization. Frequency of PIM use the inpatient setting has not been well studied.
Study Design: A retrospective cohort study.
Setting: 384 U.S. hospitals.
Synopsis: In this retrospective cohort study of 493,971 inpatients (older than 65) admitted with medical diagnoses to non-surgeons, PIM prescription was evaluated. Forty nine percent of all patients were prescribed at least one PIM, while 6% were prescribed three or more. In a multivariable model, physician specialty was associated with variation in high severity PIM (HSPIM) prescription. In comparison with internal medicine physicians, cardiologists (odds ratio [OR] 1.32) and pulmonologists (OR 1.10) were more likely to prescribe HSPIMs, while hospitalists (OR 0.90) and geriatricians (OR 0.60) were less likely. In addition, patient age older than 85 was associated with decreased HSPIM prescription (OR 0.59) compared with those younger than 85.
Compared with patients in the Midwest, patients in the South (OR 1.63) and West (OR 1.43) were more likely to prescribe HSPIMs, while those in the Northeast (OR 0.85) were less likely. Hospitals with geriatric services had less PIM use. The study couldn’t account for continuation of chronic medications and did not evaluate adverse outcomes from PIM prescribing.
Bottom line: PIM prescription to hospitalized geriatric patients is common and associated with provider and hospital characteristics.
Citation: Rothberg MB, Pekow PS, Liu F, et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3:91-102:91-102.
Is There a Benefit to Corticosteroids When Treating Bacterial Meningitis in Children?
Background: The benefit of adjuvant corticosteroids in the treatment of bacterial meningitis in children in the developed world remains unclear; recent expert guidelines reflect this uncertainty.
Study Design: Retrospective cohort study.
Setting: Twenty-seven tertiary care hospitals in the United States.
Synopsis: Researchers examined 2,780 children with a primary diagnosis of bacterial meningitis discharged from 27 tertiary care centers in the U.S. from 2001-2006. Using a propensity analysis (to control for severity of illness), the study compared those who had received adjunctive corticosteroids with those who had not, with mortality and length of study (LOS) as primary outcomes.
The median age was nine months, 8.9% of children received corticosteroids, and the overall mortality rate was 4.2%. Adjuvent corticosteroids did not reduce mortality or LOS. The outcomes were unchanged in subgroup analyses.
Although limited by its retrospective design and lack of other outcome measures (e.g., hearing loss, neurological deficits), this study provides reasonable evidence that corticosteroid use in bacterial meningitis in children may not save lives or shorten LOS. Pediatric hospitalists may not want to routinely give steroids in this setting pending large randomized-controlled trials.
Bottom line: Adjunctive corticosteroids therapy in children with bacterial meningitis may not save lives or reduce LOS.
Citation: Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055.
Should Unprotected Left Main Disease Be Treated With PCI or CABG?
Background: The current standard of care for the treatment of left main coronary artery disease is coronary-artery bypass grafting (CABG). With the advent of drug-eluting stents, there is growing interest in the use of percutaneous coronary intervention (PCI) to treat left main disease.
Study Design: Prospective observational study.
Setting: Twelve Korean cardiac centers.
Synopsis: From 2000 to 2006, patients with left main disease were treated with PCI or CABG at the discretion of the physician. Nearly 1,100 patients in each cohort were compared and evaluated for death and a composite outcome of death, myocardial infarction, or stroke. Propensity-matching was employed to control for confounders.
In the overall cohort matched by propensity score, there was no significant difference in death or the composite outcome between the PCI and CABG groups after three years. Type of stent (bare metal vs. drug-eluting) did not affect the outcome. Rates of target-vessel revascularization were significantly higher in the group that received stents.
The results are limited by the observational nature and the need for propensity analysis and yet provide an intriguing result. The standard of care for treatment of left main disease remains CABG, but clinicians may be more comfortable treating with stents while we await randomized-controlled trials.
Bottom line: In this observational study, PCI and CABG had similar outcomes in patients with left main disease.
Citation: Seung KB, Park D, Kim Y, Lee S. Stents versus coronary-artery bypass grafting for left main coronary artery disease. N Engl J Med. 2008;358:1781-1792.
Literature at a Glance
A guide to this month’s studies.
- Carotid stenting is equivalent to endarterectomy in carotid artery stenosis.
- Idiopathic ventricular fibrillation is associated with early repolarization.
- Aggressive LDL and blood pressure management may benefit diabetic patients.
- Treatment of hypertension in patients older than age 80 improves outcomes.
- Length of stay predicts mortality in pulmonary embolism patients.
- Poor symptom control and lack of family support during end-of-life hospital stays.
- Potentially inappropriate medication use in hospitalized elders is common.
- Steroids may not help in pediatric meningitis.
- PCI and CABG may be equivalent in the treatment of left main disease.
Is Stenting or Endarterectomy Best for Carotid Artery Stenosis?
Background: Patients with moderate to severe symptomatic carotid artery stenosis and those with severe asymptomatic carotid stenosis benefit from carotid endarterectomy. Carotid stenting may provide an alternative therapy, but the long-term protection against stroke compared with endarterectomy is unclear.
Study Design: Prospective randomized trial.
Setting: 29 centers in the United States.
Synopsis: This article reports the long-term (three years) follow-up of the SAPPHIRE trial, published in 2004, which compared carotid stenting to endarterectomy in patients at high surgical risk. In that trial, 334 patients randomized to either stenting or endarterectomy had similar outcomes at one year. Patients were followed for three years with death and major cardiovascular events as endpoints.
Rates of stroke at three years were approximately 10% with an overall death rate of approximately 20%. There was no difference between carotid stenting and endarterectomy with regards to death, stroke, or other cardiovascular outcome.
Notably, follow-up was not complete (78%), a specific type of stenting procedure was used, and the patient population was at high risk for surgical complications. Therefore, results may not be applicable in other centers or in other patient populations. Yet, this trial provides follow-up, long-term evidence that carotid stenting may be a viable alternative to endarterectomy in patients with carotid artery stenosis.
Bottom line: Carotid stenting and endarterectomy had similar outcomes at three years in high-risk patients with carotid artery stenosis.
Citation: Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572-1579.
Is Early Repolarization on EKG Associated with Sudden Cardiac Arrest?
Background: Electrocardiographic early repolarization, defined as elevation of the QRS-ST junction of at least 0.1mV from baseline in the inferior or lateral leads (manifested as slurring or notching), occurs in 1% to 5% of patients. It is considered benign, but experimental studies have suggested it may be arrhythmogenic.
Study Design: Prospective case-control.
Setting: 22 international tertiary care centers.
Synopsis: Case subjects were less than 60 years of age and were resuscitated after ventricular fibrillation (VF) arrest ultimately deemed idiopathic. All had normal echocardiograms, no evidence of coronary artery disease, and no repolarization abnormalities (including Brugada and long-QT). Of 206 patients, 31% had early repolarization on EKG, versus only 5% in controls without heart disease. In case subjects with prior EKGs, early repolarization was proven to be pre-existing.
The mean magnitude of J-point elevation was 2 mm in cases versus 1.2 mm in controls, and in cases this magnitude increased during later episodes of arrhythmia. Electrophysiologic mapping showed that ectopy originated at sites concordant with the location of abnormal repolarization. During five years of follow-up, arrhythmic recurrence was twice as common in cases with early repolarization.
Although long-term observational studies of persons with early repolarization have shown a benign natural course, this study may change our approach to those with syncope or a family history of sudden death.
Bottom line: Early repolarization on EKG is associated with idiopathic ventricular fibrillation.
Citation: Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358(19):2016-2023.
Does Aggressive Blood Pressure and LDL Treatment in Diabetics Affect Development of Subclinical Atherosclerosis?
Background: There is evidence to suggest more aggressive treatment of LDL cholesterol in patients with known coronary artery disease is beneficial and more aggressive blood pressure control can improve outcomes in some patient populations. However, it is unclear if patients with diabetes without cardiovascular disease would benefit from more aggressive LDL and systolic blood pressure (SBP) treatment.
Study Design: Randomized, open-label, blinded-to-end point trial.
Setting: Four centers in Oklahoma, Arizona, and South Dakota.
Synopsis: Investigators studied 499 type 2 diabetic American Indian men with no history of cardiovascular disease. Patients were randomized to receive treatment to achieve aggressive (70 mg/dL and 115 mmHg) or standard (100 mg/dL and 130 mmHg) targets for their LDL cholesterol and SBP, respectively.
At three years, the aggressive group showed decreased carotid intima-media thickness (IMT) and decreased left ventricular mass, whereas both IMT and left ventricular volume increased in the standard group. There were no differences in clinical cardiovascular events between the aggressive and standard group and both groups had lower-than-expected clinical events.
This study included no women and was limited to an American Indian population. Of note, there was an increase in adverse events related to blood pressure medications in the aggressive group. It also is unclear how the surrogates of cardiovascular disease or subclinical atherosclerosis relate to significant clinical outcomes.
Bottom line: More aggressive LDL and SBP treatment in diabetics without coronary disease decreased subclinical atherosclerosis but did not impact clinical outcomes.
Citation: Howard B, Roman M, Devereux R, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes. JAMA. 2008;299(14):1678-1689.
Should We Treat Hypertension in Patients Older Than 80?
Background: There is debate about whether treatment of hypertension in the elderly is beneficial. Numerous studies suggest blood pressure control does less to prevent strokes in patients older than 80 years than for younger patients. Moreover, other evidence shows controlling blood pressure in elderly patients may result in an increase in mortality even if there was a decreased risk of stroke.
Study Design: Randomized, double-blind, placebo-controlled trial.
Setting: 195 centers in 13 countries in Europe, China, Australasia, and North Africa.
Synopsis: This study evaluated 3,845 patients, age 80 or older, with a sustained systolic blood pressure (SBP) of 160 mmHg and randomized them to receive indapamide (sustained release) or placebo. Perindopril, or placebo, was added if necessary to achieve a target blood pressure of 150/80 mmHg. Patients who received the indapamide with or without the perindopril had lower blood pressure, lower rate of stroke, lower rate of heart failure, lower rate of death from a cardiovascular cause, and a 21% reduction in all-cause mortality (all statistically significant). There were very few adverse drug events and fewer adverse events overall in the treatment group.
Of note, exclusion criteria included a history of heart failure requiring anti-hypertensive medication, dementia, need for nursing care, an inability to stand or walk, and a creatinine more than 1.7 mg/dL. As well, the “target” SBP of 150 mmHg (which only half of the treatment group achieved) is still considered hypertensive according to the JNC 7 guidelines.
Bottom line: In some patients older than 80, treatment of hypertension may reduce the incidence of stroke, death from stroke, heart failure, and all-cause mortality.
Citation: Beckett N, Peters R, Fletcher A, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
What Is the Optimal Hospital LOS for Patients with PE?
Background: Though there are clear trends toward shorter hospital stays after pulmonary embolism (PE), especially with the introduction of low molecular weight heparin, the optimal timing of discharge and the effect of decreased length of stay (LOS) on post-discharge mortality are unknown. Furthermore, there is no risk stratification strategy used to identify low-risk patients with PE who can safely be discharged early or treated in the outpatient setting.
Study Design: Retrospective cohort study.
Setting: 186 acute care hospitals in Pennsylvania from January 2000 to November 2002.
Synopsis: Using a statewide database of 15,531 patients discharged with pulmonary embolism (PE), the authors sought to identify patient and hospital factors associated with LOS and assess whether LOS was associated with post-discharge mortality.
Findings indicate there is considerable variation in LOS for PE between and within hospitals in Pennsylvania. The median LOS for patients with PE was six days; patients with a LOS of four or fewer days had significantly higher post-discharge mortality than patients hospitalized five to eight days. More than half the patients discharged at four or fewer days were classified as high-risk, with Pulmonary Embolism Severity Index (PESI) scores of III-V (3.1% to 24.5% risk of mortality at 30 days).
Although we cannot infer causation (i.e., early discharge=death), clinicians should be aware of the results and consider severity of illness (using PESI or other criteria) in the discharge decision in patients with PE. Future prognostic models and evidence-based criteria would be helpful to identify patients with PE who can be safely discharged early.
Bottom line: Physicians may inappropriately select patients with PE for early discharge who are at increased risk of complications.
Citation: Aujesky D, Stone RA, Kim S, et al. Length of hospital stay and post-discharge mortality in patients with pulmonary embolism. Arch Intern Med. 2008;168(7):706-712.
Do Patients Have a “Good Death” in the Hospital?
Background: Despite an increasing focus on providing appropriate end-of-life care, the majority of patients in developed countries die in the hospital. The circumstances and quality of care provided at the time of death are poorly described.
Study Design: Cross-sectional survey.
Setting: 613 departments in 200 French hospitals.
Synopsis: For 3,793 in-hospital deaths, the investigators surveyed the bedside nurses about the circumstances and details of the death. Twenty-three percent of the patients were admitted for end-of-life care, 29% had a malignancy, and 50% of patients were identified as terminally ill for three days prior to their death.
A family member or relative was present in only 25% of all deaths; 20% of patients were alone at the time of death. In the last few hours of life, up to 70% of patients had symptoms of respiratory distress, while only 44% received opiate analgesia. Only 35% of nurses were satisfied with the quality of death. Satisfaction increased with presence of family members and having written protocols for care at the end of life.
This large, multicenter study has limitations but provides a concerning snapshot of death in the hospital. Hospitalists should be aggressive about symptom control at the end of life as well as attempt to ensure patients are not alone at the time of death.
Bottom line: Many patients die in the hospital in some degree of respiratory distress and without family or friends at the bedside.
Citation: Ferrand E, Jabre P, Vincent-Genod C, et al. Circumstances of death in hospitalized patients and nurses’ perceptions. Arch Intern Med. 2008;168(8):867-875.
How Common Is Potentially Inappropriate Medication Use in the Hospital?
Background: Use of potentially inappropriate medications (PIM) in the elderly based on the Beers’ List is common in nursing homes, the emergency department (ED), and outpatient settings and is associated with adverse outcomes and hospitalization. Frequency of PIM use the inpatient setting has not been well studied.
Study Design: A retrospective cohort study.
Setting: 384 U.S. hospitals.
Synopsis: In this retrospective cohort study of 493,971 inpatients (older than 65) admitted with medical diagnoses to non-surgeons, PIM prescription was evaluated. Forty nine percent of all patients were prescribed at least one PIM, while 6% were prescribed three or more. In a multivariable model, physician specialty was associated with variation in high severity PIM (HSPIM) prescription. In comparison with internal medicine physicians, cardiologists (odds ratio [OR] 1.32) and pulmonologists (OR 1.10) were more likely to prescribe HSPIMs, while hospitalists (OR 0.90) and geriatricians (OR 0.60) were less likely. In addition, patient age older than 85 was associated with decreased HSPIM prescription (OR 0.59) compared with those younger than 85.
Compared with patients in the Midwest, patients in the South (OR 1.63) and West (OR 1.43) were more likely to prescribe HSPIMs, while those in the Northeast (OR 0.85) were less likely. Hospitals with geriatric services had less PIM use. The study couldn’t account for continuation of chronic medications and did not evaluate adverse outcomes from PIM prescribing.
Bottom line: PIM prescription to hospitalized geriatric patients is common and associated with provider and hospital characteristics.
Citation: Rothberg MB, Pekow PS, Liu F, et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3:91-102:91-102.
Is There a Benefit to Corticosteroids When Treating Bacterial Meningitis in Children?
Background: The benefit of adjuvant corticosteroids in the treatment of bacterial meningitis in children in the developed world remains unclear; recent expert guidelines reflect this uncertainty.
Study Design: Retrospective cohort study.
Setting: Twenty-seven tertiary care hospitals in the United States.
Synopsis: Researchers examined 2,780 children with a primary diagnosis of bacterial meningitis discharged from 27 tertiary care centers in the U.S. from 2001-2006. Using a propensity analysis (to control for severity of illness), the study compared those who had received adjunctive corticosteroids with those who had not, with mortality and length of study (LOS) as primary outcomes.
The median age was nine months, 8.9% of children received corticosteroids, and the overall mortality rate was 4.2%. Adjuvent corticosteroids did not reduce mortality or LOS. The outcomes were unchanged in subgroup analyses.
Although limited by its retrospective design and lack of other outcome measures (e.g., hearing loss, neurological deficits), this study provides reasonable evidence that corticosteroid use in bacterial meningitis in children may not save lives or shorten LOS. Pediatric hospitalists may not want to routinely give steroids in this setting pending large randomized-controlled trials.
Bottom line: Adjunctive corticosteroids therapy in children with bacterial meningitis may not save lives or reduce LOS.
Citation: Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055.
Should Unprotected Left Main Disease Be Treated With PCI or CABG?
Background: The current standard of care for the treatment of left main coronary artery disease is coronary-artery bypass grafting (CABG). With the advent of drug-eluting stents, there is growing interest in the use of percutaneous coronary intervention (PCI) to treat left main disease.
Study Design: Prospective observational study.
Setting: Twelve Korean cardiac centers.
Synopsis: From 2000 to 2006, patients with left main disease were treated with PCI or CABG at the discretion of the physician. Nearly 1,100 patients in each cohort were compared and evaluated for death and a composite outcome of death, myocardial infarction, or stroke. Propensity-matching was employed to control for confounders.
In the overall cohort matched by propensity score, there was no significant difference in death or the composite outcome between the PCI and CABG groups after three years. Type of stent (bare metal vs. drug-eluting) did not affect the outcome. Rates of target-vessel revascularization were significantly higher in the group that received stents.
The results are limited by the observational nature and the need for propensity analysis and yet provide an intriguing result. The standard of care for treatment of left main disease remains CABG, but clinicians may be more comfortable treating with stents while we await randomized-controlled trials.
Bottom line: In this observational study, PCI and CABG had similar outcomes in patients with left main disease.
Citation: Seung KB, Park D, Kim Y, Lee S. Stents versus coronary-artery bypass grafting for left main coronary artery disease. N Engl J Med. 2008;358:1781-1792.