User login
Herpes Esophagitis in the Setting of Immunosuppression From Pemphigus Vulgaris Therapy
Pemphigus vulgaris (PV) is a chronic autoimmune intraepithelial bullous disease caused by pathogenic IgG antibodies at the intraepidermal cell-surface proteins desmoglein 1 (DSG1) and desmoglein 3 (DSG3), which are members of the cadherin superfamily of desmosomal proteins and are involved in keratinocyte adhesion. Autoantibody binding to these molecules leads to the loss of cell-cell adhesion in the epithelial suprabasilar layer, producing flaccid blisters on an erythematous base with a positive Nikolsky sign.1 The blisters frequently rupture, leaving painful nonscarring erosions with the potential for secondary infection.
The clinical phenotype of PV is directly related to the autoantibody profile. Clinically, PV often is mucosal dominant on presentation with painful oropharyngeal involvement and associated IgG antibodies against DSG3. Progression to cutaneous disease, such as on the scalp or axillae, is accompanied by a shift in IgG antibodies against both DSG1 and DSG3.2,3
Combination therapy with prednisone and mycophenolate mofetil (MMF) has proven to be an effective method of controlling the signs and symptoms of PV4; however, the immunosuppressive effects of these medications put the patient at risk for a host of opportunistic infections. Herpes simplex virus (HSV) has been associated with PV lesions of the oral mucosa, though a clear-cut relationship between these 2 entities has yet to be established.5 Herpes simplex virus has likewise been confirmed in therapy-resistant exacerbations of PV.6 Herpes esophagitis is a rare consequence of treatment with prednisone and MMF that is primarily encountered in patients with a history of solid organ transplantation7 and rarely has been reported in PV patients undergoing therapeutic immunosuppression.
Acute odynophagia in patients undergoing systemic treatment of active PV warrants prompt endoscopic evaluation to rule out esophageal pemphigus or superinfection. We report the case of a 35-year-old man with stable but poorly controlled PV who was undergoing systemic treatment and experienced rapid deterioration due to herpes esophagitis from immunosuppression.
Case Report
A 35-year-old man was referred to our clinic for evaluation of blisters on the scalp, oral mucosa, and proximal upper and lower extremities of 4 months’ duration. A biopsy performed by his primary care physician within a month of onset of symptoms was reportedly suggestive of PV; although no direct immunofluorescence had been performed, serum indirect immunofluorescence was highly positive for IgG antibodies toward DSG3 and to a lesser extent DSG1. The blisters failed to improve with a 2-week prednisone taper completed 1 month prior to presentation. The patient was not currently taking any other medications. He had a remote history of fever blisters but no other dermatologic issues.
Initial examination revealed flaccid bullae on an erythematous base involving the posterior scalp as well as tender white erosions to shallow ulcers on the tongue and hard and soft palates. A Tzanck smear (modified Wright-Giemsa stain) of these erosions confirmed acantholytic mucosal cells. Punch biopsies of lesional and perilesional skin from the scalp were obtained for histopathologic confirmation and immunofluorescence. An acantholytic dermatosis with a tombstone pattern along the basement membrane was present on hematoxylin and eosin staining, and direct immunofluorescence was positive for IgG and C3 in an intraepidermal lacelike pattern, confirming a diagnosis of PV.
Despite starting an oral regimen of high-dose corticosteroids (prednisone 80 mg once daily), no improvement was noted at 2-week follow-up. He had developed flaccid blisters on the left axillae and mildly worsened oral erosions. He also reported moderate difficulty eating due to pain with swallowing. Mycophenolate mofetil (500 mg twice daily) was added as combination therapy with the prednisone.
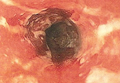
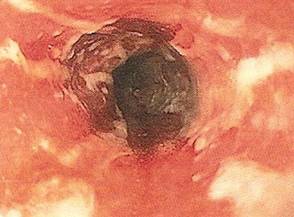
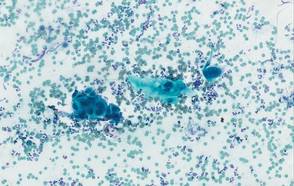
One week later, the patient was unable to eat or drink due to worsening odynophagia. He was admitted as an inpatient for treatment with intravenous methylprednisolone (120 mg every 8 hours) and MMF (1000 mg daily). The gastroenterology department was consulted and an esophagogastroduodenoscopy revealed diffuse areas of denuded and friable mucosa with an overlay of white exudate (Figure 1). Cytology performed on esophageal brushings revealed viral cytopathic changes confirming herpes esophagitis (Figure 2). No esophageal viral cultures were taken. The patient was started on intravenous acyclovir (800 mg 4 times daily), leading to rapid resolution of the odynophagia. He was discharged after 4 days with a course of oral acyclovir (400 mg 4 times daily for 14 days). Tzanck smears and HSV cultures of oral lesions performed immediately following discharge were negative. Combination therapy with MMF (500 mg twice daily) and a slow taper of prednisone (down to 5 mg once daily) was continued past 1 year without flare of his cutaneous disease.
Comment
Although PV may have been considered a fatal disease at one time, treatment with systemic steroids has made it a manageable, albeit relapsing, condition. The development of corticosteroid-sparing, adjuvant immunosuppressives such as MMF has allowed for the more aggressive treatment of this disease with fewer steroid-related side effects.4,8,9 As seen in solid organ transplant recipients who often utilize combination therapy, the use of adjuvant immunosuppressives is associated with potential complications including bone marrow suppression and an increased risk for infections.7,10
Odynophagia is among the potential complications in patients with PV and has a wide differential diagnosis. Mucosal lesions of PV previously have been associated with HSV colonization, though a causal relationship has not been corroborated.5 Herpes simplex virus is more often detected in PV patients being treated with immunosuppressive agents than in nontreated patient groups.11 Recalcitrant or suddenly exacerbated oral mucosal lesions of PV under appropriate therapy may therefore be the result of HSV superinfection, which has been deferentially referred to as pemphigus herpeticum.12 Esophageal mucosal involvement by PV also may be more common than previously thought and should be suspected in patients with active oral disease.13 Esophagitis secondary to medications or various opportunistic organisms such as Candida, cytomegalovirus, or HSV also should be ruled out in patients taking immunosuppressives.5,10
Herpes esophagitis primarily occurs in immunocompromised hosts and is well documented in the literature regarding treatment with MMF and prednisone following renal and cardiac transplantation.10 Prednisone therapy in patients with chronic obstructive pulmonary disease also has been implicated.14 Reactivation of latent HSV resulting from immunosuppression is most often described, though primary infection also is possible.15 Patients typically present with acute odynophagia progressing to dysphagia, with complications ranging from sequelae of poor oral intake to esophageal perforation and hemorrhage, but the course generally is self-limited if immune function is promptly restored. Intravenous acyclovir has been known to hasten the recovery process and improve symptoms.16 Characteristic findings on esophagogastroduodenoscopy in combination with tissue biopsy, viral culture, and/or polymerase chain reaction aid in the diagnosis of herpes esophagitis.15,16 Our patient had a grossly abnormal esophagogastroduodenoscopy with positive cytology; however, no further diagnostic workup was performed. The cytologic findings and the rapid symptomatic improvement following the initiation of acyclovir helped support HSV as the etiology.
Conclusion
We present a case of herpes esophagitis that complicated the treatment of PV with MMF and prednisone. A diagnosis of herpes esophagitis must be ruled out in patients with PV who are undergoing therapeutic immunosuppression and present with an acute episode of odynophagia that is resistant to upscaling of therapy.
- Mustasim DF, Bilic M, Hawayek LH, et al. Immunobullous diseases. J Am Acad Dermatol. 2005;52:1029-1043.
- Amagai M, Tsunoda K, Zillikens D, et al. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40(2, pt 1):167-170.
- Sirois DA, Fatahzadeh M, Roth R, et al. Diagnostic patterns and delays in pemphigus vulgaris: experience from 99 patients. Arch Dermatol. 2000;136:1569-1570.
- Strowd LC, Taylor SL, Jorizzo JL, et al. Therapeutic ladder for pemphigus vulgaris: emphasis on achieving complete remission. J Am Acad Dermatol. 2011;64:490-494.
- Nikkels AF, Delvenne P, Herfs M, et al. Occult herpes simplex virus colonization of bullous dermatitides. Am J Clin Dermatol. 2008;9:163-168.
- Hale EK, Bystryn JC. Atypical herpes simplex can mimic a flare of disease activity in patients with pemphigus vulgaris. J Eur Acad Dermatol Venereol. 1999;13:221-223.
- Smak Gregoor PJ, van Gelder T, van Riemsdijk-van Overbeeke IC, et al. Unusual presentation of herpes virus infections in renal transplant recipients exposed to high mycophenolic acid plasma concentrations. Transpl Infect Dis. 2003;5:79-83.
- Beissert S, Mimouni D, Kanwar AJ, et al. Treating pemphigus vulgaris with prednisone and mycophenolate mofetil: a multicenter, randomized, placebo-controlled trial. J Invest Dermatol. 2010;130:2041-2048.
- Yeh SW, Sami N, Ahmed RA. Treatment of pemphigus vulgaris: current and emerging options. Am J Clin Dermatol. 2005;6:327-342.
- Eisen HJ, Kobashigawa J, Keogh A, et al. Three-year results of a randomized, double-blind, controlled trial of mycophenolate mofetil versus azathioprine in cardiac transplant recipients. J Heart Lung Transplant. 2005;24:517-525.
- Marzano AV, Tourlaki A, Merlo V, et al. Herpes simplex virus infection and pemphigus. Int J Immunopathol Pharmacol. 2009;22:781-786.
- Feldmeyer L, Trüeb RM, French LE, et al. Pitfall: pemphigus herpeticatus should not be confounded with resistant pemphigus vulgaris. J Dermatolog Treat. 2010;21:311-313.
- Rao PN, Samarth A, Aurangabadkar SJ, et al. Study of upper gastrointestinal tract involvement in pemphigus by esophago-gastro-duodenoscopy. Indian J Dermatol Venereol Leprol. 2006;72:421-424.
- Wiest PM, Flanigan T, Salata RA, et al. Serious infectious complications of corticosteroid therapy for COPD. Chest. 1989;95:1180-1184.
- Lee B, Caddy G. A rare cause of dysphagia: herpes simplex esophagitis. World J Gastroenterol. 2007;13:2756-2757.
- Robertson AG, Dunn LJ, Immanuel A, et al. An unusual presentation of herpes simplex esophagitis: a nonhealing “peptic” ulcer. Endoscopy. 2009;41(suppl 2):E213.
Pemphigus vulgaris (PV) is a chronic autoimmune intraepithelial bullous disease caused by pathogenic IgG antibodies at the intraepidermal cell-surface proteins desmoglein 1 (DSG1) and desmoglein 3 (DSG3), which are members of the cadherin superfamily of desmosomal proteins and are involved in keratinocyte adhesion. Autoantibody binding to these molecules leads to the loss of cell-cell adhesion in the epithelial suprabasilar layer, producing flaccid blisters on an erythematous base with a positive Nikolsky sign.1 The blisters frequently rupture, leaving painful nonscarring erosions with the potential for secondary infection.
The clinical phenotype of PV is directly related to the autoantibody profile. Clinically, PV often is mucosal dominant on presentation with painful oropharyngeal involvement and associated IgG antibodies against DSG3. Progression to cutaneous disease, such as on the scalp or axillae, is accompanied by a shift in IgG antibodies against both DSG1 and DSG3.2,3
Combination therapy with prednisone and mycophenolate mofetil (MMF) has proven to be an effective method of controlling the signs and symptoms of PV4; however, the immunosuppressive effects of these medications put the patient at risk for a host of opportunistic infections. Herpes simplex virus (HSV) has been associated with PV lesions of the oral mucosa, though a clear-cut relationship between these 2 entities has yet to be established.5 Herpes simplex virus has likewise been confirmed in therapy-resistant exacerbations of PV.6 Herpes esophagitis is a rare consequence of treatment with prednisone and MMF that is primarily encountered in patients with a history of solid organ transplantation7 and rarely has been reported in PV patients undergoing therapeutic immunosuppression.
Acute odynophagia in patients undergoing systemic treatment of active PV warrants prompt endoscopic evaluation to rule out esophageal pemphigus or superinfection. We report the case of a 35-year-old man with stable but poorly controlled PV who was undergoing systemic treatment and experienced rapid deterioration due to herpes esophagitis from immunosuppression.
Case Report
A 35-year-old man was referred to our clinic for evaluation of blisters on the scalp, oral mucosa, and proximal upper and lower extremities of 4 months’ duration. A biopsy performed by his primary care physician within a month of onset of symptoms was reportedly suggestive of PV; although no direct immunofluorescence had been performed, serum indirect immunofluorescence was highly positive for IgG antibodies toward DSG3 and to a lesser extent DSG1. The blisters failed to improve with a 2-week prednisone taper completed 1 month prior to presentation. The patient was not currently taking any other medications. He had a remote history of fever blisters but no other dermatologic issues.
Initial examination revealed flaccid bullae on an erythematous base involving the posterior scalp as well as tender white erosions to shallow ulcers on the tongue and hard and soft palates. A Tzanck smear (modified Wright-Giemsa stain) of these erosions confirmed acantholytic mucosal cells. Punch biopsies of lesional and perilesional skin from the scalp were obtained for histopathologic confirmation and immunofluorescence. An acantholytic dermatosis with a tombstone pattern along the basement membrane was present on hematoxylin and eosin staining, and direct immunofluorescence was positive for IgG and C3 in an intraepidermal lacelike pattern, confirming a diagnosis of PV.
Despite starting an oral regimen of high-dose corticosteroids (prednisone 80 mg once daily), no improvement was noted at 2-week follow-up. He had developed flaccid blisters on the left axillae and mildly worsened oral erosions. He also reported moderate difficulty eating due to pain with swallowing. Mycophenolate mofetil (500 mg twice daily) was added as combination therapy with the prednisone.
One week later, the patient was unable to eat or drink due to worsening odynophagia. He was admitted as an inpatient for treatment with intravenous methylprednisolone (120 mg every 8 hours) and MMF (1000 mg daily). The gastroenterology department was consulted and an esophagogastroduodenoscopy revealed diffuse areas of denuded and friable mucosa with an overlay of white exudate (Figure 1). Cytology performed on esophageal brushings revealed viral cytopathic changes confirming herpes esophagitis (Figure 2). No esophageal viral cultures were taken. The patient was started on intravenous acyclovir (800 mg 4 times daily), leading to rapid resolution of the odynophagia. He was discharged after 4 days with a course of oral acyclovir (400 mg 4 times daily for 14 days). Tzanck smears and HSV cultures of oral lesions performed immediately following discharge were negative. Combination therapy with MMF (500 mg twice daily) and a slow taper of prednisone (down to 5 mg once daily) was continued past 1 year without flare of his cutaneous disease.


Comment
Although PV may have been considered a fatal disease at one time, treatment with systemic steroids has made it a manageable, albeit relapsing, condition. The development of corticosteroid-sparing, adjuvant immunosuppressives such as MMF has allowed for the more aggressive treatment of this disease with fewer steroid-related side effects.4,8,9 As seen in solid organ transplant recipients who often utilize combination therapy, the use of adjuvant immunosuppressives is associated with potential complications including bone marrow suppression and an increased risk for infections.7,10
Odynophagia is among the potential complications in patients with PV and has a wide differential diagnosis. Mucosal lesions of PV previously have been associated with HSV colonization, though a causal relationship has not been corroborated.5 Herpes simplex virus is more often detected in PV patients being treated with immunosuppressive agents than in nontreated patient groups.11 Recalcitrant or suddenly exacerbated oral mucosal lesions of PV under appropriate therapy may therefore be the result of HSV superinfection, which has been deferentially referred to as pemphigus herpeticum.12 Esophageal mucosal involvement by PV also may be more common than previously thought and should be suspected in patients with active oral disease.13 Esophagitis secondary to medications or various opportunistic organisms such as Candida, cytomegalovirus, or HSV also should be ruled out in patients taking immunosuppressives.5,10
Herpes esophagitis primarily occurs in immunocompromised hosts and is well documented in the literature regarding treatment with MMF and prednisone following renal and cardiac transplantation.10 Prednisone therapy in patients with chronic obstructive pulmonary disease also has been implicated.14 Reactivation of latent HSV resulting from immunosuppression is most often described, though primary infection also is possible.15 Patients typically present with acute odynophagia progressing to dysphagia, with complications ranging from sequelae of poor oral intake to esophageal perforation and hemorrhage, but the course generally is self-limited if immune function is promptly restored. Intravenous acyclovir has been known to hasten the recovery process and improve symptoms.16 Characteristic findings on esophagogastroduodenoscopy in combination with tissue biopsy, viral culture, and/or polymerase chain reaction aid in the diagnosis of herpes esophagitis.15,16 Our patient had a grossly abnormal esophagogastroduodenoscopy with positive cytology; however, no further diagnostic workup was performed. The cytologic findings and the rapid symptomatic improvement following the initiation of acyclovir helped support HSV as the etiology.
Conclusion
We present a case of herpes esophagitis that complicated the treatment of PV with MMF and prednisone. A diagnosis of herpes esophagitis must be ruled out in patients with PV who are undergoing therapeutic immunosuppression and present with an acute episode of odynophagia that is resistant to upscaling of therapy.
Pemphigus vulgaris (PV) is a chronic autoimmune intraepithelial bullous disease caused by pathogenic IgG antibodies at the intraepidermal cell-surface proteins desmoglein 1 (DSG1) and desmoglein 3 (DSG3), which are members of the cadherin superfamily of desmosomal proteins and are involved in keratinocyte adhesion. Autoantibody binding to these molecules leads to the loss of cell-cell adhesion in the epithelial suprabasilar layer, producing flaccid blisters on an erythematous base with a positive Nikolsky sign.1 The blisters frequently rupture, leaving painful nonscarring erosions with the potential for secondary infection.
The clinical phenotype of PV is directly related to the autoantibody profile. Clinically, PV often is mucosal dominant on presentation with painful oropharyngeal involvement and associated IgG antibodies against DSG3. Progression to cutaneous disease, such as on the scalp or axillae, is accompanied by a shift in IgG antibodies against both DSG1 and DSG3.2,3
Combination therapy with prednisone and mycophenolate mofetil (MMF) has proven to be an effective method of controlling the signs and symptoms of PV4; however, the immunosuppressive effects of these medications put the patient at risk for a host of opportunistic infections. Herpes simplex virus (HSV) has been associated with PV lesions of the oral mucosa, though a clear-cut relationship between these 2 entities has yet to be established.5 Herpes simplex virus has likewise been confirmed in therapy-resistant exacerbations of PV.6 Herpes esophagitis is a rare consequence of treatment with prednisone and MMF that is primarily encountered in patients with a history of solid organ transplantation7 and rarely has been reported in PV patients undergoing therapeutic immunosuppression.
Acute odynophagia in patients undergoing systemic treatment of active PV warrants prompt endoscopic evaluation to rule out esophageal pemphigus or superinfection. We report the case of a 35-year-old man with stable but poorly controlled PV who was undergoing systemic treatment and experienced rapid deterioration due to herpes esophagitis from immunosuppression.
Case Report
A 35-year-old man was referred to our clinic for evaluation of blisters on the scalp, oral mucosa, and proximal upper and lower extremities of 4 months’ duration. A biopsy performed by his primary care physician within a month of onset of symptoms was reportedly suggestive of PV; although no direct immunofluorescence had been performed, serum indirect immunofluorescence was highly positive for IgG antibodies toward DSG3 and to a lesser extent DSG1. The blisters failed to improve with a 2-week prednisone taper completed 1 month prior to presentation. The patient was not currently taking any other medications. He had a remote history of fever blisters but no other dermatologic issues.
Initial examination revealed flaccid bullae on an erythematous base involving the posterior scalp as well as tender white erosions to shallow ulcers on the tongue and hard and soft palates. A Tzanck smear (modified Wright-Giemsa stain) of these erosions confirmed acantholytic mucosal cells. Punch biopsies of lesional and perilesional skin from the scalp were obtained for histopathologic confirmation and immunofluorescence. An acantholytic dermatosis with a tombstone pattern along the basement membrane was present on hematoxylin and eosin staining, and direct immunofluorescence was positive for IgG and C3 in an intraepidermal lacelike pattern, confirming a diagnosis of PV.
Despite starting an oral regimen of high-dose corticosteroids (prednisone 80 mg once daily), no improvement was noted at 2-week follow-up. He had developed flaccid blisters on the left axillae and mildly worsened oral erosions. He also reported moderate difficulty eating due to pain with swallowing. Mycophenolate mofetil (500 mg twice daily) was added as combination therapy with the prednisone.
One week later, the patient was unable to eat or drink due to worsening odynophagia. He was admitted as an inpatient for treatment with intravenous methylprednisolone (120 mg every 8 hours) and MMF (1000 mg daily). The gastroenterology department was consulted and an esophagogastroduodenoscopy revealed diffuse areas of denuded and friable mucosa with an overlay of white exudate (Figure 1). Cytology performed on esophageal brushings revealed viral cytopathic changes confirming herpes esophagitis (Figure 2). No esophageal viral cultures were taken. The patient was started on intravenous acyclovir (800 mg 4 times daily), leading to rapid resolution of the odynophagia. He was discharged after 4 days with a course of oral acyclovir (400 mg 4 times daily for 14 days). Tzanck smears and HSV cultures of oral lesions performed immediately following discharge were negative. Combination therapy with MMF (500 mg twice daily) and a slow taper of prednisone (down to 5 mg once daily) was continued past 1 year without flare of his cutaneous disease.


Comment
Although PV may have been considered a fatal disease at one time, treatment with systemic steroids has made it a manageable, albeit relapsing, condition. The development of corticosteroid-sparing, adjuvant immunosuppressives such as MMF has allowed for the more aggressive treatment of this disease with fewer steroid-related side effects.4,8,9 As seen in solid organ transplant recipients who often utilize combination therapy, the use of adjuvant immunosuppressives is associated with potential complications including bone marrow suppression and an increased risk for infections.7,10
Odynophagia is among the potential complications in patients with PV and has a wide differential diagnosis. Mucosal lesions of PV previously have been associated with HSV colonization, though a causal relationship has not been corroborated.5 Herpes simplex virus is more often detected in PV patients being treated with immunosuppressive agents than in nontreated patient groups.11 Recalcitrant or suddenly exacerbated oral mucosal lesions of PV under appropriate therapy may therefore be the result of HSV superinfection, which has been deferentially referred to as pemphigus herpeticum.12 Esophageal mucosal involvement by PV also may be more common than previously thought and should be suspected in patients with active oral disease.13 Esophagitis secondary to medications or various opportunistic organisms such as Candida, cytomegalovirus, or HSV also should be ruled out in patients taking immunosuppressives.5,10
Herpes esophagitis primarily occurs in immunocompromised hosts and is well documented in the literature regarding treatment with MMF and prednisone following renal and cardiac transplantation.10 Prednisone therapy in patients with chronic obstructive pulmonary disease also has been implicated.14 Reactivation of latent HSV resulting from immunosuppression is most often described, though primary infection also is possible.15 Patients typically present with acute odynophagia progressing to dysphagia, with complications ranging from sequelae of poor oral intake to esophageal perforation and hemorrhage, but the course generally is self-limited if immune function is promptly restored. Intravenous acyclovir has been known to hasten the recovery process and improve symptoms.16 Characteristic findings on esophagogastroduodenoscopy in combination with tissue biopsy, viral culture, and/or polymerase chain reaction aid in the diagnosis of herpes esophagitis.15,16 Our patient had a grossly abnormal esophagogastroduodenoscopy with positive cytology; however, no further diagnostic workup was performed. The cytologic findings and the rapid symptomatic improvement following the initiation of acyclovir helped support HSV as the etiology.
Conclusion
We present a case of herpes esophagitis that complicated the treatment of PV with MMF and prednisone. A diagnosis of herpes esophagitis must be ruled out in patients with PV who are undergoing therapeutic immunosuppression and present with an acute episode of odynophagia that is resistant to upscaling of therapy.
- Mustasim DF, Bilic M, Hawayek LH, et al. Immunobullous diseases. J Am Acad Dermatol. 2005;52:1029-1043.
- Amagai M, Tsunoda K, Zillikens D, et al. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40(2, pt 1):167-170.
- Sirois DA, Fatahzadeh M, Roth R, et al. Diagnostic patterns and delays in pemphigus vulgaris: experience from 99 patients. Arch Dermatol. 2000;136:1569-1570.
- Strowd LC, Taylor SL, Jorizzo JL, et al. Therapeutic ladder for pemphigus vulgaris: emphasis on achieving complete remission. J Am Acad Dermatol. 2011;64:490-494.
- Nikkels AF, Delvenne P, Herfs M, et al. Occult herpes simplex virus colonization of bullous dermatitides. Am J Clin Dermatol. 2008;9:163-168.
- Hale EK, Bystryn JC. Atypical herpes simplex can mimic a flare of disease activity in patients with pemphigus vulgaris. J Eur Acad Dermatol Venereol. 1999;13:221-223.
- Smak Gregoor PJ, van Gelder T, van Riemsdijk-van Overbeeke IC, et al. Unusual presentation of herpes virus infections in renal transplant recipients exposed to high mycophenolic acid plasma concentrations. Transpl Infect Dis. 2003;5:79-83.
- Beissert S, Mimouni D, Kanwar AJ, et al. Treating pemphigus vulgaris with prednisone and mycophenolate mofetil: a multicenter, randomized, placebo-controlled trial. J Invest Dermatol. 2010;130:2041-2048.
- Yeh SW, Sami N, Ahmed RA. Treatment of pemphigus vulgaris: current and emerging options. Am J Clin Dermatol. 2005;6:327-342.
- Eisen HJ, Kobashigawa J, Keogh A, et al. Three-year results of a randomized, double-blind, controlled trial of mycophenolate mofetil versus azathioprine in cardiac transplant recipients. J Heart Lung Transplant. 2005;24:517-525.
- Marzano AV, Tourlaki A, Merlo V, et al. Herpes simplex virus infection and pemphigus. Int J Immunopathol Pharmacol. 2009;22:781-786.
- Feldmeyer L, Trüeb RM, French LE, et al. Pitfall: pemphigus herpeticatus should not be confounded with resistant pemphigus vulgaris. J Dermatolog Treat. 2010;21:311-313.
- Rao PN, Samarth A, Aurangabadkar SJ, et al. Study of upper gastrointestinal tract involvement in pemphigus by esophago-gastro-duodenoscopy. Indian J Dermatol Venereol Leprol. 2006;72:421-424.
- Wiest PM, Flanigan T, Salata RA, et al. Serious infectious complications of corticosteroid therapy for COPD. Chest. 1989;95:1180-1184.
- Lee B, Caddy G. A rare cause of dysphagia: herpes simplex esophagitis. World J Gastroenterol. 2007;13:2756-2757.
- Robertson AG, Dunn LJ, Immanuel A, et al. An unusual presentation of herpes simplex esophagitis: a nonhealing “peptic” ulcer. Endoscopy. 2009;41(suppl 2):E213.
- Mustasim DF, Bilic M, Hawayek LH, et al. Immunobullous diseases. J Am Acad Dermatol. 2005;52:1029-1043.
- Amagai M, Tsunoda K, Zillikens D, et al. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40(2, pt 1):167-170.
- Sirois DA, Fatahzadeh M, Roth R, et al. Diagnostic patterns and delays in pemphigus vulgaris: experience from 99 patients. Arch Dermatol. 2000;136:1569-1570.
- Strowd LC, Taylor SL, Jorizzo JL, et al. Therapeutic ladder for pemphigus vulgaris: emphasis on achieving complete remission. J Am Acad Dermatol. 2011;64:490-494.
- Nikkels AF, Delvenne P, Herfs M, et al. Occult herpes simplex virus colonization of bullous dermatitides. Am J Clin Dermatol. 2008;9:163-168.
- Hale EK, Bystryn JC. Atypical herpes simplex can mimic a flare of disease activity in patients with pemphigus vulgaris. J Eur Acad Dermatol Venereol. 1999;13:221-223.
- Smak Gregoor PJ, van Gelder T, van Riemsdijk-van Overbeeke IC, et al. Unusual presentation of herpes virus infections in renal transplant recipients exposed to high mycophenolic acid plasma concentrations. Transpl Infect Dis. 2003;5:79-83.
- Beissert S, Mimouni D, Kanwar AJ, et al. Treating pemphigus vulgaris with prednisone and mycophenolate mofetil: a multicenter, randomized, placebo-controlled trial. J Invest Dermatol. 2010;130:2041-2048.
- Yeh SW, Sami N, Ahmed RA. Treatment of pemphigus vulgaris: current and emerging options. Am J Clin Dermatol. 2005;6:327-342.
- Eisen HJ, Kobashigawa J, Keogh A, et al. Three-year results of a randomized, double-blind, controlled trial of mycophenolate mofetil versus azathioprine in cardiac transplant recipients. J Heart Lung Transplant. 2005;24:517-525.
- Marzano AV, Tourlaki A, Merlo V, et al. Herpes simplex virus infection and pemphigus. Int J Immunopathol Pharmacol. 2009;22:781-786.
- Feldmeyer L, Trüeb RM, French LE, et al. Pitfall: pemphigus herpeticatus should not be confounded with resistant pemphigus vulgaris. J Dermatolog Treat. 2010;21:311-313.
- Rao PN, Samarth A, Aurangabadkar SJ, et al. Study of upper gastrointestinal tract involvement in pemphigus by esophago-gastro-duodenoscopy. Indian J Dermatol Venereol Leprol. 2006;72:421-424.
- Wiest PM, Flanigan T, Salata RA, et al. Serious infectious complications of corticosteroid therapy for COPD. Chest. 1989;95:1180-1184.
- Lee B, Caddy G. A rare cause of dysphagia: herpes simplex esophagitis. World J Gastroenterol. 2007;13:2756-2757.
- Robertson AG, Dunn LJ, Immanuel A, et al. An unusual presentation of herpes simplex esophagitis: a nonhealing “peptic” ulcer. Endoscopy. 2009;41(suppl 2):E213.
Practice Points
- Pemphigus vulgaris (PV) often requires therapeutic immunosuppression for disease control.
- Acute odynophagia in the setting of systemic immunosuppression for PV requires endoscopic evaluation.