User login
Hyperpigmentation and atrophy
A 35-year-old woman sought care at our clinic for a plaque on her upper left arm. She said that it had started 3 months earlier as a small indentation, but had recently became larger and hyperpigmented. The lesion was not pruritic or painful, and she had no associated weakness or systemic symptoms. The patient denied any insect bites, instrumentation, topical ointments, or trauma to the area.
Physical examination revealed a 3.5 × 2.5 cm area of hyperpigmentation on the posterior aspect of the left arm, overlying the musculotendinous junction of the lateral head of the triceps (FIGURE 1). The lesion had an irregular border and a central region approximately 1 cm in diameter associated with a nontender subcutaneous mass that felt tethered to the skin. There was significant thinning of the subcutaneous fat beneath the hyperpigmentation relative to the normal surrounding skin. The patient had normal triceps function and a normal distal neurovascular exam.
Concerned about malignancy, we ordered computed tomography (CT) and magnetic resonance imaging (MRI) of the left arm. Both demonstrated a nonspecific density in the subcutaneous tissue, and focal indentation and architectural distortion of the skin and subcutaneous tissue in the area in question. In addition, the MRI showed skin tethering extending to the superficial myofascial layer of the posterior triceps muscle and a small superficial blood vessel (FIGURE 2).
We referred the patient to Plastic Surgery for tissue diagnosis. A punch biopsy revealed dense dermal sclerosis that could be consistent with a morphea-like process. As malignancy could not be excluded, the patient was then referred to a dermatologist to determine the need for excision.
FIGURE 1
A 3.5 × 2.5 cm lesion on the left posterior arm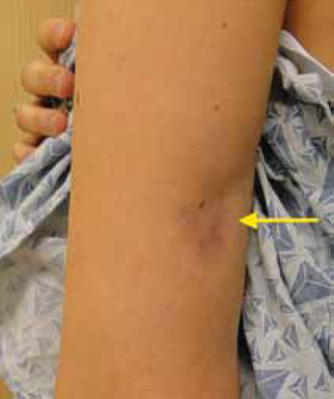
FIGURE 2
MRI reveals skin tethering to superficial myofascial layer
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Atrophy due to a steroid injection
During further discussion with the patient, she revealed that she had received a steroid injection 4 to 5 months before the lesion appeared. Although we felt reasonably certain that the steroid injection was the cause of the lesion, the patient had the lesion excised.
The most striking pathologic findings in the excised specimen were seen in the subcutaneous tissue; there was extensive fat necrosis, with abundant amorphous eosinophilic and amphophilic debris replacing, and interspersed between, adipocytes (FIGURE 3). Extensive lipomembranous changes were also seen. The constellation of pathologic findings was nonspecific.
FIGURE 3
Variation in adipocyte size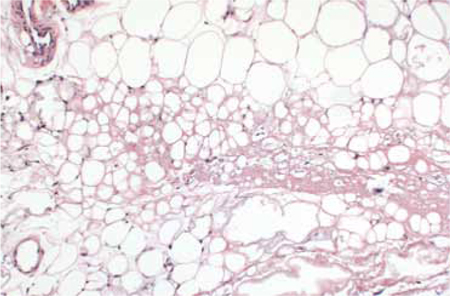
Low magnification of the excised specimen shows variation of adipocyte size, with some fat cells being replaced by amorphous eosinophilic and amphophilic material.
When treatment does harm
Steroid injections are commonly used to treat dermatologic and musculoskeletal conditions such as keloids, alopecia areata, neuromas, and inflamed bursas. However, these injections can have long-lasting dermatologic consequences such as altered pigmentation, dermal and fat atrophy, hypo- and hyperpigmentation, and telangiectasias.1-13 Localized lipodystrophy, the loss of subcutaneous fat in a localized area, can also be a result of steroid injection, as well as the injection of other drugs such as insulin or antibiotics.14
Morphea-like change, which we saw in our patient, is less common, but has also been described in the literature.1,2 Morphea presents with a single or several circumscribed indurated patches or plaques, usually with hypo- or hyperpigmentation.15
The timing of cutaneous changes due to steroid injection is variable. Case reports describe changes in pigmentation and atrophy beginning several weeks to several months after injection.9,10,12,13 This delay may occur because depot steroid preparations can remain in the skin for prolonged periods; one study demonstrated that small amounts persisted for more than a year after injection.2
Lesion with unclear etiology? Focus on the history
Because the cutaneous changes that steroids can induce are varied and nonspecific, it is important to carefully elicit any history of steroid injections when working up a patient for a cutaneous lesion of unclear etiology. Additional workup of neoplastic, infiltrative, vascular, and less commonly, infectious causes should be conducted if the etiology of such a lesion cannot be explained.
Although lesions from steroid injections are not usually evaluated with CT or MRI imaging, one study involving 2 volunteers suggested that pulsed ultrasound may be helpful in determining the long-term changes in skin thickness from steroid injection.16 Thus, it appears that radiological studies have little role in the diagnosis of steroid-induced skin changes, but may be used to raise or lower suspicion for other etiologies of cutaneous change when the diagnosis is unclear.
Healing comes in time, and sometimes, with saline
Cutaneous atrophy caused by steroid injections may resolve spontaneously within one to 2 years, or may persist.7,10,13,16,17 Treatment of persistent atrophy with normal saline infiltration has been used, and appears to be safe, tolerable, and relatively effective.17
Preventive steps to keep in mind
Attention to risk may reduce the likelihood and severity of cutaneous damage. Insoluble preparations should be used only for deep injections into joints, bursae, or muscles, and care should be taken not to track the steroid into the more superficial tissues.
More soluble preparations should be used for superficial structures.10,12 In addition, the lowest effective concentration of steroid preparation should be used, and it should not be mixed with vasoconstrictors like epinephrine.10 The anatomical location of the injection also plays a role in the extent and duration of change.10 For instance, injections into more superficial structures (eg, skin, tendons) could produce cutaneous changes that are more obvious than injections into deeper structures (eg, joints, bursae).10
Our patient
As noted earlier, our patient had the lesion excised. At follow-up one week later, she continued to progress well clinically.
CORRESPONDENCE Tia Kostas, MD, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115; [email protected]
1. Holt PJ, Marks R, Waddington E. ‘Pseudomorphoea’: a side effect of subcutaneous corticosteroid injection. Br J Dermatol. 1975;92:689-691.
2. Joshi R. Incidental finding of skin deposits of corticosteroids without associated granulomatous inflammation: report of three cases. Indian J Dermatol Venereol Leprol. 2008;74:44-46.
3. Reddy PD, Zelicof SB, Ruotolo C, et al. Interdigital neuroma. Local cutaneous changes after corticosteroid injection. Clin Orthop Relat Res. 1995;(317):185-187.
4. Stapczynski JS. Localized depigmentation after steroid injection of a ganglion cyst on the hand. Ann Emerg Med. 1991;20:807-809.
5. Okere K, Jones MC. A case of skin hypopigmentation secondary to a corticosteroid injection. South Med J. 2006;99:1393-1394.
6. Basadonna PT, Rucco V, Gasparini D, et al. Plantar fat pad atrophy after corticosteroid injection for an interdigital neuroma: a case report. Am J Phys Med Rehabil. 1999;78:283-285.
7. DiStefano V, Nixon JE. Steroid-induced skin changes following local injection. Clin Orthop Relat Res. 1972;87:254-256.
8. Friedman SJ, Butler DF, Pittelkow MR. Perilesional linear atrophy and hypopigmentation after intralesional corticosteroid therapy. Report of two cases and review of the literature. J Am Acad Dermatol. 1988;19:537-541.
9. Gallardo MJ, Johnson DA. Cutaneous hypopigmentation following a posterior sub-tenon triamcinolone injection. Am J Ophthalmol. 2004;137:779-780.
10. Jacobs MB. Local subcutaneous atrophy after corticosteroid injection. Postgrad Med. 1986;80:159-160.
11. Louis DS, Hankin FM, Eckenrode JF. Cutaneous atrophy after corticosteroid injection. Am Fam Physician. 1986;33:183-186.
12. Lund IM, Donde R, Knudsen EA. Persistent local cutaneous atrophy following corticosteroid injection for tendinitis. Rheumatol Rehabil. 1979;18:91-93.
13. Schetman D, Hambrick GW, Jr, Wilson CE. Cutaneous changes following local injection of triamcinolone. Arch Dermatol. 1963;88:820-828.
14. Myers SA, Sheedy MP. Lipodystrophy. In: Wolf KG, Lowell A, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies, Inc; 2003:586–590.
15. Falanga V, Killoran CE. Morphea. In: Wolf KG, Lowell A, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies, Inc; 2003:543–546.
16. Gomez EC, Berman B, Miller DL. Ultrasonic assessment of cutaneous atrophy caused by intradermal corticosteroids. J Dermatol Surg Oncol. 1982;8:1071-1074.
17. Shumaker PR, Rao J, Goldman MP. Treatment of local, persistent cutaneous atrophy following corticosteroid injection with normal saline infiltration. Dermatol Surg. 2005;31:1340-1343.
A 35-year-old woman sought care at our clinic for a plaque on her upper left arm. She said that it had started 3 months earlier as a small indentation, but had recently became larger and hyperpigmented. The lesion was not pruritic or painful, and she had no associated weakness or systemic symptoms. The patient denied any insect bites, instrumentation, topical ointments, or trauma to the area.
Physical examination revealed a 3.5 × 2.5 cm area of hyperpigmentation on the posterior aspect of the left arm, overlying the musculotendinous junction of the lateral head of the triceps (FIGURE 1). The lesion had an irregular border and a central region approximately 1 cm in diameter associated with a nontender subcutaneous mass that felt tethered to the skin. There was significant thinning of the subcutaneous fat beneath the hyperpigmentation relative to the normal surrounding skin. The patient had normal triceps function and a normal distal neurovascular exam.
Concerned about malignancy, we ordered computed tomography (CT) and magnetic resonance imaging (MRI) of the left arm. Both demonstrated a nonspecific density in the subcutaneous tissue, and focal indentation and architectural distortion of the skin and subcutaneous tissue in the area in question. In addition, the MRI showed skin tethering extending to the superficial myofascial layer of the posterior triceps muscle and a small superficial blood vessel (FIGURE 2).
We referred the patient to Plastic Surgery for tissue diagnosis. A punch biopsy revealed dense dermal sclerosis that could be consistent with a morphea-like process. As malignancy could not be excluded, the patient was then referred to a dermatologist to determine the need for excision.
FIGURE 1
A 3.5 × 2.5 cm lesion on the left posterior arm
FIGURE 2
MRI reveals skin tethering to superficial myofascial layer
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Atrophy due to a steroid injection
During further discussion with the patient, she revealed that she had received a steroid injection 4 to 5 months before the lesion appeared. Although we felt reasonably certain that the steroid injection was the cause of the lesion, the patient had the lesion excised.
The most striking pathologic findings in the excised specimen were seen in the subcutaneous tissue; there was extensive fat necrosis, with abundant amorphous eosinophilic and amphophilic debris replacing, and interspersed between, adipocytes (FIGURE 3). Extensive lipomembranous changes were also seen. The constellation of pathologic findings was nonspecific.
FIGURE 3
Variation in adipocyte size
Low magnification of the excised specimen shows variation of adipocyte size, with some fat cells being replaced by amorphous eosinophilic and amphophilic material.
When treatment does harm
Steroid injections are commonly used to treat dermatologic and musculoskeletal conditions such as keloids, alopecia areata, neuromas, and inflamed bursas. However, these injections can have long-lasting dermatologic consequences such as altered pigmentation, dermal and fat atrophy, hypo- and hyperpigmentation, and telangiectasias.1-13 Localized lipodystrophy, the loss of subcutaneous fat in a localized area, can also be a result of steroid injection, as well as the injection of other drugs such as insulin or antibiotics.14
Morphea-like change, which we saw in our patient, is less common, but has also been described in the literature.1,2 Morphea presents with a single or several circumscribed indurated patches or plaques, usually with hypo- or hyperpigmentation.15
The timing of cutaneous changes due to steroid injection is variable. Case reports describe changes in pigmentation and atrophy beginning several weeks to several months after injection.9,10,12,13 This delay may occur because depot steroid preparations can remain in the skin for prolonged periods; one study demonstrated that small amounts persisted for more than a year after injection.2
Lesion with unclear etiology? Focus on the history
Because the cutaneous changes that steroids can induce are varied and nonspecific, it is important to carefully elicit any history of steroid injections when working up a patient for a cutaneous lesion of unclear etiology. Additional workup of neoplastic, infiltrative, vascular, and less commonly, infectious causes should be conducted if the etiology of such a lesion cannot be explained.
Although lesions from steroid injections are not usually evaluated with CT or MRI imaging, one study involving 2 volunteers suggested that pulsed ultrasound may be helpful in determining the long-term changes in skin thickness from steroid injection.16 Thus, it appears that radiological studies have little role in the diagnosis of steroid-induced skin changes, but may be used to raise or lower suspicion for other etiologies of cutaneous change when the diagnosis is unclear.
Healing comes in time, and sometimes, with saline
Cutaneous atrophy caused by steroid injections may resolve spontaneously within one to 2 years, or may persist.7,10,13,16,17 Treatment of persistent atrophy with normal saline infiltration has been used, and appears to be safe, tolerable, and relatively effective.17
Preventive steps to keep in mind
Attention to risk may reduce the likelihood and severity of cutaneous damage. Insoluble preparations should be used only for deep injections into joints, bursae, or muscles, and care should be taken not to track the steroid into the more superficial tissues.
More soluble preparations should be used for superficial structures.10,12 In addition, the lowest effective concentration of steroid preparation should be used, and it should not be mixed with vasoconstrictors like epinephrine.10 The anatomical location of the injection also plays a role in the extent and duration of change.10 For instance, injections into more superficial structures (eg, skin, tendons) could produce cutaneous changes that are more obvious than injections into deeper structures (eg, joints, bursae).10
Our patient
As noted earlier, our patient had the lesion excised. At follow-up one week later, she continued to progress well clinically.
CORRESPONDENCE Tia Kostas, MD, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115; [email protected]
A 35-year-old woman sought care at our clinic for a plaque on her upper left arm. She said that it had started 3 months earlier as a small indentation, but had recently became larger and hyperpigmented. The lesion was not pruritic or painful, and she had no associated weakness or systemic symptoms. The patient denied any insect bites, instrumentation, topical ointments, or trauma to the area.
Physical examination revealed a 3.5 × 2.5 cm area of hyperpigmentation on the posterior aspect of the left arm, overlying the musculotendinous junction of the lateral head of the triceps (FIGURE 1). The lesion had an irregular border and a central region approximately 1 cm in diameter associated with a nontender subcutaneous mass that felt tethered to the skin. There was significant thinning of the subcutaneous fat beneath the hyperpigmentation relative to the normal surrounding skin. The patient had normal triceps function and a normal distal neurovascular exam.
Concerned about malignancy, we ordered computed tomography (CT) and magnetic resonance imaging (MRI) of the left arm. Both demonstrated a nonspecific density in the subcutaneous tissue, and focal indentation and architectural distortion of the skin and subcutaneous tissue in the area in question. In addition, the MRI showed skin tethering extending to the superficial myofascial layer of the posterior triceps muscle and a small superficial blood vessel (FIGURE 2).
We referred the patient to Plastic Surgery for tissue diagnosis. A punch biopsy revealed dense dermal sclerosis that could be consistent with a morphea-like process. As malignancy could not be excluded, the patient was then referred to a dermatologist to determine the need for excision.
FIGURE 1
A 3.5 × 2.5 cm lesion on the left posterior arm
FIGURE 2
MRI reveals skin tethering to superficial myofascial layer
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Atrophy due to a steroid injection
During further discussion with the patient, she revealed that she had received a steroid injection 4 to 5 months before the lesion appeared. Although we felt reasonably certain that the steroid injection was the cause of the lesion, the patient had the lesion excised.
The most striking pathologic findings in the excised specimen were seen in the subcutaneous tissue; there was extensive fat necrosis, with abundant amorphous eosinophilic and amphophilic debris replacing, and interspersed between, adipocytes (FIGURE 3). Extensive lipomembranous changes were also seen. The constellation of pathologic findings was nonspecific.
FIGURE 3
Variation in adipocyte size
Low magnification of the excised specimen shows variation of adipocyte size, with some fat cells being replaced by amorphous eosinophilic and amphophilic material.
When treatment does harm
Steroid injections are commonly used to treat dermatologic and musculoskeletal conditions such as keloids, alopecia areata, neuromas, and inflamed bursas. However, these injections can have long-lasting dermatologic consequences such as altered pigmentation, dermal and fat atrophy, hypo- and hyperpigmentation, and telangiectasias.1-13 Localized lipodystrophy, the loss of subcutaneous fat in a localized area, can also be a result of steroid injection, as well as the injection of other drugs such as insulin or antibiotics.14
Morphea-like change, which we saw in our patient, is less common, but has also been described in the literature.1,2 Morphea presents with a single or several circumscribed indurated patches or plaques, usually with hypo- or hyperpigmentation.15
The timing of cutaneous changes due to steroid injection is variable. Case reports describe changes in pigmentation and atrophy beginning several weeks to several months after injection.9,10,12,13 This delay may occur because depot steroid preparations can remain in the skin for prolonged periods; one study demonstrated that small amounts persisted for more than a year after injection.2
Lesion with unclear etiology? Focus on the history
Because the cutaneous changes that steroids can induce are varied and nonspecific, it is important to carefully elicit any history of steroid injections when working up a patient for a cutaneous lesion of unclear etiology. Additional workup of neoplastic, infiltrative, vascular, and less commonly, infectious causes should be conducted if the etiology of such a lesion cannot be explained.
Although lesions from steroid injections are not usually evaluated with CT or MRI imaging, one study involving 2 volunteers suggested that pulsed ultrasound may be helpful in determining the long-term changes in skin thickness from steroid injection.16 Thus, it appears that radiological studies have little role in the diagnosis of steroid-induced skin changes, but may be used to raise or lower suspicion for other etiologies of cutaneous change when the diagnosis is unclear.
Healing comes in time, and sometimes, with saline
Cutaneous atrophy caused by steroid injections may resolve spontaneously within one to 2 years, or may persist.7,10,13,16,17 Treatment of persistent atrophy with normal saline infiltration has been used, and appears to be safe, tolerable, and relatively effective.17
Preventive steps to keep in mind
Attention to risk may reduce the likelihood and severity of cutaneous damage. Insoluble preparations should be used only for deep injections into joints, bursae, or muscles, and care should be taken not to track the steroid into the more superficial tissues.
More soluble preparations should be used for superficial structures.10,12 In addition, the lowest effective concentration of steroid preparation should be used, and it should not be mixed with vasoconstrictors like epinephrine.10 The anatomical location of the injection also plays a role in the extent and duration of change.10 For instance, injections into more superficial structures (eg, skin, tendons) could produce cutaneous changes that are more obvious than injections into deeper structures (eg, joints, bursae).10
Our patient
As noted earlier, our patient had the lesion excised. At follow-up one week later, she continued to progress well clinically.
CORRESPONDENCE Tia Kostas, MD, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115; [email protected]
1. Holt PJ, Marks R, Waddington E. ‘Pseudomorphoea’: a side effect of subcutaneous corticosteroid injection. Br J Dermatol. 1975;92:689-691.
2. Joshi R. Incidental finding of skin deposits of corticosteroids without associated granulomatous inflammation: report of three cases. Indian J Dermatol Venereol Leprol. 2008;74:44-46.
3. Reddy PD, Zelicof SB, Ruotolo C, et al. Interdigital neuroma. Local cutaneous changes after corticosteroid injection. Clin Orthop Relat Res. 1995;(317):185-187.
4. Stapczynski JS. Localized depigmentation after steroid injection of a ganglion cyst on the hand. Ann Emerg Med. 1991;20:807-809.
5. Okere K, Jones MC. A case of skin hypopigmentation secondary to a corticosteroid injection. South Med J. 2006;99:1393-1394.
6. Basadonna PT, Rucco V, Gasparini D, et al. Plantar fat pad atrophy after corticosteroid injection for an interdigital neuroma: a case report. Am J Phys Med Rehabil. 1999;78:283-285.
7. DiStefano V, Nixon JE. Steroid-induced skin changes following local injection. Clin Orthop Relat Res. 1972;87:254-256.
8. Friedman SJ, Butler DF, Pittelkow MR. Perilesional linear atrophy and hypopigmentation after intralesional corticosteroid therapy. Report of two cases and review of the literature. J Am Acad Dermatol. 1988;19:537-541.
9. Gallardo MJ, Johnson DA. Cutaneous hypopigmentation following a posterior sub-tenon triamcinolone injection. Am J Ophthalmol. 2004;137:779-780.
10. Jacobs MB. Local subcutaneous atrophy after corticosteroid injection. Postgrad Med. 1986;80:159-160.
11. Louis DS, Hankin FM, Eckenrode JF. Cutaneous atrophy after corticosteroid injection. Am Fam Physician. 1986;33:183-186.
12. Lund IM, Donde R, Knudsen EA. Persistent local cutaneous atrophy following corticosteroid injection for tendinitis. Rheumatol Rehabil. 1979;18:91-93.
13. Schetman D, Hambrick GW, Jr, Wilson CE. Cutaneous changes following local injection of triamcinolone. Arch Dermatol. 1963;88:820-828.
14. Myers SA, Sheedy MP. Lipodystrophy. In: Wolf KG, Lowell A, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies, Inc; 2003:586–590.
15. Falanga V, Killoran CE. Morphea. In: Wolf KG, Lowell A, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies, Inc; 2003:543–546.
16. Gomez EC, Berman B, Miller DL. Ultrasonic assessment of cutaneous atrophy caused by intradermal corticosteroids. J Dermatol Surg Oncol. 1982;8:1071-1074.
17. Shumaker PR, Rao J, Goldman MP. Treatment of local, persistent cutaneous atrophy following corticosteroid injection with normal saline infiltration. Dermatol Surg. 2005;31:1340-1343.
1. Holt PJ, Marks R, Waddington E. ‘Pseudomorphoea’: a side effect of subcutaneous corticosteroid injection. Br J Dermatol. 1975;92:689-691.
2. Joshi R. Incidental finding of skin deposits of corticosteroids without associated granulomatous inflammation: report of three cases. Indian J Dermatol Venereol Leprol. 2008;74:44-46.
3. Reddy PD, Zelicof SB, Ruotolo C, et al. Interdigital neuroma. Local cutaneous changes after corticosteroid injection. Clin Orthop Relat Res. 1995;(317):185-187.
4. Stapczynski JS. Localized depigmentation after steroid injection of a ganglion cyst on the hand. Ann Emerg Med. 1991;20:807-809.
5. Okere K, Jones MC. A case of skin hypopigmentation secondary to a corticosteroid injection. South Med J. 2006;99:1393-1394.
6. Basadonna PT, Rucco V, Gasparini D, et al. Plantar fat pad atrophy after corticosteroid injection for an interdigital neuroma: a case report. Am J Phys Med Rehabil. 1999;78:283-285.
7. DiStefano V, Nixon JE. Steroid-induced skin changes following local injection. Clin Orthop Relat Res. 1972;87:254-256.
8. Friedman SJ, Butler DF, Pittelkow MR. Perilesional linear atrophy and hypopigmentation after intralesional corticosteroid therapy. Report of two cases and review of the literature. J Am Acad Dermatol. 1988;19:537-541.
9. Gallardo MJ, Johnson DA. Cutaneous hypopigmentation following a posterior sub-tenon triamcinolone injection. Am J Ophthalmol. 2004;137:779-780.
10. Jacobs MB. Local subcutaneous atrophy after corticosteroid injection. Postgrad Med. 1986;80:159-160.
11. Louis DS, Hankin FM, Eckenrode JF. Cutaneous atrophy after corticosteroid injection. Am Fam Physician. 1986;33:183-186.
12. Lund IM, Donde R, Knudsen EA. Persistent local cutaneous atrophy following corticosteroid injection for tendinitis. Rheumatol Rehabil. 1979;18:91-93.
13. Schetman D, Hambrick GW, Jr, Wilson CE. Cutaneous changes following local injection of triamcinolone. Arch Dermatol. 1963;88:820-828.
14. Myers SA, Sheedy MP. Lipodystrophy. In: Wolf KG, Lowell A, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies, Inc; 2003:586–590.
15. Falanga V, Killoran CE. Morphea. In: Wolf KG, Lowell A, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies, Inc; 2003:543–546.
16. Gomez EC, Berman B, Miller DL. Ultrasonic assessment of cutaneous atrophy caused by intradermal corticosteroids. J Dermatol Surg Oncol. 1982;8:1071-1074.
17. Shumaker PR, Rao J, Goldman MP. Treatment of local, persistent cutaneous atrophy following corticosteroid injection with normal saline infiltration. Dermatol Surg. 2005;31:1340-1343.