User login
Hypertension in the elderly: Some practical considerations
The management of hypertension has advanced significantly in the last few decades. But the race for more effective means to control this epidemic and its associated complications is far from won. A high percentage of patients in the United States have hypertension that is uncontrolled. Most of these belong to the most rapidly growing demographic group in the United States, ie, the elderly.
It is estimated that more than 70% of medical practice will be directed to geriatric needs in the coming years. It is therefore very important for clinicians to be comfortable with managing hypertension in the elderly.
A GROWING PROBLEM IN AN AGING POPULATION
Between 1980 and 2009, the US population age 65 and older increased from 25.6 million to 39.6 million, of which 42% are men and 58% women.1 This number is expected to reach 75 million by the year 2040. People over 85 years of age are the fastest growing subset of the US population.2 As many as 50% of people who were born recently in countries such as the United States, the United Kingdom, France, Denmark, and Japan will live to celebrate their 100th birthday.3
According to the Framingham Heart Study, by age 60 approximately 60% of the population develops hypertension, and by 70 years about 65% of men and about 75% of women have the disease. In the same study, 90% of those who were normotensive at age 55 went on to develop hypertension. The elderly also are more likely to suffer from the complications of hypertension and are more likely to have uncontrolled disease.
Compared with younger patients with similar blood pressure, elderly hypertensive patients have lower cardiac output, higher peripheral resistance, wider pulse pressure, lower intravascular volume, and lower renal blood flow.4 These age-related pathophysiologic differences must be considered when treating antihypertension in the elderly.
IS TREATING THE ELDERLY BENEFICIAL?
Most elderly hypertensive patients have multiple comorbidities, which tremendously affect the management of their hypertension. They are also more likely than younger patients to have resistant hypertension and to need multiple drugs to control their blood pressure. In the process, these frail patients are exposed to a host of drug-related adverse effects. Thus, it is relevant to question the net benefit of treatment in this age group.
Many studies have indeed shown that treating hypertension reduces the risk of stroke and other adverse cardiovascular events. A decade ago, Staessen et al,5 in a meta-analysis of more than 15,000 patients between ages 62 and 76, showed that treating isolated systolic hypertension substantially reduced morbidity and mortality rates. Moreover, a 2011 meta-analysis of randomized controlled trials in hypertensive patients age 75 and over also concluded that treatment reduced cardiovascular morbidity and mortality rates and the incidence of heart failure, even though the total mortality rate was not affected.6
Opinion on treating the very elderly (≥ 80 years of age) was divided until the results of the Hypertension in the Very Elderly trial (HYVET)7 came out in 2008. This study documented major benefits of treatment in the very elderly age group as well.
The consensus, therefore, is that it is appropriate, even imperative, to treat elderly hypertensive patients (with some cautions—see the sections that follow).
GOAL OF TREATMENT IN THE ELDERLY
Targets for blood pressure management have been based primarily on observational data in middle-aged patients. There is no such thing as an ideal blood pressure that has been derived from randomized controlled trials for any population, let alone the elderly. The generally recommended blood pressure goal of 140/90 mm Hg for elderly hypertensive patients is based on expert opinion.
Moreover, it is unclear if the same target should apply to octogenarians. According to a 2011 American College of Cardiology/American Heart Association (ACC/AHA) expert consensus report,8 an achieved systolic blood pressure of 140 to 145 mm Hg, if tolerated, can be acceptable in this age group.
An orthostatic decline in blood pressure accompanies advanced age and is an inevitable adverse effect of some antihypertensive drugs. Accordingly, systolic blood pressure lower than 130 and diastolic blood pressure lower than 70 mm Hg are best avoided in octogenarians.8 Therefore, when hypertension is complicated by coexisting conditions that require a specific blood pressure goal, it would seem reasonable to not pursue the lower target as aggressively in octogenarians as in elderly patients under age 80.
Having stated the limitations in the quality of data at hand—largely observational—it is relevant to mention the Systolic Blood Pressure Intervention trial (SPRINT).9 This ongoing randomized, multicenter trial, launched by the National Institutes of Health, is assessing whether maintaining blood pressure levels lower than current recommendations further reduces the risk of cardiovascular and kidney diseases or, in the SPRINT-MIND substudy, of age-related cognitive decline, regardless of the type of antihypertensive drug taken. Initially planning to enroll close to 10,000 participants over the age of 55 without specifying any agegroup ranges, the investigators later decided to conduct a substudy called SPRINT Senior that will enroll about 1,750 participants over the age of 75 to determine whether a lower blood pressure range will have the same beneficial effects in older adults.
Given the limitations in the quality and applicability of published data (coming from small, nonrandomized studies with no long-term follow-up), SPRINT is expected to provide the evidence needed to support standard vs aggressive hypertension control among the elderly. The trial is projected to run until late 2018.
MANAGEMENT APPROACH IN THE ELDERLY
Blood pressure should be recorded in both arms before a diagnosis is made. In a number of patients, particularly the elderly, there are significant differences in blood pressure readings between the two arms. The higher reading should be relied on and the corresponding arm used for subsequent measurements.
Lifestyle interventions
Similar to the approach in younger patients with hypertension, lifestyle interventions are the first step to managing high blood pressure in the elderly. The diet and exercise interventions in the Dietary Approaches to Stop Hypertension (DASH) trial have both been shown to lower blood pressure.10,11
Restricting sodium intake has been shown to lower blood pressure more in older adults than in younger adults. In the DASH trial,12 systolic blood pressure decreased by 8.1 mm Hg with sodium restriction in hypertensive patients age 55 to 76 years, compared with 4.8 mm Hg for adults aged 23 to 41 years. In the Trial of Nonpharmacologic Interventions in the Elderly (TONE),13 in people ages 60 to 80 who were randomized to reduce their salt intake, urinary sodium excretion was 40 mmol/day lower and blood pressure was 4.3/2.0 mm Hg lower than in a group that received usual care. Accordingly, reducing salt intake is particularly valuable for blood pressure management in the salt-sensitive elderly.14
Drug therapy
The hypertension pandemic has driven extensive pharmaceutical research, and new drugs continue to be introduced. The major classes of drugs commonly used for treating hypertension are diuretics, calcium channel blockers, and renin-angiotensin system blockers. Each class has specific benefits and adverse-effect profiles.
It is appropriate to start antihypertensive drug therapy with the lowest dose and to monitor for adverse effects, including orthostatic hypotension. The choice of drug should be guided by the patient’s comorbid conditions (Table 1) and the other drugs the patient is taking.15 If the blood pressure response is inadequate, a second drug from a different class should be added. In the same manner, a third drug from a different class should be added if the blood pressure remains outside the optimal range on two drugs.
The average elderly American is on more than six medications.16 Some of these are for high blood pressure, but others interact with antihypertensive drugs (Table 2), and some, including nonsteroidal anti-inflammatory drugs (NSAIDs) and steroids, directly affect blood pressure. Therefore, the drug regimen of an elderly hypertensive patient should be reviewed carefully at every visit. The Screening Tool of Older Person’s Prescriptions (STOPP), a list of 65 rules relating to the most common and most potentially dangerous instances of inappropriate prescribing and overprescribing in the elderly,17 has been found to be a reliable tool in this regard, with a kappa-coefficient of 0.75. Together with the Screening Tool to Alert Doctors to Right [ie, Appropriate, Indicated] Treatment (START),17 which lists 22 evidence-based prescribing indicators for common conditions in the elderly, these criteria provide clinicians with an easy screening tool to combat polypharmacy.
Given the multitude of factors that go into deciding on a specific management strategy in the elderly, it is not possible to discuss individualized care in all patients in the scope of one paper. Below, we present several case scenarios that internists commonly encounter, and suggest ways to approach each.
CASE 1: SECONDARY HYPERTENSION
A 69-year-old obese man who has hypertension of recent onset, long-standing gastroesophageal reflux disease, and benign prostatic hypertrophy comes to your office, accompanied by his wife. He has never had hypertension before. His body mass index is 34 kg/m2. On physical examination, his blood pressure is 180/112 mm Hg.
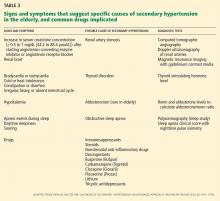
We start with this case to emphasize the importance of considering causes of secondary hypertension in all patients with the disease (Table 3).18 Further workup should be pursued in those who appear to have “inappropriate” hypertension. This could present as refractory hypertension, abrupt-onset hypertension, hypertension that is first diagnosed before age 20 or after age 60, or loss of control over previously well-controlled blood pressure. Secondary hypertension must always be considered when the history or physical examination suggests a possible cause.
Renal artery stenosis increases in incidence with age. Its prevalence is reported to be as high as 50% in elderly patients with other signs of atherosclerosis such as widespread peripheral artery disease.19
Obstructive sleep apnea also commonly coexists with hypertension and its prevalence also increases with age. In addition, elderly patients with obstructive sleep apnea have a higher incidence of cardiovascular complications, including hypertension, than middle-aged people.20 Numerous studies have found that the severity of obstructive sleep apnea corresponds with the likelihood of systemic hypertension.21–23 Randomized trials and meta-analyses have also concluded that effective treatment with continuous positive airway pressure reduces systemic blood pressure,24–27 although by less than antihypertensive medications do.
A causal relationship between obstructive sleep apnea and hypertension has not been established with certainty. It is recommended, however, that patients with resistant hypertension be screened for obstructive sleep apnea as a possible cause of their disease.
Other causes of secondary hypertension to keep in mind when evaluating patients who have inappropriate hypertension include thyroid disorders, alcohol and tobacco use, and chronic steroid or NSAID use. Pheochromocytoma and adrenal adenoma, though possible, are less prevalent in the elderly.
Case continued
Physical examination in the above patient revealed an epigastric systolic-diastolic bruit, a sign that, although not sensitive, is highly specific for renal artery stenosis, raising the suspicion of this condition. Duplex ultrasonography of the renal arteries confirmed this suspicion. The patient underwent angiography and revascularization, resulting in a distinct fall in, but not normalization of, his blood pressure.
Detecting and treating renal artery stenosis
Though we do not intend to detail the diagnostic approaches and treatments for the various causes of secondary hypertension, we need to briefly mention those for renal artery stenosis.
According to the 2006 ACC/AHA guidelines on peripheral artery disease,28 testing for renal artery stenosis is indicated only if a subsequent corrective procedure is a viable option.
Renal arteriography remains the gold standard for diagnosing renal artery stenosis. However, noninvasive imaging has largely replaced it.
Duplex Doppler ultrasonography, compared with angiography, has a sensitivity of 84% to 98% depending on operator experience, and a specificity of 62% to 99% for detecting renal artery stenosis.29 Some of its limiting factors are the time needed to do the study, its steep learning curve and operator-dependence, and interference with the results by body fat and intestinal gas.
Computed tomographic angiography has a sensitivity of over 90% for detecting renal artery stenosis, and its specificity has been shown to be as high as 99% in certain studies.29 Use of contrast can be a limiting factor in some clinical settings.
Magnetic resonance angiography also offers a sensitivity of 90% to 100% and specificities of 76% to 94% for detecting renal artery stenosis.29 On the other hand, it is costly, and the gadolinium contrast solution used is nephrotoxic, though not as toxic as the contrast used in computed tomographic angiography.
As previously stated, these imaging studies should be used only if corrective measures will be undertaken if clinically significant renal artery stenosis is found. Even in such cases, revascularization may not be curative in all cases. Its effectiveness has been compared with that of medical management alone in a number of studies.30,31 A meta-analysis32 of six key trials involving more than 1,200 patients showed no difference in systolic and diastolic blood pressures and other clinical outcomes, including all-cause mortality, between the two treatment groups over a 29-month follow-up period.
Hence, although we advise that causes of secondary hypertension be considered in cases of inappropriate hypertension, aggressive management must be pursued on a case-by-case basis.
CASE 2: DRUG ADVERSE EFFECTS
A 75-year-old Hispanic woman with a history of treated breast cancer was recently diagnosed with hypertension. Her blood pressure is controlled on amlodipine (Norvasc) 10 mg daily, and her blood pressure today is 128/80 mm Hg. Her only complaint during this office visit is some swelling of her ankles.
Edema and dihydropyridine calcium channel blockers
Like all drugs, antihypertensive medications come with their own set of adverse effects. These are more common as people age—hence the importance of identifying and effectively managing them in the elderly population.
Calcium channel blockers, especially the dihydropyridines—ie, nifedipine (Adalat), amlodipine, felodipine (Plendil), and isradipine (DynaCirc)—are known to cause peripheral vasodilation. Peripheral edema is a common dose-related effect in people on these drugs. In one study, median leg weight increased by 80 g after amlodipine 5 mg was given for 4 weeks, and by another 68 g on a 10-mg dose.33
Ankle swelling, encountered more in women, can be very bothersome. The swelling is related to hyperfiltration of fluid into the interstitial space secondary to intracapillary hypertension. Calcium channel blockers predominantly cause arteriolar dilation by paralyzing the precapillary sphincter, thereby elevating intracapillary pressure.
Traditionally, physicians have lowered the dose of the calcium channel blocker, switched to another drug, or added a diuretic to alleviate the swelling. However, giving a diuretic for edema induced by a calcium channel blocker may not relieve the edema.34
Peripheral edema is much less encountered when a calcium channel blocker is given with an inhibitor of the renin-angiotensin system.35 A meta-analysis concluded that the incidence of peripheral edema was lowered by 38% with such a combination. The same study found angiotensin-converting enzyme (ACE) inhibitors significantly more efficacious for this effect than angiotensin receptor blockers (ARBs).35
ACE inhibitors and ARBs are known to cause venodilation, thereby lowering intra-capillary pressure. It is probable that this effect helps remove the extra fluid sequestered in the capillary bed by the arteriolar dilation from the calcium channel blocker.
Pedal edema associated with use of a calcium channel blocker occurs much more commonly in the elderly than in the young. It is clearly dose-dependent, and the incidence peaks after 6 months of therapy. In the patient described above, adding a low dose of an ACE inhibitor or an ARB (if the patient is ACE inhibitor-intolerant) should relieve the swelling.
Hyponatremia and diuretics
Electrolyte imbalances are another common problem encountered in the elderly. Even though for years attention has been directed to the potassium level, hyponatremia has been equally associated with adverse effects in the elderly, such as an increased risk of fractures as shown in the Rotterdam study.36
In 180 hypertensive inpatients, mean age 76.4, Sharabi et al37 found the incidence of hyponatremia to be three times higher in women than in men (odds ratio 3.10, 95% confidence interval 2.07 to 4.67). Patients were 10 times more likely to be affected after age 65 and 14 times more likely after age 75. Most of the patients affected (74.5%) used a thiazide-type diuretic. Even though in many of the patients diuretics were used for more than 1 year before hyponatremia developed, susceptible patients—such as the frail elderly—can develop severe hyponatremia within days of starting to use a thiazide.38
Severe hyponatremia is potentially life-threatening. Most cases are caused by thiazide rather than loop diuretics.38 Thiazides inhibit electrolyte transport at the cortical diluting sites. As they decrease the glomerular filtration rate acutely, they increase proximal water reabsorption (making the plasma hypotonic), reducing water delivery distally. Loop diuretics, on the other hand, have their main effect at the thick ascending limb, reducing the osmolality at the medullary interstitium and not affecting proximal water reabsorption. Additionally, loop diuretics have a shorter half-life than thiazides, which makes hyponatremia more likely to happen with thiazides.
In patients who develop hyponatremia secondary to diuretic use, appropriate treatment includes stopping the medication, restricting water intake, and repleting electrolyte stores.38 As with any cause of chronic hyponatremia, correction must be cautiously monitored and not hastily done.
Therefore, we advise adding a thiazide diuric with caution in the elderly, and we advise avoiding thiazides in patients with high water or alcohol intake.
CASE 3: DEMENTIA AND HYPERTENSION
A 74-year-old man with long-standing hypertension, gout, and chronic obstructive pulmonary disease was recently diagnosed with Alzheimer dementia. He takes enalapril (Vasotec) 10 mg daily for his blood pressure. His blood pressure is 130/78 mm Hg.
Dementia is one of the most important and common neurologic disorders in the elderly. With the rise in average life expectancy, its magnitude has grown to cause a substantial emotional and economic burden on society and health care.
Midlife hypertension has been demonstrated to be an important modifiable risk factor for late-life cognitive decline,39 mild cognitive impairment,40 and dementia of all causes.41 It has been suggested that hypertension might be part of the pathogenesis of dementia, and targeting high blood pressure might prevent its onset.
Moreover, a significant reduction in both Alzheimer and vascular dementia was demonstrated (risk reduction 55%) with the use of a long-acting dihydropyridine calcium channel blocker (nitrendipine) in the Syst-Eur study.42 However, data from studies such as Systolic Hypertension in the Elderly Program (SHEP) and the HYVET substudy of cognitive function assessement43 showed no difference in dementia between placebo and active therapy with chlorthalidone (Hygroton) (in SHEP) or indapamide (Lozol) (in the HYVET substudy).
Disorders of calcium homeostasis are associated with the brain’s aging process. Probably, the neuroprotective effect of nitrendipine seen in Syst-Eur was due to its ability to affect this process, independent of its blood pressure-lowering effect.
In another prospective study, people over 60 years of age who complained of subjective memory loss showed a significant and positive association between memory scores and the use of calcium channel blockers (+0.14 ± 0.09 in users vs −0.12 ± 0.06 in nonusers; P = .016) independently of age, sex, white matter hyperintensities, and carotid wall cross-sectional area, all of which were associated with worse memory scores.44
Drugs that block the renin-angiotensin system have also been proposed to delay the onset and slow the progression of dementia.45 A small randomized, controlled trial suggested that centrally active ACE inhibitors—those that cross the blood-brain barrier, such as captopril (Capoten), lisinopril (Prinivil), ramipril (Altace), and fosinopril (Monopril)—slow cognitive decline in Alzheimer dementia more than non-centrally active ACE inhibitors or calcium channel blockers.46
Sink et al47 examined data from participants in the Cognition Substudy of the Cardiovascular Health Study48 on the effect of ACE inhibitors on cognitive decline. ACE inhibitors, as a class, showed no benefit in reducing the risk of dementia compared with other antihypertensive drug classes. However, centrally active ACE inhibitors, compared with other medications, were associated with a 65% reduction in cognitive decline per year of drug exposure (P = .01). Non-centrally active ACE inhibitors worsened cognitive decline.
It appears that the brain’s renin-angiotensin system plays a role in the pathogenesis of dementia. Indeed, ACE has been shown to degrade amyloid-beta protein, and its level was increased in brain tissue of Alzheimer patients postmortem.49
The relationship between blood pressure and cognitive function appears to be curvilinear, so that low blood pressure in late life is also associated with dementia and Alzheimer dementia.50 In 5,816 patients age 65 and older, Morris et al51 calculated the percentile scores of four cognitive tests according to the level of blood pressure. Patients with systolic blood pressures of 100 mm Hg, 120 mm Hg, and 180 mm Hg scored lower on the Mini Mental State Examination than those in the 140 to 160 mm Hg range. Patients with diastolic blood pressures between 80 and 90 mm Hg appeared to have the best cognitive function. This further emphasizes that blood pressure control must be pursued in the very elderly, albeit less aggressively. The MIND substudy of the SPRINT trial9 is likely to shed more light on this relationship.
When needed for optimal blood pressure control in a hypertensive patient at risk of dementia, a calcium channel blocker of the dihydropyridine type or a centrally active ACE inhibitor, or both, is preferable.
CASE 4: LABILE HYPERTENSION
A 74-year-old man with hypertension and diabetes mellitus comes to see you in the office. On physical examination, his blood pressure is 175/110 mm Hg. His blood pressure during his last visit 3 months ago was 120/75. He brings a log with him that shows random fluctuations in his blood pressure readings. He takes hydrochlorothiazide 25 mg daily for his blood pressure.
Hypertension in some patients continuously fluctuates between low and high levels. A study in Canada found that up to 15% of all adult hypertensive patients might have labile hypertension.52 In the presence of a normal average blood pressure, visit-to-visit blood pressure variability is usually considered a trivial matter. However, some but not all studies have shown that such visit-to-visit variability in blood pressure is an independent predictor of future cardiovascular events in hypertensive patients, independent of the mean systolic blood pressure.52–54
Blood pressure fluctuates from heartbeat to heartbeat, from morning to night, from winter to summer, and from sitting to standing, and it is prone to increase with exertion, stress, and other factors. But excessive fluctuations in the elderly are most likely the result of excessive stiffness of the arterial tree and a decrease in the windkessel (cushioning) function of the aorta. As a consequence, even small-volume changes in the intravascular system can trigger large blood pressure fluctuations.
There is some evidence that antihypertensive drug classes may differ in their effects on visit-to-visit blood pressure variability. In a 2010 study comparing the effects of different antihypertensive drugs on blood pressure variation, calcium channel blockers and non-loop diuretics were associated with less variation in systolic blood pressure, and calcium channel blockers reduced it the most.55
In the patient described above, switching to a low-dose calcium channel blocker with a thorough follow-up is a reasonable plan.
CASE 5: ORTHOSTATIC HYPOTENSION
A 73-year-old woman with long-standing hypertension complains of some dizziness, especially when getting out of bed in the morning. On physical examination, her blood pressure is 134/100 mm Hg sitting and 115/90 standing. She takes amlodipine 10 mg daily, enalapril 10 mg daily, and chlorthalidone 25 mg daily. Chlorthalidone had been added on her last visit 1 month before.
As a result of the increase in the number of elderly patients with hypertension and guidelines recommending better control in this age group, the number of elderly patients on anti-hypertensive drugs has risen significantly. At the same time, the elderly have increasingly presented with adverse effects of treatment.
Orthostatic hypotension is a drop in systolic pressure of 20 mm Hg or more or a drop in diastolic pressure of 10 mm Hg or more on standing, with or without symptoms. These are caused by cerebral hypoperfusion and include dizziness, lightheadedness, generalized weakness, visual blurring, and, in severe cases, syncope.
Alpha-blockers and nitrates have been most commonly implicated in causing orthostatic hypotension, due to venous pooling. Clearly, not all antihypertensive drugs are equal with regard to their venodilatory effects. Thiazide diuretics, by causing fluid volume depletion, and beta-blockers, by interfering with compensatory cardioacceleration with upright posture, are also commonly involved in causing an excessive blood pressure drop with standing.
Systolic orthostatic hypotension has been shown to be a significant and independent predictor of cardiovascular morbidity and death.56 Moreover, syncope and subsequent falls are an important cause of injury and death in the elderly.57 The clinical combination of hypertension and orthostatic hypotension is, therefore, especially challenging. A compromise between accepting a higher cardiovascular risk at either end of the spectrum with an added higher risk for fall at the lower end has to be made.
To prevent orthostatic hypotension in the elderly, it is important to avoid prescribing high-risk drugs. When starting antihypertensive therapy, a low dose should be used, and the dose should be titrated upward very slowly and cautiously. If orthostatic hypotension is suggested by the history or by the orthostatic test, which is warranted in all elderly hypertensive patients before starting or significantly altering therapy, the potential culprit drug should be withdrawn and the patient reassessed. Improved hydration, elevating the head of the bed, and taking the antihypertensive drug at night are ways to improve symptoms, but these remain largely unproven.
In this patient, the newly added chlorthalidone was stopped, and her symptoms improved.
PSEUDOHYPERTENSION
Since hypertension is defined by a numerical value, it is prudent that this value be accurate. Treating a falsely high reading or leaving a falsely low reading untreated will predispose the elderly patient to increased risk either way. One rare condition in the elderly that can give a falsely high blood pressure reading is pseudohypertension.
Pseudohypertension is a condition in which indirect blood pressure measured by the cuff method overestimates the true intra-arterial blood pressure due to marked underlying arteriosclerosis. The Osler maneuver can be used to differentiate true hypertension from pseudohypertension.58 This is performed by palpating the pulseless radial or brachial artery distal to the inflated cuff. If the artery is palpable despite being pulseless, the patient is said to be “Osler-positive” and likely has pseudohypertension.58
Pseudohypertension should be suspected if the patient has orthostatic hypotension despite normal blood pressure sitting and standing. Also, elevated blood pressure without appropriate target organ disease should raise the suspicion of pseudohypertension. Apart from the Osler maneuver, measuring the intraarterial pressure can confirm this diagnosis.
- US Census Bureau. The 2011 statistical abstract. The national data book. http://www.census.gov/compendia/statab/2011/2011edition.html. Accessed July 24, 2012.
- He W, Sengupta M, Velkoff VA, DeBarros KA. US Census Bureau. Current Population Reports, P23-209. 65+ in the United States: 2005. http://www.census.gov/prod/2006pubs/p23-209.pdf. Accessed July 24, 2012.
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet 2009; 374:1196–1208.
- Messerli FH, Sundgaard-Riise K, Ventura HO, Dunn FG, Glade LB, Frohlich ED. Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet 1983; 2:983–986.
- Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000; 355:865–872.
- Schall P, Wehling M. Treatment of arterial hypertension in the very elderly: a meta-analysis of clinical trials. Arzneimittelforschung 2011; 61:221–228.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Soc Hypertens 2011; 5:259–352.
- Wake Forest University, Winston-Salem NC. SPRINT: Systolic Blood Pressure Intervention Trial. http://www.sprinttrial.org/public/dspHome.cfm. Accessed July 24, 2012.
- Sacks FM, Svetkey LP, Vollmer WM, et al; DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001; 344:3–10.
- Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 2002; 136:493–503.
- Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ; DASH Collaborative Research Group. A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: results of the DASH-Sodium Trial. Am J Cardiol 2004; 94:222–227.
- Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med 2001; 161:685–693.
- Frisoli TM, Schmieder RE, Grodzicki T, Messerli FH. Salt and hypertension: is salt dietary reduction worth the effort? Am J Med 2012; 125:433–439.
- National High Blood Pressure Education Program. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: National Heart, Lung, and Blood Institute; 2004. http://www.ncbi.nlm.nih.gov/books/NBK9630/Accessed July 30, 2012.
- Mitka M. New guidance covers ways to prevent and treat hypertension in elderly patients. JAMA 2011; 305:2394,2398.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- Viera AJ, Neutze DM. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician 2010; 82:1471–1478.
- Rihal CS, Textor SC, Breen JF, et al. Incidental renal artery stenosis among a prospective cohort of hypertensive patients undergoing coronary angiography. Mayo Clin Proc 2002; 77:309–316.
- Wang Y, Li Y. [Clinical and polysomnographic characteristics in elderly patients with obstructive sleep apnea hypopnea syndrome]. [In Chinese] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008; 22:222–225.
- Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ 2000; 320:479–482.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med 2000; 160:2289–2295.
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002; 359:204–210.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107:68–73.
- Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive air-way pressure on blood pressure: a placebo trial. Hypertension 2000; 35:144–147.
- Sharma SK, Agrawal S, Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med 2011; 365:2277–2286.
- White CJ, Jaff MR, Haskal ZJ, et al; American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Indications for renal arteriography at the time of coronary arteriography: a science advisory from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Councils on Cardiovascular Radiology and Intervention and on Kidney in Cardiovascular Disease. Circulation 2006; 114:1892–1895.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol 2006; 47:1239–1312.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–848,W150–W151.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Kumbhani DJ, Bavry AA, Harvey JE, et al. Clinical outcomes after percutaneous revascularization versus medical management in patients with significant renal artery stenosis: a meta-analysis of randomized controlled trials. Am Heart J 2011; 161:622.e1–630.e1.
- Pedrinelli R, Dell’Omo G, Melillo E, Mariani M. Amlodipine, enalapril, and dependent leg edema in essential hypertension. Hypertension 2000; 35:621–625.
- van Hamersvelt HW, Kloke HJ, de Jong DJ, Koene RA, Huysmans FT. Oedema formation with the vasodilators nifedipine and diazoxide: direct local effect or sodium retention? J Hypertens 1996; 14:1041–1045.
- Makani H, Bangalore S, Romero J, Wever-Pinzon O, Messerli FH. Effect of renin-angiotensin system blockade on calcium channel blocker-associated peripheral edema. Am J Med 2011; 124:128–135.
- Hoorn EJ, Rivadeneira F, van Meurs JB, et al. Mild hyponatremia as a risk factor for fractures: the Rotterdam Study. J Bone Miner Res 2011; 26:1822–1828.
- Sharabi Y, Illan R, Kamari Y, et al. Diuretic induced hyponatraemia in elderly hypertensive women. J Hum Hypertens 2002; 16:631–635.
- Spital A. Diuretic-induced hyponatremia. Am J Nephrol 1999; 19:447–452.
- Knopman D, Boland LL, Mosley T, et al; Atherosclerosis Risk in Communities (ARIC) Study Investigators. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol 2007; 64:1734–1740.
- Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging 2000; 21:49–55.
- Forette F, Seux ML, Staessen JA, et al Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 1998; 352:1347–1351.
- Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 2008. www.thelancet.com/journals/laneur/article/PIIS1474-4422(08)70143-1/fulltext. Accessed August 23, 2012.
- Watfa G, Rossignol P, Kearney-Schwartz A, et al. Use of calcium channel blockers is associated with better cognitive performance in older hypertensive patients with subjective memory complaints. J Hypertens 2010; 28:2485–2493.
- Tzourio C, Anderson C, Chapman N, et al; PROGRESS Collaborative Group. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 2003; 163:1069–1075.
- Ohrui T, Tomita N, Sato-Nakagawa T, et al. Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology 2004; 63:1324–1325.
- Sink KM, Leng X, Williamson J, et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med 2009; 169:1195–1202.
- Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology 2003; 22:1–12.
- Hemming ML, Selkoe DJ. Amyloid beta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J Biol Chem 2005; 280:37644–37650.
- Nilsson SE, Read S, Berg S, Johansson B, Melander A, Lindblad U. Low systolic blood pressure is associated with impaired cognitive function in the oldest old: longitudinal observations in a population-based sample 80 years and older. Aging Clin Exp Res 2007; 19:41–47.
- Morris MC, Scherr PA, Hebert LE, et al. Association between blood pressure and cognitive function in a biracial community population of older persons. Neuroepidemiology 2002; 21:123–130.
- Joffres MR, Hamet P, Rabkin SW, Gelskey D, Hogan K, Fodor G. Prevalence, control and awareness of high blood pressure among Canadian adults. Canadian Heart Health Surveys Research Group. CMAJ 1992; 146:1997–2005.
- Schillaci G, Pucci G. The importance of instability and visit-to-visit variability of blood pressure. Expert Rev Cardiovasc Ther 2010; 8:1095–1097.
- Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-visit blood pressure variability, carotid atherosclerosis, and cardiovascular events in the European Lacidipine Study on Atherosclerosis. Circulation 2012; 126:569–578.
- Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 2010; 375:906–915.
- Fagard RH, De Cort P. Orthostatic hypotension is a more robust predictor of cardiovascular events than nighttime reverse dipping in elderly. Hypertension 2010; 56:56–61.
- Kearney F, Moore A. Treatment of combined hypertension and orthostatic hypotension in older adults: more questions than answers still remain. Expert Rev Cardiovasc Ther 2009; 7:557–560.
- Messerli FH, Ventura HO, Amodeo C. Osler’s maneuver and pseudohypertension. N Engl J Med 1985; 312:1548–1551.
The management of hypertension has advanced significantly in the last few decades. But the race for more effective means to control this epidemic and its associated complications is far from won. A high percentage of patients in the United States have hypertension that is uncontrolled. Most of these belong to the most rapidly growing demographic group in the United States, ie, the elderly.
It is estimated that more than 70% of medical practice will be directed to geriatric needs in the coming years. It is therefore very important for clinicians to be comfortable with managing hypertension in the elderly.
A GROWING PROBLEM IN AN AGING POPULATION
Between 1980 and 2009, the US population age 65 and older increased from 25.6 million to 39.6 million, of which 42% are men and 58% women.1 This number is expected to reach 75 million by the year 2040. People over 85 years of age are the fastest growing subset of the US population.2 As many as 50% of people who were born recently in countries such as the United States, the United Kingdom, France, Denmark, and Japan will live to celebrate their 100th birthday.3
According to the Framingham Heart Study, by age 60 approximately 60% of the population develops hypertension, and by 70 years about 65% of men and about 75% of women have the disease. In the same study, 90% of those who were normotensive at age 55 went on to develop hypertension. The elderly also are more likely to suffer from the complications of hypertension and are more likely to have uncontrolled disease.
Compared with younger patients with similar blood pressure, elderly hypertensive patients have lower cardiac output, higher peripheral resistance, wider pulse pressure, lower intravascular volume, and lower renal blood flow.4 These age-related pathophysiologic differences must be considered when treating antihypertension in the elderly.
IS TREATING THE ELDERLY BENEFICIAL?
Most elderly hypertensive patients have multiple comorbidities, which tremendously affect the management of their hypertension. They are also more likely than younger patients to have resistant hypertension and to need multiple drugs to control their blood pressure. In the process, these frail patients are exposed to a host of drug-related adverse effects. Thus, it is relevant to question the net benefit of treatment in this age group.
Many studies have indeed shown that treating hypertension reduces the risk of stroke and other adverse cardiovascular events. A decade ago, Staessen et al,5 in a meta-analysis of more than 15,000 patients between ages 62 and 76, showed that treating isolated systolic hypertension substantially reduced morbidity and mortality rates. Moreover, a 2011 meta-analysis of randomized controlled trials in hypertensive patients age 75 and over also concluded that treatment reduced cardiovascular morbidity and mortality rates and the incidence of heart failure, even though the total mortality rate was not affected.6
Opinion on treating the very elderly (≥ 80 years of age) was divided until the results of the Hypertension in the Very Elderly trial (HYVET)7 came out in 2008. This study documented major benefits of treatment in the very elderly age group as well.
The consensus, therefore, is that it is appropriate, even imperative, to treat elderly hypertensive patients (with some cautions—see the sections that follow).
GOAL OF TREATMENT IN THE ELDERLY
Targets for blood pressure management have been based primarily on observational data in middle-aged patients. There is no such thing as an ideal blood pressure that has been derived from randomized controlled trials for any population, let alone the elderly. The generally recommended blood pressure goal of 140/90 mm Hg for elderly hypertensive patients is based on expert opinion.
Moreover, it is unclear if the same target should apply to octogenarians. According to a 2011 American College of Cardiology/American Heart Association (ACC/AHA) expert consensus report,8 an achieved systolic blood pressure of 140 to 145 mm Hg, if tolerated, can be acceptable in this age group.
An orthostatic decline in blood pressure accompanies advanced age and is an inevitable adverse effect of some antihypertensive drugs. Accordingly, systolic blood pressure lower than 130 and diastolic blood pressure lower than 70 mm Hg are best avoided in octogenarians.8 Therefore, when hypertension is complicated by coexisting conditions that require a specific blood pressure goal, it would seem reasonable to not pursue the lower target as aggressively in octogenarians as in elderly patients under age 80.
Having stated the limitations in the quality of data at hand—largely observational—it is relevant to mention the Systolic Blood Pressure Intervention trial (SPRINT).9 This ongoing randomized, multicenter trial, launched by the National Institutes of Health, is assessing whether maintaining blood pressure levels lower than current recommendations further reduces the risk of cardiovascular and kidney diseases or, in the SPRINT-MIND substudy, of age-related cognitive decline, regardless of the type of antihypertensive drug taken. Initially planning to enroll close to 10,000 participants over the age of 55 without specifying any agegroup ranges, the investigators later decided to conduct a substudy called SPRINT Senior that will enroll about 1,750 participants over the age of 75 to determine whether a lower blood pressure range will have the same beneficial effects in older adults.
Given the limitations in the quality and applicability of published data (coming from small, nonrandomized studies with no long-term follow-up), SPRINT is expected to provide the evidence needed to support standard vs aggressive hypertension control among the elderly. The trial is projected to run until late 2018.
MANAGEMENT APPROACH IN THE ELDERLY
Blood pressure should be recorded in both arms before a diagnosis is made. In a number of patients, particularly the elderly, there are significant differences in blood pressure readings between the two arms. The higher reading should be relied on and the corresponding arm used for subsequent measurements.
Lifestyle interventions
Similar to the approach in younger patients with hypertension, lifestyle interventions are the first step to managing high blood pressure in the elderly. The diet and exercise interventions in the Dietary Approaches to Stop Hypertension (DASH) trial have both been shown to lower blood pressure.10,11
Restricting sodium intake has been shown to lower blood pressure more in older adults than in younger adults. In the DASH trial,12 systolic blood pressure decreased by 8.1 mm Hg with sodium restriction in hypertensive patients age 55 to 76 years, compared with 4.8 mm Hg for adults aged 23 to 41 years. In the Trial of Nonpharmacologic Interventions in the Elderly (TONE),13 in people ages 60 to 80 who were randomized to reduce their salt intake, urinary sodium excretion was 40 mmol/day lower and blood pressure was 4.3/2.0 mm Hg lower than in a group that received usual care. Accordingly, reducing salt intake is particularly valuable for blood pressure management in the salt-sensitive elderly.14
Drug therapy
The hypertension pandemic has driven extensive pharmaceutical research, and new drugs continue to be introduced. The major classes of drugs commonly used for treating hypertension are diuretics, calcium channel blockers, and renin-angiotensin system blockers. Each class has specific benefits and adverse-effect profiles.
It is appropriate to start antihypertensive drug therapy with the lowest dose and to monitor for adverse effects, including orthostatic hypotension. The choice of drug should be guided by the patient’s comorbid conditions (Table 1) and the other drugs the patient is taking.15 If the blood pressure response is inadequate, a second drug from a different class should be added. In the same manner, a third drug from a different class should be added if the blood pressure remains outside the optimal range on two drugs.
The average elderly American is on more than six medications.16 Some of these are for high blood pressure, but others interact with antihypertensive drugs (Table 2), and some, including nonsteroidal anti-inflammatory drugs (NSAIDs) and steroids, directly affect blood pressure. Therefore, the drug regimen of an elderly hypertensive patient should be reviewed carefully at every visit. The Screening Tool of Older Person’s Prescriptions (STOPP), a list of 65 rules relating to the most common and most potentially dangerous instances of inappropriate prescribing and overprescribing in the elderly,17 has been found to be a reliable tool in this regard, with a kappa-coefficient of 0.75. Together with the Screening Tool to Alert Doctors to Right [ie, Appropriate, Indicated] Treatment (START),17 which lists 22 evidence-based prescribing indicators for common conditions in the elderly, these criteria provide clinicians with an easy screening tool to combat polypharmacy.
Given the multitude of factors that go into deciding on a specific management strategy in the elderly, it is not possible to discuss individualized care in all patients in the scope of one paper. Below, we present several case scenarios that internists commonly encounter, and suggest ways to approach each.
CASE 1: SECONDARY HYPERTENSION
A 69-year-old obese man who has hypertension of recent onset, long-standing gastroesophageal reflux disease, and benign prostatic hypertrophy comes to your office, accompanied by his wife. He has never had hypertension before. His body mass index is 34 kg/m2. On physical examination, his blood pressure is 180/112 mm Hg.
We start with this case to emphasize the importance of considering causes of secondary hypertension in all patients with the disease (Table 3).18 Further workup should be pursued in those who appear to have “inappropriate” hypertension. This could present as refractory hypertension, abrupt-onset hypertension, hypertension that is first diagnosed before age 20 or after age 60, or loss of control over previously well-controlled blood pressure. Secondary hypertension must always be considered when the history or physical examination suggests a possible cause.
Renal artery stenosis increases in incidence with age. Its prevalence is reported to be as high as 50% in elderly patients with other signs of atherosclerosis such as widespread peripheral artery disease.19
Obstructive sleep apnea also commonly coexists with hypertension and its prevalence also increases with age. In addition, elderly patients with obstructive sleep apnea have a higher incidence of cardiovascular complications, including hypertension, than middle-aged people.20 Numerous studies have found that the severity of obstructive sleep apnea corresponds with the likelihood of systemic hypertension.21–23 Randomized trials and meta-analyses have also concluded that effective treatment with continuous positive airway pressure reduces systemic blood pressure,24–27 although by less than antihypertensive medications do.
A causal relationship between obstructive sleep apnea and hypertension has not been established with certainty. It is recommended, however, that patients with resistant hypertension be screened for obstructive sleep apnea as a possible cause of their disease.
Other causes of secondary hypertension to keep in mind when evaluating patients who have inappropriate hypertension include thyroid disorders, alcohol and tobacco use, and chronic steroid or NSAID use. Pheochromocytoma and adrenal adenoma, though possible, are less prevalent in the elderly.
Case continued
Physical examination in the above patient revealed an epigastric systolic-diastolic bruit, a sign that, although not sensitive, is highly specific for renal artery stenosis, raising the suspicion of this condition. Duplex ultrasonography of the renal arteries confirmed this suspicion. The patient underwent angiography and revascularization, resulting in a distinct fall in, but not normalization of, his blood pressure.
Detecting and treating renal artery stenosis
Though we do not intend to detail the diagnostic approaches and treatments for the various causes of secondary hypertension, we need to briefly mention those for renal artery stenosis.
According to the 2006 ACC/AHA guidelines on peripheral artery disease,28 testing for renal artery stenosis is indicated only if a subsequent corrective procedure is a viable option.
Renal arteriography remains the gold standard for diagnosing renal artery stenosis. However, noninvasive imaging has largely replaced it.
Duplex Doppler ultrasonography, compared with angiography, has a sensitivity of 84% to 98% depending on operator experience, and a specificity of 62% to 99% for detecting renal artery stenosis.29 Some of its limiting factors are the time needed to do the study, its steep learning curve and operator-dependence, and interference with the results by body fat and intestinal gas.
Computed tomographic angiography has a sensitivity of over 90% for detecting renal artery stenosis, and its specificity has been shown to be as high as 99% in certain studies.29 Use of contrast can be a limiting factor in some clinical settings.
Magnetic resonance angiography also offers a sensitivity of 90% to 100% and specificities of 76% to 94% for detecting renal artery stenosis.29 On the other hand, it is costly, and the gadolinium contrast solution used is nephrotoxic, though not as toxic as the contrast used in computed tomographic angiography.
As previously stated, these imaging studies should be used only if corrective measures will be undertaken if clinically significant renal artery stenosis is found. Even in such cases, revascularization may not be curative in all cases. Its effectiveness has been compared with that of medical management alone in a number of studies.30,31 A meta-analysis32 of six key trials involving more than 1,200 patients showed no difference in systolic and diastolic blood pressures and other clinical outcomes, including all-cause mortality, between the two treatment groups over a 29-month follow-up period.
Hence, although we advise that causes of secondary hypertension be considered in cases of inappropriate hypertension, aggressive management must be pursued on a case-by-case basis.
CASE 2: DRUG ADVERSE EFFECTS
A 75-year-old Hispanic woman with a history of treated breast cancer was recently diagnosed with hypertension. Her blood pressure is controlled on amlodipine (Norvasc) 10 mg daily, and her blood pressure today is 128/80 mm Hg. Her only complaint during this office visit is some swelling of her ankles.
Edema and dihydropyridine calcium channel blockers
Like all drugs, antihypertensive medications come with their own set of adverse effects. These are more common as people age—hence the importance of identifying and effectively managing them in the elderly population.
Calcium channel blockers, especially the dihydropyridines—ie, nifedipine (Adalat), amlodipine, felodipine (Plendil), and isradipine (DynaCirc)—are known to cause peripheral vasodilation. Peripheral edema is a common dose-related effect in people on these drugs. In one study, median leg weight increased by 80 g after amlodipine 5 mg was given for 4 weeks, and by another 68 g on a 10-mg dose.33
Ankle swelling, encountered more in women, can be very bothersome. The swelling is related to hyperfiltration of fluid into the interstitial space secondary to intracapillary hypertension. Calcium channel blockers predominantly cause arteriolar dilation by paralyzing the precapillary sphincter, thereby elevating intracapillary pressure.
Traditionally, physicians have lowered the dose of the calcium channel blocker, switched to another drug, or added a diuretic to alleviate the swelling. However, giving a diuretic for edema induced by a calcium channel blocker may not relieve the edema.34
Peripheral edema is much less encountered when a calcium channel blocker is given with an inhibitor of the renin-angiotensin system.35 A meta-analysis concluded that the incidence of peripheral edema was lowered by 38% with such a combination. The same study found angiotensin-converting enzyme (ACE) inhibitors significantly more efficacious for this effect than angiotensin receptor blockers (ARBs).35
ACE inhibitors and ARBs are known to cause venodilation, thereby lowering intra-capillary pressure. It is probable that this effect helps remove the extra fluid sequestered in the capillary bed by the arteriolar dilation from the calcium channel blocker.
Pedal edema associated with use of a calcium channel blocker occurs much more commonly in the elderly than in the young. It is clearly dose-dependent, and the incidence peaks after 6 months of therapy. In the patient described above, adding a low dose of an ACE inhibitor or an ARB (if the patient is ACE inhibitor-intolerant) should relieve the swelling.
Hyponatremia and diuretics
Electrolyte imbalances are another common problem encountered in the elderly. Even though for years attention has been directed to the potassium level, hyponatremia has been equally associated with adverse effects in the elderly, such as an increased risk of fractures as shown in the Rotterdam study.36
In 180 hypertensive inpatients, mean age 76.4, Sharabi et al37 found the incidence of hyponatremia to be three times higher in women than in men (odds ratio 3.10, 95% confidence interval 2.07 to 4.67). Patients were 10 times more likely to be affected after age 65 and 14 times more likely after age 75. Most of the patients affected (74.5%) used a thiazide-type diuretic. Even though in many of the patients diuretics were used for more than 1 year before hyponatremia developed, susceptible patients—such as the frail elderly—can develop severe hyponatremia within days of starting to use a thiazide.38
Severe hyponatremia is potentially life-threatening. Most cases are caused by thiazide rather than loop diuretics.38 Thiazides inhibit electrolyte transport at the cortical diluting sites. As they decrease the glomerular filtration rate acutely, they increase proximal water reabsorption (making the plasma hypotonic), reducing water delivery distally. Loop diuretics, on the other hand, have their main effect at the thick ascending limb, reducing the osmolality at the medullary interstitium and not affecting proximal water reabsorption. Additionally, loop diuretics have a shorter half-life than thiazides, which makes hyponatremia more likely to happen with thiazides.
In patients who develop hyponatremia secondary to diuretic use, appropriate treatment includes stopping the medication, restricting water intake, and repleting electrolyte stores.38 As with any cause of chronic hyponatremia, correction must be cautiously monitored and not hastily done.
Therefore, we advise adding a thiazide diuric with caution in the elderly, and we advise avoiding thiazides in patients with high water or alcohol intake.
CASE 3: DEMENTIA AND HYPERTENSION
A 74-year-old man with long-standing hypertension, gout, and chronic obstructive pulmonary disease was recently diagnosed with Alzheimer dementia. He takes enalapril (Vasotec) 10 mg daily for his blood pressure. His blood pressure is 130/78 mm Hg.
Dementia is one of the most important and common neurologic disorders in the elderly. With the rise in average life expectancy, its magnitude has grown to cause a substantial emotional and economic burden on society and health care.
Midlife hypertension has been demonstrated to be an important modifiable risk factor for late-life cognitive decline,39 mild cognitive impairment,40 and dementia of all causes.41 It has been suggested that hypertension might be part of the pathogenesis of dementia, and targeting high blood pressure might prevent its onset.
Moreover, a significant reduction in both Alzheimer and vascular dementia was demonstrated (risk reduction 55%) with the use of a long-acting dihydropyridine calcium channel blocker (nitrendipine) in the Syst-Eur study.42 However, data from studies such as Systolic Hypertension in the Elderly Program (SHEP) and the HYVET substudy of cognitive function assessement43 showed no difference in dementia between placebo and active therapy with chlorthalidone (Hygroton) (in SHEP) or indapamide (Lozol) (in the HYVET substudy).
Disorders of calcium homeostasis are associated with the brain’s aging process. Probably, the neuroprotective effect of nitrendipine seen in Syst-Eur was due to its ability to affect this process, independent of its blood pressure-lowering effect.
In another prospective study, people over 60 years of age who complained of subjective memory loss showed a significant and positive association between memory scores and the use of calcium channel blockers (+0.14 ± 0.09 in users vs −0.12 ± 0.06 in nonusers; P = .016) independently of age, sex, white matter hyperintensities, and carotid wall cross-sectional area, all of which were associated with worse memory scores.44
Drugs that block the renin-angiotensin system have also been proposed to delay the onset and slow the progression of dementia.45 A small randomized, controlled trial suggested that centrally active ACE inhibitors—those that cross the blood-brain barrier, such as captopril (Capoten), lisinopril (Prinivil), ramipril (Altace), and fosinopril (Monopril)—slow cognitive decline in Alzheimer dementia more than non-centrally active ACE inhibitors or calcium channel blockers.46
Sink et al47 examined data from participants in the Cognition Substudy of the Cardiovascular Health Study48 on the effect of ACE inhibitors on cognitive decline. ACE inhibitors, as a class, showed no benefit in reducing the risk of dementia compared with other antihypertensive drug classes. However, centrally active ACE inhibitors, compared with other medications, were associated with a 65% reduction in cognitive decline per year of drug exposure (P = .01). Non-centrally active ACE inhibitors worsened cognitive decline.
It appears that the brain’s renin-angiotensin system plays a role in the pathogenesis of dementia. Indeed, ACE has been shown to degrade amyloid-beta protein, and its level was increased in brain tissue of Alzheimer patients postmortem.49
The relationship between blood pressure and cognitive function appears to be curvilinear, so that low blood pressure in late life is also associated with dementia and Alzheimer dementia.50 In 5,816 patients age 65 and older, Morris et al51 calculated the percentile scores of four cognitive tests according to the level of blood pressure. Patients with systolic blood pressures of 100 mm Hg, 120 mm Hg, and 180 mm Hg scored lower on the Mini Mental State Examination than those in the 140 to 160 mm Hg range. Patients with diastolic blood pressures between 80 and 90 mm Hg appeared to have the best cognitive function. This further emphasizes that blood pressure control must be pursued in the very elderly, albeit less aggressively. The MIND substudy of the SPRINT trial9 is likely to shed more light on this relationship.
When needed for optimal blood pressure control in a hypertensive patient at risk of dementia, a calcium channel blocker of the dihydropyridine type or a centrally active ACE inhibitor, or both, is preferable.
CASE 4: LABILE HYPERTENSION
A 74-year-old man with hypertension and diabetes mellitus comes to see you in the office. On physical examination, his blood pressure is 175/110 mm Hg. His blood pressure during his last visit 3 months ago was 120/75. He brings a log with him that shows random fluctuations in his blood pressure readings. He takes hydrochlorothiazide 25 mg daily for his blood pressure.
Hypertension in some patients continuously fluctuates between low and high levels. A study in Canada found that up to 15% of all adult hypertensive patients might have labile hypertension.52 In the presence of a normal average blood pressure, visit-to-visit blood pressure variability is usually considered a trivial matter. However, some but not all studies have shown that such visit-to-visit variability in blood pressure is an independent predictor of future cardiovascular events in hypertensive patients, independent of the mean systolic blood pressure.52–54
Blood pressure fluctuates from heartbeat to heartbeat, from morning to night, from winter to summer, and from sitting to standing, and it is prone to increase with exertion, stress, and other factors. But excessive fluctuations in the elderly are most likely the result of excessive stiffness of the arterial tree and a decrease in the windkessel (cushioning) function of the aorta. As a consequence, even small-volume changes in the intravascular system can trigger large blood pressure fluctuations.
There is some evidence that antihypertensive drug classes may differ in their effects on visit-to-visit blood pressure variability. In a 2010 study comparing the effects of different antihypertensive drugs on blood pressure variation, calcium channel blockers and non-loop diuretics were associated with less variation in systolic blood pressure, and calcium channel blockers reduced it the most.55
In the patient described above, switching to a low-dose calcium channel blocker with a thorough follow-up is a reasonable plan.
CASE 5: ORTHOSTATIC HYPOTENSION
A 73-year-old woman with long-standing hypertension complains of some dizziness, especially when getting out of bed in the morning. On physical examination, her blood pressure is 134/100 mm Hg sitting and 115/90 standing. She takes amlodipine 10 mg daily, enalapril 10 mg daily, and chlorthalidone 25 mg daily. Chlorthalidone had been added on her last visit 1 month before.
As a result of the increase in the number of elderly patients with hypertension and guidelines recommending better control in this age group, the number of elderly patients on anti-hypertensive drugs has risen significantly. At the same time, the elderly have increasingly presented with adverse effects of treatment.
Orthostatic hypotension is a drop in systolic pressure of 20 mm Hg or more or a drop in diastolic pressure of 10 mm Hg or more on standing, with or without symptoms. These are caused by cerebral hypoperfusion and include dizziness, lightheadedness, generalized weakness, visual blurring, and, in severe cases, syncope.
Alpha-blockers and nitrates have been most commonly implicated in causing orthostatic hypotension, due to venous pooling. Clearly, not all antihypertensive drugs are equal with regard to their venodilatory effects. Thiazide diuretics, by causing fluid volume depletion, and beta-blockers, by interfering with compensatory cardioacceleration with upright posture, are also commonly involved in causing an excessive blood pressure drop with standing.
Systolic orthostatic hypotension has been shown to be a significant and independent predictor of cardiovascular morbidity and death.56 Moreover, syncope and subsequent falls are an important cause of injury and death in the elderly.57 The clinical combination of hypertension and orthostatic hypotension is, therefore, especially challenging. A compromise between accepting a higher cardiovascular risk at either end of the spectrum with an added higher risk for fall at the lower end has to be made.
To prevent orthostatic hypotension in the elderly, it is important to avoid prescribing high-risk drugs. When starting antihypertensive therapy, a low dose should be used, and the dose should be titrated upward very slowly and cautiously. If orthostatic hypotension is suggested by the history or by the orthostatic test, which is warranted in all elderly hypertensive patients before starting or significantly altering therapy, the potential culprit drug should be withdrawn and the patient reassessed. Improved hydration, elevating the head of the bed, and taking the antihypertensive drug at night are ways to improve symptoms, but these remain largely unproven.
In this patient, the newly added chlorthalidone was stopped, and her symptoms improved.
PSEUDOHYPERTENSION
Since hypertension is defined by a numerical value, it is prudent that this value be accurate. Treating a falsely high reading or leaving a falsely low reading untreated will predispose the elderly patient to increased risk either way. One rare condition in the elderly that can give a falsely high blood pressure reading is pseudohypertension.
Pseudohypertension is a condition in which indirect blood pressure measured by the cuff method overestimates the true intra-arterial blood pressure due to marked underlying arteriosclerosis. The Osler maneuver can be used to differentiate true hypertension from pseudohypertension.58 This is performed by palpating the pulseless radial or brachial artery distal to the inflated cuff. If the artery is palpable despite being pulseless, the patient is said to be “Osler-positive” and likely has pseudohypertension.58
Pseudohypertension should be suspected if the patient has orthostatic hypotension despite normal blood pressure sitting and standing. Also, elevated blood pressure without appropriate target organ disease should raise the suspicion of pseudohypertension. Apart from the Osler maneuver, measuring the intraarterial pressure can confirm this diagnosis.
The management of hypertension has advanced significantly in the last few decades. But the race for more effective means to control this epidemic and its associated complications is far from won. A high percentage of patients in the United States have hypertension that is uncontrolled. Most of these belong to the most rapidly growing demographic group in the United States, ie, the elderly.
It is estimated that more than 70% of medical practice will be directed to geriatric needs in the coming years. It is therefore very important for clinicians to be comfortable with managing hypertension in the elderly.
A GROWING PROBLEM IN AN AGING POPULATION
Between 1980 and 2009, the US population age 65 and older increased from 25.6 million to 39.6 million, of which 42% are men and 58% women.1 This number is expected to reach 75 million by the year 2040. People over 85 years of age are the fastest growing subset of the US population.2 As many as 50% of people who were born recently in countries such as the United States, the United Kingdom, France, Denmark, and Japan will live to celebrate their 100th birthday.3
According to the Framingham Heart Study, by age 60 approximately 60% of the population develops hypertension, and by 70 years about 65% of men and about 75% of women have the disease. In the same study, 90% of those who were normotensive at age 55 went on to develop hypertension. The elderly also are more likely to suffer from the complications of hypertension and are more likely to have uncontrolled disease.
Compared with younger patients with similar blood pressure, elderly hypertensive patients have lower cardiac output, higher peripheral resistance, wider pulse pressure, lower intravascular volume, and lower renal blood flow.4 These age-related pathophysiologic differences must be considered when treating antihypertension in the elderly.
IS TREATING THE ELDERLY BENEFICIAL?
Most elderly hypertensive patients have multiple comorbidities, which tremendously affect the management of their hypertension. They are also more likely than younger patients to have resistant hypertension and to need multiple drugs to control their blood pressure. In the process, these frail patients are exposed to a host of drug-related adverse effects. Thus, it is relevant to question the net benefit of treatment in this age group.
Many studies have indeed shown that treating hypertension reduces the risk of stroke and other adverse cardiovascular events. A decade ago, Staessen et al,5 in a meta-analysis of more than 15,000 patients between ages 62 and 76, showed that treating isolated systolic hypertension substantially reduced morbidity and mortality rates. Moreover, a 2011 meta-analysis of randomized controlled trials in hypertensive patients age 75 and over also concluded that treatment reduced cardiovascular morbidity and mortality rates and the incidence of heart failure, even though the total mortality rate was not affected.6
Opinion on treating the very elderly (≥ 80 years of age) was divided until the results of the Hypertension in the Very Elderly trial (HYVET)7 came out in 2008. This study documented major benefits of treatment in the very elderly age group as well.
The consensus, therefore, is that it is appropriate, even imperative, to treat elderly hypertensive patients (with some cautions—see the sections that follow).
GOAL OF TREATMENT IN THE ELDERLY
Targets for blood pressure management have been based primarily on observational data in middle-aged patients. There is no such thing as an ideal blood pressure that has been derived from randomized controlled trials for any population, let alone the elderly. The generally recommended blood pressure goal of 140/90 mm Hg for elderly hypertensive patients is based on expert opinion.
Moreover, it is unclear if the same target should apply to octogenarians. According to a 2011 American College of Cardiology/American Heart Association (ACC/AHA) expert consensus report,8 an achieved systolic blood pressure of 140 to 145 mm Hg, if tolerated, can be acceptable in this age group.
An orthostatic decline in blood pressure accompanies advanced age and is an inevitable adverse effect of some antihypertensive drugs. Accordingly, systolic blood pressure lower than 130 and diastolic blood pressure lower than 70 mm Hg are best avoided in octogenarians.8 Therefore, when hypertension is complicated by coexisting conditions that require a specific blood pressure goal, it would seem reasonable to not pursue the lower target as aggressively in octogenarians as in elderly patients under age 80.
Having stated the limitations in the quality of data at hand—largely observational—it is relevant to mention the Systolic Blood Pressure Intervention trial (SPRINT).9 This ongoing randomized, multicenter trial, launched by the National Institutes of Health, is assessing whether maintaining blood pressure levels lower than current recommendations further reduces the risk of cardiovascular and kidney diseases or, in the SPRINT-MIND substudy, of age-related cognitive decline, regardless of the type of antihypertensive drug taken. Initially planning to enroll close to 10,000 participants over the age of 55 without specifying any agegroup ranges, the investigators later decided to conduct a substudy called SPRINT Senior that will enroll about 1,750 participants over the age of 75 to determine whether a lower blood pressure range will have the same beneficial effects in older adults.
Given the limitations in the quality and applicability of published data (coming from small, nonrandomized studies with no long-term follow-up), SPRINT is expected to provide the evidence needed to support standard vs aggressive hypertension control among the elderly. The trial is projected to run until late 2018.
MANAGEMENT APPROACH IN THE ELDERLY
Blood pressure should be recorded in both arms before a diagnosis is made. In a number of patients, particularly the elderly, there are significant differences in blood pressure readings between the two arms. The higher reading should be relied on and the corresponding arm used for subsequent measurements.
Lifestyle interventions
Similar to the approach in younger patients with hypertension, lifestyle interventions are the first step to managing high blood pressure in the elderly. The diet and exercise interventions in the Dietary Approaches to Stop Hypertension (DASH) trial have both been shown to lower blood pressure.10,11
Restricting sodium intake has been shown to lower blood pressure more in older adults than in younger adults. In the DASH trial,12 systolic blood pressure decreased by 8.1 mm Hg with sodium restriction in hypertensive patients age 55 to 76 years, compared with 4.8 mm Hg for adults aged 23 to 41 years. In the Trial of Nonpharmacologic Interventions in the Elderly (TONE),13 in people ages 60 to 80 who were randomized to reduce their salt intake, urinary sodium excretion was 40 mmol/day lower and blood pressure was 4.3/2.0 mm Hg lower than in a group that received usual care. Accordingly, reducing salt intake is particularly valuable for blood pressure management in the salt-sensitive elderly.14
Drug therapy
The hypertension pandemic has driven extensive pharmaceutical research, and new drugs continue to be introduced. The major classes of drugs commonly used for treating hypertension are diuretics, calcium channel blockers, and renin-angiotensin system blockers. Each class has specific benefits and adverse-effect profiles.
It is appropriate to start antihypertensive drug therapy with the lowest dose and to monitor for adverse effects, including orthostatic hypotension. The choice of drug should be guided by the patient’s comorbid conditions (Table 1) and the other drugs the patient is taking.15 If the blood pressure response is inadequate, a second drug from a different class should be added. In the same manner, a third drug from a different class should be added if the blood pressure remains outside the optimal range on two drugs.
The average elderly American is on more than six medications.16 Some of these are for high blood pressure, but others interact with antihypertensive drugs (Table 2), and some, including nonsteroidal anti-inflammatory drugs (NSAIDs) and steroids, directly affect blood pressure. Therefore, the drug regimen of an elderly hypertensive patient should be reviewed carefully at every visit. The Screening Tool of Older Person’s Prescriptions (STOPP), a list of 65 rules relating to the most common and most potentially dangerous instances of inappropriate prescribing and overprescribing in the elderly,17 has been found to be a reliable tool in this regard, with a kappa-coefficient of 0.75. Together with the Screening Tool to Alert Doctors to Right [ie, Appropriate, Indicated] Treatment (START),17 which lists 22 evidence-based prescribing indicators for common conditions in the elderly, these criteria provide clinicians with an easy screening tool to combat polypharmacy.
Given the multitude of factors that go into deciding on a specific management strategy in the elderly, it is not possible to discuss individualized care in all patients in the scope of one paper. Below, we present several case scenarios that internists commonly encounter, and suggest ways to approach each.
CASE 1: SECONDARY HYPERTENSION
A 69-year-old obese man who has hypertension of recent onset, long-standing gastroesophageal reflux disease, and benign prostatic hypertrophy comes to your office, accompanied by his wife. He has never had hypertension before. His body mass index is 34 kg/m2. On physical examination, his blood pressure is 180/112 mm Hg.
We start with this case to emphasize the importance of considering causes of secondary hypertension in all patients with the disease (Table 3).18 Further workup should be pursued in those who appear to have “inappropriate” hypertension. This could present as refractory hypertension, abrupt-onset hypertension, hypertension that is first diagnosed before age 20 or after age 60, or loss of control over previously well-controlled blood pressure. Secondary hypertension must always be considered when the history or physical examination suggests a possible cause.
Renal artery stenosis increases in incidence with age. Its prevalence is reported to be as high as 50% in elderly patients with other signs of atherosclerosis such as widespread peripheral artery disease.19
Obstructive sleep apnea also commonly coexists with hypertension and its prevalence also increases with age. In addition, elderly patients with obstructive sleep apnea have a higher incidence of cardiovascular complications, including hypertension, than middle-aged people.20 Numerous studies have found that the severity of obstructive sleep apnea corresponds with the likelihood of systemic hypertension.21–23 Randomized trials and meta-analyses have also concluded that effective treatment with continuous positive airway pressure reduces systemic blood pressure,24–27 although by less than antihypertensive medications do.
A causal relationship between obstructive sleep apnea and hypertension has not been established with certainty. It is recommended, however, that patients with resistant hypertension be screened for obstructive sleep apnea as a possible cause of their disease.
Other causes of secondary hypertension to keep in mind when evaluating patients who have inappropriate hypertension include thyroid disorders, alcohol and tobacco use, and chronic steroid or NSAID use. Pheochromocytoma and adrenal adenoma, though possible, are less prevalent in the elderly.
Case continued
Physical examination in the above patient revealed an epigastric systolic-diastolic bruit, a sign that, although not sensitive, is highly specific for renal artery stenosis, raising the suspicion of this condition. Duplex ultrasonography of the renal arteries confirmed this suspicion. The patient underwent angiography and revascularization, resulting in a distinct fall in, but not normalization of, his blood pressure.
Detecting and treating renal artery stenosis
Though we do not intend to detail the diagnostic approaches and treatments for the various causes of secondary hypertension, we need to briefly mention those for renal artery stenosis.
According to the 2006 ACC/AHA guidelines on peripheral artery disease,28 testing for renal artery stenosis is indicated only if a subsequent corrective procedure is a viable option.
Renal arteriography remains the gold standard for diagnosing renal artery stenosis. However, noninvasive imaging has largely replaced it.
Duplex Doppler ultrasonography, compared with angiography, has a sensitivity of 84% to 98% depending on operator experience, and a specificity of 62% to 99% for detecting renal artery stenosis.29 Some of its limiting factors are the time needed to do the study, its steep learning curve and operator-dependence, and interference with the results by body fat and intestinal gas.
Computed tomographic angiography has a sensitivity of over 90% for detecting renal artery stenosis, and its specificity has been shown to be as high as 99% in certain studies.29 Use of contrast can be a limiting factor in some clinical settings.
Magnetic resonance angiography also offers a sensitivity of 90% to 100% and specificities of 76% to 94% for detecting renal artery stenosis.29 On the other hand, it is costly, and the gadolinium contrast solution used is nephrotoxic, though not as toxic as the contrast used in computed tomographic angiography.
As previously stated, these imaging studies should be used only if corrective measures will be undertaken if clinically significant renal artery stenosis is found. Even in such cases, revascularization may not be curative in all cases. Its effectiveness has been compared with that of medical management alone in a number of studies.30,31 A meta-analysis32 of six key trials involving more than 1,200 patients showed no difference in systolic and diastolic blood pressures and other clinical outcomes, including all-cause mortality, between the two treatment groups over a 29-month follow-up period.
Hence, although we advise that causes of secondary hypertension be considered in cases of inappropriate hypertension, aggressive management must be pursued on a case-by-case basis.
CASE 2: DRUG ADVERSE EFFECTS
A 75-year-old Hispanic woman with a history of treated breast cancer was recently diagnosed with hypertension. Her blood pressure is controlled on amlodipine (Norvasc) 10 mg daily, and her blood pressure today is 128/80 mm Hg. Her only complaint during this office visit is some swelling of her ankles.
Edema and dihydropyridine calcium channel blockers
Like all drugs, antihypertensive medications come with their own set of adverse effects. These are more common as people age—hence the importance of identifying and effectively managing them in the elderly population.
Calcium channel blockers, especially the dihydropyridines—ie, nifedipine (Adalat), amlodipine, felodipine (Plendil), and isradipine (DynaCirc)—are known to cause peripheral vasodilation. Peripheral edema is a common dose-related effect in people on these drugs. In one study, median leg weight increased by 80 g after amlodipine 5 mg was given for 4 weeks, and by another 68 g on a 10-mg dose.33
Ankle swelling, encountered more in women, can be very bothersome. The swelling is related to hyperfiltration of fluid into the interstitial space secondary to intracapillary hypertension. Calcium channel blockers predominantly cause arteriolar dilation by paralyzing the precapillary sphincter, thereby elevating intracapillary pressure.
Traditionally, physicians have lowered the dose of the calcium channel blocker, switched to another drug, or added a diuretic to alleviate the swelling. However, giving a diuretic for edema induced by a calcium channel blocker may not relieve the edema.34
Peripheral edema is much less encountered when a calcium channel blocker is given with an inhibitor of the renin-angiotensin system.35 A meta-analysis concluded that the incidence of peripheral edema was lowered by 38% with such a combination. The same study found angiotensin-converting enzyme (ACE) inhibitors significantly more efficacious for this effect than angiotensin receptor blockers (ARBs).35
ACE inhibitors and ARBs are known to cause venodilation, thereby lowering intra-capillary pressure. It is probable that this effect helps remove the extra fluid sequestered in the capillary bed by the arteriolar dilation from the calcium channel blocker.
Pedal edema associated with use of a calcium channel blocker occurs much more commonly in the elderly than in the young. It is clearly dose-dependent, and the incidence peaks after 6 months of therapy. In the patient described above, adding a low dose of an ACE inhibitor or an ARB (if the patient is ACE inhibitor-intolerant) should relieve the swelling.
Hyponatremia and diuretics
Electrolyte imbalances are another common problem encountered in the elderly. Even though for years attention has been directed to the potassium level, hyponatremia has been equally associated with adverse effects in the elderly, such as an increased risk of fractures as shown in the Rotterdam study.36
In 180 hypertensive inpatients, mean age 76.4, Sharabi et al37 found the incidence of hyponatremia to be three times higher in women than in men (odds ratio 3.10, 95% confidence interval 2.07 to 4.67). Patients were 10 times more likely to be affected after age 65 and 14 times more likely after age 75. Most of the patients affected (74.5%) used a thiazide-type diuretic. Even though in many of the patients diuretics were used for more than 1 year before hyponatremia developed, susceptible patients—such as the frail elderly—can develop severe hyponatremia within days of starting to use a thiazide.38
Severe hyponatremia is potentially life-threatening. Most cases are caused by thiazide rather than loop diuretics.38 Thiazides inhibit electrolyte transport at the cortical diluting sites. As they decrease the glomerular filtration rate acutely, they increase proximal water reabsorption (making the plasma hypotonic), reducing water delivery distally. Loop diuretics, on the other hand, have their main effect at the thick ascending limb, reducing the osmolality at the medullary interstitium and not affecting proximal water reabsorption. Additionally, loop diuretics have a shorter half-life than thiazides, which makes hyponatremia more likely to happen with thiazides.
In patients who develop hyponatremia secondary to diuretic use, appropriate treatment includes stopping the medication, restricting water intake, and repleting electrolyte stores.38 As with any cause of chronic hyponatremia, correction must be cautiously monitored and not hastily done.
Therefore, we advise adding a thiazide diuric with caution in the elderly, and we advise avoiding thiazides in patients with high water or alcohol intake.
CASE 3: DEMENTIA AND HYPERTENSION
A 74-year-old man with long-standing hypertension, gout, and chronic obstructive pulmonary disease was recently diagnosed with Alzheimer dementia. He takes enalapril (Vasotec) 10 mg daily for his blood pressure. His blood pressure is 130/78 mm Hg.
Dementia is one of the most important and common neurologic disorders in the elderly. With the rise in average life expectancy, its magnitude has grown to cause a substantial emotional and economic burden on society and health care.
Midlife hypertension has been demonstrated to be an important modifiable risk factor for late-life cognitive decline,39 mild cognitive impairment,40 and dementia of all causes.41 It has been suggested that hypertension might be part of the pathogenesis of dementia, and targeting high blood pressure might prevent its onset.
Moreover, a significant reduction in both Alzheimer and vascular dementia was demonstrated (risk reduction 55%) with the use of a long-acting dihydropyridine calcium channel blocker (nitrendipine) in the Syst-Eur study.42 However, data from studies such as Systolic Hypertension in the Elderly Program (SHEP) and the HYVET substudy of cognitive function assessement43 showed no difference in dementia between placebo and active therapy with chlorthalidone (Hygroton) (in SHEP) or indapamide (Lozol) (in the HYVET substudy).
Disorders of calcium homeostasis are associated with the brain’s aging process. Probably, the neuroprotective effect of nitrendipine seen in Syst-Eur was due to its ability to affect this process, independent of its blood pressure-lowering effect.
In another prospective study, people over 60 years of age who complained of subjective memory loss showed a significant and positive association between memory scores and the use of calcium channel blockers (+0.14 ± 0.09 in users vs −0.12 ± 0.06 in nonusers; P = .016) independently of age, sex, white matter hyperintensities, and carotid wall cross-sectional area, all of which were associated with worse memory scores.44
Drugs that block the renin-angiotensin system have also been proposed to delay the onset and slow the progression of dementia.45 A small randomized, controlled trial suggested that centrally active ACE inhibitors—those that cross the blood-brain barrier, such as captopril (Capoten), lisinopril (Prinivil), ramipril (Altace), and fosinopril (Monopril)—slow cognitive decline in Alzheimer dementia more than non-centrally active ACE inhibitors or calcium channel blockers.46
Sink et al47 examined data from participants in the Cognition Substudy of the Cardiovascular Health Study48 on the effect of ACE inhibitors on cognitive decline. ACE inhibitors, as a class, showed no benefit in reducing the risk of dementia compared with other antihypertensive drug classes. However, centrally active ACE inhibitors, compared with other medications, were associated with a 65% reduction in cognitive decline per year of drug exposure (P = .01). Non-centrally active ACE inhibitors worsened cognitive decline.
It appears that the brain’s renin-angiotensin system plays a role in the pathogenesis of dementia. Indeed, ACE has been shown to degrade amyloid-beta protein, and its level was increased in brain tissue of Alzheimer patients postmortem.49
The relationship between blood pressure and cognitive function appears to be curvilinear, so that low blood pressure in late life is also associated with dementia and Alzheimer dementia.50 In 5,816 patients age 65 and older, Morris et al51 calculated the percentile scores of four cognitive tests according to the level of blood pressure. Patients with systolic blood pressures of 100 mm Hg, 120 mm Hg, and 180 mm Hg scored lower on the Mini Mental State Examination than those in the 140 to 160 mm Hg range. Patients with diastolic blood pressures between 80 and 90 mm Hg appeared to have the best cognitive function. This further emphasizes that blood pressure control must be pursued in the very elderly, albeit less aggressively. The MIND substudy of the SPRINT trial9 is likely to shed more light on this relationship.
When needed for optimal blood pressure control in a hypertensive patient at risk of dementia, a calcium channel blocker of the dihydropyridine type or a centrally active ACE inhibitor, or both, is preferable.
CASE 4: LABILE HYPERTENSION
A 74-year-old man with hypertension and diabetes mellitus comes to see you in the office. On physical examination, his blood pressure is 175/110 mm Hg. His blood pressure during his last visit 3 months ago was 120/75. He brings a log with him that shows random fluctuations in his blood pressure readings. He takes hydrochlorothiazide 25 mg daily for his blood pressure.
Hypertension in some patients continuously fluctuates between low and high levels. A study in Canada found that up to 15% of all adult hypertensive patients might have labile hypertension.52 In the presence of a normal average blood pressure, visit-to-visit blood pressure variability is usually considered a trivial matter. However, some but not all studies have shown that such visit-to-visit variability in blood pressure is an independent predictor of future cardiovascular events in hypertensive patients, independent of the mean systolic blood pressure.52–54
Blood pressure fluctuates from heartbeat to heartbeat, from morning to night, from winter to summer, and from sitting to standing, and it is prone to increase with exertion, stress, and other factors. But excessive fluctuations in the elderly are most likely the result of excessive stiffness of the arterial tree and a decrease in the windkessel (cushioning) function of the aorta. As a consequence, even small-volume changes in the intravascular system can trigger large blood pressure fluctuations.
There is some evidence that antihypertensive drug classes may differ in their effects on visit-to-visit blood pressure variability. In a 2010 study comparing the effects of different antihypertensive drugs on blood pressure variation, calcium channel blockers and non-loop diuretics were associated with less variation in systolic blood pressure, and calcium channel blockers reduced it the most.55
In the patient described above, switching to a low-dose calcium channel blocker with a thorough follow-up is a reasonable plan.
CASE 5: ORTHOSTATIC HYPOTENSION
A 73-year-old woman with long-standing hypertension complains of some dizziness, especially when getting out of bed in the morning. On physical examination, her blood pressure is 134/100 mm Hg sitting and 115/90 standing. She takes amlodipine 10 mg daily, enalapril 10 mg daily, and chlorthalidone 25 mg daily. Chlorthalidone had been added on her last visit 1 month before.
As a result of the increase in the number of elderly patients with hypertension and guidelines recommending better control in this age group, the number of elderly patients on anti-hypertensive drugs has risen significantly. At the same time, the elderly have increasingly presented with adverse effects of treatment.
Orthostatic hypotension is a drop in systolic pressure of 20 mm Hg or more or a drop in diastolic pressure of 10 mm Hg or more on standing, with or without symptoms. These are caused by cerebral hypoperfusion and include dizziness, lightheadedness, generalized weakness, visual blurring, and, in severe cases, syncope.
Alpha-blockers and nitrates have been most commonly implicated in causing orthostatic hypotension, due to venous pooling. Clearly, not all antihypertensive drugs are equal with regard to their venodilatory effects. Thiazide diuretics, by causing fluid volume depletion, and beta-blockers, by interfering with compensatory cardioacceleration with upright posture, are also commonly involved in causing an excessive blood pressure drop with standing.
Systolic orthostatic hypotension has been shown to be a significant and independent predictor of cardiovascular morbidity and death.56 Moreover, syncope and subsequent falls are an important cause of injury and death in the elderly.57 The clinical combination of hypertension and orthostatic hypotension is, therefore, especially challenging. A compromise between accepting a higher cardiovascular risk at either end of the spectrum with an added higher risk for fall at the lower end has to be made.
To prevent orthostatic hypotension in the elderly, it is important to avoid prescribing high-risk drugs. When starting antihypertensive therapy, a low dose should be used, and the dose should be titrated upward very slowly and cautiously. If orthostatic hypotension is suggested by the history or by the orthostatic test, which is warranted in all elderly hypertensive patients before starting or significantly altering therapy, the potential culprit drug should be withdrawn and the patient reassessed. Improved hydration, elevating the head of the bed, and taking the antihypertensive drug at night are ways to improve symptoms, but these remain largely unproven.
In this patient, the newly added chlorthalidone was stopped, and her symptoms improved.
PSEUDOHYPERTENSION
Since hypertension is defined by a numerical value, it is prudent that this value be accurate. Treating a falsely high reading or leaving a falsely low reading untreated will predispose the elderly patient to increased risk either way. One rare condition in the elderly that can give a falsely high blood pressure reading is pseudohypertension.
Pseudohypertension is a condition in which indirect blood pressure measured by the cuff method overestimates the true intra-arterial blood pressure due to marked underlying arteriosclerosis. The Osler maneuver can be used to differentiate true hypertension from pseudohypertension.58 This is performed by palpating the pulseless radial or brachial artery distal to the inflated cuff. If the artery is palpable despite being pulseless, the patient is said to be “Osler-positive” and likely has pseudohypertension.58
Pseudohypertension should be suspected if the patient has orthostatic hypotension despite normal blood pressure sitting and standing. Also, elevated blood pressure without appropriate target organ disease should raise the suspicion of pseudohypertension. Apart from the Osler maneuver, measuring the intraarterial pressure can confirm this diagnosis.
- US Census Bureau. The 2011 statistical abstract. The national data book. http://www.census.gov/compendia/statab/2011/2011edition.html. Accessed July 24, 2012.
- He W, Sengupta M, Velkoff VA, DeBarros KA. US Census Bureau. Current Population Reports, P23-209. 65+ in the United States: 2005. http://www.census.gov/prod/2006pubs/p23-209.pdf. Accessed July 24, 2012.
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet 2009; 374:1196–1208.
- Messerli FH, Sundgaard-Riise K, Ventura HO, Dunn FG, Glade LB, Frohlich ED. Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet 1983; 2:983–986.
- Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000; 355:865–872.
- Schall P, Wehling M. Treatment of arterial hypertension in the very elderly: a meta-analysis of clinical trials. Arzneimittelforschung 2011; 61:221–228.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Soc Hypertens 2011; 5:259–352.
- Wake Forest University, Winston-Salem NC. SPRINT: Systolic Blood Pressure Intervention Trial. http://www.sprinttrial.org/public/dspHome.cfm. Accessed July 24, 2012.
- Sacks FM, Svetkey LP, Vollmer WM, et al; DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001; 344:3–10.
- Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 2002; 136:493–503.
- Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ; DASH Collaborative Research Group. A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: results of the DASH-Sodium Trial. Am J Cardiol 2004; 94:222–227.
- Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med 2001; 161:685–693.
- Frisoli TM, Schmieder RE, Grodzicki T, Messerli FH. Salt and hypertension: is salt dietary reduction worth the effort? Am J Med 2012; 125:433–439.
- National High Blood Pressure Education Program. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: National Heart, Lung, and Blood Institute; 2004. http://www.ncbi.nlm.nih.gov/books/NBK9630/Accessed July 30, 2012.
- Mitka M. New guidance covers ways to prevent and treat hypertension in elderly patients. JAMA 2011; 305:2394,2398.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- Viera AJ, Neutze DM. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician 2010; 82:1471–1478.
- Rihal CS, Textor SC, Breen JF, et al. Incidental renal artery stenosis among a prospective cohort of hypertensive patients undergoing coronary angiography. Mayo Clin Proc 2002; 77:309–316.
- Wang Y, Li Y. [Clinical and polysomnographic characteristics in elderly patients with obstructive sleep apnea hypopnea syndrome]. [In Chinese] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008; 22:222–225.
- Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ 2000; 320:479–482.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med 2000; 160:2289–2295.
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002; 359:204–210.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107:68–73.
- Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive air-way pressure on blood pressure: a placebo trial. Hypertension 2000; 35:144–147.
- Sharma SK, Agrawal S, Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med 2011; 365:2277–2286.
- White CJ, Jaff MR, Haskal ZJ, et al; American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Indications for renal arteriography at the time of coronary arteriography: a science advisory from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Councils on Cardiovascular Radiology and Intervention and on Kidney in Cardiovascular Disease. Circulation 2006; 114:1892–1895.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol 2006; 47:1239–1312.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–848,W150–W151.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Kumbhani DJ, Bavry AA, Harvey JE, et al. Clinical outcomes after percutaneous revascularization versus medical management in patients with significant renal artery stenosis: a meta-analysis of randomized controlled trials. Am Heart J 2011; 161:622.e1–630.e1.
- Pedrinelli R, Dell’Omo G, Melillo E, Mariani M. Amlodipine, enalapril, and dependent leg edema in essential hypertension. Hypertension 2000; 35:621–625.
- van Hamersvelt HW, Kloke HJ, de Jong DJ, Koene RA, Huysmans FT. Oedema formation with the vasodilators nifedipine and diazoxide: direct local effect or sodium retention? J Hypertens 1996; 14:1041–1045.
- Makani H, Bangalore S, Romero J, Wever-Pinzon O, Messerli FH. Effect of renin-angiotensin system blockade on calcium channel blocker-associated peripheral edema. Am J Med 2011; 124:128–135.
- Hoorn EJ, Rivadeneira F, van Meurs JB, et al. Mild hyponatremia as a risk factor for fractures: the Rotterdam Study. J Bone Miner Res 2011; 26:1822–1828.
- Sharabi Y, Illan R, Kamari Y, et al. Diuretic induced hyponatraemia in elderly hypertensive women. J Hum Hypertens 2002; 16:631–635.
- Spital A. Diuretic-induced hyponatremia. Am J Nephrol 1999; 19:447–452.
- Knopman D, Boland LL, Mosley T, et al; Atherosclerosis Risk in Communities (ARIC) Study Investigators. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol 2007; 64:1734–1740.
- Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging 2000; 21:49–55.
- Forette F, Seux ML, Staessen JA, et al Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 1998; 352:1347–1351.
- Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 2008. www.thelancet.com/journals/laneur/article/PIIS1474-4422(08)70143-1/fulltext. Accessed August 23, 2012.
- Watfa G, Rossignol P, Kearney-Schwartz A, et al. Use of calcium channel blockers is associated with better cognitive performance in older hypertensive patients with subjective memory complaints. J Hypertens 2010; 28:2485–2493.
- Tzourio C, Anderson C, Chapman N, et al; PROGRESS Collaborative Group. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 2003; 163:1069–1075.
- Ohrui T, Tomita N, Sato-Nakagawa T, et al. Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology 2004; 63:1324–1325.
- Sink KM, Leng X, Williamson J, et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med 2009; 169:1195–1202.
- Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology 2003; 22:1–12.
- Hemming ML, Selkoe DJ. Amyloid beta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J Biol Chem 2005; 280:37644–37650.
- Nilsson SE, Read S, Berg S, Johansson B, Melander A, Lindblad U. Low systolic blood pressure is associated with impaired cognitive function in the oldest old: longitudinal observations in a population-based sample 80 years and older. Aging Clin Exp Res 2007; 19:41–47.
- Morris MC, Scherr PA, Hebert LE, et al. Association between blood pressure and cognitive function in a biracial community population of older persons. Neuroepidemiology 2002; 21:123–130.
- Joffres MR, Hamet P, Rabkin SW, Gelskey D, Hogan K, Fodor G. Prevalence, control and awareness of high blood pressure among Canadian adults. Canadian Heart Health Surveys Research Group. CMAJ 1992; 146:1997–2005.
- Schillaci G, Pucci G. The importance of instability and visit-to-visit variability of blood pressure. Expert Rev Cardiovasc Ther 2010; 8:1095–1097.
- Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-visit blood pressure variability, carotid atherosclerosis, and cardiovascular events in the European Lacidipine Study on Atherosclerosis. Circulation 2012; 126:569–578.
- Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 2010; 375:906–915.
- Fagard RH, De Cort P. Orthostatic hypotension is a more robust predictor of cardiovascular events than nighttime reverse dipping in elderly. Hypertension 2010; 56:56–61.
- Kearney F, Moore A. Treatment of combined hypertension and orthostatic hypotension in older adults: more questions than answers still remain. Expert Rev Cardiovasc Ther 2009; 7:557–560.
- Messerli FH, Ventura HO, Amodeo C. Osler’s maneuver and pseudohypertension. N Engl J Med 1985; 312:1548–1551.
- US Census Bureau. The 2011 statistical abstract. The national data book. http://www.census.gov/compendia/statab/2011/2011edition.html. Accessed July 24, 2012.
- He W, Sengupta M, Velkoff VA, DeBarros KA. US Census Bureau. Current Population Reports, P23-209. 65+ in the United States: 2005. http://www.census.gov/prod/2006pubs/p23-209.pdf. Accessed July 24, 2012.
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet 2009; 374:1196–1208.
- Messerli FH, Sundgaard-Riise K, Ventura HO, Dunn FG, Glade LB, Frohlich ED. Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet 1983; 2:983–986.
- Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000; 355:865–872.
- Schall P, Wehling M. Treatment of arterial hypertension in the very elderly: a meta-analysis of clinical trials. Arzneimittelforschung 2011; 61:221–228.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Soc Hypertens 2011; 5:259–352.
- Wake Forest University, Winston-Salem NC. SPRINT: Systolic Blood Pressure Intervention Trial. http://www.sprinttrial.org/public/dspHome.cfm. Accessed July 24, 2012.
- Sacks FM, Svetkey LP, Vollmer WM, et al; DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001; 344:3–10.
- Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 2002; 136:493–503.
- Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ; DASH Collaborative Research Group. A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: results of the DASH-Sodium Trial. Am J Cardiol 2004; 94:222–227.
- Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med 2001; 161:685–693.
- Frisoli TM, Schmieder RE, Grodzicki T, Messerli FH. Salt and hypertension: is salt dietary reduction worth the effort? Am J Med 2012; 125:433–439.
- National High Blood Pressure Education Program. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: National Heart, Lung, and Blood Institute; 2004. http://www.ncbi.nlm.nih.gov/books/NBK9630/Accessed July 30, 2012.
- Mitka M. New guidance covers ways to prevent and treat hypertension in elderly patients. JAMA 2011; 305:2394,2398.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- Viera AJ, Neutze DM. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician 2010; 82:1471–1478.
- Rihal CS, Textor SC, Breen JF, et al. Incidental renal artery stenosis among a prospective cohort of hypertensive patients undergoing coronary angiography. Mayo Clin Proc 2002; 77:309–316.
- Wang Y, Li Y. [Clinical and polysomnographic characteristics in elderly patients with obstructive sleep apnea hypopnea syndrome]. [In Chinese] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008; 22:222–225.
- Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ 2000; 320:479–482.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med 2000; 160:2289–2295.
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002; 359:204–210.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107:68–73.
- Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive air-way pressure on blood pressure: a placebo trial. Hypertension 2000; 35:144–147.
- Sharma SK, Agrawal S, Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med 2011; 365:2277–2286.
- White CJ, Jaff MR, Haskal ZJ, et al; American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Indications for renal arteriography at the time of coronary arteriography: a science advisory from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Councils on Cardiovascular Radiology and Intervention and on Kidney in Cardiovascular Disease. Circulation 2006; 114:1892–1895.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol 2006; 47:1239–1312.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–848,W150–W151.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Kumbhani DJ, Bavry AA, Harvey JE, et al. Clinical outcomes after percutaneous revascularization versus medical management in patients with significant renal artery stenosis: a meta-analysis of randomized controlled trials. Am Heart J 2011; 161:622.e1–630.e1.
- Pedrinelli R, Dell’Omo G, Melillo E, Mariani M. Amlodipine, enalapril, and dependent leg edema in essential hypertension. Hypertension 2000; 35:621–625.
- van Hamersvelt HW, Kloke HJ, de Jong DJ, Koene RA, Huysmans FT. Oedema formation with the vasodilators nifedipine and diazoxide: direct local effect or sodium retention? J Hypertens 1996; 14:1041–1045.
- Makani H, Bangalore S, Romero J, Wever-Pinzon O, Messerli FH. Effect of renin-angiotensin system blockade on calcium channel blocker-associated peripheral edema. Am J Med 2011; 124:128–135.
- Hoorn EJ, Rivadeneira F, van Meurs JB, et al. Mild hyponatremia as a risk factor for fractures: the Rotterdam Study. J Bone Miner Res 2011; 26:1822–1828.
- Sharabi Y, Illan R, Kamari Y, et al. Diuretic induced hyponatraemia in elderly hypertensive women. J Hum Hypertens 2002; 16:631–635.
- Spital A. Diuretic-induced hyponatremia. Am J Nephrol 1999; 19:447–452.
- Knopman D, Boland LL, Mosley T, et al; Atherosclerosis Risk in Communities (ARIC) Study Investigators. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol 2007; 64:1734–1740.
- Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging 2000; 21:49–55.
- Forette F, Seux ML, Staessen JA, et al Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 1998; 352:1347–1351.
- Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 2008. www.thelancet.com/journals/laneur/article/PIIS1474-4422(08)70143-1/fulltext. Accessed August 23, 2012.
- Watfa G, Rossignol P, Kearney-Schwartz A, et al. Use of calcium channel blockers is associated with better cognitive performance in older hypertensive patients with subjective memory complaints. J Hypertens 2010; 28:2485–2493.
- Tzourio C, Anderson C, Chapman N, et al; PROGRESS Collaborative Group. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 2003; 163:1069–1075.
- Ohrui T, Tomita N, Sato-Nakagawa T, et al. Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology 2004; 63:1324–1325.
- Sink KM, Leng X, Williamson J, et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med 2009; 169:1195–1202.
- Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology 2003; 22:1–12.
- Hemming ML, Selkoe DJ. Amyloid beta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J Biol Chem 2005; 280:37644–37650.
- Nilsson SE, Read S, Berg S, Johansson B, Melander A, Lindblad U. Low systolic blood pressure is associated with impaired cognitive function in the oldest old: longitudinal observations in a population-based sample 80 years and older. Aging Clin Exp Res 2007; 19:41–47.
- Morris MC, Scherr PA, Hebert LE, et al. Association between blood pressure and cognitive function in a biracial community population of older persons. Neuroepidemiology 2002; 21:123–130.
- Joffres MR, Hamet P, Rabkin SW, Gelskey D, Hogan K, Fodor G. Prevalence, control and awareness of high blood pressure among Canadian adults. Canadian Heart Health Surveys Research Group. CMAJ 1992; 146:1997–2005.
- Schillaci G, Pucci G. The importance of instability and visit-to-visit variability of blood pressure. Expert Rev Cardiovasc Ther 2010; 8:1095–1097.
- Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-visit blood pressure variability, carotid atherosclerosis, and cardiovascular events in the European Lacidipine Study on Atherosclerosis. Circulation 2012; 126:569–578.
- Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 2010; 375:906–915.
- Fagard RH, De Cort P. Orthostatic hypotension is a more robust predictor of cardiovascular events than nighttime reverse dipping in elderly. Hypertension 2010; 56:56–61.
- Kearney F, Moore A. Treatment of combined hypertension and orthostatic hypotension in older adults: more questions than answers still remain. Expert Rev Cardiovasc Ther 2009; 7:557–560.
- Messerli FH, Ventura HO, Amodeo C. Osler’s maneuver and pseudohypertension. N Engl J Med 1985; 312:1548–1551.
KEY POINTS
- Therapy should be considered in all aging hypertensive patients, even the very elderly (> 80 years old).
- Most antihypertensive drugs can be used as first-line treatment in the absence of a compelling indication for a specific class, with the possible exception of alpha-blockers and beta-blockers.
- An initial goal of less than 140/90 mm Hg is reasonable in elderly patients, and an achieved systolic blood pressure of 140 to 145 mm Hg is acceptable in octogenarians.
- Start with low doses; titrate upward slowly; and monitor closely for adverse effects.
- Thiazide diuretics should be used with caution in the elderly because of the risk of hyponatremia.