User login
Continuous Cryotherapy vs Ice Following Total Shoulder Arthroplasty: A Randomized Control Trial
ABSTRACT
Postoperative pain management is an important component of total shoulder arthroplasty (TSA). Continuous cryotherapy (CC) has been proposed as a means of improving postoperative pain control. However, CC represents an increased cost not typically covered by insurance. The purpose of this study is to compare CC to plain ice (ICE) following TSA. The hypothesis was that CC would lead to lower pain scores and decreased narcotic usage during the first 2 weeks postoperatively.
A randomized controlled trial was performed to compare CC to ICE. Forty patients were randomized to receive either CC or ICE following TSA. The rehabilitation and pain control protocols were otherwise standardized. Visual analog scales (VAS) for pain, satisfaction with cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery. Narcotic usage in morphine equivalents was also recorded.
No significant differences in preoperative pain (5.9 vs 6.8; P = .121), or postoperative pain at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593) or 14 days (2.5 vs 2.7; P = .742) were observed between the CC and ICE groups. Similarly, no differences in quality of sleep, satisfaction with the cold therapy, or narcotic usage at any time interval were observed between the 2 groups.
No differences in pain control, quality of sleep, patient satisfaction, or narcotic usage were detected between CC and ICE following TSA. CC may offer convenience as an advantage, but the increased cost associated with this type of treatment may not be justified.
The number of total shoulder arthroplasties (TSAs) performed annually is increasing dramatically.1 At the same time, there has been a push toward decreased length of hospital stay and earlier mobilization following joint replacement surgery. Central to these goals is adequate pain control. Multimodal pain pathways exist, and one of the safest and cheapest methods of pain control is cold therapy, which can be accomplished with continuous cryotherapy (CC) or plain ice (ICE).
Continue to: The mechanism of cryotherapy...
The mechanism of cryotherapy for controlling pain is poorly understood. Cryotherapy reduces leukocyte migration and slows down nerve signal transmission, which reduces inflammation, thereby producing a short-term analgesic effect. Stalman and colleagues2 reported on a randomized control study that evaluated the effects of postoperative cooling after knee arthroscopy. Measurements of metabolic and inflammatory markers in the synovial membrane were used to assess whether cryotherapy provides a temperature-sensitive release of prostaglandin E2. Cryotherapy lowered the temperature in the postoperative knee, and synovial prostaglandin concentrations were correlated with temperature. Because prostaglandin is a marker of inflammation and pain, the conclusion was that postoperative cooling appeared to have an anti-inflammatory effect.
The knee literature contains multiple studies that have examined the benefits of cryotherapy after both arthroscopic and arthroplasty procedures. The clinical benefits on pain have been equivocal with some studies showing improvements using cryotherapy3,4 and others showing no difference in the treatment group.5,6
Few studies have examined cryotherapy for the shoulder. Speer and colleagues7 demonstrated that postoperative use of CC was effective in reducing recovery time after shoulder surgery. However; they did not provide an ICE comparative group and did not focus specifically on TSA. In another study, Kraeutler and colleagues8 examined only arthroscopic shoulder surgery cases in a randomized prospective trial and found no significant different between CC and ICE. They concluded that there did not appear to be a significant benefit in using CC over ICE for arthroscopic shoulder procedures.
The purpose of this study is to prospectively evaluate CC and ICE following TSA. The hypothesis was that CC leads to improved pain control, less narcotic consumption, and improved quality of sleep compared to ICE in the immediate postoperative period following TSA.
MATERIALS AND METHODS
This was a prospective randomized control study of patients undergoing TSA receiving either CC or ICE postoperatively. Institutional Review Board approval was obtained before commencement of the study. Inclusion criteria included patients aged 30 to 90 years old undergoing a primary or revision shoulder arthroplasty procedure between June 2015 and January 2016. Exclusion criteria included hemiarthroplasty procedures.
Continue to: Three patients refused...
Three patients refused to participate in the study. Enrollment was performed until 40 patients were enrolled in the study (20 patients in each group). Randomization was performed with a random number generator, and patients were assigned to a treatment group following consent to participate. Complete follow-up was available for all patients. There were 13 (65%) male patients in the CC group. The average age of the CC group at the time of surgery was 68.7 years (range). There were 11 male patients in the ICE group. The average age of the ICE group at the time of surgery was 73.2 years (range). The dominant extremity was involved in 9 (45%) patients in the CC group and in 11 patients (55%) in the ICE group. Surgical case specifics are summarized in Table 1.
Table 1. Summary of Surgical Cases
| CC group (n = 20) | ICE group (n = 20) |
Primary TSA | 7 (35%) | 9 (45%) |
Primary RSA | 12 (60%) | 9 (45%) |
Revision arthroplasty | 1 (5%) | 2 (10%) |
Abbreviations: CC, continuous cryotherapy; ICE, plain ice; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty.
All surgeries were performed by Dr. Denard. All patients received a single-shot interscalene nerve block prior to the procedure. A deltopectoral approach was utilized, and the subscapularis was managed with the peel technique.9 All patients were admitted to the hospital following surgery. Standard postoperative pain control consisted of as-needed intravenous morphine (1-2 mg every 2 hours, as needed) or an oral narcotic (hydrocodone/acetaminophen 5/325mg, 1-2 every 4 hours, as needed) which was also provided at discharge. However, total narcotic usage was recorded in morphine equivalents to account for substitutions. No non-steroidal anti-inflammatory drugs were allowed until 3 months postoperatively.
The CC group received treatment from a commercially available cryotherapy unit (Polar Care; Breg). All patients received instructions by a medical professional on how to use the unit. The unit was applied immediately postoperatively and set at a temperature of 45°F to 55°F. Patients were instructed to use the unit continuously during postoperative days 0 to 3. This cryotherapy was administered by a nurse while in the hospital but was left to the responsibility of the patient upon discharge. Patients were instructed to use the unit as needed for pain control during the day and continuously while asleep from days 4 to14.
The ICE group used standard ice packs postoperatively. The patients were instructed to apply an ice pack for 20 min every 2 hours while awake during days 0 to 3. This therapy was administered by a nurse while in the hospital but left to the responsibility of the patient upon discharge. Patients were instructed to use ice packs as needed for pain control during the day at a maximum of 20 minutes per hour on postoperative days 4 to 14. Compliance by both groups was monitored using a patient survey after hospital discharge. The number of hours that patients used either the CC or ICE per 24-hour period was recorded at 24 hours, 3 days, 7 days, and 14 days. The nursing staff recorded the number of hours of use of either cold modality for each patient prior to hospital discharge. The average length of stay as an inpatient was 1.2 days for the CC group and 1.3 days for the ICE group.
Visual analog scales (VAS) for pain, satisfaction with the cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery.
Continue to: The Wilcoxon rank-sum test...
STATISTICAL METHOD
The Wilcoxon rank-sum test was used to assess whether scores changed significantly from the preoperative period to the different postoperative time intervals, as well as to assess the values for pain, quality of sleep, and patient satisfaction. P-values <.05 were considered significant.
RESULTS
No differences were observed in the baseline characteristics between the 2 groups. Both groups showed improvements in pain, quality of sleep, and satisfaction with the cold therapy from the preoperative period to the final follow-up.
The VAS pain scores were not different between the CC and ICE groups preoperatively (5.9 vs 6.8; P = .121) or postoperatively at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593), or 14 days (2.5 vs 2.7; P = .742). Both cohorts demonstrated improved overall pain throughout the study period. These findings are summarized in Table 2.
Table 2. Summary of VAS Pain Scores With Cold Therapy
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
Preoperative | 5.9 ± 4.1 | 6.8 ± 5.3 | .121 | 3.3-8.3 |
24 hours | 4.2 ± 3.0 | 4.3 ± 3.1 | .989 | 2.9-5.7 |
3 days | 4.8 ± 2.7 | 4.7 ± 3.2 | .944 | 3.2-6.3 |
7 days | 2.9 ± 1.8 | 3.3 ± 2.5 | .593 | 2.1-4.4 |
14 days | 2.5 ± 2.1 | 2.7 ± 1.8 | .742 | 1.5-3.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
The number of morphine equivalents of pain medication was not different between the CC and ICE groups postoperatively at 24 hours (43 vs 38 mg; P = .579), 3 days (149 vs 116 mg; P = .201), 7 days (308 vs 228 mg; P = .181), or 14 days (431 vs 348 mg; P = .213). Both groups showed increased narcotic consumption from 24 hours postoperatively until the 2-week follow-up. Narcotic consumption is summarized in Table 3.
Table 3. Summary of Narcotic Consumption in Morphine Equivalents
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 43.0 ± 36.7 | 38.0 ± 42.9 | .579 | 17.9-60.1 |
3 days | 149.0 ± 106.5 | 116.3 ± 108.9 | .201 | 63.4-198.7 |
7 days | 308.1 ± 234.0 | 228 ± 258.3 | .181 | 107.1-348.9 |
14 days | 430.8 ± 384.2 | 347.5 ± 493.4 | .213 | 116.6-610.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice.
VAS for quality of sleep improved in both groups from 24 hours postoperatively until the final follow-up. However, no significant differences in sleep quality were observed between the CC and ICE groups postoperatively at 24 hours (5.1 vs 4.3; P = .382), 3 days (5.1 vs 5.3; P = .601), 7 days (6.0 vs 6.7; P = .319), or 14 days (6.5 vs 7.1; P = .348). The VAS scores for sleep quality are reported in Table 4.
Table 4. Summary of VAS Sleep Quality With Cold Therapya
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 5.1 ± 2.8 | 4.3 ± 2.4 | .382 | 3.2-6.4 |
3 days | 5.1 ± 1.9 | 5.3 ± 2.3 | .601 | 4.2-6.5 |
7 days | 6.0 ± 2.3 | 6.7 ± 2.1 | .319 | 4.9-7.7 |
14 days | 6.5 ± 2.3 | 7.1 ± 2.5 | .348 | 5.3-8.4 |
a0-10 rating with 10 being the highest possible score.
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
Continue to: Finally, VAS patient satisfaction...
Finally, VAS patient satisfaction scores were not different between the CC and ICE groups postoperatively at 24 hours (7.3 vs 6.1; P = .315), 3 days (6.1 vs 6.6; P = .698), 7 days (6.6 vs 6.9; P = .670), or 14 days (7.1 vs 6.3; P = .288).
While compliance within each group utilizing the randomly assigned cold modality was similar, the usage by the CC group was consistently higher at all time points recorded. No complications or reoperations were observed in either group.
DISCUSSION
The optimal method for managing postoperative pain from an arthroplasty procedure is controversial. This prospective randomized study attempted to confirm the hypothesis that CC infers better pain control, improves quality of sleep, and decreases narcotic usage compared to ICE in the first 2 weeks after a TSA procedure. The results of this study refuted our hypothesis, demonstrating no significant difference in pain control, satisfaction, narcotic usage, or sleep quality between the CC and ICE cohorts at all time points studied.
Studies on knees and lower extremities demonstrate equivocal results for the role CC plays in providing improved postoperative pain control. Thienpont10 evaluated CC in a randomized control trial comparing plain ice packs postoperatively in patients who underwent TKA. The author found no significant difference in VAS for pain or narcotic consumption in morphine equivalents. Thienpont10 recommended that CC not be used for outpatient knee arthroplasty as it is an additional cost that does not improve pain significantly. Healy and colleagues5 reported similar results that CC did not demonstrate a difference in narcotic requirement or pain control compared to plain ice packs, as well as no difference in local postoperative swelling or wound drainage. However, a recently published randomized trial by Su and colleagues11 comparing a cryopneumatic device and ICE with static compression in patients who underwent TKA demonstrated significantly lower narcotic consumption and increased ambulation distances in the treatment group. The treatment group consumed approximately 170 mg morphine equivalents less than the control group between discharge and the 2-week postoperative visit. In addition, a significant difference was observed in the satisfaction scores in the treatment group.11 Similarly, a meta-analysis by Raynor and colleagues12 on randomized clinical trials comparing cryotherapy to a placebo group after anterior cruciate ligament reconstruction showed that cryotherapy is associated with significantly lower postoperative pain (P = .02), but demonstrated no difference in postoperative drainage (P = .23) or range of motion (P = .25).
Although multiple studies have been published regarding the efficacy of cryotherapy after knee surgery, very few studies have compared CC to conventional ICE after shoulder surgery. A prospective randomized trial was performed by Singh and colleagues13 to compare CC vs no ICE in open and arthroscopic shoulder surgery patients. Both the open and arthroscopic groups receiving CC demonstrated significant reductions in pain frequency and more restful sleep at the 7-day, 14-day, and 21-day intervals compared to the control group. However, they did not compare the commercial unit to ICE. In contrast, a study by Kraeutler and colleagues8 randomized 46 patients to receive either CC or ICE in the setting of arthroscopic shoulder surgery. Although no significant difference was observed in morphine equivalent dosage between the 2 groups, the CC group used more pain medication on every postoperative day during the first week after surgery. They found no difference between the 2 groups with regards to narcotic consumption or pain scores. The results of this study mirror those by Kraeutler and colleagues,8 demonstrating no difference in pain scores, sleep quality, or narcotic consumption.
Continue to: With rising costs in the US...
With rising costs in the US healthcare system, a great deal of interest has developed in the application of value-based principles to healthcare. Value can be defined as a gain in benefits over the costs expended.14 The average cost for a commercial CC unit used in this study was $260. A pack of ICE is a nominal cost. Based on the results of this study, the cost of the commercial CC device may not be justified when compared to the cost of an ice pack.
The major strengths of this study are the randomized design and multiple data points during the early postoperative period. However, there are several limitations. First, we did not objectively measure compliance of either therapy and relied only on a patient survey. Usage of the commercial CC unit in hours decreased over half between days 3 and 14. This occurred despite training on the application and specific instructions. We believe this reflects “real-world” usage, but it is possible that compliance affected our results. Second, all patients in this study had a single-shot interscalene block. While this is standard at our institution, it is possible that either CC or ICE would have a more significant effect in the absence of an interscalene block. Finally, we did not evaluate final outcomes in this study and therefore cannot determine if the final outcome was different between the 2 groups. Our goal was simply to evaluate the first 2 weeks following surgery, as this is the most painful period following TSA.
CONCLUSION
There was no difference between CC and ICE in terms of pain control, quality of sleep, patient satisfaction, or narcotic consumption following TSA. CC may offer convenience advantages, but the increased cost associated with this type of unit may not be justified.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/jbjs.j.01994.
2. Stalman A, Berglund L, Dungnerc E, Arner P, Fellander-Tsai L. Temperature sensitive release of prostaglandin E2 and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2016/jbjs.j.01790.
3. Levy AS, Marmar E. The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Relat Res. 1993;297:174-178. doi:10.1097/00003086-199312000-00029.
4. Webb JM, Williams D, Ivory JP, Day S, Williamson DM. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopaedics 1998;21(1):59-61.
5. Healy WL, Seidman J, Pfeifer BA, Brown DG. Cold compressive dressing after total knee arthroplasty. Clin Orthop Relat Res. 1994;299:143-146. doi:10.1097/00003086-199402000-00019.
6. Whitelaw GP, DeMuth KA, Demos HA, Schepsis A, Jacques E. The use of Cryo/Cuff versus ice and elastic wrap in the postoperative care of knee arthroscopy patients. Am J Knee Surg. 1995;8(1):28-30.
7. Speer KP, Warren RF, Horowitz L. The efficacy of cryotherapy in the postoperative shoulder. J Shoulder Elbow Surg. 1996;5(1):62-68. doi:10.16/s1058-2746(96)80032-2.
8. Kraeutler MJ, Reynolds KA, Long C, McCarthy EC. Compressive cryotherapy versus ice- a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elbow Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
9. DeFranco MJ, Higgins LD, Warner JP. Subscapularis management in open shoulder surgery. J Am Acad Orthop Surg. 2010;18(12):707-717. doi:10.5435/00124635-201012000-00001.
10. Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty. Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
11. Su EP, Perna M, Boettner F, Mayman DJ, et al. A prospective, multicenter, randomized trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620x.94B11.30832.
12. Raynor MC, Pietrobon R, Guller U, Higgins LD. Cryotherapy after ACL reconstruction- a meta analysis. J Knee Surg. 2005;18(2):123-129. doi:10.1055/s-0030-1248169.
13. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: a prospective randomized investigation. J Shoulder Elbow Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
14. Black EM, Higgins LD, Warner JP. Value based shoulder surgery: outcomes driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1-10. doi:10.1016/j.se.2013.02.008.
ABSTRACT
Postoperative pain management is an important component of total shoulder arthroplasty (TSA). Continuous cryotherapy (CC) has been proposed as a means of improving postoperative pain control. However, CC represents an increased cost not typically covered by insurance. The purpose of this study is to compare CC to plain ice (ICE) following TSA. The hypothesis was that CC would lead to lower pain scores and decreased narcotic usage during the first 2 weeks postoperatively.
A randomized controlled trial was performed to compare CC to ICE. Forty patients were randomized to receive either CC or ICE following TSA. The rehabilitation and pain control protocols were otherwise standardized. Visual analog scales (VAS) for pain, satisfaction with cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery. Narcotic usage in morphine equivalents was also recorded.
No significant differences in preoperative pain (5.9 vs 6.8; P = .121), or postoperative pain at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593) or 14 days (2.5 vs 2.7; P = .742) were observed between the CC and ICE groups. Similarly, no differences in quality of sleep, satisfaction with the cold therapy, or narcotic usage at any time interval were observed between the 2 groups.
No differences in pain control, quality of sleep, patient satisfaction, or narcotic usage were detected between CC and ICE following TSA. CC may offer convenience as an advantage, but the increased cost associated with this type of treatment may not be justified.
The number of total shoulder arthroplasties (TSAs) performed annually is increasing dramatically.1 At the same time, there has been a push toward decreased length of hospital stay and earlier mobilization following joint replacement surgery. Central to these goals is adequate pain control. Multimodal pain pathways exist, and one of the safest and cheapest methods of pain control is cold therapy, which can be accomplished with continuous cryotherapy (CC) or plain ice (ICE).
Continue to: The mechanism of cryotherapy...
The mechanism of cryotherapy for controlling pain is poorly understood. Cryotherapy reduces leukocyte migration and slows down nerve signal transmission, which reduces inflammation, thereby producing a short-term analgesic effect. Stalman and colleagues2 reported on a randomized control study that evaluated the effects of postoperative cooling after knee arthroscopy. Measurements of metabolic and inflammatory markers in the synovial membrane were used to assess whether cryotherapy provides a temperature-sensitive release of prostaglandin E2. Cryotherapy lowered the temperature in the postoperative knee, and synovial prostaglandin concentrations were correlated with temperature. Because prostaglandin is a marker of inflammation and pain, the conclusion was that postoperative cooling appeared to have an anti-inflammatory effect.
The knee literature contains multiple studies that have examined the benefits of cryotherapy after both arthroscopic and arthroplasty procedures. The clinical benefits on pain have been equivocal with some studies showing improvements using cryotherapy3,4 and others showing no difference in the treatment group.5,6
Few studies have examined cryotherapy for the shoulder. Speer and colleagues7 demonstrated that postoperative use of CC was effective in reducing recovery time after shoulder surgery. However; they did not provide an ICE comparative group and did not focus specifically on TSA. In another study, Kraeutler and colleagues8 examined only arthroscopic shoulder surgery cases in a randomized prospective trial and found no significant different between CC and ICE. They concluded that there did not appear to be a significant benefit in using CC over ICE for arthroscopic shoulder procedures.
The purpose of this study is to prospectively evaluate CC and ICE following TSA. The hypothesis was that CC leads to improved pain control, less narcotic consumption, and improved quality of sleep compared to ICE in the immediate postoperative period following TSA.
MATERIALS AND METHODS
This was a prospective randomized control study of patients undergoing TSA receiving either CC or ICE postoperatively. Institutional Review Board approval was obtained before commencement of the study. Inclusion criteria included patients aged 30 to 90 years old undergoing a primary or revision shoulder arthroplasty procedure between June 2015 and January 2016. Exclusion criteria included hemiarthroplasty procedures.
Continue to: Three patients refused...
Three patients refused to participate in the study. Enrollment was performed until 40 patients were enrolled in the study (20 patients in each group). Randomization was performed with a random number generator, and patients were assigned to a treatment group following consent to participate. Complete follow-up was available for all patients. There were 13 (65%) male patients in the CC group. The average age of the CC group at the time of surgery was 68.7 years (range). There were 11 male patients in the ICE group. The average age of the ICE group at the time of surgery was 73.2 years (range). The dominant extremity was involved in 9 (45%) patients in the CC group and in 11 patients (55%) in the ICE group. Surgical case specifics are summarized in Table 1.
Table 1. Summary of Surgical Cases
| CC group (n = 20) | ICE group (n = 20) |
Primary TSA | 7 (35%) | 9 (45%) |
Primary RSA | 12 (60%) | 9 (45%) |
Revision arthroplasty | 1 (5%) | 2 (10%) |
Abbreviations: CC, continuous cryotherapy; ICE, plain ice; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty.
All surgeries were performed by Dr. Denard. All patients received a single-shot interscalene nerve block prior to the procedure. A deltopectoral approach was utilized, and the subscapularis was managed with the peel technique.9 All patients were admitted to the hospital following surgery. Standard postoperative pain control consisted of as-needed intravenous morphine (1-2 mg every 2 hours, as needed) or an oral narcotic (hydrocodone/acetaminophen 5/325mg, 1-2 every 4 hours, as needed) which was also provided at discharge. However, total narcotic usage was recorded in morphine equivalents to account for substitutions. No non-steroidal anti-inflammatory drugs were allowed until 3 months postoperatively.
The CC group received treatment from a commercially available cryotherapy unit (Polar Care; Breg). All patients received instructions by a medical professional on how to use the unit. The unit was applied immediately postoperatively and set at a temperature of 45°F to 55°F. Patients were instructed to use the unit continuously during postoperative days 0 to 3. This cryotherapy was administered by a nurse while in the hospital but was left to the responsibility of the patient upon discharge. Patients were instructed to use the unit as needed for pain control during the day and continuously while asleep from days 4 to14.
The ICE group used standard ice packs postoperatively. The patients were instructed to apply an ice pack for 20 min every 2 hours while awake during days 0 to 3. This therapy was administered by a nurse while in the hospital but left to the responsibility of the patient upon discharge. Patients were instructed to use ice packs as needed for pain control during the day at a maximum of 20 minutes per hour on postoperative days 4 to 14. Compliance by both groups was monitored using a patient survey after hospital discharge. The number of hours that patients used either the CC or ICE per 24-hour period was recorded at 24 hours, 3 days, 7 days, and 14 days. The nursing staff recorded the number of hours of use of either cold modality for each patient prior to hospital discharge. The average length of stay as an inpatient was 1.2 days for the CC group and 1.3 days for the ICE group.
Visual analog scales (VAS) for pain, satisfaction with the cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery.
Continue to: The Wilcoxon rank-sum test...
STATISTICAL METHOD
The Wilcoxon rank-sum test was used to assess whether scores changed significantly from the preoperative period to the different postoperative time intervals, as well as to assess the values for pain, quality of sleep, and patient satisfaction. P-values <.05 were considered significant.
RESULTS
No differences were observed in the baseline characteristics between the 2 groups. Both groups showed improvements in pain, quality of sleep, and satisfaction with the cold therapy from the preoperative period to the final follow-up.
The VAS pain scores were not different between the CC and ICE groups preoperatively (5.9 vs 6.8; P = .121) or postoperatively at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593), or 14 days (2.5 vs 2.7; P = .742). Both cohorts demonstrated improved overall pain throughout the study period. These findings are summarized in Table 2.
Table 2. Summary of VAS Pain Scores With Cold Therapy
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
Preoperative | 5.9 ± 4.1 | 6.8 ± 5.3 | .121 | 3.3-8.3 |
24 hours | 4.2 ± 3.0 | 4.3 ± 3.1 | .989 | 2.9-5.7 |
3 days | 4.8 ± 2.7 | 4.7 ± 3.2 | .944 | 3.2-6.3 |
7 days | 2.9 ± 1.8 | 3.3 ± 2.5 | .593 | 2.1-4.4 |
14 days | 2.5 ± 2.1 | 2.7 ± 1.8 | .742 | 1.5-3.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
The number of morphine equivalents of pain medication was not different between the CC and ICE groups postoperatively at 24 hours (43 vs 38 mg; P = .579), 3 days (149 vs 116 mg; P = .201), 7 days (308 vs 228 mg; P = .181), or 14 days (431 vs 348 mg; P = .213). Both groups showed increased narcotic consumption from 24 hours postoperatively until the 2-week follow-up. Narcotic consumption is summarized in Table 3.
Table 3. Summary of Narcotic Consumption in Morphine Equivalents
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 43.0 ± 36.7 | 38.0 ± 42.9 | .579 | 17.9-60.1 |
3 days | 149.0 ± 106.5 | 116.3 ± 108.9 | .201 | 63.4-198.7 |
7 days | 308.1 ± 234.0 | 228 ± 258.3 | .181 | 107.1-348.9 |
14 days | 430.8 ± 384.2 | 347.5 ± 493.4 | .213 | 116.6-610.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice.
VAS for quality of sleep improved in both groups from 24 hours postoperatively until the final follow-up. However, no significant differences in sleep quality were observed between the CC and ICE groups postoperatively at 24 hours (5.1 vs 4.3; P = .382), 3 days (5.1 vs 5.3; P = .601), 7 days (6.0 vs 6.7; P = .319), or 14 days (6.5 vs 7.1; P = .348). The VAS scores for sleep quality are reported in Table 4.
Table 4. Summary of VAS Sleep Quality With Cold Therapya
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 5.1 ± 2.8 | 4.3 ± 2.4 | .382 | 3.2-6.4 |
3 days | 5.1 ± 1.9 | 5.3 ± 2.3 | .601 | 4.2-6.5 |
7 days | 6.0 ± 2.3 | 6.7 ± 2.1 | .319 | 4.9-7.7 |
14 days | 6.5 ± 2.3 | 7.1 ± 2.5 | .348 | 5.3-8.4 |
a0-10 rating with 10 being the highest possible score.
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
Continue to: Finally, VAS patient satisfaction...
Finally, VAS patient satisfaction scores were not different between the CC and ICE groups postoperatively at 24 hours (7.3 vs 6.1; P = .315), 3 days (6.1 vs 6.6; P = .698), 7 days (6.6 vs 6.9; P = .670), or 14 days (7.1 vs 6.3; P = .288).
While compliance within each group utilizing the randomly assigned cold modality was similar, the usage by the CC group was consistently higher at all time points recorded. No complications or reoperations were observed in either group.
DISCUSSION
The optimal method for managing postoperative pain from an arthroplasty procedure is controversial. This prospective randomized study attempted to confirm the hypothesis that CC infers better pain control, improves quality of sleep, and decreases narcotic usage compared to ICE in the first 2 weeks after a TSA procedure. The results of this study refuted our hypothesis, demonstrating no significant difference in pain control, satisfaction, narcotic usage, or sleep quality between the CC and ICE cohorts at all time points studied.
Studies on knees and lower extremities demonstrate equivocal results for the role CC plays in providing improved postoperative pain control. Thienpont10 evaluated CC in a randomized control trial comparing plain ice packs postoperatively in patients who underwent TKA. The author found no significant difference in VAS for pain or narcotic consumption in morphine equivalents. Thienpont10 recommended that CC not be used for outpatient knee arthroplasty as it is an additional cost that does not improve pain significantly. Healy and colleagues5 reported similar results that CC did not demonstrate a difference in narcotic requirement or pain control compared to plain ice packs, as well as no difference in local postoperative swelling or wound drainage. However, a recently published randomized trial by Su and colleagues11 comparing a cryopneumatic device and ICE with static compression in patients who underwent TKA demonstrated significantly lower narcotic consumption and increased ambulation distances in the treatment group. The treatment group consumed approximately 170 mg morphine equivalents less than the control group between discharge and the 2-week postoperative visit. In addition, a significant difference was observed in the satisfaction scores in the treatment group.11 Similarly, a meta-analysis by Raynor and colleagues12 on randomized clinical trials comparing cryotherapy to a placebo group after anterior cruciate ligament reconstruction showed that cryotherapy is associated with significantly lower postoperative pain (P = .02), but demonstrated no difference in postoperative drainage (P = .23) or range of motion (P = .25).
Although multiple studies have been published regarding the efficacy of cryotherapy after knee surgery, very few studies have compared CC to conventional ICE after shoulder surgery. A prospective randomized trial was performed by Singh and colleagues13 to compare CC vs no ICE in open and arthroscopic shoulder surgery patients. Both the open and arthroscopic groups receiving CC demonstrated significant reductions in pain frequency and more restful sleep at the 7-day, 14-day, and 21-day intervals compared to the control group. However, they did not compare the commercial unit to ICE. In contrast, a study by Kraeutler and colleagues8 randomized 46 patients to receive either CC or ICE in the setting of arthroscopic shoulder surgery. Although no significant difference was observed in morphine equivalent dosage between the 2 groups, the CC group used more pain medication on every postoperative day during the first week after surgery. They found no difference between the 2 groups with regards to narcotic consumption or pain scores. The results of this study mirror those by Kraeutler and colleagues,8 demonstrating no difference in pain scores, sleep quality, or narcotic consumption.
Continue to: With rising costs in the US...
With rising costs in the US healthcare system, a great deal of interest has developed in the application of value-based principles to healthcare. Value can be defined as a gain in benefits over the costs expended.14 The average cost for a commercial CC unit used in this study was $260. A pack of ICE is a nominal cost. Based on the results of this study, the cost of the commercial CC device may not be justified when compared to the cost of an ice pack.
The major strengths of this study are the randomized design and multiple data points during the early postoperative period. However, there are several limitations. First, we did not objectively measure compliance of either therapy and relied only on a patient survey. Usage of the commercial CC unit in hours decreased over half between days 3 and 14. This occurred despite training on the application and specific instructions. We believe this reflects “real-world” usage, but it is possible that compliance affected our results. Second, all patients in this study had a single-shot interscalene block. While this is standard at our institution, it is possible that either CC or ICE would have a more significant effect in the absence of an interscalene block. Finally, we did not evaluate final outcomes in this study and therefore cannot determine if the final outcome was different between the 2 groups. Our goal was simply to evaluate the first 2 weeks following surgery, as this is the most painful period following TSA.
CONCLUSION
There was no difference between CC and ICE in terms of pain control, quality of sleep, patient satisfaction, or narcotic consumption following TSA. CC may offer convenience advantages, but the increased cost associated with this type of unit may not be justified.
ABSTRACT
Postoperative pain management is an important component of total shoulder arthroplasty (TSA). Continuous cryotherapy (CC) has been proposed as a means of improving postoperative pain control. However, CC represents an increased cost not typically covered by insurance. The purpose of this study is to compare CC to plain ice (ICE) following TSA. The hypothesis was that CC would lead to lower pain scores and decreased narcotic usage during the first 2 weeks postoperatively.
A randomized controlled trial was performed to compare CC to ICE. Forty patients were randomized to receive either CC or ICE following TSA. The rehabilitation and pain control protocols were otherwise standardized. Visual analog scales (VAS) for pain, satisfaction with cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery. Narcotic usage in morphine equivalents was also recorded.
No significant differences in preoperative pain (5.9 vs 6.8; P = .121), or postoperative pain at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593) or 14 days (2.5 vs 2.7; P = .742) were observed between the CC and ICE groups. Similarly, no differences in quality of sleep, satisfaction with the cold therapy, or narcotic usage at any time interval were observed between the 2 groups.
No differences in pain control, quality of sleep, patient satisfaction, or narcotic usage were detected between CC and ICE following TSA. CC may offer convenience as an advantage, but the increased cost associated with this type of treatment may not be justified.
The number of total shoulder arthroplasties (TSAs) performed annually is increasing dramatically.1 At the same time, there has been a push toward decreased length of hospital stay and earlier mobilization following joint replacement surgery. Central to these goals is adequate pain control. Multimodal pain pathways exist, and one of the safest and cheapest methods of pain control is cold therapy, which can be accomplished with continuous cryotherapy (CC) or plain ice (ICE).
Continue to: The mechanism of cryotherapy...
The mechanism of cryotherapy for controlling pain is poorly understood. Cryotherapy reduces leukocyte migration and slows down nerve signal transmission, which reduces inflammation, thereby producing a short-term analgesic effect. Stalman and colleagues2 reported on a randomized control study that evaluated the effects of postoperative cooling after knee arthroscopy. Measurements of metabolic and inflammatory markers in the synovial membrane were used to assess whether cryotherapy provides a temperature-sensitive release of prostaglandin E2. Cryotherapy lowered the temperature in the postoperative knee, and synovial prostaglandin concentrations were correlated with temperature. Because prostaglandin is a marker of inflammation and pain, the conclusion was that postoperative cooling appeared to have an anti-inflammatory effect.
The knee literature contains multiple studies that have examined the benefits of cryotherapy after both arthroscopic and arthroplasty procedures. The clinical benefits on pain have been equivocal with some studies showing improvements using cryotherapy3,4 and others showing no difference in the treatment group.5,6
Few studies have examined cryotherapy for the shoulder. Speer and colleagues7 demonstrated that postoperative use of CC was effective in reducing recovery time after shoulder surgery. However; they did not provide an ICE comparative group and did not focus specifically on TSA. In another study, Kraeutler and colleagues8 examined only arthroscopic shoulder surgery cases in a randomized prospective trial and found no significant different between CC and ICE. They concluded that there did not appear to be a significant benefit in using CC over ICE for arthroscopic shoulder procedures.
The purpose of this study is to prospectively evaluate CC and ICE following TSA. The hypothesis was that CC leads to improved pain control, less narcotic consumption, and improved quality of sleep compared to ICE in the immediate postoperative period following TSA.
MATERIALS AND METHODS
This was a prospective randomized control study of patients undergoing TSA receiving either CC or ICE postoperatively. Institutional Review Board approval was obtained before commencement of the study. Inclusion criteria included patients aged 30 to 90 years old undergoing a primary or revision shoulder arthroplasty procedure between June 2015 and January 2016. Exclusion criteria included hemiarthroplasty procedures.
Continue to: Three patients refused...
Three patients refused to participate in the study. Enrollment was performed until 40 patients were enrolled in the study (20 patients in each group). Randomization was performed with a random number generator, and patients were assigned to a treatment group following consent to participate. Complete follow-up was available for all patients. There were 13 (65%) male patients in the CC group. The average age of the CC group at the time of surgery was 68.7 years (range). There were 11 male patients in the ICE group. The average age of the ICE group at the time of surgery was 73.2 years (range). The dominant extremity was involved in 9 (45%) patients in the CC group and in 11 patients (55%) in the ICE group. Surgical case specifics are summarized in Table 1.
Table 1. Summary of Surgical Cases
| CC group (n = 20) | ICE group (n = 20) |
Primary TSA | 7 (35%) | 9 (45%) |
Primary RSA | 12 (60%) | 9 (45%) |
Revision arthroplasty | 1 (5%) | 2 (10%) |
Abbreviations: CC, continuous cryotherapy; ICE, plain ice; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty.
All surgeries were performed by Dr. Denard. All patients received a single-shot interscalene nerve block prior to the procedure. A deltopectoral approach was utilized, and the subscapularis was managed with the peel technique.9 All patients were admitted to the hospital following surgery. Standard postoperative pain control consisted of as-needed intravenous morphine (1-2 mg every 2 hours, as needed) or an oral narcotic (hydrocodone/acetaminophen 5/325mg, 1-2 every 4 hours, as needed) which was also provided at discharge. However, total narcotic usage was recorded in morphine equivalents to account for substitutions. No non-steroidal anti-inflammatory drugs were allowed until 3 months postoperatively.
The CC group received treatment from a commercially available cryotherapy unit (Polar Care; Breg). All patients received instructions by a medical professional on how to use the unit. The unit was applied immediately postoperatively and set at a temperature of 45°F to 55°F. Patients were instructed to use the unit continuously during postoperative days 0 to 3. This cryotherapy was administered by a nurse while in the hospital but was left to the responsibility of the patient upon discharge. Patients were instructed to use the unit as needed for pain control during the day and continuously while asleep from days 4 to14.
The ICE group used standard ice packs postoperatively. The patients were instructed to apply an ice pack for 20 min every 2 hours while awake during days 0 to 3. This therapy was administered by a nurse while in the hospital but left to the responsibility of the patient upon discharge. Patients were instructed to use ice packs as needed for pain control during the day at a maximum of 20 minutes per hour on postoperative days 4 to 14. Compliance by both groups was monitored using a patient survey after hospital discharge. The number of hours that patients used either the CC or ICE per 24-hour period was recorded at 24 hours, 3 days, 7 days, and 14 days. The nursing staff recorded the number of hours of use of either cold modality for each patient prior to hospital discharge. The average length of stay as an inpatient was 1.2 days for the CC group and 1.3 days for the ICE group.
Visual analog scales (VAS) for pain, satisfaction with the cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery.
Continue to: The Wilcoxon rank-sum test...
STATISTICAL METHOD
The Wilcoxon rank-sum test was used to assess whether scores changed significantly from the preoperative period to the different postoperative time intervals, as well as to assess the values for pain, quality of sleep, and patient satisfaction. P-values <.05 were considered significant.
RESULTS
No differences were observed in the baseline characteristics between the 2 groups. Both groups showed improvements in pain, quality of sleep, and satisfaction with the cold therapy from the preoperative period to the final follow-up.
The VAS pain scores were not different between the CC and ICE groups preoperatively (5.9 vs 6.8; P = .121) or postoperatively at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593), or 14 days (2.5 vs 2.7; P = .742). Both cohorts demonstrated improved overall pain throughout the study period. These findings are summarized in Table 2.
Table 2. Summary of VAS Pain Scores With Cold Therapy
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
Preoperative | 5.9 ± 4.1 | 6.8 ± 5.3 | .121 | 3.3-8.3 |
24 hours | 4.2 ± 3.0 | 4.3 ± 3.1 | .989 | 2.9-5.7 |
3 days | 4.8 ± 2.7 | 4.7 ± 3.2 | .944 | 3.2-6.3 |
7 days | 2.9 ± 1.8 | 3.3 ± 2.5 | .593 | 2.1-4.4 |
14 days | 2.5 ± 2.1 | 2.7 ± 1.8 | .742 | 1.5-3.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
The number of morphine equivalents of pain medication was not different between the CC and ICE groups postoperatively at 24 hours (43 vs 38 mg; P = .579), 3 days (149 vs 116 mg; P = .201), 7 days (308 vs 228 mg; P = .181), or 14 days (431 vs 348 mg; P = .213). Both groups showed increased narcotic consumption from 24 hours postoperatively until the 2-week follow-up. Narcotic consumption is summarized in Table 3.
Table 3. Summary of Narcotic Consumption in Morphine Equivalents
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 43.0 ± 36.7 | 38.0 ± 42.9 | .579 | 17.9-60.1 |
3 days | 149.0 ± 106.5 | 116.3 ± 108.9 | .201 | 63.4-198.7 |
7 days | 308.1 ± 234.0 | 228 ± 258.3 | .181 | 107.1-348.9 |
14 days | 430.8 ± 384.2 | 347.5 ± 493.4 | .213 | 116.6-610.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice.
VAS for quality of sleep improved in both groups from 24 hours postoperatively until the final follow-up. However, no significant differences in sleep quality were observed between the CC and ICE groups postoperatively at 24 hours (5.1 vs 4.3; P = .382), 3 days (5.1 vs 5.3; P = .601), 7 days (6.0 vs 6.7; P = .319), or 14 days (6.5 vs 7.1; P = .348). The VAS scores for sleep quality are reported in Table 4.
Table 4. Summary of VAS Sleep Quality With Cold Therapya
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 5.1 ± 2.8 | 4.3 ± 2.4 | .382 | 3.2-6.4 |
3 days | 5.1 ± 1.9 | 5.3 ± 2.3 | .601 | 4.2-6.5 |
7 days | 6.0 ± 2.3 | 6.7 ± 2.1 | .319 | 4.9-7.7 |
14 days | 6.5 ± 2.3 | 7.1 ± 2.5 | .348 | 5.3-8.4 |
a0-10 rating with 10 being the highest possible score.
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
Continue to: Finally, VAS patient satisfaction...
Finally, VAS patient satisfaction scores were not different between the CC and ICE groups postoperatively at 24 hours (7.3 vs 6.1; P = .315), 3 days (6.1 vs 6.6; P = .698), 7 days (6.6 vs 6.9; P = .670), or 14 days (7.1 vs 6.3; P = .288).
While compliance within each group utilizing the randomly assigned cold modality was similar, the usage by the CC group was consistently higher at all time points recorded. No complications or reoperations were observed in either group.
DISCUSSION
The optimal method for managing postoperative pain from an arthroplasty procedure is controversial. This prospective randomized study attempted to confirm the hypothesis that CC infers better pain control, improves quality of sleep, and decreases narcotic usage compared to ICE in the first 2 weeks after a TSA procedure. The results of this study refuted our hypothesis, demonstrating no significant difference in pain control, satisfaction, narcotic usage, or sleep quality between the CC and ICE cohorts at all time points studied.
Studies on knees and lower extremities demonstrate equivocal results for the role CC plays in providing improved postoperative pain control. Thienpont10 evaluated CC in a randomized control trial comparing plain ice packs postoperatively in patients who underwent TKA. The author found no significant difference in VAS for pain or narcotic consumption in morphine equivalents. Thienpont10 recommended that CC not be used for outpatient knee arthroplasty as it is an additional cost that does not improve pain significantly. Healy and colleagues5 reported similar results that CC did not demonstrate a difference in narcotic requirement or pain control compared to plain ice packs, as well as no difference in local postoperative swelling or wound drainage. However, a recently published randomized trial by Su and colleagues11 comparing a cryopneumatic device and ICE with static compression in patients who underwent TKA demonstrated significantly lower narcotic consumption and increased ambulation distances in the treatment group. The treatment group consumed approximately 170 mg morphine equivalents less than the control group between discharge and the 2-week postoperative visit. In addition, a significant difference was observed in the satisfaction scores in the treatment group.11 Similarly, a meta-analysis by Raynor and colleagues12 on randomized clinical trials comparing cryotherapy to a placebo group after anterior cruciate ligament reconstruction showed that cryotherapy is associated with significantly lower postoperative pain (P = .02), but demonstrated no difference in postoperative drainage (P = .23) or range of motion (P = .25).
Although multiple studies have been published regarding the efficacy of cryotherapy after knee surgery, very few studies have compared CC to conventional ICE after shoulder surgery. A prospective randomized trial was performed by Singh and colleagues13 to compare CC vs no ICE in open and arthroscopic shoulder surgery patients. Both the open and arthroscopic groups receiving CC demonstrated significant reductions in pain frequency and more restful sleep at the 7-day, 14-day, and 21-day intervals compared to the control group. However, they did not compare the commercial unit to ICE. In contrast, a study by Kraeutler and colleagues8 randomized 46 patients to receive either CC or ICE in the setting of arthroscopic shoulder surgery. Although no significant difference was observed in morphine equivalent dosage between the 2 groups, the CC group used more pain medication on every postoperative day during the first week after surgery. They found no difference between the 2 groups with regards to narcotic consumption or pain scores. The results of this study mirror those by Kraeutler and colleagues,8 demonstrating no difference in pain scores, sleep quality, or narcotic consumption.
Continue to: With rising costs in the US...
With rising costs in the US healthcare system, a great deal of interest has developed in the application of value-based principles to healthcare. Value can be defined as a gain in benefits over the costs expended.14 The average cost for a commercial CC unit used in this study was $260. A pack of ICE is a nominal cost. Based on the results of this study, the cost of the commercial CC device may not be justified when compared to the cost of an ice pack.
The major strengths of this study are the randomized design and multiple data points during the early postoperative period. However, there are several limitations. First, we did not objectively measure compliance of either therapy and relied only on a patient survey. Usage of the commercial CC unit in hours decreased over half between days 3 and 14. This occurred despite training on the application and specific instructions. We believe this reflects “real-world” usage, but it is possible that compliance affected our results. Second, all patients in this study had a single-shot interscalene block. While this is standard at our institution, it is possible that either CC or ICE would have a more significant effect in the absence of an interscalene block. Finally, we did not evaluate final outcomes in this study and therefore cannot determine if the final outcome was different between the 2 groups. Our goal was simply to evaluate the first 2 weeks following surgery, as this is the most painful period following TSA.
CONCLUSION
There was no difference between CC and ICE in terms of pain control, quality of sleep, patient satisfaction, or narcotic consumption following TSA. CC may offer convenience advantages, but the increased cost associated with this type of unit may not be justified.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/jbjs.j.01994.
2. Stalman A, Berglund L, Dungnerc E, Arner P, Fellander-Tsai L. Temperature sensitive release of prostaglandin E2 and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2016/jbjs.j.01790.
3. Levy AS, Marmar E. The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Relat Res. 1993;297:174-178. doi:10.1097/00003086-199312000-00029.
4. Webb JM, Williams D, Ivory JP, Day S, Williamson DM. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopaedics 1998;21(1):59-61.
5. Healy WL, Seidman J, Pfeifer BA, Brown DG. Cold compressive dressing after total knee arthroplasty. Clin Orthop Relat Res. 1994;299:143-146. doi:10.1097/00003086-199402000-00019.
6. Whitelaw GP, DeMuth KA, Demos HA, Schepsis A, Jacques E. The use of Cryo/Cuff versus ice and elastic wrap in the postoperative care of knee arthroscopy patients. Am J Knee Surg. 1995;8(1):28-30.
7. Speer KP, Warren RF, Horowitz L. The efficacy of cryotherapy in the postoperative shoulder. J Shoulder Elbow Surg. 1996;5(1):62-68. doi:10.16/s1058-2746(96)80032-2.
8. Kraeutler MJ, Reynolds KA, Long C, McCarthy EC. Compressive cryotherapy versus ice- a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elbow Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
9. DeFranco MJ, Higgins LD, Warner JP. Subscapularis management in open shoulder surgery. J Am Acad Orthop Surg. 2010;18(12):707-717. doi:10.5435/00124635-201012000-00001.
10. Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty. Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
11. Su EP, Perna M, Boettner F, Mayman DJ, et al. A prospective, multicenter, randomized trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620x.94B11.30832.
12. Raynor MC, Pietrobon R, Guller U, Higgins LD. Cryotherapy after ACL reconstruction- a meta analysis. J Knee Surg. 2005;18(2):123-129. doi:10.1055/s-0030-1248169.
13. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: a prospective randomized investigation. J Shoulder Elbow Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
14. Black EM, Higgins LD, Warner JP. Value based shoulder surgery: outcomes driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1-10. doi:10.1016/j.se.2013.02.008.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/jbjs.j.01994.
2. Stalman A, Berglund L, Dungnerc E, Arner P, Fellander-Tsai L. Temperature sensitive release of prostaglandin E2 and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2016/jbjs.j.01790.
3. Levy AS, Marmar E. The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Relat Res. 1993;297:174-178. doi:10.1097/00003086-199312000-00029.
4. Webb JM, Williams D, Ivory JP, Day S, Williamson DM. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopaedics 1998;21(1):59-61.
5. Healy WL, Seidman J, Pfeifer BA, Brown DG. Cold compressive dressing after total knee arthroplasty. Clin Orthop Relat Res. 1994;299:143-146. doi:10.1097/00003086-199402000-00019.
6. Whitelaw GP, DeMuth KA, Demos HA, Schepsis A, Jacques E. The use of Cryo/Cuff versus ice and elastic wrap in the postoperative care of knee arthroscopy patients. Am J Knee Surg. 1995;8(1):28-30.
7. Speer KP, Warren RF, Horowitz L. The efficacy of cryotherapy in the postoperative shoulder. J Shoulder Elbow Surg. 1996;5(1):62-68. doi:10.16/s1058-2746(96)80032-2.
8. Kraeutler MJ, Reynolds KA, Long C, McCarthy EC. Compressive cryotherapy versus ice- a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elbow Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
9. DeFranco MJ, Higgins LD, Warner JP. Subscapularis management in open shoulder surgery. J Am Acad Orthop Surg. 2010;18(12):707-717. doi:10.5435/00124635-201012000-00001.
10. Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty. Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
11. Su EP, Perna M, Boettner F, Mayman DJ, et al. A prospective, multicenter, randomized trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620x.94B11.30832.
12. Raynor MC, Pietrobon R, Guller U, Higgins LD. Cryotherapy after ACL reconstruction- a meta analysis. J Knee Surg. 2005;18(2):123-129. doi:10.1055/s-0030-1248169.
13. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: a prospective randomized investigation. J Shoulder Elbow Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
14. Black EM, Higgins LD, Warner JP. Value based shoulder surgery: outcomes driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1-10. doi:10.1016/j.se.2013.02.008.
TAKE-HOME POINTS
- CC has been proposed as a means of improving postoperative pain control.
- CC represents a cost typically not covered by insurances.
- No difference was noted between the 2 groups in quality of sleep, satisfaction with the cold therapy, or narcotic usage at any time interval.
- While CC may offer convenience advantages, the increased cost associated with this type of unit may not be justified.
- The mechanism for CC for pain control is poorly understood.
Patient-Reported Outcomes of Knotted and Knotless Glenohumeral Labral Repairs Are Equivalent
Take-Home Points
- There is no difference in PROMs following knotless or knotted labral repair.
- Operative time is shorter for knotless compared to knotted glenoid labral tears.
- Knotless constructs may be more predictable than knotted constructs biomechanically.
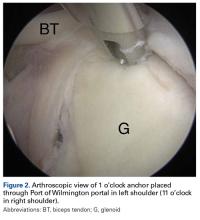
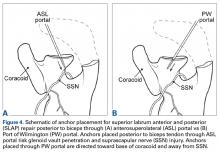
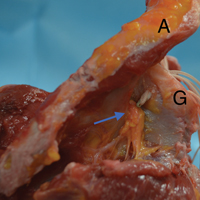
Orthopedic surgeons often encounter labral pathology, and labral tears historically have required open techniques.1-3 Arthroscopy allows for advanced visualization and treatment of shoulder lesions,4,5 including anterior, posterior, and superior labrum anterior to posterior (SLAP) lesions.6
The goal of arthroscopic labral repair is to restore joint stability while maintaining range of motion. Arthroscopically repairing the labrum with suture anchors has become the standard technique, and several studies have reported satisfactory biomechanical and clinical results.1,7-12 Surgeons traditionally have been required to tie knots for these anchors, but knot security varies significantly among experienced arthroscopic surgeons.13 In addition, knots can migrate,14 and bulky knots can cause chondral abrasion.15,16 Several manufacturers have introduced knotless anchors for soft-tissue fixation.15,17 The knotless technique provides a low-profile repair with potentially less operating time.8 These factors may warrant switching from knotted to knotless techniques if outcomes are clinically acceptable. However, few studies have compared knotted and knotless techniques for glenohumeral labral repair.8,15,18-21
We conducted a study to compare the clinical results and operative times of knotless and knotted fixation of anterior and posterior glenohumeral labral repairs and SLAP repairs. We hypothesized there would be no difference in patient-reported outcome measures (PROMs) between knotted and knotless techniques.
Methods
We retrospectively evaluated data that had been prospectively collected between 2012 and 2016 in a Surgical Outcomes System (SOS; Arthrex) database. Participation in this registry is elective, and enrollment can occur on a case-by-case basis. The database stores data on basic demographics, PROMs, and operative time. Data for our specific analysis were available for surgeries performed by 115 different surgeons. Inclusion criteria included primary isolated arthroscopic anterior, isolated posterior, and isolated SLAP repair with completely knotted or completely knotless labral repair and minimum 1-year follow-up. Exclusion criteria included hybrid knotted–knotless repair, rotator cuff repair, revision surgery, open surgery, and lack of complete follow-up data.
SOS is a proprietary registry that allows for the collection of basic patient demographics, diagnostic and operative data, and PROMs. PROMs in the SOS shoulder arthroscopy module include Veterans RAND 12-Item Health Survey (VR-12) mental health and physical health component summary scores, visual analog scale (VAS) pain scores, and American Shoulder and Elbow Surgeons (ASES) scores. For this study, PROMs were reviewed before surgery and 6 and 12 months after surgery. In addition, operative times of all procedures were collected.
For the analysis, completely knotted and completely knotless techniques were compared for anterior repair, posterior repair, and SLAP repair. A t test was used to compare the techniques on PROMs, and χ2 test was used to evaluate proportion differences. Statistical significance was set at P < .05.
Results
Anterior Labral Repairs
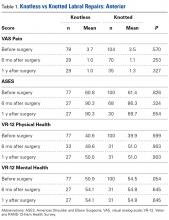
Of the 102 knotted anterior labral repairs that met the study criteria, 26 (25%) had minimum 1-year follow-up. Of the 122 knotless labral repairs, 33 (27%) had minimum 1-year follow-up. Seventy-five percent of knotted repairs and 80% of knotless repairs were performed in men. Mean (SD) age was 25.3 (11.7) years for the knotted group and 26.9 (10.6) years for the knotless group (P = .109). Anterior labral repairs did not differ in PROMs at any point (Table 1).
A mean of 2.8 anchors was used for knotted repairs, and a mean of 3.1 anchors was used for knotless repairs. Mean operative time was 75.8 minutes for knotted repairs and 67.5 minutes for knotless repairs. Mean (SD) time per anchor was 30.9 (13.9) minutes for knotted repairs and 25.6 (19.5) minutes for knotless repairs (P = .021).
Posterior Labral Repairs
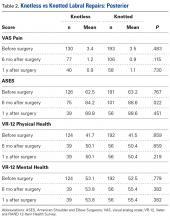
Of the 165 knotted posterior labral repairs that met the study criteria, 39 (29%) had minimum 1-year follow-up. Of the 229 knotless labral repairs, 56 (24%) had minimum 1-year follow-up. Eighty-five percent of knotted repairs and 74% of knotless repairs were performed in men. Mean (SD) age was 29.1 (12.0) years for the knotted group and 27.5 (11.9) years for the knotless group (P = .148). Posterior labral repairs did not differ in PROMs before surgery or 1 year after surgery; 6 months after surgery, these repairs differed only in ASES scores (Table 2).
A mean of 3.6 anchors was used for knotted repairs, and a mean of 3.0 anchors was used for knotless repairs. Mean operative time was 67.0 minutes for knotted repairs and 43.1 minutes for knotless repairs. Mean (SD) time per anchor was 21.1 (10.7) minutes for knotted repairs and 17.5 (14.7) minutes for knotless repairs (P = .031).
SLAP Repairs
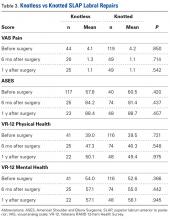
Of the 54 knotted SLAP repairs that met the study criteria, 24 (44%) had minimum 1-year follow-up. Of the 138 knotless SLAP repairs, 48 (35%) had minimum 1-year follow-up. Seventy-two percent of knotted repairs and 72% of knotless repairs were performed in men. Mean (SD) age was 32.1 (11.6) years for the knotted group and 35.0 (12.8) years for the knotless group (P = .246). SLAP repairs did not differ in PROMs at any point (Table 3).
A mean of 1.9 anchors was used for knotted repairs, and a mean of 2.1 anchors was used for knotless repairs. Mean operative time was 59.0 minutes for knotted repairs and 40.9 minutes for knotless repairs. Mean (SD) time per anchor was 36.6 (22.4) minutes for knotted repairs and 26.3 (14.0) minutes for knotless repairs (P = .080).
Discussion
Our hypothesis that there would be no difference in PROMs between knotted and knotless labral repairs was confirmed. Our findings are important because this study compared the gold standard of knotted suture anchor with the alternative knotless suture anchor in glenohumeral labral repair. These findings have several important implications for labral repair.
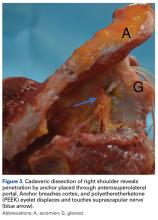
Knot tying traditionally has been used to achieve fixation with an anchor. Although simple in concept, knot tying can be challenging and its quality variable. Thal15 wrote that good-quality arthroscopic suture anchor repair is difficult to achieve because satisfactory knot tying requires significant practice with certain devices designed specifically for knot tying. Multiple surgeons have noted a significant learning curve associated with knot tying, and there is no agreement on which knot is superior.22-26 Leedle and Miller17 even suggested that, because knot tying is difficult, tying knots arthroscopically can lead to knot failure. In their study, they concluded that the knot is consistently the weakest link in suture repair of an anterior labrum construct. In a controlled laboratory study, Hanypsiak and colleagues13 found considerable knot-strength variability among expert arthroscopists. Only 65 (18%) of 365 knots tied fell within 20% of the mean for ultimate load failure, and only 128 (36%) of 365 fell within 20% of the mean for clinical failure (3 mm of displacement). These data suggested expert arthroscopists were unable to tie 5 consecutive knots of the same type consistently. Even among experts, it seems, knot strength varies significantly, and knot-strength issues may affect the rates of labral repair failure.
Multiple authors have also reported that bulky knots can cause chondral abrasion or that knots can migrate.25,27 Rhee and Ha27 reported that, when another knot (eg, a half-hitch knot) is tied to prevent knot failure, the resulting overall knot can be too bulky for a limited space, and chondral abrasion can result. In addition, regardless of size, a knot can migrate and, in its new position, start rubbing against the head of the humerus. Kim and colleagues14 found that, even when a knot is placed away from the humeral head, migration and repeated contact with the head are possible. Park and colleagues28 found that a significant number of knotted SLAP repairs required arthroscopic knot removal for relief of knot-induced pain and clicking.
Knotless constructs have several theoretical advantages over knotted constructs. Compared with a knotted technique, a knotless technique appears to provide more predictable strength, as variability in knot tying is eliminated (unpublished data). A knotless repair also has a lower profile,8 which should lead to less contact with the humeral head.19 Last, a knotless repair is more efficient—it takes less time to perform. In our study, operative time was reduced by a mean of 5.3 minutes per anchor for anterior labral repair. Assuming a mean of 3 anchors, this reduction equates to 16 minutes per case. Therefore, a surgeon who performs 25 labral repairs a year can save 6.7 hours a year. Reduced operative time benefits the patient (ie, lower risk of infection and other complications29), the surgeon, and the healthcare system (ie, cost savings). Macario30 found that operating room costs averaged $62 per minute (range, $22-$133 per minute). Therefore, saving 16 minutes per case could lead to saving $992 per case. In summary, a knotless technique appears to be clinically and financially advantageous as long as its results are the same as or better than those of a knotted technique.
A few other studies have compared knotted and knotless techniques. In a cadaveric study, Slabaugh and colleagues20 found no difference in labral height between traditional and knotless suture anchors. Leedle and Miller17 found that knotless constructs are biomechanically stronger than knotted constructs in anterior labral repair. In a level 3 clinical study, Yang and colleagues21 compared a conventional vertical knot with a knotless horizontal mattress suture in 41 patients who underwent SLAP repair. Functional outcome was no different between the 2 groups, but postoperative range of motion was improved in the knotless group. Ng and Kumar31 compared 45 patients who had knotted Bankart repair with 42 patients who had knotless Bankart repair and found no difference in functional outcome or rate of recurrent dislocation. Similarly, Kocaoglu and colleagues22 found no difference in recurrence rate between 18 patients who underwent a knotted technique for arthroscopic Bankart repair and 20 patients who underwent a knotless technique. Our findings corroborate the findings of these studies and further support the idea that there is no difference between knotted and knotless constructs with respect to PROMs.
Study Limitations
The major strength of this study was its large cohort and large population of surgeons. However, there were several study limitations. First, we could not detail specific repair techniques, such as simple or horizontal mattress orientation, and rehabilitation protocols and other variables are likely as well. Second, the repair technique was not randomized, and therefore there may have been a selection bias based on tissue quality. Although we cannot prove no bias, we think it was unlikely given that the groups were similar in age. Third, our data did not include information on range of motion or recurrent instability. Our goal was simply to evaluate PROMs among multiple surgeons using the 2 techniques. Fourth, there was substantial follow-up loss, which introduced potential selection bias. Last, there may have been conditions under which a hybrid technique with inferior knot tying, combined with a hybrid knotless construct, could have proved advantageous.
Conclusion
Our data showed that the advantages of knotless repair are not compromised in clinical situations. Although the data showed no significant difference in clinical outcomes, knotless repairs may provide surgeons with shorter surgeries, simpler constructs, less potential for chondral damage, and more consistent suture tensioning. Additional studies may further confirm these results.
1. Levy DM, Cole BJ, Bach BR Jr. History of surgical intervention of anterior shoulder instability. J Shoulder Elbow Surg. 2016;25(6):e139-e150.
2. Gill TJ, Zarins B. Open repairs for the treatment of anterior shoulder instability. Am J Sports Med. 2003;31(1):142-153.
3. Millett PJ, Clavert P, Warner JJ. Open operative treatment for anterior shoulder instability: when and why? J Bone Joint Surg Am. 2005;87(2):419-432.
4. Stein DA, Jazrawi L, Bartolozzi AR. Arthroscopic stabilization of anterior shoulder instability: a review of the literature. Arthroscopy. 2002;18(8):912-924.
5. Kim SH, Ha KI, Kim SH. Bankart repair in traumatic anterior shoulder instability: open versus arthroscopic technique. Arthroscopy. 2002;18(7):755-763.
6. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
7. Hantes M, Raoulis V. Arthroscopic findings in anterior shoulder instability. Open Orthop J. 2017;11:119-132.
8. Sileo MJ, Lee SJ, Kremenic IJ, et al. Biomechanical comparison of a knotless suture anchor with standard suture anchor in the repair of type II SLAP tears. Arthroscopy. 2009;25(4):348-354.
9. Iqbal S, Jacobs U, Akhtar A, Macfarlane RJ, Waseem M. A history of shoulder surgery. Open Orthop J. 2013;7:305-309.
10. Garofalo R, Mocci A, Moretti B, et al. Arthroscopic treatment of anterior shoulder instability using knotless suture anchors. Arthroscopy. 2005;21(11):1283-1289.
11. Kersten AD, Fabing M, Ensminger S, et al. Suture capsulorrhaphy versus capsulolabral advancement for shoulder instability. Arthroscopy. 2012;28(10):1344-1351.
12. Cole BJ, Warner JJ. Arthroscopic versus open Bankart repair for traumatic anterior shoulder instability. Clin Sports Med. 2000;19(1):19-48.
13. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984.
14. Kim SH, Ha KI, Park JH, et al. Arthroscopic posterior labral repair and capsular shift for traumatic unidirectional recurrent posterior subluxation of the shoulder. J Bone Joint Surg Am. 2003;85(8):1479-1487.
15. Thal R. Knotless suture anchor. Clin Orthop Relat Res. 2001;(390):42-51.
16. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
17. Leedle BP, Miller MD. Pullout strength of knotless suture anchors. Arthroscopy. 2005;21(1):81-85.
18. Caldwell PE 3rd, Pearson SE, D’Angelo MS. Arthroscopic knotless repair of the posterior labrum using LabralTape. Arthrosc Tech. 2016;5(2):e315-e320.
19. Tennent D, Concina C, Pearse E. Arthroscopic posterior stabilization of the shoulder using a percutaneous knotless mattress suture technique. Arthrosc Tech. 2014;3(1):e161-e164.
20. Slabaugh MA, Friel NA, Wang VM, Cole BJ. Restoring the labral height for treatment of Bankart lesions: a comparison of suture anchor constructs. Arthroscopy. 2010;26(5):587-591.
21. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849.
23. Aboalata M, Halawa A, Basyoni Y. The double Bankart bridge: a technique for restoration of the labral footprint in arthroscopic shoulder instability repair. Arthrosc Tech. 2017;6(1):e43-e47.
24. Rhee SM, Kang SY, Jang EC, Kim JY, Ha YC. Clinical outcomes after arthroscopic acetabular labral repair using knot-tying or knotless suture technique. Arch Orthop Trauma Surg. 2016;136(10):1411-1416.
25. Oh JH, Lee HK, Kim JY, Kim SH, Gong HS. Clinical and radiologic outcomes of arthroscopic glenoid labrum repair with the BioKnotless suture anchor. Am J Sports Med. 2009;37(12):2340-2348.
26. Yian E, Wang C, Millett PJ, Warner JJ. Arthroscopic repair of SLAP lesions with a BioKnotless suture anchor. Arthroscopy. 2004;20(5):547-551.
27. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: case report. J Shoulder Elbow Surg. 2006;15(3):391-393.
28. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic knot removal for failed superior labrum anterior-posterior repair secondary to knot-induced pain. Am J Sports Med. 2017;45(11):2563-2568.
29. Wang DS. Re: how slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Urol. 2016;195(5):1510-1511.
30. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
31. Ng DZ, Kumar VP. Arthroscopic Bankart repair using knot-tying versus knotless suture anchors: is there a difference? Arthroscopy. 2014;30(4):422-427.
Take-Home Points
- There is no difference in PROMs following knotless or knotted labral repair.
- Operative time is shorter for knotless compared to knotted glenoid labral tears.
- Knotless constructs may be more predictable than knotted constructs biomechanically.
Orthopedic surgeons often encounter labral pathology, and labral tears historically have required open techniques.1-3 Arthroscopy allows for advanced visualization and treatment of shoulder lesions,4,5 including anterior, posterior, and superior labrum anterior to posterior (SLAP) lesions.6
The goal of arthroscopic labral repair is to restore joint stability while maintaining range of motion. Arthroscopically repairing the labrum with suture anchors has become the standard technique, and several studies have reported satisfactory biomechanical and clinical results.1,7-12 Surgeons traditionally have been required to tie knots for these anchors, but knot security varies significantly among experienced arthroscopic surgeons.13 In addition, knots can migrate,14 and bulky knots can cause chondral abrasion.15,16 Several manufacturers have introduced knotless anchors for soft-tissue fixation.15,17 The knotless technique provides a low-profile repair with potentially less operating time.8 These factors may warrant switching from knotted to knotless techniques if outcomes are clinically acceptable. However, few studies have compared knotted and knotless techniques for glenohumeral labral repair.8,15,18-21
We conducted a study to compare the clinical results and operative times of knotless and knotted fixation of anterior and posterior glenohumeral labral repairs and SLAP repairs. We hypothesized there would be no difference in patient-reported outcome measures (PROMs) between knotted and knotless techniques.
Methods
We retrospectively evaluated data that had been prospectively collected between 2012 and 2016 in a Surgical Outcomes System (SOS; Arthrex) database. Participation in this registry is elective, and enrollment can occur on a case-by-case basis. The database stores data on basic demographics, PROMs, and operative time. Data for our specific analysis were available for surgeries performed by 115 different surgeons. Inclusion criteria included primary isolated arthroscopic anterior, isolated posterior, and isolated SLAP repair with completely knotted or completely knotless labral repair and minimum 1-year follow-up. Exclusion criteria included hybrid knotted–knotless repair, rotator cuff repair, revision surgery, open surgery, and lack of complete follow-up data.
SOS is a proprietary registry that allows for the collection of basic patient demographics, diagnostic and operative data, and PROMs. PROMs in the SOS shoulder arthroscopy module include Veterans RAND 12-Item Health Survey (VR-12) mental health and physical health component summary scores, visual analog scale (VAS) pain scores, and American Shoulder and Elbow Surgeons (ASES) scores. For this study, PROMs were reviewed before surgery and 6 and 12 months after surgery. In addition, operative times of all procedures were collected.
For the analysis, completely knotted and completely knotless techniques were compared for anterior repair, posterior repair, and SLAP repair. A t test was used to compare the techniques on PROMs, and χ2 test was used to evaluate proportion differences. Statistical significance was set at P < .05.
Results
Anterior Labral Repairs
Of the 102 knotted anterior labral repairs that met the study criteria, 26 (25%) had minimum 1-year follow-up. Of the 122 knotless labral repairs, 33 (27%) had minimum 1-year follow-up. Seventy-five percent of knotted repairs and 80% of knotless repairs were performed in men. Mean (SD) age was 25.3 (11.7) years for the knotted group and 26.9 (10.6) years for the knotless group (P = .109). Anterior labral repairs did not differ in PROMs at any point (Table 1).
A mean of 2.8 anchors was used for knotted repairs, and a mean of 3.1 anchors was used for knotless repairs. Mean operative time was 75.8 minutes for knotted repairs and 67.5 minutes for knotless repairs. Mean (SD) time per anchor was 30.9 (13.9) minutes for knotted repairs and 25.6 (19.5) minutes for knotless repairs (P = .021).
Posterior Labral Repairs
Of the 165 knotted posterior labral repairs that met the study criteria, 39 (29%) had minimum 1-year follow-up. Of the 229 knotless labral repairs, 56 (24%) had minimum 1-year follow-up. Eighty-five percent of knotted repairs and 74% of knotless repairs were performed in men. Mean (SD) age was 29.1 (12.0) years for the knotted group and 27.5 (11.9) years for the knotless group (P = .148). Posterior labral repairs did not differ in PROMs before surgery or 1 year after surgery; 6 months after surgery, these repairs differed only in ASES scores (Table 2).
A mean of 3.6 anchors was used for knotted repairs, and a mean of 3.0 anchors was used for knotless repairs. Mean operative time was 67.0 minutes for knotted repairs and 43.1 minutes for knotless repairs. Mean (SD) time per anchor was 21.1 (10.7) minutes for knotted repairs and 17.5 (14.7) minutes for knotless repairs (P = .031).
SLAP Repairs
Of the 54 knotted SLAP repairs that met the study criteria, 24 (44%) had minimum 1-year follow-up. Of the 138 knotless SLAP repairs, 48 (35%) had minimum 1-year follow-up. Seventy-two percent of knotted repairs and 72% of knotless repairs were performed in men. Mean (SD) age was 32.1 (11.6) years for the knotted group and 35.0 (12.8) years for the knotless group (P = .246). SLAP repairs did not differ in PROMs at any point (Table 3).
A mean of 1.9 anchors was used for knotted repairs, and a mean of 2.1 anchors was used for knotless repairs. Mean operative time was 59.0 minutes for knotted repairs and 40.9 minutes for knotless repairs. Mean (SD) time per anchor was 36.6 (22.4) minutes for knotted repairs and 26.3 (14.0) minutes for knotless repairs (P = .080).
Discussion
Our hypothesis that there would be no difference in PROMs between knotted and knotless labral repairs was confirmed. Our findings are important because this study compared the gold standard of knotted suture anchor with the alternative knotless suture anchor in glenohumeral labral repair. These findings have several important implications for labral repair.
Knot tying traditionally has been used to achieve fixation with an anchor. Although simple in concept, knot tying can be challenging and its quality variable. Thal15 wrote that good-quality arthroscopic suture anchor repair is difficult to achieve because satisfactory knot tying requires significant practice with certain devices designed specifically for knot tying. Multiple surgeons have noted a significant learning curve associated with knot tying, and there is no agreement on which knot is superior.22-26 Leedle and Miller17 even suggested that, because knot tying is difficult, tying knots arthroscopically can lead to knot failure. In their study, they concluded that the knot is consistently the weakest link in suture repair of an anterior labrum construct. In a controlled laboratory study, Hanypsiak and colleagues13 found considerable knot-strength variability among expert arthroscopists. Only 65 (18%) of 365 knots tied fell within 20% of the mean for ultimate load failure, and only 128 (36%) of 365 fell within 20% of the mean for clinical failure (3 mm of displacement). These data suggested expert arthroscopists were unable to tie 5 consecutive knots of the same type consistently. Even among experts, it seems, knot strength varies significantly, and knot-strength issues may affect the rates of labral repair failure.
Multiple authors have also reported that bulky knots can cause chondral abrasion or that knots can migrate.25,27 Rhee and Ha27 reported that, when another knot (eg, a half-hitch knot) is tied to prevent knot failure, the resulting overall knot can be too bulky for a limited space, and chondral abrasion can result. In addition, regardless of size, a knot can migrate and, in its new position, start rubbing against the head of the humerus. Kim and colleagues14 found that, even when a knot is placed away from the humeral head, migration and repeated contact with the head are possible. Park and colleagues28 found that a significant number of knotted SLAP repairs required arthroscopic knot removal for relief of knot-induced pain and clicking.
Knotless constructs have several theoretical advantages over knotted constructs. Compared with a knotted technique, a knotless technique appears to provide more predictable strength, as variability in knot tying is eliminated (unpublished data). A knotless repair also has a lower profile,8 which should lead to less contact with the humeral head.19 Last, a knotless repair is more efficient—it takes less time to perform. In our study, operative time was reduced by a mean of 5.3 minutes per anchor for anterior labral repair. Assuming a mean of 3 anchors, this reduction equates to 16 minutes per case. Therefore, a surgeon who performs 25 labral repairs a year can save 6.7 hours a year. Reduced operative time benefits the patient (ie, lower risk of infection and other complications29), the surgeon, and the healthcare system (ie, cost savings). Macario30 found that operating room costs averaged $62 per minute (range, $22-$133 per minute). Therefore, saving 16 minutes per case could lead to saving $992 per case. In summary, a knotless technique appears to be clinically and financially advantageous as long as its results are the same as or better than those of a knotted technique.
A few other studies have compared knotted and knotless techniques. In a cadaveric study, Slabaugh and colleagues20 found no difference in labral height between traditional and knotless suture anchors. Leedle and Miller17 found that knotless constructs are biomechanically stronger than knotted constructs in anterior labral repair. In a level 3 clinical study, Yang and colleagues21 compared a conventional vertical knot with a knotless horizontal mattress suture in 41 patients who underwent SLAP repair. Functional outcome was no different between the 2 groups, but postoperative range of motion was improved in the knotless group. Ng and Kumar31 compared 45 patients who had knotted Bankart repair with 42 patients who had knotless Bankart repair and found no difference in functional outcome or rate of recurrent dislocation. Similarly, Kocaoglu and colleagues22 found no difference in recurrence rate between 18 patients who underwent a knotted technique for arthroscopic Bankart repair and 20 patients who underwent a knotless technique. Our findings corroborate the findings of these studies and further support the idea that there is no difference between knotted and knotless constructs with respect to PROMs.
Study Limitations
The major strength of this study was its large cohort and large population of surgeons. However, there were several study limitations. First, we could not detail specific repair techniques, such as simple or horizontal mattress orientation, and rehabilitation protocols and other variables are likely as well. Second, the repair technique was not randomized, and therefore there may have been a selection bias based on tissue quality. Although we cannot prove no bias, we think it was unlikely given that the groups were similar in age. Third, our data did not include information on range of motion or recurrent instability. Our goal was simply to evaluate PROMs among multiple surgeons using the 2 techniques. Fourth, there was substantial follow-up loss, which introduced potential selection bias. Last, there may have been conditions under which a hybrid technique with inferior knot tying, combined with a hybrid knotless construct, could have proved advantageous.
Conclusion
Our data showed that the advantages of knotless repair are not compromised in clinical situations. Although the data showed no significant difference in clinical outcomes, knotless repairs may provide surgeons with shorter surgeries, simpler constructs, less potential for chondral damage, and more consistent suture tensioning. Additional studies may further confirm these results.
Take-Home Points
- There is no difference in PROMs following knotless or knotted labral repair.
- Operative time is shorter for knotless compared to knotted glenoid labral tears.
- Knotless constructs may be more predictable than knotted constructs biomechanically.
Orthopedic surgeons often encounter labral pathology, and labral tears historically have required open techniques.1-3 Arthroscopy allows for advanced visualization and treatment of shoulder lesions,4,5 including anterior, posterior, and superior labrum anterior to posterior (SLAP) lesions.6
The goal of arthroscopic labral repair is to restore joint stability while maintaining range of motion. Arthroscopically repairing the labrum with suture anchors has become the standard technique, and several studies have reported satisfactory biomechanical and clinical results.1,7-12 Surgeons traditionally have been required to tie knots for these anchors, but knot security varies significantly among experienced arthroscopic surgeons.13 In addition, knots can migrate,14 and bulky knots can cause chondral abrasion.15,16 Several manufacturers have introduced knotless anchors for soft-tissue fixation.15,17 The knotless technique provides a low-profile repair with potentially less operating time.8 These factors may warrant switching from knotted to knotless techniques if outcomes are clinically acceptable. However, few studies have compared knotted and knotless techniques for glenohumeral labral repair.8,15,18-21
We conducted a study to compare the clinical results and operative times of knotless and knotted fixation of anterior and posterior glenohumeral labral repairs and SLAP repairs. We hypothesized there would be no difference in patient-reported outcome measures (PROMs) between knotted and knotless techniques.
Methods
We retrospectively evaluated data that had been prospectively collected between 2012 and 2016 in a Surgical Outcomes System (SOS; Arthrex) database. Participation in this registry is elective, and enrollment can occur on a case-by-case basis. The database stores data on basic demographics, PROMs, and operative time. Data for our specific analysis were available for surgeries performed by 115 different surgeons. Inclusion criteria included primary isolated arthroscopic anterior, isolated posterior, and isolated SLAP repair with completely knotted or completely knotless labral repair and minimum 1-year follow-up. Exclusion criteria included hybrid knotted–knotless repair, rotator cuff repair, revision surgery, open surgery, and lack of complete follow-up data.
SOS is a proprietary registry that allows for the collection of basic patient demographics, diagnostic and operative data, and PROMs. PROMs in the SOS shoulder arthroscopy module include Veterans RAND 12-Item Health Survey (VR-12) mental health and physical health component summary scores, visual analog scale (VAS) pain scores, and American Shoulder and Elbow Surgeons (ASES) scores. For this study, PROMs were reviewed before surgery and 6 and 12 months after surgery. In addition, operative times of all procedures were collected.
For the analysis, completely knotted and completely knotless techniques were compared for anterior repair, posterior repair, and SLAP repair. A t test was used to compare the techniques on PROMs, and χ2 test was used to evaluate proportion differences. Statistical significance was set at P < .05.
Results
Anterior Labral Repairs
Of the 102 knotted anterior labral repairs that met the study criteria, 26 (25%) had minimum 1-year follow-up. Of the 122 knotless labral repairs, 33 (27%) had minimum 1-year follow-up. Seventy-five percent of knotted repairs and 80% of knotless repairs were performed in men. Mean (SD) age was 25.3 (11.7) years for the knotted group and 26.9 (10.6) years for the knotless group (P = .109). Anterior labral repairs did not differ in PROMs at any point (Table 1).
A mean of 2.8 anchors was used for knotted repairs, and a mean of 3.1 anchors was used for knotless repairs. Mean operative time was 75.8 minutes for knotted repairs and 67.5 minutes for knotless repairs. Mean (SD) time per anchor was 30.9 (13.9) minutes for knotted repairs and 25.6 (19.5) minutes for knotless repairs (P = .021).
Posterior Labral Repairs
Of the 165 knotted posterior labral repairs that met the study criteria, 39 (29%) had minimum 1-year follow-up. Of the 229 knotless labral repairs, 56 (24%) had minimum 1-year follow-up. Eighty-five percent of knotted repairs and 74% of knotless repairs were performed in men. Mean (SD) age was 29.1 (12.0) years for the knotted group and 27.5 (11.9) years for the knotless group (P = .148). Posterior labral repairs did not differ in PROMs before surgery or 1 year after surgery; 6 months after surgery, these repairs differed only in ASES scores (Table 2).
A mean of 3.6 anchors was used for knotted repairs, and a mean of 3.0 anchors was used for knotless repairs. Mean operative time was 67.0 minutes for knotted repairs and 43.1 minutes for knotless repairs. Mean (SD) time per anchor was 21.1 (10.7) minutes for knotted repairs and 17.5 (14.7) minutes for knotless repairs (P = .031).
SLAP Repairs
Of the 54 knotted SLAP repairs that met the study criteria, 24 (44%) had minimum 1-year follow-up. Of the 138 knotless SLAP repairs, 48 (35%) had minimum 1-year follow-up. Seventy-two percent of knotted repairs and 72% of knotless repairs were performed in men. Mean (SD) age was 32.1 (11.6) years for the knotted group and 35.0 (12.8) years for the knotless group (P = .246). SLAP repairs did not differ in PROMs at any point (Table 3).
A mean of 1.9 anchors was used for knotted repairs, and a mean of 2.1 anchors was used for knotless repairs. Mean operative time was 59.0 minutes for knotted repairs and 40.9 minutes for knotless repairs. Mean (SD) time per anchor was 36.6 (22.4) minutes for knotted repairs and 26.3 (14.0) minutes for knotless repairs (P = .080).
Discussion
Our hypothesis that there would be no difference in PROMs between knotted and knotless labral repairs was confirmed. Our findings are important because this study compared the gold standard of knotted suture anchor with the alternative knotless suture anchor in glenohumeral labral repair. These findings have several important implications for labral repair.
Knot tying traditionally has been used to achieve fixation with an anchor. Although simple in concept, knot tying can be challenging and its quality variable. Thal15 wrote that good-quality arthroscopic suture anchor repair is difficult to achieve because satisfactory knot tying requires significant practice with certain devices designed specifically for knot tying. Multiple surgeons have noted a significant learning curve associated with knot tying, and there is no agreement on which knot is superior.22-26 Leedle and Miller17 even suggested that, because knot tying is difficult, tying knots arthroscopically can lead to knot failure. In their study, they concluded that the knot is consistently the weakest link in suture repair of an anterior labrum construct. In a controlled laboratory study, Hanypsiak and colleagues13 found considerable knot-strength variability among expert arthroscopists. Only 65 (18%) of 365 knots tied fell within 20% of the mean for ultimate load failure, and only 128 (36%) of 365 fell within 20% of the mean for clinical failure (3 mm of displacement). These data suggested expert arthroscopists were unable to tie 5 consecutive knots of the same type consistently. Even among experts, it seems, knot strength varies significantly, and knot-strength issues may affect the rates of labral repair failure.
Multiple authors have also reported that bulky knots can cause chondral abrasion or that knots can migrate.25,27 Rhee and Ha27 reported that, when another knot (eg, a half-hitch knot) is tied to prevent knot failure, the resulting overall knot can be too bulky for a limited space, and chondral abrasion can result. In addition, regardless of size, a knot can migrate and, in its new position, start rubbing against the head of the humerus. Kim and colleagues14 found that, even when a knot is placed away from the humeral head, migration and repeated contact with the head are possible. Park and colleagues28 found that a significant number of knotted SLAP repairs required arthroscopic knot removal for relief of knot-induced pain and clicking.
Knotless constructs have several theoretical advantages over knotted constructs. Compared with a knotted technique, a knotless technique appears to provide more predictable strength, as variability in knot tying is eliminated (unpublished data). A knotless repair also has a lower profile,8 which should lead to less contact with the humeral head.19 Last, a knotless repair is more efficient—it takes less time to perform. In our study, operative time was reduced by a mean of 5.3 minutes per anchor for anterior labral repair. Assuming a mean of 3 anchors, this reduction equates to 16 minutes per case. Therefore, a surgeon who performs 25 labral repairs a year can save 6.7 hours a year. Reduced operative time benefits the patient (ie, lower risk of infection and other complications29), the surgeon, and the healthcare system (ie, cost savings). Macario30 found that operating room costs averaged $62 per minute (range, $22-$133 per minute). Therefore, saving 16 minutes per case could lead to saving $992 per case. In summary, a knotless technique appears to be clinically and financially advantageous as long as its results are the same as or better than those of a knotted technique.
A few other studies have compared knotted and knotless techniques. In a cadaveric study, Slabaugh and colleagues20 found no difference in labral height between traditional and knotless suture anchors. Leedle and Miller17 found that knotless constructs are biomechanically stronger than knotted constructs in anterior labral repair. In a level 3 clinical study, Yang and colleagues21 compared a conventional vertical knot with a knotless horizontal mattress suture in 41 patients who underwent SLAP repair. Functional outcome was no different between the 2 groups, but postoperative range of motion was improved in the knotless group. Ng and Kumar31 compared 45 patients who had knotted Bankart repair with 42 patients who had knotless Bankart repair and found no difference in functional outcome or rate of recurrent dislocation. Similarly, Kocaoglu and colleagues22 found no difference in recurrence rate between 18 patients who underwent a knotted technique for arthroscopic Bankart repair and 20 patients who underwent a knotless technique. Our findings corroborate the findings of these studies and further support the idea that there is no difference between knotted and knotless constructs with respect to PROMs.
Study Limitations
The major strength of this study was its large cohort and large population of surgeons. However, there were several study limitations. First, we could not detail specific repair techniques, such as simple or horizontal mattress orientation, and rehabilitation protocols and other variables are likely as well. Second, the repair technique was not randomized, and therefore there may have been a selection bias based on tissue quality. Although we cannot prove no bias, we think it was unlikely given that the groups were similar in age. Third, our data did not include information on range of motion or recurrent instability. Our goal was simply to evaluate PROMs among multiple surgeons using the 2 techniques. Fourth, there was substantial follow-up loss, which introduced potential selection bias. Last, there may have been conditions under which a hybrid technique with inferior knot tying, combined with a hybrid knotless construct, could have proved advantageous.
Conclusion
Our data showed that the advantages of knotless repair are not compromised in clinical situations. Although the data showed no significant difference in clinical outcomes, knotless repairs may provide surgeons with shorter surgeries, simpler constructs, less potential for chondral damage, and more consistent suture tensioning. Additional studies may further confirm these results.
1. Levy DM, Cole BJ, Bach BR Jr. History of surgical intervention of anterior shoulder instability. J Shoulder Elbow Surg. 2016;25(6):e139-e150.
2. Gill TJ, Zarins B. Open repairs for the treatment of anterior shoulder instability. Am J Sports Med. 2003;31(1):142-153.
3. Millett PJ, Clavert P, Warner JJ. Open operative treatment for anterior shoulder instability: when and why? J Bone Joint Surg Am. 2005;87(2):419-432.
4. Stein DA, Jazrawi L, Bartolozzi AR. Arthroscopic stabilization of anterior shoulder instability: a review of the literature. Arthroscopy. 2002;18(8):912-924.
5. Kim SH, Ha KI, Kim SH. Bankart repair in traumatic anterior shoulder instability: open versus arthroscopic technique. Arthroscopy. 2002;18(7):755-763.
6. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
7. Hantes M, Raoulis V. Arthroscopic findings in anterior shoulder instability. Open Orthop J. 2017;11:119-132.
8. Sileo MJ, Lee SJ, Kremenic IJ, et al. Biomechanical comparison of a knotless suture anchor with standard suture anchor in the repair of type II SLAP tears. Arthroscopy. 2009;25(4):348-354.
9. Iqbal S, Jacobs U, Akhtar A, Macfarlane RJ, Waseem M. A history of shoulder surgery. Open Orthop J. 2013;7:305-309.
10. Garofalo R, Mocci A, Moretti B, et al. Arthroscopic treatment of anterior shoulder instability using knotless suture anchors. Arthroscopy. 2005;21(11):1283-1289.
11. Kersten AD, Fabing M, Ensminger S, et al. Suture capsulorrhaphy versus capsulolabral advancement for shoulder instability. Arthroscopy. 2012;28(10):1344-1351.
12. Cole BJ, Warner JJ. Arthroscopic versus open Bankart repair for traumatic anterior shoulder instability. Clin Sports Med. 2000;19(1):19-48.
13. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984.
14. Kim SH, Ha KI, Park JH, et al. Arthroscopic posterior labral repair and capsular shift for traumatic unidirectional recurrent posterior subluxation of the shoulder. J Bone Joint Surg Am. 2003;85(8):1479-1487.
15. Thal R. Knotless suture anchor. Clin Orthop Relat Res. 2001;(390):42-51.
16. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
17. Leedle BP, Miller MD. Pullout strength of knotless suture anchors. Arthroscopy. 2005;21(1):81-85.
18. Caldwell PE 3rd, Pearson SE, D’Angelo MS. Arthroscopic knotless repair of the posterior labrum using LabralTape. Arthrosc Tech. 2016;5(2):e315-e320.
19. Tennent D, Concina C, Pearse E. Arthroscopic posterior stabilization of the shoulder using a percutaneous knotless mattress suture technique. Arthrosc Tech. 2014;3(1):e161-e164.
20. Slabaugh MA, Friel NA, Wang VM, Cole BJ. Restoring the labral height for treatment of Bankart lesions: a comparison of suture anchor constructs. Arthroscopy. 2010;26(5):587-591.
21. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849.
23. Aboalata M, Halawa A, Basyoni Y. The double Bankart bridge: a technique for restoration of the labral footprint in arthroscopic shoulder instability repair. Arthrosc Tech. 2017;6(1):e43-e47.
24. Rhee SM, Kang SY, Jang EC, Kim JY, Ha YC. Clinical outcomes after arthroscopic acetabular labral repair using knot-tying or knotless suture technique. Arch Orthop Trauma Surg. 2016;136(10):1411-1416.
25. Oh JH, Lee HK, Kim JY, Kim SH, Gong HS. Clinical and radiologic outcomes of arthroscopic glenoid labrum repair with the BioKnotless suture anchor. Am J Sports Med. 2009;37(12):2340-2348.
26. Yian E, Wang C, Millett PJ, Warner JJ. Arthroscopic repair of SLAP lesions with a BioKnotless suture anchor. Arthroscopy. 2004;20(5):547-551.
27. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: case report. J Shoulder Elbow Surg. 2006;15(3):391-393.
28. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic knot removal for failed superior labrum anterior-posterior repair secondary to knot-induced pain. Am J Sports Med. 2017;45(11):2563-2568.
29. Wang DS. Re: how slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Urol. 2016;195(5):1510-1511.
30. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
31. Ng DZ, Kumar VP. Arthroscopic Bankart repair using knot-tying versus knotless suture anchors: is there a difference? Arthroscopy. 2014;30(4):422-427.
1. Levy DM, Cole BJ, Bach BR Jr. History of surgical intervention of anterior shoulder instability. J Shoulder Elbow Surg. 2016;25(6):e139-e150.
2. Gill TJ, Zarins B. Open repairs for the treatment of anterior shoulder instability. Am J Sports Med. 2003;31(1):142-153.
3. Millett PJ, Clavert P, Warner JJ. Open operative treatment for anterior shoulder instability: when and why? J Bone Joint Surg Am. 2005;87(2):419-432.
4. Stein DA, Jazrawi L, Bartolozzi AR. Arthroscopic stabilization of anterior shoulder instability: a review of the literature. Arthroscopy. 2002;18(8):912-924.
5. Kim SH, Ha KI, Kim SH. Bankart repair in traumatic anterior shoulder instability: open versus arthroscopic technique. Arthroscopy. 2002;18(7):755-763.
6. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
7. Hantes M, Raoulis V. Arthroscopic findings in anterior shoulder instability. Open Orthop J. 2017;11:119-132.
8. Sileo MJ, Lee SJ, Kremenic IJ, et al. Biomechanical comparison of a knotless suture anchor with standard suture anchor in the repair of type II SLAP tears. Arthroscopy. 2009;25(4):348-354.
9. Iqbal S, Jacobs U, Akhtar A, Macfarlane RJ, Waseem M. A history of shoulder surgery. Open Orthop J. 2013;7:305-309.
10. Garofalo R, Mocci A, Moretti B, et al. Arthroscopic treatment of anterior shoulder instability using knotless suture anchors. Arthroscopy. 2005;21(11):1283-1289.
11. Kersten AD, Fabing M, Ensminger S, et al. Suture capsulorrhaphy versus capsulolabral advancement for shoulder instability. Arthroscopy. 2012;28(10):1344-1351.
12. Cole BJ, Warner JJ. Arthroscopic versus open Bankart repair for traumatic anterior shoulder instability. Clin Sports Med. 2000;19(1):19-48.
13. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984.
14. Kim SH, Ha KI, Park JH, et al. Arthroscopic posterior labral repair and capsular shift for traumatic unidirectional recurrent posterior subluxation of the shoulder. J Bone Joint Surg Am. 2003;85(8):1479-1487.
15. Thal R. Knotless suture anchor. Clin Orthop Relat Res. 2001;(390):42-51.
16. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
17. Leedle BP, Miller MD. Pullout strength of knotless suture anchors. Arthroscopy. 2005;21(1):81-85.
18. Caldwell PE 3rd, Pearson SE, D’Angelo MS. Arthroscopic knotless repair of the posterior labrum using LabralTape. Arthrosc Tech. 2016;5(2):e315-e320.
19. Tennent D, Concina C, Pearse E. Arthroscopic posterior stabilization of the shoulder using a percutaneous knotless mattress suture technique. Arthrosc Tech. 2014;3(1):e161-e164.
20. Slabaugh MA, Friel NA, Wang VM, Cole BJ. Restoring the labral height for treatment of Bankart lesions: a comparison of suture anchor constructs. Arthroscopy. 2010;26(5):587-591.
21. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849.
23. Aboalata M, Halawa A, Basyoni Y. The double Bankart bridge: a technique for restoration of the labral footprint in arthroscopic shoulder instability repair. Arthrosc Tech. 2017;6(1):e43-e47.
24. Rhee SM, Kang SY, Jang EC, Kim JY, Ha YC. Clinical outcomes after arthroscopic acetabular labral repair using knot-tying or knotless suture technique. Arch Orthop Trauma Surg. 2016;136(10):1411-1416.
25. Oh JH, Lee HK, Kim JY, Kim SH, Gong HS. Clinical and radiologic outcomes of arthroscopic glenoid labrum repair with the BioKnotless suture anchor. Am J Sports Med. 2009;37(12):2340-2348.
26. Yian E, Wang C, Millett PJ, Warner JJ. Arthroscopic repair of SLAP lesions with a BioKnotless suture anchor. Arthroscopy. 2004;20(5):547-551.
27. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: case report. J Shoulder Elbow Surg. 2006;15(3):391-393.
28. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic knot removal for failed superior labrum anterior-posterior repair secondary to knot-induced pain. Am J Sports Med. 2017;45(11):2563-2568.
29. Wang DS. Re: how slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Urol. 2016;195(5):1510-1511.
30. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
31. Ng DZ, Kumar VP. Arthroscopic Bankart repair using knot-tying versus knotless suture anchors: is there a difference? Arthroscopy. 2014;30(4):422-427.
Safety of Superior Labrum Anterior and Posterior (SLAP) Repair Posterior to Biceps Tendon Is Improved With a Percutaneous Approach
Take-Home Points
- Anchors placed posterior to the biceps during SLAP repair are at risk for glenoid vault penetration and/or suprascapular nerve (SSN) injury.
- Vault penetration and SSN injury are avoided by using a Port of Wilmington (PW) portal instead of an anterior portal.
- A percutaneous PW portal is safe and passes through the rotator cuff muscle only.
Since being classified by Snyder and colleagues,1 various arthroscopic techniques have been used to repair superior labrum anterior and posterior (SLAP) tears, particularly type II tears. Despite being commonly performed, repairs of SLAP lesions remain challenging. There is high variability in the rate of good/excellent functional outcomes and athletes’ return to previous level of play after SLAP repairs.2,3 Furthermore, the rate of complications after SLAP repair is as high as 5%.4
One of the most common complications of repair of a type II SLAP tear is nerve injury.4 In particular, suprascapular nerve (SSN) injury has occurred after arthroscopic repair of SLAP tears.5,6 Three cadaveric studies have demonstrated that glenoid vault penetration is common during placement of knotted anchors for SLAP repair and that the SSN is at risk during placement of these anchors.7-9 However, 2 of the 3 studies used only an anterior portal in their evaluation of anchor placement. Safety of anchor placement posterior to the biceps tendon may be improved with a percutaneous approach using a Port of Wilmington (PW) portal.10,11 No studies have evaluated the risk of glenoid vault penetration and SSN injury with shorter knotless anchors.
We conducted a study to compare a standard anterosuperolateral (ASL) portal with a percutaneous PW portal for knotless anchors placed posterior to the biceps tendon during repair of SLAP tears. We hypothesized that anchors placed through the PW portal would be less likely to penetrate the glenoid vault and would be farther from the SSN in the event of bone penetration.
Materials and Methods
Six matched pairs of fresh human cadaveric shoulders were used in this study. Each specimen included the scapula, the clavicle, and the humerus. All 6 specimens were male, and their mean age was 41.2 years (range, 23-59 years). Shoulder arthroscopy was performed for placement of SLAP anchors, and open dissection followed.
Anchor Placement
The scapula was clamped and the shoulder placed in the lateral decubitus position with 30° of abduction, 20° of forward flexion, and neutral rotation.10 A standard posterior glenohumeral viewing portal was established and a 30° arthroscope inserted. Both shoulders of each matched pair were randomly assigned to anchor placement through either an ASL portal or a PW portal. Two anchors were placed in the superior glenoid to simulate repair of a posterior SLAP tear.11 Each was a 2.9-mm short (12.5-mm) knotless anchor (BioComposite PushLock; Arthrex) that included a polyetheretherketone (PEEK) eyelet for threading sutures before anchor placement. A drill guide was inserted according to manufacturer guidelines, and a 2.9-mm drill was used to make a bone socket 18 mm deep. The anchor eyelet was loaded with suture tape (Labral Tape; Arthrex), and the anchor and suture were inserted into the socket. The sutures were left uncut to aid in anchor visualization during open dissection. On a right shoulder, the first anchor was placed just posterior to the biceps tendon, at 11 o’clock, and the second anchor about 1 cm posterior to the first, at 10 o’clock. All anchors were placed by an arthroscopy fellowship–trained shoulder surgeon. Before placement, anchor location was confirmed by another arthroscopy fellowship–trained shoulder surgeon.
The ASL portal was created, with an 18-gauge spinal needle and an outside-in technique, about 1 cm lateral to the anterolateral corner of the acromion.
In the opposite shoulder, the PW portal was created, with a percutaneous technique, about 1 cm anterior and 1 cm lateral to the posterolateral corner of the acromion. An 18-gauge spinal needle was inserted to allow a 45° angle of approach to the posterosuperior glenoid.11
Cadaveric Dissection
After anchor placement, another shoulder surgeon performed the dissection. Skin, subcutaneous tissue, deltoid, and clavicle were removed. In the percutaneous specimens, PW portal location relative to rotator cuff was recorded before cuff removal. After overlying soft tissues were removed from a specimen, the anchors were examined for glenoid vault penetration. In the setting of vault penetration, digital calipers were used to measure the shortest distance from anchor to SSN.
Results
In the ASL portal group, 8 (66.7%) of 12 anchors (4/6 at 11 o’clock, 4/6 at 10 o’clock) penetrated the medial glenoid vault.
In the PW portal group, 2 (16.7%) of 12 anchors (1/6 at 11 o’clock, 1/6 at 10 o’clock, both from a single specimen) penetrated the medial glenoid vault. Actually, in each case the eyelet and not the anchor penetrated the vault. In the penetration cases, distance to SSN was 20 mm for the 11 o’clock anchor and 8 mm for the 10 o’clock anchor (Table). Of the 6 portals, 3 passed through the supraspinatus muscle, 2 through the infraspinatus musculotendinous junction, and 1 through the infraspinatus muscle.
Discussion
Our study findings support the hypothesis that SLAP repair anchors placed posterior to the biceps tendon are more likely to remain in bone with use of a percutaneous approach relative to an ASL approach. Our findings also support the growing body of evidence that such anchors placed with an anterior approach increase the risk for SSN injury.
Three other cadaveric studies have evaluated anchor placement for SLAP repair. Chan and colleagues7 evaluated drill penetration during bone socket preparation for SLAP repair in 21 matched pairs of formalin-embalmed cadavers. A 20-mm drill was used for correspondence to a 14.5-mm anchor, though no anchors were inserted, and sockets were created in an open manner. Through a mimicked ASL portal, 1 socket was made anterior to the biceps tendon, at 1 o’clock; then, through a mimicked PW portal, 2 sockets were made posterior to the tendon, at 11 o’clock and 9 to 10 o’clock. Glenoid vault penetration occurred in 29% of the 42 anterior sockets, but only 1 anchor (2.4%) touched the SSN. Penetration did not occur with the 11 o’clock anchors. The 9 to 10 o’clock anchor was at highest risk for SSN injury (9.5%, 4 cases). The study was limited by lack of anchor placement and open creation of bone sockets in embalmed cadavers.
Koh and colleagues8 evaluated arthroscopic placement of anterior SLAP anchors in 6 matched pairs of fresh-frozen cadavers. Through an ASL portal, each 14.5-mm knotted anchor was placed anterior to the biceps tendon, at 1 o’clock. As in the study by Chan and colleagues,7 drill depth was 20 mm. Notably, anchors were seated 2 mm beyond manufacturer recommendations, and the cadavers were of Asian origin, likely indicating smaller glenoids compared to specimens from North America or Europe. All 12 anchors penetrated the glenoid vault; mean distance to SSN was 3.1 mm.
Morgan and colleagues9 compared anterior and ASL portals created for SLAP repairs in 10 matched-pair cadavers. Anchors were placed at 1 o’clock, 11 o’clock, and 10 o’clock. As in the studies by Chan and colleagues7 and Koh and colleagues,8 14.5-mm knotted anchors were used. One anterior anchor (10%) placed through an ASL portal penetrated the cortex by 1 mm, and 2 anterior anchors (20%) placed through anterior portals penetrated the cortex (1 was completely out of the bone). Overall, 65% of 11 o’clock anchors and 100% of 10 o’clock anchors violated the glenoid vault. With the 11 o’clock anchors, mean distance to SSN was 6 mm for ASL portals and 4.2 mm for anterior portals; with the 10 o’clock anchors, mean distance to SSN was 8 mm for ASL portals and 2.1 mm for anterior portals.
Overall, the results of these 3 studies suggest that, with use of ASL portals, placement of SLAP anchors anterior to the biceps tendon is safe. Using the same portals, however, anchors placed posterior to the tendon are at higher risk for glenoid vault penetration. Supporting these findings are our study’s penetration rates: 66.7% for anchors placed through ASL portals and 16.7% for anchors placed through percutaneous PW portals. The different rates are not surprising given that the coracoid process projects anterior to the glenoid and provides additional bone stock for placement of anchors anteriorly vs posteriorly. Therefore, with percutaneous PW portals, the approach angle directs the anchor toward the bone of the coracoid base. Furthermore, the SSN passes nearest the posterior aspect of the glenoid. In a study by Shishido and Kikuchi,12 the distance from the posterior rim of the glenoid to the SSN was 18 mm, and from the superior rim was 29 mm. Therefore, anchors placed with an anterior approach naturally are directed toward the SSN.
In addition to portal placement and approach angle, anchor length likely affects the risks for glenoid vault penetration and SSN injury.
One limitation of this study was the small number of cadavers, all of which were male. Female cadavers and cadavers of other ethnic origins likely have smaller glenoid vaults, and thus their inclusion would have altered our results. This issue was well described in studies mentioned in this article, and our goal was simply to compare ASL portals with percutaneous PW portals, so we think it does not change the fact that the risks for glenoid vault penetration and SSN injury are reduced with use of PW portals for anchors placed posterior to the biceps tendon.
Conclusion
This study was the first to examine glenoid vault penetration and SSN proximity with short anchors for SLAP repair. The risk for glenoid vault penetration during repair of SLAP tears posterior to the biceps tendon was reduced by anchor placement with a percutaneous posterior approach. The percutaneous posterior approach also directs the anchor away from the SSN.
Am J Orthop. 2017;46(1):E60-E64. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
2. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
3. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
4. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
5. Kim SH, Koh YG, Sung CH, Moon HK, Park YS. Iatrogenic suprascapular nerve injury after repair of type II SLAP lesion. Arthroscopy. 2010;26(7):1005-1008.
6. Yoo JC, Lee YS, Ahn JH, Park JH, Kang HJ, Koh KH. Isolated suprascapular nerve injury below the spinoglenoid notch after SLAP repair. J Shoulder Elbow Surg. 2009;18(4):e27-e29.
7. Chan H, Beaupre LA, Bouliane MJ. Injury of the suprascapular nerve during arthroscopic repair of superior labral tears: an anatomic study. J Shoulder Elbow Surg. 2010;19(5):709-715.
8. Koh KH, Park WH, Lim TK, Yoo JC. Medial perforation of the glenoid neck following SLAP repair places the suprascapular nerve at risk: a cadaveric study. J Shoulder Elbow Surg. 2011;20(2):245-250.
9. Morgan RT, Henn RF 3rd, Paryavi E, Dreese J. Injury to the suprascapular nerve during superior labrum anterior and posterior repair: is a rotator interval portal safer than an anterosuperior portal? Arthroscopy. 2014;30(11):1418-1423.
10. Lo IK, Lind CC, Burkhart SS. Glenohumeral arthroscopy portals established using an outside-in technique: neurovascular anatomy at risk. Arthroscopy. 2004;20(6):596-602.
11. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
12. Shishido H, Kikuchi S. Injury of the suprascapular nerve in shoulder surgery: an anatomic study. J Shoulder Elbow Surg. 2001;10(4):372-376.
13. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
14. Kim SH, Crater RB, Hargens AR. Movement-induced knot migration after anterior stabilization in the shoulder. Arthroscopy. 2013;29(3):485-490.
Take-Home Points
- Anchors placed posterior to the biceps during SLAP repair are at risk for glenoid vault penetration and/or suprascapular nerve (SSN) injury.
- Vault penetration and SSN injury are avoided by using a Port of Wilmington (PW) portal instead of an anterior portal.
- A percutaneous PW portal is safe and passes through the rotator cuff muscle only.
Since being classified by Snyder and colleagues,1 various arthroscopic techniques have been used to repair superior labrum anterior and posterior (SLAP) tears, particularly type II tears. Despite being commonly performed, repairs of SLAP lesions remain challenging. There is high variability in the rate of good/excellent functional outcomes and athletes’ return to previous level of play after SLAP repairs.2,3 Furthermore, the rate of complications after SLAP repair is as high as 5%.4
One of the most common complications of repair of a type II SLAP tear is nerve injury.4 In particular, suprascapular nerve (SSN) injury has occurred after arthroscopic repair of SLAP tears.5,6 Three cadaveric studies have demonstrated that glenoid vault penetration is common during placement of knotted anchors for SLAP repair and that the SSN is at risk during placement of these anchors.7-9 However, 2 of the 3 studies used only an anterior portal in their evaluation of anchor placement. Safety of anchor placement posterior to the biceps tendon may be improved with a percutaneous approach using a Port of Wilmington (PW) portal.10,11 No studies have evaluated the risk of glenoid vault penetration and SSN injury with shorter knotless anchors.
We conducted a study to compare a standard anterosuperolateral (ASL) portal with a percutaneous PW portal for knotless anchors placed posterior to the biceps tendon during repair of SLAP tears. We hypothesized that anchors placed through the PW portal would be less likely to penetrate the glenoid vault and would be farther from the SSN in the event of bone penetration.
Materials and Methods
Six matched pairs of fresh human cadaveric shoulders were used in this study. Each specimen included the scapula, the clavicle, and the humerus. All 6 specimens were male, and their mean age was 41.2 years (range, 23-59 years). Shoulder arthroscopy was performed for placement of SLAP anchors, and open dissection followed.
Anchor Placement
The scapula was clamped and the shoulder placed in the lateral decubitus position with 30° of abduction, 20° of forward flexion, and neutral rotation.10 A standard posterior glenohumeral viewing portal was established and a 30° arthroscope inserted. Both shoulders of each matched pair were randomly assigned to anchor placement through either an ASL portal or a PW portal. Two anchors were placed in the superior glenoid to simulate repair of a posterior SLAP tear.11 Each was a 2.9-mm short (12.5-mm) knotless anchor (BioComposite PushLock; Arthrex) that included a polyetheretherketone (PEEK) eyelet for threading sutures before anchor placement. A drill guide was inserted according to manufacturer guidelines, and a 2.9-mm drill was used to make a bone socket 18 mm deep. The anchor eyelet was loaded with suture tape (Labral Tape; Arthrex), and the anchor and suture were inserted into the socket. The sutures were left uncut to aid in anchor visualization during open dissection. On a right shoulder, the first anchor was placed just posterior to the biceps tendon, at 11 o’clock, and the second anchor about 1 cm posterior to the first, at 10 o’clock. All anchors were placed by an arthroscopy fellowship–trained shoulder surgeon. Before placement, anchor location was confirmed by another arthroscopy fellowship–trained shoulder surgeon.
The ASL portal was created, with an 18-gauge spinal needle and an outside-in technique, about 1 cm lateral to the anterolateral corner of the acromion.
In the opposite shoulder, the PW portal was created, with a percutaneous technique, about 1 cm anterior and 1 cm lateral to the posterolateral corner of the acromion. An 18-gauge spinal needle was inserted to allow a 45° angle of approach to the posterosuperior glenoid.11
Cadaveric Dissection
After anchor placement, another shoulder surgeon performed the dissection. Skin, subcutaneous tissue, deltoid, and clavicle were removed. In the percutaneous specimens, PW portal location relative to rotator cuff was recorded before cuff removal. After overlying soft tissues were removed from a specimen, the anchors were examined for glenoid vault penetration. In the setting of vault penetration, digital calipers were used to measure the shortest distance from anchor to SSN.
Results
In the ASL portal group, 8 (66.7%) of 12 anchors (4/6 at 11 o’clock, 4/6 at 10 o’clock) penetrated the medial glenoid vault.
In the PW portal group, 2 (16.7%) of 12 anchors (1/6 at 11 o’clock, 1/6 at 10 o’clock, both from a single specimen) penetrated the medial glenoid vault. Actually, in each case the eyelet and not the anchor penetrated the vault. In the penetration cases, distance to SSN was 20 mm for the 11 o’clock anchor and 8 mm for the 10 o’clock anchor (Table). Of the 6 portals, 3 passed through the supraspinatus muscle, 2 through the infraspinatus musculotendinous junction, and 1 through the infraspinatus muscle.
Discussion
Our study findings support the hypothesis that SLAP repair anchors placed posterior to the biceps tendon are more likely to remain in bone with use of a percutaneous approach relative to an ASL approach. Our findings also support the growing body of evidence that such anchors placed with an anterior approach increase the risk for SSN injury.
Three other cadaveric studies have evaluated anchor placement for SLAP repair. Chan and colleagues7 evaluated drill penetration during bone socket preparation for SLAP repair in 21 matched pairs of formalin-embalmed cadavers. A 20-mm drill was used for correspondence to a 14.5-mm anchor, though no anchors were inserted, and sockets were created in an open manner. Through a mimicked ASL portal, 1 socket was made anterior to the biceps tendon, at 1 o’clock; then, through a mimicked PW portal, 2 sockets were made posterior to the tendon, at 11 o’clock and 9 to 10 o’clock. Glenoid vault penetration occurred in 29% of the 42 anterior sockets, but only 1 anchor (2.4%) touched the SSN. Penetration did not occur with the 11 o’clock anchors. The 9 to 10 o’clock anchor was at highest risk for SSN injury (9.5%, 4 cases). The study was limited by lack of anchor placement and open creation of bone sockets in embalmed cadavers.
Koh and colleagues8 evaluated arthroscopic placement of anterior SLAP anchors in 6 matched pairs of fresh-frozen cadavers. Through an ASL portal, each 14.5-mm knotted anchor was placed anterior to the biceps tendon, at 1 o’clock. As in the study by Chan and colleagues,7 drill depth was 20 mm. Notably, anchors were seated 2 mm beyond manufacturer recommendations, and the cadavers were of Asian origin, likely indicating smaller glenoids compared to specimens from North America or Europe. All 12 anchors penetrated the glenoid vault; mean distance to SSN was 3.1 mm.
Morgan and colleagues9 compared anterior and ASL portals created for SLAP repairs in 10 matched-pair cadavers. Anchors were placed at 1 o’clock, 11 o’clock, and 10 o’clock. As in the studies by Chan and colleagues7 and Koh and colleagues,8 14.5-mm knotted anchors were used. One anterior anchor (10%) placed through an ASL portal penetrated the cortex by 1 mm, and 2 anterior anchors (20%) placed through anterior portals penetrated the cortex (1 was completely out of the bone). Overall, 65% of 11 o’clock anchors and 100% of 10 o’clock anchors violated the glenoid vault. With the 11 o’clock anchors, mean distance to SSN was 6 mm for ASL portals and 4.2 mm for anterior portals; with the 10 o’clock anchors, mean distance to SSN was 8 mm for ASL portals and 2.1 mm for anterior portals.
Overall, the results of these 3 studies suggest that, with use of ASL portals, placement of SLAP anchors anterior to the biceps tendon is safe. Using the same portals, however, anchors placed posterior to the tendon are at higher risk for glenoid vault penetration. Supporting these findings are our study’s penetration rates: 66.7% for anchors placed through ASL portals and 16.7% for anchors placed through percutaneous PW portals. The different rates are not surprising given that the coracoid process projects anterior to the glenoid and provides additional bone stock for placement of anchors anteriorly vs posteriorly. Therefore, with percutaneous PW portals, the approach angle directs the anchor toward the bone of the coracoid base. Furthermore, the SSN passes nearest the posterior aspect of the glenoid. In a study by Shishido and Kikuchi,12 the distance from the posterior rim of the glenoid to the SSN was 18 mm, and from the superior rim was 29 mm. Therefore, anchors placed with an anterior approach naturally are directed toward the SSN.
In addition to portal placement and approach angle, anchor length likely affects the risks for glenoid vault penetration and SSN injury.
One limitation of this study was the small number of cadavers, all of which were male. Female cadavers and cadavers of other ethnic origins likely have smaller glenoid vaults, and thus their inclusion would have altered our results. This issue was well described in studies mentioned in this article, and our goal was simply to compare ASL portals with percutaneous PW portals, so we think it does not change the fact that the risks for glenoid vault penetration and SSN injury are reduced with use of PW portals for anchors placed posterior to the biceps tendon.
Conclusion
This study was the first to examine glenoid vault penetration and SSN proximity with short anchors for SLAP repair. The risk for glenoid vault penetration during repair of SLAP tears posterior to the biceps tendon was reduced by anchor placement with a percutaneous posterior approach. The percutaneous posterior approach also directs the anchor away from the SSN.
Am J Orthop. 2017;46(1):E60-E64. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Anchors placed posterior to the biceps during SLAP repair are at risk for glenoid vault penetration and/or suprascapular nerve (SSN) injury.
- Vault penetration and SSN injury are avoided by using a Port of Wilmington (PW) portal instead of an anterior portal.
- A percutaneous PW portal is safe and passes through the rotator cuff muscle only.
Since being classified by Snyder and colleagues,1 various arthroscopic techniques have been used to repair superior labrum anterior and posterior (SLAP) tears, particularly type II tears. Despite being commonly performed, repairs of SLAP lesions remain challenging. There is high variability in the rate of good/excellent functional outcomes and athletes’ return to previous level of play after SLAP repairs.2,3 Furthermore, the rate of complications after SLAP repair is as high as 5%.4
One of the most common complications of repair of a type II SLAP tear is nerve injury.4 In particular, suprascapular nerve (SSN) injury has occurred after arthroscopic repair of SLAP tears.5,6 Three cadaveric studies have demonstrated that glenoid vault penetration is common during placement of knotted anchors for SLAP repair and that the SSN is at risk during placement of these anchors.7-9 However, 2 of the 3 studies used only an anterior portal in their evaluation of anchor placement. Safety of anchor placement posterior to the biceps tendon may be improved with a percutaneous approach using a Port of Wilmington (PW) portal.10,11 No studies have evaluated the risk of glenoid vault penetration and SSN injury with shorter knotless anchors.
We conducted a study to compare a standard anterosuperolateral (ASL) portal with a percutaneous PW portal for knotless anchors placed posterior to the biceps tendon during repair of SLAP tears. We hypothesized that anchors placed through the PW portal would be less likely to penetrate the glenoid vault and would be farther from the SSN in the event of bone penetration.
Materials and Methods
Six matched pairs of fresh human cadaveric shoulders were used in this study. Each specimen included the scapula, the clavicle, and the humerus. All 6 specimens were male, and their mean age was 41.2 years (range, 23-59 years). Shoulder arthroscopy was performed for placement of SLAP anchors, and open dissection followed.
Anchor Placement
The scapula was clamped and the shoulder placed in the lateral decubitus position with 30° of abduction, 20° of forward flexion, and neutral rotation.10 A standard posterior glenohumeral viewing portal was established and a 30° arthroscope inserted. Both shoulders of each matched pair were randomly assigned to anchor placement through either an ASL portal or a PW portal. Two anchors were placed in the superior glenoid to simulate repair of a posterior SLAP tear.11 Each was a 2.9-mm short (12.5-mm) knotless anchor (BioComposite PushLock; Arthrex) that included a polyetheretherketone (PEEK) eyelet for threading sutures before anchor placement. A drill guide was inserted according to manufacturer guidelines, and a 2.9-mm drill was used to make a bone socket 18 mm deep. The anchor eyelet was loaded with suture tape (Labral Tape; Arthrex), and the anchor and suture were inserted into the socket. The sutures were left uncut to aid in anchor visualization during open dissection. On a right shoulder, the first anchor was placed just posterior to the biceps tendon, at 11 o’clock, and the second anchor about 1 cm posterior to the first, at 10 o’clock. All anchors were placed by an arthroscopy fellowship–trained shoulder surgeon. Before placement, anchor location was confirmed by another arthroscopy fellowship–trained shoulder surgeon.
The ASL portal was created, with an 18-gauge spinal needle and an outside-in technique, about 1 cm lateral to the anterolateral corner of the acromion.
In the opposite shoulder, the PW portal was created, with a percutaneous technique, about 1 cm anterior and 1 cm lateral to the posterolateral corner of the acromion. An 18-gauge spinal needle was inserted to allow a 45° angle of approach to the posterosuperior glenoid.11
Cadaveric Dissection
After anchor placement, another shoulder surgeon performed the dissection. Skin, subcutaneous tissue, deltoid, and clavicle were removed. In the percutaneous specimens, PW portal location relative to rotator cuff was recorded before cuff removal. After overlying soft tissues were removed from a specimen, the anchors were examined for glenoid vault penetration. In the setting of vault penetration, digital calipers were used to measure the shortest distance from anchor to SSN.
Results
In the ASL portal group, 8 (66.7%) of 12 anchors (4/6 at 11 o’clock, 4/6 at 10 o’clock) penetrated the medial glenoid vault.
In the PW portal group, 2 (16.7%) of 12 anchors (1/6 at 11 o’clock, 1/6 at 10 o’clock, both from a single specimen) penetrated the medial glenoid vault. Actually, in each case the eyelet and not the anchor penetrated the vault. In the penetration cases, distance to SSN was 20 mm for the 11 o’clock anchor and 8 mm for the 10 o’clock anchor (Table). Of the 6 portals, 3 passed through the supraspinatus muscle, 2 through the infraspinatus musculotendinous junction, and 1 through the infraspinatus muscle.
Discussion
Our study findings support the hypothesis that SLAP repair anchors placed posterior to the biceps tendon are more likely to remain in bone with use of a percutaneous approach relative to an ASL approach. Our findings also support the growing body of evidence that such anchors placed with an anterior approach increase the risk for SSN injury.
Three other cadaveric studies have evaluated anchor placement for SLAP repair. Chan and colleagues7 evaluated drill penetration during bone socket preparation for SLAP repair in 21 matched pairs of formalin-embalmed cadavers. A 20-mm drill was used for correspondence to a 14.5-mm anchor, though no anchors were inserted, and sockets were created in an open manner. Through a mimicked ASL portal, 1 socket was made anterior to the biceps tendon, at 1 o’clock; then, through a mimicked PW portal, 2 sockets were made posterior to the tendon, at 11 o’clock and 9 to 10 o’clock. Glenoid vault penetration occurred in 29% of the 42 anterior sockets, but only 1 anchor (2.4%) touched the SSN. Penetration did not occur with the 11 o’clock anchors. The 9 to 10 o’clock anchor was at highest risk for SSN injury (9.5%, 4 cases). The study was limited by lack of anchor placement and open creation of bone sockets in embalmed cadavers.
Koh and colleagues8 evaluated arthroscopic placement of anterior SLAP anchors in 6 matched pairs of fresh-frozen cadavers. Through an ASL portal, each 14.5-mm knotted anchor was placed anterior to the biceps tendon, at 1 o’clock. As in the study by Chan and colleagues,7 drill depth was 20 mm. Notably, anchors were seated 2 mm beyond manufacturer recommendations, and the cadavers were of Asian origin, likely indicating smaller glenoids compared to specimens from North America or Europe. All 12 anchors penetrated the glenoid vault; mean distance to SSN was 3.1 mm.
Morgan and colleagues9 compared anterior and ASL portals created for SLAP repairs in 10 matched-pair cadavers. Anchors were placed at 1 o’clock, 11 o’clock, and 10 o’clock. As in the studies by Chan and colleagues7 and Koh and colleagues,8 14.5-mm knotted anchors were used. One anterior anchor (10%) placed through an ASL portal penetrated the cortex by 1 mm, and 2 anterior anchors (20%) placed through anterior portals penetrated the cortex (1 was completely out of the bone). Overall, 65% of 11 o’clock anchors and 100% of 10 o’clock anchors violated the glenoid vault. With the 11 o’clock anchors, mean distance to SSN was 6 mm for ASL portals and 4.2 mm for anterior portals; with the 10 o’clock anchors, mean distance to SSN was 8 mm for ASL portals and 2.1 mm for anterior portals.
Overall, the results of these 3 studies suggest that, with use of ASL portals, placement of SLAP anchors anterior to the biceps tendon is safe. Using the same portals, however, anchors placed posterior to the tendon are at higher risk for glenoid vault penetration. Supporting these findings are our study’s penetration rates: 66.7% for anchors placed through ASL portals and 16.7% for anchors placed through percutaneous PW portals. The different rates are not surprising given that the coracoid process projects anterior to the glenoid and provides additional bone stock for placement of anchors anteriorly vs posteriorly. Therefore, with percutaneous PW portals, the approach angle directs the anchor toward the bone of the coracoid base. Furthermore, the SSN passes nearest the posterior aspect of the glenoid. In a study by Shishido and Kikuchi,12 the distance from the posterior rim of the glenoid to the SSN was 18 mm, and from the superior rim was 29 mm. Therefore, anchors placed with an anterior approach naturally are directed toward the SSN.
In addition to portal placement and approach angle, anchor length likely affects the risks for glenoid vault penetration and SSN injury.
One limitation of this study was the small number of cadavers, all of which were male. Female cadavers and cadavers of other ethnic origins likely have smaller glenoid vaults, and thus their inclusion would have altered our results. This issue was well described in studies mentioned in this article, and our goal was simply to compare ASL portals with percutaneous PW portals, so we think it does not change the fact that the risks for glenoid vault penetration and SSN injury are reduced with use of PW portals for anchors placed posterior to the biceps tendon.
Conclusion
This study was the first to examine glenoid vault penetration and SSN proximity with short anchors for SLAP repair. The risk for glenoid vault penetration during repair of SLAP tears posterior to the biceps tendon was reduced by anchor placement with a percutaneous posterior approach. The percutaneous posterior approach also directs the anchor away from the SSN.
Am J Orthop. 2017;46(1):E60-E64. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
2. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
3. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
4. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
5. Kim SH, Koh YG, Sung CH, Moon HK, Park YS. Iatrogenic suprascapular nerve injury after repair of type II SLAP lesion. Arthroscopy. 2010;26(7):1005-1008.
6. Yoo JC, Lee YS, Ahn JH, Park JH, Kang HJ, Koh KH. Isolated suprascapular nerve injury below the spinoglenoid notch after SLAP repair. J Shoulder Elbow Surg. 2009;18(4):e27-e29.
7. Chan H, Beaupre LA, Bouliane MJ. Injury of the suprascapular nerve during arthroscopic repair of superior labral tears: an anatomic study. J Shoulder Elbow Surg. 2010;19(5):709-715.
8. Koh KH, Park WH, Lim TK, Yoo JC. Medial perforation of the glenoid neck following SLAP repair places the suprascapular nerve at risk: a cadaveric study. J Shoulder Elbow Surg. 2011;20(2):245-250.
9. Morgan RT, Henn RF 3rd, Paryavi E, Dreese J. Injury to the suprascapular nerve during superior labrum anterior and posterior repair: is a rotator interval portal safer than an anterosuperior portal? Arthroscopy. 2014;30(11):1418-1423.
10. Lo IK, Lind CC, Burkhart SS. Glenohumeral arthroscopy portals established using an outside-in technique: neurovascular anatomy at risk. Arthroscopy. 2004;20(6):596-602.
11. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
12. Shishido H, Kikuchi S. Injury of the suprascapular nerve in shoulder surgery: an anatomic study. J Shoulder Elbow Surg. 2001;10(4):372-376.
13. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
14. Kim SH, Crater RB, Hargens AR. Movement-induced knot migration after anterior stabilization in the shoulder. Arthroscopy. 2013;29(3):485-490.
1. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
2. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
3. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
4. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
5. Kim SH, Koh YG, Sung CH, Moon HK, Park YS. Iatrogenic suprascapular nerve injury after repair of type II SLAP lesion. Arthroscopy. 2010;26(7):1005-1008.
6. Yoo JC, Lee YS, Ahn JH, Park JH, Kang HJ, Koh KH. Isolated suprascapular nerve injury below the spinoglenoid notch after SLAP repair. J Shoulder Elbow Surg. 2009;18(4):e27-e29.
7. Chan H, Beaupre LA, Bouliane MJ. Injury of the suprascapular nerve during arthroscopic repair of superior labral tears: an anatomic study. J Shoulder Elbow Surg. 2010;19(5):709-715.
8. Koh KH, Park WH, Lim TK, Yoo JC. Medial perforation of the glenoid neck following SLAP repair places the suprascapular nerve at risk: a cadaveric study. J Shoulder Elbow Surg. 2011;20(2):245-250.
9. Morgan RT, Henn RF 3rd, Paryavi E, Dreese J. Injury to the suprascapular nerve during superior labrum anterior and posterior repair: is a rotator interval portal safer than an anterosuperior portal? Arthroscopy. 2014;30(11):1418-1423.
10. Lo IK, Lind CC, Burkhart SS. Glenohumeral arthroscopy portals established using an outside-in technique: neurovascular anatomy at risk. Arthroscopy. 2004;20(6):596-602.
11. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
12. Shishido H, Kikuchi S. Injury of the suprascapular nerve in shoulder surgery: an anatomic study. J Shoulder Elbow Surg. 2001;10(4):372-376.
13. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
14. Kim SH, Crater RB, Hargens AR. Movement-induced knot migration after anterior stabilization in the shoulder. Arthroscopy. 2013;29(3):485-490.