User login
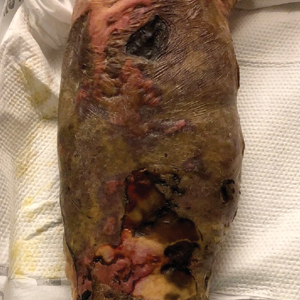
Large Leg Ulcers After Swimming in the Ocean
The Diagnosis: Vibrio vulnificus Infection
At the initial presentation, the differential diagnosis included infectious processes such as bacterial or angioinvasive fungal infections or an inflammatory process such as pyoderma gangrenosum. Blood cultures were found to be positive for pansensitive Vibrio vulnificus. He initially was treated with piperacillin-tazobactam and received surgical debridement of the affected tissues. Pathologic interpretation of the wound tissues revealed a diagnosis of necrotizing softtissue infection and positive Candida albicans growth. He received topical bacitracin on discharge as well as a 7-day course of amoxicillin-clavulanate and fluconazole. He continued to receive debridement procedures and skin grafts, followed by topical mupirocin treatment and silver sulfadiazine. He was seen 6 weeks after discharge with healing wounds and healthy-appearing granulation tissue at the base.
Our patient’s presentation of retiform purpura with stellate necrosis was consistent with a wide range of serious pathologies ranging from medium-vessel vasculitis to thromboembolic phenomena and angioinvasive fungal infections.1 Although Vibrio infection rarely is the first explanation that comes to mind when observing necrotic retiform purpura, the chronic nonhealing injury on the leg combined with the recent history of ocean swimming made V vulnificus stand out as a likely culprit. Although V vulnificus infection traditionally presents with cellulitis, edema, and hemorrhagic bulla,2 necrosis also has been observed.3 Vibrio vulnificus produces multiple virulence factors, and it is believed that these severe cutaneous symptoms are attributable to the production of a specific metalloprotease that enhances vascular permeability, thereby inducing hemorrhage within the vascular basement membrane zone.2
Vibrio vulnificus is an opportunistic bacterial pathogen associated with consumption of contaminated seafood or swimming in ocean waters with open wounds. Infections are rare, with only approximately 100 cases reported annually in the United States.4 However, V vulnificus infections have demonstrated increasing incidence in recent years, especially infections of pre-existing wounds.4,5 Risk factors for their development include age over 40 years and underlying conditions including liver disease, diabetes mellitus, and immune dysfunction.4 Vibrio vulnificus infections also demonstrate a strong male predilection, with almost 90% of infections occurring in males.4 Although the precise etiology of this sex discrepancy remains unknown, estrogen has been suggested to be a protective factor.6 Alternatively, behavioral differences also have been proposed as possible explanations for this discrepancy, with women less likely to consume seafood or go swimming. However, epidemiologic data reveal strong correlations between male sex and liver cirrhosis, a primary risk factor for V vulnificus infections, suggesting that male sex may simply be a confounding variable.7
Infections with V vulnificus are notable for their short incubation periods, with onset of symptoms occurring within 24 hours of exposure, making prompt diagnosis and treatment of high importance.8 Although rare, V vulnificus infections are associated with high mortality rates. From 1988 to 2010, nearly 600 deaths were reported secondary to V vulnificus infections.4 Wound infections carry a 17.6% fatality rate,4 while bloodborne V vulnificus infections exceed 50% fatality.8 Although sepsis secondary to V vulnificus usually is caused by ingestion of raw or undercooked shellfish, primarily oysters,4 our case highlights a rarer instance of both sepsis and localized infection stemming from ocean water exposure.
Vibrio vulnificus is an obligate halophile and therefore is found in marine environments rather than freshwater bodies. However, it rarely is isolated from bodies of water with salinities over 25 parts per thousand, such as the Mediterranean Sea; it usually is found in warmer waters, making it more common in the summer months from May to October.4 Given this proclivity for warmer environments, climate change has contributed to both a greater incidence and global distribution of V vulnificus. 9,10
Treatment of V vulnificus infections centers on antibiotic treatment, with Vibrio species generally demonstrating susceptibility to most antibiotics of human significance.11 However, some Vibrio isolates within the United States have demonstrated antibiotic resistance; 45% of a variety of clinical and environmental samples from South Carolina and Georgia demonstrated resistance to at least 3 antibiotic classes, and 17.3% resisted 8 or more classes of antibiotics.12 These included medications such as doxycycline, tetracycline, aminoglycosides, and cephalosporins—agents that normally are prescribed for V vulnificus infections. Although tetracyclines have long been touted as the preferred treatment of V vulnificus infections, the spread of antibiotic resistance may require greater reliance on alternative regimens such as combinations of cephalosporins and doxycycline or a single fluoroquinolone.13 Although rare, Vibrio infections can have rapidly fatal consequences and should be given serious consideration when evaluating patients with relevant risk factors.
The differential diagnosis included angioinvasive mucormycosis, calciphylaxis, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. Mucormycosis is a fungal infection caused by Mucorales fungi that most commonly is seen in patients with diabetes mellitus, hematologic malignancies, neutropenia, and immunocompromise.14 Calciphylaxis is a condition involving microvascular occlusion due to diffuse calcium deposition in cutaneous blood vessels. It typically presents as violaceous retiform patches and plaques commonly seen on areas such as the thighs, buttocks, or abdomen and usually is associated with chronic renal failure, hemodialysis, and/or secondary hyperparathyroidism.15 Pyoderma gangrenosum is an inflammatory condition involving neutrophilic ulceration of the skin that typically presents as ulceration with a classically undermined border. It frequently is considered a diagnosis of exclusion and therefore requires that providers rule out other causes of ulceration prior to diagnosis.16 Stevens-Johnson syndrome/toxic epidermal necrolysis is a rare drug reaction involving mucosal erosions and cutaneous detachment.17 This diagnosis is less likely given that our patient lacked mucosal involvement and did not have any notable medication exposures prior to symptom onset.
- Wysong A, Venkatesan P. An approach to the patient with retiform purpura. Dermatol Ther. 2011;24:151-172. doi:10.1111/j .1529-8019.2011.01392.x
- Miyoshi S-I. Vibrio vulnificus infection and metalloprotease. J Dermatol. 2006;33:589-595. doi:10.1111/j.1346-8138.2006.00139.x
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145. doi:10.1067 /mjd.2002.107778
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430. doi:10.1111/1462-2920.13955
- Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food —10 states, 2009. CDC website. Published April 16, 2010. Accessed November 3, 2021. https://www.cdc .gov/mmwr/preview/mmwrhtml/mm5914a2.htm
- Merkel SM, Alexander S, Zufall E, et al. Essential role for estrogen in protection against Vibrio vulnificus-induced endotoxic shock. Infect Immun. 2001;69:6119-6122. doi:10.1128/IAI.69.10.6119 -6122.2001
- Scaglione S, Kliethermes S, Cao G, et al. The epidemiology of cirrhosis in the United States: a population-based study. J Clin Gastroenterol. 2015;49:690-696. doi:10.1097/MCG.0000000000000208
- Jones M, Oliver J. Vibrio vulnificus: disease and pathogenesis [published online December 20, 2020]. Infect Immun. https://doi.org/10.1128 /IAI.01046-08
- Paz S, Bisharat N, Paz E, et al. Climate change and the emergence of Vibrio vulnificus disease in Israel. Environ Res. 2007;103:390-396. doi:10.1016/j.envres.2006.07.002
- Martinez-Urtaza J, Bowers JC, Trinanes J, et al. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res Int. 2010;43:1780-1790. doi:10.1016/j. foodres.2010.04.001
- Oliver JD. Vibrio vulnificus. In: Thompson FL, Austin B, Swings J, eds. The Biology of Vibrios. ASM Press; 2006:349-366.
- Baker-Austin C, McArthur JV, Lindell AH, et al. Multi-site analysis reveals widespread antibiotic resistance in the marine pathogen Vibrio vulnificus. Microb Ecol. 2009;57:151-159. doi:10.1007 /s00248-008-9413-8
- Elmahdi S, DaSilva LV, Parveen S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol. 2016;57:128-134. doi:10.1016/j.fm.2016.02.008
- Prasad P, Wong V, Burgin S, et al. Mucormycosis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/mucormycosis?diagnosisId=51981 &moduleId=101
- Blum A, Song P, Tan B, et al. Calciphylaxis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/calciphylaxis?diagnosisId=51241&moduleId=101
- Cohen J, Wong V, Burgin S. Pyoderma gangrenosum. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/pyoderma+gangrenosum?diagnosis Id=52242&moduleId=101
- Walls A, Burgin S. Stevens-Johnson syndrome. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/stevens-johnson+syndrome?diagnosisId=52342&moduleId=101
The Diagnosis: Vibrio vulnificus Infection
At the initial presentation, the differential diagnosis included infectious processes such as bacterial or angioinvasive fungal infections or an inflammatory process such as pyoderma gangrenosum. Blood cultures were found to be positive for pansensitive Vibrio vulnificus. He initially was treated with piperacillin-tazobactam and received surgical debridement of the affected tissues. Pathologic interpretation of the wound tissues revealed a diagnosis of necrotizing softtissue infection and positive Candida albicans growth. He received topical bacitracin on discharge as well as a 7-day course of amoxicillin-clavulanate and fluconazole. He continued to receive debridement procedures and skin grafts, followed by topical mupirocin treatment and silver sulfadiazine. He was seen 6 weeks after discharge with healing wounds and healthy-appearing granulation tissue at the base.
Our patient’s presentation of retiform purpura with stellate necrosis was consistent with a wide range of serious pathologies ranging from medium-vessel vasculitis to thromboembolic phenomena and angioinvasive fungal infections.1 Although Vibrio infection rarely is the first explanation that comes to mind when observing necrotic retiform purpura, the chronic nonhealing injury on the leg combined with the recent history of ocean swimming made V vulnificus stand out as a likely culprit. Although V vulnificus infection traditionally presents with cellulitis, edema, and hemorrhagic bulla,2 necrosis also has been observed.3 Vibrio vulnificus produces multiple virulence factors, and it is believed that these severe cutaneous symptoms are attributable to the production of a specific metalloprotease that enhances vascular permeability, thereby inducing hemorrhage within the vascular basement membrane zone.2
Vibrio vulnificus is an opportunistic bacterial pathogen associated with consumption of contaminated seafood or swimming in ocean waters with open wounds. Infections are rare, with only approximately 100 cases reported annually in the United States.4 However, V vulnificus infections have demonstrated increasing incidence in recent years, especially infections of pre-existing wounds.4,5 Risk factors for their development include age over 40 years and underlying conditions including liver disease, diabetes mellitus, and immune dysfunction.4 Vibrio vulnificus infections also demonstrate a strong male predilection, with almost 90% of infections occurring in males.4 Although the precise etiology of this sex discrepancy remains unknown, estrogen has been suggested to be a protective factor.6 Alternatively, behavioral differences also have been proposed as possible explanations for this discrepancy, with women less likely to consume seafood or go swimming. However, epidemiologic data reveal strong correlations between male sex and liver cirrhosis, a primary risk factor for V vulnificus infections, suggesting that male sex may simply be a confounding variable.7
Infections with V vulnificus are notable for their short incubation periods, with onset of symptoms occurring within 24 hours of exposure, making prompt diagnosis and treatment of high importance.8 Although rare, V vulnificus infections are associated with high mortality rates. From 1988 to 2010, nearly 600 deaths were reported secondary to V vulnificus infections.4 Wound infections carry a 17.6% fatality rate,4 while bloodborne V vulnificus infections exceed 50% fatality.8 Although sepsis secondary to V vulnificus usually is caused by ingestion of raw or undercooked shellfish, primarily oysters,4 our case highlights a rarer instance of both sepsis and localized infection stemming from ocean water exposure.
Vibrio vulnificus is an obligate halophile and therefore is found in marine environments rather than freshwater bodies. However, it rarely is isolated from bodies of water with salinities over 25 parts per thousand, such as the Mediterranean Sea; it usually is found in warmer waters, making it more common in the summer months from May to October.4 Given this proclivity for warmer environments, climate change has contributed to both a greater incidence and global distribution of V vulnificus. 9,10
Treatment of V vulnificus infections centers on antibiotic treatment, with Vibrio species generally demonstrating susceptibility to most antibiotics of human significance.11 However, some Vibrio isolates within the United States have demonstrated antibiotic resistance; 45% of a variety of clinical and environmental samples from South Carolina and Georgia demonstrated resistance to at least 3 antibiotic classes, and 17.3% resisted 8 or more classes of antibiotics.12 These included medications such as doxycycline, tetracycline, aminoglycosides, and cephalosporins—agents that normally are prescribed for V vulnificus infections. Although tetracyclines have long been touted as the preferred treatment of V vulnificus infections, the spread of antibiotic resistance may require greater reliance on alternative regimens such as combinations of cephalosporins and doxycycline or a single fluoroquinolone.13 Although rare, Vibrio infections can have rapidly fatal consequences and should be given serious consideration when evaluating patients with relevant risk factors.
The differential diagnosis included angioinvasive mucormycosis, calciphylaxis, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. Mucormycosis is a fungal infection caused by Mucorales fungi that most commonly is seen in patients with diabetes mellitus, hematologic malignancies, neutropenia, and immunocompromise.14 Calciphylaxis is a condition involving microvascular occlusion due to diffuse calcium deposition in cutaneous blood vessels. It typically presents as violaceous retiform patches and plaques commonly seen on areas such as the thighs, buttocks, or abdomen and usually is associated with chronic renal failure, hemodialysis, and/or secondary hyperparathyroidism.15 Pyoderma gangrenosum is an inflammatory condition involving neutrophilic ulceration of the skin that typically presents as ulceration with a classically undermined border. It frequently is considered a diagnosis of exclusion and therefore requires that providers rule out other causes of ulceration prior to diagnosis.16 Stevens-Johnson syndrome/toxic epidermal necrolysis is a rare drug reaction involving mucosal erosions and cutaneous detachment.17 This diagnosis is less likely given that our patient lacked mucosal involvement and did not have any notable medication exposures prior to symptom onset.
The Diagnosis: Vibrio vulnificus Infection
At the initial presentation, the differential diagnosis included infectious processes such as bacterial or angioinvasive fungal infections or an inflammatory process such as pyoderma gangrenosum. Blood cultures were found to be positive for pansensitive Vibrio vulnificus. He initially was treated with piperacillin-tazobactam and received surgical debridement of the affected tissues. Pathologic interpretation of the wound tissues revealed a diagnosis of necrotizing softtissue infection and positive Candida albicans growth. He received topical bacitracin on discharge as well as a 7-day course of amoxicillin-clavulanate and fluconazole. He continued to receive debridement procedures and skin grafts, followed by topical mupirocin treatment and silver sulfadiazine. He was seen 6 weeks after discharge with healing wounds and healthy-appearing granulation tissue at the base.
Our patient’s presentation of retiform purpura with stellate necrosis was consistent with a wide range of serious pathologies ranging from medium-vessel vasculitis to thromboembolic phenomena and angioinvasive fungal infections.1 Although Vibrio infection rarely is the first explanation that comes to mind when observing necrotic retiform purpura, the chronic nonhealing injury on the leg combined with the recent history of ocean swimming made V vulnificus stand out as a likely culprit. Although V vulnificus infection traditionally presents with cellulitis, edema, and hemorrhagic bulla,2 necrosis also has been observed.3 Vibrio vulnificus produces multiple virulence factors, and it is believed that these severe cutaneous symptoms are attributable to the production of a specific metalloprotease that enhances vascular permeability, thereby inducing hemorrhage within the vascular basement membrane zone.2
Vibrio vulnificus is an opportunistic bacterial pathogen associated with consumption of contaminated seafood or swimming in ocean waters with open wounds. Infections are rare, with only approximately 100 cases reported annually in the United States.4 However, V vulnificus infections have demonstrated increasing incidence in recent years, especially infections of pre-existing wounds.4,5 Risk factors for their development include age over 40 years and underlying conditions including liver disease, diabetes mellitus, and immune dysfunction.4 Vibrio vulnificus infections also demonstrate a strong male predilection, with almost 90% of infections occurring in males.4 Although the precise etiology of this sex discrepancy remains unknown, estrogen has been suggested to be a protective factor.6 Alternatively, behavioral differences also have been proposed as possible explanations for this discrepancy, with women less likely to consume seafood or go swimming. However, epidemiologic data reveal strong correlations between male sex and liver cirrhosis, a primary risk factor for V vulnificus infections, suggesting that male sex may simply be a confounding variable.7
Infections with V vulnificus are notable for their short incubation periods, with onset of symptoms occurring within 24 hours of exposure, making prompt diagnosis and treatment of high importance.8 Although rare, V vulnificus infections are associated with high mortality rates. From 1988 to 2010, nearly 600 deaths were reported secondary to V vulnificus infections.4 Wound infections carry a 17.6% fatality rate,4 while bloodborne V vulnificus infections exceed 50% fatality.8 Although sepsis secondary to V vulnificus usually is caused by ingestion of raw or undercooked shellfish, primarily oysters,4 our case highlights a rarer instance of both sepsis and localized infection stemming from ocean water exposure.
Vibrio vulnificus is an obligate halophile and therefore is found in marine environments rather than freshwater bodies. However, it rarely is isolated from bodies of water with salinities over 25 parts per thousand, such as the Mediterranean Sea; it usually is found in warmer waters, making it more common in the summer months from May to October.4 Given this proclivity for warmer environments, climate change has contributed to both a greater incidence and global distribution of V vulnificus. 9,10
Treatment of V vulnificus infections centers on antibiotic treatment, with Vibrio species generally demonstrating susceptibility to most antibiotics of human significance.11 However, some Vibrio isolates within the United States have demonstrated antibiotic resistance; 45% of a variety of clinical and environmental samples from South Carolina and Georgia demonstrated resistance to at least 3 antibiotic classes, and 17.3% resisted 8 or more classes of antibiotics.12 These included medications such as doxycycline, tetracycline, aminoglycosides, and cephalosporins—agents that normally are prescribed for V vulnificus infections. Although tetracyclines have long been touted as the preferred treatment of V vulnificus infections, the spread of antibiotic resistance may require greater reliance on alternative regimens such as combinations of cephalosporins and doxycycline or a single fluoroquinolone.13 Although rare, Vibrio infections can have rapidly fatal consequences and should be given serious consideration when evaluating patients with relevant risk factors.
The differential diagnosis included angioinvasive mucormycosis, calciphylaxis, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. Mucormycosis is a fungal infection caused by Mucorales fungi that most commonly is seen in patients with diabetes mellitus, hematologic malignancies, neutropenia, and immunocompromise.14 Calciphylaxis is a condition involving microvascular occlusion due to diffuse calcium deposition in cutaneous blood vessels. It typically presents as violaceous retiform patches and plaques commonly seen on areas such as the thighs, buttocks, or abdomen and usually is associated with chronic renal failure, hemodialysis, and/or secondary hyperparathyroidism.15 Pyoderma gangrenosum is an inflammatory condition involving neutrophilic ulceration of the skin that typically presents as ulceration with a classically undermined border. It frequently is considered a diagnosis of exclusion and therefore requires that providers rule out other causes of ulceration prior to diagnosis.16 Stevens-Johnson syndrome/toxic epidermal necrolysis is a rare drug reaction involving mucosal erosions and cutaneous detachment.17 This diagnosis is less likely given that our patient lacked mucosal involvement and did not have any notable medication exposures prior to symptom onset.
- Wysong A, Venkatesan P. An approach to the patient with retiform purpura. Dermatol Ther. 2011;24:151-172. doi:10.1111/j .1529-8019.2011.01392.x
- Miyoshi S-I. Vibrio vulnificus infection and metalloprotease. J Dermatol. 2006;33:589-595. doi:10.1111/j.1346-8138.2006.00139.x
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145. doi:10.1067 /mjd.2002.107778
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430. doi:10.1111/1462-2920.13955
- Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food —10 states, 2009. CDC website. Published April 16, 2010. Accessed November 3, 2021. https://www.cdc .gov/mmwr/preview/mmwrhtml/mm5914a2.htm
- Merkel SM, Alexander S, Zufall E, et al. Essential role for estrogen in protection against Vibrio vulnificus-induced endotoxic shock. Infect Immun. 2001;69:6119-6122. doi:10.1128/IAI.69.10.6119 -6122.2001
- Scaglione S, Kliethermes S, Cao G, et al. The epidemiology of cirrhosis in the United States: a population-based study. J Clin Gastroenterol. 2015;49:690-696. doi:10.1097/MCG.0000000000000208
- Jones M, Oliver J. Vibrio vulnificus: disease and pathogenesis [published online December 20, 2020]. Infect Immun. https://doi.org/10.1128 /IAI.01046-08
- Paz S, Bisharat N, Paz E, et al. Climate change and the emergence of Vibrio vulnificus disease in Israel. Environ Res. 2007;103:390-396. doi:10.1016/j.envres.2006.07.002
- Martinez-Urtaza J, Bowers JC, Trinanes J, et al. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res Int. 2010;43:1780-1790. doi:10.1016/j. foodres.2010.04.001
- Oliver JD. Vibrio vulnificus. In: Thompson FL, Austin B, Swings J, eds. The Biology of Vibrios. ASM Press; 2006:349-366.
- Baker-Austin C, McArthur JV, Lindell AH, et al. Multi-site analysis reveals widespread antibiotic resistance in the marine pathogen Vibrio vulnificus. Microb Ecol. 2009;57:151-159. doi:10.1007 /s00248-008-9413-8
- Elmahdi S, DaSilva LV, Parveen S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol. 2016;57:128-134. doi:10.1016/j.fm.2016.02.008
- Prasad P, Wong V, Burgin S, et al. Mucormycosis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/mucormycosis?diagnosisId=51981 &moduleId=101
- Blum A, Song P, Tan B, et al. Calciphylaxis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/calciphylaxis?diagnosisId=51241&moduleId=101
- Cohen J, Wong V, Burgin S. Pyoderma gangrenosum. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/pyoderma+gangrenosum?diagnosis Id=52242&moduleId=101
- Walls A, Burgin S. Stevens-Johnson syndrome. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/stevens-johnson+syndrome?diagnosisId=52342&moduleId=101
- Wysong A, Venkatesan P. An approach to the patient with retiform purpura. Dermatol Ther. 2011;24:151-172. doi:10.1111/j .1529-8019.2011.01392.x
- Miyoshi S-I. Vibrio vulnificus infection and metalloprotease. J Dermatol. 2006;33:589-595. doi:10.1111/j.1346-8138.2006.00139.x
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145. doi:10.1067 /mjd.2002.107778
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430. doi:10.1111/1462-2920.13955
- Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food —10 states, 2009. CDC website. Published April 16, 2010. Accessed November 3, 2021. https://www.cdc .gov/mmwr/preview/mmwrhtml/mm5914a2.htm
- Merkel SM, Alexander S, Zufall E, et al. Essential role for estrogen in protection against Vibrio vulnificus-induced endotoxic shock. Infect Immun. 2001;69:6119-6122. doi:10.1128/IAI.69.10.6119 -6122.2001
- Scaglione S, Kliethermes S, Cao G, et al. The epidemiology of cirrhosis in the United States: a population-based study. J Clin Gastroenterol. 2015;49:690-696. doi:10.1097/MCG.0000000000000208
- Jones M, Oliver J. Vibrio vulnificus: disease and pathogenesis [published online December 20, 2020]. Infect Immun. https://doi.org/10.1128 /IAI.01046-08
- Paz S, Bisharat N, Paz E, et al. Climate change and the emergence of Vibrio vulnificus disease in Israel. Environ Res. 2007;103:390-396. doi:10.1016/j.envres.2006.07.002
- Martinez-Urtaza J, Bowers JC, Trinanes J, et al. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res Int. 2010;43:1780-1790. doi:10.1016/j. foodres.2010.04.001
- Oliver JD. Vibrio vulnificus. In: Thompson FL, Austin B, Swings J, eds. The Biology of Vibrios. ASM Press; 2006:349-366.
- Baker-Austin C, McArthur JV, Lindell AH, et al. Multi-site analysis reveals widespread antibiotic resistance in the marine pathogen Vibrio vulnificus. Microb Ecol. 2009;57:151-159. doi:10.1007 /s00248-008-9413-8
- Elmahdi S, DaSilva LV, Parveen S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol. 2016;57:128-134. doi:10.1016/j.fm.2016.02.008
- Prasad P, Wong V, Burgin S, et al. Mucormycosis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/mucormycosis?diagnosisId=51981 &moduleId=101
- Blum A, Song P, Tan B, et al. Calciphylaxis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/calciphylaxis?diagnosisId=51241&moduleId=101
- Cohen J, Wong V, Burgin S. Pyoderma gangrenosum. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/pyoderma+gangrenosum?diagnosis Id=52242&moduleId=101
- Walls A, Burgin S. Stevens-Johnson syndrome. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/stevens-johnson+syndrome?diagnosisId=52342&moduleId=101
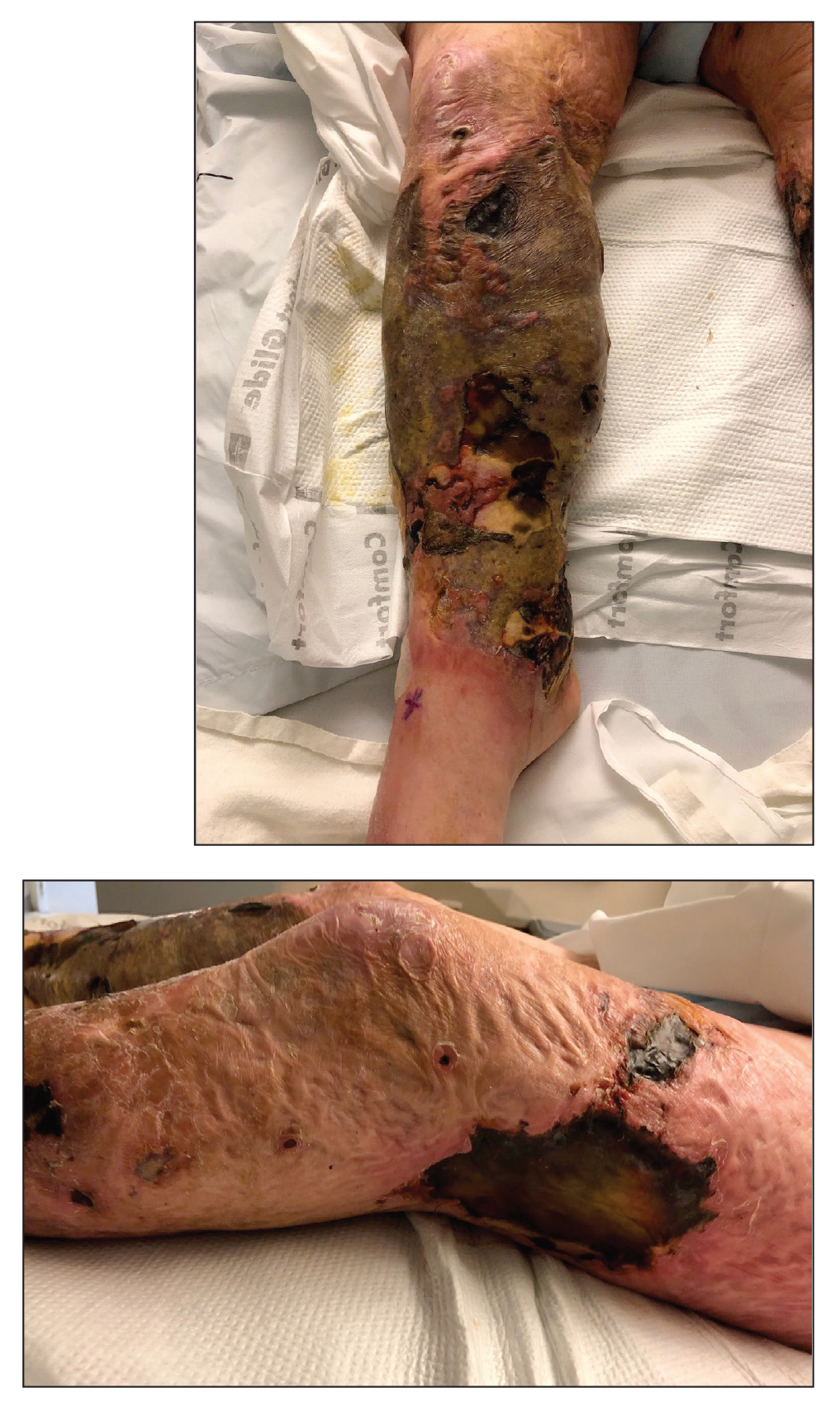
A 48-year-old man presented to the emergency department with pain in both legs after swimming in the ocean surrounding Florida 1 month prior to presentation. His medical history included skin graft treatment of burns during childhood and a chronic lower extremity ulcer that developed after trauma. He received hemodialysis for acute renal failure approximately 1 month prior to the current presentation. At the current presentation he was found to be septic and quickly developed rapidly expanding regions of retiform purpura with stellate necrosis on the legs.