User login
Malignant Transformation of an Aneurysmal Bone Cyst to Fibroblastic Osteosarcoma
Aneurysmal bone cysts (ABC) are expansile, hemorrhagic, non-neoplastic lesions that can be locally destructive1 and that can arise either de novo or secondary to another benign or malignant lesion.2 Although primary and secondary ABCs typically are benign, there are cases of malignant degeneration of primary ABCs, though the transformation arises almost exclusively in the context of prior radiation exposure.3-5 Malignant change without history of irradiation is rare; only 6 such cases have been reported.5-10 In 4 of these cases, the transformation was to osteosarcoma.5,8-10
Here we report on an ABC that degenerated into a fibroblastic osteosarcoma—the fifth such case in the medical literature. In addition to reviewing the earlier cases, we describe the radiologic and histologic underpinnings of this diagnosis and the insight that they provide into the pathogenesis of this rare process. Although the prevailing view is that ABCs are benign, it is important to know these lesions have the potential to undergo malignant transformation, even in the absence of prior radiation exposure. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
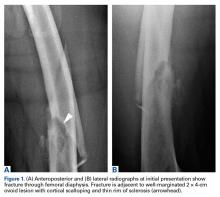
A healthy and previously asymptomatic 37-year-old man presented with thigh pain after a minor fall onto a couch. Radiographs showed a diaphyseal femoral pathologic fracture adjacent to a small but benign-appearing cystic lesion (Figures 1A, 1B).
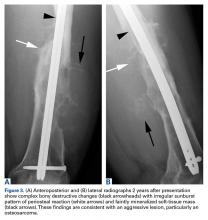
Two years later, the patient had a bicycle accident and, after 2 weeks of significantly increased thigh swelling, presented to the emergency department at the referring institution. Radiographs showed a lytic lesion in the femoral diaphysis that was highly suspicious for malignancy (Figures 3A, 3B).
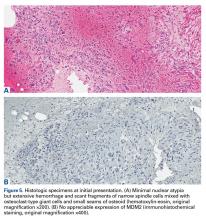
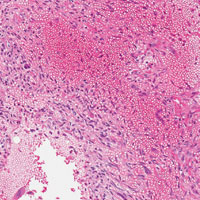
The initial biopsy specimens were evaluated at our institution and interpreted as being consistent with an ABC, with negative immunohistochemical staining for MDM2 (Figures 5A, 5B).
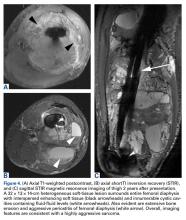
The patient underwent a 3-month course of neoadjuvant chemotherapy with cisplatin and doxorubicin. Interval-staging contrast-enhanced chest, abdomen, and pelvis computed tomography (CT) showed no evidence of metastatic disease. Preoperative MRI showed a significantly larger heterogeneous mass, now with neurovascular involvement, which precluded limb salvage.
Discussion
Aneurysmal bone cysts are expansile, hemorrhagic, locally destructive lesions that generally develop within the first 3 decades of life. Ever since they were first described by Jaffe and Lichtenstein11 in 1942, the most widely accepted theory of their pathogenesis has been that they begin as a benign reactive vascular process.12 However, more recent molecular studies by Oliveira and colleagues13 and Panoutsakopoulos and colleagues14 have demonstrated a clonal neoplastic basis for primary ABCs related to cytogenetic upregulation of oncogenes USP6 and CDH11 after translocation of 17p13 and 16q22.
Given the clonal nature of these lesions, it is surprising that malignant transformation is so rare. Until now, there have been only 4 reports of an ABC undergoing malignant degeneration to osteosarcoma without prior radiation exposure.
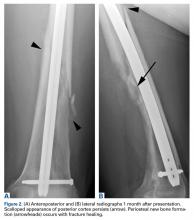
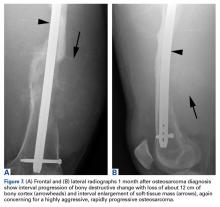
In this article, we have presented a fifth case of a primary ABC degenerating into an osteosarcoma, which in this instance was the fibroblastic subtype. This diagnosis was strongly supported by radiologic and pathologic evidence. From a radiologic perspective, imaging at initial presentation showed absolutely no suspicious features, and the same was true when follow-up radiographs were obtained, 1 month later. Although 1 month is short for a follow-up, the complete lack of radiographic changes would be highly unusual if in fact there had been a coexisting, undetected lesion as aggressive as the one that ultimately developed. Furthermore, imaging at second presentation, almost 2 years later, showed an extremely rapid evolution of findings over 1 month. Extrapolating back in time, we think this time course indicates the malignancy developed not long before its aggressive features were detected.
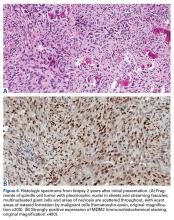
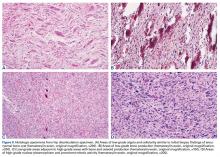
Genetic evidence suggests that most conventional high-grade osteosarcomas arise de novo from a mesenchymal precursor driven by multiple genetic aberrations. Less often, low-grade osteosarcomas progress to high-grade osteosarcomas. Amplification of 12q13-15 with resulting overexpression of MDM2 and CDK4 proteins is found in low-grade osteosarcomas and persists in examples that progress to higher-grade forms.15 Not only did review of our patient’s initial biopsy sample reveal no evidence of malignant features or abnormal mitotic activity, but the complete absence of MDM2 suggests not even a low-grade osteosarcoma was present at the time. By contrast, the second incisional biopsy specimen, 2 years later, showed markedly different histology and pronounced expression of MDM2 throughout the specimen. This finding suggests the histologically high-grade osteosarcoma did not arise de novo but rather secondarily from a low-grade osteosarcoma that had arisen from an ABC. Results of the final heterogeneous histology of the very large mass, which contained benign ABC areas indistinguishable from the initial biopsy sample, as well as areas of high-grade osteosarcoma, further support a multistep process of de-differentiation. Together, these findings are compelling evidence of malignant transformation of a primary ABC.
We acknowledge that the initial surgery performed at the outside hospital might have properly included frozen-section analysis of the biopsy material and that sampling error may have occurred during the index procedure—possibilities in the absence of complete lesional resection. In this case, however, the radiographic findings and the dominant histologic immunophenotype from medullary canal bone were both consistent with ABC and not osteosarcoma, lending support to malignant degeneration.
We have presented a fifth case of primary ABC degenerating into an osteosarcoma, now with immunohistochemical evidence supporting traditional radiologic and histologic evidence. Despite the rarity of the diagnosis, this case yields considerable insight into the pathogenetic mechanisms underlying malignant degeneration. Despite the widely held view that ABCs are benign, physicians caring for these patients must be aware that malignant transformation can occur.
Am J Orthop. 2016;45(6):E367-E372. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Donaldson WF. Aneurysmal bone cyst. J Bone Joint Surg Am. 1962;44:25-40.
2. Biesecker JL, Marcove RC, Huvos AG, Miké V. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer. 1970;26(3):615-625.
3. Aho HJ, Aho AJ, Einola S. Aneurysmal bone cyst, a study of ultrastructure and malignant transformation. Virchows Arch A Pathol Anat Histol. 1982;395(2):169-179.
4. Tillman BP, Dahlin DC, Lipscomb PR, Stewart JR. Aneurysmal bone cyst: an analysis of ninety-five cases. Mayo Clin Proc. 1968;43(7):478-495.
5. Kyriakos M, Hardy D. Malignant transformation of aneurysmal bone cyst, with an analysis of the literature. Cancer. 1991;68(8):1770-1780.
6. Mei J, Gao YS, Wang SQ, Cai XS. Malignant transformation of aneurysmal bone cysts: a case report. Chin Med J (Engl). 2009;122(1):110-112.
7. Anract P, de Pinieux G, Jeanrot C, Babinet A, Forest M, Tomeno B. Malignant fibrous histiocytoma at the site of a previously treated aneurysmal bone cyst: a case report. J Bone Joint Surg Am. 2002;84(1):106-111.
8. Hsu CC, Wang JW, Huang CH, Chen WJ. Osteosarcoma at the site of a previously treated aneurysmal bone cyst. A case report. J Bone Joint Surg Am. 2005;87(2):395-398.
9. Wuisman P, Roessner A, Blasius S, Grünert J, Vestering T, Winkelmann W. High malignant surface osteosarcoma arising at the site of a previously treated aneurysmal bone cyst. J Cancer Res Clin Oncol. 1993;119(7):375-378.
10. Brindley GW, Greene JF Jr, Frankel LS. Case reports: malignant transformation of aneurysmal bone cysts. Clin Orthop Relat Res. 2005;(438):282-287.
11. Jaffe HL, Lichtenstein L. Solitary unicameral bone cyst: with emphasis on the roentgen picture, the pathologic appearance and the pathogenesis. Arch Surg. 1942;44:1004-1025.
12. Mirra JM. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Philadelphia, PA: Lea & Febiger; 1989.
13. Oliveira AM, Chou MM, Perez-Atayde AR, Rosenberg AE. Aneurysmal bone cyst: a neoplasm driven by upregulation of the USP6 oncogene. J Clin Oncol. 2006;24(1):e1.
14. Panoutsakopoulos G, Pandis N, Kyriazoglou I, Gustafson P, Mertens F, Mandahl N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer. 1999;26(3):265-266.
15. Dujardin F, Binh MB, Bouvier C, et al. MDM2 and CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone. Mod Pathol. 2001;24(5):624-637.
Aneurysmal bone cysts (ABC) are expansile, hemorrhagic, non-neoplastic lesions that can be locally destructive1 and that can arise either de novo or secondary to another benign or malignant lesion.2 Although primary and secondary ABCs typically are benign, there are cases of malignant degeneration of primary ABCs, though the transformation arises almost exclusively in the context of prior radiation exposure.3-5 Malignant change without history of irradiation is rare; only 6 such cases have been reported.5-10 In 4 of these cases, the transformation was to osteosarcoma.5,8-10
Here we report on an ABC that degenerated into a fibroblastic osteosarcoma—the fifth such case in the medical literature. In addition to reviewing the earlier cases, we describe the radiologic and histologic underpinnings of this diagnosis and the insight that they provide into the pathogenesis of this rare process. Although the prevailing view is that ABCs are benign, it is important to know these lesions have the potential to undergo malignant transformation, even in the absence of prior radiation exposure. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy and previously asymptomatic 37-year-old man presented with thigh pain after a minor fall onto a couch. Radiographs showed a diaphyseal femoral pathologic fracture adjacent to a small but benign-appearing cystic lesion (Figures 1A, 1B).
Two years later, the patient had a bicycle accident and, after 2 weeks of significantly increased thigh swelling, presented to the emergency department at the referring institution. Radiographs showed a lytic lesion in the femoral diaphysis that was highly suspicious for malignancy (Figures 3A, 3B).
The initial biopsy specimens were evaluated at our institution and interpreted as being consistent with an ABC, with negative immunohistochemical staining for MDM2 (Figures 5A, 5B).
The patient underwent a 3-month course of neoadjuvant chemotherapy with cisplatin and doxorubicin. Interval-staging contrast-enhanced chest, abdomen, and pelvis computed tomography (CT) showed no evidence of metastatic disease. Preoperative MRI showed a significantly larger heterogeneous mass, now with neurovascular involvement, which precluded limb salvage.
Discussion
Aneurysmal bone cysts are expansile, hemorrhagic, locally destructive lesions that generally develop within the first 3 decades of life. Ever since they were first described by Jaffe and Lichtenstein11 in 1942, the most widely accepted theory of their pathogenesis has been that they begin as a benign reactive vascular process.12 However, more recent molecular studies by Oliveira and colleagues13 and Panoutsakopoulos and colleagues14 have demonstrated a clonal neoplastic basis for primary ABCs related to cytogenetic upregulation of oncogenes USP6 and CDH11 after translocation of 17p13 and 16q22.
Given the clonal nature of these lesions, it is surprising that malignant transformation is so rare. Until now, there have been only 4 reports of an ABC undergoing malignant degeneration to osteosarcoma without prior radiation exposure.
In this article, we have presented a fifth case of a primary ABC degenerating into an osteosarcoma, which in this instance was the fibroblastic subtype. This diagnosis was strongly supported by radiologic and pathologic evidence. From a radiologic perspective, imaging at initial presentation showed absolutely no suspicious features, and the same was true when follow-up radiographs were obtained, 1 month later. Although 1 month is short for a follow-up, the complete lack of radiographic changes would be highly unusual if in fact there had been a coexisting, undetected lesion as aggressive as the one that ultimately developed. Furthermore, imaging at second presentation, almost 2 years later, showed an extremely rapid evolution of findings over 1 month. Extrapolating back in time, we think this time course indicates the malignancy developed not long before its aggressive features were detected.
Genetic evidence suggests that most conventional high-grade osteosarcomas arise de novo from a mesenchymal precursor driven by multiple genetic aberrations. Less often, low-grade osteosarcomas progress to high-grade osteosarcomas. Amplification of 12q13-15 with resulting overexpression of MDM2 and CDK4 proteins is found in low-grade osteosarcomas and persists in examples that progress to higher-grade forms.15 Not only did review of our patient’s initial biopsy sample reveal no evidence of malignant features or abnormal mitotic activity, but the complete absence of MDM2 suggests not even a low-grade osteosarcoma was present at the time. By contrast, the second incisional biopsy specimen, 2 years later, showed markedly different histology and pronounced expression of MDM2 throughout the specimen. This finding suggests the histologically high-grade osteosarcoma did not arise de novo but rather secondarily from a low-grade osteosarcoma that had arisen from an ABC. Results of the final heterogeneous histology of the very large mass, which contained benign ABC areas indistinguishable from the initial biopsy sample, as well as areas of high-grade osteosarcoma, further support a multistep process of de-differentiation. Together, these findings are compelling evidence of malignant transformation of a primary ABC.
We acknowledge that the initial surgery performed at the outside hospital might have properly included frozen-section analysis of the biopsy material and that sampling error may have occurred during the index procedure—possibilities in the absence of complete lesional resection. In this case, however, the radiographic findings and the dominant histologic immunophenotype from medullary canal bone were both consistent with ABC and not osteosarcoma, lending support to malignant degeneration.
We have presented a fifth case of primary ABC degenerating into an osteosarcoma, now with immunohistochemical evidence supporting traditional radiologic and histologic evidence. Despite the rarity of the diagnosis, this case yields considerable insight into the pathogenetic mechanisms underlying malignant degeneration. Despite the widely held view that ABCs are benign, physicians caring for these patients must be aware that malignant transformation can occur.
Am J Orthop. 2016;45(6):E367-E372. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Aneurysmal bone cysts (ABC) are expansile, hemorrhagic, non-neoplastic lesions that can be locally destructive1 and that can arise either de novo or secondary to another benign or malignant lesion.2 Although primary and secondary ABCs typically are benign, there are cases of malignant degeneration of primary ABCs, though the transformation arises almost exclusively in the context of prior radiation exposure.3-5 Malignant change without history of irradiation is rare; only 6 such cases have been reported.5-10 In 4 of these cases, the transformation was to osteosarcoma.5,8-10
Here we report on an ABC that degenerated into a fibroblastic osteosarcoma—the fifth such case in the medical literature. In addition to reviewing the earlier cases, we describe the radiologic and histologic underpinnings of this diagnosis and the insight that they provide into the pathogenesis of this rare process. Although the prevailing view is that ABCs are benign, it is important to know these lesions have the potential to undergo malignant transformation, even in the absence of prior radiation exposure. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy and previously asymptomatic 37-year-old man presented with thigh pain after a minor fall onto a couch. Radiographs showed a diaphyseal femoral pathologic fracture adjacent to a small but benign-appearing cystic lesion (Figures 1A, 1B).
Two years later, the patient had a bicycle accident and, after 2 weeks of significantly increased thigh swelling, presented to the emergency department at the referring institution. Radiographs showed a lytic lesion in the femoral diaphysis that was highly suspicious for malignancy (Figures 3A, 3B).
The initial biopsy specimens were evaluated at our institution and interpreted as being consistent with an ABC, with negative immunohistochemical staining for MDM2 (Figures 5A, 5B).
The patient underwent a 3-month course of neoadjuvant chemotherapy with cisplatin and doxorubicin. Interval-staging contrast-enhanced chest, abdomen, and pelvis computed tomography (CT) showed no evidence of metastatic disease. Preoperative MRI showed a significantly larger heterogeneous mass, now with neurovascular involvement, which precluded limb salvage.
Discussion
Aneurysmal bone cysts are expansile, hemorrhagic, locally destructive lesions that generally develop within the first 3 decades of life. Ever since they were first described by Jaffe and Lichtenstein11 in 1942, the most widely accepted theory of their pathogenesis has been that they begin as a benign reactive vascular process.12 However, more recent molecular studies by Oliveira and colleagues13 and Panoutsakopoulos and colleagues14 have demonstrated a clonal neoplastic basis for primary ABCs related to cytogenetic upregulation of oncogenes USP6 and CDH11 after translocation of 17p13 and 16q22.
Given the clonal nature of these lesions, it is surprising that malignant transformation is so rare. Until now, there have been only 4 reports of an ABC undergoing malignant degeneration to osteosarcoma without prior radiation exposure.
In this article, we have presented a fifth case of a primary ABC degenerating into an osteosarcoma, which in this instance was the fibroblastic subtype. This diagnosis was strongly supported by radiologic and pathologic evidence. From a radiologic perspective, imaging at initial presentation showed absolutely no suspicious features, and the same was true when follow-up radiographs were obtained, 1 month later. Although 1 month is short for a follow-up, the complete lack of radiographic changes would be highly unusual if in fact there had been a coexisting, undetected lesion as aggressive as the one that ultimately developed. Furthermore, imaging at second presentation, almost 2 years later, showed an extremely rapid evolution of findings over 1 month. Extrapolating back in time, we think this time course indicates the malignancy developed not long before its aggressive features were detected.
Genetic evidence suggests that most conventional high-grade osteosarcomas arise de novo from a mesenchymal precursor driven by multiple genetic aberrations. Less often, low-grade osteosarcomas progress to high-grade osteosarcomas. Amplification of 12q13-15 with resulting overexpression of MDM2 and CDK4 proteins is found in low-grade osteosarcomas and persists in examples that progress to higher-grade forms.15 Not only did review of our patient’s initial biopsy sample reveal no evidence of malignant features or abnormal mitotic activity, but the complete absence of MDM2 suggests not even a low-grade osteosarcoma was present at the time. By contrast, the second incisional biopsy specimen, 2 years later, showed markedly different histology and pronounced expression of MDM2 throughout the specimen. This finding suggests the histologically high-grade osteosarcoma did not arise de novo but rather secondarily from a low-grade osteosarcoma that had arisen from an ABC. Results of the final heterogeneous histology of the very large mass, which contained benign ABC areas indistinguishable from the initial biopsy sample, as well as areas of high-grade osteosarcoma, further support a multistep process of de-differentiation. Together, these findings are compelling evidence of malignant transformation of a primary ABC.
We acknowledge that the initial surgery performed at the outside hospital might have properly included frozen-section analysis of the biopsy material and that sampling error may have occurred during the index procedure—possibilities in the absence of complete lesional resection. In this case, however, the radiographic findings and the dominant histologic immunophenotype from medullary canal bone were both consistent with ABC and not osteosarcoma, lending support to malignant degeneration.
We have presented a fifth case of primary ABC degenerating into an osteosarcoma, now with immunohistochemical evidence supporting traditional radiologic and histologic evidence. Despite the rarity of the diagnosis, this case yields considerable insight into the pathogenetic mechanisms underlying malignant degeneration. Despite the widely held view that ABCs are benign, physicians caring for these patients must be aware that malignant transformation can occur.
Am J Orthop. 2016;45(6):E367-E372. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Donaldson WF. Aneurysmal bone cyst. J Bone Joint Surg Am. 1962;44:25-40.
2. Biesecker JL, Marcove RC, Huvos AG, Miké V. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer. 1970;26(3):615-625.
3. Aho HJ, Aho AJ, Einola S. Aneurysmal bone cyst, a study of ultrastructure and malignant transformation. Virchows Arch A Pathol Anat Histol. 1982;395(2):169-179.
4. Tillman BP, Dahlin DC, Lipscomb PR, Stewart JR. Aneurysmal bone cyst: an analysis of ninety-five cases. Mayo Clin Proc. 1968;43(7):478-495.
5. Kyriakos M, Hardy D. Malignant transformation of aneurysmal bone cyst, with an analysis of the literature. Cancer. 1991;68(8):1770-1780.
6. Mei J, Gao YS, Wang SQ, Cai XS. Malignant transformation of aneurysmal bone cysts: a case report. Chin Med J (Engl). 2009;122(1):110-112.
7. Anract P, de Pinieux G, Jeanrot C, Babinet A, Forest M, Tomeno B. Malignant fibrous histiocytoma at the site of a previously treated aneurysmal bone cyst: a case report. J Bone Joint Surg Am. 2002;84(1):106-111.
8. Hsu CC, Wang JW, Huang CH, Chen WJ. Osteosarcoma at the site of a previously treated aneurysmal bone cyst. A case report. J Bone Joint Surg Am. 2005;87(2):395-398.
9. Wuisman P, Roessner A, Blasius S, Grünert J, Vestering T, Winkelmann W. High malignant surface osteosarcoma arising at the site of a previously treated aneurysmal bone cyst. J Cancer Res Clin Oncol. 1993;119(7):375-378.
10. Brindley GW, Greene JF Jr, Frankel LS. Case reports: malignant transformation of aneurysmal bone cysts. Clin Orthop Relat Res. 2005;(438):282-287.
11. Jaffe HL, Lichtenstein L. Solitary unicameral bone cyst: with emphasis on the roentgen picture, the pathologic appearance and the pathogenesis. Arch Surg. 1942;44:1004-1025.
12. Mirra JM. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Philadelphia, PA: Lea & Febiger; 1989.
13. Oliveira AM, Chou MM, Perez-Atayde AR, Rosenberg AE. Aneurysmal bone cyst: a neoplasm driven by upregulation of the USP6 oncogene. J Clin Oncol. 2006;24(1):e1.
14. Panoutsakopoulos G, Pandis N, Kyriazoglou I, Gustafson P, Mertens F, Mandahl N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer. 1999;26(3):265-266.
15. Dujardin F, Binh MB, Bouvier C, et al. MDM2 and CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone. Mod Pathol. 2001;24(5):624-637.
1. Donaldson WF. Aneurysmal bone cyst. J Bone Joint Surg Am. 1962;44:25-40.
2. Biesecker JL, Marcove RC, Huvos AG, Miké V. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer. 1970;26(3):615-625.
3. Aho HJ, Aho AJ, Einola S. Aneurysmal bone cyst, a study of ultrastructure and malignant transformation. Virchows Arch A Pathol Anat Histol. 1982;395(2):169-179.
4. Tillman BP, Dahlin DC, Lipscomb PR, Stewart JR. Aneurysmal bone cyst: an analysis of ninety-five cases. Mayo Clin Proc. 1968;43(7):478-495.
5. Kyriakos M, Hardy D. Malignant transformation of aneurysmal bone cyst, with an analysis of the literature. Cancer. 1991;68(8):1770-1780.
6. Mei J, Gao YS, Wang SQ, Cai XS. Malignant transformation of aneurysmal bone cysts: a case report. Chin Med J (Engl). 2009;122(1):110-112.
7. Anract P, de Pinieux G, Jeanrot C, Babinet A, Forest M, Tomeno B. Malignant fibrous histiocytoma at the site of a previously treated aneurysmal bone cyst: a case report. J Bone Joint Surg Am. 2002;84(1):106-111.
8. Hsu CC, Wang JW, Huang CH, Chen WJ. Osteosarcoma at the site of a previously treated aneurysmal bone cyst. A case report. J Bone Joint Surg Am. 2005;87(2):395-398.
9. Wuisman P, Roessner A, Blasius S, Grünert J, Vestering T, Winkelmann W. High malignant surface osteosarcoma arising at the site of a previously treated aneurysmal bone cyst. J Cancer Res Clin Oncol. 1993;119(7):375-378.
10. Brindley GW, Greene JF Jr, Frankel LS. Case reports: malignant transformation of aneurysmal bone cysts. Clin Orthop Relat Res. 2005;(438):282-287.
11. Jaffe HL, Lichtenstein L. Solitary unicameral bone cyst: with emphasis on the roentgen picture, the pathologic appearance and the pathogenesis. Arch Surg. 1942;44:1004-1025.
12. Mirra JM. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Philadelphia, PA: Lea & Febiger; 1989.
13. Oliveira AM, Chou MM, Perez-Atayde AR, Rosenberg AE. Aneurysmal bone cyst: a neoplasm driven by upregulation of the USP6 oncogene. J Clin Oncol. 2006;24(1):e1.
14. Panoutsakopoulos G, Pandis N, Kyriazoglou I, Gustafson P, Mertens F, Mandahl N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer. 1999;26(3):265-266.
15. Dujardin F, Binh MB, Bouvier C, et al. MDM2 and CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone. Mod Pathol. 2001;24(5):624-637.