User login
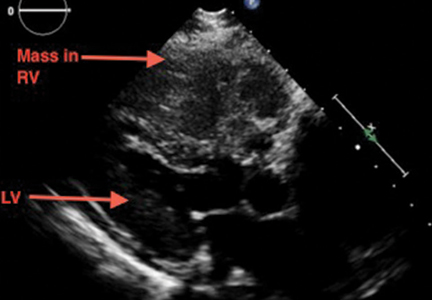
A large mass in the right ventricle: Tumor or thrombus?
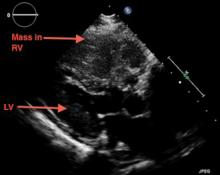
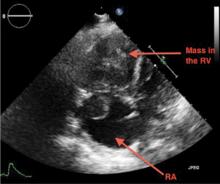
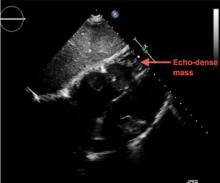
A 69-year-old woman with hypertension, diabetes mellitus, and chronic kidney disease presented with a 1-month history of worsening episodic dyspnea, lower-extremity edema, and dizziness. Two months earlier, she had been diagnosed with poorly differentiated pelvic adnexal sarcoma associated with a mature teratoma of the left ovary, and she had undergone bilateral salpingo-oophorectomy, pelvic and para-aortic lymph node dissection, and omentectomy.
Examination revealed tachypnea (23 breaths per minute) and bilateral pitting pedal edema. The neck veins were distended. There was no hepatomegaly. Results of laboratory testing were unremarkable.
EVALUATING A CARDIAC MASS
Thrombus, tumor, or vegetation?
If an intracardiac mass is discovered, we need to determine what it is.
Thrombosis is more likely if contrast echocardiography shows the mass has no stalk (thrombi almost never have a stalk), the atrial chamber is enlarged, cardiac output is low, there is stasis, the mass is avascular, and it responds to thrombolytic therapy. A giant organized thrombus can clinically mimic a tumor if it is immobile, is located close to the wall, and responds poorly to thrombolysis. A wall-motion abnormality adjacent to the mass, global hypokinesis, or a concomitant autoimmune condition such as lupus erythematosus or antiphospholipid antibody syndrome may also favor thrombosis.
Tumors in the heart are uncommon. The prevalence of primary cardiac tumors has been reported as 0.01% to 0.1% in autopsy studies. Metastases to the pericardium, myocardium, coronary arteries, or great vessels have been found at autopsy in 0.7% to 3.5% of the general population and in 9.1% of patients with known malignancy.1
Vegetations from infective endocarditis should also be considered early in the evaluation of an intracardiac mass. They can result from bacterial, fungal, or parasitic infection. Vegetations are generally irregular in appearance, mobile, and attached to a valve. Left-sided valves are generally involved, and a larger mass may indicate fungal origin. Abscess from tuberculosis may need to be considered in the appropriate setting. Whenever feasible, tissue diagnosis is desirable.
Occasionally, there may be an inflammatory component to masses detected in the setting of autoimmune disease.
CT and MRI
If echocardiography cannot clearly distinguish whether the mass is a tumor or a thrombus, MRI with gadolinium contrast is useful. MRI is superior to CT in depicting anatomic details and does not involve radiation.
Cardiac CT is increasingly used when other imaging findings are equivocal or to study a calcified mass. CT with contrast carries a small risk of contrast-induced nephropathy and has lower soft-tissue and temporal resolution. CT without contrast can detect the mass and reveal calcifications within the mass, but contrast is needed to assess the vascularity of the tumor. New-generation CT with electrocardiographic gating nearly matches MRI imaging, and CT is preferred for patients with contraindications to MRI.
CT provides additional information on the global assessment of the chest, lung and vascular structures.2 Cardiac CT and MRI help in precise anatomic delineation, characterization, and preoperative planning of treatment of a large cardiac mass.
TYPES OF CARDIAC TUMORS
Metastases account for most cardiac tumors and are often from primary cancers of the lung, breast, skin, thyroid, and kidney.
Primary cardiac tumors are most often myxomas, which are benign and generally found in the atrial chamber, solitary, with a stalk attached to the area of the fossa ovalis. Other primary cardiac tumors include sarcomas, angiosarcomas, rhabdomyosarcomas, papillary fibroelastomas, lipomas, hemangiomas, mesotheliomas, and rhabdomyomas.
TREATMENT OF CARDIAC TUMORS
For primary and secondary cardiac tumors, complete resection should be considered, provided there is no other organ involvement.3 For suspected lymphomas, image-guided biopsy should be performed before treatment.
For uncertain and diagnostically challenging cases, guided biopsy of the lesions using intracardiac echocardiography or transesophageal echocardiography has been reported to be helpful.4
Most often, the workup and management of cardiac masses calls for a team involving an internist, cardiologist, cardiothoracic surgeon, and vascular medicine specialist. Depending on the nature of the mass, the team may also include an oncologist, radiotherapist, and infectious disease specialist.
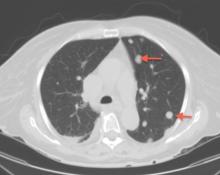
Because our patient had significant kidney disease, CT was done without contrast. However, it was not able to clearly delineate the mass in the right ventricle. Cardiac MRI was not performed. Biopsy with transesophageal or intracardiac echocardiographic guidance was not an option, as the patient’s condition was poor.
TAKE-HOME POINTS
The differential diagnosis of an intracardiac mass includes thrombus, benign or malignant tumors, and masses of infectious or inflammatory origin. While noninvasive imaging tests provide clues that can help narrow the differential diagnosis, tissue biopsy with histologic study is necessary to confirm the diagnosis. A team approach is paramount in managing cardiac masses.
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128:1790–1794.
- Exarhos DN, Tavernaraki EA, Kyratzi F, et al. Imaging of cardiac tumours and masses. Hospital Chronicles 2010; 5:1–9.
- Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors—diagnosis and surgical treatment. Dtsch Arztebl Int 2014; 111:205–211.
- Park K-I, Kim MJ, Oh JK, et al. Intracardiac echocardiography to guide biopsy for two cases of intracardiac masses. Korean Circ J 2015; 45:165–168.
A 69-year-old woman with hypertension, diabetes mellitus, and chronic kidney disease presented with a 1-month history of worsening episodic dyspnea, lower-extremity edema, and dizziness. Two months earlier, she had been diagnosed with poorly differentiated pelvic adnexal sarcoma associated with a mature teratoma of the left ovary, and she had undergone bilateral salpingo-oophorectomy, pelvic and para-aortic lymph node dissection, and omentectomy.
Examination revealed tachypnea (23 breaths per minute) and bilateral pitting pedal edema. The neck veins were distended. There was no hepatomegaly. Results of laboratory testing were unremarkable.
EVALUATING A CARDIAC MASS
Thrombus, tumor, or vegetation?
If an intracardiac mass is discovered, we need to determine what it is.
Thrombosis is more likely if contrast echocardiography shows the mass has no stalk (thrombi almost never have a stalk), the atrial chamber is enlarged, cardiac output is low, there is stasis, the mass is avascular, and it responds to thrombolytic therapy. A giant organized thrombus can clinically mimic a tumor if it is immobile, is located close to the wall, and responds poorly to thrombolysis. A wall-motion abnormality adjacent to the mass, global hypokinesis, or a concomitant autoimmune condition such as lupus erythematosus or antiphospholipid antibody syndrome may also favor thrombosis.
Tumors in the heart are uncommon. The prevalence of primary cardiac tumors has been reported as 0.01% to 0.1% in autopsy studies. Metastases to the pericardium, myocardium, coronary arteries, or great vessels have been found at autopsy in 0.7% to 3.5% of the general population and in 9.1% of patients with known malignancy.1
Vegetations from infective endocarditis should also be considered early in the evaluation of an intracardiac mass. They can result from bacterial, fungal, or parasitic infection. Vegetations are generally irregular in appearance, mobile, and attached to a valve. Left-sided valves are generally involved, and a larger mass may indicate fungal origin. Abscess from tuberculosis may need to be considered in the appropriate setting. Whenever feasible, tissue diagnosis is desirable.
Occasionally, there may be an inflammatory component to masses detected in the setting of autoimmune disease.
CT and MRI
If echocardiography cannot clearly distinguish whether the mass is a tumor or a thrombus, MRI with gadolinium contrast is useful. MRI is superior to CT in depicting anatomic details and does not involve radiation.
Cardiac CT is increasingly used when other imaging findings are equivocal or to study a calcified mass. CT with contrast carries a small risk of contrast-induced nephropathy and has lower soft-tissue and temporal resolution. CT without contrast can detect the mass and reveal calcifications within the mass, but contrast is needed to assess the vascularity of the tumor. New-generation CT with electrocardiographic gating nearly matches MRI imaging, and CT is preferred for patients with contraindications to MRI.
CT provides additional information on the global assessment of the chest, lung and vascular structures.2 Cardiac CT and MRI help in precise anatomic delineation, characterization, and preoperative planning of treatment of a large cardiac mass.
TYPES OF CARDIAC TUMORS
Metastases account for most cardiac tumors and are often from primary cancers of the lung, breast, skin, thyroid, and kidney.
Primary cardiac tumors are most often myxomas, which are benign and generally found in the atrial chamber, solitary, with a stalk attached to the area of the fossa ovalis. Other primary cardiac tumors include sarcomas, angiosarcomas, rhabdomyosarcomas, papillary fibroelastomas, lipomas, hemangiomas, mesotheliomas, and rhabdomyomas.
TREATMENT OF CARDIAC TUMORS
For primary and secondary cardiac tumors, complete resection should be considered, provided there is no other organ involvement.3 For suspected lymphomas, image-guided biopsy should be performed before treatment.
For uncertain and diagnostically challenging cases, guided biopsy of the lesions using intracardiac echocardiography or transesophageal echocardiography has been reported to be helpful.4
Most often, the workup and management of cardiac masses calls for a team involving an internist, cardiologist, cardiothoracic surgeon, and vascular medicine specialist. Depending on the nature of the mass, the team may also include an oncologist, radiotherapist, and infectious disease specialist.
Because our patient had significant kidney disease, CT was done without contrast. However, it was not able to clearly delineate the mass in the right ventricle. Cardiac MRI was not performed. Biopsy with transesophageal or intracardiac echocardiographic guidance was not an option, as the patient’s condition was poor.
TAKE-HOME POINTS
The differential diagnosis of an intracardiac mass includes thrombus, benign or malignant tumors, and masses of infectious or inflammatory origin. While noninvasive imaging tests provide clues that can help narrow the differential diagnosis, tissue biopsy with histologic study is necessary to confirm the diagnosis. A team approach is paramount in managing cardiac masses.
A 69-year-old woman with hypertension, diabetes mellitus, and chronic kidney disease presented with a 1-month history of worsening episodic dyspnea, lower-extremity edema, and dizziness. Two months earlier, she had been diagnosed with poorly differentiated pelvic adnexal sarcoma associated with a mature teratoma of the left ovary, and she had undergone bilateral salpingo-oophorectomy, pelvic and para-aortic lymph node dissection, and omentectomy.
Examination revealed tachypnea (23 breaths per minute) and bilateral pitting pedal edema. The neck veins were distended. There was no hepatomegaly. Results of laboratory testing were unremarkable.
EVALUATING A CARDIAC MASS
Thrombus, tumor, or vegetation?
If an intracardiac mass is discovered, we need to determine what it is.
Thrombosis is more likely if contrast echocardiography shows the mass has no stalk (thrombi almost never have a stalk), the atrial chamber is enlarged, cardiac output is low, there is stasis, the mass is avascular, and it responds to thrombolytic therapy. A giant organized thrombus can clinically mimic a tumor if it is immobile, is located close to the wall, and responds poorly to thrombolysis. A wall-motion abnormality adjacent to the mass, global hypokinesis, or a concomitant autoimmune condition such as lupus erythematosus or antiphospholipid antibody syndrome may also favor thrombosis.
Tumors in the heart are uncommon. The prevalence of primary cardiac tumors has been reported as 0.01% to 0.1% in autopsy studies. Metastases to the pericardium, myocardium, coronary arteries, or great vessels have been found at autopsy in 0.7% to 3.5% of the general population and in 9.1% of patients with known malignancy.1
Vegetations from infective endocarditis should also be considered early in the evaluation of an intracardiac mass. They can result from bacterial, fungal, or parasitic infection. Vegetations are generally irregular in appearance, mobile, and attached to a valve. Left-sided valves are generally involved, and a larger mass may indicate fungal origin. Abscess from tuberculosis may need to be considered in the appropriate setting. Whenever feasible, tissue diagnosis is desirable.
Occasionally, there may be an inflammatory component to masses detected in the setting of autoimmune disease.
CT and MRI
If echocardiography cannot clearly distinguish whether the mass is a tumor or a thrombus, MRI with gadolinium contrast is useful. MRI is superior to CT in depicting anatomic details and does not involve radiation.
Cardiac CT is increasingly used when other imaging findings are equivocal or to study a calcified mass. CT with contrast carries a small risk of contrast-induced nephropathy and has lower soft-tissue and temporal resolution. CT without contrast can detect the mass and reveal calcifications within the mass, but contrast is needed to assess the vascularity of the tumor. New-generation CT with electrocardiographic gating nearly matches MRI imaging, and CT is preferred for patients with contraindications to MRI.
CT provides additional information on the global assessment of the chest, lung and vascular structures.2 Cardiac CT and MRI help in precise anatomic delineation, characterization, and preoperative planning of treatment of a large cardiac mass.
TYPES OF CARDIAC TUMORS
Metastases account for most cardiac tumors and are often from primary cancers of the lung, breast, skin, thyroid, and kidney.
Primary cardiac tumors are most often myxomas, which are benign and generally found in the atrial chamber, solitary, with a stalk attached to the area of the fossa ovalis. Other primary cardiac tumors include sarcomas, angiosarcomas, rhabdomyosarcomas, papillary fibroelastomas, lipomas, hemangiomas, mesotheliomas, and rhabdomyomas.
TREATMENT OF CARDIAC TUMORS
For primary and secondary cardiac tumors, complete resection should be considered, provided there is no other organ involvement.3 For suspected lymphomas, image-guided biopsy should be performed before treatment.
For uncertain and diagnostically challenging cases, guided biopsy of the lesions using intracardiac echocardiography or transesophageal echocardiography has been reported to be helpful.4
Most often, the workup and management of cardiac masses calls for a team involving an internist, cardiologist, cardiothoracic surgeon, and vascular medicine specialist. Depending on the nature of the mass, the team may also include an oncologist, radiotherapist, and infectious disease specialist.
Because our patient had significant kidney disease, CT was done without contrast. However, it was not able to clearly delineate the mass in the right ventricle. Cardiac MRI was not performed. Biopsy with transesophageal or intracardiac echocardiographic guidance was not an option, as the patient’s condition was poor.
TAKE-HOME POINTS
The differential diagnosis of an intracardiac mass includes thrombus, benign or malignant tumors, and masses of infectious or inflammatory origin. While noninvasive imaging tests provide clues that can help narrow the differential diagnosis, tissue biopsy with histologic study is necessary to confirm the diagnosis. A team approach is paramount in managing cardiac masses.
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128:1790–1794.
- Exarhos DN, Tavernaraki EA, Kyratzi F, et al. Imaging of cardiac tumours and masses. Hospital Chronicles 2010; 5:1–9.
- Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors—diagnosis and surgical treatment. Dtsch Arztebl Int 2014; 111:205–211.
- Park K-I, Kim MJ, Oh JK, et al. Intracardiac echocardiography to guide biopsy for two cases of intracardiac masses. Korean Circ J 2015; 45:165–168.
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128:1790–1794.
- Exarhos DN, Tavernaraki EA, Kyratzi F, et al. Imaging of cardiac tumours and masses. Hospital Chronicles 2010; 5:1–9.
- Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors—diagnosis and surgical treatment. Dtsch Arztebl Int 2014; 111:205–211.
- Park K-I, Kim MJ, Oh JK, et al. Intracardiac echocardiography to guide biopsy for two cases of intracardiac masses. Korean Circ J 2015; 45:165–168.