User login
Depressed and sick with ‘nothing to live for’
CASE ‘I’ve had enough’
The psychiatry consultation team is asked to evaluate Mr. M, age 76, for a passive death wish and depression 2 months after he was admitted to the hospital after a traumatic fall.
Mr. M has several chronic medical conditions, including hypertension, type 2 diabetes mellitus, and coronary artery disease. Within 2 weeks of his admission, he developed Proteus mirabilis pneumonia and persistent respiratory failure requiring tracheostomy. Records indicate that Mr. M has told family and his treatment team, “I’m tired, just let me go.” He then developed antibiotic-induced Clostridium difficile colitis and acute renal failure requiring temporary renal replacement therapy (RRT).
Mr. M’s clinical status improves, allowing his transfer to a transitional unit, where he continues to state, “I have had enough. I’m done.” He asks for the tracheostomy tube to be removed and RRT discontinued. He is treated again for persistent C. difficile colitis and, within 2 weeks, develops hypotension, hypoxia, emesis, and abdominal distension, requiring transfer to the ICU for management of ileus.
He is stabilized with vasopressors and artificial nutritional support by nasogastric tube. Renal function improves, RRT is discontinued, and he is transferred to the general medical floor.
After a few days on the general medical floor, Mr. M develops a urinary tract infection and develops antibiotic-induced acute renal failure requiring re-initiation of RRT. A percutaneous endoscopic gastrostomy (PEG) tube is placed for nutrition when he shows little improvement with swallowing exercises. Two days after placing the PEG tube, he develops respiratory failure secondary to a left-sided pneumothorax and is transferred to the ICU for the third time, where he undergoes repeated bronchoscopies and requires pressure support ventilation.
One week later, Mr. M is weaned off the ventilator and transferred to the general medical floor with aggressive respiratory therapy, tube feeding, and RRT. Mr. M’s chart indicates that he expresses an ongoing desire to withdraw RRT, the tracheostomy, and feeding tube.
Which of the following would you consider when assessing Mr. M’s decision-making capacity (DMC)?
a) his ability to understand information relevant to treatment decision-making
b) his ability to appreciate the significance of his diagnoses and treatment options and consequences in the context of his own life circumstances
c) his ability to communicate a preference
d) his ability to reason through the relevant information to weigh the potential costs and benefits of treatment options
e) all of the above
HISTORY Guilt and regret
Mr. M reports a 30-year history of depression that has responded poorly to a variety of medications, outpatient psychotherapy, and electroconvulsive therapy. Before admission, he says, he was adherent to citalopram, 20 mg/d, and buspirone, 30 mg/d. Citalopram is continued throughout his hospitalization, although buspirone was discontinued for unknown reasons during admission.
Mr. M is undergoing hemodialysis during his initial encounter with the psychiatry team. He struggles to communicate clearly because of the tracheostomy but is alert, oriented to person and location, answers questions appropriately, maintains good eye contact, and does not demonstrate any psychomotor abnormalities. He describes his disposition as “tired,” and is on the verge of tears during the interview.
Mr. M denies physical discomfort and states, “I have just had enough. I do not want all of this done.” He clarifies that he is not suicidal and denies a history of suicidal or self-injurious behaviors.
Mr. M describes having low mood, anhedonia, and insomnia to varying degrees throughout his adult life. He also reports feeling guilt and regret about earlier experiences, but does not elaborate. He denies symptoms of panic disorder, obsessive-compulsive disorder, posttraumatic stress disorder, mania, or hypomania. He reports an episode of visual hallucinations during an earlier hospitalization, likely a symptom of delirium, but denies any recent visual disturbances.
Mr. M’s thought process is linear and logical, with intact abstract reasoning and no evidence of delusions. Attention and concentration are intact for most of the interview but diminish as he becomes fatigued. Mr. M can describe past treatments in detail and recounts the events leading to this hospitalization.
The authors’ observations
Literature on assessment of DMC recently has centered on the 4-ability model, proposed by Grisso and Appelbaum.1 With this approach, impairment to any of the 4 processes of understanding, appreciation, ability to express a choice, and ability to use reasoning to weigh treatment options could interfere with capacity to make decisions. Few studies have clarified the mechanism and degree to which depression may impair these 4 elements, making capacity assessments in a depressed patient challenging.
Preliminary evidence suggests that depression severity, not the presence of depression, determines the degree to which DMC is impaired, if at all. In several studies, depressed patients did not demonstrate more impaired DMC compared with non-depressed patients based on standardized assessments.2-4 In depressed patients who lack DMC, case reports5-7 and cross-sectional studies8 indicate that appreciation—one’s ability to comprehend the personal relevance of illness and potential consequences of treatments in the context of one’s life—is most often impaired. Other studies suggest that the ability to reason through decision-specific information and weigh the risks and benefits of treatment options is commonly impaired in depressed patients.9,10
Even when a depressed patient demonstrates the 4 elements of DMC, providers might be concerned that the patient’s preferences are skewed by the negative emotions associated with depression.11-13 In such a case, the patient’s expressed wishes might not be consistent with views and priorities that were expressed during an earlier, euthymic period.
Rather than focusing on whether cognitive elements of DMC are impaired, some experts advocate for assessing how depression might lead to “unbalanced” decision-making that is impaired by a patient’s tendency to undervalue positive outcomes and overvalue negative ones.14 Some depressed patients will decide to forego additional medical interventions because they do not see the potential benefits of treatment, view events through a negative lens, and lack hope for the future; however, studies indicate this is not typically the case.15-17
In a study of >2,500 patients age >65 with chronic medical conditions, Garrett et al15 found that those who were depressed communicated a desire for more treatment compared with non-depressed patients. Another study of patients’ wishes for life-sustaining treatment among those who had mild or moderate depression found that most patients did not express a greater desire for life-sustaining medical interventions after their depressive episode remitted. An increased desire for life-sustaining medical interventions occurred only among the most severely depressed patients.16 Similarly, Lee and Ganzini17 found that treatment preferences among patients with mild or moderate depression and serious physical illness were unchanged after the mood disorder was treated.
These findings demonstrate that a clinician charged with assessing DMC must evaluate the severity of a patient’s depression and carefully consider how mood is influencing his (her) perspective and cognitive abilities. It is important to observe how the depressed patient perceives feelings of sadness or hopelessness in the context of decision-making, and how he (she) integrates these feelings when assigning relative value to potential outcomes and alternative treatment options. Because the intensity of depression could vary over time, assessment of the depressed patient’s decision-making abilities must be viewed as a dynamic process.
Clinical application
Recent studies indicate that, although the in-hospital mortality rate for critically ill patients who develop acute renal failure is high, it is variable, ranging from 28% to 90%.18 In one study, patients who required more interventions over the course of a hospital stay (eg, mechanical ventilation, vasopressors) had an in-hospital mortality rate closer to 60% after initiating RRT.19 In a similar trial,20,21 mean survival for critically ill patients with acute renal failure was 32 days from initiation of dialysis; only 27% of these patients were alive 6 months later.21
Given his complicated hospital course, the medical team estimates that Mr. M has a reasonable chance of surviving to discharge, although his longer-term prognosis is poor.
EVALUATION Conflicting preferences
Mr. M expresses reasonable understanding of the medical indications for temporary RRT, respiratory therapy, and enteral tube feedings, and the consequences of withdrawing these interventions. He understands that the primary team recommended ongoing but temporary use of life-sustaining interventions, anticipating that he would recover from his acute medical conditions. Mr. M clearly articulates that he wants to terminate RRT knowing that this would cause a buildup of urea and other toxins, to resume eating by mouth despite the risk of aspiration, and to be allowed to die “naturally.”
Mr. M declines to speak with a clergy member, explaining that he preferred direct contact with God and had reconciled himself to the “consequences” of his actions. He reports having “nothing left to live for” and “nothing left to do.” He says that he is “tired of being a burden” to his wife and son, regrets the way he treated them in the past, and believes they would be better off without him.
Although Mr. M’s abilities to understand, reason, and express a preference are intact, the psychiatry team is concerned that depression could be influencing his perspective, thereby compromising his appreciation for the personal relevance of his request to withdraw life-sustaining treatments. The psychiatrist shares this concern with Mr. M, who voices an understanding that undertreated depression could lead him to make irreversible decisions about his medical treatment that he might not make if he were not depressed; nevertheless, he continues to state that he is “ready” to die. With his permission, the team seeks additional information from Mr. M’s family.
Mr. M’s wife recalls a conversation with her husband 5 years ago in which he said that, were he to become seriously ill, “he would want everything done.” However, she also reports that Mr. M has been expressing a passive death wish “for years,” as he was struggling with chronic medical conditions that led to recurrent hospital admissions.
“He has always been a negative person,” she adds, and confirms that he has been depressed for most of their marriage.
The conflict between Mr. M’s earlier expressed preference for full care and his current wish to withdraw life-sustaining therapies and experience a “natural death” raises significant concern that depression could explain this change in perspective. When asked about this discrepancy, Mr. M admits that he “wanted everything done” in the past, when he was younger and healthier, but his preferences changed as his chronic medical problems progressed.
OUTCOME Better mood, discharge
We encourage Mr. M to continue discussing his treatment preferences with his family, while meeting with the palliative care team to address medical conditions that could be exacerbating depression and to clarify his goals of care. The medical team and Mr. M report feeling relieved when a palliative care consult is suggested, although his wife and son ask that it be delayed until Mr. M is more medically stable. The treatment team acknowledges the competing risks of proceeding too hastily with Mr. M’s request to withdraw life-sustaining treatments because of depression, and of delaying his decision, which could prolong suffering and violate his right to refuse medical treatment.
Mr. M agrees to increase citalopram to 40 mg/d to target depressive symptoms. We monitor Mr. M for treatment response and side effects, to provide ongoing support, to facilitate communication with the medical team, and to evaluate the influence of depression on treatment preferences and decision-making.
As Mr. M is stabilized over the next 3 weeks, he begins to reply, “I’m alive,” when asked about passive death wish. His renal function improves and RRT is discontinued. Mr. M reports a slight improvement in his mood and is discharged to a skilled nursing facility, with plans for closing his tracheostomy.
The authors’ observations
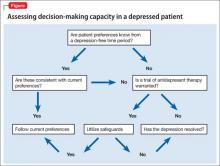
Capacity assessments can be challenging in depressed patients, often because of the uncertain role of features such as hopelessness, anhedonia, and passive death wish in the decision-making process. Depressed patients do not automatically lack DMC, and existing studies suggest that decisions regarding life-saving interventions typically are stable across time. The 4-ability model for capacity assessment is a useful starting point, but additional considerations are warranted in depressed patients with chronic illness (Figure). There is no evidence to date to guide these assessments in chronically depressed or dysthymic patients; therefore additional safeguards may be needed (Table).
In Mr. M’s case, the team’s decision to optimize depression treatment while continuing unwanted life-sustaining therapies led to improved mood and a positive health outcome. In some cases, patients do not respond quickly, if at all, to depression treatment. Also, what constitutes a reasonable attempt to treat depression, or an appropriate delay in decision-making related to life-sustaining therapies, is debatable.
When positive outcomes are not achieved or ethical dilemmas arise, health care providers could experience high moral distress.21 In Mr. M’s case, the consultation team felt moral distress because of the delayed involvement of palliative care, especially because this decision was driven by the family rather than the patient.
Related Resources
• Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-427.
• American Academy of Hospice and Palliative Medicine. www. aahpm.org.
Drug Brand Names
Buspirone • Buspar Citalopram • Celexa
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998.
2. Cohen BJ, McGarvey El, Pinkerton RC, et al. Willingness and competence of depressed and schizophrenic inpatients to consent to research. J Am Acad Psychiatry Law. 2004;32(2):134-143.
3. Lapid MI, Rummans TA, Poole KL, et al. Decisional capacity of severely depressed patients requiring electroconvulsive therapy. J ECT. 2003;19(2):67-72.
4. Appelbaum PS, Grisso T, Frank E, et al. Competence of depressed patients for consent to research. Am J Psychiatry. 1999;156(9):1380-1384.
5. Leeman CP. Depression and the right to die. Gen Hosp Psychiatry. 1999;21(2):112-115.
6. Young EW, Corby JC, Johnson R. Does depression invalidate competence? Consultants’ ethical, psychiatric, and legal considerations. Camb Q Healthc Ethics. 1993;2(4):505-515.
7. Halpern J. When concretized emotion-belief complexes derail decision-making capacity. Bioethics. 2012;26(2):108-116.
8. Grisso T, Appelbaum PS. The MacArthur Treatment Competence Study. III: abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav. 1995;19(2):149-174.
9. Bean G, Nishisato S, Rector NA, et al. The assessment of competence to make a treatment decision: an empirical approach. Can J Psychiatry. 1996;41(2):85-92.
10. Vollmann J, Bauer A, Danker-Hopfe H, et al. Competence of mentally ill patients: a comparative empirical study. Psychol Med. 2003;33(8):1463-1471.
11. Sullivan MD, Youngner SJ. Depression, competence, and the right to refuse lifesaving medical-treatment. Am J Psychiatry. 1994;151(7):971-978.
12. Meynen G. Depression, possibilities, and competence: a phenomenological perspective. Theor Med Bioeth. 2011;32(3):181-193.
13. Elliott C. Caring about risks. Are severely depressed patients competent to consent to research? Arch Gen Psychiatry. 1997;54(2):113-116.
14. Bursztajn HJ, Harding HP Jr, Gutheil TG, et al. Beyond cognition: the role of disordered affective states in impairing competence to consent to treatment. Bull Am Acad Psychiatry Law. 1991;19(4):383-388.
15. Garrett JM, Harris RP, Norburn JK, et al. Life-sustaining treatments during terminal illness: who wants what? J Gen Intern Med. 1993;8(7):361-368.
16. Ganzini L, Lee MA, Heintz RT, et al. The effect of depression treatment on elderly patients’ p for life-sustaining medical therapy. Am J Psychiatry. 1994;151(11):1631-1636.
17. Lee M, Ganzini L. The effect of recovery from depression on p for life-sustaining therapy in older patients. J Gerontol. 1994;49(1):M15-M21.
18. Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2003;30(9):2051-2058.
19. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
20. The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and p for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274(20):1591-1598.
21. Kälvemark S, Höglund AT, Hansson MG, et al. Living the conflicts-ethical dilemmas and moral distress in the health care system. Soc Sci Med. 2004;58(6):1075-1084.
CASE ‘I’ve had enough’
The psychiatry consultation team is asked to evaluate Mr. M, age 76, for a passive death wish and depression 2 months after he was admitted to the hospital after a traumatic fall.
Mr. M has several chronic medical conditions, including hypertension, type 2 diabetes mellitus, and coronary artery disease. Within 2 weeks of his admission, he developed Proteus mirabilis pneumonia and persistent respiratory failure requiring tracheostomy. Records indicate that Mr. M has told family and his treatment team, “I’m tired, just let me go.” He then developed antibiotic-induced Clostridium difficile colitis and acute renal failure requiring temporary renal replacement therapy (RRT).
Mr. M’s clinical status improves, allowing his transfer to a transitional unit, where he continues to state, “I have had enough. I’m done.” He asks for the tracheostomy tube to be removed and RRT discontinued. He is treated again for persistent C. difficile colitis and, within 2 weeks, develops hypotension, hypoxia, emesis, and abdominal distension, requiring transfer to the ICU for management of ileus.
He is stabilized with vasopressors and artificial nutritional support by nasogastric tube. Renal function improves, RRT is discontinued, and he is transferred to the general medical floor.
After a few days on the general medical floor, Mr. M develops a urinary tract infection and develops antibiotic-induced acute renal failure requiring re-initiation of RRT. A percutaneous endoscopic gastrostomy (PEG) tube is placed for nutrition when he shows little improvement with swallowing exercises. Two days after placing the PEG tube, he develops respiratory failure secondary to a left-sided pneumothorax and is transferred to the ICU for the third time, where he undergoes repeated bronchoscopies and requires pressure support ventilation.
One week later, Mr. M is weaned off the ventilator and transferred to the general medical floor with aggressive respiratory therapy, tube feeding, and RRT. Mr. M’s chart indicates that he expresses an ongoing desire to withdraw RRT, the tracheostomy, and feeding tube.
Which of the following would you consider when assessing Mr. M’s decision-making capacity (DMC)?
a) his ability to understand information relevant to treatment decision-making
b) his ability to appreciate the significance of his diagnoses and treatment options and consequences in the context of his own life circumstances
c) his ability to communicate a preference
d) his ability to reason through the relevant information to weigh the potential costs and benefits of treatment options
e) all of the above
HISTORY Guilt and regret
Mr. M reports a 30-year history of depression that has responded poorly to a variety of medications, outpatient psychotherapy, and electroconvulsive therapy. Before admission, he says, he was adherent to citalopram, 20 mg/d, and buspirone, 30 mg/d. Citalopram is continued throughout his hospitalization, although buspirone was discontinued for unknown reasons during admission.
Mr. M is undergoing hemodialysis during his initial encounter with the psychiatry team. He struggles to communicate clearly because of the tracheostomy but is alert, oriented to person and location, answers questions appropriately, maintains good eye contact, and does not demonstrate any psychomotor abnormalities. He describes his disposition as “tired,” and is on the verge of tears during the interview.
Mr. M denies physical discomfort and states, “I have just had enough. I do not want all of this done.” He clarifies that he is not suicidal and denies a history of suicidal or self-injurious behaviors.
Mr. M describes having low mood, anhedonia, and insomnia to varying degrees throughout his adult life. He also reports feeling guilt and regret about earlier experiences, but does not elaborate. He denies symptoms of panic disorder, obsessive-compulsive disorder, posttraumatic stress disorder, mania, or hypomania. He reports an episode of visual hallucinations during an earlier hospitalization, likely a symptom of delirium, but denies any recent visual disturbances.
Mr. M’s thought process is linear and logical, with intact abstract reasoning and no evidence of delusions. Attention and concentration are intact for most of the interview but diminish as he becomes fatigued. Mr. M can describe past treatments in detail and recounts the events leading to this hospitalization.
The authors’ observations
Literature on assessment of DMC recently has centered on the 4-ability model, proposed by Grisso and Appelbaum.1 With this approach, impairment to any of the 4 processes of understanding, appreciation, ability to express a choice, and ability to use reasoning to weigh treatment options could interfere with capacity to make decisions. Few studies have clarified the mechanism and degree to which depression may impair these 4 elements, making capacity assessments in a depressed patient challenging.
Preliminary evidence suggests that depression severity, not the presence of depression, determines the degree to which DMC is impaired, if at all. In several studies, depressed patients did not demonstrate more impaired DMC compared with non-depressed patients based on standardized assessments.2-4 In depressed patients who lack DMC, case reports5-7 and cross-sectional studies8 indicate that appreciation—one’s ability to comprehend the personal relevance of illness and potential consequences of treatments in the context of one’s life—is most often impaired. Other studies suggest that the ability to reason through decision-specific information and weigh the risks and benefits of treatment options is commonly impaired in depressed patients.9,10
Even when a depressed patient demonstrates the 4 elements of DMC, providers might be concerned that the patient’s preferences are skewed by the negative emotions associated with depression.11-13 In such a case, the patient’s expressed wishes might not be consistent with views and priorities that were expressed during an earlier, euthymic period.
Rather than focusing on whether cognitive elements of DMC are impaired, some experts advocate for assessing how depression might lead to “unbalanced” decision-making that is impaired by a patient’s tendency to undervalue positive outcomes and overvalue negative ones.14 Some depressed patients will decide to forego additional medical interventions because they do not see the potential benefits of treatment, view events through a negative lens, and lack hope for the future; however, studies indicate this is not typically the case.15-17
In a study of >2,500 patients age >65 with chronic medical conditions, Garrett et al15 found that those who were depressed communicated a desire for more treatment compared with non-depressed patients. Another study of patients’ wishes for life-sustaining treatment among those who had mild or moderate depression found that most patients did not express a greater desire for life-sustaining medical interventions after their depressive episode remitted. An increased desire for life-sustaining medical interventions occurred only among the most severely depressed patients.16 Similarly, Lee and Ganzini17 found that treatment preferences among patients with mild or moderate depression and serious physical illness were unchanged after the mood disorder was treated.
These findings demonstrate that a clinician charged with assessing DMC must evaluate the severity of a patient’s depression and carefully consider how mood is influencing his (her) perspective and cognitive abilities. It is important to observe how the depressed patient perceives feelings of sadness or hopelessness in the context of decision-making, and how he (she) integrates these feelings when assigning relative value to potential outcomes and alternative treatment options. Because the intensity of depression could vary over time, assessment of the depressed patient’s decision-making abilities must be viewed as a dynamic process.
Clinical application
Recent studies indicate that, although the in-hospital mortality rate for critically ill patients who develop acute renal failure is high, it is variable, ranging from 28% to 90%.18 In one study, patients who required more interventions over the course of a hospital stay (eg, mechanical ventilation, vasopressors) had an in-hospital mortality rate closer to 60% after initiating RRT.19 In a similar trial,20,21 mean survival for critically ill patients with acute renal failure was 32 days from initiation of dialysis; only 27% of these patients were alive 6 months later.21
Given his complicated hospital course, the medical team estimates that Mr. M has a reasonable chance of surviving to discharge, although his longer-term prognosis is poor.
EVALUATION Conflicting preferences
Mr. M expresses reasonable understanding of the medical indications for temporary RRT, respiratory therapy, and enteral tube feedings, and the consequences of withdrawing these interventions. He understands that the primary team recommended ongoing but temporary use of life-sustaining interventions, anticipating that he would recover from his acute medical conditions. Mr. M clearly articulates that he wants to terminate RRT knowing that this would cause a buildup of urea and other toxins, to resume eating by mouth despite the risk of aspiration, and to be allowed to die “naturally.”
Mr. M declines to speak with a clergy member, explaining that he preferred direct contact with God and had reconciled himself to the “consequences” of his actions. He reports having “nothing left to live for” and “nothing left to do.” He says that he is “tired of being a burden” to his wife and son, regrets the way he treated them in the past, and believes they would be better off without him.
Although Mr. M’s abilities to understand, reason, and express a preference are intact, the psychiatry team is concerned that depression could be influencing his perspective, thereby compromising his appreciation for the personal relevance of his request to withdraw life-sustaining treatments. The psychiatrist shares this concern with Mr. M, who voices an understanding that undertreated depression could lead him to make irreversible decisions about his medical treatment that he might not make if he were not depressed; nevertheless, he continues to state that he is “ready” to die. With his permission, the team seeks additional information from Mr. M’s family.
Mr. M’s wife recalls a conversation with her husband 5 years ago in which he said that, were he to become seriously ill, “he would want everything done.” However, she also reports that Mr. M has been expressing a passive death wish “for years,” as he was struggling with chronic medical conditions that led to recurrent hospital admissions.
“He has always been a negative person,” she adds, and confirms that he has been depressed for most of their marriage.
The conflict between Mr. M’s earlier expressed preference for full care and his current wish to withdraw life-sustaining therapies and experience a “natural death” raises significant concern that depression could explain this change in perspective. When asked about this discrepancy, Mr. M admits that he “wanted everything done” in the past, when he was younger and healthier, but his preferences changed as his chronic medical problems progressed.
OUTCOME Better mood, discharge
We encourage Mr. M to continue discussing his treatment preferences with his family, while meeting with the palliative care team to address medical conditions that could be exacerbating depression and to clarify his goals of care. The medical team and Mr. M report feeling relieved when a palliative care consult is suggested, although his wife and son ask that it be delayed until Mr. M is more medically stable. The treatment team acknowledges the competing risks of proceeding too hastily with Mr. M’s request to withdraw life-sustaining treatments because of depression, and of delaying his decision, which could prolong suffering and violate his right to refuse medical treatment.
Mr. M agrees to increase citalopram to 40 mg/d to target depressive symptoms. We monitor Mr. M for treatment response and side effects, to provide ongoing support, to facilitate communication with the medical team, and to evaluate the influence of depression on treatment preferences and decision-making.
As Mr. M is stabilized over the next 3 weeks, he begins to reply, “I’m alive,” when asked about passive death wish. His renal function improves and RRT is discontinued. Mr. M reports a slight improvement in his mood and is discharged to a skilled nursing facility, with plans for closing his tracheostomy.
The authors’ observations
Capacity assessments can be challenging in depressed patients, often because of the uncertain role of features such as hopelessness, anhedonia, and passive death wish in the decision-making process. Depressed patients do not automatically lack DMC, and existing studies suggest that decisions regarding life-saving interventions typically are stable across time. The 4-ability model for capacity assessment is a useful starting point, but additional considerations are warranted in depressed patients with chronic illness (Figure). There is no evidence to date to guide these assessments in chronically depressed or dysthymic patients; therefore additional safeguards may be needed (Table).
In Mr. M’s case, the team’s decision to optimize depression treatment while continuing unwanted life-sustaining therapies led to improved mood and a positive health outcome. In some cases, patients do not respond quickly, if at all, to depression treatment. Also, what constitutes a reasonable attempt to treat depression, or an appropriate delay in decision-making related to life-sustaining therapies, is debatable.
When positive outcomes are not achieved or ethical dilemmas arise, health care providers could experience high moral distress.21 In Mr. M’s case, the consultation team felt moral distress because of the delayed involvement of palliative care, especially because this decision was driven by the family rather than the patient.
Related Resources
• Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-427.
• American Academy of Hospice and Palliative Medicine. www. aahpm.org.
Drug Brand Names
Buspirone • Buspar Citalopram • Celexa
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE ‘I’ve had enough’
The psychiatry consultation team is asked to evaluate Mr. M, age 76, for a passive death wish and depression 2 months after he was admitted to the hospital after a traumatic fall.
Mr. M has several chronic medical conditions, including hypertension, type 2 diabetes mellitus, and coronary artery disease. Within 2 weeks of his admission, he developed Proteus mirabilis pneumonia and persistent respiratory failure requiring tracheostomy. Records indicate that Mr. M has told family and his treatment team, “I’m tired, just let me go.” He then developed antibiotic-induced Clostridium difficile colitis and acute renal failure requiring temporary renal replacement therapy (RRT).
Mr. M’s clinical status improves, allowing his transfer to a transitional unit, where he continues to state, “I have had enough. I’m done.” He asks for the tracheostomy tube to be removed and RRT discontinued. He is treated again for persistent C. difficile colitis and, within 2 weeks, develops hypotension, hypoxia, emesis, and abdominal distension, requiring transfer to the ICU for management of ileus.
He is stabilized with vasopressors and artificial nutritional support by nasogastric tube. Renal function improves, RRT is discontinued, and he is transferred to the general medical floor.
After a few days on the general medical floor, Mr. M develops a urinary tract infection and develops antibiotic-induced acute renal failure requiring re-initiation of RRT. A percutaneous endoscopic gastrostomy (PEG) tube is placed for nutrition when he shows little improvement with swallowing exercises. Two days after placing the PEG tube, he develops respiratory failure secondary to a left-sided pneumothorax and is transferred to the ICU for the third time, where he undergoes repeated bronchoscopies and requires pressure support ventilation.
One week later, Mr. M is weaned off the ventilator and transferred to the general medical floor with aggressive respiratory therapy, tube feeding, and RRT. Mr. M’s chart indicates that he expresses an ongoing desire to withdraw RRT, the tracheostomy, and feeding tube.
Which of the following would you consider when assessing Mr. M’s decision-making capacity (DMC)?
a) his ability to understand information relevant to treatment decision-making
b) his ability to appreciate the significance of his diagnoses and treatment options and consequences in the context of his own life circumstances
c) his ability to communicate a preference
d) his ability to reason through the relevant information to weigh the potential costs and benefits of treatment options
e) all of the above
HISTORY Guilt and regret
Mr. M reports a 30-year history of depression that has responded poorly to a variety of medications, outpatient psychotherapy, and electroconvulsive therapy. Before admission, he says, he was adherent to citalopram, 20 mg/d, and buspirone, 30 mg/d. Citalopram is continued throughout his hospitalization, although buspirone was discontinued for unknown reasons during admission.
Mr. M is undergoing hemodialysis during his initial encounter with the psychiatry team. He struggles to communicate clearly because of the tracheostomy but is alert, oriented to person and location, answers questions appropriately, maintains good eye contact, and does not demonstrate any psychomotor abnormalities. He describes his disposition as “tired,” and is on the verge of tears during the interview.
Mr. M denies physical discomfort and states, “I have just had enough. I do not want all of this done.” He clarifies that he is not suicidal and denies a history of suicidal or self-injurious behaviors.
Mr. M describes having low mood, anhedonia, and insomnia to varying degrees throughout his adult life. He also reports feeling guilt and regret about earlier experiences, but does not elaborate. He denies symptoms of panic disorder, obsessive-compulsive disorder, posttraumatic stress disorder, mania, or hypomania. He reports an episode of visual hallucinations during an earlier hospitalization, likely a symptom of delirium, but denies any recent visual disturbances.
Mr. M’s thought process is linear and logical, with intact abstract reasoning and no evidence of delusions. Attention and concentration are intact for most of the interview but diminish as he becomes fatigued. Mr. M can describe past treatments in detail and recounts the events leading to this hospitalization.
The authors’ observations
Literature on assessment of DMC recently has centered on the 4-ability model, proposed by Grisso and Appelbaum.1 With this approach, impairment to any of the 4 processes of understanding, appreciation, ability to express a choice, and ability to use reasoning to weigh treatment options could interfere with capacity to make decisions. Few studies have clarified the mechanism and degree to which depression may impair these 4 elements, making capacity assessments in a depressed patient challenging.
Preliminary evidence suggests that depression severity, not the presence of depression, determines the degree to which DMC is impaired, if at all. In several studies, depressed patients did not demonstrate more impaired DMC compared with non-depressed patients based on standardized assessments.2-4 In depressed patients who lack DMC, case reports5-7 and cross-sectional studies8 indicate that appreciation—one’s ability to comprehend the personal relevance of illness and potential consequences of treatments in the context of one’s life—is most often impaired. Other studies suggest that the ability to reason through decision-specific information and weigh the risks and benefits of treatment options is commonly impaired in depressed patients.9,10
Even when a depressed patient demonstrates the 4 elements of DMC, providers might be concerned that the patient’s preferences are skewed by the negative emotions associated with depression.11-13 In such a case, the patient’s expressed wishes might not be consistent with views and priorities that were expressed during an earlier, euthymic period.
Rather than focusing on whether cognitive elements of DMC are impaired, some experts advocate for assessing how depression might lead to “unbalanced” decision-making that is impaired by a patient’s tendency to undervalue positive outcomes and overvalue negative ones.14 Some depressed patients will decide to forego additional medical interventions because they do not see the potential benefits of treatment, view events through a negative lens, and lack hope for the future; however, studies indicate this is not typically the case.15-17
In a study of >2,500 patients age >65 with chronic medical conditions, Garrett et al15 found that those who were depressed communicated a desire for more treatment compared with non-depressed patients. Another study of patients’ wishes for life-sustaining treatment among those who had mild or moderate depression found that most patients did not express a greater desire for life-sustaining medical interventions after their depressive episode remitted. An increased desire for life-sustaining medical interventions occurred only among the most severely depressed patients.16 Similarly, Lee and Ganzini17 found that treatment preferences among patients with mild or moderate depression and serious physical illness were unchanged after the mood disorder was treated.
These findings demonstrate that a clinician charged with assessing DMC must evaluate the severity of a patient’s depression and carefully consider how mood is influencing his (her) perspective and cognitive abilities. It is important to observe how the depressed patient perceives feelings of sadness or hopelessness in the context of decision-making, and how he (she) integrates these feelings when assigning relative value to potential outcomes and alternative treatment options. Because the intensity of depression could vary over time, assessment of the depressed patient’s decision-making abilities must be viewed as a dynamic process.
Clinical application
Recent studies indicate that, although the in-hospital mortality rate for critically ill patients who develop acute renal failure is high, it is variable, ranging from 28% to 90%.18 In one study, patients who required more interventions over the course of a hospital stay (eg, mechanical ventilation, vasopressors) had an in-hospital mortality rate closer to 60% after initiating RRT.19 In a similar trial,20,21 mean survival for critically ill patients with acute renal failure was 32 days from initiation of dialysis; only 27% of these patients were alive 6 months later.21
Given his complicated hospital course, the medical team estimates that Mr. M has a reasonable chance of surviving to discharge, although his longer-term prognosis is poor.
EVALUATION Conflicting preferences
Mr. M expresses reasonable understanding of the medical indications for temporary RRT, respiratory therapy, and enteral tube feedings, and the consequences of withdrawing these interventions. He understands that the primary team recommended ongoing but temporary use of life-sustaining interventions, anticipating that he would recover from his acute medical conditions. Mr. M clearly articulates that he wants to terminate RRT knowing that this would cause a buildup of urea and other toxins, to resume eating by mouth despite the risk of aspiration, and to be allowed to die “naturally.”
Mr. M declines to speak with a clergy member, explaining that he preferred direct contact with God and had reconciled himself to the “consequences” of his actions. He reports having “nothing left to live for” and “nothing left to do.” He says that he is “tired of being a burden” to his wife and son, regrets the way he treated them in the past, and believes they would be better off without him.
Although Mr. M’s abilities to understand, reason, and express a preference are intact, the psychiatry team is concerned that depression could be influencing his perspective, thereby compromising his appreciation for the personal relevance of his request to withdraw life-sustaining treatments. The psychiatrist shares this concern with Mr. M, who voices an understanding that undertreated depression could lead him to make irreversible decisions about his medical treatment that he might not make if he were not depressed; nevertheless, he continues to state that he is “ready” to die. With his permission, the team seeks additional information from Mr. M’s family.
Mr. M’s wife recalls a conversation with her husband 5 years ago in which he said that, were he to become seriously ill, “he would want everything done.” However, she also reports that Mr. M has been expressing a passive death wish “for years,” as he was struggling with chronic medical conditions that led to recurrent hospital admissions.
“He has always been a negative person,” she adds, and confirms that he has been depressed for most of their marriage.
The conflict between Mr. M’s earlier expressed preference for full care and his current wish to withdraw life-sustaining therapies and experience a “natural death” raises significant concern that depression could explain this change in perspective. When asked about this discrepancy, Mr. M admits that he “wanted everything done” in the past, when he was younger and healthier, but his preferences changed as his chronic medical problems progressed.
OUTCOME Better mood, discharge
We encourage Mr. M to continue discussing his treatment preferences with his family, while meeting with the palliative care team to address medical conditions that could be exacerbating depression and to clarify his goals of care. The medical team and Mr. M report feeling relieved when a palliative care consult is suggested, although his wife and son ask that it be delayed until Mr. M is more medically stable. The treatment team acknowledges the competing risks of proceeding too hastily with Mr. M’s request to withdraw life-sustaining treatments because of depression, and of delaying his decision, which could prolong suffering and violate his right to refuse medical treatment.
Mr. M agrees to increase citalopram to 40 mg/d to target depressive symptoms. We monitor Mr. M for treatment response and side effects, to provide ongoing support, to facilitate communication with the medical team, and to evaluate the influence of depression on treatment preferences and decision-making.
As Mr. M is stabilized over the next 3 weeks, he begins to reply, “I’m alive,” when asked about passive death wish. His renal function improves and RRT is discontinued. Mr. M reports a slight improvement in his mood and is discharged to a skilled nursing facility, with plans for closing his tracheostomy.
The authors’ observations
Capacity assessments can be challenging in depressed patients, often because of the uncertain role of features such as hopelessness, anhedonia, and passive death wish in the decision-making process. Depressed patients do not automatically lack DMC, and existing studies suggest that decisions regarding life-saving interventions typically are stable across time. The 4-ability model for capacity assessment is a useful starting point, but additional considerations are warranted in depressed patients with chronic illness (Figure). There is no evidence to date to guide these assessments in chronically depressed or dysthymic patients; therefore additional safeguards may be needed (Table).
In Mr. M’s case, the team’s decision to optimize depression treatment while continuing unwanted life-sustaining therapies led to improved mood and a positive health outcome. In some cases, patients do not respond quickly, if at all, to depression treatment. Also, what constitutes a reasonable attempt to treat depression, or an appropriate delay in decision-making related to life-sustaining therapies, is debatable.
When positive outcomes are not achieved or ethical dilemmas arise, health care providers could experience high moral distress.21 In Mr. M’s case, the consultation team felt moral distress because of the delayed involvement of palliative care, especially because this decision was driven by the family rather than the patient.
Related Resources
• Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-427.
• American Academy of Hospice and Palliative Medicine. www. aahpm.org.
Drug Brand Names
Buspirone • Buspar Citalopram • Celexa
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998.
2. Cohen BJ, McGarvey El, Pinkerton RC, et al. Willingness and competence of depressed and schizophrenic inpatients to consent to research. J Am Acad Psychiatry Law. 2004;32(2):134-143.
3. Lapid MI, Rummans TA, Poole KL, et al. Decisional capacity of severely depressed patients requiring electroconvulsive therapy. J ECT. 2003;19(2):67-72.
4. Appelbaum PS, Grisso T, Frank E, et al. Competence of depressed patients for consent to research. Am J Psychiatry. 1999;156(9):1380-1384.
5. Leeman CP. Depression and the right to die. Gen Hosp Psychiatry. 1999;21(2):112-115.
6. Young EW, Corby JC, Johnson R. Does depression invalidate competence? Consultants’ ethical, psychiatric, and legal considerations. Camb Q Healthc Ethics. 1993;2(4):505-515.
7. Halpern J. When concretized emotion-belief complexes derail decision-making capacity. Bioethics. 2012;26(2):108-116.
8. Grisso T, Appelbaum PS. The MacArthur Treatment Competence Study. III: abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav. 1995;19(2):149-174.
9. Bean G, Nishisato S, Rector NA, et al. The assessment of competence to make a treatment decision: an empirical approach. Can J Psychiatry. 1996;41(2):85-92.
10. Vollmann J, Bauer A, Danker-Hopfe H, et al. Competence of mentally ill patients: a comparative empirical study. Psychol Med. 2003;33(8):1463-1471.
11. Sullivan MD, Youngner SJ. Depression, competence, and the right to refuse lifesaving medical-treatment. Am J Psychiatry. 1994;151(7):971-978.
12. Meynen G. Depression, possibilities, and competence: a phenomenological perspective. Theor Med Bioeth. 2011;32(3):181-193.
13. Elliott C. Caring about risks. Are severely depressed patients competent to consent to research? Arch Gen Psychiatry. 1997;54(2):113-116.
14. Bursztajn HJ, Harding HP Jr, Gutheil TG, et al. Beyond cognition: the role of disordered affective states in impairing competence to consent to treatment. Bull Am Acad Psychiatry Law. 1991;19(4):383-388.
15. Garrett JM, Harris RP, Norburn JK, et al. Life-sustaining treatments during terminal illness: who wants what? J Gen Intern Med. 1993;8(7):361-368.
16. Ganzini L, Lee MA, Heintz RT, et al. The effect of depression treatment on elderly patients’ p for life-sustaining medical therapy. Am J Psychiatry. 1994;151(11):1631-1636.
17. Lee M, Ganzini L. The effect of recovery from depression on p for life-sustaining therapy in older patients. J Gerontol. 1994;49(1):M15-M21.
18. Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2003;30(9):2051-2058.
19. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
20. The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and p for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274(20):1591-1598.
21. Kälvemark S, Höglund AT, Hansson MG, et al. Living the conflicts-ethical dilemmas and moral distress in the health care system. Soc Sci Med. 2004;58(6):1075-1084.
1. Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998.
2. Cohen BJ, McGarvey El, Pinkerton RC, et al. Willingness and competence of depressed and schizophrenic inpatients to consent to research. J Am Acad Psychiatry Law. 2004;32(2):134-143.
3. Lapid MI, Rummans TA, Poole KL, et al. Decisional capacity of severely depressed patients requiring electroconvulsive therapy. J ECT. 2003;19(2):67-72.
4. Appelbaum PS, Grisso T, Frank E, et al. Competence of depressed patients for consent to research. Am J Psychiatry. 1999;156(9):1380-1384.
5. Leeman CP. Depression and the right to die. Gen Hosp Psychiatry. 1999;21(2):112-115.
6. Young EW, Corby JC, Johnson R. Does depression invalidate competence? Consultants’ ethical, psychiatric, and legal considerations. Camb Q Healthc Ethics. 1993;2(4):505-515.
7. Halpern J. When concretized emotion-belief complexes derail decision-making capacity. Bioethics. 2012;26(2):108-116.
8. Grisso T, Appelbaum PS. The MacArthur Treatment Competence Study. III: abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav. 1995;19(2):149-174.
9. Bean G, Nishisato S, Rector NA, et al. The assessment of competence to make a treatment decision: an empirical approach. Can J Psychiatry. 1996;41(2):85-92.
10. Vollmann J, Bauer A, Danker-Hopfe H, et al. Competence of mentally ill patients: a comparative empirical study. Psychol Med. 2003;33(8):1463-1471.
11. Sullivan MD, Youngner SJ. Depression, competence, and the right to refuse lifesaving medical-treatment. Am J Psychiatry. 1994;151(7):971-978.
12. Meynen G. Depression, possibilities, and competence: a phenomenological perspective. Theor Med Bioeth. 2011;32(3):181-193.
13. Elliott C. Caring about risks. Are severely depressed patients competent to consent to research? Arch Gen Psychiatry. 1997;54(2):113-116.
14. Bursztajn HJ, Harding HP Jr, Gutheil TG, et al. Beyond cognition: the role of disordered affective states in impairing competence to consent to treatment. Bull Am Acad Psychiatry Law. 1991;19(4):383-388.
15. Garrett JM, Harris RP, Norburn JK, et al. Life-sustaining treatments during terminal illness: who wants what? J Gen Intern Med. 1993;8(7):361-368.
16. Ganzini L, Lee MA, Heintz RT, et al. The effect of depression treatment on elderly patients’ p for life-sustaining medical therapy. Am J Psychiatry. 1994;151(11):1631-1636.
17. Lee M, Ganzini L. The effect of recovery from depression on p for life-sustaining therapy in older patients. J Gerontol. 1994;49(1):M15-M21.
18. Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2003;30(9):2051-2058.
19. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
20. The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and p for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274(20):1591-1598.
21. Kälvemark S, Höglund AT, Hansson MG, et al. Living the conflicts-ethical dilemmas and moral distress in the health care system. Soc Sci Med. 2004;58(6):1075-1084.
Clozapine Management for Internists
Clozapine is a second‐generation antipsychotic (SGA) medication that was developed in 1959, introduced to Europe in 1971, and withdrawn from the market in 1975 due to associated concerns for potentially fatal agranulocytosis. In 1989, the US Food and Drug Administration (FDA) approved use of clozapine for the management of treatment‐resistant schizophrenia, under strict parameters for complete blood count (CBC) monitoring. Clozapine has since gained an additional FDA indication for reducing suicidal behavior in patients with schizophrenia and schizoaffective disorder,[1, 2, 3] and displayed superiority to both first generation antipsychotics and other SGA agents in reducing symptom burden.[2, 4, 5]
Clozapine's clinical benefits include lowering mortality in schizophrenia,[6] reducing deaths from ischemic heart disease,[7] curtailing substance use in individuals with psychotic disorders,[8] increasing rates of independent living and meaningful occupational activity, and reducing psychiatric hospitalizations and need for involuntary treatment.[9] Because schizophrenia, itself, is associated with a 15‐ to 20‐year decrease in average lifespan,[10] these benefits of clozapine are particularly salient. Yet the mechanism by which clozapine mitigates otherwise‐refractory psychotic symptoms is a conundrum. Structurally a tricyclic dibenzodiazepine, clozapine has relatively little effect on the dopamine D2 receptor, which has classically been thought to mediate the treatment effect of antipsychotics.[11, 12]
The unique nature of clozapine extends to its adverse effect profile. A significant percentage of patients who discontinue clozapine (17%35.4%) cite medical complications, the most common being seizures, constipation, sedation, and neutropenia.[13, 14] Yet several studies, including the landmark Clinical Antipsychotic Trials for Interventions Effectiveness (CATIE) study, have found that patients were more likely to adhere to clozapine therapy than to other antipsychotics.[2, 15] In the CATIE study, 44% of subjects taking clozapine continued the medication for 18 months, compared to 29% of individuals on olanzapine, 14% on risperidone, and 7% on quetiapine. Median time until discontinuation of clozapine was 10.5 months, significantly longer than for quetiapine (2.8 months) and olanzapine (2.7 months).[2] Because patients who experience clozapine‐related medical complications are likely to present first to the primary care or general hospital setting, internists must be aware of potential iatrogenic effects, and of their implications for psychiatric and medical care. Using case examples, we will examine both common and serious complications associated with clozapine, and discuss recommendations for management, including indications for clozapine discontinuation.
NEUROLOGICAL
Case Vignette 1
Mr. A is a 29‐year‐old man with asthma and schizophrenia who experienced a generalized tonic‐clonic seizure during treatment at a psychiatric facility. The patient started clozapine therapy 5 weeks prior, with gradual titration to 425 mg daily. Mr. A's previous medication trials included olanzapine and chlorpromazine, which rendered little improvement to his chronic auditory hallucinations. Clozapine was temporarily withheld during further neurologic workup, in which both electroencephalogram (EEG) and brain magnetic resonance imaging were unremarkable. After 60 hours, clozapine titration was reinitiated, and valproic acid was started for mood stabilization and seizure prophylaxis. Mr. A was discharged 6 weeks later on clozapine, 600 mg at bedtime, and extended‐release divalproate, 2500 mg at bedtime. The patient suffered no further seizure activity throughout hospitalization and for at least 1 year postdischarge.
Seizures complicate clozapine use in up to 5% of cases, with a dose‐dependent risk pattern.[16] Seizures are most commonly associated with serum clozapine levels above 500 g/L), but have also been reported with lower levels of clozapine and its metabolite norclozapine.[17] Though nonspecific EEG changes (ie, focal or generalized spikes, spike‐wave and polyspike discharges) have been associated with clozapine administration, they do not reliably predict seizure tendency.[17] Prophylaxis with antiepileptic drugs (AEDs) is not recommended, though AED treatment may be undertaken for patients who experience a seizure while on clozapine. When seizures occur in the context of elevated serum levels, reducing clozapine to the lowest effective dose is preferred over initiating an AED. Although this reduces the potential for exposure to anticonvulsant‐associated adverse effects, it may also introduce the risk of relapsed psychotic symptoms, and therefore requires close monitoring by a psychiatrist. For those who opt to initiate AED therapy, we recommend consideration of each medication's therapeutic and side‐effect profiles based on the patient's medical history and active symptoms. For example, in the case of Mr. A, valproate was used to target concomitant mood symptoms; likewise, patients who experience troublesome weight gain, as well as seizures, may benefit from topiramate. The occurrence of seizures does not preclude continuation of clozapine therapy, in conjunction with an AED[18] and after consideration of potential risks and benefits of use. Clozapine is not contraindicated in patients with well‐controlled epilepsy.[19]
Sedation, the most common neurologic side effect of clozapine, is also dose dependent and often abates during titration.[20] Though clozapine may induce extrapyramidal symptoms, including rigidity, tremor, and dystonia, the risk is considerably lower with clozapine than other antipsychotics, owing to a lesser affinity for D2 receptors. Associated parkinsonism should prompt consideration of dose reduction, in discussion with a psychiatrist, with concurrent monitoring of serum clozapine levels and close follow‐up for emergence of psychotic symptoms. If dose reduction is ineffective, not indicated, or not preferred by the patient, the addition of an anticholinergic medication may be considered (eg, diphenhydramine 2550 mg, benztropine 12 mg). Neuroleptic malignant syndrome, although rare, is life‐threatening and warrants immediate discontinuation of clozapine, though successful rechallenge after has been reported in case reports.[21]
CARDIAC
Case Vignette 2
Mr. B is a 34‐year‐old man with sinus tachycardia, a benign adrenal tumor, and chronic paranoid schizophrenia that had been poorly responsive to numerous antipsychotic trials. During a psychiatric hospitalization for paranoid delusions with aggressive threats toward family, Mr. B was started on clozapine and titrated to 250 mg daily. On day 16 of clozapine therapy, the patient began to experience cough, and several days later, diffuse rhonchi were noted on examination. Complete blood count revealed WBC 20.3 * 103/L, with 37% eosinophils and absolute eosinophil count of 7.51 (increased from 12%/1.90 the week before), and an electrocardiogram showed sinus tachycardia with ST‐segment changes. Mr. B was transferred to the general medical hospital for workup of presumed myocarditis.
Approximately one‐quarter of patients who take clozapine experience sinus tachycardia, which may be related to clozapine's anticholinergic effects causing rebound noradrenergic elevations[22]; persistent or problematic tachycardia may be treated using a cardio‐selective ‐blocker. Clozapine has also been linked to significant increases in systolic and diastolic blood pressure in 4% of patients (monitoring data); the risk of hypertension increases with the duration of clozapine treatment, and appears to be independent of the patient's weight.[23] Orthostatic hypotension has been reported in 9% of patients on clozapine therapy, though effects can be mitigated with gradual titration, adequate hydration, compression stockings, and patient education. Sinus tachycardia, hypertension, and orthostatic hypotension are not absolute indications to discontinue clozapine; rather, we advocate for treating these side effects while continuing clozapine treatment.[24]
Myocarditis represents the most serious cardiac side effect of clozapine.[25, 26] Although the absolute risk appears to be lower than 0.1%,[24] Kilian et al. calculated a 1000‐to‐2000fold increase in relative risk of myocarditis among patients who take clozapine, compared to the general population.[26] Most cases occur within the first month of treatment, with median time to onset of 15 days. This time course is consistent with an acute immunoglobulin Emediated hypersensitivity (type 1) reaction, and eosinophilic infiltrates have been found on autopsy, consistent with an acute drug reaction.[20]
Because of this early onset, the physician should maintain a particularly high index of suspicion in the first months of treatment, rigorously questioning patients and families about signs and symptoms of cardiac disease. If patients on clozapine present with flu‐like symptoms, fever, myalgia, dizziness, chest pain, dyspnea, tachycardia, palpitations, or other signs or symptoms of heart failure, evaluation for myocarditis should be undertaken.[25] Several centers have utilized cardiac enzymes (e.g., troponin I, troponin T, creatine kinase‐myocardial band) as a universal screen for myocarditis, though this is not a universal practice.[24] Both tachycardia and flu‐like symptoms may be associated with clozapine, particularly during the titration period, and these are normally benign symptoms requiring no intervention. If the diagnosis of myocarditis is made, however, clozapine should be stopped immediately. Myocarditis is often considered to be a contraindication to restarting clozapine, though cases have been reported of successful clozapine rechallenge in patients who had previously experienced myocarditis.[21]
Recommendations for clozapine‐associated electrocardiography (ECG) monitoring have not been standardized. Based on common clinical practice and the time course of serious cardiac complications, we recommend baseline ECG prior to the start of clozapine, with follow‐up ECG 2 to 4 weeks after clozapine initiation, and every 6 months thereafter.
GASTROINTESTINAL
Case Vignette 3
Mr. C is a 61‐year‐old man with chronic paranoid schizophrenia and a history of multiple‐state hospital admissions. He had been maintained on clozapine for 15 years, allowing him to live independently and avoid psychiatric hospitalization. Mr. C was admitted to the general medical hospital with nausea, vomiting, and an inability to tolerate oral intake. He was found to have a high‐grade small‐bowel obstruction, and all oral medications were initially discontinued. After successful management of his acute gastrointestinal presentation and discussion of potential risks and benefits of various treatment options, clozapine was reinitiated along with bulk laxative and stool softening agents.
Affecting 14% to 60% of individuals who are prescribed clozapine, constipation represents the most common associated gastrointestinal complaint.[27] For most patients, this condition is uncomfortable but nonlethal, though it has been implicated in several deaths by aspiration pneumonia and small‐bowel perforation.[28, 29] Providers must screen regularly for constipation and treat aggressively with stimulant laxatives and stool softeners,[18] while reviewing medication lists and, when possible, streamlining extraneous anticholinergic contributors. Clozapine‐prescribed individuals also frequently suffer from gastrointestinal reflux disease (GERD), for which behavioral interventions (eg, smoking cessation or remaining upright for 3 hours after meals) should be considered in addition to pharmacologic treatment with proton pump inhibitors. Clozapine therapy may be continued while constipation and GERD are managed medically.
Potentially fatal gastrointestinal hypomotility and small‐bowel obstruction are rare but well‐described complications that occur in up to 0.3% of patients who take clozapine.[27] This effect appears to be dose dependent, and higher blood levels are associated with greater severity of constipation and risk for serious hypomotility.[27] Clozapine should be withheld during treatment for such serious adverse events as ileus or small‐bowel perforation; however, once these conditions have stabilized, clozapine therapy may be reconsidered based on an analysis of potential benefits and risks. If clozapine is withheld, the internist must monitor for acute worsening of mental status, inattention, and disorientation, as clozapine withdrawal‐related delirium has been reported.[30] Ultimately, aggressive treatment of constipation in conjunction with continued clozapine therapy is the recommended course of action.[28]
Given the increased risk of ileus in the postoperative period, it is particularly important for physicians to inquire about preoperative bowel habits and assess for any existing constipation. Careful monitoring of postoperative bowel motility, along with early and aggressive management of constipation, is recommended. Concurrent administration of other constipating agents (eg, opiates, anticholinergics) should be limited to the lowest effective dose.[27] Although transaminitis, hepatitis, and pancreatitis have all been associated with clozapine in case reports, these are rare,[31] and the approach to management should be considered on a case‐by‐case basis.
HEMATOLOGIC
Case Vignette 4
Ms. D is a 38‐year‐old woman with a schizoaffective disorder who was started on clozapine after 3 other agents had failed to control her psychotic symptoms and alleviate chronic suicidal thoughts. Baseline CBC revealed serum white blood cell count (WBC) of 7800/mm3 and absolute neutrophil count (ANC) of 4700/mm3. In Ms. D's third week of clozapine use, WBC dropped to 4400/mm3 and ANC to 2200/mm3. Repeat lab draw confirmed this, prompting the treatment team to initiate twice‐weekly CBC monitoring. Ms. D's counts continued to fall, and 10 days after the initial drop, WBC was calculated at 1400/mm3 and ANC at 790/mm3. Clozapine was discontinued, and though the patient was asymptomatic, broad‐spectrum antibiotics were initiated. She received daily CBC monitoring until WBC >3000/mm3 and ANC >1500/mm3. An alternate psychotropic medication was initiated several weeks thereafter.
Neutropenia (white blood cell count 3000/mm3) is a common complication that affects approximately 3% of patients who take clozapine.[32] This may be mediated by clozapine's selective impact on the precursors of polymorphonuclear leukocytes, though the mechanism remains unknown.[33] Although neutropenia is not an absolute contraindication for clozapine therapy, guidelines recommend cessation of clozapine when the ANC drops below 1000/mm3.[34] A meta‐analysis of 112 patients who were rechallenged following neutropenia found that 69% tolerated a rechallenge without development of a subsequent dyscrasia.[21]
In the case of chemotherapy‐induced neutropenia, several case reports support the continued use of clozapine during cancer treatment[35]; this requires a written request to the pharmaceutical company that manufactures clozapine and documentation of the expected time course and contribution of chemotherapy to neutropenia.[36] Clozapine's association with neutropenia warrants close monitoring in individuals with human immunodeficiency virus (HIV) and other causes of immune compromise. Reports of clozapine continuation in HIV‐positive individuals underscore the importance of close collaboration between infectious disease and psychiatry, with specific focus on potential interactions between clozapine and antiretroviral agents and close monitoring of viral load and ANC.[37]
The most feared complication of clozapine remains agranulocytosis, defined as ANC500/mm3,[33] which occurs in up to 1% of monitored patients. In 1975, clozapine was banned worldwide after 8 fatal cases of agranulocytosis were reported in Finland.[38] The drug was reintroduced for treatment‐resistant schizophrenia with strict monitoring parameters, which has sharply reduced the death rate. One study found 12 actual deaths between 1990 and 1994, compared to the 149 predicted deaths without monitoring.[39]
The risk of agranulocytosis appears to be higher in older adults and in patients with a lower baseline WBC count. Although there are reports of delayed agranulocytosis occurring in patients after up to 19 years of treatment,[40] the incidence of leukopenia is greatest in the first year. Given this high‐risk period, mandatory monitoring is as follows: weekly WBC and neutrophil counts for the first 26 weeks, biweekly counts for the second 26 weeks, and every 4 weeks thereafter. Of note, many of the later cases of agranulocytosis appear to be related to medication coadministration, particularly with valproic acid, though no definitive link has been established.[40]
Treatment of clozapine‐induced agranulocytosis consists of immediate clozapine cessation, and consideration of initiation of prophylactic broad‐spectrum antibiotics and granulocyte colony‐stimulating factor (such as filgrastim) until the granulocyte count normalizes.[41, 42] Although few case reports describe successful clozapine rechallenge in patients with a history of agranulocytosis, the data are sparse, and current practice is to permanently discontinue clozapine if ANC falls below 1000/mm3.[21, 41]
ADDITIONAL COMPLICATIONS (METABOLIC, RENAL, URINARY)
Moderate to marked weight gain occurs in over 50% of patients treated with clozapine, with average gains of nearly 10% body weight.[43] In a 10‐year follow‐up study of patients treated with clozapine, Henderson et al. reported an average weight gain of 13 kg, with 34% percent of studied patients developing diabetes mellitus. Metabolic side effects of second‐generation antipsychotics, including clozapine, are a well‐documented and troubling phenomenon.[44] Limited evidence supports use of metformin, alongside behavioral therapy, for concerns related to glucose dysregulation.[45] Some patients have also experienced weight loss with adjunctive topiramate use, particularly if they have also suffered seizures.[46]
Urinary incontinence and nocturnal enuresis are both associated with clozapine, but are likely under‐reported because of patient and provider embarrassment; providers also may not think to ask about these specific symptoms. First‐line treatment for nocturnal enuresis is to limit fluids in the evening. Desmopressin has a controversial role in treating nocturnal enuresis owing to its risk of hyponatremia; appropriate monitoring should be implemented if this agent is used.[18]
Clozapine has been associated with acute interstitial nephritis (AIN), although this is thought to be a relatively rare side effect. Drug‐induced AIN typically appears soon after initiation and presents with the clinical triad of rash, fever, and eosinophilia. Given that weekly CBC is mandatory in the initiation phase, eosinophilia is easily detectible and may serve as a marker for potential AIN.[47]
Sialorrhea, particularly during sleep, is a bothersome condition affecting up to one‐third of patients who take clozapine.[48] Although clozapine is strongly anticholinergic, its agonist activity at the M4 muscarinic receptor and antagonism of the alpha‐2 adrenergic receptor are postulated as the mechanisms underlying hypersalivation. Sialorrhea is frequently seen early in treatment and does not appear to be dose dependent.[48] Excessive salivation is typically managed with behavioral interventions (eg, utilizing towels or other absorbent materials on top of bedding). If hypersalivation occurs during the day, chewing sugar‐free gum may increase the rate of swallowing and make symptoms less bothersome. If this does not provide adequate relief, practitioners may consider use of atropine 1% solution administered directly to the oral cavity.[49]
DRUG‐DRUG INTERACTIONS
For hospitalists, who must frequently alter existing medications or add new ones, awareness of potential drug‐drug interactions is crucial. Clozapine is metabolized by the cytochrome p450 system, with predominant metabolism through the isoenzymes 1A2, 3A4, and 2D6.[50] Common medications that induce clozapine metabolism (thereby decreasing clozapine levels) include phenytoin, phenobarbital, carbamazepine, oxcarbazepine, and corticosteroids. Conversely, stopping these medications after long‐term therapy will raise clozapine levels. Substances that inhibit clozapine metabolism (thereby increasing clozapine levels) include ciprofloxacin, erythromycin, clarithromycin, fluvoxamine, fluoxetine, paroxetine, protease inhibitors, verapamil, and grapefruit juice. We recommend caution when concurrently administering other agents that increase risk for agranulocytosis, including carbamazepine, trimethoprim‐sulfamethoxazole, sulfasalazine, and tricyclic antidepressants.
Cigarette smoking decreases clozapine blood levels by induction of CYP1A2. Patients require a 10% to 30% reduction to clozapine dose during periods of smoking cessation, including when smoking is stopped during inpatient hospitalization.[51] Nicotine replacement therapy does not induce CYP1A2 and therefore does not have a compensatory effect on clozapine levels. On discharge or resumption of smoking, patients may require an increase of their dose of clozapine to maintain adequate antipsychotic effect.
SUMMARY OF RECOMMENDATIONS
Medical complications are cited as the cause in 20% of clozapine discontinuations; most commonly, these include seizures, severe constipation, somnolence, and neutropenia. Given the high risk of psychiatric morbidity posed by discontinuation, we recommend managing mild‐moderate symptoms and side effects while continuing the drug, when possible (Table 1). We encourage hospitalists to confer with the patient's psychiatrist or the inpatient psychiatry consultation service when making changes to clozapine therapy. Specific recommendations are as follows:
- We advocate withholding clozapine administration pending medical optimization for several conditions, including: small‐bowel obstruction, neuroleptic malignant syndrome, venous thromboembolism, diabetic ketoacidosis, or hyperosmolar coma.
- Clinical scenarios requiring acute discontinuation of clozapine include agranulocytosis and myocarditis. Successful rechallenge with clozapine has been described after both conditions; at the same time, given the high morbidity and mortality of myocarditis and agranulocytosis, re‐initiation of clozapine requires an extensive risk‐benefit discussion with the patient and family, informed consent, and, in the case of agranulocytosis, approval from the national clozapine registry (Table 2).
- Although adjunctive therapy with filgrastim was initially thought to permit a clozapine rechallenge in patients with a history of agranulocytosis, case reports on this strategy have been equivocal, and further research is necessary to determine the most effective strategy for management.
| Clinical Lab/Study | Frequency of Monitoring | |
|---|---|---|
| Cardiac | Electrocardiogram | Baseline, 24 weeks after initiation, every 6 months thereafter |
| Cardiac enzymes (eg, troponin I) echocardiogram | No standard guidelines, unless clinically indicated | |
| Hematologic | Complete blood count with differential | Baseline, then weekly 26 weeks, then every 2 weeks 26 weeks, then every 4 weeks thereafter |
| Metabolic | Body mass index; circumference of waist | Baseline, then every 3 to 6 months |
| Fasting glucose | Baseline, then every 6 months | |
| Fasting lipid panel | Baseline, then yearly | |
| Neurologic | Electroencephalogram | No standard guidelines, unless clinically indicated |
| Vital signs | Heart rate, blood pressure, temperature | Baseline and at each follow‐up visit |
| Requires Acute Clozapine Discontinuation* | Clozapine Interruption During Management | Does Not Typically Require Clozapine Discontinuation |
|---|---|---|
| ||
| Agranulocytosis (ANC1.0 109/mm3) | Diabetic complications (eg, ketoacidosis, hyperosmolar coma) | Constipation |
| Cardiomyopathy (severe) | Gastrointestinal obstruction, ileus | Diabetes mellitus |
| Myocarditis | Neuroleptic malignant syndrome | Gastroesophageal Reflux |
| Venous thromboembolism | Hyperlipidemia | |
| Hypertension | ||
| Orthostatic hypotension | ||
| Sedation | ||
| Seizures | ||
| Sialorrhea | ||
| Sinus tachycardia | ||
| Urinary changes (eg, enuresis, incontinence) | ||
| Weight gain | ||
CONCLUSION
Clozapine has been a very successful treatment for patients with schizophrenia who have failed other antipsychotic therapies. However, fears of potential side effects and frequent monitoring have limited its use and led to unnecessary discontinuation. To mitigate risk for serious complications, we hope to increase hospitalists' awareness of prevention, monitoring, and treatment of side effects, and to promote comfort with circumstances that warrant continuation or discontinuation of clozapine (Table 3). The hospitalist plays a crucial role in managing these complications as well as conveying information and recommendations to primary care providers; as such, their familiarity with the medication is essential for proper management of individuals who take clozapine.
| Take‐Home Points |
|---|
| 1. Clozapine is the gold standard for treatment‐resistant schizophrenia; however, its use is limited by side effects, many of which can be successfully treated by internists. |
| 2. There are few indications for discontinuing clozapine (myocarditis, small‐bowel obstruction, agranulocytosis). The psychiatry service should be consulted in the event that clozapine is discontinued. |
| 3. Seizures are not an indication for discontinuing clozapine; instead, we recommend adding an antiepileptic drug. |
| 4. All second‐generation antipsychotics are associated with diabetes mellitus and significant weight gain. Clozapine is more highly associated with metabolic side effects than many other medications in this class. |
| 5. Sedation, sialorrhea, and constipation are common and can be managed pharmacologically and with behavioral interventions. |
Disclosure: Nothing to report.
- , , , . Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database Syst Rev. 2009(1):CD000059.
- , , , et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600–610.
- , , , et al. Randomized controlled trial of effect of prescription of clozapine versus other second‐generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull. 2006;32(4):715–723.
- , , , et al. Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151(1):20–26.
- , , , . Clozapine for the treatment‐resistant schizophrenic. A double‐blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789–796.
- , , , , , . Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82–91.
- , , , et al. 11‐year follow‐up of mortality in patients with schizophrenia: a population‐based cohort study (FIN11 study). Lancet. 2009;374(9690):620–627.
- , , , , . Clozapine use and relapses of substance use disorder among patients with co‐occurring schizophrenia and substance use disorders. Schizophr Bull. 2006;32(4):637–643.
- , , . Outcomes for schizophrenia patients with clozapine treatment: how good does it get? J Psychopharmacol. 2009;23(8):957–965.
- , , , . Morbidity and mortality in people with serious mental illness. National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council. Available at: http://www.nasmhpd.org/docs/publications/MDCdocs/Mortality%20and%20Morbidity%20Final%20Report%208.18.08.pdf. Accessed February 3, 2015.
- , . Pharmacological actions of the atypical antipsychotic drug clozapine: a review. Synapse. 1996;24(4):349–394.
- , . Clozapine. A novel antipsychotic agent. N Engl J Med. 1991;324(11):746–754.
- , . Reason for clozapine cessation. Acta Psychiatr Scand. 2012;125(1):39–44.
- , , , . Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603–613.
- , , , , , . Time to discontinuation of antipsychotic drugs in a schizophrenia cohort: influence of current treatment strategies. Ther Adv Psychopharmacol. 2014;4(6):228–239.
- , , . Clozapine‐related seizures. Neurology. 1991;41(3):369–371.
- , , , . Clozapine‐related EEG changes and seizures: dose and plasma‐level relationships. Ther Adv Psychopharmacol. 2011;1(2):47–66.
- . Review and management of clozapine side effects. J Clin Psychiatry. 2000;61(suppl 8):14–17; discussion 18–19.
- , . Epilepsy, psychosis and clozapine. Human Psychopharmacol Clin Exp. 2002;17:115–119.
- , , , , , . Response of patients with treatment‐refractory schizophrenia to clozapine within three serum level ranges. Am J Psychiatry. 1996;153(12):1579–1584.
- , , , , . When can patients with potentially life‐threatening adverse effects be rechallenged with clozapine? A systematic review of the published literature. Schizophr Res. 2012;134(2–3):180–186.
- , . Clinical profile of clozapine: adverse reactions and agranulocytosis. Psychiatr Q. 1992;63(1):51–70.
- , , , , , . Clozapine and hypertension: a chart review of 82 patients. J Clin Psychiatry. 2004;65(5):686–689.
- , , . Adverse cardiac effects associated with clozapine. J Clin Psychopharmacol. 2005;25(1):32–41.
- , , , , . Clozapine induced myocarditis: a rare but fatal complication. Int J Cardiol. 2006;112(2):e5–e6.
- , , , . Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841–1845.
- , , , . Life‐threatening clozapine‐induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry. 2008;69(5):759–768.
- , , , . Fatalities associated with clozapine‐related constipation and bowel obstruction: a literature review and two case reports. Psychosomatics. 2009;50(4):416–419.
- , , . Death from clozapine‐induced constipation: case report and literature review. Psychosomatics. 2002;43(1):71–73.
- , , , , , . Clozapine: a clinical review of adverse effects and management. Ann Clin Psychiatry. 2003;15(1):33–48.
- , , , , . Beyond white blood cell monitoring: screening in the initial phase of clozapine therapy. J Clin Psychiatry. 2012;73(10):1307–1312.
- Clozapine [package insert]. Sellersville, PA: TEVA Pharmaceuticals USA; 2013. Available at: https://www.clozapineregistry.com/insert.pdf.ashx. Accessed October 27, 2014.
- , , , , . Clozapine‐induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162–167.
- Clozaril (clozapine) prescribing information. Washington, DC: U.S. Food and Drug Administration; 2013. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/019758s069s071lbl.pdf. Accessed February 4, 2015.
- . Clozapine therapy during cancer treatment. Am J Psychiatry. 2004;161(1):175.
- , , , , . Continuation of clozapine during chemotherapy: a case report and review of literature. Psychosomatics. 2014;55(6):673–679.
- , , . Clozapine use in HIV‐infected schizophrenia patients: a case‐based discussion and review. Psychosomatics. 2009;50(6):626–632.
- , , , . Letter: clozapine and agranulocytosis. Lancet. 1975;2(7935):611.
- . Effects of the clozapine national registry system on incidence of deaths related to agranulocytosis. Psychiatr Serv. 1996;47(1):52–56.
- , . White blood cell monitoring during long‐term clozapine treatment. Am J Psychiatry. 2013;170(4):366–369.
- , , . Add‐on filgrastim during clozapine rechallenge in patients with a history of clozapine‐related granulocytopenia/agranulocytosis. Am J Psychiatry. 2009;166(2):236.
- , , . Add‐on filgrastim during clozapine rechallenge unsuccessful in preventing agranulocytosis. Gen Hosp Psychiatry. 2013;35(5):576.e11–12.
- , , , , , . Clozapine‐induced weight gain: prevalence and clinical relevance. Am J Psychiatry. 1992;149(1):68–72.
- , , , et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10‐year naturalistic study. J Clin Psychiatry. 2005;66(9):1116–1121.
- , , , et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine‐treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24‐week, randomized, double‐blind, placebo‐controlled study. J Clin Psychiatry. 2013;74(5):e424–e430.
- , , , . Topiramate for clozapine‐induced seizures. Am J Psychiatry. 2001;158(6):968–969.
- , , , , . Clozapine‐induced acute interstitial nephritis. Lancet. 1999;354(9185):1180–1181.
- , , , , . Update on the clinical efficacy and side effects of clozapine. Schizophr Bull. 1991;17(2):247–261.
- , , . Clozapine‐induced sialorrhea: pathophysiology and management strategies. Psychopharmacology. 2006;185(3):265–273.
- , , . Clozapine drug‐drug interactions: a review of the literature. Hum Psychopharm Clin. 1997;12(1):5–20.
- , , . The effect of smoking and cytochrome P450 CYP1A2 genetic polymorphism on clozapine clearance and dose requirement. Pharmacogenetics. 2003;13(3):169–172.
Clozapine is a second‐generation antipsychotic (SGA) medication that was developed in 1959, introduced to Europe in 1971, and withdrawn from the market in 1975 due to associated concerns for potentially fatal agranulocytosis. In 1989, the US Food and Drug Administration (FDA) approved use of clozapine for the management of treatment‐resistant schizophrenia, under strict parameters for complete blood count (CBC) monitoring. Clozapine has since gained an additional FDA indication for reducing suicidal behavior in patients with schizophrenia and schizoaffective disorder,[1, 2, 3] and displayed superiority to both first generation antipsychotics and other SGA agents in reducing symptom burden.[2, 4, 5]
Clozapine's clinical benefits include lowering mortality in schizophrenia,[6] reducing deaths from ischemic heart disease,[7] curtailing substance use in individuals with psychotic disorders,[8] increasing rates of independent living and meaningful occupational activity, and reducing psychiatric hospitalizations and need for involuntary treatment.[9] Because schizophrenia, itself, is associated with a 15‐ to 20‐year decrease in average lifespan,[10] these benefits of clozapine are particularly salient. Yet the mechanism by which clozapine mitigates otherwise‐refractory psychotic symptoms is a conundrum. Structurally a tricyclic dibenzodiazepine, clozapine has relatively little effect on the dopamine D2 receptor, which has classically been thought to mediate the treatment effect of antipsychotics.[11, 12]
The unique nature of clozapine extends to its adverse effect profile. A significant percentage of patients who discontinue clozapine (17%35.4%) cite medical complications, the most common being seizures, constipation, sedation, and neutropenia.[13, 14] Yet several studies, including the landmark Clinical Antipsychotic Trials for Interventions Effectiveness (CATIE) study, have found that patients were more likely to adhere to clozapine therapy than to other antipsychotics.[2, 15] In the CATIE study, 44% of subjects taking clozapine continued the medication for 18 months, compared to 29% of individuals on olanzapine, 14% on risperidone, and 7% on quetiapine. Median time until discontinuation of clozapine was 10.5 months, significantly longer than for quetiapine (2.8 months) and olanzapine (2.7 months).[2] Because patients who experience clozapine‐related medical complications are likely to present first to the primary care or general hospital setting, internists must be aware of potential iatrogenic effects, and of their implications for psychiatric and medical care. Using case examples, we will examine both common and serious complications associated with clozapine, and discuss recommendations for management, including indications for clozapine discontinuation.
NEUROLOGICAL
Case Vignette 1
Mr. A is a 29‐year‐old man with asthma and schizophrenia who experienced a generalized tonic‐clonic seizure during treatment at a psychiatric facility. The patient started clozapine therapy 5 weeks prior, with gradual titration to 425 mg daily. Mr. A's previous medication trials included olanzapine and chlorpromazine, which rendered little improvement to his chronic auditory hallucinations. Clozapine was temporarily withheld during further neurologic workup, in which both electroencephalogram (EEG) and brain magnetic resonance imaging were unremarkable. After 60 hours, clozapine titration was reinitiated, and valproic acid was started for mood stabilization and seizure prophylaxis. Mr. A was discharged 6 weeks later on clozapine, 600 mg at bedtime, and extended‐release divalproate, 2500 mg at bedtime. The patient suffered no further seizure activity throughout hospitalization and for at least 1 year postdischarge.
Seizures complicate clozapine use in up to 5% of cases, with a dose‐dependent risk pattern.[16] Seizures are most commonly associated with serum clozapine levels above 500 g/L), but have also been reported with lower levels of clozapine and its metabolite norclozapine.[17] Though nonspecific EEG changes (ie, focal or generalized spikes, spike‐wave and polyspike discharges) have been associated with clozapine administration, they do not reliably predict seizure tendency.[17] Prophylaxis with antiepileptic drugs (AEDs) is not recommended, though AED treatment may be undertaken for patients who experience a seizure while on clozapine. When seizures occur in the context of elevated serum levels, reducing clozapine to the lowest effective dose is preferred over initiating an AED. Although this reduces the potential for exposure to anticonvulsant‐associated adverse effects, it may also introduce the risk of relapsed psychotic symptoms, and therefore requires close monitoring by a psychiatrist. For those who opt to initiate AED therapy, we recommend consideration of each medication's therapeutic and side‐effect profiles based on the patient's medical history and active symptoms. For example, in the case of Mr. A, valproate was used to target concomitant mood symptoms; likewise, patients who experience troublesome weight gain, as well as seizures, may benefit from topiramate. The occurrence of seizures does not preclude continuation of clozapine therapy, in conjunction with an AED[18] and after consideration of potential risks and benefits of use. Clozapine is not contraindicated in patients with well‐controlled epilepsy.[19]
Sedation, the most common neurologic side effect of clozapine, is also dose dependent and often abates during titration.[20] Though clozapine may induce extrapyramidal symptoms, including rigidity, tremor, and dystonia, the risk is considerably lower with clozapine than other antipsychotics, owing to a lesser affinity for D2 receptors. Associated parkinsonism should prompt consideration of dose reduction, in discussion with a psychiatrist, with concurrent monitoring of serum clozapine levels and close follow‐up for emergence of psychotic symptoms. If dose reduction is ineffective, not indicated, or not preferred by the patient, the addition of an anticholinergic medication may be considered (eg, diphenhydramine 2550 mg, benztropine 12 mg). Neuroleptic malignant syndrome, although rare, is life‐threatening and warrants immediate discontinuation of clozapine, though successful rechallenge after has been reported in case reports.[21]
CARDIAC
Case Vignette 2
Mr. B is a 34‐year‐old man with sinus tachycardia, a benign adrenal tumor, and chronic paranoid schizophrenia that had been poorly responsive to numerous antipsychotic trials. During a psychiatric hospitalization for paranoid delusions with aggressive threats toward family, Mr. B was started on clozapine and titrated to 250 mg daily. On day 16 of clozapine therapy, the patient began to experience cough, and several days later, diffuse rhonchi were noted on examination. Complete blood count revealed WBC 20.3 * 103/L, with 37% eosinophils and absolute eosinophil count of 7.51 (increased from 12%/1.90 the week before), and an electrocardiogram showed sinus tachycardia with ST‐segment changes. Mr. B was transferred to the general medical hospital for workup of presumed myocarditis.
Approximately one‐quarter of patients who take clozapine experience sinus tachycardia, which may be related to clozapine's anticholinergic effects causing rebound noradrenergic elevations[22]; persistent or problematic tachycardia may be treated using a cardio‐selective ‐blocker. Clozapine has also been linked to significant increases in systolic and diastolic blood pressure in 4% of patients (monitoring data); the risk of hypertension increases with the duration of clozapine treatment, and appears to be independent of the patient's weight.[23] Orthostatic hypotension has been reported in 9% of patients on clozapine therapy, though effects can be mitigated with gradual titration, adequate hydration, compression stockings, and patient education. Sinus tachycardia, hypertension, and orthostatic hypotension are not absolute indications to discontinue clozapine; rather, we advocate for treating these side effects while continuing clozapine treatment.[24]
Myocarditis represents the most serious cardiac side effect of clozapine.[25, 26] Although the absolute risk appears to be lower than 0.1%,[24] Kilian et al. calculated a 1000‐to‐2000fold increase in relative risk of myocarditis among patients who take clozapine, compared to the general population.[26] Most cases occur within the first month of treatment, with median time to onset of 15 days. This time course is consistent with an acute immunoglobulin Emediated hypersensitivity (type 1) reaction, and eosinophilic infiltrates have been found on autopsy, consistent with an acute drug reaction.[20]
Because of this early onset, the physician should maintain a particularly high index of suspicion in the first months of treatment, rigorously questioning patients and families about signs and symptoms of cardiac disease. If patients on clozapine present with flu‐like symptoms, fever, myalgia, dizziness, chest pain, dyspnea, tachycardia, palpitations, or other signs or symptoms of heart failure, evaluation for myocarditis should be undertaken.[25] Several centers have utilized cardiac enzymes (e.g., troponin I, troponin T, creatine kinase‐myocardial band) as a universal screen for myocarditis, though this is not a universal practice.[24] Both tachycardia and flu‐like symptoms may be associated with clozapine, particularly during the titration period, and these are normally benign symptoms requiring no intervention. If the diagnosis of myocarditis is made, however, clozapine should be stopped immediately. Myocarditis is often considered to be a contraindication to restarting clozapine, though cases have been reported of successful clozapine rechallenge in patients who had previously experienced myocarditis.[21]
Recommendations for clozapine‐associated electrocardiography (ECG) monitoring have not been standardized. Based on common clinical practice and the time course of serious cardiac complications, we recommend baseline ECG prior to the start of clozapine, with follow‐up ECG 2 to 4 weeks after clozapine initiation, and every 6 months thereafter.
GASTROINTESTINAL
Case Vignette 3
Mr. C is a 61‐year‐old man with chronic paranoid schizophrenia and a history of multiple‐state hospital admissions. He had been maintained on clozapine for 15 years, allowing him to live independently and avoid psychiatric hospitalization. Mr. C was admitted to the general medical hospital with nausea, vomiting, and an inability to tolerate oral intake. He was found to have a high‐grade small‐bowel obstruction, and all oral medications were initially discontinued. After successful management of his acute gastrointestinal presentation and discussion of potential risks and benefits of various treatment options, clozapine was reinitiated along with bulk laxative and stool softening agents.
Affecting 14% to 60% of individuals who are prescribed clozapine, constipation represents the most common associated gastrointestinal complaint.[27] For most patients, this condition is uncomfortable but nonlethal, though it has been implicated in several deaths by aspiration pneumonia and small‐bowel perforation.[28, 29] Providers must screen regularly for constipation and treat aggressively with stimulant laxatives and stool softeners,[18] while reviewing medication lists and, when possible, streamlining extraneous anticholinergic contributors. Clozapine‐prescribed individuals also frequently suffer from gastrointestinal reflux disease (GERD), for which behavioral interventions (eg, smoking cessation or remaining upright for 3 hours after meals) should be considered in addition to pharmacologic treatment with proton pump inhibitors. Clozapine therapy may be continued while constipation and GERD are managed medically.
Potentially fatal gastrointestinal hypomotility and small‐bowel obstruction are rare but well‐described complications that occur in up to 0.3% of patients who take clozapine.[27] This effect appears to be dose dependent, and higher blood levels are associated with greater severity of constipation and risk for serious hypomotility.[27] Clozapine should be withheld during treatment for such serious adverse events as ileus or small‐bowel perforation; however, once these conditions have stabilized, clozapine therapy may be reconsidered based on an analysis of potential benefits and risks. If clozapine is withheld, the internist must monitor for acute worsening of mental status, inattention, and disorientation, as clozapine withdrawal‐related delirium has been reported.[30] Ultimately, aggressive treatment of constipation in conjunction with continued clozapine therapy is the recommended course of action.[28]
Given the increased risk of ileus in the postoperative period, it is particularly important for physicians to inquire about preoperative bowel habits and assess for any existing constipation. Careful monitoring of postoperative bowel motility, along with early and aggressive management of constipation, is recommended. Concurrent administration of other constipating agents (eg, opiates, anticholinergics) should be limited to the lowest effective dose.[27] Although transaminitis, hepatitis, and pancreatitis have all been associated with clozapine in case reports, these are rare,[31] and the approach to management should be considered on a case‐by‐case basis.
HEMATOLOGIC
Case Vignette 4
Ms. D is a 38‐year‐old woman with a schizoaffective disorder who was started on clozapine after 3 other agents had failed to control her psychotic symptoms and alleviate chronic suicidal thoughts. Baseline CBC revealed serum white blood cell count (WBC) of 7800/mm3 and absolute neutrophil count (ANC) of 4700/mm3. In Ms. D's third week of clozapine use, WBC dropped to 4400/mm3 and ANC to 2200/mm3. Repeat lab draw confirmed this, prompting the treatment team to initiate twice‐weekly CBC monitoring. Ms. D's counts continued to fall, and 10 days after the initial drop, WBC was calculated at 1400/mm3 and ANC at 790/mm3. Clozapine was discontinued, and though the patient was asymptomatic, broad‐spectrum antibiotics were initiated. She received daily CBC monitoring until WBC >3000/mm3 and ANC >1500/mm3. An alternate psychotropic medication was initiated several weeks thereafter.
Neutropenia (white blood cell count 3000/mm3) is a common complication that affects approximately 3% of patients who take clozapine.[32] This may be mediated by clozapine's selective impact on the precursors of polymorphonuclear leukocytes, though the mechanism remains unknown.[33] Although neutropenia is not an absolute contraindication for clozapine therapy, guidelines recommend cessation of clozapine when the ANC drops below 1000/mm3.[34] A meta‐analysis of 112 patients who were rechallenged following neutropenia found that 69% tolerated a rechallenge without development of a subsequent dyscrasia.[21]
In the case of chemotherapy‐induced neutropenia, several case reports support the continued use of clozapine during cancer treatment[35]; this requires a written request to the pharmaceutical company that manufactures clozapine and documentation of the expected time course and contribution of chemotherapy to neutropenia.[36] Clozapine's association with neutropenia warrants close monitoring in individuals with human immunodeficiency virus (HIV) and other causes of immune compromise. Reports of clozapine continuation in HIV‐positive individuals underscore the importance of close collaboration between infectious disease and psychiatry, with specific focus on potential interactions between clozapine and antiretroviral agents and close monitoring of viral load and ANC.[37]
The most feared complication of clozapine remains agranulocytosis, defined as ANC500/mm3,[33] which occurs in up to 1% of monitored patients. In 1975, clozapine was banned worldwide after 8 fatal cases of agranulocytosis were reported in Finland.[38] The drug was reintroduced for treatment‐resistant schizophrenia with strict monitoring parameters, which has sharply reduced the death rate. One study found 12 actual deaths between 1990 and 1994, compared to the 149 predicted deaths without monitoring.[39]
The risk of agranulocytosis appears to be higher in older adults and in patients with a lower baseline WBC count. Although there are reports of delayed agranulocytosis occurring in patients after up to 19 years of treatment,[40] the incidence of leukopenia is greatest in the first year. Given this high‐risk period, mandatory monitoring is as follows: weekly WBC and neutrophil counts for the first 26 weeks, biweekly counts for the second 26 weeks, and every 4 weeks thereafter. Of note, many of the later cases of agranulocytosis appear to be related to medication coadministration, particularly with valproic acid, though no definitive link has been established.[40]
Treatment of clozapine‐induced agranulocytosis consists of immediate clozapine cessation, and consideration of initiation of prophylactic broad‐spectrum antibiotics and granulocyte colony‐stimulating factor (such as filgrastim) until the granulocyte count normalizes.[41, 42] Although few case reports describe successful clozapine rechallenge in patients with a history of agranulocytosis, the data are sparse, and current practice is to permanently discontinue clozapine if ANC falls below 1000/mm3.[21, 41]
ADDITIONAL COMPLICATIONS (METABOLIC, RENAL, URINARY)
Moderate to marked weight gain occurs in over 50% of patients treated with clozapine, with average gains of nearly 10% body weight.[43] In a 10‐year follow‐up study of patients treated with clozapine, Henderson et al. reported an average weight gain of 13 kg, with 34% percent of studied patients developing diabetes mellitus. Metabolic side effects of second‐generation antipsychotics, including clozapine, are a well‐documented and troubling phenomenon.[44] Limited evidence supports use of metformin, alongside behavioral therapy, for concerns related to glucose dysregulation.[45] Some patients have also experienced weight loss with adjunctive topiramate use, particularly if they have also suffered seizures.[46]
Urinary incontinence and nocturnal enuresis are both associated with clozapine, but are likely under‐reported because of patient and provider embarrassment; providers also may not think to ask about these specific symptoms. First‐line treatment for nocturnal enuresis is to limit fluids in the evening. Desmopressin has a controversial role in treating nocturnal enuresis owing to its risk of hyponatremia; appropriate monitoring should be implemented if this agent is used.[18]
Clozapine has been associated with acute interstitial nephritis (AIN), although this is thought to be a relatively rare side effect. Drug‐induced AIN typically appears soon after initiation and presents with the clinical triad of rash, fever, and eosinophilia. Given that weekly CBC is mandatory in the initiation phase, eosinophilia is easily detectible and may serve as a marker for potential AIN.[47]
Sialorrhea, particularly during sleep, is a bothersome condition affecting up to one‐third of patients who take clozapine.[48] Although clozapine is strongly anticholinergic, its agonist activity at the M4 muscarinic receptor and antagonism of the alpha‐2 adrenergic receptor are postulated as the mechanisms underlying hypersalivation. Sialorrhea is frequently seen early in treatment and does not appear to be dose dependent.[48] Excessive salivation is typically managed with behavioral interventions (eg, utilizing towels or other absorbent materials on top of bedding). If hypersalivation occurs during the day, chewing sugar‐free gum may increase the rate of swallowing and make symptoms less bothersome. If this does not provide adequate relief, practitioners may consider use of atropine 1% solution administered directly to the oral cavity.[49]
DRUG‐DRUG INTERACTIONS
For hospitalists, who must frequently alter existing medications or add new ones, awareness of potential drug‐drug interactions is crucial. Clozapine is metabolized by the cytochrome p450 system, with predominant metabolism through the isoenzymes 1A2, 3A4, and 2D6.[50] Common medications that induce clozapine metabolism (thereby decreasing clozapine levels) include phenytoin, phenobarbital, carbamazepine, oxcarbazepine, and corticosteroids. Conversely, stopping these medications after long‐term therapy will raise clozapine levels. Substances that inhibit clozapine metabolism (thereby increasing clozapine levels) include ciprofloxacin, erythromycin, clarithromycin, fluvoxamine, fluoxetine, paroxetine, protease inhibitors, verapamil, and grapefruit juice. We recommend caution when concurrently administering other agents that increase risk for agranulocytosis, including carbamazepine, trimethoprim‐sulfamethoxazole, sulfasalazine, and tricyclic antidepressants.
Cigarette smoking decreases clozapine blood levels by induction of CYP1A2. Patients require a 10% to 30% reduction to clozapine dose during periods of smoking cessation, including when smoking is stopped during inpatient hospitalization.[51] Nicotine replacement therapy does not induce CYP1A2 and therefore does not have a compensatory effect on clozapine levels. On discharge or resumption of smoking, patients may require an increase of their dose of clozapine to maintain adequate antipsychotic effect.
SUMMARY OF RECOMMENDATIONS
Medical complications are cited as the cause in 20% of clozapine discontinuations; most commonly, these include seizures, severe constipation, somnolence, and neutropenia. Given the high risk of psychiatric morbidity posed by discontinuation, we recommend managing mild‐moderate symptoms and side effects while continuing the drug, when possible (Table 1). We encourage hospitalists to confer with the patient's psychiatrist or the inpatient psychiatry consultation service when making changes to clozapine therapy. Specific recommendations are as follows:
- We advocate withholding clozapine administration pending medical optimization for several conditions, including: small‐bowel obstruction, neuroleptic malignant syndrome, venous thromboembolism, diabetic ketoacidosis, or hyperosmolar coma.
- Clinical scenarios requiring acute discontinuation of clozapine include agranulocytosis and myocarditis. Successful rechallenge with clozapine has been described after both conditions; at the same time, given the high morbidity and mortality of myocarditis and agranulocytosis, re‐initiation of clozapine requires an extensive risk‐benefit discussion with the patient and family, informed consent, and, in the case of agranulocytosis, approval from the national clozapine registry (Table 2).
- Although adjunctive therapy with filgrastim was initially thought to permit a clozapine rechallenge in patients with a history of agranulocytosis, case reports on this strategy have been equivocal, and further research is necessary to determine the most effective strategy for management.
| Clinical Lab/Study | Frequency of Monitoring | |
|---|---|---|
| Cardiac | Electrocardiogram | Baseline, 24 weeks after initiation, every 6 months thereafter |
| Cardiac enzymes (eg, troponin I) echocardiogram | No standard guidelines, unless clinically indicated | |
| Hematologic | Complete blood count with differential | Baseline, then weekly 26 weeks, then every 2 weeks 26 weeks, then every 4 weeks thereafter |
| Metabolic | Body mass index; circumference of waist | Baseline, then every 3 to 6 months |
| Fasting glucose | Baseline, then every 6 months | |
| Fasting lipid panel | Baseline, then yearly | |
| Neurologic | Electroencephalogram | No standard guidelines, unless clinically indicated |
| Vital signs | Heart rate, blood pressure, temperature | Baseline and at each follow‐up visit |
| Requires Acute Clozapine Discontinuation* | Clozapine Interruption During Management | Does Not Typically Require Clozapine Discontinuation |
|---|---|---|
| ||
| Agranulocytosis (ANC1.0 109/mm3) | Diabetic complications (eg, ketoacidosis, hyperosmolar coma) | Constipation |
| Cardiomyopathy (severe) | Gastrointestinal obstruction, ileus | Diabetes mellitus |
| Myocarditis | Neuroleptic malignant syndrome | Gastroesophageal Reflux |
| Venous thromboembolism | Hyperlipidemia | |
| Hypertension | ||
| Orthostatic hypotension | ||
| Sedation | ||
| Seizures | ||
| Sialorrhea | ||
| Sinus tachycardia | ||
| Urinary changes (eg, enuresis, incontinence) | ||
| Weight gain | ||
CONCLUSION
Clozapine has been a very successful treatment for patients with schizophrenia who have failed other antipsychotic therapies. However, fears of potential side effects and frequent monitoring have limited its use and led to unnecessary discontinuation. To mitigate risk for serious complications, we hope to increase hospitalists' awareness of prevention, monitoring, and treatment of side effects, and to promote comfort with circumstances that warrant continuation or discontinuation of clozapine (Table 3). The hospitalist plays a crucial role in managing these complications as well as conveying information and recommendations to primary care providers; as such, their familiarity with the medication is essential for proper management of individuals who take clozapine.
| Take‐Home Points |
|---|
| 1. Clozapine is the gold standard for treatment‐resistant schizophrenia; however, its use is limited by side effects, many of which can be successfully treated by internists. |
| 2. There are few indications for discontinuing clozapine (myocarditis, small‐bowel obstruction, agranulocytosis). The psychiatry service should be consulted in the event that clozapine is discontinued. |
| 3. Seizures are not an indication for discontinuing clozapine; instead, we recommend adding an antiepileptic drug. |
| 4. All second‐generation antipsychotics are associated with diabetes mellitus and significant weight gain. Clozapine is more highly associated with metabolic side effects than many other medications in this class. |
| 5. Sedation, sialorrhea, and constipation are common and can be managed pharmacologically and with behavioral interventions. |
Disclosure: Nothing to report.
Clozapine is a second‐generation antipsychotic (SGA) medication that was developed in 1959, introduced to Europe in 1971, and withdrawn from the market in 1975 due to associated concerns for potentially fatal agranulocytosis. In 1989, the US Food and Drug Administration (FDA) approved use of clozapine for the management of treatment‐resistant schizophrenia, under strict parameters for complete blood count (CBC) monitoring. Clozapine has since gained an additional FDA indication for reducing suicidal behavior in patients with schizophrenia and schizoaffective disorder,[1, 2, 3] and displayed superiority to both first generation antipsychotics and other SGA agents in reducing symptom burden.[2, 4, 5]
Clozapine's clinical benefits include lowering mortality in schizophrenia,[6] reducing deaths from ischemic heart disease,[7] curtailing substance use in individuals with psychotic disorders,[8] increasing rates of independent living and meaningful occupational activity, and reducing psychiatric hospitalizations and need for involuntary treatment.[9] Because schizophrenia, itself, is associated with a 15‐ to 20‐year decrease in average lifespan,[10] these benefits of clozapine are particularly salient. Yet the mechanism by which clozapine mitigates otherwise‐refractory psychotic symptoms is a conundrum. Structurally a tricyclic dibenzodiazepine, clozapine has relatively little effect on the dopamine D2 receptor, which has classically been thought to mediate the treatment effect of antipsychotics.[11, 12]
The unique nature of clozapine extends to its adverse effect profile. A significant percentage of patients who discontinue clozapine (17%35.4%) cite medical complications, the most common being seizures, constipation, sedation, and neutropenia.[13, 14] Yet several studies, including the landmark Clinical Antipsychotic Trials for Interventions Effectiveness (CATIE) study, have found that patients were more likely to adhere to clozapine therapy than to other antipsychotics.[2, 15] In the CATIE study, 44% of subjects taking clozapine continued the medication for 18 months, compared to 29% of individuals on olanzapine, 14% on risperidone, and 7% on quetiapine. Median time until discontinuation of clozapine was 10.5 months, significantly longer than for quetiapine (2.8 months) and olanzapine (2.7 months).[2] Because patients who experience clozapine‐related medical complications are likely to present first to the primary care or general hospital setting, internists must be aware of potential iatrogenic effects, and of their implications for psychiatric and medical care. Using case examples, we will examine both common and serious complications associated with clozapine, and discuss recommendations for management, including indications for clozapine discontinuation.
NEUROLOGICAL
Case Vignette 1
Mr. A is a 29‐year‐old man with asthma and schizophrenia who experienced a generalized tonic‐clonic seizure during treatment at a psychiatric facility. The patient started clozapine therapy 5 weeks prior, with gradual titration to 425 mg daily. Mr. A's previous medication trials included olanzapine and chlorpromazine, which rendered little improvement to his chronic auditory hallucinations. Clozapine was temporarily withheld during further neurologic workup, in which both electroencephalogram (EEG) and brain magnetic resonance imaging were unremarkable. After 60 hours, clozapine titration was reinitiated, and valproic acid was started for mood stabilization and seizure prophylaxis. Mr. A was discharged 6 weeks later on clozapine, 600 mg at bedtime, and extended‐release divalproate, 2500 mg at bedtime. The patient suffered no further seizure activity throughout hospitalization and for at least 1 year postdischarge.
Seizures complicate clozapine use in up to 5% of cases, with a dose‐dependent risk pattern.[16] Seizures are most commonly associated with serum clozapine levels above 500 g/L), but have also been reported with lower levels of clozapine and its metabolite norclozapine.[17] Though nonspecific EEG changes (ie, focal or generalized spikes, spike‐wave and polyspike discharges) have been associated with clozapine administration, they do not reliably predict seizure tendency.[17] Prophylaxis with antiepileptic drugs (AEDs) is not recommended, though AED treatment may be undertaken for patients who experience a seizure while on clozapine. When seizures occur in the context of elevated serum levels, reducing clozapine to the lowest effective dose is preferred over initiating an AED. Although this reduces the potential for exposure to anticonvulsant‐associated adverse effects, it may also introduce the risk of relapsed psychotic symptoms, and therefore requires close monitoring by a psychiatrist. For those who opt to initiate AED therapy, we recommend consideration of each medication's therapeutic and side‐effect profiles based on the patient's medical history and active symptoms. For example, in the case of Mr. A, valproate was used to target concomitant mood symptoms; likewise, patients who experience troublesome weight gain, as well as seizures, may benefit from topiramate. The occurrence of seizures does not preclude continuation of clozapine therapy, in conjunction with an AED[18] and after consideration of potential risks and benefits of use. Clozapine is not contraindicated in patients with well‐controlled epilepsy.[19]
Sedation, the most common neurologic side effect of clozapine, is also dose dependent and often abates during titration.[20] Though clozapine may induce extrapyramidal symptoms, including rigidity, tremor, and dystonia, the risk is considerably lower with clozapine than other antipsychotics, owing to a lesser affinity for D2 receptors. Associated parkinsonism should prompt consideration of dose reduction, in discussion with a psychiatrist, with concurrent monitoring of serum clozapine levels and close follow‐up for emergence of psychotic symptoms. If dose reduction is ineffective, not indicated, or not preferred by the patient, the addition of an anticholinergic medication may be considered (eg, diphenhydramine 2550 mg, benztropine 12 mg). Neuroleptic malignant syndrome, although rare, is life‐threatening and warrants immediate discontinuation of clozapine, though successful rechallenge after has been reported in case reports.[21]
CARDIAC
Case Vignette 2
Mr. B is a 34‐year‐old man with sinus tachycardia, a benign adrenal tumor, and chronic paranoid schizophrenia that had been poorly responsive to numerous antipsychotic trials. During a psychiatric hospitalization for paranoid delusions with aggressive threats toward family, Mr. B was started on clozapine and titrated to 250 mg daily. On day 16 of clozapine therapy, the patient began to experience cough, and several days later, diffuse rhonchi were noted on examination. Complete blood count revealed WBC 20.3 * 103/L, with 37% eosinophils and absolute eosinophil count of 7.51 (increased from 12%/1.90 the week before), and an electrocardiogram showed sinus tachycardia with ST‐segment changes. Mr. B was transferred to the general medical hospital for workup of presumed myocarditis.
Approximately one‐quarter of patients who take clozapine experience sinus tachycardia, which may be related to clozapine's anticholinergic effects causing rebound noradrenergic elevations[22]; persistent or problematic tachycardia may be treated using a cardio‐selective ‐blocker. Clozapine has also been linked to significant increases in systolic and diastolic blood pressure in 4% of patients (monitoring data); the risk of hypertension increases with the duration of clozapine treatment, and appears to be independent of the patient's weight.[23] Orthostatic hypotension has been reported in 9% of patients on clozapine therapy, though effects can be mitigated with gradual titration, adequate hydration, compression stockings, and patient education. Sinus tachycardia, hypertension, and orthostatic hypotension are not absolute indications to discontinue clozapine; rather, we advocate for treating these side effects while continuing clozapine treatment.[24]
Myocarditis represents the most serious cardiac side effect of clozapine.[25, 26] Although the absolute risk appears to be lower than 0.1%,[24] Kilian et al. calculated a 1000‐to‐2000fold increase in relative risk of myocarditis among patients who take clozapine, compared to the general population.[26] Most cases occur within the first month of treatment, with median time to onset of 15 days. This time course is consistent with an acute immunoglobulin Emediated hypersensitivity (type 1) reaction, and eosinophilic infiltrates have been found on autopsy, consistent with an acute drug reaction.[20]
Because of this early onset, the physician should maintain a particularly high index of suspicion in the first months of treatment, rigorously questioning patients and families about signs and symptoms of cardiac disease. If patients on clozapine present with flu‐like symptoms, fever, myalgia, dizziness, chest pain, dyspnea, tachycardia, palpitations, or other signs or symptoms of heart failure, evaluation for myocarditis should be undertaken.[25] Several centers have utilized cardiac enzymes (e.g., troponin I, troponin T, creatine kinase‐myocardial band) as a universal screen for myocarditis, though this is not a universal practice.[24] Both tachycardia and flu‐like symptoms may be associated with clozapine, particularly during the titration period, and these are normally benign symptoms requiring no intervention. If the diagnosis of myocarditis is made, however, clozapine should be stopped immediately. Myocarditis is often considered to be a contraindication to restarting clozapine, though cases have been reported of successful clozapine rechallenge in patients who had previously experienced myocarditis.[21]
Recommendations for clozapine‐associated electrocardiography (ECG) monitoring have not been standardized. Based on common clinical practice and the time course of serious cardiac complications, we recommend baseline ECG prior to the start of clozapine, with follow‐up ECG 2 to 4 weeks after clozapine initiation, and every 6 months thereafter.
GASTROINTESTINAL
Case Vignette 3
Mr. C is a 61‐year‐old man with chronic paranoid schizophrenia and a history of multiple‐state hospital admissions. He had been maintained on clozapine for 15 years, allowing him to live independently and avoid psychiatric hospitalization. Mr. C was admitted to the general medical hospital with nausea, vomiting, and an inability to tolerate oral intake. He was found to have a high‐grade small‐bowel obstruction, and all oral medications were initially discontinued. After successful management of his acute gastrointestinal presentation and discussion of potential risks and benefits of various treatment options, clozapine was reinitiated along with bulk laxative and stool softening agents.
Affecting 14% to 60% of individuals who are prescribed clozapine, constipation represents the most common associated gastrointestinal complaint.[27] For most patients, this condition is uncomfortable but nonlethal, though it has been implicated in several deaths by aspiration pneumonia and small‐bowel perforation.[28, 29] Providers must screen regularly for constipation and treat aggressively with stimulant laxatives and stool softeners,[18] while reviewing medication lists and, when possible, streamlining extraneous anticholinergic contributors. Clozapine‐prescribed individuals also frequently suffer from gastrointestinal reflux disease (GERD), for which behavioral interventions (eg, smoking cessation or remaining upright for 3 hours after meals) should be considered in addition to pharmacologic treatment with proton pump inhibitors. Clozapine therapy may be continued while constipation and GERD are managed medically.
Potentially fatal gastrointestinal hypomotility and small‐bowel obstruction are rare but well‐described complications that occur in up to 0.3% of patients who take clozapine.[27] This effect appears to be dose dependent, and higher blood levels are associated with greater severity of constipation and risk for serious hypomotility.[27] Clozapine should be withheld during treatment for such serious adverse events as ileus or small‐bowel perforation; however, once these conditions have stabilized, clozapine therapy may be reconsidered based on an analysis of potential benefits and risks. If clozapine is withheld, the internist must monitor for acute worsening of mental status, inattention, and disorientation, as clozapine withdrawal‐related delirium has been reported.[30] Ultimately, aggressive treatment of constipation in conjunction with continued clozapine therapy is the recommended course of action.[28]
Given the increased risk of ileus in the postoperative period, it is particularly important for physicians to inquire about preoperative bowel habits and assess for any existing constipation. Careful monitoring of postoperative bowel motility, along with early and aggressive management of constipation, is recommended. Concurrent administration of other constipating agents (eg, opiates, anticholinergics) should be limited to the lowest effective dose.[27] Although transaminitis, hepatitis, and pancreatitis have all been associated with clozapine in case reports, these are rare,[31] and the approach to management should be considered on a case‐by‐case basis.
HEMATOLOGIC
Case Vignette 4
Ms. D is a 38‐year‐old woman with a schizoaffective disorder who was started on clozapine after 3 other agents had failed to control her psychotic symptoms and alleviate chronic suicidal thoughts. Baseline CBC revealed serum white blood cell count (WBC) of 7800/mm3 and absolute neutrophil count (ANC) of 4700/mm3. In Ms. D's third week of clozapine use, WBC dropped to 4400/mm3 and ANC to 2200/mm3. Repeat lab draw confirmed this, prompting the treatment team to initiate twice‐weekly CBC monitoring. Ms. D's counts continued to fall, and 10 days after the initial drop, WBC was calculated at 1400/mm3 and ANC at 790/mm3. Clozapine was discontinued, and though the patient was asymptomatic, broad‐spectrum antibiotics were initiated. She received daily CBC monitoring until WBC >3000/mm3 and ANC >1500/mm3. An alternate psychotropic medication was initiated several weeks thereafter.
Neutropenia (white blood cell count 3000/mm3) is a common complication that affects approximately 3% of patients who take clozapine.[32] This may be mediated by clozapine's selective impact on the precursors of polymorphonuclear leukocytes, though the mechanism remains unknown.[33] Although neutropenia is not an absolute contraindication for clozapine therapy, guidelines recommend cessation of clozapine when the ANC drops below 1000/mm3.[34] A meta‐analysis of 112 patients who were rechallenged following neutropenia found that 69% tolerated a rechallenge without development of a subsequent dyscrasia.[21]
In the case of chemotherapy‐induced neutropenia, several case reports support the continued use of clozapine during cancer treatment[35]; this requires a written request to the pharmaceutical company that manufactures clozapine and documentation of the expected time course and contribution of chemotherapy to neutropenia.[36] Clozapine's association with neutropenia warrants close monitoring in individuals with human immunodeficiency virus (HIV) and other causes of immune compromise. Reports of clozapine continuation in HIV‐positive individuals underscore the importance of close collaboration between infectious disease and psychiatry, with specific focus on potential interactions between clozapine and antiretroviral agents and close monitoring of viral load and ANC.[37]
The most feared complication of clozapine remains agranulocytosis, defined as ANC500/mm3,[33] which occurs in up to 1% of monitored patients. In 1975, clozapine was banned worldwide after 8 fatal cases of agranulocytosis were reported in Finland.[38] The drug was reintroduced for treatment‐resistant schizophrenia with strict monitoring parameters, which has sharply reduced the death rate. One study found 12 actual deaths between 1990 and 1994, compared to the 149 predicted deaths without monitoring.[39]
The risk of agranulocytosis appears to be higher in older adults and in patients with a lower baseline WBC count. Although there are reports of delayed agranulocytosis occurring in patients after up to 19 years of treatment,[40] the incidence of leukopenia is greatest in the first year. Given this high‐risk period, mandatory monitoring is as follows: weekly WBC and neutrophil counts for the first 26 weeks, biweekly counts for the second 26 weeks, and every 4 weeks thereafter. Of note, many of the later cases of agranulocytosis appear to be related to medication coadministration, particularly with valproic acid, though no definitive link has been established.[40]
Treatment of clozapine‐induced agranulocytosis consists of immediate clozapine cessation, and consideration of initiation of prophylactic broad‐spectrum antibiotics and granulocyte colony‐stimulating factor (such as filgrastim) until the granulocyte count normalizes.[41, 42] Although few case reports describe successful clozapine rechallenge in patients with a history of agranulocytosis, the data are sparse, and current practice is to permanently discontinue clozapine if ANC falls below 1000/mm3.[21, 41]
ADDITIONAL COMPLICATIONS (METABOLIC, RENAL, URINARY)
Moderate to marked weight gain occurs in over 50% of patients treated with clozapine, with average gains of nearly 10% body weight.[43] In a 10‐year follow‐up study of patients treated with clozapine, Henderson et al. reported an average weight gain of 13 kg, with 34% percent of studied patients developing diabetes mellitus. Metabolic side effects of second‐generation antipsychotics, including clozapine, are a well‐documented and troubling phenomenon.[44] Limited evidence supports use of metformin, alongside behavioral therapy, for concerns related to glucose dysregulation.[45] Some patients have also experienced weight loss with adjunctive topiramate use, particularly if they have also suffered seizures.[46]
Urinary incontinence and nocturnal enuresis are both associated with clozapine, but are likely under‐reported because of patient and provider embarrassment; providers also may not think to ask about these specific symptoms. First‐line treatment for nocturnal enuresis is to limit fluids in the evening. Desmopressin has a controversial role in treating nocturnal enuresis owing to its risk of hyponatremia; appropriate monitoring should be implemented if this agent is used.[18]
Clozapine has been associated with acute interstitial nephritis (AIN), although this is thought to be a relatively rare side effect. Drug‐induced AIN typically appears soon after initiation and presents with the clinical triad of rash, fever, and eosinophilia. Given that weekly CBC is mandatory in the initiation phase, eosinophilia is easily detectible and may serve as a marker for potential AIN.[47]
Sialorrhea, particularly during sleep, is a bothersome condition affecting up to one‐third of patients who take clozapine.[48] Although clozapine is strongly anticholinergic, its agonist activity at the M4 muscarinic receptor and antagonism of the alpha‐2 adrenergic receptor are postulated as the mechanisms underlying hypersalivation. Sialorrhea is frequently seen early in treatment and does not appear to be dose dependent.[48] Excessive salivation is typically managed with behavioral interventions (eg, utilizing towels or other absorbent materials on top of bedding). If hypersalivation occurs during the day, chewing sugar‐free gum may increase the rate of swallowing and make symptoms less bothersome. If this does not provide adequate relief, practitioners may consider use of atropine 1% solution administered directly to the oral cavity.[49]
DRUG‐DRUG INTERACTIONS
For hospitalists, who must frequently alter existing medications or add new ones, awareness of potential drug‐drug interactions is crucial. Clozapine is metabolized by the cytochrome p450 system, with predominant metabolism through the isoenzymes 1A2, 3A4, and 2D6.[50] Common medications that induce clozapine metabolism (thereby decreasing clozapine levels) include phenytoin, phenobarbital, carbamazepine, oxcarbazepine, and corticosteroids. Conversely, stopping these medications after long‐term therapy will raise clozapine levels. Substances that inhibit clozapine metabolism (thereby increasing clozapine levels) include ciprofloxacin, erythromycin, clarithromycin, fluvoxamine, fluoxetine, paroxetine, protease inhibitors, verapamil, and grapefruit juice. We recommend caution when concurrently administering other agents that increase risk for agranulocytosis, including carbamazepine, trimethoprim‐sulfamethoxazole, sulfasalazine, and tricyclic antidepressants.
Cigarette smoking decreases clozapine blood levels by induction of CYP1A2. Patients require a 10% to 30% reduction to clozapine dose during periods of smoking cessation, including when smoking is stopped during inpatient hospitalization.[51] Nicotine replacement therapy does not induce CYP1A2 and therefore does not have a compensatory effect on clozapine levels. On discharge or resumption of smoking, patients may require an increase of their dose of clozapine to maintain adequate antipsychotic effect.
SUMMARY OF RECOMMENDATIONS
Medical complications are cited as the cause in 20% of clozapine discontinuations; most commonly, these include seizures, severe constipation, somnolence, and neutropenia. Given the high risk of psychiatric morbidity posed by discontinuation, we recommend managing mild‐moderate symptoms and side effects while continuing the drug, when possible (Table 1). We encourage hospitalists to confer with the patient's psychiatrist or the inpatient psychiatry consultation service when making changes to clozapine therapy. Specific recommendations are as follows:
- We advocate withholding clozapine administration pending medical optimization for several conditions, including: small‐bowel obstruction, neuroleptic malignant syndrome, venous thromboembolism, diabetic ketoacidosis, or hyperosmolar coma.
- Clinical scenarios requiring acute discontinuation of clozapine include agranulocytosis and myocarditis. Successful rechallenge with clozapine has been described after both conditions; at the same time, given the high morbidity and mortality of myocarditis and agranulocytosis, re‐initiation of clozapine requires an extensive risk‐benefit discussion with the patient and family, informed consent, and, in the case of agranulocytosis, approval from the national clozapine registry (Table 2).
- Although adjunctive therapy with filgrastim was initially thought to permit a clozapine rechallenge in patients with a history of agranulocytosis, case reports on this strategy have been equivocal, and further research is necessary to determine the most effective strategy for management.
| Clinical Lab/Study | Frequency of Monitoring | |
|---|---|---|
| Cardiac | Electrocardiogram | Baseline, 24 weeks after initiation, every 6 months thereafter |
| Cardiac enzymes (eg, troponin I) echocardiogram | No standard guidelines, unless clinically indicated | |
| Hematologic | Complete blood count with differential | Baseline, then weekly 26 weeks, then every 2 weeks 26 weeks, then every 4 weeks thereafter |
| Metabolic | Body mass index; circumference of waist | Baseline, then every 3 to 6 months |
| Fasting glucose | Baseline, then every 6 months | |
| Fasting lipid panel | Baseline, then yearly | |
| Neurologic | Electroencephalogram | No standard guidelines, unless clinically indicated |
| Vital signs | Heart rate, blood pressure, temperature | Baseline and at each follow‐up visit |
| Requires Acute Clozapine Discontinuation* | Clozapine Interruption During Management | Does Not Typically Require Clozapine Discontinuation |
|---|---|---|
| ||
| Agranulocytosis (ANC1.0 109/mm3) | Diabetic complications (eg, ketoacidosis, hyperosmolar coma) | Constipation |
| Cardiomyopathy (severe) | Gastrointestinal obstruction, ileus | Diabetes mellitus |
| Myocarditis | Neuroleptic malignant syndrome | Gastroesophageal Reflux |
| Venous thromboembolism | Hyperlipidemia | |
| Hypertension | ||
| Orthostatic hypotension | ||
| Sedation | ||
| Seizures | ||
| Sialorrhea | ||
| Sinus tachycardia | ||
| Urinary changes (eg, enuresis, incontinence) | ||
| Weight gain | ||
CONCLUSION
Clozapine has been a very successful treatment for patients with schizophrenia who have failed other antipsychotic therapies. However, fears of potential side effects and frequent monitoring have limited its use and led to unnecessary discontinuation. To mitigate risk for serious complications, we hope to increase hospitalists' awareness of prevention, monitoring, and treatment of side effects, and to promote comfort with circumstances that warrant continuation or discontinuation of clozapine (Table 3). The hospitalist plays a crucial role in managing these complications as well as conveying information and recommendations to primary care providers; as such, their familiarity with the medication is essential for proper management of individuals who take clozapine.
| Take‐Home Points |
|---|
| 1. Clozapine is the gold standard for treatment‐resistant schizophrenia; however, its use is limited by side effects, many of which can be successfully treated by internists. |
| 2. There are few indications for discontinuing clozapine (myocarditis, small‐bowel obstruction, agranulocytosis). The psychiatry service should be consulted in the event that clozapine is discontinued. |
| 3. Seizures are not an indication for discontinuing clozapine; instead, we recommend adding an antiepileptic drug. |
| 4. All second‐generation antipsychotics are associated with diabetes mellitus and significant weight gain. Clozapine is more highly associated with metabolic side effects than many other medications in this class. |
| 5. Sedation, sialorrhea, and constipation are common and can be managed pharmacologically and with behavioral interventions. |
Disclosure: Nothing to report.
- , , , . Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database Syst Rev. 2009(1):CD000059.
- , , , et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600–610.
- , , , et al. Randomized controlled trial of effect of prescription of clozapine versus other second‐generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull. 2006;32(4):715–723.
- , , , et al. Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151(1):20–26.
- , , , . Clozapine for the treatment‐resistant schizophrenic. A double‐blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789–796.
- , , , , , . Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82–91.
- , , , et al. 11‐year follow‐up of mortality in patients with schizophrenia: a population‐based cohort study (FIN11 study). Lancet. 2009;374(9690):620–627.
- , , , , . Clozapine use and relapses of substance use disorder among patients with co‐occurring schizophrenia and substance use disorders. Schizophr Bull. 2006;32(4):637–643.
- , , . Outcomes for schizophrenia patients with clozapine treatment: how good does it get? J Psychopharmacol. 2009;23(8):957–965.
- , , , . Morbidity and mortality in people with serious mental illness. National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council. Available at: http://www.nasmhpd.org/docs/publications/MDCdocs/Mortality%20and%20Morbidity%20Final%20Report%208.18.08.pdf. Accessed February 3, 2015.
- , . Pharmacological actions of the atypical antipsychotic drug clozapine: a review. Synapse. 1996;24(4):349–394.
- , . Clozapine. A novel antipsychotic agent. N Engl J Med. 1991;324(11):746–754.
- , . Reason for clozapine cessation. Acta Psychiatr Scand. 2012;125(1):39–44.
- , , , . Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603–613.
- , , , , , . Time to discontinuation of antipsychotic drugs in a schizophrenia cohort: influence of current treatment strategies. Ther Adv Psychopharmacol. 2014;4(6):228–239.
- , , . Clozapine‐related seizures. Neurology. 1991;41(3):369–371.
- , , , . Clozapine‐related EEG changes and seizures: dose and plasma‐level relationships. Ther Adv Psychopharmacol. 2011;1(2):47–66.
- . Review and management of clozapine side effects. J Clin Psychiatry. 2000;61(suppl 8):14–17; discussion 18–19.
- , . Epilepsy, psychosis and clozapine. Human Psychopharmacol Clin Exp. 2002;17:115–119.
- , , , , , . Response of patients with treatment‐refractory schizophrenia to clozapine within three serum level ranges. Am J Psychiatry. 1996;153(12):1579–1584.
- , , , , . When can patients with potentially life‐threatening adverse effects be rechallenged with clozapine? A systematic review of the published literature. Schizophr Res. 2012;134(2–3):180–186.
- , . Clinical profile of clozapine: adverse reactions and agranulocytosis. Psychiatr Q. 1992;63(1):51–70.
- , , , , , . Clozapine and hypertension: a chart review of 82 patients. J Clin Psychiatry. 2004;65(5):686–689.
- , , . Adverse cardiac effects associated with clozapine. J Clin Psychopharmacol. 2005;25(1):32–41.
- , , , , . Clozapine induced myocarditis: a rare but fatal complication. Int J Cardiol. 2006;112(2):e5–e6.
- , , , . Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841–1845.
- , , , . Life‐threatening clozapine‐induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry. 2008;69(5):759–768.
- , , , . Fatalities associated with clozapine‐related constipation and bowel obstruction: a literature review and two case reports. Psychosomatics. 2009;50(4):416–419.
- , , . Death from clozapine‐induced constipation: case report and literature review. Psychosomatics. 2002;43(1):71–73.
- , , , , , . Clozapine: a clinical review of adverse effects and management. Ann Clin Psychiatry. 2003;15(1):33–48.
- , , , , . Beyond white blood cell monitoring: screening in the initial phase of clozapine therapy. J Clin Psychiatry. 2012;73(10):1307–1312.
- Clozapine [package insert]. Sellersville, PA: TEVA Pharmaceuticals USA; 2013. Available at: https://www.clozapineregistry.com/insert.pdf.ashx. Accessed October 27, 2014.
- , , , , . Clozapine‐induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162–167.
- Clozaril (clozapine) prescribing information. Washington, DC: U.S. Food and Drug Administration; 2013. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/019758s069s071lbl.pdf. Accessed February 4, 2015.
- . Clozapine therapy during cancer treatment. Am J Psychiatry. 2004;161(1):175.
- , , , , . Continuation of clozapine during chemotherapy: a case report and review of literature. Psychosomatics. 2014;55(6):673–679.
- , , . Clozapine use in HIV‐infected schizophrenia patients: a case‐based discussion and review. Psychosomatics. 2009;50(6):626–632.
- , , , . Letter: clozapine and agranulocytosis. Lancet. 1975;2(7935):611.
- . Effects of the clozapine national registry system on incidence of deaths related to agranulocytosis. Psychiatr Serv. 1996;47(1):52–56.
- , . White blood cell monitoring during long‐term clozapine treatment. Am J Psychiatry. 2013;170(4):366–369.
- , , . Add‐on filgrastim during clozapine rechallenge in patients with a history of clozapine‐related granulocytopenia/agranulocytosis. Am J Psychiatry. 2009;166(2):236.
- , , . Add‐on filgrastim during clozapine rechallenge unsuccessful in preventing agranulocytosis. Gen Hosp Psychiatry. 2013;35(5):576.e11–12.
- , , , , , . Clozapine‐induced weight gain: prevalence and clinical relevance. Am J Psychiatry. 1992;149(1):68–72.
- , , , et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10‐year naturalistic study. J Clin Psychiatry. 2005;66(9):1116–1121.
- , , , et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine‐treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24‐week, randomized, double‐blind, placebo‐controlled study. J Clin Psychiatry. 2013;74(5):e424–e430.
- , , , . Topiramate for clozapine‐induced seizures. Am J Psychiatry. 2001;158(6):968–969.
- , , , , . Clozapine‐induced acute interstitial nephritis. Lancet. 1999;354(9185):1180–1181.
- , , , , . Update on the clinical efficacy and side effects of clozapine. Schizophr Bull. 1991;17(2):247–261.
- , , . Clozapine‐induced sialorrhea: pathophysiology and management strategies. Psychopharmacology. 2006;185(3):265–273.
- , , . Clozapine drug‐drug interactions: a review of the literature. Hum Psychopharm Clin. 1997;12(1):5–20.
- , , . The effect of smoking and cytochrome P450 CYP1A2 genetic polymorphism on clozapine clearance and dose requirement. Pharmacogenetics. 2003;13(3):169–172.
- , , , . Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database Syst Rev. 2009(1):CD000059.
- , , , et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600–610.
- , , , et al. Randomized controlled trial of effect of prescription of clozapine versus other second‐generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull. 2006;32(4):715–723.
- , , , et al. Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151(1):20–26.
- , , , . Clozapine for the treatment‐resistant schizophrenic. A double‐blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789–796.
- , , , , , . Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82–91.
- , , , et al. 11‐year follow‐up of mortality in patients with schizophrenia: a population‐based cohort study (FIN11 study). Lancet. 2009;374(9690):620–627.
- , , , , . Clozapine use and relapses of substance use disorder among patients with co‐occurring schizophrenia and substance use disorders. Schizophr Bull. 2006;32(4):637–643.
- , , . Outcomes for schizophrenia patients with clozapine treatment: how good does it get? J Psychopharmacol. 2009;23(8):957–965.
- , , , . Morbidity and mortality in people with serious mental illness. National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council. Available at: http://www.nasmhpd.org/docs/publications/MDCdocs/Mortality%20and%20Morbidity%20Final%20Report%208.18.08.pdf. Accessed February 3, 2015.
- , . Pharmacological actions of the atypical antipsychotic drug clozapine: a review. Synapse. 1996;24(4):349–394.
- , . Clozapine. A novel antipsychotic agent. N Engl J Med. 1991;324(11):746–754.
- , . Reason for clozapine cessation. Acta Psychiatr Scand. 2012;125(1):39–44.
- , , , . Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603–613.
- , , , , , . Time to discontinuation of antipsychotic drugs in a schizophrenia cohort: influence of current treatment strategies. Ther Adv Psychopharmacol. 2014;4(6):228–239.
- , , . Clozapine‐related seizures. Neurology. 1991;41(3):369–371.
- , , , . Clozapine‐related EEG changes and seizures: dose and plasma‐level relationships. Ther Adv Psychopharmacol. 2011;1(2):47–66.
- . Review and management of clozapine side effects. J Clin Psychiatry. 2000;61(suppl 8):14–17; discussion 18–19.
- , . Epilepsy, psychosis and clozapine. Human Psychopharmacol Clin Exp. 2002;17:115–119.
- , , , , , . Response of patients with treatment‐refractory schizophrenia to clozapine within three serum level ranges. Am J Psychiatry. 1996;153(12):1579–1584.
- , , , , . When can patients with potentially life‐threatening adverse effects be rechallenged with clozapine? A systematic review of the published literature. Schizophr Res. 2012;134(2–3):180–186.
- , . Clinical profile of clozapine: adverse reactions and agranulocytosis. Psychiatr Q. 1992;63(1):51–70.
- , , , , , . Clozapine and hypertension: a chart review of 82 patients. J Clin Psychiatry. 2004;65(5):686–689.
- , , . Adverse cardiac effects associated with clozapine. J Clin Psychopharmacol. 2005;25(1):32–41.
- , , , , . Clozapine induced myocarditis: a rare but fatal complication. Int J Cardiol. 2006;112(2):e5–e6.
- , , , . Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841–1845.
- , , , . Life‐threatening clozapine‐induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry. 2008;69(5):759–768.
- , , , . Fatalities associated with clozapine‐related constipation and bowel obstruction: a literature review and two case reports. Psychosomatics. 2009;50(4):416–419.
- , , . Death from clozapine‐induced constipation: case report and literature review. Psychosomatics. 2002;43(1):71–73.
- , , , , , . Clozapine: a clinical review of adverse effects and management. Ann Clin Psychiatry. 2003;15(1):33–48.
- , , , , . Beyond white blood cell monitoring: screening in the initial phase of clozapine therapy. J Clin Psychiatry. 2012;73(10):1307–1312.
- Clozapine [package insert]. Sellersville, PA: TEVA Pharmaceuticals USA; 2013. Available at: https://www.clozapineregistry.com/insert.pdf.ashx. Accessed October 27, 2014.
- , , , , . Clozapine‐induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162–167.
- Clozaril (clozapine) prescribing information. Washington, DC: U.S. Food and Drug Administration; 2013. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/019758s069s071lbl.pdf. Accessed February 4, 2015.
- . Clozapine therapy during cancer treatment. Am J Psychiatry. 2004;161(1):175.
- , , , , . Continuation of clozapine during chemotherapy: a case report and review of literature. Psychosomatics. 2014;55(6):673–679.
- , , . Clozapine use in HIV‐infected schizophrenia patients: a case‐based discussion and review. Psychosomatics. 2009;50(6):626–632.
- , , , . Letter: clozapine and agranulocytosis. Lancet. 1975;2(7935):611.
- . Effects of the clozapine national registry system on incidence of deaths related to agranulocytosis. Psychiatr Serv. 1996;47(1):52–56.
- , . White blood cell monitoring during long‐term clozapine treatment. Am J Psychiatry. 2013;170(4):366–369.
- , , . Add‐on filgrastim during clozapine rechallenge in patients with a history of clozapine‐related granulocytopenia/agranulocytosis. Am J Psychiatry. 2009;166(2):236.
- , , . Add‐on filgrastim during clozapine rechallenge unsuccessful in preventing agranulocytosis. Gen Hosp Psychiatry. 2013;35(5):576.e11–12.
- , , , , , . Clozapine‐induced weight gain: prevalence and clinical relevance. Am J Psychiatry. 1992;149(1):68–72.
- , , , et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10‐year naturalistic study. J Clin Psychiatry. 2005;66(9):1116–1121.
- , , , et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine‐treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24‐week, randomized, double‐blind, placebo‐controlled study. J Clin Psychiatry. 2013;74(5):e424–e430.
- , , , . Topiramate for clozapine‐induced seizures. Am J Psychiatry. 2001;158(6):968–969.
- , , , , . Clozapine‐induced acute interstitial nephritis. Lancet. 1999;354(9185):1180–1181.
- , , , , . Update on the clinical efficacy and side effects of clozapine. Schizophr Bull. 1991;17(2):247–261.
- , , . Clozapine‐induced sialorrhea: pathophysiology and management strategies. Psychopharmacology. 2006;185(3):265–273.
- , , . Clozapine drug‐drug interactions: a review of the literature. Hum Psychopharm Clin. 1997;12(1):5–20.
- , , . The effect of smoking and cytochrome P450 CYP1A2 genetic polymorphism on clozapine clearance and dose requirement. Pharmacogenetics. 2003;13(3):169–172.