User login
A primary care guide to bipolar depression treatment
Bipolar disorder is a prevalent disorder in the primary care setting.1,2 Primary care providers therefore commonly encounter bipolar depression (BD; a major depressive episode in the context of bipolar disorder), which might be (1) an emerging depressive episode in previously undiagnosed bipolar disorder or (2) a recurrent episode during the course of chronic bipolar illness.3,4
A primary care–based collaborative model has been identified as a potential strategy for effective management of chronic mental health conditions such as bipolar disorder.5,6 However, this collaborative treatment model isn’t widely available; many patients with bipolar disorder are, in fact, treated solely by their primary care provider.
Two years ago in this journal,7 we addressed how to precisely identify an episode of BD and differentiate it from major depressive disorder (MDD; also known as unipolar depression). In this review, in addition to advancing clinical knowledge of BD, we provide:
- an overview of treatment options for BD (in contrast to the treatment of unipolar depression)
- the pharmacotherapeutic know-how to initiate and maintain treatment for uncomplicated episodes of BD.
We do not discuss management of manic, hypomanic, and mixed episodes of bipolar disorder.
How to identify bipolar depression
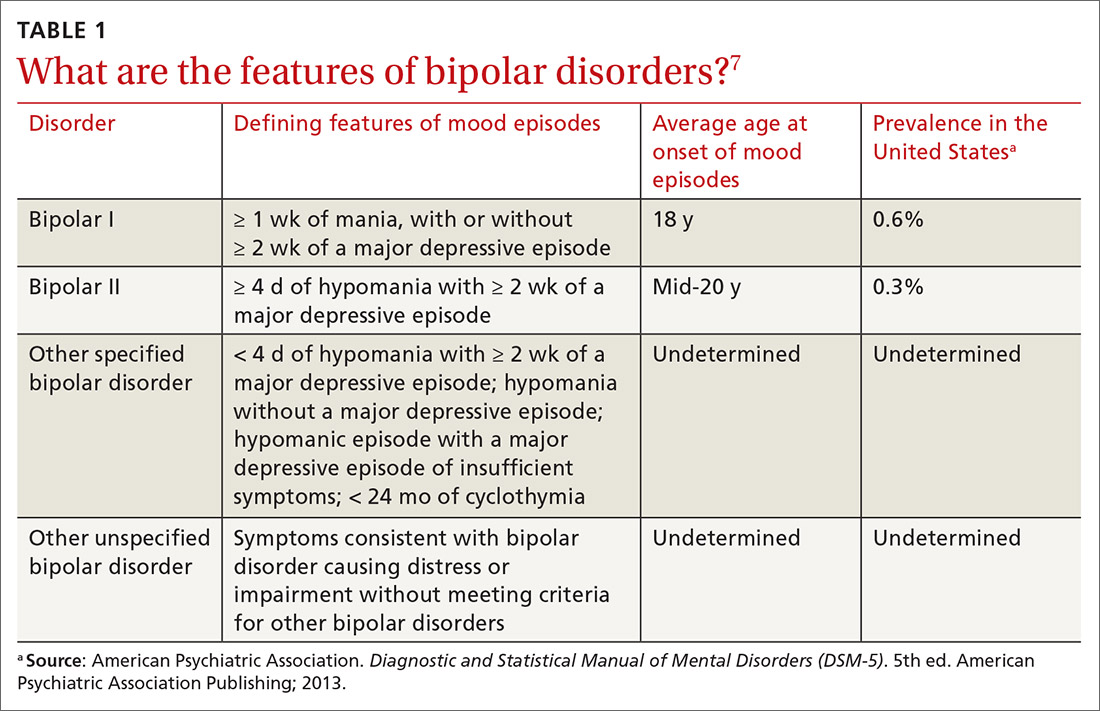
Understanding the (sometimes) unclear distinction between bipolar I and bipolar II disorders in an individual patient is key to formulating a therapeutic regimen for BD.
Bipolar I disorder consists of manic episodes, alternating (more often than not) with depressive episodes. Bipolar I usually manifests first with a depressive episode.
Bipolar II disorder manifests with depressive episodes and hypomanic episodes (but never manic episodes).
Continue to: Depressive episodes in the bipolar disorders
Depressive episodes in the bipolar disorders. Bipolar depression can be seen in the settings of both bipolar I and II disorders. When a patient presents with a manic episode, a history of depressive episodes is common (although not essential) to diagnose bipolar I; alternatively, a history of hypomania (but no prior mania) and depression is needed to make the diagnosis of bipolar II. The natural history of the bipolar disorders is therefore alternating manic and almost always depressive episodes (bipolar I) and alternating hypomanic and always depressive episodes (bipolar II).8
Symptoms of hypomanic episodes are similar to what are seen in manic episodes, but are of shorter duration (≥ 4 days [episodes of mania are at least of 1 week’s duration]), lower intensity (no psychotic symptoms), and not associated with significant functional impairment or hospitalization. Table 17 further describes the differentiating features of bipolar I and bipolar II. A history of an unequivocal manic or hypomanic episode makes the diagnosis of BD relatively easy. However, an unclear history of manic or hypomanic symptoms or episodes frequently leads to misdiagnosis or underdiagnosis of BD.
In both bipolar I and II, it is depressive symptoms and episodes that place the greatest burden on patients across the lifespan: They are the most commonly experienced features of the bipolar disorders9,10 and lead to significant distress and functional impairment11; in fact, patients with bipolar disorder spend 3 (or more) times as long in depressive episodes as in manic or hypomanic episodes.12,13 In addition, subthreshold depressive symptoms occur commonly between major mood episodes.
Failure to identify and adequately treat depressive episodes of the bipolar disorders can have serious consequences: Patients are at risk of a worsening course of illness, alcohol use disorder, substance use disorder, chronic disability, mixed states, rapid cycling of mood episodes, and suicide.
Guidelines for treating bipolar depression
Despite the similarity in presenting symptoms and signs of depressive episodes in bipolar disorders and MDD, treating episodes of BD is significantly different than treating MDD. Antidepressant monotherapy, a mainstay of treatment for MDD, has limited utility in BD (especially depressive episodes of bipolar I) because of its limited efficacy and potential to destabilize mood, lead to rapid cycling, and induce mania or hypomania. Treatment options for BD include pharmacotherapy (the primary modality), psychological intervention (a useful adjunct, described later), and electroconvulsive therapy (ECT; highly worth considering in severe or treatment-resistant cases).
Continue to: For this article...
For this article, we searched PubMed and Google Scholar for guidelines for the management of bipolar disorders in adults that were published between July 2013 (when the US Food and Drug Administration [FDA] approved lurasidone for the treatment of BD) and March 2019. Related guideline-referenced articles and clinical trials were also reviewed.
Our search identified 6 guidelines issued during the search period, developed by the:
- Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD),14
- British Association for Psychopharmacology (BAP),15
- Japanese Society of Mood Disorders (JSMD),16
- National Institute for Health and Care Excellence (NICE),17
- International College of Neuropsychopharmacology (CINP),18 and
- Royal Australian and New Zealand College of Psychiatrists.19
How to manage an episode of bipolar depression
First-line pharmacotherapeutic agents for the management of BD in acute bipolar I are listed and described in Table 2.4-19 Compared to the number of studies and reports on the management of BD in bipolar I, few studies have been conducted that specifically examine the treatment of BD in acute bipolar II. In practice, evidence from the treatment of BD in bipolar I has been extrapolated to the treatment of bipolar II depression. CANMAT–ISBD guidelines recommend quetiapine as the only first-line therapy for BD in bipolar II; JSMD, CINP, and NICE guidelines do not make distinct recommendations for treating BD in bipolar II.
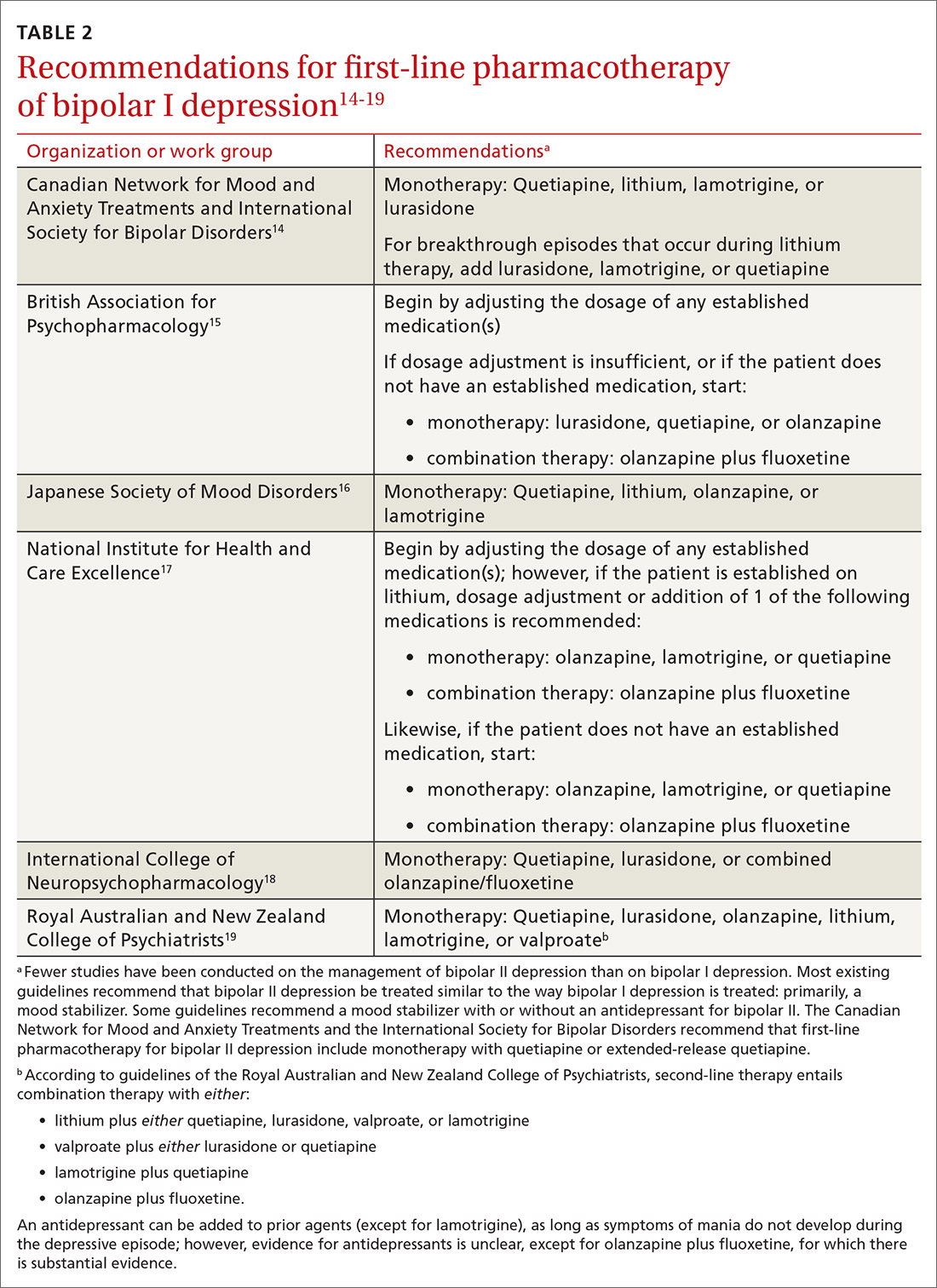
Patients who have BD can present de novo (ie, not taking any medication for bipolar disorder) or with a breakthrough episode while on maintenance medication(s). In either case, monotherapy for BD is preferred, although combinations of medications (Table 214-19) can be more effective in some cases. Treatment guidelines overlap to a high degree, especially in regard to first-line treatments, but there is variation, especially beyond first-line therapeutics.20
The top recommended medications for BD are lithium, quetiapine, olanzapine, lamotrigine, and combined olanzapine/fluoxetine. FDA-approved agents for treating acute BD specifically include quetiapine, lurasidone, and combined olanzapine/fluoxetine. Guidelines generally recommend a first step of adjusting the dosage of medications in any established regimen before changing or adding other agents. If clinical improvement is not seen using any recommended medications, psychiatric referral is recommended. See Table 321,22 for dosing and titration guidance and highlights of both common and rare but serious adverse effects.
Continue to: Recommendations, best options for acute bipolar depression
Recommendations, best options for acute bipolar depression
Start with lithium, lamotrigine, quetiapine, or lurasidone as the first-line medication at the dosages given in Table 3.21,22 Olanzapine alone, or in combination with fluoxetine, can be used when it has been determined that the medications listed above are ineffective.
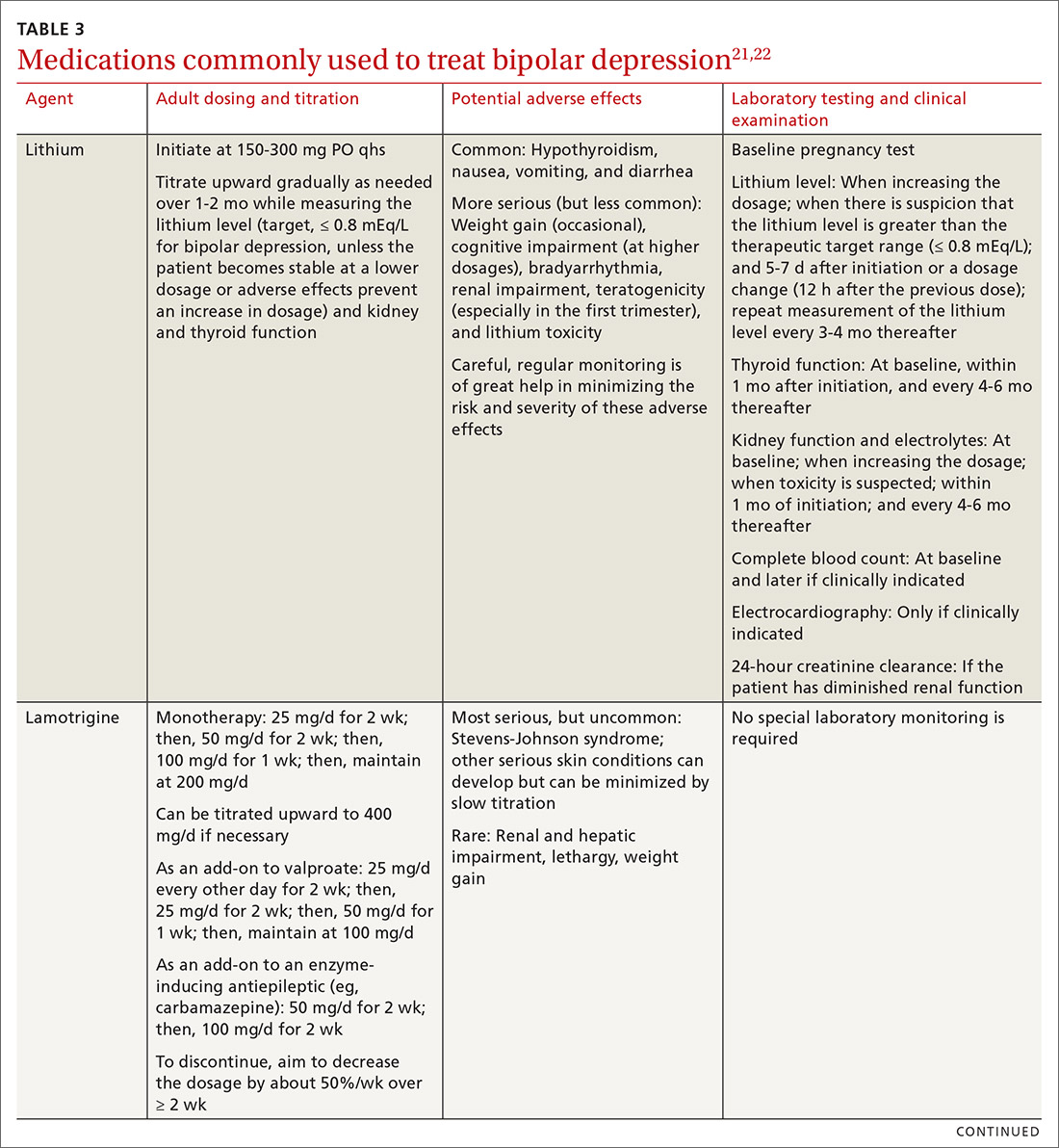
Note that lithium requires regular blood monitoring (Table 321,22). However, lithium has the advantage of strong supporting evidence of benefit in all mood episodes of bipolar disorders (depressive, manic, hypomanic), as well as maintenance, prevention of recurrence, and anti-suicidal properties.
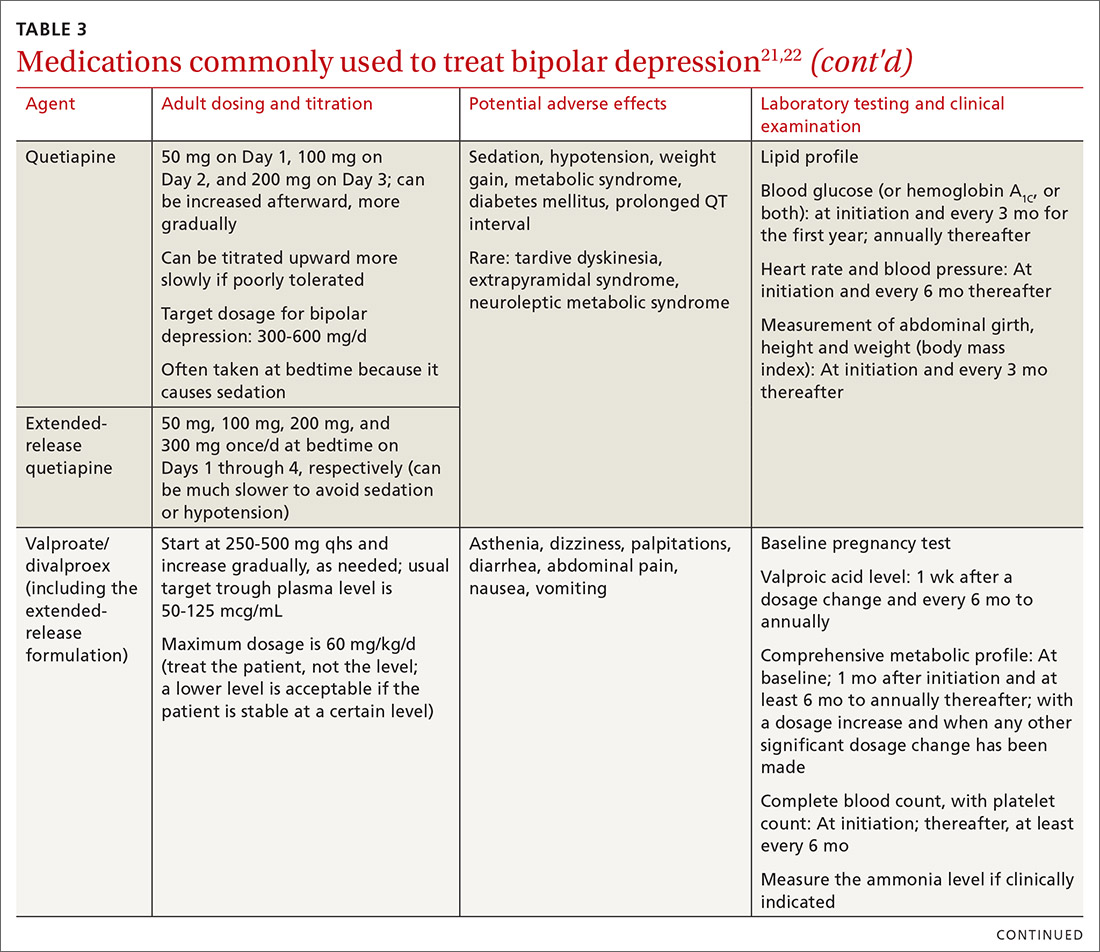
Also of note: Lurasidone is much more costly than other recommended medications because it is available only by brand name in the United States; the other agents are available as generics. Consider generic equivalents of the recommended agents when cost is an important factor, in part because of the impact that cost has on medication adherence for some patients.
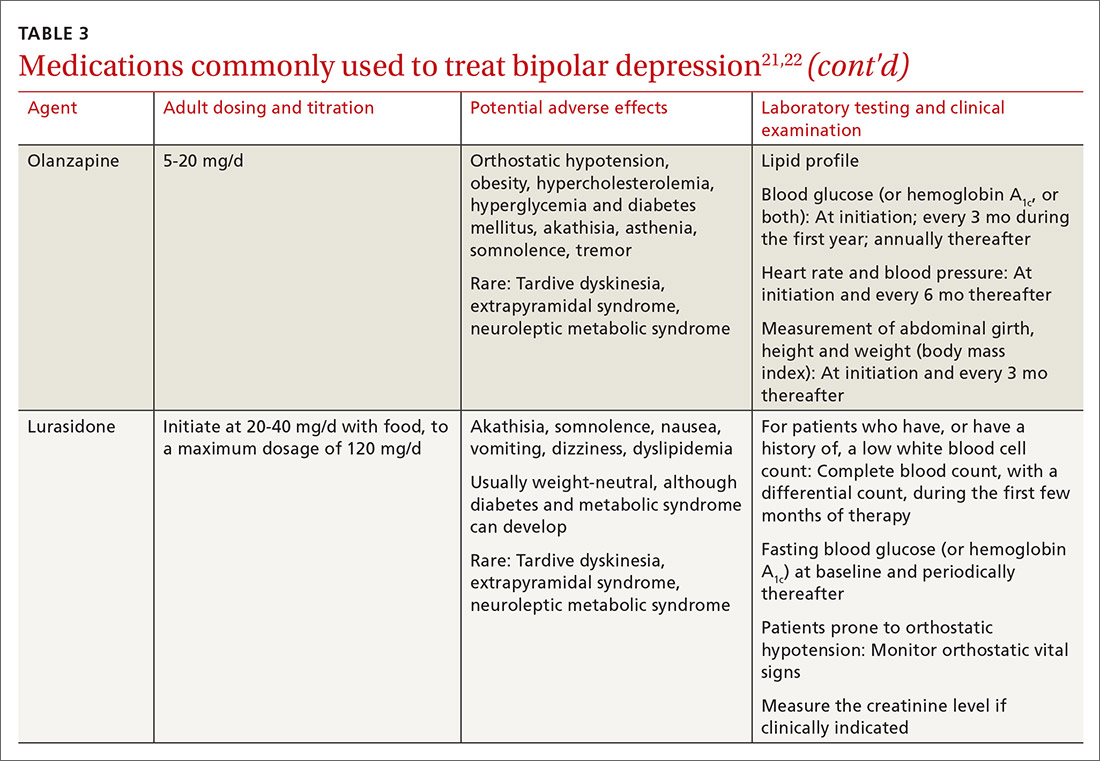
Last, olanzapine should be used later in the treatment algorithm, unless rapid control of symptoms is needed or other first-line medications are ineffective or not tolerated—given the higher propensity of the drug to produce weight gain and cause metabolic problems, including obesity and hyperglycemia.
The importance of maintenance therapy
Almost all patients with BD require maintenance treatment to prevent subsequent episodes, reduce residual symptoms, and restore functioning and quality of life. Maintenance therapy is formulated on the basis of efficacy and tolerability in the individual patient.
Continue to: As a general rule...
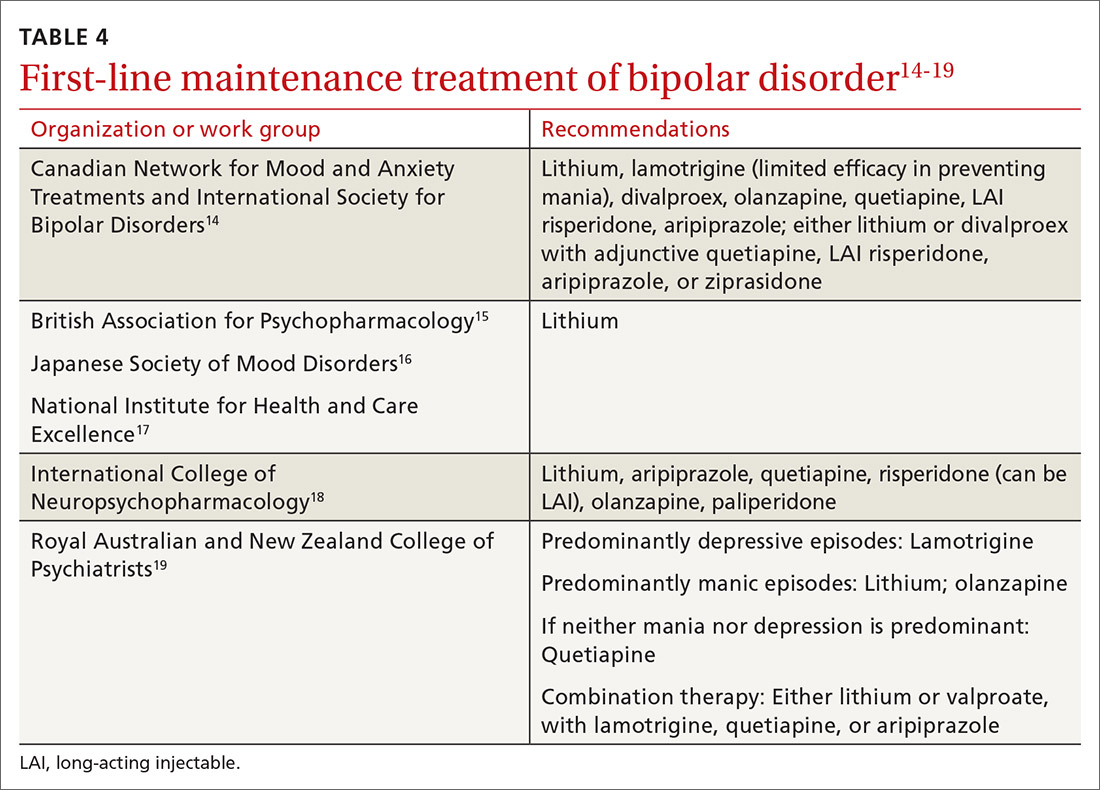
As a general rule, the strongest evidence for preventing recurrent BD episodes favors lithium—and most guidelines therefore support lithium as first-line maintenance therapy. It is important to note, however, that if a medication (or medications) successfully aborted an acute BD episode in a given patient, that agent (or agents) should be continued for maintenance purposes to prevent or minimize future episodes—generally, at the same dosage. First-line pharmacotherapeutic agents for the maintenance of bipolar disorder, and thus to prevent subsequent episodes of BD, are listed in Table 4.14-19
℞ antidepressantsin bipolar depression?
The use of antidepressants to treat BD remains a topic of ongoing deliberation. Antidepressant treatment of BD has historically raised concern for depressive relapse due to ineffectiveness and the ability of antidepressants to increase (1) the frequency of manic and hypomanic episodes23 and (2) mood instability in the form of induction of mixed states or rapid cycling. Among most authorities, the recommendation against using antidepressants for BD in both bipolar I and II is the same; however, limited evidence allows the use of antidepressant monotherapy in select cases of BD episodes in bipolar II,24,25 although not bipolar I.
The consensus in the field is that medications with mood-stabilizing effects should be considered as monotherapy before adding an antidepressant (if an antidepressant is to be added) to treat BD in bipolar II.26 In other words, if an antidepressant is to be used at all, it should be combined with a mood stabilizer or atypical antipsychotic15,27 and should probably not be used long term. The efficacy of antidepressants in treating BD in bipolar II should be assessed periodically at follow-up.
Nonpharmaceutical treatment options
Although pharmacotherapy is the mainstay of treatment of BD, adjunctive psychotherapy can be useful for treating acute BD episodes that occur during the maintenance phase of the disorder. Psychoeducation (ie, education on psychiatric illness and the importance of medication adherence), alone or in combination with interpersonal and social rhythm therapy (IPSRT), family-focused therapy (FFT), and cognitive behavioral therapy (CBT) can add to the overall efficacy of pharmacotherapy by lowering the risk of relapse and enhancing psychosocial functioning.28
IPSRT is supported by what is known as the instability model, which specifies that 3 interconnected pathways trigger recurrences of a bipolar episode: stressful life events, medication nonadherence, and social-rhythm disruption. IPSRT also uses principles of interpersonal psychotherapy that are applied in treating MDD, “arguing that improvement in interpersonal relationships can ameliorate affective symptoms and prevent their return.”29,30
Continue to: FFT
FFT focuses on communication styles between patients and their spouses and families. The goal is to improve relationship functioning. FFT is delivered to the patient and the family.
Attention to social factors. For psychotherapy to provide adequate results as an adjunct to pharmacotherapy, social stressors (eg, homelessness and financial concerns) might also need to be considered and addressed through social services or a social work consult.
NICE guidelines recommend psychological intervention (in particular, with CBT and FFT) for acute BD. CANMAT–ISBD guidelines recommend either adjunctive psychoeducation, CBT, or FFT during the maintenance phase. Again, medication is the mainstay of treatment for BD in bipolar disorders; psychotherapy has an adjunctive role—unlike the approach to treatment of MDD, in which psychotherapy can be used alone in cases of mild, or even moderate, severity.
Referral for specialty care
In the primary care setting, providers might choose to manage BD by initiating first-line pharmacotherapeutic agents or continuing established treatment regimens with necessary dosage adjustments. These patients should be monitored closely until symptoms remit.
However, it is important for the primary care provider to identify patients who need psychiatric referral. Complex presentations, severe symptoms, and poor treatment response might warrant evaluation and management by a psychiatrist. Furthermore, patients with comorbid psychotic features, catatonia, or severely debilitating depression (with or without suicidality) need referral to the emergency department.
Continue to: Electroconvulsive therapy (ECT)
Electroconvulsive therapy (ECT). Patients might also need referral to Psychiatry for ECT, which is recommended by CANMAT–ISBD and JSMD guidelines as a second-line option; by the Royal Australian and New Zealand College of Psychiatrists as a third-line option; and by BAP for cases that are resistant to conventional treatment, with or without a high risk of suicide; in pregnancy; and in life-threatening situations.15,31,32
Telemedicine. There is a considerable shortage of mental health care professionals.33,34 The fact that nearly all (96%) counties in the United States have an unmet need for prescribers of mental health services (mainly psychiatrists) makes it crucial that primary care physicians be knowledgeable and prepared to manage BD—often with infrequent psychiatry consultation or, even, without psychiatry consultation. For primary care facilities that lack access to psychiatric services, telemedicine can be used as a consultative resource.
Psychiatric consultation using telemedicine technologies has provided significant cost savings for medical centers and decreased the likelihood of hospital admission,35 thereby alleviating health care costs and improving care, as shown in a rural Kansas county study.36 Furthermore, the burden on emergency departments in several states has been significantly reduced with psychiatric consultations via interactive telemedicine technologies.37
ACKNOWLEDGEMENT
Mark Yassa, BS, provided editing assistance.
CORRESPONDENCE
Nagy Youssef, MD, PhD, Medical College of Georgia at Augusta University, Department of Psychiatry and Health Behavior, 997 St. Sebastian Way, Augusta, GA 30912; [email protected].
1. Cerimele JM, Chwastiak LA, Dodson S, et al. The prevalence of bipolar disorder in general primary care samples: a systematic review. Gen Hosp Psychiatry. 2014;36:19-25.
2. Stubbs B, Vancampfort D, Solmi M, et al. How common is bipolar disorder in general primary care attendees? A systematic review and meta-analysis investigating prevalence determined according to structured clinical assessments. Aust N Z J Psychiatry. 2016;50:631-639.
3. Carta MG, Norcini-Pala A, Moro MF, et al. Does mood disorder questionnaire identify sub-threshold bipolarity? Evidence studying worsening of quality of life. J Affect Disord. 2015;183:173-178.
4. Fonseca-Pedrero E, Ortuno-Sierra J, Paino M, et al. Screening the risk of bipolar spectrum disorders: validity evidence of the Mood Disorder Questionnaire in adolescents and young adults. Rev Psiquiatr Salud Ment. 2016;9:4-12.
5. Reilly S, Planner C, Gask L, et al. Collaborative care approaches for people with severe mental illness. Cochrane Database Syst Rev. 2013;(11):CD009531.
6. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169:790-804.
7. Aquadro E, Youssef NA. Combine these screening tools to detect bipolar depression. J Fam Pract. 2018;67:500-503.
8. Bipolar and related disorders. In: American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association Publishing; 2013:123.
9. Judd LL, Akiskal HS, Schettler PJ, et al. The comparative clinical phenotype and long term longitudinal episode course of bipolar I and II: a clinical spectrum or distinct disorders? J Affect Disord. 2013;73:19-32.
10. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530-537.
11. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68:1237-1245.
12. Judd LL, Schettler PJ, Akiskal HS, et al. Residual symptom recovery from major affective episodes in bipolar disorders and rapid episode relapse/recurrence. Arch Gen Psychiatry. 2008;65:386-394.
13. Judd LL, Schettler PJ, Solomon DA, et al. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. J Affect Disord. 2008;108:49-58.
14. Yatham LN, Kennedy SH, Parikh S, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20:97-170.
15. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30:495-553.
16. Kanba S, Kato T, Terao T, et al; Committee for Treatment Guidelines of Mood Disorders, Japanese Society of Mood Disorders, 2012. Guideline for treatment of bipolar disorder by the Japanese Society of Mood Disorders, 2012. Psychiatry Clin Neurosci. 2013;67:285-300.
17. National Institute for Health and Care Excellence. Bipolar disorder: assessment and management. Clinical Guideline CG185. September 24, 2014. www.nice.org.uk/guidance/cg185. Accessed August 17, 2020.
18. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) Treatment Guidelines for Bipolar Disorder in Adults (CINP-BD-2017), Part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20:180-195.
19. Malhi GS, Outhred T, Morris G, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Med J Aust. 2018;208:219-225.
20. Hammett S, Youssef NA. Systematic review of recent guidelines for pharmacological treatments of bipolar disorders in adults. Ann Clin Psychiatry. 2017;29:266-282.
21. Gabbard GO, ed. Gabbard’s Treatments of Psychiatric Disorders. 5th ed. American Psychiatric Association Publishing; 2014: chap 12-15.
22. National Health Service. Guidelines for the monitoring of antimanic and prophylactic medication in bipolar disorder. NHT policy number MM-G-023. 2009. http://fac.ksu.edu.sa/sites/default/files/bipolar_disorder_guidelines.pdf. Accessed August 17, 2020.
23. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653-661.
24. Amsterdam JD, Lorenzo-Luaces L, Soeller I, et al. Safety and effectiveness of continuation antidepressant versus mood stabilizer monotherapy for relapse-prevention of bipolar II depression: a randomized, double-blind, parallel-group, prospective study. J Affect Disord. 2015;185:31-37.
25. Parker G, Tully L, Olley A, et al. SSRIs as mood stabilizers for bipolar II disorder? A proof of concept study. J Affect Disord. 2006;92:205-214.
26. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170:1249-1262.
27. Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for Bipolar Disorder. Australian and New Zealand clinical practice guidelines for the treatment of bipolar disorder. Aust N Z J Psychiatry. 2004;38:280-305.
28. Chiang K-J, Tsai J-C, Liu D, et al. Efficacy of cognitive-behavioral therapy in patients with bipolar disorder: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0176849.
29. de Mello MF, de Jesus Mari J, Bacaltchuk J, et al. A systematic review of research findings on the efficacy of interpersonal therapy for depressive disorders. Eur Arch Psychiatry Clin Neurosci. 2005;255:75-82.
30. Zhou X, Teng T, Zhang Y, et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:581-601.
31. Daly JJ, Prudic J, Devanand DP, et al. ECT in bipolar and unipolar depression: differences in speed of response. Bipolar Disord. 2001;3:95-104.
32. Medda P, Perugi G, Zanello S, et al. Response to ECT in bipolar I, bipolar II and unipolar depression. J Affect Disord. 2009;118:55-59.
33. Ellis AR, Konrad TR, Thomas KC, et al. County-level estimates of mental health professional supply in the United States. Psychiatr Serv. 2009;60:1315-1322.
34. Thomas KC, Ellis AR, Konrad TR, et al. County-level estimates of mental health professional shortage in the United States. Psychiatr Serv. 2009;60:1323-1328.
35. Narasimhan M, Druss BG, Hockenberry JM, et al. Impact of a telepsychiatry program at emergency departments statewide on the quality, utilization, and costs of mental health services. Psychiatr Serv. 2015;66:1167-1172.
36. Spaulding R, Belz N, DeLurgio S, et al. Cost savings of telemedicine utilization for child psychiatry in a rural Kansas community. Telemed J E Health. 2010;16:867-871.
37. States leverage telepsychiatry solutions to ease ED crowding, accelerate care. ED Manag. 2015;27:13-17.
Bipolar disorder is a prevalent disorder in the primary care setting.1,2 Primary care providers therefore commonly encounter bipolar depression (BD; a major depressive episode in the context of bipolar disorder), which might be (1) an emerging depressive episode in previously undiagnosed bipolar disorder or (2) a recurrent episode during the course of chronic bipolar illness.3,4
A primary care–based collaborative model has been identified as a potential strategy for effective management of chronic mental health conditions such as bipolar disorder.5,6 However, this collaborative treatment model isn’t widely available; many patients with bipolar disorder are, in fact, treated solely by their primary care provider.
Two years ago in this journal,7 we addressed how to precisely identify an episode of BD and differentiate it from major depressive disorder (MDD; also known as unipolar depression). In this review, in addition to advancing clinical knowledge of BD, we provide:
- an overview of treatment options for BD (in contrast to the treatment of unipolar depression)
- the pharmacotherapeutic know-how to initiate and maintain treatment for uncomplicated episodes of BD.
We do not discuss management of manic, hypomanic, and mixed episodes of bipolar disorder.
How to identify bipolar depression
Understanding the (sometimes) unclear distinction between bipolar I and bipolar II disorders in an individual patient is key to formulating a therapeutic regimen for BD.
Bipolar I disorder consists of manic episodes, alternating (more often than not) with depressive episodes. Bipolar I usually manifests first with a depressive episode.
Bipolar II disorder manifests with depressive episodes and hypomanic episodes (but never manic episodes).
Continue to: Depressive episodes in the bipolar disorders
Depressive episodes in the bipolar disorders. Bipolar depression can be seen in the settings of both bipolar I and II disorders. When a patient presents with a manic episode, a history of depressive episodes is common (although not essential) to diagnose bipolar I; alternatively, a history of hypomania (but no prior mania) and depression is needed to make the diagnosis of bipolar II. The natural history of the bipolar disorders is therefore alternating manic and almost always depressive episodes (bipolar I) and alternating hypomanic and always depressive episodes (bipolar II).8
Symptoms of hypomanic episodes are similar to what are seen in manic episodes, but are of shorter duration (≥ 4 days [episodes of mania are at least of 1 week’s duration]), lower intensity (no psychotic symptoms), and not associated with significant functional impairment or hospitalization. Table 17 further describes the differentiating features of bipolar I and bipolar II. A history of an unequivocal manic or hypomanic episode makes the diagnosis of BD relatively easy. However, an unclear history of manic or hypomanic symptoms or episodes frequently leads to misdiagnosis or underdiagnosis of BD.

In both bipolar I and II, it is depressive symptoms and episodes that place the greatest burden on patients across the lifespan: They are the most commonly experienced features of the bipolar disorders9,10 and lead to significant distress and functional impairment11; in fact, patients with bipolar disorder spend 3 (or more) times as long in depressive episodes as in manic or hypomanic episodes.12,13 In addition, subthreshold depressive symptoms occur commonly between major mood episodes.
Failure to identify and adequately treat depressive episodes of the bipolar disorders can have serious consequences: Patients are at risk of a worsening course of illness, alcohol use disorder, substance use disorder, chronic disability, mixed states, rapid cycling of mood episodes, and suicide.
Guidelines for treating bipolar depression
Despite the similarity in presenting symptoms and signs of depressive episodes in bipolar disorders and MDD, treating episodes of BD is significantly different than treating MDD. Antidepressant monotherapy, a mainstay of treatment for MDD, has limited utility in BD (especially depressive episodes of bipolar I) because of its limited efficacy and potential to destabilize mood, lead to rapid cycling, and induce mania or hypomania. Treatment options for BD include pharmacotherapy (the primary modality), psychological intervention (a useful adjunct, described later), and electroconvulsive therapy (ECT; highly worth considering in severe or treatment-resistant cases).
Continue to: For this article...
For this article, we searched PubMed and Google Scholar for guidelines for the management of bipolar disorders in adults that were published between July 2013 (when the US Food and Drug Administration [FDA] approved lurasidone for the treatment of BD) and March 2019. Related guideline-referenced articles and clinical trials were also reviewed.
Our search identified 6 guidelines issued during the search period, developed by the:
- Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD),14
- British Association for Psychopharmacology (BAP),15
- Japanese Society of Mood Disorders (JSMD),16
- National Institute for Health and Care Excellence (NICE),17
- International College of Neuropsychopharmacology (CINP),18 and
- Royal Australian and New Zealand College of Psychiatrists.19
How to manage an episode of bipolar depression
First-line pharmacotherapeutic agents for the management of BD in acute bipolar I are listed and described in Table 2.4-19 Compared to the number of studies and reports on the management of BD in bipolar I, few studies have been conducted that specifically examine the treatment of BD in acute bipolar II. In practice, evidence from the treatment of BD in bipolar I has been extrapolated to the treatment of bipolar II depression. CANMAT–ISBD guidelines recommend quetiapine as the only first-line therapy for BD in bipolar II; JSMD, CINP, and NICE guidelines do not make distinct recommendations for treating BD in bipolar II.

Patients who have BD can present de novo (ie, not taking any medication for bipolar disorder) or with a breakthrough episode while on maintenance medication(s). In either case, monotherapy for BD is preferred, although combinations of medications (Table 214-19) can be more effective in some cases. Treatment guidelines overlap to a high degree, especially in regard to first-line treatments, but there is variation, especially beyond first-line therapeutics.20
The top recommended medications for BD are lithium, quetiapine, olanzapine, lamotrigine, and combined olanzapine/fluoxetine. FDA-approved agents for treating acute BD specifically include quetiapine, lurasidone, and combined olanzapine/fluoxetine. Guidelines generally recommend a first step of adjusting the dosage of medications in any established regimen before changing or adding other agents. If clinical improvement is not seen using any recommended medications, psychiatric referral is recommended. See Table 321,22 for dosing and titration guidance and highlights of both common and rare but serious adverse effects.
Continue to: Recommendations, best options for acute bipolar depression
Recommendations, best options for acute bipolar depression
Start with lithium, lamotrigine, quetiapine, or lurasidone as the first-line medication at the dosages given in Table 3.21,22 Olanzapine alone, or in combination with fluoxetine, can be used when it has been determined that the medications listed above are ineffective.

Note that lithium requires regular blood monitoring (Table 321,22). However, lithium has the advantage of strong supporting evidence of benefit in all mood episodes of bipolar disorders (depressive, manic, hypomanic), as well as maintenance, prevention of recurrence, and anti-suicidal properties.

Also of note: Lurasidone is much more costly than other recommended medications because it is available only by brand name in the United States; the other agents are available as generics. Consider generic equivalents of the recommended agents when cost is an important factor, in part because of the impact that cost has on medication adherence for some patients.

Last, olanzapine should be used later in the treatment algorithm, unless rapid control of symptoms is needed or other first-line medications are ineffective or not tolerated—given the higher propensity of the drug to produce weight gain and cause metabolic problems, including obesity and hyperglycemia.
The importance of maintenance therapy
Almost all patients with BD require maintenance treatment to prevent subsequent episodes, reduce residual symptoms, and restore functioning and quality of life. Maintenance therapy is formulated on the basis of efficacy and tolerability in the individual patient.
Continue to: As a general rule...
As a general rule, the strongest evidence for preventing recurrent BD episodes favors lithium—and most guidelines therefore support lithium as first-line maintenance therapy. It is important to note, however, that if a medication (or medications) successfully aborted an acute BD episode in a given patient, that agent (or agents) should be continued for maintenance purposes to prevent or minimize future episodes—generally, at the same dosage. First-line pharmacotherapeutic agents for the maintenance of bipolar disorder, and thus to prevent subsequent episodes of BD, are listed in Table 4.14-19

℞ antidepressantsin bipolar depression?
The use of antidepressants to treat BD remains a topic of ongoing deliberation. Antidepressant treatment of BD has historically raised concern for depressive relapse due to ineffectiveness and the ability of antidepressants to increase (1) the frequency of manic and hypomanic episodes23 and (2) mood instability in the form of induction of mixed states or rapid cycling. Among most authorities, the recommendation against using antidepressants for BD in both bipolar I and II is the same; however, limited evidence allows the use of antidepressant monotherapy in select cases of BD episodes in bipolar II,24,25 although not bipolar I.
The consensus in the field is that medications with mood-stabilizing effects should be considered as monotherapy before adding an antidepressant (if an antidepressant is to be added) to treat BD in bipolar II.26 In other words, if an antidepressant is to be used at all, it should be combined with a mood stabilizer or atypical antipsychotic15,27 and should probably not be used long term. The efficacy of antidepressants in treating BD in bipolar II should be assessed periodically at follow-up.
Nonpharmaceutical treatment options
Although pharmacotherapy is the mainstay of treatment of BD, adjunctive psychotherapy can be useful for treating acute BD episodes that occur during the maintenance phase of the disorder. Psychoeducation (ie, education on psychiatric illness and the importance of medication adherence), alone or in combination with interpersonal and social rhythm therapy (IPSRT), family-focused therapy (FFT), and cognitive behavioral therapy (CBT) can add to the overall efficacy of pharmacotherapy by lowering the risk of relapse and enhancing psychosocial functioning.28
IPSRT is supported by what is known as the instability model, which specifies that 3 interconnected pathways trigger recurrences of a bipolar episode: stressful life events, medication nonadherence, and social-rhythm disruption. IPSRT also uses principles of interpersonal psychotherapy that are applied in treating MDD, “arguing that improvement in interpersonal relationships can ameliorate affective symptoms and prevent their return.”29,30
Continue to: FFT
FFT focuses on communication styles between patients and their spouses and families. The goal is to improve relationship functioning. FFT is delivered to the patient and the family.
Attention to social factors. For psychotherapy to provide adequate results as an adjunct to pharmacotherapy, social stressors (eg, homelessness and financial concerns) might also need to be considered and addressed through social services or a social work consult.
NICE guidelines recommend psychological intervention (in particular, with CBT and FFT) for acute BD. CANMAT–ISBD guidelines recommend either adjunctive psychoeducation, CBT, or FFT during the maintenance phase. Again, medication is the mainstay of treatment for BD in bipolar disorders; psychotherapy has an adjunctive role—unlike the approach to treatment of MDD, in which psychotherapy can be used alone in cases of mild, or even moderate, severity.
Referral for specialty care
In the primary care setting, providers might choose to manage BD by initiating first-line pharmacotherapeutic agents or continuing established treatment regimens with necessary dosage adjustments. These patients should be monitored closely until symptoms remit.
However, it is important for the primary care provider to identify patients who need psychiatric referral. Complex presentations, severe symptoms, and poor treatment response might warrant evaluation and management by a psychiatrist. Furthermore, patients with comorbid psychotic features, catatonia, or severely debilitating depression (with or without suicidality) need referral to the emergency department.
Continue to: Electroconvulsive therapy (ECT)
Electroconvulsive therapy (ECT). Patients might also need referral to Psychiatry for ECT, which is recommended by CANMAT–ISBD and JSMD guidelines as a second-line option; by the Royal Australian and New Zealand College of Psychiatrists as a third-line option; and by BAP for cases that are resistant to conventional treatment, with or without a high risk of suicide; in pregnancy; and in life-threatening situations.15,31,32
Telemedicine. There is a considerable shortage of mental health care professionals.33,34 The fact that nearly all (96%) counties in the United States have an unmet need for prescribers of mental health services (mainly psychiatrists) makes it crucial that primary care physicians be knowledgeable and prepared to manage BD—often with infrequent psychiatry consultation or, even, without psychiatry consultation. For primary care facilities that lack access to psychiatric services, telemedicine can be used as a consultative resource.
Psychiatric consultation using telemedicine technologies has provided significant cost savings for medical centers and decreased the likelihood of hospital admission,35 thereby alleviating health care costs and improving care, as shown in a rural Kansas county study.36 Furthermore, the burden on emergency departments in several states has been significantly reduced with psychiatric consultations via interactive telemedicine technologies.37
ACKNOWLEDGEMENT
Mark Yassa, BS, provided editing assistance.
CORRESPONDENCE
Nagy Youssef, MD, PhD, Medical College of Georgia at Augusta University, Department of Psychiatry and Health Behavior, 997 St. Sebastian Way, Augusta, GA 30912; [email protected].
Bipolar disorder is a prevalent disorder in the primary care setting.1,2 Primary care providers therefore commonly encounter bipolar depression (BD; a major depressive episode in the context of bipolar disorder), which might be (1) an emerging depressive episode in previously undiagnosed bipolar disorder or (2) a recurrent episode during the course of chronic bipolar illness.3,4
A primary care–based collaborative model has been identified as a potential strategy for effective management of chronic mental health conditions such as bipolar disorder.5,6 However, this collaborative treatment model isn’t widely available; many patients with bipolar disorder are, in fact, treated solely by their primary care provider.
Two years ago in this journal,7 we addressed how to precisely identify an episode of BD and differentiate it from major depressive disorder (MDD; also known as unipolar depression). In this review, in addition to advancing clinical knowledge of BD, we provide:
- an overview of treatment options for BD (in contrast to the treatment of unipolar depression)
- the pharmacotherapeutic know-how to initiate and maintain treatment for uncomplicated episodes of BD.
We do not discuss management of manic, hypomanic, and mixed episodes of bipolar disorder.
How to identify bipolar depression
Understanding the (sometimes) unclear distinction between bipolar I and bipolar II disorders in an individual patient is key to formulating a therapeutic regimen for BD.
Bipolar I disorder consists of manic episodes, alternating (more often than not) with depressive episodes. Bipolar I usually manifests first with a depressive episode.
Bipolar II disorder manifests with depressive episodes and hypomanic episodes (but never manic episodes).
Continue to: Depressive episodes in the bipolar disorders
Depressive episodes in the bipolar disorders. Bipolar depression can be seen in the settings of both bipolar I and II disorders. When a patient presents with a manic episode, a history of depressive episodes is common (although not essential) to diagnose bipolar I; alternatively, a history of hypomania (but no prior mania) and depression is needed to make the diagnosis of bipolar II. The natural history of the bipolar disorders is therefore alternating manic and almost always depressive episodes (bipolar I) and alternating hypomanic and always depressive episodes (bipolar II).8
Symptoms of hypomanic episodes are similar to what are seen in manic episodes, but are of shorter duration (≥ 4 days [episodes of mania are at least of 1 week’s duration]), lower intensity (no psychotic symptoms), and not associated with significant functional impairment or hospitalization. Table 17 further describes the differentiating features of bipolar I and bipolar II. A history of an unequivocal manic or hypomanic episode makes the diagnosis of BD relatively easy. However, an unclear history of manic or hypomanic symptoms or episodes frequently leads to misdiagnosis or underdiagnosis of BD.

In both bipolar I and II, it is depressive symptoms and episodes that place the greatest burden on patients across the lifespan: They are the most commonly experienced features of the bipolar disorders9,10 and lead to significant distress and functional impairment11; in fact, patients with bipolar disorder spend 3 (or more) times as long in depressive episodes as in manic or hypomanic episodes.12,13 In addition, subthreshold depressive symptoms occur commonly between major mood episodes.
Failure to identify and adequately treat depressive episodes of the bipolar disorders can have serious consequences: Patients are at risk of a worsening course of illness, alcohol use disorder, substance use disorder, chronic disability, mixed states, rapid cycling of mood episodes, and suicide.
Guidelines for treating bipolar depression
Despite the similarity in presenting symptoms and signs of depressive episodes in bipolar disorders and MDD, treating episodes of BD is significantly different than treating MDD. Antidepressant monotherapy, a mainstay of treatment for MDD, has limited utility in BD (especially depressive episodes of bipolar I) because of its limited efficacy and potential to destabilize mood, lead to rapid cycling, and induce mania or hypomania. Treatment options for BD include pharmacotherapy (the primary modality), psychological intervention (a useful adjunct, described later), and electroconvulsive therapy (ECT; highly worth considering in severe or treatment-resistant cases).
Continue to: For this article...
For this article, we searched PubMed and Google Scholar for guidelines for the management of bipolar disorders in adults that were published between July 2013 (when the US Food and Drug Administration [FDA] approved lurasidone for the treatment of BD) and March 2019. Related guideline-referenced articles and clinical trials were also reviewed.
Our search identified 6 guidelines issued during the search period, developed by the:
- Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD),14
- British Association for Psychopharmacology (BAP),15
- Japanese Society of Mood Disorders (JSMD),16
- National Institute for Health and Care Excellence (NICE),17
- International College of Neuropsychopharmacology (CINP),18 and
- Royal Australian and New Zealand College of Psychiatrists.19
How to manage an episode of bipolar depression
First-line pharmacotherapeutic agents for the management of BD in acute bipolar I are listed and described in Table 2.4-19 Compared to the number of studies and reports on the management of BD in bipolar I, few studies have been conducted that specifically examine the treatment of BD in acute bipolar II. In practice, evidence from the treatment of BD in bipolar I has been extrapolated to the treatment of bipolar II depression. CANMAT–ISBD guidelines recommend quetiapine as the only first-line therapy for BD in bipolar II; JSMD, CINP, and NICE guidelines do not make distinct recommendations for treating BD in bipolar II.

Patients who have BD can present de novo (ie, not taking any medication for bipolar disorder) or with a breakthrough episode while on maintenance medication(s). In either case, monotherapy for BD is preferred, although combinations of medications (Table 214-19) can be more effective in some cases. Treatment guidelines overlap to a high degree, especially in regard to first-line treatments, but there is variation, especially beyond first-line therapeutics.20
The top recommended medications for BD are lithium, quetiapine, olanzapine, lamotrigine, and combined olanzapine/fluoxetine. FDA-approved agents for treating acute BD specifically include quetiapine, lurasidone, and combined olanzapine/fluoxetine. Guidelines generally recommend a first step of adjusting the dosage of medications in any established regimen before changing or adding other agents. If clinical improvement is not seen using any recommended medications, psychiatric referral is recommended. See Table 321,22 for dosing and titration guidance and highlights of both common and rare but serious adverse effects.
Continue to: Recommendations, best options for acute bipolar depression
Recommendations, best options for acute bipolar depression
Start with lithium, lamotrigine, quetiapine, or lurasidone as the first-line medication at the dosages given in Table 3.21,22 Olanzapine alone, or in combination with fluoxetine, can be used when it has been determined that the medications listed above are ineffective.

Note that lithium requires regular blood monitoring (Table 321,22). However, lithium has the advantage of strong supporting evidence of benefit in all mood episodes of bipolar disorders (depressive, manic, hypomanic), as well as maintenance, prevention of recurrence, and anti-suicidal properties.

Also of note: Lurasidone is much more costly than other recommended medications because it is available only by brand name in the United States; the other agents are available as generics. Consider generic equivalents of the recommended agents when cost is an important factor, in part because of the impact that cost has on medication adherence for some patients.

Last, olanzapine should be used later in the treatment algorithm, unless rapid control of symptoms is needed or other first-line medications are ineffective or not tolerated—given the higher propensity of the drug to produce weight gain and cause metabolic problems, including obesity and hyperglycemia.
The importance of maintenance therapy
Almost all patients with BD require maintenance treatment to prevent subsequent episodes, reduce residual symptoms, and restore functioning and quality of life. Maintenance therapy is formulated on the basis of efficacy and tolerability in the individual patient.
Continue to: As a general rule...
As a general rule, the strongest evidence for preventing recurrent BD episodes favors lithium—and most guidelines therefore support lithium as first-line maintenance therapy. It is important to note, however, that if a medication (or medications) successfully aborted an acute BD episode in a given patient, that agent (or agents) should be continued for maintenance purposes to prevent or minimize future episodes—generally, at the same dosage. First-line pharmacotherapeutic agents for the maintenance of bipolar disorder, and thus to prevent subsequent episodes of BD, are listed in Table 4.14-19

℞ antidepressantsin bipolar depression?
The use of antidepressants to treat BD remains a topic of ongoing deliberation. Antidepressant treatment of BD has historically raised concern for depressive relapse due to ineffectiveness and the ability of antidepressants to increase (1) the frequency of manic and hypomanic episodes23 and (2) mood instability in the form of induction of mixed states or rapid cycling. Among most authorities, the recommendation against using antidepressants for BD in both bipolar I and II is the same; however, limited evidence allows the use of antidepressant monotherapy in select cases of BD episodes in bipolar II,24,25 although not bipolar I.
The consensus in the field is that medications with mood-stabilizing effects should be considered as monotherapy before adding an antidepressant (if an antidepressant is to be added) to treat BD in bipolar II.26 In other words, if an antidepressant is to be used at all, it should be combined with a mood stabilizer or atypical antipsychotic15,27 and should probably not be used long term. The efficacy of antidepressants in treating BD in bipolar II should be assessed periodically at follow-up.
Nonpharmaceutical treatment options
Although pharmacotherapy is the mainstay of treatment of BD, adjunctive psychotherapy can be useful for treating acute BD episodes that occur during the maintenance phase of the disorder. Psychoeducation (ie, education on psychiatric illness and the importance of medication adherence), alone or in combination with interpersonal and social rhythm therapy (IPSRT), family-focused therapy (FFT), and cognitive behavioral therapy (CBT) can add to the overall efficacy of pharmacotherapy by lowering the risk of relapse and enhancing psychosocial functioning.28
IPSRT is supported by what is known as the instability model, which specifies that 3 interconnected pathways trigger recurrences of a bipolar episode: stressful life events, medication nonadherence, and social-rhythm disruption. IPSRT also uses principles of interpersonal psychotherapy that are applied in treating MDD, “arguing that improvement in interpersonal relationships can ameliorate affective symptoms and prevent their return.”29,30
Continue to: FFT
FFT focuses on communication styles between patients and their spouses and families. The goal is to improve relationship functioning. FFT is delivered to the patient and the family.
Attention to social factors. For psychotherapy to provide adequate results as an adjunct to pharmacotherapy, social stressors (eg, homelessness and financial concerns) might also need to be considered and addressed through social services or a social work consult.
NICE guidelines recommend psychological intervention (in particular, with CBT and FFT) for acute BD. CANMAT–ISBD guidelines recommend either adjunctive psychoeducation, CBT, or FFT during the maintenance phase. Again, medication is the mainstay of treatment for BD in bipolar disorders; psychotherapy has an adjunctive role—unlike the approach to treatment of MDD, in which psychotherapy can be used alone in cases of mild, or even moderate, severity.
Referral for specialty care
In the primary care setting, providers might choose to manage BD by initiating first-line pharmacotherapeutic agents or continuing established treatment regimens with necessary dosage adjustments. These patients should be monitored closely until symptoms remit.
However, it is important for the primary care provider to identify patients who need psychiatric referral. Complex presentations, severe symptoms, and poor treatment response might warrant evaluation and management by a psychiatrist. Furthermore, patients with comorbid psychotic features, catatonia, or severely debilitating depression (with or without suicidality) need referral to the emergency department.
Continue to: Electroconvulsive therapy (ECT)
Electroconvulsive therapy (ECT). Patients might also need referral to Psychiatry for ECT, which is recommended by CANMAT–ISBD and JSMD guidelines as a second-line option; by the Royal Australian and New Zealand College of Psychiatrists as a third-line option; and by BAP for cases that are resistant to conventional treatment, with or without a high risk of suicide; in pregnancy; and in life-threatening situations.15,31,32
Telemedicine. There is a considerable shortage of mental health care professionals.33,34 The fact that nearly all (96%) counties in the United States have an unmet need for prescribers of mental health services (mainly psychiatrists) makes it crucial that primary care physicians be knowledgeable and prepared to manage BD—often with infrequent psychiatry consultation or, even, without psychiatry consultation. For primary care facilities that lack access to psychiatric services, telemedicine can be used as a consultative resource.
Psychiatric consultation using telemedicine technologies has provided significant cost savings for medical centers and decreased the likelihood of hospital admission,35 thereby alleviating health care costs and improving care, as shown in a rural Kansas county study.36 Furthermore, the burden on emergency departments in several states has been significantly reduced with psychiatric consultations via interactive telemedicine technologies.37
ACKNOWLEDGEMENT
Mark Yassa, BS, provided editing assistance.
CORRESPONDENCE
Nagy Youssef, MD, PhD, Medical College of Georgia at Augusta University, Department of Psychiatry and Health Behavior, 997 St. Sebastian Way, Augusta, GA 30912; [email protected].
1. Cerimele JM, Chwastiak LA, Dodson S, et al. The prevalence of bipolar disorder in general primary care samples: a systematic review. Gen Hosp Psychiatry. 2014;36:19-25.
2. Stubbs B, Vancampfort D, Solmi M, et al. How common is bipolar disorder in general primary care attendees? A systematic review and meta-analysis investigating prevalence determined according to structured clinical assessments. Aust N Z J Psychiatry. 2016;50:631-639.
3. Carta MG, Norcini-Pala A, Moro MF, et al. Does mood disorder questionnaire identify sub-threshold bipolarity? Evidence studying worsening of quality of life. J Affect Disord. 2015;183:173-178.
4. Fonseca-Pedrero E, Ortuno-Sierra J, Paino M, et al. Screening the risk of bipolar spectrum disorders: validity evidence of the Mood Disorder Questionnaire in adolescents and young adults. Rev Psiquiatr Salud Ment. 2016;9:4-12.
5. Reilly S, Planner C, Gask L, et al. Collaborative care approaches for people with severe mental illness. Cochrane Database Syst Rev. 2013;(11):CD009531.
6. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169:790-804.
7. Aquadro E, Youssef NA. Combine these screening tools to detect bipolar depression. J Fam Pract. 2018;67:500-503.
8. Bipolar and related disorders. In: American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association Publishing; 2013:123.
9. Judd LL, Akiskal HS, Schettler PJ, et al. The comparative clinical phenotype and long term longitudinal episode course of bipolar I and II: a clinical spectrum or distinct disorders? J Affect Disord. 2013;73:19-32.
10. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530-537.
11. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68:1237-1245.
12. Judd LL, Schettler PJ, Akiskal HS, et al. Residual symptom recovery from major affective episodes in bipolar disorders and rapid episode relapse/recurrence. Arch Gen Psychiatry. 2008;65:386-394.
13. Judd LL, Schettler PJ, Solomon DA, et al. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. J Affect Disord. 2008;108:49-58.
14. Yatham LN, Kennedy SH, Parikh S, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20:97-170.
15. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30:495-553.
16. Kanba S, Kato T, Terao T, et al; Committee for Treatment Guidelines of Mood Disorders, Japanese Society of Mood Disorders, 2012. Guideline for treatment of bipolar disorder by the Japanese Society of Mood Disorders, 2012. Psychiatry Clin Neurosci. 2013;67:285-300.
17. National Institute for Health and Care Excellence. Bipolar disorder: assessment and management. Clinical Guideline CG185. September 24, 2014. www.nice.org.uk/guidance/cg185. Accessed August 17, 2020.
18. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) Treatment Guidelines for Bipolar Disorder in Adults (CINP-BD-2017), Part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20:180-195.
19. Malhi GS, Outhred T, Morris G, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Med J Aust. 2018;208:219-225.
20. Hammett S, Youssef NA. Systematic review of recent guidelines for pharmacological treatments of bipolar disorders in adults. Ann Clin Psychiatry. 2017;29:266-282.
21. Gabbard GO, ed. Gabbard’s Treatments of Psychiatric Disorders. 5th ed. American Psychiatric Association Publishing; 2014: chap 12-15.
22. National Health Service. Guidelines for the monitoring of antimanic and prophylactic medication in bipolar disorder. NHT policy number MM-G-023. 2009. http://fac.ksu.edu.sa/sites/default/files/bipolar_disorder_guidelines.pdf. Accessed August 17, 2020.
23. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653-661.
24. Amsterdam JD, Lorenzo-Luaces L, Soeller I, et al. Safety and effectiveness of continuation antidepressant versus mood stabilizer monotherapy for relapse-prevention of bipolar II depression: a randomized, double-blind, parallel-group, prospective study. J Affect Disord. 2015;185:31-37.
25. Parker G, Tully L, Olley A, et al. SSRIs as mood stabilizers for bipolar II disorder? A proof of concept study. J Affect Disord. 2006;92:205-214.
26. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170:1249-1262.
27. Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for Bipolar Disorder. Australian and New Zealand clinical practice guidelines for the treatment of bipolar disorder. Aust N Z J Psychiatry. 2004;38:280-305.
28. Chiang K-J, Tsai J-C, Liu D, et al. Efficacy of cognitive-behavioral therapy in patients with bipolar disorder: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0176849.
29. de Mello MF, de Jesus Mari J, Bacaltchuk J, et al. A systematic review of research findings on the efficacy of interpersonal therapy for depressive disorders. Eur Arch Psychiatry Clin Neurosci. 2005;255:75-82.
30. Zhou X, Teng T, Zhang Y, et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:581-601.
31. Daly JJ, Prudic J, Devanand DP, et al. ECT in bipolar and unipolar depression: differences in speed of response. Bipolar Disord. 2001;3:95-104.
32. Medda P, Perugi G, Zanello S, et al. Response to ECT in bipolar I, bipolar II and unipolar depression. J Affect Disord. 2009;118:55-59.
33. Ellis AR, Konrad TR, Thomas KC, et al. County-level estimates of mental health professional supply in the United States. Psychiatr Serv. 2009;60:1315-1322.
34. Thomas KC, Ellis AR, Konrad TR, et al. County-level estimates of mental health professional shortage in the United States. Psychiatr Serv. 2009;60:1323-1328.
35. Narasimhan M, Druss BG, Hockenberry JM, et al. Impact of a telepsychiatry program at emergency departments statewide on the quality, utilization, and costs of mental health services. Psychiatr Serv. 2015;66:1167-1172.
36. Spaulding R, Belz N, DeLurgio S, et al. Cost savings of telemedicine utilization for child psychiatry in a rural Kansas community. Telemed J E Health. 2010;16:867-871.
37. States leverage telepsychiatry solutions to ease ED crowding, accelerate care. ED Manag. 2015;27:13-17.
1. Cerimele JM, Chwastiak LA, Dodson S, et al. The prevalence of bipolar disorder in general primary care samples: a systematic review. Gen Hosp Psychiatry. 2014;36:19-25.
2. Stubbs B, Vancampfort D, Solmi M, et al. How common is bipolar disorder in general primary care attendees? A systematic review and meta-analysis investigating prevalence determined according to structured clinical assessments. Aust N Z J Psychiatry. 2016;50:631-639.
3. Carta MG, Norcini-Pala A, Moro MF, et al. Does mood disorder questionnaire identify sub-threshold bipolarity? Evidence studying worsening of quality of life. J Affect Disord. 2015;183:173-178.
4. Fonseca-Pedrero E, Ortuno-Sierra J, Paino M, et al. Screening the risk of bipolar spectrum disorders: validity evidence of the Mood Disorder Questionnaire in adolescents and young adults. Rev Psiquiatr Salud Ment. 2016;9:4-12.
5. Reilly S, Planner C, Gask L, et al. Collaborative care approaches for people with severe mental illness. Cochrane Database Syst Rev. 2013;(11):CD009531.
6. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169:790-804.
7. Aquadro E, Youssef NA. Combine these screening tools to detect bipolar depression. J Fam Pract. 2018;67:500-503.
8. Bipolar and related disorders. In: American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association Publishing; 2013:123.
9. Judd LL, Akiskal HS, Schettler PJ, et al. The comparative clinical phenotype and long term longitudinal episode course of bipolar I and II: a clinical spectrum or distinct disorders? J Affect Disord. 2013;73:19-32.
10. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530-537.
11. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68:1237-1245.
12. Judd LL, Schettler PJ, Akiskal HS, et al. Residual symptom recovery from major affective episodes in bipolar disorders and rapid episode relapse/recurrence. Arch Gen Psychiatry. 2008;65:386-394.
13. Judd LL, Schettler PJ, Solomon DA, et al. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. J Affect Disord. 2008;108:49-58.
14. Yatham LN, Kennedy SH, Parikh S, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20:97-170.
15. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30:495-553.
16. Kanba S, Kato T, Terao T, et al; Committee for Treatment Guidelines of Mood Disorders, Japanese Society of Mood Disorders, 2012. Guideline for treatment of bipolar disorder by the Japanese Society of Mood Disorders, 2012. Psychiatry Clin Neurosci. 2013;67:285-300.
17. National Institute for Health and Care Excellence. Bipolar disorder: assessment and management. Clinical Guideline CG185. September 24, 2014. www.nice.org.uk/guidance/cg185. Accessed August 17, 2020.
18. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) Treatment Guidelines for Bipolar Disorder in Adults (CINP-BD-2017), Part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20:180-195.
19. Malhi GS, Outhred T, Morris G, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Med J Aust. 2018;208:219-225.
20. Hammett S, Youssef NA. Systematic review of recent guidelines for pharmacological treatments of bipolar disorders in adults. Ann Clin Psychiatry. 2017;29:266-282.
21. Gabbard GO, ed. Gabbard’s Treatments of Psychiatric Disorders. 5th ed. American Psychiatric Association Publishing; 2014: chap 12-15.
22. National Health Service. Guidelines for the monitoring of antimanic and prophylactic medication in bipolar disorder. NHT policy number MM-G-023. 2009. http://fac.ksu.edu.sa/sites/default/files/bipolar_disorder_guidelines.pdf. Accessed August 17, 2020.
23. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653-661.
24. Amsterdam JD, Lorenzo-Luaces L, Soeller I, et al. Safety and effectiveness of continuation antidepressant versus mood stabilizer monotherapy for relapse-prevention of bipolar II depression: a randomized, double-blind, parallel-group, prospective study. J Affect Disord. 2015;185:31-37.
25. Parker G, Tully L, Olley A, et al. SSRIs as mood stabilizers for bipolar II disorder? A proof of concept study. J Affect Disord. 2006;92:205-214.
26. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170:1249-1262.
27. Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for Bipolar Disorder. Australian and New Zealand clinical practice guidelines for the treatment of bipolar disorder. Aust N Z J Psychiatry. 2004;38:280-305.
28. Chiang K-J, Tsai J-C, Liu D, et al. Efficacy of cognitive-behavioral therapy in patients with bipolar disorder: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0176849.
29. de Mello MF, de Jesus Mari J, Bacaltchuk J, et al. A systematic review of research findings on the efficacy of interpersonal therapy for depressive disorders. Eur Arch Psychiatry Clin Neurosci. 2005;255:75-82.
30. Zhou X, Teng T, Zhang Y, et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:581-601.
31. Daly JJ, Prudic J, Devanand DP, et al. ECT in bipolar and unipolar depression: differences in speed of response. Bipolar Disord. 2001;3:95-104.
32. Medda P, Perugi G, Zanello S, et al. Response to ECT in bipolar I, bipolar II and unipolar depression. J Affect Disord. 2009;118:55-59.
33. Ellis AR, Konrad TR, Thomas KC, et al. County-level estimates of mental health professional supply in the United States. Psychiatr Serv. 2009;60:1315-1322.
34. Thomas KC, Ellis AR, Konrad TR, et al. County-level estimates of mental health professional shortage in the United States. Psychiatr Serv. 2009;60:1323-1328.
35. Narasimhan M, Druss BG, Hockenberry JM, et al. Impact of a telepsychiatry program at emergency departments statewide on the quality, utilization, and costs of mental health services. Psychiatr Serv. 2015;66:1167-1172.
36. Spaulding R, Belz N, DeLurgio S, et al. Cost savings of telemedicine utilization for child psychiatry in a rural Kansas community. Telemed J E Health. 2010;16:867-871.
37. States leverage telepsychiatry solutions to ease ED crowding, accelerate care. ED Manag. 2015;27:13-17.
PRACTICE RECOMMENDATIONS
› Become knowledgeable in identifying an episode of bipolar depression and differentiating it from major depressive disorder, so as to provide effective treatment for bipolar depression. A
› Begin treatment of bipolar depression with one of the recommended first-line medications, especially lithium, lamotrigine, quetiapine, or lurasidone. A
› Treat bipolar II depression similar to the way bipolar I depression is treated: primarily, using a mood stabilizer alone or, occasionally, using a mood stabilizer plus an antidepressant. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Differentiating Alzheimer’s disease from dementia with Lewy bodies
Discuss this article at www.facebook.com/CurrentPsychiatry
Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB) are the first and second most common causes of neurodegenerative dementia, respectively.“New Alzheimer’s disease guidelines: Implications for clinicians,” Current Psychiatry, March 2012, p. 15-20; http://bit.ly/UNYikk.
The 2005 report of the DLB Consortium5 recognizes central, core, suggestive, and supportive features of DLB (Table 1).5,10 These features are considered in the context of other confounding clinical conditions and the timing of cognitive and motor symptoms. The revised DLB criteria5 require a central feature of progressive cognitive decline. “Probable DLB” is when a patient presents with 2 core features or 1 core feature and ≥1 suggestive features. A diagnosis of “possible DLB” requires 1 core feature or 1 suggestive feature in the presence of progressive cognitive decline.
Table 1
Diagnostic criteria for AD and DLB
| NIA-AA criteria for AD (2011)10 |
| Possible AD: Clinical and cognitive criteria (DSM-IV-TR) for AD are met and there is an absence of biomarkers to support the diagnosis or there is evidence of a secondary disorder that can cause dementia |
| Probable AD: Clinical and cognitive criteria for AD are met and there is documented progressive cognitive decline or abnormal biomarker(s) suggestive of AD or evidence of proven AD autosomal dominant genetic mutation (presenilin-1, presenilin-2, amyloid-β precursor protein) |
| Definite AD: Clinical criteria for probable AD are met and there is histopathologic evidence of the disorder |
| Revised clinical diagnostic criteria for DLB (2005)5 |
| Core features: Fluctuating cognition, recurrent visual hallucinations, soft motor features of parkinsonism |
| Suggestive features: REM sleep behavior disorder, severe antipsychotic sensitivity, decreased tracer uptake in striatum on SPECT dopamine transporter imaging or on myocardial scintigraphy with MIBG |
| Supportive features (common but lacking diagnostic specificity): repeated falls and syncope; transient, unexplained loss of consciousness; systematized delusions; hallucinations other than visual; relative preservation of medial temporal lobe on CT or MRI scan; decreased tracer uptake on SPECT or PET imaging in occipital regions; prominent slow waves on EEG with temporal lobe transient sharp waves |
| AD: Alzheimer’s disease; DLB: dementia with Lewy bodies; MIBG: metaiodobenzylguanidine; NIA-AA: National Institute on Aging and the Alzheimer’s Association; PET: positron emission tomography; REM: rapid eye movement; SPECT: single photon emission computed tomography |
Biomarkers for AD, but not DLB
The 2011 diagnostic criteria for AD incorporate biomarkers that can be measured in vivo and reflect speci?c features of disease-related pathophysiologic processes. Biomarkers for AD are divided into 2 categories:11
- amyloid-beta (Aβ) accumulation: abnormal tracer retention on amyloid positron emission topography (PET) imaging and low cerebrospinal fluid (CSF) Aβ42
- neuronal degeneration or injury: elevated CSF tau (total and phosphorylated tau), decreased ?uorodeoxyglucose uptake on PET in temporo-parietal cortices, and atrophy on structural MRI in the hippocampal and temporo-parietal regions.
No clinically applicable genotypic or CSF markers exist to support a DLB diagnosis, but there are many promising candidates, including elevated levels of CSF p-tau 181, CSF levels of alpha- and beta-synuclein,12 and CSF beta-glucocerebrosidase levels.13 PET mapping of brain acetylcholinesterase activity,14 123I-2β-carbomethoxy-3β- (4-iodophenyl)-N-(3-fluoropropyl)nortropane single photon emission computed tomography (SPECT) dopamine transporter (DaT) imaging15 and metaiodobenzylguanidine (MIBG) scintigraphy also are promising methods. DaT scan SPECT is FDA-approved for detecting loss of functional dopaminergic neuron terminals in the striatum and can differentiate between AD and DLB with a sensitivity and specificity of 78% to 88% and 94% to 100%, respectively.16 This test is covered by Medicare for differentiating AD and DLB.
Differences in presentation
Cognitive impairment. Contrary to the early memory impairment that characterizes AD, memory deficits in DLB usually appear later in the disease course.5 Patients with DLB manifest greater attentional, visuospatial, and executive impairments than those with AD, whereas AD causes more profound episodic (declarative) memory impairment than DLB. DLB patients show more preserved consolidation and storage of verbal information than AD patients because of less neuroanatomical and cholinergic compromise in the medial temporal lobe. There is no evidence of significant differences in remote memory, semantic memory, and language (naming and fluency).
Compromised attention in DLB may be the basis for fluctuating cognition, a characteristic of the disease. The greater attentional impairment and reaction time variability in DLB compared with AD is evident during complex tasks for attention and may be a function of the executive and visuospatial demands of the tasks.17
Executive functions critical to adaptive, goal-directed behavior are more impaired in DLB than AD. DLB patients are more susceptible to distraction and have difficulty engaging in a task and shifting from 1 task to another. This, together with a tendency for confabulation and perseveration, are signs of executive dysfunction.
Neuropsychiatric features. DLB patients are more likely than AD patients to exhibit psychiatric symptoms and have more functional impairment.18 In an analysis of autopsy-confirmed cases, hallucinations and delusions were more frequent with Lewy body pathology (75%) than AD (21%) at initial clinical evaluation.18 By the end stages of both illnesses, the degree of psychotic symptoms is comparable.19 Depression is common in DLB; whether base rates of depressed mood and major depression differ between DLB and AD is uncertain.20
Psychosis in AD can be induced by medication or delirium, or triggered by poor sensory perceptions. Psychotic symptoms occur more frequently during the moderate and advanced stages of AD, when patients present with visual hallucinations, delusions, or delusional misidentifications. As many as 10% to 20% of patients with AD experience hallucinations, typically visual. Delusions occur in 30% to 50% of AD patients, usually in the later stages of the disease. The most common delusional themes are infidelity, theft, and paranoia. Female sex is a risk factor for psychosis in AD. Delusions co-occur with aggression, anxiety, and aberrant motor behavior.
Visual hallucinations—mostly vivid, well-formed, false perceptions of insects, animals, or people—are the defining feature of DLB.21 Many patients recognize that they are experiencing visual hallucinations and can ignore them. DLB patients also may experience visual illusions, such as misperceiving household objects as living beings. Delusions—typically paranoid—are common among DLB patients, as are depression and anxiety.1 Agitation or aggressive behavior tends to occur late in the illness, if at all.
The causes of psychotic symptoms in DLB are not fully understood, but dopamine dysfunction likely is involved in hallucinations, delusions, and agitation, and serotonin dysfunction may be associated with depression and anxiety. Rapid eye movement (REM) sleep/wakefulness dysregulation, in which the dream imagery of REM sleep may occur during wakefulness, also has been proposed as a mechanism for visual hallucinations in DLB.22 In DLB, psychotic symptoms occur early and are a hallmark of this illness, whereas in AD they usually occur in the middle to late stages of the disease.
Motor symptoms. In AD, extrapyramidal symptoms (EPS) are common later in the disease, are strongly correlated with disease severity, and are a strong, independent predictor of depression severity.23 EPS are more common in DLB than in AD24 and DLB patients are at higher risk of developing EPS even with low doses of typical antipsychotics, compared with AD patients.25
Other symptoms. REM sleep behavior disorder (RBD) is characterized by enacting dreams—often violent—during REM sleep. RBD is common in DLB and many patients also have excessive daytime somnolence. Other sleep disorders in DLB include insomnia, obstructive sleep apnea, central sleep apnea, restless legs syndrome, and periodic limb movements during sleep.
In AD patients, common sleep behaviors include confusion in the early evening (“sundowning”) and frequent nighttime awakenings, often accompanied by wandering.26 Orthostatic hypotension, impotence, urinary incontinence, and constipation are common in DLB. Lack of insight concerning personal cognitive, mood, and behavioral state is highly prevalent in AD patients and more common than in DLB.
Diagnostic evaluation
Because there are no definitive clinical markers for DLB, diagnosis is based on a detailed clinical and family history from the patient and a reliable informant, as well as a physical, neurologic, and mental status examination that looks for associated noncognitive symptoms, and neuropsychological evaluation. Reasons DLB may be misdiagnosed include:
- Some “core” clinical features of DLB may not appear or may overlap with AD.
- Presence and severity of concurrent AD pathology in DLB may modify the clinical presentation, with decreased rates of hallucinations and parkinsonism and increased neurofibrillary tangles.
- Failure to reliably identify fluctuations—variations in cognition and arousal, such as periods of unresponsiveness while awake (“zoning out”), excessive daytime somnolence, and disorganized speech.27
Detecting and characterizing cognitive deficits in dementia patients using neuropsychological testing is important in establishing a clinical diagnosis, determining baseline levels of impairment, forming a prognosis, and initiating disease-specific treatments. Differences in neuropsychological findings in AD and DLB are outlined in Table 2.16,28-33 Several studies have suggested using these measures to differentiate patients with DLB from those with AD.20
Table 2
Diagnostic testing for Alzheimer’s disease and dementia with Lewy bodies
| Alzheimer’s disease | Dementia with Lewy bodies |
|---|---|
| Neuropsychological testing findings | |
| Relatively more impairment on verbal memory tasks, delayed recall, delayed recognition, and encoding and storing information.28 Dysfunction of episodic memory function | Relatively more impairment on attention or concentration, verbal fluency, visuoperceptual, visuoconstructive, visual memory tests, and frontal executive functions.28 Relatively preserved confrontation naming and verbal memory |
| MRI findings | |
| Diffuse cortical atrophy, relatively greater volume loss in hippocampus and medial temporal lobe structures (strong correlation with severity)29 | Mild generalized cerebral cortical atrophy with minimal hippocampal atrophy and relative preservation of medial temporal lobe structures30 |
| [18F]FDG PET | |
| Widespread metabolic deficits in neocortical association areas, with sparing of the basal ganglia, thalamus, cerebellum, primary sensory motor cortex, and visual cortex | Widespread cortical hypometabolism, more marked in primary visual and occipital association areas, and less severe in parietal, frontal, and anterior cingulate cortices.31 Severe cholinergic deafferentation of the neocortex, particularly in posterior cortical regions32 |
| Single photon emission computed tomography | |
| Parietotemporal hypoperfusion | Occipital hypoperfusion |
| 123I-FP-CIT SPECT (DaT scan) | |
| No significant loss of DaT | Low nigrostriatal terminal density of DaT caused by severe nigrostriatal degeneration16 |
| Myocardial scintigraphy with MIBG | |
| No significant change in MIBG uptake | Decreased MIBG uptake33 |
| 123I-FP-CIT: 123I-2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)nortropane; DaT: dopamine transporter; FDG PET: [18F]-fluoro-d-glucose positron emission tomography; MIBG: metaiodobenzylguanidine; SPECT: single photon emission computed tomography | |
Evidence is insufficient to support using electroencephalographic and polysomnographic studies when initially evaluating patients with dementia. Brain CT or MRI are recommended as part of the initial evaluation of dementia patients to exclude treatable causes of dementia and help clarify the differential diagnosis. Occipital hypometabolism and hypoperfusion demonstrated on PET and SPECT imaging have high sensitivity and specificity for differentiating AD from DLB.
To diagnose DLB more consistently, look for core features of the disease, RBD, antipsychotic hypersensitivity, and decreased striatal binding at presynaptic DaT sites.15 Abnormal (low binding) DaT activity is the most reliable diagnostic marker for DLB.34 Myocardial scintigraphy with MIBG is sensitive to pathologic changes of DLB before clinical expression and could overcome the difficulties of using clinical criteria alone to identify patients with DLB.35 MIBG scintigraphy may be preferred to DaT scan because it is less expensive and its sensitivity and specificity to DLB are independent of the presence of parkinsonism.35
For an overview of pharmacotherapy options for patients with AD or DLB, see Box 2.
Pharmacotherapy options for patients with Alzheimer’s disease (AD) or dementia with Lewy bodies (DLB) include cholinesterase inhibitors, memantine, antipsychotics, and other agents.
Cholinesterase inhibitors. Donepezil, rivastigmine, and galantamine are FDA-approved for treating AD. Their efficacy appears to be similar, so the choice of agent is based largely on cost, patient tolerability, and physician experience.
No medications are FDA-approved for treating DLB. Neocortical cholinergic activity assessed by choline acetyltransferase levels is more severely depleted in DLB than in AD, and this deficit is correlated with the presence of visual hallucinations and global severity of cognitive impairment.a Therefore, drugs that enhance central cholinergic function offer a therapeutic approach for DLB; cognitive and hallucinatory symptoms are the anticipated targets. Multiple anecdotal reports, open-label studies,b,c and 1 randomized, placebo-controlled triald suggest that cholinesterase inhibitors are efficacious in DLB, with reported benefits in cognition, fluctuations, psychotic symptoms, and parkinsonian symptoms. A 20-week randomized, double-blind, placebo-controlled multicenter studyd of patients with DLB found rivastigmine, 6 to 12 mg/d, was superior to placebo. Patients receiving rivastigmine exhibited significantly reduced anxiety, delusions, and hallucinations and significantly better performance on a computerized battery of neuropsychological tests, especially tasks that required sustained attention. Differences between rivastigmine and placebo disappeared after drug discontinuation.
Memantine is a noncompetitive antagonist of N-methyl-d-aspartate receptors that is effective in AD.e The possible involvement of glutamate in DLB has provided a rationale for treating DLB with memantine. Two randomized controlled trials in DLB found that patients treated with memantine for 24 weeks performed better on Clinical Global Impression of Change, but not on most other secondary outcome measures.f,g In both studies, memantine was well tolerated. However, other studies have noted worsening of delusions and hallucinations with memantine in DLB patients.h
Antipsychotics. Agitation is common in moderate and advanced AD. Atypical antipsychotics have been used with variable efficacy to treat agitation, but their use is associated with excess mortality. DLB patients pose a considerable therapeutic challenge because antipsychotics—the mainstay of treatment of psychosis and behavioral problems in most other disorders—can provoke severe, irreversible, and often fatal sensitivity reactions in this type of dementia.i A 2- to 3-fold increased mortality risk associated with antipsychotic sensitivity reactions in DLB is partly mediated via acute blockade of postsynaptic dopamine D2 receptors in the striatum. For severe and disabling psychosis, a trial of a cholinesterase inhibitor and/or lowering the dose of antiparkinsonian medication should be considered first. In urgent situations, small doses of an atypical antipsychotic that is least associated with parkinsonism side effects—such as quetiapine or aripiprazole—should be used.
Other treatments. Treatment of parkinsonian symptoms in DLB patients is similar to that for Parkinson’s disease, but the risk of psychotic symptoms in DLB warrants a conservative approach. Levodopa seems to be more effective than dopamine agonists and produces fewer side effects.j Rapid eye movement sleep behavior disorder often responds to low doses of clonazepam (0.25 to 1.5 mg). Depression and anxiety disorders are common in AD at all stages and their treatment is not fundamentally different than in geriatric patients without dementia. Selective serotonin reuptake inhibitors and electroconvulsive therapy have been used successfully in depressed patients with AD or DLB.k,l
Disease-modifying treatments. Researchers are evaluating an array of antiamyloid and neuroprotective therapeutic approaches for AD based on the hypothesis that amyloid-beta protein plays a pivotal role in disease onset and progression. Interventions that reduce amyloid production, limit aggregation, or increase clearance may block the cascade of events comprising AD pathogenesis. Reducing tau hyperphosphorylation, limiting oxidation and excitotoxicity, and controlling inflammation also may be beneficial strategies. Potentially neuroprotective and restorative treatments such as neurotrophins, neurotrophic factor enhancers, and stem cell-related approaches also are being investigated.
There are no large-scale studies of disease-modifying treatments for DLB. Potential areas of research include the relationship between proteasome function and a-synuclein pathology, the role of beta-synuclein, and the impact of alterations to alpha-synuclein on its propensity to aggregate.
References
a. Ballard C, Ziabreva I, Perry R, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67(11):1931-1934.
b. Beversdorf DQ, Warner JL, Davis RA, et al. Donepezil in the treatment of dementia with lewy bodies. Am J Geriatr Psychiatry. 2004;12(5):542-544.
c. Edwards K, Royall D, Hershey L, et al. Efficacy and safety of galantamine in patients with dementia with Lewy bodies: a 24-week open-label study. Dement Geriatr Cogn Disord. 2007;23(6):401-405.
d. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
e. Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317-324.
f. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
g. Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969-977.
h. Ridha BH, Josephs KA, Rossor MN. Delusions and hallucinations in dementia with Lewy bodies: worsening with memantine. Neurology. 2005;65(3):481-482.
i. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
j. Fernandez HH, Wu CK, Ott BR. Pharmacotherapy of dementia with Lewy bodies. Expert Opin Pharmacother. 2003;4(11):2027-2037.
k. Swartz M, Barak Y, Mirecki I, et al. Treating depression in Alzheimer’s disease: integration of differing guidelines. Int Psychogeriatr. 2000;12(3):353-358.
l. Takahashi S, Mizukami K, Yasuno F, et al. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics. 2009;9(2):56-61.
Related Resources
- Hanyu H, Sato T, Hirao K, et al. Differences in clinical course between dementia with Lewy bodies and Alzheimer’s disease. Eur J Neurol. 2009;16(2):212-217.
- Walker Z, McKeith I, Rodda J, et al. Comparison of cognitive decline between dementia with Lewy bodies and Alzheimer’s disease: a cohort study. BMJ Open. 2012;2:e000380.
Drug Brand Names
- Aripiprazole • Abilify
- Clonazepam • Klonopin
- Donepezil • Aricept
- Galantamine • Razadyne, Reminyl
- Levodopa • Dopar, Larodopa
- Memantine • Namenda
- Quetiapine • Seroquel
- Rivastigmine • Exelon
Disclosure
Drs. Bishnoi and Manepalli report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Grossberg serves as a consultant to Forest, Janssen, Novartis, and Pfizer. His department receives research funding from Novartis, Janssen, and Pfizer.
1. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113-1124.
2. Buracchio T, Arvanitakis Z, Gorbien M. Dementia with Lewy bodies: current concepts. Dement Geriatr Cogn Disord. 2005;20(5):306-320.
3. Fujishiro H, Iseki E, Higashi S, et al. Distribution of cerebral amyloid deposition and its relevance to clinical phenotype in Lewy body dementia. Neurosci Lett. 2010;486(1):19-23.
4. Kosaka K. Diffuse Lewy body disease. Neuropathology. 2000;20(suppl):S73-S78.
5. McKeith IG, Dickson DW, Lowe J, et al. Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872.
6. Cummings JL, Cole G. Alzheimer disease. JAMA. 2002;287(18):2335-2338.
7. Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing. 2005;34(6):561-566.
8. Bradshaw J, Saling M, Hopwood M, et al. Fluctuating cognition in dementia with Lewy bodies and Alzheimer’s disease is qualitatively distinct. J Neurol Neurosurg Psychiatry. 2004;75(3):382-387.
9. Singleton AB, Wharton A, O’Brien KK, et al. Clinical and neuropathological correlates of apolipoprotein E genotype in dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2002;14(4):167-175.
10. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
11. Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257-262.
12. Mollenhauer B, Cullen V, Kahn I, et al. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol. 2008;213(2):315-325.
13. Parnetti L, Balducci C, Pierguidi L, et al. Cerebrospinal fluid beta-glucocerebrosidase activity is reduced in dementia with Lewy bodies. Neurobiol Dis. 2009;34(3):484-486.
14. Shimada H, Hirano S, Shinotoh H, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology. 2009;73(4):273-278.
15. McKeith I, O’Brien J, Walker Z, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6(4):305-313.
16. Walker Z, Jaros E, Walker RW, et al. Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Psychiatry. 2007;78(11):1176-1181.
17. Bradshaw JM, Saling M, Anderson V, et al. Higher cortical deficits influence attentional processing in dementia with Lewy bodies, relative to patients with dementia of the Alzheimer’s type and controls. J Neurol Neurosurg Psychiatry. 2006;77(10):1129-1135.
18. Weiner MF, Hynan LS, Parikh B, et al. Can Alzheimer’s disease and dementias with Lewy bodies be distinguished clinically? J Geriatr Psychiatry Neurol. 2003;16(4):245-250.
19. Stavitsky K, Brickman AM, Scarmeas N, et al. The progression of cognition, psychiatric symptoms, and functional abilities in dementia with Lewy bodies and Alzheimer disease. Arch Neurol. 2006;63(10):1450-1456.
20. Ferman TJ, Smith GE, Boeve BF, et al. Neuropsychological differentiation of dementia with Lewy bodies from normal aging and Alzheimer’s disease. Clin Neuropsychol. 2006;20(4):623-636.
21. McKeith IG, Perry EK, Perry RH. Report of the second dementia with Lewy body international workshop: diagnosis and treatment. Consortium on Dementia with Lewy Bodies. Neurology. 1999;53(5):902-905.
22. Boeve BF, Silber MH, Ferman TJ, et al. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16(4):622-630.
23. Portet F, Scarmeas N, Cosentino S, et al. Extrapyramidal signs before and after diagnosis of incident Alzheimer disease in a prospective population study. Arch Neurol. 2009;66(9):1120-1126.
24. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
25. Tarawneh R, Galvin JE. Distinguishing Lewy body dementias from Alzheimer’s disease. Expert Rev Neurother. 2007;7(11):1499-1516.
26. Ancoli-Israel S, Klauber MR, Gillin JC, et al. Sleep in non-institutionalized Alzheimer’s disease patients. Aging (Milano). 1994;6(6):451-458.
27. Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology. 2004;62(2):181-187.
28. Salmon DP, Galasko D, Hansen LA, et al. Neuropsychological deficits associated with diffuse Lewy body disease. Brain Cogn. 1996;31(2):148-165.
29. Jack CR, Jr, Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55(4):484-489.
30. Burton EJ, Barber R, Mukaetova-Ladinska EB, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain. 2009;132(pt 1):195-203.
31. Ishii K, Soma T, Kono AK, et al. Comparison of regional brain volume and glucose metabolism between patients with mild dementia with lewy bodies and those with mild Alzheimer’s disease. J Nucl Med. 2007;48(5):704-711.
32. Klein JC, Eggers C, Kalbe E, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74(11):885-892.
33. Fujishiro H, Nakamura S, Kitazawa M, et al. Early detection of dementia with Lewy bodies in patients with amnestic mild cognitive impairment using 123I-MIBG cardiac scintigraphy. J Neurol Sci. 2012;315(1-2):115-119.
34. O’Brien JT, McKeith IG, Walker Z, et al. Diagnostic accuracy of 123I-FP-CIT SPECT in possible dementia with Lewy bodies. Br J Psychiatry. 2009;194:34-39.
35. Yoshita M, Taki J, Yokoyama K, et al. Value of 123I-MIBG radioactivity in the differential diagnosis of DLB from AD. Neurology. 2006;66(12):1850-1854.
Discuss this article at www.facebook.com/CurrentPsychiatry
Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB) are the first and second most common causes of neurodegenerative dementia, respectively.“New Alzheimer’s disease guidelines: Implications for clinicians,” Current Psychiatry, March 2012, p. 15-20; http://bit.ly/UNYikk.
The 2005 report of the DLB Consortium5 recognizes central, core, suggestive, and supportive features of DLB (Table 1).5,10 These features are considered in the context of other confounding clinical conditions and the timing of cognitive and motor symptoms. The revised DLB criteria5 require a central feature of progressive cognitive decline. “Probable DLB” is when a patient presents with 2 core features or 1 core feature and ≥1 suggestive features. A diagnosis of “possible DLB” requires 1 core feature or 1 suggestive feature in the presence of progressive cognitive decline.
Table 1
Diagnostic criteria for AD and DLB
| NIA-AA criteria for AD (2011)10 |
| Possible AD: Clinical and cognitive criteria (DSM-IV-TR) for AD are met and there is an absence of biomarkers to support the diagnosis or there is evidence of a secondary disorder that can cause dementia |
| Probable AD: Clinical and cognitive criteria for AD are met and there is documented progressive cognitive decline or abnormal biomarker(s) suggestive of AD or evidence of proven AD autosomal dominant genetic mutation (presenilin-1, presenilin-2, amyloid-β precursor protein) |
| Definite AD: Clinical criteria for probable AD are met and there is histopathologic evidence of the disorder |
| Revised clinical diagnostic criteria for DLB (2005)5 |
| Core features: Fluctuating cognition, recurrent visual hallucinations, soft motor features of parkinsonism |
| Suggestive features: REM sleep behavior disorder, severe antipsychotic sensitivity, decreased tracer uptake in striatum on SPECT dopamine transporter imaging or on myocardial scintigraphy with MIBG |
| Supportive features (common but lacking diagnostic specificity): repeated falls and syncope; transient, unexplained loss of consciousness; systematized delusions; hallucinations other than visual; relative preservation of medial temporal lobe on CT or MRI scan; decreased tracer uptake on SPECT or PET imaging in occipital regions; prominent slow waves on EEG with temporal lobe transient sharp waves |
| AD: Alzheimer’s disease; DLB: dementia with Lewy bodies; MIBG: metaiodobenzylguanidine; NIA-AA: National Institute on Aging and the Alzheimer’s Association; PET: positron emission tomography; REM: rapid eye movement; SPECT: single photon emission computed tomography |
Biomarkers for AD, but not DLB
The 2011 diagnostic criteria for AD incorporate biomarkers that can be measured in vivo and reflect speci?c features of disease-related pathophysiologic processes. Biomarkers for AD are divided into 2 categories:11
- amyloid-beta (Aβ) accumulation: abnormal tracer retention on amyloid positron emission topography (PET) imaging and low cerebrospinal fluid (CSF) Aβ42
- neuronal degeneration or injury: elevated CSF tau (total and phosphorylated tau), decreased ?uorodeoxyglucose uptake on PET in temporo-parietal cortices, and atrophy on structural MRI in the hippocampal and temporo-parietal regions.
No clinically applicable genotypic or CSF markers exist to support a DLB diagnosis, but there are many promising candidates, including elevated levels of CSF p-tau 181, CSF levels of alpha- and beta-synuclein,12 and CSF beta-glucocerebrosidase levels.13 PET mapping of brain acetylcholinesterase activity,14 123I-2β-carbomethoxy-3β- (4-iodophenyl)-N-(3-fluoropropyl)nortropane single photon emission computed tomography (SPECT) dopamine transporter (DaT) imaging15 and metaiodobenzylguanidine (MIBG) scintigraphy also are promising methods. DaT scan SPECT is FDA-approved for detecting loss of functional dopaminergic neuron terminals in the striatum and can differentiate between AD and DLB with a sensitivity and specificity of 78% to 88% and 94% to 100%, respectively.16 This test is covered by Medicare for differentiating AD and DLB.
Differences in presentation
Cognitive impairment. Contrary to the early memory impairment that characterizes AD, memory deficits in DLB usually appear later in the disease course.5 Patients with DLB manifest greater attentional, visuospatial, and executive impairments than those with AD, whereas AD causes more profound episodic (declarative) memory impairment than DLB. DLB patients show more preserved consolidation and storage of verbal information than AD patients because of less neuroanatomical and cholinergic compromise in the medial temporal lobe. There is no evidence of significant differences in remote memory, semantic memory, and language (naming and fluency).
Compromised attention in DLB may be the basis for fluctuating cognition, a characteristic of the disease. The greater attentional impairment and reaction time variability in DLB compared with AD is evident during complex tasks for attention and may be a function of the executive and visuospatial demands of the tasks.17
Executive functions critical to adaptive, goal-directed behavior are more impaired in DLB than AD. DLB patients are more susceptible to distraction and have difficulty engaging in a task and shifting from 1 task to another. This, together with a tendency for confabulation and perseveration, are signs of executive dysfunction.
Neuropsychiatric features. DLB patients are more likely than AD patients to exhibit psychiatric symptoms and have more functional impairment.18 In an analysis of autopsy-confirmed cases, hallucinations and delusions were more frequent with Lewy body pathology (75%) than AD (21%) at initial clinical evaluation.18 By the end stages of both illnesses, the degree of psychotic symptoms is comparable.19 Depression is common in DLB; whether base rates of depressed mood and major depression differ between DLB and AD is uncertain.20
Psychosis in AD can be induced by medication or delirium, or triggered by poor sensory perceptions. Psychotic symptoms occur more frequently during the moderate and advanced stages of AD, when patients present with visual hallucinations, delusions, or delusional misidentifications. As many as 10% to 20% of patients with AD experience hallucinations, typically visual. Delusions occur in 30% to 50% of AD patients, usually in the later stages of the disease. The most common delusional themes are infidelity, theft, and paranoia. Female sex is a risk factor for psychosis in AD. Delusions co-occur with aggression, anxiety, and aberrant motor behavior.
Visual hallucinations—mostly vivid, well-formed, false perceptions of insects, animals, or people—are the defining feature of DLB.21 Many patients recognize that they are experiencing visual hallucinations and can ignore them. DLB patients also may experience visual illusions, such as misperceiving household objects as living beings. Delusions—typically paranoid—are common among DLB patients, as are depression and anxiety.1 Agitation or aggressive behavior tends to occur late in the illness, if at all.
The causes of psychotic symptoms in DLB are not fully understood, but dopamine dysfunction likely is involved in hallucinations, delusions, and agitation, and serotonin dysfunction may be associated with depression and anxiety. Rapid eye movement (REM) sleep/wakefulness dysregulation, in which the dream imagery of REM sleep may occur during wakefulness, also has been proposed as a mechanism for visual hallucinations in DLB.22 In DLB, psychotic symptoms occur early and are a hallmark of this illness, whereas in AD they usually occur in the middle to late stages of the disease.
Motor symptoms. In AD, extrapyramidal symptoms (EPS) are common later in the disease, are strongly correlated with disease severity, and are a strong, independent predictor of depression severity.23 EPS are more common in DLB than in AD24 and DLB patients are at higher risk of developing EPS even with low doses of typical antipsychotics, compared with AD patients.25
Other symptoms. REM sleep behavior disorder (RBD) is characterized by enacting dreams—often violent—during REM sleep. RBD is common in DLB and many patients also have excessive daytime somnolence. Other sleep disorders in DLB include insomnia, obstructive sleep apnea, central sleep apnea, restless legs syndrome, and periodic limb movements during sleep.
In AD patients, common sleep behaviors include confusion in the early evening (“sundowning”) and frequent nighttime awakenings, often accompanied by wandering.26 Orthostatic hypotension, impotence, urinary incontinence, and constipation are common in DLB. Lack of insight concerning personal cognitive, mood, and behavioral state is highly prevalent in AD patients and more common than in DLB.
Diagnostic evaluation
Because there are no definitive clinical markers for DLB, diagnosis is based on a detailed clinical and family history from the patient and a reliable informant, as well as a physical, neurologic, and mental status examination that looks for associated noncognitive symptoms, and neuropsychological evaluation. Reasons DLB may be misdiagnosed include:
- Some “core” clinical features of DLB may not appear or may overlap with AD.
- Presence and severity of concurrent AD pathology in DLB may modify the clinical presentation, with decreased rates of hallucinations and parkinsonism and increased neurofibrillary tangles.
- Failure to reliably identify fluctuations—variations in cognition and arousal, such as periods of unresponsiveness while awake (“zoning out”), excessive daytime somnolence, and disorganized speech.27
Detecting and characterizing cognitive deficits in dementia patients using neuropsychological testing is important in establishing a clinical diagnosis, determining baseline levels of impairment, forming a prognosis, and initiating disease-specific treatments. Differences in neuropsychological findings in AD and DLB are outlined in Table 2.16,28-33 Several studies have suggested using these measures to differentiate patients with DLB from those with AD.20
Table 2
Diagnostic testing for Alzheimer’s disease and dementia with Lewy bodies
| Alzheimer’s disease | Dementia with Lewy bodies |
|---|---|
| Neuropsychological testing findings | |
| Relatively more impairment on verbal memory tasks, delayed recall, delayed recognition, and encoding and storing information.28 Dysfunction of episodic memory function | Relatively more impairment on attention or concentration, verbal fluency, visuoperceptual, visuoconstructive, visual memory tests, and frontal executive functions.28 Relatively preserved confrontation naming and verbal memory |
| MRI findings | |
| Diffuse cortical atrophy, relatively greater volume loss in hippocampus and medial temporal lobe structures (strong correlation with severity)29 | Mild generalized cerebral cortical atrophy with minimal hippocampal atrophy and relative preservation of medial temporal lobe structures30 |
| [18F]FDG PET | |
| Widespread metabolic deficits in neocortical association areas, with sparing of the basal ganglia, thalamus, cerebellum, primary sensory motor cortex, and visual cortex | Widespread cortical hypometabolism, more marked in primary visual and occipital association areas, and less severe in parietal, frontal, and anterior cingulate cortices.31 Severe cholinergic deafferentation of the neocortex, particularly in posterior cortical regions32 |
| Single photon emission computed tomography | |
| Parietotemporal hypoperfusion | Occipital hypoperfusion |
| 123I-FP-CIT SPECT (DaT scan) | |
| No significant loss of DaT | Low nigrostriatal terminal density of DaT caused by severe nigrostriatal degeneration16 |
| Myocardial scintigraphy with MIBG | |
| No significant change in MIBG uptake | Decreased MIBG uptake33 |
| 123I-FP-CIT: 123I-2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)nortropane; DaT: dopamine transporter; FDG PET: [18F]-fluoro-d-glucose positron emission tomography; MIBG: metaiodobenzylguanidine; SPECT: single photon emission computed tomography | |
Evidence is insufficient to support using electroencephalographic and polysomnographic studies when initially evaluating patients with dementia. Brain CT or MRI are recommended as part of the initial evaluation of dementia patients to exclude treatable causes of dementia and help clarify the differential diagnosis. Occipital hypometabolism and hypoperfusion demonstrated on PET and SPECT imaging have high sensitivity and specificity for differentiating AD from DLB.
To diagnose DLB more consistently, look for core features of the disease, RBD, antipsychotic hypersensitivity, and decreased striatal binding at presynaptic DaT sites.15 Abnormal (low binding) DaT activity is the most reliable diagnostic marker for DLB.34 Myocardial scintigraphy with MIBG is sensitive to pathologic changes of DLB before clinical expression and could overcome the difficulties of using clinical criteria alone to identify patients with DLB.35 MIBG scintigraphy may be preferred to DaT scan because it is less expensive and its sensitivity and specificity to DLB are independent of the presence of parkinsonism.35
For an overview of pharmacotherapy options for patients with AD or DLB, see Box 2.
Pharmacotherapy options for patients with Alzheimer’s disease (AD) or dementia with Lewy bodies (DLB) include cholinesterase inhibitors, memantine, antipsychotics, and other agents.
Cholinesterase inhibitors. Donepezil, rivastigmine, and galantamine are FDA-approved for treating AD. Their efficacy appears to be similar, so the choice of agent is based largely on cost, patient tolerability, and physician experience.
No medications are FDA-approved for treating DLB. Neocortical cholinergic activity assessed by choline acetyltransferase levels is more severely depleted in DLB than in AD, and this deficit is correlated with the presence of visual hallucinations and global severity of cognitive impairment.a Therefore, drugs that enhance central cholinergic function offer a therapeutic approach for DLB; cognitive and hallucinatory symptoms are the anticipated targets. Multiple anecdotal reports, open-label studies,b,c and 1 randomized, placebo-controlled triald suggest that cholinesterase inhibitors are efficacious in DLB, with reported benefits in cognition, fluctuations, psychotic symptoms, and parkinsonian symptoms. A 20-week randomized, double-blind, placebo-controlled multicenter studyd of patients with DLB found rivastigmine, 6 to 12 mg/d, was superior to placebo. Patients receiving rivastigmine exhibited significantly reduced anxiety, delusions, and hallucinations and significantly better performance on a computerized battery of neuropsychological tests, especially tasks that required sustained attention. Differences between rivastigmine and placebo disappeared after drug discontinuation.
Memantine is a noncompetitive antagonist of N-methyl-d-aspartate receptors that is effective in AD.e The possible involvement of glutamate in DLB has provided a rationale for treating DLB with memantine. Two randomized controlled trials in DLB found that patients treated with memantine for 24 weeks performed better on Clinical Global Impression of Change, but not on most other secondary outcome measures.f,g In both studies, memantine was well tolerated. However, other studies have noted worsening of delusions and hallucinations with memantine in DLB patients.h
Antipsychotics. Agitation is common in moderate and advanced AD. Atypical antipsychotics have been used with variable efficacy to treat agitation, but their use is associated with excess mortality. DLB patients pose a considerable therapeutic challenge because antipsychotics—the mainstay of treatment of psychosis and behavioral problems in most other disorders—can provoke severe, irreversible, and often fatal sensitivity reactions in this type of dementia.i A 2- to 3-fold increased mortality risk associated with antipsychotic sensitivity reactions in DLB is partly mediated via acute blockade of postsynaptic dopamine D2 receptors in the striatum. For severe and disabling psychosis, a trial of a cholinesterase inhibitor and/or lowering the dose of antiparkinsonian medication should be considered first. In urgent situations, small doses of an atypical antipsychotic that is least associated with parkinsonism side effects—such as quetiapine or aripiprazole—should be used.
Other treatments. Treatment of parkinsonian symptoms in DLB patients is similar to that for Parkinson’s disease, but the risk of psychotic symptoms in DLB warrants a conservative approach. Levodopa seems to be more effective than dopamine agonists and produces fewer side effects.j Rapid eye movement sleep behavior disorder often responds to low doses of clonazepam (0.25 to 1.5 mg). Depression and anxiety disorders are common in AD at all stages and their treatment is not fundamentally different than in geriatric patients without dementia. Selective serotonin reuptake inhibitors and electroconvulsive therapy have been used successfully in depressed patients with AD or DLB.k,l
Disease-modifying treatments. Researchers are evaluating an array of antiamyloid and neuroprotective therapeutic approaches for AD based on the hypothesis that amyloid-beta protein plays a pivotal role in disease onset and progression. Interventions that reduce amyloid production, limit aggregation, or increase clearance may block the cascade of events comprising AD pathogenesis. Reducing tau hyperphosphorylation, limiting oxidation and excitotoxicity, and controlling inflammation also may be beneficial strategies. Potentially neuroprotective and restorative treatments such as neurotrophins, neurotrophic factor enhancers, and stem cell-related approaches also are being investigated.
There are no large-scale studies of disease-modifying treatments for DLB. Potential areas of research include the relationship between proteasome function and a-synuclein pathology, the role of beta-synuclein, and the impact of alterations to alpha-synuclein on its propensity to aggregate.
References
a. Ballard C, Ziabreva I, Perry R, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67(11):1931-1934.
b. Beversdorf DQ, Warner JL, Davis RA, et al. Donepezil in the treatment of dementia with lewy bodies. Am J Geriatr Psychiatry. 2004;12(5):542-544.
c. Edwards K, Royall D, Hershey L, et al. Efficacy and safety of galantamine in patients with dementia with Lewy bodies: a 24-week open-label study. Dement Geriatr Cogn Disord. 2007;23(6):401-405.
d. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
e. Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317-324.
f. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
g. Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969-977.
h. Ridha BH, Josephs KA, Rossor MN. Delusions and hallucinations in dementia with Lewy bodies: worsening with memantine. Neurology. 2005;65(3):481-482.
i. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
j. Fernandez HH, Wu CK, Ott BR. Pharmacotherapy of dementia with Lewy bodies. Expert Opin Pharmacother. 2003;4(11):2027-2037.
k. Swartz M, Barak Y, Mirecki I, et al. Treating depression in Alzheimer’s disease: integration of differing guidelines. Int Psychogeriatr. 2000;12(3):353-358.
l. Takahashi S, Mizukami K, Yasuno F, et al. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics. 2009;9(2):56-61.
Related Resources
- Hanyu H, Sato T, Hirao K, et al. Differences in clinical course between dementia with Lewy bodies and Alzheimer’s disease. Eur J Neurol. 2009;16(2):212-217.
- Walker Z, McKeith I, Rodda J, et al. Comparison of cognitive decline between dementia with Lewy bodies and Alzheimer’s disease: a cohort study. BMJ Open. 2012;2:e000380.
Drug Brand Names
- Aripiprazole • Abilify
- Clonazepam • Klonopin
- Donepezil • Aricept
- Galantamine • Razadyne, Reminyl
- Levodopa • Dopar, Larodopa
- Memantine • Namenda
- Quetiapine • Seroquel
- Rivastigmine • Exelon
Disclosure
Drs. Bishnoi and Manepalli report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Grossberg serves as a consultant to Forest, Janssen, Novartis, and Pfizer. His department receives research funding from Novartis, Janssen, and Pfizer.
Discuss this article at www.facebook.com/CurrentPsychiatry
Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB) are the first and second most common causes of neurodegenerative dementia, respectively.“New Alzheimer’s disease guidelines: Implications for clinicians,” Current Psychiatry, March 2012, p. 15-20; http://bit.ly/UNYikk.
The 2005 report of the DLB Consortium5 recognizes central, core, suggestive, and supportive features of DLB (Table 1).5,10 These features are considered in the context of other confounding clinical conditions and the timing of cognitive and motor symptoms. The revised DLB criteria5 require a central feature of progressive cognitive decline. “Probable DLB” is when a patient presents with 2 core features or 1 core feature and ≥1 suggestive features. A diagnosis of “possible DLB” requires 1 core feature or 1 suggestive feature in the presence of progressive cognitive decline.
Table 1
Diagnostic criteria for AD and DLB
| NIA-AA criteria for AD (2011)10 |
| Possible AD: Clinical and cognitive criteria (DSM-IV-TR) for AD are met and there is an absence of biomarkers to support the diagnosis or there is evidence of a secondary disorder that can cause dementia |
| Probable AD: Clinical and cognitive criteria for AD are met and there is documented progressive cognitive decline or abnormal biomarker(s) suggestive of AD or evidence of proven AD autosomal dominant genetic mutation (presenilin-1, presenilin-2, amyloid-β precursor protein) |
| Definite AD: Clinical criteria for probable AD are met and there is histopathologic evidence of the disorder |
| Revised clinical diagnostic criteria for DLB (2005)5 |
| Core features: Fluctuating cognition, recurrent visual hallucinations, soft motor features of parkinsonism |
| Suggestive features: REM sleep behavior disorder, severe antipsychotic sensitivity, decreased tracer uptake in striatum on SPECT dopamine transporter imaging or on myocardial scintigraphy with MIBG |
| Supportive features (common but lacking diagnostic specificity): repeated falls and syncope; transient, unexplained loss of consciousness; systematized delusions; hallucinations other than visual; relative preservation of medial temporal lobe on CT or MRI scan; decreased tracer uptake on SPECT or PET imaging in occipital regions; prominent slow waves on EEG with temporal lobe transient sharp waves |
| AD: Alzheimer’s disease; DLB: dementia with Lewy bodies; MIBG: metaiodobenzylguanidine; NIA-AA: National Institute on Aging and the Alzheimer’s Association; PET: positron emission tomography; REM: rapid eye movement; SPECT: single photon emission computed tomography |
Biomarkers for AD, but not DLB
The 2011 diagnostic criteria for AD incorporate biomarkers that can be measured in vivo and reflect speci?c features of disease-related pathophysiologic processes. Biomarkers for AD are divided into 2 categories:11
- amyloid-beta (Aβ) accumulation: abnormal tracer retention on amyloid positron emission topography (PET) imaging and low cerebrospinal fluid (CSF) Aβ42
- neuronal degeneration or injury: elevated CSF tau (total and phosphorylated tau), decreased ?uorodeoxyglucose uptake on PET in temporo-parietal cortices, and atrophy on structural MRI in the hippocampal and temporo-parietal regions.
No clinically applicable genotypic or CSF markers exist to support a DLB diagnosis, but there are many promising candidates, including elevated levels of CSF p-tau 181, CSF levels of alpha- and beta-synuclein,12 and CSF beta-glucocerebrosidase levels.13 PET mapping of brain acetylcholinesterase activity,14 123I-2β-carbomethoxy-3β- (4-iodophenyl)-N-(3-fluoropropyl)nortropane single photon emission computed tomography (SPECT) dopamine transporter (DaT) imaging15 and metaiodobenzylguanidine (MIBG) scintigraphy also are promising methods. DaT scan SPECT is FDA-approved for detecting loss of functional dopaminergic neuron terminals in the striatum and can differentiate between AD and DLB with a sensitivity and specificity of 78% to 88% and 94% to 100%, respectively.16 This test is covered by Medicare for differentiating AD and DLB.
Differences in presentation
Cognitive impairment. Contrary to the early memory impairment that characterizes AD, memory deficits in DLB usually appear later in the disease course.5 Patients with DLB manifest greater attentional, visuospatial, and executive impairments than those with AD, whereas AD causes more profound episodic (declarative) memory impairment than DLB. DLB patients show more preserved consolidation and storage of verbal information than AD patients because of less neuroanatomical and cholinergic compromise in the medial temporal lobe. There is no evidence of significant differences in remote memory, semantic memory, and language (naming and fluency).
Compromised attention in DLB may be the basis for fluctuating cognition, a characteristic of the disease. The greater attentional impairment and reaction time variability in DLB compared with AD is evident during complex tasks for attention and may be a function of the executive and visuospatial demands of the tasks.17
Executive functions critical to adaptive, goal-directed behavior are more impaired in DLB than AD. DLB patients are more susceptible to distraction and have difficulty engaging in a task and shifting from 1 task to another. This, together with a tendency for confabulation and perseveration, are signs of executive dysfunction.
Neuropsychiatric features. DLB patients are more likely than AD patients to exhibit psychiatric symptoms and have more functional impairment.18 In an analysis of autopsy-confirmed cases, hallucinations and delusions were more frequent with Lewy body pathology (75%) than AD (21%) at initial clinical evaluation.18 By the end stages of both illnesses, the degree of psychotic symptoms is comparable.19 Depression is common in DLB; whether base rates of depressed mood and major depression differ between DLB and AD is uncertain.20
Psychosis in AD can be induced by medication or delirium, or triggered by poor sensory perceptions. Psychotic symptoms occur more frequently during the moderate and advanced stages of AD, when patients present with visual hallucinations, delusions, or delusional misidentifications. As many as 10% to 20% of patients with AD experience hallucinations, typically visual. Delusions occur in 30% to 50% of AD patients, usually in the later stages of the disease. The most common delusional themes are infidelity, theft, and paranoia. Female sex is a risk factor for psychosis in AD. Delusions co-occur with aggression, anxiety, and aberrant motor behavior.
Visual hallucinations—mostly vivid, well-formed, false perceptions of insects, animals, or people—are the defining feature of DLB.21 Many patients recognize that they are experiencing visual hallucinations and can ignore them. DLB patients also may experience visual illusions, such as misperceiving household objects as living beings. Delusions—typically paranoid—are common among DLB patients, as are depression and anxiety.1 Agitation or aggressive behavior tends to occur late in the illness, if at all.
The causes of psychotic symptoms in DLB are not fully understood, but dopamine dysfunction likely is involved in hallucinations, delusions, and agitation, and serotonin dysfunction may be associated with depression and anxiety. Rapid eye movement (REM) sleep/wakefulness dysregulation, in which the dream imagery of REM sleep may occur during wakefulness, also has been proposed as a mechanism for visual hallucinations in DLB.22 In DLB, psychotic symptoms occur early and are a hallmark of this illness, whereas in AD they usually occur in the middle to late stages of the disease.
Motor symptoms. In AD, extrapyramidal symptoms (EPS) are common later in the disease, are strongly correlated with disease severity, and are a strong, independent predictor of depression severity.23 EPS are more common in DLB than in AD24 and DLB patients are at higher risk of developing EPS even with low doses of typical antipsychotics, compared with AD patients.25
Other symptoms. REM sleep behavior disorder (RBD) is characterized by enacting dreams—often violent—during REM sleep. RBD is common in DLB and many patients also have excessive daytime somnolence. Other sleep disorders in DLB include insomnia, obstructive sleep apnea, central sleep apnea, restless legs syndrome, and periodic limb movements during sleep.
In AD patients, common sleep behaviors include confusion in the early evening (“sundowning”) and frequent nighttime awakenings, often accompanied by wandering.26 Orthostatic hypotension, impotence, urinary incontinence, and constipation are common in DLB. Lack of insight concerning personal cognitive, mood, and behavioral state is highly prevalent in AD patients and more common than in DLB.
Diagnostic evaluation
Because there are no definitive clinical markers for DLB, diagnosis is based on a detailed clinical and family history from the patient and a reliable informant, as well as a physical, neurologic, and mental status examination that looks for associated noncognitive symptoms, and neuropsychological evaluation. Reasons DLB may be misdiagnosed include:
- Some “core” clinical features of DLB may not appear or may overlap with AD.
- Presence and severity of concurrent AD pathology in DLB may modify the clinical presentation, with decreased rates of hallucinations and parkinsonism and increased neurofibrillary tangles.
- Failure to reliably identify fluctuations—variations in cognition and arousal, such as periods of unresponsiveness while awake (“zoning out”), excessive daytime somnolence, and disorganized speech.27
Detecting and characterizing cognitive deficits in dementia patients using neuropsychological testing is important in establishing a clinical diagnosis, determining baseline levels of impairment, forming a prognosis, and initiating disease-specific treatments. Differences in neuropsychological findings in AD and DLB are outlined in Table 2.16,28-33 Several studies have suggested using these measures to differentiate patients with DLB from those with AD.20
Table 2
Diagnostic testing for Alzheimer’s disease and dementia with Lewy bodies
| Alzheimer’s disease | Dementia with Lewy bodies |
|---|---|
| Neuropsychological testing findings | |
| Relatively more impairment on verbal memory tasks, delayed recall, delayed recognition, and encoding and storing information.28 Dysfunction of episodic memory function | Relatively more impairment on attention or concentration, verbal fluency, visuoperceptual, visuoconstructive, visual memory tests, and frontal executive functions.28 Relatively preserved confrontation naming and verbal memory |
| MRI findings | |
| Diffuse cortical atrophy, relatively greater volume loss in hippocampus and medial temporal lobe structures (strong correlation with severity)29 | Mild generalized cerebral cortical atrophy with minimal hippocampal atrophy and relative preservation of medial temporal lobe structures30 |
| [18F]FDG PET | |
| Widespread metabolic deficits in neocortical association areas, with sparing of the basal ganglia, thalamus, cerebellum, primary sensory motor cortex, and visual cortex | Widespread cortical hypometabolism, more marked in primary visual and occipital association areas, and less severe in parietal, frontal, and anterior cingulate cortices.31 Severe cholinergic deafferentation of the neocortex, particularly in posterior cortical regions32 |
| Single photon emission computed tomography | |
| Parietotemporal hypoperfusion | Occipital hypoperfusion |
| 123I-FP-CIT SPECT (DaT scan) | |
| No significant loss of DaT | Low nigrostriatal terminal density of DaT caused by severe nigrostriatal degeneration16 |
| Myocardial scintigraphy with MIBG | |
| No significant change in MIBG uptake | Decreased MIBG uptake33 |
| 123I-FP-CIT: 123I-2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)nortropane; DaT: dopamine transporter; FDG PET: [18F]-fluoro-d-glucose positron emission tomography; MIBG: metaiodobenzylguanidine; SPECT: single photon emission computed tomography | |
Evidence is insufficient to support using electroencephalographic and polysomnographic studies when initially evaluating patients with dementia. Brain CT or MRI are recommended as part of the initial evaluation of dementia patients to exclude treatable causes of dementia and help clarify the differential diagnosis. Occipital hypometabolism and hypoperfusion demonstrated on PET and SPECT imaging have high sensitivity and specificity for differentiating AD from DLB.
To diagnose DLB more consistently, look for core features of the disease, RBD, antipsychotic hypersensitivity, and decreased striatal binding at presynaptic DaT sites.15 Abnormal (low binding) DaT activity is the most reliable diagnostic marker for DLB.34 Myocardial scintigraphy with MIBG is sensitive to pathologic changes of DLB before clinical expression and could overcome the difficulties of using clinical criteria alone to identify patients with DLB.35 MIBG scintigraphy may be preferred to DaT scan because it is less expensive and its sensitivity and specificity to DLB are independent of the presence of parkinsonism.35
For an overview of pharmacotherapy options for patients with AD or DLB, see Box 2.
Pharmacotherapy options for patients with Alzheimer’s disease (AD) or dementia with Lewy bodies (DLB) include cholinesterase inhibitors, memantine, antipsychotics, and other agents.
Cholinesterase inhibitors. Donepezil, rivastigmine, and galantamine are FDA-approved for treating AD. Their efficacy appears to be similar, so the choice of agent is based largely on cost, patient tolerability, and physician experience.
No medications are FDA-approved for treating DLB. Neocortical cholinergic activity assessed by choline acetyltransferase levels is more severely depleted in DLB than in AD, and this deficit is correlated with the presence of visual hallucinations and global severity of cognitive impairment.a Therefore, drugs that enhance central cholinergic function offer a therapeutic approach for DLB; cognitive and hallucinatory symptoms are the anticipated targets. Multiple anecdotal reports, open-label studies,b,c and 1 randomized, placebo-controlled triald suggest that cholinesterase inhibitors are efficacious in DLB, with reported benefits in cognition, fluctuations, psychotic symptoms, and parkinsonian symptoms. A 20-week randomized, double-blind, placebo-controlled multicenter studyd of patients with DLB found rivastigmine, 6 to 12 mg/d, was superior to placebo. Patients receiving rivastigmine exhibited significantly reduced anxiety, delusions, and hallucinations and significantly better performance on a computerized battery of neuropsychological tests, especially tasks that required sustained attention. Differences between rivastigmine and placebo disappeared after drug discontinuation.
Memantine is a noncompetitive antagonist of N-methyl-d-aspartate receptors that is effective in AD.e The possible involvement of glutamate in DLB has provided a rationale for treating DLB with memantine. Two randomized controlled trials in DLB found that patients treated with memantine for 24 weeks performed better on Clinical Global Impression of Change, but not on most other secondary outcome measures.f,g In both studies, memantine was well tolerated. However, other studies have noted worsening of delusions and hallucinations with memantine in DLB patients.h
Antipsychotics. Agitation is common in moderate and advanced AD. Atypical antipsychotics have been used with variable efficacy to treat agitation, but their use is associated with excess mortality. DLB patients pose a considerable therapeutic challenge because antipsychotics—the mainstay of treatment of psychosis and behavioral problems in most other disorders—can provoke severe, irreversible, and often fatal sensitivity reactions in this type of dementia.i A 2- to 3-fold increased mortality risk associated with antipsychotic sensitivity reactions in DLB is partly mediated via acute blockade of postsynaptic dopamine D2 receptors in the striatum. For severe and disabling psychosis, a trial of a cholinesterase inhibitor and/or lowering the dose of antiparkinsonian medication should be considered first. In urgent situations, small doses of an atypical antipsychotic that is least associated with parkinsonism side effects—such as quetiapine or aripiprazole—should be used.
Other treatments. Treatment of parkinsonian symptoms in DLB patients is similar to that for Parkinson’s disease, but the risk of psychotic symptoms in DLB warrants a conservative approach. Levodopa seems to be more effective than dopamine agonists and produces fewer side effects.j Rapid eye movement sleep behavior disorder often responds to low doses of clonazepam (0.25 to 1.5 mg). Depression and anxiety disorders are common in AD at all stages and their treatment is not fundamentally different than in geriatric patients without dementia. Selective serotonin reuptake inhibitors and electroconvulsive therapy have been used successfully in depressed patients with AD or DLB.k,l
Disease-modifying treatments. Researchers are evaluating an array of antiamyloid and neuroprotective therapeutic approaches for AD based on the hypothesis that amyloid-beta protein plays a pivotal role in disease onset and progression. Interventions that reduce amyloid production, limit aggregation, or increase clearance may block the cascade of events comprising AD pathogenesis. Reducing tau hyperphosphorylation, limiting oxidation and excitotoxicity, and controlling inflammation also may be beneficial strategies. Potentially neuroprotective and restorative treatments such as neurotrophins, neurotrophic factor enhancers, and stem cell-related approaches also are being investigated.
There are no large-scale studies of disease-modifying treatments for DLB. Potential areas of research include the relationship between proteasome function and a-synuclein pathology, the role of beta-synuclein, and the impact of alterations to alpha-synuclein on its propensity to aggregate.
References
a. Ballard C, Ziabreva I, Perry R, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67(11):1931-1934.
b. Beversdorf DQ, Warner JL, Davis RA, et al. Donepezil in the treatment of dementia with lewy bodies. Am J Geriatr Psychiatry. 2004;12(5):542-544.
c. Edwards K, Royall D, Hershey L, et al. Efficacy and safety of galantamine in patients with dementia with Lewy bodies: a 24-week open-label study. Dement Geriatr Cogn Disord. 2007;23(6):401-405.
d. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
e. Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317-324.
f. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
g. Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969-977.
h. Ridha BH, Josephs KA, Rossor MN. Delusions and hallucinations in dementia with Lewy bodies: worsening with memantine. Neurology. 2005;65(3):481-482.
i. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
j. Fernandez HH, Wu CK, Ott BR. Pharmacotherapy of dementia with Lewy bodies. Expert Opin Pharmacother. 2003;4(11):2027-2037.
k. Swartz M, Barak Y, Mirecki I, et al. Treating depression in Alzheimer’s disease: integration of differing guidelines. Int Psychogeriatr. 2000;12(3):353-358.
l. Takahashi S, Mizukami K, Yasuno F, et al. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics. 2009;9(2):56-61.
Related Resources
- Hanyu H, Sato T, Hirao K, et al. Differences in clinical course between dementia with Lewy bodies and Alzheimer’s disease. Eur J Neurol. 2009;16(2):212-217.
- Walker Z, McKeith I, Rodda J, et al. Comparison of cognitive decline between dementia with Lewy bodies and Alzheimer’s disease: a cohort study. BMJ Open. 2012;2:e000380.
Drug Brand Names
- Aripiprazole • Abilify
- Clonazepam • Klonopin
- Donepezil • Aricept
- Galantamine • Razadyne, Reminyl
- Levodopa • Dopar, Larodopa
- Memantine • Namenda
- Quetiapine • Seroquel
- Rivastigmine • Exelon
Disclosure
Drs. Bishnoi and Manepalli report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Grossberg serves as a consultant to Forest, Janssen, Novartis, and Pfizer. His department receives research funding from Novartis, Janssen, and Pfizer.
1. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113-1124.
2. Buracchio T, Arvanitakis Z, Gorbien M. Dementia with Lewy bodies: current concepts. Dement Geriatr Cogn Disord. 2005;20(5):306-320.
3. Fujishiro H, Iseki E, Higashi S, et al. Distribution of cerebral amyloid deposition and its relevance to clinical phenotype in Lewy body dementia. Neurosci Lett. 2010;486(1):19-23.
4. Kosaka K. Diffuse Lewy body disease. Neuropathology. 2000;20(suppl):S73-S78.
5. McKeith IG, Dickson DW, Lowe J, et al. Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872.
6. Cummings JL, Cole G. Alzheimer disease. JAMA. 2002;287(18):2335-2338.
7. Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing. 2005;34(6):561-566.
8. Bradshaw J, Saling M, Hopwood M, et al. Fluctuating cognition in dementia with Lewy bodies and Alzheimer’s disease is qualitatively distinct. J Neurol Neurosurg Psychiatry. 2004;75(3):382-387.
9. Singleton AB, Wharton A, O’Brien KK, et al. Clinical and neuropathological correlates of apolipoprotein E genotype in dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2002;14(4):167-175.
10. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
11. Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257-262.
12. Mollenhauer B, Cullen V, Kahn I, et al. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol. 2008;213(2):315-325.
13. Parnetti L, Balducci C, Pierguidi L, et al. Cerebrospinal fluid beta-glucocerebrosidase activity is reduced in dementia with Lewy bodies. Neurobiol Dis. 2009;34(3):484-486.
14. Shimada H, Hirano S, Shinotoh H, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology. 2009;73(4):273-278.
15. McKeith I, O’Brien J, Walker Z, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6(4):305-313.
16. Walker Z, Jaros E, Walker RW, et al. Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Psychiatry. 2007;78(11):1176-1181.
17. Bradshaw JM, Saling M, Anderson V, et al. Higher cortical deficits influence attentional processing in dementia with Lewy bodies, relative to patients with dementia of the Alzheimer’s type and controls. J Neurol Neurosurg Psychiatry. 2006;77(10):1129-1135.
18. Weiner MF, Hynan LS, Parikh B, et al. Can Alzheimer’s disease and dementias with Lewy bodies be distinguished clinically? J Geriatr Psychiatry Neurol. 2003;16(4):245-250.
19. Stavitsky K, Brickman AM, Scarmeas N, et al. The progression of cognition, psychiatric symptoms, and functional abilities in dementia with Lewy bodies and Alzheimer disease. Arch Neurol. 2006;63(10):1450-1456.
20. Ferman TJ, Smith GE, Boeve BF, et al. Neuropsychological differentiation of dementia with Lewy bodies from normal aging and Alzheimer’s disease. Clin Neuropsychol. 2006;20(4):623-636.
21. McKeith IG, Perry EK, Perry RH. Report of the second dementia with Lewy body international workshop: diagnosis and treatment. Consortium on Dementia with Lewy Bodies. Neurology. 1999;53(5):902-905.
22. Boeve BF, Silber MH, Ferman TJ, et al. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16(4):622-630.
23. Portet F, Scarmeas N, Cosentino S, et al. Extrapyramidal signs before and after diagnosis of incident Alzheimer disease in a prospective population study. Arch Neurol. 2009;66(9):1120-1126.
24. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
25. Tarawneh R, Galvin JE. Distinguishing Lewy body dementias from Alzheimer’s disease. Expert Rev Neurother. 2007;7(11):1499-1516.
26. Ancoli-Israel S, Klauber MR, Gillin JC, et al. Sleep in non-institutionalized Alzheimer’s disease patients. Aging (Milano). 1994;6(6):451-458.
27. Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology. 2004;62(2):181-187.
28. Salmon DP, Galasko D, Hansen LA, et al. Neuropsychological deficits associated with diffuse Lewy body disease. Brain Cogn. 1996;31(2):148-165.
29. Jack CR, Jr, Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55(4):484-489.
30. Burton EJ, Barber R, Mukaetova-Ladinska EB, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain. 2009;132(pt 1):195-203.
31. Ishii K, Soma T, Kono AK, et al. Comparison of regional brain volume and glucose metabolism between patients with mild dementia with lewy bodies and those with mild Alzheimer’s disease. J Nucl Med. 2007;48(5):704-711.
32. Klein JC, Eggers C, Kalbe E, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74(11):885-892.
33. Fujishiro H, Nakamura S, Kitazawa M, et al. Early detection of dementia with Lewy bodies in patients with amnestic mild cognitive impairment using 123I-MIBG cardiac scintigraphy. J Neurol Sci. 2012;315(1-2):115-119.
34. O’Brien JT, McKeith IG, Walker Z, et al. Diagnostic accuracy of 123I-FP-CIT SPECT in possible dementia with Lewy bodies. Br J Psychiatry. 2009;194:34-39.
35. Yoshita M, Taki J, Yokoyama K, et al. Value of 123I-MIBG radioactivity in the differential diagnosis of DLB from AD. Neurology. 2006;66(12):1850-1854.
1. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113-1124.
2. Buracchio T, Arvanitakis Z, Gorbien M. Dementia with Lewy bodies: current concepts. Dement Geriatr Cogn Disord. 2005;20(5):306-320.
3. Fujishiro H, Iseki E, Higashi S, et al. Distribution of cerebral amyloid deposition and its relevance to clinical phenotype in Lewy body dementia. Neurosci Lett. 2010;486(1):19-23.
4. Kosaka K. Diffuse Lewy body disease. Neuropathology. 2000;20(suppl):S73-S78.
5. McKeith IG, Dickson DW, Lowe J, et al. Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872.
6. Cummings JL, Cole G. Alzheimer disease. JAMA. 2002;287(18):2335-2338.
7. Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing. 2005;34(6):561-566.
8. Bradshaw J, Saling M, Hopwood M, et al. Fluctuating cognition in dementia with Lewy bodies and Alzheimer’s disease is qualitatively distinct. J Neurol Neurosurg Psychiatry. 2004;75(3):382-387.
9. Singleton AB, Wharton A, O’Brien KK, et al. Clinical and neuropathological correlates of apolipoprotein E genotype in dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2002;14(4):167-175.
10. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
11. Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257-262.
12. Mollenhauer B, Cullen V, Kahn I, et al. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol. 2008;213(2):315-325.
13. Parnetti L, Balducci C, Pierguidi L, et al. Cerebrospinal fluid beta-glucocerebrosidase activity is reduced in dementia with Lewy bodies. Neurobiol Dis. 2009;34(3):484-486.
14. Shimada H, Hirano S, Shinotoh H, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology. 2009;73(4):273-278.
15. McKeith I, O’Brien J, Walker Z, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6(4):305-313.
16. Walker Z, Jaros E, Walker RW, et al. Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Psychiatry. 2007;78(11):1176-1181.
17. Bradshaw JM, Saling M, Anderson V, et al. Higher cortical deficits influence attentional processing in dementia with Lewy bodies, relative to patients with dementia of the Alzheimer’s type and controls. J Neurol Neurosurg Psychiatry. 2006;77(10):1129-1135.
18. Weiner MF, Hynan LS, Parikh B, et al. Can Alzheimer’s disease and dementias with Lewy bodies be distinguished clinically? J Geriatr Psychiatry Neurol. 2003;16(4):245-250.
19. Stavitsky K, Brickman AM, Scarmeas N, et al. The progression of cognition, psychiatric symptoms, and functional abilities in dementia with Lewy bodies and Alzheimer disease. Arch Neurol. 2006;63(10):1450-1456.
20. Ferman TJ, Smith GE, Boeve BF, et al. Neuropsychological differentiation of dementia with Lewy bodies from normal aging and Alzheimer’s disease. Clin Neuropsychol. 2006;20(4):623-636.
21. McKeith IG, Perry EK, Perry RH. Report of the second dementia with Lewy body international workshop: diagnosis and treatment. Consortium on Dementia with Lewy Bodies. Neurology. 1999;53(5):902-905.
22. Boeve BF, Silber MH, Ferman TJ, et al. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16(4):622-630.
23. Portet F, Scarmeas N, Cosentino S, et al. Extrapyramidal signs before and after diagnosis of incident Alzheimer disease in a prospective population study. Arch Neurol. 2009;66(9):1120-1126.
24. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
25. Tarawneh R, Galvin JE. Distinguishing Lewy body dementias from Alzheimer’s disease. Expert Rev Neurother. 2007;7(11):1499-1516.
26. Ancoli-Israel S, Klauber MR, Gillin JC, et al. Sleep in non-institutionalized Alzheimer’s disease patients. Aging (Milano). 1994;6(6):451-458.
27. Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology. 2004;62(2):181-187.
28. Salmon DP, Galasko D, Hansen LA, et al. Neuropsychological deficits associated with diffuse Lewy body disease. Brain Cogn. 1996;31(2):148-165.
29. Jack CR, Jr, Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55(4):484-489.
30. Burton EJ, Barber R, Mukaetova-Ladinska EB, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain. 2009;132(pt 1):195-203.
31. Ishii K, Soma T, Kono AK, et al. Comparison of regional brain volume and glucose metabolism between patients with mild dementia with lewy bodies and those with mild Alzheimer’s disease. J Nucl Med. 2007;48(5):704-711.
32. Klein JC, Eggers C, Kalbe E, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74(11):885-892.
33. Fujishiro H, Nakamura S, Kitazawa M, et al. Early detection of dementia with Lewy bodies in patients with amnestic mild cognitive impairment using 123I-MIBG cardiac scintigraphy. J Neurol Sci. 2012;315(1-2):115-119.
34. O’Brien JT, McKeith IG, Walker Z, et al. Diagnostic accuracy of 123I-FP-CIT SPECT in possible dementia with Lewy bodies. Br J Psychiatry. 2009;194:34-39.
35. Yoshita M, Taki J, Yokoyama K, et al. Value of 123I-MIBG radioactivity in the differential diagnosis of DLB from AD. Neurology. 2006;66(12):1850-1854.
