User login
Review of Efficacy and Safety of Spinal Cord Stimulation in Veterans
Lower back pain (LBP) affects an estimated 9.4% of the global population and has resulted in more years lived with disability than any other health condition.1 LBP affects a wide range of populations, but US veterans have been shown to have significantly higher rates of back pain than nonveterans. The National Institutes of Health reports that 65.6% of veterans experience chronic pain; 9.1% of veterans experience severe, chronic pain.2 Chronic back pain is treated by a range of methods, including medications, surgery, physical therapy (PT), patient education, and behavioral therapy.3 However, chronic neuropathic back pain has been shown to have limited responsiveness to medication.4
Neuropathic pain is caused by lesions in the somatosensory nervous system, resulting in spontaneous pain and amplified pain responses to both painful and nonpainful stimuli.5 The most common location for neuropathic pain is the back and legs. Between 10% and 40% of people who undergo lumbosacral spine surgery to treat neuropathic radicular pain will experience further neuropathic pain.6 This condition is referred to as failed back surgery syndrome or postlaminectomy syndrome (PLS). While neuropathic back pain has had limited responsiveness to medication and repeated lumbosacral spine surgery, spinal cord stimulation (SCS) has shown promise as an effective form of pain treatment for those experiencing PLS and other spine disorders.7-10 In addition, SCS therapy has had a very low incidence of complications, which may be on the decline with recent technological advancements.11 Patients with a diagnosis of PLS, LBP, or complex regional pain syndrome (CRPS) who have not responded to medications, therapy, and/or injections for ≥ 6 months were eligible for a trial of SCS therapy. Trial leads were placed via the percutaneous route with the battery strapped to the waistline for 3 to 5 days and were removed in clinic. Patients who experienced > 60% pain relief and functional improvement received a SCS implant.
The effectiveness of SCS has been demonstrated in a nonveteran population, but it has not been studied in a veteran population.12 US Department of Veterans Affairs (VA) health care coverage is different from Medicare and private insurance in that it is classified as a benefit and not insurance. The goals of treatment at the VA may include considerations in addition to feeling better, and patient presentations may not align with those in the private sector.
We hypothesize that SCS is both a safe and beneficial treatment option for veterans with chronic intractable spine and/or extremity pain. The purpose of this study was to determine the efficacy and safety of SCS in a veteran population.
Methods
The efficacy and safety of SCS was determined via a retrospective study. Inclusion criteria for the study consisted of any Southeastern Louisiana Veterans Health Care System (SLVHCS) patient who had an SCS trial and/or implant from 2008 to 2020. Eligible veterans must have had chronic pain for at least 6 months and had previously tried multiple medications, PT, transcutaneous nerve stimulation, facet injections, epidural steroid injections, or surgery without success. For medication therapy to be considered unsuccessful, it must have included acetaminophen, nonsteroidal anti-inflammatory drugs, and ≥ 1 adjuvant medication (gabapentin, duloxetine, amitriptyline, lidocaine, and menthol). A diagnosis of chronic LBP, PLS, cervical or lumbar spondylosis with radiculopathy, complex regional pain syndrome, or chronic pain syndrome was required for eligibility. Patients whose pain decreased by > 60% and had functional improvement in a 3- to 5-day trial received SCS implantation with percutaneous leads by a pain physician or paddle lead by a neurosurgeon.
The SLVHCS Institutional Review Board approved this study. Electronic health records were reviewed to determine patient age, anthropometric data, and date of SCS implantation. Patients were then called and interviewed to complete a survey. After obtaining verbal consent to the study, subjects were surveyed regarding whether the patient would recommend the procedure to peers, adverse effects (AEs) or complications, and the ability to decrease opiates if applicable. A verbal Pain Outcome Questionnaire (POQ) assessment of activities of daily living also was given during the phone interview regarding pain levels before SCS and at the time of the phone interview.13 (eAppendix available at doi:10.12788/fp.0204) Following the survey, a chart review was performed to corroborate the given AEs or complications and opiate use information. Before and after results of the POQ were compared via a paired sample t test, and P values < .05 were considered significant. Analyses were performed by IBM SPSS, version 26.
The primary outcome measure for this study was whether veterans would recommend SCS to their peers; in our view, this categorical outcome measure seemed to be more valuable to share with future patients who might be candidates for SCS. Since VA health care coverage and goals of treatment may be different from a nonveteran population, we opted to use this primary measure to decrease the possibility of confounding variables.
Secondary outcome measures included changes in POC scores, improvements in activities of daily living, and decreases in use of opioid pain medications.
POQ responses were recorded during the telephone interviews (0 to 10 scale). A paired sample t test was conducted to compare pain levels before and after SCS implant. Pain levels were gathered in the single phone call. Patient opioid usage, if applicable, was assessed by converting medications to morphine milligram equivalent dosing (MMED). Since patients who were on chronic opioids took multiple formulations, we changed the total daily dose to all morphine; for this study, morphine was considered equivalent to hydrocodone, and oxycodone was 1.5x morphine.
Results
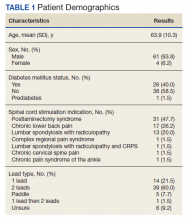
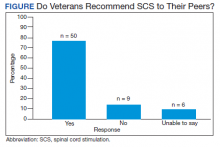
Of the 90 SLVHCS patients who received an SCS implant between 2008 and 2020, 76 were reached by telephone and 65 had their responses recorded in the study. Of the 11 patients who were not included, 5 had the SCS removed; it is unclear whether these veterans would have recommended the treatment. Four were unable to quantify pain and/or SCS effects, and 2 were excluded due to a dementia diagnosis years after the implant. The mean (SD) age of participants was 63.9 (10.3) years. Forty percent of patients had a diabetes mellitus diagnosis and 1 had prediabetes. Patients’ most common qualifying diagnosis for SCS was PLS (47.7%) followed by chronic LBP (26.2%). A percutaneous 2-lead technique was the most common type of SCS type used (60.0%) followed by 1-lead (21.5%). The most common SCS manufacturer was Boston Scientific (87.7%)(Table 1). Most veterans (76.9%) recommended SCS to their peers; 13.8% did not recommend SCS; 9.2% were undecided and stated that they were unable to recommend because they did not want to persuade a peer to get SCS (Figure).
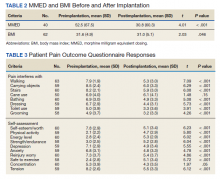
There was a statistically significant decrease in opioid use for the 40 veterans for whom pain medication was converted (P < .001)(Table 2). Six patients reported using opioids at some point but could not remember their dose, and no records were found in their chart review, so they were not included in the MMED analysis. In that group, 4 patients reported using opioids before SCS but discontinued the opioid use after SCS implantation, and 2 patients noted using opioids before SCS and concomitantly. Eighteen subjects reported no opioid use at any point before or after SCS (Table 3).
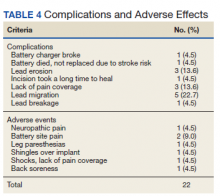
There were few life-threatening complications of SCS. Three veterans developed skin dehiscence; 2 had dehiscence at the battery/generator site, and 1 had dehiscence at the lead anchor site. Two patients with dehiscence also had morbid obesity, and the third had postoperative malnourishment. The dehiscence occurred 3 and 8 months postoperation. All 3 patients with dehiscence had the SCS explanted, though they were eager to get a new SCS implanted as soon as possible because SCS was their most successful treatment to date.
Twenty of the 64 veterans surveyed reported other complications of SCS, including lead migration, lack of pain coverage, paresthesia and numbness, soreness around generator site, SCS shocking patient when performing full thoracic spine flexion, and shingles at the battery site (Table 4). There were 11 explants among the 76 veterans contacted. The primary reason for explant was lack of pain coverage.
Patient concerns included pain with sitting in chairs due to tenderness around the implant, SCS helping with physical pain but not mental pain, SCS only working during the day and not helping with sleep, and patients lacking education regarding possible complications of SCS.
Discussion
In this nonrandomized retrospective review, SCS was shown to be an effective treatment for intractable spine and/or extremity pain. Veterans’ pain levels were significantly reduced following SCS implantation, and more than three-fourths of veterans recommended SCS to their peers. We used the recommendation of SCS to peers as the most important metric regarding the effectiveness of SCS, as this measure was felt to be more valuable to share with future patients; furthermore, categorical analysis has been shown to be more valuable than ordinal pain scales to measure pain.14 In addition to wanting to expand the available research to the general public, we wanted a measure that we could easily relay to our patient population regarding SCS.
The explant rate of 14.5% among surveyed veterans falls at the higher end of the normal ranges found in previous studies of long-term SCS outcomes.15-17 One possible reason for the higher rate is that we did not differentiate based on the reason for the explant (ie, no benefit, further surgery needed for underlying medical condition, or SCS-specific complications). Another possible contributing factor to the higher than expected explant rate is the geographic location in the New Orleans metro area; New Orleans is considered to have one of the highest rates of obesity in the United States and obesity typically has other diseases associated with it such as hypertension and diabetes mellitus.
Limitations
Limitations of the study include the relatively low number of subjects, subjective nature of the interview questions, and the patients’ answers. Typically the POQ has been used as a prospective assessment of pain; whether it is valid in a retrospective analysis is not clear. While there was a statistically significant decrease of opioid use after getting SCS, this study can only show correlation, not causation. During the study period, there has been a drastic change in opioid prescribing patterns and efforts to decrease the amount of opioids prescribed.
Subjects also were asked to rate their pain and quality of life before SCS. Some subjects had SCS implantation up to 10 years prior to the phone interview. The variable amount of time between SCS implantation and interview likely affected subjects’ responses. Chronic pain is a moving target. Patients have good days and bad days that would likely change opinions on SCS benefits on a single phone interview. Some patients needed battery replacements at the time of the interview (battery life averaged about 3 to 5 years in our study population) and were asked to report current levels of pain from the perspective of when their batteries were still functional, further affecting results.
Conclusions
SCS was shown to improve the quality of life of US veterans at SLVHCS across a wide variety of metrics, including activities of daily living, as well as mental and physical health. For veterans with chronic intractable pain who have tried and failed more conservative treatments, SCS is a great treatment.
1. Hoy DG, Smith E, Cross M, et al. The global burden of musculoskeletal conditions for 2010: an overview of methods. Ann Rheum Dis. 2014;73(6):982-989 doi:10.1136/annrheumdis-2013-204344
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
4. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. doi:10.1016/S1474-4422(14)70251-0
5. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1-32. doi:10.1146/annurev.neuro.051508.135531
6. Wilkinson HA. The Failed Back Syndrome: Etiology and Therapy. 2nd ed. Harper & Row; 1991.
7. Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132(1-2):179-188. doi:10.1016/j.pain.2007.07.028
8. North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98-107. doi:10.1227/01.neu.0000144839.65524.e0
9. Geurts JW, Smits H, Kemler MA, Brunner F, Kessels AG, van Kleef M. Spinal cord stimulation for complex regional pain syndrome type I: a prospective cohort study with long-term follow-up. Neuromodulation. 2013;16(6):523-529. doi:10.1111/ner.12024
10. Kumar K, Rizvi S, Bnurs SB. Spinal cord stimulation is effective in management of complex regional pain syndrome I: fact or fiction. Neurosurgery. 2011;69(3):566-5580. doi:10.1227/NEU.0b013e3182181e60
11. Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract. 2011;11(2):148-153. doi:10.1111/j.1533-2500.2010.00407.x
12. Veizi E, Hayek SM, North J, et al. Spinal cord stimulation (SCS) with anatomically guided (3D) neural targeting shows superior chronic axial low back pain relief compared to traditional SCS-LUMINA Study. Pain Med. 2017;18(8):1534-1548. doi:10.1093/pm/pnw286
13. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172-1186. doi:10.1016/j.jpain.2010.02.012
14. Kennedy DJ, Schneider B. Lies, damn lies, and statistic: a commentary. Pain Med. 2020;21(10):2052-2054. doi:10.1093/pm/pnaa287
15. Van Buyten JP, Wille F, Smet I, et al. Therapy-related explants after spinal cord stimulation: results of an international retrospective chart review study. Neuromodulation. 2017;20(7):642-649. doi:10.1111/ner.12642
16. Hayek SM, Veizi E, Hanes M. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center database. Neuromodulation. 2015;18(7):603-609. doi:10.1111/ner.12312
17. Pope JE, Deer TR, Falowski S, et al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation. 2017;20(6):543-552. doi:10.1111/ner.12634
Lower back pain (LBP) affects an estimated 9.4% of the global population and has resulted in more years lived with disability than any other health condition.1 LBP affects a wide range of populations, but US veterans have been shown to have significantly higher rates of back pain than nonveterans. The National Institutes of Health reports that 65.6% of veterans experience chronic pain; 9.1% of veterans experience severe, chronic pain.2 Chronic back pain is treated by a range of methods, including medications, surgery, physical therapy (PT), patient education, and behavioral therapy.3 However, chronic neuropathic back pain has been shown to have limited responsiveness to medication.4
Neuropathic pain is caused by lesions in the somatosensory nervous system, resulting in spontaneous pain and amplified pain responses to both painful and nonpainful stimuli.5 The most common location for neuropathic pain is the back and legs. Between 10% and 40% of people who undergo lumbosacral spine surgery to treat neuropathic radicular pain will experience further neuropathic pain.6 This condition is referred to as failed back surgery syndrome or postlaminectomy syndrome (PLS). While neuropathic back pain has had limited responsiveness to medication and repeated lumbosacral spine surgery, spinal cord stimulation (SCS) has shown promise as an effective form of pain treatment for those experiencing PLS and other spine disorders.7-10 In addition, SCS therapy has had a very low incidence of complications, which may be on the decline with recent technological advancements.11 Patients with a diagnosis of PLS, LBP, or complex regional pain syndrome (CRPS) who have not responded to medications, therapy, and/or injections for ≥ 6 months were eligible for a trial of SCS therapy. Trial leads were placed via the percutaneous route with the battery strapped to the waistline for 3 to 5 days and were removed in clinic. Patients who experienced > 60% pain relief and functional improvement received a SCS implant.
The effectiveness of SCS has been demonstrated in a nonveteran population, but it has not been studied in a veteran population.12 US Department of Veterans Affairs (VA) health care coverage is different from Medicare and private insurance in that it is classified as a benefit and not insurance. The goals of treatment at the VA may include considerations in addition to feeling better, and patient presentations may not align with those in the private sector.
We hypothesize that SCS is both a safe and beneficial treatment option for veterans with chronic intractable spine and/or extremity pain. The purpose of this study was to determine the efficacy and safety of SCS in a veteran population.
Methods
The efficacy and safety of SCS was determined via a retrospective study. Inclusion criteria for the study consisted of any Southeastern Louisiana Veterans Health Care System (SLVHCS) patient who had an SCS trial and/or implant from 2008 to 2020. Eligible veterans must have had chronic pain for at least 6 months and had previously tried multiple medications, PT, transcutaneous nerve stimulation, facet injections, epidural steroid injections, or surgery without success. For medication therapy to be considered unsuccessful, it must have included acetaminophen, nonsteroidal anti-inflammatory drugs, and ≥ 1 adjuvant medication (gabapentin, duloxetine, amitriptyline, lidocaine, and menthol). A diagnosis of chronic LBP, PLS, cervical or lumbar spondylosis with radiculopathy, complex regional pain syndrome, or chronic pain syndrome was required for eligibility. Patients whose pain decreased by > 60% and had functional improvement in a 3- to 5-day trial received SCS implantation with percutaneous leads by a pain physician or paddle lead by a neurosurgeon.
The SLVHCS Institutional Review Board approved this study. Electronic health records were reviewed to determine patient age, anthropometric data, and date of SCS implantation. Patients were then called and interviewed to complete a survey. After obtaining verbal consent to the study, subjects were surveyed regarding whether the patient would recommend the procedure to peers, adverse effects (AEs) or complications, and the ability to decrease opiates if applicable. A verbal Pain Outcome Questionnaire (POQ) assessment of activities of daily living also was given during the phone interview regarding pain levels before SCS and at the time of the phone interview.13 (eAppendix available at doi:10.12788/fp.0204) Following the survey, a chart review was performed to corroborate the given AEs or complications and opiate use information. Before and after results of the POQ were compared via a paired sample t test, and P values < .05 were considered significant. Analyses were performed by IBM SPSS, version 26.
The primary outcome measure for this study was whether veterans would recommend SCS to their peers; in our view, this categorical outcome measure seemed to be more valuable to share with future patients who might be candidates for SCS. Since VA health care coverage and goals of treatment may be different from a nonveteran population, we opted to use this primary measure to decrease the possibility of confounding variables.
Secondary outcome measures included changes in POC scores, improvements in activities of daily living, and decreases in use of opioid pain medications.
POQ responses were recorded during the telephone interviews (0 to 10 scale). A paired sample t test was conducted to compare pain levels before and after SCS implant. Pain levels were gathered in the single phone call. Patient opioid usage, if applicable, was assessed by converting medications to morphine milligram equivalent dosing (MMED). Since patients who were on chronic opioids took multiple formulations, we changed the total daily dose to all morphine; for this study, morphine was considered equivalent to hydrocodone, and oxycodone was 1.5x morphine.
Results
Of the 90 SLVHCS patients who received an SCS implant between 2008 and 2020, 76 were reached by telephone and 65 had their responses recorded in the study. Of the 11 patients who were not included, 5 had the SCS removed; it is unclear whether these veterans would have recommended the treatment. Four were unable to quantify pain and/or SCS effects, and 2 were excluded due to a dementia diagnosis years after the implant. The mean (SD) age of participants was 63.9 (10.3) years. Forty percent of patients had a diabetes mellitus diagnosis and 1 had prediabetes. Patients’ most common qualifying diagnosis for SCS was PLS (47.7%) followed by chronic LBP (26.2%). A percutaneous 2-lead technique was the most common type of SCS type used (60.0%) followed by 1-lead (21.5%). The most common SCS manufacturer was Boston Scientific (87.7%)(Table 1). Most veterans (76.9%) recommended SCS to their peers; 13.8% did not recommend SCS; 9.2% were undecided and stated that they were unable to recommend because they did not want to persuade a peer to get SCS (Figure).
There was a statistically significant decrease in opioid use for the 40 veterans for whom pain medication was converted (P < .001)(Table 2). Six patients reported using opioids at some point but could not remember their dose, and no records were found in their chart review, so they were not included in the MMED analysis. In that group, 4 patients reported using opioids before SCS but discontinued the opioid use after SCS implantation, and 2 patients noted using opioids before SCS and concomitantly. Eighteen subjects reported no opioid use at any point before or after SCS (Table 3).
There were few life-threatening complications of SCS. Three veterans developed skin dehiscence; 2 had dehiscence at the battery/generator site, and 1 had dehiscence at the lead anchor site. Two patients with dehiscence also had morbid obesity, and the third had postoperative malnourishment. The dehiscence occurred 3 and 8 months postoperation. All 3 patients with dehiscence had the SCS explanted, though they were eager to get a new SCS implanted as soon as possible because SCS was their most successful treatment to date.
Twenty of the 64 veterans surveyed reported other complications of SCS, including lead migration, lack of pain coverage, paresthesia and numbness, soreness around generator site, SCS shocking patient when performing full thoracic spine flexion, and shingles at the battery site (Table 4). There were 11 explants among the 76 veterans contacted. The primary reason for explant was lack of pain coverage.
Patient concerns included pain with sitting in chairs due to tenderness around the implant, SCS helping with physical pain but not mental pain, SCS only working during the day and not helping with sleep, and patients lacking education regarding possible complications of SCS.
Discussion
In this nonrandomized retrospective review, SCS was shown to be an effective treatment for intractable spine and/or extremity pain. Veterans’ pain levels were significantly reduced following SCS implantation, and more than three-fourths of veterans recommended SCS to their peers. We used the recommendation of SCS to peers as the most important metric regarding the effectiveness of SCS, as this measure was felt to be more valuable to share with future patients; furthermore, categorical analysis has been shown to be more valuable than ordinal pain scales to measure pain.14 In addition to wanting to expand the available research to the general public, we wanted a measure that we could easily relay to our patient population regarding SCS.
The explant rate of 14.5% among surveyed veterans falls at the higher end of the normal ranges found in previous studies of long-term SCS outcomes.15-17 One possible reason for the higher rate is that we did not differentiate based on the reason for the explant (ie, no benefit, further surgery needed for underlying medical condition, or SCS-specific complications). Another possible contributing factor to the higher than expected explant rate is the geographic location in the New Orleans metro area; New Orleans is considered to have one of the highest rates of obesity in the United States and obesity typically has other diseases associated with it such as hypertension and diabetes mellitus.
Limitations
Limitations of the study include the relatively low number of subjects, subjective nature of the interview questions, and the patients’ answers. Typically the POQ has been used as a prospective assessment of pain; whether it is valid in a retrospective analysis is not clear. While there was a statistically significant decrease of opioid use after getting SCS, this study can only show correlation, not causation. During the study period, there has been a drastic change in opioid prescribing patterns and efforts to decrease the amount of opioids prescribed.
Subjects also were asked to rate their pain and quality of life before SCS. Some subjects had SCS implantation up to 10 years prior to the phone interview. The variable amount of time between SCS implantation and interview likely affected subjects’ responses. Chronic pain is a moving target. Patients have good days and bad days that would likely change opinions on SCS benefits on a single phone interview. Some patients needed battery replacements at the time of the interview (battery life averaged about 3 to 5 years in our study population) and were asked to report current levels of pain from the perspective of when their batteries were still functional, further affecting results.
Conclusions
SCS was shown to improve the quality of life of US veterans at SLVHCS across a wide variety of metrics, including activities of daily living, as well as mental and physical health. For veterans with chronic intractable pain who have tried and failed more conservative treatments, SCS is a great treatment.
Lower back pain (LBP) affects an estimated 9.4% of the global population and has resulted in more years lived with disability than any other health condition.1 LBP affects a wide range of populations, but US veterans have been shown to have significantly higher rates of back pain than nonveterans. The National Institutes of Health reports that 65.6% of veterans experience chronic pain; 9.1% of veterans experience severe, chronic pain.2 Chronic back pain is treated by a range of methods, including medications, surgery, physical therapy (PT), patient education, and behavioral therapy.3 However, chronic neuropathic back pain has been shown to have limited responsiveness to medication.4
Neuropathic pain is caused by lesions in the somatosensory nervous system, resulting in spontaneous pain and amplified pain responses to both painful and nonpainful stimuli.5 The most common location for neuropathic pain is the back and legs. Between 10% and 40% of people who undergo lumbosacral spine surgery to treat neuropathic radicular pain will experience further neuropathic pain.6 This condition is referred to as failed back surgery syndrome or postlaminectomy syndrome (PLS). While neuropathic back pain has had limited responsiveness to medication and repeated lumbosacral spine surgery, spinal cord stimulation (SCS) has shown promise as an effective form of pain treatment for those experiencing PLS and other spine disorders.7-10 In addition, SCS therapy has had a very low incidence of complications, which may be on the decline with recent technological advancements.11 Patients with a diagnosis of PLS, LBP, or complex regional pain syndrome (CRPS) who have not responded to medications, therapy, and/or injections for ≥ 6 months were eligible for a trial of SCS therapy. Trial leads were placed via the percutaneous route with the battery strapped to the waistline for 3 to 5 days and were removed in clinic. Patients who experienced > 60% pain relief and functional improvement received a SCS implant.
The effectiveness of SCS has been demonstrated in a nonveteran population, but it has not been studied in a veteran population.12 US Department of Veterans Affairs (VA) health care coverage is different from Medicare and private insurance in that it is classified as a benefit and not insurance. The goals of treatment at the VA may include considerations in addition to feeling better, and patient presentations may not align with those in the private sector.
We hypothesize that SCS is both a safe and beneficial treatment option for veterans with chronic intractable spine and/or extremity pain. The purpose of this study was to determine the efficacy and safety of SCS in a veteran population.
Methods
The efficacy and safety of SCS was determined via a retrospective study. Inclusion criteria for the study consisted of any Southeastern Louisiana Veterans Health Care System (SLVHCS) patient who had an SCS trial and/or implant from 2008 to 2020. Eligible veterans must have had chronic pain for at least 6 months and had previously tried multiple medications, PT, transcutaneous nerve stimulation, facet injections, epidural steroid injections, or surgery without success. For medication therapy to be considered unsuccessful, it must have included acetaminophen, nonsteroidal anti-inflammatory drugs, and ≥ 1 adjuvant medication (gabapentin, duloxetine, amitriptyline, lidocaine, and menthol). A diagnosis of chronic LBP, PLS, cervical or lumbar spondylosis with radiculopathy, complex regional pain syndrome, or chronic pain syndrome was required for eligibility. Patients whose pain decreased by > 60% and had functional improvement in a 3- to 5-day trial received SCS implantation with percutaneous leads by a pain physician or paddle lead by a neurosurgeon.
The SLVHCS Institutional Review Board approved this study. Electronic health records were reviewed to determine patient age, anthropometric data, and date of SCS implantation. Patients were then called and interviewed to complete a survey. After obtaining verbal consent to the study, subjects were surveyed regarding whether the patient would recommend the procedure to peers, adverse effects (AEs) or complications, and the ability to decrease opiates if applicable. A verbal Pain Outcome Questionnaire (POQ) assessment of activities of daily living also was given during the phone interview regarding pain levels before SCS and at the time of the phone interview.13 (eAppendix available at doi:10.12788/fp.0204) Following the survey, a chart review was performed to corroborate the given AEs or complications and opiate use information. Before and after results of the POQ were compared via a paired sample t test, and P values < .05 were considered significant. Analyses were performed by IBM SPSS, version 26.
The primary outcome measure for this study was whether veterans would recommend SCS to their peers; in our view, this categorical outcome measure seemed to be more valuable to share with future patients who might be candidates for SCS. Since VA health care coverage and goals of treatment may be different from a nonveteran population, we opted to use this primary measure to decrease the possibility of confounding variables.
Secondary outcome measures included changes in POC scores, improvements in activities of daily living, and decreases in use of opioid pain medications.
POQ responses were recorded during the telephone interviews (0 to 10 scale). A paired sample t test was conducted to compare pain levels before and after SCS implant. Pain levels were gathered in the single phone call. Patient opioid usage, if applicable, was assessed by converting medications to morphine milligram equivalent dosing (MMED). Since patients who were on chronic opioids took multiple formulations, we changed the total daily dose to all morphine; for this study, morphine was considered equivalent to hydrocodone, and oxycodone was 1.5x morphine.
Results
Of the 90 SLVHCS patients who received an SCS implant between 2008 and 2020, 76 were reached by telephone and 65 had their responses recorded in the study. Of the 11 patients who were not included, 5 had the SCS removed; it is unclear whether these veterans would have recommended the treatment. Four were unable to quantify pain and/or SCS effects, and 2 were excluded due to a dementia diagnosis years after the implant. The mean (SD) age of participants was 63.9 (10.3) years. Forty percent of patients had a diabetes mellitus diagnosis and 1 had prediabetes. Patients’ most common qualifying diagnosis for SCS was PLS (47.7%) followed by chronic LBP (26.2%). A percutaneous 2-lead technique was the most common type of SCS type used (60.0%) followed by 1-lead (21.5%). The most common SCS manufacturer was Boston Scientific (87.7%)(Table 1). Most veterans (76.9%) recommended SCS to their peers; 13.8% did not recommend SCS; 9.2% were undecided and stated that they were unable to recommend because they did not want to persuade a peer to get SCS (Figure).
There was a statistically significant decrease in opioid use for the 40 veterans for whom pain medication was converted (P < .001)(Table 2). Six patients reported using opioids at some point but could not remember their dose, and no records were found in their chart review, so they were not included in the MMED analysis. In that group, 4 patients reported using opioids before SCS but discontinued the opioid use after SCS implantation, and 2 patients noted using opioids before SCS and concomitantly. Eighteen subjects reported no opioid use at any point before or after SCS (Table 3).
There were few life-threatening complications of SCS. Three veterans developed skin dehiscence; 2 had dehiscence at the battery/generator site, and 1 had dehiscence at the lead anchor site. Two patients with dehiscence also had morbid obesity, and the third had postoperative malnourishment. The dehiscence occurred 3 and 8 months postoperation. All 3 patients with dehiscence had the SCS explanted, though they were eager to get a new SCS implanted as soon as possible because SCS was their most successful treatment to date.
Twenty of the 64 veterans surveyed reported other complications of SCS, including lead migration, lack of pain coverage, paresthesia and numbness, soreness around generator site, SCS shocking patient when performing full thoracic spine flexion, and shingles at the battery site (Table 4). There were 11 explants among the 76 veterans contacted. The primary reason for explant was lack of pain coverage.
Patient concerns included pain with sitting in chairs due to tenderness around the implant, SCS helping with physical pain but not mental pain, SCS only working during the day and not helping with sleep, and patients lacking education regarding possible complications of SCS.
Discussion
In this nonrandomized retrospective review, SCS was shown to be an effective treatment for intractable spine and/or extremity pain. Veterans’ pain levels were significantly reduced following SCS implantation, and more than three-fourths of veterans recommended SCS to their peers. We used the recommendation of SCS to peers as the most important metric regarding the effectiveness of SCS, as this measure was felt to be more valuable to share with future patients; furthermore, categorical analysis has been shown to be more valuable than ordinal pain scales to measure pain.14 In addition to wanting to expand the available research to the general public, we wanted a measure that we could easily relay to our patient population regarding SCS.
The explant rate of 14.5% among surveyed veterans falls at the higher end of the normal ranges found in previous studies of long-term SCS outcomes.15-17 One possible reason for the higher rate is that we did not differentiate based on the reason for the explant (ie, no benefit, further surgery needed for underlying medical condition, or SCS-specific complications). Another possible contributing factor to the higher than expected explant rate is the geographic location in the New Orleans metro area; New Orleans is considered to have one of the highest rates of obesity in the United States and obesity typically has other diseases associated with it such as hypertension and diabetes mellitus.
Limitations
Limitations of the study include the relatively low number of subjects, subjective nature of the interview questions, and the patients’ answers. Typically the POQ has been used as a prospective assessment of pain; whether it is valid in a retrospective analysis is not clear. While there was a statistically significant decrease of opioid use after getting SCS, this study can only show correlation, not causation. During the study period, there has been a drastic change in opioid prescribing patterns and efforts to decrease the amount of opioids prescribed.
Subjects also were asked to rate their pain and quality of life before SCS. Some subjects had SCS implantation up to 10 years prior to the phone interview. The variable amount of time between SCS implantation and interview likely affected subjects’ responses. Chronic pain is a moving target. Patients have good days and bad days that would likely change opinions on SCS benefits on a single phone interview. Some patients needed battery replacements at the time of the interview (battery life averaged about 3 to 5 years in our study population) and were asked to report current levels of pain from the perspective of when their batteries were still functional, further affecting results.
Conclusions
SCS was shown to improve the quality of life of US veterans at SLVHCS across a wide variety of metrics, including activities of daily living, as well as mental and physical health. For veterans with chronic intractable pain who have tried and failed more conservative treatments, SCS is a great treatment.
1. Hoy DG, Smith E, Cross M, et al. The global burden of musculoskeletal conditions for 2010: an overview of methods. Ann Rheum Dis. 2014;73(6):982-989 doi:10.1136/annrheumdis-2013-204344
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
4. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. doi:10.1016/S1474-4422(14)70251-0
5. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1-32. doi:10.1146/annurev.neuro.051508.135531
6. Wilkinson HA. The Failed Back Syndrome: Etiology and Therapy. 2nd ed. Harper & Row; 1991.
7. Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132(1-2):179-188. doi:10.1016/j.pain.2007.07.028
8. North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98-107. doi:10.1227/01.neu.0000144839.65524.e0
9. Geurts JW, Smits H, Kemler MA, Brunner F, Kessels AG, van Kleef M. Spinal cord stimulation for complex regional pain syndrome type I: a prospective cohort study with long-term follow-up. Neuromodulation. 2013;16(6):523-529. doi:10.1111/ner.12024
10. Kumar K, Rizvi S, Bnurs SB. Spinal cord stimulation is effective in management of complex regional pain syndrome I: fact or fiction. Neurosurgery. 2011;69(3):566-5580. doi:10.1227/NEU.0b013e3182181e60
11. Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract. 2011;11(2):148-153. doi:10.1111/j.1533-2500.2010.00407.x
12. Veizi E, Hayek SM, North J, et al. Spinal cord stimulation (SCS) with anatomically guided (3D) neural targeting shows superior chronic axial low back pain relief compared to traditional SCS-LUMINA Study. Pain Med. 2017;18(8):1534-1548. doi:10.1093/pm/pnw286
13. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172-1186. doi:10.1016/j.jpain.2010.02.012
14. Kennedy DJ, Schneider B. Lies, damn lies, and statistic: a commentary. Pain Med. 2020;21(10):2052-2054. doi:10.1093/pm/pnaa287
15. Van Buyten JP, Wille F, Smet I, et al. Therapy-related explants after spinal cord stimulation: results of an international retrospective chart review study. Neuromodulation. 2017;20(7):642-649. doi:10.1111/ner.12642
16. Hayek SM, Veizi E, Hanes M. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center database. Neuromodulation. 2015;18(7):603-609. doi:10.1111/ner.12312
17. Pope JE, Deer TR, Falowski S, et al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation. 2017;20(6):543-552. doi:10.1111/ner.12634
1. Hoy DG, Smith E, Cross M, et al. The global burden of musculoskeletal conditions for 2010: an overview of methods. Ann Rheum Dis. 2014;73(6):982-989 doi:10.1136/annrheumdis-2013-204344
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
4. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. doi:10.1016/S1474-4422(14)70251-0
5. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1-32. doi:10.1146/annurev.neuro.051508.135531
6. Wilkinson HA. The Failed Back Syndrome: Etiology and Therapy. 2nd ed. Harper & Row; 1991.
7. Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132(1-2):179-188. doi:10.1016/j.pain.2007.07.028
8. North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98-107. doi:10.1227/01.neu.0000144839.65524.e0
9. Geurts JW, Smits H, Kemler MA, Brunner F, Kessels AG, van Kleef M. Spinal cord stimulation for complex regional pain syndrome type I: a prospective cohort study with long-term follow-up. Neuromodulation. 2013;16(6):523-529. doi:10.1111/ner.12024
10. Kumar K, Rizvi S, Bnurs SB. Spinal cord stimulation is effective in management of complex regional pain syndrome I: fact or fiction. Neurosurgery. 2011;69(3):566-5580. doi:10.1227/NEU.0b013e3182181e60
11. Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract. 2011;11(2):148-153. doi:10.1111/j.1533-2500.2010.00407.x
12. Veizi E, Hayek SM, North J, et al. Spinal cord stimulation (SCS) with anatomically guided (3D) neural targeting shows superior chronic axial low back pain relief compared to traditional SCS-LUMINA Study. Pain Med. 2017;18(8):1534-1548. doi:10.1093/pm/pnw286
13. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172-1186. doi:10.1016/j.jpain.2010.02.012
14. Kennedy DJ, Schneider B. Lies, damn lies, and statistic: a commentary. Pain Med. 2020;21(10):2052-2054. doi:10.1093/pm/pnaa287
15. Van Buyten JP, Wille F, Smet I, et al. Therapy-related explants after spinal cord stimulation: results of an international retrospective chart review study. Neuromodulation. 2017;20(7):642-649. doi:10.1111/ner.12642
16. Hayek SM, Veizi E, Hanes M. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center database. Neuromodulation. 2015;18(7):603-609. doi:10.1111/ner.12312
17. Pope JE, Deer TR, Falowski S, et al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation. 2017;20(6):543-552. doi:10.1111/ner.12634