User login
You can do more to slow the progression of heart failure
- Use B-type natriuretic peptide (BNP) levels as an aid not only in the diagnosis of heart failure (HF), but to track its progression as well (A).
- Prescribe exercise training for patients with stable heart failure; exercising at 40% to 70% of maximum capacity for 20 to 45 minutes several times a week offers benefits on par with pharmacotherapy (A).
- Consider using the Simplified Treatment Intervention to Control Hypertension (STITCH) algorithm for hypertensive patients or those who are at risk of developing HF; this step-care strategy is effective in treating hypertension, a leading cause of HF (C).
- Consult a specialist before prescribing both an angiotensin-converting enzyme (ACE) inhibitor and an angiotensin receptor blocker (ARB) for a patient with advanced HF; studies of combination therapy for this patient population have had mixed results (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Family physicians are all too familiar with heart failure (HF). This debilitating condition accounts for approximately 3.4 million outpatient visits to US physicians annually,1 and fully two-thirds of HF patients are cared for by primary care physicians.2
A host of comorbid conditions—coronary artery disease, valvular heart disease, diabetes, dyslipidemia, metabolic syndrome, obesity, chronic renal insufficiency, and hypertension chief among them—contribute to the development of HF.3 Of these, hypertension is the most important factor. In more than 75% of cases, high blood pressure precedes HF,1 and an individual’s lifetime risk of developing HF is strongly associated with poor blood pressure control.4 Hypertension is the most significant controllable factor in the management of HF as well. Because of the nexus between hypertension and HF, we encourage physicians to think of these 2 conditions as a single entity—and to recognize that a reduction of even a few millimeters of mercury can have huge clinical benefits.
This review, which highlights a recently tested hypertension algorithm along with other recent developments and long-established treatment strategies, will help you do everything possible to slow the progression of this debilitating and deadly disease.
BNP’s increasing role in evaluating heart failure
A diagnosis of HF in patients with known heart disease is based on functionality and symptoms, assessed with the help of 2 classification schemes5,6 ( TABLE 1 ) and a variety of tests. (Patients who present with the signs and symptoms of HF but no evidence of the comorbid conditions typically associated with it should be screened for other, noncardiac causes—human immunodeficiency virus, hepatitis C, hemochromatosis, hypothyroidism, and substance abuse among them.6 )
Diagnostic testing. Baseline serum chemistries include a complete blood count, urinalysis, electrolytes, magnesium, blood urea nitrogen, creatinine, and blood glucose levels, and liver and thyroid function tests.
B-type natriuretic peptide (BNP), a homeostatic marker secreted by the heart in an attempt to maintain stable blood pressure and plasma volume and avoid fluid retention, is increasingly recognized as an important aid, not only in diagnosing HF but in gauging its severity, managing symptoms, and determining the prognosis.7,8 BNP concentrations <80 pg/mL have been found to have a negative predictive value of 98%, and are also highly sensitive (98%) and specific (98%) for the diagnosis of HF.9,10
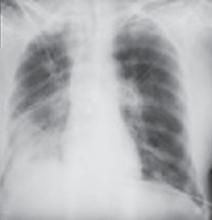
Testing may also include a 12-lead electrocardiogram as well as a posterior-anterior/lateral chest x-ray. Echocardiography is often used to evaluate left ventricular function and ejection fraction6 —a key to establishing whether the patient has systolic (reduced ejection fraction) or diastolic (preserved ejection fraction) HF.
An ejection fraction ≤40% is characteristic of systolic HF, which affects approximately 60% of patients with heart failure11 and is the focus of the following discussion of treatments.
TABLE 1
Classifying heart failure: 2 systems
| NEW YORK HEART ASSOCIATION |
| Class I: No limitation of physical activity; ordinary activity does not cause undue fatigue or dyspnea. Class II: Slight limitation of activity; comfortable at rest, but ordinary physical activity results in fatigue or dyspnea. Class III: Marked limitation in activity. Class IV: Unable to carry on any physical activity without symptoms; symptoms present even at rest. |
| AMERICAN COLLEGE OF CARDIOLOGY/AMERICAN HEART ASSOCIATION |
| Stage A: Conditions strongly associated with heart failure (HF); at high risk of HF. No identified structural or functional abnormalities of the pericardium, myocardium, or cardiac valves; no signs or symptoms of HF. Stage B: Structural heart disease strongly associated with HF, but no known signs or symptoms. Stage C: Current or prior symptoms of HF associated with underlying structural heart disease. Stage D: Advanced structural heart disease, with marked symptoms of HF at rest despite maximal medical therapy. Specialized interventions required. |
| Sources: Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels–Nomenclature and Criteria for Diagnosis. 6th ed. 1964;5 Hunt et al. Circulation. 2005.6 |
Early interventions: Get patients moving
For all patients with stable HF—and those at high risk of developingit—behavioral modification is a key component of treatment. Lifestyle intervention should be directed at weight loss and diet, including control of salt intake; increased physical activity; and smoking cessation.
Don’t shy away from exercise. Although many physicians hesitate to prescribe exercise to patients with HF, physical activity should be a routine recommendation for all but the most debilitated patients.6 Regular exercise has been shown to decrease symptoms, increase functional capacity, and improve the quality of life, with benefits comparable to those of pharmacotherapy.6,12,13
Studies of the beneficial effects of exercise were based on sustaining 40% to 70% of maximum capacity for 20 to 45 minutes, 3 to 5 days a week.6 A good walking program—of at least 30 minutes 4 to 5 days each week—should not be difficult for patients to maintain.
BP treatment guidelines: The old and the new
As noted earlier, controlling hypertension is crucial, not only to prevent HF but to attenuate its progress. But blood pressure management is suboptimal in the United States, with many patients failing to achieve recommended levels of pressure reduction. It’s been suggested that the complexity of standard treatment guidelines may be part of the problem.
STITCH step care is a newer option. Researchers designed the Simplified Treatment Intervention to Control Hypertension (STITCH) Trial, a cluster randomized study of patients at multiple family medicine clinics in Canada, to evaluate whether a simplified step-care algorithm would yield better results.
The STITCH algorithm has 4 treatment steps:
Step 1: Initiate therapy by pairing a diuretic with either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB).
Step 2: Increase combination therapy to the highest dose tolerated.
Step 3: Add a calcium channel blocker and increase to the highest tolerated dose.
Step 4: Add a non-first-line antihypertensive agent (alpha-blocker, beta-blocker, or spironolactone).
Researchers found that after 6 months, 64.7% of patients on the STITCH protocol had achieved target blood pressure, compared with 52.7% of those whose treatment was based on the Canadian Hypertension Education Program (CHEP) guidelines (P=.03).14 The CHEP protocol is similar to that of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7);15 both offer numerous options for initial treatment.16
In presenting the STITCH results at the 2007 annual meeting of the American Heart Association, the lead author described the use of a simple step-care approach as “an important way forward in the treatment of hypertension [which] may be a paradigm for managing a range of chronic diseases.”16 Yet the STITCH algorithm has yet to be widely embraced; outside of the research community, most US physicians are relying on the JNC 7 guidelines.
ACC/AHA recommendations indicate that for patients at stage A—that is, those with conditions strongly associated with, and at high risk for, HF—management of hypertension should conform to national standards such as JNC 7. The JNC 7 guidelines recommend the use of a thiazide diuretic as the initial drug of choice for patients with essential hypertension. For those with diabetes, ACE inhibitors and ARBs are the first-line antihypertensive agents of choice.
Glucose control is also essential for stage A patients with diabetes. Treatment of lipid disorders and pharmacotherapy for metabolic syndrome are also recommended for stage A patients, as needed.
Treatment escalates as HF progresses
ACE inhibitors, ARBs, and beta-blockers are the preferred pharmacologic interventions for patients at stage B—those who have structural heart disease strongly associated with HF but are not yet symptomatic. Anyone who has had a myocardial infarction (MI) should be started on a beta-blocker and an ACE inhibitor, ACC/AHA recommends, unless a contraindication exists.6 Similarly, any patient with a reduced ejection fraction should be started on an ACE inhibitor regardless of symptoms.6
The Heart Outcomes Prevention Evaluation (HOPE) study demonstrated a 23% relative risk (RR) reduction with the use of an ACE inhibitor in patients with coronary artery disease, peripheral vascular disease, or diabetes, compared with patients receiving a placebo.17 The importance of a beta-blocker was established in a subanalysis of the Survival and Ventricular Enlargement Trial (SAVE), which found that patients taking beta-blockers in addition to an ACE inhibitor had a 32% RR reduction in progression of HF, compared with patients on an ACE inhibitor alone.18
We recommend an ACE inhibitor or an ARB and a beta-blocker, when appropriate, to slow the progression of HF pathophysiology. It is important to be aware of the potential adverse effects of certain beta-blockers in patients with HF. Only 3 beta-blockers are approved for use in this patient population in the United States—bisoprolol, carvedilol, and metoprolol succinate, which have been found to provide benefits that other beta-blockers do not.6,15
Stages C and D: Tx considerations and controversies
Treatment for patients at stage C should include all components of therapy for patients at stages A and B, but with a more aggressive use of pharmacotherapy ( TABLE 2 ). Patients with stage C HF, by definition, are symptomatic, and the ACC/AHA recommendations reflect concern about their increasingly compromised status. Thus, in addition to the use of ACE inhibitors or ARBs and beta-blockers, modest use of diuretics is recommended, as needed, for fluid volume control.6 Diuretics should be used judiciously, though, with ongoing evaluation to avoid the excessive loss of potassium and magnesium, which can lead to volume depletion and lethal arrhythmias. Limiting sodium consumption is an important dietary restriction for stage C patients.
Aldosterone antagonists may also be considered on a case-by-case basis for patients with stage C HF. Due to their potassium-sparing effects, aldosterone antagonists, used in conjunction with standard therapies, may have a positive effect on electrolyte balance. Potassium levels must be carefully monitored, however, and potassium supplementation reevaluated for patients who are put on an aldosterone antagonist.19
Digitalis may also be helpful in select patients who remain symptomatic despite maximal pharmacotherapy.20 While it does not affect mortality, digitalis has been shown to reduce hospitalizations.21
ACE inhibitor-ARB combination therapy, another possible treatment for advanced HF, remains controversial. The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), detailed in “ACE inhibitors and ARBs: One or the other—not both” in the January 2009 issue of The Journal of Family Practice, evaluated use of this dual therapy; the trial was also designed to determine whether telmisartan (an ARB) is inferior to ramipril (an ACE inhibitor) in patients at high risk for vascular events.22 The researchers found that telmisartan is not, in fact, inferior to ramipril, and reported that for patients with HF, an ACE-ARB combination offers a potential benefit.
However, the clinical benefit of an ACE-ARB combination in this patient population was not clarified in this study, and may be potentially harmful. In the Valsartan Heart Failure Trial (ValHeFT), the combination of valsartan, an ARB, and an ACE inhibitor decreased hospitalizations but did not improve mortality.23 Indeed, an increase in mortality was found when an ACEARB combination was used in conjunction with beta-blockers. Because beta-blockers are indicated for routine use in patients with HF, this finding was of particular concern.
In a meta-analysis of randomized trials using both an ACE inhibitor and an ARB in patients with left ventricular dysfunction, researchers found a “marked” increase in adverse effects, including deteriorating renal function (RR=2.17), hyperkalemia (RR=4.87), and symptomatic hypotension (RR=1.05).24 Although an ACE-ARB combination may benefit a subset of patients with HF, it is best to initiate such treatment only with the guidance of an HF specialist.
TABLE 2
Treating heart failure: How the different drugs and devices rate
| STAGE | PHARMACOTHERAPY | LOE | DEVICE/INTERVENTION | LOE |
|---|---|---|---|---|
| A | Treat BP per JNC 7 ACE inhibitor or ARB for patients with vascular disease or diabetes | A | None | N/A |
| B | ACE inhibitor or ARB BB | A | None | N/A |
| C | Routine use: Diuretics ACE inhibitor BB Select use: Aldosterone antagonist ARB Digitalis | A | Consider: Biventricular pacer or ICD or both | B |
| D | Same as C | B | Consider: Heart transplant or LVAD; experimental protocols | C |
| ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BB, beta-blocker; BP, blood pressure; ICD, implantable cardioverter defibrillator; JNC 7, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; LOE, level of evidence; LVAD, left ventricular assist device. | ||||
| Adapted from: Hunt SA, et al. Circulation. 2005.6 | ||||
Beyond drug therapy: Assistive devices
Refractory end-stage HF requires a clear treatment plan, and should involve the recommendations of an HF specialist. Careful maintenance of fluid status is required, and an evaluation for cardiac transplantation may be considered.
A left ventricular assist device (LVAD) should also be considered for patients with an estimated 1-year mortality of >50%.6 LVADs are mechanical heart pumps that were initially utilized as a “bridge” to transplant, but are increasingly being used as a palliative alternative for severely ill patients.25
Other devices—an implantable cardioverter defibrillator (ICD) or a biventricular pacer—should also be considered for patients at stage D, as well as stage C patients who are at increased risk of sudden death despite maximal drug therapy.6 Patients who have had a previous MI or ventricular arrhythmia are at risk for a repeat episode.6
Use of an ICD can reduce mortality by 23% in selected patients.26 Potential candidates for the device are patients who have an ejection fraction of <30%, mild to moderate symptoms, and a life expectancy of at least 1 year.6
Biventricular pacing, also known as cardiac resynchronization therapy (CRT), has been found to improve the quality of life, functional status, and exercise capacity in some patients with advanced disease. CRT, which reduces symptoms of HF and improves cardiac function by reestablishing the mechanical sequence of ventricular activation and ventricular contraction, has also been associated with reductions in hospitalization and death from progressive HF.27,28
The Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial demonstrated a 20% reduction in the 12-month risk of death or hospitalization from any cause with CRT, and the Cardiac Resynchronization-Heart Failure (CARE-HF) trial established that patients receiving CRT had a significantly lower risk of death than those receiving medical therapy alone (40% reduction).29,30
However, not all patients with HF have problems with conduction delay that result in a dyssynchronous heart beat. CRT is indicated only for patients who are in sinus rhythm and have:
- NYHA class III or IV HF
- an ejection fraction of <35%
- a prolonged QRS complex (>120 m/sec), and
- continued symptoms despite maximal medical therapy.6
Under these criteria, approximately 10% of patients with HF would qualify for CRT.31 The restrictive criteria are due, in part, to the fact that this modality is relatively new and has been studied only in a small subset of patients.
Options for patients who are running out of them
For acutely decompensated hospitalized patients with volume overload, ultrafiltration (UF) is a useful alternative to diuretics. UF uses high pressure to “force” volume through the kidneys;32,33 the technique maximizes diuresis, and is best suited for patients who have significant renal dysfunction or are not responding to standard diuretic therapy. UF makes it easier to remove the desired amount of fluid, and has a positive impact on pulmonary wedge pressure and cardiac output.34 Its use in diuretic-resistant patients can decrease the length of stay and produce positive clinical benefits that may last up to 3 months.34
There are also a number of experimental strategies, surgical and otherwise. Among them are:
Cardiac wrap surgery, in which the heart is encased in a mesh bag attached with stitches, in an attempt to stop the progression of end-stage HF by preventing further dilation;25
Ventricular restoration surgery, a procedure in which scar tissue caused by MI is removed from the ventricular muscle and the left ventricle is reshaped and its size reduced in an attempt to restore some of the heart’s pumping ability;25 and
Enhanced external counterpulsation, or EECP, a noninvasive technique in which pressure cuffs are placed on the calves, thighs, and buttocks and inflated and deflated in an attempt to increase blood flow back to the heart.25
Correspondence
Randy Wexler, MD, MPH, FAAFP, The Ohio State University, B0902B Cramblett Hall, 456 W. 10th Avenue, Columbus, OH 43210; [email protected]
1. Rosamond W, Flegal K, Friday G, et al. American Heart Association: heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69-e171.
2. Lloyd-Jones DM, Larson MG, Leip EP, et al. Framingham heart study. Lifetime risk for developing congestive heart failure. Circulation. 2002;106:3068-3072.
3. Burt CW, Schappert SM. Ambulatory care visits to physicians offices, hospital outpatient departments, and emergency departments: United states, 1999-2000. Vital Health Stat 13. 2004;157:1-70
4. Levit K, Stranges E, Ryan K, et al. HCUP facts and figures, 2006: Statistics on Hospital-based Care in the United States. Rockville, MD: Agency for Healthcare Research and Quality, 2008. Available at: http://www.hcup-us.ahrq.gov/reports.jsp. Accessed February 9, 2009.
5. Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels–Nomenclature and Criteria for Diagnosis. 6th ed. Boston: Little, Brown and Company; 1964.
6. Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult. Circulation. 2005;112:e154-e235.
7. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74:1893-1898.
8. Maisel A, Krishnaswamy P, Nowak R, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161-167.
9. Maisel AS. B-type natriuretic peptide (BNP) levels: diagnostic and therapeutic potential. Rev Cardiovasc Med. 2001;2(suppl 2):S13-S18.
10. Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B-type natriuretic peptide (BNP) in the diagnosis of congestive heart failure in an urgent-care setting. J Am Coll Cardiol. 2001;37:379-385.
11. Philbin EF, Rocco TA, Jr, Lindenmuth NW, et al. Systolic versus diastolic heart failure in a community practice: clinical features, outcomes, and the use of angiotensin-converting enzyme inhibitors. Am J Med. 2000;109:605-613.
12. Piepoli MF, Flather M, Coats AJ. Overview of studies of exercise training in chronic heart failure: the need for a prospective randomized multicentre European trial. Eur Heart J. 1998;19:830-841.
13. Coats AJ, Adamopoulos S, Meyer TE, et al. Effects of physical training in chronic heart failure. Lancet. 1990;335:63-66.
14. Feldman RD, Zou G, Feagen BG, et al. The STITCH Investigators. The Simplified Treatment Intervention to Control Hypertension (STITCH) trial: A cluster randomized controlled trial of a step-care algorithm using initial fixed dose combination therapy for the management of hypertension. Presented at Scientific Sessions 2007 of the American Heart Association, November 4-7, 2007; Orlando, Fla.
15. Chobanian A, Bakris G, Black H, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). Hypertension. 2003;42:1206-1252.
16. Brookes L. Hypertension highlights: new drug algorithms, new drug approvals, new drugs. Available at: http://canadiancpd.medscape.com/viewarticle/568786_print. Accessed January 27, 2009.
17. Yusuf S, Sleight P, Pogue J, et al. The Heart Outcomes Prevention Evaluation Study Investigators: effects of angiotensin converting enzyme inhibitor, ramipril, on cardiovascular events in high risk patients. N Engl J Med. 2000;342:145-153.
18. Vantrimpont P, Rouleau JL, Wun CC, et al. For the SAVE Investigators. Additive beneficial effects of beta-blockers to angiotensin converting enzyme inhibitors in the Survival and Ventricular Enlargement (SAVE) study. J Am Coll Cardiol. 1997;29:229-236.
19. Juurlink DN, Mamdani M, Kopp A, et al. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652-1658.
20. Rahimtoola SH. Digitalis therapy for patients in clinical heart failure. Circulation. 2004;109:2942-2946.
21. Cayley W. Digitalis for the treatment of congestive heart failure in patients in sinus rhythm. Am Fam Physician. 2004;69:71-73.
22. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
23. Cohn JN, Tognoni G. Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667-1675.
24. Phillips CO, Kashani A, Ko DK, et al. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction. Arch Intern Med. 2007;167:1930-1936.
25. Mayo Clinic. Heart failure: treatments and drugs. January 3, 2008. Available at: http://www.mayoclinic.com/health/heart-failure.DS00061/DSECTION=treatments-and-drugs. Accessed January 30, 2009.
26. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237.
27. Blanc J-J, Bertault-Valls V, Fatemi M, et al. Mid-term benefits of left univentricular pacing in patients with congestive heart failure. Circulation. 2004;109:1741-1744.
28. Bradley DJ, Bradley EA, Baughman KL, et al. Cardiac resynchronization and death from progressive heart failure: a meta-analysis of randomized controlled trials. JAMA. 2003;289:730-740.
29. Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-2150.
30. Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539-1549.
31. Farwell D, Patel NR, Hall A, et al. How many people with heart failure are appropriate for biventricular resynchronization? Eur Heart J. 2000;21:1246-1250.
32. Bart BA, Boyle A, Bank AJ, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the relief for acutely fluid-overloaded patients with decompensated congestive heart failure (RAPD-HF). J Am Coll Cardiol. 2005;46:2043-2046.
33. Costanzo MR, Saltzberg M, O’Sullivan J, et al. Early ultrafiltration in patients with acutely decompensated heart failure and diuretic resistance. J Am Coll Cardiol. 2005;46:2047-2051.
34. Agostini PG, Marenzi GC, Lauri G, et al. Sustained improvement in functional capacity after removal of body fluid with isolated ultrafiltration in chronic cardiac insufficiency; failure of furosemide to provide same results. Am J Med. 1994;96:191-199.
- Use B-type natriuretic peptide (BNP) levels as an aid not only in the diagnosis of heart failure (HF), but to track its progression as well (A).
- Prescribe exercise training for patients with stable heart failure; exercising at 40% to 70% of maximum capacity for 20 to 45 minutes several times a week offers benefits on par with pharmacotherapy (A).
- Consider using the Simplified Treatment Intervention to Control Hypertension (STITCH) algorithm for hypertensive patients or those who are at risk of developing HF; this step-care strategy is effective in treating hypertension, a leading cause of HF (C).
- Consult a specialist before prescribing both an angiotensin-converting enzyme (ACE) inhibitor and an angiotensin receptor blocker (ARB) for a patient with advanced HF; studies of combination therapy for this patient population have had mixed results (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Family physicians are all too familiar with heart failure (HF). This debilitating condition accounts for approximately 3.4 million outpatient visits to US physicians annually,1 and fully two-thirds of HF patients are cared for by primary care physicians.2
A host of comorbid conditions—coronary artery disease, valvular heart disease, diabetes, dyslipidemia, metabolic syndrome, obesity, chronic renal insufficiency, and hypertension chief among them—contribute to the development of HF.3 Of these, hypertension is the most important factor. In more than 75% of cases, high blood pressure precedes HF,1 and an individual’s lifetime risk of developing HF is strongly associated with poor blood pressure control.4 Hypertension is the most significant controllable factor in the management of HF as well. Because of the nexus between hypertension and HF, we encourage physicians to think of these 2 conditions as a single entity—and to recognize that a reduction of even a few millimeters of mercury can have huge clinical benefits.
This review, which highlights a recently tested hypertension algorithm along with other recent developments and long-established treatment strategies, will help you do everything possible to slow the progression of this debilitating and deadly disease.
BNP’s increasing role in evaluating heart failure
A diagnosis of HF in patients with known heart disease is based on functionality and symptoms, assessed with the help of 2 classification schemes5,6 ( TABLE 1 ) and a variety of tests. (Patients who present with the signs and symptoms of HF but no evidence of the comorbid conditions typically associated with it should be screened for other, noncardiac causes—human immunodeficiency virus, hepatitis C, hemochromatosis, hypothyroidism, and substance abuse among them.6 )
Diagnostic testing. Baseline serum chemistries include a complete blood count, urinalysis, electrolytes, magnesium, blood urea nitrogen, creatinine, and blood glucose levels, and liver and thyroid function tests.
B-type natriuretic peptide (BNP), a homeostatic marker secreted by the heart in an attempt to maintain stable blood pressure and plasma volume and avoid fluid retention, is increasingly recognized as an important aid, not only in diagnosing HF but in gauging its severity, managing symptoms, and determining the prognosis.7,8 BNP concentrations <80 pg/mL have been found to have a negative predictive value of 98%, and are also highly sensitive (98%) and specific (98%) for the diagnosis of HF.9,10
Testing may also include a 12-lead electrocardiogram as well as a posterior-anterior/lateral chest x-ray. Echocardiography is often used to evaluate left ventricular function and ejection fraction6 —a key to establishing whether the patient has systolic (reduced ejection fraction) or diastolic (preserved ejection fraction) HF.
An ejection fraction ≤40% is characteristic of systolic HF, which affects approximately 60% of patients with heart failure11 and is the focus of the following discussion of treatments.
TABLE 1
Classifying heart failure: 2 systems
| NEW YORK HEART ASSOCIATION |
| Class I: No limitation of physical activity; ordinary activity does not cause undue fatigue or dyspnea. Class II: Slight limitation of activity; comfortable at rest, but ordinary physical activity results in fatigue or dyspnea. Class III: Marked limitation in activity. Class IV: Unable to carry on any physical activity without symptoms; symptoms present even at rest. |
| AMERICAN COLLEGE OF CARDIOLOGY/AMERICAN HEART ASSOCIATION |
| Stage A: Conditions strongly associated with heart failure (HF); at high risk of HF. No identified structural or functional abnormalities of the pericardium, myocardium, or cardiac valves; no signs or symptoms of HF. Stage B: Structural heart disease strongly associated with HF, but no known signs or symptoms. Stage C: Current or prior symptoms of HF associated with underlying structural heart disease. Stage D: Advanced structural heart disease, with marked symptoms of HF at rest despite maximal medical therapy. Specialized interventions required. |
| Sources: Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels–Nomenclature and Criteria for Diagnosis. 6th ed. 1964;5 Hunt et al. Circulation. 2005.6 |
Early interventions: Get patients moving
For all patients with stable HF—and those at high risk of developingit—behavioral modification is a key component of treatment. Lifestyle intervention should be directed at weight loss and diet, including control of salt intake; increased physical activity; and smoking cessation.
Don’t shy away from exercise. Although many physicians hesitate to prescribe exercise to patients with HF, physical activity should be a routine recommendation for all but the most debilitated patients.6 Regular exercise has been shown to decrease symptoms, increase functional capacity, and improve the quality of life, with benefits comparable to those of pharmacotherapy.6,12,13
Studies of the beneficial effects of exercise were based on sustaining 40% to 70% of maximum capacity for 20 to 45 minutes, 3 to 5 days a week.6 A good walking program—of at least 30 minutes 4 to 5 days each week—should not be difficult for patients to maintain.
BP treatment guidelines: The old and the new
As noted earlier, controlling hypertension is crucial, not only to prevent HF but to attenuate its progress. But blood pressure management is suboptimal in the United States, with many patients failing to achieve recommended levels of pressure reduction. It’s been suggested that the complexity of standard treatment guidelines may be part of the problem.
STITCH step care is a newer option. Researchers designed the Simplified Treatment Intervention to Control Hypertension (STITCH) Trial, a cluster randomized study of patients at multiple family medicine clinics in Canada, to evaluate whether a simplified step-care algorithm would yield better results.
The STITCH algorithm has 4 treatment steps:
Step 1: Initiate therapy by pairing a diuretic with either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB).
Step 2: Increase combination therapy to the highest dose tolerated.
Step 3: Add a calcium channel blocker and increase to the highest tolerated dose.
Step 4: Add a non-first-line antihypertensive agent (alpha-blocker, beta-blocker, or spironolactone).
Researchers found that after 6 months, 64.7% of patients on the STITCH protocol had achieved target blood pressure, compared with 52.7% of those whose treatment was based on the Canadian Hypertension Education Program (CHEP) guidelines (P=.03).14 The CHEP protocol is similar to that of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7);15 both offer numerous options for initial treatment.16
In presenting the STITCH results at the 2007 annual meeting of the American Heart Association, the lead author described the use of a simple step-care approach as “an important way forward in the treatment of hypertension [which] may be a paradigm for managing a range of chronic diseases.”16 Yet the STITCH algorithm has yet to be widely embraced; outside of the research community, most US physicians are relying on the JNC 7 guidelines.
ACC/AHA recommendations indicate that for patients at stage A—that is, those with conditions strongly associated with, and at high risk for, HF—management of hypertension should conform to national standards such as JNC 7. The JNC 7 guidelines recommend the use of a thiazide diuretic as the initial drug of choice for patients with essential hypertension. For those with diabetes, ACE inhibitors and ARBs are the first-line antihypertensive agents of choice.
Glucose control is also essential for stage A patients with diabetes. Treatment of lipid disorders and pharmacotherapy for metabolic syndrome are also recommended for stage A patients, as needed.
Treatment escalates as HF progresses
ACE inhibitors, ARBs, and beta-blockers are the preferred pharmacologic interventions for patients at stage B—those who have structural heart disease strongly associated with HF but are not yet symptomatic. Anyone who has had a myocardial infarction (MI) should be started on a beta-blocker and an ACE inhibitor, ACC/AHA recommends, unless a contraindication exists.6 Similarly, any patient with a reduced ejection fraction should be started on an ACE inhibitor regardless of symptoms.6
The Heart Outcomes Prevention Evaluation (HOPE) study demonstrated a 23% relative risk (RR) reduction with the use of an ACE inhibitor in patients with coronary artery disease, peripheral vascular disease, or diabetes, compared with patients receiving a placebo.17 The importance of a beta-blocker was established in a subanalysis of the Survival and Ventricular Enlargement Trial (SAVE), which found that patients taking beta-blockers in addition to an ACE inhibitor had a 32% RR reduction in progression of HF, compared with patients on an ACE inhibitor alone.18
We recommend an ACE inhibitor or an ARB and a beta-blocker, when appropriate, to slow the progression of HF pathophysiology. It is important to be aware of the potential adverse effects of certain beta-blockers in patients with HF. Only 3 beta-blockers are approved for use in this patient population in the United States—bisoprolol, carvedilol, and metoprolol succinate, which have been found to provide benefits that other beta-blockers do not.6,15
Stages C and D: Tx considerations and controversies
Treatment for patients at stage C should include all components of therapy for patients at stages A and B, but with a more aggressive use of pharmacotherapy ( TABLE 2 ). Patients with stage C HF, by definition, are symptomatic, and the ACC/AHA recommendations reflect concern about their increasingly compromised status. Thus, in addition to the use of ACE inhibitors or ARBs and beta-blockers, modest use of diuretics is recommended, as needed, for fluid volume control.6 Diuretics should be used judiciously, though, with ongoing evaluation to avoid the excessive loss of potassium and magnesium, which can lead to volume depletion and lethal arrhythmias. Limiting sodium consumption is an important dietary restriction for stage C patients.
Aldosterone antagonists may also be considered on a case-by-case basis for patients with stage C HF. Due to their potassium-sparing effects, aldosterone antagonists, used in conjunction with standard therapies, may have a positive effect on electrolyte balance. Potassium levels must be carefully monitored, however, and potassium supplementation reevaluated for patients who are put on an aldosterone antagonist.19
Digitalis may also be helpful in select patients who remain symptomatic despite maximal pharmacotherapy.20 While it does not affect mortality, digitalis has been shown to reduce hospitalizations.21
ACE inhibitor-ARB combination therapy, another possible treatment for advanced HF, remains controversial. The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), detailed in “ACE inhibitors and ARBs: One or the other—not both” in the January 2009 issue of The Journal of Family Practice, evaluated use of this dual therapy; the trial was also designed to determine whether telmisartan (an ARB) is inferior to ramipril (an ACE inhibitor) in patients at high risk for vascular events.22 The researchers found that telmisartan is not, in fact, inferior to ramipril, and reported that for patients with HF, an ACE-ARB combination offers a potential benefit.
However, the clinical benefit of an ACE-ARB combination in this patient population was not clarified in this study, and may be potentially harmful. In the Valsartan Heart Failure Trial (ValHeFT), the combination of valsartan, an ARB, and an ACE inhibitor decreased hospitalizations but did not improve mortality.23 Indeed, an increase in mortality was found when an ACEARB combination was used in conjunction with beta-blockers. Because beta-blockers are indicated for routine use in patients with HF, this finding was of particular concern.
In a meta-analysis of randomized trials using both an ACE inhibitor and an ARB in patients with left ventricular dysfunction, researchers found a “marked” increase in adverse effects, including deteriorating renal function (RR=2.17), hyperkalemia (RR=4.87), and symptomatic hypotension (RR=1.05).24 Although an ACE-ARB combination may benefit a subset of patients with HF, it is best to initiate such treatment only with the guidance of an HF specialist.
TABLE 2
Treating heart failure: How the different drugs and devices rate
| STAGE | PHARMACOTHERAPY | LOE | DEVICE/INTERVENTION | LOE |
|---|---|---|---|---|
| A | Treat BP per JNC 7 ACE inhibitor or ARB for patients with vascular disease or diabetes | A | None | N/A |
| B | ACE inhibitor or ARB BB | A | None | N/A |
| C | Routine use: Diuretics ACE inhibitor BB Select use: Aldosterone antagonist ARB Digitalis | A | Consider: Biventricular pacer or ICD or both | B |
| D | Same as C | B | Consider: Heart transplant or LVAD; experimental protocols | C |
| ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BB, beta-blocker; BP, blood pressure; ICD, implantable cardioverter defibrillator; JNC 7, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; LOE, level of evidence; LVAD, left ventricular assist device. | ||||
| Adapted from: Hunt SA, et al. Circulation. 2005.6 | ||||
Beyond drug therapy: Assistive devices
Refractory end-stage HF requires a clear treatment plan, and should involve the recommendations of an HF specialist. Careful maintenance of fluid status is required, and an evaluation for cardiac transplantation may be considered.
A left ventricular assist device (LVAD) should also be considered for patients with an estimated 1-year mortality of >50%.6 LVADs are mechanical heart pumps that were initially utilized as a “bridge” to transplant, but are increasingly being used as a palliative alternative for severely ill patients.25
Other devices—an implantable cardioverter defibrillator (ICD) or a biventricular pacer—should also be considered for patients at stage D, as well as stage C patients who are at increased risk of sudden death despite maximal drug therapy.6 Patients who have had a previous MI or ventricular arrhythmia are at risk for a repeat episode.6
Use of an ICD can reduce mortality by 23% in selected patients.26 Potential candidates for the device are patients who have an ejection fraction of <30%, mild to moderate symptoms, and a life expectancy of at least 1 year.6
Biventricular pacing, also known as cardiac resynchronization therapy (CRT), has been found to improve the quality of life, functional status, and exercise capacity in some patients with advanced disease. CRT, which reduces symptoms of HF and improves cardiac function by reestablishing the mechanical sequence of ventricular activation and ventricular contraction, has also been associated with reductions in hospitalization and death from progressive HF.27,28
The Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial demonstrated a 20% reduction in the 12-month risk of death or hospitalization from any cause with CRT, and the Cardiac Resynchronization-Heart Failure (CARE-HF) trial established that patients receiving CRT had a significantly lower risk of death than those receiving medical therapy alone (40% reduction).29,30
However, not all patients with HF have problems with conduction delay that result in a dyssynchronous heart beat. CRT is indicated only for patients who are in sinus rhythm and have:
- NYHA class III or IV HF
- an ejection fraction of <35%
- a prolonged QRS complex (>120 m/sec), and
- continued symptoms despite maximal medical therapy.6
Under these criteria, approximately 10% of patients with HF would qualify for CRT.31 The restrictive criteria are due, in part, to the fact that this modality is relatively new and has been studied only in a small subset of patients.
Options for patients who are running out of them
For acutely decompensated hospitalized patients with volume overload, ultrafiltration (UF) is a useful alternative to diuretics. UF uses high pressure to “force” volume through the kidneys;32,33 the technique maximizes diuresis, and is best suited for patients who have significant renal dysfunction or are not responding to standard diuretic therapy. UF makes it easier to remove the desired amount of fluid, and has a positive impact on pulmonary wedge pressure and cardiac output.34 Its use in diuretic-resistant patients can decrease the length of stay and produce positive clinical benefits that may last up to 3 months.34
There are also a number of experimental strategies, surgical and otherwise. Among them are:
Cardiac wrap surgery, in which the heart is encased in a mesh bag attached with stitches, in an attempt to stop the progression of end-stage HF by preventing further dilation;25
Ventricular restoration surgery, a procedure in which scar tissue caused by MI is removed from the ventricular muscle and the left ventricle is reshaped and its size reduced in an attempt to restore some of the heart’s pumping ability;25 and
Enhanced external counterpulsation, or EECP, a noninvasive technique in which pressure cuffs are placed on the calves, thighs, and buttocks and inflated and deflated in an attempt to increase blood flow back to the heart.25
Correspondence
Randy Wexler, MD, MPH, FAAFP, The Ohio State University, B0902B Cramblett Hall, 456 W. 10th Avenue, Columbus, OH 43210; [email protected]
- Use B-type natriuretic peptide (BNP) levels as an aid not only in the diagnosis of heart failure (HF), but to track its progression as well (A).
- Prescribe exercise training for patients with stable heart failure; exercising at 40% to 70% of maximum capacity for 20 to 45 minutes several times a week offers benefits on par with pharmacotherapy (A).
- Consider using the Simplified Treatment Intervention to Control Hypertension (STITCH) algorithm for hypertensive patients or those who are at risk of developing HF; this step-care strategy is effective in treating hypertension, a leading cause of HF (C).
- Consult a specialist before prescribing both an angiotensin-converting enzyme (ACE) inhibitor and an angiotensin receptor blocker (ARB) for a patient with advanced HF; studies of combination therapy for this patient population have had mixed results (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Family physicians are all too familiar with heart failure (HF). This debilitating condition accounts for approximately 3.4 million outpatient visits to US physicians annually,1 and fully two-thirds of HF patients are cared for by primary care physicians.2
A host of comorbid conditions—coronary artery disease, valvular heart disease, diabetes, dyslipidemia, metabolic syndrome, obesity, chronic renal insufficiency, and hypertension chief among them—contribute to the development of HF.3 Of these, hypertension is the most important factor. In more than 75% of cases, high blood pressure precedes HF,1 and an individual’s lifetime risk of developing HF is strongly associated with poor blood pressure control.4 Hypertension is the most significant controllable factor in the management of HF as well. Because of the nexus between hypertension and HF, we encourage physicians to think of these 2 conditions as a single entity—and to recognize that a reduction of even a few millimeters of mercury can have huge clinical benefits.
This review, which highlights a recently tested hypertension algorithm along with other recent developments and long-established treatment strategies, will help you do everything possible to slow the progression of this debilitating and deadly disease.
BNP’s increasing role in evaluating heart failure
A diagnosis of HF in patients with known heart disease is based on functionality and symptoms, assessed with the help of 2 classification schemes5,6 ( TABLE 1 ) and a variety of tests. (Patients who present with the signs and symptoms of HF but no evidence of the comorbid conditions typically associated with it should be screened for other, noncardiac causes—human immunodeficiency virus, hepatitis C, hemochromatosis, hypothyroidism, and substance abuse among them.6 )
Diagnostic testing. Baseline serum chemistries include a complete blood count, urinalysis, electrolytes, magnesium, blood urea nitrogen, creatinine, and blood glucose levels, and liver and thyroid function tests.
B-type natriuretic peptide (BNP), a homeostatic marker secreted by the heart in an attempt to maintain stable blood pressure and plasma volume and avoid fluid retention, is increasingly recognized as an important aid, not only in diagnosing HF but in gauging its severity, managing symptoms, and determining the prognosis.7,8 BNP concentrations <80 pg/mL have been found to have a negative predictive value of 98%, and are also highly sensitive (98%) and specific (98%) for the diagnosis of HF.9,10
Testing may also include a 12-lead electrocardiogram as well as a posterior-anterior/lateral chest x-ray. Echocardiography is often used to evaluate left ventricular function and ejection fraction6 —a key to establishing whether the patient has systolic (reduced ejection fraction) or diastolic (preserved ejection fraction) HF.
An ejection fraction ≤40% is characteristic of systolic HF, which affects approximately 60% of patients with heart failure11 and is the focus of the following discussion of treatments.
TABLE 1
Classifying heart failure: 2 systems
| NEW YORK HEART ASSOCIATION |
| Class I: No limitation of physical activity; ordinary activity does not cause undue fatigue or dyspnea. Class II: Slight limitation of activity; comfortable at rest, but ordinary physical activity results in fatigue or dyspnea. Class III: Marked limitation in activity. Class IV: Unable to carry on any physical activity without symptoms; symptoms present even at rest. |
| AMERICAN COLLEGE OF CARDIOLOGY/AMERICAN HEART ASSOCIATION |
| Stage A: Conditions strongly associated with heart failure (HF); at high risk of HF. No identified structural or functional abnormalities of the pericardium, myocardium, or cardiac valves; no signs or symptoms of HF. Stage B: Structural heart disease strongly associated with HF, but no known signs or symptoms. Stage C: Current or prior symptoms of HF associated with underlying structural heart disease. Stage D: Advanced structural heart disease, with marked symptoms of HF at rest despite maximal medical therapy. Specialized interventions required. |
| Sources: Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels–Nomenclature and Criteria for Diagnosis. 6th ed. 1964;5 Hunt et al. Circulation. 2005.6 |
Early interventions: Get patients moving
For all patients with stable HF—and those at high risk of developingit—behavioral modification is a key component of treatment. Lifestyle intervention should be directed at weight loss and diet, including control of salt intake; increased physical activity; and smoking cessation.
Don’t shy away from exercise. Although many physicians hesitate to prescribe exercise to patients with HF, physical activity should be a routine recommendation for all but the most debilitated patients.6 Regular exercise has been shown to decrease symptoms, increase functional capacity, and improve the quality of life, with benefits comparable to those of pharmacotherapy.6,12,13
Studies of the beneficial effects of exercise were based on sustaining 40% to 70% of maximum capacity for 20 to 45 minutes, 3 to 5 days a week.6 A good walking program—of at least 30 minutes 4 to 5 days each week—should not be difficult for patients to maintain.
BP treatment guidelines: The old and the new
As noted earlier, controlling hypertension is crucial, not only to prevent HF but to attenuate its progress. But blood pressure management is suboptimal in the United States, with many patients failing to achieve recommended levels of pressure reduction. It’s been suggested that the complexity of standard treatment guidelines may be part of the problem.
STITCH step care is a newer option. Researchers designed the Simplified Treatment Intervention to Control Hypertension (STITCH) Trial, a cluster randomized study of patients at multiple family medicine clinics in Canada, to evaluate whether a simplified step-care algorithm would yield better results.
The STITCH algorithm has 4 treatment steps:
Step 1: Initiate therapy by pairing a diuretic with either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB).
Step 2: Increase combination therapy to the highest dose tolerated.
Step 3: Add a calcium channel blocker and increase to the highest tolerated dose.
Step 4: Add a non-first-line antihypertensive agent (alpha-blocker, beta-blocker, or spironolactone).
Researchers found that after 6 months, 64.7% of patients on the STITCH protocol had achieved target blood pressure, compared with 52.7% of those whose treatment was based on the Canadian Hypertension Education Program (CHEP) guidelines (P=.03).14 The CHEP protocol is similar to that of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7);15 both offer numerous options for initial treatment.16
In presenting the STITCH results at the 2007 annual meeting of the American Heart Association, the lead author described the use of a simple step-care approach as “an important way forward in the treatment of hypertension [which] may be a paradigm for managing a range of chronic diseases.”16 Yet the STITCH algorithm has yet to be widely embraced; outside of the research community, most US physicians are relying on the JNC 7 guidelines.
ACC/AHA recommendations indicate that for patients at stage A—that is, those with conditions strongly associated with, and at high risk for, HF—management of hypertension should conform to national standards such as JNC 7. The JNC 7 guidelines recommend the use of a thiazide diuretic as the initial drug of choice for patients with essential hypertension. For those with diabetes, ACE inhibitors and ARBs are the first-line antihypertensive agents of choice.
Glucose control is also essential for stage A patients with diabetes. Treatment of lipid disorders and pharmacotherapy for metabolic syndrome are also recommended for stage A patients, as needed.
Treatment escalates as HF progresses
ACE inhibitors, ARBs, and beta-blockers are the preferred pharmacologic interventions for patients at stage B—those who have structural heart disease strongly associated with HF but are not yet symptomatic. Anyone who has had a myocardial infarction (MI) should be started on a beta-blocker and an ACE inhibitor, ACC/AHA recommends, unless a contraindication exists.6 Similarly, any patient with a reduced ejection fraction should be started on an ACE inhibitor regardless of symptoms.6
The Heart Outcomes Prevention Evaluation (HOPE) study demonstrated a 23% relative risk (RR) reduction with the use of an ACE inhibitor in patients with coronary artery disease, peripheral vascular disease, or diabetes, compared with patients receiving a placebo.17 The importance of a beta-blocker was established in a subanalysis of the Survival and Ventricular Enlargement Trial (SAVE), which found that patients taking beta-blockers in addition to an ACE inhibitor had a 32% RR reduction in progression of HF, compared with patients on an ACE inhibitor alone.18
We recommend an ACE inhibitor or an ARB and a beta-blocker, when appropriate, to slow the progression of HF pathophysiology. It is important to be aware of the potential adverse effects of certain beta-blockers in patients with HF. Only 3 beta-blockers are approved for use in this patient population in the United States—bisoprolol, carvedilol, and metoprolol succinate, which have been found to provide benefits that other beta-blockers do not.6,15
Stages C and D: Tx considerations and controversies
Treatment for patients at stage C should include all components of therapy for patients at stages A and B, but with a more aggressive use of pharmacotherapy ( TABLE 2 ). Patients with stage C HF, by definition, are symptomatic, and the ACC/AHA recommendations reflect concern about their increasingly compromised status. Thus, in addition to the use of ACE inhibitors or ARBs and beta-blockers, modest use of diuretics is recommended, as needed, for fluid volume control.6 Diuretics should be used judiciously, though, with ongoing evaluation to avoid the excessive loss of potassium and magnesium, which can lead to volume depletion and lethal arrhythmias. Limiting sodium consumption is an important dietary restriction for stage C patients.
Aldosterone antagonists may also be considered on a case-by-case basis for patients with stage C HF. Due to their potassium-sparing effects, aldosterone antagonists, used in conjunction with standard therapies, may have a positive effect on electrolyte balance. Potassium levels must be carefully monitored, however, and potassium supplementation reevaluated for patients who are put on an aldosterone antagonist.19
Digitalis may also be helpful in select patients who remain symptomatic despite maximal pharmacotherapy.20 While it does not affect mortality, digitalis has been shown to reduce hospitalizations.21
ACE inhibitor-ARB combination therapy, another possible treatment for advanced HF, remains controversial. The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), detailed in “ACE inhibitors and ARBs: One or the other—not both” in the January 2009 issue of The Journal of Family Practice, evaluated use of this dual therapy; the trial was also designed to determine whether telmisartan (an ARB) is inferior to ramipril (an ACE inhibitor) in patients at high risk for vascular events.22 The researchers found that telmisartan is not, in fact, inferior to ramipril, and reported that for patients with HF, an ACE-ARB combination offers a potential benefit.
However, the clinical benefit of an ACE-ARB combination in this patient population was not clarified in this study, and may be potentially harmful. In the Valsartan Heart Failure Trial (ValHeFT), the combination of valsartan, an ARB, and an ACE inhibitor decreased hospitalizations but did not improve mortality.23 Indeed, an increase in mortality was found when an ACEARB combination was used in conjunction with beta-blockers. Because beta-blockers are indicated for routine use in patients with HF, this finding was of particular concern.
In a meta-analysis of randomized trials using both an ACE inhibitor and an ARB in patients with left ventricular dysfunction, researchers found a “marked” increase in adverse effects, including deteriorating renal function (RR=2.17), hyperkalemia (RR=4.87), and symptomatic hypotension (RR=1.05).24 Although an ACE-ARB combination may benefit a subset of patients with HF, it is best to initiate such treatment only with the guidance of an HF specialist.
TABLE 2
Treating heart failure: How the different drugs and devices rate
| STAGE | PHARMACOTHERAPY | LOE | DEVICE/INTERVENTION | LOE |
|---|---|---|---|---|
| A | Treat BP per JNC 7 ACE inhibitor or ARB for patients with vascular disease or diabetes | A | None | N/A |
| B | ACE inhibitor or ARB BB | A | None | N/A |
| C | Routine use: Diuretics ACE inhibitor BB Select use: Aldosterone antagonist ARB Digitalis | A | Consider: Biventricular pacer or ICD or both | B |
| D | Same as C | B | Consider: Heart transplant or LVAD; experimental protocols | C |
| ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BB, beta-blocker; BP, blood pressure; ICD, implantable cardioverter defibrillator; JNC 7, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; LOE, level of evidence; LVAD, left ventricular assist device. | ||||
| Adapted from: Hunt SA, et al. Circulation. 2005.6 | ||||
Beyond drug therapy: Assistive devices
Refractory end-stage HF requires a clear treatment plan, and should involve the recommendations of an HF specialist. Careful maintenance of fluid status is required, and an evaluation for cardiac transplantation may be considered.
A left ventricular assist device (LVAD) should also be considered for patients with an estimated 1-year mortality of >50%.6 LVADs are mechanical heart pumps that were initially utilized as a “bridge” to transplant, but are increasingly being used as a palliative alternative for severely ill patients.25
Other devices—an implantable cardioverter defibrillator (ICD) or a biventricular pacer—should also be considered for patients at stage D, as well as stage C patients who are at increased risk of sudden death despite maximal drug therapy.6 Patients who have had a previous MI or ventricular arrhythmia are at risk for a repeat episode.6
Use of an ICD can reduce mortality by 23% in selected patients.26 Potential candidates for the device are patients who have an ejection fraction of <30%, mild to moderate symptoms, and a life expectancy of at least 1 year.6
Biventricular pacing, also known as cardiac resynchronization therapy (CRT), has been found to improve the quality of life, functional status, and exercise capacity in some patients with advanced disease. CRT, which reduces symptoms of HF and improves cardiac function by reestablishing the mechanical sequence of ventricular activation and ventricular contraction, has also been associated with reductions in hospitalization and death from progressive HF.27,28
The Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial demonstrated a 20% reduction in the 12-month risk of death or hospitalization from any cause with CRT, and the Cardiac Resynchronization-Heart Failure (CARE-HF) trial established that patients receiving CRT had a significantly lower risk of death than those receiving medical therapy alone (40% reduction).29,30
However, not all patients with HF have problems with conduction delay that result in a dyssynchronous heart beat. CRT is indicated only for patients who are in sinus rhythm and have:
- NYHA class III or IV HF
- an ejection fraction of <35%
- a prolonged QRS complex (>120 m/sec), and
- continued symptoms despite maximal medical therapy.6
Under these criteria, approximately 10% of patients with HF would qualify for CRT.31 The restrictive criteria are due, in part, to the fact that this modality is relatively new and has been studied only in a small subset of patients.
Options for patients who are running out of them
For acutely decompensated hospitalized patients with volume overload, ultrafiltration (UF) is a useful alternative to diuretics. UF uses high pressure to “force” volume through the kidneys;32,33 the technique maximizes diuresis, and is best suited for patients who have significant renal dysfunction or are not responding to standard diuretic therapy. UF makes it easier to remove the desired amount of fluid, and has a positive impact on pulmonary wedge pressure and cardiac output.34 Its use in diuretic-resistant patients can decrease the length of stay and produce positive clinical benefits that may last up to 3 months.34
There are also a number of experimental strategies, surgical and otherwise. Among them are:
Cardiac wrap surgery, in which the heart is encased in a mesh bag attached with stitches, in an attempt to stop the progression of end-stage HF by preventing further dilation;25
Ventricular restoration surgery, a procedure in which scar tissue caused by MI is removed from the ventricular muscle and the left ventricle is reshaped and its size reduced in an attempt to restore some of the heart’s pumping ability;25 and
Enhanced external counterpulsation, or EECP, a noninvasive technique in which pressure cuffs are placed on the calves, thighs, and buttocks and inflated and deflated in an attempt to increase blood flow back to the heart.25
Correspondence
Randy Wexler, MD, MPH, FAAFP, The Ohio State University, B0902B Cramblett Hall, 456 W. 10th Avenue, Columbus, OH 43210; [email protected]
1. Rosamond W, Flegal K, Friday G, et al. American Heart Association: heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69-e171.
2. Lloyd-Jones DM, Larson MG, Leip EP, et al. Framingham heart study. Lifetime risk for developing congestive heart failure. Circulation. 2002;106:3068-3072.
3. Burt CW, Schappert SM. Ambulatory care visits to physicians offices, hospital outpatient departments, and emergency departments: United states, 1999-2000. Vital Health Stat 13. 2004;157:1-70
4. Levit K, Stranges E, Ryan K, et al. HCUP facts and figures, 2006: Statistics on Hospital-based Care in the United States. Rockville, MD: Agency for Healthcare Research and Quality, 2008. Available at: http://www.hcup-us.ahrq.gov/reports.jsp. Accessed February 9, 2009.
5. Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels–Nomenclature and Criteria for Diagnosis. 6th ed. Boston: Little, Brown and Company; 1964.
6. Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult. Circulation. 2005;112:e154-e235.
7. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74:1893-1898.
8. Maisel A, Krishnaswamy P, Nowak R, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161-167.
9. Maisel AS. B-type natriuretic peptide (BNP) levels: diagnostic and therapeutic potential. Rev Cardiovasc Med. 2001;2(suppl 2):S13-S18.
10. Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B-type natriuretic peptide (BNP) in the diagnosis of congestive heart failure in an urgent-care setting. J Am Coll Cardiol. 2001;37:379-385.
11. Philbin EF, Rocco TA, Jr, Lindenmuth NW, et al. Systolic versus diastolic heart failure in a community practice: clinical features, outcomes, and the use of angiotensin-converting enzyme inhibitors. Am J Med. 2000;109:605-613.
12. Piepoli MF, Flather M, Coats AJ. Overview of studies of exercise training in chronic heart failure: the need for a prospective randomized multicentre European trial. Eur Heart J. 1998;19:830-841.
13. Coats AJ, Adamopoulos S, Meyer TE, et al. Effects of physical training in chronic heart failure. Lancet. 1990;335:63-66.
14. Feldman RD, Zou G, Feagen BG, et al. The STITCH Investigators. The Simplified Treatment Intervention to Control Hypertension (STITCH) trial: A cluster randomized controlled trial of a step-care algorithm using initial fixed dose combination therapy for the management of hypertension. Presented at Scientific Sessions 2007 of the American Heart Association, November 4-7, 2007; Orlando, Fla.
15. Chobanian A, Bakris G, Black H, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). Hypertension. 2003;42:1206-1252.
16. Brookes L. Hypertension highlights: new drug algorithms, new drug approvals, new drugs. Available at: http://canadiancpd.medscape.com/viewarticle/568786_print. Accessed January 27, 2009.
17. Yusuf S, Sleight P, Pogue J, et al. The Heart Outcomes Prevention Evaluation Study Investigators: effects of angiotensin converting enzyme inhibitor, ramipril, on cardiovascular events in high risk patients. N Engl J Med. 2000;342:145-153.
18. Vantrimpont P, Rouleau JL, Wun CC, et al. For the SAVE Investigators. Additive beneficial effects of beta-blockers to angiotensin converting enzyme inhibitors in the Survival and Ventricular Enlargement (SAVE) study. J Am Coll Cardiol. 1997;29:229-236.
19. Juurlink DN, Mamdani M, Kopp A, et al. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652-1658.
20. Rahimtoola SH. Digitalis therapy for patients in clinical heart failure. Circulation. 2004;109:2942-2946.
21. Cayley W. Digitalis for the treatment of congestive heart failure in patients in sinus rhythm. Am Fam Physician. 2004;69:71-73.
22. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
23. Cohn JN, Tognoni G. Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667-1675.
24. Phillips CO, Kashani A, Ko DK, et al. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction. Arch Intern Med. 2007;167:1930-1936.
25. Mayo Clinic. Heart failure: treatments and drugs. January 3, 2008. Available at: http://www.mayoclinic.com/health/heart-failure.DS00061/DSECTION=treatments-and-drugs. Accessed January 30, 2009.
26. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237.
27. Blanc J-J, Bertault-Valls V, Fatemi M, et al. Mid-term benefits of left univentricular pacing in patients with congestive heart failure. Circulation. 2004;109:1741-1744.
28. Bradley DJ, Bradley EA, Baughman KL, et al. Cardiac resynchronization and death from progressive heart failure: a meta-analysis of randomized controlled trials. JAMA. 2003;289:730-740.
29. Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-2150.
30. Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539-1549.
31. Farwell D, Patel NR, Hall A, et al. How many people with heart failure are appropriate for biventricular resynchronization? Eur Heart J. 2000;21:1246-1250.
32. Bart BA, Boyle A, Bank AJ, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the relief for acutely fluid-overloaded patients with decompensated congestive heart failure (RAPD-HF). J Am Coll Cardiol. 2005;46:2043-2046.
33. Costanzo MR, Saltzberg M, O’Sullivan J, et al. Early ultrafiltration in patients with acutely decompensated heart failure and diuretic resistance. J Am Coll Cardiol. 2005;46:2047-2051.
34. Agostini PG, Marenzi GC, Lauri G, et al. Sustained improvement in functional capacity after removal of body fluid with isolated ultrafiltration in chronic cardiac insufficiency; failure of furosemide to provide same results. Am J Med. 1994;96:191-199.
1. Rosamond W, Flegal K, Friday G, et al. American Heart Association: heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69-e171.
2. Lloyd-Jones DM, Larson MG, Leip EP, et al. Framingham heart study. Lifetime risk for developing congestive heart failure. Circulation. 2002;106:3068-3072.
3. Burt CW, Schappert SM. Ambulatory care visits to physicians offices, hospital outpatient departments, and emergency departments: United states, 1999-2000. Vital Health Stat 13. 2004;157:1-70
4. Levit K, Stranges E, Ryan K, et al. HCUP facts and figures, 2006: Statistics on Hospital-based Care in the United States. Rockville, MD: Agency for Healthcare Research and Quality, 2008. Available at: http://www.hcup-us.ahrq.gov/reports.jsp. Accessed February 9, 2009.
5. Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels–Nomenclature and Criteria for Diagnosis. 6th ed. Boston: Little, Brown and Company; 1964.
6. Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult. Circulation. 2005;112:e154-e235.
7. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74:1893-1898.
8. Maisel A, Krishnaswamy P, Nowak R, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161-167.
9. Maisel AS. B-type natriuretic peptide (BNP) levels: diagnostic and therapeutic potential. Rev Cardiovasc Med. 2001;2(suppl 2):S13-S18.
10. Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B-type natriuretic peptide (BNP) in the diagnosis of congestive heart failure in an urgent-care setting. J Am Coll Cardiol. 2001;37:379-385.
11. Philbin EF, Rocco TA, Jr, Lindenmuth NW, et al. Systolic versus diastolic heart failure in a community practice: clinical features, outcomes, and the use of angiotensin-converting enzyme inhibitors. Am J Med. 2000;109:605-613.
12. Piepoli MF, Flather M, Coats AJ. Overview of studies of exercise training in chronic heart failure: the need for a prospective randomized multicentre European trial. Eur Heart J. 1998;19:830-841.
13. Coats AJ, Adamopoulos S, Meyer TE, et al. Effects of physical training in chronic heart failure. Lancet. 1990;335:63-66.
14. Feldman RD, Zou G, Feagen BG, et al. The STITCH Investigators. The Simplified Treatment Intervention to Control Hypertension (STITCH) trial: A cluster randomized controlled trial of a step-care algorithm using initial fixed dose combination therapy for the management of hypertension. Presented at Scientific Sessions 2007 of the American Heart Association, November 4-7, 2007; Orlando, Fla.
15. Chobanian A, Bakris G, Black H, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). Hypertension. 2003;42:1206-1252.
16. Brookes L. Hypertension highlights: new drug algorithms, new drug approvals, new drugs. Available at: http://canadiancpd.medscape.com/viewarticle/568786_print. Accessed January 27, 2009.
17. Yusuf S, Sleight P, Pogue J, et al. The Heart Outcomes Prevention Evaluation Study Investigators: effects of angiotensin converting enzyme inhibitor, ramipril, on cardiovascular events in high risk patients. N Engl J Med. 2000;342:145-153.
18. Vantrimpont P, Rouleau JL, Wun CC, et al. For the SAVE Investigators. Additive beneficial effects of beta-blockers to angiotensin converting enzyme inhibitors in the Survival and Ventricular Enlargement (SAVE) study. J Am Coll Cardiol. 1997;29:229-236.
19. Juurlink DN, Mamdani M, Kopp A, et al. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652-1658.
20. Rahimtoola SH. Digitalis therapy for patients in clinical heart failure. Circulation. 2004;109:2942-2946.
21. Cayley W. Digitalis for the treatment of congestive heart failure in patients in sinus rhythm. Am Fam Physician. 2004;69:71-73.
22. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
23. Cohn JN, Tognoni G. Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667-1675.
24. Phillips CO, Kashani A, Ko DK, et al. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction. Arch Intern Med. 2007;167:1930-1936.
25. Mayo Clinic. Heart failure: treatments and drugs. January 3, 2008. Available at: http://www.mayoclinic.com/health/heart-failure.DS00061/DSECTION=treatments-and-drugs. Accessed January 30, 2009.
26. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237.
27. Blanc J-J, Bertault-Valls V, Fatemi M, et al. Mid-term benefits of left univentricular pacing in patients with congestive heart failure. Circulation. 2004;109:1741-1744.
28. Bradley DJ, Bradley EA, Baughman KL, et al. Cardiac resynchronization and death from progressive heart failure: a meta-analysis of randomized controlled trials. JAMA. 2003;289:730-740.
29. Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-2150.
30. Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539-1549.
31. Farwell D, Patel NR, Hall A, et al. How many people with heart failure are appropriate for biventricular resynchronization? Eur Heart J. 2000;21:1246-1250.
32. Bart BA, Boyle A, Bank AJ, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the relief for acutely fluid-overloaded patients with decompensated congestive heart failure (RAPD-HF). J Am Coll Cardiol. 2005;46:2043-2046.
33. Costanzo MR, Saltzberg M, O’Sullivan J, et al. Early ultrafiltration in patients with acutely decompensated heart failure and diuretic resistance. J Am Coll Cardiol. 2005;46:2047-2051.
34. Agostini PG, Marenzi GC, Lauri G, et al. Sustained improvement in functional capacity after removal of body fluid with isolated ultrafiltration in chronic cardiac insufficiency; failure of furosemide to provide same results. Am J Med. 1994;96:191-199.
Hypertension: Which drugs to choose for patients with cardiovascular disease
- Decreasing blood pressure by 5 mm Hg will decrease mortality due to stroke by 14%, attenuate cardiac mortality by 9%, and reduce all-cause mortality by 7% (A).
- Patients with heart failure should receive angiotensin-converting enzyme inhibitors if they are clinically tolerated (A). Beta-blockers are also recommended (A). Although aldosterone antagonists are appropriate for use in patients with heart failure (A), we recommend they are taken under the care of a cardiologist to minimize complications and to insure that a complete heart failure plan is in place (C).
- For coronary artery disease, it is now considered standard of care to add a beta-blocker to all patients post–MI that do not have severe heart block or are in cardiogenic shock (A).
- Perindopril plus indapamide should be used in all patients with a history of stroke or transient ischemic attack regardless of blood pressure (B).
Hypertension precedes more than 75% of heart failure cases and 50% of heart attacks. In 67% of first-time strokes, blood pressure exceeds 140/90 mm Hg.1 For those in heart failure or at risk of recurrent myocardial infarction (MI) or stroke, the need to reduce unrecognized or under-treated hypertension is urgent. Thankfully it is possible to select an agent that can lower blood pressure—the most important immediate goal—and confer benefit to the associated cardiovascular disorder.
Given the number of agents used for both hypertension and other cardiovascular conditions, we sought in this study to evaluate which drug classes would best achieve blood pressure treatment goals and reduce morbidity and mortality for patients with cardiac disease. In this article, you will find practical recommendations for drug selection and appropriate regimens.
Even small blood pressure reductions yield big benefits for comorbidities
Using the population of the National Health and Nutrition Examination Survey I Epidemiologic Follow Up Study, Ogden and colleagues2 found that the benefit of treating high blood pressure over a decade depended on lowering systolic blood pressure and on treating other relevant cardiovascular comorbidities. Specifically, the number needed to treat (NNT) and prevent a death was directly related to the risk stratification of patients and their initial blood pressure (TABLE W1) (LOE: 1).
In another provocative trial, the Swedish Trial in Old Patients with Hypertension-2 Study (STOP Hypertension-2)3 evaluated the use of beta-blockers, diuretics, angiotensin-converting enzyme (ACE) inhibitors, or calcium channel blockers. Results of this trial showed that an overall reduction in cardiovascular events related to the ability of a drug class to lower blood pressure (LOE: 1).3
In addition, a meta-analysis of 29 randomized trials (n=162,341) in the Blood Pressure Lowering Treatment Trialists’ Collaboration Trial (BPLTTC)4 showed that all antihypertensive drug classes significantly reduce blood pressure. This meta-analysis confirmed an overall reduction in cardiovascular events, with perceived risk reduction directly proportional to blood pressure reduction (LOE: 1). This advantage was consistent irrespective of drug class (beta-blocker, diuretic, ACE inhibitor, calcium-channel blocker, or angiotensin receptor blocker [ARB]), although different drug classes were recognized to have unique benefits specific to individual patient populations.
Should the BP target level be lowered? Recent evidence suggests that the currently accepted range for normal blood pressure may be too high.5,6
Lewington and colleagues performed a meta-analysis of 61 prospective studies with more than 1 million participants. Using a “time-dependent” correction for regression dilution, they sorted deaths in each decade of age according to estimated blood pressures at the start of the decade (LOE: 1).5 They found that with each decade of life there was a proportional decline in the risk of cardiovascular deaths when blood pressures were controlled incrementally to levels of 115 mm Hg systolic blood pressure and 75 mm Hg diastolic blood pressure (LOE: 1).5 At blood pressures below 115/75 mm Hg, no difference was observed.
In addition, Vasan and collaborators,6 using the Framingham Heart Study database (n=6859 participants), reported an increase in cardiovascular events with higher baseline levels of blood pressure. When compared with optimal blood pressure levels, those with high-normal blood pressure (130–139/85–89 mm Hg) had a risk-factor-adjusted hazard ratio for cardiovascular disease of 2.5 for women and 1.6 for men (LOE: 1).6
The “take-home” message. A small reduction in blood pressure yields a very significant risk reduction. Decreasing blood pressure by 5 mm Hg will decrease mortality due to stroke by 14%, cardiac mortality by 9%, and all cause mortality by 7% (LOE: 1).7 These data suggest that aggressive intervention to affect small changes might affect large differences in morbidity and mortality.
Antihypertensive drugs in heart failure
Angiotensin-converting enzyme inhibitors
Give all patients with heart failure an ACE inhibitor, if clinically tolerated (SOR: A). Although blood pressure control is very important to treat the physiology and neurohormonal basis of heart failure, the primary reason to use ACE inhibitors (as well as other medications) is to provide a disease modifying intervention and treat blood pressure when it is elevated. Different disease conditions require different doses to achieve the desired goal. With heart failure, the dose of an ACE inhibitor is given twice daily at typically 2 to 3 times the dose of that used for hypertension.
The evidence. In 1991, the Studies of Left Ventricular Dysfunction (SOLVD) trial8 demonstrated a 26% risk reduction for death or hospitalization due to heart failure (95% confidence interval [CI], 18–34) for those treated with the ACE inhibitor enalapril (Vasotec) (LOE: 1). The following year, the Survival and Ventricular Enlargement (SAVE)9 trial demonstrated a risk reduction of 19% for patients with a reduced ejection fraction after myocardial infarction (MI) when the ACE inhibitor captopril (Capoten) was used (LOE: 1).
The calculated NNT with an ACE inhibitor to save 1 life over 1 year is 43.10 (See TABLE W2 for a summary of clinical trials and levels of evidence.) The TABLE in this article summarizes treatment recommendations based on these studies.8-23
TABLE
Drugs of choice for hypertension and various comorbidities
| TREATMENT RECOMMENDATIONS BASED ON STUDIES/GUIDELINES | SOR |
|---|---|
| Congestive Heart Failure | |
| ACE inhibitor should be used in patients with heart failure unless a contraindication exists8,9,11,12 | A |
| Beta-blockers should be used in patients with heart failure unless a contraindication exists11-15 | A |
| ARB should be used in heart failure if patient is intolerant to ACE inhibitor12 | A |
| Aldosterone antagonists should be used in patients with severe heart failure unless a contraindication exists16,17 | A |
| Aldosterone antagonists should be prescribed in consultation with a cardiologist | C |
| Coronary Artery Disease | |
| Beta-blockers should be used in patients post-MI unless a contraindication exists11,13,18,19 | A |
| CCB should be used in patients with stable coronary artery disease unless a contraindication exists11,20 | A |
| ACE inhibitors should be used in patients with stable coronary artery disease and no left ventricular dysfunction unless a contraindication exists11,21,22 | A |
| Stroke | |
| ACE inhibitor and indapamide should be used in patients with a TIA or stroke unless a contraindication exists11,23 | B |
| ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; MI, myocardial infarction; SOR, strength of recommendation; TIA, transient ischemic attack | |
Beta-blockers
Give a beta-blocker, if tolerated, to patients in heart failure (SOR: A). Dosing has been determined by clinical trial data. In general, in order to significantly impact morbidity and mortality in congestive heart failure, the patient needs to reach a dose of 150 mg of metoprolol XL a day or 6.25 mg to 12.5 mg of carvedilol given twice daily. Ideal doses are greater than 200 mg/d of metoprolol XL or 25 mg twice daily of carvedilol.
The evidence. The Cardiac Insufficiency Bisoprolol Study (CIBIS),24 published in 1994, was a randomized, placebo-controlled, double-blind trial designed to test the efficacy of beta-blockade in the treatment of heart failure (LOE: 1). Although no difference in mortality was demonstrated between intervention and control groups, the intervention group showed improved functional status.
The Carvedilol Post Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN)13 trial (LOE: 1) evaluated patients with left ventricular dysfunction or heart failure after an MI, while the Carvedilol Prospective Randomized Cumulative Survey (COPERNICUS)14 group (LOE: 1) enrolled only patients with severe heart failure (ejection fraction <25%, NYHA class III and IV). These studies demonstrated an overall decrease in cardiovascular morbidity and mortality, as well as all-cause mortality for patients with heart failure receiving the nonspecific beta-blocker carvedilol (Coreg) (receptor blockade at β1, β2, α1). CAPRICORN produced an overall risk reduction in mortality of 2% to 3% at 1 year, resulting in the same NNT (43) over 1 year as ACE inhibitors.13 This is the only beta-blocker tested after infarction to demonstrate a mortality difference for patients with heart failure or decreased left ventricular dysfunction (ejection fraction <40%).
Hypertension specialists debate about how to approach the hypertensive patient. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Management of High Blood Pressure (JNC-7) guidelines11 call for defined goals in lowering blood pressure and a stepwise selection of drugs based on comorbidities.
Some leading hypertension experts say this is too formulaic. Dr Michael Alderman, Professor in the Department of Medicine and Epidemiology and Population Health at the Albert Einstein School of Medicine, argues “we have to get over the limitation of the straightjacket of numbers to define our actions.”25 He further asserts that “our willingness to drive blood pressure down has to be modulated by the risk the patient has and the price one has to pay to lower it. A 30% reduction in risk does not mean much if your risk is low, but if your risk is high it means a lot.”25 As such, Dr Alderman argues we should base treatment decisions on “total risk” and not the level of blood pressure. Other leaders in the field such as Mathew Weir, MD, director of the Division of Nephrology, University of Maryland, and Richard Devreaux, MD, Professor of Medicine, Division of Cardiology, Cornell University Medical Center, agree with this more individualized approach.25
But understanding the basic formula is what helps us innovate. In general, we agree that individualizing patient care is the ideal, and that some patients may not tolerate “recommended” treatment. However, it is not possible for physicians to individualize care (a highly complex undertaking) when they still lack understanding at the basic level of care. With the poor treatment of hypertension in the US, we believe that guidelines such as JNC-7 are essential to improving blood pressure control.
Hyman and Pavlik26 demonstrated that physician factors, especially lack of awareness of hypertension treatment recommendations, correlate with poor hypertension treatment. In their 2001 study that included 1200 primary care physicians, 41% of physicians were not familiar with or had not heard of the recommendations. This finding was not trivial. The importance of familiarity with JNC-7 guidelines was demonstrated when statistical analysis revealed that a working knowledge of these guidelines significantly increased adherence with published recommendations (including blood pressure control). As such, it would appear that not following the guidelines has less to do with disagreements over treatment options and more to do with understanding the value of the guidelines to basic management.
The Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF)15 concluded that the addition of extended-release metoprolol (Lopressor, a β1-adrenergic receptor blocker) for patients with heart failure demonstrated a survival benefit when compared with patients not receiving a beta-blocker (LOE: 1). One of the essential elements of this trial was the ability to achieve a dose of 200 mg of metoprolol a day. Frequently in clinical practice low-dose or even homeopathic doses are used with few data to support such use.
The Carvedilol or Metoprolol European Trial (COMET)27 of patients with heart failure suggested that nonselective neurohumoral (β and α) blockade may increase the benefit in comparison with selective β1-blockade (LOE: 1). There has been significant debate regarding the dose and the formulation of the drugs in COMET, but we advocate using doses and drug formulations that were specifically in the large prospective randomized trials (CIBIS II, the carvedilol trials, and MERIT-HF).
Angiotensin receptor blockers
Prescribe an ARB only when a patient cannot tolerate an ACE inhibitor secondary to cough or hyperkalemia.12 As these are generally used after or as an adjunct to ACE inhibitors, the usual dose is similar to that for blood pressure dosed twice daily. If patients do not tolerate ACE inhibitors, theses doses may also be higher than those used for blood pressure response alone.
The evidence. The Losartan Heart Failure Survival Study (ELITE II)28 demonstrated the benefit of the ARB losartan (Cozaar) in the treatment of heart failure, but not superiority over previously used ACE inhibitors (LOE: 1). In addition, there was no difference in renal insufficiency with one drug class compared with another. Researchers concluded that ARBs should be used only for patients intolerant to ACE inhibitors.
The Valsartan Heart Failure Trial Investigators Study (Val-HeFT)29 randomized 5010 patients to receive valsartan (Diovan) or placebo combined with standard therapy (ACE inhibitors and beta-blockers) (LOE: 1). The ARB group demonstrated a 13.2% greater reduction than placebo in the combined endpoint of morbidity and mortality (as defined by incidence of cardiac arrest and resuscitation, hospitalization for heat failure, or administration of intravenous inotropes or vasodilators for a minimum of 4 hours). However, a post-study review29 of patients who received the ARB, ACE inhibitor, and beta-blocker combination showed increased mortality.
In 2004, the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity trial (CHARM)30 found that when the ARB candesartan (Atacand) was added to standard therapy (ACE inhibitor, beta-blockers, aldosterone antagonist) there was a 33% reduction in all-cause mortality, similar to that found with beta-blockers and ACE inhibitors) (LOE: 1). In addition, the CHARM group found no increased risk when candesartan was combined with other treatments; it concluded that ARBs could be added to regimens for all patients with heart failure unless a contraindication exists.
The American College of Cardiology/American Heart Association guidelines prefer ACE inhibitors over ARBs, and recommend ARBs be used when an ACE inhibitor is not tolerated or if there are other contraindications. A low level of evidence (2b) suggests that an ARB may be added to conventional medical therapy with an increased risk of renal insufficiency and hyperkalemia (SOR: A).12
Potassium-sparing diuretics
Aldosterone antagonists are appropriate for patients with heart failure (SOR: A), though we recommend working in conjunction with a cardiologist to minimize complications and to insure that a complete heart failure plan is in place (SOR: C). Spirinolactone (Aldactone, Aldactazide) is used for physiologic purposes (as a neurohormonal regulator) and is not used for blood pressure control. Most heart failure specialists begin with a dose of 12.5 mg/d and advance to doses utilized in the clinical trials (25–50 mg/d).
The evidence. Potassium-sparing diuretics lower mortality among heart failure patients. Spironolactone works in part by reducing aldosterone levels and increasing serum potassium.
In the Randomized Aldactone Evaluation Study (RALES),16 patients with severe heart failure (ejection fraction <35%) on standard medical therapy were randomized to receive spironolactone or placebo (LOE: 1). The spironolactone group exhibited a 30% reduction in mortality compared with conventional medical therapy, and the study was ended early at 24 months.
In the Eplerenone Post Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS),17 an overall reduction in death of 8%, a decrease of sudden cardiac death by >20% (relative risk reduction), as well as an overall reduction of hospitalization for heart failure of 15% occurred in the eplerenone (Inspra) group (LOE: 1) (NNT to save 1 life in 1 year=50).
It is worth noting that the doses used in these studies were devised to alter neurohumoral regulation, and are not to be used as significant diuretics. To date, no comparative study between spironolactone and eplerenone has been undertaken.
Coronary artery disease
Beta-blockers
Prescribe a beta-blocker for every post-MI patient without severe heart block or cardiogenic shock (SOR: A).
The evidence. The Beta-Blocker Heart Attack Trial (BHAT),18 sponsored by the National Heart, Lung, and Blood Institute, was designed to evaluate the benefits of the beta-blocker propranolol (Inderal) after MI (completed more than 24 years ago, before modern medical therapy) (LOE: 1). Total mortality during the average 24-month follow-up period was 7.2% in the propranolol group and 9.8% in the placebo group. The incidence of nonfatal reinfarction was decreased by 15.6% in the treatment group.
A similar trial completed in the early 1980s was The Norwegian Multi-Center Study.19 This trial, which assessed the efficacy of timolol (Blocadren, Timolide) after MI, demonstrated a 44.6% reduction in sudden cardiac death (LOE: 1). The study group reached the same conclusion as the BHAT researchers, and recommended that beta-blockers be used following an MI to reduce reinfarction and death.
Angiotensin-converting enzyme inhibitors
Use ACE inhibitors only for stable post-MI patients without decreased left ventricular function.
The evidence. The Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE) trial31 evaluated the benefit of using an ACE inhibitor for patients with stable coronary artery disease and slightly reduced ejection fraction (>40%) (LOE: 1). There was no statistical difference in the primary endpoint (cardiac-induced death, MI, or need for revascularization) between the treatment group (21.9%) and the placebo group (22.5%).
Contrary to the findings of the PEACE trial, many other studies have shown that ACE inhibitors are beneficial for patients with coronary artery disease. In the Trandolapril Cardiac Evaluation (TRACE) study,32 patients stabilized after acute MI were randomized to receive the ACE inhibitor trandolapril (Mavik) or placebo on days 3 to 7 following infarction (LOE: 1). In the treatment group, risk of death from all causes declined 17.6%, risk of death from cardiovascular causes fell 21%, and progression to severe heart failure decreased 27%. These post-MI benefits are also supported by results of the Survival of Myocardial Infarction Long-Term Evaluation (SMILE) study (LOE: 1).33
The Heart Outcomes Prevention Evaluation Investigators (HOPE)21 (LOE: 1), and the European Trial on Reduction of Cardiac Events with Perindopril in Patients with Coronary Artery disease (EUROPA)22 (LOE: 1), demonstrated the benefits of ACE inhibitors in reducing cardiovascular events for patients with or at risk for coronary artery disease, but with normal left ventricular function. The HOPE study showed a significant reduction of events with the ACE inhibitor ramipril (Altace) (NNT=1000 patients over 4 years, resulting in a decrease of 150 events for 75 patients), whereas EUROPA demonstrated the results for the ACE inhibitor perindopril (Aceon) (NNT=50 patients over 4 years to prevent 1 major cardiovascular event).
Calcium channel blockers
Use calcium channel blockers only for stable post-MI patients without decreased left ventricular function or heart failure.
The evidence. The Comparison of Amlodipine vs Enalapril to Limit Occurrences of Thrombosis (CAMELOT)20 study compared treatment using a calcium channel blocker (amlodipine) and an ACE inhibitor (enalapril) with placebo for normotensive patients with coronary artery disease (LOE: 1). Amlodipine reduced hospitalization for angina by 42.2%, nonfatal MI by 26%, and stroke or transient ischemic attack by 50.4% (NNT=16). The study group concluded that the use of the ACE inhibitor enalapril showed “directionally similar, but smaller and nonsignificant, treatment effects.”20 There was no reduction in overall mortality.
Stroke
Prescribe the combination of perindopril and indapamide for all patients with a history of stroke or transient ischemic attack, regardless of blood pressure (SOR: B).
The evidence. The Perindopril Protection Against Recurrent Stroke Study (PROGRESS)23 was designed to evaluate the benefits of the ACE inhibitor perindopril (with the addition of indapamide at the physician’s discretion) for patients with or without hypertension who have had a transient ischemic attack or stroke (LOE: 1). Perindopril plus indapamide reduced risk of stroke by 43%, but treatment with a single agent showed risk reduction.
To see which drug classes are most often recommended in the treatment of hypertension based on underlying cardiovascular disease, we performed an initial Medline search using the key words hypertension and cardiovascular disease. This was supplemented with a search of the archives of the journals Circulation, Hypertension, Stroke, and the authors’ personal references.
All studies were evaluated using the Strength of Recommendation Taxonomy.34 Strength of recommendation (SOR) evaluates a study based on patient (not disease) oriented outcomes, and level of evidence (LOE) is based on key outcomes as well as the methodology of the study.
CORRESPONDENCE
Randy Wexler, MD, MPH, FAAFP, Assistant Professor of Clinical Family Medicine, The Ohio State University College of Medicine and Public Health, B0902B Cramblett Hall, 456 West 10th Ave, Columbus, Ohio 43201. E-mail: [email protected]
1. American Heart Association. Heart Disease and Stroke Statistics–2004 Update. Dallas, Tex: American Heart Association, 2003.
2. Ogden LG, He J, Lydick E, Whelton P. Long-term absolute benefit of lowering blood pressure in hypertensive patients according to JNC VI risk stratification. Hypertension 2000;35:539-543.
3. Hansson L, Lindholm L, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet 1999;354:1751-1756.
4. Turnbull F. Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomized trials. Lancet 2003;362:1527-1535.
5. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903-1913.
6. Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001;345:1291-1297.
7. Whelton P, He J, Appel L, et al. National High Blood Pressure Education Program Coordinating Committee. Primary prevention of hypertension: Clinical and public health advisory from the National High Blood Pressure Education Program. JAMA 2002;288:1882-1888.
8. The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293-302.
9. Pfeffer MA, Braunwald E, Moye LA, et al. The SAVE Investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. N Engl J Med 1992;327:669-677.
10. Flather MD, Yusuf S, Kobler L, et al. ACE-Inhibitor Myocardial Infarction Collaboration Group. Long-term ACE inhibitor therapy in patients with heart failure or left ventricular dysfunction: A systematic overview of data from individual patients. Lancet 2000;355:1575-1581.
11. Chobanian A, Bakris G, Black H, et al. Seventh Report of The Joint National Committee on Prevention, Detection, Evaluation, and Management of High Blood Pressure (JNC-7). Hypertension 2003;42:1206-1252.
12. Hunt S, Abraham WT, Chin M, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart failure in the Adult. A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Writing to update the 2001 guidelines for the evaluation and management of heart failure, 2005, available at www.acc.org, accessed 10-19-05.
13. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomized trial. Lancet 2001;357:1385-1390.
14. Packer M, Coats A, Fowler MB, et al. Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-1658.
15. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001-2007.
16. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patents with severe heart failure. Randomized Aldactone Evaluation Study (RALES) Investigators. N Engl J Med 1999;341:709-717.
17. Pitt B, Remme WJ, Zannad F, et al. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival (EPHESUS) Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;14:1309-1321.
18. Beta-Blocker Heart Attack (BHAT) Research Group. A randomized trial of propranalol in patients with acute myocardial infarction, I: mortality results. JAMA 1982;247:1707-1714.
19. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med 1981;304:801-807.
20. Nissen SE, Tuzcu EM, Libby P, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure. The CAMELOT Study: a randomized controlled trial. JAMA 2004;292:2217-2226.
21. The Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high risk patients. N Engl J Med 2000;342:145-153.
22. The EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease; randomized, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003;362:782-788.
23. PROGRESS Collaborative Group. Randomised trial of a perindopril based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischemic attack. Lancet 2001;358:1033-1041.
24. CIBIS Investigators and Committees. A randomized trial of beta blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS). Circulation 1994;90:1765-1773.
25. Brassuer L. Experts challenge present hypertension guidelines. Intern Med 2005;6:1-and 8.-
26. Hyman DJ, Pavlik VN. Self-reported hypertension treatment practices among primary care physicians: Blood pressure thresholds, drug choices, and the role of guidelines and evidence based medicine. Arch Intern Med 2000;160:2281-2286.
27. Poole-Wilson P, Swedberg K, Cleland J, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomized controlled trial. Lancet 2003;362:7-13.
28. Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomized trial. The Losartan Heart Failure Survival Study ELITE II. Lancet 2000;355:1582-1587.
29. Cohn JN, Tognoni G. Valsartan Heart Failure Trail (VAL-HEFT) Investigators. A randomized trial of the angiotensin receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667-1675.
30. Young JB, Dunlap ME, Pfeffer MA, et al. Mortality and morbidity reduction with candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation 2004;110:2618-2626.
31. The PEACE Trial Investigators. Angiotensin-converting enzyme inhibition in stable coronary artery disease. N Engl J Med 2004;351:2058-2068.
32. Kober L, Torp-Pedersen, Carlsen JE, et al. for the TRACE study group. A clinical trial of the angiotensin-converting enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial dysfunction. N Engl J Med 1995;333:1670-1676.
33. Ambrosioni E, Borghi C, Magnani B. For the SMILE Study Group. The effect of the angiotensin-converting enzyme inhibitor zofenopril on mortality and morbidity after myocardial infarction. N Engl J Med 1995;332:80-85.
34. Ebell MH, Siwek J, Weiss BD, et al. Strength of Recommendation Taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
- Decreasing blood pressure by 5 mm Hg will decrease mortality due to stroke by 14%, attenuate cardiac mortality by 9%, and reduce all-cause mortality by 7% (A).
- Patients with heart failure should receive angiotensin-converting enzyme inhibitors if they are clinically tolerated (A). Beta-blockers are also recommended (A). Although aldosterone antagonists are appropriate for use in patients with heart failure (A), we recommend they are taken under the care of a cardiologist to minimize complications and to insure that a complete heart failure plan is in place (C).
- For coronary artery disease, it is now considered standard of care to add a beta-blocker to all patients post–MI that do not have severe heart block or are in cardiogenic shock (A).
- Perindopril plus indapamide should be used in all patients with a history of stroke or transient ischemic attack regardless of blood pressure (B).
Hypertension precedes more than 75% of heart failure cases and 50% of heart attacks. In 67% of first-time strokes, blood pressure exceeds 140/90 mm Hg.1 For those in heart failure or at risk of recurrent myocardial infarction (MI) or stroke, the need to reduce unrecognized or under-treated hypertension is urgent. Thankfully it is possible to select an agent that can lower blood pressure—the most important immediate goal—and confer benefit to the associated cardiovascular disorder.
Given the number of agents used for both hypertension and other cardiovascular conditions, we sought in this study to evaluate which drug classes would best achieve blood pressure treatment goals and reduce morbidity and mortality for patients with cardiac disease. In this article, you will find practical recommendations for drug selection and appropriate regimens.
Even small blood pressure reductions yield big benefits for comorbidities
Using the population of the National Health and Nutrition Examination Survey I Epidemiologic Follow Up Study, Ogden and colleagues2 found that the benefit of treating high blood pressure over a decade depended on lowering systolic blood pressure and on treating other relevant cardiovascular comorbidities. Specifically, the number needed to treat (NNT) and prevent a death was directly related to the risk stratification of patients and their initial blood pressure (TABLE W1) (LOE: 1).
In another provocative trial, the Swedish Trial in Old Patients with Hypertension-2 Study (STOP Hypertension-2)3 evaluated the use of beta-blockers, diuretics, angiotensin-converting enzyme (ACE) inhibitors, or calcium channel blockers. Results of this trial showed that an overall reduction in cardiovascular events related to the ability of a drug class to lower blood pressure (LOE: 1).3
In addition, a meta-analysis of 29 randomized trials (n=162,341) in the Blood Pressure Lowering Treatment Trialists’ Collaboration Trial (BPLTTC)4 showed that all antihypertensive drug classes significantly reduce blood pressure. This meta-analysis confirmed an overall reduction in cardiovascular events, with perceived risk reduction directly proportional to blood pressure reduction (LOE: 1). This advantage was consistent irrespective of drug class (beta-blocker, diuretic, ACE inhibitor, calcium-channel blocker, or angiotensin receptor blocker [ARB]), although different drug classes were recognized to have unique benefits specific to individual patient populations.
Should the BP target level be lowered? Recent evidence suggests that the currently accepted range for normal blood pressure may be too high.5,6
Lewington and colleagues performed a meta-analysis of 61 prospective studies with more than 1 million participants. Using a “time-dependent” correction for regression dilution, they sorted deaths in each decade of age according to estimated blood pressures at the start of the decade (LOE: 1).5 They found that with each decade of life there was a proportional decline in the risk of cardiovascular deaths when blood pressures were controlled incrementally to levels of 115 mm Hg systolic blood pressure and 75 mm Hg diastolic blood pressure (LOE: 1).5 At blood pressures below 115/75 mm Hg, no difference was observed.
In addition, Vasan and collaborators,6 using the Framingham Heart Study database (n=6859 participants), reported an increase in cardiovascular events with higher baseline levels of blood pressure. When compared with optimal blood pressure levels, those with high-normal blood pressure (130–139/85–89 mm Hg) had a risk-factor-adjusted hazard ratio for cardiovascular disease of 2.5 for women and 1.6 for men (LOE: 1).6
The “take-home” message. A small reduction in blood pressure yields a very significant risk reduction. Decreasing blood pressure by 5 mm Hg will decrease mortality due to stroke by 14%, cardiac mortality by 9%, and all cause mortality by 7% (LOE: 1).7 These data suggest that aggressive intervention to affect small changes might affect large differences in morbidity and mortality.
Antihypertensive drugs in heart failure
Angiotensin-converting enzyme inhibitors
Give all patients with heart failure an ACE inhibitor, if clinically tolerated (SOR: A). Although blood pressure control is very important to treat the physiology and neurohormonal basis of heart failure, the primary reason to use ACE inhibitors (as well as other medications) is to provide a disease modifying intervention and treat blood pressure when it is elevated. Different disease conditions require different doses to achieve the desired goal. With heart failure, the dose of an ACE inhibitor is given twice daily at typically 2 to 3 times the dose of that used for hypertension.
The evidence. In 1991, the Studies of Left Ventricular Dysfunction (SOLVD) trial8 demonstrated a 26% risk reduction for death or hospitalization due to heart failure (95% confidence interval [CI], 18–34) for those treated with the ACE inhibitor enalapril (Vasotec) (LOE: 1). The following year, the Survival and Ventricular Enlargement (SAVE)9 trial demonstrated a risk reduction of 19% for patients with a reduced ejection fraction after myocardial infarction (MI) when the ACE inhibitor captopril (Capoten) was used (LOE: 1).
The calculated NNT with an ACE inhibitor to save 1 life over 1 year is 43.10 (See TABLE W2 for a summary of clinical trials and levels of evidence.) The TABLE in this article summarizes treatment recommendations based on these studies.8-23
TABLE
Drugs of choice for hypertension and various comorbidities
| TREATMENT RECOMMENDATIONS BASED ON STUDIES/GUIDELINES | SOR |
|---|---|
| Congestive Heart Failure | |
| ACE inhibitor should be used in patients with heart failure unless a contraindication exists8,9,11,12 | A |
| Beta-blockers should be used in patients with heart failure unless a contraindication exists11-15 | A |
| ARB should be used in heart failure if patient is intolerant to ACE inhibitor12 | A |
| Aldosterone antagonists should be used in patients with severe heart failure unless a contraindication exists16,17 | A |
| Aldosterone antagonists should be prescribed in consultation with a cardiologist | C |
| Coronary Artery Disease | |
| Beta-blockers should be used in patients post-MI unless a contraindication exists11,13,18,19 | A |
| CCB should be used in patients with stable coronary artery disease unless a contraindication exists11,20 | A |
| ACE inhibitors should be used in patients with stable coronary artery disease and no left ventricular dysfunction unless a contraindication exists11,21,22 | A |
| Stroke | |
| ACE inhibitor and indapamide should be used in patients with a TIA or stroke unless a contraindication exists11,23 | B |
| ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; MI, myocardial infarction; SOR, strength of recommendation; TIA, transient ischemic attack | |
Beta-blockers
Give a beta-blocker, if tolerated, to patients in heart failure (SOR: A). Dosing has been determined by clinical trial data. In general, in order to significantly impact morbidity and mortality in congestive heart failure, the patient needs to reach a dose of 150 mg of metoprolol XL a day or 6.25 mg to 12.5 mg of carvedilol given twice daily. Ideal doses are greater than 200 mg/d of metoprolol XL or 25 mg twice daily of carvedilol.
The evidence. The Cardiac Insufficiency Bisoprolol Study (CIBIS),24 published in 1994, was a randomized, placebo-controlled, double-blind trial designed to test the efficacy of beta-blockade in the treatment of heart failure (LOE: 1). Although no difference in mortality was demonstrated between intervention and control groups, the intervention group showed improved functional status.
The Carvedilol Post Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN)13 trial (LOE: 1) evaluated patients with left ventricular dysfunction or heart failure after an MI, while the Carvedilol Prospective Randomized Cumulative Survey (COPERNICUS)14 group (LOE: 1) enrolled only patients with severe heart failure (ejection fraction <25%, NYHA class III and IV). These studies demonstrated an overall decrease in cardiovascular morbidity and mortality, as well as all-cause mortality for patients with heart failure receiving the nonspecific beta-blocker carvedilol (Coreg) (receptor blockade at β1, β2, α1). CAPRICORN produced an overall risk reduction in mortality of 2% to 3% at 1 year, resulting in the same NNT (43) over 1 year as ACE inhibitors.13 This is the only beta-blocker tested after infarction to demonstrate a mortality difference for patients with heart failure or decreased left ventricular dysfunction (ejection fraction <40%).
Hypertension specialists debate about how to approach the hypertensive patient. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Management of High Blood Pressure (JNC-7) guidelines11 call for defined goals in lowering blood pressure and a stepwise selection of drugs based on comorbidities.
Some leading hypertension experts say this is too formulaic. Dr Michael Alderman, Professor in the Department of Medicine and Epidemiology and Population Health at the Albert Einstein School of Medicine, argues “we have to get over the limitation of the straightjacket of numbers to define our actions.”25 He further asserts that “our willingness to drive blood pressure down has to be modulated by the risk the patient has and the price one has to pay to lower it. A 30% reduction in risk does not mean much if your risk is low, but if your risk is high it means a lot.”25 As such, Dr Alderman argues we should base treatment decisions on “total risk” and not the level of blood pressure. Other leaders in the field such as Mathew Weir, MD, director of the Division of Nephrology, University of Maryland, and Richard Devreaux, MD, Professor of Medicine, Division of Cardiology, Cornell University Medical Center, agree with this more individualized approach.25
But understanding the basic formula is what helps us innovate. In general, we agree that individualizing patient care is the ideal, and that some patients may not tolerate “recommended” treatment. However, it is not possible for physicians to individualize care (a highly complex undertaking) when they still lack understanding at the basic level of care. With the poor treatment of hypertension in the US, we believe that guidelines such as JNC-7 are essential to improving blood pressure control.
Hyman and Pavlik26 demonstrated that physician factors, especially lack of awareness of hypertension treatment recommendations, correlate with poor hypertension treatment. In their 2001 study that included 1200 primary care physicians, 41% of physicians were not familiar with or had not heard of the recommendations. This finding was not trivial. The importance of familiarity with JNC-7 guidelines was demonstrated when statistical analysis revealed that a working knowledge of these guidelines significantly increased adherence with published recommendations (including blood pressure control). As such, it would appear that not following the guidelines has less to do with disagreements over treatment options and more to do with understanding the value of the guidelines to basic management.
The Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF)15 concluded that the addition of extended-release metoprolol (Lopressor, a β1-adrenergic receptor blocker) for patients with heart failure demonstrated a survival benefit when compared with patients not receiving a beta-blocker (LOE: 1). One of the essential elements of this trial was the ability to achieve a dose of 200 mg of metoprolol a day. Frequently in clinical practice low-dose or even homeopathic doses are used with few data to support such use.
The Carvedilol or Metoprolol European Trial (COMET)27 of patients with heart failure suggested that nonselective neurohumoral (β and α) blockade may increase the benefit in comparison with selective β1-blockade (LOE: 1). There has been significant debate regarding the dose and the formulation of the drugs in COMET, but we advocate using doses and drug formulations that were specifically in the large prospective randomized trials (CIBIS II, the carvedilol trials, and MERIT-HF).
Angiotensin receptor blockers
Prescribe an ARB only when a patient cannot tolerate an ACE inhibitor secondary to cough or hyperkalemia.12 As these are generally used after or as an adjunct to ACE inhibitors, the usual dose is similar to that for blood pressure dosed twice daily. If patients do not tolerate ACE inhibitors, theses doses may also be higher than those used for blood pressure response alone.
The evidence. The Losartan Heart Failure Survival Study (ELITE II)28 demonstrated the benefit of the ARB losartan (Cozaar) in the treatment of heart failure, but not superiority over previously used ACE inhibitors (LOE: 1). In addition, there was no difference in renal insufficiency with one drug class compared with another. Researchers concluded that ARBs should be used only for patients intolerant to ACE inhibitors.
The Valsartan Heart Failure Trial Investigators Study (Val-HeFT)29 randomized 5010 patients to receive valsartan (Diovan) or placebo combined with standard therapy (ACE inhibitors and beta-blockers) (LOE: 1). The ARB group demonstrated a 13.2% greater reduction than placebo in the combined endpoint of morbidity and mortality (as defined by incidence of cardiac arrest and resuscitation, hospitalization for heat failure, or administration of intravenous inotropes or vasodilators for a minimum of 4 hours). However, a post-study review29 of patients who received the ARB, ACE inhibitor, and beta-blocker combination showed increased mortality.
In 2004, the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity trial (CHARM)30 found that when the ARB candesartan (Atacand) was added to standard therapy (ACE inhibitor, beta-blockers, aldosterone antagonist) there was a 33% reduction in all-cause mortality, similar to that found with beta-blockers and ACE inhibitors) (LOE: 1). In addition, the CHARM group found no increased risk when candesartan was combined with other treatments; it concluded that ARBs could be added to regimens for all patients with heart failure unless a contraindication exists.
The American College of Cardiology/American Heart Association guidelines prefer ACE inhibitors over ARBs, and recommend ARBs be used when an ACE inhibitor is not tolerated or if there are other contraindications. A low level of evidence (2b) suggests that an ARB may be added to conventional medical therapy with an increased risk of renal insufficiency and hyperkalemia (SOR: A).12
Potassium-sparing diuretics
Aldosterone antagonists are appropriate for patients with heart failure (SOR: A), though we recommend working in conjunction with a cardiologist to minimize complications and to insure that a complete heart failure plan is in place (SOR: C). Spirinolactone (Aldactone, Aldactazide) is used for physiologic purposes (as a neurohormonal regulator) and is not used for blood pressure control. Most heart failure specialists begin with a dose of 12.5 mg/d and advance to doses utilized in the clinical trials (25–50 mg/d).
The evidence. Potassium-sparing diuretics lower mortality among heart failure patients. Spironolactone works in part by reducing aldosterone levels and increasing serum potassium.
In the Randomized Aldactone Evaluation Study (RALES),16 patients with severe heart failure (ejection fraction <35%) on standard medical therapy were randomized to receive spironolactone or placebo (LOE: 1). The spironolactone group exhibited a 30% reduction in mortality compared with conventional medical therapy, and the study was ended early at 24 months.
In the Eplerenone Post Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS),17 an overall reduction in death of 8%, a decrease of sudden cardiac death by >20% (relative risk reduction), as well as an overall reduction of hospitalization for heart failure of 15% occurred in the eplerenone (Inspra) group (LOE: 1) (NNT to save 1 life in 1 year=50).
It is worth noting that the doses used in these studies were devised to alter neurohumoral regulation, and are not to be used as significant diuretics. To date, no comparative study between spironolactone and eplerenone has been undertaken.
Coronary artery disease
Beta-blockers
Prescribe a beta-blocker for every post-MI patient without severe heart block or cardiogenic shock (SOR: A).
The evidence. The Beta-Blocker Heart Attack Trial (BHAT),18 sponsored by the National Heart, Lung, and Blood Institute, was designed to evaluate the benefits of the beta-blocker propranolol (Inderal) after MI (completed more than 24 years ago, before modern medical therapy) (LOE: 1). Total mortality during the average 24-month follow-up period was 7.2% in the propranolol group and 9.8% in the placebo group. The incidence of nonfatal reinfarction was decreased by 15.6% in the treatment group.
A similar trial completed in the early 1980s was The Norwegian Multi-Center Study.19 This trial, which assessed the efficacy of timolol (Blocadren, Timolide) after MI, demonstrated a 44.6% reduction in sudden cardiac death (LOE: 1). The study group reached the same conclusion as the BHAT researchers, and recommended that beta-blockers be used following an MI to reduce reinfarction and death.
Angiotensin-converting enzyme inhibitors
Use ACE inhibitors only for stable post-MI patients without decreased left ventricular function.
The evidence. The Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE) trial31 evaluated the benefit of using an ACE inhibitor for patients with stable coronary artery disease and slightly reduced ejection fraction (>40%) (LOE: 1). There was no statistical difference in the primary endpoint (cardiac-induced death, MI, or need for revascularization) between the treatment group (21.9%) and the placebo group (22.5%).
Contrary to the findings of the PEACE trial, many other studies have shown that ACE inhibitors are beneficial for patients with coronary artery disease. In the Trandolapril Cardiac Evaluation (TRACE) study,32 patients stabilized after acute MI were randomized to receive the ACE inhibitor trandolapril (Mavik) or placebo on days 3 to 7 following infarction (LOE: 1). In the treatment group, risk of death from all causes declined 17.6%, risk of death from cardiovascular causes fell 21%, and progression to severe heart failure decreased 27%. These post-MI benefits are also supported by results of the Survival of Myocardial Infarction Long-Term Evaluation (SMILE) study (LOE: 1).33
The Heart Outcomes Prevention Evaluation Investigators (HOPE)21 (LOE: 1), and the European Trial on Reduction of Cardiac Events with Perindopril in Patients with Coronary Artery disease (EUROPA)22 (LOE: 1), demonstrated the benefits of ACE inhibitors in reducing cardiovascular events for patients with or at risk for coronary artery disease, but with normal left ventricular function. The HOPE study showed a significant reduction of events with the ACE inhibitor ramipril (Altace) (NNT=1000 patients over 4 years, resulting in a decrease of 150 events for 75 patients), whereas EUROPA demonstrated the results for the ACE inhibitor perindopril (Aceon) (NNT=50 patients over 4 years to prevent 1 major cardiovascular event).
Calcium channel blockers
Use calcium channel blockers only for stable post-MI patients without decreased left ventricular function or heart failure.
The evidence. The Comparison of Amlodipine vs Enalapril to Limit Occurrences of Thrombosis (CAMELOT)20 study compared treatment using a calcium channel blocker (amlodipine) and an ACE inhibitor (enalapril) with placebo for normotensive patients with coronary artery disease (LOE: 1). Amlodipine reduced hospitalization for angina by 42.2%, nonfatal MI by 26%, and stroke or transient ischemic attack by 50.4% (NNT=16). The study group concluded that the use of the ACE inhibitor enalapril showed “directionally similar, but smaller and nonsignificant, treatment effects.”20 There was no reduction in overall mortality.
Stroke
Prescribe the combination of perindopril and indapamide for all patients with a history of stroke or transient ischemic attack, regardless of blood pressure (SOR: B).
The evidence. The Perindopril Protection Against Recurrent Stroke Study (PROGRESS)23 was designed to evaluate the benefits of the ACE inhibitor perindopril (with the addition of indapamide at the physician’s discretion) for patients with or without hypertension who have had a transient ischemic attack or stroke (LOE: 1). Perindopril plus indapamide reduced risk of stroke by 43%, but treatment with a single agent showed risk reduction.
To see which drug classes are most often recommended in the treatment of hypertension based on underlying cardiovascular disease, we performed an initial Medline search using the key words hypertension and cardiovascular disease. This was supplemented with a search of the archives of the journals Circulation, Hypertension, Stroke, and the authors’ personal references.
All studies were evaluated using the Strength of Recommendation Taxonomy.34 Strength of recommendation (SOR) evaluates a study based on patient (not disease) oriented outcomes, and level of evidence (LOE) is based on key outcomes as well as the methodology of the study.
CORRESPONDENCE
Randy Wexler, MD, MPH, FAAFP, Assistant Professor of Clinical Family Medicine, The Ohio State University College of Medicine and Public Health, B0902B Cramblett Hall, 456 West 10th Ave, Columbus, Ohio 43201. E-mail: [email protected]
- Decreasing blood pressure by 5 mm Hg will decrease mortality due to stroke by 14%, attenuate cardiac mortality by 9%, and reduce all-cause mortality by 7% (A).
- Patients with heart failure should receive angiotensin-converting enzyme inhibitors if they are clinically tolerated (A). Beta-blockers are also recommended (A). Although aldosterone antagonists are appropriate for use in patients with heart failure (A), we recommend they are taken under the care of a cardiologist to minimize complications and to insure that a complete heart failure plan is in place (C).
- For coronary artery disease, it is now considered standard of care to add a beta-blocker to all patients post–MI that do not have severe heart block or are in cardiogenic shock (A).
- Perindopril plus indapamide should be used in all patients with a history of stroke or transient ischemic attack regardless of blood pressure (B).
Hypertension precedes more than 75% of heart failure cases and 50% of heart attacks. In 67% of first-time strokes, blood pressure exceeds 140/90 mm Hg.1 For those in heart failure or at risk of recurrent myocardial infarction (MI) or stroke, the need to reduce unrecognized or under-treated hypertension is urgent. Thankfully it is possible to select an agent that can lower blood pressure—the most important immediate goal—and confer benefit to the associated cardiovascular disorder.
Given the number of agents used for both hypertension and other cardiovascular conditions, we sought in this study to evaluate which drug classes would best achieve blood pressure treatment goals and reduce morbidity and mortality for patients with cardiac disease. In this article, you will find practical recommendations for drug selection and appropriate regimens.
Even small blood pressure reductions yield big benefits for comorbidities
Using the population of the National Health and Nutrition Examination Survey I Epidemiologic Follow Up Study, Ogden and colleagues2 found that the benefit of treating high blood pressure over a decade depended on lowering systolic blood pressure and on treating other relevant cardiovascular comorbidities. Specifically, the number needed to treat (NNT) and prevent a death was directly related to the risk stratification of patients and their initial blood pressure (TABLE W1) (LOE: 1).
In another provocative trial, the Swedish Trial in Old Patients with Hypertension-2 Study (STOP Hypertension-2)3 evaluated the use of beta-blockers, diuretics, angiotensin-converting enzyme (ACE) inhibitors, or calcium channel blockers. Results of this trial showed that an overall reduction in cardiovascular events related to the ability of a drug class to lower blood pressure (LOE: 1).3
In addition, a meta-analysis of 29 randomized trials (n=162,341) in the Blood Pressure Lowering Treatment Trialists’ Collaboration Trial (BPLTTC)4 showed that all antihypertensive drug classes significantly reduce blood pressure. This meta-analysis confirmed an overall reduction in cardiovascular events, with perceived risk reduction directly proportional to blood pressure reduction (LOE: 1). This advantage was consistent irrespective of drug class (beta-blocker, diuretic, ACE inhibitor, calcium-channel blocker, or angiotensin receptor blocker [ARB]), although different drug classes were recognized to have unique benefits specific to individual patient populations.
Should the BP target level be lowered? Recent evidence suggests that the currently accepted range for normal blood pressure may be too high.5,6
Lewington and colleagues performed a meta-analysis of 61 prospective studies with more than 1 million participants. Using a “time-dependent” correction for regression dilution, they sorted deaths in each decade of age according to estimated blood pressures at the start of the decade (LOE: 1).5 They found that with each decade of life there was a proportional decline in the risk of cardiovascular deaths when blood pressures were controlled incrementally to levels of 115 mm Hg systolic blood pressure and 75 mm Hg diastolic blood pressure (LOE: 1).5 At blood pressures below 115/75 mm Hg, no difference was observed.
In addition, Vasan and collaborators,6 using the Framingham Heart Study database (n=6859 participants), reported an increase in cardiovascular events with higher baseline levels of blood pressure. When compared with optimal blood pressure levels, those with high-normal blood pressure (130–139/85–89 mm Hg) had a risk-factor-adjusted hazard ratio for cardiovascular disease of 2.5 for women and 1.6 for men (LOE: 1).6
The “take-home” message. A small reduction in blood pressure yields a very significant risk reduction. Decreasing blood pressure by 5 mm Hg will decrease mortality due to stroke by 14%, cardiac mortality by 9%, and all cause mortality by 7% (LOE: 1).7 These data suggest that aggressive intervention to affect small changes might affect large differences in morbidity and mortality.
Antihypertensive drugs in heart failure
Angiotensin-converting enzyme inhibitors
Give all patients with heart failure an ACE inhibitor, if clinically tolerated (SOR: A). Although blood pressure control is very important to treat the physiology and neurohormonal basis of heart failure, the primary reason to use ACE inhibitors (as well as other medications) is to provide a disease modifying intervention and treat blood pressure when it is elevated. Different disease conditions require different doses to achieve the desired goal. With heart failure, the dose of an ACE inhibitor is given twice daily at typically 2 to 3 times the dose of that used for hypertension.
The evidence. In 1991, the Studies of Left Ventricular Dysfunction (SOLVD) trial8 demonstrated a 26% risk reduction for death or hospitalization due to heart failure (95% confidence interval [CI], 18–34) for those treated with the ACE inhibitor enalapril (Vasotec) (LOE: 1). The following year, the Survival and Ventricular Enlargement (SAVE)9 trial demonstrated a risk reduction of 19% for patients with a reduced ejection fraction after myocardial infarction (MI) when the ACE inhibitor captopril (Capoten) was used (LOE: 1).
The calculated NNT with an ACE inhibitor to save 1 life over 1 year is 43.10 (See TABLE W2 for a summary of clinical trials and levels of evidence.) The TABLE in this article summarizes treatment recommendations based on these studies.8-23
TABLE
Drugs of choice for hypertension and various comorbidities
| TREATMENT RECOMMENDATIONS BASED ON STUDIES/GUIDELINES | SOR |
|---|---|
| Congestive Heart Failure | |
| ACE inhibitor should be used in patients with heart failure unless a contraindication exists8,9,11,12 | A |
| Beta-blockers should be used in patients with heart failure unless a contraindication exists11-15 | A |
| ARB should be used in heart failure if patient is intolerant to ACE inhibitor12 | A |
| Aldosterone antagonists should be used in patients with severe heart failure unless a contraindication exists16,17 | A |
| Aldosterone antagonists should be prescribed in consultation with a cardiologist | C |
| Coronary Artery Disease | |
| Beta-blockers should be used in patients post-MI unless a contraindication exists11,13,18,19 | A |
| CCB should be used in patients with stable coronary artery disease unless a contraindication exists11,20 | A |
| ACE inhibitors should be used in patients with stable coronary artery disease and no left ventricular dysfunction unless a contraindication exists11,21,22 | A |
| Stroke | |
| ACE inhibitor and indapamide should be used in patients with a TIA or stroke unless a contraindication exists11,23 | B |
| ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; MI, myocardial infarction; SOR, strength of recommendation; TIA, transient ischemic attack | |
Beta-blockers
Give a beta-blocker, if tolerated, to patients in heart failure (SOR: A). Dosing has been determined by clinical trial data. In general, in order to significantly impact morbidity and mortality in congestive heart failure, the patient needs to reach a dose of 150 mg of metoprolol XL a day or 6.25 mg to 12.5 mg of carvedilol given twice daily. Ideal doses are greater than 200 mg/d of metoprolol XL or 25 mg twice daily of carvedilol.
The evidence. The Cardiac Insufficiency Bisoprolol Study (CIBIS),24 published in 1994, was a randomized, placebo-controlled, double-blind trial designed to test the efficacy of beta-blockade in the treatment of heart failure (LOE: 1). Although no difference in mortality was demonstrated between intervention and control groups, the intervention group showed improved functional status.
The Carvedilol Post Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN)13 trial (LOE: 1) evaluated patients with left ventricular dysfunction or heart failure after an MI, while the Carvedilol Prospective Randomized Cumulative Survey (COPERNICUS)14 group (LOE: 1) enrolled only patients with severe heart failure (ejection fraction <25%, NYHA class III and IV). These studies demonstrated an overall decrease in cardiovascular morbidity and mortality, as well as all-cause mortality for patients with heart failure receiving the nonspecific beta-blocker carvedilol (Coreg) (receptor blockade at β1, β2, α1). CAPRICORN produced an overall risk reduction in mortality of 2% to 3% at 1 year, resulting in the same NNT (43) over 1 year as ACE inhibitors.13 This is the only beta-blocker tested after infarction to demonstrate a mortality difference for patients with heart failure or decreased left ventricular dysfunction (ejection fraction <40%).
Hypertension specialists debate about how to approach the hypertensive patient. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Management of High Blood Pressure (JNC-7) guidelines11 call for defined goals in lowering blood pressure and a stepwise selection of drugs based on comorbidities.
Some leading hypertension experts say this is too formulaic. Dr Michael Alderman, Professor in the Department of Medicine and Epidemiology and Population Health at the Albert Einstein School of Medicine, argues “we have to get over the limitation of the straightjacket of numbers to define our actions.”25 He further asserts that “our willingness to drive blood pressure down has to be modulated by the risk the patient has and the price one has to pay to lower it. A 30% reduction in risk does not mean much if your risk is low, but if your risk is high it means a lot.”25 As such, Dr Alderman argues we should base treatment decisions on “total risk” and not the level of blood pressure. Other leaders in the field such as Mathew Weir, MD, director of the Division of Nephrology, University of Maryland, and Richard Devreaux, MD, Professor of Medicine, Division of Cardiology, Cornell University Medical Center, agree with this more individualized approach.25
But understanding the basic formula is what helps us innovate. In general, we agree that individualizing patient care is the ideal, and that some patients may not tolerate “recommended” treatment. However, it is not possible for physicians to individualize care (a highly complex undertaking) when they still lack understanding at the basic level of care. With the poor treatment of hypertension in the US, we believe that guidelines such as JNC-7 are essential to improving blood pressure control.
Hyman and Pavlik26 demonstrated that physician factors, especially lack of awareness of hypertension treatment recommendations, correlate with poor hypertension treatment. In their 2001 study that included 1200 primary care physicians, 41% of physicians were not familiar with or had not heard of the recommendations. This finding was not trivial. The importance of familiarity with JNC-7 guidelines was demonstrated when statistical analysis revealed that a working knowledge of these guidelines significantly increased adherence with published recommendations (including blood pressure control). As such, it would appear that not following the guidelines has less to do with disagreements over treatment options and more to do with understanding the value of the guidelines to basic management.
The Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF)15 concluded that the addition of extended-release metoprolol (Lopressor, a β1-adrenergic receptor blocker) for patients with heart failure demonstrated a survival benefit when compared with patients not receiving a beta-blocker (LOE: 1). One of the essential elements of this trial was the ability to achieve a dose of 200 mg of metoprolol a day. Frequently in clinical practice low-dose or even homeopathic doses are used with few data to support such use.
The Carvedilol or Metoprolol European Trial (COMET)27 of patients with heart failure suggested that nonselective neurohumoral (β and α) blockade may increase the benefit in comparison with selective β1-blockade (LOE: 1). There has been significant debate regarding the dose and the formulation of the drugs in COMET, but we advocate using doses and drug formulations that were specifically in the large prospective randomized trials (CIBIS II, the carvedilol trials, and MERIT-HF).
Angiotensin receptor blockers
Prescribe an ARB only when a patient cannot tolerate an ACE inhibitor secondary to cough or hyperkalemia.12 As these are generally used after or as an adjunct to ACE inhibitors, the usual dose is similar to that for blood pressure dosed twice daily. If patients do not tolerate ACE inhibitors, theses doses may also be higher than those used for blood pressure response alone.
The evidence. The Losartan Heart Failure Survival Study (ELITE II)28 demonstrated the benefit of the ARB losartan (Cozaar) in the treatment of heart failure, but not superiority over previously used ACE inhibitors (LOE: 1). In addition, there was no difference in renal insufficiency with one drug class compared with another. Researchers concluded that ARBs should be used only for patients intolerant to ACE inhibitors.
The Valsartan Heart Failure Trial Investigators Study (Val-HeFT)29 randomized 5010 patients to receive valsartan (Diovan) or placebo combined with standard therapy (ACE inhibitors and beta-blockers) (LOE: 1). The ARB group demonstrated a 13.2% greater reduction than placebo in the combined endpoint of morbidity and mortality (as defined by incidence of cardiac arrest and resuscitation, hospitalization for heat failure, or administration of intravenous inotropes or vasodilators for a minimum of 4 hours). However, a post-study review29 of patients who received the ARB, ACE inhibitor, and beta-blocker combination showed increased mortality.
In 2004, the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity trial (CHARM)30 found that when the ARB candesartan (Atacand) was added to standard therapy (ACE inhibitor, beta-blockers, aldosterone antagonist) there was a 33% reduction in all-cause mortality, similar to that found with beta-blockers and ACE inhibitors) (LOE: 1). In addition, the CHARM group found no increased risk when candesartan was combined with other treatments; it concluded that ARBs could be added to regimens for all patients with heart failure unless a contraindication exists.
The American College of Cardiology/American Heart Association guidelines prefer ACE inhibitors over ARBs, and recommend ARBs be used when an ACE inhibitor is not tolerated or if there are other contraindications. A low level of evidence (2b) suggests that an ARB may be added to conventional medical therapy with an increased risk of renal insufficiency and hyperkalemia (SOR: A).12
Potassium-sparing diuretics
Aldosterone antagonists are appropriate for patients with heart failure (SOR: A), though we recommend working in conjunction with a cardiologist to minimize complications and to insure that a complete heart failure plan is in place (SOR: C). Spirinolactone (Aldactone, Aldactazide) is used for physiologic purposes (as a neurohormonal regulator) and is not used for blood pressure control. Most heart failure specialists begin with a dose of 12.5 mg/d and advance to doses utilized in the clinical trials (25–50 mg/d).
The evidence. Potassium-sparing diuretics lower mortality among heart failure patients. Spironolactone works in part by reducing aldosterone levels and increasing serum potassium.
In the Randomized Aldactone Evaluation Study (RALES),16 patients with severe heart failure (ejection fraction <35%) on standard medical therapy were randomized to receive spironolactone or placebo (LOE: 1). The spironolactone group exhibited a 30% reduction in mortality compared with conventional medical therapy, and the study was ended early at 24 months.
In the Eplerenone Post Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS),17 an overall reduction in death of 8%, a decrease of sudden cardiac death by >20% (relative risk reduction), as well as an overall reduction of hospitalization for heart failure of 15% occurred in the eplerenone (Inspra) group (LOE: 1) (NNT to save 1 life in 1 year=50).
It is worth noting that the doses used in these studies were devised to alter neurohumoral regulation, and are not to be used as significant diuretics. To date, no comparative study between spironolactone and eplerenone has been undertaken.
Coronary artery disease
Beta-blockers
Prescribe a beta-blocker for every post-MI patient without severe heart block or cardiogenic shock (SOR: A).
The evidence. The Beta-Blocker Heart Attack Trial (BHAT),18 sponsored by the National Heart, Lung, and Blood Institute, was designed to evaluate the benefits of the beta-blocker propranolol (Inderal) after MI (completed more than 24 years ago, before modern medical therapy) (LOE: 1). Total mortality during the average 24-month follow-up period was 7.2% in the propranolol group and 9.8% in the placebo group. The incidence of nonfatal reinfarction was decreased by 15.6% in the treatment group.
A similar trial completed in the early 1980s was The Norwegian Multi-Center Study.19 This trial, which assessed the efficacy of timolol (Blocadren, Timolide) after MI, demonstrated a 44.6% reduction in sudden cardiac death (LOE: 1). The study group reached the same conclusion as the BHAT researchers, and recommended that beta-blockers be used following an MI to reduce reinfarction and death.
Angiotensin-converting enzyme inhibitors
Use ACE inhibitors only for stable post-MI patients without decreased left ventricular function.
The evidence. The Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE) trial31 evaluated the benefit of using an ACE inhibitor for patients with stable coronary artery disease and slightly reduced ejection fraction (>40%) (LOE: 1). There was no statistical difference in the primary endpoint (cardiac-induced death, MI, or need for revascularization) between the treatment group (21.9%) and the placebo group (22.5%).
Contrary to the findings of the PEACE trial, many other studies have shown that ACE inhibitors are beneficial for patients with coronary artery disease. In the Trandolapril Cardiac Evaluation (TRACE) study,32 patients stabilized after acute MI were randomized to receive the ACE inhibitor trandolapril (Mavik) or placebo on days 3 to 7 following infarction (LOE: 1). In the treatment group, risk of death from all causes declined 17.6%, risk of death from cardiovascular causes fell 21%, and progression to severe heart failure decreased 27%. These post-MI benefits are also supported by results of the Survival of Myocardial Infarction Long-Term Evaluation (SMILE) study (LOE: 1).33
The Heart Outcomes Prevention Evaluation Investigators (HOPE)21 (LOE: 1), and the European Trial on Reduction of Cardiac Events with Perindopril in Patients with Coronary Artery disease (EUROPA)22 (LOE: 1), demonstrated the benefits of ACE inhibitors in reducing cardiovascular events for patients with or at risk for coronary artery disease, but with normal left ventricular function. The HOPE study showed a significant reduction of events with the ACE inhibitor ramipril (Altace) (NNT=1000 patients over 4 years, resulting in a decrease of 150 events for 75 patients), whereas EUROPA demonstrated the results for the ACE inhibitor perindopril (Aceon) (NNT=50 patients over 4 years to prevent 1 major cardiovascular event).
Calcium channel blockers
Use calcium channel blockers only for stable post-MI patients without decreased left ventricular function or heart failure.
The evidence. The Comparison of Amlodipine vs Enalapril to Limit Occurrences of Thrombosis (CAMELOT)20 study compared treatment using a calcium channel blocker (amlodipine) and an ACE inhibitor (enalapril) with placebo for normotensive patients with coronary artery disease (LOE: 1). Amlodipine reduced hospitalization for angina by 42.2%, nonfatal MI by 26%, and stroke or transient ischemic attack by 50.4% (NNT=16). The study group concluded that the use of the ACE inhibitor enalapril showed “directionally similar, but smaller and nonsignificant, treatment effects.”20 There was no reduction in overall mortality.
Stroke
Prescribe the combination of perindopril and indapamide for all patients with a history of stroke or transient ischemic attack, regardless of blood pressure (SOR: B).
The evidence. The Perindopril Protection Against Recurrent Stroke Study (PROGRESS)23 was designed to evaluate the benefits of the ACE inhibitor perindopril (with the addition of indapamide at the physician’s discretion) for patients with or without hypertension who have had a transient ischemic attack or stroke (LOE: 1). Perindopril plus indapamide reduced risk of stroke by 43%, but treatment with a single agent showed risk reduction.
To see which drug classes are most often recommended in the treatment of hypertension based on underlying cardiovascular disease, we performed an initial Medline search using the key words hypertension and cardiovascular disease. This was supplemented with a search of the archives of the journals Circulation, Hypertension, Stroke, and the authors’ personal references.
All studies were evaluated using the Strength of Recommendation Taxonomy.34 Strength of recommendation (SOR) evaluates a study based on patient (not disease) oriented outcomes, and level of evidence (LOE) is based on key outcomes as well as the methodology of the study.
CORRESPONDENCE
Randy Wexler, MD, MPH, FAAFP, Assistant Professor of Clinical Family Medicine, The Ohio State University College of Medicine and Public Health, B0902B Cramblett Hall, 456 West 10th Ave, Columbus, Ohio 43201. E-mail: [email protected]
1. American Heart Association. Heart Disease and Stroke Statistics–2004 Update. Dallas, Tex: American Heart Association, 2003.
2. Ogden LG, He J, Lydick E, Whelton P. Long-term absolute benefit of lowering blood pressure in hypertensive patients according to JNC VI risk stratification. Hypertension 2000;35:539-543.
3. Hansson L, Lindholm L, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet 1999;354:1751-1756.
4. Turnbull F. Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomized trials. Lancet 2003;362:1527-1535.
5. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903-1913.
6. Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001;345:1291-1297.
7. Whelton P, He J, Appel L, et al. National High Blood Pressure Education Program Coordinating Committee. Primary prevention of hypertension: Clinical and public health advisory from the National High Blood Pressure Education Program. JAMA 2002;288:1882-1888.
8. The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293-302.
9. Pfeffer MA, Braunwald E, Moye LA, et al. The SAVE Investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. N Engl J Med 1992;327:669-677.
10. Flather MD, Yusuf S, Kobler L, et al. ACE-Inhibitor Myocardial Infarction Collaboration Group. Long-term ACE inhibitor therapy in patients with heart failure or left ventricular dysfunction: A systematic overview of data from individual patients. Lancet 2000;355:1575-1581.
11. Chobanian A, Bakris G, Black H, et al. Seventh Report of The Joint National Committee on Prevention, Detection, Evaluation, and Management of High Blood Pressure (JNC-7). Hypertension 2003;42:1206-1252.
12. Hunt S, Abraham WT, Chin M, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart failure in the Adult. A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Writing to update the 2001 guidelines for the evaluation and management of heart failure, 2005, available at www.acc.org, accessed 10-19-05.
13. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomized trial. Lancet 2001;357:1385-1390.
14. Packer M, Coats A, Fowler MB, et al. Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-1658.
15. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001-2007.
16. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patents with severe heart failure. Randomized Aldactone Evaluation Study (RALES) Investigators. N Engl J Med 1999;341:709-717.
17. Pitt B, Remme WJ, Zannad F, et al. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival (EPHESUS) Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;14:1309-1321.
18. Beta-Blocker Heart Attack (BHAT) Research Group. A randomized trial of propranalol in patients with acute myocardial infarction, I: mortality results. JAMA 1982;247:1707-1714.
19. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med 1981;304:801-807.
20. Nissen SE, Tuzcu EM, Libby P, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure. The CAMELOT Study: a randomized controlled trial. JAMA 2004;292:2217-2226.
21. The Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high risk patients. N Engl J Med 2000;342:145-153.
22. The EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease; randomized, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003;362:782-788.
23. PROGRESS Collaborative Group. Randomised trial of a perindopril based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischemic attack. Lancet 2001;358:1033-1041.
24. CIBIS Investigators and Committees. A randomized trial of beta blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS). Circulation 1994;90:1765-1773.
25. Brassuer L. Experts challenge present hypertension guidelines. Intern Med 2005;6:1-and 8.-
26. Hyman DJ, Pavlik VN. Self-reported hypertension treatment practices among primary care physicians: Blood pressure thresholds, drug choices, and the role of guidelines and evidence based medicine. Arch Intern Med 2000;160:2281-2286.
27. Poole-Wilson P, Swedberg K, Cleland J, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomized controlled trial. Lancet 2003;362:7-13.
28. Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomized trial. The Losartan Heart Failure Survival Study ELITE II. Lancet 2000;355:1582-1587.
29. Cohn JN, Tognoni G. Valsartan Heart Failure Trail (VAL-HEFT) Investigators. A randomized trial of the angiotensin receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667-1675.
30. Young JB, Dunlap ME, Pfeffer MA, et al. Mortality and morbidity reduction with candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation 2004;110:2618-2626.
31. The PEACE Trial Investigators. Angiotensin-converting enzyme inhibition in stable coronary artery disease. N Engl J Med 2004;351:2058-2068.
32. Kober L, Torp-Pedersen, Carlsen JE, et al. for the TRACE study group. A clinical trial of the angiotensin-converting enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial dysfunction. N Engl J Med 1995;333:1670-1676.
33. Ambrosioni E, Borghi C, Magnani B. For the SMILE Study Group. The effect of the angiotensin-converting enzyme inhibitor zofenopril on mortality and morbidity after myocardial infarction. N Engl J Med 1995;332:80-85.
34. Ebell MH, Siwek J, Weiss BD, et al. Strength of Recommendation Taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
1. American Heart Association. Heart Disease and Stroke Statistics–2004 Update. Dallas, Tex: American Heart Association, 2003.
2. Ogden LG, He J, Lydick E, Whelton P. Long-term absolute benefit of lowering blood pressure in hypertensive patients according to JNC VI risk stratification. Hypertension 2000;35:539-543.
3. Hansson L, Lindholm L, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet 1999;354:1751-1756.
4. Turnbull F. Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomized trials. Lancet 2003;362:1527-1535.
5. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903-1913.
6. Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001;345:1291-1297.
7. Whelton P, He J, Appel L, et al. National High Blood Pressure Education Program Coordinating Committee. Primary prevention of hypertension: Clinical and public health advisory from the National High Blood Pressure Education Program. JAMA 2002;288:1882-1888.
8. The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293-302.
9. Pfeffer MA, Braunwald E, Moye LA, et al. The SAVE Investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. N Engl J Med 1992;327:669-677.
10. Flather MD, Yusuf S, Kobler L, et al. ACE-Inhibitor Myocardial Infarction Collaboration Group. Long-term ACE inhibitor therapy in patients with heart failure or left ventricular dysfunction: A systematic overview of data from individual patients. Lancet 2000;355:1575-1581.
11. Chobanian A, Bakris G, Black H, et al. Seventh Report of The Joint National Committee on Prevention, Detection, Evaluation, and Management of High Blood Pressure (JNC-7). Hypertension 2003;42:1206-1252.
12. Hunt S, Abraham WT, Chin M, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart failure in the Adult. A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Writing to update the 2001 guidelines for the evaluation and management of heart failure, 2005, available at www.acc.org, accessed 10-19-05.
13. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomized trial. Lancet 2001;357:1385-1390.
14. Packer M, Coats A, Fowler MB, et al. Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-1658.
15. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001-2007.
16. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patents with severe heart failure. Randomized Aldactone Evaluation Study (RALES) Investigators. N Engl J Med 1999;341:709-717.
17. Pitt B, Remme WJ, Zannad F, et al. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival (EPHESUS) Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;14:1309-1321.
18. Beta-Blocker Heart Attack (BHAT) Research Group. A randomized trial of propranalol in patients with acute myocardial infarction, I: mortality results. JAMA 1982;247:1707-1714.
19. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med 1981;304:801-807.
20. Nissen SE, Tuzcu EM, Libby P, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure. The CAMELOT Study: a randomized controlled trial. JAMA 2004;292:2217-2226.
21. The Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high risk patients. N Engl J Med 2000;342:145-153.
22. The EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease; randomized, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003;362:782-788.
23. PROGRESS Collaborative Group. Randomised trial of a perindopril based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischemic attack. Lancet 2001;358:1033-1041.
24. CIBIS Investigators and Committees. A randomized trial of beta blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS). Circulation 1994;90:1765-1773.
25. Brassuer L. Experts challenge present hypertension guidelines. Intern Med 2005;6:1-and 8.-
26. Hyman DJ, Pavlik VN. Self-reported hypertension treatment practices among primary care physicians: Blood pressure thresholds, drug choices, and the role of guidelines and evidence based medicine. Arch Intern Med 2000;160:2281-2286.
27. Poole-Wilson P, Swedberg K, Cleland J, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomized controlled trial. Lancet 2003;362:7-13.
28. Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomized trial. The Losartan Heart Failure Survival Study ELITE II. Lancet 2000;355:1582-1587.
29. Cohn JN, Tognoni G. Valsartan Heart Failure Trail (VAL-HEFT) Investigators. A randomized trial of the angiotensin receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667-1675.
30. Young JB, Dunlap ME, Pfeffer MA, et al. Mortality and morbidity reduction with candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation 2004;110:2618-2626.
31. The PEACE Trial Investigators. Angiotensin-converting enzyme inhibition in stable coronary artery disease. N Engl J Med 2004;351:2058-2068.
32. Kober L, Torp-Pedersen, Carlsen JE, et al. for the TRACE study group. A clinical trial of the angiotensin-converting enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial dysfunction. N Engl J Med 1995;333:1670-1676.
33. Ambrosioni E, Borghi C, Magnani B. For the SMILE Study Group. The effect of the angiotensin-converting enzyme inhibitor zofenopril on mortality and morbidity after myocardial infarction. N Engl J Med 1995;332:80-85.
34. Ebell MH, Siwek J, Weiss BD, et al. Strength of Recommendation Taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.