User login
The Effectiveness of Magnet Therapy for Treatment of Wrist Pain Attributed to Carpal Tunnel Syndrome
We conducted a double-blind placebo-controlled randomized clinical trial in which 30 patients with pain attributed to carpal tunnel syndrome had either a 1000 gauss magnet or a placebo metal disk applied to the carpal tunnel area using a Velcro wrap for a period of 45 minutes. Pain was measured on a visual analogue scale using 0 and 10 as anchors.
Presenting symptoms including numbness, tingling, burning, and pain did not differ significantly between the 2 groups. There was significant pain reduction across the 45-minute period for both groups. However, t test comparisons found no significant differences between the groups for beginning pain, pain at 15 minutes, pain at 30 minutes, or pain at 45 minutes. The use of a magnet for reducing pain attributed to carpal tunnel syndrome was no more effective than use of the placebo device.
Four recent randomized trials have provided conflicting results concerning the efficacy of magnets in relieving pain. Two double-blind randomized trials have found that magnets relieve pain in postpolio subjects1 and in patients with postoperative wounds.2 However, double-blind randomized studies of magnet therapy for treatment of low back pain3 and foot pain4 showed no benefit.
In an attempt to find alternate forms of therapy,5,6 many chronic sufferers of carpal tunnel syndrome have resorted to using magnets to alleviate their symptoms. The purpose of our study was to determine the efficacy of magnet therapy on pain attributed to carpal tunnel syndrome when compared with a placebo device.
Methods
Subjects
We contacted 160 patients who had wrist pain attributed to carpal tunnel syndrome by their primary care physicians. These patients were identified from the billing databases at a university-operated family practice clinic and a rural private practitioner’s office. The inclusion criteria for participation were presence of chronic wrist pain in the area of the carpal tunnel and the willingness to accept randomization into treatment or control group. Individuals were excluded before randomization if the source of pain had been attributed to some cause other than carpal tunnel syndrome, if they had taken pain medication within 4 hours of beginning treatment, if their body mass index was greater than 35, or if they were not experiencing pain at the time treatment was started.
Treatment intervention
The magnets and placebo devices used in our study were custom made by Medical Magnetics of Houston, Texas. The devices consisted of 5 stacked magnetic pads. Four of these were flexible (2500 gauss, residual induction). The fifth pad was a neodymium disk (10,000 gauss, residual induction). The flexible pads were 1.7 inches in diameter, and the neodymium disk was 0.5 inches in diameter. All 5 pads were glued together to form a single unit. Actual magnetic energy was determined to be 1000 gauss at the surface of the center of the magnet, and depth of penetration was estimated to be adequate for the carpal tunnel area. The placebo disks appeared identical to the magnets. Each magnet and placebo was labeled with a computer-generated random number, wrapped in foam, and boxed individually. Individual boxes were selected at the time of the patient appointment without regard for the order or numerical identifier, which served as a blinding device. Codes identifying placebo or control were not broken until the completion of the study.
After giving written consent, patients were asked to complete a short questionnaire collecting demographic and symptom information. They were then asked to rate the pain at the most painful point in the wrist using the visual analog scale (VAS) of the McGill Pain Questionnaire.7 The VAS consisted of a standard length line labeled 0 on the left and 10 on the right. The patient was instructed to place a mark on that line at the appropriate position relative to the degree of pain experienced (0 = no pain; 10 = the worst pain ever experienced). The distance of the mark from 0 was then measured in millimeters to provide the pain score. A new pain scale was provided for each measurement, and patients were not allowed to view previous measurements, to insure the objectivity of the patient’s pain perception.
A device, either magnet or placebo, was then placed on the wrist overlying the carpal tunnel. The device was secured with foam and a wrist bracelet fastened with Velcro. Each patient was then asked to remain seated and to keep the device in place for the next 45 minutes. This time period was selected based on the experience of the postpolio pain trial.1 Throughout the 45 minutes a research assistant observed the patients to ensure that they did not tamper with their device. The patients were asked to rate their pain on the VAS at 15-minute intervals. After 45 minutes the device was removed, and the patient again rated his or her pain on a VAS.
Patients were sent home with a postcard that served as a 2-week follow-up. Two weeks after treatment patients rated their current pain, maximum pain over the 2-week period, and typical pain over the 2-week period, using the previously described VAS.
Data analysis
Previous research on the effect of magnets on pain has shown reduction in pain on a 10-point VAS ranging from 1.1 to 4.4 points with standard deviations of 1.6 and 3.1, respectively.1 Corresponding sample sizes to detect these differences would range from 34 per group to 8 per group. Standard sample size formulas for power equal to 0.80, ( equal to 0.05, and a standard deviation of 2.5 estimated that a sample size of 15 per group could detect a difference of 2.6 points between groups.
Data were analyzed using chi-square analysis for categorical data, paired t tests for within group comparisons, and independent t tests for between group comparisons on age and pain. Confirmation of normal distributions for the VAS variables was made using the Kolmogorov-Smirnov goodness-of-fit test.
Results
Of the 160 patients contacted by mail, 45 replied, 38 qualified for participation, and 30 patients completed the 45-minute treatment protocol: 15 with a magnetic device and 15 with a placebo. Descriptive statistics for the 2 groups are provided in Table 1. Groups did not differ significantly in age or any of the presenting symptoms including numbness, tingling, burning, and pain. There were no men in the magnet group and 4 in the placebo group ( P =.01).
Table 2 contains the mean pain scores for both groups at different points in time. There were no significant differences for any of the pain variables. Twenty of the participants in this study completed a 2-week follow-up questionnaire, 10 in each group. There were no significant differences between groups in the pain at 2 weeks post-treatment, the greatest pain experienced during the 2 weeks, and the typical pain experienced during the 2 weeks. The mean pain score at 2 weeks post-treatment and their typical pain across the 2 weeks had not returned to their baseline pain levels measured before device application.
The Figure shows the pain trend across the 45-minute treatment for both groups. The steep decline across each pain measurement period was almost identical for each group but illustrates the significant pain relief provided by both the magnet and the placebo devices. Paired t test analysis revealed that the mean change between pre- and post-treatment was -2.4 ( P =.004) for the magnet group and -2.4 ( P =.003) for the placebo group.
TABLE 1
BASELINE CHARACTERISTICS OF THE STUDY GROUPS
| Characteristic | Magnet N (%) | Placebo N (%) | P |
| Mean age, years, N (SD) | 50.7 (15.5) | 48.5 (11.7) | .67* |
| Women | 15 (100) | 11 (73) | .01† |
| Repetitive work | 11 (73) | 13 (87) | .36† |
| Numbness | |||
| None | 5 (33) | 7 (49) | .13† |
| Some | 2 (13) | 5 (33) | |
| A great deal | 8 (53) | 3 (20) | |
| Tingling | |||
| None | 8 (47) | 9 (60) | .68† |
| Some | 2 (13) | 3 (20) | |
| A great deal | 5 (33) | 3 (20) | |
| Burning | |||
| None | 12 (80) | 11 (73) | .22† |
| Some | 0 (0) | 2 (13) | |
| A great deal | 3 (20) | 2 (13) | |
| Pain | |||
| None | 5 (33) | 6 (40) | .25† |
| Some | 3 (20) | 6 (40) | |
| A great deal | 7 (47) | 3 (20) | |
| *t test analysis | |||
| † Chi-square analysis | |||
| SD denotes standard deviation. | |||
TABLE 2
COMPARISON OF GROUP VISUAL ANALOG SCALE MEANS BEFORE, DURING, AND AFTER DEVICE APPLICATION
| Pain Score | Magnet Mean (SD) | Placebo Mean (SD) | Difference (95% CI)* |
|---|---|---|---|
| Pretreatment pain† | 5.9 (2.6) | 5.0 (2.4) | 0.9 (-.90 to 2.84) |
| Pain at 15 minutes† | 4.5 (2.6) | 3.9 (2.8) | 0.6 (-1.49 to 2.47) |
| Pain at 30 minutes† | 3.7 (2.6) | 3.2 (2.6) | 0.5 (-1.47 to 2.36) |
| Post-treatment pain† | 3.6 (3.1) | 2.6 (2.7) | 1.0 (-1.21 to 3.15) |
| Total pain decrease† | -2.4 (2.7) | -2.4 (2.6) | 0.0 (-2.02 to 1.97) |
| Pain at 2 week follow-up‡ | 4.3 (2.9) | 4.3 (3.5) | 0.0 (-3.0 to 3.03) |
| Greatest pain during 2 weeks‡ | 5.5 (2.7) | 4.9 (2.8) | 0.6 (-2.07 to 3.15) |
| Typical pain during 2 weeks‡ | 4.1 (2.7) | 3.7 (2.4) | 0.4 (-1.99 to 2.83) |
| *95% confidence interval for the difference between the mean pain scores. None of the differences were statistically significant. | |||
| SD denotes standard deviation; CI, confidence interval. | |||
| † N=150 | |||
| ‡ N=100 | |||
FIGURE
PAIN TREND BY GROUP
Discussion
The delivery of a unipolar static magnetic field through a magnetized device directly applied to the point of greatest wrist pain resulted in no significant difference in relief of pain when compared with an identical placebo device. However, both magnet and placebo produced a significant decrease in pain during the 45-minute application that was still detectable at the 2-week follow-up. The decrease in pain observed in both experimental and control groups could be attributed to a variety of causes. Most likely, this is a placebo effect due to the patients’ belief in the efficacy of the device. Also, it is possible that pressure over the area of pain, due to application of the bracelet, somehow reduces the amount of pain experienced.
A limitation of this study is the small sample size. It is possible that a larger study would detect small improvements in outcomes, but it is questionable whether these would be clinically significant.
Conclusions
Collacott and colleagues3 found that magnets were not effective in treating low back pain. Although they proposed that the depth of the pain source might have played a role in the outcome of their research project, such an issue would not be a significant factor in our study because of the relatively short distance from the surface of the wrist to the median nerve. Future research might include a measure of belief in magnets as healing devices to determine the impact of the placebo device. The addition of another arm of the study to include magnet placement adjacent to, but not touching, the point of pain to determine the pressure effect might be interesting. Although this study did not show magnets to be more effective than the placebo, the reduction in pain with this simple intervention was remarkable.
Acknowledgments
Funding for this project was provided by The Oklahoma Center for Family Medicine Research, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma; Dr. James Mold, director. Thanks to Deborah Cacy, PhD, for her assistance in the development of our project.
1. Vallbona C, Hazlewood CF, Jurida G. Response of pain to static magnetic fields in postpolio patients: a double-blind pilot study. Arch Phys Med Rehabil 1997;78:1200-03.
2. Man D, Man B, Plosker H. The influence of permanent magnetic field therapy on wound healing in suction lipectomy patients: a double-blind study. Plastic Reconstruct Surg 1999;104:2261-66.
3. Collacott EA, Zimmerman JT, White DW, Rindone JP. Bipolar permanent magnets for the treatment of chronic low back pain. JAMA 1999;283:1322-25.
4. Caselli MA, Clark N, Lazarus S, Velez Z, Venegas L. Evaluation of magnetic foil and PPT insoles in the treatment of heel pain. J Am Podiatr Med Assoc 1997;87:11-16.
5. Lawrence MD, Rosch PJ, Plowden J. Magnet therapy. Rocklin, Calif: Prima Publishers; 1998.
6. Howells B. Magnet therapy’s strong attractions. Available online: outside.starwave.com/magazine/0897/9708bodypres.html.
7. Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975;1:277-99.
We conducted a double-blind placebo-controlled randomized clinical trial in which 30 patients with pain attributed to carpal tunnel syndrome had either a 1000 gauss magnet or a placebo metal disk applied to the carpal tunnel area using a Velcro wrap for a period of 45 minutes. Pain was measured on a visual analogue scale using 0 and 10 as anchors.
Presenting symptoms including numbness, tingling, burning, and pain did not differ significantly between the 2 groups. There was significant pain reduction across the 45-minute period for both groups. However, t test comparisons found no significant differences between the groups for beginning pain, pain at 15 minutes, pain at 30 minutes, or pain at 45 minutes. The use of a magnet for reducing pain attributed to carpal tunnel syndrome was no more effective than use of the placebo device.
Four recent randomized trials have provided conflicting results concerning the efficacy of magnets in relieving pain. Two double-blind randomized trials have found that magnets relieve pain in postpolio subjects1 and in patients with postoperative wounds.2 However, double-blind randomized studies of magnet therapy for treatment of low back pain3 and foot pain4 showed no benefit.
In an attempt to find alternate forms of therapy,5,6 many chronic sufferers of carpal tunnel syndrome have resorted to using magnets to alleviate their symptoms. The purpose of our study was to determine the efficacy of magnet therapy on pain attributed to carpal tunnel syndrome when compared with a placebo device.
Methods
Subjects
We contacted 160 patients who had wrist pain attributed to carpal tunnel syndrome by their primary care physicians. These patients were identified from the billing databases at a university-operated family practice clinic and a rural private practitioner’s office. The inclusion criteria for participation were presence of chronic wrist pain in the area of the carpal tunnel and the willingness to accept randomization into treatment or control group. Individuals were excluded before randomization if the source of pain had been attributed to some cause other than carpal tunnel syndrome, if they had taken pain medication within 4 hours of beginning treatment, if their body mass index was greater than 35, or if they were not experiencing pain at the time treatment was started.
Treatment intervention
The magnets and placebo devices used in our study were custom made by Medical Magnetics of Houston, Texas. The devices consisted of 5 stacked magnetic pads. Four of these were flexible (2500 gauss, residual induction). The fifth pad was a neodymium disk (10,000 gauss, residual induction). The flexible pads were 1.7 inches in diameter, and the neodymium disk was 0.5 inches in diameter. All 5 pads were glued together to form a single unit. Actual magnetic energy was determined to be 1000 gauss at the surface of the center of the magnet, and depth of penetration was estimated to be adequate for the carpal tunnel area. The placebo disks appeared identical to the magnets. Each magnet and placebo was labeled with a computer-generated random number, wrapped in foam, and boxed individually. Individual boxes were selected at the time of the patient appointment without regard for the order or numerical identifier, which served as a blinding device. Codes identifying placebo or control were not broken until the completion of the study.
After giving written consent, patients were asked to complete a short questionnaire collecting demographic and symptom information. They were then asked to rate the pain at the most painful point in the wrist using the visual analog scale (VAS) of the McGill Pain Questionnaire.7 The VAS consisted of a standard length line labeled 0 on the left and 10 on the right. The patient was instructed to place a mark on that line at the appropriate position relative to the degree of pain experienced (0 = no pain; 10 = the worst pain ever experienced). The distance of the mark from 0 was then measured in millimeters to provide the pain score. A new pain scale was provided for each measurement, and patients were not allowed to view previous measurements, to insure the objectivity of the patient’s pain perception.
A device, either magnet or placebo, was then placed on the wrist overlying the carpal tunnel. The device was secured with foam and a wrist bracelet fastened with Velcro. Each patient was then asked to remain seated and to keep the device in place for the next 45 minutes. This time period was selected based on the experience of the postpolio pain trial.1 Throughout the 45 minutes a research assistant observed the patients to ensure that they did not tamper with their device. The patients were asked to rate their pain on the VAS at 15-minute intervals. After 45 minutes the device was removed, and the patient again rated his or her pain on a VAS.
Patients were sent home with a postcard that served as a 2-week follow-up. Two weeks after treatment patients rated their current pain, maximum pain over the 2-week period, and typical pain over the 2-week period, using the previously described VAS.
Data analysis
Previous research on the effect of magnets on pain has shown reduction in pain on a 10-point VAS ranging from 1.1 to 4.4 points with standard deviations of 1.6 and 3.1, respectively.1 Corresponding sample sizes to detect these differences would range from 34 per group to 8 per group. Standard sample size formulas for power equal to 0.80, ( equal to 0.05, and a standard deviation of 2.5 estimated that a sample size of 15 per group could detect a difference of 2.6 points between groups.
Data were analyzed using chi-square analysis for categorical data, paired t tests for within group comparisons, and independent t tests for between group comparisons on age and pain. Confirmation of normal distributions for the VAS variables was made using the Kolmogorov-Smirnov goodness-of-fit test.
Results
Of the 160 patients contacted by mail, 45 replied, 38 qualified for participation, and 30 patients completed the 45-minute treatment protocol: 15 with a magnetic device and 15 with a placebo. Descriptive statistics for the 2 groups are provided in Table 1. Groups did not differ significantly in age or any of the presenting symptoms including numbness, tingling, burning, and pain. There were no men in the magnet group and 4 in the placebo group ( P =.01).
Table 2 contains the mean pain scores for both groups at different points in time. There were no significant differences for any of the pain variables. Twenty of the participants in this study completed a 2-week follow-up questionnaire, 10 in each group. There were no significant differences between groups in the pain at 2 weeks post-treatment, the greatest pain experienced during the 2 weeks, and the typical pain experienced during the 2 weeks. The mean pain score at 2 weeks post-treatment and their typical pain across the 2 weeks had not returned to their baseline pain levels measured before device application.
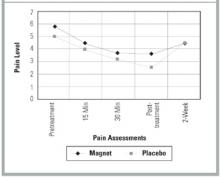
The Figure shows the pain trend across the 45-minute treatment for both groups. The steep decline across each pain measurement period was almost identical for each group but illustrates the significant pain relief provided by both the magnet and the placebo devices. Paired t test analysis revealed that the mean change between pre- and post-treatment was -2.4 ( P =.004) for the magnet group and -2.4 ( P =.003) for the placebo group.
TABLE 1
BASELINE CHARACTERISTICS OF THE STUDY GROUPS
| Characteristic | Magnet N (%) | Placebo N (%) | P |
| Mean age, years, N (SD) | 50.7 (15.5) | 48.5 (11.7) | .67* |
| Women | 15 (100) | 11 (73) | .01† |
| Repetitive work | 11 (73) | 13 (87) | .36† |
| Numbness | |||
| None | 5 (33) | 7 (49) | .13† |
| Some | 2 (13) | 5 (33) | |
| A great deal | 8 (53) | 3 (20) | |
| Tingling | |||
| None | 8 (47) | 9 (60) | .68† |
| Some | 2 (13) | 3 (20) | |
| A great deal | 5 (33) | 3 (20) | |
| Burning | |||
| None | 12 (80) | 11 (73) | .22† |
| Some | 0 (0) | 2 (13) | |
| A great deal | 3 (20) | 2 (13) | |
| Pain | |||
| None | 5 (33) | 6 (40) | .25† |
| Some | 3 (20) | 6 (40) | |
| A great deal | 7 (47) | 3 (20) | |
| *t test analysis | |||
| † Chi-square analysis | |||
| SD denotes standard deviation. | |||
TABLE 2
COMPARISON OF GROUP VISUAL ANALOG SCALE MEANS BEFORE, DURING, AND AFTER DEVICE APPLICATION
| Pain Score | Magnet Mean (SD) | Placebo Mean (SD) | Difference (95% CI)* |
|---|---|---|---|
| Pretreatment pain† | 5.9 (2.6) | 5.0 (2.4) | 0.9 (-.90 to 2.84) |
| Pain at 15 minutes† | 4.5 (2.6) | 3.9 (2.8) | 0.6 (-1.49 to 2.47) |
| Pain at 30 minutes† | 3.7 (2.6) | 3.2 (2.6) | 0.5 (-1.47 to 2.36) |
| Post-treatment pain† | 3.6 (3.1) | 2.6 (2.7) | 1.0 (-1.21 to 3.15) |
| Total pain decrease† | -2.4 (2.7) | -2.4 (2.6) | 0.0 (-2.02 to 1.97) |
| Pain at 2 week follow-up‡ | 4.3 (2.9) | 4.3 (3.5) | 0.0 (-3.0 to 3.03) |
| Greatest pain during 2 weeks‡ | 5.5 (2.7) | 4.9 (2.8) | 0.6 (-2.07 to 3.15) |
| Typical pain during 2 weeks‡ | 4.1 (2.7) | 3.7 (2.4) | 0.4 (-1.99 to 2.83) |
| *95% confidence interval for the difference between the mean pain scores. None of the differences were statistically significant. | |||
| SD denotes standard deviation; CI, confidence interval. | |||
| † N=150 | |||
| ‡ N=100 | |||
FIGURE
PAIN TREND BY GROUP
Discussion
The delivery of a unipolar static magnetic field through a magnetized device directly applied to the point of greatest wrist pain resulted in no significant difference in relief of pain when compared with an identical placebo device. However, both magnet and placebo produced a significant decrease in pain during the 45-minute application that was still detectable at the 2-week follow-up. The decrease in pain observed in both experimental and control groups could be attributed to a variety of causes. Most likely, this is a placebo effect due to the patients’ belief in the efficacy of the device. Also, it is possible that pressure over the area of pain, due to application of the bracelet, somehow reduces the amount of pain experienced.
A limitation of this study is the small sample size. It is possible that a larger study would detect small improvements in outcomes, but it is questionable whether these would be clinically significant.
Conclusions
Collacott and colleagues3 found that magnets were not effective in treating low back pain. Although they proposed that the depth of the pain source might have played a role in the outcome of their research project, such an issue would not be a significant factor in our study because of the relatively short distance from the surface of the wrist to the median nerve. Future research might include a measure of belief in magnets as healing devices to determine the impact of the placebo device. The addition of another arm of the study to include magnet placement adjacent to, but not touching, the point of pain to determine the pressure effect might be interesting. Although this study did not show magnets to be more effective than the placebo, the reduction in pain with this simple intervention was remarkable.
Acknowledgments
Funding for this project was provided by The Oklahoma Center for Family Medicine Research, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma; Dr. James Mold, director. Thanks to Deborah Cacy, PhD, for her assistance in the development of our project.
We conducted a double-blind placebo-controlled randomized clinical trial in which 30 patients with pain attributed to carpal tunnel syndrome had either a 1000 gauss magnet or a placebo metal disk applied to the carpal tunnel area using a Velcro wrap for a period of 45 minutes. Pain was measured on a visual analogue scale using 0 and 10 as anchors.
Presenting symptoms including numbness, tingling, burning, and pain did not differ significantly between the 2 groups. There was significant pain reduction across the 45-minute period for both groups. However, t test comparisons found no significant differences between the groups for beginning pain, pain at 15 minutes, pain at 30 minutes, or pain at 45 minutes. The use of a magnet for reducing pain attributed to carpal tunnel syndrome was no more effective than use of the placebo device.
Four recent randomized trials have provided conflicting results concerning the efficacy of magnets in relieving pain. Two double-blind randomized trials have found that magnets relieve pain in postpolio subjects1 and in patients with postoperative wounds.2 However, double-blind randomized studies of magnet therapy for treatment of low back pain3 and foot pain4 showed no benefit.
In an attempt to find alternate forms of therapy,5,6 many chronic sufferers of carpal tunnel syndrome have resorted to using magnets to alleviate their symptoms. The purpose of our study was to determine the efficacy of magnet therapy on pain attributed to carpal tunnel syndrome when compared with a placebo device.
Methods
Subjects
We contacted 160 patients who had wrist pain attributed to carpal tunnel syndrome by their primary care physicians. These patients were identified from the billing databases at a university-operated family practice clinic and a rural private practitioner’s office. The inclusion criteria for participation were presence of chronic wrist pain in the area of the carpal tunnel and the willingness to accept randomization into treatment or control group. Individuals were excluded before randomization if the source of pain had been attributed to some cause other than carpal tunnel syndrome, if they had taken pain medication within 4 hours of beginning treatment, if their body mass index was greater than 35, or if they were not experiencing pain at the time treatment was started.
Treatment intervention
The magnets and placebo devices used in our study were custom made by Medical Magnetics of Houston, Texas. The devices consisted of 5 stacked magnetic pads. Four of these were flexible (2500 gauss, residual induction). The fifth pad was a neodymium disk (10,000 gauss, residual induction). The flexible pads were 1.7 inches in diameter, and the neodymium disk was 0.5 inches in diameter. All 5 pads were glued together to form a single unit. Actual magnetic energy was determined to be 1000 gauss at the surface of the center of the magnet, and depth of penetration was estimated to be adequate for the carpal tunnel area. The placebo disks appeared identical to the magnets. Each magnet and placebo was labeled with a computer-generated random number, wrapped in foam, and boxed individually. Individual boxes were selected at the time of the patient appointment without regard for the order or numerical identifier, which served as a blinding device. Codes identifying placebo or control were not broken until the completion of the study.
After giving written consent, patients were asked to complete a short questionnaire collecting demographic and symptom information. They were then asked to rate the pain at the most painful point in the wrist using the visual analog scale (VAS) of the McGill Pain Questionnaire.7 The VAS consisted of a standard length line labeled 0 on the left and 10 on the right. The patient was instructed to place a mark on that line at the appropriate position relative to the degree of pain experienced (0 = no pain; 10 = the worst pain ever experienced). The distance of the mark from 0 was then measured in millimeters to provide the pain score. A new pain scale was provided for each measurement, and patients were not allowed to view previous measurements, to insure the objectivity of the patient’s pain perception.
A device, either magnet or placebo, was then placed on the wrist overlying the carpal tunnel. The device was secured with foam and a wrist bracelet fastened with Velcro. Each patient was then asked to remain seated and to keep the device in place for the next 45 minutes. This time period was selected based on the experience of the postpolio pain trial.1 Throughout the 45 minutes a research assistant observed the patients to ensure that they did not tamper with their device. The patients were asked to rate their pain on the VAS at 15-minute intervals. After 45 minutes the device was removed, and the patient again rated his or her pain on a VAS.
Patients were sent home with a postcard that served as a 2-week follow-up. Two weeks after treatment patients rated their current pain, maximum pain over the 2-week period, and typical pain over the 2-week period, using the previously described VAS.
Data analysis
Previous research on the effect of magnets on pain has shown reduction in pain on a 10-point VAS ranging from 1.1 to 4.4 points with standard deviations of 1.6 and 3.1, respectively.1 Corresponding sample sizes to detect these differences would range from 34 per group to 8 per group. Standard sample size formulas for power equal to 0.80, ( equal to 0.05, and a standard deviation of 2.5 estimated that a sample size of 15 per group could detect a difference of 2.6 points between groups.
Data were analyzed using chi-square analysis for categorical data, paired t tests for within group comparisons, and independent t tests for between group comparisons on age and pain. Confirmation of normal distributions for the VAS variables was made using the Kolmogorov-Smirnov goodness-of-fit test.
Results
Of the 160 patients contacted by mail, 45 replied, 38 qualified for participation, and 30 patients completed the 45-minute treatment protocol: 15 with a magnetic device and 15 with a placebo. Descriptive statistics for the 2 groups are provided in Table 1. Groups did not differ significantly in age or any of the presenting symptoms including numbness, tingling, burning, and pain. There were no men in the magnet group and 4 in the placebo group ( P =.01).
Table 2 contains the mean pain scores for both groups at different points in time. There were no significant differences for any of the pain variables. Twenty of the participants in this study completed a 2-week follow-up questionnaire, 10 in each group. There were no significant differences between groups in the pain at 2 weeks post-treatment, the greatest pain experienced during the 2 weeks, and the typical pain experienced during the 2 weeks. The mean pain score at 2 weeks post-treatment and their typical pain across the 2 weeks had not returned to their baseline pain levels measured before device application.
The Figure shows the pain trend across the 45-minute treatment for both groups. The steep decline across each pain measurement period was almost identical for each group but illustrates the significant pain relief provided by both the magnet and the placebo devices. Paired t test analysis revealed that the mean change between pre- and post-treatment was -2.4 ( P =.004) for the magnet group and -2.4 ( P =.003) for the placebo group.
TABLE 1
BASELINE CHARACTERISTICS OF THE STUDY GROUPS
| Characteristic | Magnet N (%) | Placebo N (%) | P |
| Mean age, years, N (SD) | 50.7 (15.5) | 48.5 (11.7) | .67* |
| Women | 15 (100) | 11 (73) | .01† |
| Repetitive work | 11 (73) | 13 (87) | .36† |
| Numbness | |||
| None | 5 (33) | 7 (49) | .13† |
| Some | 2 (13) | 5 (33) | |
| A great deal | 8 (53) | 3 (20) | |
| Tingling | |||
| None | 8 (47) | 9 (60) | .68† |
| Some | 2 (13) | 3 (20) | |
| A great deal | 5 (33) | 3 (20) | |
| Burning | |||
| None | 12 (80) | 11 (73) | .22† |
| Some | 0 (0) | 2 (13) | |
| A great deal | 3 (20) | 2 (13) | |
| Pain | |||
| None | 5 (33) | 6 (40) | .25† |
| Some | 3 (20) | 6 (40) | |
| A great deal | 7 (47) | 3 (20) | |
| *t test analysis | |||
| † Chi-square analysis | |||
| SD denotes standard deviation. | |||
TABLE 2
COMPARISON OF GROUP VISUAL ANALOG SCALE MEANS BEFORE, DURING, AND AFTER DEVICE APPLICATION
| Pain Score | Magnet Mean (SD) | Placebo Mean (SD) | Difference (95% CI)* |
|---|---|---|---|
| Pretreatment pain† | 5.9 (2.6) | 5.0 (2.4) | 0.9 (-.90 to 2.84) |
| Pain at 15 minutes† | 4.5 (2.6) | 3.9 (2.8) | 0.6 (-1.49 to 2.47) |
| Pain at 30 minutes† | 3.7 (2.6) | 3.2 (2.6) | 0.5 (-1.47 to 2.36) |
| Post-treatment pain† | 3.6 (3.1) | 2.6 (2.7) | 1.0 (-1.21 to 3.15) |
| Total pain decrease† | -2.4 (2.7) | -2.4 (2.6) | 0.0 (-2.02 to 1.97) |
| Pain at 2 week follow-up‡ | 4.3 (2.9) | 4.3 (3.5) | 0.0 (-3.0 to 3.03) |
| Greatest pain during 2 weeks‡ | 5.5 (2.7) | 4.9 (2.8) | 0.6 (-2.07 to 3.15) |
| Typical pain during 2 weeks‡ | 4.1 (2.7) | 3.7 (2.4) | 0.4 (-1.99 to 2.83) |
| *95% confidence interval for the difference between the mean pain scores. None of the differences were statistically significant. | |||
| SD denotes standard deviation; CI, confidence interval. | |||
| † N=150 | |||
| ‡ N=100 | |||
FIGURE
PAIN TREND BY GROUP
Discussion
The delivery of a unipolar static magnetic field through a magnetized device directly applied to the point of greatest wrist pain resulted in no significant difference in relief of pain when compared with an identical placebo device. However, both magnet and placebo produced a significant decrease in pain during the 45-minute application that was still detectable at the 2-week follow-up. The decrease in pain observed in both experimental and control groups could be attributed to a variety of causes. Most likely, this is a placebo effect due to the patients’ belief in the efficacy of the device. Also, it is possible that pressure over the area of pain, due to application of the bracelet, somehow reduces the amount of pain experienced.
A limitation of this study is the small sample size. It is possible that a larger study would detect small improvements in outcomes, but it is questionable whether these would be clinically significant.
Conclusions
Collacott and colleagues3 found that magnets were not effective in treating low back pain. Although they proposed that the depth of the pain source might have played a role in the outcome of their research project, such an issue would not be a significant factor in our study because of the relatively short distance from the surface of the wrist to the median nerve. Future research might include a measure of belief in magnets as healing devices to determine the impact of the placebo device. The addition of another arm of the study to include magnet placement adjacent to, but not touching, the point of pain to determine the pressure effect might be interesting. Although this study did not show magnets to be more effective than the placebo, the reduction in pain with this simple intervention was remarkable.
Acknowledgments
Funding for this project was provided by The Oklahoma Center for Family Medicine Research, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma; Dr. James Mold, director. Thanks to Deborah Cacy, PhD, for her assistance in the development of our project.
1. Vallbona C, Hazlewood CF, Jurida G. Response of pain to static magnetic fields in postpolio patients: a double-blind pilot study. Arch Phys Med Rehabil 1997;78:1200-03.
2. Man D, Man B, Plosker H. The influence of permanent magnetic field therapy on wound healing in suction lipectomy patients: a double-blind study. Plastic Reconstruct Surg 1999;104:2261-66.
3. Collacott EA, Zimmerman JT, White DW, Rindone JP. Bipolar permanent magnets for the treatment of chronic low back pain. JAMA 1999;283:1322-25.
4. Caselli MA, Clark N, Lazarus S, Velez Z, Venegas L. Evaluation of magnetic foil and PPT insoles in the treatment of heel pain. J Am Podiatr Med Assoc 1997;87:11-16.
5. Lawrence MD, Rosch PJ, Plowden J. Magnet therapy. Rocklin, Calif: Prima Publishers; 1998.
6. Howells B. Magnet therapy’s strong attractions. Available online: outside.starwave.com/magazine/0897/9708bodypres.html.
7. Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975;1:277-99.
1. Vallbona C, Hazlewood CF, Jurida G. Response of pain to static magnetic fields in postpolio patients: a double-blind pilot study. Arch Phys Med Rehabil 1997;78:1200-03.
2. Man D, Man B, Plosker H. The influence of permanent magnetic field therapy on wound healing in suction lipectomy patients: a double-blind study. Plastic Reconstruct Surg 1999;104:2261-66.
3. Collacott EA, Zimmerman JT, White DW, Rindone JP. Bipolar permanent magnets for the treatment of chronic low back pain. JAMA 1999;283:1322-25.
4. Caselli MA, Clark N, Lazarus S, Velez Z, Venegas L. Evaluation of magnetic foil and PPT insoles in the treatment of heel pain. J Am Podiatr Med Assoc 1997;87:11-16.
5. Lawrence MD, Rosch PJ, Plowden J. Magnet therapy. Rocklin, Calif: Prima Publishers; 1998.
6. Howells B. Magnet therapy’s strong attractions. Available online: outside.starwave.com/magazine/0897/9708bodypres.html.
7. Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975;1:277-99.