User login
VA Home Telehealth Program for Initiating and Optimizing Heart Failure Guideline-Directed Medical Therapy
Heart failure (HF) is a chronic, progressive condition that is characterized by the heart’s inability to effectively pump blood throughout the body. In 2018, approximately 6.2 million US adults had HF, and 13.4% of all death certificates noted HF as a precipitating factor.1 Patients not receiving appropriate guideline-directed medical therapy (GDMT) face a 29% excess mortality risk over a 2-year period.2 Each additional GDMT included in a patient’s regimen significantly reduces all-cause mortality.3
The Change the Management of Patients with Heart Failure (CHAMP) registry reports that only about 1% of patients with HF are prescribed 3 agents from contemporary GDMT at target doses, highlighting the need for optimizing clinicians’ approaches to GDMT.4 Similarly, The Get With The Guidelines Heart Failure Registry has noted that only 20.2% of patients with HF with reduced ejection fraction (HFrEF) are prescribed a sodium-glucose cotransporter 2 inhibitor (SGLT2i) following hospital discharge for HFrEF exacerbation.5 Overall, treatment rates with GDMT saw limited improvement between 2013 and 2019, with no significant difference between groups in mortality, indicating the need for optimized methods to encourage the initiation of GDMT.6
Remote monitoring and telecare are novel ways to improve GDMT rates in those with HFrEF. However, data are inconsistent regarding the impact of remote HF monitoring and improvements in GDMT or HF-related outcomes.6-10 The modalities of remote monitoring for GDMT vary among studies, but the potential for telehealth monitoring to improve GDMT, thereby potentially reducing HF-related hospitalizations, is clear.
Telemonitoring has demonstrated improved participant adherence with weight monitoring, although the withdrawal rate was high, and has the potential to reduce all-cause mortality and HF-related hospitalizations.11,12 Telemonitoring for GDMT optimization led to an increase in the proportion of patients who achieved optimal GDMT doses, a decrease in the time to dose optimization, and a reduction in the number of clinic visits.13 Remote GDMT titration was accomplished in the general patient population with HFrEF; however, in populations already followed by cardiologists or HF specialists, remote optimization strategies did not yield different proportions of GDMT use.14 The aim of this study was to assess the impact of the home telehealth (HT) monitoring program on the initiation and optimization of HF GDMT among veterans with HFrEF at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan.
Methods
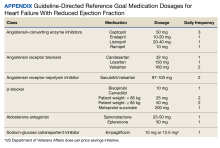
This was a single-center retrospective study of Computerized Patient Record System (CPRS)data. Patients at the VAAAHS were evaluated if they were diagnosed with HFrEF and were eligible for enrollment in the HT monitoring program. Eligibility criteria included a diagnosis of stage C HF, irrespective of EF, and a history of any HF-related hospitalization. We focused on patients with HFrEF due to stronger guideline-based recommendations for certain pharmacotherapies as compared with HF with mildly reduced ejection fraction (HFmrEF) and preserved ejection fraction (HFpEF). Initial patient data for HT enrolling were accessed using the Heart Failure Dashboard via the US Department of Veterans Affairs (VA) Academic Detailing Service. The target daily doses of typical agents used in HFrEF GDMT are listed in the Appendix.
The HT program is an embedded model in which HT nurses receive remote data from the patient and triage that with the VAAAHS cardiology team. Patients’ questions, concerns, and/or vital signs are recovered remotely. In this model, nurses are embedded in the cardiology team, working with the cardiologists, cardiology clinical pharmacist, and/or cardiology nurse practitioners to make medication interventions. Data are recorded with an HT device, including weight, blood pressure (BP), heart rate, and pulse oximetry. HT nurses are also available to the patient via phone or video. The program uses a 180-day disease management protocol for HF via remote device, enabling the patient to answer questions and receive education on their disease daily. Responses to questions and data are then reviewed by an HT nurse remotely during business hours and triaged as appropriate with the cardiology team. Data can be communicated to the cardiology team via the patient record, eliminating the need for the cardiology team to use the proprietary portal affiliated with the HT device.
Study Sample
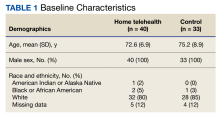
Patient information was obtained from a list of 417 patients eligible for enrollment in the HT program; the list was sent to the HT program for review and enrollment. Patient data were extracted from the VAAAHS HF Dashboard and included all patients with HFrEF and available data on the platform. The sample for the retrospective chart review included 40 adults who had HFrEF, defined as a left ventricular EF (LVEF) of ≤ 40% as evidenced by a transthoracic echocardiogram or cardiac magnetic resonance imaging. These patients were contacted and agreed to enroll in the HT monitoring program. The HT program population was compared against a control group of 33 patients who were ineligible for the HT program. Patients were deemed ineligible for HT if they resided in a nursing home, lacked a VAAAHS primary care clinician, or declined participation in the HT program.
Procedures
Patients who declined participation in the HT program followed the standard of care, which was limited to visits with primary care clinicians and/or cardiologists as per the follow-up plan. Patient data were collected over 12 months. The study was approved by the VAAAHS Institutional Review Board (reference number, 1703034), Research and Development Committee, and Research Administration.
Primary and Secondary Goals
The primary goal of the study was to assess the impact of the HT program on drug interventions, specifically initiating and titrating HFrEF pharmacotherapies. Interventions were based on GDMT with known mortality- and morbidity-reducing properties when used at their maximum tolerated doses, including angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor-neprilysin inhibitor (ARNi), or angiotensin receptor blockers (ARB), with a preference for ARNi, β-blockers for HFrEF (metoprolol succinate, bisoprolol, or carvedilol), aldosterone antagonists, and SGLT2is.
Secondary goals included HF-related hospitalizations, medication adherence, time to enrollment in HT, time to laboratory analysis after the initiation or titration of an ACEi/ARB/ARNi or aldosterone antagonist, and time enrolled in the HT program. Patients were considered adherent if their drug refill history showed consistent fills of their medications. The χ2 test was used for total interventions made during the study period and Fisher exact test for all others.
Results
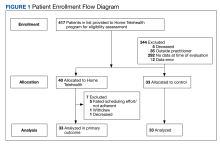
Patient data were collected between July 2022 and June 2023. All 73 patients were male, and the mean age in the HT group (n = 40) was 72.6 years and 75.2 years for the control group (n = 33). Overall, the baseline demographics were similar between the groups (Table 1). Of 40 patients screened for enrollment in the HT program, 33 were included in the analysis (Figure 1).
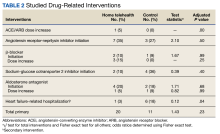
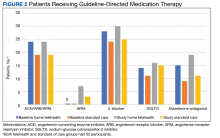
At baseline, the HT group included more individuals than the control group on ACEi/ARB/ARNi (24 vs 19, respectively), β-blocker (28 vs 24, respectively), SGLT2i (14 vs 11, respectively), and aldosterone antagonist (15 vs 9, respectively) (Figure 2). There were 20 interventions made in the HT group compared with 11 therapy changes in the control arm during the study (odds ratio, 1.43; P = .23) (Table 2). In the HT group, 1 patient achieved an ACEi target dose, 3 patients achieved a β-blocker target dose, and 7 achieved a target dose of spironolactone (titration is not required for SGLT2i therapy and is counted as target dose). In the HT group, 17 patients were on ≥ 3 recommended agents, while 9 patients were taking 4 agents. Seven of 20 HT group interventions resulted in titration to the target dose. In the control group, no patients achieved an ARNi target dose, 3 patients achieved a β-blocker target dose, and 2 patients achieved a spironolactone antagonist target dose. In the control arm, 7 patients were on ≥ 3 GDMTs, and 2 were taking 4 agents. No patient in either group achieved a target dose of 4 agents. Five of 11 control group interventions resulted in initiation or titration of GDMT to the target dose.
Of the 40 HT group patients, 7 were excluded from analysis (3 failed to schedule HT, 1 was at a long-term care facility, 1 was nonadherent, 1 declined participation, and 1 died) and 33 remained in the program for a mean (SD) 5.3 (3.5) months. Death rates were tracked during the study: 1 patient died in the HT group and 3 in the control group.
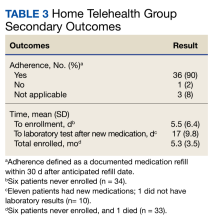
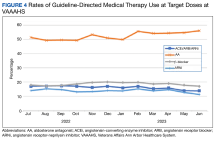
We analyzed the overall percentage of VAAAHS patients with HFrEF who were on appropriate GDMT. Given the ongoing drive to improve HF-related outcomes, HT interventions could not be compared to a static population, so the HT and control patients were compared with the rates of GDMT at VAAAHS, which was available in the Academic Detailing Service Heart Failure Dashboard (Figure 3). Titration and optimization rates were also compared (Figure 4). From July 2022 to June 2023, ARNi use increased by 16.6%, aldosterone antagonist by 6.8%, and β-blockers by 2.4%. Target doses of GDMTs were more difficult to achieve in the hospital system. There was an increase in aldosterone antagonist target dose achievement by 4.7%, but overall there were decreases in target doses in other GDMTs: ACEi/ARB/ARNi target dose use decreased by 3.2%, ARNi target dose use decreased by 2.7% and target β-blocker use decreased by 0.9%.
Discussion
Telehealth yielded clinically important interventions, with some titrations bringing patients to their target doses of medications for HFrEF. The 20 interventions made in the HT group can be largely attributed to the nurses’ efforts to alert clinicians to drug titrations or ACEi/ARB to ARNi transitions. Although the findings were not statistically significant, the difference in the number of drug therapy changes supports the use of the HT program for a GDMT optimization strategy. Patients may be difficult to titrate secondary to adverse effects that make medication initiation or titration inappropriate, such as hypotension and hyperkalemia, although this was not observed in this small sample size. Considering a mean HT enrollment of 5.3 months, many patients had adequate disease assessment and medication titration. Given that patients are discharged from the service once deemed appropriate, this decreases the burden on the patient and increases the utility and implementation of the HT program for other patients.
A surprising finding of this study was the lower rate of HF-related hospitalizations in the HT group. Although not the primary subject of interest in the study, it reinforced the importance of close health care professional follow-up for patients living with HF. Telehealth may improve communication and shared decision making over medication use. Because the finding was unanticipated, the rate of diuretic adjustments was not tracked.
Patients were reevaluated every 6 months for willingness to continue the program, adherence, and clinical needs. These results are similar to those of other trials that demonstrated improved rates of GDMT in the setting of pharmacist- or nurse-led HF treatment optimization.15,16 These positive results differ from other trials incorporating remote monitoring regarding patient continuation in HT programs. For example, in a study by Ding and colleagues, the withdrawal rate from their monitoring service was about 22%, while in our study only 1 patient withdrew from the HT program.11
The HT program resulted in fewer hospitalizations than the control arm. There were 6 HF-related hospitalizations in the control group, although 5 involved a single patient. Typically, such a patient would be encouraged to follow HT monitoring after just 1 HF-related hospitalization; however, the patient declined to participate.
Early optimization of GDMT in patients who were recently discharged from the hospital for an HF-related hospitalization yields a reduction in hospital rehospitalization.17 GDMT optimization has unequivocal benefits in HF outcomes. Unfortunately, the issues surrounding methodologies on how to best optimize GDMT are lacking. While HT has been found to be feasible in the aid of optimizing medical therapy, the TIM-HF trial concluded that remote monitoring services had no significant benefit in reducing mortality.7,8 On the other hand, the OptiLink HF Study showed that when clinicians respond to remote monitoring prompts from fluid index threshold crossing alerts, these interventions are associated with significantly improved clinical outcomes in patients with implantable cardioverter-defibrillators and advanced HF.9 In contrast to previous trials, the AMULET trial showed that remote monitoring compared with standard care significantly reduced the risk of HF hospitalization or cardiovascular death during the 12-month follow-up among patients with HF and LVEF ≤ 49% after an episode of acute exacerbation.10 Additionally, patients who received skilled home health services and participated in remote monitoring for their chronic HF experienced a reduction in all-cause 30-day readmission.18
Given the contrasting evidence regarding remote monitoring and variable modalities of implementing interventions, we investigated whether HT monitoring yields improvements in GDMT optimization. We found that HT nurses were able to nearly double the rate of interventions for patients with HFrEF. The HT program in providing expanded services will require adequate staffing responsibilities and support. The HT program is geared toward following a large, diverse patient population, such as those with chronic obstructive pulmonary disease, hypertension, and HF. We only evaluated services for patients with HFrEF, but the program also follows patients with HfmrEF and HfpEF. These patients were not included as GDMT optimization differs for patients with an LVEF > 40%.19,20
The lower rates of achieving target doses of GDMTs were likely obstructed by continuous use of initial drug doses and further limited by lack of follow-up. When compared with the rest of the VAAAHS, there was a greater effort to increase ARNi use in the HT group as 7 of 33 patients (21%) were started on ARNi compared with a background increase of ARNi use of 17%. There was a lower mortality rate observed in the HT group compared with the control group. One patient in each group died of unrelated causes, while 2 deaths in the control group were due to worsening HF. The difference in mortality is likely multifactorial, possibly related to the control group’s greater disease burden or higher mean age (75.2 years vs 72.6 years).
Limitations
This was an observational cohort design, which is subject to bias. Thus, the findings of this study are entirely hypothesis-generating and a randomized controlled trial would be necessary for clearer results. Second, low numbers of participants may have skewed the data set. Given the observational nature of the study, this nonetheless is a positive finding to support the HT program for assisting with HF monitoring and prompting drug interventions. Due to the low number of participants, a single patient may have skewed the results with 5 hospitalizations.
Conclusions
This pilot study demonstrates the applicability of HT monitoring to optimize veteran HFrEF GDMT. The HT program yielded numerically relevant interventions and fewer HF-related hospitalizations compared with the control arm. The study shows the feasibility of the program to safely optimize GDMT toward their target doses and may serve as an additional catalyst to further develop HT programs specifically targeted toward HF monitoring and management. Cost-savings analyses would likely need to demonstrate the cost utility of such a service.
We thank the home telehealth nursing staff for their assistance in data collection and enrollment of patients into the monitoring program.
1. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153-e639. doi:10.1161/CIR.0000000000001052
2. McCullough PA, Mehta HS, Barker CM, et al. Mortality and guideline-directed medical therapy in real-world heart failure patients with reduced ejection fraction. Clin Cardiol. 2021;44(9):1192-1198. doi:10.1002/clc.23664
3. Tromp J, Ouwerkerk W, van Veldhuisen DJ, et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2022;10(2):73-84. doi:10.1016/j.jchf.2021.09.004
4. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF Registry. J Am Coll Cardiol. 2018;72(4):351-366. doi:10.1016/j.jacc.2018.04.070
5. Pierce JB, Vaduganathan M, Fonarow GC, et al. Contemporary use of sodium-glucose cotransporter-2 inhibitor therapy among patients hospitalized for heart failure with reduced ejection fraction in the US: The Get With The Guidelines-Heart Failure Registry. JAMA Cardiol. 2023;8(7):652-661. doi:10.1001/jamacardio.2023.1266
6. Sandhu AT, Kohsaka S, Turakhia MP, Lewis EF, Heidenreich PA. Evaluation of quality of care for US veterans with recent-onset heart failure with reduced ejection fraction. JAMA Cardiol. 2022;7(2):130-139. doi:10.1001/jamacardio.2021.4585 7. Rahimi K, Nazarzadeh M, Pinho-Gomes AC, et al. Home monitoring with technology-supported management in chronic heart failure: a randomised trial. Heart. 2020;106(20):1573-1578. doi:10.1136/heartjnl-2020-316773 8. Koehler F, Winkler S, Schieber M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123(17):1873-1880. doi:10.1161/CIRCULATIONAHA.111.018473
9. Wintrich J, Pavlicek V, Brachmann J, et al. Remote monitoring with appropriate reaction to alerts was associated with improved outcomes in chronic heart failure: results from the OptiLink HF study. Circ Arrhythm Electrophysiol. 2021;14(1):e008693. doi:10.1161/CIRCEP.120.008693
10. Krzesinski P, Jankowska EA, Siebert J, et al. Effects of an outpatient intervention comprising nurse-led non-invasive assessments, telemedicine support and remote cardiologists’ decisions in patients with heart failure (AMULET study): a randomised controlled trial. Eur J Heart Fail. 2022;24(3):565-577. doi:10.1002/ejhf.2358
11. Ding H, Jayasena R, Chen SH, et al. The effects of telemonitoring on patient compliance with self-management recommendations and outcomes of the innovative telemonitoring enhanced care program for chronic heart failure: randomized controlled trial. J Med Internet Res. 2020;22(7):e17559. doi:10.2196/17559
12. Kitsiou S, Pare G, Jaana M. Effects of home telemonitoring interventions on patients with chronic heart failure: an overview of systematic reviews. J Med Internet Res. 2015;17(3):e63. doi:10.2196/jmir.4174
13. Artanian V, Ross HJ, Rac VE, O’Sullivan M, Brahmbhatt DH, Seto E. Impact of remote titration combined with telemonitoring on the optimization of guideline-directed medical therapy for patients with heart failure: internal pilot of a randomized controlled trial. JMIR Cardio. 2020;4(1):e21962. doi:10.2196/21962
14. Desai AS, Maclean T, Blood AJ, et al. Remote optimization of guideline-directed medical therapy in patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2020;5(12):1430-1434. doi:10.1001/jamacardio.2020.3757
15. Patil T, Ali S, Kaur A, et al. Impact of pharmacist-led heart failure clinic on optimization of guideline-directed medical therapy (PHARM-HF). J Cardiovasc Transl Res. 2022;15(6):1424-1435. doi:10.1007/s12265-022-10262-9
16. Zheng J, Mednick T, Heidenreich PA, Sandhu AT. Pharmacist- and nurse-led medical optimization in heart failure: a systematic review and meta-analysis. J Card Fail. 2023;29(7):1000-1013. doi:10.1016/j.cardfail.2023.03.012
17. Mebazaa A, Davison B, Chioncel O, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022;400(10367):1938-1952. doi:10.1016/S0140-6736(22)02076-1
18. O’Connor M, Asdornwised U, Dempsey ML, et al. Using telehealth to reduce all-cause 30-day hospital readmissions among heart failure patients receiving skilled home health services. Appl Clin Inform. 2016;7(2):238-47. doi:10.4338/ACI-2015-11-SOA-0157
19. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e876-e894. doi:10.1161/CIR.0000000000001062
20. Kittleson MM, Panjrath GS, Amancherla K, et al. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023;81(18):1835-1878. doi:10.1016/j.jacc.2023.03.393
Heart failure (HF) is a chronic, progressive condition that is characterized by the heart’s inability to effectively pump blood throughout the body. In 2018, approximately 6.2 million US adults had HF, and 13.4% of all death certificates noted HF as a precipitating factor.1 Patients not receiving appropriate guideline-directed medical therapy (GDMT) face a 29% excess mortality risk over a 2-year period.2 Each additional GDMT included in a patient’s regimen significantly reduces all-cause mortality.3
The Change the Management of Patients with Heart Failure (CHAMP) registry reports that only about 1% of patients with HF are prescribed 3 agents from contemporary GDMT at target doses, highlighting the need for optimizing clinicians’ approaches to GDMT.4 Similarly, The Get With The Guidelines Heart Failure Registry has noted that only 20.2% of patients with HF with reduced ejection fraction (HFrEF) are prescribed a sodium-glucose cotransporter 2 inhibitor (SGLT2i) following hospital discharge for HFrEF exacerbation.5 Overall, treatment rates with GDMT saw limited improvement between 2013 and 2019, with no significant difference between groups in mortality, indicating the need for optimized methods to encourage the initiation of GDMT.6
Remote monitoring and telecare are novel ways to improve GDMT rates in those with HFrEF. However, data are inconsistent regarding the impact of remote HF monitoring and improvements in GDMT or HF-related outcomes.6-10 The modalities of remote monitoring for GDMT vary among studies, but the potential for telehealth monitoring to improve GDMT, thereby potentially reducing HF-related hospitalizations, is clear.
Telemonitoring has demonstrated improved participant adherence with weight monitoring, although the withdrawal rate was high, and has the potential to reduce all-cause mortality and HF-related hospitalizations.11,12 Telemonitoring for GDMT optimization led to an increase in the proportion of patients who achieved optimal GDMT doses, a decrease in the time to dose optimization, and a reduction in the number of clinic visits.13 Remote GDMT titration was accomplished in the general patient population with HFrEF; however, in populations already followed by cardiologists or HF specialists, remote optimization strategies did not yield different proportions of GDMT use.14 The aim of this study was to assess the impact of the home telehealth (HT) monitoring program on the initiation and optimization of HF GDMT among veterans with HFrEF at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan.
Methods
This was a single-center retrospective study of Computerized Patient Record System (CPRS)data. Patients at the VAAAHS were evaluated if they were diagnosed with HFrEF and were eligible for enrollment in the HT monitoring program. Eligibility criteria included a diagnosis of stage C HF, irrespective of EF, and a history of any HF-related hospitalization. We focused on patients with HFrEF due to stronger guideline-based recommendations for certain pharmacotherapies as compared with HF with mildly reduced ejection fraction (HFmrEF) and preserved ejection fraction (HFpEF). Initial patient data for HT enrolling were accessed using the Heart Failure Dashboard via the US Department of Veterans Affairs (VA) Academic Detailing Service. The target daily doses of typical agents used in HFrEF GDMT are listed in the Appendix.
The HT program is an embedded model in which HT nurses receive remote data from the patient and triage that with the VAAAHS cardiology team. Patients’ questions, concerns, and/or vital signs are recovered remotely. In this model, nurses are embedded in the cardiology team, working with the cardiologists, cardiology clinical pharmacist, and/or cardiology nurse practitioners to make medication interventions. Data are recorded with an HT device, including weight, blood pressure (BP), heart rate, and pulse oximetry. HT nurses are also available to the patient via phone or video. The program uses a 180-day disease management protocol for HF via remote device, enabling the patient to answer questions and receive education on their disease daily. Responses to questions and data are then reviewed by an HT nurse remotely during business hours and triaged as appropriate with the cardiology team. Data can be communicated to the cardiology team via the patient record, eliminating the need for the cardiology team to use the proprietary portal affiliated with the HT device.
Study Sample
Patient information was obtained from a list of 417 patients eligible for enrollment in the HT program; the list was sent to the HT program for review and enrollment. Patient data were extracted from the VAAAHS HF Dashboard and included all patients with HFrEF and available data on the platform. The sample for the retrospective chart review included 40 adults who had HFrEF, defined as a left ventricular EF (LVEF) of ≤ 40% as evidenced by a transthoracic echocardiogram or cardiac magnetic resonance imaging. These patients were contacted and agreed to enroll in the HT monitoring program. The HT program population was compared against a control group of 33 patients who were ineligible for the HT program. Patients were deemed ineligible for HT if they resided in a nursing home, lacked a VAAAHS primary care clinician, or declined participation in the HT program.
Procedures
Patients who declined participation in the HT program followed the standard of care, which was limited to visits with primary care clinicians and/or cardiologists as per the follow-up plan. Patient data were collected over 12 months. The study was approved by the VAAAHS Institutional Review Board (reference number, 1703034), Research and Development Committee, and Research Administration.
Primary and Secondary Goals
The primary goal of the study was to assess the impact of the HT program on drug interventions, specifically initiating and titrating HFrEF pharmacotherapies. Interventions were based on GDMT with known mortality- and morbidity-reducing properties when used at their maximum tolerated doses, including angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor-neprilysin inhibitor (ARNi), or angiotensin receptor blockers (ARB), with a preference for ARNi, β-blockers for HFrEF (metoprolol succinate, bisoprolol, or carvedilol), aldosterone antagonists, and SGLT2is.
Secondary goals included HF-related hospitalizations, medication adherence, time to enrollment in HT, time to laboratory analysis after the initiation or titration of an ACEi/ARB/ARNi or aldosterone antagonist, and time enrolled in the HT program. Patients were considered adherent if their drug refill history showed consistent fills of their medications. The χ2 test was used for total interventions made during the study period and Fisher exact test for all others.
Results
Patient data were collected between July 2022 and June 2023. All 73 patients were male, and the mean age in the HT group (n = 40) was 72.6 years and 75.2 years for the control group (n = 33). Overall, the baseline demographics were similar between the groups (Table 1). Of 40 patients screened for enrollment in the HT program, 33 were included in the analysis (Figure 1).
At baseline, the HT group included more individuals than the control group on ACEi/ARB/ARNi (24 vs 19, respectively), β-blocker (28 vs 24, respectively), SGLT2i (14 vs 11, respectively), and aldosterone antagonist (15 vs 9, respectively) (Figure 2). There were 20 interventions made in the HT group compared with 11 therapy changes in the control arm during the study (odds ratio, 1.43; P = .23) (Table 2). In the HT group, 1 patient achieved an ACEi target dose, 3 patients achieved a β-blocker target dose, and 7 achieved a target dose of spironolactone (titration is not required for SGLT2i therapy and is counted as target dose). In the HT group, 17 patients were on ≥ 3 recommended agents, while 9 patients were taking 4 agents. Seven of 20 HT group interventions resulted in titration to the target dose. In the control group, no patients achieved an ARNi target dose, 3 patients achieved a β-blocker target dose, and 2 patients achieved a spironolactone antagonist target dose. In the control arm, 7 patients were on ≥ 3 GDMTs, and 2 were taking 4 agents. No patient in either group achieved a target dose of 4 agents. Five of 11 control group interventions resulted in initiation or titration of GDMT to the target dose.
Of the 40 HT group patients, 7 were excluded from analysis (3 failed to schedule HT, 1 was at a long-term care facility, 1 was nonadherent, 1 declined participation, and 1 died) and 33 remained in the program for a mean (SD) 5.3 (3.5) months. Death rates were tracked during the study: 1 patient died in the HT group and 3 in the control group.
We analyzed the overall percentage of VAAAHS patients with HFrEF who were on appropriate GDMT. Given the ongoing drive to improve HF-related outcomes, HT interventions could not be compared to a static population, so the HT and control patients were compared with the rates of GDMT at VAAAHS, which was available in the Academic Detailing Service Heart Failure Dashboard (Figure 3). Titration and optimization rates were also compared (Figure 4). From July 2022 to June 2023, ARNi use increased by 16.6%, aldosterone antagonist by 6.8%, and β-blockers by 2.4%. Target doses of GDMTs were more difficult to achieve in the hospital system. There was an increase in aldosterone antagonist target dose achievement by 4.7%, but overall there were decreases in target doses in other GDMTs: ACEi/ARB/ARNi target dose use decreased by 3.2%, ARNi target dose use decreased by 2.7% and target β-blocker use decreased by 0.9%.
Discussion
Telehealth yielded clinically important interventions, with some titrations bringing patients to their target doses of medications for HFrEF. The 20 interventions made in the HT group can be largely attributed to the nurses’ efforts to alert clinicians to drug titrations or ACEi/ARB to ARNi transitions. Although the findings were not statistically significant, the difference in the number of drug therapy changes supports the use of the HT program for a GDMT optimization strategy. Patients may be difficult to titrate secondary to adverse effects that make medication initiation or titration inappropriate, such as hypotension and hyperkalemia, although this was not observed in this small sample size. Considering a mean HT enrollment of 5.3 months, many patients had adequate disease assessment and medication titration. Given that patients are discharged from the service once deemed appropriate, this decreases the burden on the patient and increases the utility and implementation of the HT program for other patients.
A surprising finding of this study was the lower rate of HF-related hospitalizations in the HT group. Although not the primary subject of interest in the study, it reinforced the importance of close health care professional follow-up for patients living with HF. Telehealth may improve communication and shared decision making over medication use. Because the finding was unanticipated, the rate of diuretic adjustments was not tracked.
Patients were reevaluated every 6 months for willingness to continue the program, adherence, and clinical needs. These results are similar to those of other trials that demonstrated improved rates of GDMT in the setting of pharmacist- or nurse-led HF treatment optimization.15,16 These positive results differ from other trials incorporating remote monitoring regarding patient continuation in HT programs. For example, in a study by Ding and colleagues, the withdrawal rate from their monitoring service was about 22%, while in our study only 1 patient withdrew from the HT program.11
The HT program resulted in fewer hospitalizations than the control arm. There were 6 HF-related hospitalizations in the control group, although 5 involved a single patient. Typically, such a patient would be encouraged to follow HT monitoring after just 1 HF-related hospitalization; however, the patient declined to participate.
Early optimization of GDMT in patients who were recently discharged from the hospital for an HF-related hospitalization yields a reduction in hospital rehospitalization.17 GDMT optimization has unequivocal benefits in HF outcomes. Unfortunately, the issues surrounding methodologies on how to best optimize GDMT are lacking. While HT has been found to be feasible in the aid of optimizing medical therapy, the TIM-HF trial concluded that remote monitoring services had no significant benefit in reducing mortality.7,8 On the other hand, the OptiLink HF Study showed that when clinicians respond to remote monitoring prompts from fluid index threshold crossing alerts, these interventions are associated with significantly improved clinical outcomes in patients with implantable cardioverter-defibrillators and advanced HF.9 In contrast to previous trials, the AMULET trial showed that remote monitoring compared with standard care significantly reduced the risk of HF hospitalization or cardiovascular death during the 12-month follow-up among patients with HF and LVEF ≤ 49% after an episode of acute exacerbation.10 Additionally, patients who received skilled home health services and participated in remote monitoring for their chronic HF experienced a reduction in all-cause 30-day readmission.18
Given the contrasting evidence regarding remote monitoring and variable modalities of implementing interventions, we investigated whether HT monitoring yields improvements in GDMT optimization. We found that HT nurses were able to nearly double the rate of interventions for patients with HFrEF. The HT program in providing expanded services will require adequate staffing responsibilities and support. The HT program is geared toward following a large, diverse patient population, such as those with chronic obstructive pulmonary disease, hypertension, and HF. We only evaluated services for patients with HFrEF, but the program also follows patients with HfmrEF and HfpEF. These patients were not included as GDMT optimization differs for patients with an LVEF > 40%.19,20
The lower rates of achieving target doses of GDMTs were likely obstructed by continuous use of initial drug doses and further limited by lack of follow-up. When compared with the rest of the VAAAHS, there was a greater effort to increase ARNi use in the HT group as 7 of 33 patients (21%) were started on ARNi compared with a background increase of ARNi use of 17%. There was a lower mortality rate observed in the HT group compared with the control group. One patient in each group died of unrelated causes, while 2 deaths in the control group were due to worsening HF. The difference in mortality is likely multifactorial, possibly related to the control group’s greater disease burden or higher mean age (75.2 years vs 72.6 years).
Limitations
This was an observational cohort design, which is subject to bias. Thus, the findings of this study are entirely hypothesis-generating and a randomized controlled trial would be necessary for clearer results. Second, low numbers of participants may have skewed the data set. Given the observational nature of the study, this nonetheless is a positive finding to support the HT program for assisting with HF monitoring and prompting drug interventions. Due to the low number of participants, a single patient may have skewed the results with 5 hospitalizations.
Conclusions
This pilot study demonstrates the applicability of HT monitoring to optimize veteran HFrEF GDMT. The HT program yielded numerically relevant interventions and fewer HF-related hospitalizations compared with the control arm. The study shows the feasibility of the program to safely optimize GDMT toward their target doses and may serve as an additional catalyst to further develop HT programs specifically targeted toward HF monitoring and management. Cost-savings analyses would likely need to demonstrate the cost utility of such a service.
We thank the home telehealth nursing staff for their assistance in data collection and enrollment of patients into the monitoring program.
Heart failure (HF) is a chronic, progressive condition that is characterized by the heart’s inability to effectively pump blood throughout the body. In 2018, approximately 6.2 million US adults had HF, and 13.4% of all death certificates noted HF as a precipitating factor.1 Patients not receiving appropriate guideline-directed medical therapy (GDMT) face a 29% excess mortality risk over a 2-year period.2 Each additional GDMT included in a patient’s regimen significantly reduces all-cause mortality.3
The Change the Management of Patients with Heart Failure (CHAMP) registry reports that only about 1% of patients with HF are prescribed 3 agents from contemporary GDMT at target doses, highlighting the need for optimizing clinicians’ approaches to GDMT.4 Similarly, The Get With The Guidelines Heart Failure Registry has noted that only 20.2% of patients with HF with reduced ejection fraction (HFrEF) are prescribed a sodium-glucose cotransporter 2 inhibitor (SGLT2i) following hospital discharge for HFrEF exacerbation.5 Overall, treatment rates with GDMT saw limited improvement between 2013 and 2019, with no significant difference between groups in mortality, indicating the need for optimized methods to encourage the initiation of GDMT.6
Remote monitoring and telecare are novel ways to improve GDMT rates in those with HFrEF. However, data are inconsistent regarding the impact of remote HF monitoring and improvements in GDMT or HF-related outcomes.6-10 The modalities of remote monitoring for GDMT vary among studies, but the potential for telehealth monitoring to improve GDMT, thereby potentially reducing HF-related hospitalizations, is clear.
Telemonitoring has demonstrated improved participant adherence with weight monitoring, although the withdrawal rate was high, and has the potential to reduce all-cause mortality and HF-related hospitalizations.11,12 Telemonitoring for GDMT optimization led to an increase in the proportion of patients who achieved optimal GDMT doses, a decrease in the time to dose optimization, and a reduction in the number of clinic visits.13 Remote GDMT titration was accomplished in the general patient population with HFrEF; however, in populations already followed by cardiologists or HF specialists, remote optimization strategies did not yield different proportions of GDMT use.14 The aim of this study was to assess the impact of the home telehealth (HT) monitoring program on the initiation and optimization of HF GDMT among veterans with HFrEF at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan.
Methods
This was a single-center retrospective study of Computerized Patient Record System (CPRS)data. Patients at the VAAAHS were evaluated if they were diagnosed with HFrEF and were eligible for enrollment in the HT monitoring program. Eligibility criteria included a diagnosis of stage C HF, irrespective of EF, and a history of any HF-related hospitalization. We focused on patients with HFrEF due to stronger guideline-based recommendations for certain pharmacotherapies as compared with HF with mildly reduced ejection fraction (HFmrEF) and preserved ejection fraction (HFpEF). Initial patient data for HT enrolling were accessed using the Heart Failure Dashboard via the US Department of Veterans Affairs (VA) Academic Detailing Service. The target daily doses of typical agents used in HFrEF GDMT are listed in the Appendix.
The HT program is an embedded model in which HT nurses receive remote data from the patient and triage that with the VAAAHS cardiology team. Patients’ questions, concerns, and/or vital signs are recovered remotely. In this model, nurses are embedded in the cardiology team, working with the cardiologists, cardiology clinical pharmacist, and/or cardiology nurse practitioners to make medication interventions. Data are recorded with an HT device, including weight, blood pressure (BP), heart rate, and pulse oximetry. HT nurses are also available to the patient via phone or video. The program uses a 180-day disease management protocol for HF via remote device, enabling the patient to answer questions and receive education on their disease daily. Responses to questions and data are then reviewed by an HT nurse remotely during business hours and triaged as appropriate with the cardiology team. Data can be communicated to the cardiology team via the patient record, eliminating the need for the cardiology team to use the proprietary portal affiliated with the HT device.
Study Sample
Patient information was obtained from a list of 417 patients eligible for enrollment in the HT program; the list was sent to the HT program for review and enrollment. Patient data were extracted from the VAAAHS HF Dashboard and included all patients with HFrEF and available data on the platform. The sample for the retrospective chart review included 40 adults who had HFrEF, defined as a left ventricular EF (LVEF) of ≤ 40% as evidenced by a transthoracic echocardiogram or cardiac magnetic resonance imaging. These patients were contacted and agreed to enroll in the HT monitoring program. The HT program population was compared against a control group of 33 patients who were ineligible for the HT program. Patients were deemed ineligible for HT if they resided in a nursing home, lacked a VAAAHS primary care clinician, or declined participation in the HT program.
Procedures
Patients who declined participation in the HT program followed the standard of care, which was limited to visits with primary care clinicians and/or cardiologists as per the follow-up plan. Patient data were collected over 12 months. The study was approved by the VAAAHS Institutional Review Board (reference number, 1703034), Research and Development Committee, and Research Administration.
Primary and Secondary Goals
The primary goal of the study was to assess the impact of the HT program on drug interventions, specifically initiating and titrating HFrEF pharmacotherapies. Interventions were based on GDMT with known mortality- and morbidity-reducing properties when used at their maximum tolerated doses, including angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor-neprilysin inhibitor (ARNi), or angiotensin receptor blockers (ARB), with a preference for ARNi, β-blockers for HFrEF (metoprolol succinate, bisoprolol, or carvedilol), aldosterone antagonists, and SGLT2is.
Secondary goals included HF-related hospitalizations, medication adherence, time to enrollment in HT, time to laboratory analysis after the initiation or titration of an ACEi/ARB/ARNi or aldosterone antagonist, and time enrolled in the HT program. Patients were considered adherent if their drug refill history showed consistent fills of their medications. The χ2 test was used for total interventions made during the study period and Fisher exact test for all others.
Results
Patient data were collected between July 2022 and June 2023. All 73 patients were male, and the mean age in the HT group (n = 40) was 72.6 years and 75.2 years for the control group (n = 33). Overall, the baseline demographics were similar between the groups (Table 1). Of 40 patients screened for enrollment in the HT program, 33 were included in the analysis (Figure 1).
At baseline, the HT group included more individuals than the control group on ACEi/ARB/ARNi (24 vs 19, respectively), β-blocker (28 vs 24, respectively), SGLT2i (14 vs 11, respectively), and aldosterone antagonist (15 vs 9, respectively) (Figure 2). There were 20 interventions made in the HT group compared with 11 therapy changes in the control arm during the study (odds ratio, 1.43; P = .23) (Table 2). In the HT group, 1 patient achieved an ACEi target dose, 3 patients achieved a β-blocker target dose, and 7 achieved a target dose of spironolactone (titration is not required for SGLT2i therapy and is counted as target dose). In the HT group, 17 patients were on ≥ 3 recommended agents, while 9 patients were taking 4 agents. Seven of 20 HT group interventions resulted in titration to the target dose. In the control group, no patients achieved an ARNi target dose, 3 patients achieved a β-blocker target dose, and 2 patients achieved a spironolactone antagonist target dose. In the control arm, 7 patients were on ≥ 3 GDMTs, and 2 were taking 4 agents. No patient in either group achieved a target dose of 4 agents. Five of 11 control group interventions resulted in initiation or titration of GDMT to the target dose.
Of the 40 HT group patients, 7 were excluded from analysis (3 failed to schedule HT, 1 was at a long-term care facility, 1 was nonadherent, 1 declined participation, and 1 died) and 33 remained in the program for a mean (SD) 5.3 (3.5) months. Death rates were tracked during the study: 1 patient died in the HT group and 3 in the control group.
We analyzed the overall percentage of VAAAHS patients with HFrEF who were on appropriate GDMT. Given the ongoing drive to improve HF-related outcomes, HT interventions could not be compared to a static population, so the HT and control patients were compared with the rates of GDMT at VAAAHS, which was available in the Academic Detailing Service Heart Failure Dashboard (Figure 3). Titration and optimization rates were also compared (Figure 4). From July 2022 to June 2023, ARNi use increased by 16.6%, aldosterone antagonist by 6.8%, and β-blockers by 2.4%. Target doses of GDMTs were more difficult to achieve in the hospital system. There was an increase in aldosterone antagonist target dose achievement by 4.7%, but overall there were decreases in target doses in other GDMTs: ACEi/ARB/ARNi target dose use decreased by 3.2%, ARNi target dose use decreased by 2.7% and target β-blocker use decreased by 0.9%.
Discussion
Telehealth yielded clinically important interventions, with some titrations bringing patients to their target doses of medications for HFrEF. The 20 interventions made in the HT group can be largely attributed to the nurses’ efforts to alert clinicians to drug titrations or ACEi/ARB to ARNi transitions. Although the findings were not statistically significant, the difference in the number of drug therapy changes supports the use of the HT program for a GDMT optimization strategy. Patients may be difficult to titrate secondary to adverse effects that make medication initiation or titration inappropriate, such as hypotension and hyperkalemia, although this was not observed in this small sample size. Considering a mean HT enrollment of 5.3 months, many patients had adequate disease assessment and medication titration. Given that patients are discharged from the service once deemed appropriate, this decreases the burden on the patient and increases the utility and implementation of the HT program for other patients.
A surprising finding of this study was the lower rate of HF-related hospitalizations in the HT group. Although not the primary subject of interest in the study, it reinforced the importance of close health care professional follow-up for patients living with HF. Telehealth may improve communication and shared decision making over medication use. Because the finding was unanticipated, the rate of diuretic adjustments was not tracked.
Patients were reevaluated every 6 months for willingness to continue the program, adherence, and clinical needs. These results are similar to those of other trials that demonstrated improved rates of GDMT in the setting of pharmacist- or nurse-led HF treatment optimization.15,16 These positive results differ from other trials incorporating remote monitoring regarding patient continuation in HT programs. For example, in a study by Ding and colleagues, the withdrawal rate from their monitoring service was about 22%, while in our study only 1 patient withdrew from the HT program.11
The HT program resulted in fewer hospitalizations than the control arm. There were 6 HF-related hospitalizations in the control group, although 5 involved a single patient. Typically, such a patient would be encouraged to follow HT monitoring after just 1 HF-related hospitalization; however, the patient declined to participate.
Early optimization of GDMT in patients who were recently discharged from the hospital for an HF-related hospitalization yields a reduction in hospital rehospitalization.17 GDMT optimization has unequivocal benefits in HF outcomes. Unfortunately, the issues surrounding methodologies on how to best optimize GDMT are lacking. While HT has been found to be feasible in the aid of optimizing medical therapy, the TIM-HF trial concluded that remote monitoring services had no significant benefit in reducing mortality.7,8 On the other hand, the OptiLink HF Study showed that when clinicians respond to remote monitoring prompts from fluid index threshold crossing alerts, these interventions are associated with significantly improved clinical outcomes in patients with implantable cardioverter-defibrillators and advanced HF.9 In contrast to previous trials, the AMULET trial showed that remote monitoring compared with standard care significantly reduced the risk of HF hospitalization or cardiovascular death during the 12-month follow-up among patients with HF and LVEF ≤ 49% after an episode of acute exacerbation.10 Additionally, patients who received skilled home health services and participated in remote monitoring for their chronic HF experienced a reduction in all-cause 30-day readmission.18
Given the contrasting evidence regarding remote monitoring and variable modalities of implementing interventions, we investigated whether HT monitoring yields improvements in GDMT optimization. We found that HT nurses were able to nearly double the rate of interventions for patients with HFrEF. The HT program in providing expanded services will require adequate staffing responsibilities and support. The HT program is geared toward following a large, diverse patient population, such as those with chronic obstructive pulmonary disease, hypertension, and HF. We only evaluated services for patients with HFrEF, but the program also follows patients with HfmrEF and HfpEF. These patients were not included as GDMT optimization differs for patients with an LVEF > 40%.19,20
The lower rates of achieving target doses of GDMTs were likely obstructed by continuous use of initial drug doses and further limited by lack of follow-up. When compared with the rest of the VAAAHS, there was a greater effort to increase ARNi use in the HT group as 7 of 33 patients (21%) were started on ARNi compared with a background increase of ARNi use of 17%. There was a lower mortality rate observed in the HT group compared with the control group. One patient in each group died of unrelated causes, while 2 deaths in the control group were due to worsening HF. The difference in mortality is likely multifactorial, possibly related to the control group’s greater disease burden or higher mean age (75.2 years vs 72.6 years).
Limitations
This was an observational cohort design, which is subject to bias. Thus, the findings of this study are entirely hypothesis-generating and a randomized controlled trial would be necessary for clearer results. Second, low numbers of participants may have skewed the data set. Given the observational nature of the study, this nonetheless is a positive finding to support the HT program for assisting with HF monitoring and prompting drug interventions. Due to the low number of participants, a single patient may have skewed the results with 5 hospitalizations.
Conclusions
This pilot study demonstrates the applicability of HT monitoring to optimize veteran HFrEF GDMT. The HT program yielded numerically relevant interventions and fewer HF-related hospitalizations compared with the control arm. The study shows the feasibility of the program to safely optimize GDMT toward their target doses and may serve as an additional catalyst to further develop HT programs specifically targeted toward HF monitoring and management. Cost-savings analyses would likely need to demonstrate the cost utility of such a service.
We thank the home telehealth nursing staff for their assistance in data collection and enrollment of patients into the monitoring program.
1. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153-e639. doi:10.1161/CIR.0000000000001052
2. McCullough PA, Mehta HS, Barker CM, et al. Mortality and guideline-directed medical therapy in real-world heart failure patients with reduced ejection fraction. Clin Cardiol. 2021;44(9):1192-1198. doi:10.1002/clc.23664
3. Tromp J, Ouwerkerk W, van Veldhuisen DJ, et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2022;10(2):73-84. doi:10.1016/j.jchf.2021.09.004
4. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF Registry. J Am Coll Cardiol. 2018;72(4):351-366. doi:10.1016/j.jacc.2018.04.070
5. Pierce JB, Vaduganathan M, Fonarow GC, et al. Contemporary use of sodium-glucose cotransporter-2 inhibitor therapy among patients hospitalized for heart failure with reduced ejection fraction in the US: The Get With The Guidelines-Heart Failure Registry. JAMA Cardiol. 2023;8(7):652-661. doi:10.1001/jamacardio.2023.1266
6. Sandhu AT, Kohsaka S, Turakhia MP, Lewis EF, Heidenreich PA. Evaluation of quality of care for US veterans with recent-onset heart failure with reduced ejection fraction. JAMA Cardiol. 2022;7(2):130-139. doi:10.1001/jamacardio.2021.4585 7. Rahimi K, Nazarzadeh M, Pinho-Gomes AC, et al. Home monitoring with technology-supported management in chronic heart failure: a randomised trial. Heart. 2020;106(20):1573-1578. doi:10.1136/heartjnl-2020-316773 8. Koehler F, Winkler S, Schieber M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123(17):1873-1880. doi:10.1161/CIRCULATIONAHA.111.018473
9. Wintrich J, Pavlicek V, Brachmann J, et al. Remote monitoring with appropriate reaction to alerts was associated with improved outcomes in chronic heart failure: results from the OptiLink HF study. Circ Arrhythm Electrophysiol. 2021;14(1):e008693. doi:10.1161/CIRCEP.120.008693
10. Krzesinski P, Jankowska EA, Siebert J, et al. Effects of an outpatient intervention comprising nurse-led non-invasive assessments, telemedicine support and remote cardiologists’ decisions in patients with heart failure (AMULET study): a randomised controlled trial. Eur J Heart Fail. 2022;24(3):565-577. doi:10.1002/ejhf.2358
11. Ding H, Jayasena R, Chen SH, et al. The effects of telemonitoring on patient compliance with self-management recommendations and outcomes of the innovative telemonitoring enhanced care program for chronic heart failure: randomized controlled trial. J Med Internet Res. 2020;22(7):e17559. doi:10.2196/17559
12. Kitsiou S, Pare G, Jaana M. Effects of home telemonitoring interventions on patients with chronic heart failure: an overview of systematic reviews. J Med Internet Res. 2015;17(3):e63. doi:10.2196/jmir.4174
13. Artanian V, Ross HJ, Rac VE, O’Sullivan M, Brahmbhatt DH, Seto E. Impact of remote titration combined with telemonitoring on the optimization of guideline-directed medical therapy for patients with heart failure: internal pilot of a randomized controlled trial. JMIR Cardio. 2020;4(1):e21962. doi:10.2196/21962
14. Desai AS, Maclean T, Blood AJ, et al. Remote optimization of guideline-directed medical therapy in patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2020;5(12):1430-1434. doi:10.1001/jamacardio.2020.3757
15. Patil T, Ali S, Kaur A, et al. Impact of pharmacist-led heart failure clinic on optimization of guideline-directed medical therapy (PHARM-HF). J Cardiovasc Transl Res. 2022;15(6):1424-1435. doi:10.1007/s12265-022-10262-9
16. Zheng J, Mednick T, Heidenreich PA, Sandhu AT. Pharmacist- and nurse-led medical optimization in heart failure: a systematic review and meta-analysis. J Card Fail. 2023;29(7):1000-1013. doi:10.1016/j.cardfail.2023.03.012
17. Mebazaa A, Davison B, Chioncel O, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022;400(10367):1938-1952. doi:10.1016/S0140-6736(22)02076-1
18. O’Connor M, Asdornwised U, Dempsey ML, et al. Using telehealth to reduce all-cause 30-day hospital readmissions among heart failure patients receiving skilled home health services. Appl Clin Inform. 2016;7(2):238-47. doi:10.4338/ACI-2015-11-SOA-0157
19. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e876-e894. doi:10.1161/CIR.0000000000001062
20. Kittleson MM, Panjrath GS, Amancherla K, et al. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023;81(18):1835-1878. doi:10.1016/j.jacc.2023.03.393
1. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153-e639. doi:10.1161/CIR.0000000000001052
2. McCullough PA, Mehta HS, Barker CM, et al. Mortality and guideline-directed medical therapy in real-world heart failure patients with reduced ejection fraction. Clin Cardiol. 2021;44(9):1192-1198. doi:10.1002/clc.23664
3. Tromp J, Ouwerkerk W, van Veldhuisen DJ, et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2022;10(2):73-84. doi:10.1016/j.jchf.2021.09.004
4. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF Registry. J Am Coll Cardiol. 2018;72(4):351-366. doi:10.1016/j.jacc.2018.04.070
5. Pierce JB, Vaduganathan M, Fonarow GC, et al. Contemporary use of sodium-glucose cotransporter-2 inhibitor therapy among patients hospitalized for heart failure with reduced ejection fraction in the US: The Get With The Guidelines-Heart Failure Registry. JAMA Cardiol. 2023;8(7):652-661. doi:10.1001/jamacardio.2023.1266
6. Sandhu AT, Kohsaka S, Turakhia MP, Lewis EF, Heidenreich PA. Evaluation of quality of care for US veterans with recent-onset heart failure with reduced ejection fraction. JAMA Cardiol. 2022;7(2):130-139. doi:10.1001/jamacardio.2021.4585 7. Rahimi K, Nazarzadeh M, Pinho-Gomes AC, et al. Home monitoring with technology-supported management in chronic heart failure: a randomised trial. Heart. 2020;106(20):1573-1578. doi:10.1136/heartjnl-2020-316773 8. Koehler F, Winkler S, Schieber M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123(17):1873-1880. doi:10.1161/CIRCULATIONAHA.111.018473
9. Wintrich J, Pavlicek V, Brachmann J, et al. Remote monitoring with appropriate reaction to alerts was associated with improved outcomes in chronic heart failure: results from the OptiLink HF study. Circ Arrhythm Electrophysiol. 2021;14(1):e008693. doi:10.1161/CIRCEP.120.008693
10. Krzesinski P, Jankowska EA, Siebert J, et al. Effects of an outpatient intervention comprising nurse-led non-invasive assessments, telemedicine support and remote cardiologists’ decisions in patients with heart failure (AMULET study): a randomised controlled trial. Eur J Heart Fail. 2022;24(3):565-577. doi:10.1002/ejhf.2358
11. Ding H, Jayasena R, Chen SH, et al. The effects of telemonitoring on patient compliance with self-management recommendations and outcomes of the innovative telemonitoring enhanced care program for chronic heart failure: randomized controlled trial. J Med Internet Res. 2020;22(7):e17559. doi:10.2196/17559
12. Kitsiou S, Pare G, Jaana M. Effects of home telemonitoring interventions on patients with chronic heart failure: an overview of systematic reviews. J Med Internet Res. 2015;17(3):e63. doi:10.2196/jmir.4174
13. Artanian V, Ross HJ, Rac VE, O’Sullivan M, Brahmbhatt DH, Seto E. Impact of remote titration combined with telemonitoring on the optimization of guideline-directed medical therapy for patients with heart failure: internal pilot of a randomized controlled trial. JMIR Cardio. 2020;4(1):e21962. doi:10.2196/21962
14. Desai AS, Maclean T, Blood AJ, et al. Remote optimization of guideline-directed medical therapy in patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2020;5(12):1430-1434. doi:10.1001/jamacardio.2020.3757
15. Patil T, Ali S, Kaur A, et al. Impact of pharmacist-led heart failure clinic on optimization of guideline-directed medical therapy (PHARM-HF). J Cardiovasc Transl Res. 2022;15(6):1424-1435. doi:10.1007/s12265-022-10262-9
16. Zheng J, Mednick T, Heidenreich PA, Sandhu AT. Pharmacist- and nurse-led medical optimization in heart failure: a systematic review and meta-analysis. J Card Fail. 2023;29(7):1000-1013. doi:10.1016/j.cardfail.2023.03.012
17. Mebazaa A, Davison B, Chioncel O, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022;400(10367):1938-1952. doi:10.1016/S0140-6736(22)02076-1
18. O’Connor M, Asdornwised U, Dempsey ML, et al. Using telehealth to reduce all-cause 30-day hospital readmissions among heart failure patients receiving skilled home health services. Appl Clin Inform. 2016;7(2):238-47. doi:10.4338/ACI-2015-11-SOA-0157
19. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e876-e894. doi:10.1161/CIR.0000000000001062
20. Kittleson MM, Panjrath GS, Amancherla K, et al. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023;81(18):1835-1878. doi:10.1016/j.jacc.2023.03.393