User login
Ready to Go Home? Assessment of Shared Mental Models of the Patient and Discharging Team Regarding Readiness for Hospital Discharge
Preparing patients for hospital discharge requires multiple tasks that cross professional boundaries. Clinician’s roles may be ambiguous, and responsibility for a safe high-quality discharge is often diffused among the team rather than being defined as the core responsibility of a single member.1-8 Without a shared understanding of patient resources and tasks involved in anticipatory planning, lapses in discharge preparation can occur, which places patients at increased risk for harm after hospitalization.3-7 As a result, organizations like the Centers for Medicare & Medicaid Services (CMS) have called for team-based patient-centered discharge planning.8 Yet to develop more effective team-based discharge planning interventions, a more nuanced understanding of how healthcare teams work together is needed.2,3,9
Shared mental models (SMMs) provide a useful theoretical framework and measurement approach for examining how interprofessional teams coordinate complex tasks like hospital discharge.10-13 SMMs represent the team members’ collective understanding and organized knowledge of key elements needed for teams to perform effectively.9-11 Validated questionnaires can be used to measure two key properties of SMMs: the degree to which team members have a similar understanding of the situation at hand (team SMM convergence) and to what extent this understanding is aligned with the patient (team-patient SMM convergence).10,11 Researchers have found that teams with higher-quality SMMs have a better understanding of what is happening and why, have clearer expectations of their roles and tasks, and can better predict what might happen next.10,12 As a result, these teams more effectively coordinate actions and adapt to task demands even in cases of high complexity, uncertainty, and stress.10-13 Prior studies examining healthcare teams in emergency departments,14-16 critical care units,17,18 and operating rooms19 suggest high-quality SMMs are needed to safely care for patients.13 Yet there has been limited evaluation of SMMs in general internal medicine, much less during hospital discharge.9,13 The purpose of this study was to examine SMMs for a critical task of the inpatient team: developing a shared understanding of the patient’s readiness for hospital discharge.20,21
METHODS
Design
We used a cross-sectional survey design at a single Midwestern community hospital to determine inpatient care teams’ SMMs of patient hospital discharge readiness. This study is part of a larger mixed-methods evaluation of interprofessional hospital discharge teamwork in older adult patients at risk for a poor transition to home.9 Data were collected using questionnaires from patients and their team (nurse, coordinator, and physician) within 4 hours of the patient leaving the hospital. First, we measured the teams’ assessment, team convergence, and team-patient convergence on patient readiness for discharge from the hospital. Then, after identifying relevant potential predictors from the literature, we developed regression models to predict the teams’ assessment, team convergence, and team-patient convergence of discharge readiness based on these variables. Our local institutional review board approved this study.
Sample and Participants
We used a convenience sampling approach to identify eligible discharge events consisting of the patient and care team.9 We focused on patients at high-risk for poor hospital-to-home transitions.3,22 Eligible events included older patients (≥65 years) who were discharged home without home health or hospice services and admitted with a primary diagnosis of heart failure, acute myocardial infarction, hip replacement, knee replacement, pneumonia, or chronic obstructive pulmonary disease. Patient exclusion criteria included inability to complete study forms because of mental incapacity or a language barrier. Discharge team member inclusion criteria included the bedside nurse, attending physician, and coordinator (a unit-dedicated discharge nurse or social worker) caring for the patient participant at the time of hospital discharge. Each discharge team was unique: The same three individuals could not be included as a “team” for more than one discharge event, although individual members could be included as a part of other teams with a different set of individuals. Appendix A provides an enrollment flowchart.
Conceptual Framework
We applied the SMM conceptual framework to the context of hospital discharge. As shown in the Figure, SMMs are examined at the team level and contain the critical knowledge held by the team to be effective.15,16 From a patient-centered perspective, patients are considered the expert on how ready they feel to be discharged home.20,23,24 In this case, the SMM content is the discharge team members’ shared assessment of how ready the patient is for hospital discharge (Figure, B).10 Convergence is the degree of agreement among individual mental models.10-13 In this study we examined two types of convergence: (1) team convergence, or the team members degree of agreement on the patient’s readiness for discharge (Figure, C), and (2) team-patient convergence, or the degree to which the team’s SMM aligns with the patient’s mental model (Figure, D).10-13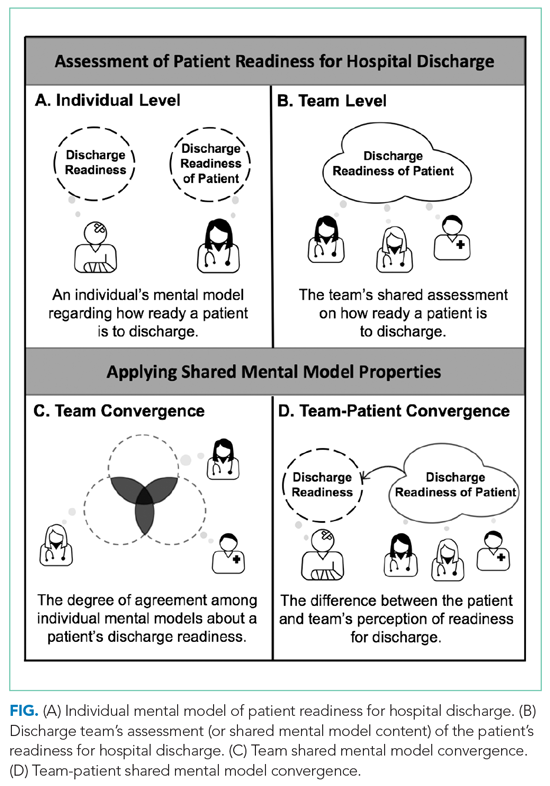
Measures and Variables
Readiness for Hospital Discharge Scales/Short Form
We used parallel clinician and patient versions of the Readiness for Hospital Discharge Scale/Short Form (RHDS/SF)25-28 to determine the teams’ assessment of discharge readiness, team SMM convergence, and team-patient SMM convergence.
The RHDS/SF scales are 8-item validated instruments that use a Likert scale (0 for not ready to 10 for totally ready) to assess the individual clinician’s or patient’s perceptions of how ready the patient is to be discharged.20,25,27 The RHDS/SF instruments include four dimensions conceptualized as crucial to patient readiness for discharge and important to anticipatory planning: (1) Personal Status, physical-emotional state of the patient before discharge; (2) Knowledge, perceived adequacy of information needed to respond to common posthospitalization concerns/problems; (3) Coping Ability, perceived ability to self-manage health care needs; and (4) Expected Support, emotional-physical assistance available (Appendix B).20,25,27 The RHDS/SF instruments’ results are calculated as a mean of item scores, with higher individual scores indicating the rater assessed the patient as being more ready for hospital discharge.20 The RHDS/SF scales have undergone rigorous psychometric testing and are linked to patient outcomes (eg, readmissions, emergency room visits, patient coping difficulties after discharge, and patient-rated quality of preparation for posthospital care).20,25-28 For example, predictive validity assessments for adult medical-surgical patients found lower Nurse-RHDS/SF scores are associated with a six- to ninefold increase in 30-day readmission risk.20
Contextual Variables
We reviewed the literature to identify potential patient and system factors associated with adverse transitional care outcomes1-8 and/or higher quality SMMs in other settings.10-19 For example, patient characteristics included age, principal diagnosis, length of stay, number of comorbidities, and cognition impairment (using the Short Portable Mini Mental Status Questionnaire29).2,22,30 Examples of system factor include teamwork and communication quality1-6 on day of discharge, as well as educational background and experience of clinicians on the team.31-33 We adapted a validated survey using 7-point Likert scale questions to determine teamwork quality and communication quality during individual patent discharges.33 Appendix C provides descriptions of all variables.
Data Collection
Patient recruitment occurred from February to October 2017 in a single community hospital in Iowa.9 We identified potentially eligible events in collaboration with the unit charge nurses. Patients were screened 24 to 48 hours prior to anticipated day of discharge; those interested/eligible underwent informed consent procedures.9 We collected data from the patient and their corresponding bedside nurse, coordinator, and attending physician on the day of discharge. After the discharge order was placed and care instructions were provided, the patient completed a demographic survey, Short Portable Mini Mental Status Questionnaire, and the Patient-RHDS/SF. Individual team members completed a survey with the demographic information, their respective versions of RHDS/SF, and day-of-discharge teamwork-related questions. On average, the survey took clinicians less than 5 minutes to complete. We performed a chart review to determine additional patient characteristics such as principal diagnosis, length of stay, and number of comorbidities.
Data Analysis
Team Assessment of Patient Discharge Readiness
The teams’ shared assessment (SMM content) was determined by averaging the members’ individual scores on the Clinician-RHDS/SF.34 Discharge events with higher team assessments indicated the team perceived the patient as being readier for hospital discharge. Guided by prior research, we examined the RHDS/SF scores as a continuous variable and as a four-level categorical variable of readiness: low (<7), moderate (7-7.9), high (8-8.9), and very high (9-10).20
Team SMM Convergence
To determine the teams’ convergence on patient discharge readiness, we calculated an adjusted interrater agreement index (r*wg(j))35,36 for each team using the individual clinicians’ scores on the RHDS/SF. These convergence values were categorized into four agreement levels: low agreement (<0.7), moderate agreement (0.7-0.79), high agreement (0.8-0.89), and very high agreement (0.9-1). See Appendix D for the r*wg(j) equation.35,36
Team-Patient SMM Convergence
To determine the team-patient SMM convergence, we subtracted the team’s assessment of patient discharge readiness from the Patient-RHDS/SF score. We used a one-unit change on the RHDS/SF (1 point on the 0-10 scale) as a meaningful difference between the patient’s self-assessment and teams’ assessment on readiness for hospital discharge. This definition for divergence aligns with prior RHDS psychometric testing studies20,27 and research examining convergence between patient and nurse assessments.28 For example, Weiss and colleagues27 found a 1-point decrease in the RN-RHDS/SF item mean was associated with a 45% increase in likelihood of postdischarge utilization (hospital readmission and emergency room department visits). Therefore, we defined convergence of team-patient SMMs (or similar patient and team scores) as those with an absolute difference score less than 1 point, whereas teams with low team-patient SMM convergence (or divergent patient and team scores) were defined as having an absolute difference score greater than 1 point.
Prediction Models
For the exploratory aim, we first examined the bivariate relationship between the outcome variables (discharge teams’ assessment of patient readiness, team convergence, and team-patient convergence) and the identified contextual variables. We also checked for potential collinearity among the explanatory variables. Then we used a linear stepwise regression procedure to identify factors associated with each continuous outcome variable. Due to the small sample size, we performed separate backward stepwise regression selection analyses for the three outcomes of interest. The candidate explanatory variables were evaluated using P < .20 for model entry. Final models were evaluated using leave-one-out cross validation. STATA (v.15.1, StataCorp; 2017) was used for analysis.
RESULTS
Sample
A total of 64 discharge events were included in this study. All discharge teams had a unique composition including 64 patients and varying combinations of 56 individual nurses (n = 27), physicians (n = 23), and coordinators (n = 6). Each event had three team members (ie, a nurse, a coordinator, and a physician) with no missing data. The majority of the 64 patient participants were White, retired, had a high school education, and lived in their own home with only one other person (Table 1).
Interprofessional Teams’ SMM of Readiness for Hospital Discharge
While the majority of teams perceived patients had high readiness for hospital discharge (mean, 8.5 out of 10; SD, 0.91), patients scores were nearly a full point lower (mean, 7.7; SD, 1.6; Table 2). The largest difference across categories was in the low-readiness category with 27% of patient scores falling into this category vs only 9.4% of discharge team mean scores. The mean SMM convergence of team perception of patients’ readiness for discharge was 0.90 (SD, 0.10); however, scores ranged from 0.66 (low agreement) to 1 (complete agreement). The average SMM team-patient convergence, or the discrepancy between the discharge team mean scores and the patient total scores across domains, was 1.16 (SD, 0.82). Of the 64 discharge events, 42.2% had similar team-patient perceptions of readiness for discharge, 9.4% had the patient reporting higher readiness for discharge than the team, and 48.4% had a team assessment rating of higher readiness for discharge than the patient’s self-assessment.
Prediction Models
In the exploratory analysis, we created individual linear regression models to predict the teams’ assessment, team convergence, and team-patient convergence for readiness of hospital discharge (Table 3; Appendix E). Factors associated with the teams’ assessment of discharge readiness included whether the patient was married and had less cognitive impairment, both of which were positively related to a higher-rated readiness among teams. An important system factor was higher quality of communication among team members, which was positively associated with teams’ assessment of patient discharge readiness. In contrast, only patient factors—married patients and those with a principal diagnosis of heart failure—were associated with more convergent team SMMs. Team-patient convergence was positively associated with two patient factors: marital status (married) and fewer comorbidities. However, team-patient convergence was also associated with two system factors: teams with a bachelor’s level–trained nurse (compared with a nurse with an associate degree ) and teams reporting a higher quality of teamwork on day of discharge.

DISCUSSION
Our study applied novel approaches to explore the interprofessional teams’ understanding of discharge readiness, a concept known to be an important predictor of patient outcomes after discharge, including readmission.20,28 We found that discharge teams frequently had poor quality SMMs of hospital discharge readiness. Despite having a discharge order and receiving home care instructions, one in four patients reported low readiness for hospital discharge. Additionally, discharge teams frequently overestimated patient’s readiness for hospital discharge. Misalignment on patient readiness for discharge occurred both within the discharge team (ie, low team convergence) and between patients and their care teams (ie, low team-patient convergence). The potential importance of this disagreement is substantiated by prior work suggesting that divergence in readiness ratings between nurses and patients are associated with postdischarge coping difficulties.28
Previous readiness for discharge has been measured from the perspective of the patient,20,21,27,28 nurse,20,25-28 and physician,37 yet rarely has the teams’ perspective been examined. We add to this literature by measuring the team’s perspective, as well as agreement between team and patient, on the individual patient’s readiness for discharge. Notably, we found that higher-quality communication is positively related to teams’ assessment of discharge readiness, with teams that reported higher quality teamwork having more convergent team-patient SMMs. Our results support many qualitative studies identifying communication and teamwork as major factors in teams’ effectiveness in discharge planning.1-7,9 However, given the small sample size in this study, additional research is needed to further understand these relationships, as well as link SMMs to patient outcomes such as hospital readmission.
In an attempt to improve discharge planning, hospitals are increasingly assessing readiness for discharge as a low-intensity, low-cost intervention.26,27 Yet, recent evidence suggests that readiness assessments alone have minimal impact on reducing hospital readmissions.26 To be successful, these assessments likely depend on quality interprofessional communication and ensuring the patient’s voice is incorporated into the discharge decision process.26 However, there have been few ways to effectively evaluate these types of team interventions.9 Measuring SMM properties holds promise for identifying specific team mechanisms that may influence the effectiveness and fidelity of interventions for team-based discharge planning. As our findings indicate, SMMs provide a theoretical and methodological basis for evaluating if readiness for discharge was team based (convergence among team members) and patient centered (convergence among team assessment and patient self-assessment). Researchers and improvement scientists can use the approach outlined to evaluate team-based patient-centered interventions for hospital discharge planning.9
This study provides a unique contribution to the growing work in the team science of SMMs.9,10 We rigorously evaluated SMMs of key stakeholders (patients and their interprofessional team) in “real-time” clinical practice using a patient-centered assessment linked with postdischarge outcomes.20,27,28 However, it is still unknown how much convergence is needed (and with whom) to safely discharge patients.13 Prior studies suggest highly convergent SMMs increase team performance when they are also accurate.10-13 Convergence alone should not be sought because this may reflect groupthink or clinical inertia.10,15 To improve discharge team performance over time,10‑13 it is important to assess not only patient’s readiness on the day of discharge but also how prepared the patient actually was for the recovery period following acute care. In the larger mixed-methods study, we found that teams’ with more convergent SMMs on teamwork quality were associated with patient’s reported quality of transition 30-days after discharge.9 Together, these findings further highlight the importance of aligning patient and interprofessional team members perspectives during the discharge planning, as well as providing clinicians with regular feedback about patient’s postdischarge experiences and outcomes.
To optimize team performance, the discharge planning process must be considered from an interprofessional team perspective as it functions in real-world practice settings. There are increasing pressures to discharge patients “quicker and sicker,” to simplify and standardize clinical process, and to provide patient-centered care.3,5-8 Without thoughtful interventions to facilitate communication during discharge planning, these pressures likely reinforce inaccurate assumptions regarding the work of fellow team members and force teams to think “fast” instead of “slow.”38-40 One approach to overcome such barriers is to focus on building a high-quality interprofessional SMM around discharge readiness. For example, the RHDS/SF questions could be integrated into the electronic medical records, displayed on dashboards, and discussed regularly during discharge rounds. In particular, to strengthen the team’s SMM and quality of teamwork, together the staff can ask three practical questions (Appendix F): (1) Do we think the patient is ready for discharge? (2) To what extent do we all agree the patient is ready for discharge? (3) Does our assessment of discharge readiness match the patient’s? During this high-risk transition point, asking these questions might allow the team to move from thinking fast to thinking slowly so they can more effectively identify heuristics they may be using inaccurately, prevent blind spots, and move toward high reliability.10,13,18,38-40
This study has limitations. First, events were recruited from patients with any of only six conditions at a single hospital. Other settings, patient condition types, or team compositions of other clinicians may differ in results. Second, in this study the SMM content was focused on readiness for hospital discharge among four key stakeholders. It is possible other SMM content needs to be shared among the interprofessional discharge team (eg, caregivers’ perspectives,2,6-8 resource availability,3-6 clinicians’ roles4,9) or additional members should be included (eg, physical therapists, nursing assistants, home health consultants, or primary care clinicians). Although this study focused on a patient-centered outcome (Patient-RHDS/SF), we did not examine other important outcomes such as hospital readmission. Additionally, due to the small sample size, these results have limited generalizability and should be interpreted with caution. Last, we limited data collection to the day of hospital discharge; future studies might consider assessing discharge readiness throughout hospitalization.
CONCLUSION
Readying patients for hospital discharge is a time-sensitivehigh-risk task requiring multiple healthcare professionals to concurrently assess patient needs, formulate an anticipatory care plan, provide education, and arrange for postdischarge needs.20,21 Despite this, few studies have analyzed teamwork aspects to understand how these transitions could be improved.9 By piloting SMM measurement and describing factors that affect SMMs, we provide a step toward identifying and evaluating strategies to assist interprofessional care teams in preparing patients for a safe, high-quality, patient-centered hospital discharge.
Presentations
This work was presented at the Midwest Nursing Research Society’s 2018 Annual Research Conference in Cleveland, Ohio, as well as at AcademyHealth’s 2019 Annual Research Meeting in Washington, District of Columbia.
- Greysen SR, Schiliro D, Horwitz LI, Curry L, Bradley E. “Out of sight, out of mind”: house staff perceptions of quality-limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376-381.https://doi.org/10.1002/%20jhm.1928
- Fuji KT, Abbott AA, Norris JF. Exploring care transitions from patient, caregiver, and health-care provider perspectives. Clin Nurs Res. 2013;22(3):258-274. https://doi.org/10.1177/1054773812465084
- Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. https://doi.org/10.1002/jhm.228
- Waring J, Bishop S, Marshall F. A qualitative study of professional and career perceptions of the threats to safe hospital discharge for stroke and hip fracture patients in the English National Health Service. BMC Health Serv Res. 2016;16:297. https://doi.org/10.1186/s12913-016-1568-2
- Nosbusch JM, Weiss ME, Bobay KL. An integrated review of the literature on challenges confronting the acute care staff nurse in discharge planning. J Clin Nurs. 2011;20(5-6):754-774. https://doi.org/10.1111/j.1365-2702.2010.03257.x
- Ashbrook L, Mourad M, Sehgal N. Communicating discharge instructions to patients: a survey of nurse, intern, and hospitalist practices. J Hosp Med. 2013;8(1):36-41. https://doi.org/10.1002/jhm.1986
- Prusaczyk B, Kripalani S, Dhand A. Networks of hospital discharge planning teams and readmissions. J Interprof Care. 2019;33(1):85-92. https://doi.org/1 0.1080/13561820.2018.1515193
- Centers for Medicare & Medicaid Services. Medicare and Medicaid Programs; Revisions to Requirements for Discharge Planning for Hospitals, Critical Access Hospitals, and Home Health Agencies, and Hospital and Critical Access Hospital Changes to Promote Innovation, Flexibility, and Improvement in Patient Care. Septemeber 30, 2019. Federal Register. Accessed May 24, 2021. https://www.federalregister.gov/documents/2019/09/30/2019-20732/medicare-and-medicaid-programs-revisions-to-requirements-for-discharge-planning-for-hospitals
- Manges K, Groves PS, Farag A, Peterson R, Harton J, Greysen SR. A mixed methods study examining teamwork shared mental models of interprofessional teams during hospital discharge. BMJ Qual Saf. 2020;29(6):499-508. https://doi.org/10.1136/bmjqs-2019-009716
- Mohammed S, Ferzandi L, Hamilton K. Metaphor no more: a 15-year review of the team mental model construct. J Manage. 2010;36(4):876-910. https:// doi.org/10.1177%2F0149206309356804
- Langan-Fox J, Code S, Langfield-Smith K. Team mental models: techniques, methods, and analytic approaches. Hum Factors. 2000;42(2);242-271. https:// doi.org/10.1518/001872000779656534
- Lim BC, Klein KJ. Team mental models and team performance: a field study of the effects of team mental model similarity and accuracy. J Organ Behav. 2006;27(4):403-418. https://doi.org/10.1002/job.387
- Burtscher MJ, Manser T. Team mental models and their potential to improve teamwork and safety: a review and implications for future research in healthcare. Safety Sci. 2012;50(5):1344-1354. https://doi.org/10.1016/j. ssci.2011.12.033
- Calder LA, Mastoras G, Rahimpour M, et al. Team communication patterns in emergency resuscitation: a mixed methods qualitative analysis. Int J Emerg Med. 2017:10(1):24. https://doi.org/10.1186/s12245-017-0149-4
- Westli HK, Johnsen BH, Eid J, Rasten I, Brattebø G. Teamwork skills, shared mental models, and performance in simulated trauma teams: an independent group design. Scand J Trauma Resusc Emerg Med. 2010;18:47. https:// doi.org/10.1186/1757-7241-18-47
- Johnsen BH, Westli HK, Espevik R, Wisborg R, Brattebø G. High-performing trauma teams: frequency of behavioral markers of a shared mental model displayed by team leaders and quality of medical performance. Scand J Trauma Resusc Emerg Med. 2017;25(1):109. https://doi.org/10.1186/s13049- 017-0452-3
- Custer JW, White E, Fackler JC, et al. A qualitative study of expert and team cognition on complex patients in the pediatric intensive care unit. Pediatr Crit Care Med. 2012;13(3):278-284. https://doi.org/10.1097/ pcc.0b013e31822f1766
- Cutrer WB, Thammasitboon S. Team mental model creation as a mechanism to decrease errors in the intensive care unit. Pediatr Crit Care Med. 2012;13(3):354-356. https://doi.org/10.1097/pcc.0b013e3182388994
- Gjeraa K, Dieckmann P, Spanager L, et al. Exploring shared mental models of surgical teams in video-assisted thoracoscopic surgery lobectomy. Ann Thorac Surg. 2019;107(3):954-961. https://doi.org/10.1016/j.athoracsur.2018.08.010
- Weiss ME, Costa LL, Yakusheva O, Bobay KL. Validation of patient and nurse short forms of the Readiness for Hospital Discharge Scale and their relationship to return to the hospital. Health Serv Res. 2014;49(1):304-317. https:// doi.org/10.1111/1475-6773.12092
- Galvin EC, Wills T, Coffey A. Readiness for hospital discharge: a concept analysis. J Adv Nurs. 2017;73(11):2547-2557. https://doi.org/10.1111/jan.13324
- Hospital Readmissions Reduction Program (HRRP). Centers for Medicare & Medicaid Services. Updated August 11, 2020. Accessed January 6, 2020. https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/value-based-programs/hrrp/hospital-readmission-reduction-program.html
- Epstein RM, Fiscella K, Lesser CS, Stange KC. Why the nation needs a policy push on patient-centered health care. Health Aff (Millwood). 2010;29(8):1489- 1495. https://doi.org/10.1377/hlthaff.2009.0888
- Graumlich JF, Novotny NL, Aldag JC. Brief scale measuring patient preparedness for hospital discharge to home: psychometric properties. J Hosp Med. 2008;3(6):446-454. https://doi.org/10.1002/jhm.316
- Bobay KL, Weiss ME, Oswald D, Yakusheva O. Validation of the Registered Nurse Assessment of Readiness for Hospital Discharge scale. Nurs Res. 2018;67(4):305-313. https://doi.org/10.1097/nnr.0000000000000293
- Weiss ME, Yakusheva O, Bobay K, et al. Effect of implementing discharge readiness assessment in adult medical-surgical units on 30-Day return to hospital: the READI randomized clinical trial. JAMA Netw Open. 2019;2(1):e187387. https://doi.org/10.1001/jamanetworkopen.2018.7387
- Weiss ME, Yakusheva O, Bobay K. Nurse and patient perceptions of discharge readiness in relation to postdischarge utilization. Med Care. 2010;48(5):482-486. https://doi.org/10.1097/mlr.0b013e3181d5feae
- Wallace AS, Perkhounkova Y, Bohr NL, Chung SJ. Readiness for hospital discharge, health literacy, and social living status. Clin Nurs Res. 2016;25(5):494- 511. https://doi.org/10.1177/1054773815624380
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433- 441. https://doi.org/10.1111/j.1532-5415.1975.tb00927.x
- Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688-1698. https://doi.org/10.1001/jama.2011.1515
- Aiken LH, Cimiotti JP, Sloane DM, Smith HL, Flynn L, Neff DF. Effects of nurse staffing and nurse education on patient deaths in hospitals with different nurse work environments. J Nurs Adm. 2012;42(10 Suppl):S10-S16. https:// doi.org/10.1097/01.nna.0000420390.87789.67
- Goodwin JS, Salameh H, Zhou J, Singh S, Kuo YF, Nattinger AB. Association of hospitalist years of experience with mortality in the hospitalized Medicare population. JAMA Intern Med. 2018;178(2):196-203. https://doi. org/10.1001/jamainternmed.2017.7049
- Millward LJ, Jeffries N. The team survey: a tool for health care team development. J Adv Nurs. 2001;35(2):276-287. https://doi.org/10.1046/j. 1365-2648.2001.01844.x
- Klein KJ, Kozlowski SW. From micro to meso: critical steps in conceptualizing and conducting multilevel research. Organ Res Methods. 2000;3(3):211-236. https://doi.org/10.1177/109442810033001
- Lindell MK, Brandt CJ, Whitney DJ. A revised index of interrater agreement for multi-item ratings of a single target. Appl Psychol Meas. 1999;23(2):127- 135. https://doi.org/10.1177%2F01466219922031257
- O’Neill TA. An overview of interrater agreement on Likert scales for researchers and practitioners. Front Psychol. 2017;8:777. https://doi.org/10.3389/ fpsyg.2017.00777
- Sullivan B, Ming D, Boggan JC, et al. An evaluation of physician predictions of discharge on a general medicine service. J Hosp Med. 2015;10(12):808- 810. https://doi.org/10.1002/jhm.2439
- Kahneman D. Thinking, fast and slow. Doubleday; 2011.
- Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22(Suppl 2):ii58-ii64. https://doi. org/10.1136/bmjqs-2012-001712
- Burke RE, Leonard C, Lee M, et al. Cognitive biases influence decision-making regarding postacute care in a skilled nursing facility. J Hosp Med. 2020:15(1)22-27. https://doi.org/10.12788/jhm.3273
Preparing patients for hospital discharge requires multiple tasks that cross professional boundaries. Clinician’s roles may be ambiguous, and responsibility for a safe high-quality discharge is often diffused among the team rather than being defined as the core responsibility of a single member.1-8 Without a shared understanding of patient resources and tasks involved in anticipatory planning, lapses in discharge preparation can occur, which places patients at increased risk for harm after hospitalization.3-7 As a result, organizations like the Centers for Medicare & Medicaid Services (CMS) have called for team-based patient-centered discharge planning.8 Yet to develop more effective team-based discharge planning interventions, a more nuanced understanding of how healthcare teams work together is needed.2,3,9
Shared mental models (SMMs) provide a useful theoretical framework and measurement approach for examining how interprofessional teams coordinate complex tasks like hospital discharge.10-13 SMMs represent the team members’ collective understanding and organized knowledge of key elements needed for teams to perform effectively.9-11 Validated questionnaires can be used to measure two key properties of SMMs: the degree to which team members have a similar understanding of the situation at hand (team SMM convergence) and to what extent this understanding is aligned with the patient (team-patient SMM convergence).10,11 Researchers have found that teams with higher-quality SMMs have a better understanding of what is happening and why, have clearer expectations of their roles and tasks, and can better predict what might happen next.10,12 As a result, these teams more effectively coordinate actions and adapt to task demands even in cases of high complexity, uncertainty, and stress.10-13 Prior studies examining healthcare teams in emergency departments,14-16 critical care units,17,18 and operating rooms19 suggest high-quality SMMs are needed to safely care for patients.13 Yet there has been limited evaluation of SMMs in general internal medicine, much less during hospital discharge.9,13 The purpose of this study was to examine SMMs for a critical task of the inpatient team: developing a shared understanding of the patient’s readiness for hospital discharge.20,21
METHODS
Design
We used a cross-sectional survey design at a single Midwestern community hospital to determine inpatient care teams’ SMMs of patient hospital discharge readiness. This study is part of a larger mixed-methods evaluation of interprofessional hospital discharge teamwork in older adult patients at risk for a poor transition to home.9 Data were collected using questionnaires from patients and their team (nurse, coordinator, and physician) within 4 hours of the patient leaving the hospital. First, we measured the teams’ assessment, team convergence, and team-patient convergence on patient readiness for discharge from the hospital. Then, after identifying relevant potential predictors from the literature, we developed regression models to predict the teams’ assessment, team convergence, and team-patient convergence of discharge readiness based on these variables. Our local institutional review board approved this study.
Sample and Participants
We used a convenience sampling approach to identify eligible discharge events consisting of the patient and care team.9 We focused on patients at high-risk for poor hospital-to-home transitions.3,22 Eligible events included older patients (≥65 years) who were discharged home without home health or hospice services and admitted with a primary diagnosis of heart failure, acute myocardial infarction, hip replacement, knee replacement, pneumonia, or chronic obstructive pulmonary disease. Patient exclusion criteria included inability to complete study forms because of mental incapacity or a language barrier. Discharge team member inclusion criteria included the bedside nurse, attending physician, and coordinator (a unit-dedicated discharge nurse or social worker) caring for the patient participant at the time of hospital discharge. Each discharge team was unique: The same three individuals could not be included as a “team” for more than one discharge event, although individual members could be included as a part of other teams with a different set of individuals. Appendix A provides an enrollment flowchart.
Conceptual Framework
We applied the SMM conceptual framework to the context of hospital discharge. As shown in the Figure, SMMs are examined at the team level and contain the critical knowledge held by the team to be effective.15,16 From a patient-centered perspective, patients are considered the expert on how ready they feel to be discharged home.20,23,24 In this case, the SMM content is the discharge team members’ shared assessment of how ready the patient is for hospital discharge (Figure, B).10 Convergence is the degree of agreement among individual mental models.10-13 In this study we examined two types of convergence: (1) team convergence, or the team members degree of agreement on the patient’s readiness for discharge (Figure, C), and (2) team-patient convergence, or the degree to which the team’s SMM aligns with the patient’s mental model (Figure, D).10-13
Measures and Variables
Readiness for Hospital Discharge Scales/Short Form
We used parallel clinician and patient versions of the Readiness for Hospital Discharge Scale/Short Form (RHDS/SF)25-28 to determine the teams’ assessment of discharge readiness, team SMM convergence, and team-patient SMM convergence.
The RHDS/SF scales are 8-item validated instruments that use a Likert scale (0 for not ready to 10 for totally ready) to assess the individual clinician’s or patient’s perceptions of how ready the patient is to be discharged.20,25,27 The RHDS/SF instruments include four dimensions conceptualized as crucial to patient readiness for discharge and important to anticipatory planning: (1) Personal Status, physical-emotional state of the patient before discharge; (2) Knowledge, perceived adequacy of information needed to respond to common posthospitalization concerns/problems; (3) Coping Ability, perceived ability to self-manage health care needs; and (4) Expected Support, emotional-physical assistance available (Appendix B).20,25,27 The RHDS/SF instruments’ results are calculated as a mean of item scores, with higher individual scores indicating the rater assessed the patient as being more ready for hospital discharge.20 The RHDS/SF scales have undergone rigorous psychometric testing and are linked to patient outcomes (eg, readmissions, emergency room visits, patient coping difficulties after discharge, and patient-rated quality of preparation for posthospital care).20,25-28 For example, predictive validity assessments for adult medical-surgical patients found lower Nurse-RHDS/SF scores are associated with a six- to ninefold increase in 30-day readmission risk.20
Contextual Variables
We reviewed the literature to identify potential patient and system factors associated with adverse transitional care outcomes1-8 and/or higher quality SMMs in other settings.10-19 For example, patient characteristics included age, principal diagnosis, length of stay, number of comorbidities, and cognition impairment (using the Short Portable Mini Mental Status Questionnaire29).2,22,30 Examples of system factor include teamwork and communication quality1-6 on day of discharge, as well as educational background and experience of clinicians on the team.31-33 We adapted a validated survey using 7-point Likert scale questions to determine teamwork quality and communication quality during individual patent discharges.33 Appendix C provides descriptions of all variables.
Data Collection
Patient recruitment occurred from February to October 2017 in a single community hospital in Iowa.9 We identified potentially eligible events in collaboration with the unit charge nurses. Patients were screened 24 to 48 hours prior to anticipated day of discharge; those interested/eligible underwent informed consent procedures.9 We collected data from the patient and their corresponding bedside nurse, coordinator, and attending physician on the day of discharge. After the discharge order was placed and care instructions were provided, the patient completed a demographic survey, Short Portable Mini Mental Status Questionnaire, and the Patient-RHDS/SF. Individual team members completed a survey with the demographic information, their respective versions of RHDS/SF, and day-of-discharge teamwork-related questions. On average, the survey took clinicians less than 5 minutes to complete. We performed a chart review to determine additional patient characteristics such as principal diagnosis, length of stay, and number of comorbidities.
Data Analysis
Team Assessment of Patient Discharge Readiness
The teams’ shared assessment (SMM content) was determined by averaging the members’ individual scores on the Clinician-RHDS/SF.34 Discharge events with higher team assessments indicated the team perceived the patient as being readier for hospital discharge. Guided by prior research, we examined the RHDS/SF scores as a continuous variable and as a four-level categorical variable of readiness: low (<7), moderate (7-7.9), high (8-8.9), and very high (9-10).20
Team SMM Convergence
To determine the teams’ convergence on patient discharge readiness, we calculated an adjusted interrater agreement index (r*wg(j))35,36 for each team using the individual clinicians’ scores on the RHDS/SF. These convergence values were categorized into four agreement levels: low agreement (<0.7), moderate agreement (0.7-0.79), high agreement (0.8-0.89), and very high agreement (0.9-1). See Appendix D for the r*wg(j) equation.35,36
Team-Patient SMM Convergence
To determine the team-patient SMM convergence, we subtracted the team’s assessment of patient discharge readiness from the Patient-RHDS/SF score. We used a one-unit change on the RHDS/SF (1 point on the 0-10 scale) as a meaningful difference between the patient’s self-assessment and teams’ assessment on readiness for hospital discharge. This definition for divergence aligns with prior RHDS psychometric testing studies20,27 and research examining convergence between patient and nurse assessments.28 For example, Weiss and colleagues27 found a 1-point decrease in the RN-RHDS/SF item mean was associated with a 45% increase in likelihood of postdischarge utilization (hospital readmission and emergency room department visits). Therefore, we defined convergence of team-patient SMMs (or similar patient and team scores) as those with an absolute difference score less than 1 point, whereas teams with low team-patient SMM convergence (or divergent patient and team scores) were defined as having an absolute difference score greater than 1 point.
Prediction Models
For the exploratory aim, we first examined the bivariate relationship between the outcome variables (discharge teams’ assessment of patient readiness, team convergence, and team-patient convergence) and the identified contextual variables. We also checked for potential collinearity among the explanatory variables. Then we used a linear stepwise regression procedure to identify factors associated with each continuous outcome variable. Due to the small sample size, we performed separate backward stepwise regression selection analyses for the three outcomes of interest. The candidate explanatory variables were evaluated using P < .20 for model entry. Final models were evaluated using leave-one-out cross validation. STATA (v.15.1, StataCorp; 2017) was used for analysis.
RESULTS
Sample
A total of 64 discharge events were included in this study. All discharge teams had a unique composition including 64 patients and varying combinations of 56 individual nurses (n = 27), physicians (n = 23), and coordinators (n = 6). Each event had three team members (ie, a nurse, a coordinator, and a physician) with no missing data. The majority of the 64 patient participants were White, retired, had a high school education, and lived in their own home with only one other person (Table 1).

Interprofessional Teams’ SMM of Readiness for Hospital Discharge
While the majority of teams perceived patients had high readiness for hospital discharge (mean, 8.5 out of 10; SD, 0.91), patients scores were nearly a full point lower (mean, 7.7; SD, 1.6; Table 2). The largest difference across categories was in the low-readiness category with 27% of patient scores falling into this category vs only 9.4% of discharge team mean scores. The mean SMM convergence of team perception of patients’ readiness for discharge was 0.90 (SD, 0.10); however, scores ranged from 0.66 (low agreement) to 1 (complete agreement). The average SMM team-patient convergence, or the discrepancy between the discharge team mean scores and the patient total scores across domains, was 1.16 (SD, 0.82). Of the 64 discharge events, 42.2% had similar team-patient perceptions of readiness for discharge, 9.4% had the patient reporting higher readiness for discharge than the team, and 48.4% had a team assessment rating of higher readiness for discharge than the patient’s self-assessment.

Prediction Models
In the exploratory analysis, we created individual linear regression models to predict the teams’ assessment, team convergence, and team-patient convergence for readiness of hospital discharge (Table 3; Appendix E). Factors associated with the teams’ assessment of discharge readiness included whether the patient was married and had less cognitive impairment, both of which were positively related to a higher-rated readiness among teams. An important system factor was higher quality of communication among team members, which was positively associated with teams’ assessment of patient discharge readiness. In contrast, only patient factors—married patients and those with a principal diagnosis of heart failure—were associated with more convergent team SMMs. Team-patient convergence was positively associated with two patient factors: marital status (married) and fewer comorbidities. However, team-patient convergence was also associated with two system factors: teams with a bachelor’s level–trained nurse (compared with a nurse with an associate degree ) and teams reporting a higher quality of teamwork on day of discharge.

DISCUSSION
Our study applied novel approaches to explore the interprofessional teams’ understanding of discharge readiness, a concept known to be an important predictor of patient outcomes after discharge, including readmission.20,28 We found that discharge teams frequently had poor quality SMMs of hospital discharge readiness. Despite having a discharge order and receiving home care instructions, one in four patients reported low readiness for hospital discharge. Additionally, discharge teams frequently overestimated patient’s readiness for hospital discharge. Misalignment on patient readiness for discharge occurred both within the discharge team (ie, low team convergence) and between patients and their care teams (ie, low team-patient convergence). The potential importance of this disagreement is substantiated by prior work suggesting that divergence in readiness ratings between nurses and patients are associated with postdischarge coping difficulties.28
Previous readiness for discharge has been measured from the perspective of the patient,20,21,27,28 nurse,20,25-28 and physician,37 yet rarely has the teams’ perspective been examined. We add to this literature by measuring the team’s perspective, as well as agreement between team and patient, on the individual patient’s readiness for discharge. Notably, we found that higher-quality communication is positively related to teams’ assessment of discharge readiness, with teams that reported higher quality teamwork having more convergent team-patient SMMs. Our results support many qualitative studies identifying communication and teamwork as major factors in teams’ effectiveness in discharge planning.1-7,9 However, given the small sample size in this study, additional research is needed to further understand these relationships, as well as link SMMs to patient outcomes such as hospital readmission.
In an attempt to improve discharge planning, hospitals are increasingly assessing readiness for discharge as a low-intensity, low-cost intervention.26,27 Yet, recent evidence suggests that readiness assessments alone have minimal impact on reducing hospital readmissions.26 To be successful, these assessments likely depend on quality interprofessional communication and ensuring the patient’s voice is incorporated into the discharge decision process.26 However, there have been few ways to effectively evaluate these types of team interventions.9 Measuring SMM properties holds promise for identifying specific team mechanisms that may influence the effectiveness and fidelity of interventions for team-based discharge planning. As our findings indicate, SMMs provide a theoretical and methodological basis for evaluating if readiness for discharge was team based (convergence among team members) and patient centered (convergence among team assessment and patient self-assessment). Researchers and improvement scientists can use the approach outlined to evaluate team-based patient-centered interventions for hospital discharge planning.9
This study provides a unique contribution to the growing work in the team science of SMMs.9,10 We rigorously evaluated SMMs of key stakeholders (patients and their interprofessional team) in “real-time” clinical practice using a patient-centered assessment linked with postdischarge outcomes.20,27,28 However, it is still unknown how much convergence is needed (and with whom) to safely discharge patients.13 Prior studies suggest highly convergent SMMs increase team performance when they are also accurate.10-13 Convergence alone should not be sought because this may reflect groupthink or clinical inertia.10,15 To improve discharge team performance over time,10‑13 it is important to assess not only patient’s readiness on the day of discharge but also how prepared the patient actually was for the recovery period following acute care. In the larger mixed-methods study, we found that teams’ with more convergent SMMs on teamwork quality were associated with patient’s reported quality of transition 30-days after discharge.9 Together, these findings further highlight the importance of aligning patient and interprofessional team members perspectives during the discharge planning, as well as providing clinicians with regular feedback about patient’s postdischarge experiences and outcomes.
To optimize team performance, the discharge planning process must be considered from an interprofessional team perspective as it functions in real-world practice settings. There are increasing pressures to discharge patients “quicker and sicker,” to simplify and standardize clinical process, and to provide patient-centered care.3,5-8 Without thoughtful interventions to facilitate communication during discharge planning, these pressures likely reinforce inaccurate assumptions regarding the work of fellow team members and force teams to think “fast” instead of “slow.”38-40 One approach to overcome such barriers is to focus on building a high-quality interprofessional SMM around discharge readiness. For example, the RHDS/SF questions could be integrated into the electronic medical records, displayed on dashboards, and discussed regularly during discharge rounds. In particular, to strengthen the team’s SMM and quality of teamwork, together the staff can ask three practical questions (Appendix F): (1) Do we think the patient is ready for discharge? (2) To what extent do we all agree the patient is ready for discharge? (3) Does our assessment of discharge readiness match the patient’s? During this high-risk transition point, asking these questions might allow the team to move from thinking fast to thinking slowly so they can more effectively identify heuristics they may be using inaccurately, prevent blind spots, and move toward high reliability.10,13,18,38-40
This study has limitations. First, events were recruited from patients with any of only six conditions at a single hospital. Other settings, patient condition types, or team compositions of other clinicians may differ in results. Second, in this study the SMM content was focused on readiness for hospital discharge among four key stakeholders. It is possible other SMM content needs to be shared among the interprofessional discharge team (eg, caregivers’ perspectives,2,6-8 resource availability,3-6 clinicians’ roles4,9) or additional members should be included (eg, physical therapists, nursing assistants, home health consultants, or primary care clinicians). Although this study focused on a patient-centered outcome (Patient-RHDS/SF), we did not examine other important outcomes such as hospital readmission. Additionally, due to the small sample size, these results have limited generalizability and should be interpreted with caution. Last, we limited data collection to the day of hospital discharge; future studies might consider assessing discharge readiness throughout hospitalization.
CONCLUSION
Readying patients for hospital discharge is a time-sensitivehigh-risk task requiring multiple healthcare professionals to concurrently assess patient needs, formulate an anticipatory care plan, provide education, and arrange for postdischarge needs.20,21 Despite this, few studies have analyzed teamwork aspects to understand how these transitions could be improved.9 By piloting SMM measurement and describing factors that affect SMMs, we provide a step toward identifying and evaluating strategies to assist interprofessional care teams in preparing patients for a safe, high-quality, patient-centered hospital discharge.
Presentations
This work was presented at the Midwest Nursing Research Society’s 2018 Annual Research Conference in Cleveland, Ohio, as well as at AcademyHealth’s 2019 Annual Research Meeting in Washington, District of Columbia.
Preparing patients for hospital discharge requires multiple tasks that cross professional boundaries. Clinician’s roles may be ambiguous, and responsibility for a safe high-quality discharge is often diffused among the team rather than being defined as the core responsibility of a single member.1-8 Without a shared understanding of patient resources and tasks involved in anticipatory planning, lapses in discharge preparation can occur, which places patients at increased risk for harm after hospitalization.3-7 As a result, organizations like the Centers for Medicare & Medicaid Services (CMS) have called for team-based patient-centered discharge planning.8 Yet to develop more effective team-based discharge planning interventions, a more nuanced understanding of how healthcare teams work together is needed.2,3,9
Shared mental models (SMMs) provide a useful theoretical framework and measurement approach for examining how interprofessional teams coordinate complex tasks like hospital discharge.10-13 SMMs represent the team members’ collective understanding and organized knowledge of key elements needed for teams to perform effectively.9-11 Validated questionnaires can be used to measure two key properties of SMMs: the degree to which team members have a similar understanding of the situation at hand (team SMM convergence) and to what extent this understanding is aligned with the patient (team-patient SMM convergence).10,11 Researchers have found that teams with higher-quality SMMs have a better understanding of what is happening and why, have clearer expectations of their roles and tasks, and can better predict what might happen next.10,12 As a result, these teams more effectively coordinate actions and adapt to task demands even in cases of high complexity, uncertainty, and stress.10-13 Prior studies examining healthcare teams in emergency departments,14-16 critical care units,17,18 and operating rooms19 suggest high-quality SMMs are needed to safely care for patients.13 Yet there has been limited evaluation of SMMs in general internal medicine, much less during hospital discharge.9,13 The purpose of this study was to examine SMMs for a critical task of the inpatient team: developing a shared understanding of the patient’s readiness for hospital discharge.20,21
METHODS
Design
We used a cross-sectional survey design at a single Midwestern community hospital to determine inpatient care teams’ SMMs of patient hospital discharge readiness. This study is part of a larger mixed-methods evaluation of interprofessional hospital discharge teamwork in older adult patients at risk for a poor transition to home.9 Data were collected using questionnaires from patients and their team (nurse, coordinator, and physician) within 4 hours of the patient leaving the hospital. First, we measured the teams’ assessment, team convergence, and team-patient convergence on patient readiness for discharge from the hospital. Then, after identifying relevant potential predictors from the literature, we developed regression models to predict the teams’ assessment, team convergence, and team-patient convergence of discharge readiness based on these variables. Our local institutional review board approved this study.
Sample and Participants
We used a convenience sampling approach to identify eligible discharge events consisting of the patient and care team.9 We focused on patients at high-risk for poor hospital-to-home transitions.3,22 Eligible events included older patients (≥65 years) who were discharged home without home health or hospice services and admitted with a primary diagnosis of heart failure, acute myocardial infarction, hip replacement, knee replacement, pneumonia, or chronic obstructive pulmonary disease. Patient exclusion criteria included inability to complete study forms because of mental incapacity or a language barrier. Discharge team member inclusion criteria included the bedside nurse, attending physician, and coordinator (a unit-dedicated discharge nurse or social worker) caring for the patient participant at the time of hospital discharge. Each discharge team was unique: The same three individuals could not be included as a “team” for more than one discharge event, although individual members could be included as a part of other teams with a different set of individuals. Appendix A provides an enrollment flowchart.
Conceptual Framework
We applied the SMM conceptual framework to the context of hospital discharge. As shown in the Figure, SMMs are examined at the team level and contain the critical knowledge held by the team to be effective.15,16 From a patient-centered perspective, patients are considered the expert on how ready they feel to be discharged home.20,23,24 In this case, the SMM content is the discharge team members’ shared assessment of how ready the patient is for hospital discharge (Figure, B).10 Convergence is the degree of agreement among individual mental models.10-13 In this study we examined two types of convergence: (1) team convergence, or the team members degree of agreement on the patient’s readiness for discharge (Figure, C), and (2) team-patient convergence, or the degree to which the team’s SMM aligns with the patient’s mental model (Figure, D).10-13
Measures and Variables
Readiness for Hospital Discharge Scales/Short Form
We used parallel clinician and patient versions of the Readiness for Hospital Discharge Scale/Short Form (RHDS/SF)25-28 to determine the teams’ assessment of discharge readiness, team SMM convergence, and team-patient SMM convergence.
The RHDS/SF scales are 8-item validated instruments that use a Likert scale (0 for not ready to 10 for totally ready) to assess the individual clinician’s or patient’s perceptions of how ready the patient is to be discharged.20,25,27 The RHDS/SF instruments include four dimensions conceptualized as crucial to patient readiness for discharge and important to anticipatory planning: (1) Personal Status, physical-emotional state of the patient before discharge; (2) Knowledge, perceived adequacy of information needed to respond to common posthospitalization concerns/problems; (3) Coping Ability, perceived ability to self-manage health care needs; and (4) Expected Support, emotional-physical assistance available (Appendix B).20,25,27 The RHDS/SF instruments’ results are calculated as a mean of item scores, with higher individual scores indicating the rater assessed the patient as being more ready for hospital discharge.20 The RHDS/SF scales have undergone rigorous psychometric testing and are linked to patient outcomes (eg, readmissions, emergency room visits, patient coping difficulties after discharge, and patient-rated quality of preparation for posthospital care).20,25-28 For example, predictive validity assessments for adult medical-surgical patients found lower Nurse-RHDS/SF scores are associated with a six- to ninefold increase in 30-day readmission risk.20
Contextual Variables
We reviewed the literature to identify potential patient and system factors associated with adverse transitional care outcomes1-8 and/or higher quality SMMs in other settings.10-19 For example, patient characteristics included age, principal diagnosis, length of stay, number of comorbidities, and cognition impairment (using the Short Portable Mini Mental Status Questionnaire29).2,22,30 Examples of system factor include teamwork and communication quality1-6 on day of discharge, as well as educational background and experience of clinicians on the team.31-33 We adapted a validated survey using 7-point Likert scale questions to determine teamwork quality and communication quality during individual patent discharges.33 Appendix C provides descriptions of all variables.
Data Collection
Patient recruitment occurred from February to October 2017 in a single community hospital in Iowa.9 We identified potentially eligible events in collaboration with the unit charge nurses. Patients were screened 24 to 48 hours prior to anticipated day of discharge; those interested/eligible underwent informed consent procedures.9 We collected data from the patient and their corresponding bedside nurse, coordinator, and attending physician on the day of discharge. After the discharge order was placed and care instructions were provided, the patient completed a demographic survey, Short Portable Mini Mental Status Questionnaire, and the Patient-RHDS/SF. Individual team members completed a survey with the demographic information, their respective versions of RHDS/SF, and day-of-discharge teamwork-related questions. On average, the survey took clinicians less than 5 minutes to complete. We performed a chart review to determine additional patient characteristics such as principal diagnosis, length of stay, and number of comorbidities.
Data Analysis
Team Assessment of Patient Discharge Readiness
The teams’ shared assessment (SMM content) was determined by averaging the members’ individual scores on the Clinician-RHDS/SF.34 Discharge events with higher team assessments indicated the team perceived the patient as being readier for hospital discharge. Guided by prior research, we examined the RHDS/SF scores as a continuous variable and as a four-level categorical variable of readiness: low (<7), moderate (7-7.9), high (8-8.9), and very high (9-10).20
Team SMM Convergence
To determine the teams’ convergence on patient discharge readiness, we calculated an adjusted interrater agreement index (r*wg(j))35,36 for each team using the individual clinicians’ scores on the RHDS/SF. These convergence values were categorized into four agreement levels: low agreement (<0.7), moderate agreement (0.7-0.79), high agreement (0.8-0.89), and very high agreement (0.9-1). See Appendix D for the r*wg(j) equation.35,36
Team-Patient SMM Convergence
To determine the team-patient SMM convergence, we subtracted the team’s assessment of patient discharge readiness from the Patient-RHDS/SF score. We used a one-unit change on the RHDS/SF (1 point on the 0-10 scale) as a meaningful difference between the patient’s self-assessment and teams’ assessment on readiness for hospital discharge. This definition for divergence aligns with prior RHDS psychometric testing studies20,27 and research examining convergence between patient and nurse assessments.28 For example, Weiss and colleagues27 found a 1-point decrease in the RN-RHDS/SF item mean was associated with a 45% increase in likelihood of postdischarge utilization (hospital readmission and emergency room department visits). Therefore, we defined convergence of team-patient SMMs (or similar patient and team scores) as those with an absolute difference score less than 1 point, whereas teams with low team-patient SMM convergence (or divergent patient and team scores) were defined as having an absolute difference score greater than 1 point.
Prediction Models
For the exploratory aim, we first examined the bivariate relationship between the outcome variables (discharge teams’ assessment of patient readiness, team convergence, and team-patient convergence) and the identified contextual variables. We also checked for potential collinearity among the explanatory variables. Then we used a linear stepwise regression procedure to identify factors associated with each continuous outcome variable. Due to the small sample size, we performed separate backward stepwise regression selection analyses for the three outcomes of interest. The candidate explanatory variables were evaluated using P < .20 for model entry. Final models were evaluated using leave-one-out cross validation. STATA (v.15.1, StataCorp; 2017) was used for analysis.
RESULTS
Sample
A total of 64 discharge events were included in this study. All discharge teams had a unique composition including 64 patients and varying combinations of 56 individual nurses (n = 27), physicians (n = 23), and coordinators (n = 6). Each event had three team members (ie, a nurse, a coordinator, and a physician) with no missing data. The majority of the 64 patient participants were White, retired, had a high school education, and lived in their own home with only one other person (Table 1).

Interprofessional Teams’ SMM of Readiness for Hospital Discharge
While the majority of teams perceived patients had high readiness for hospital discharge (mean, 8.5 out of 10; SD, 0.91), patients scores were nearly a full point lower (mean, 7.7; SD, 1.6; Table 2). The largest difference across categories was in the low-readiness category with 27% of patient scores falling into this category vs only 9.4% of discharge team mean scores. The mean SMM convergence of team perception of patients’ readiness for discharge was 0.90 (SD, 0.10); however, scores ranged from 0.66 (low agreement) to 1 (complete agreement). The average SMM team-patient convergence, or the discrepancy between the discharge team mean scores and the patient total scores across domains, was 1.16 (SD, 0.82). Of the 64 discharge events, 42.2% had similar team-patient perceptions of readiness for discharge, 9.4% had the patient reporting higher readiness for discharge than the team, and 48.4% had a team assessment rating of higher readiness for discharge than the patient’s self-assessment.

Prediction Models
In the exploratory analysis, we created individual linear regression models to predict the teams’ assessment, team convergence, and team-patient convergence for readiness of hospital discharge (Table 3; Appendix E). Factors associated with the teams’ assessment of discharge readiness included whether the patient was married and had less cognitive impairment, both of which were positively related to a higher-rated readiness among teams. An important system factor was higher quality of communication among team members, which was positively associated with teams’ assessment of patient discharge readiness. In contrast, only patient factors—married patients and those with a principal diagnosis of heart failure—were associated with more convergent team SMMs. Team-patient convergence was positively associated with two patient factors: marital status (married) and fewer comorbidities. However, team-patient convergence was also associated with two system factors: teams with a bachelor’s level–trained nurse (compared with a nurse with an associate degree ) and teams reporting a higher quality of teamwork on day of discharge.

DISCUSSION
Our study applied novel approaches to explore the interprofessional teams’ understanding of discharge readiness, a concept known to be an important predictor of patient outcomes after discharge, including readmission.20,28 We found that discharge teams frequently had poor quality SMMs of hospital discharge readiness. Despite having a discharge order and receiving home care instructions, one in four patients reported low readiness for hospital discharge. Additionally, discharge teams frequently overestimated patient’s readiness for hospital discharge. Misalignment on patient readiness for discharge occurred both within the discharge team (ie, low team convergence) and between patients and their care teams (ie, low team-patient convergence). The potential importance of this disagreement is substantiated by prior work suggesting that divergence in readiness ratings between nurses and patients are associated with postdischarge coping difficulties.28
Previous readiness for discharge has been measured from the perspective of the patient,20,21,27,28 nurse,20,25-28 and physician,37 yet rarely has the teams’ perspective been examined. We add to this literature by measuring the team’s perspective, as well as agreement between team and patient, on the individual patient’s readiness for discharge. Notably, we found that higher-quality communication is positively related to teams’ assessment of discharge readiness, with teams that reported higher quality teamwork having more convergent team-patient SMMs. Our results support many qualitative studies identifying communication and teamwork as major factors in teams’ effectiveness in discharge planning.1-7,9 However, given the small sample size in this study, additional research is needed to further understand these relationships, as well as link SMMs to patient outcomes such as hospital readmission.
In an attempt to improve discharge planning, hospitals are increasingly assessing readiness for discharge as a low-intensity, low-cost intervention.26,27 Yet, recent evidence suggests that readiness assessments alone have minimal impact on reducing hospital readmissions.26 To be successful, these assessments likely depend on quality interprofessional communication and ensuring the patient’s voice is incorporated into the discharge decision process.26 However, there have been few ways to effectively evaluate these types of team interventions.9 Measuring SMM properties holds promise for identifying specific team mechanisms that may influence the effectiveness and fidelity of interventions for team-based discharge planning. As our findings indicate, SMMs provide a theoretical and methodological basis for evaluating if readiness for discharge was team based (convergence among team members) and patient centered (convergence among team assessment and patient self-assessment). Researchers and improvement scientists can use the approach outlined to evaluate team-based patient-centered interventions for hospital discharge planning.9
This study provides a unique contribution to the growing work in the team science of SMMs.9,10 We rigorously evaluated SMMs of key stakeholders (patients and their interprofessional team) in “real-time” clinical practice using a patient-centered assessment linked with postdischarge outcomes.20,27,28 However, it is still unknown how much convergence is needed (and with whom) to safely discharge patients.13 Prior studies suggest highly convergent SMMs increase team performance when they are also accurate.10-13 Convergence alone should not be sought because this may reflect groupthink or clinical inertia.10,15 To improve discharge team performance over time,10‑13 it is important to assess not only patient’s readiness on the day of discharge but also how prepared the patient actually was for the recovery period following acute care. In the larger mixed-methods study, we found that teams’ with more convergent SMMs on teamwork quality were associated with patient’s reported quality of transition 30-days after discharge.9 Together, these findings further highlight the importance of aligning patient and interprofessional team members perspectives during the discharge planning, as well as providing clinicians with regular feedback about patient’s postdischarge experiences and outcomes.
To optimize team performance, the discharge planning process must be considered from an interprofessional team perspective as it functions in real-world practice settings. There are increasing pressures to discharge patients “quicker and sicker,” to simplify and standardize clinical process, and to provide patient-centered care.3,5-8 Without thoughtful interventions to facilitate communication during discharge planning, these pressures likely reinforce inaccurate assumptions regarding the work of fellow team members and force teams to think “fast” instead of “slow.”38-40 One approach to overcome such barriers is to focus on building a high-quality interprofessional SMM around discharge readiness. For example, the RHDS/SF questions could be integrated into the electronic medical records, displayed on dashboards, and discussed regularly during discharge rounds. In particular, to strengthen the team’s SMM and quality of teamwork, together the staff can ask three practical questions (Appendix F): (1) Do we think the patient is ready for discharge? (2) To what extent do we all agree the patient is ready for discharge? (3) Does our assessment of discharge readiness match the patient’s? During this high-risk transition point, asking these questions might allow the team to move from thinking fast to thinking slowly so they can more effectively identify heuristics they may be using inaccurately, prevent blind spots, and move toward high reliability.10,13,18,38-40
This study has limitations. First, events were recruited from patients with any of only six conditions at a single hospital. Other settings, patient condition types, or team compositions of other clinicians may differ in results. Second, in this study the SMM content was focused on readiness for hospital discharge among four key stakeholders. It is possible other SMM content needs to be shared among the interprofessional discharge team (eg, caregivers’ perspectives,2,6-8 resource availability,3-6 clinicians’ roles4,9) or additional members should be included (eg, physical therapists, nursing assistants, home health consultants, or primary care clinicians). Although this study focused on a patient-centered outcome (Patient-RHDS/SF), we did not examine other important outcomes such as hospital readmission. Additionally, due to the small sample size, these results have limited generalizability and should be interpreted with caution. Last, we limited data collection to the day of hospital discharge; future studies might consider assessing discharge readiness throughout hospitalization.
CONCLUSION
Readying patients for hospital discharge is a time-sensitivehigh-risk task requiring multiple healthcare professionals to concurrently assess patient needs, formulate an anticipatory care plan, provide education, and arrange for postdischarge needs.20,21 Despite this, few studies have analyzed teamwork aspects to understand how these transitions could be improved.9 By piloting SMM measurement and describing factors that affect SMMs, we provide a step toward identifying and evaluating strategies to assist interprofessional care teams in preparing patients for a safe, high-quality, patient-centered hospital discharge.
Presentations
This work was presented at the Midwest Nursing Research Society’s 2018 Annual Research Conference in Cleveland, Ohio, as well as at AcademyHealth’s 2019 Annual Research Meeting in Washington, District of Columbia.
- Greysen SR, Schiliro D, Horwitz LI, Curry L, Bradley E. “Out of sight, out of mind”: house staff perceptions of quality-limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376-381.https://doi.org/10.1002/%20jhm.1928
- Fuji KT, Abbott AA, Norris JF. Exploring care transitions from patient, caregiver, and health-care provider perspectives. Clin Nurs Res. 2013;22(3):258-274. https://doi.org/10.1177/1054773812465084
- Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. https://doi.org/10.1002/jhm.228
- Waring J, Bishop S, Marshall F. A qualitative study of professional and career perceptions of the threats to safe hospital discharge for stroke and hip fracture patients in the English National Health Service. BMC Health Serv Res. 2016;16:297. https://doi.org/10.1186/s12913-016-1568-2
- Nosbusch JM, Weiss ME, Bobay KL. An integrated review of the literature on challenges confronting the acute care staff nurse in discharge planning. J Clin Nurs. 2011;20(5-6):754-774. https://doi.org/10.1111/j.1365-2702.2010.03257.x
- Ashbrook L, Mourad M, Sehgal N. Communicating discharge instructions to patients: a survey of nurse, intern, and hospitalist practices. J Hosp Med. 2013;8(1):36-41. https://doi.org/10.1002/jhm.1986
- Prusaczyk B, Kripalani S, Dhand A. Networks of hospital discharge planning teams and readmissions. J Interprof Care. 2019;33(1):85-92. https://doi.org/1 0.1080/13561820.2018.1515193
- Centers for Medicare & Medicaid Services. Medicare and Medicaid Programs; Revisions to Requirements for Discharge Planning for Hospitals, Critical Access Hospitals, and Home Health Agencies, and Hospital and Critical Access Hospital Changes to Promote Innovation, Flexibility, and Improvement in Patient Care. Septemeber 30, 2019. Federal Register. Accessed May 24, 2021. https://www.federalregister.gov/documents/2019/09/30/2019-20732/medicare-and-medicaid-programs-revisions-to-requirements-for-discharge-planning-for-hospitals
- Manges K, Groves PS, Farag A, Peterson R, Harton J, Greysen SR. A mixed methods study examining teamwork shared mental models of interprofessional teams during hospital discharge. BMJ Qual Saf. 2020;29(6):499-508. https://doi.org/10.1136/bmjqs-2019-009716
- Mohammed S, Ferzandi L, Hamilton K. Metaphor no more: a 15-year review of the team mental model construct. J Manage. 2010;36(4):876-910. https:// doi.org/10.1177%2F0149206309356804
- Langan-Fox J, Code S, Langfield-Smith K. Team mental models: techniques, methods, and analytic approaches. Hum Factors. 2000;42(2);242-271. https:// doi.org/10.1518/001872000779656534
- Lim BC, Klein KJ. Team mental models and team performance: a field study of the effects of team mental model similarity and accuracy. J Organ Behav. 2006;27(4):403-418. https://doi.org/10.1002/job.387
- Burtscher MJ, Manser T. Team mental models and their potential to improve teamwork and safety: a review and implications for future research in healthcare. Safety Sci. 2012;50(5):1344-1354. https://doi.org/10.1016/j. ssci.2011.12.033
- Calder LA, Mastoras G, Rahimpour M, et al. Team communication patterns in emergency resuscitation: a mixed methods qualitative analysis. Int J Emerg Med. 2017:10(1):24. https://doi.org/10.1186/s12245-017-0149-4
- Westli HK, Johnsen BH, Eid J, Rasten I, Brattebø G. Teamwork skills, shared mental models, and performance in simulated trauma teams: an independent group design. Scand J Trauma Resusc Emerg Med. 2010;18:47. https:// doi.org/10.1186/1757-7241-18-47
- Johnsen BH, Westli HK, Espevik R, Wisborg R, Brattebø G. High-performing trauma teams: frequency of behavioral markers of a shared mental model displayed by team leaders and quality of medical performance. Scand J Trauma Resusc Emerg Med. 2017;25(1):109. https://doi.org/10.1186/s13049- 017-0452-3
- Custer JW, White E, Fackler JC, et al. A qualitative study of expert and team cognition on complex patients in the pediatric intensive care unit. Pediatr Crit Care Med. 2012;13(3):278-284. https://doi.org/10.1097/ pcc.0b013e31822f1766
- Cutrer WB, Thammasitboon S. Team mental model creation as a mechanism to decrease errors in the intensive care unit. Pediatr Crit Care Med. 2012;13(3):354-356. https://doi.org/10.1097/pcc.0b013e3182388994
- Gjeraa K, Dieckmann P, Spanager L, et al. Exploring shared mental models of surgical teams in video-assisted thoracoscopic surgery lobectomy. Ann Thorac Surg. 2019;107(3):954-961. https://doi.org/10.1016/j.athoracsur.2018.08.010
- Weiss ME, Costa LL, Yakusheva O, Bobay KL. Validation of patient and nurse short forms of the Readiness for Hospital Discharge Scale and their relationship to return to the hospital. Health Serv Res. 2014;49(1):304-317. https:// doi.org/10.1111/1475-6773.12092
- Galvin EC, Wills T, Coffey A. Readiness for hospital discharge: a concept analysis. J Adv Nurs. 2017;73(11):2547-2557. https://doi.org/10.1111/jan.13324
- Hospital Readmissions Reduction Program (HRRP). Centers for Medicare & Medicaid Services. Updated August 11, 2020. Accessed January 6, 2020. https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/value-based-programs/hrrp/hospital-readmission-reduction-program.html
- Epstein RM, Fiscella K, Lesser CS, Stange KC. Why the nation needs a policy push on patient-centered health care. Health Aff (Millwood). 2010;29(8):1489- 1495. https://doi.org/10.1377/hlthaff.2009.0888
- Graumlich JF, Novotny NL, Aldag JC. Brief scale measuring patient preparedness for hospital discharge to home: psychometric properties. J Hosp Med. 2008;3(6):446-454. https://doi.org/10.1002/jhm.316
- Bobay KL, Weiss ME, Oswald D, Yakusheva O. Validation of the Registered Nurse Assessment of Readiness for Hospital Discharge scale. Nurs Res. 2018;67(4):305-313. https://doi.org/10.1097/nnr.0000000000000293
- Weiss ME, Yakusheva O, Bobay K, et al. Effect of implementing discharge readiness assessment in adult medical-surgical units on 30-Day return to hospital: the READI randomized clinical trial. JAMA Netw Open. 2019;2(1):e187387. https://doi.org/10.1001/jamanetworkopen.2018.7387
- Weiss ME, Yakusheva O, Bobay K. Nurse and patient perceptions of discharge readiness in relation to postdischarge utilization. Med Care. 2010;48(5):482-486. https://doi.org/10.1097/mlr.0b013e3181d5feae
- Wallace AS, Perkhounkova Y, Bohr NL, Chung SJ. Readiness for hospital discharge, health literacy, and social living status. Clin Nurs Res. 2016;25(5):494- 511. https://doi.org/10.1177/1054773815624380
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433- 441. https://doi.org/10.1111/j.1532-5415.1975.tb00927.x
- Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688-1698. https://doi.org/10.1001/jama.2011.1515
- Aiken LH, Cimiotti JP, Sloane DM, Smith HL, Flynn L, Neff DF. Effects of nurse staffing and nurse education on patient deaths in hospitals with different nurse work environments. J Nurs Adm. 2012;42(10 Suppl):S10-S16. https:// doi.org/10.1097/01.nna.0000420390.87789.67
- Goodwin JS, Salameh H, Zhou J, Singh S, Kuo YF, Nattinger AB. Association of hospitalist years of experience with mortality in the hospitalized Medicare population. JAMA Intern Med. 2018;178(2):196-203. https://doi. org/10.1001/jamainternmed.2017.7049
- Millward LJ, Jeffries N. The team survey: a tool for health care team development. J Adv Nurs. 2001;35(2):276-287. https://doi.org/10.1046/j. 1365-2648.2001.01844.x
- Klein KJ, Kozlowski SW. From micro to meso: critical steps in conceptualizing and conducting multilevel research. Organ Res Methods. 2000;3(3):211-236. https://doi.org/10.1177/109442810033001
- Lindell MK, Brandt CJ, Whitney DJ. A revised index of interrater agreement for multi-item ratings of a single target. Appl Psychol Meas. 1999;23(2):127- 135. https://doi.org/10.1177%2F01466219922031257
- O’Neill TA. An overview of interrater agreement on Likert scales for researchers and practitioners. Front Psychol. 2017;8:777. https://doi.org/10.3389/ fpsyg.2017.00777
- Sullivan B, Ming D, Boggan JC, et al. An evaluation of physician predictions of discharge on a general medicine service. J Hosp Med. 2015;10(12):808- 810. https://doi.org/10.1002/jhm.2439
- Kahneman D. Thinking, fast and slow. Doubleday; 2011.
- Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22(Suppl 2):ii58-ii64. https://doi. org/10.1136/bmjqs-2012-001712
- Burke RE, Leonard C, Lee M, et al. Cognitive biases influence decision-making regarding postacute care in a skilled nursing facility. J Hosp Med. 2020:15(1)22-27. https://doi.org/10.12788/jhm.3273
- Greysen SR, Schiliro D, Horwitz LI, Curry L, Bradley E. “Out of sight, out of mind”: house staff perceptions of quality-limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376-381.https://doi.org/10.1002/%20jhm.1928
- Fuji KT, Abbott AA, Norris JF. Exploring care transitions from patient, caregiver, and health-care provider perspectives. Clin Nurs Res. 2013;22(3):258-274. https://doi.org/10.1177/1054773812465084
- Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. https://doi.org/10.1002/jhm.228
- Waring J, Bishop S, Marshall F. A qualitative study of professional and career perceptions of the threats to safe hospital discharge for stroke and hip fracture patients in the English National Health Service. BMC Health Serv Res. 2016;16:297. https://doi.org/10.1186/s12913-016-1568-2
- Nosbusch JM, Weiss ME, Bobay KL. An integrated review of the literature on challenges confronting the acute care staff nurse in discharge planning. J Clin Nurs. 2011;20(5-6):754-774. https://doi.org/10.1111/j.1365-2702.2010.03257.x
- Ashbrook L, Mourad M, Sehgal N. Communicating discharge instructions to patients: a survey of nurse, intern, and hospitalist practices. J Hosp Med. 2013;8(1):36-41. https://doi.org/10.1002/jhm.1986
- Prusaczyk B, Kripalani S, Dhand A. Networks of hospital discharge planning teams and readmissions. J Interprof Care. 2019;33(1):85-92. https://doi.org/1 0.1080/13561820.2018.1515193
- Centers for Medicare & Medicaid Services. Medicare and Medicaid Programs; Revisions to Requirements for Discharge Planning for Hospitals, Critical Access Hospitals, and Home Health Agencies, and Hospital and Critical Access Hospital Changes to Promote Innovation, Flexibility, and Improvement in Patient Care. Septemeber 30, 2019. Federal Register. Accessed May 24, 2021. https://www.federalregister.gov/documents/2019/09/30/2019-20732/medicare-and-medicaid-programs-revisions-to-requirements-for-discharge-planning-for-hospitals
- Manges K, Groves PS, Farag A, Peterson R, Harton J, Greysen SR. A mixed methods study examining teamwork shared mental models of interprofessional teams during hospital discharge. BMJ Qual Saf. 2020;29(6):499-508. https://doi.org/10.1136/bmjqs-2019-009716
- Mohammed S, Ferzandi L, Hamilton K. Metaphor no more: a 15-year review of the team mental model construct. J Manage. 2010;36(4):876-910. https:// doi.org/10.1177%2F0149206309356804
- Langan-Fox J, Code S, Langfield-Smith K. Team mental models: techniques, methods, and analytic approaches. Hum Factors. 2000;42(2);242-271. https:// doi.org/10.1518/001872000779656534
- Lim BC, Klein KJ. Team mental models and team performance: a field study of the effects of team mental model similarity and accuracy. J Organ Behav. 2006;27(4):403-418. https://doi.org/10.1002/job.387
- Burtscher MJ, Manser T. Team mental models and their potential to improve teamwork and safety: a review and implications for future research in healthcare. Safety Sci. 2012;50(5):1344-1354. https://doi.org/10.1016/j. ssci.2011.12.033
- Calder LA, Mastoras G, Rahimpour M, et al. Team communication patterns in emergency resuscitation: a mixed methods qualitative analysis. Int J Emerg Med. 2017:10(1):24. https://doi.org/10.1186/s12245-017-0149-4
- Westli HK, Johnsen BH, Eid J, Rasten I, Brattebø G. Teamwork skills, shared mental models, and performance in simulated trauma teams: an independent group design. Scand J Trauma Resusc Emerg Med. 2010;18:47. https:// doi.org/10.1186/1757-7241-18-47
- Johnsen BH, Westli HK, Espevik R, Wisborg R, Brattebø G. High-performing trauma teams: frequency of behavioral markers of a shared mental model displayed by team leaders and quality of medical performance. Scand J Trauma Resusc Emerg Med. 2017;25(1):109. https://doi.org/10.1186/s13049- 017-0452-3
- Custer JW, White E, Fackler JC, et al. A qualitative study of expert and team cognition on complex patients in the pediatric intensive care unit. Pediatr Crit Care Med. 2012;13(3):278-284. https://doi.org/10.1097/ pcc.0b013e31822f1766
- Cutrer WB, Thammasitboon S. Team mental model creation as a mechanism to decrease errors in the intensive care unit. Pediatr Crit Care Med. 2012;13(3):354-356. https://doi.org/10.1097/pcc.0b013e3182388994
- Gjeraa K, Dieckmann P, Spanager L, et al. Exploring shared mental models of surgical teams in video-assisted thoracoscopic surgery lobectomy. Ann Thorac Surg. 2019;107(3):954-961. https://doi.org/10.1016/j.athoracsur.2018.08.010
- Weiss ME, Costa LL, Yakusheva O, Bobay KL. Validation of patient and nurse short forms of the Readiness for Hospital Discharge Scale and their relationship to return to the hospital. Health Serv Res. 2014;49(1):304-317. https:// doi.org/10.1111/1475-6773.12092
- Galvin EC, Wills T, Coffey A. Readiness for hospital discharge: a concept analysis. J Adv Nurs. 2017;73(11):2547-2557. https://doi.org/10.1111/jan.13324
- Hospital Readmissions Reduction Program (HRRP). Centers for Medicare & Medicaid Services. Updated August 11, 2020. Accessed January 6, 2020. https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/value-based-programs/hrrp/hospital-readmission-reduction-program.html
- Epstein RM, Fiscella K, Lesser CS, Stange KC. Why the nation needs a policy push on patient-centered health care. Health Aff (Millwood). 2010;29(8):1489- 1495. https://doi.org/10.1377/hlthaff.2009.0888
- Graumlich JF, Novotny NL, Aldag JC. Brief scale measuring patient preparedness for hospital discharge to home: psychometric properties. J Hosp Med. 2008;3(6):446-454. https://doi.org/10.1002/jhm.316
- Bobay KL, Weiss ME, Oswald D, Yakusheva O. Validation of the Registered Nurse Assessment of Readiness for Hospital Discharge scale. Nurs Res. 2018;67(4):305-313. https://doi.org/10.1097/nnr.0000000000000293
- Weiss ME, Yakusheva O, Bobay K, et al. Effect of implementing discharge readiness assessment in adult medical-surgical units on 30-Day return to hospital: the READI randomized clinical trial. JAMA Netw Open. 2019;2(1):e187387. https://doi.org/10.1001/jamanetworkopen.2018.7387
- Weiss ME, Yakusheva O, Bobay K. Nurse and patient perceptions of discharge readiness in relation to postdischarge utilization. Med Care. 2010;48(5):482-486. https://doi.org/10.1097/mlr.0b013e3181d5feae
- Wallace AS, Perkhounkova Y, Bohr NL, Chung SJ. Readiness for hospital discharge, health literacy, and social living status. Clin Nurs Res. 2016;25(5):494- 511. https://doi.org/10.1177/1054773815624380
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433- 441. https://doi.org/10.1111/j.1532-5415.1975.tb00927.x
- Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688-1698. https://doi.org/10.1001/jama.2011.1515
- Aiken LH, Cimiotti JP, Sloane DM, Smith HL, Flynn L, Neff DF. Effects of nurse staffing and nurse education on patient deaths in hospitals with different nurse work environments. J Nurs Adm. 2012;42(10 Suppl):S10-S16. https:// doi.org/10.1097/01.nna.0000420390.87789.67
- Goodwin JS, Salameh H, Zhou J, Singh S, Kuo YF, Nattinger AB. Association of hospitalist years of experience with mortality in the hospitalized Medicare population. JAMA Intern Med. 2018;178(2):196-203. https://doi. org/10.1001/jamainternmed.2017.7049
- Millward LJ, Jeffries N. The team survey: a tool for health care team development. J Adv Nurs. 2001;35(2):276-287. https://doi.org/10.1046/j. 1365-2648.2001.01844.x
- Klein KJ, Kozlowski SW. From micro to meso: critical steps in conceptualizing and conducting multilevel research. Organ Res Methods. 2000;3(3):211-236. https://doi.org/10.1177/109442810033001
- Lindell MK, Brandt CJ, Whitney DJ. A revised index of interrater agreement for multi-item ratings of a single target. Appl Psychol Meas. 1999;23(2):127- 135. https://doi.org/10.1177%2F01466219922031257
- O’Neill TA. An overview of interrater agreement on Likert scales for researchers and practitioners. Front Psychol. 2017;8:777. https://doi.org/10.3389/ fpsyg.2017.00777
- Sullivan B, Ming D, Boggan JC, et al. An evaluation of physician predictions of discharge on a general medicine service. J Hosp Med. 2015;10(12):808- 810. https://doi.org/10.1002/jhm.2439
- Kahneman D. Thinking, fast and slow. Doubleday; 2011.
- Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22(Suppl 2):ii58-ii64. https://doi. org/10.1136/bmjqs-2012-001712
- Burke RE, Leonard C, Lee M, et al. Cognitive biases influence decision-making regarding postacute care in a skilled nursing facility. J Hosp Med. 2020:15(1)22-27. https://doi.org/10.12788/jhm.3273
© 2020 Society of Hospital Medicine
Perspectives of Clinicians, Staff, and Veterans in Transitioning Veterans from non-VA Hospitals to Primary Care in a Single VA Healthcare System
As the VA moves toward increased utilization of non-VA care, it is crucial to understand and address the challenges of transitional care faced by dual-use veterans to provide high-quality care that improves healthcare outcomes.7,11,12 The VA implemented a shift in policy from the Veterans Access, Choice, and Accountability Act of 2014 (Public Law 113-146; “Choice Act”) to the VA Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act beginning June 6, 2019.13,14 Under the MISSION Act, veterans have more ways to access healthcare within the VA’s network and through approved non-VA medical providers in the community known as “community care providers.”15 This shift expanded the existing VA Choice Act of 2014, where the program allowed those veterans who are unable to schedule an appointment within 30 days of their preferred date or the clinically appropriate date, or on the basis of their place of residence, to elect to receive care from eligible non-VA healthcare entities or providers.14,15 These efforts to better serve veterans by increasing non-VA care might present added care coordination challenges for patients and their providers when they seek care in the VA.
High-quality transitional care prevents poor outcomes such as hospital readmissions.16-18 When communication and coordination across healthcare delivery systems are lacking, patients and their families often find themselves at risk for adverse events.19,20 Past research shows that patients have fewer adverse events when they receive comprehensive postdischarge care, including instructions on medications and self-care, symptom recognition and management, and reminders to attend follow-up appointments.17,21,22 Although researchers have identified the components of effective transitional care,23 barriers persist. The communication and collaboration needed to provide coordinated care across healthcare delivery systems are difficult due to the lack of standardized approaches between systems.24 Consequently, follow-up care may be delayed or missed altogether. To our knowledge, there is no published research identifying transitional care challenges for clinicians, staff, and veterans in transitioning from non-VA hospitals to a VA primary care setting.
The objective of this quality assessment was to understand VA and non-VA hospital clinicians’ and staff as well as veterans’ perspectives of the barriers and facilitators to providing high-quality transitional care.
METHODS
Study Design
We conducted a qualitative assessment within the VA Eastern Colorado Health Care System, an urban tertiary medical center, as well as urban and rural non-VA hospitals used by veterans. Semi-structured interview guides informed by the practical robust implementation and sustainability (PRISM) model, the Lean approach, and the Ideal Transitions of Care Bridge were used.25-27 We explored the PRISM domains such as recipient’s characteristics, the interaction with the external environment, and the implementation and sustainability infrastructure to inform the design and implementation of the intervention.25 The Lean approach included methods to optimize processes by maximizing efficiency and minimizing waste.26 The Ideal Transitions of Care Bridge was used to identify the domains in transitions of care such as discharge planning, communication of information, and care coordination.27
Setting and Participants
We identified the top 10 non-VA hospitals serving the most urban and rural veterans in 2015 using VA administrative data. Purposive sampling was used to ensure that urban and rural non-VA hospitals and different roles within these hospitals were represented. VA clinicians and staff were selected from the Denver VA Medical Center, a tertiary hospital within the Eastern Colorado Health Care System and one VA Community-Based Outpatient Clinic (CBOC) that primarily serves rural veterans. The Denver VA Medical Center has three clinics staffed by Patient Aligned Care Teams (PACTs), a model built on the concept of Patient-Centered Medical Home.28 Hospital leadership were initially approached for permission to recruit their staff and to be involved as key informants, and all agreed. To ensure representativeness, diversity of roles was recruited, including PACT primary care physicians, nurses, and other staff members such as medical assistants and administrators. Veterans were approached for sampling if they were discharged from a non-VA hospital during June–September 2015 and used the VA for primary care. This was to ensure that they remembered the process they went through postdischarge at the time of the interview.
Data Collection and Analysis
The evaluation team members (RA, EL, and MM) conducted the interviews from November 2015 to July 2016. Clinicians, staff, and veterans were asked semi-structured questions about their experiences and their role in transitioning VA patients across systems (see Appendix for interview guides). Veterans were asked to describe their experience and satisfaction with the current postdischarge transition process. We stopped the interviews when we reached data saturation.29
Interviews were audio-recorded, transcribed verbatim, and validated (transcribed interviews were double-checked against recording) to ensure data quality and accuracy. Coding was guided by a conventional content analysis technique30, 31 using a deductive and inductive coding approach.31 The deductive coding approach was drawn from the Ideal Transitions of Care Bridge and PRISM domains. 32,33 Two evaluation team members (RA and EL) defined the initial code book by independently coding the first three interviews, worked to clarify the meanings of emergent codes, and came to a consensus when disagreements occurred. Next, a priori codes were added by team members to include the PRISM domains. These PRISM domains included the implementation and sustainability infrastructure, the external environment, the characteristics of intervention recipients, and the organizational and patient perspectives of an intervention.
Additional emergent codes were added to the code book and agreed upon by team members (RA, EL, and MM). Consistent with previously used methods, consensus building was achieved by identifying and resolving differences by discussing with team members (RA, EL, MM, CB, and RB).29 Codes were examined and organized into themes by team members.29,34-36 This process was continued until no new themes were identified. Results were reviewed by all evaluation team members to assess thoroughness and comprehensiveness.34,35 In addition, team members triangulated the findings with VA and non-VA participants to ensure validity and reduce researcher bias.29,37
RESULTS
We conducted a total of 70 interviews with 23 VA and 29 non-VA hospital clinicians and staff and 18 veterans (Table 1). Overall, we found that there was no standardized process for transitioning veterans across healthcare delivery systems. Participants reported that transitions were often inefficient when non-VA hospitals could not (1) identify patients as veterans and notify VA primary care of discharge; (2) transfer non-VA hospital medical records to VA primary care; (3) obtain follow-up care appointments with VA primary care; and (4) write VA formulary medications for veterans to fill at VA pharmacies. In addition, participants discussed about facilitators and suggestions to overcome these inefficiencies and improve transitional care (Table2). We mapped the identified barriers as well as the suggestions for improvement to the PRISM and the Ideal Transitions of Care Bridge domains (Table 3).
Unable to Identify Patients as Veterans and Notify VA Primary Care of Discharge
VA and non-VA participants reported difficulty in communicating about veterans’ hospitalizations and discharge follow-up needs across systems. Non-VA clinicians referenced difficulty in identifying patients as veterans to communicate with VA, except in instances where the VA is a payor, while VA providers described feeling largely uninformed of the veterans non-VA hospitalization. For non-VA clinicians, the lack of a systematic method for veteran identification often left them to inadvertently identify veteran status by asking about their primary care clinicians and insurance and even through an offhanded comment made by the veteran. If a veteran was identified, non-VA clinicians described being uncertain about the best way to notify VA primary care of the patient’s impending discharge. Veterans described instances of the non-VA hospital knowing their veteran status upon admission, but accounts varied on whether the non-VA hospital notified the VA primary care of their hospitalization (Table 2, Theme 1).
Unable to Transfer Non-VA Hospital Medical Records to VA Primary Care
VA clinicians discussed about the challenges associated with obtaining the veteran’s medical record from the non-VA hospitals, and when it was received, it was often incomplete information and significantly delayed. They described relying on the veteran’s description of the care received, which was not complete or accurate information needed to make clinical judgment or coordinate follow-up care. Non-VA clinicians mentioned about trying several methods for transferring the medical record to VA primary care, including discharge summary via electronic system and sometimes solely relying on patients to deliver discharge paperwork to their primary care clinicians. In instances where non-VA hospitals sent discharge paperwork to VA, there was no way for non-VA hospitals to verify whether the faxed electronic medical record was received by the VA hospital. Most of the veterans discussed receiving written postdischarge instructions to take to their VA primary care clinicians; however, they were unsure whether the VA primary care received their medical record or any other information from the non-VA hospital (Table 2, Theme 2).
Unable to Obtain Follow-Up Care Appointments with VA Primary Care
All participants described how difficult it was to obtain a follow-up appointment for veterans with VA primary care. This often resulted in delayed follow-up care. VA clinicians also shared that a non-VA hospitalization can be the impetus for a veteran to seek care at the VA for the very first time. Once eligibility is determined, the veteran is assigned a VA primary care clinician. This process may take up to six weeks, and in the meantime, the veteran is scheduled in VA urgent care for immediate postdischarge care. This lag in primary care assignment creates delayed and fragmented care (Table 2, Theme 3).
Non-VA clinicians, administrators, and staff also discussed the difficulties in scheduling follow-up care with VA primary care. Although discharge paperwork instructed patients to see their VA clinicians, there was no process in place for non-VA clinicians to confirm whether the follow-up care was received due to lack of bilateral communication. In addition, veterans discussed the inefficiencies in scheduling follow-up appointments with VA clinicians where attempts to follow-up with primary care clinicians took eight weeks or more. Several veterans described walking into the clinic without an appointment asking to be seen postdischarge or utilizing the VA emergency department for follow-up care after discharge from a non-VA hospital. Veterans admitted utilizing the VA emergency department for nonemergent reasons such as filling their prescriptions because they are unable to see a VA PCP in a timely manner (Table 2, Theme 3).
Unable to Write VA Formulary Medications for Veterans to Fill at VA Pharmacies
All participants described the difficulties in obtaining medications at VA pharmacies when prescribed by the non-VA hospital clinicians. VA clinicians often had to reassess, and rewrite prescriptions written by clinicians, causing delays. Moreover, rural VA clinicians described lack of VA pharmacies in their locations, where veterans had to mail order medications, causing further delays in needed medications. Non-VA clinicians echoed these frustrations. They noted that veterans were confused about their VA pharmacy benefits as well as the need for the non-VA clinicians to follow VA formulary guidelines. Veterans expressed that it was especially challenging to physically go to the VA pharmacy to pick up medications after discharge due to lack of transportation, limited VA pharmacy hours, and long wait times. Several veterans discussed paying for their prescriptions out of pocket even though they had VA pharmacy benefits because it was more convenient to use the non-VA pharmacy. In other instances, veterans discussed going to a VA emergency department and waiting for hours to have their non-VA clinician prescription rewritten by a VA clinician (Table 2, Theme 4).
Facilitators of the Current Transition Process
Several participants provided examples of when transitional care communication between systems occurred seamlessly. VA staff and veterans noted that the VA increased the availability of urgent care appointments, which allowed for timelier postacute care follow-up appointments. Non-VA hospital clinicians also noted the availability of additional appointment slots but stated that they did not learn about these additional appointments directly from the VA. Instead, they learned of these through medical residents caring for patients at both VA and non-VA hospitals. One VA CBOC designated two nurses to care for walk-in veterans for their postdischarge follow-up needs. Some VA participants also noted that the VA Call Center Nurses occasionally called veterans upon discharge to schedule a follow-up appointment and facilitated timely care.
Participants from a VA CBOC discussed being part of a Community Transitions Consortium aimed at identifying high-utilizing patients (veteran and nonveteran) and improving communication across systems. The consortium members discussed each facility’s transition-of-care process, described having access to local non-VA hospital medical records and a backline phone number at the non-VA hospitals to coordinate transitional care. This allowed the VA clinicians to learn about non-VA hospital processes and veteran needs.
Suggestions for Improving the Transitional Care Process
VA and non-VA clinicians suggested hiring a VA liaison, preferably with a clinical background to facilitate care coordination across healthcare systems. They recommended that this person work closely with VA primary care, strengthen the relationship with non-VA hospitals, and help veterans learn more about the transition-of-care processes. Topics discussed for veteran education included how to (1) access their primary care tea
Veterans agreed that improvements to the current process should include an efficient system for obtaining medications and the ability to schedule timely follow-up appointments. Furthermore, veterans wanted education about the VA transition-of-care process following a non-VA hospitalization, including payment and VA notification processes (Table 2, Theme 5).
DISCUSSION
Participants described the current transitional care process as inefficient with specific barriers that have negative consequences on patient care and clinician and staff work processes. They described difficulties in obtaining medications prescribed by non-VA clinicians from VA pharmacies, delays in follow-up appointments at the VA, and lack of bilateral communication between systems and medical record transfer. Participants also provided concrete suggestions to improving the current process, including a care coordinator with clinical background. These findings are important in the context of VA increasing veteran access to care in the community.
Despite an increasing emphasis on veteran access to non-VA care as a result of the VA strategic goals and several new programs,7,12,13 there has not been a close examination of the current transition-of-care process from non-VA hospitals to VA primary care. Several studies have shown that the period following a hospitalization is especially vulnerable and associated with adverse events such as readmission, high cost, and death.12,31,32 Our findings agree with previous research that identified medical record transfer across systems as one of the most challenging issues contributing to deficits in communication between care teams.33 In addition, our study brought into focus the significant challenges faced by veterans in obtaining medications post non-VA hospital discharge. Addressing these key barriers in transitional care will improve the quality, safety, and value of healthcare in the current transition process.38,39
Based on our findings, our participants’ concern in transitional care can be addressed in various ways. First, as veterans are increasingly receiving care in the community, identifying their veteran status early on in the non-VA hospital setting could help in improved, real time communication with the VA. This could be done by updating patient intake forms to ask patients whether they are veterans or not. Second, VA policy-level changes should work to provide veterans access to non-VA pharmacy benefits equivalent to the access patients are receiving for hospital, specialty, and outpatient care. Third, patient and provider satisfaction for dual-use veterans should be examined closely. Although participants expressed frustration with the overall transitions of care from non-VA hospitals to VA primary care setting, influence of this on the Quadruple Aim-improving patient outcomes, experience, and reducing clinician and staff burnout should be examined closely.40 Fourth, evidence-based interventions such as nurse-led transitional care programs that have proven helpful in reducing adverse outcomes in both VA and non-VA settings will be useful to implement.41-45 Such programs could be located in the VA, and a care coordinator role could help facilitate transitional care needs for veterans by working with multiple non-VA hospitals.
The limitations of this study are that the perspectives shared by these participants may not represent all VA and non-VA hospitals as well as veterans’ experiences with transition of care. In addition, the study was conducted in one state and the findings may not be applicable to other healthcare systems. However, our study highlighted the consistent challenges of receiving care across VA and other hospital systems. Two strengths of this study are that it was conducted by multidisciplinary research team members with expertise in qualitative research, clinical care, and implementation science and that we obtained convergent information from VA, non-VA, and veteran participants.
Our current transition-of-care process has several shortcomings. There was a clear agreement on barriers, facilitators, and suggestions for improving the current transitions-of-care process among VA and non-VA hospital participants, as well as from veterans who experienced transitions across different delivery systems. Transitioning veterans to VA primary care following a non-VA hospitalization is a crucial first step for improving care for veterans and reducing adverse outcomes such as avoidable hospital readmissions and death.
These results describe the inefficiencies experienced by patients, clinicians, and staff and their suggestions to alleviate these barriers for optimal continuum of care. To avoid frustration and inefficiencies, the increased emphasis of providing non-VA care for veterans should consider the challenges experienced in transitional care and the opportunities for increased coordination of care.
1. Borowsky SJ, Cowper DC. Dual use of VA and non-VA primary care. J Gen Intern Med. 1999;14(5):274-280. https://doi.org/10.1046/j.1525-1497.1999.00335.x.
2. Charlton ME, Mengeling MA, Schlichting JA, et al. Veteran use of health care systems in rural states. Comparing VA and Non-VA health care use among privately insured veterans under age 65. J Rural Health. 2016;32(4):407-417. https://doi.org/10.1111/jrh.12206.
3. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161. https://doi.org/10.7326/0003-4819-138-3-200302040-00007.
4. Nguyen KA, Haggstrom DA, Ofner S, et al. Medication use among veterans across health care systems. Appl Clin Inform. 2017;26(1):235-249. https://doi.org/10.4338/ACI-2016-10-RA-0184.
5. Nayar P, Apenteng B, Yu F, Woodbridge P, Fetrick A. Rural veterans’ perspectives of dual care. J Commun Health. 2013;38(1):70-77. https://doi.org/10.1007/s10900-012-9583-7.
6. West AN, Charlton ME. Insured veterans’ use of VA and Non-VA health care in a rural state. J Rural Health. 2016;32(4):387-396. https://doi.org/10.1111/jrh.12196.
7. Gellad WF. The veterans choice act and dual health system use. J Gen Intern Med. 2016;31(2):153-154. https://doi.org/10.1007/s11606-015-3492-2.
8. Axon RN, Gebregziabher M, Everett CJ, Heidenreich P, Hunt KJ. Dual health care system use is associated with higher rates of hospitalization and hospital readmission among veterans with heart failure. Am Heart J. 2016;174:157-163. https://doi.org/10.1016/j.ahj.2015.09.023.
9. Humensky J, Carretta H, de Groot K, et al. Service utilization of veterans dually eligible for VA and medicare fee-for-service: 1999–2004. Medicare Medicaid Res Rev. 2012;2(3). https://doi.org/10.5600/mmrr.002.03.A06.
10. West AN, Charlton ME, Vaughan-Sarrazin M. Dual use of VA and non-VA hospitals by veterans with multiple hospitalizations. BMC Health Serv Res. 2015;15(1):431. https://doi.org/10.1186/s12913-015-1069-8.
11. Gaglioti A, Cozad A, Wittrock S, et al. Non-VA primary care providers’ perspectives on comanagement for rural veterans. Mil Med. 2014;179(11):1236-1243. https://doi.org/10.7205/MILMED-D-13-00342.
12. Department of Veterans Affairs. Expanded access to non-VA care through the veterans choice program. Final rule. Fed Regist. 2018;83(92):21893-21897.
13. Shuster B. Text-H.R.3236-114th Congress. Surface Transportation and Veterans Health Care Choice Improvement Act of 2015.. https://www.congress.gov/bill/114th-congress/house-bill/3236/text/pl. Accessed April 16, 2017; 2015-2016.
14. Veterans Affairs Mission Act. MISSIONAct.va.gov Available at. https://missionact.va.gov/. Accessed August 9, 2019.
15. Veterans Choice Program (VCP). Community care. https://www.va.gov/COMMUNITYCARE/programs/veterans/VCP/index.asp. Accessed August 9, 2019.
16. A Decade of Transitional Care Research with Vulnerable Elder… : journal of cardiovascular nursing. LWW. http://journals.lww.com/jcnjournal/Fulltext/2000/04000/A_Decade_of_Transitional_Care_Research_with.4.aspx. Accessed April 16, 2017.
17. Coleman EA, Boult C. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51(4):556-557. https://doi.org/10.1046/j.1532-5415.2003.51186.x.
18. Krichbaum K. GAPN postacute care coordination improves hip fracture outcomes. West J Nurs Res. 2007;29(5):523-544. https://doi.org/10.1177/0193945906293817.
19. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. https://doi.org/10.1002/jhm.228.
20. Coleman EA, Mahoney E, Parry C. Assessing the quality of preparation for posthospital care from the patient’s perspective: the care transitions measure. Med Care. 2005;43(3):246-255. https://doi.org/10.1097/00005650-200503000-00007.
21. Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The importance of transitional care in achieving health reform. Health Aff (Millwood). 2011;30(4):746-754. https://doi.org/10.1377/hlthaff.2011.0041.
22. Naylor MD, Brooten DA, Campbell RL, et al. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675-684. https://doi.org/10.1111/j.1532-5415.2004.52202.x.
23. Snow V, Beck D, Budnitz T, et al. Transitions of care consensus policy statement: American College of Physicians, Society of General Internal Medicine, society of hospital medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364-370. https://doi.org/10.1002/jhm.510.
24. Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549-555. https://doi.org/10.1046/j.1532-5415.2003.51185.x.
25. Feldstein AC, Glasgow RE. A practical, robust implementation and sustainability model (PRISM) for integrating research findings into practice. Jt Comm J Qual Patient Saf. 2008;34(4):228-243. https://doi.org/10.1016/S1553-7250(08)34030-6.
26. Schweikhart SA, Dembe AE. The applicability of lean and six sigma techniques to clinical and translational research. J Investig Med. 2009;57(7):748-755. https://doi.org/10.2310/JIM.0b013e3181b91b3a.
27. Burke RE, Kripalani S, Vasilevskis EE, Schnipper JL. Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102-109. https://doi.org/10.1002/jhm.1990.
28. Patient Aligned Care Team (PACT)-Patient Care. Services. https://www.patientcare.va.gov/primarycare/PACT.asp. Accessed November 20, 2017.
29. Morse JM. Critical analysis of strategies for determining rigor in qualitative inquiry. Qual Health Res. 2015;25(9):1212-1222. https://doi.org/10.1177/1049732315588501.
30. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288. https://doi.org/10.1177/1049732305276687.
31. Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. Int J Qual Methods. 2006;5(1):80-92. https://doi.org/10.1177/160940690600500107.
32. Ayele RA, Lawrence E, McCreight M, et al. Study protocol: improving the transition of care from a non-network hospital back to the patient’s medical home. BMC Health Serv Res. 2017;17(1):123. https://doi.org/10.1186/s12913-017-2048-z.
33. Burke RE, Kripalani S, Vasilevskis EE, Schnipper JL. Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102-109. https://doi.org/10.1002/jhm.1990.
34. Qualitative research & evaluation methods. https://us.sagepub.com/en-us/nam/qualitative-research-evaluation-methods/book232962. Accessed April 16, 2017. SAGE Publications Inc.
35. Curry LA, Nembhard IM, Bradley EH. Qualitative and mixed methods provide unique contributions to outcomes research. Circulation. 2009;119(10):1442-1452. https://doi.org/10.1161/CIRCULATIONAHA.107.742775.
36. Creswell JW, Hanson WE, Clark Plano VL, Morales A. Qualitative research designs: selection and implementation. Couns Psychol. 2007;35(2):236-264. https://doi.org/10.1177/0011000006287390.
37. Carter N, Bryant-Lukosius D, DiCenso A, Blythe J, Neville AJ. The use of triangulation in qualitative research. Oncol Nurs Forum. 2014;41(5):545-547. https://doi.org/10.1188/14.ONF.545-547.
38. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. https://doi.org/10.1056/NEJMp1212324.
39. Improving Care Transitions. Health affairs-health policy briefs. http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=76. Accessed August 13, 2016.
40. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-576. https://doi.org/10.1370/afm.1713.
41. Burke RE, Kelley L, Gunzburger E, et al. Improving transitions of care for veterans transferred to tertiary VA medical centers. Am J Med Qual. 2018;33(2):147-153. https://doi.org/10.1177/1062860617715508.
42. Capp R, Misky GJ, Lindrooth RC, et al. Coordination program reduced acute care use and increased primary care visits among frequent emergency care users. Health Aff (Millwood). 2017;36(10):1705-1711. https://doi.org/10.1377/hlthaff.2017.0612.
43. Kind AJH, Brenny-Fitzpatrick M, Leahy-Gross K, et al. Harnessing protocolized adaptation in dissemination: successful implementation and sustainment of the veterans affairs coordinated-transitional care program in a non-veterans affairs hospital. J Am Geriatr Soc. 2016;64(2):409-416. https://doi.org/10.1111/jgs.13935.
44. Kind AJH, Jensen L, Barczi S, et al. Low-cost transitional care with nurse managers making mostly phone contact With patients cut rehospitalization at a VA Hospital. Health Aff. 2012;31(12):2659-2668. https://doi.org/10.1377/hlthaff.2012.0366.
45. Reese RL, Clement SA, Syeda S, et al. Coordinated-transitional care for veterans with heart failure and chronic lung disease. J Am Geriatr Soc. 2019;67(7):1502-1507. https://doi.org/10.1111/jgs.15978.
As the VA moves toward increased utilization of non-VA care, it is crucial to understand and address the challenges of transitional care faced by dual-use veterans to provide high-quality care that improves healthcare outcomes.7,11,12 The VA implemented a shift in policy from the Veterans Access, Choice, and Accountability Act of 2014 (Public Law 113-146; “Choice Act”) to the VA Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act beginning June 6, 2019.13,14 Under the MISSION Act, veterans have more ways to access healthcare within the VA’s network and through approved non-VA medical providers in the community known as “community care providers.”15 This shift expanded the existing VA Choice Act of 2014, where the program allowed those veterans who are unable to schedule an appointment within 30 days of their preferred date or the clinically appropriate date, or on the basis of their place of residence, to elect to receive care from eligible non-VA healthcare entities or providers.14,15 These efforts to better serve veterans by increasing non-VA care might present added care coordination challenges for patients and their providers when they seek care in the VA.
High-quality transitional care prevents poor outcomes such as hospital readmissions.16-18 When communication and coordination across healthcare delivery systems are lacking, patients and their families often find themselves at risk for adverse events.19,20 Past research shows that patients have fewer adverse events when they receive comprehensive postdischarge care, including instructions on medications and self-care, symptom recognition and management, and reminders to attend follow-up appointments.17,21,22 Although researchers have identified the components of effective transitional care,23 barriers persist. The communication and collaboration needed to provide coordinated care across healthcare delivery systems are difficult due to the lack of standardized approaches between systems.24 Consequently, follow-up care may be delayed or missed altogether. To our knowledge, there is no published research identifying transitional care challenges for clinicians, staff, and veterans in transitioning from non-VA hospitals to a VA primary care setting.
The objective of this quality assessment was to understand VA and non-VA hospital clinicians’ and staff as well as veterans’ perspectives of the barriers and facilitators to providing high-quality transitional care.
METHODS
Study Design
We conducted a qualitative assessment within the VA Eastern Colorado Health Care System, an urban tertiary medical center, as well as urban and rural non-VA hospitals used by veterans. Semi-structured interview guides informed by the practical robust implementation and sustainability (PRISM) model, the Lean approach, and the Ideal Transitions of Care Bridge were used.25-27 We explored the PRISM domains such as recipient’s characteristics, the interaction with the external environment, and the implementation and sustainability infrastructure to inform the design and implementation of the intervention.25 The Lean approach included methods to optimize processes by maximizing efficiency and minimizing waste.26 The Ideal Transitions of Care Bridge was used to identify the domains in transitions of care such as discharge planning, communication of information, and care coordination.27
Setting and Participants
We identified the top 10 non-VA hospitals serving the most urban and rural veterans in 2015 using VA administrative data. Purposive sampling was used to ensure that urban and rural non-VA hospitals and different roles within these hospitals were represented. VA clinicians and staff were selected from the Denver VA Medical Center, a tertiary hospital within the Eastern Colorado Health Care System and one VA Community-Based Outpatient Clinic (CBOC) that primarily serves rural veterans. The Denver VA Medical Center has three clinics staffed by Patient Aligned Care Teams (PACTs), a model built on the concept of Patient-Centered Medical Home.28 Hospital leadership were initially approached for permission to recruit their staff and to be involved as key informants, and all agreed. To ensure representativeness, diversity of roles was recruited, including PACT primary care physicians, nurses, and other staff members such as medical assistants and administrators. Veterans were approached for sampling if they were discharged from a non-VA hospital during June–September 2015 and used the VA for primary care. This was to ensure that they remembered the process they went through postdischarge at the time of the interview.
Data Collection and Analysis
The evaluation team members (RA, EL, and MM) conducted the interviews from November 2015 to July 2016. Clinicians, staff, and veterans were asked semi-structured questions about their experiences and their role in transitioning VA patients across systems (see Appendix for interview guides). Veterans were asked to describe their experience and satisfaction with the current postdischarge transition process. We stopped the interviews when we reached data saturation.29
Interviews were audio-recorded, transcribed verbatim, and validated (transcribed interviews were double-checked against recording) to ensure data quality and accuracy. Coding was guided by a conventional content analysis technique30, 31 using a deductive and inductive coding approach.31 The deductive coding approach was drawn from the Ideal Transitions of Care Bridge and PRISM domains. 32,33 Two evaluation team members (RA and EL) defined the initial code book by independently coding the first three interviews, worked to clarify the meanings of emergent codes, and came to a consensus when disagreements occurred. Next, a priori codes were added by team members to include the PRISM domains. These PRISM domains included the implementation and sustainability infrastructure, the external environment, the characteristics of intervention recipients, and the organizational and patient perspectives of an intervention.
Additional emergent codes were added to the code book and agreed upon by team members (RA, EL, and MM). Consistent with previously used methods, consensus building was achieved by identifying and resolving differences by discussing with team members (RA, EL, MM, CB, and RB).29 Codes were examined and organized into themes by team members.29,34-36 This process was continued until no new themes were identified. Results were reviewed by all evaluation team members to assess thoroughness and comprehensiveness.34,35 In addition, team members triangulated the findings with VA and non-VA participants to ensure validity and reduce researcher bias.29,37
RESULTS
We conducted a total of 70 interviews with 23 VA and 29 non-VA hospital clinicians and staff and 18 veterans (Table 1). Overall, we found that there was no standardized process for transitioning veterans across healthcare delivery systems. Participants reported that transitions were often inefficient when non-VA hospitals could not (1) identify patients as veterans and notify VA primary care of discharge; (2) transfer non-VA hospital medical records to VA primary care; (3) obtain follow-up care appointments with VA primary care; and (4) write VA formulary medications for veterans to fill at VA pharmacies. In addition, participants discussed about facilitators and suggestions to overcome these inefficiencies and improve transitional care (Table2). We mapped the identified barriers as well as the suggestions for improvement to the PRISM and the Ideal Transitions of Care Bridge domains (Table 3).
Unable to Identify Patients as Veterans and Notify VA Primary Care of Discharge
VA and non-VA participants reported difficulty in communicating about veterans’ hospitalizations and discharge follow-up needs across systems. Non-VA clinicians referenced difficulty in identifying patients as veterans to communicate with VA, except in instances where the VA is a payor, while VA providers described feeling largely uninformed of the veterans non-VA hospitalization. For non-VA clinicians, the lack of a systematic method for veteran identification often left them to inadvertently identify veteran status by asking about their primary care clinicians and insurance and even through an offhanded comment made by the veteran. If a veteran was identified, non-VA clinicians described being uncertain about the best way to notify VA primary care of the patient’s impending discharge. Veterans described instances of the non-VA hospital knowing their veteran status upon admission, but accounts varied on whether the non-VA hospital notified the VA primary care of their hospitalization (Table 2, Theme 1).
Unable to Transfer Non-VA Hospital Medical Records to VA Primary Care
VA clinicians discussed about the challenges associated with obtaining the veteran’s medical record from the non-VA hospitals, and when it was received, it was often incomplete information and significantly delayed. They described relying on the veteran’s description of the care received, which was not complete or accurate information needed to make clinical judgment or coordinate follow-up care. Non-VA clinicians mentioned about trying several methods for transferring the medical record to VA primary care, including discharge summary via electronic system and sometimes solely relying on patients to deliver discharge paperwork to their primary care clinicians. In instances where non-VA hospitals sent discharge paperwork to VA, there was no way for non-VA hospitals to verify whether the faxed electronic medical record was received by the VA hospital. Most of the veterans discussed receiving written postdischarge instructions to take to their VA primary care clinicians; however, they were unsure whether the VA primary care received their medical record or any other information from the non-VA hospital (Table 2, Theme 2).
Unable to Obtain Follow-Up Care Appointments with VA Primary Care
All participants described how difficult it was to obtain a follow-up appointment for veterans with VA primary care. This often resulted in delayed follow-up care. VA clinicians also shared that a non-VA hospitalization can be the impetus for a veteran to seek care at the VA for the very first time. Once eligibility is determined, the veteran is assigned a VA primary care clinician. This process may take up to six weeks, and in the meantime, the veteran is scheduled in VA urgent care for immediate postdischarge care. This lag in primary care assignment creates delayed and fragmented care (Table 2, Theme 3).
Non-VA clinicians, administrators, and staff also discussed the difficulties in scheduling follow-up care with VA primary care. Although discharge paperwork instructed patients to see their VA clinicians, there was no process in place for non-VA clinicians to confirm whether the follow-up care was received due to lack of bilateral communication. In addition, veterans discussed the inefficiencies in scheduling follow-up appointments with VA clinicians where attempts to follow-up with primary care clinicians took eight weeks or more. Several veterans described walking into the clinic without an appointment asking to be seen postdischarge or utilizing the VA emergency department for follow-up care after discharge from a non-VA hospital. Veterans admitted utilizing the VA emergency department for nonemergent reasons such as filling their prescriptions because they are unable to see a VA PCP in a timely manner (Table 2, Theme 3).
Unable to Write VA Formulary Medications for Veterans to Fill at VA Pharmacies
All participants described the difficulties in obtaining medications at VA pharmacies when prescribed by the non-VA hospital clinicians. VA clinicians often had to reassess, and rewrite prescriptions written by clinicians, causing delays. Moreover, rural VA clinicians described lack of VA pharmacies in their locations, where veterans had to mail order medications, causing further delays in needed medications. Non-VA clinicians echoed these frustrations. They noted that veterans were confused about their VA pharmacy benefits as well as the need for the non-VA clinicians to follow VA formulary guidelines. Veterans expressed that it was especially challenging to physically go to the VA pharmacy to pick up medications after discharge due to lack of transportation, limited VA pharmacy hours, and long wait times. Several veterans discussed paying for their prescriptions out of pocket even though they had VA pharmacy benefits because it was more convenient to use the non-VA pharmacy. In other instances, veterans discussed going to a VA emergency department and waiting for hours to have their non-VA clinician prescription rewritten by a VA clinician (Table 2, Theme 4).
Facilitators of the Current Transition Process
Several participants provided examples of when transitional care communication between systems occurred seamlessly. VA staff and veterans noted that the VA increased the availability of urgent care appointments, which allowed for timelier postacute care follow-up appointments. Non-VA hospital clinicians also noted the availability of additional appointment slots but stated that they did not learn about these additional appointments directly from the VA. Instead, they learned of these through medical residents caring for patients at both VA and non-VA hospitals. One VA CBOC designated two nurses to care for walk-in veterans for their postdischarge follow-up needs. Some VA participants also noted that the VA Call Center Nurses occasionally called veterans upon discharge to schedule a follow-up appointment and facilitated timely care.
Participants from a VA CBOC discussed being part of a Community Transitions Consortium aimed at identifying high-utilizing patients (veteran and nonveteran) and improving communication across systems. The consortium members discussed each facility’s transition-of-care process, described having access to local non-VA hospital medical records and a backline phone number at the non-VA hospitals to coordinate transitional care. This allowed the VA clinicians to learn about non-VA hospital processes and veteran needs.
Suggestions for Improving the Transitional Care Process
VA and non-VA clinicians suggested hiring a VA liaison, preferably with a clinical background to facilitate care coordination across healthcare systems. They recommended that this person work closely with VA primary care, strengthen the relationship with non-VA hospitals, and help veterans learn more about the transition-of-care processes. Topics discussed for veteran education included how to (1) access their primary care tea
Veterans agreed that improvements to the current process should include an efficient system for obtaining medications and the ability to schedule timely follow-up appointments. Furthermore, veterans wanted education about the VA transition-of-care process following a non-VA hospitalization, including payment and VA notification processes (Table 2, Theme 5).
DISCUSSION
Participants described the current transitional care process as inefficient with specific barriers that have negative consequences on patient care and clinician and staff work processes. They described difficulties in obtaining medications prescribed by non-VA clinicians from VA pharmacies, delays in follow-up appointments at the VA, and lack of bilateral communication between systems and medical record transfer. Participants also provided concrete suggestions to improving the current process, including a care coordinator with clinical background. These findings are important in the context of VA increasing veteran access to care in the community.
Despite an increasing emphasis on veteran access to non-VA care as a result of the VA strategic goals and several new programs,7,12,13 there has not been a close examination of the current transition-of-care process from non-VA hospitals to VA primary care. Several studies have shown that the period following a hospitalization is especially vulnerable and associated with adverse events such as readmission, high cost, and death.12,31,32 Our findings agree with previous research that identified medical record transfer across systems as one of the most challenging issues contributing to deficits in communication between care teams.33 In addition, our study brought into focus the significant challenges faced by veterans in obtaining medications post non-VA hospital discharge. Addressing these key barriers in transitional care will improve the quality, safety, and value of healthcare in the current transition process.38,39
Based on our findings, our participants’ concern in transitional care can be addressed in various ways. First, as veterans are increasingly receiving care in the community, identifying their veteran status early on in the non-VA hospital setting could help in improved, real time communication with the VA. This could be done by updating patient intake forms to ask patients whether they are veterans or not. Second, VA policy-level changes should work to provide veterans access to non-VA pharmacy benefits equivalent to the access patients are receiving for hospital, specialty, and outpatient care. Third, patient and provider satisfaction for dual-use veterans should be examined closely. Although participants expressed frustration with the overall transitions of care from non-VA hospitals to VA primary care setting, influence of this on the Quadruple Aim-improving patient outcomes, experience, and reducing clinician and staff burnout should be examined closely.40 Fourth, evidence-based interventions such as nurse-led transitional care programs that have proven helpful in reducing adverse outcomes in both VA and non-VA settings will be useful to implement.41-45 Such programs could be located in the VA, and a care coordinator role could help facilitate transitional care needs for veterans by working with multiple non-VA hospitals.
The limitations of this study are that the perspectives shared by these participants may not represent all VA and non-VA hospitals as well as veterans’ experiences with transition of care. In addition, the study was conducted in one state and the findings may not be applicable to other healthcare systems. However, our study highlighted the consistent challenges of receiving care across VA and other hospital systems. Two strengths of this study are that it was conducted by multidisciplinary research team members with expertise in qualitative research, clinical care, and implementation science and that we obtained convergent information from VA, non-VA, and veteran participants.
Our current transition-of-care process has several shortcomings. There was a clear agreement on barriers, facilitators, and suggestions for improving the current transitions-of-care process among VA and non-VA hospital participants, as well as from veterans who experienced transitions across different delivery systems. Transitioning veterans to VA primary care following a non-VA hospitalization is a crucial first step for improving care for veterans and reducing adverse outcomes such as avoidable hospital readmissions and death.
These results describe the inefficiencies experienced by patients, clinicians, and staff and their suggestions to alleviate these barriers for optimal continuum of care. To avoid frustration and inefficiencies, the increased emphasis of providing non-VA care for veterans should consider the challenges experienced in transitional care and the opportunities for increased coordination of care.
As the VA moves toward increased utilization of non-VA care, it is crucial to understand and address the challenges of transitional care faced by dual-use veterans to provide high-quality care that improves healthcare outcomes.7,11,12 The VA implemented a shift in policy from the Veterans Access, Choice, and Accountability Act of 2014 (Public Law 113-146; “Choice Act”) to the VA Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act beginning June 6, 2019.13,14 Under the MISSION Act, veterans have more ways to access healthcare within the VA’s network and through approved non-VA medical providers in the community known as “community care providers.”15 This shift expanded the existing VA Choice Act of 2014, where the program allowed those veterans who are unable to schedule an appointment within 30 days of their preferred date or the clinically appropriate date, or on the basis of their place of residence, to elect to receive care from eligible non-VA healthcare entities or providers.14,15 These efforts to better serve veterans by increasing non-VA care might present added care coordination challenges for patients and their providers when they seek care in the VA.
High-quality transitional care prevents poor outcomes such as hospital readmissions.16-18 When communication and coordination across healthcare delivery systems are lacking, patients and their families often find themselves at risk for adverse events.19,20 Past research shows that patients have fewer adverse events when they receive comprehensive postdischarge care, including instructions on medications and self-care, symptom recognition and management, and reminders to attend follow-up appointments.17,21,22 Although researchers have identified the components of effective transitional care,23 barriers persist. The communication and collaboration needed to provide coordinated care across healthcare delivery systems are difficult due to the lack of standardized approaches between systems.24 Consequently, follow-up care may be delayed or missed altogether. To our knowledge, there is no published research identifying transitional care challenges for clinicians, staff, and veterans in transitioning from non-VA hospitals to a VA primary care setting.
The objective of this quality assessment was to understand VA and non-VA hospital clinicians’ and staff as well as veterans’ perspectives of the barriers and facilitators to providing high-quality transitional care.
METHODS
Study Design
We conducted a qualitative assessment within the VA Eastern Colorado Health Care System, an urban tertiary medical center, as well as urban and rural non-VA hospitals used by veterans. Semi-structured interview guides informed by the practical robust implementation and sustainability (PRISM) model, the Lean approach, and the Ideal Transitions of Care Bridge were used.25-27 We explored the PRISM domains such as recipient’s characteristics, the interaction with the external environment, and the implementation and sustainability infrastructure to inform the design and implementation of the intervention.25 The Lean approach included methods to optimize processes by maximizing efficiency and minimizing waste.26 The Ideal Transitions of Care Bridge was used to identify the domains in transitions of care such as discharge planning, communication of information, and care coordination.27
Setting and Participants
We identified the top 10 non-VA hospitals serving the most urban and rural veterans in 2015 using VA administrative data. Purposive sampling was used to ensure that urban and rural non-VA hospitals and different roles within these hospitals were represented. VA clinicians and staff were selected from the Denver VA Medical Center, a tertiary hospital within the Eastern Colorado Health Care System and one VA Community-Based Outpatient Clinic (CBOC) that primarily serves rural veterans. The Denver VA Medical Center has three clinics staffed by Patient Aligned Care Teams (PACTs), a model built on the concept of Patient-Centered Medical Home.28 Hospital leadership were initially approached for permission to recruit their staff and to be involved as key informants, and all agreed. To ensure representativeness, diversity of roles was recruited, including PACT primary care physicians, nurses, and other staff members such as medical assistants and administrators. Veterans were approached for sampling if they were discharged from a non-VA hospital during June–September 2015 and used the VA for primary care. This was to ensure that they remembered the process they went through postdischarge at the time of the interview.
Data Collection and Analysis
The evaluation team members (RA, EL, and MM) conducted the interviews from November 2015 to July 2016. Clinicians, staff, and veterans were asked semi-structured questions about their experiences and their role in transitioning VA patients across systems (see Appendix for interview guides). Veterans were asked to describe their experience and satisfaction with the current postdischarge transition process. We stopped the interviews when we reached data saturation.29
Interviews were audio-recorded, transcribed verbatim, and validated (transcribed interviews were double-checked against recording) to ensure data quality and accuracy. Coding was guided by a conventional content analysis technique30, 31 using a deductive and inductive coding approach.31 The deductive coding approach was drawn from the Ideal Transitions of Care Bridge and PRISM domains. 32,33 Two evaluation team members (RA and EL) defined the initial code book by independently coding the first three interviews, worked to clarify the meanings of emergent codes, and came to a consensus when disagreements occurred. Next, a priori codes were added by team members to include the PRISM domains. These PRISM domains included the implementation and sustainability infrastructure, the external environment, the characteristics of intervention recipients, and the organizational and patient perspectives of an intervention.
Additional emergent codes were added to the code book and agreed upon by team members (RA, EL, and MM). Consistent with previously used methods, consensus building was achieved by identifying and resolving differences by discussing with team members (RA, EL, MM, CB, and RB).29 Codes were examined and organized into themes by team members.29,34-36 This process was continued until no new themes were identified. Results were reviewed by all evaluation team members to assess thoroughness and comprehensiveness.34,35 In addition, team members triangulated the findings with VA and non-VA participants to ensure validity and reduce researcher bias.29,37
RESULTS
We conducted a total of 70 interviews with 23 VA and 29 non-VA hospital clinicians and staff and 18 veterans (Table 1). Overall, we found that there was no standardized process for transitioning veterans across healthcare delivery systems. Participants reported that transitions were often inefficient when non-VA hospitals could not (1) identify patients as veterans and notify VA primary care of discharge; (2) transfer non-VA hospital medical records to VA primary care; (3) obtain follow-up care appointments with VA primary care; and (4) write VA formulary medications for veterans to fill at VA pharmacies. In addition, participants discussed about facilitators and suggestions to overcome these inefficiencies and improve transitional care (Table2). We mapped the identified barriers as well as the suggestions for improvement to the PRISM and the Ideal Transitions of Care Bridge domains (Table 3).
Unable to Identify Patients as Veterans and Notify VA Primary Care of Discharge
VA and non-VA participants reported difficulty in communicating about veterans’ hospitalizations and discharge follow-up needs across systems. Non-VA clinicians referenced difficulty in identifying patients as veterans to communicate with VA, except in instances where the VA is a payor, while VA providers described feeling largely uninformed of the veterans non-VA hospitalization. For non-VA clinicians, the lack of a systematic method for veteran identification often left them to inadvertently identify veteran status by asking about their primary care clinicians and insurance and even through an offhanded comment made by the veteran. If a veteran was identified, non-VA clinicians described being uncertain about the best way to notify VA primary care of the patient’s impending discharge. Veterans described instances of the non-VA hospital knowing their veteran status upon admission, but accounts varied on whether the non-VA hospital notified the VA primary care of their hospitalization (Table 2, Theme 1).
Unable to Transfer Non-VA Hospital Medical Records to VA Primary Care
VA clinicians discussed about the challenges associated with obtaining the veteran’s medical record from the non-VA hospitals, and when it was received, it was often incomplete information and significantly delayed. They described relying on the veteran’s description of the care received, which was not complete or accurate information needed to make clinical judgment or coordinate follow-up care. Non-VA clinicians mentioned about trying several methods for transferring the medical record to VA primary care, including discharge summary via electronic system and sometimes solely relying on patients to deliver discharge paperwork to their primary care clinicians. In instances where non-VA hospitals sent discharge paperwork to VA, there was no way for non-VA hospitals to verify whether the faxed electronic medical record was received by the VA hospital. Most of the veterans discussed receiving written postdischarge instructions to take to their VA primary care clinicians; however, they were unsure whether the VA primary care received their medical record or any other information from the non-VA hospital (Table 2, Theme 2).
Unable to Obtain Follow-Up Care Appointments with VA Primary Care
All participants described how difficult it was to obtain a follow-up appointment for veterans with VA primary care. This often resulted in delayed follow-up care. VA clinicians also shared that a non-VA hospitalization can be the impetus for a veteran to seek care at the VA for the very first time. Once eligibility is determined, the veteran is assigned a VA primary care clinician. This process may take up to six weeks, and in the meantime, the veteran is scheduled in VA urgent care for immediate postdischarge care. This lag in primary care assignment creates delayed and fragmented care (Table 2, Theme 3).
Non-VA clinicians, administrators, and staff also discussed the difficulties in scheduling follow-up care with VA primary care. Although discharge paperwork instructed patients to see their VA clinicians, there was no process in place for non-VA clinicians to confirm whether the follow-up care was received due to lack of bilateral communication. In addition, veterans discussed the inefficiencies in scheduling follow-up appointments with VA clinicians where attempts to follow-up with primary care clinicians took eight weeks or more. Several veterans described walking into the clinic without an appointment asking to be seen postdischarge or utilizing the VA emergency department for follow-up care after discharge from a non-VA hospital. Veterans admitted utilizing the VA emergency department for nonemergent reasons such as filling their prescriptions because they are unable to see a VA PCP in a timely manner (Table 2, Theme 3).
Unable to Write VA Formulary Medications for Veterans to Fill at VA Pharmacies
All participants described the difficulties in obtaining medications at VA pharmacies when prescribed by the non-VA hospital clinicians. VA clinicians often had to reassess, and rewrite prescriptions written by clinicians, causing delays. Moreover, rural VA clinicians described lack of VA pharmacies in their locations, where veterans had to mail order medications, causing further delays in needed medications. Non-VA clinicians echoed these frustrations. They noted that veterans were confused about their VA pharmacy benefits as well as the need for the non-VA clinicians to follow VA formulary guidelines. Veterans expressed that it was especially challenging to physically go to the VA pharmacy to pick up medications after discharge due to lack of transportation, limited VA pharmacy hours, and long wait times. Several veterans discussed paying for their prescriptions out of pocket even though they had VA pharmacy benefits because it was more convenient to use the non-VA pharmacy. In other instances, veterans discussed going to a VA emergency department and waiting for hours to have their non-VA clinician prescription rewritten by a VA clinician (Table 2, Theme 4).
Facilitators of the Current Transition Process
Several participants provided examples of when transitional care communication between systems occurred seamlessly. VA staff and veterans noted that the VA increased the availability of urgent care appointments, which allowed for timelier postacute care follow-up appointments. Non-VA hospital clinicians also noted the availability of additional appointment slots but stated that they did not learn about these additional appointments directly from the VA. Instead, they learned of these through medical residents caring for patients at both VA and non-VA hospitals. One VA CBOC designated two nurses to care for walk-in veterans for their postdischarge follow-up needs. Some VA participants also noted that the VA Call Center Nurses occasionally called veterans upon discharge to schedule a follow-up appointment and facilitated timely care.
Participants from a VA CBOC discussed being part of a Community Transitions Consortium aimed at identifying high-utilizing patients (veteran and nonveteran) and improving communication across systems. The consortium members discussed each facility’s transition-of-care process, described having access to local non-VA hospital medical records and a backline phone number at the non-VA hospitals to coordinate transitional care. This allowed the VA clinicians to learn about non-VA hospital processes and veteran needs.
Suggestions for Improving the Transitional Care Process
VA and non-VA clinicians suggested hiring a VA liaison, preferably with a clinical background to facilitate care coordination across healthcare systems. They recommended that this person work closely with VA primary care, strengthen the relationship with non-VA hospitals, and help veterans learn more about the transition-of-care processes. Topics discussed for veteran education included how to (1) access their primary care tea
Veterans agreed that improvements to the current process should include an efficient system for obtaining medications and the ability to schedule timely follow-up appointments. Furthermore, veterans wanted education about the VA transition-of-care process following a non-VA hospitalization, including payment and VA notification processes (Table 2, Theme 5).
DISCUSSION
Participants described the current transitional care process as inefficient with specific barriers that have negative consequences on patient care and clinician and staff work processes. They described difficulties in obtaining medications prescribed by non-VA clinicians from VA pharmacies, delays in follow-up appointments at the VA, and lack of bilateral communication between systems and medical record transfer. Participants also provided concrete suggestions to improving the current process, including a care coordinator with clinical background. These findings are important in the context of VA increasing veteran access to care in the community.
Despite an increasing emphasis on veteran access to non-VA care as a result of the VA strategic goals and several new programs,7,12,13 there has not been a close examination of the current transition-of-care process from non-VA hospitals to VA primary care. Several studies have shown that the period following a hospitalization is especially vulnerable and associated with adverse events such as readmission, high cost, and death.12,31,32 Our findings agree with previous research that identified medical record transfer across systems as one of the most challenging issues contributing to deficits in communication between care teams.33 In addition, our study brought into focus the significant challenges faced by veterans in obtaining medications post non-VA hospital discharge. Addressing these key barriers in transitional care will improve the quality, safety, and value of healthcare in the current transition process.38,39
Based on our findings, our participants’ concern in transitional care can be addressed in various ways. First, as veterans are increasingly receiving care in the community, identifying their veteran status early on in the non-VA hospital setting could help in improved, real time communication with the VA. This could be done by updating patient intake forms to ask patients whether they are veterans or not. Second, VA policy-level changes should work to provide veterans access to non-VA pharmacy benefits equivalent to the access patients are receiving for hospital, specialty, and outpatient care. Third, patient and provider satisfaction for dual-use veterans should be examined closely. Although participants expressed frustration with the overall transitions of care from non-VA hospitals to VA primary care setting, influence of this on the Quadruple Aim-improving patient outcomes, experience, and reducing clinician and staff burnout should be examined closely.40 Fourth, evidence-based interventions such as nurse-led transitional care programs that have proven helpful in reducing adverse outcomes in both VA and non-VA settings will be useful to implement.41-45 Such programs could be located in the VA, and a care coordinator role could help facilitate transitional care needs for veterans by working with multiple non-VA hospitals.
The limitations of this study are that the perspectives shared by these participants may not represent all VA and non-VA hospitals as well as veterans’ experiences with transition of care. In addition, the study was conducted in one state and the findings may not be applicable to other healthcare systems. However, our study highlighted the consistent challenges of receiving care across VA and other hospital systems. Two strengths of this study are that it was conducted by multidisciplinary research team members with expertise in qualitative research, clinical care, and implementation science and that we obtained convergent information from VA, non-VA, and veteran participants.
Our current transition-of-care process has several shortcomings. There was a clear agreement on barriers, facilitators, and suggestions for improving the current transitions-of-care process among VA and non-VA hospital participants, as well as from veterans who experienced transitions across different delivery systems. Transitioning veterans to VA primary care following a non-VA hospitalization is a crucial first step for improving care for veterans and reducing adverse outcomes such as avoidable hospital readmissions and death.
These results describe the inefficiencies experienced by patients, clinicians, and staff and their suggestions to alleviate these barriers for optimal continuum of care. To avoid frustration and inefficiencies, the increased emphasis of providing non-VA care for veterans should consider the challenges experienced in transitional care and the opportunities for increased coordination of care.
1. Borowsky SJ, Cowper DC. Dual use of VA and non-VA primary care. J Gen Intern Med. 1999;14(5):274-280. https://doi.org/10.1046/j.1525-1497.1999.00335.x.
2. Charlton ME, Mengeling MA, Schlichting JA, et al. Veteran use of health care systems in rural states. Comparing VA and Non-VA health care use among privately insured veterans under age 65. J Rural Health. 2016;32(4):407-417. https://doi.org/10.1111/jrh.12206.
3. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161. https://doi.org/10.7326/0003-4819-138-3-200302040-00007.
4. Nguyen KA, Haggstrom DA, Ofner S, et al. Medication use among veterans across health care systems. Appl Clin Inform. 2017;26(1):235-249. https://doi.org/10.4338/ACI-2016-10-RA-0184.
5. Nayar P, Apenteng B, Yu F, Woodbridge P, Fetrick A. Rural veterans’ perspectives of dual care. J Commun Health. 2013;38(1):70-77. https://doi.org/10.1007/s10900-012-9583-7.
6. West AN, Charlton ME. Insured veterans’ use of VA and Non-VA health care in a rural state. J Rural Health. 2016;32(4):387-396. https://doi.org/10.1111/jrh.12196.
7. Gellad WF. The veterans choice act and dual health system use. J Gen Intern Med. 2016;31(2):153-154. https://doi.org/10.1007/s11606-015-3492-2.
8. Axon RN, Gebregziabher M, Everett CJ, Heidenreich P, Hunt KJ. Dual health care system use is associated with higher rates of hospitalization and hospital readmission among veterans with heart failure. Am Heart J. 2016;174:157-163. https://doi.org/10.1016/j.ahj.2015.09.023.
9. Humensky J, Carretta H, de Groot K, et al. Service utilization of veterans dually eligible for VA and medicare fee-for-service: 1999–2004. Medicare Medicaid Res Rev. 2012;2(3). https://doi.org/10.5600/mmrr.002.03.A06.
10. West AN, Charlton ME, Vaughan-Sarrazin M. Dual use of VA and non-VA hospitals by veterans with multiple hospitalizations. BMC Health Serv Res. 2015;15(1):431. https://doi.org/10.1186/s12913-015-1069-8.
11. Gaglioti A, Cozad A, Wittrock S, et al. Non-VA primary care providers’ perspectives on comanagement for rural veterans. Mil Med. 2014;179(11):1236-1243. https://doi.org/10.7205/MILMED-D-13-00342.
12. Department of Veterans Affairs. Expanded access to non-VA care through the veterans choice program. Final rule. Fed Regist. 2018;83(92):21893-21897.
13. Shuster B. Text-H.R.3236-114th Congress. Surface Transportation and Veterans Health Care Choice Improvement Act of 2015.. https://www.congress.gov/bill/114th-congress/house-bill/3236/text/pl. Accessed April 16, 2017; 2015-2016.
14. Veterans Affairs Mission Act. MISSIONAct.va.gov Available at. https://missionact.va.gov/. Accessed August 9, 2019.
15. Veterans Choice Program (VCP). Community care. https://www.va.gov/COMMUNITYCARE/programs/veterans/VCP/index.asp. Accessed August 9, 2019.
16. A Decade of Transitional Care Research with Vulnerable Elder… : journal of cardiovascular nursing. LWW. http://journals.lww.com/jcnjournal/Fulltext/2000/04000/A_Decade_of_Transitional_Care_Research_with.4.aspx. Accessed April 16, 2017.
17. Coleman EA, Boult C. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51(4):556-557. https://doi.org/10.1046/j.1532-5415.2003.51186.x.
18. Krichbaum K. GAPN postacute care coordination improves hip fracture outcomes. West J Nurs Res. 2007;29(5):523-544. https://doi.org/10.1177/0193945906293817.
19. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. https://doi.org/10.1002/jhm.228.
20. Coleman EA, Mahoney E, Parry C. Assessing the quality of preparation for posthospital care from the patient’s perspective: the care transitions measure. Med Care. 2005;43(3):246-255. https://doi.org/10.1097/00005650-200503000-00007.
21. Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The importance of transitional care in achieving health reform. Health Aff (Millwood). 2011;30(4):746-754. https://doi.org/10.1377/hlthaff.2011.0041.
22. Naylor MD, Brooten DA, Campbell RL, et al. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675-684. https://doi.org/10.1111/j.1532-5415.2004.52202.x.
23. Snow V, Beck D, Budnitz T, et al. Transitions of care consensus policy statement: American College of Physicians, Society of General Internal Medicine, society of hospital medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364-370. https://doi.org/10.1002/jhm.510.
24. Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549-555. https://doi.org/10.1046/j.1532-5415.2003.51185.x.
25. Feldstein AC, Glasgow RE. A practical, robust implementation and sustainability model (PRISM) for integrating research findings into practice. Jt Comm J Qual Patient Saf. 2008;34(4):228-243. https://doi.org/10.1016/S1553-7250(08)34030-6.
26. Schweikhart SA, Dembe AE. The applicability of lean and six sigma techniques to clinical and translational research. J Investig Med. 2009;57(7):748-755. https://doi.org/10.2310/JIM.0b013e3181b91b3a.
27. Burke RE, Kripalani S, Vasilevskis EE, Schnipper JL. Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102-109. https://doi.org/10.1002/jhm.1990.
28. Patient Aligned Care Team (PACT)-Patient Care. Services. https://www.patientcare.va.gov/primarycare/PACT.asp. Accessed November 20, 2017.
29. Morse JM. Critical analysis of strategies for determining rigor in qualitative inquiry. Qual Health Res. 2015;25(9):1212-1222. https://doi.org/10.1177/1049732315588501.
30. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288. https://doi.org/10.1177/1049732305276687.
31. Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. Int J Qual Methods. 2006;5(1):80-92. https://doi.org/10.1177/160940690600500107.
32. Ayele RA, Lawrence E, McCreight M, et al. Study protocol: improving the transition of care from a non-network hospital back to the patient’s medical home. BMC Health Serv Res. 2017;17(1):123. https://doi.org/10.1186/s12913-017-2048-z.
33. Burke RE, Kripalani S, Vasilevskis EE, Schnipper JL. Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102-109. https://doi.org/10.1002/jhm.1990.
34. Qualitative research & evaluation methods. https://us.sagepub.com/en-us/nam/qualitative-research-evaluation-methods/book232962. Accessed April 16, 2017. SAGE Publications Inc.
35. Curry LA, Nembhard IM, Bradley EH. Qualitative and mixed methods provide unique contributions to outcomes research. Circulation. 2009;119(10):1442-1452. https://doi.org/10.1161/CIRCULATIONAHA.107.742775.
36. Creswell JW, Hanson WE, Clark Plano VL, Morales A. Qualitative research designs: selection and implementation. Couns Psychol. 2007;35(2):236-264. https://doi.org/10.1177/0011000006287390.
37. Carter N, Bryant-Lukosius D, DiCenso A, Blythe J, Neville AJ. The use of triangulation in qualitative research. Oncol Nurs Forum. 2014;41(5):545-547. https://doi.org/10.1188/14.ONF.545-547.
38. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. https://doi.org/10.1056/NEJMp1212324.
39. Improving Care Transitions. Health affairs-health policy briefs. http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=76. Accessed August 13, 2016.
40. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-576. https://doi.org/10.1370/afm.1713.
41. Burke RE, Kelley L, Gunzburger E, et al. Improving transitions of care for veterans transferred to tertiary VA medical centers. Am J Med Qual. 2018;33(2):147-153. https://doi.org/10.1177/1062860617715508.
42. Capp R, Misky GJ, Lindrooth RC, et al. Coordination program reduced acute care use and increased primary care visits among frequent emergency care users. Health Aff (Millwood). 2017;36(10):1705-1711. https://doi.org/10.1377/hlthaff.2017.0612.
43. Kind AJH, Brenny-Fitzpatrick M, Leahy-Gross K, et al. Harnessing protocolized adaptation in dissemination: successful implementation and sustainment of the veterans affairs coordinated-transitional care program in a non-veterans affairs hospital. J Am Geriatr Soc. 2016;64(2):409-416. https://doi.org/10.1111/jgs.13935.
44. Kind AJH, Jensen L, Barczi S, et al. Low-cost transitional care with nurse managers making mostly phone contact With patients cut rehospitalization at a VA Hospital. Health Aff. 2012;31(12):2659-2668. https://doi.org/10.1377/hlthaff.2012.0366.
45. Reese RL, Clement SA, Syeda S, et al. Coordinated-transitional care for veterans with heart failure and chronic lung disease. J Am Geriatr Soc. 2019;67(7):1502-1507. https://doi.org/10.1111/jgs.15978.
1. Borowsky SJ, Cowper DC. Dual use of VA and non-VA primary care. J Gen Intern Med. 1999;14(5):274-280. https://doi.org/10.1046/j.1525-1497.1999.00335.x.
2. Charlton ME, Mengeling MA, Schlichting JA, et al. Veteran use of health care systems in rural states. Comparing VA and Non-VA health care use among privately insured veterans under age 65. J Rural Health. 2016;32(4):407-417. https://doi.org/10.1111/jrh.12206.
3. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161. https://doi.org/10.7326/0003-4819-138-3-200302040-00007.
4. Nguyen KA, Haggstrom DA, Ofner S, et al. Medication use among veterans across health care systems. Appl Clin Inform. 2017;26(1):235-249. https://doi.org/10.4338/ACI-2016-10-RA-0184.
5. Nayar P, Apenteng B, Yu F, Woodbridge P, Fetrick A. Rural veterans’ perspectives of dual care. J Commun Health. 2013;38(1):70-77. https://doi.org/10.1007/s10900-012-9583-7.
6. West AN, Charlton ME. Insured veterans’ use of VA and Non-VA health care in a rural state. J Rural Health. 2016;32(4):387-396. https://doi.org/10.1111/jrh.12196.
7. Gellad WF. The veterans choice act and dual health system use. J Gen Intern Med. 2016;31(2):153-154. https://doi.org/10.1007/s11606-015-3492-2.
8. Axon RN, Gebregziabher M, Everett CJ, Heidenreich P, Hunt KJ. Dual health care system use is associated with higher rates of hospitalization and hospital readmission among veterans with heart failure. Am Heart J. 2016;174:157-163. https://doi.org/10.1016/j.ahj.2015.09.023.
9. Humensky J, Carretta H, de Groot K, et al. Service utilization of veterans dually eligible for VA and medicare fee-for-service: 1999–2004. Medicare Medicaid Res Rev. 2012;2(3). https://doi.org/10.5600/mmrr.002.03.A06.
10. West AN, Charlton ME, Vaughan-Sarrazin M. Dual use of VA and non-VA hospitals by veterans with multiple hospitalizations. BMC Health Serv Res. 2015;15(1):431. https://doi.org/10.1186/s12913-015-1069-8.
11. Gaglioti A, Cozad A, Wittrock S, et al. Non-VA primary care providers’ perspectives on comanagement for rural veterans. Mil Med. 2014;179(11):1236-1243. https://doi.org/10.7205/MILMED-D-13-00342.
12. Department of Veterans Affairs. Expanded access to non-VA care through the veterans choice program. Final rule. Fed Regist. 2018;83(92):21893-21897.
13. Shuster B. Text-H.R.3236-114th Congress. Surface Transportation and Veterans Health Care Choice Improvement Act of 2015.. https://www.congress.gov/bill/114th-congress/house-bill/3236/text/pl. Accessed April 16, 2017; 2015-2016.
14. Veterans Affairs Mission Act. MISSIONAct.va.gov Available at. https://missionact.va.gov/. Accessed August 9, 2019.
15. Veterans Choice Program (VCP). Community care. https://www.va.gov/COMMUNITYCARE/programs/veterans/VCP/index.asp. Accessed August 9, 2019.
16. A Decade of Transitional Care Research with Vulnerable Elder… : journal of cardiovascular nursing. LWW. http://journals.lww.com/jcnjournal/Fulltext/2000/04000/A_Decade_of_Transitional_Care_Research_with.4.aspx. Accessed April 16, 2017.
17. Coleman EA, Boult C. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51(4):556-557. https://doi.org/10.1046/j.1532-5415.2003.51186.x.
18. Krichbaum K. GAPN postacute care coordination improves hip fracture outcomes. West J Nurs Res. 2007;29(5):523-544. https://doi.org/10.1177/0193945906293817.
19. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. https://doi.org/10.1002/jhm.228.
20. Coleman EA, Mahoney E, Parry C. Assessing the quality of preparation for posthospital care from the patient’s perspective: the care transitions measure. Med Care. 2005;43(3):246-255. https://doi.org/10.1097/00005650-200503000-00007.
21. Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The importance of transitional care in achieving health reform. Health Aff (Millwood). 2011;30(4):746-754. https://doi.org/10.1377/hlthaff.2011.0041.
22. Naylor MD, Brooten DA, Campbell RL, et al. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675-684. https://doi.org/10.1111/j.1532-5415.2004.52202.x.
23. Snow V, Beck D, Budnitz T, et al. Transitions of care consensus policy statement: American College of Physicians, Society of General Internal Medicine, society of hospital medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364-370. https://doi.org/10.1002/jhm.510.
24. Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549-555. https://doi.org/10.1046/j.1532-5415.2003.51185.x.
25. Feldstein AC, Glasgow RE. A practical, robust implementation and sustainability model (PRISM) for integrating research findings into practice. Jt Comm J Qual Patient Saf. 2008;34(4):228-243. https://doi.org/10.1016/S1553-7250(08)34030-6.
26. Schweikhart SA, Dembe AE. The applicability of lean and six sigma techniques to clinical and translational research. J Investig Med. 2009;57(7):748-755. https://doi.org/10.2310/JIM.0b013e3181b91b3a.
27. Burke RE, Kripalani S, Vasilevskis EE, Schnipper JL. Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102-109. https://doi.org/10.1002/jhm.1990.
28. Patient Aligned Care Team (PACT)-Patient Care. Services. https://www.patientcare.va.gov/primarycare/PACT.asp. Accessed November 20, 2017.
29. Morse JM. Critical analysis of strategies for determining rigor in qualitative inquiry. Qual Health Res. 2015;25(9):1212-1222. https://doi.org/10.1177/1049732315588501.
30. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288. https://doi.org/10.1177/1049732305276687.
31. Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. Int J Qual Methods. 2006;5(1):80-92. https://doi.org/10.1177/160940690600500107.
32. Ayele RA, Lawrence E, McCreight M, et al. Study protocol: improving the transition of care from a non-network hospital back to the patient’s medical home. BMC Health Serv Res. 2017;17(1):123. https://doi.org/10.1186/s12913-017-2048-z.
33. Burke RE, Kripalani S, Vasilevskis EE, Schnipper JL. Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102-109. https://doi.org/10.1002/jhm.1990.
34. Qualitative research & evaluation methods. https://us.sagepub.com/en-us/nam/qualitative-research-evaluation-methods/book232962. Accessed April 16, 2017. SAGE Publications Inc.
35. Curry LA, Nembhard IM, Bradley EH. Qualitative and mixed methods provide unique contributions to outcomes research. Circulation. 2009;119(10):1442-1452. https://doi.org/10.1161/CIRCULATIONAHA.107.742775.
36. Creswell JW, Hanson WE, Clark Plano VL, Morales A. Qualitative research designs: selection and implementation. Couns Psychol. 2007;35(2):236-264. https://doi.org/10.1177/0011000006287390.
37. Carter N, Bryant-Lukosius D, DiCenso A, Blythe J, Neville AJ. The use of triangulation in qualitative research. Oncol Nurs Forum. 2014;41(5):545-547. https://doi.org/10.1188/14.ONF.545-547.
38. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. https://doi.org/10.1056/NEJMp1212324.
39. Improving Care Transitions. Health affairs-health policy briefs. http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=76. Accessed August 13, 2016.
40. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-576. https://doi.org/10.1370/afm.1713.
41. Burke RE, Kelley L, Gunzburger E, et al. Improving transitions of care for veterans transferred to tertiary VA medical centers. Am J Med Qual. 2018;33(2):147-153. https://doi.org/10.1177/1062860617715508.
42. Capp R, Misky GJ, Lindrooth RC, et al. Coordination program reduced acute care use and increased primary care visits among frequent emergency care users. Health Aff (Millwood). 2017;36(10):1705-1711. https://doi.org/10.1377/hlthaff.2017.0612.
43. Kind AJH, Brenny-Fitzpatrick M, Leahy-Gross K, et al. Harnessing protocolized adaptation in dissemination: successful implementation and sustainment of the veterans affairs coordinated-transitional care program in a non-veterans affairs hospital. J Am Geriatr Soc. 2016;64(2):409-416. https://doi.org/10.1111/jgs.13935.
44. Kind AJH, Jensen L, Barczi S, et al. Low-cost transitional care with nurse managers making mostly phone contact With patients cut rehospitalization at a VA Hospital. Health Aff. 2012;31(12):2659-2668. https://doi.org/10.1377/hlthaff.2012.0366.
45. Reese RL, Clement SA, Syeda S, et al. Coordinated-transitional care for veterans with heart failure and chronic lung disease. J Am Geriatr Soc. 2019;67(7):1502-1507. https://doi.org/10.1111/jgs.15978.
© 2020 Society of Hospital Medicine
Cognitive Biases Influence Decision-Making Regarding Postacute Care in a Skilled Nursing Facility
The combination of decreasing hospital lengths of stay and increasing age and comorbidity of the United States population is a principal driver of the increased use of postacute care in the US.1-3 Postacute care refers to care in long-term acute care hospitals, inpatient rehabilitation facilities, skilled nursing facilities (SNFs), and care provided by home health agencies after an acute hospitalization. In 2016, 43% of Medicare beneficiaries received postacute care after hospital discharge at the cost of $60 billion annually; nearly half of these received care in an SNF.4 Increasing recognition of the significant cost and poor outcomes of postacute care led to payment reforms, such as bundled payments, that incentivized less expensive forms of postacute care and improvements in outcomes.5-9 Early evaluations suggested that hospitals are sensitive to these reforms and responded by significantly decreasing SNF utilization.10,11 It remains unclear whether this was safe and effective.
In this context, increased attention to how hospital clinicians and hospitalized patients decide whether to use postacute care (and what form to use) is appropriate since the effect of payment reforms could negatively impact vulnerable populations of older adults without adequate protection.12 Suboptimal decision-making can drive both overuse and inappropriate underuse of this expensive medical resource. Initial evidence suggests that patients and clinicians are poorly equipped to make high-quality decisions about postacute care, with significant deficits in both the decision-making process and content.13-16 While these gaps are important to address, they may only be part of the problem. The fields of cognitive psychology and behavioral economics have revealed new insights into decision-making, demonstrating that people deviate from rational decision-making in predictable ways, termed decision heuristics, or cognitive biases.17 This growing field of research suggests heuristics or biases play important roles in decision-making and determining behavior, particularly in situations where there may be little information provided and the patient is stressed, tired, and ill—precisely like deciding on postacute care.18 However, it is currently unknown whether cognitive biases are at play when making hospital discharge decisions.
We sought to identify the most salient heuristics or cognitive biases patients may utilize when making decisions about postacute care at the end of their hospitalization and ways clinicians may contribute to these biases. The overall goal was to derive insights for improving postacute care decision-making.
METHODS
Study Design
We conducted a secondary analysis on interviews with hospital and SNF clinicians as well as patients and their caregivers who were either leaving the hospital for an SNF or newly arrived in an SNF from the hospital to understand if cognitive biases were present and how they manifested themselves in a real-world clinical context.19 These interviews were part of a larger qualitative study that sought to understand how clinicians, patients, and their caregivers made decisions about postacute care, particularly related to SNFs.13,14 This study represents the analysis of all our interviews, specifically examining decision-making bias. Participating sites, clinical roles, and both patient and caregiver characteristics (Table 1) in our cohort have been previously described.13,14
Analysis
We used a team-based approach to framework analysis, which has been used in other decision-making studies14, including those measuring cognitive bias.20 A limitation in cognitive bias research is the lack of a standardized list or categorization of cognitive biases. We reviewed prior systematic17,21 and narrative reviews18,22, as well as prior studies describing examples of cognitive biases playing a role in decision-making about therapy20 to construct a list of possible cognitive biases to evaluate and narrow these a priori to potential biases relevant to the decision about postacute care based on our prior work (Table 2).
We applied this framework to analyze transcripts through an iterative process of deductive coding and reviewing across four reviewers (ML, RA, AL, CL) and a hospitalist physician with expertise leading qualitative studies (REB).
Intercoder consensus was built through team discussion by resolving points of disagreement.23 Consistency of coding was regularly checked by having more than one investigator code individual manuscripts and comparing coding, and discrepancies were resolved through team discussion. We triangulated the data (shared our preliminary results) using a larger study team, including an expert in behavioral economics (SRG), physicians at study sites (EC, RA), and an anthropologist with expertise in qualitative methods (CL). We did this to ensure credibility (to what extent the findings are credible or believable) and confirmability of findings (ensuring the findings are based on participant narratives rather than researcher biases).
RESULTS
We reviewed a total of 105 interviews with 25 hospital clinicians, 20 SNF clinicians, 21 patients and 14 caregivers in the hospital, and 15 patients and 10 caregivers in the SNF setting (Table 1). We found authority bias/halo effect; default/status quo bias, anchoring bias, and framing was commonly present in decision-making about postacute care in a SNF, whereas there were few if any examples of ambiguity aversion, availability heuristic, confirmation bias, optimism bias, or false consensus effect (Table 2).
Authority Bias/Halo Effect
While most patients deferred to their inpatient teams when it came to decision-making, this effect seemed to differ across VA and non-VA settings. Veterans expressed a higher degree of potential authority bias regarding the VA as an institution, whereas older adults in non-VA settings saw physicians as the authority figure making decisions in their best interests.
Veterans expressed confidence in the VA regarding both whether to go to a SNF and where to go:
“The VA wouldn’t license [an SNF] if they didn’t have a good reputation for care, cleanliness, things of that nature” (Veteran, VA CLC)
“I just knew the VA would have my best interests at heart” (Veteran, VA CLC)
Their caregivers expressed similar confidence:
“I’m not gonna decide [on whether the patient they care for goes to postacute care], like I told you, that’s totally up to the VA. I have trust and faith in them…so wherever they send him, that’s where he’s going” (Caregiver, VA hospital)
In some cases, this perspective was closer to the halo effect: a positive experience with the care provider or the care team led the decision-makers to believe that their recommendations about postacute care would be similarly positive.
“I think we were very trusting in the sense that whatever happened the last time around, he survived it…they took care of him…he got back home, and he started his life again, you know, so why would we question what they’re telling us to do? (Caregiver, VA hospital)
In contrast to Veterans, non-Veteran patients seemed to experience authority bias when it came to the inpatient team.
“Well, I’d like to know more about the PTs [Physical Therapists] there, but I assume since they were recommended, they will be good.” (Patient, University hospital)
This perspective was especially apparent when it came to physicians:
“The level of trust that they [patients] put in their doctor is gonna outweigh what anyone else would say.” (Clinical liaison, SNF)
“[In response to a question about influences on the decision to go to rehab] I don’t…that’s not my decision to make, that’s the doctor’s decision.” (Patient, University hospital)
“They said so…[the doctor] said I needed to go to rehab, so I guess I do because it’s the doctor’s decision.” (Patient, University hospital)
Default/Status quo Bias
In a related way, patients and caregivers with exposure to a SNF seemed to default to the same SNF with which they had previous experience. This bias seems to be primarily related to knowing what to expect.
“He thinks it’s [a particular SNF] the right place for him now…he was there before and he knew, again, it was the right place for him to be” (Caregiver, VA hospital)
“It’s the only one I’ve ever been in…but they have a lot of activities; you have a lot of freedom, staff was good” (Patient, VA hospital)
“I’ve been [to this SNF] before and I kind of know what the program involves…so it was kind of like going home, not, going home is the wrong way to put it…I mean coming here is like something I know, you know, I didn’t need anybody to explain it to me.” (Patient, VA hospital)
“Anybody that’s been to [SNF], that would be their choice to go back to, and I guess I must’ve liked it that first time because I asked to go back again.” (Patient, University hospital)
Anchoring Bias
While anchoring bias was less frequent, it came up in two domains: first, related to costs of care, and second, related to facility characteristics. Costs came up most frequently for Veterans who preferred to move their care to the VA for cost reasons, which appeared in these cases to overshadow other considerations:
“I kept emphasizing that the VA could do all the same things at a lot more reasonable price. The whole purpose of having the VA is for the Veteran, so that…we can get the healthcare that we need at a more reasonable [sic] or a reasonable price.” (Veteran, CLC)
“I think the CLC [VA SNF] is going to take care of her probably the same way any other facility of its type would, unless she were in a private facility, but you know, that costs a lot more money.” (Caregiver, VA hospital)
Patients occasionally had striking responses to particular characteristics of SNFs, regardless of whether this was a central feature or related to their rehabilitation:
“The social worker comes and talks to me about the nursing home where cats are running around, you know, to infect my leg or spin their little cat hairs into my lungs and make my asthma worse…I’m going to have to beg the nurses or the aides or the family or somebody to clean the cat…” (Veteran, VA hospital)
Framing
Framing was the strongest theme among clinician interviews in our sample. Clinicians most frequently described the SNF as a place where patients could recover function (a positive frame), explaining risks (eg, rehospitalization) associated with alternative postacute care options besides the SNF in great detail.
“Aside from explaining the benefits of going and…having that 24-hour care, having the therapies provided to them [the patients], talking about them getting stronger, phrasing it in such a way that patients sometimes are more agreeable, like not calling it a skilled nursing facility, calling it a rehab you know, for them to get physically stronger so they can be the most independent that they can once they do go home, and also explaining … we think that this would be the best plan to prevent them from coming back to the hospital, so those are some of the things that we’ll mention to patients to try and educate them and get them to be agreeable for placement.” (Social worker, University hospital)
Clinicians avoided negative associations with “nursing home” (even though all SNFs are nursing homes) and tended to use more positive frames such as “rehabilitation facility.”
“Use the word rehab….we definitely use the word rehab, to get more therapy, to go home; it’s not a, we really emphasize it’s not a nursing home, it’s not to go to stay forever.” (Physical therapist, safety-net hospital)
Clinicians used a frame of “safety” when discussing the SNF and used a frame of “risk” when discussing alternative postacute care options such as returning home. We did not find examples of clinicians discussing similar risks in going to a SNF even for risks, such as falling, which exist in both settings.
“I’ve talked to them primarily on an avenue of safety because I think people want and they value independence, they value making sure they can get home, but you know, a lot of the times they understand safety is, it can be a concern and outlining that our goal is to make sure that they’re safe and they stay home, and I tend to broach the subject saying that our therapists believe that they might not be safe at home in the moment, but they have potential goals to be safe later on if we continue therapy. I really highlight safety being the major driver of our discussion.” (Physician, VA hospital)
In some cases, framing was so overt that other risk-mitigating options (eg, home healthcare) are not discussed.
“I definitely tend to explain the ideal first. I’m not going to bring up home care when we really think somebody should go to rehab, however, once people say I don’t want to do that, I’m not going, then that’s when I’m like OK, well, let’s talk to the doctors, but we can see about other supports in the home.” (Social worker, VA hospital)
DISCUSSION
In a large sample of patients and their caregivers, as well as multidisciplinary clinicians at three different hospitals and three SNFs, we found authority bias/halo effect and framing biases were most common and seemed most impactful. Default/status quo bias and anchoring bias were also present in decision-making about a SNF. The combination of authority bias/halo effect and framing biases could synergistically interact to augment the likelihood of patients accepting a SNF for postacute care. Patients who had been to a SNF before seemed more likely to choose the SNF they had experienced previously even if they had no other postacute care experiences, and could be highly influenced by isolated characteristics of that facility (such as the physical environment or cost of care).
It is important to mention that cognitive biases do not necessarily have a negative impact: indeed, as Kahneman and Tversky point out, these are useful heuristics from “fast” thinking that are often effective.24 For example, clinicians may be trying to act in the best interests of the patient when framing the decision in terms of regaining function and averting loss of safety and independence. However, the evidence base regarding the outcomes of an SNF versus other postacute options is not robust, and this decision-making is complex. While this decision was most commonly framed in terms of rehabilitation and returning home, the fact that only about half of patients have returned to the community by 100 days4 was not discussed in any interview. In fact, initial evidence suggests replacing the SNF with home healthcare in patients with hip and knee arthroplasty may reduce costs without worsening clinical outcomes.6 However, across a broader population, SNFs significantly reduce 30-day readmissions when directly compared with home healthcare, but other clinical outcomes are similar.25 This evidence suggests that the “right” postacute care option for an individual patient is not clear, highlighting a key role biases may play in decision-making. Further, the nebulous concept of “safety” could introduce potential disparities related to social determinants of health.12 The observed inclination to accept an SNF with which the individual had prior experience may be influenced by the acceptability of this choice because of personal factors or prior research, even if it also represents a bias by limiting the consideration of current alternatives.
Our findings complement those of others in the literature which have also identified profound gaps in discharge decision-making among patients and clinicians,13-16,26-31 though to our knowledge the role of cognitive biases in these decisions has not been explored. This study also addresses gaps in the cognitive bias literature, including the need for real-world data rather than hypothetical vignettes,17 and evaluation of treatment and management decisions rather than diagnoses, which have been more commonly studied.21
These findings have implications for both individual clinicians and healthcare institutions. In the immediate term, these findings may serve as a call to discharging clinicians to modulate language and “debias” their conversations with patients about care after discharge.18,22 Shared decision-making requires an informed choice by patients based on their goals and values; framing a decision in a way that puts the clinician’s goals or values (eg, safety) ahead of patient values (eg, independence and autonomy) or limits disclosure (eg, a “rehab” is a nursing home) in the hope of influencing choice may be more consistent with framing bias and less with shared decision-making.14 Although controversy exists about the best way to “debias” oneself,32 self-awareness of bias is increasingly recognized across healthcare venues as critical to improving care for vulnerable populations.33 The use of data rather than vignettes may be a useful debiasing strategy, although the limitations of currently available data (eg, capturing nursing home quality) are increasingly recognized.34 From a policy and health system perspective, cognitive biases should be integrated into the development of decision aids to facilitate informed, shared, and high-quality decision-making that incorporates patient values, and perhaps “nudges” from behavioral economics to assist patients in choosing the right postdischarge care for them. Such nudges use principles of framing to influence care without restricting choice.35 As the science informing best practice regarding postacute care improves, identifying the “right” postdischarge care may become easier and recommendations more evidence-based.36
Strengths of the study include a large, diverse sample of patients, caregivers, and clinicians in both the hospital and SNF setting. Also, we used a team-based analysis with an experienced team and a deep knowledge of the data, including triangulation with clinicians to verify results. However, all hospitals and SNFs were located in a single metropolitan area, and responses may vary by region or population density. All three hospitals have housestaff teaching programs, and at the time of the interviews all three community SNFs were “five-star” facilities on the Nursing Home Compare website; results may be different at community hospitals or other SNFs. Hospitalists were the only physician group sampled in the hospital as they provide the majority of inpatient care to older adults; geriatricians, in particular, may have had different perspectives. Since we intended to explore whether cognitive biases were present overall, we did not evaluate whether cognitive biases differed by role or subgroup (by clinician type, patient, or caregiver), but this may be a promising area to explore in future work. Many cognitive biases have been described, and there are likely additional biases we did not identify. To confirm the generalizability of these findings, they should be studied in a larger, more generalizable sample of respondents in future work.
Cognitive biases play an important role in patient decision-making about postacute care, particularly regarding SNF care. As postacute care undergoes a transformation spurred by payment reforms, it is more important than ever to ensure that patients understand their choices at hospital discharge and can make a high-quality decision consistent with their goals.
1. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Rise of post-acute care facilities as a discharge destination of US hospitalizations. JAMA Intern Med. 2015;175(2):295-296. https://doi.org/10.1001/jamainternmed.2014.6383.
2. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Patient and hospitalization characteristics associated with increased postacute care facility discharges from US hospitals. Med Care. 2015;53(6):492-500. https://doi.org/10.1097/MLR.0000000000000359.
3. Werner RM, Konetzka RT. Trends in post-acute care use among medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616-1617. https://doi.org/10.1001/jama.2018.2408.
4. Medicare Payment Advisory Commission June 2018 Report to Congress. http://www.medpac.gov/docs/default-source/reports/jun18_ch5_medpacreport_sec.pdf?sfvrsn=0. Accessed November 9, 2018.
5. Burke RE, Cumbler E, Coleman EA, Levy C. Post-acute care reform: implications and opportunities for hospitalists. J Hosp Med. 2017;12(1):46-51. https://doi.org/10.1002/jhm.2673.
6. Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316(12):1267-1278. https://doi.org/10.1001/jama.2016.12717.
7. Navathe AS, Troxel AB, Liao JM, et al. Cost of joint replacement using bundled payment models. JAMA Intern Med. 2017;177(2):214-222. https://doi.org/10.1001/jamainternmed.2016.8263.
8. Kennedy G, Lewis VA, Kundu S, Mousqués J, Colla CH. Accountable care organizations and post-acute care: a focus on preferred SNF networks. Med Care Res Rev MCRR. 2018;1077558718781117. https://doi.org/10.1177/1077558718781117.
9. Chandra A, Dalton MA, Holmes J. Large increases in spending on postacute care in Medicare point to the potential for cost savings in these settings. Health Aff Proj Hope. 2013;32(5):864-872. https://doi.org/10.1377/hlthaff.2012.1262.
10. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the Medicare shared savings program. JAMA Intern Med. 2017;177(4):518-526. https://doi.org/10.1001/jamainternmed.2016.9115.
11. Zhu JM, Patel V, Shea JA, Neuman MD, Werner RM. Hospitals using bundled payment report reducing skilled nursing facility use and improving care integration. Health Aff Proj Hope. 2018;37(8):1282-1289. https://doi.org/10.1377/hlthaff.2018.0257.
12. Burke RE, Ibrahim SA. Discharge destination and disparities in postoperative care. JAMA. 2018;319(16):1653-1654. https://doi.org/10.1001/jama.2017.21884.
13. Burke RE, Lawrence E, Ladebue A, et al. How hospital clinicians select patients for skilled nursing facilities. J Am Geriatr Soc. 2017;65(11):2466-2472. https://doi.org/10.1111/jgs.14954.
14. Burke RE, Jones J, Lawrence E, et al. Evaluating the quality of patient decision-making regarding post-acute care. J Gen Intern Med. 2018;33(5):678-684. https://doi.org/10.1007/s11606-017-4298-1.
15. Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc. 2017;65(11):2459-2465. https://doi.org/10.1111/jgs.14988.
16. Tyler DA, Gadbois EA, McHugh JP, Shield RR, Winblad U, Mor V. Patients are not given quality-of-care data about skilled nursing facilities when discharged from hospitals. Health Aff. 2017;36(8):1385-1391. https://doi.org/10.1377/hlthaff.2017.0155.
17. Blumenthal-Barby JS, Krieger H. Cognitive biases and heuristics in medical decision making: a critical review using a systematic search strategy. Med Decis Mak Int J Soc Med Decis Mak. 2015;35(4):539-557. https://doi.org/10.1177/0272989X14547740.
18. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22 Suppl 2:ii58-ii64. https://doi.org/10.1136/bmjqs-2012-001712.
19. Hinds PS, Vogel RJ, Clarke-Steffen L. The possibilities and pitfalls of doing a secondary analysis of a qualitative data set. Qual Health Res. 1997;7(3):408-424. https://doi.org/10.1177/104973239700700306.
20. Magid M, Mcllvennan CK, Jones J, et al. Exploring cognitive bias in destination therapy left ventricular assist device decision making: a retrospective qualitative framework analysis. Am Heart J. 2016;180:64-73. https://doi.org/10.1016/j.ahj.2016.06.024.
21. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16(1):138. https://doi.org/10.1186/s12911-016-0377-1.
22. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: impediments to and strategies for change. BMJ Qual Saf. 2013;22 Suppl 2:ii65-ii72. https://doi.org/10.1136/bmjqs-2012-001713.
23. Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42(4):1758-1772. https://doi.org/10.1111/j.1475-6773.2006.00684.x.
24. Thinking, Fast and Slow. Daniel Kahneman. Macmillan. US Macmillan. https://us.macmillan.com/thinkingfastandslow/danielkahneman/9780374533557. Accessed February 5, 2019.
25. Werner RM, Konetzka RT, Coe NB. Does type of post-acute care matter? The effect of hospital discharge to home with home health care versus to skilled nursing facility. JAMA Intern Med. In press.
26. Jones J, Lawrence E, Ladebue A, Leonard C, Ayele R, Burke RE. Nurses’ role in managing “The Fit” of older adults in skilled nursing facilities. J Gerontol Nurs. 2017;43(12):11-20. https://doi.org/10.3928/00989134-20171110-06.
27. Lawrence E, Casler J-J, Jones J, et al. Variability in skilled nursing facility screening and admission processes: implications for value-based purchasing. Health Care Manage Rev. 2018. https://doi.org/10.1097/HMR.0000000000000225.
28. Ayele R, Jones J, Ladebue A, et al. Perceived costs of care influence post-acute care choices by clinicians, patients, and caregivers. J Am Geriatr Soc. 2019. https://doi.org/10.1111/jgs.15768.
29. Sefcik JS, Nock RH, Flores EJ, et al. Patient preferences for information on post-acute care services. Res Gerontol Nurs. 2016;9(4):175-182. https://doi.org/10.3928/19404921-20160120-01.
30. Konetzka RT, Perraillon MC. Use of nursing home compare website appears limited by lack of awareness and initial mistrust of the data. Health Aff Proj Hope. 2016;35(4):706-713. https://doi.org/10.1377/hlthaff.2015.1377.
31. Schapira MM, Shea JA, Duey KA, Kleiman C, Werner RM. The nursing home compare report card: perceptions of residents and caregivers regarding quality ratings and nursing home choice. Health Serv Res. 2016;51 Suppl 2:1212-1228. https://doi.org/10.1111/1475-6773.12458.
32. Dhaliwal G. Premature closure? Not so fast. BMJ Qual Saf. 2017;26(2):87-89. https://doi.org/10.1136/bmjqs-2016-005267.
33. Masters C, Robinson D, Faulkner S, Patterson E, McIlraith T, Ansari A. Addressing biases in patient care with the 5Rs of cultural humility, a clinician coaching tool. J Gen Intern Med. 2019;34(4):627-630. https://doi.org/10.1007/s11606-018-4814-y.
34. Burke RE, Werner RM. Quality measurement and nursing homes: measuring what matters. BMJ Qual Saf. 2019;28(7);520-523. https://doi.org/10.1136/bmjqs-2019-009447.
35. Patel MS, Volpp KG, Asch DA. Nudge units to improve the delivery of health care. N Engl J Med. 2018;378(3):214-216. https://doi.org/10.1056/NEJMp1712984.
36. Jenq GY, Tinetti ME. Post–acute care: who belongs where? JAMA Intern Med. 2015;175(2):296-297. https://doi.org/10.1001/jamainternmed.2014.4298.
The combination of decreasing hospital lengths of stay and increasing age and comorbidity of the United States population is a principal driver of the increased use of postacute care in the US.1-3 Postacute care refers to care in long-term acute care hospitals, inpatient rehabilitation facilities, skilled nursing facilities (SNFs), and care provided by home health agencies after an acute hospitalization. In 2016, 43% of Medicare beneficiaries received postacute care after hospital discharge at the cost of $60 billion annually; nearly half of these received care in an SNF.4 Increasing recognition of the significant cost and poor outcomes of postacute care led to payment reforms, such as bundled payments, that incentivized less expensive forms of postacute care and improvements in outcomes.5-9 Early evaluations suggested that hospitals are sensitive to these reforms and responded by significantly decreasing SNF utilization.10,11 It remains unclear whether this was safe and effective.
In this context, increased attention to how hospital clinicians and hospitalized patients decide whether to use postacute care (and what form to use) is appropriate since the effect of payment reforms could negatively impact vulnerable populations of older adults without adequate protection.12 Suboptimal decision-making can drive both overuse and inappropriate underuse of this expensive medical resource. Initial evidence suggests that patients and clinicians are poorly equipped to make high-quality decisions about postacute care, with significant deficits in both the decision-making process and content.13-16 While these gaps are important to address, they may only be part of the problem. The fields of cognitive psychology and behavioral economics have revealed new insights into decision-making, demonstrating that people deviate from rational decision-making in predictable ways, termed decision heuristics, or cognitive biases.17 This growing field of research suggests heuristics or biases play important roles in decision-making and determining behavior, particularly in situations where there may be little information provided and the patient is stressed, tired, and ill—precisely like deciding on postacute care.18 However, it is currently unknown whether cognitive biases are at play when making hospital discharge decisions.
We sought to identify the most salient heuristics or cognitive biases patients may utilize when making decisions about postacute care at the end of their hospitalization and ways clinicians may contribute to these biases. The overall goal was to derive insights for improving postacute care decision-making.
METHODS
Study Design
We conducted a secondary analysis on interviews with hospital and SNF clinicians as well as patients and their caregivers who were either leaving the hospital for an SNF or newly arrived in an SNF from the hospital to understand if cognitive biases were present and how they manifested themselves in a real-world clinical context.19 These interviews were part of a larger qualitative study that sought to understand how clinicians, patients, and their caregivers made decisions about postacute care, particularly related to SNFs.13,14 This study represents the analysis of all our interviews, specifically examining decision-making bias. Participating sites, clinical roles, and both patient and caregiver characteristics (Table 1) in our cohort have been previously described.13,14
Analysis
We used a team-based approach to framework analysis, which has been used in other decision-making studies14, including those measuring cognitive bias.20 A limitation in cognitive bias research is the lack of a standardized list or categorization of cognitive biases. We reviewed prior systematic17,21 and narrative reviews18,22, as well as prior studies describing examples of cognitive biases playing a role in decision-making about therapy20 to construct a list of possible cognitive biases to evaluate and narrow these a priori to potential biases relevant to the decision about postacute care based on our prior work (Table 2).
We applied this framework to analyze transcripts through an iterative process of deductive coding and reviewing across four reviewers (ML, RA, AL, CL) and a hospitalist physician with expertise leading qualitative studies (REB).
Intercoder consensus was built through team discussion by resolving points of disagreement.23 Consistency of coding was regularly checked by having more than one investigator code individual manuscripts and comparing coding, and discrepancies were resolved through team discussion. We triangulated the data (shared our preliminary results) using a larger study team, including an expert in behavioral economics (SRG), physicians at study sites (EC, RA), and an anthropologist with expertise in qualitative methods (CL). We did this to ensure credibility (to what extent the findings are credible or believable) and confirmability of findings (ensuring the findings are based on participant narratives rather than researcher biases).
RESULTS
We reviewed a total of 105 interviews with 25 hospital clinicians, 20 SNF clinicians, 21 patients and 14 caregivers in the hospital, and 15 patients and 10 caregivers in the SNF setting (Table 1). We found authority bias/halo effect; default/status quo bias, anchoring bias, and framing was commonly present in decision-making about postacute care in a SNF, whereas there were few if any examples of ambiguity aversion, availability heuristic, confirmation bias, optimism bias, or false consensus effect (Table 2).
Authority Bias/Halo Effect
While most patients deferred to their inpatient teams when it came to decision-making, this effect seemed to differ across VA and non-VA settings. Veterans expressed a higher degree of potential authority bias regarding the VA as an institution, whereas older adults in non-VA settings saw physicians as the authority figure making decisions in their best interests.
Veterans expressed confidence in the VA regarding both whether to go to a SNF and where to go:
“The VA wouldn’t license [an SNF] if they didn’t have a good reputation for care, cleanliness, things of that nature” (Veteran, VA CLC)
“I just knew the VA would have my best interests at heart” (Veteran, VA CLC)
Their caregivers expressed similar confidence:
“I’m not gonna decide [on whether the patient they care for goes to postacute care], like I told you, that’s totally up to the VA. I have trust and faith in them…so wherever they send him, that’s where he’s going” (Caregiver, VA hospital)
In some cases, this perspective was closer to the halo effect: a positive experience with the care provider or the care team led the decision-makers to believe that their recommendations about postacute care would be similarly positive.
“I think we were very trusting in the sense that whatever happened the last time around, he survived it…they took care of him…he got back home, and he started his life again, you know, so why would we question what they’re telling us to do? (Caregiver, VA hospital)
In contrast to Veterans, non-Veteran patients seemed to experience authority bias when it came to the inpatient team.
“Well, I’d like to know more about the PTs [Physical Therapists] there, but I assume since they were recommended, they will be good.” (Patient, University hospital)
This perspective was especially apparent when it came to physicians:
“The level of trust that they [patients] put in their doctor is gonna outweigh what anyone else would say.” (Clinical liaison, SNF)
“[In response to a question about influences on the decision to go to rehab] I don’t…that’s not my decision to make, that’s the doctor’s decision.” (Patient, University hospital)
“They said so…[the doctor] said I needed to go to rehab, so I guess I do because it’s the doctor’s decision.” (Patient, University hospital)
Default/Status quo Bias
In a related way, patients and caregivers with exposure to a SNF seemed to default to the same SNF with which they had previous experience. This bias seems to be primarily related to knowing what to expect.
“He thinks it’s [a particular SNF] the right place for him now…he was there before and he knew, again, it was the right place for him to be” (Caregiver, VA hospital)
“It’s the only one I’ve ever been in…but they have a lot of activities; you have a lot of freedom, staff was good” (Patient, VA hospital)
“I’ve been [to this SNF] before and I kind of know what the program involves…so it was kind of like going home, not, going home is the wrong way to put it…I mean coming here is like something I know, you know, I didn’t need anybody to explain it to me.” (Patient, VA hospital)
“Anybody that’s been to [SNF], that would be their choice to go back to, and I guess I must’ve liked it that first time because I asked to go back again.” (Patient, University hospital)
Anchoring Bias
While anchoring bias was less frequent, it came up in two domains: first, related to costs of care, and second, related to facility characteristics. Costs came up most frequently for Veterans who preferred to move their care to the VA for cost reasons, which appeared in these cases to overshadow other considerations:
“I kept emphasizing that the VA could do all the same things at a lot more reasonable price. The whole purpose of having the VA is for the Veteran, so that…we can get the healthcare that we need at a more reasonable [sic] or a reasonable price.” (Veteran, CLC)
“I think the CLC [VA SNF] is going to take care of her probably the same way any other facility of its type would, unless she were in a private facility, but you know, that costs a lot more money.” (Caregiver, VA hospital)
Patients occasionally had striking responses to particular characteristics of SNFs, regardless of whether this was a central feature or related to their rehabilitation:
“The social worker comes and talks to me about the nursing home where cats are running around, you know, to infect my leg or spin their little cat hairs into my lungs and make my asthma worse…I’m going to have to beg the nurses or the aides or the family or somebody to clean the cat…” (Veteran, VA hospital)
Framing
Framing was the strongest theme among clinician interviews in our sample. Clinicians most frequently described the SNF as a place where patients could recover function (a positive frame), explaining risks (eg, rehospitalization) associated with alternative postacute care options besides the SNF in great detail.
“Aside from explaining the benefits of going and…having that 24-hour care, having the therapies provided to them [the patients], talking about them getting stronger, phrasing it in such a way that patients sometimes are more agreeable, like not calling it a skilled nursing facility, calling it a rehab you know, for them to get physically stronger so they can be the most independent that they can once they do go home, and also explaining … we think that this would be the best plan to prevent them from coming back to the hospital, so those are some of the things that we’ll mention to patients to try and educate them and get them to be agreeable for placement.” (Social worker, University hospital)
Clinicians avoided negative associations with “nursing home” (even though all SNFs are nursing homes) and tended to use more positive frames such as “rehabilitation facility.”
“Use the word rehab….we definitely use the word rehab, to get more therapy, to go home; it’s not a, we really emphasize it’s not a nursing home, it’s not to go to stay forever.” (Physical therapist, safety-net hospital)
Clinicians used a frame of “safety” when discussing the SNF and used a frame of “risk” when discussing alternative postacute care options such as returning home. We did not find examples of clinicians discussing similar risks in going to a SNF even for risks, such as falling, which exist in both settings.
“I’ve talked to them primarily on an avenue of safety because I think people want and they value independence, they value making sure they can get home, but you know, a lot of the times they understand safety is, it can be a concern and outlining that our goal is to make sure that they’re safe and they stay home, and I tend to broach the subject saying that our therapists believe that they might not be safe at home in the moment, but they have potential goals to be safe later on if we continue therapy. I really highlight safety being the major driver of our discussion.” (Physician, VA hospital)
In some cases, framing was so overt that other risk-mitigating options (eg, home healthcare) are not discussed.
“I definitely tend to explain the ideal first. I’m not going to bring up home care when we really think somebody should go to rehab, however, once people say I don’t want to do that, I’m not going, then that’s when I’m like OK, well, let’s talk to the doctors, but we can see about other supports in the home.” (Social worker, VA hospital)
DISCUSSION
In a large sample of patients and their caregivers, as well as multidisciplinary clinicians at three different hospitals and three SNFs, we found authority bias/halo effect and framing biases were most common and seemed most impactful. Default/status quo bias and anchoring bias were also present in decision-making about a SNF. The combination of authority bias/halo effect and framing biases could synergistically interact to augment the likelihood of patients accepting a SNF for postacute care. Patients who had been to a SNF before seemed more likely to choose the SNF they had experienced previously even if they had no other postacute care experiences, and could be highly influenced by isolated characteristics of that facility (such as the physical environment or cost of care).
It is important to mention that cognitive biases do not necessarily have a negative impact: indeed, as Kahneman and Tversky point out, these are useful heuristics from “fast” thinking that are often effective.24 For example, clinicians may be trying to act in the best interests of the patient when framing the decision in terms of regaining function and averting loss of safety and independence. However, the evidence base regarding the outcomes of an SNF versus other postacute options is not robust, and this decision-making is complex. While this decision was most commonly framed in terms of rehabilitation and returning home, the fact that only about half of patients have returned to the community by 100 days4 was not discussed in any interview. In fact, initial evidence suggests replacing the SNF with home healthcare in patients with hip and knee arthroplasty may reduce costs without worsening clinical outcomes.6 However, across a broader population, SNFs significantly reduce 30-day readmissions when directly compared with home healthcare, but other clinical outcomes are similar.25 This evidence suggests that the “right” postacute care option for an individual patient is not clear, highlighting a key role biases may play in decision-making. Further, the nebulous concept of “safety” could introduce potential disparities related to social determinants of health.12 The observed inclination to accept an SNF with which the individual had prior experience may be influenced by the acceptability of this choice because of personal factors or prior research, even if it also represents a bias by limiting the consideration of current alternatives.
Our findings complement those of others in the literature which have also identified profound gaps in discharge decision-making among patients and clinicians,13-16,26-31 though to our knowledge the role of cognitive biases in these decisions has not been explored. This study also addresses gaps in the cognitive bias literature, including the need for real-world data rather than hypothetical vignettes,17 and evaluation of treatment and management decisions rather than diagnoses, which have been more commonly studied.21
These findings have implications for both individual clinicians and healthcare institutions. In the immediate term, these findings may serve as a call to discharging clinicians to modulate language and “debias” their conversations with patients about care after discharge.18,22 Shared decision-making requires an informed choice by patients based on their goals and values; framing a decision in a way that puts the clinician’s goals or values (eg, safety) ahead of patient values (eg, independence and autonomy) or limits disclosure (eg, a “rehab” is a nursing home) in the hope of influencing choice may be more consistent with framing bias and less with shared decision-making.14 Although controversy exists about the best way to “debias” oneself,32 self-awareness of bias is increasingly recognized across healthcare venues as critical to improving care for vulnerable populations.33 The use of data rather than vignettes may be a useful debiasing strategy, although the limitations of currently available data (eg, capturing nursing home quality) are increasingly recognized.34 From a policy and health system perspective, cognitive biases should be integrated into the development of decision aids to facilitate informed, shared, and high-quality decision-making that incorporates patient values, and perhaps “nudges” from behavioral economics to assist patients in choosing the right postdischarge care for them. Such nudges use principles of framing to influence care without restricting choice.35 As the science informing best practice regarding postacute care improves, identifying the “right” postdischarge care may become easier and recommendations more evidence-based.36
Strengths of the study include a large, diverse sample of patients, caregivers, and clinicians in both the hospital and SNF setting. Also, we used a team-based analysis with an experienced team and a deep knowledge of the data, including triangulation with clinicians to verify results. However, all hospitals and SNFs were located in a single metropolitan area, and responses may vary by region or population density. All three hospitals have housestaff teaching programs, and at the time of the interviews all three community SNFs were “five-star” facilities on the Nursing Home Compare website; results may be different at community hospitals or other SNFs. Hospitalists were the only physician group sampled in the hospital as they provide the majority of inpatient care to older adults; geriatricians, in particular, may have had different perspectives. Since we intended to explore whether cognitive biases were present overall, we did not evaluate whether cognitive biases differed by role or subgroup (by clinician type, patient, or caregiver), but this may be a promising area to explore in future work. Many cognitive biases have been described, and there are likely additional biases we did not identify. To confirm the generalizability of these findings, they should be studied in a larger, more generalizable sample of respondents in future work.
Cognitive biases play an important role in patient decision-making about postacute care, particularly regarding SNF care. As postacute care undergoes a transformation spurred by payment reforms, it is more important than ever to ensure that patients understand their choices at hospital discharge and can make a high-quality decision consistent with their goals.
The combination of decreasing hospital lengths of stay and increasing age and comorbidity of the United States population is a principal driver of the increased use of postacute care in the US.1-3 Postacute care refers to care in long-term acute care hospitals, inpatient rehabilitation facilities, skilled nursing facilities (SNFs), and care provided by home health agencies after an acute hospitalization. In 2016, 43% of Medicare beneficiaries received postacute care after hospital discharge at the cost of $60 billion annually; nearly half of these received care in an SNF.4 Increasing recognition of the significant cost and poor outcomes of postacute care led to payment reforms, such as bundled payments, that incentivized less expensive forms of postacute care and improvements in outcomes.5-9 Early evaluations suggested that hospitals are sensitive to these reforms and responded by significantly decreasing SNF utilization.10,11 It remains unclear whether this was safe and effective.
In this context, increased attention to how hospital clinicians and hospitalized patients decide whether to use postacute care (and what form to use) is appropriate since the effect of payment reforms could negatively impact vulnerable populations of older adults without adequate protection.12 Suboptimal decision-making can drive both overuse and inappropriate underuse of this expensive medical resource. Initial evidence suggests that patients and clinicians are poorly equipped to make high-quality decisions about postacute care, with significant deficits in both the decision-making process and content.13-16 While these gaps are important to address, they may only be part of the problem. The fields of cognitive psychology and behavioral economics have revealed new insights into decision-making, demonstrating that people deviate from rational decision-making in predictable ways, termed decision heuristics, or cognitive biases.17 This growing field of research suggests heuristics or biases play important roles in decision-making and determining behavior, particularly in situations where there may be little information provided and the patient is stressed, tired, and ill—precisely like deciding on postacute care.18 However, it is currently unknown whether cognitive biases are at play when making hospital discharge decisions.
We sought to identify the most salient heuristics or cognitive biases patients may utilize when making decisions about postacute care at the end of their hospitalization and ways clinicians may contribute to these biases. The overall goal was to derive insights for improving postacute care decision-making.
METHODS
Study Design
We conducted a secondary analysis on interviews with hospital and SNF clinicians as well as patients and their caregivers who were either leaving the hospital for an SNF or newly arrived in an SNF from the hospital to understand if cognitive biases were present and how they manifested themselves in a real-world clinical context.19 These interviews were part of a larger qualitative study that sought to understand how clinicians, patients, and their caregivers made decisions about postacute care, particularly related to SNFs.13,14 This study represents the analysis of all our interviews, specifically examining decision-making bias. Participating sites, clinical roles, and both patient and caregiver characteristics (Table 1) in our cohort have been previously described.13,14
Analysis
We used a team-based approach to framework analysis, which has been used in other decision-making studies14, including those measuring cognitive bias.20 A limitation in cognitive bias research is the lack of a standardized list or categorization of cognitive biases. We reviewed prior systematic17,21 and narrative reviews18,22, as well as prior studies describing examples of cognitive biases playing a role in decision-making about therapy20 to construct a list of possible cognitive biases to evaluate and narrow these a priori to potential biases relevant to the decision about postacute care based on our prior work (Table 2).
We applied this framework to analyze transcripts through an iterative process of deductive coding and reviewing across four reviewers (ML, RA, AL, CL) and a hospitalist physician with expertise leading qualitative studies (REB).
Intercoder consensus was built through team discussion by resolving points of disagreement.23 Consistency of coding was regularly checked by having more than one investigator code individual manuscripts and comparing coding, and discrepancies were resolved through team discussion. We triangulated the data (shared our preliminary results) using a larger study team, including an expert in behavioral economics (SRG), physicians at study sites (EC, RA), and an anthropologist with expertise in qualitative methods (CL). We did this to ensure credibility (to what extent the findings are credible or believable) and confirmability of findings (ensuring the findings are based on participant narratives rather than researcher biases).
RESULTS
We reviewed a total of 105 interviews with 25 hospital clinicians, 20 SNF clinicians, 21 patients and 14 caregivers in the hospital, and 15 patients and 10 caregivers in the SNF setting (Table 1). We found authority bias/halo effect; default/status quo bias, anchoring bias, and framing was commonly present in decision-making about postacute care in a SNF, whereas there were few if any examples of ambiguity aversion, availability heuristic, confirmation bias, optimism bias, or false consensus effect (Table 2).
Authority Bias/Halo Effect
While most patients deferred to their inpatient teams when it came to decision-making, this effect seemed to differ across VA and non-VA settings. Veterans expressed a higher degree of potential authority bias regarding the VA as an institution, whereas older adults in non-VA settings saw physicians as the authority figure making decisions in their best interests.
Veterans expressed confidence in the VA regarding both whether to go to a SNF and where to go:
“The VA wouldn’t license [an SNF] if they didn’t have a good reputation for care, cleanliness, things of that nature” (Veteran, VA CLC)
“I just knew the VA would have my best interests at heart” (Veteran, VA CLC)
Their caregivers expressed similar confidence:
“I’m not gonna decide [on whether the patient they care for goes to postacute care], like I told you, that’s totally up to the VA. I have trust and faith in them…so wherever they send him, that’s where he’s going” (Caregiver, VA hospital)
In some cases, this perspective was closer to the halo effect: a positive experience with the care provider or the care team led the decision-makers to believe that their recommendations about postacute care would be similarly positive.
“I think we were very trusting in the sense that whatever happened the last time around, he survived it…they took care of him…he got back home, and he started his life again, you know, so why would we question what they’re telling us to do? (Caregiver, VA hospital)
In contrast to Veterans, non-Veteran patients seemed to experience authority bias when it came to the inpatient team.
“Well, I’d like to know more about the PTs [Physical Therapists] there, but I assume since they were recommended, they will be good.” (Patient, University hospital)
This perspective was especially apparent when it came to physicians:
“The level of trust that they [patients] put in their doctor is gonna outweigh what anyone else would say.” (Clinical liaison, SNF)
“[In response to a question about influences on the decision to go to rehab] I don’t…that’s not my decision to make, that’s the doctor’s decision.” (Patient, University hospital)
“They said so…[the doctor] said I needed to go to rehab, so I guess I do because it’s the doctor’s decision.” (Patient, University hospital)
Default/Status quo Bias
In a related way, patients and caregivers with exposure to a SNF seemed to default to the same SNF with which they had previous experience. This bias seems to be primarily related to knowing what to expect.
“He thinks it’s [a particular SNF] the right place for him now…he was there before and he knew, again, it was the right place for him to be” (Caregiver, VA hospital)
“It’s the only one I’ve ever been in…but they have a lot of activities; you have a lot of freedom, staff was good” (Patient, VA hospital)
“I’ve been [to this SNF] before and I kind of know what the program involves…so it was kind of like going home, not, going home is the wrong way to put it…I mean coming here is like something I know, you know, I didn’t need anybody to explain it to me.” (Patient, VA hospital)
“Anybody that’s been to [SNF], that would be their choice to go back to, and I guess I must’ve liked it that first time because I asked to go back again.” (Patient, University hospital)
Anchoring Bias
While anchoring bias was less frequent, it came up in two domains: first, related to costs of care, and second, related to facility characteristics. Costs came up most frequently for Veterans who preferred to move their care to the VA for cost reasons, which appeared in these cases to overshadow other considerations:
“I kept emphasizing that the VA could do all the same things at a lot more reasonable price. The whole purpose of having the VA is for the Veteran, so that…we can get the healthcare that we need at a more reasonable [sic] or a reasonable price.” (Veteran, CLC)
“I think the CLC [VA SNF] is going to take care of her probably the same way any other facility of its type would, unless she were in a private facility, but you know, that costs a lot more money.” (Caregiver, VA hospital)
Patients occasionally had striking responses to particular characteristics of SNFs, regardless of whether this was a central feature or related to their rehabilitation:
“The social worker comes and talks to me about the nursing home where cats are running around, you know, to infect my leg or spin their little cat hairs into my lungs and make my asthma worse…I’m going to have to beg the nurses or the aides or the family or somebody to clean the cat…” (Veteran, VA hospital)
Framing
Framing was the strongest theme among clinician interviews in our sample. Clinicians most frequently described the SNF as a place where patients could recover function (a positive frame), explaining risks (eg, rehospitalization) associated with alternative postacute care options besides the SNF in great detail.
“Aside from explaining the benefits of going and…having that 24-hour care, having the therapies provided to them [the patients], talking about them getting stronger, phrasing it in such a way that patients sometimes are more agreeable, like not calling it a skilled nursing facility, calling it a rehab you know, for them to get physically stronger so they can be the most independent that they can once they do go home, and also explaining … we think that this would be the best plan to prevent them from coming back to the hospital, so those are some of the things that we’ll mention to patients to try and educate them and get them to be agreeable for placement.” (Social worker, University hospital)
Clinicians avoided negative associations with “nursing home” (even though all SNFs are nursing homes) and tended to use more positive frames such as “rehabilitation facility.”
“Use the word rehab….we definitely use the word rehab, to get more therapy, to go home; it’s not a, we really emphasize it’s not a nursing home, it’s not to go to stay forever.” (Physical therapist, safety-net hospital)
Clinicians used a frame of “safety” when discussing the SNF and used a frame of “risk” when discussing alternative postacute care options such as returning home. We did not find examples of clinicians discussing similar risks in going to a SNF even for risks, such as falling, which exist in both settings.
“I’ve talked to them primarily on an avenue of safety because I think people want and they value independence, they value making sure they can get home, but you know, a lot of the times they understand safety is, it can be a concern and outlining that our goal is to make sure that they’re safe and they stay home, and I tend to broach the subject saying that our therapists believe that they might not be safe at home in the moment, but they have potential goals to be safe later on if we continue therapy. I really highlight safety being the major driver of our discussion.” (Physician, VA hospital)
In some cases, framing was so overt that other risk-mitigating options (eg, home healthcare) are not discussed.
“I definitely tend to explain the ideal first. I’m not going to bring up home care when we really think somebody should go to rehab, however, once people say I don’t want to do that, I’m not going, then that’s when I’m like OK, well, let’s talk to the doctors, but we can see about other supports in the home.” (Social worker, VA hospital)
DISCUSSION
In a large sample of patients and their caregivers, as well as multidisciplinary clinicians at three different hospitals and three SNFs, we found authority bias/halo effect and framing biases were most common and seemed most impactful. Default/status quo bias and anchoring bias were also present in decision-making about a SNF. The combination of authority bias/halo effect and framing biases could synergistically interact to augment the likelihood of patients accepting a SNF for postacute care. Patients who had been to a SNF before seemed more likely to choose the SNF they had experienced previously even if they had no other postacute care experiences, and could be highly influenced by isolated characteristics of that facility (such as the physical environment or cost of care).
It is important to mention that cognitive biases do not necessarily have a negative impact: indeed, as Kahneman and Tversky point out, these are useful heuristics from “fast” thinking that are often effective.24 For example, clinicians may be trying to act in the best interests of the patient when framing the decision in terms of regaining function and averting loss of safety and independence. However, the evidence base regarding the outcomes of an SNF versus other postacute options is not robust, and this decision-making is complex. While this decision was most commonly framed in terms of rehabilitation and returning home, the fact that only about half of patients have returned to the community by 100 days4 was not discussed in any interview. In fact, initial evidence suggests replacing the SNF with home healthcare in patients with hip and knee arthroplasty may reduce costs without worsening clinical outcomes.6 However, across a broader population, SNFs significantly reduce 30-day readmissions when directly compared with home healthcare, but other clinical outcomes are similar.25 This evidence suggests that the “right” postacute care option for an individual patient is not clear, highlighting a key role biases may play in decision-making. Further, the nebulous concept of “safety” could introduce potential disparities related to social determinants of health.12 The observed inclination to accept an SNF with which the individual had prior experience may be influenced by the acceptability of this choice because of personal factors or prior research, even if it also represents a bias by limiting the consideration of current alternatives.
Our findings complement those of others in the literature which have also identified profound gaps in discharge decision-making among patients and clinicians,13-16,26-31 though to our knowledge the role of cognitive biases in these decisions has not been explored. This study also addresses gaps in the cognitive bias literature, including the need for real-world data rather than hypothetical vignettes,17 and evaluation of treatment and management decisions rather than diagnoses, which have been more commonly studied.21
These findings have implications for both individual clinicians and healthcare institutions. In the immediate term, these findings may serve as a call to discharging clinicians to modulate language and “debias” their conversations with patients about care after discharge.18,22 Shared decision-making requires an informed choice by patients based on their goals and values; framing a decision in a way that puts the clinician’s goals or values (eg, safety) ahead of patient values (eg, independence and autonomy) or limits disclosure (eg, a “rehab” is a nursing home) in the hope of influencing choice may be more consistent with framing bias and less with shared decision-making.14 Although controversy exists about the best way to “debias” oneself,32 self-awareness of bias is increasingly recognized across healthcare venues as critical to improving care for vulnerable populations.33 The use of data rather than vignettes may be a useful debiasing strategy, although the limitations of currently available data (eg, capturing nursing home quality) are increasingly recognized.34 From a policy and health system perspective, cognitive biases should be integrated into the development of decision aids to facilitate informed, shared, and high-quality decision-making that incorporates patient values, and perhaps “nudges” from behavioral economics to assist patients in choosing the right postdischarge care for them. Such nudges use principles of framing to influence care without restricting choice.35 As the science informing best practice regarding postacute care improves, identifying the “right” postdischarge care may become easier and recommendations more evidence-based.36
Strengths of the study include a large, diverse sample of patients, caregivers, and clinicians in both the hospital and SNF setting. Also, we used a team-based analysis with an experienced team and a deep knowledge of the data, including triangulation with clinicians to verify results. However, all hospitals and SNFs were located in a single metropolitan area, and responses may vary by region or population density. All three hospitals have housestaff teaching programs, and at the time of the interviews all three community SNFs were “five-star” facilities on the Nursing Home Compare website; results may be different at community hospitals or other SNFs. Hospitalists were the only physician group sampled in the hospital as they provide the majority of inpatient care to older adults; geriatricians, in particular, may have had different perspectives. Since we intended to explore whether cognitive biases were present overall, we did not evaluate whether cognitive biases differed by role or subgroup (by clinician type, patient, or caregiver), but this may be a promising area to explore in future work. Many cognitive biases have been described, and there are likely additional biases we did not identify. To confirm the generalizability of these findings, they should be studied in a larger, more generalizable sample of respondents in future work.
Cognitive biases play an important role in patient decision-making about postacute care, particularly regarding SNF care. As postacute care undergoes a transformation spurred by payment reforms, it is more important than ever to ensure that patients understand their choices at hospital discharge and can make a high-quality decision consistent with their goals.
1. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Rise of post-acute care facilities as a discharge destination of US hospitalizations. JAMA Intern Med. 2015;175(2):295-296. https://doi.org/10.1001/jamainternmed.2014.6383.
2. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Patient and hospitalization characteristics associated with increased postacute care facility discharges from US hospitals. Med Care. 2015;53(6):492-500. https://doi.org/10.1097/MLR.0000000000000359.
3. Werner RM, Konetzka RT. Trends in post-acute care use among medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616-1617. https://doi.org/10.1001/jama.2018.2408.
4. Medicare Payment Advisory Commission June 2018 Report to Congress. http://www.medpac.gov/docs/default-source/reports/jun18_ch5_medpacreport_sec.pdf?sfvrsn=0. Accessed November 9, 2018.
5. Burke RE, Cumbler E, Coleman EA, Levy C. Post-acute care reform: implications and opportunities for hospitalists. J Hosp Med. 2017;12(1):46-51. https://doi.org/10.1002/jhm.2673.
6. Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316(12):1267-1278. https://doi.org/10.1001/jama.2016.12717.
7. Navathe AS, Troxel AB, Liao JM, et al. Cost of joint replacement using bundled payment models. JAMA Intern Med. 2017;177(2):214-222. https://doi.org/10.1001/jamainternmed.2016.8263.
8. Kennedy G, Lewis VA, Kundu S, Mousqués J, Colla CH. Accountable care organizations and post-acute care: a focus on preferred SNF networks. Med Care Res Rev MCRR. 2018;1077558718781117. https://doi.org/10.1177/1077558718781117.
9. Chandra A, Dalton MA, Holmes J. Large increases in spending on postacute care in Medicare point to the potential for cost savings in these settings. Health Aff Proj Hope. 2013;32(5):864-872. https://doi.org/10.1377/hlthaff.2012.1262.
10. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the Medicare shared savings program. JAMA Intern Med. 2017;177(4):518-526. https://doi.org/10.1001/jamainternmed.2016.9115.
11. Zhu JM, Patel V, Shea JA, Neuman MD, Werner RM. Hospitals using bundled payment report reducing skilled nursing facility use and improving care integration. Health Aff Proj Hope. 2018;37(8):1282-1289. https://doi.org/10.1377/hlthaff.2018.0257.
12. Burke RE, Ibrahim SA. Discharge destination and disparities in postoperative care. JAMA. 2018;319(16):1653-1654. https://doi.org/10.1001/jama.2017.21884.
13. Burke RE, Lawrence E, Ladebue A, et al. How hospital clinicians select patients for skilled nursing facilities. J Am Geriatr Soc. 2017;65(11):2466-2472. https://doi.org/10.1111/jgs.14954.
14. Burke RE, Jones J, Lawrence E, et al. Evaluating the quality of patient decision-making regarding post-acute care. J Gen Intern Med. 2018;33(5):678-684. https://doi.org/10.1007/s11606-017-4298-1.
15. Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc. 2017;65(11):2459-2465. https://doi.org/10.1111/jgs.14988.
16. Tyler DA, Gadbois EA, McHugh JP, Shield RR, Winblad U, Mor V. Patients are not given quality-of-care data about skilled nursing facilities when discharged from hospitals. Health Aff. 2017;36(8):1385-1391. https://doi.org/10.1377/hlthaff.2017.0155.
17. Blumenthal-Barby JS, Krieger H. Cognitive biases and heuristics in medical decision making: a critical review using a systematic search strategy. Med Decis Mak Int J Soc Med Decis Mak. 2015;35(4):539-557. https://doi.org/10.1177/0272989X14547740.
18. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22 Suppl 2:ii58-ii64. https://doi.org/10.1136/bmjqs-2012-001712.
19. Hinds PS, Vogel RJ, Clarke-Steffen L. The possibilities and pitfalls of doing a secondary analysis of a qualitative data set. Qual Health Res. 1997;7(3):408-424. https://doi.org/10.1177/104973239700700306.
20. Magid M, Mcllvennan CK, Jones J, et al. Exploring cognitive bias in destination therapy left ventricular assist device decision making: a retrospective qualitative framework analysis. Am Heart J. 2016;180:64-73. https://doi.org/10.1016/j.ahj.2016.06.024.
21. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16(1):138. https://doi.org/10.1186/s12911-016-0377-1.
22. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: impediments to and strategies for change. BMJ Qual Saf. 2013;22 Suppl 2:ii65-ii72. https://doi.org/10.1136/bmjqs-2012-001713.
23. Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42(4):1758-1772. https://doi.org/10.1111/j.1475-6773.2006.00684.x.
24. Thinking, Fast and Slow. Daniel Kahneman. Macmillan. US Macmillan. https://us.macmillan.com/thinkingfastandslow/danielkahneman/9780374533557. Accessed February 5, 2019.
25. Werner RM, Konetzka RT, Coe NB. Does type of post-acute care matter? The effect of hospital discharge to home with home health care versus to skilled nursing facility. JAMA Intern Med. In press.
26. Jones J, Lawrence E, Ladebue A, Leonard C, Ayele R, Burke RE. Nurses’ role in managing “The Fit” of older adults in skilled nursing facilities. J Gerontol Nurs. 2017;43(12):11-20. https://doi.org/10.3928/00989134-20171110-06.
27. Lawrence E, Casler J-J, Jones J, et al. Variability in skilled nursing facility screening and admission processes: implications for value-based purchasing. Health Care Manage Rev. 2018. https://doi.org/10.1097/HMR.0000000000000225.
28. Ayele R, Jones J, Ladebue A, et al. Perceived costs of care influence post-acute care choices by clinicians, patients, and caregivers. J Am Geriatr Soc. 2019. https://doi.org/10.1111/jgs.15768.
29. Sefcik JS, Nock RH, Flores EJ, et al. Patient preferences for information on post-acute care services. Res Gerontol Nurs. 2016;9(4):175-182. https://doi.org/10.3928/19404921-20160120-01.
30. Konetzka RT, Perraillon MC. Use of nursing home compare website appears limited by lack of awareness and initial mistrust of the data. Health Aff Proj Hope. 2016;35(4):706-713. https://doi.org/10.1377/hlthaff.2015.1377.
31. Schapira MM, Shea JA, Duey KA, Kleiman C, Werner RM. The nursing home compare report card: perceptions of residents and caregivers regarding quality ratings and nursing home choice. Health Serv Res. 2016;51 Suppl 2:1212-1228. https://doi.org/10.1111/1475-6773.12458.
32. Dhaliwal G. Premature closure? Not so fast. BMJ Qual Saf. 2017;26(2):87-89. https://doi.org/10.1136/bmjqs-2016-005267.
33. Masters C, Robinson D, Faulkner S, Patterson E, McIlraith T, Ansari A. Addressing biases in patient care with the 5Rs of cultural humility, a clinician coaching tool. J Gen Intern Med. 2019;34(4):627-630. https://doi.org/10.1007/s11606-018-4814-y.
34. Burke RE, Werner RM. Quality measurement and nursing homes: measuring what matters. BMJ Qual Saf. 2019;28(7);520-523. https://doi.org/10.1136/bmjqs-2019-009447.
35. Patel MS, Volpp KG, Asch DA. Nudge units to improve the delivery of health care. N Engl J Med. 2018;378(3):214-216. https://doi.org/10.1056/NEJMp1712984.
36. Jenq GY, Tinetti ME. Post–acute care: who belongs where? JAMA Intern Med. 2015;175(2):296-297. https://doi.org/10.1001/jamainternmed.2014.4298.
1. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Rise of post-acute care facilities as a discharge destination of US hospitalizations. JAMA Intern Med. 2015;175(2):295-296. https://doi.org/10.1001/jamainternmed.2014.6383.
2. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Patient and hospitalization characteristics associated with increased postacute care facility discharges from US hospitals. Med Care. 2015;53(6):492-500. https://doi.org/10.1097/MLR.0000000000000359.
3. Werner RM, Konetzka RT. Trends in post-acute care use among medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616-1617. https://doi.org/10.1001/jama.2018.2408.
4. Medicare Payment Advisory Commission June 2018 Report to Congress. http://www.medpac.gov/docs/default-source/reports/jun18_ch5_medpacreport_sec.pdf?sfvrsn=0. Accessed November 9, 2018.
5. Burke RE, Cumbler E, Coleman EA, Levy C. Post-acute care reform: implications and opportunities for hospitalists. J Hosp Med. 2017;12(1):46-51. https://doi.org/10.1002/jhm.2673.
6. Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316(12):1267-1278. https://doi.org/10.1001/jama.2016.12717.
7. Navathe AS, Troxel AB, Liao JM, et al. Cost of joint replacement using bundled payment models. JAMA Intern Med. 2017;177(2):214-222. https://doi.org/10.1001/jamainternmed.2016.8263.
8. Kennedy G, Lewis VA, Kundu S, Mousqués J, Colla CH. Accountable care organizations and post-acute care: a focus on preferred SNF networks. Med Care Res Rev MCRR. 2018;1077558718781117. https://doi.org/10.1177/1077558718781117.
9. Chandra A, Dalton MA, Holmes J. Large increases in spending on postacute care in Medicare point to the potential for cost savings in these settings. Health Aff Proj Hope. 2013;32(5):864-872. https://doi.org/10.1377/hlthaff.2012.1262.
10. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the Medicare shared savings program. JAMA Intern Med. 2017;177(4):518-526. https://doi.org/10.1001/jamainternmed.2016.9115.
11. Zhu JM, Patel V, Shea JA, Neuman MD, Werner RM. Hospitals using bundled payment report reducing skilled nursing facility use and improving care integration. Health Aff Proj Hope. 2018;37(8):1282-1289. https://doi.org/10.1377/hlthaff.2018.0257.
12. Burke RE, Ibrahim SA. Discharge destination and disparities in postoperative care. JAMA. 2018;319(16):1653-1654. https://doi.org/10.1001/jama.2017.21884.
13. Burke RE, Lawrence E, Ladebue A, et al. How hospital clinicians select patients for skilled nursing facilities. J Am Geriatr Soc. 2017;65(11):2466-2472. https://doi.org/10.1111/jgs.14954.
14. Burke RE, Jones J, Lawrence E, et al. Evaluating the quality of patient decision-making regarding post-acute care. J Gen Intern Med. 2018;33(5):678-684. https://doi.org/10.1007/s11606-017-4298-1.
15. Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc. 2017;65(11):2459-2465. https://doi.org/10.1111/jgs.14988.
16. Tyler DA, Gadbois EA, McHugh JP, Shield RR, Winblad U, Mor V. Patients are not given quality-of-care data about skilled nursing facilities when discharged from hospitals. Health Aff. 2017;36(8):1385-1391. https://doi.org/10.1377/hlthaff.2017.0155.
17. Blumenthal-Barby JS, Krieger H. Cognitive biases and heuristics in medical decision making: a critical review using a systematic search strategy. Med Decis Mak Int J Soc Med Decis Mak. 2015;35(4):539-557. https://doi.org/10.1177/0272989X14547740.
18. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22 Suppl 2:ii58-ii64. https://doi.org/10.1136/bmjqs-2012-001712.
19. Hinds PS, Vogel RJ, Clarke-Steffen L. The possibilities and pitfalls of doing a secondary analysis of a qualitative data set. Qual Health Res. 1997;7(3):408-424. https://doi.org/10.1177/104973239700700306.
20. Magid M, Mcllvennan CK, Jones J, et al. Exploring cognitive bias in destination therapy left ventricular assist device decision making: a retrospective qualitative framework analysis. Am Heart J. 2016;180:64-73. https://doi.org/10.1016/j.ahj.2016.06.024.
21. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16(1):138. https://doi.org/10.1186/s12911-016-0377-1.
22. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: impediments to and strategies for change. BMJ Qual Saf. 2013;22 Suppl 2:ii65-ii72. https://doi.org/10.1136/bmjqs-2012-001713.
23. Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42(4):1758-1772. https://doi.org/10.1111/j.1475-6773.2006.00684.x.
24. Thinking, Fast and Slow. Daniel Kahneman. Macmillan. US Macmillan. https://us.macmillan.com/thinkingfastandslow/danielkahneman/9780374533557. Accessed February 5, 2019.
25. Werner RM, Konetzka RT, Coe NB. Does type of post-acute care matter? The effect of hospital discharge to home with home health care versus to skilled nursing facility. JAMA Intern Med. In press.
26. Jones J, Lawrence E, Ladebue A, Leonard C, Ayele R, Burke RE. Nurses’ role in managing “The Fit” of older adults in skilled nursing facilities. J Gerontol Nurs. 2017;43(12):11-20. https://doi.org/10.3928/00989134-20171110-06.
27. Lawrence E, Casler J-J, Jones J, et al. Variability in skilled nursing facility screening and admission processes: implications for value-based purchasing. Health Care Manage Rev. 2018. https://doi.org/10.1097/HMR.0000000000000225.
28. Ayele R, Jones J, Ladebue A, et al. Perceived costs of care influence post-acute care choices by clinicians, patients, and caregivers. J Am Geriatr Soc. 2019. https://doi.org/10.1111/jgs.15768.
29. Sefcik JS, Nock RH, Flores EJ, et al. Patient preferences for information on post-acute care services. Res Gerontol Nurs. 2016;9(4):175-182. https://doi.org/10.3928/19404921-20160120-01.
30. Konetzka RT, Perraillon MC. Use of nursing home compare website appears limited by lack of awareness and initial mistrust of the data. Health Aff Proj Hope. 2016;35(4):706-713. https://doi.org/10.1377/hlthaff.2015.1377.
31. Schapira MM, Shea JA, Duey KA, Kleiman C, Werner RM. The nursing home compare report card: perceptions of residents and caregivers regarding quality ratings and nursing home choice. Health Serv Res. 2016;51 Suppl 2:1212-1228. https://doi.org/10.1111/1475-6773.12458.
32. Dhaliwal G. Premature closure? Not so fast. BMJ Qual Saf. 2017;26(2):87-89. https://doi.org/10.1136/bmjqs-2016-005267.
33. Masters C, Robinson D, Faulkner S, Patterson E, McIlraith T, Ansari A. Addressing biases in patient care with the 5Rs of cultural humility, a clinician coaching tool. J Gen Intern Med. 2019;34(4):627-630. https://doi.org/10.1007/s11606-018-4814-y.
34. Burke RE, Werner RM. Quality measurement and nursing homes: measuring what matters. BMJ Qual Saf. 2019;28(7);520-523. https://doi.org/10.1136/bmjqs-2019-009447.
35. Patel MS, Volpp KG, Asch DA. Nudge units to improve the delivery of health care. N Engl J Med. 2018;378(3):214-216. https://doi.org/10.1056/NEJMp1712984.
36. Jenq GY, Tinetti ME. Post–acute care: who belongs where? JAMA Intern Med. 2015;175(2):296-297. https://doi.org/10.1001/jamainternmed.2014.4298.
© 2020 Society of Hospital Medicine
Waiting for Godot: The Quest to Promote Scholarship in Hospital Medicine
Twenty years into the hospitalist movement, the proven formula for developing high-quality scholarly output in a hospital medicine group remains elusive. In this issue of the Journal of Hospital Medicine, McKinney et al. describe a new model in which an academic research coach—a PhD-trained researcher with 50% protected time to assist with hospitalist scholarly activities—is utilized to support scholarship.1 Built on the premise that most hospitalist faculty do not have research training and many are embarking on their first academic project, the research coach was available to engage hospitalists at any stage of scholarship from conceptualizing an idea, to submitting one’s first IRB, to data analysis, and grant and manuscript submission. This innovation (and the financial investment required) provides an opportunity to consider how to facilitate scholarship and measure its value in hospital medicine groups.
Academic institutions are built on the premise that scholarship—and research in particular—is of equal value to clinical care and teaching; a perspective that is commonly enshrined in promotion criteria that require scholarship for career advancement. While hospitalists are competent to begin clinical practice and transfer their knowledge to others at the conclusion of their residency, most are not prepared to lead research programs or create academic products from their clinical innovations, quality improvement, or medical education work. Yet, particularly for hospitalists who choose to practice in an academic setting, the leadership of their Section, Division, or Department may naturally expect scholarship to occur, similar to other clinical disciplines. In our experience as the directors of research and faculty development in our hospital medicine group, meeting this expectation requires recognizing that faculty development and scholarship development are intertwined and there must be an investment in both.
We believe that faculty development is required—but not sufficient—for the development of high-quality scholarship. In order for hospitalists to generate new knowledge in clinical, educational, quality improvement, and research domains, they must acquire a new skill set after residency training. These skills can be gained in different formats and time frames such as dedicated hospital medicine fellowships, internal faculty development programs, external programs (eg, Academic Hospitalist Academy), and/or individual mentorship. Descriptions of internal faculty development programs have unfortunately been limited to a single institutions with uncertain generalizability.2,3 One could argue that faculty development may even be more important in hospital medicine than in clinical subspecialties given the relative youth of the field and the experience level of the entry-level faculty. Pediatric hospital medicine may be farthest along in faculty development and scholarship development after becoming a distinct subspecialty recognized by the American Board of Pediatrics and American Board of Medical Specialties; pediatric hospitalists must now complete fellowship training after residency before independent practice.4 Importantly, completion of a scholarly product that advances the field is a required component of the pediatric hospital medicine fellowship curricular framework.5 Regardless of what infrastructure a hospital medicine group chooses to build, there is a growing realization that faculty development must be firmly in place in order for scholarship to flourish.
In addition to junior faculty development, there is also a need for scholarship development to translate new skills into products of scholarship. For example, a well-published senior faculty member still may need statistical assistance and a midcareer hospitalist who leads quality improvement may struggle to write an effective manuscript to disseminate their findings. McKinney et al.’s innovation seems intended to meet this need, and the just-in-time and menu-style nature of the academic research coach resource is unique and novel. One can imagine how this approach to increasing scholarship productivity could be effective and utilized by busy junior, midcareer, and senior hospitalists alike. As the authors point out, this model attempts to mitigate the drawbacks that other models for enhancing hospitalist scholarship have faced, such as relying on physician scientists as mentors, holding works-in-progress or research seminars, or funding a consulting statistician. A well-trained scientist who meets hospitalists “where they are” is appealing when placed in the context of an effective faculty development program that enables faculty to take advantage of this resource. We hope that future evaluations of this promising innovation will include a comparison group to measure the effect of the academic research coach and demonstrate a return on the financial investment supporting the academic research coach.
Measuring return on investment requires defining the value of scholarship in hospital medicine. Some things that are easy to measure and have valence for traditional academic productivity are captured in the McKinney manuscript: the number of abstracts, papers, and grants.
Measuring the value of scholarship in hospital medicine touches very near to the core of the value proposition of hospital medicine overall as a specialty. Without high-quality scholarship that demonstrates the influence of hospitalists in improving systems, leading change, educating learners, and advocating for the needs of our patients, why continue to invest in this model? We are struck every year at the Society of Hospital Medicine national conference about how much innovation hospitalists are leading – and how little is systematically evaluated or disseminated. In Beckett’s “Waiting for Godot,” Vladimir and Estragon talk about life and wait for Godot who, of course, never arrives. Instead of patiently waiting for more scholarship to arrive, we suggest that hospital medicine leaders follow the lead of McKinney et al. and take action by investing in it.
Disclosures
The views expressed are those of the authors and not necessarily those of the Department of Veterans Affairs.
Funding
Dr. Burke is funded by a VA HSR&D Career Development Award.
1. McKinney CM, Mookherjee S, Fihn SD, Gallagher TH. An academic research coach: an innovative approach to increasing scholarly productivity in medicine. J Hosp Med. 2019;14(8):457-461. https://doi.org/10.12788/jhm.3194.
2. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161-166. https://doi.org/10.1002/jhm.845.
3. Seymann GB, Southern W, Burger A, et al. Features of successful academic hospitalist programs: Insights from the SCHOLAR (SuCcessful HOspitaLists in academics and research) project. J Hosp Med. 2016;11(10):708-713. https://doi.org/10.1002/jhm.2603.
4. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: a proposed new subspecialty. Pediatrics. 2017;139(3):e20161823. https://doi.org/10.1542/peds.2016-1823.
5. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698.
6. Wachter RM, Goldman L. Zero to 50,000 - the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
Twenty years into the hospitalist movement, the proven formula for developing high-quality scholarly output in a hospital medicine group remains elusive. In this issue of the Journal of Hospital Medicine, McKinney et al. describe a new model in which an academic research coach—a PhD-trained researcher with 50% protected time to assist with hospitalist scholarly activities—is utilized to support scholarship.1 Built on the premise that most hospitalist faculty do not have research training and many are embarking on their first academic project, the research coach was available to engage hospitalists at any stage of scholarship from conceptualizing an idea, to submitting one’s first IRB, to data analysis, and grant and manuscript submission. This innovation (and the financial investment required) provides an opportunity to consider how to facilitate scholarship and measure its value in hospital medicine groups.
Academic institutions are built on the premise that scholarship—and research in particular—is of equal value to clinical care and teaching; a perspective that is commonly enshrined in promotion criteria that require scholarship for career advancement. While hospitalists are competent to begin clinical practice and transfer their knowledge to others at the conclusion of their residency, most are not prepared to lead research programs or create academic products from their clinical innovations, quality improvement, or medical education work. Yet, particularly for hospitalists who choose to practice in an academic setting, the leadership of their Section, Division, or Department may naturally expect scholarship to occur, similar to other clinical disciplines. In our experience as the directors of research and faculty development in our hospital medicine group, meeting this expectation requires recognizing that faculty development and scholarship development are intertwined and there must be an investment in both.
We believe that faculty development is required—but not sufficient—for the development of high-quality scholarship. In order for hospitalists to generate new knowledge in clinical, educational, quality improvement, and research domains, they must acquire a new skill set after residency training. These skills can be gained in different formats and time frames such as dedicated hospital medicine fellowships, internal faculty development programs, external programs (eg, Academic Hospitalist Academy), and/or individual mentorship. Descriptions of internal faculty development programs have unfortunately been limited to a single institutions with uncertain generalizability.2,3 One could argue that faculty development may even be more important in hospital medicine than in clinical subspecialties given the relative youth of the field and the experience level of the entry-level faculty. Pediatric hospital medicine may be farthest along in faculty development and scholarship development after becoming a distinct subspecialty recognized by the American Board of Pediatrics and American Board of Medical Specialties; pediatric hospitalists must now complete fellowship training after residency before independent practice.4 Importantly, completion of a scholarly product that advances the field is a required component of the pediatric hospital medicine fellowship curricular framework.5 Regardless of what infrastructure a hospital medicine group chooses to build, there is a growing realization that faculty development must be firmly in place in order for scholarship to flourish.
In addition to junior faculty development, there is also a need for scholarship development to translate new skills into products of scholarship. For example, a well-published senior faculty member still may need statistical assistance and a midcareer hospitalist who leads quality improvement may struggle to write an effective manuscript to disseminate their findings. McKinney et al.’s innovation seems intended to meet this need, and the just-in-time and menu-style nature of the academic research coach resource is unique and novel. One can imagine how this approach to increasing scholarship productivity could be effective and utilized by busy junior, midcareer, and senior hospitalists alike. As the authors point out, this model attempts to mitigate the drawbacks that other models for enhancing hospitalist scholarship have faced, such as relying on physician scientists as mentors, holding works-in-progress or research seminars, or funding a consulting statistician. A well-trained scientist who meets hospitalists “where they are” is appealing when placed in the context of an effective faculty development program that enables faculty to take advantage of this resource. We hope that future evaluations of this promising innovation will include a comparison group to measure the effect of the academic research coach and demonstrate a return on the financial investment supporting the academic research coach.
Measuring return on investment requires defining the value of scholarship in hospital medicine. Some things that are easy to measure and have valence for traditional academic productivity are captured in the McKinney manuscript: the number of abstracts, papers, and grants.
Measuring the value of scholarship in hospital medicine touches very near to the core of the value proposition of hospital medicine overall as a specialty. Without high-quality scholarship that demonstrates the influence of hospitalists in improving systems, leading change, educating learners, and advocating for the needs of our patients, why continue to invest in this model? We are struck every year at the Society of Hospital Medicine national conference about how much innovation hospitalists are leading – and how little is systematically evaluated or disseminated. In Beckett’s “Waiting for Godot,” Vladimir and Estragon talk about life and wait for Godot who, of course, never arrives. Instead of patiently waiting for more scholarship to arrive, we suggest that hospital medicine leaders follow the lead of McKinney et al. and take action by investing in it.
Disclosures
The views expressed are those of the authors and not necessarily those of the Department of Veterans Affairs.
Funding
Dr. Burke is funded by a VA HSR&D Career Development Award.
Twenty years into the hospitalist movement, the proven formula for developing high-quality scholarly output in a hospital medicine group remains elusive. In this issue of the Journal of Hospital Medicine, McKinney et al. describe a new model in which an academic research coach—a PhD-trained researcher with 50% protected time to assist with hospitalist scholarly activities—is utilized to support scholarship.1 Built on the premise that most hospitalist faculty do not have research training and many are embarking on their first academic project, the research coach was available to engage hospitalists at any stage of scholarship from conceptualizing an idea, to submitting one’s first IRB, to data analysis, and grant and manuscript submission. This innovation (and the financial investment required) provides an opportunity to consider how to facilitate scholarship and measure its value in hospital medicine groups.
Academic institutions are built on the premise that scholarship—and research in particular—is of equal value to clinical care and teaching; a perspective that is commonly enshrined in promotion criteria that require scholarship for career advancement. While hospitalists are competent to begin clinical practice and transfer their knowledge to others at the conclusion of their residency, most are not prepared to lead research programs or create academic products from their clinical innovations, quality improvement, or medical education work. Yet, particularly for hospitalists who choose to practice in an academic setting, the leadership of their Section, Division, or Department may naturally expect scholarship to occur, similar to other clinical disciplines. In our experience as the directors of research and faculty development in our hospital medicine group, meeting this expectation requires recognizing that faculty development and scholarship development are intertwined and there must be an investment in both.
We believe that faculty development is required—but not sufficient—for the development of high-quality scholarship. In order for hospitalists to generate new knowledge in clinical, educational, quality improvement, and research domains, they must acquire a new skill set after residency training. These skills can be gained in different formats and time frames such as dedicated hospital medicine fellowships, internal faculty development programs, external programs (eg, Academic Hospitalist Academy), and/or individual mentorship. Descriptions of internal faculty development programs have unfortunately been limited to a single institutions with uncertain generalizability.2,3 One could argue that faculty development may even be more important in hospital medicine than in clinical subspecialties given the relative youth of the field and the experience level of the entry-level faculty. Pediatric hospital medicine may be farthest along in faculty development and scholarship development after becoming a distinct subspecialty recognized by the American Board of Pediatrics and American Board of Medical Specialties; pediatric hospitalists must now complete fellowship training after residency before independent practice.4 Importantly, completion of a scholarly product that advances the field is a required component of the pediatric hospital medicine fellowship curricular framework.5 Regardless of what infrastructure a hospital medicine group chooses to build, there is a growing realization that faculty development must be firmly in place in order for scholarship to flourish.
In addition to junior faculty development, there is also a need for scholarship development to translate new skills into products of scholarship. For example, a well-published senior faculty member still may need statistical assistance and a midcareer hospitalist who leads quality improvement may struggle to write an effective manuscript to disseminate their findings. McKinney et al.’s innovation seems intended to meet this need, and the just-in-time and menu-style nature of the academic research coach resource is unique and novel. One can imagine how this approach to increasing scholarship productivity could be effective and utilized by busy junior, midcareer, and senior hospitalists alike. As the authors point out, this model attempts to mitigate the drawbacks that other models for enhancing hospitalist scholarship have faced, such as relying on physician scientists as mentors, holding works-in-progress or research seminars, or funding a consulting statistician. A well-trained scientist who meets hospitalists “where they are” is appealing when placed in the context of an effective faculty development program that enables faculty to take advantage of this resource. We hope that future evaluations of this promising innovation will include a comparison group to measure the effect of the academic research coach and demonstrate a return on the financial investment supporting the academic research coach.
Measuring return on investment requires defining the value of scholarship in hospital medicine. Some things that are easy to measure and have valence for traditional academic productivity are captured in the McKinney manuscript: the number of abstracts, papers, and grants.
Measuring the value of scholarship in hospital medicine touches very near to the core of the value proposition of hospital medicine overall as a specialty. Without high-quality scholarship that demonstrates the influence of hospitalists in improving systems, leading change, educating learners, and advocating for the needs of our patients, why continue to invest in this model? We are struck every year at the Society of Hospital Medicine national conference about how much innovation hospitalists are leading – and how little is systematically evaluated or disseminated. In Beckett’s “Waiting for Godot,” Vladimir and Estragon talk about life and wait for Godot who, of course, never arrives. Instead of patiently waiting for more scholarship to arrive, we suggest that hospital medicine leaders follow the lead of McKinney et al. and take action by investing in it.
Disclosures
The views expressed are those of the authors and not necessarily those of the Department of Veterans Affairs.
Funding
Dr. Burke is funded by a VA HSR&D Career Development Award.
1. McKinney CM, Mookherjee S, Fihn SD, Gallagher TH. An academic research coach: an innovative approach to increasing scholarly productivity in medicine. J Hosp Med. 2019;14(8):457-461. https://doi.org/10.12788/jhm.3194.
2. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161-166. https://doi.org/10.1002/jhm.845.
3. Seymann GB, Southern W, Burger A, et al. Features of successful academic hospitalist programs: Insights from the SCHOLAR (SuCcessful HOspitaLists in academics and research) project. J Hosp Med. 2016;11(10):708-713. https://doi.org/10.1002/jhm.2603.
4. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: a proposed new subspecialty. Pediatrics. 2017;139(3):e20161823. https://doi.org/10.1542/peds.2016-1823.
5. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698.
6. Wachter RM, Goldman L. Zero to 50,000 - the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
1. McKinney CM, Mookherjee S, Fihn SD, Gallagher TH. An academic research coach: an innovative approach to increasing scholarly productivity in medicine. J Hosp Med. 2019;14(8):457-461. https://doi.org/10.12788/jhm.3194.
2. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161-166. https://doi.org/10.1002/jhm.845.
3. Seymann GB, Southern W, Burger A, et al. Features of successful academic hospitalist programs: Insights from the SCHOLAR (SuCcessful HOspitaLists in academics and research) project. J Hosp Med. 2016;11(10):708-713. https://doi.org/10.1002/jhm.2603.
4. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: a proposed new subspecialty. Pediatrics. 2017;139(3):e20161823. https://doi.org/10.1542/peds.2016-1823.
5. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698.
6. Wachter RM, Goldman L. Zero to 50,000 - the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
© 2019 Society of Hospital Medicine




