User login
Swollen toes
A 15-month-old black male was brought to the pediatric emergency department by his grandmother because she was concerned about his 2 swollen big toes. The patient’s grandmother said that the swelling began 36 hours prior and that her grandson’s big toes had continued to increase in size. She denied trauma, bites, or unusual exposures and said that although her grandson had been fussier than usual that day, he was eating and drinking normally and had normal urine output.
The patient had a history of developmental delay, but was otherwise healthy. He had no rashes, and there was no recent history of vomiting, diarrhea, difficulty breathing, or fever.
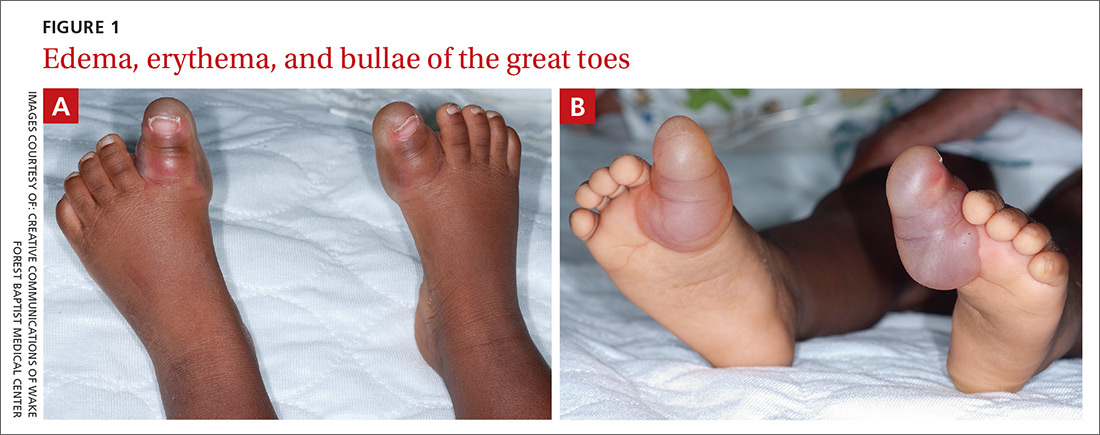
Examination of the patient’s skin revealed diffuse edema and erythema of the bilateral great toes (FIGURE 1A), with large overlying bullae extending from the dorsal surface of the base of the great toes around to the plantar (volar) surface of the foot (FIGURE 1B). The bullae on the plantar surface were approximately 4 cm long, extending from the tip of the toes proximally to the region of the head of the first metatarsal.
The patient’s vital signs were notable for a rectal temperature of 100.2° F and a heart rate of 180 beats per minute.
Initial lab tests included a complete blood count (CBC), blood cultures, and urinalysis with urine culture. The CBC revealed a white blood count of 27,000/mcL (normal: 6000-17,500/mcL). Both wound culture and herpes simplex viral culture were negative. An intranasal surveillance culture for methicillin-resistant Staphylococcus aureus (MRSA) was also negative.
Given the patient’s fever and leukocytosis, a 100-mg dose of intravenous clindamycin was administered.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Blistering distal dactylitis
We made a clinical diagnosis of blistering distal dactylitis (BDD), a condition typically caused by infection with Gram-positive bacteria. BDD is generally described as a localized infection of the volar fat pads of one or more fingers. The infection may also occur more proximally on the hand or involve the thumbs or toes.1
Who’s at risk? BDD occurs among children ages 2 to 16 years, although it has been reported in infants as young as 6 months and in adults. No cases have occurred among the elderly.2-7
The most common etiologic agents are group A beta-hemolytic Streptococci. Less commonly reported agents include Staphylococcus aureus, S. epidermidis, group B Streptococci, and MRSA.1,6,8 The presence of multiple bullae may be predictive of infection with S. aureus.9
A clinical diagnosis
Diagnosis is usually made on clinical grounds based on the presence of large, tense, superficial, and typically painful bullae, the base of which may be erythematous. Culture of the blister fluid and the base of an unroofed blister may confirm the presence of a Streptococcus or Staphylococcus species.
Lab tests are typically not required to confirm a diagnosis of BDD. However, wound cultures of blister fluid, rapid antigen testing for group A beta-hemolytic Streptococci, and viral culture or polymerase chain reaction testing for herpes simplex virus may be considered.
Rule these conditions out
Lesions similar to those seen with BDD can be caused by the following infections and irritants:4,5,8
Herpetic whitlow is caused by a herpes simplex virus infection. It presents as a cluster of painful vesicles or ulcers with an erythematous base on the distal part of a finger or toe.
Bullous impetigo is the result of a staphylococcal infection, which produces an epidermolytic toxin leading to bulla formation. Lesions may occur anywhere on the body but are most common on the face.
Irritant or allergic contact dermatitis results from an external topical exposure and is typically localized to the area of contact. The reaction is an eczematous eruption that may include bullae.
Treatment is typically empiric
Treatment of BDD includes wound care with wet-to-dry saline dressings, incision and drainage of the bulla(e), and a systemic beta-lactamase-resistant antibiotic. Topical antibiotics alone are not recommended.7
Our patient was transitioned from intravenous to oral clindamycin, 100 mg every 8 hours, and the bullae were incised and drained. His leukocytosis resolved within 24 hours, and he continued to do well. At follow-up one week later, the patient’s blisters were healing well, and he was playful and eating and drinking normally.
CORRESPONDENCE
C. Randall Clinch, DO, MS, Wake Forest University School of Medicine, 1 Medical Center Blvd, Winston-Salem, NC 27157; [email protected].
1. Hays GC, Mullard JE. Blistering distal dactylitis: a clinically recognizable streptococcal infection. Pediatrics. 1975;56:129-131.
2. Schneider JA, Parlette HL 3rd. Blistering distal dactylitis: a manifestation of group A beta-hemolytic streptococcal infection. Arch Dermatol. 1982;118:879-880.
3. Scheinfeld NS. Is blistering distal dactylitis a variant of bullous impetigo? Clin Exp Dermatol. 2007;32:314-316.
4. Kollipara R, Downing C, Lee M, et al. Blistering distal dactylitis in an adult. J Cutan Med Surg. 2015;19:397-399.
5. Fretzayas A, Moustaki M, Tsagris V, et al. MRSA blistering distal dactylitis and review of reported cases. Pediatr Dermatol. 2011;28:433-435.
6. Lyon M, Doehring MC. Blistering distal dactylitis: a case series in children under nine months of age. J Emerg Med. 2004;26:421-423.
7. Frieden IJ. Blistering dactylitis caused by group B streptococci. Pediatr Dermatol. 1989;6:300-302.
8. Woroszylski A, Durán C, Tamayo L, et al. Staphylococcal blistering dactylitis: report of two patients. Pediatr Dermatol. 1996;13:292-293.
9. Norcross MC Jr, Mitchell DF. Blistering distal dactylitis caused by Staphylococcus aureus. Cutis. 1993;51:353-354 .
A 15-month-old black male was brought to the pediatric emergency department by his grandmother because she was concerned about his 2 swollen big toes. The patient’s grandmother said that the swelling began 36 hours prior and that her grandson’s big toes had continued to increase in size. She denied trauma, bites, or unusual exposures and said that although her grandson had been fussier than usual that day, he was eating and drinking normally and had normal urine output.
The patient had a history of developmental delay, but was otherwise healthy. He had no rashes, and there was no recent history of vomiting, diarrhea, difficulty breathing, or fever.
Examination of the patient’s skin revealed diffuse edema and erythema of the bilateral great toes (FIGURE 1A), with large overlying bullae extending from the dorsal surface of the base of the great toes around to the plantar (volar) surface of the foot (FIGURE 1B). The bullae on the plantar surface were approximately 4 cm long, extending from the tip of the toes proximally to the region of the head of the first metatarsal.

The patient’s vital signs were notable for a rectal temperature of 100.2° F and a heart rate of 180 beats per minute.
Initial lab tests included a complete blood count (CBC), blood cultures, and urinalysis with urine culture. The CBC revealed a white blood count of 27,000/mcL (normal: 6000-17,500/mcL). Both wound culture and herpes simplex viral culture were negative. An intranasal surveillance culture for methicillin-resistant Staphylococcus aureus (MRSA) was also negative.
Given the patient’s fever and leukocytosis, a 100-mg dose of intravenous clindamycin was administered.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Blistering distal dactylitis
We made a clinical diagnosis of blistering distal dactylitis (BDD), a condition typically caused by infection with Gram-positive bacteria. BDD is generally described as a localized infection of the volar fat pads of one or more fingers. The infection may also occur more proximally on the hand or involve the thumbs or toes.1
Who’s at risk? BDD occurs among children ages 2 to 16 years, although it has been reported in infants as young as 6 months and in adults. No cases have occurred among the elderly.2-7
The most common etiologic agents are group A beta-hemolytic Streptococci. Less commonly reported agents include Staphylococcus aureus, S. epidermidis, group B Streptococci, and MRSA.1,6,8 The presence of multiple bullae may be predictive of infection with S. aureus.9
A clinical diagnosis
Diagnosis is usually made on clinical grounds based on the presence of large, tense, superficial, and typically painful bullae, the base of which may be erythematous. Culture of the blister fluid and the base of an unroofed blister may confirm the presence of a Streptococcus or Staphylococcus species.
Lab tests are typically not required to confirm a diagnosis of BDD. However, wound cultures of blister fluid, rapid antigen testing for group A beta-hemolytic Streptococci, and viral culture or polymerase chain reaction testing for herpes simplex virus may be considered.
Rule these conditions out
Lesions similar to those seen with BDD can be caused by the following infections and irritants:4,5,8
Herpetic whitlow is caused by a herpes simplex virus infection. It presents as a cluster of painful vesicles or ulcers with an erythematous base on the distal part of a finger or toe.
Bullous impetigo is the result of a staphylococcal infection, which produces an epidermolytic toxin leading to bulla formation. Lesions may occur anywhere on the body but are most common on the face.
Irritant or allergic contact dermatitis results from an external topical exposure and is typically localized to the area of contact. The reaction is an eczematous eruption that may include bullae.
Treatment is typically empiric
Treatment of BDD includes wound care with wet-to-dry saline dressings, incision and drainage of the bulla(e), and a systemic beta-lactamase-resistant antibiotic. Topical antibiotics alone are not recommended.7
Our patient was transitioned from intravenous to oral clindamycin, 100 mg every 8 hours, and the bullae were incised and drained. His leukocytosis resolved within 24 hours, and he continued to do well. At follow-up one week later, the patient’s blisters were healing well, and he was playful and eating and drinking normally.
CORRESPONDENCE
C. Randall Clinch, DO, MS, Wake Forest University School of Medicine, 1 Medical Center Blvd, Winston-Salem, NC 27157; [email protected].
A 15-month-old black male was brought to the pediatric emergency department by his grandmother because she was concerned about his 2 swollen big toes. The patient’s grandmother said that the swelling began 36 hours prior and that her grandson’s big toes had continued to increase in size. She denied trauma, bites, or unusual exposures and said that although her grandson had been fussier than usual that day, he was eating and drinking normally and had normal urine output.
The patient had a history of developmental delay, but was otherwise healthy. He had no rashes, and there was no recent history of vomiting, diarrhea, difficulty breathing, or fever.
Examination of the patient’s skin revealed diffuse edema and erythema of the bilateral great toes (FIGURE 1A), with large overlying bullae extending from the dorsal surface of the base of the great toes around to the plantar (volar) surface of the foot (FIGURE 1B). The bullae on the plantar surface were approximately 4 cm long, extending from the tip of the toes proximally to the region of the head of the first metatarsal.

The patient’s vital signs were notable for a rectal temperature of 100.2° F and a heart rate of 180 beats per minute.
Initial lab tests included a complete blood count (CBC), blood cultures, and urinalysis with urine culture. The CBC revealed a white blood count of 27,000/mcL (normal: 6000-17,500/mcL). Both wound culture and herpes simplex viral culture were negative. An intranasal surveillance culture for methicillin-resistant Staphylococcus aureus (MRSA) was also negative.
Given the patient’s fever and leukocytosis, a 100-mg dose of intravenous clindamycin was administered.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Blistering distal dactylitis
We made a clinical diagnosis of blistering distal dactylitis (BDD), a condition typically caused by infection with Gram-positive bacteria. BDD is generally described as a localized infection of the volar fat pads of one or more fingers. The infection may also occur more proximally on the hand or involve the thumbs or toes.1
Who’s at risk? BDD occurs among children ages 2 to 16 years, although it has been reported in infants as young as 6 months and in adults. No cases have occurred among the elderly.2-7
The most common etiologic agents are group A beta-hemolytic Streptococci. Less commonly reported agents include Staphylococcus aureus, S. epidermidis, group B Streptococci, and MRSA.1,6,8 The presence of multiple bullae may be predictive of infection with S. aureus.9
A clinical diagnosis
Diagnosis is usually made on clinical grounds based on the presence of large, tense, superficial, and typically painful bullae, the base of which may be erythematous. Culture of the blister fluid and the base of an unroofed blister may confirm the presence of a Streptococcus or Staphylococcus species.
Lab tests are typically not required to confirm a diagnosis of BDD. However, wound cultures of blister fluid, rapid antigen testing for group A beta-hemolytic Streptococci, and viral culture or polymerase chain reaction testing for herpes simplex virus may be considered.
Rule these conditions out
Lesions similar to those seen with BDD can be caused by the following infections and irritants:4,5,8
Herpetic whitlow is caused by a herpes simplex virus infection. It presents as a cluster of painful vesicles or ulcers with an erythematous base on the distal part of a finger or toe.
Bullous impetigo is the result of a staphylococcal infection, which produces an epidermolytic toxin leading to bulla formation. Lesions may occur anywhere on the body but are most common on the face.
Irritant or allergic contact dermatitis results from an external topical exposure and is typically localized to the area of contact. The reaction is an eczematous eruption that may include bullae.
Treatment is typically empiric
Treatment of BDD includes wound care with wet-to-dry saline dressings, incision and drainage of the bulla(e), and a systemic beta-lactamase-resistant antibiotic. Topical antibiotics alone are not recommended.7
Our patient was transitioned from intravenous to oral clindamycin, 100 mg every 8 hours, and the bullae were incised and drained. His leukocytosis resolved within 24 hours, and he continued to do well. At follow-up one week later, the patient’s blisters were healing well, and he was playful and eating and drinking normally.
CORRESPONDENCE
C. Randall Clinch, DO, MS, Wake Forest University School of Medicine, 1 Medical Center Blvd, Winston-Salem, NC 27157; [email protected].
1. Hays GC, Mullard JE. Blistering distal dactylitis: a clinically recognizable streptococcal infection. Pediatrics. 1975;56:129-131.
2. Schneider JA, Parlette HL 3rd. Blistering distal dactylitis: a manifestation of group A beta-hemolytic streptococcal infection. Arch Dermatol. 1982;118:879-880.
3. Scheinfeld NS. Is blistering distal dactylitis a variant of bullous impetigo? Clin Exp Dermatol. 2007;32:314-316.
4. Kollipara R, Downing C, Lee M, et al. Blistering distal dactylitis in an adult. J Cutan Med Surg. 2015;19:397-399.
5. Fretzayas A, Moustaki M, Tsagris V, et al. MRSA blistering distal dactylitis and review of reported cases. Pediatr Dermatol. 2011;28:433-435.
6. Lyon M, Doehring MC. Blistering distal dactylitis: a case series in children under nine months of age. J Emerg Med. 2004;26:421-423.
7. Frieden IJ. Blistering dactylitis caused by group B streptococci. Pediatr Dermatol. 1989;6:300-302.
8. Woroszylski A, Durán C, Tamayo L, et al. Staphylococcal blistering dactylitis: report of two patients. Pediatr Dermatol. 1996;13:292-293.
9. Norcross MC Jr, Mitchell DF. Blistering distal dactylitis caused by Staphylococcus aureus. Cutis. 1993;51:353-354 .
1. Hays GC, Mullard JE. Blistering distal dactylitis: a clinically recognizable streptococcal infection. Pediatrics. 1975;56:129-131.
2. Schneider JA, Parlette HL 3rd. Blistering distal dactylitis: a manifestation of group A beta-hemolytic streptococcal infection. Arch Dermatol. 1982;118:879-880.
3. Scheinfeld NS. Is blistering distal dactylitis a variant of bullous impetigo? Clin Exp Dermatol. 2007;32:314-316.
4. Kollipara R, Downing C, Lee M, et al. Blistering distal dactylitis in an adult. J Cutan Med Surg. 2015;19:397-399.
5. Fretzayas A, Moustaki M, Tsagris V, et al. MRSA blistering distal dactylitis and review of reported cases. Pediatr Dermatol. 2011;28:433-435.
6. Lyon M, Doehring MC. Blistering distal dactylitis: a case series in children under nine months of age. J Emerg Med. 2004;26:421-423.
7. Frieden IJ. Blistering dactylitis caused by group B streptococci. Pediatr Dermatol. 1989;6:300-302.
8. Woroszylski A, Durán C, Tamayo L, et al. Staphylococcal blistering dactylitis: report of two patients. Pediatr Dermatol. 1996;13:292-293.
9. Norcross MC Jr, Mitchell DF. Blistering distal dactylitis caused by Staphylococcus aureus. Cutis. 1993;51:353-354 .
Do patients with type 2 diabetes who aren’t taking insulin benefit from self-monitoring blood glucose?
YES, UNDER SOME CIRCUMSTANCES. Patients with type 2 diabetes who aren’t on insulin and perform self- monitoring of blood glucose (SMBG) show small but significant reductions in hemoglobin A1c (HbA1c) at 6 months but not at 12 months (strength of recommendation [SOR]: B, systematic reviews and meta-analyses of disease-oriented evidence).
Patients with a baseline HbA1c <8% who self-monitor don’t reduce their HbA1c levels, but patients with a baseline HbA1c >8% do (SOR: B, systematic reviews and meta-analyses of disease-oriented evidence).
More frequent SMBG—4 to 7 times weekly—doesn’t reduce HbA1c more than less frequent self-monitoring—1 or 2 times a week (SOR: B, a systematic review and meta-analysis of disease-oriented evidence).
Evidence summary
A 2012 Cochrane review and meta-analysis of 9 RCTs found that 1261 patients who used SMBG showed a small but statistically significant decrease in HbA1c at 6 months compared with 1063 controls. In 2 other RCTs, patients using SMBG showed a nonsignificant decrease in HbA1c compared with control subjects at 12 months (TABLE 1).1
TABLE 1
Difference in HbA1c by duration of self-monitoring*
| Study | Duration (months) | Total number of patients | Number of patients using SMBG | Number of patients not using SMBG | Mean HbA1c difference | 95% CI for average HbA1c reduction |
|---|---|---|---|---|---|---|
| Meta-analysis1 | 6 | 2324 | 1261 | 1063 | –0.26 | –0.39 to –0.13 Test overall effect Z=3.99 (P<.0001) |
| Meta-analysis2 | 6 | 1563 | 858 | 705 | –0.21 | –0.38 to –0.04 (P value NR) |
| Meta-analysis1 | 12 | 493 | 323 | 170 | –0.13 | –0.31 to 0.04 Test overall effect Z=1.50 (P=.13) |
| Meta-analysis2 | 12 | 648 | 391 | 257 | –0.16 | –0.38 to 0.05 (NS) |
| CI, confidence interval; HbA1c, hemoglobin A1c; NR, not reported; NS, not significant; SMBG, self-monitoring blood glucose. *Mean HbA1c was not reported for either of the 2 studies described here. | ||||||
Another meta-analysis reported similar findings. The study grouped 9 RCTs based on the duration of SMBG and examined the change in HbA1c from baseline. In 5 of the trials, SMBG for 6 months was associated with a small decrease in HbA1c, but in the other 4, SMBG for longer than one year didn’t significantly change HbA1c levels.2
Baseline values make a difference
A meta-analysis of 9 RCTs demonstrated that SMBG was marginally superior to non-SMBG for reducing HbA1c when the baseline value was >8%. SMBG didn’t lower HbA1c in patients with a baseline HbA1c <8%. The greatest change in HbA1c occurred in patients with baseline values >10% (TABLE 2).3
In another meta-analysis, 12 of 15 RCTs found SMBG to be better than non-SMBG at reducing HbA1c when the baseline was >8%.4
TABLE 2
Patients benefit from self-monitoring when baseline HbA1c is >8%
| Study | Duration (months) | Number of patients | Baseline HbA1c | Mean HbA1c difference | 95% CI for average HbA1c reduction |
|---|---|---|---|---|---|
| Meta-analysis3 | 6-12 | SMBG=301 Controls=150 | <8% | –0.15 | –0.33 to 0.03 (NS) |
| Meta-analysis4 | 6-12 | SMBG=386 Controls=390 | <8% | –0.21 | –0.37 to –0.05 (NS) |
| Meta-analysis3 | 6-12 | SMBG=964 Controls=920 | 8%-10% | –0.27 | –0.40 to –0.14 (P<.0001) |
| Meta-analysis4 | 4-12 | SMBG=1154 Controls=1156 | ≥8% | –0.38 | –0.58 to –0.18 (P value NR) |
| Meta-analysis3 | 4-7 | SMBG=29 Controls=33 | >10% | –1.23 | –2.31 to –0.14 (P<.03) |
| CI, confidence interval; HbA1c, hemoglobin A1c; NR, not reported; NS, not significant; SMBG, self-monitoring blood glucose. | |||||
Limitations of studies
Limitations of the studies (TABLES 1 AND 2) reviewed included methodological quality,1,3 limited patient compliance reporting,3 heterogeneity,1,2,4 and small sample size.2,3
More frequent self-monitoring has no effect
A systematic review of 4 RCTs with a total of 637 patients compared frequent SMBG (4-7 times a week) with less frequent self-monitoring (1-2 times a week) for periods ranging from 3 to 12 months and found no difference in reduction of values (HbA1c reduction difference between the groups = –0.21; 95% confidence interval, –0.57 to 0.15).4
Recommendations
The American Diabetes Association advocates SMBG as a guide for patients who use oral or medical nutrition therapies for diabetes. Patients should receive initial instruction in SMBG and routine follow-up evaluation of their technique and ability to use data to adjust therapy.5
The American Association of Clinical Endocrinologists (AACE) advises that SMBG can be initiated at the same time as medical therapy, lifestyle modification, specific diabetes education, or dietary consultation. If HbA1c levels are above target, the AACE recommends more frequent SMBG: preprandially, 2 hours postprandially, occasionally between 2 am and 3 am, during illness, or anytime a low glucose level is suspected.6
1. Malanda UL, Welschen LM, Riphagen II, et al. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012;(1):CD005060.-
2. Towfigh A, Romanova M, Weinreb JE, et al. Self-monitoring of blood glucose levels in patients with type 2 diabetes mellitus not taking insulin: Am J Manag Care. 2008;14:468-475.
3. Poolsup N, Suksomboon N, Rattanasookchit S. Meta-analysis of the benefits of self-monitoring of blood glucose on glycemic control in type 2 diabetes patients: an update. Diabetes Technol Ther. 2009;11:775-784.
4. Allemann S, Houriet C, Diem P, et al. Self-monitoring of blood glucose in non-insulin treated patients with type 2 diabetes: Curr Med Res Opin. 2009;25:2903-2913.
5. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
6. Rodbard HW, Blonde L, Braithwaite SS, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(suppl 1):1-68.
YES, UNDER SOME CIRCUMSTANCES. Patients with type 2 diabetes who aren’t on insulin and perform self- monitoring of blood glucose (SMBG) show small but significant reductions in hemoglobin A1c (HbA1c) at 6 months but not at 12 months (strength of recommendation [SOR]: B, systematic reviews and meta-analyses of disease-oriented evidence).
Patients with a baseline HbA1c <8% who self-monitor don’t reduce their HbA1c levels, but patients with a baseline HbA1c >8% do (SOR: B, systematic reviews and meta-analyses of disease-oriented evidence).
More frequent SMBG—4 to 7 times weekly—doesn’t reduce HbA1c more than less frequent self-monitoring—1 or 2 times a week (SOR: B, a systematic review and meta-analysis of disease-oriented evidence).
Evidence summary
A 2012 Cochrane review and meta-analysis of 9 RCTs found that 1261 patients who used SMBG showed a small but statistically significant decrease in HbA1c at 6 months compared with 1063 controls. In 2 other RCTs, patients using SMBG showed a nonsignificant decrease in HbA1c compared with control subjects at 12 months (TABLE 1).1
TABLE 1
Difference in HbA1c by duration of self-monitoring*
| Study | Duration (months) | Total number of patients | Number of patients using SMBG | Number of patients not using SMBG | Mean HbA1c difference | 95% CI for average HbA1c reduction |
|---|---|---|---|---|---|---|
| Meta-analysis1 | 6 | 2324 | 1261 | 1063 | –0.26 | –0.39 to –0.13 Test overall effect Z=3.99 (P<.0001) |
| Meta-analysis2 | 6 | 1563 | 858 | 705 | –0.21 | –0.38 to –0.04 (P value NR) |
| Meta-analysis1 | 12 | 493 | 323 | 170 | –0.13 | –0.31 to 0.04 Test overall effect Z=1.50 (P=.13) |
| Meta-analysis2 | 12 | 648 | 391 | 257 | –0.16 | –0.38 to 0.05 (NS) |
| CI, confidence interval; HbA1c, hemoglobin A1c; NR, not reported; NS, not significant; SMBG, self-monitoring blood glucose. *Mean HbA1c was not reported for either of the 2 studies described here. | ||||||
Another meta-analysis reported similar findings. The study grouped 9 RCTs based on the duration of SMBG and examined the change in HbA1c from baseline. In 5 of the trials, SMBG for 6 months was associated with a small decrease in HbA1c, but in the other 4, SMBG for longer than one year didn’t significantly change HbA1c levels.2
Baseline values make a difference
A meta-analysis of 9 RCTs demonstrated that SMBG was marginally superior to non-SMBG for reducing HbA1c when the baseline value was >8%. SMBG didn’t lower HbA1c in patients with a baseline HbA1c <8%. The greatest change in HbA1c occurred in patients with baseline values >10% (TABLE 2).3
In another meta-analysis, 12 of 15 RCTs found SMBG to be better than non-SMBG at reducing HbA1c when the baseline was >8%.4
TABLE 2
Patients benefit from self-monitoring when baseline HbA1c is >8%
| Study | Duration (months) | Number of patients | Baseline HbA1c | Mean HbA1c difference | 95% CI for average HbA1c reduction |
|---|---|---|---|---|---|
| Meta-analysis3 | 6-12 | SMBG=301 Controls=150 | <8% | –0.15 | –0.33 to 0.03 (NS) |
| Meta-analysis4 | 6-12 | SMBG=386 Controls=390 | <8% | –0.21 | –0.37 to –0.05 (NS) |
| Meta-analysis3 | 6-12 | SMBG=964 Controls=920 | 8%-10% | –0.27 | –0.40 to –0.14 (P<.0001) |
| Meta-analysis4 | 4-12 | SMBG=1154 Controls=1156 | ≥8% | –0.38 | –0.58 to –0.18 (P value NR) |
| Meta-analysis3 | 4-7 | SMBG=29 Controls=33 | >10% | –1.23 | –2.31 to –0.14 (P<.03) |
| CI, confidence interval; HbA1c, hemoglobin A1c; NR, not reported; NS, not significant; SMBG, self-monitoring blood glucose. | |||||
Limitations of studies
Limitations of the studies (TABLES 1 AND 2) reviewed included methodological quality,1,3 limited patient compliance reporting,3 heterogeneity,1,2,4 and small sample size.2,3
More frequent self-monitoring has no effect
A systematic review of 4 RCTs with a total of 637 patients compared frequent SMBG (4-7 times a week) with less frequent self-monitoring (1-2 times a week) for periods ranging from 3 to 12 months and found no difference in reduction of values (HbA1c reduction difference between the groups = –0.21; 95% confidence interval, –0.57 to 0.15).4
Recommendations
The American Diabetes Association advocates SMBG as a guide for patients who use oral or medical nutrition therapies for diabetes. Patients should receive initial instruction in SMBG and routine follow-up evaluation of their technique and ability to use data to adjust therapy.5
The American Association of Clinical Endocrinologists (AACE) advises that SMBG can be initiated at the same time as medical therapy, lifestyle modification, specific diabetes education, or dietary consultation. If HbA1c levels are above target, the AACE recommends more frequent SMBG: preprandially, 2 hours postprandially, occasionally between 2 am and 3 am, during illness, or anytime a low glucose level is suspected.6
YES, UNDER SOME CIRCUMSTANCES. Patients with type 2 diabetes who aren’t on insulin and perform self- monitoring of blood glucose (SMBG) show small but significant reductions in hemoglobin A1c (HbA1c) at 6 months but not at 12 months (strength of recommendation [SOR]: B, systematic reviews and meta-analyses of disease-oriented evidence).
Patients with a baseline HbA1c <8% who self-monitor don’t reduce their HbA1c levels, but patients with a baseline HbA1c >8% do (SOR: B, systematic reviews and meta-analyses of disease-oriented evidence).
More frequent SMBG—4 to 7 times weekly—doesn’t reduce HbA1c more than less frequent self-monitoring—1 or 2 times a week (SOR: B, a systematic review and meta-analysis of disease-oriented evidence).
Evidence summary
A 2012 Cochrane review and meta-analysis of 9 RCTs found that 1261 patients who used SMBG showed a small but statistically significant decrease in HbA1c at 6 months compared with 1063 controls. In 2 other RCTs, patients using SMBG showed a nonsignificant decrease in HbA1c compared with control subjects at 12 months (TABLE 1).1
TABLE 1
Difference in HbA1c by duration of self-monitoring*
| Study | Duration (months) | Total number of patients | Number of patients using SMBG | Number of patients not using SMBG | Mean HbA1c difference | 95% CI for average HbA1c reduction |
|---|---|---|---|---|---|---|
| Meta-analysis1 | 6 | 2324 | 1261 | 1063 | –0.26 | –0.39 to –0.13 Test overall effect Z=3.99 (P<.0001) |
| Meta-analysis2 | 6 | 1563 | 858 | 705 | –0.21 | –0.38 to –0.04 (P value NR) |
| Meta-analysis1 | 12 | 493 | 323 | 170 | –0.13 | –0.31 to 0.04 Test overall effect Z=1.50 (P=.13) |
| Meta-analysis2 | 12 | 648 | 391 | 257 | –0.16 | –0.38 to 0.05 (NS) |
| CI, confidence interval; HbA1c, hemoglobin A1c; NR, not reported; NS, not significant; SMBG, self-monitoring blood glucose. *Mean HbA1c was not reported for either of the 2 studies described here. | ||||||
Another meta-analysis reported similar findings. The study grouped 9 RCTs based on the duration of SMBG and examined the change in HbA1c from baseline. In 5 of the trials, SMBG for 6 months was associated with a small decrease in HbA1c, but in the other 4, SMBG for longer than one year didn’t significantly change HbA1c levels.2
Baseline values make a difference
A meta-analysis of 9 RCTs demonstrated that SMBG was marginally superior to non-SMBG for reducing HbA1c when the baseline value was >8%. SMBG didn’t lower HbA1c in patients with a baseline HbA1c <8%. The greatest change in HbA1c occurred in patients with baseline values >10% (TABLE 2).3
In another meta-analysis, 12 of 15 RCTs found SMBG to be better than non-SMBG at reducing HbA1c when the baseline was >8%.4
TABLE 2
Patients benefit from self-monitoring when baseline HbA1c is >8%
| Study | Duration (months) | Number of patients | Baseline HbA1c | Mean HbA1c difference | 95% CI for average HbA1c reduction |
|---|---|---|---|---|---|
| Meta-analysis3 | 6-12 | SMBG=301 Controls=150 | <8% | –0.15 | –0.33 to 0.03 (NS) |
| Meta-analysis4 | 6-12 | SMBG=386 Controls=390 | <8% | –0.21 | –0.37 to –0.05 (NS) |
| Meta-analysis3 | 6-12 | SMBG=964 Controls=920 | 8%-10% | –0.27 | –0.40 to –0.14 (P<.0001) |
| Meta-analysis4 | 4-12 | SMBG=1154 Controls=1156 | ≥8% | –0.38 | –0.58 to –0.18 (P value NR) |
| Meta-analysis3 | 4-7 | SMBG=29 Controls=33 | >10% | –1.23 | –2.31 to –0.14 (P<.03) |
| CI, confidence interval; HbA1c, hemoglobin A1c; NR, not reported; NS, not significant; SMBG, self-monitoring blood glucose. | |||||
Limitations of studies
Limitations of the studies (TABLES 1 AND 2) reviewed included methodological quality,1,3 limited patient compliance reporting,3 heterogeneity,1,2,4 and small sample size.2,3
More frequent self-monitoring has no effect
A systematic review of 4 RCTs with a total of 637 patients compared frequent SMBG (4-7 times a week) with less frequent self-monitoring (1-2 times a week) for periods ranging from 3 to 12 months and found no difference in reduction of values (HbA1c reduction difference between the groups = –0.21; 95% confidence interval, –0.57 to 0.15).4
Recommendations
The American Diabetes Association advocates SMBG as a guide for patients who use oral or medical nutrition therapies for diabetes. Patients should receive initial instruction in SMBG and routine follow-up evaluation of their technique and ability to use data to adjust therapy.5
The American Association of Clinical Endocrinologists (AACE) advises that SMBG can be initiated at the same time as medical therapy, lifestyle modification, specific diabetes education, or dietary consultation. If HbA1c levels are above target, the AACE recommends more frequent SMBG: preprandially, 2 hours postprandially, occasionally between 2 am and 3 am, during illness, or anytime a low glucose level is suspected.6
1. Malanda UL, Welschen LM, Riphagen II, et al. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012;(1):CD005060.-
2. Towfigh A, Romanova M, Weinreb JE, et al. Self-monitoring of blood glucose levels in patients with type 2 diabetes mellitus not taking insulin: Am J Manag Care. 2008;14:468-475.
3. Poolsup N, Suksomboon N, Rattanasookchit S. Meta-analysis of the benefits of self-monitoring of blood glucose on glycemic control in type 2 diabetes patients: an update. Diabetes Technol Ther. 2009;11:775-784.
4. Allemann S, Houriet C, Diem P, et al. Self-monitoring of blood glucose in non-insulin treated patients with type 2 diabetes: Curr Med Res Opin. 2009;25:2903-2913.
5. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
6. Rodbard HW, Blonde L, Braithwaite SS, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(suppl 1):1-68.
1. Malanda UL, Welschen LM, Riphagen II, et al. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012;(1):CD005060.-
2. Towfigh A, Romanova M, Weinreb JE, et al. Self-monitoring of blood glucose levels in patients with type 2 diabetes mellitus not taking insulin: Am J Manag Care. 2008;14:468-475.
3. Poolsup N, Suksomboon N, Rattanasookchit S. Meta-analysis of the benefits of self-monitoring of blood glucose on glycemic control in type 2 diabetes patients: an update. Diabetes Technol Ther. 2009;11:775-784.
4. Allemann S, Houriet C, Diem P, et al. Self-monitoring of blood glucose in non-insulin treated patients with type 2 diabetes: Curr Med Res Opin. 2009;25:2903-2913.
5. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
6. Rodbard HW, Blonde L, Braithwaite SS, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(suppl 1):1-68.
Evidence-based answers from the Family Physicians Inquiries Network
Which women should we screen for gestational diabetes mellitus?
IT’S UNCLEAR which women we should screen. No randomized controlled trials (RCTs) demonstrate that either universal screening or risk factor screening for gestational diabetes mellitus (GDM) prevents maternal and fetal adverse outcomes.
That said, the common practice of universal screening is more sensitive than screening based on risk factors (strength of recommendation [SOR]: B, 1 randomized trial and 3 retrospective cohort studies without patient-oriented outcomes). Historic risk factors are poor predictors of GDM in a current pregnancy (SOR: C, 1 retrospective cohort study without patient-oriented outcomes).
Evidence summary
No RCTs have evaluated the risks, benefits, and clinical outcomes of screening for GDM. A review of universal screening compared with risk factor screening included 2 retrospective studies, 1 observational cohort study, and 1 nonconcurrent cohort study.1-4
Risk factor screening misses women with GDM
All 4 studies clearly show that risk factor screening would miss patients with GDM.1-4 Two studies found that the detection rate of GDM increases when universal screening is performed.1,4
One observational study in a multiethnic cohort concluded that risk factor screening missed 30% of patients with GDM and that universal screening increased the detection rate from 8.3% to 12.6% (P=.001) compared with risk factor screening.1 Similarly, a retrospective study of 147 pregnant women with GDM found that risk factor screening would have missed 23%.2
Universal screening diagnoses GDM earlier than risk factor screening
One prospective randomized study that compared universal screening (using a 50-g 1-hour glucose challenge test) in 1853 women with risk factor screening in 1299 women demonstrated that nearly half of those with GDM had no historical risk factors and would have been missed by risk factor screening in a low-prevalence, mostly Caucasian sample. The prevalence was 2.7% in the universal screening group vs 1.45% in the risk factor screening group (P<.03). Universal screening diagnosed GDM earlier than risk factor screening (mean gestation 30 ± 2.6 weeks vs 33 ± 3.7 weeks; P<.05).3
Need for insulin is similar, with and without GDM risk factors
A retrospective cohort study demonstrated that risk factor screening misses 43% of women with GDM. The study also showed that women with GDM who had identifiable risk factors and women without identifiable risk factors were equally likely to require insulin to control their GDM. Adverse birth outcomes such as macrosomia and shoulder dystocia or cesarean section were similar in patients with and without risk factors for GDM.4
Macrosomia and primary C-section increase along with glucose intolerance
The prospective cohort Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study of 23,316 women at 15 centers in 9 countries used the 2-hour 75-g oral glucose tolerance test at 24 to 32 weeks’ gestation to clarify the risks of adverse outcomes associated with varying degrees of maternal glucose intolerance. The study found a linear increase in the risk of macrosomia and primary cesarean section as glucose intolerance levels increased from normal to the gestational diabetes range.5
Recommendations
The US Preventive Services Task Force states that evidence is insufficient to advise for or against routine screening for GDM.6
The American College of Obstetricians and Gynecologists considers universal glucose challenge screening for GDM to be the most sensitive approach, but notes that some pregnant women at low risk may be less likely to benefit from testing.7
The Cochrane review protocol states that universally accepted screening is controversial because of a lack of clearly defined, universally accepted screening criteria and uncertainty about the severity of glucose intolerance at which treatment is beneficial.8
1. Cosson E, Benchimol M, Carbillon L, et al. Universal rather than selective screening for gestational diabetes mellitus may improve fetal outcomes. Diabetes Metab. 2006;32:140-146.
2. Baliutaviciene D, Petrenko V, Zalinkevicius R. Selective or universal diagnostic testing for gestational diabetes mellitus. Int J Gynaecol Obstet. 2002;78:207-211.
3. Griffin ME, Coffey M, Johnson H, et al. Universal vs. risk factor-based screening for gestational diabetes mellitus: detection rates, gestation at diagnosis and outcome. Diabetes Med. 2000;17:26-32.
4. Weeks JW, Major CA, de Veciana M, et al. Gestational diabetes: does the presence of risk factors influence perinatal outcome? Am J Obstet Gynecol. 1994;171:1003-1007.
5. HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
6. US Preventive Services Task Force. Screening for gestational diabetes mellitus: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148:759-765.
7. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstet Gynecol. 2001;98:525-538.
8. Tieu J, Crowther CA, Middleton P, et al. Screening for gestational diabetes mellitus for improving maternal and infant health (Protocol). Cochrane Database Syst Rev. 2008;(2):CD007222.-
IT’S UNCLEAR which women we should screen. No randomized controlled trials (RCTs) demonstrate that either universal screening or risk factor screening for gestational diabetes mellitus (GDM) prevents maternal and fetal adverse outcomes.
That said, the common practice of universal screening is more sensitive than screening based on risk factors (strength of recommendation [SOR]: B, 1 randomized trial and 3 retrospective cohort studies without patient-oriented outcomes). Historic risk factors are poor predictors of GDM in a current pregnancy (SOR: C, 1 retrospective cohort study without patient-oriented outcomes).
Evidence summary
No RCTs have evaluated the risks, benefits, and clinical outcomes of screening for GDM. A review of universal screening compared with risk factor screening included 2 retrospective studies, 1 observational cohort study, and 1 nonconcurrent cohort study.1-4
Risk factor screening misses women with GDM
All 4 studies clearly show that risk factor screening would miss patients with GDM.1-4 Two studies found that the detection rate of GDM increases when universal screening is performed.1,4
One observational study in a multiethnic cohort concluded that risk factor screening missed 30% of patients with GDM and that universal screening increased the detection rate from 8.3% to 12.6% (P=.001) compared with risk factor screening.1 Similarly, a retrospective study of 147 pregnant women with GDM found that risk factor screening would have missed 23%.2
Universal screening diagnoses GDM earlier than risk factor screening
One prospective randomized study that compared universal screening (using a 50-g 1-hour glucose challenge test) in 1853 women with risk factor screening in 1299 women demonstrated that nearly half of those with GDM had no historical risk factors and would have been missed by risk factor screening in a low-prevalence, mostly Caucasian sample. The prevalence was 2.7% in the universal screening group vs 1.45% in the risk factor screening group (P<.03). Universal screening diagnosed GDM earlier than risk factor screening (mean gestation 30 ± 2.6 weeks vs 33 ± 3.7 weeks; P<.05).3
Need for insulin is similar, with and without GDM risk factors
A retrospective cohort study demonstrated that risk factor screening misses 43% of women with GDM. The study also showed that women with GDM who had identifiable risk factors and women without identifiable risk factors were equally likely to require insulin to control their GDM. Adverse birth outcomes such as macrosomia and shoulder dystocia or cesarean section were similar in patients with and without risk factors for GDM.4
Macrosomia and primary C-section increase along with glucose intolerance
The prospective cohort Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study of 23,316 women at 15 centers in 9 countries used the 2-hour 75-g oral glucose tolerance test at 24 to 32 weeks’ gestation to clarify the risks of adverse outcomes associated with varying degrees of maternal glucose intolerance. The study found a linear increase in the risk of macrosomia and primary cesarean section as glucose intolerance levels increased from normal to the gestational diabetes range.5
Recommendations
The US Preventive Services Task Force states that evidence is insufficient to advise for or against routine screening for GDM.6
The American College of Obstetricians and Gynecologists considers universal glucose challenge screening for GDM to be the most sensitive approach, but notes that some pregnant women at low risk may be less likely to benefit from testing.7
The Cochrane review protocol states that universally accepted screening is controversial because of a lack of clearly defined, universally accepted screening criteria and uncertainty about the severity of glucose intolerance at which treatment is beneficial.8
IT’S UNCLEAR which women we should screen. No randomized controlled trials (RCTs) demonstrate that either universal screening or risk factor screening for gestational diabetes mellitus (GDM) prevents maternal and fetal adverse outcomes.
That said, the common practice of universal screening is more sensitive than screening based on risk factors (strength of recommendation [SOR]: B, 1 randomized trial and 3 retrospective cohort studies without patient-oriented outcomes). Historic risk factors are poor predictors of GDM in a current pregnancy (SOR: C, 1 retrospective cohort study without patient-oriented outcomes).
Evidence summary
No RCTs have evaluated the risks, benefits, and clinical outcomes of screening for GDM. A review of universal screening compared with risk factor screening included 2 retrospective studies, 1 observational cohort study, and 1 nonconcurrent cohort study.1-4
Risk factor screening misses women with GDM
All 4 studies clearly show that risk factor screening would miss patients with GDM.1-4 Two studies found that the detection rate of GDM increases when universal screening is performed.1,4
One observational study in a multiethnic cohort concluded that risk factor screening missed 30% of patients with GDM and that universal screening increased the detection rate from 8.3% to 12.6% (P=.001) compared with risk factor screening.1 Similarly, a retrospective study of 147 pregnant women with GDM found that risk factor screening would have missed 23%.2
Universal screening diagnoses GDM earlier than risk factor screening
One prospective randomized study that compared universal screening (using a 50-g 1-hour glucose challenge test) in 1853 women with risk factor screening in 1299 women demonstrated that nearly half of those with GDM had no historical risk factors and would have been missed by risk factor screening in a low-prevalence, mostly Caucasian sample. The prevalence was 2.7% in the universal screening group vs 1.45% in the risk factor screening group (P<.03). Universal screening diagnosed GDM earlier than risk factor screening (mean gestation 30 ± 2.6 weeks vs 33 ± 3.7 weeks; P<.05).3
Need for insulin is similar, with and without GDM risk factors
A retrospective cohort study demonstrated that risk factor screening misses 43% of women with GDM. The study also showed that women with GDM who had identifiable risk factors and women without identifiable risk factors were equally likely to require insulin to control their GDM. Adverse birth outcomes such as macrosomia and shoulder dystocia or cesarean section were similar in patients with and without risk factors for GDM.4
Macrosomia and primary C-section increase along with glucose intolerance
The prospective cohort Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study of 23,316 women at 15 centers in 9 countries used the 2-hour 75-g oral glucose tolerance test at 24 to 32 weeks’ gestation to clarify the risks of adverse outcomes associated with varying degrees of maternal glucose intolerance. The study found a linear increase in the risk of macrosomia and primary cesarean section as glucose intolerance levels increased from normal to the gestational diabetes range.5
Recommendations
The US Preventive Services Task Force states that evidence is insufficient to advise for or against routine screening for GDM.6
The American College of Obstetricians and Gynecologists considers universal glucose challenge screening for GDM to be the most sensitive approach, but notes that some pregnant women at low risk may be less likely to benefit from testing.7
The Cochrane review protocol states that universally accepted screening is controversial because of a lack of clearly defined, universally accepted screening criteria and uncertainty about the severity of glucose intolerance at which treatment is beneficial.8
1. Cosson E, Benchimol M, Carbillon L, et al. Universal rather than selective screening for gestational diabetes mellitus may improve fetal outcomes. Diabetes Metab. 2006;32:140-146.
2. Baliutaviciene D, Petrenko V, Zalinkevicius R. Selective or universal diagnostic testing for gestational diabetes mellitus. Int J Gynaecol Obstet. 2002;78:207-211.
3. Griffin ME, Coffey M, Johnson H, et al. Universal vs. risk factor-based screening for gestational diabetes mellitus: detection rates, gestation at diagnosis and outcome. Diabetes Med. 2000;17:26-32.
4. Weeks JW, Major CA, de Veciana M, et al. Gestational diabetes: does the presence of risk factors influence perinatal outcome? Am J Obstet Gynecol. 1994;171:1003-1007.
5. HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
6. US Preventive Services Task Force. Screening for gestational diabetes mellitus: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148:759-765.
7. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstet Gynecol. 2001;98:525-538.
8. Tieu J, Crowther CA, Middleton P, et al. Screening for gestational diabetes mellitus for improving maternal and infant health (Protocol). Cochrane Database Syst Rev. 2008;(2):CD007222.-
1. Cosson E, Benchimol M, Carbillon L, et al. Universal rather than selective screening for gestational diabetes mellitus may improve fetal outcomes. Diabetes Metab. 2006;32:140-146.
2. Baliutaviciene D, Petrenko V, Zalinkevicius R. Selective or universal diagnostic testing for gestational diabetes mellitus. Int J Gynaecol Obstet. 2002;78:207-211.
3. Griffin ME, Coffey M, Johnson H, et al. Universal vs. risk factor-based screening for gestational diabetes mellitus: detection rates, gestation at diagnosis and outcome. Diabetes Med. 2000;17:26-32.
4. Weeks JW, Major CA, de Veciana M, et al. Gestational diabetes: does the presence of risk factors influence perinatal outcome? Am J Obstet Gynecol. 1994;171:1003-1007.
5. HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
6. US Preventive Services Task Force. Screening for gestational diabetes mellitus: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148:759-765.
7. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstet Gynecol. 2001;98:525-538.
8. Tieu J, Crowther CA, Middleton P, et al. Screening for gestational diabetes mellitus for improving maternal and infant health (Protocol). Cochrane Database Syst Rev. 2008;(2):CD007222.-
Evidence-based answers from the Family Physicians Inquiries Network