User login
A new generation of drug-eluting stents: Indications and outcomes of bioresorbable vascular scaffolds
The development of a new generation of drug-eluting stents (DES) has had a dramatic impact on the number of stents used for percutaneous transluminal coronary angioplasty for the treatment of coronary artery disease (CAD). But even second- and third-generation DES fall short when compared with coronary artery bypass grafting (CABG) with regards to the need for repeat reavascularization. CABG is advantageous because it bypasses the entire disease segment of the vessel. Thus for multivessel complex CAD, it is still considered the best choice. Nevertheless, most patients prefer the less-invasive option of stents, so practitioners need to provide the best stent available.
There are 3 primary criteria for DES selection:
- Efficacy for a broad range of patients and lesion complexities that primarily provides consistency in improving measures of angiographic and clinical efficacy
- Safety as determined by the following:
- Enable healing and promote endothelialization
- Permit functional endothelium
- Obtaining complete apposition
- Reduction or elimination of late and very late stent thrombosis
- Minimizing the need for long-term dual antiplatelet therapy
- Performance provided by reliable delivery capabilities to the lesion site.
GREAT EXPECTATIONS
New DES must be shown to be superior to previous generation stents. Although preclinical endothelialization and other mechanistic surrogates are good enough to claim an improvement, the traditional method is to compare clinical outcomes with the new stent versus the existing stent in a randomized clinical trial.
PROBLEMS WITH DURABLE POLYMER STENTS
Complications with durable polymer DES have included increased local inflammation and neoatherosclerosis. There are reports of subacute stent thrombosis due to lack of adequate expansion and stent apposition. Also reported was late thrombosis, resulting in increased rates of myocardial infarction and death.
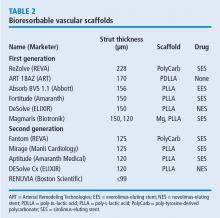
These issues motivated engineers to improve and iterate the DES technology. One important technological change is the decrease in strut thickness from 140 µm to as low as 60 µm. The thickness of the polymer coating also has been reduced. The polymer became thinner, more biocompatible, and in some stents, only abluminal. Further developments were to substitute the durable polymer with a biodegradable polymer and perhaps even design a polymer-free stent.
BIORESORBABLE POLYMERS EMERGE
The time course for resorption of bioresorbable polymers ranges from 2 to 15 months, but they all degrade, which should improve long-term outcomes. A meta-analysis of data from the LEADERS trial and ISAR-TEST 3 and 4 found that the bioresorbable polymer stents were associated with significantly lower rates of target-lesion revascularization (P = .029) and stent thrombosis (P = .015) than durable polymer DES at 4 years after implantation.1 Those results led to the notion that stents with a biodegradable polymer would result in lower rates of stent thrombosis than durable polymer stents; however, that was not the case when stents with biodegradable polymers were compared with second-generation DES.
In the COMPARE II trial,2 the rates of stent thrombosis and target-lesion revascularization were not statistically different for the thick-strut biodegradable polymer biolimus-eluting stent (Nobori) compared with the second-generation thin-strut permanent-polymer stents (Xience). In the CENTURY II trial,3 a third-generation biodegradable sirolimus-eluting stent (Ultimaster) had stent thrombosis rates similar to those of a durable polymer everolimus-eluting stent (Xience) 300 days after insertion (4.36% vs 5.27%, respectively). Target-lesion revascularization rates were also about the same for the stents. In the EVOLVE II trial comparing the thin-strut biodegradable everolimus-eluting stent (Synergy) vs the thin-strut permanent-polymer everolimus-eluting stent (Promus), the 12-month target lesion failure rates for the stents were essentially the same.4
THE RATIONALE FOR BIORESORBABLE STENTS
Another approach was to use biodegradable scaffolds that will be eliminating from the vessel wall once it “completes the job.” The main bioresorbable materials used were polylactic acid or biodegradable metal-like magnesium. These materials pose a technological challenge. While the biodegradable scaffolds are completely eliminated overtime, they still need to equate the performance of best-in-class drug-eluting stent with respect to efficacy and safety. After the Absorb everolimus-eluting BVS system (Absorb BVS) was launched in Europe, initial studies showed scaffold-related thrombosis rates as high as 3.4%.5–7 That compares with 0.4% for second-generation DES—a troubling result for a new technology.
Rates of restenosis and stent thrombosis are similar for bioresorbable stents and standard durable polymer stents. But what are the potential added benefits of bioresorbable stents? And will they improve patient outcomes?
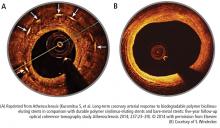
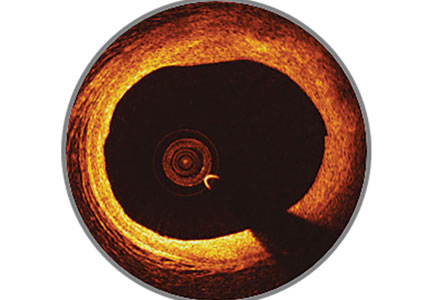
Bioresorbable stents certainly appeal to patients who do not want a permanent, rigid, metallic implant. Also appealing are the proposed benefits of restoration of vasomotion, late luminal enlargement, preservation of CABG targets, and relief of angina. Whether bioresorbable stents improve these outcomes has not been established. Currently, there is no long-term evidence of reduced rates of adverse events, although in 1 study, optical coherence tomography images recorded 10 years after implantation of the first bioresorbable stents showed a pristine vessel with no signs of the struts.8
Several facts are known about the Absorb BVS:
- Preclinical evidence shows complete resorption and return of vascular function, but this takes 3 to 4 years.
- Imaging data at 5 years from the Absorb cohort B trial show complete resorption of struts, lumen preservation, return of function, and plaque regression.9
- In ABSORB III, the pivotal US trial, the stent was within the primary end point showing noninferiority in safety and effectiveness compared with Xience in the first year.10
- Absorb clinical trials in Japan and China confirmed ABSORB III results.
- Meta-analysis (> 3,300 patients) confirmed safety and effectiveness of Absorb.11
- Real-world Absorb clinical evidence continues to show improving outcomes with optimized implant techniques.
- Absorb stent was approved by the US Food and Drug Administration (FDA) in July 2016; more than 150,000 have been implanted worldwide.
The increased rates of target-lesion revascularization and stent thrombosis were likely attributable to inserting the stents into small-diameter vessels that are probably too small for the Absorb BVS. When small vessels (< 2.25 mm) are eliminated from the analysis, the rates were as follows.
Results for vessels > 2.25 mm:
- Target-lesion revascularization: 6.7 % vs 5.5%
- Stent thrombosis: 0.9% vs 0.6%.
- Results for small vessels (< 2.25 mm):
- Target-lesion revascularization: 12.9% vs 8.3%
- Stent thrombosis: 4.6% vs 1.5%.
The lesson is that the Absorb BVS should not be placed in arteries smaller than 2.25 mm in diameter.
ABSORB II STUDY RESULTS RAISE QUESTIONS
Another concern was uncovered in July 2016 when results were published from the ABSORB II trial on vasomotor reactivity at 3 years.13 This clinical trial randomized 501 patients in a 2:1 ratio to the Absorb BVS or the Xience DES at 46 sites outside the United States. Assessment for changes in mean lumen diameter between pre- and post-nitrate administration showed no differences between the groups; thus, the Absorb BVS did not achieve a level of superior vasomotor reactivity. There was vasomotor reactivity probably because the surrogate marker was angiographic follow-up and not intravascular ultrasound or tomography.
Further, the coprimary end point of angiographic late luminal loss at 3 years did not meet its noninferiority standard. The Absorb BVS was expected to have lower rates of late lumen loss because the struts are gone and there is less new intimal formation; however, at 3 years, that was not the case.
The rate of acute stent thrombosis also was alarming: 8 cases for Absorb BVS versus none for Xience. This caused alarm, raising the question of why it was happening in these patients 2 to 3 years after implantation.
Animal studies investigating the association of thicker struts and increased thrombogenicity have reported that the 157-µm BVS had much more platelet buildup and thrombogenicity than a 120-µm biomatrix stent. The 74-µm Synergy stent had even lower rates of thrombosis. The reason for increased thrombogenicity with thicker struts requires further study.
Also, an analysis of the secondary cardiac end points at 3 years in ABSORB II found no clinical patient-oriented differences between the Absorb BVS and the Xience stent (20.8% vs 24.0%, respectively; P = .44). However, rates of device-oriented clinical end points were significantly higher for Absorb BVS (10.4% vs 4.9%; P = .043).13
Clearly, the results for Absorb BVS in this study were not positive. One explanation is suboptimal implantation techniques that did not appose the polymer to the wall. A few years ago, focus shifted to an optimal technique for scaffold deployment, which included predilation, appropriate sizing of the scaffold to the size of the vessel, and postdilation with the intention of embedding the polymer in the vessel wall. Multiple studies have reported fewer incidents of stent thrombosis with the implementation of this protocol.14
Further studies have continued to report increased rates of late scaffold thrombosis in follow-ups of 30 days to 3 years. This resulted in an advisory letter from the FDA focused on appropriate clinical use of the device and withdrawal of ABSORB from commercial use in Europe and Australia.
BIORESORBABLE SCAFFOLDS PIPELINE
This is questionable because one has to believe in the vulnerable plaque theory, which assumes potential eruption of plaques. The Absorb can actually seal a thin cap atheroma and necrotic core over time. It seems that this technology can cause some late lumen enlargement and seal an existing plaque, which may have implications for the future.
SUMMARY
This is the current state of the Absorb BVS:
- More than 150,000 implanted globally
- Received FDA approval in July 2016
- Should not be used in small vessels (ie, lumen diameter < 2.25 mm)
- Thrombosis rates 2 to 3 years after implantation are of concern
- Focusing on appropriate surgical implantation technique can improve outcomes.
Overall, use of bioresorbable stent technology is intriguing. While there is ongoing patient preference for bioresorbable technology, clinical trial results raise the question of whether bioresorbable scaffolds are inferior to best-in-class DES. Improving the scaffold technology and the implantation techniques may equate the short-term outcome of the bioresorbable scaffolds with metallic stents with the hope that over time (when the scaffold is gone), the advantage will be with the bioresorbable scaffolds. Meanwhile, the technology is still seeking its best clinical utility, and a matching performance to the best-in-class DES.
Time will tell whether 5 to 10 years after implantation, BRS technology will outperform durable metallic stents.
- Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J 2012; 33:1214–1222.
- Smits PC, Hofma S, Togni M, et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet 2013; 381:651–660.
- Saito S, Valdes-Chavarri M, Richardt G, et al; for the CENTURY II Investigators. A randomized, prospective, intercontinental evaluation of a bioresorbable polymer sirolimus-eluting coronary stent system: the CENTURY II (Clinical Evaluation of New Terumo Drug-Eluting Coronary Stent System in the Treatment of Patients with Coronary Artery Disease) trial. Eur Heart J 2014; 35:2021–2031.
- Kereiakes DJ, Meredith IT, Windecker S, et al. Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent: the EVOLVE II randomized trial. Circ Cardiovasc Interv 2015; 8:e002372. doi: 10.1161/CIRCINTERVENTIONS.114.002372
- Kraak RP, Hassell ME, Grundeken MJ, et al. Initial experience and clinical evaluation of the Absorb bioresorbable vascular scaffold (BVS) in real-world practice: the AMC Single Centre Real World PCI Registry. EuroIntervention 2015; 10:1160–1168.
- Capodanno D, Gori T, Nef H, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention 2015; 10:1144–1153.
- Ielasi A, Cortese B, Varricchio A, et al. Immediate and midterm outcomes following primary PCI with bioresorbable vascular scaffold implantation in patients with ST-segment myocardial infarction: insights from the multicentre “Registro ABSORB Italiano” (RAI registry). EuroIntervention 2015; 11:157–162.
- Onuma Y, Piazza N, Ormiston JA, Serruys PW. Everolimus-eluting bioabsorbable stent—Abbott Vascular programme. EuroIntervention 2009; 5(suppl F):F98–F102.
- De Bruyne B, Toth GG, Onuma Y, Serruys PW. ABSORB cohort B trial: five year angiographic results of the ABSORB everolimus eluting bioresorbable vascular scaffold. J Am Coll Cardiol 2014; 64(suppl):B181. Abstract TCT 619.
- Ellis SG, Kereiakes DJ, Metzger DC, et al; for the ABSORB III Investigators. Everolimus-eluting bioresorbable scaffolds for coronary artery disease. N Engl J Med 2015; 373:1905–1915.
- Stone GW, Gao R, Kimura T, et al. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease: a patient-level, pooled meta-analysis. Lancet 2016; 387:1277–1289.
- Kuramitsu S, Sonoda S, Yokoi H, et al. Long-term coronary arterial response to biodegradable polymer biolimus-eluting stents in comparison with durable polymer sirolimus-eluting stents and bare-metal stents: five-year follow-up optical coherence tomography study. Atherosclerosis 2014; 237:23–29.
- Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016; 388:2479–2491.
- Puricel S, Cuculi F, Weissner M, et al. Bioresorbable coronary scaffold thrombosis: multicenter comprehensive analysis of clinical presentation, mechanisms, and predictors. J Am Coll Cardiol 2016; 67:921–931.
The development of a new generation of drug-eluting stents (DES) has had a dramatic impact on the number of stents used for percutaneous transluminal coronary angioplasty for the treatment of coronary artery disease (CAD). But even second- and third-generation DES fall short when compared with coronary artery bypass grafting (CABG) with regards to the need for repeat reavascularization. CABG is advantageous because it bypasses the entire disease segment of the vessel. Thus for multivessel complex CAD, it is still considered the best choice. Nevertheless, most patients prefer the less-invasive option of stents, so practitioners need to provide the best stent available.
There are 3 primary criteria for DES selection:
- Efficacy for a broad range of patients and lesion complexities that primarily provides consistency in improving measures of angiographic and clinical efficacy
- Safety as determined by the following:
- Enable healing and promote endothelialization
- Permit functional endothelium
- Obtaining complete apposition
- Reduction or elimination of late and very late stent thrombosis
- Minimizing the need for long-term dual antiplatelet therapy
- Performance provided by reliable delivery capabilities to the lesion site.
GREAT EXPECTATIONS
New DES must be shown to be superior to previous generation stents. Although preclinical endothelialization and other mechanistic surrogates are good enough to claim an improvement, the traditional method is to compare clinical outcomes with the new stent versus the existing stent in a randomized clinical trial.
PROBLEMS WITH DURABLE POLYMER STENTS
Complications with durable polymer DES have included increased local inflammation and neoatherosclerosis. There are reports of subacute stent thrombosis due to lack of adequate expansion and stent apposition. Also reported was late thrombosis, resulting in increased rates of myocardial infarction and death.
These issues motivated engineers to improve and iterate the DES technology. One important technological change is the decrease in strut thickness from 140 µm to as low as 60 µm. The thickness of the polymer coating also has been reduced. The polymer became thinner, more biocompatible, and in some stents, only abluminal. Further developments were to substitute the durable polymer with a biodegradable polymer and perhaps even design a polymer-free stent.
BIORESORBABLE POLYMERS EMERGE
The time course for resorption of bioresorbable polymers ranges from 2 to 15 months, but they all degrade, which should improve long-term outcomes. A meta-analysis of data from the LEADERS trial and ISAR-TEST 3 and 4 found that the bioresorbable polymer stents were associated with significantly lower rates of target-lesion revascularization (P = .029) and stent thrombosis (P = .015) than durable polymer DES at 4 years after implantation.1 Those results led to the notion that stents with a biodegradable polymer would result in lower rates of stent thrombosis than durable polymer stents; however, that was not the case when stents with biodegradable polymers were compared with second-generation DES.
In the COMPARE II trial,2 the rates of stent thrombosis and target-lesion revascularization were not statistically different for the thick-strut biodegradable polymer biolimus-eluting stent (Nobori) compared with the second-generation thin-strut permanent-polymer stents (Xience). In the CENTURY II trial,3 a third-generation biodegradable sirolimus-eluting stent (Ultimaster) had stent thrombosis rates similar to those of a durable polymer everolimus-eluting stent (Xience) 300 days after insertion (4.36% vs 5.27%, respectively). Target-lesion revascularization rates were also about the same for the stents. In the EVOLVE II trial comparing the thin-strut biodegradable everolimus-eluting stent (Synergy) vs the thin-strut permanent-polymer everolimus-eluting stent (Promus), the 12-month target lesion failure rates for the stents were essentially the same.4
THE RATIONALE FOR BIORESORBABLE STENTS
Another approach was to use biodegradable scaffolds that will be eliminating from the vessel wall once it “completes the job.” The main bioresorbable materials used were polylactic acid or biodegradable metal-like magnesium. These materials pose a technological challenge. While the biodegradable scaffolds are completely eliminated overtime, they still need to equate the performance of best-in-class drug-eluting stent with respect to efficacy and safety. After the Absorb everolimus-eluting BVS system (Absorb BVS) was launched in Europe, initial studies showed scaffold-related thrombosis rates as high as 3.4%.5–7 That compares with 0.4% for second-generation DES—a troubling result for a new technology.
Rates of restenosis and stent thrombosis are similar for bioresorbable stents and standard durable polymer stents. But what are the potential added benefits of bioresorbable stents? And will they improve patient outcomes?
Bioresorbable stents certainly appeal to patients who do not want a permanent, rigid, metallic implant. Also appealing are the proposed benefits of restoration of vasomotion, late luminal enlargement, preservation of CABG targets, and relief of angina. Whether bioresorbable stents improve these outcomes has not been established. Currently, there is no long-term evidence of reduced rates of adverse events, although in 1 study, optical coherence tomography images recorded 10 years after implantation of the first bioresorbable stents showed a pristine vessel with no signs of the struts.8
Several facts are known about the Absorb BVS:
- Preclinical evidence shows complete resorption and return of vascular function, but this takes 3 to 4 years.
- Imaging data at 5 years from the Absorb cohort B trial show complete resorption of struts, lumen preservation, return of function, and plaque regression.9
- In ABSORB III, the pivotal US trial, the stent was within the primary end point showing noninferiority in safety and effectiveness compared with Xience in the first year.10
- Absorb clinical trials in Japan and China confirmed ABSORB III results.
- Meta-analysis (> 3,300 patients) confirmed safety and effectiveness of Absorb.11
- Real-world Absorb clinical evidence continues to show improving outcomes with optimized implant techniques.
- Absorb stent was approved by the US Food and Drug Administration (FDA) in July 2016; more than 150,000 have been implanted worldwide.
The increased rates of target-lesion revascularization and stent thrombosis were likely attributable to inserting the stents into small-diameter vessels that are probably too small for the Absorb BVS. When small vessels (< 2.25 mm) are eliminated from the analysis, the rates were as follows.
Results for vessels > 2.25 mm:
- Target-lesion revascularization: 6.7 % vs 5.5%
- Stent thrombosis: 0.9% vs 0.6%.
- Results for small vessels (< 2.25 mm):
- Target-lesion revascularization: 12.9% vs 8.3%
- Stent thrombosis: 4.6% vs 1.5%.
The lesson is that the Absorb BVS should not be placed in arteries smaller than 2.25 mm in diameter.
ABSORB II STUDY RESULTS RAISE QUESTIONS
Another concern was uncovered in July 2016 when results were published from the ABSORB II trial on vasomotor reactivity at 3 years.13 This clinical trial randomized 501 patients in a 2:1 ratio to the Absorb BVS or the Xience DES at 46 sites outside the United States. Assessment for changes in mean lumen diameter between pre- and post-nitrate administration showed no differences between the groups; thus, the Absorb BVS did not achieve a level of superior vasomotor reactivity. There was vasomotor reactivity probably because the surrogate marker was angiographic follow-up and not intravascular ultrasound or tomography.
Further, the coprimary end point of angiographic late luminal loss at 3 years did not meet its noninferiority standard. The Absorb BVS was expected to have lower rates of late lumen loss because the struts are gone and there is less new intimal formation; however, at 3 years, that was not the case.
The rate of acute stent thrombosis also was alarming: 8 cases for Absorb BVS versus none for Xience. This caused alarm, raising the question of why it was happening in these patients 2 to 3 years after implantation.
Animal studies investigating the association of thicker struts and increased thrombogenicity have reported that the 157-µm BVS had much more platelet buildup and thrombogenicity than a 120-µm biomatrix stent. The 74-µm Synergy stent had even lower rates of thrombosis. The reason for increased thrombogenicity with thicker struts requires further study.
Also, an analysis of the secondary cardiac end points at 3 years in ABSORB II found no clinical patient-oriented differences between the Absorb BVS and the Xience stent (20.8% vs 24.0%, respectively; P = .44). However, rates of device-oriented clinical end points were significantly higher for Absorb BVS (10.4% vs 4.9%; P = .043).13
Clearly, the results for Absorb BVS in this study were not positive. One explanation is suboptimal implantation techniques that did not appose the polymer to the wall. A few years ago, focus shifted to an optimal technique for scaffold deployment, which included predilation, appropriate sizing of the scaffold to the size of the vessel, and postdilation with the intention of embedding the polymer in the vessel wall. Multiple studies have reported fewer incidents of stent thrombosis with the implementation of this protocol.14
Further studies have continued to report increased rates of late scaffold thrombosis in follow-ups of 30 days to 3 years. This resulted in an advisory letter from the FDA focused on appropriate clinical use of the device and withdrawal of ABSORB from commercial use in Europe and Australia.
BIORESORBABLE SCAFFOLDS PIPELINE
This is questionable because one has to believe in the vulnerable plaque theory, which assumes potential eruption of plaques. The Absorb can actually seal a thin cap atheroma and necrotic core over time. It seems that this technology can cause some late lumen enlargement and seal an existing plaque, which may have implications for the future.
SUMMARY
This is the current state of the Absorb BVS:
- More than 150,000 implanted globally
- Received FDA approval in July 2016
- Should not be used in small vessels (ie, lumen diameter < 2.25 mm)
- Thrombosis rates 2 to 3 years after implantation are of concern
- Focusing on appropriate surgical implantation technique can improve outcomes.
Overall, use of bioresorbable stent technology is intriguing. While there is ongoing patient preference for bioresorbable technology, clinical trial results raise the question of whether bioresorbable scaffolds are inferior to best-in-class DES. Improving the scaffold technology and the implantation techniques may equate the short-term outcome of the bioresorbable scaffolds with metallic stents with the hope that over time (when the scaffold is gone), the advantage will be with the bioresorbable scaffolds. Meanwhile, the technology is still seeking its best clinical utility, and a matching performance to the best-in-class DES.
Time will tell whether 5 to 10 years after implantation, BRS technology will outperform durable metallic stents.
The development of a new generation of drug-eluting stents (DES) has had a dramatic impact on the number of stents used for percutaneous transluminal coronary angioplasty for the treatment of coronary artery disease (CAD). But even second- and third-generation DES fall short when compared with coronary artery bypass grafting (CABG) with regards to the need for repeat reavascularization. CABG is advantageous because it bypasses the entire disease segment of the vessel. Thus for multivessel complex CAD, it is still considered the best choice. Nevertheless, most patients prefer the less-invasive option of stents, so practitioners need to provide the best stent available.
There are 3 primary criteria for DES selection:
- Efficacy for a broad range of patients and lesion complexities that primarily provides consistency in improving measures of angiographic and clinical efficacy
- Safety as determined by the following:
- Enable healing and promote endothelialization
- Permit functional endothelium
- Obtaining complete apposition
- Reduction or elimination of late and very late stent thrombosis
- Minimizing the need for long-term dual antiplatelet therapy
- Performance provided by reliable delivery capabilities to the lesion site.
GREAT EXPECTATIONS
New DES must be shown to be superior to previous generation stents. Although preclinical endothelialization and other mechanistic surrogates are good enough to claim an improvement, the traditional method is to compare clinical outcomes with the new stent versus the existing stent in a randomized clinical trial.
PROBLEMS WITH DURABLE POLYMER STENTS
Complications with durable polymer DES have included increased local inflammation and neoatherosclerosis. There are reports of subacute stent thrombosis due to lack of adequate expansion and stent apposition. Also reported was late thrombosis, resulting in increased rates of myocardial infarction and death.
These issues motivated engineers to improve and iterate the DES technology. One important technological change is the decrease in strut thickness from 140 µm to as low as 60 µm. The thickness of the polymer coating also has been reduced. The polymer became thinner, more biocompatible, and in some stents, only abluminal. Further developments were to substitute the durable polymer with a biodegradable polymer and perhaps even design a polymer-free stent.
BIORESORBABLE POLYMERS EMERGE
The time course for resorption of bioresorbable polymers ranges from 2 to 15 months, but they all degrade, which should improve long-term outcomes. A meta-analysis of data from the LEADERS trial and ISAR-TEST 3 and 4 found that the bioresorbable polymer stents were associated with significantly lower rates of target-lesion revascularization (P = .029) and stent thrombosis (P = .015) than durable polymer DES at 4 years after implantation.1 Those results led to the notion that stents with a biodegradable polymer would result in lower rates of stent thrombosis than durable polymer stents; however, that was not the case when stents with biodegradable polymers were compared with second-generation DES.
In the COMPARE II trial,2 the rates of stent thrombosis and target-lesion revascularization were not statistically different for the thick-strut biodegradable polymer biolimus-eluting stent (Nobori) compared with the second-generation thin-strut permanent-polymer stents (Xience). In the CENTURY II trial,3 a third-generation biodegradable sirolimus-eluting stent (Ultimaster) had stent thrombosis rates similar to those of a durable polymer everolimus-eluting stent (Xience) 300 days after insertion (4.36% vs 5.27%, respectively). Target-lesion revascularization rates were also about the same for the stents. In the EVOLVE II trial comparing the thin-strut biodegradable everolimus-eluting stent (Synergy) vs the thin-strut permanent-polymer everolimus-eluting stent (Promus), the 12-month target lesion failure rates for the stents were essentially the same.4
THE RATIONALE FOR BIORESORBABLE STENTS
Another approach was to use biodegradable scaffolds that will be eliminating from the vessel wall once it “completes the job.” The main bioresorbable materials used were polylactic acid or biodegradable metal-like magnesium. These materials pose a technological challenge. While the biodegradable scaffolds are completely eliminated overtime, they still need to equate the performance of best-in-class drug-eluting stent with respect to efficacy and safety. After the Absorb everolimus-eluting BVS system (Absorb BVS) was launched in Europe, initial studies showed scaffold-related thrombosis rates as high as 3.4%.5–7 That compares with 0.4% for second-generation DES—a troubling result for a new technology.
Rates of restenosis and stent thrombosis are similar for bioresorbable stents and standard durable polymer stents. But what are the potential added benefits of bioresorbable stents? And will they improve patient outcomes?
Bioresorbable stents certainly appeal to patients who do not want a permanent, rigid, metallic implant. Also appealing are the proposed benefits of restoration of vasomotion, late luminal enlargement, preservation of CABG targets, and relief of angina. Whether bioresorbable stents improve these outcomes has not been established. Currently, there is no long-term evidence of reduced rates of adverse events, although in 1 study, optical coherence tomography images recorded 10 years after implantation of the first bioresorbable stents showed a pristine vessel with no signs of the struts.8
Several facts are known about the Absorb BVS:
- Preclinical evidence shows complete resorption and return of vascular function, but this takes 3 to 4 years.
- Imaging data at 5 years from the Absorb cohort B trial show complete resorption of struts, lumen preservation, return of function, and plaque regression.9
- In ABSORB III, the pivotal US trial, the stent was within the primary end point showing noninferiority in safety and effectiveness compared with Xience in the first year.10
- Absorb clinical trials in Japan and China confirmed ABSORB III results.
- Meta-analysis (> 3,300 patients) confirmed safety and effectiveness of Absorb.11
- Real-world Absorb clinical evidence continues to show improving outcomes with optimized implant techniques.
- Absorb stent was approved by the US Food and Drug Administration (FDA) in July 2016; more than 150,000 have been implanted worldwide.
The increased rates of target-lesion revascularization and stent thrombosis were likely attributable to inserting the stents into small-diameter vessels that are probably too small for the Absorb BVS. When small vessels (< 2.25 mm) are eliminated from the analysis, the rates were as follows.
Results for vessels > 2.25 mm:
- Target-lesion revascularization: 6.7 % vs 5.5%
- Stent thrombosis: 0.9% vs 0.6%.
- Results for small vessels (< 2.25 mm):
- Target-lesion revascularization: 12.9% vs 8.3%
- Stent thrombosis: 4.6% vs 1.5%.
The lesson is that the Absorb BVS should not be placed in arteries smaller than 2.25 mm in diameter.
ABSORB II STUDY RESULTS RAISE QUESTIONS
Another concern was uncovered in July 2016 when results were published from the ABSORB II trial on vasomotor reactivity at 3 years.13 This clinical trial randomized 501 patients in a 2:1 ratio to the Absorb BVS or the Xience DES at 46 sites outside the United States. Assessment for changes in mean lumen diameter between pre- and post-nitrate administration showed no differences between the groups; thus, the Absorb BVS did not achieve a level of superior vasomotor reactivity. There was vasomotor reactivity probably because the surrogate marker was angiographic follow-up and not intravascular ultrasound or tomography.
Further, the coprimary end point of angiographic late luminal loss at 3 years did not meet its noninferiority standard. The Absorb BVS was expected to have lower rates of late lumen loss because the struts are gone and there is less new intimal formation; however, at 3 years, that was not the case.
The rate of acute stent thrombosis also was alarming: 8 cases for Absorb BVS versus none for Xience. This caused alarm, raising the question of why it was happening in these patients 2 to 3 years after implantation.
Animal studies investigating the association of thicker struts and increased thrombogenicity have reported that the 157-µm BVS had much more platelet buildup and thrombogenicity than a 120-µm biomatrix stent. The 74-µm Synergy stent had even lower rates of thrombosis. The reason for increased thrombogenicity with thicker struts requires further study.
Also, an analysis of the secondary cardiac end points at 3 years in ABSORB II found no clinical patient-oriented differences between the Absorb BVS and the Xience stent (20.8% vs 24.0%, respectively; P = .44). However, rates of device-oriented clinical end points were significantly higher for Absorb BVS (10.4% vs 4.9%; P = .043).13
Clearly, the results for Absorb BVS in this study were not positive. One explanation is suboptimal implantation techniques that did not appose the polymer to the wall. A few years ago, focus shifted to an optimal technique for scaffold deployment, which included predilation, appropriate sizing of the scaffold to the size of the vessel, and postdilation with the intention of embedding the polymer in the vessel wall. Multiple studies have reported fewer incidents of stent thrombosis with the implementation of this protocol.14
Further studies have continued to report increased rates of late scaffold thrombosis in follow-ups of 30 days to 3 years. This resulted in an advisory letter from the FDA focused on appropriate clinical use of the device and withdrawal of ABSORB from commercial use in Europe and Australia.
BIORESORBABLE SCAFFOLDS PIPELINE
This is questionable because one has to believe in the vulnerable plaque theory, which assumes potential eruption of plaques. The Absorb can actually seal a thin cap atheroma and necrotic core over time. It seems that this technology can cause some late lumen enlargement and seal an existing plaque, which may have implications for the future.
SUMMARY
This is the current state of the Absorb BVS:
- More than 150,000 implanted globally
- Received FDA approval in July 2016
- Should not be used in small vessels (ie, lumen diameter < 2.25 mm)
- Thrombosis rates 2 to 3 years after implantation are of concern
- Focusing on appropriate surgical implantation technique can improve outcomes.
Overall, use of bioresorbable stent technology is intriguing. While there is ongoing patient preference for bioresorbable technology, clinical trial results raise the question of whether bioresorbable scaffolds are inferior to best-in-class DES. Improving the scaffold technology and the implantation techniques may equate the short-term outcome of the bioresorbable scaffolds with metallic stents with the hope that over time (when the scaffold is gone), the advantage will be with the bioresorbable scaffolds. Meanwhile, the technology is still seeking its best clinical utility, and a matching performance to the best-in-class DES.
Time will tell whether 5 to 10 years after implantation, BRS technology will outperform durable metallic stents.
- Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J 2012; 33:1214–1222.
- Smits PC, Hofma S, Togni M, et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet 2013; 381:651–660.
- Saito S, Valdes-Chavarri M, Richardt G, et al; for the CENTURY II Investigators. A randomized, prospective, intercontinental evaluation of a bioresorbable polymer sirolimus-eluting coronary stent system: the CENTURY II (Clinical Evaluation of New Terumo Drug-Eluting Coronary Stent System in the Treatment of Patients with Coronary Artery Disease) trial. Eur Heart J 2014; 35:2021–2031.
- Kereiakes DJ, Meredith IT, Windecker S, et al. Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent: the EVOLVE II randomized trial. Circ Cardiovasc Interv 2015; 8:e002372. doi: 10.1161/CIRCINTERVENTIONS.114.002372
- Kraak RP, Hassell ME, Grundeken MJ, et al. Initial experience and clinical evaluation of the Absorb bioresorbable vascular scaffold (BVS) in real-world practice: the AMC Single Centre Real World PCI Registry. EuroIntervention 2015; 10:1160–1168.
- Capodanno D, Gori T, Nef H, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention 2015; 10:1144–1153.
- Ielasi A, Cortese B, Varricchio A, et al. Immediate and midterm outcomes following primary PCI with bioresorbable vascular scaffold implantation in patients with ST-segment myocardial infarction: insights from the multicentre “Registro ABSORB Italiano” (RAI registry). EuroIntervention 2015; 11:157–162.
- Onuma Y, Piazza N, Ormiston JA, Serruys PW. Everolimus-eluting bioabsorbable stent—Abbott Vascular programme. EuroIntervention 2009; 5(suppl F):F98–F102.
- De Bruyne B, Toth GG, Onuma Y, Serruys PW. ABSORB cohort B trial: five year angiographic results of the ABSORB everolimus eluting bioresorbable vascular scaffold. J Am Coll Cardiol 2014; 64(suppl):B181. Abstract TCT 619.
- Ellis SG, Kereiakes DJ, Metzger DC, et al; for the ABSORB III Investigators. Everolimus-eluting bioresorbable scaffolds for coronary artery disease. N Engl J Med 2015; 373:1905–1915.
- Stone GW, Gao R, Kimura T, et al. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease: a patient-level, pooled meta-analysis. Lancet 2016; 387:1277–1289.
- Kuramitsu S, Sonoda S, Yokoi H, et al. Long-term coronary arterial response to biodegradable polymer biolimus-eluting stents in comparison with durable polymer sirolimus-eluting stents and bare-metal stents: five-year follow-up optical coherence tomography study. Atherosclerosis 2014; 237:23–29.
- Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016; 388:2479–2491.
- Puricel S, Cuculi F, Weissner M, et al. Bioresorbable coronary scaffold thrombosis: multicenter comprehensive analysis of clinical presentation, mechanisms, and predictors. J Am Coll Cardiol 2016; 67:921–931.
- Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J 2012; 33:1214–1222.
- Smits PC, Hofma S, Togni M, et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet 2013; 381:651–660.
- Saito S, Valdes-Chavarri M, Richardt G, et al; for the CENTURY II Investigators. A randomized, prospective, intercontinental evaluation of a bioresorbable polymer sirolimus-eluting coronary stent system: the CENTURY II (Clinical Evaluation of New Terumo Drug-Eluting Coronary Stent System in the Treatment of Patients with Coronary Artery Disease) trial. Eur Heart J 2014; 35:2021–2031.
- Kereiakes DJ, Meredith IT, Windecker S, et al. Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent: the EVOLVE II randomized trial. Circ Cardiovasc Interv 2015; 8:e002372. doi: 10.1161/CIRCINTERVENTIONS.114.002372
- Kraak RP, Hassell ME, Grundeken MJ, et al. Initial experience and clinical evaluation of the Absorb bioresorbable vascular scaffold (BVS) in real-world practice: the AMC Single Centre Real World PCI Registry. EuroIntervention 2015; 10:1160–1168.
- Capodanno D, Gori T, Nef H, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention 2015; 10:1144–1153.
- Ielasi A, Cortese B, Varricchio A, et al. Immediate and midterm outcomes following primary PCI with bioresorbable vascular scaffold implantation in patients with ST-segment myocardial infarction: insights from the multicentre “Registro ABSORB Italiano” (RAI registry). EuroIntervention 2015; 11:157–162.
- Onuma Y, Piazza N, Ormiston JA, Serruys PW. Everolimus-eluting bioabsorbable stent—Abbott Vascular programme. EuroIntervention 2009; 5(suppl F):F98–F102.
- De Bruyne B, Toth GG, Onuma Y, Serruys PW. ABSORB cohort B trial: five year angiographic results of the ABSORB everolimus eluting bioresorbable vascular scaffold. J Am Coll Cardiol 2014; 64(suppl):B181. Abstract TCT 619.
- Ellis SG, Kereiakes DJ, Metzger DC, et al; for the ABSORB III Investigators. Everolimus-eluting bioresorbable scaffolds for coronary artery disease. N Engl J Med 2015; 373:1905–1915.
- Stone GW, Gao R, Kimura T, et al. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease: a patient-level, pooled meta-analysis. Lancet 2016; 387:1277–1289.
- Kuramitsu S, Sonoda S, Yokoi H, et al. Long-term coronary arterial response to biodegradable polymer biolimus-eluting stents in comparison with durable polymer sirolimus-eluting stents and bare-metal stents: five-year follow-up optical coherence tomography study. Atherosclerosis 2014; 237:23–29.
- Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016; 388:2479–2491.
- Puricel S, Cuculi F, Weissner M, et al. Bioresorbable coronary scaffold thrombosis: multicenter comprehensive analysis of clinical presentation, mechanisms, and predictors. J Am Coll Cardiol 2016; 67:921–931.
KEY POINTS
- Complications with first-generation durable polymer DES—stent thrombosis and restenosis with target lesion revascularization—led to the development of bioresorbable stents.
- Bioresorbable and durable polymer metallic DES have similar rates of efficacy and of stent thrombosis.
- Bioresorbable DES should be placed in appropriate patient populations and lesion subsets, and limited to arteries larger than 2.25 mm.