User login
A Rising Tide: No Hospital Is an Island Unto Itself in the Era of COVID-19
The early phase of the COVID-19 pandemic was an extraordinarily uncertain, yet innovative, time.1 Few data describe site-level effects of the many adaptations made to deal with surging case numbers, but studies of larger hospital referral regions (HRR) provide important clues.
In this issue of the Journal of Hospital Medicine, Janke et al2 describe how availability of hospital resources in a region relate to COVID-19 mortality between March and June 2020.The authors’ findings suggest that, at least for early periods of the pandemic, having more intensive care unit (ICU), hospital bed, or nursing capacity per COVID-19 case was associated with lower mortality, while physician availability was not. Moreover, months later there were no associations between service or physician availability and HRR COVID-19 mortality. The authors observed variations in mortality rates in places commonly thought to have been overwhelmed early in the pandemic (April 2020), as well as in cities (Boston, Philadelphia, Hartford, Detroit, and Camden, New Jersey) that had a less prominent place in the news at that time.
Larger hospitals tend to have the resources necessary to make wholesale changes when preparing for a pandemic wave. Thus, Janke et al’s results may not have fully captured the pandemic’s potential impact in settings with fewer resources or in smaller hospitals, which are currently being overwhelmed.3
The number of cases and hospitalizations in this third wave of COVID-19 continues to rise, and the strain on healthcare resources has been felt across entire regions, making the results of this study even more salient. Hospital outcomes for COVID-19 are sensitive to limitations in physical locations (number of beds, ICU capacity) and nursing capacity. Nurses more often are assigned specifically to a bed or unit, and the number of patients per nurse is limited by state or local statute. Innovations such as COVID-19 field hospitals or redeploying existing beds (eg, converting postanesthesia care units to ICUs) offset physically constrained resources.4 On the other hand, lower acuity in this phase of the pandemic (eg, fewer ICU admissions) and shorter lengths of stay may produce higher turnover, producing more workforce stress, regardless of bed availability.
Early work of our COVID-19 collaborative5 suggests that the focus on localizing patients to geographic units or teams has given way to strategies that utilize more flexible team and bed-finding approaches. Clinical care has evolved to focus on more aggressive discharge strategies, with remote monitoring and hospital-at-home capabilities. Overall, the pandemic is providing fodder for future studies examining interaction between case volumes, physician and nurse availability, and evolution in clinical care practices. Most critically, it provides an opportunity to study health system flexibility and robustness with a lens that incorporates a view of the hospital and its surroundings as tightly related parts of care delivery. Because if there is one thing the pandemic is teaching us, it is that, more than ever, no hospital can be an island unto itself, and each hospital is part of a larger ecosystem where rising tides are felt throughout.
1. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
2. Janke AT, Mei H, Rothenberg C, Becher RD, Lin Z, Venkatesh AK. Analysis of hospital resource availability and COVID-19 mortality across the United States. J Hosp Med. 2021;16(4):211-214.
3. Achenbach J, Brulliard K, Shammas B, Dupree J. Hospitals in nearly every region report a flood of covid-19 patients. Washington Post. October 26, 2020. Accessed March 4, 2021. https://www.washingtonpost.com/health/covid-hospitals-record-patients/2020/10/26/0bc362cc-17b2-11eb-befb-8864259bd2d8_story.html
4. Chaudhary MJ, Howell E, Ficke JR, et al. Caring for patients at a COVID-19 field hospital. J Hosp Med. 2021;16(2):117-119. https://doi.org/10.12788/jhm.3551
5. Welcome to the COVID-19 response working team knowledge base. HOMERun Hospital Medicine Reengineering Network COVID-19 Collaboration. Accessed March 4, 2021. https://www.hospitalinnovate.org/covid19/
The early phase of the COVID-19 pandemic was an extraordinarily uncertain, yet innovative, time.1 Few data describe site-level effects of the many adaptations made to deal with surging case numbers, but studies of larger hospital referral regions (HRR) provide important clues.
In this issue of the Journal of Hospital Medicine, Janke et al2 describe how availability of hospital resources in a region relate to COVID-19 mortality between March and June 2020.The authors’ findings suggest that, at least for early periods of the pandemic, having more intensive care unit (ICU), hospital bed, or nursing capacity per COVID-19 case was associated with lower mortality, while physician availability was not. Moreover, months later there were no associations between service or physician availability and HRR COVID-19 mortality. The authors observed variations in mortality rates in places commonly thought to have been overwhelmed early in the pandemic (April 2020), as well as in cities (Boston, Philadelphia, Hartford, Detroit, and Camden, New Jersey) that had a less prominent place in the news at that time.
Larger hospitals tend to have the resources necessary to make wholesale changes when preparing for a pandemic wave. Thus, Janke et al’s results may not have fully captured the pandemic’s potential impact in settings with fewer resources or in smaller hospitals, which are currently being overwhelmed.3
The number of cases and hospitalizations in this third wave of COVID-19 continues to rise, and the strain on healthcare resources has been felt across entire regions, making the results of this study even more salient. Hospital outcomes for COVID-19 are sensitive to limitations in physical locations (number of beds, ICU capacity) and nursing capacity. Nurses more often are assigned specifically to a bed or unit, and the number of patients per nurse is limited by state or local statute. Innovations such as COVID-19 field hospitals or redeploying existing beds (eg, converting postanesthesia care units to ICUs) offset physically constrained resources.4 On the other hand, lower acuity in this phase of the pandemic (eg, fewer ICU admissions) and shorter lengths of stay may produce higher turnover, producing more workforce stress, regardless of bed availability.
Early work of our COVID-19 collaborative5 suggests that the focus on localizing patients to geographic units or teams has given way to strategies that utilize more flexible team and bed-finding approaches. Clinical care has evolved to focus on more aggressive discharge strategies, with remote monitoring and hospital-at-home capabilities. Overall, the pandemic is providing fodder for future studies examining interaction between case volumes, physician and nurse availability, and evolution in clinical care practices. Most critically, it provides an opportunity to study health system flexibility and robustness with a lens that incorporates a view of the hospital and its surroundings as tightly related parts of care delivery. Because if there is one thing the pandemic is teaching us, it is that, more than ever, no hospital can be an island unto itself, and each hospital is part of a larger ecosystem where rising tides are felt throughout.
The early phase of the COVID-19 pandemic was an extraordinarily uncertain, yet innovative, time.1 Few data describe site-level effects of the many adaptations made to deal with surging case numbers, but studies of larger hospital referral regions (HRR) provide important clues.
In this issue of the Journal of Hospital Medicine, Janke et al2 describe how availability of hospital resources in a region relate to COVID-19 mortality between March and June 2020.The authors’ findings suggest that, at least for early periods of the pandemic, having more intensive care unit (ICU), hospital bed, or nursing capacity per COVID-19 case was associated with lower mortality, while physician availability was not. Moreover, months later there were no associations between service or physician availability and HRR COVID-19 mortality. The authors observed variations in mortality rates in places commonly thought to have been overwhelmed early in the pandemic (April 2020), as well as in cities (Boston, Philadelphia, Hartford, Detroit, and Camden, New Jersey) that had a less prominent place in the news at that time.
Larger hospitals tend to have the resources necessary to make wholesale changes when preparing for a pandemic wave. Thus, Janke et al’s results may not have fully captured the pandemic’s potential impact in settings with fewer resources or in smaller hospitals, which are currently being overwhelmed.3
The number of cases and hospitalizations in this third wave of COVID-19 continues to rise, and the strain on healthcare resources has been felt across entire regions, making the results of this study even more salient. Hospital outcomes for COVID-19 are sensitive to limitations in physical locations (number of beds, ICU capacity) and nursing capacity. Nurses more often are assigned specifically to a bed or unit, and the number of patients per nurse is limited by state or local statute. Innovations such as COVID-19 field hospitals or redeploying existing beds (eg, converting postanesthesia care units to ICUs) offset physically constrained resources.4 On the other hand, lower acuity in this phase of the pandemic (eg, fewer ICU admissions) and shorter lengths of stay may produce higher turnover, producing more workforce stress, regardless of bed availability.
Early work of our COVID-19 collaborative5 suggests that the focus on localizing patients to geographic units or teams has given way to strategies that utilize more flexible team and bed-finding approaches. Clinical care has evolved to focus on more aggressive discharge strategies, with remote monitoring and hospital-at-home capabilities. Overall, the pandemic is providing fodder for future studies examining interaction between case volumes, physician and nurse availability, and evolution in clinical care practices. Most critically, it provides an opportunity to study health system flexibility and robustness with a lens that incorporates a view of the hospital and its surroundings as tightly related parts of care delivery. Because if there is one thing the pandemic is teaching us, it is that, more than ever, no hospital can be an island unto itself, and each hospital is part of a larger ecosystem where rising tides are felt throughout.
1. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
2. Janke AT, Mei H, Rothenberg C, Becher RD, Lin Z, Venkatesh AK. Analysis of hospital resource availability and COVID-19 mortality across the United States. J Hosp Med. 2021;16(4):211-214.
3. Achenbach J, Brulliard K, Shammas B, Dupree J. Hospitals in nearly every region report a flood of covid-19 patients. Washington Post. October 26, 2020. Accessed March 4, 2021. https://www.washingtonpost.com/health/covid-hospitals-record-patients/2020/10/26/0bc362cc-17b2-11eb-befb-8864259bd2d8_story.html
4. Chaudhary MJ, Howell E, Ficke JR, et al. Caring for patients at a COVID-19 field hospital. J Hosp Med. 2021;16(2):117-119. https://doi.org/10.12788/jhm.3551
5. Welcome to the COVID-19 response working team knowledge base. HOMERun Hospital Medicine Reengineering Network COVID-19 Collaboration. Accessed March 4, 2021. https://www.hospitalinnovate.org/covid19/
1. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
2. Janke AT, Mei H, Rothenberg C, Becher RD, Lin Z, Venkatesh AK. Analysis of hospital resource availability and COVID-19 mortality across the United States. J Hosp Med. 2021;16(4):211-214.
3. Achenbach J, Brulliard K, Shammas B, Dupree J. Hospitals in nearly every region report a flood of covid-19 patients. Washington Post. October 26, 2020. Accessed March 4, 2021. https://www.washingtonpost.com/health/covid-hospitals-record-patients/2020/10/26/0bc362cc-17b2-11eb-befb-8864259bd2d8_story.html
4. Chaudhary MJ, Howell E, Ficke JR, et al. Caring for patients at a COVID-19 field hospital. J Hosp Med. 2021;16(2):117-119. https://doi.org/10.12788/jhm.3551
5. Welcome to the COVID-19 response working team knowledge base. HOMERun Hospital Medicine Reengineering Network COVID-19 Collaboration. Accessed March 4, 2021. https://www.hospitalinnovate.org/covid19/
© 2021 Society of Hospital Medicine
Dearth of Hospitalist Investigators in Academic Medicine: A Call to Action
In their report celebrating the increase in the number of hospitalists from a few hundred in the 1990s to more than 50,000 in 2016, Drs Robert Wachter and Lee Goldman also noted the stunted growth of productive hospital medicine research programs, which presents a challenge to academic credibility in hospital medicine.1 Given the substantial increase in the number of hospitalists over the past two decades, we surveyed adult academic hospital medicine groups to quantify the number of hospitalist clinician investigators and identify gaps in resources for researchers. The number of clinician investigators supported at academic medical centers (AMCs) remains disturbingly low despite the rapid growth of our specialty. Some programs also reported a lack of access to fundamental research services. We report selected results from our survey and provide recommendations to support and facilitate the development of clinician investigators in hospital medicine.
DEARTH OF CLINICIAN INVESTIGATORS IN HOSPITAL MEDICINE
We performed a survey of hospital medicine programs at AMCs in the United States through the Hospital Medicine Reengineering Network (HOMERuN), a hospital medicine research collaborative that facilitates and conducts multisite research studies.2 The purpose of this survey was to obtain a profile of adult academic hospital medicine groups. Surveys were distributed via email to directors and/or senior leaders of each hospital medicine group between January and August 2019. In the survey, a clinician investigator was defined as “faculty whose primary nonclinical focus is scientific papers and grant writing.”
We received responses from 43 of the 86 invitees (50%), each of whom represented a unique hospital medicine group; 41 of the representatives responded to the questions concerning available research services. Collectively, these 43 programs represented 2,503 hospitalists. There were 79 clinician investigators reported among all surveyed hospital medicine groups (3.1% of all hospitalists). The median number of clinician investigators per hospital medicine group was 0 (range 0-12) (Appendix Figure 1), and 22 of 43 (51.2%) hospital medicine groups reported having no clinician investigators. Two of the hospital medicine groups, however, reported having 12 clinician investigators at their respective institutions, comprising nearly one third of the total number of clinician investigators reported in the survey.
Many of the programs reported lack of access to resources such as research assistants (56.1%) and dedicated research fellowships (53.7%) (Appendix Figure 2). A number of groups reported a need for more support for various junior faculty development activities, including research mentoring (53.5%), networking with other researchers (60.5%), and access to clinical data from multiple sites (62.8%).
One of the limitations of this survey was the manner in which the participating hospital medicine groups were chosen. Selection was based on groups affiliated with HOMERuN; among those chosen were highly visible US AMCs, including 70% of the top 20 AMCs based on National Institutes of Health (NIH) funding.3 Therefore, our results likely overestimate the research presence of hospital medicine across all AMCs in the United States.
LACK OF GROWTH OVER TIME: CONTEXTUALIZATION AND IMPLICATIONS
Despite the substantial growth of hospital medicine over the past 2 decades, there has been no proportional increase in the number of hospitalist clinician investigators, with earlier surveys also demonstrating low numbers.4,5 Along with the survey by Chopra and colleagues published in 2019,6 our survey provides an additional contemporary appraisal of research activities for adult academic hospital medicine groups. In the survey by Chopra et al, only 54% (15 of 28) of responding programs reported having any faculty with research as their major activity (ie, >50% effort), and 3% of total faculty reported having funding for >50% effort toward research.6 Our study expands upon these findings by providing more detailed data on the number of clinician investigators per hospital medicine group. Results of our survey showed a concentration of hospitalists within a small number of programs, which may have contributed to the observed lack of growth. We also expand on prior work by identifying a lack of resources and services to support hospitalist researchers.
The findings of our survey have important implications for the field of hospital medicine. Without a critical mass of hospitalist clinician investigators, the quality of research that addresses important questions in our field will suffer. It will also limit academic credibility of the field, as well as individual academic achievement; previous studies have consistently demonstrated that few hospitalists at AMCs achieve the rank of associate or full professor.5-9
POTENTIAL EXPLANATIONS FOR LACK OF RESEARCH GROWTH
The results of our study additionally offer possible explanations for the dearth of clinician investigators in hospital medicine. The limited access to research resources and fellowship training identified in our survey are critical domains that must be addressed in order to develop successful academic hospital medicine programs.4,6,8,10
Regarding dedicated hospital medicine research fellowships, there are only a handful across the country. The small number of existing research fellowships only have one or two fellows per year, and these positions often go unfilled because of a lack of applicants and lower salaries compared to full-time clinical positions.11 The lack of applicants for adult hospital medicine fellowship positions is also integrally linked to board certification requirements. Unlike pediatric hospital medicine where additional fellowship training is required to become board-certified, no such fellowship is required in adult hospital medicine. In pediatrics, this requirement has led to a rapid increase in the number of fellowships with scholarly work requirements (more than 60 fellowships, plus additional programs in development) and greater standardization among training experiences.12,13
The lack of fellowship applicants may also stem from the fact that many trainees are not aware of a potential career as a hospitalist clinician investigator due to limited exposure to this career at most AMCs. Our results revealed that nearly half of sites in our survey had zero clinician investigators, depriving trainees at these programs of role models and thus perpetuating a negative feedback loop. Lastly, although unfilled fellowship positions may indicate that demand is a larger problem than supply, it is also true that fellowship programs generate their own demand through recruitment efforts and the gradual establishment of a positive reputation.
Another potential explanation could relate to the development of hospital medicine in response to rising clinical demands at hospitals: compared with other medical specialties, AMCs may regard hospitalists as being clinicians first and academicians second.1,7,10 Also, hospitalists may be perceived as being beholden to hospitals and less engaged with their surrounding communities than other general medicine fields. With a small footprint in health equity research, academic hospital medicine may be less of a draw to generalists interested in pursuing this area of research. Further, there are very few underrepresented in medicine (URiM) hospital medicine research faculty.5
Another challenge to the career development of hospitalist researchers is the lack of available funding for the type of research typically conducted by hospitalists (eg, rigorous quality improvement implementation and evaluation, optimizing best evidence-based care delivery models, evaluation of patient safety in the hospital setting). As hospitalists tend to be system-level thinkers, this lack of funding may steer potential researchers away from externally funded research careers and into hospital operations and quality improvement positions. Also, unlike other medical specialties, there is no dedicated NIH funding source for hospital medicine research (eg, cardiology and the National Heart, Lung, and Blood Institute), placing hospitalists at a disadvantage in seeking funding compared to subspecialists.
STRATEGIES TO ENHANCE RESEARCH PRESENCE
We recommend several approaches—ones that should be pursued simultaneously—to increase the number of clinician investigators in hospital medicine. First, hospital medicine groups and their respective divisions, departments, and hospitals should allocate funding to support research resources; this includes investing in research assistants, data analysts, statisticians, and administrative support. Through the funding of such research infrastructure programs, AMCs could incentivize hospitalists to research best approaches to improve the value of healthcare delivery, ultimately leading to cost savings.
With 60% of respondents identifying the need for improved access to data across multiple sites, our survey also emphasizes the requirement for further collaboration among hospital medicine groups. Such collaboration could lead to high-powered observational studies and the evaluation of interventions across multiple sites, thus improving the generalizability of study findings.
The Society of Hospital Medicine (SHM) and its research committee can continue to expand the research footprint of hospital medicine. To date, the committee has achieved this by highlighting hospitalist research activity at the SHM Annual Conference Scientific Abstract and Poster Competition and developing a visiting professorship exchange program. In addition to these efforts, SHM could foster collaboration and networking between institutions, as well as take advantage of the current political push for expanded Medicare access by lobbying for robust funding for the Agency for Healthcare Research and Quality, which could provide more opportunities for hospitalists to study the effects of healthcare policy reform on the delivery of inpatient care.
Another strategy to increase the number of hospitalist clinician investigators is to expand hospital medicine research fellowships and recruit trainees for these programs. Fellowships could be internally funded wherein a fellow’s clinical productivity is used to offset the costs associated with obtaining advanced degrees. As an incentive to encourage applicants to temporarily forego a full-time clinical salary during fellowship, hospital medicine groups could offer expanded moonlighting opportunities and contribute to repayment of medical school loans. Hospital medicine groups should also advocate for NIH-funded T32 or K12 training grants for hospital medicine. (There are, however, challenges with this approach because the number of T32 spots per NIH institute is usually fixed). The success of academic emergency medicine offers a precedent for such efforts: After the development of a K12 research training program in emergency medicine, the number of NIH-sponsored principal investigators in this specialty increased by 40% in 6 years.14 Additionally, now that fellowships are required for the pediatric hospital medicine clinician investigators, it would be revealing to track the growth of this workforce.12,13
Structured and formalized mentorship is an essential part of the development of clinician investigators in hospital medicine.4,7,8,10 One successful strategy for mentorship has been the partnering of hospital medicine groups with faculty of general internal medicine and other subspecialty divisions with robust research programs.7,8,15 In addition to developing sustainable mentorship programs, hospital medicine researchers must increase their visibility to trainees. Therefore, it is essential that the majority of academic hospital medicine groups not only hire clinician investigators but also invest in their development, rather than rely on the few programs that have several such faculty members. With this strategy, we could dramatically increase the number of hospitalist clinician investigators from a diverse background of training institutions.
SHM could also play a greater role in organizing events for networking and mentoring for trainees and medical students interested in pursuing a career in hospital medicine research. It is also critically important that hospital medicine groups actively recruit, retain, and develop URiM hospital medicine research faculty in order to attract talented researchers and actively participate in the necessary effort to mitigate the inequities prevalent throughout our healthcare system.
CONCLUSION
Despite the growth of hospital medicine over the past decade, there remains a dearth of hospitalist clinician investigators at major AMCs in the United States. This may be due in part to lack of research resources and mentorship within hospital medicine groups. We believe that investment in these resources, expanded funding opportunities, mentorship development, research fellowship programs, and greater exposure of trainees to hospitalist researchers are solutions that should be strongly considered to develop hospitalist clinician investigators.
Acknowledgments
The authors thank HOMERuN executive committee members, including Grant Fletcher, MD, James Harrison, PhD, BSC, MPH, Peter K. Lindenauer, MD, Melissa Mattison, MD, David Meltzer, MD, PhD, Joshua Metlay, MD, PhD, Jennifer Myers, MD, Sumant Ranji, MD, Gregory Ruhnke, MD, MPH, Edmondo Robinson, MD, MBA, and Neil Sehgal, MPH PhD, for their assistance in developing the survey. They also thank Tiffany Lee, MA, for her project management assistance for HOMERuN.
1. Wachter RM, Goldman L. Zero to 50,000 – The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
3. Roskoski R Jr, Parslow TG. Ranking Tables of NIH funding to US medical schools in 2019. Blue Ridge Institute for Medical Research. Published 2020. Updated July 14, 2020. Accessed July 30, 2020. http://www.brimr.org/NIH_Awards/2019/NIH_Awards_2019.htm
4. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
5. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the society of general internal medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
6. Chopra V, Burden M, Jones CD, et al; Society of Hospital Medicine Research Committee. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
7. Seymann GB, Southern W, Burger A, et al. Features of successful academic hospitalist programs: insights from the SCHOLAR (SuCcessful HOspitaLists in academics and research) project. J Hosp Med. 2016;11(10):708-713. https://doi.org/10.1002/jhm.2603
8. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836
9. Dang Do AN, Munchhof AM, Terry C, Emmett T, Kara A. Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148-154. https://doi.org/10.1002/jhm.2148
10. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161-166. https://doi.org/10.1002/jhm.845
11. Ranji SR, Rosenman DJ, Amin AN, Kripalani S. Hospital medicine fellowships: works in progress. Am J Med. 2006;119(1):72.e1-72.e7. https://doi.org/10.1016/j.amjmed.2005.07.061
12. Shah NH, Rhim HJ, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: a survey of program directors. J Hosp Med. 2016;11(5):324-328. https://doi.org/10.1002/jhm.2571
13. Jerardi KE, Fisher E, Rassbach C, et al; Council of Pediatric Hospital Medicine Fellowship Directors. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698
14. Lewis RJ, Neumar RW. Research in emergency medicine: building the investigator pipeline. Ann Emerg Med. 2018;72(6):691-695. https://doi.org/10.1016/j.annemergmed.2018.10.019
15. Flanders SA, Kaufman SR, Nallamothu BK, Saint S. The University of Michigan Specialist-Hospitalist Allied Research Program: jumpstarting hospital medicine research. J Hosp Med. 2008;3(4):308-313. https://doi.org/10.1002/jhm.342
In their report celebrating the increase in the number of hospitalists from a few hundred in the 1990s to more than 50,000 in 2016, Drs Robert Wachter and Lee Goldman also noted the stunted growth of productive hospital medicine research programs, which presents a challenge to academic credibility in hospital medicine.1 Given the substantial increase in the number of hospitalists over the past two decades, we surveyed adult academic hospital medicine groups to quantify the number of hospitalist clinician investigators and identify gaps in resources for researchers. The number of clinician investigators supported at academic medical centers (AMCs) remains disturbingly low despite the rapid growth of our specialty. Some programs also reported a lack of access to fundamental research services. We report selected results from our survey and provide recommendations to support and facilitate the development of clinician investigators in hospital medicine.
DEARTH OF CLINICIAN INVESTIGATORS IN HOSPITAL MEDICINE
We performed a survey of hospital medicine programs at AMCs in the United States through the Hospital Medicine Reengineering Network (HOMERuN), a hospital medicine research collaborative that facilitates and conducts multisite research studies.2 The purpose of this survey was to obtain a profile of adult academic hospital medicine groups. Surveys were distributed via email to directors and/or senior leaders of each hospital medicine group between January and August 2019. In the survey, a clinician investigator was defined as “faculty whose primary nonclinical focus is scientific papers and grant writing.”
We received responses from 43 of the 86 invitees (50%), each of whom represented a unique hospital medicine group; 41 of the representatives responded to the questions concerning available research services. Collectively, these 43 programs represented 2,503 hospitalists. There were 79 clinician investigators reported among all surveyed hospital medicine groups (3.1% of all hospitalists). The median number of clinician investigators per hospital medicine group was 0 (range 0-12) (Appendix Figure 1), and 22 of 43 (51.2%) hospital medicine groups reported having no clinician investigators. Two of the hospital medicine groups, however, reported having 12 clinician investigators at their respective institutions, comprising nearly one third of the total number of clinician investigators reported in the survey.
Many of the programs reported lack of access to resources such as research assistants (56.1%) and dedicated research fellowships (53.7%) (Appendix Figure 2). A number of groups reported a need for more support for various junior faculty development activities, including research mentoring (53.5%), networking with other researchers (60.5%), and access to clinical data from multiple sites (62.8%).
One of the limitations of this survey was the manner in which the participating hospital medicine groups were chosen. Selection was based on groups affiliated with HOMERuN; among those chosen were highly visible US AMCs, including 70% of the top 20 AMCs based on National Institutes of Health (NIH) funding.3 Therefore, our results likely overestimate the research presence of hospital medicine across all AMCs in the United States.
LACK OF GROWTH OVER TIME: CONTEXTUALIZATION AND IMPLICATIONS
Despite the substantial growth of hospital medicine over the past 2 decades, there has been no proportional increase in the number of hospitalist clinician investigators, with earlier surveys also demonstrating low numbers.4,5 Along with the survey by Chopra and colleagues published in 2019,6 our survey provides an additional contemporary appraisal of research activities for adult academic hospital medicine groups. In the survey by Chopra et al, only 54% (15 of 28) of responding programs reported having any faculty with research as their major activity (ie, >50% effort), and 3% of total faculty reported having funding for >50% effort toward research.6 Our study expands upon these findings by providing more detailed data on the number of clinician investigators per hospital medicine group. Results of our survey showed a concentration of hospitalists within a small number of programs, which may have contributed to the observed lack of growth. We also expand on prior work by identifying a lack of resources and services to support hospitalist researchers.
The findings of our survey have important implications for the field of hospital medicine. Without a critical mass of hospitalist clinician investigators, the quality of research that addresses important questions in our field will suffer. It will also limit academic credibility of the field, as well as individual academic achievement; previous studies have consistently demonstrated that few hospitalists at AMCs achieve the rank of associate or full professor.5-9
POTENTIAL EXPLANATIONS FOR LACK OF RESEARCH GROWTH
The results of our study additionally offer possible explanations for the dearth of clinician investigators in hospital medicine. The limited access to research resources and fellowship training identified in our survey are critical domains that must be addressed in order to develop successful academic hospital medicine programs.4,6,8,10
Regarding dedicated hospital medicine research fellowships, there are only a handful across the country. The small number of existing research fellowships only have one or two fellows per year, and these positions often go unfilled because of a lack of applicants and lower salaries compared to full-time clinical positions.11 The lack of applicants for adult hospital medicine fellowship positions is also integrally linked to board certification requirements. Unlike pediatric hospital medicine where additional fellowship training is required to become board-certified, no such fellowship is required in adult hospital medicine. In pediatrics, this requirement has led to a rapid increase in the number of fellowships with scholarly work requirements (more than 60 fellowships, plus additional programs in development) and greater standardization among training experiences.12,13
The lack of fellowship applicants may also stem from the fact that many trainees are not aware of a potential career as a hospitalist clinician investigator due to limited exposure to this career at most AMCs. Our results revealed that nearly half of sites in our survey had zero clinician investigators, depriving trainees at these programs of role models and thus perpetuating a negative feedback loop. Lastly, although unfilled fellowship positions may indicate that demand is a larger problem than supply, it is also true that fellowship programs generate their own demand through recruitment efforts and the gradual establishment of a positive reputation.
Another potential explanation could relate to the development of hospital medicine in response to rising clinical demands at hospitals: compared with other medical specialties, AMCs may regard hospitalists as being clinicians first and academicians second.1,7,10 Also, hospitalists may be perceived as being beholden to hospitals and less engaged with their surrounding communities than other general medicine fields. With a small footprint in health equity research, academic hospital medicine may be less of a draw to generalists interested in pursuing this area of research. Further, there are very few underrepresented in medicine (URiM) hospital medicine research faculty.5
Another challenge to the career development of hospitalist researchers is the lack of available funding for the type of research typically conducted by hospitalists (eg, rigorous quality improvement implementation and evaluation, optimizing best evidence-based care delivery models, evaluation of patient safety in the hospital setting). As hospitalists tend to be system-level thinkers, this lack of funding may steer potential researchers away from externally funded research careers and into hospital operations and quality improvement positions. Also, unlike other medical specialties, there is no dedicated NIH funding source for hospital medicine research (eg, cardiology and the National Heart, Lung, and Blood Institute), placing hospitalists at a disadvantage in seeking funding compared to subspecialists.
STRATEGIES TO ENHANCE RESEARCH PRESENCE
We recommend several approaches—ones that should be pursued simultaneously—to increase the number of clinician investigators in hospital medicine. First, hospital medicine groups and their respective divisions, departments, and hospitals should allocate funding to support research resources; this includes investing in research assistants, data analysts, statisticians, and administrative support. Through the funding of such research infrastructure programs, AMCs could incentivize hospitalists to research best approaches to improve the value of healthcare delivery, ultimately leading to cost savings.
With 60% of respondents identifying the need for improved access to data across multiple sites, our survey also emphasizes the requirement for further collaboration among hospital medicine groups. Such collaboration could lead to high-powered observational studies and the evaluation of interventions across multiple sites, thus improving the generalizability of study findings.
The Society of Hospital Medicine (SHM) and its research committee can continue to expand the research footprint of hospital medicine. To date, the committee has achieved this by highlighting hospitalist research activity at the SHM Annual Conference Scientific Abstract and Poster Competition and developing a visiting professorship exchange program. In addition to these efforts, SHM could foster collaboration and networking between institutions, as well as take advantage of the current political push for expanded Medicare access by lobbying for robust funding for the Agency for Healthcare Research and Quality, which could provide more opportunities for hospitalists to study the effects of healthcare policy reform on the delivery of inpatient care.
Another strategy to increase the number of hospitalist clinician investigators is to expand hospital medicine research fellowships and recruit trainees for these programs. Fellowships could be internally funded wherein a fellow’s clinical productivity is used to offset the costs associated with obtaining advanced degrees. As an incentive to encourage applicants to temporarily forego a full-time clinical salary during fellowship, hospital medicine groups could offer expanded moonlighting opportunities and contribute to repayment of medical school loans. Hospital medicine groups should also advocate for NIH-funded T32 or K12 training grants for hospital medicine. (There are, however, challenges with this approach because the number of T32 spots per NIH institute is usually fixed). The success of academic emergency medicine offers a precedent for such efforts: After the development of a K12 research training program in emergency medicine, the number of NIH-sponsored principal investigators in this specialty increased by 40% in 6 years.14 Additionally, now that fellowships are required for the pediatric hospital medicine clinician investigators, it would be revealing to track the growth of this workforce.12,13
Structured and formalized mentorship is an essential part of the development of clinician investigators in hospital medicine.4,7,8,10 One successful strategy for mentorship has been the partnering of hospital medicine groups with faculty of general internal medicine and other subspecialty divisions with robust research programs.7,8,15 In addition to developing sustainable mentorship programs, hospital medicine researchers must increase their visibility to trainees. Therefore, it is essential that the majority of academic hospital medicine groups not only hire clinician investigators but also invest in their development, rather than rely on the few programs that have several such faculty members. With this strategy, we could dramatically increase the number of hospitalist clinician investigators from a diverse background of training institutions.
SHM could also play a greater role in organizing events for networking and mentoring for trainees and medical students interested in pursuing a career in hospital medicine research. It is also critically important that hospital medicine groups actively recruit, retain, and develop URiM hospital medicine research faculty in order to attract talented researchers and actively participate in the necessary effort to mitigate the inequities prevalent throughout our healthcare system.
CONCLUSION
Despite the growth of hospital medicine over the past decade, there remains a dearth of hospitalist clinician investigators at major AMCs in the United States. This may be due in part to lack of research resources and mentorship within hospital medicine groups. We believe that investment in these resources, expanded funding opportunities, mentorship development, research fellowship programs, and greater exposure of trainees to hospitalist researchers are solutions that should be strongly considered to develop hospitalist clinician investigators.
Acknowledgments
The authors thank HOMERuN executive committee members, including Grant Fletcher, MD, James Harrison, PhD, BSC, MPH, Peter K. Lindenauer, MD, Melissa Mattison, MD, David Meltzer, MD, PhD, Joshua Metlay, MD, PhD, Jennifer Myers, MD, Sumant Ranji, MD, Gregory Ruhnke, MD, MPH, Edmondo Robinson, MD, MBA, and Neil Sehgal, MPH PhD, for their assistance in developing the survey. They also thank Tiffany Lee, MA, for her project management assistance for HOMERuN.
In their report celebrating the increase in the number of hospitalists from a few hundred in the 1990s to more than 50,000 in 2016, Drs Robert Wachter and Lee Goldman also noted the stunted growth of productive hospital medicine research programs, which presents a challenge to academic credibility in hospital medicine.1 Given the substantial increase in the number of hospitalists over the past two decades, we surveyed adult academic hospital medicine groups to quantify the number of hospitalist clinician investigators and identify gaps in resources for researchers. The number of clinician investigators supported at academic medical centers (AMCs) remains disturbingly low despite the rapid growth of our specialty. Some programs also reported a lack of access to fundamental research services. We report selected results from our survey and provide recommendations to support and facilitate the development of clinician investigators in hospital medicine.
DEARTH OF CLINICIAN INVESTIGATORS IN HOSPITAL MEDICINE
We performed a survey of hospital medicine programs at AMCs in the United States through the Hospital Medicine Reengineering Network (HOMERuN), a hospital medicine research collaborative that facilitates and conducts multisite research studies.2 The purpose of this survey was to obtain a profile of adult academic hospital medicine groups. Surveys were distributed via email to directors and/or senior leaders of each hospital medicine group between January and August 2019. In the survey, a clinician investigator was defined as “faculty whose primary nonclinical focus is scientific papers and grant writing.”
We received responses from 43 of the 86 invitees (50%), each of whom represented a unique hospital medicine group; 41 of the representatives responded to the questions concerning available research services. Collectively, these 43 programs represented 2,503 hospitalists. There were 79 clinician investigators reported among all surveyed hospital medicine groups (3.1% of all hospitalists). The median number of clinician investigators per hospital medicine group was 0 (range 0-12) (Appendix Figure 1), and 22 of 43 (51.2%) hospital medicine groups reported having no clinician investigators. Two of the hospital medicine groups, however, reported having 12 clinician investigators at their respective institutions, comprising nearly one third of the total number of clinician investigators reported in the survey.
Many of the programs reported lack of access to resources such as research assistants (56.1%) and dedicated research fellowships (53.7%) (Appendix Figure 2). A number of groups reported a need for more support for various junior faculty development activities, including research mentoring (53.5%), networking with other researchers (60.5%), and access to clinical data from multiple sites (62.8%).
One of the limitations of this survey was the manner in which the participating hospital medicine groups were chosen. Selection was based on groups affiliated with HOMERuN; among those chosen were highly visible US AMCs, including 70% of the top 20 AMCs based on National Institutes of Health (NIH) funding.3 Therefore, our results likely overestimate the research presence of hospital medicine across all AMCs in the United States.
LACK OF GROWTH OVER TIME: CONTEXTUALIZATION AND IMPLICATIONS
Despite the substantial growth of hospital medicine over the past 2 decades, there has been no proportional increase in the number of hospitalist clinician investigators, with earlier surveys also demonstrating low numbers.4,5 Along with the survey by Chopra and colleagues published in 2019,6 our survey provides an additional contemporary appraisal of research activities for adult academic hospital medicine groups. In the survey by Chopra et al, only 54% (15 of 28) of responding programs reported having any faculty with research as their major activity (ie, >50% effort), and 3% of total faculty reported having funding for >50% effort toward research.6 Our study expands upon these findings by providing more detailed data on the number of clinician investigators per hospital medicine group. Results of our survey showed a concentration of hospitalists within a small number of programs, which may have contributed to the observed lack of growth. We also expand on prior work by identifying a lack of resources and services to support hospitalist researchers.
The findings of our survey have important implications for the field of hospital medicine. Without a critical mass of hospitalist clinician investigators, the quality of research that addresses important questions in our field will suffer. It will also limit academic credibility of the field, as well as individual academic achievement; previous studies have consistently demonstrated that few hospitalists at AMCs achieve the rank of associate or full professor.5-9
POTENTIAL EXPLANATIONS FOR LACK OF RESEARCH GROWTH
The results of our study additionally offer possible explanations for the dearth of clinician investigators in hospital medicine. The limited access to research resources and fellowship training identified in our survey are critical domains that must be addressed in order to develop successful academic hospital medicine programs.4,6,8,10
Regarding dedicated hospital medicine research fellowships, there are only a handful across the country. The small number of existing research fellowships only have one or two fellows per year, and these positions often go unfilled because of a lack of applicants and lower salaries compared to full-time clinical positions.11 The lack of applicants for adult hospital medicine fellowship positions is also integrally linked to board certification requirements. Unlike pediatric hospital medicine where additional fellowship training is required to become board-certified, no such fellowship is required in adult hospital medicine. In pediatrics, this requirement has led to a rapid increase in the number of fellowships with scholarly work requirements (more than 60 fellowships, plus additional programs in development) and greater standardization among training experiences.12,13
The lack of fellowship applicants may also stem from the fact that many trainees are not aware of a potential career as a hospitalist clinician investigator due to limited exposure to this career at most AMCs. Our results revealed that nearly half of sites in our survey had zero clinician investigators, depriving trainees at these programs of role models and thus perpetuating a negative feedback loop. Lastly, although unfilled fellowship positions may indicate that demand is a larger problem than supply, it is also true that fellowship programs generate their own demand through recruitment efforts and the gradual establishment of a positive reputation.
Another potential explanation could relate to the development of hospital medicine in response to rising clinical demands at hospitals: compared with other medical specialties, AMCs may regard hospitalists as being clinicians first and academicians second.1,7,10 Also, hospitalists may be perceived as being beholden to hospitals and less engaged with their surrounding communities than other general medicine fields. With a small footprint in health equity research, academic hospital medicine may be less of a draw to generalists interested in pursuing this area of research. Further, there are very few underrepresented in medicine (URiM) hospital medicine research faculty.5
Another challenge to the career development of hospitalist researchers is the lack of available funding for the type of research typically conducted by hospitalists (eg, rigorous quality improvement implementation and evaluation, optimizing best evidence-based care delivery models, evaluation of patient safety in the hospital setting). As hospitalists tend to be system-level thinkers, this lack of funding may steer potential researchers away from externally funded research careers and into hospital operations and quality improvement positions. Also, unlike other medical specialties, there is no dedicated NIH funding source for hospital medicine research (eg, cardiology and the National Heart, Lung, and Blood Institute), placing hospitalists at a disadvantage in seeking funding compared to subspecialists.
STRATEGIES TO ENHANCE RESEARCH PRESENCE
We recommend several approaches—ones that should be pursued simultaneously—to increase the number of clinician investigators in hospital medicine. First, hospital medicine groups and their respective divisions, departments, and hospitals should allocate funding to support research resources; this includes investing in research assistants, data analysts, statisticians, and administrative support. Through the funding of such research infrastructure programs, AMCs could incentivize hospitalists to research best approaches to improve the value of healthcare delivery, ultimately leading to cost savings.
With 60% of respondents identifying the need for improved access to data across multiple sites, our survey also emphasizes the requirement for further collaboration among hospital medicine groups. Such collaboration could lead to high-powered observational studies and the evaluation of interventions across multiple sites, thus improving the generalizability of study findings.
The Society of Hospital Medicine (SHM) and its research committee can continue to expand the research footprint of hospital medicine. To date, the committee has achieved this by highlighting hospitalist research activity at the SHM Annual Conference Scientific Abstract and Poster Competition and developing a visiting professorship exchange program. In addition to these efforts, SHM could foster collaboration and networking between institutions, as well as take advantage of the current political push for expanded Medicare access by lobbying for robust funding for the Agency for Healthcare Research and Quality, which could provide more opportunities for hospitalists to study the effects of healthcare policy reform on the delivery of inpatient care.
Another strategy to increase the number of hospitalist clinician investigators is to expand hospital medicine research fellowships and recruit trainees for these programs. Fellowships could be internally funded wherein a fellow’s clinical productivity is used to offset the costs associated with obtaining advanced degrees. As an incentive to encourage applicants to temporarily forego a full-time clinical salary during fellowship, hospital medicine groups could offer expanded moonlighting opportunities and contribute to repayment of medical school loans. Hospital medicine groups should also advocate for NIH-funded T32 or K12 training grants for hospital medicine. (There are, however, challenges with this approach because the number of T32 spots per NIH institute is usually fixed). The success of academic emergency medicine offers a precedent for such efforts: After the development of a K12 research training program in emergency medicine, the number of NIH-sponsored principal investigators in this specialty increased by 40% in 6 years.14 Additionally, now that fellowships are required for the pediatric hospital medicine clinician investigators, it would be revealing to track the growth of this workforce.12,13
Structured and formalized mentorship is an essential part of the development of clinician investigators in hospital medicine.4,7,8,10 One successful strategy for mentorship has been the partnering of hospital medicine groups with faculty of general internal medicine and other subspecialty divisions with robust research programs.7,8,15 In addition to developing sustainable mentorship programs, hospital medicine researchers must increase their visibility to trainees. Therefore, it is essential that the majority of academic hospital medicine groups not only hire clinician investigators but also invest in their development, rather than rely on the few programs that have several such faculty members. With this strategy, we could dramatically increase the number of hospitalist clinician investigators from a diverse background of training institutions.
SHM could also play a greater role in organizing events for networking and mentoring for trainees and medical students interested in pursuing a career in hospital medicine research. It is also critically important that hospital medicine groups actively recruit, retain, and develop URiM hospital medicine research faculty in order to attract talented researchers and actively participate in the necessary effort to mitigate the inequities prevalent throughout our healthcare system.
CONCLUSION
Despite the growth of hospital medicine over the past decade, there remains a dearth of hospitalist clinician investigators at major AMCs in the United States. This may be due in part to lack of research resources and mentorship within hospital medicine groups. We believe that investment in these resources, expanded funding opportunities, mentorship development, research fellowship programs, and greater exposure of trainees to hospitalist researchers are solutions that should be strongly considered to develop hospitalist clinician investigators.
Acknowledgments
The authors thank HOMERuN executive committee members, including Grant Fletcher, MD, James Harrison, PhD, BSC, MPH, Peter K. Lindenauer, MD, Melissa Mattison, MD, David Meltzer, MD, PhD, Joshua Metlay, MD, PhD, Jennifer Myers, MD, Sumant Ranji, MD, Gregory Ruhnke, MD, MPH, Edmondo Robinson, MD, MBA, and Neil Sehgal, MPH PhD, for their assistance in developing the survey. They also thank Tiffany Lee, MA, for her project management assistance for HOMERuN.
1. Wachter RM, Goldman L. Zero to 50,000 – The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
3. Roskoski R Jr, Parslow TG. Ranking Tables of NIH funding to US medical schools in 2019. Blue Ridge Institute for Medical Research. Published 2020. Updated July 14, 2020. Accessed July 30, 2020. http://www.brimr.org/NIH_Awards/2019/NIH_Awards_2019.htm
4. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
5. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the society of general internal medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
6. Chopra V, Burden M, Jones CD, et al; Society of Hospital Medicine Research Committee. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
7. Seymann GB, Southern W, Burger A, et al. Features of successful academic hospitalist programs: insights from the SCHOLAR (SuCcessful HOspitaLists in academics and research) project. J Hosp Med. 2016;11(10):708-713. https://doi.org/10.1002/jhm.2603
8. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836
9. Dang Do AN, Munchhof AM, Terry C, Emmett T, Kara A. Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148-154. https://doi.org/10.1002/jhm.2148
10. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161-166. https://doi.org/10.1002/jhm.845
11. Ranji SR, Rosenman DJ, Amin AN, Kripalani S. Hospital medicine fellowships: works in progress. Am J Med. 2006;119(1):72.e1-72.e7. https://doi.org/10.1016/j.amjmed.2005.07.061
12. Shah NH, Rhim HJ, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: a survey of program directors. J Hosp Med. 2016;11(5):324-328. https://doi.org/10.1002/jhm.2571
13. Jerardi KE, Fisher E, Rassbach C, et al; Council of Pediatric Hospital Medicine Fellowship Directors. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698
14. Lewis RJ, Neumar RW. Research in emergency medicine: building the investigator pipeline. Ann Emerg Med. 2018;72(6):691-695. https://doi.org/10.1016/j.annemergmed.2018.10.019
15. Flanders SA, Kaufman SR, Nallamothu BK, Saint S. The University of Michigan Specialist-Hospitalist Allied Research Program: jumpstarting hospital medicine research. J Hosp Med. 2008;3(4):308-313. https://doi.org/10.1002/jhm.342
1. Wachter RM, Goldman L. Zero to 50,000 – The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
3. Roskoski R Jr, Parslow TG. Ranking Tables of NIH funding to US medical schools in 2019. Blue Ridge Institute for Medical Research. Published 2020. Updated July 14, 2020. Accessed July 30, 2020. http://www.brimr.org/NIH_Awards/2019/NIH_Awards_2019.htm
4. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
5. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the society of general internal medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
6. Chopra V, Burden M, Jones CD, et al; Society of Hospital Medicine Research Committee. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
7. Seymann GB, Southern W, Burger A, et al. Features of successful academic hospitalist programs: insights from the SCHOLAR (SuCcessful HOspitaLists in academics and research) project. J Hosp Med. 2016;11(10):708-713. https://doi.org/10.1002/jhm.2603
8. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836
9. Dang Do AN, Munchhof AM, Terry C, Emmett T, Kara A. Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148-154. https://doi.org/10.1002/jhm.2148
10. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161-166. https://doi.org/10.1002/jhm.845
11. Ranji SR, Rosenman DJ, Amin AN, Kripalani S. Hospital medicine fellowships: works in progress. Am J Med. 2006;119(1):72.e1-72.e7. https://doi.org/10.1016/j.amjmed.2005.07.061
12. Shah NH, Rhim HJ, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: a survey of program directors. J Hosp Med. 2016;11(5):324-328. https://doi.org/10.1002/jhm.2571
13. Jerardi KE, Fisher E, Rassbach C, et al; Council of Pediatric Hospital Medicine Fellowship Directors. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698
14. Lewis RJ, Neumar RW. Research in emergency medicine: building the investigator pipeline. Ann Emerg Med. 2018;72(6):691-695. https://doi.org/10.1016/j.annemergmed.2018.10.019
15. Flanders SA, Kaufman SR, Nallamothu BK, Saint S. The University of Michigan Specialist-Hospitalist Allied Research Program: jumpstarting hospital medicine research. J Hosp Med. 2008;3(4):308-313. https://doi.org/10.1002/jhm.342
© 2021 Society of Hospital Medicine
Hospital Ward Adaptation During the COVID-19 Pandemic: A National Survey of Academic Medical Centers
The coronavirus disease of 2019 (COVID-19) pandemic has resulted in a surge in hospitalizations of patients with a novel, serious, and highly contagious infectious disease for which there is yet no proven treatment. Currently, much of the focus has been on intensive care unit (ICU) and ventilator capacity for the sickest of these patients who develop respiratory failure. However, most hospitalized patients are being cared for in general medical units.1 Some evidence exists to describe adaptations to capacity needs outside of medical wards,2-4 but few studies have specifically addressed the ward setting. Therefore, there is a pressing need for evidence to describe how to expand capacity and deliver medical ward–based care.
To better understand how inpatient care in the United States is adapting to the COVID-19 pandemic, we surveyed 72 sites participating in the Hospital Medicine Reengineering Network (HOMERuN), a national consortium of hospital medicine groups.5 We report results of this survey, carried out between April 3 and April 5, 2020.
METHODS
Sites and Subjects
HOMERuN is a collaborative network of hospitalists from across the United States whose primary goal is to catalyze research and share best practices across hospital medicine groups. Using surveys of Hospital Medicine leaders, targeted medical record review, and other methods, HOMERuN’s funded research interests to date have included care transitions, workforce issues, patient and family engagement, and diagnostic errors. Sites participating in HOMERuN sites are relatively large urban academic medical centers (Appendix).
Survey Development and Deployment
We designed a focused survey that aimed to provide a snapshot of evolving operational and clinical aspects of COVID-19 care (Appendix). Domains included COVID-19 testing turnaround times, personal protective equipment (PPE) stewardship,6 features of respiratory isolation units (RIUs; ie, dedicated units for patients with known or suspected COVID-19), and observed effects on clinical care. We tested the instrument to ensure feasibility and clarity internally, performed brief cognitive testing with several hospital medicine leaders in HOMERuN, then disseminated the survey by email on April 3, with two follow-up emails on 2 subsequent days. Our study was deemed non–human subjects research by the University of California, San Francisco, Committee on Human Research. Descriptive statistics were used to characterize survey responses.
RESULTS
Of 72 hospitals surveyed, 51 (71%) responded. Mean hospital bed count was 940, three were safety-net hospitals, and one was a community-based teaching center; responding and nonresponding hospitals did not differ significantly in terms of bed count (Appendix).
Health System Adaptations, Testing, and PPE Status
Nearly all responding hospitals (46 of 51; 90%) had RIUs for patients with known or suspected COVID-19 (Table 1). Nearly all hospitals took steps to keep potentially sick healthcare providers from infecting others (eg, staying home if sick or exposed). Among respondents, 32% had rapid response teams, 24% had respiratory therapy teams, and 29% had case management teams that were dedicated to COVID-19 care. Thirty-two (63%) had developed models, such as ethics or palliative care consult services, to assist with difficult resource-allocation decisions (eg, how to prioritize ventilator use if demand exceeded supply). Twenty-three (45%) had developed post-acute care monitoring programs dedicated to COVID-19 patients.

At the time of our survey, only 2 sites (4%) reported COVID-19 test time turnaround under 1 hour, and 15 (30%) reported turnaround in less than 6 hours. Of the 29 sites able to provide estimates of PPE stockpile, 14 (48%) reported a supply of 2 weeks or less. The most common approaches to PPE stewardship focused on reuse of masks and face shields if not obviously soiled, centralizing PPE distribution, and disinfecting or sterilizing masks. Ten sites (20%) were utilizing 3-D printed masks, while 10% used homemade face shields or masks.
Characteristics of COVID-19 RIUs
Forty-six hospitals (90% of all respondents) in our cohort had developed RIUs at the time of survey administration. The earliest RIU implementation date was February 10, 2020, and the most recent was launched on the day of our survey. Admission to RIUs was primarily based on clinical factors associated with known or suspected COVID-19 infection (Table 2). The number of non–critical care RIU beds among locations at that time ranged from 10 or less to more than 50. The mean number of hospitalist attendings caring for patients in the RIUs was 10.2, with a mean 4.1 advanced practice providers, 5.5 residents, and 0 medical students. The number of planned patients per attending was typically 5 to 15. Nurses and physicians typically rounded separately. Medical distancing (eg, reducing patient room entry) was accomplished most commonly by grouped timing of medication administration (76% of sites), video links to room outside of rounding times (54% of sites), the use of video or telemedicine during rounds (17%), and clustering of activities such as medication administration or phlebotomy. The most common criteria prompting discharge from the RIU were a negative COVID-19 test (59%) and hospital discharge (57%), though comments from many respondents suggested that discharge criteria were changing rapidly.

Effects of Isolation Measures on In-Room Encounters and Diagnostic Processes
More than 90% of sites reported decreases in in-room encounter frequency across all provider types whether as a result of policies in place or not. Reductions were reported among hospitalists, advanced practice providers, residents, consultants, and therapists (Table 3). Reduced room entry most often resulted from an established or developing policy, but many noted reduced room entry without formal policies in place. Nearly all sites reported moving specialty consultations to phone or video evaluations. Diagnostic error was commonly reported, with missed non–COVID-19 medical diagnoses among COVID-19 infected patients being reported by 22 sites (46%) and missed COVID-19 diagnoses in patients admitted for other reasons by 22 sites (45%).

DISCUSSION
In this study of medical wards at academic medical centers, we found that, in response to the COVID-19 pandemic, hospitals made several changes in a short period of time to adapt to the crisis. These included implementation and rapid expansion of dedicated RIUs, greatly expanded use of inpatient telehealth for patient assessments and consultation, implementation of other approaches to minimize room entry (such as grouping in-room activities), and deployment of ethics consultation services to help manage issues around potential scarcity of life-saving measures such as ventilators. We also found that availability of PPE and timely testing was limited. Finally, a large proportion of sites reported potential diagnostic problems in the assessment of both patients suspected and those not suspected of having COVID-19.
RIUs are emerging as a primary modality for caring for non-ICU COVID-19 patients, though they never involved medical students; we hope the role of students in particular will increase as new models of training emerge in response to the pandemic.7 In contrast, telemedicine evolved rapidly to hold a substantial role in RIUs, with both ward and specialty teams using video visit technology to communicate with patients. COVID-19 has been viewed as a perfect use case for outpatient telemedicine,8 and a growing number of studies are examining its outpatient use9,10; however, to date, somewhat less attention has been paid to inpatient deployment. Although our data suggest telemedicine has found a prominent place in RIUs, it remains to be seen whether it is associated with differences in patient or provider outcomes. For example, deficiencies in the physical examination, limited face-to-face contact, and lack of physical presence could all affect the patient–provider relationship, patient engagement, and the accuracy of the diagnostic process.
Our data suggest the possibility of missing non–COVID-19 diagnoses in patients suspected of COVID-19 and missing COVID-19 in those admitted for nonrespiratory reasons. The latter may be addressed as routine COVID-19 screening of admitted patients becomes commonplace. For the former, however, it is possible that physicians are “anchoring” their thinking on COVID-19 to the exclusion of other diagnoses, that physicians are not fully aware of complications unique to COVID-19 infection (such as thromboembolism), and/or that the above-mentioned limitations of telemedicine have decreased diagnostic performance.
Although PPE stockpile data were not easily available for some sites, a distressingly large number reported stockpiles of 2 weeks or less, with reuse being the most common approach to extending PPE supply. We also found it concerning that 43% of hospital leaders did not know their stockpile data; we believe this is an important question that hospital leaders need to be asking. Most sites in our study reported test turnaround times of longer than 6 hours; lack of rapid COVID-19 testing further stresses PPE stockpile and may slow patients’ transition out of the RIU or discharge to home.
Our study has several limitations, including the evolving nature of the pandemic and rapid adaptations of care systems in the pandemic’s surge phase. However, we attempted to frame our questions in ways that provided a focused snapshot of care. Furthermore, respondents may not have had exhaustive knowledge of their institution’s COVID-19 response strategies, but most were the directors of their hospitalist services, and we encouraged the respondents to confer with others to gather high-fidelity data. Finally, as a survey of large academic medical centers, our results may not apply to nonacademic centers.
Approaches to caring for non-ICU patients during the COVID-19 pandemic are rapidly evolving. Expansion of RIUs and developing the workforce to support them has been a primary focus, with rapid innovation in use of technology emerging as a critical adaptation while PPE limitations persist and needs for “medical distancing” continue to grow. Although rates of missed COVID-19 diagnoses will likely be reduced with testing and systems improvements, physicians and systems will also need to consider how to utilize emerging technology in ways that can improve clinical care and provider safety while aiding diagnostic thinking. This survey illustrates the rapid adaptations made by our hospitals in response to the pandemic; ongoing adaptation will likely be needed to optimally care for hospitalized patients with COVID-19 while the pandemic continues to evolve.
Acknowledgment
Thanks to members of the HOMERuN COVID-19 Collaborative Group: Baylor Scott & White Medical Center – Temple, Texas - Tresa McNeal MD; Beth Israel Deaconess Medical Center - Shani Herzig MD MPH, Joseph Li MD, Julius Yang MD PhD; Brigham and Women’s Hospital - Christopher Roy MD, Jeffrey Schnipper MD MPH; Cedars-Sinai Medical Center - Ed Seferian MD, ; ChristianaCare - Surekha Bhamidipati MD; Cleveland Clinic - Matthew Pappas MD MPH; Dartmouth-Hitchcock Medical Center - Jonathan Lurie MD MS; Dell Medical School at The University of Texas at Austin - Chris Moriates MD, Luci Leykum MD MBA MSc; Denver Health and Hospitals Authority - Diana Mancini MD; Emory University Hospital - Dan Hunt MD; Johns Hopkins Hospital - Daniel J Brotman MD, Zishan K Siddiqui MD, Shaker Eid MD MBA; Maine Medical Center - Daniel A Meyer MD, Robert Trowbridge MD; Massachusetts General Hospital - Melissa Mattison MD; Mayo Clinic Rochester – Caroline Burton MD, Sagar Dugani MD PhD; Medical College of Wisconsin - Sanjay Bhandari MD; Miriam Hospital - Kwame Dapaah-Afriyie MD MBA; Mount Sinai Hospital - Andrew Dunn MD; NorthShore - David Lovinger MD; Northwestern Memorial Hospital - Kevin O’Leary MD MS; Ohio State University Wexner Medical Center - Eric Schumacher DO; Oregon Health & Science University - Angela Alday MD; Penn Medicine - Ryan Greysen MD MHS MA; Rutgers- Robert Wood Johnson University Hospital - Michael Steinberg MD MPH; Stanford University School of Medicine - Neera Ahuja MD; Tulane Hospital and University Medical Center - Geraldine Ménard MD; UC San Diego Health - Ian Jenkins MD; UC Los Angeles Health - Michael Lazarus MD, Magdalena E. Ptaszny, MD; UC San Francisco Health - Bradley A Sharpe, MD, Margaret Fang MD MPH; UK HealthCare - Mark Williams MD MHM, John Romond MD; University of Chicago – David Meltzer MD PhD, Gregory Ruhnke MD; University of Colorado - Marisha Burden MD; University of Florida - Nila Radhakrishnan MD; University of Iowa Hospitals and Clinics - Kevin Glenn MD MS; University of Miami - Efren Manjarrez MD; University of Michigan - Vineet Chopra MD MSc, Valerie Vaughn MD MSc; University of Missouri-Columbia Hospital - Hasan Naqvi MD; University of Nebraska Medical Center - Chad Vokoun MD; University of North Carolina at Chapel Hill - David Hemsey MD; University of Pittsburgh Medical Center - Gena Marie Walker MD; University of Vermont Medical Center - Steven Grant MD; University of Washington Medical Center - Christopher Kim MD MBA, Andrew White MD; University of Washington-Harborview Medical Center - Maralyssa Bann MD; University of Wisconsin Hospital and Clinics - David Sterken MD, Farah Kaiksow MD MPP, Ann Sheehy MD MS, Jordan Kenik MD MPH; UW Northwest Campus - Ben Wolpaw MD; Vanderbilt University Medical Center - Sunil Kripalani MD MSc, Eduard E Vasilevskis MD, Kathleene T Wooldridge MD MPH; Wake Forest Baptist Health - Erik Summers MD; Washington University St. Louis - Michael Lin MD; Weill Cornell - Justin Choi MD; Yale New Haven Hospital - William Cushing MA, Chris Sankey MD; Zuckerberg San Francisco General Hospital - Sumant Ranji MD.
1. Institute for Health Metrics and Evaluation. COVID-19 Projections: United States of America. 2020. Accessed May 5, 2020. https://covid19.healthdata.org/united-states-of-america
2. Iserson KV. Alternative care sites: an option in disasters. West J Emerg Med. 2020;21(3):484‐489. https://doi.org/10.5811/westjem.2020.4.47552
3. Paganini M, Conti A, Weinstein E, Della Corte F, Ragazzoni L. Translating COVID-19 pandemic surge theory to practice in the emergency department: how to expand structure [online first]. Disaster Med Public Health Prep. 2020:1-10. https://doi.org/10.1017/dmp.2020.57
4. Kumaraiah D, Yip N, Ivascu N, Hill L. Innovative ICU Physician Care Models: Covid-19 Pandemic at NewYork-Presbyterian. NEJM: Catalyst. April 28, 2020. Accessed May 5, 2020. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0158
5. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
6. Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.5317
7. Bauchner H, Sharfstein J. A bold response to the COVID-19 pandemic: medical students, national service, and public health [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6166
8. Hollander JE, Carr BG. Virtually perfect? telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679‐1681. https://doi.org/10.1056/nejmp2003539
9. Hau YS, Kim JK, Hur J, Chang MC. How about actively using telemedicine during the COVID-19 pandemic? J Med Syst. 2020;44(6):108. https://doi.org/10.1007/s10916-020-01580-z
10. Smith WR, Atala AJ, Terlecki RP, Kelly EE, Matthews CA. Implementation guide for rapid integration of an outpatient telemedicine program during the COVID-19 pandemic [online first]. J Am Coll Surg. 2020. https://doi.org/10.1016/j.jamcollsurg.2020.04.030
The coronavirus disease of 2019 (COVID-19) pandemic has resulted in a surge in hospitalizations of patients with a novel, serious, and highly contagious infectious disease for which there is yet no proven treatment. Currently, much of the focus has been on intensive care unit (ICU) and ventilator capacity for the sickest of these patients who develop respiratory failure. However, most hospitalized patients are being cared for in general medical units.1 Some evidence exists to describe adaptations to capacity needs outside of medical wards,2-4 but few studies have specifically addressed the ward setting. Therefore, there is a pressing need for evidence to describe how to expand capacity and deliver medical ward–based care.
To better understand how inpatient care in the United States is adapting to the COVID-19 pandemic, we surveyed 72 sites participating in the Hospital Medicine Reengineering Network (HOMERuN), a national consortium of hospital medicine groups.5 We report results of this survey, carried out between April 3 and April 5, 2020.
METHODS
Sites and Subjects
HOMERuN is a collaborative network of hospitalists from across the United States whose primary goal is to catalyze research and share best practices across hospital medicine groups. Using surveys of Hospital Medicine leaders, targeted medical record review, and other methods, HOMERuN’s funded research interests to date have included care transitions, workforce issues, patient and family engagement, and diagnostic errors. Sites participating in HOMERuN sites are relatively large urban academic medical centers (Appendix).
Survey Development and Deployment
We designed a focused survey that aimed to provide a snapshot of evolving operational and clinical aspects of COVID-19 care (Appendix). Domains included COVID-19 testing turnaround times, personal protective equipment (PPE) stewardship,6 features of respiratory isolation units (RIUs; ie, dedicated units for patients with known or suspected COVID-19), and observed effects on clinical care. We tested the instrument to ensure feasibility and clarity internally, performed brief cognitive testing with several hospital medicine leaders in HOMERuN, then disseminated the survey by email on April 3, with two follow-up emails on 2 subsequent days. Our study was deemed non–human subjects research by the University of California, San Francisco, Committee on Human Research. Descriptive statistics were used to characterize survey responses.
RESULTS
Of 72 hospitals surveyed, 51 (71%) responded. Mean hospital bed count was 940, three were safety-net hospitals, and one was a community-based teaching center; responding and nonresponding hospitals did not differ significantly in terms of bed count (Appendix).
Health System Adaptations, Testing, and PPE Status
Nearly all responding hospitals (46 of 51; 90%) had RIUs for patients with known or suspected COVID-19 (Table 1). Nearly all hospitals took steps to keep potentially sick healthcare providers from infecting others (eg, staying home if sick or exposed). Among respondents, 32% had rapid response teams, 24% had respiratory therapy teams, and 29% had case management teams that were dedicated to COVID-19 care. Thirty-two (63%) had developed models, such as ethics or palliative care consult services, to assist with difficult resource-allocation decisions (eg, how to prioritize ventilator use if demand exceeded supply). Twenty-three (45%) had developed post-acute care monitoring programs dedicated to COVID-19 patients.

At the time of our survey, only 2 sites (4%) reported COVID-19 test time turnaround under 1 hour, and 15 (30%) reported turnaround in less than 6 hours. Of the 29 sites able to provide estimates of PPE stockpile, 14 (48%) reported a supply of 2 weeks or less. The most common approaches to PPE stewardship focused on reuse of masks and face shields if not obviously soiled, centralizing PPE distribution, and disinfecting or sterilizing masks. Ten sites (20%) were utilizing 3-D printed masks, while 10% used homemade face shields or masks.
Characteristics of COVID-19 RIUs
Forty-six hospitals (90% of all respondents) in our cohort had developed RIUs at the time of survey administration. The earliest RIU implementation date was February 10, 2020, and the most recent was launched on the day of our survey. Admission to RIUs was primarily based on clinical factors associated with known or suspected COVID-19 infection (Table 2). The number of non–critical care RIU beds among locations at that time ranged from 10 or less to more than 50. The mean number of hospitalist attendings caring for patients in the RIUs was 10.2, with a mean 4.1 advanced practice providers, 5.5 residents, and 0 medical students. The number of planned patients per attending was typically 5 to 15. Nurses and physicians typically rounded separately. Medical distancing (eg, reducing patient room entry) was accomplished most commonly by grouped timing of medication administration (76% of sites), video links to room outside of rounding times (54% of sites), the use of video or telemedicine during rounds (17%), and clustering of activities such as medication administration or phlebotomy. The most common criteria prompting discharge from the RIU were a negative COVID-19 test (59%) and hospital discharge (57%), though comments from many respondents suggested that discharge criteria were changing rapidly.

Effects of Isolation Measures on In-Room Encounters and Diagnostic Processes
More than 90% of sites reported decreases in in-room encounter frequency across all provider types whether as a result of policies in place or not. Reductions were reported among hospitalists, advanced practice providers, residents, consultants, and therapists (Table 3). Reduced room entry most often resulted from an established or developing policy, but many noted reduced room entry without formal policies in place. Nearly all sites reported moving specialty consultations to phone or video evaluations. Diagnostic error was commonly reported, with missed non–COVID-19 medical diagnoses among COVID-19 infected patients being reported by 22 sites (46%) and missed COVID-19 diagnoses in patients admitted for other reasons by 22 sites (45%).

DISCUSSION
In this study of medical wards at academic medical centers, we found that, in response to the COVID-19 pandemic, hospitals made several changes in a short period of time to adapt to the crisis. These included implementation and rapid expansion of dedicated RIUs, greatly expanded use of inpatient telehealth for patient assessments and consultation, implementation of other approaches to minimize room entry (such as grouping in-room activities), and deployment of ethics consultation services to help manage issues around potential scarcity of life-saving measures such as ventilators. We also found that availability of PPE and timely testing was limited. Finally, a large proportion of sites reported potential diagnostic problems in the assessment of both patients suspected and those not suspected of having COVID-19.
RIUs are emerging as a primary modality for caring for non-ICU COVID-19 patients, though they never involved medical students; we hope the role of students in particular will increase as new models of training emerge in response to the pandemic.7 In contrast, telemedicine evolved rapidly to hold a substantial role in RIUs, with both ward and specialty teams using video visit technology to communicate with patients. COVID-19 has been viewed as a perfect use case for outpatient telemedicine,8 and a growing number of studies are examining its outpatient use9,10; however, to date, somewhat less attention has been paid to inpatient deployment. Although our data suggest telemedicine has found a prominent place in RIUs, it remains to be seen whether it is associated with differences in patient or provider outcomes. For example, deficiencies in the physical examination, limited face-to-face contact, and lack of physical presence could all affect the patient–provider relationship, patient engagement, and the accuracy of the diagnostic process.
Our data suggest the possibility of missing non–COVID-19 diagnoses in patients suspected of COVID-19 and missing COVID-19 in those admitted for nonrespiratory reasons. The latter may be addressed as routine COVID-19 screening of admitted patients becomes commonplace. For the former, however, it is possible that physicians are “anchoring” their thinking on COVID-19 to the exclusion of other diagnoses, that physicians are not fully aware of complications unique to COVID-19 infection (such as thromboembolism), and/or that the above-mentioned limitations of telemedicine have decreased diagnostic performance.
Although PPE stockpile data were not easily available for some sites, a distressingly large number reported stockpiles of 2 weeks or less, with reuse being the most common approach to extending PPE supply. We also found it concerning that 43% of hospital leaders did not know their stockpile data; we believe this is an important question that hospital leaders need to be asking. Most sites in our study reported test turnaround times of longer than 6 hours; lack of rapid COVID-19 testing further stresses PPE stockpile and may slow patients’ transition out of the RIU or discharge to home.
Our study has several limitations, including the evolving nature of the pandemic and rapid adaptations of care systems in the pandemic’s surge phase. However, we attempted to frame our questions in ways that provided a focused snapshot of care. Furthermore, respondents may not have had exhaustive knowledge of their institution’s COVID-19 response strategies, but most were the directors of their hospitalist services, and we encouraged the respondents to confer with others to gather high-fidelity data. Finally, as a survey of large academic medical centers, our results may not apply to nonacademic centers.
Approaches to caring for non-ICU patients during the COVID-19 pandemic are rapidly evolving. Expansion of RIUs and developing the workforce to support them has been a primary focus, with rapid innovation in use of technology emerging as a critical adaptation while PPE limitations persist and needs for “medical distancing” continue to grow. Although rates of missed COVID-19 diagnoses will likely be reduced with testing and systems improvements, physicians and systems will also need to consider how to utilize emerging technology in ways that can improve clinical care and provider safety while aiding diagnostic thinking. This survey illustrates the rapid adaptations made by our hospitals in response to the pandemic; ongoing adaptation will likely be needed to optimally care for hospitalized patients with COVID-19 while the pandemic continues to evolve.
Acknowledgment
Thanks to members of the HOMERuN COVID-19 Collaborative Group: Baylor Scott & White Medical Center – Temple, Texas - Tresa McNeal MD; Beth Israel Deaconess Medical Center - Shani Herzig MD MPH, Joseph Li MD, Julius Yang MD PhD; Brigham and Women’s Hospital - Christopher Roy MD, Jeffrey Schnipper MD MPH; Cedars-Sinai Medical Center - Ed Seferian MD, ; ChristianaCare - Surekha Bhamidipati MD; Cleveland Clinic - Matthew Pappas MD MPH; Dartmouth-Hitchcock Medical Center - Jonathan Lurie MD MS; Dell Medical School at The University of Texas at Austin - Chris Moriates MD, Luci Leykum MD MBA MSc; Denver Health and Hospitals Authority - Diana Mancini MD; Emory University Hospital - Dan Hunt MD; Johns Hopkins Hospital - Daniel J Brotman MD, Zishan K Siddiqui MD, Shaker Eid MD MBA; Maine Medical Center - Daniel A Meyer MD, Robert Trowbridge MD; Massachusetts General Hospital - Melissa Mattison MD; Mayo Clinic Rochester – Caroline Burton MD, Sagar Dugani MD PhD; Medical College of Wisconsin - Sanjay Bhandari MD; Miriam Hospital - Kwame Dapaah-Afriyie MD MBA; Mount Sinai Hospital - Andrew Dunn MD; NorthShore - David Lovinger MD; Northwestern Memorial Hospital - Kevin O’Leary MD MS; Ohio State University Wexner Medical Center - Eric Schumacher DO; Oregon Health & Science University - Angela Alday MD; Penn Medicine - Ryan Greysen MD MHS MA; Rutgers- Robert Wood Johnson University Hospital - Michael Steinberg MD MPH; Stanford University School of Medicine - Neera Ahuja MD; Tulane Hospital and University Medical Center - Geraldine Ménard MD; UC San Diego Health - Ian Jenkins MD; UC Los Angeles Health - Michael Lazarus MD, Magdalena E. Ptaszny, MD; UC San Francisco Health - Bradley A Sharpe, MD, Margaret Fang MD MPH; UK HealthCare - Mark Williams MD MHM, John Romond MD; University of Chicago – David Meltzer MD PhD, Gregory Ruhnke MD; University of Colorado - Marisha Burden MD; University of Florida - Nila Radhakrishnan MD; University of Iowa Hospitals and Clinics - Kevin Glenn MD MS; University of Miami - Efren Manjarrez MD; University of Michigan - Vineet Chopra MD MSc, Valerie Vaughn MD MSc; University of Missouri-Columbia Hospital - Hasan Naqvi MD; University of Nebraska Medical Center - Chad Vokoun MD; University of North Carolina at Chapel Hill - David Hemsey MD; University of Pittsburgh Medical Center - Gena Marie Walker MD; University of Vermont Medical Center - Steven Grant MD; University of Washington Medical Center - Christopher Kim MD MBA, Andrew White MD; University of Washington-Harborview Medical Center - Maralyssa Bann MD; University of Wisconsin Hospital and Clinics - David Sterken MD, Farah Kaiksow MD MPP, Ann Sheehy MD MS, Jordan Kenik MD MPH; UW Northwest Campus - Ben Wolpaw MD; Vanderbilt University Medical Center - Sunil Kripalani MD MSc, Eduard E Vasilevskis MD, Kathleene T Wooldridge MD MPH; Wake Forest Baptist Health - Erik Summers MD; Washington University St. Louis - Michael Lin MD; Weill Cornell - Justin Choi MD; Yale New Haven Hospital - William Cushing MA, Chris Sankey MD; Zuckerberg San Francisco General Hospital - Sumant Ranji MD.
The coronavirus disease of 2019 (COVID-19) pandemic has resulted in a surge in hospitalizations of patients with a novel, serious, and highly contagious infectious disease for which there is yet no proven treatment. Currently, much of the focus has been on intensive care unit (ICU) and ventilator capacity for the sickest of these patients who develop respiratory failure. However, most hospitalized patients are being cared for in general medical units.1 Some evidence exists to describe adaptations to capacity needs outside of medical wards,2-4 but few studies have specifically addressed the ward setting. Therefore, there is a pressing need for evidence to describe how to expand capacity and deliver medical ward–based care.
To better understand how inpatient care in the United States is adapting to the COVID-19 pandemic, we surveyed 72 sites participating in the Hospital Medicine Reengineering Network (HOMERuN), a national consortium of hospital medicine groups.5 We report results of this survey, carried out between April 3 and April 5, 2020.
METHODS
Sites and Subjects
HOMERuN is a collaborative network of hospitalists from across the United States whose primary goal is to catalyze research and share best practices across hospital medicine groups. Using surveys of Hospital Medicine leaders, targeted medical record review, and other methods, HOMERuN’s funded research interests to date have included care transitions, workforce issues, patient and family engagement, and diagnostic errors. Sites participating in HOMERuN sites are relatively large urban academic medical centers (Appendix).
Survey Development and Deployment
We designed a focused survey that aimed to provide a snapshot of evolving operational and clinical aspects of COVID-19 care (Appendix). Domains included COVID-19 testing turnaround times, personal protective equipment (PPE) stewardship,6 features of respiratory isolation units (RIUs; ie, dedicated units for patients with known or suspected COVID-19), and observed effects on clinical care. We tested the instrument to ensure feasibility and clarity internally, performed brief cognitive testing with several hospital medicine leaders in HOMERuN, then disseminated the survey by email on April 3, with two follow-up emails on 2 subsequent days. Our study was deemed non–human subjects research by the University of California, San Francisco, Committee on Human Research. Descriptive statistics were used to characterize survey responses.
RESULTS
Of 72 hospitals surveyed, 51 (71%) responded. Mean hospital bed count was 940, three were safety-net hospitals, and one was a community-based teaching center; responding and nonresponding hospitals did not differ significantly in terms of bed count (Appendix).
Health System Adaptations, Testing, and PPE Status
Nearly all responding hospitals (46 of 51; 90%) had RIUs for patients with known or suspected COVID-19 (Table 1). Nearly all hospitals took steps to keep potentially sick healthcare providers from infecting others (eg, staying home if sick or exposed). Among respondents, 32% had rapid response teams, 24% had respiratory therapy teams, and 29% had case management teams that were dedicated to COVID-19 care. Thirty-two (63%) had developed models, such as ethics or palliative care consult services, to assist with difficult resource-allocation decisions (eg, how to prioritize ventilator use if demand exceeded supply). Twenty-three (45%) had developed post-acute care monitoring programs dedicated to COVID-19 patients.

At the time of our survey, only 2 sites (4%) reported COVID-19 test time turnaround under 1 hour, and 15 (30%) reported turnaround in less than 6 hours. Of the 29 sites able to provide estimates of PPE stockpile, 14 (48%) reported a supply of 2 weeks or less. The most common approaches to PPE stewardship focused on reuse of masks and face shields if not obviously soiled, centralizing PPE distribution, and disinfecting or sterilizing masks. Ten sites (20%) were utilizing 3-D printed masks, while 10% used homemade face shields or masks.
Characteristics of COVID-19 RIUs
Forty-six hospitals (90% of all respondents) in our cohort had developed RIUs at the time of survey administration. The earliest RIU implementation date was February 10, 2020, and the most recent was launched on the day of our survey. Admission to RIUs was primarily based on clinical factors associated with known or suspected COVID-19 infection (Table 2). The number of non–critical care RIU beds among locations at that time ranged from 10 or less to more than 50. The mean number of hospitalist attendings caring for patients in the RIUs was 10.2, with a mean 4.1 advanced practice providers, 5.5 residents, and 0 medical students. The number of planned patients per attending was typically 5 to 15. Nurses and physicians typically rounded separately. Medical distancing (eg, reducing patient room entry) was accomplished most commonly by grouped timing of medication administration (76% of sites), video links to room outside of rounding times (54% of sites), the use of video or telemedicine during rounds (17%), and clustering of activities such as medication administration or phlebotomy. The most common criteria prompting discharge from the RIU were a negative COVID-19 test (59%) and hospital discharge (57%), though comments from many respondents suggested that discharge criteria were changing rapidly.

Effects of Isolation Measures on In-Room Encounters and Diagnostic Processes
More than 90% of sites reported decreases in in-room encounter frequency across all provider types whether as a result of policies in place or not. Reductions were reported among hospitalists, advanced practice providers, residents, consultants, and therapists (Table 3). Reduced room entry most often resulted from an established or developing policy, but many noted reduced room entry without formal policies in place. Nearly all sites reported moving specialty consultations to phone or video evaluations. Diagnostic error was commonly reported, with missed non–COVID-19 medical diagnoses among COVID-19 infected patients being reported by 22 sites (46%) and missed COVID-19 diagnoses in patients admitted for other reasons by 22 sites (45%).

DISCUSSION
In this study of medical wards at academic medical centers, we found that, in response to the COVID-19 pandemic, hospitals made several changes in a short period of time to adapt to the crisis. These included implementation and rapid expansion of dedicated RIUs, greatly expanded use of inpatient telehealth for patient assessments and consultation, implementation of other approaches to minimize room entry (such as grouping in-room activities), and deployment of ethics consultation services to help manage issues around potential scarcity of life-saving measures such as ventilators. We also found that availability of PPE and timely testing was limited. Finally, a large proportion of sites reported potential diagnostic problems in the assessment of both patients suspected and those not suspected of having COVID-19.
RIUs are emerging as a primary modality for caring for non-ICU COVID-19 patients, though they never involved medical students; we hope the role of students in particular will increase as new models of training emerge in response to the pandemic.7 In contrast, telemedicine evolved rapidly to hold a substantial role in RIUs, with both ward and specialty teams using video visit technology to communicate with patients. COVID-19 has been viewed as a perfect use case for outpatient telemedicine,8 and a growing number of studies are examining its outpatient use9,10; however, to date, somewhat less attention has been paid to inpatient deployment. Although our data suggest telemedicine has found a prominent place in RIUs, it remains to be seen whether it is associated with differences in patient or provider outcomes. For example, deficiencies in the physical examination, limited face-to-face contact, and lack of physical presence could all affect the patient–provider relationship, patient engagement, and the accuracy of the diagnostic process.
Our data suggest the possibility of missing non–COVID-19 diagnoses in patients suspected of COVID-19 and missing COVID-19 in those admitted for nonrespiratory reasons. The latter may be addressed as routine COVID-19 screening of admitted patients becomes commonplace. For the former, however, it is possible that physicians are “anchoring” their thinking on COVID-19 to the exclusion of other diagnoses, that physicians are not fully aware of complications unique to COVID-19 infection (such as thromboembolism), and/or that the above-mentioned limitations of telemedicine have decreased diagnostic performance.
Although PPE stockpile data were not easily available for some sites, a distressingly large number reported stockpiles of 2 weeks or less, with reuse being the most common approach to extending PPE supply. We also found it concerning that 43% of hospital leaders did not know their stockpile data; we believe this is an important question that hospital leaders need to be asking. Most sites in our study reported test turnaround times of longer than 6 hours; lack of rapid COVID-19 testing further stresses PPE stockpile and may slow patients’ transition out of the RIU or discharge to home.
Our study has several limitations, including the evolving nature of the pandemic and rapid adaptations of care systems in the pandemic’s surge phase. However, we attempted to frame our questions in ways that provided a focused snapshot of care. Furthermore, respondents may not have had exhaustive knowledge of their institution’s COVID-19 response strategies, but most were the directors of their hospitalist services, and we encouraged the respondents to confer with others to gather high-fidelity data. Finally, as a survey of large academic medical centers, our results may not apply to nonacademic centers.
Approaches to caring for non-ICU patients during the COVID-19 pandemic are rapidly evolving. Expansion of RIUs and developing the workforce to support them has been a primary focus, with rapid innovation in use of technology emerging as a critical adaptation while PPE limitations persist and needs for “medical distancing” continue to grow. Although rates of missed COVID-19 diagnoses will likely be reduced with testing and systems improvements, physicians and systems will also need to consider how to utilize emerging technology in ways that can improve clinical care and provider safety while aiding diagnostic thinking. This survey illustrates the rapid adaptations made by our hospitals in response to the pandemic; ongoing adaptation will likely be needed to optimally care for hospitalized patients with COVID-19 while the pandemic continues to evolve.
Acknowledgment
Thanks to members of the HOMERuN COVID-19 Collaborative Group: Baylor Scott & White Medical Center – Temple, Texas - Tresa McNeal MD; Beth Israel Deaconess Medical Center - Shani Herzig MD MPH, Joseph Li MD, Julius Yang MD PhD; Brigham and Women’s Hospital - Christopher Roy MD, Jeffrey Schnipper MD MPH; Cedars-Sinai Medical Center - Ed Seferian MD, ; ChristianaCare - Surekha Bhamidipati MD; Cleveland Clinic - Matthew Pappas MD MPH; Dartmouth-Hitchcock Medical Center - Jonathan Lurie MD MS; Dell Medical School at The University of Texas at Austin - Chris Moriates MD, Luci Leykum MD MBA MSc; Denver Health and Hospitals Authority - Diana Mancini MD; Emory University Hospital - Dan Hunt MD; Johns Hopkins Hospital - Daniel J Brotman MD, Zishan K Siddiqui MD, Shaker Eid MD MBA; Maine Medical Center - Daniel A Meyer MD, Robert Trowbridge MD; Massachusetts General Hospital - Melissa Mattison MD; Mayo Clinic Rochester – Caroline Burton MD, Sagar Dugani MD PhD; Medical College of Wisconsin - Sanjay Bhandari MD; Miriam Hospital - Kwame Dapaah-Afriyie MD MBA; Mount Sinai Hospital - Andrew Dunn MD; NorthShore - David Lovinger MD; Northwestern Memorial Hospital - Kevin O’Leary MD MS; Ohio State University Wexner Medical Center - Eric Schumacher DO; Oregon Health & Science University - Angela Alday MD; Penn Medicine - Ryan Greysen MD MHS MA; Rutgers- Robert Wood Johnson University Hospital - Michael Steinberg MD MPH; Stanford University School of Medicine - Neera Ahuja MD; Tulane Hospital and University Medical Center - Geraldine Ménard MD; UC San Diego Health - Ian Jenkins MD; UC Los Angeles Health - Michael Lazarus MD, Magdalena E. Ptaszny, MD; UC San Francisco Health - Bradley A Sharpe, MD, Margaret Fang MD MPH; UK HealthCare - Mark Williams MD MHM, John Romond MD; University of Chicago – David Meltzer MD PhD, Gregory Ruhnke MD; University of Colorado - Marisha Burden MD; University of Florida - Nila Radhakrishnan MD; University of Iowa Hospitals and Clinics - Kevin Glenn MD MS; University of Miami - Efren Manjarrez MD; University of Michigan - Vineet Chopra MD MSc, Valerie Vaughn MD MSc; University of Missouri-Columbia Hospital - Hasan Naqvi MD; University of Nebraska Medical Center - Chad Vokoun MD; University of North Carolina at Chapel Hill - David Hemsey MD; University of Pittsburgh Medical Center - Gena Marie Walker MD; University of Vermont Medical Center - Steven Grant MD; University of Washington Medical Center - Christopher Kim MD MBA, Andrew White MD; University of Washington-Harborview Medical Center - Maralyssa Bann MD; University of Wisconsin Hospital and Clinics - David Sterken MD, Farah Kaiksow MD MPP, Ann Sheehy MD MS, Jordan Kenik MD MPH; UW Northwest Campus - Ben Wolpaw MD; Vanderbilt University Medical Center - Sunil Kripalani MD MSc, Eduard E Vasilevskis MD, Kathleene T Wooldridge MD MPH; Wake Forest Baptist Health - Erik Summers MD; Washington University St. Louis - Michael Lin MD; Weill Cornell - Justin Choi MD; Yale New Haven Hospital - William Cushing MA, Chris Sankey MD; Zuckerberg San Francisco General Hospital - Sumant Ranji MD.
1. Institute for Health Metrics and Evaluation. COVID-19 Projections: United States of America. 2020. Accessed May 5, 2020. https://covid19.healthdata.org/united-states-of-america
2. Iserson KV. Alternative care sites: an option in disasters. West J Emerg Med. 2020;21(3):484‐489. https://doi.org/10.5811/westjem.2020.4.47552
3. Paganini M, Conti A, Weinstein E, Della Corte F, Ragazzoni L. Translating COVID-19 pandemic surge theory to practice in the emergency department: how to expand structure [online first]. Disaster Med Public Health Prep. 2020:1-10. https://doi.org/10.1017/dmp.2020.57
4. Kumaraiah D, Yip N, Ivascu N, Hill L. Innovative ICU Physician Care Models: Covid-19 Pandemic at NewYork-Presbyterian. NEJM: Catalyst. April 28, 2020. Accessed May 5, 2020. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0158
5. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
6. Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.5317
7. Bauchner H, Sharfstein J. A bold response to the COVID-19 pandemic: medical students, national service, and public health [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6166
8. Hollander JE, Carr BG. Virtually perfect? telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679‐1681. https://doi.org/10.1056/nejmp2003539
9. Hau YS, Kim JK, Hur J, Chang MC. How about actively using telemedicine during the COVID-19 pandemic? J Med Syst. 2020;44(6):108. https://doi.org/10.1007/s10916-020-01580-z
10. Smith WR, Atala AJ, Terlecki RP, Kelly EE, Matthews CA. Implementation guide for rapid integration of an outpatient telemedicine program during the COVID-19 pandemic [online first]. J Am Coll Surg. 2020. https://doi.org/10.1016/j.jamcollsurg.2020.04.030
1. Institute for Health Metrics and Evaluation. COVID-19 Projections: United States of America. 2020. Accessed May 5, 2020. https://covid19.healthdata.org/united-states-of-america
2. Iserson KV. Alternative care sites: an option in disasters. West J Emerg Med. 2020;21(3):484‐489. https://doi.org/10.5811/westjem.2020.4.47552
3. Paganini M, Conti A, Weinstein E, Della Corte F, Ragazzoni L. Translating COVID-19 pandemic surge theory to practice in the emergency department: how to expand structure [online first]. Disaster Med Public Health Prep. 2020:1-10. https://doi.org/10.1017/dmp.2020.57
4. Kumaraiah D, Yip N, Ivascu N, Hill L. Innovative ICU Physician Care Models: Covid-19 Pandemic at NewYork-Presbyterian. NEJM: Catalyst. April 28, 2020. Accessed May 5, 2020. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0158
5. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
6. Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.5317
7. Bauchner H, Sharfstein J. A bold response to the COVID-19 pandemic: medical students, national service, and public health [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6166
8. Hollander JE, Carr BG. Virtually perfect? telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679‐1681. https://doi.org/10.1056/nejmp2003539
9. Hau YS, Kim JK, Hur J, Chang MC. How about actively using telemedicine during the COVID-19 pandemic? J Med Syst. 2020;44(6):108. https://doi.org/10.1007/s10916-020-01580-z
10. Smith WR, Atala AJ, Terlecki RP, Kelly EE, Matthews CA. Implementation guide for rapid integration of an outpatient telemedicine program during the COVID-19 pandemic [online first]. J Am Coll Surg. 2020. https://doi.org/10.1016/j.jamcollsurg.2020.04.030
© 2020 Society of Hospital Medicine
Establishing an Orthopedic Excess Hospital Days in Acute Care Program
Total joint arthroplasty (TJA) procedures currently account for more Medicare expenses than any other inpatient procedure.1 In 2015, Centers for Medicare & Medicaid Services (CMS) announced the Comprehensive Care for Joint Replacement (CJR) model in which hospitals are paid one bundled payment for all related items and services utilized within a 90-day episode of care.2 Recent studies have suggested that the best opportunity to lower episode costs appears to be in the post-acute care setting and reducing readmissions.1,3
Surgical comanagement, which provides shared management of surgical patients between surgeons and hospitalists, is typically used in orthopedic surgery, neurosurgery, vascular surgery, and general surgery.4 Among patients with at least one medical comorbidity, surgical comanagement decreases length of stay (LOS), 30-day readmission rate for medical causes, and the proportion of patients with at least two medical consultants.5,6 Not all studies have shown that comanagement is beneficial. Maxwell et al found no significant differences in mortality or morbidity among hip fracture patients who did or did not receive comanagement7; however, comanaged patients were older and had more significant comorbidities, and there was no standard definition of comanagement among the participating institutions.
Comanagement after patients are discharged is a concept that has not been previously published but may become important with the Bundled Payments for Care Improvement initiative and high costs of excess days in acute care (EDAC). Hospitalists may be able to continue their work after discharge as part of the 90-day episode of care.8 TJA patients often have comorbidities, and surgical site infections and cardiovascular events are the most common causes of 30-day TJA readmissions.9
At our institution, 25% of TJA patients who presented to the Emergency Department (ED) within 90 days of surgery required a stay of less than 48 hours for conditions that did not require inpatient level of care. In addition, 50% of readmissions were secondary to medical complications. We also found significant variation in the management of common postoperative complications, such as postoperative fever, dislocation, anemia, and shortness of breath, especially among the different service lines caring for these patients. Therefore, we developed an Orthopedic EDAC program to reduce readmissions and to implement standardized admission algorithms and evidenced-based treatment protocols for common postoperative problems.
METHODS
Setting/Participants
We included patients who underwent total knee arthroplasty (TKA), total hip arthroplasty (THA), revision TKA, or revision THA from April 1, 2017, to September 30, 2018, at an urban teaching hospital. Patients were followed for 90 days after discharge. Factors such as age, sex, race, primary payer, Medicare Severity-Diagnosis Related Group (MS-DRG), discharge destination (home, home with home health, skilled nursing facility [SNF], acute rehab, other), and EDAC LOS were compared. An interdisciplinary committee comprising representatives from orthopedic surgery, hospital medicine, emergency medicine, and case management formulated observation criteria for the Orthopedic EDAC program. To be eligible for inclusion, observation patients had to have re-presented within 90 days from their initial surgery, could not be safely discharged home immediately from the ED, and did not require inpatient level of care. Patients qualifying for orthopedic observation were assigned rooms on the orthopedic wards to maintain continuity with nursing, physical therapy/occupational therapy, and case management staff. The University of Pennsylvania institutional review board reviewed this study and determined the project to be exempt.
Study Design
The Figure shows the admitting algorithm for TJA patients re-presenting within 90 days of their surgery. The ED evaluated the eligible patients; if they were not able to discharge the patient home, they notified the orthopedic resident on call for evaluation. Eligible diagnoses for the orthopedic observation in which orthopedics was the primary service included the need for postoperative pain control, fever (without signs or symptoms of sepsis), deep venous thrombosis or pulmonary embolism without hemodynamic instability, hemodynamically stable hypovolemia, symptomatic anemia secondary to acute blood loss anemia following surgery, and postoperative nausea, vomiting, constipation, ileus, and cellulitis. Eligible diagnoses for medical observation on the Medicine service included mild exacerbations of chronic obstructive pulmonary disease (COPD), syncope, upper respiratory tract infections, chest pain, delirium, and other exacerbation of medical problems. Full admission to Orthopedics included patients with wound infections requiring surgical washout, periprosthetic fractures/hematoma requiring operative management, and wound dehiscence requiring repair. All other readmissions requiring a stay of 48 or more hours were admitted to the medical or subspecialty medical service lines (eg, internal medicine, family medicine, geriatrics, cardiology, or pulmonary critical care).
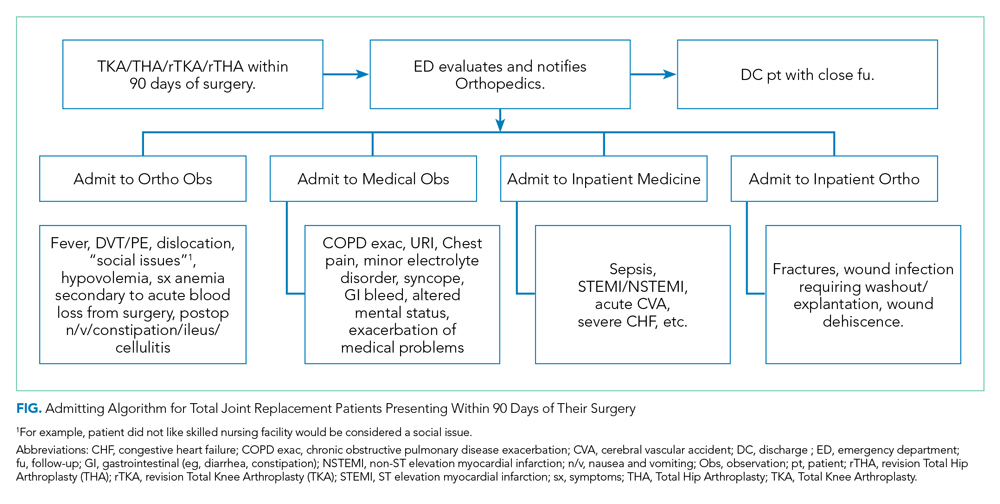
Development of Evidence-Based Algorithms
Patients who re-presented to acute care (for either observation stays or readmissions) were treated based on standardized algorithms. The interdisciplinary work group developed evidence-based evaluation and treatment plans for common postoperative problems, including postoperative fever, postoperative shortness of breath, and postoperative septic joints. This was based on a comprehensive literature review and consensus among emergency medicine, hospital medicine, and orthopedic surgery. Appendix 1 illustrates an example of a standardized algorithm for the workup of hypoxia.
Definition of Readmissions and EDAC
Readmission and observation stays were flagged on re-presentation, and reasons for readmission or observation status were analyzed. Observation cutoffs of “successful” (<48 hours) vs “unsuccessful” (≥48 hours and/or conversion to inpatient status) were based on the CMS Two-Midnight Rule in accordance with past studies.10 Readmissions were defined as patients who required an acute stay of 48 or more hours within 90 days of discharge from their original surgical stay. Patients admitted under observation status who required a stay of less than 48 hours did not count as a readmission but did count toward EDAC.
We acknowledge that our definition of Orthopedic EDAC is not the same as CMS’s definition of EDAC for other conditions such as congestive heart failure, which includes hours in observation, readmissions, and ED visits. We focused on studying and reducing days in the hospital (observation status and readmissions), and our intervention was not intended to prevent issues that would cause patients to present to the ED. Therefore, including ED visits in our operational definition of EDAC would add an unnecessary source of confounding that would bias our results toward the null hypothesis.
Data Collection and Data Analysis
The Orthopedic EDAC program was implemented on October 1, 2017, based on the above triage and treatment plans. We analyzed demographic and outcome data (readmissions, LOS, time in observation status, reason for readmission/observation status) for 6 months prior (April 1, 2017, to September 30, 2017) and 1 year after (October 1, 2017, to September 30, 2018). Microsoft Excel (Jones, 2013) was used for data analysis. Paired t-test with P < .05 was predefined as significant.
Eligible patients were identified from previous admission diagnoses obtained through Vizient, which is a collaboration of academic medical centers that maintains a hospital discharge data set (the Clinical Data Base/Resource Manager CDB/RM). It included patient demographics, discharge diagnoses, procedures, and outcomes.11 The Vizient database is a respected source of data and has been used for several scholarly studies.10-12 We queried the Vizient Clinical Data Base/Resource Manager v. 8.12.0.11 (Vizient Inc., Irvine, TX) for the following data from both before and after the program’s implementation: disposition, LOS, insurance information, gender, type of surgery, MS-DRG, and race.
The five included MS-DRGs represented major hip and knee joint replacements with and without major comorbid conditions (MCCs; MS-DRG 469 and MS-DRG 470, respectively) and revision hip or knee replacement with MCCs, with comorbid conditions (CCs), and without MCCs or CCs (MS-DRG 466, MS-DRG 467, and MS-DRG 468, respectively). MCCs included but were not limited to decubitus ulcer, severe malnutrition, quadriplegia, and end-stage renal disease. Examples of CCs included transplant patients, lymphoma, leukemia, and malignancies (except breast or prostate), based on CMS definitions.13
RESULTS
Table 1 compares the demographics of the pre-implementation and post-implementation periods. There were a total of 2,662 admissions (799 before program implementation and 1,863 after). TKA and THA patients without MCCs (MS-DRG 470) accounted for 80% of patients during both periods. In both periods, approximately 60% of patients were female, 50% of patients were White, 40% were Black, and 10% were another race. The mean age was 63.6 years old. Most patients had Medicare or commercial insurance. Discharge destinations were similar during both periods.
Table 2 illustrates how the patients who re-presented to acute care were triaged based on the algorithm described in the Figure. Among the 64 patients who re-presented during the pre-implementation period, there were no observation stays; there were 38 patients who were placed under medicine inpatient services. During post-implementation, there were 48 patients (29 on orthopedics, 17 on medicine, and 2 on other service lines) who were admitted under observation status. Twenty-three patients were discharged on observation status. Of those patients, 20 were admitted to orthopedic observation and 3 patients to medicine observation. Among the 71 patients who re-presented during the post-implementation period, 40.8% (29 patients) were admitted to inpatient orthopedic services, and 17 patients were readmitted to medicine services (24.9%). Among re-presenting patients, 70% were admitted to orthopedics inpatient and observation combined, in contrast to just 35% during the pre-implementation period.
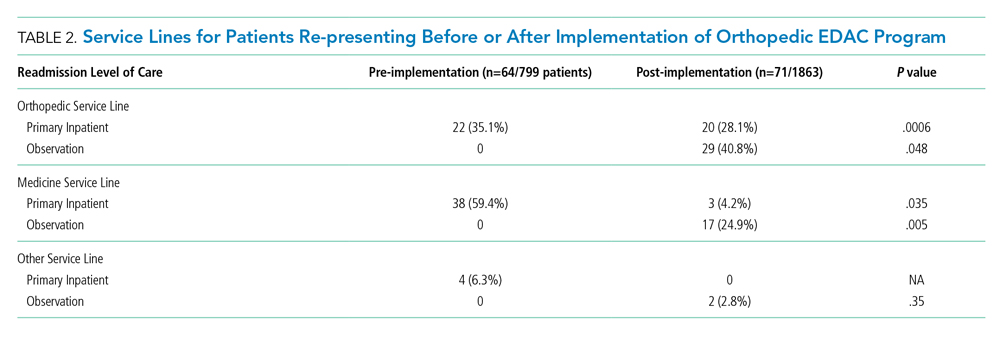
Readmissions decreased from 6.1% during pre-implementation to 2% during post-implementation (P = .004). In addition, the LOS for patients re-presenting during post-implementation was significantly lower than it was during pre-implementation. Table 3 details the associated LOS based on study period and readmission diagnosis. The aggregate LOS for all readmissions decreased from 7.75 days to 4.73 days (P = .005). The LOS decreased across all realms of readmission diagnoses. An outlier with an LOS greater than 100 days was removed from the pre-implementation group.
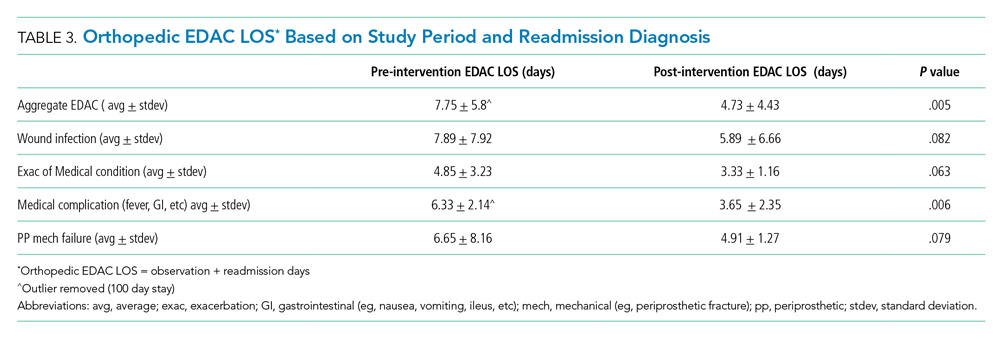
Appendix 2 further looked at patients who had observation orders, reasons for observation stay, and which patients were able to be discharged on observation status. Patients with medical complications such as fever and urinary tract infection were more likely to be discharged on observation status than were patients with wound drainage or redness that was concerning for a periprosthetic joint infection.
DISCUSSION
To our knowledge, this is the first description of a published Orthopedic EDAC program using orthopedic observation, standardized admitting and treatment algorithms, and comanagement of patients who re-presented after their original surgery. The development of an Orthopedic EDAC program at our hospital with comanagement was successful in reducing readmissions, decreasing LOS for readmitted patients, and increasing continuity of care. A number of points require more elaboration.
The Orthopedic EDAC program’s improvement in both reducing readmissions and decreasing LOS for EDAC (including days for observation and readmissions) was not caused by simply shifting patients with shorter LOS from inpatient to observation because the inpatients did not have a longer LOS. We had lower Orthopedic EDAC during the post-implementation vs pre-implementation even when considering EDAC in terms of both observation and readmissions. The decrease in readmissions is not only from the patients that were discharged on observation status, but also a result of other concurrent interventions, such as encouraging discharge to home rather than to rehabilitation facilities and more rigorous preoperative optimization.
The national rates of 30- and 90-day readmissions after primary TKA were 4% (95% CI, 3.8%-4.0%) and 7% (95% CI, 6.8%-7.2%), respectively,10 and the average cost of readmission for medical causes was $22,775 for THA and $11,682 for TKA.12 If one considers the 23 “saved readmissions” with 12 surgical complications and 11 medical complications, we “saved” roughly $591,105. Also, with the decrease in LOS for each readmission for any cause from 7.75 days to 4.73 days, the 48 readmissions had a 150 day lower LOS overall. With the average hospital day costing $2,289/day at nonprofit hospitals,13 there are additional cost savings of $343,350 overall. Therefore, the grand total estimated savings during this pilot was $934,455.
The decrease in post-implementation LOS vs pre-implementation LOS was likely multifactorial. The Orthopedic EDAC program improved continuity of care with orthopedic surgery and support staff (registered nurses, social workers, physical therapists) and utilized standardized protocols for work-up of common postoperative problems. These evidence-based protocols reduced waste that resulted in less testing with fewer incidental findings and side effects. The clinical history and patient circumstance did not need to be reestablished and tests did not need to be duplicated, which led to decreased LOS. Observation status allowed us to return patients to SNFs without the tedious procedure of insurance reauthorization and reevaluation by physical therapy and occupational therapy. Other factors such as “discharge before noon” and early physical therapy services ongoing during post-implementation also contributed to the decreased LOS.
Our Orthopedic EDAC program did not deliberately place patients on observation status who met full inpatient criteria solely to decrease the readmission rate. Our average LOS on observation status was 26 hours. In contrast, a study of observation stays at another tertiary academic medical center showed longer LOS: The average observation LOS was 33.3 hours with 44.4% of stays less than 24 hours and 16.5% greater than 48 hours.11 The use of EDAC hours in our study, which included both observation hours and readmission hours, made our impact more than simply a shifting of readmissions to observation stays.
It is important to utilize observation stays as they were intended—ie, stays requiring less than 48 hours. Over the past 10 years, the incidence and duration of observation stays has increased significantly while readmissions have decreased.14,15 Observation status has serious financial implications, and it is estimated that 10% of observation stays end up costing the patient more than an inpatient stay would and patients must pay 20% of services after the Part B deductible.16,17 In addition, Medicare beneficiaries have no cap on costs for an observation stay.16 Therefore, it is important to determine which patients and diagnoses are best suited for observation status. We found that younger patients without comorbidities who came from home and presented with complications such as fever and syncope were most likely to be successfully discharged on observation status with the Orthopedic EDAC program. SNF patients on observation status in particular may have large hospital bills because they often require 3 midnight stays but do not meet inpatient level of care and are thus not covered as inpatients.18
The Orthopedic EDAC program emphasized continuity of care with the primary orthopedic surgery team. Prior to implementation, orthopedics was often not even notified when their patients were in the ED or readmitted because the prevailing practice was that once surgery was completed, the surgeon’s job was done. Post-implementation, orthopedics was called for every bundled patient re-presenting within 90 days after a TJA. The triage protocol (Figure) was agreed upon prior to implementation by orthopedics, hospital medicine, and emergency medicine. Orthopedic attendings wanted to play a larger role and more strongly influence care of their patients on re-presentation because these attendings had become frustrated with the great disparities in work-up when patients went to various other services instead. Pre-implementation, many patients admitted to the primary orthopedic service had lower acuity, and they tended to be younger and have less medical complexity. Post-implementation, primary orthopedic services took care of more patients under observation status and those with “mechanical” complications that required surgery.
It is important to note that, while comanagement is common preoperatively and immediately postoperatively, studies of comanaged patients on re-presentation have apparently not been previously published. In addition, a recent study by Maxwell et al found that patients who were comanaged perioperatively had higher mortality and morbidity than did patients who were not comanaged.7 These findings reflect the need for more studies to be done to best optimize the use of comanagement. Comanagement as part of the Orthopedic EDAC program at our institution was successful in keeping patients who re-presented on the orthopedic service, decreasing LOS, and decreasing readmissions.
The study has some limitations. First, this was a retrospective study, so confounding variables may not be completely eliminated. Second, our study was conducted at a single center for total joint arthroplasty and did not consider other orthopedic conditions; however, our readmission numbers and demographics are similar to past studies. Third, we had small numbers of readmissions and observation patients, which resulted in a small effect size; however, our intervention demonstrated significant changes in LOS and readmissions. Fourth, our data is based on prior billing and coding, which may not always be accurate or inclusive. Fifth, we did not have THA or TKA patients on overnight recovery status or same day surgeries during either period studied; however, we are developing infrastructure to implement this in the future. Finally, ED visit data was not readily available to us, so we were not able to calculate the traditional EDAC. Despite these limitations, this study provides an important look at how an Orthopedic EDAC program can decrease readmissions, decrease LOS, and improve continuity of care in patients undergoing TJA.
CONCLUSION
An Orthopedic EDAC program with comanagement may decrease readmissions, improve continuity of care on re-presentation, and decrease LOS for total joint arthroplasty patients who presented after initial surgery and lead to substantial cost savings.
Disclosures
The authors have no potential conflicts to disclose. Dr Greysen was supported by a career development award from the National Institute on Aging (K23AG045338).
1. Hawker GA, Badley EM, Croxford R, et al. A population based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47(7):732-741. https://doi.org/10.1097/MLR.0b013e3181934553
2. Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63-70. https://doi.org/10.2147/RMHP.S130341
3. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskeletal Med. 2017;10(3):370-377. https://doi.org/10.1007/s12178-017-9423-6
4. The Society of Hospital Medicine. The Evolution of Co-Management. 2017. Accessed October 30, 2019. https://www.hospitalmedicine.org/globalassets/practice-management/practice-management-pdf/pm-19-0004-co-management-white-paper_minor-update-m.pdf
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Fitzgerald SJ, Palmer TC, Kraay MJ. Improved perioperative care of elective joint replacement patients: the impact of an orthopedic perioperative hospitalist. J Arthroplasty. 2018;33(8):2387-2391. https://doi,org/10.1016/j.arth.2018.03.029
7. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3343
8. Centers for Medicare & Medicaid Services. Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services; Final Rule. November 24, 2015. https://www.govinfo.gov/content/pkg/FR-2015-11-24/pdf/2015-29438.pdf
9. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468. https://doi.org/10.1016/j.arth.2013.07.039
10. ICD-10-CM/PCS MS-DRG v37.0 Definitions Manual. Accessed April 27, 2020. https://www.cms.gov/icd10m/version37-fullcode-cms/fullcode_cms/P0031.html
11. Chaudhary NS, Donnelly JP, Wang HE. Racial differences in sepsis mortality at United States academic medical center-affiliated hospitals. Crit Care Med. 2018;46(6):878-883. https://doi.org/10.1097/CCM.0000000000003020
12. Clair AJ, Evangelista PJ, Lajam CM, Slover JD, Bosco JA, Iorio R. Cost analysis of total joint arthroplasty readmissions in a Bundled Payment Care Improvement Initiative. J Arthroplasty. 2016;31(9):1862-1865.
13. Kaiser Family Foundation. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. Accessed April 27, 2020. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
14. Goldstein JN, Zhang Z, Schwartz JS, Hicks LS. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2018;131(1):101.e9-101.e15. https://doi.org/10.1016/j.amjmed.2017.07.013
15. Lind KD, Noel-Miller CM, Sangaralingham LR, et al. Increasing trends in the use of hospital observation services for older Medicare Advantage and privately insured patients. Med Care Res Rev. 2019;76(2):229-239. https://doi.org/10.1177/1077558717718026
16. Sabbatini AK, Wright B. Excluding observation stays from readmission rates - what quality measures are missing. N Engl J Med. 2018;378(22):2062-2065. https://doi.org/10.1056/NEJMp1800732
17. Gabayan GZ, Doyle B, Liang, L, Donkor K, Huang, D, Sarkisian CA. Who has an unsuccessful observation care stay? Healthcare (Basel). 2018;6(4):138. https://doi.org/10.3390/healthcare6040138
18. Fang M, Hume E, Ibrahim S. Race, Bundled payment policy, and discharge destination after TKA: the experience of an urban academic hospital. Geriatr Orthop Surg Rehabil. 2018. https://doi.org/10.1177/2151459318803222
Total joint arthroplasty (TJA) procedures currently account for more Medicare expenses than any other inpatient procedure.1 In 2015, Centers for Medicare & Medicaid Services (CMS) announced the Comprehensive Care for Joint Replacement (CJR) model in which hospitals are paid one bundled payment for all related items and services utilized within a 90-day episode of care.2 Recent studies have suggested that the best opportunity to lower episode costs appears to be in the post-acute care setting and reducing readmissions.1,3
Surgical comanagement, which provides shared management of surgical patients between surgeons and hospitalists, is typically used in orthopedic surgery, neurosurgery, vascular surgery, and general surgery.4 Among patients with at least one medical comorbidity, surgical comanagement decreases length of stay (LOS), 30-day readmission rate for medical causes, and the proportion of patients with at least two medical consultants.5,6 Not all studies have shown that comanagement is beneficial. Maxwell et al found no significant differences in mortality or morbidity among hip fracture patients who did or did not receive comanagement7; however, comanaged patients were older and had more significant comorbidities, and there was no standard definition of comanagement among the participating institutions.
Comanagement after patients are discharged is a concept that has not been previously published but may become important with the Bundled Payments for Care Improvement initiative and high costs of excess days in acute care (EDAC). Hospitalists may be able to continue their work after discharge as part of the 90-day episode of care.8 TJA patients often have comorbidities, and surgical site infections and cardiovascular events are the most common causes of 30-day TJA readmissions.9
At our institution, 25% of TJA patients who presented to the Emergency Department (ED) within 90 days of surgery required a stay of less than 48 hours for conditions that did not require inpatient level of care. In addition, 50% of readmissions were secondary to medical complications. We also found significant variation in the management of common postoperative complications, such as postoperative fever, dislocation, anemia, and shortness of breath, especially among the different service lines caring for these patients. Therefore, we developed an Orthopedic EDAC program to reduce readmissions and to implement standardized admission algorithms and evidenced-based treatment protocols for common postoperative problems.
METHODS
Setting/Participants
We included patients who underwent total knee arthroplasty (TKA), total hip arthroplasty (THA), revision TKA, or revision THA from April 1, 2017, to September 30, 2018, at an urban teaching hospital. Patients were followed for 90 days after discharge. Factors such as age, sex, race, primary payer, Medicare Severity-Diagnosis Related Group (MS-DRG), discharge destination (home, home with home health, skilled nursing facility [SNF], acute rehab, other), and EDAC LOS were compared. An interdisciplinary committee comprising representatives from orthopedic surgery, hospital medicine, emergency medicine, and case management formulated observation criteria for the Orthopedic EDAC program. To be eligible for inclusion, observation patients had to have re-presented within 90 days from their initial surgery, could not be safely discharged home immediately from the ED, and did not require inpatient level of care. Patients qualifying for orthopedic observation were assigned rooms on the orthopedic wards to maintain continuity with nursing, physical therapy/occupational therapy, and case management staff. The University of Pennsylvania institutional review board reviewed this study and determined the project to be exempt.
Study Design
The Figure shows the admitting algorithm for TJA patients re-presenting within 90 days of their surgery. The ED evaluated the eligible patients; if they were not able to discharge the patient home, they notified the orthopedic resident on call for evaluation. Eligible diagnoses for the orthopedic observation in which orthopedics was the primary service included the need for postoperative pain control, fever (without signs or symptoms of sepsis), deep venous thrombosis or pulmonary embolism without hemodynamic instability, hemodynamically stable hypovolemia, symptomatic anemia secondary to acute blood loss anemia following surgery, and postoperative nausea, vomiting, constipation, ileus, and cellulitis. Eligible diagnoses for medical observation on the Medicine service included mild exacerbations of chronic obstructive pulmonary disease (COPD), syncope, upper respiratory tract infections, chest pain, delirium, and other exacerbation of medical problems. Full admission to Orthopedics included patients with wound infections requiring surgical washout, periprosthetic fractures/hematoma requiring operative management, and wound dehiscence requiring repair. All other readmissions requiring a stay of 48 or more hours were admitted to the medical or subspecialty medical service lines (eg, internal medicine, family medicine, geriatrics, cardiology, or pulmonary critical care).

Development of Evidence-Based Algorithms
Patients who re-presented to acute care (for either observation stays or readmissions) were treated based on standardized algorithms. The interdisciplinary work group developed evidence-based evaluation and treatment plans for common postoperative problems, including postoperative fever, postoperative shortness of breath, and postoperative septic joints. This was based on a comprehensive literature review and consensus among emergency medicine, hospital medicine, and orthopedic surgery. Appendix 1 illustrates an example of a standardized algorithm for the workup of hypoxia.
Definition of Readmissions and EDAC
Readmission and observation stays were flagged on re-presentation, and reasons for readmission or observation status were analyzed. Observation cutoffs of “successful” (<48 hours) vs “unsuccessful” (≥48 hours and/or conversion to inpatient status) were based on the CMS Two-Midnight Rule in accordance with past studies.10 Readmissions were defined as patients who required an acute stay of 48 or more hours within 90 days of discharge from their original surgical stay. Patients admitted under observation status who required a stay of less than 48 hours did not count as a readmission but did count toward EDAC.
We acknowledge that our definition of Orthopedic EDAC is not the same as CMS’s definition of EDAC for other conditions such as congestive heart failure, which includes hours in observation, readmissions, and ED visits. We focused on studying and reducing days in the hospital (observation status and readmissions), and our intervention was not intended to prevent issues that would cause patients to present to the ED. Therefore, including ED visits in our operational definition of EDAC would add an unnecessary source of confounding that would bias our results toward the null hypothesis.
Data Collection and Data Analysis
The Orthopedic EDAC program was implemented on October 1, 2017, based on the above triage and treatment plans. We analyzed demographic and outcome data (readmissions, LOS, time in observation status, reason for readmission/observation status) for 6 months prior (April 1, 2017, to September 30, 2017) and 1 year after (October 1, 2017, to September 30, 2018). Microsoft Excel (Jones, 2013) was used for data analysis. Paired t-test with P < .05 was predefined as significant.
Eligible patients were identified from previous admission diagnoses obtained through Vizient, which is a collaboration of academic medical centers that maintains a hospital discharge data set (the Clinical Data Base/Resource Manager CDB/RM). It included patient demographics, discharge diagnoses, procedures, and outcomes.11 The Vizient database is a respected source of data and has been used for several scholarly studies.10-12 We queried the Vizient Clinical Data Base/Resource Manager v. 8.12.0.11 (Vizient Inc., Irvine, TX) for the following data from both before and after the program’s implementation: disposition, LOS, insurance information, gender, type of surgery, MS-DRG, and race.
The five included MS-DRGs represented major hip and knee joint replacements with and without major comorbid conditions (MCCs; MS-DRG 469 and MS-DRG 470, respectively) and revision hip or knee replacement with MCCs, with comorbid conditions (CCs), and without MCCs or CCs (MS-DRG 466, MS-DRG 467, and MS-DRG 468, respectively). MCCs included but were not limited to decubitus ulcer, severe malnutrition, quadriplegia, and end-stage renal disease. Examples of CCs included transplant patients, lymphoma, leukemia, and malignancies (except breast or prostate), based on CMS definitions.13
RESULTS
Table 1 compares the demographics of the pre-implementation and post-implementation periods. There were a total of 2,662 admissions (799 before program implementation and 1,863 after). TKA and THA patients without MCCs (MS-DRG 470) accounted for 80% of patients during both periods. In both periods, approximately 60% of patients were female, 50% of patients were White, 40% were Black, and 10% were another race. The mean age was 63.6 years old. Most patients had Medicare or commercial insurance. Discharge destinations were similar during both periods.
Table 2 illustrates how the patients who re-presented to acute care were triaged based on the algorithm described in the Figure. Among the 64 patients who re-presented during the pre-implementation period, there were no observation stays; there were 38 patients who were placed under medicine inpatient services. During post-implementation, there were 48 patients (29 on orthopedics, 17 on medicine, and 2 on other service lines) who were admitted under observation status. Twenty-three patients were discharged on observation status. Of those patients, 20 were admitted to orthopedic observation and 3 patients to medicine observation. Among the 71 patients who re-presented during the post-implementation period, 40.8% (29 patients) were admitted to inpatient orthopedic services, and 17 patients were readmitted to medicine services (24.9%). Among re-presenting patients, 70% were admitted to orthopedics inpatient and observation combined, in contrast to just 35% during the pre-implementation period.

Readmissions decreased from 6.1% during pre-implementation to 2% during post-implementation (P = .004). In addition, the LOS for patients re-presenting during post-implementation was significantly lower than it was during pre-implementation. Table 3 details the associated LOS based on study period and readmission diagnosis. The aggregate LOS for all readmissions decreased from 7.75 days to 4.73 days (P = .005). The LOS decreased across all realms of readmission diagnoses. An outlier with an LOS greater than 100 days was removed from the pre-implementation group.

Appendix 2 further looked at patients who had observation orders, reasons for observation stay, and which patients were able to be discharged on observation status. Patients with medical complications such as fever and urinary tract infection were more likely to be discharged on observation status than were patients with wound drainage or redness that was concerning for a periprosthetic joint infection.
DISCUSSION
To our knowledge, this is the first description of a published Orthopedic EDAC program using orthopedic observation, standardized admitting and treatment algorithms, and comanagement of patients who re-presented after their original surgery. The development of an Orthopedic EDAC program at our hospital with comanagement was successful in reducing readmissions, decreasing LOS for readmitted patients, and increasing continuity of care. A number of points require more elaboration.
The Orthopedic EDAC program’s improvement in both reducing readmissions and decreasing LOS for EDAC (including days for observation and readmissions) was not caused by simply shifting patients with shorter LOS from inpatient to observation because the inpatients did not have a longer LOS. We had lower Orthopedic EDAC during the post-implementation vs pre-implementation even when considering EDAC in terms of both observation and readmissions. The decrease in readmissions is not only from the patients that were discharged on observation status, but also a result of other concurrent interventions, such as encouraging discharge to home rather than to rehabilitation facilities and more rigorous preoperative optimization.
The national rates of 30- and 90-day readmissions after primary TKA were 4% (95% CI, 3.8%-4.0%) and 7% (95% CI, 6.8%-7.2%), respectively,10 and the average cost of readmission for medical causes was $22,775 for THA and $11,682 for TKA.12 If one considers the 23 “saved readmissions” with 12 surgical complications and 11 medical complications, we “saved” roughly $591,105. Also, with the decrease in LOS for each readmission for any cause from 7.75 days to 4.73 days, the 48 readmissions had a 150 day lower LOS overall. With the average hospital day costing $2,289/day at nonprofit hospitals,13 there are additional cost savings of $343,350 overall. Therefore, the grand total estimated savings during this pilot was $934,455.
The decrease in post-implementation LOS vs pre-implementation LOS was likely multifactorial. The Orthopedic EDAC program improved continuity of care with orthopedic surgery and support staff (registered nurses, social workers, physical therapists) and utilized standardized protocols for work-up of common postoperative problems. These evidence-based protocols reduced waste that resulted in less testing with fewer incidental findings and side effects. The clinical history and patient circumstance did not need to be reestablished and tests did not need to be duplicated, which led to decreased LOS. Observation status allowed us to return patients to SNFs without the tedious procedure of insurance reauthorization and reevaluation by physical therapy and occupational therapy. Other factors such as “discharge before noon” and early physical therapy services ongoing during post-implementation also contributed to the decreased LOS.
Our Orthopedic EDAC program did not deliberately place patients on observation status who met full inpatient criteria solely to decrease the readmission rate. Our average LOS on observation status was 26 hours. In contrast, a study of observation stays at another tertiary academic medical center showed longer LOS: The average observation LOS was 33.3 hours with 44.4% of stays less than 24 hours and 16.5% greater than 48 hours.11 The use of EDAC hours in our study, which included both observation hours and readmission hours, made our impact more than simply a shifting of readmissions to observation stays.
It is important to utilize observation stays as they were intended—ie, stays requiring less than 48 hours. Over the past 10 years, the incidence and duration of observation stays has increased significantly while readmissions have decreased.14,15 Observation status has serious financial implications, and it is estimated that 10% of observation stays end up costing the patient more than an inpatient stay would and patients must pay 20% of services after the Part B deductible.16,17 In addition, Medicare beneficiaries have no cap on costs for an observation stay.16 Therefore, it is important to determine which patients and diagnoses are best suited for observation status. We found that younger patients without comorbidities who came from home and presented with complications such as fever and syncope were most likely to be successfully discharged on observation status with the Orthopedic EDAC program. SNF patients on observation status in particular may have large hospital bills because they often require 3 midnight stays but do not meet inpatient level of care and are thus not covered as inpatients.18
The Orthopedic EDAC program emphasized continuity of care with the primary orthopedic surgery team. Prior to implementation, orthopedics was often not even notified when their patients were in the ED or readmitted because the prevailing practice was that once surgery was completed, the surgeon’s job was done. Post-implementation, orthopedics was called for every bundled patient re-presenting within 90 days after a TJA. The triage protocol (Figure) was agreed upon prior to implementation by orthopedics, hospital medicine, and emergency medicine. Orthopedic attendings wanted to play a larger role and more strongly influence care of their patients on re-presentation because these attendings had become frustrated with the great disparities in work-up when patients went to various other services instead. Pre-implementation, many patients admitted to the primary orthopedic service had lower acuity, and they tended to be younger and have less medical complexity. Post-implementation, primary orthopedic services took care of more patients under observation status and those with “mechanical” complications that required surgery.
It is important to note that, while comanagement is common preoperatively and immediately postoperatively, studies of comanaged patients on re-presentation have apparently not been previously published. In addition, a recent study by Maxwell et al found that patients who were comanaged perioperatively had higher mortality and morbidity than did patients who were not comanaged.7 These findings reflect the need for more studies to be done to best optimize the use of comanagement. Comanagement as part of the Orthopedic EDAC program at our institution was successful in keeping patients who re-presented on the orthopedic service, decreasing LOS, and decreasing readmissions.
The study has some limitations. First, this was a retrospective study, so confounding variables may not be completely eliminated. Second, our study was conducted at a single center for total joint arthroplasty and did not consider other orthopedic conditions; however, our readmission numbers and demographics are similar to past studies. Third, we had small numbers of readmissions and observation patients, which resulted in a small effect size; however, our intervention demonstrated significant changes in LOS and readmissions. Fourth, our data is based on prior billing and coding, which may not always be accurate or inclusive. Fifth, we did not have THA or TKA patients on overnight recovery status or same day surgeries during either period studied; however, we are developing infrastructure to implement this in the future. Finally, ED visit data was not readily available to us, so we were not able to calculate the traditional EDAC. Despite these limitations, this study provides an important look at how an Orthopedic EDAC program can decrease readmissions, decrease LOS, and improve continuity of care in patients undergoing TJA.
CONCLUSION
An Orthopedic EDAC program with comanagement may decrease readmissions, improve continuity of care on re-presentation, and decrease LOS for total joint arthroplasty patients who presented after initial surgery and lead to substantial cost savings.
Disclosures
The authors have no potential conflicts to disclose. Dr Greysen was supported by a career development award from the National Institute on Aging (K23AG045338).
Total joint arthroplasty (TJA) procedures currently account for more Medicare expenses than any other inpatient procedure.1 In 2015, Centers for Medicare & Medicaid Services (CMS) announced the Comprehensive Care for Joint Replacement (CJR) model in which hospitals are paid one bundled payment for all related items and services utilized within a 90-day episode of care.2 Recent studies have suggested that the best opportunity to lower episode costs appears to be in the post-acute care setting and reducing readmissions.1,3
Surgical comanagement, which provides shared management of surgical patients between surgeons and hospitalists, is typically used in orthopedic surgery, neurosurgery, vascular surgery, and general surgery.4 Among patients with at least one medical comorbidity, surgical comanagement decreases length of stay (LOS), 30-day readmission rate for medical causes, and the proportion of patients with at least two medical consultants.5,6 Not all studies have shown that comanagement is beneficial. Maxwell et al found no significant differences in mortality or morbidity among hip fracture patients who did or did not receive comanagement7; however, comanaged patients were older and had more significant comorbidities, and there was no standard definition of comanagement among the participating institutions.
Comanagement after patients are discharged is a concept that has not been previously published but may become important with the Bundled Payments for Care Improvement initiative and high costs of excess days in acute care (EDAC). Hospitalists may be able to continue their work after discharge as part of the 90-day episode of care.8 TJA patients often have comorbidities, and surgical site infections and cardiovascular events are the most common causes of 30-day TJA readmissions.9
At our institution, 25% of TJA patients who presented to the Emergency Department (ED) within 90 days of surgery required a stay of less than 48 hours for conditions that did not require inpatient level of care. In addition, 50% of readmissions were secondary to medical complications. We also found significant variation in the management of common postoperative complications, such as postoperative fever, dislocation, anemia, and shortness of breath, especially among the different service lines caring for these patients. Therefore, we developed an Orthopedic EDAC program to reduce readmissions and to implement standardized admission algorithms and evidenced-based treatment protocols for common postoperative problems.
METHODS
Setting/Participants
We included patients who underwent total knee arthroplasty (TKA), total hip arthroplasty (THA), revision TKA, or revision THA from April 1, 2017, to September 30, 2018, at an urban teaching hospital. Patients were followed for 90 days after discharge. Factors such as age, sex, race, primary payer, Medicare Severity-Diagnosis Related Group (MS-DRG), discharge destination (home, home with home health, skilled nursing facility [SNF], acute rehab, other), and EDAC LOS were compared. An interdisciplinary committee comprising representatives from orthopedic surgery, hospital medicine, emergency medicine, and case management formulated observation criteria for the Orthopedic EDAC program. To be eligible for inclusion, observation patients had to have re-presented within 90 days from their initial surgery, could not be safely discharged home immediately from the ED, and did not require inpatient level of care. Patients qualifying for orthopedic observation were assigned rooms on the orthopedic wards to maintain continuity with nursing, physical therapy/occupational therapy, and case management staff. The University of Pennsylvania institutional review board reviewed this study and determined the project to be exempt.
Study Design
The Figure shows the admitting algorithm for TJA patients re-presenting within 90 days of their surgery. The ED evaluated the eligible patients; if they were not able to discharge the patient home, they notified the orthopedic resident on call for evaluation. Eligible diagnoses for the orthopedic observation in which orthopedics was the primary service included the need for postoperative pain control, fever (without signs or symptoms of sepsis), deep venous thrombosis or pulmonary embolism without hemodynamic instability, hemodynamically stable hypovolemia, symptomatic anemia secondary to acute blood loss anemia following surgery, and postoperative nausea, vomiting, constipation, ileus, and cellulitis. Eligible diagnoses for medical observation on the Medicine service included mild exacerbations of chronic obstructive pulmonary disease (COPD), syncope, upper respiratory tract infections, chest pain, delirium, and other exacerbation of medical problems. Full admission to Orthopedics included patients with wound infections requiring surgical washout, periprosthetic fractures/hematoma requiring operative management, and wound dehiscence requiring repair. All other readmissions requiring a stay of 48 or more hours were admitted to the medical or subspecialty medical service lines (eg, internal medicine, family medicine, geriatrics, cardiology, or pulmonary critical care).

Development of Evidence-Based Algorithms
Patients who re-presented to acute care (for either observation stays or readmissions) were treated based on standardized algorithms. The interdisciplinary work group developed evidence-based evaluation and treatment plans for common postoperative problems, including postoperative fever, postoperative shortness of breath, and postoperative septic joints. This was based on a comprehensive literature review and consensus among emergency medicine, hospital medicine, and orthopedic surgery. Appendix 1 illustrates an example of a standardized algorithm for the workup of hypoxia.
Definition of Readmissions and EDAC
Readmission and observation stays were flagged on re-presentation, and reasons for readmission or observation status were analyzed. Observation cutoffs of “successful” (<48 hours) vs “unsuccessful” (≥48 hours and/or conversion to inpatient status) were based on the CMS Two-Midnight Rule in accordance with past studies.10 Readmissions were defined as patients who required an acute stay of 48 or more hours within 90 days of discharge from their original surgical stay. Patients admitted under observation status who required a stay of less than 48 hours did not count as a readmission but did count toward EDAC.
We acknowledge that our definition of Orthopedic EDAC is not the same as CMS’s definition of EDAC for other conditions such as congestive heart failure, which includes hours in observation, readmissions, and ED visits. We focused on studying and reducing days in the hospital (observation status and readmissions), and our intervention was not intended to prevent issues that would cause patients to present to the ED. Therefore, including ED visits in our operational definition of EDAC would add an unnecessary source of confounding that would bias our results toward the null hypothesis.
Data Collection and Data Analysis
The Orthopedic EDAC program was implemented on October 1, 2017, based on the above triage and treatment plans. We analyzed demographic and outcome data (readmissions, LOS, time in observation status, reason for readmission/observation status) for 6 months prior (April 1, 2017, to September 30, 2017) and 1 year after (October 1, 2017, to September 30, 2018). Microsoft Excel (Jones, 2013) was used for data analysis. Paired t-test with P < .05 was predefined as significant.
Eligible patients were identified from previous admission diagnoses obtained through Vizient, which is a collaboration of academic medical centers that maintains a hospital discharge data set (the Clinical Data Base/Resource Manager CDB/RM). It included patient demographics, discharge diagnoses, procedures, and outcomes.11 The Vizient database is a respected source of data and has been used for several scholarly studies.10-12 We queried the Vizient Clinical Data Base/Resource Manager v. 8.12.0.11 (Vizient Inc., Irvine, TX) for the following data from both before and after the program’s implementation: disposition, LOS, insurance information, gender, type of surgery, MS-DRG, and race.
The five included MS-DRGs represented major hip and knee joint replacements with and without major comorbid conditions (MCCs; MS-DRG 469 and MS-DRG 470, respectively) and revision hip or knee replacement with MCCs, with comorbid conditions (CCs), and without MCCs or CCs (MS-DRG 466, MS-DRG 467, and MS-DRG 468, respectively). MCCs included but were not limited to decubitus ulcer, severe malnutrition, quadriplegia, and end-stage renal disease. Examples of CCs included transplant patients, lymphoma, leukemia, and malignancies (except breast or prostate), based on CMS definitions.13
RESULTS
Table 1 compares the demographics of the pre-implementation and post-implementation periods. There were a total of 2,662 admissions (799 before program implementation and 1,863 after). TKA and THA patients without MCCs (MS-DRG 470) accounted for 80% of patients during both periods. In both periods, approximately 60% of patients were female, 50% of patients were White, 40% were Black, and 10% were another race. The mean age was 63.6 years old. Most patients had Medicare or commercial insurance. Discharge destinations were similar during both periods.
Table 2 illustrates how the patients who re-presented to acute care were triaged based on the algorithm described in the Figure. Among the 64 patients who re-presented during the pre-implementation period, there were no observation stays; there were 38 patients who were placed under medicine inpatient services. During post-implementation, there were 48 patients (29 on orthopedics, 17 on medicine, and 2 on other service lines) who were admitted under observation status. Twenty-three patients were discharged on observation status. Of those patients, 20 were admitted to orthopedic observation and 3 patients to medicine observation. Among the 71 patients who re-presented during the post-implementation period, 40.8% (29 patients) were admitted to inpatient orthopedic services, and 17 patients were readmitted to medicine services (24.9%). Among re-presenting patients, 70% were admitted to orthopedics inpatient and observation combined, in contrast to just 35% during the pre-implementation period.

Readmissions decreased from 6.1% during pre-implementation to 2% during post-implementation (P = .004). In addition, the LOS for patients re-presenting during post-implementation was significantly lower than it was during pre-implementation. Table 3 details the associated LOS based on study period and readmission diagnosis. The aggregate LOS for all readmissions decreased from 7.75 days to 4.73 days (P = .005). The LOS decreased across all realms of readmission diagnoses. An outlier with an LOS greater than 100 days was removed from the pre-implementation group.

Appendix 2 further looked at patients who had observation orders, reasons for observation stay, and which patients were able to be discharged on observation status. Patients with medical complications such as fever and urinary tract infection were more likely to be discharged on observation status than were patients with wound drainage or redness that was concerning for a periprosthetic joint infection.
DISCUSSION
To our knowledge, this is the first description of a published Orthopedic EDAC program using orthopedic observation, standardized admitting and treatment algorithms, and comanagement of patients who re-presented after their original surgery. The development of an Orthopedic EDAC program at our hospital with comanagement was successful in reducing readmissions, decreasing LOS for readmitted patients, and increasing continuity of care. A number of points require more elaboration.
The Orthopedic EDAC program’s improvement in both reducing readmissions and decreasing LOS for EDAC (including days for observation and readmissions) was not caused by simply shifting patients with shorter LOS from inpatient to observation because the inpatients did not have a longer LOS. We had lower Orthopedic EDAC during the post-implementation vs pre-implementation even when considering EDAC in terms of both observation and readmissions. The decrease in readmissions is not only from the patients that were discharged on observation status, but also a result of other concurrent interventions, such as encouraging discharge to home rather than to rehabilitation facilities and more rigorous preoperative optimization.
The national rates of 30- and 90-day readmissions after primary TKA were 4% (95% CI, 3.8%-4.0%) and 7% (95% CI, 6.8%-7.2%), respectively,10 and the average cost of readmission for medical causes was $22,775 for THA and $11,682 for TKA.12 If one considers the 23 “saved readmissions” with 12 surgical complications and 11 medical complications, we “saved” roughly $591,105. Also, with the decrease in LOS for each readmission for any cause from 7.75 days to 4.73 days, the 48 readmissions had a 150 day lower LOS overall. With the average hospital day costing $2,289/day at nonprofit hospitals,13 there are additional cost savings of $343,350 overall. Therefore, the grand total estimated savings during this pilot was $934,455.
The decrease in post-implementation LOS vs pre-implementation LOS was likely multifactorial. The Orthopedic EDAC program improved continuity of care with orthopedic surgery and support staff (registered nurses, social workers, physical therapists) and utilized standardized protocols for work-up of common postoperative problems. These evidence-based protocols reduced waste that resulted in less testing with fewer incidental findings and side effects. The clinical history and patient circumstance did not need to be reestablished and tests did not need to be duplicated, which led to decreased LOS. Observation status allowed us to return patients to SNFs without the tedious procedure of insurance reauthorization and reevaluation by physical therapy and occupational therapy. Other factors such as “discharge before noon” and early physical therapy services ongoing during post-implementation also contributed to the decreased LOS.
Our Orthopedic EDAC program did not deliberately place patients on observation status who met full inpatient criteria solely to decrease the readmission rate. Our average LOS on observation status was 26 hours. In contrast, a study of observation stays at another tertiary academic medical center showed longer LOS: The average observation LOS was 33.3 hours with 44.4% of stays less than 24 hours and 16.5% greater than 48 hours.11 The use of EDAC hours in our study, which included both observation hours and readmission hours, made our impact more than simply a shifting of readmissions to observation stays.
It is important to utilize observation stays as they were intended—ie, stays requiring less than 48 hours. Over the past 10 years, the incidence and duration of observation stays has increased significantly while readmissions have decreased.14,15 Observation status has serious financial implications, and it is estimated that 10% of observation stays end up costing the patient more than an inpatient stay would and patients must pay 20% of services after the Part B deductible.16,17 In addition, Medicare beneficiaries have no cap on costs for an observation stay.16 Therefore, it is important to determine which patients and diagnoses are best suited for observation status. We found that younger patients without comorbidities who came from home and presented with complications such as fever and syncope were most likely to be successfully discharged on observation status with the Orthopedic EDAC program. SNF patients on observation status in particular may have large hospital bills because they often require 3 midnight stays but do not meet inpatient level of care and are thus not covered as inpatients.18
The Orthopedic EDAC program emphasized continuity of care with the primary orthopedic surgery team. Prior to implementation, orthopedics was often not even notified when their patients were in the ED or readmitted because the prevailing practice was that once surgery was completed, the surgeon’s job was done. Post-implementation, orthopedics was called for every bundled patient re-presenting within 90 days after a TJA. The triage protocol (Figure) was agreed upon prior to implementation by orthopedics, hospital medicine, and emergency medicine. Orthopedic attendings wanted to play a larger role and more strongly influence care of their patients on re-presentation because these attendings had become frustrated with the great disparities in work-up when patients went to various other services instead. Pre-implementation, many patients admitted to the primary orthopedic service had lower acuity, and they tended to be younger and have less medical complexity. Post-implementation, primary orthopedic services took care of more patients under observation status and those with “mechanical” complications that required surgery.
It is important to note that, while comanagement is common preoperatively and immediately postoperatively, studies of comanaged patients on re-presentation have apparently not been previously published. In addition, a recent study by Maxwell et al found that patients who were comanaged perioperatively had higher mortality and morbidity than did patients who were not comanaged.7 These findings reflect the need for more studies to be done to best optimize the use of comanagement. Comanagement as part of the Orthopedic EDAC program at our institution was successful in keeping patients who re-presented on the orthopedic service, decreasing LOS, and decreasing readmissions.
The study has some limitations. First, this was a retrospective study, so confounding variables may not be completely eliminated. Second, our study was conducted at a single center for total joint arthroplasty and did not consider other orthopedic conditions; however, our readmission numbers and demographics are similar to past studies. Third, we had small numbers of readmissions and observation patients, which resulted in a small effect size; however, our intervention demonstrated significant changes in LOS and readmissions. Fourth, our data is based on prior billing and coding, which may not always be accurate or inclusive. Fifth, we did not have THA or TKA patients on overnight recovery status or same day surgeries during either period studied; however, we are developing infrastructure to implement this in the future. Finally, ED visit data was not readily available to us, so we were not able to calculate the traditional EDAC. Despite these limitations, this study provides an important look at how an Orthopedic EDAC program can decrease readmissions, decrease LOS, and improve continuity of care in patients undergoing TJA.
CONCLUSION
An Orthopedic EDAC program with comanagement may decrease readmissions, improve continuity of care on re-presentation, and decrease LOS for total joint arthroplasty patients who presented after initial surgery and lead to substantial cost savings.
Disclosures
The authors have no potential conflicts to disclose. Dr Greysen was supported by a career development award from the National Institute on Aging (K23AG045338).
1. Hawker GA, Badley EM, Croxford R, et al. A population based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47(7):732-741. https://doi.org/10.1097/MLR.0b013e3181934553
2. Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63-70. https://doi.org/10.2147/RMHP.S130341
3. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskeletal Med. 2017;10(3):370-377. https://doi.org/10.1007/s12178-017-9423-6
4. The Society of Hospital Medicine. The Evolution of Co-Management. 2017. Accessed October 30, 2019. https://www.hospitalmedicine.org/globalassets/practice-management/practice-management-pdf/pm-19-0004-co-management-white-paper_minor-update-m.pdf
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Fitzgerald SJ, Palmer TC, Kraay MJ. Improved perioperative care of elective joint replacement patients: the impact of an orthopedic perioperative hospitalist. J Arthroplasty. 2018;33(8):2387-2391. https://doi,org/10.1016/j.arth.2018.03.029
7. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3343
8. Centers for Medicare & Medicaid Services. Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services; Final Rule. November 24, 2015. https://www.govinfo.gov/content/pkg/FR-2015-11-24/pdf/2015-29438.pdf
9. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468. https://doi.org/10.1016/j.arth.2013.07.039
10. ICD-10-CM/PCS MS-DRG v37.0 Definitions Manual. Accessed April 27, 2020. https://www.cms.gov/icd10m/version37-fullcode-cms/fullcode_cms/P0031.html
11. Chaudhary NS, Donnelly JP, Wang HE. Racial differences in sepsis mortality at United States academic medical center-affiliated hospitals. Crit Care Med. 2018;46(6):878-883. https://doi.org/10.1097/CCM.0000000000003020
12. Clair AJ, Evangelista PJ, Lajam CM, Slover JD, Bosco JA, Iorio R. Cost analysis of total joint arthroplasty readmissions in a Bundled Payment Care Improvement Initiative. J Arthroplasty. 2016;31(9):1862-1865.
13. Kaiser Family Foundation. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. Accessed April 27, 2020. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
14. Goldstein JN, Zhang Z, Schwartz JS, Hicks LS. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2018;131(1):101.e9-101.e15. https://doi.org/10.1016/j.amjmed.2017.07.013
15. Lind KD, Noel-Miller CM, Sangaralingham LR, et al. Increasing trends in the use of hospital observation services for older Medicare Advantage and privately insured patients. Med Care Res Rev. 2019;76(2):229-239. https://doi.org/10.1177/1077558717718026
16. Sabbatini AK, Wright B. Excluding observation stays from readmission rates - what quality measures are missing. N Engl J Med. 2018;378(22):2062-2065. https://doi.org/10.1056/NEJMp1800732
17. Gabayan GZ, Doyle B, Liang, L, Donkor K, Huang, D, Sarkisian CA. Who has an unsuccessful observation care stay? Healthcare (Basel). 2018;6(4):138. https://doi.org/10.3390/healthcare6040138
18. Fang M, Hume E, Ibrahim S. Race, Bundled payment policy, and discharge destination after TKA: the experience of an urban academic hospital. Geriatr Orthop Surg Rehabil. 2018. https://doi.org/10.1177/2151459318803222
1. Hawker GA, Badley EM, Croxford R, et al. A population based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47(7):732-741. https://doi.org/10.1097/MLR.0b013e3181934553
2. Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63-70. https://doi.org/10.2147/RMHP.S130341
3. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskeletal Med. 2017;10(3):370-377. https://doi.org/10.1007/s12178-017-9423-6
4. The Society of Hospital Medicine. The Evolution of Co-Management. 2017. Accessed October 30, 2019. https://www.hospitalmedicine.org/globalassets/practice-management/practice-management-pdf/pm-19-0004-co-management-white-paper_minor-update-m.pdf
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Fitzgerald SJ, Palmer TC, Kraay MJ. Improved perioperative care of elective joint replacement patients: the impact of an orthopedic perioperative hospitalist. J Arthroplasty. 2018;33(8):2387-2391. https://doi,org/10.1016/j.arth.2018.03.029
7. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3343
8. Centers for Medicare & Medicaid Services. Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services; Final Rule. November 24, 2015. https://www.govinfo.gov/content/pkg/FR-2015-11-24/pdf/2015-29438.pdf
9. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468. https://doi.org/10.1016/j.arth.2013.07.039
10. ICD-10-CM/PCS MS-DRG v37.0 Definitions Manual. Accessed April 27, 2020. https://www.cms.gov/icd10m/version37-fullcode-cms/fullcode_cms/P0031.html
11. Chaudhary NS, Donnelly JP, Wang HE. Racial differences in sepsis mortality at United States academic medical center-affiliated hospitals. Crit Care Med. 2018;46(6):878-883. https://doi.org/10.1097/CCM.0000000000003020
12. Clair AJ, Evangelista PJ, Lajam CM, Slover JD, Bosco JA, Iorio R. Cost analysis of total joint arthroplasty readmissions in a Bundled Payment Care Improvement Initiative. J Arthroplasty. 2016;31(9):1862-1865.
13. Kaiser Family Foundation. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. Accessed April 27, 2020. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
14. Goldstein JN, Zhang Z, Schwartz JS, Hicks LS. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2018;131(1):101.e9-101.e15. https://doi.org/10.1016/j.amjmed.2017.07.013
15. Lind KD, Noel-Miller CM, Sangaralingham LR, et al. Increasing trends in the use of hospital observation services for older Medicare Advantage and privately insured patients. Med Care Res Rev. 2019;76(2):229-239. https://doi.org/10.1177/1077558717718026
16. Sabbatini AK, Wright B. Excluding observation stays from readmission rates - what quality measures are missing. N Engl J Med. 2018;378(22):2062-2065. https://doi.org/10.1056/NEJMp1800732
17. Gabayan GZ, Doyle B, Liang, L, Donkor K, Huang, D, Sarkisian CA. Who has an unsuccessful observation care stay? Healthcare (Basel). 2018;6(4):138. https://doi.org/10.3390/healthcare6040138
18. Fang M, Hume E, Ibrahim S. Race, Bundled payment policy, and discharge destination after TKA: the experience of an urban academic hospital. Geriatr Orthop Surg Rehabil. 2018. https://doi.org/10.1177/2151459318803222
© 2020 Society of Hospital Medicine
Nudging Providers to Improve Sleep for Hospitalized Patients
It is 5:45
In this edition of the Journal of Hospital Medicine, Arora et al. present a single-center, pre–post analysis of an intervention designed to improve sleep for hospitalized patients.5 The SIESTA (Sleep for Inpatients: Empowering Staff to Act) intervention was composed of the following three components: provider education on patient sleep, Electronic Health Record (EHR) promotion of sleep-friendly order entry, and empowerment of nurses to actively protect patient sleep. Education and changes to order entry were implemented in two hospital units, but only one received the additional nurse-empowerment intervention. Results were compared for six months pre- and post-intervention. Although the authors found increases in sleep-friendly orders in both units, nighttime room entries and patient-reported sleep disturbance decreased only in the nurse-empowerment unit.
Previous studies assessing both pharmacologic sleep aids as well as bundled nonpharmacologic interventions have demonstrated mixed results and focused primarily on ICU populations.6,7 What sets this study apart from prior interventions aimed at improving patient sleep is the novelty and implications of their successful intervention. In this study, the authors used the EHR and nursing huddles to “nudge” providers to protect their patients’ sleep. The “nudge” concept, first studied in behavioral economics and more recently applied to healthcare, represents ways to present choices that positively influence behavior without restricting options.8 This study incorporates two distinct nudges, one that utilized the EMR to adjust the default timing of orders for vital sign procurement and delivery of VTE-prophylaxis, and another that made sleep part of the default checklist for nursing huddles. This study suggests that nudges altered both physician and nurse behavior and encouraged improvements in process measures, if not clinical outcomes, around patient sleep.
A key insight and strength of this study was to engage and empower nurses to promote better sleep for patients. In particular, nurses in the sleep-enhanced unit suggested—during the course of the intervention—that sleep protection be added as a default item in daily huddles. As illustrated in the Figure, the timing of this suggestion corresponded with an inflection point in reducing patient room disruptions at night. This simple, low-cost nudge sustained sleep improvement while the effect of the initial higher-cost intervention using pocket cards and posters had begun to fade. This is not a randomized clinical trial, but rather a pragmatic assessment of a rigorous quality improvement initiative. Although more follow-up time, particularly after the nurse-empowerment intervention was adjusted, would be helpful to assess the durability of their intervention, we applaud the authors for demonstrating adaptability and efforts for ongoing engagement, as is needed in real-world quality improvement initiatives.
There are additional factors that disrupt patient sleep that were not targeted in this study but could very well respond to nudges. Recently, Wesselius et al. showed that patient-reported nocturnal awakenings were frequently due to toilet visits and awakening by hospital staff.9 Perhaps nudges could be implemented to reduce unnecessary overnight intravenous fluids, prevent late dosing of diuretics, and delay the default timing of standard morning phlebotomy orders.
Although this study by Arora et al. makes a very meaningful contribution to the literature on sleep and hospitalization, it also raises unanswered questions.5 First and foremost, while the pragmatic nature of this study should inspire other hospitals to attempt similar sleep promotion interventions, the use of a pre–post design (rather than a randomized, control design) leaves room for future studies to explore causality more rigorously. Second, although this study has demonstrated significant uptake in standardized order sets to improve sleep (and a corresponding decrease in patient-reported disruptions), future studies should also explore more distal and more challenging outcomes of care. These could include length of stay, incidence of delirium (especially in older adults), and frequency of readmission after discharge. Finally, more longitudinal data to explore the sustainability of order set usage and reported or observed interruptions would be useful to guide hospitals that would like to follow the example set by the SIESTA study.
Notwithstanding these limitations, there is an incredible opportunity for nudges and technology to combine to change the paradigms of clinical care. One of the outcomes of this study was to reduce nocturnal room entry for clinical tasks such as obtaining vital signs. It is worth considering whether providers even need to enter patient rooms to obtain vital signs. The technology now exists to measure vitals passively and continuously via low-impact wearable devices. Milani et al. employed the use of such devices, as well as other techniques, including red-enriched light and sensors that warned staff in clinical areas when noises exceeded acceptable thresholds for sleep, and demonstrated decreases in hospital length of stay and readmission rates.4
Arora et al. present a compelling study of utilizing nudges to influence physician and nurse behavior.5 They show that rigorous quality improvement initiatives can be studied and disseminated in a compelling manner. Their study calls appropriate attention to the need for improving patient sleep and provides us with additional tools that can be used in these efforts. Future research is needed to determine whether the changes observed in process measures will translate into meaningful effects on clinical outcomes and to continue to identify ways to curb some of the toxicities of hospital care.
Disclosures
The authors have nothing to disclose.
1. Krumholz HM. Post hospital syndrome: A condition of generalized risk. N Engl J Med. 2013;368(2):100-102. doi: 10.1056/NEJMp1212324. PubMed
2. Pisani MA, Friese RS, Gehlback BK, Schwab RJ, Weinhouse GL, Jones SF. Sleep in the intensive care unit. Am J Respir Crit Care Med. 2015;191(7):731-738. doi: 10.1164/rccm.201411-2099CI. PubMed
3. Judson T, Johnson K, Bieraugel K, et al. Sleep is vital: improving sleep by reducing unnecessary nocturnal vital signs [abstract]. https://www.shmabstracts.com/abstract/sleep-is-vital-improving-sleep-by-reducing-unnecessary-nocturnal-vital-signs/
4. Milani RV, Bober RM, Lavie CJ, Wilt JK, Milani AR, White CJ. Reducing hospital toxicity: impact on patient outcomes. Am J Med. 2018;131(8):961-966. doi: 10.1016/j.amjmed.2018.04.013. PubMed
5. Arora VM, Machado N, Anderson SL, Desai N, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019:14(1):38-41. doi: 10.12788/jhm.3091
6. Hu RF, Jiang XY, Chen J, et al. Non-pharmacologic treatments for sleep promotion in the intensive care unit. Cochrane Database Syst Rev. 2015(10):CD008808. doi: 10.1002/14651858.CD008808.pub2.
7. Lewis SR, Pritchard MW, Schofield-Robinson OJ, Alderson P, Smith AF. Melatonin for the promotion of sleep in adults in the intensive care unit. Cochrane Database Syst Rev. 2018;(5):CD012455. doi: 10.1002/14651858.CD012455.pub2. PubMed
8. Patel MS, Volpp KG, Asch DA. Nudge units to improve the delivery of health care. N Engl J Med. 2018;378:214-216. doi: 10.1056/NEJMp1712984. PubMed
9. Wesselius HM, van den Ende ES, Alsma J, et al. Quality and quantity of sleep and factor associated with sleep disturbance in hospitalized patients. JAMA Intern Med. 2018;178(9):1201-1208. doi: 10.1001/jamainternmed.2018.2669. PubMed
It is 5:45
In this edition of the Journal of Hospital Medicine, Arora et al. present a single-center, pre–post analysis of an intervention designed to improve sleep for hospitalized patients.5 The SIESTA (Sleep for Inpatients: Empowering Staff to Act) intervention was composed of the following three components: provider education on patient sleep, Electronic Health Record (EHR) promotion of sleep-friendly order entry, and empowerment of nurses to actively protect patient sleep. Education and changes to order entry were implemented in two hospital units, but only one received the additional nurse-empowerment intervention. Results were compared for six months pre- and post-intervention. Although the authors found increases in sleep-friendly orders in both units, nighttime room entries and patient-reported sleep disturbance decreased only in the nurse-empowerment unit.
Previous studies assessing both pharmacologic sleep aids as well as bundled nonpharmacologic interventions have demonstrated mixed results and focused primarily on ICU populations.6,7 What sets this study apart from prior interventions aimed at improving patient sleep is the novelty and implications of their successful intervention. In this study, the authors used the EHR and nursing huddles to “nudge” providers to protect their patients’ sleep. The “nudge” concept, first studied in behavioral economics and more recently applied to healthcare, represents ways to present choices that positively influence behavior without restricting options.8 This study incorporates two distinct nudges, one that utilized the EMR to adjust the default timing of orders for vital sign procurement and delivery of VTE-prophylaxis, and another that made sleep part of the default checklist for nursing huddles. This study suggests that nudges altered both physician and nurse behavior and encouraged improvements in process measures, if not clinical outcomes, around patient sleep.
A key insight and strength of this study was to engage and empower nurses to promote better sleep for patients. In particular, nurses in the sleep-enhanced unit suggested—during the course of the intervention—that sleep protection be added as a default item in daily huddles. As illustrated in the Figure, the timing of this suggestion corresponded with an inflection point in reducing patient room disruptions at night. This simple, low-cost nudge sustained sleep improvement while the effect of the initial higher-cost intervention using pocket cards and posters had begun to fade. This is not a randomized clinical trial, but rather a pragmatic assessment of a rigorous quality improvement initiative. Although more follow-up time, particularly after the nurse-empowerment intervention was adjusted, would be helpful to assess the durability of their intervention, we applaud the authors for demonstrating adaptability and efforts for ongoing engagement, as is needed in real-world quality improvement initiatives.
There are additional factors that disrupt patient sleep that were not targeted in this study but could very well respond to nudges. Recently, Wesselius et al. showed that patient-reported nocturnal awakenings were frequently due to toilet visits and awakening by hospital staff.9 Perhaps nudges could be implemented to reduce unnecessary overnight intravenous fluids, prevent late dosing of diuretics, and delay the default timing of standard morning phlebotomy orders.
Although this study by Arora et al. makes a very meaningful contribution to the literature on sleep and hospitalization, it also raises unanswered questions.5 First and foremost, while the pragmatic nature of this study should inspire other hospitals to attempt similar sleep promotion interventions, the use of a pre–post design (rather than a randomized, control design) leaves room for future studies to explore causality more rigorously. Second, although this study has demonstrated significant uptake in standardized order sets to improve sleep (and a corresponding decrease in patient-reported disruptions), future studies should also explore more distal and more challenging outcomes of care. These could include length of stay, incidence of delirium (especially in older adults), and frequency of readmission after discharge. Finally, more longitudinal data to explore the sustainability of order set usage and reported or observed interruptions would be useful to guide hospitals that would like to follow the example set by the SIESTA study.
Notwithstanding these limitations, there is an incredible opportunity for nudges and technology to combine to change the paradigms of clinical care. One of the outcomes of this study was to reduce nocturnal room entry for clinical tasks such as obtaining vital signs. It is worth considering whether providers even need to enter patient rooms to obtain vital signs. The technology now exists to measure vitals passively and continuously via low-impact wearable devices. Milani et al. employed the use of such devices, as well as other techniques, including red-enriched light and sensors that warned staff in clinical areas when noises exceeded acceptable thresholds for sleep, and demonstrated decreases in hospital length of stay and readmission rates.4
Arora et al. present a compelling study of utilizing nudges to influence physician and nurse behavior.5 They show that rigorous quality improvement initiatives can be studied and disseminated in a compelling manner. Their study calls appropriate attention to the need for improving patient sleep and provides us with additional tools that can be used in these efforts. Future research is needed to determine whether the changes observed in process measures will translate into meaningful effects on clinical outcomes and to continue to identify ways to curb some of the toxicities of hospital care.
Disclosures
The authors have nothing to disclose.
It is 5:45
In this edition of the Journal of Hospital Medicine, Arora et al. present a single-center, pre–post analysis of an intervention designed to improve sleep for hospitalized patients.5 The SIESTA (Sleep for Inpatients: Empowering Staff to Act) intervention was composed of the following three components: provider education on patient sleep, Electronic Health Record (EHR) promotion of sleep-friendly order entry, and empowerment of nurses to actively protect patient sleep. Education and changes to order entry were implemented in two hospital units, but only one received the additional nurse-empowerment intervention. Results were compared for six months pre- and post-intervention. Although the authors found increases in sleep-friendly orders in both units, nighttime room entries and patient-reported sleep disturbance decreased only in the nurse-empowerment unit.
Previous studies assessing both pharmacologic sleep aids as well as bundled nonpharmacologic interventions have demonstrated mixed results and focused primarily on ICU populations.6,7 What sets this study apart from prior interventions aimed at improving patient sleep is the novelty and implications of their successful intervention. In this study, the authors used the EHR and nursing huddles to “nudge” providers to protect their patients’ sleep. The “nudge” concept, first studied in behavioral economics and more recently applied to healthcare, represents ways to present choices that positively influence behavior without restricting options.8 This study incorporates two distinct nudges, one that utilized the EMR to adjust the default timing of orders for vital sign procurement and delivery of VTE-prophylaxis, and another that made sleep part of the default checklist for nursing huddles. This study suggests that nudges altered both physician and nurse behavior and encouraged improvements in process measures, if not clinical outcomes, around patient sleep.
A key insight and strength of this study was to engage and empower nurses to promote better sleep for patients. In particular, nurses in the sleep-enhanced unit suggested—during the course of the intervention—that sleep protection be added as a default item in daily huddles. As illustrated in the Figure, the timing of this suggestion corresponded with an inflection point in reducing patient room disruptions at night. This simple, low-cost nudge sustained sleep improvement while the effect of the initial higher-cost intervention using pocket cards and posters had begun to fade. This is not a randomized clinical trial, but rather a pragmatic assessment of a rigorous quality improvement initiative. Although more follow-up time, particularly after the nurse-empowerment intervention was adjusted, would be helpful to assess the durability of their intervention, we applaud the authors for demonstrating adaptability and efforts for ongoing engagement, as is needed in real-world quality improvement initiatives.
There are additional factors that disrupt patient sleep that were not targeted in this study but could very well respond to nudges. Recently, Wesselius et al. showed that patient-reported nocturnal awakenings were frequently due to toilet visits and awakening by hospital staff.9 Perhaps nudges could be implemented to reduce unnecessary overnight intravenous fluids, prevent late dosing of diuretics, and delay the default timing of standard morning phlebotomy orders.
Although this study by Arora et al. makes a very meaningful contribution to the literature on sleep and hospitalization, it also raises unanswered questions.5 First and foremost, while the pragmatic nature of this study should inspire other hospitals to attempt similar sleep promotion interventions, the use of a pre–post design (rather than a randomized, control design) leaves room for future studies to explore causality more rigorously. Second, although this study has demonstrated significant uptake in standardized order sets to improve sleep (and a corresponding decrease in patient-reported disruptions), future studies should also explore more distal and more challenging outcomes of care. These could include length of stay, incidence of delirium (especially in older adults), and frequency of readmission after discharge. Finally, more longitudinal data to explore the sustainability of order set usage and reported or observed interruptions would be useful to guide hospitals that would like to follow the example set by the SIESTA study.
Notwithstanding these limitations, there is an incredible opportunity for nudges and technology to combine to change the paradigms of clinical care. One of the outcomes of this study was to reduce nocturnal room entry for clinical tasks such as obtaining vital signs. It is worth considering whether providers even need to enter patient rooms to obtain vital signs. The technology now exists to measure vitals passively and continuously via low-impact wearable devices. Milani et al. employed the use of such devices, as well as other techniques, including red-enriched light and sensors that warned staff in clinical areas when noises exceeded acceptable thresholds for sleep, and demonstrated decreases in hospital length of stay and readmission rates.4
Arora et al. present a compelling study of utilizing nudges to influence physician and nurse behavior.5 They show that rigorous quality improvement initiatives can be studied and disseminated in a compelling manner. Their study calls appropriate attention to the need for improving patient sleep and provides us with additional tools that can be used in these efforts. Future research is needed to determine whether the changes observed in process measures will translate into meaningful effects on clinical outcomes and to continue to identify ways to curb some of the toxicities of hospital care.
Disclosures
The authors have nothing to disclose.
1. Krumholz HM. Post hospital syndrome: A condition of generalized risk. N Engl J Med. 2013;368(2):100-102. doi: 10.1056/NEJMp1212324. PubMed
2. Pisani MA, Friese RS, Gehlback BK, Schwab RJ, Weinhouse GL, Jones SF. Sleep in the intensive care unit. Am J Respir Crit Care Med. 2015;191(7):731-738. doi: 10.1164/rccm.201411-2099CI. PubMed
3. Judson T, Johnson K, Bieraugel K, et al. Sleep is vital: improving sleep by reducing unnecessary nocturnal vital signs [abstract]. https://www.shmabstracts.com/abstract/sleep-is-vital-improving-sleep-by-reducing-unnecessary-nocturnal-vital-signs/
4. Milani RV, Bober RM, Lavie CJ, Wilt JK, Milani AR, White CJ. Reducing hospital toxicity: impact on patient outcomes. Am J Med. 2018;131(8):961-966. doi: 10.1016/j.amjmed.2018.04.013. PubMed
5. Arora VM, Machado N, Anderson SL, Desai N, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019:14(1):38-41. doi: 10.12788/jhm.3091
6. Hu RF, Jiang XY, Chen J, et al. Non-pharmacologic treatments for sleep promotion in the intensive care unit. Cochrane Database Syst Rev. 2015(10):CD008808. doi: 10.1002/14651858.CD008808.pub2.
7. Lewis SR, Pritchard MW, Schofield-Robinson OJ, Alderson P, Smith AF. Melatonin for the promotion of sleep in adults in the intensive care unit. Cochrane Database Syst Rev. 2018;(5):CD012455. doi: 10.1002/14651858.CD012455.pub2. PubMed
8. Patel MS, Volpp KG, Asch DA. Nudge units to improve the delivery of health care. N Engl J Med. 2018;378:214-216. doi: 10.1056/NEJMp1712984. PubMed
9. Wesselius HM, van den Ende ES, Alsma J, et al. Quality and quantity of sleep and factor associated with sleep disturbance in hospitalized patients. JAMA Intern Med. 2018;178(9):1201-1208. doi: 10.1001/jamainternmed.2018.2669. PubMed
1. Krumholz HM. Post hospital syndrome: A condition of generalized risk. N Engl J Med. 2013;368(2):100-102. doi: 10.1056/NEJMp1212324. PubMed
2. Pisani MA, Friese RS, Gehlback BK, Schwab RJ, Weinhouse GL, Jones SF. Sleep in the intensive care unit. Am J Respir Crit Care Med. 2015;191(7):731-738. doi: 10.1164/rccm.201411-2099CI. PubMed
3. Judson T, Johnson K, Bieraugel K, et al. Sleep is vital: improving sleep by reducing unnecessary nocturnal vital signs [abstract]. https://www.shmabstracts.com/abstract/sleep-is-vital-improving-sleep-by-reducing-unnecessary-nocturnal-vital-signs/
4. Milani RV, Bober RM, Lavie CJ, Wilt JK, Milani AR, White CJ. Reducing hospital toxicity: impact on patient outcomes. Am J Med. 2018;131(8):961-966. doi: 10.1016/j.amjmed.2018.04.013. PubMed
5. Arora VM, Machado N, Anderson SL, Desai N, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019:14(1):38-41. doi: 10.12788/jhm.3091
6. Hu RF, Jiang XY, Chen J, et al. Non-pharmacologic treatments for sleep promotion in the intensive care unit. Cochrane Database Syst Rev. 2015(10):CD008808. doi: 10.1002/14651858.CD008808.pub2.
7. Lewis SR, Pritchard MW, Schofield-Robinson OJ, Alderson P, Smith AF. Melatonin for the promotion of sleep in adults in the intensive care unit. Cochrane Database Syst Rev. 2018;(5):CD012455. doi: 10.1002/14651858.CD012455.pub2. PubMed
8. Patel MS, Volpp KG, Asch DA. Nudge units to improve the delivery of health care. N Engl J Med. 2018;378:214-216. doi: 10.1056/NEJMp1712984. PubMed
9. Wesselius HM, van den Ende ES, Alsma J, et al. Quality and quantity of sleep and factor associated with sleep disturbance in hospitalized patients. JAMA Intern Med. 2018;178(9):1201-1208. doi: 10.1001/jamainternmed.2018.2669. PubMed
© 2019 Society of Hospital Medicine
