User login
Dearth of Hospitalist Investigators in Academic Medicine: A Call to Action
In their report celebrating the increase in the number of hospitalists from a few hundred in the 1990s to more than 50,000 in 2016, Drs Robert Wachter and Lee Goldman also noted the stunted growth of productive hospital medicine research programs, which presents a challenge to academic credibility in hospital medicine.1 Given the substantial increase in the number of hospitalists over the past two decades, we surveyed adult academic hospital medicine groups to quantify the number of hospitalist clinician investigators and identify gaps in resources for researchers. The number of clinician investigators supported at academic medical centers (AMCs) remains disturbingly low despite the rapid growth of our specialty. Some programs also reported a lack of access to fundamental research services. We report selected results from our survey and provide recommendations to support and facilitate the development of clinician investigators in hospital medicine.
DEARTH OF CLINICIAN INVESTIGATORS IN HOSPITAL MEDICINE
We performed a survey of hospital medicine programs at AMCs in the United States through the Hospital Medicine Reengineering Network (HOMERuN), a hospital medicine research collaborative that facilitates and conducts multisite research studies.2 The purpose of this survey was to obtain a profile of adult academic hospital medicine groups. Surveys were distributed via email to directors and/or senior leaders of each hospital medicine group between January and August 2019. In the survey, a clinician investigator was defined as “faculty whose primary nonclinical focus is scientific papers and grant writing.”
We received responses from 43 of the 86 invitees (50%), each of whom represented a unique hospital medicine group; 41 of the representatives responded to the questions concerning available research services. Collectively, these 43 programs represented 2,503 hospitalists. There were 79 clinician investigators reported among all surveyed hospital medicine groups (3.1% of all hospitalists). The median number of clinician investigators per hospital medicine group was 0 (range 0-12) (Appendix Figure 1), and 22 of 43 (51.2%) hospital medicine groups reported having no clinician investigators. Two of the hospital medicine groups, however, reported having 12 clinician investigators at their respective institutions, comprising nearly one third of the total number of clinician investigators reported in the survey.
Many of the programs reported lack of access to resources such as research assistants (56.1%) and dedicated research fellowships (53.7%) (Appendix Figure 2). A number of groups reported a need for more support for various junior faculty development activities, including research mentoring (53.5%), networking with other researchers (60.5%), and access to clinical data from multiple sites (62.8%).
One of the limitations of this survey was the manner in which the participating hospital medicine groups were chosen. Selection was based on groups affiliated with HOMERuN; among those chosen were highly visible US AMCs, including 70% of the top 20 AMCs based on National Institutes of Health (NIH) funding.3 Therefore, our results likely overestimate the research presence of hospital medicine across all AMCs in the United States.
LACK OF GROWTH OVER TIME: CONTEXTUALIZATION AND IMPLICATIONS
Despite the substantial growth of hospital medicine over the past 2 decades, there has been no proportional increase in the number of hospitalist clinician investigators, with earlier surveys also demonstrating low numbers.4,5 Along with the survey by Chopra and colleagues published in 2019,6 our survey provides an additional contemporary appraisal of research activities for adult academic hospital medicine groups. In the survey by Chopra et al, only 54% (15 of 28) of responding programs reported having any faculty with research as their major activity (ie, >50% effort), and 3% of total faculty reported having funding for >50% effort toward research.6 Our study expands upon these findings by providing more detailed data on the number of clinician investigators per hospital medicine group. Results of our survey showed a concentration of hospitalists within a small number of programs, which may have contributed to the observed lack of growth. We also expand on prior work by identifying a lack of resources and services to support hospitalist researchers.
The findings of our survey have important implications for the field of hospital medicine. Without a critical mass of hospitalist clinician investigators, the quality of research that addresses important questions in our field will suffer. It will also limit academic credibility of the field, as well as individual academic achievement; previous studies have consistently demonstrated that few hospitalists at AMCs achieve the rank of associate or full professor.5-9
POTENTIAL EXPLANATIONS FOR LACK OF RESEARCH GROWTH
The results of our study additionally offer possible explanations for the dearth of clinician investigators in hospital medicine. The limited access to research resources and fellowship training identified in our survey are critical domains that must be addressed in order to develop successful academic hospital medicine programs.4,6,8,10
Regarding dedicated hospital medicine research fellowships, there are only a handful across the country. The small number of existing research fellowships only have one or two fellows per year, and these positions often go unfilled because of a lack of applicants and lower salaries compared to full-time clinical positions.11 The lack of applicants for adult hospital medicine fellowship positions is also integrally linked to board certification requirements. Unlike pediatric hospital medicine where additional fellowship training is required to become board-certified, no such fellowship is required in adult hospital medicine. In pediatrics, this requirement has led to a rapid increase in the number of fellowships with scholarly work requirements (more than 60 fellowships, plus additional programs in development) and greater standardization among training experiences.12,13
The lack of fellowship applicants may also stem from the fact that many trainees are not aware of a potential career as a hospitalist clinician investigator due to limited exposure to this career at most AMCs. Our results revealed that nearly half of sites in our survey had zero clinician investigators, depriving trainees at these programs of role models and thus perpetuating a negative feedback loop. Lastly, although unfilled fellowship positions may indicate that demand is a larger problem than supply, it is also true that fellowship programs generate their own demand through recruitment efforts and the gradual establishment of a positive reputation.
Another potential explanation could relate to the development of hospital medicine in response to rising clinical demands at hospitals: compared with other medical specialties, AMCs may regard hospitalists as being clinicians first and academicians second.1,7,10 Also, hospitalists may be perceived as being beholden to hospitals and less engaged with their surrounding communities than other general medicine fields. With a small footprint in health equity research, academic hospital medicine may be less of a draw to generalists interested in pursuing this area of research. Further, there are very few underrepresented in medicine (URiM) hospital medicine research faculty.5
Another challenge to the career development of hospitalist researchers is the lack of available funding for the type of research typically conducted by hospitalists (eg, rigorous quality improvement implementation and evaluation, optimizing best evidence-based care delivery models, evaluation of patient safety in the hospital setting). As hospitalists tend to be system-level thinkers, this lack of funding may steer potential researchers away from externally funded research careers and into hospital operations and quality improvement positions. Also, unlike other medical specialties, there is no dedicated NIH funding source for hospital medicine research (eg, cardiology and the National Heart, Lung, and Blood Institute), placing hospitalists at a disadvantage in seeking funding compared to subspecialists.
STRATEGIES TO ENHANCE RESEARCH PRESENCE
We recommend several approaches—ones that should be pursued simultaneously—to increase the number of clinician investigators in hospital medicine. First, hospital medicine groups and their respective divisions, departments, and hospitals should allocate funding to support research resources; this includes investing in research assistants, data analysts, statisticians, and administrative support. Through the funding of such research infrastructure programs, AMCs could incentivize hospitalists to research best approaches to improve the value of healthcare delivery, ultimately leading to cost savings.
With 60% of respondents identifying the need for improved access to data across multiple sites, our survey also emphasizes the requirement for further collaboration among hospital medicine groups. Such collaboration could lead to high-powered observational studies and the evaluation of interventions across multiple sites, thus improving the generalizability of study findings.
The Society of Hospital Medicine (SHM) and its research committee can continue to expand the research footprint of hospital medicine. To date, the committee has achieved this by highlighting hospitalist research activity at the SHM Annual Conference Scientific Abstract and Poster Competition and developing a visiting professorship exchange program. In addition to these efforts, SHM could foster collaboration and networking between institutions, as well as take advantage of the current political push for expanded Medicare access by lobbying for robust funding for the Agency for Healthcare Research and Quality, which could provide more opportunities for hospitalists to study the effects of healthcare policy reform on the delivery of inpatient care.
Another strategy to increase the number of hospitalist clinician investigators is to expand hospital medicine research fellowships and recruit trainees for these programs. Fellowships could be internally funded wherein a fellow’s clinical productivity is used to offset the costs associated with obtaining advanced degrees. As an incentive to encourage applicants to temporarily forego a full-time clinical salary during fellowship, hospital medicine groups could offer expanded moonlighting opportunities and contribute to repayment of medical school loans. Hospital medicine groups should also advocate for NIH-funded T32 or K12 training grants for hospital medicine. (There are, however, challenges with this approach because the number of T32 spots per NIH institute is usually fixed). The success of academic emergency medicine offers a precedent for such efforts: After the development of a K12 research training program in emergency medicine, the number of NIH-sponsored principal investigators in this specialty increased by 40% in 6 years.14 Additionally, now that fellowships are required for the pediatric hospital medicine clinician investigators, it would be revealing to track the growth of this workforce.12,13
Structured and formalized mentorship is an essential part of the development of clinician investigators in hospital medicine.4,7,8,10 One successful strategy for mentorship has been the partnering of hospital medicine groups with faculty of general internal medicine and other subspecialty divisions with robust research programs.7,8,15 In addition to developing sustainable mentorship programs, hospital medicine researchers must increase their visibility to trainees. Therefore, it is essential that the majority of academic hospital medicine groups not only hire clinician investigators but also invest in their development, rather than rely on the few programs that have several such faculty members. With this strategy, we could dramatically increase the number of hospitalist clinician investigators from a diverse background of training institutions.
SHM could also play a greater role in organizing events for networking and mentoring for trainees and medical students interested in pursuing a career in hospital medicine research. It is also critically important that hospital medicine groups actively recruit, retain, and develop URiM hospital medicine research faculty in order to attract talented researchers and actively participate in the necessary effort to mitigate the inequities prevalent throughout our healthcare system.
CONCLUSION
Despite the growth of hospital medicine over the past decade, there remains a dearth of hospitalist clinician investigators at major AMCs in the United States. This may be due in part to lack of research resources and mentorship within hospital medicine groups. We believe that investment in these resources, expanded funding opportunities, mentorship development, research fellowship programs, and greater exposure of trainees to hospitalist researchers are solutions that should be strongly considered to develop hospitalist clinician investigators.
Acknowledgments
The authors thank HOMERuN executive committee members, including Grant Fletcher, MD, James Harrison, PhD, BSC, MPH, Peter K. Lindenauer, MD, Melissa Mattison, MD, David Meltzer, MD, PhD, Joshua Metlay, MD, PhD, Jennifer Myers, MD, Sumant Ranji, MD, Gregory Ruhnke, MD, MPH, Edmondo Robinson, MD, MBA, and Neil Sehgal, MPH PhD, for their assistance in developing the survey. They also thank Tiffany Lee, MA, for her project management assistance for HOMERuN.
1. Wachter RM, Goldman L. Zero to 50,000 – The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
3. Roskoski R Jr, Parslow TG. Ranking Tables of NIH funding to US medical schools in 2019. Blue Ridge Institute for Medical Research. Published 2020. Updated July 14, 2020. Accessed July 30, 2020. http://www.brimr.org/NIH_Awards/2019/NIH_Awards_2019.htm
4. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
5. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the society of general internal medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
6. Chopra V, Burden M, Jones CD, et al; Society of Hospital Medicine Research Committee. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
7. Seymann GB, Southern W, Burger A, et al. Features of successful academic hospitalist programs: insights from the SCHOLAR (SuCcessful HOspitaLists in academics and research) project. J Hosp Med. 2016;11(10):708-713. https://doi.org/10.1002/jhm.2603
8. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836
9. Dang Do AN, Munchhof AM, Terry C, Emmett T, Kara A. Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148-154. https://doi.org/10.1002/jhm.2148
10. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161-166. https://doi.org/10.1002/jhm.845
11. Ranji SR, Rosenman DJ, Amin AN, Kripalani S. Hospital medicine fellowships: works in progress. Am J Med. 2006;119(1):72.e1-72.e7. https://doi.org/10.1016/j.amjmed.2005.07.061
12. Shah NH, Rhim HJ, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: a survey of program directors. J Hosp Med. 2016;11(5):324-328. https://doi.org/10.1002/jhm.2571
13. Jerardi KE, Fisher E, Rassbach C, et al; Council of Pediatric Hospital Medicine Fellowship Directors. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698
14. Lewis RJ, Neumar RW. Research in emergency medicine: building the investigator pipeline. Ann Emerg Med. 2018;72(6):691-695. https://doi.org/10.1016/j.annemergmed.2018.10.019
15. Flanders SA, Kaufman SR, Nallamothu BK, Saint S. The University of Michigan Specialist-Hospitalist Allied Research Program: jumpstarting hospital medicine research. J Hosp Med. 2008;3(4):308-313. https://doi.org/10.1002/jhm.342
In their report celebrating the increase in the number of hospitalists from a few hundred in the 1990s to more than 50,000 in 2016, Drs Robert Wachter and Lee Goldman also noted the stunted growth of productive hospital medicine research programs, which presents a challenge to academic credibility in hospital medicine.1 Given the substantial increase in the number of hospitalists over the past two decades, we surveyed adult academic hospital medicine groups to quantify the number of hospitalist clinician investigators and identify gaps in resources for researchers. The number of clinician investigators supported at academic medical centers (AMCs) remains disturbingly low despite the rapid growth of our specialty. Some programs also reported a lack of access to fundamental research services. We report selected results from our survey and provide recommendations to support and facilitate the development of clinician investigators in hospital medicine.
DEARTH OF CLINICIAN INVESTIGATORS IN HOSPITAL MEDICINE
We performed a survey of hospital medicine programs at AMCs in the United States through the Hospital Medicine Reengineering Network (HOMERuN), a hospital medicine research collaborative that facilitates and conducts multisite research studies.2 The purpose of this survey was to obtain a profile of adult academic hospital medicine groups. Surveys were distributed via email to directors and/or senior leaders of each hospital medicine group between January and August 2019. In the survey, a clinician investigator was defined as “faculty whose primary nonclinical focus is scientific papers and grant writing.”
We received responses from 43 of the 86 invitees (50%), each of whom represented a unique hospital medicine group; 41 of the representatives responded to the questions concerning available research services. Collectively, these 43 programs represented 2,503 hospitalists. There were 79 clinician investigators reported among all surveyed hospital medicine groups (3.1% of all hospitalists). The median number of clinician investigators per hospital medicine group was 0 (range 0-12) (Appendix Figure 1), and 22 of 43 (51.2%) hospital medicine groups reported having no clinician investigators. Two of the hospital medicine groups, however, reported having 12 clinician investigators at their respective institutions, comprising nearly one third of the total number of clinician investigators reported in the survey.
Many of the programs reported lack of access to resources such as research assistants (56.1%) and dedicated research fellowships (53.7%) (Appendix Figure 2). A number of groups reported a need for more support for various junior faculty development activities, including research mentoring (53.5%), networking with other researchers (60.5%), and access to clinical data from multiple sites (62.8%).
One of the limitations of this survey was the manner in which the participating hospital medicine groups were chosen. Selection was based on groups affiliated with HOMERuN; among those chosen were highly visible US AMCs, including 70% of the top 20 AMCs based on National Institutes of Health (NIH) funding.3 Therefore, our results likely overestimate the research presence of hospital medicine across all AMCs in the United States.
LACK OF GROWTH OVER TIME: CONTEXTUALIZATION AND IMPLICATIONS
Despite the substantial growth of hospital medicine over the past 2 decades, there has been no proportional increase in the number of hospitalist clinician investigators, with earlier surveys also demonstrating low numbers.4,5 Along with the survey by Chopra and colleagues published in 2019,6 our survey provides an additional contemporary appraisal of research activities for adult academic hospital medicine groups. In the survey by Chopra et al, only 54% (15 of 28) of responding programs reported having any faculty with research as their major activity (ie, >50% effort), and 3% of total faculty reported having funding for >50% effort toward research.6 Our study expands upon these findings by providing more detailed data on the number of clinician investigators per hospital medicine group. Results of our survey showed a concentration of hospitalists within a small number of programs, which may have contributed to the observed lack of growth. We also expand on prior work by identifying a lack of resources and services to support hospitalist researchers.
The findings of our survey have important implications for the field of hospital medicine. Without a critical mass of hospitalist clinician investigators, the quality of research that addresses important questions in our field will suffer. It will also limit academic credibility of the field, as well as individual academic achievement; previous studies have consistently demonstrated that few hospitalists at AMCs achieve the rank of associate or full professor.5-9
POTENTIAL EXPLANATIONS FOR LACK OF RESEARCH GROWTH
The results of our study additionally offer possible explanations for the dearth of clinician investigators in hospital medicine. The limited access to research resources and fellowship training identified in our survey are critical domains that must be addressed in order to develop successful academic hospital medicine programs.4,6,8,10
Regarding dedicated hospital medicine research fellowships, there are only a handful across the country. The small number of existing research fellowships only have one or two fellows per year, and these positions often go unfilled because of a lack of applicants and lower salaries compared to full-time clinical positions.11 The lack of applicants for adult hospital medicine fellowship positions is also integrally linked to board certification requirements. Unlike pediatric hospital medicine where additional fellowship training is required to become board-certified, no such fellowship is required in adult hospital medicine. In pediatrics, this requirement has led to a rapid increase in the number of fellowships with scholarly work requirements (more than 60 fellowships, plus additional programs in development) and greater standardization among training experiences.12,13
The lack of fellowship applicants may also stem from the fact that many trainees are not aware of a potential career as a hospitalist clinician investigator due to limited exposure to this career at most AMCs. Our results revealed that nearly half of sites in our survey had zero clinician investigators, depriving trainees at these programs of role models and thus perpetuating a negative feedback loop. Lastly, although unfilled fellowship positions may indicate that demand is a larger problem than supply, it is also true that fellowship programs generate their own demand through recruitment efforts and the gradual establishment of a positive reputation.
Another potential explanation could relate to the development of hospital medicine in response to rising clinical demands at hospitals: compared with other medical specialties, AMCs may regard hospitalists as being clinicians first and academicians second.1,7,10 Also, hospitalists may be perceived as being beholden to hospitals and less engaged with their surrounding communities than other general medicine fields. With a small footprint in health equity research, academic hospital medicine may be less of a draw to generalists interested in pursuing this area of research. Further, there are very few underrepresented in medicine (URiM) hospital medicine research faculty.5
Another challenge to the career development of hospitalist researchers is the lack of available funding for the type of research typically conducted by hospitalists (eg, rigorous quality improvement implementation and evaluation, optimizing best evidence-based care delivery models, evaluation of patient safety in the hospital setting). As hospitalists tend to be system-level thinkers, this lack of funding may steer potential researchers away from externally funded research careers and into hospital operations and quality improvement positions. Also, unlike other medical specialties, there is no dedicated NIH funding source for hospital medicine research (eg, cardiology and the National Heart, Lung, and Blood Institute), placing hospitalists at a disadvantage in seeking funding compared to subspecialists.
STRATEGIES TO ENHANCE RESEARCH PRESENCE
We recommend several approaches—ones that should be pursued simultaneously—to increase the number of clinician investigators in hospital medicine. First, hospital medicine groups and their respective divisions, departments, and hospitals should allocate funding to support research resources; this includes investing in research assistants, data analysts, statisticians, and administrative support. Through the funding of such research infrastructure programs, AMCs could incentivize hospitalists to research best approaches to improve the value of healthcare delivery, ultimately leading to cost savings.
With 60% of respondents identifying the need for improved access to data across multiple sites, our survey also emphasizes the requirement for further collaboration among hospital medicine groups. Such collaboration could lead to high-powered observational studies and the evaluation of interventions across multiple sites, thus improving the generalizability of study findings.
The Society of Hospital Medicine (SHM) and its research committee can continue to expand the research footprint of hospital medicine. To date, the committee has achieved this by highlighting hospitalist research activity at the SHM Annual Conference Scientific Abstract and Poster Competition and developing a visiting professorship exchange program. In addition to these efforts, SHM could foster collaboration and networking between institutions, as well as take advantage of the current political push for expanded Medicare access by lobbying for robust funding for the Agency for Healthcare Research and Quality, which could provide more opportunities for hospitalists to study the effects of healthcare policy reform on the delivery of inpatient care.
Another strategy to increase the number of hospitalist clinician investigators is to expand hospital medicine research fellowships and recruit trainees for these programs. Fellowships could be internally funded wherein a fellow’s clinical productivity is used to offset the costs associated with obtaining advanced degrees. As an incentive to encourage applicants to temporarily forego a full-time clinical salary during fellowship, hospital medicine groups could offer expanded moonlighting opportunities and contribute to repayment of medical school loans. Hospital medicine groups should also advocate for NIH-funded T32 or K12 training grants for hospital medicine. (There are, however, challenges with this approach because the number of T32 spots per NIH institute is usually fixed). The success of academic emergency medicine offers a precedent for such efforts: After the development of a K12 research training program in emergency medicine, the number of NIH-sponsored principal investigators in this specialty increased by 40% in 6 years.14 Additionally, now that fellowships are required for the pediatric hospital medicine clinician investigators, it would be revealing to track the growth of this workforce.12,13
Structured and formalized mentorship is an essential part of the development of clinician investigators in hospital medicine.4,7,8,10 One successful strategy for mentorship has been the partnering of hospital medicine groups with faculty of general internal medicine and other subspecialty divisions with robust research programs.7,8,15 In addition to developing sustainable mentorship programs, hospital medicine researchers must increase their visibility to trainees. Therefore, it is essential that the majority of academic hospital medicine groups not only hire clinician investigators but also invest in their development, rather than rely on the few programs that have several such faculty members. With this strategy, we could dramatically increase the number of hospitalist clinician investigators from a diverse background of training institutions.
SHM could also play a greater role in organizing events for networking and mentoring for trainees and medical students interested in pursuing a career in hospital medicine research. It is also critically important that hospital medicine groups actively recruit, retain, and develop URiM hospital medicine research faculty in order to attract talented researchers and actively participate in the necessary effort to mitigate the inequities prevalent throughout our healthcare system.
CONCLUSION
Despite the growth of hospital medicine over the past decade, there remains a dearth of hospitalist clinician investigators at major AMCs in the United States. This may be due in part to lack of research resources and mentorship within hospital medicine groups. We believe that investment in these resources, expanded funding opportunities, mentorship development, research fellowship programs, and greater exposure of trainees to hospitalist researchers are solutions that should be strongly considered to develop hospitalist clinician investigators.
Acknowledgments
The authors thank HOMERuN executive committee members, including Grant Fletcher, MD, James Harrison, PhD, BSC, MPH, Peter K. Lindenauer, MD, Melissa Mattison, MD, David Meltzer, MD, PhD, Joshua Metlay, MD, PhD, Jennifer Myers, MD, Sumant Ranji, MD, Gregory Ruhnke, MD, MPH, Edmondo Robinson, MD, MBA, and Neil Sehgal, MPH PhD, for their assistance in developing the survey. They also thank Tiffany Lee, MA, for her project management assistance for HOMERuN.
In their report celebrating the increase in the number of hospitalists from a few hundred in the 1990s to more than 50,000 in 2016, Drs Robert Wachter and Lee Goldman also noted the stunted growth of productive hospital medicine research programs, which presents a challenge to academic credibility in hospital medicine.1 Given the substantial increase in the number of hospitalists over the past two decades, we surveyed adult academic hospital medicine groups to quantify the number of hospitalist clinician investigators and identify gaps in resources for researchers. The number of clinician investigators supported at academic medical centers (AMCs) remains disturbingly low despite the rapid growth of our specialty. Some programs also reported a lack of access to fundamental research services. We report selected results from our survey and provide recommendations to support and facilitate the development of clinician investigators in hospital medicine.
DEARTH OF CLINICIAN INVESTIGATORS IN HOSPITAL MEDICINE
We performed a survey of hospital medicine programs at AMCs in the United States through the Hospital Medicine Reengineering Network (HOMERuN), a hospital medicine research collaborative that facilitates and conducts multisite research studies.2 The purpose of this survey was to obtain a profile of adult academic hospital medicine groups. Surveys were distributed via email to directors and/or senior leaders of each hospital medicine group between January and August 2019. In the survey, a clinician investigator was defined as “faculty whose primary nonclinical focus is scientific papers and grant writing.”
We received responses from 43 of the 86 invitees (50%), each of whom represented a unique hospital medicine group; 41 of the representatives responded to the questions concerning available research services. Collectively, these 43 programs represented 2,503 hospitalists. There were 79 clinician investigators reported among all surveyed hospital medicine groups (3.1% of all hospitalists). The median number of clinician investigators per hospital medicine group was 0 (range 0-12) (Appendix Figure 1), and 22 of 43 (51.2%) hospital medicine groups reported having no clinician investigators. Two of the hospital medicine groups, however, reported having 12 clinician investigators at their respective institutions, comprising nearly one third of the total number of clinician investigators reported in the survey.
Many of the programs reported lack of access to resources such as research assistants (56.1%) and dedicated research fellowships (53.7%) (Appendix Figure 2). A number of groups reported a need for more support for various junior faculty development activities, including research mentoring (53.5%), networking with other researchers (60.5%), and access to clinical data from multiple sites (62.8%).
One of the limitations of this survey was the manner in which the participating hospital medicine groups were chosen. Selection was based on groups affiliated with HOMERuN; among those chosen were highly visible US AMCs, including 70% of the top 20 AMCs based on National Institutes of Health (NIH) funding.3 Therefore, our results likely overestimate the research presence of hospital medicine across all AMCs in the United States.
LACK OF GROWTH OVER TIME: CONTEXTUALIZATION AND IMPLICATIONS
Despite the substantial growth of hospital medicine over the past 2 decades, there has been no proportional increase in the number of hospitalist clinician investigators, with earlier surveys also demonstrating low numbers.4,5 Along with the survey by Chopra and colleagues published in 2019,6 our survey provides an additional contemporary appraisal of research activities for adult academic hospital medicine groups. In the survey by Chopra et al, only 54% (15 of 28) of responding programs reported having any faculty with research as their major activity (ie, >50% effort), and 3% of total faculty reported having funding for >50% effort toward research.6 Our study expands upon these findings by providing more detailed data on the number of clinician investigators per hospital medicine group. Results of our survey showed a concentration of hospitalists within a small number of programs, which may have contributed to the observed lack of growth. We also expand on prior work by identifying a lack of resources and services to support hospitalist researchers.
The findings of our survey have important implications for the field of hospital medicine. Without a critical mass of hospitalist clinician investigators, the quality of research that addresses important questions in our field will suffer. It will also limit academic credibility of the field, as well as individual academic achievement; previous studies have consistently demonstrated that few hospitalists at AMCs achieve the rank of associate or full professor.5-9
POTENTIAL EXPLANATIONS FOR LACK OF RESEARCH GROWTH
The results of our study additionally offer possible explanations for the dearth of clinician investigators in hospital medicine. The limited access to research resources and fellowship training identified in our survey are critical domains that must be addressed in order to develop successful academic hospital medicine programs.4,6,8,10
Regarding dedicated hospital medicine research fellowships, there are only a handful across the country. The small number of existing research fellowships only have one or two fellows per year, and these positions often go unfilled because of a lack of applicants and lower salaries compared to full-time clinical positions.11 The lack of applicants for adult hospital medicine fellowship positions is also integrally linked to board certification requirements. Unlike pediatric hospital medicine where additional fellowship training is required to become board-certified, no such fellowship is required in adult hospital medicine. In pediatrics, this requirement has led to a rapid increase in the number of fellowships with scholarly work requirements (more than 60 fellowships, plus additional programs in development) and greater standardization among training experiences.12,13
The lack of fellowship applicants may also stem from the fact that many trainees are not aware of a potential career as a hospitalist clinician investigator due to limited exposure to this career at most AMCs. Our results revealed that nearly half of sites in our survey had zero clinician investigators, depriving trainees at these programs of role models and thus perpetuating a negative feedback loop. Lastly, although unfilled fellowship positions may indicate that demand is a larger problem than supply, it is also true that fellowship programs generate their own demand through recruitment efforts and the gradual establishment of a positive reputation.
Another potential explanation could relate to the development of hospital medicine in response to rising clinical demands at hospitals: compared with other medical specialties, AMCs may regard hospitalists as being clinicians first and academicians second.1,7,10 Also, hospitalists may be perceived as being beholden to hospitals and less engaged with their surrounding communities than other general medicine fields. With a small footprint in health equity research, academic hospital medicine may be less of a draw to generalists interested in pursuing this area of research. Further, there are very few underrepresented in medicine (URiM) hospital medicine research faculty.5
Another challenge to the career development of hospitalist researchers is the lack of available funding for the type of research typically conducted by hospitalists (eg, rigorous quality improvement implementation and evaluation, optimizing best evidence-based care delivery models, evaluation of patient safety in the hospital setting). As hospitalists tend to be system-level thinkers, this lack of funding may steer potential researchers away from externally funded research careers and into hospital operations and quality improvement positions. Also, unlike other medical specialties, there is no dedicated NIH funding source for hospital medicine research (eg, cardiology and the National Heart, Lung, and Blood Institute), placing hospitalists at a disadvantage in seeking funding compared to subspecialists.
STRATEGIES TO ENHANCE RESEARCH PRESENCE
We recommend several approaches—ones that should be pursued simultaneously—to increase the number of clinician investigators in hospital medicine. First, hospital medicine groups and their respective divisions, departments, and hospitals should allocate funding to support research resources; this includes investing in research assistants, data analysts, statisticians, and administrative support. Through the funding of such research infrastructure programs, AMCs could incentivize hospitalists to research best approaches to improve the value of healthcare delivery, ultimately leading to cost savings.
With 60% of respondents identifying the need for improved access to data across multiple sites, our survey also emphasizes the requirement for further collaboration among hospital medicine groups. Such collaboration could lead to high-powered observational studies and the evaluation of interventions across multiple sites, thus improving the generalizability of study findings.
The Society of Hospital Medicine (SHM) and its research committee can continue to expand the research footprint of hospital medicine. To date, the committee has achieved this by highlighting hospitalist research activity at the SHM Annual Conference Scientific Abstract and Poster Competition and developing a visiting professorship exchange program. In addition to these efforts, SHM could foster collaboration and networking between institutions, as well as take advantage of the current political push for expanded Medicare access by lobbying for robust funding for the Agency for Healthcare Research and Quality, which could provide more opportunities for hospitalists to study the effects of healthcare policy reform on the delivery of inpatient care.
Another strategy to increase the number of hospitalist clinician investigators is to expand hospital medicine research fellowships and recruit trainees for these programs. Fellowships could be internally funded wherein a fellow’s clinical productivity is used to offset the costs associated with obtaining advanced degrees. As an incentive to encourage applicants to temporarily forego a full-time clinical salary during fellowship, hospital medicine groups could offer expanded moonlighting opportunities and contribute to repayment of medical school loans. Hospital medicine groups should also advocate for NIH-funded T32 or K12 training grants for hospital medicine. (There are, however, challenges with this approach because the number of T32 spots per NIH institute is usually fixed). The success of academic emergency medicine offers a precedent for such efforts: After the development of a K12 research training program in emergency medicine, the number of NIH-sponsored principal investigators in this specialty increased by 40% in 6 years.14 Additionally, now that fellowships are required for the pediatric hospital medicine clinician investigators, it would be revealing to track the growth of this workforce.12,13
Structured and formalized mentorship is an essential part of the development of clinician investigators in hospital medicine.4,7,8,10 One successful strategy for mentorship has been the partnering of hospital medicine groups with faculty of general internal medicine and other subspecialty divisions with robust research programs.7,8,15 In addition to developing sustainable mentorship programs, hospital medicine researchers must increase their visibility to trainees. Therefore, it is essential that the majority of academic hospital medicine groups not only hire clinician investigators but also invest in their development, rather than rely on the few programs that have several such faculty members. With this strategy, we could dramatically increase the number of hospitalist clinician investigators from a diverse background of training institutions.
SHM could also play a greater role in organizing events for networking and mentoring for trainees and medical students interested in pursuing a career in hospital medicine research. It is also critically important that hospital medicine groups actively recruit, retain, and develop URiM hospital medicine research faculty in order to attract talented researchers and actively participate in the necessary effort to mitigate the inequities prevalent throughout our healthcare system.
CONCLUSION
Despite the growth of hospital medicine over the past decade, there remains a dearth of hospitalist clinician investigators at major AMCs in the United States. This may be due in part to lack of research resources and mentorship within hospital medicine groups. We believe that investment in these resources, expanded funding opportunities, mentorship development, research fellowship programs, and greater exposure of trainees to hospitalist researchers are solutions that should be strongly considered to develop hospitalist clinician investigators.
Acknowledgments
The authors thank HOMERuN executive committee members, including Grant Fletcher, MD, James Harrison, PhD, BSC, MPH, Peter K. Lindenauer, MD, Melissa Mattison, MD, David Meltzer, MD, PhD, Joshua Metlay, MD, PhD, Jennifer Myers, MD, Sumant Ranji, MD, Gregory Ruhnke, MD, MPH, Edmondo Robinson, MD, MBA, and Neil Sehgal, MPH PhD, for their assistance in developing the survey. They also thank Tiffany Lee, MA, for her project management assistance for HOMERuN.
1. Wachter RM, Goldman L. Zero to 50,000 – The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
3. Roskoski R Jr, Parslow TG. Ranking Tables of NIH funding to US medical schools in 2019. Blue Ridge Institute for Medical Research. Published 2020. Updated July 14, 2020. Accessed July 30, 2020. http://www.brimr.org/NIH_Awards/2019/NIH_Awards_2019.htm
4. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
5. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the society of general internal medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
6. Chopra V, Burden M, Jones CD, et al; Society of Hospital Medicine Research Committee. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
7. Seymann GB, Southern W, Burger A, et al. Features of successful academic hospitalist programs: insights from the SCHOLAR (SuCcessful HOspitaLists in academics and research) project. J Hosp Med. 2016;11(10):708-713. https://doi.org/10.1002/jhm.2603
8. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836
9. Dang Do AN, Munchhof AM, Terry C, Emmett T, Kara A. Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148-154. https://doi.org/10.1002/jhm.2148
10. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161-166. https://doi.org/10.1002/jhm.845
11. Ranji SR, Rosenman DJ, Amin AN, Kripalani S. Hospital medicine fellowships: works in progress. Am J Med. 2006;119(1):72.e1-72.e7. https://doi.org/10.1016/j.amjmed.2005.07.061
12. Shah NH, Rhim HJ, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: a survey of program directors. J Hosp Med. 2016;11(5):324-328. https://doi.org/10.1002/jhm.2571
13. Jerardi KE, Fisher E, Rassbach C, et al; Council of Pediatric Hospital Medicine Fellowship Directors. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698
14. Lewis RJ, Neumar RW. Research in emergency medicine: building the investigator pipeline. Ann Emerg Med. 2018;72(6):691-695. https://doi.org/10.1016/j.annemergmed.2018.10.019
15. Flanders SA, Kaufman SR, Nallamothu BK, Saint S. The University of Michigan Specialist-Hospitalist Allied Research Program: jumpstarting hospital medicine research. J Hosp Med. 2008;3(4):308-313. https://doi.org/10.1002/jhm.342
1. Wachter RM, Goldman L. Zero to 50,000 – The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
3. Roskoski R Jr, Parslow TG. Ranking Tables of NIH funding to US medical schools in 2019. Blue Ridge Institute for Medical Research. Published 2020. Updated July 14, 2020. Accessed July 30, 2020. http://www.brimr.org/NIH_Awards/2019/NIH_Awards_2019.htm
4. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
5. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the society of general internal medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
6. Chopra V, Burden M, Jones CD, et al; Society of Hospital Medicine Research Committee. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
7. Seymann GB, Southern W, Burger A, et al. Features of successful academic hospitalist programs: insights from the SCHOLAR (SuCcessful HOspitaLists in academics and research) project. J Hosp Med. 2016;11(10):708-713. https://doi.org/10.1002/jhm.2603
8. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836
9. Dang Do AN, Munchhof AM, Terry C, Emmett T, Kara A. Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148-154. https://doi.org/10.1002/jhm.2148
10. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161-166. https://doi.org/10.1002/jhm.845
11. Ranji SR, Rosenman DJ, Amin AN, Kripalani S. Hospital medicine fellowships: works in progress. Am J Med. 2006;119(1):72.e1-72.e7. https://doi.org/10.1016/j.amjmed.2005.07.061
12. Shah NH, Rhim HJ, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: a survey of program directors. J Hosp Med. 2016;11(5):324-328. https://doi.org/10.1002/jhm.2571
13. Jerardi KE, Fisher E, Rassbach C, et al; Council of Pediatric Hospital Medicine Fellowship Directors. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698
14. Lewis RJ, Neumar RW. Research in emergency medicine: building the investigator pipeline. Ann Emerg Med. 2018;72(6):691-695. https://doi.org/10.1016/j.annemergmed.2018.10.019
15. Flanders SA, Kaufman SR, Nallamothu BK, Saint S. The University of Michigan Specialist-Hospitalist Allied Research Program: jumpstarting hospital medicine research. J Hosp Med. 2008;3(4):308-313. https://doi.org/10.1002/jhm.342
© 2021 Society of Hospital Medicine
The Effects of a Multifaceted Intervention to Improve Care Transitions Within an Accountable Care Organization: Results of a Stepped-Wedge Cluster-Randomized Trial
The Effects of a Multifaceted Intervention to Improve Care Transitions Within an Accountable Care Organization: Results of a Stepped-Wedge Cluster-Randomized Trial

This work is licensed under a Creative Commons Attribution 4.0 International License
Transitions from the hospital to the ambulatory setting are high-risk periods for patients in terms of adverse events, poor clinical outcomes, and readmission. Processes of care during care transitions are suboptimal, including poor communication among inpatient providers, patients, and ambulatory providers1,2; suboptimal communication of postdischarge plans of care to patients and their ability to carry out these plans3; medication discrepancies and nonadherence after discharge4; and lack of timely follow-up with ambulatory providers.5 Healthcare organizations continue to struggle with the question of which interventions to implement and how best to implement them.
Interventions to improve care transitions typically focus on readmission rates, but some studies have focused on postdischarge adverse events, defined as injuries in the 30 days after discharge caused by medical management rather than underlying disease processes.2 These adverse events cause psychological distress, out-of-pocket expenses, decreases in functional status, and caregiver burden. An estimated 20% of hospitalized patients suffer a postdischarge adverse event.1,2 Approximately two-thirds of these may be preventable or ameliorable.
The advent of Accountable Care Organizations (ACOs), defined as “groups of doctors, hospitals, and other health care providers who come together voluntarily to give coordinated high quality care to their patients,” creates an opportunity for improvements in patient safety during care transitions.6 Another opportunity has been the advent of Patient-Centered Medical Homes (PCMH), consisting of patient-oriented, comprehensive, team-based primary care enhanced by health information technology and population-based disease management tools.7,8 In theory, a hospital-PCMH collaboration within an ACO can improve transitional interventions since optimal communication and collaboration are more likely when both inpatient and primary care providers (PCPs) share infrastructure and are similarly incentivized. The objectives of this study were to design and implement a collaborative hospital-PCMH care transitions intervention within an ACO and evaluate its effects.
METHODS
This study was a two-arm, single-blind (blinded outcomes assessor), stepped-wedge, multisite cluster-randomized clinical trial (NCT02130570) approved by the institutional review board of Partners HealthCare.
Study Design and Randomization
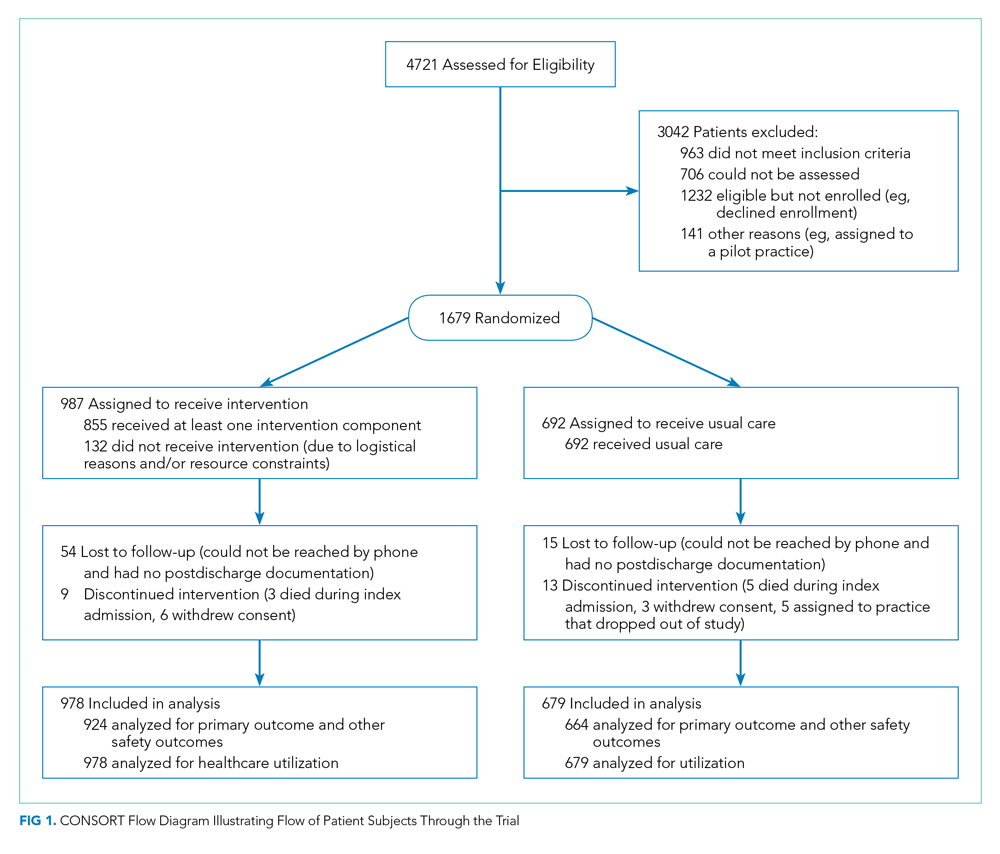
The study employed a “stepped-wedge” design, which is a cluster-randomized study design in which an intervention is sequentially rolled out to different groups at different, prespecified, randomly determined times.9 Each cluster (in this case, each primary care practice) served as its own control, while still allowing for adjustment for temporal trends. Originally, 18 practices participated, but one withdrew due to the low number of patients enrolled in the study, leaving 17 clusters and 16 sequences; see Figure 1 of Appendix 1 for a full description of the sample size and timeline for each cluster. Practices were not aware of this timeline until after recruitment.
Study Setting and Participants
Conducted within a large Pioneer ACO in Boston and funded by the Patient-Centered Outcomes Research Institute (PCORI), the Partners-PCORI Transitions Study was designed as a “real-world” quality improvement project. Potential participants were adult patients who were admitted to medical and surgical services of two large academic hospitals (Hospital A and Hospital B) affiliated with an ACO, who were likely to be discharged back to the community, and whose PCP belonged to a primary care practice that was affiliated with the ACO, agreed to participate, and were designated PCMHs or on their way to being designated by meeting certain criteria: electronic health record, patient portal, team-based care, practice redesign, care management, and identification of high-risk patients. See Study Protocol (Appendix 2) for detailed patient and primary care practice inclusion criteria.
Patient Enrollment
Study staff screened participants from a daily automated list of patients admitted the day before, using medical records to determine eligibility, which was then confirmed by the patient’s nurse. Exclusion criteria included likely discharge to a location other than home, being in police custody, lack of a telephone, being homeless, previous enrollment in the study, and being unable to communicate in English or Spanish. Allocation to study arm was concealed until the patient or proxy provided informed written consent. The research assistant administered questionnaires to all study subjects to assess potential confounders and functional status 1 month prior to admission (
Intervention
The intervention was based on a conceptual model of an ideal discharge11 that we developed based on work by Naylor et al,12 work by Coleman and Berenson,3 best practices in medication reconciliation and information transfer according to our own research,13-15 the best examples of interventions to improve the discharge process,12,16,17 and a systematic review of discharge interventions.18 Some of the factors necessary for an ideal care transition include complete, organized, and timely documentation of the patient’s hospital course and postdischarge plan; effective discharge planning; coordination of care among the patient’s providers; methods to ensure medication safety; advanced care planning in appropriate patients; and education and “coaching” of patients and their caregivers so they learn how to manage their conditions. The final multifaceted intervention addressed each component of the ideal discharge and included inpatient and outpatient components (Table 1 and Table 1 of Appendix 1).

Patient and Public Involvement in Research
As with all PCORI-funded studies, this study involved a patient-family advisory council (PFAC). Our PFAC included six recently hospitalized patients or caregivers of recently hospitalized patients. The PFAC participated in monthly meetings throughout the study period. They helped inform the research questions, including confirmation that the endpoints were patient centered, and provided valuable input for the design of the intervention and the patient-facing components of the data collection instruments. They also interviewed several patient participants in the study regarding their experiences with the intervention. Lastly, they helped develop plans for dissemination of study results to the public.19
We also formed a steering committee consisting of physician, nursing, pharmacy, information technology, and administrative leadership representing primary care, inpatient care, and transitional care at both hospitals and Partners Healthcare. PFAC members took turns participating in quarterly steering committee meetings.
Evolution of the Intervention and Implementation
The intervention was iteratively refined during the course of the study in response to input from the PFAC, steering committee, and members of the intervention team; cases of adverse events and readmissions from patients despite being in the intervention arm; exit interviews of patients who had recently completed the intervention; and informal feedback from inpatient and outpatient clinicians. For example, we learned that the more complicated a patient’s conditions are, the sooner the clinical team wanted them to be seen after discharge. However, these patients were also less likely to feel well enough to keep that appointment. Therefore, the timing of follow-up of appointments needed to be a negotiation among the inpatient team, the patient, any caregivers, and the outpatient provider. PFAC members also emphasized that patients wanted one person to trust and to be the “point person” during a complicated transition such as hospital discharge.
At the same time, the intervention components evolved because of factors outside our control (eg, resource limitations). In keeping with the real-world nature of the research, the aim was for the intervention to be internally supported because incentives were theoretically more aligned with improvement of care transitions under the ACO model. By design, the PCORI contract only paid for limited parts of the intervention, such as a nurse practitioner to act as the discharge advocate at one hospital, overtime costs of inpatient pharmacists, and project manager time to facilitate inpatient-outpatient provider communication. (See Table 1 of Appendix 1 for details about the modifications to the intervention.)
Lastly, in keeping with PCORI’s methodology standards for studies of complex interventions,20 we strove to standardize the intervention by function across hospitals, units, and practices, while still allowing for local adaptation in the form. In other words, rather than specifying exactly how a task (eg, medication counseling) needed to be performed, the study design offered sites flexibility in how they implemented the task given their available personnel and institutional culture.
Intervention Fidelity
To determine the extent to which each patient in the intervention arm received each intervention component, a project manager unblinded to treatment arm reviewed the electronic medical record for documentation of each component implemented by providers (eg, inpatient pharmacists, outpatient nurses). Because each intervention component produced documentation, this provided an accurate assessment of intervention fidelity, ie, the extent to which the intervention was implemented as intended.
Outcome Assessment
Postdischarge Follow-up
Based on previous studies,2,21 a trained research assistant attempted to contact all study subjects 30 days (±5 days) after discharge and administered a questionnaire to identify any new or worsening symptoms since discharge, any healthcare use since discharge, and functional status in the previous week. Follow-up questions used branching logic to determine the relationship of any new or worsening symptoms to medications or other aspects of medical management. Research assistants followed up any positive responses with directed medical record review for objective findings, diagnoses, treatments, and responses. If patients could not be reached after five attempts, the research assistant instead conducted a thorough review of the outpatient medical record alone for provider reports of any new or worsening symptoms noted during follow-up within the 30-day postdischarge period. Research assistants also reviewed laboratory test results in all patients for evidence of postdischarge renal failure, elevated liver function tests, or new/worsening anemia.
Hospital Readmissions
We measured nonelective hospital readmissions within 30 days of discharge using a combination of administrative data for hospitalizations within the ACO network plus patient report during the 30-day phone call for all other readmissions.22
Adjudication of Outcomes
Adverse events and preventable adverse events: All cases of new or worsening symptoms or signs, along with all supporting documentation, were then presented to teams of two trained blinded physician adjudicators through application of methods established in previous studies.4,21 Each of the two adjudicators independently reviewed the information, along with the medical record, and completed a standardized form to confirm or deny the presence of any adverse events (ie, patient injury due to medical management) and to classify the type of event (eg, adverse drug event, hospital-acquired infection, procedural complication, diagnostic or management error), the severity and duration of the event, and whether the event was preventable or ameliorable. The two adjudicators then met to resolve any differences in their findings and come to consensus.
Preventable readmissions: If patients were readmitted to either study hospital, we conducted an evaluation, based on previous studies,23 to determine if and how the readmission could have been prevented including (a) a standardized patient and caregiver interview to identify possible problems with the transitions process and (b) an email questionnaire to the patient’s PCP and the inpatient teams who cared for the patient during the index admission and readmission regarding possible deficiencies with the transitions process. As with adverse event adjudications, two physician adjudicators worked independently to classify the preventability of the readmission and then met to come to consensus. Conflicts were resolved by a third adjudicator.
Analysis Plan
To evaluate the effects of the intervention on the primary outcome, the number of postdischarge adverse events per patient, we used multivariable Poisson regression, with study arm as the main predictor. A similar approach was used to evaluate the number of new or worsening postdischarge signs or symptoms and the number of preventable adverse events per patient. We used an intention-to-treat analysis: If a practice did not start the intervention when they were scheduled to, based on our randomization, we counted all patients in that practice admitted after that point as intervention patients. We adjusted for patient demographics, clinical characteristics, month, inpatient unit, and primary care practice as fixed effects. We clustered by PCP using general linear models. Intervention effects were expressed as both unadjusted and adjusted incidence rate ratios (IRRs). We also conducted a limited number of subgroup analyses, determined a priori, to determine whether the intervention was more effective in certain patient populations; we used interaction terms (intervention × subgroup) to determine the statistical significance of any effect modification.
To evaluate the effects of the intervention on nonelective readmissions and preventable readmissions, we used a similar approach, using multivariable logistic regression. Postdischarge functional status, adjusted for status prior to admission, was analyzed using multivariable linear regression and random effects by primary care practice. The general linear mixed model (GLIMMIX) procedure in the SAS 9.3 statistical package (SAS Institute) was used to carry out all analyses.
Power and Sample Size
We assumed a baseline rate of postdischarge adverse events of 0.30 per patient.21 We conservatively assumed an effect size of a change from 0.30 in the control group to 0.23 in the intervention group (a relative reduction of 22%, which was based on studies of preventability rates23 and close to the minimum clinically important difference). Based on prior studies,4,22 we assumed an intraclass correlation coefficient of 0.01 with an average cluster size of seven patients per PCP. Assuming a 10% loss to follow-up rate and an alpha of 0.05, we targeted a sample size of 1,800 patients to achieve 80% power, with one-third of the patients in the usual care arm and two-thirds in the intervention arm.
RESULTS
We enrolled 18 PCMH primary care practices to participate in the study, including 8 from Hospital A (out of 13 approached), 8 from Hospital B (out of 11), and 2 from other ACO practices (out of 9) (plus two pilot practices). Reasons for not participating included not having dedicated personnel to play the role of the responsible outpatient clinician, undergoing recent turn-over in practice leadership, and not having enough patients admitted to the two hospitals. One practice only enrolled 5 patients in the study and withdrew from participation, which left 17 practices.
Study Patients
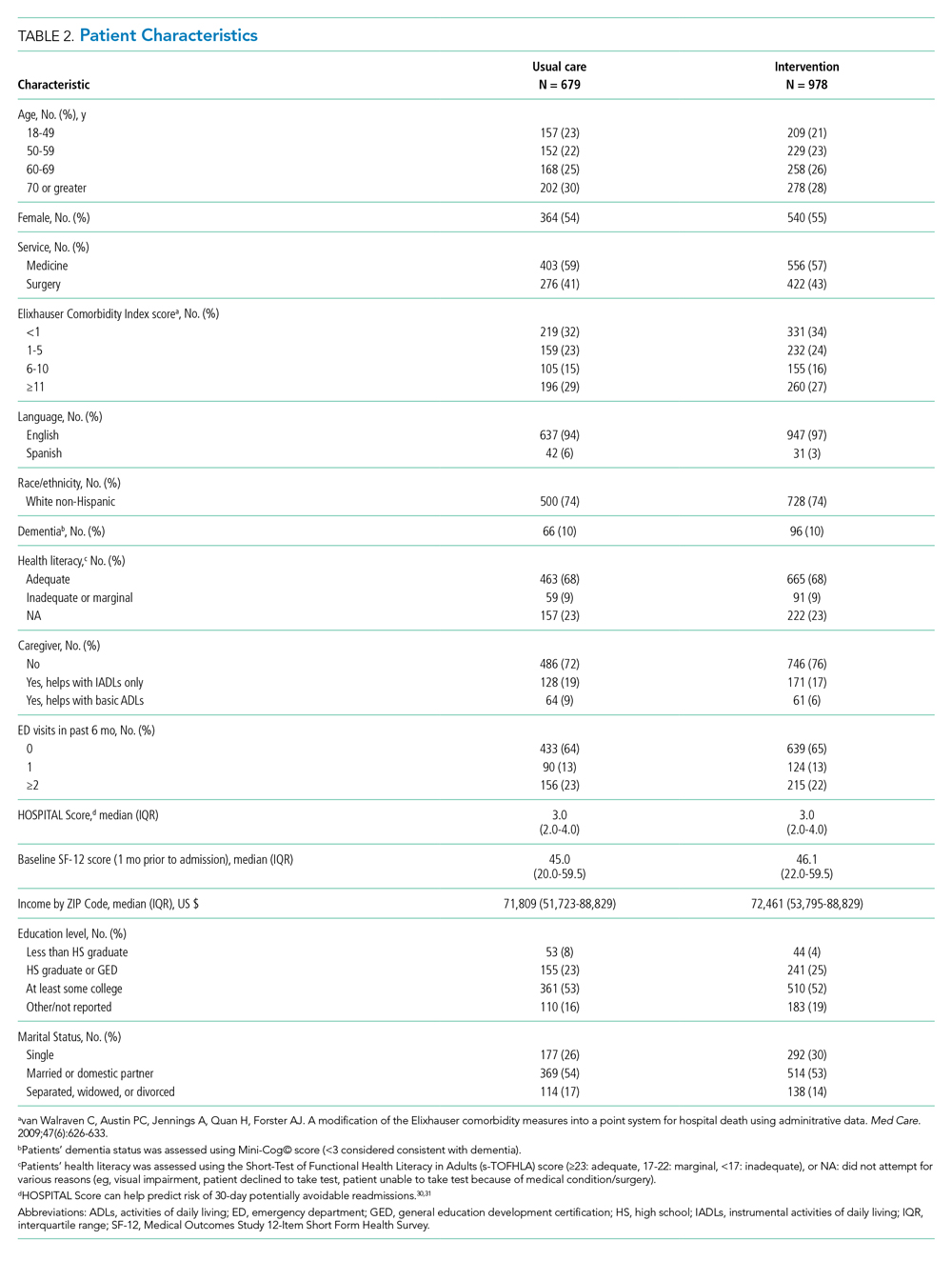
We enrolled 1,679 patients (Figure 1). Reasons for nonenrollment included being unable to complete the screen prior to discharge, not meeting inclusion criteria or meeting exclusion criteria, being assigned to a pilot practice, and declining informed written consent. The baseline characteristics of enrolled patients are presented in Table 2. Differences between the two study arms were small. About 47% of the cohort was not reachable by phone after five attempts for the 30-day phone call, but only 69 (4.1%) were truly lost to follow-up because they were unreachable by phone and had no documentation in the electronic medical record in the 30-days after discharge.
Intervention Fidelity
The majority of patients did not receive most intervention components, even those components that were supposed to be delivered to all intervention patients (Table 3). A minority of patients were referred to visiting nurse services and to the home pharmacy program. However, 855 patients (87%) in the intervention arm received at least one intervention component.

Outcome Measures
The intervention was associated with a statistically significant reduction in several of the outcomes of interest, including the primary outcome, number of postdischarge adverse events (45% reduction), and new or worsening postdischarge signs or symptoms (22% reduction), as well as preventable postdischarge adverse events (58% reduction) (Table 4). There was a nonsignificant difference in functional status. There was no significant effect on total nonelective or on preventable readmission rates. When analyzed by type of adverse event, the intervention was associated with a reduction in adverse drug events and in procedural complications (Table 2 of Appendix 1). Of note, there was no significant difference in the proportion of patients with at least one adverse event whether the outcome was determined by phone call and medical record review (49%) or medical record review alone (51%) (P = .48).
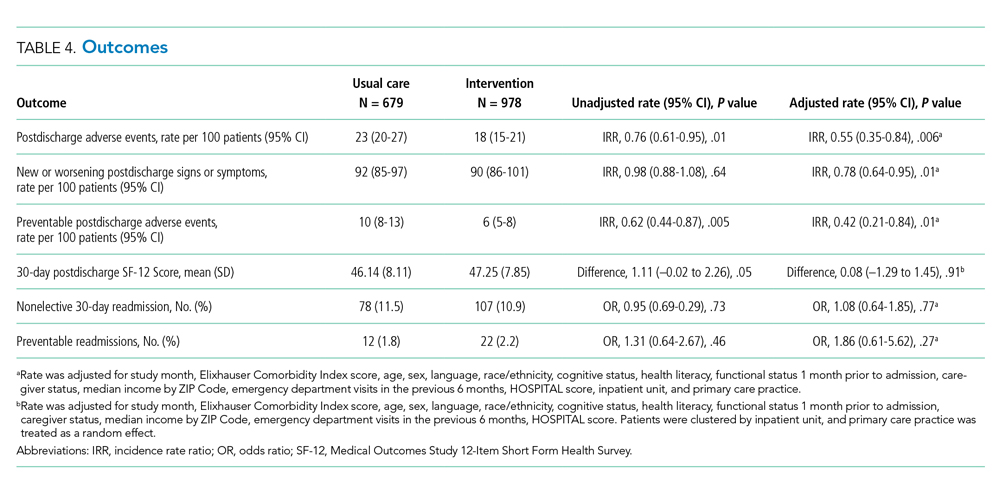
In subgroup analyses, there was no evidence of effect modification by service, hospital, patient age, readmission risk, health literacy, or comorbidity score (Table 3 of Appendix 1). Table 4 of Appendix 1 provides examples of postdischarge adverse events seen in the usual care arm that might have been prevented in the intervention.
DISCUSSION
This intervention was associated with a reduction in postdischarge adverse events. The relative improvement in each outcome aligned with the hypothesized sensitivity to change: the smallest improvement was seen in new or worsening signs or symptoms, followed by postdischarge adverse events and then by preventable postdischarge adverse events. The intervention was not associated with a difference in readmissions. The lack of effect on hospital readmissions may have been caused by the low proportion of readmissions that are preventable, as well as low intervention fidelity and lack of resources to implement facets such as postdischarge coaching, an evidence-based intervention that was never adopted.16,23 One lesson of this study is that it may be easier to reduce postdischarge injury (still an important outcome) than readmissions.
Putting this study in context, we should note that the literature on interventions to improve care transitions is mixed.18 While there are several reports of successful interventions, there are many reports of unsuccessful ones, often using similar components. Success is often the result of adequate resources and attention to myriad details regarding implementation.24 The intervention in our study likely contributed to improvements in patient and caregiver engagement in the hospital, enhancements of communication between inpatient and outpatient clinicians, and implementation of pharmacist-led interventions to improve medication safety. Regarding the latter, several prior studies have shown the benefits of pharmacist interventions in decreasing postdischarge adverse drug events.4,25,26 Therefore, even an intervention with incomplete intervention fidelity can reduce postdischarge adverse events, especially because adverse drug events make up the majority of adverse events.1,2,21
Perhaps the biggest lesson we learned was regarding the limitations of the hospital-led ACO model to incentivize sufficient up-front investments in transitional care interventions. By design, we chose a real-world approach in which interventions were integrated with existing ACO efforts, which were paid for internally by the institution. As a result, many of the interventions had to be scaled back because of resource constraints. The ACO model theoretically incentivizes more integrated care, but this may not always be true in practice. Emerging evidence suggests that physician group–led ACOs are associated with lower costs and use compared with hospital-led ACOs, likely because of more aligned incentives in physician group–led ACOs to reduce use of inpatient care.27,28
An unresolved question is whether the ideal implementation approach is to protect the time of existing clinical personnel to carry out transitional care tasks or to hire external personnel to do these tasks. We purposely spread the intervention over several clinician types to minimize the additional burden on any one of them, minimize additional costs, and play to each clinician’s expertise, but in retrospect, this may not have been the right approach. By providing additional personnel with dedicated time, interest, and training in care transitions, the intervention may be delivered with higher quantitative and qualitative fidelity, and it could create a single point of contact for patients, which was considered highly desirable by our PFAC.
This study has several limitations. A large proportion of patients (44%) were unavailable for postdischarge phone calls. However, we were able to perform medical record review for worsening signs (eg, lab abnormalities) and symptoms (as reported by patients’ providers) in the postdischarge period and adjudicate them for adverse events for all but 69 of these patients. Because all these patients had ACO-affiliated PCPs, we would expect most of their utilization to have been within the system and, therefore, to be present in the medical record. The proportion of patients with at least one adverse event did not vary by the method of follow-up, which suggests that this issue is an unlikely source of bias. Assessment of readmission was imperfect because we do not have statewide or national data. However, our combination of administrative data for Partners readmissions plus self-report for non-Partners readmission has been shown to be fairly complete in previous studies.29 Adjudicators could not be fully blinded to intervention status due to the lack of blinding of admission date. We did not calculate a kappa value for interrater reliability of individual assessments of adverse events; rather, coming to consensus among the two adjudicators was part of the process. In only a handful of cases was a third adjudicator required. Lastly, this study was conducted at two academic medical centers and their affiliated primary care clinics, which potentially limits generalizability; however, the results are likely generalizable to other ACOs that include major academic medical centers.
CONCLUSION
In conclusion, in this real-world clinical trial, we designed, implemented, and iteratively refined a multifaceted intervention to improve care transitions within a hospital-based academic ACO. Evolution of the intervention components was the result of stakeholder input, experience with the intervention, and ACO resource constraints. The intervention reduced postdischarge adverse events. However, across the ACO network, intervention fidelity was low, and this may have contributed to the lack of effect on readmission rates. ACOs that implement interventions without hiring new personnel or protecting the time of existing personnel to conduct transitional tasks are likely to face the same challenges of low fidelity.
Acknowledgments
The authors would like to acknowledge the many people who worked on designing, implementing, and evaluating this intervention, including but not limited to: Natasha Isaac, Hilary Heyison, Jacqueline Minahan, Molly O’Reilly, Michelle Potter, Nailah Khoory, Maureen Fagan, David Bates, Laura Carr, Joseph Frolkis, Eric Weil, Jacqueline Somerville, Stephanie Ahmed, Marcy Bergeron, Jessica Smith, and Jane Millett. We would also like to thank the members of our Patient-Family Advisory Council: Maureen Fagan, Karen Spikes, Margie Hodges, Win Hodges, Aureldon Henderson, Dena Salzberg, and Kay Bander.
1. Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345-349.
2. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. https://doi.org/10.7326/0003-4819-138-3-200302040-00007
3. Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141(7):533-536. https://doi.org/10.7326/0003-4819-141-7-200410050-00009
4. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565-571. https://doi.org/10.1001/archinte.166.5.565
5. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/nejmsa0803563
6. Accountable Care Organizations (ACOs). Centers for Medicare & Medicaid Services. 2012. Updated February 11, 2020. Accessed July 15, 2012. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ACO/index.html?redirect=/ACO/
7. Bates DW, Bitton A. The future of health information technology in the patient-centered medical home. Health Aff (Millwood). 2010;29(4):614-621. https://doi.org/10.1377/hlthaff.2010.0007
8. Bitton A, Martin C, Landon BE. A nationwide survey of patient centered medical home demonstration projects. J Gen Intern Med. 2010;25(6):584-592. https://doi.org/10.1007/s11606-010-1262-8
9. Brown C, Lilford R. Evaluating service delivery interventions to enhance patient safety. BMJ. 2008;337:a2764. https://doi.org/10.1136/bmj.a2764
10. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. https://doi.org/10.1097/00005650-199603000-00003
11. Burke RE, Kripalani S, Vasilevskis EE, Schnipper JL. Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102-109. https://doi.org/10.1002/jhm.1990
12. Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613-620. https://doi.org/10.1001/jama.281.7.613
13. Gandara E, Ungar J, Lee J, Chan-Macrae M, O’Malley T, Schnipper JL. Discharge documentation of patients discharged to subacute facilities: a three-year quality improvement process across an integrated health care system. Jt Comm J Qual Patient Saf. 2010;36(6):243-251. https://doi.org/10.1016/s1553-7250(10)36039-9
14. Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414-1422. https://doi.org/10.1007/s11606-008-0687-9
15. Schnipper JL, Hamann C, Ndumele CD, et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster-randomized trial. Arch Intern Med. 2009;169(8):771-780. https://doi.org/10.1001/archinternmed.2009.51
16. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. https://doi.org/10.1001/archinte.166.17.1822
17. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178-187. https://doi.org/10.7326/0003-4819-150-3-200902030-00007
18. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. https://doi.org/10.7326/0003-4819-155-8-201110180-00008
19. Schnipper J, Levine C. The important thing to do before leaving the hospital: many patients and families forget, which can lead to complications later. Next Avenue. October 22, 2019. Accessed September 10, 2020. https://www.nextavenue.org/before-leaving-hospital/
20. PCORI Methodology Standards: Standards for Studies of Complex Interventions. Patient-Centered Outcomes Research Institute; November 12, 2015. Updated: February 26, 2019. Accessed June 3, 2019. https://www.pcori.org/research-results/about-our-research/research-methodology/pcori-methodology-standards#Complex
21. Tsilimingras D, Schnipper J, Duke A, et al. Post-discharge adverse events among urban and rural patients of an urban community hospital: a prospective cohort study. J Gen Intern Med. 2015;30(8):1164-1171. https://doi.org/10.1007/s11606-015-3260-3
22. Kripalani S, Roumie CL, Dalal AK, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1-10. https://doi.org/10.7326/0003-4819-157-1-201207030-00003
23. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. https://doi.org/10.1001/jamainternmed.2015.7863
24. Vasilevskis EE, Kripalani S, Ong MK, et al. Variability in implementation of interventions aimed at reducing readmissions among patients with heart failure: a survey of teaching hospitals. Acad Med. 2016;91(4):522-529. https://doi.org/10.1097/acm.0000000000000994
25. Gardella JE, Cardwell TB, Nnadi M. Improving medication safety with accurate preadmission medication lists and postdischarge education. Jt Comm J Qual Patient Saf. 2012;38(10):452-458. https://doi.org/10.1016/s1553-7250(12)38060-4
26. Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955-964. https://doi.org/10.1001/archinte.166.9.955
27. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early performance of accountable care organizations in Medicare. N Engl J Med. 2016;374(24):2357-2366. https://doi.org/10.1056/nejmsa1600142
28. McWilliams JM, Hatfield LA, Landon BE, Hamed P, Chernew ME. Medicare spending after 3 years of the Medicare Shared Savings Program. N Engl J Med. 2018;379(12):1139-1149. https://doi.org/10.1056/nejmsa1803388
29. Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211-219. https://doi.org/10.1007/s11606-009-1196-1
30. Donzé JD, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632-638. https://doi.org/10.001/jamainternmed.2013.3023
31. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502. https://doi.org/10.1001/jamainternmed.2015.8462
Transitions from the hospital to the ambulatory setting are high-risk periods for patients in terms of adverse events, poor clinical outcomes, and readmission. Processes of care during care transitions are suboptimal, including poor communication among inpatient providers, patients, and ambulatory providers1,2; suboptimal communication of postdischarge plans of care to patients and their ability to carry out these plans3; medication discrepancies and nonadherence after discharge4; and lack of timely follow-up with ambulatory providers.5 Healthcare organizations continue to struggle with the question of which interventions to implement and how best to implement them.
Interventions to improve care transitions typically focus on readmission rates, but some studies have focused on postdischarge adverse events, defined as injuries in the 30 days after discharge caused by medical management rather than underlying disease processes.2 These adverse events cause psychological distress, out-of-pocket expenses, decreases in functional status, and caregiver burden. An estimated 20% of hospitalized patients suffer a postdischarge adverse event.1,2 Approximately two-thirds of these may be preventable or ameliorable.
The advent of Accountable Care Organizations (ACOs), defined as “groups of doctors, hospitals, and other health care providers who come together voluntarily to give coordinated high quality care to their patients,” creates an opportunity for improvements in patient safety during care transitions.6 Another opportunity has been the advent of Patient-Centered Medical Homes (PCMH), consisting of patient-oriented, comprehensive, team-based primary care enhanced by health information technology and population-based disease management tools.7,8 In theory, a hospital-PCMH collaboration within an ACO can improve transitional interventions since optimal communication and collaboration are more likely when both inpatient and primary care providers (PCPs) share infrastructure and are similarly incentivized. The objectives of this study were to design and implement a collaborative hospital-PCMH care transitions intervention within an ACO and evaluate its effects.
METHODS
This study was a two-arm, single-blind (blinded outcomes assessor), stepped-wedge, multisite cluster-randomized clinical trial (NCT02130570) approved by the institutional review board of Partners HealthCare.
Study Design and Randomization
The study employed a “stepped-wedge” design, which is a cluster-randomized study design in which an intervention is sequentially rolled out to different groups at different, prespecified, randomly determined times.9 Each cluster (in this case, each primary care practice) served as its own control, while still allowing for adjustment for temporal trends. Originally, 18 practices participated, but one withdrew due to the low number of patients enrolled in the study, leaving 17 clusters and 16 sequences; see Figure 1 of Appendix 1 for a full description of the sample size and timeline for each cluster. Practices were not aware of this timeline until after recruitment.

Study Setting and Participants
Conducted within a large Pioneer ACO in Boston and funded by the Patient-Centered Outcomes Research Institute (PCORI), the Partners-PCORI Transitions Study was designed as a “real-world” quality improvement project. Potential participants were adult patients who were admitted to medical and surgical services of two large academic hospitals (Hospital A and Hospital B) affiliated with an ACO, who were likely to be discharged back to the community, and whose PCP belonged to a primary care practice that was affiliated with the ACO, agreed to participate, and were designated PCMHs or on their way to being designated by meeting certain criteria: electronic health record, patient portal, team-based care, practice redesign, care management, and identification of high-risk patients. See Study Protocol (Appendix 2) for detailed patient and primary care practice inclusion criteria.
Patient Enrollment
Study staff screened participants from a daily automated list of patients admitted the day before, using medical records to determine eligibility, which was then confirmed by the patient’s nurse. Exclusion criteria included likely discharge to a location other than home, being in police custody, lack of a telephone, being homeless, previous enrollment in the study, and being unable to communicate in English or Spanish. Allocation to study arm was concealed until the patient or proxy provided informed written consent. The research assistant administered questionnaires to all study subjects to assess potential confounders and functional status 1 month prior to admission (
Intervention
The intervention was based on a conceptual model of an ideal discharge11 that we developed based on work by Naylor et al,12 work by Coleman and Berenson,3 best practices in medication reconciliation and information transfer according to our own research,13-15 the best examples of interventions to improve the discharge process,12,16,17 and a systematic review of discharge interventions.18 Some of the factors necessary for an ideal care transition include complete, organized, and timely documentation of the patient’s hospital course and postdischarge plan; effective discharge planning; coordination of care among the patient’s providers; methods to ensure medication safety; advanced care planning in appropriate patients; and education and “coaching” of patients and their caregivers so they learn how to manage their conditions. The final multifaceted intervention addressed each component of the ideal discharge and included inpatient and outpatient components (Table 1 and Table 1 of Appendix 1).

Patient and Public Involvement in Research
As with all PCORI-funded studies, this study involved a patient-family advisory council (PFAC). Our PFAC included six recently hospitalized patients or caregivers of recently hospitalized patients. The PFAC participated in monthly meetings throughout the study period. They helped inform the research questions, including confirmation that the endpoints were patient centered, and provided valuable input for the design of the intervention and the patient-facing components of the data collection instruments. They also interviewed several patient participants in the study regarding their experiences with the intervention. Lastly, they helped develop plans for dissemination of study results to the public.19
We also formed a steering committee consisting of physician, nursing, pharmacy, information technology, and administrative leadership representing primary care, inpatient care, and transitional care at both hospitals and Partners Healthcare. PFAC members took turns participating in quarterly steering committee meetings.
Evolution of the Intervention and Implementation
The intervention was iteratively refined during the course of the study in response to input from the PFAC, steering committee, and members of the intervention team; cases of adverse events and readmissions from patients despite being in the intervention arm; exit interviews of patients who had recently completed the intervention; and informal feedback from inpatient and outpatient clinicians. For example, we learned that the more complicated a patient’s conditions are, the sooner the clinical team wanted them to be seen after discharge. However, these patients were also less likely to feel well enough to keep that appointment. Therefore, the timing of follow-up of appointments needed to be a negotiation among the inpatient team, the patient, any caregivers, and the outpatient provider. PFAC members also emphasized that patients wanted one person to trust and to be the “point person” during a complicated transition such as hospital discharge.
At the same time, the intervention components evolved because of factors outside our control (eg, resource limitations). In keeping with the real-world nature of the research, the aim was for the intervention to be internally supported because incentives were theoretically more aligned with improvement of care transitions under the ACO model. By design, the PCORI contract only paid for limited parts of the intervention, such as a nurse practitioner to act as the discharge advocate at one hospital, overtime costs of inpatient pharmacists, and project manager time to facilitate inpatient-outpatient provider communication. (See Table 1 of Appendix 1 for details about the modifications to the intervention.)
Lastly, in keeping with PCORI’s methodology standards for studies of complex interventions,20 we strove to standardize the intervention by function across hospitals, units, and practices, while still allowing for local adaptation in the form. In other words, rather than specifying exactly how a task (eg, medication counseling) needed to be performed, the study design offered sites flexibility in how they implemented the task given their available personnel and institutional culture.
Intervention Fidelity
To determine the extent to which each patient in the intervention arm received each intervention component, a project manager unblinded to treatment arm reviewed the electronic medical record for documentation of each component implemented by providers (eg, inpatient pharmacists, outpatient nurses). Because each intervention component produced documentation, this provided an accurate assessment of intervention fidelity, ie, the extent to which the intervention was implemented as intended.
Outcome Assessment
Postdischarge Follow-up
Based on previous studies,2,21 a trained research assistant attempted to contact all study subjects 30 days (±5 days) after discharge and administered a questionnaire to identify any new or worsening symptoms since discharge, any healthcare use since discharge, and functional status in the previous week. Follow-up questions used branching logic to determine the relationship of any new or worsening symptoms to medications or other aspects of medical management. Research assistants followed up any positive responses with directed medical record review for objective findings, diagnoses, treatments, and responses. If patients could not be reached after five attempts, the research assistant instead conducted a thorough review of the outpatient medical record alone for provider reports of any new or worsening symptoms noted during follow-up within the 30-day postdischarge period. Research assistants also reviewed laboratory test results in all patients for evidence of postdischarge renal failure, elevated liver function tests, or new/worsening anemia.
Hospital Readmissions
We measured nonelective hospital readmissions within 30 days of discharge using a combination of administrative data for hospitalizations within the ACO network plus patient report during the 30-day phone call for all other readmissions.22
Adjudication of Outcomes
Adverse events and preventable adverse events: All cases of new or worsening symptoms or signs, along with all supporting documentation, were then presented to teams of two trained blinded physician adjudicators through application of methods established in previous studies.4,21 Each of the two adjudicators independently reviewed the information, along with the medical record, and completed a standardized form to confirm or deny the presence of any adverse events (ie, patient injury due to medical management) and to classify the type of event (eg, adverse drug event, hospital-acquired infection, procedural complication, diagnostic or management error), the severity and duration of the event, and whether the event was preventable or ameliorable. The two adjudicators then met to resolve any differences in their findings and come to consensus.
Preventable readmissions: If patients were readmitted to either study hospital, we conducted an evaluation, based on previous studies,23 to determine if and how the readmission could have been prevented including (a) a standardized patient and caregiver interview to identify possible problems with the transitions process and (b) an email questionnaire to the patient’s PCP and the inpatient teams who cared for the patient during the index admission and readmission regarding possible deficiencies with the transitions process. As with adverse event adjudications, two physician adjudicators worked independently to classify the preventability of the readmission and then met to come to consensus. Conflicts were resolved by a third adjudicator.
Analysis Plan
To evaluate the effects of the intervention on the primary outcome, the number of postdischarge adverse events per patient, we used multivariable Poisson regression, with study arm as the main predictor. A similar approach was used to evaluate the number of new or worsening postdischarge signs or symptoms and the number of preventable adverse events per patient. We used an intention-to-treat analysis: If a practice did not start the intervention when they were scheduled to, based on our randomization, we counted all patients in that practice admitted after that point as intervention patients. We adjusted for patient demographics, clinical characteristics, month, inpatient unit, and primary care practice as fixed effects. We clustered by PCP using general linear models. Intervention effects were expressed as both unadjusted and adjusted incidence rate ratios (IRRs). We also conducted a limited number of subgroup analyses, determined a priori, to determine whether the intervention was more effective in certain patient populations; we used interaction terms (intervention × subgroup) to determine the statistical significance of any effect modification.
To evaluate the effects of the intervention on nonelective readmissions and preventable readmissions, we used a similar approach, using multivariable logistic regression. Postdischarge functional status, adjusted for status prior to admission, was analyzed using multivariable linear regression and random effects by primary care practice. The general linear mixed model (GLIMMIX) procedure in the SAS 9.3 statistical package (SAS Institute) was used to carry out all analyses.
Power and Sample Size
We assumed a baseline rate of postdischarge adverse events of 0.30 per patient.21 We conservatively assumed an effect size of a change from 0.30 in the control group to 0.23 in the intervention group (a relative reduction of 22%, which was based on studies of preventability rates23 and close to the minimum clinically important difference). Based on prior studies,4,22 we assumed an intraclass correlation coefficient of 0.01 with an average cluster size of seven patients per PCP. Assuming a 10% loss to follow-up rate and an alpha of 0.05, we targeted a sample size of 1,800 patients to achieve 80% power, with one-third of the patients in the usual care arm and two-thirds in the intervention arm.
RESULTS
We enrolled 18 PCMH primary care practices to participate in the study, including 8 from Hospital A (out of 13 approached), 8 from Hospital B (out of 11), and 2 from other ACO practices (out of 9) (plus two pilot practices). Reasons for not participating included not having dedicated personnel to play the role of the responsible outpatient clinician, undergoing recent turn-over in practice leadership, and not having enough patients admitted to the two hospitals. One practice only enrolled 5 patients in the study and withdrew from participation, which left 17 practices.
Study Patients
We enrolled 1,679 patients (Figure 1). Reasons for nonenrollment included being unable to complete the screen prior to discharge, not meeting inclusion criteria or meeting exclusion criteria, being assigned to a pilot practice, and declining informed written consent. The baseline characteristics of enrolled patients are presented in Table 2. Differences between the two study arms were small. About 47% of the cohort was not reachable by phone after five attempts for the 30-day phone call, but only 69 (4.1%) were truly lost to follow-up because they were unreachable by phone and had no documentation in the electronic medical record in the 30-days after discharge.

Intervention Fidelity
The majority of patients did not receive most intervention components, even those components that were supposed to be delivered to all intervention patients (Table 3). A minority of patients were referred to visiting nurse services and to the home pharmacy program. However, 855 patients (87%) in the intervention arm received at least one intervention component.

Outcome Measures
The intervention was associated with a statistically significant reduction in several of the outcomes of interest, including the primary outcome, number of postdischarge adverse events (45% reduction), and new or worsening postdischarge signs or symptoms (22% reduction), as well as preventable postdischarge adverse events (58% reduction) (Table 4). There was a nonsignificant difference in functional status. There was no significant effect on total nonelective or on preventable readmission rates. When analyzed by type of adverse event, the intervention was associated with a reduction in adverse drug events and in procedural complications (Table 2 of Appendix 1). Of note, there was no significant difference in the proportion of patients with at least one adverse event whether the outcome was determined by phone call and medical record review (49%) or medical record review alone (51%) (P = .48).

In subgroup analyses, there was no evidence of effect modification by service, hospital, patient age, readmission risk, health literacy, or comorbidity score (Table 3 of Appendix 1). Table 4 of Appendix 1 provides examples of postdischarge adverse events seen in the usual care arm that might have been prevented in the intervention.
DISCUSSION
This intervention was associated with a reduction in postdischarge adverse events. The relative improvement in each outcome aligned with the hypothesized sensitivity to change: the smallest improvement was seen in new or worsening signs or symptoms, followed by postdischarge adverse events and then by preventable postdischarge adverse events. The intervention was not associated with a difference in readmissions. The lack of effect on hospital readmissions may have been caused by the low proportion of readmissions that are preventable, as well as low intervention fidelity and lack of resources to implement facets such as postdischarge coaching, an evidence-based intervention that was never adopted.16,23 One lesson of this study is that it may be easier to reduce postdischarge injury (still an important outcome) than readmissions.
Putting this study in context, we should note that the literature on interventions to improve care transitions is mixed.18 While there are several reports of successful interventions, there are many reports of unsuccessful ones, often using similar components. Success is often the result of adequate resources and attention to myriad details regarding implementation.24 The intervention in our study likely contributed to improvements in patient and caregiver engagement in the hospital, enhancements of communication between inpatient and outpatient clinicians, and implementation of pharmacist-led interventions to improve medication safety. Regarding the latter, several prior studies have shown the benefits of pharmacist interventions in decreasing postdischarge adverse drug events.4,25,26 Therefore, even an intervention with incomplete intervention fidelity can reduce postdischarge adverse events, especially because adverse drug events make up the majority of adverse events.1,2,21
Perhaps the biggest lesson we learned was regarding the limitations of the hospital-led ACO model to incentivize sufficient up-front investments in transitional care interventions. By design, we chose a real-world approach in which interventions were integrated with existing ACO efforts, which were paid for internally by the institution. As a result, many of the interventions had to be scaled back because of resource constraints. The ACO model theoretically incentivizes more integrated care, but this may not always be true in practice. Emerging evidence suggests that physician group–led ACOs are associated with lower costs and use compared with hospital-led ACOs, likely because of more aligned incentives in physician group–led ACOs to reduce use of inpatient care.27,28
An unresolved question is whether the ideal implementation approach is to protect the time of existing clinical personnel to carry out transitional care tasks or to hire external personnel to do these tasks. We purposely spread the intervention over several clinician types to minimize the additional burden on any one of them, minimize additional costs, and play to each clinician’s expertise, but in retrospect, this may not have been the right approach. By providing additional personnel with dedicated time, interest, and training in care transitions, the intervention may be delivered with higher quantitative and qualitative fidelity, and it could create a single point of contact for patients, which was considered highly desirable by our PFAC.
This study has several limitations. A large proportion of patients (44%) were unavailable for postdischarge phone calls. However, we were able to perform medical record review for worsening signs (eg, lab abnormalities) and symptoms (as reported by patients’ providers) in the postdischarge period and adjudicate them for adverse events for all but 69 of these patients. Because all these patients had ACO-affiliated PCPs, we would expect most of their utilization to have been within the system and, therefore, to be present in the medical record. The proportion of patients with at least one adverse event did not vary by the method of follow-up, which suggests that this issue is an unlikely source of bias. Assessment of readmission was imperfect because we do not have statewide or national data. However, our combination of administrative data for Partners readmissions plus self-report for non-Partners readmission has been shown to be fairly complete in previous studies.29 Adjudicators could not be fully blinded to intervention status due to the lack of blinding of admission date. We did not calculate a kappa value for interrater reliability of individual assessments of adverse events; rather, coming to consensus among the two adjudicators was part of the process. In only a handful of cases was a third adjudicator required. Lastly, this study was conducted at two academic medical centers and their affiliated primary care clinics, which potentially limits generalizability; however, the results are likely generalizable to other ACOs that include major academic medical centers.
CONCLUSION
In conclusion, in this real-world clinical trial, we designed, implemented, and iteratively refined a multifaceted intervention to improve care transitions within a hospital-based academic ACO. Evolution of the intervention components was the result of stakeholder input, experience with the intervention, and ACO resource constraints. The intervention reduced postdischarge adverse events. However, across the ACO network, intervention fidelity was low, and this may have contributed to the lack of effect on readmission rates. ACOs that implement interventions without hiring new personnel or protecting the time of existing personnel to conduct transitional tasks are likely to face the same challenges of low fidelity.
Acknowledgments
The authors would like to acknowledge the many people who worked on designing, implementing, and evaluating this intervention, including but not limited to: Natasha Isaac, Hilary Heyison, Jacqueline Minahan, Molly O’Reilly, Michelle Potter, Nailah Khoory, Maureen Fagan, David Bates, Laura Carr, Joseph Frolkis, Eric Weil, Jacqueline Somerville, Stephanie Ahmed, Marcy Bergeron, Jessica Smith, and Jane Millett. We would also like to thank the members of our Patient-Family Advisory Council: Maureen Fagan, Karen Spikes, Margie Hodges, Win Hodges, Aureldon Henderson, Dena Salzberg, and Kay Bander.
Transitions from the hospital to the ambulatory setting are high-risk periods for patients in terms of adverse events, poor clinical outcomes, and readmission. Processes of care during care transitions are suboptimal, including poor communication among inpatient providers, patients, and ambulatory providers1,2; suboptimal communication of postdischarge plans of care to patients and their ability to carry out these plans3; medication discrepancies and nonadherence after discharge4; and lack of timely follow-up with ambulatory providers.5 Healthcare organizations continue to struggle with the question of which interventions to implement and how best to implement them.
Interventions to improve care transitions typically focus on readmission rates, but some studies have focused on postdischarge adverse events, defined as injuries in the 30 days after discharge caused by medical management rather than underlying disease processes.2 These adverse events cause psychological distress, out-of-pocket expenses, decreases in functional status, and caregiver burden. An estimated 20% of hospitalized patients suffer a postdischarge adverse event.1,2 Approximately two-thirds of these may be preventable or ameliorable.
The advent of Accountable Care Organizations (ACOs), defined as “groups of doctors, hospitals, and other health care providers who come together voluntarily to give coordinated high quality care to their patients,” creates an opportunity for improvements in patient safety during care transitions.6 Another opportunity has been the advent of Patient-Centered Medical Homes (PCMH), consisting of patient-oriented, comprehensive, team-based primary care enhanced by health information technology and population-based disease management tools.7,8 In theory, a hospital-PCMH collaboration within an ACO can improve transitional interventions since optimal communication and collaboration are more likely when both inpatient and primary care providers (PCPs) share infrastructure and are similarly incentivized. The objectives of this study were to design and implement a collaborative hospital-PCMH care transitions intervention within an ACO and evaluate its effects.
METHODS
This study was a two-arm, single-blind (blinded outcomes assessor), stepped-wedge, multisite cluster-randomized clinical trial (NCT02130570) approved by the institutional review board of Partners HealthCare.
Study Design and Randomization
The study employed a “stepped-wedge” design, which is a cluster-randomized study design in which an intervention is sequentially rolled out to different groups at different, prespecified, randomly determined times.9 Each cluster (in this case, each primary care practice) served as its own control, while still allowing for adjustment for temporal trends. Originally, 18 practices participated, but one withdrew due to the low number of patients enrolled in the study, leaving 17 clusters and 16 sequences; see Figure 1 of Appendix 1 for a full description of the sample size and timeline for each cluster. Practices were not aware of this timeline until after recruitment.

Study Setting and Participants
Conducted within a large Pioneer ACO in Boston and funded by the Patient-Centered Outcomes Research Institute (PCORI), the Partners-PCORI Transitions Study was designed as a “real-world” quality improvement project. Potential participants were adult patients who were admitted to medical and surgical services of two large academic hospitals (Hospital A and Hospital B) affiliated with an ACO, who were likely to be discharged back to the community, and whose PCP belonged to a primary care practice that was affiliated with the ACO, agreed to participate, and were designated PCMHs or on their way to being designated by meeting certain criteria: electronic health record, patient portal, team-based care, practice redesign, care management, and identification of high-risk patients. See Study Protocol (Appendix 2) for detailed patient and primary care practice inclusion criteria.
Patient Enrollment
Study staff screened participants from a daily automated list of patients admitted the day before, using medical records to determine eligibility, which was then confirmed by the patient’s nurse. Exclusion criteria included likely discharge to a location other than home, being in police custody, lack of a telephone, being homeless, previous enrollment in the study, and being unable to communicate in English or Spanish. Allocation to study arm was concealed until the patient or proxy provided informed written consent. The research assistant administered questionnaires to all study subjects to assess potential confounders and functional status 1 month prior to admission (
Intervention
The intervention was based on a conceptual model of an ideal discharge11 that we developed based on work by Naylor et al,12 work by Coleman and Berenson,3 best practices in medication reconciliation and information transfer according to our own research,13-15 the best examples of interventions to improve the discharge process,12,16,17 and a systematic review of discharge interventions.18 Some of the factors necessary for an ideal care transition include complete, organized, and timely documentation of the patient’s hospital course and postdischarge plan; effective discharge planning; coordination of care among the patient’s providers; methods to ensure medication safety; advanced care planning in appropriate patients; and education and “coaching” of patients and their caregivers so they learn how to manage their conditions. The final multifaceted intervention addressed each component of the ideal discharge and included inpatient and outpatient components (Table 1 and Table 1 of Appendix 1).

Patient and Public Involvement in Research
As with all PCORI-funded studies, this study involved a patient-family advisory council (PFAC). Our PFAC included six recently hospitalized patients or caregivers of recently hospitalized patients. The PFAC participated in monthly meetings throughout the study period. They helped inform the research questions, including confirmation that the endpoints were patient centered, and provided valuable input for the design of the intervention and the patient-facing components of the data collection instruments. They also interviewed several patient participants in the study regarding their experiences with the intervention. Lastly, they helped develop plans for dissemination of study results to the public.19
We also formed a steering committee consisting of physician, nursing, pharmacy, information technology, and administrative leadership representing primary care, inpatient care, and transitional care at both hospitals and Partners Healthcare. PFAC members took turns participating in quarterly steering committee meetings.
Evolution of the Intervention and Implementation
The intervention was iteratively refined during the course of the study in response to input from the PFAC, steering committee, and members of the intervention team; cases of adverse events and readmissions from patients despite being in the intervention arm; exit interviews of patients who had recently completed the intervention; and informal feedback from inpatient and outpatient clinicians. For example, we learned that the more complicated a patient’s conditions are, the sooner the clinical team wanted them to be seen after discharge. However, these patients were also less likely to feel well enough to keep that appointment. Therefore, the timing of follow-up of appointments needed to be a negotiation among the inpatient team, the patient, any caregivers, and the outpatient provider. PFAC members also emphasized that patients wanted one person to trust and to be the “point person” during a complicated transition such as hospital discharge.
At the same time, the intervention components evolved because of factors outside our control (eg, resource limitations). In keeping with the real-world nature of the research, the aim was for the intervention to be internally supported because incentives were theoretically more aligned with improvement of care transitions under the ACO model. By design, the PCORI contract only paid for limited parts of the intervention, such as a nurse practitioner to act as the discharge advocate at one hospital, overtime costs of inpatient pharmacists, and project manager time to facilitate inpatient-outpatient provider communication. (See Table 1 of Appendix 1 for details about the modifications to the intervention.)
Lastly, in keeping with PCORI’s methodology standards for studies of complex interventions,20 we strove to standardize the intervention by function across hospitals, units, and practices, while still allowing for local adaptation in the form. In other words, rather than specifying exactly how a task (eg, medication counseling) needed to be performed, the study design offered sites flexibility in how they implemented the task given their available personnel and institutional culture.
Intervention Fidelity
To determine the extent to which each patient in the intervention arm received each intervention component, a project manager unblinded to treatment arm reviewed the electronic medical record for documentation of each component implemented by providers (eg, inpatient pharmacists, outpatient nurses). Because each intervention component produced documentation, this provided an accurate assessment of intervention fidelity, ie, the extent to which the intervention was implemented as intended.
Outcome Assessment
Postdischarge Follow-up
Based on previous studies,2,21 a trained research assistant attempted to contact all study subjects 30 days (±5 days) after discharge and administered a questionnaire to identify any new or worsening symptoms since discharge, any healthcare use since discharge, and functional status in the previous week. Follow-up questions used branching logic to determine the relationship of any new or worsening symptoms to medications or other aspects of medical management. Research assistants followed up any positive responses with directed medical record review for objective findings, diagnoses, treatments, and responses. If patients could not be reached after five attempts, the research assistant instead conducted a thorough review of the outpatient medical record alone for provider reports of any new or worsening symptoms noted during follow-up within the 30-day postdischarge period. Research assistants also reviewed laboratory test results in all patients for evidence of postdischarge renal failure, elevated liver function tests, or new/worsening anemia.
Hospital Readmissions
We measured nonelective hospital readmissions within 30 days of discharge using a combination of administrative data for hospitalizations within the ACO network plus patient report during the 30-day phone call for all other readmissions.22
Adjudication of Outcomes
Adverse events and preventable adverse events: All cases of new or worsening symptoms or signs, along with all supporting documentation, were then presented to teams of two trained blinded physician adjudicators through application of methods established in previous studies.4,21 Each of the two adjudicators independently reviewed the information, along with the medical record, and completed a standardized form to confirm or deny the presence of any adverse events (ie, patient injury due to medical management) and to classify the type of event (eg, adverse drug event, hospital-acquired infection, procedural complication, diagnostic or management error), the severity and duration of the event, and whether the event was preventable or ameliorable. The two adjudicators then met to resolve any differences in their findings and come to consensus.
Preventable readmissions: If patients were readmitted to either study hospital, we conducted an evaluation, based on previous studies,23 to determine if and how the readmission could have been prevented including (a) a standardized patient and caregiver interview to identify possible problems with the transitions process and (b) an email questionnaire to the patient’s PCP and the inpatient teams who cared for the patient during the index admission and readmission regarding possible deficiencies with the transitions process. As with adverse event adjudications, two physician adjudicators worked independently to classify the preventability of the readmission and then met to come to consensus. Conflicts were resolved by a third adjudicator.
Analysis Plan
To evaluate the effects of the intervention on the primary outcome, the number of postdischarge adverse events per patient, we used multivariable Poisson regression, with study arm as the main predictor. A similar approach was used to evaluate the number of new or worsening postdischarge signs or symptoms and the number of preventable adverse events per patient. We used an intention-to-treat analysis: If a practice did not start the intervention when they were scheduled to, based on our randomization, we counted all patients in that practice admitted after that point as intervention patients. We adjusted for patient demographics, clinical characteristics, month, inpatient unit, and primary care practice as fixed effects. We clustered by PCP using general linear models. Intervention effects were expressed as both unadjusted and adjusted incidence rate ratios (IRRs). We also conducted a limited number of subgroup analyses, determined a priori, to determine whether the intervention was more effective in certain patient populations; we used interaction terms (intervention × subgroup) to determine the statistical significance of any effect modification.
To evaluate the effects of the intervention on nonelective readmissions and preventable readmissions, we used a similar approach, using multivariable logistic regression. Postdischarge functional status, adjusted for status prior to admission, was analyzed using multivariable linear regression and random effects by primary care practice. The general linear mixed model (GLIMMIX) procedure in the SAS 9.3 statistical package (SAS Institute) was used to carry out all analyses.
Power and Sample Size
We assumed a baseline rate of postdischarge adverse events of 0.30 per patient.21 We conservatively assumed an effect size of a change from 0.30 in the control group to 0.23 in the intervention group (a relative reduction of 22%, which was based on studies of preventability rates23 and close to the minimum clinically important difference). Based on prior studies,4,22 we assumed an intraclass correlation coefficient of 0.01 with an average cluster size of seven patients per PCP. Assuming a 10% loss to follow-up rate and an alpha of 0.05, we targeted a sample size of 1,800 patients to achieve 80% power, with one-third of the patients in the usual care arm and two-thirds in the intervention arm.
RESULTS
We enrolled 18 PCMH primary care practices to participate in the study, including 8 from Hospital A (out of 13 approached), 8 from Hospital B (out of 11), and 2 from other ACO practices (out of 9) (plus two pilot practices). Reasons for not participating included not having dedicated personnel to play the role of the responsible outpatient clinician, undergoing recent turn-over in practice leadership, and not having enough patients admitted to the two hospitals. One practice only enrolled 5 patients in the study and withdrew from participation, which left 17 practices.
Study Patients
We enrolled 1,679 patients (Figure 1). Reasons for nonenrollment included being unable to complete the screen prior to discharge, not meeting inclusion criteria or meeting exclusion criteria, being assigned to a pilot practice, and declining informed written consent. The baseline characteristics of enrolled patients are presented in Table 2. Differences between the two study arms were small. About 47% of the cohort was not reachable by phone after five attempts for the 30-day phone call, but only 69 (4.1%) were truly lost to follow-up because they were unreachable by phone and had no documentation in the electronic medical record in the 30-days after discharge.

Intervention Fidelity
The majority of patients did not receive most intervention components, even those components that were supposed to be delivered to all intervention patients (Table 3). A minority of patients were referred to visiting nurse services and to the home pharmacy program. However, 855 patients (87%) in the intervention arm received at least one intervention component.

Outcome Measures
The intervention was associated with a statistically significant reduction in several of the outcomes of interest, including the primary outcome, number of postdischarge adverse events (45% reduction), and new or worsening postdischarge signs or symptoms (22% reduction), as well as preventable postdischarge adverse events (58% reduction) (Table 4). There was a nonsignificant difference in functional status. There was no significant effect on total nonelective or on preventable readmission rates. When analyzed by type of adverse event, the intervention was associated with a reduction in adverse drug events and in procedural complications (Table 2 of Appendix 1). Of note, there was no significant difference in the proportion of patients with at least one adverse event whether the outcome was determined by phone call and medical record review (49%) or medical record review alone (51%) (P = .48).

In subgroup analyses, there was no evidence of effect modification by service, hospital, patient age, readmission risk, health literacy, or comorbidity score (Table 3 of Appendix 1). Table 4 of Appendix 1 provides examples of postdischarge adverse events seen in the usual care arm that might have been prevented in the intervention.
DISCUSSION
This intervention was associated with a reduction in postdischarge adverse events. The relative improvement in each outcome aligned with the hypothesized sensitivity to change: the smallest improvement was seen in new or worsening signs or symptoms, followed by postdischarge adverse events and then by preventable postdischarge adverse events. The intervention was not associated with a difference in readmissions. The lack of effect on hospital readmissions may have been caused by the low proportion of readmissions that are preventable, as well as low intervention fidelity and lack of resources to implement facets such as postdischarge coaching, an evidence-based intervention that was never adopted.16,23 One lesson of this study is that it may be easier to reduce postdischarge injury (still an important outcome) than readmissions.
Putting this study in context, we should note that the literature on interventions to improve care transitions is mixed.18 While there are several reports of successful interventions, there are many reports of unsuccessful ones, often using similar components. Success is often the result of adequate resources and attention to myriad details regarding implementation.24 The intervention in our study likely contributed to improvements in patient and caregiver engagement in the hospital, enhancements of communication between inpatient and outpatient clinicians, and implementation of pharmacist-led interventions to improve medication safety. Regarding the latter, several prior studies have shown the benefits of pharmacist interventions in decreasing postdischarge adverse drug events.4,25,26 Therefore, even an intervention with incomplete intervention fidelity can reduce postdischarge adverse events, especially because adverse drug events make up the majority of adverse events.1,2,21
Perhaps the biggest lesson we learned was regarding the limitations of the hospital-led ACO model to incentivize sufficient up-front investments in transitional care interventions. By design, we chose a real-world approach in which interventions were integrated with existing ACO efforts, which were paid for internally by the institution. As a result, many of the interventions had to be scaled back because of resource constraints. The ACO model theoretically incentivizes more integrated care, but this may not always be true in practice. Emerging evidence suggests that physician group–led ACOs are associated with lower costs and use compared with hospital-led ACOs, likely because of more aligned incentives in physician group–led ACOs to reduce use of inpatient care.27,28
An unresolved question is whether the ideal implementation approach is to protect the time of existing clinical personnel to carry out transitional care tasks or to hire external personnel to do these tasks. We purposely spread the intervention over several clinician types to minimize the additional burden on any one of them, minimize additional costs, and play to each clinician’s expertise, but in retrospect, this may not have been the right approach. By providing additional personnel with dedicated time, interest, and training in care transitions, the intervention may be delivered with higher quantitative and qualitative fidelity, and it could create a single point of contact for patients, which was considered highly desirable by our PFAC.
This study has several limitations. A large proportion of patients (44%) were unavailable for postdischarge phone calls. However, we were able to perform medical record review for worsening signs (eg, lab abnormalities) and symptoms (as reported by patients’ providers) in the postdischarge period and adjudicate them for adverse events for all but 69 of these patients. Because all these patients had ACO-affiliated PCPs, we would expect most of their utilization to have been within the system and, therefore, to be present in the medical record. The proportion of patients with at least one adverse event did not vary by the method of follow-up, which suggests that this issue is an unlikely source of bias. Assessment of readmission was imperfect because we do not have statewide or national data. However, our combination of administrative data for Partners readmissions plus self-report for non-Partners readmission has been shown to be fairly complete in previous studies.29 Adjudicators could not be fully blinded to intervention status due to the lack of blinding of admission date. We did not calculate a kappa value for interrater reliability of individual assessments of adverse events; rather, coming to consensus among the two adjudicators was part of the process. In only a handful of cases was a third adjudicator required. Lastly, this study was conducted at two academic medical centers and their affiliated primary care clinics, which potentially limits generalizability; however, the results are likely generalizable to other ACOs that include major academic medical centers.
CONCLUSION
In conclusion, in this real-world clinical trial, we designed, implemented, and iteratively refined a multifaceted intervention to improve care transitions within a hospital-based academic ACO. Evolution of the intervention components was the result of stakeholder input, experience with the intervention, and ACO resource constraints. The intervention reduced postdischarge adverse events. However, across the ACO network, intervention fidelity was low, and this may have contributed to the lack of effect on readmission rates. ACOs that implement interventions without hiring new personnel or protecting the time of existing personnel to conduct transitional tasks are likely to face the same challenges of low fidelity.
Acknowledgments
The authors would like to acknowledge the many people who worked on designing, implementing, and evaluating this intervention, including but not limited to: Natasha Isaac, Hilary Heyison, Jacqueline Minahan, Molly O’Reilly, Michelle Potter, Nailah Khoory, Maureen Fagan, David Bates, Laura Carr, Joseph Frolkis, Eric Weil, Jacqueline Somerville, Stephanie Ahmed, Marcy Bergeron, Jessica Smith, and Jane Millett. We would also like to thank the members of our Patient-Family Advisory Council: Maureen Fagan, Karen Spikes, Margie Hodges, Win Hodges, Aureldon Henderson, Dena Salzberg, and Kay Bander.
1. Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345-349.
2. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. https://doi.org/10.7326/0003-4819-138-3-200302040-00007
3. Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141(7):533-536. https://doi.org/10.7326/0003-4819-141-7-200410050-00009
4. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565-571. https://doi.org/10.1001/archinte.166.5.565
5. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/nejmsa0803563
6. Accountable Care Organizations (ACOs). Centers for Medicare & Medicaid Services. 2012. Updated February 11, 2020. Accessed July 15, 2012. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ACO/index.html?redirect=/ACO/
7. Bates DW, Bitton A. The future of health information technology in the patient-centered medical home. Health Aff (Millwood). 2010;29(4):614-621. https://doi.org/10.1377/hlthaff.2010.0007
8. Bitton A, Martin C, Landon BE. A nationwide survey of patient centered medical home demonstration projects. J Gen Intern Med. 2010;25(6):584-592. https://doi.org/10.1007/s11606-010-1262-8
9. Brown C, Lilford R. Evaluating service delivery interventions to enhance patient safety. BMJ. 2008;337:a2764. https://doi.org/10.1136/bmj.a2764
10. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. https://doi.org/10.1097/00005650-199603000-00003
11. Burke RE, Kripalani S, Vasilevskis EE, Schnipper JL. Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102-109. https://doi.org/10.1002/jhm.1990
12. Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613-620. https://doi.org/10.1001/jama.281.7.613
13. Gandara E, Ungar J, Lee J, Chan-Macrae M, O’Malley T, Schnipper JL. Discharge documentation of patients discharged to subacute facilities: a three-year quality improvement process across an integrated health care system. Jt Comm J Qual Patient Saf. 2010;36(6):243-251. https://doi.org/10.1016/s1553-7250(10)36039-9
14. Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414-1422. https://doi.org/10.1007/s11606-008-0687-9
15. Schnipper JL, Hamann C, Ndumele CD, et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster-randomized trial. Arch Intern Med. 2009;169(8):771-780. https://doi.org/10.1001/archinternmed.2009.51
16. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. https://doi.org/10.1001/archinte.166.17.1822
17. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178-187. https://doi.org/10.7326/0003-4819-150-3-200902030-00007
18. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. https://doi.org/10.7326/0003-4819-155-8-201110180-00008
19. Schnipper J, Levine C. The important thing to do before leaving the hospital: many patients and families forget, which can lead to complications later. Next Avenue. October 22, 2019. Accessed September 10, 2020. https://www.nextavenue.org/before-leaving-hospital/
20. PCORI Methodology Standards: Standards for Studies of Complex Interventions. Patient-Centered Outcomes Research Institute; November 12, 2015. Updated: February 26, 2019. Accessed June 3, 2019. https://www.pcori.org/research-results/about-our-research/research-methodology/pcori-methodology-standards#Complex
21. Tsilimingras D, Schnipper J, Duke A, et al. Post-discharge adverse events among urban and rural patients of an urban community hospital: a prospective cohort study. J Gen Intern Med. 2015;30(8):1164-1171. https://doi.org/10.1007/s11606-015-3260-3
22. Kripalani S, Roumie CL, Dalal AK, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1-10. https://doi.org/10.7326/0003-4819-157-1-201207030-00003
23. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. https://doi.org/10.1001/jamainternmed.2015.7863
24. Vasilevskis EE, Kripalani S, Ong MK, et al. Variability in implementation of interventions aimed at reducing readmissions among patients with heart failure: a survey of teaching hospitals. Acad Med. 2016;91(4):522-529. https://doi.org/10.1097/acm.0000000000000994
25. Gardella JE, Cardwell TB, Nnadi M. Improving medication safety with accurate preadmission medication lists and postdischarge education. Jt Comm J Qual Patient Saf. 2012;38(10):452-458. https://doi.org/10.1016/s1553-7250(12)38060-4
26. Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955-964. https://doi.org/10.1001/archinte.166.9.955
27. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early performance of accountable care organizations in Medicare. N Engl J Med. 2016;374(24):2357-2366. https://doi.org/10.1056/nejmsa1600142
28. McWilliams JM, Hatfield LA, Landon BE, Hamed P, Chernew ME. Medicare spending after 3 years of the Medicare Shared Savings Program. N Engl J Med. 2018;379(12):1139-1149. https://doi.org/10.1056/nejmsa1803388
29. Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211-219. https://doi.org/10.1007/s11606-009-1196-1
30. Donzé JD, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632-638. https://doi.org/10.001/jamainternmed.2013.3023
31. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502. https://doi.org/10.1001/jamainternmed.2015.8462
1. Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345-349.
2. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. https://doi.org/10.7326/0003-4819-138-3-200302040-00007
3. Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141(7):533-536. https://doi.org/10.7326/0003-4819-141-7-200410050-00009
4. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565-571. https://doi.org/10.1001/archinte.166.5.565
5. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/nejmsa0803563
6. Accountable Care Organizations (ACOs). Centers for Medicare & Medicaid Services. 2012. Updated February 11, 2020. Accessed July 15, 2012. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ACO/index.html?redirect=/ACO/
7. Bates DW, Bitton A. The future of health information technology in the patient-centered medical home. Health Aff (Millwood). 2010;29(4):614-621. https://doi.org/10.1377/hlthaff.2010.0007
8. Bitton A, Martin C, Landon BE. A nationwide survey of patient centered medical home demonstration projects. J Gen Intern Med. 2010;25(6):584-592. https://doi.org/10.1007/s11606-010-1262-8
9. Brown C, Lilford R. Evaluating service delivery interventions to enhance patient safety. BMJ. 2008;337:a2764. https://doi.org/10.1136/bmj.a2764
10. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. https://doi.org/10.1097/00005650-199603000-00003
11. Burke RE, Kripalani S, Vasilevskis EE, Schnipper JL. Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102-109. https://doi.org/10.1002/jhm.1990
12. Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613-620. https://doi.org/10.1001/jama.281.7.613
13. Gandara E, Ungar J, Lee J, Chan-Macrae M, O’Malley T, Schnipper JL. Discharge documentation of patients discharged to subacute facilities: a three-year quality improvement process across an integrated health care system. Jt Comm J Qual Patient Saf. 2010;36(6):243-251. https://doi.org/10.1016/s1553-7250(10)36039-9
14. Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414-1422. https://doi.org/10.1007/s11606-008-0687-9
15. Schnipper JL, Hamann C, Ndumele CD, et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster-randomized trial. Arch Intern Med. 2009;169(8):771-780. https://doi.org/10.1001/archinternmed.2009.51
16. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. https://doi.org/10.1001/archinte.166.17.1822
17. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178-187. https://doi.org/10.7326/0003-4819-150-3-200902030-00007
18. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. https://doi.org/10.7326/0003-4819-155-8-201110180-00008
19. Schnipper J, Levine C. The important thing to do before leaving the hospital: many patients and families forget, which can lead to complications later. Next Avenue. October 22, 2019. Accessed September 10, 2020. https://www.nextavenue.org/before-leaving-hospital/
20. PCORI Methodology Standards: Standards for Studies of Complex Interventions. Patient-Centered Outcomes Research Institute; November 12, 2015. Updated: February 26, 2019. Accessed June 3, 2019. https://www.pcori.org/research-results/about-our-research/research-methodology/pcori-methodology-standards#Complex
21. Tsilimingras D, Schnipper J, Duke A, et al. Post-discharge adverse events among urban and rural patients of an urban community hospital: a prospective cohort study. J Gen Intern Med. 2015;30(8):1164-1171. https://doi.org/10.1007/s11606-015-3260-3
22. Kripalani S, Roumie CL, Dalal AK, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1-10. https://doi.org/10.7326/0003-4819-157-1-201207030-00003
23. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. https://doi.org/10.1001/jamainternmed.2015.7863
24. Vasilevskis EE, Kripalani S, Ong MK, et al. Variability in implementation of interventions aimed at reducing readmissions among patients with heart failure: a survey of teaching hospitals. Acad Med. 2016;91(4):522-529. https://doi.org/10.1097/acm.0000000000000994
25. Gardella JE, Cardwell TB, Nnadi M. Improving medication safety with accurate preadmission medication lists and postdischarge education. Jt Comm J Qual Patient Saf. 2012;38(10):452-458. https://doi.org/10.1016/s1553-7250(12)38060-4
26. Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955-964. https://doi.org/10.1001/archinte.166.9.955
27. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early performance of accountable care organizations in Medicare. N Engl J Med. 2016;374(24):2357-2366. https://doi.org/10.1056/nejmsa1600142
28. McWilliams JM, Hatfield LA, Landon BE, Hamed P, Chernew ME. Medicare spending after 3 years of the Medicare Shared Savings Program. N Engl J Med. 2018;379(12):1139-1149. https://doi.org/10.1056/nejmsa1803388
29. Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211-219. https://doi.org/10.1007/s11606-009-1196-1
30. Donzé JD, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632-638. https://doi.org/10.001/jamainternmed.2013.3023
31. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502. https://doi.org/10.1001/jamainternmed.2015.8462
The Effects of a Multifaceted Intervention to Improve Care Transitions Within an Accountable Care Organization: Results of a Stepped-Wedge Cluster-Randomized Trial

This work is licensed under a Creative Commons Attribution 4.0 International License
The Effects of a Multifaceted Intervention to Improve Care Transitions Within an Accountable Care Organization: Results of a Stepped-Wedge Cluster-Randomized Trial

This work is licensed under a Creative Commons Attribution 4.0 International License
© 2021 Society of Hospital Medicine
Email: [email protected]; Telephone: 617-732-7812; Twitter: @LipikaSamalMD; @drjschnip.
An On-Treatment Analysis of the MARQUIS Study: Interventions to Improve Inpatient Medication Reconciliation
Unintentional medication discrepancies in the hospital setting are common and contribute to adverse drug events, resulting in patient harm.1 Discrepancies can be resolved by implementing high-quality medication reconciliation, but there are insufficient data to guide hospitals as to which interventions are most effective at improving medication reconciliation processes and reducing harm.2 We recently reported that implementation of a best practices toolkit reduced total medication discrepancies in the Multi-Center Medication Reconciliation Quality Improvement Study (MARQUIS).3 This report describes the effect of individual toolkit components on rates of medication discrepancies with the potential for patient harm.
METHODS
Detailed descriptions of the intervention toolkit and study design of MARQUIS are published.4,5 Briefly, MARQUIS was a pragmatic, mentored, quality improvement (QI) study in which five hospitals in the United States implemented interventions from a best practices toolkit to improve medication reconciliation on noncritical care medical and surgical units from September 2011 to July 2014. We used a mentored implementation approach, in which each site identified the leaders of their local quality improvement team (ie, mentees) who received mentorship from a trained physician with QI and medication safety experience.6 Mentors conducted monthly calls with their mentees and two site visits. Sites adapted and implemented one or more components from the MARQUIS toolkit, a compilation of evidence-based best practices in medication reconciliation.5,7
The primary outcome was unintentional medication discrepancies in admission and discharge orders with the potential for causing harm, as previously described.4 Trained study pharmacists at each site took “gold standard” medication histories on a random sample of up to 22 patients per month. These medications were then compared with admission and discharge medication orders, and all unintentional discrepancies were identified. The discrepancies were then adjudicated by physicians blinded to the treatment arm, who confirmed whether discrepancies were unintentional and carried the potential for patient harm.
We employed a modification of a stepped wedge methodology to measure the incremental effect of implementing nine different intervention components, introduced at different sites over the course of the study, on the number of potentially harmful discrepancies per patient. These analyses were restricted to the postimplementation period on hospital units that implemented at least one intervention. All interventions conducted at each site were categorized by component, including dates of implementation. Each intervention component could be applied more than once per site (eg, when involving a new group of providers) or implemented on a new hospital unit or service, in which case, all dates were included in the analysis. We conducted a multivariable Poisson regression (with time divided into months) adjusted for patient factors, season, and site, with the number of potentially harmful discrepancies as the dependent variable, and the total number of gold standard medications as a model offset. The model was designed to analyze changes in the y-intercept each time an intervention component was either implemented or spread and assumed the change in the y-intercept was the same for each of these events for any given component. The model also assumes that combinations of interventions had independent additive effects.
RESULTS
Across the five participating sites, 1,648 patients were enrolled from September 2011 to July 2014. This number included 613 patients during the preimplementation period and 1,035 patients during the postimplementation period, of which 791 were on intervention units and comprised the study population. Table 1 displays the intervention components implemented by site. Sites implemented between one and seven components. The most frequently implemented intervention component was training existing staff to take the best possible medication histories (BPMHs), implemented at four sites. The regression results are displayed in Table 2. Three interventions were associated with significant decreases in potentially harmful discrepancy rates: (1) clearly defining roles and responsibilities and communicating this with clinical staff (hazard ratio [HR] 0.53, 95% CI: 0.32–0.87); (2) training existing staff to perform discharge medication reconciliation and patient counseling (HR 0.64, 95% CI: 0.46–0.89); and (3) hiring additional staff to perform discharge medication reconciliation and patient counseling (HR 0.48, 95% CI: 0.31–0.77). Two interventions were associated with significant increases in potentially harmful discrepancy rates: training existing staff to take BPMHs (HR 1.38, 95% CI: 1.21–1.57) and implementing a new electronic health record (EHR; HR 2.21, 95% CI: 1.64–2.97).
DISCUSSION
We noted that three intervention components were associated with decreased rates of unintentional medication discrepancies with potential for harm, whereas two were associated with increased rates. The components with a beneficial effect were not surprising. A prior qualitative study demonstrated the confusion related to clinicians’ roles and responsibilities during medication reconciliation; therefore, clear delineations should reduce rework and improve the medication reconciliation process.8 Other studies have shown the benefits of pharmacist involvement in the inpatient setting, particularly in reducing errors at discharge.9 However, we did not anticipate that training staff to take BPMHs would be detrimental. Possible reasons for this finding that are based on direct observations by mentors at site visits or noted during monthly calls include (1) training personnel on this task without certification of competency may not sufficiently improve their skills, leading instead to diffusion of responsibility; (2) training personnel without sufficient time to perform the task well (eg, frontline nurses with many other responsibilities) may be counterproductive compared with training a few personnel with time dedicated to this task; and (3) training existing personnel in history-taking may have been used to delay the necessary hiring of more staff to take BPMHs. Future studies could address several of these shortcomings in both the design and implementation of medication history-training intervention components.
Several reasons may explain the association we found between implementing a new EHR and increased rates of discrepancies. Based on mentors’ experiences, we suspect it is because sitewide EHR implementation requires significant resources, time, and effort. Therefore, sitewide EHR implementation pulls attention away from a focus on medication safety
Our study has several limitations. We conducted an on-treatment analysis, which may be confounded by characteristics of sites that chose to implement different intervention components; however, we adjusted for sites in the analysis. Some results are based on a limited number of sites implementing an intervention component (eg, defining roles and responsibilities). Although this was a longitudinal study, and we adjusted for seasonal effects, it is possible that temporal trends and cointerventions confounded our results. The adjudication of discrepancies for the potential for harm was somewhat subjective, although we used a rigorous process to ensure the reliability of adjudication, as in prior studies.3,14 As in the main analysis of the MARQUIS study, this analysis did not measure intervention fidelity.
Based on these analyses and the literature base, we recommend that hospitals focus first on hiring and training dedicated staff (usually pharmacists) to assist with medication reconciliation at discharge.7 Hospitals should also be aware of potential increases in medication discrepancies when implementing a large vendor EHR across their institution. Further work is needed on the best ways to mitigate these adverse effects, at both the design and local site levels. Finally, the effect of medication history training on discrepancies warrants further study.
Disclosures
SK has served as a consultant to Verustat, a remote health monitoring company. All other authors have no disclosures or conflicts of interests.
Funding
This study was supported by the Agency for Healthcare Research and Quality (grant number: R18 HS019598). JLS has received funding from (1) Mallinckrodt Pharmaceuticals for an investigator-initiated study of opioid-related adverse drug events in postsurgical patients; (2) Horizon Blue Cross Blue Shield for an honorarium and travel expenses for workshop on medication reconciliation; (3) Island Peer Review Organization for honorarium and travel expenses for workshop on medication reconciliation; and, (4) Portola Pharmaceuticals for investigator-initiated study of inpatients who decline subcutaneous medications for venous thromboembolism prophylaxis. ASM was funded by a VA HSR&D Career Development Award (12-168).
Trial Registration
ClinicalTrials.gov NCT01337063
1. Cornish PL, Knowles SR, Marchesano R, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165(4):424-429. https://doi.org/10.1001/archinte.165.4.424.
2. Kaboli PJ, Fernandes O. Medication reconciliation: moving forward. Arch Intern Med. 2012;172(14):1069-1070. https://doi.org/10.1001/archinternmed.2012.2667. PubMed
3. Schnipper JL, Mixon A, Stein J, et al. Effects of a multifaceted medication reconciliation quality improvement intervention on patient safety: final results of the MARQUIS study. BMJ Qual Saf. 2018;27(12):954-964. https://doi.org/10.1136/bmjqs-2018-008233.
4. Salanitro AH, Kripalani S, Resnic J, et al. Rational and design of the Multicenter Medication Reconciliation Quality Improvement Study (MARQUIS). BMC Health Serv Res. 2013;13:230. https://doi.org/10.1186/1472-6963-13-230.
5. Mueller SK, Kripalani S, Stein J, et al. Development of a toolkit to disseminate best practices in inpatient medication reconciliation. Jt Comm J Qual Patient Saf. 2013;39(8):371-382. https://10.1016/S1553-7250(13)39051-5.
6. Maynard GA, Budnitz TL, Nickel WK, et al. 2011 John M. Eisenberg patient safety and quality awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf. 2012;38(7):301-310. https://doi.org/10.1016/S1553-7250(12)38040-9.
7. Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069. https://doi.org/10.1001/archinternmed.2012.2246.
8. Vogelsmeier A, Pepper GA, Oderda L, Weir C. Medication reconciliation: a qualitative analysis of clinicians’ perceptions. Res Social Adm Pharm. 2013;9(4):419-430. https://doi.org/10.1016/j.sapharm.2012.08.002.
9. Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955-964. https://doi.org/10.1001/archinte.166.9.955.
10. Plaisant C, Wu J, Hettinger AZ, Powsner S, Shneiderman B. Novel user interface design for medication reconciliation: an evaluation of Twinlist. J Am Med Inform Assoc. 2015;22(2):340-349. https://doi.org/10.1093/jamia/ocu021.
11. Bassi J, Lau F, Bardal S. Use of information technology in medication reconciliation: a scoping review. Ann Pharmacother. 2010;44(5):885-897. https://doi.org/10.1345/aph.1M699.
12. Marien S, Krug B, Spinewine A. Electronic tools to support medication reconciliation: a systematic review. J Am Med Inform Assoc. 2017;24(1):227-240. https://doi.org/10.1093/jamia/ocw068.
13. Agrawal A. Medication errors: prevention using information technology systems. Br J Clin Pharmacol. 2009;67(6):681-686. https://doi.org/10.1111/j.1365-2125.2009.03427.x.
14. Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414-1422. https://doi.org/10.1007/s11606-008-0687-9.
Unintentional medication discrepancies in the hospital setting are common and contribute to adverse drug events, resulting in patient harm.1 Discrepancies can be resolved by implementing high-quality medication reconciliation, but there are insufficient data to guide hospitals as to which interventions are most effective at improving medication reconciliation processes and reducing harm.2 We recently reported that implementation of a best practices toolkit reduced total medication discrepancies in the Multi-Center Medication Reconciliation Quality Improvement Study (MARQUIS).3 This report describes the effect of individual toolkit components on rates of medication discrepancies with the potential for patient harm.
METHODS
Detailed descriptions of the intervention toolkit and study design of MARQUIS are published.4,5 Briefly, MARQUIS was a pragmatic, mentored, quality improvement (QI) study in which five hospitals in the United States implemented interventions from a best practices toolkit to improve medication reconciliation on noncritical care medical and surgical units from September 2011 to July 2014. We used a mentored implementation approach, in which each site identified the leaders of their local quality improvement team (ie, mentees) who received mentorship from a trained physician with QI and medication safety experience.6 Mentors conducted monthly calls with their mentees and two site visits. Sites adapted and implemented one or more components from the MARQUIS toolkit, a compilation of evidence-based best practices in medication reconciliation.5,7
The primary outcome was unintentional medication discrepancies in admission and discharge orders with the potential for causing harm, as previously described.4 Trained study pharmacists at each site took “gold standard” medication histories on a random sample of up to 22 patients per month. These medications were then compared with admission and discharge medication orders, and all unintentional discrepancies were identified. The discrepancies were then adjudicated by physicians blinded to the treatment arm, who confirmed whether discrepancies were unintentional and carried the potential for patient harm.
We employed a modification of a stepped wedge methodology to measure the incremental effect of implementing nine different intervention components, introduced at different sites over the course of the study, on the number of potentially harmful discrepancies per patient. These analyses were restricted to the postimplementation period on hospital units that implemented at least one intervention. All interventions conducted at each site were categorized by component, including dates of implementation. Each intervention component could be applied more than once per site (eg, when involving a new group of providers) or implemented on a new hospital unit or service, in which case, all dates were included in the analysis. We conducted a multivariable Poisson regression (with time divided into months) adjusted for patient factors, season, and site, with the number of potentially harmful discrepancies as the dependent variable, and the total number of gold standard medications as a model offset. The model was designed to analyze changes in the y-intercept each time an intervention component was either implemented or spread and assumed the change in the y-intercept was the same for each of these events for any given component. The model also assumes that combinations of interventions had independent additive effects.
RESULTS
Across the five participating sites, 1,648 patients were enrolled from September 2011 to July 2014. This number included 613 patients during the preimplementation period and 1,035 patients during the postimplementation period, of which 791 were on intervention units and comprised the study population. Table 1 displays the intervention components implemented by site. Sites implemented between one and seven components. The most frequently implemented intervention component was training existing staff to take the best possible medication histories (BPMHs), implemented at four sites. The regression results are displayed in Table 2. Three interventions were associated with significant decreases in potentially harmful discrepancy rates: (1) clearly defining roles and responsibilities and communicating this with clinical staff (hazard ratio [HR] 0.53, 95% CI: 0.32–0.87); (2) training existing staff to perform discharge medication reconciliation and patient counseling (HR 0.64, 95% CI: 0.46–0.89); and (3) hiring additional staff to perform discharge medication reconciliation and patient counseling (HR 0.48, 95% CI: 0.31–0.77). Two interventions were associated with significant increases in potentially harmful discrepancy rates: training existing staff to take BPMHs (HR 1.38, 95% CI: 1.21–1.57) and implementing a new electronic health record (EHR; HR 2.21, 95% CI: 1.64–2.97).
DISCUSSION
We noted that three intervention components were associated with decreased rates of unintentional medication discrepancies with potential for harm, whereas two were associated with increased rates. The components with a beneficial effect were not surprising. A prior qualitative study demonstrated the confusion related to clinicians’ roles and responsibilities during medication reconciliation; therefore, clear delineations should reduce rework and improve the medication reconciliation process.8 Other studies have shown the benefits of pharmacist involvement in the inpatient setting, particularly in reducing errors at discharge.9 However, we did not anticipate that training staff to take BPMHs would be detrimental. Possible reasons for this finding that are based on direct observations by mentors at site visits or noted during monthly calls include (1) training personnel on this task without certification of competency may not sufficiently improve their skills, leading instead to diffusion of responsibility; (2) training personnel without sufficient time to perform the task well (eg, frontline nurses with many other responsibilities) may be counterproductive compared with training a few personnel with time dedicated to this task; and (3) training existing personnel in history-taking may have been used to delay the necessary hiring of more staff to take BPMHs. Future studies could address several of these shortcomings in both the design and implementation of medication history-training intervention components.
Several reasons may explain the association we found between implementing a new EHR and increased rates of discrepancies. Based on mentors’ experiences, we suspect it is because sitewide EHR implementation requires significant resources, time, and effort. Therefore, sitewide EHR implementation pulls attention away from a focus on medication safety
Our study has several limitations. We conducted an on-treatment analysis, which may be confounded by characteristics of sites that chose to implement different intervention components; however, we adjusted for sites in the analysis. Some results are based on a limited number of sites implementing an intervention component (eg, defining roles and responsibilities). Although this was a longitudinal study, and we adjusted for seasonal effects, it is possible that temporal trends and cointerventions confounded our results. The adjudication of discrepancies for the potential for harm was somewhat subjective, although we used a rigorous process to ensure the reliability of adjudication, as in prior studies.3,14 As in the main analysis of the MARQUIS study, this analysis did not measure intervention fidelity.
Based on these analyses and the literature base, we recommend that hospitals focus first on hiring and training dedicated staff (usually pharmacists) to assist with medication reconciliation at discharge.7 Hospitals should also be aware of potential increases in medication discrepancies when implementing a large vendor EHR across their institution. Further work is needed on the best ways to mitigate these adverse effects, at both the design and local site levels. Finally, the effect of medication history training on discrepancies warrants further study.
Disclosures
SK has served as a consultant to Verustat, a remote health monitoring company. All other authors have no disclosures or conflicts of interests.
Funding
This study was supported by the Agency for Healthcare Research and Quality (grant number: R18 HS019598). JLS has received funding from (1) Mallinckrodt Pharmaceuticals for an investigator-initiated study of opioid-related adverse drug events in postsurgical patients; (2) Horizon Blue Cross Blue Shield for an honorarium and travel expenses for workshop on medication reconciliation; (3) Island Peer Review Organization for honorarium and travel expenses for workshop on medication reconciliation; and, (4) Portola Pharmaceuticals for investigator-initiated study of inpatients who decline subcutaneous medications for venous thromboembolism prophylaxis. ASM was funded by a VA HSR&D Career Development Award (12-168).
Trial Registration
ClinicalTrials.gov NCT01337063
Unintentional medication discrepancies in the hospital setting are common and contribute to adverse drug events, resulting in patient harm.1 Discrepancies can be resolved by implementing high-quality medication reconciliation, but there are insufficient data to guide hospitals as to which interventions are most effective at improving medication reconciliation processes and reducing harm.2 We recently reported that implementation of a best practices toolkit reduced total medication discrepancies in the Multi-Center Medication Reconciliation Quality Improvement Study (MARQUIS).3 This report describes the effect of individual toolkit components on rates of medication discrepancies with the potential for patient harm.
METHODS
Detailed descriptions of the intervention toolkit and study design of MARQUIS are published.4,5 Briefly, MARQUIS was a pragmatic, mentored, quality improvement (QI) study in which five hospitals in the United States implemented interventions from a best practices toolkit to improve medication reconciliation on noncritical care medical and surgical units from September 2011 to July 2014. We used a mentored implementation approach, in which each site identified the leaders of their local quality improvement team (ie, mentees) who received mentorship from a trained physician with QI and medication safety experience.6 Mentors conducted monthly calls with their mentees and two site visits. Sites adapted and implemented one or more components from the MARQUIS toolkit, a compilation of evidence-based best practices in medication reconciliation.5,7
The primary outcome was unintentional medication discrepancies in admission and discharge orders with the potential for causing harm, as previously described.4 Trained study pharmacists at each site took “gold standard” medication histories on a random sample of up to 22 patients per month. These medications were then compared with admission and discharge medication orders, and all unintentional discrepancies were identified. The discrepancies were then adjudicated by physicians blinded to the treatment arm, who confirmed whether discrepancies were unintentional and carried the potential for patient harm.
We employed a modification of a stepped wedge methodology to measure the incremental effect of implementing nine different intervention components, introduced at different sites over the course of the study, on the number of potentially harmful discrepancies per patient. These analyses were restricted to the postimplementation period on hospital units that implemented at least one intervention. All interventions conducted at each site were categorized by component, including dates of implementation. Each intervention component could be applied more than once per site (eg, when involving a new group of providers) or implemented on a new hospital unit or service, in which case, all dates were included in the analysis. We conducted a multivariable Poisson regression (with time divided into months) adjusted for patient factors, season, and site, with the number of potentially harmful discrepancies as the dependent variable, and the total number of gold standard medications as a model offset. The model was designed to analyze changes in the y-intercept each time an intervention component was either implemented or spread and assumed the change in the y-intercept was the same for each of these events for any given component. The model also assumes that combinations of interventions had independent additive effects.
RESULTS
Across the five participating sites, 1,648 patients were enrolled from September 2011 to July 2014. This number included 613 patients during the preimplementation period and 1,035 patients during the postimplementation period, of which 791 were on intervention units and comprised the study population. Table 1 displays the intervention components implemented by site. Sites implemented between one and seven components. The most frequently implemented intervention component was training existing staff to take the best possible medication histories (BPMHs), implemented at four sites. The regression results are displayed in Table 2. Three interventions were associated with significant decreases in potentially harmful discrepancy rates: (1) clearly defining roles and responsibilities and communicating this with clinical staff (hazard ratio [HR] 0.53, 95% CI: 0.32–0.87); (2) training existing staff to perform discharge medication reconciliation and patient counseling (HR 0.64, 95% CI: 0.46–0.89); and (3) hiring additional staff to perform discharge medication reconciliation and patient counseling (HR 0.48, 95% CI: 0.31–0.77). Two interventions were associated with significant increases in potentially harmful discrepancy rates: training existing staff to take BPMHs (HR 1.38, 95% CI: 1.21–1.57) and implementing a new electronic health record (EHR; HR 2.21, 95% CI: 1.64–2.97).
DISCUSSION
We noted that three intervention components were associated with decreased rates of unintentional medication discrepancies with potential for harm, whereas two were associated with increased rates. The components with a beneficial effect were not surprising. A prior qualitative study demonstrated the confusion related to clinicians’ roles and responsibilities during medication reconciliation; therefore, clear delineations should reduce rework and improve the medication reconciliation process.8 Other studies have shown the benefits of pharmacist involvement in the inpatient setting, particularly in reducing errors at discharge.9 However, we did not anticipate that training staff to take BPMHs would be detrimental. Possible reasons for this finding that are based on direct observations by mentors at site visits or noted during monthly calls include (1) training personnel on this task without certification of competency may not sufficiently improve their skills, leading instead to diffusion of responsibility; (2) training personnel without sufficient time to perform the task well (eg, frontline nurses with many other responsibilities) may be counterproductive compared with training a few personnel with time dedicated to this task; and (3) training existing personnel in history-taking may have been used to delay the necessary hiring of more staff to take BPMHs. Future studies could address several of these shortcomings in both the design and implementation of medication history-training intervention components.
Several reasons may explain the association we found between implementing a new EHR and increased rates of discrepancies. Based on mentors’ experiences, we suspect it is because sitewide EHR implementation requires significant resources, time, and effort. Therefore, sitewide EHR implementation pulls attention away from a focus on medication safety
Our study has several limitations. We conducted an on-treatment analysis, which may be confounded by characteristics of sites that chose to implement different intervention components; however, we adjusted for sites in the analysis. Some results are based on a limited number of sites implementing an intervention component (eg, defining roles and responsibilities). Although this was a longitudinal study, and we adjusted for seasonal effects, it is possible that temporal trends and cointerventions confounded our results. The adjudication of discrepancies for the potential for harm was somewhat subjective, although we used a rigorous process to ensure the reliability of adjudication, as in prior studies.3,14 As in the main analysis of the MARQUIS study, this analysis did not measure intervention fidelity.
Based on these analyses and the literature base, we recommend that hospitals focus first on hiring and training dedicated staff (usually pharmacists) to assist with medication reconciliation at discharge.7 Hospitals should also be aware of potential increases in medication discrepancies when implementing a large vendor EHR across their institution. Further work is needed on the best ways to mitigate these adverse effects, at both the design and local site levels. Finally, the effect of medication history training on discrepancies warrants further study.
Disclosures
SK has served as a consultant to Verustat, a remote health monitoring company. All other authors have no disclosures or conflicts of interests.
Funding
This study was supported by the Agency for Healthcare Research and Quality (grant number: R18 HS019598). JLS has received funding from (1) Mallinckrodt Pharmaceuticals for an investigator-initiated study of opioid-related adverse drug events in postsurgical patients; (2) Horizon Blue Cross Blue Shield for an honorarium and travel expenses for workshop on medication reconciliation; (3) Island Peer Review Organization for honorarium and travel expenses for workshop on medication reconciliation; and, (4) Portola Pharmaceuticals for investigator-initiated study of inpatients who decline subcutaneous medications for venous thromboembolism prophylaxis. ASM was funded by a VA HSR&D Career Development Award (12-168).
Trial Registration
ClinicalTrials.gov NCT01337063
1. Cornish PL, Knowles SR, Marchesano R, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165(4):424-429. https://doi.org/10.1001/archinte.165.4.424.
2. Kaboli PJ, Fernandes O. Medication reconciliation: moving forward. Arch Intern Med. 2012;172(14):1069-1070. https://doi.org/10.1001/archinternmed.2012.2667. PubMed
3. Schnipper JL, Mixon A, Stein J, et al. Effects of a multifaceted medication reconciliation quality improvement intervention on patient safety: final results of the MARQUIS study. BMJ Qual Saf. 2018;27(12):954-964. https://doi.org/10.1136/bmjqs-2018-008233.
4. Salanitro AH, Kripalani S, Resnic J, et al. Rational and design of the Multicenter Medication Reconciliation Quality Improvement Study (MARQUIS). BMC Health Serv Res. 2013;13:230. https://doi.org/10.1186/1472-6963-13-230.
5. Mueller SK, Kripalani S, Stein J, et al. Development of a toolkit to disseminate best practices in inpatient medication reconciliation. Jt Comm J Qual Patient Saf. 2013;39(8):371-382. https://10.1016/S1553-7250(13)39051-5.
6. Maynard GA, Budnitz TL, Nickel WK, et al. 2011 John M. Eisenberg patient safety and quality awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf. 2012;38(7):301-310. https://doi.org/10.1016/S1553-7250(12)38040-9.
7. Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069. https://doi.org/10.1001/archinternmed.2012.2246.
8. Vogelsmeier A, Pepper GA, Oderda L, Weir C. Medication reconciliation: a qualitative analysis of clinicians’ perceptions. Res Social Adm Pharm. 2013;9(4):419-430. https://doi.org/10.1016/j.sapharm.2012.08.002.
9. Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955-964. https://doi.org/10.1001/archinte.166.9.955.
10. Plaisant C, Wu J, Hettinger AZ, Powsner S, Shneiderman B. Novel user interface design for medication reconciliation: an evaluation of Twinlist. J Am Med Inform Assoc. 2015;22(2):340-349. https://doi.org/10.1093/jamia/ocu021.
11. Bassi J, Lau F, Bardal S. Use of information technology in medication reconciliation: a scoping review. Ann Pharmacother. 2010;44(5):885-897. https://doi.org/10.1345/aph.1M699.
12. Marien S, Krug B, Spinewine A. Electronic tools to support medication reconciliation: a systematic review. J Am Med Inform Assoc. 2017;24(1):227-240. https://doi.org/10.1093/jamia/ocw068.
13. Agrawal A. Medication errors: prevention using information technology systems. Br J Clin Pharmacol. 2009;67(6):681-686. https://doi.org/10.1111/j.1365-2125.2009.03427.x.
14. Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414-1422. https://doi.org/10.1007/s11606-008-0687-9.
1. Cornish PL, Knowles SR, Marchesano R, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165(4):424-429. https://doi.org/10.1001/archinte.165.4.424.
2. Kaboli PJ, Fernandes O. Medication reconciliation: moving forward. Arch Intern Med. 2012;172(14):1069-1070. https://doi.org/10.1001/archinternmed.2012.2667. PubMed
3. Schnipper JL, Mixon A, Stein J, et al. Effects of a multifaceted medication reconciliation quality improvement intervention on patient safety: final results of the MARQUIS study. BMJ Qual Saf. 2018;27(12):954-964. https://doi.org/10.1136/bmjqs-2018-008233.
4. Salanitro AH, Kripalani S, Resnic J, et al. Rational and design of the Multicenter Medication Reconciliation Quality Improvement Study (MARQUIS). BMC Health Serv Res. 2013;13:230. https://doi.org/10.1186/1472-6963-13-230.
5. Mueller SK, Kripalani S, Stein J, et al. Development of a toolkit to disseminate best practices in inpatient medication reconciliation. Jt Comm J Qual Patient Saf. 2013;39(8):371-382. https://10.1016/S1553-7250(13)39051-5.
6. Maynard GA, Budnitz TL, Nickel WK, et al. 2011 John M. Eisenberg patient safety and quality awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf. 2012;38(7):301-310. https://doi.org/10.1016/S1553-7250(12)38040-9.
7. Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069. https://doi.org/10.1001/archinternmed.2012.2246.
8. Vogelsmeier A, Pepper GA, Oderda L, Weir C. Medication reconciliation: a qualitative analysis of clinicians’ perceptions. Res Social Adm Pharm. 2013;9(4):419-430. https://doi.org/10.1016/j.sapharm.2012.08.002.
9. Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955-964. https://doi.org/10.1001/archinte.166.9.955.
10. Plaisant C, Wu J, Hettinger AZ, Powsner S, Shneiderman B. Novel user interface design for medication reconciliation: an evaluation of Twinlist. J Am Med Inform Assoc. 2015;22(2):340-349. https://doi.org/10.1093/jamia/ocu021.
11. Bassi J, Lau F, Bardal S. Use of information technology in medication reconciliation: a scoping review. Ann Pharmacother. 2010;44(5):885-897. https://doi.org/10.1345/aph.1M699.
12. Marien S, Krug B, Spinewine A. Electronic tools to support medication reconciliation: a systematic review. J Am Med Inform Assoc. 2017;24(1):227-240. https://doi.org/10.1093/jamia/ocw068.
13. Agrawal A. Medication errors: prevention using information technology systems. Br J Clin Pharmacol. 2009;67(6):681-686. https://doi.org/10.1111/j.1365-2125.2009.03427.x.
14. Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414-1422. https://doi.org/10.1007/s11606-008-0687-9.
© 2019 Society of Hospital Medicine
Information Transfer to Rehabilitation
Effective communication among physicians during the hospital discharge process is critical to patient care. Patients are at high risk of having an adverse drug event,1 readmission, or death2 during the transition from hospital to home.3 Ineffective communication between inpatient and outpatient providers has been implicated as a leading cause of adverse events.35 Conversely, efforts to improve communication have been shown to improve compliance with follow‐up tests and decrease readmission rates.6, 7 Recently, the absence of several specific data elements in discharge documentation have been shown to be common and to have potential for patient harm, including test results that are pending at the time of discharge.8, 9 Unexplained discrepancies between preadmission and discharge medication regimens are also common and potentially dangerous.1
According to the Joint Commission for Accreditation of Healthcare Organizations (TJC), the following elements should be included in discharge summaries: the reason for hospitalization; significant findings; procedures performed and care, treatment, and services provided; the patient's condition at discharge; and information provided to the patient and family, as appropriate.10 TJC also advocates medication reconciliation, a process of identifying the most accurate list of all medications a patient is takingincluding name, dosage, frequency, and routeand using this list to provide correct medications for patients anywhere within the health care system.11
Despite the importance of complete communication among providers at hospital discharge, a recent systematic review showed that discharge summaries often lacked important information such as diagnostic test results (missing from 33%‐63%), treatment or hospital course (7%‐22%), discharge medications (2%‐40%), test results pending at discharge (65%), patient or family counseling (90%‐92%), and follow‐up plans (2%‐43%).1
Most of the studies addressing this issue have evaluated communication pitfalls between acute care hospitals and primary care physicians among patients discharged home.17 In contrast, the quality of discharge documentation among patients discharged to rehabilitation centers and other subacute care facilities has been less well studied, perhaps due to relatively smaller numbers of patients discharged to such facilities. This communication is as or more important because these patients are potentially more vulnerable and their medical conditions more active than for patients discharged home.12 Furthermore, discharge information from acute care hospitals will often form the basis for admission orders at subacute facilities. Last, these patients will have a second transition in care (from subacute facility to home) whose quality is dependent at least in part on the quality of communication during the first transition.
The aim of this study was to evaluate the quality of information transfer among patients discharged from acute hospitals to subacute facilities across an integrated healthcare delivery system. The long‐term goals of this effort were to determine the areas most in need of improvement, to guide interventions to address these problems, and to track improvements in these measures over time as interventions are implemented and refined.
Methods
This observational study was conducted as part of a quality improvement project evaluating the quality of information provided during the discharge process across Partners Health Care System. The institutional review boards of the participating institutions approved the study.
Study Sample
We evaluated a sample of discharge documentation packets (eg, discharge summaries, discharge orders, nursing instructions, care coordination, and physical/occupational therapy notes) of patients discharged from all 5 acute care hospitals of the Partners Healthcare System to 30 subacute facilities (rehabilitation hospitals and skilled nursing facilities) from March 2005 through June 2007.
For reviewers at acute sites, discharge documentation packets were randomly selected each quarter using a random number generator within Microsoft Excel (Microsoft, Redmond, WA). At subacute sites, reviewers selected which packets to review, although they were encouraged to review all of them. Random selection of packets could not be achieved at subacute sites because reviews took place on the day of admission to the subacute facility. All reviewers received 1 hour of training on how to evaluate discharge packets, including review of a standardized teaching packet with 1 of the coauthors (J.L.S. or T.O.).
Two of the 5 acute care hospitals in the study are academic medical centers and the other 3 are community hospitals. Reviewers were a mix of trained medical residents or nurse practitioners at acute sites and admitting physicians or nurse practitioners at receiving subacute sites.
Fifty packets were reviewed per acute site per quarter. This provided roughly 10% precision around our estimates (ie, if compliance with a measure were 80%, the 95% confidence interval around this estimate would be 70%‐90%). This sample size is consistent with those used to obtain other national benchmarks, such as those for National Hospital Quality Measures, which generally require at least 35 cases per quarter.13
Measures
A multidisciplinary team at Partners derived, reviewed, and refined a minimum data set required to appropriately care for patients during the first 72 hours after transfer from an acute care hospital to a subacute facility. Several of these measures are required by TJC. Other measures were either modifications of TJC measures made to facilitate uniform data collection (eg, history and physical examination at admission instead of significant findings) or additional data elements (not required by TJC) felt to be important to patient care based on the medical literature and interviews with receiving providers at subacute facilities. All measures were refined by the multidisciplinary team with input from additional subspecialists as needed (see Table 1 for the final list of measures).
| Reason(s) for Admission | |
|---|---|
| Joint Commission requirements | A focused history |
| A focused physical exam | |
| Pertinent past medical history | |
| Treatment rendered | |
| Discharge diagnosis(es) | |
| Condition on discharge | |
| Discharge summary | |
| Any information missing | |
| Non‐Joint Commission requirements | |
| Medication information | Discharge medications |
| Drug allergies | |
| Preadmission medication information | |
| Explanation for any differences between preadmission and discharge medications | |
| Test results information | Latest pertinent laboratory results |
| Pertinent radiology results | |
| Test results pending at time of transfer | |
| Overall assessment | Were management and follow‐up plans adequately described? |
| Did you uncover a significant condition not mentioned in the discharge packet? | |
Data Collection
After reviewing the entire discharge documentation packet, reviewers completed a survey concerning the inclusion of the required data elements. Surveys were completed online using Perseus Survey Solutions 6.0 (Perseus Development Corp., Braintree, MA) in the month following discharge (for reviewers at acute care sites) or within 24 hours of admission to the subacute facility (for reviewers at subacute sites). To verify the accuracy and completeness of packets, reviewers at acute sites were instructed to compare the discharge documentation to a review of the inpatient medical record. Similarly, reviewers at subacute sites were instructed to complete their evaluations after admitting each patient to their facility.
Outcomes
The primary outcome was the proportion of packets that contained each data element. In addition, we calculated the proportion of packets that contained all applicable elements required by TJC and all applicable data elements measured in the study. Last, we evaluated two global (albeit subjective) measures of satisfaction with the packet: Were management and follow‐up plans adequately described? (both components needed to be adequately described to get credit for this question) and Did you uncover a significant condition not mentioned in the discharge packet? Significant conditions were defined as active medical problems requiring management during or immediately following the hospitalization.
Statistical Analysis
Results were calculated as proportions, odds ratios, and 95% confidence intervals (CI), using SAS version 9.1 (SAS Institute, Inc., Cary, NC). Simple logistic regression was used to compare inclusion of data elements between medical and surgical services and between academic medical centers and community hospitals. To evaluate interrater reliability, 2 reviewers (both at acute sites) independently evaluated 29 randomly chosen charts, each with 12 data elements.
Results
A total of 1501 discharge documentation packets were reviewed, including 980 patients (65%) from a medical unit and 521 patients (35%) from a surgical unit. Based on 2007 data, these packets represent approximately 4% of all eligible discharges to subacute facilities. Patients discharged from 1 of the 2 academic medical centers represented 44% of the sample. A total of 644 discharge packets (43%) were reviewed at acute sites and 814 packets (54%) were reviewed at subacute sites. Information about reviewer site was missing in 43 discharge packets (3%). For the 29 charts independently reviewed by 2 reviewers, there was complete agreement for 331 out of 348 data elements (95.1%).
Only 1055 (70%) discharge summaries had all the information required by TJC (Table 2). Physical examination at admission (a component of significant findings, as noted above) and condition at discharge were the 2 elements most often missing. The defect‐free rate varied by site, with a range of 61% to 76% across the 5 acute care hospitals (data not shown).
| Sample Size | Missing [n (%)] | 95% CI Missing % | |
|---|---|---|---|
| |||
| Joint Commission requirements | |||
| Reason(s) for admission | 1497 | 14 (0.9) | 0.41.4 |
| A focused history | 1493 | 65 (4.4) | 3.35.3 |
| A focused physical exam | 1493 | 170 (11.4) | 9.713 |
| Pertinent past medical history | 1494 | 69 (4.6) | 3.55.6 |
| Treatment rendered | 1494 | 33 (2.2) | 1.42.9 |
| Discharge diagnosis(es) | 1480 | 53 (3.6) | 2.64.5 |
| Condition on discharge | 1462 | 208 (14.2) | 12.416.0 |
| Discharge summary | 1475 | 90 (6.1) | 4.87.3 |
| Any information missing | 1501 | 447 (29.7) | 27.432.0 |
| Non‐Joint Commission requirements | |||
| Medication information | |||
| Discharge medications | 1491 | 19 (1.3) | 0.71.8 |
| Drug allergies | 1470 | 88 (6.0) | 4.77.2 |
| Preadmission medication information | 1460 | 297 (20.3) | 18.322.4 |
| Explanation for any differences between preadmission and discharge medications | 1060 | 374 (35.3) | 32.038.1 |
| Test results information | |||
| Latest pertinent lab results | 1460 | 261 (17.9) | 15.919.8 |
| Pertinent radiology results | 1303 | 139 (10.7) | 912.4 |
| Test results pending at time of transfer | 341 | 160 (47.2) | 41.952.5 |
| Overall assessment | |||
| Were management and follow‐up plans adequately described? | 1461 | No (%): 161 (11.1) | 95% CI No %: 9.512.7 |
| Did you uncover a significant condition not mentioned in the discharge packet? | 1469 | Yes (%): 162 (11.0) | 95% CI Yes %: 9.413.0 |
| All applicable elements present | 1501 | 503 (33.5) | 31.135.9 |
The rates of inclusion of other (non‐TJC required) data elements are shown in Table 2. Most often missing were preadmission medication regimens, any documented reason for any difference between preadmission and discharge medications, pertinent laboratory results, and an adequate follow‐up plan (including who to follow up with, when to follow‐up, and a list of tasks to be accomplished at the follow‐up visit). Notation regarding significant test results that were pending at the time of transfer was missing in 160 of 341 applicable patients (47%), and in 162 patients (11%), physicians uncovered a significant condition that was not mentioned in the discharge documentation. Only 503 (33.5%) discharge documentation packets had all applicable measures present. In addition, the discharge summary was not received at all on the day of discharge according to the receiving site in 90 patients (6%).
Reviewers were asked in a separate question which missing data were necessary for patient care. Data elements most often cited were explanations for any medication discrepancies and test results pending at the time of the hospital discharge.
Community hospitals had a higher rate of inclusion of TJC‐required data elements when compared to academic medical centers (Table 3). Also, among non‐TJC required data elements, inclusion rates were higher among the community hospitals, especially regarding information about medication discrepancies, pending test results, and follow‐up information (Table 3).
| Total (n) | All Elements Present [n (%)] | OR (95% CI) | |
|---|---|---|---|
| |||
| Joint Commission requirements | |||
| Hospital type | |||
| Community hospitals | 949 | 826 (87) | 2.7 (2.13.6) |
| Academic medical centers | 541 | 384 (71) | Ref. |
| Service | |||
| Medical services | 1013 | 745 (73) | 1.3 (1.01.7) |
| Surgical services | 488 | 332 (68) | Ref. |
| Explanation for any medication discrepancies | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 718 | 550 (76) | 5.0 (3.86.5) |
| Academic medical centers | 342 | 136 (39) | Ref. |
| Service | |||
| Medical services | 754 | 529 (70) | 2.2 (1.72.9) |
| Surgical services | 306 | 157 (51) | Ref. |
| Test results pending at time of transfer | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 172 | 109 (63) | 2.4 (1.53.7) |
| Academic medical centers | 169 | 71 (42) | Ref. |
| Service | |||
| Medical services | 227 | 146 (64) | 4.2 (2.66.9) |
| Surgical services | 114 | 34 (30) | Ref. |
| Follow‐up plans adequately described | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 968 | 883 (91) | 1.7 (1.22.4) |
| Academic medical centers | 543 | 466 (85) | Ref. |
| Service | |||
| Medical services | 983 | 862 (87) | 0.67 (0.51.0) |
| Surgical services | 478 | 437 (91) | Ref. |
Although no differences were found between medical and surgical services regarding compliance with TJC requirements, a difference was noted in documentation of explanations of medication discrepancies and pending test results, with medical services performing better in both measures (Table 3).
In general, reviewers at subacute sites more often evaluated packets as deficient than reviewers at acute sites, up to an absolute difference of 33% in the proportion of missing data, depending on the data element (see Appendix, Table 1).
Discussion
Our study evaluated the completeness of documentation in the discharge summaries of patients discharged from acute care to subacute care facilities. Our results for the inclusion of TJC‐required data elements were similar to those quoted in the literature for patients discharged home.6 Our results also demonstrated a high rate of other missing data elements that are arguably of equal or greater importance, including reasons for discrepancies between preadmission and discharge medication regimens and tests that are pending at the time of discharge.1, 8, 9 Our results also demonstrated the relatively poorer performance of academic centers compared to community hospitals regarding inclusion of information about medication reconciliation, follow‐up, pending test results, and complete information required by TJC. Finally, we found that patients discharged from surgical services more often lacked documentation of medication discrepancies and pending test results compared with patients from medical services.
To our knowledge, this is one of the first studies looking at the quality of information transfer in patients discharged to subacute care facilities. The results of this study are not surprising given the known problems with general information transfer at hospital discharge.1 The fact that community hospitals provided more complete information than academic medical centers for certain data elements may be due to the difference between residents and more senior physicians preparing discharge documentation. Such differences could reflect differences in experience, training, and degree of appreciation for the importance of discharge documentation, and/or restrictions in work hours among residents (eg, resulting in time‐pressure to complete discharge summaries and/or summaries being written by residents who know the patients less well). These hypotheses deserve further exploration. The differences between medical and surgical services should also be validated and explored in other healthcare systems, including both academic and community settings.
The results of this study should be viewed in light of the study's limitations. Packets evaluated by reviewers at subacute facilities were chosen by the reviewers and may not have been representative of all patients received by that facility (in contrast to those reviewed at the acute sites, which were chosen at random and more likely to be representative, although we did not formally test for this). It is possible that reviewers at subacute sites selected the worst discharge documentation packets for evaluation. Second, evaluations by reviewers at subacute sites did not distinguish between information missing from discharge documentation and failure to receive the documentation at all from the acute care hospital (again in contrast to reviewers at acute sites, who always had access to the documentation). Lastly, reviewers at acute and subacute sites may have graded packets differently due to their different clinical perspectives. These 3 factors may explain the relatively poorer results of discharge packets reviewed by reviewers at subacute sites. Further study would be needed to distinguish among these possibilities (eg, having acute and subacute reviewers answer the same questions for the same discharge packets to allow us to measure interrater reliability between the different kinds of reviewers; explicitly asking subacute reviewers about receipt of each piece of documentation; comparing the distribution of diagnosis‐related group [DRG] codes and hospital length of stay in evaluated vs. total discharge packets as a measure of representativeness). We also cannot rule out the possibility of reviewer bias, but all reviewers were trained in a standardized fashion and we know that reliability of assessments were high, at least among reviewers at acute sites. Last, we did not measure actual or potential adverse events caused by these information deficits.
As part of a Partners‐wide initiative to improve transitions in care, the results were presented to the administrations of each of the 5 acute care hospitals. The Partners High Performance Medicine Transition team then began work with a steering committee (composed of representatives from each hospital) to address these deficiencies. Since then, the hospitals have taken several steps to improve the quality of information transfer for discharged patients, including the following:
Technological improvements to the hospitals' discharge ordering systems to actively solicit and/or autoimport the required information into discharge documentation.
Creation of discharge templates to record the required information on paper.
Provision of feedback to clinicians and their service chiefs regarding the ongoing quality of their discharge documentation.
Creation of an online Partners‐wide curriculum on discharge summary authorship, with a mandatory quiz to be taken by all incoming clinicians.
In conclusion, we found room for improvement in the inclusion of data elements required for the safe transfer of patients from acute hospitals to subacute facilities, especially in areas such as medication reconciliation, pending test results, and adequate follow‐up plans. We also found variation by site and type of service. For patients discharged to rehabilitation and other subacute facilities, improvement is needed in the communication of clinically relevant information to those providing continuing care.
Appendix
| JCAHO Indicators | Reviews from Sub‐Acute Sites (N = 814)* | Reviews from Acute Sites (N = 644)* | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample Size | Missing N | % | 95% CI | Sample Size | Missing | % | 95% CI | |
| ||||||||
| Reason(s) for admission | 812 | 9 | 1.1 | 0.41.8 | 643 | 4 | 0.6 | 0.011.2 |
| A focused history | 810 | 49 | 6.1 | 4.47.7 | 642 | 16 | 2.5 | 1.33.7 |
| A focused physical exam | 810 | 131 | 16.2 | 13.718.7 | 641 | 34 | 5.3 | 3.67.0 |
| Pertinent past medical history | 810 | 50 | 6.2 | 4.57.8 | 642 | 14 | 22.0 | 1.13.3 |
| Treatment rendered | 811 | 29 | 3.6 | 2.34.9 | 641 | 4 | 0.6 | 0.011.2 |
| Discharge diagnosis(es) | 806 | 59 | 7.3 | 5.59.1 | 630 | 7 | 1.1 | 0.31.9 |
| Condition on discharge | 800 | 92 | 11.5 | 9.313.7 | 622 | 109 | 17.5 | 14.520.5 |
| Discharge summary | 809 | 77 | 9.5 | 7.511.5 | 624 | 11 | 1.8 | 0.72.8 |
| Any information missing | ||||||||
| Medication Information | Sample Size | Missing | % | 95% CI | Sample Size | Missing | % | 95% CI |
| Discharge medications | 811 | 12 | 1.5 | 0.72.3 | 638 | 6 | 0.9 | 0.21.7 |
| Drug allergies | 811 | 47 | 5.8 | 4.27.4 | 639 | 35 | 5.5 | 3.77.2 |
| Explanation for any differences between preadmission and discharge medications | 542 | 275 | 50.7 | 46.555 | 498 | 88 | 17.7 | 14.321.0 |
| Test results information | Sample Size | Missing | % | 95% CI | Sample Size | Missing | % | 95% CI |
| Latest pertinent lab results | 790 | 178 | 22.5 | 19.625.4 | 629 | 73 | 11.6 | 9.114.1 |
| Pertinent radiology results | 668 | 110 | 16.5 | 13.719.3 | 601 | 27 | 4.5 | 2.86.2 |
| Test results pending at time of transfer | 183 | 87 | 47.5 | 40.354.8 | 152 | 73 | 48.0 | 40.156.0 |
| Management Information | Sample Size | No | % | 95% CI | Sample Size | No | % | 95% CI |
| Were management and follow‐up plans adequately described? | 794 | 121 | 15.2 | 12.717.7 | 631 | 79 | 12.5 | 9.915.1 |
| Sample Size | Yes | % | 95% CI | Sample Size | Yes | % | 95% CI | |
| Did you uncover a significant condition not mentioned in the discharge packet? | 793 | 117 | 14.8 | 12.317.2 | 635 | 38 | 6.0 | 4.47.8 |
- , , , et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- , , , .Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.1989;19:624–631.
- , , , , , .Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- , , , .Effect of discharge summary availability during post‐discharge visits on hospital readmission.JGen Intern Med.2002;17:186–192.
- , , , .Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18:646–651.
- , , , .Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- , , , et al.Impact of a standardized communication system on continuity of care between family physicians and the emergency department.CJEM.2007;9:79–86.
- , , , et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- , , .Tying up loose ends: discharging patients with unresolved medical issues.Arch Intern Med.2007;167:1305–1311.
- Standard IM.6.10: Hospital Accreditation Standards.Oakbrook Terrace, IL:Joint Commission on Accreditation of Healthcare Organizations;2006:338–340.
- Joint Commission on Accreditation of Healthcare Organizations. Joint Commission national patient safety goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals. Accessed July 2009.
- , .Effectiveness of multidisciplinary rehabilitation services in post acute care: state‐of‐the‐science. A review.Arch Phys Med Rehabil.2007;88:1526–1534.
- Joint Commission on Accreditation of Healthcare Organizations. Specification Manual for National Hospital Quality Measures: Population and Sampling Specifications Version 2.4. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Current+NHQM+Manual.htm. Accessed July 2009.
Effective communication among physicians during the hospital discharge process is critical to patient care. Patients are at high risk of having an adverse drug event,1 readmission, or death2 during the transition from hospital to home.3 Ineffective communication between inpatient and outpatient providers has been implicated as a leading cause of adverse events.35 Conversely, efforts to improve communication have been shown to improve compliance with follow‐up tests and decrease readmission rates.6, 7 Recently, the absence of several specific data elements in discharge documentation have been shown to be common and to have potential for patient harm, including test results that are pending at the time of discharge.8, 9 Unexplained discrepancies between preadmission and discharge medication regimens are also common and potentially dangerous.1
According to the Joint Commission for Accreditation of Healthcare Organizations (TJC), the following elements should be included in discharge summaries: the reason for hospitalization; significant findings; procedures performed and care, treatment, and services provided; the patient's condition at discharge; and information provided to the patient and family, as appropriate.10 TJC also advocates medication reconciliation, a process of identifying the most accurate list of all medications a patient is takingincluding name, dosage, frequency, and routeand using this list to provide correct medications for patients anywhere within the health care system.11
Despite the importance of complete communication among providers at hospital discharge, a recent systematic review showed that discharge summaries often lacked important information such as diagnostic test results (missing from 33%‐63%), treatment or hospital course (7%‐22%), discharge medications (2%‐40%), test results pending at discharge (65%), patient or family counseling (90%‐92%), and follow‐up plans (2%‐43%).1
Most of the studies addressing this issue have evaluated communication pitfalls between acute care hospitals and primary care physicians among patients discharged home.17 In contrast, the quality of discharge documentation among patients discharged to rehabilitation centers and other subacute care facilities has been less well studied, perhaps due to relatively smaller numbers of patients discharged to such facilities. This communication is as or more important because these patients are potentially more vulnerable and their medical conditions more active than for patients discharged home.12 Furthermore, discharge information from acute care hospitals will often form the basis for admission orders at subacute facilities. Last, these patients will have a second transition in care (from subacute facility to home) whose quality is dependent at least in part on the quality of communication during the first transition.
The aim of this study was to evaluate the quality of information transfer among patients discharged from acute hospitals to subacute facilities across an integrated healthcare delivery system. The long‐term goals of this effort were to determine the areas most in need of improvement, to guide interventions to address these problems, and to track improvements in these measures over time as interventions are implemented and refined.
Methods
This observational study was conducted as part of a quality improvement project evaluating the quality of information provided during the discharge process across Partners Health Care System. The institutional review boards of the participating institutions approved the study.
Study Sample
We evaluated a sample of discharge documentation packets (eg, discharge summaries, discharge orders, nursing instructions, care coordination, and physical/occupational therapy notes) of patients discharged from all 5 acute care hospitals of the Partners Healthcare System to 30 subacute facilities (rehabilitation hospitals and skilled nursing facilities) from March 2005 through June 2007.
For reviewers at acute sites, discharge documentation packets were randomly selected each quarter using a random number generator within Microsoft Excel (Microsoft, Redmond, WA). At subacute sites, reviewers selected which packets to review, although they were encouraged to review all of them. Random selection of packets could not be achieved at subacute sites because reviews took place on the day of admission to the subacute facility. All reviewers received 1 hour of training on how to evaluate discharge packets, including review of a standardized teaching packet with 1 of the coauthors (J.L.S. or T.O.).
Two of the 5 acute care hospitals in the study are academic medical centers and the other 3 are community hospitals. Reviewers were a mix of trained medical residents or nurse practitioners at acute sites and admitting physicians or nurse practitioners at receiving subacute sites.
Fifty packets were reviewed per acute site per quarter. This provided roughly 10% precision around our estimates (ie, if compliance with a measure were 80%, the 95% confidence interval around this estimate would be 70%‐90%). This sample size is consistent with those used to obtain other national benchmarks, such as those for National Hospital Quality Measures, which generally require at least 35 cases per quarter.13
Measures
A multidisciplinary team at Partners derived, reviewed, and refined a minimum data set required to appropriately care for patients during the first 72 hours after transfer from an acute care hospital to a subacute facility. Several of these measures are required by TJC. Other measures were either modifications of TJC measures made to facilitate uniform data collection (eg, history and physical examination at admission instead of significant findings) or additional data elements (not required by TJC) felt to be important to patient care based on the medical literature and interviews with receiving providers at subacute facilities. All measures were refined by the multidisciplinary team with input from additional subspecialists as needed (see Table 1 for the final list of measures).
| Reason(s) for Admission | |
|---|---|
| Joint Commission requirements | A focused history |
| A focused physical exam | |
| Pertinent past medical history | |
| Treatment rendered | |
| Discharge diagnosis(es) | |
| Condition on discharge | |
| Discharge summary | |
| Any information missing | |
| Non‐Joint Commission requirements | |
| Medication information | Discharge medications |
| Drug allergies | |
| Preadmission medication information | |
| Explanation for any differences between preadmission and discharge medications | |
| Test results information | Latest pertinent laboratory results |
| Pertinent radiology results | |
| Test results pending at time of transfer | |
| Overall assessment | Were management and follow‐up plans adequately described? |
| Did you uncover a significant condition not mentioned in the discharge packet? | |
Data Collection
After reviewing the entire discharge documentation packet, reviewers completed a survey concerning the inclusion of the required data elements. Surveys were completed online using Perseus Survey Solutions 6.0 (Perseus Development Corp., Braintree, MA) in the month following discharge (for reviewers at acute care sites) or within 24 hours of admission to the subacute facility (for reviewers at subacute sites). To verify the accuracy and completeness of packets, reviewers at acute sites were instructed to compare the discharge documentation to a review of the inpatient medical record. Similarly, reviewers at subacute sites were instructed to complete their evaluations after admitting each patient to their facility.
Outcomes
The primary outcome was the proportion of packets that contained each data element. In addition, we calculated the proportion of packets that contained all applicable elements required by TJC and all applicable data elements measured in the study. Last, we evaluated two global (albeit subjective) measures of satisfaction with the packet: Were management and follow‐up plans adequately described? (both components needed to be adequately described to get credit for this question) and Did you uncover a significant condition not mentioned in the discharge packet? Significant conditions were defined as active medical problems requiring management during or immediately following the hospitalization.
Statistical Analysis
Results were calculated as proportions, odds ratios, and 95% confidence intervals (CI), using SAS version 9.1 (SAS Institute, Inc., Cary, NC). Simple logistic regression was used to compare inclusion of data elements between medical and surgical services and between academic medical centers and community hospitals. To evaluate interrater reliability, 2 reviewers (both at acute sites) independently evaluated 29 randomly chosen charts, each with 12 data elements.
Results
A total of 1501 discharge documentation packets were reviewed, including 980 patients (65%) from a medical unit and 521 patients (35%) from a surgical unit. Based on 2007 data, these packets represent approximately 4% of all eligible discharges to subacute facilities. Patients discharged from 1 of the 2 academic medical centers represented 44% of the sample. A total of 644 discharge packets (43%) were reviewed at acute sites and 814 packets (54%) were reviewed at subacute sites. Information about reviewer site was missing in 43 discharge packets (3%). For the 29 charts independently reviewed by 2 reviewers, there was complete agreement for 331 out of 348 data elements (95.1%).
Only 1055 (70%) discharge summaries had all the information required by TJC (Table 2). Physical examination at admission (a component of significant findings, as noted above) and condition at discharge were the 2 elements most often missing. The defect‐free rate varied by site, with a range of 61% to 76% across the 5 acute care hospitals (data not shown).
| Sample Size | Missing [n (%)] | 95% CI Missing % | |
|---|---|---|---|
| |||
| Joint Commission requirements | |||
| Reason(s) for admission | 1497 | 14 (0.9) | 0.41.4 |
| A focused history | 1493 | 65 (4.4) | 3.35.3 |
| A focused physical exam | 1493 | 170 (11.4) | 9.713 |
| Pertinent past medical history | 1494 | 69 (4.6) | 3.55.6 |
| Treatment rendered | 1494 | 33 (2.2) | 1.42.9 |
| Discharge diagnosis(es) | 1480 | 53 (3.6) | 2.64.5 |
| Condition on discharge | 1462 | 208 (14.2) | 12.416.0 |
| Discharge summary | 1475 | 90 (6.1) | 4.87.3 |
| Any information missing | 1501 | 447 (29.7) | 27.432.0 |
| Non‐Joint Commission requirements | |||
| Medication information | |||
| Discharge medications | 1491 | 19 (1.3) | 0.71.8 |
| Drug allergies | 1470 | 88 (6.0) | 4.77.2 |
| Preadmission medication information | 1460 | 297 (20.3) | 18.322.4 |
| Explanation for any differences between preadmission and discharge medications | 1060 | 374 (35.3) | 32.038.1 |
| Test results information | |||
| Latest pertinent lab results | 1460 | 261 (17.9) | 15.919.8 |
| Pertinent radiology results | 1303 | 139 (10.7) | 912.4 |
| Test results pending at time of transfer | 341 | 160 (47.2) | 41.952.5 |
| Overall assessment | |||
| Were management and follow‐up plans adequately described? | 1461 | No (%): 161 (11.1) | 95% CI No %: 9.512.7 |
| Did you uncover a significant condition not mentioned in the discharge packet? | 1469 | Yes (%): 162 (11.0) | 95% CI Yes %: 9.413.0 |
| All applicable elements present | 1501 | 503 (33.5) | 31.135.9 |
The rates of inclusion of other (non‐TJC required) data elements are shown in Table 2. Most often missing were preadmission medication regimens, any documented reason for any difference between preadmission and discharge medications, pertinent laboratory results, and an adequate follow‐up plan (including who to follow up with, when to follow‐up, and a list of tasks to be accomplished at the follow‐up visit). Notation regarding significant test results that were pending at the time of transfer was missing in 160 of 341 applicable patients (47%), and in 162 patients (11%), physicians uncovered a significant condition that was not mentioned in the discharge documentation. Only 503 (33.5%) discharge documentation packets had all applicable measures present. In addition, the discharge summary was not received at all on the day of discharge according to the receiving site in 90 patients (6%).
Reviewers were asked in a separate question which missing data were necessary for patient care. Data elements most often cited were explanations for any medication discrepancies and test results pending at the time of the hospital discharge.
Community hospitals had a higher rate of inclusion of TJC‐required data elements when compared to academic medical centers (Table 3). Also, among non‐TJC required data elements, inclusion rates were higher among the community hospitals, especially regarding information about medication discrepancies, pending test results, and follow‐up information (Table 3).
| Total (n) | All Elements Present [n (%)] | OR (95% CI) | |
|---|---|---|---|
| |||
| Joint Commission requirements | |||
| Hospital type | |||
| Community hospitals | 949 | 826 (87) | 2.7 (2.13.6) |
| Academic medical centers | 541 | 384 (71) | Ref. |
| Service | |||
| Medical services | 1013 | 745 (73) | 1.3 (1.01.7) |
| Surgical services | 488 | 332 (68) | Ref. |
| Explanation for any medication discrepancies | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 718 | 550 (76) | 5.0 (3.86.5) |
| Academic medical centers | 342 | 136 (39) | Ref. |
| Service | |||
| Medical services | 754 | 529 (70) | 2.2 (1.72.9) |
| Surgical services | 306 | 157 (51) | Ref. |
| Test results pending at time of transfer | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 172 | 109 (63) | 2.4 (1.53.7) |
| Academic medical centers | 169 | 71 (42) | Ref. |
| Service | |||
| Medical services | 227 | 146 (64) | 4.2 (2.66.9) |
| Surgical services | 114 | 34 (30) | Ref. |
| Follow‐up plans adequately described | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 968 | 883 (91) | 1.7 (1.22.4) |
| Academic medical centers | 543 | 466 (85) | Ref. |
| Service | |||
| Medical services | 983 | 862 (87) | 0.67 (0.51.0) |
| Surgical services | 478 | 437 (91) | Ref. |
Although no differences were found between medical and surgical services regarding compliance with TJC requirements, a difference was noted in documentation of explanations of medication discrepancies and pending test results, with medical services performing better in both measures (Table 3).
In general, reviewers at subacute sites more often evaluated packets as deficient than reviewers at acute sites, up to an absolute difference of 33% in the proportion of missing data, depending on the data element (see Appendix, Table 1).
Discussion
Our study evaluated the completeness of documentation in the discharge summaries of patients discharged from acute care to subacute care facilities. Our results for the inclusion of TJC‐required data elements were similar to those quoted in the literature for patients discharged home.6 Our results also demonstrated a high rate of other missing data elements that are arguably of equal or greater importance, including reasons for discrepancies between preadmission and discharge medication regimens and tests that are pending at the time of discharge.1, 8, 9 Our results also demonstrated the relatively poorer performance of academic centers compared to community hospitals regarding inclusion of information about medication reconciliation, follow‐up, pending test results, and complete information required by TJC. Finally, we found that patients discharged from surgical services more often lacked documentation of medication discrepancies and pending test results compared with patients from medical services.
To our knowledge, this is one of the first studies looking at the quality of information transfer in patients discharged to subacute care facilities. The results of this study are not surprising given the known problems with general information transfer at hospital discharge.1 The fact that community hospitals provided more complete information than academic medical centers for certain data elements may be due to the difference between residents and more senior physicians preparing discharge documentation. Such differences could reflect differences in experience, training, and degree of appreciation for the importance of discharge documentation, and/or restrictions in work hours among residents (eg, resulting in time‐pressure to complete discharge summaries and/or summaries being written by residents who know the patients less well). These hypotheses deserve further exploration. The differences between medical and surgical services should also be validated and explored in other healthcare systems, including both academic and community settings.
The results of this study should be viewed in light of the study's limitations. Packets evaluated by reviewers at subacute facilities were chosen by the reviewers and may not have been representative of all patients received by that facility (in contrast to those reviewed at the acute sites, which were chosen at random and more likely to be representative, although we did not formally test for this). It is possible that reviewers at subacute sites selected the worst discharge documentation packets for evaluation. Second, evaluations by reviewers at subacute sites did not distinguish between information missing from discharge documentation and failure to receive the documentation at all from the acute care hospital (again in contrast to reviewers at acute sites, who always had access to the documentation). Lastly, reviewers at acute and subacute sites may have graded packets differently due to their different clinical perspectives. These 3 factors may explain the relatively poorer results of discharge packets reviewed by reviewers at subacute sites. Further study would be needed to distinguish among these possibilities (eg, having acute and subacute reviewers answer the same questions for the same discharge packets to allow us to measure interrater reliability between the different kinds of reviewers; explicitly asking subacute reviewers about receipt of each piece of documentation; comparing the distribution of diagnosis‐related group [DRG] codes and hospital length of stay in evaluated vs. total discharge packets as a measure of representativeness). We also cannot rule out the possibility of reviewer bias, but all reviewers were trained in a standardized fashion and we know that reliability of assessments were high, at least among reviewers at acute sites. Last, we did not measure actual or potential adverse events caused by these information deficits.
As part of a Partners‐wide initiative to improve transitions in care, the results were presented to the administrations of each of the 5 acute care hospitals. The Partners High Performance Medicine Transition team then began work with a steering committee (composed of representatives from each hospital) to address these deficiencies. Since then, the hospitals have taken several steps to improve the quality of information transfer for discharged patients, including the following:
Technological improvements to the hospitals' discharge ordering systems to actively solicit and/or autoimport the required information into discharge documentation.
Creation of discharge templates to record the required information on paper.
Provision of feedback to clinicians and their service chiefs regarding the ongoing quality of their discharge documentation.
Creation of an online Partners‐wide curriculum on discharge summary authorship, with a mandatory quiz to be taken by all incoming clinicians.
In conclusion, we found room for improvement in the inclusion of data elements required for the safe transfer of patients from acute hospitals to subacute facilities, especially in areas such as medication reconciliation, pending test results, and adequate follow‐up plans. We also found variation by site and type of service. For patients discharged to rehabilitation and other subacute facilities, improvement is needed in the communication of clinically relevant information to those providing continuing care.
Appendix
| JCAHO Indicators | Reviews from Sub‐Acute Sites (N = 814)* | Reviews from Acute Sites (N = 644)* | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample Size | Missing N | % | 95% CI | Sample Size | Missing | % | 95% CI | |
| ||||||||
| Reason(s) for admission | 812 | 9 | 1.1 | 0.41.8 | 643 | 4 | 0.6 | 0.011.2 |
| A focused history | 810 | 49 | 6.1 | 4.47.7 | 642 | 16 | 2.5 | 1.33.7 |
| A focused physical exam | 810 | 131 | 16.2 | 13.718.7 | 641 | 34 | 5.3 | 3.67.0 |
| Pertinent past medical history | 810 | 50 | 6.2 | 4.57.8 | 642 | 14 | 22.0 | 1.13.3 |
| Treatment rendered | 811 | 29 | 3.6 | 2.34.9 | 641 | 4 | 0.6 | 0.011.2 |
| Discharge diagnosis(es) | 806 | 59 | 7.3 | 5.59.1 | 630 | 7 | 1.1 | 0.31.9 |
| Condition on discharge | 800 | 92 | 11.5 | 9.313.7 | 622 | 109 | 17.5 | 14.520.5 |
| Discharge summary | 809 | 77 | 9.5 | 7.511.5 | 624 | 11 | 1.8 | 0.72.8 |
| Any information missing | ||||||||
| Medication Information | Sample Size | Missing | % | 95% CI | Sample Size | Missing | % | 95% CI |
| Discharge medications | 811 | 12 | 1.5 | 0.72.3 | 638 | 6 | 0.9 | 0.21.7 |
| Drug allergies | 811 | 47 | 5.8 | 4.27.4 | 639 | 35 | 5.5 | 3.77.2 |
| Explanation for any differences between preadmission and discharge medications | 542 | 275 | 50.7 | 46.555 | 498 | 88 | 17.7 | 14.321.0 |
| Test results information | Sample Size | Missing | % | 95% CI | Sample Size | Missing | % | 95% CI |
| Latest pertinent lab results | 790 | 178 | 22.5 | 19.625.4 | 629 | 73 | 11.6 | 9.114.1 |
| Pertinent radiology results | 668 | 110 | 16.5 | 13.719.3 | 601 | 27 | 4.5 | 2.86.2 |
| Test results pending at time of transfer | 183 | 87 | 47.5 | 40.354.8 | 152 | 73 | 48.0 | 40.156.0 |
| Management Information | Sample Size | No | % | 95% CI | Sample Size | No | % | 95% CI |
| Were management and follow‐up plans adequately described? | 794 | 121 | 15.2 | 12.717.7 | 631 | 79 | 12.5 | 9.915.1 |
| Sample Size | Yes | % | 95% CI | Sample Size | Yes | % | 95% CI | |
| Did you uncover a significant condition not mentioned in the discharge packet? | 793 | 117 | 14.8 | 12.317.2 | 635 | 38 | 6.0 | 4.47.8 |
Effective communication among physicians during the hospital discharge process is critical to patient care. Patients are at high risk of having an adverse drug event,1 readmission, or death2 during the transition from hospital to home.3 Ineffective communication between inpatient and outpatient providers has been implicated as a leading cause of adverse events.35 Conversely, efforts to improve communication have been shown to improve compliance with follow‐up tests and decrease readmission rates.6, 7 Recently, the absence of several specific data elements in discharge documentation have been shown to be common and to have potential for patient harm, including test results that are pending at the time of discharge.8, 9 Unexplained discrepancies between preadmission and discharge medication regimens are also common and potentially dangerous.1
According to the Joint Commission for Accreditation of Healthcare Organizations (TJC), the following elements should be included in discharge summaries: the reason for hospitalization; significant findings; procedures performed and care, treatment, and services provided; the patient's condition at discharge; and information provided to the patient and family, as appropriate.10 TJC also advocates medication reconciliation, a process of identifying the most accurate list of all medications a patient is takingincluding name, dosage, frequency, and routeand using this list to provide correct medications for patients anywhere within the health care system.11
Despite the importance of complete communication among providers at hospital discharge, a recent systematic review showed that discharge summaries often lacked important information such as diagnostic test results (missing from 33%‐63%), treatment or hospital course (7%‐22%), discharge medications (2%‐40%), test results pending at discharge (65%), patient or family counseling (90%‐92%), and follow‐up plans (2%‐43%).1
Most of the studies addressing this issue have evaluated communication pitfalls between acute care hospitals and primary care physicians among patients discharged home.17 In contrast, the quality of discharge documentation among patients discharged to rehabilitation centers and other subacute care facilities has been less well studied, perhaps due to relatively smaller numbers of patients discharged to such facilities. This communication is as or more important because these patients are potentially more vulnerable and their medical conditions more active than for patients discharged home.12 Furthermore, discharge information from acute care hospitals will often form the basis for admission orders at subacute facilities. Last, these patients will have a second transition in care (from subacute facility to home) whose quality is dependent at least in part on the quality of communication during the first transition.
The aim of this study was to evaluate the quality of information transfer among patients discharged from acute hospitals to subacute facilities across an integrated healthcare delivery system. The long‐term goals of this effort were to determine the areas most in need of improvement, to guide interventions to address these problems, and to track improvements in these measures over time as interventions are implemented and refined.
Methods
This observational study was conducted as part of a quality improvement project evaluating the quality of information provided during the discharge process across Partners Health Care System. The institutional review boards of the participating institutions approved the study.
Study Sample
We evaluated a sample of discharge documentation packets (eg, discharge summaries, discharge orders, nursing instructions, care coordination, and physical/occupational therapy notes) of patients discharged from all 5 acute care hospitals of the Partners Healthcare System to 30 subacute facilities (rehabilitation hospitals and skilled nursing facilities) from March 2005 through June 2007.
For reviewers at acute sites, discharge documentation packets were randomly selected each quarter using a random number generator within Microsoft Excel (Microsoft, Redmond, WA). At subacute sites, reviewers selected which packets to review, although they were encouraged to review all of them. Random selection of packets could not be achieved at subacute sites because reviews took place on the day of admission to the subacute facility. All reviewers received 1 hour of training on how to evaluate discharge packets, including review of a standardized teaching packet with 1 of the coauthors (J.L.S. or T.O.).
Two of the 5 acute care hospitals in the study are academic medical centers and the other 3 are community hospitals. Reviewers were a mix of trained medical residents or nurse practitioners at acute sites and admitting physicians or nurse practitioners at receiving subacute sites.
Fifty packets were reviewed per acute site per quarter. This provided roughly 10% precision around our estimates (ie, if compliance with a measure were 80%, the 95% confidence interval around this estimate would be 70%‐90%). This sample size is consistent with those used to obtain other national benchmarks, such as those for National Hospital Quality Measures, which generally require at least 35 cases per quarter.13
Measures
A multidisciplinary team at Partners derived, reviewed, and refined a minimum data set required to appropriately care for patients during the first 72 hours after transfer from an acute care hospital to a subacute facility. Several of these measures are required by TJC. Other measures were either modifications of TJC measures made to facilitate uniform data collection (eg, history and physical examination at admission instead of significant findings) or additional data elements (not required by TJC) felt to be important to patient care based on the medical literature and interviews with receiving providers at subacute facilities. All measures were refined by the multidisciplinary team with input from additional subspecialists as needed (see Table 1 for the final list of measures).
| Reason(s) for Admission | |
|---|---|
| Joint Commission requirements | A focused history |
| A focused physical exam | |
| Pertinent past medical history | |
| Treatment rendered | |
| Discharge diagnosis(es) | |
| Condition on discharge | |
| Discharge summary | |
| Any information missing | |
| Non‐Joint Commission requirements | |
| Medication information | Discharge medications |
| Drug allergies | |
| Preadmission medication information | |
| Explanation for any differences between preadmission and discharge medications | |
| Test results information | Latest pertinent laboratory results |
| Pertinent radiology results | |
| Test results pending at time of transfer | |
| Overall assessment | Were management and follow‐up plans adequately described? |
| Did you uncover a significant condition not mentioned in the discharge packet? | |
Data Collection
After reviewing the entire discharge documentation packet, reviewers completed a survey concerning the inclusion of the required data elements. Surveys were completed online using Perseus Survey Solutions 6.0 (Perseus Development Corp., Braintree, MA) in the month following discharge (for reviewers at acute care sites) or within 24 hours of admission to the subacute facility (for reviewers at subacute sites). To verify the accuracy and completeness of packets, reviewers at acute sites were instructed to compare the discharge documentation to a review of the inpatient medical record. Similarly, reviewers at subacute sites were instructed to complete their evaluations after admitting each patient to their facility.
Outcomes
The primary outcome was the proportion of packets that contained each data element. In addition, we calculated the proportion of packets that contained all applicable elements required by TJC and all applicable data elements measured in the study. Last, we evaluated two global (albeit subjective) measures of satisfaction with the packet: Were management and follow‐up plans adequately described? (both components needed to be adequately described to get credit for this question) and Did you uncover a significant condition not mentioned in the discharge packet? Significant conditions were defined as active medical problems requiring management during or immediately following the hospitalization.
Statistical Analysis
Results were calculated as proportions, odds ratios, and 95% confidence intervals (CI), using SAS version 9.1 (SAS Institute, Inc., Cary, NC). Simple logistic regression was used to compare inclusion of data elements between medical and surgical services and between academic medical centers and community hospitals. To evaluate interrater reliability, 2 reviewers (both at acute sites) independently evaluated 29 randomly chosen charts, each with 12 data elements.
Results
A total of 1501 discharge documentation packets were reviewed, including 980 patients (65%) from a medical unit and 521 patients (35%) from a surgical unit. Based on 2007 data, these packets represent approximately 4% of all eligible discharges to subacute facilities. Patients discharged from 1 of the 2 academic medical centers represented 44% of the sample. A total of 644 discharge packets (43%) were reviewed at acute sites and 814 packets (54%) were reviewed at subacute sites. Information about reviewer site was missing in 43 discharge packets (3%). For the 29 charts independently reviewed by 2 reviewers, there was complete agreement for 331 out of 348 data elements (95.1%).
Only 1055 (70%) discharge summaries had all the information required by TJC (Table 2). Physical examination at admission (a component of significant findings, as noted above) and condition at discharge were the 2 elements most often missing. The defect‐free rate varied by site, with a range of 61% to 76% across the 5 acute care hospitals (data not shown).
| Sample Size | Missing [n (%)] | 95% CI Missing % | |
|---|---|---|---|
| |||
| Joint Commission requirements | |||
| Reason(s) for admission | 1497 | 14 (0.9) | 0.41.4 |
| A focused history | 1493 | 65 (4.4) | 3.35.3 |
| A focused physical exam | 1493 | 170 (11.4) | 9.713 |
| Pertinent past medical history | 1494 | 69 (4.6) | 3.55.6 |
| Treatment rendered | 1494 | 33 (2.2) | 1.42.9 |
| Discharge diagnosis(es) | 1480 | 53 (3.6) | 2.64.5 |
| Condition on discharge | 1462 | 208 (14.2) | 12.416.0 |
| Discharge summary | 1475 | 90 (6.1) | 4.87.3 |
| Any information missing | 1501 | 447 (29.7) | 27.432.0 |
| Non‐Joint Commission requirements | |||
| Medication information | |||
| Discharge medications | 1491 | 19 (1.3) | 0.71.8 |
| Drug allergies | 1470 | 88 (6.0) | 4.77.2 |
| Preadmission medication information | 1460 | 297 (20.3) | 18.322.4 |
| Explanation for any differences between preadmission and discharge medications | 1060 | 374 (35.3) | 32.038.1 |
| Test results information | |||
| Latest pertinent lab results | 1460 | 261 (17.9) | 15.919.8 |
| Pertinent radiology results | 1303 | 139 (10.7) | 912.4 |
| Test results pending at time of transfer | 341 | 160 (47.2) | 41.952.5 |
| Overall assessment | |||
| Were management and follow‐up plans adequately described? | 1461 | No (%): 161 (11.1) | 95% CI No %: 9.512.7 |
| Did you uncover a significant condition not mentioned in the discharge packet? | 1469 | Yes (%): 162 (11.0) | 95% CI Yes %: 9.413.0 |
| All applicable elements present | 1501 | 503 (33.5) | 31.135.9 |
The rates of inclusion of other (non‐TJC required) data elements are shown in Table 2. Most often missing were preadmission medication regimens, any documented reason for any difference between preadmission and discharge medications, pertinent laboratory results, and an adequate follow‐up plan (including who to follow up with, when to follow‐up, and a list of tasks to be accomplished at the follow‐up visit). Notation regarding significant test results that were pending at the time of transfer was missing in 160 of 341 applicable patients (47%), and in 162 patients (11%), physicians uncovered a significant condition that was not mentioned in the discharge documentation. Only 503 (33.5%) discharge documentation packets had all applicable measures present. In addition, the discharge summary was not received at all on the day of discharge according to the receiving site in 90 patients (6%).
Reviewers were asked in a separate question which missing data were necessary for patient care. Data elements most often cited were explanations for any medication discrepancies and test results pending at the time of the hospital discharge.
Community hospitals had a higher rate of inclusion of TJC‐required data elements when compared to academic medical centers (Table 3). Also, among non‐TJC required data elements, inclusion rates were higher among the community hospitals, especially regarding information about medication discrepancies, pending test results, and follow‐up information (Table 3).
| Total (n) | All Elements Present [n (%)] | OR (95% CI) | |
|---|---|---|---|
| |||
| Joint Commission requirements | |||
| Hospital type | |||
| Community hospitals | 949 | 826 (87) | 2.7 (2.13.6) |
| Academic medical centers | 541 | 384 (71) | Ref. |
| Service | |||
| Medical services | 1013 | 745 (73) | 1.3 (1.01.7) |
| Surgical services | 488 | 332 (68) | Ref. |
| Explanation for any medication discrepancies | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 718 | 550 (76) | 5.0 (3.86.5) |
| Academic medical centers | 342 | 136 (39) | Ref. |
| Service | |||
| Medical services | 754 | 529 (70) | 2.2 (1.72.9) |
| Surgical services | 306 | 157 (51) | Ref. |
| Test results pending at time of transfer | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 172 | 109 (63) | 2.4 (1.53.7) |
| Academic medical centers | 169 | 71 (42) | Ref. |
| Service | |||
| Medical services | 227 | 146 (64) | 4.2 (2.66.9) |
| Surgical services | 114 | 34 (30) | Ref. |
| Follow‐up plans adequately described | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 968 | 883 (91) | 1.7 (1.22.4) |
| Academic medical centers | 543 | 466 (85) | Ref. |
| Service | |||
| Medical services | 983 | 862 (87) | 0.67 (0.51.0) |
| Surgical services | 478 | 437 (91) | Ref. |
Although no differences were found between medical and surgical services regarding compliance with TJC requirements, a difference was noted in documentation of explanations of medication discrepancies and pending test results, with medical services performing better in both measures (Table 3).
In general, reviewers at subacute sites more often evaluated packets as deficient than reviewers at acute sites, up to an absolute difference of 33% in the proportion of missing data, depending on the data element (see Appendix, Table 1).
Discussion
Our study evaluated the completeness of documentation in the discharge summaries of patients discharged from acute care to subacute care facilities. Our results for the inclusion of TJC‐required data elements were similar to those quoted in the literature for patients discharged home.6 Our results also demonstrated a high rate of other missing data elements that are arguably of equal or greater importance, including reasons for discrepancies between preadmission and discharge medication regimens and tests that are pending at the time of discharge.1, 8, 9 Our results also demonstrated the relatively poorer performance of academic centers compared to community hospitals regarding inclusion of information about medication reconciliation, follow‐up, pending test results, and complete information required by TJC. Finally, we found that patients discharged from surgical services more often lacked documentation of medication discrepancies and pending test results compared with patients from medical services.
To our knowledge, this is one of the first studies looking at the quality of information transfer in patients discharged to subacute care facilities. The results of this study are not surprising given the known problems with general information transfer at hospital discharge.1 The fact that community hospitals provided more complete information than academic medical centers for certain data elements may be due to the difference between residents and more senior physicians preparing discharge documentation. Such differences could reflect differences in experience, training, and degree of appreciation for the importance of discharge documentation, and/or restrictions in work hours among residents (eg, resulting in time‐pressure to complete discharge summaries and/or summaries being written by residents who know the patients less well). These hypotheses deserve further exploration. The differences between medical and surgical services should also be validated and explored in other healthcare systems, including both academic and community settings.
The results of this study should be viewed in light of the study's limitations. Packets evaluated by reviewers at subacute facilities were chosen by the reviewers and may not have been representative of all patients received by that facility (in contrast to those reviewed at the acute sites, which were chosen at random and more likely to be representative, although we did not formally test for this). It is possible that reviewers at subacute sites selected the worst discharge documentation packets for evaluation. Second, evaluations by reviewers at subacute sites did not distinguish between information missing from discharge documentation and failure to receive the documentation at all from the acute care hospital (again in contrast to reviewers at acute sites, who always had access to the documentation). Lastly, reviewers at acute and subacute sites may have graded packets differently due to their different clinical perspectives. These 3 factors may explain the relatively poorer results of discharge packets reviewed by reviewers at subacute sites. Further study would be needed to distinguish among these possibilities (eg, having acute and subacute reviewers answer the same questions for the same discharge packets to allow us to measure interrater reliability between the different kinds of reviewers; explicitly asking subacute reviewers about receipt of each piece of documentation; comparing the distribution of diagnosis‐related group [DRG] codes and hospital length of stay in evaluated vs. total discharge packets as a measure of representativeness). We also cannot rule out the possibility of reviewer bias, but all reviewers were trained in a standardized fashion and we know that reliability of assessments were high, at least among reviewers at acute sites. Last, we did not measure actual or potential adverse events caused by these information deficits.
As part of a Partners‐wide initiative to improve transitions in care, the results were presented to the administrations of each of the 5 acute care hospitals. The Partners High Performance Medicine Transition team then began work with a steering committee (composed of representatives from each hospital) to address these deficiencies. Since then, the hospitals have taken several steps to improve the quality of information transfer for discharged patients, including the following:
Technological improvements to the hospitals' discharge ordering systems to actively solicit and/or autoimport the required information into discharge documentation.
Creation of discharge templates to record the required information on paper.
Provision of feedback to clinicians and their service chiefs regarding the ongoing quality of their discharge documentation.
Creation of an online Partners‐wide curriculum on discharge summary authorship, with a mandatory quiz to be taken by all incoming clinicians.
In conclusion, we found room for improvement in the inclusion of data elements required for the safe transfer of patients from acute hospitals to subacute facilities, especially in areas such as medication reconciliation, pending test results, and adequate follow‐up plans. We also found variation by site and type of service. For patients discharged to rehabilitation and other subacute facilities, improvement is needed in the communication of clinically relevant information to those providing continuing care.
Appendix
| JCAHO Indicators | Reviews from Sub‐Acute Sites (N = 814)* | Reviews from Acute Sites (N = 644)* | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample Size | Missing N | % | 95% CI | Sample Size | Missing | % | 95% CI | |
| ||||||||
| Reason(s) for admission | 812 | 9 | 1.1 | 0.41.8 | 643 | 4 | 0.6 | 0.011.2 |
| A focused history | 810 | 49 | 6.1 | 4.47.7 | 642 | 16 | 2.5 | 1.33.7 |
| A focused physical exam | 810 | 131 | 16.2 | 13.718.7 | 641 | 34 | 5.3 | 3.67.0 |
| Pertinent past medical history | 810 | 50 | 6.2 | 4.57.8 | 642 | 14 | 22.0 | 1.13.3 |
| Treatment rendered | 811 | 29 | 3.6 | 2.34.9 | 641 | 4 | 0.6 | 0.011.2 |
| Discharge diagnosis(es) | 806 | 59 | 7.3 | 5.59.1 | 630 | 7 | 1.1 | 0.31.9 |
| Condition on discharge | 800 | 92 | 11.5 | 9.313.7 | 622 | 109 | 17.5 | 14.520.5 |
| Discharge summary | 809 | 77 | 9.5 | 7.511.5 | 624 | 11 | 1.8 | 0.72.8 |
| Any information missing | ||||||||
| Medication Information | Sample Size | Missing | % | 95% CI | Sample Size | Missing | % | 95% CI |
| Discharge medications | 811 | 12 | 1.5 | 0.72.3 | 638 | 6 | 0.9 | 0.21.7 |
| Drug allergies | 811 | 47 | 5.8 | 4.27.4 | 639 | 35 | 5.5 | 3.77.2 |
| Explanation for any differences between preadmission and discharge medications | 542 | 275 | 50.7 | 46.555 | 498 | 88 | 17.7 | 14.321.0 |
| Test results information | Sample Size | Missing | % | 95% CI | Sample Size | Missing | % | 95% CI |
| Latest pertinent lab results | 790 | 178 | 22.5 | 19.625.4 | 629 | 73 | 11.6 | 9.114.1 |
| Pertinent radiology results | 668 | 110 | 16.5 | 13.719.3 | 601 | 27 | 4.5 | 2.86.2 |
| Test results pending at time of transfer | 183 | 87 | 47.5 | 40.354.8 | 152 | 73 | 48.0 | 40.156.0 |
| Management Information | Sample Size | No | % | 95% CI | Sample Size | No | % | 95% CI |
| Were management and follow‐up plans adequately described? | 794 | 121 | 15.2 | 12.717.7 | 631 | 79 | 12.5 | 9.915.1 |
| Sample Size | Yes | % | 95% CI | Sample Size | Yes | % | 95% CI | |
| Did you uncover a significant condition not mentioned in the discharge packet? | 793 | 117 | 14.8 | 12.317.2 | 635 | 38 | 6.0 | 4.47.8 |
- , , , et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- , , , .Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.1989;19:624–631.
- , , , , , .Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- , , , .Effect of discharge summary availability during post‐discharge visits on hospital readmission.JGen Intern Med.2002;17:186–192.
- , , , .Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18:646–651.
- , , , .Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- , , , et al.Impact of a standardized communication system on continuity of care between family physicians and the emergency department.CJEM.2007;9:79–86.
- , , , et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- , , .Tying up loose ends: discharging patients with unresolved medical issues.Arch Intern Med.2007;167:1305–1311.
- Standard IM.6.10: Hospital Accreditation Standards.Oakbrook Terrace, IL:Joint Commission on Accreditation of Healthcare Organizations;2006:338–340.
- Joint Commission on Accreditation of Healthcare Organizations. Joint Commission national patient safety goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals. Accessed July 2009.
- , .Effectiveness of multidisciplinary rehabilitation services in post acute care: state‐of‐the‐science. A review.Arch Phys Med Rehabil.2007;88:1526–1534.
- Joint Commission on Accreditation of Healthcare Organizations. Specification Manual for National Hospital Quality Measures: Population and Sampling Specifications Version 2.4. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Current+NHQM+Manual.htm. Accessed July 2009.
- , , , et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- , , , .Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.1989;19:624–631.
- , , , , , .Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- , , , .Effect of discharge summary availability during post‐discharge visits on hospital readmission.JGen Intern Med.2002;17:186–192.
- , , , .Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18:646–651.
- , , , .Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- , , , et al.Impact of a standardized communication system on continuity of care between family physicians and the emergency department.CJEM.2007;9:79–86.
- , , , et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- , , .Tying up loose ends: discharging patients with unresolved medical issues.Arch Intern Med.2007;167:1305–1311.
- Standard IM.6.10: Hospital Accreditation Standards.Oakbrook Terrace, IL:Joint Commission on Accreditation of Healthcare Organizations;2006:338–340.
- Joint Commission on Accreditation of Healthcare Organizations. Joint Commission national patient safety goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals. Accessed July 2009.
- , .Effectiveness of multidisciplinary rehabilitation services in post acute care: state‐of‐the‐science. A review.Arch Phys Med Rehabil.2007;88:1526–1534.
- Joint Commission on Accreditation of Healthcare Organizations. Specification Manual for National Hospital Quality Measures: Population and Sampling Specifications Version 2.4. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Current+NHQM+Manual.htm. Accessed July 2009.
Copyright © 2009 Society of Hospital Medicine

