User login
Neurocognitive Deficits and Cerebral Desaturation During Shoulder Arthroscopy With Patient in Beach-Chair Position: A Review of the Current Literature
The beach-chair position (BCP) is commonly used for both arthroscopic and open shoulder surgery. This technique positions the shoulder in an anatomical upright position, facilitating shoulder access and visualization.1 Compared with the lateral decubitus position, the BCP also improves airway access, reduces bleeding, and lessens the risk for brachial plexus injury.2
Despite the advantages of using the BCP, there have been multiple reports of catastrophic neurologic complications, including severe brain damage and death, in relatively healthy patients without any known risk factors.3-6 The definitive etiology of these complications remains unclear, but it has been hypothesized that BCP use may be an independent risk factor for cerebrovascular ischemia,1,5-16 as the upright position can cause hypotension leading to increased risk for cerebral hypoperfusion.7-11,17 Reducing cerebral perfusion pressure below critical thresholds may result in permanent neurologic injury.4-6,14 Therefore, monitoring of cerebral perfusion and optimization of intraoperative cerebral oxygenation have been recommended to help avoid potential neurologic complications. However, a direct relationship between intraoperative cerebral desaturation events (CDEs) and postoperative neurocognitive deficits has not been definitively established.1,9-12
To put into perspective the importance of detecting and preventing CDEs and neurologic complications, we can consider the incidence of fatal pulmonary embolism associated with total joint arthroplasty. Although the incidence is very low, about 0.1% to 2.0%, some form of venous thromboembolism prophylaxis is the standard of care for helping prevent this serious complication. Similarly, catastrophic neurologic complications of upright shoulder arthroscopy are very rare, but it is still important to consider measures that help minimize them.
We reviewed the literature for the incidence of postoperative neurocognitive deficits, number of reported neurocognitive complications, and incidence of intraoperative CDEs in patients who underwent arthroscopic shoulder surgery in the BCP.
Methods
Dr. Salazar and Dr. Hazel independently searched the Medline, Cochrane, and Embase databases for case series, prospective studies, and cohort studies that reported neurocognitive complications associated with the BCP and the incidence of intraoperative CDEs. The authors used beach chair, desaturation, near infrared spectroscopy, and shoulder as medical subject headings (MeSH). In addition, bibliographies of retrieved articles were checked for studies that the search terms may have missed. Eighty-one publications were identified and reviewed for possible inclusion.
Next, the same 2 authors reviewed the titles and abstracts for relevance and determined which articles had potential to contribute to the study. Only English-language publications were considered for inclusion. To review the incidence of postoperative neurocognitive deficits, we included only those studies with more than 25 patients, documentation of postoperative complications, and arthroscopic shoulder surgery performed with the patient in the seated, semi-upright, or BCP. Only studies with at least 25 patients were used in order to increase the power and improve the level of evidence. To review reported cases of neurocognitive complications, we included all relevant case reports and case series. To review the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive deficits, we included studies that reported on use of intraoperative cerebral perfusion monitoring. Modalities used in these studies included near infrared spectroscopy, electroencephalography, and invasive blood pressure monitoring calculated at the brain level. Studies were excluded if they did not involve arthroscopic shoulder surgery or were not conducted with human subjects.
Information recorded for each study included general information such as author and publication year, type of study, number of patients enrolled, type of intraoperative monitoring, anesthesia protocol, number of patients with CDEs, and number of patients with neurocognitive complications after surgery.
Results
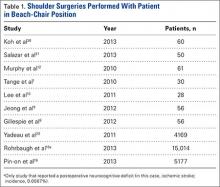
Our search identified 81 publications for potential inclusion. Our first aim was to identify the overall incidence of reported neurocognitive deficits after arthroscopic shoulder surgery with the patient in the BCP. We identified 10 studies (Table 1) that met the inclusion criteria. Among the 24,701 patients in these 10 studies, there was only 1 reported case of neurocognitive deficit after surgery, in a mixed prospective-retrospective study of 15,014 cases by Rohrbaugh and colleagues.18 The deficit they reported was an ischemic cerebral vascular accident. The 0.0067% incidence in their study demonstrates how rare the complication is. Two large retrospective studies (Ns = 4169 and 5177 patients) found no postoperative neurocognitive complications.19,20 Only 3 studies performed formal postoperative cognitive testing. Salazar and colleagues21 used the Repeatable Battery for the Assessment of Neuropsychological Status before and after surgery, and Gillespie and colleagues8 and Lee and colleagues10 used the Mini–Mental State Examination before and after surgery. Total incidence of reported neurocognitive deficits from our review was 0.004% (1/24,701).
Our second aim was to review all reported cases of neurocognitive complications after arthroscopic shoulder surgery with the patient in the BCP. We identified 4 publications that fit our inclusion criteria (Table 2). Pohl and Cullen6 described 4 cases of ischemic brain injury after arthroscopic shoulder surgery with the patient in the BCP. Age range was 47 to 57 years. Specific intraoperative cerebral monitoring was not used. However, these patients had several episodes of intraoperative hypotension (systolic blood pressures, 80-90 mm Hg), measured with a traditional blood pressure cuff on the arm. In general, these patients had minimal cerebrovascular risk factors and no known preexisting cerebrovascular disease. Drummond and colleagues22 described an ischemic stroke in a 50-year-old man after arthroscopic subacromial decompression and open rotator cuff repair that resulted in unresolved right hemiplegia. Subsequent diagnostic investigation revealed an asymmetry of the circle of Willis resulting in limited flow to the left anterior and middle cerebral artery distributions. Bhatti and Enneking3 reported the case of a 64-year-old man who lost vision in the right eye immediately after arthroscopic rotator cuff repair. His vision improved spontaneously the next morning and continued to improve over the next 6 months—he regained 20/20 vision with some residual optic neuropathy.
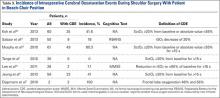
Our third aim was to determine the incidence of intraoperative CDEs during arthroscopic shoulder surgery with the patient in the BCP. Incidence of CDEs varied widely among the 7 studies reviewed (Table 3). Minimum incidence of intraoperative CDE was 0% in a cohort of 30 patients,1 and maximum incidence was 80% in a study of 61 patients,12 all of whom underwent elective arthroscopic shoulder surgery in the BCP. Although there was wide variability in CDE incidence, the studies were consistent with respect to their definition of a CDE. Most authors used a decrease in regional cerebral tissue oxygen saturation of 20% or more from baseline, or an absolute value up to 55%, to define a CDE. None of the 7 studies reviewed reported a clinically significant adverse neurocognitive event.
Discussion
Of concern, there have been several surveys, case reports, and small case series of previously healthy patients who had no known risk factors, underwent arthroscopic shoulder surgery in the BCP, and developed unanticipated postoperative neurologic complications.4-6,14 Beach-chair positioning during surgical procedures has been implicated as a contributing factor leading to cerebral hypoperfusion with potential for cerebral ischemia.1,12,23 These changes in cerebral perfusion pressure are thought to be the major determinant of poor neurologic outcomes. Such reports have exposed the potential need for heightened vigilance, alternative anesthesia techniques, and improved monitoring, though the exact etiology of the central nervous system injuries in this patient population is incompletely understood and is likely multifactorial. Therefore, in this study we wanted to determine the incidence of postoperative neurocognitive deficits and review all reported cases of neurocognitive complications in patients who have undergone arthroscopic shoulder surgery in the BCP. In addition, we wanted to define the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive complications.
According to our review, the incidence of postoperative neurocognitive complications after surgery in the BCP is 0.004% (1/24,701). However, this finding is based only on what has been reported; the true incidence is not known. It is also important to note that the incidence of neurocognitive deficits after many other types of surgery is not known and that surgery itself may be a risk factor for postoperative neurocognitive deficits.24 In their retrospective review of 15,014 patients who underwent arthroscopic shoulder surgery in the BCP at a single institution over an 11-year period, Rohrbaugh and colleagues18 found an overall postoperative complication rate of 0.37% and a 0.0067% incidence of neurocognitive deficits. One patient in the series was given a diagnosis of ischemic stroke on the basis of neurologic deficits that occurred 24 hours after surgery. Yadeau and colleagues20 found no postoperative neurocognitive complications in a mixed prospective-retrospective study of 4169 patients—3000 identified retrospectively, 1169 prospectively—who underwent arthroscopic shoulder surgery in the BCP at an ambulatory surgery center. Pin-on and colleagues19 reported on a series of 5177 orthopedic and neurosurgical patients who underwent surgery in the BCP. In those who had arthroscopic shoulder surgery, intraoperative systolic blood pressures obtained from an arterial line referenced to heart level decreased a mean (SD) of 14.4% (12.7%), whereas in those whose pressures were obtained from a noninvasive blood pressure cuff referenced to heart level decreased 19.3% (12.6%). However, the authors reported no incidence of postoperative stroke or neurologic deficits.
Although uncommon, perioperative cerebral ischemic accidents are potentially devastating for patients, their families, and the health care professionals involved. These events have tremendous economic, social, professional, and medicolegal implications, with perioperative stroke being particularly morbid. Perioperative stroke has a mortality rate of 60%, versus 15% to 46% for stroke in general.25,26 In 2005, Pohl and Cullen6 published a landmark article on a series of 4 relatively healthy middle-aged patients who were at low risk for stroke but had catastrophic neurocognitive complications (including 1 death) after arthroscopic shoulder surgery in the BCP. Bhatti and Enneking3 described a case of acute postoperative vision loss and ophthalmoplegia attributed to intraoperative hypotension leading to ischemia in a patient who underwent an elective shoulder arthroscopic procedure in the BCP. These reports prompted multiple investigations into the physiologic hemodynamic changes associated with surgery in the BCP and the treatment strategies used to improve patient safety.
In the normal physiologic state, the sympathetic nervous system is activated when a person assumes the seated position. The result is increased systemic vascular resistance and heart rate alterations to maintain cardiac output and mean arterial pressure. In anesthetized patients, this response is blunted by the vasodilatory effects of intravenous and volatile anesthetics. Multiple studies have demonstrated substantial hemodynamic changes in both awake and anesthetized patients during the maneuver from the supine position to the seated position1,27,28; these changes include diminished cardiac index, stroke volume, and arterial pressure.17 The data underscore the need for attentiveness and accurate monitoring of cerebral perfusion when the transition is made from the supine position to the BCP, particularly in the early phase of surgery and in high-risk patients.
Knowledge of these hemodynamic changes has led several authors to recommend additional intraoperative monitoring of cerebral perfusion. Monitoring techniques have included use of invasive blood pressure monitoring adjusted to brain level, cerebral oximetry using near infrared spectroscopy, and electroencephalography. However, the clinical relevance of intraoperative CDEs in isolation is not well understood.1,6,7,23 In addition, cost and availability of additional advanced monitoring likely factor into why it is not more commonly used. For this patient population, the severity, frequency, and duration of desaturation that causes cerebral ischemia and the relationship with postoperative neurocognitive deficits remain undefined.
The incidence of CDEs in patients being monitored with near infrared spectroscopy while undergoing elective arthroscopic shoulder surgery in the BCP varies widely, from 0% to 80% (mean, 41%).1,4,7,10,12,21 Magnitude and duration of cerebral ischemia required to produce neurocognitive dysfunction in this patient population remain unidentified as well. In conscious patients, a 20% reduction in frontal lobe oxygenation is associated with clinical manifestations of cerebral hypoperfusion, such as syncope.15,29 As none of the patients in the studies we reviewed experienced any sort of deficit, we cannot definitively state there is a correlation between CDE occurrence and neurocognitive deficit.
One limitation of our investigation is that it was a systemic review, and thus there was substantial heterogeneity in the methods and designs of the studies included in the analysis. Among the different series, there was variability in multiple aspects of the study design, including type of anesthetic, patient inclusion criteria, type of surgery, type of intraoperative cerebral perfusion monitoring, and type of neurocognitive testing. As a result, comparing the groups was difficult, and the generalizability of our findings may be limited. In addition, it is difficult to accurately establish incidence and comprehensively review these events because of the paucity of literature.
Conclusion
Neurocognitive complications after shoulder arthroscopy with the patient in the BCP are extremely rare but potentially devastating events that can affect healthy patients with no preexisting cerebrovascular risk factors. Our review indicated the incidence of permanent neurologic deficit after arthroscopy in the BCP may be as low as 0.004%. The exact etiology of such complications is not clear. Basic science research and large prospective studies are needed to identify the clinically relevant thresholds of magnitude, duration, and frequency of intraoperative CDEs in order to establish their relationship with postoperative neurocognitive complications. Such large studies may also elucidate modifiable patient-specific risk factors and establish the most sensitive, safe, and cost-effective intraoperative monitoring tools. Current literature suggests that accurate intraoperative monitoring of cerebral perfusion, alternatives to general anesthesia, and prudent use of intraoperative blood pressure control may improve patient safety.
1. Tange K, Kinoshita H, Minonishi T, et al. Cerebral oxygenation in the beach chair position before and during general anesthesia. Minerva Anestesiol. 2010;76(7):485-490.
2. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
3. Bhatti MT, Enneking FK. Visual loss and ophthalmoplegia after shoulder surgery. Anesth Analg. 2003;96(3):899-902.
4. Friedman DJ, Parnes NZ, Zimmer Z, Higgins LD, Warner JJ. Prevalence of cerebrovascular events during shoulder surgery and association with patient position. Orthopedics. 2009;32(4).
5. Papadonikolakis A, Wiesler ER, Olympio MA, Poehling GG. Avoiding catastrophic complications of stroke and death related to shoulder surgery in the sitting position. Arthroscopy. 2008;24(4):481-482.
6. Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17(6):463-469.
7. Dippmann C, Winge S, Nielsen HB. Severe cerebral desaturation during shoulder arthroscopy in the beach-chair position. Arthroscopy. 2010;26(9 suppl):S148-S150.
8. Gillespie R, Shishani Y, Streit J, et al. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am. 2012;94(14):1284-1290.
9. Jeong H, Lee SH, Jang EA, Chung SS, Lee J, Yoo KY. Haemodynamics and cerebral oxygenation during arthroscopic shoulder surgery in beach chair position under general anaesthesia. Acta Anaesthesiol Scand. 2012;56(7):872-879.
10. Lee JH, Min KT, Chun YM, Kim EJ, Choi SH. Effects of beach-chair position and induced hypotension on cerebral oxygen saturation in patients undergoing arthroscopic shoulder surgery. Arthroscopy. 2011;27(7):889-894.
11. Moerman AT, De Hert SG, Jacobs TF, De Wilde LF, Wouters PF. Cerebral oxygen desaturation during beach chair position. Eur J Anaesthesiol. 2012;29(2):82-87.
12. Murphy GS, Szokol JW, Marymont JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111(2):496-505.
13. Peruto CM, Ciccotti MG, Cohen SB. Shoulder arthroscopy positioning: lateral decubitus versus beach chair. Arthroscopy. 2009;25(8):891-896.
14. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
15. Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. 2000;93(4):964-970.
16. Smythe PR, Samra SK. Monitors of cerebral oxygenation. Anesthesiol Clin North Am. 2002;20(2):293-313.
17. Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34(5):375-386.
18. Rohrbaugh M, Kentor ML, Orebaugh SL, Williams B. Outcomes of shoulder surgery in the sitting position with interscalene nerve block: a single-center series. Reg Anesth Pain Med. 2013;38(1):28-33.
19. Pin-on P, Schroeder D, Munis J. The hemodynamic management of 5177 neurosurgical and orthopedic patients who underwent surgery in the sitting or “beach chair” position without incidence of adverse neurologic events. Anesth Analg. 2013;116(6):1317-1324.
20. Yadeau JT, Casciano M, Liu SS, et al. Stroke, regional anesthesia in the sitting position, and hypotension: a review of 4169 ambulatory surgery patients. Reg Anesth Pain Med. 2011;36(5):430-435.
21. Salazar D, Sears BW, Aghdasi B, et al. Cerebral desaturation events during shoulder arthroscopy in the beach chair position: patient risk factors and neurocognitive effects. J Shoulder Elbow Surg. 2013;22(9):1228-1235.
22. Drummond JC, Lee RR, Howell JP Jr. Focal cerebral ischemia after surgery in the “beach chair” position: the role of a congenital variation of circle of Willis anatomy. Anesth Analg. 2012;114(6):1301-1303.
23. Fischer GW, Torrillo TM, Weiner MM, Rosenblatt MA. The use of cerebral oximetry as a monitor of the adequacy of cerebral perfusion in a patient undergoing shoulder surgery in the beach chair position. Pain Pract. 2009;9(4):304-307.
24. Wong GY, Warner DO, Schroeder DR, et al. Risk of surgery and anesthesia for ischemic stroke. Anesthesiology. 2000;92(2):425-432.
25. Knapp RB, Topkins MJ, Artusio JF Jr. The cerebrovascular accident and coronary occlusion in anesthesia. JAMA. 1962;182:332-334.
26. Landercasper J, Merz BJ, Cogbill TH, et al. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125(8):986-989.
27. Fuchs G, Schwarz G, Kulier A, Litscher G. The influence of positioning on spectroscopic measurements of brain oxygenation. J Neurosurg Anesthesiol. 2000;12(2):75-80.
28. Lovell AT, Owen-Reece H, Elwell CE, Smith M, Goldstone JC. Continuous measurement of cerebral oxygenation by near infrared spectroscopy during induction of anesthesia. Anesth Analg. 1999;88(3):554-558.
29. Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58(6):541-560.
30. Koh JL, Levin SD, Chehab EL, Murphy GS. Neer award 2012: cerebral oxygenation in the beach chair position: a prospective study on the effect of general anesthesia compared with regional anesthesia and sedation. J Shoulder Elbow Surg. 2013;22:1325-1331.
The beach-chair position (BCP) is commonly used for both arthroscopic and open shoulder surgery. This technique positions the shoulder in an anatomical upright position, facilitating shoulder access and visualization.1 Compared with the lateral decubitus position, the BCP also improves airway access, reduces bleeding, and lessens the risk for brachial plexus injury.2
Despite the advantages of using the BCP, there have been multiple reports of catastrophic neurologic complications, including severe brain damage and death, in relatively healthy patients without any known risk factors.3-6 The definitive etiology of these complications remains unclear, but it has been hypothesized that BCP use may be an independent risk factor for cerebrovascular ischemia,1,5-16 as the upright position can cause hypotension leading to increased risk for cerebral hypoperfusion.7-11,17 Reducing cerebral perfusion pressure below critical thresholds may result in permanent neurologic injury.4-6,14 Therefore, monitoring of cerebral perfusion and optimization of intraoperative cerebral oxygenation have been recommended to help avoid potential neurologic complications. However, a direct relationship between intraoperative cerebral desaturation events (CDEs) and postoperative neurocognitive deficits has not been definitively established.1,9-12
To put into perspective the importance of detecting and preventing CDEs and neurologic complications, we can consider the incidence of fatal pulmonary embolism associated with total joint arthroplasty. Although the incidence is very low, about 0.1% to 2.0%, some form of venous thromboembolism prophylaxis is the standard of care for helping prevent this serious complication. Similarly, catastrophic neurologic complications of upright shoulder arthroscopy are very rare, but it is still important to consider measures that help minimize them.
We reviewed the literature for the incidence of postoperative neurocognitive deficits, number of reported neurocognitive complications, and incidence of intraoperative CDEs in patients who underwent arthroscopic shoulder surgery in the BCP.
Methods
Dr. Salazar and Dr. Hazel independently searched the Medline, Cochrane, and Embase databases for case series, prospective studies, and cohort studies that reported neurocognitive complications associated with the BCP and the incidence of intraoperative CDEs. The authors used beach chair, desaturation, near infrared spectroscopy, and shoulder as medical subject headings (MeSH). In addition, bibliographies of retrieved articles were checked for studies that the search terms may have missed. Eighty-one publications were identified and reviewed for possible inclusion.
Next, the same 2 authors reviewed the titles and abstracts for relevance and determined which articles had potential to contribute to the study. Only English-language publications were considered for inclusion. To review the incidence of postoperative neurocognitive deficits, we included only those studies with more than 25 patients, documentation of postoperative complications, and arthroscopic shoulder surgery performed with the patient in the seated, semi-upright, or BCP. Only studies with at least 25 patients were used in order to increase the power and improve the level of evidence. To review reported cases of neurocognitive complications, we included all relevant case reports and case series. To review the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive deficits, we included studies that reported on use of intraoperative cerebral perfusion monitoring. Modalities used in these studies included near infrared spectroscopy, electroencephalography, and invasive blood pressure monitoring calculated at the brain level. Studies were excluded if they did not involve arthroscopic shoulder surgery or were not conducted with human subjects.
Information recorded for each study included general information such as author and publication year, type of study, number of patients enrolled, type of intraoperative monitoring, anesthesia protocol, number of patients with CDEs, and number of patients with neurocognitive complications after surgery.
Results
Our search identified 81 publications for potential inclusion. Our first aim was to identify the overall incidence of reported neurocognitive deficits after arthroscopic shoulder surgery with the patient in the BCP. We identified 10 studies (Table 1) that met the inclusion criteria. Among the 24,701 patients in these 10 studies, there was only 1 reported case of neurocognitive deficit after surgery, in a mixed prospective-retrospective study of 15,014 cases by Rohrbaugh and colleagues.18 The deficit they reported was an ischemic cerebral vascular accident. The 0.0067% incidence in their study demonstrates how rare the complication is. Two large retrospective studies (Ns = 4169 and 5177 patients) found no postoperative neurocognitive complications.19,20 Only 3 studies performed formal postoperative cognitive testing. Salazar and colleagues21 used the Repeatable Battery for the Assessment of Neuropsychological Status before and after surgery, and Gillespie and colleagues8 and Lee and colleagues10 used the Mini–Mental State Examination before and after surgery. Total incidence of reported neurocognitive deficits from our review was 0.004% (1/24,701).
Our second aim was to review all reported cases of neurocognitive complications after arthroscopic shoulder surgery with the patient in the BCP. We identified 4 publications that fit our inclusion criteria (Table 2). Pohl and Cullen6 described 4 cases of ischemic brain injury after arthroscopic shoulder surgery with the patient in the BCP. Age range was 47 to 57 years. Specific intraoperative cerebral monitoring was not used. However, these patients had several episodes of intraoperative hypotension (systolic blood pressures, 80-90 mm Hg), measured with a traditional blood pressure cuff on the arm. In general, these patients had minimal cerebrovascular risk factors and no known preexisting cerebrovascular disease. Drummond and colleagues22 described an ischemic stroke in a 50-year-old man after arthroscopic subacromial decompression and open rotator cuff repair that resulted in unresolved right hemiplegia. Subsequent diagnostic investigation revealed an asymmetry of the circle of Willis resulting in limited flow to the left anterior and middle cerebral artery distributions. Bhatti and Enneking3 reported the case of a 64-year-old man who lost vision in the right eye immediately after arthroscopic rotator cuff repair. His vision improved spontaneously the next morning and continued to improve over the next 6 months—he regained 20/20 vision with some residual optic neuropathy.
Our third aim was to determine the incidence of intraoperative CDEs during arthroscopic shoulder surgery with the patient in the BCP. Incidence of CDEs varied widely among the 7 studies reviewed (Table 3). Minimum incidence of intraoperative CDE was 0% in a cohort of 30 patients,1 and maximum incidence was 80% in a study of 61 patients,12 all of whom underwent elective arthroscopic shoulder surgery in the BCP. Although there was wide variability in CDE incidence, the studies were consistent with respect to their definition of a CDE. Most authors used a decrease in regional cerebral tissue oxygen saturation of 20% or more from baseline, or an absolute value up to 55%, to define a CDE. None of the 7 studies reviewed reported a clinically significant adverse neurocognitive event.
Discussion
Of concern, there have been several surveys, case reports, and small case series of previously healthy patients who had no known risk factors, underwent arthroscopic shoulder surgery in the BCP, and developed unanticipated postoperative neurologic complications.4-6,14 Beach-chair positioning during surgical procedures has been implicated as a contributing factor leading to cerebral hypoperfusion with potential for cerebral ischemia.1,12,23 These changes in cerebral perfusion pressure are thought to be the major determinant of poor neurologic outcomes. Such reports have exposed the potential need for heightened vigilance, alternative anesthesia techniques, and improved monitoring, though the exact etiology of the central nervous system injuries in this patient population is incompletely understood and is likely multifactorial. Therefore, in this study we wanted to determine the incidence of postoperative neurocognitive deficits and review all reported cases of neurocognitive complications in patients who have undergone arthroscopic shoulder surgery in the BCP. In addition, we wanted to define the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive complications.
According to our review, the incidence of postoperative neurocognitive complications after surgery in the BCP is 0.004% (1/24,701). However, this finding is based only on what has been reported; the true incidence is not known. It is also important to note that the incidence of neurocognitive deficits after many other types of surgery is not known and that surgery itself may be a risk factor for postoperative neurocognitive deficits.24 In their retrospective review of 15,014 patients who underwent arthroscopic shoulder surgery in the BCP at a single institution over an 11-year period, Rohrbaugh and colleagues18 found an overall postoperative complication rate of 0.37% and a 0.0067% incidence of neurocognitive deficits. One patient in the series was given a diagnosis of ischemic stroke on the basis of neurologic deficits that occurred 24 hours after surgery. Yadeau and colleagues20 found no postoperative neurocognitive complications in a mixed prospective-retrospective study of 4169 patients—3000 identified retrospectively, 1169 prospectively—who underwent arthroscopic shoulder surgery in the BCP at an ambulatory surgery center. Pin-on and colleagues19 reported on a series of 5177 orthopedic and neurosurgical patients who underwent surgery in the BCP. In those who had arthroscopic shoulder surgery, intraoperative systolic blood pressures obtained from an arterial line referenced to heart level decreased a mean (SD) of 14.4% (12.7%), whereas in those whose pressures were obtained from a noninvasive blood pressure cuff referenced to heart level decreased 19.3% (12.6%). However, the authors reported no incidence of postoperative stroke or neurologic deficits.
Although uncommon, perioperative cerebral ischemic accidents are potentially devastating for patients, their families, and the health care professionals involved. These events have tremendous economic, social, professional, and medicolegal implications, with perioperative stroke being particularly morbid. Perioperative stroke has a mortality rate of 60%, versus 15% to 46% for stroke in general.25,26 In 2005, Pohl and Cullen6 published a landmark article on a series of 4 relatively healthy middle-aged patients who were at low risk for stroke but had catastrophic neurocognitive complications (including 1 death) after arthroscopic shoulder surgery in the BCP. Bhatti and Enneking3 described a case of acute postoperative vision loss and ophthalmoplegia attributed to intraoperative hypotension leading to ischemia in a patient who underwent an elective shoulder arthroscopic procedure in the BCP. These reports prompted multiple investigations into the physiologic hemodynamic changes associated with surgery in the BCP and the treatment strategies used to improve patient safety.
In the normal physiologic state, the sympathetic nervous system is activated when a person assumes the seated position. The result is increased systemic vascular resistance and heart rate alterations to maintain cardiac output and mean arterial pressure. In anesthetized patients, this response is blunted by the vasodilatory effects of intravenous and volatile anesthetics. Multiple studies have demonstrated substantial hemodynamic changes in both awake and anesthetized patients during the maneuver from the supine position to the seated position1,27,28; these changes include diminished cardiac index, stroke volume, and arterial pressure.17 The data underscore the need for attentiveness and accurate monitoring of cerebral perfusion when the transition is made from the supine position to the BCP, particularly in the early phase of surgery and in high-risk patients.
Knowledge of these hemodynamic changes has led several authors to recommend additional intraoperative monitoring of cerebral perfusion. Monitoring techniques have included use of invasive blood pressure monitoring adjusted to brain level, cerebral oximetry using near infrared spectroscopy, and electroencephalography. However, the clinical relevance of intraoperative CDEs in isolation is not well understood.1,6,7,23 In addition, cost and availability of additional advanced monitoring likely factor into why it is not more commonly used. For this patient population, the severity, frequency, and duration of desaturation that causes cerebral ischemia and the relationship with postoperative neurocognitive deficits remain undefined.
The incidence of CDEs in patients being monitored with near infrared spectroscopy while undergoing elective arthroscopic shoulder surgery in the BCP varies widely, from 0% to 80% (mean, 41%).1,4,7,10,12,21 Magnitude and duration of cerebral ischemia required to produce neurocognitive dysfunction in this patient population remain unidentified as well. In conscious patients, a 20% reduction in frontal lobe oxygenation is associated with clinical manifestations of cerebral hypoperfusion, such as syncope.15,29 As none of the patients in the studies we reviewed experienced any sort of deficit, we cannot definitively state there is a correlation between CDE occurrence and neurocognitive deficit.
One limitation of our investigation is that it was a systemic review, and thus there was substantial heterogeneity in the methods and designs of the studies included in the analysis. Among the different series, there was variability in multiple aspects of the study design, including type of anesthetic, patient inclusion criteria, type of surgery, type of intraoperative cerebral perfusion monitoring, and type of neurocognitive testing. As a result, comparing the groups was difficult, and the generalizability of our findings may be limited. In addition, it is difficult to accurately establish incidence and comprehensively review these events because of the paucity of literature.
Conclusion
Neurocognitive complications after shoulder arthroscopy with the patient in the BCP are extremely rare but potentially devastating events that can affect healthy patients with no preexisting cerebrovascular risk factors. Our review indicated the incidence of permanent neurologic deficit after arthroscopy in the BCP may be as low as 0.004%. The exact etiology of such complications is not clear. Basic science research and large prospective studies are needed to identify the clinically relevant thresholds of magnitude, duration, and frequency of intraoperative CDEs in order to establish their relationship with postoperative neurocognitive complications. Such large studies may also elucidate modifiable patient-specific risk factors and establish the most sensitive, safe, and cost-effective intraoperative monitoring tools. Current literature suggests that accurate intraoperative monitoring of cerebral perfusion, alternatives to general anesthesia, and prudent use of intraoperative blood pressure control may improve patient safety.
The beach-chair position (BCP) is commonly used for both arthroscopic and open shoulder surgery. This technique positions the shoulder in an anatomical upright position, facilitating shoulder access and visualization.1 Compared with the lateral decubitus position, the BCP also improves airway access, reduces bleeding, and lessens the risk for brachial plexus injury.2
Despite the advantages of using the BCP, there have been multiple reports of catastrophic neurologic complications, including severe brain damage and death, in relatively healthy patients without any known risk factors.3-6 The definitive etiology of these complications remains unclear, but it has been hypothesized that BCP use may be an independent risk factor for cerebrovascular ischemia,1,5-16 as the upright position can cause hypotension leading to increased risk for cerebral hypoperfusion.7-11,17 Reducing cerebral perfusion pressure below critical thresholds may result in permanent neurologic injury.4-6,14 Therefore, monitoring of cerebral perfusion and optimization of intraoperative cerebral oxygenation have been recommended to help avoid potential neurologic complications. However, a direct relationship between intraoperative cerebral desaturation events (CDEs) and postoperative neurocognitive deficits has not been definitively established.1,9-12
To put into perspective the importance of detecting and preventing CDEs and neurologic complications, we can consider the incidence of fatal pulmonary embolism associated with total joint arthroplasty. Although the incidence is very low, about 0.1% to 2.0%, some form of venous thromboembolism prophylaxis is the standard of care for helping prevent this serious complication. Similarly, catastrophic neurologic complications of upright shoulder arthroscopy are very rare, but it is still important to consider measures that help minimize them.
We reviewed the literature for the incidence of postoperative neurocognitive deficits, number of reported neurocognitive complications, and incidence of intraoperative CDEs in patients who underwent arthroscopic shoulder surgery in the BCP.
Methods
Dr. Salazar and Dr. Hazel independently searched the Medline, Cochrane, and Embase databases for case series, prospective studies, and cohort studies that reported neurocognitive complications associated with the BCP and the incidence of intraoperative CDEs. The authors used beach chair, desaturation, near infrared spectroscopy, and shoulder as medical subject headings (MeSH). In addition, bibliographies of retrieved articles were checked for studies that the search terms may have missed. Eighty-one publications were identified and reviewed for possible inclusion.
Next, the same 2 authors reviewed the titles and abstracts for relevance and determined which articles had potential to contribute to the study. Only English-language publications were considered for inclusion. To review the incidence of postoperative neurocognitive deficits, we included only those studies with more than 25 patients, documentation of postoperative complications, and arthroscopic shoulder surgery performed with the patient in the seated, semi-upright, or BCP. Only studies with at least 25 patients were used in order to increase the power and improve the level of evidence. To review reported cases of neurocognitive complications, we included all relevant case reports and case series. To review the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive deficits, we included studies that reported on use of intraoperative cerebral perfusion monitoring. Modalities used in these studies included near infrared spectroscopy, electroencephalography, and invasive blood pressure monitoring calculated at the brain level. Studies were excluded if they did not involve arthroscopic shoulder surgery or were not conducted with human subjects.
Information recorded for each study included general information such as author and publication year, type of study, number of patients enrolled, type of intraoperative monitoring, anesthesia protocol, number of patients with CDEs, and number of patients with neurocognitive complications after surgery.
Results
Our search identified 81 publications for potential inclusion. Our first aim was to identify the overall incidence of reported neurocognitive deficits after arthroscopic shoulder surgery with the patient in the BCP. We identified 10 studies (Table 1) that met the inclusion criteria. Among the 24,701 patients in these 10 studies, there was only 1 reported case of neurocognitive deficit after surgery, in a mixed prospective-retrospective study of 15,014 cases by Rohrbaugh and colleagues.18 The deficit they reported was an ischemic cerebral vascular accident. The 0.0067% incidence in their study demonstrates how rare the complication is. Two large retrospective studies (Ns = 4169 and 5177 patients) found no postoperative neurocognitive complications.19,20 Only 3 studies performed formal postoperative cognitive testing. Salazar and colleagues21 used the Repeatable Battery for the Assessment of Neuropsychological Status before and after surgery, and Gillespie and colleagues8 and Lee and colleagues10 used the Mini–Mental State Examination before and after surgery. Total incidence of reported neurocognitive deficits from our review was 0.004% (1/24,701).
Our second aim was to review all reported cases of neurocognitive complications after arthroscopic shoulder surgery with the patient in the BCP. We identified 4 publications that fit our inclusion criteria (Table 2). Pohl and Cullen6 described 4 cases of ischemic brain injury after arthroscopic shoulder surgery with the patient in the BCP. Age range was 47 to 57 years. Specific intraoperative cerebral monitoring was not used. However, these patients had several episodes of intraoperative hypotension (systolic blood pressures, 80-90 mm Hg), measured with a traditional blood pressure cuff on the arm. In general, these patients had minimal cerebrovascular risk factors and no known preexisting cerebrovascular disease. Drummond and colleagues22 described an ischemic stroke in a 50-year-old man after arthroscopic subacromial decompression and open rotator cuff repair that resulted in unresolved right hemiplegia. Subsequent diagnostic investigation revealed an asymmetry of the circle of Willis resulting in limited flow to the left anterior and middle cerebral artery distributions. Bhatti and Enneking3 reported the case of a 64-year-old man who lost vision in the right eye immediately after arthroscopic rotator cuff repair. His vision improved spontaneously the next morning and continued to improve over the next 6 months—he regained 20/20 vision with some residual optic neuropathy.
Our third aim was to determine the incidence of intraoperative CDEs during arthroscopic shoulder surgery with the patient in the BCP. Incidence of CDEs varied widely among the 7 studies reviewed (Table 3). Minimum incidence of intraoperative CDE was 0% in a cohort of 30 patients,1 and maximum incidence was 80% in a study of 61 patients,12 all of whom underwent elective arthroscopic shoulder surgery in the BCP. Although there was wide variability in CDE incidence, the studies were consistent with respect to their definition of a CDE. Most authors used a decrease in regional cerebral tissue oxygen saturation of 20% or more from baseline, or an absolute value up to 55%, to define a CDE. None of the 7 studies reviewed reported a clinically significant adverse neurocognitive event.
Discussion
Of concern, there have been several surveys, case reports, and small case series of previously healthy patients who had no known risk factors, underwent arthroscopic shoulder surgery in the BCP, and developed unanticipated postoperative neurologic complications.4-6,14 Beach-chair positioning during surgical procedures has been implicated as a contributing factor leading to cerebral hypoperfusion with potential for cerebral ischemia.1,12,23 These changes in cerebral perfusion pressure are thought to be the major determinant of poor neurologic outcomes. Such reports have exposed the potential need for heightened vigilance, alternative anesthesia techniques, and improved monitoring, though the exact etiology of the central nervous system injuries in this patient population is incompletely understood and is likely multifactorial. Therefore, in this study we wanted to determine the incidence of postoperative neurocognitive deficits and review all reported cases of neurocognitive complications in patients who have undergone arthroscopic shoulder surgery in the BCP. In addition, we wanted to define the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive complications.
According to our review, the incidence of postoperative neurocognitive complications after surgery in the BCP is 0.004% (1/24,701). However, this finding is based only on what has been reported; the true incidence is not known. It is also important to note that the incidence of neurocognitive deficits after many other types of surgery is not known and that surgery itself may be a risk factor for postoperative neurocognitive deficits.24 In their retrospective review of 15,014 patients who underwent arthroscopic shoulder surgery in the BCP at a single institution over an 11-year period, Rohrbaugh and colleagues18 found an overall postoperative complication rate of 0.37% and a 0.0067% incidence of neurocognitive deficits. One patient in the series was given a diagnosis of ischemic stroke on the basis of neurologic deficits that occurred 24 hours after surgery. Yadeau and colleagues20 found no postoperative neurocognitive complications in a mixed prospective-retrospective study of 4169 patients—3000 identified retrospectively, 1169 prospectively—who underwent arthroscopic shoulder surgery in the BCP at an ambulatory surgery center. Pin-on and colleagues19 reported on a series of 5177 orthopedic and neurosurgical patients who underwent surgery in the BCP. In those who had arthroscopic shoulder surgery, intraoperative systolic blood pressures obtained from an arterial line referenced to heart level decreased a mean (SD) of 14.4% (12.7%), whereas in those whose pressures were obtained from a noninvasive blood pressure cuff referenced to heart level decreased 19.3% (12.6%). However, the authors reported no incidence of postoperative stroke or neurologic deficits.
Although uncommon, perioperative cerebral ischemic accidents are potentially devastating for patients, their families, and the health care professionals involved. These events have tremendous economic, social, professional, and medicolegal implications, with perioperative stroke being particularly morbid. Perioperative stroke has a mortality rate of 60%, versus 15% to 46% for stroke in general.25,26 In 2005, Pohl and Cullen6 published a landmark article on a series of 4 relatively healthy middle-aged patients who were at low risk for stroke but had catastrophic neurocognitive complications (including 1 death) after arthroscopic shoulder surgery in the BCP. Bhatti and Enneking3 described a case of acute postoperative vision loss and ophthalmoplegia attributed to intraoperative hypotension leading to ischemia in a patient who underwent an elective shoulder arthroscopic procedure in the BCP. These reports prompted multiple investigations into the physiologic hemodynamic changes associated with surgery in the BCP and the treatment strategies used to improve patient safety.
In the normal physiologic state, the sympathetic nervous system is activated when a person assumes the seated position. The result is increased systemic vascular resistance and heart rate alterations to maintain cardiac output and mean arterial pressure. In anesthetized patients, this response is blunted by the vasodilatory effects of intravenous and volatile anesthetics. Multiple studies have demonstrated substantial hemodynamic changes in both awake and anesthetized patients during the maneuver from the supine position to the seated position1,27,28; these changes include diminished cardiac index, stroke volume, and arterial pressure.17 The data underscore the need for attentiveness and accurate monitoring of cerebral perfusion when the transition is made from the supine position to the BCP, particularly in the early phase of surgery and in high-risk patients.
Knowledge of these hemodynamic changes has led several authors to recommend additional intraoperative monitoring of cerebral perfusion. Monitoring techniques have included use of invasive blood pressure monitoring adjusted to brain level, cerebral oximetry using near infrared spectroscopy, and electroencephalography. However, the clinical relevance of intraoperative CDEs in isolation is not well understood.1,6,7,23 In addition, cost and availability of additional advanced monitoring likely factor into why it is not more commonly used. For this patient population, the severity, frequency, and duration of desaturation that causes cerebral ischemia and the relationship with postoperative neurocognitive deficits remain undefined.
The incidence of CDEs in patients being monitored with near infrared spectroscopy while undergoing elective arthroscopic shoulder surgery in the BCP varies widely, from 0% to 80% (mean, 41%).1,4,7,10,12,21 Magnitude and duration of cerebral ischemia required to produce neurocognitive dysfunction in this patient population remain unidentified as well. In conscious patients, a 20% reduction in frontal lobe oxygenation is associated with clinical manifestations of cerebral hypoperfusion, such as syncope.15,29 As none of the patients in the studies we reviewed experienced any sort of deficit, we cannot definitively state there is a correlation between CDE occurrence and neurocognitive deficit.
One limitation of our investigation is that it was a systemic review, and thus there was substantial heterogeneity in the methods and designs of the studies included in the analysis. Among the different series, there was variability in multiple aspects of the study design, including type of anesthetic, patient inclusion criteria, type of surgery, type of intraoperative cerebral perfusion monitoring, and type of neurocognitive testing. As a result, comparing the groups was difficult, and the generalizability of our findings may be limited. In addition, it is difficult to accurately establish incidence and comprehensively review these events because of the paucity of literature.
Conclusion
Neurocognitive complications after shoulder arthroscopy with the patient in the BCP are extremely rare but potentially devastating events that can affect healthy patients with no preexisting cerebrovascular risk factors. Our review indicated the incidence of permanent neurologic deficit after arthroscopy in the BCP may be as low as 0.004%. The exact etiology of such complications is not clear. Basic science research and large prospective studies are needed to identify the clinically relevant thresholds of magnitude, duration, and frequency of intraoperative CDEs in order to establish their relationship with postoperative neurocognitive complications. Such large studies may also elucidate modifiable patient-specific risk factors and establish the most sensitive, safe, and cost-effective intraoperative monitoring tools. Current literature suggests that accurate intraoperative monitoring of cerebral perfusion, alternatives to general anesthesia, and prudent use of intraoperative blood pressure control may improve patient safety.
1. Tange K, Kinoshita H, Minonishi T, et al. Cerebral oxygenation in the beach chair position before and during general anesthesia. Minerva Anestesiol. 2010;76(7):485-490.
2. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
3. Bhatti MT, Enneking FK. Visual loss and ophthalmoplegia after shoulder surgery. Anesth Analg. 2003;96(3):899-902.
4. Friedman DJ, Parnes NZ, Zimmer Z, Higgins LD, Warner JJ. Prevalence of cerebrovascular events during shoulder surgery and association with patient position. Orthopedics. 2009;32(4).
5. Papadonikolakis A, Wiesler ER, Olympio MA, Poehling GG. Avoiding catastrophic complications of stroke and death related to shoulder surgery in the sitting position. Arthroscopy. 2008;24(4):481-482.
6. Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17(6):463-469.
7. Dippmann C, Winge S, Nielsen HB. Severe cerebral desaturation during shoulder arthroscopy in the beach-chair position. Arthroscopy. 2010;26(9 suppl):S148-S150.
8. Gillespie R, Shishani Y, Streit J, et al. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am. 2012;94(14):1284-1290.
9. Jeong H, Lee SH, Jang EA, Chung SS, Lee J, Yoo KY. Haemodynamics and cerebral oxygenation during arthroscopic shoulder surgery in beach chair position under general anaesthesia. Acta Anaesthesiol Scand. 2012;56(7):872-879.
10. Lee JH, Min KT, Chun YM, Kim EJ, Choi SH. Effects of beach-chair position and induced hypotension on cerebral oxygen saturation in patients undergoing arthroscopic shoulder surgery. Arthroscopy. 2011;27(7):889-894.
11. Moerman AT, De Hert SG, Jacobs TF, De Wilde LF, Wouters PF. Cerebral oxygen desaturation during beach chair position. Eur J Anaesthesiol. 2012;29(2):82-87.
12. Murphy GS, Szokol JW, Marymont JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111(2):496-505.
13. Peruto CM, Ciccotti MG, Cohen SB. Shoulder arthroscopy positioning: lateral decubitus versus beach chair. Arthroscopy. 2009;25(8):891-896.
14. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
15. Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. 2000;93(4):964-970.
16. Smythe PR, Samra SK. Monitors of cerebral oxygenation. Anesthesiol Clin North Am. 2002;20(2):293-313.
17. Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34(5):375-386.
18. Rohrbaugh M, Kentor ML, Orebaugh SL, Williams B. Outcomes of shoulder surgery in the sitting position with interscalene nerve block: a single-center series. Reg Anesth Pain Med. 2013;38(1):28-33.
19. Pin-on P, Schroeder D, Munis J. The hemodynamic management of 5177 neurosurgical and orthopedic patients who underwent surgery in the sitting or “beach chair” position without incidence of adverse neurologic events. Anesth Analg. 2013;116(6):1317-1324.
20. Yadeau JT, Casciano M, Liu SS, et al. Stroke, regional anesthesia in the sitting position, and hypotension: a review of 4169 ambulatory surgery patients. Reg Anesth Pain Med. 2011;36(5):430-435.
21. Salazar D, Sears BW, Aghdasi B, et al. Cerebral desaturation events during shoulder arthroscopy in the beach chair position: patient risk factors and neurocognitive effects. J Shoulder Elbow Surg. 2013;22(9):1228-1235.
22. Drummond JC, Lee RR, Howell JP Jr. Focal cerebral ischemia after surgery in the “beach chair” position: the role of a congenital variation of circle of Willis anatomy. Anesth Analg. 2012;114(6):1301-1303.
23. Fischer GW, Torrillo TM, Weiner MM, Rosenblatt MA. The use of cerebral oximetry as a monitor of the adequacy of cerebral perfusion in a patient undergoing shoulder surgery in the beach chair position. Pain Pract. 2009;9(4):304-307.
24. Wong GY, Warner DO, Schroeder DR, et al. Risk of surgery and anesthesia for ischemic stroke. Anesthesiology. 2000;92(2):425-432.
25. Knapp RB, Topkins MJ, Artusio JF Jr. The cerebrovascular accident and coronary occlusion in anesthesia. JAMA. 1962;182:332-334.
26. Landercasper J, Merz BJ, Cogbill TH, et al. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125(8):986-989.
27. Fuchs G, Schwarz G, Kulier A, Litscher G. The influence of positioning on spectroscopic measurements of brain oxygenation. J Neurosurg Anesthesiol. 2000;12(2):75-80.
28. Lovell AT, Owen-Reece H, Elwell CE, Smith M, Goldstone JC. Continuous measurement of cerebral oxygenation by near infrared spectroscopy during induction of anesthesia. Anesth Analg. 1999;88(3):554-558.
29. Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58(6):541-560.
30. Koh JL, Levin SD, Chehab EL, Murphy GS. Neer award 2012: cerebral oxygenation in the beach chair position: a prospective study on the effect of general anesthesia compared with regional anesthesia and sedation. J Shoulder Elbow Surg. 2013;22:1325-1331.
1. Tange K, Kinoshita H, Minonishi T, et al. Cerebral oxygenation in the beach chair position before and during general anesthesia. Minerva Anestesiol. 2010;76(7):485-490.
2. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
3. Bhatti MT, Enneking FK. Visual loss and ophthalmoplegia after shoulder surgery. Anesth Analg. 2003;96(3):899-902.
4. Friedman DJ, Parnes NZ, Zimmer Z, Higgins LD, Warner JJ. Prevalence of cerebrovascular events during shoulder surgery and association with patient position. Orthopedics. 2009;32(4).
5. Papadonikolakis A, Wiesler ER, Olympio MA, Poehling GG. Avoiding catastrophic complications of stroke and death related to shoulder surgery in the sitting position. Arthroscopy. 2008;24(4):481-482.
6. Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17(6):463-469.
7. Dippmann C, Winge S, Nielsen HB. Severe cerebral desaturation during shoulder arthroscopy in the beach-chair position. Arthroscopy. 2010;26(9 suppl):S148-S150.
8. Gillespie R, Shishani Y, Streit J, et al. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am. 2012;94(14):1284-1290.
9. Jeong H, Lee SH, Jang EA, Chung SS, Lee J, Yoo KY. Haemodynamics and cerebral oxygenation during arthroscopic shoulder surgery in beach chair position under general anaesthesia. Acta Anaesthesiol Scand. 2012;56(7):872-879.
10. Lee JH, Min KT, Chun YM, Kim EJ, Choi SH. Effects of beach-chair position and induced hypotension on cerebral oxygen saturation in patients undergoing arthroscopic shoulder surgery. Arthroscopy. 2011;27(7):889-894.
11. Moerman AT, De Hert SG, Jacobs TF, De Wilde LF, Wouters PF. Cerebral oxygen desaturation during beach chair position. Eur J Anaesthesiol. 2012;29(2):82-87.
12. Murphy GS, Szokol JW, Marymont JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111(2):496-505.
13. Peruto CM, Ciccotti MG, Cohen SB. Shoulder arthroscopy positioning: lateral decubitus versus beach chair. Arthroscopy. 2009;25(8):891-896.
14. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
15. Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. 2000;93(4):964-970.
16. Smythe PR, Samra SK. Monitors of cerebral oxygenation. Anesthesiol Clin North Am. 2002;20(2):293-313.
17. Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34(5):375-386.
18. Rohrbaugh M, Kentor ML, Orebaugh SL, Williams B. Outcomes of shoulder surgery in the sitting position with interscalene nerve block: a single-center series. Reg Anesth Pain Med. 2013;38(1):28-33.
19. Pin-on P, Schroeder D, Munis J. The hemodynamic management of 5177 neurosurgical and orthopedic patients who underwent surgery in the sitting or “beach chair” position without incidence of adverse neurologic events. Anesth Analg. 2013;116(6):1317-1324.
20. Yadeau JT, Casciano M, Liu SS, et al. Stroke, regional anesthesia in the sitting position, and hypotension: a review of 4169 ambulatory surgery patients. Reg Anesth Pain Med. 2011;36(5):430-435.
21. Salazar D, Sears BW, Aghdasi B, et al. Cerebral desaturation events during shoulder arthroscopy in the beach chair position: patient risk factors and neurocognitive effects. J Shoulder Elbow Surg. 2013;22(9):1228-1235.
22. Drummond JC, Lee RR, Howell JP Jr. Focal cerebral ischemia after surgery in the “beach chair” position: the role of a congenital variation of circle of Willis anatomy. Anesth Analg. 2012;114(6):1301-1303.
23. Fischer GW, Torrillo TM, Weiner MM, Rosenblatt MA. The use of cerebral oximetry as a monitor of the adequacy of cerebral perfusion in a patient undergoing shoulder surgery in the beach chair position. Pain Pract. 2009;9(4):304-307.
24. Wong GY, Warner DO, Schroeder DR, et al. Risk of surgery and anesthesia for ischemic stroke. Anesthesiology. 2000;92(2):425-432.
25. Knapp RB, Topkins MJ, Artusio JF Jr. The cerebrovascular accident and coronary occlusion in anesthesia. JAMA. 1962;182:332-334.
26. Landercasper J, Merz BJ, Cogbill TH, et al. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125(8):986-989.
27. Fuchs G, Schwarz G, Kulier A, Litscher G. The influence of positioning on spectroscopic measurements of brain oxygenation. J Neurosurg Anesthesiol. 2000;12(2):75-80.
28. Lovell AT, Owen-Reece H, Elwell CE, Smith M, Goldstone JC. Continuous measurement of cerebral oxygenation by near infrared spectroscopy during induction of anesthesia. Anesth Analg. 1999;88(3):554-558.
29. Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58(6):541-560.
30. Koh JL, Levin SD, Chehab EL, Murphy GS. Neer award 2012: cerebral oxygenation in the beach chair position: a prospective study on the effect of general anesthesia compared with regional anesthesia and sedation. J Shoulder Elbow Surg. 2013;22:1325-1331.
Isolated Avulsion of Extensor Carpi Radialis Longus and Brachioradialis Origins: A Case Report and Surgical Repair Technique
The literature includes only 2 case reports of bony avulsion fracture of the origin of the brachioradialis1,2 and, up until now, no case reports of isolated avulsion of the extensor carpi radialis longus and brachioradialis origins from the lateral epicondyle and lateral supracondylar ridge. In this article, we report the case of a 31-year-old man who sustained this injury during a fall onto his outstretched right hand, and we present our surgical repair technique. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 31-year-old right hand–dominant garbage truck worker sustained a right elbow injury and presented 2 months later. He described slipping and falling onto his outstretched right hand while doing his work. He could not describe the exact mechanism or action or position of the arm at time of impact but thought he tried to catch himself on the truck during the fall. At time of injury, he had immediate pain and swelling to the lateral aspect of the right elbow and difficulty when he attempted lifting. He denied antecedent elbow symptoms before the injury. After evaluation by an outside occupational medicine physician, he engaged in treatment consisting of activity modification and physical therapy, including range-of-motion (ROM) exercises and iontophoresis. This course of management failed to completely relieve his symptoms, and he was unable to return to work.
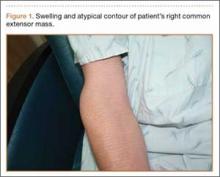
The patient presented to our institution 9 weeks after injury with complaints of pain along the lateral aspect of the elbow, painful flexion-extension, and continued swelling. The pain had been unrelieved with anti-inflammatory medications and opioids. Physical examination revealed tenderness and swelling along the lateral epicondyle and extensor mass of the right elbow. The patient had tenderness, marked weakness, and a palpable soft-tissue defect at the origin of the extensor mass with resisted extension of the wrist (Figure 1). Elbow ROM was from 20° to 120° of flexion, 60° of pronation, and 60° of supination. No varus or valgus instability was present about the elbow. Radiographs did not show any fracture or dislocation. Magnetic resonance imaging (MRI) did not definitively show extensor tendon avulsion but did identify signal change of the common extensor tendon (Figures 2A, 2B). Advanced imaging was inconclusive, but, given the patient’s history and physical examination findings, he was diagnosed with an avulsion injury of the origin of the extensor mass of the right elbow.
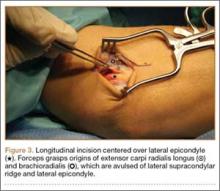
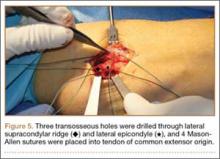
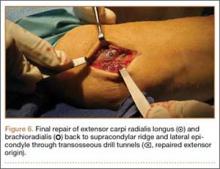
The patient was brought to the operating room, administered general anesthesia, and placed supine on the operating table with a tourniquet on the upper arm. A lateral 4.5-cm incision was made centered over the lateral epicondyle. The origin of the extensor mass was exposed, and isolated avulsions of the extensor carpi radialis longus and the brachioradialis were identified (Figures 3, 4). Underlying the avulsed sleeve of tissue, the origin of the extensor carpi radialis brevis was found intact. The lateral supracondylar ridge and the lateral epicondyle of the humerus were débrided, and 3 transosseous holes were drilled (using a 2.3-mm bit) through the lateral epicondyle. Four Mason-Allen sutures were placed into the tendon of the common extensor origin using No. 2 braided polyester suture (Ethibond Excel, Ethicon) (Figure 5). The tendon was reduced down to the native footprint, and the sutures were passed through the drill holes and tied down securely (Figure 6). The skin was then closed using layered 4-0 absorbable monofilament suture (Monocryl, Ethicon). The patient was placed in a posterior mold plaster splint with 90° of elbow flexion and with the wrist in 30° of extension.
On postoperative day 3, the patient was seen for a wound check and was placed in a long-arm fiberglass cast (90° of elbow flexion, forearm in neutral, 25° of wrist extension) for immobilization. One week after surgery, he was transitioned to a removable thermoplastic splint, and physical therapy for ROM was initiated. He was allowed therapist-guided active extension of the elbow and flexion of the wrist but was restricted to passive flexion of the elbow and extension of the wrist. Seven weeks after surgery, passive ROM about the elbow was measured, and he was found to have 120° of flexion, 0° extension, 80° pronation, and 80° supination. At 12 weeks, the physical therapy regimen was advanced to include muscle strengthening and active wrist extension and elbow flexion. At 16 weeks, the wrist extensors demonstrated 5/5 strength (Medical Research Council grading system), and the patient was cleared for full activity and weight-bearing without restriction. He returned to work pain-free and without restrictions 18 weeks after surgery. At 2-year follow-up, he had a Mayo performance elbow score of 100 and an Oxford elbow score of 48.3,4 He had full active ROM, full strength, and no subjective pain and was back doing heavy lifting at his job.
Discussion
The brachioradialis, extensor carpi radialis longus, and extensor carpi radialis brevis originate from the anterolateral aspect of the lateral column of the distal humeral metaphysis and form the dorsal mobile wad. The origin of the brachioradialis is about 7 cm in length and begins about 10 to 11 cm above the elbow.5 The origin and insertions of the mobile wad, specifically the brachioradialis, provide a tremendous mechanical advantage with respect to elbow flexion against resistance, particularly with the forearm in the pronated and semipronated positions.6 With the elbow in 30° of flexion, a force 3 times the body weight can be encountered during strenuous lifting.6,7 We hypothesized these large forces likely led to this injury pattern in the patient we have described.
The literature includes 2 case reports of avulsion fracture of the brachioradialis muscle from its origin on the lateral supracondylar humeral ridge.1,2 To our knowledge, however, there have been no reports of pure avulsion. In our patient’s case, there was no bony fracture, but rather avulsion of the extensor carpi radialis longus and brachioradialis at their origin, with the underlying fibers of the extensor carpi radialis brevis remaining in continuity. Because of the rarity of this injury pattern, there was a significant delay in diagnosis. On initial presentation, the differential diagnosis for lateral elbow pain and tenderness included occult fracture, intracapsular plica, osteochondritis dissecans lesion, radial tunnel syndrome, lateral or posterolateral instability, and lateral epicondylitis. Given the absence of antecedent elbow symptoms before the injury, the dynamic soft-tissue asymmetry of the mobile wad with wrist extension, and the palpable soft-tissue defect, we thought the presentation was inconsistent with a simple inflammatory or overuse syndrome, such as lateral epicondylitis. In addition, the physical examination findings were inconsistent with radial tunnel syndrome or disruption of the lateral collateral ligament complex. Elbow MRI did not show an occult fracture, plica, or osteochondritis dissecans lesion but did reveal joint effusion and signal change in the common extensor tendon origin. Interestingly, MRI did not definitively show a tear of the mobile wad. This may be explained by the fact that the fibers of the underlying extensor carpi radialis brevis remained intact. Also potentially involved are the static nature of MRI and potentially suboptimal sequencing and axis of acquisition resulting from the relative infrequency of imaging this joint at certain health care institutions. Our case demonstrates the limitations of MRI in this setting and highlights the need for a detailed history and thorough physical examination for diagnosis.
Funk and colleagues8 used electromyography (EMG) to study the activity of the elbow musculature in uninjured subjects. EMG data were obtained with the elbow joint subjected to resisted flexion, extension, abduction, and adduction. During resisted elbow flexion, there was an increasing amount of activity in the extensor carpi radialis with larger angles of elbow flexion. In addition, the brachioradialis demonstrated the most muscle activity of any of the elbow flexors with 90° or more of elbow flexion and forearm pronation, as opposed to other positions in which the brachialis was the primary flexor. For this reason, we hypothesized that our patient’s forearm was pronated and his elbow flexed to 90° or more when he braced for impact. The ensuing injury resulted from a violent eccentric contraction that caused extensive rupture of the lateral elbow musculature from its broad origin. With the forearm in supination or neutral position, we would have expected a possible injury to the distal biceps as opposed to the brachioradialis and extensor carpi radialis.
In our patient, this injury caused much functional disability, especially with elbow flexion and wrist extension. We hypothesized that, for the muscles to function properly, anatomical restoration would have to be achieved at their known footprint to maintain their mechanical advantage. Therefore, surgical intervention was indicated in our patient, an active laborer. Given the absence of an osseous fracture fragment in this injury pattern, healing must occur at the bone–tendon interface. As tendinous healing is more tenuous and protracted than osseous healing, we preferred transosseous repair. We believed that better tendon-to-bone healing would be possible with drilled osseous tunnels rather than with suture anchors. New studies describing alternative successful methods of treatment would add to our limited body of knowledge regarding this rare injury.
Conclusion
This is the first report of avulsion of the extensor carpi radialis longus and brachioradialis from their origins. Given the biomechanics and anatomy of the dorsal mobile wad, we posit that our patient’s injury occurred when he fell onto his outstretched hand secondary to overwhelming eccentric muscle contracture at time of impact. This injury caused significant upper extremity dysfunction, and surgical intervention was required.
1. Guettler JH, Mayo DB. Avulsion fracture of the origin of the brachioradialis muscle. Am J Orthop. 2001;30(9):693-694.
2. Marchant MH Jr, Gambardella RA, Podesta L. Superficial radial nerve injury after avulsion fracture of the brachioradialis muscle origin in a professional lacrosse player: a case report. J Shoulder Elbow Surg. 2009;18(6):e9-e12.
3. Dawson J, Doll H, Boller I, et al. The development and validation of a patient-reported questionnaire to assess outcomes of elbow surgery. J Bone Joint Surg Br. 2008;90(4):466-473.
4. Sathyamoorthy P, Kemp GJ, Rawal A, Rayner V, Frostick SP. Development and validation of an elbow score. Rheumatology. 2004;43(11):1434-1440.
5. Freehafer AA, Peckham PH, Keith MW, Mendelson LS. The brachioradialis: anatomy, properties, and value for tendon transfer in the tetraplegic. J Hand Surg Am. 1988;13(1):99-104.
6. Morrey BF, Sanchez-Sotelo J. The Elbow and Its Disorders. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2009.
7. Nakazawa K, Kawakami Y, Fukunaga T, Yano H, Miyashita M. Differences in activation patterns in elbow flexor muscles during isometric, concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1993;66(3):214-220.
8. Funk DA, An KN, Morrey BF, Daube JR. Electromyographic analysis of muscles across the elbow joint. J Orthop Res. 1987;5(4):529-538.
The literature includes only 2 case reports of bony avulsion fracture of the origin of the brachioradialis1,2 and, up until now, no case reports of isolated avulsion of the extensor carpi radialis longus and brachioradialis origins from the lateral epicondyle and lateral supracondylar ridge. In this article, we report the case of a 31-year-old man who sustained this injury during a fall onto his outstretched right hand, and we present our surgical repair technique. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 31-year-old right hand–dominant garbage truck worker sustained a right elbow injury and presented 2 months later. He described slipping and falling onto his outstretched right hand while doing his work. He could not describe the exact mechanism or action or position of the arm at time of impact but thought he tried to catch himself on the truck during the fall. At time of injury, he had immediate pain and swelling to the lateral aspect of the right elbow and difficulty when he attempted lifting. He denied antecedent elbow symptoms before the injury. After evaluation by an outside occupational medicine physician, he engaged in treatment consisting of activity modification and physical therapy, including range-of-motion (ROM) exercises and iontophoresis. This course of management failed to completely relieve his symptoms, and he was unable to return to work.
The patient presented to our institution 9 weeks after injury with complaints of pain along the lateral aspect of the elbow, painful flexion-extension, and continued swelling. The pain had been unrelieved with anti-inflammatory medications and opioids. Physical examination revealed tenderness and swelling along the lateral epicondyle and extensor mass of the right elbow. The patient had tenderness, marked weakness, and a palpable soft-tissue defect at the origin of the extensor mass with resisted extension of the wrist (Figure 1). Elbow ROM was from 20° to 120° of flexion, 60° of pronation, and 60° of supination. No varus or valgus instability was present about the elbow. Radiographs did not show any fracture or dislocation. Magnetic resonance imaging (MRI) did not definitively show extensor tendon avulsion but did identify signal change of the common extensor tendon (Figures 2A, 2B). Advanced imaging was inconclusive, but, given the patient’s history and physical examination findings, he was diagnosed with an avulsion injury of the origin of the extensor mass of the right elbow.
The patient was brought to the operating room, administered general anesthesia, and placed supine on the operating table with a tourniquet on the upper arm. A lateral 4.5-cm incision was made centered over the lateral epicondyle. The origin of the extensor mass was exposed, and isolated avulsions of the extensor carpi radialis longus and the brachioradialis were identified (Figures 3, 4). Underlying the avulsed sleeve of tissue, the origin of the extensor carpi radialis brevis was found intact. The lateral supracondylar ridge and the lateral epicondyle of the humerus were débrided, and 3 transosseous holes were drilled (using a 2.3-mm bit) through the lateral epicondyle. Four Mason-Allen sutures were placed into the tendon of the common extensor origin using No. 2 braided polyester suture (Ethibond Excel, Ethicon) (Figure 5). The tendon was reduced down to the native footprint, and the sutures were passed through the drill holes and tied down securely (Figure 6). The skin was then closed using layered 4-0 absorbable monofilament suture (Monocryl, Ethicon). The patient was placed in a posterior mold plaster splint with 90° of elbow flexion and with the wrist in 30° of extension.
On postoperative day 3, the patient was seen for a wound check and was placed in a long-arm fiberglass cast (90° of elbow flexion, forearm in neutral, 25° of wrist extension) for immobilization. One week after surgery, he was transitioned to a removable thermoplastic splint, and physical therapy for ROM was initiated. He was allowed therapist-guided active extension of the elbow and flexion of the wrist but was restricted to passive flexion of the elbow and extension of the wrist. Seven weeks after surgery, passive ROM about the elbow was measured, and he was found to have 120° of flexion, 0° extension, 80° pronation, and 80° supination. At 12 weeks, the physical therapy regimen was advanced to include muscle strengthening and active wrist extension and elbow flexion. At 16 weeks, the wrist extensors demonstrated 5/5 strength (Medical Research Council grading system), and the patient was cleared for full activity and weight-bearing without restriction. He returned to work pain-free and without restrictions 18 weeks after surgery. At 2-year follow-up, he had a Mayo performance elbow score of 100 and an Oxford elbow score of 48.3,4 He had full active ROM, full strength, and no subjective pain and was back doing heavy lifting at his job.
Discussion
The brachioradialis, extensor carpi radialis longus, and extensor carpi radialis brevis originate from the anterolateral aspect of the lateral column of the distal humeral metaphysis and form the dorsal mobile wad. The origin of the brachioradialis is about 7 cm in length and begins about 10 to 11 cm above the elbow.5 The origin and insertions of the mobile wad, specifically the brachioradialis, provide a tremendous mechanical advantage with respect to elbow flexion against resistance, particularly with the forearm in the pronated and semipronated positions.6 With the elbow in 30° of flexion, a force 3 times the body weight can be encountered during strenuous lifting.6,7 We hypothesized these large forces likely led to this injury pattern in the patient we have described.
The literature includes 2 case reports of avulsion fracture of the brachioradialis muscle from its origin on the lateral supracondylar humeral ridge.1,2 To our knowledge, however, there have been no reports of pure avulsion. In our patient’s case, there was no bony fracture, but rather avulsion of the extensor carpi radialis longus and brachioradialis at their origin, with the underlying fibers of the extensor carpi radialis brevis remaining in continuity. Because of the rarity of this injury pattern, there was a significant delay in diagnosis. On initial presentation, the differential diagnosis for lateral elbow pain and tenderness included occult fracture, intracapsular plica, osteochondritis dissecans lesion, radial tunnel syndrome, lateral or posterolateral instability, and lateral epicondylitis. Given the absence of antecedent elbow symptoms before the injury, the dynamic soft-tissue asymmetry of the mobile wad with wrist extension, and the palpable soft-tissue defect, we thought the presentation was inconsistent with a simple inflammatory or overuse syndrome, such as lateral epicondylitis. In addition, the physical examination findings were inconsistent with radial tunnel syndrome or disruption of the lateral collateral ligament complex. Elbow MRI did not show an occult fracture, plica, or osteochondritis dissecans lesion but did reveal joint effusion and signal change in the common extensor tendon origin. Interestingly, MRI did not definitively show a tear of the mobile wad. This may be explained by the fact that the fibers of the underlying extensor carpi radialis brevis remained intact. Also potentially involved are the static nature of MRI and potentially suboptimal sequencing and axis of acquisition resulting from the relative infrequency of imaging this joint at certain health care institutions. Our case demonstrates the limitations of MRI in this setting and highlights the need for a detailed history and thorough physical examination for diagnosis.
Funk and colleagues8 used electromyography (EMG) to study the activity of the elbow musculature in uninjured subjects. EMG data were obtained with the elbow joint subjected to resisted flexion, extension, abduction, and adduction. During resisted elbow flexion, there was an increasing amount of activity in the extensor carpi radialis with larger angles of elbow flexion. In addition, the brachioradialis demonstrated the most muscle activity of any of the elbow flexors with 90° or more of elbow flexion and forearm pronation, as opposed to other positions in which the brachialis was the primary flexor. For this reason, we hypothesized that our patient’s forearm was pronated and his elbow flexed to 90° or more when he braced for impact. The ensuing injury resulted from a violent eccentric contraction that caused extensive rupture of the lateral elbow musculature from its broad origin. With the forearm in supination or neutral position, we would have expected a possible injury to the distal biceps as opposed to the brachioradialis and extensor carpi radialis.
In our patient, this injury caused much functional disability, especially with elbow flexion and wrist extension. We hypothesized that, for the muscles to function properly, anatomical restoration would have to be achieved at their known footprint to maintain their mechanical advantage. Therefore, surgical intervention was indicated in our patient, an active laborer. Given the absence of an osseous fracture fragment in this injury pattern, healing must occur at the bone–tendon interface. As tendinous healing is more tenuous and protracted than osseous healing, we preferred transosseous repair. We believed that better tendon-to-bone healing would be possible with drilled osseous tunnels rather than with suture anchors. New studies describing alternative successful methods of treatment would add to our limited body of knowledge regarding this rare injury.
Conclusion
This is the first report of avulsion of the extensor carpi radialis longus and brachioradialis from their origins. Given the biomechanics and anatomy of the dorsal mobile wad, we posit that our patient’s injury occurred when he fell onto his outstretched hand secondary to overwhelming eccentric muscle contracture at time of impact. This injury caused significant upper extremity dysfunction, and surgical intervention was required.
The literature includes only 2 case reports of bony avulsion fracture of the origin of the brachioradialis1,2 and, up until now, no case reports of isolated avulsion of the extensor carpi radialis longus and brachioradialis origins from the lateral epicondyle and lateral supracondylar ridge. In this article, we report the case of a 31-year-old man who sustained this injury during a fall onto his outstretched right hand, and we present our surgical repair technique. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 31-year-old right hand–dominant garbage truck worker sustained a right elbow injury and presented 2 months later. He described slipping and falling onto his outstretched right hand while doing his work. He could not describe the exact mechanism or action or position of the arm at time of impact but thought he tried to catch himself on the truck during the fall. At time of injury, he had immediate pain and swelling to the lateral aspect of the right elbow and difficulty when he attempted lifting. He denied antecedent elbow symptoms before the injury. After evaluation by an outside occupational medicine physician, he engaged in treatment consisting of activity modification and physical therapy, including range-of-motion (ROM) exercises and iontophoresis. This course of management failed to completely relieve his symptoms, and he was unable to return to work.
The patient presented to our institution 9 weeks after injury with complaints of pain along the lateral aspect of the elbow, painful flexion-extension, and continued swelling. The pain had been unrelieved with anti-inflammatory medications and opioids. Physical examination revealed tenderness and swelling along the lateral epicondyle and extensor mass of the right elbow. The patient had tenderness, marked weakness, and a palpable soft-tissue defect at the origin of the extensor mass with resisted extension of the wrist (Figure 1). Elbow ROM was from 20° to 120° of flexion, 60° of pronation, and 60° of supination. No varus or valgus instability was present about the elbow. Radiographs did not show any fracture or dislocation. Magnetic resonance imaging (MRI) did not definitively show extensor tendon avulsion but did identify signal change of the common extensor tendon (Figures 2A, 2B). Advanced imaging was inconclusive, but, given the patient’s history and physical examination findings, he was diagnosed with an avulsion injury of the origin of the extensor mass of the right elbow.
The patient was brought to the operating room, administered general anesthesia, and placed supine on the operating table with a tourniquet on the upper arm. A lateral 4.5-cm incision was made centered over the lateral epicondyle. The origin of the extensor mass was exposed, and isolated avulsions of the extensor carpi radialis longus and the brachioradialis were identified (Figures 3, 4). Underlying the avulsed sleeve of tissue, the origin of the extensor carpi radialis brevis was found intact. The lateral supracondylar ridge and the lateral epicondyle of the humerus were débrided, and 3 transosseous holes were drilled (using a 2.3-mm bit) through the lateral epicondyle. Four Mason-Allen sutures were placed into the tendon of the common extensor origin using No. 2 braided polyester suture (Ethibond Excel, Ethicon) (Figure 5). The tendon was reduced down to the native footprint, and the sutures were passed through the drill holes and tied down securely (Figure 6). The skin was then closed using layered 4-0 absorbable monofilament suture (Monocryl, Ethicon). The patient was placed in a posterior mold plaster splint with 90° of elbow flexion and with the wrist in 30° of extension.
On postoperative day 3, the patient was seen for a wound check and was placed in a long-arm fiberglass cast (90° of elbow flexion, forearm in neutral, 25° of wrist extension) for immobilization. One week after surgery, he was transitioned to a removable thermoplastic splint, and physical therapy for ROM was initiated. He was allowed therapist-guided active extension of the elbow and flexion of the wrist but was restricted to passive flexion of the elbow and extension of the wrist. Seven weeks after surgery, passive ROM about the elbow was measured, and he was found to have 120° of flexion, 0° extension, 80° pronation, and 80° supination. At 12 weeks, the physical therapy regimen was advanced to include muscle strengthening and active wrist extension and elbow flexion. At 16 weeks, the wrist extensors demonstrated 5/5 strength (Medical Research Council grading system), and the patient was cleared for full activity and weight-bearing without restriction. He returned to work pain-free and without restrictions 18 weeks after surgery. At 2-year follow-up, he had a Mayo performance elbow score of 100 and an Oxford elbow score of 48.3,4 He had full active ROM, full strength, and no subjective pain and was back doing heavy lifting at his job.
Discussion
The brachioradialis, extensor carpi radialis longus, and extensor carpi radialis brevis originate from the anterolateral aspect of the lateral column of the distal humeral metaphysis and form the dorsal mobile wad. The origin of the brachioradialis is about 7 cm in length and begins about 10 to 11 cm above the elbow.5 The origin and insertions of the mobile wad, specifically the brachioradialis, provide a tremendous mechanical advantage with respect to elbow flexion against resistance, particularly with the forearm in the pronated and semipronated positions.6 With the elbow in 30° of flexion, a force 3 times the body weight can be encountered during strenuous lifting.6,7 We hypothesized these large forces likely led to this injury pattern in the patient we have described.
The literature includes 2 case reports of avulsion fracture of the brachioradialis muscle from its origin on the lateral supracondylar humeral ridge.1,2 To our knowledge, however, there have been no reports of pure avulsion. In our patient’s case, there was no bony fracture, but rather avulsion of the extensor carpi radialis longus and brachioradialis at their origin, with the underlying fibers of the extensor carpi radialis brevis remaining in continuity. Because of the rarity of this injury pattern, there was a significant delay in diagnosis. On initial presentation, the differential diagnosis for lateral elbow pain and tenderness included occult fracture, intracapsular plica, osteochondritis dissecans lesion, radial tunnel syndrome, lateral or posterolateral instability, and lateral epicondylitis. Given the absence of antecedent elbow symptoms before the injury, the dynamic soft-tissue asymmetry of the mobile wad with wrist extension, and the palpable soft-tissue defect, we thought the presentation was inconsistent with a simple inflammatory or overuse syndrome, such as lateral epicondylitis. In addition, the physical examination findings were inconsistent with radial tunnel syndrome or disruption of the lateral collateral ligament complex. Elbow MRI did not show an occult fracture, plica, or osteochondritis dissecans lesion but did reveal joint effusion and signal change in the common extensor tendon origin. Interestingly, MRI did not definitively show a tear of the mobile wad. This may be explained by the fact that the fibers of the underlying extensor carpi radialis brevis remained intact. Also potentially involved are the static nature of MRI and potentially suboptimal sequencing and axis of acquisition resulting from the relative infrequency of imaging this joint at certain health care institutions. Our case demonstrates the limitations of MRI in this setting and highlights the need for a detailed history and thorough physical examination for diagnosis.
Funk and colleagues8 used electromyography (EMG) to study the activity of the elbow musculature in uninjured subjects. EMG data were obtained with the elbow joint subjected to resisted flexion, extension, abduction, and adduction. During resisted elbow flexion, there was an increasing amount of activity in the extensor carpi radialis with larger angles of elbow flexion. In addition, the brachioradialis demonstrated the most muscle activity of any of the elbow flexors with 90° or more of elbow flexion and forearm pronation, as opposed to other positions in which the brachialis was the primary flexor. For this reason, we hypothesized that our patient’s forearm was pronated and his elbow flexed to 90° or more when he braced for impact. The ensuing injury resulted from a violent eccentric contraction that caused extensive rupture of the lateral elbow musculature from its broad origin. With the forearm in supination or neutral position, we would have expected a possible injury to the distal biceps as opposed to the brachioradialis and extensor carpi radialis.
In our patient, this injury caused much functional disability, especially with elbow flexion and wrist extension. We hypothesized that, for the muscles to function properly, anatomical restoration would have to be achieved at their known footprint to maintain their mechanical advantage. Therefore, surgical intervention was indicated in our patient, an active laborer. Given the absence of an osseous fracture fragment in this injury pattern, healing must occur at the bone–tendon interface. As tendinous healing is more tenuous and protracted than osseous healing, we preferred transosseous repair. We believed that better tendon-to-bone healing would be possible with drilled osseous tunnels rather than with suture anchors. New studies describing alternative successful methods of treatment would add to our limited body of knowledge regarding this rare injury.
Conclusion
This is the first report of avulsion of the extensor carpi radialis longus and brachioradialis from their origins. Given the biomechanics and anatomy of the dorsal mobile wad, we posit that our patient’s injury occurred when he fell onto his outstretched hand secondary to overwhelming eccentric muscle contracture at time of impact. This injury caused significant upper extremity dysfunction, and surgical intervention was required.
1. Guettler JH, Mayo DB. Avulsion fracture of the origin of the brachioradialis muscle. Am J Orthop. 2001;30(9):693-694.
2. Marchant MH Jr, Gambardella RA, Podesta L. Superficial radial nerve injury after avulsion fracture of the brachioradialis muscle origin in a professional lacrosse player: a case report. J Shoulder Elbow Surg. 2009;18(6):e9-e12.
3. Dawson J, Doll H, Boller I, et al. The development and validation of a patient-reported questionnaire to assess outcomes of elbow surgery. J Bone Joint Surg Br. 2008;90(4):466-473.
4. Sathyamoorthy P, Kemp GJ, Rawal A, Rayner V, Frostick SP. Development and validation of an elbow score. Rheumatology. 2004;43(11):1434-1440.
5. Freehafer AA, Peckham PH, Keith MW, Mendelson LS. The brachioradialis: anatomy, properties, and value for tendon transfer in the tetraplegic. J Hand Surg Am. 1988;13(1):99-104.
6. Morrey BF, Sanchez-Sotelo J. The Elbow and Its Disorders. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2009.
7. Nakazawa K, Kawakami Y, Fukunaga T, Yano H, Miyashita M. Differences in activation patterns in elbow flexor muscles during isometric, concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1993;66(3):214-220.
8. Funk DA, An KN, Morrey BF, Daube JR. Electromyographic analysis of muscles across the elbow joint. J Orthop Res. 1987;5(4):529-538.
1. Guettler JH, Mayo DB. Avulsion fracture of the origin of the brachioradialis muscle. Am J Orthop. 2001;30(9):693-694.
2. Marchant MH Jr, Gambardella RA, Podesta L. Superficial radial nerve injury after avulsion fracture of the brachioradialis muscle origin in a professional lacrosse player: a case report. J Shoulder Elbow Surg. 2009;18(6):e9-e12.
3. Dawson J, Doll H, Boller I, et al. The development and validation of a patient-reported questionnaire to assess outcomes of elbow surgery. J Bone Joint Surg Br. 2008;90(4):466-473.
4. Sathyamoorthy P, Kemp GJ, Rawal A, Rayner V, Frostick SP. Development and validation of an elbow score. Rheumatology. 2004;43(11):1434-1440.
5. Freehafer AA, Peckham PH, Keith MW, Mendelson LS. The brachioradialis: anatomy, properties, and value for tendon transfer in the tetraplegic. J Hand Surg Am. 1988;13(1):99-104.
6. Morrey BF, Sanchez-Sotelo J. The Elbow and Its Disorders. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2009.
7. Nakazawa K, Kawakami Y, Fukunaga T, Yano H, Miyashita M. Differences in activation patterns in elbow flexor muscles during isometric, concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1993;66(3):214-220.
8. Funk DA, An KN, Morrey BF, Daube JR. Electromyographic analysis of muscles across the elbow joint. J Orthop Res. 1987;5(4):529-538.