User login
How accurate are point-of-care urine drug screens in patients taking chronic opioid therapy?
EVIDENCE SUMMARY
A 2011 blinded diagnostic accuracy study of 1000 adult chronic pain patients in an interventional pain management program in the United States compared POC immunoassay urine drug testing with LC-MS.1 The immunoassay index test can be performed in the office with rapid results. The LC-MS reference test requires that the urine sample be sent to a lab.
Study participants were 37% male and 63% female, average age 51 years. Of the 1000 patients, 920 were prescribed opioids. Morphine, hydrocodone, codeine, and hydromorphone (morphine group) were tested with cutoff values of 300 ng/mL for POC testing and 50 ng/mL for LC-MS. Cutoffs for methadone were 300 ng/mL for POC and 100 ng/mL for LC-MS. For oxycodone, they were 100 ng/mL for POC and 50 ng/mL for LC-MS.
Methadone had the highest sensitivity and specificity at 96% and 99%, with a false-negative rate of 3.9% and a false-positive rate of 1.2%. It also had the highest agreement between the 2 testing methods at 99%. The morphine group had a sensitivity of 92%, specificity of 93%, false-negative rate of 7.8%, false-positive rate of 6.9%, and 93% test agreement. Oxycodone showed the lowest sensitivity at 75%; it had a specificity of 92%, a false-negative rate of 25%, a false-positive rate of 7.7%, and 90% test agreement.
More false negatives than with LC-MS
A 2010 blinded diagnostic accuracy study of 4200 adults treated with opioids for chronic pain compared immunoassay urine testing with LC-MS for opioids, benzodiazepines, marijuana, cocaine, and methamphetamine between October and November 2008.2 Urine samples were tested using both methods simultaneously on split specimens. Cutoff values for methadone, codeine, hydrocodone, hydromorphone, and morphine were 50 ng/mL on LC-MS. Immunoassay relative activity—the difference between the immunoassay and the LC-MS cutoffs—was 300 for methadone, 180 for codeine, 1700 for hydrocodone, 4000 for hydromorphone, and 300 for morphine.
Of the 3414 samples submitted for opiate testing, 2191 tested positive using immunoassay and 2233 tested positive using LC-MS for a total of 42 false-negative results with immunoassay. The positive rate (percentage of samples testing positive by LC-MS) was 65%, and the false-negative rate was 1.9%. Methadone testing produced 17 false-negative results; the positive rate was 10%, and the false-negative rate was 6.1%. The immunoassay false-positive results occurred in patients taking hydromorphone and hydrocodone.
The study was limited by lack of demographic information on the participants.
1. Manchikanti L, Malla Y, Wargo B, et al. Comparative evaluation of the accuracy of immunoassay with liquid chromatography tandem mass spectrometry of urine drug testing opioids and illicit drugs in chronic pain patients. Pain Physician. 2011;14:175–187.
2. Pesce A, Rosenthal M, West R, et al. An evaluation of the diagnostic accuracy of liquid chromatography-tandem mass spectrometry versus immunoassay drug testing in pain patients. Pain Physician. 2010;13:273–281.
EVIDENCE SUMMARY
A 2011 blinded diagnostic accuracy study of 1000 adult chronic pain patients in an interventional pain management program in the United States compared POC immunoassay urine drug testing with LC-MS.1 The immunoassay index test can be performed in the office with rapid results. The LC-MS reference test requires that the urine sample be sent to a lab.
Study participants were 37% male and 63% female, average age 51 years. Of the 1000 patients, 920 were prescribed opioids. Morphine, hydrocodone, codeine, and hydromorphone (morphine group) were tested with cutoff values of 300 ng/mL for POC testing and 50 ng/mL for LC-MS. Cutoffs for methadone were 300 ng/mL for POC and 100 ng/mL for LC-MS. For oxycodone, they were 100 ng/mL for POC and 50 ng/mL for LC-MS.
Methadone had the highest sensitivity and specificity at 96% and 99%, with a false-negative rate of 3.9% and a false-positive rate of 1.2%. It also had the highest agreement between the 2 testing methods at 99%. The morphine group had a sensitivity of 92%, specificity of 93%, false-negative rate of 7.8%, false-positive rate of 6.9%, and 93% test agreement. Oxycodone showed the lowest sensitivity at 75%; it had a specificity of 92%, a false-negative rate of 25%, a false-positive rate of 7.7%, and 90% test agreement.
More false negatives than with LC-MS
A 2010 blinded diagnostic accuracy study of 4200 adults treated with opioids for chronic pain compared immunoassay urine testing with LC-MS for opioids, benzodiazepines, marijuana, cocaine, and methamphetamine between October and November 2008.2 Urine samples were tested using both methods simultaneously on split specimens. Cutoff values for methadone, codeine, hydrocodone, hydromorphone, and morphine were 50 ng/mL on LC-MS. Immunoassay relative activity—the difference between the immunoassay and the LC-MS cutoffs—was 300 for methadone, 180 for codeine, 1700 for hydrocodone, 4000 for hydromorphone, and 300 for morphine.
Of the 3414 samples submitted for opiate testing, 2191 tested positive using immunoassay and 2233 tested positive using LC-MS for a total of 42 false-negative results with immunoassay. The positive rate (percentage of samples testing positive by LC-MS) was 65%, and the false-negative rate was 1.9%. Methadone testing produced 17 false-negative results; the positive rate was 10%, and the false-negative rate was 6.1%. The immunoassay false-positive results occurred in patients taking hydromorphone and hydrocodone.
The study was limited by lack of demographic information on the participants.
EVIDENCE SUMMARY
A 2011 blinded diagnostic accuracy study of 1000 adult chronic pain patients in an interventional pain management program in the United States compared POC immunoassay urine drug testing with LC-MS.1 The immunoassay index test can be performed in the office with rapid results. The LC-MS reference test requires that the urine sample be sent to a lab.
Study participants were 37% male and 63% female, average age 51 years. Of the 1000 patients, 920 were prescribed opioids. Morphine, hydrocodone, codeine, and hydromorphone (morphine group) were tested with cutoff values of 300 ng/mL for POC testing and 50 ng/mL for LC-MS. Cutoffs for methadone were 300 ng/mL for POC and 100 ng/mL for LC-MS. For oxycodone, they were 100 ng/mL for POC and 50 ng/mL for LC-MS.
Methadone had the highest sensitivity and specificity at 96% and 99%, with a false-negative rate of 3.9% and a false-positive rate of 1.2%. It also had the highest agreement between the 2 testing methods at 99%. The morphine group had a sensitivity of 92%, specificity of 93%, false-negative rate of 7.8%, false-positive rate of 6.9%, and 93% test agreement. Oxycodone showed the lowest sensitivity at 75%; it had a specificity of 92%, a false-negative rate of 25%, a false-positive rate of 7.7%, and 90% test agreement.
More false negatives than with LC-MS
A 2010 blinded diagnostic accuracy study of 4200 adults treated with opioids for chronic pain compared immunoassay urine testing with LC-MS for opioids, benzodiazepines, marijuana, cocaine, and methamphetamine between October and November 2008.2 Urine samples were tested using both methods simultaneously on split specimens. Cutoff values for methadone, codeine, hydrocodone, hydromorphone, and morphine were 50 ng/mL on LC-MS. Immunoassay relative activity—the difference between the immunoassay and the LC-MS cutoffs—was 300 for methadone, 180 for codeine, 1700 for hydrocodone, 4000 for hydromorphone, and 300 for morphine.
Of the 3414 samples submitted for opiate testing, 2191 tested positive using immunoassay and 2233 tested positive using LC-MS for a total of 42 false-negative results with immunoassay. The positive rate (percentage of samples testing positive by LC-MS) was 65%, and the false-negative rate was 1.9%. Methadone testing produced 17 false-negative results; the positive rate was 10%, and the false-negative rate was 6.1%. The immunoassay false-positive results occurred in patients taking hydromorphone and hydrocodone.
The study was limited by lack of demographic information on the participants.
1. Manchikanti L, Malla Y, Wargo B, et al. Comparative evaluation of the accuracy of immunoassay with liquid chromatography tandem mass spectrometry of urine drug testing opioids and illicit drugs in chronic pain patients. Pain Physician. 2011;14:175–187.
2. Pesce A, Rosenthal M, West R, et al. An evaluation of the diagnostic accuracy of liquid chromatography-tandem mass spectrometry versus immunoassay drug testing in pain patients. Pain Physician. 2010;13:273–281.
1. Manchikanti L, Malla Y, Wargo B, et al. Comparative evaluation of the accuracy of immunoassay with liquid chromatography tandem mass spectrometry of urine drug testing opioids and illicit drugs in chronic pain patients. Pain Physician. 2011;14:175–187.
2. Pesce A, Rosenthal M, West R, et al. An evaluation of the diagnostic accuracy of liquid chromatography-tandem mass spectrometry versus immunoassay drug testing in pain patients. Pain Physician. 2010;13:273–281.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
In adults treated with opioids for chronic pain, point-of-care (POC) urine drug screens (immunoassays) for detecting opioids show a false-negative rate of 1.9%, a sensitivity of 92%, and a specificity of 93% compared with the gold-standard liquid chromatography tandem mass spectrometry (LC-MS). Oxycodone has the highest rate of false-negative results at 25%; methadone has the lowest rate at 4% to 6% (strength of recommendation [SOR]: A, 2 blinded diagnostic accuracy studies with similar results).
Does vitamin D without calcium reduce fracture risk?
EVIDENCE SUMMARY
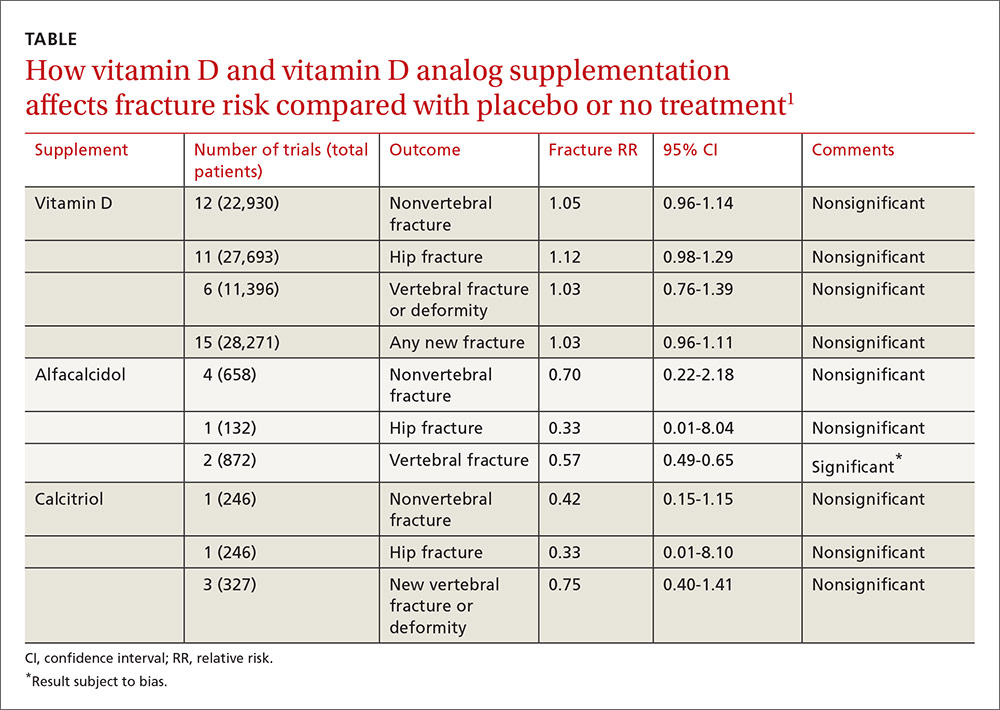
A 2014 meta-analysis of 15 trials (quasi-random and RCT) with a total of 28,271 patients that compared the effect of vitamin D on fracture risk with placebo or no treatment, found no benefit for vitamin D supplementation (TABLE).1 Patients lived in community and nursing home settings and ranged in age from 50 to 85 years; 24% to 100% were female.
Only 3 trials required patients to have had a previous fracture. Exclusions included: diseases affecting bone metabolism, cognitive impairment, drugs affecting bone metabolism (bisphosphonates, selective estrogen receptor modulators, and corticosteroids), renal failure, hypercalcemia, nephrolithiasis, and decreased mobility (recent stroke recovery and Parkinson’s disease).
Formulations of vitamin D included cholecalciferol (D3) 400 to 2000 IU/d for 4 months to 5 years or 100,000 to 500,000 IU every 3 to 12 months for 1 to 5 years; calcifediol (25(OH)D3) 600 IU/d for 4 years; and ergocalciferol (D2) 400 IU/d for 2 years or 3000 to 300,000 IU every 3 to 12 months for 10 months to 3 years.
Vitamin D analogs generally have no benefit either
The same meta-analysis compared vitamin D analogs to placebo or no treatment (8 trials, quasi-random and RCT, 1743 patients) on the risk of fracture, again finding no benefit in all but one case. Included patients were mostly by referral to tertiary or university hospitals and outpatient community settings.
Most of the studies included only a small number of patients (about 200), with the largest study having 740 patients. The age range was 50 to 77 years, and 50% to 100% were female. Most of the trials required patients to have osteoporosis or vitamin D deficiency with a previous vertebral deformity on imaging. Study exclusions included osteomalacia, malabsorption, hyperparathyroidism, active kidney stones, history of hypercalciuria, cancer, incurable disease, dementia, severe chronic illness (renal or liver failure), recent stroke or fracture, and drugs that affect bone metabolism.
Vitamin D analogs were given as alfacalcidol (1-alphahydroxyvitamin D3) 0.5 mcg twice daily or 1 mcg/d for 36 weeks to 2 years or calcitriol (1,25-dihydroxyvitamin D3) 0.25 to 1 mcg once or twice daily for one to 3 years. Researchers found a significant reduction in vertebral (but not nonvertebral or hip) fractures with alfacalcidol, but the finding occurred in a single trial that was assessed by the authors of the meta-analysis as subject to bias.
Supplementation doesn’t affect mortality, but does have some side effects
Patients taking vitamin D or an analog with or without calcium showed no difference in risk of death compared with patients taking placebo (29 trials, 71,032 patients; relative risk [RR]=0.97; 95% confidence interval [CI], 0.93-1.01).
Patients taking vitamin D or an analog were more likely than controls to have mild hypercalcemia, with an average increase of 2.7 mmol/L (21 trials, 17,124 patients; RR=2.28; 95% CI, 1.57-3.31). Patients taking calcitriol had the highest risk (4 trials, 988 patients; RR=4.41; 95% CI, 2.14-9.09).
Gastrointestinal adverse effects (4% increase) and renal calculi or mild renal insufficiency (16% increase) were more common with vitamin D and analogs than placebo (GI adverse effects: 15 trials, 47,761 patients; RR=1.04; 95% CI, 1.00-1.08; renal calculi or mild renal insufficiency: 11 trials, 46,548 patients; RR=1.16; 95% CI, 1.02-1.33).
RECOMMENDATIONS
There are no guidelines recommending vitamin D supplementation without calcium to prevent fracture.
1. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
EVIDENCE SUMMARY
A 2014 meta-analysis of 15 trials (quasi-random and RCT) with a total of 28,271 patients that compared the effect of vitamin D on fracture risk with placebo or no treatment, found no benefit for vitamin D supplementation (TABLE).1 Patients lived in community and nursing home settings and ranged in age from 50 to 85 years; 24% to 100% were female.
Only 3 trials required patients to have had a previous fracture. Exclusions included: diseases affecting bone metabolism, cognitive impairment, drugs affecting bone metabolism (bisphosphonates, selective estrogen receptor modulators, and corticosteroids), renal failure, hypercalcemia, nephrolithiasis, and decreased mobility (recent stroke recovery and Parkinson’s disease).
Formulations of vitamin D included cholecalciferol (D3) 400 to 2000 IU/d for 4 months to 5 years or 100,000 to 500,000 IU every 3 to 12 months for 1 to 5 years; calcifediol (25(OH)D3) 600 IU/d for 4 years; and ergocalciferol (D2) 400 IU/d for 2 years or 3000 to 300,000 IU every 3 to 12 months for 10 months to 3 years.

Vitamin D analogs generally have no benefit either
The same meta-analysis compared vitamin D analogs to placebo or no treatment (8 trials, quasi-random and RCT, 1743 patients) on the risk of fracture, again finding no benefit in all but one case. Included patients were mostly by referral to tertiary or university hospitals and outpatient community settings.
Most of the studies included only a small number of patients (about 200), with the largest study having 740 patients. The age range was 50 to 77 years, and 50% to 100% were female. Most of the trials required patients to have osteoporosis or vitamin D deficiency with a previous vertebral deformity on imaging. Study exclusions included osteomalacia, malabsorption, hyperparathyroidism, active kidney stones, history of hypercalciuria, cancer, incurable disease, dementia, severe chronic illness (renal or liver failure), recent stroke or fracture, and drugs that affect bone metabolism.
Vitamin D analogs were given as alfacalcidol (1-alphahydroxyvitamin D3) 0.5 mcg twice daily or 1 mcg/d for 36 weeks to 2 years or calcitriol (1,25-dihydroxyvitamin D3) 0.25 to 1 mcg once or twice daily for one to 3 years. Researchers found a significant reduction in vertebral (but not nonvertebral or hip) fractures with alfacalcidol, but the finding occurred in a single trial that was assessed by the authors of the meta-analysis as subject to bias.
Supplementation doesn’t affect mortality, but does have some side effects
Patients taking vitamin D or an analog with or without calcium showed no difference in risk of death compared with patients taking placebo (29 trials, 71,032 patients; relative risk [RR]=0.97; 95% confidence interval [CI], 0.93-1.01).
Patients taking vitamin D or an analog were more likely than controls to have mild hypercalcemia, with an average increase of 2.7 mmol/L (21 trials, 17,124 patients; RR=2.28; 95% CI, 1.57-3.31). Patients taking calcitriol had the highest risk (4 trials, 988 patients; RR=4.41; 95% CI, 2.14-9.09).
Gastrointestinal adverse effects (4% increase) and renal calculi or mild renal insufficiency (16% increase) were more common with vitamin D and analogs than placebo (GI adverse effects: 15 trials, 47,761 patients; RR=1.04; 95% CI, 1.00-1.08; renal calculi or mild renal insufficiency: 11 trials, 46,548 patients; RR=1.16; 95% CI, 1.02-1.33).
RECOMMENDATIONS
There are no guidelines recommending vitamin D supplementation without calcium to prevent fracture.
EVIDENCE SUMMARY
A 2014 meta-analysis of 15 trials (quasi-random and RCT) with a total of 28,271 patients that compared the effect of vitamin D on fracture risk with placebo or no treatment, found no benefit for vitamin D supplementation (TABLE).1 Patients lived in community and nursing home settings and ranged in age from 50 to 85 years; 24% to 100% were female.
Only 3 trials required patients to have had a previous fracture. Exclusions included: diseases affecting bone metabolism, cognitive impairment, drugs affecting bone metabolism (bisphosphonates, selective estrogen receptor modulators, and corticosteroids), renal failure, hypercalcemia, nephrolithiasis, and decreased mobility (recent stroke recovery and Parkinson’s disease).
Formulations of vitamin D included cholecalciferol (D3) 400 to 2000 IU/d for 4 months to 5 years or 100,000 to 500,000 IU every 3 to 12 months for 1 to 5 years; calcifediol (25(OH)D3) 600 IU/d for 4 years; and ergocalciferol (D2) 400 IU/d for 2 years or 3000 to 300,000 IU every 3 to 12 months for 10 months to 3 years.

Vitamin D analogs generally have no benefit either
The same meta-analysis compared vitamin D analogs to placebo or no treatment (8 trials, quasi-random and RCT, 1743 patients) on the risk of fracture, again finding no benefit in all but one case. Included patients were mostly by referral to tertiary or university hospitals and outpatient community settings.
Most of the studies included only a small number of patients (about 200), with the largest study having 740 patients. The age range was 50 to 77 years, and 50% to 100% were female. Most of the trials required patients to have osteoporosis or vitamin D deficiency with a previous vertebral deformity on imaging. Study exclusions included osteomalacia, malabsorption, hyperparathyroidism, active kidney stones, history of hypercalciuria, cancer, incurable disease, dementia, severe chronic illness (renal or liver failure), recent stroke or fracture, and drugs that affect bone metabolism.
Vitamin D analogs were given as alfacalcidol (1-alphahydroxyvitamin D3) 0.5 mcg twice daily or 1 mcg/d for 36 weeks to 2 years or calcitriol (1,25-dihydroxyvitamin D3) 0.25 to 1 mcg once or twice daily for one to 3 years. Researchers found a significant reduction in vertebral (but not nonvertebral or hip) fractures with alfacalcidol, but the finding occurred in a single trial that was assessed by the authors of the meta-analysis as subject to bias.
Supplementation doesn’t affect mortality, but does have some side effects
Patients taking vitamin D or an analog with or without calcium showed no difference in risk of death compared with patients taking placebo (29 trials, 71,032 patients; relative risk [RR]=0.97; 95% confidence interval [CI], 0.93-1.01).
Patients taking vitamin D or an analog were more likely than controls to have mild hypercalcemia, with an average increase of 2.7 mmol/L (21 trials, 17,124 patients; RR=2.28; 95% CI, 1.57-3.31). Patients taking calcitriol had the highest risk (4 trials, 988 patients; RR=4.41; 95% CI, 2.14-9.09).
Gastrointestinal adverse effects (4% increase) and renal calculi or mild renal insufficiency (16% increase) were more common with vitamin D and analogs than placebo (GI adverse effects: 15 trials, 47,761 patients; RR=1.04; 95% CI, 1.00-1.08; renal calculi or mild renal insufficiency: 11 trials, 46,548 patients; RR=1.16; 95% CI, 1.02-1.33).
RECOMMENDATIONS
There are no guidelines recommending vitamin D supplementation without calcium to prevent fracture.
1. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
1. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
No. Supplemental vitamin D without calcium—in doses averaging as much as 800 IU per day—doesn’t reduce the risk of hip, vertebral, or nonvertebral fractures in postmenopausal women and older men (strength of recommendation [SOR]: A, large, high-quality meta-analysis of randomized or quasi-randomized placebo-controlled trials).
The vitamin D analogs alfacalcidol and calcitriol also don’t reduce hip or nonvertebral fractures (SOR: A, multiple randomized, controlled trials [RCTs]), although alfacalcidol (but not calcitriol) does reduce vertebral fractures by 43% (SOR: B, one RCT and one quasi-randomized trial with potential for bias)
Vitamin D supplementation, with or without calcium, doesn’t affect mortality. It does double the risk of mild hypercalcemia (about 2.7 mmol/L increase), raise the risk of renal calculi or mild renal insufficiency by 16%, and slightly increase (4%) gastrointestinal adverse effects (SOR: A, meta-analysis of RCTs or quasi-randomized trials).
Is nonoperative therapy as effective as surgery for meniscal injuries?
Yes. There is no significant difference in symptom or functional improvement between adult patients with symptomatic meniscal injury who are treated with operative vs nonoperative therapy (strength of recommendation: A, consistent randomized controlled trials [RCTs]).
Both approaches resulted in function and pain improvement
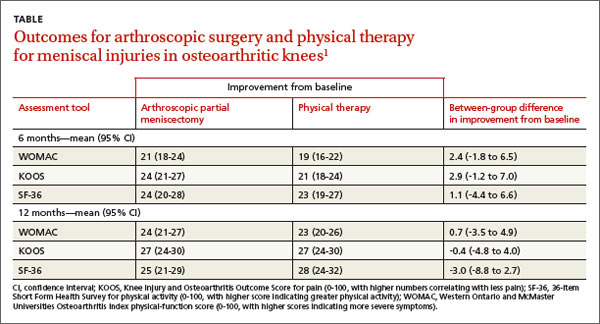
A 2013 multicenter RCT evaluated 351 adults, 45 years and older, with a meniscal tear and mild to moderate osteoarthritis confirmed by imaging, for functional improvement by physical therapy alone compared with arthroscopic partial meniscectomy and physical therapy.1
At the beginning of the study and 6 and 12 months after treatment, researchers assessed symptoms using the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index physical-function score (0-100, with higher scores indicating more severe symptoms), the Knee Injury and Osteoarthritis Outcome Score (KOOS) for pain (0-100, with higher numbers correlating with less pain), and the 36-item Short Form Health Survey (SF-36) for physical activity (0-100, with higher scores indicating greater physical activity).
Modified intention to treat analysis showed no significant difference in function and pain improvement at 6 and 12 months between patients with meniscal injury who underwent arthroscopic repair and physical therapy and patients who underwent physical therapy alone (TABLE1). A limitation of the study was the crossover of 30% of patients from the nonoperative group to the operative group.
No differences found in Tx outcomes for nontraumatic tears
A 2007 prospective RCT evaluated 90 adults ages 45 to 64 with nontraumatic meniscal tears confirmed by magnetic resonance imaging for improvement in knee pain and function with arthroscopic treatment and supervised exercise (AE) or supervised exercise (E) alone.2 Knee pain and function were assessed before intervention, after 8 weeks, and after 6 months of treatment using 3 surveys: the KOOS, the Lysholm Knee Scoring Scale (LKSS; 0-100, with higher scores correlating with good knee function), and the Visual Analogue Scale (VAS) for knee pain (0-10, with 0 indicating no pain and 10 indicating maximum pain).
The KOOS revealed that at 8 weeks and 6 months both groups had significant improvement from the initial evaluation in all subscale scores. In the AE group, the 8-week pain score increased from a baseline of 56 to 89 (P<.001) and remained at 89 at 6 months (P<.001). For the E group, the 8-week pain score improved from a baseline of 62 to 86 (P<.001) and continued at 86 after 6 months (P<.001).
The LKSS score for both groups showed significant improvement from baseline at 8 weeks: 34% of the AE group and 42% of the E group scored higher than 91 (P<.001).
VAS scores showed a significant decrease in pain at 8 weeks for both the AE and E groups: beginning median value for both groups was 5.5 and decreased to 1.0 at 8 weeks and 6 months (P<.001).
The authors concluded that both groups improved significantly from initial evaluation regardless of treatment method and that no statistically significant difference existed between treatment results.
1. Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368:1675-1684.
2. Herrlin S, Hallander M, Wange P, et al. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surg Sports Traumatol Arthrosc. 2007;15:393-401.
Yes. There is no significant difference in symptom or functional improvement between adult patients with symptomatic meniscal injury who are treated with operative vs nonoperative therapy (strength of recommendation: A, consistent randomized controlled trials [RCTs]).
Both approaches resulted in function and pain improvement
A 2013 multicenter RCT evaluated 351 adults, 45 years and older, with a meniscal tear and mild to moderate osteoarthritis confirmed by imaging, for functional improvement by physical therapy alone compared with arthroscopic partial meniscectomy and physical therapy.1
At the beginning of the study and 6 and 12 months after treatment, researchers assessed symptoms using the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index physical-function score (0-100, with higher scores indicating more severe symptoms), the Knee Injury and Osteoarthritis Outcome Score (KOOS) for pain (0-100, with higher numbers correlating with less pain), and the 36-item Short Form Health Survey (SF-36) for physical activity (0-100, with higher scores indicating greater physical activity).
Modified intention to treat analysis showed no significant difference in function and pain improvement at 6 and 12 months between patients with meniscal injury who underwent arthroscopic repair and physical therapy and patients who underwent physical therapy alone (TABLE1). A limitation of the study was the crossover of 30% of patients from the nonoperative group to the operative group.
No differences found in Tx outcomes for nontraumatic tears
A 2007 prospective RCT evaluated 90 adults ages 45 to 64 with nontraumatic meniscal tears confirmed by magnetic resonance imaging for improvement in knee pain and function with arthroscopic treatment and supervised exercise (AE) or supervised exercise (E) alone.2 Knee pain and function were assessed before intervention, after 8 weeks, and after 6 months of treatment using 3 surveys: the KOOS, the Lysholm Knee Scoring Scale (LKSS; 0-100, with higher scores correlating with good knee function), and the Visual Analogue Scale (VAS) for knee pain (0-10, with 0 indicating no pain and 10 indicating maximum pain).

The KOOS revealed that at 8 weeks and 6 months both groups had significant improvement from the initial evaluation in all subscale scores. In the AE group, the 8-week pain score increased from a baseline of 56 to 89 (P<.001) and remained at 89 at 6 months (P<.001). For the E group, the 8-week pain score improved from a baseline of 62 to 86 (P<.001) and continued at 86 after 6 months (P<.001).
The LKSS score for both groups showed significant improvement from baseline at 8 weeks: 34% of the AE group and 42% of the E group scored higher than 91 (P<.001).
VAS scores showed a significant decrease in pain at 8 weeks for both the AE and E groups: beginning median value for both groups was 5.5 and decreased to 1.0 at 8 weeks and 6 months (P<.001).
The authors concluded that both groups improved significantly from initial evaluation regardless of treatment method and that no statistically significant difference existed between treatment results.
Yes. There is no significant difference in symptom or functional improvement between adult patients with symptomatic meniscal injury who are treated with operative vs nonoperative therapy (strength of recommendation: A, consistent randomized controlled trials [RCTs]).
Both approaches resulted in function and pain improvement
A 2013 multicenter RCT evaluated 351 adults, 45 years and older, with a meniscal tear and mild to moderate osteoarthritis confirmed by imaging, for functional improvement by physical therapy alone compared with arthroscopic partial meniscectomy and physical therapy.1
At the beginning of the study and 6 and 12 months after treatment, researchers assessed symptoms using the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index physical-function score (0-100, with higher scores indicating more severe symptoms), the Knee Injury and Osteoarthritis Outcome Score (KOOS) for pain (0-100, with higher numbers correlating with less pain), and the 36-item Short Form Health Survey (SF-36) for physical activity (0-100, with higher scores indicating greater physical activity).
Modified intention to treat analysis showed no significant difference in function and pain improvement at 6 and 12 months between patients with meniscal injury who underwent arthroscopic repair and physical therapy and patients who underwent physical therapy alone (TABLE1). A limitation of the study was the crossover of 30% of patients from the nonoperative group to the operative group.
No differences found in Tx outcomes for nontraumatic tears
A 2007 prospective RCT evaluated 90 adults ages 45 to 64 with nontraumatic meniscal tears confirmed by magnetic resonance imaging for improvement in knee pain and function with arthroscopic treatment and supervised exercise (AE) or supervised exercise (E) alone.2 Knee pain and function were assessed before intervention, after 8 weeks, and after 6 months of treatment using 3 surveys: the KOOS, the Lysholm Knee Scoring Scale (LKSS; 0-100, with higher scores correlating with good knee function), and the Visual Analogue Scale (VAS) for knee pain (0-10, with 0 indicating no pain and 10 indicating maximum pain).

The KOOS revealed that at 8 weeks and 6 months both groups had significant improvement from the initial evaluation in all subscale scores. In the AE group, the 8-week pain score increased from a baseline of 56 to 89 (P<.001) and remained at 89 at 6 months (P<.001). For the E group, the 8-week pain score improved from a baseline of 62 to 86 (P<.001) and continued at 86 after 6 months (P<.001).
The LKSS score for both groups showed significant improvement from baseline at 8 weeks: 34% of the AE group and 42% of the E group scored higher than 91 (P<.001).
VAS scores showed a significant decrease in pain at 8 weeks for both the AE and E groups: beginning median value for both groups was 5.5 and decreased to 1.0 at 8 weeks and 6 months (P<.001).
The authors concluded that both groups improved significantly from initial evaluation regardless of treatment method and that no statistically significant difference existed between treatment results.
1. Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368:1675-1684.
2. Herrlin S, Hallander M, Wange P, et al. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surg Sports Traumatol Arthrosc. 2007;15:393-401.
1. Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368:1675-1684.
2. Herrlin S, Hallander M, Wange P, et al. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surg Sports Traumatol Arthrosc. 2007;15:393-401.
Evidence-based answers from the Family Physicians Inquiries Network

