User login
Follow-up of Abnormal Metanephrine and Catecholamine Testing: Chasing Missed Neuroendocrine Tumors
From the Department of Medicine, Tufts Medical Center, Boston, MA.
Abstract
- Objective: To measure the frequency of missed pheochromocytoma test results and identify factors related to the risk of failed follow-up.
- Methods: We performed a retrospective review of the medical record to identify patients with abnormal urine or serum metanephrine or catecholamine test results over a 3-year period. We then searched the electronic medical record for documentation that the responsible physician was aware of the test results. We surveyed the physicians in cases where there were abnormal results and no documented follow-up to assess their awareness of the results and any follow-up actions they may have taken.
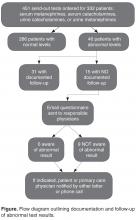
- Results: During the 3-year look-back period, 451 send-out tests for 332 patients were ordered for serum metanephrines, serum catecholamines, or urine catecholamines and/or metanephrines. Fifty-five tests affecting 46 patients returned with either moderately (n = 41) or critically elevated values (n = 5). Fifteen of these patients were inpatients when the tests were ordered, and 31 were outpatients. In 15 of 46 abnormal cases, there was no documentation in the electronic medical record that the responsible physician was aware of the result. Of the 15 cases without documentation, 6 of the responsible physicians in such cases were aware of the results.
- Conclusion: One-third of patients with abnormal lab testing for pheochromocytoma did not have clearly documented follow-up in the electronic medical record, and the majority of physicians in such cases were not aware of the results. Changes to the processes at health care institutions and reference laboratories are needed to improve follow-up of send-out lab results.
Delayed or missed follow-up of laboratory tests is a major source of medical harm [1–5]. Testing performed in both the inpatient and outpatient settings is susceptible to lost follow-up, in part because medical testing is a complex process that is vulnerable to multiple process-of-care failures [1,5–7]. In previous studies, the rate of missed follow-up of abnormal medical test results has ranged from 1% to 75% [6]. Laboratory test follow-up is a particularly challenging problem as patients transition between care settings [8,9]. In a study of 86 patients at one academic medical center, Moore and colleagues found that over a 1-year period, 41% of patients who had laboratory tests pending at the time of discharge had no documented follow-up for at least one of those tests [9]. More recently, Roy and colleagues reported that nearly half of 2644 patients discharged from general medicine hospitalist services at 2 academic tertiary care centers had pending laboratory or radiographic results. Nine percent of the pending results were potentially actionable, and a follow-up survey from the study revealed that 61% of physicians were unaware of pending results [10]. Similar findings have been reported in ambulatory care [5,8,11].
Among the universe of laboratory tests, tests performed at reference laboratories outside of the hospital or clinic where care is rendered (ie, “send-out” tests) are particularly susceptible to lost follow-up [12,13]. Because many of these tests are expensive and infrequently ordered, it is most feasible and economical for hospitals and clinics to transport these samples to regional or national laboratories for specialized testing [14,15]. Examples include the serotonin release assay, certain rheumatologic studies, cancer genetics, and advanced endocrine testing. Send-out testing poses several potential risks including accidental ordering of the wrong test, processing or transportation delays, failure of the outside laboratory to receive the specimen, failures of results reporting by the reference laboratory, incorrect result entry into the electronic medical record upon receipt, failure of the clinician to receive or note the result, or failure of clinician to interpret or act on the result [12,13,15]. Although previous studies have identified risk factors associated with missed abnormal test results [1], none to our knowledge have assessed the particular risks associated with samples processed at reference laboratories.
A critical event at our hospital involved a young woman who presented with respiratory failure attributed to a community-acquired pneumonia and systolic congestive heart failure that was thought to be related to her acute illness. Serum and urine metanephrines were ordered in the intensive care unit given the possibility that heart failure in a young patient could be attributed to an occult neuroendocrine tumor. The patient improved clinically and was discharged. Because the discharging service was unaware that the metanephrine tests had been ordered and were being processed at a national reference laboratory, they did not follow up on the test result or include it as pending in the discharge summary. Fortunately, the patient’s primary care physician discovered that the metanephrine levels were elevated and referred the patient for endocrine evaluation and definitive treatment.
Given the risk represented by pending send-out tests raised by this episode, we performed a retrospective study to identify other cases of missed abnormal send-out tests for metanephrines and catecholamines for in- and outpatients over the previous 3 years. We also sought to identify factors that increased the risk of failed follow-up.
Methods
Subjects and Setting
We studied adult in- and outpatients who received care at a 415-bed Boston-based academic medical center.
Project Design and Data Collection
We performed a retrospective record review of a cohort of patients with abnormal send-out laboratory tests for metanephrines and catecholamines. We collected laboratory reports of all results of urine and serum metanephrine and catecholamine tests performed from 1 January 2012 through 31 December 2014. All tests were performed at and reported by Quest Diagnostics in Chantilly, Virginia. The relevant tests were identified using a query of the online Quest Diagnostics system to extract all laboratory results for serum metanephrines, serum catecholamines, urine metanephrines, and urine catecholamines that resulted during this period. Reports were PDF files that were printed and reviewed manually. (Of note, providers typically view lab results directly in the electronic medical record. Reports were extracted from the Quest Diagnostics system for study purposes only.)
We used the reference ranges supplied by the laboratory to sort results into: normal levels, moderately elevated levels (1 to 4 times the upper limit of normal), and critically elevated levels (greater than 4 times the upper limit of normal). A physician (RZ) then reviewed the electronic medical record of each patient with moderately or critically elevated results for evidence that the responsible physician was aware of the results and had documented a follow-up plan. Documentation of physician awareness and follow-up was ascertained by notation and interpretation of the test result in either a discharge summary from the index admission or in an outpatient clinic note. The responsible physician was defined as the ordering physician for tests ordered in ambulatory care and the attending physician at time of discharge for inpatients. In cases where no documentation was identified in the medical record, the responsible physicians received an email questionnaire that asked (1) if they were aware of the abnormal result, (2) if aware of the result, did they notify the primary care physician or referring physician, and (3) if they were aware of any further follow-up or intervention.
Analysis
We stratified the cases into those with normal and abnormal labs values, and then further by those that did and did not have documentation of results and follow-up in the medical record. We then further stratified cases into those in which the responsible physician was aware and those in which they were unaware. If unaware, the patient was contacted directly by the risk management department, primarily for patient safety purposes. If we were unable to contact the patient, the patient’s listed primary care physician was contacted directly. We then performed qualitative analysis of the cases with abnormal results and no documented follow-up, with the goal of identifying common themes.
Results
During the 3-year look-back period, 451 send-out tests for 332 patients were ordered for serum metanephrines, serum catecholamines, urine catecholamines, or metanephrines. Fifty-five tests affecting 46 patients returned with either moderately or critically elevated values, while 396 results affecting 286 patients returned within the reference range. Five patients had critically elevated values and 41 patients had moderately elevated values. Fifteen were inpatients when the tests were ordered and 31 were outpatients.
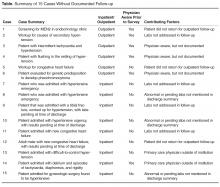
In the survey of the responsible physicians in the 15 cases with no follow-up, all 15 physicians responded. Six were aware of the abnormal result and 9 were not (Figure). Five of the 6 cases in which the physician was aware were outpatients. Eight of the 9 cases in which the physician was not aware were inpatients. In 4 of 15 abnormal cases with no follow-up, the patient was seen at a follow-up appointment but the lab results were not addressed. In 3 of 15 abnormal cases with no follow-up, the patient did not return for a planned follow-up appointment. In 3 of 15 abnormal cases with no follow-up, the physician was aware and addressed the results, but did not document that the results were addressed (all 3 were outpatient cases). In 3 of 15 abnormal cases with no follow-up, lab results for inpatients were pending at time of discharge and there was no documentation of pending results in the designated space for this in the discharge summary. In 2 of 15 abnormal cases with no follow-up, the patient was followed by a primary care physician outside of our institution. In 7 cases, the patient had multiple subspecialists involved in their care. All undocumented abnormal levels were addressed by our institution, either by contacting the patient or primary care physician, or by determining that the abnormality was not clinically relevant.
Discussion
We identified cases in which patients had abnormal results on tests used to diagnose neuroendocrine tumors such as pheochromocytoma over a 3-year period and sought evidence that a responsible clinician had followed up on the abnormal results. In one-third of abnormal test results, we found no documentation in the medical record that the responsible clinician was aware of the result or had communicated it to another clinician or the patient. This occurred most often in cases in which metanephrine and/or catecholamine levels were pending at the time of hospital discharge, and when a patient who was discharged from the hospital or seen in clinic did not return for a scheduled follow-up appointment. When we followed up with the responsible physician, only 6 in 15 were aware of the abnormal results and had either concluded that they were not clinically significant or had addressed the issue without completing documentation.
Previous research has identified vulnerabilities in the follow-up of send-out test results that exceed the challenges with tests performed in-house. These include that send-out tests inherently have more steps and require more manual processes [8], and that these tests are more prone to delay, misinterpretation, and poor documentation. Reference laboratories usually provide non-structured reporting of results, often in the form of paper or PDF files. This can make it difficult for receiving hospitals or clinics to incorporate information into the electronic medical record or to build clinical reminders or alerts for ordering clinicians. Additionally, these data elements are often cryptic in that they provide reference values without necessarily setting parameters for abnormalities. This is a case in point with metanephrine and catecholamine testing, as the results are often variable and poorly reproducible and difficult for clinicians to interpret. There are different cutoffs for moderately elevated and critically elevated values, and how to proceed with patients with moderately elevated values is not clear and may require the expertise of subspecialists. Our study confirmed several issues surrounding vulnerabilities of send-out lab testing.
As a single-institution project with a small cohort of subjects, the generalizability of this project may be limited. However, some process-of-care vulnerabilities noted here are similar to those reported in previous research studies [8]. In addition, hospitals and clinics send specimens to a limited number of regional and national reference laboratories. The challenges that our clinicians encountered in managing these results are likely to be challenges in many other organizations. Also, while our study was limited to tests done to evaluate for pheochromocytoma, our findings are likely applicable to other reference laboratory tests.
Send-out labs continue to represent a major source of lost follow-up and potential patient harm. Creating systems with effective and timely alerts for providers will be useful in preventing missed follow-up. Our study found a lack of clear guidelines designating responsibility for pending lab results, which has been found across institutions in previous studies [8]. Since we conducted this project, our institution has reminded clinicians that discharging attendings are responsible for pending lab results at time of discharge and has developed an automated electronic method for delivering these results. Similar policy interventions at other institutions have shown promise [16]. We hope this will minimize the number of lab results, including those of send-out labs, which are not acted upon in a timely manner. However, other issues, including data interface with the electronic medical record and patients with abnormal results being lost to follow-up, remain barriers for our institution to address.
There are several immediate steps that could be taken by health care organizations and reference labs to reduce patient harm as a result of send-out labs that are not followed up. First, health care organizations can develop better integration between electronic records and lab processing for send-out labs, as well as more electronic alerts. This may help to notify ordering physicians after patients have been discharged and the case may not be front of mind. Reference labs should create robust electronic systems to transmit results as electronic data elements so that health care organizations can easily incorporate results into their electronic medical records, and develop notification systems that flag out-of-bound values. Secure online lab results for send-outs may shorten the delay in reporting. Additionally, creating clear policies establishing the responsible provider is crucial, as has been found by previous research by Singh and others [11,15].
In conclusion, send-out labs are vulnerable to lost follow-up. It is crucial for clinicians to be aware of all send-out lab results and to document their interpretation of abnormal results. Developing policies and systems to facilitate timely follow-up will help to reduce potential patient harm related to send-out labs.
Corresponding author: Richard Zamore, MD, MPH, Tufts Medical Center, 800 Washington St., Boston, MA 02111, [email protected].
Financial disclosures: None.
1. Callen J, Georgiou A, Li J, Westbrook JI, et al. The safety implications of missed test results for hospitalised patients: a systematic review. BMJ Qual Saf 2011;20:194–9.
2. Wahls TL, Cram PM. The frequency of missed test results and associated treatment delays in a highly computerized health system. BMC Fam Pract 2007;8:32.
3. Bates DW, Leape LL. Doing better with critical test results. Jt Comm J Qual Patient Saf 2005;31:66–7.
4. Schiff GD, Kim S, Krosnjar N, et al. Missed hypothyroidism diagnosis uncovered by linking laboratory and pharmacy data. Arch Intern Med 2005;165:574.
5. Singh H, Thomas EJ, Sittig DF, et al. Notification of abnormal lab test results in an electronic medical record: do any safety concerns remain? Am J Med 2010;123:238–44.
6. Hickner J, Graham DG, Elder NC, et al. Testing process errors and their harms and consequences reported from family medicine practices: a study of the American Academy of Family Physicians National Research Network. Qual Saf Health Care 2008;17:194–200.
7. Casalino LP, Dunham D, Chin MH, et al. Frequency of failure to inform patients of clinically significant outpatient test results. Arch Intern Med 2009;169:1123–9.
8. Callen JL, Westbrook JI, Georgiou A, et al. Failure to follow-up test results for ambulatory patients: a systematic review. J Gen Intern Med 2012;27:1334–48.
9. Moore C, Wisnivesky J, Williams S, et al. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med 2003;18:646–51.
10. Roy CL, Poon EG, Karson AS, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med 2005;143:121–8.
11. Singh H, Wilson L, Reis B, et al. Ten strategies to improve management of abnormal test result alerts in the electronic health record. J Patient Saf 2010;6:121–3.
12. Dickerson JA, Cole B, Astion ML. Ten ways to improve the quality of send-out testing. Clin Lab News 2012;38:12–3.
13. Cole B, Dickerson JA, Graber ML, et al. A prospective tool for risk assessment of sendout testing. Clin Chim Acta 2014;434:1–5.
14. MacMillan D, Lewandrowski E, Lewandrowski K. An analysis of reference laboratory (send out) testing: an 8-year experience in a large academic medical center. Clin Leadersh Manag Rev 2004;18:216–9.
15. Krasowski MD, Chudzik D, Dolezal A, et al. Promoting improved utilization of laboratory testing through changes in an electronic medical record: experience at an academic medical center. BMC Med Inform Decis Mak 2015;15:11.
16. Singh H, Arora HS, Vij MS, et al. Communication outcomes of critical imaging results in a computerized notification system. J Am Med Inform Assoc 2007;14:459–66.
From the Department of Medicine, Tufts Medical Center, Boston, MA.
Abstract
- Objective: To measure the frequency of missed pheochromocytoma test results and identify factors related to the risk of failed follow-up.
- Methods: We performed a retrospective review of the medical record to identify patients with abnormal urine or serum metanephrine or catecholamine test results over a 3-year period. We then searched the electronic medical record for documentation that the responsible physician was aware of the test results. We surveyed the physicians in cases where there were abnormal results and no documented follow-up to assess their awareness of the results and any follow-up actions they may have taken.
- Results: During the 3-year look-back period, 451 send-out tests for 332 patients were ordered for serum metanephrines, serum catecholamines, or urine catecholamines and/or metanephrines. Fifty-five tests affecting 46 patients returned with either moderately (n = 41) or critically elevated values (n = 5). Fifteen of these patients were inpatients when the tests were ordered, and 31 were outpatients. In 15 of 46 abnormal cases, there was no documentation in the electronic medical record that the responsible physician was aware of the result. Of the 15 cases without documentation, 6 of the responsible physicians in such cases were aware of the results.
- Conclusion: One-third of patients with abnormal lab testing for pheochromocytoma did not have clearly documented follow-up in the electronic medical record, and the majority of physicians in such cases were not aware of the results. Changes to the processes at health care institutions and reference laboratories are needed to improve follow-up of send-out lab results.
Delayed or missed follow-up of laboratory tests is a major source of medical harm [1–5]. Testing performed in both the inpatient and outpatient settings is susceptible to lost follow-up, in part because medical testing is a complex process that is vulnerable to multiple process-of-care failures [1,5–7]. In previous studies, the rate of missed follow-up of abnormal medical test results has ranged from 1% to 75% [6]. Laboratory test follow-up is a particularly challenging problem as patients transition between care settings [8,9]. In a study of 86 patients at one academic medical center, Moore and colleagues found that over a 1-year period, 41% of patients who had laboratory tests pending at the time of discharge had no documented follow-up for at least one of those tests [9]. More recently, Roy and colleagues reported that nearly half of 2644 patients discharged from general medicine hospitalist services at 2 academic tertiary care centers had pending laboratory or radiographic results. Nine percent of the pending results were potentially actionable, and a follow-up survey from the study revealed that 61% of physicians were unaware of pending results [10]. Similar findings have been reported in ambulatory care [5,8,11].
Among the universe of laboratory tests, tests performed at reference laboratories outside of the hospital or clinic where care is rendered (ie, “send-out” tests) are particularly susceptible to lost follow-up [12,13]. Because many of these tests are expensive and infrequently ordered, it is most feasible and economical for hospitals and clinics to transport these samples to regional or national laboratories for specialized testing [14,15]. Examples include the serotonin release assay, certain rheumatologic studies, cancer genetics, and advanced endocrine testing. Send-out testing poses several potential risks including accidental ordering of the wrong test, processing or transportation delays, failure of the outside laboratory to receive the specimen, failures of results reporting by the reference laboratory, incorrect result entry into the electronic medical record upon receipt, failure of the clinician to receive or note the result, or failure of clinician to interpret or act on the result [12,13,15]. Although previous studies have identified risk factors associated with missed abnormal test results [1], none to our knowledge have assessed the particular risks associated with samples processed at reference laboratories.
A critical event at our hospital involved a young woman who presented with respiratory failure attributed to a community-acquired pneumonia and systolic congestive heart failure that was thought to be related to her acute illness. Serum and urine metanephrines were ordered in the intensive care unit given the possibility that heart failure in a young patient could be attributed to an occult neuroendocrine tumor. The patient improved clinically and was discharged. Because the discharging service was unaware that the metanephrine tests had been ordered and were being processed at a national reference laboratory, they did not follow up on the test result or include it as pending in the discharge summary. Fortunately, the patient’s primary care physician discovered that the metanephrine levels were elevated and referred the patient for endocrine evaluation and definitive treatment.
Given the risk represented by pending send-out tests raised by this episode, we performed a retrospective study to identify other cases of missed abnormal send-out tests for metanephrines and catecholamines for in- and outpatients over the previous 3 years. We also sought to identify factors that increased the risk of failed follow-up.
Methods
Subjects and Setting
We studied adult in- and outpatients who received care at a 415-bed Boston-based academic medical center.
Project Design and Data Collection
We performed a retrospective record review of a cohort of patients with abnormal send-out laboratory tests for metanephrines and catecholamines. We collected laboratory reports of all results of urine and serum metanephrine and catecholamine tests performed from 1 January 2012 through 31 December 2014. All tests were performed at and reported by Quest Diagnostics in Chantilly, Virginia. The relevant tests were identified using a query of the online Quest Diagnostics system to extract all laboratory results for serum metanephrines, serum catecholamines, urine metanephrines, and urine catecholamines that resulted during this period. Reports were PDF files that were printed and reviewed manually. (Of note, providers typically view lab results directly in the electronic medical record. Reports were extracted from the Quest Diagnostics system for study purposes only.)
We used the reference ranges supplied by the laboratory to sort results into: normal levels, moderately elevated levels (1 to 4 times the upper limit of normal), and critically elevated levels (greater than 4 times the upper limit of normal). A physician (RZ) then reviewed the electronic medical record of each patient with moderately or critically elevated results for evidence that the responsible physician was aware of the results and had documented a follow-up plan. Documentation of physician awareness and follow-up was ascertained by notation and interpretation of the test result in either a discharge summary from the index admission or in an outpatient clinic note. The responsible physician was defined as the ordering physician for tests ordered in ambulatory care and the attending physician at time of discharge for inpatients. In cases where no documentation was identified in the medical record, the responsible physicians received an email questionnaire that asked (1) if they were aware of the abnormal result, (2) if aware of the result, did they notify the primary care physician or referring physician, and (3) if they were aware of any further follow-up or intervention.
Analysis
We stratified the cases into those with normal and abnormal labs values, and then further by those that did and did not have documentation of results and follow-up in the medical record. We then further stratified cases into those in which the responsible physician was aware and those in which they were unaware. If unaware, the patient was contacted directly by the risk management department, primarily for patient safety purposes. If we were unable to contact the patient, the patient’s listed primary care physician was contacted directly. We then performed qualitative analysis of the cases with abnormal results and no documented follow-up, with the goal of identifying common themes.
Results
During the 3-year look-back period, 451 send-out tests for 332 patients were ordered for serum metanephrines, serum catecholamines, urine catecholamines, or metanephrines. Fifty-five tests affecting 46 patients returned with either moderately or critically elevated values, while 396 results affecting 286 patients returned within the reference range. Five patients had critically elevated values and 41 patients had moderately elevated values. Fifteen were inpatients when the tests were ordered and 31 were outpatients.
In the survey of the responsible physicians in the 15 cases with no follow-up, all 15 physicians responded. Six were aware of the abnormal result and 9 were not (Figure). Five of the 6 cases in which the physician was aware were outpatients. Eight of the 9 cases in which the physician was not aware were inpatients. In 4 of 15 abnormal cases with no follow-up, the patient was seen at a follow-up appointment but the lab results were not addressed. In 3 of 15 abnormal cases with no follow-up, the patient did not return for a planned follow-up appointment. In 3 of 15 abnormal cases with no follow-up, the physician was aware and addressed the results, but did not document that the results were addressed (all 3 were outpatient cases). In 3 of 15 abnormal cases with no follow-up, lab results for inpatients were pending at time of discharge and there was no documentation of pending results in the designated space for this in the discharge summary. In 2 of 15 abnormal cases with no follow-up, the patient was followed by a primary care physician outside of our institution. In 7 cases, the patient had multiple subspecialists involved in their care. All undocumented abnormal levels were addressed by our institution, either by contacting the patient or primary care physician, or by determining that the abnormality was not clinically relevant.
Discussion
We identified cases in which patients had abnormal results on tests used to diagnose neuroendocrine tumors such as pheochromocytoma over a 3-year period and sought evidence that a responsible clinician had followed up on the abnormal results. In one-third of abnormal test results, we found no documentation in the medical record that the responsible clinician was aware of the result or had communicated it to another clinician or the patient. This occurred most often in cases in which metanephrine and/or catecholamine levels were pending at the time of hospital discharge, and when a patient who was discharged from the hospital or seen in clinic did not return for a scheduled follow-up appointment. When we followed up with the responsible physician, only 6 in 15 were aware of the abnormal results and had either concluded that they were not clinically significant or had addressed the issue without completing documentation.
Previous research has identified vulnerabilities in the follow-up of send-out test results that exceed the challenges with tests performed in-house. These include that send-out tests inherently have more steps and require more manual processes [8], and that these tests are more prone to delay, misinterpretation, and poor documentation. Reference laboratories usually provide non-structured reporting of results, often in the form of paper or PDF files. This can make it difficult for receiving hospitals or clinics to incorporate information into the electronic medical record or to build clinical reminders or alerts for ordering clinicians. Additionally, these data elements are often cryptic in that they provide reference values without necessarily setting parameters for abnormalities. This is a case in point with metanephrine and catecholamine testing, as the results are often variable and poorly reproducible and difficult for clinicians to interpret. There are different cutoffs for moderately elevated and critically elevated values, and how to proceed with patients with moderately elevated values is not clear and may require the expertise of subspecialists. Our study confirmed several issues surrounding vulnerabilities of send-out lab testing.
As a single-institution project with a small cohort of subjects, the generalizability of this project may be limited. However, some process-of-care vulnerabilities noted here are similar to those reported in previous research studies [8]. In addition, hospitals and clinics send specimens to a limited number of regional and national reference laboratories. The challenges that our clinicians encountered in managing these results are likely to be challenges in many other organizations. Also, while our study was limited to tests done to evaluate for pheochromocytoma, our findings are likely applicable to other reference laboratory tests.
Send-out labs continue to represent a major source of lost follow-up and potential patient harm. Creating systems with effective and timely alerts for providers will be useful in preventing missed follow-up. Our study found a lack of clear guidelines designating responsibility for pending lab results, which has been found across institutions in previous studies [8]. Since we conducted this project, our institution has reminded clinicians that discharging attendings are responsible for pending lab results at time of discharge and has developed an automated electronic method for delivering these results. Similar policy interventions at other institutions have shown promise [16]. We hope this will minimize the number of lab results, including those of send-out labs, which are not acted upon in a timely manner. However, other issues, including data interface with the electronic medical record and patients with abnormal results being lost to follow-up, remain barriers for our institution to address.
There are several immediate steps that could be taken by health care organizations and reference labs to reduce patient harm as a result of send-out labs that are not followed up. First, health care organizations can develop better integration between electronic records and lab processing for send-out labs, as well as more electronic alerts. This may help to notify ordering physicians after patients have been discharged and the case may not be front of mind. Reference labs should create robust electronic systems to transmit results as electronic data elements so that health care organizations can easily incorporate results into their electronic medical records, and develop notification systems that flag out-of-bound values. Secure online lab results for send-outs may shorten the delay in reporting. Additionally, creating clear policies establishing the responsible provider is crucial, as has been found by previous research by Singh and others [11,15].
In conclusion, send-out labs are vulnerable to lost follow-up. It is crucial for clinicians to be aware of all send-out lab results and to document their interpretation of abnormal results. Developing policies and systems to facilitate timely follow-up will help to reduce potential patient harm related to send-out labs.
Corresponding author: Richard Zamore, MD, MPH, Tufts Medical Center, 800 Washington St., Boston, MA 02111, [email protected].
Financial disclosures: None.
From the Department of Medicine, Tufts Medical Center, Boston, MA.
Abstract
- Objective: To measure the frequency of missed pheochromocytoma test results and identify factors related to the risk of failed follow-up.
- Methods: We performed a retrospective review of the medical record to identify patients with abnormal urine or serum metanephrine or catecholamine test results over a 3-year period. We then searched the electronic medical record for documentation that the responsible physician was aware of the test results. We surveyed the physicians in cases where there were abnormal results and no documented follow-up to assess their awareness of the results and any follow-up actions they may have taken.
- Results: During the 3-year look-back period, 451 send-out tests for 332 patients were ordered for serum metanephrines, serum catecholamines, or urine catecholamines and/or metanephrines. Fifty-five tests affecting 46 patients returned with either moderately (n = 41) or critically elevated values (n = 5). Fifteen of these patients were inpatients when the tests were ordered, and 31 were outpatients. In 15 of 46 abnormal cases, there was no documentation in the electronic medical record that the responsible physician was aware of the result. Of the 15 cases without documentation, 6 of the responsible physicians in such cases were aware of the results.
- Conclusion: One-third of patients with abnormal lab testing for pheochromocytoma did not have clearly documented follow-up in the electronic medical record, and the majority of physicians in such cases were not aware of the results. Changes to the processes at health care institutions and reference laboratories are needed to improve follow-up of send-out lab results.
Delayed or missed follow-up of laboratory tests is a major source of medical harm [1–5]. Testing performed in both the inpatient and outpatient settings is susceptible to lost follow-up, in part because medical testing is a complex process that is vulnerable to multiple process-of-care failures [1,5–7]. In previous studies, the rate of missed follow-up of abnormal medical test results has ranged from 1% to 75% [6]. Laboratory test follow-up is a particularly challenging problem as patients transition between care settings [8,9]. In a study of 86 patients at one academic medical center, Moore and colleagues found that over a 1-year period, 41% of patients who had laboratory tests pending at the time of discharge had no documented follow-up for at least one of those tests [9]. More recently, Roy and colleagues reported that nearly half of 2644 patients discharged from general medicine hospitalist services at 2 academic tertiary care centers had pending laboratory or radiographic results. Nine percent of the pending results were potentially actionable, and a follow-up survey from the study revealed that 61% of physicians were unaware of pending results [10]. Similar findings have been reported in ambulatory care [5,8,11].
Among the universe of laboratory tests, tests performed at reference laboratories outside of the hospital or clinic where care is rendered (ie, “send-out” tests) are particularly susceptible to lost follow-up [12,13]. Because many of these tests are expensive and infrequently ordered, it is most feasible and economical for hospitals and clinics to transport these samples to regional or national laboratories for specialized testing [14,15]. Examples include the serotonin release assay, certain rheumatologic studies, cancer genetics, and advanced endocrine testing. Send-out testing poses several potential risks including accidental ordering of the wrong test, processing or transportation delays, failure of the outside laboratory to receive the specimen, failures of results reporting by the reference laboratory, incorrect result entry into the electronic medical record upon receipt, failure of the clinician to receive or note the result, or failure of clinician to interpret or act on the result [12,13,15]. Although previous studies have identified risk factors associated with missed abnormal test results [1], none to our knowledge have assessed the particular risks associated with samples processed at reference laboratories.
A critical event at our hospital involved a young woman who presented with respiratory failure attributed to a community-acquired pneumonia and systolic congestive heart failure that was thought to be related to her acute illness. Serum and urine metanephrines were ordered in the intensive care unit given the possibility that heart failure in a young patient could be attributed to an occult neuroendocrine tumor. The patient improved clinically and was discharged. Because the discharging service was unaware that the metanephrine tests had been ordered and were being processed at a national reference laboratory, they did not follow up on the test result or include it as pending in the discharge summary. Fortunately, the patient’s primary care physician discovered that the metanephrine levels were elevated and referred the patient for endocrine evaluation and definitive treatment.
Given the risk represented by pending send-out tests raised by this episode, we performed a retrospective study to identify other cases of missed abnormal send-out tests for metanephrines and catecholamines for in- and outpatients over the previous 3 years. We also sought to identify factors that increased the risk of failed follow-up.
Methods
Subjects and Setting
We studied adult in- and outpatients who received care at a 415-bed Boston-based academic medical center.
Project Design and Data Collection
We performed a retrospective record review of a cohort of patients with abnormal send-out laboratory tests for metanephrines and catecholamines. We collected laboratory reports of all results of urine and serum metanephrine and catecholamine tests performed from 1 January 2012 through 31 December 2014. All tests were performed at and reported by Quest Diagnostics in Chantilly, Virginia. The relevant tests were identified using a query of the online Quest Diagnostics system to extract all laboratory results for serum metanephrines, serum catecholamines, urine metanephrines, and urine catecholamines that resulted during this period. Reports were PDF files that were printed and reviewed manually. (Of note, providers typically view lab results directly in the electronic medical record. Reports were extracted from the Quest Diagnostics system for study purposes only.)
We used the reference ranges supplied by the laboratory to sort results into: normal levels, moderately elevated levels (1 to 4 times the upper limit of normal), and critically elevated levels (greater than 4 times the upper limit of normal). A physician (RZ) then reviewed the electronic medical record of each patient with moderately or critically elevated results for evidence that the responsible physician was aware of the results and had documented a follow-up plan. Documentation of physician awareness and follow-up was ascertained by notation and interpretation of the test result in either a discharge summary from the index admission or in an outpatient clinic note. The responsible physician was defined as the ordering physician for tests ordered in ambulatory care and the attending physician at time of discharge for inpatients. In cases where no documentation was identified in the medical record, the responsible physicians received an email questionnaire that asked (1) if they were aware of the abnormal result, (2) if aware of the result, did they notify the primary care physician or referring physician, and (3) if they were aware of any further follow-up or intervention.
Analysis
We stratified the cases into those with normal and abnormal labs values, and then further by those that did and did not have documentation of results and follow-up in the medical record. We then further stratified cases into those in which the responsible physician was aware and those in which they were unaware. If unaware, the patient was contacted directly by the risk management department, primarily for patient safety purposes. If we were unable to contact the patient, the patient’s listed primary care physician was contacted directly. We then performed qualitative analysis of the cases with abnormal results and no documented follow-up, with the goal of identifying common themes.
Results
During the 3-year look-back period, 451 send-out tests for 332 patients were ordered for serum metanephrines, serum catecholamines, urine catecholamines, or metanephrines. Fifty-five tests affecting 46 patients returned with either moderately or critically elevated values, while 396 results affecting 286 patients returned within the reference range. Five patients had critically elevated values and 41 patients had moderately elevated values. Fifteen were inpatients when the tests were ordered and 31 were outpatients.
In the survey of the responsible physicians in the 15 cases with no follow-up, all 15 physicians responded. Six were aware of the abnormal result and 9 were not (Figure). Five of the 6 cases in which the physician was aware were outpatients. Eight of the 9 cases in which the physician was not aware were inpatients. In 4 of 15 abnormal cases with no follow-up, the patient was seen at a follow-up appointment but the lab results were not addressed. In 3 of 15 abnormal cases with no follow-up, the patient did not return for a planned follow-up appointment. In 3 of 15 abnormal cases with no follow-up, the physician was aware and addressed the results, but did not document that the results were addressed (all 3 were outpatient cases). In 3 of 15 abnormal cases with no follow-up, lab results for inpatients were pending at time of discharge and there was no documentation of pending results in the designated space for this in the discharge summary. In 2 of 15 abnormal cases with no follow-up, the patient was followed by a primary care physician outside of our institution. In 7 cases, the patient had multiple subspecialists involved in their care. All undocumented abnormal levels were addressed by our institution, either by contacting the patient or primary care physician, or by determining that the abnormality was not clinically relevant.
Discussion
We identified cases in which patients had abnormal results on tests used to diagnose neuroendocrine tumors such as pheochromocytoma over a 3-year period and sought evidence that a responsible clinician had followed up on the abnormal results. In one-third of abnormal test results, we found no documentation in the medical record that the responsible clinician was aware of the result or had communicated it to another clinician or the patient. This occurred most often in cases in which metanephrine and/or catecholamine levels were pending at the time of hospital discharge, and when a patient who was discharged from the hospital or seen in clinic did not return for a scheduled follow-up appointment. When we followed up with the responsible physician, only 6 in 15 were aware of the abnormal results and had either concluded that they were not clinically significant or had addressed the issue without completing documentation.
Previous research has identified vulnerabilities in the follow-up of send-out test results that exceed the challenges with tests performed in-house. These include that send-out tests inherently have more steps and require more manual processes [8], and that these tests are more prone to delay, misinterpretation, and poor documentation. Reference laboratories usually provide non-structured reporting of results, often in the form of paper or PDF files. This can make it difficult for receiving hospitals or clinics to incorporate information into the electronic medical record or to build clinical reminders or alerts for ordering clinicians. Additionally, these data elements are often cryptic in that they provide reference values without necessarily setting parameters for abnormalities. This is a case in point with metanephrine and catecholamine testing, as the results are often variable and poorly reproducible and difficult for clinicians to interpret. There are different cutoffs for moderately elevated and critically elevated values, and how to proceed with patients with moderately elevated values is not clear and may require the expertise of subspecialists. Our study confirmed several issues surrounding vulnerabilities of send-out lab testing.
As a single-institution project with a small cohort of subjects, the generalizability of this project may be limited. However, some process-of-care vulnerabilities noted here are similar to those reported in previous research studies [8]. In addition, hospitals and clinics send specimens to a limited number of regional and national reference laboratories. The challenges that our clinicians encountered in managing these results are likely to be challenges in many other organizations. Also, while our study was limited to tests done to evaluate for pheochromocytoma, our findings are likely applicable to other reference laboratory tests.
Send-out labs continue to represent a major source of lost follow-up and potential patient harm. Creating systems with effective and timely alerts for providers will be useful in preventing missed follow-up. Our study found a lack of clear guidelines designating responsibility for pending lab results, which has been found across institutions in previous studies [8]. Since we conducted this project, our institution has reminded clinicians that discharging attendings are responsible for pending lab results at time of discharge and has developed an automated electronic method for delivering these results. Similar policy interventions at other institutions have shown promise [16]. We hope this will minimize the number of lab results, including those of send-out labs, which are not acted upon in a timely manner. However, other issues, including data interface with the electronic medical record and patients with abnormal results being lost to follow-up, remain barriers for our institution to address.
There are several immediate steps that could be taken by health care organizations and reference labs to reduce patient harm as a result of send-out labs that are not followed up. First, health care organizations can develop better integration between electronic records and lab processing for send-out labs, as well as more electronic alerts. This may help to notify ordering physicians after patients have been discharged and the case may not be front of mind. Reference labs should create robust electronic systems to transmit results as electronic data elements so that health care organizations can easily incorporate results into their electronic medical records, and develop notification systems that flag out-of-bound values. Secure online lab results for send-outs may shorten the delay in reporting. Additionally, creating clear policies establishing the responsible provider is crucial, as has been found by previous research by Singh and others [11,15].
In conclusion, send-out labs are vulnerable to lost follow-up. It is crucial for clinicians to be aware of all send-out lab results and to document their interpretation of abnormal results. Developing policies and systems to facilitate timely follow-up will help to reduce potential patient harm related to send-out labs.
Corresponding author: Richard Zamore, MD, MPH, Tufts Medical Center, 800 Washington St., Boston, MA 02111, [email protected].
Financial disclosures: None.
1. Callen J, Georgiou A, Li J, Westbrook JI, et al. The safety implications of missed test results for hospitalised patients: a systematic review. BMJ Qual Saf 2011;20:194–9.
2. Wahls TL, Cram PM. The frequency of missed test results and associated treatment delays in a highly computerized health system. BMC Fam Pract 2007;8:32.
3. Bates DW, Leape LL. Doing better with critical test results. Jt Comm J Qual Patient Saf 2005;31:66–7.
4. Schiff GD, Kim S, Krosnjar N, et al. Missed hypothyroidism diagnosis uncovered by linking laboratory and pharmacy data. Arch Intern Med 2005;165:574.
5. Singh H, Thomas EJ, Sittig DF, et al. Notification of abnormal lab test results in an electronic medical record: do any safety concerns remain? Am J Med 2010;123:238–44.
6. Hickner J, Graham DG, Elder NC, et al. Testing process errors and their harms and consequences reported from family medicine practices: a study of the American Academy of Family Physicians National Research Network. Qual Saf Health Care 2008;17:194–200.
7. Casalino LP, Dunham D, Chin MH, et al. Frequency of failure to inform patients of clinically significant outpatient test results. Arch Intern Med 2009;169:1123–9.
8. Callen JL, Westbrook JI, Georgiou A, et al. Failure to follow-up test results for ambulatory patients: a systematic review. J Gen Intern Med 2012;27:1334–48.
9. Moore C, Wisnivesky J, Williams S, et al. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med 2003;18:646–51.
10. Roy CL, Poon EG, Karson AS, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med 2005;143:121–8.
11. Singh H, Wilson L, Reis B, et al. Ten strategies to improve management of abnormal test result alerts in the electronic health record. J Patient Saf 2010;6:121–3.
12. Dickerson JA, Cole B, Astion ML. Ten ways to improve the quality of send-out testing. Clin Lab News 2012;38:12–3.
13. Cole B, Dickerson JA, Graber ML, et al. A prospective tool for risk assessment of sendout testing. Clin Chim Acta 2014;434:1–5.
14. MacMillan D, Lewandrowski E, Lewandrowski K. An analysis of reference laboratory (send out) testing: an 8-year experience in a large academic medical center. Clin Leadersh Manag Rev 2004;18:216–9.
15. Krasowski MD, Chudzik D, Dolezal A, et al. Promoting improved utilization of laboratory testing through changes in an electronic medical record: experience at an academic medical center. BMC Med Inform Decis Mak 2015;15:11.
16. Singh H, Arora HS, Vij MS, et al. Communication outcomes of critical imaging results in a computerized notification system. J Am Med Inform Assoc 2007;14:459–66.
1. Callen J, Georgiou A, Li J, Westbrook JI, et al. The safety implications of missed test results for hospitalised patients: a systematic review. BMJ Qual Saf 2011;20:194–9.
2. Wahls TL, Cram PM. The frequency of missed test results and associated treatment delays in a highly computerized health system. BMC Fam Pract 2007;8:32.
3. Bates DW, Leape LL. Doing better with critical test results. Jt Comm J Qual Patient Saf 2005;31:66–7.
4. Schiff GD, Kim S, Krosnjar N, et al. Missed hypothyroidism diagnosis uncovered by linking laboratory and pharmacy data. Arch Intern Med 2005;165:574.
5. Singh H, Thomas EJ, Sittig DF, et al. Notification of abnormal lab test results in an electronic medical record: do any safety concerns remain? Am J Med 2010;123:238–44.
6. Hickner J, Graham DG, Elder NC, et al. Testing process errors and their harms and consequences reported from family medicine practices: a study of the American Academy of Family Physicians National Research Network. Qual Saf Health Care 2008;17:194–200.
7. Casalino LP, Dunham D, Chin MH, et al. Frequency of failure to inform patients of clinically significant outpatient test results. Arch Intern Med 2009;169:1123–9.
8. Callen JL, Westbrook JI, Georgiou A, et al. Failure to follow-up test results for ambulatory patients: a systematic review. J Gen Intern Med 2012;27:1334–48.
9. Moore C, Wisnivesky J, Williams S, et al. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med 2003;18:646–51.
10. Roy CL, Poon EG, Karson AS, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med 2005;143:121–8.
11. Singh H, Wilson L, Reis B, et al. Ten strategies to improve management of abnormal test result alerts in the electronic health record. J Patient Saf 2010;6:121–3.
12. Dickerson JA, Cole B, Astion ML. Ten ways to improve the quality of send-out testing. Clin Lab News 2012;38:12–3.
13. Cole B, Dickerson JA, Graber ML, et al. A prospective tool for risk assessment of sendout testing. Clin Chim Acta 2014;434:1–5.
14. MacMillan D, Lewandrowski E, Lewandrowski K. An analysis of reference laboratory (send out) testing: an 8-year experience in a large academic medical center. Clin Leadersh Manag Rev 2004;18:216–9.
15. Krasowski MD, Chudzik D, Dolezal A, et al. Promoting improved utilization of laboratory testing through changes in an electronic medical record: experience at an academic medical center. BMC Med Inform Decis Mak 2015;15:11.
16. Singh H, Arora HS, Vij MS, et al. Communication outcomes of critical imaging results in a computerized notification system. J Am Med Inform Assoc 2007;14:459–66.
Asking for Help
There is little scientific evidence about professional help‐seeking behavior among resident physicians. Although junior physicians have many sources of information available to them in the course of clinical practiceprint materials, internet resources, curbside consultations, and advice from senior residents and facultywe have little empirical knowledge about when, why, and how physician trainees ask for help.
To study this phenomenon, we examined the use of a medical procedure service (MPS) by resident physicians. The MPS is an inpatient service at a Boston teaching hospital that provides education, supervision, and evaluation of internal medicine residents who perform common bedside procedures; it has been described previously.1 Residents who call the MPS review an online curriculum with self‐assessment quizzes, perform procedures with faculty supervision and feedback, and assess their own performance using online checklists. This program has been available to internal medicine residents since 2002. In a previous study, we found that residents reported greater comfort performing bedside procedures when they used the procedure service, when the operator was a postgraduate year (PGY)2 or PGY3 resident (compared to PGY1 residents), and while placing central venous catheters (CVCs) (compared to thoracenteses).2
The goal of the current study was to examine help‐seeking behavior among resident physicians as they placed CVCs and performed thoracenteses. We interpreted the decision to use the MPS to indicate that the resident successfully sought and received assistance from pulmonary attending physicians or interventional pulmonary fellows. We hypothesized that: (1) residents earlier in their training would choose to use the procedure service due to their relative lack of experience; (2) they would seek consultation when the procedure was performed in high‐risk patients, as indicated by the number of comorbidities, presence of medications that increase the risk of bleeding, and treatment in an intensive care unit; and (3) residents would be less likely to call the MPS for urgent or emergent situations, when timely assistance may be difficult to obtain. To examine the potentially confounding influence of procedures supervised by non‐MPS physicians, we also investigated differences between informally supervised procedures (i.e. by a non‐MPS attending or fellow) and unsupervised procedures (i.e. no attending or fellow supervision) to determine whether any significant differences in their characteristics existed.
Methods
Study Site
We studied CVC placement and thoracenteses performed by internal medicine residents at a 556‐bed Boston teaching hospital in 2003‐2004. During the 9‐month study period, 63 PGY1 residents (16 in a 1‐year preliminary program) and 95 PGY2 and PGY3 residents were enrolled in the program.
The MPS was staffed by hospitalists and pulmonologists skilled in teaching and performing 4 common inpatient procedures: CVC placement, thoracentesis, lumbar puncture, and paracentesis. We chose to study only the first 2 procedures because supervision of CVCs and thoracenteses by pulmonologists was available 24 hours a day in this initial year of the MPS. The other procedures were supervised by hospitalists during business hours only at the time. Ultrasound guidance was available for all procedures, supervised or not. At the time of the study, the residency program recommended consulting the MPS for procedures, but this was not mandatory. A resident electing to use the MPS to supervise a procedure on her own patient would page the MPS physician. If she were performing a procedure for the first time, she was required to review an online multimedia curriculum and complete a 5‐question cognitive test. She would then perform the procedure while supervised by the MPS physician, who would complete a checklist evaluation of the resident's performance online. All residents performing procedures, regardless of use of the MPS, would also complete procedure logs online to document procedural experience for the American Board of Internal Medicine requirements.
Study Design and Data Sources
We prospectively collected data from resident procedure logs from July 2003 through April 2004. We elicited the following information from the residents for each procedure: name of operator, year of training, date of procedure, patient's medical record number, name of attending or fellow supervisor, procedure, immediate complications (pneumothorax, bleeding, other, or none), self‐reported level of urgency (emergent, urgent, elective), time of day, procedure location, and the number of such procedures completed previously. We categorized level of supervision as: (1) MPS‐supervised if a pulmonary attending or interventional pulmonary fellow were listed as the supervisor (entailing formal faculty development as MPS faculty, resident use of the curriculum, and completion of faculty evaluations with structured feedback); (2) informally supervised if nonpulmonary attendings or fellows were involved (who may not supervise the entire procedure and would not complete a faculty evaluation); and (3) unsupervised if a resident physician or no supervisor was identified. Faculty development involved a single training session with the interventional pulmonary fellows and attendings and focused on optimal procedural teaching. During the session, we described the structure of the MPS, provided the curricular materials available to the residents, and reviewed the faculty evaluation forms in depth.
We abstracted patient characteristics (age, race/ethnicity, type of insurance, length of stay) from the electronic medical record. We performed retrospective chart reviews to record patient comorbidities (as defined by modified Deyo criteria3), to determine the number of medications associated with the risk of bleeding (such as anticoagulants and antiplatelet agents), and to discover complications that arose after the procedure was logged, including delayed bleeding, pneumothorax, or infection (localized site infection or line‐related bloodstream infection).
Data Analyses
We tabulated characteristics of residents (training level, gender, and self‐reported number of procedures), procedures (procedure type, procedure location, level of urgency, time of day), and patients (number of comorbidities and number of medications that promote bleeding) by use of the MPS. We combined resident‐reported (ie, immediate) complications and delayed complications (identified on retrospective chart review), stratified by use of the MPS. We also performed a subgroup analysis of non‐MPS procedures by comparing resident, procedure, and patient characteristics by presence or absence of informal supervision.
We created a univariable logistic regression model to examine factors associated with elective use of the MPS. We dichotomized the following independent variables: resident characteristics (PGY status, female gender, first time performing the procedure), patient characteristics (nonwhite race, female gender, Medicaid recipient, 3 or more comorbidities, any bleeding medication), and procedure characteristics (intensive care unit procedures, nonelective procedures, procedures performed between 11 PM and 8 AM). We also included 2 patient‐related interval variables (age and length of stay) in the univariable logistic regression model. We created a multivariable logistic regression model with backward elimination (P < 0.05) using the same independent variables as in the univariable analyses, to identify factors associated with use of the MPS, clustering by resident. We repeated this method to create a multivariable model to examine factors associated with the use of informal supervision among non‐MPS procedures. Analyses used Stata 7.0 (StataCorp, College Station, TX).
The study protocol was approved in advance by the hospital investigational review board.
Results
Resident Characteristics
Sixty‐nine residents reported procedures during the 9‐month study period (Table 1). Thirty (43%) residents were PGY1 and 36 (52%) were female. Twelve (17%) residents performed the procedure for the first time.
| Total residents, n (%) | 69 (100) |
|---|---|
| |
| Training year, n (%) | |
| PGY1 | 30 (43) |
| PGY2 | 23 (33) |
| PGY3 | 16 (23) |
| Gender, n (%) | |
| Female | 36 (52) |
| Male | 33 (48) |
| Self‐reported number of prior procedures, n (%)* | |
| 0 | 12 (17) |
| 1‐5 | 26 (38) |
| >6 | 31 (45) |
Patient Characteristics
The 134 patients in the study had a mean age of 65.6 years. One‐half of patients were female, and 34% were nonwhite. The principal insurer was Medicare (57%); 24% were privately insured, and 17% received Medicaid. The mean length of stay was 18.4 days (range, 0‐98 days).
MPS and Non‐MPS Procedures
As detailed in the bivariate analyses in Table 2, residents performed 191 procedures (156 CVCs and 35 thoracenteses). PGY1 residents performed approximately one‐half of the 79 MPS procedures. Fifty‐one (65%) of the 79 MPS procedures were CVC placements and 28 (35%) were thoracenteses (P < 0.001). MPS procedures were less often performed in the emergency department than non‐MPS procedures (1% versus 21%, P < 0.001). There was no significant difference in the percentage of MPS and non‐MPS procedures by time of day. Patients whose procedures were supervised by the MPS had on average 3.0 comorbidities, while patients who underwent non‐MPS procedures had 2.6 comorbidities (P = 0.02). Complications occurred in 11 (14%) of MPS and 22 (20%) of non‐MPS procedures, a statistically nonsignificant difference.
| MPS | No MPS | P Value* | |
|---|---|---|---|
| |||
| Total procedures, n (%) | 79 (100) | 112 (100) | |
| Resident characteristics | |||
| Resident level, n (%) | 0.77 | ||
| PGY1 | 42 (53) | 60 (54) | |
| PGY2 | 22 (28) | 34 (30) | |
| PGY3 | 15 (19) | 18 (16) | |
| Gender, n (%) | 0.50 | ||
| Female | 42 (53) | 54 (48) | |
| Male | 37 (47) | 58 (52) | |
| Self‐reported number of prior procedures, n (%) | 0.11 | ||
| 0 | 14 (18) | 13 (12) | |
| 1‐5 | 40 (51) | 47 (42) | |
| >6 | 25 (32) | 52 (46) | |
| Procedure characteristics | |||
| Procedure, n (%) | 0.001 | ||
| Central venous catheter | 51 (65) | 105 (94) | |
| Thoracentesis | 28 (35) | 7 (6) | |
| Location, n (%) | 0.001 | ||
| Ward | 18 (23) | 17 (15) | |
| Emergency department | 1 (1) | 23 (21) | |
| Intensive care unit | 53 (67) | 71 (63) | |
| Other | 7 (9) | 1 (1) | |
| Urgency, n (%) | 0.001 | ||
| Elective | 32 (41) | 22 (20) | |
| Urgent | 42 (53) | 78 (70) | |
| Emergent | 5 (6) | 12 (11) | |
| Time of day, n (%) | 0.33 | ||
| 8 AM to 5 PM | 50 (63) | 65 (58) | |
| 5 PM to 11 PM | 15 (19) | 20 (18) | |
| 11 PM to 8 AM | 14 (18) | 27 (24) | |
| Complications or problems, n (%) | 0.54 | ||
| Bleeding | 1 (1) | 6 (5) | |
| Pneumothorax | 1 (1) | 2 (2) | |
| Infection | 5 (6) | 5 (4) | |
| Other∥ | 4 (5) | 9 (8) | |
| None | 68 (86) | 90 (80) | |
| Patient characteristics | |||
| Number of comorbidities, mean (SD, range) | 3.0 (1.4, 0‐7) | 2.6 (1.6, 0‐7) | 0.02 |
| Number of medications associated with bleeding risk, mean (SD, range)# | 1.1 (1.0, 0‐3) | 1.1 (0.9, 0‐3) | 0.90 |
In the univariable analysis, the only variable associated with elective use of the MPS was the presence of 3 or more comorbidities (oodds ratio [OR], 2.3; 95% confidence interval [CI], 1.2‐4.1). In the multivariable analysis, residents were more likely to use the MPS when patients had 3 or more comorbidities (OR, 2.1; 95% CI, 1.2‐3.5) and less likely to use the MPS when procedures were either urgent or emergent (OR, 0.4; 95% CI, 0.2‐0.8).
Unsupervised and Informally Supervised Procedures
Table 3 shows the results of the bivariate analyses of the characteristics of the 112 procedures that were unsupervised or supervised by non‐MPS physicians. Twenty‐seven (24%) were informally supervised by nonpulmonary attendings. Residents who had performed more than 6 procedures previously were more likely to be informally supervised than not supervised at all (P = 0.001). More informally supervised procedures were performed in the emergency department (41%) than in other settings (P = 0.01). There were no significant differences in year of training, gender, urgency, time of day, complications, comorbidities, or bleeding medications.
| Informal Supervision | No Supervision | P Value* | |
|---|---|---|---|
| |||
| Total procedures, n (%) | 27 (100) | 85 (100) | |
| Resident characteristics | |||
| Resident level, n (%) | 0.13 | ||
| PGY1 | 10 (37) | 50 (59) | |
| PGY2 | 12 (44) | 22 (26) | |
| PGY3 | 5 (19) | 13 (15) | |
| Gender, n (%) | 0.99 | ||
| Female | 13 (48) | 41 (48) | |
| Male | 14 (52) | 44 (52) | |
| Self‐reported number of prior procedures, n (%) | 0.001 | ||
| 0 | 2 (7) | 11 (13) | |
| 1‐5 | 4 (15) | 43 (51) | |
| >6 | 21 (78) | 31 (36) | |
| Procedure characteristics | |||
| Procedure, n (%) | 0.53 | ||
| Central venous catheter | 26 (96) | 79 (93) | |
| Thoracentesis | 1 (4) | 6 (7) | |
| Location, n (%) | 0.01 | ||
| Ward | 1 (4) | 16 (19) | |
| Emergency department | 11 (41) | 12 (14) | |
| Intensive care unit | 15 (56) | 56 (66) | |
| Other | 0 (0) | 1 (1) | |
| Urgency, n (%) | 0.33 | ||
| Elective | 4 (15) | 18 (21) | |
| Urgent | 19 (70) | 59 (69) | |
| Emergent | 4 (15) | 8 (9) | |
| Time of Day, n (%) | 0.11 | ||
| 8 AM to 5 PM | 13 (48) | 52 (61) | |
| 5 PM to 11 PM | 4 (15) | 16 (19) | |
| 11 PM to 8 AM | 10 (37) | 17 (20) | |
| Complications or problems, n (%) | 0.45 | ||
| Bleeding | 0 (0) | 6 (7) | |
| Pneumothorax | 0 (0) | 2 (2) | |
| Infection | 0 (0) | 5 (6) | |
| Other∥ | 2 (7) | 7 (8) | |
| None | 25 (93) | 65 (76) | |
| Patient characteristics | |||
| Number of comorbidities, mean (SD, range) | 2.2 (1.3, 1‐5) | 2.7 (1.7, 0‐7) | 0.22 |
| Number of medications associated with bleeding risk, mean (SD, range)# | 0.9 (.93, 0‐3) | 1.1 (0.9, 0‐3) | 0.24 |
In the multivariable analysis, the only factor associated with the use of informal supervision (rather than absent supervision) was patient gender; informal supervision was less likely with female patients (OR, 0.3; 95% CI, 0.1‐0.8).
Discussion
To understand professional help‐seeking behavior by internal medicine resident physicians, we studied factors associated with the use of a MPS for performing 2 common bedside procedures. We found that residents used the MPS more often when they performed procedures on patients with more comorbidities and less often during urgent or emergent procedures.
These results are consistent with our hypothesis that residents use formal supervision when caring for high‐risk patients. We had also hypothesized that they would seek the MPS for patients on medications that increase the risk of bleeding, but this was not borne out. One possible explanation is that invasive procedures on anticoagulated patients may be deferred or avoided. Additionally, we did not collect prothrombin times nor platelet count, which may represent better proxies for coagulopathy. Our hypothesis that residents would not seek the MPS for urgent and emergent procedures was confirmed; the time delay between contacting the faculty member and performing the procedure may have inhibited or obviated consultation of the MPS. We hypothesized that interns would use the MPS preferentially; we found instead that level of training did not influence use of the MPS. A resident early in training may struggle with the balance between autonomy and supervision, wanting instead to establish himself as able to solve clinical problems independently and by seeking consultation only as a last resort. Alternatively, interns may be primarily supervised by their residents and may seek expert assistance only for particularly challenging or high‐risk cases. Additionally, as newcomers to the training program, they may not be well acquainted with the role and availability of the service (although periodic announcements were made throughout the year). Our examination of procedures not supervised by the MPS showed that informally supervised and unsupervised procedures are quite similar to each other; the inverse relationship between informal supervision and patient gender is difficult to explain and may be spurious.
To our knowledge, only 1 author has postulated a theoretical foundation for help‐seeking in trainees, depicted in the context of the patient‐resident‐attending triadic relationship.4 The mature help‐seeker, whether patient, resident, or attending, is willing to confront problems, receptive to new information, able to acknowledge dependence on expertise, and able to apply new input with self‐reliance. However, little is known about how this model manifests itself empirically in professional help‐seeking or what the optimal conditions of faculty supervision are. One observational study suggested that faculty who spent more time on hospital floors created environments with higher resident satisfaction scores, higher perceived quality of patient care, and, paradoxically, increased perceptions of autonomy.5 These results are consistent with our previous work showing that residents' comfort with bedside procedures increased with use of the MPS.2 In the related field of consultation medicine, 2 studies6, 7 showed that family practitioners prefer to consult internal medicine subspecialists over general internists. One of these studies7 demonstrated that the primary need was for a consultant with technical (ie, procedural) skills. Our use of MPS faculty who are specifically skilled in performing medical procedures appears to be consistent with this observation that specific technical expertise is valued over general supervision or guidance.
How can we best design formal procedural supervision programs that allow residents to obtain help when they need it? In addition to fostering mature help‐seeking behavior, help‐giving requires: (1) an environment that encourages help‐seeking; (2) a mechanism to provide assistance when and where it is needed; (3) supervisors with technical expertise; and (4) supervision that supports learning, skill acquisition, and graduated autonomy. It is difficult to devise mechanisms that include all of these elements. For instance, 24‐hour per day faculty coverage may be logistically challenging and expensive. Physicians with technical expertise may not be good teachers despite faculty development on procedural teaching. Obstacles to successful help‐seeking may include differences in residents' and supervisors' perceptions about the need for supervision. For example, a supervisor may be available and willing to assist, but the resident may feel capable of performing independently. When assistance is provided, residents and supervisors may differ in their perceptions of the quality of supervision.8 Ultimately, any educational intervention to increase supervision must confront a cultural norm of self‐sufficiency among many residency programs, in which managing a situation without assistance is equated with competence. To address this issue, our hospital has mandated the use of the MPS for all bedside procedures since 2005 and staffed the program 24 hours a day, in recognition of the potential risk of procedural complications9, 10 among inexperienced trainees.
This study has several limitations. We had a small number of thoracenteses. The study was not designed or powered to examine differences in complication rates among MPS and non‐MPS procedures. Because we represent a single institution, our findings may not be generalizable to other teaching hospitals or nonteaching settings. Our data on procedure characteristics were ascertained through resident self‐reports and, though typically submitted in a timely way, are subject to recall bias. In particular, discrepancies in the reported level of urgency may have affected our results about the time‐dependent nature of help‐seeking. Additionally, our findings about the types of patients about which residents seek consultation are somewhat at odds; use of the modified Deyo criteria to adjust for clinical severity weighs chronic conditions heavily and may translate into complication risk, but the level of urgency may better reflect the acuity of the clinical presentation. We could not distinguish between resident‐supervised procedures and those performed without supervision because of limited data. We also acknowledge the possibility that some non‐MPS faculty (classified for the study as informal supervisors) may serendipitously provide an equal quality of supervision that our MPS faculty did, by being present throughout the procedure and giving structured and valuable feedback.
Nevertheless, our results suggest that many residents do seek formal help appropriately when they perform procedures on the sickest patients, recognizing the risk and technical difficulty associated with bedside procedures in these patients. Our results also point to a greater area of inquiry: how do we optimally address the help‐seeking needs among trainee physicians? How do we create an environment in which help‐seeking is encouraged? How do we overcome the logistical barriers of providing timely assistance to residents, particularly at times of greatest need (urgent or emergent procedures)? How do we confront a longstanding culture in which independence is equated with competence, especially as it relates to procedural skills? A better understanding of how the widespread availability of programs like our MPS would affect the residents' use of supervision in general may guide the design of resident curricula and the development of mechanisms to ensure safe and effective clinical care.
- ,,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19:510–513.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–e24.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- .Parallel process in the family medicine system: issues and challenges for resident training.Fam Med.1990;22:312–319.
- ,,,,.Increased faculty presence on inpatient teaching services.Mayo Clin Proc.2004;79:332–336.
- ,,,.Principles of effective consultation: an update for the 21st‐century consultant.Arch Intern Med.2007;167:271–275.
- ,.Family physician consultation/referral patterns.J Am Board Fam Pract.1988;1:106–111.
- ,,, et al.Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder.J Am Coll Surg.2006;202:746–752.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324:377–384.
- ,,,.Impact of procedure‐related complications on patient outcome on a general medicine service.J Gen Intern Med.1994;9:66–70.
There is little scientific evidence about professional help‐seeking behavior among resident physicians. Although junior physicians have many sources of information available to them in the course of clinical practiceprint materials, internet resources, curbside consultations, and advice from senior residents and facultywe have little empirical knowledge about when, why, and how physician trainees ask for help.
To study this phenomenon, we examined the use of a medical procedure service (MPS) by resident physicians. The MPS is an inpatient service at a Boston teaching hospital that provides education, supervision, and evaluation of internal medicine residents who perform common bedside procedures; it has been described previously.1 Residents who call the MPS review an online curriculum with self‐assessment quizzes, perform procedures with faculty supervision and feedback, and assess their own performance using online checklists. This program has been available to internal medicine residents since 2002. In a previous study, we found that residents reported greater comfort performing bedside procedures when they used the procedure service, when the operator was a postgraduate year (PGY)2 or PGY3 resident (compared to PGY1 residents), and while placing central venous catheters (CVCs) (compared to thoracenteses).2
The goal of the current study was to examine help‐seeking behavior among resident physicians as they placed CVCs and performed thoracenteses. We interpreted the decision to use the MPS to indicate that the resident successfully sought and received assistance from pulmonary attending physicians or interventional pulmonary fellows. We hypothesized that: (1) residents earlier in their training would choose to use the procedure service due to their relative lack of experience; (2) they would seek consultation when the procedure was performed in high‐risk patients, as indicated by the number of comorbidities, presence of medications that increase the risk of bleeding, and treatment in an intensive care unit; and (3) residents would be less likely to call the MPS for urgent or emergent situations, when timely assistance may be difficult to obtain. To examine the potentially confounding influence of procedures supervised by non‐MPS physicians, we also investigated differences between informally supervised procedures (i.e. by a non‐MPS attending or fellow) and unsupervised procedures (i.e. no attending or fellow supervision) to determine whether any significant differences in their characteristics existed.
Methods
Study Site
We studied CVC placement and thoracenteses performed by internal medicine residents at a 556‐bed Boston teaching hospital in 2003‐2004. During the 9‐month study period, 63 PGY1 residents (16 in a 1‐year preliminary program) and 95 PGY2 and PGY3 residents were enrolled in the program.
The MPS was staffed by hospitalists and pulmonologists skilled in teaching and performing 4 common inpatient procedures: CVC placement, thoracentesis, lumbar puncture, and paracentesis. We chose to study only the first 2 procedures because supervision of CVCs and thoracenteses by pulmonologists was available 24 hours a day in this initial year of the MPS. The other procedures were supervised by hospitalists during business hours only at the time. Ultrasound guidance was available for all procedures, supervised or not. At the time of the study, the residency program recommended consulting the MPS for procedures, but this was not mandatory. A resident electing to use the MPS to supervise a procedure on her own patient would page the MPS physician. If she were performing a procedure for the first time, she was required to review an online multimedia curriculum and complete a 5‐question cognitive test. She would then perform the procedure while supervised by the MPS physician, who would complete a checklist evaluation of the resident's performance online. All residents performing procedures, regardless of use of the MPS, would also complete procedure logs online to document procedural experience for the American Board of Internal Medicine requirements.
Study Design and Data Sources
We prospectively collected data from resident procedure logs from July 2003 through April 2004. We elicited the following information from the residents for each procedure: name of operator, year of training, date of procedure, patient's medical record number, name of attending or fellow supervisor, procedure, immediate complications (pneumothorax, bleeding, other, or none), self‐reported level of urgency (emergent, urgent, elective), time of day, procedure location, and the number of such procedures completed previously. We categorized level of supervision as: (1) MPS‐supervised if a pulmonary attending or interventional pulmonary fellow were listed as the supervisor (entailing formal faculty development as MPS faculty, resident use of the curriculum, and completion of faculty evaluations with structured feedback); (2) informally supervised if nonpulmonary attendings or fellows were involved (who may not supervise the entire procedure and would not complete a faculty evaluation); and (3) unsupervised if a resident physician or no supervisor was identified. Faculty development involved a single training session with the interventional pulmonary fellows and attendings and focused on optimal procedural teaching. During the session, we described the structure of the MPS, provided the curricular materials available to the residents, and reviewed the faculty evaluation forms in depth.
We abstracted patient characteristics (age, race/ethnicity, type of insurance, length of stay) from the electronic medical record. We performed retrospective chart reviews to record patient comorbidities (as defined by modified Deyo criteria3), to determine the number of medications associated with the risk of bleeding (such as anticoagulants and antiplatelet agents), and to discover complications that arose after the procedure was logged, including delayed bleeding, pneumothorax, or infection (localized site infection or line‐related bloodstream infection).
Data Analyses
We tabulated characteristics of residents (training level, gender, and self‐reported number of procedures), procedures (procedure type, procedure location, level of urgency, time of day), and patients (number of comorbidities and number of medications that promote bleeding) by use of the MPS. We combined resident‐reported (ie, immediate) complications and delayed complications (identified on retrospective chart review), stratified by use of the MPS. We also performed a subgroup analysis of non‐MPS procedures by comparing resident, procedure, and patient characteristics by presence or absence of informal supervision.
We created a univariable logistic regression model to examine factors associated with elective use of the MPS. We dichotomized the following independent variables: resident characteristics (PGY status, female gender, first time performing the procedure), patient characteristics (nonwhite race, female gender, Medicaid recipient, 3 or more comorbidities, any bleeding medication), and procedure characteristics (intensive care unit procedures, nonelective procedures, procedures performed between 11 PM and 8 AM). We also included 2 patient‐related interval variables (age and length of stay) in the univariable logistic regression model. We created a multivariable logistic regression model with backward elimination (P < 0.05) using the same independent variables as in the univariable analyses, to identify factors associated with use of the MPS, clustering by resident. We repeated this method to create a multivariable model to examine factors associated with the use of informal supervision among non‐MPS procedures. Analyses used Stata 7.0 (StataCorp, College Station, TX).
The study protocol was approved in advance by the hospital investigational review board.
Results
Resident Characteristics
Sixty‐nine residents reported procedures during the 9‐month study period (Table 1). Thirty (43%) residents were PGY1 and 36 (52%) were female. Twelve (17%) residents performed the procedure for the first time.
| Total residents, n (%) | 69 (100) |
|---|---|
| |
| Training year, n (%) | |
| PGY1 | 30 (43) |
| PGY2 | 23 (33) |
| PGY3 | 16 (23) |
| Gender, n (%) | |
| Female | 36 (52) |
| Male | 33 (48) |
| Self‐reported number of prior procedures, n (%)* | |
| 0 | 12 (17) |
| 1‐5 | 26 (38) |
| >6 | 31 (45) |
Patient Characteristics
The 134 patients in the study had a mean age of 65.6 years. One‐half of patients were female, and 34% were nonwhite. The principal insurer was Medicare (57%); 24% were privately insured, and 17% received Medicaid. The mean length of stay was 18.4 days (range, 0‐98 days).
MPS and Non‐MPS Procedures
As detailed in the bivariate analyses in Table 2, residents performed 191 procedures (156 CVCs and 35 thoracenteses). PGY1 residents performed approximately one‐half of the 79 MPS procedures. Fifty‐one (65%) of the 79 MPS procedures were CVC placements and 28 (35%) were thoracenteses (P < 0.001). MPS procedures were less often performed in the emergency department than non‐MPS procedures (1% versus 21%, P < 0.001). There was no significant difference in the percentage of MPS and non‐MPS procedures by time of day. Patients whose procedures were supervised by the MPS had on average 3.0 comorbidities, while patients who underwent non‐MPS procedures had 2.6 comorbidities (P = 0.02). Complications occurred in 11 (14%) of MPS and 22 (20%) of non‐MPS procedures, a statistically nonsignificant difference.
| MPS | No MPS | P Value* | |
|---|---|---|---|
| |||
| Total procedures, n (%) | 79 (100) | 112 (100) | |
| Resident characteristics | |||
| Resident level, n (%) | 0.77 | ||
| PGY1 | 42 (53) | 60 (54) | |
| PGY2 | 22 (28) | 34 (30) | |
| PGY3 | 15 (19) | 18 (16) | |
| Gender, n (%) | 0.50 | ||
| Female | 42 (53) | 54 (48) | |
| Male | 37 (47) | 58 (52) | |
| Self‐reported number of prior procedures, n (%) | 0.11 | ||
| 0 | 14 (18) | 13 (12) | |
| 1‐5 | 40 (51) | 47 (42) | |
| >6 | 25 (32) | 52 (46) | |
| Procedure characteristics | |||
| Procedure, n (%) | 0.001 | ||
| Central venous catheter | 51 (65) | 105 (94) | |
| Thoracentesis | 28 (35) | 7 (6) | |
| Location, n (%) | 0.001 | ||
| Ward | 18 (23) | 17 (15) | |
| Emergency department | 1 (1) | 23 (21) | |
| Intensive care unit | 53 (67) | 71 (63) | |
| Other | 7 (9) | 1 (1) | |
| Urgency, n (%) | 0.001 | ||
| Elective | 32 (41) | 22 (20) | |
| Urgent | 42 (53) | 78 (70) | |
| Emergent | 5 (6) | 12 (11) | |
| Time of day, n (%) | 0.33 | ||
| 8 AM to 5 PM | 50 (63) | 65 (58) | |
| 5 PM to 11 PM | 15 (19) | 20 (18) | |
| 11 PM to 8 AM | 14 (18) | 27 (24) | |
| Complications or problems, n (%) | 0.54 | ||
| Bleeding | 1 (1) | 6 (5) | |
| Pneumothorax | 1 (1) | 2 (2) | |
| Infection | 5 (6) | 5 (4) | |
| Other∥ | 4 (5) | 9 (8) | |
| None | 68 (86) | 90 (80) | |
| Patient characteristics | |||
| Number of comorbidities, mean (SD, range) | 3.0 (1.4, 0‐7) | 2.6 (1.6, 0‐7) | 0.02 |
| Number of medications associated with bleeding risk, mean (SD, range)# | 1.1 (1.0, 0‐3) | 1.1 (0.9, 0‐3) | 0.90 |
In the univariable analysis, the only variable associated with elective use of the MPS was the presence of 3 or more comorbidities (oodds ratio [OR], 2.3; 95% confidence interval [CI], 1.2‐4.1). In the multivariable analysis, residents were more likely to use the MPS when patients had 3 or more comorbidities (OR, 2.1; 95% CI, 1.2‐3.5) and less likely to use the MPS when procedures were either urgent or emergent (OR, 0.4; 95% CI, 0.2‐0.8).
Unsupervised and Informally Supervised Procedures
Table 3 shows the results of the bivariate analyses of the characteristics of the 112 procedures that were unsupervised or supervised by non‐MPS physicians. Twenty‐seven (24%) were informally supervised by nonpulmonary attendings. Residents who had performed more than 6 procedures previously were more likely to be informally supervised than not supervised at all (P = 0.001). More informally supervised procedures were performed in the emergency department (41%) than in other settings (P = 0.01). There were no significant differences in year of training, gender, urgency, time of day, complications, comorbidities, or bleeding medications.
| Informal Supervision | No Supervision | P Value* | |
|---|---|---|---|
| |||
| Total procedures, n (%) | 27 (100) | 85 (100) | |
| Resident characteristics | |||
| Resident level, n (%) | 0.13 | ||
| PGY1 | 10 (37) | 50 (59) | |
| PGY2 | 12 (44) | 22 (26) | |
| PGY3 | 5 (19) | 13 (15) | |
| Gender, n (%) | 0.99 | ||
| Female | 13 (48) | 41 (48) | |
| Male | 14 (52) | 44 (52) | |
| Self‐reported number of prior procedures, n (%) | 0.001 | ||
| 0 | 2 (7) | 11 (13) | |
| 1‐5 | 4 (15) | 43 (51) | |
| >6 | 21 (78) | 31 (36) | |
| Procedure characteristics | |||
| Procedure, n (%) | 0.53 | ||
| Central venous catheter | 26 (96) | 79 (93) | |
| Thoracentesis | 1 (4) | 6 (7) | |
| Location, n (%) | 0.01 | ||
| Ward | 1 (4) | 16 (19) | |
| Emergency department | 11 (41) | 12 (14) | |
| Intensive care unit | 15 (56) | 56 (66) | |
| Other | 0 (0) | 1 (1) | |
| Urgency, n (%) | 0.33 | ||
| Elective | 4 (15) | 18 (21) | |
| Urgent | 19 (70) | 59 (69) | |
| Emergent | 4 (15) | 8 (9) | |
| Time of Day, n (%) | 0.11 | ||
| 8 AM to 5 PM | 13 (48) | 52 (61) | |
| 5 PM to 11 PM | 4 (15) | 16 (19) | |
| 11 PM to 8 AM | 10 (37) | 17 (20) | |
| Complications or problems, n (%) | 0.45 | ||
| Bleeding | 0 (0) | 6 (7) | |
| Pneumothorax | 0 (0) | 2 (2) | |
| Infection | 0 (0) | 5 (6) | |
| Other∥ | 2 (7) | 7 (8) | |
| None | 25 (93) | 65 (76) | |
| Patient characteristics | |||
| Number of comorbidities, mean (SD, range) | 2.2 (1.3, 1‐5) | 2.7 (1.7, 0‐7) | 0.22 |
| Number of medications associated with bleeding risk, mean (SD, range)# | 0.9 (.93, 0‐3) | 1.1 (0.9, 0‐3) | 0.24 |
In the multivariable analysis, the only factor associated with the use of informal supervision (rather than absent supervision) was patient gender; informal supervision was less likely with female patients (OR, 0.3; 95% CI, 0.1‐0.8).
Discussion
To understand professional help‐seeking behavior by internal medicine resident physicians, we studied factors associated with the use of a MPS for performing 2 common bedside procedures. We found that residents used the MPS more often when they performed procedures on patients with more comorbidities and less often during urgent or emergent procedures.
These results are consistent with our hypothesis that residents use formal supervision when caring for high‐risk patients. We had also hypothesized that they would seek the MPS for patients on medications that increase the risk of bleeding, but this was not borne out. One possible explanation is that invasive procedures on anticoagulated patients may be deferred or avoided. Additionally, we did not collect prothrombin times nor platelet count, which may represent better proxies for coagulopathy. Our hypothesis that residents would not seek the MPS for urgent and emergent procedures was confirmed; the time delay between contacting the faculty member and performing the procedure may have inhibited or obviated consultation of the MPS. We hypothesized that interns would use the MPS preferentially; we found instead that level of training did not influence use of the MPS. A resident early in training may struggle with the balance between autonomy and supervision, wanting instead to establish himself as able to solve clinical problems independently and by seeking consultation only as a last resort. Alternatively, interns may be primarily supervised by their residents and may seek expert assistance only for particularly challenging or high‐risk cases. Additionally, as newcomers to the training program, they may not be well acquainted with the role and availability of the service (although periodic announcements were made throughout the year). Our examination of procedures not supervised by the MPS showed that informally supervised and unsupervised procedures are quite similar to each other; the inverse relationship between informal supervision and patient gender is difficult to explain and may be spurious.
To our knowledge, only 1 author has postulated a theoretical foundation for help‐seeking in trainees, depicted in the context of the patient‐resident‐attending triadic relationship.4 The mature help‐seeker, whether patient, resident, or attending, is willing to confront problems, receptive to new information, able to acknowledge dependence on expertise, and able to apply new input with self‐reliance. However, little is known about how this model manifests itself empirically in professional help‐seeking or what the optimal conditions of faculty supervision are. One observational study suggested that faculty who spent more time on hospital floors created environments with higher resident satisfaction scores, higher perceived quality of patient care, and, paradoxically, increased perceptions of autonomy.5 These results are consistent with our previous work showing that residents' comfort with bedside procedures increased with use of the MPS.2 In the related field of consultation medicine, 2 studies6, 7 showed that family practitioners prefer to consult internal medicine subspecialists over general internists. One of these studies7 demonstrated that the primary need was for a consultant with technical (ie, procedural) skills. Our use of MPS faculty who are specifically skilled in performing medical procedures appears to be consistent with this observation that specific technical expertise is valued over general supervision or guidance.
How can we best design formal procedural supervision programs that allow residents to obtain help when they need it? In addition to fostering mature help‐seeking behavior, help‐giving requires: (1) an environment that encourages help‐seeking; (2) a mechanism to provide assistance when and where it is needed; (3) supervisors with technical expertise; and (4) supervision that supports learning, skill acquisition, and graduated autonomy. It is difficult to devise mechanisms that include all of these elements. For instance, 24‐hour per day faculty coverage may be logistically challenging and expensive. Physicians with technical expertise may not be good teachers despite faculty development on procedural teaching. Obstacles to successful help‐seeking may include differences in residents' and supervisors' perceptions about the need for supervision. For example, a supervisor may be available and willing to assist, but the resident may feel capable of performing independently. When assistance is provided, residents and supervisors may differ in their perceptions of the quality of supervision.8 Ultimately, any educational intervention to increase supervision must confront a cultural norm of self‐sufficiency among many residency programs, in which managing a situation without assistance is equated with competence. To address this issue, our hospital has mandated the use of the MPS for all bedside procedures since 2005 and staffed the program 24 hours a day, in recognition of the potential risk of procedural complications9, 10 among inexperienced trainees.
This study has several limitations. We had a small number of thoracenteses. The study was not designed or powered to examine differences in complication rates among MPS and non‐MPS procedures. Because we represent a single institution, our findings may not be generalizable to other teaching hospitals or nonteaching settings. Our data on procedure characteristics were ascertained through resident self‐reports and, though typically submitted in a timely way, are subject to recall bias. In particular, discrepancies in the reported level of urgency may have affected our results about the time‐dependent nature of help‐seeking. Additionally, our findings about the types of patients about which residents seek consultation are somewhat at odds; use of the modified Deyo criteria to adjust for clinical severity weighs chronic conditions heavily and may translate into complication risk, but the level of urgency may better reflect the acuity of the clinical presentation. We could not distinguish between resident‐supervised procedures and those performed without supervision because of limited data. We also acknowledge the possibility that some non‐MPS faculty (classified for the study as informal supervisors) may serendipitously provide an equal quality of supervision that our MPS faculty did, by being present throughout the procedure and giving structured and valuable feedback.
Nevertheless, our results suggest that many residents do seek formal help appropriately when they perform procedures on the sickest patients, recognizing the risk and technical difficulty associated with bedside procedures in these patients. Our results also point to a greater area of inquiry: how do we optimally address the help‐seeking needs among trainee physicians? How do we create an environment in which help‐seeking is encouraged? How do we overcome the logistical barriers of providing timely assistance to residents, particularly at times of greatest need (urgent or emergent procedures)? How do we confront a longstanding culture in which independence is equated with competence, especially as it relates to procedural skills? A better understanding of how the widespread availability of programs like our MPS would affect the residents' use of supervision in general may guide the design of resident curricula and the development of mechanisms to ensure safe and effective clinical care.
There is little scientific evidence about professional help‐seeking behavior among resident physicians. Although junior physicians have many sources of information available to them in the course of clinical practiceprint materials, internet resources, curbside consultations, and advice from senior residents and facultywe have little empirical knowledge about when, why, and how physician trainees ask for help.
To study this phenomenon, we examined the use of a medical procedure service (MPS) by resident physicians. The MPS is an inpatient service at a Boston teaching hospital that provides education, supervision, and evaluation of internal medicine residents who perform common bedside procedures; it has been described previously.1 Residents who call the MPS review an online curriculum with self‐assessment quizzes, perform procedures with faculty supervision and feedback, and assess their own performance using online checklists. This program has been available to internal medicine residents since 2002. In a previous study, we found that residents reported greater comfort performing bedside procedures when they used the procedure service, when the operator was a postgraduate year (PGY)2 or PGY3 resident (compared to PGY1 residents), and while placing central venous catheters (CVCs) (compared to thoracenteses).2
The goal of the current study was to examine help‐seeking behavior among resident physicians as they placed CVCs and performed thoracenteses. We interpreted the decision to use the MPS to indicate that the resident successfully sought and received assistance from pulmonary attending physicians or interventional pulmonary fellows. We hypothesized that: (1) residents earlier in their training would choose to use the procedure service due to their relative lack of experience; (2) they would seek consultation when the procedure was performed in high‐risk patients, as indicated by the number of comorbidities, presence of medications that increase the risk of bleeding, and treatment in an intensive care unit; and (3) residents would be less likely to call the MPS for urgent or emergent situations, when timely assistance may be difficult to obtain. To examine the potentially confounding influence of procedures supervised by non‐MPS physicians, we also investigated differences between informally supervised procedures (i.e. by a non‐MPS attending or fellow) and unsupervised procedures (i.e. no attending or fellow supervision) to determine whether any significant differences in their characteristics existed.
Methods
Study Site
We studied CVC placement and thoracenteses performed by internal medicine residents at a 556‐bed Boston teaching hospital in 2003‐2004. During the 9‐month study period, 63 PGY1 residents (16 in a 1‐year preliminary program) and 95 PGY2 and PGY3 residents were enrolled in the program.
The MPS was staffed by hospitalists and pulmonologists skilled in teaching and performing 4 common inpatient procedures: CVC placement, thoracentesis, lumbar puncture, and paracentesis. We chose to study only the first 2 procedures because supervision of CVCs and thoracenteses by pulmonologists was available 24 hours a day in this initial year of the MPS. The other procedures were supervised by hospitalists during business hours only at the time. Ultrasound guidance was available for all procedures, supervised or not. At the time of the study, the residency program recommended consulting the MPS for procedures, but this was not mandatory. A resident electing to use the MPS to supervise a procedure on her own patient would page the MPS physician. If she were performing a procedure for the first time, she was required to review an online multimedia curriculum and complete a 5‐question cognitive test. She would then perform the procedure while supervised by the MPS physician, who would complete a checklist evaluation of the resident's performance online. All residents performing procedures, regardless of use of the MPS, would also complete procedure logs online to document procedural experience for the American Board of Internal Medicine requirements.
Study Design and Data Sources
We prospectively collected data from resident procedure logs from July 2003 through April 2004. We elicited the following information from the residents for each procedure: name of operator, year of training, date of procedure, patient's medical record number, name of attending or fellow supervisor, procedure, immediate complications (pneumothorax, bleeding, other, or none), self‐reported level of urgency (emergent, urgent, elective), time of day, procedure location, and the number of such procedures completed previously. We categorized level of supervision as: (1) MPS‐supervised if a pulmonary attending or interventional pulmonary fellow were listed as the supervisor (entailing formal faculty development as MPS faculty, resident use of the curriculum, and completion of faculty evaluations with structured feedback); (2) informally supervised if nonpulmonary attendings or fellows were involved (who may not supervise the entire procedure and would not complete a faculty evaluation); and (3) unsupervised if a resident physician or no supervisor was identified. Faculty development involved a single training session with the interventional pulmonary fellows and attendings and focused on optimal procedural teaching. During the session, we described the structure of the MPS, provided the curricular materials available to the residents, and reviewed the faculty evaluation forms in depth.
We abstracted patient characteristics (age, race/ethnicity, type of insurance, length of stay) from the electronic medical record. We performed retrospective chart reviews to record patient comorbidities (as defined by modified Deyo criteria3), to determine the number of medications associated with the risk of bleeding (such as anticoagulants and antiplatelet agents), and to discover complications that arose after the procedure was logged, including delayed bleeding, pneumothorax, or infection (localized site infection or line‐related bloodstream infection).
Data Analyses
We tabulated characteristics of residents (training level, gender, and self‐reported number of procedures), procedures (procedure type, procedure location, level of urgency, time of day), and patients (number of comorbidities and number of medications that promote bleeding) by use of the MPS. We combined resident‐reported (ie, immediate) complications and delayed complications (identified on retrospective chart review), stratified by use of the MPS. We also performed a subgroup analysis of non‐MPS procedures by comparing resident, procedure, and patient characteristics by presence or absence of informal supervision.
We created a univariable logistic regression model to examine factors associated with elective use of the MPS. We dichotomized the following independent variables: resident characteristics (PGY status, female gender, first time performing the procedure), patient characteristics (nonwhite race, female gender, Medicaid recipient, 3 or more comorbidities, any bleeding medication), and procedure characteristics (intensive care unit procedures, nonelective procedures, procedures performed between 11 PM and 8 AM). We also included 2 patient‐related interval variables (age and length of stay) in the univariable logistic regression model. We created a multivariable logistic regression model with backward elimination (P < 0.05) using the same independent variables as in the univariable analyses, to identify factors associated with use of the MPS, clustering by resident. We repeated this method to create a multivariable model to examine factors associated with the use of informal supervision among non‐MPS procedures. Analyses used Stata 7.0 (StataCorp, College Station, TX).
The study protocol was approved in advance by the hospital investigational review board.
Results
Resident Characteristics
Sixty‐nine residents reported procedures during the 9‐month study period (Table 1). Thirty (43%) residents were PGY1 and 36 (52%) were female. Twelve (17%) residents performed the procedure for the first time.
| Total residents, n (%) | 69 (100) |
|---|---|
| |
| Training year, n (%) | |
| PGY1 | 30 (43) |
| PGY2 | 23 (33) |
| PGY3 | 16 (23) |
| Gender, n (%) | |
| Female | 36 (52) |
| Male | 33 (48) |
| Self‐reported number of prior procedures, n (%)* | |
| 0 | 12 (17) |
| 1‐5 | 26 (38) |
| >6 | 31 (45) |
Patient Characteristics
The 134 patients in the study had a mean age of 65.6 years. One‐half of patients were female, and 34% were nonwhite. The principal insurer was Medicare (57%); 24% were privately insured, and 17% received Medicaid. The mean length of stay was 18.4 days (range, 0‐98 days).
MPS and Non‐MPS Procedures
As detailed in the bivariate analyses in Table 2, residents performed 191 procedures (156 CVCs and 35 thoracenteses). PGY1 residents performed approximately one‐half of the 79 MPS procedures. Fifty‐one (65%) of the 79 MPS procedures were CVC placements and 28 (35%) were thoracenteses (P < 0.001). MPS procedures were less often performed in the emergency department than non‐MPS procedures (1% versus 21%, P < 0.001). There was no significant difference in the percentage of MPS and non‐MPS procedures by time of day. Patients whose procedures were supervised by the MPS had on average 3.0 comorbidities, while patients who underwent non‐MPS procedures had 2.6 comorbidities (P = 0.02). Complications occurred in 11 (14%) of MPS and 22 (20%) of non‐MPS procedures, a statistically nonsignificant difference.
| MPS | No MPS | P Value* | |
|---|---|---|---|
| |||
| Total procedures, n (%) | 79 (100) | 112 (100) | |
| Resident characteristics | |||
| Resident level, n (%) | 0.77 | ||
| PGY1 | 42 (53) | 60 (54) | |
| PGY2 | 22 (28) | 34 (30) | |
| PGY3 | 15 (19) | 18 (16) | |
| Gender, n (%) | 0.50 | ||
| Female | 42 (53) | 54 (48) | |
| Male | 37 (47) | 58 (52) | |
| Self‐reported number of prior procedures, n (%) | 0.11 | ||
| 0 | 14 (18) | 13 (12) | |
| 1‐5 | 40 (51) | 47 (42) | |
| >6 | 25 (32) | 52 (46) | |
| Procedure characteristics | |||
| Procedure, n (%) | 0.001 | ||
| Central venous catheter | 51 (65) | 105 (94) | |
| Thoracentesis | 28 (35) | 7 (6) | |
| Location, n (%) | 0.001 | ||
| Ward | 18 (23) | 17 (15) | |
| Emergency department | 1 (1) | 23 (21) | |
| Intensive care unit | 53 (67) | 71 (63) | |
| Other | 7 (9) | 1 (1) | |
| Urgency, n (%) | 0.001 | ||
| Elective | 32 (41) | 22 (20) | |
| Urgent | 42 (53) | 78 (70) | |
| Emergent | 5 (6) | 12 (11) | |
| Time of day, n (%) | 0.33 | ||
| 8 AM to 5 PM | 50 (63) | 65 (58) | |
| 5 PM to 11 PM | 15 (19) | 20 (18) | |
| 11 PM to 8 AM | 14 (18) | 27 (24) | |
| Complications or problems, n (%) | 0.54 | ||
| Bleeding | 1 (1) | 6 (5) | |
| Pneumothorax | 1 (1) | 2 (2) | |
| Infection | 5 (6) | 5 (4) | |
| Other∥ | 4 (5) | 9 (8) | |
| None | 68 (86) | 90 (80) | |
| Patient characteristics | |||
| Number of comorbidities, mean (SD, range) | 3.0 (1.4, 0‐7) | 2.6 (1.6, 0‐7) | 0.02 |
| Number of medications associated with bleeding risk, mean (SD, range)# | 1.1 (1.0, 0‐3) | 1.1 (0.9, 0‐3) | 0.90 |
In the univariable analysis, the only variable associated with elective use of the MPS was the presence of 3 or more comorbidities (oodds ratio [OR], 2.3; 95% confidence interval [CI], 1.2‐4.1). In the multivariable analysis, residents were more likely to use the MPS when patients had 3 or more comorbidities (OR, 2.1; 95% CI, 1.2‐3.5) and less likely to use the MPS when procedures were either urgent or emergent (OR, 0.4; 95% CI, 0.2‐0.8).
Unsupervised and Informally Supervised Procedures
Table 3 shows the results of the bivariate analyses of the characteristics of the 112 procedures that were unsupervised or supervised by non‐MPS physicians. Twenty‐seven (24%) were informally supervised by nonpulmonary attendings. Residents who had performed more than 6 procedures previously were more likely to be informally supervised than not supervised at all (P = 0.001). More informally supervised procedures were performed in the emergency department (41%) than in other settings (P = 0.01). There were no significant differences in year of training, gender, urgency, time of day, complications, comorbidities, or bleeding medications.
| Informal Supervision | No Supervision | P Value* | |
|---|---|---|---|
| |||
| Total procedures, n (%) | 27 (100) | 85 (100) | |
| Resident characteristics | |||
| Resident level, n (%) | 0.13 | ||
| PGY1 | 10 (37) | 50 (59) | |
| PGY2 | 12 (44) | 22 (26) | |
| PGY3 | 5 (19) | 13 (15) | |
| Gender, n (%) | 0.99 | ||
| Female | 13 (48) | 41 (48) | |
| Male | 14 (52) | 44 (52) | |
| Self‐reported number of prior procedures, n (%) | 0.001 | ||
| 0 | 2 (7) | 11 (13) | |
| 1‐5 | 4 (15) | 43 (51) | |
| >6 | 21 (78) | 31 (36) | |
| Procedure characteristics | |||
| Procedure, n (%) | 0.53 | ||
| Central venous catheter | 26 (96) | 79 (93) | |
| Thoracentesis | 1 (4) | 6 (7) | |
| Location, n (%) | 0.01 | ||
| Ward | 1 (4) | 16 (19) | |
| Emergency department | 11 (41) | 12 (14) | |
| Intensive care unit | 15 (56) | 56 (66) | |
| Other | 0 (0) | 1 (1) | |
| Urgency, n (%) | 0.33 | ||
| Elective | 4 (15) | 18 (21) | |
| Urgent | 19 (70) | 59 (69) | |
| Emergent | 4 (15) | 8 (9) | |
| Time of Day, n (%) | 0.11 | ||
| 8 AM to 5 PM | 13 (48) | 52 (61) | |
| 5 PM to 11 PM | 4 (15) | 16 (19) | |
| 11 PM to 8 AM | 10 (37) | 17 (20) | |
| Complications or problems, n (%) | 0.45 | ||
| Bleeding | 0 (0) | 6 (7) | |
| Pneumothorax | 0 (0) | 2 (2) | |
| Infection | 0 (0) | 5 (6) | |
| Other∥ | 2 (7) | 7 (8) | |
| None | 25 (93) | 65 (76) | |
| Patient characteristics | |||
| Number of comorbidities, mean (SD, range) | 2.2 (1.3, 1‐5) | 2.7 (1.7, 0‐7) | 0.22 |
| Number of medications associated with bleeding risk, mean (SD, range)# | 0.9 (.93, 0‐3) | 1.1 (0.9, 0‐3) | 0.24 |
In the multivariable analysis, the only factor associated with the use of informal supervision (rather than absent supervision) was patient gender; informal supervision was less likely with female patients (OR, 0.3; 95% CI, 0.1‐0.8).
Discussion
To understand professional help‐seeking behavior by internal medicine resident physicians, we studied factors associated with the use of a MPS for performing 2 common bedside procedures. We found that residents used the MPS more often when they performed procedures on patients with more comorbidities and less often during urgent or emergent procedures.
These results are consistent with our hypothesis that residents use formal supervision when caring for high‐risk patients. We had also hypothesized that they would seek the MPS for patients on medications that increase the risk of bleeding, but this was not borne out. One possible explanation is that invasive procedures on anticoagulated patients may be deferred or avoided. Additionally, we did not collect prothrombin times nor platelet count, which may represent better proxies for coagulopathy. Our hypothesis that residents would not seek the MPS for urgent and emergent procedures was confirmed; the time delay between contacting the faculty member and performing the procedure may have inhibited or obviated consultation of the MPS. We hypothesized that interns would use the MPS preferentially; we found instead that level of training did not influence use of the MPS. A resident early in training may struggle with the balance between autonomy and supervision, wanting instead to establish himself as able to solve clinical problems independently and by seeking consultation only as a last resort. Alternatively, interns may be primarily supervised by their residents and may seek expert assistance only for particularly challenging or high‐risk cases. Additionally, as newcomers to the training program, they may not be well acquainted with the role and availability of the service (although periodic announcements were made throughout the year). Our examination of procedures not supervised by the MPS showed that informally supervised and unsupervised procedures are quite similar to each other; the inverse relationship between informal supervision and patient gender is difficult to explain and may be spurious.
To our knowledge, only 1 author has postulated a theoretical foundation for help‐seeking in trainees, depicted in the context of the patient‐resident‐attending triadic relationship.4 The mature help‐seeker, whether patient, resident, or attending, is willing to confront problems, receptive to new information, able to acknowledge dependence on expertise, and able to apply new input with self‐reliance. However, little is known about how this model manifests itself empirically in professional help‐seeking or what the optimal conditions of faculty supervision are. One observational study suggested that faculty who spent more time on hospital floors created environments with higher resident satisfaction scores, higher perceived quality of patient care, and, paradoxically, increased perceptions of autonomy.5 These results are consistent with our previous work showing that residents' comfort with bedside procedures increased with use of the MPS.2 In the related field of consultation medicine, 2 studies6, 7 showed that family practitioners prefer to consult internal medicine subspecialists over general internists. One of these studies7 demonstrated that the primary need was for a consultant with technical (ie, procedural) skills. Our use of MPS faculty who are specifically skilled in performing medical procedures appears to be consistent with this observation that specific technical expertise is valued over general supervision or guidance.
How can we best design formal procedural supervision programs that allow residents to obtain help when they need it? In addition to fostering mature help‐seeking behavior, help‐giving requires: (1) an environment that encourages help‐seeking; (2) a mechanism to provide assistance when and where it is needed; (3) supervisors with technical expertise; and (4) supervision that supports learning, skill acquisition, and graduated autonomy. It is difficult to devise mechanisms that include all of these elements. For instance, 24‐hour per day faculty coverage may be logistically challenging and expensive. Physicians with technical expertise may not be good teachers despite faculty development on procedural teaching. Obstacles to successful help‐seeking may include differences in residents' and supervisors' perceptions about the need for supervision. For example, a supervisor may be available and willing to assist, but the resident may feel capable of performing independently. When assistance is provided, residents and supervisors may differ in their perceptions of the quality of supervision.8 Ultimately, any educational intervention to increase supervision must confront a cultural norm of self‐sufficiency among many residency programs, in which managing a situation without assistance is equated with competence. To address this issue, our hospital has mandated the use of the MPS for all bedside procedures since 2005 and staffed the program 24 hours a day, in recognition of the potential risk of procedural complications9, 10 among inexperienced trainees.
This study has several limitations. We had a small number of thoracenteses. The study was not designed or powered to examine differences in complication rates among MPS and non‐MPS procedures. Because we represent a single institution, our findings may not be generalizable to other teaching hospitals or nonteaching settings. Our data on procedure characteristics were ascertained through resident self‐reports and, though typically submitted in a timely way, are subject to recall bias. In particular, discrepancies in the reported level of urgency may have affected our results about the time‐dependent nature of help‐seeking. Additionally, our findings about the types of patients about which residents seek consultation are somewhat at odds; use of the modified Deyo criteria to adjust for clinical severity weighs chronic conditions heavily and may translate into complication risk, but the level of urgency may better reflect the acuity of the clinical presentation. We could not distinguish between resident‐supervised procedures and those performed without supervision because of limited data. We also acknowledge the possibility that some non‐MPS faculty (classified for the study as informal supervisors) may serendipitously provide an equal quality of supervision that our MPS faculty did, by being present throughout the procedure and giving structured and valuable feedback.
Nevertheless, our results suggest that many residents do seek formal help appropriately when they perform procedures on the sickest patients, recognizing the risk and technical difficulty associated with bedside procedures in these patients. Our results also point to a greater area of inquiry: how do we optimally address the help‐seeking needs among trainee physicians? How do we create an environment in which help‐seeking is encouraged? How do we overcome the logistical barriers of providing timely assistance to residents, particularly at times of greatest need (urgent or emergent procedures)? How do we confront a longstanding culture in which independence is equated with competence, especially as it relates to procedural skills? A better understanding of how the widespread availability of programs like our MPS would affect the residents' use of supervision in general may guide the design of resident curricula and the development of mechanisms to ensure safe and effective clinical care.
- ,,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19:510–513.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–e24.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- .Parallel process in the family medicine system: issues and challenges for resident training.Fam Med.1990;22:312–319.
- ,,,,.Increased faculty presence on inpatient teaching services.Mayo Clin Proc.2004;79:332–336.
- ,,,.Principles of effective consultation: an update for the 21st‐century consultant.Arch Intern Med.2007;167:271–275.
- ,.Family physician consultation/referral patterns.J Am Board Fam Pract.1988;1:106–111.
- ,,, et al.Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder.J Am Coll Surg.2006;202:746–752.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324:377–384.
- ,,,.Impact of procedure‐related complications on patient outcome on a general medicine service.J Gen Intern Med.1994;9:66–70.
- ,,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19:510–513.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–e24.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- .Parallel process in the family medicine system: issues and challenges for resident training.Fam Med.1990;22:312–319.
- ,,,,.Increased faculty presence on inpatient teaching services.Mayo Clin Proc.2004;79:332–336.
- ,,,.Principles of effective consultation: an update for the 21st‐century consultant.Arch Intern Med.2007;167:271–275.
- ,.Family physician consultation/referral patterns.J Am Board Fam Pract.1988;1:106–111.
- ,,, et al.Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder.J Am Coll Surg.2006;202:746–752.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324:377–384.
- ,,,.Impact of procedure‐related complications on patient outcome on a general medicine service.J Gen Intern Med.1994;9:66–70.
Copyright © 2009 Society of Hospital Medicine