User login
Continuous Glucose Monitoring vs Fingerstick Monitoring for Hemoglobin A1c Control in Veterans
In the United States, 1 in 4 veterans lives with type 2 diabetes mellitus (T2DM), double the rate of the general population.1 Medications are important for the treatment of T2DM and preventing complications that may develop if not properly managed. Common classes of medications for diabetes include biguanides, sodiumglucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 inhibitors, thiazolidinediones, sulfonylureas, and insulin. The selection of treatment depends on patient-specific factors including hemoglobin A1c (HbA1c) goal, potential effects on weight, risk of hypoglycemia, and comorbidities such as atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.2
HbA1c level reflects the mean blood glucose over the previous 3 months and serves as an indication of diabetes control. In patients with diabetes, it is recommended that HbA1c is checked ≥ 2 times annually for those meeting treatment goals, or more often if the patient needs to adjust medications to reach their HbA1c goal. The goal HbA1c level for most adults with diabetes is < 7%.3 This target can be adjusted based on age, comorbidities, or other patient factors. It is generally recommended that frequent glucose monitoring is not needed for patients with T2DM who are only taking oral agents and/or noninsulin injectables. However, for those on insulin regimens, it is advised to monitor glucose closely, with even more frequent testing for those with an intensive insulin regimen.3
Most patients with diabetes use fingerstick testing to self-monitor their blood glucose. However, continuous glucose monitors (CGMs) are becoming widely available and offer a solution to those who do not have the ability to check their glucose multiple times a day and throughout the night. The American Diabetes Association recommends that the frequency and timing of blood glucose monitoring, or the consideration of CGM use, should be based on the specific needs and goals of each patient.3 Guidelines also encourage those on intensive insulin regimens to check glucose levels when fasting, before and after meals, prior to exercise, and when hypoglycemia or hyperglycemia is suspected. Frequent testing can become a burden for patients, whereas once a CGM sensor is placed, it can be worn for 10 to 14 days. CGMs are also capable of transmitting glucose readings every 1 to 15 minutes to a receiver or mobile phone, allowing for further adaptability to a patient’s lifestyle.3
CGMs work by measuring the interstitial glucose with a small filament sensor and have demonstrated accuracy when compared to blood glucose readings. The ability of a CGM to accurately reflect HbA1c levels is a potential benefit, reducing the need for frequent testing to determine whether patients have achieved glycemic control.4 Another benefit of a CGM is the ease of sharing data; patient accounts can be linked with a health care site, allowing clinicians to access glucose data even if the patient is not able to be seen in clinic. This allows health care practitioners (HCPs) to more efficiently tailor medications and optimize regimens based on patient-specific data that was not available by fingerstick testing alone.
Vigersky and colleagues provided one of the few studies on the long-term effects of CGM in patients managing T2DM through diet and exercise alone, oral medications, or basal insulin and found significant improvement in HbA1c after only 3 months of CGM use.5
An important aspect of CGM use is the ability to alert the patient to low blood glucose readings, which can be dangerous for those unaware of hypoglycemia. Many studies have investigated the association between CGM use and acute metabolic events, demonstrating the potential for CGMs to prevent these emergencies. Karter and colleagues found a reduction in emergency department visits and hospitalizations for hypoglycemia associated with the use of CGMs in patients with type 1 DM (T1DM) and T2DM.6
There have been few studies on the use of CGM in veterans. Langford and colleagues found a reduction of HbA1c among veterans with T2DM using CGMs. However, > 50% of the patients in the study were not receiving insulin therapy, which currently is a US Department of Veterans Affairs (VA) CGM criteria for use.7 While current studies provide evidence that supports improvement in HbA1c levels with the use of CGMs, data are lacking for veterans with T2DM taking insulin. There is also minimal research that indicates which patients should be offered a CGM. The objective of this study was to evaluate glycemic control in veterans with T2DM on insulin using a CGM who were previously monitoring blood glucose with fingerstick testing. Secondary endpoints were explored to identify subgroups that may benefit from a CGM and other potential advantages of CGMs.
Methods
This was a retrospective study of veterans who transitioned from fingerstick testing to CGM for glucose monitoring. Each veteran served as their own control to limit confounding variables when comparing HbA1c levels. Veterans with an active or suspended CGM order were identified by reviewing outpatient prescription data. All data collection and analysis were done within the Veterans Affairs Sioux Falls Health Care System.
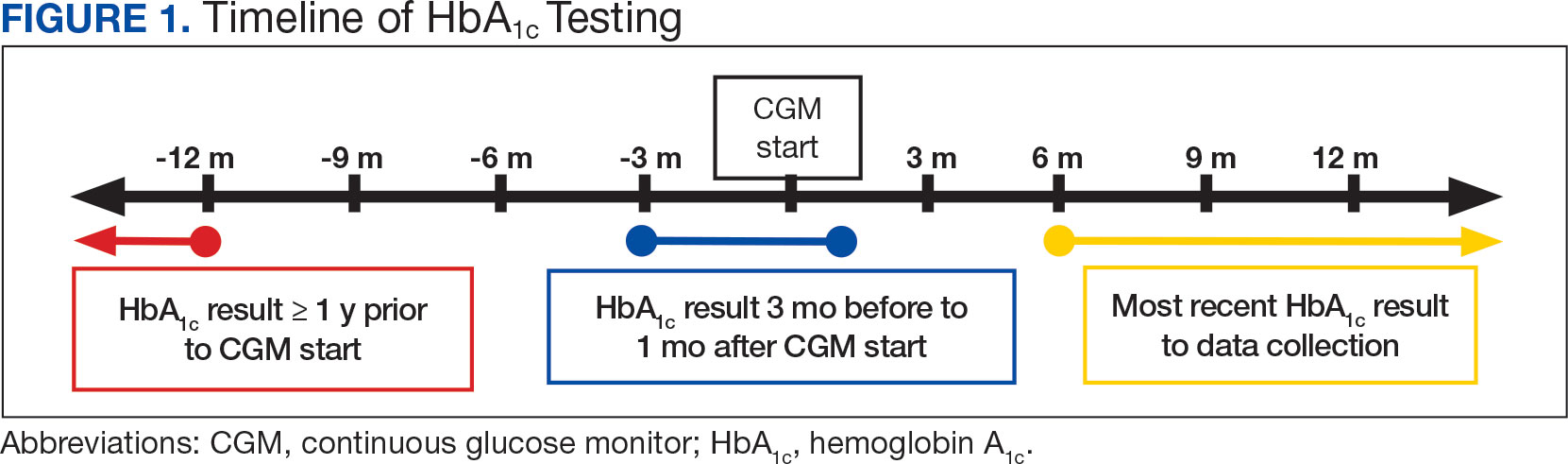
The primary objective of this study was to assess glycemic control from the use of a CGM by evaluating the change in HbA1c after transitioning to a CGM compared to the change in HbA1c with standard fingerstick monitoring. Three HbA1c values were collected for each veteran: before starting CGM, at initiation, and following CGM initiation (Figure 1). CGM start date was the date the CGM prescription order was placed. The pre-CGM HbA1c level was ≥ 1 year prior to the CGM start date or the HbA1c closest to 1 year. The start CGM HbA1c level was within 3 months before or 1 month after the CGM start date. The post-CGM HbA1c level was the most recent time of data collection and at least 6 months after CGM initiation. The change in HbA1c from fingerstick glucose monitoring was the difference between the pre-CGM and start CGM values. The change in HbA1c from use of a CGM was the difference between start CGM and post-CGM values, which were compared to determine HbA1c reduction from CGM use.
This study also explored secondary outcomes including changes in HbA1c by prescriber type, differences in HbA1c reduction based on age, and changes in diabetes medications, including total daily insulin doses. For secondary outcomes, diabetes medication information and the total daily dose of insulin were gathered at the start of CGM use and at the time of data collection. The most recent CGM order prescribed was also collected.
Veterans were included if they were aged ≥ 18 years, had an active order for a CGM, T2DM diagnosis, an insulin prescription, and previously used test strips for glucose monitoring. Patients with T1DM, those who accessed CGMs or care in the community, and patients without HbA1c values pre-CGM, were excluded.
Statistical Analysis
The primary endpoint of change in HbA1c level before and after CGM use was compared using a paired t test. A 0.5% change in HbA1c was considered clinically significant, as suggested in other studies.8,9 P < .05 was considered statistically significant. Analysis for continuous baseline characteristics, including age and total daily insulin, were reported as mean values. Nominal characteristics including sex, race, diabetes medications, and prescriber type are reported as percentages.
Results
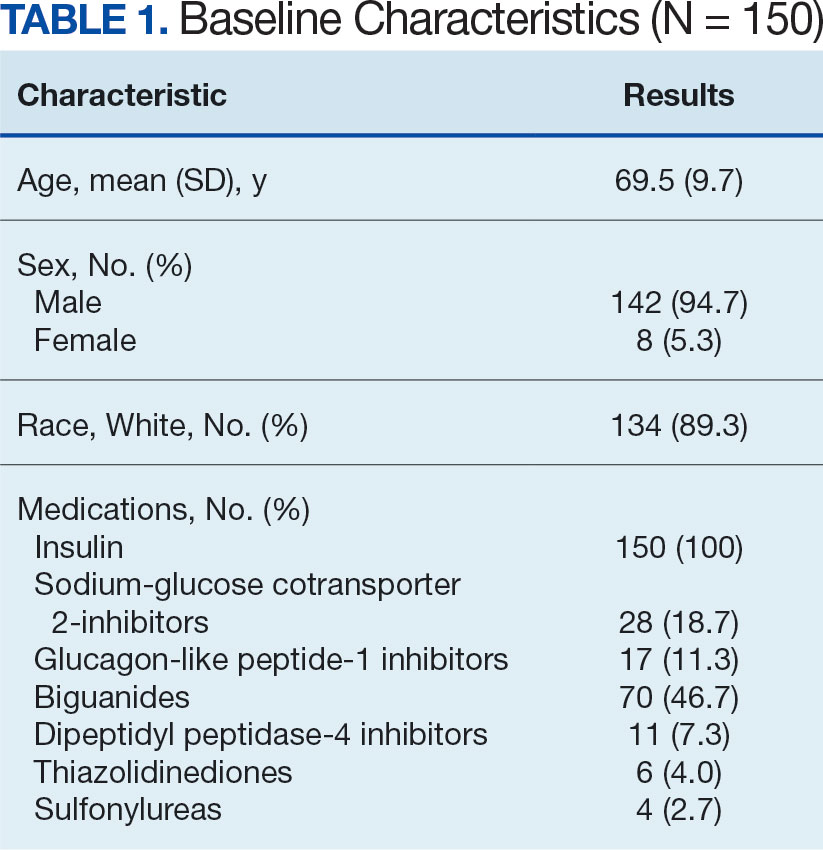
A total of 402 veterans were identified with an active CGM at the time of initial data collection in January 2024 and 175 met inclusion criteria. Sixty patients were excluded due to diabetes managed through a community HCP, 38 had T1DM, and 129 lacked HbA1c within all specified time periods. The 175 veterans were randomized, and 150 were selected to perform a chart review for data collection. The mean age was 70 years, most were male and identified as White (Table 1). The majority of patients were managed by endocrinology (53.3%), followed by primary care (24.0%), and pharmacy (22.7%) (Table 2). The mean baseline HbA1c was 8.6%.
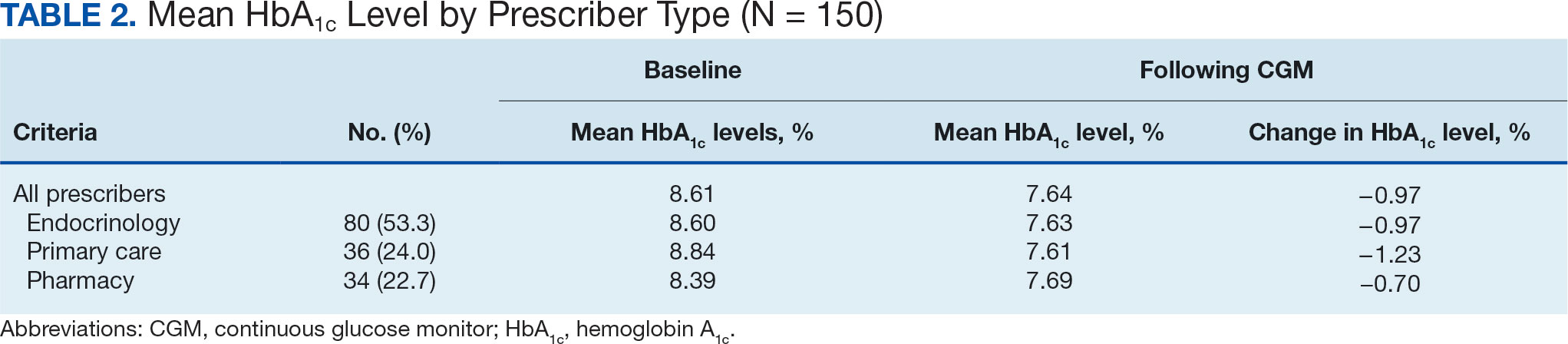
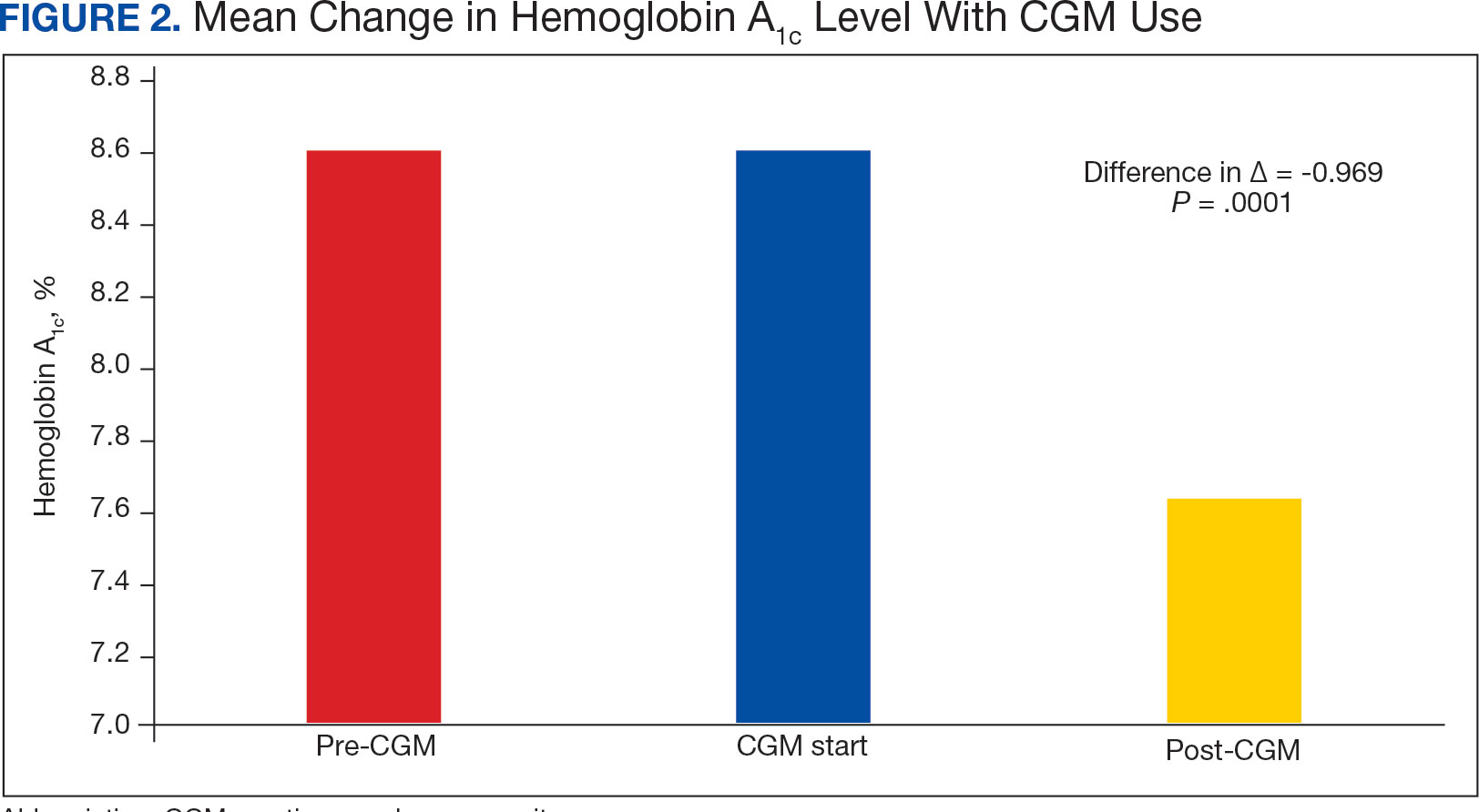
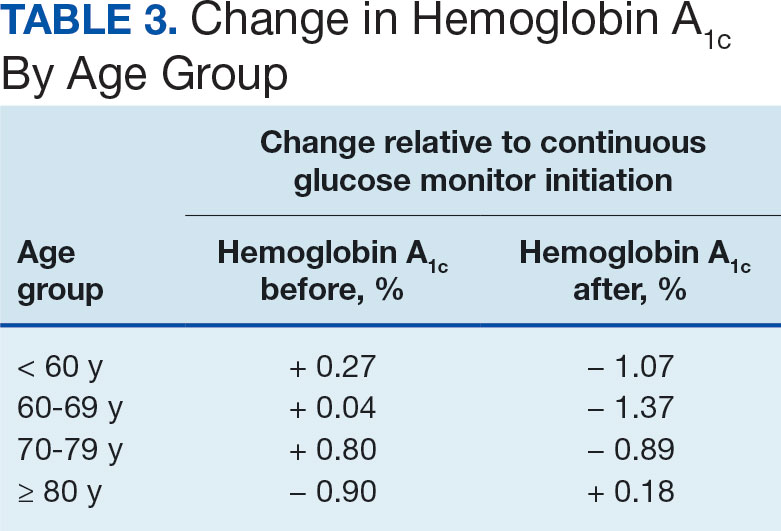
The difference in HbA1c before and after use of CGM was -0.97% (P = .0001). Prior to use of a CGM the change in HbA1c was minimal, with an increase of 0.003% with the use of selfmonitoring glucose. After use of a CGM, HbA1c decreased by 0.971%. This reduction in HbA1c would also be considered clinically significant as the change was > 0.5%. The mean pre-, at start, and post-CGM HbA1c levels were 8.6%, 8.6%, and 7.6%, respectively (Figure 2). Pharmacy prescribers had a 0.7% reduction in HbA1c post-CGM, the least of all prescribers. While most age groups saw a reduction in HbA1c, those aged ≥ 80 years had an increase of 0.18% (Table 3). There was an overall mean reduction in insulin of 22 units, which was similar between all prescribers.
Discussion
The primary endpoint of difference in change of HbA1c before and after CGM use was found to be statistically and clinically significant, with a nearly 1% reduction in HbA1c, which was similar to the reduction found by Vigersky and colleagues. 5 Across all prescribers, post-CGM HbA1c levels were similar; however, patients with CGM prescribed by pharmacists had the smallest change in HbA1c. VA pharmacists primarily assess veterans taking insulin who have HbA1c levels that are below the goal with the aim of decreasing insulin to reduce the risk of hypoglycemia, which could result in increased HbA1c levels. This may also explain the observed increase in post-CGM HbA1c levels in patients aged ≥ 80 years. Patients under the care of pharmacists also had baseline mean HbA1c levels that were lower than primary care and endocrinology prescribers and were closer to their HbA1c goal at baseline, which likely was reflected in the smaller reduction in post-CGM HbA1c level.
While there was a decrease in HbA1c levels with CGM use, there were also changes to medications during this timeframe that also may have impacted HbA1c levels. The most common diabetes medications started during CGM use were GLP-1 agonists and SGLT2-inhibitors. Additionally, there was a reduction in the total daily dose of insulin in the study population. These results demonstrate the potential benefits of CGMs for prescribers who take advantage of the CGM glucose data available to assist with medication adjustments. Another consideration for differences in changes of HbA1c among prescriber types is the opportunity for more frequent follow- up visits with pharmacy or endocrinology compared with primary care. If veterans are followed more closely, it may be associated with improved HbA1c control. Further research investigating changes in HbA1c levels based on followup frequency may be useful.
Strengths and Limitations
The crossover design was a strength of this study. This design reduced confounding variables by having veterans serve as their own controls. In addition, the collection of multiple secondary outcomes adds to the knowledge base for future studies. This study focused on a unique population of veterans with T2DM who were taking insulin, an area that previously had very little data available to determine the benefits of CGM use.
Although the use of a CGM showed statistical significance in lowering HbA1c, many veterans were started on new diabetes medication during the period of CGM use, which also likely contributed to the reduction in HbA1c and may have confounded the results. The study was limited by its small population size due to time constraints of chart reviews and the limited generalizability of results outside of the VA system. The majority of patients were from a single site, male and identified as White, which may not be reflective of other VA and community health care systems. It was also noted that the time from the initiation of CGM use to the most recent HbA1c level varied from 6 months to several years. Additionally, veterans managed by community-based HCPs with complex diabetes cases were excluded.
Conclusions
This study demonstrated a clinically and statistically significant reduction in HbA1c with the use of a CGM compared to fingerstick monitoring in veterans with T2DM who were being treated with insulin. The change in post-CGM HbA1c levels across prescribers was similar. In the subgroup analysis of change in HbA1c among age groups, there was a lower HbA1c reduction in individuals aged ≥ 80 years. The results from this study support the idea that CGM use may be beneficial for patients who require a reduction in HbA1c by allowing more precise adjustments to medications and optimization of therapy, as well as the potential to reduce insulin requirements, which is especially valuable in the older adult veteran population.
- US Department of Veterans Affairs. VA supports veterans who have type 2 diabetes. VA News. Accessed September 30, 2024. https://news.va.gov/107579/va-supports-veterans-who-have-type-2-diabetes/
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140- S157. doi:10.2337/dc23-S009
- ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- Miller E, Gavin JR, Kruger DF, Brunton SA. Continuous glucose monitoring: optimizing diabetes care: executive summary. Clin Diabetes. 2022;40(4):394-398. doi:10.2337/cd22-0043
- Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. doi:10.2337/dc11-1438
- Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325(22):2273-2284. doi:10.1001/JAMA.2021.6530
- Langford SN, Lane M, Karounos D. Continuous blood glucose monitoring outcomes in veterans with type 2 diabetes. Fed Pract. 2021;38(Suppl 4):S14-S17. doi:10.12788/fp.0189
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394. doi:10.1007/s11606-013-2595-x.
- Little RR, Rohlfing CL, Sacks DB; National Glycohemoglobin Standardization Program (NGSP) steering committee. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205-214. doi:10.1373/clinchem.2010.148841
In the United States, 1 in 4 veterans lives with type 2 diabetes mellitus (T2DM), double the rate of the general population.1 Medications are important for the treatment of T2DM and preventing complications that may develop if not properly managed. Common classes of medications for diabetes include biguanides, sodiumglucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 inhibitors, thiazolidinediones, sulfonylureas, and insulin. The selection of treatment depends on patient-specific factors including hemoglobin A1c (HbA1c) goal, potential effects on weight, risk of hypoglycemia, and comorbidities such as atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.2
HbA1c level reflects the mean blood glucose over the previous 3 months and serves as an indication of diabetes control. In patients with diabetes, it is recommended that HbA1c is checked ≥ 2 times annually for those meeting treatment goals, or more often if the patient needs to adjust medications to reach their HbA1c goal. The goal HbA1c level for most adults with diabetes is < 7%.3 This target can be adjusted based on age, comorbidities, or other patient factors. It is generally recommended that frequent glucose monitoring is not needed for patients with T2DM who are only taking oral agents and/or noninsulin injectables. However, for those on insulin regimens, it is advised to monitor glucose closely, with even more frequent testing for those with an intensive insulin regimen.3
Most patients with diabetes use fingerstick testing to self-monitor their blood glucose. However, continuous glucose monitors (CGMs) are becoming widely available and offer a solution to those who do not have the ability to check their glucose multiple times a day and throughout the night. The American Diabetes Association recommends that the frequency and timing of blood glucose monitoring, or the consideration of CGM use, should be based on the specific needs and goals of each patient.3 Guidelines also encourage those on intensive insulin regimens to check glucose levels when fasting, before and after meals, prior to exercise, and when hypoglycemia or hyperglycemia is suspected. Frequent testing can become a burden for patients, whereas once a CGM sensor is placed, it can be worn for 10 to 14 days. CGMs are also capable of transmitting glucose readings every 1 to 15 minutes to a receiver or mobile phone, allowing for further adaptability to a patient’s lifestyle.3
CGMs work by measuring the interstitial glucose with a small filament sensor and have demonstrated accuracy when compared to blood glucose readings. The ability of a CGM to accurately reflect HbA1c levels is a potential benefit, reducing the need for frequent testing to determine whether patients have achieved glycemic control.4 Another benefit of a CGM is the ease of sharing data; patient accounts can be linked with a health care site, allowing clinicians to access glucose data even if the patient is not able to be seen in clinic. This allows health care practitioners (HCPs) to more efficiently tailor medications and optimize regimens based on patient-specific data that was not available by fingerstick testing alone.
Vigersky and colleagues provided one of the few studies on the long-term effects of CGM in patients managing T2DM through diet and exercise alone, oral medications, or basal insulin and found significant improvement in HbA1c after only 3 months of CGM use.5
An important aspect of CGM use is the ability to alert the patient to low blood glucose readings, which can be dangerous for those unaware of hypoglycemia. Many studies have investigated the association between CGM use and acute metabolic events, demonstrating the potential for CGMs to prevent these emergencies. Karter and colleagues found a reduction in emergency department visits and hospitalizations for hypoglycemia associated with the use of CGMs in patients with type 1 DM (T1DM) and T2DM.6
There have been few studies on the use of CGM in veterans. Langford and colleagues found a reduction of HbA1c among veterans with T2DM using CGMs. However, > 50% of the patients in the study were not receiving insulin therapy, which currently is a US Department of Veterans Affairs (VA) CGM criteria for use.7 While current studies provide evidence that supports improvement in HbA1c levels with the use of CGMs, data are lacking for veterans with T2DM taking insulin. There is also minimal research that indicates which patients should be offered a CGM. The objective of this study was to evaluate glycemic control in veterans with T2DM on insulin using a CGM who were previously monitoring blood glucose with fingerstick testing. Secondary endpoints were explored to identify subgroups that may benefit from a CGM and other potential advantages of CGMs.
Methods
This was a retrospective study of veterans who transitioned from fingerstick testing to CGM for glucose monitoring. Each veteran served as their own control to limit confounding variables when comparing HbA1c levels. Veterans with an active or suspended CGM order were identified by reviewing outpatient prescription data. All data collection and analysis were done within the Veterans Affairs Sioux Falls Health Care System.
The primary objective of this study was to assess glycemic control from the use of a CGM by evaluating the change in HbA1c after transitioning to a CGM compared to the change in HbA1c with standard fingerstick monitoring. Three HbA1c values were collected for each veteran: before starting CGM, at initiation, and following CGM initiation (Figure 1). CGM start date was the date the CGM prescription order was placed. The pre-CGM HbA1c level was ≥ 1 year prior to the CGM start date or the HbA1c closest to 1 year. The start CGM HbA1c level was within 3 months before or 1 month after the CGM start date. The post-CGM HbA1c level was the most recent time of data collection and at least 6 months after CGM initiation. The change in HbA1c from fingerstick glucose monitoring was the difference between the pre-CGM and start CGM values. The change in HbA1c from use of a CGM was the difference between start CGM and post-CGM values, which were compared to determine HbA1c reduction from CGM use.

This study also explored secondary outcomes including changes in HbA1c by prescriber type, differences in HbA1c reduction based on age, and changes in diabetes medications, including total daily insulin doses. For secondary outcomes, diabetes medication information and the total daily dose of insulin were gathered at the start of CGM use and at the time of data collection. The most recent CGM order prescribed was also collected.
Veterans were included if they were aged ≥ 18 years, had an active order for a CGM, T2DM diagnosis, an insulin prescription, and previously used test strips for glucose monitoring. Patients with T1DM, those who accessed CGMs or care in the community, and patients without HbA1c values pre-CGM, were excluded.
Statistical Analysis
The primary endpoint of change in HbA1c level before and after CGM use was compared using a paired t test. A 0.5% change in HbA1c was considered clinically significant, as suggested in other studies.8,9 P < .05 was considered statistically significant. Analysis for continuous baseline characteristics, including age and total daily insulin, were reported as mean values. Nominal characteristics including sex, race, diabetes medications, and prescriber type are reported as percentages.
Results
A total of 402 veterans were identified with an active CGM at the time of initial data collection in January 2024 and 175 met inclusion criteria. Sixty patients were excluded due to diabetes managed through a community HCP, 38 had T1DM, and 129 lacked HbA1c within all specified time periods. The 175 veterans were randomized, and 150 were selected to perform a chart review for data collection. The mean age was 70 years, most were male and identified as White (Table 1). The majority of patients were managed by endocrinology (53.3%), followed by primary care (24.0%), and pharmacy (22.7%) (Table 2). The mean baseline HbA1c was 8.6%.


The difference in HbA1c before and after use of CGM was -0.97% (P = .0001). Prior to use of a CGM the change in HbA1c was minimal, with an increase of 0.003% with the use of selfmonitoring glucose. After use of a CGM, HbA1c decreased by 0.971%. This reduction in HbA1c would also be considered clinically significant as the change was > 0.5%. The mean pre-, at start, and post-CGM HbA1c levels were 8.6%, 8.6%, and 7.6%, respectively (Figure 2). Pharmacy prescribers had a 0.7% reduction in HbA1c post-CGM, the least of all prescribers. While most age groups saw a reduction in HbA1c, those aged ≥ 80 years had an increase of 0.18% (Table 3). There was an overall mean reduction in insulin of 22 units, which was similar between all prescribers.


Discussion
The primary endpoint of difference in change of HbA1c before and after CGM use was found to be statistically and clinically significant, with a nearly 1% reduction in HbA1c, which was similar to the reduction found by Vigersky and colleagues. 5 Across all prescribers, post-CGM HbA1c levels were similar; however, patients with CGM prescribed by pharmacists had the smallest change in HbA1c. VA pharmacists primarily assess veterans taking insulin who have HbA1c levels that are below the goal with the aim of decreasing insulin to reduce the risk of hypoglycemia, which could result in increased HbA1c levels. This may also explain the observed increase in post-CGM HbA1c levels in patients aged ≥ 80 years. Patients under the care of pharmacists also had baseline mean HbA1c levels that were lower than primary care and endocrinology prescribers and were closer to their HbA1c goal at baseline, which likely was reflected in the smaller reduction in post-CGM HbA1c level.
While there was a decrease in HbA1c levels with CGM use, there were also changes to medications during this timeframe that also may have impacted HbA1c levels. The most common diabetes medications started during CGM use were GLP-1 agonists and SGLT2-inhibitors. Additionally, there was a reduction in the total daily dose of insulin in the study population. These results demonstrate the potential benefits of CGMs for prescribers who take advantage of the CGM glucose data available to assist with medication adjustments. Another consideration for differences in changes of HbA1c among prescriber types is the opportunity for more frequent follow- up visits with pharmacy or endocrinology compared with primary care. If veterans are followed more closely, it may be associated with improved HbA1c control. Further research investigating changes in HbA1c levels based on followup frequency may be useful.
Strengths and Limitations
The crossover design was a strength of this study. This design reduced confounding variables by having veterans serve as their own controls. In addition, the collection of multiple secondary outcomes adds to the knowledge base for future studies. This study focused on a unique population of veterans with T2DM who were taking insulin, an area that previously had very little data available to determine the benefits of CGM use.
Although the use of a CGM showed statistical significance in lowering HbA1c, many veterans were started on new diabetes medication during the period of CGM use, which also likely contributed to the reduction in HbA1c and may have confounded the results. The study was limited by its small population size due to time constraints of chart reviews and the limited generalizability of results outside of the VA system. The majority of patients were from a single site, male and identified as White, which may not be reflective of other VA and community health care systems. It was also noted that the time from the initiation of CGM use to the most recent HbA1c level varied from 6 months to several years. Additionally, veterans managed by community-based HCPs with complex diabetes cases were excluded.
Conclusions
This study demonstrated a clinically and statistically significant reduction in HbA1c with the use of a CGM compared to fingerstick monitoring in veterans with T2DM who were being treated with insulin. The change in post-CGM HbA1c levels across prescribers was similar. In the subgroup analysis of change in HbA1c among age groups, there was a lower HbA1c reduction in individuals aged ≥ 80 years. The results from this study support the idea that CGM use may be beneficial for patients who require a reduction in HbA1c by allowing more precise adjustments to medications and optimization of therapy, as well as the potential to reduce insulin requirements, which is especially valuable in the older adult veteran population.
In the United States, 1 in 4 veterans lives with type 2 diabetes mellitus (T2DM), double the rate of the general population.1 Medications are important for the treatment of T2DM and preventing complications that may develop if not properly managed. Common classes of medications for diabetes include biguanides, sodiumglucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 inhibitors, thiazolidinediones, sulfonylureas, and insulin. The selection of treatment depends on patient-specific factors including hemoglobin A1c (HbA1c) goal, potential effects on weight, risk of hypoglycemia, and comorbidities such as atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.2
HbA1c level reflects the mean blood glucose over the previous 3 months and serves as an indication of diabetes control. In patients with diabetes, it is recommended that HbA1c is checked ≥ 2 times annually for those meeting treatment goals, or more often if the patient needs to adjust medications to reach their HbA1c goal. The goal HbA1c level for most adults with diabetes is < 7%.3 This target can be adjusted based on age, comorbidities, or other patient factors. It is generally recommended that frequent glucose monitoring is not needed for patients with T2DM who are only taking oral agents and/or noninsulin injectables. However, for those on insulin regimens, it is advised to monitor glucose closely, with even more frequent testing for those with an intensive insulin regimen.3
Most patients with diabetes use fingerstick testing to self-monitor their blood glucose. However, continuous glucose monitors (CGMs) are becoming widely available and offer a solution to those who do not have the ability to check their glucose multiple times a day and throughout the night. The American Diabetes Association recommends that the frequency and timing of blood glucose monitoring, or the consideration of CGM use, should be based on the specific needs and goals of each patient.3 Guidelines also encourage those on intensive insulin regimens to check glucose levels when fasting, before and after meals, prior to exercise, and when hypoglycemia or hyperglycemia is suspected. Frequent testing can become a burden for patients, whereas once a CGM sensor is placed, it can be worn for 10 to 14 days. CGMs are also capable of transmitting glucose readings every 1 to 15 minutes to a receiver or mobile phone, allowing for further adaptability to a patient’s lifestyle.3
CGMs work by measuring the interstitial glucose with a small filament sensor and have demonstrated accuracy when compared to blood glucose readings. The ability of a CGM to accurately reflect HbA1c levels is a potential benefit, reducing the need for frequent testing to determine whether patients have achieved glycemic control.4 Another benefit of a CGM is the ease of sharing data; patient accounts can be linked with a health care site, allowing clinicians to access glucose data even if the patient is not able to be seen in clinic. This allows health care practitioners (HCPs) to more efficiently tailor medications and optimize regimens based on patient-specific data that was not available by fingerstick testing alone.
Vigersky and colleagues provided one of the few studies on the long-term effects of CGM in patients managing T2DM through diet and exercise alone, oral medications, or basal insulin and found significant improvement in HbA1c after only 3 months of CGM use.5
An important aspect of CGM use is the ability to alert the patient to low blood glucose readings, which can be dangerous for those unaware of hypoglycemia. Many studies have investigated the association between CGM use and acute metabolic events, demonstrating the potential for CGMs to prevent these emergencies. Karter and colleagues found a reduction in emergency department visits and hospitalizations for hypoglycemia associated with the use of CGMs in patients with type 1 DM (T1DM) and T2DM.6
There have been few studies on the use of CGM in veterans. Langford and colleagues found a reduction of HbA1c among veterans with T2DM using CGMs. However, > 50% of the patients in the study were not receiving insulin therapy, which currently is a US Department of Veterans Affairs (VA) CGM criteria for use.7 While current studies provide evidence that supports improvement in HbA1c levels with the use of CGMs, data are lacking for veterans with T2DM taking insulin. There is also minimal research that indicates which patients should be offered a CGM. The objective of this study was to evaluate glycemic control in veterans with T2DM on insulin using a CGM who were previously monitoring blood glucose with fingerstick testing. Secondary endpoints were explored to identify subgroups that may benefit from a CGM and other potential advantages of CGMs.
Methods
This was a retrospective study of veterans who transitioned from fingerstick testing to CGM for glucose monitoring. Each veteran served as their own control to limit confounding variables when comparing HbA1c levels. Veterans with an active or suspended CGM order were identified by reviewing outpatient prescription data. All data collection and analysis were done within the Veterans Affairs Sioux Falls Health Care System.
The primary objective of this study was to assess glycemic control from the use of a CGM by evaluating the change in HbA1c after transitioning to a CGM compared to the change in HbA1c with standard fingerstick monitoring. Three HbA1c values were collected for each veteran: before starting CGM, at initiation, and following CGM initiation (Figure 1). CGM start date was the date the CGM prescription order was placed. The pre-CGM HbA1c level was ≥ 1 year prior to the CGM start date or the HbA1c closest to 1 year. The start CGM HbA1c level was within 3 months before or 1 month after the CGM start date. The post-CGM HbA1c level was the most recent time of data collection and at least 6 months after CGM initiation. The change in HbA1c from fingerstick glucose monitoring was the difference between the pre-CGM and start CGM values. The change in HbA1c from use of a CGM was the difference between start CGM and post-CGM values, which were compared to determine HbA1c reduction from CGM use.

This study also explored secondary outcomes including changes in HbA1c by prescriber type, differences in HbA1c reduction based on age, and changes in diabetes medications, including total daily insulin doses. For secondary outcomes, diabetes medication information and the total daily dose of insulin were gathered at the start of CGM use and at the time of data collection. The most recent CGM order prescribed was also collected.
Veterans were included if they were aged ≥ 18 years, had an active order for a CGM, T2DM diagnosis, an insulin prescription, and previously used test strips for glucose monitoring. Patients with T1DM, those who accessed CGMs or care in the community, and patients without HbA1c values pre-CGM, were excluded.
Statistical Analysis
The primary endpoint of change in HbA1c level before and after CGM use was compared using a paired t test. A 0.5% change in HbA1c was considered clinically significant, as suggested in other studies.8,9 P < .05 was considered statistically significant. Analysis for continuous baseline characteristics, including age and total daily insulin, were reported as mean values. Nominal characteristics including sex, race, diabetes medications, and prescriber type are reported as percentages.
Results
A total of 402 veterans were identified with an active CGM at the time of initial data collection in January 2024 and 175 met inclusion criteria. Sixty patients were excluded due to diabetes managed through a community HCP, 38 had T1DM, and 129 lacked HbA1c within all specified time periods. The 175 veterans were randomized, and 150 were selected to perform a chart review for data collection. The mean age was 70 years, most were male and identified as White (Table 1). The majority of patients were managed by endocrinology (53.3%), followed by primary care (24.0%), and pharmacy (22.7%) (Table 2). The mean baseline HbA1c was 8.6%.


The difference in HbA1c before and after use of CGM was -0.97% (P = .0001). Prior to use of a CGM the change in HbA1c was minimal, with an increase of 0.003% with the use of selfmonitoring glucose. After use of a CGM, HbA1c decreased by 0.971%. This reduction in HbA1c would also be considered clinically significant as the change was > 0.5%. The mean pre-, at start, and post-CGM HbA1c levels were 8.6%, 8.6%, and 7.6%, respectively (Figure 2). Pharmacy prescribers had a 0.7% reduction in HbA1c post-CGM, the least of all prescribers. While most age groups saw a reduction in HbA1c, those aged ≥ 80 years had an increase of 0.18% (Table 3). There was an overall mean reduction in insulin of 22 units, which was similar between all prescribers.


Discussion
The primary endpoint of difference in change of HbA1c before and after CGM use was found to be statistically and clinically significant, with a nearly 1% reduction in HbA1c, which was similar to the reduction found by Vigersky and colleagues. 5 Across all prescribers, post-CGM HbA1c levels were similar; however, patients with CGM prescribed by pharmacists had the smallest change in HbA1c. VA pharmacists primarily assess veterans taking insulin who have HbA1c levels that are below the goal with the aim of decreasing insulin to reduce the risk of hypoglycemia, which could result in increased HbA1c levels. This may also explain the observed increase in post-CGM HbA1c levels in patients aged ≥ 80 years. Patients under the care of pharmacists also had baseline mean HbA1c levels that were lower than primary care and endocrinology prescribers and were closer to their HbA1c goal at baseline, which likely was reflected in the smaller reduction in post-CGM HbA1c level.
While there was a decrease in HbA1c levels with CGM use, there were also changes to medications during this timeframe that also may have impacted HbA1c levels. The most common diabetes medications started during CGM use were GLP-1 agonists and SGLT2-inhibitors. Additionally, there was a reduction in the total daily dose of insulin in the study population. These results demonstrate the potential benefits of CGMs for prescribers who take advantage of the CGM glucose data available to assist with medication adjustments. Another consideration for differences in changes of HbA1c among prescriber types is the opportunity for more frequent follow- up visits with pharmacy or endocrinology compared with primary care. If veterans are followed more closely, it may be associated with improved HbA1c control. Further research investigating changes in HbA1c levels based on followup frequency may be useful.
Strengths and Limitations
The crossover design was a strength of this study. This design reduced confounding variables by having veterans serve as their own controls. In addition, the collection of multiple secondary outcomes adds to the knowledge base for future studies. This study focused on a unique population of veterans with T2DM who were taking insulin, an area that previously had very little data available to determine the benefits of CGM use.
Although the use of a CGM showed statistical significance in lowering HbA1c, many veterans were started on new diabetes medication during the period of CGM use, which also likely contributed to the reduction in HbA1c and may have confounded the results. The study was limited by its small population size due to time constraints of chart reviews and the limited generalizability of results outside of the VA system. The majority of patients were from a single site, male and identified as White, which may not be reflective of other VA and community health care systems. It was also noted that the time from the initiation of CGM use to the most recent HbA1c level varied from 6 months to several years. Additionally, veterans managed by community-based HCPs with complex diabetes cases were excluded.
Conclusions
This study demonstrated a clinically and statistically significant reduction in HbA1c with the use of a CGM compared to fingerstick monitoring in veterans with T2DM who were being treated with insulin. The change in post-CGM HbA1c levels across prescribers was similar. In the subgroup analysis of change in HbA1c among age groups, there was a lower HbA1c reduction in individuals aged ≥ 80 years. The results from this study support the idea that CGM use may be beneficial for patients who require a reduction in HbA1c by allowing more precise adjustments to medications and optimization of therapy, as well as the potential to reduce insulin requirements, which is especially valuable in the older adult veteran population.
- US Department of Veterans Affairs. VA supports veterans who have type 2 diabetes. VA News. Accessed September 30, 2024. https://news.va.gov/107579/va-supports-veterans-who-have-type-2-diabetes/
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140- S157. doi:10.2337/dc23-S009
- ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- Miller E, Gavin JR, Kruger DF, Brunton SA. Continuous glucose monitoring: optimizing diabetes care: executive summary. Clin Diabetes. 2022;40(4):394-398. doi:10.2337/cd22-0043
- Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. doi:10.2337/dc11-1438
- Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325(22):2273-2284. doi:10.1001/JAMA.2021.6530
- Langford SN, Lane M, Karounos D. Continuous blood glucose monitoring outcomes in veterans with type 2 diabetes. Fed Pract. 2021;38(Suppl 4):S14-S17. doi:10.12788/fp.0189
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394. doi:10.1007/s11606-013-2595-x.
- Little RR, Rohlfing CL, Sacks DB; National Glycohemoglobin Standardization Program (NGSP) steering committee. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205-214. doi:10.1373/clinchem.2010.148841
- US Department of Veterans Affairs. VA supports veterans who have type 2 diabetes. VA News. Accessed September 30, 2024. https://news.va.gov/107579/va-supports-veterans-who-have-type-2-diabetes/
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140- S157. doi:10.2337/dc23-S009
- ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- Miller E, Gavin JR, Kruger DF, Brunton SA. Continuous glucose monitoring: optimizing diabetes care: executive summary. Clin Diabetes. 2022;40(4):394-398. doi:10.2337/cd22-0043
- Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. doi:10.2337/dc11-1438
- Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325(22):2273-2284. doi:10.1001/JAMA.2021.6530
- Langford SN, Lane M, Karounos D. Continuous blood glucose monitoring outcomes in veterans with type 2 diabetes. Fed Pract. 2021;38(Suppl 4):S14-S17. doi:10.12788/fp.0189
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394. doi:10.1007/s11606-013-2595-x.
- Little RR, Rohlfing CL, Sacks DB; National Glycohemoglobin Standardization Program (NGSP) steering committee. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205-214. doi:10.1373/clinchem.2010.148841
Proton Pump Inhibitor Use and Risk of Dementia in the Veteran Population (FULL)
Proton pump inhibitors (PPIs) have become the mainstay of therapy in the treatment of acid-related disorders since their introduction in 1989. Due to their high potency, excellent tolerability, and generic availability, PPIs have largely replaced histamine-2 receptor antagonists for gastric problems. Since they were first released on the market, the use of PPIs has continued to rise in both the hospital and primary care settings.1 However, this rapid growth has led to the concern of overutilization. A study conducted at the Department of Veterans Affairs (VA) Ann Arbor Health Care System found that out of 946 patients in the ambulatory care setting taking PPIs, only 35% were appropriately prescribed PPIs.2
Although the short-term adverse effects of PPI use seem minimal, chronic PPI use consequences are a growing concern. Chronic PPI use is associated with increased risks of osteoporosis, pneumonia, and Clostridium difficile infections.3 Another long-term risk that has been associated with chronic PPI use is dementia. Dementia is a cognitive syndrome that is characterized by a progressive decline beyond what is expected in normal aging in 1 or more of the cognitive domains of memory, language, orientation, learning capacity, executive function, or social cognition.4 Because it interferes with activities of daily living, dementia is a major cause of disability in the elderly and is an immense burden for caregivers. Currently, about 47 million people globally live with dementia.5 This number is projected to nearly triple by 2050 to 132 million.5 With no cure, identification of risk factors and creation of protective measures are critical in decreasing the prevalence of dementia.
Although the exact pathophysiology behind the link between PPIs and dementia is unknown, several theories exist. One such theory is that PPI-induced vitamin B12 deficiency leads to cognitive decline.6,7 Another theory suggests that PPIs can directly cause dementia by inhibiting enzymes that normally degrade β amyloid.8 This leads to increased levels of β-amyloid plaques, which is a known characteristic of dementia patients. This theory is derived from animal studies that have shown increased amyloid levels in the brains of mice given PPIs.8
Current studies are conflicting regarding the association between PPIs and dementia. Two German prospective, cohort studies found statistically significant increased risks of dementia in patients taking PPIs with hazard ratios (HR) of 1.38 (95% CI, 1.04-1.83) and 1.44 (95% CI, 1.36-1.52), respectively.9,10 A study conducted in Taiwan also found an increased risk of dementia among PPI users with a HR of 1.22 (95% CI, 1.05-1.42).11 On the contrary, other studies have failed to show an increased risk of dementia with PPI use. In fact, Goldstein and colleagues found a decreased risk of dementia in PPI users with a HR of 0.78 (95% CI, 0.76-0.93).12 This study was an observational study conducted in the US using data from the National Alzheimer’s Coordinating Center database.12 Another recent retrospective study conducted in Finland showed that PPI use was not associated with a significantly increased risk of Alzheimer disease.13
Much is unknown about the cause of dementia, and no curative treatment exists. Investigation into potential risk factors for dementia can lead to the development of preventative measures, which can lead to significant improvement in quality of life for both patients and caregivers. Current studies regarding the association between PPIs and dementia are conflicting, and to our knowledge, no study analyzing the effects of PPIs and dementia has been conducted within the veteran population specifically. The objective of the current study is to investigate the association between PPI use and dementia in the veteran population.
Methods
This study is a retrospective, cohort, single-center, chart review study conducted at the Sioux Falls Veteran Affairs Health Care System (SFVAHCS). Data were extracted from the VA electronic health record (EHR) from January 1, 2005 through December 31, 2015. The study included both currently living and deceased veterans who received ≥ 2 documented outpatient visits at the SFVAHCS during the study time frame. Patients also had to be aged ≥ 60 years at the start of the study period. Patients were excluded if they received only a ≤ 30-day PPI prescription. Patients with dementia related to head trauma, acute intoxication, or other known diseases were excluded.
To analyze the primary endpoint of association between PPI use and dementia, the study compared the rate of dementia in a cohort of veterans who had received an outpatient prescription for a PPI within the study time frame vs the rate of dementia in a random, equal number of veterans who had never been prescribed PPIs within the study time frame. In this study, veterans were classified as having dementia if they had a diagnosis of dementia based on ICD-9 or ICD-10 codes (Table 1), or if they had been prescribed medications used to treat dementia (donepezil, ergoloid mesylates, galantamine, memantine, and rivastigmine).
Secondary endpoints included analysis of the effects of PPI agent, PPI dose, and PPI duration on the risk of dementia. For the PPI dose analysis, cumulative doses were converted into defined daily doses (DDDs) using the World Health Organization calculation to equalize the different potencies of PPI agents (Table 2).14 In addition, the effect of PPI use on vitamin B12 levels was analyzed as an exploratory endpoint to investigate the hypothesis that PPI may be associated with vitamin B12 deficiency, which in turn may be associated with dementia.6,7
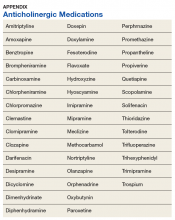
Baseline characteristics were collected to determine the variability between the treatment and control group. Data collected included age, gender, past medical history of diseases that may increase risk of dementia, and anticholinergic drug use. Anticholinergic drugs were included if they were classified as having “definite anticholinergic effects” based on the Aging Brain Care Anticholinergic Burden Scale (Appendix).15
Statistical Analysis
The primary endpoint was analyzed using a χ2 for association test. For the secondary endpoints, a χ2 for association test was used for endpoints with nominal data, and the Mood median test was used for endpoints with continuous data. The exploratory endpoint analyzing vitamin B12 levels was analyzed with the Mood median test. A P value of < .05 was defined as being statistically significant. Power analysis was not performed since all veterans who met the criteria were included in the study.
Results
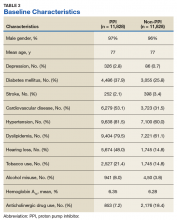
Records of 23,656 veterans were included in the study with 11,828 veterans in both the PPI cohort and the non-PPI cohort (Table 3).
Primary Endpoint
Within the PPI group, 1,119 (9.5%) veterans had dementia compared with only 740 (6.3%) veterans in the non-PPI group. There was a statistically significant association between PPI use and dementia (P < .001). These results yielded an odds ratio of 1.55 for dementia risk in PPI users vs nonusers and a relative risk increase of 51.4% for dementia risk with PPI use compared with no PPI use.
Secondary Endpoints
Users of rabeprazole had the highest rate of dementia (12.8%), followed by lansoprazole (10.9%), omeprazole (9.7%), esomeprazole (7.7%), and pantoprazole (7.0%). The rate of dementia for non-PPI users was 6.3% (P < .001). The median cumulative doses of PPIs were not significant: 597 DDDs (95% CI, 540-630) in the dementia group vs 570 DDDs (95% CI, 540-624) in the nondementia group (P = .79). The median cumulative duration of PPI use in the dementia group was 4.6 years (95% CI, 4.25-4.92) vs 5.3 years (95% CI, 5.08-5.42) in the nondementia group (P < .001).
Exploratory Endpoint
The median B12 level in the PPI group was 521 pg/mL (95% CI, 509-533) compared with 480 pg/mL (95% CI, 465-496) in the non-PPI group (P < .001). However, both groups fell within the normal range for vitamin B12 (200-900 pg/mL).16
Discussion
The aim of this study was to determine whether an association existed between PPI use and dementia. This study showed a statistically significant association between PPI use and dementia within the veteran population. This study also showed a significant association between specific PPI agents and dementia. When analyzing the individual PPI agents, the rabeprazole group yielded the strongest relationship. However, this study was not powered to evaluate and compare risks of dementia between individual PPI agents. More data are needed to determine statistical and clinical significance of associations between individual PPI agents and risk of dementia.
The veterans with dementia had a higher median cumulative PPI dose than did the veterans without dementia; however, the results were not statistically significant. Therefore, the data cannot correlate higher doses of PPI use to increased risk of dementia.
The cumulative duration of PPI use was statistically significant but opposite of the expected outcome. The dementia group had a lower median lifetime duration of PPI use compared with that of the nondementia group. It is difficult to determine the reason for this outcome, but it seems that for this study population, a longer duration of PPI use was not associated with an increased risk of dementia.
Finally, the exploratory endpoint analyzed vitamin B12 levels, since it has been shown that PPI use can lead to vitamin B12 deficiency and that B12 deficiency can lead to dementia.6-8 This study found that the dementia group had significantly higher vitamin B12 levels than the nondementia group. These data suggest that PPI use may not be associated with vitamin B12 deficiency. However, it is important to note that this study was unable to collect data on the use of vitamin B12 supplementation due to the unreliability of over-the-counter (OTC) and non-VA medication use records. Therefore, it is possible that the PPI group had higher rates of B12 deficiency but were effectively treated with B12 supplementation. More research is needed to determine the exact relationship between PPI use, vitamin B12 deficiency, and dementia risk.
Strengths/Limitations
Strengths of this study that support its findings include the large population size. Additionally, the use of the VA EHR allowed for a complete drug dispensing history to be collected, which improves reliability of the data.
This study also had some limitations. First, the causal relationship of PPI use and dementia cannot be proven using a retrospective cohort design. This study’s design can show association, but it cannot prove causation. Also, due to the retrospective design, exposure to PPI use could not be randomized; thus, correlation between PPI use and dementia may be explained by confounding variables that are not captured within this study. This is especially true since the baseline characteristics were not equally distributed between the 2 groups. In fact, the PPI group had higher rates of many clinical comorbidities. This imbalance may have skewed the results of the primary endpoint. Lastly, OTC PPI use and non-VA PPI prescriptions were not available. Therefore, some of the patients included in the non-PPI group may have been PPI users if they received PPIs from OTC or non-VA sources, which could skew the results.
Conclusion
This study showed a significant association between PPI use and dementia within the veteran study population. The study also showed a significant association between PPI use and dementia within the secondary endpoint of individual PPI agent. Higher cumulative dose and duration of PPI use did not seem to increase risk of dementia. Finally, PPI use was not associated with significantly low vitamin B12 levels. More studies are needed to determine causation of dementia and its risk factors.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System.
1. Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19-24.
2. Heidelbaugh J, Goldberg K, Inadomi J. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16(9):e228-e234.
3. Heidelbaugh JJ, Kim AH, Chang R. Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219-232.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, (DSM-5). American Psychiatric Association: Washington, DC; 2013.
5. World Health Organization. Dementia. http://www.who.int/mediacentre/factsheets/fs362/en/. Published December 12, 2017. Accessed March 10, 2019.
6. Vogiatzoglou A, Smith AD, Nurk E, et al. Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E ε4: the Hordaland Homocysteine Study. Psychosom Med. 2013;75(1):20-29.
7. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
8. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58837.
9. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
10. Gomm W, von Holt K, Thomé F, et al. Association between proton pump inhibitors with risk of dementia. A pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
11. Tai SY, Chien CY, Wu DC, et al. Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One. 2017;12(2):e0171006.
12. Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65(9):1969-1674.
13. Taipale H, Tolppanen AM, Tiihonen M. Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer’s disease. Am J Gastroenterol. 2017;112(12):1801-1808.
14. World Health Organization Collaborating Centre for Drug Statistics Methodology. Definition and general considerations. https://www.whocc.no/ddd/definition_and_general_considera/. Updated February 7, 2018. Accessed March 13, 2019.
15. Indiana University Center for Aging Research, Aging Brain Program. Anticholinergic cognitive burden scale. http://www.idhca.org/wp-content/uploads/2018/02/DESAI_ACB_scale_-_Legal_size_paper.pdf. Updated 2012. Accessed March 10, 2019.
16. US National Library of Medicine, MedlinePlus. Vitamin B12 level. https://medlineplus.gov/ency/article/003705.htm. Updated March 7, 2019. Accessed March 13, 2019.
Proton pump inhibitors (PPIs) have become the mainstay of therapy in the treatment of acid-related disorders since their introduction in 1989. Due to their high potency, excellent tolerability, and generic availability, PPIs have largely replaced histamine-2 receptor antagonists for gastric problems. Since they were first released on the market, the use of PPIs has continued to rise in both the hospital and primary care settings.1 However, this rapid growth has led to the concern of overutilization. A study conducted at the Department of Veterans Affairs (VA) Ann Arbor Health Care System found that out of 946 patients in the ambulatory care setting taking PPIs, only 35% were appropriately prescribed PPIs.2
Although the short-term adverse effects of PPI use seem minimal, chronic PPI use consequences are a growing concern. Chronic PPI use is associated with increased risks of osteoporosis, pneumonia, and Clostridium difficile infections.3 Another long-term risk that has been associated with chronic PPI use is dementia. Dementia is a cognitive syndrome that is characterized by a progressive decline beyond what is expected in normal aging in 1 or more of the cognitive domains of memory, language, orientation, learning capacity, executive function, or social cognition.4 Because it interferes with activities of daily living, dementia is a major cause of disability in the elderly and is an immense burden for caregivers. Currently, about 47 million people globally live with dementia.5 This number is projected to nearly triple by 2050 to 132 million.5 With no cure, identification of risk factors and creation of protective measures are critical in decreasing the prevalence of dementia.
Although the exact pathophysiology behind the link between PPIs and dementia is unknown, several theories exist. One such theory is that PPI-induced vitamin B12 deficiency leads to cognitive decline.6,7 Another theory suggests that PPIs can directly cause dementia by inhibiting enzymes that normally degrade β amyloid.8 This leads to increased levels of β-amyloid plaques, which is a known characteristic of dementia patients. This theory is derived from animal studies that have shown increased amyloid levels in the brains of mice given PPIs.8
Current studies are conflicting regarding the association between PPIs and dementia. Two German prospective, cohort studies found statistically significant increased risks of dementia in patients taking PPIs with hazard ratios (HR) of 1.38 (95% CI, 1.04-1.83) and 1.44 (95% CI, 1.36-1.52), respectively.9,10 A study conducted in Taiwan also found an increased risk of dementia among PPI users with a HR of 1.22 (95% CI, 1.05-1.42).11 On the contrary, other studies have failed to show an increased risk of dementia with PPI use. In fact, Goldstein and colleagues found a decreased risk of dementia in PPI users with a HR of 0.78 (95% CI, 0.76-0.93).12 This study was an observational study conducted in the US using data from the National Alzheimer’s Coordinating Center database.12 Another recent retrospective study conducted in Finland showed that PPI use was not associated with a significantly increased risk of Alzheimer disease.13
Much is unknown about the cause of dementia, and no curative treatment exists. Investigation into potential risk factors for dementia can lead to the development of preventative measures, which can lead to significant improvement in quality of life for both patients and caregivers. Current studies regarding the association between PPIs and dementia are conflicting, and to our knowledge, no study analyzing the effects of PPIs and dementia has been conducted within the veteran population specifically. The objective of the current study is to investigate the association between PPI use and dementia in the veteran population.
Methods
This study is a retrospective, cohort, single-center, chart review study conducted at the Sioux Falls Veteran Affairs Health Care System (SFVAHCS). Data were extracted from the VA electronic health record (EHR) from January 1, 2005 through December 31, 2015. The study included both currently living and deceased veterans who received ≥ 2 documented outpatient visits at the SFVAHCS during the study time frame. Patients also had to be aged ≥ 60 years at the start of the study period. Patients were excluded if they received only a ≤ 30-day PPI prescription. Patients with dementia related to head trauma, acute intoxication, or other known diseases were excluded.
To analyze the primary endpoint of association between PPI use and dementia, the study compared the rate of dementia in a cohort of veterans who had received an outpatient prescription for a PPI within the study time frame vs the rate of dementia in a random, equal number of veterans who had never been prescribed PPIs within the study time frame. In this study, veterans were classified as having dementia if they had a diagnosis of dementia based on ICD-9 or ICD-10 codes (Table 1), or if they had been prescribed medications used to treat dementia (donepezil, ergoloid mesylates, galantamine, memantine, and rivastigmine).
Secondary endpoints included analysis of the effects of PPI agent, PPI dose, and PPI duration on the risk of dementia. For the PPI dose analysis, cumulative doses were converted into defined daily doses (DDDs) using the World Health Organization calculation to equalize the different potencies of PPI agents (Table 2).14 In addition, the effect of PPI use on vitamin B12 levels was analyzed as an exploratory endpoint to investigate the hypothesis that PPI may be associated with vitamin B12 deficiency, which in turn may be associated with dementia.6,7
Baseline characteristics were collected to determine the variability between the treatment and control group. Data collected included age, gender, past medical history of diseases that may increase risk of dementia, and anticholinergic drug use. Anticholinergic drugs were included if they were classified as having “definite anticholinergic effects” based on the Aging Brain Care Anticholinergic Burden Scale (Appendix).15
Statistical Analysis
The primary endpoint was analyzed using a χ2 for association test. For the secondary endpoints, a χ2 for association test was used for endpoints with nominal data, and the Mood median test was used for endpoints with continuous data. The exploratory endpoint analyzing vitamin B12 levels was analyzed with the Mood median test. A P value of < .05 was defined as being statistically significant. Power analysis was not performed since all veterans who met the criteria were included in the study.
Results
Records of 23,656 veterans were included in the study with 11,828 veterans in both the PPI cohort and the non-PPI cohort (Table 3).
Primary Endpoint
Within the PPI group, 1,119 (9.5%) veterans had dementia compared with only 740 (6.3%) veterans in the non-PPI group. There was a statistically significant association between PPI use and dementia (P < .001). These results yielded an odds ratio of 1.55 for dementia risk in PPI users vs nonusers and a relative risk increase of 51.4% for dementia risk with PPI use compared with no PPI use.
Secondary Endpoints
Users of rabeprazole had the highest rate of dementia (12.8%), followed by lansoprazole (10.9%), omeprazole (9.7%), esomeprazole (7.7%), and pantoprazole (7.0%). The rate of dementia for non-PPI users was 6.3% (P < .001). The median cumulative doses of PPIs were not significant: 597 DDDs (95% CI, 540-630) in the dementia group vs 570 DDDs (95% CI, 540-624) in the nondementia group (P = .79). The median cumulative duration of PPI use in the dementia group was 4.6 years (95% CI, 4.25-4.92) vs 5.3 years (95% CI, 5.08-5.42) in the nondementia group (P < .001).
Exploratory Endpoint
The median B12 level in the PPI group was 521 pg/mL (95% CI, 509-533) compared with 480 pg/mL (95% CI, 465-496) in the non-PPI group (P < .001). However, both groups fell within the normal range for vitamin B12 (200-900 pg/mL).16
Discussion
The aim of this study was to determine whether an association existed between PPI use and dementia. This study showed a statistically significant association between PPI use and dementia within the veteran population. This study also showed a significant association between specific PPI agents and dementia. When analyzing the individual PPI agents, the rabeprazole group yielded the strongest relationship. However, this study was not powered to evaluate and compare risks of dementia between individual PPI agents. More data are needed to determine statistical and clinical significance of associations between individual PPI agents and risk of dementia.
The veterans with dementia had a higher median cumulative PPI dose than did the veterans without dementia; however, the results were not statistically significant. Therefore, the data cannot correlate higher doses of PPI use to increased risk of dementia.
The cumulative duration of PPI use was statistically significant but opposite of the expected outcome. The dementia group had a lower median lifetime duration of PPI use compared with that of the nondementia group. It is difficult to determine the reason for this outcome, but it seems that for this study population, a longer duration of PPI use was not associated with an increased risk of dementia.
Finally, the exploratory endpoint analyzed vitamin B12 levels, since it has been shown that PPI use can lead to vitamin B12 deficiency and that B12 deficiency can lead to dementia.6-8 This study found that the dementia group had significantly higher vitamin B12 levels than the nondementia group. These data suggest that PPI use may not be associated with vitamin B12 deficiency. However, it is important to note that this study was unable to collect data on the use of vitamin B12 supplementation due to the unreliability of over-the-counter (OTC) and non-VA medication use records. Therefore, it is possible that the PPI group had higher rates of B12 deficiency but were effectively treated with B12 supplementation. More research is needed to determine the exact relationship between PPI use, vitamin B12 deficiency, and dementia risk.
Strengths/Limitations
Strengths of this study that support its findings include the large population size. Additionally, the use of the VA EHR allowed for a complete drug dispensing history to be collected, which improves reliability of the data.
This study also had some limitations. First, the causal relationship of PPI use and dementia cannot be proven using a retrospective cohort design. This study’s design can show association, but it cannot prove causation. Also, due to the retrospective design, exposure to PPI use could not be randomized; thus, correlation between PPI use and dementia may be explained by confounding variables that are not captured within this study. This is especially true since the baseline characteristics were not equally distributed between the 2 groups. In fact, the PPI group had higher rates of many clinical comorbidities. This imbalance may have skewed the results of the primary endpoint. Lastly, OTC PPI use and non-VA PPI prescriptions were not available. Therefore, some of the patients included in the non-PPI group may have been PPI users if they received PPIs from OTC or non-VA sources, which could skew the results.
Conclusion
This study showed a significant association between PPI use and dementia within the veteran study population. The study also showed a significant association between PPI use and dementia within the secondary endpoint of individual PPI agent. Higher cumulative dose and duration of PPI use did not seem to increase risk of dementia. Finally, PPI use was not associated with significantly low vitamin B12 levels. More studies are needed to determine causation of dementia and its risk factors.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System.
Proton pump inhibitors (PPIs) have become the mainstay of therapy in the treatment of acid-related disorders since their introduction in 1989. Due to their high potency, excellent tolerability, and generic availability, PPIs have largely replaced histamine-2 receptor antagonists for gastric problems. Since they were first released on the market, the use of PPIs has continued to rise in both the hospital and primary care settings.1 However, this rapid growth has led to the concern of overutilization. A study conducted at the Department of Veterans Affairs (VA) Ann Arbor Health Care System found that out of 946 patients in the ambulatory care setting taking PPIs, only 35% were appropriately prescribed PPIs.2
Although the short-term adverse effects of PPI use seem minimal, chronic PPI use consequences are a growing concern. Chronic PPI use is associated with increased risks of osteoporosis, pneumonia, and Clostridium difficile infections.3 Another long-term risk that has been associated with chronic PPI use is dementia. Dementia is a cognitive syndrome that is characterized by a progressive decline beyond what is expected in normal aging in 1 or more of the cognitive domains of memory, language, orientation, learning capacity, executive function, or social cognition.4 Because it interferes with activities of daily living, dementia is a major cause of disability in the elderly and is an immense burden for caregivers. Currently, about 47 million people globally live with dementia.5 This number is projected to nearly triple by 2050 to 132 million.5 With no cure, identification of risk factors and creation of protective measures are critical in decreasing the prevalence of dementia.
Although the exact pathophysiology behind the link between PPIs and dementia is unknown, several theories exist. One such theory is that PPI-induced vitamin B12 deficiency leads to cognitive decline.6,7 Another theory suggests that PPIs can directly cause dementia by inhibiting enzymes that normally degrade β amyloid.8 This leads to increased levels of β-amyloid plaques, which is a known characteristic of dementia patients. This theory is derived from animal studies that have shown increased amyloid levels in the brains of mice given PPIs.8
Current studies are conflicting regarding the association between PPIs and dementia. Two German prospective, cohort studies found statistically significant increased risks of dementia in patients taking PPIs with hazard ratios (HR) of 1.38 (95% CI, 1.04-1.83) and 1.44 (95% CI, 1.36-1.52), respectively.9,10 A study conducted in Taiwan also found an increased risk of dementia among PPI users with a HR of 1.22 (95% CI, 1.05-1.42).11 On the contrary, other studies have failed to show an increased risk of dementia with PPI use. In fact, Goldstein and colleagues found a decreased risk of dementia in PPI users with a HR of 0.78 (95% CI, 0.76-0.93).12 This study was an observational study conducted in the US using data from the National Alzheimer’s Coordinating Center database.12 Another recent retrospective study conducted in Finland showed that PPI use was not associated with a significantly increased risk of Alzheimer disease.13
Much is unknown about the cause of dementia, and no curative treatment exists. Investigation into potential risk factors for dementia can lead to the development of preventative measures, which can lead to significant improvement in quality of life for both patients and caregivers. Current studies regarding the association between PPIs and dementia are conflicting, and to our knowledge, no study analyzing the effects of PPIs and dementia has been conducted within the veteran population specifically. The objective of the current study is to investigate the association between PPI use and dementia in the veteran population.
Methods
This study is a retrospective, cohort, single-center, chart review study conducted at the Sioux Falls Veteran Affairs Health Care System (SFVAHCS). Data were extracted from the VA electronic health record (EHR) from January 1, 2005 through December 31, 2015. The study included both currently living and deceased veterans who received ≥ 2 documented outpatient visits at the SFVAHCS during the study time frame. Patients also had to be aged ≥ 60 years at the start of the study period. Patients were excluded if they received only a ≤ 30-day PPI prescription. Patients with dementia related to head trauma, acute intoxication, or other known diseases were excluded.
To analyze the primary endpoint of association between PPI use and dementia, the study compared the rate of dementia in a cohort of veterans who had received an outpatient prescription for a PPI within the study time frame vs the rate of dementia in a random, equal number of veterans who had never been prescribed PPIs within the study time frame. In this study, veterans were classified as having dementia if they had a diagnosis of dementia based on ICD-9 or ICD-10 codes (Table 1), or if they had been prescribed medications used to treat dementia (donepezil, ergoloid mesylates, galantamine, memantine, and rivastigmine).
Secondary endpoints included analysis of the effects of PPI agent, PPI dose, and PPI duration on the risk of dementia. For the PPI dose analysis, cumulative doses were converted into defined daily doses (DDDs) using the World Health Organization calculation to equalize the different potencies of PPI agents (Table 2).14 In addition, the effect of PPI use on vitamin B12 levels was analyzed as an exploratory endpoint to investigate the hypothesis that PPI may be associated with vitamin B12 deficiency, which in turn may be associated with dementia.6,7
Baseline characteristics were collected to determine the variability between the treatment and control group. Data collected included age, gender, past medical history of diseases that may increase risk of dementia, and anticholinergic drug use. Anticholinergic drugs were included if they were classified as having “definite anticholinergic effects” based on the Aging Brain Care Anticholinergic Burden Scale (Appendix).15
Statistical Analysis
The primary endpoint was analyzed using a χ2 for association test. For the secondary endpoints, a χ2 for association test was used for endpoints with nominal data, and the Mood median test was used for endpoints with continuous data. The exploratory endpoint analyzing vitamin B12 levels was analyzed with the Mood median test. A P value of < .05 was defined as being statistically significant. Power analysis was not performed since all veterans who met the criteria were included in the study.
Results
Records of 23,656 veterans were included in the study with 11,828 veterans in both the PPI cohort and the non-PPI cohort (Table 3).
Primary Endpoint
Within the PPI group, 1,119 (9.5%) veterans had dementia compared with only 740 (6.3%) veterans in the non-PPI group. There was a statistically significant association between PPI use and dementia (P < .001). These results yielded an odds ratio of 1.55 for dementia risk in PPI users vs nonusers and a relative risk increase of 51.4% for dementia risk with PPI use compared with no PPI use.
Secondary Endpoints
Users of rabeprazole had the highest rate of dementia (12.8%), followed by lansoprazole (10.9%), omeprazole (9.7%), esomeprazole (7.7%), and pantoprazole (7.0%). The rate of dementia for non-PPI users was 6.3% (P < .001). The median cumulative doses of PPIs were not significant: 597 DDDs (95% CI, 540-630) in the dementia group vs 570 DDDs (95% CI, 540-624) in the nondementia group (P = .79). The median cumulative duration of PPI use in the dementia group was 4.6 years (95% CI, 4.25-4.92) vs 5.3 years (95% CI, 5.08-5.42) in the nondementia group (P < .001).
Exploratory Endpoint
The median B12 level in the PPI group was 521 pg/mL (95% CI, 509-533) compared with 480 pg/mL (95% CI, 465-496) in the non-PPI group (P < .001). However, both groups fell within the normal range for vitamin B12 (200-900 pg/mL).16
Discussion
The aim of this study was to determine whether an association existed between PPI use and dementia. This study showed a statistically significant association between PPI use and dementia within the veteran population. This study also showed a significant association between specific PPI agents and dementia. When analyzing the individual PPI agents, the rabeprazole group yielded the strongest relationship. However, this study was not powered to evaluate and compare risks of dementia between individual PPI agents. More data are needed to determine statistical and clinical significance of associations between individual PPI agents and risk of dementia.
The veterans with dementia had a higher median cumulative PPI dose than did the veterans without dementia; however, the results were not statistically significant. Therefore, the data cannot correlate higher doses of PPI use to increased risk of dementia.
The cumulative duration of PPI use was statistically significant but opposite of the expected outcome. The dementia group had a lower median lifetime duration of PPI use compared with that of the nondementia group. It is difficult to determine the reason for this outcome, but it seems that for this study population, a longer duration of PPI use was not associated with an increased risk of dementia.
Finally, the exploratory endpoint analyzed vitamin B12 levels, since it has been shown that PPI use can lead to vitamin B12 deficiency and that B12 deficiency can lead to dementia.6-8 This study found that the dementia group had significantly higher vitamin B12 levels than the nondementia group. These data suggest that PPI use may not be associated with vitamin B12 deficiency. However, it is important to note that this study was unable to collect data on the use of vitamin B12 supplementation due to the unreliability of over-the-counter (OTC) and non-VA medication use records. Therefore, it is possible that the PPI group had higher rates of B12 deficiency but were effectively treated with B12 supplementation. More research is needed to determine the exact relationship between PPI use, vitamin B12 deficiency, and dementia risk.
Strengths/Limitations
Strengths of this study that support its findings include the large population size. Additionally, the use of the VA EHR allowed for a complete drug dispensing history to be collected, which improves reliability of the data.
This study also had some limitations. First, the causal relationship of PPI use and dementia cannot be proven using a retrospective cohort design. This study’s design can show association, but it cannot prove causation. Also, due to the retrospective design, exposure to PPI use could not be randomized; thus, correlation between PPI use and dementia may be explained by confounding variables that are not captured within this study. This is especially true since the baseline characteristics were not equally distributed between the 2 groups. In fact, the PPI group had higher rates of many clinical comorbidities. This imbalance may have skewed the results of the primary endpoint. Lastly, OTC PPI use and non-VA PPI prescriptions were not available. Therefore, some of the patients included in the non-PPI group may have been PPI users if they received PPIs from OTC or non-VA sources, which could skew the results.
Conclusion
This study showed a significant association between PPI use and dementia within the veteran study population. The study also showed a significant association between PPI use and dementia within the secondary endpoint of individual PPI agent. Higher cumulative dose and duration of PPI use did not seem to increase risk of dementia. Finally, PPI use was not associated with significantly low vitamin B12 levels. More studies are needed to determine causation of dementia and its risk factors.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System.
1. Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19-24.
2. Heidelbaugh J, Goldberg K, Inadomi J. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16(9):e228-e234.
3. Heidelbaugh JJ, Kim AH, Chang R. Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219-232.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, (DSM-5). American Psychiatric Association: Washington, DC; 2013.
5. World Health Organization. Dementia. http://www.who.int/mediacentre/factsheets/fs362/en/. Published December 12, 2017. Accessed March 10, 2019.
6. Vogiatzoglou A, Smith AD, Nurk E, et al. Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E ε4: the Hordaland Homocysteine Study. Psychosom Med. 2013;75(1):20-29.
7. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
8. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58837.
9. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
10. Gomm W, von Holt K, Thomé F, et al. Association between proton pump inhibitors with risk of dementia. A pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
11. Tai SY, Chien CY, Wu DC, et al. Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One. 2017;12(2):e0171006.
12. Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65(9):1969-1674.
13. Taipale H, Tolppanen AM, Tiihonen M. Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer’s disease. Am J Gastroenterol. 2017;112(12):1801-1808.
14. World Health Organization Collaborating Centre for Drug Statistics Methodology. Definition and general considerations. https://www.whocc.no/ddd/definition_and_general_considera/. Updated February 7, 2018. Accessed March 13, 2019.
15. Indiana University Center for Aging Research, Aging Brain Program. Anticholinergic cognitive burden scale. http://www.idhca.org/wp-content/uploads/2018/02/DESAI_ACB_scale_-_Legal_size_paper.pdf. Updated 2012. Accessed March 10, 2019.
16. US National Library of Medicine, MedlinePlus. Vitamin B12 level. https://medlineplus.gov/ency/article/003705.htm. Updated March 7, 2019. Accessed March 13, 2019.
1. Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19-24.
2. Heidelbaugh J, Goldberg K, Inadomi J. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16(9):e228-e234.
3. Heidelbaugh JJ, Kim AH, Chang R. Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219-232.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, (DSM-5). American Psychiatric Association: Washington, DC; 2013.
5. World Health Organization. Dementia. http://www.who.int/mediacentre/factsheets/fs362/en/. Published December 12, 2017. Accessed March 10, 2019.
6. Vogiatzoglou A, Smith AD, Nurk E, et al. Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E ε4: the Hordaland Homocysteine Study. Psychosom Med. 2013;75(1):20-29.
7. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
8. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58837.
9. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
10. Gomm W, von Holt K, Thomé F, et al. Association between proton pump inhibitors with risk of dementia. A pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
11. Tai SY, Chien CY, Wu DC, et al. Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One. 2017;12(2):e0171006.
12. Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65(9):1969-1674.
13. Taipale H, Tolppanen AM, Tiihonen M. Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer’s disease. Am J Gastroenterol. 2017;112(12):1801-1808.
14. World Health Organization Collaborating Centre for Drug Statistics Methodology. Definition and general considerations. https://www.whocc.no/ddd/definition_and_general_considera/. Updated February 7, 2018. Accessed March 13, 2019.
15. Indiana University Center for Aging Research, Aging Brain Program. Anticholinergic cognitive burden scale. http://www.idhca.org/wp-content/uploads/2018/02/DESAI_ACB_scale_-_Legal_size_paper.pdf. Updated 2012. Accessed March 10, 2019.
16. US National Library of Medicine, MedlinePlus. Vitamin B12 level. https://medlineplus.gov/ency/article/003705.htm. Updated March 7, 2019. Accessed March 13, 2019.