User login
Clinical Progress Note: Point-of-Care Ultrasound Applications in COVID-19
COVID-19, the disease caused by the novel coronavirus SARS-CoV-2, was declared a pandemic on March 11, 2020. Although most patients (81%) develop mild illness, 14% develop severe illness, and 5% develop critical illness, including acute respiratory failure, septic shock, and multiorgan dysfunction.1
Point-of-care ultrasound (POCUS), or bedside ultrasound performed by a clinician caring for the patient, is being used to support the diagnosis and serially monitor patients with COVID-19. We performed a literature search of electronically discoverable peer-reviewed publications on POCUS use in COVID-19 from December 1, 2019, to April 10, 2020. We review key POCUS applications that are most relevant to frontline providers in the care of COVID-19 patients.
LUNG AND PLEURAL ULTRASOUND
Diagnosing COVID-19 disease by polymerase chain reaction is limited by availability of testing, delays in test positivity (mean 5.1 days), and high false-negative rate early in the course of the disease (sensitivity 81%).2 Chest computed tomography (CT) scans are often requested during the initial evaluation of suspected COVID-19, but the American College of Radiology has recommend against the routine use of CT scans for diagnosing COVID-19.3
The diagnostic accuracy of lung ultrasound (LUS) has been shown to be similar to chest CT scans in patients presenting with respiratory complaints, such as dyspnea and hypoxemia, caused by non–COVID-19 pneumonia (sensitivity, 85%; specificity, 93%).4 Normal LUS findings correlate well with CT chest scans showing absence of typical ground glass opacities. This negative predictive value is very important.5 However, early in the course of COVID-19, similar to CT scans, LUS may be normal during the first 5 days or in patients with mild disease.2 Unique advantages of LUS in COVID-19 include immediate availability of results, repeatability over time, and performance at the bedside, which avoids transportation of patients to radiology suites and disinfection of large imaging equipment.
LUS findings in COVID-19 include (a) an irregular, thickened pleural line, (b) B-lines in various patterns (discrete and confluent), (c) small subpleural consolidations, and (d) absence of pleural effusions (Figure). Bilateral, multifocal disease is common, while lobar alveolar consolidation is less common.6,7 In addition to supporting the initial diagnosis, LUS is being used to serially monitor hospitalized COVID-19 patients. As lung interstitial fluid content increases, discrete B-lines become confluent, and the number of affected lung zones increases, which can guide decisions about escalation of care. LUS is often used to guide decisions about prone ventilation, extracorporeal membrane oxygenation, and weaning from mechanical ventilation in acute respiratory failure of non–COVID-19 patients,8 and these concepts are being applied to COVID-19 patients. During recovery, reappearance of A-lines can be seen, but normalization of the LUS pattern is gradual over several weeks based on our experience and one report.9 Multiple LUS protocols examining 6 to 12 lung zones have been published prior to the COVID-19 pandemic. We recommend continuing to use an institutional protocol and evaluating at least one to two rib interspaces on the anterior, lateral, and posterior chest wall.

FOCUSED CARDIAC ULTRASOUND
Myriad cardiac complications have been described in COVID-19 – including acute coronary syndrome, myocarditis, cardiomyopathy with heart failure, and arrhythmias – secondary to increased cardiac stress from hypoxia, direct myocardial infection, or indirect injury from a hyperinflammatory response. Mortality is higher in patients with hypertension, diabetes, and coronary artery disease.10,11 Cardiac POCUS is being used to evaluate COVID-19 patients when troponin and B-type natriuretic peptide (BNP) are elevated or when there are hemodynamic or electrocardiogram changes. Given the high incidence of venous thromboembolism (VTE) in COVID-19,12 cardiac POCUS is being used to rapidly assess for right ventricular (RV) dysfunction and acute pulmonary hypertension.
The American Society of Echocardiography has recommended the use of cardiac POCUS by frontline providers for detection or characterization of preexisting cardiovascular disease, early identification of worsening cardiac function, serial monitoring and examination, and elucidation of cardiovascular pathologies associated with COVID-19.13 Sharing cardiac POCUS images in real time or through an image archive can reduce the need for consultative echocardiography, which ultimately reduces staff exposure, conserves personal protective equipment, and reduces need for decontamination of echocardiographic equipment.
The minimum cardiac POCUS views recommended in COVID-19 patients include the parasternal long-axis and short-axis views (midventricular level), either the apical or subcostal four-chamber view, and the subcostal long-axis view of the inferior vena cava.13 The goal of a cardiac POCUS exam is to qualitatively assess left ventricular (LV) systolic function, RV size and contractility, gross valvular and regional wall motion abnormalities, and pericardial effusion. In prone position ventilation, the swimmer’s position with one arm elevated above the shoulder may permit acquisition of apical views. Finally, integrated cardiopulmonary ultrasonography, including evaluation for deep vein thrombosis (DVT; see below), is ideal for proper characterization of underlying LV and RV function, volume status, and titration of vasopressor and inotropic support.
VENOUS THROMBOEMBOLISM
COVID-19 has been associated with a proinflammatory and hypercoagulable state with elevated
A POCUS exam for LE DVT consists of two-dimensional venous compression alone and yields results similar to formal vascular studies in both critically ill and noncritically ill patients. Because proximal LE thrombi have the highest risk of embolization, evaluation of the common femoral vein, femoral vein, and popliteal vein is most important.15 Either inability to compress a vein completely with wall-to-wall apposition or visualization of echogenic thrombus within the vein is diagnostic of DVT. Acute thrombi are gelatinous and may appear anechoic, while subacute or chronic thrombi are echogenic, but all veins with a DVT will not compress completely.
VASCULAR ACCESS
Ultrasound guidance for central venous catheter (CVC) insertion has been shown to increase procedure success rates and decrease mechanical complications, primarily arterial puncture and pneumothorax. Similarly, higher success rates and fewer insertion attempts have been observed with ultrasound-guided peripheral intravenous line and arterial line placement.17 Ultrasound-guided PIV placement can reduce referrals for midlines and peripherally inserted central catheters in hospitalized patients.18
In COVID-19 patients, use of ultrasound guidance for vascular access has distinct advantages. First, given the high incidence of DVT in COVID-19 patients,12 POCUS allows preprocedural evaluation of the target vessel for thrombosis, as well as anatomic variations and stenosis. Second, visualizing the needle tip and guidewire within the target vein prior to dilation nearly eliminates the risk of arterial puncture and inadvertent arterial dilation, which is particularly important in COVID-19 patients receiving high-dose prophylactic or therapeutic anticoagulation. Third, when inserting internal jugular and subclavian CVCs, visualization of normal lung sliding before and after the procedure safely rules out pneumothorax. However, if lung sliding is not seen before the procedure, it cannot be used to rule out pneumothorax afterward. Additionally, visualizing absence of the catheter tip in the right atrium and presence of a rapid atrial swirl sign within 2 seconds of briskly injecting 10 mL of saline confirms catheter tip placement near the superior vena cava/right atrial junction, which can eliminate the need for a postprocedure chest radiograph.17
ENDOTRACHEAL INTUBATION
POCUS can be used to rapidly confirm endotracheal tube (ETT) placement, which can reduce reliance on postintubation chest radiographs. A meta-analysis of prospective and randomized trials showed transtracheal ultrasonography had high sensitivity (98.7%) and specificity (97.1%) for confirming tracheal placement of ETTs.19 Confirming endotracheal intubation involves two steps: First, a linear transducer is placed transversely over the suprasternal notch to visualize the ETT passing through the trachea, and not the esophagus, during insertion. Second, after the ETT cuff has been inflated, bilateral lung sliding should be seen in sync with the respiratory cycle if the ETT is in the trachea. Absent lung sliding, but preserved lung pulse, on the anterior hemithorax is likely caused by main stem bronchial intubation, and withdrawing the ETT until bilateral lung sliding is seen confirms tracheal placement. Additionally, the following steps are recommended to reduce the risk of exposure to healthcare workers: minimizing use of bag-valve-mask ventilation, performing rapid sequence intubation using video laryngoscopy, and connecting the ETT to the ventilator immediately.
ULTRASOUND DEVICES AND DISINFECTION
Important considerations when selecting an ultrasound machine for use in COVID-19 patients include image quality, portability, functionality, and ease of disinfection. Advantages of handheld devices include portability and ease of disinfection, whereas cart-based systems generally have better image quality and functionality. To minimize the risk of cross contamination, an ultrasound machine should be dedicated exclusively for use on patients with confirmed COVID-19 and not shared with patients with suspected COVID-19.20 To minimize exposure to COVID-19 patients, frontline providers should perform POCUS exams only when findings may change management, and timing of the exam and views acquired should be selected deliberately.
Ultrasound machine disinfection should be integrated into routine donning and doffing procedures. When possible, both handheld and cart-based machines should be draped with protective covers during aerosol-generating procedures. Single use ultrasound gel packets are recommended in order to decrease the risk of nosocomial infection.20 After every use of an ultrasound machine on intact skin or for percutaneous procedures, low-level disinfection should be performed with an Environmental Protection Agency–recommended product that is effective against coronavirus.
Some ultrasound manufacturers have added teleultrasound software that allows remote training of novice POCUS users and remote guidance in actual patient care. Teleultrasound can be utilized to share images in real time with consultants or expert providers.
CONCLUSION
POCUS is uniquely poised to improve patient care during the COVID-19 pandemic. POCUS can be used to support the diagnosis of COVID-19 patients and monitor patients with confirmed disease. Common POCUS applications used in COVID-19 patients include evaluation of the lungs, heart, and deep veins, as well as performance of bedside procedures. Ultrasound machine portability and disinfection are important considerations in COVID-19 patients.
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. https://doi.org/10.1001/jama.2020.2648.
2. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. https://doi.org/10.1148/radiol.2020200642.
3. American College of Radiology. ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. March 11, 2020. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed April 10, 2020.
4. Alzahrani SA, Al-Salamah MA, Al-Madani WH, Elbarbary MA. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit Ultrasound J. 2017;9(1):6. https://doi.org/10.1186/s13089-017-0059-y.
5. Hew M, Corcoran JP, Harriss EK, Rahman NM, Mallett S. The diagnostic accuracy of chest ultrasound for CT-detected radiographic consolidation in hospitalised adults with acute respiratory failure: a systematic review. BMJ Open. 2015;5(5):e007838. https://doi.org/10.1136/bmjopen-2015-007838.
6. Peng QY, Wang XT, Zhang LN; Chinese Critical Care Ultrasound Study Group. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020. https://doi.org/10.1007/s00134-020-05996-6.
7. Huang Y, Wang S, Liu Y, et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19). Soc Sci Res Netw (SSRN). 2020. http://doi.org/10.2139/ssrn.3544750.
8. Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung ultrasound for critically ill patients. Am J Respir Crit Care Med. 2019;199(6):701-714. https://doi.org/10.1164/rccm.201802-0236ci.
9. Ji L, Cao C, Lv Q, Li Y, Xie M. Serial bedside lung ultrasonography in a critically ill COVID-19 patient. Qjm. 2020. https://doi.org/10.1093/qjmed/hcaa141.
10. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020. https://doi.org/10.1001/jamacardio.2020.1286.
11. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;e201017. https://doi.org/10.1001/jamacardio.2020.1017.
12. Klok F, Kruip M, van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Throm Res. 2020. https://doi.org/10.1016/j.thromres.2020.04.013.
13. Johri AM, Galen B, Kirkpatrick J, Lanspa M, Mulvagh S, Thamman R. ASE statement on point-of-care ultrasound (POCUS) during the 2019 novel coronavirus pandemic. J Am Soc Echocardiogr. 2020. https://doi.org/10.1016/j.echo.2020.04.017.
14. American Society of Hematology. COVID-19 and Pulmonary Embolism: Frequently Asked Questions. April 9, 2020. https://www.hematology.org/covid-19/covid-19-and-pulmonary-embolism. Accessed April 10, 2020.
15. Fischer EA, Kinnear B, Sall D, et al. Hospitalist-Operated Compression Ultrasonography: a Point-of-Care Ultrasound Study (HOCUS-POCUS). J Gen Intern Med. 2019;34(10):2062-2067. https://doi.org/10.1007/s11606-019-05120-5.
16. Tavazzi G, Civardi L, Caneva L, Mongodi S, Mojoli F. Thrombotic events in SARS-CoV-2 patients: an urgent call for ultrasound screening. Intensive Care Med. 2020;1-3. https://doi.org/10.1007/s00134-020-06040-3.
17. Franco-Sadud R, Schnobrich D, Mathews BK, et al. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287.
18. Galen B, Baron S, Young S, Hall A, Berger-Spivack L, Southern W. Reducing peripherally inserted central catheters and midline catheters by training nurses in ultrasound-guided peripheral intravenous catheter placement. BMJ Qual Saf. 2020;29(3):245-249. https://doi.org/10.1136/bmjqs-2019-009923.
19. Gottlieb M, Holladay D, Peksa GD. Ultrasonography for the confirmation of endotracheal tube intubation: a systematic review and meta-analysis. Ann Emerg Med. 2018;72(6):627-636. https://doi.org/10.1016/j.annemergmed.2018.06.024.
20. Abramowicz J, Basseal J. WFUMB Position Statement: how to perform a safe ultrasound examination and clean equipment in the context of COVID-19. Ultrasound Med Biol. 2020. https://doi.org/10.1016/j.ultrasmedbio.2020.03.033.
COVID-19, the disease caused by the novel coronavirus SARS-CoV-2, was declared a pandemic on March 11, 2020. Although most patients (81%) develop mild illness, 14% develop severe illness, and 5% develop critical illness, including acute respiratory failure, septic shock, and multiorgan dysfunction.1
Point-of-care ultrasound (POCUS), or bedside ultrasound performed by a clinician caring for the patient, is being used to support the diagnosis and serially monitor patients with COVID-19. We performed a literature search of electronically discoverable peer-reviewed publications on POCUS use in COVID-19 from December 1, 2019, to April 10, 2020. We review key POCUS applications that are most relevant to frontline providers in the care of COVID-19 patients.
LUNG AND PLEURAL ULTRASOUND
Diagnosing COVID-19 disease by polymerase chain reaction is limited by availability of testing, delays in test positivity (mean 5.1 days), and high false-negative rate early in the course of the disease (sensitivity 81%).2 Chest computed tomography (CT) scans are often requested during the initial evaluation of suspected COVID-19, but the American College of Radiology has recommend against the routine use of CT scans for diagnosing COVID-19.3
The diagnostic accuracy of lung ultrasound (LUS) has been shown to be similar to chest CT scans in patients presenting with respiratory complaints, such as dyspnea and hypoxemia, caused by non–COVID-19 pneumonia (sensitivity, 85%; specificity, 93%).4 Normal LUS findings correlate well with CT chest scans showing absence of typical ground glass opacities. This negative predictive value is very important.5 However, early in the course of COVID-19, similar to CT scans, LUS may be normal during the first 5 days or in patients with mild disease.2 Unique advantages of LUS in COVID-19 include immediate availability of results, repeatability over time, and performance at the bedside, which avoids transportation of patients to radiology suites and disinfection of large imaging equipment.
LUS findings in COVID-19 include (a) an irregular, thickened pleural line, (b) B-lines in various patterns (discrete and confluent), (c) small subpleural consolidations, and (d) absence of pleural effusions (Figure). Bilateral, multifocal disease is common, while lobar alveolar consolidation is less common.6,7 In addition to supporting the initial diagnosis, LUS is being used to serially monitor hospitalized COVID-19 patients. As lung interstitial fluid content increases, discrete B-lines become confluent, and the number of affected lung zones increases, which can guide decisions about escalation of care. LUS is often used to guide decisions about prone ventilation, extracorporeal membrane oxygenation, and weaning from mechanical ventilation in acute respiratory failure of non–COVID-19 patients,8 and these concepts are being applied to COVID-19 patients. During recovery, reappearance of A-lines can be seen, but normalization of the LUS pattern is gradual over several weeks based on our experience and one report.9 Multiple LUS protocols examining 6 to 12 lung zones have been published prior to the COVID-19 pandemic. We recommend continuing to use an institutional protocol and evaluating at least one to two rib interspaces on the anterior, lateral, and posterior chest wall.

FOCUSED CARDIAC ULTRASOUND
Myriad cardiac complications have been described in COVID-19 – including acute coronary syndrome, myocarditis, cardiomyopathy with heart failure, and arrhythmias – secondary to increased cardiac stress from hypoxia, direct myocardial infection, or indirect injury from a hyperinflammatory response. Mortality is higher in patients with hypertension, diabetes, and coronary artery disease.10,11 Cardiac POCUS is being used to evaluate COVID-19 patients when troponin and B-type natriuretic peptide (BNP) are elevated or when there are hemodynamic or electrocardiogram changes. Given the high incidence of venous thromboembolism (VTE) in COVID-19,12 cardiac POCUS is being used to rapidly assess for right ventricular (RV) dysfunction and acute pulmonary hypertension.
The American Society of Echocardiography has recommended the use of cardiac POCUS by frontline providers for detection or characterization of preexisting cardiovascular disease, early identification of worsening cardiac function, serial monitoring and examination, and elucidation of cardiovascular pathologies associated with COVID-19.13 Sharing cardiac POCUS images in real time or through an image archive can reduce the need for consultative echocardiography, which ultimately reduces staff exposure, conserves personal protective equipment, and reduces need for decontamination of echocardiographic equipment.
The minimum cardiac POCUS views recommended in COVID-19 patients include the parasternal long-axis and short-axis views (midventricular level), either the apical or subcostal four-chamber view, and the subcostal long-axis view of the inferior vena cava.13 The goal of a cardiac POCUS exam is to qualitatively assess left ventricular (LV) systolic function, RV size and contractility, gross valvular and regional wall motion abnormalities, and pericardial effusion. In prone position ventilation, the swimmer’s position with one arm elevated above the shoulder may permit acquisition of apical views. Finally, integrated cardiopulmonary ultrasonography, including evaluation for deep vein thrombosis (DVT; see below), is ideal for proper characterization of underlying LV and RV function, volume status, and titration of vasopressor and inotropic support.
VENOUS THROMBOEMBOLISM
COVID-19 has been associated with a proinflammatory and hypercoagulable state with elevated
A POCUS exam for LE DVT consists of two-dimensional venous compression alone and yields results similar to formal vascular studies in both critically ill and noncritically ill patients. Because proximal LE thrombi have the highest risk of embolization, evaluation of the common femoral vein, femoral vein, and popliteal vein is most important.15 Either inability to compress a vein completely with wall-to-wall apposition or visualization of echogenic thrombus within the vein is diagnostic of DVT. Acute thrombi are gelatinous and may appear anechoic, while subacute or chronic thrombi are echogenic, but all veins with a DVT will not compress completely.
VASCULAR ACCESS
Ultrasound guidance for central venous catheter (CVC) insertion has been shown to increase procedure success rates and decrease mechanical complications, primarily arterial puncture and pneumothorax. Similarly, higher success rates and fewer insertion attempts have been observed with ultrasound-guided peripheral intravenous line and arterial line placement.17 Ultrasound-guided PIV placement can reduce referrals for midlines and peripherally inserted central catheters in hospitalized patients.18
In COVID-19 patients, use of ultrasound guidance for vascular access has distinct advantages. First, given the high incidence of DVT in COVID-19 patients,12 POCUS allows preprocedural evaluation of the target vessel for thrombosis, as well as anatomic variations and stenosis. Second, visualizing the needle tip and guidewire within the target vein prior to dilation nearly eliminates the risk of arterial puncture and inadvertent arterial dilation, which is particularly important in COVID-19 patients receiving high-dose prophylactic or therapeutic anticoagulation. Third, when inserting internal jugular and subclavian CVCs, visualization of normal lung sliding before and after the procedure safely rules out pneumothorax. However, if lung sliding is not seen before the procedure, it cannot be used to rule out pneumothorax afterward. Additionally, visualizing absence of the catheter tip in the right atrium and presence of a rapid atrial swirl sign within 2 seconds of briskly injecting 10 mL of saline confirms catheter tip placement near the superior vena cava/right atrial junction, which can eliminate the need for a postprocedure chest radiograph.17
ENDOTRACHEAL INTUBATION
POCUS can be used to rapidly confirm endotracheal tube (ETT) placement, which can reduce reliance on postintubation chest radiographs. A meta-analysis of prospective and randomized trials showed transtracheal ultrasonography had high sensitivity (98.7%) and specificity (97.1%) for confirming tracheal placement of ETTs.19 Confirming endotracheal intubation involves two steps: First, a linear transducer is placed transversely over the suprasternal notch to visualize the ETT passing through the trachea, and not the esophagus, during insertion. Second, after the ETT cuff has been inflated, bilateral lung sliding should be seen in sync with the respiratory cycle if the ETT is in the trachea. Absent lung sliding, but preserved lung pulse, on the anterior hemithorax is likely caused by main stem bronchial intubation, and withdrawing the ETT until bilateral lung sliding is seen confirms tracheal placement. Additionally, the following steps are recommended to reduce the risk of exposure to healthcare workers: minimizing use of bag-valve-mask ventilation, performing rapid sequence intubation using video laryngoscopy, and connecting the ETT to the ventilator immediately.
ULTRASOUND DEVICES AND DISINFECTION
Important considerations when selecting an ultrasound machine for use in COVID-19 patients include image quality, portability, functionality, and ease of disinfection. Advantages of handheld devices include portability and ease of disinfection, whereas cart-based systems generally have better image quality and functionality. To minimize the risk of cross contamination, an ultrasound machine should be dedicated exclusively for use on patients with confirmed COVID-19 and not shared with patients with suspected COVID-19.20 To minimize exposure to COVID-19 patients, frontline providers should perform POCUS exams only when findings may change management, and timing of the exam and views acquired should be selected deliberately.
Ultrasound machine disinfection should be integrated into routine donning and doffing procedures. When possible, both handheld and cart-based machines should be draped with protective covers during aerosol-generating procedures. Single use ultrasound gel packets are recommended in order to decrease the risk of nosocomial infection.20 After every use of an ultrasound machine on intact skin or for percutaneous procedures, low-level disinfection should be performed with an Environmental Protection Agency–recommended product that is effective against coronavirus.
Some ultrasound manufacturers have added teleultrasound software that allows remote training of novice POCUS users and remote guidance in actual patient care. Teleultrasound can be utilized to share images in real time with consultants or expert providers.
CONCLUSION
POCUS is uniquely poised to improve patient care during the COVID-19 pandemic. POCUS can be used to support the diagnosis of COVID-19 patients and monitor patients with confirmed disease. Common POCUS applications used in COVID-19 patients include evaluation of the lungs, heart, and deep veins, as well as performance of bedside procedures. Ultrasound machine portability and disinfection are important considerations in COVID-19 patients.
COVID-19, the disease caused by the novel coronavirus SARS-CoV-2, was declared a pandemic on March 11, 2020. Although most patients (81%) develop mild illness, 14% develop severe illness, and 5% develop critical illness, including acute respiratory failure, septic shock, and multiorgan dysfunction.1
Point-of-care ultrasound (POCUS), or bedside ultrasound performed by a clinician caring for the patient, is being used to support the diagnosis and serially monitor patients with COVID-19. We performed a literature search of electronically discoverable peer-reviewed publications on POCUS use in COVID-19 from December 1, 2019, to April 10, 2020. We review key POCUS applications that are most relevant to frontline providers in the care of COVID-19 patients.
LUNG AND PLEURAL ULTRASOUND
Diagnosing COVID-19 disease by polymerase chain reaction is limited by availability of testing, delays in test positivity (mean 5.1 days), and high false-negative rate early in the course of the disease (sensitivity 81%).2 Chest computed tomography (CT) scans are often requested during the initial evaluation of suspected COVID-19, but the American College of Radiology has recommend against the routine use of CT scans for diagnosing COVID-19.3
The diagnostic accuracy of lung ultrasound (LUS) has been shown to be similar to chest CT scans in patients presenting with respiratory complaints, such as dyspnea and hypoxemia, caused by non–COVID-19 pneumonia (sensitivity, 85%; specificity, 93%).4 Normal LUS findings correlate well with CT chest scans showing absence of typical ground glass opacities. This negative predictive value is very important.5 However, early in the course of COVID-19, similar to CT scans, LUS may be normal during the first 5 days or in patients with mild disease.2 Unique advantages of LUS in COVID-19 include immediate availability of results, repeatability over time, and performance at the bedside, which avoids transportation of patients to radiology suites and disinfection of large imaging equipment.
LUS findings in COVID-19 include (a) an irregular, thickened pleural line, (b) B-lines in various patterns (discrete and confluent), (c) small subpleural consolidations, and (d) absence of pleural effusions (Figure). Bilateral, multifocal disease is common, while lobar alveolar consolidation is less common.6,7 In addition to supporting the initial diagnosis, LUS is being used to serially monitor hospitalized COVID-19 patients. As lung interstitial fluid content increases, discrete B-lines become confluent, and the number of affected lung zones increases, which can guide decisions about escalation of care. LUS is often used to guide decisions about prone ventilation, extracorporeal membrane oxygenation, and weaning from mechanical ventilation in acute respiratory failure of non–COVID-19 patients,8 and these concepts are being applied to COVID-19 patients. During recovery, reappearance of A-lines can be seen, but normalization of the LUS pattern is gradual over several weeks based on our experience and one report.9 Multiple LUS protocols examining 6 to 12 lung zones have been published prior to the COVID-19 pandemic. We recommend continuing to use an institutional protocol and evaluating at least one to two rib interspaces on the anterior, lateral, and posterior chest wall.

FOCUSED CARDIAC ULTRASOUND
Myriad cardiac complications have been described in COVID-19 – including acute coronary syndrome, myocarditis, cardiomyopathy with heart failure, and arrhythmias – secondary to increased cardiac stress from hypoxia, direct myocardial infection, or indirect injury from a hyperinflammatory response. Mortality is higher in patients with hypertension, diabetes, and coronary artery disease.10,11 Cardiac POCUS is being used to evaluate COVID-19 patients when troponin and B-type natriuretic peptide (BNP) are elevated or when there are hemodynamic or electrocardiogram changes. Given the high incidence of venous thromboembolism (VTE) in COVID-19,12 cardiac POCUS is being used to rapidly assess for right ventricular (RV) dysfunction and acute pulmonary hypertension.
The American Society of Echocardiography has recommended the use of cardiac POCUS by frontline providers for detection or characterization of preexisting cardiovascular disease, early identification of worsening cardiac function, serial monitoring and examination, and elucidation of cardiovascular pathologies associated with COVID-19.13 Sharing cardiac POCUS images in real time or through an image archive can reduce the need for consultative echocardiography, which ultimately reduces staff exposure, conserves personal protective equipment, and reduces need for decontamination of echocardiographic equipment.
The minimum cardiac POCUS views recommended in COVID-19 patients include the parasternal long-axis and short-axis views (midventricular level), either the apical or subcostal four-chamber view, and the subcostal long-axis view of the inferior vena cava.13 The goal of a cardiac POCUS exam is to qualitatively assess left ventricular (LV) systolic function, RV size and contractility, gross valvular and regional wall motion abnormalities, and pericardial effusion. In prone position ventilation, the swimmer’s position with one arm elevated above the shoulder may permit acquisition of apical views. Finally, integrated cardiopulmonary ultrasonography, including evaluation for deep vein thrombosis (DVT; see below), is ideal for proper characterization of underlying LV and RV function, volume status, and titration of vasopressor and inotropic support.
VENOUS THROMBOEMBOLISM
COVID-19 has been associated with a proinflammatory and hypercoagulable state with elevated
A POCUS exam for LE DVT consists of two-dimensional venous compression alone and yields results similar to formal vascular studies in both critically ill and noncritically ill patients. Because proximal LE thrombi have the highest risk of embolization, evaluation of the common femoral vein, femoral vein, and popliteal vein is most important.15 Either inability to compress a vein completely with wall-to-wall apposition or visualization of echogenic thrombus within the vein is diagnostic of DVT. Acute thrombi are gelatinous and may appear anechoic, while subacute or chronic thrombi are echogenic, but all veins with a DVT will not compress completely.
VASCULAR ACCESS
Ultrasound guidance for central venous catheter (CVC) insertion has been shown to increase procedure success rates and decrease mechanical complications, primarily arterial puncture and pneumothorax. Similarly, higher success rates and fewer insertion attempts have been observed with ultrasound-guided peripheral intravenous line and arterial line placement.17 Ultrasound-guided PIV placement can reduce referrals for midlines and peripherally inserted central catheters in hospitalized patients.18
In COVID-19 patients, use of ultrasound guidance for vascular access has distinct advantages. First, given the high incidence of DVT in COVID-19 patients,12 POCUS allows preprocedural evaluation of the target vessel for thrombosis, as well as anatomic variations and stenosis. Second, visualizing the needle tip and guidewire within the target vein prior to dilation nearly eliminates the risk of arterial puncture and inadvertent arterial dilation, which is particularly important in COVID-19 patients receiving high-dose prophylactic or therapeutic anticoagulation. Third, when inserting internal jugular and subclavian CVCs, visualization of normal lung sliding before and after the procedure safely rules out pneumothorax. However, if lung sliding is not seen before the procedure, it cannot be used to rule out pneumothorax afterward. Additionally, visualizing absence of the catheter tip in the right atrium and presence of a rapid atrial swirl sign within 2 seconds of briskly injecting 10 mL of saline confirms catheter tip placement near the superior vena cava/right atrial junction, which can eliminate the need for a postprocedure chest radiograph.17
ENDOTRACHEAL INTUBATION
POCUS can be used to rapidly confirm endotracheal tube (ETT) placement, which can reduce reliance on postintubation chest radiographs. A meta-analysis of prospective and randomized trials showed transtracheal ultrasonography had high sensitivity (98.7%) and specificity (97.1%) for confirming tracheal placement of ETTs.19 Confirming endotracheal intubation involves two steps: First, a linear transducer is placed transversely over the suprasternal notch to visualize the ETT passing through the trachea, and not the esophagus, during insertion. Second, after the ETT cuff has been inflated, bilateral lung sliding should be seen in sync with the respiratory cycle if the ETT is in the trachea. Absent lung sliding, but preserved lung pulse, on the anterior hemithorax is likely caused by main stem bronchial intubation, and withdrawing the ETT until bilateral lung sliding is seen confirms tracheal placement. Additionally, the following steps are recommended to reduce the risk of exposure to healthcare workers: minimizing use of bag-valve-mask ventilation, performing rapid sequence intubation using video laryngoscopy, and connecting the ETT to the ventilator immediately.
ULTRASOUND DEVICES AND DISINFECTION
Important considerations when selecting an ultrasound machine for use in COVID-19 patients include image quality, portability, functionality, and ease of disinfection. Advantages of handheld devices include portability and ease of disinfection, whereas cart-based systems generally have better image quality and functionality. To minimize the risk of cross contamination, an ultrasound machine should be dedicated exclusively for use on patients with confirmed COVID-19 and not shared with patients with suspected COVID-19.20 To minimize exposure to COVID-19 patients, frontline providers should perform POCUS exams only when findings may change management, and timing of the exam and views acquired should be selected deliberately.
Ultrasound machine disinfection should be integrated into routine donning and doffing procedures. When possible, both handheld and cart-based machines should be draped with protective covers during aerosol-generating procedures. Single use ultrasound gel packets are recommended in order to decrease the risk of nosocomial infection.20 After every use of an ultrasound machine on intact skin or for percutaneous procedures, low-level disinfection should be performed with an Environmental Protection Agency–recommended product that is effective against coronavirus.
Some ultrasound manufacturers have added teleultrasound software that allows remote training of novice POCUS users and remote guidance in actual patient care. Teleultrasound can be utilized to share images in real time with consultants or expert providers.
CONCLUSION
POCUS is uniquely poised to improve patient care during the COVID-19 pandemic. POCUS can be used to support the diagnosis of COVID-19 patients and monitor patients with confirmed disease. Common POCUS applications used in COVID-19 patients include evaluation of the lungs, heart, and deep veins, as well as performance of bedside procedures. Ultrasound machine portability and disinfection are important considerations in COVID-19 patients.
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. https://doi.org/10.1001/jama.2020.2648.
2. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. https://doi.org/10.1148/radiol.2020200642.
3. American College of Radiology. ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. March 11, 2020. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed April 10, 2020.
4. Alzahrani SA, Al-Salamah MA, Al-Madani WH, Elbarbary MA. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit Ultrasound J. 2017;9(1):6. https://doi.org/10.1186/s13089-017-0059-y.
5. Hew M, Corcoran JP, Harriss EK, Rahman NM, Mallett S. The diagnostic accuracy of chest ultrasound for CT-detected radiographic consolidation in hospitalised adults with acute respiratory failure: a systematic review. BMJ Open. 2015;5(5):e007838. https://doi.org/10.1136/bmjopen-2015-007838.
6. Peng QY, Wang XT, Zhang LN; Chinese Critical Care Ultrasound Study Group. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020. https://doi.org/10.1007/s00134-020-05996-6.
7. Huang Y, Wang S, Liu Y, et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19). Soc Sci Res Netw (SSRN). 2020. http://doi.org/10.2139/ssrn.3544750.
8. Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung ultrasound for critically ill patients. Am J Respir Crit Care Med. 2019;199(6):701-714. https://doi.org/10.1164/rccm.201802-0236ci.
9. Ji L, Cao C, Lv Q, Li Y, Xie M. Serial bedside lung ultrasonography in a critically ill COVID-19 patient. Qjm. 2020. https://doi.org/10.1093/qjmed/hcaa141.
10. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020. https://doi.org/10.1001/jamacardio.2020.1286.
11. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;e201017. https://doi.org/10.1001/jamacardio.2020.1017.
12. Klok F, Kruip M, van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Throm Res. 2020. https://doi.org/10.1016/j.thromres.2020.04.013.
13. Johri AM, Galen B, Kirkpatrick J, Lanspa M, Mulvagh S, Thamman R. ASE statement on point-of-care ultrasound (POCUS) during the 2019 novel coronavirus pandemic. J Am Soc Echocardiogr. 2020. https://doi.org/10.1016/j.echo.2020.04.017.
14. American Society of Hematology. COVID-19 and Pulmonary Embolism: Frequently Asked Questions. April 9, 2020. https://www.hematology.org/covid-19/covid-19-and-pulmonary-embolism. Accessed April 10, 2020.
15. Fischer EA, Kinnear B, Sall D, et al. Hospitalist-Operated Compression Ultrasonography: a Point-of-Care Ultrasound Study (HOCUS-POCUS). J Gen Intern Med. 2019;34(10):2062-2067. https://doi.org/10.1007/s11606-019-05120-5.
16. Tavazzi G, Civardi L, Caneva L, Mongodi S, Mojoli F. Thrombotic events in SARS-CoV-2 patients: an urgent call for ultrasound screening. Intensive Care Med. 2020;1-3. https://doi.org/10.1007/s00134-020-06040-3.
17. Franco-Sadud R, Schnobrich D, Mathews BK, et al. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287.
18. Galen B, Baron S, Young S, Hall A, Berger-Spivack L, Southern W. Reducing peripherally inserted central catheters and midline catheters by training nurses in ultrasound-guided peripheral intravenous catheter placement. BMJ Qual Saf. 2020;29(3):245-249. https://doi.org/10.1136/bmjqs-2019-009923.
19. Gottlieb M, Holladay D, Peksa GD. Ultrasonography for the confirmation of endotracheal tube intubation: a systematic review and meta-analysis. Ann Emerg Med. 2018;72(6):627-636. https://doi.org/10.1016/j.annemergmed.2018.06.024.
20. Abramowicz J, Basseal J. WFUMB Position Statement: how to perform a safe ultrasound examination and clean equipment in the context of COVID-19. Ultrasound Med Biol. 2020. https://doi.org/10.1016/j.ultrasmedbio.2020.03.033.
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. https://doi.org/10.1001/jama.2020.2648.
2. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. https://doi.org/10.1148/radiol.2020200642.
3. American College of Radiology. ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. March 11, 2020. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed April 10, 2020.
4. Alzahrani SA, Al-Salamah MA, Al-Madani WH, Elbarbary MA. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit Ultrasound J. 2017;9(1):6. https://doi.org/10.1186/s13089-017-0059-y.
5. Hew M, Corcoran JP, Harriss EK, Rahman NM, Mallett S. The diagnostic accuracy of chest ultrasound for CT-detected radiographic consolidation in hospitalised adults with acute respiratory failure: a systematic review. BMJ Open. 2015;5(5):e007838. https://doi.org/10.1136/bmjopen-2015-007838.
6. Peng QY, Wang XT, Zhang LN; Chinese Critical Care Ultrasound Study Group. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020. https://doi.org/10.1007/s00134-020-05996-6.
7. Huang Y, Wang S, Liu Y, et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19). Soc Sci Res Netw (SSRN). 2020. http://doi.org/10.2139/ssrn.3544750.
8. Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung ultrasound for critically ill patients. Am J Respir Crit Care Med. 2019;199(6):701-714. https://doi.org/10.1164/rccm.201802-0236ci.
9. Ji L, Cao C, Lv Q, Li Y, Xie M. Serial bedside lung ultrasonography in a critically ill COVID-19 patient. Qjm. 2020. https://doi.org/10.1093/qjmed/hcaa141.
10. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020. https://doi.org/10.1001/jamacardio.2020.1286.
11. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;e201017. https://doi.org/10.1001/jamacardio.2020.1017.
12. Klok F, Kruip M, van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Throm Res. 2020. https://doi.org/10.1016/j.thromres.2020.04.013.
13. Johri AM, Galen B, Kirkpatrick J, Lanspa M, Mulvagh S, Thamman R. ASE statement on point-of-care ultrasound (POCUS) during the 2019 novel coronavirus pandemic. J Am Soc Echocardiogr. 2020. https://doi.org/10.1016/j.echo.2020.04.017.
14. American Society of Hematology. COVID-19 and Pulmonary Embolism: Frequently Asked Questions. April 9, 2020. https://www.hematology.org/covid-19/covid-19-and-pulmonary-embolism. Accessed April 10, 2020.
15. Fischer EA, Kinnear B, Sall D, et al. Hospitalist-Operated Compression Ultrasonography: a Point-of-Care Ultrasound Study (HOCUS-POCUS). J Gen Intern Med. 2019;34(10):2062-2067. https://doi.org/10.1007/s11606-019-05120-5.
16. Tavazzi G, Civardi L, Caneva L, Mongodi S, Mojoli F. Thrombotic events in SARS-CoV-2 patients: an urgent call for ultrasound screening. Intensive Care Med. 2020;1-3. https://doi.org/10.1007/s00134-020-06040-3.
17. Franco-Sadud R, Schnobrich D, Mathews BK, et al. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287.
18. Galen B, Baron S, Young S, Hall A, Berger-Spivack L, Southern W. Reducing peripherally inserted central catheters and midline catheters by training nurses in ultrasound-guided peripheral intravenous catheter placement. BMJ Qual Saf. 2020;29(3):245-249. https://doi.org/10.1136/bmjqs-2019-009923.
19. Gottlieb M, Holladay D, Peksa GD. Ultrasonography for the confirmation of endotracheal tube intubation: a systematic review and meta-analysis. Ann Emerg Med. 2018;72(6):627-636. https://doi.org/10.1016/j.annemergmed.2018.06.024.
20. Abramowicz J, Basseal J. WFUMB Position Statement: how to perform a safe ultrasound examination and clean equipment in the context of COVID-19. Ultrasound Med Biol. 2020. https://doi.org/10.1016/j.ultrasmedbio.2020.03.033.
© 2020 Society of Hospital Medicine
The Design and Evaluation of the Comprehensive Hospitalist Assessment and Mentorship with Portfolios (CHAMP) Ultrasound Program
Point-of-care ultrasound (POCUS) is a valuable tool to assist in the diagnosis and treatment of many common diseases.1-11 Its use has increased in clinical settings over the years, primarily because of more portable, economical, high-quality devices and training availability.12 POCUS improves procedural success and guides the diagnostic management of hospitalized patients.2,9-12 Literature details the training of medical students,13,14 residents,15-21 and providers in emergency medicine22 and critical care,23,24 as well as focused cardiac training with hospitalists.25-27 However, no literature exists describing a comprehensive longitudinal training program for hospitalists or skills retention.
This document details the hospital medicine department’s ultrasound training program from Regions Hospital, part of HealthPartners in Saint Paul, Minnesota, a large tertiary care medical center. We describe the development and effectiveness of the Comprehensive Hospitalist Assessment and Mentorship with Portfolios (CHAMP) Ultrasound Program. This approach is intended to support the development of POCUS training programs at other organizations.
The aim of the program was to build a comprehensive bedside ultrasound training paradigm for hospitalists. The primary objective of the study was to assess the program’s effect on skills over time. Secondary objectives were confidence ratings in the use of ultrasound and with various patient care realms (volume management, quality of physical exam, and ability to narrow the differential diagnosis). We hypothesized there would be higher retention of ultrasound skills in those who completed portfolios and/or monthly scanning sessions as well as increased confidence through all secondary outcome measures (see below).
MATERIALS AND METHODS
This was a retrospective descriptive report of hospitalists who entered the CHAMP Ultrasound Program. Study participants were providers from the 454-bed Regions Hospital in Saint Paul, Minnesota. The study was deemed exempt by the HealthPartners Institutional Review Board. Three discrete 3-day courses and two 1-day in-person courses held at the Regions Hospital Simulation Center (Saint Paul, Minnesota) were studied.
Program Description
In 2014, a working group was developed in the hospital medicine department to support the hospital-wide POCUS committee with a charter to provide standardized training for providers to complete credentialing.28 The goal of the hospital medicine ultrasound program was to establish the use of ultrasound by credentialed hospitalists into well-defined applications integrated into the practice of hospital medicine. Two providers were selected to lead the efforts and completed additional training through the American College of Chest Physicians (CHEST) Certificate of Completion Program.29 An overall director was designated with the responsibilities delineated in supplementary Appendix 1. This director provided leadership on group practice, protocols, and equipment, creating the organizational framework for success with the training program. The hospital medicine training program had a 3-day in-person component built off the CHEST Critical Care Ultrasonography Program.24 The curriculum was adapted from the American College of Chest Physicians/Société de Réanimation de Langue Française Statement on Competence in Critical Care Ultrasonography.30 See Table 1 for the components of the training program.
Online Modules
3-Day In-Person Course with Assessments
The 3-day course provided 6 hours of didactics, 8 hours of image interpretation, and 9 hours of hands-on instruction (supplementary Appendix 4). Hospitalists first attended a large group didactic, followed by divided groups in image interpretation and hands-on scanning.24
Didactics were provided in a room with a 2-screen set up. Providers used 1 screen to present primary content and the other for simultaneously scanning a human model.
Image interpretation sessions were interactive smaller group learning forums in which participants reviewed high-yield images related to the care of hospital medicine patients and received feedback. Approximately 45 videos with normal and abnormal findings were reviewed during each session.
The hands-on scanning component was accomplished with human models and a faculty-to-participant ratio between 1:2 and
Portfolios
Refresher Training: 1-Day In-Person Course with Assessments and Monthly Scanning Sessions (Optional)
Only hospitalists who completed the 3-day course were eligible to take the 1-day in-person refresher course (supplementary Appendix 5). The first half of the course incorporated scanning with live human models, while the second half of the course had scanning with hospitalized patients focusing on pathology (pleural effusion, hydronephrosis, reduced left ventricular function, etc.). The course was offered at 3, 6, and 12 months after the initial 3-day course.
Monthly scanning sessions occurred for 2 hours every third Friday and were also available prior to the 1-day refresher. The first 90 minutes had a hands-on scanning component with hospitalized patients with faculty supervision (1:2 ratio). The last 30 minutes had an image interpretation component.
Assessments
Knowledge and skills assessment were adapted from the CHEST model (supplementary Appendix 6).24 Before and after the 3-day and 1-day in-person courses, the same hands-on skills assessment with a checklist was provided (supplementary Appendix 7). Before and after the 3-day course, a written knowledge assessment with case-based image interpretation was provided (supplementary Appendix 6).
Measurement
Participant demographic and clinical information was collected at the initial 3-day course for all participants, including age, gender, specialty, years of experience, and number and type of ultrasound procedures personally conducted or supervised in the past year. For skills assessment, a 20-item dichotomous checklist was developed and scored as done correctly or not done/done incorrectly. This same assessment was provided both before and after each of the 3-day and 1-day courses. A 20-question image-based knowledge assessment was also developed and administered both before and after the 3-day course only. The same 20-item checklist was used for the final skills examination. However, a new more detailed 50-question examination was written for the final examination after the portfolio of images was complete. Self-reported measures were confidence in the use of ultrasound, volume management, quality of physical exam, and ability to narrow the differential diagnosis. Confidence in ultrasound use, confidence in volume management, and quality of physical exam were assessed by using a questionnaire both before and after the 3-day course and 1-day course. Participants rated confidence and quality on a 5-point scale, 1 being least confident and 5 being most confident.
Statistical Analysis
Demographics of the included hospitalist population and pre and post 3-day assessments, including knowledge score, skills score, confidence in ultrasound use, confidence in volume management, and quality of physical exam, were summarized. Values for all assessment variables are presented as percentages. Confidence scores were reported as a percentage of the Likert scale (eg, 4/5 was reported as 80%). Skills and written examinations were expressed as percentages of items correct. Data were reported as median and interquartile range or means and standard deviation based on variable distributions. Differences between pre- and postvalues for 3-day course variables were assessed by using 2-sample paired Wilcoxon signed rank tests with a 95% confidence level.
For the subset of hospitalists who also completed the 1-day course, pre and post 1-day course assessments, including skills score, confidence in ultrasound use, confidence in volume management, and quality of physical exam, were summarized. Differences between pre- and postvalues for 1-day assessment variables were assessed by using 2-sample paired Wilcoxon signed rank tests with a 95% confidence level.
Multiple linear regression was performed with the change in skills assessment score from postcompletion of the 3-day course to precompletion of the 1-day course as the dependent variable. Hospitalists were split into 2 age groups (30-39 and 40-49) for the purpose of this analysis. The percent of monthly scanning sessions attended, age category, timing of 1-day course, and percent portfolio were assessed as possible predictors of the skills score by using simple linear regression with a P = .05 cutoff. A final model was chosen based on predictors significant in simple linear regression and included the percent of the portfolio completed and attendance of monthly scanning sessions.
RESULTS
Demographics
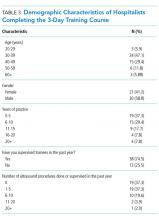
3-Day In-Person Course
For the 53 hospitalists who completed skills-based assessments, performance increased significantly after the 3-day course. Knowledge scores also increased significantly from preassessment to postassessment. Self-reported confidence ratings for ultrasound use, confidence in volume management, and quality of physical exam all increased significantly from preassessment to postassessment (Table 2).
Refresher Training: 1-Day In-Person Course
Because the refresher training was encouraged but not required, only 25 of 53 hospitalists, 23 with complete data, completed the 1-day course. For the 23 hospitalists who completed skills-based assessments before and after the 1-day course, mean skills scores increased significantly (Table 2). Self-reported confidence ratings for ultrasound use, confidence in volume management, and quality of physical exam all increased significantly from preassessment to postassessment (Table 2).
Monthly Scanning Sessions and Portfolio Development
The skills retention from initial course to refresher course by portfolio completion and monthly scanning sessions is shown in Table 2. Multiple regression analysis showed that for every 10% increase in the percent of monthly sessions attended, the mean change in skills score was 3.7% (P = .017), and for every 10% increase in the percent of portfolio completed, the mean change in skills score was 2.5% (P = .04), showing that both monthly scanning session attendance and portfolio completion are significantly predictive of skills retention over time.
Final Assessments
DISCUSSION
This is the first description of a successful longitudinal training program with assessments in POCUS for hospital medicine providers that shows an increase in skill retention with the use of a follow-up course and bedside scanning.
The CHAMP Ultrasound Program was developed to provide hospital medicine clinicians with a specialty focused in-house training pathway in POCUS and to assist in sustained skills acquisition by providing opportunities for regular feedback and practice. Practice with regular expert feedback is a critical aspect to develop and maintain skills in POCUS.32,33 Arntfield34 described the utility of remote supervision with feedback for ultrasound training in critical care, which demonstrated varying learning curves in the submission of portfolio images.35,36 The CHAMP Ultrasound training program provided expert oversight, longitudinal supervision, and feedback for course participants. The educational method of mastery learning was employed by setting minimum standards and allowing learners to practice until they met that standard.37-39
This unique program is made possible by the availability of expert-level faculty. Assessment scores improved with an initial 3-day course; however, they also decayed over time, most prominently with hospitalists that did not continue with POCUS scanning after their initial course. Ironically, those who performed more ultrasounds in the year prior to beginning the 3-day course had lower confidence ratings, likely explained by their awareness of their limitations and opportunities for improvement. The incorporation of refresher training to supplement the core 3-day course and portfolio development are key additions that differentiate this training program. These additions and the demonstration of successful training make this a durable pathway for other hospitalist programs. There are many workshops and short courses for medical students, residents, and practicing providers in POCUS.40-43 However, without an opportunity for longitudinal supervision and feedback, there is a noted decrease in the skills for participants. The refresher training with its 2 components (1-day in-person course and monthly scanning sessions) provides evidence of the value of mentored training.
In the initial program development, refresher training was encouraged but optional. We intentionally tracked those that completed refresher training compared with those that did not. Based on the results showing significant skills retention among those attending some form of refresher training, the program is planned to change to make this a requirement. We recommend refresher training within 12 months of the initial introductory course. There were several hospitalists that were unable to accommodate taking a full-day refresher course and, therefore, monthly scanning sessions were provided as an alternative.
The main limitation of the study is that it was completed in a single hospital system with available training mentors in POCUS. This gave us the ability to perform longitudinal training but may make this less reproducible in other hospital systems. Another limitation is that our course participants did not complete the pre- and postknowledge assessments for the refresher training components of the program, though they did for the initial 3-day course. Our pre- and postassessments have not been externally shown to produce valid data, though they are based on the already validated CHEST ultrasound data.44
Finally, our CHAMP Ultrasound Program required a significant time commitment by both faculty and learners. A relatively small percentage of hospitalists have completed the final assessments. The reasons are multifactorial, including program rigor, desire by certain hospitalists to know the basics but not pursue more expertise, and the challenges of developing a skillset that takes dedicated practice over time. We have aimed to address these barriers by providing additional hands-on scanning opportunities, giving timely feedback with portfolios, and obtaining more ultrasound machines. We expect more hospitalists to complete the final assessments in the coming year as evidenced by portfolio submissions to the shared online portal and many choosing to attend either the monthly scanning sessions and/or the 1-day course. We recognize that other institutions may need to adapt our program to suit their local environment.
CONCLUSION
A comprehensive longitudinal ultrasound training program including competency assessments significantly improved ultrasound acquisition skills with hospitalists. Those attending monthly scanning sessions and participating in the portfolio completion as well as a refresher course significantly retained and augmented their skills.
Acknowledgments
The authors would like to acknowledge Kelly Logue, Jason Robertson, MD, Jerome Siy, MD, Shauna Baer, and Jack Dressen for their support in the development and implementation of the POCUS program in hospital medicine.
Disclosure
The authors do not have any relevant financial disclosures to report.
1. Spevack R, Al Shukairi M, Jayaraman D, Dankoff J, Rudski L, Lipes J. Serial lung and IVC ultrasound in the assessment of congestive heart failure. Crit Ultrasound J. 2017;9:7-13. PubMed
2. Soni NJ, Franco R, Velez M, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015 Dec;10(12):811-816. PubMed
3. Boyd JH, Sirounis D, Maizel J, Slama M. Echocardiography as a guide for fluid management. Crit Care. 2016;20(1):274-280. PubMed
4. Mantuani D, Frazee BW, Fahimi J, Nagdev A. Point-of-care multi-organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. West J Emerg Med. 2016;17(1):46-53. PubMed
5. Glockner E, Christ M, Geier F, et al. Accuracy of Point-of-Care B-Line Lung Ultrasound in Comparison to NT-ProBNP for Screening Acute Heart Failure. Ultrasound Int Open. 2016;2(3):E90-E92. PubMed
6. Bhagra A, Tierney DM, Sekiguchi H, Soni NH. Point-of-Care Ultrasonography for Primary Care Physicians and General Internists. Mayo Clin Proc. 2016 Dec;91(12):1811-1827. PubMed
7. Crisp JG, Lovato LM, Jang TB. Compression ultrasonography of the lower extremity with portable vascular ultrasonography can accurately detect deep venous thrombosis in the emergency department. Ann Emerg Med. 2010;56(6):601-610. PubMed
8. Squire BT, Fox JC, Anderson C. ABSCESS: Applied bedside sonography for convenient. Evaluation of superficial soft tissue infections. Acad Emerg Med. 2005;12(7):601-606. PubMed
9. Narasimhan M, Koenig SJ, Mayo PH. A Whole-Body Approach to Point of Care Ultrasound. Chest. 2016;150(4):772-776. PubMed
10. Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16-25. PubMed
11. Soni NJ, Arntfield R, Kory P. Point of Care Ultrasound. Philadelphia: Elsevier Saunders; 2015.
12. Moore CL, Copel JA. Point-of-Care Ultrasonography. N Engl J Med. 2011;364(8):749-757. PubMed
13. Rempell JS, Saldana F, DiSalvo D, et al. Pilot Point-of-Care Ultrasound Curriculum at Harvard Medical School: Early Experience. West J Emerg Med. 2016;17(6):734-740. doi:10.5811/westjem.2016.8.31387. PubMed
14. Heiberg J, Hansen LS, Wemmelund K, et al. Point-of-Care Clinical Ultrasound for Medical Students. Ultrasound Int Open. 2015;1(2):E58-E66. doi:10.1055/s-0035-1565173. PubMed
15. Razi R, Estrada JR, Doll J, Spencer KT. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24(12):1319-1324. PubMed
16. Alexander JH, Peterson ED, Chen AY, Harding TM, Adams DB, Kisslo JA Jr. Feasibility of point-of-care echocardiography by internal medicine house staff. Am Heart J. 2004;147(3):476-481. PubMed
17. Hellmann DB, Whiting-O’Keefe Q, Shapiro EP, Martin LD, Martire C, Ziegelstein RC. The rate at which residents learn to use hand-held echocardiography at the bedside. Am J Med. 2005;118(9):1010-1018. PubMed
18. Kimura BJ, Amundson SA, Phan JN, Agan DL, Shaw DJ. Observations during development of an internal medicine residency training program in cardiovascular limited ultrasound examination. J Hosp Med. 2012;7(7):537-542. PubMed
19. Akhtar S, Theodoro D, Gaspari R, et al. Resident training in emergency ultrasound: consensus recommendations from the 2008 Council of Emergency Medicine Residency Directors Conference. Acad Emerg Med. 2009;16(s2):S32-S36. PubMed
, , , , , . Can emergency medicine residents detect acute deep venous thrombosis with a limited, two-site ultrasound examination? J Emerg Med. 2007;32(2):197-200. PubMed
, , , . Resident-performed compression ultrasonography for the detection of proximal deep vein thrombosis: fast and accurate. Acad Emerg Med. 2004;11(3):319-322. PubMed
22. Mandavia D, Aragona J, Chan L, et al. Ultrasound training for emergency physicians—a prospective study. Acad Emerg Med. 2000;7(9):1008-1014. PubMed
23. Koenig SJ, Narasimhan M, Mayo PH. Thoracic ultrasonography for the pulmonary specialist. Chest. 2011;140(5):1332-1341. doi: 10.1378/chest.11-0348. PubMed
24. Greenstein YY, Littauer R, Narasimhan M, Mayo PH, Koenig SJ. Effectiveness of a Critical Care Ultrasonography Course. Chest. 2017;151(1):34-40. doi:10.1016/j.chest.2016.08.1465. PubMed
25. Martin LD, Howell EE, Ziegelstein RC, Martire C, Shapiro EP, Hellmann DB. Hospitalist performance of cardiac hand-carried ultrasound after focused training. Am J Med. 2007;120(11):1000-1004. PubMed
26. Martin LD, Howell EE, Ziegelstein RC, et al.
27. Lucas BP, Candotti C, Margeta B, et al. Diagnostic accuracy of hospitalist-performed hand-carried ultrasound echocardiography after a brief training program. J Hosp Med. 2009;4(6):340-349. PubMed
28.
29. Critical Care Ultrasonography Certificate of Completion Program. American College of Chest Physicians. http://www.chestnet.org/Education/Advanced-Clinical-Training/Certificate-of-Completion-Program/Critical-Care-Ultrasonography. Accessed March 30, 2017
30. Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/Société de Réanimation de Langue Française statement on competence in critical care ultrasonography. Chest. 2009;135(4):1050-1060. PubMed
31. Donlon TF, Angoff WH. The scholastic aptitude test. The College Board Admissions Testing Program; 1971:15-47.
32. Ericsson KA, Lehmann AC. Expert and exceptional performance: Evidence of maximal adaptation to task constraints. Annu Rev Psychol. 1996;47:273-305. PubMed
33. Ericcson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100(3):363-406.
34. Arntfield RT. The utility of remote supervision with feedback as a method to deliver high-volume critical care ultrasound training. J Crit Care. 2015;30(2):441.e1-e6. PubMed
35. Ma OJ, Gaddis G, Norvell JG, Subramanian S. How fast is the focused assessment with sonography for trauma examination learning curve? Emerg Med Australas. 2008;20(1):32-37. PubMed
36. Gaspari RJ, Dickman E, Blehar D. Learning curve of bedside ultrasound of the gallbladder. J Emerg Med. 2009;37(1):51-66. doi:10.1016/j.jemermed.2007.10.070. PubMed
37. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wane DB. Use of simulation-based mastery learning to improve quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009:4(7):397-403. PubMed
38. McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. A critical review of simulation-based mastery learning with translational outcomes. Med Educ. 2014:48(4):375-385. PubMed
39. Guskey TR. The essential elements of mastery learning. J Classroom Interac. 1987;22:19-22.
40. Ultrasound Institute. Introduction to Primary Care Ultrasound. University of South Carolina School of Medicine. http://ultrasoundinstitute.med.sc.edu/UIcme.asp. Accessed October 24, 2017.
41. Society of Critical Care Medicine. Live Critical Care Ultrasound: Adult. http://www.sccm.org/Education-Center/Ultrasound/Pages/Fundamentals.aspx. Accessed October 24, 2017.
42. Castlefest Ultrasound Event. Castlefest 2018. http://castlefest2018.com/. Accessed October 24, 2017.
43. Office of Continuing Medical Education. Point of Care Ultrasound Workshop. UT Health San Antonio Joe R. & Teresa Lozano Long School of Medicine. http://cme.uthscsa.edu/ultrasound.asp. Accessed October 24, 2017.
44. Patrawalla P, Eisen LA, Shiloh A, et al. Development and Validation of an Assessment Tool for Competency in Critical Care Ultrasound. J Grad Med Educ. 2015;7(4):567-573. PubMed
Point-of-care ultrasound (POCUS) is a valuable tool to assist in the diagnosis and treatment of many common diseases.1-11 Its use has increased in clinical settings over the years, primarily because of more portable, economical, high-quality devices and training availability.12 POCUS improves procedural success and guides the diagnostic management of hospitalized patients.2,9-12 Literature details the training of medical students,13,14 residents,15-21 and providers in emergency medicine22 and critical care,23,24 as well as focused cardiac training with hospitalists.25-27 However, no literature exists describing a comprehensive longitudinal training program for hospitalists or skills retention.
This document details the hospital medicine department’s ultrasound training program from Regions Hospital, part of HealthPartners in Saint Paul, Minnesota, a large tertiary care medical center. We describe the development and effectiveness of the Comprehensive Hospitalist Assessment and Mentorship with Portfolios (CHAMP) Ultrasound Program. This approach is intended to support the development of POCUS training programs at other organizations.
The aim of the program was to build a comprehensive bedside ultrasound training paradigm for hospitalists. The primary objective of the study was to assess the program’s effect on skills over time. Secondary objectives were confidence ratings in the use of ultrasound and with various patient care realms (volume management, quality of physical exam, and ability to narrow the differential diagnosis). We hypothesized there would be higher retention of ultrasound skills in those who completed portfolios and/or monthly scanning sessions as well as increased confidence through all secondary outcome measures (see below).
MATERIALS AND METHODS
This was a retrospective descriptive report of hospitalists who entered the CHAMP Ultrasound Program. Study participants were providers from the 454-bed Regions Hospital in Saint Paul, Minnesota. The study was deemed exempt by the HealthPartners Institutional Review Board. Three discrete 3-day courses and two 1-day in-person courses held at the Regions Hospital Simulation Center (Saint Paul, Minnesota) were studied.
Program Description
In 2014, a working group was developed in the hospital medicine department to support the hospital-wide POCUS committee with a charter to provide standardized training for providers to complete credentialing.28 The goal of the hospital medicine ultrasound program was to establish the use of ultrasound by credentialed hospitalists into well-defined applications integrated into the practice of hospital medicine. Two providers were selected to lead the efforts and completed additional training through the American College of Chest Physicians (CHEST) Certificate of Completion Program.29 An overall director was designated with the responsibilities delineated in supplementary Appendix 1. This director provided leadership on group practice, protocols, and equipment, creating the organizational framework for success with the training program. The hospital medicine training program had a 3-day in-person component built off the CHEST Critical Care Ultrasonography Program.24 The curriculum was adapted from the American College of Chest Physicians/Société de Réanimation de Langue Française Statement on Competence in Critical Care Ultrasonography.30 See Table 1 for the components of the training program.
Online Modules
3-Day In-Person Course with Assessments
The 3-day course provided 6 hours of didactics, 8 hours of image interpretation, and 9 hours of hands-on instruction (supplementary Appendix 4). Hospitalists first attended a large group didactic, followed by divided groups in image interpretation and hands-on scanning.24
Didactics were provided in a room with a 2-screen set up. Providers used 1 screen to present primary content and the other for simultaneously scanning a human model.
Image interpretation sessions were interactive smaller group learning forums in which participants reviewed high-yield images related to the care of hospital medicine patients and received feedback. Approximately 45 videos with normal and abnormal findings were reviewed during each session.
The hands-on scanning component was accomplished with human models and a faculty-to-participant ratio between 1:2 and
Portfolios
Refresher Training: 1-Day In-Person Course with Assessments and Monthly Scanning Sessions (Optional)
Only hospitalists who completed the 3-day course were eligible to take the 1-day in-person refresher course (supplementary Appendix 5). The first half of the course incorporated scanning with live human models, while the second half of the course had scanning with hospitalized patients focusing on pathology (pleural effusion, hydronephrosis, reduced left ventricular function, etc.). The course was offered at 3, 6, and 12 months after the initial 3-day course.
Monthly scanning sessions occurred for 2 hours every third Friday and were also available prior to the 1-day refresher. The first 90 minutes had a hands-on scanning component with hospitalized patients with faculty supervision (1:2 ratio). The last 30 minutes had an image interpretation component.
Assessments
Knowledge and skills assessment were adapted from the CHEST model (supplementary Appendix 6).24 Before and after the 3-day and 1-day in-person courses, the same hands-on skills assessment with a checklist was provided (supplementary Appendix 7). Before and after the 3-day course, a written knowledge assessment with case-based image interpretation was provided (supplementary Appendix 6).
Measurement
Participant demographic and clinical information was collected at the initial 3-day course for all participants, including age, gender, specialty, years of experience, and number and type of ultrasound procedures personally conducted or supervised in the past year. For skills assessment, a 20-item dichotomous checklist was developed and scored as done correctly or not done/done incorrectly. This same assessment was provided both before and after each of the 3-day and 1-day courses. A 20-question image-based knowledge assessment was also developed and administered both before and after the 3-day course only. The same 20-item checklist was used for the final skills examination. However, a new more detailed 50-question examination was written for the final examination after the portfolio of images was complete. Self-reported measures were confidence in the use of ultrasound, volume management, quality of physical exam, and ability to narrow the differential diagnosis. Confidence in ultrasound use, confidence in volume management, and quality of physical exam were assessed by using a questionnaire both before and after the 3-day course and 1-day course. Participants rated confidence and quality on a 5-point scale, 1 being least confident and 5 being most confident.
Statistical Analysis
Demographics of the included hospitalist population and pre and post 3-day assessments, including knowledge score, skills score, confidence in ultrasound use, confidence in volume management, and quality of physical exam, were summarized. Values for all assessment variables are presented as percentages. Confidence scores were reported as a percentage of the Likert scale (eg, 4/5 was reported as 80%). Skills and written examinations were expressed as percentages of items correct. Data were reported as median and interquartile range or means and standard deviation based on variable distributions. Differences between pre- and postvalues for 3-day course variables were assessed by using 2-sample paired Wilcoxon signed rank tests with a 95% confidence level.
For the subset of hospitalists who also completed the 1-day course, pre and post 1-day course assessments, including skills score, confidence in ultrasound use, confidence in volume management, and quality of physical exam, were summarized. Differences between pre- and postvalues for 1-day assessment variables were assessed by using 2-sample paired Wilcoxon signed rank tests with a 95% confidence level.
Multiple linear regression was performed with the change in skills assessment score from postcompletion of the 3-day course to precompletion of the 1-day course as the dependent variable. Hospitalists were split into 2 age groups (30-39 and 40-49) for the purpose of this analysis. The percent of monthly scanning sessions attended, age category, timing of 1-day course, and percent portfolio were assessed as possible predictors of the skills score by using simple linear regression with a P = .05 cutoff. A final model was chosen based on predictors significant in simple linear regression and included the percent of the portfolio completed and attendance of monthly scanning sessions.
RESULTS
Demographics
3-Day In-Person Course
For the 53 hospitalists who completed skills-based assessments, performance increased significantly after the 3-day course. Knowledge scores also increased significantly from preassessment to postassessment. Self-reported confidence ratings for ultrasound use, confidence in volume management, and quality of physical exam all increased significantly from preassessment to postassessment (Table 2).
Refresher Training: 1-Day In-Person Course
Because the refresher training was encouraged but not required, only 25 of 53 hospitalists, 23 with complete data, completed the 1-day course. For the 23 hospitalists who completed skills-based assessments before and after the 1-day course, mean skills scores increased significantly (Table 2). Self-reported confidence ratings for ultrasound use, confidence in volume management, and quality of physical exam all increased significantly from preassessment to postassessment (Table 2).
Monthly Scanning Sessions and Portfolio Development
The skills retention from initial course to refresher course by portfolio completion and monthly scanning sessions is shown in Table 2. Multiple regression analysis showed that for every 10% increase in the percent of monthly sessions attended, the mean change in skills score was 3.7% (P = .017), and for every 10% increase in the percent of portfolio completed, the mean change in skills score was 2.5% (P = .04), showing that both monthly scanning session attendance and portfolio completion are significantly predictive of skills retention over time.
Final Assessments
DISCUSSION
This is the first description of a successful longitudinal training program with assessments in POCUS for hospital medicine providers that shows an increase in skill retention with the use of a follow-up course and bedside scanning.
The CHAMP Ultrasound Program was developed to provide hospital medicine clinicians with a specialty focused in-house training pathway in POCUS and to assist in sustained skills acquisition by providing opportunities for regular feedback and practice. Practice with regular expert feedback is a critical aspect to develop and maintain skills in POCUS.32,33 Arntfield34 described the utility of remote supervision with feedback for ultrasound training in critical care, which demonstrated varying learning curves in the submission of portfolio images.35,36 The CHAMP Ultrasound training program provided expert oversight, longitudinal supervision, and feedback for course participants. The educational method of mastery learning was employed by setting minimum standards and allowing learners to practice until they met that standard.37-39
This unique program is made possible by the availability of expert-level faculty. Assessment scores improved with an initial 3-day course; however, they also decayed over time, most prominently with hospitalists that did not continue with POCUS scanning after their initial course. Ironically, those who performed more ultrasounds in the year prior to beginning the 3-day course had lower confidence ratings, likely explained by their awareness of their limitations and opportunities for improvement. The incorporation of refresher training to supplement the core 3-day course and portfolio development are key additions that differentiate this training program. These additions and the demonstration of successful training make this a durable pathway for other hospitalist programs. There are many workshops and short courses for medical students, residents, and practicing providers in POCUS.40-43 However, without an opportunity for longitudinal supervision and feedback, there is a noted decrease in the skills for participants. The refresher training with its 2 components (1-day in-person course and monthly scanning sessions) provides evidence of the value of mentored training.
In the initial program development, refresher training was encouraged but optional. We intentionally tracked those that completed refresher training compared with those that did not. Based on the results showing significant skills retention among those attending some form of refresher training, the program is planned to change to make this a requirement. We recommend refresher training within 12 months of the initial introductory course. There were several hospitalists that were unable to accommodate taking a full-day refresher course and, therefore, monthly scanning sessions were provided as an alternative.
The main limitation of the study is that it was completed in a single hospital system with available training mentors in POCUS. This gave us the ability to perform longitudinal training but may make this less reproducible in other hospital systems. Another limitation is that our course participants did not complete the pre- and postknowledge assessments for the refresher training components of the program, though they did for the initial 3-day course. Our pre- and postassessments have not been externally shown to produce valid data, though they are based on the already validated CHEST ultrasound data.44
Finally, our CHAMP Ultrasound Program required a significant time commitment by both faculty and learners. A relatively small percentage of hospitalists have completed the final assessments. The reasons are multifactorial, including program rigor, desire by certain hospitalists to know the basics but not pursue more expertise, and the challenges of developing a skillset that takes dedicated practice over time. We have aimed to address these barriers by providing additional hands-on scanning opportunities, giving timely feedback with portfolios, and obtaining more ultrasound machines. We expect more hospitalists to complete the final assessments in the coming year as evidenced by portfolio submissions to the shared online portal and many choosing to attend either the monthly scanning sessions and/or the 1-day course. We recognize that other institutions may need to adapt our program to suit their local environment.
CONCLUSION
A comprehensive longitudinal ultrasound training program including competency assessments significantly improved ultrasound acquisition skills with hospitalists. Those attending monthly scanning sessions and participating in the portfolio completion as well as a refresher course significantly retained and augmented their skills.
Acknowledgments
The authors would like to acknowledge Kelly Logue, Jason Robertson, MD, Jerome Siy, MD, Shauna Baer, and Jack Dressen for their support in the development and implementation of the POCUS program in hospital medicine.
Disclosure
The authors do not have any relevant financial disclosures to report.
Point-of-care ultrasound (POCUS) is a valuable tool to assist in the diagnosis and treatment of many common diseases.1-11 Its use has increased in clinical settings over the years, primarily because of more portable, economical, high-quality devices and training availability.12 POCUS improves procedural success and guides the diagnostic management of hospitalized patients.2,9-12 Literature details the training of medical students,13,14 residents,15-21 and providers in emergency medicine22 and critical care,23,24 as well as focused cardiac training with hospitalists.25-27 However, no literature exists describing a comprehensive longitudinal training program for hospitalists or skills retention.
This document details the hospital medicine department’s ultrasound training program from Regions Hospital, part of HealthPartners in Saint Paul, Minnesota, a large tertiary care medical center. We describe the development and effectiveness of the Comprehensive Hospitalist Assessment and Mentorship with Portfolios (CHAMP) Ultrasound Program. This approach is intended to support the development of POCUS training programs at other organizations.
The aim of the program was to build a comprehensive bedside ultrasound training paradigm for hospitalists. The primary objective of the study was to assess the program’s effect on skills over time. Secondary objectives were confidence ratings in the use of ultrasound and with various patient care realms (volume management, quality of physical exam, and ability to narrow the differential diagnosis). We hypothesized there would be higher retention of ultrasound skills in those who completed portfolios and/or monthly scanning sessions as well as increased confidence through all secondary outcome measures (see below).
MATERIALS AND METHODS
This was a retrospective descriptive report of hospitalists who entered the CHAMP Ultrasound Program. Study participants were providers from the 454-bed Regions Hospital in Saint Paul, Minnesota. The study was deemed exempt by the HealthPartners Institutional Review Board. Three discrete 3-day courses and two 1-day in-person courses held at the Regions Hospital Simulation Center (Saint Paul, Minnesota) were studied.
Program Description
In 2014, a working group was developed in the hospital medicine department to support the hospital-wide POCUS committee with a charter to provide standardized training for providers to complete credentialing.28 The goal of the hospital medicine ultrasound program was to establish the use of ultrasound by credentialed hospitalists into well-defined applications integrated into the practice of hospital medicine. Two providers were selected to lead the efforts and completed additional training through the American College of Chest Physicians (CHEST) Certificate of Completion Program.29 An overall director was designated with the responsibilities delineated in supplementary Appendix 1. This director provided leadership on group practice, protocols, and equipment, creating the organizational framework for success with the training program. The hospital medicine training program had a 3-day in-person component built off the CHEST Critical Care Ultrasonography Program.24 The curriculum was adapted from the American College of Chest Physicians/Société de Réanimation de Langue Française Statement on Competence in Critical Care Ultrasonography.30 See Table 1 for the components of the training program.
Online Modules
3-Day In-Person Course with Assessments
The 3-day course provided 6 hours of didactics, 8 hours of image interpretation, and 9 hours of hands-on instruction (supplementary Appendix 4). Hospitalists first attended a large group didactic, followed by divided groups in image interpretation and hands-on scanning.24
Didactics were provided in a room with a 2-screen set up. Providers used 1 screen to present primary content and the other for simultaneously scanning a human model.
Image interpretation sessions were interactive smaller group learning forums in which participants reviewed high-yield images related to the care of hospital medicine patients and received feedback. Approximately 45 videos with normal and abnormal findings were reviewed during each session.
The hands-on scanning component was accomplished with human models and a faculty-to-participant ratio between 1:2 and
Portfolios
Refresher Training: 1-Day In-Person Course with Assessments and Monthly Scanning Sessions (Optional)
Only hospitalists who completed the 3-day course were eligible to take the 1-day in-person refresher course (supplementary Appendix 5). The first half of the course incorporated scanning with live human models, while the second half of the course had scanning with hospitalized patients focusing on pathology (pleural effusion, hydronephrosis, reduced left ventricular function, etc.). The course was offered at 3, 6, and 12 months after the initial 3-day course.
Monthly scanning sessions occurred for 2 hours every third Friday and were also available prior to the 1-day refresher. The first 90 minutes had a hands-on scanning component with hospitalized patients with faculty supervision (1:2 ratio). The last 30 minutes had an image interpretation component.
Assessments
Knowledge and skills assessment were adapted from the CHEST model (supplementary Appendix 6).24 Before and after the 3-day and 1-day in-person courses, the same hands-on skills assessment with a checklist was provided (supplementary Appendix 7). Before and after the 3-day course, a written knowledge assessment with case-based image interpretation was provided (supplementary Appendix 6).
Measurement
Participant demographic and clinical information was collected at the initial 3-day course for all participants, including age, gender, specialty, years of experience, and number and type of ultrasound procedures personally conducted or supervised in the past year. For skills assessment, a 20-item dichotomous checklist was developed and scored as done correctly or not done/done incorrectly. This same assessment was provided both before and after each of the 3-day and 1-day courses. A 20-question image-based knowledge assessment was also developed and administered both before and after the 3-day course only. The same 20-item checklist was used for the final skills examination. However, a new more detailed 50-question examination was written for the final examination after the portfolio of images was complete. Self-reported measures were confidence in the use of ultrasound, volume management, quality of physical exam, and ability to narrow the differential diagnosis. Confidence in ultrasound use, confidence in volume management, and quality of physical exam were assessed by using a questionnaire both before and after the 3-day course and 1-day course. Participants rated confidence and quality on a 5-point scale, 1 being least confident and 5 being most confident.
Statistical Analysis
Demographics of the included hospitalist population and pre and post 3-day assessments, including knowledge score, skills score, confidence in ultrasound use, confidence in volume management, and quality of physical exam, were summarized. Values for all assessment variables are presented as percentages. Confidence scores were reported as a percentage of the Likert scale (eg, 4/5 was reported as 80%). Skills and written examinations were expressed as percentages of items correct. Data were reported as median and interquartile range or means and standard deviation based on variable distributions. Differences between pre- and postvalues for 3-day course variables were assessed by using 2-sample paired Wilcoxon signed rank tests with a 95% confidence level.
For the subset of hospitalists who also completed the 1-day course, pre and post 1-day course assessments, including skills score, confidence in ultrasound use, confidence in volume management, and quality of physical exam, were summarized. Differences between pre- and postvalues for 1-day assessment variables were assessed by using 2-sample paired Wilcoxon signed rank tests with a 95% confidence level.
Multiple linear regression was performed with the change in skills assessment score from postcompletion of the 3-day course to precompletion of the 1-day course as the dependent variable. Hospitalists were split into 2 age groups (30-39 and 40-49) for the purpose of this analysis. The percent of monthly scanning sessions attended, age category, timing of 1-day course, and percent portfolio were assessed as possible predictors of the skills score by using simple linear regression with a P = .05 cutoff. A final model was chosen based on predictors significant in simple linear regression and included the percent of the portfolio completed and attendance of monthly scanning sessions.
RESULTS
Demographics
3-Day In-Person Course
For the 53 hospitalists who completed skills-based assessments, performance increased significantly after the 3-day course. Knowledge scores also increased significantly from preassessment to postassessment. Self-reported confidence ratings for ultrasound use, confidence in volume management, and quality of physical exam all increased significantly from preassessment to postassessment (Table 2).
Refresher Training: 1-Day In-Person Course
Because the refresher training was encouraged but not required, only 25 of 53 hospitalists, 23 with complete data, completed the 1-day course. For the 23 hospitalists who completed skills-based assessments before and after the 1-day course, mean skills scores increased significantly (Table 2). Self-reported confidence ratings for ultrasound use, confidence in volume management, and quality of physical exam all increased significantly from preassessment to postassessment (Table 2).
Monthly Scanning Sessions and Portfolio Development
The skills retention from initial course to refresher course by portfolio completion and monthly scanning sessions is shown in Table 2. Multiple regression analysis showed that for every 10% increase in the percent of monthly sessions attended, the mean change in skills score was 3.7% (P = .017), and for every 10% increase in the percent of portfolio completed, the mean change in skills score was 2.5% (P = .04), showing that both monthly scanning session attendance and portfolio completion are significantly predictive of skills retention over time.
Final Assessments
DISCUSSION
This is the first description of a successful longitudinal training program with assessments in POCUS for hospital medicine providers that shows an increase in skill retention with the use of a follow-up course and bedside scanning.
The CHAMP Ultrasound Program was developed to provide hospital medicine clinicians with a specialty focused in-house training pathway in POCUS and to assist in sustained skills acquisition by providing opportunities for regular feedback and practice. Practice with regular expert feedback is a critical aspect to develop and maintain skills in POCUS.32,33 Arntfield34 described the utility of remote supervision with feedback for ultrasound training in critical care, which demonstrated varying learning curves in the submission of portfolio images.35,36 The CHAMP Ultrasound training program provided expert oversight, longitudinal supervision, and feedback for course participants. The educational method of mastery learning was employed by setting minimum standards and allowing learners to practice until they met that standard.37-39
This unique program is made possible by the availability of expert-level faculty. Assessment scores improved with an initial 3-day course; however, they also decayed over time, most prominently with hospitalists that did not continue with POCUS scanning after their initial course. Ironically, those who performed more ultrasounds in the year prior to beginning the 3-day course had lower confidence ratings, likely explained by their awareness of their limitations and opportunities for improvement. The incorporation of refresher training to supplement the core 3-day course and portfolio development are key additions that differentiate this training program. These additions and the demonstration of successful training make this a durable pathway for other hospitalist programs. There are many workshops and short courses for medical students, residents, and practicing providers in POCUS.40-43 However, without an opportunity for longitudinal supervision and feedback, there is a noted decrease in the skills for participants. The refresher training with its 2 components (1-day in-person course and monthly scanning sessions) provides evidence of the value of mentored training.
In the initial program development, refresher training was encouraged but optional. We intentionally tracked those that completed refresher training compared with those that did not. Based on the results showing significant skills retention among those attending some form of refresher training, the program is planned to change to make this a requirement. We recommend refresher training within 12 months of the initial introductory course. There were several hospitalists that were unable to accommodate taking a full-day refresher course and, therefore, monthly scanning sessions were provided as an alternative.
The main limitation of the study is that it was completed in a single hospital system with available training mentors in POCUS. This gave us the ability to perform longitudinal training but may make this less reproducible in other hospital systems. Another limitation is that our course participants did not complete the pre- and postknowledge assessments for the refresher training components of the program, though they did for the initial 3-day course. Our pre- and postassessments have not been externally shown to produce valid data, though they are based on the already validated CHEST ultrasound data.44
Finally, our CHAMP Ultrasound Program required a significant time commitment by both faculty and learners. A relatively small percentage of hospitalists have completed the final assessments. The reasons are multifactorial, including program rigor, desire by certain hospitalists to know the basics but not pursue more expertise, and the challenges of developing a skillset that takes dedicated practice over time. We have aimed to address these barriers by providing additional hands-on scanning opportunities, giving timely feedback with portfolios, and obtaining more ultrasound machines. We expect more hospitalists to complete the final assessments in the coming year as evidenced by portfolio submissions to the shared online portal and many choosing to attend either the monthly scanning sessions and/or the 1-day course. We recognize that other institutions may need to adapt our program to suit their local environment.
CONCLUSION
A comprehensive longitudinal ultrasound training program including competency assessments significantly improved ultrasound acquisition skills with hospitalists. Those attending monthly scanning sessions and participating in the portfolio completion as well as a refresher course significantly retained and augmented their skills.
Acknowledgments
The authors would like to acknowledge Kelly Logue, Jason Robertson, MD, Jerome Siy, MD, Shauna Baer, and Jack Dressen for their support in the development and implementation of the POCUS program in hospital medicine.
Disclosure
The authors do not have any relevant financial disclosures to report.
1. Spevack R, Al Shukairi M, Jayaraman D, Dankoff J, Rudski L, Lipes J. Serial lung and IVC ultrasound in the assessment of congestive heart failure. Crit Ultrasound J. 2017;9:7-13. PubMed
2. Soni NJ, Franco R, Velez M, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015 Dec;10(12):811-816. PubMed
3. Boyd JH, Sirounis D, Maizel J, Slama M. Echocardiography as a guide for fluid management. Crit Care. 2016;20(1):274-280. PubMed
4. Mantuani D, Frazee BW, Fahimi J, Nagdev A. Point-of-care multi-organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. West J Emerg Med. 2016;17(1):46-53. PubMed
5. Glockner E, Christ M, Geier F, et al. Accuracy of Point-of-Care B-Line Lung Ultrasound in Comparison to NT-ProBNP for Screening Acute Heart Failure. Ultrasound Int Open. 2016;2(3):E90-E92. PubMed
6. Bhagra A, Tierney DM, Sekiguchi H, Soni NH. Point-of-Care Ultrasonography for Primary Care Physicians and General Internists. Mayo Clin Proc. 2016 Dec;91(12):1811-1827. PubMed
7. Crisp JG, Lovato LM, Jang TB. Compression ultrasonography of the lower extremity with portable vascular ultrasonography can accurately detect deep venous thrombosis in the emergency department. Ann Emerg Med. 2010;56(6):601-610. PubMed
8. Squire BT, Fox JC, Anderson C. ABSCESS: Applied bedside sonography for convenient. Evaluation of superficial soft tissue infections. Acad Emerg Med. 2005;12(7):601-606. PubMed
9. Narasimhan M, Koenig SJ, Mayo PH. A Whole-Body Approach to Point of Care Ultrasound. Chest. 2016;150(4):772-776. PubMed
10. Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16-25. PubMed
11. Soni NJ, Arntfield R, Kory P. Point of Care Ultrasound. Philadelphia: Elsevier Saunders; 2015.
12. Moore CL, Copel JA. Point-of-Care Ultrasonography. N Engl J Med. 2011;364(8):749-757. PubMed
13. Rempell JS, Saldana F, DiSalvo D, et al. Pilot Point-of-Care Ultrasound Curriculum at Harvard Medical School: Early Experience. West J Emerg Med. 2016;17(6):734-740. doi:10.5811/westjem.2016.8.31387. PubMed
14. Heiberg J, Hansen LS, Wemmelund K, et al. Point-of-Care Clinical Ultrasound for Medical Students. Ultrasound Int Open. 2015;1(2):E58-E66. doi:10.1055/s-0035-1565173. PubMed
15. Razi R, Estrada JR, Doll J, Spencer KT. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24(12):1319-1324. PubMed
16. Alexander JH, Peterson ED, Chen AY, Harding TM, Adams DB, Kisslo JA Jr. Feasibility of point-of-care echocardiography by internal medicine house staff. Am Heart J. 2004;147(3):476-481. PubMed
17. Hellmann DB, Whiting-O’Keefe Q, Shapiro EP, Martin LD, Martire C, Ziegelstein RC. The rate at which residents learn to use hand-held echocardiography at the bedside. Am J Med. 2005;118(9):1010-1018. PubMed
18. Kimura BJ, Amundson SA, Phan JN, Agan DL, Shaw DJ. Observations during development of an internal medicine residency training program in cardiovascular limited ultrasound examination. J Hosp Med. 2012;7(7):537-542. PubMed
19. Akhtar S, Theodoro D, Gaspari R, et al. Resident training in emergency ultrasound: consensus recommendations from the 2008 Council of Emergency Medicine Residency Directors Conference. Acad Emerg Med. 2009;16(s2):S32-S36. PubMed
, , , , , . Can emergency medicine residents detect acute deep venous thrombosis with a limited, two-site ultrasound examination? J Emerg Med. 2007;32(2):197-200. PubMed
, , , . Resident-performed compression ultrasonography for the detection of proximal deep vein thrombosis: fast and accurate. Acad Emerg Med. 2004;11(3):319-322. PubMed
22. Mandavia D, Aragona J, Chan L, et al. Ultrasound training for emergency physicians—a prospective study. Acad Emerg Med. 2000;7(9):1008-1014. PubMed
23. Koenig SJ, Narasimhan M, Mayo PH. Thoracic ultrasonography for the pulmonary specialist. Chest. 2011;140(5):1332-1341. doi: 10.1378/chest.11-0348. PubMed
24. Greenstein YY, Littauer R, Narasimhan M, Mayo PH, Koenig SJ. Effectiveness of a Critical Care Ultrasonography Course. Chest. 2017;151(1):34-40. doi:10.1016/j.chest.2016.08.1465. PubMed
25. Martin LD, Howell EE, Ziegelstein RC, Martire C, Shapiro EP, Hellmann DB. Hospitalist performance of cardiac hand-carried ultrasound after focused training. Am J Med. 2007;120(11):1000-1004. PubMed
26. Martin LD, Howell EE, Ziegelstein RC, et al.
27. Lucas BP, Candotti C, Margeta B, et al. Diagnostic accuracy of hospitalist-performed hand-carried ultrasound echocardiography after a brief training program. J Hosp Med. 2009;4(6):340-349. PubMed
28.
29. Critical Care Ultrasonography Certificate of Completion Program. American College of Chest Physicians. http://www.chestnet.org/Education/Advanced-Clinical-Training/Certificate-of-Completion-Program/Critical-Care-Ultrasonography. Accessed March 30, 2017
30. Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/Société de Réanimation de Langue Française statement on competence in critical care ultrasonography. Chest. 2009;135(4):1050-1060. PubMed
31. Donlon TF, Angoff WH. The scholastic aptitude test. The College Board Admissions Testing Program; 1971:15-47.
32. Ericsson KA, Lehmann AC. Expert and exceptional performance: Evidence of maximal adaptation to task constraints. Annu Rev Psychol. 1996;47:273-305. PubMed
33. Ericcson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100(3):363-406.
34. Arntfield RT. The utility of remote supervision with feedback as a method to deliver high-volume critical care ultrasound training. J Crit Care. 2015;30(2):441.e1-e6. PubMed
35. Ma OJ, Gaddis G, Norvell JG, Subramanian S. How fast is the focused assessment with sonography for trauma examination learning curve? Emerg Med Australas. 2008;20(1):32-37. PubMed
36. Gaspari RJ, Dickman E, Blehar D. Learning curve of bedside ultrasound of the gallbladder. J Emerg Med. 2009;37(1):51-66. doi:10.1016/j.jemermed.2007.10.070. PubMed
37. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wane DB. Use of simulation-based mastery learning to improve quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009:4(7):397-403. PubMed
38. McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. A critical review of simulation-based mastery learning with translational outcomes. Med Educ. 2014:48(4):375-385. PubMed
39. Guskey TR. The essential elements of mastery learning. J Classroom Interac. 1987;22:19-22.
40. Ultrasound Institute. Introduction to Primary Care Ultrasound. University of South Carolina School of Medicine. http://ultrasoundinstitute.med.sc.edu/UIcme.asp. Accessed October 24, 2017.
41. Society of Critical Care Medicine. Live Critical Care Ultrasound: Adult. http://www.sccm.org/Education-Center/Ultrasound/Pages/Fundamentals.aspx. Accessed October 24, 2017.
42. Castlefest Ultrasound Event. Castlefest 2018. http://castlefest2018.com/. Accessed October 24, 2017.
43. Office of Continuing Medical Education. Point of Care Ultrasound Workshop. UT Health San Antonio Joe R. & Teresa Lozano Long School of Medicine. http://cme.uthscsa.edu/ultrasound.asp. Accessed October 24, 2017.
44. Patrawalla P, Eisen LA, Shiloh A, et al. Development and Validation of an Assessment Tool for Competency in Critical Care Ultrasound. J Grad Med Educ. 2015;7(4):567-573. PubMed
1. Spevack R, Al Shukairi M, Jayaraman D, Dankoff J, Rudski L, Lipes J. Serial lung and IVC ultrasound in the assessment of congestive heart failure. Crit Ultrasound J. 2017;9:7-13. PubMed
2. Soni NJ, Franco R, Velez M, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015 Dec;10(12):811-816. PubMed
3. Boyd JH, Sirounis D, Maizel J, Slama M. Echocardiography as a guide for fluid management. Crit Care. 2016;20(1):274-280. PubMed
4. Mantuani D, Frazee BW, Fahimi J, Nagdev A. Point-of-care multi-organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. West J Emerg Med. 2016;17(1):46-53. PubMed
5. Glockner E, Christ M, Geier F, et al. Accuracy of Point-of-Care B-Line Lung Ultrasound in Comparison to NT-ProBNP for Screening Acute Heart Failure. Ultrasound Int Open. 2016;2(3):E90-E92. PubMed
6. Bhagra A, Tierney DM, Sekiguchi H, Soni NH. Point-of-Care Ultrasonography for Primary Care Physicians and General Internists. Mayo Clin Proc. 2016 Dec;91(12):1811-1827. PubMed
7. Crisp JG, Lovato LM, Jang TB. Compression ultrasonography of the lower extremity with portable vascular ultrasonography can accurately detect deep venous thrombosis in the emergency department. Ann Emerg Med. 2010;56(6):601-610. PubMed
8. Squire BT, Fox JC, Anderson C. ABSCESS: Applied bedside sonography for convenient. Evaluation of superficial soft tissue infections. Acad Emerg Med. 2005;12(7):601-606. PubMed
9. Narasimhan M, Koenig SJ, Mayo PH. A Whole-Body Approach to Point of Care Ultrasound. Chest. 2016;150(4):772-776. PubMed
10. Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16-25. PubMed
11. Soni NJ, Arntfield R, Kory P. Point of Care Ultrasound. Philadelphia: Elsevier Saunders; 2015.
12. Moore CL, Copel JA. Point-of-Care Ultrasonography. N Engl J Med. 2011;364(8):749-757. PubMed
13. Rempell JS, Saldana F, DiSalvo D, et al. Pilot Point-of-Care Ultrasound Curriculum at Harvard Medical School: Early Experience. West J Emerg Med. 2016;17(6):734-740. doi:10.5811/westjem.2016.8.31387. PubMed
14. Heiberg J, Hansen LS, Wemmelund K, et al. Point-of-Care Clinical Ultrasound for Medical Students. Ultrasound Int Open. 2015;1(2):E58-E66. doi:10.1055/s-0035-1565173. PubMed
15. Razi R, Estrada JR, Doll J, Spencer KT. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24(12):1319-1324. PubMed
16. Alexander JH, Peterson ED, Chen AY, Harding TM, Adams DB, Kisslo JA Jr. Feasibility of point-of-care echocardiography by internal medicine house staff. Am Heart J. 2004;147(3):476-481. PubMed
17. Hellmann DB, Whiting-O’Keefe Q, Shapiro EP, Martin LD, Martire C, Ziegelstein RC. The rate at which residents learn to use hand-held echocardiography at the bedside. Am J Med. 2005;118(9):1010-1018. PubMed
18. Kimura BJ, Amundson SA, Phan JN, Agan DL, Shaw DJ. Observations during development of an internal medicine residency training program in cardiovascular limited ultrasound examination. J Hosp Med. 2012;7(7):537-542. PubMed
19. Akhtar S, Theodoro D, Gaspari R, et al. Resident training in emergency ultrasound: consensus recommendations from the 2008 Council of Emergency Medicine Residency Directors Conference. Acad Emerg Med. 2009;16(s2):S32-S36. PubMed
, , , , , . Can emergency medicine residents detect acute deep venous thrombosis with a limited, two-site ultrasound examination? J Emerg Med. 2007;32(2):197-200. PubMed
, , , . Resident-performed compression ultrasonography for the detection of proximal deep vein thrombosis: fast and accurate. Acad Emerg Med. 2004;11(3):319-322. PubMed
22. Mandavia D, Aragona J, Chan L, et al. Ultrasound training for emergency physicians—a prospective study. Acad Emerg Med. 2000;7(9):1008-1014. PubMed
23. Koenig SJ, Narasimhan M, Mayo PH. Thoracic ultrasonography for the pulmonary specialist. Chest. 2011;140(5):1332-1341. doi: 10.1378/chest.11-0348. PubMed
24. Greenstein YY, Littauer R, Narasimhan M, Mayo PH, Koenig SJ. Effectiveness of a Critical Care Ultrasonography Course. Chest. 2017;151(1):34-40. doi:10.1016/j.chest.2016.08.1465. PubMed
25. Martin LD, Howell EE, Ziegelstein RC, Martire C, Shapiro EP, Hellmann DB. Hospitalist performance of cardiac hand-carried ultrasound after focused training. Am J Med. 2007;120(11):1000-1004. PubMed
26. Martin LD, Howell EE, Ziegelstein RC, et al.
27. Lucas BP, Candotti C, Margeta B, et al. Diagnostic accuracy of hospitalist-performed hand-carried ultrasound echocardiography after a brief training program. J Hosp Med. 2009;4(6):340-349. PubMed
28.
29. Critical Care Ultrasonography Certificate of Completion Program. American College of Chest Physicians. http://www.chestnet.org/Education/Advanced-Clinical-Training/Certificate-of-Completion-Program/Critical-Care-Ultrasonography. Accessed March 30, 2017
30. Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/Société de Réanimation de Langue Française statement on competence in critical care ultrasonography. Chest. 2009;135(4):1050-1060. PubMed
31. Donlon TF, Angoff WH. The scholastic aptitude test. The College Board Admissions Testing Program; 1971:15-47.
32. Ericsson KA, Lehmann AC. Expert and exceptional performance: Evidence of maximal adaptation to task constraints. Annu Rev Psychol. 1996;47:273-305. PubMed
33. Ericcson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100(3):363-406.
34. Arntfield RT. The utility of remote supervision with feedback as a method to deliver high-volume critical care ultrasound training. J Crit Care. 2015;30(2):441.e1-e6. PubMed
35. Ma OJ, Gaddis G, Norvell JG, Subramanian S. How fast is the focused assessment with sonography for trauma examination learning curve? Emerg Med Australas. 2008;20(1):32-37. PubMed
36. Gaspari RJ, Dickman E, Blehar D. Learning curve of bedside ultrasound of the gallbladder. J Emerg Med. 2009;37(1):51-66. doi:10.1016/j.jemermed.2007.10.070. PubMed
37. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wane DB. Use of simulation-based mastery learning to improve quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009:4(7):397-403. PubMed
38. McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. A critical review of simulation-based mastery learning with translational outcomes. Med Educ. 2014:48(4):375-385. PubMed
39. Guskey TR. The essential elements of mastery learning. J Classroom Interac. 1987;22:19-22.
40. Ultrasound Institute. Introduction to Primary Care Ultrasound. University of South Carolina School of Medicine. http://ultrasoundinstitute.med.sc.edu/UIcme.asp. Accessed October 24, 2017.
41. Society of Critical Care Medicine. Live Critical Care Ultrasound: Adult. http://www.sccm.org/Education-Center/Ultrasound/Pages/Fundamentals.aspx. Accessed October 24, 2017.
42. Castlefest Ultrasound Event. Castlefest 2018. http://castlefest2018.com/. Accessed October 24, 2017.
43. Office of Continuing Medical Education. Point of Care Ultrasound Workshop. UT Health San Antonio Joe R. & Teresa Lozano Long School of Medicine. http://cme.uthscsa.edu/ultrasound.asp. Accessed October 24, 2017.
44. Patrawalla P, Eisen LA, Shiloh A, et al. Development and Validation of an Assessment Tool for Competency in Critical Care Ultrasound. J Grad Med Educ. 2015;7(4):567-573. PubMed
© 2018 Society of Hospital Medicine
Ultrasound: bread and butter of intensivists
Ultrasound use in ICUs is gaining momentum as more critical care physicians realize the effectiveness of point-of-care, goal-directed ultrasounds in the management of their patients, both for procedural guidance and as diagnostic tools.
Through well-designed studies and numerous critical care ultrasound hands-on courses offered through the ACCP and others, the opportunities to acquire the cognitive aspect of ultrasound, image acquisition, and interpretation skills continue to grow.
To this end, CHEST has launched a new video-based ultrasound case-based series called Ultrasound Corner. Its focus is to bridge image acquisition, interpretation skills, and its application to the critically ill patient. While the intensivist may gain proficiency in ultrasound image acquisition and interpretation with relative ease, its application to a critically ill patient may be more challenging.
This video-based ultrasound case format may fill a need for our readers by combining the clinical case scenario and physical exam with an appropriate logical, goal-directed ultrasound exam. Case patient video images will be compared with normal patient video images, allowing the intensivist immediate distinction. Videos will be accompanied by both labeled still images and voice narration to further illustrate the main teaching point of each case.
While rare and fascinating case reports interest both readers and editors for publication, Ultrasound Corner will focus on common, everyday clinical situations and the application of goal-directed ultrasound for diagnosis and management.
The rapid assessment of patients presenting with cardiopulmonary failure is the bread and butter of all intensivists; these cases are intended to provide guidance with ultrasonography to categorize shock states (cardiogenic, distributive, obstructive, etc) and to search for an etiology of respiratory failure using thoracic ultrasound. Interested members may also take an active role in this new series by submitting their video-based ultrasound cases for publication in CHEST (http://journal.publications.chestnet.org/ss/forauthors.aspx).
email address On Twitter
Ultrasound use in ICUs is gaining momentum as more critical care physicians realize the effectiveness of point-of-care, goal-directed ultrasounds in the management of their patients, both for procedural guidance and as diagnostic tools.
Through well-designed studies and numerous critical care ultrasound hands-on courses offered through the ACCP and others, the opportunities to acquire the cognitive aspect of ultrasound, image acquisition, and interpretation skills continue to grow.
To this end, CHEST has launched a new video-based ultrasound case-based series called Ultrasound Corner. Its focus is to bridge image acquisition, interpretation skills, and its application to the critically ill patient. While the intensivist may gain proficiency in ultrasound image acquisition and interpretation with relative ease, its application to a critically ill patient may be more challenging.
This video-based ultrasound case format may fill a need for our readers by combining the clinical case scenario and physical exam with an appropriate logical, goal-directed ultrasound exam. Case patient video images will be compared with normal patient video images, allowing the intensivist immediate distinction. Videos will be accompanied by both labeled still images and voice narration to further illustrate the main teaching point of each case.
While rare and fascinating case reports interest both readers and editors for publication, Ultrasound Corner will focus on common, everyday clinical situations and the application of goal-directed ultrasound for diagnosis and management.
The rapid assessment of patients presenting with cardiopulmonary failure is the bread and butter of all intensivists; these cases are intended to provide guidance with ultrasonography to categorize shock states (cardiogenic, distributive, obstructive, etc) and to search for an etiology of respiratory failure using thoracic ultrasound. Interested members may also take an active role in this new series by submitting their video-based ultrasound cases for publication in CHEST (http://journal.publications.chestnet.org/ss/forauthors.aspx).
email address On Twitter
Ultrasound use in ICUs is gaining momentum as more critical care physicians realize the effectiveness of point-of-care, goal-directed ultrasounds in the management of their patients, both for procedural guidance and as diagnostic tools.
Through well-designed studies and numerous critical care ultrasound hands-on courses offered through the ACCP and others, the opportunities to acquire the cognitive aspect of ultrasound, image acquisition, and interpretation skills continue to grow.
To this end, CHEST has launched a new video-based ultrasound case-based series called Ultrasound Corner. Its focus is to bridge image acquisition, interpretation skills, and its application to the critically ill patient. While the intensivist may gain proficiency in ultrasound image acquisition and interpretation with relative ease, its application to a critically ill patient may be more challenging.
This video-based ultrasound case format may fill a need for our readers by combining the clinical case scenario and physical exam with an appropriate logical, goal-directed ultrasound exam. Case patient video images will be compared with normal patient video images, allowing the intensivist immediate distinction. Videos will be accompanied by both labeled still images and voice narration to further illustrate the main teaching point of each case.
While rare and fascinating case reports interest both readers and editors for publication, Ultrasound Corner will focus on common, everyday clinical situations and the application of goal-directed ultrasound for diagnosis and management.
The rapid assessment of patients presenting with cardiopulmonary failure is the bread and butter of all intensivists; these cases are intended to provide guidance with ultrasonography to categorize shock states (cardiogenic, distributive, obstructive, etc) and to search for an etiology of respiratory failure using thoracic ultrasound. Interested members may also take an active role in this new series by submitting their video-based ultrasound cases for publication in CHEST (http://journal.publications.chestnet.org/ss/forauthors.aspx).
email address On Twitter