User login
Avoid transcutaneous biopsy in adrenocortical carcinoma
SAN FRANCISCO – Transcutaneous biopsy of adrenocortical carcinomas did not improve diagnosis or survival, and potentially harmed 11% of patients, in a study of 74 patients.
The study fills a gap behind general recommendations against biopsy of single adrenal masses in the absence of another known malignancy or metastasis, which had been based on fears that biopsy would spread the tumor but lacked clinical evidence, Andrew Williams said in a poster presentation at the annual meeting of the Endocrine Society.
Among 74 patients with adrenocortical carcinoma who had undergone transcutaneous biopsy of an adrenal mass between 1991 and 2011, the sensitivity of the biopsy for the final pathological diagnosis of the disease was 84% at best and as low as 51% in community settings, the retrospective review of electronic medical records found.
Among the 36 patients with stage I-III disease (confined to the adrenal gland) at the time of diagnosis, the biopsy did not significantly change choices regarding adjuvant therapy, compared with 254 patients with stage I-III disease who did not undergo transcutaneous biopsy. The baseline characteristics of these two groups were similar except that patients in the biopsy group were significantly less likely to be secreting hormones (28%) compared with the no-biopsy group (59%).
"Single adrenal masses with malignant characteristics invariably should be treated surgically, making biopsy an unnecessary procedure," reported Mr. Williams, a medical student at the University of Michigan, Ann Arbor, and his associates.
Complications from transcutaneous biopsy in 11% of all patients consisted mainly of bleeding-related events but with one potentially fatal event – needle track metastasis in one patient.
"Single adrenal masses with malignant characteristics invariably should be treated surgically, making biopsy an unnecessary procedure."
Rates of overall and tumor-free survival did not differ significantly between patients who did or did not undergo transcutaneous biopsy in either univariate analysis or multivariate analyses adjusting for age, sex, cortisol production, and adjuvant therapies.
Adrenocortical carcinomas are rare cancers with high rates of recurrence and mortality. Cumulative survival rates in the study decreased steadily to roughly 80% at 1 year, 60% at 3 years, and less than 50% at 5 years.
"Biopsy of adrenocortical carcinomas at stages confined to the adrenal gland does not significantly influence survival," Mr. Williams concluded in the poster. "Adrenal biopsy should only be considered in patients with metastasized disease and unclear tumor origin, only if it may alter the therapeutic approach and only if the adrenal is the easiest site to biopsy."
Patients who underwent biopsy in the study had a median age of 52 years (ranging from 17 to 77 years), and 54% were female. The cohort was 84% white.
Mr. Williams reported having no financial disclosures. Coinvestigator Dr. Gary D. Hammer, also at the University of Michigan, disclosed financial associations with Atterocor, Orphagen Pharmaceuticals, Embera NeuroTherapeutics, HRA Pharma, Corcept Therapeutics, Isis, and OSI-Astella.
On Twitter @sherryboschert
SAN FRANCISCO – Transcutaneous biopsy of adrenocortical carcinomas did not improve diagnosis or survival, and potentially harmed 11% of patients, in a study of 74 patients.
The study fills a gap behind general recommendations against biopsy of single adrenal masses in the absence of another known malignancy or metastasis, which had been based on fears that biopsy would spread the tumor but lacked clinical evidence, Andrew Williams said in a poster presentation at the annual meeting of the Endocrine Society.
Among 74 patients with adrenocortical carcinoma who had undergone transcutaneous biopsy of an adrenal mass between 1991 and 2011, the sensitivity of the biopsy for the final pathological diagnosis of the disease was 84% at best and as low as 51% in community settings, the retrospective review of electronic medical records found.
Among the 36 patients with stage I-III disease (confined to the adrenal gland) at the time of diagnosis, the biopsy did not significantly change choices regarding adjuvant therapy, compared with 254 patients with stage I-III disease who did not undergo transcutaneous biopsy. The baseline characteristics of these two groups were similar except that patients in the biopsy group were significantly less likely to be secreting hormones (28%) compared with the no-biopsy group (59%).
"Single adrenal masses with malignant characteristics invariably should be treated surgically, making biopsy an unnecessary procedure," reported Mr. Williams, a medical student at the University of Michigan, Ann Arbor, and his associates.
Complications from transcutaneous biopsy in 11% of all patients consisted mainly of bleeding-related events but with one potentially fatal event – needle track metastasis in one patient.
"Single adrenal masses with malignant characteristics invariably should be treated surgically, making biopsy an unnecessary procedure."
Rates of overall and tumor-free survival did not differ significantly between patients who did or did not undergo transcutaneous biopsy in either univariate analysis or multivariate analyses adjusting for age, sex, cortisol production, and adjuvant therapies.
Adrenocortical carcinomas are rare cancers with high rates of recurrence and mortality. Cumulative survival rates in the study decreased steadily to roughly 80% at 1 year, 60% at 3 years, and less than 50% at 5 years.
"Biopsy of adrenocortical carcinomas at stages confined to the adrenal gland does not significantly influence survival," Mr. Williams concluded in the poster. "Adrenal biopsy should only be considered in patients with metastasized disease and unclear tumor origin, only if it may alter the therapeutic approach and only if the adrenal is the easiest site to biopsy."
Patients who underwent biopsy in the study had a median age of 52 years (ranging from 17 to 77 years), and 54% were female. The cohort was 84% white.
Mr. Williams reported having no financial disclosures. Coinvestigator Dr. Gary D. Hammer, also at the University of Michigan, disclosed financial associations with Atterocor, Orphagen Pharmaceuticals, Embera NeuroTherapeutics, HRA Pharma, Corcept Therapeutics, Isis, and OSI-Astella.
On Twitter @sherryboschert
SAN FRANCISCO – Transcutaneous biopsy of adrenocortical carcinomas did not improve diagnosis or survival, and potentially harmed 11% of patients, in a study of 74 patients.
The study fills a gap behind general recommendations against biopsy of single adrenal masses in the absence of another known malignancy or metastasis, which had been based on fears that biopsy would spread the tumor but lacked clinical evidence, Andrew Williams said in a poster presentation at the annual meeting of the Endocrine Society.
Among 74 patients with adrenocortical carcinoma who had undergone transcutaneous biopsy of an adrenal mass between 1991 and 2011, the sensitivity of the biopsy for the final pathological diagnosis of the disease was 84% at best and as low as 51% in community settings, the retrospective review of electronic medical records found.
Among the 36 patients with stage I-III disease (confined to the adrenal gland) at the time of diagnosis, the biopsy did not significantly change choices regarding adjuvant therapy, compared with 254 patients with stage I-III disease who did not undergo transcutaneous biopsy. The baseline characteristics of these two groups were similar except that patients in the biopsy group were significantly less likely to be secreting hormones (28%) compared with the no-biopsy group (59%).
"Single adrenal masses with malignant characteristics invariably should be treated surgically, making biopsy an unnecessary procedure," reported Mr. Williams, a medical student at the University of Michigan, Ann Arbor, and his associates.
Complications from transcutaneous biopsy in 11% of all patients consisted mainly of bleeding-related events but with one potentially fatal event – needle track metastasis in one patient.
"Single adrenal masses with malignant characteristics invariably should be treated surgically, making biopsy an unnecessary procedure."
Rates of overall and tumor-free survival did not differ significantly between patients who did or did not undergo transcutaneous biopsy in either univariate analysis or multivariate analyses adjusting for age, sex, cortisol production, and adjuvant therapies.
Adrenocortical carcinomas are rare cancers with high rates of recurrence and mortality. Cumulative survival rates in the study decreased steadily to roughly 80% at 1 year, 60% at 3 years, and less than 50% at 5 years.
"Biopsy of adrenocortical carcinomas at stages confined to the adrenal gland does not significantly influence survival," Mr. Williams concluded in the poster. "Adrenal biopsy should only be considered in patients with metastasized disease and unclear tumor origin, only if it may alter the therapeutic approach and only if the adrenal is the easiest site to biopsy."
Patients who underwent biopsy in the study had a median age of 52 years (ranging from 17 to 77 years), and 54% were female. The cohort was 84% white.
Mr. Williams reported having no financial disclosures. Coinvestigator Dr. Gary D. Hammer, also at the University of Michigan, disclosed financial associations with Atterocor, Orphagen Pharmaceuticals, Embera NeuroTherapeutics, HRA Pharma, Corcept Therapeutics, Isis, and OSI-Astella.
On Twitter @sherryboschert
AT ENDO 2013
Major finding: Transcutaneous biopsy was 51%-84% sensitive for adrenocortical carcinoma, depending on where it was done, and caused complications in 11% of patients.
Data source: Retrospective study of records for 74 patients with adrenocortical carcinoma who underwent transcutaneous biopsy between 1991 and 2011, and a comparison of 290 patients with stage I-III disease with or without biopsy.
Disclosures: Mr. Williams reported having no financial disclosures. Dr. Gary D. Hammer disclosed financial associations with Atterocor, Orphagen Pharmaceuticals, Embera NeuroTherapeutics, HRA Pharma, Corcept Therapeutics, Isis, and OSI-Astella.
Vitrification efficiently preserves oocytes, embryos
SAN FRANCISCO – Recent data show that vitrification of oocytes or embryos can be as efficient as using slow-frozen or fresh oocytes for in vitro fertilization, and so far it appears to be just as safe.
Oocytes from 22 women aged 32-39 years that were randomly allocated for fresh embryo transfer (204 oocytes) or vitrification and warming before transfer (186 oocytes) produced similar rates of fertilization, good-quality embryos at day 3, and blastocysts at day 5 or 6, Zsolt Peter Nagy, Ph.D., reported at the UCLA annual in vitro fertilization and embryo transfer update 2013.
Eighty percent of vitrified oocytes survived freezing and warming. Fertilization succeeded in 75% of fresh oocytes and 67% of vitrified oocytes, a slight difference that was not statistically significant. On day 3 of in vitro fertilization (IVF), 50% of fresh oocytes and 48% of vitrified oocytes produced good-quality embryos; by days 5 or 6, 53% of fresh embryos and 55% of vitrified embryos had produced blastocysts, results that did not differ significantly between groups, said Dr. Nagy, scientific and laboratory director of Reproductive Biology Associates, Atlanta.
The study at his institution found that 11 of the 186 vitrified oocytes (6%) achieved a live birth (Fertil. Steril. 2013;99:1891-7).
Maternal age did affect outcomes. Among 11 women aged 30-36 years, 56% of oocytes produced a good embryo by day 3 of IVF, compared with 40% of oocytes from 11 women aged 37-39 years, a significant difference.
The findings support previous data by other investigators on 224 oocytes, 120 of which underwent intracytoplasmic sperm injection (ICSI) as fresh oocytes and 124 of which underwent vitrification and warming before ICSI. Fertilization rates were statistically similar (83% and 77%, respectively). Excellent-quality embryos were produced by 52% in the fresh ICSI group and 52% in the vitrification group. The mean maternal age in the study was 36 years (Hum. Reprod. 2010;25:66-73).
The 39 embryo transfers in the vitrification group resulted in 15 clinical pregnancies (38% per cycle and per embryo transfer) and an implantation rate of 20%. Twelve pregnancies beyond 12 weeks’ gestation were ongoing (30% per cycle and 31% per embryo transfer).
Separate data from Molecular Biometrics suggest that vitrification is efficient for freezing donor oocytes in egg banks, Dr. Nagy said. Among 11,553 eggs from 342 donors that were vitrified, 7,063 were warmed and 90% survived warming, leading to a clinical pregnancy rate of 57% and an implantation rate of 43%.
Cryopreserving eggs has advantages over fresh ovum donation programs, which have long waiting lists, require more complex synchronization between the donor and recipient, offer relatively limited choice, and have other logistical disadvantages. "A couple of years ago, most of us were doing slow freezing" for cryopreservation, Dr. Nagy said. "Many of us have changed to vitrification because we have realized that it is more efficient than slow freezing."
Before, two to four embryos were needed to achieve a clinical pregnancy under slow-freeze methods at his institution, but only one or two are needed using vitrification, he said.
Oocyte cryopreservation programs make elective single-embryo transfer (eSET) a viable option, Dr. Nagy said. In 98 recipients of eSET, 52% achieved a clinical pregnancy, compared with clinical pregnancies in 72% of 109 elective double-embryo transfers, but 51% of the double-embryo transfers resulted in multiple pregnancies, a retrospective study of data from his institution showed. Among nonelective double-embryo transfers, 52% achieved clinical pregnancies, with multiple pregnancies in 30% of recipients.
Double vitrification also appears to be efficient for egg/embryo banking – cryopreserving an oocyte, thawing it, and then freezing the resulting embryo to later be warmed and transferred. One study that compared 471 warming cycles of double-vitrified embryos with 2,629 warming cycles of vitrified embryos derived from fresh oocytes found overall embryo survival rates of 97% and 96%, respectively, with a delivery rate per warming cycle of 34% in the double-vitrified group and 31% in the single-vitrified group (Fertil. Steril. 2013;99:1623-30).
At Dr. Nagy’s institution, 99% of 190 warmed double-vitrified embryos have survived, leading to a 53% clinical pregnancy rate, a 39% implantation rate, and 33 live births so far. "It shows that if you do the procedure correctly, you are able to preserve the viability of those embryos," he said.
The rate of congenital anomalies in live births from donor eggs cryopreserved at his institution does not seem to be significantly higher than the rate in live births from fresh donor eggs – 3 congenital anomalies among 91 live births using fresh donor eggs and 5 congenital anomalies in 338 live births using cryopreserved donor eggs. Case reports from 1986 to 2008 suggest there’s no increased incidence of birth defects using vitrification compared with slow-freeze methods for cryopreservation.
In the future, registry data should provide further evidence of the safety of vitrification, he said.
Dr. Nagy reported having financial associations with Molecular Biometrics, Origio, and other companies.
On Twitter @sherryboschert
SAN FRANCISCO – Recent data show that vitrification of oocytes or embryos can be as efficient as using slow-frozen or fresh oocytes for in vitro fertilization, and so far it appears to be just as safe.
Oocytes from 22 women aged 32-39 years that were randomly allocated for fresh embryo transfer (204 oocytes) or vitrification and warming before transfer (186 oocytes) produced similar rates of fertilization, good-quality embryos at day 3, and blastocysts at day 5 or 6, Zsolt Peter Nagy, Ph.D., reported at the UCLA annual in vitro fertilization and embryo transfer update 2013.
Eighty percent of vitrified oocytes survived freezing and warming. Fertilization succeeded in 75% of fresh oocytes and 67% of vitrified oocytes, a slight difference that was not statistically significant. On day 3 of in vitro fertilization (IVF), 50% of fresh oocytes and 48% of vitrified oocytes produced good-quality embryos; by days 5 or 6, 53% of fresh embryos and 55% of vitrified embryos had produced blastocysts, results that did not differ significantly between groups, said Dr. Nagy, scientific and laboratory director of Reproductive Biology Associates, Atlanta.
The study at his institution found that 11 of the 186 vitrified oocytes (6%) achieved a live birth (Fertil. Steril. 2013;99:1891-7).
Maternal age did affect outcomes. Among 11 women aged 30-36 years, 56% of oocytes produced a good embryo by day 3 of IVF, compared with 40% of oocytes from 11 women aged 37-39 years, a significant difference.
The findings support previous data by other investigators on 224 oocytes, 120 of which underwent intracytoplasmic sperm injection (ICSI) as fresh oocytes and 124 of which underwent vitrification and warming before ICSI. Fertilization rates were statistically similar (83% and 77%, respectively). Excellent-quality embryos were produced by 52% in the fresh ICSI group and 52% in the vitrification group. The mean maternal age in the study was 36 years (Hum. Reprod. 2010;25:66-73).
The 39 embryo transfers in the vitrification group resulted in 15 clinical pregnancies (38% per cycle and per embryo transfer) and an implantation rate of 20%. Twelve pregnancies beyond 12 weeks’ gestation were ongoing (30% per cycle and 31% per embryo transfer).
Separate data from Molecular Biometrics suggest that vitrification is efficient for freezing donor oocytes in egg banks, Dr. Nagy said. Among 11,553 eggs from 342 donors that were vitrified, 7,063 were warmed and 90% survived warming, leading to a clinical pregnancy rate of 57% and an implantation rate of 43%.
Cryopreserving eggs has advantages over fresh ovum donation programs, which have long waiting lists, require more complex synchronization between the donor and recipient, offer relatively limited choice, and have other logistical disadvantages. "A couple of years ago, most of us were doing slow freezing" for cryopreservation, Dr. Nagy said. "Many of us have changed to vitrification because we have realized that it is more efficient than slow freezing."
Before, two to four embryos were needed to achieve a clinical pregnancy under slow-freeze methods at his institution, but only one or two are needed using vitrification, he said.
Oocyte cryopreservation programs make elective single-embryo transfer (eSET) a viable option, Dr. Nagy said. In 98 recipients of eSET, 52% achieved a clinical pregnancy, compared with clinical pregnancies in 72% of 109 elective double-embryo transfers, but 51% of the double-embryo transfers resulted in multiple pregnancies, a retrospective study of data from his institution showed. Among nonelective double-embryo transfers, 52% achieved clinical pregnancies, with multiple pregnancies in 30% of recipients.
Double vitrification also appears to be efficient for egg/embryo banking – cryopreserving an oocyte, thawing it, and then freezing the resulting embryo to later be warmed and transferred. One study that compared 471 warming cycles of double-vitrified embryos with 2,629 warming cycles of vitrified embryos derived from fresh oocytes found overall embryo survival rates of 97% and 96%, respectively, with a delivery rate per warming cycle of 34% in the double-vitrified group and 31% in the single-vitrified group (Fertil. Steril. 2013;99:1623-30).
At Dr. Nagy’s institution, 99% of 190 warmed double-vitrified embryos have survived, leading to a 53% clinical pregnancy rate, a 39% implantation rate, and 33 live births so far. "It shows that if you do the procedure correctly, you are able to preserve the viability of those embryos," he said.
The rate of congenital anomalies in live births from donor eggs cryopreserved at his institution does not seem to be significantly higher than the rate in live births from fresh donor eggs – 3 congenital anomalies among 91 live births using fresh donor eggs and 5 congenital anomalies in 338 live births using cryopreserved donor eggs. Case reports from 1986 to 2008 suggest there’s no increased incidence of birth defects using vitrification compared with slow-freeze methods for cryopreservation.
In the future, registry data should provide further evidence of the safety of vitrification, he said.
Dr. Nagy reported having financial associations with Molecular Biometrics, Origio, and other companies.
On Twitter @sherryboschert
SAN FRANCISCO – Recent data show that vitrification of oocytes or embryos can be as efficient as using slow-frozen or fresh oocytes for in vitro fertilization, and so far it appears to be just as safe.
Oocytes from 22 women aged 32-39 years that were randomly allocated for fresh embryo transfer (204 oocytes) or vitrification and warming before transfer (186 oocytes) produced similar rates of fertilization, good-quality embryos at day 3, and blastocysts at day 5 or 6, Zsolt Peter Nagy, Ph.D., reported at the UCLA annual in vitro fertilization and embryo transfer update 2013.
Eighty percent of vitrified oocytes survived freezing and warming. Fertilization succeeded in 75% of fresh oocytes and 67% of vitrified oocytes, a slight difference that was not statistically significant. On day 3 of in vitro fertilization (IVF), 50% of fresh oocytes and 48% of vitrified oocytes produced good-quality embryos; by days 5 or 6, 53% of fresh embryos and 55% of vitrified embryos had produced blastocysts, results that did not differ significantly between groups, said Dr. Nagy, scientific and laboratory director of Reproductive Biology Associates, Atlanta.
The study at his institution found that 11 of the 186 vitrified oocytes (6%) achieved a live birth (Fertil. Steril. 2013;99:1891-7).
Maternal age did affect outcomes. Among 11 women aged 30-36 years, 56% of oocytes produced a good embryo by day 3 of IVF, compared with 40% of oocytes from 11 women aged 37-39 years, a significant difference.
The findings support previous data by other investigators on 224 oocytes, 120 of which underwent intracytoplasmic sperm injection (ICSI) as fresh oocytes and 124 of which underwent vitrification and warming before ICSI. Fertilization rates were statistically similar (83% and 77%, respectively). Excellent-quality embryos were produced by 52% in the fresh ICSI group and 52% in the vitrification group. The mean maternal age in the study was 36 years (Hum. Reprod. 2010;25:66-73).
The 39 embryo transfers in the vitrification group resulted in 15 clinical pregnancies (38% per cycle and per embryo transfer) and an implantation rate of 20%. Twelve pregnancies beyond 12 weeks’ gestation were ongoing (30% per cycle and 31% per embryo transfer).
Separate data from Molecular Biometrics suggest that vitrification is efficient for freezing donor oocytes in egg banks, Dr. Nagy said. Among 11,553 eggs from 342 donors that were vitrified, 7,063 were warmed and 90% survived warming, leading to a clinical pregnancy rate of 57% and an implantation rate of 43%.
Cryopreserving eggs has advantages over fresh ovum donation programs, which have long waiting lists, require more complex synchronization between the donor and recipient, offer relatively limited choice, and have other logistical disadvantages. "A couple of years ago, most of us were doing slow freezing" for cryopreservation, Dr. Nagy said. "Many of us have changed to vitrification because we have realized that it is more efficient than slow freezing."
Before, two to four embryos were needed to achieve a clinical pregnancy under slow-freeze methods at his institution, but only one or two are needed using vitrification, he said.
Oocyte cryopreservation programs make elective single-embryo transfer (eSET) a viable option, Dr. Nagy said. In 98 recipients of eSET, 52% achieved a clinical pregnancy, compared with clinical pregnancies in 72% of 109 elective double-embryo transfers, but 51% of the double-embryo transfers resulted in multiple pregnancies, a retrospective study of data from his institution showed. Among nonelective double-embryo transfers, 52% achieved clinical pregnancies, with multiple pregnancies in 30% of recipients.
Double vitrification also appears to be efficient for egg/embryo banking – cryopreserving an oocyte, thawing it, and then freezing the resulting embryo to later be warmed and transferred. One study that compared 471 warming cycles of double-vitrified embryos with 2,629 warming cycles of vitrified embryos derived from fresh oocytes found overall embryo survival rates of 97% and 96%, respectively, with a delivery rate per warming cycle of 34% in the double-vitrified group and 31% in the single-vitrified group (Fertil. Steril. 2013;99:1623-30).
At Dr. Nagy’s institution, 99% of 190 warmed double-vitrified embryos have survived, leading to a 53% clinical pregnancy rate, a 39% implantation rate, and 33 live births so far. "It shows that if you do the procedure correctly, you are able to preserve the viability of those embryos," he said.
The rate of congenital anomalies in live births from donor eggs cryopreserved at his institution does not seem to be significantly higher than the rate in live births from fresh donor eggs – 3 congenital anomalies among 91 live births using fresh donor eggs and 5 congenital anomalies in 338 live births using cryopreserved donor eggs. Case reports from 1986 to 2008 suggest there’s no increased incidence of birth defects using vitrification compared with slow-freeze methods for cryopreservation.
In the future, registry data should provide further evidence of the safety of vitrification, he said.
Dr. Nagy reported having financial associations with Molecular Biometrics, Origio, and other companies.
On Twitter @sherryboschert
AT A MEETING ON IVF AND EMBRYO TRANSFER
Switching nonadherent to denosumab improved bone density
SAN FRANCISCO – Switching to denosumab boosted bone mineral density more than did switching to ibandronate or risedronate in high-risk osteoporosis patients who were not adherent to oral bisphosphonate therapy, an assessment of data from 1,576 patients in two studies found.
The open-label studies included postmenopausal women who previously had been prescribed oral bisphosphonate therapy for osteoporosis but had either stopped the drug or were insufficiently adherent to therapy, by a score of less than six on the Osteoporosis-Specific Morisky Medication Adherence Scale.
One study randomized patients to denosumab (Prolia) 60 mg subcutaneously every 6 months or ibandronate (Boniva) 150 mg orally every month.
The other study randomized patients to the same denosumab regimen or risedronate (Actonel) 150 mg orally per month, taken as a 75-mg tablet on each of two consecutive days.
BMD measurements on 1,576 women in the studies showed significantly greater increases at 12 months at the hip, femoral neck, and spine in patients receiving denosumab, compared with either of the oral bisphosphonates, Dr. Christopher Recknor and his associates reported at the annual meeting of the Endocrine Society.
Bone density in patients on denosumab increased at the hip by about 2.3% in one study and 2% in the other, increased at the femoral neck by roughly 1.7% in one study and 1.5% in the other, and increased at the spine by 4% in one study and 3.5% in the other. In comparison, BMDs in patients on ibandronate increased by roughly 1.1% at the hip, 0.7% at the femoral neck, and 2% at the spine. BMDs in patients on risedronate increased by about 0.4% at the hip and 1% at the spine but decreased by 0.1% at the femoral neck.
The changes in bone density did not differ significantly between the 33% of patients who had had a prior fragility fracture and patients with no history of fragility fracture, with one exception: The gains in femoral neck BMD from denosumab compared with ibandronate were significantly larger in patients with a prior fracture, compared with those with no prior fracture, reported Dr. Recknor, medical director of the United Osteoporosis Centers, Gainesville, Ga.
In patients with a prior fragility fracture, femoral neck BMD increased by about 2.2% on denosumab and by 0.1% on ibandronate, compared with gains in patients with no prior fracture of 1.5% on denosumab and 1% on ibandronate.
The study defined fragility fractures as those not involving the skull, facial bones, fingers, and toes and not associated with severe trauma or pathological fractures.
In the lower-risk patients without a prior fracture, "that is where Boniva does not seem to work very well" in comparison with switching to another oral bisphosphonate, Dr. Recknor said in an interview. For high-risk patients with a prior fracture, however, "I’m even more convinced that if I’ve got somebody who’s at high risk and they’re noncompliant" with bisphosphonate therapy, "giving Prolia would be the way to go."
Even if the bisphosphonate regimen is switched to monthly dosing to try to improve adherence, "the bone density increases are just not going to be that great," he said.
BMDs at baseline did not differ significantly between treatment groups or between patients with or without a prior fragility fracture.
Infections, though not serious ones, were reported in 24% of patients on denosumab in each study and in 19% of patients on ibandronate and 21% of patients on risedronate. There were no cases of atypical femoral fracture, delayed fracture healing, or osteonecrosis of the jaw.
The study was funded by Amgen, which markets denosumab, and by GlaxoSmithKline. Dr. Recknor disclosed ties with Amgen, Novartis Pharmaceuticals, Ion Med Systems, Eli Lilly, Merck, and UCB. Some coinvestigators were employees of Amgen and others reported financial ties with these companies and/or Warner Chilcott, which markets risedronate; Roche Pharmaceuticals, which markets ibandronate through Genentech; Tarsa, Wyeth, Takeda, Nycomed, Servier, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – Switching to denosumab boosted bone mineral density more than did switching to ibandronate or risedronate in high-risk osteoporosis patients who were not adherent to oral bisphosphonate therapy, an assessment of data from 1,576 patients in two studies found.
The open-label studies included postmenopausal women who previously had been prescribed oral bisphosphonate therapy for osteoporosis but had either stopped the drug or were insufficiently adherent to therapy, by a score of less than six on the Osteoporosis-Specific Morisky Medication Adherence Scale.
One study randomized patients to denosumab (Prolia) 60 mg subcutaneously every 6 months or ibandronate (Boniva) 150 mg orally every month.
The other study randomized patients to the same denosumab regimen or risedronate (Actonel) 150 mg orally per month, taken as a 75-mg tablet on each of two consecutive days.
BMD measurements on 1,576 women in the studies showed significantly greater increases at 12 months at the hip, femoral neck, and spine in patients receiving denosumab, compared with either of the oral bisphosphonates, Dr. Christopher Recknor and his associates reported at the annual meeting of the Endocrine Society.
Bone density in patients on denosumab increased at the hip by about 2.3% in one study and 2% in the other, increased at the femoral neck by roughly 1.7% in one study and 1.5% in the other, and increased at the spine by 4% in one study and 3.5% in the other. In comparison, BMDs in patients on ibandronate increased by roughly 1.1% at the hip, 0.7% at the femoral neck, and 2% at the spine. BMDs in patients on risedronate increased by about 0.4% at the hip and 1% at the spine but decreased by 0.1% at the femoral neck.
The changes in bone density did not differ significantly between the 33% of patients who had had a prior fragility fracture and patients with no history of fragility fracture, with one exception: The gains in femoral neck BMD from denosumab compared with ibandronate were significantly larger in patients with a prior fracture, compared with those with no prior fracture, reported Dr. Recknor, medical director of the United Osteoporosis Centers, Gainesville, Ga.
In patients with a prior fragility fracture, femoral neck BMD increased by about 2.2% on denosumab and by 0.1% on ibandronate, compared with gains in patients with no prior fracture of 1.5% on denosumab and 1% on ibandronate.
The study defined fragility fractures as those not involving the skull, facial bones, fingers, and toes and not associated with severe trauma or pathological fractures.
In the lower-risk patients without a prior fracture, "that is where Boniva does not seem to work very well" in comparison with switching to another oral bisphosphonate, Dr. Recknor said in an interview. For high-risk patients with a prior fracture, however, "I’m even more convinced that if I’ve got somebody who’s at high risk and they’re noncompliant" with bisphosphonate therapy, "giving Prolia would be the way to go."
Even if the bisphosphonate regimen is switched to monthly dosing to try to improve adherence, "the bone density increases are just not going to be that great," he said.
BMDs at baseline did not differ significantly between treatment groups or between patients with or without a prior fragility fracture.
Infections, though not serious ones, were reported in 24% of patients on denosumab in each study and in 19% of patients on ibandronate and 21% of patients on risedronate. There were no cases of atypical femoral fracture, delayed fracture healing, or osteonecrosis of the jaw.
The study was funded by Amgen, which markets denosumab, and by GlaxoSmithKline. Dr. Recknor disclosed ties with Amgen, Novartis Pharmaceuticals, Ion Med Systems, Eli Lilly, Merck, and UCB. Some coinvestigators were employees of Amgen and others reported financial ties with these companies and/or Warner Chilcott, which markets risedronate; Roche Pharmaceuticals, which markets ibandronate through Genentech; Tarsa, Wyeth, Takeda, Nycomed, Servier, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – Switching to denosumab boosted bone mineral density more than did switching to ibandronate or risedronate in high-risk osteoporosis patients who were not adherent to oral bisphosphonate therapy, an assessment of data from 1,576 patients in two studies found.
The open-label studies included postmenopausal women who previously had been prescribed oral bisphosphonate therapy for osteoporosis but had either stopped the drug or were insufficiently adherent to therapy, by a score of less than six on the Osteoporosis-Specific Morisky Medication Adherence Scale.
One study randomized patients to denosumab (Prolia) 60 mg subcutaneously every 6 months or ibandronate (Boniva) 150 mg orally every month.
The other study randomized patients to the same denosumab regimen or risedronate (Actonel) 150 mg orally per month, taken as a 75-mg tablet on each of two consecutive days.
BMD measurements on 1,576 women in the studies showed significantly greater increases at 12 months at the hip, femoral neck, and spine in patients receiving denosumab, compared with either of the oral bisphosphonates, Dr. Christopher Recknor and his associates reported at the annual meeting of the Endocrine Society.
Bone density in patients on denosumab increased at the hip by about 2.3% in one study and 2% in the other, increased at the femoral neck by roughly 1.7% in one study and 1.5% in the other, and increased at the spine by 4% in one study and 3.5% in the other. In comparison, BMDs in patients on ibandronate increased by roughly 1.1% at the hip, 0.7% at the femoral neck, and 2% at the spine. BMDs in patients on risedronate increased by about 0.4% at the hip and 1% at the spine but decreased by 0.1% at the femoral neck.
The changes in bone density did not differ significantly between the 33% of patients who had had a prior fragility fracture and patients with no history of fragility fracture, with one exception: The gains in femoral neck BMD from denosumab compared with ibandronate were significantly larger in patients with a prior fracture, compared with those with no prior fracture, reported Dr. Recknor, medical director of the United Osteoporosis Centers, Gainesville, Ga.
In patients with a prior fragility fracture, femoral neck BMD increased by about 2.2% on denosumab and by 0.1% on ibandronate, compared with gains in patients with no prior fracture of 1.5% on denosumab and 1% on ibandronate.
The study defined fragility fractures as those not involving the skull, facial bones, fingers, and toes and not associated with severe trauma or pathological fractures.
In the lower-risk patients without a prior fracture, "that is where Boniva does not seem to work very well" in comparison with switching to another oral bisphosphonate, Dr. Recknor said in an interview. For high-risk patients with a prior fracture, however, "I’m even more convinced that if I’ve got somebody who’s at high risk and they’re noncompliant" with bisphosphonate therapy, "giving Prolia would be the way to go."
Even if the bisphosphonate regimen is switched to monthly dosing to try to improve adherence, "the bone density increases are just not going to be that great," he said.
BMDs at baseline did not differ significantly between treatment groups or between patients with or without a prior fragility fracture.
Infections, though not serious ones, were reported in 24% of patients on denosumab in each study and in 19% of patients on ibandronate and 21% of patients on risedronate. There were no cases of atypical femoral fracture, delayed fracture healing, or osteonecrosis of the jaw.
The study was funded by Amgen, which markets denosumab, and by GlaxoSmithKline. Dr. Recknor disclosed ties with Amgen, Novartis Pharmaceuticals, Ion Med Systems, Eli Lilly, Merck, and UCB. Some coinvestigators were employees of Amgen and others reported financial ties with these companies and/or Warner Chilcott, which markets risedronate; Roche Pharmaceuticals, which markets ibandronate through Genentech; Tarsa, Wyeth, Takeda, Nycomed, Servier, and Pfizer.
On Twitter @sherryboschert
AT ENDO 2013
Major finding: Bone density increased at 1 year by 2.3% and 2% in the hip, 1.7% and 1.5% at the femoral neck, and 4% and 3.5% at the spine on denosumab compared with 1.1% at the hip, 0.7% at the femoral neck, and 2% at the spine on ibandronate and 0.4% at the hip and 0.4% at the spine with slightly decreased density at the femoral neck on risedronate.
Data source: Secondary analysis of data from two open-label, randomized studies of 1,576 postmenopausal women who had been insufficiently adherent to oral bisphosphonate therapy.
Disclosures: The study was funded by Amgen, which markets denosumab, and by GlaxoSmithKline. Dr. Recknor disclosed ties with Amgen, Novartis Pharmaceuticals, Ion Med Systems, Eli Lilly, Merck, and UCB. Some coinvestigators were employees of Amgen and others reported financial ties with these companies and/or Warner Chilcott, which markets risedronate; Roche Pharmaceuticals, which markets ibandronate through Genentech; Tarsa, Wyeth, Takeda, Nycomed, Servier, and Pfizer.
Get umbilical artery systolic-to-diastolic ratio in intrauterine growth restriction
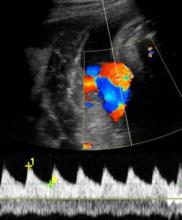
SAN FRANCISCO – An umbilical artery systolic-to-diastolic ratio of less than 3 as measured on weekly Doppler ultrasounds in a fetus with 30 weeks’ or more gestation and suspected intrauterine growth restriction suggests that the fetus probably is doing okay, Dr. Vickie A. Feldstein said.
That "ballpark guideline" is most helpful if physicians at your institution have agreed to use the umbilical artery systolic/diastolic (S/D) ratio as the parameter for assessing fetuses with intrauterine growth restriction (IUGR) and have agreed on which anatomical location is preferred for the ultrasound interrogation, so that there is some uniformity in how results are presented and interpreted, she said.
The recent Practice Bulletin No. 134 from the American College of Obstetricians and Gynecologists recommended in May 2013 that if the ultrasonographically estimated fetal weight is below the 10th percentile for gestational age, further evaluation should be considered, such as Doppler blood flow studies of the umbilical artery (Obstet. Gynecol. 2013;121:1122-33).
The medical literature describes several Doppler ultrasound parameters that could be used in suspected IUGR, including the umbilical artery S/D ratio, the resistance index, or the pulsatility index. They’re all about the same phenomenon, which is measuring resistance to perfusion in the placenta as reflected in the interrogation of the umbilical artery, Dr. Feldstein said at a meeting on antepartum and intrapartum management sponsored by the University of California, San Francisco.
"I think a report that includes all of them would make our heads spin," said Dr. Feldstein, professor of clinical radiology at the university. She and her colleagues use the S/D ratio, calculated by using calipers to measure the peak of systole on umbilical artery Doppler ultrasound and dividing that by the measure of end diastole.
"I don’t care how fast the flow actually is, I care about the character of the flow, the relative difference between systole and diastole," she said. The ratio reflects the status of placental circulation. It normally is high early in pregnancy and decreases as gestation advances, placental resistance decreases, and there is more forward flow during diastole.
If separate umbilical artery Doppler tracings yield discrepant S/D ratios, that may reflect normal variability or be due to changes in fetal heart rate. "A significant change in heart rate might change the S/D ratio quite a bit," she said. Or, an ultrasound filter set too low can produce noise in the tracing that might alter where you place the calipers.
The location along the umbilical cord that the sonographer samples also can affect measurements. The medical literature is full of suggestions about where to sample. Dr. Feldstein recommends sampling toward the placenta, if possible, which will reflect resistance to perfusion in the placenta.
"We’ve found the cord insertion, typically, so we know where to look," she said. "The farther away from the placenta you go, you’re adding resistance of the cord to your tracing."
She encouraged obstetricians to talk to the people who do Doppler at their institutions "to decide together how you want this done, how you want it reported, and what parameter you want used, so you don’t overwhelm yourselves with excess information."
If the S/D ratio is a bit above 3 in a third-trimester fetus with IUGR but there’s decent diastolic flow, "don’t sweat the small stuff," she suggested. As the S/D ratio goes higher and higher, however, placental insufficiency (and resistance) increases and forward flow decreases, and can become absent or even reversed end-diastolic flow.
An absence of diastolic flow is associated with a 60%-70% loss of vasculature, "a really significant abnormality in the placenta," she said. With reversed diastolic flow, the odds of perinatal death increase more than fivefold.
A recent study of 1,116 fetuses with IUGR showed that an abnormal umbilical artery Doppler ultrasound tracing (defined by pulsatility index or the absence or reversal of end-diastolic flow) was significantly associated with adverse outcomes irrespective of estimated fetal weight or abdominal circumference (Am. J. Obstet. Gynecol. 2013;208:e1-6 [doi: 10.1016/j.ajog.2013.02.007]).
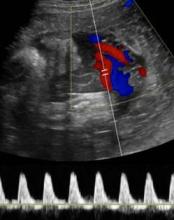
When Dr. Feldstein sees an abnormal umbilical artery Doppler tracing, she samples the fetal middle cerebral artery by Doppler ultrasound. The middle cerebral artery S/D ratio should always be higher than the umbilical artery SD ratio, with a typical middle cerebral artery S/D ratio greater than 4 after 30 weeks’ gestation.
A fetus in trouble with IUGR will respond by lowering cerebral vascular resistance to maintain blood flow to its brain, decreasing the middle cerebral artery S/D ratio in what’s known as a "brain-sparing" wave form. Although there’s nothing that can be done for the fetus at this point, she said, it can be helpful to know that brain-sparing in growth-restricted fetuses was associated with increased risk for abnormal neurobehavioral outcomes in a controlled study of 126 preterm infants (Ultrasound Obstet. Gynecol. 2011;38:288-94).
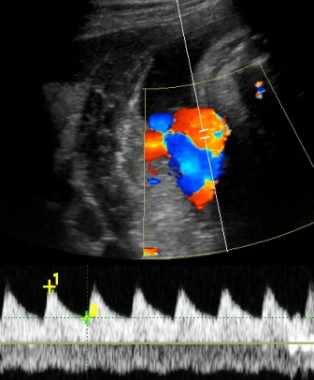
"It’s a sign of distress," she said. Until recently, clinicians would follow that fetus carefully with various kinds of testing and watch for reverse diastolic flow in the umbilical artery, which is a sign of significant compromise, hypoxemia, and possible death. Today, a finding of brain-sparing next leads Dr. Feldstein to interrogate the ductus venosus in the liver, which is "the hardest to do, but it can be done," she said.
The ductus venosus flow should be phasic but continuous, in a pattern called the "a wave" reflecting forward, continuous flow even during right atrial contractions. If the flow reverses backward into the ductus venosus during right atrial contractions, that’s a sign of cardiac compromise, severe hypoxia, and right ventricular dysfunction, associated with high risk of morbidity and mortality.
Locating the ductus venosus for sampling can be tricky, in part because it is so close to hepatic veins, but if the ductus venosus flow is abnormal enough, there’s a shortcut that is easier to do: Sample the umbilical vein. Abnormal phasicity in umbilical vein pulsation may reflect ductus venosus flow reversal.
A previous study reported that the risk for perinatal mortality increased to nearly 6% with an elevated umbilical artery S/D ratio, to more than 11% with absent or reversed diastolic flow in the uterine artery, and to 39% with an abnormal ductus venosus wave form (Ultrasound Obstet. Gynecol. 2003;22:240-5). In a 2010 Cochrane Review of 18 studies that included more than 10,000 women with high-risk pregnancies, Doppler ultrasound was associated with a 29% reduction in the rate of perinatal deaths (1.2% with Doppler and 1.7% without); analysis showed that using Doppler on 203 high-risk pregnancies would avoid 1 perinatal death (Cochrane Database Syst. Rev. 2010 Jan. 20). "So, there’s a significant impact and not that much excess work," Dr. Feldstein said.
She reported having no financial disclosures.
[email protected]
On Twitter @sherryboschert
SAN FRANCISCO – An umbilical artery systolic-to-diastolic ratio of less than 3 as measured on weekly Doppler ultrasounds in a fetus with 30 weeks’ or more gestation and suspected intrauterine growth restriction suggests that the fetus probably is doing okay, Dr. Vickie A. Feldstein said.
That "ballpark guideline" is most helpful if physicians at your institution have agreed to use the umbilical artery systolic/diastolic (S/D) ratio as the parameter for assessing fetuses with intrauterine growth restriction (IUGR) and have agreed on which anatomical location is preferred for the ultrasound interrogation, so that there is some uniformity in how results are presented and interpreted, she said.
The recent Practice Bulletin No. 134 from the American College of Obstetricians and Gynecologists recommended in May 2013 that if the ultrasonographically estimated fetal weight is below the 10th percentile for gestational age, further evaluation should be considered, such as Doppler blood flow studies of the umbilical artery (Obstet. Gynecol. 2013;121:1122-33).
The medical literature describes several Doppler ultrasound parameters that could be used in suspected IUGR, including the umbilical artery S/D ratio, the resistance index, or the pulsatility index. They’re all about the same phenomenon, which is measuring resistance to perfusion in the placenta as reflected in the interrogation of the umbilical artery, Dr. Feldstein said at a meeting on antepartum and intrapartum management sponsored by the University of California, San Francisco.
"I think a report that includes all of them would make our heads spin," said Dr. Feldstein, professor of clinical radiology at the university. She and her colleagues use the S/D ratio, calculated by using calipers to measure the peak of systole on umbilical artery Doppler ultrasound and dividing that by the measure of end diastole.
"I don’t care how fast the flow actually is, I care about the character of the flow, the relative difference between systole and diastole," she said. The ratio reflects the status of placental circulation. It normally is high early in pregnancy and decreases as gestation advances, placental resistance decreases, and there is more forward flow during diastole.
If separate umbilical artery Doppler tracings yield discrepant S/D ratios, that may reflect normal variability or be due to changes in fetal heart rate. "A significant change in heart rate might change the S/D ratio quite a bit," she said. Or, an ultrasound filter set too low can produce noise in the tracing that might alter where you place the calipers.
The location along the umbilical cord that the sonographer samples also can affect measurements. The medical literature is full of suggestions about where to sample. Dr. Feldstein recommends sampling toward the placenta, if possible, which will reflect resistance to perfusion in the placenta.
"We’ve found the cord insertion, typically, so we know where to look," she said. "The farther away from the placenta you go, you’re adding resistance of the cord to your tracing."
She encouraged obstetricians to talk to the people who do Doppler at their institutions "to decide together how you want this done, how you want it reported, and what parameter you want used, so you don’t overwhelm yourselves with excess information."
If the S/D ratio is a bit above 3 in a third-trimester fetus with IUGR but there’s decent diastolic flow, "don’t sweat the small stuff," she suggested. As the S/D ratio goes higher and higher, however, placental insufficiency (and resistance) increases and forward flow decreases, and can become absent or even reversed end-diastolic flow.
An absence of diastolic flow is associated with a 60%-70% loss of vasculature, "a really significant abnormality in the placenta," she said. With reversed diastolic flow, the odds of perinatal death increase more than fivefold.
A recent study of 1,116 fetuses with IUGR showed that an abnormal umbilical artery Doppler ultrasound tracing (defined by pulsatility index or the absence or reversal of end-diastolic flow) was significantly associated with adverse outcomes irrespective of estimated fetal weight or abdominal circumference (Am. J. Obstet. Gynecol. 2013;208:e1-6 [doi: 10.1016/j.ajog.2013.02.007]).
When Dr. Feldstein sees an abnormal umbilical artery Doppler tracing, she samples the fetal middle cerebral artery by Doppler ultrasound. The middle cerebral artery S/D ratio should always be higher than the umbilical artery SD ratio, with a typical middle cerebral artery S/D ratio greater than 4 after 30 weeks’ gestation.
A fetus in trouble with IUGR will respond by lowering cerebral vascular resistance to maintain blood flow to its brain, decreasing the middle cerebral artery S/D ratio in what’s known as a "brain-sparing" wave form. Although there’s nothing that can be done for the fetus at this point, she said, it can be helpful to know that brain-sparing in growth-restricted fetuses was associated with increased risk for abnormal neurobehavioral outcomes in a controlled study of 126 preterm infants (Ultrasound Obstet. Gynecol. 2011;38:288-94).
"It’s a sign of distress," she said. Until recently, clinicians would follow that fetus carefully with various kinds of testing and watch for reverse diastolic flow in the umbilical artery, which is a sign of significant compromise, hypoxemia, and possible death. Today, a finding of brain-sparing next leads Dr. Feldstein to interrogate the ductus venosus in the liver, which is "the hardest to do, but it can be done," she said.
The ductus venosus flow should be phasic but continuous, in a pattern called the "a wave" reflecting forward, continuous flow even during right atrial contractions. If the flow reverses backward into the ductus venosus during right atrial contractions, that’s a sign of cardiac compromise, severe hypoxia, and right ventricular dysfunction, associated with high risk of morbidity and mortality.
Locating the ductus venosus for sampling can be tricky, in part because it is so close to hepatic veins, but if the ductus venosus flow is abnormal enough, there’s a shortcut that is easier to do: Sample the umbilical vein. Abnormal phasicity in umbilical vein pulsation may reflect ductus venosus flow reversal.
A previous study reported that the risk for perinatal mortality increased to nearly 6% with an elevated umbilical artery S/D ratio, to more than 11% with absent or reversed diastolic flow in the uterine artery, and to 39% with an abnormal ductus venosus wave form (Ultrasound Obstet. Gynecol. 2003;22:240-5). In a 2010 Cochrane Review of 18 studies that included more than 10,000 women with high-risk pregnancies, Doppler ultrasound was associated with a 29% reduction in the rate of perinatal deaths (1.2% with Doppler and 1.7% without); analysis showed that using Doppler on 203 high-risk pregnancies would avoid 1 perinatal death (Cochrane Database Syst. Rev. 2010 Jan. 20). "So, there’s a significant impact and not that much excess work," Dr. Feldstein said.
She reported having no financial disclosures.
[email protected]
On Twitter @sherryboschert
SAN FRANCISCO – An umbilical artery systolic-to-diastolic ratio of less than 3 as measured on weekly Doppler ultrasounds in a fetus with 30 weeks’ or more gestation and suspected intrauterine growth restriction suggests that the fetus probably is doing okay, Dr. Vickie A. Feldstein said.
That "ballpark guideline" is most helpful if physicians at your institution have agreed to use the umbilical artery systolic/diastolic (S/D) ratio as the parameter for assessing fetuses with intrauterine growth restriction (IUGR) and have agreed on which anatomical location is preferred for the ultrasound interrogation, so that there is some uniformity in how results are presented and interpreted, she said.
The recent Practice Bulletin No. 134 from the American College of Obstetricians and Gynecologists recommended in May 2013 that if the ultrasonographically estimated fetal weight is below the 10th percentile for gestational age, further evaluation should be considered, such as Doppler blood flow studies of the umbilical artery (Obstet. Gynecol. 2013;121:1122-33).
The medical literature describes several Doppler ultrasound parameters that could be used in suspected IUGR, including the umbilical artery S/D ratio, the resistance index, or the pulsatility index. They’re all about the same phenomenon, which is measuring resistance to perfusion in the placenta as reflected in the interrogation of the umbilical artery, Dr. Feldstein said at a meeting on antepartum and intrapartum management sponsored by the University of California, San Francisco.
"I think a report that includes all of them would make our heads spin," said Dr. Feldstein, professor of clinical radiology at the university. She and her colleagues use the S/D ratio, calculated by using calipers to measure the peak of systole on umbilical artery Doppler ultrasound and dividing that by the measure of end diastole.
"I don’t care how fast the flow actually is, I care about the character of the flow, the relative difference between systole and diastole," she said. The ratio reflects the status of placental circulation. It normally is high early in pregnancy and decreases as gestation advances, placental resistance decreases, and there is more forward flow during diastole.
If separate umbilical artery Doppler tracings yield discrepant S/D ratios, that may reflect normal variability or be due to changes in fetal heart rate. "A significant change in heart rate might change the S/D ratio quite a bit," she said. Or, an ultrasound filter set too low can produce noise in the tracing that might alter where you place the calipers.
The location along the umbilical cord that the sonographer samples also can affect measurements. The medical literature is full of suggestions about where to sample. Dr. Feldstein recommends sampling toward the placenta, if possible, which will reflect resistance to perfusion in the placenta.
"We’ve found the cord insertion, typically, so we know where to look," she said. "The farther away from the placenta you go, you’re adding resistance of the cord to your tracing."
She encouraged obstetricians to talk to the people who do Doppler at their institutions "to decide together how you want this done, how you want it reported, and what parameter you want used, so you don’t overwhelm yourselves with excess information."
If the S/D ratio is a bit above 3 in a third-trimester fetus with IUGR but there’s decent diastolic flow, "don’t sweat the small stuff," she suggested. As the S/D ratio goes higher and higher, however, placental insufficiency (and resistance) increases and forward flow decreases, and can become absent or even reversed end-diastolic flow.
An absence of diastolic flow is associated with a 60%-70% loss of vasculature, "a really significant abnormality in the placenta," she said. With reversed diastolic flow, the odds of perinatal death increase more than fivefold.
A recent study of 1,116 fetuses with IUGR showed that an abnormal umbilical artery Doppler ultrasound tracing (defined by pulsatility index or the absence or reversal of end-diastolic flow) was significantly associated with adverse outcomes irrespective of estimated fetal weight or abdominal circumference (Am. J. Obstet. Gynecol. 2013;208:e1-6 [doi: 10.1016/j.ajog.2013.02.007]).
When Dr. Feldstein sees an abnormal umbilical artery Doppler tracing, she samples the fetal middle cerebral artery by Doppler ultrasound. The middle cerebral artery S/D ratio should always be higher than the umbilical artery SD ratio, with a typical middle cerebral artery S/D ratio greater than 4 after 30 weeks’ gestation.
A fetus in trouble with IUGR will respond by lowering cerebral vascular resistance to maintain blood flow to its brain, decreasing the middle cerebral artery S/D ratio in what’s known as a "brain-sparing" wave form. Although there’s nothing that can be done for the fetus at this point, she said, it can be helpful to know that brain-sparing in growth-restricted fetuses was associated with increased risk for abnormal neurobehavioral outcomes in a controlled study of 126 preterm infants (Ultrasound Obstet. Gynecol. 2011;38:288-94).
"It’s a sign of distress," she said. Until recently, clinicians would follow that fetus carefully with various kinds of testing and watch for reverse diastolic flow in the umbilical artery, which is a sign of significant compromise, hypoxemia, and possible death. Today, a finding of brain-sparing next leads Dr. Feldstein to interrogate the ductus venosus in the liver, which is "the hardest to do, but it can be done," she said.
The ductus venosus flow should be phasic but continuous, in a pattern called the "a wave" reflecting forward, continuous flow even during right atrial contractions. If the flow reverses backward into the ductus venosus during right atrial contractions, that’s a sign of cardiac compromise, severe hypoxia, and right ventricular dysfunction, associated with high risk of morbidity and mortality.
Locating the ductus venosus for sampling can be tricky, in part because it is so close to hepatic veins, but if the ductus venosus flow is abnormal enough, there’s a shortcut that is easier to do: Sample the umbilical vein. Abnormal phasicity in umbilical vein pulsation may reflect ductus venosus flow reversal.
A previous study reported that the risk for perinatal mortality increased to nearly 6% with an elevated umbilical artery S/D ratio, to more than 11% with absent or reversed diastolic flow in the uterine artery, and to 39% with an abnormal ductus venosus wave form (Ultrasound Obstet. Gynecol. 2003;22:240-5). In a 2010 Cochrane Review of 18 studies that included more than 10,000 women with high-risk pregnancies, Doppler ultrasound was associated with a 29% reduction in the rate of perinatal deaths (1.2% with Doppler and 1.7% without); analysis showed that using Doppler on 203 high-risk pregnancies would avoid 1 perinatal death (Cochrane Database Syst. Rev. 2010 Jan. 20). "So, there’s a significant impact and not that much excess work," Dr. Feldstein said.
She reported having no financial disclosures.
[email protected]
On Twitter @sherryboschert
EXPERT ANALYSIS FROM A MEETING ON ANTEPARTUM AND INTRAPARTUM MANAGEMENT
Egg banks save patients time, not money
SAN FRANCISCO – Achieving a pregnancy using frozen oocytes costs nearly $27,000, a survey of U.S. egg banks suggests.
Compared with using fresh oocytes from a matched donor, the frozen-egg route is "not really cost-saving as far as the patient is concerned, but it’s quicker and they don’t have to be matched, necessarily, to another person," Dr. Richard J. Paulson said at the UCLA annual in vitro fertilization and embryo transfer update 2013.
He and his associates surveyed all seven commercial egg banks that they could find in the United States in April 2012, six of which used vitrification as their freezing technique and one of which used slow-freeze techniques. The cost per oocyte averaged $2,225. The egg banks estimated their "efficiency" – the clinical pregnancy rate per oocyte (calculated as the number of pregnancies divided by the number of oocytes obtained, multiplied by 100) – as 10% using slow freezing or averaging 7.5% using vitrification.
"If efficiency is 7.5% per oocyte, you would need about 12 eggs to make one baby. Twelve eggs at $2,225 brings that to around $27,000 to get a pregnancy from frozen eggs," said Dr. Paulson, professor of obstetrics and gynecology and chief of the division of reproductive endocrinology and infertility at the University of Southern California in Los Angeles.
The efficiency rates are not as high as hoped for, but probably acceptable. "We’re not sure if we’re going to get to 24%. That seems optimistic to me, but even 10% is not bad if you have that kind of reproducibility," he said.
Commercial egg banks came in one of three operational models. A majority are affiliated with a single assisted reproductive technology (ART) clinic and supply oocytes only to that clinic’s patients. Two or three ART clinics may jointly create a shared commercial egg bank. Last, a few commercial egg banks ship cryopreserved oocytes all over the country and all over the world.
In the survey, the egg banks recommended using a minimum of four oocytes (in slow-freeze cryopreservation) or six to seven oocytes (in vitrification) per patient to attempt pregnancy. At the time, the banks reported having 3,130 oocytes from 294 donors in storage, and said 8,780 oocytes had been used for in vitro fertilization (IVF) to achieve 602 pregnancies (Fertil. Steril. 2013;99:827-31).
The survey’s results already are out of date because the field of egg banks is moving so quickly, Dr. Paulson said. For example, the number of pregnancies from frozen eggs probably has increased 10-fold, he said. "I’m guessing that right now there are perhaps 5,000-6,000 pregnancies in the world from egg freezing."
The egg banks in the survey had been in existence for 1-8 years.
Donor screening at all seven egg banks included psychological and medical screening, genetic counseling, estradiol/follicle-stimulating hormone (FSH) testing, antral follicle count testing, and routine CT cancer testing. Four egg banks also screened donors by anti-Müllerian hormone testing, five conducted routine fragile X carrier testing, and two performed routine karyotyping.
Dr. Paulson shared statistics from his institution’s experience with oocyte cryopreservation and embryo transfer. Ten of 18 women who were recipients of their own previously frozen eggs delivered live births (56%), as did three of four women who used frozen eggs that were not their own (75%).
This year is the 30th anniversary of the first oocyte donation for a successful pregnancy, Dr. Paulson said. The American Society for Reproductive Medicine released a guideline in 2013 saying that mature oocyte cryopreservation via vitrification should no longer be considered experimental (Fertil. Steril. 2013;99:37-43).
Advances in the field and the advantages of IVF using cryopreserved oocytes make egg banks the future for egg donations, he said.
Dr. Paulson is a speaker for Ferring Pharmaceuticals and an adviser to Cooper Surgical.
[email protected]
On Twitter @sherryboschert
SAN FRANCISCO – Achieving a pregnancy using frozen oocytes costs nearly $27,000, a survey of U.S. egg banks suggests.
Compared with using fresh oocytes from a matched donor, the frozen-egg route is "not really cost-saving as far as the patient is concerned, but it’s quicker and they don’t have to be matched, necessarily, to another person," Dr. Richard J. Paulson said at the UCLA annual in vitro fertilization and embryo transfer update 2013.
He and his associates surveyed all seven commercial egg banks that they could find in the United States in April 2012, six of which used vitrification as their freezing technique and one of which used slow-freeze techniques. The cost per oocyte averaged $2,225. The egg banks estimated their "efficiency" – the clinical pregnancy rate per oocyte (calculated as the number of pregnancies divided by the number of oocytes obtained, multiplied by 100) – as 10% using slow freezing or averaging 7.5% using vitrification.
"If efficiency is 7.5% per oocyte, you would need about 12 eggs to make one baby. Twelve eggs at $2,225 brings that to around $27,000 to get a pregnancy from frozen eggs," said Dr. Paulson, professor of obstetrics and gynecology and chief of the division of reproductive endocrinology and infertility at the University of Southern California in Los Angeles.
The efficiency rates are not as high as hoped for, but probably acceptable. "We’re not sure if we’re going to get to 24%. That seems optimistic to me, but even 10% is not bad if you have that kind of reproducibility," he said.
Commercial egg banks came in one of three operational models. A majority are affiliated with a single assisted reproductive technology (ART) clinic and supply oocytes only to that clinic’s patients. Two or three ART clinics may jointly create a shared commercial egg bank. Last, a few commercial egg banks ship cryopreserved oocytes all over the country and all over the world.
In the survey, the egg banks recommended using a minimum of four oocytes (in slow-freeze cryopreservation) or six to seven oocytes (in vitrification) per patient to attempt pregnancy. At the time, the banks reported having 3,130 oocytes from 294 donors in storage, and said 8,780 oocytes had been used for in vitro fertilization (IVF) to achieve 602 pregnancies (Fertil. Steril. 2013;99:827-31).
The survey’s results already are out of date because the field of egg banks is moving so quickly, Dr. Paulson said. For example, the number of pregnancies from frozen eggs probably has increased 10-fold, he said. "I’m guessing that right now there are perhaps 5,000-6,000 pregnancies in the world from egg freezing."
The egg banks in the survey had been in existence for 1-8 years.
Donor screening at all seven egg banks included psychological and medical screening, genetic counseling, estradiol/follicle-stimulating hormone (FSH) testing, antral follicle count testing, and routine CT cancer testing. Four egg banks also screened donors by anti-Müllerian hormone testing, five conducted routine fragile X carrier testing, and two performed routine karyotyping.
Dr. Paulson shared statistics from his institution’s experience with oocyte cryopreservation and embryo transfer. Ten of 18 women who were recipients of their own previously frozen eggs delivered live births (56%), as did three of four women who used frozen eggs that were not their own (75%).
This year is the 30th anniversary of the first oocyte donation for a successful pregnancy, Dr. Paulson said. The American Society for Reproductive Medicine released a guideline in 2013 saying that mature oocyte cryopreservation via vitrification should no longer be considered experimental (Fertil. Steril. 2013;99:37-43).
Advances in the field and the advantages of IVF using cryopreserved oocytes make egg banks the future for egg donations, he said.
Dr. Paulson is a speaker for Ferring Pharmaceuticals and an adviser to Cooper Surgical.
[email protected]
On Twitter @sherryboschert
SAN FRANCISCO – Achieving a pregnancy using frozen oocytes costs nearly $27,000, a survey of U.S. egg banks suggests.
Compared with using fresh oocytes from a matched donor, the frozen-egg route is "not really cost-saving as far as the patient is concerned, but it’s quicker and they don’t have to be matched, necessarily, to another person," Dr. Richard J. Paulson said at the UCLA annual in vitro fertilization and embryo transfer update 2013.
He and his associates surveyed all seven commercial egg banks that they could find in the United States in April 2012, six of which used vitrification as their freezing technique and one of which used slow-freeze techniques. The cost per oocyte averaged $2,225. The egg banks estimated their "efficiency" – the clinical pregnancy rate per oocyte (calculated as the number of pregnancies divided by the number of oocytes obtained, multiplied by 100) – as 10% using slow freezing or averaging 7.5% using vitrification.
"If efficiency is 7.5% per oocyte, you would need about 12 eggs to make one baby. Twelve eggs at $2,225 brings that to around $27,000 to get a pregnancy from frozen eggs," said Dr. Paulson, professor of obstetrics and gynecology and chief of the division of reproductive endocrinology and infertility at the University of Southern California in Los Angeles.
The efficiency rates are not as high as hoped for, but probably acceptable. "We’re not sure if we’re going to get to 24%. That seems optimistic to me, but even 10% is not bad if you have that kind of reproducibility," he said.
Commercial egg banks came in one of three operational models. A majority are affiliated with a single assisted reproductive technology (ART) clinic and supply oocytes only to that clinic’s patients. Two or three ART clinics may jointly create a shared commercial egg bank. Last, a few commercial egg banks ship cryopreserved oocytes all over the country and all over the world.
In the survey, the egg banks recommended using a minimum of four oocytes (in slow-freeze cryopreservation) or six to seven oocytes (in vitrification) per patient to attempt pregnancy. At the time, the banks reported having 3,130 oocytes from 294 donors in storage, and said 8,780 oocytes had been used for in vitro fertilization (IVF) to achieve 602 pregnancies (Fertil. Steril. 2013;99:827-31).
The survey’s results already are out of date because the field of egg banks is moving so quickly, Dr. Paulson said. For example, the number of pregnancies from frozen eggs probably has increased 10-fold, he said. "I’m guessing that right now there are perhaps 5,000-6,000 pregnancies in the world from egg freezing."
The egg banks in the survey had been in existence for 1-8 years.
Donor screening at all seven egg banks included psychological and medical screening, genetic counseling, estradiol/follicle-stimulating hormone (FSH) testing, antral follicle count testing, and routine CT cancer testing. Four egg banks also screened donors by anti-Müllerian hormone testing, five conducted routine fragile X carrier testing, and two performed routine karyotyping.
Dr. Paulson shared statistics from his institution’s experience with oocyte cryopreservation and embryo transfer. Ten of 18 women who were recipients of their own previously frozen eggs delivered live births (56%), as did three of four women who used frozen eggs that were not their own (75%).
This year is the 30th anniversary of the first oocyte donation for a successful pregnancy, Dr. Paulson said. The American Society for Reproductive Medicine released a guideline in 2013 saying that mature oocyte cryopreservation via vitrification should no longer be considered experimental (Fertil. Steril. 2013;99:37-43).
Advances in the field and the advantages of IVF using cryopreserved oocytes make egg banks the future for egg donations, he said.
Dr. Paulson is a speaker for Ferring Pharmaceuticals and an adviser to Cooper Surgical.
[email protected]
On Twitter @sherryboschert
AT A MEETING ON IVF AND EMBRYO TRANSFER
Major finding: Pregnancy using frozen oocytes requires an average of 12 eggs at a cost of $2,225 per egg and an efficiency rate of 7.5%-10%, for a total cost of approximately $27,000 per pregnancy.
Data source: Survey of all seven U.S. commercial egg banks in existence in April 2012.
Disclosures: Dr. Paulson is a speaker for Ferring Pharmaceuticals and an adviser to Cooper Surgical.
Oocyte vitrification aids fertility after cancer
SAN FRANCISCO – Controlled ovarian hyperstimulation and oocyte vitrification were similarly successful in preserving fertility in women with or without cancer, based on a retrospective study of 5 years of experience at one European center.
Among 475 oncologic patients and 560 patients with no cancer, 1,080 oocyte vitrification cycles were performed, 33% of them in women with cancer and 67% for nonmedical reasons. So far, 30 women have returned to use their frozen eggs (4 cancer patients and 26 noncancer patients), resulting in 20 pregnancies and 6 live births (in 1 cancer patient and 5 noncancer patients), with 8 pregnancies ongoing, Dr. Antonio Pellicer said at the UCLA annual in vitro fertilization and embryo transfer update 2013.
Some differences between the patient groups were seen. The total dose of gonadotropins used and serum estradiol levels were lower in patients who’d had cancer, said Dr. Pellicer, professor of obstetrics and gynecology and director of the Instituto Valenciano de Infertilidad at the University of Valencia (Spain) (Fertil. Steril. 2013;99:1994-9).
The quality of the oocytes seemed similar between groups, but fewer eggs can be retrieved after controlled ovarian stimulation in patients with cancer because some cancers affect the ovaries. Previous work by Dr. Pellicer and his associates showed that a mean of two fewer oocytes are retrieved from patients with hormone-dependent breast cancer, compared with noncancer controls.
Oocyte vitrification is a safe, promising method for fertility preservation not only in patients with cancer but in noncancer patients as well, he said. Approximately 91% of the nonmedical reasons for oocyte vitrification at his center were "social" reasons, Dr. Pellicer said. "This is a procedure that is increasing more and more."
A majority of women treated for breast cancer at his institution lose some ovarian function. Fertility preservation in female cancer patients can take three forms: medical protection of the gonads, ovarian cortex freezing and transplantation, and/or oocyte and embryo vitrification.
Five years of experience with reimplantation of frozen and then thawed ovarian tissue at three European centers showed that 11 of 60 patients who underwent orthotropic reimplantations of cryopreserved ovarian tissue became pregnant, and 6 patients so far have been delivered of 12 healthy infants (Fertil. Steril. 2013;99:1503-13).
The centers in the study included Dr. Pellicer’s institution and centers in Belgium and Denmark. "All of us have done between 800 and 1,000" cases of harvesting and freezing ovarian tissue. Cryopreservation of ovarian tissue may be the only option available to preserve fertility in prepubertal girls facing cancer treatment and for women who cannot delay the start of chemotherapy.
"The technique is promising and improving. I think there is room for it to continue in this direction for women who need fertility preservation," Dr. Pellicer said.
The concern with ovarian tissue cryopreservation has been that it may transplant neoplastic cells. Previous studies by other investigators, however, suggest that this risk is true in patients with leukemias but not in patients with breast cancer or non-Hodgkin’s lymphoma, he said.
Dr. Pellicer is a shareholder in Unisense. In his report on oocyte vitrification for patients with cancer, Merck Serono–Spain provided the medication for oncologic patients.
On Twitter @sherryboschert
SAN FRANCISCO – Controlled ovarian hyperstimulation and oocyte vitrification were similarly successful in preserving fertility in women with or without cancer, based on a retrospective study of 5 years of experience at one European center.
Among 475 oncologic patients and 560 patients with no cancer, 1,080 oocyte vitrification cycles were performed, 33% of them in women with cancer and 67% for nonmedical reasons. So far, 30 women have returned to use their frozen eggs (4 cancer patients and 26 noncancer patients), resulting in 20 pregnancies and 6 live births (in 1 cancer patient and 5 noncancer patients), with 8 pregnancies ongoing, Dr. Antonio Pellicer said at the UCLA annual in vitro fertilization and embryo transfer update 2013.
Some differences between the patient groups were seen. The total dose of gonadotropins used and serum estradiol levels were lower in patients who’d had cancer, said Dr. Pellicer, professor of obstetrics and gynecology and director of the Instituto Valenciano de Infertilidad at the University of Valencia (Spain) (Fertil. Steril. 2013;99:1994-9).
The quality of the oocytes seemed similar between groups, but fewer eggs can be retrieved after controlled ovarian stimulation in patients with cancer because some cancers affect the ovaries. Previous work by Dr. Pellicer and his associates showed that a mean of two fewer oocytes are retrieved from patients with hormone-dependent breast cancer, compared with noncancer controls.
Oocyte vitrification is a safe, promising method for fertility preservation not only in patients with cancer but in noncancer patients as well, he said. Approximately 91% of the nonmedical reasons for oocyte vitrification at his center were "social" reasons, Dr. Pellicer said. "This is a procedure that is increasing more and more."
A majority of women treated for breast cancer at his institution lose some ovarian function. Fertility preservation in female cancer patients can take three forms: medical protection of the gonads, ovarian cortex freezing and transplantation, and/or oocyte and embryo vitrification.
Five years of experience with reimplantation of frozen and then thawed ovarian tissue at three European centers showed that 11 of 60 patients who underwent orthotropic reimplantations of cryopreserved ovarian tissue became pregnant, and 6 patients so far have been delivered of 12 healthy infants (Fertil. Steril. 2013;99:1503-13).
The centers in the study included Dr. Pellicer’s institution and centers in Belgium and Denmark. "All of us have done between 800 and 1,000" cases of harvesting and freezing ovarian tissue. Cryopreservation of ovarian tissue may be the only option available to preserve fertility in prepubertal girls facing cancer treatment and for women who cannot delay the start of chemotherapy.
"The technique is promising and improving. I think there is room for it to continue in this direction for women who need fertility preservation," Dr. Pellicer said.
The concern with ovarian tissue cryopreservation has been that it may transplant neoplastic cells. Previous studies by other investigators, however, suggest that this risk is true in patients with leukemias but not in patients with breast cancer or non-Hodgkin’s lymphoma, he said.
Dr. Pellicer is a shareholder in Unisense. In his report on oocyte vitrification for patients with cancer, Merck Serono–Spain provided the medication for oncologic patients.
On Twitter @sherryboschert
SAN FRANCISCO – Controlled ovarian hyperstimulation and oocyte vitrification were similarly successful in preserving fertility in women with or without cancer, based on a retrospective study of 5 years of experience at one European center.
Among 475 oncologic patients and 560 patients with no cancer, 1,080 oocyte vitrification cycles were performed, 33% of them in women with cancer and 67% for nonmedical reasons. So far, 30 women have returned to use their frozen eggs (4 cancer patients and 26 noncancer patients), resulting in 20 pregnancies and 6 live births (in 1 cancer patient and 5 noncancer patients), with 8 pregnancies ongoing, Dr. Antonio Pellicer said at the UCLA annual in vitro fertilization and embryo transfer update 2013.
Some differences between the patient groups were seen. The total dose of gonadotropins used and serum estradiol levels were lower in patients who’d had cancer, said Dr. Pellicer, professor of obstetrics and gynecology and director of the Instituto Valenciano de Infertilidad at the University of Valencia (Spain) (Fertil. Steril. 2013;99:1994-9).
The quality of the oocytes seemed similar between groups, but fewer eggs can be retrieved after controlled ovarian stimulation in patients with cancer because some cancers affect the ovaries. Previous work by Dr. Pellicer and his associates showed that a mean of two fewer oocytes are retrieved from patients with hormone-dependent breast cancer, compared with noncancer controls.
Oocyte vitrification is a safe, promising method for fertility preservation not only in patients with cancer but in noncancer patients as well, he said. Approximately 91% of the nonmedical reasons for oocyte vitrification at his center were "social" reasons, Dr. Pellicer said. "This is a procedure that is increasing more and more."
A majority of women treated for breast cancer at his institution lose some ovarian function. Fertility preservation in female cancer patients can take three forms: medical protection of the gonads, ovarian cortex freezing and transplantation, and/or oocyte and embryo vitrification.
Five years of experience with reimplantation of frozen and then thawed ovarian tissue at three European centers showed that 11 of 60 patients who underwent orthotropic reimplantations of cryopreserved ovarian tissue became pregnant, and 6 patients so far have been delivered of 12 healthy infants (Fertil. Steril. 2013;99:1503-13).
The centers in the study included Dr. Pellicer’s institution and centers in Belgium and Denmark. "All of us have done between 800 and 1,000" cases of harvesting and freezing ovarian tissue. Cryopreservation of ovarian tissue may be the only option available to preserve fertility in prepubertal girls facing cancer treatment and for women who cannot delay the start of chemotherapy.
"The technique is promising and improving. I think there is room for it to continue in this direction for women who need fertility preservation," Dr. Pellicer said.
The concern with ovarian tissue cryopreservation has been that it may transplant neoplastic cells. Previous studies by other investigators, however, suggest that this risk is true in patients with leukemias but not in patients with breast cancer or non-Hodgkin’s lymphoma, he said.
Dr. Pellicer is a shareholder in Unisense. In his report on oocyte vitrification for patients with cancer, Merck Serono–Spain provided the medication for oncologic patients.
On Twitter @sherryboschert
AT A MEETING ON IVF AND EMBRYO TRANSFER
Major finding: Among 26 noncancer patients and 4 cancer patients who reclaimed their frozen oocytes, 5 noncancer patients and 1 cancer patient gave birth to live newborns; 8 pregnancies are ongoing.
Data source: A review of 5 years of experience with controlled ovarian hyperstimulation and oocyte vitrification at one European center.
Disclosures: Dr. Pellicer is a shareholder in Unisense. In his report on oocyte vitrification for patients with cancer, Merck Serono–Spain provided the medication for oncologic patients.
Identify monochorionic twins to guide pregnancy management
SAN FRANCISCO – The physician is responsible for knowing the chorionicity of a pregnant patient’s twins, so if you don’t offer diagnostic ultrasound or can’t make the diagnosis yourself, refer the patient to someone who can.
Knowing the chorionicity is essential because "it literally will define how you manage the pregnancy," Dr. Larry Rand said at a meeting on antepartum and intrapartum management sponsored by the University of California, San Francisco.
"This is a critical, critical concept," said Dr. Rand, director of perinatal services for the Fetal Treatment Program at the university. The chorionicity "should be the very first question that you ask yourself when you have a patient with twins."
You don’t need to be an expert on monochorionic twins; you just need to know if the patient is at high risk because of monochorionicity. Determining the chorionicity of twins before 14 weeks’ gestation is considered the standard of care, he stressed, and a physician who doesn’t know that twins are monochorionic could be liable if something goes wrong.
If the chorionicity is undetermined on your office ultrasound, refer the patient and request a chorionicity determination, he advised.
"This is one of the times that I have to say that ultrasound makes all the difference," Dr. Rand said. "The single most important ultrasound finding in the entire pregnancy is going to be the chorionicity."
A patient deserves to know whether her twins are monochorionic because she needs to be counseled appropriately about the risks. "It’s not the radiologist’s responsibility," he said. "The obstetrician is responsible for knowing the effect of chorionicity." Make sure to document that you have either determined the chorionicity yourself or have asked for an exam to assess chorionicity.
The rate of congenital anomalies in monochorionic twins, for example, is similar to the rate seen in diabetic mothers. This "changes the kind of tests you order and the things you see" compared with dichorionic twins, he said. Level II ultrasound examinations of anatomy that would be done in high-risk cases such as diabetic pregnancies, for example, should be done for monochorionic twins, whose elevated risk for anomalies may be due to an imperfect splitting of the single egg from which they came.
Compared with dichorionic twins, monochorionic twins also have increased risk for neurologic injury, including an eightfold increased incidence of cerebral palsy, and for preterm birth, spontaneous abortion, intrauterine growth restriction and growth discordance, intrauterine fetal demise, and twin-to-twin transfusion syndrome. Specialized centers can treat twin-to-twin transfusion syndrome with laser therapy if patients are diagnosed and referred in a timely manner – which is the reason for more-frequent screening of monochorionic twins. (See management recommendations below.)
Aterio-arterial (AA) anastomosis, a type of vascular connection within the placenta, can be detected on antenatal ultrasound. If present, the risk of twin-to-twin transfusion syndrome decreases 10-fold and the risk of intrauterine fetal demise decreases sevenfold. "It’s very helpful prognostically," he said.
Although AA anastomosis is not a new concept, it’s only recently that obstetrical imaging has evolved to identify it on antenatal ultrasound, which is "so helpful for counseling and management," said Dr. Rand, also the Lynne and Marc Benioff Endowed Chair in Maternal and Fetal Medicine at the university.
The major fear in monochorionic twins is intrauterine fetal demise of one twin, which leaves the surviving twin with a 10%-20% risk of death and a 20%-40% risk of long-term neurologic injury if the second twin survives. The difference even comes into play with genetic counseling. Aneuploidy risk in monochorionic twins is similar to risk in singletons (because they originated from a single egg). For invasive testing, one chorionic villus sampling covers both monochorionic twins (because there’s only one placenta to sample), but amniocentesis typically is performed on each amniotic sac. Some physicians sample just one amniotic sac for amniocentesis, thinking that it should cover both twins because they’re identical, but there are extremely rare cases of subtle differences that would not be detected by single-sac sampling, he said.
The other outcomes differ between monochorionic and dichorionic twins because the splitting of an egg can be imperfect; monochorionic twins share "plumbing" and vascular connections, and they have to share the resources of one placenta, Dr. Rand explained.
Before 14 weeks’ gestation, ultrasound is 99% sensitive and 100% specific for chorionicity, and it’s an easy assessment to do. In the second trimester, it gets trickier, which is why determining chorionicity early is so important, he said.
Dr. Rand gave several recommendations for management of monochorionic twins. In the first trimester, he said, determine the chorionicity, check the nuchal translucency, and assess fetal growth.
In the second trimester, conduct level II (high risk) anatomical surveys and echocardiograms to screen for fetal cardiac disease (as you would in a diabetic mother). Establish a minimum surveillance schedule of at least every 2 weeks for amniotic fluid between 16 and 28 weeks’ gestation, and every 4 weeks for fetal growth between 16 and 32 weeks’ gestation.
In the third trimester, continue the surveillance of amniotic fluid and fetal growth. Consider performing non–stress tests and Doppler ultrasounds. There’s debate about the optimal gestational age for delivery of monochorionic twins, between 36 and 38 weeks. "We’re not sure when to deliver," Dr. Rand said, but he recommends delivering monochorionic twins who’ve had issues with growth, fluid, or other aspects during pregnancy closer to 36 weeks and delivering twins with no issues closer to 38 weeks. "Monochorionicity is not, in and of itself, an indication for cesarean delivery," he added.
Approximately 75% of twins are dizygotic (from two separate eggs, each fertilized by sperm) and thus dichorionic. Among the 25% of twin pregnancies that are monozygotic (from one egg), the egg splits more slowly in 75% of cases, creating monochorionic twins that share a placenta and circulation. In the other 25% of monozygotic twins, the egg splits quickly, within 3 days, yielding two separate units and creating dichorionic twins, each with their own placenta.
Dr. Rand reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – The physician is responsible for knowing the chorionicity of a pregnant patient’s twins, so if you don’t offer diagnostic ultrasound or can’t make the diagnosis yourself, refer the patient to someone who can.
Knowing the chorionicity is essential because "it literally will define how you manage the pregnancy," Dr. Larry Rand said at a meeting on antepartum and intrapartum management sponsored by the University of California, San Francisco.
"This is a critical, critical concept," said Dr. Rand, director of perinatal services for the Fetal Treatment Program at the university. The chorionicity "should be the very first question that you ask yourself when you have a patient with twins."
You don’t need to be an expert on monochorionic twins; you just need to know if the patient is at high risk because of monochorionicity. Determining the chorionicity of twins before 14 weeks’ gestation is considered the standard of care, he stressed, and a physician who doesn’t know that twins are monochorionic could be liable if something goes wrong.
If the chorionicity is undetermined on your office ultrasound, refer the patient and request a chorionicity determination, he advised.
"This is one of the times that I have to say that ultrasound makes all the difference," Dr. Rand said. "The single most important ultrasound finding in the entire pregnancy is going to be the chorionicity."
A patient deserves to know whether her twins are monochorionic because she needs to be counseled appropriately about the risks. "It’s not the radiologist’s responsibility," he said. "The obstetrician is responsible for knowing the effect of chorionicity." Make sure to document that you have either determined the chorionicity yourself or have asked for an exam to assess chorionicity.
The rate of congenital anomalies in monochorionic twins, for example, is similar to the rate seen in diabetic mothers. This "changes the kind of tests you order and the things you see" compared with dichorionic twins, he said. Level II ultrasound examinations of anatomy that would be done in high-risk cases such as diabetic pregnancies, for example, should be done for monochorionic twins, whose elevated risk for anomalies may be due to an imperfect splitting of the single egg from which they came.
Compared with dichorionic twins, monochorionic twins also have increased risk for neurologic injury, including an eightfold increased incidence of cerebral palsy, and for preterm birth, spontaneous abortion, intrauterine growth restriction and growth discordance, intrauterine fetal demise, and twin-to-twin transfusion syndrome. Specialized centers can treat twin-to-twin transfusion syndrome with laser therapy if patients are diagnosed and referred in a timely manner – which is the reason for more-frequent screening of monochorionic twins. (See management recommendations below.)
Aterio-arterial (AA) anastomosis, a type of vascular connection within the placenta, can be detected on antenatal ultrasound. If present, the risk of twin-to-twin transfusion syndrome decreases 10-fold and the risk of intrauterine fetal demise decreases sevenfold. "It’s very helpful prognostically," he said.
Although AA anastomosis is not a new concept, it’s only recently that obstetrical imaging has evolved to identify it on antenatal ultrasound, which is "so helpful for counseling and management," said Dr. Rand, also the Lynne and Marc Benioff Endowed Chair in Maternal and Fetal Medicine at the university.
The major fear in monochorionic twins is intrauterine fetal demise of one twin, which leaves the surviving twin with a 10%-20% risk of death and a 20%-40% risk of long-term neurologic injury if the second twin survives. The difference even comes into play with genetic counseling. Aneuploidy risk in monochorionic twins is similar to risk in singletons (because they originated from a single egg). For invasive testing, one chorionic villus sampling covers both monochorionic twins (because there’s only one placenta to sample), but amniocentesis typically is performed on each amniotic sac. Some physicians sample just one amniotic sac for amniocentesis, thinking that it should cover both twins because they’re identical, but there are extremely rare cases of subtle differences that would not be detected by single-sac sampling, he said.
The other outcomes differ between monochorionic and dichorionic twins because the splitting of an egg can be imperfect; monochorionic twins share "plumbing" and vascular connections, and they have to share the resources of one placenta, Dr. Rand explained.
Before 14 weeks’ gestation, ultrasound is 99% sensitive and 100% specific for chorionicity, and it’s an easy assessment to do. In the second trimester, it gets trickier, which is why determining chorionicity early is so important, he said.
Dr. Rand gave several recommendations for management of monochorionic twins. In the first trimester, he said, determine the chorionicity, check the nuchal translucency, and assess fetal growth.
In the second trimester, conduct level II (high risk) anatomical surveys and echocardiograms to screen for fetal cardiac disease (as you would in a diabetic mother). Establish a minimum surveillance schedule of at least every 2 weeks for amniotic fluid between 16 and 28 weeks’ gestation, and every 4 weeks for fetal growth between 16 and 32 weeks’ gestation.
In the third trimester, continue the surveillance of amniotic fluid and fetal growth. Consider performing non–stress tests and Doppler ultrasounds. There’s debate about the optimal gestational age for delivery of monochorionic twins, between 36 and 38 weeks. "We’re not sure when to deliver," Dr. Rand said, but he recommends delivering monochorionic twins who’ve had issues with growth, fluid, or other aspects during pregnancy closer to 36 weeks and delivering twins with no issues closer to 38 weeks. "Monochorionicity is not, in and of itself, an indication for cesarean delivery," he added.
Approximately 75% of twins are dizygotic (from two separate eggs, each fertilized by sperm) and thus dichorionic. Among the 25% of twin pregnancies that are monozygotic (from one egg), the egg splits more slowly in 75% of cases, creating monochorionic twins that share a placenta and circulation. In the other 25% of monozygotic twins, the egg splits quickly, within 3 days, yielding two separate units and creating dichorionic twins, each with their own placenta.
Dr. Rand reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – The physician is responsible for knowing the chorionicity of a pregnant patient’s twins, so if you don’t offer diagnostic ultrasound or can’t make the diagnosis yourself, refer the patient to someone who can.
Knowing the chorionicity is essential because "it literally will define how you manage the pregnancy," Dr. Larry Rand said at a meeting on antepartum and intrapartum management sponsored by the University of California, San Francisco.
"This is a critical, critical concept," said Dr. Rand, director of perinatal services for the Fetal Treatment Program at the university. The chorionicity "should be the very first question that you ask yourself when you have a patient with twins."
You don’t need to be an expert on monochorionic twins; you just need to know if the patient is at high risk because of monochorionicity. Determining the chorionicity of twins before 14 weeks’ gestation is considered the standard of care, he stressed, and a physician who doesn’t know that twins are monochorionic could be liable if something goes wrong.
If the chorionicity is undetermined on your office ultrasound, refer the patient and request a chorionicity determination, he advised.
"This is one of the times that I have to say that ultrasound makes all the difference," Dr. Rand said. "The single most important ultrasound finding in the entire pregnancy is going to be the chorionicity."
A patient deserves to know whether her twins are monochorionic because she needs to be counseled appropriately about the risks. "It’s not the radiologist’s responsibility," he said. "The obstetrician is responsible for knowing the effect of chorionicity." Make sure to document that you have either determined the chorionicity yourself or have asked for an exam to assess chorionicity.
The rate of congenital anomalies in monochorionic twins, for example, is similar to the rate seen in diabetic mothers. This "changes the kind of tests you order and the things you see" compared with dichorionic twins, he said. Level II ultrasound examinations of anatomy that would be done in high-risk cases such as diabetic pregnancies, for example, should be done for monochorionic twins, whose elevated risk for anomalies may be due to an imperfect splitting of the single egg from which they came.
Compared with dichorionic twins, monochorionic twins also have increased risk for neurologic injury, including an eightfold increased incidence of cerebral palsy, and for preterm birth, spontaneous abortion, intrauterine growth restriction and growth discordance, intrauterine fetal demise, and twin-to-twin transfusion syndrome. Specialized centers can treat twin-to-twin transfusion syndrome with laser therapy if patients are diagnosed and referred in a timely manner – which is the reason for more-frequent screening of monochorionic twins. (See management recommendations below.)
Aterio-arterial (AA) anastomosis, a type of vascular connection within the placenta, can be detected on antenatal ultrasound. If present, the risk of twin-to-twin transfusion syndrome decreases 10-fold and the risk of intrauterine fetal demise decreases sevenfold. "It’s very helpful prognostically," he said.
Although AA anastomosis is not a new concept, it’s only recently that obstetrical imaging has evolved to identify it on antenatal ultrasound, which is "so helpful for counseling and management," said Dr. Rand, also the Lynne and Marc Benioff Endowed Chair in Maternal and Fetal Medicine at the university.
The major fear in monochorionic twins is intrauterine fetal demise of one twin, which leaves the surviving twin with a 10%-20% risk of death and a 20%-40% risk of long-term neurologic injury if the second twin survives. The difference even comes into play with genetic counseling. Aneuploidy risk in monochorionic twins is similar to risk in singletons (because they originated from a single egg). For invasive testing, one chorionic villus sampling covers both monochorionic twins (because there’s only one placenta to sample), but amniocentesis typically is performed on each amniotic sac. Some physicians sample just one amniotic sac for amniocentesis, thinking that it should cover both twins because they’re identical, but there are extremely rare cases of subtle differences that would not be detected by single-sac sampling, he said.
The other outcomes differ between monochorionic and dichorionic twins because the splitting of an egg can be imperfect; monochorionic twins share "plumbing" and vascular connections, and they have to share the resources of one placenta, Dr. Rand explained.
Before 14 weeks’ gestation, ultrasound is 99% sensitive and 100% specific for chorionicity, and it’s an easy assessment to do. In the second trimester, it gets trickier, which is why determining chorionicity early is so important, he said.
Dr. Rand gave several recommendations for management of monochorionic twins. In the first trimester, he said, determine the chorionicity, check the nuchal translucency, and assess fetal growth.
In the second trimester, conduct level II (high risk) anatomical surveys and echocardiograms to screen for fetal cardiac disease (as you would in a diabetic mother). Establish a minimum surveillance schedule of at least every 2 weeks for amniotic fluid between 16 and 28 weeks’ gestation, and every 4 weeks for fetal growth between 16 and 32 weeks’ gestation.
In the third trimester, continue the surveillance of amniotic fluid and fetal growth. Consider performing non–stress tests and Doppler ultrasounds. There’s debate about the optimal gestational age for delivery of monochorionic twins, between 36 and 38 weeks. "We’re not sure when to deliver," Dr. Rand said, but he recommends delivering monochorionic twins who’ve had issues with growth, fluid, or other aspects during pregnancy closer to 36 weeks and delivering twins with no issues closer to 38 weeks. "Monochorionicity is not, in and of itself, an indication for cesarean delivery," he added.
Approximately 75% of twins are dizygotic (from two separate eggs, each fertilized by sperm) and thus dichorionic. Among the 25% of twin pregnancies that are monozygotic (from one egg), the egg splits more slowly in 75% of cases, creating monochorionic twins that share a placenta and circulation. In the other 25% of monozygotic twins, the egg splits quickly, within 3 days, yielding two separate units and creating dichorionic twins, each with their own placenta.
Dr. Rand reported having no financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM A MEETING ON ANTEPARTUM AND INTRAPARTUM MANAGEMENT
Noninvasive prenatal testing could be secondary screen
SAN FRANCISCO – Using noninvasive prenatal DNA testing as a secondary screen after conventional prenatal testing could decrease the number of amniocenteses by more than 90%, reduce fetal losses, and improve the ratio of Down syndrome cases detected per amniocentesis, according to Dr. Mary E. Norton.
On the other hand, using noninvasive prenatal testing as the primary screen would increase the rate of detecting trisomy 13, 18, or 21 by a bit, but many women will have unsuccessful test results and will go on to have amniocentesis, negatively affecting the fetal loss rate and the ratio of Down syndrome detected per amniocentesis, she said.
For these and other reasons, it’s premature to abandon current prenatal screening for noninvasive prenatal testing, she said at a meeting on antepartum and intrapartum management sponsored by the University of California, San Francisco.
She used available data to compare three hypothetical scenarios in which 2.9 million pregnant women would be screened and 5,110 pregnancies would be affected by trisomy 13, 18, or 21. The women would be screened by conventional prenatal testing alone, by current screening methods followed by noninvasive prenatal testing, or solely by the noninvasive Digital Analysis of Selected Regions (DANSR) assay that can identify chromosome abnormalities by evaluating specific fragments of maternal cell-free DNA.
Approximately 145,000 of the women would have a positive screen under current testing or with current testing plus noninvasive testing as a secondary screen, but only 45,710 would have a positive screen with noninvasive testing as the primary screen, she estimated. The number of trisomy 13, 18, or 21 cases identified would be 4,667 under scenario one or two and slightly higher – 5,100 – using the noninvasive screening test primarily, said Dr. Norton, professor of obstetrics and gynecology at the university. Current screening can detect many more problems than can noninvasive screening, so 2,004 other abnormalities would be detected using current methods, compared with none using noninvasive testing.
A proportion of patients undergoing noninvasive prenatal screening would have no test result because the sequencing failed to work or not enough DNA was present to get a result – 4,350 women undergoing noninvasive testing as a secondary screen and 87,000 women with noninvasive testing as the primary screen, she estimated.
The total number of amniocenteses would be 145,000 under current testing (one for every positive screen), but would be reduced to 11,047 if noninvasive testing was used as a secondary screen to detect aneuploidies. With noninvasive testing as the primary screen, 67,460 women would undergo amniocentesis. That would result in 435 fetal losses with current testing alone, 33 with current testing and secondary noninvasive prenatal testing, or 202 fetal losses with noninvasive testing as the primary screen.
Eighteen amniocenteses would have to be performed to detect one case of Down syndrome with current prenatal testing alone. With noninvasive testing as a secondary screen, every two amniocenteses would detect a case of Down syndrome. With noninvasive testing as the primary screen, 13 amniocenteses would be needed to detect one case of Down syndrome, Dr. Norton said.
The relative benefits of each scenario remain controversial and may vary by each patient’s level of risk. Further study is needed before the standard of care in prenatal screening is changed.
"Prenatal screening is changing at a rapid pace," Dr. Norton said. Even experts are struggling with how best to incorporate all the new information and new tools. "It’s very exciting times, but confusing even for those of us who practice in the field," she commented.
Dr. Norton has received research funding from Ariosa Diagnostics and CellScape, which are involved in prenatal diagnosis products.
On Twitter @sherryboschert
SAN FRANCISCO – Using noninvasive prenatal DNA testing as a secondary screen after conventional prenatal testing could decrease the number of amniocenteses by more than 90%, reduce fetal losses, and improve the ratio of Down syndrome cases detected per amniocentesis, according to Dr. Mary E. Norton.
On the other hand, using noninvasive prenatal testing as the primary screen would increase the rate of detecting trisomy 13, 18, or 21 by a bit, but many women will have unsuccessful test results and will go on to have amniocentesis, negatively affecting the fetal loss rate and the ratio of Down syndrome detected per amniocentesis, she said.
For these and other reasons, it’s premature to abandon current prenatal screening for noninvasive prenatal testing, she said at a meeting on antepartum and intrapartum management sponsored by the University of California, San Francisco.
She used available data to compare three hypothetical scenarios in which 2.9 million pregnant women would be screened and 5,110 pregnancies would be affected by trisomy 13, 18, or 21. The women would be screened by conventional prenatal testing alone, by current screening methods followed by noninvasive prenatal testing, or solely by the noninvasive Digital Analysis of Selected Regions (DANSR) assay that can identify chromosome abnormalities by evaluating specific fragments of maternal cell-free DNA.
Approximately 145,000 of the women would have a positive screen under current testing or with current testing plus noninvasive testing as a secondary screen, but only 45,710 would have a positive screen with noninvasive testing as the primary screen, she estimated. The number of trisomy 13, 18, or 21 cases identified would be 4,667 under scenario one or two and slightly higher – 5,100 – using the noninvasive screening test primarily, said Dr. Norton, professor of obstetrics and gynecology at the university. Current screening can detect many more problems than can noninvasive screening, so 2,004 other abnormalities would be detected using current methods, compared with none using noninvasive testing.
A proportion of patients undergoing noninvasive prenatal screening would have no test result because the sequencing failed to work or not enough DNA was present to get a result – 4,350 women undergoing noninvasive testing as a secondary screen and 87,000 women with noninvasive testing as the primary screen, she estimated.
The total number of amniocenteses would be 145,000 under current testing (one for every positive screen), but would be reduced to 11,047 if noninvasive testing was used as a secondary screen to detect aneuploidies. With noninvasive testing as the primary screen, 67,460 women would undergo amniocentesis. That would result in 435 fetal losses with current testing alone, 33 with current testing and secondary noninvasive prenatal testing, or 202 fetal losses with noninvasive testing as the primary screen.
Eighteen amniocenteses would have to be performed to detect one case of Down syndrome with current prenatal testing alone. With noninvasive testing as a secondary screen, every two amniocenteses would detect a case of Down syndrome. With noninvasive testing as the primary screen, 13 amniocenteses would be needed to detect one case of Down syndrome, Dr. Norton said.
The relative benefits of each scenario remain controversial and may vary by each patient’s level of risk. Further study is needed before the standard of care in prenatal screening is changed.
"Prenatal screening is changing at a rapid pace," Dr. Norton said. Even experts are struggling with how best to incorporate all the new information and new tools. "It’s very exciting times, but confusing even for those of us who practice in the field," she commented.
Dr. Norton has received research funding from Ariosa Diagnostics and CellScape, which are involved in prenatal diagnosis products.
On Twitter @sherryboschert
SAN FRANCISCO – Using noninvasive prenatal DNA testing as a secondary screen after conventional prenatal testing could decrease the number of amniocenteses by more than 90%, reduce fetal losses, and improve the ratio of Down syndrome cases detected per amniocentesis, according to Dr. Mary E. Norton.
On the other hand, using noninvasive prenatal testing as the primary screen would increase the rate of detecting trisomy 13, 18, or 21 by a bit, but many women will have unsuccessful test results and will go on to have amniocentesis, negatively affecting the fetal loss rate and the ratio of Down syndrome detected per amniocentesis, she said.
For these and other reasons, it’s premature to abandon current prenatal screening for noninvasive prenatal testing, she said at a meeting on antepartum and intrapartum management sponsored by the University of California, San Francisco.
She used available data to compare three hypothetical scenarios in which 2.9 million pregnant women would be screened and 5,110 pregnancies would be affected by trisomy 13, 18, or 21. The women would be screened by conventional prenatal testing alone, by current screening methods followed by noninvasive prenatal testing, or solely by the noninvasive Digital Analysis of Selected Regions (DANSR) assay that can identify chromosome abnormalities by evaluating specific fragments of maternal cell-free DNA.
Approximately 145,000 of the women would have a positive screen under current testing or with current testing plus noninvasive testing as a secondary screen, but only 45,710 would have a positive screen with noninvasive testing as the primary screen, she estimated. The number of trisomy 13, 18, or 21 cases identified would be 4,667 under scenario one or two and slightly higher – 5,100 – using the noninvasive screening test primarily, said Dr. Norton, professor of obstetrics and gynecology at the university. Current screening can detect many more problems than can noninvasive screening, so 2,004 other abnormalities would be detected using current methods, compared with none using noninvasive testing.
A proportion of patients undergoing noninvasive prenatal screening would have no test result because the sequencing failed to work or not enough DNA was present to get a result – 4,350 women undergoing noninvasive testing as a secondary screen and 87,000 women with noninvasive testing as the primary screen, she estimated.
The total number of amniocenteses would be 145,000 under current testing (one for every positive screen), but would be reduced to 11,047 if noninvasive testing was used as a secondary screen to detect aneuploidies. With noninvasive testing as the primary screen, 67,460 women would undergo amniocentesis. That would result in 435 fetal losses with current testing alone, 33 with current testing and secondary noninvasive prenatal testing, or 202 fetal losses with noninvasive testing as the primary screen.
Eighteen amniocenteses would have to be performed to detect one case of Down syndrome with current prenatal testing alone. With noninvasive testing as a secondary screen, every two amniocenteses would detect a case of Down syndrome. With noninvasive testing as the primary screen, 13 amniocenteses would be needed to detect one case of Down syndrome, Dr. Norton said.
The relative benefits of each scenario remain controversial and may vary by each patient’s level of risk. Further study is needed before the standard of care in prenatal screening is changed.
"Prenatal screening is changing at a rapid pace," Dr. Norton said. Even experts are struggling with how best to incorporate all the new information and new tools. "It’s very exciting times, but confusing even for those of us who practice in the field," she commented.
Dr. Norton has received research funding from Ariosa Diagnostics and CellScape, which are involved in prenatal diagnosis products.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM A MEETING ON ANTEPARTUM AND INTRAPARTUM MANAGEMENT
Radioisotope therapy slowed metastatic pheochromocytoma, paraganglioma
SAN FRANCISCO – A majority of 22 patients with metastatic, inoperable pheochromocytoma or paraganglioma had stable disease or complete resolution 6 months after receiving the first dose of treatment with the radioisotope 131I-meta-iodobenzylguanidine.
Treatment with 131I-meta-iodobenzylguanidine (131I-MIBG) appears to be safe and was associated with disease stabilization or improvement, which makes it a useful therapeutic tool either alone or with external beam radiotherapy and chemotherapy for patients with metastatic pheochromocytoma or paraganglioma, according to Dr. Matthew A. Rutherford of Glasgow (Scotland) Royal Infirmary and his associates.
In general, approximately 10% of cases of pheochromocytoma and paraganglioma metastasize. Optimal treatment of these cases has been controversial as the tumors are rare and there is no standard therapy.
The researchers reviewed the experience of one tertiary center in offering treatment with 131I-MIBG from 1986 to 2011.
The 12 patients with metastatic pheochromocytoma and 10 patients with metastatic paraganglioma received a total of 68 doses of 131I-MIBG, a type of radioactive iodine. The average dose was 9,835 MBq (range, 5,000-11,300).
One year after starting therapy, 16 of the 22 patients were alive. The average survival time after the first dose of 131I-MIBG was more than 7 years (86 months), Dr. Rutherford reported in a featured poster presentation at the Endocrine Society’s Annual Meeting.
At the 6-month follow-up after starting 131I-MIBG treatment, one patient had complete resolution of disease as assessed by symptoms, biochemical assessment of catecholamine excretion rate, and tumor size, Dr. Rutherford reported.
Four patients (18%) had partial responses in symptoms. A partial biochemical or tumor response, defined as greater than a 50% reduction in catecholamine excretion rate or tumor bulk, was seen in one patient (5%) biochemically and three patients (14%) by tumor size.
Stable disease was defined as no change in symptoms, biochemical markers, or tumor bulk, or less than a 25% increase in tumor bulk or catecholamine excretion rate. Thirteen patients had stable symptoms, 11 had stable biochemical results, and 13 had stable tumor size.
Progressive disease was identified by increased symptoms in two patients, by a greater than a 25% increase in catecholamine excretion rate in two patients, and by a greater than 50% increase in tumor size in two patients. There were no 6-month data for two patients regarding symptoms, for seven patients regarding biochemical assessment, and for three patients regarding tumor bulk.
Eleven (50%) reported having no adverse effects from 131I-MIBG, seven (32%) had nausea and vomiting, and five (23%) developed transient bone marrow defects. There were no severe adverse events related to treatment.
Fourteen patients had no treatment other than 131I-MIBG (64%), while five patients also underwent chemotherapy, one had radiotherapy, and two patients underwent chemoradiotherapy.
Bone was the most common site of metastasis (nine patients), followed by lymph nodes (eight patients) and liver (five patients), with local invasion in two patients.
The patients’ mean age was 45 years (range, 15-77 years). Nine of the 22 were male. They were followed according to a defined protocol, with repeat hormonal evaluation and imaging within 6 months of starting 131I-MIBG treatment when possible.
Genetic testing identified four patients who were positive for the succinate dehydrogenase B gene (SDHB) and four with no identified genetic mutation. The other 14 patients were not tested for genetic mutations; 7 of them were older than the institution’s age cut-off for routine genetic testing.
Dr. Rutherford reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – A majority of 22 patients with metastatic, inoperable pheochromocytoma or paraganglioma had stable disease or complete resolution 6 months after receiving the first dose of treatment with the radioisotope 131I-meta-iodobenzylguanidine.
Treatment with 131I-meta-iodobenzylguanidine (131I-MIBG) appears to be safe and was associated with disease stabilization or improvement, which makes it a useful therapeutic tool either alone or with external beam radiotherapy and chemotherapy for patients with metastatic pheochromocytoma or paraganglioma, according to Dr. Matthew A. Rutherford of Glasgow (Scotland) Royal Infirmary and his associates.
In general, approximately 10% of cases of pheochromocytoma and paraganglioma metastasize. Optimal treatment of these cases has been controversial as the tumors are rare and there is no standard therapy.
The researchers reviewed the experience of one tertiary center in offering treatment with 131I-MIBG from 1986 to 2011.
The 12 patients with metastatic pheochromocytoma and 10 patients with metastatic paraganglioma received a total of 68 doses of 131I-MIBG, a type of radioactive iodine. The average dose was 9,835 MBq (range, 5,000-11,300).
One year after starting therapy, 16 of the 22 patients were alive. The average survival time after the first dose of 131I-MIBG was more than 7 years (86 months), Dr. Rutherford reported in a featured poster presentation at the Endocrine Society’s Annual Meeting.
At the 6-month follow-up after starting 131I-MIBG treatment, one patient had complete resolution of disease as assessed by symptoms, biochemical assessment of catecholamine excretion rate, and tumor size, Dr. Rutherford reported.
Four patients (18%) had partial responses in symptoms. A partial biochemical or tumor response, defined as greater than a 50% reduction in catecholamine excretion rate or tumor bulk, was seen in one patient (5%) biochemically and three patients (14%) by tumor size.
Stable disease was defined as no change in symptoms, biochemical markers, or tumor bulk, or less than a 25% increase in tumor bulk or catecholamine excretion rate. Thirteen patients had stable symptoms, 11 had stable biochemical results, and 13 had stable tumor size.
Progressive disease was identified by increased symptoms in two patients, by a greater than a 25% increase in catecholamine excretion rate in two patients, and by a greater than 50% increase in tumor size in two patients. There were no 6-month data for two patients regarding symptoms, for seven patients regarding biochemical assessment, and for three patients regarding tumor bulk.
Eleven (50%) reported having no adverse effects from 131I-MIBG, seven (32%) had nausea and vomiting, and five (23%) developed transient bone marrow defects. There were no severe adverse events related to treatment.
Fourteen patients had no treatment other than 131I-MIBG (64%), while five patients also underwent chemotherapy, one had radiotherapy, and two patients underwent chemoradiotherapy.
Bone was the most common site of metastasis (nine patients), followed by lymph nodes (eight patients) and liver (five patients), with local invasion in two patients.
The patients’ mean age was 45 years (range, 15-77 years). Nine of the 22 were male. They were followed according to a defined protocol, with repeat hormonal evaluation and imaging within 6 months of starting 131I-MIBG treatment when possible.
Genetic testing identified four patients who were positive for the succinate dehydrogenase B gene (SDHB) and four with no identified genetic mutation. The other 14 patients were not tested for genetic mutations; 7 of them were older than the institution’s age cut-off for routine genetic testing.
Dr. Rutherford reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – A majority of 22 patients with metastatic, inoperable pheochromocytoma or paraganglioma had stable disease or complete resolution 6 months after receiving the first dose of treatment with the radioisotope 131I-meta-iodobenzylguanidine.
Treatment with 131I-meta-iodobenzylguanidine (131I-MIBG) appears to be safe and was associated with disease stabilization or improvement, which makes it a useful therapeutic tool either alone or with external beam radiotherapy and chemotherapy for patients with metastatic pheochromocytoma or paraganglioma, according to Dr. Matthew A. Rutherford of Glasgow (Scotland) Royal Infirmary and his associates.
In general, approximately 10% of cases of pheochromocytoma and paraganglioma metastasize. Optimal treatment of these cases has been controversial as the tumors are rare and there is no standard therapy.
The researchers reviewed the experience of one tertiary center in offering treatment with 131I-MIBG from 1986 to 2011.
The 12 patients with metastatic pheochromocytoma and 10 patients with metastatic paraganglioma received a total of 68 doses of 131I-MIBG, a type of radioactive iodine. The average dose was 9,835 MBq (range, 5,000-11,300).
One year after starting therapy, 16 of the 22 patients were alive. The average survival time after the first dose of 131I-MIBG was more than 7 years (86 months), Dr. Rutherford reported in a featured poster presentation at the Endocrine Society’s Annual Meeting.
At the 6-month follow-up after starting 131I-MIBG treatment, one patient had complete resolution of disease as assessed by symptoms, biochemical assessment of catecholamine excretion rate, and tumor size, Dr. Rutherford reported.
Four patients (18%) had partial responses in symptoms. A partial biochemical or tumor response, defined as greater than a 50% reduction in catecholamine excretion rate or tumor bulk, was seen in one patient (5%) biochemically and three patients (14%) by tumor size.
Stable disease was defined as no change in symptoms, biochemical markers, or tumor bulk, or less than a 25% increase in tumor bulk or catecholamine excretion rate. Thirteen patients had stable symptoms, 11 had stable biochemical results, and 13 had stable tumor size.
Progressive disease was identified by increased symptoms in two patients, by a greater than a 25% increase in catecholamine excretion rate in two patients, and by a greater than 50% increase in tumor size in two patients. There were no 6-month data for two patients regarding symptoms, for seven patients regarding biochemical assessment, and for three patients regarding tumor bulk.
Eleven (50%) reported having no adverse effects from 131I-MIBG, seven (32%) had nausea and vomiting, and five (23%) developed transient bone marrow defects. There were no severe adverse events related to treatment.
Fourteen patients had no treatment other than 131I-MIBG (64%), while five patients also underwent chemotherapy, one had radiotherapy, and two patients underwent chemoradiotherapy.
Bone was the most common site of metastasis (nine patients), followed by lymph nodes (eight patients) and liver (five patients), with local invasion in two patients.
The patients’ mean age was 45 years (range, 15-77 years). Nine of the 22 were male. They were followed according to a defined protocol, with repeat hormonal evaluation and imaging within 6 months of starting 131I-MIBG treatment when possible.
Genetic testing identified four patients who were positive for the succinate dehydrogenase B gene (SDHB) and four with no identified genetic mutation. The other 14 patients were not tested for genetic mutations; 7 of them were older than the institution’s age cut-off for routine genetic testing.
Dr. Rutherford reported having no financial disclosures.
On Twitter @sherryboschert
AT ENDO 2013
Major finding: Disease completely resolved in one patient and was stable or improved in a majority of patients 6 months after starting 131I-MIBG therapy.
Data source: Review of 22 patients treated with 131I-MIBG for metastatic pheochromocytoma or paraganglioma at one tertiary care center in 1986-2011.
Disclosures: Dr. Rutherford reported having no financial disclosures.
HIV postexposure prophylaxis guidelines revised
Revised federal guidelines for managing health care workers who are exposed to HIV recommend using at least three drugs for prophylaxis in all cases instead of assessing a person’s risk to decide on the number of drugs, among other updates to the guidelines.
Changes in the recommended drugs in preferred postexposure prophylaxis (PEP) regimens should make them easier to tolerate than earlier regimens and may increase the proportion of health care workers who are able to complete the 28-day treatment, Dr. David T. Kuhar and his associates said in the new document from the U.S. Public Health Service.
Another change is that follow-up HIV testing in health care workers on PEP can be shortened to 4 months instead of 6 months if a newer fourth-generation combination HIV p24 antigen/HIV antibody test is used, the guidelines state. The document, which updates 2005 guidelines, was published online in the journal Infection Control and Hospital Epidemiology (2013 Aug. 7 [doi: 10.1086/672271]).
The updates are based on expert opinion in a working group comprised of representatives from the Centers for Disease Control and Prevention (CDC), the National Institutes of Health, the Food and Drug Administration, and the Health Resources and Services Administration, in consultation with a panel of experts, wrote Dr. Kuhar of the CDC and his associates.
The principles of PEP remain the same: Occupational exposures to HIV in health care workers should be considered urgent medical concerns, and should be reported and managed promptly under your institution’s procedures. Determine the HIV status of the patient whose blood or other bodily fluids potentially exposed the health care worker to HIV, if possible, to guide the need for PEP. Start PEP as soon as possible (within hours) after the exposure if PEP is indicated. Expert consultation is recommended, but PEP initiation should not be delayed while waiting for a consult. Close follow-up should start within 72 hours of HIV exposure and include counseling, baseline and follow-up HIV testing, and monitoring for drug toxicity, according to the guidelines.
Clinicians can consult experts locally or can call the National Clinicians’ Post-Exposure Prophylaxis Hotline (PEPline) at 888-448-4911.
The PEPline received approximately 10,000 calls in 2012 from clinicians about potential occupational exposures to HIV, said Dr. Ronald Goldschmidt of the department of family and community medicine at the University of California, San Francisco. He is director of the university’s National HIV/AIDS Clinicians’ Consultation Center, which includes the PEPline. Dr. Goldschmidt was a member of the panel of experts that consulted on the guidelines update.
Several new antiretroviral agents have been approved since the last update to the guidelines in 2005, and more information has become available about the use and side effects of the PEP medications. Clinicians had struggled with the 2005 version’s recommendations to assess the level of risk of HIV transmission in individual exposure incidents, creating problems in deciding whether to use two or three or more drugs in PEP regiments.
The new guidelines’ preferred HIV PEP regimen is oral raltegravir (Isentress) 400 mg twice daily and once-daily Truvada (a fixed-dose combination tablet containing 300 mg of tenofovir and 200 mg of emtricitabine). "Preparation of this PEP regimen in single-dose ‘starter packets,’ which are kept on hand at sites expected to manage occupational exposures to HIV, may facilitate timely initiation of PEP," the guidelines state.
Dr. Goldschmidt cautioned that Truvada should not be used before checking for preexisting renal problems. If there’s a history of renal problems, consult an HIV expert or consider a different regimen, he said.
An appendix to the document lists recommended and contraindicated alternatives.
Although there are no new definitive data showing greater efficacy with three-drug regimens for PEP compared with two-drug regimens for occupational HIV exposures, the new recommendation to use at least three drugs rests on studies showing greater reduction of viral burden in HIV-infected patients on three antiretroviral drugs instead of two, concerns about drug resistance in source patients of occupational exposures, and better safety and tolerability with some of the newer medications. The guidelines add a caveat that two-drug PEP regimens might be considered in consultation with an expert if antiretrovirals aren’t easily available or because of adherence or toxicity issues.
None of the antiretroviral agents have been approved by the Food and Drug Administration for use as PEP.
The previous complicated recommendations to assess a health care worker’s level of risk after a potential exposure to HIV were enshrined in a 2001 version of the guidelines, Dr. Goldschmidt said. The 2005 revision updated the recommended drugs but didn’t simplify the risk assessment. The current update makes decision-making less confusing by recommending at least a three-drug regimen for all occupational PEP cases and using better-tolerated drugs.
"With the simplification at this point, they’re throwing away those old tables that required clinicians to assess risk" and decreased the likelihood that PEP would be administered appropriately and completed, he said.
Separate guidelines have been published previously for management of nonoccupational HIV exposure (sexual, pediatric, or perinatal exposures).
Dr. Kuhar reported having no financial disclosures. Some of his coauthors reported financial associations with Bristol-Myers Squibb, Janssen, and other companies. Dr. Goldschmidt reported having no financial disclosures.
Exposed health care workers and those managing their exposures will now have more PEP options to consider. This offers the benefit of being able to individualize therapy to the individual; however, for providers with less experience in managing HIV exposures, having these options may be more confusing or intimidating. For that reason, expert resources like the HIV PEPline (888-448-4911) will be increasingly important.
These regimens will be more expensive. There aren’t any data to suggest that these three-drug regimens will be more effective in PEP. Here’s why the guideline authors said they made the change:

|
|
"The recommendation for consistent use of three-drug HIV PEP regimens reflects (1) studies demonstrating superior effectiveness of three drugs in reducing viral burden in HIV-infected persons compared with two agents; (2) concerns about source patient drug resistance to agents commonly used for PEP; (3) the safety and tolerability of new HIV drugs, and (4) the potential for improved PEP regimen adherence due to newer medications that are likely to have fewer side effects. Clinicians facing challenges such as antiretroviral medication availability, potential adherence and toxicity issues, and others associated with a three-drug PEP regimen might still consider a two-drug PEP regimen in consultation with an expert."
Any time you use more drugs, there is an opportunity for side effects. While the medications recommended here are generally safe and well tolerated, careful monitoring for side effects will be important for individuals receiving PEP.
In addition their improved tolerability, I think the other rationale for recommending these exact regimens is based on these regimens being less likely to have resistance.
Dr. C. Bradley Hare is medical director of the HIV/AIDS Clinic at San Francisco General Hospital. He has been a consultant or speaker for the following companies that make HIV medications: Gilead, Bristol-Myers Squibb, Janssen Pharmaceuticals, Merck, and AbbVie Pharmaceuticals.
Exposed health care workers and those managing their exposures will now have more PEP options to consider. This offers the benefit of being able to individualize therapy to the individual; however, for providers with less experience in managing HIV exposures, having these options may be more confusing or intimidating. For that reason, expert resources like the HIV PEPline (888-448-4911) will be increasingly important.
These regimens will be more expensive. There aren’t any data to suggest that these three-drug regimens will be more effective in PEP. Here’s why the guideline authors said they made the change:

|
|
"The recommendation for consistent use of three-drug HIV PEP regimens reflects (1) studies demonstrating superior effectiveness of three drugs in reducing viral burden in HIV-infected persons compared with two agents; (2) concerns about source patient drug resistance to agents commonly used for PEP; (3) the safety and tolerability of new HIV drugs, and (4) the potential for improved PEP regimen adherence due to newer medications that are likely to have fewer side effects. Clinicians facing challenges such as antiretroviral medication availability, potential adherence and toxicity issues, and others associated with a three-drug PEP regimen might still consider a two-drug PEP regimen in consultation with an expert."
Any time you use more drugs, there is an opportunity for side effects. While the medications recommended here are generally safe and well tolerated, careful monitoring for side effects will be important for individuals receiving PEP.
In addition their improved tolerability, I think the other rationale for recommending these exact regimens is based on these regimens being less likely to have resistance.
Dr. C. Bradley Hare is medical director of the HIV/AIDS Clinic at San Francisco General Hospital. He has been a consultant or speaker for the following companies that make HIV medications: Gilead, Bristol-Myers Squibb, Janssen Pharmaceuticals, Merck, and AbbVie Pharmaceuticals.
Exposed health care workers and those managing their exposures will now have more PEP options to consider. This offers the benefit of being able to individualize therapy to the individual; however, for providers with less experience in managing HIV exposures, having these options may be more confusing or intimidating. For that reason, expert resources like the HIV PEPline (888-448-4911) will be increasingly important.
These regimens will be more expensive. There aren’t any data to suggest that these three-drug regimens will be more effective in PEP. Here’s why the guideline authors said they made the change:

|
|
"The recommendation for consistent use of three-drug HIV PEP regimens reflects (1) studies demonstrating superior effectiveness of three drugs in reducing viral burden in HIV-infected persons compared with two agents; (2) concerns about source patient drug resistance to agents commonly used for PEP; (3) the safety and tolerability of new HIV drugs, and (4) the potential for improved PEP regimen adherence due to newer medications that are likely to have fewer side effects. Clinicians facing challenges such as antiretroviral medication availability, potential adherence and toxicity issues, and others associated with a three-drug PEP regimen might still consider a two-drug PEP regimen in consultation with an expert."
Any time you use more drugs, there is an opportunity for side effects. While the medications recommended here are generally safe and well tolerated, careful monitoring for side effects will be important for individuals receiving PEP.
In addition their improved tolerability, I think the other rationale for recommending these exact regimens is based on these regimens being less likely to have resistance.
Dr. C. Bradley Hare is medical director of the HIV/AIDS Clinic at San Francisco General Hospital. He has been a consultant or speaker for the following companies that make HIV medications: Gilead, Bristol-Myers Squibb, Janssen Pharmaceuticals, Merck, and AbbVie Pharmaceuticals.
Revised federal guidelines for managing health care workers who are exposed to HIV recommend using at least three drugs for prophylaxis in all cases instead of assessing a person’s risk to decide on the number of drugs, among other updates to the guidelines.
Changes in the recommended drugs in preferred postexposure prophylaxis (PEP) regimens should make them easier to tolerate than earlier regimens and may increase the proportion of health care workers who are able to complete the 28-day treatment, Dr. David T. Kuhar and his associates said in the new document from the U.S. Public Health Service.
Another change is that follow-up HIV testing in health care workers on PEP can be shortened to 4 months instead of 6 months if a newer fourth-generation combination HIV p24 antigen/HIV antibody test is used, the guidelines state. The document, which updates 2005 guidelines, was published online in the journal Infection Control and Hospital Epidemiology (2013 Aug. 7 [doi: 10.1086/672271]).
The updates are based on expert opinion in a working group comprised of representatives from the Centers for Disease Control and Prevention (CDC), the National Institutes of Health, the Food and Drug Administration, and the Health Resources and Services Administration, in consultation with a panel of experts, wrote Dr. Kuhar of the CDC and his associates.
The principles of PEP remain the same: Occupational exposures to HIV in health care workers should be considered urgent medical concerns, and should be reported and managed promptly under your institution’s procedures. Determine the HIV status of the patient whose blood or other bodily fluids potentially exposed the health care worker to HIV, if possible, to guide the need for PEP. Start PEP as soon as possible (within hours) after the exposure if PEP is indicated. Expert consultation is recommended, but PEP initiation should not be delayed while waiting for a consult. Close follow-up should start within 72 hours of HIV exposure and include counseling, baseline and follow-up HIV testing, and monitoring for drug toxicity, according to the guidelines.
Clinicians can consult experts locally or can call the National Clinicians’ Post-Exposure Prophylaxis Hotline (PEPline) at 888-448-4911.
The PEPline received approximately 10,000 calls in 2012 from clinicians about potential occupational exposures to HIV, said Dr. Ronald Goldschmidt of the department of family and community medicine at the University of California, San Francisco. He is director of the university’s National HIV/AIDS Clinicians’ Consultation Center, which includes the PEPline. Dr. Goldschmidt was a member of the panel of experts that consulted on the guidelines update.
Several new antiretroviral agents have been approved since the last update to the guidelines in 2005, and more information has become available about the use and side effects of the PEP medications. Clinicians had struggled with the 2005 version’s recommendations to assess the level of risk of HIV transmission in individual exposure incidents, creating problems in deciding whether to use two or three or more drugs in PEP regiments.
The new guidelines’ preferred HIV PEP regimen is oral raltegravir (Isentress) 400 mg twice daily and once-daily Truvada (a fixed-dose combination tablet containing 300 mg of tenofovir and 200 mg of emtricitabine). "Preparation of this PEP regimen in single-dose ‘starter packets,’ which are kept on hand at sites expected to manage occupational exposures to HIV, may facilitate timely initiation of PEP," the guidelines state.
Dr. Goldschmidt cautioned that Truvada should not be used before checking for preexisting renal problems. If there’s a history of renal problems, consult an HIV expert or consider a different regimen, he said.
An appendix to the document lists recommended and contraindicated alternatives.
Although there are no new definitive data showing greater efficacy with three-drug regimens for PEP compared with two-drug regimens for occupational HIV exposures, the new recommendation to use at least three drugs rests on studies showing greater reduction of viral burden in HIV-infected patients on three antiretroviral drugs instead of two, concerns about drug resistance in source patients of occupational exposures, and better safety and tolerability with some of the newer medications. The guidelines add a caveat that two-drug PEP regimens might be considered in consultation with an expert if antiretrovirals aren’t easily available or because of adherence or toxicity issues.
None of the antiretroviral agents have been approved by the Food and Drug Administration for use as PEP.
The previous complicated recommendations to assess a health care worker’s level of risk after a potential exposure to HIV were enshrined in a 2001 version of the guidelines, Dr. Goldschmidt said. The 2005 revision updated the recommended drugs but didn’t simplify the risk assessment. The current update makes decision-making less confusing by recommending at least a three-drug regimen for all occupational PEP cases and using better-tolerated drugs.
"With the simplification at this point, they’re throwing away those old tables that required clinicians to assess risk" and decreased the likelihood that PEP would be administered appropriately and completed, he said.
Separate guidelines have been published previously for management of nonoccupational HIV exposure (sexual, pediatric, or perinatal exposures).
Dr. Kuhar reported having no financial disclosures. Some of his coauthors reported financial associations with Bristol-Myers Squibb, Janssen, and other companies. Dr. Goldschmidt reported having no financial disclosures.
Revised federal guidelines for managing health care workers who are exposed to HIV recommend using at least three drugs for prophylaxis in all cases instead of assessing a person’s risk to decide on the number of drugs, among other updates to the guidelines.
Changes in the recommended drugs in preferred postexposure prophylaxis (PEP) regimens should make them easier to tolerate than earlier regimens and may increase the proportion of health care workers who are able to complete the 28-day treatment, Dr. David T. Kuhar and his associates said in the new document from the U.S. Public Health Service.
Another change is that follow-up HIV testing in health care workers on PEP can be shortened to 4 months instead of 6 months if a newer fourth-generation combination HIV p24 antigen/HIV antibody test is used, the guidelines state. The document, which updates 2005 guidelines, was published online in the journal Infection Control and Hospital Epidemiology (2013 Aug. 7 [doi: 10.1086/672271]).
The updates are based on expert opinion in a working group comprised of representatives from the Centers for Disease Control and Prevention (CDC), the National Institutes of Health, the Food and Drug Administration, and the Health Resources and Services Administration, in consultation with a panel of experts, wrote Dr. Kuhar of the CDC and his associates.
The principles of PEP remain the same: Occupational exposures to HIV in health care workers should be considered urgent medical concerns, and should be reported and managed promptly under your institution’s procedures. Determine the HIV status of the patient whose blood or other bodily fluids potentially exposed the health care worker to HIV, if possible, to guide the need for PEP. Start PEP as soon as possible (within hours) after the exposure if PEP is indicated. Expert consultation is recommended, but PEP initiation should not be delayed while waiting for a consult. Close follow-up should start within 72 hours of HIV exposure and include counseling, baseline and follow-up HIV testing, and monitoring for drug toxicity, according to the guidelines.
Clinicians can consult experts locally or can call the National Clinicians’ Post-Exposure Prophylaxis Hotline (PEPline) at 888-448-4911.
The PEPline received approximately 10,000 calls in 2012 from clinicians about potential occupational exposures to HIV, said Dr. Ronald Goldschmidt of the department of family and community medicine at the University of California, San Francisco. He is director of the university’s National HIV/AIDS Clinicians’ Consultation Center, which includes the PEPline. Dr. Goldschmidt was a member of the panel of experts that consulted on the guidelines update.
Several new antiretroviral agents have been approved since the last update to the guidelines in 2005, and more information has become available about the use and side effects of the PEP medications. Clinicians had struggled with the 2005 version’s recommendations to assess the level of risk of HIV transmission in individual exposure incidents, creating problems in deciding whether to use two or three or more drugs in PEP regiments.
The new guidelines’ preferred HIV PEP regimen is oral raltegravir (Isentress) 400 mg twice daily and once-daily Truvada (a fixed-dose combination tablet containing 300 mg of tenofovir and 200 mg of emtricitabine). "Preparation of this PEP regimen in single-dose ‘starter packets,’ which are kept on hand at sites expected to manage occupational exposures to HIV, may facilitate timely initiation of PEP," the guidelines state.
Dr. Goldschmidt cautioned that Truvada should not be used before checking for preexisting renal problems. If there’s a history of renal problems, consult an HIV expert or consider a different regimen, he said.
An appendix to the document lists recommended and contraindicated alternatives.
Although there are no new definitive data showing greater efficacy with three-drug regimens for PEP compared with two-drug regimens for occupational HIV exposures, the new recommendation to use at least three drugs rests on studies showing greater reduction of viral burden in HIV-infected patients on three antiretroviral drugs instead of two, concerns about drug resistance in source patients of occupational exposures, and better safety and tolerability with some of the newer medications. The guidelines add a caveat that two-drug PEP regimens might be considered in consultation with an expert if antiretrovirals aren’t easily available or because of adherence or toxicity issues.
None of the antiretroviral agents have been approved by the Food and Drug Administration for use as PEP.
The previous complicated recommendations to assess a health care worker’s level of risk after a potential exposure to HIV were enshrined in a 2001 version of the guidelines, Dr. Goldschmidt said. The 2005 revision updated the recommended drugs but didn’t simplify the risk assessment. The current update makes decision-making less confusing by recommending at least a three-drug regimen for all occupational PEP cases and using better-tolerated drugs.
"With the simplification at this point, they’re throwing away those old tables that required clinicians to assess risk" and decreased the likelihood that PEP would be administered appropriately and completed, he said.
Separate guidelines have been published previously for management of nonoccupational HIV exposure (sexual, pediatric, or perinatal exposures).
Dr. Kuhar reported having no financial disclosures. Some of his coauthors reported financial associations with Bristol-Myers Squibb, Janssen, and other companies. Dr. Goldschmidt reported having no financial disclosures.
FROM INFECTION CONTROL AND HOSPITAL EPIDEMIOLOGY
Major finding: All PEP regimens for health care workers should include at least three drugs from a new list of easier-to-tolerate regimens, and follow-up HIV testing can be shortened to 4 months if a newer fourth-generation combination HIV p24 antigen/HIV antibody test is used.
Data source: Updated guidelines by the U.S. Public Health Service, based on expert opinion.
Disclosures: Dr. Kuhar reported having no financial disclosures. Some of his coauthors reported financial associations with Bristol-Myers Squibb, Janssen, and other companies. Dr. Goldschmidt reported having no financial disclosures.