User login
Genetic assessment for CHD: Case-specific, stepwise
Congenital heart defects (CHDs) are etiologically heterogeneous, but in recent years it has become clear that genetics plays a larger role in the development of CHDs than was previously thought. Research has been shifting from a focus on risk – estimating the magnitude of increased risk, for instance, based on maternal or familial risk factors – to a focus on the etiology of cardiac defects.
In practice, advances in genetic testing technologies have made the underlying causes of CHDs increasingly detectable. Chromosomal microarray analysis (CMA) – technology that detects significantly more and smaller changes in the amount of chromosomal material than traditional karyotype – has been proven to increase the diagnostic yield in cases of isolated CHDs and CHDs with extracardiac anomalies. Targeted next-generation sequencing also is now available as an additional approach in selective cases, and a clinically viable option for whole-exome sequencing is fast approaching.
For researchers, genetic evaluation carries the potential to unravel remaining mysteries about underlying causes of CHDs – to provide pathological insights and identify potential therapeutic targets. Currently, about 6 % of the total pie of presumed genetic determinants of CHDs is attributed to chromosomal anomalies, 10% to copy number variants, and 12% to single-gene defects. The remaining 72% of etiology, approximately, is undetermined.
As Helen Taussig, MD, (known as the founder of pediatric cardiology) once said, common cardiac malformations occurring in otherwise “normal” individuals “must be genetic in origin.”1 Greater use of genetic testing – and in particular, of whole-exome sequencing – will drive down this “undetermined” piece of the genetics pie.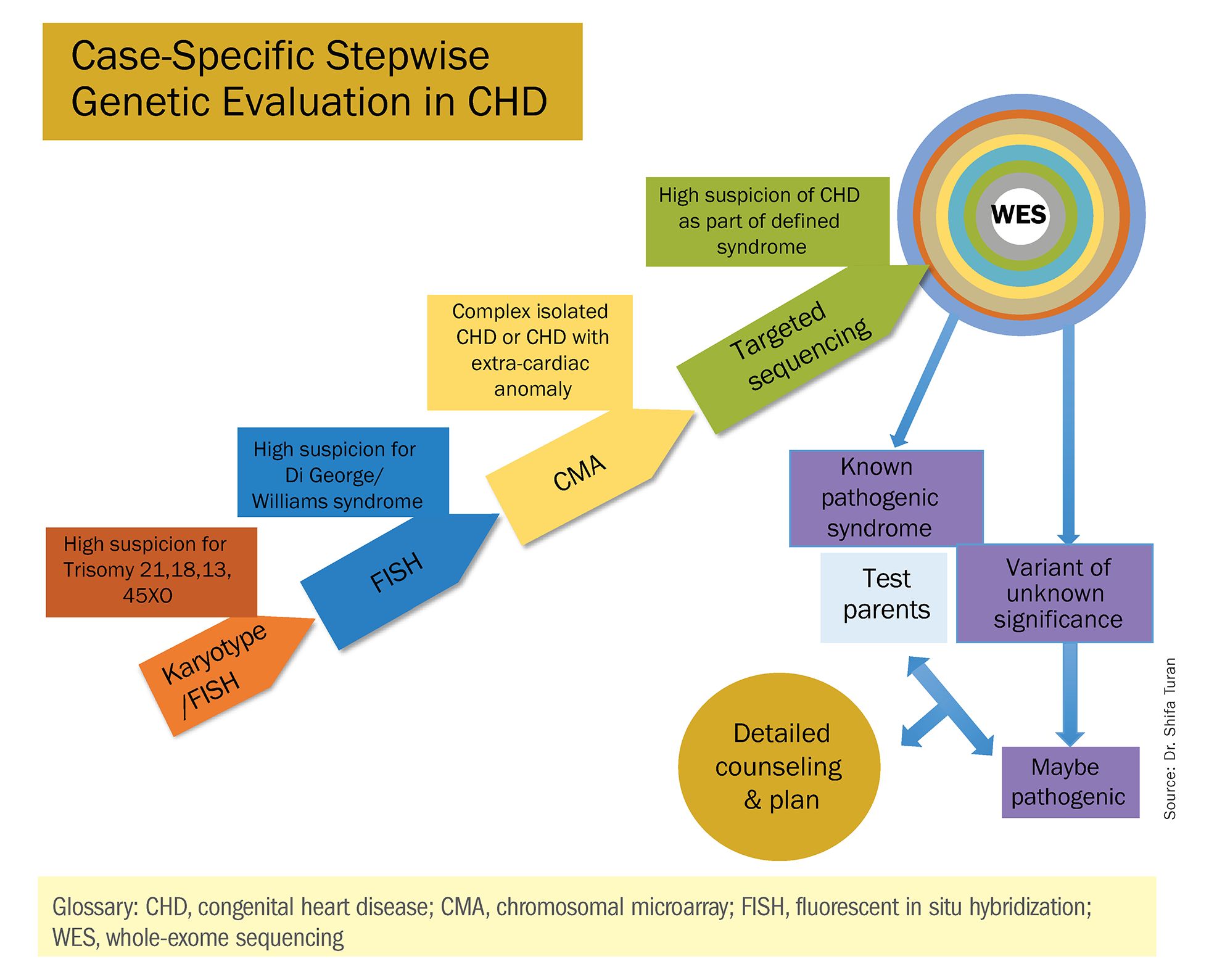
For clinicians and patients, prenatal genetic evaluation can inform clinical management, guiding decisions on the mode, timing, and location of delivery. Genetic assessments help guide the neonatal health care team in taking optimal care of the infant, and the surgeon in preparing for neonatal surgeries and postsurgical complications.
In a recent analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database, prenatal diagnosis was associated with a lower overall prevalence of major preoperative risk factors for cardiac surgery.2 Surgical outcomes themselves also have been shown to be better after the prenatal diagnosis of complex CHDs, mainly because of improvements in perioperative care.3
When genetic etiology is elucidated, the cardiologist also is better able to counsel patients about anticipated challenges – such as the propensity, with certain genetic variants of CHD, to develop neurodevelopmental delays or other cardiac complications – and to target patient follow-up. Patients also can make informed decisions about termination or continuation of a current pregnancy and about family planning in the future.
Fortunately, advances in genetics technology have paralleled technological advancements in ultrasound. As I discussed in part one of this two-part Master Class series, it is now possible to detect many major CHDs well before 16 weeks’ gestation. Checking the structure of the fetal heart at the first-trimester screening and sonography (11-14 weeks of gestation) offers the opportunity for early genetic assessment, counseling, and planning when anomalies are detected.
A personalized approach
There has been growing interest in recent years in CMA for the prenatal genetic workup of CHDs. Microarray targets chromosomal regions at a much higher resolution than traditional karyotype. Traditional karyotype assesses both changes in chromosome number as well as more subtle structural changes such as chromosomal deletions and duplications. CMA finds what traditional karyotype identifies, but in addition, it identifies much smaller, clinically relevant chromosomal deletions and duplications that are not detected by karyotype performed with or without fluorescence in-situ hybridization (FISH). FISH uses DNA probes that carry fluorescent tags to detect chromosomal DNA.
At our center, we studied the prenatal genetic test results of 145 fetuses diagnosed with CHDs. Each case involved FISH for aneuploidy/karyotype, followed by CMA in cases of a negative karyotype result. CMA increased the diagnostic yield in cases of CHD by 19.8% overall – 17.4% in cases of isolated CHD and 24.5% in cases of CHD plus extracardiac anomalies.4
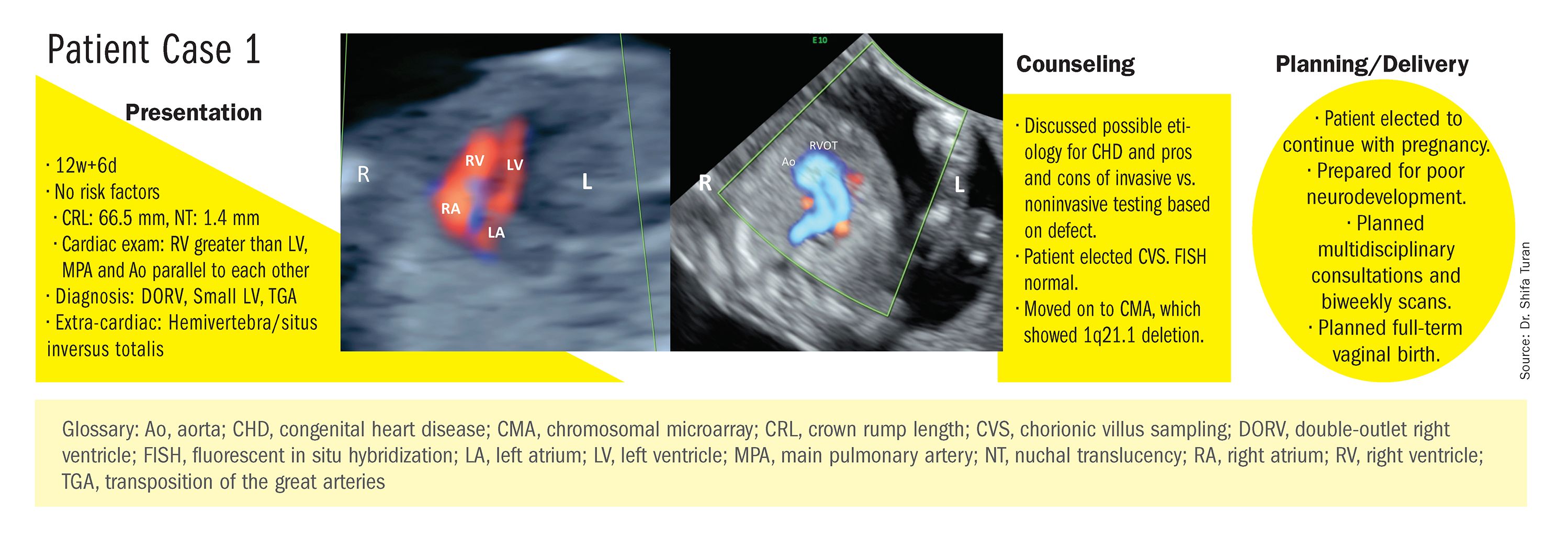
Indeed, although a microarray costs more and takes an additional 2 weeks to run, CMA should be strongly considered as first-line testing for the prenatal genetic evaluation of fetuses with major structural cardiac abnormalities detected by ultrasound. However, there still are cases in which a karyotype might be sufficient. For instance, if I see that a fetus has an atrial-ventricular septal defect on a prenatal ultrasound, and there are markers for trisomy 21, 13, or 18, or Turner’s syndrome (45 XO), I usually recommend a karyotype or FISH rather than an initial CMA. If the karyotype is abnormal – which is likely in such a scenario – there isn’t a need for more extensive testing.
Similarly, when there is high suspicion for DiGeorge syndrome (the 22q11.2 deletion, which often includes cleft palate and aortic arch abnormalities), usually it is most appropriate to perform a FISH test.
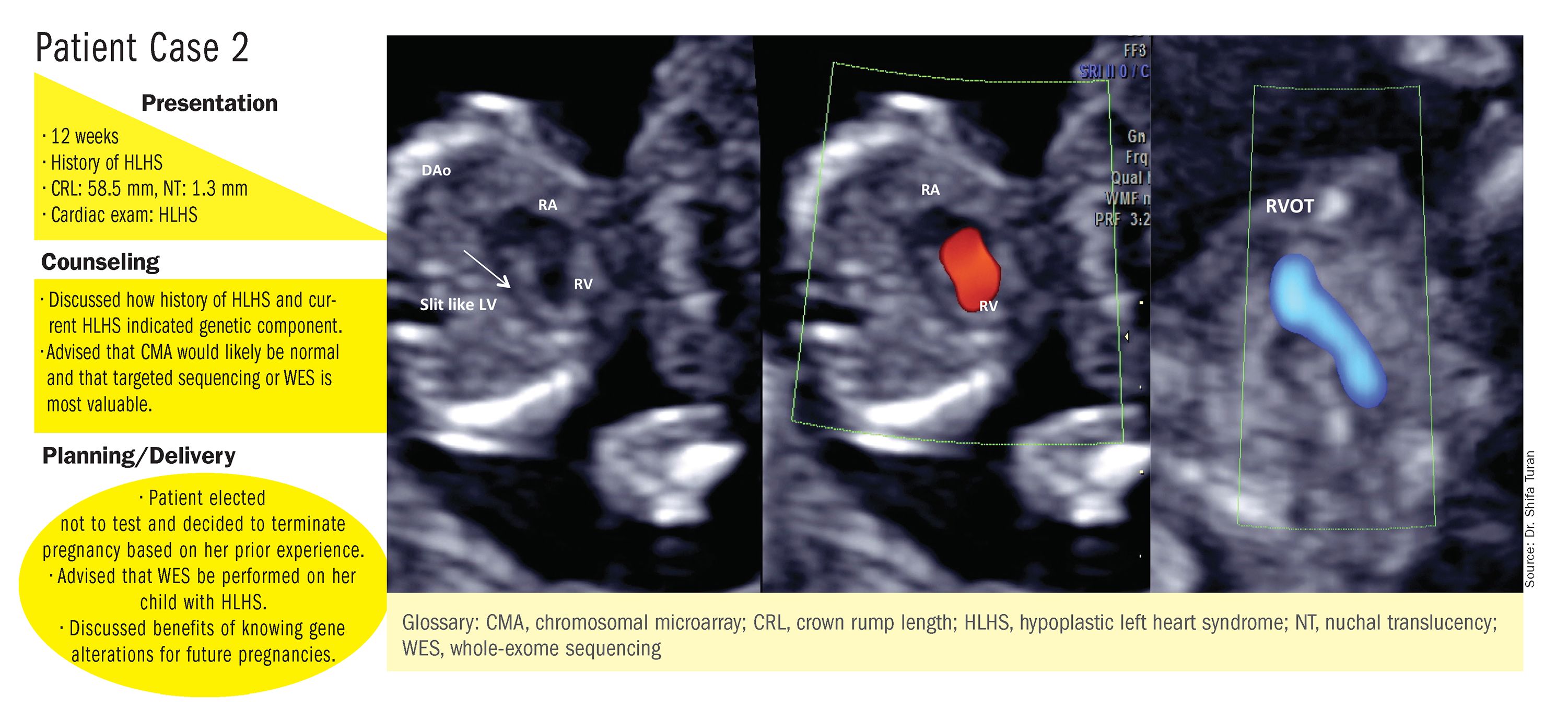
CMA is the preferred first modality, however, when prenatal imaging suggests severe CHD – for instance, when there are signs of hypoplastic left heart syndrome or tetralogy of Fallot (a conotruncal defect) – or complex CHD with extracardiac anomalies. In these cases, there is a high likelihood of detecting a small deletion or duplication that would be missed with karyotype.
In the past decade, karyotype and CMA have become the major methods used in our practice. However, targeted next‐generation sequencing and whole‐exome sequencing may become more widely used because these technologies enable rapid analysis of a large number of gene sequences and facilitate discovery of novel causative genes in many genetic diseases that cause CHDs.
Currently, targeted next-generation sequencing has mainly been used in the postnatal setting, and there are limited data available on its prenatal use. Compared with whole-exome sequencing, which sequences all of the protein-coding regions of the genome, targeted next-generation sequencing panels select regions of genes that are known to be associated with diseases of interest.
For CHDs, some perinatal centers have begun using a customized gene panel that targets 77 CHD-associated genes. This particular panel has been shown to be useful in addition to current methods and is an effective tool for prenatal genetic diagnosis.5
Whole-exome sequencing is currently expensive and time consuming. While sometimes it is used in the postnatal context, it is not yet part of routine practice as a prenatal diagnostic tool. As technology advances this will change – early in the next decade, I believe. For now, whole-exome sequencing may be an option for some patients who want to know more when severe CHD is evident on ultrasound and there are negative results from CMA or targeted sequencing. We have diagnosed some rare genetic syndromes using whole-exome sequencing; these diagnoses helped us to better manage the pregnancies.
These choices are part of the case-specific, stepwise approach to genetic evaluation that we take in our fetal heart program. Our goal is to pursue information that will be accurate and valuable for the patient and clinicians, in the most cost-effective and timely manner.
Limitations of noninvasive screening
In our fetal heart program we see increasing numbers of referred patients who have chosen noninvasive cell-free fetal DNA screening (cfDNA) after a cardiac anomaly is detected on ultrasound examination, and who believe that their “low risk” results demonstrate very little or no risk of CHD. Many of these patients express a belief that noninvasive testing is highly sensitive and accurate for fetal anomalies, including CHDs, and are not easily convinced of the value of other genetic tests.
We recently conducted a retrospective chart analysis (unpublished) in which we found that 41% of cases of CHD with abnormal genetics results were not detectable by cfDNA screening.
In the case of atrial-ventricular septal defects and conotruncal abnormalities that often are more associated with common aneuploidies (trisomy 21, 18, 13, and 45 XO), a “high-risk” result from cfDNA screening may offer the family and cardiology/neonatal team some guidance, but a “low-risk” result does not eliminate the risk of a microarray abnormality and thus may provide false reassurance.
Other research has shown that noninvasive screening will miss up to 7.3% of karyotype abnormalities in pregnancies at high risk for common aneuploidies.6
While invasive testing poses a very small risk of miscarriage, it is hard without such testing to elucidate the potential genetic etiologies of CHDs and truly understand the problems. We must take time to thoughtfully counsel patients who decline invasive testing about the limitations of cfDNA screening for CHDs and other anomalies.
Dr. Turan is an associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. Dr. Turan reported that she has no disclosures relevant to this Master Class. Email her at [email protected].
References
1. J Am Coll Cardiol. 1988 Oct;12(4):1079-86.
2. Pediatr Cardiol. 2019 Mar;40(3):489-96.
3. Ann Pediatr Cardiol. 2017 May-Aug;10(2):126-30.
4. Eur J Obstet Gynecol Reprod Biol 2018;221:172-76.
5. Ultrasound Obstet Gynecol. 2018 Aug;52(2):205-11.
6. PLoS One. 2016 Jan 15;11(1):e0146794.
Congenital heart defects (CHDs) are etiologically heterogeneous, but in recent years it has become clear that genetics plays a larger role in the development of CHDs than was previously thought. Research has been shifting from a focus on risk – estimating the magnitude of increased risk, for instance, based on maternal or familial risk factors – to a focus on the etiology of cardiac defects.
In practice, advances in genetic testing technologies have made the underlying causes of CHDs increasingly detectable. Chromosomal microarray analysis (CMA) – technology that detects significantly more and smaller changes in the amount of chromosomal material than traditional karyotype – has been proven to increase the diagnostic yield in cases of isolated CHDs and CHDs with extracardiac anomalies. Targeted next-generation sequencing also is now available as an additional approach in selective cases, and a clinically viable option for whole-exome sequencing is fast approaching.
For researchers, genetic evaluation carries the potential to unravel remaining mysteries about underlying causes of CHDs – to provide pathological insights and identify potential therapeutic targets. Currently, about 6 % of the total pie of presumed genetic determinants of CHDs is attributed to chromosomal anomalies, 10% to copy number variants, and 12% to single-gene defects. The remaining 72% of etiology, approximately, is undetermined.
As Helen Taussig, MD, (known as the founder of pediatric cardiology) once said, common cardiac malformations occurring in otherwise “normal” individuals “must be genetic in origin.”1 Greater use of genetic testing – and in particular, of whole-exome sequencing – will drive down this “undetermined” piece of the genetics pie.
For clinicians and patients, prenatal genetic evaluation can inform clinical management, guiding decisions on the mode, timing, and location of delivery. Genetic assessments help guide the neonatal health care team in taking optimal care of the infant, and the surgeon in preparing for neonatal surgeries and postsurgical complications.
In a recent analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database, prenatal diagnosis was associated with a lower overall prevalence of major preoperative risk factors for cardiac surgery.2 Surgical outcomes themselves also have been shown to be better after the prenatal diagnosis of complex CHDs, mainly because of improvements in perioperative care.3
When genetic etiology is elucidated, the cardiologist also is better able to counsel patients about anticipated challenges – such as the propensity, with certain genetic variants of CHD, to develop neurodevelopmental delays or other cardiac complications – and to target patient follow-up. Patients also can make informed decisions about termination or continuation of a current pregnancy and about family planning in the future.
Fortunately, advances in genetics technology have paralleled technological advancements in ultrasound. As I discussed in part one of this two-part Master Class series, it is now possible to detect many major CHDs well before 16 weeks’ gestation. Checking the structure of the fetal heart at the first-trimester screening and sonography (11-14 weeks of gestation) offers the opportunity for early genetic assessment, counseling, and planning when anomalies are detected.
A personalized approach
There has been growing interest in recent years in CMA for the prenatal genetic workup of CHDs. Microarray targets chromosomal regions at a much higher resolution than traditional karyotype. Traditional karyotype assesses both changes in chromosome number as well as more subtle structural changes such as chromosomal deletions and duplications. CMA finds what traditional karyotype identifies, but in addition, it identifies much smaller, clinically relevant chromosomal deletions and duplications that are not detected by karyotype performed with or without fluorescence in-situ hybridization (FISH). FISH uses DNA probes that carry fluorescent tags to detect chromosomal DNA.

At our center, we studied the prenatal genetic test results of 145 fetuses diagnosed with CHDs. Each case involved FISH for aneuploidy/karyotype, followed by CMA in cases of a negative karyotype result. CMA increased the diagnostic yield in cases of CHD by 19.8% overall – 17.4% in cases of isolated CHD and 24.5% in cases of CHD plus extracardiac anomalies.4
Indeed, although a microarray costs more and takes an additional 2 weeks to run, CMA should be strongly considered as first-line testing for the prenatal genetic evaluation of fetuses with major structural cardiac abnormalities detected by ultrasound. However, there still are cases in which a karyotype might be sufficient. For instance, if I see that a fetus has an atrial-ventricular septal defect on a prenatal ultrasound, and there are markers for trisomy 21, 13, or 18, or Turner’s syndrome (45 XO), I usually recommend a karyotype or FISH rather than an initial CMA. If the karyotype is abnormal – which is likely in such a scenario – there isn’t a need for more extensive testing.
Similarly, when there is high suspicion for DiGeorge syndrome (the 22q11.2 deletion, which often includes cleft palate and aortic arch abnormalities), usually it is most appropriate to perform a FISH test.
CMA is the preferred first modality, however, when prenatal imaging suggests severe CHD – for instance, when there are signs of hypoplastic left heart syndrome or tetralogy of Fallot (a conotruncal defect) – or complex CHD with extracardiac anomalies. In these cases, there is a high likelihood of detecting a small deletion or duplication that would be missed with karyotype.
In the past decade, karyotype and CMA have become the major methods used in our practice. However, targeted next‐generation sequencing and whole‐exome sequencing may become more widely used because these technologies enable rapid analysis of a large number of gene sequences and facilitate discovery of novel causative genes in many genetic diseases that cause CHDs.
Currently, targeted next-generation sequencing has mainly been used in the postnatal setting, and there are limited data available on its prenatal use. Compared with whole-exome sequencing, which sequences all of the protein-coding regions of the genome, targeted next-generation sequencing panels select regions of genes that are known to be associated with diseases of interest.
For CHDs, some perinatal centers have begun using a customized gene panel that targets 77 CHD-associated genes. This particular panel has been shown to be useful in addition to current methods and is an effective tool for prenatal genetic diagnosis.5
Whole-exome sequencing is currently expensive and time consuming. While sometimes it is used in the postnatal context, it is not yet part of routine practice as a prenatal diagnostic tool. As technology advances this will change – early in the next decade, I believe. For now, whole-exome sequencing may be an option for some patients who want to know more when severe CHD is evident on ultrasound and there are negative results from CMA or targeted sequencing. We have diagnosed some rare genetic syndromes using whole-exome sequencing; these diagnoses helped us to better manage the pregnancies.

These choices are part of the case-specific, stepwise approach to genetic evaluation that we take in our fetal heart program. Our goal is to pursue information that will be accurate and valuable for the patient and clinicians, in the most cost-effective and timely manner.
Limitations of noninvasive screening
In our fetal heart program we see increasing numbers of referred patients who have chosen noninvasive cell-free fetal DNA screening (cfDNA) after a cardiac anomaly is detected on ultrasound examination, and who believe that their “low risk” results demonstrate very little or no risk of CHD. Many of these patients express a belief that noninvasive testing is highly sensitive and accurate for fetal anomalies, including CHDs, and are not easily convinced of the value of other genetic tests.
We recently conducted a retrospective chart analysis (unpublished) in which we found that 41% of cases of CHD with abnormal genetics results were not detectable by cfDNA screening.
In the case of atrial-ventricular septal defects and conotruncal abnormalities that often are more associated with common aneuploidies (trisomy 21, 18, 13, and 45 XO), a “high-risk” result from cfDNA screening may offer the family and cardiology/neonatal team some guidance, but a “low-risk” result does not eliminate the risk of a microarray abnormality and thus may provide false reassurance.
Other research has shown that noninvasive screening will miss up to 7.3% of karyotype abnormalities in pregnancies at high risk for common aneuploidies.6
While invasive testing poses a very small risk of miscarriage, it is hard without such testing to elucidate the potential genetic etiologies of CHDs and truly understand the problems. We must take time to thoughtfully counsel patients who decline invasive testing about the limitations of cfDNA screening for CHDs and other anomalies.
Dr. Turan is an associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. Dr. Turan reported that she has no disclosures relevant to this Master Class. Email her at [email protected].
References
1. J Am Coll Cardiol. 1988 Oct;12(4):1079-86.
2. Pediatr Cardiol. 2019 Mar;40(3):489-96.
3. Ann Pediatr Cardiol. 2017 May-Aug;10(2):126-30.
4. Eur J Obstet Gynecol Reprod Biol 2018;221:172-76.
5. Ultrasound Obstet Gynecol. 2018 Aug;52(2):205-11.
6. PLoS One. 2016 Jan 15;11(1):e0146794.
Congenital heart defects (CHDs) are etiologically heterogeneous, but in recent years it has become clear that genetics plays a larger role in the development of CHDs than was previously thought. Research has been shifting from a focus on risk – estimating the magnitude of increased risk, for instance, based on maternal or familial risk factors – to a focus on the etiology of cardiac defects.
In practice, advances in genetic testing technologies have made the underlying causes of CHDs increasingly detectable. Chromosomal microarray analysis (CMA) – technology that detects significantly more and smaller changes in the amount of chromosomal material than traditional karyotype – has been proven to increase the diagnostic yield in cases of isolated CHDs and CHDs with extracardiac anomalies. Targeted next-generation sequencing also is now available as an additional approach in selective cases, and a clinically viable option for whole-exome sequencing is fast approaching.
For researchers, genetic evaluation carries the potential to unravel remaining mysteries about underlying causes of CHDs – to provide pathological insights and identify potential therapeutic targets. Currently, about 6 % of the total pie of presumed genetic determinants of CHDs is attributed to chromosomal anomalies, 10% to copy number variants, and 12% to single-gene defects. The remaining 72% of etiology, approximately, is undetermined.
As Helen Taussig, MD, (known as the founder of pediatric cardiology) once said, common cardiac malformations occurring in otherwise “normal” individuals “must be genetic in origin.”1 Greater use of genetic testing – and in particular, of whole-exome sequencing – will drive down this “undetermined” piece of the genetics pie.
For clinicians and patients, prenatal genetic evaluation can inform clinical management, guiding decisions on the mode, timing, and location of delivery. Genetic assessments help guide the neonatal health care team in taking optimal care of the infant, and the surgeon in preparing for neonatal surgeries and postsurgical complications.
In a recent analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database, prenatal diagnosis was associated with a lower overall prevalence of major preoperative risk factors for cardiac surgery.2 Surgical outcomes themselves also have been shown to be better after the prenatal diagnosis of complex CHDs, mainly because of improvements in perioperative care.3
When genetic etiology is elucidated, the cardiologist also is better able to counsel patients about anticipated challenges – such as the propensity, with certain genetic variants of CHD, to develop neurodevelopmental delays or other cardiac complications – and to target patient follow-up. Patients also can make informed decisions about termination or continuation of a current pregnancy and about family planning in the future.
Fortunately, advances in genetics technology have paralleled technological advancements in ultrasound. As I discussed in part one of this two-part Master Class series, it is now possible to detect many major CHDs well before 16 weeks’ gestation. Checking the structure of the fetal heart at the first-trimester screening and sonography (11-14 weeks of gestation) offers the opportunity for early genetic assessment, counseling, and planning when anomalies are detected.
A personalized approach
There has been growing interest in recent years in CMA for the prenatal genetic workup of CHDs. Microarray targets chromosomal regions at a much higher resolution than traditional karyotype. Traditional karyotype assesses both changes in chromosome number as well as more subtle structural changes such as chromosomal deletions and duplications. CMA finds what traditional karyotype identifies, but in addition, it identifies much smaller, clinically relevant chromosomal deletions and duplications that are not detected by karyotype performed with or without fluorescence in-situ hybridization (FISH). FISH uses DNA probes that carry fluorescent tags to detect chromosomal DNA.

At our center, we studied the prenatal genetic test results of 145 fetuses diagnosed with CHDs. Each case involved FISH for aneuploidy/karyotype, followed by CMA in cases of a negative karyotype result. CMA increased the diagnostic yield in cases of CHD by 19.8% overall – 17.4% in cases of isolated CHD and 24.5% in cases of CHD plus extracardiac anomalies.4
Indeed, although a microarray costs more and takes an additional 2 weeks to run, CMA should be strongly considered as first-line testing for the prenatal genetic evaluation of fetuses with major structural cardiac abnormalities detected by ultrasound. However, there still are cases in which a karyotype might be sufficient. For instance, if I see that a fetus has an atrial-ventricular septal defect on a prenatal ultrasound, and there are markers for trisomy 21, 13, or 18, or Turner’s syndrome (45 XO), I usually recommend a karyotype or FISH rather than an initial CMA. If the karyotype is abnormal – which is likely in such a scenario – there isn’t a need for more extensive testing.
Similarly, when there is high suspicion for DiGeorge syndrome (the 22q11.2 deletion, which often includes cleft palate and aortic arch abnormalities), usually it is most appropriate to perform a FISH test.
CMA is the preferred first modality, however, when prenatal imaging suggests severe CHD – for instance, when there are signs of hypoplastic left heart syndrome or tetralogy of Fallot (a conotruncal defect) – or complex CHD with extracardiac anomalies. In these cases, there is a high likelihood of detecting a small deletion or duplication that would be missed with karyotype.
In the past decade, karyotype and CMA have become the major methods used in our practice. However, targeted next‐generation sequencing and whole‐exome sequencing may become more widely used because these technologies enable rapid analysis of a large number of gene sequences and facilitate discovery of novel causative genes in many genetic diseases that cause CHDs.
Currently, targeted next-generation sequencing has mainly been used in the postnatal setting, and there are limited data available on its prenatal use. Compared with whole-exome sequencing, which sequences all of the protein-coding regions of the genome, targeted next-generation sequencing panels select regions of genes that are known to be associated with diseases of interest.
For CHDs, some perinatal centers have begun using a customized gene panel that targets 77 CHD-associated genes. This particular panel has been shown to be useful in addition to current methods and is an effective tool for prenatal genetic diagnosis.5
Whole-exome sequencing is currently expensive and time consuming. While sometimes it is used in the postnatal context, it is not yet part of routine practice as a prenatal diagnostic tool. As technology advances this will change – early in the next decade, I believe. For now, whole-exome sequencing may be an option for some patients who want to know more when severe CHD is evident on ultrasound and there are negative results from CMA or targeted sequencing. We have diagnosed some rare genetic syndromes using whole-exome sequencing; these diagnoses helped us to better manage the pregnancies.

These choices are part of the case-specific, stepwise approach to genetic evaluation that we take in our fetal heart program. Our goal is to pursue information that will be accurate and valuable for the patient and clinicians, in the most cost-effective and timely manner.
Limitations of noninvasive screening
In our fetal heart program we see increasing numbers of referred patients who have chosen noninvasive cell-free fetal DNA screening (cfDNA) after a cardiac anomaly is detected on ultrasound examination, and who believe that their “low risk” results demonstrate very little or no risk of CHD. Many of these patients express a belief that noninvasive testing is highly sensitive and accurate for fetal anomalies, including CHDs, and are not easily convinced of the value of other genetic tests.
We recently conducted a retrospective chart analysis (unpublished) in which we found that 41% of cases of CHD with abnormal genetics results were not detectable by cfDNA screening.
In the case of atrial-ventricular septal defects and conotruncal abnormalities that often are more associated with common aneuploidies (trisomy 21, 18, 13, and 45 XO), a “high-risk” result from cfDNA screening may offer the family and cardiology/neonatal team some guidance, but a “low-risk” result does not eliminate the risk of a microarray abnormality and thus may provide false reassurance.
Other research has shown that noninvasive screening will miss up to 7.3% of karyotype abnormalities in pregnancies at high risk for common aneuploidies.6
While invasive testing poses a very small risk of miscarriage, it is hard without such testing to elucidate the potential genetic etiologies of CHDs and truly understand the problems. We must take time to thoughtfully counsel patients who decline invasive testing about the limitations of cfDNA screening for CHDs and other anomalies.
Dr. Turan is an associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. Dr. Turan reported that she has no disclosures relevant to this Master Class. Email her at [email protected].
References
1. J Am Coll Cardiol. 1988 Oct;12(4):1079-86.
2. Pediatr Cardiol. 2019 Mar;40(3):489-96.
3. Ann Pediatr Cardiol. 2017 May-Aug;10(2):126-30.
4. Eur J Obstet Gynecol Reprod Biol 2018;221:172-76.
5. Ultrasound Obstet Gynecol. 2018 Aug;52(2):205-11.
6. PLoS One. 2016 Jan 15;11(1):e0146794.
The benefits of first-trimester fetal heart evaluation
The fetal heart typically is examined during the routine 18-20 week obstetric ultrasound screening, and pregnancies with abnormalities on this routine scan are referred for detailed fetal echocardiography. Per multiple practice guidelines, patients deemed to be at high risk of congenital heart defects (CHDs) are referred for fetal echocardiography as well between 18 and 24 weeks’ gestation.
However, with technological advancements in ultrasound, it is possible for obstetricians to detect many major CHDs well before 16 weeks’ gestation. First-trimester fetal heart assessment – and early detection of CHDs – has numerous advantages: It enables early genetic testing, early decision making about continuation or termination of pregnancy, and earlier planning for appropriate management during and after pregnancy. Perioperative outcomes are improved.
At least 75% of CHDs occur in pregnancies with no identifiable maternal, familial, or fetal risk factors. It only seems fitting, therefore, that we check the structure of the fetal heart in all women at the time of their first-trimester screening and sonography at 11-14 weeks. In addition to a determination of fetal viability and gestational age, nuchal translucency measurement, and a check of basic anatomy, .
The value of early detection
Women who have diabetes, congenital defects, in vitro fertilization pregnancies, twin and multiple pregnancies, and certain medication and drug exposures are at high risk for their fetus having a CHD and should undergo fetal echocardiography. Lupus, Sjögren’s, and other medical disorders also are risk factors, as are abnormal biochemical test results.
During the last 10 years, the first-trimester fetal heart evaluation has been performed for all patients who come for a first-trimester screening scan at the University of Maryland’s fetal heart program, part of the Center for Advanced Fetal Care. Approximately 45% of indications for detailed first-trimester fetal heart evaluation have been driven by maternal history, and almost 40% by abnormal basic first-trimester ultrasound findings such as increased nuchal translucency, tricuspid regurgitation, abnormal ductus venosus blood flow, and other structural anomalies.
An estimated 50%-60% of serious cardiac malformations can be detected with a four-chamber heart view during routine first-trimester ultrasound. When the outflow tract relationship and three-vessel views also are examined in the first trimester – as is now recommended in guidelines for second-trimester protocols – an estimated 85%-95% of major CHDs can be detected. One should see the great arteries originating from the left and right sides and crisscrossing each other by a transabdominal scan, or by a transvaginal scan if the transabdominal approach fails to show these features of the fetal heart.
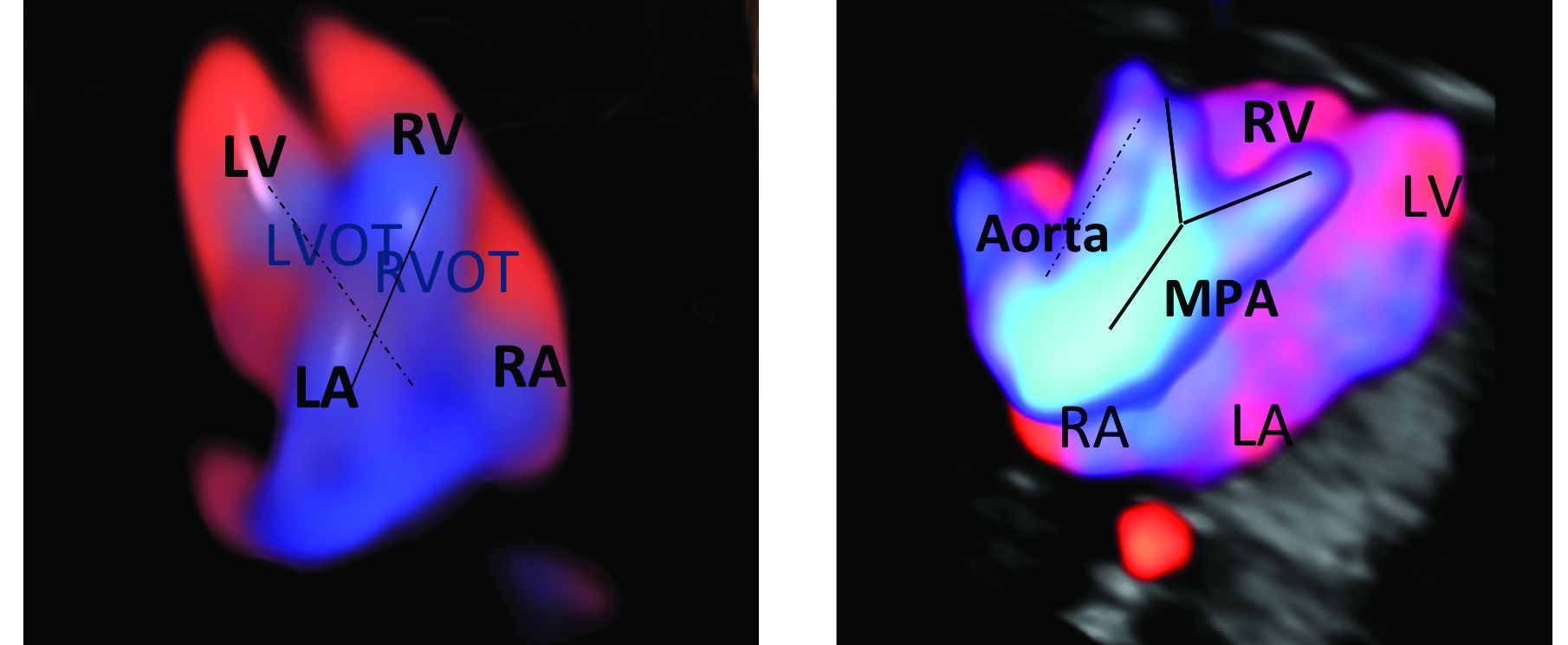
Early sonography not only has been shown to have a high sensitivity but also a specificity of greater than 95% in identifying CHDs. Multiple studies also have demonstrated high negative predictive values in cases with normal findings.1
When defects seen or suspected on routine obstetric ultrasound are then confirmed and diagnosed with detailed fetal echocardiography, women are counseled about outcomes, management options, and mortality – and some patients will choose to terminate their pregnancies.
Psychologically, for the mother, earlier termination is less traumatic. A cross-sectional study of 254 women conducted 2-7 years after pregnancy termination for fetal anomalies found that advanced gestational age at termination was associated with higher levels of grief and posttraumatic stress symptoms, and that long-term psychological morbidity was rare when termination occurred before 14 weeks’ gestation.2 Others studies have shown similar results, with grief and posttraumatic stress time shorter with earlier termination.
First-trimester termination also involves significantly less maternal morbidity and risk, as shown in a retrospective study of 844 patients who underwent a termination of pregnancy after a positive amniocentesis or chorionic villus sampling. Hemorrhages, transfusions, infections, and other complications were significantly higher in second-trimester terminations than in earlier terminations.3
Early fetal heart evaluation can reassure high-risk patients – and low-risk patients as well – when a normal four-chamber heart and great arteries are seen. And when defects are spotted, early evaluation allows appropriate time to test for associated chromosomal abnormalities and genetic syndromes, which in turn improves management. It also gives patients and providers more time to plan and prepare for delivery, surgery, and other specific needs at delivery and after birth.
In our fetal heart program, patients are cared for by a multidisciplinary team of perinatologists with special expertise in the fetal heart, geneticists, cardiologists, cardiac surgeons, and neonatologists. Perioperative outcomes are improved when CHDs are diagnosed prenatally. One meta-analysis showed that prenatal diagnosis reduced the risk of death prior to planned cardiac surgery by about one-fourth relative to patients with a comparable postnatal diagnosis.4
Prenatal diagnosis appears to have generally been improving, although rates remain too low overall. According to the National Institute for Cardiovascular Outcomes Research, which collects data from centers across the United Kingdom and Republic of Ireland, prenatal detection rates of CHDs requiring a procedure in the first year of life moved from about 25% in 2004-2005 to just over 50% between 2010 and 2016.5 More complex lesions, such as hypoplastic left heart syndrome, were more likely to be detected prenatally (80%).
Trends in the United States appear to be similar. A study utilizing the Society of Thoracic Surgeons Congenital Heart Surgery Database found that prenatal detection increased from 26% in 2006 to 42% in 2012.6
A first-trimester evaluation cannot replace the second-trimester echocardiography that currently is performed for high-risk patients, because a small percentage of CHDs – aortic coarctation, valve stenosis, mild tetralogy of Fallot, and hypoplastic left heart, for instance – have the potential to evolve past the first trimester. High-risk patients whose first-trimester evaluations are normal still should undergo another evaluation at 18-20 weeks. The fetal heart completes its embryologic development over the first 8 weeks of gestation, and the majority of CHDs are present at the time of the first-trimester screening (11-14 weeks).
Early evaluation of the fetal heart does not appear to be impacted by obesity. We compared the early evaluation of fetal heart landmarks using two-dimensional sonography with color/power Doppler in obese and nonobese women and found that there were no significant differences in experienced sonographers’ ability to evaluate the four-chamber view, outflow tract relationship, and transverse arches views.
In about 6% of obese women, the evaluation at 11-14 weeks’ gestation required additional imaging with transvaginal sonography. The chances of needing transvaginal ultrasound rose as body mass index rose.1 The median scan time was only 5 minutes longer in the obese group, however, so there is no reason that obesity should be a contraindication to look at the fetal heart.
In fact, it is extremely important that we do early fetal heart evaluations in women who are obese, because the risk of having a fetus with CHD is increasingly being found to be higher in obese women, and because fetal heart assessment with transvaginal ultrasound is an option only in early gestation, when the fetal heart is within the depth of penetration of the vaginal probe. With advancing gestational age, a combined abdominal/transvaginal approach becomes increasingly difficult. Our study also demonstrated a dose-response relationship between maternal obesity and CHD risk.
Preexisting diabetes mellitus, which can occur in conjunction with obesity, has been found to increase the risk for all types of CHDs, especially conotruncal abnormalities. While the pathophysiology is not completely understood, elevated oxidative stress is believed to be the primary trigger.7
First-trimester echocardiography benefits
Patients referred to our fetal heart program for detailed first-trimester fetal heart evaluation – again, a significant number of whom have been found on standard 2-D ultrasound to have increased nuchal translucency thickness or other abnormalities – undergo a four-dimensional fetal echocardiographic technique that utilizes spatiotemporal image correlation and tomographic ultrasound imaging display (STIC-TUI echo) along with color Doppler. The heart is swept from top to bottom in about 10 seconds, and tomographic ultrasound imaging is used offline, after the patient leaves, to develop volume datasets that simultaneously display multiple cross-sectional images.
This method has been implemented into our routine scan at the first trimester as well, and all of our staff have been trained to perform it. Obtaining STIC-TUI by color Doppler allows us to assess all of the important landmarks of the cardiac anatomy in one picture.
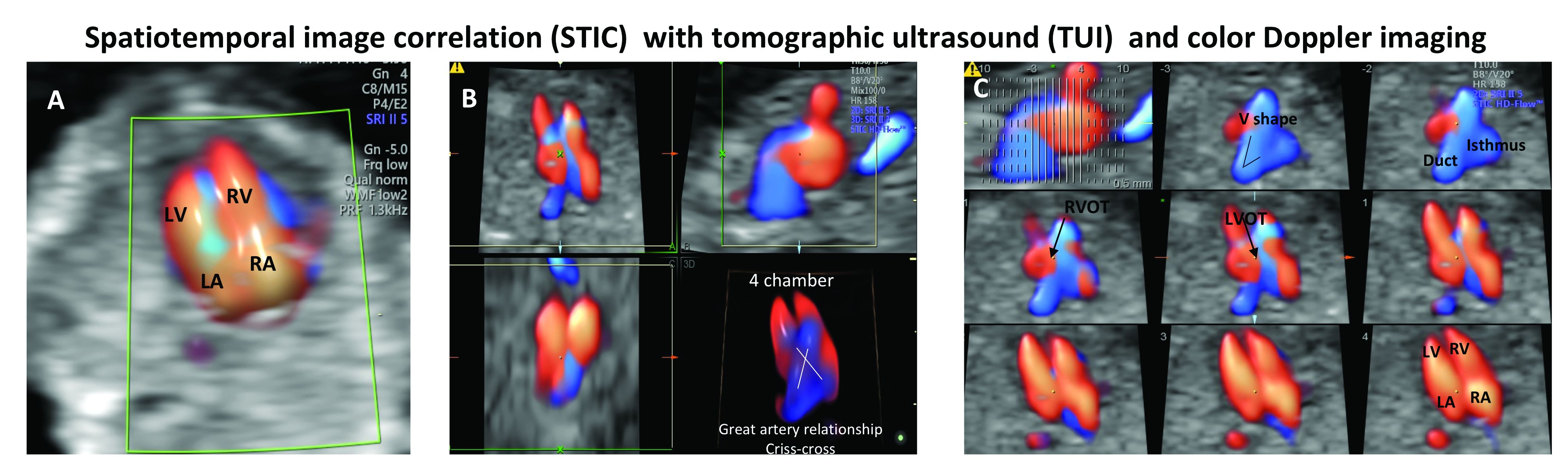
In a prospective study of 164 fetuses from 152 patients, we found that first-trimester STIC-TUI echo had 91% sensitivity and 100% specificity for the detection of CHD. Most anomalies were evident in the four-chamber view plane of the TUI display, and the rest were diagnosed in the outflow tract planes. Two cases of CHD missed by this first-trimester evaluation were diagnosed on second-trimester echo and neither involved a major CHD.8
Dr. Turan is associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland, Baltimore.
References
1. J Ultrasound Med. 2019 May;38(5):1269-77.
2. Prenat Diagn. 2005 Mar;25(3):253-60.
3. J Perinat Med. 2018 May 24;46(4):373-8.
4. Ultrasound Obstet Gynecol. 2015 Jun;45(6):631-8.
5. National Congenital Heart Disease Audit Report 2013-2016.
6. Pediatrics. 2015. doi: 10.1542/peds.2014-3783.
7. Echocardiography. 2018 Feb;35(2):244-57.
8. Ultrasound Obstet Gynecol. 2014 Nov;44(5):562-7.
The fetal heart typically is examined during the routine 18-20 week obstetric ultrasound screening, and pregnancies with abnormalities on this routine scan are referred for detailed fetal echocardiography. Per multiple practice guidelines, patients deemed to be at high risk of congenital heart defects (CHDs) are referred for fetal echocardiography as well between 18 and 24 weeks’ gestation.
However, with technological advancements in ultrasound, it is possible for obstetricians to detect many major CHDs well before 16 weeks’ gestation. First-trimester fetal heart assessment – and early detection of CHDs – has numerous advantages: It enables early genetic testing, early decision making about continuation or termination of pregnancy, and earlier planning for appropriate management during and after pregnancy. Perioperative outcomes are improved.
At least 75% of CHDs occur in pregnancies with no identifiable maternal, familial, or fetal risk factors. It only seems fitting, therefore, that we check the structure of the fetal heart in all women at the time of their first-trimester screening and sonography at 11-14 weeks. In addition to a determination of fetal viability and gestational age, nuchal translucency measurement, and a check of basic anatomy, .
The value of early detection
Women who have diabetes, congenital defects, in vitro fertilization pregnancies, twin and multiple pregnancies, and certain medication and drug exposures are at high risk for their fetus having a CHD and should undergo fetal echocardiography. Lupus, Sjögren’s, and other medical disorders also are risk factors, as are abnormal biochemical test results.
During the last 10 years, the first-trimester fetal heart evaluation has been performed for all patients who come for a first-trimester screening scan at the University of Maryland’s fetal heart program, part of the Center for Advanced Fetal Care. Approximately 45% of indications for detailed first-trimester fetal heart evaluation have been driven by maternal history, and almost 40% by abnormal basic first-trimester ultrasound findings such as increased nuchal translucency, tricuspid regurgitation, abnormal ductus venosus blood flow, and other structural anomalies.
An estimated 50%-60% of serious cardiac malformations can be detected with a four-chamber heart view during routine first-trimester ultrasound. When the outflow tract relationship and three-vessel views also are examined in the first trimester – as is now recommended in guidelines for second-trimester protocols – an estimated 85%-95% of major CHDs can be detected. One should see the great arteries originating from the left and right sides and crisscrossing each other by a transabdominal scan, or by a transvaginal scan if the transabdominal approach fails to show these features of the fetal heart.

Early sonography not only has been shown to have a high sensitivity but also a specificity of greater than 95% in identifying CHDs. Multiple studies also have demonstrated high negative predictive values in cases with normal findings.1
When defects seen or suspected on routine obstetric ultrasound are then confirmed and diagnosed with detailed fetal echocardiography, women are counseled about outcomes, management options, and mortality – and some patients will choose to terminate their pregnancies.
Psychologically, for the mother, earlier termination is less traumatic. A cross-sectional study of 254 women conducted 2-7 years after pregnancy termination for fetal anomalies found that advanced gestational age at termination was associated with higher levels of grief and posttraumatic stress symptoms, and that long-term psychological morbidity was rare when termination occurred before 14 weeks’ gestation.2 Others studies have shown similar results, with grief and posttraumatic stress time shorter with earlier termination.
First-trimester termination also involves significantly less maternal morbidity and risk, as shown in a retrospective study of 844 patients who underwent a termination of pregnancy after a positive amniocentesis or chorionic villus sampling. Hemorrhages, transfusions, infections, and other complications were significantly higher in second-trimester terminations than in earlier terminations.3
Early fetal heart evaluation can reassure high-risk patients – and low-risk patients as well – when a normal four-chamber heart and great arteries are seen. And when defects are spotted, early evaluation allows appropriate time to test for associated chromosomal abnormalities and genetic syndromes, which in turn improves management. It also gives patients and providers more time to plan and prepare for delivery, surgery, and other specific needs at delivery and after birth.
In our fetal heart program, patients are cared for by a multidisciplinary team of perinatologists with special expertise in the fetal heart, geneticists, cardiologists, cardiac surgeons, and neonatologists. Perioperative outcomes are improved when CHDs are diagnosed prenatally. One meta-analysis showed that prenatal diagnosis reduced the risk of death prior to planned cardiac surgery by about one-fourth relative to patients with a comparable postnatal diagnosis.4
Prenatal diagnosis appears to have generally been improving, although rates remain too low overall. According to the National Institute for Cardiovascular Outcomes Research, which collects data from centers across the United Kingdom and Republic of Ireland, prenatal detection rates of CHDs requiring a procedure in the first year of life moved from about 25% in 2004-2005 to just over 50% between 2010 and 2016.5 More complex lesions, such as hypoplastic left heart syndrome, were more likely to be detected prenatally (80%).
Trends in the United States appear to be similar. A study utilizing the Society of Thoracic Surgeons Congenital Heart Surgery Database found that prenatal detection increased from 26% in 2006 to 42% in 2012.6
A first-trimester evaluation cannot replace the second-trimester echocardiography that currently is performed for high-risk patients, because a small percentage of CHDs – aortic coarctation, valve stenosis, mild tetralogy of Fallot, and hypoplastic left heart, for instance – have the potential to evolve past the first trimester. High-risk patients whose first-trimester evaluations are normal still should undergo another evaluation at 18-20 weeks. The fetal heart completes its embryologic development over the first 8 weeks of gestation, and the majority of CHDs are present at the time of the first-trimester screening (11-14 weeks).
Early evaluation of the fetal heart does not appear to be impacted by obesity. We compared the early evaluation of fetal heart landmarks using two-dimensional sonography with color/power Doppler in obese and nonobese women and found that there were no significant differences in experienced sonographers’ ability to evaluate the four-chamber view, outflow tract relationship, and transverse arches views.
In about 6% of obese women, the evaluation at 11-14 weeks’ gestation required additional imaging with transvaginal sonography. The chances of needing transvaginal ultrasound rose as body mass index rose.1 The median scan time was only 5 minutes longer in the obese group, however, so there is no reason that obesity should be a contraindication to look at the fetal heart.
In fact, it is extremely important that we do early fetal heart evaluations in women who are obese, because the risk of having a fetus with CHD is increasingly being found to be higher in obese women, and because fetal heart assessment with transvaginal ultrasound is an option only in early gestation, when the fetal heart is within the depth of penetration of the vaginal probe. With advancing gestational age, a combined abdominal/transvaginal approach becomes increasingly difficult. Our study also demonstrated a dose-response relationship between maternal obesity and CHD risk.
Preexisting diabetes mellitus, which can occur in conjunction with obesity, has been found to increase the risk for all types of CHDs, especially conotruncal abnormalities. While the pathophysiology is not completely understood, elevated oxidative stress is believed to be the primary trigger.7
First-trimester echocardiography benefits
Patients referred to our fetal heart program for detailed first-trimester fetal heart evaluation – again, a significant number of whom have been found on standard 2-D ultrasound to have increased nuchal translucency thickness or other abnormalities – undergo a four-dimensional fetal echocardiographic technique that utilizes spatiotemporal image correlation and tomographic ultrasound imaging display (STIC-TUI echo) along with color Doppler. The heart is swept from top to bottom in about 10 seconds, and tomographic ultrasound imaging is used offline, after the patient leaves, to develop volume datasets that simultaneously display multiple cross-sectional images.
This method has been implemented into our routine scan at the first trimester as well, and all of our staff have been trained to perform it. Obtaining STIC-TUI by color Doppler allows us to assess all of the important landmarks of the cardiac anatomy in one picture.

In a prospective study of 164 fetuses from 152 patients, we found that first-trimester STIC-TUI echo had 91% sensitivity and 100% specificity for the detection of CHD. Most anomalies were evident in the four-chamber view plane of the TUI display, and the rest were diagnosed in the outflow tract planes. Two cases of CHD missed by this first-trimester evaluation were diagnosed on second-trimester echo and neither involved a major CHD.8
Dr. Turan is associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland, Baltimore.
References
1. J Ultrasound Med. 2019 May;38(5):1269-77.
2. Prenat Diagn. 2005 Mar;25(3):253-60.
3. J Perinat Med. 2018 May 24;46(4):373-8.
4. Ultrasound Obstet Gynecol. 2015 Jun;45(6):631-8.
5. National Congenital Heart Disease Audit Report 2013-2016.
6. Pediatrics. 2015. doi: 10.1542/peds.2014-3783.
7. Echocardiography. 2018 Feb;35(2):244-57.
8. Ultrasound Obstet Gynecol. 2014 Nov;44(5):562-7.
The fetal heart typically is examined during the routine 18-20 week obstetric ultrasound screening, and pregnancies with abnormalities on this routine scan are referred for detailed fetal echocardiography. Per multiple practice guidelines, patients deemed to be at high risk of congenital heart defects (CHDs) are referred for fetal echocardiography as well between 18 and 24 weeks’ gestation.
However, with technological advancements in ultrasound, it is possible for obstetricians to detect many major CHDs well before 16 weeks’ gestation. First-trimester fetal heart assessment – and early detection of CHDs – has numerous advantages: It enables early genetic testing, early decision making about continuation or termination of pregnancy, and earlier planning for appropriate management during and after pregnancy. Perioperative outcomes are improved.
At least 75% of CHDs occur in pregnancies with no identifiable maternal, familial, or fetal risk factors. It only seems fitting, therefore, that we check the structure of the fetal heart in all women at the time of their first-trimester screening and sonography at 11-14 weeks. In addition to a determination of fetal viability and gestational age, nuchal translucency measurement, and a check of basic anatomy, .
The value of early detection
Women who have diabetes, congenital defects, in vitro fertilization pregnancies, twin and multiple pregnancies, and certain medication and drug exposures are at high risk for their fetus having a CHD and should undergo fetal echocardiography. Lupus, Sjögren’s, and other medical disorders also are risk factors, as are abnormal biochemical test results.
During the last 10 years, the first-trimester fetal heart evaluation has been performed for all patients who come for a first-trimester screening scan at the University of Maryland’s fetal heart program, part of the Center for Advanced Fetal Care. Approximately 45% of indications for detailed first-trimester fetal heart evaluation have been driven by maternal history, and almost 40% by abnormal basic first-trimester ultrasound findings such as increased nuchal translucency, tricuspid regurgitation, abnormal ductus venosus blood flow, and other structural anomalies.
An estimated 50%-60% of serious cardiac malformations can be detected with a four-chamber heart view during routine first-trimester ultrasound. When the outflow tract relationship and three-vessel views also are examined in the first trimester – as is now recommended in guidelines for second-trimester protocols – an estimated 85%-95% of major CHDs can be detected. One should see the great arteries originating from the left and right sides and crisscrossing each other by a transabdominal scan, or by a transvaginal scan if the transabdominal approach fails to show these features of the fetal heart.

Early sonography not only has been shown to have a high sensitivity but also a specificity of greater than 95% in identifying CHDs. Multiple studies also have demonstrated high negative predictive values in cases with normal findings.1
When defects seen or suspected on routine obstetric ultrasound are then confirmed and diagnosed with detailed fetal echocardiography, women are counseled about outcomes, management options, and mortality – and some patients will choose to terminate their pregnancies.
Psychologically, for the mother, earlier termination is less traumatic. A cross-sectional study of 254 women conducted 2-7 years after pregnancy termination for fetal anomalies found that advanced gestational age at termination was associated with higher levels of grief and posttraumatic stress symptoms, and that long-term psychological morbidity was rare when termination occurred before 14 weeks’ gestation.2 Others studies have shown similar results, with grief and posttraumatic stress time shorter with earlier termination.
First-trimester termination also involves significantly less maternal morbidity and risk, as shown in a retrospective study of 844 patients who underwent a termination of pregnancy after a positive amniocentesis or chorionic villus sampling. Hemorrhages, transfusions, infections, and other complications were significantly higher in second-trimester terminations than in earlier terminations.3
Early fetal heart evaluation can reassure high-risk patients – and low-risk patients as well – when a normal four-chamber heart and great arteries are seen. And when defects are spotted, early evaluation allows appropriate time to test for associated chromosomal abnormalities and genetic syndromes, which in turn improves management. It also gives patients and providers more time to plan and prepare for delivery, surgery, and other specific needs at delivery and after birth.
In our fetal heart program, patients are cared for by a multidisciplinary team of perinatologists with special expertise in the fetal heart, geneticists, cardiologists, cardiac surgeons, and neonatologists. Perioperative outcomes are improved when CHDs are diagnosed prenatally. One meta-analysis showed that prenatal diagnosis reduced the risk of death prior to planned cardiac surgery by about one-fourth relative to patients with a comparable postnatal diagnosis.4
Prenatal diagnosis appears to have generally been improving, although rates remain too low overall. According to the National Institute for Cardiovascular Outcomes Research, which collects data from centers across the United Kingdom and Republic of Ireland, prenatal detection rates of CHDs requiring a procedure in the first year of life moved from about 25% in 2004-2005 to just over 50% between 2010 and 2016.5 More complex lesions, such as hypoplastic left heart syndrome, were more likely to be detected prenatally (80%).
Trends in the United States appear to be similar. A study utilizing the Society of Thoracic Surgeons Congenital Heart Surgery Database found that prenatal detection increased from 26% in 2006 to 42% in 2012.6
A first-trimester evaluation cannot replace the second-trimester echocardiography that currently is performed for high-risk patients, because a small percentage of CHDs – aortic coarctation, valve stenosis, mild tetralogy of Fallot, and hypoplastic left heart, for instance – have the potential to evolve past the first trimester. High-risk patients whose first-trimester evaluations are normal still should undergo another evaluation at 18-20 weeks. The fetal heart completes its embryologic development over the first 8 weeks of gestation, and the majority of CHDs are present at the time of the first-trimester screening (11-14 weeks).
Early evaluation of the fetal heart does not appear to be impacted by obesity. We compared the early evaluation of fetal heart landmarks using two-dimensional sonography with color/power Doppler in obese and nonobese women and found that there were no significant differences in experienced sonographers’ ability to evaluate the four-chamber view, outflow tract relationship, and transverse arches views.
In about 6% of obese women, the evaluation at 11-14 weeks’ gestation required additional imaging with transvaginal sonography. The chances of needing transvaginal ultrasound rose as body mass index rose.1 The median scan time was only 5 minutes longer in the obese group, however, so there is no reason that obesity should be a contraindication to look at the fetal heart.
In fact, it is extremely important that we do early fetal heart evaluations in women who are obese, because the risk of having a fetus with CHD is increasingly being found to be higher in obese women, and because fetal heart assessment with transvaginal ultrasound is an option only in early gestation, when the fetal heart is within the depth of penetration of the vaginal probe. With advancing gestational age, a combined abdominal/transvaginal approach becomes increasingly difficult. Our study also demonstrated a dose-response relationship between maternal obesity and CHD risk.
Preexisting diabetes mellitus, which can occur in conjunction with obesity, has been found to increase the risk for all types of CHDs, especially conotruncal abnormalities. While the pathophysiology is not completely understood, elevated oxidative stress is believed to be the primary trigger.7
First-trimester echocardiography benefits
Patients referred to our fetal heart program for detailed first-trimester fetal heart evaluation – again, a significant number of whom have been found on standard 2-D ultrasound to have increased nuchal translucency thickness or other abnormalities – undergo a four-dimensional fetal echocardiographic technique that utilizes spatiotemporal image correlation and tomographic ultrasound imaging display (STIC-TUI echo) along with color Doppler. The heart is swept from top to bottom in about 10 seconds, and tomographic ultrasound imaging is used offline, after the patient leaves, to develop volume datasets that simultaneously display multiple cross-sectional images.
This method has been implemented into our routine scan at the first trimester as well, and all of our staff have been trained to perform it. Obtaining STIC-TUI by color Doppler allows us to assess all of the important landmarks of the cardiac anatomy in one picture.

In a prospective study of 164 fetuses from 152 patients, we found that first-trimester STIC-TUI echo had 91% sensitivity and 100% specificity for the detection of CHD. Most anomalies were evident in the four-chamber view plane of the TUI display, and the rest were diagnosed in the outflow tract planes. Two cases of CHD missed by this first-trimester evaluation were diagnosed on second-trimester echo and neither involved a major CHD.8
Dr. Turan is associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland, Baltimore.
References
1. J Ultrasound Med. 2019 May;38(5):1269-77.
2. Prenat Diagn. 2005 Mar;25(3):253-60.
3. J Perinat Med. 2018 May 24;46(4):373-8.
4. Ultrasound Obstet Gynecol. 2015 Jun;45(6):631-8.
5. National Congenital Heart Disease Audit Report 2013-2016.
6. Pediatrics. 2015. doi: 10.1542/peds.2014-3783.
7. Echocardiography. 2018 Feb;35(2):244-57.
8. Ultrasound Obstet Gynecol. 2014 Nov;44(5):562-7.

