User login
Palifermin-Associated Cutaneous Papular Rash of the Head and Neck
To the Editor:
Palifermin is a recombinant keratinocyte growth factor (KGF) approved by the US Food and Drug Administration to prevent oral mucositis following radiation therapy or chemotherapy. Cutaneous reactions associated with palifermin have been reported.1-5 One case described a distinctive polymorphous eruption in a patient treated with palifermin.6 On histologic analysis, papules demonstrated findings similar to verrucae, with evidence of papillomatosis, hypergranulosis, and hyperorthokeratosis. Given its mechanism of action as a KGF, it was concluded that these findings were likely the direct result of palifermin.6 We report a similar case of a patient who was given palifermin prior to an autologous stem cell transplant. Histopathologic analysis confirmed epidermal dysmaturation and marked hypergranulosis. We present this case to expand the paucity of data on palifermin-associated cutaneous reactions.
A 63-year-old man with a history of psoriasis, eczema, and relapsed diffuse large B-cell lymphoma was admitted to the hospital for routine management of an autologous stem cell transplant with a conditioning regimen involving thiotepa, busulfan, and cyclophosphamide. The patient had completed a 3-day course of palifermin 1 day prior to the current presentation. On admission, he developed a pruritic erythematous rash over the face and axillae. Within 24 hours, the facial rash progressed with appreciable edema, and he reported difficulty opening his eyes. He denied any fever, nausea, vomiting, diarrhea, or increased fatigue. He also denied use of any other medications other than starting a course of prophylactic trimethoprim-sulfamethoxazole 3 times weekly 2 months prior to admission.
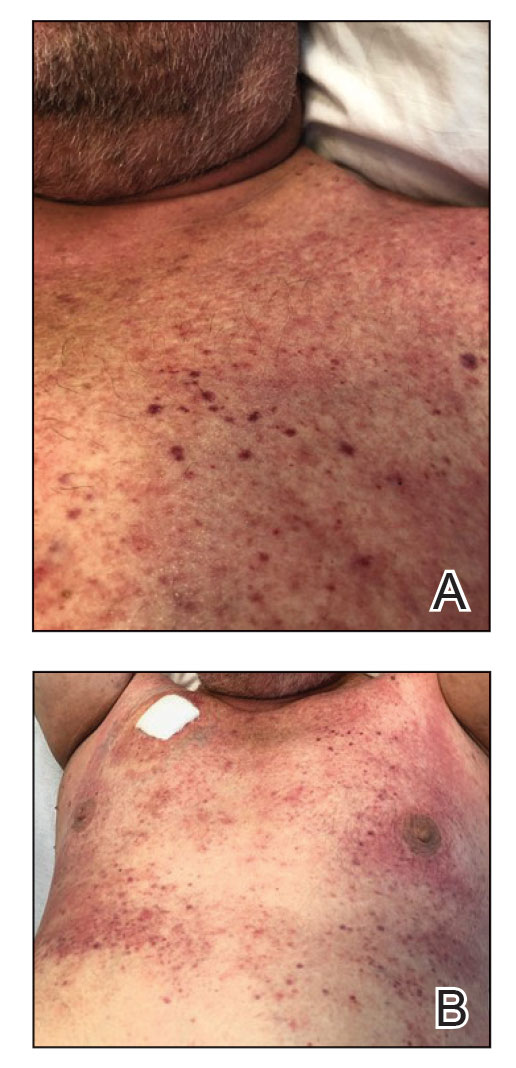
Diffuse blanching erythema with a well-demarcated linear border was noted along the lower anterior neck extending to the posterior hairline. There was notable edema but no evidence of pustules or overlying scale. Similar areas of blanchable erythema were present along the axillae and inguinal folds. There also were flesh-colored to pink papules within the axillary vaults and on the back that occasionally coalesced into plaques. There was no involvement of the mucous membranes or acral sites.
A complete blood cell count with differential and a comprehensive metabolic profile largely were unremarkable. A potassium hydroxide preparation of the face and groin was negative for hyphae and Demodex mites. Histopathologic analysis from a punch biopsy of a representative papule from the posterior neck demonstrated epidermal dysmaturation with marked thickening of the granular cell layer with notably large keratohyalin granules (Figure 1).

In the setting of treatment with thiotepa, we recommended supportive care with cool compresses rather than topical medication because he was neutropenic, and we wanted to avoid further immunosuppression or toxicity. By 24 hours after completing the course of palifermin, the patient experienced complete resolution of the rash. At his request, the trial of palifermin was restarted 10 days into conditioning therapy. A similar rash with less facial edema but more prominent involvement of the chest appeared 3 days into the retrial (Figure 2). The medication was discontinued, which resulted in resolution of the rash. Again, the patient remained afebrile without involvement of the mucous membranes. Liver enzyme and creatinine levels remained within reference range.Eosinophilia and the level of atypical lymphocytes could not be assessed because of leukopenia in the setting of recent chemotherapy. The rash self-resolved in 4 days.
Palifermin is a recombinant form of human KGF that is more stable than the endogenous form but retains all vital properties of the protein.5-7 Similar to other growth factors, KGF induces differentiation, proliferation, and migration of cells in vivo.8 However, it uniquely produces a targeted effect on epithelial cells in the skin, oral mucosa, lungs, gastrointestinal tract, and genitourinary system.7-9
Palifermin was approved by the US Food and Drug Administration in 2004 for the prevention and treatment of severe oral mucositis in patients receiving myelotoxic therapy prior to stem cell transplantation.7,9 Severe mucositis occurs in approximately 70% to 80% of patients receiving radiation or chemotherapy-based conditioning treatments.4,7 Compared to placebo, palifermin has been shown to greatly reduce the incidence of Grade 4 oral mucositis, defined as severe enough to prevent alimentation.10
The proliferative effect of palifermin on the oral mucosa is beneficial to patients but likely is the driving force behind its cutaneous adverse effects. A nonspecific rash is the most commonly cited treatment-related adverse event associated with palifermin, occurring in approximately 62% of patients.5,7,9
Our case is a rare report of a palifermin-associated cutaneous reaction. Previous cases have cited the occurrence of palmoplantar erythrodysesthesias, papulopustular eruptions involving the face and chest, and a papular rash involving the dorsal hands and intertriginous areas.1-4 Another report documented a “mild rash” but failed to further characterize the morphology or the body site involved.5
In 2009, King et al6 reported the occurrence of a lichen planus–like eruption involving the intertriginous regions and of white oral plaques in a patient treated with palifermin. Hematoxylin and eosin staining of a representative lesion in that patient demonstrated an appearance similar to that of verrucae, including papillomatosis, hypergranulosis, and hyperorthokeratosis.
King et al6 expanded analysis of the reaction to include immunohistochemical study, using targeted antibody stains for cytokeratin 5/6 and Ki-67 protein. Staining with Ki-67 showed dramatically increased activity within basilar and suprabasilar keratinocytes in a biopsy taken at the height of the reaction. Biopsy specimens obtained when the eruption was clinically resolving—2 days after the first biopsy—showed decreased Ki-67 staining. These findings taken together suggest a direct causal effect of palifermin inducing hyperkeratotic changes appreciated on examination of treated patients.6
We present this case to add to current data regarding palifermin-induced cutaneous changes. Unique to our patient was a strikingly well-demarcated rash confined to the head and neck. Although a photosensitive eruption due to trimethoprim-sulfamethoxazole is conceivable, the fixed time course of the eruption—corresponding to (1) initiation and discontinuation of palifermin and (2) histologic findings—led us to conclude that this self-limited eruption likely was due to palifermin.
- Gorcey L, Lewin JM, Trufant J, et al. Papular eruption associated with palifermin. J Am Acad Dermatol. 2014;71:E101-E102. doi:10.1016/j.jaad.2014.04.006
- Grzegorczyk-Jaz´win´ska A, Kozak I, Karakulska-Prystupiuk E, et al. Transient oral cavity and skin complications after mucositis preventing therapy (palifermin) in a patient after allogeneic PBSCT. case history. Adv Med Sci. 2006;51(suppl 1):66-68.
- Keijzer A, Huijgens PC, van de Loosdrecht AA. Palifermin and palmar–plantar erythrodysesthesia. Br J Haematol. 2007;136:856-857. doi:10.1111/j.1365-2141.2007.06509.x
- Sibelt LAG, Aboosy N, van der Velden WJFM, et al. Palifermin-induced flexural hyperpigmentation: a clinical and histological study of five cases. Br J Dermatol. 2008;159:1200-1203. doi:10.1111/j.1365-2133.2008.08816.x
- Keefe D, Lees J, Horvath N. Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer. 2006;14:580-582. doi:10.1007/s00520-006-0048-3
- King B, Knopp E, Galan A, et al. Palifermin-associated papular eruption. Arch Dermatol. 2009;145:179-182. doi:10.1001/archdermatol.2008.548
- Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598. doi: 10.1056/NEJMoa040125
- Rubin JS, Bottaro DP, Chedid M, et al. Keratinocyte growth factor. Cell Biol Int. 1995;19:399-411. doi:10.1006/cbir.1995.1085
- McDonnell AM, Lenz KL. Palifermin: role in the prevention of chemotherapy- and radiation-induced mucositis. Ann Pharmacother. 2007;41:86-94. doi:10.1345/aph.1G473
- Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi:10.3389/fonc.2017.00089
To the Editor:
Palifermin is a recombinant keratinocyte growth factor (KGF) approved by the US Food and Drug Administration to prevent oral mucositis following radiation therapy or chemotherapy. Cutaneous reactions associated with palifermin have been reported.1-5 One case described a distinctive polymorphous eruption in a patient treated with palifermin.6 On histologic analysis, papules demonstrated findings similar to verrucae, with evidence of papillomatosis, hypergranulosis, and hyperorthokeratosis. Given its mechanism of action as a KGF, it was concluded that these findings were likely the direct result of palifermin.6 We report a similar case of a patient who was given palifermin prior to an autologous stem cell transplant. Histopathologic analysis confirmed epidermal dysmaturation and marked hypergranulosis. We present this case to expand the paucity of data on palifermin-associated cutaneous reactions.
A 63-year-old man with a history of psoriasis, eczema, and relapsed diffuse large B-cell lymphoma was admitted to the hospital for routine management of an autologous stem cell transplant with a conditioning regimen involving thiotepa, busulfan, and cyclophosphamide. The patient had completed a 3-day course of palifermin 1 day prior to the current presentation. On admission, he developed a pruritic erythematous rash over the face and axillae. Within 24 hours, the facial rash progressed with appreciable edema, and he reported difficulty opening his eyes. He denied any fever, nausea, vomiting, diarrhea, or increased fatigue. He also denied use of any other medications other than starting a course of prophylactic trimethoprim-sulfamethoxazole 3 times weekly 2 months prior to admission.
Diffuse blanching erythema with a well-demarcated linear border was noted along the lower anterior neck extending to the posterior hairline. There was notable edema but no evidence of pustules or overlying scale. Similar areas of blanchable erythema were present along the axillae and inguinal folds. There also were flesh-colored to pink papules within the axillary vaults and on the back that occasionally coalesced into plaques. There was no involvement of the mucous membranes or acral sites.
A complete blood cell count with differential and a comprehensive metabolic profile largely were unremarkable. A potassium hydroxide preparation of the face and groin was negative for hyphae and Demodex mites. Histopathologic analysis from a punch biopsy of a representative papule from the posterior neck demonstrated epidermal dysmaturation with marked thickening of the granular cell layer with notably large keratohyalin granules (Figure 1).

In the setting of treatment with thiotepa, we recommended supportive care with cool compresses rather than topical medication because he was neutropenic, and we wanted to avoid further immunosuppression or toxicity. By 24 hours after completing the course of palifermin, the patient experienced complete resolution of the rash. At his request, the trial of palifermin was restarted 10 days into conditioning therapy. A similar rash with less facial edema but more prominent involvement of the chest appeared 3 days into the retrial (Figure 2). The medication was discontinued, which resulted in resolution of the rash. Again, the patient remained afebrile without involvement of the mucous membranes. Liver enzyme and creatinine levels remained within reference range.Eosinophilia and the level of atypical lymphocytes could not be assessed because of leukopenia in the setting of recent chemotherapy. The rash self-resolved in 4 days.

Palifermin is a recombinant form of human KGF that is more stable than the endogenous form but retains all vital properties of the protein.5-7 Similar to other growth factors, KGF induces differentiation, proliferation, and migration of cells in vivo.8 However, it uniquely produces a targeted effect on epithelial cells in the skin, oral mucosa, lungs, gastrointestinal tract, and genitourinary system.7-9
Palifermin was approved by the US Food and Drug Administration in 2004 for the prevention and treatment of severe oral mucositis in patients receiving myelotoxic therapy prior to stem cell transplantation.7,9 Severe mucositis occurs in approximately 70% to 80% of patients receiving radiation or chemotherapy-based conditioning treatments.4,7 Compared to placebo, palifermin has been shown to greatly reduce the incidence of Grade 4 oral mucositis, defined as severe enough to prevent alimentation.10
The proliferative effect of palifermin on the oral mucosa is beneficial to patients but likely is the driving force behind its cutaneous adverse effects. A nonspecific rash is the most commonly cited treatment-related adverse event associated with palifermin, occurring in approximately 62% of patients.5,7,9
Our case is a rare report of a palifermin-associated cutaneous reaction. Previous cases have cited the occurrence of palmoplantar erythrodysesthesias, papulopustular eruptions involving the face and chest, and a papular rash involving the dorsal hands and intertriginous areas.1-4 Another report documented a “mild rash” but failed to further characterize the morphology or the body site involved.5
In 2009, King et al6 reported the occurrence of a lichen planus–like eruption involving the intertriginous regions and of white oral plaques in a patient treated with palifermin. Hematoxylin and eosin staining of a representative lesion in that patient demonstrated an appearance similar to that of verrucae, including papillomatosis, hypergranulosis, and hyperorthokeratosis.
King et al6 expanded analysis of the reaction to include immunohistochemical study, using targeted antibody stains for cytokeratin 5/6 and Ki-67 protein. Staining with Ki-67 showed dramatically increased activity within basilar and suprabasilar keratinocytes in a biopsy taken at the height of the reaction. Biopsy specimens obtained when the eruption was clinically resolving—2 days after the first biopsy—showed decreased Ki-67 staining. These findings taken together suggest a direct causal effect of palifermin inducing hyperkeratotic changes appreciated on examination of treated patients.6
We present this case to add to current data regarding palifermin-induced cutaneous changes. Unique to our patient was a strikingly well-demarcated rash confined to the head and neck. Although a photosensitive eruption due to trimethoprim-sulfamethoxazole is conceivable, the fixed time course of the eruption—corresponding to (1) initiation and discontinuation of palifermin and (2) histologic findings—led us to conclude that this self-limited eruption likely was due to palifermin.
To the Editor:
Palifermin is a recombinant keratinocyte growth factor (KGF) approved by the US Food and Drug Administration to prevent oral mucositis following radiation therapy or chemotherapy. Cutaneous reactions associated with palifermin have been reported.1-5 One case described a distinctive polymorphous eruption in a patient treated with palifermin.6 On histologic analysis, papules demonstrated findings similar to verrucae, with evidence of papillomatosis, hypergranulosis, and hyperorthokeratosis. Given its mechanism of action as a KGF, it was concluded that these findings were likely the direct result of palifermin.6 We report a similar case of a patient who was given palifermin prior to an autologous stem cell transplant. Histopathologic analysis confirmed epidermal dysmaturation and marked hypergranulosis. We present this case to expand the paucity of data on palifermin-associated cutaneous reactions.
A 63-year-old man with a history of psoriasis, eczema, and relapsed diffuse large B-cell lymphoma was admitted to the hospital for routine management of an autologous stem cell transplant with a conditioning regimen involving thiotepa, busulfan, and cyclophosphamide. The patient had completed a 3-day course of palifermin 1 day prior to the current presentation. On admission, he developed a pruritic erythematous rash over the face and axillae. Within 24 hours, the facial rash progressed with appreciable edema, and he reported difficulty opening his eyes. He denied any fever, nausea, vomiting, diarrhea, or increased fatigue. He also denied use of any other medications other than starting a course of prophylactic trimethoprim-sulfamethoxazole 3 times weekly 2 months prior to admission.
Diffuse blanching erythema with a well-demarcated linear border was noted along the lower anterior neck extending to the posterior hairline. There was notable edema but no evidence of pustules or overlying scale. Similar areas of blanchable erythema were present along the axillae and inguinal folds. There also were flesh-colored to pink papules within the axillary vaults and on the back that occasionally coalesced into plaques. There was no involvement of the mucous membranes or acral sites.
A complete blood cell count with differential and a comprehensive metabolic profile largely were unremarkable. A potassium hydroxide preparation of the face and groin was negative for hyphae and Demodex mites. Histopathologic analysis from a punch biopsy of a representative papule from the posterior neck demonstrated epidermal dysmaturation with marked thickening of the granular cell layer with notably large keratohyalin granules (Figure 1).

In the setting of treatment with thiotepa, we recommended supportive care with cool compresses rather than topical medication because he was neutropenic, and we wanted to avoid further immunosuppression or toxicity. By 24 hours after completing the course of palifermin, the patient experienced complete resolution of the rash. At his request, the trial of palifermin was restarted 10 days into conditioning therapy. A similar rash with less facial edema but more prominent involvement of the chest appeared 3 days into the retrial (Figure 2). The medication was discontinued, which resulted in resolution of the rash. Again, the patient remained afebrile without involvement of the mucous membranes. Liver enzyme and creatinine levels remained within reference range.Eosinophilia and the level of atypical lymphocytes could not be assessed because of leukopenia in the setting of recent chemotherapy. The rash self-resolved in 4 days.

Palifermin is a recombinant form of human KGF that is more stable than the endogenous form but retains all vital properties of the protein.5-7 Similar to other growth factors, KGF induces differentiation, proliferation, and migration of cells in vivo.8 However, it uniquely produces a targeted effect on epithelial cells in the skin, oral mucosa, lungs, gastrointestinal tract, and genitourinary system.7-9
Palifermin was approved by the US Food and Drug Administration in 2004 for the prevention and treatment of severe oral mucositis in patients receiving myelotoxic therapy prior to stem cell transplantation.7,9 Severe mucositis occurs in approximately 70% to 80% of patients receiving radiation or chemotherapy-based conditioning treatments.4,7 Compared to placebo, palifermin has been shown to greatly reduce the incidence of Grade 4 oral mucositis, defined as severe enough to prevent alimentation.10
The proliferative effect of palifermin on the oral mucosa is beneficial to patients but likely is the driving force behind its cutaneous adverse effects. A nonspecific rash is the most commonly cited treatment-related adverse event associated with palifermin, occurring in approximately 62% of patients.5,7,9
Our case is a rare report of a palifermin-associated cutaneous reaction. Previous cases have cited the occurrence of palmoplantar erythrodysesthesias, papulopustular eruptions involving the face and chest, and a papular rash involving the dorsal hands and intertriginous areas.1-4 Another report documented a “mild rash” but failed to further characterize the morphology or the body site involved.5
In 2009, King et al6 reported the occurrence of a lichen planus–like eruption involving the intertriginous regions and of white oral plaques in a patient treated with palifermin. Hematoxylin and eosin staining of a representative lesion in that patient demonstrated an appearance similar to that of verrucae, including papillomatosis, hypergranulosis, and hyperorthokeratosis.
King et al6 expanded analysis of the reaction to include immunohistochemical study, using targeted antibody stains for cytokeratin 5/6 and Ki-67 protein. Staining with Ki-67 showed dramatically increased activity within basilar and suprabasilar keratinocytes in a biopsy taken at the height of the reaction. Biopsy specimens obtained when the eruption was clinically resolving—2 days after the first biopsy—showed decreased Ki-67 staining. These findings taken together suggest a direct causal effect of palifermin inducing hyperkeratotic changes appreciated on examination of treated patients.6
We present this case to add to current data regarding palifermin-induced cutaneous changes. Unique to our patient was a strikingly well-demarcated rash confined to the head and neck. Although a photosensitive eruption due to trimethoprim-sulfamethoxazole is conceivable, the fixed time course of the eruption—corresponding to (1) initiation and discontinuation of palifermin and (2) histologic findings—led us to conclude that this self-limited eruption likely was due to palifermin.
- Gorcey L, Lewin JM, Trufant J, et al. Papular eruption associated with palifermin. J Am Acad Dermatol. 2014;71:E101-E102. doi:10.1016/j.jaad.2014.04.006
- Grzegorczyk-Jaz´win´ska A, Kozak I, Karakulska-Prystupiuk E, et al. Transient oral cavity and skin complications after mucositis preventing therapy (palifermin) in a patient after allogeneic PBSCT. case history. Adv Med Sci. 2006;51(suppl 1):66-68.
- Keijzer A, Huijgens PC, van de Loosdrecht AA. Palifermin and palmar–plantar erythrodysesthesia. Br J Haematol. 2007;136:856-857. doi:10.1111/j.1365-2141.2007.06509.x
- Sibelt LAG, Aboosy N, van der Velden WJFM, et al. Palifermin-induced flexural hyperpigmentation: a clinical and histological study of five cases. Br J Dermatol. 2008;159:1200-1203. doi:10.1111/j.1365-2133.2008.08816.x
- Keefe D, Lees J, Horvath N. Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer. 2006;14:580-582. doi:10.1007/s00520-006-0048-3
- King B, Knopp E, Galan A, et al. Palifermin-associated papular eruption. Arch Dermatol. 2009;145:179-182. doi:10.1001/archdermatol.2008.548
- Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598. doi: 10.1056/NEJMoa040125
- Rubin JS, Bottaro DP, Chedid M, et al. Keratinocyte growth factor. Cell Biol Int. 1995;19:399-411. doi:10.1006/cbir.1995.1085
- McDonnell AM, Lenz KL. Palifermin: role in the prevention of chemotherapy- and radiation-induced mucositis. Ann Pharmacother. 2007;41:86-94. doi:10.1345/aph.1G473
- Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi:10.3389/fonc.2017.00089
- Gorcey L, Lewin JM, Trufant J, et al. Papular eruption associated with palifermin. J Am Acad Dermatol. 2014;71:E101-E102. doi:10.1016/j.jaad.2014.04.006
- Grzegorczyk-Jaz´win´ska A, Kozak I, Karakulska-Prystupiuk E, et al. Transient oral cavity and skin complications after mucositis preventing therapy (palifermin) in a patient after allogeneic PBSCT. case history. Adv Med Sci. 2006;51(suppl 1):66-68.
- Keijzer A, Huijgens PC, van de Loosdrecht AA. Palifermin and palmar–plantar erythrodysesthesia. Br J Haematol. 2007;136:856-857. doi:10.1111/j.1365-2141.2007.06509.x
- Sibelt LAG, Aboosy N, van der Velden WJFM, et al. Palifermin-induced flexural hyperpigmentation: a clinical and histological study of five cases. Br J Dermatol. 2008;159:1200-1203. doi:10.1111/j.1365-2133.2008.08816.x
- Keefe D, Lees J, Horvath N. Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer. 2006;14:580-582. doi:10.1007/s00520-006-0048-3
- King B, Knopp E, Galan A, et al. Palifermin-associated papular eruption. Arch Dermatol. 2009;145:179-182. doi:10.1001/archdermatol.2008.548
- Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598. doi: 10.1056/NEJMoa040125
- Rubin JS, Bottaro DP, Chedid M, et al. Keratinocyte growth factor. Cell Biol Int. 1995;19:399-411. doi:10.1006/cbir.1995.1085
- McDonnell AM, Lenz KL. Palifermin: role in the prevention of chemotherapy- and radiation-induced mucositis. Ann Pharmacother. 2007;41:86-94. doi:10.1345/aph.1G473
- Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi:10.3389/fonc.2017.00089
Practice Points
- Palifermin is a recombinant keratinocyte growth factor that is US Food and Drug Administration approved to prevent oral mucositis in patients undergoing chemotherapy or radiation therapy.
- Histologically, the rash can resemble verrucae with evidence of hypergranulosis, hyperorthokeratosis, and papillomatosis.
- Cutaneous reactions have been reported with use of palifermin and generally are benign and self-limited with removal of the offending agent.
