User login
Scarring alopecia in a woman with psoriasis
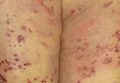
A 57-year-old African American woman came to our dermatology clinic to reestablish care. She had a long history of plaque psoriasis involving her trunk and extremities. More recently, she had developed progressive hair loss, which her previous physician had attributed to the psoriasis. Before this visit, our patient had been treating her psoriasis with topical clobetasol and calcipotriene.
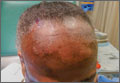
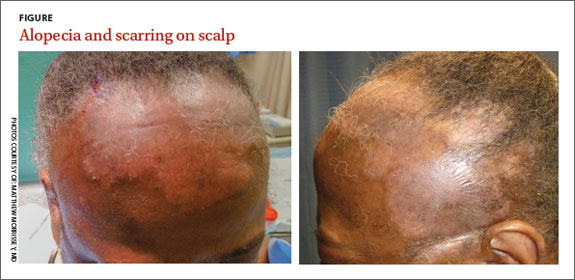
A physical exam revealed multiple welldemarcated, erythematous, scaly plaques consistent with plaque psoriasis on her trunk and extremities. She also said her scalp was itchy, and we noted significant cicatricial (scarring) alopecia of the scalp, with faint perifollicular erythema, that was predominantly affecting the frontotemporal region (FIGURE). We performed a scalp biopsy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lichen planopilaris
We suspected that this was not simply a case of plaque psoriasis because psoriasis of the scalp only causes non-cicatricial alopecia.1 Biopsy results confirmed that while the patient did have plaque psoriasis on her scalp, there was also evidence of peri-infundibular fibrosis and inflammation at the junction of the epidermis and dermis along the follicular epithelium. These 2 findings are pathognomonic for lichen planopilaris (LPP).
An uncommon diagnosis
Although its exact incidence and prevalence are unknown, LPP appears to be uncommon.2 The condition typically presents in adults ages 25 to 70, and is more common in women than in men.2 There is no known association between LPP and psoriasis.
Clinically, LPP manifests as cicatricial hair loss, often in a band-like fashion that can coalesce into larger, reticulated patterns.1 In addition to the scalp, LPP can affect other hair-bearing areas, such as the eyelids (lashes, brows), body, axillae, or pubic region.3,4 It is typically accompanied by burning and itching, and commonly presents with perifollicular erythema.1
LPP is thought to be the result of an immune-mediated lymphocytic inflammatory process that produces follicular hyperkeratosis, surrounding erythema, overlying scale, and, eventually, fibrosis and loss of the hair follicle.3,5
LPP has 3 variants: classic LPP, which typically affects the vertex and parietal areas of the scalp; frontal fibrosing alopecia, which is characterized by frontotemporal hair loss in a band-like pattern (as in our patient’s case); and Graham-Little syndrome, which can include cicatricial alopecia of the scalp and non-cicatricial alopecia of the axillary and pubic areas.3 Postmenopausal women appear to be at heightened risk for frontal fibrosing LPP.4
Differential diagnosis includes other types of scarring hair loss
The differential diagnosis for LPP includes discoid lupus erythematosus (DLE), central centrifugal cicatricial alopecia (CCCA), folliculitis decalvans, and dissecting cellulitis.1
DLE typically causes discrete, indurated lesions of central hypopigmentation (erythematous when active), along with slight atrophy and a rim of hyperpigmentation. This is in contrast to the perifollicular erythema and lack of atrophy that you’ll see in LPP. In addition, patients with DLE will have telangiectasias, while those with LPP will not.
CCCA is typically non-inflammatory, but can sometimes have symptoms such as mild itching. As the name implies, the hair loss associated with this disease starts in the central scalp and works its way centrifugally to the periphery, whereas in LPP, the alopecia can be patchy or diffuse, or can involve only the frontal scalp.2
Folliculitis decalvans is a form of scarring alopecia characterized by inflammatory perifollicular papules and pustules. Such lesions would not be observed in a patient with LPP. Bacterial culture will identify Staphylococcus aureus in most patients with untreated folliculitis decalvans.6
Dissecting cellulitis presents with tender, fluctuant nodules on the scalp that commonly suppurate and drain. The scarring hair loss that results could be mistaken for LPP, but a history of active, inflamed, nodular lesions will help to distinguish this condition from LPP.
Do a punch biopsy next to a patch of alopecia
A biopsy is required to confirm the diagnosis of LPP.2 A 4 mm punch biopsy should be performed, and optimally, 2 adjacent biopsies are taken so that they can be sectioned both vertically and horizontally.2 The biopsy should be done adjacent to a patch of alopecia that still has most of the hair follicles present. This is important because a biopsy of an area of scalp completely scarred with no remaining hair follicles will not demonstrate the pathognomonic patterns of inflammation that will allow for an accurate diagnosis.
In early-stage LPP, histopathology will reveal a lichenoid interface inflammation with hypergranulosis, hyperkeratosis, and hyperacanthosis, whereas in later stages, inflammation may be minimal or absent, with fibrous tracts taking the place of destroyed hair follicles.7
Steroids have produced the best treatment outcomes
LPP has an unpredictable course.2 Currently, there is no cure, and in areas where follicle destruction has occurred, normal hair growth cannot be restored.2 Therefore, treatment should focus on preventing progression and improving symptoms. It is imperative to manage patients’ expectations when dealing with cicatricial hair loss to ensure that they understand the likely outcomes.
Topical corticosteroids alone have shown some efficacy in treating LPP, but intralesional corticosteroids and oral glucocorticosteroids have resulted in better outcomes.4 Typical doses for intralesional triamcinolone are up to 1 mL of 10 mg/mL to 40 mg/mL per treatment session, with a one-month interval between treatments. Oral steroids can be used to initially control the disease and would require approximately 1 mg/kg/d of prednisone tapered over 3 to 4 weeks.
Adverse effects. Intralesional steroids can cause atrophy at the injection site and oral steroids can have rebound effects after an oral regimen is completed. This is in addition to other known adverse effects, such as insomnia and mood changes.4
Hydroxychloroquine has been reported to help arrest progression of, and control symptoms of, LPP with minimal adverse effects; a typical dosage is 200 mg twice a day.4 The 5-alpha-reductase inhibitors finasteride and dutasteride, which inhibit the conversion of testosterone to its more active form of dihydrotestosterone, have also shown similar efficacy.4
Finasteride can be used at a dose of 1 mg/d to 5 mg/d, and dutasteride is most effective at 0.5 to 2.5 mg/d.8 In a preliminary trial, pioglitazone (a peroxisome proliferatoractivated receptor gamma [PPAR-gamma] agonist) showed promise as a new treatment modality for LPP, perhaps because tissue expression of PPAR-gamma is decreased in LPP.9
A reasonable approach to therapy is to follow a stepwise increase from topical or intralesional corticosteroids to oral glucocorticosteroids, then to hydroxychloroquine or finasteride/dutasteride. The addition of a PPAR-gamma agonist can be added at any stage as adjunct therapy. A referral to a dermatologist may be necessary for refractory cases.
We started our patient on topical clobetasol 0.05% foam, which decreased her pruritus. However, we counseled her that we did not expect hair to regrow in the areas where she’d experienced hair loss. We continue to monitor her, and she would be a candidate for systemic therapy if the topical corticosteroid does not continue to control her disease.
CORRESPONDENCE
Simon Ritchie, MD, San Antonio Military Health System, 59MDSP/SGO7D, 2200 Berquist Drive, Suite 1, Lackland AFB, TX 78236; [email protected]
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Edinburgh, Scotland: Mosby; 2004:214,252,841,855-856,860-861.
2. Shapiro J, Otberg N. Lichen planopilaris. UpToDate Web site. Available at: http://www.uptodate.com/contents/lichen-planopilaris. Accessed June 2, 2015.
3. Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
4. Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37.
5. Mobini N, Tam S, Kamino H. Possible role of the bulge region in the pathogenesis of inflammatory scarring alopecia: lichen planopilaris as the prototype. J Cutan Pathol. 2005;32:675-679.
6. Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244.
7. Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin Cutan Med Surg. 2009;28:3-10.
8. Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
9. Baibergenova A, Walsh S. Use of pioglitazone in patients with lichen planopilaris. J Cutan Med Surg. 2012;16:97-100.
A 57-year-old African American woman came to our dermatology clinic to reestablish care. She had a long history of plaque psoriasis involving her trunk and extremities. More recently, she had developed progressive hair loss, which her previous physician had attributed to the psoriasis. Before this visit, our patient had been treating her psoriasis with topical clobetasol and calcipotriene.
A physical exam revealed multiple welldemarcated, erythematous, scaly plaques consistent with plaque psoriasis on her trunk and extremities. She also said her scalp was itchy, and we noted significant cicatricial (scarring) alopecia of the scalp, with faint perifollicular erythema, that was predominantly affecting the frontotemporal region (FIGURE). We performed a scalp biopsy.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lichen planopilaris
We suspected that this was not simply a case of plaque psoriasis because psoriasis of the scalp only causes non-cicatricial alopecia.1 Biopsy results confirmed that while the patient did have plaque psoriasis on her scalp, there was also evidence of peri-infundibular fibrosis and inflammation at the junction of the epidermis and dermis along the follicular epithelium. These 2 findings are pathognomonic for lichen planopilaris (LPP).
An uncommon diagnosis
Although its exact incidence and prevalence are unknown, LPP appears to be uncommon.2 The condition typically presents in adults ages 25 to 70, and is more common in women than in men.2 There is no known association between LPP and psoriasis.
Clinically, LPP manifests as cicatricial hair loss, often in a band-like fashion that can coalesce into larger, reticulated patterns.1 In addition to the scalp, LPP can affect other hair-bearing areas, such as the eyelids (lashes, brows), body, axillae, or pubic region.3,4 It is typically accompanied by burning and itching, and commonly presents with perifollicular erythema.1
LPP is thought to be the result of an immune-mediated lymphocytic inflammatory process that produces follicular hyperkeratosis, surrounding erythema, overlying scale, and, eventually, fibrosis and loss of the hair follicle.3,5
LPP has 3 variants: classic LPP, which typically affects the vertex and parietal areas of the scalp; frontal fibrosing alopecia, which is characterized by frontotemporal hair loss in a band-like pattern (as in our patient’s case); and Graham-Little syndrome, which can include cicatricial alopecia of the scalp and non-cicatricial alopecia of the axillary and pubic areas.3 Postmenopausal women appear to be at heightened risk for frontal fibrosing LPP.4
Differential diagnosis includes other types of scarring hair loss
The differential diagnosis for LPP includes discoid lupus erythematosus (DLE), central centrifugal cicatricial alopecia (CCCA), folliculitis decalvans, and dissecting cellulitis.1
DLE typically causes discrete, indurated lesions of central hypopigmentation (erythematous when active), along with slight atrophy and a rim of hyperpigmentation. This is in contrast to the perifollicular erythema and lack of atrophy that you’ll see in LPP. In addition, patients with DLE will have telangiectasias, while those with LPP will not.
CCCA is typically non-inflammatory, but can sometimes have symptoms such as mild itching. As the name implies, the hair loss associated with this disease starts in the central scalp and works its way centrifugally to the periphery, whereas in LPP, the alopecia can be patchy or diffuse, or can involve only the frontal scalp.2
Folliculitis decalvans is a form of scarring alopecia characterized by inflammatory perifollicular papules and pustules. Such lesions would not be observed in a patient with LPP. Bacterial culture will identify Staphylococcus aureus in most patients with untreated folliculitis decalvans.6
Dissecting cellulitis presents with tender, fluctuant nodules on the scalp that commonly suppurate and drain. The scarring hair loss that results could be mistaken for LPP, but a history of active, inflamed, nodular lesions will help to distinguish this condition from LPP.
Do a punch biopsy next to a patch of alopecia
A biopsy is required to confirm the diagnosis of LPP.2 A 4 mm punch biopsy should be performed, and optimally, 2 adjacent biopsies are taken so that they can be sectioned both vertically and horizontally.2 The biopsy should be done adjacent to a patch of alopecia that still has most of the hair follicles present. This is important because a biopsy of an area of scalp completely scarred with no remaining hair follicles will not demonstrate the pathognomonic patterns of inflammation that will allow for an accurate diagnosis.
In early-stage LPP, histopathology will reveal a lichenoid interface inflammation with hypergranulosis, hyperkeratosis, and hyperacanthosis, whereas in later stages, inflammation may be minimal or absent, with fibrous tracts taking the place of destroyed hair follicles.7
Steroids have produced the best treatment outcomes
LPP has an unpredictable course.2 Currently, there is no cure, and in areas where follicle destruction has occurred, normal hair growth cannot be restored.2 Therefore, treatment should focus on preventing progression and improving symptoms. It is imperative to manage patients’ expectations when dealing with cicatricial hair loss to ensure that they understand the likely outcomes.
Topical corticosteroids alone have shown some efficacy in treating LPP, but intralesional corticosteroids and oral glucocorticosteroids have resulted in better outcomes.4 Typical doses for intralesional triamcinolone are up to 1 mL of 10 mg/mL to 40 mg/mL per treatment session, with a one-month interval between treatments. Oral steroids can be used to initially control the disease and would require approximately 1 mg/kg/d of prednisone tapered over 3 to 4 weeks.
Adverse effects. Intralesional steroids can cause atrophy at the injection site and oral steroids can have rebound effects after an oral regimen is completed. This is in addition to other known adverse effects, such as insomnia and mood changes.4
Hydroxychloroquine has been reported to help arrest progression of, and control symptoms of, LPP with minimal adverse effects; a typical dosage is 200 mg twice a day.4 The 5-alpha-reductase inhibitors finasteride and dutasteride, which inhibit the conversion of testosterone to its more active form of dihydrotestosterone, have also shown similar efficacy.4
Finasteride can be used at a dose of 1 mg/d to 5 mg/d, and dutasteride is most effective at 0.5 to 2.5 mg/d.8 In a preliminary trial, pioglitazone (a peroxisome proliferatoractivated receptor gamma [PPAR-gamma] agonist) showed promise as a new treatment modality for LPP, perhaps because tissue expression of PPAR-gamma is decreased in LPP.9
A reasonable approach to therapy is to follow a stepwise increase from topical or intralesional corticosteroids to oral glucocorticosteroids, then to hydroxychloroquine or finasteride/dutasteride. The addition of a PPAR-gamma agonist can be added at any stage as adjunct therapy. A referral to a dermatologist may be necessary for refractory cases.
We started our patient on topical clobetasol 0.05% foam, which decreased her pruritus. However, we counseled her that we did not expect hair to regrow in the areas where she’d experienced hair loss. We continue to monitor her, and she would be a candidate for systemic therapy if the topical corticosteroid does not continue to control her disease.
CORRESPONDENCE
Simon Ritchie, MD, San Antonio Military Health System, 59MDSP/SGO7D, 2200 Berquist Drive, Suite 1, Lackland AFB, TX 78236; [email protected]
A 57-year-old African American woman came to our dermatology clinic to reestablish care. She had a long history of plaque psoriasis involving her trunk and extremities. More recently, she had developed progressive hair loss, which her previous physician had attributed to the psoriasis. Before this visit, our patient had been treating her psoriasis with topical clobetasol and calcipotriene.
A physical exam revealed multiple welldemarcated, erythematous, scaly plaques consistent with plaque psoriasis on her trunk and extremities. She also said her scalp was itchy, and we noted significant cicatricial (scarring) alopecia of the scalp, with faint perifollicular erythema, that was predominantly affecting the frontotemporal region (FIGURE). We performed a scalp biopsy.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lichen planopilaris
We suspected that this was not simply a case of plaque psoriasis because psoriasis of the scalp only causes non-cicatricial alopecia.1 Biopsy results confirmed that while the patient did have plaque psoriasis on her scalp, there was also evidence of peri-infundibular fibrosis and inflammation at the junction of the epidermis and dermis along the follicular epithelium. These 2 findings are pathognomonic for lichen planopilaris (LPP).
An uncommon diagnosis
Although its exact incidence and prevalence are unknown, LPP appears to be uncommon.2 The condition typically presents in adults ages 25 to 70, and is more common in women than in men.2 There is no known association between LPP and psoriasis.
Clinically, LPP manifests as cicatricial hair loss, often in a band-like fashion that can coalesce into larger, reticulated patterns.1 In addition to the scalp, LPP can affect other hair-bearing areas, such as the eyelids (lashes, brows), body, axillae, or pubic region.3,4 It is typically accompanied by burning and itching, and commonly presents with perifollicular erythema.1
LPP is thought to be the result of an immune-mediated lymphocytic inflammatory process that produces follicular hyperkeratosis, surrounding erythema, overlying scale, and, eventually, fibrosis and loss of the hair follicle.3,5
LPP has 3 variants: classic LPP, which typically affects the vertex and parietal areas of the scalp; frontal fibrosing alopecia, which is characterized by frontotemporal hair loss in a band-like pattern (as in our patient’s case); and Graham-Little syndrome, which can include cicatricial alopecia of the scalp and non-cicatricial alopecia of the axillary and pubic areas.3 Postmenopausal women appear to be at heightened risk for frontal fibrosing LPP.4
Differential diagnosis includes other types of scarring hair loss
The differential diagnosis for LPP includes discoid lupus erythematosus (DLE), central centrifugal cicatricial alopecia (CCCA), folliculitis decalvans, and dissecting cellulitis.1
DLE typically causes discrete, indurated lesions of central hypopigmentation (erythematous when active), along with slight atrophy and a rim of hyperpigmentation. This is in contrast to the perifollicular erythema and lack of atrophy that you’ll see in LPP. In addition, patients with DLE will have telangiectasias, while those with LPP will not.
CCCA is typically non-inflammatory, but can sometimes have symptoms such as mild itching. As the name implies, the hair loss associated with this disease starts in the central scalp and works its way centrifugally to the periphery, whereas in LPP, the alopecia can be patchy or diffuse, or can involve only the frontal scalp.2
Folliculitis decalvans is a form of scarring alopecia characterized by inflammatory perifollicular papules and pustules. Such lesions would not be observed in a patient with LPP. Bacterial culture will identify Staphylococcus aureus in most patients with untreated folliculitis decalvans.6
Dissecting cellulitis presents with tender, fluctuant nodules on the scalp that commonly suppurate and drain. The scarring hair loss that results could be mistaken for LPP, but a history of active, inflamed, nodular lesions will help to distinguish this condition from LPP.
Do a punch biopsy next to a patch of alopecia
A biopsy is required to confirm the diagnosis of LPP.2 A 4 mm punch biopsy should be performed, and optimally, 2 adjacent biopsies are taken so that they can be sectioned both vertically and horizontally.2 The biopsy should be done adjacent to a patch of alopecia that still has most of the hair follicles present. This is important because a biopsy of an area of scalp completely scarred with no remaining hair follicles will not demonstrate the pathognomonic patterns of inflammation that will allow for an accurate diagnosis.
In early-stage LPP, histopathology will reveal a lichenoid interface inflammation with hypergranulosis, hyperkeratosis, and hyperacanthosis, whereas in later stages, inflammation may be minimal or absent, with fibrous tracts taking the place of destroyed hair follicles.7
Steroids have produced the best treatment outcomes
LPP has an unpredictable course.2 Currently, there is no cure, and in areas where follicle destruction has occurred, normal hair growth cannot be restored.2 Therefore, treatment should focus on preventing progression and improving symptoms. It is imperative to manage patients’ expectations when dealing with cicatricial hair loss to ensure that they understand the likely outcomes.
Topical corticosteroids alone have shown some efficacy in treating LPP, but intralesional corticosteroids and oral glucocorticosteroids have resulted in better outcomes.4 Typical doses for intralesional triamcinolone are up to 1 mL of 10 mg/mL to 40 mg/mL per treatment session, with a one-month interval between treatments. Oral steroids can be used to initially control the disease and would require approximately 1 mg/kg/d of prednisone tapered over 3 to 4 weeks.
Adverse effects. Intralesional steroids can cause atrophy at the injection site and oral steroids can have rebound effects after an oral regimen is completed. This is in addition to other known adverse effects, such as insomnia and mood changes.4
Hydroxychloroquine has been reported to help arrest progression of, and control symptoms of, LPP with minimal adverse effects; a typical dosage is 200 mg twice a day.4 The 5-alpha-reductase inhibitors finasteride and dutasteride, which inhibit the conversion of testosterone to its more active form of dihydrotestosterone, have also shown similar efficacy.4
Finasteride can be used at a dose of 1 mg/d to 5 mg/d, and dutasteride is most effective at 0.5 to 2.5 mg/d.8 In a preliminary trial, pioglitazone (a peroxisome proliferatoractivated receptor gamma [PPAR-gamma] agonist) showed promise as a new treatment modality for LPP, perhaps because tissue expression of PPAR-gamma is decreased in LPP.9
A reasonable approach to therapy is to follow a stepwise increase from topical or intralesional corticosteroids to oral glucocorticosteroids, then to hydroxychloroquine or finasteride/dutasteride. The addition of a PPAR-gamma agonist can be added at any stage as adjunct therapy. A referral to a dermatologist may be necessary for refractory cases.
We started our patient on topical clobetasol 0.05% foam, which decreased her pruritus. However, we counseled her that we did not expect hair to regrow in the areas where she’d experienced hair loss. We continue to monitor her, and she would be a candidate for systemic therapy if the topical corticosteroid does not continue to control her disease.
CORRESPONDENCE
Simon Ritchie, MD, San Antonio Military Health System, 59MDSP/SGO7D, 2200 Berquist Drive, Suite 1, Lackland AFB, TX 78236; [email protected]
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Edinburgh, Scotland: Mosby; 2004:214,252,841,855-856,860-861.
2. Shapiro J, Otberg N. Lichen planopilaris. UpToDate Web site. Available at: http://www.uptodate.com/contents/lichen-planopilaris. Accessed June 2, 2015.
3. Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
4. Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37.
5. Mobini N, Tam S, Kamino H. Possible role of the bulge region in the pathogenesis of inflammatory scarring alopecia: lichen planopilaris as the prototype. J Cutan Pathol. 2005;32:675-679.
6. Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244.
7. Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin Cutan Med Surg. 2009;28:3-10.
8. Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
9. Baibergenova A, Walsh S. Use of pioglitazone in patients with lichen planopilaris. J Cutan Med Surg. 2012;16:97-100.
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Edinburgh, Scotland: Mosby; 2004:214,252,841,855-856,860-861.
2. Shapiro J, Otberg N. Lichen planopilaris. UpToDate Web site. Available at: http://www.uptodate.com/contents/lichen-planopilaris. Accessed June 2, 2015.
3. Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
4. Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37.
5. Mobini N, Tam S, Kamino H. Possible role of the bulge region in the pathogenesis of inflammatory scarring alopecia: lichen planopilaris as the prototype. J Cutan Pathol. 2005;32:675-679.
6. Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244.
7. Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin Cutan Med Surg. 2009;28:3-10.
8. Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
9. Baibergenova A, Walsh S. Use of pioglitazone in patients with lichen planopilaris. J Cutan Med Surg. 2012;16:97-100.