User login
Periprocedural Bridging Anticoagulation
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but that may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Oral anticoagulation (OAC) is commonly prescribed to patients with atrial fibrillation, venous thromboembolism (VTE), and mechanical heart valves (MHVs) for primary and secondary thromboembolism prevention. When patients require surgery or an invasive procedure, “bridging” anticoagulants (eg, enoxaparin) are commonly administered during the period of OAC interruption to reduce thromboembolic risk. This practice stems from small observational studies and expert opinion, which influenced several clinical guidelines despite the lack of high-quality evidence. Although prospective randomized trials of periprocedural bridging in patients with VTE and MHVs are lacking, available evidence is consistent with findings from the BRIDGE trial, which guides the following general recommendations: (1) avoid unnecessary periprocedural interruptions of OAC, especially for low bleeding risk procedures; (2) avoid the administration of periprocedural bridging anticoagulation in patients with low to moderate thromboembolic risk; (3) in patients with high thromboembolic risk, individually assess the patient-specific and procedure-specific bleeding risks versus thromboembolic risks.
A 75-year-old man with a history of hypertension, diabetes mellitus, and atrial fibrillation is admitted for surgical repair of a comminuted intertrochanteric left hip fracture. He suffered a mechanical ground-level fall without loss of consciousness. At baseline, he denies any chest pain, dyspnea on exertion, or recent change in his exercise tolerance. A physical examination is notable for stable vital signs, irregular cardiac rhythm, and a shortened and externally rotated left lower extremity with exquisite tenderness to palpation and range of motion. The patient is taking warfarin for stroke prophylaxis based on a CHA2DS2VaSc score of 4 points. The international normalized ratio (INR) is 1.9 upon admission, and surgery is planned within 48 hours, once the patient is “medically cleared.” Will this patient benefit from periprocedural bridging anticoagulation?
WHY YOU MIGHT THINK PERIPROCEDURAL “BRIDGING” ANTICOAGULATION IS HELPFUL
OAC is commonly prescribed to patients with atrial fibrillation, venous thromboembolism (VTE), and mechanical heart valves (MHVs) for the primary or secondary prevention of thromboembolic events, with more than 35 million prescriptions written annually in the United States alone.1 Many of these patients will require a temporary interruption of their OAC for surgery or an invasive procedure.2 As a result, patients may be treated with short-acting, or “bridging,” anticoagulants, such as low-molecular-weight heparin (LMWH), to minimize the duration of anticoagulation interruption and theoretically reduce their thromboembolic risk. The rationale for bridging stemmed from small observational studies and expert opinion that perceived the estimated thromboembolic risk to be higher than the estimated bleeding risk.3-5 One such example estimated that the VTE risk increased 100-fold postoperatively, whereas heparin administration only doubled the bleeding risk.3 Furthermore, clinical practice guidelines published from the American Heart Association, American College of Cardiology, European Heart Rhythm Society, and American College of Chest Physicians recommend when and how to initiate bridging anticoagulation. Clinicians have widely adopted these recommendations despite an acknowledged paucity of high-quality supporting evidence.6,7
WHY PERIPROCEDURAL “BRIDGING” ANTICOAGULATION IS MORE HARMFUL THAN HELPFUL
Periprocedural Anticoagulation Interruption is Often Not Indicated
Patients undergoing a surgical or invasive procedure may require an interruption of OAC to minimize the periprocedural bleeding risk. The decision to interrupt OAC should generally be based on the procedure-specific bleeding risk. Procedures with low bleeding risk such as cataract surgery, dermatologic biopsy (including Mohs), arthrocentesis, diagnostic gastrointestinal endoscopy, and cardiac pacemaker implantation can be performed safely without OAC interruption.5,7 Despite evidence supporting the safety of periprocedural OAC continuation, unnecessary OAC interruptions remain commonplace and are associated with increased adverse outcomes.8 The BRUISE CONTROL trial compared uninterrupted OAC to interrupted OAC with periprocedural bridging for cardiac pacemaker or defibrillator implantation in a moderate to high thromboembolic risk population. The uninterrupted OAC group experienced significantly fewer pocket hematomas, hematoma evacuations, and prolonged hospitalizations (relative risk [RR] 0.19-0.24; P < .05) without significantly increased thromboembolic events, highlighting the potential benefits of this approach.9
Nevertheless, many surgical and invasive procedures do warrant OAC interruption due to the inherent bleeding risk of the procedure or other logistical considerations. Procedures associated with an increased bleeding risk include urologic surgery (except laser lithotripsy), surgery on highly vascular organs (eg, kidney, liver, spleen), bowel resection, cardiac surgery, and intracranial or spinal surgery.7 Alternatively, some procedures with acceptably low bleeding risk (eg, colonoscopy) are routinely performed during an OAC interruption due to the fact that a high bleeding risk intervention may be necessary during the procedure (eg, polypectomy). This approach may be preferable when a significant amount of preparation is required (eg, bowel preparation) and may be a more efficient use of healthcare resources by avoiding repeat procedures.
Bridging Anticoagulation Does Not Significantly Reduce Thromboembolic Events
BRIDGE was a randomized, double-blind, placebo-controlled trial among patients with atrial fibrillation (n = 1884) requiring OAC interruption for mostly low-risk, ambulatory surgeries or invasive procedures (eg, gastrointestinal endoscopy, cardiac catheterization). Notably, thromboembolism events were rare, and there was no significant difference in thromboembolism events between patients randomized to placebo or bridging with LMWH (0.4% vs 0.3%, respectively; P = .73).14 However, the proportion of patients enrolled with the highest thromboembolic risk (ie, CHADS2 score 5-6 or prior transient ischemic attack and/or stroke) was low, potentially indicating an underestimated benefit in these patients. Major bleeding was significantly reduced in patients forgoing bridging anticoagulation (1.3% vs 3.2%; RR 0.41; 95% confidence interval, 0.20-0.78; P = .005), although bleeding occurred more frequently than thromboembolism in both groups.
Even though randomized trials assessing the safety and efficacy of bridging for VTE or MHVs have not been completed, evidence is not entirely lacking.16,17 A rigorous observational study limited to a VTE cohort (deep vein thrombosis of upper or lower extremity and/or pulmonary embolism) analyzed the effects of bridging in patients with a surgical or invasive procedure-related OAC interruption. Patients were stratified according to the American College of Chest Physicians perioperative guideline risk-stratification schema, and most VTE events (≥93%) occurred more than 12 months prior to OAC interruption.7 Importantly, the study found a nonsignificant difference in thromboembolism events between patients who were bridged and those who were not (0.0% vs 0.2%, respectively; P = .56), a very low overall thromboembolism event rate (0.2%), and a lack of correlation between events and risk-stratification category.17 In other words, all thromboembolic events occurred in the low- and moderate-risk groups, which include patients who do not warrant bridging under current guidelines. Clinically relevant bleeding occurred in 17 (0.9%) of 1812 patients studied. Notably, 15 (2.7%) of 555 patients receiving bridging suffered clinically relevant bleeding as compared with 2 (0.2%) of 1257 patients forgoing bridging anticoagulation.
The Bleeding Risk of Bridging Anticoagulation Often Outweighs the Potential Benefits
The early observational studies on LMWH bridging demonstrated that thromboembolic events are infrequent (0.4%-0.9%), whereas major bleeding events occur up to 7 times more often (0.7%-6.7%).10-12 The BRIDGE trial demonstrated comparably low thromboembolic events (0.3%). In the patients treated with bridging LMWH, major bleeding (3.2%) occurred 10 times more frequently than thromboembolism.14 Likewise, in a VTE cohort study, Clark et al.17 demonstrated “a 17-fold higher risk of bleeding without a significant difference in the rate of recurrent VTE” in patients bridged with heparin as compared with those who were not. Considering that recurrent VTE and major bleeding events have similar case-fatality rates,18 these increases in major bleeding events without reductions in thromboembolic events unmistakably tip the risk–benefit balance sharply towards an increased risk of harm.
When is bridging anticoagulation potentially helpful?
WHAT SHOULD YOU DO INSTEAD?
First, determine whether periprocedural OAC interruption is necessary for patients on chronic OAC due to atrial fibrillation, VTE, or MHVs. Avoid unwarranted OAC interruption by discussing the need for OAC interruptions with the surgeon or proceduralist, especially if the surgery is associated with a low bleeding risk and the patient has a high thromboembolic risk. When a periprocedural OAC interruption is justified, bridging should be avoided in the majority of patients, especially those with low to moderate thromboembolic risk or increased bleeding risk according to current risk-stratification schema.7,15,19
Periprocedural management of direct oral anticoagulants (DOACs) is different than that of warfarin. The duration of DOAC interruption is determined by the procedural bleeding risk, drug half-life, and a patient’s creatinine clearance. Although the pharmacokinetics of DOACs generally allow for brief interruptions (eg, 24-48 hours), longer interruptions (eg, 96-120 hours) are warranted prior to high bleeding risk procedures, when drug half-life is prolonged (ie, dabigatran), and in patients with renal impairment. Parenteral bridging anticoagulation is not recommended during brief DOAC interruptions, and substituting a DOAC in place of LMWH for bridging is not advised. The 2017 American College of Cardiology Expert Consensus Decision Pathway provides periprocedural OAC interruption guidance for atrial fibrillation, with many principles applicable to other OAC indications.15We developed an institutional guideline that provides clinicians a structured approach to bridging OAC that steers them away from inappropriate bridging and helps them make decisions when evidence is lacking. Shared decision-making represents another effective method for well-informed patients and clinicians to arrive at a mutually agreed upon bridging decision.
RECOMMENDATIONS
- Avoid unnecessary periprocedural interruptions of OAC, especially for procedures with a low bleeding risk.
- Avoid the administration of bridging anticoagulation in patients with low to moderate thromboembolic risk during periprocedural OAC interruptions.
- In patients with a high thromboembolic risk, an individualized assessment of the patient-specific and procedure-specific bleeding risks versus the thromboembolic risks is necessary when considering bridging anticoagulation administration.
CONCLUSION
Returning to the opening case, the patient requires an anticoagulation interruption and INR correction prior to surgery. Because the CHA2DS2VaSc score of 4 does not categorize him as a high thromboembolic risk, bridging anticoagulation should be avoided. In the majority of patients on OAC, bridging anticoagulation does not reduce thromboembolic events and is associated with increased major bleeding. Unnecessary anticoagulation interruptions should be avoided for procedures associated with low bleeding risk. Bridging should not be administered to the majority of patients requiring a periprocedural anticoagulation interruption.
Do
Disclosure: The authors report no conflicts of interest relevant to this article to disclose.
1. Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5(5):615-621. PubMed
2. Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131(5):488-494. PubMed
3. Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med. 1997;336(21):1506-1511. PubMed
4. Eckman MH. “Bridging on the river Kwai”: the perioperative management of anticoagulation therapy. Med Decis Making. 2005;25(4):370-373. PubMed
5. Dunn AS, Turpie AG. Perioperative management of patients receiving oral anticoagulants: a systematic review. Arch Intern Med. 2003;163(8):901-908. PubMed
6. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071-2104. PubMed
7. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-e350S. PubMed
8. Gerson LB, Gage BF, Owens DK, Triadafilopoulos G. Effect and outcomes of the ASGE guidelines on the periendoscopic management of patients who take anticoagulants. Am J Gastroenterol. 2000;95(7):1717-1724. PubMed
9. Birnie DH, Healey JS, Wells GA, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. 2013;368(22):2084-2093. PubMed
10. Douketis JD, Johnson JA, Turpie AG. Low-molecular-weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen. Arch Intern Med. 2004;164(12):1319-1326. PubMed
11. Spyropoulos AC, Turpie AG, Dunn AS, et al. Clinical outcomes with unfractionated heparin or low-molecular-weight heparin as bridging therapy in patients on long-term oral anticoagulants: the REGIMEN registry. J Thromb Haemost. 2006;4(6):1246-1252. PubMed
12. Kovacs MJ, Kearon C, Rodger M, et al. Single-arm study of bridging therapy with low-molecular-weight heparin for patients at risk of arterial embolism who require temporary interruption of warfarin. Circulation. 2004;110(12):1658-1663. PubMed
13. Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126(13):1630-1639. PubMed
14. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N Engl J Med. 2015;373(9):823-833. PubMed
15. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC Expert Consensus Decision Pathway for Periprocedural Management of Anticoagulation in Patients With Nonvalvular Atrial Fibrillation: A Report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol. 2017;69(7):871-898. PubMed
16. Daniels PR, McBane RD, Litin SC, et al. Peri-procedural anticoagulation management of mechanical prosthetic heart valve patients. Thromb Res. 2009;124(3):300-305. PubMed
17. Clark NP, Witt DM, Davies LE, et al. Bleeding, Recurrent Venous Thromboembolism, and Mortality Risks During Warfarin Interruption for Invasive Procedures. JAMA Intern Med. 2015;175(7):1163-1168. PubMed
18. Carrier M, Le Gal G, Wells PS, Rodger MA. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010;152(9):578-589. PubMed
19. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159-e1195. PubMed
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but that may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Oral anticoagulation (OAC) is commonly prescribed to patients with atrial fibrillation, venous thromboembolism (VTE), and mechanical heart valves (MHVs) for primary and secondary thromboembolism prevention. When patients require surgery or an invasive procedure, “bridging” anticoagulants (eg, enoxaparin) are commonly administered during the period of OAC interruption to reduce thromboembolic risk. This practice stems from small observational studies and expert opinion, which influenced several clinical guidelines despite the lack of high-quality evidence. Although prospective randomized trials of periprocedural bridging in patients with VTE and MHVs are lacking, available evidence is consistent with findings from the BRIDGE trial, which guides the following general recommendations: (1) avoid unnecessary periprocedural interruptions of OAC, especially for low bleeding risk procedures; (2) avoid the administration of periprocedural bridging anticoagulation in patients with low to moderate thromboembolic risk; (3) in patients with high thromboembolic risk, individually assess the patient-specific and procedure-specific bleeding risks versus thromboembolic risks.
A 75-year-old man with a history of hypertension, diabetes mellitus, and atrial fibrillation is admitted for surgical repair of a comminuted intertrochanteric left hip fracture. He suffered a mechanical ground-level fall without loss of consciousness. At baseline, he denies any chest pain, dyspnea on exertion, or recent change in his exercise tolerance. A physical examination is notable for stable vital signs, irregular cardiac rhythm, and a shortened and externally rotated left lower extremity with exquisite tenderness to palpation and range of motion. The patient is taking warfarin for stroke prophylaxis based on a CHA2DS2VaSc score of 4 points. The international normalized ratio (INR) is 1.9 upon admission, and surgery is planned within 48 hours, once the patient is “medically cleared.” Will this patient benefit from periprocedural bridging anticoagulation?
WHY YOU MIGHT THINK PERIPROCEDURAL “BRIDGING” ANTICOAGULATION IS HELPFUL
OAC is commonly prescribed to patients with atrial fibrillation, venous thromboembolism (VTE), and mechanical heart valves (MHVs) for the primary or secondary prevention of thromboembolic events, with more than 35 million prescriptions written annually in the United States alone.1 Many of these patients will require a temporary interruption of their OAC for surgery or an invasive procedure.2 As a result, patients may be treated with short-acting, or “bridging,” anticoagulants, such as low-molecular-weight heparin (LMWH), to minimize the duration of anticoagulation interruption and theoretically reduce their thromboembolic risk. The rationale for bridging stemmed from small observational studies and expert opinion that perceived the estimated thromboembolic risk to be higher than the estimated bleeding risk.3-5 One such example estimated that the VTE risk increased 100-fold postoperatively, whereas heparin administration only doubled the bleeding risk.3 Furthermore, clinical practice guidelines published from the American Heart Association, American College of Cardiology, European Heart Rhythm Society, and American College of Chest Physicians recommend when and how to initiate bridging anticoagulation. Clinicians have widely adopted these recommendations despite an acknowledged paucity of high-quality supporting evidence.6,7
WHY PERIPROCEDURAL “BRIDGING” ANTICOAGULATION IS MORE HARMFUL THAN HELPFUL
Periprocedural Anticoagulation Interruption is Often Not Indicated
Patients undergoing a surgical or invasive procedure may require an interruption of OAC to minimize the periprocedural bleeding risk. The decision to interrupt OAC should generally be based on the procedure-specific bleeding risk. Procedures with low bleeding risk such as cataract surgery, dermatologic biopsy (including Mohs), arthrocentesis, diagnostic gastrointestinal endoscopy, and cardiac pacemaker implantation can be performed safely without OAC interruption.5,7 Despite evidence supporting the safety of periprocedural OAC continuation, unnecessary OAC interruptions remain commonplace and are associated with increased adverse outcomes.8 The BRUISE CONTROL trial compared uninterrupted OAC to interrupted OAC with periprocedural bridging for cardiac pacemaker or defibrillator implantation in a moderate to high thromboembolic risk population. The uninterrupted OAC group experienced significantly fewer pocket hematomas, hematoma evacuations, and prolonged hospitalizations (relative risk [RR] 0.19-0.24; P < .05) without significantly increased thromboembolic events, highlighting the potential benefits of this approach.9
Nevertheless, many surgical and invasive procedures do warrant OAC interruption due to the inherent bleeding risk of the procedure or other logistical considerations. Procedures associated with an increased bleeding risk include urologic surgery (except laser lithotripsy), surgery on highly vascular organs (eg, kidney, liver, spleen), bowel resection, cardiac surgery, and intracranial or spinal surgery.7 Alternatively, some procedures with acceptably low bleeding risk (eg, colonoscopy) are routinely performed during an OAC interruption due to the fact that a high bleeding risk intervention may be necessary during the procedure (eg, polypectomy). This approach may be preferable when a significant amount of preparation is required (eg, bowel preparation) and may be a more efficient use of healthcare resources by avoiding repeat procedures.
Bridging Anticoagulation Does Not Significantly Reduce Thromboembolic Events
BRIDGE was a randomized, double-blind, placebo-controlled trial among patients with atrial fibrillation (n = 1884) requiring OAC interruption for mostly low-risk, ambulatory surgeries or invasive procedures (eg, gastrointestinal endoscopy, cardiac catheterization). Notably, thromboembolism events were rare, and there was no significant difference in thromboembolism events between patients randomized to placebo or bridging with LMWH (0.4% vs 0.3%, respectively; P = .73).14 However, the proportion of patients enrolled with the highest thromboembolic risk (ie, CHADS2 score 5-6 or prior transient ischemic attack and/or stroke) was low, potentially indicating an underestimated benefit in these patients. Major bleeding was significantly reduced in patients forgoing bridging anticoagulation (1.3% vs 3.2%; RR 0.41; 95% confidence interval, 0.20-0.78; P = .005), although bleeding occurred more frequently than thromboembolism in both groups.
Even though randomized trials assessing the safety and efficacy of bridging for VTE or MHVs have not been completed, evidence is not entirely lacking.16,17 A rigorous observational study limited to a VTE cohort (deep vein thrombosis of upper or lower extremity and/or pulmonary embolism) analyzed the effects of bridging in patients with a surgical or invasive procedure-related OAC interruption. Patients were stratified according to the American College of Chest Physicians perioperative guideline risk-stratification schema, and most VTE events (≥93%) occurred more than 12 months prior to OAC interruption.7 Importantly, the study found a nonsignificant difference in thromboembolism events between patients who were bridged and those who were not (0.0% vs 0.2%, respectively; P = .56), a very low overall thromboembolism event rate (0.2%), and a lack of correlation between events and risk-stratification category.17 In other words, all thromboembolic events occurred in the low- and moderate-risk groups, which include patients who do not warrant bridging under current guidelines. Clinically relevant bleeding occurred in 17 (0.9%) of 1812 patients studied. Notably, 15 (2.7%) of 555 patients receiving bridging suffered clinically relevant bleeding as compared with 2 (0.2%) of 1257 patients forgoing bridging anticoagulation.
The Bleeding Risk of Bridging Anticoagulation Often Outweighs the Potential Benefits
The early observational studies on LMWH bridging demonstrated that thromboembolic events are infrequent (0.4%-0.9%), whereas major bleeding events occur up to 7 times more often (0.7%-6.7%).10-12 The BRIDGE trial demonstrated comparably low thromboembolic events (0.3%). In the patients treated with bridging LMWH, major bleeding (3.2%) occurred 10 times more frequently than thromboembolism.14 Likewise, in a VTE cohort study, Clark et al.17 demonstrated “a 17-fold higher risk of bleeding without a significant difference in the rate of recurrent VTE” in patients bridged with heparin as compared with those who were not. Considering that recurrent VTE and major bleeding events have similar case-fatality rates,18 these increases in major bleeding events without reductions in thromboembolic events unmistakably tip the risk–benefit balance sharply towards an increased risk of harm.
When is bridging anticoagulation potentially helpful?
WHAT SHOULD YOU DO INSTEAD?
First, determine whether periprocedural OAC interruption is necessary for patients on chronic OAC due to atrial fibrillation, VTE, or MHVs. Avoid unwarranted OAC interruption by discussing the need for OAC interruptions with the surgeon or proceduralist, especially if the surgery is associated with a low bleeding risk and the patient has a high thromboembolic risk. When a periprocedural OAC interruption is justified, bridging should be avoided in the majority of patients, especially those with low to moderate thromboembolic risk or increased bleeding risk according to current risk-stratification schema.7,15,19
Periprocedural management of direct oral anticoagulants (DOACs) is different than that of warfarin. The duration of DOAC interruption is determined by the procedural bleeding risk, drug half-life, and a patient’s creatinine clearance. Although the pharmacokinetics of DOACs generally allow for brief interruptions (eg, 24-48 hours), longer interruptions (eg, 96-120 hours) are warranted prior to high bleeding risk procedures, when drug half-life is prolonged (ie, dabigatran), and in patients with renal impairment. Parenteral bridging anticoagulation is not recommended during brief DOAC interruptions, and substituting a DOAC in place of LMWH for bridging is not advised. The 2017 American College of Cardiology Expert Consensus Decision Pathway provides periprocedural OAC interruption guidance for atrial fibrillation, with many principles applicable to other OAC indications.15We developed an institutional guideline that provides clinicians a structured approach to bridging OAC that steers them away from inappropriate bridging and helps them make decisions when evidence is lacking. Shared decision-making represents another effective method for well-informed patients and clinicians to arrive at a mutually agreed upon bridging decision.
RECOMMENDATIONS
- Avoid unnecessary periprocedural interruptions of OAC, especially for procedures with a low bleeding risk.
- Avoid the administration of bridging anticoagulation in patients with low to moderate thromboembolic risk during periprocedural OAC interruptions.
- In patients with a high thromboembolic risk, an individualized assessment of the patient-specific and procedure-specific bleeding risks versus the thromboembolic risks is necessary when considering bridging anticoagulation administration.
CONCLUSION
Returning to the opening case, the patient requires an anticoagulation interruption and INR correction prior to surgery. Because the CHA2DS2VaSc score of 4 does not categorize him as a high thromboembolic risk, bridging anticoagulation should be avoided. In the majority of patients on OAC, bridging anticoagulation does not reduce thromboembolic events and is associated with increased major bleeding. Unnecessary anticoagulation interruptions should be avoided for procedures associated with low bleeding risk. Bridging should not be administered to the majority of patients requiring a periprocedural anticoagulation interruption.
Do
Disclosure: The authors report no conflicts of interest relevant to this article to disclose.
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but that may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Oral anticoagulation (OAC) is commonly prescribed to patients with atrial fibrillation, venous thromboembolism (VTE), and mechanical heart valves (MHVs) for primary and secondary thromboembolism prevention. When patients require surgery or an invasive procedure, “bridging” anticoagulants (eg, enoxaparin) are commonly administered during the period of OAC interruption to reduce thromboembolic risk. This practice stems from small observational studies and expert opinion, which influenced several clinical guidelines despite the lack of high-quality evidence. Although prospective randomized trials of periprocedural bridging in patients with VTE and MHVs are lacking, available evidence is consistent with findings from the BRIDGE trial, which guides the following general recommendations: (1) avoid unnecessary periprocedural interruptions of OAC, especially for low bleeding risk procedures; (2) avoid the administration of periprocedural bridging anticoagulation in patients with low to moderate thromboembolic risk; (3) in patients with high thromboembolic risk, individually assess the patient-specific and procedure-specific bleeding risks versus thromboembolic risks.
A 75-year-old man with a history of hypertension, diabetes mellitus, and atrial fibrillation is admitted for surgical repair of a comminuted intertrochanteric left hip fracture. He suffered a mechanical ground-level fall without loss of consciousness. At baseline, he denies any chest pain, dyspnea on exertion, or recent change in his exercise tolerance. A physical examination is notable for stable vital signs, irregular cardiac rhythm, and a shortened and externally rotated left lower extremity with exquisite tenderness to palpation and range of motion. The patient is taking warfarin for stroke prophylaxis based on a CHA2DS2VaSc score of 4 points. The international normalized ratio (INR) is 1.9 upon admission, and surgery is planned within 48 hours, once the patient is “medically cleared.” Will this patient benefit from periprocedural bridging anticoagulation?
WHY YOU MIGHT THINK PERIPROCEDURAL “BRIDGING” ANTICOAGULATION IS HELPFUL
OAC is commonly prescribed to patients with atrial fibrillation, venous thromboembolism (VTE), and mechanical heart valves (MHVs) for the primary or secondary prevention of thromboembolic events, with more than 35 million prescriptions written annually in the United States alone.1 Many of these patients will require a temporary interruption of their OAC for surgery or an invasive procedure.2 As a result, patients may be treated with short-acting, or “bridging,” anticoagulants, such as low-molecular-weight heparin (LMWH), to minimize the duration of anticoagulation interruption and theoretically reduce their thromboembolic risk. The rationale for bridging stemmed from small observational studies and expert opinion that perceived the estimated thromboembolic risk to be higher than the estimated bleeding risk.3-5 One such example estimated that the VTE risk increased 100-fold postoperatively, whereas heparin administration only doubled the bleeding risk.3 Furthermore, clinical practice guidelines published from the American Heart Association, American College of Cardiology, European Heart Rhythm Society, and American College of Chest Physicians recommend when and how to initiate bridging anticoagulation. Clinicians have widely adopted these recommendations despite an acknowledged paucity of high-quality supporting evidence.6,7
WHY PERIPROCEDURAL “BRIDGING” ANTICOAGULATION IS MORE HARMFUL THAN HELPFUL
Periprocedural Anticoagulation Interruption is Often Not Indicated
Patients undergoing a surgical or invasive procedure may require an interruption of OAC to minimize the periprocedural bleeding risk. The decision to interrupt OAC should generally be based on the procedure-specific bleeding risk. Procedures with low bleeding risk such as cataract surgery, dermatologic biopsy (including Mohs), arthrocentesis, diagnostic gastrointestinal endoscopy, and cardiac pacemaker implantation can be performed safely without OAC interruption.5,7 Despite evidence supporting the safety of periprocedural OAC continuation, unnecessary OAC interruptions remain commonplace and are associated with increased adverse outcomes.8 The BRUISE CONTROL trial compared uninterrupted OAC to interrupted OAC with periprocedural bridging for cardiac pacemaker or defibrillator implantation in a moderate to high thromboembolic risk population. The uninterrupted OAC group experienced significantly fewer pocket hematomas, hematoma evacuations, and prolonged hospitalizations (relative risk [RR] 0.19-0.24; P < .05) without significantly increased thromboembolic events, highlighting the potential benefits of this approach.9
Nevertheless, many surgical and invasive procedures do warrant OAC interruption due to the inherent bleeding risk of the procedure or other logistical considerations. Procedures associated with an increased bleeding risk include urologic surgery (except laser lithotripsy), surgery on highly vascular organs (eg, kidney, liver, spleen), bowel resection, cardiac surgery, and intracranial or spinal surgery.7 Alternatively, some procedures with acceptably low bleeding risk (eg, colonoscopy) are routinely performed during an OAC interruption due to the fact that a high bleeding risk intervention may be necessary during the procedure (eg, polypectomy). This approach may be preferable when a significant amount of preparation is required (eg, bowel preparation) and may be a more efficient use of healthcare resources by avoiding repeat procedures.
Bridging Anticoagulation Does Not Significantly Reduce Thromboembolic Events
BRIDGE was a randomized, double-blind, placebo-controlled trial among patients with atrial fibrillation (n = 1884) requiring OAC interruption for mostly low-risk, ambulatory surgeries or invasive procedures (eg, gastrointestinal endoscopy, cardiac catheterization). Notably, thromboembolism events were rare, and there was no significant difference in thromboembolism events between patients randomized to placebo or bridging with LMWH (0.4% vs 0.3%, respectively; P = .73).14 However, the proportion of patients enrolled with the highest thromboembolic risk (ie, CHADS2 score 5-6 or prior transient ischemic attack and/or stroke) was low, potentially indicating an underestimated benefit in these patients. Major bleeding was significantly reduced in patients forgoing bridging anticoagulation (1.3% vs 3.2%; RR 0.41; 95% confidence interval, 0.20-0.78; P = .005), although bleeding occurred more frequently than thromboembolism in both groups.
Even though randomized trials assessing the safety and efficacy of bridging for VTE or MHVs have not been completed, evidence is not entirely lacking.16,17 A rigorous observational study limited to a VTE cohort (deep vein thrombosis of upper or lower extremity and/or pulmonary embolism) analyzed the effects of bridging in patients with a surgical or invasive procedure-related OAC interruption. Patients were stratified according to the American College of Chest Physicians perioperative guideline risk-stratification schema, and most VTE events (≥93%) occurred more than 12 months prior to OAC interruption.7 Importantly, the study found a nonsignificant difference in thromboembolism events between patients who were bridged and those who were not (0.0% vs 0.2%, respectively; P = .56), a very low overall thromboembolism event rate (0.2%), and a lack of correlation between events and risk-stratification category.17 In other words, all thromboembolic events occurred in the low- and moderate-risk groups, which include patients who do not warrant bridging under current guidelines. Clinically relevant bleeding occurred in 17 (0.9%) of 1812 patients studied. Notably, 15 (2.7%) of 555 patients receiving bridging suffered clinically relevant bleeding as compared with 2 (0.2%) of 1257 patients forgoing bridging anticoagulation.
The Bleeding Risk of Bridging Anticoagulation Often Outweighs the Potential Benefits
The early observational studies on LMWH bridging demonstrated that thromboembolic events are infrequent (0.4%-0.9%), whereas major bleeding events occur up to 7 times more often (0.7%-6.7%).10-12 The BRIDGE trial demonstrated comparably low thromboembolic events (0.3%). In the patients treated with bridging LMWH, major bleeding (3.2%) occurred 10 times more frequently than thromboembolism.14 Likewise, in a VTE cohort study, Clark et al.17 demonstrated “a 17-fold higher risk of bleeding without a significant difference in the rate of recurrent VTE” in patients bridged with heparin as compared with those who were not. Considering that recurrent VTE and major bleeding events have similar case-fatality rates,18 these increases in major bleeding events without reductions in thromboembolic events unmistakably tip the risk–benefit balance sharply towards an increased risk of harm.
When is bridging anticoagulation potentially helpful?
WHAT SHOULD YOU DO INSTEAD?
First, determine whether periprocedural OAC interruption is necessary for patients on chronic OAC due to atrial fibrillation, VTE, or MHVs. Avoid unwarranted OAC interruption by discussing the need for OAC interruptions with the surgeon or proceduralist, especially if the surgery is associated with a low bleeding risk and the patient has a high thromboembolic risk. When a periprocedural OAC interruption is justified, bridging should be avoided in the majority of patients, especially those with low to moderate thromboembolic risk or increased bleeding risk according to current risk-stratification schema.7,15,19
Periprocedural management of direct oral anticoagulants (DOACs) is different than that of warfarin. The duration of DOAC interruption is determined by the procedural bleeding risk, drug half-life, and a patient’s creatinine clearance. Although the pharmacokinetics of DOACs generally allow for brief interruptions (eg, 24-48 hours), longer interruptions (eg, 96-120 hours) are warranted prior to high bleeding risk procedures, when drug half-life is prolonged (ie, dabigatran), and in patients with renal impairment. Parenteral bridging anticoagulation is not recommended during brief DOAC interruptions, and substituting a DOAC in place of LMWH for bridging is not advised. The 2017 American College of Cardiology Expert Consensus Decision Pathway provides periprocedural OAC interruption guidance for atrial fibrillation, with many principles applicable to other OAC indications.15We developed an institutional guideline that provides clinicians a structured approach to bridging OAC that steers them away from inappropriate bridging and helps them make decisions when evidence is lacking. Shared decision-making represents another effective method for well-informed patients and clinicians to arrive at a mutually agreed upon bridging decision.
RECOMMENDATIONS
- Avoid unnecessary periprocedural interruptions of OAC, especially for procedures with a low bleeding risk.
- Avoid the administration of bridging anticoagulation in patients with low to moderate thromboembolic risk during periprocedural OAC interruptions.
- In patients with a high thromboembolic risk, an individualized assessment of the patient-specific and procedure-specific bleeding risks versus the thromboembolic risks is necessary when considering bridging anticoagulation administration.
CONCLUSION
Returning to the opening case, the patient requires an anticoagulation interruption and INR correction prior to surgery. Because the CHA2DS2VaSc score of 4 does not categorize him as a high thromboembolic risk, bridging anticoagulation should be avoided. In the majority of patients on OAC, bridging anticoagulation does not reduce thromboembolic events and is associated with increased major bleeding. Unnecessary anticoagulation interruptions should be avoided for procedures associated with low bleeding risk. Bridging should not be administered to the majority of patients requiring a periprocedural anticoagulation interruption.
Do
Disclosure: The authors report no conflicts of interest relevant to this article to disclose.
1. Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5(5):615-621. PubMed
2. Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131(5):488-494. PubMed
3. Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med. 1997;336(21):1506-1511. PubMed
4. Eckman MH. “Bridging on the river Kwai”: the perioperative management of anticoagulation therapy. Med Decis Making. 2005;25(4):370-373. PubMed
5. Dunn AS, Turpie AG. Perioperative management of patients receiving oral anticoagulants: a systematic review. Arch Intern Med. 2003;163(8):901-908. PubMed
6. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071-2104. PubMed
7. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-e350S. PubMed
8. Gerson LB, Gage BF, Owens DK, Triadafilopoulos G. Effect and outcomes of the ASGE guidelines on the periendoscopic management of patients who take anticoagulants. Am J Gastroenterol. 2000;95(7):1717-1724. PubMed
9. Birnie DH, Healey JS, Wells GA, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. 2013;368(22):2084-2093. PubMed
10. Douketis JD, Johnson JA, Turpie AG. Low-molecular-weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen. Arch Intern Med. 2004;164(12):1319-1326. PubMed
11. Spyropoulos AC, Turpie AG, Dunn AS, et al. Clinical outcomes with unfractionated heparin or low-molecular-weight heparin as bridging therapy in patients on long-term oral anticoagulants: the REGIMEN registry. J Thromb Haemost. 2006;4(6):1246-1252. PubMed
12. Kovacs MJ, Kearon C, Rodger M, et al. Single-arm study of bridging therapy with low-molecular-weight heparin for patients at risk of arterial embolism who require temporary interruption of warfarin. Circulation. 2004;110(12):1658-1663. PubMed
13. Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126(13):1630-1639. PubMed
14. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N Engl J Med. 2015;373(9):823-833. PubMed
15. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC Expert Consensus Decision Pathway for Periprocedural Management of Anticoagulation in Patients With Nonvalvular Atrial Fibrillation: A Report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol. 2017;69(7):871-898. PubMed
16. Daniels PR, McBane RD, Litin SC, et al. Peri-procedural anticoagulation management of mechanical prosthetic heart valve patients. Thromb Res. 2009;124(3):300-305. PubMed
17. Clark NP, Witt DM, Davies LE, et al. Bleeding, Recurrent Venous Thromboembolism, and Mortality Risks During Warfarin Interruption for Invasive Procedures. JAMA Intern Med. 2015;175(7):1163-1168. PubMed
18. Carrier M, Le Gal G, Wells PS, Rodger MA. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010;152(9):578-589. PubMed
19. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159-e1195. PubMed
1. Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5(5):615-621. PubMed
2. Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131(5):488-494. PubMed
3. Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med. 1997;336(21):1506-1511. PubMed
4. Eckman MH. “Bridging on the river Kwai”: the perioperative management of anticoagulation therapy. Med Decis Making. 2005;25(4):370-373. PubMed
5. Dunn AS, Turpie AG. Perioperative management of patients receiving oral anticoagulants: a systematic review. Arch Intern Med. 2003;163(8):901-908. PubMed
6. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071-2104. PubMed
7. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-e350S. PubMed
8. Gerson LB, Gage BF, Owens DK, Triadafilopoulos G. Effect and outcomes of the ASGE guidelines on the periendoscopic management of patients who take anticoagulants. Am J Gastroenterol. 2000;95(7):1717-1724. PubMed
9. Birnie DH, Healey JS, Wells GA, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. 2013;368(22):2084-2093. PubMed
10. Douketis JD, Johnson JA, Turpie AG. Low-molecular-weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen. Arch Intern Med. 2004;164(12):1319-1326. PubMed
11. Spyropoulos AC, Turpie AG, Dunn AS, et al. Clinical outcomes with unfractionated heparin or low-molecular-weight heparin as bridging therapy in patients on long-term oral anticoagulants: the REGIMEN registry. J Thromb Haemost. 2006;4(6):1246-1252. PubMed
12. Kovacs MJ, Kearon C, Rodger M, et al. Single-arm study of bridging therapy with low-molecular-weight heparin for patients at risk of arterial embolism who require temporary interruption of warfarin. Circulation. 2004;110(12):1658-1663. PubMed
13. Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126(13):1630-1639. PubMed
14. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N Engl J Med. 2015;373(9):823-833. PubMed
15. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC Expert Consensus Decision Pathway for Periprocedural Management of Anticoagulation in Patients With Nonvalvular Atrial Fibrillation: A Report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol. 2017;69(7):871-898. PubMed
16. Daniels PR, McBane RD, Litin SC, et al. Peri-procedural anticoagulation management of mechanical prosthetic heart valve patients. Thromb Res. 2009;124(3):300-305. PubMed
17. Clark NP, Witt DM, Davies LE, et al. Bleeding, Recurrent Venous Thromboembolism, and Mortality Risks During Warfarin Interruption for Invasive Procedures. JAMA Intern Med. 2015;175(7):1163-1168. PubMed
18. Carrier M, Le Gal G, Wells PS, Rodger MA. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010;152(9):578-589. PubMed
19. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159-e1195. PubMed
© 2018 Society of Hospital Medicine
Patterns and Appropriateness of Thrombophilia Testing in an Academic Medical Center
Thrombophilia is a prothrombotic state, either acquired or inherited, leading to a thrombotic predisposition.1 The most common heritable thrombophilias include factor V Leiden (FVL) and prothrombin G20210A. The most common acquired thrombophilia is the presence of phospholipid antibodies.1 Thrombotic risk varies with thrombophilia type. For example, deficiencies of antithrombin, protein C and protein S, and the presence of phospholipid antibodies, confer higher risk than FVL and prothrombin G20210A.2-5 Other thrombophilias (eg, methylenetetrahydrofolate reductase mutation, increased factor VIII activity) are relatively uncommon and/or their impact on thrombosis risk appears to be either minimal or unknown.1-6 There is little clinical evidence that testing for thrombophilia impacts subsequent thrombosis prevention.5,7,8 Multiple clinical guidelines and medical societies recommend against the routine and indiscriminate use of thrombophilia testing.8-13 In general, thrombophilia testing should be considered only if the result would lead to changes in anticoagulant initiation, intensity, and/or duration, or might inform interventions to prevent thrombosis in asymptomatic family members.8-13 However, thrombophilia testing rarely changes the acute management of a thrombotic event and may have harmful effects on patients and their family members because positive results may unnecessarily increase anxiety and negative results may provide false reassurance.6,14-18 The cost-effectiveness of thrombophilia testing is unknown. Economic models have sought to quantify cost-effectiveness, but conclusions from these studies are limited.7
The utility of thrombophilia testing in emergency department (ED) and inpatient settings is further limited because patients are often treated and discharged before thrombophilia test results are available. Additionally, in these settings, multiple factors increase the risk of false-positive or false-negative results (eg, acute thrombosis, acute illness, pregnancy, and anticoagulant therapy).19,20 The purpose of this study was to systematically assess thrombophilia testing patterns in the ED and hospitalized patients at an academic medical center and to quantify the proportion of tests associated with minimal clinical utility. We hypothesize that the majority of thrombophilia tests completed in the inpatient setting are associated with minimal clinical utility.
METHODS
Setting and Patients
This study was conducted at University of Utah Health Care (UUHC) University Hospital, a 488-bed academic medical center with a level I trauma center, primary stroke center, and 50-bed ED. Laboratory services for UUHC, including thrombophilia testing, are provided by a national reference laboratory, Associated Regional and University Pathologists Laboratories. This study included patients ≥18 years of age who received thrombophilia testing (Supplementary Table 1) during an ED visit or inpatient admission at University Hospital between July 1, 2014 and December 31, 2014. There were no exclusion criteria. An institutional electronic data repository was used to identify patients matching inclusion criteria. All study activities were reviewed and approved by the UUHC Institutional Review Board with a waiver of informed consent.
Outcomes
An electronic database query was used to identify patients, collect patient demographic information, and collect test characteristics. Each patient’s electronic medical record was manually reviewed to collect all other outcomes. Indication for thrombophilia testing was identified by manual review of provider notes. Thrombophilia tests occurring in situations associated with minimal clinical utility were defined as tests meeting at least one of the following criteria: patient discharged before test results were available for review; test type not recommended by published guidelines or by UUHC Thrombosis Service physicians for thrombophilia testing (Supplementary Table 2); test performed in situations associated with decreased accuracy; test was a duplicate test as a result of different thrombophilia panels containing identical tests; and test followed a provoked venous thromboembolism (VTE). Testing in situations associated with decreased accuracy are summarized in Supplementary Table 3 and included at least one of the following at the time of the test: anticoagulant therapy, acute thrombosis, pregnant or <8 weeks postpartum, and receiving estrogen-containing medications. Only test types known to be affected by the respective situation were included. Testing following a provoked VTE was defined as testing prompted by an acute thrombosis and performed within 3 months following major surgery (defined administratively as any surgery performed in an operating room), during pregnancy, <8 weeks postpartum, or while on estrogen-containing medications. Thrombophilia testing during anticoagulant therapy was defined as testing within 4 half-lives of anticoagulant administration based on medication administration records. Anticoagulant therapy changes were identified by comparing prior-to-admission and discharge medication lists.
Data Analysis
Patient and laboratory characteristics were summarized using descriptive statistics, including mean and standard deviation (SD) for continuous variables and proportions for categorical variables. Data analysis was performed using Excel (Version 2013, Microsoft Corporation. Redmond, Washington).
RESULTS
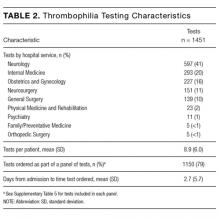
During the 6-month study period, 163 patients received at least 1 thrombophilia test during an ED visit or inpatient admission. Patient characteristics are summarized in Table 1. Tested patients were most commonly inpatients (96%) and female (71%). A total of 1451 thrombophilia tests were performed with a mean (± SD) of 8.9 ± 6.0 tests per patient. Testing characteristics are summarized in Table 2. Of the 39 different test types performed, the most commonly ordered were cardiolipin IgG and IgM antibodies (9% each), lupus anticoagulant (9%), and β2-glycoprotein 1 IgG and IgM antibodies (8% each). When combined with testing for phosphatidyl antibodies, antiphospholipid tests accounted for 70% of all tests. Overall, 134 (9%) test results were positive. The mean time for results to become available was 2.2 ± 2.5 days. The frequency of test types with corresponding positivity rates and mean time for results to become available are summarized in Supplementary Table 4.
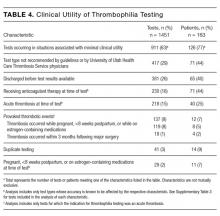
The indications for thrombophilia testing are summarized in Table 3. Ischemic stroke was the most common indication for testing (50% of tests; 35% of patients), followed by VTE (21% of tests; 21% of patients), and pregnancy-related conditions (eg, preeclampsia, intrauterine fetal demise; 15% of tests; 25% of patients). Overall, 911 tests (63%) occurred in situations associated with minimal clinical utility, with 126 patients (77%) receiving at least one of these tests (Table 4).
Anticoagulant therapy was changed in 43 patients (26%) in the following ways: initiated in 35 patients (21%), transitioned to a different anticoagulant in 6 patients (4%), and discontinued in 2 patients (1%). Of the 35 patients initiating anticoagulant therapy, 29 had documented thrombosis (24 had VTE, 4 had cerebral venous sinus thrombosis [CVST], and 1 had basilar artery thrombosis). Overall, 2 instances were identified in which initiation of anticoagulant therapy at discharge was in response to thrombophilia test results. In the first instance, warfarin without a parenteral anticoagulant bridge was initiated for a 54-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG antibodies, lupus anticoagulant, and protein S deficiency. In the second instance, warfarin with an enoxaparin bridge was initiated for a 26-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG and IgM antibodies, cardiolipin IgG antibodies, lupus anticoagulant, protein C deficiency, and antithrombin deficiency. Of the 163 patients receiving thrombophilia testing, only 2 patients (1%) had clear documentation of being offered genetic consultation.
DISCUSSION
In this retrospective analysis, 1451 thrombophilia tests were performed in 163 patients over 6 months. Tested patients were relatively young, which is likely explained by the number of patients tested for pregnancy-related conditions and the fact that a stroke or VTE in younger patients more frequently prompted providers to suspect thrombophilia. Nearly three-fourths of patients were female, which is likely due to testing for pregnancy-related conditions and possibly diagnostic suspicion bias given the comparative predilection of antiphospholipid syndrome for women. The patient characteristics in our study are consistent with other studies evaluating thrombophilia testing.21,22
Thrombophilia testing was most frequently prompted by stroke, VTE, and pregnancy-related conditions. Only 26% of patients had acute thrombosis identified during the admission, primarily because of the high proportion of tests for cryptogenic strokes and pregnancy-related conditions. Thrombophilia testing is recommended in patients who have had a stroke when the stroke is considered to be cryptogenic after a standard stroke evaluation.23 Thrombophilia testing in pregnancy-related conditions is controversial but is often considered in situations such as stillbirths with severe placental pathology and/or significant growth restriction, or in mothers with a personal or family history of thrombosis.24 The proportion of testing for pregnancy-related conditions may be greater than at other institutions because UUHC Maternal Fetal Medicine is a referral center for women with conditions associated with hypercoagulability. Anticoagulant therapy was initiated in 21% of patients, but specifically in response to thrombophilia testing in only 2 instances; in most cases, anticoagulant therapy was initiated regardless of thrombophilia test results.
The results of this study confirm our hypothesis because the majority of thrombophilia tests occurred in situations associated with minimal clinical utility. Testing in these situations was not isolated to specific patients or medical services because 77% of tested patients received at least 1 test associated with minimal clinical utility. Our study took a conservative approach in defining scenarios associated with minimal clinical utility because other situations can also affect testing accuracy (eg, hepatic disease, nephrotic syndrome) but were not included in our analysis of this outcome.
The results of this study highlight opportunities to improve thrombophilia testing practices at our institution and may be generalizable to institutions with similar testing patterns. Because multiple medical services order thrombophilia tests, strategies to improve testing practices are still being determined. The results of this study can serve as a baseline for comparison after strategies are implemented. The most common situation associated with minimal clinical utility was the use of test types not generally recommended by guidelines or UUHC Thrombosis Service physicians for thrombophilia testing (eg, β2-glycoprotein 1 IgA antibodies, phosphatidyl antibodies). We intend to require a hematology or thrombosis specialty consult prior to ordering these tests. This intervention alone could potentially decrease unnecessary testing by a third. Another consideration is to require a specialty consult prior to any inpatient thrombophilia testing. This strategy has been found to decrease inappropriate testing at other institutions.21 We also intend to streamline available thrombophilia testing panels because a poorly designed panel could lead to ordering of multiple tests associated with minimal clinical utility. At least 12 different thrombophilia panels are currently available in our computerized physician order entry system (see Supplementary Table 5). We hypothesize that current panel designs contribute to providers inadvertently ordering unintended or duplicate tests and that reducing the number of available panels and clearly delineating what tests are contained in each panel is likely to reduce unnecessary testing. Other strategies being considered include using electronic clinical decision support tools, implementing strict ordering criteria for all inpatient testing, and establishing a thrombosis stewardship program.
Our study was unique in at least 2 ways. First, previous studies describing thrombophilia testing have described testing patterns for patients with specific indications (eg, VTE), whereas our study described all thrombophilia tests regardless of indication. This allows for testing pattern comparisons across indications and medical services, increasing the generalizability of our results. Second, this study quantifies tests occurring in situations associated with a practical definition of minimal clinical utility.
Our study has several limitations: (1) Many variables were reliant on provider notes and other documentation, which allows for potential misclassification of variables. (2) It was not always possible to determine the ultimate utility of each test in clinical management decisions, and our study did not investigate the impact of thrombophilia testing on duration of anticoagulant therapy. Additionally, select situations could benefit from testing regardless if anticoagulant therapy is altered (eg, informing contraceptive choices). (3) Testing performed following a provoked acute thrombosis was defined as testing within 3 months following administratively defined major surgery. This definition could have included some minor procedures that do not substantially increase VTE risk, resulting in underestimated clinical utility. (4) The UUHC University Hospital serves as a referral hospital for a large geographical area, and investigators did not have access to outpatient records for a large proportion of discharged patients. As a result, frequency of repeat testing could not be assessed, possibly resulting in overestimated clinical utility. (5) In categorizing indications for testing, testing for CVST was subcategorized under testing for ischemic stroke based on presenting symptoms rather than on underlying pathophysiology. The rationale for this categorization is that patients with CVST were often tested based on presenting symptoms. Additionally, tests for CVST were ordered by the neurology service, which also ordered tests for all other ischemic stroke indications. (6) The purpose of our study was to investigate the subset of the hospital’s patient population that received thrombophilia testing, and patients were identified by tests received and not by diagnosis codes. As a result, we are unable to provide the proportion of total patients treated at the hospital for specific conditions who were tested (eg, the proportion of stroke patients that received thrombophilia testing). (7) Current practice guidelines do not recommend testing for phosphatidyl antibodies, even when traditional antiphospholipid testing is negative.25-27 Although expert panels continue to explore associations between phosphatidyl antibodies and pregnancy morbidity and thrombotic events, the low level of evidence is insufficient to guide clinical management.28 Therefore, we categorized all phosphatidyl testing as associated with minimal clinical utility.
CONCLUSIONS
In a large academic medical center, the majority of tests occurred in situations associated with minimal clinical utility. Strategies to improve thrombophilia testing practices are needed in order to minimize potentially inappropriate testing, provide more cost-effective care, and promote value-driven outcomes.
Disclosure
S.W. received financial support for this submitted work via a Bristol-Myers-Squibb grant. G.F. received financial support from Portola Pharmaceuticals for consulting and lectures that were not related to this submitted work.
1. Franco RF, Reitsma PH. Genetic risk factors of venous thrombosis. Hum Genet. 2001;109(4):369-384. PubMed
2. Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med. 1995;332(14):912-917. PubMed
3. Koster T, Rosendaal FR, de Ronde H, Briët E, Vandenbroucke JP, Bertina RM. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden Thrombophilia Study. Lancet. 1993;342(8886-8887):1503-1506. PubMed
4. Margaglione M, Brancaccio V, Giuliani N, et al. Increased risk for venous thrombosis in carriers of the prothrombin G-->A20210 gene variant. Ann Intern Med. 1998;129(2):89-93. PubMed
5. De Stefano V, Martinelli I, Mannucci PM, et al. The risk of recurrent deep venous thrombosis among heterozygous carriers of both factor V Leiden and the G20210A prothrombin mutation. N Engl J Med. 1999;341:801-806. PubMed
6. Dickey TL. Can thrombophilia testing help to prevent recurrent VTE? Part 2. JAAPA. 2002;15(12):23-24, 27-29. PubMed
7. Simpson EL, Stevenson MD, Rawdin A, Papaioannou D. Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis. Health Technol Assess. 2009;13(2):iii, ix-x, 1-91. PubMed
8. National Institute for Health and Clinical Excellence. Venous thromboembolic disease: the management of venous thromboembolic diseases and the role of thrombophilia testing. NICE clinical guideline 144. https://www.nice.org.uk/guidance/cg144. Accessed on June 30, 2017.
9. Evalution of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: routine testing for factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13(1):67-76.
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e419S-494S. PubMed
11. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220. PubMed
12. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Hematology Am Soc Hematol Educ Program. 2013;2013:9-14. PubMed
13. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
14. Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361. PubMed
15. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1-7. PubMed
16. Miles JS, Miletich JP, Goldhaber SZ, Hennekens CH, Ridker PM. G20210A mutation in the prothrombin gene and the risk of recurrent venous thromboembolism. J Am Coll Cardiol. 2001;37(1):215-218. PubMed
17. Eichinger S, Weltermann A, Mannhalter C, et al. The risk of recurrent venous thromboembolism in heterozygous carriers of factor V Leiden and a first spontaneous venous thromboembolism. Arch Intern Med. 2002;162(20):2357-2360. PubMed
18. Mazzolai L, Duchosal MA. Hereditary thrombophilia and venous thromboembolism: critical evaluation of the clinical implications of screening. Eur J Vasc Endovasc Surg. 2007;34(4):483-488. PubMed
19. Merriman L, Greaves M. Testing for thrombophilia: an evidence‐based approach. Postgrad Med J. 2006;82(973):699-704. PubMed
20. Favaloro EJ, McDonald D, Lippi G. Laboratory investigation of thrombophilia: the good, the bad, and the ugly. Semin Thromb Hemost. 2009;35(7):695-710. PubMed
21. Shen YM, Tsai J, Taiwo E, et al. Analysis of thrombophilia test ordering practices at an academic center: a proposal for appropriate testing to reduce harm and cost. PLoS One. 2016;11(5):e0155326. PubMed
22. Meyer MR, Witt DM, Delate T, et al. Thrombophilia testing patterns amongst patients with acute venous thromboembolism. Thromb Res. 2015;136(6):1160-1164. PubMed
23. Saver JL. Clinical practice: cryptogenic stroke. N Engl J Med. 2016;374(21):2065-2074. PubMed
24. ACOG practice bulletin no. 102: management of stillbirth. Obstet Gynecol. 2009;113(3):748-761. PubMed
25. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295-306. PubMed
26. Keeling D, Mackie I, Moore GW, Greer IA, Greaves M, British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157(1):47-58. PubMed
27. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice bulletin no. 132: antiphospholipid syndrome. Obstet Gynecol. 2012;120(6):1514-1521. PubMed
28. Bertolaccini ML, Amengual O, Andreoli L, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev. 2014;13(9):917-930. PubMed
Thrombophilia is a prothrombotic state, either acquired or inherited, leading to a thrombotic predisposition.1 The most common heritable thrombophilias include factor V Leiden (FVL) and prothrombin G20210A. The most common acquired thrombophilia is the presence of phospholipid antibodies.1 Thrombotic risk varies with thrombophilia type. For example, deficiencies of antithrombin, protein C and protein S, and the presence of phospholipid antibodies, confer higher risk than FVL and prothrombin G20210A.2-5 Other thrombophilias (eg, methylenetetrahydrofolate reductase mutation, increased factor VIII activity) are relatively uncommon and/or their impact on thrombosis risk appears to be either minimal or unknown.1-6 There is little clinical evidence that testing for thrombophilia impacts subsequent thrombosis prevention.5,7,8 Multiple clinical guidelines and medical societies recommend against the routine and indiscriminate use of thrombophilia testing.8-13 In general, thrombophilia testing should be considered only if the result would lead to changes in anticoagulant initiation, intensity, and/or duration, or might inform interventions to prevent thrombosis in asymptomatic family members.8-13 However, thrombophilia testing rarely changes the acute management of a thrombotic event and may have harmful effects on patients and their family members because positive results may unnecessarily increase anxiety and negative results may provide false reassurance.6,14-18 The cost-effectiveness of thrombophilia testing is unknown. Economic models have sought to quantify cost-effectiveness, but conclusions from these studies are limited.7
The utility of thrombophilia testing in emergency department (ED) and inpatient settings is further limited because patients are often treated and discharged before thrombophilia test results are available. Additionally, in these settings, multiple factors increase the risk of false-positive or false-negative results (eg, acute thrombosis, acute illness, pregnancy, and anticoagulant therapy).19,20 The purpose of this study was to systematically assess thrombophilia testing patterns in the ED and hospitalized patients at an academic medical center and to quantify the proportion of tests associated with minimal clinical utility. We hypothesize that the majority of thrombophilia tests completed in the inpatient setting are associated with minimal clinical utility.
METHODS
Setting and Patients
This study was conducted at University of Utah Health Care (UUHC) University Hospital, a 488-bed academic medical center with a level I trauma center, primary stroke center, and 50-bed ED. Laboratory services for UUHC, including thrombophilia testing, are provided by a national reference laboratory, Associated Regional and University Pathologists Laboratories. This study included patients ≥18 years of age who received thrombophilia testing (Supplementary Table 1) during an ED visit or inpatient admission at University Hospital between July 1, 2014 and December 31, 2014. There were no exclusion criteria. An institutional electronic data repository was used to identify patients matching inclusion criteria. All study activities were reviewed and approved by the UUHC Institutional Review Board with a waiver of informed consent.
Outcomes
An electronic database query was used to identify patients, collect patient demographic information, and collect test characteristics. Each patient’s electronic medical record was manually reviewed to collect all other outcomes. Indication for thrombophilia testing was identified by manual review of provider notes. Thrombophilia tests occurring in situations associated with minimal clinical utility were defined as tests meeting at least one of the following criteria: patient discharged before test results were available for review; test type not recommended by published guidelines or by UUHC Thrombosis Service physicians for thrombophilia testing (Supplementary Table 2); test performed in situations associated with decreased accuracy; test was a duplicate test as a result of different thrombophilia panels containing identical tests; and test followed a provoked venous thromboembolism (VTE). Testing in situations associated with decreased accuracy are summarized in Supplementary Table 3 and included at least one of the following at the time of the test: anticoagulant therapy, acute thrombosis, pregnant or <8 weeks postpartum, and receiving estrogen-containing medications. Only test types known to be affected by the respective situation were included. Testing following a provoked VTE was defined as testing prompted by an acute thrombosis and performed within 3 months following major surgery (defined administratively as any surgery performed in an operating room), during pregnancy, <8 weeks postpartum, or while on estrogen-containing medications. Thrombophilia testing during anticoagulant therapy was defined as testing within 4 half-lives of anticoagulant administration based on medication administration records. Anticoagulant therapy changes were identified by comparing prior-to-admission and discharge medication lists.
Data Analysis
Patient and laboratory characteristics were summarized using descriptive statistics, including mean and standard deviation (SD) for continuous variables and proportions for categorical variables. Data analysis was performed using Excel (Version 2013, Microsoft Corporation. Redmond, Washington).
RESULTS
During the 6-month study period, 163 patients received at least 1 thrombophilia test during an ED visit or inpatient admission. Patient characteristics are summarized in Table 1. Tested patients were most commonly inpatients (96%) and female (71%). A total of 1451 thrombophilia tests were performed with a mean (± SD) of 8.9 ± 6.0 tests per patient. Testing characteristics are summarized in Table 2. Of the 39 different test types performed, the most commonly ordered were cardiolipin IgG and IgM antibodies (9% each), lupus anticoagulant (9%), and β2-glycoprotein 1 IgG and IgM antibodies (8% each). When combined with testing for phosphatidyl antibodies, antiphospholipid tests accounted for 70% of all tests. Overall, 134 (9%) test results were positive. The mean time for results to become available was 2.2 ± 2.5 days. The frequency of test types with corresponding positivity rates and mean time for results to become available are summarized in Supplementary Table 4.
The indications for thrombophilia testing are summarized in Table 3. Ischemic stroke was the most common indication for testing (50% of tests; 35% of patients), followed by VTE (21% of tests; 21% of patients), and pregnancy-related conditions (eg, preeclampsia, intrauterine fetal demise; 15% of tests; 25% of patients). Overall, 911 tests (63%) occurred in situations associated with minimal clinical utility, with 126 patients (77%) receiving at least one of these tests (Table 4).
Anticoagulant therapy was changed in 43 patients (26%) in the following ways: initiated in 35 patients (21%), transitioned to a different anticoagulant in 6 patients (4%), and discontinued in 2 patients (1%). Of the 35 patients initiating anticoagulant therapy, 29 had documented thrombosis (24 had VTE, 4 had cerebral venous sinus thrombosis [CVST], and 1 had basilar artery thrombosis). Overall, 2 instances were identified in which initiation of anticoagulant therapy at discharge was in response to thrombophilia test results. In the first instance, warfarin without a parenteral anticoagulant bridge was initiated for a 54-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG antibodies, lupus anticoagulant, and protein S deficiency. In the second instance, warfarin with an enoxaparin bridge was initiated for a 26-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG and IgM antibodies, cardiolipin IgG antibodies, lupus anticoagulant, protein C deficiency, and antithrombin deficiency. Of the 163 patients receiving thrombophilia testing, only 2 patients (1%) had clear documentation of being offered genetic consultation.
DISCUSSION
In this retrospective analysis, 1451 thrombophilia tests were performed in 163 patients over 6 months. Tested patients were relatively young, which is likely explained by the number of patients tested for pregnancy-related conditions and the fact that a stroke or VTE in younger patients more frequently prompted providers to suspect thrombophilia. Nearly three-fourths of patients were female, which is likely due to testing for pregnancy-related conditions and possibly diagnostic suspicion bias given the comparative predilection of antiphospholipid syndrome for women. The patient characteristics in our study are consistent with other studies evaluating thrombophilia testing.21,22
Thrombophilia testing was most frequently prompted by stroke, VTE, and pregnancy-related conditions. Only 26% of patients had acute thrombosis identified during the admission, primarily because of the high proportion of tests for cryptogenic strokes and pregnancy-related conditions. Thrombophilia testing is recommended in patients who have had a stroke when the stroke is considered to be cryptogenic after a standard stroke evaluation.23 Thrombophilia testing in pregnancy-related conditions is controversial but is often considered in situations such as stillbirths with severe placental pathology and/or significant growth restriction, or in mothers with a personal or family history of thrombosis.24 The proportion of testing for pregnancy-related conditions may be greater than at other institutions because UUHC Maternal Fetal Medicine is a referral center for women with conditions associated with hypercoagulability. Anticoagulant therapy was initiated in 21% of patients, but specifically in response to thrombophilia testing in only 2 instances; in most cases, anticoagulant therapy was initiated regardless of thrombophilia test results.
The results of this study confirm our hypothesis because the majority of thrombophilia tests occurred in situations associated with minimal clinical utility. Testing in these situations was not isolated to specific patients or medical services because 77% of tested patients received at least 1 test associated with minimal clinical utility. Our study took a conservative approach in defining scenarios associated with minimal clinical utility because other situations can also affect testing accuracy (eg, hepatic disease, nephrotic syndrome) but were not included in our analysis of this outcome.
The results of this study highlight opportunities to improve thrombophilia testing practices at our institution and may be generalizable to institutions with similar testing patterns. Because multiple medical services order thrombophilia tests, strategies to improve testing practices are still being determined. The results of this study can serve as a baseline for comparison after strategies are implemented. The most common situation associated with minimal clinical utility was the use of test types not generally recommended by guidelines or UUHC Thrombosis Service physicians for thrombophilia testing (eg, β2-glycoprotein 1 IgA antibodies, phosphatidyl antibodies). We intend to require a hematology or thrombosis specialty consult prior to ordering these tests. This intervention alone could potentially decrease unnecessary testing by a third. Another consideration is to require a specialty consult prior to any inpatient thrombophilia testing. This strategy has been found to decrease inappropriate testing at other institutions.21 We also intend to streamline available thrombophilia testing panels because a poorly designed panel could lead to ordering of multiple tests associated with minimal clinical utility. At least 12 different thrombophilia panels are currently available in our computerized physician order entry system (see Supplementary Table 5). We hypothesize that current panel designs contribute to providers inadvertently ordering unintended or duplicate tests and that reducing the number of available panels and clearly delineating what tests are contained in each panel is likely to reduce unnecessary testing. Other strategies being considered include using electronic clinical decision support tools, implementing strict ordering criteria for all inpatient testing, and establishing a thrombosis stewardship program.
Our study was unique in at least 2 ways. First, previous studies describing thrombophilia testing have described testing patterns for patients with specific indications (eg, VTE), whereas our study described all thrombophilia tests regardless of indication. This allows for testing pattern comparisons across indications and medical services, increasing the generalizability of our results. Second, this study quantifies tests occurring in situations associated with a practical definition of minimal clinical utility.
Our study has several limitations: (1) Many variables were reliant on provider notes and other documentation, which allows for potential misclassification of variables. (2) It was not always possible to determine the ultimate utility of each test in clinical management decisions, and our study did not investigate the impact of thrombophilia testing on duration of anticoagulant therapy. Additionally, select situations could benefit from testing regardless if anticoagulant therapy is altered (eg, informing contraceptive choices). (3) Testing performed following a provoked acute thrombosis was defined as testing within 3 months following administratively defined major surgery. This definition could have included some minor procedures that do not substantially increase VTE risk, resulting in underestimated clinical utility. (4) The UUHC University Hospital serves as a referral hospital for a large geographical area, and investigators did not have access to outpatient records for a large proportion of discharged patients. As a result, frequency of repeat testing could not be assessed, possibly resulting in overestimated clinical utility. (5) In categorizing indications for testing, testing for CVST was subcategorized under testing for ischemic stroke based on presenting symptoms rather than on underlying pathophysiology. The rationale for this categorization is that patients with CVST were often tested based on presenting symptoms. Additionally, tests for CVST were ordered by the neurology service, which also ordered tests for all other ischemic stroke indications. (6) The purpose of our study was to investigate the subset of the hospital’s patient population that received thrombophilia testing, and patients were identified by tests received and not by diagnosis codes. As a result, we are unable to provide the proportion of total patients treated at the hospital for specific conditions who were tested (eg, the proportion of stroke patients that received thrombophilia testing). (7) Current practice guidelines do not recommend testing for phosphatidyl antibodies, even when traditional antiphospholipid testing is negative.25-27 Although expert panels continue to explore associations between phosphatidyl antibodies and pregnancy morbidity and thrombotic events, the low level of evidence is insufficient to guide clinical management.28 Therefore, we categorized all phosphatidyl testing as associated with minimal clinical utility.
CONCLUSIONS
In a large academic medical center, the majority of tests occurred in situations associated with minimal clinical utility. Strategies to improve thrombophilia testing practices are needed in order to minimize potentially inappropriate testing, provide more cost-effective care, and promote value-driven outcomes.
Disclosure
S.W. received financial support for this submitted work via a Bristol-Myers-Squibb grant. G.F. received financial support from Portola Pharmaceuticals for consulting and lectures that were not related to this submitted work.
Thrombophilia is a prothrombotic state, either acquired or inherited, leading to a thrombotic predisposition.1 The most common heritable thrombophilias include factor V Leiden (FVL) and prothrombin G20210A. The most common acquired thrombophilia is the presence of phospholipid antibodies.1 Thrombotic risk varies with thrombophilia type. For example, deficiencies of antithrombin, protein C and protein S, and the presence of phospholipid antibodies, confer higher risk than FVL and prothrombin G20210A.2-5 Other thrombophilias (eg, methylenetetrahydrofolate reductase mutation, increased factor VIII activity) are relatively uncommon and/or their impact on thrombosis risk appears to be either minimal or unknown.1-6 There is little clinical evidence that testing for thrombophilia impacts subsequent thrombosis prevention.5,7,8 Multiple clinical guidelines and medical societies recommend against the routine and indiscriminate use of thrombophilia testing.8-13 In general, thrombophilia testing should be considered only if the result would lead to changes in anticoagulant initiation, intensity, and/or duration, or might inform interventions to prevent thrombosis in asymptomatic family members.8-13 However, thrombophilia testing rarely changes the acute management of a thrombotic event and may have harmful effects on patients and their family members because positive results may unnecessarily increase anxiety and negative results may provide false reassurance.6,14-18 The cost-effectiveness of thrombophilia testing is unknown. Economic models have sought to quantify cost-effectiveness, but conclusions from these studies are limited.7
The utility of thrombophilia testing in emergency department (ED) and inpatient settings is further limited because patients are often treated and discharged before thrombophilia test results are available. Additionally, in these settings, multiple factors increase the risk of false-positive or false-negative results (eg, acute thrombosis, acute illness, pregnancy, and anticoagulant therapy).19,20 The purpose of this study was to systematically assess thrombophilia testing patterns in the ED and hospitalized patients at an academic medical center and to quantify the proportion of tests associated with minimal clinical utility. We hypothesize that the majority of thrombophilia tests completed in the inpatient setting are associated with minimal clinical utility.
METHODS
Setting and Patients
This study was conducted at University of Utah Health Care (UUHC) University Hospital, a 488-bed academic medical center with a level I trauma center, primary stroke center, and 50-bed ED. Laboratory services for UUHC, including thrombophilia testing, are provided by a national reference laboratory, Associated Regional and University Pathologists Laboratories. This study included patients ≥18 years of age who received thrombophilia testing (Supplementary Table 1) during an ED visit or inpatient admission at University Hospital between July 1, 2014 and December 31, 2014. There were no exclusion criteria. An institutional electronic data repository was used to identify patients matching inclusion criteria. All study activities were reviewed and approved by the UUHC Institutional Review Board with a waiver of informed consent.
Outcomes
An electronic database query was used to identify patients, collect patient demographic information, and collect test characteristics. Each patient’s electronic medical record was manually reviewed to collect all other outcomes. Indication for thrombophilia testing was identified by manual review of provider notes. Thrombophilia tests occurring in situations associated with minimal clinical utility were defined as tests meeting at least one of the following criteria: patient discharged before test results were available for review; test type not recommended by published guidelines or by UUHC Thrombosis Service physicians for thrombophilia testing (Supplementary Table 2); test performed in situations associated with decreased accuracy; test was a duplicate test as a result of different thrombophilia panels containing identical tests; and test followed a provoked venous thromboembolism (VTE). Testing in situations associated with decreased accuracy are summarized in Supplementary Table 3 and included at least one of the following at the time of the test: anticoagulant therapy, acute thrombosis, pregnant or <8 weeks postpartum, and receiving estrogen-containing medications. Only test types known to be affected by the respective situation were included. Testing following a provoked VTE was defined as testing prompted by an acute thrombosis and performed within 3 months following major surgery (defined administratively as any surgery performed in an operating room), during pregnancy, <8 weeks postpartum, or while on estrogen-containing medications. Thrombophilia testing during anticoagulant therapy was defined as testing within 4 half-lives of anticoagulant administration based on medication administration records. Anticoagulant therapy changes were identified by comparing prior-to-admission and discharge medication lists.
Data Analysis
Patient and laboratory characteristics were summarized using descriptive statistics, including mean and standard deviation (SD) for continuous variables and proportions for categorical variables. Data analysis was performed using Excel (Version 2013, Microsoft Corporation. Redmond, Washington).
RESULTS
During the 6-month study period, 163 patients received at least 1 thrombophilia test during an ED visit or inpatient admission. Patient characteristics are summarized in Table 1. Tested patients were most commonly inpatients (96%) and female (71%). A total of 1451 thrombophilia tests were performed with a mean (± SD) of 8.9 ± 6.0 tests per patient. Testing characteristics are summarized in Table 2. Of the 39 different test types performed, the most commonly ordered were cardiolipin IgG and IgM antibodies (9% each), lupus anticoagulant (9%), and β2-glycoprotein 1 IgG and IgM antibodies (8% each). When combined with testing for phosphatidyl antibodies, antiphospholipid tests accounted for 70% of all tests. Overall, 134 (9%) test results were positive. The mean time for results to become available was 2.2 ± 2.5 days. The frequency of test types with corresponding positivity rates and mean time for results to become available are summarized in Supplementary Table 4.
The indications for thrombophilia testing are summarized in Table 3. Ischemic stroke was the most common indication for testing (50% of tests; 35% of patients), followed by VTE (21% of tests; 21% of patients), and pregnancy-related conditions (eg, preeclampsia, intrauterine fetal demise; 15% of tests; 25% of patients). Overall, 911 tests (63%) occurred in situations associated with minimal clinical utility, with 126 patients (77%) receiving at least one of these tests (Table 4).
Anticoagulant therapy was changed in 43 patients (26%) in the following ways: initiated in 35 patients (21%), transitioned to a different anticoagulant in 6 patients (4%), and discontinued in 2 patients (1%). Of the 35 patients initiating anticoagulant therapy, 29 had documented thrombosis (24 had VTE, 4 had cerebral venous sinus thrombosis [CVST], and 1 had basilar artery thrombosis). Overall, 2 instances were identified in which initiation of anticoagulant therapy at discharge was in response to thrombophilia test results. In the first instance, warfarin without a parenteral anticoagulant bridge was initiated for a 54-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG antibodies, lupus anticoagulant, and protein S deficiency. In the second instance, warfarin with an enoxaparin bridge was initiated for a 26-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG and IgM antibodies, cardiolipin IgG antibodies, lupus anticoagulant, protein C deficiency, and antithrombin deficiency. Of the 163 patients receiving thrombophilia testing, only 2 patients (1%) had clear documentation of being offered genetic consultation.
DISCUSSION
In this retrospective analysis, 1451 thrombophilia tests were performed in 163 patients over 6 months. Tested patients were relatively young, which is likely explained by the number of patients tested for pregnancy-related conditions and the fact that a stroke or VTE in younger patients more frequently prompted providers to suspect thrombophilia. Nearly three-fourths of patients were female, which is likely due to testing for pregnancy-related conditions and possibly diagnostic suspicion bias given the comparative predilection of antiphospholipid syndrome for women. The patient characteristics in our study are consistent with other studies evaluating thrombophilia testing.21,22
Thrombophilia testing was most frequently prompted by stroke, VTE, and pregnancy-related conditions. Only 26% of patients had acute thrombosis identified during the admission, primarily because of the high proportion of tests for cryptogenic strokes and pregnancy-related conditions. Thrombophilia testing is recommended in patients who have had a stroke when the stroke is considered to be cryptogenic after a standard stroke evaluation.23 Thrombophilia testing in pregnancy-related conditions is controversial but is often considered in situations such as stillbirths with severe placental pathology and/or significant growth restriction, or in mothers with a personal or family history of thrombosis.24 The proportion of testing for pregnancy-related conditions may be greater than at other institutions because UUHC Maternal Fetal Medicine is a referral center for women with conditions associated with hypercoagulability. Anticoagulant therapy was initiated in 21% of patients, but specifically in response to thrombophilia testing in only 2 instances; in most cases, anticoagulant therapy was initiated regardless of thrombophilia test results.
The results of this study confirm our hypothesis because the majority of thrombophilia tests occurred in situations associated with minimal clinical utility. Testing in these situations was not isolated to specific patients or medical services because 77% of tested patients received at least 1 test associated with minimal clinical utility. Our study took a conservative approach in defining scenarios associated with minimal clinical utility because other situations can also affect testing accuracy (eg, hepatic disease, nephrotic syndrome) but were not included in our analysis of this outcome.
The results of this study highlight opportunities to improve thrombophilia testing practices at our institution and may be generalizable to institutions with similar testing patterns. Because multiple medical services order thrombophilia tests, strategies to improve testing practices are still being determined. The results of this study can serve as a baseline for comparison after strategies are implemented. The most common situation associated with minimal clinical utility was the use of test types not generally recommended by guidelines or UUHC Thrombosis Service physicians for thrombophilia testing (eg, β2-glycoprotein 1 IgA antibodies, phosphatidyl antibodies). We intend to require a hematology or thrombosis specialty consult prior to ordering these tests. This intervention alone could potentially decrease unnecessary testing by a third. Another consideration is to require a specialty consult prior to any inpatient thrombophilia testing. This strategy has been found to decrease inappropriate testing at other institutions.21 We also intend to streamline available thrombophilia testing panels because a poorly designed panel could lead to ordering of multiple tests associated with minimal clinical utility. At least 12 different thrombophilia panels are currently available in our computerized physician order entry system (see Supplementary Table 5). We hypothesize that current panel designs contribute to providers inadvertently ordering unintended or duplicate tests and that reducing the number of available panels and clearly delineating what tests are contained in each panel is likely to reduce unnecessary testing. Other strategies being considered include using electronic clinical decision support tools, implementing strict ordering criteria for all inpatient testing, and establishing a thrombosis stewardship program.
Our study was unique in at least 2 ways. First, previous studies describing thrombophilia testing have described testing patterns for patients with specific indications (eg, VTE), whereas our study described all thrombophilia tests regardless of indication. This allows for testing pattern comparisons across indications and medical services, increasing the generalizability of our results. Second, this study quantifies tests occurring in situations associated with a practical definition of minimal clinical utility.
Our study has several limitations: (1) Many variables were reliant on provider notes and other documentation, which allows for potential misclassification of variables. (2) It was not always possible to determine the ultimate utility of each test in clinical management decisions, and our study did not investigate the impact of thrombophilia testing on duration of anticoagulant therapy. Additionally, select situations could benefit from testing regardless if anticoagulant therapy is altered (eg, informing contraceptive choices). (3) Testing performed following a provoked acute thrombosis was defined as testing within 3 months following administratively defined major surgery. This definition could have included some minor procedures that do not substantially increase VTE risk, resulting in underestimated clinical utility. (4) The UUHC University Hospital serves as a referral hospital for a large geographical area, and investigators did not have access to outpatient records for a large proportion of discharged patients. As a result, frequency of repeat testing could not be assessed, possibly resulting in overestimated clinical utility. (5) In categorizing indications for testing, testing for CVST was subcategorized under testing for ischemic stroke based on presenting symptoms rather than on underlying pathophysiology. The rationale for this categorization is that patients with CVST were often tested based on presenting symptoms. Additionally, tests for CVST were ordered by the neurology service, which also ordered tests for all other ischemic stroke indications. (6) The purpose of our study was to investigate the subset of the hospital’s patient population that received thrombophilia testing, and patients were identified by tests received and not by diagnosis codes. As a result, we are unable to provide the proportion of total patients treated at the hospital for specific conditions who were tested (eg, the proportion of stroke patients that received thrombophilia testing). (7) Current practice guidelines do not recommend testing for phosphatidyl antibodies, even when traditional antiphospholipid testing is negative.25-27 Although expert panels continue to explore associations between phosphatidyl antibodies and pregnancy morbidity and thrombotic events, the low level of evidence is insufficient to guide clinical management.28 Therefore, we categorized all phosphatidyl testing as associated with minimal clinical utility.
CONCLUSIONS
In a large academic medical center, the majority of tests occurred in situations associated with minimal clinical utility. Strategies to improve thrombophilia testing practices are needed in order to minimize potentially inappropriate testing, provide more cost-effective care, and promote value-driven outcomes.
Disclosure
S.W. received financial support for this submitted work via a Bristol-Myers-Squibb grant. G.F. received financial support from Portola Pharmaceuticals for consulting and lectures that were not related to this submitted work.
1. Franco RF, Reitsma PH. Genetic risk factors of venous thrombosis. Hum Genet. 2001;109(4):369-384. PubMed
2. Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med. 1995;332(14):912-917. PubMed
3. Koster T, Rosendaal FR, de Ronde H, Briët E, Vandenbroucke JP, Bertina RM. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden Thrombophilia Study. Lancet. 1993;342(8886-8887):1503-1506. PubMed
4. Margaglione M, Brancaccio V, Giuliani N, et al. Increased risk for venous thrombosis in carriers of the prothrombin G-->A20210 gene variant. Ann Intern Med. 1998;129(2):89-93. PubMed
5. De Stefano V, Martinelli I, Mannucci PM, et al. The risk of recurrent deep venous thrombosis among heterozygous carriers of both factor V Leiden and the G20210A prothrombin mutation. N Engl J Med. 1999;341:801-806. PubMed
6. Dickey TL. Can thrombophilia testing help to prevent recurrent VTE? Part 2. JAAPA. 2002;15(12):23-24, 27-29. PubMed
7. Simpson EL, Stevenson MD, Rawdin A, Papaioannou D. Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis. Health Technol Assess. 2009;13(2):iii, ix-x, 1-91. PubMed
8. National Institute for Health and Clinical Excellence. Venous thromboembolic disease: the management of venous thromboembolic diseases and the role of thrombophilia testing. NICE clinical guideline 144. https://www.nice.org.uk/guidance/cg144. Accessed on June 30, 2017.
9. Evalution of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: routine testing for factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13(1):67-76.
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e419S-494S. PubMed
11. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220. PubMed
12. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Hematology Am Soc Hematol Educ Program. 2013;2013:9-14. PubMed
13. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
14. Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361. PubMed
15. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1-7. PubMed
16. Miles JS, Miletich JP, Goldhaber SZ, Hennekens CH, Ridker PM. G20210A mutation in the prothrombin gene and the risk of recurrent venous thromboembolism. J Am Coll Cardiol. 2001;37(1):215-218. PubMed
17. Eichinger S, Weltermann A, Mannhalter C, et al. The risk of recurrent venous thromboembolism in heterozygous carriers of factor V Leiden and a first spontaneous venous thromboembolism. Arch Intern Med. 2002;162(20):2357-2360. PubMed
18. Mazzolai L, Duchosal MA. Hereditary thrombophilia and venous thromboembolism: critical evaluation of the clinical implications of screening. Eur J Vasc Endovasc Surg. 2007;34(4):483-488. PubMed
19. Merriman L, Greaves M. Testing for thrombophilia: an evidence‐based approach. Postgrad Med J. 2006;82(973):699-704. PubMed
20. Favaloro EJ, McDonald D, Lippi G. Laboratory investigation of thrombophilia: the good, the bad, and the ugly. Semin Thromb Hemost. 2009;35(7):695-710. PubMed
21. Shen YM, Tsai J, Taiwo E, et al. Analysis of thrombophilia test ordering practices at an academic center: a proposal for appropriate testing to reduce harm and cost. PLoS One. 2016;11(5):e0155326. PubMed
22. Meyer MR, Witt DM, Delate T, et al. Thrombophilia testing patterns amongst patients with acute venous thromboembolism. Thromb Res. 2015;136(6):1160-1164. PubMed
23. Saver JL. Clinical practice: cryptogenic stroke. N Engl J Med. 2016;374(21):2065-2074. PubMed
24. ACOG practice bulletin no. 102: management of stillbirth. Obstet Gynecol. 2009;113(3):748-761. PubMed
25. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295-306. PubMed
26. Keeling D, Mackie I, Moore GW, Greer IA, Greaves M, British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157(1):47-58. PubMed
27. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice bulletin no. 132: antiphospholipid syndrome. Obstet Gynecol. 2012;120(6):1514-1521. PubMed
28. Bertolaccini ML, Amengual O, Andreoli L, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev. 2014;13(9):917-930. PubMed
1. Franco RF, Reitsma PH. Genetic risk factors of venous thrombosis. Hum Genet. 2001;109(4):369-384. PubMed
2. Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med. 1995;332(14):912-917. PubMed
3. Koster T, Rosendaal FR, de Ronde H, Briët E, Vandenbroucke JP, Bertina RM. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden Thrombophilia Study. Lancet. 1993;342(8886-8887):1503-1506. PubMed
4. Margaglione M, Brancaccio V, Giuliani N, et al. Increased risk for venous thrombosis in carriers of the prothrombin G-->A20210 gene variant. Ann Intern Med. 1998;129(2):89-93. PubMed
5. De Stefano V, Martinelli I, Mannucci PM, et al. The risk of recurrent deep venous thrombosis among heterozygous carriers of both factor V Leiden and the G20210A prothrombin mutation. N Engl J Med. 1999;341:801-806. PubMed
6. Dickey TL. Can thrombophilia testing help to prevent recurrent VTE? Part 2. JAAPA. 2002;15(12):23-24, 27-29. PubMed
7. Simpson EL, Stevenson MD, Rawdin A, Papaioannou D. Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis. Health Technol Assess. 2009;13(2):iii, ix-x, 1-91. PubMed
8. National Institute for Health and Clinical Excellence. Venous thromboembolic disease: the management of venous thromboembolic diseases and the role of thrombophilia testing. NICE clinical guideline 144. https://www.nice.org.uk/guidance/cg144. Accessed on June 30, 2017.
9. Evalution of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: routine testing for factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13(1):67-76.
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e419S-494S. PubMed
11. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220. PubMed
12. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Hematology Am Soc Hematol Educ Program. 2013;2013:9-14. PubMed
13. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
14. Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361. PubMed
15. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1-7. PubMed
16. Miles JS, Miletich JP, Goldhaber SZ, Hennekens CH, Ridker PM. G20210A mutation in the prothrombin gene and the risk of recurrent venous thromboembolism. J Am Coll Cardiol. 2001;37(1):215-218. PubMed
17. Eichinger S, Weltermann A, Mannhalter C, et al. The risk of recurrent venous thromboembolism in heterozygous carriers of factor V Leiden and a first spontaneous venous thromboembolism. Arch Intern Med. 2002;162(20):2357-2360. PubMed
18. Mazzolai L, Duchosal MA. Hereditary thrombophilia and venous thromboembolism: critical evaluation of the clinical implications of screening. Eur J Vasc Endovasc Surg. 2007;34(4):483-488. PubMed
19. Merriman L, Greaves M. Testing for thrombophilia: an evidence‐based approach. Postgrad Med J. 2006;82(973):699-704. PubMed
20. Favaloro EJ, McDonald D, Lippi G. Laboratory investigation of thrombophilia: the good, the bad, and the ugly. Semin Thromb Hemost. 2009;35(7):695-710. PubMed
21. Shen YM, Tsai J, Taiwo E, et al. Analysis of thrombophilia test ordering practices at an academic center: a proposal for appropriate testing to reduce harm and cost. PLoS One. 2016;11(5):e0155326. PubMed
22. Meyer MR, Witt DM, Delate T, et al. Thrombophilia testing patterns amongst patients with acute venous thromboembolism. Thromb Res. 2015;136(6):1160-1164. PubMed
23. Saver JL. Clinical practice: cryptogenic stroke. N Engl J Med. 2016;374(21):2065-2074. PubMed
24. ACOG practice bulletin no. 102: management of stillbirth. Obstet Gynecol. 2009;113(3):748-761. PubMed
25. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295-306. PubMed
26. Keeling D, Mackie I, Moore GW, Greer IA, Greaves M, British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157(1):47-58. PubMed
27. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice bulletin no. 132: antiphospholipid syndrome. Obstet Gynecol. 2012;120(6):1514-1521. PubMed
28. Bertolaccini ML, Amengual O, Andreoli L, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev. 2014;13(9):917-930. PubMed
© 2017 Society of Hospital Medicine