User login
Clinical Progress Note: Intravenous Human Albumin in Patients With Cirrhosis
The burden of chronic liver disease (CLD) in the United States is growing, and it is currently the fourth leading cause of death in adults aged 45 to 64 years.1 From 2012 to 2016, there were 538,720 hospitalizations in the United States for patients with cirrhosis, with almost a quarter having at least one cirrhosis-related complication. Inpatient hospitalizations for cirrhosis contribute to healthcare resource utilization, with a mean cost per CLD-related hospitalization of $16,271, and the presence of cirrhosis results in higher mortality and cost burden.1
In hospitalized patients with decompensated cirrhosis with ascites, intravenous human albumin (HA) infusion has been utilized for decades for a variety of indications. Current guidance by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommends the use of albumin for the prevention of paracentesis-induced circulatory dysfunction (PICD) for the prevention of kidney injury in spontaneous bacterial peritonitis (SBP) and for the diagnosis and treatment of hepatorenal syndrome (HRS).2,3 There have been several major trials in recent years studying the use of HA for other indications in patients with cirrhosis, and the Society of Critical Care Medicine (SCCM) updated their guidelines in 2020 to recommend HA administration in resuscitation of critically ill patients with liver failure with hypoalbuminemia.4This Clinical Progress Note addresses the use of albumin in hospitalized patients with cirrhosis, focusing on current indications and discussing potential uses published after the 2018 EASL guidelines. We conducted a literature search via the PubMed database. The authors began by using the Medical Subject Heading (MeSH) terms albumins/administration AND dosage; organization AND administration; adverse effects; and therapeutic use combined with liver cirrhosis as a MeSH major topic, which yielded 107 English-language articles published in the previous 10 years, and MeSH major topics of albumins and liver cirrhosis, which yielded 461 English-language articles, with 178 published in the previous 10 years. The search results were reviewed for applicability to albumin strategies for patients with cirrhosis.
CURRENT EVIDENCE-BASED INDICATIONS FOR USE OF ALBUMIN IN PATIENTS WITH CIRRHOSIS
There are three widely accepted and evidence-based indications for HA infusion in patients with cirrhosis, considered standard of care (Table).
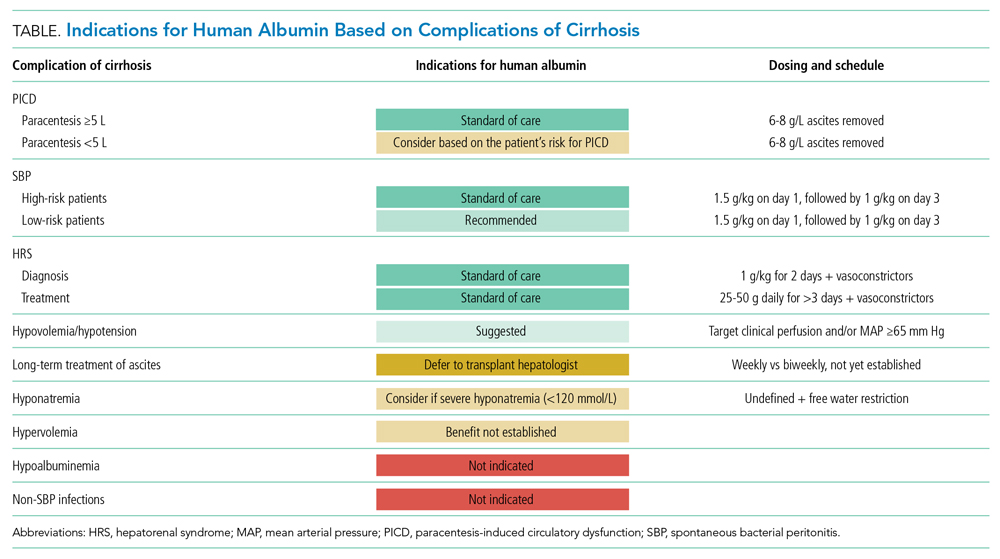
Prevention of PICD
Therapeutic large-volume paracentesis (LVP) leads to a rise in plasma renin activity (PRA) centrally through several mechanisms and is not impacted by the rate of ascites removal.5 LVP relieves abdominal pressure, increasing venous return to the heart and cardiac output, and the corresponding drop in systemic vascular resistance with splanchnic vasodilation decreases effective circulating volume and activates the renin-angiotensin system. This PRA activation and circulatory dysfunction are associated with reaccumulating ascites, renal impairment, hypervolemic hyponatremia, and increased mortality.6 A large meta-analysis of 17 trials with 1225 patients found that HA infusion improves outcomes and reduces mortality for patients undergoing LVP (odds ratio [OR], 0.64; 95% CI, 0.41-0.98), reduces the risk of PICD more than other volume expanders tested, and lowers the incidence of hyponatremia.6 More recently, in 2017, Kütting et al7 analyzed 21 trials with 1277 patients and did not observe a significant mortality benefit for HA after LVP (OR, 0.78; 95% CI, 0.55-1.11). However, negative outcomes such as rise in PRA (OR, 0.53; 95% CI, 0.29-0.97) and hyponatremia (OR, 0.62; 95% CI, 0.42-0.94) were prevented. Guidelines recommend HA after LVP ≥5 L to prevent PICD, with a replacement volume of 6 to 8 g of albumin per liter of ascitic fluid removed.2,3 Some patients may be at higher risk for PICD with less ascites removed, and the AASLD supports the use of HA to prevent PICD after smaller-volume paracentesis in patients who are already hypotensive (systolic blood pressure <90 mm Hg) or hyponatremic (<130 mmol/L), or have acute kidney injury.3
Spontaneous Bacterial Peritonitis
Spontaneous bacterial peritonitis is diagnosed by paracentesis, defined as ascitic neutrophil count ≥250 cells/µL with or without bacterascites (positive bacteriological culture). Bacterascites may be a precursor to the development of SBP, with the fluid neutrophil count of ≥250 determining the need for SBP treatment.2 SBP can lead to circulatory dysfunction, hepatic encephalopathy, and HRS. Treating SBP with HA in addition to antibiotics reduces the risk of kidney injury compared with antibiotics alone (OR for kidney injury with antibiotics alone, 4.6; 95% CI, 1.3-16.1) and also reduces the risk of death (OR for mortality with antibiotics alone, 4.5; 95% CI, 1.0-20.9).8 The AASLD recommends albumin in addition to antibiotics in SBP to prevent HRS and acute kidney injury, and high-risk patients who already have kidney dysfunction (creatinine >1 mg/dL) or jaundice (total bilirubin >5 mg/dL) are more likely to benefit from albumin. The treatment schedule is 25% HA at 1.5 g/kg on day 1 and 1 g/kg on day 3.3 The EASL recommends administering HA to all patients with cirrhosis with SBP regardless of renal or liver indices. They acknowledge, however, that the incidence of SBP-associated acute kidney injury will be low in patients without severe hepatic disease or baseline renal impairment.2
Hepatorenal Syndrome
Albumin combined with vasoconstrictors is effective in treating HRS with a response rate of 20% to 80% (average, 50%).3 Vasoactive medications can include combination midodrine and octreotide or norepinephrine (or terlipressin outside of the United States). In patients with suspected HRS, the recommended dosing of 25% HA is 1 g/kg (to a maximum of 100 g of albumin) on day 1 and then 40 to 50 g daily for at least 3 days after the diagnosis is confirmed.3 The optimal duration of therapy beyond 3 days of combined therapy with midodrine, albumin, and octreotide is not established. Terlipressin treatment is recommended for a maximum of 14 days in cases of partial response or nonresponse in renal recovery.2
INDICATIONS FOR ALBUMIN WITHOUT CLEAR EVIDENCE OF EFFICACY
Hypoalbuminemia
Albumin administration to raise serum albumin levels in hospitalized patients has been a common practice. However, new evidence suggests that treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis does not protect patients from risk and causes harm. The Albumin To prevenT Infection in chronic liveR (ATTIRE) trial, published in 2021, randomly assigned 777 patients across 35 centers in the United Kingdom to receive daily 20% HA to target a serum albumin level of 3.0 g/dL vs standard care, including HA for established indications.2,3 The primary end point was a composite of infection, kidney dysfunction, and death within 3 to 15 days of initiating treatment. There were no differences in the primary end point; secondary end points of death at 28 days, 3 months, or 6 months; or duration of hospitalization. The treatment group received 10 times more albumin than the control group and reported more adverse events, including pulmonary edema.9
Long-Term Treatment in Patients With Ascites
The human Albumin for the treatmeNt of aScites in patients With hEpatic ciRrhosis (ANSWER) trial, published in 2018, found improved 18-month survival in patients with cirrhosis and ascites treated with diuretics who received long-term albumin. This was an open-label trial of 431 patients at 33 sites in Italy, and the treatment arm received weekly infusions of 40 g of 20% HA. They observed a 38% reduction in mortality hazard ratio and half the number of hospital days annually.10 Based on these data and those from a 2006 Italian study with similar design and results, the Italian Association for the Study of the Liver (AISF) strongly recommends long-term albumin treatment in patients with cirrhosis with ascites.11 The lead author on the ANSWER trial also authored the AISF statement, although this recommendation has not been adopted by the EASL or the AASLD.
Conversely, the Midodrine and Albumin for CirrHoTic patients (MACHT) trial, also published in 2018, randomly assigned 173 patients with ascites awaiting liver transplant to receive 40 g of HA every 15 days and midodrine in addition to standard care vs placebo. MACHT found no difference in mortality or complications at 1 year.12
Long-term albumin therapy as a preventive measure may be a disease modifier, taking advantage of the pleiotropic effects of albumin, though the differing conclusions from ANSWER and MACHT necessitate additional trials. The ongoing PRECIOSA study in Spain is assessing dosage and schedule for this therapy.13
Augmenting Diuresis
Loop diuretics are highly protein-bound, and, with hypoalbuminemia, there is less effective drug delivered to the site of action. One clinical approach is to augment diuretics with concomitant HA infusion. This approach is not supported by strong evidence or guidelines.
Hyponatremia
In a retrospective cohort study of 2435 hospitalized patients with cirrhosis, 1126 of whom had hyponatremia, those patients with sodium <130 mmol/L who received HA were more likely to have resolution of hyponatremia to >135 mmol/L. This was associated with improved 30-day survival.14 From this observational data, the AASLD supports the use of albumin combined with extreme fluid restriction (<1000 mL/d) for patients with severe hyponatremia (<120 mmol/L).3
Non-SBP Infections
A 2019 meta-analysis found no evidence of a benefit of HA for bacterial infections other than SBP. However, only three trials encompassing 407 patients met the inclusion criteria.15
NEW GUIDELINE-SUGGESTED USE FOR ALBUMIN IN PATIENTS WITH CIRRHOSIS
SCCM Guideline Update: Hypoalbuminemia and Hypotension
The 2020 SCCM Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU “suggest using albumin for resuscitation of patients [with liver failure] over other fluids, especially when serum albumin is low (<3 g/dL).” Acute-on-chronic liver failure is decompensation of cirrhosis combined with organ dysfunction (eg, coagulopathy, encephalopathy, kidney injury), a scenario that is frequently encountered by hospitalists outside of intensive care settings. In hypotensive patients with cirrhosis, the SCCM recommends administering albumin to a target mean arterial pressure of 65 mm Hg or otherwise adequate perfusion. This new recommendation is conditional, based on expert consensus, and derives from low-quality evidence, with acknowledgement that “costs may be prohibitive.”4
While the ATTIRE study demonstrated no benefit in treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis, the 2020 SCCM guidelines, released prior to the publication of the ATTIRE study, focused on more acutely ill patients. In the ATTIRE study, only 2% to 3% of the study population was in an intensive care unit.4,9 The use of albumin infusion in the critically ill, hypoalbuminemic, hypotensive patient is not well studied, and the SCCM acknowledges the lack of supportive evidence for this practice in their guideline statement.
CONCLUSION
The three cardinal clinical indications for human albumin in patients with cirrhosis—prevention of PICD after LVP, in SBP, and for HRS—remain supported by the literature and guidelines, with the most recent guidance adding more nuance in patient selection based on individual risk (Table). With the publication of several large-scale studies in the past few years and a 2021 update to the AASLD guidance statement, clinicians have more evidence to guide their use of HA in patients with cirrhosis. In particular, the practice of treating isolated hypoalbuminemia with HA is no longer supported by the best evidence and is potentially harmful. A professional society recommendation to preferentially use albumin as a resuscitation fluid in hypoalbuminemia was made without the benefit of the results of the 2021 ATTIRE trial. On the horizon, additional results from ongoing and upcoming studies exploring concepts of effective albumin concentration and the pleiotropic properties of HA will impact the use of this therapy in hospitalized patients with cirrhosis.
1. Hirode G, Saab S, Wong RJ. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open. 2020;3(4):e201997. https://doi.org/10.1001/jamanetworkopen.2020.1997
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406-460. https://doi.org/10.1016/j.jhep.2018.03.024
3. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
4. Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. 2020;48(3):e173-e191. https://doi.org/10.1097/CCM.0000000000004192
5. Elsabaawy MM, Abdelhamid SR, Alsebaey A, et al. The impact of paracentesis flow rate in patients with liver cirrhosis on the development of paracentesis induced circulatory dysfunction. Clin Mol Hepatol. 2015;21(4):365-371. https://doi.org/10.3350/cmh.2015.21.4.365
6. Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55(4):1172-1181. https://doi.org/10.1002/hep.24786
7. Kütting F, Schubert J, Franklin J, et al. Insufficient evidence of benefit regarding mortality due to albumin substitution in HCC-free cirrhotic patients undergoing large volume paracentesis. J Gastroenterol Hepatol. 2017;32(2):327-338. https://doi.org/10.1111/jgh.13421
8. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
9. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. https://doi.org/10.1056/NEJMoa2022166
10. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417-2429. https://doi.org/10.1016/S0140-6736(18)30840-7
11. Caraceni P, Angeli P, Prati D, et al. AISF-SIMTI position paper on the appropriate use of albumin in patients with liver cirrhosis: a 2020 update. Blood Transfus. 2021;19(1):9-13. https://doi.org/10.2450/2020.0414-20
12. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69(6):1250-1259. https://doi.org/10.1016/j.jhep.2018.08.006
13. Fernández J, Clària J, Amorós A, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157(1):149-162. https://doi.org/10.1053/j.gastro.2019.03.021
14. Bajaj JS, Tandon P, O’Leary JG, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol. 2018;113(9):1339. https://doi.org/10.1038/s41395-018-0119-3
15. Leão GS, Neto GJ, Jotz RdF, de Mattos AA, de Mattos ÂZ. Albumin for cirrhotic patients with extraperitoneal infections: a meta-analysis. J Gastroenterol Hepatol. 2019;34(12):2071-2076. https://doi.org/10.1111/jgh.14791
The burden of chronic liver disease (CLD) in the United States is growing, and it is currently the fourth leading cause of death in adults aged 45 to 64 years.1 From 2012 to 2016, there were 538,720 hospitalizations in the United States for patients with cirrhosis, with almost a quarter having at least one cirrhosis-related complication. Inpatient hospitalizations for cirrhosis contribute to healthcare resource utilization, with a mean cost per CLD-related hospitalization of $16,271, and the presence of cirrhosis results in higher mortality and cost burden.1
In hospitalized patients with decompensated cirrhosis with ascites, intravenous human albumin (HA) infusion has been utilized for decades for a variety of indications. Current guidance by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommends the use of albumin for the prevention of paracentesis-induced circulatory dysfunction (PICD) for the prevention of kidney injury in spontaneous bacterial peritonitis (SBP) and for the diagnosis and treatment of hepatorenal syndrome (HRS).2,3 There have been several major trials in recent years studying the use of HA for other indications in patients with cirrhosis, and the Society of Critical Care Medicine (SCCM) updated their guidelines in 2020 to recommend HA administration in resuscitation of critically ill patients with liver failure with hypoalbuminemia.4This Clinical Progress Note addresses the use of albumin in hospitalized patients with cirrhosis, focusing on current indications and discussing potential uses published after the 2018 EASL guidelines. We conducted a literature search via the PubMed database. The authors began by using the Medical Subject Heading (MeSH) terms albumins/administration AND dosage; organization AND administration; adverse effects; and therapeutic use combined with liver cirrhosis as a MeSH major topic, which yielded 107 English-language articles published in the previous 10 years, and MeSH major topics of albumins and liver cirrhosis, which yielded 461 English-language articles, with 178 published in the previous 10 years. The search results were reviewed for applicability to albumin strategies for patients with cirrhosis.
CURRENT EVIDENCE-BASED INDICATIONS FOR USE OF ALBUMIN IN PATIENTS WITH CIRRHOSIS
There are three widely accepted and evidence-based indications for HA infusion in patients with cirrhosis, considered standard of care (Table).

Prevention of PICD
Therapeutic large-volume paracentesis (LVP) leads to a rise in plasma renin activity (PRA) centrally through several mechanisms and is not impacted by the rate of ascites removal.5 LVP relieves abdominal pressure, increasing venous return to the heart and cardiac output, and the corresponding drop in systemic vascular resistance with splanchnic vasodilation decreases effective circulating volume and activates the renin-angiotensin system. This PRA activation and circulatory dysfunction are associated with reaccumulating ascites, renal impairment, hypervolemic hyponatremia, and increased mortality.6 A large meta-analysis of 17 trials with 1225 patients found that HA infusion improves outcomes and reduces mortality for patients undergoing LVP (odds ratio [OR], 0.64; 95% CI, 0.41-0.98), reduces the risk of PICD more than other volume expanders tested, and lowers the incidence of hyponatremia.6 More recently, in 2017, Kütting et al7 analyzed 21 trials with 1277 patients and did not observe a significant mortality benefit for HA after LVP (OR, 0.78; 95% CI, 0.55-1.11). However, negative outcomes such as rise in PRA (OR, 0.53; 95% CI, 0.29-0.97) and hyponatremia (OR, 0.62; 95% CI, 0.42-0.94) were prevented. Guidelines recommend HA after LVP ≥5 L to prevent PICD, with a replacement volume of 6 to 8 g of albumin per liter of ascitic fluid removed.2,3 Some patients may be at higher risk for PICD with less ascites removed, and the AASLD supports the use of HA to prevent PICD after smaller-volume paracentesis in patients who are already hypotensive (systolic blood pressure <90 mm Hg) or hyponatremic (<130 mmol/L), or have acute kidney injury.3
Spontaneous Bacterial Peritonitis
Spontaneous bacterial peritonitis is diagnosed by paracentesis, defined as ascitic neutrophil count ≥250 cells/µL with or without bacterascites (positive bacteriological culture). Bacterascites may be a precursor to the development of SBP, with the fluid neutrophil count of ≥250 determining the need for SBP treatment.2 SBP can lead to circulatory dysfunction, hepatic encephalopathy, and HRS. Treating SBP with HA in addition to antibiotics reduces the risk of kidney injury compared with antibiotics alone (OR for kidney injury with antibiotics alone, 4.6; 95% CI, 1.3-16.1) and also reduces the risk of death (OR for mortality with antibiotics alone, 4.5; 95% CI, 1.0-20.9).8 The AASLD recommends albumin in addition to antibiotics in SBP to prevent HRS and acute kidney injury, and high-risk patients who already have kidney dysfunction (creatinine >1 mg/dL) or jaundice (total bilirubin >5 mg/dL) are more likely to benefit from albumin. The treatment schedule is 25% HA at 1.5 g/kg on day 1 and 1 g/kg on day 3.3 The EASL recommends administering HA to all patients with cirrhosis with SBP regardless of renal or liver indices. They acknowledge, however, that the incidence of SBP-associated acute kidney injury will be low in patients without severe hepatic disease or baseline renal impairment.2
Hepatorenal Syndrome
Albumin combined with vasoconstrictors is effective in treating HRS with a response rate of 20% to 80% (average, 50%).3 Vasoactive medications can include combination midodrine and octreotide or norepinephrine (or terlipressin outside of the United States). In patients with suspected HRS, the recommended dosing of 25% HA is 1 g/kg (to a maximum of 100 g of albumin) on day 1 and then 40 to 50 g daily for at least 3 days after the diagnosis is confirmed.3 The optimal duration of therapy beyond 3 days of combined therapy with midodrine, albumin, and octreotide is not established. Terlipressin treatment is recommended for a maximum of 14 days in cases of partial response or nonresponse in renal recovery.2
INDICATIONS FOR ALBUMIN WITHOUT CLEAR EVIDENCE OF EFFICACY
Hypoalbuminemia
Albumin administration to raise serum albumin levels in hospitalized patients has been a common practice. However, new evidence suggests that treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis does not protect patients from risk and causes harm. The Albumin To prevenT Infection in chronic liveR (ATTIRE) trial, published in 2021, randomly assigned 777 patients across 35 centers in the United Kingdom to receive daily 20% HA to target a serum albumin level of 3.0 g/dL vs standard care, including HA for established indications.2,3 The primary end point was a composite of infection, kidney dysfunction, and death within 3 to 15 days of initiating treatment. There were no differences in the primary end point; secondary end points of death at 28 days, 3 months, or 6 months; or duration of hospitalization. The treatment group received 10 times more albumin than the control group and reported more adverse events, including pulmonary edema.9
Long-Term Treatment in Patients With Ascites
The human Albumin for the treatmeNt of aScites in patients With hEpatic ciRrhosis (ANSWER) trial, published in 2018, found improved 18-month survival in patients with cirrhosis and ascites treated with diuretics who received long-term albumin. This was an open-label trial of 431 patients at 33 sites in Italy, and the treatment arm received weekly infusions of 40 g of 20% HA. They observed a 38% reduction in mortality hazard ratio and half the number of hospital days annually.10 Based on these data and those from a 2006 Italian study with similar design and results, the Italian Association for the Study of the Liver (AISF) strongly recommends long-term albumin treatment in patients with cirrhosis with ascites.11 The lead author on the ANSWER trial also authored the AISF statement, although this recommendation has not been adopted by the EASL or the AASLD.
Conversely, the Midodrine and Albumin for CirrHoTic patients (MACHT) trial, also published in 2018, randomly assigned 173 patients with ascites awaiting liver transplant to receive 40 g of HA every 15 days and midodrine in addition to standard care vs placebo. MACHT found no difference in mortality or complications at 1 year.12
Long-term albumin therapy as a preventive measure may be a disease modifier, taking advantage of the pleiotropic effects of albumin, though the differing conclusions from ANSWER and MACHT necessitate additional trials. The ongoing PRECIOSA study in Spain is assessing dosage and schedule for this therapy.13
Augmenting Diuresis
Loop diuretics are highly protein-bound, and, with hypoalbuminemia, there is less effective drug delivered to the site of action. One clinical approach is to augment diuretics with concomitant HA infusion. This approach is not supported by strong evidence or guidelines.
Hyponatremia
In a retrospective cohort study of 2435 hospitalized patients with cirrhosis, 1126 of whom had hyponatremia, those patients with sodium <130 mmol/L who received HA were more likely to have resolution of hyponatremia to >135 mmol/L. This was associated with improved 30-day survival.14 From this observational data, the AASLD supports the use of albumin combined with extreme fluid restriction (<1000 mL/d) for patients with severe hyponatremia (<120 mmol/L).3
Non-SBP Infections
A 2019 meta-analysis found no evidence of a benefit of HA for bacterial infections other than SBP. However, only three trials encompassing 407 patients met the inclusion criteria.15
NEW GUIDELINE-SUGGESTED USE FOR ALBUMIN IN PATIENTS WITH CIRRHOSIS
SCCM Guideline Update: Hypoalbuminemia and Hypotension
The 2020 SCCM Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU “suggest using albumin for resuscitation of patients [with liver failure] over other fluids, especially when serum albumin is low (<3 g/dL).” Acute-on-chronic liver failure is decompensation of cirrhosis combined with organ dysfunction (eg, coagulopathy, encephalopathy, kidney injury), a scenario that is frequently encountered by hospitalists outside of intensive care settings. In hypotensive patients with cirrhosis, the SCCM recommends administering albumin to a target mean arterial pressure of 65 mm Hg or otherwise adequate perfusion. This new recommendation is conditional, based on expert consensus, and derives from low-quality evidence, with acknowledgement that “costs may be prohibitive.”4
While the ATTIRE study demonstrated no benefit in treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis, the 2020 SCCM guidelines, released prior to the publication of the ATTIRE study, focused on more acutely ill patients. In the ATTIRE study, only 2% to 3% of the study population was in an intensive care unit.4,9 The use of albumin infusion in the critically ill, hypoalbuminemic, hypotensive patient is not well studied, and the SCCM acknowledges the lack of supportive evidence for this practice in their guideline statement.
CONCLUSION
The three cardinal clinical indications for human albumin in patients with cirrhosis—prevention of PICD after LVP, in SBP, and for HRS—remain supported by the literature and guidelines, with the most recent guidance adding more nuance in patient selection based on individual risk (Table). With the publication of several large-scale studies in the past few years and a 2021 update to the AASLD guidance statement, clinicians have more evidence to guide their use of HA in patients with cirrhosis. In particular, the practice of treating isolated hypoalbuminemia with HA is no longer supported by the best evidence and is potentially harmful. A professional society recommendation to preferentially use albumin as a resuscitation fluid in hypoalbuminemia was made without the benefit of the results of the 2021 ATTIRE trial. On the horizon, additional results from ongoing and upcoming studies exploring concepts of effective albumin concentration and the pleiotropic properties of HA will impact the use of this therapy in hospitalized patients with cirrhosis.
The burden of chronic liver disease (CLD) in the United States is growing, and it is currently the fourth leading cause of death in adults aged 45 to 64 years.1 From 2012 to 2016, there were 538,720 hospitalizations in the United States for patients with cirrhosis, with almost a quarter having at least one cirrhosis-related complication. Inpatient hospitalizations for cirrhosis contribute to healthcare resource utilization, with a mean cost per CLD-related hospitalization of $16,271, and the presence of cirrhosis results in higher mortality and cost burden.1
In hospitalized patients with decompensated cirrhosis with ascites, intravenous human albumin (HA) infusion has been utilized for decades for a variety of indications. Current guidance by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommends the use of albumin for the prevention of paracentesis-induced circulatory dysfunction (PICD) for the prevention of kidney injury in spontaneous bacterial peritonitis (SBP) and for the diagnosis and treatment of hepatorenal syndrome (HRS).2,3 There have been several major trials in recent years studying the use of HA for other indications in patients with cirrhosis, and the Society of Critical Care Medicine (SCCM) updated their guidelines in 2020 to recommend HA administration in resuscitation of critically ill patients with liver failure with hypoalbuminemia.4This Clinical Progress Note addresses the use of albumin in hospitalized patients with cirrhosis, focusing on current indications and discussing potential uses published after the 2018 EASL guidelines. We conducted a literature search via the PubMed database. The authors began by using the Medical Subject Heading (MeSH) terms albumins/administration AND dosage; organization AND administration; adverse effects; and therapeutic use combined with liver cirrhosis as a MeSH major topic, which yielded 107 English-language articles published in the previous 10 years, and MeSH major topics of albumins and liver cirrhosis, which yielded 461 English-language articles, with 178 published in the previous 10 years. The search results were reviewed for applicability to albumin strategies for patients with cirrhosis.
CURRENT EVIDENCE-BASED INDICATIONS FOR USE OF ALBUMIN IN PATIENTS WITH CIRRHOSIS
There are three widely accepted and evidence-based indications for HA infusion in patients with cirrhosis, considered standard of care (Table).

Prevention of PICD
Therapeutic large-volume paracentesis (LVP) leads to a rise in plasma renin activity (PRA) centrally through several mechanisms and is not impacted by the rate of ascites removal.5 LVP relieves abdominal pressure, increasing venous return to the heart and cardiac output, and the corresponding drop in systemic vascular resistance with splanchnic vasodilation decreases effective circulating volume and activates the renin-angiotensin system. This PRA activation and circulatory dysfunction are associated with reaccumulating ascites, renal impairment, hypervolemic hyponatremia, and increased mortality.6 A large meta-analysis of 17 trials with 1225 patients found that HA infusion improves outcomes and reduces mortality for patients undergoing LVP (odds ratio [OR], 0.64; 95% CI, 0.41-0.98), reduces the risk of PICD more than other volume expanders tested, and lowers the incidence of hyponatremia.6 More recently, in 2017, Kütting et al7 analyzed 21 trials with 1277 patients and did not observe a significant mortality benefit for HA after LVP (OR, 0.78; 95% CI, 0.55-1.11). However, negative outcomes such as rise in PRA (OR, 0.53; 95% CI, 0.29-0.97) and hyponatremia (OR, 0.62; 95% CI, 0.42-0.94) were prevented. Guidelines recommend HA after LVP ≥5 L to prevent PICD, with a replacement volume of 6 to 8 g of albumin per liter of ascitic fluid removed.2,3 Some patients may be at higher risk for PICD with less ascites removed, and the AASLD supports the use of HA to prevent PICD after smaller-volume paracentesis in patients who are already hypotensive (systolic blood pressure <90 mm Hg) or hyponatremic (<130 mmol/L), or have acute kidney injury.3
Spontaneous Bacterial Peritonitis
Spontaneous bacterial peritonitis is diagnosed by paracentesis, defined as ascitic neutrophil count ≥250 cells/µL with or without bacterascites (positive bacteriological culture). Bacterascites may be a precursor to the development of SBP, with the fluid neutrophil count of ≥250 determining the need for SBP treatment.2 SBP can lead to circulatory dysfunction, hepatic encephalopathy, and HRS. Treating SBP with HA in addition to antibiotics reduces the risk of kidney injury compared with antibiotics alone (OR for kidney injury with antibiotics alone, 4.6; 95% CI, 1.3-16.1) and also reduces the risk of death (OR for mortality with antibiotics alone, 4.5; 95% CI, 1.0-20.9).8 The AASLD recommends albumin in addition to antibiotics in SBP to prevent HRS and acute kidney injury, and high-risk patients who already have kidney dysfunction (creatinine >1 mg/dL) or jaundice (total bilirubin >5 mg/dL) are more likely to benefit from albumin. The treatment schedule is 25% HA at 1.5 g/kg on day 1 and 1 g/kg on day 3.3 The EASL recommends administering HA to all patients with cirrhosis with SBP regardless of renal or liver indices. They acknowledge, however, that the incidence of SBP-associated acute kidney injury will be low in patients without severe hepatic disease or baseline renal impairment.2
Hepatorenal Syndrome
Albumin combined with vasoconstrictors is effective in treating HRS with a response rate of 20% to 80% (average, 50%).3 Vasoactive medications can include combination midodrine and octreotide or norepinephrine (or terlipressin outside of the United States). In patients with suspected HRS, the recommended dosing of 25% HA is 1 g/kg (to a maximum of 100 g of albumin) on day 1 and then 40 to 50 g daily for at least 3 days after the diagnosis is confirmed.3 The optimal duration of therapy beyond 3 days of combined therapy with midodrine, albumin, and octreotide is not established. Terlipressin treatment is recommended for a maximum of 14 days in cases of partial response or nonresponse in renal recovery.2
INDICATIONS FOR ALBUMIN WITHOUT CLEAR EVIDENCE OF EFFICACY
Hypoalbuminemia
Albumin administration to raise serum albumin levels in hospitalized patients has been a common practice. However, new evidence suggests that treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis does not protect patients from risk and causes harm. The Albumin To prevenT Infection in chronic liveR (ATTIRE) trial, published in 2021, randomly assigned 777 patients across 35 centers in the United Kingdom to receive daily 20% HA to target a serum albumin level of 3.0 g/dL vs standard care, including HA for established indications.2,3 The primary end point was a composite of infection, kidney dysfunction, and death within 3 to 15 days of initiating treatment. There were no differences in the primary end point; secondary end points of death at 28 days, 3 months, or 6 months; or duration of hospitalization. The treatment group received 10 times more albumin than the control group and reported more adverse events, including pulmonary edema.9
Long-Term Treatment in Patients With Ascites
The human Albumin for the treatmeNt of aScites in patients With hEpatic ciRrhosis (ANSWER) trial, published in 2018, found improved 18-month survival in patients with cirrhosis and ascites treated with diuretics who received long-term albumin. This was an open-label trial of 431 patients at 33 sites in Italy, and the treatment arm received weekly infusions of 40 g of 20% HA. They observed a 38% reduction in mortality hazard ratio and half the number of hospital days annually.10 Based on these data and those from a 2006 Italian study with similar design and results, the Italian Association for the Study of the Liver (AISF) strongly recommends long-term albumin treatment in patients with cirrhosis with ascites.11 The lead author on the ANSWER trial also authored the AISF statement, although this recommendation has not been adopted by the EASL or the AASLD.
Conversely, the Midodrine and Albumin for CirrHoTic patients (MACHT) trial, also published in 2018, randomly assigned 173 patients with ascites awaiting liver transplant to receive 40 g of HA every 15 days and midodrine in addition to standard care vs placebo. MACHT found no difference in mortality or complications at 1 year.12
Long-term albumin therapy as a preventive measure may be a disease modifier, taking advantage of the pleiotropic effects of albumin, though the differing conclusions from ANSWER and MACHT necessitate additional trials. The ongoing PRECIOSA study in Spain is assessing dosage and schedule for this therapy.13
Augmenting Diuresis
Loop diuretics are highly protein-bound, and, with hypoalbuminemia, there is less effective drug delivered to the site of action. One clinical approach is to augment diuretics with concomitant HA infusion. This approach is not supported by strong evidence or guidelines.
Hyponatremia
In a retrospective cohort study of 2435 hospitalized patients with cirrhosis, 1126 of whom had hyponatremia, those patients with sodium <130 mmol/L who received HA were more likely to have resolution of hyponatremia to >135 mmol/L. This was associated with improved 30-day survival.14 From this observational data, the AASLD supports the use of albumin combined with extreme fluid restriction (<1000 mL/d) for patients with severe hyponatremia (<120 mmol/L).3
Non-SBP Infections
A 2019 meta-analysis found no evidence of a benefit of HA for bacterial infections other than SBP. However, only three trials encompassing 407 patients met the inclusion criteria.15
NEW GUIDELINE-SUGGESTED USE FOR ALBUMIN IN PATIENTS WITH CIRRHOSIS
SCCM Guideline Update: Hypoalbuminemia and Hypotension
The 2020 SCCM Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU “suggest using albumin for resuscitation of patients [with liver failure] over other fluids, especially when serum albumin is low (<3 g/dL).” Acute-on-chronic liver failure is decompensation of cirrhosis combined with organ dysfunction (eg, coagulopathy, encephalopathy, kidney injury), a scenario that is frequently encountered by hospitalists outside of intensive care settings. In hypotensive patients with cirrhosis, the SCCM recommends administering albumin to a target mean arterial pressure of 65 mm Hg or otherwise adequate perfusion. This new recommendation is conditional, based on expert consensus, and derives from low-quality evidence, with acknowledgement that “costs may be prohibitive.”4
While the ATTIRE study demonstrated no benefit in treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis, the 2020 SCCM guidelines, released prior to the publication of the ATTIRE study, focused on more acutely ill patients. In the ATTIRE study, only 2% to 3% of the study population was in an intensive care unit.4,9 The use of albumin infusion in the critically ill, hypoalbuminemic, hypotensive patient is not well studied, and the SCCM acknowledges the lack of supportive evidence for this practice in their guideline statement.
CONCLUSION
The three cardinal clinical indications for human albumin in patients with cirrhosis—prevention of PICD after LVP, in SBP, and for HRS—remain supported by the literature and guidelines, with the most recent guidance adding more nuance in patient selection based on individual risk (Table). With the publication of several large-scale studies in the past few years and a 2021 update to the AASLD guidance statement, clinicians have more evidence to guide their use of HA in patients with cirrhosis. In particular, the practice of treating isolated hypoalbuminemia with HA is no longer supported by the best evidence and is potentially harmful. A professional society recommendation to preferentially use albumin as a resuscitation fluid in hypoalbuminemia was made without the benefit of the results of the 2021 ATTIRE trial. On the horizon, additional results from ongoing and upcoming studies exploring concepts of effective albumin concentration and the pleiotropic properties of HA will impact the use of this therapy in hospitalized patients with cirrhosis.
1. Hirode G, Saab S, Wong RJ. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open. 2020;3(4):e201997. https://doi.org/10.1001/jamanetworkopen.2020.1997
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406-460. https://doi.org/10.1016/j.jhep.2018.03.024
3. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
4. Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. 2020;48(3):e173-e191. https://doi.org/10.1097/CCM.0000000000004192
5. Elsabaawy MM, Abdelhamid SR, Alsebaey A, et al. The impact of paracentesis flow rate in patients with liver cirrhosis on the development of paracentesis induced circulatory dysfunction. Clin Mol Hepatol. 2015;21(4):365-371. https://doi.org/10.3350/cmh.2015.21.4.365
6. Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55(4):1172-1181. https://doi.org/10.1002/hep.24786
7. Kütting F, Schubert J, Franklin J, et al. Insufficient evidence of benefit regarding mortality due to albumin substitution in HCC-free cirrhotic patients undergoing large volume paracentesis. J Gastroenterol Hepatol. 2017;32(2):327-338. https://doi.org/10.1111/jgh.13421
8. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
9. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. https://doi.org/10.1056/NEJMoa2022166
10. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417-2429. https://doi.org/10.1016/S0140-6736(18)30840-7
11. Caraceni P, Angeli P, Prati D, et al. AISF-SIMTI position paper on the appropriate use of albumin in patients with liver cirrhosis: a 2020 update. Blood Transfus. 2021;19(1):9-13. https://doi.org/10.2450/2020.0414-20
12. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69(6):1250-1259. https://doi.org/10.1016/j.jhep.2018.08.006
13. Fernández J, Clària J, Amorós A, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157(1):149-162. https://doi.org/10.1053/j.gastro.2019.03.021
14. Bajaj JS, Tandon P, O’Leary JG, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol. 2018;113(9):1339. https://doi.org/10.1038/s41395-018-0119-3
15. Leão GS, Neto GJ, Jotz RdF, de Mattos AA, de Mattos ÂZ. Albumin for cirrhotic patients with extraperitoneal infections: a meta-analysis. J Gastroenterol Hepatol. 2019;34(12):2071-2076. https://doi.org/10.1111/jgh.14791
1. Hirode G, Saab S, Wong RJ. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open. 2020;3(4):e201997. https://doi.org/10.1001/jamanetworkopen.2020.1997
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406-460. https://doi.org/10.1016/j.jhep.2018.03.024
3. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
4. Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. 2020;48(3):e173-e191. https://doi.org/10.1097/CCM.0000000000004192
5. Elsabaawy MM, Abdelhamid SR, Alsebaey A, et al. The impact of paracentesis flow rate in patients with liver cirrhosis on the development of paracentesis induced circulatory dysfunction. Clin Mol Hepatol. 2015;21(4):365-371. https://doi.org/10.3350/cmh.2015.21.4.365
6. Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55(4):1172-1181. https://doi.org/10.1002/hep.24786
7. Kütting F, Schubert J, Franklin J, et al. Insufficient evidence of benefit regarding mortality due to albumin substitution in HCC-free cirrhotic patients undergoing large volume paracentesis. J Gastroenterol Hepatol. 2017;32(2):327-338. https://doi.org/10.1111/jgh.13421
8. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
9. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. https://doi.org/10.1056/NEJMoa2022166
10. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417-2429. https://doi.org/10.1016/S0140-6736(18)30840-7
11. Caraceni P, Angeli P, Prati D, et al. AISF-SIMTI position paper on the appropriate use of albumin in patients with liver cirrhosis: a 2020 update. Blood Transfus. 2021;19(1):9-13. https://doi.org/10.2450/2020.0414-20
12. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69(6):1250-1259. https://doi.org/10.1016/j.jhep.2018.08.006
13. Fernández J, Clària J, Amorós A, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157(1):149-162. https://doi.org/10.1053/j.gastro.2019.03.021
14. Bajaj JS, Tandon P, O’Leary JG, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol. 2018;113(9):1339. https://doi.org/10.1038/s41395-018-0119-3
15. Leão GS, Neto GJ, Jotz RdF, de Mattos AA, de Mattos ÂZ. Albumin for cirrhotic patients with extraperitoneal infections: a meta-analysis. J Gastroenterol Hepatol. 2019;34(12):2071-2076. https://doi.org/10.1111/jgh.14791
© 2021 Society of Hospital Medicine
Reducing Overuse of Proton Pump Inhibitors for Stress Ulcer Prophylaxis and Nonvariceal Gastrointestinal Bleeding in the Hospital: A Narrative Review and Implementation Guide
Proton pump inhibitors (PPIs) are among the most commonly used drugs worldwide to treat dyspepsia and prevent gastrointestinal bleeding (GIB).1 Between 40% and 70% of hospitalized patients receive acid-suppressive therapy (AST; defined as PPIs or histamine-receptor antagonists), and nearly half of these are initiated during the inpatient stay.2,3 While up to 50% of inpatients who received a new AST were discharged on these medications,2 there were no evidence-based indications for a majority of the prescriptions.2,3
Growing evidence shows that PPIs are overutilized and may be associated with wide-ranging adverse events, such as acute and chronic kidney disease,4Clostridium difficile infection,5 hypomagnesemia,6 and fractures.7 Because of the widespread overuse and the potential harm associated with PPIs, a concerted effort to promote their appropriate use in the inpatient setting is necessary. It is important to note that reducing the use of PPIs does not increase the risks of GIB or worsening dyspepsia. Rather, reducing overuse of PPIs lowers the risk of harm to patients. The efforts to reduce overuse, however, are complex and difficult.
This article summarizes evidence regarding interventions to reduce overuse and offers an implementation guide based on this evidence. This guide promotes value-based quality improvement and provides a blueprint for implementing an institution-wide program to reduce PPI overuse in the inpatient setting. We begin with a discussion about quality initiatives to reduce PPI overuse, followed by a review of the safety outcomes associated with reduced use of PPIs.
METHODS
A focused search of the US National Library of Medicine’s PubMed database was performed to identify English-language articles published between 2000 and 2018 that addressed strategies to reduce PPI overuse for stress ulcer prophylaxis (SUP) and nonvariceal GIB. The following search terms were used: PPI and inappropriate use; acid-suppressive therapy and inappropriate use; PPI and discontinuation; acid-suppressive (or suppressant) therapy and discontinuation; SUP and cost; and histamine receptor antagonist and PPI. Inpatient or outpatient studies of patients aged 18 years or older were considered for inclusion in this narrative review, and all study types were included. The primary exclusion criterion was patients aged younger than 18 years. A manual review of the full text of the retrieved articles was performed and references were reviewed for missed citations.
RESULTS
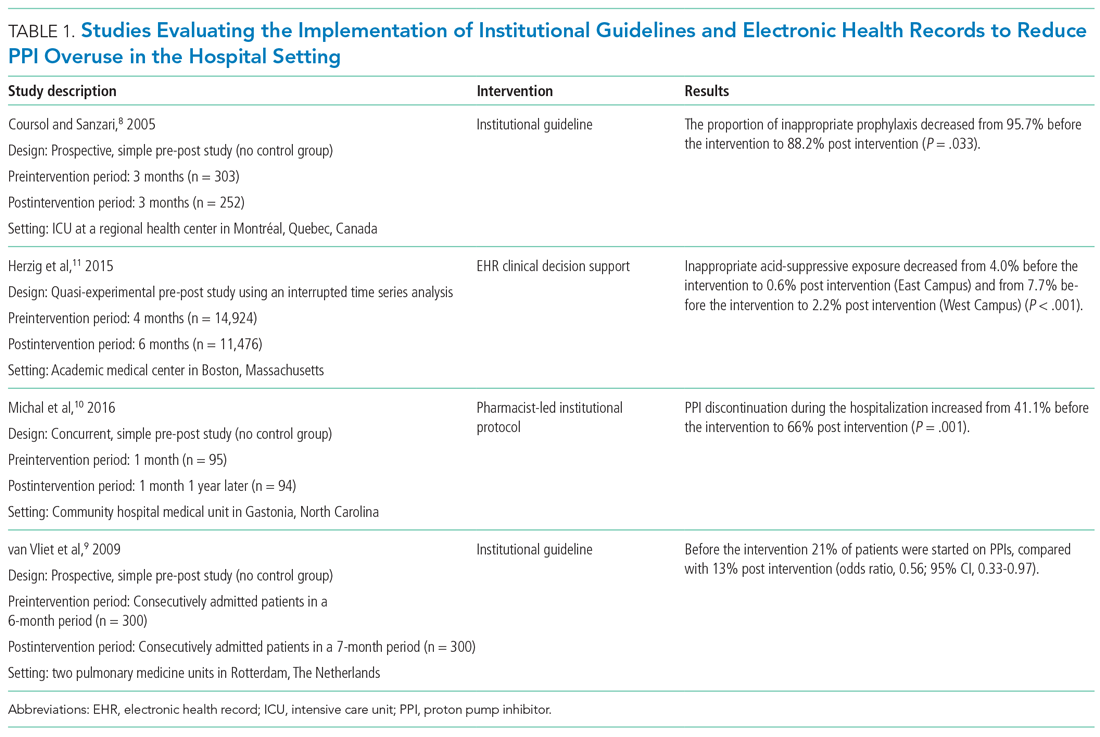
We identified a total of 1,497 unique citations through our initial search. After performing a manual review, we excluded 1,483 of the references and added an additional 2, resulting in 16 articles selected for inclusion. The selected articles addressed interventions falling into three main groupings: implementation of institutional guidelines with or without electronic health record (EHR)–based decision support, educational interventions alone, and multifaceted interventions. Each of these interventions is discussed in the sections that follow. Table 1, Table 2, and Table 3 summarize the results of the studies included in our narrative review.
QUALITY INITIATIVES TO REDUCE PPI OVERUSE
Institutional Guidelines With or Without EHR-Based Decision Support
Table 1 summarizes institutional guidelines, with or without EHR-based decision support, to reduce inappropriate PPI use. The implementation of institutional guidelines for the appropriate reduction of PPI use has had some success. Coursol and Sanzari evaluated the impact of a treatment algorithm on the appropriateness of prescriptions for SUP in the intensive care unit (ICU).8 Risk factors of patients in this study included mechanical ventilation for 48 hours, coagulopathy for 24 hours, postoperative transplant, severe burns, active gastrointestinal (GI) disease, multiple trauma, multiple organ failure, and septicemia. The three treatment options chosen for the algorithm were intravenous (IV) famotidine (if the oral route was unavailable or impractical), omeprazole tablets (if oral access was available), and omeprazole suspension (in cases of dysphagia and presence of nasogastric or orogastric tube). After implementation of the treatment algorithm, the proportion of inappropriate prophylaxis decreased from 95.7% to 88.2% (P = .033), and the cost per patient decreased from $11.11 to $8.49 Canadian dollars (P = .003).
Van Vliet et al implemented a clinical practice guideline listing specific criteria for prescribing a PPI.9 Their criteria included the presence of gastric or duodenal ulcer and use of a nonsteroidal anti-inflammatory drug (NSAID) or aspirin, plus at least one additional risk factor (eg, history of gastroduodenal hemorrhage or age >70 years). The proportion of patients started on PPIs during hospitalization decreased from 21% to 13% (odds ratio, 0.56; 95% CI, 0.33-0.97).
Michal et al utilized an institutional pharmacist-driven protocol that stipulated criteria for appropriate PPI use (eg, upper GIB, mechanical ventilation, peptic ulcer disease, gastroesophageal reflux disease, coagulopathy).10 Pharmacists in the study evaluated patients for PPI appropriateness and recommended changes in medication or discontinuation of use. This institutional intervention decreased PPI use in non-ICU hospitalized adults. Discontinuation of PPIs increased from 41% of patients in the preintervention group to 66% of patients in the postintervention group (P = .001).
In addition to implementing guidelines and intervention strategies, institutions have also adopted changes to the EHR to reduce inappropriate PPI use. Herzig et al utilized a computerized clinical decision support intervention to decrease SUP in non-ICU hospitalized patients.11 Of the available response options for acid-suppressive medication, when SUP was chosen as the only indication for PPI use a prompt alerted the clinician that “[SUP] is not recommended for patients outside the [ICU]”; the alert resulted in a significant reduction in AST for the sole purpose of SUP. With this intervention, the percentage of patients who had any inappropriate acid-suppressive exposure decreased from 4.0% to 0.6% (P < .001).
EDUCATION
Table 2 summarizes educational interventions to reduce inappropriate PPI use.
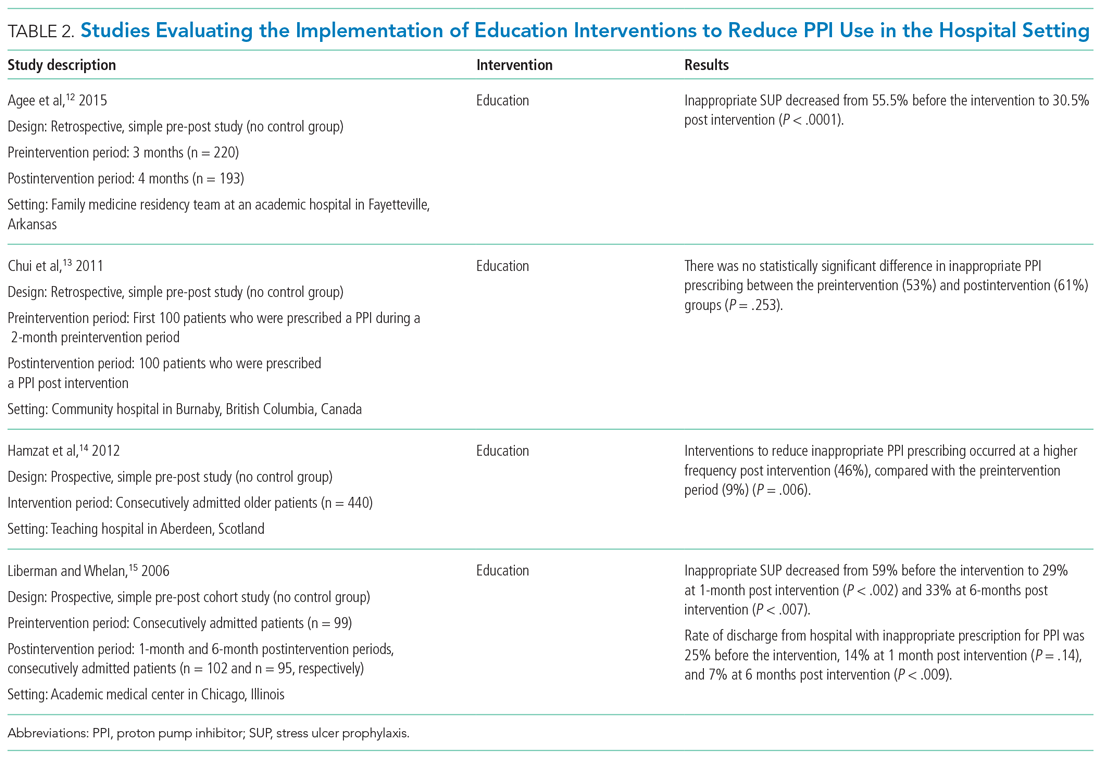
Agee et al employed a pharmacist-led educational seminar that described SUP indications, risks, and costs.12 Inappropriate SUP prescriptions decreased from 55.5% to 30.5% after the intervention (P < .0001). However, there was no reduction in the percentage of patients discharged on inappropriate AST.
Chui et al performed an intervention with academic detailing wherein a one-on-one visit with a physician took place, providing education to improve physician prescribing behavior.13 In this study, academic detailing focused on the most common instances for which PPIs were inappropriately utilized at that hospital (eg, surgical prophylaxis, anemia). Inappropriate use of double-dose PPIs was also targeted. Despite these efforts, no significant difference in inappropriate PPI prescribing was observed post intervention.
Hamzat et al implemented an educational strategy to reduce inappropriate PPI prescribing during hospital stays, which included dissemination of fliers, posters, emails, and presentations over a 4-week period.14 Educational efforts targeted clinical pharmacists, nurses, physicians, and patients. Appropriate indications for PPI use in this study included peptic ulcer disease (current or previous), H pylori infection, and treatment or prevention of an NSAID-induced ulcer. The primary outcome was a reduction in PPI dose or discontinuation of PPI during the hospital admission, which increased from 9% in the preintervention (pre-education) phase to 43% during the intervention (education) phase and to 46% in the postintervention (posteducation) phase (P = .006).
Liberman and Whelan also implemented an educational intervention among internal medicine residents to reduce inappropriate use of SUP; this intervention was based on practice-based learning and improvement methodology.15 They noted that the rate of inappropriate prophylaxis with AST decreased from 59% preintervention to 33% post intervention (P < .007).
MULTIFACETED APPROACHES
Table 3 summarizes several multifaceted approaches aimed at reducing inappropriate PPI use. Belfield et al utilized an intervention consisting of an institutional guideline review, education, and monitoring of AST by clinical pharmacists to reduce inappropriate use of PPI for SUP.16 With this intervention, the primary outcome of total inappropriate days of AST during hospitalization decreased from 279 to 116 (48% relative reduction in risk, P < .01, across 142 patients studied). Furthermore, inappropriate AST prescriptions at discharge decreased from 32% to 8% (P = .006). The one case of GIB noted in this study occurred in the control group.
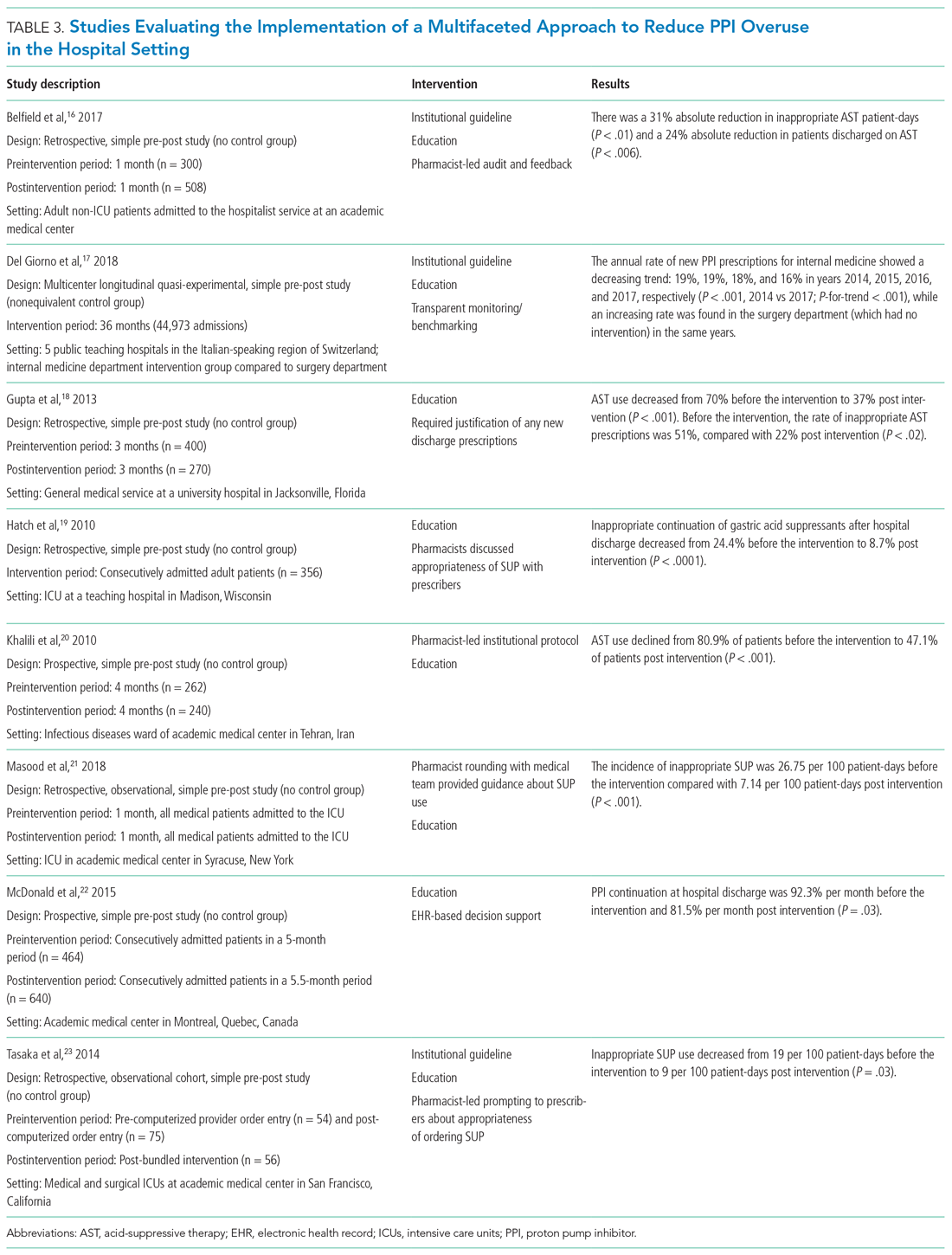
Del Giorno et al combined audit and feedback with education to reduce new PPI prescriptions at the time of discharge from the hospital.17 The educational component of this intervention included guidance regarding potentially inappropriate PPI use and associated side effects and targeted multiple departments in the hospital. This intervention led to a sustained reduction in new PPI prescriptions at discharge during the 3-year study period. The annual rate of new PPI prescriptions was 19%, 19%, 18%, and 16% in years 2014, 2015, 2016, and 2017, respectively, in the internal medicine department (postintervention group), compared with rates of 30%, 29%, 36%, 36% (P < .001) for the same years in the surgery department (control group).
Education and the use of medication reconciliation forms on admission and discharge were utilized by Gupta et al to reduce inappropriate AST in hospitalized patients from 51% prior to intervention to 22% post intervention (P < .001).18 Furthermore, the proportion of patients discharged on inappropriate AST decreased from 69% to 20% (P < .001).
Hatch et al also used educational resources and pharmacist-led medication reconciliation to reduce use of SUP.19 Before the intervention, 24.4% of patients were continued on SUP after hospital discharge in the absence of a clear indication for use; post intervention, 11% of patients were continued on SUP after hospital discharge (of these patients, 8.7% had no clear indication for use). This represented a 64.4% decrease in inappropriately prescribed SUP after discharge (P < .0001).
Khalili et al combined an educational intervention with an institutional guideline in an infectious disease ward to reduce inappropriate use of SUP.20 This intervention reduced the inappropriate use of AST from 80.9% before the intervention to 47.1% post intervention (P < .001).
Masood et al implemented two interventions wherein pharmacists reviewed SUP indications for each patient during daily team rounds, and ICU residents and fellows received education about indications for SUP and the implemented initiative on a bimonthly basis.21 Inappropriate AST decreased from 26.75 to 7.14 prescriptions per 100 patient-days of care (P < .001).
McDonald et al combined education with a web-based quality improvement tool to reduce inappropriate exit prescriptions for PPIs.22 The proportion of PPIs discontinued at hospital discharge increased from 7.7% per month to 18.5% per month (P = .03).
Finally, the initiative implemented by Tasaka et al to reduce overutilization of SUP included an institutional guideline, a pharmacist-led intervention, and an institutional education and awareness campaign.23 Their initiative led to a reduction in inappropriate SUP both at the time of transfer out of the ICU (8% before intervention, 4% post intervention, P = .54) and at the time of discharge from the hospital (7% before intervention, 0% post intervention, P = .22).
REDUCING PPI USE AND SAFETY OUTCOMES
Proton pump inhibitors are often initiated in the hospital setting, with up to half of these new prescriptions continued at discharge.2,24,25 Inappropriate prescriptions for PPIs expose patients to excess risk of long-term adverse events.26 De-escalating PPIs, however, raises concern among clinicians and patients for potential recurrence of dyspepsia and GIB. There is limited evidence regarding long-term safety outcomes (including GIB) following the discontinuation of PPIs deemed to have been inappropriately initiated in the hospital. In view of this, clinicians should educate and monitor individual patients for symptom relapse to ensure timely and appropriate resumption of AST.
LIMITATIONS
Our literature search for this narrative review and implementation guide has limitations. First, the time frame we included (2000-2018) may have excluded relevant articles published before our starting year. We did not include articles published before 2000 based on concerns these might contain outdated information. Also, there may have been incomplete retrieval of relevant studies/articles due to the labor-intensive nature involved in determining whether PPI prescriptions are appropriate or inappropriate.
We noted that interventional studies aimed at reducing overuse of PPIs were often limited by a low number of participants; these studies were also more likely to be single-center interventions, which limits generalizability. In addition, the studies often had low methodological rigor and lacked randomization or controls. Moreover, to fully evaluate the sustainability of interventions, some of the studies had a limited postimplementation period. For multifaceted interventions, the efficacy of individual components of the interventions was not clearly evaluated. Moreover, there was a high risk of bias in many of the included studies. Some of the larger studies used overall AST prescriptions as a surrogate for more appropriate use. It would be advantageous for a site to perform a pilot study that provides well-defined parameters for appropriate prescribing, and then correlate with the total number of prescriptions (automated and much easier) thereafter. Further, although the evidence regarding appropriate PPI use for SUP and GIB has shifted rapidly in recent years, society guidelines have not been updated to reflect this change. As such, quality improvement interventions have predominantly focused on reducing PPI use for the indications reflected by these guidelines.
IMPLEMENTATION BLUEPRINT
The following are our recommendations for successfully implementing an evidence-based, institution-wide initiative to promote the appropriate use of PPIs during hospitalization. These recommendations are informed by the evidence review and reflect the consensus of the combined committees coauthoring this review.
For an initiative to succeed, participation from multiple disciplines is necessary to formulate local guidelines and design and implement interventions. Such an interdisciplinary approach requires advocates to closely monitor and evaluate the program; sustainability will be greatly facilitated by the active engagement of key stakeholders, including the hospital’s executive administration, supply chain, pharmacists, and gastroenterologists. Lack of adequate buy-in on the part of key stakeholders is a barrier to the success of any intervention. Accordingly, before selecting a particular intervention, it is important to understand local factors driving the overuse of PPI.
1. Develop evidence-based institutional guidelines for both SUP and nonvariceal upper GIB through an interdisciplinary workgroup.
- Establish an interdisciplinary group including, but not limited to, pharmacists, hospitalists, gastroenterologists, and intensivists so that changes in practice will be widely adopted as institutional policy.
- Incorporate the best evidence and clearly convey appropriate and inappropriate uses.
2. Integrate changes to the EHR.
- If possible, the EHR should be leveraged to implement changes in PPI ordering practices.
- While integrating changes to the EHR, it is important to consider informatics and implementation science, since the utility of hard stops and best practice alerts has been questioned in the setting of operational inefficiencies and alert fatigue.
- Options for integrating changes to the EHR include the following:
- Create an ordering pathway that provides clinical decision support for PPI use.
- Incorporate a best practice alert in the EMR to notify clinicians of institutional guidelines when they initiate an order for PPI outside of the pathway.
- Consider restricting the authority to order IV PPIs by requiring a code or password or implement another means of using the EHR to limit the supply of PPI.
- Limit the duration of IV PPI by requiring daily renewal of IV PPI dosing or by altering the period of time that use of IV PPI is permitted (eg, 48 to 72 hours).
- PPIs should be removed from any current order sets that include medications for SUP.
3. Foster pharmacy-driven interventions.
- Consider requiring pharmacist approval for IV PPIs.
- Pharmacist-led review and feedback to clinicians for discontinuation of inappropriate PPIs can be effective in decreasing inappropriate utilization.
4. Provide education, audit data, and obtain feedback.
- Data auditing is needed to measure the efficacy of interventions. Outcome measures may include the number of non-ICU and ICU patients who are started on a PPI during an admission; the audit should be continued through discharge. A process measure may be the number of pharmacist calls for inappropriate PPIs. A balancing measure would be ulcer-specific upper GIB in patients who do not receive SUP during their admission. (Upper GIB from other etiologies, such as varices, portal hypertensive gastropathy, and Mallory-Weiss tear would not be affected by PPI SUP.)
- Run or control charts should be utilized, and data should be shared with project champions and ordering clinicians—in real time if possible.
- Project champions should provide feedback to colleagues; they should also work with hospital leadership to develop new strategies to improve adherence.
- Provide ongoing education about appropriate indications for PPIs and potential adverse effects associated with their use. Whenever possible, point-of-care or just-in-time teaching is the preferred format.
CONCLUSION
Excessive use of PPIs during hospitalization is prevalent; however, quality improvement interventions can be effective in achieving sustainable reductions in overuse. There is a need for the American College of Gastroenterology to revisit and update their guidelines for management of patients with ulcer bleeding to include stronger evidence-based recommendations on the proper use of PPIs.27 These updated guidelines could be used to update the implementation blueprint.
Quality improvement teams have an opportunity to use the principles of value-based healthcare to reduce inappropriate PPI use. By following the blueprint outlined in this article, institutions can safely and effectively tailor the use of PPIs to suitable patients in the appropriate settings. Reduction of PPI overuse can be employed as an institutional catalyst to promote implementation of further value-based measures to improve efficiency and quality of patient care.
1. Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Exp Rev Clin Pharmacol. 2018;11(11):1123-1134. https://doi.org/10.1080/17512433.2018.1531703
2. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122. https://doi.org/10.1111/j.1572-0241.2000.03259.x
3. Ahrens D, Behrens G, Himmel W, Kochen MM, Chenot JF. Appropriateness of proton pump inhibitor recommendations at hospital discharge and continuation in primary care. Int J Clin Pract. 2012;66(8):767-773. https://doi.org/10.1111/j.1742-1241.2012.02973.x
4. Moledina DG, Perazella MA. PPIs and kidney disease: from AIN to CKD. J Nephrol. 2016;29(5):611-616. https://doi.org/10.1007/s40620-016-0309-2
5. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019. https://doi.org/10.1038/ajg.2012.108
6. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37(7):1237-1241. https://doi.org/10.3109/0886022x.2015.1057800
7. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947-2953. https://doi.org/10.1001/jama.296.24.2947
8. Coursol CJ, Sanzari SE. Impact of stress ulcer prophylaxis algorithm study. Ann Pharmacother. 2005;39(5):810-816. https://doi.org/10.1345/aph.1d129
9. van Vliet EPM, Steyerberg EW, Otten HJ, et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther. 2009;29(2):213-221. https://doi.org/10.1111/j.1365-2036.2008.03875.x
10. Michal J, Henry T, Street C. Impact of a pharmacist-driven protocol to decrease proton pump inhibitor use in non-intensive care hospitalized adults. Am J Health Syst Pharm. 2016;73(17 Suppl 4):S126-S132. https://doi.org/10.2146/ajhp150519
11. Herzig SJ, Guess JR, Feinbloom DB, et al. Improving appropriateness of acid-suppressive medication use via computerized clinical decision support. J Hosp Med. 2015;10(1):41-45. https://doi.org/10.1002/jhm.2260
12. Agee C, Coulter L, Hudson J. Effects of pharmacy resident led education on resident physician prescribing habits associated with stress ulcer prophylaxis in non-intensive care unit patients. Am J Health Syst Pharm. 2015;72(11 Suppl 1):S48-S52. https://doi.org/10.2146/sp150013
13. Chui D, Young F, Tejani AM, Dillon EC. Impact of academic detailing on proton pump inhibitor prescribing behaviour in a community hospital. Can Pharm J (Ott). 2011;144(2):66-71. https://doi.org/10.3821/1913-701X-144.2.66
14. Hamzat H, Sun H, Ford JC, Macleod J, Soiza RL, Mangoni AA. Inappropriate prescribing of proton pump inhibitors in older patients: effects of an educational strategy. Drugs Aging. 2012;29(8):681-690. https://doi.org/10.1007/bf03262283
15. Liberman JD, Whelan CT. Brief report: Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice-based educational intervention. J Gen Intern Med. 2006;21(5):498-500. https://doi.org/10.1111/j.1525-1497.2006.00435.x
16. Belfield KD, Kuyumjian AG, Teran R, Amadi M, Blatt M, Bicking K. Impact of a collaborative strategy to reduce the inappropriate use of acid suppressive therapy in non-intensive care unit patients. Ann Pharmacother. 2017;51(7):577-583. https://doi.org/10.1177/1060028017698797
17. Del Giorno R, Ceschi A, Pironi M, Zasa A, Greco A, Gabutti L. Multifaceted intervention to curb in-hospital over-prescription of proton pump inhibitors: a longitudinal multicenter quasi-experimental before-and-after study. Eur J Intern Med. 2018;50:52-59. https://doi.org/10.1016/j.ejim.2017.11.002
18. Gupta R, Marshall J, Munoz JC, Kottoor R, Jamal MM, Vega KJ. Decreased acid suppression therapy overuse after education and medication reconciliation. Int J Clin Pract. 2013;67(1):60-65. https://doi.org/10.1111/ijcp.12046
19. Hatch JB, Schulz L, Fish JT. Stress ulcer prophylaxis: reducing non-indicated prescribing after hospital discharge. Ann Pharmacother. 2010;44(10):1565-1571. https://doi.org/10.1345/aph.1p167
20. Khalili H, Dashti-Khavidaki S, Hossein Talasaz AH, Tabeefar H, Hendoiee N. Descriptive analysis of a clinical pharmacy intervention to improve the appropriate use of stress ulcer prophylaxis in a hospital infectious disease ward. J Manag Care Pharm. 2010;16(2):114-121. https://doi.org/10.18553/jmcp.2010.16.2.114
21. Masood U, Sharma A, Bhatti Z, et al. A successful pharmacist-based quality initiative to reduce inappropriate stress ulcer prophylaxis use in an academic medical intensive care unit. Inquiry. 2018;55:46958018759116. https://doi.org/10.1177/0046958018759116
22. McDonald EG, Jones J, Green L, Jayaraman D, Lee TC. Reduction of inappropriate exit prescriptions for proton pump inhibitors: a before-after study using education paired with a web-based quality-improvement tool. J Hosp Med. 2015;10(5):281-286. https://doi.org/10.1002/jhm.2330
23. Tasaka CL, Burg C, VanOsdol SJ, et al. An interprofessional approach to reducing the overutilization of stress ulcer prophylaxis in adult medical and surgical intensive care units. Ann Pharmacother. 2014;48(4):462-469. https://doi.org/10.1177/1060028013517088
24. Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther. 2005;21(10):1203-1209. https://doi.org/10.1111/j.1365-2036.2005.02454.x
25. Pham CQ, Regal RE, Bostwick TR, Knauf KS. Acid suppressive therapy use on an inpatient internal medicine service. Ann Pharmacother. 2006;40(7-8):1261-1266. https://doi.org/10.1345/aph.1g703
26. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors [editorial]. JAMA Intern Med. 2016;176(2):172-174. https://doi.org/10.1001/jamainternmed.2015.7927
27. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345-360; quiz 361. https://doi.org/10.1038/ajg.2011.480
Proton pump inhibitors (PPIs) are among the most commonly used drugs worldwide to treat dyspepsia and prevent gastrointestinal bleeding (GIB).1 Between 40% and 70% of hospitalized patients receive acid-suppressive therapy (AST; defined as PPIs or histamine-receptor antagonists), and nearly half of these are initiated during the inpatient stay.2,3 While up to 50% of inpatients who received a new AST were discharged on these medications,2 there were no evidence-based indications for a majority of the prescriptions.2,3
Growing evidence shows that PPIs are overutilized and may be associated with wide-ranging adverse events, such as acute and chronic kidney disease,4Clostridium difficile infection,5 hypomagnesemia,6 and fractures.7 Because of the widespread overuse and the potential harm associated with PPIs, a concerted effort to promote their appropriate use in the inpatient setting is necessary. It is important to note that reducing the use of PPIs does not increase the risks of GIB or worsening dyspepsia. Rather, reducing overuse of PPIs lowers the risk of harm to patients. The efforts to reduce overuse, however, are complex and difficult.
This article summarizes evidence regarding interventions to reduce overuse and offers an implementation guide based on this evidence. This guide promotes value-based quality improvement and provides a blueprint for implementing an institution-wide program to reduce PPI overuse in the inpatient setting. We begin with a discussion about quality initiatives to reduce PPI overuse, followed by a review of the safety outcomes associated with reduced use of PPIs.
METHODS
A focused search of the US National Library of Medicine’s PubMed database was performed to identify English-language articles published between 2000 and 2018 that addressed strategies to reduce PPI overuse for stress ulcer prophylaxis (SUP) and nonvariceal GIB. The following search terms were used: PPI and inappropriate use; acid-suppressive therapy and inappropriate use; PPI and discontinuation; acid-suppressive (or suppressant) therapy and discontinuation; SUP and cost; and histamine receptor antagonist and PPI. Inpatient or outpatient studies of patients aged 18 years or older were considered for inclusion in this narrative review, and all study types were included. The primary exclusion criterion was patients aged younger than 18 years. A manual review of the full text of the retrieved articles was performed and references were reviewed for missed citations.
RESULTS
We identified a total of 1,497 unique citations through our initial search. After performing a manual review, we excluded 1,483 of the references and added an additional 2, resulting in 16 articles selected for inclusion. The selected articles addressed interventions falling into three main groupings: implementation of institutional guidelines with or without electronic health record (EHR)–based decision support, educational interventions alone, and multifaceted interventions. Each of these interventions is discussed in the sections that follow. Table 1, Table 2, and Table 3 summarize the results of the studies included in our narrative review.
QUALITY INITIATIVES TO REDUCE PPI OVERUSE
Institutional Guidelines With or Without EHR-Based Decision Support
Table 1 summarizes institutional guidelines, with or without EHR-based decision support, to reduce inappropriate PPI use. The implementation of institutional guidelines for the appropriate reduction of PPI use has had some success. Coursol and Sanzari evaluated the impact of a treatment algorithm on the appropriateness of prescriptions for SUP in the intensive care unit (ICU).8 Risk factors of patients in this study included mechanical ventilation for 48 hours, coagulopathy for 24 hours, postoperative transplant, severe burns, active gastrointestinal (GI) disease, multiple trauma, multiple organ failure, and septicemia. The three treatment options chosen for the algorithm were intravenous (IV) famotidine (if the oral route was unavailable or impractical), omeprazole tablets (if oral access was available), and omeprazole suspension (in cases of dysphagia and presence of nasogastric or orogastric tube). After implementation of the treatment algorithm, the proportion of inappropriate prophylaxis decreased from 95.7% to 88.2% (P = .033), and the cost per patient decreased from $11.11 to $8.49 Canadian dollars (P = .003).

Van Vliet et al implemented a clinical practice guideline listing specific criteria for prescribing a PPI.9 Their criteria included the presence of gastric or duodenal ulcer and use of a nonsteroidal anti-inflammatory drug (NSAID) or aspirin, plus at least one additional risk factor (eg, history of gastroduodenal hemorrhage or age >70 years). The proportion of patients started on PPIs during hospitalization decreased from 21% to 13% (odds ratio, 0.56; 95% CI, 0.33-0.97).
Michal et al utilized an institutional pharmacist-driven protocol that stipulated criteria for appropriate PPI use (eg, upper GIB, mechanical ventilation, peptic ulcer disease, gastroesophageal reflux disease, coagulopathy).10 Pharmacists in the study evaluated patients for PPI appropriateness and recommended changes in medication or discontinuation of use. This institutional intervention decreased PPI use in non-ICU hospitalized adults. Discontinuation of PPIs increased from 41% of patients in the preintervention group to 66% of patients in the postintervention group (P = .001).
In addition to implementing guidelines and intervention strategies, institutions have also adopted changes to the EHR to reduce inappropriate PPI use. Herzig et al utilized a computerized clinical decision support intervention to decrease SUP in non-ICU hospitalized patients.11 Of the available response options for acid-suppressive medication, when SUP was chosen as the only indication for PPI use a prompt alerted the clinician that “[SUP] is not recommended for patients outside the [ICU]”; the alert resulted in a significant reduction in AST for the sole purpose of SUP. With this intervention, the percentage of patients who had any inappropriate acid-suppressive exposure decreased from 4.0% to 0.6% (P < .001).
EDUCATION
Table 2 summarizes educational interventions to reduce inappropriate PPI use.

Agee et al employed a pharmacist-led educational seminar that described SUP indications, risks, and costs.12 Inappropriate SUP prescriptions decreased from 55.5% to 30.5% after the intervention (P < .0001). However, there was no reduction in the percentage of patients discharged on inappropriate AST.
Chui et al performed an intervention with academic detailing wherein a one-on-one visit with a physician took place, providing education to improve physician prescribing behavior.13 In this study, academic detailing focused on the most common instances for which PPIs were inappropriately utilized at that hospital (eg, surgical prophylaxis, anemia). Inappropriate use of double-dose PPIs was also targeted. Despite these efforts, no significant difference in inappropriate PPI prescribing was observed post intervention.
Hamzat et al implemented an educational strategy to reduce inappropriate PPI prescribing during hospital stays, which included dissemination of fliers, posters, emails, and presentations over a 4-week period.14 Educational efforts targeted clinical pharmacists, nurses, physicians, and patients. Appropriate indications for PPI use in this study included peptic ulcer disease (current or previous), H pylori infection, and treatment or prevention of an NSAID-induced ulcer. The primary outcome was a reduction in PPI dose or discontinuation of PPI during the hospital admission, which increased from 9% in the preintervention (pre-education) phase to 43% during the intervention (education) phase and to 46% in the postintervention (posteducation) phase (P = .006).
Liberman and Whelan also implemented an educational intervention among internal medicine residents to reduce inappropriate use of SUP; this intervention was based on practice-based learning and improvement methodology.15 They noted that the rate of inappropriate prophylaxis with AST decreased from 59% preintervention to 33% post intervention (P < .007).
MULTIFACETED APPROACHES
Table 3 summarizes several multifaceted approaches aimed at reducing inappropriate PPI use. Belfield et al utilized an intervention consisting of an institutional guideline review, education, and monitoring of AST by clinical pharmacists to reduce inappropriate use of PPI for SUP.16 With this intervention, the primary outcome of total inappropriate days of AST during hospitalization decreased from 279 to 116 (48% relative reduction in risk, P < .01, across 142 patients studied). Furthermore, inappropriate AST prescriptions at discharge decreased from 32% to 8% (P = .006). The one case of GIB noted in this study occurred in the control group.

Del Giorno et al combined audit and feedback with education to reduce new PPI prescriptions at the time of discharge from the hospital.17 The educational component of this intervention included guidance regarding potentially inappropriate PPI use and associated side effects and targeted multiple departments in the hospital. This intervention led to a sustained reduction in new PPI prescriptions at discharge during the 3-year study period. The annual rate of new PPI prescriptions was 19%, 19%, 18%, and 16% in years 2014, 2015, 2016, and 2017, respectively, in the internal medicine department (postintervention group), compared with rates of 30%, 29%, 36%, 36% (P < .001) for the same years in the surgery department (control group).
Education and the use of medication reconciliation forms on admission and discharge were utilized by Gupta et al to reduce inappropriate AST in hospitalized patients from 51% prior to intervention to 22% post intervention (P < .001).18 Furthermore, the proportion of patients discharged on inappropriate AST decreased from 69% to 20% (P < .001).
Hatch et al also used educational resources and pharmacist-led medication reconciliation to reduce use of SUP.19 Before the intervention, 24.4% of patients were continued on SUP after hospital discharge in the absence of a clear indication for use; post intervention, 11% of patients were continued on SUP after hospital discharge (of these patients, 8.7% had no clear indication for use). This represented a 64.4% decrease in inappropriately prescribed SUP after discharge (P < .0001).
Khalili et al combined an educational intervention with an institutional guideline in an infectious disease ward to reduce inappropriate use of SUP.20 This intervention reduced the inappropriate use of AST from 80.9% before the intervention to 47.1% post intervention (P < .001).
Masood et al implemented two interventions wherein pharmacists reviewed SUP indications for each patient during daily team rounds, and ICU residents and fellows received education about indications for SUP and the implemented initiative on a bimonthly basis.21 Inappropriate AST decreased from 26.75 to 7.14 prescriptions per 100 patient-days of care (P < .001).
McDonald et al combined education with a web-based quality improvement tool to reduce inappropriate exit prescriptions for PPIs.22 The proportion of PPIs discontinued at hospital discharge increased from 7.7% per month to 18.5% per month (P = .03).
Finally, the initiative implemented by Tasaka et al to reduce overutilization of SUP included an institutional guideline, a pharmacist-led intervention, and an institutional education and awareness campaign.23 Their initiative led to a reduction in inappropriate SUP both at the time of transfer out of the ICU (8% before intervention, 4% post intervention, P = .54) and at the time of discharge from the hospital (7% before intervention, 0% post intervention, P = .22).
REDUCING PPI USE AND SAFETY OUTCOMES
Proton pump inhibitors are often initiated in the hospital setting, with up to half of these new prescriptions continued at discharge.2,24,25 Inappropriate prescriptions for PPIs expose patients to excess risk of long-term adverse events.26 De-escalating PPIs, however, raises concern among clinicians and patients for potential recurrence of dyspepsia and GIB. There is limited evidence regarding long-term safety outcomes (including GIB) following the discontinuation of PPIs deemed to have been inappropriately initiated in the hospital. In view of this, clinicians should educate and monitor individual patients for symptom relapse to ensure timely and appropriate resumption of AST.
LIMITATIONS
Our literature search for this narrative review and implementation guide has limitations. First, the time frame we included (2000-2018) may have excluded relevant articles published before our starting year. We did not include articles published before 2000 based on concerns these might contain outdated information. Also, there may have been incomplete retrieval of relevant studies/articles due to the labor-intensive nature involved in determining whether PPI prescriptions are appropriate or inappropriate.
We noted that interventional studies aimed at reducing overuse of PPIs were often limited by a low number of participants; these studies were also more likely to be single-center interventions, which limits generalizability. In addition, the studies often had low methodological rigor and lacked randomization or controls. Moreover, to fully evaluate the sustainability of interventions, some of the studies had a limited postimplementation period. For multifaceted interventions, the efficacy of individual components of the interventions was not clearly evaluated. Moreover, there was a high risk of bias in many of the included studies. Some of the larger studies used overall AST prescriptions as a surrogate for more appropriate use. It would be advantageous for a site to perform a pilot study that provides well-defined parameters for appropriate prescribing, and then correlate with the total number of prescriptions (automated and much easier) thereafter. Further, although the evidence regarding appropriate PPI use for SUP and GIB has shifted rapidly in recent years, society guidelines have not been updated to reflect this change. As such, quality improvement interventions have predominantly focused on reducing PPI use for the indications reflected by these guidelines.
IMPLEMENTATION BLUEPRINT
The following are our recommendations for successfully implementing an evidence-based, institution-wide initiative to promote the appropriate use of PPIs during hospitalization. These recommendations are informed by the evidence review and reflect the consensus of the combined committees coauthoring this review.
For an initiative to succeed, participation from multiple disciplines is necessary to formulate local guidelines and design and implement interventions. Such an interdisciplinary approach requires advocates to closely monitor and evaluate the program; sustainability will be greatly facilitated by the active engagement of key stakeholders, including the hospital’s executive administration, supply chain, pharmacists, and gastroenterologists. Lack of adequate buy-in on the part of key stakeholders is a barrier to the success of any intervention. Accordingly, before selecting a particular intervention, it is important to understand local factors driving the overuse of PPI.
1. Develop evidence-based institutional guidelines for both SUP and nonvariceal upper GIB through an interdisciplinary workgroup.
- Establish an interdisciplinary group including, but not limited to, pharmacists, hospitalists, gastroenterologists, and intensivists so that changes in practice will be widely adopted as institutional policy.
- Incorporate the best evidence and clearly convey appropriate and inappropriate uses.
2. Integrate changes to the EHR.
- If possible, the EHR should be leveraged to implement changes in PPI ordering practices.
- While integrating changes to the EHR, it is important to consider informatics and implementation science, since the utility of hard stops and best practice alerts has been questioned in the setting of operational inefficiencies and alert fatigue.
- Options for integrating changes to the EHR include the following:
- Create an ordering pathway that provides clinical decision support for PPI use.
- Incorporate a best practice alert in the EMR to notify clinicians of institutional guidelines when they initiate an order for PPI outside of the pathway.
- Consider restricting the authority to order IV PPIs by requiring a code or password or implement another means of using the EHR to limit the supply of PPI.
- Limit the duration of IV PPI by requiring daily renewal of IV PPI dosing or by altering the period of time that use of IV PPI is permitted (eg, 48 to 72 hours).
- PPIs should be removed from any current order sets that include medications for SUP.
3. Foster pharmacy-driven interventions.
- Consider requiring pharmacist approval for IV PPIs.
- Pharmacist-led review and feedback to clinicians for discontinuation of inappropriate PPIs can be effective in decreasing inappropriate utilization.
4. Provide education, audit data, and obtain feedback.
- Data auditing is needed to measure the efficacy of interventions. Outcome measures may include the number of non-ICU and ICU patients who are started on a PPI during an admission; the audit should be continued through discharge. A process measure may be the number of pharmacist calls for inappropriate PPIs. A balancing measure would be ulcer-specific upper GIB in patients who do not receive SUP during their admission. (Upper GIB from other etiologies, such as varices, portal hypertensive gastropathy, and Mallory-Weiss tear would not be affected by PPI SUP.)
- Run or control charts should be utilized, and data should be shared with project champions and ordering clinicians—in real time if possible.
- Project champions should provide feedback to colleagues; they should also work with hospital leadership to develop new strategies to improve adherence.
- Provide ongoing education about appropriate indications for PPIs and potential adverse effects associated with their use. Whenever possible, point-of-care or just-in-time teaching is the preferred format.
CONCLUSION
Excessive use of PPIs during hospitalization is prevalent; however, quality improvement interventions can be effective in achieving sustainable reductions in overuse. There is a need for the American College of Gastroenterology to revisit and update their guidelines for management of patients with ulcer bleeding to include stronger evidence-based recommendations on the proper use of PPIs.27 These updated guidelines could be used to update the implementation blueprint.
Quality improvement teams have an opportunity to use the principles of value-based healthcare to reduce inappropriate PPI use. By following the blueprint outlined in this article, institutions can safely and effectively tailor the use of PPIs to suitable patients in the appropriate settings. Reduction of PPI overuse can be employed as an institutional catalyst to promote implementation of further value-based measures to improve efficiency and quality of patient care.
Proton pump inhibitors (PPIs) are among the most commonly used drugs worldwide to treat dyspepsia and prevent gastrointestinal bleeding (GIB).1 Between 40% and 70% of hospitalized patients receive acid-suppressive therapy (AST; defined as PPIs or histamine-receptor antagonists), and nearly half of these are initiated during the inpatient stay.2,3 While up to 50% of inpatients who received a new AST were discharged on these medications,2 there were no evidence-based indications for a majority of the prescriptions.2,3
Growing evidence shows that PPIs are overutilized and may be associated with wide-ranging adverse events, such as acute and chronic kidney disease,4Clostridium difficile infection,5 hypomagnesemia,6 and fractures.7 Because of the widespread overuse and the potential harm associated with PPIs, a concerted effort to promote their appropriate use in the inpatient setting is necessary. It is important to note that reducing the use of PPIs does not increase the risks of GIB or worsening dyspepsia. Rather, reducing overuse of PPIs lowers the risk of harm to patients. The efforts to reduce overuse, however, are complex and difficult.
This article summarizes evidence regarding interventions to reduce overuse and offers an implementation guide based on this evidence. This guide promotes value-based quality improvement and provides a blueprint for implementing an institution-wide program to reduce PPI overuse in the inpatient setting. We begin with a discussion about quality initiatives to reduce PPI overuse, followed by a review of the safety outcomes associated with reduced use of PPIs.
METHODS
A focused search of the US National Library of Medicine’s PubMed database was performed to identify English-language articles published between 2000 and 2018 that addressed strategies to reduce PPI overuse for stress ulcer prophylaxis (SUP) and nonvariceal GIB. The following search terms were used: PPI and inappropriate use; acid-suppressive therapy and inappropriate use; PPI and discontinuation; acid-suppressive (or suppressant) therapy and discontinuation; SUP and cost; and histamine receptor antagonist and PPI. Inpatient or outpatient studies of patients aged 18 years or older were considered for inclusion in this narrative review, and all study types were included. The primary exclusion criterion was patients aged younger than 18 years. A manual review of the full text of the retrieved articles was performed and references were reviewed for missed citations.
RESULTS
We identified a total of 1,497 unique citations through our initial search. After performing a manual review, we excluded 1,483 of the references and added an additional 2, resulting in 16 articles selected for inclusion. The selected articles addressed interventions falling into three main groupings: implementation of institutional guidelines with or without electronic health record (EHR)–based decision support, educational interventions alone, and multifaceted interventions. Each of these interventions is discussed in the sections that follow. Table 1, Table 2, and Table 3 summarize the results of the studies included in our narrative review.
QUALITY INITIATIVES TO REDUCE PPI OVERUSE
Institutional Guidelines With or Without EHR-Based Decision Support
Table 1 summarizes institutional guidelines, with or without EHR-based decision support, to reduce inappropriate PPI use. The implementation of institutional guidelines for the appropriate reduction of PPI use has had some success. Coursol and Sanzari evaluated the impact of a treatment algorithm on the appropriateness of prescriptions for SUP in the intensive care unit (ICU).8 Risk factors of patients in this study included mechanical ventilation for 48 hours, coagulopathy for 24 hours, postoperative transplant, severe burns, active gastrointestinal (GI) disease, multiple trauma, multiple organ failure, and septicemia. The three treatment options chosen for the algorithm were intravenous (IV) famotidine (if the oral route was unavailable or impractical), omeprazole tablets (if oral access was available), and omeprazole suspension (in cases of dysphagia and presence of nasogastric or orogastric tube). After implementation of the treatment algorithm, the proportion of inappropriate prophylaxis decreased from 95.7% to 88.2% (P = .033), and the cost per patient decreased from $11.11 to $8.49 Canadian dollars (P = .003).

Van Vliet et al implemented a clinical practice guideline listing specific criteria for prescribing a PPI.9 Their criteria included the presence of gastric or duodenal ulcer and use of a nonsteroidal anti-inflammatory drug (NSAID) or aspirin, plus at least one additional risk factor (eg, history of gastroduodenal hemorrhage or age >70 years). The proportion of patients started on PPIs during hospitalization decreased from 21% to 13% (odds ratio, 0.56; 95% CI, 0.33-0.97).
Michal et al utilized an institutional pharmacist-driven protocol that stipulated criteria for appropriate PPI use (eg, upper GIB, mechanical ventilation, peptic ulcer disease, gastroesophageal reflux disease, coagulopathy).10 Pharmacists in the study evaluated patients for PPI appropriateness and recommended changes in medication or discontinuation of use. This institutional intervention decreased PPI use in non-ICU hospitalized adults. Discontinuation of PPIs increased from 41% of patients in the preintervention group to 66% of patients in the postintervention group (P = .001).
In addition to implementing guidelines and intervention strategies, institutions have also adopted changes to the EHR to reduce inappropriate PPI use. Herzig et al utilized a computerized clinical decision support intervention to decrease SUP in non-ICU hospitalized patients.11 Of the available response options for acid-suppressive medication, when SUP was chosen as the only indication for PPI use a prompt alerted the clinician that “[SUP] is not recommended for patients outside the [ICU]”; the alert resulted in a significant reduction in AST for the sole purpose of SUP. With this intervention, the percentage of patients who had any inappropriate acid-suppressive exposure decreased from 4.0% to 0.6% (P < .001).
EDUCATION
Table 2 summarizes educational interventions to reduce inappropriate PPI use.

Agee et al employed a pharmacist-led educational seminar that described SUP indications, risks, and costs.12 Inappropriate SUP prescriptions decreased from 55.5% to 30.5% after the intervention (P < .0001). However, there was no reduction in the percentage of patients discharged on inappropriate AST.
Chui et al performed an intervention with academic detailing wherein a one-on-one visit with a physician took place, providing education to improve physician prescribing behavior.13 In this study, academic detailing focused on the most common instances for which PPIs were inappropriately utilized at that hospital (eg, surgical prophylaxis, anemia). Inappropriate use of double-dose PPIs was also targeted. Despite these efforts, no significant difference in inappropriate PPI prescribing was observed post intervention.
Hamzat et al implemented an educational strategy to reduce inappropriate PPI prescribing during hospital stays, which included dissemination of fliers, posters, emails, and presentations over a 4-week period.14 Educational efforts targeted clinical pharmacists, nurses, physicians, and patients. Appropriate indications for PPI use in this study included peptic ulcer disease (current or previous), H pylori infection, and treatment or prevention of an NSAID-induced ulcer. The primary outcome was a reduction in PPI dose or discontinuation of PPI during the hospital admission, which increased from 9% in the preintervention (pre-education) phase to 43% during the intervention (education) phase and to 46% in the postintervention (posteducation) phase (P = .006).
Liberman and Whelan also implemented an educational intervention among internal medicine residents to reduce inappropriate use of SUP; this intervention was based on practice-based learning and improvement methodology.15 They noted that the rate of inappropriate prophylaxis with AST decreased from 59% preintervention to 33% post intervention (P < .007).
MULTIFACETED APPROACHES
Table 3 summarizes several multifaceted approaches aimed at reducing inappropriate PPI use. Belfield et al utilized an intervention consisting of an institutional guideline review, education, and monitoring of AST by clinical pharmacists to reduce inappropriate use of PPI for SUP.16 With this intervention, the primary outcome of total inappropriate days of AST during hospitalization decreased from 279 to 116 (48% relative reduction in risk, P < .01, across 142 patients studied). Furthermore, inappropriate AST prescriptions at discharge decreased from 32% to 8% (P = .006). The one case of GIB noted in this study occurred in the control group.

Del Giorno et al combined audit and feedback with education to reduce new PPI prescriptions at the time of discharge from the hospital.17 The educational component of this intervention included guidance regarding potentially inappropriate PPI use and associated side effects and targeted multiple departments in the hospital. This intervention led to a sustained reduction in new PPI prescriptions at discharge during the 3-year study period. The annual rate of new PPI prescriptions was 19%, 19%, 18%, and 16% in years 2014, 2015, 2016, and 2017, respectively, in the internal medicine department (postintervention group), compared with rates of 30%, 29%, 36%, 36% (P < .001) for the same years in the surgery department (control group).
Education and the use of medication reconciliation forms on admission and discharge were utilized by Gupta et al to reduce inappropriate AST in hospitalized patients from 51% prior to intervention to 22% post intervention (P < .001).18 Furthermore, the proportion of patients discharged on inappropriate AST decreased from 69% to 20% (P < .001).
Hatch et al also used educational resources and pharmacist-led medication reconciliation to reduce use of SUP.19 Before the intervention, 24.4% of patients were continued on SUP after hospital discharge in the absence of a clear indication for use; post intervention, 11% of patients were continued on SUP after hospital discharge (of these patients, 8.7% had no clear indication for use). This represented a 64.4% decrease in inappropriately prescribed SUP after discharge (P < .0001).
Khalili et al combined an educational intervention with an institutional guideline in an infectious disease ward to reduce inappropriate use of SUP.20 This intervention reduced the inappropriate use of AST from 80.9% before the intervention to 47.1% post intervention (P < .001).
Masood et al implemented two interventions wherein pharmacists reviewed SUP indications for each patient during daily team rounds, and ICU residents and fellows received education about indications for SUP and the implemented initiative on a bimonthly basis.21 Inappropriate AST decreased from 26.75 to 7.14 prescriptions per 100 patient-days of care (P < .001).
McDonald et al combined education with a web-based quality improvement tool to reduce inappropriate exit prescriptions for PPIs.22 The proportion of PPIs discontinued at hospital discharge increased from 7.7% per month to 18.5% per month (P = .03).
Finally, the initiative implemented by Tasaka et al to reduce overutilization of SUP included an institutional guideline, a pharmacist-led intervention, and an institutional education and awareness campaign.23 Their initiative led to a reduction in inappropriate SUP both at the time of transfer out of the ICU (8% before intervention, 4% post intervention, P = .54) and at the time of discharge from the hospital (7% before intervention, 0% post intervention, P = .22).
REDUCING PPI USE AND SAFETY OUTCOMES
Proton pump inhibitors are often initiated in the hospital setting, with up to half of these new prescriptions continued at discharge.2,24,25 Inappropriate prescriptions for PPIs expose patients to excess risk of long-term adverse events.26 De-escalating PPIs, however, raises concern among clinicians and patients for potential recurrence of dyspepsia and GIB. There is limited evidence regarding long-term safety outcomes (including GIB) following the discontinuation of PPIs deemed to have been inappropriately initiated in the hospital. In view of this, clinicians should educate and monitor individual patients for symptom relapse to ensure timely and appropriate resumption of AST.
LIMITATIONS
Our literature search for this narrative review and implementation guide has limitations. First, the time frame we included (2000-2018) may have excluded relevant articles published before our starting year. We did not include articles published before 2000 based on concerns these might contain outdated information. Also, there may have been incomplete retrieval of relevant studies/articles due to the labor-intensive nature involved in determining whether PPI prescriptions are appropriate or inappropriate.
We noted that interventional studies aimed at reducing overuse of PPIs were often limited by a low number of participants; these studies were also more likely to be single-center interventions, which limits generalizability. In addition, the studies often had low methodological rigor and lacked randomization or controls. Moreover, to fully evaluate the sustainability of interventions, some of the studies had a limited postimplementation period. For multifaceted interventions, the efficacy of individual components of the interventions was not clearly evaluated. Moreover, there was a high risk of bias in many of the included studies. Some of the larger studies used overall AST prescriptions as a surrogate for more appropriate use. It would be advantageous for a site to perform a pilot study that provides well-defined parameters for appropriate prescribing, and then correlate with the total number of prescriptions (automated and much easier) thereafter. Further, although the evidence regarding appropriate PPI use for SUP and GIB has shifted rapidly in recent years, society guidelines have not been updated to reflect this change. As such, quality improvement interventions have predominantly focused on reducing PPI use for the indications reflected by these guidelines.
IMPLEMENTATION BLUEPRINT
The following are our recommendations for successfully implementing an evidence-based, institution-wide initiative to promote the appropriate use of PPIs during hospitalization. These recommendations are informed by the evidence review and reflect the consensus of the combined committees coauthoring this review.
For an initiative to succeed, participation from multiple disciplines is necessary to formulate local guidelines and design and implement interventions. Such an interdisciplinary approach requires advocates to closely monitor and evaluate the program; sustainability will be greatly facilitated by the active engagement of key stakeholders, including the hospital’s executive administration, supply chain, pharmacists, and gastroenterologists. Lack of adequate buy-in on the part of key stakeholders is a barrier to the success of any intervention. Accordingly, before selecting a particular intervention, it is important to understand local factors driving the overuse of PPI.
1. Develop evidence-based institutional guidelines for both SUP and nonvariceal upper GIB through an interdisciplinary workgroup.
- Establish an interdisciplinary group including, but not limited to, pharmacists, hospitalists, gastroenterologists, and intensivists so that changes in practice will be widely adopted as institutional policy.
- Incorporate the best evidence and clearly convey appropriate and inappropriate uses.
2. Integrate changes to the EHR.
- If possible, the EHR should be leveraged to implement changes in PPI ordering practices.
- While integrating changes to the EHR, it is important to consider informatics and implementation science, since the utility of hard stops and best practice alerts has been questioned in the setting of operational inefficiencies and alert fatigue.
- Options for integrating changes to the EHR include the following:
- Create an ordering pathway that provides clinical decision support for PPI use.
- Incorporate a best practice alert in the EMR to notify clinicians of institutional guidelines when they initiate an order for PPI outside of the pathway.
- Consider restricting the authority to order IV PPIs by requiring a code or password or implement another means of using the EHR to limit the supply of PPI.
- Limit the duration of IV PPI by requiring daily renewal of IV PPI dosing or by altering the period of time that use of IV PPI is permitted (eg, 48 to 72 hours).
- PPIs should be removed from any current order sets that include medications for SUP.
3. Foster pharmacy-driven interventions.
- Consider requiring pharmacist approval for IV PPIs.
- Pharmacist-led review and feedback to clinicians for discontinuation of inappropriate PPIs can be effective in decreasing inappropriate utilization.
4. Provide education, audit data, and obtain feedback.
- Data auditing is needed to measure the efficacy of interventions. Outcome measures may include the number of non-ICU and ICU patients who are started on a PPI during an admission; the audit should be continued through discharge. A process measure may be the number of pharmacist calls for inappropriate PPIs. A balancing measure would be ulcer-specific upper GIB in patients who do not receive SUP during their admission. (Upper GIB from other etiologies, such as varices, portal hypertensive gastropathy, and Mallory-Weiss tear would not be affected by PPI SUP.)
- Run or control charts should be utilized, and data should be shared with project champions and ordering clinicians—in real time if possible.
- Project champions should provide feedback to colleagues; they should also work with hospital leadership to develop new strategies to improve adherence.
- Provide ongoing education about appropriate indications for PPIs and potential adverse effects associated with their use. Whenever possible, point-of-care or just-in-time teaching is the preferred format.
CONCLUSION
Excessive use of PPIs during hospitalization is prevalent; however, quality improvement interventions can be effective in achieving sustainable reductions in overuse. There is a need for the American College of Gastroenterology to revisit and update their guidelines for management of patients with ulcer bleeding to include stronger evidence-based recommendations on the proper use of PPIs.27 These updated guidelines could be used to update the implementation blueprint.
Quality improvement teams have an opportunity to use the principles of value-based healthcare to reduce inappropriate PPI use. By following the blueprint outlined in this article, institutions can safely and effectively tailor the use of PPIs to suitable patients in the appropriate settings. Reduction of PPI overuse can be employed as an institutional catalyst to promote implementation of further value-based measures to improve efficiency and quality of patient care.
1. Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Exp Rev Clin Pharmacol. 2018;11(11):1123-1134. https://doi.org/10.1080/17512433.2018.1531703
2. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122. https://doi.org/10.1111/j.1572-0241.2000.03259.x
3. Ahrens D, Behrens G, Himmel W, Kochen MM, Chenot JF. Appropriateness of proton pump inhibitor recommendations at hospital discharge and continuation in primary care. Int J Clin Pract. 2012;66(8):767-773. https://doi.org/10.1111/j.1742-1241.2012.02973.x
4. Moledina DG, Perazella MA. PPIs and kidney disease: from AIN to CKD. J Nephrol. 2016;29(5):611-616. https://doi.org/10.1007/s40620-016-0309-2
5. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019. https://doi.org/10.1038/ajg.2012.108
6. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37(7):1237-1241. https://doi.org/10.3109/0886022x.2015.1057800
7. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947-2953. https://doi.org/10.1001/jama.296.24.2947
8. Coursol CJ, Sanzari SE. Impact of stress ulcer prophylaxis algorithm study. Ann Pharmacother. 2005;39(5):810-816. https://doi.org/10.1345/aph.1d129
9. van Vliet EPM, Steyerberg EW, Otten HJ, et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther. 2009;29(2):213-221. https://doi.org/10.1111/j.1365-2036.2008.03875.x
10. Michal J, Henry T, Street C. Impact of a pharmacist-driven protocol to decrease proton pump inhibitor use in non-intensive care hospitalized adults. Am J Health Syst Pharm. 2016;73(17 Suppl 4):S126-S132. https://doi.org/10.2146/ajhp150519
11. Herzig SJ, Guess JR, Feinbloom DB, et al. Improving appropriateness of acid-suppressive medication use via computerized clinical decision support. J Hosp Med. 2015;10(1):41-45. https://doi.org/10.1002/jhm.2260
12. Agee C, Coulter L, Hudson J. Effects of pharmacy resident led education on resident physician prescribing habits associated with stress ulcer prophylaxis in non-intensive care unit patients. Am J Health Syst Pharm. 2015;72(11 Suppl 1):S48-S52. https://doi.org/10.2146/sp150013
13. Chui D, Young F, Tejani AM, Dillon EC. Impact of academic detailing on proton pump inhibitor prescribing behaviour in a community hospital. Can Pharm J (Ott). 2011;144(2):66-71. https://doi.org/10.3821/1913-701X-144.2.66
14. Hamzat H, Sun H, Ford JC, Macleod J, Soiza RL, Mangoni AA. Inappropriate prescribing of proton pump inhibitors in older patients: effects of an educational strategy. Drugs Aging. 2012;29(8):681-690. https://doi.org/10.1007/bf03262283
15. Liberman JD, Whelan CT. Brief report: Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice-based educational intervention. J Gen Intern Med. 2006;21(5):498-500. https://doi.org/10.1111/j.1525-1497.2006.00435.x
16. Belfield KD, Kuyumjian AG, Teran R, Amadi M, Blatt M, Bicking K. Impact of a collaborative strategy to reduce the inappropriate use of acid suppressive therapy in non-intensive care unit patients. Ann Pharmacother. 2017;51(7):577-583. https://doi.org/10.1177/1060028017698797
17. Del Giorno R, Ceschi A, Pironi M, Zasa A, Greco A, Gabutti L. Multifaceted intervention to curb in-hospital over-prescription of proton pump inhibitors: a longitudinal multicenter quasi-experimental before-and-after study. Eur J Intern Med. 2018;50:52-59. https://doi.org/10.1016/j.ejim.2017.11.002
18. Gupta R, Marshall J, Munoz JC, Kottoor R, Jamal MM, Vega KJ. Decreased acid suppression therapy overuse after education and medication reconciliation. Int J Clin Pract. 2013;67(1):60-65. https://doi.org/10.1111/ijcp.12046
19. Hatch JB, Schulz L, Fish JT. Stress ulcer prophylaxis: reducing non-indicated prescribing after hospital discharge. Ann Pharmacother. 2010;44(10):1565-1571. https://doi.org/10.1345/aph.1p167
20. Khalili H, Dashti-Khavidaki S, Hossein Talasaz AH, Tabeefar H, Hendoiee N. Descriptive analysis of a clinical pharmacy intervention to improve the appropriate use of stress ulcer prophylaxis in a hospital infectious disease ward. J Manag Care Pharm. 2010;16(2):114-121. https://doi.org/10.18553/jmcp.2010.16.2.114
21. Masood U, Sharma A, Bhatti Z, et al. A successful pharmacist-based quality initiative to reduce inappropriate stress ulcer prophylaxis use in an academic medical intensive care unit. Inquiry. 2018;55:46958018759116. https://doi.org/10.1177/0046958018759116
22. McDonald EG, Jones J, Green L, Jayaraman D, Lee TC. Reduction of inappropriate exit prescriptions for proton pump inhibitors: a before-after study using education paired with a web-based quality-improvement tool. J Hosp Med. 2015;10(5):281-286. https://doi.org/10.1002/jhm.2330
23. Tasaka CL, Burg C, VanOsdol SJ, et al. An interprofessional approach to reducing the overutilization of stress ulcer prophylaxis in adult medical and surgical intensive care units. Ann Pharmacother. 2014;48(4):462-469. https://doi.org/10.1177/1060028013517088
24. Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther. 2005;21(10):1203-1209. https://doi.org/10.1111/j.1365-2036.2005.02454.x
25. Pham CQ, Regal RE, Bostwick TR, Knauf KS. Acid suppressive therapy use on an inpatient internal medicine service. Ann Pharmacother. 2006;40(7-8):1261-1266. https://doi.org/10.1345/aph.1g703
26. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors [editorial]. JAMA Intern Med. 2016;176(2):172-174. https://doi.org/10.1001/jamainternmed.2015.7927
27. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345-360; quiz 361. https://doi.org/10.1038/ajg.2011.480
1. Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Exp Rev Clin Pharmacol. 2018;11(11):1123-1134. https://doi.org/10.1080/17512433.2018.1531703
2. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122. https://doi.org/10.1111/j.1572-0241.2000.03259.x
3. Ahrens D, Behrens G, Himmel W, Kochen MM, Chenot JF. Appropriateness of proton pump inhibitor recommendations at hospital discharge and continuation in primary care. Int J Clin Pract. 2012;66(8):767-773. https://doi.org/10.1111/j.1742-1241.2012.02973.x
4. Moledina DG, Perazella MA. PPIs and kidney disease: from AIN to CKD. J Nephrol. 2016;29(5):611-616. https://doi.org/10.1007/s40620-016-0309-2
5. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019. https://doi.org/10.1038/ajg.2012.108
6. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37(7):1237-1241. https://doi.org/10.3109/0886022x.2015.1057800
7. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947-2953. https://doi.org/10.1001/jama.296.24.2947
8. Coursol CJ, Sanzari SE. Impact of stress ulcer prophylaxis algorithm study. Ann Pharmacother. 2005;39(5):810-816. https://doi.org/10.1345/aph.1d129
9. van Vliet EPM, Steyerberg EW, Otten HJ, et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther. 2009;29(2):213-221. https://doi.org/10.1111/j.1365-2036.2008.03875.x
10. Michal J, Henry T, Street C. Impact of a pharmacist-driven protocol to decrease proton pump inhibitor use in non-intensive care hospitalized adults. Am J Health Syst Pharm. 2016;73(17 Suppl 4):S126-S132. https://doi.org/10.2146/ajhp150519
11. Herzig SJ, Guess JR, Feinbloom DB, et al. Improving appropriateness of acid-suppressive medication use via computerized clinical decision support. J Hosp Med. 2015;10(1):41-45. https://doi.org/10.1002/jhm.2260
12. Agee C, Coulter L, Hudson J. Effects of pharmacy resident led education on resident physician prescribing habits associated with stress ulcer prophylaxis in non-intensive care unit patients. Am J Health Syst Pharm. 2015;72(11 Suppl 1):S48-S52. https://doi.org/10.2146/sp150013
13. Chui D, Young F, Tejani AM, Dillon EC. Impact of academic detailing on proton pump inhibitor prescribing behaviour in a community hospital. Can Pharm J (Ott). 2011;144(2):66-71. https://doi.org/10.3821/1913-701X-144.2.66
14. Hamzat H, Sun H, Ford JC, Macleod J, Soiza RL, Mangoni AA. Inappropriate prescribing of proton pump inhibitors in older patients: effects of an educational strategy. Drugs Aging. 2012;29(8):681-690. https://doi.org/10.1007/bf03262283
15. Liberman JD, Whelan CT. Brief report: Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice-based educational intervention. J Gen Intern Med. 2006;21(5):498-500. https://doi.org/10.1111/j.1525-1497.2006.00435.x
16. Belfield KD, Kuyumjian AG, Teran R, Amadi M, Blatt M, Bicking K. Impact of a collaborative strategy to reduce the inappropriate use of acid suppressive therapy in non-intensive care unit patients. Ann Pharmacother. 2017;51(7):577-583. https://doi.org/10.1177/1060028017698797
17. Del Giorno R, Ceschi A, Pironi M, Zasa A, Greco A, Gabutti L. Multifaceted intervention to curb in-hospital over-prescription of proton pump inhibitors: a longitudinal multicenter quasi-experimental before-and-after study. Eur J Intern Med. 2018;50:52-59. https://doi.org/10.1016/j.ejim.2017.11.002
18. Gupta R, Marshall J, Munoz JC, Kottoor R, Jamal MM, Vega KJ. Decreased acid suppression therapy overuse after education and medication reconciliation. Int J Clin Pract. 2013;67(1):60-65. https://doi.org/10.1111/ijcp.12046
19. Hatch JB, Schulz L, Fish JT. Stress ulcer prophylaxis: reducing non-indicated prescribing after hospital discharge. Ann Pharmacother. 2010;44(10):1565-1571. https://doi.org/10.1345/aph.1p167
20. Khalili H, Dashti-Khavidaki S, Hossein Talasaz AH, Tabeefar H, Hendoiee N. Descriptive analysis of a clinical pharmacy intervention to improve the appropriate use of stress ulcer prophylaxis in a hospital infectious disease ward. J Manag Care Pharm. 2010;16(2):114-121. https://doi.org/10.18553/jmcp.2010.16.2.114
21. Masood U, Sharma A, Bhatti Z, et al. A successful pharmacist-based quality initiative to reduce inappropriate stress ulcer prophylaxis use in an academic medical intensive care unit. Inquiry. 2018;55:46958018759116. https://doi.org/10.1177/0046958018759116
22. McDonald EG, Jones J, Green L, Jayaraman D, Lee TC. Reduction of inappropriate exit prescriptions for proton pump inhibitors: a before-after study using education paired with a web-based quality-improvement tool. J Hosp Med. 2015;10(5):281-286. https://doi.org/10.1002/jhm.2330
23. Tasaka CL, Burg C, VanOsdol SJ, et al. An interprofessional approach to reducing the overutilization of stress ulcer prophylaxis in adult medical and surgical intensive care units. Ann Pharmacother. 2014;48(4):462-469. https://doi.org/10.1177/1060028013517088
24. Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther. 2005;21(10):1203-1209. https://doi.org/10.1111/j.1365-2036.2005.02454.x
25. Pham CQ, Regal RE, Bostwick TR, Knauf KS. Acid suppressive therapy use on an inpatient internal medicine service. Ann Pharmacother. 2006;40(7-8):1261-1266. https://doi.org/10.1345/aph.1g703
26. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors [editorial]. JAMA Intern Med. 2016;176(2):172-174. https://doi.org/10.1001/jamainternmed.2015.7927
27. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345-360; quiz 361. https://doi.org/10.1038/ajg.2011.480
© 2021 Society of Hospital Medicine
Anticoagulant therapy for AFib in patients with end-stage renal disease
Warfarin or apixaban are sensible options
Case
A 78-year-old woman with end-stage renal disease (ESRD) is hospitalized with cellulitis and is incidentally found to be in atrial fibrillation. She does not have a history of mitral stenosis, nor does she have a prosthetic valve. She does have a history of hypertension, diabetes, and prior stroke without residual deficits.
After counseling her about the risk of stroke associated with atrial fibrillation (AFib) she makes it clear she is interested in pharmacologic therapy to minimize her risk of stroke and asks what medication you would recommend for anticoagulation.
Brief overview of the issue
Anticoagulation for AFib is indicated for stroke prophylaxis in patients with an elevated risk of stroke. The CHA2DS2-VASc score is useful in calculating an individual patient’s risk of stroke and as a decision tool to determine who would benefit from anticoagulation, and it is recommended in the American Heart Association guidelines.1
Low-risk patients (CHA2DS2-VASc score of 0 in men or 1 in women) should not be started on anticoagulation for stroke prophylaxis. For anyone with a risk factor, other than being female, anticoagulation is indicated and should be considered.
The guideline recommends anticoagulant therapy, not antiplatelet agents. For most of the recent past, this has meant a vitamin K antagonist (warfarin) or sometimes a low-molecular-weight heparin injected subcutaneously. Over the past decade, however, with the approval of multiple direct oral anticoagulants (DOACs), nonwarfarin oral anticoagulation has grown in popularity as the prophylactic medication of choice.2
While the data for patients with preserved renal function is robust, there is far less data to guide decision making for patients with end-stage renal disease.
Overview of the data
Until the introduction of DOACs, warfarin was the main agent used for stroke prophylaxis in patients with end-stage kidney disease and AFib. Professional guidelines favored warfarin for these patients who were mostly excluded from DOAC trials. Specialized conferences also looked at this issue.
The Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference, which reviewed chronic kidney disease and arrhythmias, noted that there were no randomized controlled trials that examined the efficacy and safety of anticoagulation in chronic kidney disease patients with estimated creatinine clearance less than 30 mL/min. They remarked that there was insufficient high-quality evidence to recommend warfarin for the prevention of stroke in patients with AFib and dialysis-dependent chronic kidney disease.
Since, according to other trials, DOACs had better safety profiles in other populations, the conference noted that lower-dose apixaban (2.5 mg orally twice daily) or rivaroxaban (15 mg daily) may be considered in this population until clinical safety data were available. Furthermore, the conference recommended that these patients be treated with a multidisciplinary approach in regards to anticoagulation and have an annual reevaluation of treatment goals, along with a risk-benefit assessment.3
Since the publication of the 2018 AHA guidelines and the guidance document that resulted from the KDIGO conference, additional research has been published comparing anticoagulation with a DOAC versus warfarin for AFib in patients with ESRD.
“Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States” was an observational, retrospective, cohort study that compared outcomes in dialysis patients who took warfarin for AFib with those who took apixaban.4 Patients’ data was taken from the U.S. Renal Data System database and were included in the final analysis if they had ESRD, a recent diagnosis of AFib or atrial flutter, and a new prescription for either warfarin or apixaban. Outcome measures were stroke or systemic embolism, major bleeding (critical site, transfusion, or death), gastrointestinal bleeding, intracranial bleeding, or death. Drug usage and compliance were assessed using Medicare Part D prescription information.
A total of 25,523 patients met the inclusion/exclusion criteria and had taken either warfarin (n = 23,172) or apixaban (n = 2,351). To account for selection bias in these cohorts, a subset of the warfarin patients was selected based on prognostic score matching. The prognostic score was calculated from the baseline characteristics (which included age, stroke history, diabetes, smoking, antiplatelet medication, liver disease, prior bleeding, and CHA2DS2-VASc score). Kaplan-Meier and Cox regression analysis were used to give hazard ratios and 95% confidence intervals for each outcome measure. Prespecified subgroup analyses were conducted to compare apixaban doses, where 44% were prescribed 5 mg b.i.d. and 56% were prescribed 2.5 mg b.i.d..
In the study, patients in the apixaban group had a significantly lower risk of major bleeding as compared with the warfarin group (HR, 0.72; 95% CI, 0.59-0.87; P less than .001) with overall high rates of major bleeding in both groups at 19.7 and 22.9 per 100 patient-years in the apixaban group and warfarin group, respectively. There was no difference in the rate of stroke/systemic embolism between patients receiving apixaban and warfarin (HR, 0.88; 95% CI, 0.69-1.12; P = .29). There was a nonsignificant trend toward decreased risk of GI bleeding in the apixaban group and no significant differences between the groups in the rates of intracranial bleeding. Apixaban was also associated with a nonsignificant trend toward lower risk of mortality (HR, 0.85; 95% CI, 0.71-1.01; P = .06).
Notably, censoring rates because of expired prescriptions or a 1-month gap between prescriptions were high in both groups and the majority of censoring occurred within the first 12 months. Additionally, in dose specific analyses, patients receiving the 5-mg, twice-daily dose were found to have statistically significant decreases in risk of stroke/systemic embolism (P = .035) and mortality (P = .005) as compared with the 2.5-mg, twice-daily dose without significant differences in GI or intracranial bleeding.
There are three ongoing, open-label, randomized, controlled trials examining anticoagulation for nonvalvular AFib in patients with ESRD on hemodialysis with two comparing apixaban to warfarin (or derivative) and the other warfarin versus no anticoagulation.5 All trials are in adult patients with documented AFib and CHA2DS2-VASc score of at least 2. AKADIA (Germany based) plans to enroll 222 patients and compares a vitamin K antagonist (INR goal, 2-3) with 2.5-mg b.i.d. apixaban patients with ESRD on hemodialysis for at least 3 months with primary outcome of major and clinically relevant nonmajor bleeding and secondary outcome of thromboembolic events, as well as apixaban levels pre- and post hemodialysis.
RENAL-AF (U.S. based) plans to enrolled 762 patients and compares 5-mg b.i.d. apixaban (with 2.5 mg for selected patients) with warfarin in people of chronic hemodialysis with primary outcome of days to first major or clinically relevant nonmajor bleeding event and secondary outcome of stroke, systemic embolism, mortality, adherence and plasma apixaban levels. AVKDIAL (France based) plans to enroll 855 patients and compares no anticoagulation with vitamin K antagonists in patients on hemodialysis for at least 1 month, with primary outcome of cumulative incidence of severe bleeding and thrombosis.
Application of the data to our original case
Our patient is Medicare age with ESRD and newly diagnosed nonvalvular AFib. Recent data suggests apixaban could be used for stroke prevention instead of the prior standard of care, warfarin. This approach is supported in the 2019 guidelines.1
Patients with ESRD have an increased risk of bleeding and apixaban was shown to have less bleeding complications than warfarin in this analysis. However, only standard-dose apixaban was associated with a statistically significant lower risk of stroke/systemic embolism, major bleeding, and death. Reduced-dose apixaban had a lower risk of major bleeding but no difference for stroke/systemic embolism or death. Reduced-dose apixaban is used for patients who have two out of the following three criteria: aged at least 80 years, weight of at least 60 kg, and creatinine of at least 1.5 mg/dL. Therefore, many Medicare-age patients with ESRD would not be indicated for the dose of apixaban that was shown to improve the most important outcomes of stroke/SE and death.
It may still be beneficial to use apixaban in this patient since it appears to work as well as warfarin for stroke/systemic embolism prevention with less bleeding complications.
Bottom line
For patients who have decided to pursue an anticoagulation strategy for stroke prevention in AFib and have end-stage renal disease, either warfarin or apixaban are sensible options.
Dr. Farber is a medical instructor at Duke University Health System in Durham, N.C. Dr. Stafford is a medical instructor at Duke University. Dr. Sata is assistant professor of medicine at Duke University. Dr. Abdo and Dr. Menon are hospitalists at Duke University. Dr. Brooks is assistant professor of medicine at Duke University. Dr. Wachter is associate medical director at Duke Regional Hospital and assistant professor of medicine at Duke University. Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and assistant professor of medicine at Duke University.
References
1. January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
2. Lippi G et al. Direct oral anticoagulants: Analysis of worldwide use and popularity using Google Trends. Ann Transl Med. 2017 Aug; 5(16):322. doi: 10.21037/atm.2017.06.65.
3. Turakhia MP et al. Chronic kidney disease and arrhythmias: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J. 2018 Jun 21;39(24):2314-25. doi: 10.1093/eurheartj/ehy060.
4. Siontis KC et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018 Oct 9;138(15):1519-29. doi: 10.1161/CIRCULATIONAHA.118.035418.
5. Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit-to-risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Key points
- According to 2019 American Heart Association guidelines, warfarin or apixaban are reasonable options for stroke prevention for patients who have end-stage renal disease and who plan for anticoagulation because of atrial fibrillation.
- Recent observational data suggests that apixaban may be safer than warfarin in this population.
- Several randomized, controlled trials are ongoing that may help determine the optimal agent to use in this setting.
- Until more definitive data is available, a reasonable approach is to discuss the risks and benefits of various treatment strategies with patients, and engage a multidisciplinary team (cardiologist, nephrologist, primary care provider, pharmacist) in the decision making process.
Additional reading
January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit to risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Garlo KG et al. Demystifying the benefits and harms of anticoagulation for atrial fibrillation in chronic kidney disease. Clin J Am Soc Nephrol 2019;14:125-36. doi: 10.2215/CJN.06430518.
Quiz
Two days ago you admitted a 72-year-old woman with end-stage renal disease on dialysis who had developed new-onset atrial fibrillation causing a mild acute diastolic congestive heart failure exacerbation. Transthoracic ECG showed a preserved left ventricular ejection fraction and no significant valvular disease. After two sessions of dialysis in the hospital and initiation of a beta-blocker for control of her heart rate, she is stable and ready for discharge. Her discharge weight is 75 kg.
Which of the following recommendations should you make to this patient regarding anticoagulation for prevention of stroke and systemic embolism from atrial fibrillation?
A. Take warfarin with a international normalized ratio goal of 2.5.
B. Take apixaban 2.5 mg twice a day.
C. Take apixaban 5 mg twice a day.
D. Discuss the risks/benefits of various treatment approaches with the patient, and involve the hospital pharmacist as well as the patient’s nephrologist, cardiologist, and/or primary care provider in the decision making process to reach a consensus and to ensure a safe follow-up plan.
The best answer is D. While A, B, and C are all reasonable approaches based on the available data and current guidelines, the best approach is to involve the patient and the multidisciplinary team in the decision making process. When more clinical trial data becomes available in the future, the optimal approach to managing patients such as this one may become clearer, but until then it makes sense to take into account individual patient characteristics and patient preferences.
Warfarin or apixaban are sensible options
Warfarin or apixaban are sensible options
Case
A 78-year-old woman with end-stage renal disease (ESRD) is hospitalized with cellulitis and is incidentally found to be in atrial fibrillation. She does not have a history of mitral stenosis, nor does she have a prosthetic valve. She does have a history of hypertension, diabetes, and prior stroke without residual deficits.
After counseling her about the risk of stroke associated with atrial fibrillation (AFib) she makes it clear she is interested in pharmacologic therapy to minimize her risk of stroke and asks what medication you would recommend for anticoagulation.
Brief overview of the issue
Anticoagulation for AFib is indicated for stroke prophylaxis in patients with an elevated risk of stroke. The CHA2DS2-VASc score is useful in calculating an individual patient’s risk of stroke and as a decision tool to determine who would benefit from anticoagulation, and it is recommended in the American Heart Association guidelines.1
Low-risk patients (CHA2DS2-VASc score of 0 in men or 1 in women) should not be started on anticoagulation for stroke prophylaxis. For anyone with a risk factor, other than being female, anticoagulation is indicated and should be considered.
The guideline recommends anticoagulant therapy, not antiplatelet agents. For most of the recent past, this has meant a vitamin K antagonist (warfarin) or sometimes a low-molecular-weight heparin injected subcutaneously. Over the past decade, however, with the approval of multiple direct oral anticoagulants (DOACs), nonwarfarin oral anticoagulation has grown in popularity as the prophylactic medication of choice.2
While the data for patients with preserved renal function is robust, there is far less data to guide decision making for patients with end-stage renal disease.
Overview of the data
Until the introduction of DOACs, warfarin was the main agent used for stroke prophylaxis in patients with end-stage kidney disease and AFib. Professional guidelines favored warfarin for these patients who were mostly excluded from DOAC trials. Specialized conferences also looked at this issue.
The Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference, which reviewed chronic kidney disease and arrhythmias, noted that there were no randomized controlled trials that examined the efficacy and safety of anticoagulation in chronic kidney disease patients with estimated creatinine clearance less than 30 mL/min. They remarked that there was insufficient high-quality evidence to recommend warfarin for the prevention of stroke in patients with AFib and dialysis-dependent chronic kidney disease.
Since, according to other trials, DOACs had better safety profiles in other populations, the conference noted that lower-dose apixaban (2.5 mg orally twice daily) or rivaroxaban (15 mg daily) may be considered in this population until clinical safety data were available. Furthermore, the conference recommended that these patients be treated with a multidisciplinary approach in regards to anticoagulation and have an annual reevaluation of treatment goals, along with a risk-benefit assessment.3
Since the publication of the 2018 AHA guidelines and the guidance document that resulted from the KDIGO conference, additional research has been published comparing anticoagulation with a DOAC versus warfarin for AFib in patients with ESRD.
“Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States” was an observational, retrospective, cohort study that compared outcomes in dialysis patients who took warfarin for AFib with those who took apixaban.4 Patients’ data was taken from the U.S. Renal Data System database and were included in the final analysis if they had ESRD, a recent diagnosis of AFib or atrial flutter, and a new prescription for either warfarin or apixaban. Outcome measures were stroke or systemic embolism, major bleeding (critical site, transfusion, or death), gastrointestinal bleeding, intracranial bleeding, or death. Drug usage and compliance were assessed using Medicare Part D prescription information.
A total of 25,523 patients met the inclusion/exclusion criteria and had taken either warfarin (n = 23,172) or apixaban (n = 2,351). To account for selection bias in these cohorts, a subset of the warfarin patients was selected based on prognostic score matching. The prognostic score was calculated from the baseline characteristics (which included age, stroke history, diabetes, smoking, antiplatelet medication, liver disease, prior bleeding, and CHA2DS2-VASc score). Kaplan-Meier and Cox regression analysis were used to give hazard ratios and 95% confidence intervals for each outcome measure. Prespecified subgroup analyses were conducted to compare apixaban doses, where 44% were prescribed 5 mg b.i.d. and 56% were prescribed 2.5 mg b.i.d..
In the study, patients in the apixaban group had a significantly lower risk of major bleeding as compared with the warfarin group (HR, 0.72; 95% CI, 0.59-0.87; P less than .001) with overall high rates of major bleeding in both groups at 19.7 and 22.9 per 100 patient-years in the apixaban group and warfarin group, respectively. There was no difference in the rate of stroke/systemic embolism between patients receiving apixaban and warfarin (HR, 0.88; 95% CI, 0.69-1.12; P = .29). There was a nonsignificant trend toward decreased risk of GI bleeding in the apixaban group and no significant differences between the groups in the rates of intracranial bleeding. Apixaban was also associated with a nonsignificant trend toward lower risk of mortality (HR, 0.85; 95% CI, 0.71-1.01; P = .06).
Notably, censoring rates because of expired prescriptions or a 1-month gap between prescriptions were high in both groups and the majority of censoring occurred within the first 12 months. Additionally, in dose specific analyses, patients receiving the 5-mg, twice-daily dose were found to have statistically significant decreases in risk of stroke/systemic embolism (P = .035) and mortality (P = .005) as compared with the 2.5-mg, twice-daily dose without significant differences in GI or intracranial bleeding.
There are three ongoing, open-label, randomized, controlled trials examining anticoagulation for nonvalvular AFib in patients with ESRD on hemodialysis with two comparing apixaban to warfarin (or derivative) and the other warfarin versus no anticoagulation.5 All trials are in adult patients with documented AFib and CHA2DS2-VASc score of at least 2. AKADIA (Germany based) plans to enroll 222 patients and compares a vitamin K antagonist (INR goal, 2-3) with 2.5-mg b.i.d. apixaban patients with ESRD on hemodialysis for at least 3 months with primary outcome of major and clinically relevant nonmajor bleeding and secondary outcome of thromboembolic events, as well as apixaban levels pre- and post hemodialysis.
RENAL-AF (U.S. based) plans to enrolled 762 patients and compares 5-mg b.i.d. apixaban (with 2.5 mg for selected patients) with warfarin in people of chronic hemodialysis with primary outcome of days to first major or clinically relevant nonmajor bleeding event and secondary outcome of stroke, systemic embolism, mortality, adherence and plasma apixaban levels. AVKDIAL (France based) plans to enroll 855 patients and compares no anticoagulation with vitamin K antagonists in patients on hemodialysis for at least 1 month, with primary outcome of cumulative incidence of severe bleeding and thrombosis.
Application of the data to our original case
Our patient is Medicare age with ESRD and newly diagnosed nonvalvular AFib. Recent data suggests apixaban could be used for stroke prevention instead of the prior standard of care, warfarin. This approach is supported in the 2019 guidelines.1
Patients with ESRD have an increased risk of bleeding and apixaban was shown to have less bleeding complications than warfarin in this analysis. However, only standard-dose apixaban was associated with a statistically significant lower risk of stroke/systemic embolism, major bleeding, and death. Reduced-dose apixaban had a lower risk of major bleeding but no difference for stroke/systemic embolism or death. Reduced-dose apixaban is used for patients who have two out of the following three criteria: aged at least 80 years, weight of at least 60 kg, and creatinine of at least 1.5 mg/dL. Therefore, many Medicare-age patients with ESRD would not be indicated for the dose of apixaban that was shown to improve the most important outcomes of stroke/SE and death.
It may still be beneficial to use apixaban in this patient since it appears to work as well as warfarin for stroke/systemic embolism prevention with less bleeding complications.
Bottom line
For patients who have decided to pursue an anticoagulation strategy for stroke prevention in AFib and have end-stage renal disease, either warfarin or apixaban are sensible options.
Dr. Farber is a medical instructor at Duke University Health System in Durham, N.C. Dr. Stafford is a medical instructor at Duke University. Dr. Sata is assistant professor of medicine at Duke University. Dr. Abdo and Dr. Menon are hospitalists at Duke University. Dr. Brooks is assistant professor of medicine at Duke University. Dr. Wachter is associate medical director at Duke Regional Hospital and assistant professor of medicine at Duke University. Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and assistant professor of medicine at Duke University.
References
1. January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
2. Lippi G et al. Direct oral anticoagulants: Analysis of worldwide use and popularity using Google Trends. Ann Transl Med. 2017 Aug; 5(16):322. doi: 10.21037/atm.2017.06.65.
3. Turakhia MP et al. Chronic kidney disease and arrhythmias: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J. 2018 Jun 21;39(24):2314-25. doi: 10.1093/eurheartj/ehy060.
4. Siontis KC et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018 Oct 9;138(15):1519-29. doi: 10.1161/CIRCULATIONAHA.118.035418.
5. Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit-to-risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Key points
- According to 2019 American Heart Association guidelines, warfarin or apixaban are reasonable options for stroke prevention for patients who have end-stage renal disease and who plan for anticoagulation because of atrial fibrillation.
- Recent observational data suggests that apixaban may be safer than warfarin in this population.
- Several randomized, controlled trials are ongoing that may help determine the optimal agent to use in this setting.
- Until more definitive data is available, a reasonable approach is to discuss the risks and benefits of various treatment strategies with patients, and engage a multidisciplinary team (cardiologist, nephrologist, primary care provider, pharmacist) in the decision making process.
Additional reading
January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit to risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Garlo KG et al. Demystifying the benefits and harms of anticoagulation for atrial fibrillation in chronic kidney disease. Clin J Am Soc Nephrol 2019;14:125-36. doi: 10.2215/CJN.06430518.
Quiz
Two days ago you admitted a 72-year-old woman with end-stage renal disease on dialysis who had developed new-onset atrial fibrillation causing a mild acute diastolic congestive heart failure exacerbation. Transthoracic ECG showed a preserved left ventricular ejection fraction and no significant valvular disease. After two sessions of dialysis in the hospital and initiation of a beta-blocker for control of her heart rate, she is stable and ready for discharge. Her discharge weight is 75 kg.
Which of the following recommendations should you make to this patient regarding anticoagulation for prevention of stroke and systemic embolism from atrial fibrillation?
A. Take warfarin with a international normalized ratio goal of 2.5.
B. Take apixaban 2.5 mg twice a day.
C. Take apixaban 5 mg twice a day.
D. Discuss the risks/benefits of various treatment approaches with the patient, and involve the hospital pharmacist as well as the patient’s nephrologist, cardiologist, and/or primary care provider in the decision making process to reach a consensus and to ensure a safe follow-up plan.
The best answer is D. While A, B, and C are all reasonable approaches based on the available data and current guidelines, the best approach is to involve the patient and the multidisciplinary team in the decision making process. When more clinical trial data becomes available in the future, the optimal approach to managing patients such as this one may become clearer, but until then it makes sense to take into account individual patient characteristics and patient preferences.
Case
A 78-year-old woman with end-stage renal disease (ESRD) is hospitalized with cellulitis and is incidentally found to be in atrial fibrillation. She does not have a history of mitral stenosis, nor does she have a prosthetic valve. She does have a history of hypertension, diabetes, and prior stroke without residual deficits.
After counseling her about the risk of stroke associated with atrial fibrillation (AFib) she makes it clear she is interested in pharmacologic therapy to minimize her risk of stroke and asks what medication you would recommend for anticoagulation.
Brief overview of the issue
Anticoagulation for AFib is indicated for stroke prophylaxis in patients with an elevated risk of stroke. The CHA2DS2-VASc score is useful in calculating an individual patient’s risk of stroke and as a decision tool to determine who would benefit from anticoagulation, and it is recommended in the American Heart Association guidelines.1
Low-risk patients (CHA2DS2-VASc score of 0 in men or 1 in women) should not be started on anticoagulation for stroke prophylaxis. For anyone with a risk factor, other than being female, anticoagulation is indicated and should be considered.
The guideline recommends anticoagulant therapy, not antiplatelet agents. For most of the recent past, this has meant a vitamin K antagonist (warfarin) or sometimes a low-molecular-weight heparin injected subcutaneously. Over the past decade, however, with the approval of multiple direct oral anticoagulants (DOACs), nonwarfarin oral anticoagulation has grown in popularity as the prophylactic medication of choice.2
While the data for patients with preserved renal function is robust, there is far less data to guide decision making for patients with end-stage renal disease.
Overview of the data
Until the introduction of DOACs, warfarin was the main agent used for stroke prophylaxis in patients with end-stage kidney disease and AFib. Professional guidelines favored warfarin for these patients who were mostly excluded from DOAC trials. Specialized conferences also looked at this issue.
The Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference, which reviewed chronic kidney disease and arrhythmias, noted that there were no randomized controlled trials that examined the efficacy and safety of anticoagulation in chronic kidney disease patients with estimated creatinine clearance less than 30 mL/min. They remarked that there was insufficient high-quality evidence to recommend warfarin for the prevention of stroke in patients with AFib and dialysis-dependent chronic kidney disease.
Since, according to other trials, DOACs had better safety profiles in other populations, the conference noted that lower-dose apixaban (2.5 mg orally twice daily) or rivaroxaban (15 mg daily) may be considered in this population until clinical safety data were available. Furthermore, the conference recommended that these patients be treated with a multidisciplinary approach in regards to anticoagulation and have an annual reevaluation of treatment goals, along with a risk-benefit assessment.3
Since the publication of the 2018 AHA guidelines and the guidance document that resulted from the KDIGO conference, additional research has been published comparing anticoagulation with a DOAC versus warfarin for AFib in patients with ESRD.
“Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States” was an observational, retrospective, cohort study that compared outcomes in dialysis patients who took warfarin for AFib with those who took apixaban.4 Patients’ data was taken from the U.S. Renal Data System database and were included in the final analysis if they had ESRD, a recent diagnosis of AFib or atrial flutter, and a new prescription for either warfarin or apixaban. Outcome measures were stroke or systemic embolism, major bleeding (critical site, transfusion, or death), gastrointestinal bleeding, intracranial bleeding, or death. Drug usage and compliance were assessed using Medicare Part D prescription information.
A total of 25,523 patients met the inclusion/exclusion criteria and had taken either warfarin (n = 23,172) or apixaban (n = 2,351). To account for selection bias in these cohorts, a subset of the warfarin patients was selected based on prognostic score matching. The prognostic score was calculated from the baseline characteristics (which included age, stroke history, diabetes, smoking, antiplatelet medication, liver disease, prior bleeding, and CHA2DS2-VASc score). Kaplan-Meier and Cox regression analysis were used to give hazard ratios and 95% confidence intervals for each outcome measure. Prespecified subgroup analyses were conducted to compare apixaban doses, where 44% were prescribed 5 mg b.i.d. and 56% were prescribed 2.5 mg b.i.d..
In the study, patients in the apixaban group had a significantly lower risk of major bleeding as compared with the warfarin group (HR, 0.72; 95% CI, 0.59-0.87; P less than .001) with overall high rates of major bleeding in both groups at 19.7 and 22.9 per 100 patient-years in the apixaban group and warfarin group, respectively. There was no difference in the rate of stroke/systemic embolism between patients receiving apixaban and warfarin (HR, 0.88; 95% CI, 0.69-1.12; P = .29). There was a nonsignificant trend toward decreased risk of GI bleeding in the apixaban group and no significant differences between the groups in the rates of intracranial bleeding. Apixaban was also associated with a nonsignificant trend toward lower risk of mortality (HR, 0.85; 95% CI, 0.71-1.01; P = .06).
Notably, censoring rates because of expired prescriptions or a 1-month gap between prescriptions were high in both groups and the majority of censoring occurred within the first 12 months. Additionally, in dose specific analyses, patients receiving the 5-mg, twice-daily dose were found to have statistically significant decreases in risk of stroke/systemic embolism (P = .035) and mortality (P = .005) as compared with the 2.5-mg, twice-daily dose without significant differences in GI or intracranial bleeding.
There are three ongoing, open-label, randomized, controlled trials examining anticoagulation for nonvalvular AFib in patients with ESRD on hemodialysis with two comparing apixaban to warfarin (or derivative) and the other warfarin versus no anticoagulation.5 All trials are in adult patients with documented AFib and CHA2DS2-VASc score of at least 2. AKADIA (Germany based) plans to enroll 222 patients and compares a vitamin K antagonist (INR goal, 2-3) with 2.5-mg b.i.d. apixaban patients with ESRD on hemodialysis for at least 3 months with primary outcome of major and clinically relevant nonmajor bleeding and secondary outcome of thromboembolic events, as well as apixaban levels pre- and post hemodialysis.
RENAL-AF (U.S. based) plans to enrolled 762 patients and compares 5-mg b.i.d. apixaban (with 2.5 mg for selected patients) with warfarin in people of chronic hemodialysis with primary outcome of days to first major or clinically relevant nonmajor bleeding event and secondary outcome of stroke, systemic embolism, mortality, adherence and plasma apixaban levels. AVKDIAL (France based) plans to enroll 855 patients and compares no anticoagulation with vitamin K antagonists in patients on hemodialysis for at least 1 month, with primary outcome of cumulative incidence of severe bleeding and thrombosis.
Application of the data to our original case
Our patient is Medicare age with ESRD and newly diagnosed nonvalvular AFib. Recent data suggests apixaban could be used for stroke prevention instead of the prior standard of care, warfarin. This approach is supported in the 2019 guidelines.1
Patients with ESRD have an increased risk of bleeding and apixaban was shown to have less bleeding complications than warfarin in this analysis. However, only standard-dose apixaban was associated with a statistically significant lower risk of stroke/systemic embolism, major bleeding, and death. Reduced-dose apixaban had a lower risk of major bleeding but no difference for stroke/systemic embolism or death. Reduced-dose apixaban is used for patients who have two out of the following three criteria: aged at least 80 years, weight of at least 60 kg, and creatinine of at least 1.5 mg/dL. Therefore, many Medicare-age patients with ESRD would not be indicated for the dose of apixaban that was shown to improve the most important outcomes of stroke/SE and death.
It may still be beneficial to use apixaban in this patient since it appears to work as well as warfarin for stroke/systemic embolism prevention with less bleeding complications.
Bottom line
For patients who have decided to pursue an anticoagulation strategy for stroke prevention in AFib and have end-stage renal disease, either warfarin or apixaban are sensible options.
Dr. Farber is a medical instructor at Duke University Health System in Durham, N.C. Dr. Stafford is a medical instructor at Duke University. Dr. Sata is assistant professor of medicine at Duke University. Dr. Abdo and Dr. Menon are hospitalists at Duke University. Dr. Brooks is assistant professor of medicine at Duke University. Dr. Wachter is associate medical director at Duke Regional Hospital and assistant professor of medicine at Duke University. Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and assistant professor of medicine at Duke University.
References
1. January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
2. Lippi G et al. Direct oral anticoagulants: Analysis of worldwide use and popularity using Google Trends. Ann Transl Med. 2017 Aug; 5(16):322. doi: 10.21037/atm.2017.06.65.
3. Turakhia MP et al. Chronic kidney disease and arrhythmias: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J. 2018 Jun 21;39(24):2314-25. doi: 10.1093/eurheartj/ehy060.
4. Siontis KC et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018 Oct 9;138(15):1519-29. doi: 10.1161/CIRCULATIONAHA.118.035418.
5. Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit-to-risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Key points
- According to 2019 American Heart Association guidelines, warfarin or apixaban are reasonable options for stroke prevention for patients who have end-stage renal disease and who plan for anticoagulation because of atrial fibrillation.
- Recent observational data suggests that apixaban may be safer than warfarin in this population.
- Several randomized, controlled trials are ongoing that may help determine the optimal agent to use in this setting.
- Until more definitive data is available, a reasonable approach is to discuss the risks and benefits of various treatment strategies with patients, and engage a multidisciplinary team (cardiologist, nephrologist, primary care provider, pharmacist) in the decision making process.
Additional reading
January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit to risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Garlo KG et al. Demystifying the benefits and harms of anticoagulation for atrial fibrillation in chronic kidney disease. Clin J Am Soc Nephrol 2019;14:125-36. doi: 10.2215/CJN.06430518.
Quiz
Two days ago you admitted a 72-year-old woman with end-stage renal disease on dialysis who had developed new-onset atrial fibrillation causing a mild acute diastolic congestive heart failure exacerbation. Transthoracic ECG showed a preserved left ventricular ejection fraction and no significant valvular disease. After two sessions of dialysis in the hospital and initiation of a beta-blocker for control of her heart rate, she is stable and ready for discharge. Her discharge weight is 75 kg.
Which of the following recommendations should you make to this patient regarding anticoagulation for prevention of stroke and systemic embolism from atrial fibrillation?
A. Take warfarin with a international normalized ratio goal of 2.5.
B. Take apixaban 2.5 mg twice a day.
C. Take apixaban 5 mg twice a day.
D. Discuss the risks/benefits of various treatment approaches with the patient, and involve the hospital pharmacist as well as the patient’s nephrologist, cardiologist, and/or primary care provider in the decision making process to reach a consensus and to ensure a safe follow-up plan.
The best answer is D. While A, B, and C are all reasonable approaches based on the available data and current guidelines, the best approach is to involve the patient and the multidisciplinary team in the decision making process. When more clinical trial data becomes available in the future, the optimal approach to managing patients such as this one may become clearer, but until then it makes sense to take into account individual patient characteristics and patient preferences.
Does supplemental oxygen help COPD patients who have chronic stable moderate hypoxia?
New study a departure from previous research
Case
An 85-year-old man with long-standing chronic obstructive pulmonary disease (COPD) has a witnessed aspiration event while undergoing an outpatient procedure requiring conscious sedation. He is admitted to the hospital for observation overnight. The next morning, he feels well, but his oxygen saturation dips to 85% with ambulation. He reports this is not new for him, but he vehemently does not want supplemental oxygen.
Background
Patients with COPD and severe resting hypoxemia – arterial oxygen partial pressure less than or equal to 55 mm Hg or peripheral capillary oxygen saturation (SpO2) less than or equal to 88% – commonly are prescribed supplemental oxygen. The evidence supporting this practice is limited to two small trials from the 1970s that showed a survival benefit of long-term oxygen therapy (LTOT) in this population,1,2 but these trials may not be generalizable to patients today.
For patients with COPD and mild to moderate resting hypoxemia (SpO2, 89%-93%) or patients with exercise-induced hypoxemia, LTOT has not been shown to improve survival, although it may improve symptoms of dyspnea, exercise tolerance, and other patient reported outcomes. Given the costs, risks, and burdens associated with LTOT, a high-quality clinical trial assessing the effects of LTOT on clinically meaningful outcomes, such as survival or hospitalization, in patients with COPD and moderate hypoxemia has been long overdue.
Overview of the data
The utility of long-term treatment with supplemental oxygen in patients with stable COPD and moderate resting or exercise-induced desaturation was examined by the Long-Term Oxygen Treatment Trial (LOTT) Research Group. Results were published in the New England Journal of Medicine in October 2016 in the article, “A Randomized Trial of Long-Term Oxygen for COPD with Moderate Desaturation.”3
The study was initially designed to test whether the use of supplemental oxygen would lead to longer time until death as compared with no supplemental oxygen in the subgroup of COPD patients with stable disease and moderate resting desaturation (defined as resting SpO2 of 89%-93%). However, because of an enrollment of only 34 patients after 7 months, the trial was redesigned to include exercise-induced desaturation (defined as SpO2 of greater than or equal to 80% for at least 5 minutes, and less than 90% for at least 10 seconds, on a 6-minute walk test) and the secondary outcome of all-cause hospitalization. Hospitalization for any cause was combined with mortality into a new composite primary outcome.
This study was a randomized, controlled trial which enrolled patients at a total of 14 regional clinical centers and their associated sites for a total of 42 centers in the United States. The experimental arm consisted of a long term supplemental oxygen group, and the control group did not receive long term supplemental oxygen. Patients were assigned to groups in a 1:1 ratio and the study was not blinded. Patients with moderate resting desaturation were prescribed 24 hour oxygen at 2 L/min, and patients with moderate exercise-induced desaturation were prescribed oxygen at 2 L/min during exercise and sleep only. The primary outcome was a composite outcome of time until death or time until first hospitalization for any cause. There were multiple secondary outcomes, including incidence of COPD exacerbation, incidence of severe resting desaturation and severe exercise-induced desaturation, quality of life, sleep quality, depression and anxiety, adherence to regimen, 6-minute walk distance, spirometric measurements, risk of cardiovascular disease, and neurocognitive function.
Data were gathered via yearly visits, biannual telephone interviews, and questionnaires mailed at 4 months and 16 months. Adherence was assessed by inquiring about oxygen use every 4 months. If patients in the supplemental oxygen group used stationary oxygen concentrators, logs of meter readings were kept as well. The necessary final sample size was calculated using a time to composite event survival model with the use of the log-rank test statistic.
A total of 738 patients were enrolled in the trial between January 2009 and September 2015 and were followed for 1-6 years. A total of 97% of participants had at least 1 year of follow-up. Out of the 738 randomized patients, 133 (18%) had only resting desaturations, 319 (43%) had only exercise-induced desaturations, and 286 (39%) had both resting and exercise-induced desaturations. Baseline characteristics including age, sex, race, smoking status, quality of life scores, resting SpO2, and nadir SpO2 during the 6-minute walk test were similar between the two groups. The only significant difference noted by the authors between the two groups was a lower BODE (body mass index, airflow obstruction, dyspnea, and exercise) index, which was lower in the group with no supplemental oxygen.
In the time-to-event analysis, there was no significant difference between the two groups in the time to death or first hospitalization (hazard ratio, 0.94; 95% confidence interval, 0.79-1.12; P = .52). There were no significant differences in the rates of all hospitalizations (rate ratio, 1.01; 95% CI, 0.91-1.13), COPD exacerbations (RR 1.08; 95% CI, 0.98-1.19), and COPD related hospitalizations (RR, 0.99; 95% CI, 0.83-1.17). There were also no differences between the experimental and control groups in quality of life, lung function, and 6-minute walk distance. There were no significant differences in the subgroups classified by desaturation profile, sex, race, nadir SpO2 during the 6-minute walk test, and forced expiratory volume in 1 second.
The findings in this study show that, in the subgroup of chronic obstructive pulmonary disease patients with stable COPD and moderate resting or exercise-induced desaturation, supplemental oxygen did not affect the time to death or first hospitalization, time to death, time to first hospitalization, time to first COPD exacerbation, time to first hospitalization for a COPD exacerbation, rate of all hospitalizations, rate of all COPD exacerbations, or changes in metrics surrounding quality of life, anxiety/depression, or functional status. This supports earlier studies that demonstrated that long-term treatment with oxygen does not result in longer survival than does no long-term treatment with oxygen in patients with COPD and resting SpO2 of more than 88%.
The results of this study are a departure from previous studies that had shown improved mortality in patients with COPD and severe desaturation who were treated with LTOT. The authors hypothesized that this may have been caused by physiological effects of oxygen saturation on pulmonary vasoconstriction, release of mediators, and ventilator drive, which occur at an O2 saturation of 88% or less and may be more significant in patients with chronic hypoxemia. This trial also contrasted previous studies that had shown that oxygen therapy may reduce dyspnea in COPD patients with mild or no hypoxia because the LOTT trial showed no improvement in quality of life, anxiety, and depression measures in patients treated with long-term oxygen as compared with those treated with no oxygen.
Some limitations of the study included the absence of highly symptomatic patients or patients who the providers believed were too ill to participate, the effect of the unblinded nature of the study on outcomes that were patient reported, the lack of assessment of immediate effects of oxygen on exercise performance or symptoms, possible variability in amount of oxygen delivered, and the fact that patients may have overestimated their oxygen use.
In patients with stable COPD and moderate resting or exercise induced desaturation, long-term supplemental oxygen did not provide any benefit in regard to time until death or first hospitalization or any of the other measured outcomes.
Application of data to the case
Our patient has stable COPD and had only moderate exercise-induced desaturation. Long-term supplemental oxygen would not produce a benefit for him.
This study shows us that it would not increase his survival at this point; however, if he were to have worsening exercise-induced or new resting desaturation at some point in the future, supplemental oxygen would then be beneficial. At this point supplemental oxygen would not even affect his rate of hospitalization for COPD- or non-COPD–related reasons. Perhaps most importantly, adding oxygen therapy would not affect his overall quality of life, including his functional status and mood.
Bottom line
The addition of supplemental oxygen is not helpful for patients with COPD who have chronic stable moderate hypoxia.
Dr. Farber is a medical instructor in the Duke University Health System in Durham, N.C. Dr. Sata is a medical instructor in the Duke University Hospital. Dr. Wachter is an assistant professor of medicine at Duke University. Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and an assistant professor of medicine at Duke University.
References
1. Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Ann Intern Med. 1980 Sep;93(3):391-8.
2. Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: Report of the Medical Research Council Working Party. Lancet 1981 Mar 28;1(8222):681-6.
3. Long-term oxygen treatment trial research group et al. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016 Oct 27;375(17):1617-27.
Additional reading
Stoller JK et al. Oxygen therapy for patients with COPD: Current evidence and the Long-term Oxygen Treatment Trial. Chest. 2010 July;138:179-87.
Qaseem A et al. Diagnosis and management of stable chronic obstructive pulmonary disease: A clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011 Aug 2;155(3):179-91.
Ameer F et al. Ambulatory oxygen for people with chronic obstructive pulmonary disease who are not hypoxaemic at rest. Cochrane Database Syst Rev. 2014 Jun 24;(6):CD000238.
Vestbo J et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013 Feb 15;187(4):347-65.
Quiz: Does this patient need oxygen?
You are caring for a 72-year-old man with stable COPD who was admitted for cellulitis. He is improving clinically on appropriate antibiotics, and he has been stable on room air every time you examine him. The nurse pages you on the day of discharge – a Sunday – informing you that his oxygen saturation dropped to 88% while he was walking the halls this morning. She asks whether he needs to stay in the hospital so you can arrange home supplemental oxygen therapy. What should you do?
A. Keep him in the hospital until you can arrange home oxygen therapy.
B. Discharge him home Sunday but have the oxygen company go out to his house first thing on Monday.
C. Discharge him home without supplemental oxygen therapy.
D. Check an arterial blood gas to help decide if you should set up oxygen therapy.
The answer is C. He meets the description of stable COPD with mild to moderate exercise-induced desaturation. The LOTT trial supports our clinical decision that he would not benefit from supplemental oxygen therapy at this point.
Key Points
- Long-term oxygen therapy (LTOT) is beneficial in patients with COPD and severe resting hypoxemia (arterial oxygen partial pressure ≤ 55 mm Hg or SpO2 ≤ 88%) and should be prescribed to improve survival in this population.
- Patients with COPD and mild to moderate resting hypoxemia or exercised-induced hypoxemia should not be routinely prescribed LTOT given the associated costs, risks, and burdens and the lack of evidence of benefit.
New study a departure from previous research
New study a departure from previous research
Case
An 85-year-old man with long-standing chronic obstructive pulmonary disease (COPD) has a witnessed aspiration event while undergoing an outpatient procedure requiring conscious sedation. He is admitted to the hospital for observation overnight. The next morning, he feels well, but his oxygen saturation dips to 85% with ambulation. He reports this is not new for him, but he vehemently does not want supplemental oxygen.
Background
Patients with COPD and severe resting hypoxemia – arterial oxygen partial pressure less than or equal to 55 mm Hg or peripheral capillary oxygen saturation (SpO2) less than or equal to 88% – commonly are prescribed supplemental oxygen. The evidence supporting this practice is limited to two small trials from the 1970s that showed a survival benefit of long-term oxygen therapy (LTOT) in this population,1,2 but these trials may not be generalizable to patients today.
For patients with COPD and mild to moderate resting hypoxemia (SpO2, 89%-93%) or patients with exercise-induced hypoxemia, LTOT has not been shown to improve survival, although it may improve symptoms of dyspnea, exercise tolerance, and other patient reported outcomes. Given the costs, risks, and burdens associated with LTOT, a high-quality clinical trial assessing the effects of LTOT on clinically meaningful outcomes, such as survival or hospitalization, in patients with COPD and moderate hypoxemia has been long overdue.
Overview of the data
The utility of long-term treatment with supplemental oxygen in patients with stable COPD and moderate resting or exercise-induced desaturation was examined by the Long-Term Oxygen Treatment Trial (LOTT) Research Group. Results were published in the New England Journal of Medicine in October 2016 in the article, “A Randomized Trial of Long-Term Oxygen for COPD with Moderate Desaturation.”3
The study was initially designed to test whether the use of supplemental oxygen would lead to longer time until death as compared with no supplemental oxygen in the subgroup of COPD patients with stable disease and moderate resting desaturation (defined as resting SpO2 of 89%-93%). However, because of an enrollment of only 34 patients after 7 months, the trial was redesigned to include exercise-induced desaturation (defined as SpO2 of greater than or equal to 80% for at least 5 minutes, and less than 90% for at least 10 seconds, on a 6-minute walk test) and the secondary outcome of all-cause hospitalization. Hospitalization for any cause was combined with mortality into a new composite primary outcome.
This study was a randomized, controlled trial which enrolled patients at a total of 14 regional clinical centers and their associated sites for a total of 42 centers in the United States. The experimental arm consisted of a long term supplemental oxygen group, and the control group did not receive long term supplemental oxygen. Patients were assigned to groups in a 1:1 ratio and the study was not blinded. Patients with moderate resting desaturation were prescribed 24 hour oxygen at 2 L/min, and patients with moderate exercise-induced desaturation were prescribed oxygen at 2 L/min during exercise and sleep only. The primary outcome was a composite outcome of time until death or time until first hospitalization for any cause. There were multiple secondary outcomes, including incidence of COPD exacerbation, incidence of severe resting desaturation and severe exercise-induced desaturation, quality of life, sleep quality, depression and anxiety, adherence to regimen, 6-minute walk distance, spirometric measurements, risk of cardiovascular disease, and neurocognitive function.
Data were gathered via yearly visits, biannual telephone interviews, and questionnaires mailed at 4 months and 16 months. Adherence was assessed by inquiring about oxygen use every 4 months. If patients in the supplemental oxygen group used stationary oxygen concentrators, logs of meter readings were kept as well. The necessary final sample size was calculated using a time to composite event survival model with the use of the log-rank test statistic.
A total of 738 patients were enrolled in the trial between January 2009 and September 2015 and were followed for 1-6 years. A total of 97% of participants had at least 1 year of follow-up. Out of the 738 randomized patients, 133 (18%) had only resting desaturations, 319 (43%) had only exercise-induced desaturations, and 286 (39%) had both resting and exercise-induced desaturations. Baseline characteristics including age, sex, race, smoking status, quality of life scores, resting SpO2, and nadir SpO2 during the 6-minute walk test were similar between the two groups. The only significant difference noted by the authors between the two groups was a lower BODE (body mass index, airflow obstruction, dyspnea, and exercise) index, which was lower in the group with no supplemental oxygen.
In the time-to-event analysis, there was no significant difference between the two groups in the time to death or first hospitalization (hazard ratio, 0.94; 95% confidence interval, 0.79-1.12; P = .52). There were no significant differences in the rates of all hospitalizations (rate ratio, 1.01; 95% CI, 0.91-1.13), COPD exacerbations (RR 1.08; 95% CI, 0.98-1.19), and COPD related hospitalizations (RR, 0.99; 95% CI, 0.83-1.17). There were also no differences between the experimental and control groups in quality of life, lung function, and 6-minute walk distance. There were no significant differences in the subgroups classified by desaturation profile, sex, race, nadir SpO2 during the 6-minute walk test, and forced expiratory volume in 1 second.
The findings in this study show that, in the subgroup of chronic obstructive pulmonary disease patients with stable COPD and moderate resting or exercise-induced desaturation, supplemental oxygen did not affect the time to death or first hospitalization, time to death, time to first hospitalization, time to first COPD exacerbation, time to first hospitalization for a COPD exacerbation, rate of all hospitalizations, rate of all COPD exacerbations, or changes in metrics surrounding quality of life, anxiety/depression, or functional status. This supports earlier studies that demonstrated that long-term treatment with oxygen does not result in longer survival than does no long-term treatment with oxygen in patients with COPD and resting SpO2 of more than 88%.
The results of this study are a departure from previous studies that had shown improved mortality in patients with COPD and severe desaturation who were treated with LTOT. The authors hypothesized that this may have been caused by physiological effects of oxygen saturation on pulmonary vasoconstriction, release of mediators, and ventilator drive, which occur at an O2 saturation of 88% or less and may be more significant in patients with chronic hypoxemia. This trial also contrasted previous studies that had shown that oxygen therapy may reduce dyspnea in COPD patients with mild or no hypoxia because the LOTT trial showed no improvement in quality of life, anxiety, and depression measures in patients treated with long-term oxygen as compared with those treated with no oxygen.
Some limitations of the study included the absence of highly symptomatic patients or patients who the providers believed were too ill to participate, the effect of the unblinded nature of the study on outcomes that were patient reported, the lack of assessment of immediate effects of oxygen on exercise performance or symptoms, possible variability in amount of oxygen delivered, and the fact that patients may have overestimated their oxygen use.
In patients with stable COPD and moderate resting or exercise induced desaturation, long-term supplemental oxygen did not provide any benefit in regard to time until death or first hospitalization or any of the other measured outcomes.
Application of data to the case
Our patient has stable COPD and had only moderate exercise-induced desaturation. Long-term supplemental oxygen would not produce a benefit for him.
This study shows us that it would not increase his survival at this point; however, if he were to have worsening exercise-induced or new resting desaturation at some point in the future, supplemental oxygen would then be beneficial. At this point supplemental oxygen would not even affect his rate of hospitalization for COPD- or non-COPD–related reasons. Perhaps most importantly, adding oxygen therapy would not affect his overall quality of life, including his functional status and mood.
Bottom line
The addition of supplemental oxygen is not helpful for patients with COPD who have chronic stable moderate hypoxia.
Dr. Farber is a medical instructor in the Duke University Health System in Durham, N.C. Dr. Sata is a medical instructor in the Duke University Hospital. Dr. Wachter is an assistant professor of medicine at Duke University. Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and an assistant professor of medicine at Duke University.
References
1. Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Ann Intern Med. 1980 Sep;93(3):391-8.
2. Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: Report of the Medical Research Council Working Party. Lancet 1981 Mar 28;1(8222):681-6.
3. Long-term oxygen treatment trial research group et al. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016 Oct 27;375(17):1617-27.
Additional reading
Stoller JK et al. Oxygen therapy for patients with COPD: Current evidence and the Long-term Oxygen Treatment Trial. Chest. 2010 July;138:179-87.
Qaseem A et al. Diagnosis and management of stable chronic obstructive pulmonary disease: A clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011 Aug 2;155(3):179-91.
Ameer F et al. Ambulatory oxygen for people with chronic obstructive pulmonary disease who are not hypoxaemic at rest. Cochrane Database Syst Rev. 2014 Jun 24;(6):CD000238.
Vestbo J et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013 Feb 15;187(4):347-65.
Quiz: Does this patient need oxygen?
You are caring for a 72-year-old man with stable COPD who was admitted for cellulitis. He is improving clinically on appropriate antibiotics, and he has been stable on room air every time you examine him. The nurse pages you on the day of discharge – a Sunday – informing you that his oxygen saturation dropped to 88% while he was walking the halls this morning. She asks whether he needs to stay in the hospital so you can arrange home supplemental oxygen therapy. What should you do?
A. Keep him in the hospital until you can arrange home oxygen therapy.
B. Discharge him home Sunday but have the oxygen company go out to his house first thing on Monday.
C. Discharge him home without supplemental oxygen therapy.
D. Check an arterial blood gas to help decide if you should set up oxygen therapy.
The answer is C. He meets the description of stable COPD with mild to moderate exercise-induced desaturation. The LOTT trial supports our clinical decision that he would not benefit from supplemental oxygen therapy at this point.
Key Points
- Long-term oxygen therapy (LTOT) is beneficial in patients with COPD and severe resting hypoxemia (arterial oxygen partial pressure ≤ 55 mm Hg or SpO2 ≤ 88%) and should be prescribed to improve survival in this population.
- Patients with COPD and mild to moderate resting hypoxemia or exercised-induced hypoxemia should not be routinely prescribed LTOT given the associated costs, risks, and burdens and the lack of evidence of benefit.
Case
An 85-year-old man with long-standing chronic obstructive pulmonary disease (COPD) has a witnessed aspiration event while undergoing an outpatient procedure requiring conscious sedation. He is admitted to the hospital for observation overnight. The next morning, he feels well, but his oxygen saturation dips to 85% with ambulation. He reports this is not new for him, but he vehemently does not want supplemental oxygen.
Background
Patients with COPD and severe resting hypoxemia – arterial oxygen partial pressure less than or equal to 55 mm Hg or peripheral capillary oxygen saturation (SpO2) less than or equal to 88% – commonly are prescribed supplemental oxygen. The evidence supporting this practice is limited to two small trials from the 1970s that showed a survival benefit of long-term oxygen therapy (LTOT) in this population,1,2 but these trials may not be generalizable to patients today.
For patients with COPD and mild to moderate resting hypoxemia (SpO2, 89%-93%) or patients with exercise-induced hypoxemia, LTOT has not been shown to improve survival, although it may improve symptoms of dyspnea, exercise tolerance, and other patient reported outcomes. Given the costs, risks, and burdens associated with LTOT, a high-quality clinical trial assessing the effects of LTOT on clinically meaningful outcomes, such as survival or hospitalization, in patients with COPD and moderate hypoxemia has been long overdue.
Overview of the data
The utility of long-term treatment with supplemental oxygen in patients with stable COPD and moderate resting or exercise-induced desaturation was examined by the Long-Term Oxygen Treatment Trial (LOTT) Research Group. Results were published in the New England Journal of Medicine in October 2016 in the article, “A Randomized Trial of Long-Term Oxygen for COPD with Moderate Desaturation.”3
The study was initially designed to test whether the use of supplemental oxygen would lead to longer time until death as compared with no supplemental oxygen in the subgroup of COPD patients with stable disease and moderate resting desaturation (defined as resting SpO2 of 89%-93%). However, because of an enrollment of only 34 patients after 7 months, the trial was redesigned to include exercise-induced desaturation (defined as SpO2 of greater than or equal to 80% for at least 5 minutes, and less than 90% for at least 10 seconds, on a 6-minute walk test) and the secondary outcome of all-cause hospitalization. Hospitalization for any cause was combined with mortality into a new composite primary outcome.
This study was a randomized, controlled trial which enrolled patients at a total of 14 regional clinical centers and their associated sites for a total of 42 centers in the United States. The experimental arm consisted of a long term supplemental oxygen group, and the control group did not receive long term supplemental oxygen. Patients were assigned to groups in a 1:1 ratio and the study was not blinded. Patients with moderate resting desaturation were prescribed 24 hour oxygen at 2 L/min, and patients with moderate exercise-induced desaturation were prescribed oxygen at 2 L/min during exercise and sleep only. The primary outcome was a composite outcome of time until death or time until first hospitalization for any cause. There were multiple secondary outcomes, including incidence of COPD exacerbation, incidence of severe resting desaturation and severe exercise-induced desaturation, quality of life, sleep quality, depression and anxiety, adherence to regimen, 6-minute walk distance, spirometric measurements, risk of cardiovascular disease, and neurocognitive function.
Data were gathered via yearly visits, biannual telephone interviews, and questionnaires mailed at 4 months and 16 months. Adherence was assessed by inquiring about oxygen use every 4 months. If patients in the supplemental oxygen group used stationary oxygen concentrators, logs of meter readings were kept as well. The necessary final sample size was calculated using a time to composite event survival model with the use of the log-rank test statistic.
A total of 738 patients were enrolled in the trial between January 2009 and September 2015 and were followed for 1-6 years. A total of 97% of participants had at least 1 year of follow-up. Out of the 738 randomized patients, 133 (18%) had only resting desaturations, 319 (43%) had only exercise-induced desaturations, and 286 (39%) had both resting and exercise-induced desaturations. Baseline characteristics including age, sex, race, smoking status, quality of life scores, resting SpO2, and nadir SpO2 during the 6-minute walk test were similar between the two groups. The only significant difference noted by the authors between the two groups was a lower BODE (body mass index, airflow obstruction, dyspnea, and exercise) index, which was lower in the group with no supplemental oxygen.
In the time-to-event analysis, there was no significant difference between the two groups in the time to death or first hospitalization (hazard ratio, 0.94; 95% confidence interval, 0.79-1.12; P = .52). There were no significant differences in the rates of all hospitalizations (rate ratio, 1.01; 95% CI, 0.91-1.13), COPD exacerbations (RR 1.08; 95% CI, 0.98-1.19), and COPD related hospitalizations (RR, 0.99; 95% CI, 0.83-1.17). There were also no differences between the experimental and control groups in quality of life, lung function, and 6-minute walk distance. There were no significant differences in the subgroups classified by desaturation profile, sex, race, nadir SpO2 during the 6-minute walk test, and forced expiratory volume in 1 second.
The findings in this study show that, in the subgroup of chronic obstructive pulmonary disease patients with stable COPD and moderate resting or exercise-induced desaturation, supplemental oxygen did not affect the time to death or first hospitalization, time to death, time to first hospitalization, time to first COPD exacerbation, time to first hospitalization for a COPD exacerbation, rate of all hospitalizations, rate of all COPD exacerbations, or changes in metrics surrounding quality of life, anxiety/depression, or functional status. This supports earlier studies that demonstrated that long-term treatment with oxygen does not result in longer survival than does no long-term treatment with oxygen in patients with COPD and resting SpO2 of more than 88%.
The results of this study are a departure from previous studies that had shown improved mortality in patients with COPD and severe desaturation who were treated with LTOT. The authors hypothesized that this may have been caused by physiological effects of oxygen saturation on pulmonary vasoconstriction, release of mediators, and ventilator drive, which occur at an O2 saturation of 88% or less and may be more significant in patients with chronic hypoxemia. This trial also contrasted previous studies that had shown that oxygen therapy may reduce dyspnea in COPD patients with mild or no hypoxia because the LOTT trial showed no improvement in quality of life, anxiety, and depression measures in patients treated with long-term oxygen as compared with those treated with no oxygen.
Some limitations of the study included the absence of highly symptomatic patients or patients who the providers believed were too ill to participate, the effect of the unblinded nature of the study on outcomes that were patient reported, the lack of assessment of immediate effects of oxygen on exercise performance or symptoms, possible variability in amount of oxygen delivered, and the fact that patients may have overestimated their oxygen use.
In patients with stable COPD and moderate resting or exercise induced desaturation, long-term supplemental oxygen did not provide any benefit in regard to time until death or first hospitalization or any of the other measured outcomes.
Application of data to the case
Our patient has stable COPD and had only moderate exercise-induced desaturation. Long-term supplemental oxygen would not produce a benefit for him.
This study shows us that it would not increase his survival at this point; however, if he were to have worsening exercise-induced or new resting desaturation at some point in the future, supplemental oxygen would then be beneficial. At this point supplemental oxygen would not even affect his rate of hospitalization for COPD- or non-COPD–related reasons. Perhaps most importantly, adding oxygen therapy would not affect his overall quality of life, including his functional status and mood.
Bottom line
The addition of supplemental oxygen is not helpful for patients with COPD who have chronic stable moderate hypoxia.
Dr. Farber is a medical instructor in the Duke University Health System in Durham, N.C. Dr. Sata is a medical instructor in the Duke University Hospital. Dr. Wachter is an assistant professor of medicine at Duke University. Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and an assistant professor of medicine at Duke University.
References
1. Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Ann Intern Med. 1980 Sep;93(3):391-8.
2. Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: Report of the Medical Research Council Working Party. Lancet 1981 Mar 28;1(8222):681-6.
3. Long-term oxygen treatment trial research group et al. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016 Oct 27;375(17):1617-27.
Additional reading
Stoller JK et al. Oxygen therapy for patients with COPD: Current evidence and the Long-term Oxygen Treatment Trial. Chest. 2010 July;138:179-87.
Qaseem A et al. Diagnosis and management of stable chronic obstructive pulmonary disease: A clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011 Aug 2;155(3):179-91.
Ameer F et al. Ambulatory oxygen for people with chronic obstructive pulmonary disease who are not hypoxaemic at rest. Cochrane Database Syst Rev. 2014 Jun 24;(6):CD000238.
Vestbo J et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013 Feb 15;187(4):347-65.
Quiz: Does this patient need oxygen?
You are caring for a 72-year-old man with stable COPD who was admitted for cellulitis. He is improving clinically on appropriate antibiotics, and he has been stable on room air every time you examine him. The nurse pages you on the day of discharge – a Sunday – informing you that his oxygen saturation dropped to 88% while he was walking the halls this morning. She asks whether he needs to stay in the hospital so you can arrange home supplemental oxygen therapy. What should you do?
A. Keep him in the hospital until you can arrange home oxygen therapy.
B. Discharge him home Sunday but have the oxygen company go out to his house first thing on Monday.
C. Discharge him home without supplemental oxygen therapy.
D. Check an arterial blood gas to help decide if you should set up oxygen therapy.
The answer is C. He meets the description of stable COPD with mild to moderate exercise-induced desaturation. The LOTT trial supports our clinical decision that he would not benefit from supplemental oxygen therapy at this point.
Key Points
- Long-term oxygen therapy (LTOT) is beneficial in patients with COPD and severe resting hypoxemia (arterial oxygen partial pressure ≤ 55 mm Hg or SpO2 ≤ 88%) and should be prescribed to improve survival in this population.
- Patients with COPD and mild to moderate resting hypoxemia or exercised-induced hypoxemia should not be routinely prescribed LTOT given the associated costs, risks, and burdens and the lack of evidence of benefit.
Treat sleep apnea with positive airway pressure, but don’t expect it to prevent heart attacks
Clinical question: In patients with sleep apnea, does using positive airway pressure (PAP) treatment prevent adverse cardiovascular events and death?
Background: Previous observational studies have suggested that untreated sleep apnea is a factor in cardiopulmonary morbidity as well as cerebrovascular events. Guidelines advise its use for prevention of cerebrovascular events. However, not enough is known from trials about its impact on prevention of cardiovascular events.
Synopsis: The authors analyzed 10 randomized-controlled trials encompassing 7,266 patients with sleep apnea. They examined instances of major adverse cardiovascular events (MACE; acute coronary syndrome, stroke, cardiovascular death) as well as hospitalization for unstable angina and all-cause deaths, among others. They found no association between treatment with positive airway pressure and MACEs (169 events vs. 187 events, with a relative risk of 0.77; 95% confidence interval, 0.53-1.13) or all-cause death (324 events vs. 289 events, RR 1.13; 95% CI,0.99-1.29).
Bottom line: Positive airway pressure treatment for patients with sleep apnea is not an intervention to prevent cardiovascular morbidity.
Citation: Yu J et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea. JAMA. 2017 Jul 11;318(2):156-66.
Dr. Sata is a medical instructor, Duke University Hospital.
Clinical question: In patients with sleep apnea, does using positive airway pressure (PAP) treatment prevent adverse cardiovascular events and death?
Background: Previous observational studies have suggested that untreated sleep apnea is a factor in cardiopulmonary morbidity as well as cerebrovascular events. Guidelines advise its use for prevention of cerebrovascular events. However, not enough is known from trials about its impact on prevention of cardiovascular events.
Synopsis: The authors analyzed 10 randomized-controlled trials encompassing 7,266 patients with sleep apnea. They examined instances of major adverse cardiovascular events (MACE; acute coronary syndrome, stroke, cardiovascular death) as well as hospitalization for unstable angina and all-cause deaths, among others. They found no association between treatment with positive airway pressure and MACEs (169 events vs. 187 events, with a relative risk of 0.77; 95% confidence interval, 0.53-1.13) or all-cause death (324 events vs. 289 events, RR 1.13; 95% CI,0.99-1.29).
Bottom line: Positive airway pressure treatment for patients with sleep apnea is not an intervention to prevent cardiovascular morbidity.
Citation: Yu J et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea. JAMA. 2017 Jul 11;318(2):156-66.
Dr. Sata is a medical instructor, Duke University Hospital.
Clinical question: In patients with sleep apnea, does using positive airway pressure (PAP) treatment prevent adverse cardiovascular events and death?
Background: Previous observational studies have suggested that untreated sleep apnea is a factor in cardiopulmonary morbidity as well as cerebrovascular events. Guidelines advise its use for prevention of cerebrovascular events. However, not enough is known from trials about its impact on prevention of cardiovascular events.
Synopsis: The authors analyzed 10 randomized-controlled trials encompassing 7,266 patients with sleep apnea. They examined instances of major adverse cardiovascular events (MACE; acute coronary syndrome, stroke, cardiovascular death) as well as hospitalization for unstable angina and all-cause deaths, among others. They found no association between treatment with positive airway pressure and MACEs (169 events vs. 187 events, with a relative risk of 0.77; 95% confidence interval, 0.53-1.13) or all-cause death (324 events vs. 289 events, RR 1.13; 95% CI,0.99-1.29).
Bottom line: Positive airway pressure treatment for patients with sleep apnea is not an intervention to prevent cardiovascular morbidity.
Citation: Yu J et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea. JAMA. 2017 Jul 11;318(2):156-66.
Dr. Sata is a medical instructor, Duke University Hospital.
You aren’t (necessarily) a walking petri dish!
Clinical question: Does exposure to a patient with a multidrug-resistant organism result in colonization of a health care provider?
Background: Multidrug-resistant organisms (MDROs) are growing threats in our hospitals, particularly vancomycin-resistant enterococci (VRE) and resistant gram-negative bacteria. The role of the health care team in preventing infection transmission is paramount. If a team member who is caring for a patient with an MDRO or handling lab specimens becomes colonized with these bacteria, he or she could potentially transmit them to the next patient.
Setting: Large academic research hospital.
Synopsis: Staff submitted self-collected rectal swabs, which were then cultured for MDROs. 379 health care personnel (which they defined as having had self-reported exposure to MDROs) were compared with 376 staff members in the control group, who reported no exposure to MDROs. There was a nonsignificant difference between growth of multidrug-resistant organisms between the groups (4.0% vs 3.2%).
Bottom line: This study suggests that occupational exposure to an MDRO does not result in subsequent colonization of the health care provider and may not be a major risk factor for nosocomial transmission.
Citation: Decker BK et al. Healthcare personnel intestinal colonization with multidrug-resistant organisms. Clin Microbiol Infect. 2017 May 12. pii:S1198-743X(17)30270-7.
Dr. Sata is a medical instructor, Duke University Hospital.
Clinical question: Does exposure to a patient with a multidrug-resistant organism result in colonization of a health care provider?
Background: Multidrug-resistant organisms (MDROs) are growing threats in our hospitals, particularly vancomycin-resistant enterococci (VRE) and resistant gram-negative bacteria. The role of the health care team in preventing infection transmission is paramount. If a team member who is caring for a patient with an MDRO or handling lab specimens becomes colonized with these bacteria, he or she could potentially transmit them to the next patient.
Setting: Large academic research hospital.
Synopsis: Staff submitted self-collected rectal swabs, which were then cultured for MDROs. 379 health care personnel (which they defined as having had self-reported exposure to MDROs) were compared with 376 staff members in the control group, who reported no exposure to MDROs. There was a nonsignificant difference between growth of multidrug-resistant organisms between the groups (4.0% vs 3.2%).
Bottom line: This study suggests that occupational exposure to an MDRO does not result in subsequent colonization of the health care provider and may not be a major risk factor for nosocomial transmission.
Citation: Decker BK et al. Healthcare personnel intestinal colonization with multidrug-resistant organisms. Clin Microbiol Infect. 2017 May 12. pii:S1198-743X(17)30270-7.
Dr. Sata is a medical instructor, Duke University Hospital.
Clinical question: Does exposure to a patient with a multidrug-resistant organism result in colonization of a health care provider?
Background: Multidrug-resistant organisms (MDROs) are growing threats in our hospitals, particularly vancomycin-resistant enterococci (VRE) and resistant gram-negative bacteria. The role of the health care team in preventing infection transmission is paramount. If a team member who is caring for a patient with an MDRO or handling lab specimens becomes colonized with these bacteria, he or she could potentially transmit them to the next patient.
Setting: Large academic research hospital.
Synopsis: Staff submitted self-collected rectal swabs, which were then cultured for MDROs. 379 health care personnel (which they defined as having had self-reported exposure to MDROs) were compared with 376 staff members in the control group, who reported no exposure to MDROs. There was a nonsignificant difference between growth of multidrug-resistant organisms between the groups (4.0% vs 3.2%).
Bottom line: This study suggests that occupational exposure to an MDRO does not result in subsequent colonization of the health care provider and may not be a major risk factor for nosocomial transmission.
Citation: Decker BK et al. Healthcare personnel intestinal colonization with multidrug-resistant organisms. Clin Microbiol Infect. 2017 May 12. pii:S1198-743X(17)30270-7.
Dr. Sata is a medical instructor, Duke University Hospital.
Metformin Continues to Be First-Line Therapy for Type 2 Diabetes
Clinical question: Which medications are most safe and effective at managing type 2 diabetes?
Background: Patients and practitioners need an updated review of the evidence to select the optimal medication for type 2 diabetes management.
Study design: Systematic review.
Synopsis: The authors reviewed 179 trials and 25 observational studies. When comparing metformin to sulfonylureas, metformin was associated with less cardiovascular mortality.
However, when trying to make comparisons based on all-cause mortality or microvascular complications, the evidence is limited. Improvements in hemoglobin A1c levels are similar when comparing different monotherapy options, and low blood sugar was most common with sulfonylureas. The short duration of many trials limits the ability to provide better data on long-term outcomes.
Bottom line: Metformin remains the first-line agent for type 2 diabetes management.
Citation: Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systemic review and meta-analysis. Ann Intern Med. 2016;164(1):740-751.
Short Take
Patients Discharge Readiness May Not Be Adequately Assessed and/or Addressed During Hospitalization
Prospective observational study found unresolved barriers to discharge were common in at least 90% of patients. Patients frequently cited issues including unresolved pain, lack of understanding around discharge plans, and ability to provide self-care.
Citation: Harrison JD, Greysen RS, Jacolbia R, Nguyen A, Auerbach AD. Not ready, not set…discharge: patient-reported barriers to discharge readiness at an academic medical center [published online ahead of print April 15, 2016]. J Hosp Med. doi:10.1002/jhm.2591.
Clinical question: Which medications are most safe and effective at managing type 2 diabetes?
Background: Patients and practitioners need an updated review of the evidence to select the optimal medication for type 2 diabetes management.
Study design: Systematic review.
Synopsis: The authors reviewed 179 trials and 25 observational studies. When comparing metformin to sulfonylureas, metformin was associated with less cardiovascular mortality.
However, when trying to make comparisons based on all-cause mortality or microvascular complications, the evidence is limited. Improvements in hemoglobin A1c levels are similar when comparing different monotherapy options, and low blood sugar was most common with sulfonylureas. The short duration of many trials limits the ability to provide better data on long-term outcomes.
Bottom line: Metformin remains the first-line agent for type 2 diabetes management.
Citation: Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systemic review and meta-analysis. Ann Intern Med. 2016;164(1):740-751.
Short Take
Patients Discharge Readiness May Not Be Adequately Assessed and/or Addressed During Hospitalization
Prospective observational study found unresolved barriers to discharge were common in at least 90% of patients. Patients frequently cited issues including unresolved pain, lack of understanding around discharge plans, and ability to provide self-care.
Citation: Harrison JD, Greysen RS, Jacolbia R, Nguyen A, Auerbach AD. Not ready, not set…discharge: patient-reported barriers to discharge readiness at an academic medical center [published online ahead of print April 15, 2016]. J Hosp Med. doi:10.1002/jhm.2591.
Clinical question: Which medications are most safe and effective at managing type 2 diabetes?
Background: Patients and practitioners need an updated review of the evidence to select the optimal medication for type 2 diabetes management.
Study design: Systematic review.
Synopsis: The authors reviewed 179 trials and 25 observational studies. When comparing metformin to sulfonylureas, metformin was associated with less cardiovascular mortality.
However, when trying to make comparisons based on all-cause mortality or microvascular complications, the evidence is limited. Improvements in hemoglobin A1c levels are similar when comparing different monotherapy options, and low blood sugar was most common with sulfonylureas. The short duration of many trials limits the ability to provide better data on long-term outcomes.
Bottom line: Metformin remains the first-line agent for type 2 diabetes management.
Citation: Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systemic review and meta-analysis. Ann Intern Med. 2016;164(1):740-751.
Short Take
Patients Discharge Readiness May Not Be Adequately Assessed and/or Addressed During Hospitalization
Prospective observational study found unresolved barriers to discharge were common in at least 90% of patients. Patients frequently cited issues including unresolved pain, lack of understanding around discharge plans, and ability to provide self-care.
Citation: Harrison JD, Greysen RS, Jacolbia R, Nguyen A, Auerbach AD. Not ready, not set…discharge: patient-reported barriers to discharge readiness at an academic medical center [published online ahead of print April 15, 2016]. J Hosp Med. doi:10.1002/jhm.2591.
Reevaluating Cardiovascular Risk after TIA
Clinical question: What is the prognosis of patients who have a TIA or minor stroke?
Background: Prior studies had estimated the risk in the three months following a TIA or minor stroke of having a stroke or acute coronary syndrome (ACS) as 12% to 20%, but this may not reflect the risk of modern patients receiving the current standards of care.
Study design: Prospective observational registry of patients with recent TIA or minor stroke.
Setting: International, including 21 countries.
Synopsis: Adults with recent TIA or minor stroke were included in this multi-center, international registry, and one-year outcomes were reported. At one year, the Kaplan-Meier estimated event rate for the combined outcome of stroke, ACS, or death from cardiovascular causes was 6.2%. The risk of the cardiovascular events was found to be lower than previously reported, suggesting an improvement in outcomes with current interventions. Elevated ABCD2 score, infarction seen on brain imaging, and large-artery atherosclerosis were each associated with higher risk.
Bottom line: Elevated ABCD2 score, brain imaging findings, and large-artery atherosclerosis suggest increased risk for recurrent stroke.
Citation: Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374(16):1533-1542.
Clinical question: What is the prognosis of patients who have a TIA or minor stroke?
Background: Prior studies had estimated the risk in the three months following a TIA or minor stroke of having a stroke or acute coronary syndrome (ACS) as 12% to 20%, but this may not reflect the risk of modern patients receiving the current standards of care.
Study design: Prospective observational registry of patients with recent TIA or minor stroke.
Setting: International, including 21 countries.
Synopsis: Adults with recent TIA or minor stroke were included in this multi-center, international registry, and one-year outcomes were reported. At one year, the Kaplan-Meier estimated event rate for the combined outcome of stroke, ACS, or death from cardiovascular causes was 6.2%. The risk of the cardiovascular events was found to be lower than previously reported, suggesting an improvement in outcomes with current interventions. Elevated ABCD2 score, infarction seen on brain imaging, and large-artery atherosclerosis were each associated with higher risk.
Bottom line: Elevated ABCD2 score, brain imaging findings, and large-artery atherosclerosis suggest increased risk for recurrent stroke.
Citation: Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374(16):1533-1542.
Clinical question: What is the prognosis of patients who have a TIA or minor stroke?
Background: Prior studies had estimated the risk in the three months following a TIA or minor stroke of having a stroke or acute coronary syndrome (ACS) as 12% to 20%, but this may not reflect the risk of modern patients receiving the current standards of care.
Study design: Prospective observational registry of patients with recent TIA or minor stroke.
Setting: International, including 21 countries.
Synopsis: Adults with recent TIA or minor stroke were included in this multi-center, international registry, and one-year outcomes were reported. At one year, the Kaplan-Meier estimated event rate for the combined outcome of stroke, ACS, or death from cardiovascular causes was 6.2%. The risk of the cardiovascular events was found to be lower than previously reported, suggesting an improvement in outcomes with current interventions. Elevated ABCD2 score, infarction seen on brain imaging, and large-artery atherosclerosis were each associated with higher risk.
Bottom line: Elevated ABCD2 score, brain imaging findings, and large-artery atherosclerosis suggest increased risk for recurrent stroke.
Citation: Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374(16):1533-1542.
Single Dose of Dexamethasone Not an Alternative to ‘Steroid Burst’ for Acute Asthma Treatment
Clinical question: Is one dose of dexamethasone comparable to five days of prednisone for treating mild-to-moderate asthma exacerbations?
Background: Corticosteroids are the mainstay of initial treatment for asthma exacerbations. The National Heart, Lung, and Blood Institute recommends a minimum of five days of prednisone, though studies have shown incomplete adherence to prolonged therapies. Dexamethasone has a longer duration of action than prednisone.
Study design: Randomized, controlled, double-blinded trial.
Setting: Urban, safety-net, teaching hospital.
Synopsis: The study included 376 adults ages 18–55 presenting to the emergency department for a mild-to-moderate asthma exacerbation who were randomized to two treatment courses of corticosteroids: one 12 mg dose of oral dexamethasone followed by four days of placebo versus five days of 60 mg of oral prednisone. Two weeks later, a telephone survey asked if they had relapsed and had to seek medical attention. This study did not show noninferiority of the dexamethasone option compared to the standard of care. Specifically, it showed a 12.1% relapse rate in the dexamethasone group versus a 9.8% relapse rate for prednisone (95% CI, -4.1% to 8.6%).
This was a small study looking at adults without other chronic lung diseases or diabetes. The authors did not include those patients who were either lost to follow-up (20% of those initially randomized) or ultimately admitted after their emergency department course.
Hospitalists who care for patients with asthma should look to the current standards of corticosteroid selection and duration to minimize clinical relapses and possibly readmissions.
Bottom line: One large dose of dexamethasone is inferior to the standard five days of prednisone for treating acute asthma exacerbations in adults.
Citation: Rehrer MW, Liu B, Rodriguez M, Lam J, Alter HJ. A randomized controlled noninferiority trial of single dose of oral dexamethasone versus 5 days of oral prednisone in acute adult asthma [published online ahead of print April 14, 2016]. Ann Emerg Med. doi:10.1016/j.annemergmed.2016.03.017.
Short Take
Guideline Recommends ED Asthma Management Associated with Shorter Inpatient Stay
Observational study found ED treatment concordance with four guideline-based processes for acute asthma treatment (inhaled beta-agonists, inhaled anticholinergics, systemic corticosteroids, and avoidance of methylxanthines) is associated with a 17% shorter hospital length of stay.
Citation: Hasegawa K, Brenner BE, Nowak RM, et al. Association of guideline-concordant acute asthma care in the emergency department with shorter hospital length of stay: a multicenter observational study. Acad Emerg Med. 2016;23(5):616-622.
Clinical question: Is one dose of dexamethasone comparable to five days of prednisone for treating mild-to-moderate asthma exacerbations?
Background: Corticosteroids are the mainstay of initial treatment for asthma exacerbations. The National Heart, Lung, and Blood Institute recommends a minimum of five days of prednisone, though studies have shown incomplete adherence to prolonged therapies. Dexamethasone has a longer duration of action than prednisone.
Study design: Randomized, controlled, double-blinded trial.
Setting: Urban, safety-net, teaching hospital.
Synopsis: The study included 376 adults ages 18–55 presenting to the emergency department for a mild-to-moderate asthma exacerbation who were randomized to two treatment courses of corticosteroids: one 12 mg dose of oral dexamethasone followed by four days of placebo versus five days of 60 mg of oral prednisone. Two weeks later, a telephone survey asked if they had relapsed and had to seek medical attention. This study did not show noninferiority of the dexamethasone option compared to the standard of care. Specifically, it showed a 12.1% relapse rate in the dexamethasone group versus a 9.8% relapse rate for prednisone (95% CI, -4.1% to 8.6%).
This was a small study looking at adults without other chronic lung diseases or diabetes. The authors did not include those patients who were either lost to follow-up (20% of those initially randomized) or ultimately admitted after their emergency department course.
Hospitalists who care for patients with asthma should look to the current standards of corticosteroid selection and duration to minimize clinical relapses and possibly readmissions.
Bottom line: One large dose of dexamethasone is inferior to the standard five days of prednisone for treating acute asthma exacerbations in adults.
Citation: Rehrer MW, Liu B, Rodriguez M, Lam J, Alter HJ. A randomized controlled noninferiority trial of single dose of oral dexamethasone versus 5 days of oral prednisone in acute adult asthma [published online ahead of print April 14, 2016]. Ann Emerg Med. doi:10.1016/j.annemergmed.2016.03.017.
Short Take
Guideline Recommends ED Asthma Management Associated with Shorter Inpatient Stay
Observational study found ED treatment concordance with four guideline-based processes for acute asthma treatment (inhaled beta-agonists, inhaled anticholinergics, systemic corticosteroids, and avoidance of methylxanthines) is associated with a 17% shorter hospital length of stay.
Citation: Hasegawa K, Brenner BE, Nowak RM, et al. Association of guideline-concordant acute asthma care in the emergency department with shorter hospital length of stay: a multicenter observational study. Acad Emerg Med. 2016;23(5):616-622.
Clinical question: Is one dose of dexamethasone comparable to five days of prednisone for treating mild-to-moderate asthma exacerbations?
Background: Corticosteroids are the mainstay of initial treatment for asthma exacerbations. The National Heart, Lung, and Blood Institute recommends a minimum of five days of prednisone, though studies have shown incomplete adherence to prolonged therapies. Dexamethasone has a longer duration of action than prednisone.
Study design: Randomized, controlled, double-blinded trial.
Setting: Urban, safety-net, teaching hospital.
Synopsis: The study included 376 adults ages 18–55 presenting to the emergency department for a mild-to-moderate asthma exacerbation who were randomized to two treatment courses of corticosteroids: one 12 mg dose of oral dexamethasone followed by four days of placebo versus five days of 60 mg of oral prednisone. Two weeks later, a telephone survey asked if they had relapsed and had to seek medical attention. This study did not show noninferiority of the dexamethasone option compared to the standard of care. Specifically, it showed a 12.1% relapse rate in the dexamethasone group versus a 9.8% relapse rate for prednisone (95% CI, -4.1% to 8.6%).
This was a small study looking at adults without other chronic lung diseases or diabetes. The authors did not include those patients who were either lost to follow-up (20% of those initially randomized) or ultimately admitted after their emergency department course.
Hospitalists who care for patients with asthma should look to the current standards of corticosteroid selection and duration to minimize clinical relapses and possibly readmissions.
Bottom line: One large dose of dexamethasone is inferior to the standard five days of prednisone for treating acute asthma exacerbations in adults.
Citation: Rehrer MW, Liu B, Rodriguez M, Lam J, Alter HJ. A randomized controlled noninferiority trial of single dose of oral dexamethasone versus 5 days of oral prednisone in acute adult asthma [published online ahead of print April 14, 2016]. Ann Emerg Med. doi:10.1016/j.annemergmed.2016.03.017.
Short Take
Guideline Recommends ED Asthma Management Associated with Shorter Inpatient Stay
Observational study found ED treatment concordance with four guideline-based processes for acute asthma treatment (inhaled beta-agonists, inhaled anticholinergics, systemic corticosteroids, and avoidance of methylxanthines) is associated with a 17% shorter hospital length of stay.
Citation: Hasegawa K, Brenner BE, Nowak RM, et al. Association of guideline-concordant acute asthma care in the emergency department with shorter hospital length of stay: a multicenter observational study. Acad Emerg Med. 2016;23(5):616-622.
Interhospital Transfer Handoff Practice Variance at U.S. Tertiary Care Centers
Clinical question: How do interhospital handoff practices differ among U.S. tertiary care centers, and what challenges and innovations have providers encountered?
Background: Little has been studied regarding interhospital transfers. Many centers differ in the processes they follow, and well-delineated national guidelines are lacking. Adverse events occur in up to 30% of transfers. Standardization of these handoffs has been shown to reduce preventable errors and near misses.
Study design: Survey of convenience sample of institutions.
Setting: Transfer center directors from 32 tertiary care centers in the U.S.
Synopsis: The authors surveyed directors of 32 transfer centers between 2013 and 2015. Hospitals were selected from a nationally ranked list as well as those comparable to the authors’ own institutions. The median number of patients transferred per month was 700.
Only 23% of hospitals surveyed identified significant EHR interoperability. Almost all required three-way recorded discussion between transfer center staff and referring and accepting physicians. Only 29% had available objective clinical information to share. Only 23% recorded a three-way nursing handoff, and only 32% used their EHR to document the transfer process and share clinical information among providers.
Innovations included electronic transfer notes, a standardized system of feedback to referring hospitals, automatic internal review for adverse events and delayed transfers, and use of a scorecard with key measures shared with stakeholders.
Barriers noted included complexity, acuity, and lack of continuity. Increased use of EHRs, checklists, and common processes were identified as best practices.
Limitations of the study included reliance on verbal qualitative data, a single investigator doing most of the discussions, and possible sampling bias.
Bottom line: Interhospital transfer practices at academic tertiary care centers vary widely, and optimizing and aligning practices between sending and receiving hospitals may improve efficiency and patient outcomes.
References: Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11(6):413-417.
Clinical question: How do interhospital handoff practices differ among U.S. tertiary care centers, and what challenges and innovations have providers encountered?
Background: Little has been studied regarding interhospital transfers. Many centers differ in the processes they follow, and well-delineated national guidelines are lacking. Adverse events occur in up to 30% of transfers. Standardization of these handoffs has been shown to reduce preventable errors and near misses.
Study design: Survey of convenience sample of institutions.
Setting: Transfer center directors from 32 tertiary care centers in the U.S.
Synopsis: The authors surveyed directors of 32 transfer centers between 2013 and 2015. Hospitals were selected from a nationally ranked list as well as those comparable to the authors’ own institutions. The median number of patients transferred per month was 700.
Only 23% of hospitals surveyed identified significant EHR interoperability. Almost all required three-way recorded discussion between transfer center staff and referring and accepting physicians. Only 29% had available objective clinical information to share. Only 23% recorded a three-way nursing handoff, and only 32% used their EHR to document the transfer process and share clinical information among providers.
Innovations included electronic transfer notes, a standardized system of feedback to referring hospitals, automatic internal review for adverse events and delayed transfers, and use of a scorecard with key measures shared with stakeholders.
Barriers noted included complexity, acuity, and lack of continuity. Increased use of EHRs, checklists, and common processes were identified as best practices.
Limitations of the study included reliance on verbal qualitative data, a single investigator doing most of the discussions, and possible sampling bias.
Bottom line: Interhospital transfer practices at academic tertiary care centers vary widely, and optimizing and aligning practices between sending and receiving hospitals may improve efficiency and patient outcomes.
References: Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11(6):413-417.
Clinical question: How do interhospital handoff practices differ among U.S. tertiary care centers, and what challenges and innovations have providers encountered?
Background: Little has been studied regarding interhospital transfers. Many centers differ in the processes they follow, and well-delineated national guidelines are lacking. Adverse events occur in up to 30% of transfers. Standardization of these handoffs has been shown to reduce preventable errors and near misses.
Study design: Survey of convenience sample of institutions.
Setting: Transfer center directors from 32 tertiary care centers in the U.S.
Synopsis: The authors surveyed directors of 32 transfer centers between 2013 and 2015. Hospitals were selected from a nationally ranked list as well as those comparable to the authors’ own institutions. The median number of patients transferred per month was 700.
Only 23% of hospitals surveyed identified significant EHR interoperability. Almost all required three-way recorded discussion between transfer center staff and referring and accepting physicians. Only 29% had available objective clinical information to share. Only 23% recorded a three-way nursing handoff, and only 32% used their EHR to document the transfer process and share clinical information among providers.
Innovations included electronic transfer notes, a standardized system of feedback to referring hospitals, automatic internal review for adverse events and delayed transfers, and use of a scorecard with key measures shared with stakeholders.
Barriers noted included complexity, acuity, and lack of continuity. Increased use of EHRs, checklists, and common processes were identified as best practices.
Limitations of the study included reliance on verbal qualitative data, a single investigator doing most of the discussions, and possible sampling bias.
Bottom line: Interhospital transfer practices at academic tertiary care centers vary widely, and optimizing and aligning practices between sending and receiving hospitals may improve efficiency and patient outcomes.
References: Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11(6):413-417.















