User login
Fatigue, arthalgia, amenorrhea—Dx?
THE CASE
A 46-year-old Caucasian female with a history of epilepsy came into our family medicine center complaining of weakness, fatigue, and arthralgia that made it difficult for her to walk. She’d had these symptoms for 6 months and reported having amenorrhea and hot flashes for the past 2 years.
The patient’s blood pressure was 133/72 mm Hg, heart rate was 82 beats per min, and respiratory rate was 20 breaths per min. Her skin was dry without hyperpigmentation, and her sclerae were anicteric. A musculoskeletal examination revealed tenderness of the metacarpophalangeal and metatarsophalangeal joints without edema, deformity, or evidence of synovitis.
She had no history of skin bronzing, jaundice, transfusions, hepatitis, abdominal pain, or diabetes and denied using tobacco, alcohol, or illicit drugs. Her medications included lamotrigine (250 mg BID) and over-the-counter iron supplementation. She had no family history of rheumatoid arthritis, lupus, cirrhosis, hemochromatosis, or other liver disease. Her mother died from colorectal cancer and her father’s cause of death was unknown; her sisters did not have any medical issues. The patient’s lab tests were normal, except for the following: aspartate aminotransferase, 89 U/L (normal, 13-45 U/L); alanine aminotransferase, 80 U/L (normal, 5-57 U/L); and alkaline phosphatase, 132 U/L (normal, 39-117 U/L). Her coagulation panel revealed a prothrombin time of 13.1 seconds, and an international normalized ratio of 1.3. Serology was negative for hepatitis A, B, and C. Additional testing revealed the following: ferritin, 4014.1 ng/dL (normal, 7-282 ng/dL); iron, 210 mg/dL (normal, 40-170 mg/dL); total iron binding capacity, 258 mg/dL (normal, 260-445 mg/dL); and transferrin saturation, 81% (normal, 20%-55%).
Abdominal ultrasonography revealed gallstones, an enlarged spleen, a dilated portal vein, and a fatty liver consistent with cirrhosis. X-rays showed soft-tissue swelling and demineralization in her hands consistent with osteopenia and degenerative arthritis in both feet.
THE DIAGNOSIS
Based on our patient’s complaints of fatigue, weakness, arthralgia, and amenorrhea, as well as her abnormal iron levels, we suspected hereditary hemochromatosis (HH). We ordered HFE genotyping, and the results indicated that the patient was homozygous for the C282Y mutation, confirming our diagnosis.
DISCUSSION
HH is an autosomal recessive disorder of iron homeostasis characterized by increased gastrointestinal iron absorption and tissue deposition of iron. It is caused by mutations in the HFE gene (C282Y or H63D) located on chromosome 6 (locus 6p21) and commonly seen in Northern European Caucasians.1 Approximately 85% of patients with HH are homozygous for C282Y; the H63D mutation can cause HH when in the presence of a single C282Y mutation.1 Men manifest HH symptoms usually between the ages of 40 and 60 years,2 although women may be affected at a later age than men because physiologic blood loss from menstruation and parturition limit the rate at which excess iron is accumulated.2
|
Signs and symptoms of HH include depression, fatigue, restless legs syndrome, weakness, and weight loss.3 In advanced HH, patients may develop progressive skin pigmentation or bronzing, and hypogonadism. Advanced HH can affect the patient’s organs, including the pancreas (diabetes), liver (hepatomegaly, abnormal liver function tests), pituitary gland (amenorrhea, decreased libido, erectile dysfunction), and heart (arrhythmias, congestive heart failure), as well as the musculoskeletal system (joint pain).3,4 The spleen can also be affected after cirrhosis develops. Cirrhosis, hepatocellular carcinoma, and cardiomyopathy can reduce life expectancy.4
Testing for HH
Because symptoms of HH are common and nonspecific, a high degree of clinical suspicion is required for early diagnosis. The differential diagnosis includes conditions related to chronic liver disease or iron overload (TABLE).5 If the diagnosis goes undetected until complications arise, the risk of morbidity and mortality are greatly increased.5
If HH is suspected, serum ferritin concentration and fasting serum transferrin saturation (the ratio of serum iron level to total iron-binding capacity × 100) are recommended as initial tests.5 The normal range of transferrin saturation for males is 15% to 50% and the normal range for females is 12% to 45%. If the transferrin saturation exceeds 50% in women or 60% in men, further evaluation is warranted (FIGURE 1).6,7 The sensitivity and specificity of elevated transferrin saturation for HH are 92% and 93%, respectively.5 These transferrin saturation cutoffs don’t apply to patients with a history of frequent blood transfusion (ie, patients with sickle cell disease or thalassemia).
Additional testing for patients in whom you suspect HH includes:
• a complete blood count, metabolic panel, and coagulation panel
• hepatitis serologies
• imaging (abdominal ultrasound, skeletal radiographs, echocardiogram, abdominal magnetic resonance imaging [MRI])
• a liver biopsy with iron staining and quantitative iron measurements.
The gold standard. Performing a liver biopsy to measure hepatic iron concentration by staining is considered the gold standard test for HH.8 But since genetic testing has become more readily available, liver biopsies aren’t widely used to confirm the diagnosis.8 The diagnosis of HH usually is confirmed by molecular testing for the C282Y and H63D mutations. Liver biopsy may be recommended to document the degree of fibrosis in all homozygotes over age 40 with elevated serum transaminase levels, clinical evidence of liver disease, or a serum ferritin level >1000 mcg/L.7
Phlebotomy helps lower iron levels
Treatment should not be delayed until symptoms develop.3 The mainstay of therapy is phlebotomy.9 If phlebotomy is started before the onset of organ damage, patients can anticipate a normal lifespan.9 Without treatment death may occur from cirrhosis, hepatocellular carcinoma, or cardiomyopathy.
Removal of 1 unit of red blood cells (450-500 mL) results in the loss of approximately 200 mg of iron. Serum ferritin level testing is the most reliable and least expensive method to monitor therapy.9 Iron depletion is complete when the serum ferritin level is 10 to 20 g/L, when the hemoglobin concentration is <11 g/dL, or the hematocrit is <33% for >3 weeks. HH patients need to undergo lifelong phlebotomy to maintain a serum ferritin level <50 g/L. Encourage patients to take in an adequate amount of dietary protein, vitamin B12, and folate to support the accelerated level of erythropoiesis that occurs during therapy.9
Chelation therapy is reserved for patients with advanced disease (eg, those with organ damage) or those who do not respond to phlebotomy.10 Deferoxamine given intravenously (IV) or subcutaneously has been the standard chelation agent. It’s usually administered by continuous subcutaneous infusion using a battery-operated pump at a dose of 40 mg/kg/d for 8 to 12 hours nightly for 5 to 7 nights weekly. A dose of approximately 2 g per 24 hours usually achieves maximal urinary iron excretion.
The use of deferoxamine therapy is limited by cost as well as the need for parenteral therapy, discomfort, inconvenience, and neurotoxicity.5 The US Food and Drug Administration recently approved an oral ironchelating agent, deferasirox, for the treatment of secondary iron overload due to ineffective erythropoiesis. Studies are ongoing to evaluate its potential use in HH.5,9
Our patient’s outcome
Our patient declined liver biopsy and her sisters declined HFE genotyping. Our patient did, however, complete 7 phlebotomies over 4 months. Two months later, she reported shortness of breath during exertion, leg swelling, and palpitations. A chest x-ray revealed a right-sided pleural effusion and an electrocardiogram showed atrial fibrillation with rapid ventricular response. Our patient was admitted for telemetry monitoring and started on diltiazem IV. Echocardiogram showed a restrictive cardiomyopathy, with an ejection fraction of 15% (normal range >55%).
Six weeks later, her ejection fraction decreased to 10%. An MRI of her abdomen showed iron deposition in her liver, pancreas, and lymph nodes (FIGURE 2). She was started on deferoxamine IV and transferred to the coronary care unit for 3 weeks. She was discharged with a diagnosis of class IV heart failure and admitted 2 weeks later for exacerbation of heart failure symptoms. She did not want to pursue a heart transplant. Her condition deteriorated and she expired after a fatal cardiac arrhythmia.
THE TAKEAWAY
Patients with abnormal iron studies and those with evidence of liver disease should be evaluated for HH5 (strength of recommendation [SOR]: A). Fasting serum transferrin saturation and serum ferritin concentration are recommended as initial tests for HH11 (SOR C). Liver biopsy is the gold standard for diagnosis of HH, but the diagnosis usually is confirmed by genetic testing8 (SOR C). Phlebotomy is the mainstay of therapy9 (SOR B). Chelation therapy is reserved for patients with advanced disease or for those who do not respond to phlebotomy10 (SOR C).
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
1. Matthews AL, Grimes SJ, Wiesner GL, et al. Clinical consult: iron overload--hereditary hemochromatosis. Prim Care. 2004;31:767-770,xii-xiii.
2. Gochee PA, Powell LW. What’s new in hemochromatosis. Curr Opin Hematol. 2001;8:98-104.
3. Niederau C, Fischer R, Sonnenberg A, et al. Survival and causes of death in cirrhotic patients with primary hemochromatosis. N Engl J Med. 1985;313:1256-1262.
4. Adams PC. Hemochromatosis. Clin Liver Dis. 2004;8:735-753,vii.
5. Bacon BR, Adams PC, Kowdley KV, et al; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:328-343.
6. Brandhagen DJ, Fairbanks VF, Baldus W. Recognition and management of hereditary hemochromatosis. Am Fam Physician. 2002;65:853-860.
7. Hash RB. Hereditary hemochromatosis. J Am Board Fam Pract. 2001;14:266-273.
8. Qaseem A, Aronson M, Fitterman N, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for hereditary hemochromatosis: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2005;143:517-521.
9. Brissot P, de Bels F. Current approaches to the management of hemochromatosis. Hematology Am Soc Hematol Educ Program. 2006:36-41.
10. US Preventive Services Task Force. Screening for hemochromatosis: recommendation statement. Ann Intern Med. 2006;145:204-208.
11. Borwein S, Ghent CN, Valberg LS. Diagnostic efficacy of screening tests for hereditary hemochromatosis. Can Med Assoc J. 1984;131:895-901.
THE CASE
A 46-year-old Caucasian female with a history of epilepsy came into our family medicine center complaining of weakness, fatigue, and arthralgia that made it difficult for her to walk. She’d had these symptoms for 6 months and reported having amenorrhea and hot flashes for the past 2 years.
The patient’s blood pressure was 133/72 mm Hg, heart rate was 82 beats per min, and respiratory rate was 20 breaths per min. Her skin was dry without hyperpigmentation, and her sclerae were anicteric. A musculoskeletal examination revealed tenderness of the metacarpophalangeal and metatarsophalangeal joints without edema, deformity, or evidence of synovitis.
She had no history of skin bronzing, jaundice, transfusions, hepatitis, abdominal pain, or diabetes and denied using tobacco, alcohol, or illicit drugs. Her medications included lamotrigine (250 mg BID) and over-the-counter iron supplementation. She had no family history of rheumatoid arthritis, lupus, cirrhosis, hemochromatosis, or other liver disease. Her mother died from colorectal cancer and her father’s cause of death was unknown; her sisters did not have any medical issues. The patient’s lab tests were normal, except for the following: aspartate aminotransferase, 89 U/L (normal, 13-45 U/L); alanine aminotransferase, 80 U/L (normal, 5-57 U/L); and alkaline phosphatase, 132 U/L (normal, 39-117 U/L). Her coagulation panel revealed a prothrombin time of 13.1 seconds, and an international normalized ratio of 1.3. Serology was negative for hepatitis A, B, and C. Additional testing revealed the following: ferritin, 4014.1 ng/dL (normal, 7-282 ng/dL); iron, 210 mg/dL (normal, 40-170 mg/dL); total iron binding capacity, 258 mg/dL (normal, 260-445 mg/dL); and transferrin saturation, 81% (normal, 20%-55%).
Abdominal ultrasonography revealed gallstones, an enlarged spleen, a dilated portal vein, and a fatty liver consistent with cirrhosis. X-rays showed soft-tissue swelling and demineralization in her hands consistent with osteopenia and degenerative arthritis in both feet.
THE DIAGNOSIS
Based on our patient’s complaints of fatigue, weakness, arthralgia, and amenorrhea, as well as her abnormal iron levels, we suspected hereditary hemochromatosis (HH). We ordered HFE genotyping, and the results indicated that the patient was homozygous for the C282Y mutation, confirming our diagnosis.
DISCUSSION
HH is an autosomal recessive disorder of iron homeostasis characterized by increased gastrointestinal iron absorption and tissue deposition of iron. It is caused by mutations in the HFE gene (C282Y or H63D) located on chromosome 6 (locus 6p21) and commonly seen in Northern European Caucasians.1 Approximately 85% of patients with HH are homozygous for C282Y; the H63D mutation can cause HH when in the presence of a single C282Y mutation.1 Men manifest HH symptoms usually between the ages of 40 and 60 years,2 although women may be affected at a later age than men because physiologic blood loss from menstruation and parturition limit the rate at which excess iron is accumulated.2
|
Signs and symptoms of HH include depression, fatigue, restless legs syndrome, weakness, and weight loss.3 In advanced HH, patients may develop progressive skin pigmentation or bronzing, and hypogonadism. Advanced HH can affect the patient’s organs, including the pancreas (diabetes), liver (hepatomegaly, abnormal liver function tests), pituitary gland (amenorrhea, decreased libido, erectile dysfunction), and heart (arrhythmias, congestive heart failure), as well as the musculoskeletal system (joint pain).3,4 The spleen can also be affected after cirrhosis develops. Cirrhosis, hepatocellular carcinoma, and cardiomyopathy can reduce life expectancy.4
Testing for HH
Because symptoms of HH are common and nonspecific, a high degree of clinical suspicion is required for early diagnosis. The differential diagnosis includes conditions related to chronic liver disease or iron overload (TABLE).5 If the diagnosis goes undetected until complications arise, the risk of morbidity and mortality are greatly increased.5
If HH is suspected, serum ferritin concentration and fasting serum transferrin saturation (the ratio of serum iron level to total iron-binding capacity × 100) are recommended as initial tests.5 The normal range of transferrin saturation for males is 15% to 50% and the normal range for females is 12% to 45%. If the transferrin saturation exceeds 50% in women or 60% in men, further evaluation is warranted (FIGURE 1).6,7 The sensitivity and specificity of elevated transferrin saturation for HH are 92% and 93%, respectively.5 These transferrin saturation cutoffs don’t apply to patients with a history of frequent blood transfusion (ie, patients with sickle cell disease or thalassemia).
Additional testing for patients in whom you suspect HH includes:
• a complete blood count, metabolic panel, and coagulation panel
• hepatitis serologies
• imaging (abdominal ultrasound, skeletal radiographs, echocardiogram, abdominal magnetic resonance imaging [MRI])
• a liver biopsy with iron staining and quantitative iron measurements.
The gold standard. Performing a liver biopsy to measure hepatic iron concentration by staining is considered the gold standard test for HH.8 But since genetic testing has become more readily available, liver biopsies aren’t widely used to confirm the diagnosis.8 The diagnosis of HH usually is confirmed by molecular testing for the C282Y and H63D mutations. Liver biopsy may be recommended to document the degree of fibrosis in all homozygotes over age 40 with elevated serum transaminase levels, clinical evidence of liver disease, or a serum ferritin level >1000 mcg/L.7
Phlebotomy helps lower iron levels
Treatment should not be delayed until symptoms develop.3 The mainstay of therapy is phlebotomy.9 If phlebotomy is started before the onset of organ damage, patients can anticipate a normal lifespan.9 Without treatment death may occur from cirrhosis, hepatocellular carcinoma, or cardiomyopathy.
Removal of 1 unit of red blood cells (450-500 mL) results in the loss of approximately 200 mg of iron. Serum ferritin level testing is the most reliable and least expensive method to monitor therapy.9 Iron depletion is complete when the serum ferritin level is 10 to 20 g/L, when the hemoglobin concentration is <11 g/dL, or the hematocrit is <33% for >3 weeks. HH patients need to undergo lifelong phlebotomy to maintain a serum ferritin level <50 g/L. Encourage patients to take in an adequate amount of dietary protein, vitamin B12, and folate to support the accelerated level of erythropoiesis that occurs during therapy.9
Chelation therapy is reserved for patients with advanced disease (eg, those with organ damage) or those who do not respond to phlebotomy.10 Deferoxamine given intravenously (IV) or subcutaneously has been the standard chelation agent. It’s usually administered by continuous subcutaneous infusion using a battery-operated pump at a dose of 40 mg/kg/d for 8 to 12 hours nightly for 5 to 7 nights weekly. A dose of approximately 2 g per 24 hours usually achieves maximal urinary iron excretion.
The use of deferoxamine therapy is limited by cost as well as the need for parenteral therapy, discomfort, inconvenience, and neurotoxicity.5 The US Food and Drug Administration recently approved an oral ironchelating agent, deferasirox, for the treatment of secondary iron overload due to ineffective erythropoiesis. Studies are ongoing to evaluate its potential use in HH.5,9
Our patient’s outcome
Our patient declined liver biopsy and her sisters declined HFE genotyping. Our patient did, however, complete 7 phlebotomies over 4 months. Two months later, she reported shortness of breath during exertion, leg swelling, and palpitations. A chest x-ray revealed a right-sided pleural effusion and an electrocardiogram showed atrial fibrillation with rapid ventricular response. Our patient was admitted for telemetry monitoring and started on diltiazem IV. Echocardiogram showed a restrictive cardiomyopathy, with an ejection fraction of 15% (normal range >55%).
Six weeks later, her ejection fraction decreased to 10%. An MRI of her abdomen showed iron deposition in her liver, pancreas, and lymph nodes (FIGURE 2). She was started on deferoxamine IV and transferred to the coronary care unit for 3 weeks. She was discharged with a diagnosis of class IV heart failure and admitted 2 weeks later for exacerbation of heart failure symptoms. She did not want to pursue a heart transplant. Her condition deteriorated and she expired after a fatal cardiac arrhythmia.
THE TAKEAWAY
Patients with abnormal iron studies and those with evidence of liver disease should be evaluated for HH5 (strength of recommendation [SOR]: A). Fasting serum transferrin saturation and serum ferritin concentration are recommended as initial tests for HH11 (SOR C). Liver biopsy is the gold standard for diagnosis of HH, but the diagnosis usually is confirmed by genetic testing8 (SOR C). Phlebotomy is the mainstay of therapy9 (SOR B). Chelation therapy is reserved for patients with advanced disease or for those who do not respond to phlebotomy10 (SOR C).
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
THE CASE
A 46-year-old Caucasian female with a history of epilepsy came into our family medicine center complaining of weakness, fatigue, and arthralgia that made it difficult for her to walk. She’d had these symptoms for 6 months and reported having amenorrhea and hot flashes for the past 2 years.
The patient’s blood pressure was 133/72 mm Hg, heart rate was 82 beats per min, and respiratory rate was 20 breaths per min. Her skin was dry without hyperpigmentation, and her sclerae were anicteric. A musculoskeletal examination revealed tenderness of the metacarpophalangeal and metatarsophalangeal joints without edema, deformity, or evidence of synovitis.
She had no history of skin bronzing, jaundice, transfusions, hepatitis, abdominal pain, or diabetes and denied using tobacco, alcohol, or illicit drugs. Her medications included lamotrigine (250 mg BID) and over-the-counter iron supplementation. She had no family history of rheumatoid arthritis, lupus, cirrhosis, hemochromatosis, or other liver disease. Her mother died from colorectal cancer and her father’s cause of death was unknown; her sisters did not have any medical issues. The patient’s lab tests were normal, except for the following: aspartate aminotransferase, 89 U/L (normal, 13-45 U/L); alanine aminotransferase, 80 U/L (normal, 5-57 U/L); and alkaline phosphatase, 132 U/L (normal, 39-117 U/L). Her coagulation panel revealed a prothrombin time of 13.1 seconds, and an international normalized ratio of 1.3. Serology was negative for hepatitis A, B, and C. Additional testing revealed the following: ferritin, 4014.1 ng/dL (normal, 7-282 ng/dL); iron, 210 mg/dL (normal, 40-170 mg/dL); total iron binding capacity, 258 mg/dL (normal, 260-445 mg/dL); and transferrin saturation, 81% (normal, 20%-55%).
Abdominal ultrasonography revealed gallstones, an enlarged spleen, a dilated portal vein, and a fatty liver consistent with cirrhosis. X-rays showed soft-tissue swelling and demineralization in her hands consistent with osteopenia and degenerative arthritis in both feet.
THE DIAGNOSIS
Based on our patient’s complaints of fatigue, weakness, arthralgia, and amenorrhea, as well as her abnormal iron levels, we suspected hereditary hemochromatosis (HH). We ordered HFE genotyping, and the results indicated that the patient was homozygous for the C282Y mutation, confirming our diagnosis.
DISCUSSION
HH is an autosomal recessive disorder of iron homeostasis characterized by increased gastrointestinal iron absorption and tissue deposition of iron. It is caused by mutations in the HFE gene (C282Y or H63D) located on chromosome 6 (locus 6p21) and commonly seen in Northern European Caucasians.1 Approximately 85% of patients with HH are homozygous for C282Y; the H63D mutation can cause HH when in the presence of a single C282Y mutation.1 Men manifest HH symptoms usually between the ages of 40 and 60 years,2 although women may be affected at a later age than men because physiologic blood loss from menstruation and parturition limit the rate at which excess iron is accumulated.2
|
Signs and symptoms of HH include depression, fatigue, restless legs syndrome, weakness, and weight loss.3 In advanced HH, patients may develop progressive skin pigmentation or bronzing, and hypogonadism. Advanced HH can affect the patient’s organs, including the pancreas (diabetes), liver (hepatomegaly, abnormal liver function tests), pituitary gland (amenorrhea, decreased libido, erectile dysfunction), and heart (arrhythmias, congestive heart failure), as well as the musculoskeletal system (joint pain).3,4 The spleen can also be affected after cirrhosis develops. Cirrhosis, hepatocellular carcinoma, and cardiomyopathy can reduce life expectancy.4
Testing for HH
Because symptoms of HH are common and nonspecific, a high degree of clinical suspicion is required for early diagnosis. The differential diagnosis includes conditions related to chronic liver disease or iron overload (TABLE).5 If the diagnosis goes undetected until complications arise, the risk of morbidity and mortality are greatly increased.5
If HH is suspected, serum ferritin concentration and fasting serum transferrin saturation (the ratio of serum iron level to total iron-binding capacity × 100) are recommended as initial tests.5 The normal range of transferrin saturation for males is 15% to 50% and the normal range for females is 12% to 45%. If the transferrin saturation exceeds 50% in women or 60% in men, further evaluation is warranted (FIGURE 1).6,7 The sensitivity and specificity of elevated transferrin saturation for HH are 92% and 93%, respectively.5 These transferrin saturation cutoffs don’t apply to patients with a history of frequent blood transfusion (ie, patients with sickle cell disease or thalassemia).
Additional testing for patients in whom you suspect HH includes:
• a complete blood count, metabolic panel, and coagulation panel
• hepatitis serologies
• imaging (abdominal ultrasound, skeletal radiographs, echocardiogram, abdominal magnetic resonance imaging [MRI])
• a liver biopsy with iron staining and quantitative iron measurements.
The gold standard. Performing a liver biopsy to measure hepatic iron concentration by staining is considered the gold standard test for HH.8 But since genetic testing has become more readily available, liver biopsies aren’t widely used to confirm the diagnosis.8 The diagnosis of HH usually is confirmed by molecular testing for the C282Y and H63D mutations. Liver biopsy may be recommended to document the degree of fibrosis in all homozygotes over age 40 with elevated serum transaminase levels, clinical evidence of liver disease, or a serum ferritin level >1000 mcg/L.7
Phlebotomy helps lower iron levels
Treatment should not be delayed until symptoms develop.3 The mainstay of therapy is phlebotomy.9 If phlebotomy is started before the onset of organ damage, patients can anticipate a normal lifespan.9 Without treatment death may occur from cirrhosis, hepatocellular carcinoma, or cardiomyopathy.
Removal of 1 unit of red blood cells (450-500 mL) results in the loss of approximately 200 mg of iron. Serum ferritin level testing is the most reliable and least expensive method to monitor therapy.9 Iron depletion is complete when the serum ferritin level is 10 to 20 g/L, when the hemoglobin concentration is <11 g/dL, or the hematocrit is <33% for >3 weeks. HH patients need to undergo lifelong phlebotomy to maintain a serum ferritin level <50 g/L. Encourage patients to take in an adequate amount of dietary protein, vitamin B12, and folate to support the accelerated level of erythropoiesis that occurs during therapy.9
Chelation therapy is reserved for patients with advanced disease (eg, those with organ damage) or those who do not respond to phlebotomy.10 Deferoxamine given intravenously (IV) or subcutaneously has been the standard chelation agent. It’s usually administered by continuous subcutaneous infusion using a battery-operated pump at a dose of 40 mg/kg/d for 8 to 12 hours nightly for 5 to 7 nights weekly. A dose of approximately 2 g per 24 hours usually achieves maximal urinary iron excretion.
The use of deferoxamine therapy is limited by cost as well as the need for parenteral therapy, discomfort, inconvenience, and neurotoxicity.5 The US Food and Drug Administration recently approved an oral ironchelating agent, deferasirox, for the treatment of secondary iron overload due to ineffective erythropoiesis. Studies are ongoing to evaluate its potential use in HH.5,9
Our patient’s outcome
Our patient declined liver biopsy and her sisters declined HFE genotyping. Our patient did, however, complete 7 phlebotomies over 4 months. Two months later, she reported shortness of breath during exertion, leg swelling, and palpitations. A chest x-ray revealed a right-sided pleural effusion and an electrocardiogram showed atrial fibrillation with rapid ventricular response. Our patient was admitted for telemetry monitoring and started on diltiazem IV. Echocardiogram showed a restrictive cardiomyopathy, with an ejection fraction of 15% (normal range >55%).
Six weeks later, her ejection fraction decreased to 10%. An MRI of her abdomen showed iron deposition in her liver, pancreas, and lymph nodes (FIGURE 2). She was started on deferoxamine IV and transferred to the coronary care unit for 3 weeks. She was discharged with a diagnosis of class IV heart failure and admitted 2 weeks later for exacerbation of heart failure symptoms. She did not want to pursue a heart transplant. Her condition deteriorated and she expired after a fatal cardiac arrhythmia.
THE TAKEAWAY
Patients with abnormal iron studies and those with evidence of liver disease should be evaluated for HH5 (strength of recommendation [SOR]: A). Fasting serum transferrin saturation and serum ferritin concentration are recommended as initial tests for HH11 (SOR C). Liver biopsy is the gold standard for diagnosis of HH, but the diagnosis usually is confirmed by genetic testing8 (SOR C). Phlebotomy is the mainstay of therapy9 (SOR B). Chelation therapy is reserved for patients with advanced disease or for those who do not respond to phlebotomy10 (SOR C).
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
1. Matthews AL, Grimes SJ, Wiesner GL, et al. Clinical consult: iron overload--hereditary hemochromatosis. Prim Care. 2004;31:767-770,xii-xiii.
2. Gochee PA, Powell LW. What’s new in hemochromatosis. Curr Opin Hematol. 2001;8:98-104.
3. Niederau C, Fischer R, Sonnenberg A, et al. Survival and causes of death in cirrhotic patients with primary hemochromatosis. N Engl J Med. 1985;313:1256-1262.
4. Adams PC. Hemochromatosis. Clin Liver Dis. 2004;8:735-753,vii.
5. Bacon BR, Adams PC, Kowdley KV, et al; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:328-343.
6. Brandhagen DJ, Fairbanks VF, Baldus W. Recognition and management of hereditary hemochromatosis. Am Fam Physician. 2002;65:853-860.
7. Hash RB. Hereditary hemochromatosis. J Am Board Fam Pract. 2001;14:266-273.
8. Qaseem A, Aronson M, Fitterman N, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for hereditary hemochromatosis: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2005;143:517-521.
9. Brissot P, de Bels F. Current approaches to the management of hemochromatosis. Hematology Am Soc Hematol Educ Program. 2006:36-41.
10. US Preventive Services Task Force. Screening for hemochromatosis: recommendation statement. Ann Intern Med. 2006;145:204-208.
11. Borwein S, Ghent CN, Valberg LS. Diagnostic efficacy of screening tests for hereditary hemochromatosis. Can Med Assoc J. 1984;131:895-901.
1. Matthews AL, Grimes SJ, Wiesner GL, et al. Clinical consult: iron overload--hereditary hemochromatosis. Prim Care. 2004;31:767-770,xii-xiii.
2. Gochee PA, Powell LW. What’s new in hemochromatosis. Curr Opin Hematol. 2001;8:98-104.
3. Niederau C, Fischer R, Sonnenberg A, et al. Survival and causes of death in cirrhotic patients with primary hemochromatosis. N Engl J Med. 1985;313:1256-1262.
4. Adams PC. Hemochromatosis. Clin Liver Dis. 2004;8:735-753,vii.
5. Bacon BR, Adams PC, Kowdley KV, et al; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:328-343.
6. Brandhagen DJ, Fairbanks VF, Baldus W. Recognition and management of hereditary hemochromatosis. Am Fam Physician. 2002;65:853-860.
7. Hash RB. Hereditary hemochromatosis. J Am Board Fam Pract. 2001;14:266-273.
8. Qaseem A, Aronson M, Fitterman N, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for hereditary hemochromatosis: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2005;143:517-521.
9. Brissot P, de Bels F. Current approaches to the management of hemochromatosis. Hematology Am Soc Hematol Educ Program. 2006:36-41.
10. US Preventive Services Task Force. Screening for hemochromatosis: recommendation statement. Ann Intern Med. 2006;145:204-208.
11. Borwein S, Ghent CN, Valberg LS. Diagnostic efficacy of screening tests for hereditary hemochromatosis. Can Med Assoc J. 1984;131:895-901.
Comparison of thin versus standard esophagogastroduodenoscopy
OBJECTIVE: To compare the tolerance, feasibility, and safety of ultrathin esophagogastroduodenoscopy (EGD) in unsedated patients with conventional EGD in sedated patients.
STUDY DESIGN: This was an unblinded, randomized controlled trial.
POPULATION: Diagnostic EGD was performed on 72 adult outpatients at a US Air Force community hospital residency. Patients were randomized to either ultrathin or conventional EGD (n = 33 and 39, respectively).
OUTCOMES MEASURED: Patients reported their tolerance of the procedure (pain, choking, gagging, and anxiety; scale 0–10), and the endoscopist reported the effectiveness of the procedure (successful intubation, reaching duodenum, retroflexion, and duration of examination and recovery) and safety (complications).
RESULTS: No statistically significant difference was noted between the 2 groups in mean procedure time or pain during the procedure. Mean (± standard error) recovery time was approximately halved in the ultrathin group vs the conventional group (21.5 ± 2.3 min vs 55.4 ± 2.3 min, P < .0001). Although patients undergoing ultrathin EGD had higher mean gagging and choking scores, they had lower mean anxiety scores. Of 33 patients randomized to the unsedated ultrathin EGD procedure, 29 completed the protocol. The retroflexion maneuver was completed in 85% of patients in the ultrathin EGD group and 100% of patients in the conventional EGD group (P = .017). No statistically significant difference was noted between groups as to the likelihood of reaching the second portion of the duodenum (97% vs 100%).
CONCLUSIONS: Most patients tolerate ultrathin EGD with significantly shorter recovery time and less overall anxiety than with the conventioanl procedure. Techniques to reduce gagging and choking associated with ultrathin EGD may improve patient acceptance and tolerability. Adoption of ultrathin EGD by primary care physicians may decrease cost, time, and inconvenience while increasing access to EGD for many patients.
- Most patients tolerate unsedated, ultrathin esophagogastroduodenoscopy (EGD).
- The recovery time is approximately halved for ultrathin unsedated EGDs.
- Patients undergoing unsedated EGD report more gagging and choking than do patients having the sedated examination.
- Patients receiving sedated upper endoscopy report more anxiety than those receiving an unsedated examination.
- Once credentialed in upper endoscopy, physicians do not require further training or skills to perform ultrathin EGD.
Hacker et al1 reported that 3 times as many patients prefer esophagogastroduodenoscopy (EGD) to upper gastrointestinal roentgenography. EGD is safe, with complication rates between 5 and 10 per 10,000 procedures.2 However, only 2% of US family physicians perform upper endoscopy.3
In the United States, conventional EGD is usually performed under conscious sedation to reduce discomfort and anxiety.4 Conscious sedation has potential negative aspects, including costs and side effects,5 increased risks of respiratory depression,2 and, very rarely, mortality.5 Indirect costs related to lost work are unmeasured. All these factors may decrease patient tolerance or acceptance of conventional EGD with conscious sedation.
In the last decade, an ultrathin (5.3–5.9 mm diameter) fiberoptic endoscope was developed. Shaker et al6 used an ultrathin endoscope to perform transnasal endoscopy of the gastrointestinal tract. Since then, several prospective studies have evaluated ultrathin EGD.7-9 One study compared ultrathin with conventional EGD, although both groups of subjects were unsedated.10 Studies comparing EGD techniques were performed in large medical centers by gastroenterologists.7-17 The purpose of this study was to assess the feasibility, safety, and patient tolerance of unsedated ultrathin EGD by generalists in a community setting.
Methods
Outpatients (aged 20–80 years) from a US Air Force family practice residency were referred to a family practice endoscopist for further evaluation of dyspepsia, heartburn, and epigastric pain. Exclusion criteria included pregnancy, evidence of acute gastrointestinal hemorrhage, potential need for therapeutic endoscopy, a medically unstable patient (eg, recent myocardial infarction or stroke), coagulopathy, unstable angina, severe chronic obstructive pulmonary disease, and severe aortic stenosis. The local institutional review board approved the study.
An Olympus GIF-N230 gastrointestinal videoscope (Olympus America Inc, Melville, NY) with an outer diameter of 6.0 mm, an accessory channel of 2.0 mm, a working length of 122 cm, tip deflection of 180° (up and down) and 160° (right and left), with a field of view of 120° was used for the ultrathin procedures. An Olympus GIF-130 standard gastroscope (Olympus America Inc) with an outer diameter of 9.8 mm, an accessory channel of 2.8 mm, a working length of 103 cm, tip deflection of 210° up, 90° down, and 100° right and left, with a field of view of 120°, was used for the conventional procedures. Both endoscopes are forward-viewing (Figure 1).
After standard informed consent was obtained, the endoscopist made a phone call to access a computer-generated patient list for randomization. No patient withdrew after randomization. The endoscopist (T.W.) performed all endoscopies in the gastrointestinal suite with the patient in the left lateral position. An intravenous line was started and tetracaine 2% was sprayed in the posterior pharynx; pulse oximetry, cardiac monitoring, and verbal reassurance by the endoscopist were provided to patients in both groups. Sedation was slowly titrated in increments of 25 mg meperidine (or 50 mg fentanyl if the patient was allergic to meperidine) and 0.5 mg midazolam until a suitable level of sedation was obtained for endoscopy. Biopsy samples were obtained when indicated. Patients who were unable to tolerate the unsedated examination were given intravenous sedation, and the examination was completed using ultrathin EGD.
Upon discharge from the recovery area, all patients completed a questionnaire regarding their tolerance of the procedure. Patients completed 10-point visual scales indicating pain, choking, gagging, and anxiety both during the insertion of the endoscope and for the remainder of the examination. Patients were asked if they would choose to have the procedure again if endoscopy were indicated in the future. After the procedure, the endoscopist completed a questionnaire assessing the completeness of the examination (eg, whether the endoscope was advanced to the second portion of the duodenum and retroflexion was performed). Indications for the procedure, demographics, clinical findings, complications, the duration of the examination, and recovery duration were noted.
This study was planned to achieve a power of 0.80 to detect changes of 2.0 on tolerance scores between the study groups. Statistical analyses included the independent t-test, Fisher exact test, Mann-Whitney U test, and chi-square test. Multivariate regression analysis and analysis of variance were used to assess the effect that sex may have had on patients’ tolerance scores. Analysis was by intention to treat.
FIGURE 1
Ultrathin endoscope, GIF-N230 (left); standard endoscope, GIF-130 (right)
Results
Of 80 outpatients eligible for the study, 8 (10%) declined entry before randomization. Of 72 remaining, 33 were randomized to ultrathin EGD and 39 to the conventional procedure. The 2 groups were evenly matched for age, race, body mass index (BMI), indication, and EGD findings (Tables 1 and 2). There were more women in the conventional group (80% vs 55%, P = .041; Table 1).
During endoscope insertion, patients undergoing ultrathin EGD had higher mean gagging and choking scores, but lower anxiety scores. For the remainder of the procedure, ultrathin EGD patients had higher gagging scores but no statistically significant differences were noted between groups for pain, choking, or anxiety (Table 2). Twenty-nine patients (88%) assigned to the ultrathin EGD group completed the unsedated examination, and 32 (97%) were willing to repeat an unsedated procedure with the ultrathin endoscope in the future. The mean (± standard error) dose of meperidine was 48.8 ± 2.3 mg; 2 patients required fentanyl 62.5 ± 12.5 mg and midazolam 2.2 ± 0.1 mg. Of the 4 patients allocated to the ultrathin group, which required sedation, the mean dose of meperidine was 50 ± 0 mg and mida-zolam 2.8 ± 0.5 mg. The time required for sedation in the conventional group was 4.1 ± 0.6 min and in the ultrathin EGD group was 5.5 ± 0.5 min (P = .280).
The second portion of the duodenum was reached as often with the ultrathin endoscope as with the conventional apparatus (Table 2). However, retroflexion was achieved less often with ultrathin EGD than with conventional EGD (85% vs 100%, P = .017). Although examination times did not differ between groups, the recovery time was significantly shorter with ultrathin EGD (21.5 ± 2.3 min vs 55.4 ± 2.3 min, P < .0001). No complications were noted in either group. Analysis of variance and multiple regression analysis showed no statistically significant difference in the tolerance scores by sex.
TABLE 1
Patient demographics
| Characteristic | Ultrathin EGD (n = 33) | Conventional EGD (n = 39) | P |
|---|---|---|---|
| Age, y (mean ± SE) | 49.8 ± 2.9 | 46.9 ± 2.2 | .406 |
| Female (%) | 55 | 80 | .041 |
| Race (%) | |||
| Caucasian | 7 | 44 | .123 |
| African American | 27 | 39 | |
| Other | 6 | 18 | |
| Body mass index (mean ± SE) | 28.8 ± 0.88 | 28.7 ± 0.81 | .966 |
| EGD, esophagogastroduodenoscopy; SE, standard error. | |||
TABLE 2
Indications and esophagogastroduodenoscopy findings
| Ultrathin EGD | Conventional EGD | P | |
|---|---|---|---|
| Indications, n (%) | |||
| GERD | 26 (79) | 22 (56) | .050 |
| Abdominal pain | 9 (27) | 18 (46) | .143 |
| Dyspepsia | 2 (6) | 7 (18) | .166 |
| EGD findings, n (%) | |||
| Esophagitis | 9 (27) | 6 (15) | .254 |
| Hiatal hernia | 15 (45) | 13 (33) | .338 |
| Gastritis | 20 (61) | 29 (74) | .310 |
| Gastric ulcer | 1 (3) | 4 (10) | .366 |
| Duodenal ulcer | 0 | 0 | |
| CLO test positive | 4 (13) | 11 (28) | .150 |
| Patient tolerance, score (mean ±SE) | |||
| During insertion | |||
| Anxiety | 3.2 ± 0.47 | 5.7 ± 0.45 | <.0001 |
| Pain | 2.0 ± 0.34 | 1.4 ± 0.29 | .574 |
| Choking | 3.0 ± 0.42 | 1.0 ± 0.32 | .022 |
| Gagging | 4.2 ± 0.45 | 1.3 ± 0.34 | <.0001 |
| During procedure | |||
| Anxiety | 3.1 ± 0.48 | 2.5 ± 0.43 | .350 |
| Pain | 1.3 ± 0.31 | 1.2 ± 0.27 | .771 |
| Choking | 1.7 ± 0.33 | 1.0 ± 0.25 | .081 |
| Gagging | 2.4 ± 0.35 | 1.2 ± 0.28 | .007 |
| Technical aspects of procedure | |||
| To second portion of duodenum (%) | 97 | 100 | .458 |
| Retroflexed (%) | 85 | 100 | .017 |
| Duration of examination, min (mean ± SE) | 18.2 ± 0.93 | 17.5 ± 1.1 | .632 |
| Duration of recovery, min (mean ± SE) | 21.5 ± 2.3 | 55.4 ± 2.3 | <.0001 |
| EGD, esophagogastroduodenoscopy; GERD, gastroesophageal reflux disease; SE, standard error. | |||
Discussion
We examined differences in patients’ experiences during EGD when a relatively thin scope was used without sedation vs a conventional wider scope with sedation. We expected the ultrathin scope to be preferable to both patients and physicians because of the reduced risk and lower cost associated with an unsedated procedure performed in an out-patient setting.
This study has major implications for family physicians. First, ultrathin EGD requires less recovery time than the conventional procedure. In addition, unsedated endoscopy does not require continuous cardiopulmonary monitoring.18 In contrast, conventional EGD generally requires a minimum of 2 support personnel: 1 to assist the endoscopist and 1 to monitor vital signs. A third assistant is occasionally needed to monitor patients in recovery. Ultrathin EGD requires only 1 assistant. A thorough exploration of cost savings associated with ultrathin EGD was beyond the scope of this study. A recent study13 found that ultrathin EGD required less procedure time, less time in the procedure room, and less recovery time, with a cost savings of US $125 per procedure. Second, EGD is traditionally limited to being perforen type=med in gastrointestinal suites. Our findings suggested that most patients can tolerate ultrathin EGD in an outpatient setting, thereby offering easier access to the procedure.
Increased gagging and choking associated with the ultrathin device suggests that its deployment will require techniques to reduce gagging. Transnasal upper endoscopy appears to cause less gagging and choking,8,9,11,14-17,19 but has not been studied in the family practice setting. Other techniques to determine pharyngeal sensitivity are needed.20,21 One study10 found that ultrathin EGD was tolerated better than conventional EGD for unsedated examinations; investigators22 identified younger age and higher levels of pre-endoscopic anxiety as predictors of patient intolerance of unsedated endoscopy.
Although the success rate of retroflexion and duodenal intubation has not been reported in other studies6-9,11,12,14-17,19,23 of ultrathin EGD, we could not perform retroflexion in 15% of subjects in the ultrathin EGD group. Inability to retroflex was secondary to patient intolerance and increased instrument flexibility. In these patients, the endoscopist might switch to a normal diameter scope; however, in a large national study using a standard diameter endoscope, retroflexion was not performed in 7% of patients.24 The most frequent contribution of retroflexion is the identification of a dysfunctional lower esophageal sphincter. In our experience, a small fundal polyp and a large diverticulum in the cardia would be missed in the absence of this maneuver. In contrast, we were unable to intubate the duodenum in 3% of patients undergoing ultrathin EGD. Rodney and colleagues24 cited in their national study that with use of the standard endoscope, duodenal intubation was not achieved in 7% of patients. With increased experience with the ultrathin device, endoscopists may be able to develop techniques to overcome the increased flexibility (eg, using the biopsy forceps in the accessory channel to increase rigidity).
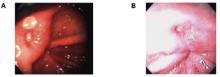
Although we found no significant differences in the proportion of clinical findings between the 2 groups, the findings may have been different had we been able to retroflex the scope in the ultrathin EGD group. The diagnostic accuracy,11,17 image quality,11,23 and adequacy of the smaller biopsy specimen for pathologic diagnosis9,25,26 for ultrathin EGD have been reviewed and consistently determined to be clinically acceptable. Image quality of the 2 techniques is comparable (Figure 2). Although the biopsy specimens obtained with the ultrathin endoscope were smaller than samples of tissue obtained with the conventional device, CLO test positivity did not differ between the groups.
Conventional EGD required more recovery time and was associated with significantly higher anxiety. It is possible that the relatively higher anxiety experienced by patients in the conventional EGD group can be explained by fear of loss of control, fear about risks related to sedation, or a combination of psychosocial factors. One limitation in the conventional EGD group was the potential bias of the seda-tion when patients responded to the postrecovery surveys. Future studies may control for this sedation-effect bias by repeated measures over a period of a few days. Another limitation of our study was that the verbal reassurance offered to patients before and during endoscopy was nonscripted and may have influenced tolerance scores. A third limitation was that the patient questionnaire was given to patients by the endoscopist, thereby possibly introducing a social desirability bias. Finally, the small sample size limited the ability to detect differences that may be clinically meaningful.
Ultrathin EGD costs less, provides similar results, and has acceptable tolerability compared with conventional EGD. Once they are EGD credentialed, clinicians do not require further training or skills to perform the procedure with the ultrathin device. As more family physicians feel comfortable performing EGD in an outpatient setting, more patients will have access to this important procedure.
FIGURE 2
View of gastric ulcer with GIF-N230 (A) and GIF-130 (B)
Acknowledgments
The investigators acknowledge Olympus for the use of a GIF-N230 gastrointestinal videoscope, which was used for the ultrathin procedures. We also acknowledge Staff Sergeant Ron O’Dell for his photograph of the endoscopes.
1. Hacker JFD, Chobanian SJ, Johnson DA, Winters C, Jr, Cattau EL, J. Patient preference in upper gastrointestinal studies: roentgenography versus endoscopy. South Med J 1987;80:1091-3.
2. Zurad EG Indications, Contraindications, and Complications of Upper Gastrointestinal Endoscopy. Kansas City, KS: American Academy of Family Physicians; 1994.
3. American Academy of Family Physicians Practice Profile II Survey. Leawood, KS: American Academy of Family Physicians; 1998.
4. Daneshmend TK, Bell GD, Logan RF. Sedation for upper gastrointestinal endoscopy: results of a nationwide survey. Gut 1991;32:12-5.
5. Mokhashi MS, Hawes RH. Struggling toward easier endoscopy [editorial]. Gastrointest Endosc 1998;48:432-40.
6. Shaker R. Unsedated trans-nasal pharyngoesophagogastroduodenoscopy (T-EGD): technique. Gastrointest Endosc 1994;40:346-8.
7. De Gregorio BT, Poorman JC, Katon RM. Peroral ultrathin endoscopy in adult patients. Gastrointest Endosc 1997;45:303-6.
8. Rey JF, Duforest D, Marek TA. Prospective comparison of nasal versus oral insertion of a thin video endoscope in healthy volunteers. Endoscopy 1996;28:422-4.
9. Dumortier J, Ponchon T, Scoazec JY, et al. Prospective evaluation of transnasal esophagogastroduodenoscopy: feasibility and study on performance and tolerance. Gastrointest Endosc 1999;49(3 Pt 1):285-91.
10. Mulcahy HE, Riches A, Kiely M, Farthing MJ, Fairclough PD. A prospective controlled trial of an ultrathin versus a conventional endoscope in unsedated upper gastrointestinal endoscopy. Endoscopy 2001;33:311-6.
11. Zaman A, Hahn M, Hapke R, Knigge K, Fennerty MB, Katon RM. A randomized trial of peroral versus transnasal unsedated endoscopy using an ultrathin videoendoscope. Gastrointest Endosc 1999;49(3 Pt 1):279-84.
12. Zaman A, Hapke R, Sahagun G, Katon RM. Unsedated peroral endoscopy with a video ultrathin endoscope: patient acceptance, tolerance, and diagnostic accuracy. Am J Gastroenterol 1998;93:1260-3.
13. Gorelick AB, Inadomi JM, Barnett JL. Unsedated small-caliber esophagogastroduodenoscopy (EGD): less expensive and less time-consuming than conventional EGD. J Clin Gastroenterol 2001;33:210-4.
14. Craig A, Hanlon J, Dent J, Schoeman M. A comparison of transnasal and transoral endoscopy with small-diameter endoscopes in unsedated patients. Gastrointest Endosc 1999;49(3 Pt 1):292-6.
15. Nozaki R, Fujiyoshi T, Tamura M, Tsuchiya A, Takagi K, Takano M. Evaluation of small caliber transnasal panendoscopes for upper GI screening examination. Dig Endosc 1995;7:155-9.
16. Campo R, Montserrat A, Brullet E. Transnasal gastroscopy compared to conventional gastroscopy: a randomized study of feasibility, safety, and tolerance. Endoscopy 1998;30:448-52.
17. Dean R, Dua K, Massey B, Berger W, Hogan WJ, Shaker R. A comparative study of unsedated transnasal esophagogastroduodenoscopy and conventional EGD. Gastrointest Endosc 1996;44:422-4.
18. Banks MR, Kumar PJ, Mulcahy HE. Pulse oximetry saturation levels during routine unsedated diagnostic upper gastrointestinal endoscopy. Scand J Gastroenterol 2001;36:105-9.
19. Bampton PA, Reid DP, Johnson RD, Fitch RJ, Dent J. A comparison of transnasal and transoral oesophagogastroduodenoscopy. J Gastroenterol Hepatol 1998;13:579-84.
20. Ladas SD, Tassios PS, Raptis SA. A simple test for predicting patients’ tolerance of upper gastrointestinal endoscopy. Endoscopy 1997;29:430.
21. Ladas SD, Raptis SA. Selection of patients for upper gastrointestinal endoscopy without sedation. The finger-throat test. Ital J Gastroenterol 1986;18:162-5.
22. Mulcahy HE, Kelly P, Banks MR, et al. Factors associated with tolerance to, and discomfort with, unsedated diagnostic gastroscopy. Scand J Gastroenterol 2001;36:1352-7.
23. Sorbi D, Gostout CJ, Henry J, Lindor KD. Unsedated small-caliber esophagogastroduodenoscopy (EGD) versus conventional EGD: a comparative study. Gastroenterology 1999;117:1301-7.
24. Rodney WM, Weber JR, Swedberg JA, et al. Esophago-gastroduodenoscopy by family physicians phase II: a national multisite study of 2,500 procedures. Fam Pract Res J 1993;13:121-31.
25. Saeian K, Townsend WF, Rochling FA, et al. Unsedated transnasal EGD: an alternative approach to conventional esophagogastroduodenoscopy for documenting Helicobacter pylori eradication. Gastrointest Endosc 1999;49(3 Pt 1):297-301.
26. Yousfi MM, El-Zimaity HM, Cole RA, Genta RM, Graham DY. Detection of Helicobacter pylori by rapid urease tests: is biopsy size a critical variable? Gastrointest Endosc 1996;43:222-4.
OBJECTIVE: To compare the tolerance, feasibility, and safety of ultrathin esophagogastroduodenoscopy (EGD) in unsedated patients with conventional EGD in sedated patients.
STUDY DESIGN: This was an unblinded, randomized controlled trial.
POPULATION: Diagnostic EGD was performed on 72 adult outpatients at a US Air Force community hospital residency. Patients were randomized to either ultrathin or conventional EGD (n = 33 and 39, respectively).
OUTCOMES MEASURED: Patients reported their tolerance of the procedure (pain, choking, gagging, and anxiety; scale 0–10), and the endoscopist reported the effectiveness of the procedure (successful intubation, reaching duodenum, retroflexion, and duration of examination and recovery) and safety (complications).
RESULTS: No statistically significant difference was noted between the 2 groups in mean procedure time or pain during the procedure. Mean (± standard error) recovery time was approximately halved in the ultrathin group vs the conventional group (21.5 ± 2.3 min vs 55.4 ± 2.3 min, P < .0001). Although patients undergoing ultrathin EGD had higher mean gagging and choking scores, they had lower mean anxiety scores. Of 33 patients randomized to the unsedated ultrathin EGD procedure, 29 completed the protocol. The retroflexion maneuver was completed in 85% of patients in the ultrathin EGD group and 100% of patients in the conventional EGD group (P = .017). No statistically significant difference was noted between groups as to the likelihood of reaching the second portion of the duodenum (97% vs 100%).
CONCLUSIONS: Most patients tolerate ultrathin EGD with significantly shorter recovery time and less overall anxiety than with the conventioanl procedure. Techniques to reduce gagging and choking associated with ultrathin EGD may improve patient acceptance and tolerability. Adoption of ultrathin EGD by primary care physicians may decrease cost, time, and inconvenience while increasing access to EGD for many patients.
- Most patients tolerate unsedated, ultrathin esophagogastroduodenoscopy (EGD).
- The recovery time is approximately halved for ultrathin unsedated EGDs.
- Patients undergoing unsedated EGD report more gagging and choking than do patients having the sedated examination.
- Patients receiving sedated upper endoscopy report more anxiety than those receiving an unsedated examination.
- Once credentialed in upper endoscopy, physicians do not require further training or skills to perform ultrathin EGD.
Hacker et al1 reported that 3 times as many patients prefer esophagogastroduodenoscopy (EGD) to upper gastrointestinal roentgenography. EGD is safe, with complication rates between 5 and 10 per 10,000 procedures.2 However, only 2% of US family physicians perform upper endoscopy.3
In the United States, conventional EGD is usually performed under conscious sedation to reduce discomfort and anxiety.4 Conscious sedation has potential negative aspects, including costs and side effects,5 increased risks of respiratory depression,2 and, very rarely, mortality.5 Indirect costs related to lost work are unmeasured. All these factors may decrease patient tolerance or acceptance of conventional EGD with conscious sedation.
In the last decade, an ultrathin (5.3–5.9 mm diameter) fiberoptic endoscope was developed. Shaker et al6 used an ultrathin endoscope to perform transnasal endoscopy of the gastrointestinal tract. Since then, several prospective studies have evaluated ultrathin EGD.7-9 One study compared ultrathin with conventional EGD, although both groups of subjects were unsedated.10 Studies comparing EGD techniques were performed in large medical centers by gastroenterologists.7-17 The purpose of this study was to assess the feasibility, safety, and patient tolerance of unsedated ultrathin EGD by generalists in a community setting.
Methods
Outpatients (aged 20–80 years) from a US Air Force family practice residency were referred to a family practice endoscopist for further evaluation of dyspepsia, heartburn, and epigastric pain. Exclusion criteria included pregnancy, evidence of acute gastrointestinal hemorrhage, potential need for therapeutic endoscopy, a medically unstable patient (eg, recent myocardial infarction or stroke), coagulopathy, unstable angina, severe chronic obstructive pulmonary disease, and severe aortic stenosis. The local institutional review board approved the study.
An Olympus GIF-N230 gastrointestinal videoscope (Olympus America Inc, Melville, NY) with an outer diameter of 6.0 mm, an accessory channel of 2.0 mm, a working length of 122 cm, tip deflection of 180° (up and down) and 160° (right and left), with a field of view of 120° was used for the ultrathin procedures. An Olympus GIF-130 standard gastroscope (Olympus America Inc) with an outer diameter of 9.8 mm, an accessory channel of 2.8 mm, a working length of 103 cm, tip deflection of 210° up, 90° down, and 100° right and left, with a field of view of 120°, was used for the conventional procedures. Both endoscopes are forward-viewing (Figure 1).
After standard informed consent was obtained, the endoscopist made a phone call to access a computer-generated patient list for randomization. No patient withdrew after randomization. The endoscopist (T.W.) performed all endoscopies in the gastrointestinal suite with the patient in the left lateral position. An intravenous line was started and tetracaine 2% was sprayed in the posterior pharynx; pulse oximetry, cardiac monitoring, and verbal reassurance by the endoscopist were provided to patients in both groups. Sedation was slowly titrated in increments of 25 mg meperidine (or 50 mg fentanyl if the patient was allergic to meperidine) and 0.5 mg midazolam until a suitable level of sedation was obtained for endoscopy. Biopsy samples were obtained when indicated. Patients who were unable to tolerate the unsedated examination were given intravenous sedation, and the examination was completed using ultrathin EGD.
Upon discharge from the recovery area, all patients completed a questionnaire regarding their tolerance of the procedure. Patients completed 10-point visual scales indicating pain, choking, gagging, and anxiety both during the insertion of the endoscope and for the remainder of the examination. Patients were asked if they would choose to have the procedure again if endoscopy were indicated in the future. After the procedure, the endoscopist completed a questionnaire assessing the completeness of the examination (eg, whether the endoscope was advanced to the second portion of the duodenum and retroflexion was performed). Indications for the procedure, demographics, clinical findings, complications, the duration of the examination, and recovery duration were noted.
This study was planned to achieve a power of 0.80 to detect changes of 2.0 on tolerance scores between the study groups. Statistical analyses included the independent t-test, Fisher exact test, Mann-Whitney U test, and chi-square test. Multivariate regression analysis and analysis of variance were used to assess the effect that sex may have had on patients’ tolerance scores. Analysis was by intention to treat.
FIGURE 1
Ultrathin endoscope, GIF-N230 (left); standard endoscope, GIF-130 (right)
Results
Of 80 outpatients eligible for the study, 8 (10%) declined entry before randomization. Of 72 remaining, 33 were randomized to ultrathin EGD and 39 to the conventional procedure. The 2 groups were evenly matched for age, race, body mass index (BMI), indication, and EGD findings (Tables 1 and 2). There were more women in the conventional group (80% vs 55%, P = .041; Table 1).
During endoscope insertion, patients undergoing ultrathin EGD had higher mean gagging and choking scores, but lower anxiety scores. For the remainder of the procedure, ultrathin EGD patients had higher gagging scores but no statistically significant differences were noted between groups for pain, choking, or anxiety (Table 2). Twenty-nine patients (88%) assigned to the ultrathin EGD group completed the unsedated examination, and 32 (97%) were willing to repeat an unsedated procedure with the ultrathin endoscope in the future. The mean (± standard error) dose of meperidine was 48.8 ± 2.3 mg; 2 patients required fentanyl 62.5 ± 12.5 mg and midazolam 2.2 ± 0.1 mg. Of the 4 patients allocated to the ultrathin group, which required sedation, the mean dose of meperidine was 50 ± 0 mg and mida-zolam 2.8 ± 0.5 mg. The time required for sedation in the conventional group was 4.1 ± 0.6 min and in the ultrathin EGD group was 5.5 ± 0.5 min (P = .280).
The second portion of the duodenum was reached as often with the ultrathin endoscope as with the conventional apparatus (Table 2). However, retroflexion was achieved less often with ultrathin EGD than with conventional EGD (85% vs 100%, P = .017). Although examination times did not differ between groups, the recovery time was significantly shorter with ultrathin EGD (21.5 ± 2.3 min vs 55.4 ± 2.3 min, P < .0001). No complications were noted in either group. Analysis of variance and multiple regression analysis showed no statistically significant difference in the tolerance scores by sex.
TABLE 1
Patient demographics
| Characteristic | Ultrathin EGD (n = 33) | Conventional EGD (n = 39) | P |
|---|---|---|---|
| Age, y (mean ± SE) | 49.8 ± 2.9 | 46.9 ± 2.2 | .406 |
| Female (%) | 55 | 80 | .041 |
| Race (%) | |||
| Caucasian | 7 | 44 | .123 |
| African American | 27 | 39 | |
| Other | 6 | 18 | |
| Body mass index (mean ± SE) | 28.8 ± 0.88 | 28.7 ± 0.81 | .966 |
| EGD, esophagogastroduodenoscopy; SE, standard error. | |||
TABLE 2
Indications and esophagogastroduodenoscopy findings
| Ultrathin EGD | Conventional EGD | P | |
|---|---|---|---|
| Indications, n (%) | |||
| GERD | 26 (79) | 22 (56) | .050 |
| Abdominal pain | 9 (27) | 18 (46) | .143 |
| Dyspepsia | 2 (6) | 7 (18) | .166 |
| EGD findings, n (%) | |||
| Esophagitis | 9 (27) | 6 (15) | .254 |
| Hiatal hernia | 15 (45) | 13 (33) | .338 |
| Gastritis | 20 (61) | 29 (74) | .310 |
| Gastric ulcer | 1 (3) | 4 (10) | .366 |
| Duodenal ulcer | 0 | 0 | |
| CLO test positive | 4 (13) | 11 (28) | .150 |
| Patient tolerance, score (mean ±SE) | |||
| During insertion | |||
| Anxiety | 3.2 ± 0.47 | 5.7 ± 0.45 | <.0001 |
| Pain | 2.0 ± 0.34 | 1.4 ± 0.29 | .574 |
| Choking | 3.0 ± 0.42 | 1.0 ± 0.32 | .022 |
| Gagging | 4.2 ± 0.45 | 1.3 ± 0.34 | <.0001 |
| During procedure | |||
| Anxiety | 3.1 ± 0.48 | 2.5 ± 0.43 | .350 |
| Pain | 1.3 ± 0.31 | 1.2 ± 0.27 | .771 |
| Choking | 1.7 ± 0.33 | 1.0 ± 0.25 | .081 |
| Gagging | 2.4 ± 0.35 | 1.2 ± 0.28 | .007 |
| Technical aspects of procedure | |||
| To second portion of duodenum (%) | 97 | 100 | .458 |
| Retroflexed (%) | 85 | 100 | .017 |
| Duration of examination, min (mean ± SE) | 18.2 ± 0.93 | 17.5 ± 1.1 | .632 |
| Duration of recovery, min (mean ± SE) | 21.5 ± 2.3 | 55.4 ± 2.3 | <.0001 |
| EGD, esophagogastroduodenoscopy; GERD, gastroesophageal reflux disease; SE, standard error. | |||
Discussion
We examined differences in patients’ experiences during EGD when a relatively thin scope was used without sedation vs a conventional wider scope with sedation. We expected the ultrathin scope to be preferable to both patients and physicians because of the reduced risk and lower cost associated with an unsedated procedure performed in an out-patient setting.
This study has major implications for family physicians. First, ultrathin EGD requires less recovery time than the conventional procedure. In addition, unsedated endoscopy does not require continuous cardiopulmonary monitoring.18 In contrast, conventional EGD generally requires a minimum of 2 support personnel: 1 to assist the endoscopist and 1 to monitor vital signs. A third assistant is occasionally needed to monitor patients in recovery. Ultrathin EGD requires only 1 assistant. A thorough exploration of cost savings associated with ultrathin EGD was beyond the scope of this study. A recent study13 found that ultrathin EGD required less procedure time, less time in the procedure room, and less recovery time, with a cost savings of US $125 per procedure. Second, EGD is traditionally limited to being perforen type=med in gastrointestinal suites. Our findings suggested that most patients can tolerate ultrathin EGD in an outpatient setting, thereby offering easier access to the procedure.
Increased gagging and choking associated with the ultrathin device suggests that its deployment will require techniques to reduce gagging. Transnasal upper endoscopy appears to cause less gagging and choking,8,9,11,14-17,19 but has not been studied in the family practice setting. Other techniques to determine pharyngeal sensitivity are needed.20,21 One study10 found that ultrathin EGD was tolerated better than conventional EGD for unsedated examinations; investigators22 identified younger age and higher levels of pre-endoscopic anxiety as predictors of patient intolerance of unsedated endoscopy.
Although the success rate of retroflexion and duodenal intubation has not been reported in other studies6-9,11,12,14-17,19,23 of ultrathin EGD, we could not perform retroflexion in 15% of subjects in the ultrathin EGD group. Inability to retroflex was secondary to patient intolerance and increased instrument flexibility. In these patients, the endoscopist might switch to a normal diameter scope; however, in a large national study using a standard diameter endoscope, retroflexion was not performed in 7% of patients.24 The most frequent contribution of retroflexion is the identification of a dysfunctional lower esophageal sphincter. In our experience, a small fundal polyp and a large diverticulum in the cardia would be missed in the absence of this maneuver. In contrast, we were unable to intubate the duodenum in 3% of patients undergoing ultrathin EGD. Rodney and colleagues24 cited in their national study that with use of the standard endoscope, duodenal intubation was not achieved in 7% of patients. With increased experience with the ultrathin device, endoscopists may be able to develop techniques to overcome the increased flexibility (eg, using the biopsy forceps in the accessory channel to increase rigidity).
Although we found no significant differences in the proportion of clinical findings between the 2 groups, the findings may have been different had we been able to retroflex the scope in the ultrathin EGD group. The diagnostic accuracy,11,17 image quality,11,23 and adequacy of the smaller biopsy specimen for pathologic diagnosis9,25,26 for ultrathin EGD have been reviewed and consistently determined to be clinically acceptable. Image quality of the 2 techniques is comparable (Figure 2). Although the biopsy specimens obtained with the ultrathin endoscope were smaller than samples of tissue obtained with the conventional device, CLO test positivity did not differ between the groups.
Conventional EGD required more recovery time and was associated with significantly higher anxiety. It is possible that the relatively higher anxiety experienced by patients in the conventional EGD group can be explained by fear of loss of control, fear about risks related to sedation, or a combination of psychosocial factors. One limitation in the conventional EGD group was the potential bias of the seda-tion when patients responded to the postrecovery surveys. Future studies may control for this sedation-effect bias by repeated measures over a period of a few days. Another limitation of our study was that the verbal reassurance offered to patients before and during endoscopy was nonscripted and may have influenced tolerance scores. A third limitation was that the patient questionnaire was given to patients by the endoscopist, thereby possibly introducing a social desirability bias. Finally, the small sample size limited the ability to detect differences that may be clinically meaningful.
Ultrathin EGD costs less, provides similar results, and has acceptable tolerability compared with conventional EGD. Once they are EGD credentialed, clinicians do not require further training or skills to perform the procedure with the ultrathin device. As more family physicians feel comfortable performing EGD in an outpatient setting, more patients will have access to this important procedure.
FIGURE 2
View of gastric ulcer with GIF-N230 (A) and GIF-130 (B)
Acknowledgments
The investigators acknowledge Olympus for the use of a GIF-N230 gastrointestinal videoscope, which was used for the ultrathin procedures. We also acknowledge Staff Sergeant Ron O’Dell for his photograph of the endoscopes.
OBJECTIVE: To compare the tolerance, feasibility, and safety of ultrathin esophagogastroduodenoscopy (EGD) in unsedated patients with conventional EGD in sedated patients.
STUDY DESIGN: This was an unblinded, randomized controlled trial.
POPULATION: Diagnostic EGD was performed on 72 adult outpatients at a US Air Force community hospital residency. Patients were randomized to either ultrathin or conventional EGD (n = 33 and 39, respectively).
OUTCOMES MEASURED: Patients reported their tolerance of the procedure (pain, choking, gagging, and anxiety; scale 0–10), and the endoscopist reported the effectiveness of the procedure (successful intubation, reaching duodenum, retroflexion, and duration of examination and recovery) and safety (complications).
RESULTS: No statistically significant difference was noted between the 2 groups in mean procedure time or pain during the procedure. Mean (± standard error) recovery time was approximately halved in the ultrathin group vs the conventional group (21.5 ± 2.3 min vs 55.4 ± 2.3 min, P < .0001). Although patients undergoing ultrathin EGD had higher mean gagging and choking scores, they had lower mean anxiety scores. Of 33 patients randomized to the unsedated ultrathin EGD procedure, 29 completed the protocol. The retroflexion maneuver was completed in 85% of patients in the ultrathin EGD group and 100% of patients in the conventional EGD group (P = .017). No statistically significant difference was noted between groups as to the likelihood of reaching the second portion of the duodenum (97% vs 100%).
CONCLUSIONS: Most patients tolerate ultrathin EGD with significantly shorter recovery time and less overall anxiety than with the conventioanl procedure. Techniques to reduce gagging and choking associated with ultrathin EGD may improve patient acceptance and tolerability. Adoption of ultrathin EGD by primary care physicians may decrease cost, time, and inconvenience while increasing access to EGD for many patients.
- Most patients tolerate unsedated, ultrathin esophagogastroduodenoscopy (EGD).
- The recovery time is approximately halved for ultrathin unsedated EGDs.
- Patients undergoing unsedated EGD report more gagging and choking than do patients having the sedated examination.
- Patients receiving sedated upper endoscopy report more anxiety than those receiving an unsedated examination.
- Once credentialed in upper endoscopy, physicians do not require further training or skills to perform ultrathin EGD.
Hacker et al1 reported that 3 times as many patients prefer esophagogastroduodenoscopy (EGD) to upper gastrointestinal roentgenography. EGD is safe, with complication rates between 5 and 10 per 10,000 procedures.2 However, only 2% of US family physicians perform upper endoscopy.3
In the United States, conventional EGD is usually performed under conscious sedation to reduce discomfort and anxiety.4 Conscious sedation has potential negative aspects, including costs and side effects,5 increased risks of respiratory depression,2 and, very rarely, mortality.5 Indirect costs related to lost work are unmeasured. All these factors may decrease patient tolerance or acceptance of conventional EGD with conscious sedation.
In the last decade, an ultrathin (5.3–5.9 mm diameter) fiberoptic endoscope was developed. Shaker et al6 used an ultrathin endoscope to perform transnasal endoscopy of the gastrointestinal tract. Since then, several prospective studies have evaluated ultrathin EGD.7-9 One study compared ultrathin with conventional EGD, although both groups of subjects were unsedated.10 Studies comparing EGD techniques were performed in large medical centers by gastroenterologists.7-17 The purpose of this study was to assess the feasibility, safety, and patient tolerance of unsedated ultrathin EGD by generalists in a community setting.
Methods
Outpatients (aged 20–80 years) from a US Air Force family practice residency were referred to a family practice endoscopist for further evaluation of dyspepsia, heartburn, and epigastric pain. Exclusion criteria included pregnancy, evidence of acute gastrointestinal hemorrhage, potential need for therapeutic endoscopy, a medically unstable patient (eg, recent myocardial infarction or stroke), coagulopathy, unstable angina, severe chronic obstructive pulmonary disease, and severe aortic stenosis. The local institutional review board approved the study.
An Olympus GIF-N230 gastrointestinal videoscope (Olympus America Inc, Melville, NY) with an outer diameter of 6.0 mm, an accessory channel of 2.0 mm, a working length of 122 cm, tip deflection of 180° (up and down) and 160° (right and left), with a field of view of 120° was used for the ultrathin procedures. An Olympus GIF-130 standard gastroscope (Olympus America Inc) with an outer diameter of 9.8 mm, an accessory channel of 2.8 mm, a working length of 103 cm, tip deflection of 210° up, 90° down, and 100° right and left, with a field of view of 120°, was used for the conventional procedures. Both endoscopes are forward-viewing (Figure 1).
After standard informed consent was obtained, the endoscopist made a phone call to access a computer-generated patient list for randomization. No patient withdrew after randomization. The endoscopist (T.W.) performed all endoscopies in the gastrointestinal suite with the patient in the left lateral position. An intravenous line was started and tetracaine 2% was sprayed in the posterior pharynx; pulse oximetry, cardiac monitoring, and verbal reassurance by the endoscopist were provided to patients in both groups. Sedation was slowly titrated in increments of 25 mg meperidine (or 50 mg fentanyl if the patient was allergic to meperidine) and 0.5 mg midazolam until a suitable level of sedation was obtained for endoscopy. Biopsy samples were obtained when indicated. Patients who were unable to tolerate the unsedated examination were given intravenous sedation, and the examination was completed using ultrathin EGD.
Upon discharge from the recovery area, all patients completed a questionnaire regarding their tolerance of the procedure. Patients completed 10-point visual scales indicating pain, choking, gagging, and anxiety both during the insertion of the endoscope and for the remainder of the examination. Patients were asked if they would choose to have the procedure again if endoscopy were indicated in the future. After the procedure, the endoscopist completed a questionnaire assessing the completeness of the examination (eg, whether the endoscope was advanced to the second portion of the duodenum and retroflexion was performed). Indications for the procedure, demographics, clinical findings, complications, the duration of the examination, and recovery duration were noted.
This study was planned to achieve a power of 0.80 to detect changes of 2.0 on tolerance scores between the study groups. Statistical analyses included the independent t-test, Fisher exact test, Mann-Whitney U test, and chi-square test. Multivariate regression analysis and analysis of variance were used to assess the effect that sex may have had on patients’ tolerance scores. Analysis was by intention to treat.
FIGURE 1
Ultrathin endoscope, GIF-N230 (left); standard endoscope, GIF-130 (right)
Results
Of 80 outpatients eligible for the study, 8 (10%) declined entry before randomization. Of 72 remaining, 33 were randomized to ultrathin EGD and 39 to the conventional procedure. The 2 groups were evenly matched for age, race, body mass index (BMI), indication, and EGD findings (Tables 1 and 2). There were more women in the conventional group (80% vs 55%, P = .041; Table 1).
During endoscope insertion, patients undergoing ultrathin EGD had higher mean gagging and choking scores, but lower anxiety scores. For the remainder of the procedure, ultrathin EGD patients had higher gagging scores but no statistically significant differences were noted between groups for pain, choking, or anxiety (Table 2). Twenty-nine patients (88%) assigned to the ultrathin EGD group completed the unsedated examination, and 32 (97%) were willing to repeat an unsedated procedure with the ultrathin endoscope in the future. The mean (± standard error) dose of meperidine was 48.8 ± 2.3 mg; 2 patients required fentanyl 62.5 ± 12.5 mg and midazolam 2.2 ± 0.1 mg. Of the 4 patients allocated to the ultrathin group, which required sedation, the mean dose of meperidine was 50 ± 0 mg and mida-zolam 2.8 ± 0.5 mg. The time required for sedation in the conventional group was 4.1 ± 0.6 min and in the ultrathin EGD group was 5.5 ± 0.5 min (P = .280).
The second portion of the duodenum was reached as often with the ultrathin endoscope as with the conventional apparatus (Table 2). However, retroflexion was achieved less often with ultrathin EGD than with conventional EGD (85% vs 100%, P = .017). Although examination times did not differ between groups, the recovery time was significantly shorter with ultrathin EGD (21.5 ± 2.3 min vs 55.4 ± 2.3 min, P < .0001). No complications were noted in either group. Analysis of variance and multiple regression analysis showed no statistically significant difference in the tolerance scores by sex.
TABLE 1
Patient demographics
| Characteristic | Ultrathin EGD (n = 33) | Conventional EGD (n = 39) | P |
|---|---|---|---|
| Age, y (mean ± SE) | 49.8 ± 2.9 | 46.9 ± 2.2 | .406 |
| Female (%) | 55 | 80 | .041 |
| Race (%) | |||
| Caucasian | 7 | 44 | .123 |
| African American | 27 | 39 | |
| Other | 6 | 18 | |
| Body mass index (mean ± SE) | 28.8 ± 0.88 | 28.7 ± 0.81 | .966 |
| EGD, esophagogastroduodenoscopy; SE, standard error. | |||
TABLE 2
Indications and esophagogastroduodenoscopy findings
| Ultrathin EGD | Conventional EGD | P | |
|---|---|---|---|
| Indications, n (%) | |||
| GERD | 26 (79) | 22 (56) | .050 |
| Abdominal pain | 9 (27) | 18 (46) | .143 |
| Dyspepsia | 2 (6) | 7 (18) | .166 |
| EGD findings, n (%) | |||
| Esophagitis | 9 (27) | 6 (15) | .254 |
| Hiatal hernia | 15 (45) | 13 (33) | .338 |
| Gastritis | 20 (61) | 29 (74) | .310 |
| Gastric ulcer | 1 (3) | 4 (10) | .366 |
| Duodenal ulcer | 0 | 0 | |
| CLO test positive | 4 (13) | 11 (28) | .150 |
| Patient tolerance, score (mean ±SE) | |||
| During insertion | |||
| Anxiety | 3.2 ± 0.47 | 5.7 ± 0.45 | <.0001 |
| Pain | 2.0 ± 0.34 | 1.4 ± 0.29 | .574 |
| Choking | 3.0 ± 0.42 | 1.0 ± 0.32 | .022 |
| Gagging | 4.2 ± 0.45 | 1.3 ± 0.34 | <.0001 |
| During procedure | |||
| Anxiety | 3.1 ± 0.48 | 2.5 ± 0.43 | .350 |
| Pain | 1.3 ± 0.31 | 1.2 ± 0.27 | .771 |
| Choking | 1.7 ± 0.33 | 1.0 ± 0.25 | .081 |
| Gagging | 2.4 ± 0.35 | 1.2 ± 0.28 | .007 |
| Technical aspects of procedure | |||
| To second portion of duodenum (%) | 97 | 100 | .458 |
| Retroflexed (%) | 85 | 100 | .017 |
| Duration of examination, min (mean ± SE) | 18.2 ± 0.93 | 17.5 ± 1.1 | .632 |
| Duration of recovery, min (mean ± SE) | 21.5 ± 2.3 | 55.4 ± 2.3 | <.0001 |
| EGD, esophagogastroduodenoscopy; GERD, gastroesophageal reflux disease; SE, standard error. | |||
Discussion
We examined differences in patients’ experiences during EGD when a relatively thin scope was used without sedation vs a conventional wider scope with sedation. We expected the ultrathin scope to be preferable to both patients and physicians because of the reduced risk and lower cost associated with an unsedated procedure performed in an out-patient setting.
This study has major implications for family physicians. First, ultrathin EGD requires less recovery time than the conventional procedure. In addition, unsedated endoscopy does not require continuous cardiopulmonary monitoring.18 In contrast, conventional EGD generally requires a minimum of 2 support personnel: 1 to assist the endoscopist and 1 to monitor vital signs. A third assistant is occasionally needed to monitor patients in recovery. Ultrathin EGD requires only 1 assistant. A thorough exploration of cost savings associated with ultrathin EGD was beyond the scope of this study. A recent study13 found that ultrathin EGD required less procedure time, less time in the procedure room, and less recovery time, with a cost savings of US $125 per procedure. Second, EGD is traditionally limited to being perforen type=med in gastrointestinal suites. Our findings suggested that most patients can tolerate ultrathin EGD in an outpatient setting, thereby offering easier access to the procedure.
Increased gagging and choking associated with the ultrathin device suggests that its deployment will require techniques to reduce gagging. Transnasal upper endoscopy appears to cause less gagging and choking,8,9,11,14-17,19 but has not been studied in the family practice setting. Other techniques to determine pharyngeal sensitivity are needed.20,21 One study10 found that ultrathin EGD was tolerated better than conventional EGD for unsedated examinations; investigators22 identified younger age and higher levels of pre-endoscopic anxiety as predictors of patient intolerance of unsedated endoscopy.
Although the success rate of retroflexion and duodenal intubation has not been reported in other studies6-9,11,12,14-17,19,23 of ultrathin EGD, we could not perform retroflexion in 15% of subjects in the ultrathin EGD group. Inability to retroflex was secondary to patient intolerance and increased instrument flexibility. In these patients, the endoscopist might switch to a normal diameter scope; however, in a large national study using a standard diameter endoscope, retroflexion was not performed in 7% of patients.24 The most frequent contribution of retroflexion is the identification of a dysfunctional lower esophageal sphincter. In our experience, a small fundal polyp and a large diverticulum in the cardia would be missed in the absence of this maneuver. In contrast, we were unable to intubate the duodenum in 3% of patients undergoing ultrathin EGD. Rodney and colleagues24 cited in their national study that with use of the standard endoscope, duodenal intubation was not achieved in 7% of patients. With increased experience with the ultrathin device, endoscopists may be able to develop techniques to overcome the increased flexibility (eg, using the biopsy forceps in the accessory channel to increase rigidity).
Although we found no significant differences in the proportion of clinical findings between the 2 groups, the findings may have been different had we been able to retroflex the scope in the ultrathin EGD group. The diagnostic accuracy,11,17 image quality,11,23 and adequacy of the smaller biopsy specimen for pathologic diagnosis9,25,26 for ultrathin EGD have been reviewed and consistently determined to be clinically acceptable. Image quality of the 2 techniques is comparable (Figure 2). Although the biopsy specimens obtained with the ultrathin endoscope were smaller than samples of tissue obtained with the conventional device, CLO test positivity did not differ between the groups.
Conventional EGD required more recovery time and was associated with significantly higher anxiety. It is possible that the relatively higher anxiety experienced by patients in the conventional EGD group can be explained by fear of loss of control, fear about risks related to sedation, or a combination of psychosocial factors. One limitation in the conventional EGD group was the potential bias of the seda-tion when patients responded to the postrecovery surveys. Future studies may control for this sedation-effect bias by repeated measures over a period of a few days. Another limitation of our study was that the verbal reassurance offered to patients before and during endoscopy was nonscripted and may have influenced tolerance scores. A third limitation was that the patient questionnaire was given to patients by the endoscopist, thereby possibly introducing a social desirability bias. Finally, the small sample size limited the ability to detect differences that may be clinically meaningful.
Ultrathin EGD costs less, provides similar results, and has acceptable tolerability compared with conventional EGD. Once they are EGD credentialed, clinicians do not require further training or skills to perform the procedure with the ultrathin device. As more family physicians feel comfortable performing EGD in an outpatient setting, more patients will have access to this important procedure.
FIGURE 2
View of gastric ulcer with GIF-N230 (A) and GIF-130 (B)
Acknowledgments
The investigators acknowledge Olympus for the use of a GIF-N230 gastrointestinal videoscope, which was used for the ultrathin procedures. We also acknowledge Staff Sergeant Ron O’Dell for his photograph of the endoscopes.
1. Hacker JFD, Chobanian SJ, Johnson DA, Winters C, Jr, Cattau EL, J. Patient preference in upper gastrointestinal studies: roentgenography versus endoscopy. South Med J 1987;80:1091-3.
2. Zurad EG Indications, Contraindications, and Complications of Upper Gastrointestinal Endoscopy. Kansas City, KS: American Academy of Family Physicians; 1994.
3. American Academy of Family Physicians Practice Profile II Survey. Leawood, KS: American Academy of Family Physicians; 1998.
4. Daneshmend TK, Bell GD, Logan RF. Sedation for upper gastrointestinal endoscopy: results of a nationwide survey. Gut 1991;32:12-5.
5. Mokhashi MS, Hawes RH. Struggling toward easier endoscopy [editorial]. Gastrointest Endosc 1998;48:432-40.
6. Shaker R. Unsedated trans-nasal pharyngoesophagogastroduodenoscopy (T-EGD): technique. Gastrointest Endosc 1994;40:346-8.
7. De Gregorio BT, Poorman JC, Katon RM. Peroral ultrathin endoscopy in adult patients. Gastrointest Endosc 1997;45:303-6.
8. Rey JF, Duforest D, Marek TA. Prospective comparison of nasal versus oral insertion of a thin video endoscope in healthy volunteers. Endoscopy 1996;28:422-4.
9. Dumortier J, Ponchon T, Scoazec JY, et al. Prospective evaluation of transnasal esophagogastroduodenoscopy: feasibility and study on performance and tolerance. Gastrointest Endosc 1999;49(3 Pt 1):285-91.
10. Mulcahy HE, Riches A, Kiely M, Farthing MJ, Fairclough PD. A prospective controlled trial of an ultrathin versus a conventional endoscope in unsedated upper gastrointestinal endoscopy. Endoscopy 2001;33:311-6.
11. Zaman A, Hahn M, Hapke R, Knigge K, Fennerty MB, Katon RM. A randomized trial of peroral versus transnasal unsedated endoscopy using an ultrathin videoendoscope. Gastrointest Endosc 1999;49(3 Pt 1):279-84.
12. Zaman A, Hapke R, Sahagun G, Katon RM. Unsedated peroral endoscopy with a video ultrathin endoscope: patient acceptance, tolerance, and diagnostic accuracy. Am J Gastroenterol 1998;93:1260-3.
13. Gorelick AB, Inadomi JM, Barnett JL. Unsedated small-caliber esophagogastroduodenoscopy (EGD): less expensive and less time-consuming than conventional EGD. J Clin Gastroenterol 2001;33:210-4.
14. Craig A, Hanlon J, Dent J, Schoeman M. A comparison of transnasal and transoral endoscopy with small-diameter endoscopes in unsedated patients. Gastrointest Endosc 1999;49(3 Pt 1):292-6.
15. Nozaki R, Fujiyoshi T, Tamura M, Tsuchiya A, Takagi K, Takano M. Evaluation of small caliber transnasal panendoscopes for upper GI screening examination. Dig Endosc 1995;7:155-9.
16. Campo R, Montserrat A, Brullet E. Transnasal gastroscopy compared to conventional gastroscopy: a randomized study of feasibility, safety, and tolerance. Endoscopy 1998;30:448-52.
17. Dean R, Dua K, Massey B, Berger W, Hogan WJ, Shaker R. A comparative study of unsedated transnasal esophagogastroduodenoscopy and conventional EGD. Gastrointest Endosc 1996;44:422-4.
18. Banks MR, Kumar PJ, Mulcahy HE. Pulse oximetry saturation levels during routine unsedated diagnostic upper gastrointestinal endoscopy. Scand J Gastroenterol 2001;36:105-9.
19. Bampton PA, Reid DP, Johnson RD, Fitch RJ, Dent J. A comparison of transnasal and transoral oesophagogastroduodenoscopy. J Gastroenterol Hepatol 1998;13:579-84.
20. Ladas SD, Tassios PS, Raptis SA. A simple test for predicting patients’ tolerance of upper gastrointestinal endoscopy. Endoscopy 1997;29:430.
21. Ladas SD, Raptis SA. Selection of patients for upper gastrointestinal endoscopy without sedation. The finger-throat test. Ital J Gastroenterol 1986;18:162-5.
22. Mulcahy HE, Kelly P, Banks MR, et al. Factors associated with tolerance to, and discomfort with, unsedated diagnostic gastroscopy. Scand J Gastroenterol 2001;36:1352-7.
23. Sorbi D, Gostout CJ, Henry J, Lindor KD. Unsedated small-caliber esophagogastroduodenoscopy (EGD) versus conventional EGD: a comparative study. Gastroenterology 1999;117:1301-7.
24. Rodney WM, Weber JR, Swedberg JA, et al. Esophago-gastroduodenoscopy by family physicians phase II: a national multisite study of 2,500 procedures. Fam Pract Res J 1993;13:121-31.
25. Saeian K, Townsend WF, Rochling FA, et al. Unsedated transnasal EGD: an alternative approach to conventional esophagogastroduodenoscopy for documenting Helicobacter pylori eradication. Gastrointest Endosc 1999;49(3 Pt 1):297-301.
26. Yousfi MM, El-Zimaity HM, Cole RA, Genta RM, Graham DY. Detection of Helicobacter pylori by rapid urease tests: is biopsy size a critical variable? Gastrointest Endosc 1996;43:222-4.
1. Hacker JFD, Chobanian SJ, Johnson DA, Winters C, Jr, Cattau EL, J. Patient preference in upper gastrointestinal studies: roentgenography versus endoscopy. South Med J 1987;80:1091-3.
2. Zurad EG Indications, Contraindications, and Complications of Upper Gastrointestinal Endoscopy. Kansas City, KS: American Academy of Family Physicians; 1994.
3. American Academy of Family Physicians Practice Profile II Survey. Leawood, KS: American Academy of Family Physicians; 1998.
4. Daneshmend TK, Bell GD, Logan RF. Sedation for upper gastrointestinal endoscopy: results of a nationwide survey. Gut 1991;32:12-5.
5. Mokhashi MS, Hawes RH. Struggling toward easier endoscopy [editorial]. Gastrointest Endosc 1998;48:432-40.
6. Shaker R. Unsedated trans-nasal pharyngoesophagogastroduodenoscopy (T-EGD): technique. Gastrointest Endosc 1994;40:346-8.
7. De Gregorio BT, Poorman JC, Katon RM. Peroral ultrathin endoscopy in adult patients. Gastrointest Endosc 1997;45:303-6.
8. Rey JF, Duforest D, Marek TA. Prospective comparison of nasal versus oral insertion of a thin video endoscope in healthy volunteers. Endoscopy 1996;28:422-4.
9. Dumortier J, Ponchon T, Scoazec JY, et al. Prospective evaluation of transnasal esophagogastroduodenoscopy: feasibility and study on performance and tolerance. Gastrointest Endosc 1999;49(3 Pt 1):285-91.
10. Mulcahy HE, Riches A, Kiely M, Farthing MJ, Fairclough PD. A prospective controlled trial of an ultrathin versus a conventional endoscope in unsedated upper gastrointestinal endoscopy. Endoscopy 2001;33:311-6.
11. Zaman A, Hahn M, Hapke R, Knigge K, Fennerty MB, Katon RM. A randomized trial of peroral versus transnasal unsedated endoscopy using an ultrathin videoendoscope. Gastrointest Endosc 1999;49(3 Pt 1):279-84.
12. Zaman A, Hapke R, Sahagun G, Katon RM. Unsedated peroral endoscopy with a video ultrathin endoscope: patient acceptance, tolerance, and diagnostic accuracy. Am J Gastroenterol 1998;93:1260-3.
13. Gorelick AB, Inadomi JM, Barnett JL. Unsedated small-caliber esophagogastroduodenoscopy (EGD): less expensive and less time-consuming than conventional EGD. J Clin Gastroenterol 2001;33:210-4.
14. Craig A, Hanlon J, Dent J, Schoeman M. A comparison of transnasal and transoral endoscopy with small-diameter endoscopes in unsedated patients. Gastrointest Endosc 1999;49(3 Pt 1):292-6.
15. Nozaki R, Fujiyoshi T, Tamura M, Tsuchiya A, Takagi K, Takano M. Evaluation of small caliber transnasal panendoscopes for upper GI screening examination. Dig Endosc 1995;7:155-9.
16. Campo R, Montserrat A, Brullet E. Transnasal gastroscopy compared to conventional gastroscopy: a randomized study of feasibility, safety, and tolerance. Endoscopy 1998;30:448-52.
17. Dean R, Dua K, Massey B, Berger W, Hogan WJ, Shaker R. A comparative study of unsedated transnasal esophagogastroduodenoscopy and conventional EGD. Gastrointest Endosc 1996;44:422-4.
18. Banks MR, Kumar PJ, Mulcahy HE. Pulse oximetry saturation levels during routine unsedated diagnostic upper gastrointestinal endoscopy. Scand J Gastroenterol 2001;36:105-9.
19. Bampton PA, Reid DP, Johnson RD, Fitch RJ, Dent J. A comparison of transnasal and transoral oesophagogastroduodenoscopy. J Gastroenterol Hepatol 1998;13:579-84.
20. Ladas SD, Tassios PS, Raptis SA. A simple test for predicting patients’ tolerance of upper gastrointestinal endoscopy. Endoscopy 1997;29:430.
21. Ladas SD, Raptis SA. Selection of patients for upper gastrointestinal endoscopy without sedation. The finger-throat test. Ital J Gastroenterol 1986;18:162-5.
22. Mulcahy HE, Kelly P, Banks MR, et al. Factors associated with tolerance to, and discomfort with, unsedated diagnostic gastroscopy. Scand J Gastroenterol 2001;36:1352-7.
23. Sorbi D, Gostout CJ, Henry J, Lindor KD. Unsedated small-caliber esophagogastroduodenoscopy (EGD) versus conventional EGD: a comparative study. Gastroenterology 1999;117:1301-7.
24. Rodney WM, Weber JR, Swedberg JA, et al. Esophago-gastroduodenoscopy by family physicians phase II: a national multisite study of 2,500 procedures. Fam Pract Res J 1993;13:121-31.
25. Saeian K, Townsend WF, Rochling FA, et al. Unsedated transnasal EGD: an alternative approach to conventional esophagogastroduodenoscopy for documenting Helicobacter pylori eradication. Gastrointest Endosc 1999;49(3 Pt 1):297-301.
26. Yousfi MM, El-Zimaity HM, Cole RA, Genta RM, Graham DY. Detection of Helicobacter pylori by rapid urease tests: is biopsy size a critical variable? Gastrointest Endosc 1996;43:222-4.