User login
Neuroendocrine dysfunction following mild TBI: When to screen for it
› Consider neuroendocrine dysfunction (NED) following confirmed traumatic brain injury of any severity when symptoms suggestive of NED persist for >3 months after injury. A
› Order blood studies to detect deficiencies in pituitary and other key hormones when NED is suspected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The Centers for Disease Control and Prevention (CDC) reports that >1.7 million cases of traumatic brain injury (TBI) occur annually in the United States.1 More than 266,000 military service members sustained at least one TBI from 2000 to 2012.2 Most TBIs (80%-85%), military and civilian, are classified as mild (mTBI), and most mTBI patients (80%-85%) experience a complete functional recovery within 3 months of injury.1,3 The remaining 15% to 20% of mTBI patients experience persistent symptoms and difficulty in rehabilitation, particularly if there are concomitant disorders, such as post-traumatic stress disorder (PTSD), sleep disorders, acute stress disorder, substance abuse disorder, and depression.4,5 Symptoms that mTBI and these other disorders have in common can make differential diagnosis difficult, requiring a high degree of clinical awareness by primary care providers. An additional concern following mTBI is neuroendocrine dysfunction (NED). This association has not been widely discussed and therefore may go largely undiagnosed.6 Consider NED in the setting of prolonged symptoms or in patients experiencing difficulty with rehabilitation following mTBI.7,8
NED following mTBI is more common than once thought
The term “neuroendocrine dysfunction,” as discussed in this article, refers to a variety of conditions caused by imbalances in the body’s hormone production directly related to the pituitary, hypothalamus, and their axes following TBI. Until the past decade, the incidence of TBI-associated pituitary dysfunction was thought to be an uncommon event, usually associated with catastrophic head injuries. Studies of NED in TBI patients focused primarily on moderate or severe TBI, usually from motor vehicle incidents, falls, and assaults.7 Other research has since shown that NED occurs more commonly than once believed.9 And while the risk of NED may be higher for patients who sustain more severe brain injuries, NED also occurs in mTBI.7,9,10,11 Interestingly, a recent literature review indicated that the incidence of NED in mTBI was 16.8%, while the incidence with moderate TBI was reported at 10.9%.7 Other research has noted that the incidence of NED in mTBI may be as high as 42%.9,12 No evidence suggests that the severity of NED is related to a specific hormonal dysfunction, nor is there evidence that NED may be associated with a specific mechanism of injury.
Pituitary anatomy is susceptible to injury and dysfunction
The anatomic and physiologic complexities of the hypothalamus and pituitary gland increase their susceptibility to injury from TBI. The pituitary gland is connected to the hypothalamus by a blood vessel-containing stalk, making the pituitary gland—particularly the anterior portion—susceptible to damage during a head injury.13 The hypothalamus secretes thyrotropin-releasing hormone (TRH) and luteinizing-releasing hormone (LRH) to stimulate or suppress the production of anterior pituitary gland hormones, which in turn stimulate the release of hormones and other substances from target organs. Anterior pituitary hormones are growth hormone (GH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), adrenocorticotropic hormone (ACTH), thyroid-stimulating hormone (TSH), and prolactin (PRL). The posterior pituitary secretes oxytocin and vasopressin, also known as antidiuretic hormone (ADH).13
Impact from a direct blow with an object or from a concussive blast can cause focal trauma or rotational shearing of tissue internally. Resultant vascular injury, rupture, cerebral edema, vasospasm, pituitary swelling, or inflammation may then initiate an endocrine response that drives a cascade of complex hormonal processes.5,7,8 Anterior pituitary deficiencies account for the majority of chronic neuroendocrine disorders following mTBI. GH and gonadotropin deficiencies are the most common, but TSH deficiency (secondary hypothyroidism) and ACTH deficiency (adrenal insufficiency) may occur as well, although in <10% of cases with TBI associated NED.12
Clinical features of NED mimic those of other conditions
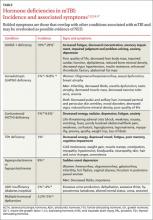
The symptoms of NED include fatigue, insomnia, impaired cognition, memory loss, difficulty concentrating, and emotional and mood disturbances (TABLE).7,12,14-17 Various combinations of these symptoms may occur and are similar to those of other post-mTBI conditions, such as sleep problems, postconcussive syndrome (PCS), and memory and attention difficulties.18 The onset of NED may be immediate (eg, in diabetes insipidus [DI] or syndrome of inappropriate antidiuretic hormone [SIADH], which are very rare in mTBI) and potentially life-threatening (eg, in sodium and potassium imbalances), or may be nonspecific and take years to manifest.6,10,15,19 Additionally, symptoms of NED may spontaneously resolve or persist. Studies have demonstrated pituitary dysfunction in the acute postinjury phase as well as its development as late as 2 to 3 years after injury.7,8,11,20
Due to the range of symptoms related to the combinations of possible hormonal derangements, NED can be an elusive diagnosis and may have a deleterious effect on individuals who sustain TBI.12 For example, an undiagnosed GH deficiency—which can result in increased abdominal fat mass and decreased lean muscle mass as well as impaired cardiac function, dyslipidemia, and insulin resistance—makes it more difficult for an affected individual either to recover from additional injuries or to maintain fitness. Considering NED may avoid a delay in diagnosis and improve prognosis.7,8,20
Findings leading to recommendations on diagnosis
Primary care providers, military and civilian alike, can benefit from the findings and recommendations of an expert panel assembled by the Defense Centers of Excellence (DCoE) for Psychological Health and Traumatic Brain Injury to address NED in mTBI. The panel that convened in December 2010 included experts representing the military services, the Department of Veterans Affairs, DCoE, and civilian sectors. Based on the group’s recommendations combined with literature review findings, the DCoE developed a clinical recommendation to encourage primary care providers to consider screening for NED in patients with persistent symptoms following mTBI.21 Key findings and issues identified by the group included the following:
• The most frequent mechanism of injury in the military deployed population is blast-induced TBI. Such injury could occur in the civilian population at construction blast sites or in factories producing or using highly flammable substances.
• The prevalence of any anterior pituitary hormone deficiency is as high as 30% to hormone deficiency is as high as 30% to 80% at 24 to 36 months post injury.
• The prevalence of posterior pituitary hormone deficiency is as high as 4% to 7% at 12 months post injury.
• The anterior pituitary hormones most frequently affected in survivors of TBI are ACTH, gonadotropin, prolactin, and GH.
• In 2004 Agha et al,22 reported >28% of survivors of TBI had at least one anterior pituitary hormone deficiency.
• According to research by Agha et al23 in 2005, >20% of survivors of TBI developed DI; those who developed DI, either acutely or permanently, were more likely to have sustained a severe TBI.
• The development of pituitary dysfunction is independent of the severity of TBI.
• In 2005, civilian guidelines4 recommended screening for pituitary dysfunction in all patients who sustained a moderate to severe TBI.
• In 2010, civilian guidelines7 recommended screening for pituitary dysfunction in patients who sustained a mild TBI.
When to screen for NED after TBI
Given the complexities described—including the similarity of NED to other post-mTBI medical diagnoses and such concomitant disorders as a sleep disorder, memory difficulties, depression, PTSD, and PCS24—consider NED in the primary care setting following confirmed TBI of any severity level when symptoms suggestive of NED persist for >3 months following injury or appear up to 36 months later.7,8,12,20
Order a lab evaluation of blood levels for cortisol (drawn at 8 am), LH, FSH, PRL, insulin-like growth factor-1, TSH, free thyroxine-4, and testosterone for men (8 am) and estradiol for women (8 am). With frankly abnormal lab results or with borderline results and strong clinical suspicion for NED, refer for further endocrinology workup (FIGURE).21 Earlier diagnosis of NED results in more rapid improvement of symptoms and an improved prognosis.7,8,20 Postinjury screening for NED should be one component of a thorough clinical evaluation by a qualified provider, and not used in isolation for clinical decision making. NED screening should not be routinely ordered during the early stages of mTBI, defined as <3 months postinjury.
Provider awareness and willingness to include NED screening in a timely manner, and to refer to specialty services as indicated for symptoms that may be sleep related or psychiatric in nature, may increase the opportunities for early treatment, better rehabilitation outcomes, and better overall quality of life.
Looking ahead
While the DCoE expert group made recommendations on screening for NED in the military combat population, they also acknowledged that NED diagnosis and treatment would benefit from additional areas of research:
• the effect of GH replacement (for GH-deficient patients or as prophylaxis for all TBI patients) on rehabilitation response and quality of life
• the role of multiple TBIs on long-term cognition and possible premature aging
• the role of NED over time
• biomarkers for diagnosis
• factors affecting resiliency
• resiliency in the context of increased or decreased susceptibility to the development of an acute clinical syndrome, as well as susceptibility in developing the spectrum of consequences of TBI.
The research areas given the highest priority by the group were incidence and prevalence studies of pituitary dysfunction after TBI in the combat military population, including pre- and postdeployment rates of dysfunction and the incidence of comorbidities. Also of benefit would be a retrospective study of the consequences of pituitary dysfunction that additionally addresses the effects of comorbid conditions commonly associated with TBI. Considering the rapid expansion in the field of mTBI, additional research and provider awareness concerning early identification and treatments may improve the outcomes for those with persistent mTBI symptoms.
CORRESPONDENCE
Theres A. West, DNP, APN, BC, Defense and Veterans Brain Injury Center, 1335 East West Highway, 6th floor, Silver Spring, MD 20910; [email protected]
1. Injury prevention & control: Traumatic brain injury national TBI estimates. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/traumaticbraininjury/statistics.html. Accessed August 12, 2013.
2. Armed Forces Surveillance Center. DoD Worldwide Numbers for TBI. Defense and Veterans Brain Injury Centers Web site. Available at: www.dvbic.org/dod-worldwide-Numbers-tbi. Accessed August 12, 2013.
3. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury (mTBI). Available at: http://www.healthquality.va.gov/management_of_concussion_mtbi.asp. Published April 2009. Accessed August 12, 2013.
4. Ghigo E, Masel B, Aimaretti G, et al. Consensus guidelines on screening for hypopituitarism following traumatic brain injury. Brain Inj. 2005;19:711-724.
5. Krahulik D, Zapletalova J, Frysak Z, et al. Dysfunction of hypothalamic-hyperphysical axis after traumatic brain injury in adults. J Neurosurg. 2010;113:581-584.
6. Behan LA, Phillips J, Thompson CJ, et al. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008;79:753-759.
7. Tanriverdi F, Unluhizarci K, Kelestimur F. Pituitary function in subjects with mild traumatic brain injury: a review of literature and proposal of a screening strategy. Pituitary. 2010;13:146-153.
8. Bondanelli M, Ambrosio MR, Zatelli MC, et al. Hypopituitarism after traumatic brain injury. Eur J Endocrinol. 2005;152:679-691.
9. Wilkinson CW, Pagulayan KF, Petrie EC, et al. High prevalence of chronic pituitary and target-hormone abnormalities after blast related mild traumatic brain injury. Front Neurol. 2012;3:11.
10. Bondanelli M, Ambrosio MR, Cavazzini L, et al. Anterior pituitary function may predict functional and cognitive outcome in patients with traumatic brain injury undergoing rehabilitation. J Neurotrauma. 2007;24:1687-1697.
11. Benvenga S, Campenmi A, Ruggeri R, et al. Hypopituitarism secondary to head trauma. J Clin Endocrinol Metab. 2000;85:1353-1361.
12. Schneider H, Kreitschman-Andermahr I, Ghigo E, et al. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA. 2007;298:1429-1438.
13. Amar AP, Weiss MH. Pituitary anatomy and physiology. Neurosurg Clin N Am. 2003;14:11-23.
14. Tanriverdi F, Unluhizarci K, Kocyigit I, et al. Brief communication: Pituitary volume and function in competing and retired male boxers. Ann Intern Med. 2008;148:827-831.
15. Bondanelli M, De Marinis L, Ambrosio MR, et al. Occurrence of pituitary dysfunction following traumatic brain injury. J Neurotrauma. 2004;21:685-696.
16. Klose M, Watt T, Brennum J, et al. Posttraumatic hypopituitarism is associated with an unfavorable body composition and lipid profile, and decreased quality of life in 12 months after injury. J Clin Endocrinol Metab. 2007;92:3861-3868.
17. Aimeretti G, Ambrosio MR, Di Somma C, et al. Residual pituitary function after brain injury-induced hypopituitaryism: a prospective 12-month study. J Clin Endocrinol Metab. 2005;90:6085-6092.
18. Agha A, Phillips J, Thompson CJ. Hypopituitarism following traumatic brain injury. Br J Neurosurg. 2007;21:210-216.
19. Cohan P, Wang C, McArthur DL, et al. Acute secondary adrenal insufficiency after traumatic brain injury: a prospective study. Crit Care Med. 2005;33:2358-2366.
20. Rothman MS, Arciniegas DS, Filley CM, et al. The neuroendocrine effects of traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2007;19:363-372.
21. Defense Centers of Excellence. Neuroendocrine screening post mild TBI clinical recommendation. Available at: http://www.dcoe.mil/Content/Navigation/Documents/DCoE_TBI_NED_Reference_Card.pdf. Published February 2012. Accessed August 12, 2013.
22. Agha A, Rogers B, Mylotte D, et al. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin Endocrinol (Oxf). 2004;60:584-591.
23. Agha A, Phillips J, O’Kelly P, et al. The natural history of post-traumatic hypopituitarism: implications for assessment and treatment. Am J Med. 2005;118:1416.
24. Guerrero AF, Alfonso A. Traumatic brain injury related hypopituitarism: a review and recommendations for screening combat veterans. Mil Med. 2010;175:574-580.
› Consider neuroendocrine dysfunction (NED) following confirmed traumatic brain injury of any severity when symptoms suggestive of NED persist for >3 months after injury. A
› Order blood studies to detect deficiencies in pituitary and other key hormones when NED is suspected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The Centers for Disease Control and Prevention (CDC) reports that >1.7 million cases of traumatic brain injury (TBI) occur annually in the United States.1 More than 266,000 military service members sustained at least one TBI from 2000 to 2012.2 Most TBIs (80%-85%), military and civilian, are classified as mild (mTBI), and most mTBI patients (80%-85%) experience a complete functional recovery within 3 months of injury.1,3 The remaining 15% to 20% of mTBI patients experience persistent symptoms and difficulty in rehabilitation, particularly if there are concomitant disorders, such as post-traumatic stress disorder (PTSD), sleep disorders, acute stress disorder, substance abuse disorder, and depression.4,5 Symptoms that mTBI and these other disorders have in common can make differential diagnosis difficult, requiring a high degree of clinical awareness by primary care providers. An additional concern following mTBI is neuroendocrine dysfunction (NED). This association has not been widely discussed and therefore may go largely undiagnosed.6 Consider NED in the setting of prolonged symptoms or in patients experiencing difficulty with rehabilitation following mTBI.7,8
NED following mTBI is more common than once thought
The term “neuroendocrine dysfunction,” as discussed in this article, refers to a variety of conditions caused by imbalances in the body’s hormone production directly related to the pituitary, hypothalamus, and their axes following TBI. Until the past decade, the incidence of TBI-associated pituitary dysfunction was thought to be an uncommon event, usually associated with catastrophic head injuries. Studies of NED in TBI patients focused primarily on moderate or severe TBI, usually from motor vehicle incidents, falls, and assaults.7 Other research has since shown that NED occurs more commonly than once believed.9 And while the risk of NED may be higher for patients who sustain more severe brain injuries, NED also occurs in mTBI.7,9,10,11 Interestingly, a recent literature review indicated that the incidence of NED in mTBI was 16.8%, while the incidence with moderate TBI was reported at 10.9%.7 Other research has noted that the incidence of NED in mTBI may be as high as 42%.9,12 No evidence suggests that the severity of NED is related to a specific hormonal dysfunction, nor is there evidence that NED may be associated with a specific mechanism of injury.
Pituitary anatomy is susceptible to injury and dysfunction
The anatomic and physiologic complexities of the hypothalamus and pituitary gland increase their susceptibility to injury from TBI. The pituitary gland is connected to the hypothalamus by a blood vessel-containing stalk, making the pituitary gland—particularly the anterior portion—susceptible to damage during a head injury.13 The hypothalamus secretes thyrotropin-releasing hormone (TRH) and luteinizing-releasing hormone (LRH) to stimulate or suppress the production of anterior pituitary gland hormones, which in turn stimulate the release of hormones and other substances from target organs. Anterior pituitary hormones are growth hormone (GH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), adrenocorticotropic hormone (ACTH), thyroid-stimulating hormone (TSH), and prolactin (PRL). The posterior pituitary secretes oxytocin and vasopressin, also known as antidiuretic hormone (ADH).13
Impact from a direct blow with an object or from a concussive blast can cause focal trauma or rotational shearing of tissue internally. Resultant vascular injury, rupture, cerebral edema, vasospasm, pituitary swelling, or inflammation may then initiate an endocrine response that drives a cascade of complex hormonal processes.5,7,8 Anterior pituitary deficiencies account for the majority of chronic neuroendocrine disorders following mTBI. GH and gonadotropin deficiencies are the most common, but TSH deficiency (secondary hypothyroidism) and ACTH deficiency (adrenal insufficiency) may occur as well, although in <10% of cases with TBI associated NED.12
Clinical features of NED mimic those of other conditions
The symptoms of NED include fatigue, insomnia, impaired cognition, memory loss, difficulty concentrating, and emotional and mood disturbances (TABLE).7,12,14-17 Various combinations of these symptoms may occur and are similar to those of other post-mTBI conditions, such as sleep problems, postconcussive syndrome (PCS), and memory and attention difficulties.18 The onset of NED may be immediate (eg, in diabetes insipidus [DI] or syndrome of inappropriate antidiuretic hormone [SIADH], which are very rare in mTBI) and potentially life-threatening (eg, in sodium and potassium imbalances), or may be nonspecific and take years to manifest.6,10,15,19 Additionally, symptoms of NED may spontaneously resolve or persist. Studies have demonstrated pituitary dysfunction in the acute postinjury phase as well as its development as late as 2 to 3 years after injury.7,8,11,20
Due to the range of symptoms related to the combinations of possible hormonal derangements, NED can be an elusive diagnosis and may have a deleterious effect on individuals who sustain TBI.12 For example, an undiagnosed GH deficiency—which can result in increased abdominal fat mass and decreased lean muscle mass as well as impaired cardiac function, dyslipidemia, and insulin resistance—makes it more difficult for an affected individual either to recover from additional injuries or to maintain fitness. Considering NED may avoid a delay in diagnosis and improve prognosis.7,8,20
Findings leading to recommendations on diagnosis
Primary care providers, military and civilian alike, can benefit from the findings and recommendations of an expert panel assembled by the Defense Centers of Excellence (DCoE) for Psychological Health and Traumatic Brain Injury to address NED in mTBI. The panel that convened in December 2010 included experts representing the military services, the Department of Veterans Affairs, DCoE, and civilian sectors. Based on the group’s recommendations combined with literature review findings, the DCoE developed a clinical recommendation to encourage primary care providers to consider screening for NED in patients with persistent symptoms following mTBI.21 Key findings and issues identified by the group included the following:
• The most frequent mechanism of injury in the military deployed population is blast-induced TBI. Such injury could occur in the civilian population at construction blast sites or in factories producing or using highly flammable substances.
• The prevalence of any anterior pituitary hormone deficiency is as high as 30% to hormone deficiency is as high as 30% to 80% at 24 to 36 months post injury.
• The prevalence of posterior pituitary hormone deficiency is as high as 4% to 7% at 12 months post injury.
• The anterior pituitary hormones most frequently affected in survivors of TBI are ACTH, gonadotropin, prolactin, and GH.
• In 2004 Agha et al,22 reported >28% of survivors of TBI had at least one anterior pituitary hormone deficiency.
• According to research by Agha et al23 in 2005, >20% of survivors of TBI developed DI; those who developed DI, either acutely or permanently, were more likely to have sustained a severe TBI.
• The development of pituitary dysfunction is independent of the severity of TBI.
• In 2005, civilian guidelines4 recommended screening for pituitary dysfunction in all patients who sustained a moderate to severe TBI.
• In 2010, civilian guidelines7 recommended screening for pituitary dysfunction in patients who sustained a mild TBI.
When to screen for NED after TBI
Given the complexities described—including the similarity of NED to other post-mTBI medical diagnoses and such concomitant disorders as a sleep disorder, memory difficulties, depression, PTSD, and PCS24—consider NED in the primary care setting following confirmed TBI of any severity level when symptoms suggestive of NED persist for >3 months following injury or appear up to 36 months later.7,8,12,20
Order a lab evaluation of blood levels for cortisol (drawn at 8 am), LH, FSH, PRL, insulin-like growth factor-1, TSH, free thyroxine-4, and testosterone for men (8 am) and estradiol for women (8 am). With frankly abnormal lab results or with borderline results and strong clinical suspicion for NED, refer for further endocrinology workup (FIGURE).21 Earlier diagnosis of NED results in more rapid improvement of symptoms and an improved prognosis.7,8,20 Postinjury screening for NED should be one component of a thorough clinical evaluation by a qualified provider, and not used in isolation for clinical decision making. NED screening should not be routinely ordered during the early stages of mTBI, defined as <3 months postinjury.
Provider awareness and willingness to include NED screening in a timely manner, and to refer to specialty services as indicated for symptoms that may be sleep related or psychiatric in nature, may increase the opportunities for early treatment, better rehabilitation outcomes, and better overall quality of life.
Looking ahead
While the DCoE expert group made recommendations on screening for NED in the military combat population, they also acknowledged that NED diagnosis and treatment would benefit from additional areas of research:
• the effect of GH replacement (for GH-deficient patients or as prophylaxis for all TBI patients) on rehabilitation response and quality of life
• the role of multiple TBIs on long-term cognition and possible premature aging
• the role of NED over time
• biomarkers for diagnosis
• factors affecting resiliency
• resiliency in the context of increased or decreased susceptibility to the development of an acute clinical syndrome, as well as susceptibility in developing the spectrum of consequences of TBI.
The research areas given the highest priority by the group were incidence and prevalence studies of pituitary dysfunction after TBI in the combat military population, including pre- and postdeployment rates of dysfunction and the incidence of comorbidities. Also of benefit would be a retrospective study of the consequences of pituitary dysfunction that additionally addresses the effects of comorbid conditions commonly associated with TBI. Considering the rapid expansion in the field of mTBI, additional research and provider awareness concerning early identification and treatments may improve the outcomes for those with persistent mTBI symptoms.
CORRESPONDENCE
Theres A. West, DNP, APN, BC, Defense and Veterans Brain Injury Center, 1335 East West Highway, 6th floor, Silver Spring, MD 20910; [email protected]
› Consider neuroendocrine dysfunction (NED) following confirmed traumatic brain injury of any severity when symptoms suggestive of NED persist for >3 months after injury. A
› Order blood studies to detect deficiencies in pituitary and other key hormones when NED is suspected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The Centers for Disease Control and Prevention (CDC) reports that >1.7 million cases of traumatic brain injury (TBI) occur annually in the United States.1 More than 266,000 military service members sustained at least one TBI from 2000 to 2012.2 Most TBIs (80%-85%), military and civilian, are classified as mild (mTBI), and most mTBI patients (80%-85%) experience a complete functional recovery within 3 months of injury.1,3 The remaining 15% to 20% of mTBI patients experience persistent symptoms and difficulty in rehabilitation, particularly if there are concomitant disorders, such as post-traumatic stress disorder (PTSD), sleep disorders, acute stress disorder, substance abuse disorder, and depression.4,5 Symptoms that mTBI and these other disorders have in common can make differential diagnosis difficult, requiring a high degree of clinical awareness by primary care providers. An additional concern following mTBI is neuroendocrine dysfunction (NED). This association has not been widely discussed and therefore may go largely undiagnosed.6 Consider NED in the setting of prolonged symptoms or in patients experiencing difficulty with rehabilitation following mTBI.7,8
NED following mTBI is more common than once thought
The term “neuroendocrine dysfunction,” as discussed in this article, refers to a variety of conditions caused by imbalances in the body’s hormone production directly related to the pituitary, hypothalamus, and their axes following TBI. Until the past decade, the incidence of TBI-associated pituitary dysfunction was thought to be an uncommon event, usually associated with catastrophic head injuries. Studies of NED in TBI patients focused primarily on moderate or severe TBI, usually from motor vehicle incidents, falls, and assaults.7 Other research has since shown that NED occurs more commonly than once believed.9 And while the risk of NED may be higher for patients who sustain more severe brain injuries, NED also occurs in mTBI.7,9,10,11 Interestingly, a recent literature review indicated that the incidence of NED in mTBI was 16.8%, while the incidence with moderate TBI was reported at 10.9%.7 Other research has noted that the incidence of NED in mTBI may be as high as 42%.9,12 No evidence suggests that the severity of NED is related to a specific hormonal dysfunction, nor is there evidence that NED may be associated with a specific mechanism of injury.
Pituitary anatomy is susceptible to injury and dysfunction
The anatomic and physiologic complexities of the hypothalamus and pituitary gland increase their susceptibility to injury from TBI. The pituitary gland is connected to the hypothalamus by a blood vessel-containing stalk, making the pituitary gland—particularly the anterior portion—susceptible to damage during a head injury.13 The hypothalamus secretes thyrotropin-releasing hormone (TRH) and luteinizing-releasing hormone (LRH) to stimulate or suppress the production of anterior pituitary gland hormones, which in turn stimulate the release of hormones and other substances from target organs. Anterior pituitary hormones are growth hormone (GH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), adrenocorticotropic hormone (ACTH), thyroid-stimulating hormone (TSH), and prolactin (PRL). The posterior pituitary secretes oxytocin and vasopressin, also known as antidiuretic hormone (ADH).13
Impact from a direct blow with an object or from a concussive blast can cause focal trauma or rotational shearing of tissue internally. Resultant vascular injury, rupture, cerebral edema, vasospasm, pituitary swelling, or inflammation may then initiate an endocrine response that drives a cascade of complex hormonal processes.5,7,8 Anterior pituitary deficiencies account for the majority of chronic neuroendocrine disorders following mTBI. GH and gonadotropin deficiencies are the most common, but TSH deficiency (secondary hypothyroidism) and ACTH deficiency (adrenal insufficiency) may occur as well, although in <10% of cases with TBI associated NED.12
Clinical features of NED mimic those of other conditions
The symptoms of NED include fatigue, insomnia, impaired cognition, memory loss, difficulty concentrating, and emotional and mood disturbances (TABLE).7,12,14-17 Various combinations of these symptoms may occur and are similar to those of other post-mTBI conditions, such as sleep problems, postconcussive syndrome (PCS), and memory and attention difficulties.18 The onset of NED may be immediate (eg, in diabetes insipidus [DI] or syndrome of inappropriate antidiuretic hormone [SIADH], which are very rare in mTBI) and potentially life-threatening (eg, in sodium and potassium imbalances), or may be nonspecific and take years to manifest.6,10,15,19 Additionally, symptoms of NED may spontaneously resolve or persist. Studies have demonstrated pituitary dysfunction in the acute postinjury phase as well as its development as late as 2 to 3 years after injury.7,8,11,20
Due to the range of symptoms related to the combinations of possible hormonal derangements, NED can be an elusive diagnosis and may have a deleterious effect on individuals who sustain TBI.12 For example, an undiagnosed GH deficiency—which can result in increased abdominal fat mass and decreased lean muscle mass as well as impaired cardiac function, dyslipidemia, and insulin resistance—makes it more difficult for an affected individual either to recover from additional injuries or to maintain fitness. Considering NED may avoid a delay in diagnosis and improve prognosis.7,8,20
Findings leading to recommendations on diagnosis
Primary care providers, military and civilian alike, can benefit from the findings and recommendations of an expert panel assembled by the Defense Centers of Excellence (DCoE) for Psychological Health and Traumatic Brain Injury to address NED in mTBI. The panel that convened in December 2010 included experts representing the military services, the Department of Veterans Affairs, DCoE, and civilian sectors. Based on the group’s recommendations combined with literature review findings, the DCoE developed a clinical recommendation to encourage primary care providers to consider screening for NED in patients with persistent symptoms following mTBI.21 Key findings and issues identified by the group included the following:
• The most frequent mechanism of injury in the military deployed population is blast-induced TBI. Such injury could occur in the civilian population at construction blast sites or in factories producing or using highly flammable substances.
• The prevalence of any anterior pituitary hormone deficiency is as high as 30% to hormone deficiency is as high as 30% to 80% at 24 to 36 months post injury.
• The prevalence of posterior pituitary hormone deficiency is as high as 4% to 7% at 12 months post injury.
• The anterior pituitary hormones most frequently affected in survivors of TBI are ACTH, gonadotropin, prolactin, and GH.
• In 2004 Agha et al,22 reported >28% of survivors of TBI had at least one anterior pituitary hormone deficiency.
• According to research by Agha et al23 in 2005, >20% of survivors of TBI developed DI; those who developed DI, either acutely or permanently, were more likely to have sustained a severe TBI.
• The development of pituitary dysfunction is independent of the severity of TBI.
• In 2005, civilian guidelines4 recommended screening for pituitary dysfunction in all patients who sustained a moderate to severe TBI.
• In 2010, civilian guidelines7 recommended screening for pituitary dysfunction in patients who sustained a mild TBI.
When to screen for NED after TBI
Given the complexities described—including the similarity of NED to other post-mTBI medical diagnoses and such concomitant disorders as a sleep disorder, memory difficulties, depression, PTSD, and PCS24—consider NED in the primary care setting following confirmed TBI of any severity level when symptoms suggestive of NED persist for >3 months following injury or appear up to 36 months later.7,8,12,20
Order a lab evaluation of blood levels for cortisol (drawn at 8 am), LH, FSH, PRL, insulin-like growth factor-1, TSH, free thyroxine-4, and testosterone for men (8 am) and estradiol for women (8 am). With frankly abnormal lab results or with borderline results and strong clinical suspicion for NED, refer for further endocrinology workup (FIGURE).21 Earlier diagnosis of NED results in more rapid improvement of symptoms and an improved prognosis.7,8,20 Postinjury screening for NED should be one component of a thorough clinical evaluation by a qualified provider, and not used in isolation for clinical decision making. NED screening should not be routinely ordered during the early stages of mTBI, defined as <3 months postinjury.
Provider awareness and willingness to include NED screening in a timely manner, and to refer to specialty services as indicated for symptoms that may be sleep related or psychiatric in nature, may increase the opportunities for early treatment, better rehabilitation outcomes, and better overall quality of life.
Looking ahead
While the DCoE expert group made recommendations on screening for NED in the military combat population, they also acknowledged that NED diagnosis and treatment would benefit from additional areas of research:
• the effect of GH replacement (for GH-deficient patients or as prophylaxis for all TBI patients) on rehabilitation response and quality of life
• the role of multiple TBIs on long-term cognition and possible premature aging
• the role of NED over time
• biomarkers for diagnosis
• factors affecting resiliency
• resiliency in the context of increased or decreased susceptibility to the development of an acute clinical syndrome, as well as susceptibility in developing the spectrum of consequences of TBI.
The research areas given the highest priority by the group were incidence and prevalence studies of pituitary dysfunction after TBI in the combat military population, including pre- and postdeployment rates of dysfunction and the incidence of comorbidities. Also of benefit would be a retrospective study of the consequences of pituitary dysfunction that additionally addresses the effects of comorbid conditions commonly associated with TBI. Considering the rapid expansion in the field of mTBI, additional research and provider awareness concerning early identification and treatments may improve the outcomes for those with persistent mTBI symptoms.
CORRESPONDENCE
Theres A. West, DNP, APN, BC, Defense and Veterans Brain Injury Center, 1335 East West Highway, 6th floor, Silver Spring, MD 20910; [email protected]
1. Injury prevention & control: Traumatic brain injury national TBI estimates. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/traumaticbraininjury/statistics.html. Accessed August 12, 2013.
2. Armed Forces Surveillance Center. DoD Worldwide Numbers for TBI. Defense and Veterans Brain Injury Centers Web site. Available at: www.dvbic.org/dod-worldwide-Numbers-tbi. Accessed August 12, 2013.
3. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury (mTBI). Available at: http://www.healthquality.va.gov/management_of_concussion_mtbi.asp. Published April 2009. Accessed August 12, 2013.
4. Ghigo E, Masel B, Aimaretti G, et al. Consensus guidelines on screening for hypopituitarism following traumatic brain injury. Brain Inj. 2005;19:711-724.
5. Krahulik D, Zapletalova J, Frysak Z, et al. Dysfunction of hypothalamic-hyperphysical axis after traumatic brain injury in adults. J Neurosurg. 2010;113:581-584.
6. Behan LA, Phillips J, Thompson CJ, et al. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008;79:753-759.
7. Tanriverdi F, Unluhizarci K, Kelestimur F. Pituitary function in subjects with mild traumatic brain injury: a review of literature and proposal of a screening strategy. Pituitary. 2010;13:146-153.
8. Bondanelli M, Ambrosio MR, Zatelli MC, et al. Hypopituitarism after traumatic brain injury. Eur J Endocrinol. 2005;152:679-691.
9. Wilkinson CW, Pagulayan KF, Petrie EC, et al. High prevalence of chronic pituitary and target-hormone abnormalities after blast related mild traumatic brain injury. Front Neurol. 2012;3:11.
10. Bondanelli M, Ambrosio MR, Cavazzini L, et al. Anterior pituitary function may predict functional and cognitive outcome in patients with traumatic brain injury undergoing rehabilitation. J Neurotrauma. 2007;24:1687-1697.
11. Benvenga S, Campenmi A, Ruggeri R, et al. Hypopituitarism secondary to head trauma. J Clin Endocrinol Metab. 2000;85:1353-1361.
12. Schneider H, Kreitschman-Andermahr I, Ghigo E, et al. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA. 2007;298:1429-1438.
13. Amar AP, Weiss MH. Pituitary anatomy and physiology. Neurosurg Clin N Am. 2003;14:11-23.
14. Tanriverdi F, Unluhizarci K, Kocyigit I, et al. Brief communication: Pituitary volume and function in competing and retired male boxers. Ann Intern Med. 2008;148:827-831.
15. Bondanelli M, De Marinis L, Ambrosio MR, et al. Occurrence of pituitary dysfunction following traumatic brain injury. J Neurotrauma. 2004;21:685-696.
16. Klose M, Watt T, Brennum J, et al. Posttraumatic hypopituitarism is associated with an unfavorable body composition and lipid profile, and decreased quality of life in 12 months after injury. J Clin Endocrinol Metab. 2007;92:3861-3868.
17. Aimeretti G, Ambrosio MR, Di Somma C, et al. Residual pituitary function after brain injury-induced hypopituitaryism: a prospective 12-month study. J Clin Endocrinol Metab. 2005;90:6085-6092.
18. Agha A, Phillips J, Thompson CJ. Hypopituitarism following traumatic brain injury. Br J Neurosurg. 2007;21:210-216.
19. Cohan P, Wang C, McArthur DL, et al. Acute secondary adrenal insufficiency after traumatic brain injury: a prospective study. Crit Care Med. 2005;33:2358-2366.
20. Rothman MS, Arciniegas DS, Filley CM, et al. The neuroendocrine effects of traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2007;19:363-372.
21. Defense Centers of Excellence. Neuroendocrine screening post mild TBI clinical recommendation. Available at: http://www.dcoe.mil/Content/Navigation/Documents/DCoE_TBI_NED_Reference_Card.pdf. Published February 2012. Accessed August 12, 2013.
22. Agha A, Rogers B, Mylotte D, et al. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin Endocrinol (Oxf). 2004;60:584-591.
23. Agha A, Phillips J, O’Kelly P, et al. The natural history of post-traumatic hypopituitarism: implications for assessment and treatment. Am J Med. 2005;118:1416.
24. Guerrero AF, Alfonso A. Traumatic brain injury related hypopituitarism: a review and recommendations for screening combat veterans. Mil Med. 2010;175:574-580.
1. Injury prevention & control: Traumatic brain injury national TBI estimates. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/traumaticbraininjury/statistics.html. Accessed August 12, 2013.
2. Armed Forces Surveillance Center. DoD Worldwide Numbers for TBI. Defense and Veterans Brain Injury Centers Web site. Available at: www.dvbic.org/dod-worldwide-Numbers-tbi. Accessed August 12, 2013.
3. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury (mTBI). Available at: http://www.healthquality.va.gov/management_of_concussion_mtbi.asp. Published April 2009. Accessed August 12, 2013.
4. Ghigo E, Masel B, Aimaretti G, et al. Consensus guidelines on screening for hypopituitarism following traumatic brain injury. Brain Inj. 2005;19:711-724.
5. Krahulik D, Zapletalova J, Frysak Z, et al. Dysfunction of hypothalamic-hyperphysical axis after traumatic brain injury in adults. J Neurosurg. 2010;113:581-584.
6. Behan LA, Phillips J, Thompson CJ, et al. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008;79:753-759.
7. Tanriverdi F, Unluhizarci K, Kelestimur F. Pituitary function in subjects with mild traumatic brain injury: a review of literature and proposal of a screening strategy. Pituitary. 2010;13:146-153.
8. Bondanelli M, Ambrosio MR, Zatelli MC, et al. Hypopituitarism after traumatic brain injury. Eur J Endocrinol. 2005;152:679-691.
9. Wilkinson CW, Pagulayan KF, Petrie EC, et al. High prevalence of chronic pituitary and target-hormone abnormalities after blast related mild traumatic brain injury. Front Neurol. 2012;3:11.
10. Bondanelli M, Ambrosio MR, Cavazzini L, et al. Anterior pituitary function may predict functional and cognitive outcome in patients with traumatic brain injury undergoing rehabilitation. J Neurotrauma. 2007;24:1687-1697.
11. Benvenga S, Campenmi A, Ruggeri R, et al. Hypopituitarism secondary to head trauma. J Clin Endocrinol Metab. 2000;85:1353-1361.
12. Schneider H, Kreitschman-Andermahr I, Ghigo E, et al. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA. 2007;298:1429-1438.
13. Amar AP, Weiss MH. Pituitary anatomy and physiology. Neurosurg Clin N Am. 2003;14:11-23.
14. Tanriverdi F, Unluhizarci K, Kocyigit I, et al. Brief communication: Pituitary volume and function in competing and retired male boxers. Ann Intern Med. 2008;148:827-831.
15. Bondanelli M, De Marinis L, Ambrosio MR, et al. Occurrence of pituitary dysfunction following traumatic brain injury. J Neurotrauma. 2004;21:685-696.
16. Klose M, Watt T, Brennum J, et al. Posttraumatic hypopituitarism is associated with an unfavorable body composition and lipid profile, and decreased quality of life in 12 months after injury. J Clin Endocrinol Metab. 2007;92:3861-3868.
17. Aimeretti G, Ambrosio MR, Di Somma C, et al. Residual pituitary function after brain injury-induced hypopituitaryism: a prospective 12-month study. J Clin Endocrinol Metab. 2005;90:6085-6092.
18. Agha A, Phillips J, Thompson CJ. Hypopituitarism following traumatic brain injury. Br J Neurosurg. 2007;21:210-216.
19. Cohan P, Wang C, McArthur DL, et al. Acute secondary adrenal insufficiency after traumatic brain injury: a prospective study. Crit Care Med. 2005;33:2358-2366.
20. Rothman MS, Arciniegas DS, Filley CM, et al. The neuroendocrine effects of traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2007;19:363-372.
21. Defense Centers of Excellence. Neuroendocrine screening post mild TBI clinical recommendation. Available at: http://www.dcoe.mil/Content/Navigation/Documents/DCoE_TBI_NED_Reference_Card.pdf. Published February 2012. Accessed August 12, 2013.
22. Agha A, Rogers B, Mylotte D, et al. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin Endocrinol (Oxf). 2004;60:584-591.
23. Agha A, Phillips J, O’Kelly P, et al. The natural history of post-traumatic hypopituitarism: implications for assessment and treatment. Am J Med. 2005;118:1416.
24. Guerrero AF, Alfonso A. Traumatic brain injury related hypopituitarism: a review and recommendations for screening combat veterans. Mil Med. 2010;175:574-580.