User login
Analysis of Direct Costs of Outpatient Arthroscopic Rotator Cuff Repair
Musculoskeletal disorders, the leading cause of disability in the United States,1 account for more than half of all persons reporting missing a workday because of a medical condition.2 Shoulder disorders in particular play a significant role in the burden of musculoskeletal disorders and cost of care. In 2008, 18.9 million adults (8.2% of the US adult population) reported chronic shoulder pain.1 Among shoulder disorders, rotator cuff pathology is the most common cause of shoulder-related disability found by orthopedic surgeons.3 Rotator cuff surgery (RCS) is one of the most commonly performed orthopedic surgical procedures, and surgery volume is on the rise. One study found a 141% increase in rotator cuff repairs between the years 1996 (~41 per 100,000 population) and 2006 (~98 per 100,000 population).4
US health care costs are also increasing. In 2011, $2.7 trillion was spent on health care, representing 17.9% of the national gross domestic product (GDP). According to projections, costs will rise to $4.6 trillion by 2020.5 In particular, as patients continue to live longer and remain more active into their later years, the costs of treating and managing musculoskeletal disorders become more important from a public policy standpoint. In 2006, the cost of treating musculoskeletal disorders alone was $576 billion, representing 4.5% of that year’s GDP.2
Paramount in this era of rising costs is the idea of maximizing the value of health care dollars. Health care economists Porter and Teisberg6 defined value as patient health outcomes achieved per dollar of cost expended in a care cycle (diagnosis, treatment, ongoing management) for a particular disease or disorder. For proper management of value, outcomes and costs for an entire cycle of care must be determined. From a practical standpoint, this first requires determining the true cost of a care cycle—dollars spent on personnel, equipment, materials, and other resources required to deliver a particular service—rather than the amount charged or reimbursed for providing the service in question.7
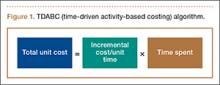
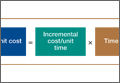
Kaplan and Anderson8,9 described the TDABC (time-driven activity-based costing) algorithm for calculating the cost of delivering a service based on 2 parameters: unit cost of a particular resource, and time required to supply it. These parameters apply to material costs and labor costs. In the medical setting, the TDABC algorithm can be applied by defining a care delivery value chain for each aspect of patient care and then multiplying incremental cost per unit time by time required to deliver that resource (Figure 1). Tabulating the overall unit cost for each resource then yields the overall cost of the care cycle. Clinical outcomes data can then be determined and used to calculate overall value for the patient care cycle.
In the study reported here, we used the TDABC algorithm to calculate the direct financial costs of surgical treatment of rotator cuff tears confirmed by magnetic resonance imaging (MRI) in an academic medical center.
Methods
Per our institution’s Office for the Protection of Research Subjects, institutional review board (IRB) approval is required only for projects using “human subjects” as defined by federal policy. In the present study, no private information could be identified, and all data were obtained from hospital billing records without intervention or interaction with individual patients. Accordingly, IRB approval was deemed unnecessary for our economic cost analysis.
Billing records of a single academic fellowship-trained sports surgeon were reviewed to identify patients who underwent primary repair of an MRI-confirmed rotator cuff tear between April 1, 2009, and July 31, 2012. Patients who had undergone prior shoulder surgery of any type were excluded from the study. Operative reports were reviewed, and exact surgical procedures performed were noted. The operating surgeon selected the specific repair techniques, including single- or double-row repair, with emphasis on restoring footprint coverage and avoiding overtensioning.
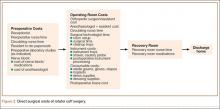
All surgeries were performed in an outpatient surgical center owned and operated by the surgeon’s home university. Surgeries were performed by the attending physician assisted by a senior orthopedic resident. The RCS care cycle was divided into 3 phases (Figure 2):
1. Preoperative. Patient’s interaction with receptionist in surgery center, time with preoperative nurse and circulating nurse in preoperative area, resident check-in time, and time placing preoperative nerve block and consumable materials used during block placement.
2. Operative. Time in operating room with surgical team for RCS, consumable materials used during surgery (eg, anchors, shavers, drapes), anesthetic medications, shoulder abduction pillow placed on completion of surgery, and cost of instrument processing.
3. Postoperative. Time in postoperative recovery area with recovery room nursing staff.
Time in each portion of the care cycle was directly observed and tabulated by hospital volunteers in the surgery center. Institutional billing data were used to identify material resources consumed, and the actual cost paid by the hospital for these resources was obtained from internal records. Mean hourly salary data and standard benefit rates were obtained for surgery center staff. Attending physician salary was extrapolated from published mean market salary data for academic physicians and mean hours worked,10,11 and resident physician costs were tabulated from publically available institutional payroll data and average resident work hours at our institution. These cost data and times were then used to tabulate total cost for the RCS care cycle using the TDABC algorithm.
Results
We identified 28 shoulders in 26 patients (mean age, 54.5 years) who met the inclusion criteria. Of these 28 shoulders, 18 (64.3%) had an isolated supraspinatus tear, 8 (28.6%) had combined supraspinatus and infraspinatus tears, 1 (3.6%) had combined supraspinatus and subscapularis tears, and 1 (3.6%) had an isolated infraspinatus tear. Demographic data are listed in Table 1.
All patients received an interscalene nerve block in the preoperative area before being brought into the operating room. In our analysis, we included nerve block supply costs and the anesthesiologist’s mean time placing the nerve block.
In all cases, primary rotator cuff repair was performed with suture anchors (Parcus Medical) with the patient in the lateral decubitus position. In 13 (46%) of the 28 shoulders, this repair was described as “complex,” requiring double-row technique. Subacromial decompression and bursectomy were performed in addition to the rotator cuff repair. Labral débridement was performed in 23 patients, synovectomy in 10, biceps tenodesis with anchor (Smith & Nephew) in 1, and biceps tenotomy in 1. Mean time in operating room was 148 minutes; mean time in postoperative recovery unit was 105 minutes.
Directly observing the care cycle, hospital volunteers found that patients spent a mean of 15 minutes with the receptionist when they arrived in the outpatient surgical center, 25 minutes with nurses for check-in in the preoperative holding area, and 10 minutes with the anesthesiology resident and 15 minutes with the orthopedic surgery resident for preoperative evaluation and paperwork. Mean nerve block time was 20 minutes. Mean electrocardiogram (ECG) time (12 patients) was 15 minutes. The surgical technician spent a mean time of 20 minutes setting up the operating room before the patient was brought in and 15 minutes cleaning up after the patient was transferred to the recovery room. Costs of postoperative care in the recovery room were based on a 2:1 patient-to-nurse ratio, as is the standard practice in our outpatient surgery center.
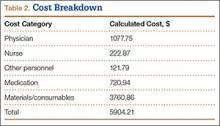
Using the times mentioned and our hospital’s salary data—including standard hospital benefits rates of 33.5% for nonphysicians and 17.65% for physicians—we determined, using the TDABC algorithm, a direct cost of $5904.21 for this process cycle, excluding hospital overhead and indirect costs. Table 2 provides the overall cost breakdown. Compared with the direct economic cost, the mean hospital charge to insurers for the procedure was $31,459.35. Mean reimbursement from insurers was $9679.08.
Overall attending and resident physician costs were $1077.75, which consisted of $623.66 for the surgeon and $454.09 for the anesthesiologist (included placement of nerve block and administration of anesthesia during surgery). Preoperative bloodwork was obtained in 23 cases, adding a mean cost of $111.04 after adjusting for standard hospital markup. Preoperative ECG was performed in 12 cases, for an added mean cost of $7.30 based on the TDABC algorithm.
We also broke down costs by care cycle phase. The preoperative phase, excluding the preoperative laboratory studies and ECGs (not performed in all cases), cost $134.34 (2.3% of total costs); the operative phase cost $5718.01 (96.8% of total costs); and the postoperative phase cost $51.86 (0.9% of total costs). Within the operative phase, the cost of consumables (specifically, suture anchors) was the main cost driver. Mean anchor cost per case was $3432.67. “Complex” tears involving a double-row repair averaged $4570.25 in anchor cost per patient, as compared with $2522.60 in anchor costs for simple repairs.
Discussion
US health care costs continue to increase unsustainably, with rising pressure on hospitals and providers to deliver the highest value for each health care dollar. The present study is the first to calculate (using the TDABC algorithm) the direct economic cost ($5904.21) of the entire RCS care cycle at a university-based outpatient surgery center. Rent, utility costs, administrative costs, overhead, and other indirect costs at the surgery center were not included in this cost analysis, as they would be incurred irrespective of type of surgery performed. As such, our data isolate the procedure-specific costs of rotator cuff repair in order to provide a more meaningful comparison for other institutions, where indirect costs may be different.
In the literature, rigorous economic analysis of shoulder pathology is sparse. Kuye and colleagues12 systematically reviewed economic evaluations in shoulder surgery for the period 1980–2010 and noted more than 50% of the papers were published between 2005 and 2010.12 They also noted the poor quality of these studies and concluded more rigorous economic evaluations are needed to help justify the rising costs of shoulder-related treatments.
Several studies have directly evaluated costs associated with RCS. Cordasco and colleagues13 detailed the success of open rotator cuff repair as an outpatient procedure—noting its 43% cost savings ($4300 for outpatient vs $7500 for inpatient) and high patient satisfaction—using hospital charge data for operating room time, supplies, instruments, and postoperative slings. Churchill and Ghorai14 evaluated costs of mini-open and arthroscopic rotator cuff repairs in a statewide database and estimated the arthroscopic repair cost at $8985, compared with $7841 for the mini-open repair. They used reported hospital charge data, which were not itemized and did not include physician professional fees. Adla and colleagues,15 in a similar analysis of open versus arthroscopic cuff repair, estimated direct material costs of $1609.50 (arthroscopic) and $360.75 (open); these figures were converted from 2005 UK currency using the exchange rate cited in their paper. Salaries of surgeon, anesthesiologist, and other operating room personnel were said to be included in the operating room cost, but the authors’ paper did not include these data.
Two studies directly estimated the costs of arthroscopic rotator cuff repair. Hearnden and Tennent16 calculated the cost of RCS at their UK institution to be £2672, which included cost of operating room consumable materials, medication, and salaries of operating room personnel, including surgeon and anesthesiologist. Using online currency conversion from 2008 exchange rates and adjusting for inflation gave a corresponding US cost of $5449.63.17 Vitale and colleagues18 prospectively calculated costs of arthroscopic rotator cuff repair over a 1-year period using a cost-to-charge ratio from tabulated inpatient charges, procedure charges, and physician fees and payments abstracted from medical records, hospital billing, and administrative databases. Mean total cost for this cycle was $10,605.20, which included several costs (physical therapy, radiologist fees) not included in the present study. These studies, though more comprehensive than prior work, did not capture the entire cycle of surgical care.
Our study was designed to provide initial data on the direct costs of arthroscopic repair of the rotator cuff for the entire process cycle. Our overall cost estimate of $5904.21 differs significantly from prior work—not unexpected given the completely different cost methodology used.
Our study had several limitations. First, it was a single-surgeon evaluation, and a number of operating room variables (eg, use of adjunct instrumentation such as radiofrequency probes, differences in draping preferences) as well as surgeon volume in performing rotator cuff repairs might have substantially affected the reproducibility and generalizability of our data. Similarly, the large number of adjunctive procedures (eg, subacromial decompression, labral débridement) performed in conjunction with the rotator cuff repairs added operative time and therefore increased overall cost. Double-row repairs added operative time and increased the cost of consumable materials as well. Differences in surgeon preference for suture anchors may also be important, as anchors are a major cost driver and can vary significantly between vendors and institutions. Tear-related variables (eg, tear size, tear chronicity, degree of fatty cuff degeneration) were not controlled for and might have significantly affected operative time and associated cost. Resident involvement in the surgical procedure and anesthesia process in an academic setting prolongs surgical time and thus directly impacts costs.
In addition, we used the patient’s time in the operating room as a proxy for actual surgical time, as this was the only reliable and reproducible data point available in our electronic medical record. As such, an unquantifiable amount of surgeon time may have been overallocated to our cost estimate for time spent inducing anesthesia, positioning, helping take the patient off the operating table, and so on. However, as typical surgeon practice is to be involved in these tasks in the operating room, the possible overestimate of surgeon cost is likely minimal.
Our salary data for the TDABC algorithm were based on national averages for work hours and gross income for physicians and on hospital-based wage structure and may not be generalizable to other institutions. There may also be regional differences in work hours and salaries, which in turn would factor into a different per-minute cost for surgeon and anesthesiologist, depending on the exact geographic area where the surgery is performed. Costs may be higher at institutions that use certified nurse anesthetists rather than resident physicians because of the salary differences between these practitioners.
Moreover, the time that patients spend in the holding area—waiting to go into surgery and, after surgery, waiting for their ride home, for their prescriptions to be ready, and so forth—is an important variable to consider from a cost standpoint. However, as this time varied significantly and involved minimal contact with hospital personnel, we excluded its associated costs from our analysis. Similarly, and as already noted, hospital overhead and other indirect costs were excluded from analysis as well.
Conclusion
Using the TDABC algorithm, we found a direct economic cost of $5904.21 for RCS at our academic outpatient surgical center, with anchor cost the main cost driver. Judicious use of consumable resources is a key focus for cost containment in arthroscopic shoulder surgery, particularly with respect to implantable suture anchors. However, in the setting of more complex tears that require multiple anchors in a double-row repair construct, our pilot data may be useful to hospitals and surgery centers negotiating procedural reimbursement for the increased cost of complex repairs. Use of the TDABC algorithm for RCS and other procedures may also help in identifying opportunities to deliver more cost-effective health care.
1. American Academy of Orthopaedic Surgeons. The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011.
2. National health expenditure data. Centers for Medicare & Medicare Services website. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/index.html. Updated May 5, 2014. Accessed December 1, 2015.
3. Tashjian RZ. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31(4):589-604.
4. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
5. Black EM, Higgins LD, Warner JJ. Value-based shoulder surgery: practicing outcomes-driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1000-1009.
6. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
7. Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46-52, 54, 56-61 passim.
8. Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev. 2004;82(11):131-138, 150.
9. Kaplan RS, Anderson SR. Time-Driven Activity-Based Costing: A Simpler and More Powerful Path to Higher Profits. Boston, MA: Harvard Business Review Press; 2007.
10. American Academy of Orthopaedic Surgeons. Orthopaedic Practice in the U.S. 2012. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2012.
11. Medical Group Management Association. Physician Compensation and Production Survey: 2012 Report Based on 2011 Data. Englewood, CO: Medical Group Management Association; 2012.
12. Kuye IO, Jain NB, Warner L, Herndon JH, Warner JJ. Economic evaluations in shoulder pathologies: a systematic review of the literature. J Shoulder Elbow Surg. 2012;21(3):367-375.
13. Cordasco FA, McGinley BJ, Charlton T. Rotator cuff repair as an outpatient procedure. J Shoulder Elbow Surg. 2000;9(1):27-30.
14. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
15. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
16. Hearnden A, Tennent D. The cost of shoulder arthroscopy: a comparison with national tariff. Ann R Coll Surg Engl. 2008;90(7):587-591.
17. Xrates currency conversion. http://www.x-rates.com/historical/?from=GBP&amount=1&date=2015-12-03. Accessed December 13, 2015.
18. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
Musculoskeletal disorders, the leading cause of disability in the United States,1 account for more than half of all persons reporting missing a workday because of a medical condition.2 Shoulder disorders in particular play a significant role in the burden of musculoskeletal disorders and cost of care. In 2008, 18.9 million adults (8.2% of the US adult population) reported chronic shoulder pain.1 Among shoulder disorders, rotator cuff pathology is the most common cause of shoulder-related disability found by orthopedic surgeons.3 Rotator cuff surgery (RCS) is one of the most commonly performed orthopedic surgical procedures, and surgery volume is on the rise. One study found a 141% increase in rotator cuff repairs between the years 1996 (~41 per 100,000 population) and 2006 (~98 per 100,000 population).4
US health care costs are also increasing. In 2011, $2.7 trillion was spent on health care, representing 17.9% of the national gross domestic product (GDP). According to projections, costs will rise to $4.6 trillion by 2020.5 In particular, as patients continue to live longer and remain more active into their later years, the costs of treating and managing musculoskeletal disorders become more important from a public policy standpoint. In 2006, the cost of treating musculoskeletal disorders alone was $576 billion, representing 4.5% of that year’s GDP.2
Paramount in this era of rising costs is the idea of maximizing the value of health care dollars. Health care economists Porter and Teisberg6 defined value as patient health outcomes achieved per dollar of cost expended in a care cycle (diagnosis, treatment, ongoing management) for a particular disease or disorder. For proper management of value, outcomes and costs for an entire cycle of care must be determined. From a practical standpoint, this first requires determining the true cost of a care cycle—dollars spent on personnel, equipment, materials, and other resources required to deliver a particular service—rather than the amount charged or reimbursed for providing the service in question.7
Kaplan and Anderson8,9 described the TDABC (time-driven activity-based costing) algorithm for calculating the cost of delivering a service based on 2 parameters: unit cost of a particular resource, and time required to supply it. These parameters apply to material costs and labor costs. In the medical setting, the TDABC algorithm can be applied by defining a care delivery value chain for each aspect of patient care and then multiplying incremental cost per unit time by time required to deliver that resource (Figure 1). Tabulating the overall unit cost for each resource then yields the overall cost of the care cycle. Clinical outcomes data can then be determined and used to calculate overall value for the patient care cycle.
In the study reported here, we used the TDABC algorithm to calculate the direct financial costs of surgical treatment of rotator cuff tears confirmed by magnetic resonance imaging (MRI) in an academic medical center.
Methods
Per our institution’s Office for the Protection of Research Subjects, institutional review board (IRB) approval is required only for projects using “human subjects” as defined by federal policy. In the present study, no private information could be identified, and all data were obtained from hospital billing records without intervention or interaction with individual patients. Accordingly, IRB approval was deemed unnecessary for our economic cost analysis.
Billing records of a single academic fellowship-trained sports surgeon were reviewed to identify patients who underwent primary repair of an MRI-confirmed rotator cuff tear between April 1, 2009, and July 31, 2012. Patients who had undergone prior shoulder surgery of any type were excluded from the study. Operative reports were reviewed, and exact surgical procedures performed were noted. The operating surgeon selected the specific repair techniques, including single- or double-row repair, with emphasis on restoring footprint coverage and avoiding overtensioning.
All surgeries were performed in an outpatient surgical center owned and operated by the surgeon’s home university. Surgeries were performed by the attending physician assisted by a senior orthopedic resident. The RCS care cycle was divided into 3 phases (Figure 2):
1. Preoperative. Patient’s interaction with receptionist in surgery center, time with preoperative nurse and circulating nurse in preoperative area, resident check-in time, and time placing preoperative nerve block and consumable materials used during block placement.
2. Operative. Time in operating room with surgical team for RCS, consumable materials used during surgery (eg, anchors, shavers, drapes), anesthetic medications, shoulder abduction pillow placed on completion of surgery, and cost of instrument processing.
3. Postoperative. Time in postoperative recovery area with recovery room nursing staff.
Time in each portion of the care cycle was directly observed and tabulated by hospital volunteers in the surgery center. Institutional billing data were used to identify material resources consumed, and the actual cost paid by the hospital for these resources was obtained from internal records. Mean hourly salary data and standard benefit rates were obtained for surgery center staff. Attending physician salary was extrapolated from published mean market salary data for academic physicians and mean hours worked,10,11 and resident physician costs were tabulated from publically available institutional payroll data and average resident work hours at our institution. These cost data and times were then used to tabulate total cost for the RCS care cycle using the TDABC algorithm.
Results
We identified 28 shoulders in 26 patients (mean age, 54.5 years) who met the inclusion criteria. Of these 28 shoulders, 18 (64.3%) had an isolated supraspinatus tear, 8 (28.6%) had combined supraspinatus and infraspinatus tears, 1 (3.6%) had combined supraspinatus and subscapularis tears, and 1 (3.6%) had an isolated infraspinatus tear. Demographic data are listed in Table 1.
All patients received an interscalene nerve block in the preoperative area before being brought into the operating room. In our analysis, we included nerve block supply costs and the anesthesiologist’s mean time placing the nerve block.
In all cases, primary rotator cuff repair was performed with suture anchors (Parcus Medical) with the patient in the lateral decubitus position. In 13 (46%) of the 28 shoulders, this repair was described as “complex,” requiring double-row technique. Subacromial decompression and bursectomy were performed in addition to the rotator cuff repair. Labral débridement was performed in 23 patients, synovectomy in 10, biceps tenodesis with anchor (Smith & Nephew) in 1, and biceps tenotomy in 1. Mean time in operating room was 148 minutes; mean time in postoperative recovery unit was 105 minutes.
Directly observing the care cycle, hospital volunteers found that patients spent a mean of 15 minutes with the receptionist when they arrived in the outpatient surgical center, 25 minutes with nurses for check-in in the preoperative holding area, and 10 minutes with the anesthesiology resident and 15 minutes with the orthopedic surgery resident for preoperative evaluation and paperwork. Mean nerve block time was 20 minutes. Mean electrocardiogram (ECG) time (12 patients) was 15 minutes. The surgical technician spent a mean time of 20 minutes setting up the operating room before the patient was brought in and 15 minutes cleaning up after the patient was transferred to the recovery room. Costs of postoperative care in the recovery room were based on a 2:1 patient-to-nurse ratio, as is the standard practice in our outpatient surgery center.
Using the times mentioned and our hospital’s salary data—including standard hospital benefits rates of 33.5% for nonphysicians and 17.65% for physicians—we determined, using the TDABC algorithm, a direct cost of $5904.21 for this process cycle, excluding hospital overhead and indirect costs. Table 2 provides the overall cost breakdown. Compared with the direct economic cost, the mean hospital charge to insurers for the procedure was $31,459.35. Mean reimbursement from insurers was $9679.08.
Overall attending and resident physician costs were $1077.75, which consisted of $623.66 for the surgeon and $454.09 for the anesthesiologist (included placement of nerve block and administration of anesthesia during surgery). Preoperative bloodwork was obtained in 23 cases, adding a mean cost of $111.04 after adjusting for standard hospital markup. Preoperative ECG was performed in 12 cases, for an added mean cost of $7.30 based on the TDABC algorithm.
We also broke down costs by care cycle phase. The preoperative phase, excluding the preoperative laboratory studies and ECGs (not performed in all cases), cost $134.34 (2.3% of total costs); the operative phase cost $5718.01 (96.8% of total costs); and the postoperative phase cost $51.86 (0.9% of total costs). Within the operative phase, the cost of consumables (specifically, suture anchors) was the main cost driver. Mean anchor cost per case was $3432.67. “Complex” tears involving a double-row repair averaged $4570.25 in anchor cost per patient, as compared with $2522.60 in anchor costs for simple repairs.
Discussion
US health care costs continue to increase unsustainably, with rising pressure on hospitals and providers to deliver the highest value for each health care dollar. The present study is the first to calculate (using the TDABC algorithm) the direct economic cost ($5904.21) of the entire RCS care cycle at a university-based outpatient surgery center. Rent, utility costs, administrative costs, overhead, and other indirect costs at the surgery center were not included in this cost analysis, as they would be incurred irrespective of type of surgery performed. As such, our data isolate the procedure-specific costs of rotator cuff repair in order to provide a more meaningful comparison for other institutions, where indirect costs may be different.
In the literature, rigorous economic analysis of shoulder pathology is sparse. Kuye and colleagues12 systematically reviewed economic evaluations in shoulder surgery for the period 1980–2010 and noted more than 50% of the papers were published between 2005 and 2010.12 They also noted the poor quality of these studies and concluded more rigorous economic evaluations are needed to help justify the rising costs of shoulder-related treatments.
Several studies have directly evaluated costs associated with RCS. Cordasco and colleagues13 detailed the success of open rotator cuff repair as an outpatient procedure—noting its 43% cost savings ($4300 for outpatient vs $7500 for inpatient) and high patient satisfaction—using hospital charge data for operating room time, supplies, instruments, and postoperative slings. Churchill and Ghorai14 evaluated costs of mini-open and arthroscopic rotator cuff repairs in a statewide database and estimated the arthroscopic repair cost at $8985, compared with $7841 for the mini-open repair. They used reported hospital charge data, which were not itemized and did not include physician professional fees. Adla and colleagues,15 in a similar analysis of open versus arthroscopic cuff repair, estimated direct material costs of $1609.50 (arthroscopic) and $360.75 (open); these figures were converted from 2005 UK currency using the exchange rate cited in their paper. Salaries of surgeon, anesthesiologist, and other operating room personnel were said to be included in the operating room cost, but the authors’ paper did not include these data.
Two studies directly estimated the costs of arthroscopic rotator cuff repair. Hearnden and Tennent16 calculated the cost of RCS at their UK institution to be £2672, which included cost of operating room consumable materials, medication, and salaries of operating room personnel, including surgeon and anesthesiologist. Using online currency conversion from 2008 exchange rates and adjusting for inflation gave a corresponding US cost of $5449.63.17 Vitale and colleagues18 prospectively calculated costs of arthroscopic rotator cuff repair over a 1-year period using a cost-to-charge ratio from tabulated inpatient charges, procedure charges, and physician fees and payments abstracted from medical records, hospital billing, and administrative databases. Mean total cost for this cycle was $10,605.20, which included several costs (physical therapy, radiologist fees) not included in the present study. These studies, though more comprehensive than prior work, did not capture the entire cycle of surgical care.
Our study was designed to provide initial data on the direct costs of arthroscopic repair of the rotator cuff for the entire process cycle. Our overall cost estimate of $5904.21 differs significantly from prior work—not unexpected given the completely different cost methodology used.
Our study had several limitations. First, it was a single-surgeon evaluation, and a number of operating room variables (eg, use of adjunct instrumentation such as radiofrequency probes, differences in draping preferences) as well as surgeon volume in performing rotator cuff repairs might have substantially affected the reproducibility and generalizability of our data. Similarly, the large number of adjunctive procedures (eg, subacromial decompression, labral débridement) performed in conjunction with the rotator cuff repairs added operative time and therefore increased overall cost. Double-row repairs added operative time and increased the cost of consumable materials as well. Differences in surgeon preference for suture anchors may also be important, as anchors are a major cost driver and can vary significantly between vendors and institutions. Tear-related variables (eg, tear size, tear chronicity, degree of fatty cuff degeneration) were not controlled for and might have significantly affected operative time and associated cost. Resident involvement in the surgical procedure and anesthesia process in an academic setting prolongs surgical time and thus directly impacts costs.
In addition, we used the patient’s time in the operating room as a proxy for actual surgical time, as this was the only reliable and reproducible data point available in our electronic medical record. As such, an unquantifiable amount of surgeon time may have been overallocated to our cost estimate for time spent inducing anesthesia, positioning, helping take the patient off the operating table, and so on. However, as typical surgeon practice is to be involved in these tasks in the operating room, the possible overestimate of surgeon cost is likely minimal.
Our salary data for the TDABC algorithm were based on national averages for work hours and gross income for physicians and on hospital-based wage structure and may not be generalizable to other institutions. There may also be regional differences in work hours and salaries, which in turn would factor into a different per-minute cost for surgeon and anesthesiologist, depending on the exact geographic area where the surgery is performed. Costs may be higher at institutions that use certified nurse anesthetists rather than resident physicians because of the salary differences between these practitioners.
Moreover, the time that patients spend in the holding area—waiting to go into surgery and, after surgery, waiting for their ride home, for their prescriptions to be ready, and so forth—is an important variable to consider from a cost standpoint. However, as this time varied significantly and involved minimal contact with hospital personnel, we excluded its associated costs from our analysis. Similarly, and as already noted, hospital overhead and other indirect costs were excluded from analysis as well.
Conclusion
Using the TDABC algorithm, we found a direct economic cost of $5904.21 for RCS at our academic outpatient surgical center, with anchor cost the main cost driver. Judicious use of consumable resources is a key focus for cost containment in arthroscopic shoulder surgery, particularly with respect to implantable suture anchors. However, in the setting of more complex tears that require multiple anchors in a double-row repair construct, our pilot data may be useful to hospitals and surgery centers negotiating procedural reimbursement for the increased cost of complex repairs. Use of the TDABC algorithm for RCS and other procedures may also help in identifying opportunities to deliver more cost-effective health care.
Musculoskeletal disorders, the leading cause of disability in the United States,1 account for more than half of all persons reporting missing a workday because of a medical condition.2 Shoulder disorders in particular play a significant role in the burden of musculoskeletal disorders and cost of care. In 2008, 18.9 million adults (8.2% of the US adult population) reported chronic shoulder pain.1 Among shoulder disorders, rotator cuff pathology is the most common cause of shoulder-related disability found by orthopedic surgeons.3 Rotator cuff surgery (RCS) is one of the most commonly performed orthopedic surgical procedures, and surgery volume is on the rise. One study found a 141% increase in rotator cuff repairs between the years 1996 (~41 per 100,000 population) and 2006 (~98 per 100,000 population).4
US health care costs are also increasing. In 2011, $2.7 trillion was spent on health care, representing 17.9% of the national gross domestic product (GDP). According to projections, costs will rise to $4.6 trillion by 2020.5 In particular, as patients continue to live longer and remain more active into their later years, the costs of treating and managing musculoskeletal disorders become more important from a public policy standpoint. In 2006, the cost of treating musculoskeletal disorders alone was $576 billion, representing 4.5% of that year’s GDP.2
Paramount in this era of rising costs is the idea of maximizing the value of health care dollars. Health care economists Porter and Teisberg6 defined value as patient health outcomes achieved per dollar of cost expended in a care cycle (diagnosis, treatment, ongoing management) for a particular disease or disorder. For proper management of value, outcomes and costs for an entire cycle of care must be determined. From a practical standpoint, this first requires determining the true cost of a care cycle—dollars spent on personnel, equipment, materials, and other resources required to deliver a particular service—rather than the amount charged or reimbursed for providing the service in question.7
Kaplan and Anderson8,9 described the TDABC (time-driven activity-based costing) algorithm for calculating the cost of delivering a service based on 2 parameters: unit cost of a particular resource, and time required to supply it. These parameters apply to material costs and labor costs. In the medical setting, the TDABC algorithm can be applied by defining a care delivery value chain for each aspect of patient care and then multiplying incremental cost per unit time by time required to deliver that resource (Figure 1). Tabulating the overall unit cost for each resource then yields the overall cost of the care cycle. Clinical outcomes data can then be determined and used to calculate overall value for the patient care cycle.
In the study reported here, we used the TDABC algorithm to calculate the direct financial costs of surgical treatment of rotator cuff tears confirmed by magnetic resonance imaging (MRI) in an academic medical center.
Methods
Per our institution’s Office for the Protection of Research Subjects, institutional review board (IRB) approval is required only for projects using “human subjects” as defined by federal policy. In the present study, no private information could be identified, and all data were obtained from hospital billing records without intervention or interaction with individual patients. Accordingly, IRB approval was deemed unnecessary for our economic cost analysis.
Billing records of a single academic fellowship-trained sports surgeon were reviewed to identify patients who underwent primary repair of an MRI-confirmed rotator cuff tear between April 1, 2009, and July 31, 2012. Patients who had undergone prior shoulder surgery of any type were excluded from the study. Operative reports were reviewed, and exact surgical procedures performed were noted. The operating surgeon selected the specific repair techniques, including single- or double-row repair, with emphasis on restoring footprint coverage and avoiding overtensioning.
All surgeries were performed in an outpatient surgical center owned and operated by the surgeon’s home university. Surgeries were performed by the attending physician assisted by a senior orthopedic resident. The RCS care cycle was divided into 3 phases (Figure 2):
1. Preoperative. Patient’s interaction with receptionist in surgery center, time with preoperative nurse and circulating nurse in preoperative area, resident check-in time, and time placing preoperative nerve block and consumable materials used during block placement.
2. Operative. Time in operating room with surgical team for RCS, consumable materials used during surgery (eg, anchors, shavers, drapes), anesthetic medications, shoulder abduction pillow placed on completion of surgery, and cost of instrument processing.
3. Postoperative. Time in postoperative recovery area with recovery room nursing staff.
Time in each portion of the care cycle was directly observed and tabulated by hospital volunteers in the surgery center. Institutional billing data were used to identify material resources consumed, and the actual cost paid by the hospital for these resources was obtained from internal records. Mean hourly salary data and standard benefit rates were obtained for surgery center staff. Attending physician salary was extrapolated from published mean market salary data for academic physicians and mean hours worked,10,11 and resident physician costs were tabulated from publically available institutional payroll data and average resident work hours at our institution. These cost data and times were then used to tabulate total cost for the RCS care cycle using the TDABC algorithm.
Results
We identified 28 shoulders in 26 patients (mean age, 54.5 years) who met the inclusion criteria. Of these 28 shoulders, 18 (64.3%) had an isolated supraspinatus tear, 8 (28.6%) had combined supraspinatus and infraspinatus tears, 1 (3.6%) had combined supraspinatus and subscapularis tears, and 1 (3.6%) had an isolated infraspinatus tear. Demographic data are listed in Table 1.
All patients received an interscalene nerve block in the preoperative area before being brought into the operating room. In our analysis, we included nerve block supply costs and the anesthesiologist’s mean time placing the nerve block.
In all cases, primary rotator cuff repair was performed with suture anchors (Parcus Medical) with the patient in the lateral decubitus position. In 13 (46%) of the 28 shoulders, this repair was described as “complex,” requiring double-row technique. Subacromial decompression and bursectomy were performed in addition to the rotator cuff repair. Labral débridement was performed in 23 patients, synovectomy in 10, biceps tenodesis with anchor (Smith & Nephew) in 1, and biceps tenotomy in 1. Mean time in operating room was 148 minutes; mean time in postoperative recovery unit was 105 minutes.
Directly observing the care cycle, hospital volunteers found that patients spent a mean of 15 minutes with the receptionist when they arrived in the outpatient surgical center, 25 minutes with nurses for check-in in the preoperative holding area, and 10 minutes with the anesthesiology resident and 15 minutes with the orthopedic surgery resident for preoperative evaluation and paperwork. Mean nerve block time was 20 minutes. Mean electrocardiogram (ECG) time (12 patients) was 15 minutes. The surgical technician spent a mean time of 20 minutes setting up the operating room before the patient was brought in and 15 minutes cleaning up after the patient was transferred to the recovery room. Costs of postoperative care in the recovery room were based on a 2:1 patient-to-nurse ratio, as is the standard practice in our outpatient surgery center.
Using the times mentioned and our hospital’s salary data—including standard hospital benefits rates of 33.5% for nonphysicians and 17.65% for physicians—we determined, using the TDABC algorithm, a direct cost of $5904.21 for this process cycle, excluding hospital overhead and indirect costs. Table 2 provides the overall cost breakdown. Compared with the direct economic cost, the mean hospital charge to insurers for the procedure was $31,459.35. Mean reimbursement from insurers was $9679.08.
Overall attending and resident physician costs were $1077.75, which consisted of $623.66 for the surgeon and $454.09 for the anesthesiologist (included placement of nerve block and administration of anesthesia during surgery). Preoperative bloodwork was obtained in 23 cases, adding a mean cost of $111.04 after adjusting for standard hospital markup. Preoperative ECG was performed in 12 cases, for an added mean cost of $7.30 based on the TDABC algorithm.
We also broke down costs by care cycle phase. The preoperative phase, excluding the preoperative laboratory studies and ECGs (not performed in all cases), cost $134.34 (2.3% of total costs); the operative phase cost $5718.01 (96.8% of total costs); and the postoperative phase cost $51.86 (0.9% of total costs). Within the operative phase, the cost of consumables (specifically, suture anchors) was the main cost driver. Mean anchor cost per case was $3432.67. “Complex” tears involving a double-row repair averaged $4570.25 in anchor cost per patient, as compared with $2522.60 in anchor costs for simple repairs.
Discussion
US health care costs continue to increase unsustainably, with rising pressure on hospitals and providers to deliver the highest value for each health care dollar. The present study is the first to calculate (using the TDABC algorithm) the direct economic cost ($5904.21) of the entire RCS care cycle at a university-based outpatient surgery center. Rent, utility costs, administrative costs, overhead, and other indirect costs at the surgery center were not included in this cost analysis, as they would be incurred irrespective of type of surgery performed. As such, our data isolate the procedure-specific costs of rotator cuff repair in order to provide a more meaningful comparison for other institutions, where indirect costs may be different.
In the literature, rigorous economic analysis of shoulder pathology is sparse. Kuye and colleagues12 systematically reviewed economic evaluations in shoulder surgery for the period 1980–2010 and noted more than 50% of the papers were published between 2005 and 2010.12 They also noted the poor quality of these studies and concluded more rigorous economic evaluations are needed to help justify the rising costs of shoulder-related treatments.
Several studies have directly evaluated costs associated with RCS. Cordasco and colleagues13 detailed the success of open rotator cuff repair as an outpatient procedure—noting its 43% cost savings ($4300 for outpatient vs $7500 for inpatient) and high patient satisfaction—using hospital charge data for operating room time, supplies, instruments, and postoperative slings. Churchill and Ghorai14 evaluated costs of mini-open and arthroscopic rotator cuff repairs in a statewide database and estimated the arthroscopic repair cost at $8985, compared with $7841 for the mini-open repair. They used reported hospital charge data, which were not itemized and did not include physician professional fees. Adla and colleagues,15 in a similar analysis of open versus arthroscopic cuff repair, estimated direct material costs of $1609.50 (arthroscopic) and $360.75 (open); these figures were converted from 2005 UK currency using the exchange rate cited in their paper. Salaries of surgeon, anesthesiologist, and other operating room personnel were said to be included in the operating room cost, but the authors’ paper did not include these data.
Two studies directly estimated the costs of arthroscopic rotator cuff repair. Hearnden and Tennent16 calculated the cost of RCS at their UK institution to be £2672, which included cost of operating room consumable materials, medication, and salaries of operating room personnel, including surgeon and anesthesiologist. Using online currency conversion from 2008 exchange rates and adjusting for inflation gave a corresponding US cost of $5449.63.17 Vitale and colleagues18 prospectively calculated costs of arthroscopic rotator cuff repair over a 1-year period using a cost-to-charge ratio from tabulated inpatient charges, procedure charges, and physician fees and payments abstracted from medical records, hospital billing, and administrative databases. Mean total cost for this cycle was $10,605.20, which included several costs (physical therapy, radiologist fees) not included in the present study. These studies, though more comprehensive than prior work, did not capture the entire cycle of surgical care.
Our study was designed to provide initial data on the direct costs of arthroscopic repair of the rotator cuff for the entire process cycle. Our overall cost estimate of $5904.21 differs significantly from prior work—not unexpected given the completely different cost methodology used.
Our study had several limitations. First, it was a single-surgeon evaluation, and a number of operating room variables (eg, use of adjunct instrumentation such as radiofrequency probes, differences in draping preferences) as well as surgeon volume in performing rotator cuff repairs might have substantially affected the reproducibility and generalizability of our data. Similarly, the large number of adjunctive procedures (eg, subacromial decompression, labral débridement) performed in conjunction with the rotator cuff repairs added operative time and therefore increased overall cost. Double-row repairs added operative time and increased the cost of consumable materials as well. Differences in surgeon preference for suture anchors may also be important, as anchors are a major cost driver and can vary significantly between vendors and institutions. Tear-related variables (eg, tear size, tear chronicity, degree of fatty cuff degeneration) were not controlled for and might have significantly affected operative time and associated cost. Resident involvement in the surgical procedure and anesthesia process in an academic setting prolongs surgical time and thus directly impacts costs.
In addition, we used the patient’s time in the operating room as a proxy for actual surgical time, as this was the only reliable and reproducible data point available in our electronic medical record. As such, an unquantifiable amount of surgeon time may have been overallocated to our cost estimate for time spent inducing anesthesia, positioning, helping take the patient off the operating table, and so on. However, as typical surgeon practice is to be involved in these tasks in the operating room, the possible overestimate of surgeon cost is likely minimal.
Our salary data for the TDABC algorithm were based on national averages for work hours and gross income for physicians and on hospital-based wage structure and may not be generalizable to other institutions. There may also be regional differences in work hours and salaries, which in turn would factor into a different per-minute cost for surgeon and anesthesiologist, depending on the exact geographic area where the surgery is performed. Costs may be higher at institutions that use certified nurse anesthetists rather than resident physicians because of the salary differences between these practitioners.
Moreover, the time that patients spend in the holding area—waiting to go into surgery and, after surgery, waiting for their ride home, for their prescriptions to be ready, and so forth—is an important variable to consider from a cost standpoint. However, as this time varied significantly and involved minimal contact with hospital personnel, we excluded its associated costs from our analysis. Similarly, and as already noted, hospital overhead and other indirect costs were excluded from analysis as well.
Conclusion
Using the TDABC algorithm, we found a direct economic cost of $5904.21 for RCS at our academic outpatient surgical center, with anchor cost the main cost driver. Judicious use of consumable resources is a key focus for cost containment in arthroscopic shoulder surgery, particularly with respect to implantable suture anchors. However, in the setting of more complex tears that require multiple anchors in a double-row repair construct, our pilot data may be useful to hospitals and surgery centers negotiating procedural reimbursement for the increased cost of complex repairs. Use of the TDABC algorithm for RCS and other procedures may also help in identifying opportunities to deliver more cost-effective health care.
1. American Academy of Orthopaedic Surgeons. The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011.
2. National health expenditure data. Centers for Medicare & Medicare Services website. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/index.html. Updated May 5, 2014. Accessed December 1, 2015.
3. Tashjian RZ. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31(4):589-604.
4. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
5. Black EM, Higgins LD, Warner JJ. Value-based shoulder surgery: practicing outcomes-driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1000-1009.
6. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
7. Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46-52, 54, 56-61 passim.
8. Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev. 2004;82(11):131-138, 150.
9. Kaplan RS, Anderson SR. Time-Driven Activity-Based Costing: A Simpler and More Powerful Path to Higher Profits. Boston, MA: Harvard Business Review Press; 2007.
10. American Academy of Orthopaedic Surgeons. Orthopaedic Practice in the U.S. 2012. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2012.
11. Medical Group Management Association. Physician Compensation and Production Survey: 2012 Report Based on 2011 Data. Englewood, CO: Medical Group Management Association; 2012.
12. Kuye IO, Jain NB, Warner L, Herndon JH, Warner JJ. Economic evaluations in shoulder pathologies: a systematic review of the literature. J Shoulder Elbow Surg. 2012;21(3):367-375.
13. Cordasco FA, McGinley BJ, Charlton T. Rotator cuff repair as an outpatient procedure. J Shoulder Elbow Surg. 2000;9(1):27-30.
14. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
15. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
16. Hearnden A, Tennent D. The cost of shoulder arthroscopy: a comparison with national tariff. Ann R Coll Surg Engl. 2008;90(7):587-591.
17. Xrates currency conversion. http://www.x-rates.com/historical/?from=GBP&amount=1&date=2015-12-03. Accessed December 13, 2015.
18. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
1. American Academy of Orthopaedic Surgeons. The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011.
2. National health expenditure data. Centers for Medicare & Medicare Services website. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/index.html. Updated May 5, 2014. Accessed December 1, 2015.
3. Tashjian RZ. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31(4):589-604.
4. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
5. Black EM, Higgins LD, Warner JJ. Value-based shoulder surgery: practicing outcomes-driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1000-1009.
6. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
7. Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46-52, 54, 56-61 passim.
8. Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev. 2004;82(11):131-138, 150.
9. Kaplan RS, Anderson SR. Time-Driven Activity-Based Costing: A Simpler and More Powerful Path to Higher Profits. Boston, MA: Harvard Business Review Press; 2007.
10. American Academy of Orthopaedic Surgeons. Orthopaedic Practice in the U.S. 2012. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2012.
11. Medical Group Management Association. Physician Compensation and Production Survey: 2012 Report Based on 2011 Data. Englewood, CO: Medical Group Management Association; 2012.
12. Kuye IO, Jain NB, Warner L, Herndon JH, Warner JJ. Economic evaluations in shoulder pathologies: a systematic review of the literature. J Shoulder Elbow Surg. 2012;21(3):367-375.
13. Cordasco FA, McGinley BJ, Charlton T. Rotator cuff repair as an outpatient procedure. J Shoulder Elbow Surg. 2000;9(1):27-30.
14. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
15. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
16. Hearnden A, Tennent D. The cost of shoulder arthroscopy: a comparison with national tariff. Ann R Coll Surg Engl. 2008;90(7):587-591.
17. Xrates currency conversion. http://www.x-rates.com/historical/?from=GBP&amount=1&date=2015-12-03. Accessed December 13, 2015.
18. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
Fish Oil and Osteoarthritis: Current Evidence
First-line treatments for osteoarthritis (OA) are targeted at the inflammatory reaction that occurs after breakdown of articular cartilage through regular use of nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections, or surgical intervention. Associated activity restrictions and chronic pain have spurred a search for alternative treatments, commonly daily supplements such as glucosamine, chondroitin, and fish oil, to name a select few of the innumerable products reported to benefit patients with OA.
Background
Fish oil is 1 of the 2 most popular supplements among patients with OA. However, its effectiveness and precise benefit are still debated,1,2 and there is confusion about the definition of the product, the nature of investigations into its effectiveness, and the standardization of research unique to OA. Most fish oil research relates to patients with rheumatoid arthritis (RA). The anti-inflammatory benefits seen in patients with RA are generally applied to characterize fish oils as anti-inflammatory agents with a logical benefit in reducing OA symptoms. However, there is a dearth of independent and focused clinical results justifying that assumption. Further, lack of federal regulation of the supplement industry hinders conducting generalizable studies regarding medical benefit in a regulated and verified dose and form.3
The benefits of fish oil in RA treatment are well supported and accepted. In patients with RA, daily fish oil supplementation has been shown to reduce use of other medications and improve pain scores reported by both physicians and patients.4-10 The clinical efficacy of fish oil use in RA has been determined to be “reasonably strong,” with multiple studies confirming suppression of inflammatory cytokines in vitro and in vivo.11,12 The mechanism by which the inflammatory processes are augmented by fish oil supplementation suggests potential benefit to patients with OA, though review articles as recent as 2011 have concluded that research in that capacity is not sufficient to warrant recommendation.13,14
Most studies of OA-specific use of fish oils have been conducted in in vitro models. Treatment of bovine chondrocytes with omega-3 fatty acids causes reductions in inflammatory markers induced by interleukin 1, one of several proinflammatory cytokines that induce inflammation in OA at the gene and plasma levels, and these reductions have been reproduced.15-17 Although a preventive benefit was found in a study of pig medial collateral ligament fibroblasts, findings of later studies have been inconsistent.18 It also appears that fish oils may alter lipid composition in membranes, favoring incorporation of anti-inflammatory precursor n-3 fatty acids over proinflammatory precursor n-6 fatty acids in these model systems.19,20
Animal in vivo models have also been used to describe the effects of fish oil supplementation on OA. Assessment of dogs with OA before and after supplementation with the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) revealed improvement in clinical signs observed by owners, improvement in weight-bearing measured by veterinary clinicians, and decreased use of NSAIDs.21-24
Fish oil studies using osteoarthritic cartilage samples harvested during surgical procedures have demonstrated results consistent with other model systems described thus far. They have demonstrated a dose-dependent decrease in induced inflammatory destruction of tissue associated with fish oil supplementation. In addition, finding a lack of cellular toxicity, they have validated the safety of supplements.25,26 Proposed but unproven mechanisms for the anti-inflammatory actions of EPA and DHA include competition with n-6 fatty acids; presence of resolvins (anti-inflammatory molecules derived from EPA and DHA); presence of n-3 products that compete with proinflammatory molecules for receptors; reduction in gene expression of cytokines, cyclo-oxygenase 2, and degrading proteinases; interference in the signaling pathways of inflammation; and reduction in lymphocyte proliferation.26,27
Reduction in the n-6/n-3 ratio has been correlated with reduced inflammatory conditions such as OA, stemming from the epidemiologic evidence that higher n-3 intake in Eastern diets and lower intake of n-6 result in a lower incidence of these diseases.18,28,29 Studies have found sufficient evidence to suggest that this ratio has a role in OA, though not sufficient to recommend supplement use over diet modification.19 One study demonstrated an ability to favorably alter bone marrow lipid composition with n-3 fatty acid supplementation.10
The evidence leads to a conclusion of anti-inflammatory benefits from fish oils in these abstracted models. The multitude of basic science studies conducted on the anti-inflammatory properties of omega-3 fatty acids, only briefly reviewed here, supports the potential benefits colloquially ascribed to fish oil in the treatment of OA yet also implies the need for human clinical trials to address these properties clinically.
We reviewed the literature to address claims that fish oil supplementation can prevent or decrease severity of OA. We hypothesized there would be insufficient clinical studies to justify recommending supplementation to patients. Of note, the degree of heterogeneity in the evidence precluded performing a meta-analysis with any statistical validity.
Literature Review
In the PubMed database, we targeted the subject of fish oils and OA by using search terms that included omega-3, DHA, EPA, and alpha-linolenic acid. The MedLine and Google Scholar databases were searched as well. Results were limited to those reported in English and involving human subjects and clinical trials; results were excluded if they primarily involved patients with RA. Studies cited or mentioned in articles found through the PubMed search were evaluated according to the criteria mentioned, such that all relevant articles available at time of search are thought to be included, and these articles represent a reasonable presentation of the available evidence.
Findings
Our search revealed 6 clinical trials in which omega-3–containing supplements were used in the treatment of human OA with differing endpoints. We reviewed these trials in detail. One study, which used alteration of bone marrow lipids as an endpoint, was included for completeness of the evaluation of the relevant evidence.20 In addition, the study by Wang and colleagues,30 who assessed patients without clinical evidence of OA for development of bone marrow lesions, was reviewed. This study was deemed relevant to examine the process by which n-3 fatty acids alter knee structure, as subsequent risk of OA has not been elucidated, and effects on bone marrow lesions may indeed have a direct impact on the OA process. Results of the trials that were identified were varied between no significant difference in OA symptoms between treatment and control groups, implied benefits, and substantial benefits.
The first clinical study of omega-3 supplementation in OA treatment was conducted in 1992.31 The study compared 10 g of cod liver oil (containing 786 mg of EPA) with 10 g of olive oil, both taken daily over 24 weeks by 86 patients with OA. Effects were assessed by NSAID use (recorded in patient diary) and pain score (evaluated by clinician) every 4 weeks. The trial found no significant difference in effects between the oils.
Wang and colleagues30 used a food questionnaire to measure the n-3 intake of 293 healthy adults and quantified their bone marrow lesions after 10 years in an effort to describe how n-3 intake correlates with development of OA or pre-OA lesions. Higher intake of n-6 fatty acids was positively associated with presence of bone marrow lesions; n-3 intake had no association.
In a study of 84 patients who had joint replacement, Pritchett20 evaluated lipid alterations resulting from a regimen of 3 g of fish oil containing 11% DHA daily for a 6-month trial period, measuring lipids before and after the trial period. Pritchett20 found a 20% increase in long-chain fatty acids and a corresponding decrease in saturated fatty acids, as measured in bone marrow.
The supplement Phytalgic (Phythea Laboratories), which is advertised for OA, includes n-3 fatty acids, n-6 fatty acids, extract from Urtica dioica (the common nettle), zinc, and vitamin E. In a study by Jacquet and colleagues,32 this supplement was given 3 times daily over 3 separate 4-week periods to 81 patients with knee or hip OA. Measuring NSAID use with patient diaries and assessing pain with the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index every 4 weeks for 12 weeks, the authors found a significant decrease in NSAID use and, according to WOMAC results, a more than 50% reduction in pain and stiffness, and improved function.
One study compared the effects of glucosamine with and without omega-3 fatty acids in 182 patients with knee or hip OA.33 Each day, patients took 500 mg of glucosamine plus 3 capsules each containing either 444 mg of omega-3 fatty acids or 444 mg of an oil mixture. Pain was assessed with visual analog scale and the WOMAC scale 3 times over the 26-week study. More than 90% reductions in morning stiffness and pain were found for the combination of fish oil and glucosamine.
The Multicenter Osteoarthritis Study (MOST), published in February 2012, demonstrated that plasma levels of n-3 and n-6 polyunsaturated fatty acids (PUFAs) may be related to knee structural findings.34 This study confirmed that dietary modification of n-3 and n-6 PUFAs altered plasma concentration predictably. Higher DHA intake was associated with less evidence of OA on patellofemoral cartilage, though no association was found on tibiofemoral cartilage.34
Discussion
The lack of human clinical trials detailing the effects of fish oil supplementation in patients with OA is arguably the most significant hindrance to fish oil being routinely recommended. Since 1992, only 6 studies have addressed this topic, and their endpoints and results were inconsistent. These interventional trials had their limitations, including short duration, insufficient dosage, inappropriate n-3 choice, dietary interactions, genotype, and medication interactions.18 The present review is limited as well, by the quantity of evidence on the topic and by the focus (of the majority of the studies) on short-term alterations in pain and mobility instead of on disease-modifying potential. Short-term evaluation is unlikely to capture such an effect, which may require long-term supplementation to become evident.
The results of the study by Stammers and colleagues31 must be examined critically, as the likelihood of detection bias is high. Highly subjective assessments of effect, lack of standardized NSAID treatments, and limitations in patient numbers and disease severity raise concerns about validity. In addition, confounding variables (eg, medication interactions, alternative treatments, olive oil use) undermine the design. It is therefore difficult to interpret the results of this trial.
The study by Wang and colleagues30 did not involve supplementation, and intake was assessed only with food frequency questionnaires. It is therefore difficult to apply their results or findings to this review. In addition, the authors did not obtain baseline magnetic resonance imaging for comparison with that obtained at study completion—that is, they did not address any subclinical disease before dietary recording.
Pritchett20 acknowledged study limitations of small sample size and use of 1 subject as both patient and control. Although the study seemed to demonstrate that omega-3 supplementation augmented the lipid profile of joints, it did not directly demonstrate improvement in or prevention of OA. Identification of bone marrow lesions is not definitive proof of OA but an alteration that may correlate with development. The logical supposition is that altering the local environment may alter development of disease within that environment, though this is not proven.
An article reviewing the Phytalgic study highlighted the suspect nature of its results—claims that the supplement is 76% more effective than gold-standard corticosteroid injection.35 Also highlighted were lack of confirmed mechanism, questionable control, detection bias caused by aftertaste, and the high attrition rate in the placebo group. It is difficult to apply these results to fish oil supplementation, as Phytalgic contains other potentially confounding substances.
Of note, the findings of MOST were observational; n-3 and n-6 levels were not altered or supplemented. Altered disease process was demonstrated in patellofemoral cartilage but not in tibiofemoral cartilage in the same patient. The inconsistencies may be explained by the observational nature of the study and the lack of supplementation that would have produced a more significant increase in n-3 PUFA levels and thus more uniform conclusions, if in fact n-3 PUFAs were the significant factor in the altered cartilage structure. Although supportive of a preventive or disease-altering benefit, the results do not speak to supplementation.
Perhaps the most convincing evidence supporting fish oil for OA comes from a 2009 study by Gruenwald and colleagues.33 However, this 2-supplement study addressing synergy was financed by Seven Seas, a company with industry ties. The study was not placebo-controlled and was registered only after completion. The authors omitted baseline values, apparently did not correct for baseline in the statistical analysis, and did not report the distribution of results. The implication is that the results were overstated, or that, at minimum, the supporting data were not reported. Nevertheless, this study demonstrated benefits consistent with the animal and human laboratory studies. However, research is needed to repeat and validate these results, elucidate the mechanism of action, and quantify the benefit unique to fish oil.
Conclusion
Despite the overwhelming popularity of fish oil supplements and the assumption of benefit for patients with arthritis, there appears to be insufficient clinical evidence to justify use of fish oils in the treatment or prevention of OA. Possible efficacy in laboratory and animal studies has yet to be sufficiently observed and verified in clinical trials. Although it is impossible to refute the promise of these agents as beneficial adjuncts to anti-inflammatory regimens, there remains a need for significant, well-designed clinical trials to evaluate the efficacy, safety, and clinical parameters of omega-3 fatty acids in a standardized form before they can in good faith be recommended to patients with OA.
1. Jordan KM, Sawyer S, Coakley HE, Smith HE, Cooper C, Arden NK. The use of conventional and complementary treatments for knee osteoarthritis in the community. Rheumatology. 2003;43(3):381-384.
2. Vista ES, Lau CS. What about supplements for osteoarthritis? A critical and evidenced-based review. Int J Rheum Dis. 2011;14(2):152-158.
3. European Food Safety Authority Panel on Biological Hazards (BIOHAZ). Scientific opinion on fish oil for human consumption. Food hygiene, including rancidity. EFSA J. 2010;8(10):1874.
4. Berbert AA, Kondo CR, Almendra CL, Matsuo T, Dichi I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition. 2005;21(2):131-136.
5. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 suppl):1505S-1519S.
6. Calder PC, Zurier RB. Polyunsaturated fatty acids and rheumatoid arthritis. Curr Opin Clin Nutr Metab Care. 2001;4(2):115-121.
7. Kremer JM. Effects of modulation of inflammatory and immune parameters in patients with rheumatic and inflammatory disease receiving dietary supplementation of n-3 and n-6 fatty acids. Lipids. 1996;31(suppl):S243-S247.
8. Kremer JM, Jubiz W, Michalek A, et al. Fish oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987:106(4):497-503.
9. Kremer JM, Lawrence DA, Jubiz W, et al. Dietary fish oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. 1990;33(6):810-820.
10. Nielsen GL, Faarvang KL, Thomsen BS, et al. The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a randomized, double blind trial. Eur J Clin Invest. 1992;22(10):687-691.
11. Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129(1-2):210-223.
12. van der Tempel H, Tulleken JE, Limburg PC, Muskiet FA, van Rijswijk MH. Effects of fish oil supplementation in rheumatoid arthritis. Ann Rheum Dis. 1990;49(2):76-80.
13. Rosenbaum CC, O’Mathúna DP, Chavez M, Shields K. Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern Ther Health Med. 2010;16(2):32-40.
14. Sanghi D, Avasthi S, Srivastava RN, Singh A. Nutritional factors and osteoarthritis: a review article. Internet J Med Update. 2009;4(1).
15. Curtis CL, Hughes CE, Flannery CR, Little CB, Harwood JL, Caterson B. n-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J Biol Chem. 2000;275(2):721-724.
16. Curtis CL, Rees SG, Cramp J, et al. Effects of fatty acids on cartilage metabolism. Proc Nutr Soc. 2002;61(3):381-389.
17. Zainal Z, Longman AJ, Hurst S, et al. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthritis Cartilage. 2009;17(7):896-905.
18. Hankenson KD, Watkins BA, Schoenlein IA, Allen KG, Turek JJ. Omega-3 fatty acids enhance ligament fibroblast collagen formation in association with changes in interleukin-6 production. Proc Soc Exp Biol Med. 2000;223(1):88-95.
19. Melanson KJ. Diet, nutrition and osteoarthritis. Am J Lifestyle Med. 2007;1(4):260-263.
20. Pritchett JW. Statins and dietary fish oils improve lipid composition in bone marrow and joints. Clin Orthop Relat Res. 2007;(456):233-237.
21. Roush JK, Cross AR, Renberg WC, et al. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(1):67-73.
22. Roush JK, Dodd CE, Fritsch DA, et al. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J Am Vet Med Assoc. 2010;236(1):59-66.
23. Fritsch DA, Allen TA, Dodd CE, et al. A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(5):535-539.
24. Fritsch DA, Allen TA, Dodd CE, et al. Dose-titration effects of fish oil in osteoarthritic dogs. J Vet Intern Med. 2010;24(5):1020-1026.
25. Curtis CL, Rees SG, Little CB, et al. Pathologic indicators of degradation and inflammation in human osteoarthritic cartilage are abrogated by exposure to n-3 fatty acids. Arthritis Rheum. 2002;46(6):1544-1553.
26. Shen CL, Dunn DM, Henry JH, Li Y, Watkins BA. Decreased production of inflammatory mediators in human osteoarthritic chondrocytes by conjugated linoleic acids. Lipids. 2004;39(2):161-166.
27. Hurst S, Zainal Z, Caterson B, Hughes CE, Harwood JL. Dietary fatty acids and arthritis. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4-6):315-318.
28. Cleland LG, Hill CL, James MJ. Diet and arthritis. Baillieres Clin Rheumatol. 1995;9(4):771-785.
29. Maresz K, Meus K, Porwolik B. Krill oil: background and benefits. Int Sci Health Found. 2010;1-11.
30. Wang Y, Wluka AE, Hodge AM, et al. Effect of fatty acids on bone marrow lesions and knee cartilage in healthy, middle-aged subjects without clinical knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(5):579-583.
31. Stammers T, Sibbald B, Freeling P. Efficacy of cod liver oil as an adjunct to non-steroidal anti-inflammatory drug treatment in the management of osteoarthritis in general practice. Ann Rheum Dis. 1992;51(1):128-129.
32. Jacquet A, Girodet PO, Pariente A, Forest K, Mallet L, Moore N. Phytalgic, a food supplement, vs placebo in patients with osteoarthritis of the knee or hip: a randomised double-blind placebo-controlled clinical trial. Arthritis Res Ther. 2009;11(6):R192.
33. Gruenwald J, Petzold E, Busch R, Petzold HP, Graubaum HJ. Effect of glucosamine sulfate with or without omega-3 fatty acids in patients with osteoarthritis. Adv Ther. 2009;26(9):858-871.
34. Baker KR, Matthan NR, Lichtenstein AH, et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis Cartilage. 2012;20(5):382-387.
35. Christensen R, Bliddal H. Is Phytalgic® a goldmine for osteoarthritis patients or is there something fishy about this neutraceutical? A summary of findings and risk-of-bias assessment. Arthritis Res Ther. 2010;12(1):105.
First-line treatments for osteoarthritis (OA) are targeted at the inflammatory reaction that occurs after breakdown of articular cartilage through regular use of nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections, or surgical intervention. Associated activity restrictions and chronic pain have spurred a search for alternative treatments, commonly daily supplements such as glucosamine, chondroitin, and fish oil, to name a select few of the innumerable products reported to benefit patients with OA.
Background
Fish oil is 1 of the 2 most popular supplements among patients with OA. However, its effectiveness and precise benefit are still debated,1,2 and there is confusion about the definition of the product, the nature of investigations into its effectiveness, and the standardization of research unique to OA. Most fish oil research relates to patients with rheumatoid arthritis (RA). The anti-inflammatory benefits seen in patients with RA are generally applied to characterize fish oils as anti-inflammatory agents with a logical benefit in reducing OA symptoms. However, there is a dearth of independent and focused clinical results justifying that assumption. Further, lack of federal regulation of the supplement industry hinders conducting generalizable studies regarding medical benefit in a regulated and verified dose and form.3
The benefits of fish oil in RA treatment are well supported and accepted. In patients with RA, daily fish oil supplementation has been shown to reduce use of other medications and improve pain scores reported by both physicians and patients.4-10 The clinical efficacy of fish oil use in RA has been determined to be “reasonably strong,” with multiple studies confirming suppression of inflammatory cytokines in vitro and in vivo.11,12 The mechanism by which the inflammatory processes are augmented by fish oil supplementation suggests potential benefit to patients with OA, though review articles as recent as 2011 have concluded that research in that capacity is not sufficient to warrant recommendation.13,14
Most studies of OA-specific use of fish oils have been conducted in in vitro models. Treatment of bovine chondrocytes with omega-3 fatty acids causes reductions in inflammatory markers induced by interleukin 1, one of several proinflammatory cytokines that induce inflammation in OA at the gene and plasma levels, and these reductions have been reproduced.15-17 Although a preventive benefit was found in a study of pig medial collateral ligament fibroblasts, findings of later studies have been inconsistent.18 It also appears that fish oils may alter lipid composition in membranes, favoring incorporation of anti-inflammatory precursor n-3 fatty acids over proinflammatory precursor n-6 fatty acids in these model systems.19,20
Animal in vivo models have also been used to describe the effects of fish oil supplementation on OA. Assessment of dogs with OA before and after supplementation with the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) revealed improvement in clinical signs observed by owners, improvement in weight-bearing measured by veterinary clinicians, and decreased use of NSAIDs.21-24
Fish oil studies using osteoarthritic cartilage samples harvested during surgical procedures have demonstrated results consistent with other model systems described thus far. They have demonstrated a dose-dependent decrease in induced inflammatory destruction of tissue associated with fish oil supplementation. In addition, finding a lack of cellular toxicity, they have validated the safety of supplements.25,26 Proposed but unproven mechanisms for the anti-inflammatory actions of EPA and DHA include competition with n-6 fatty acids; presence of resolvins (anti-inflammatory molecules derived from EPA and DHA); presence of n-3 products that compete with proinflammatory molecules for receptors; reduction in gene expression of cytokines, cyclo-oxygenase 2, and degrading proteinases; interference in the signaling pathways of inflammation; and reduction in lymphocyte proliferation.26,27
Reduction in the n-6/n-3 ratio has been correlated with reduced inflammatory conditions such as OA, stemming from the epidemiologic evidence that higher n-3 intake in Eastern diets and lower intake of n-6 result in a lower incidence of these diseases.18,28,29 Studies have found sufficient evidence to suggest that this ratio has a role in OA, though not sufficient to recommend supplement use over diet modification.19 One study demonstrated an ability to favorably alter bone marrow lipid composition with n-3 fatty acid supplementation.10
The evidence leads to a conclusion of anti-inflammatory benefits from fish oils in these abstracted models. The multitude of basic science studies conducted on the anti-inflammatory properties of omega-3 fatty acids, only briefly reviewed here, supports the potential benefits colloquially ascribed to fish oil in the treatment of OA yet also implies the need for human clinical trials to address these properties clinically.
We reviewed the literature to address claims that fish oil supplementation can prevent or decrease severity of OA. We hypothesized there would be insufficient clinical studies to justify recommending supplementation to patients. Of note, the degree of heterogeneity in the evidence precluded performing a meta-analysis with any statistical validity.
Literature Review
In the PubMed database, we targeted the subject of fish oils and OA by using search terms that included omega-3, DHA, EPA, and alpha-linolenic acid. The MedLine and Google Scholar databases were searched as well. Results were limited to those reported in English and involving human subjects and clinical trials; results were excluded if they primarily involved patients with RA. Studies cited or mentioned in articles found through the PubMed search were evaluated according to the criteria mentioned, such that all relevant articles available at time of search are thought to be included, and these articles represent a reasonable presentation of the available evidence.
Findings
Our search revealed 6 clinical trials in which omega-3–containing supplements were used in the treatment of human OA with differing endpoints. We reviewed these trials in detail. One study, which used alteration of bone marrow lipids as an endpoint, was included for completeness of the evaluation of the relevant evidence.20 In addition, the study by Wang and colleagues,30 who assessed patients without clinical evidence of OA for development of bone marrow lesions, was reviewed. This study was deemed relevant to examine the process by which n-3 fatty acids alter knee structure, as subsequent risk of OA has not been elucidated, and effects on bone marrow lesions may indeed have a direct impact on the OA process. Results of the trials that were identified were varied between no significant difference in OA symptoms between treatment and control groups, implied benefits, and substantial benefits.
The first clinical study of omega-3 supplementation in OA treatment was conducted in 1992.31 The study compared 10 g of cod liver oil (containing 786 mg of EPA) with 10 g of olive oil, both taken daily over 24 weeks by 86 patients with OA. Effects were assessed by NSAID use (recorded in patient diary) and pain score (evaluated by clinician) every 4 weeks. The trial found no significant difference in effects between the oils.
Wang and colleagues30 used a food questionnaire to measure the n-3 intake of 293 healthy adults and quantified their bone marrow lesions after 10 years in an effort to describe how n-3 intake correlates with development of OA or pre-OA lesions. Higher intake of n-6 fatty acids was positively associated with presence of bone marrow lesions; n-3 intake had no association.
In a study of 84 patients who had joint replacement, Pritchett20 evaluated lipid alterations resulting from a regimen of 3 g of fish oil containing 11% DHA daily for a 6-month trial period, measuring lipids before and after the trial period. Pritchett20 found a 20% increase in long-chain fatty acids and a corresponding decrease in saturated fatty acids, as measured in bone marrow.
The supplement Phytalgic (Phythea Laboratories), which is advertised for OA, includes n-3 fatty acids, n-6 fatty acids, extract from Urtica dioica (the common nettle), zinc, and vitamin E. In a study by Jacquet and colleagues,32 this supplement was given 3 times daily over 3 separate 4-week periods to 81 patients with knee or hip OA. Measuring NSAID use with patient diaries and assessing pain with the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index every 4 weeks for 12 weeks, the authors found a significant decrease in NSAID use and, according to WOMAC results, a more than 50% reduction in pain and stiffness, and improved function.
One study compared the effects of glucosamine with and without omega-3 fatty acids in 182 patients with knee or hip OA.33 Each day, patients took 500 mg of glucosamine plus 3 capsules each containing either 444 mg of omega-3 fatty acids or 444 mg of an oil mixture. Pain was assessed with visual analog scale and the WOMAC scale 3 times over the 26-week study. More than 90% reductions in morning stiffness and pain were found for the combination of fish oil and glucosamine.
The Multicenter Osteoarthritis Study (MOST), published in February 2012, demonstrated that plasma levels of n-3 and n-6 polyunsaturated fatty acids (PUFAs) may be related to knee structural findings.34 This study confirmed that dietary modification of n-3 and n-6 PUFAs altered plasma concentration predictably. Higher DHA intake was associated with less evidence of OA on patellofemoral cartilage, though no association was found on tibiofemoral cartilage.34
Discussion
The lack of human clinical trials detailing the effects of fish oil supplementation in patients with OA is arguably the most significant hindrance to fish oil being routinely recommended. Since 1992, only 6 studies have addressed this topic, and their endpoints and results were inconsistent. These interventional trials had their limitations, including short duration, insufficient dosage, inappropriate n-3 choice, dietary interactions, genotype, and medication interactions.18 The present review is limited as well, by the quantity of evidence on the topic and by the focus (of the majority of the studies) on short-term alterations in pain and mobility instead of on disease-modifying potential. Short-term evaluation is unlikely to capture such an effect, which may require long-term supplementation to become evident.
The results of the study by Stammers and colleagues31 must be examined critically, as the likelihood of detection bias is high. Highly subjective assessments of effect, lack of standardized NSAID treatments, and limitations in patient numbers and disease severity raise concerns about validity. In addition, confounding variables (eg, medication interactions, alternative treatments, olive oil use) undermine the design. It is therefore difficult to interpret the results of this trial.
The study by Wang and colleagues30 did not involve supplementation, and intake was assessed only with food frequency questionnaires. It is therefore difficult to apply their results or findings to this review. In addition, the authors did not obtain baseline magnetic resonance imaging for comparison with that obtained at study completion—that is, they did not address any subclinical disease before dietary recording.
Pritchett20 acknowledged study limitations of small sample size and use of 1 subject as both patient and control. Although the study seemed to demonstrate that omega-3 supplementation augmented the lipid profile of joints, it did not directly demonstrate improvement in or prevention of OA. Identification of bone marrow lesions is not definitive proof of OA but an alteration that may correlate with development. The logical supposition is that altering the local environment may alter development of disease within that environment, though this is not proven.
An article reviewing the Phytalgic study highlighted the suspect nature of its results—claims that the supplement is 76% more effective than gold-standard corticosteroid injection.35 Also highlighted were lack of confirmed mechanism, questionable control, detection bias caused by aftertaste, and the high attrition rate in the placebo group. It is difficult to apply these results to fish oil supplementation, as Phytalgic contains other potentially confounding substances.
Of note, the findings of MOST were observational; n-3 and n-6 levels were not altered or supplemented. Altered disease process was demonstrated in patellofemoral cartilage but not in tibiofemoral cartilage in the same patient. The inconsistencies may be explained by the observational nature of the study and the lack of supplementation that would have produced a more significant increase in n-3 PUFA levels and thus more uniform conclusions, if in fact n-3 PUFAs were the significant factor in the altered cartilage structure. Although supportive of a preventive or disease-altering benefit, the results do not speak to supplementation.
Perhaps the most convincing evidence supporting fish oil for OA comes from a 2009 study by Gruenwald and colleagues.33 However, this 2-supplement study addressing synergy was financed by Seven Seas, a company with industry ties. The study was not placebo-controlled and was registered only after completion. The authors omitted baseline values, apparently did not correct for baseline in the statistical analysis, and did not report the distribution of results. The implication is that the results were overstated, or that, at minimum, the supporting data were not reported. Nevertheless, this study demonstrated benefits consistent with the animal and human laboratory studies. However, research is needed to repeat and validate these results, elucidate the mechanism of action, and quantify the benefit unique to fish oil.
Conclusion
Despite the overwhelming popularity of fish oil supplements and the assumption of benefit for patients with arthritis, there appears to be insufficient clinical evidence to justify use of fish oils in the treatment or prevention of OA. Possible efficacy in laboratory and animal studies has yet to be sufficiently observed and verified in clinical trials. Although it is impossible to refute the promise of these agents as beneficial adjuncts to anti-inflammatory regimens, there remains a need for significant, well-designed clinical trials to evaluate the efficacy, safety, and clinical parameters of omega-3 fatty acids in a standardized form before they can in good faith be recommended to patients with OA.
First-line treatments for osteoarthritis (OA) are targeted at the inflammatory reaction that occurs after breakdown of articular cartilage through regular use of nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections, or surgical intervention. Associated activity restrictions and chronic pain have spurred a search for alternative treatments, commonly daily supplements such as glucosamine, chondroitin, and fish oil, to name a select few of the innumerable products reported to benefit patients with OA.
Background
Fish oil is 1 of the 2 most popular supplements among patients with OA. However, its effectiveness and precise benefit are still debated,1,2 and there is confusion about the definition of the product, the nature of investigations into its effectiveness, and the standardization of research unique to OA. Most fish oil research relates to patients with rheumatoid arthritis (RA). The anti-inflammatory benefits seen in patients with RA are generally applied to characterize fish oils as anti-inflammatory agents with a logical benefit in reducing OA symptoms. However, there is a dearth of independent and focused clinical results justifying that assumption. Further, lack of federal regulation of the supplement industry hinders conducting generalizable studies regarding medical benefit in a regulated and verified dose and form.3
The benefits of fish oil in RA treatment are well supported and accepted. In patients with RA, daily fish oil supplementation has been shown to reduce use of other medications and improve pain scores reported by both physicians and patients.4-10 The clinical efficacy of fish oil use in RA has been determined to be “reasonably strong,” with multiple studies confirming suppression of inflammatory cytokines in vitro and in vivo.11,12 The mechanism by which the inflammatory processes are augmented by fish oil supplementation suggests potential benefit to patients with OA, though review articles as recent as 2011 have concluded that research in that capacity is not sufficient to warrant recommendation.13,14
Most studies of OA-specific use of fish oils have been conducted in in vitro models. Treatment of bovine chondrocytes with omega-3 fatty acids causes reductions in inflammatory markers induced by interleukin 1, one of several proinflammatory cytokines that induce inflammation in OA at the gene and plasma levels, and these reductions have been reproduced.15-17 Although a preventive benefit was found in a study of pig medial collateral ligament fibroblasts, findings of later studies have been inconsistent.18 It also appears that fish oils may alter lipid composition in membranes, favoring incorporation of anti-inflammatory precursor n-3 fatty acids over proinflammatory precursor n-6 fatty acids in these model systems.19,20
Animal in vivo models have also been used to describe the effects of fish oil supplementation on OA. Assessment of dogs with OA before and after supplementation with the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) revealed improvement in clinical signs observed by owners, improvement in weight-bearing measured by veterinary clinicians, and decreased use of NSAIDs.21-24
Fish oil studies using osteoarthritic cartilage samples harvested during surgical procedures have demonstrated results consistent with other model systems described thus far. They have demonstrated a dose-dependent decrease in induced inflammatory destruction of tissue associated with fish oil supplementation. In addition, finding a lack of cellular toxicity, they have validated the safety of supplements.25,26 Proposed but unproven mechanisms for the anti-inflammatory actions of EPA and DHA include competition with n-6 fatty acids; presence of resolvins (anti-inflammatory molecules derived from EPA and DHA); presence of n-3 products that compete with proinflammatory molecules for receptors; reduction in gene expression of cytokines, cyclo-oxygenase 2, and degrading proteinases; interference in the signaling pathways of inflammation; and reduction in lymphocyte proliferation.26,27
Reduction in the n-6/n-3 ratio has been correlated with reduced inflammatory conditions such as OA, stemming from the epidemiologic evidence that higher n-3 intake in Eastern diets and lower intake of n-6 result in a lower incidence of these diseases.18,28,29 Studies have found sufficient evidence to suggest that this ratio has a role in OA, though not sufficient to recommend supplement use over diet modification.19 One study demonstrated an ability to favorably alter bone marrow lipid composition with n-3 fatty acid supplementation.10
The evidence leads to a conclusion of anti-inflammatory benefits from fish oils in these abstracted models. The multitude of basic science studies conducted on the anti-inflammatory properties of omega-3 fatty acids, only briefly reviewed here, supports the potential benefits colloquially ascribed to fish oil in the treatment of OA yet also implies the need for human clinical trials to address these properties clinically.
We reviewed the literature to address claims that fish oil supplementation can prevent or decrease severity of OA. We hypothesized there would be insufficient clinical studies to justify recommending supplementation to patients. Of note, the degree of heterogeneity in the evidence precluded performing a meta-analysis with any statistical validity.
Literature Review
In the PubMed database, we targeted the subject of fish oils and OA by using search terms that included omega-3, DHA, EPA, and alpha-linolenic acid. The MedLine and Google Scholar databases were searched as well. Results were limited to those reported in English and involving human subjects and clinical trials; results were excluded if they primarily involved patients with RA. Studies cited or mentioned in articles found through the PubMed search were evaluated according to the criteria mentioned, such that all relevant articles available at time of search are thought to be included, and these articles represent a reasonable presentation of the available evidence.
Findings
Our search revealed 6 clinical trials in which omega-3–containing supplements were used in the treatment of human OA with differing endpoints. We reviewed these trials in detail. One study, which used alteration of bone marrow lipids as an endpoint, was included for completeness of the evaluation of the relevant evidence.20 In addition, the study by Wang and colleagues,30 who assessed patients without clinical evidence of OA for development of bone marrow lesions, was reviewed. This study was deemed relevant to examine the process by which n-3 fatty acids alter knee structure, as subsequent risk of OA has not been elucidated, and effects on bone marrow lesions may indeed have a direct impact on the OA process. Results of the trials that were identified were varied between no significant difference in OA symptoms between treatment and control groups, implied benefits, and substantial benefits.
The first clinical study of omega-3 supplementation in OA treatment was conducted in 1992.31 The study compared 10 g of cod liver oil (containing 786 mg of EPA) with 10 g of olive oil, both taken daily over 24 weeks by 86 patients with OA. Effects were assessed by NSAID use (recorded in patient diary) and pain score (evaluated by clinician) every 4 weeks. The trial found no significant difference in effects between the oils.
Wang and colleagues30 used a food questionnaire to measure the n-3 intake of 293 healthy adults and quantified their bone marrow lesions after 10 years in an effort to describe how n-3 intake correlates with development of OA or pre-OA lesions. Higher intake of n-6 fatty acids was positively associated with presence of bone marrow lesions; n-3 intake had no association.
In a study of 84 patients who had joint replacement, Pritchett20 evaluated lipid alterations resulting from a regimen of 3 g of fish oil containing 11% DHA daily for a 6-month trial period, measuring lipids before and after the trial period. Pritchett20 found a 20% increase in long-chain fatty acids and a corresponding decrease in saturated fatty acids, as measured in bone marrow.
The supplement Phytalgic (Phythea Laboratories), which is advertised for OA, includes n-3 fatty acids, n-6 fatty acids, extract from Urtica dioica (the common nettle), zinc, and vitamin E. In a study by Jacquet and colleagues,32 this supplement was given 3 times daily over 3 separate 4-week periods to 81 patients with knee or hip OA. Measuring NSAID use with patient diaries and assessing pain with the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index every 4 weeks for 12 weeks, the authors found a significant decrease in NSAID use and, according to WOMAC results, a more than 50% reduction in pain and stiffness, and improved function.
One study compared the effects of glucosamine with and without omega-3 fatty acids in 182 patients with knee or hip OA.33 Each day, patients took 500 mg of glucosamine plus 3 capsules each containing either 444 mg of omega-3 fatty acids or 444 mg of an oil mixture. Pain was assessed with visual analog scale and the WOMAC scale 3 times over the 26-week study. More than 90% reductions in morning stiffness and pain were found for the combination of fish oil and glucosamine.
The Multicenter Osteoarthritis Study (MOST), published in February 2012, demonstrated that plasma levels of n-3 and n-6 polyunsaturated fatty acids (PUFAs) may be related to knee structural findings.34 This study confirmed that dietary modification of n-3 and n-6 PUFAs altered plasma concentration predictably. Higher DHA intake was associated with less evidence of OA on patellofemoral cartilage, though no association was found on tibiofemoral cartilage.34
Discussion
The lack of human clinical trials detailing the effects of fish oil supplementation in patients with OA is arguably the most significant hindrance to fish oil being routinely recommended. Since 1992, only 6 studies have addressed this topic, and their endpoints and results were inconsistent. These interventional trials had their limitations, including short duration, insufficient dosage, inappropriate n-3 choice, dietary interactions, genotype, and medication interactions.18 The present review is limited as well, by the quantity of evidence on the topic and by the focus (of the majority of the studies) on short-term alterations in pain and mobility instead of on disease-modifying potential. Short-term evaluation is unlikely to capture such an effect, which may require long-term supplementation to become evident.
The results of the study by Stammers and colleagues31 must be examined critically, as the likelihood of detection bias is high. Highly subjective assessments of effect, lack of standardized NSAID treatments, and limitations in patient numbers and disease severity raise concerns about validity. In addition, confounding variables (eg, medication interactions, alternative treatments, olive oil use) undermine the design. It is therefore difficult to interpret the results of this trial.
The study by Wang and colleagues30 did not involve supplementation, and intake was assessed only with food frequency questionnaires. It is therefore difficult to apply their results or findings to this review. In addition, the authors did not obtain baseline magnetic resonance imaging for comparison with that obtained at study completion—that is, they did not address any subclinical disease before dietary recording.
Pritchett20 acknowledged study limitations of small sample size and use of 1 subject as both patient and control. Although the study seemed to demonstrate that omega-3 supplementation augmented the lipid profile of joints, it did not directly demonstrate improvement in or prevention of OA. Identification of bone marrow lesions is not definitive proof of OA but an alteration that may correlate with development. The logical supposition is that altering the local environment may alter development of disease within that environment, though this is not proven.
An article reviewing the Phytalgic study highlighted the suspect nature of its results—claims that the supplement is 76% more effective than gold-standard corticosteroid injection.35 Also highlighted were lack of confirmed mechanism, questionable control, detection bias caused by aftertaste, and the high attrition rate in the placebo group. It is difficult to apply these results to fish oil supplementation, as Phytalgic contains other potentially confounding substances.
Of note, the findings of MOST were observational; n-3 and n-6 levels were not altered or supplemented. Altered disease process was demonstrated in patellofemoral cartilage but not in tibiofemoral cartilage in the same patient. The inconsistencies may be explained by the observational nature of the study and the lack of supplementation that would have produced a more significant increase in n-3 PUFA levels and thus more uniform conclusions, if in fact n-3 PUFAs were the significant factor in the altered cartilage structure. Although supportive of a preventive or disease-altering benefit, the results do not speak to supplementation.
Perhaps the most convincing evidence supporting fish oil for OA comes from a 2009 study by Gruenwald and colleagues.33 However, this 2-supplement study addressing synergy was financed by Seven Seas, a company with industry ties. The study was not placebo-controlled and was registered only after completion. The authors omitted baseline values, apparently did not correct for baseline in the statistical analysis, and did not report the distribution of results. The implication is that the results were overstated, or that, at minimum, the supporting data were not reported. Nevertheless, this study demonstrated benefits consistent with the animal and human laboratory studies. However, research is needed to repeat and validate these results, elucidate the mechanism of action, and quantify the benefit unique to fish oil.
Conclusion
Despite the overwhelming popularity of fish oil supplements and the assumption of benefit for patients with arthritis, there appears to be insufficient clinical evidence to justify use of fish oils in the treatment or prevention of OA. Possible efficacy in laboratory and animal studies has yet to be sufficiently observed and verified in clinical trials. Although it is impossible to refute the promise of these agents as beneficial adjuncts to anti-inflammatory regimens, there remains a need for significant, well-designed clinical trials to evaluate the efficacy, safety, and clinical parameters of omega-3 fatty acids in a standardized form before they can in good faith be recommended to patients with OA.
1. Jordan KM, Sawyer S, Coakley HE, Smith HE, Cooper C, Arden NK. The use of conventional and complementary treatments for knee osteoarthritis in the community. Rheumatology. 2003;43(3):381-384.
2. Vista ES, Lau CS. What about supplements for osteoarthritis? A critical and evidenced-based review. Int J Rheum Dis. 2011;14(2):152-158.
3. European Food Safety Authority Panel on Biological Hazards (BIOHAZ). Scientific opinion on fish oil for human consumption. Food hygiene, including rancidity. EFSA J. 2010;8(10):1874.
4. Berbert AA, Kondo CR, Almendra CL, Matsuo T, Dichi I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition. 2005;21(2):131-136.
5. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 suppl):1505S-1519S.
6. Calder PC, Zurier RB. Polyunsaturated fatty acids and rheumatoid arthritis. Curr Opin Clin Nutr Metab Care. 2001;4(2):115-121.
7. Kremer JM. Effects of modulation of inflammatory and immune parameters in patients with rheumatic and inflammatory disease receiving dietary supplementation of n-3 and n-6 fatty acids. Lipids. 1996;31(suppl):S243-S247.
8. Kremer JM, Jubiz W, Michalek A, et al. Fish oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987:106(4):497-503.
9. Kremer JM, Lawrence DA, Jubiz W, et al. Dietary fish oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. 1990;33(6):810-820.
10. Nielsen GL, Faarvang KL, Thomsen BS, et al. The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a randomized, double blind trial. Eur J Clin Invest. 1992;22(10):687-691.
11. Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129(1-2):210-223.
12. van der Tempel H, Tulleken JE, Limburg PC, Muskiet FA, van Rijswijk MH. Effects of fish oil supplementation in rheumatoid arthritis. Ann Rheum Dis. 1990;49(2):76-80.
13. Rosenbaum CC, O’Mathúna DP, Chavez M, Shields K. Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern Ther Health Med. 2010;16(2):32-40.
14. Sanghi D, Avasthi S, Srivastava RN, Singh A. Nutritional factors and osteoarthritis: a review article. Internet J Med Update. 2009;4(1).
15. Curtis CL, Hughes CE, Flannery CR, Little CB, Harwood JL, Caterson B. n-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J Biol Chem. 2000;275(2):721-724.
16. Curtis CL, Rees SG, Cramp J, et al. Effects of fatty acids on cartilage metabolism. Proc Nutr Soc. 2002;61(3):381-389.
17. Zainal Z, Longman AJ, Hurst S, et al. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthritis Cartilage. 2009;17(7):896-905.
18. Hankenson KD, Watkins BA, Schoenlein IA, Allen KG, Turek JJ. Omega-3 fatty acids enhance ligament fibroblast collagen formation in association with changes in interleukin-6 production. Proc Soc Exp Biol Med. 2000;223(1):88-95.
19. Melanson KJ. Diet, nutrition and osteoarthritis. Am J Lifestyle Med. 2007;1(4):260-263.
20. Pritchett JW. Statins and dietary fish oils improve lipid composition in bone marrow and joints. Clin Orthop Relat Res. 2007;(456):233-237.
21. Roush JK, Cross AR, Renberg WC, et al. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(1):67-73.
22. Roush JK, Dodd CE, Fritsch DA, et al. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J Am Vet Med Assoc. 2010;236(1):59-66.
23. Fritsch DA, Allen TA, Dodd CE, et al. A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(5):535-539.
24. Fritsch DA, Allen TA, Dodd CE, et al. Dose-titration effects of fish oil in osteoarthritic dogs. J Vet Intern Med. 2010;24(5):1020-1026.
25. Curtis CL, Rees SG, Little CB, et al. Pathologic indicators of degradation and inflammation in human osteoarthritic cartilage are abrogated by exposure to n-3 fatty acids. Arthritis Rheum. 2002;46(6):1544-1553.
26. Shen CL, Dunn DM, Henry JH, Li Y, Watkins BA. Decreased production of inflammatory mediators in human osteoarthritic chondrocytes by conjugated linoleic acids. Lipids. 2004;39(2):161-166.
27. Hurst S, Zainal Z, Caterson B, Hughes CE, Harwood JL. Dietary fatty acids and arthritis. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4-6):315-318.
28. Cleland LG, Hill CL, James MJ. Diet and arthritis. Baillieres Clin Rheumatol. 1995;9(4):771-785.
29. Maresz K, Meus K, Porwolik B. Krill oil: background and benefits. Int Sci Health Found. 2010;1-11.
30. Wang Y, Wluka AE, Hodge AM, et al. Effect of fatty acids on bone marrow lesions and knee cartilage in healthy, middle-aged subjects without clinical knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(5):579-583.
31. Stammers T, Sibbald B, Freeling P. Efficacy of cod liver oil as an adjunct to non-steroidal anti-inflammatory drug treatment in the management of osteoarthritis in general practice. Ann Rheum Dis. 1992;51(1):128-129.
32. Jacquet A, Girodet PO, Pariente A, Forest K, Mallet L, Moore N. Phytalgic, a food supplement, vs placebo in patients with osteoarthritis of the knee or hip: a randomised double-blind placebo-controlled clinical trial. Arthritis Res Ther. 2009;11(6):R192.
33. Gruenwald J, Petzold E, Busch R, Petzold HP, Graubaum HJ. Effect of glucosamine sulfate with or without omega-3 fatty acids in patients with osteoarthritis. Adv Ther. 2009;26(9):858-871.
34. Baker KR, Matthan NR, Lichtenstein AH, et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis Cartilage. 2012;20(5):382-387.
35. Christensen R, Bliddal H. Is Phytalgic® a goldmine for osteoarthritis patients or is there something fishy about this neutraceutical? A summary of findings and risk-of-bias assessment. Arthritis Res Ther. 2010;12(1):105.
1. Jordan KM, Sawyer S, Coakley HE, Smith HE, Cooper C, Arden NK. The use of conventional and complementary treatments for knee osteoarthritis in the community. Rheumatology. 2003;43(3):381-384.
2. Vista ES, Lau CS. What about supplements for osteoarthritis? A critical and evidenced-based review. Int J Rheum Dis. 2011;14(2):152-158.
3. European Food Safety Authority Panel on Biological Hazards (BIOHAZ). Scientific opinion on fish oil for human consumption. Food hygiene, including rancidity. EFSA J. 2010;8(10):1874.
4. Berbert AA, Kondo CR, Almendra CL, Matsuo T, Dichi I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition. 2005;21(2):131-136.
5. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 suppl):1505S-1519S.
6. Calder PC, Zurier RB. Polyunsaturated fatty acids and rheumatoid arthritis. Curr Opin Clin Nutr Metab Care. 2001;4(2):115-121.
7. Kremer JM. Effects of modulation of inflammatory and immune parameters in patients with rheumatic and inflammatory disease receiving dietary supplementation of n-3 and n-6 fatty acids. Lipids. 1996;31(suppl):S243-S247.
8. Kremer JM, Jubiz W, Michalek A, et al. Fish oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987:106(4):497-503.
9. Kremer JM, Lawrence DA, Jubiz W, et al. Dietary fish oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. 1990;33(6):810-820.
10. Nielsen GL, Faarvang KL, Thomsen BS, et al. The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a randomized, double blind trial. Eur J Clin Invest. 1992;22(10):687-691.
11. Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129(1-2):210-223.
12. van der Tempel H, Tulleken JE, Limburg PC, Muskiet FA, van Rijswijk MH. Effects of fish oil supplementation in rheumatoid arthritis. Ann Rheum Dis. 1990;49(2):76-80.
13. Rosenbaum CC, O’Mathúna DP, Chavez M, Shields K. Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern Ther Health Med. 2010;16(2):32-40.
14. Sanghi D, Avasthi S, Srivastava RN, Singh A. Nutritional factors and osteoarthritis: a review article. Internet J Med Update. 2009;4(1).
15. Curtis CL, Hughes CE, Flannery CR, Little CB, Harwood JL, Caterson B. n-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J Biol Chem. 2000;275(2):721-724.
16. Curtis CL, Rees SG, Cramp J, et al. Effects of fatty acids on cartilage metabolism. Proc Nutr Soc. 2002;61(3):381-389.
17. Zainal Z, Longman AJ, Hurst S, et al. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthritis Cartilage. 2009;17(7):896-905.
18. Hankenson KD, Watkins BA, Schoenlein IA, Allen KG, Turek JJ. Omega-3 fatty acids enhance ligament fibroblast collagen formation in association with changes in interleukin-6 production. Proc Soc Exp Biol Med. 2000;223(1):88-95.
19. Melanson KJ. Diet, nutrition and osteoarthritis. Am J Lifestyle Med. 2007;1(4):260-263.
20. Pritchett JW. Statins and dietary fish oils improve lipid composition in bone marrow and joints. Clin Orthop Relat Res. 2007;(456):233-237.
21. Roush JK, Cross AR, Renberg WC, et al. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(1):67-73.
22. Roush JK, Dodd CE, Fritsch DA, et al. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J Am Vet Med Assoc. 2010;236(1):59-66.
23. Fritsch DA, Allen TA, Dodd CE, et al. A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(5):535-539.
24. Fritsch DA, Allen TA, Dodd CE, et al. Dose-titration effects of fish oil in osteoarthritic dogs. J Vet Intern Med. 2010;24(5):1020-1026.
25. Curtis CL, Rees SG, Little CB, et al. Pathologic indicators of degradation and inflammation in human osteoarthritic cartilage are abrogated by exposure to n-3 fatty acids. Arthritis Rheum. 2002;46(6):1544-1553.
26. Shen CL, Dunn DM, Henry JH, Li Y, Watkins BA. Decreased production of inflammatory mediators in human osteoarthritic chondrocytes by conjugated linoleic acids. Lipids. 2004;39(2):161-166.
27. Hurst S, Zainal Z, Caterson B, Hughes CE, Harwood JL. Dietary fatty acids and arthritis. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4-6):315-318.
28. Cleland LG, Hill CL, James MJ. Diet and arthritis. Baillieres Clin Rheumatol. 1995;9(4):771-785.
29. Maresz K, Meus K, Porwolik B. Krill oil: background and benefits. Int Sci Health Found. 2010;1-11.
30. Wang Y, Wluka AE, Hodge AM, et al. Effect of fatty acids on bone marrow lesions and knee cartilage in healthy, middle-aged subjects without clinical knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(5):579-583.
31. Stammers T, Sibbald B, Freeling P. Efficacy of cod liver oil as an adjunct to non-steroidal anti-inflammatory drug treatment in the management of osteoarthritis in general practice. Ann Rheum Dis. 1992;51(1):128-129.
32. Jacquet A, Girodet PO, Pariente A, Forest K, Mallet L, Moore N. Phytalgic, a food supplement, vs placebo in patients with osteoarthritis of the knee or hip: a randomised double-blind placebo-controlled clinical trial. Arthritis Res Ther. 2009;11(6):R192.
33. Gruenwald J, Petzold E, Busch R, Petzold HP, Graubaum HJ. Effect of glucosamine sulfate with or without omega-3 fatty acids in patients with osteoarthritis. Adv Ther. 2009;26(9):858-871.
34. Baker KR, Matthan NR, Lichtenstein AH, et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis Cartilage. 2012;20(5):382-387.
35. Christensen R, Bliddal H. Is Phytalgic® a goldmine for osteoarthritis patients or is there something fishy about this neutraceutical? A summary of findings and risk-of-bias assessment. Arthritis Res Ther. 2010;12(1):105.