User login
Use of Comprehensive Geriatric Assessment in Oncology Patients to Guide Treatment Decisions and Predict Chemotherapy Toxicity
Age is a well recognized risk factor for cancer development. The population of older Americans is growing, and by 2030, 20% of the US population will be aged ≥ 65 years.1 While 25% of all new cancer cases are diagnosed in people aged 65 to 74 years, more than half of cancers occur in individuals aged ≥ 70 years, with even higher rates in those aged ≥ 75 years.2 Although cancer rates have declined slightly overall among people aged ≥ 65 years, this population still has an 11-fold increased incidence of cancer compared with that of younger individuals.3 With a rapidly growing older population, there will be increasing demand for cancer care.
Treatment of cancer in older individuals often is complicated by medical comorbidities, frailty, and poor functional status. Distinguishing patients who can tolerate aggressive therapy from those who require less intensive therapy can be challenging. Age-related physiologic changes predispose older adults to an increased risk of therapy-related toxicities, resulting in suboptimal therapeutic benefit and substantial morbidity. For example, cardiovascular changes can lead to reduction of the cardiac functional reserve, which can increase the risk of congestive heart failure. Similarly, decline in renal function leads to an increased potential for nephrotoxicity.4 Although patients may be of the same chronologic age, their performance, functional, and biologic status may be quite variable; thus, tolerance to aggressive treatment is not easily predicted. The comprehensive geriatric assessment (CGA) may be used as a global assessment tool to risk stratify older patients prior to oncologic treatment decisions.
Health care providers (HCPs), including physician assistants, nurse practitioners, clinical nurse specialists, nurses, and physicians, routinely participate in every aspect of cancer care by ordering and interpreting diagnostic tests, addressing comorbidities, managing symptoms, and discussing cancer treatment recommendations. HCPs in oncology will continue to play a vital role in the coordination and management of older patients with cancer. However, in general, CGA has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools.
What Is Geriatric Assessment?
Geriatric assessment is a multidisciplinary, multidimensional process aimed at detecting medical, psychosocial, and functional issues of older adults that are not identified by traditional performance status measures alone. It provides guidance for management of identified problems and improvement in quality of life.6 CGA was developed by geriatricians and multidisciplinary care teams to evaluate the domains of functional, nutritional, cognitive, psychosocial, and economic status; comorbidities; geriatric syndromes; and mood, and it has been tested in both clinics and hospitals.7 Although such assessment requires additional time and resources, its goals are to identify areas of vulnerability, assist in clinical decisions of treatable health problems, and guide therapeutic interventions.6 In oncology practice, the assessment not only addresses these global issues, but also is critical in predicting toxicity and survival outcomes in older oncology patients.
Components of CGA
Advancing age brings many physiologic, psychosocial, and functional challenges, and a cancer diagnosis only adds to these issues. CGA provides a system of assessing older and/or frail patients with cancer through specific domains to identify issues that are not apparent on routine evaluation in a clinic setting before and during chemotherapy treatments. These domains include comorbidity, polypharmacy, functional status, cognition, psychological and social status, and nutrition.8
Comorbidity
The prevalence of multiple medical problems and comorbidities, including cancer, among people aged > 65 years is increasing.9 Studies have shown that two-thirds of patients with cancer had ≥ 2 medical conditions, and nearly one quarter had ≥ 4 medical conditions.10 In older adults, common comorbidities include cardiovascular disease, hypertension, diabetes mellitus, and dementia. These comorbidities can impact treatment decisions, increase the risk of disease, impact treatment-related complications, and affect a patient’s life expectancy.11 Assessing comorbidities is essential to CGA and is done using the Charlson Comorbidity Index and/or the Cumulative Illness Rating Scale.12
The Charlson Comorbidity Index was originally designed to predict 1-year mortality on the basis of a weighted composite score for the following categories: cardiovascular, endocrine, pulmonary, neurologic, renal, hepatic, gastrointestinal, and neoplastic disease.13 It is now the most widely used comorbidity index and has been adapted and verified as applicable and valid for predicting the outcomes and risk of death from many comorbid diseases.14 The Cumulative Illness Rating Scale has been validated as a predictor for readmission for hospitalized older adults, hospitalization within 1 year in a residential setting, and long-term mortality when assessed in inpatient and residential settings.15
Polypharmacy
Polypharmacy (use of ≥ 5 medications) is common in older patients regardless of cancer diagnosis and is often instead defined as “the use of multiple drugs or more than are medically necessary.”16 The use of multiple medications, including those not indicated for existing medical conditions (such as over‐the‐counter, herbal, and complementary/alternative medicines, which patients often fail to declare to their specialist, doctor, or pharmacist) adds to the potential negative aspects of polypharmacy that affect older patients.17
Patients with cancer usually are prescribed an extensive number of medicines, both for the disease and for supportive care, which can increase the chance of drug-drug interactions and adverse reactions.18 While these issues certainly affect quality of life, they also may influence chemotherapy treatment and potentially impact survival. Studies have shown that the presence of polypharmacy has been associated with higher numbers of comorbidities, increased use of inappropriate medications, poor performance status, decline in functional status, and poor survival.18
Functional Status
Although Eastern Cooperative Oncology Group (ECOG) performance status and Karnofsky Performance Status are commonly used by oncologists, these guidelines are limited in focus and do not reliably measure functional status in older patients. Functional status is determined by the ability to perform daily acts of self-care, which includes assessment of activities of daily living (ADLs) and instrumental activities of daily living (IADLs). ADLs refer to such tasks as bathing, dressing, eating, mobility, balance, and toileting.19 IADLs include the ability to perform activities required to live within a community and include shopping, transportation, managing finances, medication management, cooking, and cleaning.11
Physical functionality also can be assessed by measures such as gait speed, grip strength, balance, and lower extremity strength. These are more sensitive and shown to be associated with worse clinical outcomes.20 Grip strength and gait speed, as assessed by the Timed Up and Go test or the Short Physical Performance Battery measure strength and balance.12 Reduction in gait speed and/or grip strength are associated with adverse clinical outcomes and increased risk of mortality.21 Patients with cancer who have difficulty with ADLs are at increased risk for falls, which can limit their functional independence, compromise cancer therapy, and increase the risk of chemotherapy toxicities.11 Impaired hearing and poor vision are added factors that can be barriers to cancer treatment.
Cognition
Cognitive impairment in patients with cancer is becoming more of an issue for oncology HCPs as both cancer and cognitive decline are more common with advancing age. Cognition in cancer patients is important for understanding their diagnosis, prognosis, treatment options, and adherence. Impaired cognition can affect decision making regarding treatment options and administration. Cognition can be assessed through validated screening tools such as the Mini-Mental State Examination and Mini-Cog.11
Psychological and Social Status
A cancer diagnosis has a major impact on the mental and emotional state of patients and family members. Clinically significant anxiety has been reported in approximately 21% of older patients with cancer, and the incidence of depression ranges from 17 to 26%.22 In older patients with, psychologic distress can impact cancer treatment, resulting in less definitive therapy and poorer outcomes.23 All patients with cancer should be screened for psychologic distress using standardized methods, such as the Geriatric Depression Scale or the General Anxiety Disorder-7 scale.24 A positive screen should lead to additional assessments that evaluate the severity of depression and other comorbid psychological problems and medical conditions.
Social isolation and loneliness are factors that can affect both depression and anxiety. Older patients with cancer are at risk for decreased social activities and are already challenged with issues related to home care, comorbidities, functional status, and caregiver support.23 Therefore, it is important to assess the social interactions of an older and/or frail patient with cancer and use social work assistance to address needs for supportive services.
Nutrition
Nutrition is important in any patient with cancer undergoing chemotherapy treatment. However, it is of greater importance in older adults, as malnutrition and weight loss are negative prognostic factors that correlate with poor tolerance to chemotherapy treatment, decline in quality of life, and increased mortality.25 The Mini-Nutritional Assessment is a widely used validated tool to assess nutritional status and risk of malnutrition.11 This tool can help identify those older and/or frail patients with cancer with impaired nutritional status and aid in instituting corrective measures to treat or prevent malnutrition.
Effectiveness of CGA
Multiple randomized controlled clinical trials assessing the effectiveness of CGA have been conducted over the past 3 decades with overall positive outcomes related to its value.26 Benefits of CGA can include overall improved medical care, avoidance of hospitalization or nursing home placement, identification of cognitive impairment, and prevention of geriatric syndrome (a range of conditions representing multiple organ impairment in older adults).27
In oncology, CGA is particularly beneficial, as it can identify issues in nearly 70% of patients that may not be apparent through traditional oncology assessment.28 A systematic review of 36 studies assessing the prognostic value of CGA in elderly patients with cancer receiving chemotherapy concluded that impaired performance and functional status as well as a frail and vulnerable profile are important predictors of severe chemotherapy-related toxicity and are associated with a higher risk of mortality.29 Therefore, CGA should be an integral part of the evaluation of older and/or frail patients with cancer prior to chemotherapy consideration.
Several screening tools have been developed using information from CGA to assess the risk of severe toxicities. The most commonly used tools for predicting toxicity include the Cancer and Aging Research Group (CARG) chemotoxicity calculator and the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH).30,31 Although these tools are readily available to facilitate CGA, and despite their proven beneficial outcome and recommended usage by national guidelines, implementation of these tools in routine oncology practice has been challenging and slow to spread. Unless these recommended interventions are effectively implemented, the benefits of CGA cannot be realized. With the expected surge in the number of older patients with cancer, hopefully this will change.
Geriatric Assessment Screening Tools
A screening tool recommended for use in older and/or frail patients with cancer allows for a brief assessment to help clinicians identify patients in need of further evaluation by CGA and to provides information on treatment-related toxicities, functional decline, and survival.32 The predictive value and utility of geriatric assessment screening tools have been repeatedly proven to identify older and/or frail adults at risk for treatment-related toxicities.12 The CARG and the CRASH are validated screening tools used in identifying patients at higher risk for chemotherapy toxicity. These screening tools are intended to provide guidance to the clinical oncology practitioner on risk stratification of chemotherapy toxicity in older patients with cancer.33
Both of these screening tools provide similar predictive performance for chemotherapy toxicity in older patients with cancer.34 However, the CARG tool seems to have the advantage of using more data that had already been obtained during regular office visits and is clear and easy to use clinically. The CRASH tool is slightly more involved, as it uses multiple geriatric instruments to determine the predictive risk of both hematologic and nonhematologic toxicities of chemotherapy.
CARG Chemotoxicity Calculator
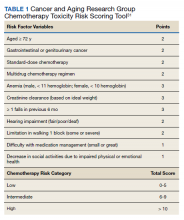
Hurria and colleagues originally developed the CARG tool from data obtained through a prospective multicenter study involving 500 patients with cancer aged ≥ 65 years.35 They concluded that chemotherapy-related toxicity is common in older adults, with 53% of patients sustaining grade 3 or 4 treatment-related toxicities and 2% treatment-related mortality.12 This predictive model for chemotherapy-related toxicity used 11 variables, both objective (obtained during a regular clinical encounter: age, tumor type, chemotherapy dosing, number of drugs, creatinine, and hemoglobin) and subjective (completed by patient: number of falls, social support, the ability to take medications, hearing impairment, and physical performance), to determine at-risk patients (Table 1).31
Compared with standard performance status measures in oncology practice, the CARG model was better able to predict chemotherapy-related toxicities. In 2016, Hurria and colleagues published the results of an updated external validation study with a cohort of 250 older patients with cancer receiving chemotherapy that confirmed the prediction of chemotherapy toxicity using the CARG screening tool in this population.31 An appealing feature of this tool is the free online accessibility and the expedited manner in which screening can be conducted.
CRASH Score
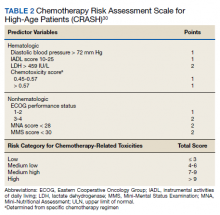
The CRASH score was derived from the results of a prospective, multicenter study of 518 patients aged ≥ 70 years who were assessed on 24 parameters prior to starting chemotherapy.30 A total of 64% of patients experienced significant toxicities, including 32% with grade 4 hematologic toxicity and 56% with grade 3 or 4 nonhematologic toxicity. The hematologic and nonhematologic toxicity risks are the 2 categories that comprise the CRASH score. Both baseline patient variables and chemotherapy regimen are incorporated into an 8-item assessment profile that determines the risk categories (Table 2).30
Increased risk of hematologic toxicities was associated with increased diastolic blood pressure, increased lactate dehydrogenase, need for assistance with IADL, and increased toxicity potential of the chemotherapy regimen. Nonhematologic toxicities were associated with ECOG performance score, Mini Mental Status Examination and Mini-Nutritional Assessment, and increased toxicity of the chemotherapy regimen.12 Patient scores are stratified into 4 risk categories: low, medium-low, medium-high, and high.30 Like the CARG tool, the CRASH screening tool also is available as a free online resource and can be used in everyday clinical practice to assess older and/or frail adults with cancer.
Conclusions
In older adults, cancer may significantly impact the natural course of concurrent comorbidities due to physiologic and functional changes. These vulnerabilities predispose older patients with cancer to an increased risk of adverse outcomes, including treatment-related toxicities.36 Given the rapidly aging population, it is critical for oncology clinical teams to be prepared to assess for, prevent, and manage issues for older adults that could impact outcomes, including complications and toxicities from chemotherapy.35 Studies have reported that 78 to 93% of older oncology patients have at least 1 geriatric impairment that could potentially impact oncology treatment plans.37,38 This supports the utility of CGA as a global assessment tool to risk stratify older and/or frail patients prior to deciding on subsequent oncologic treatment approaches.5 In fact, major cooperative groups sponsored by the National Cancer Institute, such as the Alliance for Clinical Trials in Oncology, are including CGA as part of some of their treatment trials. CGA was conducted as part of a multicenter cooperative group study in older patients with acute myeloid leukemia prior to inpatient intensive induction chemotherapy and was determined to be feasible and useful in clinical trials and practice.39
Despite the increasing evidence for benefits of CGA, it has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools. Although oncology providers routinely participate in every aspect of cancer care and play a vital role in the coordination and management of older patients with cancer, CGA implementation into routine clinical practice has been slow in part due to lack of knowledge and training regarding the use of GA tools.
Oncology providers can easily incorporate CGA screening tools into the history and physical examination process for older patients with cancer, which will add an important dimension to these patient evaluations. Oncology providers are not only well positioned to administer these screening tools, but also can lead the field in developing innovative ways for effective implementation in busy routine oncology clinics. However, to be successful, oncology providers must be knowledgeable about these tools and understand their utility in guiding treatment decisions and improving quality of care in older patients with cancer.
1. Sharless NE. The challenging landscape of cancer and aging: charting a way forward. Published January 24, 2018. Accessed April 16, 2021. https://www.cancer.gov/news-events/cancer-currents-blog/2018/sharpless-aging-cancer-research
2. National Cancer Institute. Age and cancer risk. Updated March 5, 2021. Accessed April 16, 2021. https://www.cancer.gov/about-cancer/causes-prevention/risk/age
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551 4. Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11(6):449-460. doi:10.1097/00130404-200511000-00004
5. Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol. 2013;24(5):1306-1312. doi:10.1093/annonc/mds619
6. Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. What every oncologist should know about geriatric assessment for older patients with cancer: Young International Society of Geriatric Oncology position paper. J Oncol Pract. 2018;14(2):85-94. doi:10.1200/JOP.2017.026435
7. Cohen HJ. Evolution of geriatric assessment in oncology. J Oncol Pract. 2018;14(2):95-96. doi:10.1200/JOP.18.00017
8. Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603. doi:10.1200/JCO.2013.54.8347
9. American Cancer Society. Cancer facts & figures 2019. Accessed April 16, 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
10. Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249-257. doi:10.1016/j.jgo.2015.12.002
11. Korc-Grodzicki B, Holmes HM, Shahrokni A. Geriatric assessment for oncologists. Cancer Biol Med. 2015;12(4):261-274. doi:10.7497/j.issn.2095-3941.2015.0082
12. Li D, Soto-Perez-de-Celis E, Hurria A. Geriatric assessment and tools for predicting treatment toxicity in older adults with cancer. Cancer J. 2017;23(4):206-210. doi:10.1097/PPO.0000000000000269
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
14. Huang Y, Gou R, Diao Y, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58-66. doi:10.1631/jzus.B1300109
15. Osborn KP IV, Nothelle S, Slaven JE, Montz K, Hui S, Torke AM. Cumulative Illness Rating Scale (CIRS) can be used to predict hospital outcomes in older adults. J Geriatric Med Gerontol. 2017;3(2). doi:10.23937/2469-5858/1510030
16. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65. doi:10.1517/14740338.2013.827660
17. Shrestha S, Shrestha S, Khanal S. Polypharmacy in elderly cancer patients: challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med. 2019;2(1):42-49. doi:10.1002/agm2.12051
18. Sharma M, Loh KP, Nightingale G, Mohile SG, Holmes HM. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol. 2016;7(5):346-353. doi:10.1016/j.jgo.2016.07.010
19. Norburn JE, Bernard SL, Konrad TR, et al. Self-care and assistance from others in coping with functional status limitations among a national sample of older adults. J Gerontol B Psychol Sci Soc Sci. 1995;50(2):S101-S109. doi:10.1093/geronb/50b.2.s101
20. Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc. 2016;64(1):144-150. doi:10.1111/jgs.13871
21. Owusu C, Berger NA. Comprehensive geriatric assessment in the older cancer patient: coming of age in clinical cancer care. Clin Pract (Lond). 2014;11(6):749-762. doi:10.2217/cpr.14.72
22. Weiss Wiesel TR, Nelson CJ, Tew WP, et al. The relationship between age, anxiety, and depression in older adults with cancer. Psychooncology. 2015;24(6):712-717. doi:10.1002/pon.3638
23. Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19(6):e305-e316. doi:10.1016/S1470-2045(18)30348-6
24. Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605-1619. doi:10.1200/JCO.2013.52.4611
25. Muscaritoli M, Lucia S, Farcomeni A, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. 2017;8(45):79884-79886. doi:10.18632/oncotarget.20168
26. Ekdahl AW, Axmon A, Sandberg M, Steen Carlsson K. Is care based on comprehensive geriatric assessment with mobile teams better than usual care? A study protocol of a randomised controlled trial (the GerMoT study). BMJ Open. 2018;8(10)e23969. doi:10.1136/bmjopen-2018-023969
27. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
28. Hernandez Torres C, Hsu T. Comprehensive geriatric assessment in the older adult with cancer: a review. Eur Urol Focus. 2017;3(4-5):330-339. doi:10.1016/j.euf.2017.10.010
29. Janssens K, Specenier P. The prognostic value of the comprehensive geriatric assessment (CGA) in elderly cancer patients (ECP) treated with chemotherapy (CT): a systematic review. Eur J Cancer. 2017;72(1):S164-S165. doi:10.1016/S0959-8049(17)30611-1
30. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer. 2012;118(13):3377-3386. doi:10.1002/cncr.26646
31. Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366-2371. doi:10.1200/JCO.2015.65.4327
32. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288-300. doi:10.1093/annonc/mdu210
33. Schiefen JK, Madsen LT, Dains JE. Instruments that predict oncology treatment risk in the senior population. J Adv Pract Oncol. 2017;8(5):528-533.
34. Ortland I, Mendel Ott M, Kowar M, et al. Comparing the performance of the CARG and the CRASH score for predicting toxicity in older patients with cancer. J Geriatr Oncol. 2020;11(6):997-1005. doi:10.1016/j.jgo.2019.12.016
35. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465. doi:10.1200/JCO.2011.34.7625
36. Mohile SG, Velarde C, Hurria A, et al. Geriatric assessment-guided care processes for older adults: a Delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13(9):1120-1130. doi:10.6004/jnccn.2015.0137
37. Schiphorst AHW, Ten Bokkel Huinink D, Breumelhof R, Burgmans JPJ, Pronk A, Hamaker ME. Geriatric consultation can aid in complex treatment decisions for elderly cancer patients. Eur J Cancer Care (Engl). 2016;25(3):365-370. doi:10.1111/ecc.12349
38. Schulkes KJG, Souwer ETD, Hamaker ME, et al. The effect of a geriatric assessment on treatment decisions for patients with lung cancer. Lung. 2017;195(2):225-231. doi:10.1007/s00408-017-9983-7
39. Klepin HD, Ritchie E, Major-Elechi B, et al. Geriatric assessment among older adults receiving intensive therapy for acute myeloid leukemia: report of CALGB 361006 (Alliance). J Geriatr Oncol. 2020;11(1):107-113. doi:10.1016/j.jgo.2019.10.002
Age is a well recognized risk factor for cancer development. The population of older Americans is growing, and by 2030, 20% of the US population will be aged ≥ 65 years.1 While 25% of all new cancer cases are diagnosed in people aged 65 to 74 years, more than half of cancers occur in individuals aged ≥ 70 years, with even higher rates in those aged ≥ 75 years.2 Although cancer rates have declined slightly overall among people aged ≥ 65 years, this population still has an 11-fold increased incidence of cancer compared with that of younger individuals.3 With a rapidly growing older population, there will be increasing demand for cancer care.
Treatment of cancer in older individuals often is complicated by medical comorbidities, frailty, and poor functional status. Distinguishing patients who can tolerate aggressive therapy from those who require less intensive therapy can be challenging. Age-related physiologic changes predispose older adults to an increased risk of therapy-related toxicities, resulting in suboptimal therapeutic benefit and substantial morbidity. For example, cardiovascular changes can lead to reduction of the cardiac functional reserve, which can increase the risk of congestive heart failure. Similarly, decline in renal function leads to an increased potential for nephrotoxicity.4 Although patients may be of the same chronologic age, their performance, functional, and biologic status may be quite variable; thus, tolerance to aggressive treatment is not easily predicted. The comprehensive geriatric assessment (CGA) may be used as a global assessment tool to risk stratify older patients prior to oncologic treatment decisions.
Health care providers (HCPs), including physician assistants, nurse practitioners, clinical nurse specialists, nurses, and physicians, routinely participate in every aspect of cancer care by ordering and interpreting diagnostic tests, addressing comorbidities, managing symptoms, and discussing cancer treatment recommendations. HCPs in oncology will continue to play a vital role in the coordination and management of older patients with cancer. However, in general, CGA has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools.
What Is Geriatric Assessment?
Geriatric assessment is a multidisciplinary, multidimensional process aimed at detecting medical, psychosocial, and functional issues of older adults that are not identified by traditional performance status measures alone. It provides guidance for management of identified problems and improvement in quality of life.6 CGA was developed by geriatricians and multidisciplinary care teams to evaluate the domains of functional, nutritional, cognitive, psychosocial, and economic status; comorbidities; geriatric syndromes; and mood, and it has been tested in both clinics and hospitals.7 Although such assessment requires additional time and resources, its goals are to identify areas of vulnerability, assist in clinical decisions of treatable health problems, and guide therapeutic interventions.6 In oncology practice, the assessment not only addresses these global issues, but also is critical in predicting toxicity and survival outcomes in older oncology patients.
Components of CGA
Advancing age brings many physiologic, psychosocial, and functional challenges, and a cancer diagnosis only adds to these issues. CGA provides a system of assessing older and/or frail patients with cancer through specific domains to identify issues that are not apparent on routine evaluation in a clinic setting before and during chemotherapy treatments. These domains include comorbidity, polypharmacy, functional status, cognition, psychological and social status, and nutrition.8
Comorbidity
The prevalence of multiple medical problems and comorbidities, including cancer, among people aged > 65 years is increasing.9 Studies have shown that two-thirds of patients with cancer had ≥ 2 medical conditions, and nearly one quarter had ≥ 4 medical conditions.10 In older adults, common comorbidities include cardiovascular disease, hypertension, diabetes mellitus, and dementia. These comorbidities can impact treatment decisions, increase the risk of disease, impact treatment-related complications, and affect a patient’s life expectancy.11 Assessing comorbidities is essential to CGA and is done using the Charlson Comorbidity Index and/or the Cumulative Illness Rating Scale.12
The Charlson Comorbidity Index was originally designed to predict 1-year mortality on the basis of a weighted composite score for the following categories: cardiovascular, endocrine, pulmonary, neurologic, renal, hepatic, gastrointestinal, and neoplastic disease.13 It is now the most widely used comorbidity index and has been adapted and verified as applicable and valid for predicting the outcomes and risk of death from many comorbid diseases.14 The Cumulative Illness Rating Scale has been validated as a predictor for readmission for hospitalized older adults, hospitalization within 1 year in a residential setting, and long-term mortality when assessed in inpatient and residential settings.15
Polypharmacy
Polypharmacy (use of ≥ 5 medications) is common in older patients regardless of cancer diagnosis and is often instead defined as “the use of multiple drugs or more than are medically necessary.”16 The use of multiple medications, including those not indicated for existing medical conditions (such as over‐the‐counter, herbal, and complementary/alternative medicines, which patients often fail to declare to their specialist, doctor, or pharmacist) adds to the potential negative aspects of polypharmacy that affect older patients.17
Patients with cancer usually are prescribed an extensive number of medicines, both for the disease and for supportive care, which can increase the chance of drug-drug interactions and adverse reactions.18 While these issues certainly affect quality of life, they also may influence chemotherapy treatment and potentially impact survival. Studies have shown that the presence of polypharmacy has been associated with higher numbers of comorbidities, increased use of inappropriate medications, poor performance status, decline in functional status, and poor survival.18
Functional Status
Although Eastern Cooperative Oncology Group (ECOG) performance status and Karnofsky Performance Status are commonly used by oncologists, these guidelines are limited in focus and do not reliably measure functional status in older patients. Functional status is determined by the ability to perform daily acts of self-care, which includes assessment of activities of daily living (ADLs) and instrumental activities of daily living (IADLs). ADLs refer to such tasks as bathing, dressing, eating, mobility, balance, and toileting.19 IADLs include the ability to perform activities required to live within a community and include shopping, transportation, managing finances, medication management, cooking, and cleaning.11
Physical functionality also can be assessed by measures such as gait speed, grip strength, balance, and lower extremity strength. These are more sensitive and shown to be associated with worse clinical outcomes.20 Grip strength and gait speed, as assessed by the Timed Up and Go test or the Short Physical Performance Battery measure strength and balance.12 Reduction in gait speed and/or grip strength are associated with adverse clinical outcomes and increased risk of mortality.21 Patients with cancer who have difficulty with ADLs are at increased risk for falls, which can limit their functional independence, compromise cancer therapy, and increase the risk of chemotherapy toxicities.11 Impaired hearing and poor vision are added factors that can be barriers to cancer treatment.
Cognition
Cognitive impairment in patients with cancer is becoming more of an issue for oncology HCPs as both cancer and cognitive decline are more common with advancing age. Cognition in cancer patients is important for understanding their diagnosis, prognosis, treatment options, and adherence. Impaired cognition can affect decision making regarding treatment options and administration. Cognition can be assessed through validated screening tools such as the Mini-Mental State Examination and Mini-Cog.11
Psychological and Social Status
A cancer diagnosis has a major impact on the mental and emotional state of patients and family members. Clinically significant anxiety has been reported in approximately 21% of older patients with cancer, and the incidence of depression ranges from 17 to 26%.22 In older patients with, psychologic distress can impact cancer treatment, resulting in less definitive therapy and poorer outcomes.23 All patients with cancer should be screened for psychologic distress using standardized methods, such as the Geriatric Depression Scale or the General Anxiety Disorder-7 scale.24 A positive screen should lead to additional assessments that evaluate the severity of depression and other comorbid psychological problems and medical conditions.
Social isolation and loneliness are factors that can affect both depression and anxiety. Older patients with cancer are at risk for decreased social activities and are already challenged with issues related to home care, comorbidities, functional status, and caregiver support.23 Therefore, it is important to assess the social interactions of an older and/or frail patient with cancer and use social work assistance to address needs for supportive services.
Nutrition
Nutrition is important in any patient with cancer undergoing chemotherapy treatment. However, it is of greater importance in older adults, as malnutrition and weight loss are negative prognostic factors that correlate with poor tolerance to chemotherapy treatment, decline in quality of life, and increased mortality.25 The Mini-Nutritional Assessment is a widely used validated tool to assess nutritional status and risk of malnutrition.11 This tool can help identify those older and/or frail patients with cancer with impaired nutritional status and aid in instituting corrective measures to treat or prevent malnutrition.
Effectiveness of CGA
Multiple randomized controlled clinical trials assessing the effectiveness of CGA have been conducted over the past 3 decades with overall positive outcomes related to its value.26 Benefits of CGA can include overall improved medical care, avoidance of hospitalization or nursing home placement, identification of cognitive impairment, and prevention of geriatric syndrome (a range of conditions representing multiple organ impairment in older adults).27
In oncology, CGA is particularly beneficial, as it can identify issues in nearly 70% of patients that may not be apparent through traditional oncology assessment.28 A systematic review of 36 studies assessing the prognostic value of CGA in elderly patients with cancer receiving chemotherapy concluded that impaired performance and functional status as well as a frail and vulnerable profile are important predictors of severe chemotherapy-related toxicity and are associated with a higher risk of mortality.29 Therefore, CGA should be an integral part of the evaluation of older and/or frail patients with cancer prior to chemotherapy consideration.
Several screening tools have been developed using information from CGA to assess the risk of severe toxicities. The most commonly used tools for predicting toxicity include the Cancer and Aging Research Group (CARG) chemotoxicity calculator and the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH).30,31 Although these tools are readily available to facilitate CGA, and despite their proven beneficial outcome and recommended usage by national guidelines, implementation of these tools in routine oncology practice has been challenging and slow to spread. Unless these recommended interventions are effectively implemented, the benefits of CGA cannot be realized. With the expected surge in the number of older patients with cancer, hopefully this will change.
Geriatric Assessment Screening Tools
A screening tool recommended for use in older and/or frail patients with cancer allows for a brief assessment to help clinicians identify patients in need of further evaluation by CGA and to provides information on treatment-related toxicities, functional decline, and survival.32 The predictive value and utility of geriatric assessment screening tools have been repeatedly proven to identify older and/or frail adults at risk for treatment-related toxicities.12 The CARG and the CRASH are validated screening tools used in identifying patients at higher risk for chemotherapy toxicity. These screening tools are intended to provide guidance to the clinical oncology practitioner on risk stratification of chemotherapy toxicity in older patients with cancer.33
Both of these screening tools provide similar predictive performance for chemotherapy toxicity in older patients with cancer.34 However, the CARG tool seems to have the advantage of using more data that had already been obtained during regular office visits and is clear and easy to use clinically. The CRASH tool is slightly more involved, as it uses multiple geriatric instruments to determine the predictive risk of both hematologic and nonhematologic toxicities of chemotherapy.
CARG Chemotoxicity Calculator
Hurria and colleagues originally developed the CARG tool from data obtained through a prospective multicenter study involving 500 patients with cancer aged ≥ 65 years.35 They concluded that chemotherapy-related toxicity is common in older adults, with 53% of patients sustaining grade 3 or 4 treatment-related toxicities and 2% treatment-related mortality.12 This predictive model for chemotherapy-related toxicity used 11 variables, both objective (obtained during a regular clinical encounter: age, tumor type, chemotherapy dosing, number of drugs, creatinine, and hemoglobin) and subjective (completed by patient: number of falls, social support, the ability to take medications, hearing impairment, and physical performance), to determine at-risk patients (Table 1).31
Compared with standard performance status measures in oncology practice, the CARG model was better able to predict chemotherapy-related toxicities. In 2016, Hurria and colleagues published the results of an updated external validation study with a cohort of 250 older patients with cancer receiving chemotherapy that confirmed the prediction of chemotherapy toxicity using the CARG screening tool in this population.31 An appealing feature of this tool is the free online accessibility and the expedited manner in which screening can be conducted.
CRASH Score
The CRASH score was derived from the results of a prospective, multicenter study of 518 patients aged ≥ 70 years who were assessed on 24 parameters prior to starting chemotherapy.30 A total of 64% of patients experienced significant toxicities, including 32% with grade 4 hematologic toxicity and 56% with grade 3 or 4 nonhematologic toxicity. The hematologic and nonhematologic toxicity risks are the 2 categories that comprise the CRASH score. Both baseline patient variables and chemotherapy regimen are incorporated into an 8-item assessment profile that determines the risk categories (Table 2).30
Increased risk of hematologic toxicities was associated with increased diastolic blood pressure, increased lactate dehydrogenase, need for assistance with IADL, and increased toxicity potential of the chemotherapy regimen. Nonhematologic toxicities were associated with ECOG performance score, Mini Mental Status Examination and Mini-Nutritional Assessment, and increased toxicity of the chemotherapy regimen.12 Patient scores are stratified into 4 risk categories: low, medium-low, medium-high, and high.30 Like the CARG tool, the CRASH screening tool also is available as a free online resource and can be used in everyday clinical practice to assess older and/or frail adults with cancer.
Conclusions
In older adults, cancer may significantly impact the natural course of concurrent comorbidities due to physiologic and functional changes. These vulnerabilities predispose older patients with cancer to an increased risk of adverse outcomes, including treatment-related toxicities.36 Given the rapidly aging population, it is critical for oncology clinical teams to be prepared to assess for, prevent, and manage issues for older adults that could impact outcomes, including complications and toxicities from chemotherapy.35 Studies have reported that 78 to 93% of older oncology patients have at least 1 geriatric impairment that could potentially impact oncology treatment plans.37,38 This supports the utility of CGA as a global assessment tool to risk stratify older and/or frail patients prior to deciding on subsequent oncologic treatment approaches.5 In fact, major cooperative groups sponsored by the National Cancer Institute, such as the Alliance for Clinical Trials in Oncology, are including CGA as part of some of their treatment trials. CGA was conducted as part of a multicenter cooperative group study in older patients with acute myeloid leukemia prior to inpatient intensive induction chemotherapy and was determined to be feasible and useful in clinical trials and practice.39
Despite the increasing evidence for benefits of CGA, it has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools. Although oncology providers routinely participate in every aspect of cancer care and play a vital role in the coordination and management of older patients with cancer, CGA implementation into routine clinical practice has been slow in part due to lack of knowledge and training regarding the use of GA tools.
Oncology providers can easily incorporate CGA screening tools into the history and physical examination process for older patients with cancer, which will add an important dimension to these patient evaluations. Oncology providers are not only well positioned to administer these screening tools, but also can lead the field in developing innovative ways for effective implementation in busy routine oncology clinics. However, to be successful, oncology providers must be knowledgeable about these tools and understand their utility in guiding treatment decisions and improving quality of care in older patients with cancer.
Age is a well recognized risk factor for cancer development. The population of older Americans is growing, and by 2030, 20% of the US population will be aged ≥ 65 years.1 While 25% of all new cancer cases are diagnosed in people aged 65 to 74 years, more than half of cancers occur in individuals aged ≥ 70 years, with even higher rates in those aged ≥ 75 years.2 Although cancer rates have declined slightly overall among people aged ≥ 65 years, this population still has an 11-fold increased incidence of cancer compared with that of younger individuals.3 With a rapidly growing older population, there will be increasing demand for cancer care.
Treatment of cancer in older individuals often is complicated by medical comorbidities, frailty, and poor functional status. Distinguishing patients who can tolerate aggressive therapy from those who require less intensive therapy can be challenging. Age-related physiologic changes predispose older adults to an increased risk of therapy-related toxicities, resulting in suboptimal therapeutic benefit and substantial morbidity. For example, cardiovascular changes can lead to reduction of the cardiac functional reserve, which can increase the risk of congestive heart failure. Similarly, decline in renal function leads to an increased potential for nephrotoxicity.4 Although patients may be of the same chronologic age, their performance, functional, and biologic status may be quite variable; thus, tolerance to aggressive treatment is not easily predicted. The comprehensive geriatric assessment (CGA) may be used as a global assessment tool to risk stratify older patients prior to oncologic treatment decisions.
Health care providers (HCPs), including physician assistants, nurse practitioners, clinical nurse specialists, nurses, and physicians, routinely participate in every aspect of cancer care by ordering and interpreting diagnostic tests, addressing comorbidities, managing symptoms, and discussing cancer treatment recommendations. HCPs in oncology will continue to play a vital role in the coordination and management of older patients with cancer. However, in general, CGA has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools.
What Is Geriatric Assessment?
Geriatric assessment is a multidisciplinary, multidimensional process aimed at detecting medical, psychosocial, and functional issues of older adults that are not identified by traditional performance status measures alone. It provides guidance for management of identified problems and improvement in quality of life.6 CGA was developed by geriatricians and multidisciplinary care teams to evaluate the domains of functional, nutritional, cognitive, psychosocial, and economic status; comorbidities; geriatric syndromes; and mood, and it has been tested in both clinics and hospitals.7 Although such assessment requires additional time and resources, its goals are to identify areas of vulnerability, assist in clinical decisions of treatable health problems, and guide therapeutic interventions.6 In oncology practice, the assessment not only addresses these global issues, but also is critical in predicting toxicity and survival outcomes in older oncology patients.
Components of CGA
Advancing age brings many physiologic, psychosocial, and functional challenges, and a cancer diagnosis only adds to these issues. CGA provides a system of assessing older and/or frail patients with cancer through specific domains to identify issues that are not apparent on routine evaluation in a clinic setting before and during chemotherapy treatments. These domains include comorbidity, polypharmacy, functional status, cognition, psychological and social status, and nutrition.8
Comorbidity
The prevalence of multiple medical problems and comorbidities, including cancer, among people aged > 65 years is increasing.9 Studies have shown that two-thirds of patients with cancer had ≥ 2 medical conditions, and nearly one quarter had ≥ 4 medical conditions.10 In older adults, common comorbidities include cardiovascular disease, hypertension, diabetes mellitus, and dementia. These comorbidities can impact treatment decisions, increase the risk of disease, impact treatment-related complications, and affect a patient’s life expectancy.11 Assessing comorbidities is essential to CGA and is done using the Charlson Comorbidity Index and/or the Cumulative Illness Rating Scale.12
The Charlson Comorbidity Index was originally designed to predict 1-year mortality on the basis of a weighted composite score for the following categories: cardiovascular, endocrine, pulmonary, neurologic, renal, hepatic, gastrointestinal, and neoplastic disease.13 It is now the most widely used comorbidity index and has been adapted and verified as applicable and valid for predicting the outcomes and risk of death from many comorbid diseases.14 The Cumulative Illness Rating Scale has been validated as a predictor for readmission for hospitalized older adults, hospitalization within 1 year in a residential setting, and long-term mortality when assessed in inpatient and residential settings.15
Polypharmacy
Polypharmacy (use of ≥ 5 medications) is common in older patients regardless of cancer diagnosis and is often instead defined as “the use of multiple drugs or more than are medically necessary.”16 The use of multiple medications, including those not indicated for existing medical conditions (such as over‐the‐counter, herbal, and complementary/alternative medicines, which patients often fail to declare to their specialist, doctor, or pharmacist) adds to the potential negative aspects of polypharmacy that affect older patients.17
Patients with cancer usually are prescribed an extensive number of medicines, both for the disease and for supportive care, which can increase the chance of drug-drug interactions and adverse reactions.18 While these issues certainly affect quality of life, they also may influence chemotherapy treatment and potentially impact survival. Studies have shown that the presence of polypharmacy has been associated with higher numbers of comorbidities, increased use of inappropriate medications, poor performance status, decline in functional status, and poor survival.18
Functional Status
Although Eastern Cooperative Oncology Group (ECOG) performance status and Karnofsky Performance Status are commonly used by oncologists, these guidelines are limited in focus and do not reliably measure functional status in older patients. Functional status is determined by the ability to perform daily acts of self-care, which includes assessment of activities of daily living (ADLs) and instrumental activities of daily living (IADLs). ADLs refer to such tasks as bathing, dressing, eating, mobility, balance, and toileting.19 IADLs include the ability to perform activities required to live within a community and include shopping, transportation, managing finances, medication management, cooking, and cleaning.11
Physical functionality also can be assessed by measures such as gait speed, grip strength, balance, and lower extremity strength. These are more sensitive and shown to be associated with worse clinical outcomes.20 Grip strength and gait speed, as assessed by the Timed Up and Go test or the Short Physical Performance Battery measure strength and balance.12 Reduction in gait speed and/or grip strength are associated with adverse clinical outcomes and increased risk of mortality.21 Patients with cancer who have difficulty with ADLs are at increased risk for falls, which can limit their functional independence, compromise cancer therapy, and increase the risk of chemotherapy toxicities.11 Impaired hearing and poor vision are added factors that can be barriers to cancer treatment.
Cognition
Cognitive impairment in patients with cancer is becoming more of an issue for oncology HCPs as both cancer and cognitive decline are more common with advancing age. Cognition in cancer patients is important for understanding their diagnosis, prognosis, treatment options, and adherence. Impaired cognition can affect decision making regarding treatment options and administration. Cognition can be assessed through validated screening tools such as the Mini-Mental State Examination and Mini-Cog.11
Psychological and Social Status
A cancer diagnosis has a major impact on the mental and emotional state of patients and family members. Clinically significant anxiety has been reported in approximately 21% of older patients with cancer, and the incidence of depression ranges from 17 to 26%.22 In older patients with, psychologic distress can impact cancer treatment, resulting in less definitive therapy and poorer outcomes.23 All patients with cancer should be screened for psychologic distress using standardized methods, such as the Geriatric Depression Scale or the General Anxiety Disorder-7 scale.24 A positive screen should lead to additional assessments that evaluate the severity of depression and other comorbid psychological problems and medical conditions.
Social isolation and loneliness are factors that can affect both depression and anxiety. Older patients with cancer are at risk for decreased social activities and are already challenged with issues related to home care, comorbidities, functional status, and caregiver support.23 Therefore, it is important to assess the social interactions of an older and/or frail patient with cancer and use social work assistance to address needs for supportive services.
Nutrition
Nutrition is important in any patient with cancer undergoing chemotherapy treatment. However, it is of greater importance in older adults, as malnutrition and weight loss are negative prognostic factors that correlate with poor tolerance to chemotherapy treatment, decline in quality of life, and increased mortality.25 The Mini-Nutritional Assessment is a widely used validated tool to assess nutritional status and risk of malnutrition.11 This tool can help identify those older and/or frail patients with cancer with impaired nutritional status and aid in instituting corrective measures to treat or prevent malnutrition.
Effectiveness of CGA
Multiple randomized controlled clinical trials assessing the effectiveness of CGA have been conducted over the past 3 decades with overall positive outcomes related to its value.26 Benefits of CGA can include overall improved medical care, avoidance of hospitalization or nursing home placement, identification of cognitive impairment, and prevention of geriatric syndrome (a range of conditions representing multiple organ impairment in older adults).27
In oncology, CGA is particularly beneficial, as it can identify issues in nearly 70% of patients that may not be apparent through traditional oncology assessment.28 A systematic review of 36 studies assessing the prognostic value of CGA in elderly patients with cancer receiving chemotherapy concluded that impaired performance and functional status as well as a frail and vulnerable profile are important predictors of severe chemotherapy-related toxicity and are associated with a higher risk of mortality.29 Therefore, CGA should be an integral part of the evaluation of older and/or frail patients with cancer prior to chemotherapy consideration.
Several screening tools have been developed using information from CGA to assess the risk of severe toxicities. The most commonly used tools for predicting toxicity include the Cancer and Aging Research Group (CARG) chemotoxicity calculator and the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH).30,31 Although these tools are readily available to facilitate CGA, and despite their proven beneficial outcome and recommended usage by national guidelines, implementation of these tools in routine oncology practice has been challenging and slow to spread. Unless these recommended interventions are effectively implemented, the benefits of CGA cannot be realized. With the expected surge in the number of older patients with cancer, hopefully this will change.
Geriatric Assessment Screening Tools
A screening tool recommended for use in older and/or frail patients with cancer allows for a brief assessment to help clinicians identify patients in need of further evaluation by CGA and to provides information on treatment-related toxicities, functional decline, and survival.32 The predictive value and utility of geriatric assessment screening tools have been repeatedly proven to identify older and/or frail adults at risk for treatment-related toxicities.12 The CARG and the CRASH are validated screening tools used in identifying patients at higher risk for chemotherapy toxicity. These screening tools are intended to provide guidance to the clinical oncology practitioner on risk stratification of chemotherapy toxicity in older patients with cancer.33
Both of these screening tools provide similar predictive performance for chemotherapy toxicity in older patients with cancer.34 However, the CARG tool seems to have the advantage of using more data that had already been obtained during regular office visits and is clear and easy to use clinically. The CRASH tool is slightly more involved, as it uses multiple geriatric instruments to determine the predictive risk of both hematologic and nonhematologic toxicities of chemotherapy.
CARG Chemotoxicity Calculator
Hurria and colleagues originally developed the CARG tool from data obtained through a prospective multicenter study involving 500 patients with cancer aged ≥ 65 years.35 They concluded that chemotherapy-related toxicity is common in older adults, with 53% of patients sustaining grade 3 or 4 treatment-related toxicities and 2% treatment-related mortality.12 This predictive model for chemotherapy-related toxicity used 11 variables, both objective (obtained during a regular clinical encounter: age, tumor type, chemotherapy dosing, number of drugs, creatinine, and hemoglobin) and subjective (completed by patient: number of falls, social support, the ability to take medications, hearing impairment, and physical performance), to determine at-risk patients (Table 1).31
Compared with standard performance status measures in oncology practice, the CARG model was better able to predict chemotherapy-related toxicities. In 2016, Hurria and colleagues published the results of an updated external validation study with a cohort of 250 older patients with cancer receiving chemotherapy that confirmed the prediction of chemotherapy toxicity using the CARG screening tool in this population.31 An appealing feature of this tool is the free online accessibility and the expedited manner in which screening can be conducted.
CRASH Score
The CRASH score was derived from the results of a prospective, multicenter study of 518 patients aged ≥ 70 years who were assessed on 24 parameters prior to starting chemotherapy.30 A total of 64% of patients experienced significant toxicities, including 32% with grade 4 hematologic toxicity and 56% with grade 3 or 4 nonhematologic toxicity. The hematologic and nonhematologic toxicity risks are the 2 categories that comprise the CRASH score. Both baseline patient variables and chemotherapy regimen are incorporated into an 8-item assessment profile that determines the risk categories (Table 2).30
Increased risk of hematologic toxicities was associated with increased diastolic blood pressure, increased lactate dehydrogenase, need for assistance with IADL, and increased toxicity potential of the chemotherapy regimen. Nonhematologic toxicities were associated with ECOG performance score, Mini Mental Status Examination and Mini-Nutritional Assessment, and increased toxicity of the chemotherapy regimen.12 Patient scores are stratified into 4 risk categories: low, medium-low, medium-high, and high.30 Like the CARG tool, the CRASH screening tool also is available as a free online resource and can be used in everyday clinical practice to assess older and/or frail adults with cancer.
Conclusions
In older adults, cancer may significantly impact the natural course of concurrent comorbidities due to physiologic and functional changes. These vulnerabilities predispose older patients with cancer to an increased risk of adverse outcomes, including treatment-related toxicities.36 Given the rapidly aging population, it is critical for oncology clinical teams to be prepared to assess for, prevent, and manage issues for older adults that could impact outcomes, including complications and toxicities from chemotherapy.35 Studies have reported that 78 to 93% of older oncology patients have at least 1 geriatric impairment that could potentially impact oncology treatment plans.37,38 This supports the utility of CGA as a global assessment tool to risk stratify older and/or frail patients prior to deciding on subsequent oncologic treatment approaches.5 In fact, major cooperative groups sponsored by the National Cancer Institute, such as the Alliance for Clinical Trials in Oncology, are including CGA as part of some of their treatment trials. CGA was conducted as part of a multicenter cooperative group study in older patients with acute myeloid leukemia prior to inpatient intensive induction chemotherapy and was determined to be feasible and useful in clinical trials and practice.39
Despite the increasing evidence for benefits of CGA, it has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools. Although oncology providers routinely participate in every aspect of cancer care and play a vital role in the coordination and management of older patients with cancer, CGA implementation into routine clinical practice has been slow in part due to lack of knowledge and training regarding the use of GA tools.
Oncology providers can easily incorporate CGA screening tools into the history and physical examination process for older patients with cancer, which will add an important dimension to these patient evaluations. Oncology providers are not only well positioned to administer these screening tools, but also can lead the field in developing innovative ways for effective implementation in busy routine oncology clinics. However, to be successful, oncology providers must be knowledgeable about these tools and understand their utility in guiding treatment decisions and improving quality of care in older patients with cancer.
1. Sharless NE. The challenging landscape of cancer and aging: charting a way forward. Published January 24, 2018. Accessed April 16, 2021. https://www.cancer.gov/news-events/cancer-currents-blog/2018/sharpless-aging-cancer-research
2. National Cancer Institute. Age and cancer risk. Updated March 5, 2021. Accessed April 16, 2021. https://www.cancer.gov/about-cancer/causes-prevention/risk/age
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551 4. Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11(6):449-460. doi:10.1097/00130404-200511000-00004
5. Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol. 2013;24(5):1306-1312. doi:10.1093/annonc/mds619
6. Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. What every oncologist should know about geriatric assessment for older patients with cancer: Young International Society of Geriatric Oncology position paper. J Oncol Pract. 2018;14(2):85-94. doi:10.1200/JOP.2017.026435
7. Cohen HJ. Evolution of geriatric assessment in oncology. J Oncol Pract. 2018;14(2):95-96. doi:10.1200/JOP.18.00017
8. Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603. doi:10.1200/JCO.2013.54.8347
9. American Cancer Society. Cancer facts & figures 2019. Accessed April 16, 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
10. Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249-257. doi:10.1016/j.jgo.2015.12.002
11. Korc-Grodzicki B, Holmes HM, Shahrokni A. Geriatric assessment for oncologists. Cancer Biol Med. 2015;12(4):261-274. doi:10.7497/j.issn.2095-3941.2015.0082
12. Li D, Soto-Perez-de-Celis E, Hurria A. Geriatric assessment and tools for predicting treatment toxicity in older adults with cancer. Cancer J. 2017;23(4):206-210. doi:10.1097/PPO.0000000000000269
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
14. Huang Y, Gou R, Diao Y, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58-66. doi:10.1631/jzus.B1300109
15. Osborn KP IV, Nothelle S, Slaven JE, Montz K, Hui S, Torke AM. Cumulative Illness Rating Scale (CIRS) can be used to predict hospital outcomes in older adults. J Geriatric Med Gerontol. 2017;3(2). doi:10.23937/2469-5858/1510030
16. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65. doi:10.1517/14740338.2013.827660
17. Shrestha S, Shrestha S, Khanal S. Polypharmacy in elderly cancer patients: challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med. 2019;2(1):42-49. doi:10.1002/agm2.12051
18. Sharma M, Loh KP, Nightingale G, Mohile SG, Holmes HM. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol. 2016;7(5):346-353. doi:10.1016/j.jgo.2016.07.010
19. Norburn JE, Bernard SL, Konrad TR, et al. Self-care and assistance from others in coping with functional status limitations among a national sample of older adults. J Gerontol B Psychol Sci Soc Sci. 1995;50(2):S101-S109. doi:10.1093/geronb/50b.2.s101
20. Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc. 2016;64(1):144-150. doi:10.1111/jgs.13871
21. Owusu C, Berger NA. Comprehensive geriatric assessment in the older cancer patient: coming of age in clinical cancer care. Clin Pract (Lond). 2014;11(6):749-762. doi:10.2217/cpr.14.72
22. Weiss Wiesel TR, Nelson CJ, Tew WP, et al. The relationship between age, anxiety, and depression in older adults with cancer. Psychooncology. 2015;24(6):712-717. doi:10.1002/pon.3638
23. Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19(6):e305-e316. doi:10.1016/S1470-2045(18)30348-6
24. Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605-1619. doi:10.1200/JCO.2013.52.4611
25. Muscaritoli M, Lucia S, Farcomeni A, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. 2017;8(45):79884-79886. doi:10.18632/oncotarget.20168
26. Ekdahl AW, Axmon A, Sandberg M, Steen Carlsson K. Is care based on comprehensive geriatric assessment with mobile teams better than usual care? A study protocol of a randomised controlled trial (the GerMoT study). BMJ Open. 2018;8(10)e23969. doi:10.1136/bmjopen-2018-023969
27. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
28. Hernandez Torres C, Hsu T. Comprehensive geriatric assessment in the older adult with cancer: a review. Eur Urol Focus. 2017;3(4-5):330-339. doi:10.1016/j.euf.2017.10.010
29. Janssens K, Specenier P. The prognostic value of the comprehensive geriatric assessment (CGA) in elderly cancer patients (ECP) treated with chemotherapy (CT): a systematic review. Eur J Cancer. 2017;72(1):S164-S165. doi:10.1016/S0959-8049(17)30611-1
30. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer. 2012;118(13):3377-3386. doi:10.1002/cncr.26646
31. Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366-2371. doi:10.1200/JCO.2015.65.4327
32. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288-300. doi:10.1093/annonc/mdu210
33. Schiefen JK, Madsen LT, Dains JE. Instruments that predict oncology treatment risk in the senior population. J Adv Pract Oncol. 2017;8(5):528-533.
34. Ortland I, Mendel Ott M, Kowar M, et al. Comparing the performance of the CARG and the CRASH score for predicting toxicity in older patients with cancer. J Geriatr Oncol. 2020;11(6):997-1005. doi:10.1016/j.jgo.2019.12.016
35. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465. doi:10.1200/JCO.2011.34.7625
36. Mohile SG, Velarde C, Hurria A, et al. Geriatric assessment-guided care processes for older adults: a Delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13(9):1120-1130. doi:10.6004/jnccn.2015.0137
37. Schiphorst AHW, Ten Bokkel Huinink D, Breumelhof R, Burgmans JPJ, Pronk A, Hamaker ME. Geriatric consultation can aid in complex treatment decisions for elderly cancer patients. Eur J Cancer Care (Engl). 2016;25(3):365-370. doi:10.1111/ecc.12349
38. Schulkes KJG, Souwer ETD, Hamaker ME, et al. The effect of a geriatric assessment on treatment decisions for patients with lung cancer. Lung. 2017;195(2):225-231. doi:10.1007/s00408-017-9983-7
39. Klepin HD, Ritchie E, Major-Elechi B, et al. Geriatric assessment among older adults receiving intensive therapy for acute myeloid leukemia: report of CALGB 361006 (Alliance). J Geriatr Oncol. 2020;11(1):107-113. doi:10.1016/j.jgo.2019.10.002
1. Sharless NE. The challenging landscape of cancer and aging: charting a way forward. Published January 24, 2018. Accessed April 16, 2021. https://www.cancer.gov/news-events/cancer-currents-blog/2018/sharpless-aging-cancer-research
2. National Cancer Institute. Age and cancer risk. Updated March 5, 2021. Accessed April 16, 2021. https://www.cancer.gov/about-cancer/causes-prevention/risk/age
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551 4. Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11(6):449-460. doi:10.1097/00130404-200511000-00004
5. Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol. 2013;24(5):1306-1312. doi:10.1093/annonc/mds619
6. Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. What every oncologist should know about geriatric assessment for older patients with cancer: Young International Society of Geriatric Oncology position paper. J Oncol Pract. 2018;14(2):85-94. doi:10.1200/JOP.2017.026435
7. Cohen HJ. Evolution of geriatric assessment in oncology. J Oncol Pract. 2018;14(2):95-96. doi:10.1200/JOP.18.00017
8. Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603. doi:10.1200/JCO.2013.54.8347
9. American Cancer Society. Cancer facts & figures 2019. Accessed April 16, 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
10. Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249-257. doi:10.1016/j.jgo.2015.12.002
11. Korc-Grodzicki B, Holmes HM, Shahrokni A. Geriatric assessment for oncologists. Cancer Biol Med. 2015;12(4):261-274. doi:10.7497/j.issn.2095-3941.2015.0082
12. Li D, Soto-Perez-de-Celis E, Hurria A. Geriatric assessment and tools for predicting treatment toxicity in older adults with cancer. Cancer J. 2017;23(4):206-210. doi:10.1097/PPO.0000000000000269
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
14. Huang Y, Gou R, Diao Y, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58-66. doi:10.1631/jzus.B1300109
15. Osborn KP IV, Nothelle S, Slaven JE, Montz K, Hui S, Torke AM. Cumulative Illness Rating Scale (CIRS) can be used to predict hospital outcomes in older adults. J Geriatric Med Gerontol. 2017;3(2). doi:10.23937/2469-5858/1510030
16. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65. doi:10.1517/14740338.2013.827660
17. Shrestha S, Shrestha S, Khanal S. Polypharmacy in elderly cancer patients: challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med. 2019;2(1):42-49. doi:10.1002/agm2.12051
18. Sharma M, Loh KP, Nightingale G, Mohile SG, Holmes HM. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol. 2016;7(5):346-353. doi:10.1016/j.jgo.2016.07.010
19. Norburn JE, Bernard SL, Konrad TR, et al. Self-care and assistance from others in coping with functional status limitations among a national sample of older adults. J Gerontol B Psychol Sci Soc Sci. 1995;50(2):S101-S109. doi:10.1093/geronb/50b.2.s101
20. Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc. 2016;64(1):144-150. doi:10.1111/jgs.13871
21. Owusu C, Berger NA. Comprehensive geriatric assessment in the older cancer patient: coming of age in clinical cancer care. Clin Pract (Lond). 2014;11(6):749-762. doi:10.2217/cpr.14.72
22. Weiss Wiesel TR, Nelson CJ, Tew WP, et al. The relationship between age, anxiety, and depression in older adults with cancer. Psychooncology. 2015;24(6):712-717. doi:10.1002/pon.3638
23. Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19(6):e305-e316. doi:10.1016/S1470-2045(18)30348-6
24. Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605-1619. doi:10.1200/JCO.2013.52.4611
25. Muscaritoli M, Lucia S, Farcomeni A, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. 2017;8(45):79884-79886. doi:10.18632/oncotarget.20168
26. Ekdahl AW, Axmon A, Sandberg M, Steen Carlsson K. Is care based on comprehensive geriatric assessment with mobile teams better than usual care? A study protocol of a randomised controlled trial (the GerMoT study). BMJ Open. 2018;8(10)e23969. doi:10.1136/bmjopen-2018-023969
27. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
28. Hernandez Torres C, Hsu T. Comprehensive geriatric assessment in the older adult with cancer: a review. Eur Urol Focus. 2017;3(4-5):330-339. doi:10.1016/j.euf.2017.10.010
29. Janssens K, Specenier P. The prognostic value of the comprehensive geriatric assessment (CGA) in elderly cancer patients (ECP) treated with chemotherapy (CT): a systematic review. Eur J Cancer. 2017;72(1):S164-S165. doi:10.1016/S0959-8049(17)30611-1
30. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer. 2012;118(13):3377-3386. doi:10.1002/cncr.26646
31. Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366-2371. doi:10.1200/JCO.2015.65.4327
32. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288-300. doi:10.1093/annonc/mdu210
33. Schiefen JK, Madsen LT, Dains JE. Instruments that predict oncology treatment risk in the senior population. J Adv Pract Oncol. 2017;8(5):528-533.
34. Ortland I, Mendel Ott M, Kowar M, et al. Comparing the performance of the CARG and the CRASH score for predicting toxicity in older patients with cancer. J Geriatr Oncol. 2020;11(6):997-1005. doi:10.1016/j.jgo.2019.12.016
35. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465. doi:10.1200/JCO.2011.34.7625
36. Mohile SG, Velarde C, Hurria A, et al. Geriatric assessment-guided care processes for older adults: a Delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13(9):1120-1130. doi:10.6004/jnccn.2015.0137
37. Schiphorst AHW, Ten Bokkel Huinink D, Breumelhof R, Burgmans JPJ, Pronk A, Hamaker ME. Geriatric consultation can aid in complex treatment decisions for elderly cancer patients. Eur J Cancer Care (Engl). 2016;25(3):365-370. doi:10.1111/ecc.12349
38. Schulkes KJG, Souwer ETD, Hamaker ME, et al. The effect of a geriatric assessment on treatment decisions for patients with lung cancer. Lung. 2017;195(2):225-231. doi:10.1007/s00408-017-9983-7
39. Klepin HD, Ritchie E, Major-Elechi B, et al. Geriatric assessment among older adults receiving intensive therapy for acute myeloid leukemia: report of CALGB 361006 (Alliance). J Geriatr Oncol. 2020;11(1):107-113. doi:10.1016/j.jgo.2019.10.002