User login
Trends in Intravenous Magnesium Use and Outcomes for Status Asthmaticus in Children’s Hospitals from 2010 to 2017
For severe asthma exacerbations unresponsive to initial treatment, expert consensus guidelines from 2007 recommend consideration for adjunct treatments (magnesium or heliox) to decrease the likelihood of intubation.1 Over the last decade, data have emerged suggesting that intravenous (IV) magnesium may be more effective for reduction of hospital admission rates.2 Pooled meta-analyses have demonstrated improved pulmonary function and reduction of hospital admission by as much as 68% in children when IV magnesium is administered in the emergency department (ED), although the evidence is extremely limited because of a small number of studies (three) and small sample size (115 children).2-5
Though these data suggest that use of IV magnesium may reduce admission rates, a study of pediatric emergency medicine (PEM) physicians in US and Canada reported reluctance regarding use for this purpose. While PEM physicians reported awareness of the literature on admission prevention, they estimated that fewer than 5% of their patients receiving IV magnesium were discharged home.6 Their practice was generally limited to using IV magnesium in children with impending respiratory failure for the purpose of reducing intensive care unit (ICU) admission and not hospitalization.6 PEM physicians’ reluctance to use IV magnesium was related to the lack of strong available evidence supporting the impact of IV magnesium on outcomes, such as admission, and gaps in the literature about its dosing and safety profile.
The goal of this study was to assess the prevailing trends in IV magnesium use across US children’s hospitals and to assess the relationship of IV magnesium use to admission rate, length of stay (LOS), readmission rate, and ICU admission rate. We hypothesized that IV magnesium use might have increased following publication of studies demonstrating an association between IV magnesium use and fewer admissions.
METHODS
Study Design, Setting, and Participants
This is a retrospective cohort study of asthma (All Patient Refined Diagnosis Related Group 141) hospitalizations for patients less than 18 years old presenting to 35 tertiary care children’s hospitals from January 1, 2010, to December 31, 2017, included in the Pediatric Health Information System (PHIS; Children’s Hospital Association, Lenexa, Kansas) database. The PHIS database is an administrative database that contains demographics, International Classification of Diseases 9th and 10th Revision diagnoses and procedures, and daily billing records for all inpatient, observation, ED, and ambulatory surgery encounters. All data were deidentified prior to inclusion in the database and tracking of patients across ED and inpatient visits was achieved through an encrypted and unique patient identifier. Children transferred from other hospitals were excluded because we could not verify IV magnesium use prior to transfer. For hospitals to be included, they were required to provide continuous data throughout the study period.
Main Outcome Measure
The main outcome was exposure to IV magnesium as determined by billing information available in the PHIS database.
Patient Demographics
We assessed patients’ demographic characteristics, including age (younger than 5 years, 5-11 years, and 12-17 years), sex, race/ethnicity, and insurance status.
Healthcare Utilization and Hospital Characteristics
We assessed healthcare utilization using geometric mean LOS, proportion of patients admitted to the hospital and to the ICU, and the proportion of patients with a 7-day all-cause readmission. In addition, we divided hospitals into three equal groups based on their annual inpatient asthma volume (<300, 300-850, >850 cases per year).
Statistical Analysis
We compared demographic and clinical characteristics across patients receiving IV magnesium with those who did not receive it with use of chi-square tests for categorical variables and Wilcoxon rank sum test for continuous variables. We calculated annual IV magnesium use rates for each hospital and modeled the average annual rate with a general linear model in order to assess change over time. We used Pearson product moment correlation to compare the annual proportion of magnesium use and healthcare utilization measures, including geometric mean LOS, the proportion of patients using the inpatient wards or the ICU, and the proportion of cases with a 7-day all-cause readmission. Geometric mean LOS was used to normalize the compounding effect of non–normally distributed arithmetic mean LOS. A sensitivity analysis was performed stratifying IV magnesium use over time by hospital inpatient volume. Data were analyzed using SAS version 9.4 (SAS Institute, Cary, North Carolina), and P values < .05 were considered statistically significant.
RESULTS
Study Population
A total of 878,188 encounters with acute asthma exacerbation met the inclusion criteria, with 65,558 (7.5%) receiving IV magnesium (Table). Of those receiving IV magnesium, 90% were admitted to the hospital. There were statistically significant differences in IV magnesium use when compared by age, race/ethnicity, and payer type, but not gender. IV magnesium use was significantly associated with older age (more than 5 years old), non-Hispanic black race, ED visit in the year prior to admission, longer hospital LOS, and higher ICU admission rate.

Trends in Intravenous Magnesium Use
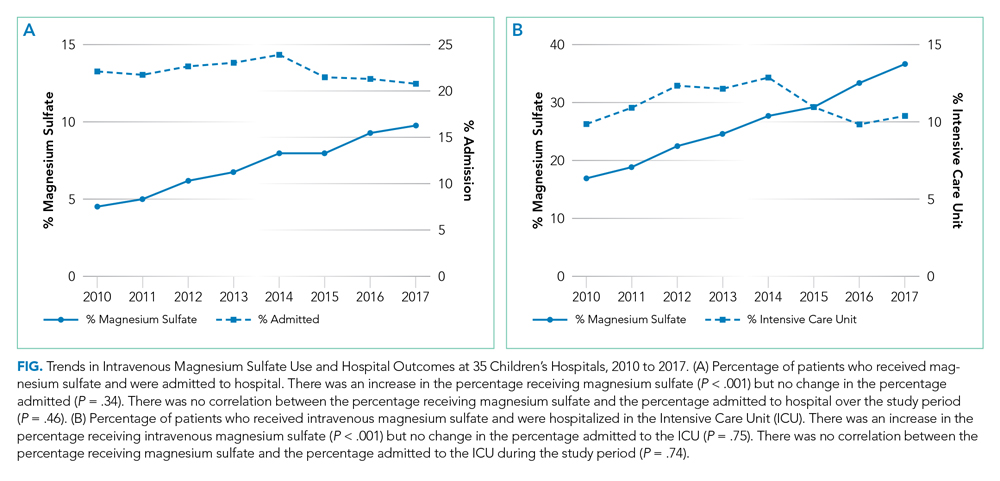
IV magnesium use among hospitalized children more than doubled from 2010 to 2017 (17% vs 36%). Low-volume hospitals had a lower frequency of IV magnesium use, compared with the moderate- and high-volume hospitals. The growth rate per year of IV magnesium use was greater in high- and moderate-volume hospitals (3.4% and 2.9% per year, respectively), compared with the low-volume hospitals (1.2% per year; P = .04).
Trends in Intravenous Magnesium Use and Hospital Outcomes
The trend in IV magnesium use was not associated with a statistically significant change in the inpatient and ICU admission rate or in the 7-day all-cause readmission rate (Figure and Appendix Figure). Although IV magnesium use increased over time, LOS decreased significantly during the same period (1.6 days in 2010 vs 1.4 days in 2017; P < .001). When analyzed by hospital volume, no significant associations were found in the inpatient admission, ICU admission, and 7-day readmission rate.
DISCUSSION
The use of IV magnesium has significantly increased in US children’s hospitals over the last 8 years, especially among those hospitalized following an ED evaluation. Over this interval, trends in inpatient and ICU admission rate, as well as 7-day all-cause readmission rate, for asthma did not change, while LOS decreased. These findings contrast with a recent Cochrane review that summarized the efficacy of IV magnesium for reducing admission rate in few small trials.2
Our study findings are more consistent with prior survey findings that IV magnesium does not reduce hospitalization and that ED physicians tend to use IV magnesium in severe asthma exacerbation for its potential therapeutic benefits because of bronchodilator and anti-inflammatory effect.6,7 Similar to PEM physicians’ estimates, only 10% of patients receiving IV magnesium were discharged home in our study.
IV magnesium use is higher in high-volume hospitals than in moderate- and low-volume ones. One potential explanation is that high- and moderate-volume hospitals may see a higher volume of children with severe or impending respiratory failure and, therefore, are more likely to use IV magnesium than the low-volume hospitals are. Alternatively, physician adoption of magnesium use for lower-acuity asthma exacerbations could vary by hospital volume.
Trend analyses of outcomes suggest that increase in IV magnesium use was not associated with an increase in inpatient and ICU admission rate or with 7-day all-cause readmission rate, although LOS reduced. LOS might be reduced because of various quality improvement initiatives (eg, discharging patients after every 3 hours albuterol treatments or respiratory therapist–driven protocols) and might not be related to IV magnesium use.8,9 To this point, a recent study of a respiratory assessment score–matched cohort did not find any therapeutic benefit of IV magnesium with severe asthma exacerbation when receiving continuous albuterol therapy on a pediatric ward.5 Perhaps future studies could explore estimating the outcome by performing comparative effectiveness studies between those with severe asthma exacerbation who did or did not receive IV magnesium. Additionally, randomized controlled trials comparing IV magnesium and standard therapy and its effects on outcomes, such as hospitalization, LOS, association with asthma chronicity, and previous oral steroid use, might provide further insight to inform clinical practice.
Certain study limitations should be noted. The study cohort included children’s hospitals only, and it is possible that care at nonchildren’s hospitals for asthma differs. PHIS dataset used in this study does not allow determination of where and when IV magnesium was given, the severity of asthma exacerbation, or the chronicity of baseline disease. Moreover, PHIS hospitals include centers in large cities, and other competing children’s hospitals may provide other tertiary care that could affect the readmission data calculation. Lastly, the temporal associations between IV magnesium use and outcomes reported in this study should not be used as evidence or lack of evidence for the effectiveness of magnesium given the limitations of the observational study design and dataset used.
In conclusion, IV magnesium use in management of asthma exacerbation in children across the United States has significantly increased. The increase occurred disproportionately in high-volume hospitals and was not associated with changes in admission rate, ICU admission rate, or 7-day all-cause readmission rate, although LOS has decreased over time.
Disclosures
The authors have no financial relationships relevant to this article or conflicts of interest to disclose.
This paper was a platform presentation at annual meetings of Pediatric Academic Societies 2019; accepted for presentation at annual meeting of Pediatric Hospital Medicine, July 2019.
Funding Source
No funding was secured for this study.
1. National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Maryland: National Heart, Lung, and Blood Institute; 2007. https://www.ncbi.nlm.nih.gov/books/NBK7232/.
2. Griffiths B, Kew KM. Intravenous magnesium sulfate for treating children with acute asthma in the emergency department. Cochrane Database Syst Rev. 2016;4(4):CD011050. https://doi.org/10.1002/14651858.CD011050.pub2.
3. Shan Z, Rong Y, Yang W, et al. Intravenous and nebulized magnesium sulfate for treating acute asthma in adults and children: a systematic review and meta-analysis. Respir Med. 2013;107(3):321-330. https://doi.org/10.1016/j.med.2012.12.001.
4. Rower J, Liu X, Yu T, Mundorff M, Sherwin C, Johnson M. Clinical pharmacokinetics of magnesium sulfate in treatment of children with severe acute asthma. Eur J Clin Pharmacol. 2017;73(3):325-331. https://doi.org/10.1007/s00228-016-2165-3.
5. Desanti R, Agasthya N, Hunter K, Hussain M. The effectiveness of magnesium sulfate for status asthmaticus outside intensive care unit. Pediatric Pulmonol. 2018;53(7):866-871. https://doi.org/10.1002/ppul.24013.Epub 2018.
6. Schuh S, Macias C, Freedman S, et al. North American practice patterns of intravenous magnesium sulfate in severe acute asthma exacerbations. Acad Emerg Med. 2010;17(11):1189-1196. https://doi.org/10.1111/j.1553-2712.2010.00913.x.
7. Cheuk DK, Chau TC, Lee SL. A meta-analysis on intravenous magnesium sulphate for treating acute asthma. Arch Dis Child. 2005;90(1):74-77. https://doi.org/10.1136/adc.2004.050005.
8. Lo HY, Messer A, Loveless J, et al. Discharging asthma patients on 3-hour β-agonist treatments: a quality improvement project. Hosp Pediatr. 2018;8(12):733-739. https://doi.org/10.1542/hpeds.2018-0072.
9. Magruder TG, Narayanan S, Walley S, et al. Improving inpatient asthma management: the implementation and evaluation of pediatric asthma clinical pathway. Pediatr Qual Saf. 2017;2(5);e041. https://doi.org/10.1097/pq9.0000000000000041.
For severe asthma exacerbations unresponsive to initial treatment, expert consensus guidelines from 2007 recommend consideration for adjunct treatments (magnesium or heliox) to decrease the likelihood of intubation.1 Over the last decade, data have emerged suggesting that intravenous (IV) magnesium may be more effective for reduction of hospital admission rates.2 Pooled meta-analyses have demonstrated improved pulmonary function and reduction of hospital admission by as much as 68% in children when IV magnesium is administered in the emergency department (ED), although the evidence is extremely limited because of a small number of studies (three) and small sample size (115 children).2-5
Though these data suggest that use of IV magnesium may reduce admission rates, a study of pediatric emergency medicine (PEM) physicians in US and Canada reported reluctance regarding use for this purpose. While PEM physicians reported awareness of the literature on admission prevention, they estimated that fewer than 5% of their patients receiving IV magnesium were discharged home.6 Their practice was generally limited to using IV magnesium in children with impending respiratory failure for the purpose of reducing intensive care unit (ICU) admission and not hospitalization.6 PEM physicians’ reluctance to use IV magnesium was related to the lack of strong available evidence supporting the impact of IV magnesium on outcomes, such as admission, and gaps in the literature about its dosing and safety profile.
The goal of this study was to assess the prevailing trends in IV magnesium use across US children’s hospitals and to assess the relationship of IV magnesium use to admission rate, length of stay (LOS), readmission rate, and ICU admission rate. We hypothesized that IV magnesium use might have increased following publication of studies demonstrating an association between IV magnesium use and fewer admissions.
METHODS
Study Design, Setting, and Participants
This is a retrospective cohort study of asthma (All Patient Refined Diagnosis Related Group 141) hospitalizations for patients less than 18 years old presenting to 35 tertiary care children’s hospitals from January 1, 2010, to December 31, 2017, included in the Pediatric Health Information System (PHIS; Children’s Hospital Association, Lenexa, Kansas) database. The PHIS database is an administrative database that contains demographics, International Classification of Diseases 9th and 10th Revision diagnoses and procedures, and daily billing records for all inpatient, observation, ED, and ambulatory surgery encounters. All data were deidentified prior to inclusion in the database and tracking of patients across ED and inpatient visits was achieved through an encrypted and unique patient identifier. Children transferred from other hospitals were excluded because we could not verify IV magnesium use prior to transfer. For hospitals to be included, they were required to provide continuous data throughout the study period.
Main Outcome Measure
The main outcome was exposure to IV magnesium as determined by billing information available in the PHIS database.
Patient Demographics
We assessed patients’ demographic characteristics, including age (younger than 5 years, 5-11 years, and 12-17 years), sex, race/ethnicity, and insurance status.
Healthcare Utilization and Hospital Characteristics
We assessed healthcare utilization using geometric mean LOS, proportion of patients admitted to the hospital and to the ICU, and the proportion of patients with a 7-day all-cause readmission. In addition, we divided hospitals into three equal groups based on their annual inpatient asthma volume (<300, 300-850, >850 cases per year).
Statistical Analysis
We compared demographic and clinical characteristics across patients receiving IV magnesium with those who did not receive it with use of chi-square tests for categorical variables and Wilcoxon rank sum test for continuous variables. We calculated annual IV magnesium use rates for each hospital and modeled the average annual rate with a general linear model in order to assess change over time. We used Pearson product moment correlation to compare the annual proportion of magnesium use and healthcare utilization measures, including geometric mean LOS, the proportion of patients using the inpatient wards or the ICU, and the proportion of cases with a 7-day all-cause readmission. Geometric mean LOS was used to normalize the compounding effect of non–normally distributed arithmetic mean LOS. A sensitivity analysis was performed stratifying IV magnesium use over time by hospital inpatient volume. Data were analyzed using SAS version 9.4 (SAS Institute, Cary, North Carolina), and P values < .05 were considered statistically significant.
RESULTS
Study Population
A total of 878,188 encounters with acute asthma exacerbation met the inclusion criteria, with 65,558 (7.5%) receiving IV magnesium (Table). Of those receiving IV magnesium, 90% were admitted to the hospital. There were statistically significant differences in IV magnesium use when compared by age, race/ethnicity, and payer type, but not gender. IV magnesium use was significantly associated with older age (more than 5 years old), non-Hispanic black race, ED visit in the year prior to admission, longer hospital LOS, and higher ICU admission rate.

Trends in Intravenous Magnesium Use
IV magnesium use among hospitalized children more than doubled from 2010 to 2017 (17% vs 36%). Low-volume hospitals had a lower frequency of IV magnesium use, compared with the moderate- and high-volume hospitals. The growth rate per year of IV magnesium use was greater in high- and moderate-volume hospitals (3.4% and 2.9% per year, respectively), compared with the low-volume hospitals (1.2% per year; P = .04).
Trends in Intravenous Magnesium Use and Hospital Outcomes
The trend in IV magnesium use was not associated with a statistically significant change in the inpatient and ICU admission rate or in the 7-day all-cause readmission rate (Figure and Appendix Figure). Although IV magnesium use increased over time, LOS decreased significantly during the same period (1.6 days in 2010 vs 1.4 days in 2017; P < .001). When analyzed by hospital volume, no significant associations were found in the inpatient admission, ICU admission, and 7-day readmission rate.

DISCUSSION
The use of IV magnesium has significantly increased in US children’s hospitals over the last 8 years, especially among those hospitalized following an ED evaluation. Over this interval, trends in inpatient and ICU admission rate, as well as 7-day all-cause readmission rate, for asthma did not change, while LOS decreased. These findings contrast with a recent Cochrane review that summarized the efficacy of IV magnesium for reducing admission rate in few small trials.2
Our study findings are more consistent with prior survey findings that IV magnesium does not reduce hospitalization and that ED physicians tend to use IV magnesium in severe asthma exacerbation for its potential therapeutic benefits because of bronchodilator and anti-inflammatory effect.6,7 Similar to PEM physicians’ estimates, only 10% of patients receiving IV magnesium were discharged home in our study.
IV magnesium use is higher in high-volume hospitals than in moderate- and low-volume ones. One potential explanation is that high- and moderate-volume hospitals may see a higher volume of children with severe or impending respiratory failure and, therefore, are more likely to use IV magnesium than the low-volume hospitals are. Alternatively, physician adoption of magnesium use for lower-acuity asthma exacerbations could vary by hospital volume.
Trend analyses of outcomes suggest that increase in IV magnesium use was not associated with an increase in inpatient and ICU admission rate or with 7-day all-cause readmission rate, although LOS reduced. LOS might be reduced because of various quality improvement initiatives (eg, discharging patients after every 3 hours albuterol treatments or respiratory therapist–driven protocols) and might not be related to IV magnesium use.8,9 To this point, a recent study of a respiratory assessment score–matched cohort did not find any therapeutic benefit of IV magnesium with severe asthma exacerbation when receiving continuous albuterol therapy on a pediatric ward.5 Perhaps future studies could explore estimating the outcome by performing comparative effectiveness studies between those with severe asthma exacerbation who did or did not receive IV magnesium. Additionally, randomized controlled trials comparing IV magnesium and standard therapy and its effects on outcomes, such as hospitalization, LOS, association with asthma chronicity, and previous oral steroid use, might provide further insight to inform clinical practice.
Certain study limitations should be noted. The study cohort included children’s hospitals only, and it is possible that care at nonchildren’s hospitals for asthma differs. PHIS dataset used in this study does not allow determination of where and when IV magnesium was given, the severity of asthma exacerbation, or the chronicity of baseline disease. Moreover, PHIS hospitals include centers in large cities, and other competing children’s hospitals may provide other tertiary care that could affect the readmission data calculation. Lastly, the temporal associations between IV magnesium use and outcomes reported in this study should not be used as evidence or lack of evidence for the effectiveness of magnesium given the limitations of the observational study design and dataset used.
In conclusion, IV magnesium use in management of asthma exacerbation in children across the United States has significantly increased. The increase occurred disproportionately in high-volume hospitals and was not associated with changes in admission rate, ICU admission rate, or 7-day all-cause readmission rate, although LOS has decreased over time.
Disclosures
The authors have no financial relationships relevant to this article or conflicts of interest to disclose.
This paper was a platform presentation at annual meetings of Pediatric Academic Societies 2019; accepted for presentation at annual meeting of Pediatric Hospital Medicine, July 2019.
Funding Source
No funding was secured for this study.
For severe asthma exacerbations unresponsive to initial treatment, expert consensus guidelines from 2007 recommend consideration for adjunct treatments (magnesium or heliox) to decrease the likelihood of intubation.1 Over the last decade, data have emerged suggesting that intravenous (IV) magnesium may be more effective for reduction of hospital admission rates.2 Pooled meta-analyses have demonstrated improved pulmonary function and reduction of hospital admission by as much as 68% in children when IV magnesium is administered in the emergency department (ED), although the evidence is extremely limited because of a small number of studies (three) and small sample size (115 children).2-5
Though these data suggest that use of IV magnesium may reduce admission rates, a study of pediatric emergency medicine (PEM) physicians in US and Canada reported reluctance regarding use for this purpose. While PEM physicians reported awareness of the literature on admission prevention, they estimated that fewer than 5% of their patients receiving IV magnesium were discharged home.6 Their practice was generally limited to using IV magnesium in children with impending respiratory failure for the purpose of reducing intensive care unit (ICU) admission and not hospitalization.6 PEM physicians’ reluctance to use IV magnesium was related to the lack of strong available evidence supporting the impact of IV magnesium on outcomes, such as admission, and gaps in the literature about its dosing and safety profile.
The goal of this study was to assess the prevailing trends in IV magnesium use across US children’s hospitals and to assess the relationship of IV magnesium use to admission rate, length of stay (LOS), readmission rate, and ICU admission rate. We hypothesized that IV magnesium use might have increased following publication of studies demonstrating an association between IV magnesium use and fewer admissions.
METHODS
Study Design, Setting, and Participants
This is a retrospective cohort study of asthma (All Patient Refined Diagnosis Related Group 141) hospitalizations for patients less than 18 years old presenting to 35 tertiary care children’s hospitals from January 1, 2010, to December 31, 2017, included in the Pediatric Health Information System (PHIS; Children’s Hospital Association, Lenexa, Kansas) database. The PHIS database is an administrative database that contains demographics, International Classification of Diseases 9th and 10th Revision diagnoses and procedures, and daily billing records for all inpatient, observation, ED, and ambulatory surgery encounters. All data were deidentified prior to inclusion in the database and tracking of patients across ED and inpatient visits was achieved through an encrypted and unique patient identifier. Children transferred from other hospitals were excluded because we could not verify IV magnesium use prior to transfer. For hospitals to be included, they were required to provide continuous data throughout the study period.
Main Outcome Measure
The main outcome was exposure to IV magnesium as determined by billing information available in the PHIS database.
Patient Demographics
We assessed patients’ demographic characteristics, including age (younger than 5 years, 5-11 years, and 12-17 years), sex, race/ethnicity, and insurance status.
Healthcare Utilization and Hospital Characteristics
We assessed healthcare utilization using geometric mean LOS, proportion of patients admitted to the hospital and to the ICU, and the proportion of patients with a 7-day all-cause readmission. In addition, we divided hospitals into three equal groups based on their annual inpatient asthma volume (<300, 300-850, >850 cases per year).
Statistical Analysis
We compared demographic and clinical characteristics across patients receiving IV magnesium with those who did not receive it with use of chi-square tests for categorical variables and Wilcoxon rank sum test for continuous variables. We calculated annual IV magnesium use rates for each hospital and modeled the average annual rate with a general linear model in order to assess change over time. We used Pearson product moment correlation to compare the annual proportion of magnesium use and healthcare utilization measures, including geometric mean LOS, the proportion of patients using the inpatient wards or the ICU, and the proportion of cases with a 7-day all-cause readmission. Geometric mean LOS was used to normalize the compounding effect of non–normally distributed arithmetic mean LOS. A sensitivity analysis was performed stratifying IV magnesium use over time by hospital inpatient volume. Data were analyzed using SAS version 9.4 (SAS Institute, Cary, North Carolina), and P values < .05 were considered statistically significant.
RESULTS
Study Population
A total of 878,188 encounters with acute asthma exacerbation met the inclusion criteria, with 65,558 (7.5%) receiving IV magnesium (Table). Of those receiving IV magnesium, 90% were admitted to the hospital. There were statistically significant differences in IV magnesium use when compared by age, race/ethnicity, and payer type, but not gender. IV magnesium use was significantly associated with older age (more than 5 years old), non-Hispanic black race, ED visit in the year prior to admission, longer hospital LOS, and higher ICU admission rate.

Trends in Intravenous Magnesium Use
IV magnesium use among hospitalized children more than doubled from 2010 to 2017 (17% vs 36%). Low-volume hospitals had a lower frequency of IV magnesium use, compared with the moderate- and high-volume hospitals. The growth rate per year of IV magnesium use was greater in high- and moderate-volume hospitals (3.4% and 2.9% per year, respectively), compared with the low-volume hospitals (1.2% per year; P = .04).
Trends in Intravenous Magnesium Use and Hospital Outcomes
The trend in IV magnesium use was not associated with a statistically significant change in the inpatient and ICU admission rate or in the 7-day all-cause readmission rate (Figure and Appendix Figure). Although IV magnesium use increased over time, LOS decreased significantly during the same period (1.6 days in 2010 vs 1.4 days in 2017; P < .001). When analyzed by hospital volume, no significant associations were found in the inpatient admission, ICU admission, and 7-day readmission rate.

DISCUSSION
The use of IV magnesium has significantly increased in US children’s hospitals over the last 8 years, especially among those hospitalized following an ED evaluation. Over this interval, trends in inpatient and ICU admission rate, as well as 7-day all-cause readmission rate, for asthma did not change, while LOS decreased. These findings contrast with a recent Cochrane review that summarized the efficacy of IV magnesium for reducing admission rate in few small trials.2
Our study findings are more consistent with prior survey findings that IV magnesium does not reduce hospitalization and that ED physicians tend to use IV magnesium in severe asthma exacerbation for its potential therapeutic benefits because of bronchodilator and anti-inflammatory effect.6,7 Similar to PEM physicians’ estimates, only 10% of patients receiving IV magnesium were discharged home in our study.
IV magnesium use is higher in high-volume hospitals than in moderate- and low-volume ones. One potential explanation is that high- and moderate-volume hospitals may see a higher volume of children with severe or impending respiratory failure and, therefore, are more likely to use IV magnesium than the low-volume hospitals are. Alternatively, physician adoption of magnesium use for lower-acuity asthma exacerbations could vary by hospital volume.
Trend analyses of outcomes suggest that increase in IV magnesium use was not associated with an increase in inpatient and ICU admission rate or with 7-day all-cause readmission rate, although LOS reduced. LOS might be reduced because of various quality improvement initiatives (eg, discharging patients after every 3 hours albuterol treatments or respiratory therapist–driven protocols) and might not be related to IV magnesium use.8,9 To this point, a recent study of a respiratory assessment score–matched cohort did not find any therapeutic benefit of IV magnesium with severe asthma exacerbation when receiving continuous albuterol therapy on a pediatric ward.5 Perhaps future studies could explore estimating the outcome by performing comparative effectiveness studies between those with severe asthma exacerbation who did or did not receive IV magnesium. Additionally, randomized controlled trials comparing IV magnesium and standard therapy and its effects on outcomes, such as hospitalization, LOS, association with asthma chronicity, and previous oral steroid use, might provide further insight to inform clinical practice.
Certain study limitations should be noted. The study cohort included children’s hospitals only, and it is possible that care at nonchildren’s hospitals for asthma differs. PHIS dataset used in this study does not allow determination of where and when IV magnesium was given, the severity of asthma exacerbation, or the chronicity of baseline disease. Moreover, PHIS hospitals include centers in large cities, and other competing children’s hospitals may provide other tertiary care that could affect the readmission data calculation. Lastly, the temporal associations between IV magnesium use and outcomes reported in this study should not be used as evidence or lack of evidence for the effectiveness of magnesium given the limitations of the observational study design and dataset used.
In conclusion, IV magnesium use in management of asthma exacerbation in children across the United States has significantly increased. The increase occurred disproportionately in high-volume hospitals and was not associated with changes in admission rate, ICU admission rate, or 7-day all-cause readmission rate, although LOS has decreased over time.
Disclosures
The authors have no financial relationships relevant to this article or conflicts of interest to disclose.
This paper was a platform presentation at annual meetings of Pediatric Academic Societies 2019; accepted for presentation at annual meeting of Pediatric Hospital Medicine, July 2019.
Funding Source
No funding was secured for this study.
1. National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Maryland: National Heart, Lung, and Blood Institute; 2007. https://www.ncbi.nlm.nih.gov/books/NBK7232/.
2. Griffiths B, Kew KM. Intravenous magnesium sulfate for treating children with acute asthma in the emergency department. Cochrane Database Syst Rev. 2016;4(4):CD011050. https://doi.org/10.1002/14651858.CD011050.pub2.
3. Shan Z, Rong Y, Yang W, et al. Intravenous and nebulized magnesium sulfate for treating acute asthma in adults and children: a systematic review and meta-analysis. Respir Med. 2013;107(3):321-330. https://doi.org/10.1016/j.med.2012.12.001.
4. Rower J, Liu X, Yu T, Mundorff M, Sherwin C, Johnson M. Clinical pharmacokinetics of magnesium sulfate in treatment of children with severe acute asthma. Eur J Clin Pharmacol. 2017;73(3):325-331. https://doi.org/10.1007/s00228-016-2165-3.
5. Desanti R, Agasthya N, Hunter K, Hussain M. The effectiveness of magnesium sulfate for status asthmaticus outside intensive care unit. Pediatric Pulmonol. 2018;53(7):866-871. https://doi.org/10.1002/ppul.24013.Epub 2018.
6. Schuh S, Macias C, Freedman S, et al. North American practice patterns of intravenous magnesium sulfate in severe acute asthma exacerbations. Acad Emerg Med. 2010;17(11):1189-1196. https://doi.org/10.1111/j.1553-2712.2010.00913.x.
7. Cheuk DK, Chau TC, Lee SL. A meta-analysis on intravenous magnesium sulphate for treating acute asthma. Arch Dis Child. 2005;90(1):74-77. https://doi.org/10.1136/adc.2004.050005.
8. Lo HY, Messer A, Loveless J, et al. Discharging asthma patients on 3-hour β-agonist treatments: a quality improvement project. Hosp Pediatr. 2018;8(12):733-739. https://doi.org/10.1542/hpeds.2018-0072.
9. Magruder TG, Narayanan S, Walley S, et al. Improving inpatient asthma management: the implementation and evaluation of pediatric asthma clinical pathway. Pediatr Qual Saf. 2017;2(5);e041. https://doi.org/10.1097/pq9.0000000000000041.
1. National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Maryland: National Heart, Lung, and Blood Institute; 2007. https://www.ncbi.nlm.nih.gov/books/NBK7232/.
2. Griffiths B, Kew KM. Intravenous magnesium sulfate for treating children with acute asthma in the emergency department. Cochrane Database Syst Rev. 2016;4(4):CD011050. https://doi.org/10.1002/14651858.CD011050.pub2.
3. Shan Z, Rong Y, Yang W, et al. Intravenous and nebulized magnesium sulfate for treating acute asthma in adults and children: a systematic review and meta-analysis. Respir Med. 2013;107(3):321-330. https://doi.org/10.1016/j.med.2012.12.001.
4. Rower J, Liu X, Yu T, Mundorff M, Sherwin C, Johnson M. Clinical pharmacokinetics of magnesium sulfate in treatment of children with severe acute asthma. Eur J Clin Pharmacol. 2017;73(3):325-331. https://doi.org/10.1007/s00228-016-2165-3.
5. Desanti R, Agasthya N, Hunter K, Hussain M. The effectiveness of magnesium sulfate for status asthmaticus outside intensive care unit. Pediatric Pulmonol. 2018;53(7):866-871. https://doi.org/10.1002/ppul.24013.Epub 2018.
6. Schuh S, Macias C, Freedman S, et al. North American practice patterns of intravenous magnesium sulfate in severe acute asthma exacerbations. Acad Emerg Med. 2010;17(11):1189-1196. https://doi.org/10.1111/j.1553-2712.2010.00913.x.
7. Cheuk DK, Chau TC, Lee SL. A meta-analysis on intravenous magnesium sulphate for treating acute asthma. Arch Dis Child. 2005;90(1):74-77. https://doi.org/10.1136/adc.2004.050005.
8. Lo HY, Messer A, Loveless J, et al. Discharging asthma patients on 3-hour β-agonist treatments: a quality improvement project. Hosp Pediatr. 2018;8(12):733-739. https://doi.org/10.1542/hpeds.2018-0072.
9. Magruder TG, Narayanan S, Walley S, et al. Improving inpatient asthma management: the implementation and evaluation of pediatric asthma clinical pathway. Pediatr Qual Saf. 2017;2(5);e041. https://doi.org/10.1097/pq9.0000000000000041.
©2020 Society of Hospital Medicine
1.03 Common Clinical Diagnoses and Conditions: Acute Respiratory Failure
Introduction
Respiratory failure is defined by inadequate gas exchange by the respiratory system that results in ineffective alveolar ventilation and/or oxygenation. Acute respiratory failure is more common in children than adults and is the primary cause of cardiopulmonary arrest in children. The differential diagnosis for acute respiratory failure in children is extensive, as failure may stem from any portion of the respiratory system or be a consequence of systemic disease. Pediatric hospitalists frequently encounter children with conditions affecting the respiratory system and should be able to anticipate, identify, and treat acute respiratory distress and acute respiratory failure in children, including those with chronic respiratory conditions and other comorbidities.
Knowledge
Pediatric hospitalists should be able to:
- Describe the structure and function respiratory system components, including upper and lower airways, muscles of respiration, and central and peripheral regulation systems.
- Explain developmental differences that contribute to acute respiratory failure in infants and young children, including upper airway size, lower airway growth and development, diaphragmatic muscle reserve, chest wall compliance, and respiratory regulatory center maturity.
- Discuss the basic principles of respiratory physiology, including the alveolar gas equation, minute ventilation, and alveolar-arterial gradient.
- Summarize the five causes of hypoxemia: ventilation-perfusion mismatch, hypoventilation, right to left shunt, diffusion impairment, and low inspired oxygen.
- Construct an age-based differential diagnosis for acute respiratory distress in children.
- List causes of poor respiratory muscle function, attending to age, neuromuscular disorders, central nervous system dysfunction, nerve injury, and others.
- Discuss comorbidities that place children at higher risk for acute respiratory failure.
- Summarize evaluation, monitoring, and treatment options for patients with worsening respiratory status, including mental status assessment, blood gas analysis, medications, and respiratory support.
- Describe the signs and symptoms of impending acute respiratory failure, including criteria for transfer to a higher level of care.
- Discuss the advantages and disadvantages of different supplemental oxygen delivery devices for children with and without medical complexity, such as low flow and heated high-flow nasal cannula, simple mask, partial rebreather or non-rebreather, and tracheostomy collar or mask.
- Summarize the modalities commonly available to support the airway and adequate gas exchange in children with worsening respiratory distress, including nasopharyngeal or oropharyngeal airways, bag-valve-mask ventilation, bi-level positive airway pressure, continuous positive airway pressure, endotracheal tube, and laryngeal-mask-airway intubation.
- Describe criteria for, risks of, and complications due to endotracheal or laryngeal-mask-airway intubation, including strategies to reduce these risks.
- Compare and contrast optimal treatment strategies for acute respiratory failure in children with common acute respiratory conditions, including asthma, bronchiolitis, croup, and pneumonia.
Skills
Pediatric hospitalists should be able to:
- Perform and teach other health care providers to perform a thorough respiratory assessment of a child with acute respiratory distress.
- Identify early warning signs of acute respiratory distress and institute corrective actions and therapies to avert further deterioration.
- Identify patients with comorbidities and other risk factors for progression to acute respiratory failure.
- Order appropriate monitoring and relevant testing (such as radiographs and blood gases) and correctly interpret their results.
- Diagnose and initiate medical management for systemic causes of acute respiratory failure.
- Identify signs and symptoms of impending acute respiratory failure and activate local emergency response teams and/or transfer patients to an appropriate site with critical care services in a safe and efficient manner.
- Initiate oxygen supplementation via oxygen delivery devices and escalate as required to manage hypoxia and/or acute respiratory distress.
- Stabilize the airway, using non-invasive airway management techniques independently and invasive airway management in collaboration with other services.
- Demonstrate proficiency in basic management of patients with chronic respiratory support needs.
- Identify patients requiring subspecialty care and obtain timely consults.
Attitudes
Pediatric hospitalists should be able to:
- Acknowledge the importance of collaboration with patients, the family/caregivers, hospital staff, and subspecialists to ensure family-centered, coordinated hospital care for children with conditions at risk for acute respiratory failure.
- Realize the value of providing consultation for healthcare providers in community settings to ensure transport of patients to higher acuity settings as needed.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in educational programs for the family/caregivers, hospital staff, and other healthcare providers regarding recognition of signs and symptoms of acute respiratory distress in children, particularly those at higher risk for acute respiratory failure.
- Work with hospital administration, hospital staff, subspecialists, and others to develop, implement, and assess outcomes of intervention strategies such as rapid response teams and early warning scores for hospitalized patients with deterioration in respiratory status in order to prevent adverse outcomes.
- Work with hospital administration, hospital staff, pharmacy, and others to ensure availability of medications and appropriately sized equipment for use in the management of acute respiratory failure in children.
1. Samson RA, Schexnayder SM, Hazinski MF, et al. Part 3 Systematic approach to the seriously ill or injured child, Part 6 Recognition of Respiratory Distress and Failure, and Part 7 Management of Respiratory Distress and Failure. In: Pediatric Advanced Life Support: Provider Manual. Dallas, TX: American Heart Association; 2016;29-68; 113-170.
2. Hammer J. Acute respiratory failure in children. Paediatr Respir Rev. 2013; 14:64-69. https://doi.org/10.1016/j.prrv.2013.02.001.
Introduction
Respiratory failure is defined by inadequate gas exchange by the respiratory system that results in ineffective alveolar ventilation and/or oxygenation. Acute respiratory failure is more common in children than adults and is the primary cause of cardiopulmonary arrest in children. The differential diagnosis for acute respiratory failure in children is extensive, as failure may stem from any portion of the respiratory system or be a consequence of systemic disease. Pediatric hospitalists frequently encounter children with conditions affecting the respiratory system and should be able to anticipate, identify, and treat acute respiratory distress and acute respiratory failure in children, including those with chronic respiratory conditions and other comorbidities.
Knowledge
Pediatric hospitalists should be able to:
- Describe the structure and function respiratory system components, including upper and lower airways, muscles of respiration, and central and peripheral regulation systems.
- Explain developmental differences that contribute to acute respiratory failure in infants and young children, including upper airway size, lower airway growth and development, diaphragmatic muscle reserve, chest wall compliance, and respiratory regulatory center maturity.
- Discuss the basic principles of respiratory physiology, including the alveolar gas equation, minute ventilation, and alveolar-arterial gradient.
- Summarize the five causes of hypoxemia: ventilation-perfusion mismatch, hypoventilation, right to left shunt, diffusion impairment, and low inspired oxygen.
- Construct an age-based differential diagnosis for acute respiratory distress in children.
- List causes of poor respiratory muscle function, attending to age, neuromuscular disorders, central nervous system dysfunction, nerve injury, and others.
- Discuss comorbidities that place children at higher risk for acute respiratory failure.
- Summarize evaluation, monitoring, and treatment options for patients with worsening respiratory status, including mental status assessment, blood gas analysis, medications, and respiratory support.
- Describe the signs and symptoms of impending acute respiratory failure, including criteria for transfer to a higher level of care.
- Discuss the advantages and disadvantages of different supplemental oxygen delivery devices for children with and without medical complexity, such as low flow and heated high-flow nasal cannula, simple mask, partial rebreather or non-rebreather, and tracheostomy collar or mask.
- Summarize the modalities commonly available to support the airway and adequate gas exchange in children with worsening respiratory distress, including nasopharyngeal or oropharyngeal airways, bag-valve-mask ventilation, bi-level positive airway pressure, continuous positive airway pressure, endotracheal tube, and laryngeal-mask-airway intubation.
- Describe criteria for, risks of, and complications due to endotracheal or laryngeal-mask-airway intubation, including strategies to reduce these risks.
- Compare and contrast optimal treatment strategies for acute respiratory failure in children with common acute respiratory conditions, including asthma, bronchiolitis, croup, and pneumonia.
Skills
Pediatric hospitalists should be able to:
- Perform and teach other health care providers to perform a thorough respiratory assessment of a child with acute respiratory distress.
- Identify early warning signs of acute respiratory distress and institute corrective actions and therapies to avert further deterioration.
- Identify patients with comorbidities and other risk factors for progression to acute respiratory failure.
- Order appropriate monitoring and relevant testing (such as radiographs and blood gases) and correctly interpret their results.
- Diagnose and initiate medical management for systemic causes of acute respiratory failure.
- Identify signs and symptoms of impending acute respiratory failure and activate local emergency response teams and/or transfer patients to an appropriate site with critical care services in a safe and efficient manner.
- Initiate oxygen supplementation via oxygen delivery devices and escalate as required to manage hypoxia and/or acute respiratory distress.
- Stabilize the airway, using non-invasive airway management techniques independently and invasive airway management in collaboration with other services.
- Demonstrate proficiency in basic management of patients with chronic respiratory support needs.
- Identify patients requiring subspecialty care and obtain timely consults.
Attitudes
Pediatric hospitalists should be able to:
- Acknowledge the importance of collaboration with patients, the family/caregivers, hospital staff, and subspecialists to ensure family-centered, coordinated hospital care for children with conditions at risk for acute respiratory failure.
- Realize the value of providing consultation for healthcare providers in community settings to ensure transport of patients to higher acuity settings as needed.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in educational programs for the family/caregivers, hospital staff, and other healthcare providers regarding recognition of signs and symptoms of acute respiratory distress in children, particularly those at higher risk for acute respiratory failure.
- Work with hospital administration, hospital staff, subspecialists, and others to develop, implement, and assess outcomes of intervention strategies such as rapid response teams and early warning scores for hospitalized patients with deterioration in respiratory status in order to prevent adverse outcomes.
- Work with hospital administration, hospital staff, pharmacy, and others to ensure availability of medications and appropriately sized equipment for use in the management of acute respiratory failure in children.
Introduction
Respiratory failure is defined by inadequate gas exchange by the respiratory system that results in ineffective alveolar ventilation and/or oxygenation. Acute respiratory failure is more common in children than adults and is the primary cause of cardiopulmonary arrest in children. The differential diagnosis for acute respiratory failure in children is extensive, as failure may stem from any portion of the respiratory system or be a consequence of systemic disease. Pediatric hospitalists frequently encounter children with conditions affecting the respiratory system and should be able to anticipate, identify, and treat acute respiratory distress and acute respiratory failure in children, including those with chronic respiratory conditions and other comorbidities.
Knowledge
Pediatric hospitalists should be able to:
- Describe the structure and function respiratory system components, including upper and lower airways, muscles of respiration, and central and peripheral regulation systems.
- Explain developmental differences that contribute to acute respiratory failure in infants and young children, including upper airway size, lower airway growth and development, diaphragmatic muscle reserve, chest wall compliance, and respiratory regulatory center maturity.
- Discuss the basic principles of respiratory physiology, including the alveolar gas equation, minute ventilation, and alveolar-arterial gradient.
- Summarize the five causes of hypoxemia: ventilation-perfusion mismatch, hypoventilation, right to left shunt, diffusion impairment, and low inspired oxygen.
- Construct an age-based differential diagnosis for acute respiratory distress in children.
- List causes of poor respiratory muscle function, attending to age, neuromuscular disorders, central nervous system dysfunction, nerve injury, and others.
- Discuss comorbidities that place children at higher risk for acute respiratory failure.
- Summarize evaluation, monitoring, and treatment options for patients with worsening respiratory status, including mental status assessment, blood gas analysis, medications, and respiratory support.
- Describe the signs and symptoms of impending acute respiratory failure, including criteria for transfer to a higher level of care.
- Discuss the advantages and disadvantages of different supplemental oxygen delivery devices for children with and without medical complexity, such as low flow and heated high-flow nasal cannula, simple mask, partial rebreather or non-rebreather, and tracheostomy collar or mask.
- Summarize the modalities commonly available to support the airway and adequate gas exchange in children with worsening respiratory distress, including nasopharyngeal or oropharyngeal airways, bag-valve-mask ventilation, bi-level positive airway pressure, continuous positive airway pressure, endotracheal tube, and laryngeal-mask-airway intubation.
- Describe criteria for, risks of, and complications due to endotracheal or laryngeal-mask-airway intubation, including strategies to reduce these risks.
- Compare and contrast optimal treatment strategies for acute respiratory failure in children with common acute respiratory conditions, including asthma, bronchiolitis, croup, and pneumonia.
Skills
Pediatric hospitalists should be able to:
- Perform and teach other health care providers to perform a thorough respiratory assessment of a child with acute respiratory distress.
- Identify early warning signs of acute respiratory distress and institute corrective actions and therapies to avert further deterioration.
- Identify patients with comorbidities and other risk factors for progression to acute respiratory failure.
- Order appropriate monitoring and relevant testing (such as radiographs and blood gases) and correctly interpret their results.
- Diagnose and initiate medical management for systemic causes of acute respiratory failure.
- Identify signs and symptoms of impending acute respiratory failure and activate local emergency response teams and/or transfer patients to an appropriate site with critical care services in a safe and efficient manner.
- Initiate oxygen supplementation via oxygen delivery devices and escalate as required to manage hypoxia and/or acute respiratory distress.
- Stabilize the airway, using non-invasive airway management techniques independently and invasive airway management in collaboration with other services.
- Demonstrate proficiency in basic management of patients with chronic respiratory support needs.
- Identify patients requiring subspecialty care and obtain timely consults.
Attitudes
Pediatric hospitalists should be able to:
- Acknowledge the importance of collaboration with patients, the family/caregivers, hospital staff, and subspecialists to ensure family-centered, coordinated hospital care for children with conditions at risk for acute respiratory failure.
- Realize the value of providing consultation for healthcare providers in community settings to ensure transport of patients to higher acuity settings as needed.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in educational programs for the family/caregivers, hospital staff, and other healthcare providers regarding recognition of signs and symptoms of acute respiratory distress in children, particularly those at higher risk for acute respiratory failure.
- Work with hospital administration, hospital staff, subspecialists, and others to develop, implement, and assess outcomes of intervention strategies such as rapid response teams and early warning scores for hospitalized patients with deterioration in respiratory status in order to prevent adverse outcomes.
- Work with hospital administration, hospital staff, pharmacy, and others to ensure availability of medications and appropriately sized equipment for use in the management of acute respiratory failure in children.
1. Samson RA, Schexnayder SM, Hazinski MF, et al. Part 3 Systematic approach to the seriously ill or injured child, Part 6 Recognition of Respiratory Distress and Failure, and Part 7 Management of Respiratory Distress and Failure. In: Pediatric Advanced Life Support: Provider Manual. Dallas, TX: American Heart Association; 2016;29-68; 113-170.
2. Hammer J. Acute respiratory failure in children. Paediatr Respir Rev. 2013; 14:64-69. https://doi.org/10.1016/j.prrv.2013.02.001.
1. Samson RA, Schexnayder SM, Hazinski MF, et al. Part 3 Systematic approach to the seriously ill or injured child, Part 6 Recognition of Respiratory Distress and Failure, and Part 7 Management of Respiratory Distress and Failure. In: Pediatric Advanced Life Support: Provider Manual. Dallas, TX: American Heart Association; 2016;29-68; 113-170.
2. Hammer J. Acute respiratory failure in children. Paediatr Respir Rev. 2013; 14:64-69. https://doi.org/10.1016/j.prrv.2013.02.001.
LOS in Children With Medical Complexity
Children with medical complexity (CMC) have complex and chronic health conditions that often involve multiple organ systems and severely affect cognitive and physical functioning. Although the prevalence of CMC is low (1% of all children), they account for nearly one‐fifth of all pediatric admissions and one‐half of all hospital days and charges in the United States.[1] Over the last decade, CMC have had a particularly large and increasing impact in tertiary‐care children's hospitals.[1, 2] The Institute of Medicine has identified CMC as a priority population for a revised healthcare system.[3]
Medical homes, hospitals, health plans, states, federal agencies, and others are striving to reduce excessive hospital use in CMC because of its high cost.[4, 5, 6] Containing length of stay (LOS)an increasingly used indicator of the time sensitiveness and efficiency of hospital careis a common aim across these initiatives. CMC have longer hospitalizations than children without medical complexity. Speculated reasons for this are that CMC tend to have (1) higher severity of acute illnesses (eg, pneumonia, cellulitis), (2) prolonged recovery time in the hospital, and (3) higher risk of adverse events in the hospital. Moreover, hospital clinicians caring for CMC often find it difficult to determine discharge readiness, given that many CMC do not return to a completely healthy baseline.[7]
Little is known about long LOS in CMC, including which CMC have the highest risk of experiencing such stays and which stays might have the greatest opportunity to be shortened. Patient characteristics associated with prolonged length of stay have been studied extensively for many pediatric conditions (eg, asthma).[8, 9, 10, 11, 12, 13, 14] However, most of these studies excluded CMC. Therefore, the objectives of this study were to examine (1) the prevalence of long LOS in CMC, (2) patient characteristics associated with long LOS, and (3) hospital‐to‐hospital variation in prevalence of long LOS hospitalizations.
METHODS
Study Design and Data Source
This study is a multicenter, retrospective cohort analysis of the Pediatric Health Information System (PHIS). PHIS is an administrative database of 44 not for profit, tertiary care pediatric hospitals affiliated with the Children's Hospital Association (CHA) (Overland Park, KS). PHIS contains data regarding patient demographics, diagnoses, and procedures (with International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM] codes), All‐Patient Refined Diagnostic Related Groups version 30 (APR‐DRGs) (3M Health Information Systems, Salt Lake City, UT), and service lines that aggregate the APR‐DRGs into 38 distinct groups. Data quality and reliability are assured through CHA and participating hospitals. In accordance with the policies of the Cincinnati Children's Hospital Medical Center Institutional Review Board, this study of deidentified data was not considered human subjects research.
Study Population
Inclusion Criteria
Children discharged following an observation or inpatient admission from a hospital participating in the PHIS database between January 1, 2013 and December 31, 2014 were eligible for inclusion if they were considered medically complex. Medical complexity was defined using Clinical Risk Groups (CRGs) version 1.8, developed by 3M Health Information Systems and the National Association of Children's Hospitals and Related Institutions. CRGs were used to assign each hospitalized patient to 1 of 9 mutually exclusive chronicity groups according to the presence, type, and severity of chronic conditions.[15, 16, 17, 18] Each patient's CRG designation was based on 2 years of previous hospital encounters.
As defined in prior studies and definitional frameworks of CMC,[1] patients belonging to CRG group 6 (significant chronic disease in 2 organ systems), CRG group 7 (dominant chronic disease in 3 organ systems), and CRG group 9 (catastrophic condition) were considered medically complex.[17, 19] Patients with malignancies (CRG group 8) were not included for analysis because they are a unique population with anticipated, long hospital stays. Patients with CRG group 5, representing those with chronic conditions affecting a single body system, were also not included because most do not have attributes consistent with medical complexity.
Exclusion Criteria
We used the APR‐DRG system, which leverages ICD‐9‐CM codes to identify the health problem most responsible for the hospitalization, to refine the study cohort. We excluded hospitalizations that were classified by the APR‐DRG system as neonatal, as we did not wish to focus on LOS in the neonatal intensive care unit (ICU) or for birth admissions. Similarly, hospitalizations for chemotherapy (APR‐DRG 693) or malignancy (identified with previously used ICD‐9‐CM codes)[20] were also excluded because long LOS is anticipated. We also excluded hospitalizations for medical rehabilitation (APR‐DRG 860).
Outcome Measures
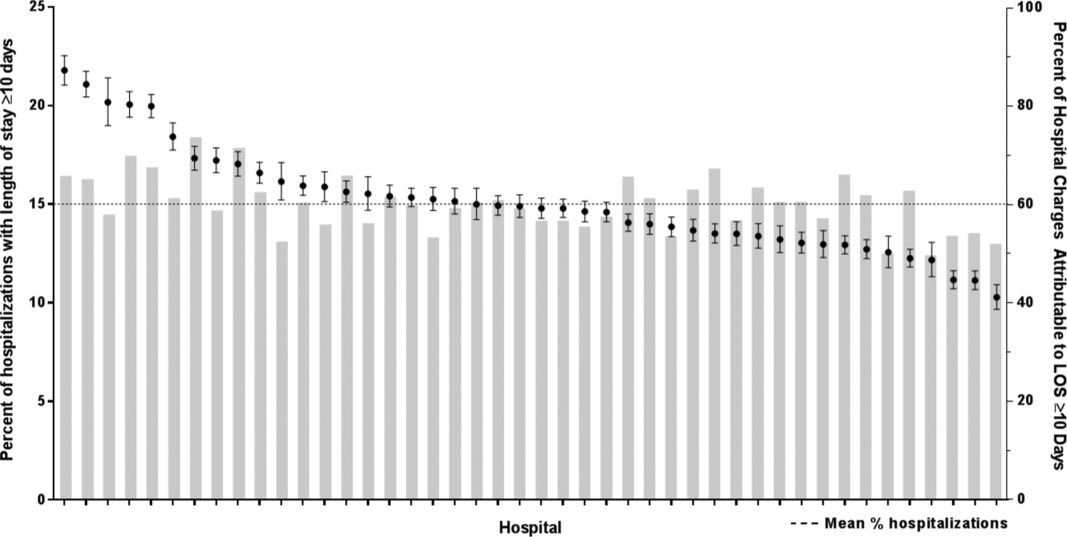
The primary outcome measure was long LOS, defined as LOS 10 days. The cut point of LOS 10 days represents the 90th percentile of LOS for all children, with and without medical complexity, hospitalized during 2013 to 2014. LOS 10 days has previously been used as a threshold of long LOS.[21] For hospitalizations involving transfer at admission from another acute care facility, LOS was measured from the date of transfer. We also assessed hospitals' cost attributable to long LOS admissions.
Patient Demographics and Clinical Characteristics
We measured demographic characteristics including age, gender, race/ethnicity, insurance type, and distance traveled (the linear distance between the centroid of the patient's home ZIP code and the centroid of the hospital's ZIP code). Clinical characteristics included CRG classification, complex chronic condition (CCC), and dependence on medical technology. CCCs are defined as any medical condition that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or 1 system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center.[20] Medical technology included devices used to optimize the health and functioning of the child (eg, gastrostomy, tracheostomy, cerebrospinal fluid shunt).[22]
Hospitalization Characteristics
Characteristics of the hospitalization included transfer from an outside facility, ICU admission, surgical procedure (using surgical APR‐DRGs), and discharge disposition (home, skilled nursing facility, home health services, death, other). Cost of the hospitalization was estimated in the PHIS from charges using hospital and year‐specific ratios of cost to charge.
Statistical Analysis
Continuous data (eg, distance from hospital to home residence) were described with median and interquartile ranges (IQR) because they were not normally distributed. Categorical data (eg, type of chronic condition) were described with counts and frequencies. In bivariate analyses, demographic, clinical, and hospitalization characteristics were stratified by LOS (long LOS vs LOS 10 days), and compared using 2 statistics or Wilcoxon rank sum tests as appropriate.
We modeled the likelihood of experiencing a long LOS using generalized linear mixed effects models with a random hospital intercept and discharge‐level fixed effects for age, gender, payor, CCC type, ICU utilization, transfer status, a medical/surgical admission indicator derived from the APR‐DRG, and CRG assigned to each hospitalization. To examine hospital‐to‐hospital variability, we generated hospital risk‐adjusted rates of long LOS from these models. Similar models and hospital risk‐adjusted rates were built for a post hoc correlational analysis of 30‐day all cause readmission, where hospitals' rates and percent of long LOS were compared with a Pearson correlation coefficient. Also, for our multivariable models, we performed a sensitivity analysis using an alternative definition of long LOS as 4 days (the 75th percentile of LOS for all children, with and without medical complexity, hospitalized during 20132014). All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), and P values 0.05 were considered statistically significant.
RESULTS
Study Population
There were 954,018 hospitalizations of 217,163 CMC at 44 children's hospitals included for analysis. Forty‐seven percent of hospitalizations were for females, 49.4% for non‐Hispanic whites, and 61.1% for children with government insurance. Fifteen percent (n = 142,082) had a long LOS of 10 days. The median (IQR) LOS of hospitalizations 10 days versus 10 days were 2 (IQR, 14) and 16 days (IQR, 1226), respectively. Long LOS hospitalizations accounted for 61.1% (3.7 million) hospital days and 61.8% ($13.7 billion) of total hospitalization costs for all CMC in the cohort (Table 1).
| Characteristic | Overall (n = 954,018) | Length of Stay | |
|---|---|---|---|
| 10 Days (n = 811,936) | 10 Days (n = 142,082) | ||
| |||
| Age at admission, y, % | |||
| 1 | 14.6 | 12.7 | 25.7 |
| 14 | 27.1 | 27.9 | 22.4 |
| 59 | 20.1 | 21.0 | 14.9 |
| 1018 | 33.6 | 34.0 | 31.7 |
| 18+ | 4.6 | 4.4 | 5.4 |
| Gender, % | |||
| Female | 47.0 | 46.9 | 47.5 |
| Race/ethnicity, % | |||
| Non‐Hispanic white | 49.4 | 49.4 | 49.4 |
| Non‐Hispanic black | 23.1 | 23.8 | 19.3 |
| Hispanic | 18.2 | 17.8 | 20.4 |
| Asian | 2.0 | 1.9 | 2.3 |
| Other | 7.4 | 7.1 | 8.6 |
| Complex chronic condition, % | |||
| Any | 79.5 | 77.3 | 91.8 |
| Technology assistance | 37.1 | 34.1 | 54.2 |
| Gastrointestinal | 30.0 | 27.2 | 45.9 |
| Neuromuscular | 28.2 | 27.7 | 30.9 |
| Cardiovascular | 16.8 | 14.5 | 29.9 |
| Respiratory | 14.1 | 11.5 | 29.4 |
| Congenital/genetic defect | 17.2 | 16.7 | 20.2 |
| Metabolic | 9.9 | 8.9 | 15.4 |
| Renal | 10.1 | 9.5 | 13.8 |
| Hematology/emmmunodeficiency | 11.7 | 12.0 | 10.0 |
| Neonatal | 3.8 | 3.1 | 7.7 |
| Transplantation | 4.5 | 4.2 | 6.7 |
| Clinical risk group, % | |||
| Chronic condition in 2 systems | 68.4 | 71.2 | 53.9 |
| Catastrophic chronic condition | 31.4 | 28.8 | 46.1 |
| Distance from hospital to home residence in miles, median [IQR] | 16.2 [7.440.4] | 15.8 [7.338.7] | 19.1 [8.552.6] |
| Transferred from outside hospital (%) | 6.5 | 5.3 | 13.6 |
| Admitted for surgery, % | 23.4 | 20.7 | 38.7 |
| Use of intensive care, % | 19.6 | 14.9 | 46.5 |
| Discharge disposition, % | |||
| Home | 91.2 | 92.9 | 81.4 |
| Home healthcare | 4.5 | 3.5 | 9.9 |
| Other | 2.9 | 2.6 | 4.5 |
| Postacute care facility | 1.1 | 0.8 | 3.1 |
| Died | 0.4 | 0.3 | 1.1 |
| Payor, % | |||
| Government | 61.1 | 60.6 | 63.5 |
| Private | 33.2 | 33.6 | 30.9 |
| Other | 5.7 | 5.7 | 5.7 |
| Hospital resource use | |||
| Median length of stay [IQR] | 3 [16] | 2 [14] | 16 [1226] |
| Median hospital cost [IQR] | $8,144 [$4,122$18,447] | $6,689 [$3,685$12,395] | $49,207 [$29,444$95,738] |
| Total hospital cost, $, billions | $22.2 | $8.5 | $13.7 |
Demographics and Clinical Characteristics of Children With and Without Long LOS
Compared with hospitalized CMC with LOS 10 days, a higher percentage of hospitalizations with LOS 10 days were CMC age 1 year (25.7% vs 12.7%, P 0.001) and Hispanic (20.4% vs 17.8%, P 0.001). CMC hospitalizations with a long LOS had a higher percentage of any CCC (91.8% vs 77.3%, P 0.001); the most common CCCs were gastrointestinal (45.9%), neuromuscular (30.9%), and cardiovascular (29.9%). Hospitalizations of CMC with a long LOS had a higher percentage of a catastrophic chronic condition (46.1% vs 28.8%, P 0.001) and technology dependence (46.1% vs 28.8%, P 0.001) (Table 1).
Hospitalization Characteristics of Children With and Without Long LOS
Compared with hospitalizations of CMC with LOS 10 days, hospitalizations of CMC with a long LOS more often involved transfer in from another hospital at admission (13.6% vs 5.3%, P 0.001). CMC hospital stays with a long LOS more often involved surgery (38.7% vs 20.7%, P 0.001) and use of intensive care (46.5% vs 14.9%; P 0.001). A higher percentage of CMC with long LOS were discharged with home health services (9.9% vs 3.5%; P 0.001) (Table 1).
The most common admitting diagnoses and CCCs for hospitalizations of CMC with long LOS are presented in Table 2. The two most prevalent APR‐DRGs in CMC hospitalizations lasting 10 days or longer were cystic fibrosis (10.7%) and respiratory system disease with ventilator support (5.5%). The two most common chronic condition characteristics represented among long CMC hospitalizations were gastrointestinal devices (eg, gastrostomy tube) (39.7%) and heart and great vessel malformations (eg, tetralogy of Fallot) (12.8%). The 5 most common CCC subcategories, as listed in Table 2, account for nearly 100% of the patients with long LOS hospitalizations.
| |
| Most common reason for admission* | |
| Cystic fibrosis | 10.7% |
| Respiratory system diagnosis with ventilator support 96+ hours | 5.5% |
| Malfunction, reaction, and complication of cardiac or vascular device or procedure | 2.8% |
| Craniotomy except for trauma | 2.6% |
| Major small and large bowel procedures | 2.3% |
| Most common complex chronic condition | |
| Gastrointestinal devices | 39.7% |
| Heart and great vessel malformations | 12.8% |
| Cystic fibrosis | 12.5% |
| Dysrhythmias | 11.0% |
| Respiratory devices | 10.7% |
Multivariable Analysis of Characteristics Associated With Long LOS
In multivariable analysis, the highest likelihood of long LOS was experienced by children who received care in the ICU (odds ratio [OR]: 3.5, 95% confidence interval [CI]: 3.43.5), who had a respiratory CCC (OR: 2.7, 95% CI: 2.62.7), and who were transferred from another acute care hospital at admission (OR: 2.1, 95% CI: 2.0, 2.1). The likelihood of long LOS was also higher in children 1 year of age (OR: 1.2, 95% CI: 1.21.3), and Hispanic children (OR: 1.1, 95% CI 1.0‐1.10) (Table 3). Similar multivariable findings were observed in sensitivity analysis using the 75th percentile of LOS (4 days) as the model outcome.
| Characteristic | Odds Ratio (95% CI) of LOS 10 Days | P Value |
|---|---|---|
| ||
| Use of intensive care | 3.5 (3.4‐3.5) | 0.001 |
| Transfer from another acute‐care hospital | 2.1 (2.0‐2.1) | 0.001 |
| Procedure/surgery | 1.8 (1.8‐1.9) | 0.001 |
| Complex chronic condition | ||
| Respiratory | 2.7 (2.6‐2.7) | 0.001 |
| Gastrointestinal | 1.8 (1.8‐1.8) | 0.001 |
| Metabolic | 1.7 (1.7‐1.7) | 0.001 |
| Cardiovascular | 1.6 (1.5‐1.6) | 0.001 |
| Neonatal | 1.5 (1.5‐1.5) | 0.001 |
| Renal | 1.4 (1.4‐1.4) | 0.001 |
| Transplant | 1.4 (1.4‐1.4) | 0.001 |
| Hematology and immunodeficiency | 1.3 (1.3‐1.3) | 0.001 |
| Technology assistance | 1.1 (1.1, 1.1) | 0.001 |
| Neuromuscular | 0.9 (0.9‐0.9) | 0.001 |
| Congenital or genetic defect | 0.8 (0.8‐0.8) | 0.001 |
| Age at admission, y | ||
| 1 | 1.2 (1.2‐1.3) | 0.001 |
| 14 | 0.5 (0.5‐0.5) | 0.001 |
| 59 | 0.6 (0.6‐0.6) | 0.001 |
| 1018 | 0.9 (0.9‐0.9) | 0.001 |
| 18+ | Reference | |
| Male | 0.9 (0.9‐0.9) | 0.001 |
| Race/ethnicity | ||
| Non‐Hispanic black | 0.9 (0.9‐0.9) | 0.001 |
| Hispanic | 1.1 (1.0‐1.1) | 0.001 |
| Asian | 1.0 (1.0‐1.1) | 0.3 |
| Other | 1.1 (1.1‐1.1) | 0.001 |
| Non‐Hispanic white | Reference | |
| Payor | ||
| Private | 0.9 (0.8 0.9) | 0.001 |
| Other | 1.0 (1.0‐1.0) | 0.4 |
| Government | Reference | |
| Season | ||
| Spring | 1.0 (1.0 1.0) | 0.001 |
| Summer | 0.9 (0.9‐0.9) | 0.001 |
| Fall | 1.0 (0.9‐1.0) | 0.001 |
| Winter | Reference | |
Variation in the Prevalence of Long LOS Across Children's Hospitals
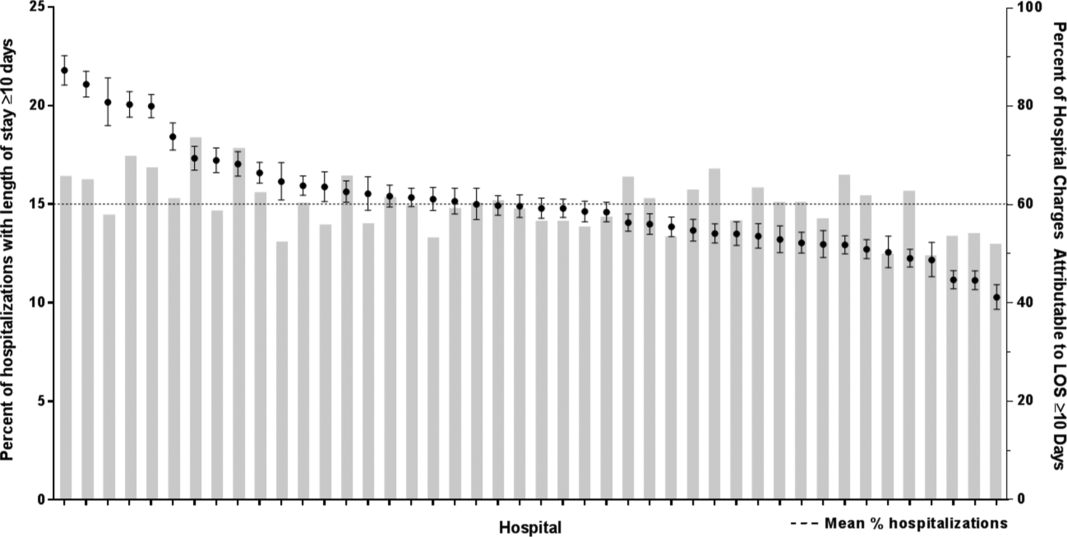
After controlling for demographic, clinical, and hospital characteristics associated with long LOS, there was significant (P 0.001) variation in the prevalence of long LOS for CMC across children's hospitals in the cohort (range, 10.3%21.8%) (Figure 1). Twelve (27%) hospitals had a significantly (P 0.001) higher prevalence of long LOS for their hospitalized CMC, compared to the mean. Eighteen (41%) had a significantly (P 0.001) lower prevalence of long LOS for their hospitalized CMC. There was also significant variation across hospitals with respect to cost, with 49.7% to 73.7% of all hospital costs of CMC attributed to long LOS hospitalizations. Finally, there was indirect correlation with the prevalence of LOS across hospitals and the hospitals' 30‐day readmission rate ( = 0.3; P = 0.04). As the prevalence of long LOS increased, the readmission rate decreased.
DISCUSSION
The main findings from this study suggest that a small percentage of CMC experiencing long LOS account for the majority of hospital bed days and cost of all hospitalized CMC in children's hospitals. The likelihood of long LOS varies significantly by CMC's age, race/ethnicity, and payor as well as by type and number of chronic conditions. Among CMC with long LOS, the use of gastrointestinal devices such as gastrostomy tubes, as well as congenital heart disease, were highly prevalent. In multivariable analysis, the characteristics most strongly associated with LOS 10 days were use of the ICU, respiratory complex chronic condition, and transfer from another medical facility at admission. After adjusting for these factors, there was significant variation in the prevalence of LOS 10 days for CMC across children's hospitals.
Although it is well known that CMC as a whole have a major impact on resource use in children's hospitals, this study reveals that 15% of hospitalizations of CMC account for 62% of all hospital costs of CMC. That is, a small fraction of hospitalizations of CMC is largely responsible for the significant financial impact of hospital resource use. To date, most clinical efforts and policies striving to reduce hospital use in CMC have focused on avoiding readmissions or index hospital admissions entirely, rather than improving the efficiency of hospital care after admission occurs.[23, 24, 25, 26] In the adult population, the impact of long LOS on hospital costs has been recognized, and several Medicare incentive programs have focused on in‐hospital timeliness and efficiency. As a result, LOS in Medicare beneficiaries has decreased dramatically over the past 2 decades.[27, 28, 29, 30] Optimizing the efficiency of hospital care for CMC may be an important goal to pursue, especially with precedent set in the adult literature.
Perhaps the substantial variation across hospitals in the prevalence of long LOS in CMC indicates opportunity to improve the efficiency of their inpatient care. This variation was not due to differences across hospitals' case mix of CMC. Further investigation is needed to determine how much of it is due to differences in quality of care. Clinical practice guidelines for hospital treatment of common illnesses usually exclude CMC. In our clinical experience across 9 children's hospitals, we have experienced varying approaches to setting discharge goals (ie, parameters on how healthy the child needs to be to ensure a successful hospital discharge) for CMC.[31] When the goals are absent or not clearly articulated, they can contribute to a prolonged hospitalization. Some families of CMC report significant issues when working with pediatric hospital staff to assess their child's discharge readiness.[7, 32, 33] In addition, there is significant variation across states and regions in access to and quality of post‐discharge health services (eg, home nursing, postacute care, durable medical equipment).[34, 35] In some areas, many CMC are not actively involved with their primary care physician.[5] These issues might also influence the ability of some children's hospitals to efficiently discharge CMC to a safe and supportive post‐discharge environment. Further examination of hospital outliersthose with the lowest and highest percentage of CMC hospitalizations with long LOSmay reveal opportunities to identify and spread best practices.
The demographic and clinical factors associated with long LOS in the present study, including age, ICU use, and transfer from another hospital, might help hospitals target which CMC have the greatest risk for experiencing long LOS. We found that infants age 1 year had longer LOS when compared with older children. Similar to our findings, younger‐aged children hospitalized with bronchiolitis have longer LOS.[36] Certainly, infants with medical complexity, in general, are a high‐acuity population with the potential for rapid clinical deterioration during an acute illness. Prolonged hospitalization for treatment and stabilization may be expected for many of them. Additional investigation is warranted to examine ICU use in CMC, and whether ICU admission or duration can be safely prevented or abbreviated. Opportunities to assess the quality of transfers into children's hospitals of CMC admitted to outside hospitals may be necessary. A study of pediatric burn patients reported that patients initially stabilized at a facility that was not a burn center and subsequently transferred to a burn center had a longer LOS than patients solely treated at a designated burn center.[37] Furthermore, events during transport itself may adversely impact the stability of an already fragile patient. Interventions to optimize the quality of care provided by transport teams have resulted in decreased LOS at the receiving hospital.[38]
This study's findings should be considered in the context of several limitations. Absent a gold‐standard definition of long LOS, we used the distribution of LOS across patients to inform our methods; LOS at the 90th percentile was selected as long. Although our sensitivity analysis using LOS at the 75th percentile produced similar findings, other cut points in LOS might be associated with different results. The study is not positioned to determine how much of the reported LOS was excessive, unnecessary, or preventable. The study findings may not generalize to types of hospitals not contained in PHIS (eg, nonchildren's hospitals and community hospitals). We did not focus on the impact of a new diagnosis (eg, new chronic illness) or acute in‐hospital event (eg, nosocomial infection) on prolonged LOS; future studies should investigate these clinical events with LOS.
PHIS does not contain information regarding characteristics that could influence LOS, including the children's social and familial attributes, transportation availability, home equipment needs, and local availability of postacute care facilities. Moreover, PHIS does not contain information about the hospital discharge procedures, process, or personnel across hospitals, which could influence LOS. Future studies on prolonged LOS should consider assessing this information. Because of the large sample size of hospitalizations included, the statistical power for the analyses was strong, rendering it possible that some findings that were statistically significant might have modest clinical significance (eg, relationship of Hispanic ethnicity with prolonged LOS). We could not determine why a positive correlation was not observed between hospitals' long LOS prevalence and their percentage of cost associated with long LOS; future studies should investigate the reasons for this finding.
Despite these limitations, the findings of the present study highlight the significance of long LOS in hospitalized CMC. These long hospitalizations account for a significant proportion of all hospital costs for this important population of children. The prevalence of long LOS for CMC varies considerably across children's hospitals, even after accounting for the case mix. Efforts to curtail hospital resource use and costs for CMC may benefit from focus on long LOS.
- , , , et al. Inpatient growth and resource use in 28 children's hospitals: a longitudinal, multi‐institutional study. JAMA Pediatr. 2013;167(2):170–177.
- , , , et al. Children with complex chronic conditions in inpatient hospital settings in the united states. Pediatrics. 2010;126(4):647–655.
- , . Meeting the health care needs of persons with disabilities. Milbank Q. 2002;80(2):381–391.
- , , , et al. Effect of an enhanced medical home on serious illness and cost of care among high‐risk children with chronic illness: a randomized clinical trial. JAMA. 2014;312(24):2640–2648.
- , , , et al. Children with medical complexity and Medicaid: spending and cost savings. Health Aff Proj Hope. 2014;33(12):2199–2206.
- Children's Hospital Association. CARE Award. Available at: https://www.childrenshospitals.org/Programs‐and‐Services/Quality‐Improvement‐and‐Measurement/CARE‐Award. Accessed December 18, 2015.
- , , , et al. Hospital readmission and parent perceptions of their child's hospital discharge. Int J Qual Health Care. 2013;25(5):573–581.
- , , , et al. Weekend matters: Friday and Saturday admissions are associated with prolonged hospitalization of children. Clin Pediatr (Phila). 2013;52(9):875–878.
- , , , . Attributable cost and length of stay for central line‐associated bloodstream infections. Pediatrics. 2014;133(6):e1525–e1532.
- , , , et al. Effect of healthcare‐acquired infection on length of hospital stay and cost. Infect Control Hosp Epidemiol. 2007;28(3):280–292.
- , , , , . Hospital utilization and costs among children with influenza, 2003. Am J Prev Med. 2009;36(4):292–296.
- , , , . Charges and lengths of stay attributable to adverse patient‐care events using pediatric‐specific quality indicators: a multicenter study of freestanding children's hospitals. Pediatrics. 2008;121(6):e1653–e1659.
- , , , , . Variation in resource utilization for the management of uncomplicated community‐acquired pneumonia across community and children's hospitals. J Pediatr. 2014;165(3):585–591.
- , , , , . Variation and outcomes associated with direct hospital admission among children with pneumonia in the United States. JAMA Pediatr. 2014;168(9):829–836.
- , , , et al. Clinical Risk Groups (CRGs): a classification system for risk‐adjusted capitation‐based payment and health care management. Med Care. 2004;42(1):81–90.
- , , , et al. Identifying children with lifelong chronic conditions for care coordination by using hospital discharge data. Acad Pediatr. 2010;10(6):417–423.
- , , , , . Profile of medical charges for children by health status group and severity level in a Washington State Health Plan. Health Serv Res. 2004;39(1):73–89.
- , , , . Using medical billing data to evaluate chronically ill children over time. J Ambulatory Care Manage. 2006;29(4):283–290.
- , , , , , . Medical complexity and pediatric emergency department and inpatient utilization. Pediatrics. 2013;131(2):e559–e565.
- , , , , . Pediatric complex chronic conditions classification system version 2: updated for ICD‐10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199.
- . Analyzing intensive care unit length of stay data: problems and possible solutions. Crit Care Med. 1997;25(9):1594–1600.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- . Hospital readmissions and repeat emergency department visits among children with medical complexity: an integrative review. J Pediatr Nurs. 2013;28(4):316–339.
- , , , . Hospital readmission in children with complex chronic conditions discharged from subacute care. Hosp Pediatr. 2014;4(3):153–158.
- , , , et al. Preventing hospitalizations in children with medical complexity: a systematic review. Pediatrics. 2014;134(6):e1628–e1647.
- , , , . Hospital readmissions for newly discharged pediatric home mechanical ventilation patients. Pediatr Pulmonol. 2012;47(4):409–414.
- , , , et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2008. JAMA. 2011;305(15):1560–1567.
- , , , et al. Trends in length of stay and short‐term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303(21):2141–2147.
- U.S. Department of Health and Human Services. CMS Statistics 2013. Available at: https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/CMS‐Statistics‐Reference‐Booklet/Downloads/CMS_Stats_2013_final.pdf. Published August 2013. Accessed October 6, 2015.
- Centers for Medicare and Medicaid Services. Evaluation of the premier hospital quality incentive demonstration. Available at: https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Reports/downloads/Premier_ExecSum_2010.pdf. Published March 3, 2009. Accessed September 18, 2015.
- , , , et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955–962; quiz 965–966.
- , , , , . Parent and provider perspectives on pediatric readmissions: what can we learn about readiness for discharge? Hosp Pediatr. 2015;5(11):559–565.
- , . Preventing readmissions in children: how do we do that? Hosp Pediatr. 2015;5(11):602–604.
- , , . Pediatric post‐acute hospital care: striving for identity and value. Hosp Pediatr. 2015;5(10):548–551.
- , , , et al. Pediatric hospital discharges to home health and postacute facility care: a national study. JAMA Pediatr. 2016;170(4):326–333.
- , , , et al. Bronchiolitis: clinical characteristics associated with hospitalization and length of stay. Pediatr Emerg Care. 2012;28(2):99–103.
- , , , , . The effect of transfers between health care facilities on costs and length of stay for pediatric burn patients. J Burn Care Res. 2015;36(1):178–183.
- , , , et al. Goal‐directed resuscitative interventions during pediatric interfacility transport. Crit Care Med. 2015;43(8):1692–1698.
Children with medical complexity (CMC) have complex and chronic health conditions that often involve multiple organ systems and severely affect cognitive and physical functioning. Although the prevalence of CMC is low (1% of all children), they account for nearly one‐fifth of all pediatric admissions and one‐half of all hospital days and charges in the United States.[1] Over the last decade, CMC have had a particularly large and increasing impact in tertiary‐care children's hospitals.[1, 2] The Institute of Medicine has identified CMC as a priority population for a revised healthcare system.[3]
Medical homes, hospitals, health plans, states, federal agencies, and others are striving to reduce excessive hospital use in CMC because of its high cost.[4, 5, 6] Containing length of stay (LOS)an increasingly used indicator of the time sensitiveness and efficiency of hospital careis a common aim across these initiatives. CMC have longer hospitalizations than children without medical complexity. Speculated reasons for this are that CMC tend to have (1) higher severity of acute illnesses (eg, pneumonia, cellulitis), (2) prolonged recovery time in the hospital, and (3) higher risk of adverse events in the hospital. Moreover, hospital clinicians caring for CMC often find it difficult to determine discharge readiness, given that many CMC do not return to a completely healthy baseline.[7]
Little is known about long LOS in CMC, including which CMC have the highest risk of experiencing such stays and which stays might have the greatest opportunity to be shortened. Patient characteristics associated with prolonged length of stay have been studied extensively for many pediatric conditions (eg, asthma).[8, 9, 10, 11, 12, 13, 14] However, most of these studies excluded CMC. Therefore, the objectives of this study were to examine (1) the prevalence of long LOS in CMC, (2) patient characteristics associated with long LOS, and (3) hospital‐to‐hospital variation in prevalence of long LOS hospitalizations.
METHODS
Study Design and Data Source
This study is a multicenter, retrospective cohort analysis of the Pediatric Health Information System (PHIS). PHIS is an administrative database of 44 not for profit, tertiary care pediatric hospitals affiliated with the Children's Hospital Association (CHA) (Overland Park, KS). PHIS contains data regarding patient demographics, diagnoses, and procedures (with International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM] codes), All‐Patient Refined Diagnostic Related Groups version 30 (APR‐DRGs) (3M Health Information Systems, Salt Lake City, UT), and service lines that aggregate the APR‐DRGs into 38 distinct groups. Data quality and reliability are assured through CHA and participating hospitals. In accordance with the policies of the Cincinnati Children's Hospital Medical Center Institutional Review Board, this study of deidentified data was not considered human subjects research.
Study Population
Inclusion Criteria
Children discharged following an observation or inpatient admission from a hospital participating in the PHIS database between January 1, 2013 and December 31, 2014 were eligible for inclusion if they were considered medically complex. Medical complexity was defined using Clinical Risk Groups (CRGs) version 1.8, developed by 3M Health Information Systems and the National Association of Children's Hospitals and Related Institutions. CRGs were used to assign each hospitalized patient to 1 of 9 mutually exclusive chronicity groups according to the presence, type, and severity of chronic conditions.[15, 16, 17, 18] Each patient's CRG designation was based on 2 years of previous hospital encounters.
As defined in prior studies and definitional frameworks of CMC,[1] patients belonging to CRG group 6 (significant chronic disease in 2 organ systems), CRG group 7 (dominant chronic disease in 3 organ systems), and CRG group 9 (catastrophic condition) were considered medically complex.[17, 19] Patients with malignancies (CRG group 8) were not included for analysis because they are a unique population with anticipated, long hospital stays. Patients with CRG group 5, representing those with chronic conditions affecting a single body system, were also not included because most do not have attributes consistent with medical complexity.
Exclusion Criteria
We used the APR‐DRG system, which leverages ICD‐9‐CM codes to identify the health problem most responsible for the hospitalization, to refine the study cohort. We excluded hospitalizations that were classified by the APR‐DRG system as neonatal, as we did not wish to focus on LOS in the neonatal intensive care unit (ICU) or for birth admissions. Similarly, hospitalizations for chemotherapy (APR‐DRG 693) or malignancy (identified with previously used ICD‐9‐CM codes)[20] were also excluded because long LOS is anticipated. We also excluded hospitalizations for medical rehabilitation (APR‐DRG 860).
Outcome Measures
The primary outcome measure was long LOS, defined as LOS 10 days. The cut point of LOS 10 days represents the 90th percentile of LOS for all children, with and without medical complexity, hospitalized during 2013 to 2014. LOS 10 days has previously been used as a threshold of long LOS.[21] For hospitalizations involving transfer at admission from another acute care facility, LOS was measured from the date of transfer. We also assessed hospitals' cost attributable to long LOS admissions.
Patient Demographics and Clinical Characteristics
We measured demographic characteristics including age, gender, race/ethnicity, insurance type, and distance traveled (the linear distance between the centroid of the patient's home ZIP code and the centroid of the hospital's ZIP code). Clinical characteristics included CRG classification, complex chronic condition (CCC), and dependence on medical technology. CCCs are defined as any medical condition that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or 1 system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center.[20] Medical technology included devices used to optimize the health and functioning of the child (eg, gastrostomy, tracheostomy, cerebrospinal fluid shunt).[22]
Hospitalization Characteristics
Characteristics of the hospitalization included transfer from an outside facility, ICU admission, surgical procedure (using surgical APR‐DRGs), and discharge disposition (home, skilled nursing facility, home health services, death, other). Cost of the hospitalization was estimated in the PHIS from charges using hospital and year‐specific ratios of cost to charge.
Statistical Analysis
Continuous data (eg, distance from hospital to home residence) were described with median and interquartile ranges (IQR) because they were not normally distributed. Categorical data (eg, type of chronic condition) were described with counts and frequencies. In bivariate analyses, demographic, clinical, and hospitalization characteristics were stratified by LOS (long LOS vs LOS 10 days), and compared using 2 statistics or Wilcoxon rank sum tests as appropriate.
We modeled the likelihood of experiencing a long LOS using generalized linear mixed effects models with a random hospital intercept and discharge‐level fixed effects for age, gender, payor, CCC type, ICU utilization, transfer status, a medical/surgical admission indicator derived from the APR‐DRG, and CRG assigned to each hospitalization. To examine hospital‐to‐hospital variability, we generated hospital risk‐adjusted rates of long LOS from these models. Similar models and hospital risk‐adjusted rates were built for a post hoc correlational analysis of 30‐day all cause readmission, where hospitals' rates and percent of long LOS were compared with a Pearson correlation coefficient. Also, for our multivariable models, we performed a sensitivity analysis using an alternative definition of long LOS as 4 days (the 75th percentile of LOS for all children, with and without medical complexity, hospitalized during 20132014). All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), and P values 0.05 were considered statistically significant.
RESULTS
Study Population
There were 954,018 hospitalizations of 217,163 CMC at 44 children's hospitals included for analysis. Forty‐seven percent of hospitalizations were for females, 49.4% for non‐Hispanic whites, and 61.1% for children with government insurance. Fifteen percent (n = 142,082) had a long LOS of 10 days. The median (IQR) LOS of hospitalizations 10 days versus 10 days were 2 (IQR, 14) and 16 days (IQR, 1226), respectively. Long LOS hospitalizations accounted for 61.1% (3.7 million) hospital days and 61.8% ($13.7 billion) of total hospitalization costs for all CMC in the cohort (Table 1).
| Characteristic | Overall (n = 954,018) | Length of Stay | |
|---|---|---|---|
| 10 Days (n = 811,936) | 10 Days (n = 142,082) | ||
| |||
| Age at admission, y, % | |||
| 1 | 14.6 | 12.7 | 25.7 |
| 14 | 27.1 | 27.9 | 22.4 |
| 59 | 20.1 | 21.0 | 14.9 |
| 1018 | 33.6 | 34.0 | 31.7 |
| 18+ | 4.6 | 4.4 | 5.4 |
| Gender, % | |||
| Female | 47.0 | 46.9 | 47.5 |
| Race/ethnicity, % | |||
| Non‐Hispanic white | 49.4 | 49.4 | 49.4 |
| Non‐Hispanic black | 23.1 | 23.8 | 19.3 |
| Hispanic | 18.2 | 17.8 | 20.4 |
| Asian | 2.0 | 1.9 | 2.3 |
| Other | 7.4 | 7.1 | 8.6 |
| Complex chronic condition, % | |||
| Any | 79.5 | 77.3 | 91.8 |
| Technology assistance | 37.1 | 34.1 | 54.2 |
| Gastrointestinal | 30.0 | 27.2 | 45.9 |
| Neuromuscular | 28.2 | 27.7 | 30.9 |
| Cardiovascular | 16.8 | 14.5 | 29.9 |
| Respiratory | 14.1 | 11.5 | 29.4 |
| Congenital/genetic defect | 17.2 | 16.7 | 20.2 |
| Metabolic | 9.9 | 8.9 | 15.4 |
| Renal | 10.1 | 9.5 | 13.8 |
| Hematology/emmmunodeficiency | 11.7 | 12.0 | 10.0 |
| Neonatal | 3.8 | 3.1 | 7.7 |
| Transplantation | 4.5 | 4.2 | 6.7 |
| Clinical risk group, % | |||
| Chronic condition in 2 systems | 68.4 | 71.2 | 53.9 |
| Catastrophic chronic condition | 31.4 | 28.8 | 46.1 |
| Distance from hospital to home residence in miles, median [IQR] | 16.2 [7.440.4] | 15.8 [7.338.7] | 19.1 [8.552.6] |
| Transferred from outside hospital (%) | 6.5 | 5.3 | 13.6 |
| Admitted for surgery, % | 23.4 | 20.7 | 38.7 |
| Use of intensive care, % | 19.6 | 14.9 | 46.5 |
| Discharge disposition, % | |||
| Home | 91.2 | 92.9 | 81.4 |
| Home healthcare | 4.5 | 3.5 | 9.9 |
| Other | 2.9 | 2.6 | 4.5 |
| Postacute care facility | 1.1 | 0.8 | 3.1 |
| Died | 0.4 | 0.3 | 1.1 |
| Payor, % | |||
| Government | 61.1 | 60.6 | 63.5 |
| Private | 33.2 | 33.6 | 30.9 |
| Other | 5.7 | 5.7 | 5.7 |
| Hospital resource use | |||
| Median length of stay [IQR] | 3 [16] | 2 [14] | 16 [1226] |
| Median hospital cost [IQR] | $8,144 [$4,122$18,447] | $6,689 [$3,685$12,395] | $49,207 [$29,444$95,738] |
| Total hospital cost, $, billions | $22.2 | $8.5 | $13.7 |
Demographics and Clinical Characteristics of Children With and Without Long LOS
Compared with hospitalized CMC with LOS 10 days, a higher percentage of hospitalizations with LOS 10 days were CMC age 1 year (25.7% vs 12.7%, P 0.001) and Hispanic (20.4% vs 17.8%, P 0.001). CMC hospitalizations with a long LOS had a higher percentage of any CCC (91.8% vs 77.3%, P 0.001); the most common CCCs were gastrointestinal (45.9%), neuromuscular (30.9%), and cardiovascular (29.9%). Hospitalizations of CMC with a long LOS had a higher percentage of a catastrophic chronic condition (46.1% vs 28.8%, P 0.001) and technology dependence (46.1% vs 28.8%, P 0.001) (Table 1).
Hospitalization Characteristics of Children With and Without Long LOS
Compared with hospitalizations of CMC with LOS 10 days, hospitalizations of CMC with a long LOS more often involved transfer in from another hospital at admission (13.6% vs 5.3%, P 0.001). CMC hospital stays with a long LOS more often involved surgery (38.7% vs 20.7%, P 0.001) and use of intensive care (46.5% vs 14.9%; P 0.001). A higher percentage of CMC with long LOS were discharged with home health services (9.9% vs 3.5%; P 0.001) (Table 1).
The most common admitting diagnoses and CCCs for hospitalizations of CMC with long LOS are presented in Table 2. The two most prevalent APR‐DRGs in CMC hospitalizations lasting 10 days or longer were cystic fibrosis (10.7%) and respiratory system disease with ventilator support (5.5%). The two most common chronic condition characteristics represented among long CMC hospitalizations were gastrointestinal devices (eg, gastrostomy tube) (39.7%) and heart and great vessel malformations (eg, tetralogy of Fallot) (12.8%). The 5 most common CCC subcategories, as listed in Table 2, account for nearly 100% of the patients with long LOS hospitalizations.
| |
| Most common reason for admission* | |
| Cystic fibrosis | 10.7% |
| Respiratory system diagnosis with ventilator support 96+ hours | 5.5% |
| Malfunction, reaction, and complication of cardiac or vascular device or procedure | 2.8% |
| Craniotomy except for trauma | 2.6% |
| Major small and large bowel procedures | 2.3% |
| Most common complex chronic condition | |
| Gastrointestinal devices | 39.7% |
| Heart and great vessel malformations | 12.8% |
| Cystic fibrosis | 12.5% |
| Dysrhythmias | 11.0% |
| Respiratory devices | 10.7% |
Multivariable Analysis of Characteristics Associated With Long LOS
In multivariable analysis, the highest likelihood of long LOS was experienced by children who received care in the ICU (odds ratio [OR]: 3.5, 95% confidence interval [CI]: 3.43.5), who had a respiratory CCC (OR: 2.7, 95% CI: 2.62.7), and who were transferred from another acute care hospital at admission (OR: 2.1, 95% CI: 2.0, 2.1). The likelihood of long LOS was also higher in children 1 year of age (OR: 1.2, 95% CI: 1.21.3), and Hispanic children (OR: 1.1, 95% CI 1.0‐1.10) (Table 3). Similar multivariable findings were observed in sensitivity analysis using the 75th percentile of LOS (4 days) as the model outcome.
| Characteristic | Odds Ratio (95% CI) of LOS 10 Days | P Value |
|---|---|---|
| ||
| Use of intensive care | 3.5 (3.4‐3.5) | 0.001 |
| Transfer from another acute‐care hospital | 2.1 (2.0‐2.1) | 0.001 |
| Procedure/surgery | 1.8 (1.8‐1.9) | 0.001 |
| Complex chronic condition | ||
| Respiratory | 2.7 (2.6‐2.7) | 0.001 |
| Gastrointestinal | 1.8 (1.8‐1.8) | 0.001 |
| Metabolic | 1.7 (1.7‐1.7) | 0.001 |
| Cardiovascular | 1.6 (1.5‐1.6) | 0.001 |
| Neonatal | 1.5 (1.5‐1.5) | 0.001 |
| Renal | 1.4 (1.4‐1.4) | 0.001 |
| Transplant | 1.4 (1.4‐1.4) | 0.001 |
| Hematology and immunodeficiency | 1.3 (1.3‐1.3) | 0.001 |
| Technology assistance | 1.1 (1.1, 1.1) | 0.001 |
| Neuromuscular | 0.9 (0.9‐0.9) | 0.001 |
| Congenital or genetic defect | 0.8 (0.8‐0.8) | 0.001 |
| Age at admission, y | ||
| 1 | 1.2 (1.2‐1.3) | 0.001 |
| 14 | 0.5 (0.5‐0.5) | 0.001 |
| 59 | 0.6 (0.6‐0.6) | 0.001 |
| 1018 | 0.9 (0.9‐0.9) | 0.001 |
| 18+ | Reference | |
| Male | 0.9 (0.9‐0.9) | 0.001 |
| Race/ethnicity | ||
| Non‐Hispanic black | 0.9 (0.9‐0.9) | 0.001 |
| Hispanic | 1.1 (1.0‐1.1) | 0.001 |
| Asian | 1.0 (1.0‐1.1) | 0.3 |
| Other | 1.1 (1.1‐1.1) | 0.001 |
| Non‐Hispanic white | Reference | |
| Payor | ||
| Private | 0.9 (0.8 0.9) | 0.001 |
| Other | 1.0 (1.0‐1.0) | 0.4 |
| Government | Reference | |
| Season | ||
| Spring | 1.0 (1.0 1.0) | 0.001 |
| Summer | 0.9 (0.9‐0.9) | 0.001 |
| Fall | 1.0 (0.9‐1.0) | 0.001 |
| Winter | Reference | |
Variation in the Prevalence of Long LOS Across Children's Hospitals
After controlling for demographic, clinical, and hospital characteristics associated with long LOS, there was significant (P 0.001) variation in the prevalence of long LOS for CMC across children's hospitals in the cohort (range, 10.3%21.8%) (Figure 1). Twelve (27%) hospitals had a significantly (P 0.001) higher prevalence of long LOS for their hospitalized CMC, compared to the mean. Eighteen (41%) had a significantly (P 0.001) lower prevalence of long LOS for their hospitalized CMC. There was also significant variation across hospitals with respect to cost, with 49.7% to 73.7% of all hospital costs of CMC attributed to long LOS hospitalizations. Finally, there was indirect correlation with the prevalence of LOS across hospitals and the hospitals' 30‐day readmission rate ( = 0.3; P = 0.04). As the prevalence of long LOS increased, the readmission rate decreased.

DISCUSSION
The main findings from this study suggest that a small percentage of CMC experiencing long LOS account for the majority of hospital bed days and cost of all hospitalized CMC in children's hospitals. The likelihood of long LOS varies significantly by CMC's age, race/ethnicity, and payor as well as by type and number of chronic conditions. Among CMC with long LOS, the use of gastrointestinal devices such as gastrostomy tubes, as well as congenital heart disease, were highly prevalent. In multivariable analysis, the characteristics most strongly associated with LOS 10 days were use of the ICU, respiratory complex chronic condition, and transfer from another medical facility at admission. After adjusting for these factors, there was significant variation in the prevalence of LOS 10 days for CMC across children's hospitals.
Although it is well known that CMC as a whole have a major impact on resource use in children's hospitals, this study reveals that 15% of hospitalizations of CMC account for 62% of all hospital costs of CMC. That is, a small fraction of hospitalizations of CMC is largely responsible for the significant financial impact of hospital resource use. To date, most clinical efforts and policies striving to reduce hospital use in CMC have focused on avoiding readmissions or index hospital admissions entirely, rather than improving the efficiency of hospital care after admission occurs.[23, 24, 25, 26] In the adult population, the impact of long LOS on hospital costs has been recognized, and several Medicare incentive programs have focused on in‐hospital timeliness and efficiency. As a result, LOS in Medicare beneficiaries has decreased dramatically over the past 2 decades.[27, 28, 29, 30] Optimizing the efficiency of hospital care for CMC may be an important goal to pursue, especially with precedent set in the adult literature.
Perhaps the substantial variation across hospitals in the prevalence of long LOS in CMC indicates opportunity to improve the efficiency of their inpatient care. This variation was not due to differences across hospitals' case mix of CMC. Further investigation is needed to determine how much of it is due to differences in quality of care. Clinical practice guidelines for hospital treatment of common illnesses usually exclude CMC. In our clinical experience across 9 children's hospitals, we have experienced varying approaches to setting discharge goals (ie, parameters on how healthy the child needs to be to ensure a successful hospital discharge) for CMC.[31] When the goals are absent or not clearly articulated, they can contribute to a prolonged hospitalization. Some families of CMC report significant issues when working with pediatric hospital staff to assess their child's discharge readiness.[7, 32, 33] In addition, there is significant variation across states and regions in access to and quality of post‐discharge health services (eg, home nursing, postacute care, durable medical equipment).[34, 35] In some areas, many CMC are not actively involved with their primary care physician.[5] These issues might also influence the ability of some children's hospitals to efficiently discharge CMC to a safe and supportive post‐discharge environment. Further examination of hospital outliersthose with the lowest and highest percentage of CMC hospitalizations with long LOSmay reveal opportunities to identify and spread best practices.
The demographic and clinical factors associated with long LOS in the present study, including age, ICU use, and transfer from another hospital, might help hospitals target which CMC have the greatest risk for experiencing long LOS. We found that infants age 1 year had longer LOS when compared with older children. Similar to our findings, younger‐aged children hospitalized with bronchiolitis have longer LOS.[36] Certainly, infants with medical complexity, in general, are a high‐acuity population with the potential for rapid clinical deterioration during an acute illness. Prolonged hospitalization for treatment and stabilization may be expected for many of them. Additional investigation is warranted to examine ICU use in CMC, and whether ICU admission or duration can be safely prevented or abbreviated. Opportunities to assess the quality of transfers into children's hospitals of CMC admitted to outside hospitals may be necessary. A study of pediatric burn patients reported that patients initially stabilized at a facility that was not a burn center and subsequently transferred to a burn center had a longer LOS than patients solely treated at a designated burn center.[37] Furthermore, events during transport itself may adversely impact the stability of an already fragile patient. Interventions to optimize the quality of care provided by transport teams have resulted in decreased LOS at the receiving hospital.[38]
This study's findings should be considered in the context of several limitations. Absent a gold‐standard definition of long LOS, we used the distribution of LOS across patients to inform our methods; LOS at the 90th percentile was selected as long. Although our sensitivity analysis using LOS at the 75th percentile produced similar findings, other cut points in LOS might be associated with different results. The study is not positioned to determine how much of the reported LOS was excessive, unnecessary, or preventable. The study findings may not generalize to types of hospitals not contained in PHIS (eg, nonchildren's hospitals and community hospitals). We did not focus on the impact of a new diagnosis (eg, new chronic illness) or acute in‐hospital event (eg, nosocomial infection) on prolonged LOS; future studies should investigate these clinical events with LOS.
PHIS does not contain information regarding characteristics that could influence LOS, including the children's social and familial attributes, transportation availability, home equipment needs, and local availability of postacute care facilities. Moreover, PHIS does not contain information about the hospital discharge procedures, process, or personnel across hospitals, which could influence LOS. Future studies on prolonged LOS should consider assessing this information. Because of the large sample size of hospitalizations included, the statistical power for the analyses was strong, rendering it possible that some findings that were statistically significant might have modest clinical significance (eg, relationship of Hispanic ethnicity with prolonged LOS). We could not determine why a positive correlation was not observed between hospitals' long LOS prevalence and their percentage of cost associated with long LOS; future studies should investigate the reasons for this finding.
Despite these limitations, the findings of the present study highlight the significance of long LOS in hospitalized CMC. These long hospitalizations account for a significant proportion of all hospital costs for this important population of children. The prevalence of long LOS for CMC varies considerably across children's hospitals, even after accounting for the case mix. Efforts to curtail hospital resource use and costs for CMC may benefit from focus on long LOS.
Children with medical complexity (CMC) have complex and chronic health conditions that often involve multiple organ systems and severely affect cognitive and physical functioning. Although the prevalence of CMC is low (1% of all children), they account for nearly one‐fifth of all pediatric admissions and one‐half of all hospital days and charges in the United States.[1] Over the last decade, CMC have had a particularly large and increasing impact in tertiary‐care children's hospitals.[1, 2] The Institute of Medicine has identified CMC as a priority population for a revised healthcare system.[3]
Medical homes, hospitals, health plans, states, federal agencies, and others are striving to reduce excessive hospital use in CMC because of its high cost.[4, 5, 6] Containing length of stay (LOS)an increasingly used indicator of the time sensitiveness and efficiency of hospital careis a common aim across these initiatives. CMC have longer hospitalizations than children without medical complexity. Speculated reasons for this are that CMC tend to have (1) higher severity of acute illnesses (eg, pneumonia, cellulitis), (2) prolonged recovery time in the hospital, and (3) higher risk of adverse events in the hospital. Moreover, hospital clinicians caring for CMC often find it difficult to determine discharge readiness, given that many CMC do not return to a completely healthy baseline.[7]
Little is known about long LOS in CMC, including which CMC have the highest risk of experiencing such stays and which stays might have the greatest opportunity to be shortened. Patient characteristics associated with prolonged length of stay have been studied extensively for many pediatric conditions (eg, asthma).[8, 9, 10, 11, 12, 13, 14] However, most of these studies excluded CMC. Therefore, the objectives of this study were to examine (1) the prevalence of long LOS in CMC, (2) patient characteristics associated with long LOS, and (3) hospital‐to‐hospital variation in prevalence of long LOS hospitalizations.
METHODS
Study Design and Data Source
This study is a multicenter, retrospective cohort analysis of the Pediatric Health Information System (PHIS). PHIS is an administrative database of 44 not for profit, tertiary care pediatric hospitals affiliated with the Children's Hospital Association (CHA) (Overland Park, KS). PHIS contains data regarding patient demographics, diagnoses, and procedures (with International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM] codes), All‐Patient Refined Diagnostic Related Groups version 30 (APR‐DRGs) (3M Health Information Systems, Salt Lake City, UT), and service lines that aggregate the APR‐DRGs into 38 distinct groups. Data quality and reliability are assured through CHA and participating hospitals. In accordance with the policies of the Cincinnati Children's Hospital Medical Center Institutional Review Board, this study of deidentified data was not considered human subjects research.
Study Population
Inclusion Criteria
Children discharged following an observation or inpatient admission from a hospital participating in the PHIS database between January 1, 2013 and December 31, 2014 were eligible for inclusion if they were considered medically complex. Medical complexity was defined using Clinical Risk Groups (CRGs) version 1.8, developed by 3M Health Information Systems and the National Association of Children's Hospitals and Related Institutions. CRGs were used to assign each hospitalized patient to 1 of 9 mutually exclusive chronicity groups according to the presence, type, and severity of chronic conditions.[15, 16, 17, 18] Each patient's CRG designation was based on 2 years of previous hospital encounters.
As defined in prior studies and definitional frameworks of CMC,[1] patients belonging to CRG group 6 (significant chronic disease in 2 organ systems), CRG group 7 (dominant chronic disease in 3 organ systems), and CRG group 9 (catastrophic condition) were considered medically complex.[17, 19] Patients with malignancies (CRG group 8) were not included for analysis because they are a unique population with anticipated, long hospital stays. Patients with CRG group 5, representing those with chronic conditions affecting a single body system, were also not included because most do not have attributes consistent with medical complexity.
Exclusion Criteria
We used the APR‐DRG system, which leverages ICD‐9‐CM codes to identify the health problem most responsible for the hospitalization, to refine the study cohort. We excluded hospitalizations that were classified by the APR‐DRG system as neonatal, as we did not wish to focus on LOS in the neonatal intensive care unit (ICU) or for birth admissions. Similarly, hospitalizations for chemotherapy (APR‐DRG 693) or malignancy (identified with previously used ICD‐9‐CM codes)[20] were also excluded because long LOS is anticipated. We also excluded hospitalizations for medical rehabilitation (APR‐DRG 860).
Outcome Measures
The primary outcome measure was long LOS, defined as LOS 10 days. The cut point of LOS 10 days represents the 90th percentile of LOS for all children, with and without medical complexity, hospitalized during 2013 to 2014. LOS 10 days has previously been used as a threshold of long LOS.[21] For hospitalizations involving transfer at admission from another acute care facility, LOS was measured from the date of transfer. We also assessed hospitals' cost attributable to long LOS admissions.
Patient Demographics and Clinical Characteristics
We measured demographic characteristics including age, gender, race/ethnicity, insurance type, and distance traveled (the linear distance between the centroid of the patient's home ZIP code and the centroid of the hospital's ZIP code). Clinical characteristics included CRG classification, complex chronic condition (CCC), and dependence on medical technology. CCCs are defined as any medical condition that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or 1 system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center.[20] Medical technology included devices used to optimize the health and functioning of the child (eg, gastrostomy, tracheostomy, cerebrospinal fluid shunt).[22]
Hospitalization Characteristics
Characteristics of the hospitalization included transfer from an outside facility, ICU admission, surgical procedure (using surgical APR‐DRGs), and discharge disposition (home, skilled nursing facility, home health services, death, other). Cost of the hospitalization was estimated in the PHIS from charges using hospital and year‐specific ratios of cost to charge.
Statistical Analysis
Continuous data (eg, distance from hospital to home residence) were described with median and interquartile ranges (IQR) because they were not normally distributed. Categorical data (eg, type of chronic condition) were described with counts and frequencies. In bivariate analyses, demographic, clinical, and hospitalization characteristics were stratified by LOS (long LOS vs LOS 10 days), and compared using 2 statistics or Wilcoxon rank sum tests as appropriate.
We modeled the likelihood of experiencing a long LOS using generalized linear mixed effects models with a random hospital intercept and discharge‐level fixed effects for age, gender, payor, CCC type, ICU utilization, transfer status, a medical/surgical admission indicator derived from the APR‐DRG, and CRG assigned to each hospitalization. To examine hospital‐to‐hospital variability, we generated hospital risk‐adjusted rates of long LOS from these models. Similar models and hospital risk‐adjusted rates were built for a post hoc correlational analysis of 30‐day all cause readmission, where hospitals' rates and percent of long LOS were compared with a Pearson correlation coefficient. Also, for our multivariable models, we performed a sensitivity analysis using an alternative definition of long LOS as 4 days (the 75th percentile of LOS for all children, with and without medical complexity, hospitalized during 20132014). All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), and P values 0.05 were considered statistically significant.
RESULTS
Study Population
There were 954,018 hospitalizations of 217,163 CMC at 44 children's hospitals included for analysis. Forty‐seven percent of hospitalizations were for females, 49.4% for non‐Hispanic whites, and 61.1% for children with government insurance. Fifteen percent (n = 142,082) had a long LOS of 10 days. The median (IQR) LOS of hospitalizations 10 days versus 10 days were 2 (IQR, 14) and 16 days (IQR, 1226), respectively. Long LOS hospitalizations accounted for 61.1% (3.7 million) hospital days and 61.8% ($13.7 billion) of total hospitalization costs for all CMC in the cohort (Table 1).
| Characteristic | Overall (n = 954,018) | Length of Stay | |
|---|---|---|---|
| 10 Days (n = 811,936) | 10 Days (n = 142,082) | ||
| |||
| Age at admission, y, % | |||
| 1 | 14.6 | 12.7 | 25.7 |
| 14 | 27.1 | 27.9 | 22.4 |
| 59 | 20.1 | 21.0 | 14.9 |
| 1018 | 33.6 | 34.0 | 31.7 |
| 18+ | 4.6 | 4.4 | 5.4 |
| Gender, % | |||
| Female | 47.0 | 46.9 | 47.5 |
| Race/ethnicity, % | |||
| Non‐Hispanic white | 49.4 | 49.4 | 49.4 |
| Non‐Hispanic black | 23.1 | 23.8 | 19.3 |
| Hispanic | 18.2 | 17.8 | 20.4 |
| Asian | 2.0 | 1.9 | 2.3 |
| Other | 7.4 | 7.1 | 8.6 |
| Complex chronic condition, % | |||
| Any | 79.5 | 77.3 | 91.8 |
| Technology assistance | 37.1 | 34.1 | 54.2 |
| Gastrointestinal | 30.0 | 27.2 | 45.9 |
| Neuromuscular | 28.2 | 27.7 | 30.9 |
| Cardiovascular | 16.8 | 14.5 | 29.9 |
| Respiratory | 14.1 | 11.5 | 29.4 |
| Congenital/genetic defect | 17.2 | 16.7 | 20.2 |
| Metabolic | 9.9 | 8.9 | 15.4 |
| Renal | 10.1 | 9.5 | 13.8 |
| Hematology/emmmunodeficiency | 11.7 | 12.0 | 10.0 |
| Neonatal | 3.8 | 3.1 | 7.7 |
| Transplantation | 4.5 | 4.2 | 6.7 |
| Clinical risk group, % | |||
| Chronic condition in 2 systems | 68.4 | 71.2 | 53.9 |
| Catastrophic chronic condition | 31.4 | 28.8 | 46.1 |
| Distance from hospital to home residence in miles, median [IQR] | 16.2 [7.440.4] | 15.8 [7.338.7] | 19.1 [8.552.6] |
| Transferred from outside hospital (%) | 6.5 | 5.3 | 13.6 |
| Admitted for surgery, % | 23.4 | 20.7 | 38.7 |
| Use of intensive care, % | 19.6 | 14.9 | 46.5 |
| Discharge disposition, % | |||
| Home | 91.2 | 92.9 | 81.4 |
| Home healthcare | 4.5 | 3.5 | 9.9 |
| Other | 2.9 | 2.6 | 4.5 |
| Postacute care facility | 1.1 | 0.8 | 3.1 |
| Died | 0.4 | 0.3 | 1.1 |
| Payor, % | |||
| Government | 61.1 | 60.6 | 63.5 |
| Private | 33.2 | 33.6 | 30.9 |
| Other | 5.7 | 5.7 | 5.7 |
| Hospital resource use | |||
| Median length of stay [IQR] | 3 [16] | 2 [14] | 16 [1226] |
| Median hospital cost [IQR] | $8,144 [$4,122$18,447] | $6,689 [$3,685$12,395] | $49,207 [$29,444$95,738] |
| Total hospital cost, $, billions | $22.2 | $8.5 | $13.7 |
Demographics and Clinical Characteristics of Children With and Without Long LOS
Compared with hospitalized CMC with LOS 10 days, a higher percentage of hospitalizations with LOS 10 days were CMC age 1 year (25.7% vs 12.7%, P 0.001) and Hispanic (20.4% vs 17.8%, P 0.001). CMC hospitalizations with a long LOS had a higher percentage of any CCC (91.8% vs 77.3%, P 0.001); the most common CCCs were gastrointestinal (45.9%), neuromuscular (30.9%), and cardiovascular (29.9%). Hospitalizations of CMC with a long LOS had a higher percentage of a catastrophic chronic condition (46.1% vs 28.8%, P 0.001) and technology dependence (46.1% vs 28.8%, P 0.001) (Table 1).
Hospitalization Characteristics of Children With and Without Long LOS
Compared with hospitalizations of CMC with LOS 10 days, hospitalizations of CMC with a long LOS more often involved transfer in from another hospital at admission (13.6% vs 5.3%, P 0.001). CMC hospital stays with a long LOS more often involved surgery (38.7% vs 20.7%, P 0.001) and use of intensive care (46.5% vs 14.9%; P 0.001). A higher percentage of CMC with long LOS were discharged with home health services (9.9% vs 3.5%; P 0.001) (Table 1).
The most common admitting diagnoses and CCCs for hospitalizations of CMC with long LOS are presented in Table 2. The two most prevalent APR‐DRGs in CMC hospitalizations lasting 10 days or longer were cystic fibrosis (10.7%) and respiratory system disease with ventilator support (5.5%). The two most common chronic condition characteristics represented among long CMC hospitalizations were gastrointestinal devices (eg, gastrostomy tube) (39.7%) and heart and great vessel malformations (eg, tetralogy of Fallot) (12.8%). The 5 most common CCC subcategories, as listed in Table 2, account for nearly 100% of the patients with long LOS hospitalizations.
| |
| Most common reason for admission* | |
| Cystic fibrosis | 10.7% |
| Respiratory system diagnosis with ventilator support 96+ hours | 5.5% |
| Malfunction, reaction, and complication of cardiac or vascular device or procedure | 2.8% |
| Craniotomy except for trauma | 2.6% |
| Major small and large bowel procedures | 2.3% |
| Most common complex chronic condition | |
| Gastrointestinal devices | 39.7% |
| Heart and great vessel malformations | 12.8% |
| Cystic fibrosis | 12.5% |
| Dysrhythmias | 11.0% |
| Respiratory devices | 10.7% |
Multivariable Analysis of Characteristics Associated With Long LOS
In multivariable analysis, the highest likelihood of long LOS was experienced by children who received care in the ICU (odds ratio [OR]: 3.5, 95% confidence interval [CI]: 3.43.5), who had a respiratory CCC (OR: 2.7, 95% CI: 2.62.7), and who were transferred from another acute care hospital at admission (OR: 2.1, 95% CI: 2.0, 2.1). The likelihood of long LOS was also higher in children 1 year of age (OR: 1.2, 95% CI: 1.21.3), and Hispanic children (OR: 1.1, 95% CI 1.0‐1.10) (Table 3). Similar multivariable findings were observed in sensitivity analysis using the 75th percentile of LOS (4 days) as the model outcome.
| Characteristic | Odds Ratio (95% CI) of LOS 10 Days | P Value |
|---|---|---|
| ||
| Use of intensive care | 3.5 (3.4‐3.5) | 0.001 |
| Transfer from another acute‐care hospital | 2.1 (2.0‐2.1) | 0.001 |
| Procedure/surgery | 1.8 (1.8‐1.9) | 0.001 |
| Complex chronic condition | ||
| Respiratory | 2.7 (2.6‐2.7) | 0.001 |
| Gastrointestinal | 1.8 (1.8‐1.8) | 0.001 |
| Metabolic | 1.7 (1.7‐1.7) | 0.001 |
| Cardiovascular | 1.6 (1.5‐1.6) | 0.001 |
| Neonatal | 1.5 (1.5‐1.5) | 0.001 |
| Renal | 1.4 (1.4‐1.4) | 0.001 |
| Transplant | 1.4 (1.4‐1.4) | 0.001 |
| Hematology and immunodeficiency | 1.3 (1.3‐1.3) | 0.001 |
| Technology assistance | 1.1 (1.1, 1.1) | 0.001 |
| Neuromuscular | 0.9 (0.9‐0.9) | 0.001 |
| Congenital or genetic defect | 0.8 (0.8‐0.8) | 0.001 |
| Age at admission, y | ||
| 1 | 1.2 (1.2‐1.3) | 0.001 |
| 14 | 0.5 (0.5‐0.5) | 0.001 |
| 59 | 0.6 (0.6‐0.6) | 0.001 |
| 1018 | 0.9 (0.9‐0.9) | 0.001 |
| 18+ | Reference | |
| Male | 0.9 (0.9‐0.9) | 0.001 |
| Race/ethnicity | ||
| Non‐Hispanic black | 0.9 (0.9‐0.9) | 0.001 |
| Hispanic | 1.1 (1.0‐1.1) | 0.001 |
| Asian | 1.0 (1.0‐1.1) | 0.3 |
| Other | 1.1 (1.1‐1.1) | 0.001 |
| Non‐Hispanic white | Reference | |
| Payor | ||
| Private | 0.9 (0.8 0.9) | 0.001 |
| Other | 1.0 (1.0‐1.0) | 0.4 |
| Government | Reference | |
| Season | ||
| Spring | 1.0 (1.0 1.0) | 0.001 |
| Summer | 0.9 (0.9‐0.9) | 0.001 |
| Fall | 1.0 (0.9‐1.0) | 0.001 |
| Winter | Reference | |
Variation in the Prevalence of Long LOS Across Children's Hospitals
After controlling for demographic, clinical, and hospital characteristics associated with long LOS, there was significant (P 0.001) variation in the prevalence of long LOS for CMC across children's hospitals in the cohort (range, 10.3%21.8%) (Figure 1). Twelve (27%) hospitals had a significantly (P 0.001) higher prevalence of long LOS for their hospitalized CMC, compared to the mean. Eighteen (41%) had a significantly (P 0.001) lower prevalence of long LOS for their hospitalized CMC. There was also significant variation across hospitals with respect to cost, with 49.7% to 73.7% of all hospital costs of CMC attributed to long LOS hospitalizations. Finally, there was indirect correlation with the prevalence of LOS across hospitals and the hospitals' 30‐day readmission rate ( = 0.3; P = 0.04). As the prevalence of long LOS increased, the readmission rate decreased.

DISCUSSION
The main findings from this study suggest that a small percentage of CMC experiencing long LOS account for the majority of hospital bed days and cost of all hospitalized CMC in children's hospitals. The likelihood of long LOS varies significantly by CMC's age, race/ethnicity, and payor as well as by type and number of chronic conditions. Among CMC with long LOS, the use of gastrointestinal devices such as gastrostomy tubes, as well as congenital heart disease, were highly prevalent. In multivariable analysis, the characteristics most strongly associated with LOS 10 days were use of the ICU, respiratory complex chronic condition, and transfer from another medical facility at admission. After adjusting for these factors, there was significant variation in the prevalence of LOS 10 days for CMC across children's hospitals.
Although it is well known that CMC as a whole have a major impact on resource use in children's hospitals, this study reveals that 15% of hospitalizations of CMC account for 62% of all hospital costs of CMC. That is, a small fraction of hospitalizations of CMC is largely responsible for the significant financial impact of hospital resource use. To date, most clinical efforts and policies striving to reduce hospital use in CMC have focused on avoiding readmissions or index hospital admissions entirely, rather than improving the efficiency of hospital care after admission occurs.[23, 24, 25, 26] In the adult population, the impact of long LOS on hospital costs has been recognized, and several Medicare incentive programs have focused on in‐hospital timeliness and efficiency. As a result, LOS in Medicare beneficiaries has decreased dramatically over the past 2 decades.[27, 28, 29, 30] Optimizing the efficiency of hospital care for CMC may be an important goal to pursue, especially with precedent set in the adult literature.
Perhaps the substantial variation across hospitals in the prevalence of long LOS in CMC indicates opportunity to improve the efficiency of their inpatient care. This variation was not due to differences across hospitals' case mix of CMC. Further investigation is needed to determine how much of it is due to differences in quality of care. Clinical practice guidelines for hospital treatment of common illnesses usually exclude CMC. In our clinical experience across 9 children's hospitals, we have experienced varying approaches to setting discharge goals (ie, parameters on how healthy the child needs to be to ensure a successful hospital discharge) for CMC.[31] When the goals are absent or not clearly articulated, they can contribute to a prolonged hospitalization. Some families of CMC report significant issues when working with pediatric hospital staff to assess their child's discharge readiness.[7, 32, 33] In addition, there is significant variation across states and regions in access to and quality of post‐discharge health services (eg, home nursing, postacute care, durable medical equipment).[34, 35] In some areas, many CMC are not actively involved with their primary care physician.[5] These issues might also influence the ability of some children's hospitals to efficiently discharge CMC to a safe and supportive post‐discharge environment. Further examination of hospital outliersthose with the lowest and highest percentage of CMC hospitalizations with long LOSmay reveal opportunities to identify and spread best practices.
The demographic and clinical factors associated with long LOS in the present study, including age, ICU use, and transfer from another hospital, might help hospitals target which CMC have the greatest risk for experiencing long LOS. We found that infants age 1 year had longer LOS when compared with older children. Similar to our findings, younger‐aged children hospitalized with bronchiolitis have longer LOS.[36] Certainly, infants with medical complexity, in general, are a high‐acuity population with the potential for rapid clinical deterioration during an acute illness. Prolonged hospitalization for treatment and stabilization may be expected for many of them. Additional investigation is warranted to examine ICU use in CMC, and whether ICU admission or duration can be safely prevented or abbreviated. Opportunities to assess the quality of transfers into children's hospitals of CMC admitted to outside hospitals may be necessary. A study of pediatric burn patients reported that patients initially stabilized at a facility that was not a burn center and subsequently transferred to a burn center had a longer LOS than patients solely treated at a designated burn center.[37] Furthermore, events during transport itself may adversely impact the stability of an already fragile patient. Interventions to optimize the quality of care provided by transport teams have resulted in decreased LOS at the receiving hospital.[38]
This study's findings should be considered in the context of several limitations. Absent a gold‐standard definition of long LOS, we used the distribution of LOS across patients to inform our methods; LOS at the 90th percentile was selected as long. Although our sensitivity analysis using LOS at the 75th percentile produced similar findings, other cut points in LOS might be associated with different results. The study is not positioned to determine how much of the reported LOS was excessive, unnecessary, or preventable. The study findings may not generalize to types of hospitals not contained in PHIS (eg, nonchildren's hospitals and community hospitals). We did not focus on the impact of a new diagnosis (eg, new chronic illness) or acute in‐hospital event (eg, nosocomial infection) on prolonged LOS; future studies should investigate these clinical events with LOS.
PHIS does not contain information regarding characteristics that could influence LOS, including the children's social and familial attributes, transportation availability, home equipment needs, and local availability of postacute care facilities. Moreover, PHIS does not contain information about the hospital discharge procedures, process, or personnel across hospitals, which could influence LOS. Future studies on prolonged LOS should consider assessing this information. Because of the large sample size of hospitalizations included, the statistical power for the analyses was strong, rendering it possible that some findings that were statistically significant might have modest clinical significance (eg, relationship of Hispanic ethnicity with prolonged LOS). We could not determine why a positive correlation was not observed between hospitals' long LOS prevalence and their percentage of cost associated with long LOS; future studies should investigate the reasons for this finding.
Despite these limitations, the findings of the present study highlight the significance of long LOS in hospitalized CMC. These long hospitalizations account for a significant proportion of all hospital costs for this important population of children. The prevalence of long LOS for CMC varies considerably across children's hospitals, even after accounting for the case mix. Efforts to curtail hospital resource use and costs for CMC may benefit from focus on long LOS.
- , , , et al. Inpatient growth and resource use in 28 children's hospitals: a longitudinal, multi‐institutional study. JAMA Pediatr. 2013;167(2):170–177.
- , , , et al. Children with complex chronic conditions in inpatient hospital settings in the united states. Pediatrics. 2010;126(4):647–655.
- , . Meeting the health care needs of persons with disabilities. Milbank Q. 2002;80(2):381–391.
- , , , et al. Effect of an enhanced medical home on serious illness and cost of care among high‐risk children with chronic illness: a randomized clinical trial. JAMA. 2014;312(24):2640–2648.
- , , , et al. Children with medical complexity and Medicaid: spending and cost savings. Health Aff Proj Hope. 2014;33(12):2199–2206.
- Children's Hospital Association. CARE Award. Available at: https://www.childrenshospitals.org/Programs‐and‐Services/Quality‐Improvement‐and‐Measurement/CARE‐Award. Accessed December 18, 2015.
- , , , et al. Hospital readmission and parent perceptions of their child's hospital discharge. Int J Qual Health Care. 2013;25(5):573–581.
- , , , et al. Weekend matters: Friday and Saturday admissions are associated with prolonged hospitalization of children. Clin Pediatr (Phila). 2013;52(9):875–878.
- , , , . Attributable cost and length of stay for central line‐associated bloodstream infections. Pediatrics. 2014;133(6):e1525–e1532.
- , , , et al. Effect of healthcare‐acquired infection on length of hospital stay and cost. Infect Control Hosp Epidemiol. 2007;28(3):280–292.
- , , , , . Hospital utilization and costs among children with influenza, 2003. Am J Prev Med. 2009;36(4):292–296.
- , , , . Charges and lengths of stay attributable to adverse patient‐care events using pediatric‐specific quality indicators: a multicenter study of freestanding children's hospitals. Pediatrics. 2008;121(6):e1653–e1659.
- , , , , . Variation in resource utilization for the management of uncomplicated community‐acquired pneumonia across community and children's hospitals. J Pediatr. 2014;165(3):585–591.
- , , , , . Variation and outcomes associated with direct hospital admission among children with pneumonia in the United States. JAMA Pediatr. 2014;168(9):829–836.
- , , , et al. Clinical Risk Groups (CRGs): a classification system for risk‐adjusted capitation‐based payment and health care management. Med Care. 2004;42(1):81–90.
- , , , et al. Identifying children with lifelong chronic conditions for care coordination by using hospital discharge data. Acad Pediatr. 2010;10(6):417–423.
- , , , , . Profile of medical charges for children by health status group and severity level in a Washington State Health Plan. Health Serv Res. 2004;39(1):73–89.
- , , , . Using medical billing data to evaluate chronically ill children over time. J Ambulatory Care Manage. 2006;29(4):283–290.
- , , , , , . Medical complexity and pediatric emergency department and inpatient utilization. Pediatrics. 2013;131(2):e559–e565.
- , , , , . Pediatric complex chronic conditions classification system version 2: updated for ICD‐10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199.
- . Analyzing intensive care unit length of stay data: problems and possible solutions. Crit Care Med. 1997;25(9):1594–1600.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- . Hospital readmissions and repeat emergency department visits among children with medical complexity: an integrative review. J Pediatr Nurs. 2013;28(4):316–339.
- , , , . Hospital readmission in children with complex chronic conditions discharged from subacute care. Hosp Pediatr. 2014;4(3):153–158.
- , , , et al. Preventing hospitalizations in children with medical complexity: a systematic review. Pediatrics. 2014;134(6):e1628–e1647.
- , , , . Hospital readmissions for newly discharged pediatric home mechanical ventilation patients. Pediatr Pulmonol. 2012;47(4):409–414.
- , , , et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2008. JAMA. 2011;305(15):1560–1567.
- , , , et al. Trends in length of stay and short‐term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303(21):2141–2147.
- U.S. Department of Health and Human Services. CMS Statistics 2013. Available at: https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/CMS‐Statistics‐Reference‐Booklet/Downloads/CMS_Stats_2013_final.pdf. Published August 2013. Accessed October 6, 2015.
- Centers for Medicare and Medicaid Services. Evaluation of the premier hospital quality incentive demonstration. Available at: https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Reports/downloads/Premier_ExecSum_2010.pdf. Published March 3, 2009. Accessed September 18, 2015.
- , , , et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955–962; quiz 965–966.
- , , , , . Parent and provider perspectives on pediatric readmissions: what can we learn about readiness for discharge? Hosp Pediatr. 2015;5(11):559–565.
- , . Preventing readmissions in children: how do we do that? Hosp Pediatr. 2015;5(11):602–604.
- , , . Pediatric post‐acute hospital care: striving for identity and value. Hosp Pediatr. 2015;5(10):548–551.
- , , , et al. Pediatric hospital discharges to home health and postacute facility care: a national study. JAMA Pediatr. 2016;170(4):326–333.
- , , , et al. Bronchiolitis: clinical characteristics associated with hospitalization and length of stay. Pediatr Emerg Care. 2012;28(2):99–103.
- , , , , . The effect of transfers between health care facilities on costs and length of stay for pediatric burn patients. J Burn Care Res. 2015;36(1):178–183.
- , , , et al. Goal‐directed resuscitative interventions during pediatric interfacility transport. Crit Care Med. 2015;43(8):1692–1698.
- , , , et al. Inpatient growth and resource use in 28 children's hospitals: a longitudinal, multi‐institutional study. JAMA Pediatr. 2013;167(2):170–177.
- , , , et al. Children with complex chronic conditions in inpatient hospital settings in the united states. Pediatrics. 2010;126(4):647–655.
- , . Meeting the health care needs of persons with disabilities. Milbank Q. 2002;80(2):381–391.
- , , , et al. Effect of an enhanced medical home on serious illness and cost of care among high‐risk children with chronic illness: a randomized clinical trial. JAMA. 2014;312(24):2640–2648.
- , , , et al. Children with medical complexity and Medicaid: spending and cost savings. Health Aff Proj Hope. 2014;33(12):2199–2206.
- Children's Hospital Association. CARE Award. Available at: https://www.childrenshospitals.org/Programs‐and‐Services/Quality‐Improvement‐and‐Measurement/CARE‐Award. Accessed December 18, 2015.
- , , , et al. Hospital readmission and parent perceptions of their child's hospital discharge. Int J Qual Health Care. 2013;25(5):573–581.
- , , , et al. Weekend matters: Friday and Saturday admissions are associated with prolonged hospitalization of children. Clin Pediatr (Phila). 2013;52(9):875–878.
- , , , . Attributable cost and length of stay for central line‐associated bloodstream infections. Pediatrics. 2014;133(6):e1525–e1532.
- , , , et al. Effect of healthcare‐acquired infection on length of hospital stay and cost. Infect Control Hosp Epidemiol. 2007;28(3):280–292.
- , , , , . Hospital utilization and costs among children with influenza, 2003. Am J Prev Med. 2009;36(4):292–296.
- , , , . Charges and lengths of stay attributable to adverse patient‐care events using pediatric‐specific quality indicators: a multicenter study of freestanding children's hospitals. Pediatrics. 2008;121(6):e1653–e1659.
- , , , , . Variation in resource utilization for the management of uncomplicated community‐acquired pneumonia across community and children's hospitals. J Pediatr. 2014;165(3):585–591.
- , , , , . Variation and outcomes associated with direct hospital admission among children with pneumonia in the United States. JAMA Pediatr. 2014;168(9):829–836.
- , , , et al. Clinical Risk Groups (CRGs): a classification system for risk‐adjusted capitation‐based payment and health care management. Med Care. 2004;42(1):81–90.
- , , , et al. Identifying children with lifelong chronic conditions for care coordination by using hospital discharge data. Acad Pediatr. 2010;10(6):417–423.
- , , , , . Profile of medical charges for children by health status group and severity level in a Washington State Health Plan. Health Serv Res. 2004;39(1):73–89.
- , , , . Using medical billing data to evaluate chronically ill children over time. J Ambulatory Care Manage. 2006;29(4):283–290.
- , , , , , . Medical complexity and pediatric emergency department and inpatient utilization. Pediatrics. 2013;131(2):e559–e565.
- , , , , . Pediatric complex chronic conditions classification system version 2: updated for ICD‐10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199.
- . Analyzing intensive care unit length of stay data: problems and possible solutions. Crit Care Med. 1997;25(9):1594–1600.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- . Hospital readmissions and repeat emergency department visits among children with medical complexity: an integrative review. J Pediatr Nurs. 2013;28(4):316–339.
- , , , . Hospital readmission in children with complex chronic conditions discharged from subacute care. Hosp Pediatr. 2014;4(3):153–158.
- , , , et al. Preventing hospitalizations in children with medical complexity: a systematic review. Pediatrics. 2014;134(6):e1628–e1647.
- , , , . Hospital readmissions for newly discharged pediatric home mechanical ventilation patients. Pediatr Pulmonol. 2012;47(4):409–414.
- , , , et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2008. JAMA. 2011;305(15):1560–1567.
- , , , et al. Trends in length of stay and short‐term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303(21):2141–2147.
- U.S. Department of Health and Human Services. CMS Statistics 2013. Available at: https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/CMS‐Statistics‐Reference‐Booklet/Downloads/CMS_Stats_2013_final.pdf. Published August 2013. Accessed October 6, 2015.
- Centers for Medicare and Medicaid Services. Evaluation of the premier hospital quality incentive demonstration. Available at: https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Reports/downloads/Premier_ExecSum_2010.pdf. Published March 3, 2009. Accessed September 18, 2015.
- , , , et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955–962; quiz 965–966.
- , , , , . Parent and provider perspectives on pediatric readmissions: what can we learn about readiness for discharge? Hosp Pediatr. 2015;5(11):559–565.
- , . Preventing readmissions in children: how do we do that? Hosp Pediatr. 2015;5(11):602–604.
- , , . Pediatric post‐acute hospital care: striving for identity and value. Hosp Pediatr. 2015;5(10):548–551.
- , , , et al. Pediatric hospital discharges to home health and postacute facility care: a national study. JAMA Pediatr. 2016;170(4):326–333.
- , , , et al. Bronchiolitis: clinical characteristics associated with hospitalization and length of stay. Pediatr Emerg Care. 2012;28(2):99–103.
- , , , , . The effect of transfers between health care facilities on costs and length of stay for pediatric burn patients. J Burn Care Res. 2015;36(1):178–183.
- , , , et al. Goal‐directed resuscitative interventions during pediatric interfacility transport. Crit Care Med. 2015;43(8):1692–1698.
How to Manage Family-Centered Rounds
From the Department of Pediatrics, George Washington University and Children’s National Medical Center, Washington, DC (Dr. Kern), the Department of Pediatrics, Children’s Hospital Los Angeles and University of Southern California Keck School of Medicine, Los Angeles, CA (Dr. Gay), and the Department of Pediatrics, University of Texas Southwestern Medical Center and Children’s Health System, Dallas, TX (Dr. Mittal).
Abstract
- Objective: To present a model for operationalizing successful family-centered rounds (FCRs).
- Methods: Literature review and experience with leading FCR workshops at national meetings.
- Results: FCRs are multidisciplinary rounds that involve patients and families in decision-making. The model has gained substantial momentum nationally and is widely practiced in US pediatric hospitals. Many quality improvement–related FCR benefits have been identified, including improved parental satisfaction, communication, team-based practice, incorporation of practice guidelines, prevention of medication errors, and improved trainee and staff education and satisfaction. Physical and time constraints, variability in attending FCR style and teaching style, lack of FCR structure and process, specific and sensitive patient conditions, and language barriers are key challenges to implementing FCRs. Operationalizing a successful FCR program requires key stakeholders developing and defining a FCR process and structure, including developing a strong faculty development program.
- Conclusion: FCR benefits for a health care system are many. Key stakeholders involvement, developing FCR "ground rules," troubleshooting FCR barriers, and developing a strong faculty development program are key to managing successful FCRs.
The practice of medicine is a team sport and no team is complete without the patient and family being an integral part of it. Over the past 15 years, health care and the practice of medicine has slowly moved away from physician-centered care to patient- and family-centered care (FCC). This change has been a gradual shift in our culture and FCC has become a widely adopted philosophy within the US health care system [1]. FCC has been recognized and embraced by numerous medical and professional societies, including the Institute of Medicine (IOM), the American Academy of Pediatrics (AAP), and family advocacy organizations such as Family Voices and the Institute for Patient- and Family-Centered Care [1,2]. At its most basic, “family-centered care” occurs when patients/families and medical providers partner together to formulate medical plans that are built upon the sharing of open and unbiased information and that account for the diversity and individual strengths and needs of each patient and family unit [3]. FCC in the inpatient setting for hospitalized patients is most exemplified by the practice of family-centered (bedside) rounds, or FCRs [1].
Interestingly, FCC as a philosophy of care developed during a time when bedside rounds, and by extension clinical teaching, moved away from the bedside. Rounds are an integral part of how work is done in the inpatient setting. They come in many different flavors, from “pre-rounds” to “card-flip rounds” to “attending rounds,” “table/conference room rounds,” “hallway rounds,” “bedside rounds,” and the aforementioned family-centered rounds. In the first half of the 20th century,the majority of teaching rounds took place at the patient’s bedside, in the model advocated by Sir William Osler [4]. Indeed, as Dr. Osler wrote in 1903, “there should be no teaching without a patient for a text, and the best teaching is that taught by the patient himself” [5]. By the late 1970s through the mid-1990s, however, the proportion of clinical teaching occurring at the bedside had decreased to as low as 16% [6–8]. Many reasons behind the change have been speculated, including faculty comfort with lecture-based teaching and desire to control the content of teaching discussions, as well as technological advancement necessitating access to computers during case review.
In contrast, the patient-and family-centered movement began in the mid-20th century as a response to the separation trauma experienced by hospitalized children and their families [9]. Hospitals responded by liberalizing their visiting policies and encouraging direct care-giving by parents. FCC was further bolstered by consumer-led movements in the 1960s and 1970s, and by federal legislation in the 1980s targeting children with special health care needs. FCC gained national recognition in 2001 when the Institute of Medicine emphasized that involving patients and families in health care decisions increased the quality of their care [2]. Subsequently, the AAP endorsed FCC as a guiding approach to pediatric care in their 2003 report “Family-centered care and the pediatrician’s role” [1]. As part of this report, the AAP recommended that bedside presentations with active engagement of families become the standard of care. FCRs developed at several children’s hospitals in the US in the following years, with the first conceptual model of FCR published by Muething et al in 2007 [10].
Definition of Family-Centered Rounds
While no consensus definition of FCR exists, the most frequently cited description comes from Sisterhen et al who describe FCR as “interdisciplinary work rounds at the bedside in which the patient and family share in the control of the management plan as well as in the evaluation of the process itself” [11]. Three key features should be noted in this definition. First, FCR requires the active participation of family members, not merely their presence. In this way, patient and family voices are heard and their preferences solicited with respect to clinical decision-making. Second, FCR take place at the bedside, in alignment with the 2003 AAP policy statement that standard practice should be to conduct attending rounds with full case presentations in patient rooms in the presence of family. Third, FCR are typically interdisciplinary, involving patients and their families, physicians and trainees, nurses, and other ancillary staff (such as interpreters, case managers, and pharmacists) [1,10,11,12].
Since the IOM report, FCRs have gained substantial national momentum. A PRIS (Pediatric Research in Inpatient Setting) network study in 2010 published the first survey of pediatric hospitalist rounding practices in the US and Canada [12]. The study reported that 44% of pediatric hospitalists conducted FCRs, and about a quarter conducted rounds as hallway rounds or sit down rounds. Academic hospitalists were significantly more likely to conduct FCRs compared with non-academic (48% vs. 31%; P < 0.05) hospitalists. In accordance with Muething et al’s experience with FCRs in the Cincinnati model, the survey respondents did not associate FCR with prolonged rounding duration [10,12]. FCRs were also associated with greater bedside nurse participation [12]. Given the momentum behind FCC and the oft-cited benefits of FCR, it can only be presumed that the number of pediatric hospitals conducting FCR has significantly increased since the PRIS study was published in 2010.
FCRs Can Improve Quality of Care for Hospitalized Children
FCRs bring together multiple stakeholders involved in the patient’s care in the same place at the same time everyday. This allows for shared-decision making, identification of medical teams by families, and allows for direct and open communication between parents and medical teams [1,10–12]. The key stakeholders on a FCR team include the patient and family members and the medical team. The medical team includes attending physician, fellow, resident, and students, bedside nurse, care coordinator/case manager and other ancillary services. Although not enough data is available on who should attend rounds, case mangers and bedside nurse along with medical team and patients and families were found to be crucial in the general inpatient setting [12].
Integrating FCRs into the daily workflow in the inpatient setting provides several benefits for patients and families and the medical team, including trainees. Improvements in family-centered care principles, parental satisfaction, interdisciplinary team communication, efficiency, patient safety, and resident and medical student education have been reported consistently [9–23].
FCR Benefits for Patients and Families
Muething et al described increased patient-family satis-faction with higher levels of family participation in rounds and earlier discharge times [10]. On FCRs, families report having the opportunity to communicate directly with the entire care team, clarify misinformation and better understand care plans including discharge goals, leading to higher levels family satisfaction [10,14,24]. Both English and limited-English-proficient families report positive experiences with FCRs [21–23]. Families express appreciation with learning opportunities on FCRs, as well as the opportunity to serve as teachers to the medical team [14,16,21]. Families reported comfort with trainees being on rounds and appreciated seeing the medical personnel working as team [21]. They also report trust, comfort, and accountability towards the system and providers as they saw them working together as teams. They felt respected and involved as the medical teams involved them during rounds. Parents also report comfort with diversity of providers and feel that having multidisciplinary and diverse teams help with cultural competencies. Parents appreciated trainees being led by attending physician and felt that attending FCRs made them understand the medical process and the steps involved in caring for their child. They also reported that attending FCRs helps trainees learn about answering the kind of questions that parents usually ask. Contrary to the popular belief, parental participation has not increased the duration of FCRs and parental presence during rounds decreases time spent discussing each patient [14,25].
FCRs and Staff Satisfaction
Staff satisfaction with FCRs has been consistently high [13,14,18–23]. Nursing and medical staffs report valuing FCRs as they foster a sense of teamwork, improve understanding of the patient’s care plan and enhance communication between the care team and families [14]. FCRs significantly increase bedside nurse participation during rounds [12]. Presence of nursing and ancillary staff on FCRs improves efficiency by providing valuable information and helping address discharge goal [10]. Anecdotal data suggests that FCRs reduces number of pages trainees receive from nurses.
FCRs and Outcomes
FCRs have been perceived to improve in patient safety including errors in history taking and miscommunication, and incorrect information; and promote medication reconciliation, safety and adherence [17,20,21]. FCRs have shown to improve patient satisfaction, communication, and coordination of care and trainee education [10,14,21].
Educational Benefits of FCRs
families (Table 1) [26].
FCR Benefits for Hospitals and Health Care Systems
As health care prepares to fully adopt reforms and shift from volume-based to value-based payment systems, creating value in every patient encounter is vital. Conducting daily FCRs provide an dynamic venue for hospitals where daily rounds can incorporate evidence-based practice guidelines, prevent medication errors, ensure safety, reduce unnecessary tests and treatments, and improve transparency and accountability in care. This model can help hospital financially by meeting key quality and safety metrics and also help provide cost effective care through use and reinforcement of clinical pathways during rounds.
FCR Barriers
While many hospitals have adopted FCRs, many barriers to FCR implementation exist [10–14,18–23] (Table 1). Understanding these barriers and overcoming them are crucial for successful implementation. Conducting FCRs involve many aspects of care that happen during rounds. These include discussions about history, physical examinations, labs, and other tests; clinical decision-making and communication between parents and providers; team communication; teaching of trainees; discharge planning; and coordination of care [20]. Given all these aspects of care involved during rounds, being able to conduct multidisciplinary rounds in a timely and efficient way can be a challenge in a busy and dynamic inpatient setting.
Key identified FCR barriers have included physical constraints such as small patient rooms, large team size, patients being on multiple floors or units, infection control precautions leading to increased time involved with teams gowning and gloving; lack of training on FCRs for trainees and faculty; language and cultural barriers; family/patient concerns of privacy/disclosure of sensitive information; trainee’s fears of not appearing knowledgeable in front of families; and variability in attending physicians’ teaching style and approach to FCR [10–15,21].
Operationalizing Successful FCRs
Forming FCR Steering Committee: Developing Ground Rules
While there are many barriers to conducting efficient FCRs there are some that are unique to each institution. Therefore, for those institutions planning to initiate FCRs, the first step might be to form a FCR steering committee of key stakeholders who could review the current state, do a needs assessment for initiating FCRs, develop a structured and standardized FCR process and revise the FCR process periodically to meet the needs of the dynamic inpatient setting [10,12,14].
Defining and Identifying the FCR Process: Who, Where, and When of FCRs
The steering committee should clearly define FCRs and identify what FCRs would involve. For example, should FCRs involve complete case presentations and discussion in front of the parent or focused relevent H&P in a language that the parent understands? The steering committee should identify key elements/aspects of FCRs that would happen on daily rounds. For example: how should each patient receive information about FCRs? Should FCRs be offered to all patients? Do patients have options to opt-in or opt-out of FCRs on a daily basis or a one-time basis? Who should attend FCRs? For example, other than medical team, the bedside nurse and case manager should attend FCRs on a general pediatric service. Should the team round based on nursing assignments or resident assignments or in the order of room numbers? What should a typical rounding encounter involve? For example, each encounter should begin with the intern knocking on the door, asking parental permission for FCR team to enter the room, who should present, who should lead the rounds (the senior resident or the attending), who should stand where in the room? What should each encounter involve—for example, case presentation and discussion, parental involvement in decision-making, clarification of any parental questions, plan for that day, criteria for discharge and discharge needs assessment, teaching of resident and students, use of lay language etc. How should each rounding encounter end? Should the intern ask if parents have additional questions? It is important that the steering committee clearly identify these minute rounding details. Additionally, the committee should identify the rounding wards/area, the timing and duration of FCRs, how information about FCRs will be shared with patients and families, how trainees and attendees will be educated about FCRs and when are FCRs appropriate and when not. Defining the process early through stakeholder identification can reduce variability and create some standardization yet allow for individual style variations within the constraints of standardization. This will help reduced attending variability, which was cited as the most common FCR barrier by trainees.
As Seltz et al described, Latino families reported positive experiences with FCRs when a Spanish-speaking provider was involved. However, they report less satisfaction with telephone interpreters and did not feel empowered at times on FCRs due to language differences [23]. Addressing the language needs based on demographics and cultural needs will promote greater acceptance of FCRs [23].
Identifying and Defining Trainee Role
Participating in the FCR can create anxiety for medical students and residents. Therefore, educating them about the FCR process and structure beforehand and clearly defining roles can help them conceptualize their roles and expectation and ease their anxiety with FCRs. This will require the steering committee to collaboratively discuss how each encounter would look during FCR from a trainee’s perspective. Who will present the case? The third- year medical student versus the fourth-year medical student or the intern or based on case allocations? How should the case be presented? Should it be short and pointed presentation versus complete history and physical examination on each patient? How long should an encounter last on a new patient and on a follow-up patient? Who will examine the patient? The student who is presenting the case, the attending, the intern who overlooks the student, or the senior resident? Who will answer the follow-up questions from a parent initially? Should the senior resident lead the team under the attending guidance? How will the senior resident be prepared for morning rounds? Using lay language when talking to parents should be encouraged and taught to trainees routinely during FCRs.
Identifying and Defining Clinical Teaching Styles
Faculty Development Program and Importance of “Safe Environment”
Developing an educational program to train faculty, trainee and staff about FCRs can help streamline FCRs. Conducting FCRs is a cultural change and focusing on early adopters is crucial. Muething et al’s model showed better acceptance of FCRs by interns than by senior residents. Being patient during change management is key to successful implementation. Anecdotal discussions during PAS workshops suggests that on an average programs have required 3 years to get significant buy-in and streamlining of FCRs [10,12].
Suboptimal attending behavior such as attending variability in the FCRs process and teaching strategies have been reported as FCR barriers [14,21]. Residents report attending physician as an important factor determining success of FCRs. As attending physicians typically are the leaders of the FCRs team, training faculty about conducting effective and efficient FCRs is crucial to successful FCRs. [12,21]. Key aspects of faculty development should include: (1) education about the FCR standard process for the institution, (2) importance of time management during rounds, including tips and strategies to be efficient, (3) teaching styles during FCRs, including demonstrating role modeling, and (4) direct observation of trainees and individual and team feedback to streamline FCRs. Role-plays or simulated FCRs might be a venue to explore for faculty development on FCRs [14,21].
Creating a “safe environment” during FCRs where each person feels comfortable and secure is vital to team work [7,12,21]. Often trainees are apprehensive or afraid due to medical hierarchy and this might prevent developing a teaching and learning environment. Trainees fear not appearing knowledgeable in front of families and student rotate too often to adapt to different attending styles [21]. Therefore, reassuring trainees that the goal of FCRs is to conduct daily inpatient rounding to ensure key aspects of FCRs are met without disrespecting and insulting any person on rounds and clarifying and reassuring trainees that their fear of not appearing knowledgeable is real and it will be respected, might help create a safe environment where FCR teams are not only conducting the daily ritual of inpatient rounding, and teaching but also ensuring that trainees are enjoying being the clinician and physicians that they want to be. Therefore, attending role modeling is crucial and it is no surprise that in multiple studies variability in attending rounding and teaching style was identified consistently as a FCR barrier.
Preparing for Daily FCRS: Team Work, Efficiency, and Time Management
Conducting daily timely and efficient rounds require daily preparation by teams. Prior to FCRs, teams should know about all of the patients on whom FCRs will be conducted including those who refused FCRs, if any. This can be done via a pre-round or card-flip rounding method where the teams discuss key diagnoses, indication for admission, and identify any outliers to conducting FCRs such as sensitive patient condition, patients refused FCRs, etc. Some institutions have incorporated these at “morning check out” or at morning “huddles.” These help faculty avoid any last minute surprises during rounds and helps with time management during FCRs [12]. Faculty can then plan on some anticipated “teaching moments” before rounds to keep the rounds flowing, for example, a physical exam finding, a clarifying history that can clinch a diagnoses, a clinical pearl, a complex medical case where the parent might share their story and knowledge, an interesting interpretation of a lab, an x-ray or MRI finding. Faculties are multitasking during FCRs by diagnosing and managing patient and learners and leading effective efficient and timely rounds where parental questions are answered, orders are written, to-do work is identified, discharge planning and care coordination is done and trainees stay focused and attend noon conference on time. This requires thoughtful planning before starting FCRs. Time management and managing priorities is key to positive team experiences of FCRs. Both starting and ending FCRs on time should be emphasized and reinforced continually.
Nurse Preparation for FCRs
Nurses are the frontline providers and educating them about FCRs process can help them better explain FCRs to patients and families. Nurses often know the minute details such as timing of an MRI, if the patient has vomited in the morning, or when the parents are coming, etc. This important information sharing during FCRs can help team prepare for the day and provide patients and families’ expectations for the day. Nursing participation can also enhance their knowledge about the thought process behind decisions and care plans and avoid additional time paging house staff to obtain clarification [12–15,21].
Trainee Preparation for FCRs
While pediatric residents do report that FCRs leads to fewer requests for clarifications from families and nurses after FCRs, many still harbor concerns about the time required for FCRs and the overall efficiency of rounds [14]. Educating trainees about the FCR process and explaining why FCRs are beneficial can help alleviate trainee anxiety around FCRs. Involving trainees in the FCR communication and creating a safe and nurturing environment during FCRs can further reduce trainee anxiety [21]. Parents who have attended FCRs with trainees report understanding that trainees are in training and that they have felt comfortable to see attending physician lead the trainees.
FCRs and Technology
Use of technology during FCRs can be helpful to write orders in real time, follow-up and share lab values and or imaging study with parents or teach students. The increasing use of technology on FCRs, such as computers and handheld devices, can help with rounding and teaching; however, it also has the potential to be a distractor and requires that the medical team remain vigilant that the patient and family are the focus of FCRs [26].
Efficiency Pearls
Certain strategies can be utilized to keep FCRs efficient:
- Orient the FCR team about FCR process
- Identify rounding sequence for the day so team can move efficiently between rooms. Identifying potential discharges for the following morning and discharging those patients before rounds can reduce rounding census and provide additional rounding time. Teams can identify approximate time spent in each room based on census, as rounding time is constant.
- Starting and ending FCRs at the allocated time is key to success of FCRs. Sometimes this might require the attending and senior resident splitting the last 1–2 patients to finish rounds on time.
- Prepare students and interns for effective and efficient yet complete presentations during rounds that reflect their knowledge and thought process rather than presenting the entire H&P.
- Keep teaching during rounds focused. As a resident reported, “attendings should keep it short and not go off on a half hour lecture during FCRs. On FCRs I want to hear bam…bam…bam! tidbits, little hints, clinical pearls. Things that you would not know and only see and know when you were there in the room [21].”
- Encourage and teach senior residents’ role as a leader and teacher [21].
- With a situation requiring more time talking to families, request to go back later in the afternoon so as to stay on track on FCR time.
- Faculty can review lab results and history and physical findings on new admissions before rounds to avoid surprises during FCRs and to save time. This can be done during pre-round/card flip/or morning huddle.
Limitations
This article is based on the authors’ review of literature, experience in conducting FCRs, and experience from leading and attending FCR-related workshops at annual pediatric academic societies’ meetings and annual pediatric hospital medicine meetings between 2010 and 2015. There are several limitations to this work. Firstly, the majority of FCR literature is based on perceptions and are not measured outcomes. In addition, how FCRs will apply on services with complex patients needs more study. Different institutions have different physical constraints as well as sociodemographic and cultural factors that might affect FCRs. Daily census among hospitals varies and rounding duration may vary for them.
Conclusion
Family-centered rounds are widely accepted among pediatric hospitalists in the US. Reported benefits of FCRs include improved parent satisfaction, communication, better team communication, improved patient safety and better education for trainees. Many barriers to efficient FCRs exist, and for programs planning to incorporate FCRs in their daily rounds it is crucial to understand FCR benefits and barriers and assess their current state, including physical environment, when planning FCRs. Having a period to plan for FCR implementation through key stakeholder involvement helps define FCR process and lay down a conceptual model suited to individual organization. Educating the team members including families about FCRs and developing a strong faculty development program can further strengthen FCR implementation. Special focus should be given to time management, teaching styles during FCRs, and creating a safe and nurturing environment for FCRs to succeed.
Corresponding author: Vineeta Mittal, MD, MBA, 1935 Medical District Dr., Dallas, TX 75235, [email protected].
1. American Academy of Pediatrics Committee on Hospital Care. Family-centered care and the pediatrician’s role. Pediatrics 2003;112:691–7.
2. Institute of Medicine, Committee on Quality Health Care in America. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: The National Academies Press; 2001.
3. Kuo DZ, Joutrow AJ, Arango P, et al. Family-centered care: current applications and future directions in pediatric health care. Matern Child Health J 2012;16:297–305.
4. Reichsman F, Browning FE, Hinshaw JR. Observations of undergraduate clinical teaching in action. J Med Educ 1964;39:147–63.
5. Osler W. On the need of a radical reform in our methods of teaching senior students. Med News 1903;82:49–53.
6. Collins GF, Cassie JM, Dagget CJ. The role of the attending physician in clinical training. J Med Educ 1978;53:429–31.
7. Lacombe MA. On bedside teaching. Ann Intern Med 1997;126:217–20.
8. Linfors EW, Neelon FA. Sounding board. The case of bedside rounds. N Engl J Med 1980;303:1230–3.
9. Jolley J, Shields J. The evolution of family-centered care. J Pediatr Nursing 2009;42:164–70.
10. Muething SE, Kotagal UR, Schoettker PJ, et al. Family-centered rounds: a new approach to patient care and teaching. Pediatrics 2007;119:829–32.
11. Sisterhen LL, Blaszak RT, Woods MB, Smith CE. Defining family-centered rounds. Teach Learn Med 2007;19:319–22.
12. Mittal V, Sigrest T, Ottolini M, et al. Family-centered rounds on pediatric wards: a PRIS network survey of Canadian and US hospitalists. Pediatrics 2010;126:37–43.
13. Rosen P, Stenger E, Bochkoris M, et al. Family-centered multidisciplinary rounds enhance the team approach in pediatrics. Pediatrics 2009;123:e603–8.
14. Rappaport DI, Ketterer TA, Nilforoshan V, Sharif I. Family-centered rounds: views of families, nurses, trainees, and attending physicians. Clin Pediatr (Phila) 2012;51:260–6.
15. Young HN, Schumacher JB, Moreno MA, et al. Medical student self-efficacy with family-centered care during bedside rounds. Acad Med 2012;87:767–75.
16. Beck J, Meyer R, Kind T, Bhansali B. The importance of situational awareness: a qualitative study of family members’ and nurses’ perspectives on teaching during family-centered rounds. Acad Med 2015 Jul 21. Epub ahead of print.
17. Benjamin J, Cox E, Trapskin P, et al. Family-initiated dialogue about medicaitons during family-centered rounds. Pediatrics 2015;135:94–100.
18. Cox E, Schumacher J, Young H, et al. Medical student outcomes after family-centered bedside rounds. Acad Pediatri 2011;11:403–8.
19. Latta LC, Dick R, Parry C, Tamura GS. Parental responses to involvement in rounds on a pediatric inpatient unit at a teaching hospital: a qualitative study. Acad Med 2008;83:292–7.
20. Mittal V. Family-centered rounds. Pediatr Clin North Am 2014;61:663–70.
21. Mittal V, Krieger E, Lee B, et al. Pediatric residents’ perspectives on family-centered rounds - a qualitative study at 2 children’s hospitals. J Grad Med Educ 2013;5:81–7.
22. Lion KC, Mangione-Smith R, Martyn M, et al. Comprehension on family-centered rounds for limited English proficient families. Acad Pediatr 2013;13:236–42.
23. Seltz LB, Zimmer L, Ochoa-Nunez L, et al. Latino families’ experiences with family-centered rounds at an academic children’s hospital. Acad Pediatr 2011;11:432–8.
24. Kuo DZ, Sisterhen LL, Sigrest TE, et al. Family experiences and pediatric health services use associated with family-centered rounds. Pediatrics 2012;130:299–305.
25. Bhansali P, Birch S, Campbell JK, et al. A time-motion study of inpatient rounds using a family-centered rounds model. Hosp Pediatr 2013;3:31–8.
26. Kern J, Bhansali P. Handheld electronic device use by pediatric hospitalists on family centered rounds. J Med Syst 2016;40:9.
From the Department of Pediatrics, George Washington University and Children’s National Medical Center, Washington, DC (Dr. Kern), the Department of Pediatrics, Children’s Hospital Los Angeles and University of Southern California Keck School of Medicine, Los Angeles, CA (Dr. Gay), and the Department of Pediatrics, University of Texas Southwestern Medical Center and Children’s Health System, Dallas, TX (Dr. Mittal).
Abstract
- Objective: To present a model for operationalizing successful family-centered rounds (FCRs).
- Methods: Literature review and experience with leading FCR workshops at national meetings.
- Results: FCRs are multidisciplinary rounds that involve patients and families in decision-making. The model has gained substantial momentum nationally and is widely practiced in US pediatric hospitals. Many quality improvement–related FCR benefits have been identified, including improved parental satisfaction, communication, team-based practice, incorporation of practice guidelines, prevention of medication errors, and improved trainee and staff education and satisfaction. Physical and time constraints, variability in attending FCR style and teaching style, lack of FCR structure and process, specific and sensitive patient conditions, and language barriers are key challenges to implementing FCRs. Operationalizing a successful FCR program requires key stakeholders developing and defining a FCR process and structure, including developing a strong faculty development program.
- Conclusion: FCR benefits for a health care system are many. Key stakeholders involvement, developing FCR "ground rules," troubleshooting FCR barriers, and developing a strong faculty development program are key to managing successful FCRs.
The practice of medicine is a team sport and no team is complete without the patient and family being an integral part of it. Over the past 15 years, health care and the practice of medicine has slowly moved away from physician-centered care to patient- and family-centered care (FCC). This change has been a gradual shift in our culture and FCC has become a widely adopted philosophy within the US health care system [1]. FCC has been recognized and embraced by numerous medical and professional societies, including the Institute of Medicine (IOM), the American Academy of Pediatrics (AAP), and family advocacy organizations such as Family Voices and the Institute for Patient- and Family-Centered Care [1,2]. At its most basic, “family-centered care” occurs when patients/families and medical providers partner together to formulate medical plans that are built upon the sharing of open and unbiased information and that account for the diversity and individual strengths and needs of each patient and family unit [3]. FCC in the inpatient setting for hospitalized patients is most exemplified by the practice of family-centered (bedside) rounds, or FCRs [1].
Interestingly, FCC as a philosophy of care developed during a time when bedside rounds, and by extension clinical teaching, moved away from the bedside. Rounds are an integral part of how work is done in the inpatient setting. They come in many different flavors, from “pre-rounds” to “card-flip rounds” to “attending rounds,” “table/conference room rounds,” “hallway rounds,” “bedside rounds,” and the aforementioned family-centered rounds. In the first half of the 20th century,the majority of teaching rounds took place at the patient’s bedside, in the model advocated by Sir William Osler [4]. Indeed, as Dr. Osler wrote in 1903, “there should be no teaching without a patient for a text, and the best teaching is that taught by the patient himself” [5]. By the late 1970s through the mid-1990s, however, the proportion of clinical teaching occurring at the bedside had decreased to as low as 16% [6–8]. Many reasons behind the change have been speculated, including faculty comfort with lecture-based teaching and desire to control the content of teaching discussions, as well as technological advancement necessitating access to computers during case review.
In contrast, the patient-and family-centered movement began in the mid-20th century as a response to the separation trauma experienced by hospitalized children and their families [9]. Hospitals responded by liberalizing their visiting policies and encouraging direct care-giving by parents. FCC was further bolstered by consumer-led movements in the 1960s and 1970s, and by federal legislation in the 1980s targeting children with special health care needs. FCC gained national recognition in 2001 when the Institute of Medicine emphasized that involving patients and families in health care decisions increased the quality of their care [2]. Subsequently, the AAP endorsed FCC as a guiding approach to pediatric care in their 2003 report “Family-centered care and the pediatrician’s role” [1]. As part of this report, the AAP recommended that bedside presentations with active engagement of families become the standard of care. FCRs developed at several children’s hospitals in the US in the following years, with the first conceptual model of FCR published by Muething et al in 2007 [10].
Definition of Family-Centered Rounds
While no consensus definition of FCR exists, the most frequently cited description comes from Sisterhen et al who describe FCR as “interdisciplinary work rounds at the bedside in which the patient and family share in the control of the management plan as well as in the evaluation of the process itself” [11]. Three key features should be noted in this definition. First, FCR requires the active participation of family members, not merely their presence. In this way, patient and family voices are heard and their preferences solicited with respect to clinical decision-making. Second, FCR take place at the bedside, in alignment with the 2003 AAP policy statement that standard practice should be to conduct attending rounds with full case presentations in patient rooms in the presence of family. Third, FCR are typically interdisciplinary, involving patients and their families, physicians and trainees, nurses, and other ancillary staff (such as interpreters, case managers, and pharmacists) [1,10,11,12].
Since the IOM report, FCRs have gained substantial national momentum. A PRIS (Pediatric Research in Inpatient Setting) network study in 2010 published the first survey of pediatric hospitalist rounding practices in the US and Canada [12]. The study reported that 44% of pediatric hospitalists conducted FCRs, and about a quarter conducted rounds as hallway rounds or sit down rounds. Academic hospitalists were significantly more likely to conduct FCRs compared with non-academic (48% vs. 31%; P < 0.05) hospitalists. In accordance with Muething et al’s experience with FCRs in the Cincinnati model, the survey respondents did not associate FCR with prolonged rounding duration [10,12]. FCRs were also associated with greater bedside nurse participation [12]. Given the momentum behind FCC and the oft-cited benefits of FCR, it can only be presumed that the number of pediatric hospitals conducting FCR has significantly increased since the PRIS study was published in 2010.
FCRs Can Improve Quality of Care for Hospitalized Children
FCRs bring together multiple stakeholders involved in the patient’s care in the same place at the same time everyday. This allows for shared-decision making, identification of medical teams by families, and allows for direct and open communication between parents and medical teams [1,10–12]. The key stakeholders on a FCR team include the patient and family members and the medical team. The medical team includes attending physician, fellow, resident, and students, bedside nurse, care coordinator/case manager and other ancillary services. Although not enough data is available on who should attend rounds, case mangers and bedside nurse along with medical team and patients and families were found to be crucial in the general inpatient setting [12].
Integrating FCRs into the daily workflow in the inpatient setting provides several benefits for patients and families and the medical team, including trainees. Improvements in family-centered care principles, parental satisfaction, interdisciplinary team communication, efficiency, patient safety, and resident and medical student education have been reported consistently [9–23].
FCR Benefits for Patients and Families
Muething et al described increased patient-family satis-faction with higher levels of family participation in rounds and earlier discharge times [10]. On FCRs, families report having the opportunity to communicate directly with the entire care team, clarify misinformation and better understand care plans including discharge goals, leading to higher levels family satisfaction [10,14,24]. Both English and limited-English-proficient families report positive experiences with FCRs [21–23]. Families express appreciation with learning opportunities on FCRs, as well as the opportunity to serve as teachers to the medical team [14,16,21]. Families reported comfort with trainees being on rounds and appreciated seeing the medical personnel working as team [21]. They also report trust, comfort, and accountability towards the system and providers as they saw them working together as teams. They felt respected and involved as the medical teams involved them during rounds. Parents also report comfort with diversity of providers and feel that having multidisciplinary and diverse teams help with cultural competencies. Parents appreciated trainees being led by attending physician and felt that attending FCRs made them understand the medical process and the steps involved in caring for their child. They also reported that attending FCRs helps trainees learn about answering the kind of questions that parents usually ask. Contrary to the popular belief, parental participation has not increased the duration of FCRs and parental presence during rounds decreases time spent discussing each patient [14,25].
FCRs and Staff Satisfaction
Staff satisfaction with FCRs has been consistently high [13,14,18–23]. Nursing and medical staffs report valuing FCRs as they foster a sense of teamwork, improve understanding of the patient’s care plan and enhance communication between the care team and families [14]. FCRs significantly increase bedside nurse participation during rounds [12]. Presence of nursing and ancillary staff on FCRs improves efficiency by providing valuable information and helping address discharge goal [10]. Anecdotal data suggests that FCRs reduces number of pages trainees receive from nurses.
FCRs and Outcomes
FCRs have been perceived to improve in patient safety including errors in history taking and miscommunication, and incorrect information; and promote medication reconciliation, safety and adherence [17,20,21]. FCRs have shown to improve patient satisfaction, communication, and coordination of care and trainee education [10,14,21].
Educational Benefits of FCRs
families (Table 1) [26].
FCR Benefits for Hospitals and Health Care Systems
As health care prepares to fully adopt reforms and shift from volume-based to value-based payment systems, creating value in every patient encounter is vital. Conducting daily FCRs provide an dynamic venue for hospitals where daily rounds can incorporate evidence-based practice guidelines, prevent medication errors, ensure safety, reduce unnecessary tests and treatments, and improve transparency and accountability in care. This model can help hospital financially by meeting key quality and safety metrics and also help provide cost effective care through use and reinforcement of clinical pathways during rounds.
FCR Barriers
While many hospitals have adopted FCRs, many barriers to FCR implementation exist [10–14,18–23] (Table 1). Understanding these barriers and overcoming them are crucial for successful implementation. Conducting FCRs involve many aspects of care that happen during rounds. These include discussions about history, physical examinations, labs, and other tests; clinical decision-making and communication between parents and providers; team communication; teaching of trainees; discharge planning; and coordination of care [20]. Given all these aspects of care involved during rounds, being able to conduct multidisciplinary rounds in a timely and efficient way can be a challenge in a busy and dynamic inpatient setting.
Key identified FCR barriers have included physical constraints such as small patient rooms, large team size, patients being on multiple floors or units, infection control precautions leading to increased time involved with teams gowning and gloving; lack of training on FCRs for trainees and faculty; language and cultural barriers; family/patient concerns of privacy/disclosure of sensitive information; trainee’s fears of not appearing knowledgeable in front of families; and variability in attending physicians’ teaching style and approach to FCR [10–15,21].
Operationalizing Successful FCRs
Forming FCR Steering Committee: Developing Ground Rules
While there are many barriers to conducting efficient FCRs there are some that are unique to each institution. Therefore, for those institutions planning to initiate FCRs, the first step might be to form a FCR steering committee of key stakeholders who could review the current state, do a needs assessment for initiating FCRs, develop a structured and standardized FCR process and revise the FCR process periodically to meet the needs of the dynamic inpatient setting [10,12,14].
Defining and Identifying the FCR Process: Who, Where, and When of FCRs
The steering committee should clearly define FCRs and identify what FCRs would involve. For example, should FCRs involve complete case presentations and discussion in front of the parent or focused relevent H&P in a language that the parent understands? The steering committee should identify key elements/aspects of FCRs that would happen on daily rounds. For example: how should each patient receive information about FCRs? Should FCRs be offered to all patients? Do patients have options to opt-in or opt-out of FCRs on a daily basis or a one-time basis? Who should attend FCRs? For example, other than medical team, the bedside nurse and case manager should attend FCRs on a general pediatric service. Should the team round based on nursing assignments or resident assignments or in the order of room numbers? What should a typical rounding encounter involve? For example, each encounter should begin with the intern knocking on the door, asking parental permission for FCR team to enter the room, who should present, who should lead the rounds (the senior resident or the attending), who should stand where in the room? What should each encounter involve—for example, case presentation and discussion, parental involvement in decision-making, clarification of any parental questions, plan for that day, criteria for discharge and discharge needs assessment, teaching of resident and students, use of lay language etc. How should each rounding encounter end? Should the intern ask if parents have additional questions? It is important that the steering committee clearly identify these minute rounding details. Additionally, the committee should identify the rounding wards/area, the timing and duration of FCRs, how information about FCRs will be shared with patients and families, how trainees and attendees will be educated about FCRs and when are FCRs appropriate and when not. Defining the process early through stakeholder identification can reduce variability and create some standardization yet allow for individual style variations within the constraints of standardization. This will help reduced attending variability, which was cited as the most common FCR barrier by trainees.
As Seltz et al described, Latino families reported positive experiences with FCRs when a Spanish-speaking provider was involved. However, they report less satisfaction with telephone interpreters and did not feel empowered at times on FCRs due to language differences [23]. Addressing the language needs based on demographics and cultural needs will promote greater acceptance of FCRs [23].
Identifying and Defining Trainee Role
Participating in the FCR can create anxiety for medical students and residents. Therefore, educating them about the FCR process and structure beforehand and clearly defining roles can help them conceptualize their roles and expectation and ease their anxiety with FCRs. This will require the steering committee to collaboratively discuss how each encounter would look during FCR from a trainee’s perspective. Who will present the case? The third- year medical student versus the fourth-year medical student or the intern or based on case allocations? How should the case be presented? Should it be short and pointed presentation versus complete history and physical examination on each patient? How long should an encounter last on a new patient and on a follow-up patient? Who will examine the patient? The student who is presenting the case, the attending, the intern who overlooks the student, or the senior resident? Who will answer the follow-up questions from a parent initially? Should the senior resident lead the team under the attending guidance? How will the senior resident be prepared for morning rounds? Using lay language when talking to parents should be encouraged and taught to trainees routinely during FCRs.
Identifying and Defining Clinical Teaching Styles
Faculty Development Program and Importance of “Safe Environment”
Developing an educational program to train faculty, trainee and staff about FCRs can help streamline FCRs. Conducting FCRs is a cultural change and focusing on early adopters is crucial. Muething et al’s model showed better acceptance of FCRs by interns than by senior residents. Being patient during change management is key to successful implementation. Anecdotal discussions during PAS workshops suggests that on an average programs have required 3 years to get significant buy-in and streamlining of FCRs [10,12].
Suboptimal attending behavior such as attending variability in the FCRs process and teaching strategies have been reported as FCR barriers [14,21]. Residents report attending physician as an important factor determining success of FCRs. As attending physicians typically are the leaders of the FCRs team, training faculty about conducting effective and efficient FCRs is crucial to successful FCRs. [12,21]. Key aspects of faculty development should include: (1) education about the FCR standard process for the institution, (2) importance of time management during rounds, including tips and strategies to be efficient, (3) teaching styles during FCRs, including demonstrating role modeling, and (4) direct observation of trainees and individual and team feedback to streamline FCRs. Role-plays or simulated FCRs might be a venue to explore for faculty development on FCRs [14,21].
Creating a “safe environment” during FCRs where each person feels comfortable and secure is vital to team work [7,12,21]. Often trainees are apprehensive or afraid due to medical hierarchy and this might prevent developing a teaching and learning environment. Trainees fear not appearing knowledgeable in front of families and student rotate too often to adapt to different attending styles [21]. Therefore, reassuring trainees that the goal of FCRs is to conduct daily inpatient rounding to ensure key aspects of FCRs are met without disrespecting and insulting any person on rounds and clarifying and reassuring trainees that their fear of not appearing knowledgeable is real and it will be respected, might help create a safe environment where FCR teams are not only conducting the daily ritual of inpatient rounding, and teaching but also ensuring that trainees are enjoying being the clinician and physicians that they want to be. Therefore, attending role modeling is crucial and it is no surprise that in multiple studies variability in attending rounding and teaching style was identified consistently as a FCR barrier.
Preparing for Daily FCRS: Team Work, Efficiency, and Time Management
Conducting daily timely and efficient rounds require daily preparation by teams. Prior to FCRs, teams should know about all of the patients on whom FCRs will be conducted including those who refused FCRs, if any. This can be done via a pre-round or card-flip rounding method where the teams discuss key diagnoses, indication for admission, and identify any outliers to conducting FCRs such as sensitive patient condition, patients refused FCRs, etc. Some institutions have incorporated these at “morning check out” or at morning “huddles.” These help faculty avoid any last minute surprises during rounds and helps with time management during FCRs [12]. Faculty can then plan on some anticipated “teaching moments” before rounds to keep the rounds flowing, for example, a physical exam finding, a clarifying history that can clinch a diagnoses, a clinical pearl, a complex medical case where the parent might share their story and knowledge, an interesting interpretation of a lab, an x-ray or MRI finding. Faculties are multitasking during FCRs by diagnosing and managing patient and learners and leading effective efficient and timely rounds where parental questions are answered, orders are written, to-do work is identified, discharge planning and care coordination is done and trainees stay focused and attend noon conference on time. This requires thoughtful planning before starting FCRs. Time management and managing priorities is key to positive team experiences of FCRs. Both starting and ending FCRs on time should be emphasized and reinforced continually.
Nurse Preparation for FCRs
Nurses are the frontline providers and educating them about FCRs process can help them better explain FCRs to patients and families. Nurses often know the minute details such as timing of an MRI, if the patient has vomited in the morning, or when the parents are coming, etc. This important information sharing during FCRs can help team prepare for the day and provide patients and families’ expectations for the day. Nursing participation can also enhance their knowledge about the thought process behind decisions and care plans and avoid additional time paging house staff to obtain clarification [12–15,21].
Trainee Preparation for FCRs
While pediatric residents do report that FCRs leads to fewer requests for clarifications from families and nurses after FCRs, many still harbor concerns about the time required for FCRs and the overall efficiency of rounds [14]. Educating trainees about the FCR process and explaining why FCRs are beneficial can help alleviate trainee anxiety around FCRs. Involving trainees in the FCR communication and creating a safe and nurturing environment during FCRs can further reduce trainee anxiety [21]. Parents who have attended FCRs with trainees report understanding that trainees are in training and that they have felt comfortable to see attending physician lead the trainees.
FCRs and Technology
Use of technology during FCRs can be helpful to write orders in real time, follow-up and share lab values and or imaging study with parents or teach students. The increasing use of technology on FCRs, such as computers and handheld devices, can help with rounding and teaching; however, it also has the potential to be a distractor and requires that the medical team remain vigilant that the patient and family are the focus of FCRs [26].
Efficiency Pearls
Certain strategies can be utilized to keep FCRs efficient:
- Orient the FCR team about FCR process
- Identify rounding sequence for the day so team can move efficiently between rooms. Identifying potential discharges for the following morning and discharging those patients before rounds can reduce rounding census and provide additional rounding time. Teams can identify approximate time spent in each room based on census, as rounding time is constant.
- Starting and ending FCRs at the allocated time is key to success of FCRs. Sometimes this might require the attending and senior resident splitting the last 1–2 patients to finish rounds on time.
- Prepare students and interns for effective and efficient yet complete presentations during rounds that reflect their knowledge and thought process rather than presenting the entire H&P.
- Keep teaching during rounds focused. As a resident reported, “attendings should keep it short and not go off on a half hour lecture during FCRs. On FCRs I want to hear bam…bam…bam! tidbits, little hints, clinical pearls. Things that you would not know and only see and know when you were there in the room [21].”
- Encourage and teach senior residents’ role as a leader and teacher [21].
- With a situation requiring more time talking to families, request to go back later in the afternoon so as to stay on track on FCR time.
- Faculty can review lab results and history and physical findings on new admissions before rounds to avoid surprises during FCRs and to save time. This can be done during pre-round/card flip/or morning huddle.
Limitations
This article is based on the authors’ review of literature, experience in conducting FCRs, and experience from leading and attending FCR-related workshops at annual pediatric academic societies’ meetings and annual pediatric hospital medicine meetings between 2010 and 2015. There are several limitations to this work. Firstly, the majority of FCR literature is based on perceptions and are not measured outcomes. In addition, how FCRs will apply on services with complex patients needs more study. Different institutions have different physical constraints as well as sociodemographic and cultural factors that might affect FCRs. Daily census among hospitals varies and rounding duration may vary for them.
Conclusion
Family-centered rounds are widely accepted among pediatric hospitalists in the US. Reported benefits of FCRs include improved parent satisfaction, communication, better team communication, improved patient safety and better education for trainees. Many barriers to efficient FCRs exist, and for programs planning to incorporate FCRs in their daily rounds it is crucial to understand FCR benefits and barriers and assess their current state, including physical environment, when planning FCRs. Having a period to plan for FCR implementation through key stakeholder involvement helps define FCR process and lay down a conceptual model suited to individual organization. Educating the team members including families about FCRs and developing a strong faculty development program can further strengthen FCR implementation. Special focus should be given to time management, teaching styles during FCRs, and creating a safe and nurturing environment for FCRs to succeed.
Corresponding author: Vineeta Mittal, MD, MBA, 1935 Medical District Dr., Dallas, TX 75235, [email protected].
From the Department of Pediatrics, George Washington University and Children’s National Medical Center, Washington, DC (Dr. Kern), the Department of Pediatrics, Children’s Hospital Los Angeles and University of Southern California Keck School of Medicine, Los Angeles, CA (Dr. Gay), and the Department of Pediatrics, University of Texas Southwestern Medical Center and Children’s Health System, Dallas, TX (Dr. Mittal).
Abstract
- Objective: To present a model for operationalizing successful family-centered rounds (FCRs).
- Methods: Literature review and experience with leading FCR workshops at national meetings.
- Results: FCRs are multidisciplinary rounds that involve patients and families in decision-making. The model has gained substantial momentum nationally and is widely practiced in US pediatric hospitals. Many quality improvement–related FCR benefits have been identified, including improved parental satisfaction, communication, team-based practice, incorporation of practice guidelines, prevention of medication errors, and improved trainee and staff education and satisfaction. Physical and time constraints, variability in attending FCR style and teaching style, lack of FCR structure and process, specific and sensitive patient conditions, and language barriers are key challenges to implementing FCRs. Operationalizing a successful FCR program requires key stakeholders developing and defining a FCR process and structure, including developing a strong faculty development program.
- Conclusion: FCR benefits for a health care system are many. Key stakeholders involvement, developing FCR "ground rules," troubleshooting FCR barriers, and developing a strong faculty development program are key to managing successful FCRs.
The practice of medicine is a team sport and no team is complete without the patient and family being an integral part of it. Over the past 15 years, health care and the practice of medicine has slowly moved away from physician-centered care to patient- and family-centered care (FCC). This change has been a gradual shift in our culture and FCC has become a widely adopted philosophy within the US health care system [1]. FCC has been recognized and embraced by numerous medical and professional societies, including the Institute of Medicine (IOM), the American Academy of Pediatrics (AAP), and family advocacy organizations such as Family Voices and the Institute for Patient- and Family-Centered Care [1,2]. At its most basic, “family-centered care” occurs when patients/families and medical providers partner together to formulate medical plans that are built upon the sharing of open and unbiased information and that account for the diversity and individual strengths and needs of each patient and family unit [3]. FCC in the inpatient setting for hospitalized patients is most exemplified by the practice of family-centered (bedside) rounds, or FCRs [1].
Interestingly, FCC as a philosophy of care developed during a time when bedside rounds, and by extension clinical teaching, moved away from the bedside. Rounds are an integral part of how work is done in the inpatient setting. They come in many different flavors, from “pre-rounds” to “card-flip rounds” to “attending rounds,” “table/conference room rounds,” “hallway rounds,” “bedside rounds,” and the aforementioned family-centered rounds. In the first half of the 20th century,the majority of teaching rounds took place at the patient’s bedside, in the model advocated by Sir William Osler [4]. Indeed, as Dr. Osler wrote in 1903, “there should be no teaching without a patient for a text, and the best teaching is that taught by the patient himself” [5]. By the late 1970s through the mid-1990s, however, the proportion of clinical teaching occurring at the bedside had decreased to as low as 16% [6–8]. Many reasons behind the change have been speculated, including faculty comfort with lecture-based teaching and desire to control the content of teaching discussions, as well as technological advancement necessitating access to computers during case review.
In contrast, the patient-and family-centered movement began in the mid-20th century as a response to the separation trauma experienced by hospitalized children and their families [9]. Hospitals responded by liberalizing their visiting policies and encouraging direct care-giving by parents. FCC was further bolstered by consumer-led movements in the 1960s and 1970s, and by federal legislation in the 1980s targeting children with special health care needs. FCC gained national recognition in 2001 when the Institute of Medicine emphasized that involving patients and families in health care decisions increased the quality of their care [2]. Subsequently, the AAP endorsed FCC as a guiding approach to pediatric care in their 2003 report “Family-centered care and the pediatrician’s role” [1]. As part of this report, the AAP recommended that bedside presentations with active engagement of families become the standard of care. FCRs developed at several children’s hospitals in the US in the following years, with the first conceptual model of FCR published by Muething et al in 2007 [10].
Definition of Family-Centered Rounds
While no consensus definition of FCR exists, the most frequently cited description comes from Sisterhen et al who describe FCR as “interdisciplinary work rounds at the bedside in which the patient and family share in the control of the management plan as well as in the evaluation of the process itself” [11]. Three key features should be noted in this definition. First, FCR requires the active participation of family members, not merely their presence. In this way, patient and family voices are heard and their preferences solicited with respect to clinical decision-making. Second, FCR take place at the bedside, in alignment with the 2003 AAP policy statement that standard practice should be to conduct attending rounds with full case presentations in patient rooms in the presence of family. Third, FCR are typically interdisciplinary, involving patients and their families, physicians and trainees, nurses, and other ancillary staff (such as interpreters, case managers, and pharmacists) [1,10,11,12].
Since the IOM report, FCRs have gained substantial national momentum. A PRIS (Pediatric Research in Inpatient Setting) network study in 2010 published the first survey of pediatric hospitalist rounding practices in the US and Canada [12]. The study reported that 44% of pediatric hospitalists conducted FCRs, and about a quarter conducted rounds as hallway rounds or sit down rounds. Academic hospitalists were significantly more likely to conduct FCRs compared with non-academic (48% vs. 31%; P < 0.05) hospitalists. In accordance with Muething et al’s experience with FCRs in the Cincinnati model, the survey respondents did not associate FCR with prolonged rounding duration [10,12]. FCRs were also associated with greater bedside nurse participation [12]. Given the momentum behind FCC and the oft-cited benefits of FCR, it can only be presumed that the number of pediatric hospitals conducting FCR has significantly increased since the PRIS study was published in 2010.
FCRs Can Improve Quality of Care for Hospitalized Children
FCRs bring together multiple stakeholders involved in the patient’s care in the same place at the same time everyday. This allows for shared-decision making, identification of medical teams by families, and allows for direct and open communication between parents and medical teams [1,10–12]. The key stakeholders on a FCR team include the patient and family members and the medical team. The medical team includes attending physician, fellow, resident, and students, bedside nurse, care coordinator/case manager and other ancillary services. Although not enough data is available on who should attend rounds, case mangers and bedside nurse along with medical team and patients and families were found to be crucial in the general inpatient setting [12].
Integrating FCRs into the daily workflow in the inpatient setting provides several benefits for patients and families and the medical team, including trainees. Improvements in family-centered care principles, parental satisfaction, interdisciplinary team communication, efficiency, patient safety, and resident and medical student education have been reported consistently [9–23].
FCR Benefits for Patients and Families
Muething et al described increased patient-family satis-faction with higher levels of family participation in rounds and earlier discharge times [10]. On FCRs, families report having the opportunity to communicate directly with the entire care team, clarify misinformation and better understand care plans including discharge goals, leading to higher levels family satisfaction [10,14,24]. Both English and limited-English-proficient families report positive experiences with FCRs [21–23]. Families express appreciation with learning opportunities on FCRs, as well as the opportunity to serve as teachers to the medical team [14,16,21]. Families reported comfort with trainees being on rounds and appreciated seeing the medical personnel working as team [21]. They also report trust, comfort, and accountability towards the system and providers as they saw them working together as teams. They felt respected and involved as the medical teams involved them during rounds. Parents also report comfort with diversity of providers and feel that having multidisciplinary and diverse teams help with cultural competencies. Parents appreciated trainees being led by attending physician and felt that attending FCRs made them understand the medical process and the steps involved in caring for their child. They also reported that attending FCRs helps trainees learn about answering the kind of questions that parents usually ask. Contrary to the popular belief, parental participation has not increased the duration of FCRs and parental presence during rounds decreases time spent discussing each patient [14,25].
FCRs and Staff Satisfaction
Staff satisfaction with FCRs has been consistently high [13,14,18–23]. Nursing and medical staffs report valuing FCRs as they foster a sense of teamwork, improve understanding of the patient’s care plan and enhance communication between the care team and families [14]. FCRs significantly increase bedside nurse participation during rounds [12]. Presence of nursing and ancillary staff on FCRs improves efficiency by providing valuable information and helping address discharge goal [10]. Anecdotal data suggests that FCRs reduces number of pages trainees receive from nurses.
FCRs and Outcomes
FCRs have been perceived to improve in patient safety including errors in history taking and miscommunication, and incorrect information; and promote medication reconciliation, safety and adherence [17,20,21]. FCRs have shown to improve patient satisfaction, communication, and coordination of care and trainee education [10,14,21].
Educational Benefits of FCRs
families (Table 1) [26].
FCR Benefits for Hospitals and Health Care Systems
As health care prepares to fully adopt reforms and shift from volume-based to value-based payment systems, creating value in every patient encounter is vital. Conducting daily FCRs provide an dynamic venue for hospitals where daily rounds can incorporate evidence-based practice guidelines, prevent medication errors, ensure safety, reduce unnecessary tests and treatments, and improve transparency and accountability in care. This model can help hospital financially by meeting key quality and safety metrics and also help provide cost effective care through use and reinforcement of clinical pathways during rounds.
FCR Barriers
While many hospitals have adopted FCRs, many barriers to FCR implementation exist [10–14,18–23] (Table 1). Understanding these barriers and overcoming them are crucial for successful implementation. Conducting FCRs involve many aspects of care that happen during rounds. These include discussions about history, physical examinations, labs, and other tests; clinical decision-making and communication between parents and providers; team communication; teaching of trainees; discharge planning; and coordination of care [20]. Given all these aspects of care involved during rounds, being able to conduct multidisciplinary rounds in a timely and efficient way can be a challenge in a busy and dynamic inpatient setting.
Key identified FCR barriers have included physical constraints such as small patient rooms, large team size, patients being on multiple floors or units, infection control precautions leading to increased time involved with teams gowning and gloving; lack of training on FCRs for trainees and faculty; language and cultural barriers; family/patient concerns of privacy/disclosure of sensitive information; trainee’s fears of not appearing knowledgeable in front of families; and variability in attending physicians’ teaching style and approach to FCR [10–15,21].
Operationalizing Successful FCRs
Forming FCR Steering Committee: Developing Ground Rules
While there are many barriers to conducting efficient FCRs there are some that are unique to each institution. Therefore, for those institutions planning to initiate FCRs, the first step might be to form a FCR steering committee of key stakeholders who could review the current state, do a needs assessment for initiating FCRs, develop a structured and standardized FCR process and revise the FCR process periodically to meet the needs of the dynamic inpatient setting [10,12,14].
Defining and Identifying the FCR Process: Who, Where, and When of FCRs
The steering committee should clearly define FCRs and identify what FCRs would involve. For example, should FCRs involve complete case presentations and discussion in front of the parent or focused relevent H&P in a language that the parent understands? The steering committee should identify key elements/aspects of FCRs that would happen on daily rounds. For example: how should each patient receive information about FCRs? Should FCRs be offered to all patients? Do patients have options to opt-in or opt-out of FCRs on a daily basis or a one-time basis? Who should attend FCRs? For example, other than medical team, the bedside nurse and case manager should attend FCRs on a general pediatric service. Should the team round based on nursing assignments or resident assignments or in the order of room numbers? What should a typical rounding encounter involve? For example, each encounter should begin with the intern knocking on the door, asking parental permission for FCR team to enter the room, who should present, who should lead the rounds (the senior resident or the attending), who should stand where in the room? What should each encounter involve—for example, case presentation and discussion, parental involvement in decision-making, clarification of any parental questions, plan for that day, criteria for discharge and discharge needs assessment, teaching of resident and students, use of lay language etc. How should each rounding encounter end? Should the intern ask if parents have additional questions? It is important that the steering committee clearly identify these minute rounding details. Additionally, the committee should identify the rounding wards/area, the timing and duration of FCRs, how information about FCRs will be shared with patients and families, how trainees and attendees will be educated about FCRs and when are FCRs appropriate and when not. Defining the process early through stakeholder identification can reduce variability and create some standardization yet allow for individual style variations within the constraints of standardization. This will help reduced attending variability, which was cited as the most common FCR barrier by trainees.
As Seltz et al described, Latino families reported positive experiences with FCRs when a Spanish-speaking provider was involved. However, they report less satisfaction with telephone interpreters and did not feel empowered at times on FCRs due to language differences [23]. Addressing the language needs based on demographics and cultural needs will promote greater acceptance of FCRs [23].
Identifying and Defining Trainee Role
Participating in the FCR can create anxiety for medical students and residents. Therefore, educating them about the FCR process and structure beforehand and clearly defining roles can help them conceptualize their roles and expectation and ease their anxiety with FCRs. This will require the steering committee to collaboratively discuss how each encounter would look during FCR from a trainee’s perspective. Who will present the case? The third- year medical student versus the fourth-year medical student or the intern or based on case allocations? How should the case be presented? Should it be short and pointed presentation versus complete history and physical examination on each patient? How long should an encounter last on a new patient and on a follow-up patient? Who will examine the patient? The student who is presenting the case, the attending, the intern who overlooks the student, or the senior resident? Who will answer the follow-up questions from a parent initially? Should the senior resident lead the team under the attending guidance? How will the senior resident be prepared for morning rounds? Using lay language when talking to parents should be encouraged and taught to trainees routinely during FCRs.
Identifying and Defining Clinical Teaching Styles
Faculty Development Program and Importance of “Safe Environment”
Developing an educational program to train faculty, trainee and staff about FCRs can help streamline FCRs. Conducting FCRs is a cultural change and focusing on early adopters is crucial. Muething et al’s model showed better acceptance of FCRs by interns than by senior residents. Being patient during change management is key to successful implementation. Anecdotal discussions during PAS workshops suggests that on an average programs have required 3 years to get significant buy-in and streamlining of FCRs [10,12].
Suboptimal attending behavior such as attending variability in the FCRs process and teaching strategies have been reported as FCR barriers [14,21]. Residents report attending physician as an important factor determining success of FCRs. As attending physicians typically are the leaders of the FCRs team, training faculty about conducting effective and efficient FCRs is crucial to successful FCRs. [12,21]. Key aspects of faculty development should include: (1) education about the FCR standard process for the institution, (2) importance of time management during rounds, including tips and strategies to be efficient, (3) teaching styles during FCRs, including demonstrating role modeling, and (4) direct observation of trainees and individual and team feedback to streamline FCRs. Role-plays or simulated FCRs might be a venue to explore for faculty development on FCRs [14,21].
Creating a “safe environment” during FCRs where each person feels comfortable and secure is vital to team work [7,12,21]. Often trainees are apprehensive or afraid due to medical hierarchy and this might prevent developing a teaching and learning environment. Trainees fear not appearing knowledgeable in front of families and student rotate too often to adapt to different attending styles [21]. Therefore, reassuring trainees that the goal of FCRs is to conduct daily inpatient rounding to ensure key aspects of FCRs are met without disrespecting and insulting any person on rounds and clarifying and reassuring trainees that their fear of not appearing knowledgeable is real and it will be respected, might help create a safe environment where FCR teams are not only conducting the daily ritual of inpatient rounding, and teaching but also ensuring that trainees are enjoying being the clinician and physicians that they want to be. Therefore, attending role modeling is crucial and it is no surprise that in multiple studies variability in attending rounding and teaching style was identified consistently as a FCR barrier.
Preparing for Daily FCRS: Team Work, Efficiency, and Time Management
Conducting daily timely and efficient rounds require daily preparation by teams. Prior to FCRs, teams should know about all of the patients on whom FCRs will be conducted including those who refused FCRs, if any. This can be done via a pre-round or card-flip rounding method where the teams discuss key diagnoses, indication for admission, and identify any outliers to conducting FCRs such as sensitive patient condition, patients refused FCRs, etc. Some institutions have incorporated these at “morning check out” or at morning “huddles.” These help faculty avoid any last minute surprises during rounds and helps with time management during FCRs [12]. Faculty can then plan on some anticipated “teaching moments” before rounds to keep the rounds flowing, for example, a physical exam finding, a clarifying history that can clinch a diagnoses, a clinical pearl, a complex medical case where the parent might share their story and knowledge, an interesting interpretation of a lab, an x-ray or MRI finding. Faculties are multitasking during FCRs by diagnosing and managing patient and learners and leading effective efficient and timely rounds where parental questions are answered, orders are written, to-do work is identified, discharge planning and care coordination is done and trainees stay focused and attend noon conference on time. This requires thoughtful planning before starting FCRs. Time management and managing priorities is key to positive team experiences of FCRs. Both starting and ending FCRs on time should be emphasized and reinforced continually.
Nurse Preparation for FCRs
Nurses are the frontline providers and educating them about FCRs process can help them better explain FCRs to patients and families. Nurses often know the minute details such as timing of an MRI, if the patient has vomited in the morning, or when the parents are coming, etc. This important information sharing during FCRs can help team prepare for the day and provide patients and families’ expectations for the day. Nursing participation can also enhance their knowledge about the thought process behind decisions and care plans and avoid additional time paging house staff to obtain clarification [12–15,21].
Trainee Preparation for FCRs
While pediatric residents do report that FCRs leads to fewer requests for clarifications from families and nurses after FCRs, many still harbor concerns about the time required for FCRs and the overall efficiency of rounds [14]. Educating trainees about the FCR process and explaining why FCRs are beneficial can help alleviate trainee anxiety around FCRs. Involving trainees in the FCR communication and creating a safe and nurturing environment during FCRs can further reduce trainee anxiety [21]. Parents who have attended FCRs with trainees report understanding that trainees are in training and that they have felt comfortable to see attending physician lead the trainees.
FCRs and Technology
Use of technology during FCRs can be helpful to write orders in real time, follow-up and share lab values and or imaging study with parents or teach students. The increasing use of technology on FCRs, such as computers and handheld devices, can help with rounding and teaching; however, it also has the potential to be a distractor and requires that the medical team remain vigilant that the patient and family are the focus of FCRs [26].
Efficiency Pearls
Certain strategies can be utilized to keep FCRs efficient:
- Orient the FCR team about FCR process
- Identify rounding sequence for the day so team can move efficiently between rooms. Identifying potential discharges for the following morning and discharging those patients before rounds can reduce rounding census and provide additional rounding time. Teams can identify approximate time spent in each room based on census, as rounding time is constant.
- Starting and ending FCRs at the allocated time is key to success of FCRs. Sometimes this might require the attending and senior resident splitting the last 1–2 patients to finish rounds on time.
- Prepare students and interns for effective and efficient yet complete presentations during rounds that reflect their knowledge and thought process rather than presenting the entire H&P.
- Keep teaching during rounds focused. As a resident reported, “attendings should keep it short and not go off on a half hour lecture during FCRs. On FCRs I want to hear bam…bam…bam! tidbits, little hints, clinical pearls. Things that you would not know and only see and know when you were there in the room [21].”
- Encourage and teach senior residents’ role as a leader and teacher [21].
- With a situation requiring more time talking to families, request to go back later in the afternoon so as to stay on track on FCR time.
- Faculty can review lab results and history and physical findings on new admissions before rounds to avoid surprises during FCRs and to save time. This can be done during pre-round/card flip/or morning huddle.
Limitations
This article is based on the authors’ review of literature, experience in conducting FCRs, and experience from leading and attending FCR-related workshops at annual pediatric academic societies’ meetings and annual pediatric hospital medicine meetings between 2010 and 2015. There are several limitations to this work. Firstly, the majority of FCR literature is based on perceptions and are not measured outcomes. In addition, how FCRs will apply on services with complex patients needs more study. Different institutions have different physical constraints as well as sociodemographic and cultural factors that might affect FCRs. Daily census among hospitals varies and rounding duration may vary for them.
Conclusion
Family-centered rounds are widely accepted among pediatric hospitalists in the US. Reported benefits of FCRs include improved parent satisfaction, communication, better team communication, improved patient safety and better education for trainees. Many barriers to efficient FCRs exist, and for programs planning to incorporate FCRs in their daily rounds it is crucial to understand FCR benefits and barriers and assess their current state, including physical environment, when planning FCRs. Having a period to plan for FCR implementation through key stakeholder involvement helps define FCR process and lay down a conceptual model suited to individual organization. Educating the team members including families about FCRs and developing a strong faculty development program can further strengthen FCR implementation. Special focus should be given to time management, teaching styles during FCRs, and creating a safe and nurturing environment for FCRs to succeed.
Corresponding author: Vineeta Mittal, MD, MBA, 1935 Medical District Dr., Dallas, TX 75235, [email protected].
1. American Academy of Pediatrics Committee on Hospital Care. Family-centered care and the pediatrician’s role. Pediatrics 2003;112:691–7.
2. Institute of Medicine, Committee on Quality Health Care in America. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: The National Academies Press; 2001.
3. Kuo DZ, Joutrow AJ, Arango P, et al. Family-centered care: current applications and future directions in pediatric health care. Matern Child Health J 2012;16:297–305.
4. Reichsman F, Browning FE, Hinshaw JR. Observations of undergraduate clinical teaching in action. J Med Educ 1964;39:147–63.
5. Osler W. On the need of a radical reform in our methods of teaching senior students. Med News 1903;82:49–53.
6. Collins GF, Cassie JM, Dagget CJ. The role of the attending physician in clinical training. J Med Educ 1978;53:429–31.
7. Lacombe MA. On bedside teaching. Ann Intern Med 1997;126:217–20.
8. Linfors EW, Neelon FA. Sounding board. The case of bedside rounds. N Engl J Med 1980;303:1230–3.
9. Jolley J, Shields J. The evolution of family-centered care. J Pediatr Nursing 2009;42:164–70.
10. Muething SE, Kotagal UR, Schoettker PJ, et al. Family-centered rounds: a new approach to patient care and teaching. Pediatrics 2007;119:829–32.
11. Sisterhen LL, Blaszak RT, Woods MB, Smith CE. Defining family-centered rounds. Teach Learn Med 2007;19:319–22.
12. Mittal V, Sigrest T, Ottolini M, et al. Family-centered rounds on pediatric wards: a PRIS network survey of Canadian and US hospitalists. Pediatrics 2010;126:37–43.
13. Rosen P, Stenger E, Bochkoris M, et al. Family-centered multidisciplinary rounds enhance the team approach in pediatrics. Pediatrics 2009;123:e603–8.
14. Rappaport DI, Ketterer TA, Nilforoshan V, Sharif I. Family-centered rounds: views of families, nurses, trainees, and attending physicians. Clin Pediatr (Phila) 2012;51:260–6.
15. Young HN, Schumacher JB, Moreno MA, et al. Medical student self-efficacy with family-centered care during bedside rounds. Acad Med 2012;87:767–75.
16. Beck J, Meyer R, Kind T, Bhansali B. The importance of situational awareness: a qualitative study of family members’ and nurses’ perspectives on teaching during family-centered rounds. Acad Med 2015 Jul 21. Epub ahead of print.
17. Benjamin J, Cox E, Trapskin P, et al. Family-initiated dialogue about medicaitons during family-centered rounds. Pediatrics 2015;135:94–100.
18. Cox E, Schumacher J, Young H, et al. Medical student outcomes after family-centered bedside rounds. Acad Pediatri 2011;11:403–8.
19. Latta LC, Dick R, Parry C, Tamura GS. Parental responses to involvement in rounds on a pediatric inpatient unit at a teaching hospital: a qualitative study. Acad Med 2008;83:292–7.
20. Mittal V. Family-centered rounds. Pediatr Clin North Am 2014;61:663–70.
21. Mittal V, Krieger E, Lee B, et al. Pediatric residents’ perspectives on family-centered rounds - a qualitative study at 2 children’s hospitals. J Grad Med Educ 2013;5:81–7.
22. Lion KC, Mangione-Smith R, Martyn M, et al. Comprehension on family-centered rounds for limited English proficient families. Acad Pediatr 2013;13:236–42.
23. Seltz LB, Zimmer L, Ochoa-Nunez L, et al. Latino families’ experiences with family-centered rounds at an academic children’s hospital. Acad Pediatr 2011;11:432–8.
24. Kuo DZ, Sisterhen LL, Sigrest TE, et al. Family experiences and pediatric health services use associated with family-centered rounds. Pediatrics 2012;130:299–305.
25. Bhansali P, Birch S, Campbell JK, et al. A time-motion study of inpatient rounds using a family-centered rounds model. Hosp Pediatr 2013;3:31–8.
26. Kern J, Bhansali P. Handheld electronic device use by pediatric hospitalists on family centered rounds. J Med Syst 2016;40:9.
1. American Academy of Pediatrics Committee on Hospital Care. Family-centered care and the pediatrician’s role. Pediatrics 2003;112:691–7.
2. Institute of Medicine, Committee on Quality Health Care in America. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: The National Academies Press; 2001.
3. Kuo DZ, Joutrow AJ, Arango P, et al. Family-centered care: current applications and future directions in pediatric health care. Matern Child Health J 2012;16:297–305.
4. Reichsman F, Browning FE, Hinshaw JR. Observations of undergraduate clinical teaching in action. J Med Educ 1964;39:147–63.
5. Osler W. On the need of a radical reform in our methods of teaching senior students. Med News 1903;82:49–53.
6. Collins GF, Cassie JM, Dagget CJ. The role of the attending physician in clinical training. J Med Educ 1978;53:429–31.
7. Lacombe MA. On bedside teaching. Ann Intern Med 1997;126:217–20.
8. Linfors EW, Neelon FA. Sounding board. The case of bedside rounds. N Engl J Med 1980;303:1230–3.
9. Jolley J, Shields J. The evolution of family-centered care. J Pediatr Nursing 2009;42:164–70.
10. Muething SE, Kotagal UR, Schoettker PJ, et al. Family-centered rounds: a new approach to patient care and teaching. Pediatrics 2007;119:829–32.
11. Sisterhen LL, Blaszak RT, Woods MB, Smith CE. Defining family-centered rounds. Teach Learn Med 2007;19:319–22.
12. Mittal V, Sigrest T, Ottolini M, et al. Family-centered rounds on pediatric wards: a PRIS network survey of Canadian and US hospitalists. Pediatrics 2010;126:37–43.
13. Rosen P, Stenger E, Bochkoris M, et al. Family-centered multidisciplinary rounds enhance the team approach in pediatrics. Pediatrics 2009;123:e603–8.
14. Rappaport DI, Ketterer TA, Nilforoshan V, Sharif I. Family-centered rounds: views of families, nurses, trainees, and attending physicians. Clin Pediatr (Phila) 2012;51:260–6.
15. Young HN, Schumacher JB, Moreno MA, et al. Medical student self-efficacy with family-centered care during bedside rounds. Acad Med 2012;87:767–75.
16. Beck J, Meyer R, Kind T, Bhansali B. The importance of situational awareness: a qualitative study of family members’ and nurses’ perspectives on teaching during family-centered rounds. Acad Med 2015 Jul 21. Epub ahead of print.
17. Benjamin J, Cox E, Trapskin P, et al. Family-initiated dialogue about medicaitons during family-centered rounds. Pediatrics 2015;135:94–100.
18. Cox E, Schumacher J, Young H, et al. Medical student outcomes after family-centered bedside rounds. Acad Pediatri 2011;11:403–8.
19. Latta LC, Dick R, Parry C, Tamura GS. Parental responses to involvement in rounds on a pediatric inpatient unit at a teaching hospital: a qualitative study. Acad Med 2008;83:292–7.
20. Mittal V. Family-centered rounds. Pediatr Clin North Am 2014;61:663–70.
21. Mittal V, Krieger E, Lee B, et al. Pediatric residents’ perspectives on family-centered rounds - a qualitative study at 2 children’s hospitals. J Grad Med Educ 2013;5:81–7.
22. Lion KC, Mangione-Smith R, Martyn M, et al. Comprehension on family-centered rounds for limited English proficient families. Acad Pediatr 2013;13:236–42.
23. Seltz LB, Zimmer L, Ochoa-Nunez L, et al. Latino families’ experiences with family-centered rounds at an academic children’s hospital. Acad Pediatr 2011;11:432–8.
24. Kuo DZ, Sisterhen LL, Sigrest TE, et al. Family experiences and pediatric health services use associated with family-centered rounds. Pediatrics 2012;130:299–305.
25. Bhansali P, Birch S, Campbell JK, et al. A time-motion study of inpatient rounds using a family-centered rounds model. Hosp Pediatr 2013;3:31–8.
26. Kern J, Bhansali P. Handheld electronic device use by pediatric hospitalists on family centered rounds. J Med Syst 2016;40:9.