User login
Investigating the etiology of recurrent pregnancy loss
With attention to the timing of loss
Introduction: Reassurance through pregnancy loss and workups
Pregnancy loss is not an uncommon complication but it can be associated with significant stress among parents and loved ones when it occurs. Especially when recurrent, it also becomes a medical dilemma for physicians and nurses because the cause is not always obvious immediately, and even with exploration, the cause may not always be found.
First and foremost, it is important that physicians provide counseling and reassurance to families experiencing loss, and that they encourage a level of patience while the investigation for loss is done. Investigations tend not to be linear. One must look at a number of diagnostic areas including genetics, anatomy, immunology, and infections.
Even with an extensive workup, what often is found are potential associations rather than precise causes. For instance, one may find that certain antibodies or certain conditions are present, or that certain anatomic structures are abnormal. While such findings are not necessarily causative, there are therapeutic interventions that we can offer to address many of the conditions (e.g., surgical correction of the septate uterus, and low-dose aspirin and heparin for antiphospholipid syndrome).
Less than 1% of couples experience recurrent pregnancy loss (traditionally defined as three or more losses), so parents who experience one loss should be given reassurance that their loss was likely a sporadic miscarriage and that chances of recurrence will be low. Even as workups proceed, reassurance is important.
For this month’s Master Class in Obstetrics we’ve invited Wendy L. Kinzler, MD, and Anthony Vintzileos, MD, both of whom have expertise in the area of recurrent pregnancy loss, to review the potential causes and the management approach. They focus on the first trimester, when genetic causes predominate, and the early second trimester, when undetected uterine factors can be at play. They explain that the gestational age at which recurrent losses occur is an important factor in decoding etiology and management.
Dr. Kinzler is associate dean, graduate medical education, and professor of obstetrics and gynecology at NYU Long Island School of Medicine, Mineola, N.Y., and Dr. Vintzileos is chief patient safety officer for obstetrics, Northwell Health–Western Region, and professor in the department of obstetrics and gynecology in the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y. Dr. Kinzler and Dr. Vintzileos reported no relevant disclosures.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Investigating the etiology of recurrent pregnancy loss
Pregnancy loss is defined as a loss occurring at < 20 weeks’ gestation.
Consideration of the timing of the pregnancy loss can provide a useful guide to this evaluation, as etiologies vary depending on when in gestation losses occur. In this Master Class, we will address the evaluation of pregnancy loss at < 20 weeks’ gestation, with particular attention to first trimester and early second trimester causes. Literature on the role of the cervix and intra-amniotic infection in midtrimester loss is extensive and will not be covered here.
Although early first trimester miscarriage is common, occurring in approximately 15% of clinically recognized pregnancies, only 2%-3% of couples experience two or more miscarriages and 0.5%-1% experience three or more.
When to begin a diagnostic workup should be part of a shared decision-making process, taking into consideration future family planning, parity, number of previous losses, and notably, the gestational age at which loss(es) occurred. Recurrence rates for first trimester miscarriage are similar after two versus three consecutive losses and either situation usually prompts an evaluation, whereas any second-trimester loss should be evaluated.
Increasingly, we are appreciating the value of a more targeted, gestational age–driven approach to the evaluation of pregnancy loss in an attempt to provide grieving parents with useful information without subjecting them to a wide array of expensive and unnecessary tests.
Genetic causes
The earlier the pregnancy loss, the more likely it is the result of abnormal fetal genetics. Genetic factors should be considered as the most likely cause for first trimester pregnancy losses (especially those occurring at < 10 weeks’ gestation), the most frequent being autosomal trisomies or monosomy X. The vast majority of trisomy conceptuses are sporadic in nature and are related to the natural aging process of the ovary (increasing the rate of meiotic nondisjunction).
If fetal aneuploidy is identified in a pregnancy loss, couples can be counseled about the definitive cause of the loss and can be counseled about recurrence based on age-related risks and/or tests of ovarian reserve. Recurrent pregnancy loss (RPL) is only rarely associated with a parental translocation (< 5%). Testing parental karyotypes should be reserved for cases in which the fetal karyotypes are unknown or in which an unbalanced translocation was identified in the fetus.
When a first trimester pregnancy loss is diagnosed, chorionic villus sampling (CVS) with microarray testing is the most reliable and comprehensive method for evaluating potential genetic causes. It provides valuable information even when cells are not viable and reduces the risk of maternal cell contamination – two significant limitations to standard karyotype analysis performed on tissue passed spontaneously or at the time of D&C. Studies of products of conception (POC) testing with microarray have documented the detection of abnormalities in an additional 10%-15% of cases compared with traditional karyotype analysis.
When CVS is not feasible, testing maternal serum for cell-free DNA is a reasonable alternative. In a prospective cohort study of 50 maternal blood samples taken after fetal demise, 76% of samples yielded cell-free DNA results, meaning fetal fractions were within the detectable range. The higher the gestational age at the time of loss, the higher the chance of obtaining a result: Findings in the study were possible in 88% of samples when the gestational age was 8 weeks or greater, and in 53% of cases involving a lower gestational age. The time from demise to blood draw did not affect the likelihood of obtaining a result (Obstet Gynecol. 2015 Jun;125[6]:1321-29).
When neither CVS nor cell-free DNA analysis is feasible, analysis of either spontaneously passed tissue or tissue obtained at the time of a D&C may still be worthwhile. Maternal cell contamination, of course, is the main downside.
A paper published in 2020 documented the value of refocusing the initial workup. Researchers reported that 57% of 1,400 cases of recurrent pregnancy loss went unexplained using the 2012 ASRM guidelines, which included parental karyotyping but not POC cytogenetic analysis. When parental karyotypes were omitted from the initial workup and POC analysis with 24-chromosome microarray was added, at least one potential explanation for loss could be identified in 92% of cases. Only 8% were left “unexplained” (Curr Opin Obstet Gynecol. 2020 Oct;32[5]:371-9).
When genetics are ruled out
Issues that are top of mind when we lack genetic information or when genetic causes are ruled out include maternal metabolic disorders (uncontrolled diabetes, obesity, uncontrolled thyroid disease), environmental exposures (smoking), uterine abnormalities, and antiphospholipid syndrome.
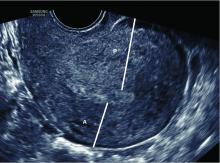
Thorough evaluation of the uterine cavity after recurrent first trimester miscarriage – or after any second trimester loss – is too often a missing element of investigation. It is a vital component of the evaluation, and information about uterine structure is easily obtained.
A saline infusion sonogram (SIS) allows us to look at the external contour of the uterus, assess the myometrium for muscular abnormalities, visualize the uterine lining, and assess uterine orientation. Performed in the nonpregnant state, and ideally coupled with 3D technology, this relatively simple test can identify congenital uterine anomalies, intracavitary abnormalities (such as fibroids, polyps, or synechiae) which can surgically be removed prior to another pregnancy, a retroverted uterus that may be predisposed to incarceration during pregnancy, and other potentially impactful conditions, such as adenomyosis.
Structural anomalies
Congenital uterine anomalies are associated with first trimester miscarriage, second trimester pregnancy loss, and preterm birth. A uterine septum is of particular concern for early miscarriage, as the early embryo can implant on the relatively avascular septum.
Other congenital uterine anomalies (bicornuate, didelphys, unicornuate) can be associated with concomitant cervical structural abnormalities leading to cervical insufficiency and/or result in pathologic uterine stretch of a space-limited cavity, leading to midtrimester loss or preterm birth. The diagnosis of these anomalies is an important part of the evaluation of pregnancy loss, as it can guide monitoring in future pregnancies, or can be surgically corrected, as in the case of a uterine septum, significantly improving pregnancy outcomes.
A short cervix can result either congenitally or from injury or trauma and may also be associated with cervical insufficiency and miscarriage. It can be evaluated and monitored by ultrasound and, in some cases, treated by surgical cerclage. Pregnancy losses due to cervical insufficiency usually occur after 16 weeks of gestation and frequently are associated with intra-amniotic infections.
Incarcerated uterus and adenomyosis
Other uterine factors that can contribute to pregnancy loss and that are largely underdiagnosed or undiagnosed are an incarcerated retroverted uterus and adenomyosis.
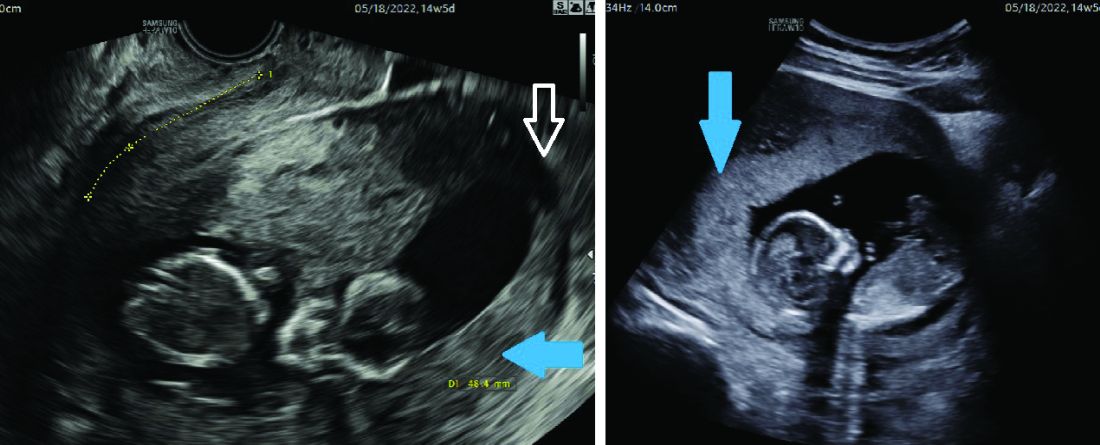
Most of the time, a retroverted uterus naturally assumes an anteverted position by the late first trimester or early second trimester, allowing for continued growth of the uterus and developing fetus. In approximately 10% of cases, however, the retroverted uterus becomes “stuck” or incarcerated in the posterior pelvis. This is more likely if there are large uterine fibroids or in the presence of pelvic adhesions due to endometriosis or previous pelvic surgery.
When this occurs, the fundus is wedged on the sacral promontory (may cause pelvic discomfort and constipation) and the cervix is markedly displaced anteriorly under the pubic symphysis (causing bladder outlet obstruction and urinary retention).
It is critical that ob.gyns. and emergency medicine providers are aware of this condition, which typically presents between 12 and 16 weeks’ gestation. The most frequent complaint is lower abdominal discomfort due to distended bladder and inability to void, which usually leads to bladder catheterization with drainage of large amounts of urine. An incarcerated uterus can predispose to pregnancy loss during this time (few other conditions cause loss during this time window), presumably due to impaired uterine artery blood flow.
Once the diagnosis is made, uterine incarceration may be corrected by elevating the gravid uterus out of the pelvis either manually, or by using a transvaginal ultrasound probe. (The latter minimally invasive approach was described in March at the American Institute of Ultrasound in Medicine’s annual conference by Martin Chavez, MD, and coinvestigators. More invasive approaches are rarely required but include CO2 intraperitoneal insufflation, as used prior to laparoscopy, or laparotomy.
The later in gestation the condition is allowed to persist, the less likely correction will be possible due to the enlarging fundus. Correction between 14 and 16 weeks, or earlier if symptoms develop, is recommended.
Adenomyosis, another poorly understood condition impacting pregnancy outcomes, has been associated with increased rates of miscarriage after in vitro fertilization (in addition to lower implantation rates); a meta-analysis published almost a decade ago found a risk ratio of miscarriage of 2.12 (95% confidence interval, 1.2-3.75) in women with adenomyosis versus those without (Hum Reprod. 2014 May;29[5]:964-77). However, outside of reproductive endocrinology, its impact on pregnancy outcomes in the obstetrical literature is not well recognized.
Although more research is necessary, we believe that adenomyosis should be considered a risk factor for pregnancy loss in the second trimester. The presence of endometrial glands within the myometrium, predisposing for an inflammatory environment, can lead to abnormal implantation, poor uterine distensibility, sterile inflammation, and early cervical insufficiency. As the prevalence of adenomyosis increases with age and maternal ages are increasing, this is an important condition to consider.
Diagnosis is typically made with MRI (although pathology of a hysterectomy specimen is the gold standard). Ultrasound findings consistent with adenomyosis are not routinely assessed and have not been studied in a gravid uterus. Nonetheless, a heightened sense of awareness about this possible contributor to pregnancy loss is very important.
A word about antiphospholipid syndrome
Antiphospholipid syndrome can cause a variety of adverse pregnancy outcomes, including first and second trimester pregnancy loss, fetal demise, fetal growth restriction, preeclampsia, preterm birth, and maternal thromboembolism. The classical presentation of miscarriage due to untreated antiphospholipid antibody syndrome is early severe fetal growth restriction, oligohydramnios, and IUFD in the second trimester.
The diagnosis requires at least one clinical criterion and one laboratory criterion. The mere presence of low level antibodies does not make the diagnosis of antiphospholipid antibody syndrome, and care should be taken to consider both the clinical and laboratory diagnostic criteria to make an accurate diagnosis.
When present, close maternal and fetal surveillance and a combination of low-dose aspirin and heparin are mainstays of treatment. The majority of studies suggest that low-molecular weight heparin (LMWH) and unfractionated heparin have comparable clinical efficacy. However, if a recurrent loss is experienced despite treatment with LMWH, the use of unfractionated heparin in a subsequent pregnancy should be considered.
With attention to the timing of loss
With attention to the timing of loss
Introduction: Reassurance through pregnancy loss and workups
Pregnancy loss is not an uncommon complication but it can be associated with significant stress among parents and loved ones when it occurs. Especially when recurrent, it also becomes a medical dilemma for physicians and nurses because the cause is not always obvious immediately, and even with exploration, the cause may not always be found.
First and foremost, it is important that physicians provide counseling and reassurance to families experiencing loss, and that they encourage a level of patience while the investigation for loss is done. Investigations tend not to be linear. One must look at a number of diagnostic areas including genetics, anatomy, immunology, and infections.
Even with an extensive workup, what often is found are potential associations rather than precise causes. For instance, one may find that certain antibodies or certain conditions are present, or that certain anatomic structures are abnormal. While such findings are not necessarily causative, there are therapeutic interventions that we can offer to address many of the conditions (e.g., surgical correction of the septate uterus, and low-dose aspirin and heparin for antiphospholipid syndrome).
Less than 1% of couples experience recurrent pregnancy loss (traditionally defined as three or more losses), so parents who experience one loss should be given reassurance that their loss was likely a sporadic miscarriage and that chances of recurrence will be low. Even as workups proceed, reassurance is important.
For this month’s Master Class in Obstetrics we’ve invited Wendy L. Kinzler, MD, and Anthony Vintzileos, MD, both of whom have expertise in the area of recurrent pregnancy loss, to review the potential causes and the management approach. They focus on the first trimester, when genetic causes predominate, and the early second trimester, when undetected uterine factors can be at play. They explain that the gestational age at which recurrent losses occur is an important factor in decoding etiology and management.
Dr. Kinzler is associate dean, graduate medical education, and professor of obstetrics and gynecology at NYU Long Island School of Medicine, Mineola, N.Y., and Dr. Vintzileos is chief patient safety officer for obstetrics, Northwell Health–Western Region, and professor in the department of obstetrics and gynecology in the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y. Dr. Kinzler and Dr. Vintzileos reported no relevant disclosures.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Investigating the etiology of recurrent pregnancy loss
Pregnancy loss is defined as a loss occurring at < 20 weeks’ gestation.
Consideration of the timing of the pregnancy loss can provide a useful guide to this evaluation, as etiologies vary depending on when in gestation losses occur. In this Master Class, we will address the evaluation of pregnancy loss at < 20 weeks’ gestation, with particular attention to first trimester and early second trimester causes. Literature on the role of the cervix and intra-amniotic infection in midtrimester loss is extensive and will not be covered here.
Although early first trimester miscarriage is common, occurring in approximately 15% of clinically recognized pregnancies, only 2%-3% of couples experience two or more miscarriages and 0.5%-1% experience three or more.
When to begin a diagnostic workup should be part of a shared decision-making process, taking into consideration future family planning, parity, number of previous losses, and notably, the gestational age at which loss(es) occurred. Recurrence rates for first trimester miscarriage are similar after two versus three consecutive losses and either situation usually prompts an evaluation, whereas any second-trimester loss should be evaluated.
Increasingly, we are appreciating the value of a more targeted, gestational age–driven approach to the evaluation of pregnancy loss in an attempt to provide grieving parents with useful information without subjecting them to a wide array of expensive and unnecessary tests.
Genetic causes
The earlier the pregnancy loss, the more likely it is the result of abnormal fetal genetics. Genetic factors should be considered as the most likely cause for first trimester pregnancy losses (especially those occurring at < 10 weeks’ gestation), the most frequent being autosomal trisomies or monosomy X. The vast majority of trisomy conceptuses are sporadic in nature and are related to the natural aging process of the ovary (increasing the rate of meiotic nondisjunction).
If fetal aneuploidy is identified in a pregnancy loss, couples can be counseled about the definitive cause of the loss and can be counseled about recurrence based on age-related risks and/or tests of ovarian reserve. Recurrent pregnancy loss (RPL) is only rarely associated with a parental translocation (< 5%). Testing parental karyotypes should be reserved for cases in which the fetal karyotypes are unknown or in which an unbalanced translocation was identified in the fetus.
When a first trimester pregnancy loss is diagnosed, chorionic villus sampling (CVS) with microarray testing is the most reliable and comprehensive method for evaluating potential genetic causes. It provides valuable information even when cells are not viable and reduces the risk of maternal cell contamination – two significant limitations to standard karyotype analysis performed on tissue passed spontaneously or at the time of D&C. Studies of products of conception (POC) testing with microarray have documented the detection of abnormalities in an additional 10%-15% of cases compared with traditional karyotype analysis.
When CVS is not feasible, testing maternal serum for cell-free DNA is a reasonable alternative. In a prospective cohort study of 50 maternal blood samples taken after fetal demise, 76% of samples yielded cell-free DNA results, meaning fetal fractions were within the detectable range. The higher the gestational age at the time of loss, the higher the chance of obtaining a result: Findings in the study were possible in 88% of samples when the gestational age was 8 weeks or greater, and in 53% of cases involving a lower gestational age. The time from demise to blood draw did not affect the likelihood of obtaining a result (Obstet Gynecol. 2015 Jun;125[6]:1321-29).
When neither CVS nor cell-free DNA analysis is feasible, analysis of either spontaneously passed tissue or tissue obtained at the time of a D&C may still be worthwhile. Maternal cell contamination, of course, is the main downside.
A paper published in 2020 documented the value of refocusing the initial workup. Researchers reported that 57% of 1,400 cases of recurrent pregnancy loss went unexplained using the 2012 ASRM guidelines, which included parental karyotyping but not POC cytogenetic analysis. When parental karyotypes were omitted from the initial workup and POC analysis with 24-chromosome microarray was added, at least one potential explanation for loss could be identified in 92% of cases. Only 8% were left “unexplained” (Curr Opin Obstet Gynecol. 2020 Oct;32[5]:371-9).
When genetics are ruled out
Issues that are top of mind when we lack genetic information or when genetic causes are ruled out include maternal metabolic disorders (uncontrolled diabetes, obesity, uncontrolled thyroid disease), environmental exposures (smoking), uterine abnormalities, and antiphospholipid syndrome.
Thorough evaluation of the uterine cavity after recurrent first trimester miscarriage – or after any second trimester loss – is too often a missing element of investigation. It is a vital component of the evaluation, and information about uterine structure is easily obtained.
A saline infusion sonogram (SIS) allows us to look at the external contour of the uterus, assess the myometrium for muscular abnormalities, visualize the uterine lining, and assess uterine orientation. Performed in the nonpregnant state, and ideally coupled with 3D technology, this relatively simple test can identify congenital uterine anomalies, intracavitary abnormalities (such as fibroids, polyps, or synechiae) which can surgically be removed prior to another pregnancy, a retroverted uterus that may be predisposed to incarceration during pregnancy, and other potentially impactful conditions, such as adenomyosis.
Structural anomalies
Congenital uterine anomalies are associated with first trimester miscarriage, second trimester pregnancy loss, and preterm birth. A uterine septum is of particular concern for early miscarriage, as the early embryo can implant on the relatively avascular septum.
Other congenital uterine anomalies (bicornuate, didelphys, unicornuate) can be associated with concomitant cervical structural abnormalities leading to cervical insufficiency and/or result in pathologic uterine stretch of a space-limited cavity, leading to midtrimester loss or preterm birth. The diagnosis of these anomalies is an important part of the evaluation of pregnancy loss, as it can guide monitoring in future pregnancies, or can be surgically corrected, as in the case of a uterine septum, significantly improving pregnancy outcomes.
A short cervix can result either congenitally or from injury or trauma and may also be associated with cervical insufficiency and miscarriage. It can be evaluated and monitored by ultrasound and, in some cases, treated by surgical cerclage. Pregnancy losses due to cervical insufficiency usually occur after 16 weeks of gestation and frequently are associated with intra-amniotic infections.
Incarcerated uterus and adenomyosis
Other uterine factors that can contribute to pregnancy loss and that are largely underdiagnosed or undiagnosed are an incarcerated retroverted uterus and adenomyosis.

Most of the time, a retroverted uterus naturally assumes an anteverted position by the late first trimester or early second trimester, allowing for continued growth of the uterus and developing fetus. In approximately 10% of cases, however, the retroverted uterus becomes “stuck” or incarcerated in the posterior pelvis. This is more likely if there are large uterine fibroids or in the presence of pelvic adhesions due to endometriosis or previous pelvic surgery.
When this occurs, the fundus is wedged on the sacral promontory (may cause pelvic discomfort and constipation) and the cervix is markedly displaced anteriorly under the pubic symphysis (causing bladder outlet obstruction and urinary retention).
It is critical that ob.gyns. and emergency medicine providers are aware of this condition, which typically presents between 12 and 16 weeks’ gestation. The most frequent complaint is lower abdominal discomfort due to distended bladder and inability to void, which usually leads to bladder catheterization with drainage of large amounts of urine. An incarcerated uterus can predispose to pregnancy loss during this time (few other conditions cause loss during this time window), presumably due to impaired uterine artery blood flow.
Once the diagnosis is made, uterine incarceration may be corrected by elevating the gravid uterus out of the pelvis either manually, or by using a transvaginal ultrasound probe. (The latter minimally invasive approach was described in March at the American Institute of Ultrasound in Medicine’s annual conference by Martin Chavez, MD, and coinvestigators. More invasive approaches are rarely required but include CO2 intraperitoneal insufflation, as used prior to laparoscopy, or laparotomy.
The later in gestation the condition is allowed to persist, the less likely correction will be possible due to the enlarging fundus. Correction between 14 and 16 weeks, or earlier if symptoms develop, is recommended.
Adenomyosis, another poorly understood condition impacting pregnancy outcomes, has been associated with increased rates of miscarriage after in vitro fertilization (in addition to lower implantation rates); a meta-analysis published almost a decade ago found a risk ratio of miscarriage of 2.12 (95% confidence interval, 1.2-3.75) in women with adenomyosis versus those without (Hum Reprod. 2014 May;29[5]:964-77). However, outside of reproductive endocrinology, its impact on pregnancy outcomes in the obstetrical literature is not well recognized.
Although more research is necessary, we believe that adenomyosis should be considered a risk factor for pregnancy loss in the second trimester. The presence of endometrial glands within the myometrium, predisposing for an inflammatory environment, can lead to abnormal implantation, poor uterine distensibility, sterile inflammation, and early cervical insufficiency. As the prevalence of adenomyosis increases with age and maternal ages are increasing, this is an important condition to consider.
Diagnosis is typically made with MRI (although pathology of a hysterectomy specimen is the gold standard). Ultrasound findings consistent with adenomyosis are not routinely assessed and have not been studied in a gravid uterus. Nonetheless, a heightened sense of awareness about this possible contributor to pregnancy loss is very important.
A word about antiphospholipid syndrome
Antiphospholipid syndrome can cause a variety of adverse pregnancy outcomes, including first and second trimester pregnancy loss, fetal demise, fetal growth restriction, preeclampsia, preterm birth, and maternal thromboembolism. The classical presentation of miscarriage due to untreated antiphospholipid antibody syndrome is early severe fetal growth restriction, oligohydramnios, and IUFD in the second trimester.
The diagnosis requires at least one clinical criterion and one laboratory criterion. The mere presence of low level antibodies does not make the diagnosis of antiphospholipid antibody syndrome, and care should be taken to consider both the clinical and laboratory diagnostic criteria to make an accurate diagnosis.
When present, close maternal and fetal surveillance and a combination of low-dose aspirin and heparin are mainstays of treatment. The majority of studies suggest that low-molecular weight heparin (LMWH) and unfractionated heparin have comparable clinical efficacy. However, if a recurrent loss is experienced despite treatment with LMWH, the use of unfractionated heparin in a subsequent pregnancy should be considered.
Introduction: Reassurance through pregnancy loss and workups
Pregnancy loss is not an uncommon complication but it can be associated with significant stress among parents and loved ones when it occurs. Especially when recurrent, it also becomes a medical dilemma for physicians and nurses because the cause is not always obvious immediately, and even with exploration, the cause may not always be found.
First and foremost, it is important that physicians provide counseling and reassurance to families experiencing loss, and that they encourage a level of patience while the investigation for loss is done. Investigations tend not to be linear. One must look at a number of diagnostic areas including genetics, anatomy, immunology, and infections.
Even with an extensive workup, what often is found are potential associations rather than precise causes. For instance, one may find that certain antibodies or certain conditions are present, or that certain anatomic structures are abnormal. While such findings are not necessarily causative, there are therapeutic interventions that we can offer to address many of the conditions (e.g., surgical correction of the septate uterus, and low-dose aspirin and heparin for antiphospholipid syndrome).
Less than 1% of couples experience recurrent pregnancy loss (traditionally defined as three or more losses), so parents who experience one loss should be given reassurance that their loss was likely a sporadic miscarriage and that chances of recurrence will be low. Even as workups proceed, reassurance is important.
For this month’s Master Class in Obstetrics we’ve invited Wendy L. Kinzler, MD, and Anthony Vintzileos, MD, both of whom have expertise in the area of recurrent pregnancy loss, to review the potential causes and the management approach. They focus on the first trimester, when genetic causes predominate, and the early second trimester, when undetected uterine factors can be at play. They explain that the gestational age at which recurrent losses occur is an important factor in decoding etiology and management.
Dr. Kinzler is associate dean, graduate medical education, and professor of obstetrics and gynecology at NYU Long Island School of Medicine, Mineola, N.Y., and Dr. Vintzileos is chief patient safety officer for obstetrics, Northwell Health–Western Region, and professor in the department of obstetrics and gynecology in the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y. Dr. Kinzler and Dr. Vintzileos reported no relevant disclosures.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Investigating the etiology of recurrent pregnancy loss
Pregnancy loss is defined as a loss occurring at < 20 weeks’ gestation.
Consideration of the timing of the pregnancy loss can provide a useful guide to this evaluation, as etiologies vary depending on when in gestation losses occur. In this Master Class, we will address the evaluation of pregnancy loss at < 20 weeks’ gestation, with particular attention to first trimester and early second trimester causes. Literature on the role of the cervix and intra-amniotic infection in midtrimester loss is extensive and will not be covered here.
Although early first trimester miscarriage is common, occurring in approximately 15% of clinically recognized pregnancies, only 2%-3% of couples experience two or more miscarriages and 0.5%-1% experience three or more.
When to begin a diagnostic workup should be part of a shared decision-making process, taking into consideration future family planning, parity, number of previous losses, and notably, the gestational age at which loss(es) occurred. Recurrence rates for first trimester miscarriage are similar after two versus three consecutive losses and either situation usually prompts an evaluation, whereas any second-trimester loss should be evaluated.
Increasingly, we are appreciating the value of a more targeted, gestational age–driven approach to the evaluation of pregnancy loss in an attempt to provide grieving parents with useful information without subjecting them to a wide array of expensive and unnecessary tests.
Genetic causes
The earlier the pregnancy loss, the more likely it is the result of abnormal fetal genetics. Genetic factors should be considered as the most likely cause for first trimester pregnancy losses (especially those occurring at < 10 weeks’ gestation), the most frequent being autosomal trisomies or monosomy X. The vast majority of trisomy conceptuses are sporadic in nature and are related to the natural aging process of the ovary (increasing the rate of meiotic nondisjunction).
If fetal aneuploidy is identified in a pregnancy loss, couples can be counseled about the definitive cause of the loss and can be counseled about recurrence based on age-related risks and/or tests of ovarian reserve. Recurrent pregnancy loss (RPL) is only rarely associated with a parental translocation (< 5%). Testing parental karyotypes should be reserved for cases in which the fetal karyotypes are unknown or in which an unbalanced translocation was identified in the fetus.
When a first trimester pregnancy loss is diagnosed, chorionic villus sampling (CVS) with microarray testing is the most reliable and comprehensive method for evaluating potential genetic causes. It provides valuable information even when cells are not viable and reduces the risk of maternal cell contamination – two significant limitations to standard karyotype analysis performed on tissue passed spontaneously or at the time of D&C. Studies of products of conception (POC) testing with microarray have documented the detection of abnormalities in an additional 10%-15% of cases compared with traditional karyotype analysis.
When CVS is not feasible, testing maternal serum for cell-free DNA is a reasonable alternative. In a prospective cohort study of 50 maternal blood samples taken after fetal demise, 76% of samples yielded cell-free DNA results, meaning fetal fractions were within the detectable range. The higher the gestational age at the time of loss, the higher the chance of obtaining a result: Findings in the study were possible in 88% of samples when the gestational age was 8 weeks or greater, and in 53% of cases involving a lower gestational age. The time from demise to blood draw did not affect the likelihood of obtaining a result (Obstet Gynecol. 2015 Jun;125[6]:1321-29).
When neither CVS nor cell-free DNA analysis is feasible, analysis of either spontaneously passed tissue or tissue obtained at the time of a D&C may still be worthwhile. Maternal cell contamination, of course, is the main downside.
A paper published in 2020 documented the value of refocusing the initial workup. Researchers reported that 57% of 1,400 cases of recurrent pregnancy loss went unexplained using the 2012 ASRM guidelines, which included parental karyotyping but not POC cytogenetic analysis. When parental karyotypes were omitted from the initial workup and POC analysis with 24-chromosome microarray was added, at least one potential explanation for loss could be identified in 92% of cases. Only 8% were left “unexplained” (Curr Opin Obstet Gynecol. 2020 Oct;32[5]:371-9).
When genetics are ruled out
Issues that are top of mind when we lack genetic information or when genetic causes are ruled out include maternal metabolic disorders (uncontrolled diabetes, obesity, uncontrolled thyroid disease), environmental exposures (smoking), uterine abnormalities, and antiphospholipid syndrome.
Thorough evaluation of the uterine cavity after recurrent first trimester miscarriage – or after any second trimester loss – is too often a missing element of investigation. It is a vital component of the evaluation, and information about uterine structure is easily obtained.
A saline infusion sonogram (SIS) allows us to look at the external contour of the uterus, assess the myometrium for muscular abnormalities, visualize the uterine lining, and assess uterine orientation. Performed in the nonpregnant state, and ideally coupled with 3D technology, this relatively simple test can identify congenital uterine anomalies, intracavitary abnormalities (such as fibroids, polyps, or synechiae) which can surgically be removed prior to another pregnancy, a retroverted uterus that may be predisposed to incarceration during pregnancy, and other potentially impactful conditions, such as adenomyosis.
Structural anomalies
Congenital uterine anomalies are associated with first trimester miscarriage, second trimester pregnancy loss, and preterm birth. A uterine septum is of particular concern for early miscarriage, as the early embryo can implant on the relatively avascular septum.
Other congenital uterine anomalies (bicornuate, didelphys, unicornuate) can be associated with concomitant cervical structural abnormalities leading to cervical insufficiency and/or result in pathologic uterine stretch of a space-limited cavity, leading to midtrimester loss or preterm birth. The diagnosis of these anomalies is an important part of the evaluation of pregnancy loss, as it can guide monitoring in future pregnancies, or can be surgically corrected, as in the case of a uterine septum, significantly improving pregnancy outcomes.
A short cervix can result either congenitally or from injury or trauma and may also be associated with cervical insufficiency and miscarriage. It can be evaluated and monitored by ultrasound and, in some cases, treated by surgical cerclage. Pregnancy losses due to cervical insufficiency usually occur after 16 weeks of gestation and frequently are associated with intra-amniotic infections.
Incarcerated uterus and adenomyosis
Other uterine factors that can contribute to pregnancy loss and that are largely underdiagnosed or undiagnosed are an incarcerated retroverted uterus and adenomyosis.

Most of the time, a retroverted uterus naturally assumes an anteverted position by the late first trimester or early second trimester, allowing for continued growth of the uterus and developing fetus. In approximately 10% of cases, however, the retroverted uterus becomes “stuck” or incarcerated in the posterior pelvis. This is more likely if there are large uterine fibroids or in the presence of pelvic adhesions due to endometriosis or previous pelvic surgery.
When this occurs, the fundus is wedged on the sacral promontory (may cause pelvic discomfort and constipation) and the cervix is markedly displaced anteriorly under the pubic symphysis (causing bladder outlet obstruction and urinary retention).
It is critical that ob.gyns. and emergency medicine providers are aware of this condition, which typically presents between 12 and 16 weeks’ gestation. The most frequent complaint is lower abdominal discomfort due to distended bladder and inability to void, which usually leads to bladder catheterization with drainage of large amounts of urine. An incarcerated uterus can predispose to pregnancy loss during this time (few other conditions cause loss during this time window), presumably due to impaired uterine artery blood flow.
Once the diagnosis is made, uterine incarceration may be corrected by elevating the gravid uterus out of the pelvis either manually, or by using a transvaginal ultrasound probe. (The latter minimally invasive approach was described in March at the American Institute of Ultrasound in Medicine’s annual conference by Martin Chavez, MD, and coinvestigators. More invasive approaches are rarely required but include CO2 intraperitoneal insufflation, as used prior to laparoscopy, or laparotomy.
The later in gestation the condition is allowed to persist, the less likely correction will be possible due to the enlarging fundus. Correction between 14 and 16 weeks, or earlier if symptoms develop, is recommended.
Adenomyosis, another poorly understood condition impacting pregnancy outcomes, has been associated with increased rates of miscarriage after in vitro fertilization (in addition to lower implantation rates); a meta-analysis published almost a decade ago found a risk ratio of miscarriage of 2.12 (95% confidence interval, 1.2-3.75) in women with adenomyosis versus those without (Hum Reprod. 2014 May;29[5]:964-77). However, outside of reproductive endocrinology, its impact on pregnancy outcomes in the obstetrical literature is not well recognized.
Although more research is necessary, we believe that adenomyosis should be considered a risk factor for pregnancy loss in the second trimester. The presence of endometrial glands within the myometrium, predisposing for an inflammatory environment, can lead to abnormal implantation, poor uterine distensibility, sterile inflammation, and early cervical insufficiency. As the prevalence of adenomyosis increases with age and maternal ages are increasing, this is an important condition to consider.
Diagnosis is typically made with MRI (although pathology of a hysterectomy specimen is the gold standard). Ultrasound findings consistent with adenomyosis are not routinely assessed and have not been studied in a gravid uterus. Nonetheless, a heightened sense of awareness about this possible contributor to pregnancy loss is very important.
A word about antiphospholipid syndrome
Antiphospholipid syndrome can cause a variety of adverse pregnancy outcomes, including first and second trimester pregnancy loss, fetal demise, fetal growth restriction, preeclampsia, preterm birth, and maternal thromboembolism. The classical presentation of miscarriage due to untreated antiphospholipid antibody syndrome is early severe fetal growth restriction, oligohydramnios, and IUFD in the second trimester.
The diagnosis requires at least one clinical criterion and one laboratory criterion. The mere presence of low level antibodies does not make the diagnosis of antiphospholipid antibody syndrome, and care should be taken to consider both the clinical and laboratory diagnostic criteria to make an accurate diagnosis.
When present, close maternal and fetal surveillance and a combination of low-dose aspirin and heparin are mainstays of treatment. The majority of studies suggest that low-molecular weight heparin (LMWH) and unfractionated heparin have comparable clinical efficacy. However, if a recurrent loss is experienced despite treatment with LMWH, the use of unfractionated heparin in a subsequent pregnancy should be considered.