User login
Invasive Penile Squamous Cell Carcinoma
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
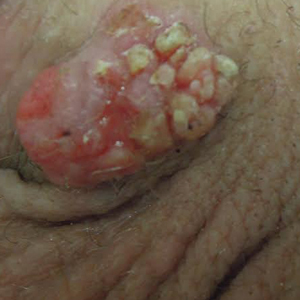
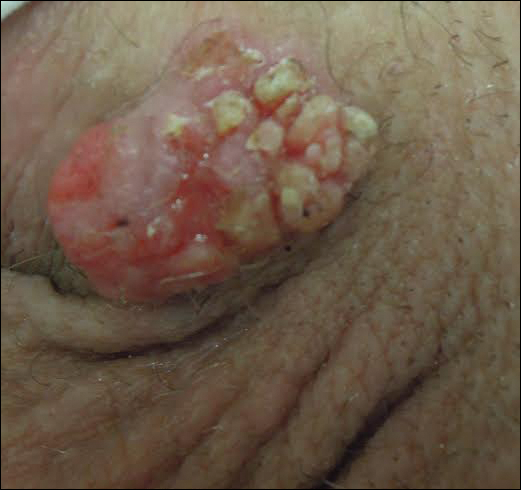
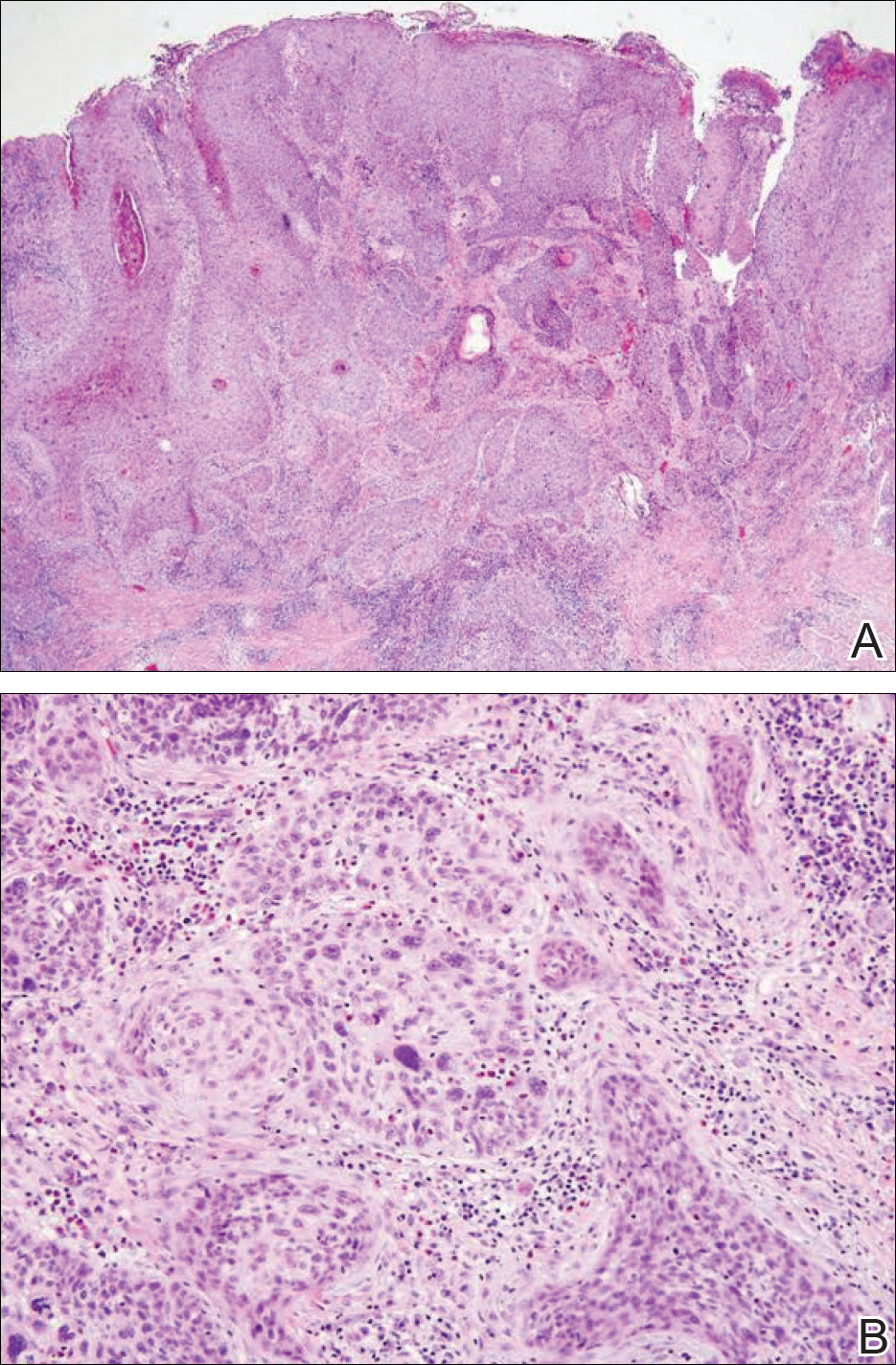
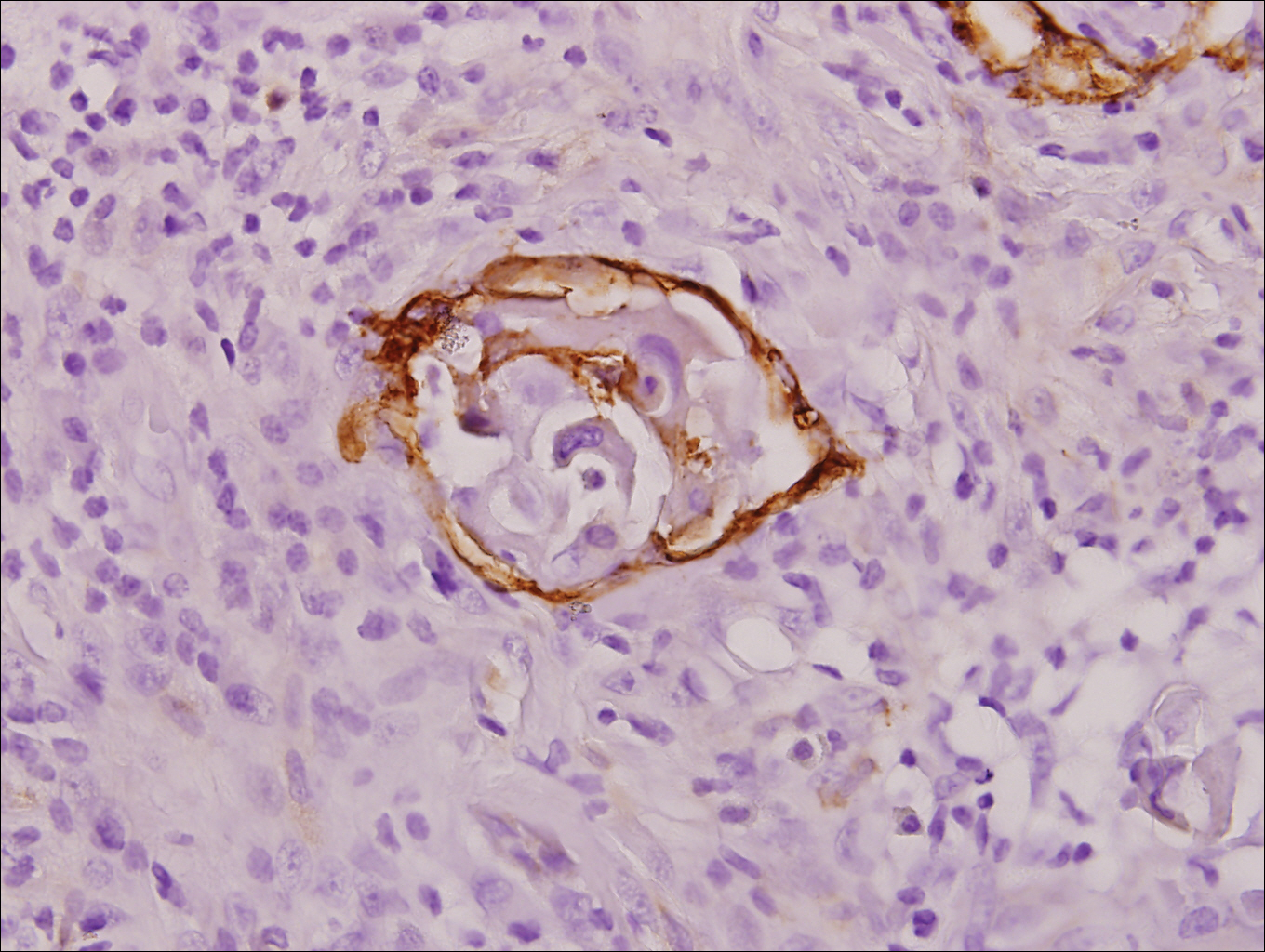
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.
Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.



Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.



Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
Practice Points
- Invasive penile squamous cell carcinoma (PSCC) is a rare malignancy with considerable morbidity and mortality that typically does not present in young men.
- Delayed or incorrect diagnosis of PSCC can have a devastating outcome; therefore, physicians should maintain a high index of clinical suspicion for PSCC in patients presenting with penile lesions, particularly in young or middle-aged patients.