User login
A shot in the arm: Boost your knowledge about immunizations for psychiatric patients
Patients with chronic, severe mental illness live much shorter lives than the general population. The 25-year loss in life expectancy for people with chronic mental illness has been attributed to higher rates of cardiovascular disease driven by increased smoking, obesity, poverty, and poor nutrition.1 These individuals also face the added burden of struggling with a psychiatric condition that often interferes with their ability to make optimal preventative health decisions, including staying up to date on vaccinations.2 A recent review from Toronto, Canada, found that the influenza vaccination rates among homeless adults with mental illness—a population at high risk of respiratory illness—was only 6.7% compared with 31.1% for the general population of Ontario.3
Mental health professionals may serve as the only contacts to offer medical care to this vulnerable population, leading some psychiatric leaders to advocate that psychiatrists be considered primary care providers within accountable care organizations. Because most vaccines are easily available, mental health professionals should know about key immunizations to guide their patients accordingly.
In the United States, approximately 45,000 adults die annually from vaccine-preventable diseases, the majority from influenza.4 When combined with the most recent Adult Immunization Schedule and general recommendations adapted from the CDC,5,6 the mnemonic ARM SHOT allows for a quick assessment of risk factors to guide administration and education about most vaccinations (Table 1). ARM SHOT involves assessing the following components of an individual’s health status and living arrangements to determine one’s risk of contracting communicable diseases:
- Age
- Risk of exposure
- Medical conditions (comorbidities)
- Substance use history
- HIV status or other immunocompromised states
- Occupancy, or living arrangements
- Tobacco use.

We recommend keeping a copy of the Adult Immunization Schedule (age ≥19) and/or the immunization schedule for children and adolescents (age ≤18) close for quick reference. Here, we provide a case and then explore how each component of the ARM SHOT mnemonic applies in decision-making.
Case Evaluating risk, assess needs
Ms. W, age 24, has bipolar I disorder, most recently manic with psychotic features. She presents for follow-up in clinic after a 5-day hospitalization for mania and comorbid alcohol use disorder. Her medical comorbidities include asthma and active tobacco use. She is taking lurasidone, 20 mg/d, and lithium, 900 mg/d. Her case manager is working to place Ms. W in a residential substance use disorder treatment program. Ms. W is on a waiting list to establish care with a primary care physician and has a history of poor engagement with medical services in general; prior attempts to place her with a primary care physician failed.
In advance of Ms. W’s transfer to a residential treatment facility, you have been asked to place a Mantoux screening test for tuberculosis (purified protein derivative), which raises the important question about her susceptibility to infectious diseases in general. To protect Ms. W from preventable diseases for which vaccines are available, you review the ARM SHOT mnemonic to broadly assess her candidacy for vaccinations.
Age
Age may be the most important determinant of a patient’s need for vaccination (Table 2). The CDC immunization schedules account for age-specific risks for diseases, complications, and responses to vaccination (Figure 1).6
Influenza vaccination. Adults can have an intramuscular or intradermal inactivated influenza vaccination yearly in the fall or winter, unless they have an allergy to a vaccine component such as egg protein. Those with such an allergy can receive a recombinant influenza vaccine. Until the 2016 to 2017 flu season, an intranasal mist of live, attenuated influenza vaccine was available to healthy, non-pregnant women, ages 2 to 49, without high-risk medical conditions. However, the CDC dropped its recommendation for this vaccine because data showed it did not effectively prevent the flu.7 Individuals age ≥65 can receive either the standard- or high-dose inactivated influenza vaccination. The latter contains 4 times the amount of antigen with the intention of triggering a stronger immune response in older adults.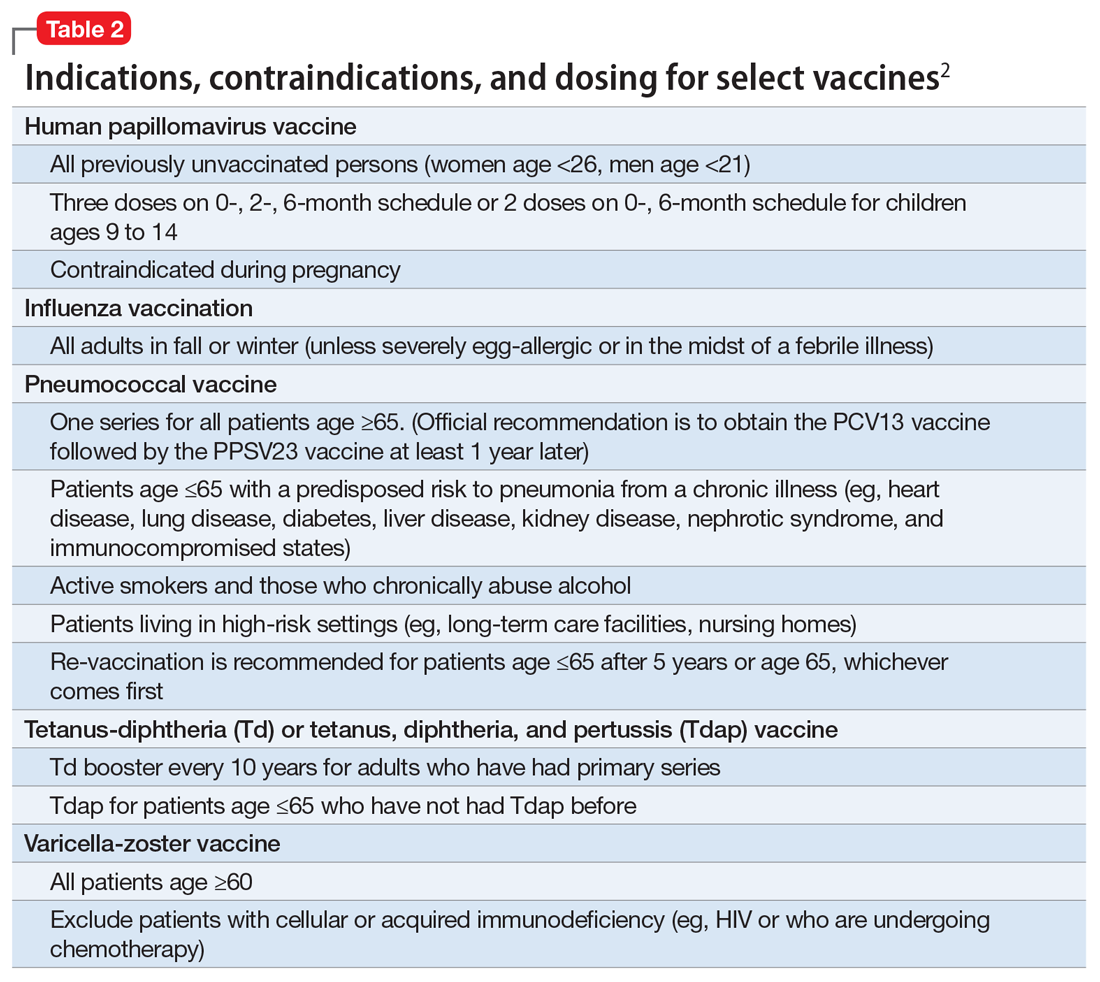
Pneumonia immunization. All patients age ≥65 should receive vaccinations for Streptococcus pneumoniae and its variants in the form of one 13-valent pneumococcal conjugate vaccine and, at least 1 year later, one 23-valent pneumococcal polysaccharide vaccine (PPSV23). Immunization reduces the morbidity and mortality from pneumococcal illness by decreasing the burden of a pneumonia, bacteremia, or meningitis infection. Adults, ages 19 to 64, with a chronic disease (referred to as “special populations” in CDC tables), such as diabetes, heart or lung disease, alcoholism, or cirrhosis, or those who smoke cigarettes, should receive PPSV23 with a second dose administered at least 5 years after the first. The CDC recommends a 1-time re-vaccination at age 65 for patients if >5 years have passed since the last PPSV23 and if the patient was younger than age 65 at the time of primary vaccine for S. pneumoniae. This can be a rather tricky clinical situation; the health care provider should verify a patient’s immunization history to ensure that she (he) is receiving only necessary vaccines. However, when the history cannot be verified, err on the side of inclusion, because risks are minimal.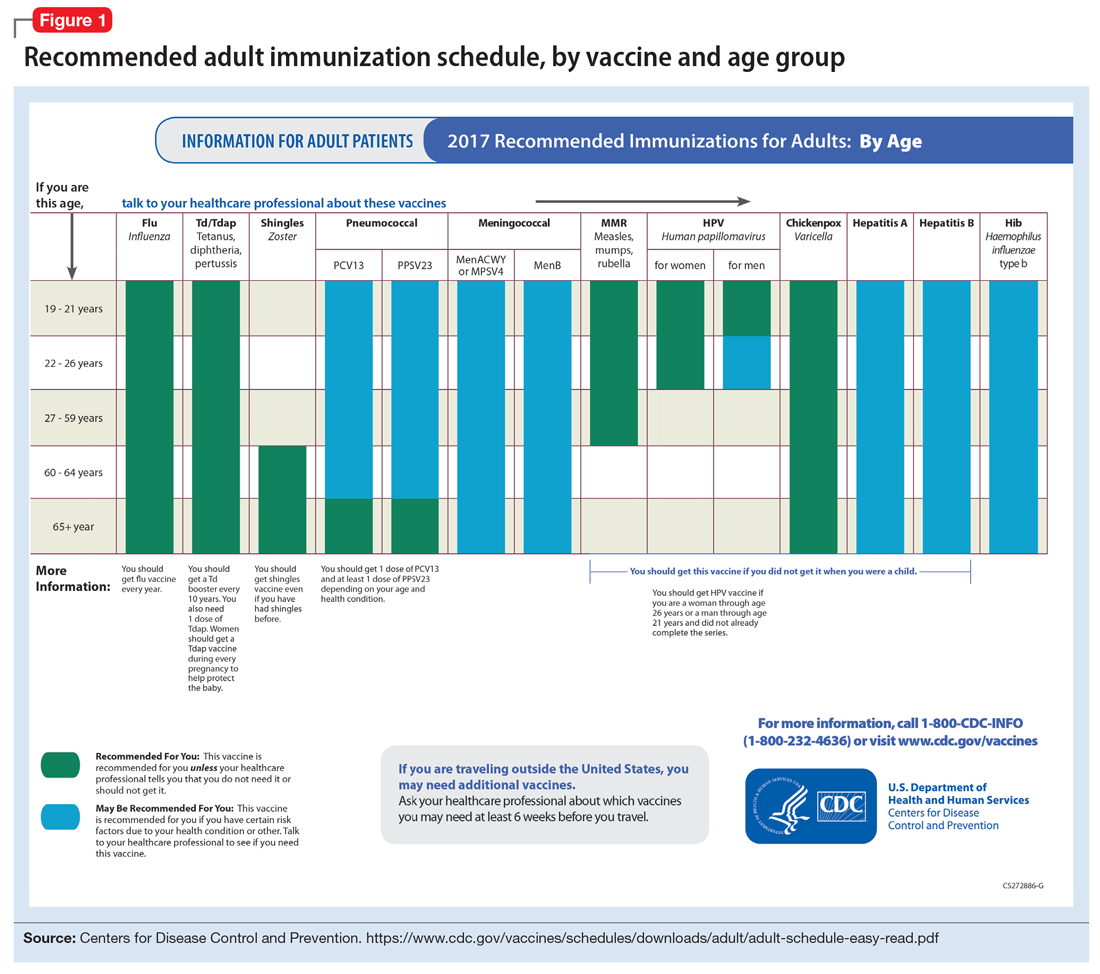
Shingles vaccination. Adults age ≥60 who are not immunocompromised should receive a single dose of live attenuated vaccine from varicella-zoster virus (VZV) to limit the risk of shingles from a prior chickenpox infection. The vaccine is approximately 66.5% effective at preventing postherpetic neuralgia for up to 4.9 years. Individuals as young as age 50 may have the vaccine because the risk of herpes zoster radically increases from then on,8 although most insurers only cover VZV vaccination after age 60.
Tetanus, diphtheria, and acellular pertussis (Tdap) vaccine. All adults should complete the 3-dose primary vaccination series for tetanus, diphtheria, and pertussis (also known as whooping cough) and this should include 1 dose of Tdap. Administration of the primary series is staged so that the second dose is given 4 weeks after the initial dose and the final dose 6 to 12 months after the first dose. After receiving the primary series, adults should receive a tetanus-diphtheria booster dose every 10 years. For adults ages 19 to 64, the Advisory Committee on Immunization Practices (ACIP) recommends 1 dose of Tdap in place of a booster vaccination to decrease the transmission risk of pertussis to vulnerable persons, especially children.
Human papillomavirus (HPV) immunization. The ACIP recommendation9 has been for children to receive routine vaccination for the 4 major strains of HPV—strains 6, 11, 16, and 18—starting at ages 11 to 12 to confer protection from HPV-associated diseases, such as genital warts, oropharyngeal cancer, and anal cancer; cancers of the cervix, vulva, and vagina in women; and penile cancer in men. Ideally, the vaccines are administered prior to HPV exposure from sexual contact. The quadrivalent HPV vaccine is safe and is administered as a 3-dose series, with the second and third doses given 2 and 6 months, respectively, after the initial dose. Adolescent girls also have the option of a bivalent HPV vaccine.
In 2016, the FDA approved a 9-valent HPV vaccine, a simpler 2-dose schedule for children ages 9 to 14 (2 doses at least 6 months apart). Leading cancer centers have endorsed this vaccine based on strong comparative data with the 3-dose regimen.10 For those not previously vaccinated, the HPV vaccine is available for women ages 13 to 26 and for men ages 13 to 21 (although men ages 22 to 26 can receive the vaccine, and it is recommended for men who have sex with men [MSM]). Women do not require Papanicolaou, serum pregnancy, HPV DNA, or HPV antibody tests prior to vaccination. If a woman becomes pregnant, remaining doses of the vaccine should be postponed until after delivery. Women still need to follow recommendations for cervical cancer screening because the HPV vaccine does not cover all genital strains of the virus. For sexually active individuals who might have HPV or genital warts, immunization has no clinical effect except to prevent other HPV strains.
Measles, mumps, and rubella (MMR) vaccine. All adults should receive, at minimum, 1 dose of MMR vaccination unless serological immunity can be verified or if contraindicated. Two doses of the vaccine are recommended for students attending post-high school institutions, health care personnel, and international travelers because they are at higher risk for exposure and transmission of measles and mumps. Individuals born before 1957 are considered immune to measles and mumps. A measles outbreak from December 2014 to February 201511 highlighted the importance of maintaining one’s immunity status for MMR.
Case continued
Based on Ms. W’s age, she should be offered vaccinations for influenza and opportunities to receive vaccinations for HPV, Tdap (the primary series, a Tdap or Td booster), and MMR, if appropriate and not completed previously.
Risk of exposure
Certain behaviors will increase the risk of exposure to and transmission of diseases communicable by blood and other bodily fluids (Table 3). These behaviors include needle injections (eg, during use of illicit drugs) and sexual activity with multiple partners, including MSM or promiscuity/impulsivity during a manic episode. A common consequence of risky behaviors is comorbid infection of HIV and viral hepatitis for those with substance use disorder or those who engage in high-risk sexual practices.12,13
Hepatitis B virus (HBV) immunization. Vaccination is one of the most effective ways to prevent HBV infection, which is why it is offered to all health care workers. HBV immunization is a 3-dose series in which the second and third doses are given 1 and 6 months after the initial doses, respectively. In addition to certain medical risk factors or conditions that indicate HBV vaccination, people should be offered the vaccine if they are in a higher risk occupation, travel, are of Asian or Pacific Islander ethnicity from an endemic area, or have any present or suspected sexually transmitted diseases.
Hepatitis A virus (HAV) vaccination. HAV is transmitted via fecal–oral routes, often from contaminated water or food, or through household or sexual contact with an infected person. Individuals should receive the HAV vaccine if they use illicit drugs by any route of administration, work with primates infected with HAV, travel to countries with unknown or high rates of HAV, or have chronic liver disease (ie, hepatitis, alcohol use disorder, or non-alcoholic fatty liver disease) or clotting deficiencies. The CDC Health Information for International Travel, commonly called the “Yellow Book,” publishes vaccination recommendations for those who plan travel to specific countries.14
Case continued
Ms. W’s history of mania (if such episodes included increased sexual activity) and substance use would make her a candidate for the HBV and HAV vaccinations and could also strengthen our previous recommendation that she receive the HPV vaccination.
Medical conditions
Patients with certain medical conditions may have difficulty fighting infections or become more susceptible to morbidity and mortality from coinfection with vaccine-preventable illnesses. Secondary effects of psychotropic medications that may carry implications for vaccine recommendations (eg, risk of agranulocytosis and impaired cell-medicated immunity with mirtazapine and clozapine or renal impairment from lithium use) are of particular concern in psychiatric patients.2
To help care for these patients, the CDC has developed a “medical conditions” schedule (Figure 2). This schedule makes vaccination recommendations for those with a weakened immune system, including patients with HIV, chronic obstructive pulmonary disease (COPD), diabetes, hepatitis, asplenia, end-stage renal disease, cardiac disease, and pregnancy.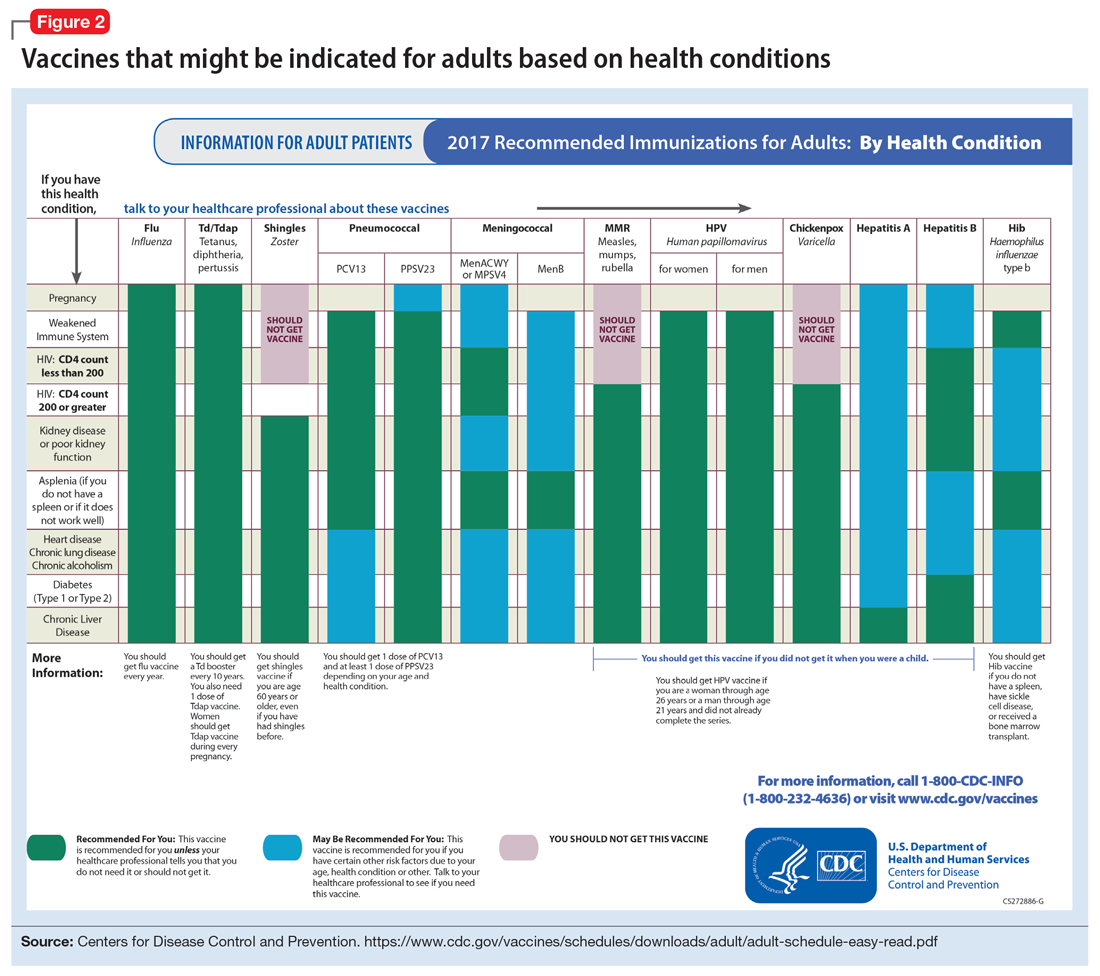
Because patients with psychiatric illness face a greater risk of heart disease and diabetes, these conditions may warrant special reference on the schedule. The increased cardiometabolic risk factors in these patients may be due in part to genetics, socioeconomic status, lifestyle behaviors, and medications to treat their mental illness (eg, antipsychotics). Patients with bipolar disorder or schizophrenia in particular tend to have higher rates of COPD (mainly from chronic bronchitis) and asthma than the general population.12 Pay special attention to the indications schedule for those with chronic lung disease, especially patients who continue to smoke cigarettes.
Case continued
Because of Ms. W’s asthma, the CDC schedule recommends ensuring she is up to date on her influenza, pneumococcal, and Tdap vaccinations.
Substance use
Patients with combined psychiatric and substance use disorders (“dual diagnosis”) have lower rates of receiving preventive care than patients with either condition alone.15 Substance use can be behaviorally disinhibiting, leading to increased risk of exposures from sexual contact or other risky activities. The use of illicit substances can provide a nidus for infection depending on the route of administration and can result in negative effects on organ systems, compromising one’s ability to ward off infection.
Patients who use any illicit drugs, regardless of the method of delivery, should be recommended for HAV vaccination. For those with alcohol use disorder and/or chronic liver disease, and/or seeking treatment for substance use, hepatitis B screening and vaccination is recommended.
Case continued
From a substance use perspective, discussion of vaccination status for both hepatitis A and B would be important for Ms. W.
HIV or immunocompromised
Persons with severe mental illness have high rates of HIV, with almost 8 times the risk of exposure, compared with the general population due to myriad reasons, including greater rates of substance abuse, higher risk sexual behavior, and lack of awareness of HIV transmission.12,13 Patients with mental illness are also at risk of leukopenia and agranulocytosis from certain drugs used to treat their conditions, such as clozapine.
Pregnancy is a challenge for women with mental illness because of the pharmacologic risk and immune-system compromise to the mother and baby. A pregnant woman who has HIV with a CD4 count <200, or has a weakened immune system from an organ transplant or a similar condition, is a candidate for certain vaccines based on the Adult Immunization Schedule (Figure 2). However, these patients should avoid live vaccines, such as the intranasal mist of live influenza, MMR, VZV, and varicella, to avoid illness from these inoculations.
Case continued
Ms. W should undergo testing for pregnancy and HIV (and preferably other sexually transmitted infections per general preventive health guidelines) before receiving any live vaccinations.
Occupancy
Aside from direct transmission of bodily fluids, infectious diseases also can spread through droplets/secretions from the throat and respiratory tract. Close quarters or lengthy contact enhances communicability by droplets, and therefore people who reside in a communal living space (eg, individuals in substance use treatment facilities or those who reside in a nursing home) are most susceptible.
The bacterial disease Neisseria meningitidis (meningococcus) can spread through droplets and can cause pneumonia, bacteremia, and meningitis. Vaccination is indicated, and in some states is mandated, for college students who live in residence halls and missed routine vaccination by age 16. Meningococcus conjugate vaccine is administered in 2 doses; each dose may be given at least 2 months apart for those with HIV, asplenia, or persistent complement-related disorders. A single dose may be recommended for travelers to areas where meningococcal disease is hyperendemic or epidemic, military recruits, or microbiologists. For those age ≥55 and older, meningococcal polysaccharide vaccine is recommended over meningococcal conjugate vaccine.
Influenza, MMR, diphtheria, pertussis, and pneumococcus also spread through droplet contact.
Case continued
If Ms. W had not previously received the meningococcus vaccine as part of adolescent immunizations, she could benefit from this vaccine because she plans to enter a residential substance use disorder treatment program.
Tobacco use
Patients with psychiatric illness are twice as likely to smoke compared with the general population.16 Adult smokers, especially those with chronic lung disease, are at higher risk for influenza and pneumococcal-related illness; they should be vaccinated against these illnesses regardless of age (as discussed in the “Age” section).
Case continued
Because she smokes, Ms. W should receive counseling on vaccinations, such as influenza and pneumonia, to lessen her risk of respiratory illnesses and downstream sepsis.
Conclusion
Ms. W’s case represents an unfortunately all-too-common scenario where her multifaceted biopsychosocial circumstances place her at high risk for vaccine-preventable conditions. Her weight is recorded and laboratory work ordered to evaluate her pregnancy status, blood counts, lipids, complete metabolic panel, lithium level, and HIV status. Fortunately, she had received her series of MMR, meningococcal, and Tdap vaccinations when she was younger. Influenza, HPV, HAV, HBV, and pneumococcal vaccinations were all recommended to her, all of which can be given on the same day (HAV and HBV often are available as a combined vaccine). Ms. W receives a renewal of her psychiatric medications and counseling on healthy living habits (eg, diet and exercise, quitting tobacco and alcohol use, and safe sex practices) and the importance of immunizations.
Vaccination is 1 of the 10 great public health achievements of the 20th century when one considers how immunization of vaccine-preventable diseases has reduced morbidity, mortality, and health-associated costs.17 As mental health professionals, we can help pass on the direct and indirect benefits of immunizations to an often underserved and undertreated population to help improve their health outcomes and quality of life.
1. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
2. Raj YP, Lloyd L. Adult immunizations. In: McCarron RM, Xiong GL, Keenan GR, et al, eds. Preventive medical care in psychiatry. Arlington, VA: American Psychiatric Publishing. 2015;215-227.
3. Young S, Dosani N, Whisler A, et al. Influenza vaccination rates among homeless adults with mental illness in Toronto. J Prim Care Community Health. 2015;6(3):211-214.
4. Kroger AT, Atkinson WL, Marcues EK, et al; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). General recommendations on immunization: recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-15):1-48.
5. Centers for Disease Control and Prevention. Recommended Adult Immunization by Vaccine and Age Group. http://www.cdc.gov/vaccines/schedules/hcp/adult.html. Updated February 27, 2017. Accessed February 1, 2017.
6. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(2):1-64.
7. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. https://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Updated June 22, 2016. Accessed February 1, 2017.
8. Hales CM, Harpaz, R, Ortega-Sanchez I, et al; Centers for Disease Control and Prevention. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63(33):729-731.
9. Petrosky E, Bocchini Jr JA, Hariri S, et al; Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccine recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11)300-304.
10. Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316(22):2411-2421.
11. Zipprich J, Winter K, Hacker J, et al; Centers for Disease Control and Prevention (CDC). Measles outbreak—California, December 2014-February 2015. MMWR Morb Mortal Wkly Rep. 2015;64(6):153-154.
12. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
13. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
14. Centers for Disease for Control and Prevention. CDC yellow book 2018: health information for international travel. New York, NY: Oxford University Press; 2017.
15. Druss BG, Rosenheck RA, Desai MM, et al. Quality of preventive medical care for patients with mental disorders. Med Care. 2002;40(2):129-136.
16. Lasser K, Boyd J, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606-2610.
17. Centers for Disease Control and Prevention (CDC). Ten great public health achievements—United States, 2001-2010. MMWR Morb Mortal Wkly Rep. 2011;60(19);619-623.
Patients with chronic, severe mental illness live much shorter lives than the general population. The 25-year loss in life expectancy for people with chronic mental illness has been attributed to higher rates of cardiovascular disease driven by increased smoking, obesity, poverty, and poor nutrition.1 These individuals also face the added burden of struggling with a psychiatric condition that often interferes with their ability to make optimal preventative health decisions, including staying up to date on vaccinations.2 A recent review from Toronto, Canada, found that the influenza vaccination rates among homeless adults with mental illness—a population at high risk of respiratory illness—was only 6.7% compared with 31.1% for the general population of Ontario.3
Mental health professionals may serve as the only contacts to offer medical care to this vulnerable population, leading some psychiatric leaders to advocate that psychiatrists be considered primary care providers within accountable care organizations. Because most vaccines are easily available, mental health professionals should know about key immunizations to guide their patients accordingly.
In the United States, approximately 45,000 adults die annually from vaccine-preventable diseases, the majority from influenza.4 When combined with the most recent Adult Immunization Schedule and general recommendations adapted from the CDC,5,6 the mnemonic ARM SHOT allows for a quick assessment of risk factors to guide administration and education about most vaccinations (Table 1). ARM SHOT involves assessing the following components of an individual’s health status and living arrangements to determine one’s risk of contracting communicable diseases:
- Age
- Risk of exposure
- Medical conditions (comorbidities)
- Substance use history
- HIV status or other immunocompromised states
- Occupancy, or living arrangements
- Tobacco use.

We recommend keeping a copy of the Adult Immunization Schedule (age ≥19) and/or the immunization schedule for children and adolescents (age ≤18) close for quick reference. Here, we provide a case and then explore how each component of the ARM SHOT mnemonic applies in decision-making.
Case Evaluating risk, assess needs
Ms. W, age 24, has bipolar I disorder, most recently manic with psychotic features. She presents for follow-up in clinic after a 5-day hospitalization for mania and comorbid alcohol use disorder. Her medical comorbidities include asthma and active tobacco use. She is taking lurasidone, 20 mg/d, and lithium, 900 mg/d. Her case manager is working to place Ms. W in a residential substance use disorder treatment program. Ms. W is on a waiting list to establish care with a primary care physician and has a history of poor engagement with medical services in general; prior attempts to place her with a primary care physician failed.
In advance of Ms. W’s transfer to a residential treatment facility, you have been asked to place a Mantoux screening test for tuberculosis (purified protein derivative), which raises the important question about her susceptibility to infectious diseases in general. To protect Ms. W from preventable diseases for which vaccines are available, you review the ARM SHOT mnemonic to broadly assess her candidacy for vaccinations.
Age
Age may be the most important determinant of a patient’s need for vaccination (Table 2). The CDC immunization schedules account for age-specific risks for diseases, complications, and responses to vaccination (Figure 1).6
Influenza vaccination. Adults can have an intramuscular or intradermal inactivated influenza vaccination yearly in the fall or winter, unless they have an allergy to a vaccine component such as egg protein. Those with such an allergy can receive a recombinant influenza vaccine. Until the 2016 to 2017 flu season, an intranasal mist of live, attenuated influenza vaccine was available to healthy, non-pregnant women, ages 2 to 49, without high-risk medical conditions. However, the CDC dropped its recommendation for this vaccine because data showed it did not effectively prevent the flu.7 Individuals age ≥65 can receive either the standard- or high-dose inactivated influenza vaccination. The latter contains 4 times the amount of antigen with the intention of triggering a stronger immune response in older adults.
Pneumonia immunization. All patients age ≥65 should receive vaccinations for Streptococcus pneumoniae and its variants in the form of one 13-valent pneumococcal conjugate vaccine and, at least 1 year later, one 23-valent pneumococcal polysaccharide vaccine (PPSV23). Immunization reduces the morbidity and mortality from pneumococcal illness by decreasing the burden of a pneumonia, bacteremia, or meningitis infection. Adults, ages 19 to 64, with a chronic disease (referred to as “special populations” in CDC tables), such as diabetes, heart or lung disease, alcoholism, or cirrhosis, or those who smoke cigarettes, should receive PPSV23 with a second dose administered at least 5 years after the first. The CDC recommends a 1-time re-vaccination at age 65 for patients if >5 years have passed since the last PPSV23 and if the patient was younger than age 65 at the time of primary vaccine for S. pneumoniae. This can be a rather tricky clinical situation; the health care provider should verify a patient’s immunization history to ensure that she (he) is receiving only necessary vaccines. However, when the history cannot be verified, err on the side of inclusion, because risks are minimal.
Shingles vaccination. Adults age ≥60 who are not immunocompromised should receive a single dose of live attenuated vaccine from varicella-zoster virus (VZV) to limit the risk of shingles from a prior chickenpox infection. The vaccine is approximately 66.5% effective at preventing postherpetic neuralgia for up to 4.9 years. Individuals as young as age 50 may have the vaccine because the risk of herpes zoster radically increases from then on,8 although most insurers only cover VZV vaccination after age 60.
Tetanus, diphtheria, and acellular pertussis (Tdap) vaccine. All adults should complete the 3-dose primary vaccination series for tetanus, diphtheria, and pertussis (also known as whooping cough) and this should include 1 dose of Tdap. Administration of the primary series is staged so that the second dose is given 4 weeks after the initial dose and the final dose 6 to 12 months after the first dose. After receiving the primary series, adults should receive a tetanus-diphtheria booster dose every 10 years. For adults ages 19 to 64, the Advisory Committee on Immunization Practices (ACIP) recommends 1 dose of Tdap in place of a booster vaccination to decrease the transmission risk of pertussis to vulnerable persons, especially children.
Human papillomavirus (HPV) immunization. The ACIP recommendation9 has been for children to receive routine vaccination for the 4 major strains of HPV—strains 6, 11, 16, and 18—starting at ages 11 to 12 to confer protection from HPV-associated diseases, such as genital warts, oropharyngeal cancer, and anal cancer; cancers of the cervix, vulva, and vagina in women; and penile cancer in men. Ideally, the vaccines are administered prior to HPV exposure from sexual contact. The quadrivalent HPV vaccine is safe and is administered as a 3-dose series, with the second and third doses given 2 and 6 months, respectively, after the initial dose. Adolescent girls also have the option of a bivalent HPV vaccine.
In 2016, the FDA approved a 9-valent HPV vaccine, a simpler 2-dose schedule for children ages 9 to 14 (2 doses at least 6 months apart). Leading cancer centers have endorsed this vaccine based on strong comparative data with the 3-dose regimen.10 For those not previously vaccinated, the HPV vaccine is available for women ages 13 to 26 and for men ages 13 to 21 (although men ages 22 to 26 can receive the vaccine, and it is recommended for men who have sex with men [MSM]). Women do not require Papanicolaou, serum pregnancy, HPV DNA, or HPV antibody tests prior to vaccination. If a woman becomes pregnant, remaining doses of the vaccine should be postponed until after delivery. Women still need to follow recommendations for cervical cancer screening because the HPV vaccine does not cover all genital strains of the virus. For sexually active individuals who might have HPV or genital warts, immunization has no clinical effect except to prevent other HPV strains.
Measles, mumps, and rubella (MMR) vaccine. All adults should receive, at minimum, 1 dose of MMR vaccination unless serological immunity can be verified or if contraindicated. Two doses of the vaccine are recommended for students attending post-high school institutions, health care personnel, and international travelers because they are at higher risk for exposure and transmission of measles and mumps. Individuals born before 1957 are considered immune to measles and mumps. A measles outbreak from December 2014 to February 201511 highlighted the importance of maintaining one’s immunity status for MMR.
Case continued
Based on Ms. W’s age, she should be offered vaccinations for influenza and opportunities to receive vaccinations for HPV, Tdap (the primary series, a Tdap or Td booster), and MMR, if appropriate and not completed previously.
Risk of exposure
Certain behaviors will increase the risk of exposure to and transmission of diseases communicable by blood and other bodily fluids (Table 3). These behaviors include needle injections (eg, during use of illicit drugs) and sexual activity with multiple partners, including MSM or promiscuity/impulsivity during a manic episode. A common consequence of risky behaviors is comorbid infection of HIV and viral hepatitis for those with substance use disorder or those who engage in high-risk sexual practices.12,13
Hepatitis B virus (HBV) immunization. Vaccination is one of the most effective ways to prevent HBV infection, which is why it is offered to all health care workers. HBV immunization is a 3-dose series in which the second and third doses are given 1 and 6 months after the initial doses, respectively. In addition to certain medical risk factors or conditions that indicate HBV vaccination, people should be offered the vaccine if they are in a higher risk occupation, travel, are of Asian or Pacific Islander ethnicity from an endemic area, or have any present or suspected sexually transmitted diseases.
Hepatitis A virus (HAV) vaccination. HAV is transmitted via fecal–oral routes, often from contaminated water or food, or through household or sexual contact with an infected person. Individuals should receive the HAV vaccine if they use illicit drugs by any route of administration, work with primates infected with HAV, travel to countries with unknown or high rates of HAV, or have chronic liver disease (ie, hepatitis, alcohol use disorder, or non-alcoholic fatty liver disease) or clotting deficiencies. The CDC Health Information for International Travel, commonly called the “Yellow Book,” publishes vaccination recommendations for those who plan travel to specific countries.14
Case continued
Ms. W’s history of mania (if such episodes included increased sexual activity) and substance use would make her a candidate for the HBV and HAV vaccinations and could also strengthen our previous recommendation that she receive the HPV vaccination.
Medical conditions
Patients with certain medical conditions may have difficulty fighting infections or become more susceptible to morbidity and mortality from coinfection with vaccine-preventable illnesses. Secondary effects of psychotropic medications that may carry implications for vaccine recommendations (eg, risk of agranulocytosis and impaired cell-medicated immunity with mirtazapine and clozapine or renal impairment from lithium use) are of particular concern in psychiatric patients.2
To help care for these patients, the CDC has developed a “medical conditions” schedule (Figure 2). This schedule makes vaccination recommendations for those with a weakened immune system, including patients with HIV, chronic obstructive pulmonary disease (COPD), diabetes, hepatitis, asplenia, end-stage renal disease, cardiac disease, and pregnancy.
Because patients with psychiatric illness face a greater risk of heart disease and diabetes, these conditions may warrant special reference on the schedule. The increased cardiometabolic risk factors in these patients may be due in part to genetics, socioeconomic status, lifestyle behaviors, and medications to treat their mental illness (eg, antipsychotics). Patients with bipolar disorder or schizophrenia in particular tend to have higher rates of COPD (mainly from chronic bronchitis) and asthma than the general population.12 Pay special attention to the indications schedule for those with chronic lung disease, especially patients who continue to smoke cigarettes.
Case continued
Because of Ms. W’s asthma, the CDC schedule recommends ensuring she is up to date on her influenza, pneumococcal, and Tdap vaccinations.
Substance use
Patients with combined psychiatric and substance use disorders (“dual diagnosis”) have lower rates of receiving preventive care than patients with either condition alone.15 Substance use can be behaviorally disinhibiting, leading to increased risk of exposures from sexual contact or other risky activities. The use of illicit substances can provide a nidus for infection depending on the route of administration and can result in negative effects on organ systems, compromising one’s ability to ward off infection.
Patients who use any illicit drugs, regardless of the method of delivery, should be recommended for HAV vaccination. For those with alcohol use disorder and/or chronic liver disease, and/or seeking treatment for substance use, hepatitis B screening and vaccination is recommended.
Case continued
From a substance use perspective, discussion of vaccination status for both hepatitis A and B would be important for Ms. W.
HIV or immunocompromised
Persons with severe mental illness have high rates of HIV, with almost 8 times the risk of exposure, compared with the general population due to myriad reasons, including greater rates of substance abuse, higher risk sexual behavior, and lack of awareness of HIV transmission.12,13 Patients with mental illness are also at risk of leukopenia and agranulocytosis from certain drugs used to treat their conditions, such as clozapine.
Pregnancy is a challenge for women with mental illness because of the pharmacologic risk and immune-system compromise to the mother and baby. A pregnant woman who has HIV with a CD4 count <200, or has a weakened immune system from an organ transplant or a similar condition, is a candidate for certain vaccines based on the Adult Immunization Schedule (Figure 2). However, these patients should avoid live vaccines, such as the intranasal mist of live influenza, MMR, VZV, and varicella, to avoid illness from these inoculations.
Case continued
Ms. W should undergo testing for pregnancy and HIV (and preferably other sexually transmitted infections per general preventive health guidelines) before receiving any live vaccinations.
Occupancy
Aside from direct transmission of bodily fluids, infectious diseases also can spread through droplets/secretions from the throat and respiratory tract. Close quarters or lengthy contact enhances communicability by droplets, and therefore people who reside in a communal living space (eg, individuals in substance use treatment facilities or those who reside in a nursing home) are most susceptible.
The bacterial disease Neisseria meningitidis (meningococcus) can spread through droplets and can cause pneumonia, bacteremia, and meningitis. Vaccination is indicated, and in some states is mandated, for college students who live in residence halls and missed routine vaccination by age 16. Meningococcus conjugate vaccine is administered in 2 doses; each dose may be given at least 2 months apart for those with HIV, asplenia, or persistent complement-related disorders. A single dose may be recommended for travelers to areas where meningococcal disease is hyperendemic or epidemic, military recruits, or microbiologists. For those age ≥55 and older, meningococcal polysaccharide vaccine is recommended over meningococcal conjugate vaccine.
Influenza, MMR, diphtheria, pertussis, and pneumococcus also spread through droplet contact.
Case continued
If Ms. W had not previously received the meningococcus vaccine as part of adolescent immunizations, she could benefit from this vaccine because she plans to enter a residential substance use disorder treatment program.
Tobacco use
Patients with psychiatric illness are twice as likely to smoke compared with the general population.16 Adult smokers, especially those with chronic lung disease, are at higher risk for influenza and pneumococcal-related illness; they should be vaccinated against these illnesses regardless of age (as discussed in the “Age” section).
Case continued
Because she smokes, Ms. W should receive counseling on vaccinations, such as influenza and pneumonia, to lessen her risk of respiratory illnesses and downstream sepsis.
Conclusion
Ms. W’s case represents an unfortunately all-too-common scenario where her multifaceted biopsychosocial circumstances place her at high risk for vaccine-preventable conditions. Her weight is recorded and laboratory work ordered to evaluate her pregnancy status, blood counts, lipids, complete metabolic panel, lithium level, and HIV status. Fortunately, she had received her series of MMR, meningococcal, and Tdap vaccinations when she was younger. Influenza, HPV, HAV, HBV, and pneumococcal vaccinations were all recommended to her, all of which can be given on the same day (HAV and HBV often are available as a combined vaccine). Ms. W receives a renewal of her psychiatric medications and counseling on healthy living habits (eg, diet and exercise, quitting tobacco and alcohol use, and safe sex practices) and the importance of immunizations.
Vaccination is 1 of the 10 great public health achievements of the 20th century when one considers how immunization of vaccine-preventable diseases has reduced morbidity, mortality, and health-associated costs.17 As mental health professionals, we can help pass on the direct and indirect benefits of immunizations to an often underserved and undertreated population to help improve their health outcomes and quality of life.
Patients with chronic, severe mental illness live much shorter lives than the general population. The 25-year loss in life expectancy for people with chronic mental illness has been attributed to higher rates of cardiovascular disease driven by increased smoking, obesity, poverty, and poor nutrition.1 These individuals also face the added burden of struggling with a psychiatric condition that often interferes with their ability to make optimal preventative health decisions, including staying up to date on vaccinations.2 A recent review from Toronto, Canada, found that the influenza vaccination rates among homeless adults with mental illness—a population at high risk of respiratory illness—was only 6.7% compared with 31.1% for the general population of Ontario.3
Mental health professionals may serve as the only contacts to offer medical care to this vulnerable population, leading some psychiatric leaders to advocate that psychiatrists be considered primary care providers within accountable care organizations. Because most vaccines are easily available, mental health professionals should know about key immunizations to guide their patients accordingly.
In the United States, approximately 45,000 adults die annually from vaccine-preventable diseases, the majority from influenza.4 When combined with the most recent Adult Immunization Schedule and general recommendations adapted from the CDC,5,6 the mnemonic ARM SHOT allows for a quick assessment of risk factors to guide administration and education about most vaccinations (Table 1). ARM SHOT involves assessing the following components of an individual’s health status and living arrangements to determine one’s risk of contracting communicable diseases:
- Age
- Risk of exposure
- Medical conditions (comorbidities)
- Substance use history
- HIV status or other immunocompromised states
- Occupancy, or living arrangements
- Tobacco use.

We recommend keeping a copy of the Adult Immunization Schedule (age ≥19) and/or the immunization schedule for children and adolescents (age ≤18) close for quick reference. Here, we provide a case and then explore how each component of the ARM SHOT mnemonic applies in decision-making.
Case Evaluating risk, assess needs
Ms. W, age 24, has bipolar I disorder, most recently manic with psychotic features. She presents for follow-up in clinic after a 5-day hospitalization for mania and comorbid alcohol use disorder. Her medical comorbidities include asthma and active tobacco use. She is taking lurasidone, 20 mg/d, and lithium, 900 mg/d. Her case manager is working to place Ms. W in a residential substance use disorder treatment program. Ms. W is on a waiting list to establish care with a primary care physician and has a history of poor engagement with medical services in general; prior attempts to place her with a primary care physician failed.
In advance of Ms. W’s transfer to a residential treatment facility, you have been asked to place a Mantoux screening test for tuberculosis (purified protein derivative), which raises the important question about her susceptibility to infectious diseases in general. To protect Ms. W from preventable diseases for which vaccines are available, you review the ARM SHOT mnemonic to broadly assess her candidacy for vaccinations.
Age
Age may be the most important determinant of a patient’s need for vaccination (Table 2). The CDC immunization schedules account for age-specific risks for diseases, complications, and responses to vaccination (Figure 1).6
Influenza vaccination. Adults can have an intramuscular or intradermal inactivated influenza vaccination yearly in the fall or winter, unless they have an allergy to a vaccine component such as egg protein. Those with such an allergy can receive a recombinant influenza vaccine. Until the 2016 to 2017 flu season, an intranasal mist of live, attenuated influenza vaccine was available to healthy, non-pregnant women, ages 2 to 49, without high-risk medical conditions. However, the CDC dropped its recommendation for this vaccine because data showed it did not effectively prevent the flu.7 Individuals age ≥65 can receive either the standard- or high-dose inactivated influenza vaccination. The latter contains 4 times the amount of antigen with the intention of triggering a stronger immune response in older adults.
Pneumonia immunization. All patients age ≥65 should receive vaccinations for Streptococcus pneumoniae and its variants in the form of one 13-valent pneumococcal conjugate vaccine and, at least 1 year later, one 23-valent pneumococcal polysaccharide vaccine (PPSV23). Immunization reduces the morbidity and mortality from pneumococcal illness by decreasing the burden of a pneumonia, bacteremia, or meningitis infection. Adults, ages 19 to 64, with a chronic disease (referred to as “special populations” in CDC tables), such as diabetes, heart or lung disease, alcoholism, or cirrhosis, or those who smoke cigarettes, should receive PPSV23 with a second dose administered at least 5 years after the first. The CDC recommends a 1-time re-vaccination at age 65 for patients if >5 years have passed since the last PPSV23 and if the patient was younger than age 65 at the time of primary vaccine for S. pneumoniae. This can be a rather tricky clinical situation; the health care provider should verify a patient’s immunization history to ensure that she (he) is receiving only necessary vaccines. However, when the history cannot be verified, err on the side of inclusion, because risks are minimal.
Shingles vaccination. Adults age ≥60 who are not immunocompromised should receive a single dose of live attenuated vaccine from varicella-zoster virus (VZV) to limit the risk of shingles from a prior chickenpox infection. The vaccine is approximately 66.5% effective at preventing postherpetic neuralgia for up to 4.9 years. Individuals as young as age 50 may have the vaccine because the risk of herpes zoster radically increases from then on,8 although most insurers only cover VZV vaccination after age 60.
Tetanus, diphtheria, and acellular pertussis (Tdap) vaccine. All adults should complete the 3-dose primary vaccination series for tetanus, diphtheria, and pertussis (also known as whooping cough) and this should include 1 dose of Tdap. Administration of the primary series is staged so that the second dose is given 4 weeks after the initial dose and the final dose 6 to 12 months after the first dose. After receiving the primary series, adults should receive a tetanus-diphtheria booster dose every 10 years. For adults ages 19 to 64, the Advisory Committee on Immunization Practices (ACIP) recommends 1 dose of Tdap in place of a booster vaccination to decrease the transmission risk of pertussis to vulnerable persons, especially children.
Human papillomavirus (HPV) immunization. The ACIP recommendation9 has been for children to receive routine vaccination for the 4 major strains of HPV—strains 6, 11, 16, and 18—starting at ages 11 to 12 to confer protection from HPV-associated diseases, such as genital warts, oropharyngeal cancer, and anal cancer; cancers of the cervix, vulva, and vagina in women; and penile cancer in men. Ideally, the vaccines are administered prior to HPV exposure from sexual contact. The quadrivalent HPV vaccine is safe and is administered as a 3-dose series, with the second and third doses given 2 and 6 months, respectively, after the initial dose. Adolescent girls also have the option of a bivalent HPV vaccine.
In 2016, the FDA approved a 9-valent HPV vaccine, a simpler 2-dose schedule for children ages 9 to 14 (2 doses at least 6 months apart). Leading cancer centers have endorsed this vaccine based on strong comparative data with the 3-dose regimen.10 For those not previously vaccinated, the HPV vaccine is available for women ages 13 to 26 and for men ages 13 to 21 (although men ages 22 to 26 can receive the vaccine, and it is recommended for men who have sex with men [MSM]). Women do not require Papanicolaou, serum pregnancy, HPV DNA, or HPV antibody tests prior to vaccination. If a woman becomes pregnant, remaining doses of the vaccine should be postponed until after delivery. Women still need to follow recommendations for cervical cancer screening because the HPV vaccine does not cover all genital strains of the virus. For sexually active individuals who might have HPV or genital warts, immunization has no clinical effect except to prevent other HPV strains.
Measles, mumps, and rubella (MMR) vaccine. All adults should receive, at minimum, 1 dose of MMR vaccination unless serological immunity can be verified or if contraindicated. Two doses of the vaccine are recommended for students attending post-high school institutions, health care personnel, and international travelers because they are at higher risk for exposure and transmission of measles and mumps. Individuals born before 1957 are considered immune to measles and mumps. A measles outbreak from December 2014 to February 201511 highlighted the importance of maintaining one’s immunity status for MMR.
Case continued
Based on Ms. W’s age, she should be offered vaccinations for influenza and opportunities to receive vaccinations for HPV, Tdap (the primary series, a Tdap or Td booster), and MMR, if appropriate and not completed previously.
Risk of exposure
Certain behaviors will increase the risk of exposure to and transmission of diseases communicable by blood and other bodily fluids (Table 3). These behaviors include needle injections (eg, during use of illicit drugs) and sexual activity with multiple partners, including MSM or promiscuity/impulsivity during a manic episode. A common consequence of risky behaviors is comorbid infection of HIV and viral hepatitis for those with substance use disorder or those who engage in high-risk sexual practices.12,13
Hepatitis B virus (HBV) immunization. Vaccination is one of the most effective ways to prevent HBV infection, which is why it is offered to all health care workers. HBV immunization is a 3-dose series in which the second and third doses are given 1 and 6 months after the initial doses, respectively. In addition to certain medical risk factors or conditions that indicate HBV vaccination, people should be offered the vaccine if they are in a higher risk occupation, travel, are of Asian or Pacific Islander ethnicity from an endemic area, or have any present or suspected sexually transmitted diseases.
Hepatitis A virus (HAV) vaccination. HAV is transmitted via fecal–oral routes, often from contaminated water or food, or through household or sexual contact with an infected person. Individuals should receive the HAV vaccine if they use illicit drugs by any route of administration, work with primates infected with HAV, travel to countries with unknown or high rates of HAV, or have chronic liver disease (ie, hepatitis, alcohol use disorder, or non-alcoholic fatty liver disease) or clotting deficiencies. The CDC Health Information for International Travel, commonly called the “Yellow Book,” publishes vaccination recommendations for those who plan travel to specific countries.14
Case continued
Ms. W’s history of mania (if such episodes included increased sexual activity) and substance use would make her a candidate for the HBV and HAV vaccinations and could also strengthen our previous recommendation that she receive the HPV vaccination.
Medical conditions
Patients with certain medical conditions may have difficulty fighting infections or become more susceptible to morbidity and mortality from coinfection with vaccine-preventable illnesses. Secondary effects of psychotropic medications that may carry implications for vaccine recommendations (eg, risk of agranulocytosis and impaired cell-medicated immunity with mirtazapine and clozapine or renal impairment from lithium use) are of particular concern in psychiatric patients.2
To help care for these patients, the CDC has developed a “medical conditions” schedule (Figure 2). This schedule makes vaccination recommendations for those with a weakened immune system, including patients with HIV, chronic obstructive pulmonary disease (COPD), diabetes, hepatitis, asplenia, end-stage renal disease, cardiac disease, and pregnancy.
Because patients with psychiatric illness face a greater risk of heart disease and diabetes, these conditions may warrant special reference on the schedule. The increased cardiometabolic risk factors in these patients may be due in part to genetics, socioeconomic status, lifestyle behaviors, and medications to treat their mental illness (eg, antipsychotics). Patients with bipolar disorder or schizophrenia in particular tend to have higher rates of COPD (mainly from chronic bronchitis) and asthma than the general population.12 Pay special attention to the indications schedule for those with chronic lung disease, especially patients who continue to smoke cigarettes.
Case continued
Because of Ms. W’s asthma, the CDC schedule recommends ensuring she is up to date on her influenza, pneumococcal, and Tdap vaccinations.
Substance use
Patients with combined psychiatric and substance use disorders (“dual diagnosis”) have lower rates of receiving preventive care than patients with either condition alone.15 Substance use can be behaviorally disinhibiting, leading to increased risk of exposures from sexual contact or other risky activities. The use of illicit substances can provide a nidus for infection depending on the route of administration and can result in negative effects on organ systems, compromising one’s ability to ward off infection.
Patients who use any illicit drugs, regardless of the method of delivery, should be recommended for HAV vaccination. For those with alcohol use disorder and/or chronic liver disease, and/or seeking treatment for substance use, hepatitis B screening and vaccination is recommended.
Case continued
From a substance use perspective, discussion of vaccination status for both hepatitis A and B would be important for Ms. W.
HIV or immunocompromised
Persons with severe mental illness have high rates of HIV, with almost 8 times the risk of exposure, compared with the general population due to myriad reasons, including greater rates of substance abuse, higher risk sexual behavior, and lack of awareness of HIV transmission.12,13 Patients with mental illness are also at risk of leukopenia and agranulocytosis from certain drugs used to treat their conditions, such as clozapine.
Pregnancy is a challenge for women with mental illness because of the pharmacologic risk and immune-system compromise to the mother and baby. A pregnant woman who has HIV with a CD4 count <200, or has a weakened immune system from an organ transplant or a similar condition, is a candidate for certain vaccines based on the Adult Immunization Schedule (Figure 2). However, these patients should avoid live vaccines, such as the intranasal mist of live influenza, MMR, VZV, and varicella, to avoid illness from these inoculations.
Case continued
Ms. W should undergo testing for pregnancy and HIV (and preferably other sexually transmitted infections per general preventive health guidelines) before receiving any live vaccinations.
Occupancy
Aside from direct transmission of bodily fluids, infectious diseases also can spread through droplets/secretions from the throat and respiratory tract. Close quarters or lengthy contact enhances communicability by droplets, and therefore people who reside in a communal living space (eg, individuals in substance use treatment facilities or those who reside in a nursing home) are most susceptible.
The bacterial disease Neisseria meningitidis (meningococcus) can spread through droplets and can cause pneumonia, bacteremia, and meningitis. Vaccination is indicated, and in some states is mandated, for college students who live in residence halls and missed routine vaccination by age 16. Meningococcus conjugate vaccine is administered in 2 doses; each dose may be given at least 2 months apart for those with HIV, asplenia, or persistent complement-related disorders. A single dose may be recommended for travelers to areas where meningococcal disease is hyperendemic or epidemic, military recruits, or microbiologists. For those age ≥55 and older, meningococcal polysaccharide vaccine is recommended over meningococcal conjugate vaccine.
Influenza, MMR, diphtheria, pertussis, and pneumococcus also spread through droplet contact.
Case continued
If Ms. W had not previously received the meningococcus vaccine as part of adolescent immunizations, she could benefit from this vaccine because she plans to enter a residential substance use disorder treatment program.
Tobacco use
Patients with psychiatric illness are twice as likely to smoke compared with the general population.16 Adult smokers, especially those with chronic lung disease, are at higher risk for influenza and pneumococcal-related illness; they should be vaccinated against these illnesses regardless of age (as discussed in the “Age” section).
Case continued
Because she smokes, Ms. W should receive counseling on vaccinations, such as influenza and pneumonia, to lessen her risk of respiratory illnesses and downstream sepsis.
Conclusion
Ms. W’s case represents an unfortunately all-too-common scenario where her multifaceted biopsychosocial circumstances place her at high risk for vaccine-preventable conditions. Her weight is recorded and laboratory work ordered to evaluate her pregnancy status, blood counts, lipids, complete metabolic panel, lithium level, and HIV status. Fortunately, she had received her series of MMR, meningococcal, and Tdap vaccinations when she was younger. Influenza, HPV, HAV, HBV, and pneumococcal vaccinations were all recommended to her, all of which can be given on the same day (HAV and HBV often are available as a combined vaccine). Ms. W receives a renewal of her psychiatric medications and counseling on healthy living habits (eg, diet and exercise, quitting tobacco and alcohol use, and safe sex practices) and the importance of immunizations.
Vaccination is 1 of the 10 great public health achievements of the 20th century when one considers how immunization of vaccine-preventable diseases has reduced morbidity, mortality, and health-associated costs.17 As mental health professionals, we can help pass on the direct and indirect benefits of immunizations to an often underserved and undertreated population to help improve their health outcomes and quality of life.
1. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
2. Raj YP, Lloyd L. Adult immunizations. In: McCarron RM, Xiong GL, Keenan GR, et al, eds. Preventive medical care in psychiatry. Arlington, VA: American Psychiatric Publishing. 2015;215-227.
3. Young S, Dosani N, Whisler A, et al. Influenza vaccination rates among homeless adults with mental illness in Toronto. J Prim Care Community Health. 2015;6(3):211-214.
4. Kroger AT, Atkinson WL, Marcues EK, et al; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). General recommendations on immunization: recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-15):1-48.
5. Centers for Disease Control and Prevention. Recommended Adult Immunization by Vaccine and Age Group. http://www.cdc.gov/vaccines/schedules/hcp/adult.html. Updated February 27, 2017. Accessed February 1, 2017.
6. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(2):1-64.
7. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. https://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Updated June 22, 2016. Accessed February 1, 2017.
8. Hales CM, Harpaz, R, Ortega-Sanchez I, et al; Centers for Disease Control and Prevention. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63(33):729-731.
9. Petrosky E, Bocchini Jr JA, Hariri S, et al; Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccine recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11)300-304.
10. Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316(22):2411-2421.
11. Zipprich J, Winter K, Hacker J, et al; Centers for Disease Control and Prevention (CDC). Measles outbreak—California, December 2014-February 2015. MMWR Morb Mortal Wkly Rep. 2015;64(6):153-154.
12. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
13. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
14. Centers for Disease for Control and Prevention. CDC yellow book 2018: health information for international travel. New York, NY: Oxford University Press; 2017.
15. Druss BG, Rosenheck RA, Desai MM, et al. Quality of preventive medical care for patients with mental disorders. Med Care. 2002;40(2):129-136.
16. Lasser K, Boyd J, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606-2610.
17. Centers for Disease Control and Prevention (CDC). Ten great public health achievements—United States, 2001-2010. MMWR Morb Mortal Wkly Rep. 2011;60(19);619-623.
1. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
2. Raj YP, Lloyd L. Adult immunizations. In: McCarron RM, Xiong GL, Keenan GR, et al, eds. Preventive medical care in psychiatry. Arlington, VA: American Psychiatric Publishing. 2015;215-227.
3. Young S, Dosani N, Whisler A, et al. Influenza vaccination rates among homeless adults with mental illness in Toronto. J Prim Care Community Health. 2015;6(3):211-214.
4. Kroger AT, Atkinson WL, Marcues EK, et al; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). General recommendations on immunization: recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-15):1-48.
5. Centers for Disease Control and Prevention. Recommended Adult Immunization by Vaccine and Age Group. http://www.cdc.gov/vaccines/schedules/hcp/adult.html. Updated February 27, 2017. Accessed February 1, 2017.
6. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(2):1-64.
7. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. https://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Updated June 22, 2016. Accessed February 1, 2017.
8. Hales CM, Harpaz, R, Ortega-Sanchez I, et al; Centers for Disease Control and Prevention. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63(33):729-731.
9. Petrosky E, Bocchini Jr JA, Hariri S, et al; Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccine recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11)300-304.
10. Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316(22):2411-2421.
11. Zipprich J, Winter K, Hacker J, et al; Centers for Disease Control and Prevention (CDC). Measles outbreak—California, December 2014-February 2015. MMWR Morb Mortal Wkly Rep. 2015;64(6):153-154.
12. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
13. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
14. Centers for Disease for Control and Prevention. CDC yellow book 2018: health information for international travel. New York, NY: Oxford University Press; 2017.
15. Druss BG, Rosenheck RA, Desai MM, et al. Quality of preventive medical care for patients with mental disorders. Med Care. 2002;40(2):129-136.
16. Lasser K, Boyd J, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606-2610.
17. Centers for Disease Control and Prevention (CDC). Ten great public health achievements—United States, 2001-2010. MMWR Morb Mortal Wkly Rep. 2011;60(19);619-623.
Memory problems: How best to assess and address
Memory disorders
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Clearing up confusion
“Mr. Smith seems somewhat confused today” is one of the most serious and concerning pre-visit reports you can receive from your staff or the patient’s family. Such a descriptor can be confusing—pardon the pun—not only for the patient, but to even seasoned mental health providers.
The term confusion can be code for diagnoses ranging from deliriuma to a progressive neurocognitive disorder (NCD) such as major NCD due to Alzheimer’s disease (AD), or even a more challenging problem such as beclouded dementia (delirium superimposed on dementia/NCD). It is essential for all mental health professionals to have an evidence-based approach when encountering signs or symptoms of confusion.
aICD-10 code R41.0 encompasses Confusion, Other Specified Delirium, or Unspecified Delirium.
CASE REPORT
Ms. T, age 62, has hypothyroidism and bipolar I disorder, most recently depressed, with comorbid generalized anxiety disorder. She has been taking lithium, 600 mg/d, to control her mood symptoms. Her daughter-in-law reports that Ms. T has been exhibiting increasing signs of confusion. During the office evaluation, Ms. T minimizes her symptoms, only describing mild issues with forgetfulness while cooking and concern over increasing anxiety. Her daughter-in-law plays a voicemail message from earlier in the week, in which Ms. T’s speech is halting, disorganized, and in a word, confused. I decide to use the mnemonic decision chart MR. MIND (Table 1) to get to the bottom of her recent confusion.
Measure cognition
It is nice to receive advanced warning about a cognitive change or a change in activities of daily living; however, many patients present with subtle, sub-acute changes that are more difficult to assess. When encountering a broad symptom such as “confusion”—which has an equally broad differential diagnosis—systematic assessment of the current cognitive state compared with the patient’s baseline becomes the first order of business. However, this requires that the patient has had a baseline cognitive assessment.
In my practice, I often administer one of the validated neurocognitive screening instruments when a patient first begins care—even a brief test such as the Mini- Cog (3-item recall plus clock drawing test), which is comparable to longer screening tests at least for NCD/dementia.1 During a presentation for confusion, a more detailed neurocognitive assessment instrument would be recommended, allowing one to marry the clinical impression with a validated, objective measure. Formal neuropsychological testing by a clinical neuropsychologist is the gold standard, but such testing is time-consuming and expensive and often not readily available. The screening instrument I use for a more thorough evaluation depends on the clinical scenario.
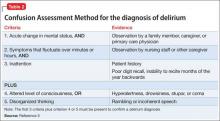
The Six-Item Screener is used in some emergency settings because it is short but boasts a higher sensitivity than the Mini- Cog (94% vs 75%) with similar specificity when screening for cognitive impairment.2 The Mini-Mental State Examination (MMSE) is a valuable instrument, although, recently, the Saint Louis University Mental Status Examination has been thought to be better at detecting mild NCD than the MMSE; more data are needed to substantiate this claim.3 The Montreal Cognitive Assessment is another validated screening tool that has been shown to be superior to the MMSE in terms of screening for mild cognitive impairment.4 The best delirium-specific assessment tool is the Confusion Assessment Method (Table 2).5
Ms. T’s MMSE score was 26/30, down from 29/30 at baseline. Her score fell below the cutoff score of 27 for mild cognitive impairment for someone with at least 8 years of completed education. Her results were abnormal mainly in the memory domain (3-item recall), raising the question of a possible prodromal state of AD although the acute nature of the change made delirium or mild NCD high in the differential.
Review medications
A review of the medication list is not just a Joint Commission mandate (medication reconciliation during each encounter) but is important whenever confusion is noted. Polypharmacy can be a concern, but is not as concerning as the class of medication prescribed, particularly anticholinergic and sedative medications in patients age >65. The Drug Burden Index can be helpful in assessing this risk.6 Medications such as the benzodiazepine-receptor agonists, tricyclic antidepressants, and antipsychotics should be discontinued if possible, keeping in mind that the addition or subtraction of medications must be done prudently and only after reviewing the evidence and in consultation with the patient. A detailed medication review is as important for confused outpatients as it is for an inpatient case (steps 2 and 3 of the inpatient algorithm outlined in Table 3).7
In Ms. T’s case, the primary concern on her medication list was that her medical team was prescribing levothyroxine, 112 mcg/d, and desiccated thyroid (combination thyroxine and triiodothyronine in the form of 20 mg Armour Thyroid), despite a lack of data for such combination therapy. Earlier, I had discontinued lorazepam, leaving lithium, 600 mg/d, quetiapine, 400 mg/d, and escitalopram, 10 mg/d, as her remaining psychotropics. Her other medications included atorvastatin, 40 mg/d, for hyper-lipidemia and metformin, 750 mg/d, for type 2 diabetes mellitus.
Medical illness
An organic basis must rank high in the differential diagnosis if medications are not the culprit. There are myriad medical disorders that can lead to confusion (Table 4).8 In an outpatient psychiatric setting, laboratory and radiology testing might not be readily available. It then becomes important to collaborate with a patient’s medical team if any of the following are met:
•there is high suspicion of a medical cause
•there could be delays in performing a medical workup
•a physical examination is needed.
Laboratory work-up should include:
•comprehensive metabolic panel (CMP) to assess for electrolyte derangements and liver or kidney disease
•urinalysis if there are signs of urinary tract infection (low threshold for testing in patients age >65 even if they are asymptomatic)
•urine drug screen or serum alcohol level if substance use is suspected
•complete blood count (CBC) if there are reports of infection (white blood cell count) or blood loss/bruising to ensure that anemia or thrombocytopenia is not playing a role
•thyroid-stimulating hormone (TSH) because thyroid disorders can cause neuropsychiatric as well as somatic symptoms.9
Other laboratory testing could be valuable depending on the clinical scenario. These include tests such as:
•drug level monitoring (lithium, valproic acid, etc.) to assess for toxicity
•HIV and rapid plasma reagin for suspected sexually transmitted infections
•vitamin levels in patients with poor nutrition or post bariatric surgery
•erythrocyte sedimentation rate or C-reactive protein, or both, if there are signs of inflammation
•bacterial culture if blood or tissue infection is a concern.
Esoteric tests include ceruloplasmin (Wilson’s disease), heavy metals screen, and even tests such as anti-gliadin antibodies because the prevalence of gluten sensitivity and celiac disease appear to be on the rise and have been associated with neuropsychiatric problems including encephalopathy.10
Brain imaging is an important consideration when a medical differential diagnosis for confusion is formulated. Unfortunately, there is little evidence-based guidance as to when brain imaging should be performed, often leading to overuse of tests such as CT, especially in emergency settings when confusion is noted. From a clinical standpoint, a head CT scan often is best ordered for patients who demonstrate an acute change in mental status, are age >70, are receiving anticoagulation, or have sustained trauma to the head. The key concern would be intracranial hemorrhage. However, some data suggest that the best use of head CT is for patients who have an impaired level of consciousness or a new focal neurologic deficit.11
Apart from more acute changes, a brain MRI study is more helpful than a head CT when evaluating the brain parenchyma for more sub-acute diagnoses such as multiple sclerosis or a brain tumor. T2-weighted hyperintensities seen on an MRI are thought to predict an increased risk of stroke, dementia, and death.
Their discovery should prompt a detailed evaluation for risk factors of stroke and dementia/NCD.12
In Ms. T’s case, she was taking lithium, so it was logical to obtain a trough lithium level 12 hours after the last dose and to check kidney function (serum creatinine to estimate the glomerular filtration rate), which were in the therapeutic/normal range. Her serum lithium level was 0.7 mEq/L. Brain imaging was not ordered, but several other labs (CMP, CBC, hemoglobin A1c [HgbA1c], and TSH) were drawn. These labs were notable for HgbA1c of 5.1% (normal <5.7%) and TSH of 0.5 mIU/L (normal level, 1.5 mIU/L), which is low for someone taking thyroid replacement.
I requested that Ms. T stop Armour Thyroid to address the suppressed TSH. I also requested that she stop metformin because, although hypoglycemia from metformin monotherapy is uncommon, it can happen in older patients. Hypoglycemia associated with metformin also can occur in situations when caloric intake is deficient or when metformin is used in combination with other drugs such as sulfonylureas (ie, glipizide), beta-adrenergic blocking drugs, angiotensin-converting enzyme inhibitors, or even nonsteroidal anti-inflammatory drugs.13
Identifying overlapping psychiatric (or psychological) illness
Symptoms of depression, anxiety, psychosis, and even dissociation can present as confusion. The term pseudodementia describes patients who exhibit cognitive symptoms consistent with NCD but could improve once the underlying mood, thought, anxiety, or personality disorder is treated.
For example, a patient with depression typically exhibits neurovegetative symptoms—such as poor sleep or appetite— amotivation, and low energy. All of these can lead to abrupt-onset cognitive changes, which are a hallmark of pseudodementia rather than the more insidious pattern of mild NCD. In cases of pseudodementia, neurocognitive testing will show impairment that often rapidly improves after the primary psychiatric (or psychological) issue is rectified. Making a diagnosis of pseudodementia at the initial presentation is difficult because neurocognitive tests such as the MMSE often fail to separate depression from true cognitive changes.14 Such a diagnosis typically requires hindsight. Yet, one must also keep in mind that pseudodementia may be part of a NCD prodrome.15
Conversion disorder as well as the dissociative disorders and substance-related disorders are notorious for causing confusion. In Ms. T’s case, pseudodementia stemming from her underlying bipolar disorder and anxiety figured prominently in the differential diagnosis, but she did not have any other overt psychopathology, personality disorder, or signs of malingering to further complicate her picture.
Notebook. I recommend that my patients keep a small notebook to record medical data ranging from blood pressure and glycemic measurements to details about sleep and dietary intake. Such data comprise the necessary metrics to properly assess target conditions and then track changes once treatment is initiated. This exercise not only yields much-needed detail about the patient’s condition for the clinician; the act of journaling also can be therapeutic for the writer through a process known as experimental disclosure, in which writing down one’s thoughts and observations has a positive impact on the writer’s physical health and psychology.16
Diagnosis. The first rule in medicine (perhaps the second, behind primum non nocere) is to determine what you are treating before beginning treatment (decernite quid tractemus, prius cura ministrandi, for Latin buffs). This means trying to fashion the best diagnostic label, even if it is merely a place-holder, while assessment of the confused state continues. DSM-5 has attempted to remove stigma from several neuropsychiatric disorders. On the cognition front, the new name for dementia is “neurocognitive disorder (NCD),” the umbrella term that focuses on the decline from a previous level of cognitive functioning. NCD has been divided into mild or major cognitive impairment headings either “with” or “without behavioral disturbance” subspecifiers.17
Aside from NCD, there are several other diagnoses in the differential for confusion. Delirium remains the most prominent and focuses on disturbances in attention and orientation that develops over a short period of time, with a change seen in an additional cognitive domain, such as memory, but not in the context of a severely reduced level of arousal such as coma. Subjective cognitive impairment (SCI) is when subjective complaints of cognitive impairment are hallmark compared with objective findings—with evidence suggesting that the presence of SCI could predict a 4.5 times higher rate of developing mild cognitive impairment (MCI) over 7 years.18 MCI was originally used to describe the early prodrome of AD, minus functional decline.
Treatment
After even a provisional diagnosis comes the final, all-important challenge: treating the neuropsychiatric symptoms (NPS) of the confused patient. NPS are nearly universal in NCD/delirium throughout the course of illness. There are no FDA-approved treatments for the NPS associated with these conditions. In terms of treating delirium, the best approach is to treat the underlying medical condition. For control of behavior, which can range from agitated to psychotic to hypoactive, nonpharmacotherapeutic interventions are paramount; they include making sure that the patient is at the appropriate level of care, which, for the confused outpatient, could mean hospitalization. Ensuring proper nutrition, hydration, sensory care (hearing aids, glasses, etc.), and stability in ambulation must be done before considering pharmacotherapy.
Antipsychotic use has been the mainstay of drug treatment of behavioral dyscontrol. Haloperidol has been the traditional go-to medication because there is no evidence that low-dose haloperidol (<3 mg/d) has any different efficacy compared with the atypical antipsychotics or has a greater frequency of adverse drug effects. However, high-dose haloperidol (>4.5 mg/d) was associated with a greater incidence of adverse effects, mainly parkinsonism, than atypical antipsychotics.19 Neither the typical nor atypical antipsychotics have shown mortality benefit—the real outcome measure of interest.
In terms of treating major (or minor) NCD, there are only 2 FDA-approved medication classes: cholinesterase inhibitors (donepezil, galantamine, rivastigmine, etc.) and memantine. However, these medication classes—even when combined together—have only shown marginal benefit in terms of improving cognition. Worse, even when given early in the course of illness they do not reduce the rate of NCD. For pseudodementia, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors tend to form the mainstay of treating underlying depression or anxiety leading to cognitive changes. Preliminary data suggest that some SSRIs might improve cognition in terms of processing speed, verbal learning, and memory.20 More studies are needed before definitive conclusions can be drawn.
For the confused patient, a personalized therapeutic program, in which multiple interventions are considered at once (targeting all areas of the patient’s life) is gaining research traction. For example, a novel, comprehensive program involving multiple modalities designed to achieve metabolic enhancement for neurodegeneration (MEND) recently has shown robust benefit for patients with AD, MCI, and SCI.21 Using an individual approach to improve diet, activity, sleep, metabolic status including body mass index, and several other markers that affect neural plasticity, researchers demonstrated symptom improvement in 9 of 10 study patients.
Yet, some of the interventions, such as the use of statins for hyperlipidemia, remain controversial, with some studies suggesting that they help cognition,22,23 and others showing no association.24 The researchers caution that further research is warranted before costly dementia prevention trials with statins are undertaken. It does not appear that there are current MEND-type research projects in delirium but it’s to be hoped that we will see these in the future.
In the case of Ms. T, the cause of delirium vs mild NCD was thought to be multifactorial. Discontinuing Armour Thyroid and metformin—symptoms of hypoglycemia emerged as a leading concern—were simple adjustments that led to resolution of the most concerning elements of her confusion. She continued her other psychotropics, although there might be mild residual cognitive issues that warrant close observation.
Related Resources
• Lin JS, O’Connor E, Rossum RC, et al. Screening for cognitive impairment in older adults: an evidence update for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013.
• Grover S, Kate N. Assessment scales for delirium: a review. World J Psychiatry. 2012;2(4):58-70.
Drug Brand Names
Atorvastatin • Lipitor Lithium • Eskalith, Lithobid
Donepezil • Aricept Lorazepam • Ativan
Escitalopram • Lexapro Memantine • Namenda
Flumazenil • Romazicon Metformin • Glucophage
Galantamine • Razadyne Naloxone • Narcan
Glipizide • Glucotrol Physostigmine • Antilirium
Haloperidol • Haldol Quetiapine • Seroquel
Levothyroxine • Levoxyl, Synthroid Rivastigmine • Exelon
Lithium • Eskalith, Lithobid Valproic acid • Depakene
Disclosure
Dr. Raj is a speaker for Actavis Pharmaceuticals, AstraZeneca, and Merck.
1. Borson S, Scanlan JM, Chen P, et al. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10):1451-1454.
2. Wilber ST, Lofgren SD, Mager TG, et al. An evaluation of two screening tools for cognitive impairment in older emergency department patients. Acad Emerg Med. 2005;12(7):612-616.
3. Tariq SH, Tumosa N, Chibnall JT, et al. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
4. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695- 699.
5. Inouye S, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Int Med. 1990;113(12):941-948.
6. Hillmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167(8):781-787.
7. Raj YP. Psychiatric emergencies. In: Jiang W, Gagliardi JP, Krishnan KR, eds. Clinician’s guide to psychiatric care. New York, NY: Oxford University Press; 2009:33-40.
8. Liptzin B. Clinical diagnosis and management of delirium. In: Stoudemire A, Fogel BS, Greenberg DB, eds. Psychiatric care of the medical patient. 2nd ed. New York, NY: Oxford University Press; 2000:581-596.
9. Raj YP. Subclinical hypothyroidism: merely monitor or time to treat? Current Psychiatry. 2009;8(2):47-48.
10. Poloni N, Vender S, Bolla E, et al. Gluten encephalopathy with psychiatric onset: case report. Clin Pract Epidemiol Ment Health. 2009;5:16.
11. Naughton BJ, Moran M, Ghaly Y, et al. Computed tomography scanning and delirium in elder patients. Acad Emerg Med. 1997;4(12):1107-1110.
12. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666.
13. Zitzmann S, Reimann IR, Schmechel H. Severe hypoglycemia in an elderly patient treated with metformin. Int J Clin Pharmacol Ther. 2002;40(3):108-110.
14. Benson AD, Slavin MJ, Tran TT, et al. Screening for early Alzheimer’s Disease: is there still a role for the Mini-Mental State Examination? Prim Care Companion J Clin Psychiatry. 2005;7(2):62-69.
15. Brown WA. Pseudodementia: issues in diagnosis. Psychiatric Times. http://www.psychiatrictimes.com/ pseudodementia-issues-diagnosis. Published April 9, 2005. Accessed February 2, 2015.
16. Frattaroli J. Experimental disclosure and its moderators: a meta-analysis. Psychol Bull. 2006;132(6):823-865.
17. Stetka BS, Correll CU. A guide to DSM-5: neurocognitive disorder. Medscape. http://www.medscape.com/ viewarticle/803884_13. Published May 21, 2013. Accessed October 30, 2014.
18. Reisberg B, Sulman MD, Torossian C, et al. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6(1):11-24.
19. Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;(2):CD005594.
20. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Intern Clin Psychopharmacol. 2012;27(4):215-223.
21. Bredesen DE. Reversal of cognitive decline: a novel therapeutic program. Aging (Albany NY). 2014;6(9):707-717.
22. Sparks DL, Kryscio RJ, Sabbagh MN, et al. Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr Alzheimer Res. 2008;5(4):416-421.
23. Andrade C, Radhakrishnan R. The prevention and treatment of cognitive decline and dementia: an overview of recent research on experimental treatments. Indian J Psychiatry. 2009;51(1):12-25.
24. Zandi PP, Sparks DL, Khachaturian AS, et al. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry. 2005;62(2):217-224.
“Mr. Smith seems somewhat confused today” is one of the most serious and concerning pre-visit reports you can receive from your staff or the patient’s family. Such a descriptor can be confusing—pardon the pun—not only for the patient, but to even seasoned mental health providers.
The term confusion can be code for diagnoses ranging from deliriuma to a progressive neurocognitive disorder (NCD) such as major NCD due to Alzheimer’s disease (AD), or even a more challenging problem such as beclouded dementia (delirium superimposed on dementia/NCD). It is essential for all mental health professionals to have an evidence-based approach when encountering signs or symptoms of confusion.
aICD-10 code R41.0 encompasses Confusion, Other Specified Delirium, or Unspecified Delirium.
CASE REPORT
Ms. T, age 62, has hypothyroidism and bipolar I disorder, most recently depressed, with comorbid generalized anxiety disorder. She has been taking lithium, 600 mg/d, to control her mood symptoms. Her daughter-in-law reports that Ms. T has been exhibiting increasing signs of confusion. During the office evaluation, Ms. T minimizes her symptoms, only describing mild issues with forgetfulness while cooking and concern over increasing anxiety. Her daughter-in-law plays a voicemail message from earlier in the week, in which Ms. T’s speech is halting, disorganized, and in a word, confused. I decide to use the mnemonic decision chart MR. MIND (Table 1) to get to the bottom of her recent confusion.
Measure cognition
It is nice to receive advanced warning about a cognitive change or a change in activities of daily living; however, many patients present with subtle, sub-acute changes that are more difficult to assess. When encountering a broad symptom such as “confusion”—which has an equally broad differential diagnosis—systematic assessment of the current cognitive state compared with the patient’s baseline becomes the first order of business. However, this requires that the patient has had a baseline cognitive assessment.
In my practice, I often administer one of the validated neurocognitive screening instruments when a patient first begins care—even a brief test such as the Mini- Cog (3-item recall plus clock drawing test), which is comparable to longer screening tests at least for NCD/dementia.1 During a presentation for confusion, a more detailed neurocognitive assessment instrument would be recommended, allowing one to marry the clinical impression with a validated, objective measure. Formal neuropsychological testing by a clinical neuropsychologist is the gold standard, but such testing is time-consuming and expensive and often not readily available. The screening instrument I use for a more thorough evaluation depends on the clinical scenario.
The Six-Item Screener is used in some emergency settings because it is short but boasts a higher sensitivity than the Mini- Cog (94% vs 75%) with similar specificity when screening for cognitive impairment.2 The Mini-Mental State Examination (MMSE) is a valuable instrument, although, recently, the Saint Louis University Mental Status Examination has been thought to be better at detecting mild NCD than the MMSE; more data are needed to substantiate this claim.3 The Montreal Cognitive Assessment is another validated screening tool that has been shown to be superior to the MMSE in terms of screening for mild cognitive impairment.4 The best delirium-specific assessment tool is the Confusion Assessment Method (Table 2).5
Ms. T’s MMSE score was 26/30, down from 29/30 at baseline. Her score fell below the cutoff score of 27 for mild cognitive impairment for someone with at least 8 years of completed education. Her results were abnormal mainly in the memory domain (3-item recall), raising the question of a possible prodromal state of AD although the acute nature of the change made delirium or mild NCD high in the differential.
Review medications
A review of the medication list is not just a Joint Commission mandate (medication reconciliation during each encounter) but is important whenever confusion is noted. Polypharmacy can be a concern, but is not as concerning as the class of medication prescribed, particularly anticholinergic and sedative medications in patients age >65. The Drug Burden Index can be helpful in assessing this risk.6 Medications such as the benzodiazepine-receptor agonists, tricyclic antidepressants, and antipsychotics should be discontinued if possible, keeping in mind that the addition or subtraction of medications must be done prudently and only after reviewing the evidence and in consultation with the patient. A detailed medication review is as important for confused outpatients as it is for an inpatient case (steps 2 and 3 of the inpatient algorithm outlined in Table 3).7
In Ms. T’s case, the primary concern on her medication list was that her medical team was prescribing levothyroxine, 112 mcg/d, and desiccated thyroid (combination thyroxine and triiodothyronine in the form of 20 mg Armour Thyroid), despite a lack of data for such combination therapy. Earlier, I had discontinued lorazepam, leaving lithium, 600 mg/d, quetiapine, 400 mg/d, and escitalopram, 10 mg/d, as her remaining psychotropics. Her other medications included atorvastatin, 40 mg/d, for hyper-lipidemia and metformin, 750 mg/d, for type 2 diabetes mellitus.
Medical illness
An organic basis must rank high in the differential diagnosis if medications are not the culprit. There are myriad medical disorders that can lead to confusion (Table 4).8 In an outpatient psychiatric setting, laboratory and radiology testing might not be readily available. It then becomes important to collaborate with a patient’s medical team if any of the following are met:
•there is high suspicion of a medical cause
•there could be delays in performing a medical workup
•a physical examination is needed.
Laboratory work-up should include:
•comprehensive metabolic panel (CMP) to assess for electrolyte derangements and liver or kidney disease
•urinalysis if there are signs of urinary tract infection (low threshold for testing in patients age >65 even if they are asymptomatic)
•urine drug screen or serum alcohol level if substance use is suspected
•complete blood count (CBC) if there are reports of infection (white blood cell count) or blood loss/bruising to ensure that anemia or thrombocytopenia is not playing a role
•thyroid-stimulating hormone (TSH) because thyroid disorders can cause neuropsychiatric as well as somatic symptoms.9
Other laboratory testing could be valuable depending on the clinical scenario. These include tests such as:
•drug level monitoring (lithium, valproic acid, etc.) to assess for toxicity
•HIV and rapid plasma reagin for suspected sexually transmitted infections
•vitamin levels in patients with poor nutrition or post bariatric surgery
•erythrocyte sedimentation rate or C-reactive protein, or both, if there are signs of inflammation
•bacterial culture if blood or tissue infection is a concern.
Esoteric tests include ceruloplasmin (Wilson’s disease), heavy metals screen, and even tests such as anti-gliadin antibodies because the prevalence of gluten sensitivity and celiac disease appear to be on the rise and have been associated with neuropsychiatric problems including encephalopathy.10
Brain imaging is an important consideration when a medical differential diagnosis for confusion is formulated. Unfortunately, there is little evidence-based guidance as to when brain imaging should be performed, often leading to overuse of tests such as CT, especially in emergency settings when confusion is noted. From a clinical standpoint, a head CT scan often is best ordered for patients who demonstrate an acute change in mental status, are age >70, are receiving anticoagulation, or have sustained trauma to the head. The key concern would be intracranial hemorrhage. However, some data suggest that the best use of head CT is for patients who have an impaired level of consciousness or a new focal neurologic deficit.11
Apart from more acute changes, a brain MRI study is more helpful than a head CT when evaluating the brain parenchyma for more sub-acute diagnoses such as multiple sclerosis or a brain tumor. T2-weighted hyperintensities seen on an MRI are thought to predict an increased risk of stroke, dementia, and death.
Their discovery should prompt a detailed evaluation for risk factors of stroke and dementia/NCD.12
In Ms. T’s case, she was taking lithium, so it was logical to obtain a trough lithium level 12 hours after the last dose and to check kidney function (serum creatinine to estimate the glomerular filtration rate), which were in the therapeutic/normal range. Her serum lithium level was 0.7 mEq/L. Brain imaging was not ordered, but several other labs (CMP, CBC, hemoglobin A1c [HgbA1c], and TSH) were drawn. These labs were notable for HgbA1c of 5.1% (normal <5.7%) and TSH of 0.5 mIU/L (normal level, 1.5 mIU/L), which is low for someone taking thyroid replacement.
I requested that Ms. T stop Armour Thyroid to address the suppressed TSH. I also requested that she stop metformin because, although hypoglycemia from metformin monotherapy is uncommon, it can happen in older patients. Hypoglycemia associated with metformin also can occur in situations when caloric intake is deficient or when metformin is used in combination with other drugs such as sulfonylureas (ie, glipizide), beta-adrenergic blocking drugs, angiotensin-converting enzyme inhibitors, or even nonsteroidal anti-inflammatory drugs.13
Identifying overlapping psychiatric (or psychological) illness
Symptoms of depression, anxiety, psychosis, and even dissociation can present as confusion. The term pseudodementia describes patients who exhibit cognitive symptoms consistent with NCD but could improve once the underlying mood, thought, anxiety, or personality disorder is treated.
For example, a patient with depression typically exhibits neurovegetative symptoms—such as poor sleep or appetite— amotivation, and low energy. All of these can lead to abrupt-onset cognitive changes, which are a hallmark of pseudodementia rather than the more insidious pattern of mild NCD. In cases of pseudodementia, neurocognitive testing will show impairment that often rapidly improves after the primary psychiatric (or psychological) issue is rectified. Making a diagnosis of pseudodementia at the initial presentation is difficult because neurocognitive tests such as the MMSE often fail to separate depression from true cognitive changes.14 Such a diagnosis typically requires hindsight. Yet, one must also keep in mind that pseudodementia may be part of a NCD prodrome.15
Conversion disorder as well as the dissociative disorders and substance-related disorders are notorious for causing confusion. In Ms. T’s case, pseudodementia stemming from her underlying bipolar disorder and anxiety figured prominently in the differential diagnosis, but she did not have any other overt psychopathology, personality disorder, or signs of malingering to further complicate her picture.
Notebook. I recommend that my patients keep a small notebook to record medical data ranging from blood pressure and glycemic measurements to details about sleep and dietary intake. Such data comprise the necessary metrics to properly assess target conditions and then track changes once treatment is initiated. This exercise not only yields much-needed detail about the patient’s condition for the clinician; the act of journaling also can be therapeutic for the writer through a process known as experimental disclosure, in which writing down one’s thoughts and observations has a positive impact on the writer’s physical health and psychology.16
Diagnosis. The first rule in medicine (perhaps the second, behind primum non nocere) is to determine what you are treating before beginning treatment (decernite quid tractemus, prius cura ministrandi, for Latin buffs). This means trying to fashion the best diagnostic label, even if it is merely a place-holder, while assessment of the confused state continues. DSM-5 has attempted to remove stigma from several neuropsychiatric disorders. On the cognition front, the new name for dementia is “neurocognitive disorder (NCD),” the umbrella term that focuses on the decline from a previous level of cognitive functioning. NCD has been divided into mild or major cognitive impairment headings either “with” or “without behavioral disturbance” subspecifiers.17
Aside from NCD, there are several other diagnoses in the differential for confusion. Delirium remains the most prominent and focuses on disturbances in attention and orientation that develops over a short period of time, with a change seen in an additional cognitive domain, such as memory, but not in the context of a severely reduced level of arousal such as coma. Subjective cognitive impairment (SCI) is when subjective complaints of cognitive impairment are hallmark compared with objective findings—with evidence suggesting that the presence of SCI could predict a 4.5 times higher rate of developing mild cognitive impairment (MCI) over 7 years.18 MCI was originally used to describe the early prodrome of AD, minus functional decline.
Treatment
After even a provisional diagnosis comes the final, all-important challenge: treating the neuropsychiatric symptoms (NPS) of the confused patient. NPS are nearly universal in NCD/delirium throughout the course of illness. There are no FDA-approved treatments for the NPS associated with these conditions. In terms of treating delirium, the best approach is to treat the underlying medical condition. For control of behavior, which can range from agitated to psychotic to hypoactive, nonpharmacotherapeutic interventions are paramount; they include making sure that the patient is at the appropriate level of care, which, for the confused outpatient, could mean hospitalization. Ensuring proper nutrition, hydration, sensory care (hearing aids, glasses, etc.), and stability in ambulation must be done before considering pharmacotherapy.
Antipsychotic use has been the mainstay of drug treatment of behavioral dyscontrol. Haloperidol has been the traditional go-to medication because there is no evidence that low-dose haloperidol (<3 mg/d) has any different efficacy compared with the atypical antipsychotics or has a greater frequency of adverse drug effects. However, high-dose haloperidol (>4.5 mg/d) was associated with a greater incidence of adverse effects, mainly parkinsonism, than atypical antipsychotics.19 Neither the typical nor atypical antipsychotics have shown mortality benefit—the real outcome measure of interest.
In terms of treating major (or minor) NCD, there are only 2 FDA-approved medication classes: cholinesterase inhibitors (donepezil, galantamine, rivastigmine, etc.) and memantine. However, these medication classes—even when combined together—have only shown marginal benefit in terms of improving cognition. Worse, even when given early in the course of illness they do not reduce the rate of NCD. For pseudodementia, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors tend to form the mainstay of treating underlying depression or anxiety leading to cognitive changes. Preliminary data suggest that some SSRIs might improve cognition in terms of processing speed, verbal learning, and memory.20 More studies are needed before definitive conclusions can be drawn.
For the confused patient, a personalized therapeutic program, in which multiple interventions are considered at once (targeting all areas of the patient’s life) is gaining research traction. For example, a novel, comprehensive program involving multiple modalities designed to achieve metabolic enhancement for neurodegeneration (MEND) recently has shown robust benefit for patients with AD, MCI, and SCI.21 Using an individual approach to improve diet, activity, sleep, metabolic status including body mass index, and several other markers that affect neural plasticity, researchers demonstrated symptom improvement in 9 of 10 study patients.
Yet, some of the interventions, such as the use of statins for hyperlipidemia, remain controversial, with some studies suggesting that they help cognition,22,23 and others showing no association.24 The researchers caution that further research is warranted before costly dementia prevention trials with statins are undertaken. It does not appear that there are current MEND-type research projects in delirium but it’s to be hoped that we will see these in the future.
In the case of Ms. T, the cause of delirium vs mild NCD was thought to be multifactorial. Discontinuing Armour Thyroid and metformin—symptoms of hypoglycemia emerged as a leading concern—were simple adjustments that led to resolution of the most concerning elements of her confusion. She continued her other psychotropics, although there might be mild residual cognitive issues that warrant close observation.
Related Resources
• Lin JS, O’Connor E, Rossum RC, et al. Screening for cognitive impairment in older adults: an evidence update for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013.
• Grover S, Kate N. Assessment scales for delirium: a review. World J Psychiatry. 2012;2(4):58-70.
Drug Brand Names
Atorvastatin • Lipitor Lithium • Eskalith, Lithobid
Donepezil • Aricept Lorazepam • Ativan
Escitalopram • Lexapro Memantine • Namenda
Flumazenil • Romazicon Metformin • Glucophage
Galantamine • Razadyne Naloxone • Narcan
Glipizide • Glucotrol Physostigmine • Antilirium
Haloperidol • Haldol Quetiapine • Seroquel
Levothyroxine • Levoxyl, Synthroid Rivastigmine • Exelon
Lithium • Eskalith, Lithobid Valproic acid • Depakene
Disclosure
Dr. Raj is a speaker for Actavis Pharmaceuticals, AstraZeneca, and Merck.
“Mr. Smith seems somewhat confused today” is one of the most serious and concerning pre-visit reports you can receive from your staff or the patient’s family. Such a descriptor can be confusing—pardon the pun—not only for the patient, but to even seasoned mental health providers.
The term confusion can be code for diagnoses ranging from deliriuma to a progressive neurocognitive disorder (NCD) such as major NCD due to Alzheimer’s disease (AD), or even a more challenging problem such as beclouded dementia (delirium superimposed on dementia/NCD). It is essential for all mental health professionals to have an evidence-based approach when encountering signs or symptoms of confusion.
aICD-10 code R41.0 encompasses Confusion, Other Specified Delirium, or Unspecified Delirium.
CASE REPORT
Ms. T, age 62, has hypothyroidism and bipolar I disorder, most recently depressed, with comorbid generalized anxiety disorder. She has been taking lithium, 600 mg/d, to control her mood symptoms. Her daughter-in-law reports that Ms. T has been exhibiting increasing signs of confusion. During the office evaluation, Ms. T minimizes her symptoms, only describing mild issues with forgetfulness while cooking and concern over increasing anxiety. Her daughter-in-law plays a voicemail message from earlier in the week, in which Ms. T’s speech is halting, disorganized, and in a word, confused. I decide to use the mnemonic decision chart MR. MIND (Table 1) to get to the bottom of her recent confusion.
Measure cognition
It is nice to receive advanced warning about a cognitive change or a change in activities of daily living; however, many patients present with subtle, sub-acute changes that are more difficult to assess. When encountering a broad symptom such as “confusion”—which has an equally broad differential diagnosis—systematic assessment of the current cognitive state compared with the patient’s baseline becomes the first order of business. However, this requires that the patient has had a baseline cognitive assessment.
In my practice, I often administer one of the validated neurocognitive screening instruments when a patient first begins care—even a brief test such as the Mini- Cog (3-item recall plus clock drawing test), which is comparable to longer screening tests at least for NCD/dementia.1 During a presentation for confusion, a more detailed neurocognitive assessment instrument would be recommended, allowing one to marry the clinical impression with a validated, objective measure. Formal neuropsychological testing by a clinical neuropsychologist is the gold standard, but such testing is time-consuming and expensive and often not readily available. The screening instrument I use for a more thorough evaluation depends on the clinical scenario.
The Six-Item Screener is used in some emergency settings because it is short but boasts a higher sensitivity than the Mini- Cog (94% vs 75%) with similar specificity when screening for cognitive impairment.2 The Mini-Mental State Examination (MMSE) is a valuable instrument, although, recently, the Saint Louis University Mental Status Examination has been thought to be better at detecting mild NCD than the MMSE; more data are needed to substantiate this claim.3 The Montreal Cognitive Assessment is another validated screening tool that has been shown to be superior to the MMSE in terms of screening for mild cognitive impairment.4 The best delirium-specific assessment tool is the Confusion Assessment Method (Table 2).5
Ms. T’s MMSE score was 26/30, down from 29/30 at baseline. Her score fell below the cutoff score of 27 for mild cognitive impairment for someone with at least 8 years of completed education. Her results were abnormal mainly in the memory domain (3-item recall), raising the question of a possible prodromal state of AD although the acute nature of the change made delirium or mild NCD high in the differential.
Review medications
A review of the medication list is not just a Joint Commission mandate (medication reconciliation during each encounter) but is important whenever confusion is noted. Polypharmacy can be a concern, but is not as concerning as the class of medication prescribed, particularly anticholinergic and sedative medications in patients age >65. The Drug Burden Index can be helpful in assessing this risk.6 Medications such as the benzodiazepine-receptor agonists, tricyclic antidepressants, and antipsychotics should be discontinued if possible, keeping in mind that the addition or subtraction of medications must be done prudently and only after reviewing the evidence and in consultation with the patient. A detailed medication review is as important for confused outpatients as it is for an inpatient case (steps 2 and 3 of the inpatient algorithm outlined in Table 3).7
In Ms. T’s case, the primary concern on her medication list was that her medical team was prescribing levothyroxine, 112 mcg/d, and desiccated thyroid (combination thyroxine and triiodothyronine in the form of 20 mg Armour Thyroid), despite a lack of data for such combination therapy. Earlier, I had discontinued lorazepam, leaving lithium, 600 mg/d, quetiapine, 400 mg/d, and escitalopram, 10 mg/d, as her remaining psychotropics. Her other medications included atorvastatin, 40 mg/d, for hyper-lipidemia and metformin, 750 mg/d, for type 2 diabetes mellitus.
Medical illness
An organic basis must rank high in the differential diagnosis if medications are not the culprit. There are myriad medical disorders that can lead to confusion (Table 4).8 In an outpatient psychiatric setting, laboratory and radiology testing might not be readily available. It then becomes important to collaborate with a patient’s medical team if any of the following are met:
•there is high suspicion of a medical cause
•there could be delays in performing a medical workup
•a physical examination is needed.
Laboratory work-up should include:
•comprehensive metabolic panel (CMP) to assess for electrolyte derangements and liver or kidney disease
•urinalysis if there are signs of urinary tract infection (low threshold for testing in patients age >65 even if they are asymptomatic)
•urine drug screen or serum alcohol level if substance use is suspected
•complete blood count (CBC) if there are reports of infection (white blood cell count) or blood loss/bruising to ensure that anemia or thrombocytopenia is not playing a role
•thyroid-stimulating hormone (TSH) because thyroid disorders can cause neuropsychiatric as well as somatic symptoms.9
Other laboratory testing could be valuable depending on the clinical scenario. These include tests such as:
•drug level monitoring (lithium, valproic acid, etc.) to assess for toxicity
•HIV and rapid plasma reagin for suspected sexually transmitted infections
•vitamin levels in patients with poor nutrition or post bariatric surgery
•erythrocyte sedimentation rate or C-reactive protein, or both, if there are signs of inflammation
•bacterial culture if blood or tissue infection is a concern.
Esoteric tests include ceruloplasmin (Wilson’s disease), heavy metals screen, and even tests such as anti-gliadin antibodies because the prevalence of gluten sensitivity and celiac disease appear to be on the rise and have been associated with neuropsychiatric problems including encephalopathy.10
Brain imaging is an important consideration when a medical differential diagnosis for confusion is formulated. Unfortunately, there is little evidence-based guidance as to when brain imaging should be performed, often leading to overuse of tests such as CT, especially in emergency settings when confusion is noted. From a clinical standpoint, a head CT scan often is best ordered for patients who demonstrate an acute change in mental status, are age >70, are receiving anticoagulation, or have sustained trauma to the head. The key concern would be intracranial hemorrhage. However, some data suggest that the best use of head CT is for patients who have an impaired level of consciousness or a new focal neurologic deficit.11
Apart from more acute changes, a brain MRI study is more helpful than a head CT when evaluating the brain parenchyma for more sub-acute diagnoses such as multiple sclerosis or a brain tumor. T2-weighted hyperintensities seen on an MRI are thought to predict an increased risk of stroke, dementia, and death.
Their discovery should prompt a detailed evaluation for risk factors of stroke and dementia/NCD.12
In Ms. T’s case, she was taking lithium, so it was logical to obtain a trough lithium level 12 hours after the last dose and to check kidney function (serum creatinine to estimate the glomerular filtration rate), which were in the therapeutic/normal range. Her serum lithium level was 0.7 mEq/L. Brain imaging was not ordered, but several other labs (CMP, CBC, hemoglobin A1c [HgbA1c], and TSH) were drawn. These labs were notable for HgbA1c of 5.1% (normal <5.7%) and TSH of 0.5 mIU/L (normal level, 1.5 mIU/L), which is low for someone taking thyroid replacement.
I requested that Ms. T stop Armour Thyroid to address the suppressed TSH. I also requested that she stop metformin because, although hypoglycemia from metformin monotherapy is uncommon, it can happen in older patients. Hypoglycemia associated with metformin also can occur in situations when caloric intake is deficient or when metformin is used in combination with other drugs such as sulfonylureas (ie, glipizide), beta-adrenergic blocking drugs, angiotensin-converting enzyme inhibitors, or even nonsteroidal anti-inflammatory drugs.13
Identifying overlapping psychiatric (or psychological) illness
Symptoms of depression, anxiety, psychosis, and even dissociation can present as confusion. The term pseudodementia describes patients who exhibit cognitive symptoms consistent with NCD but could improve once the underlying mood, thought, anxiety, or personality disorder is treated.
For example, a patient with depression typically exhibits neurovegetative symptoms—such as poor sleep or appetite— amotivation, and low energy. All of these can lead to abrupt-onset cognitive changes, which are a hallmark of pseudodementia rather than the more insidious pattern of mild NCD. In cases of pseudodementia, neurocognitive testing will show impairment that often rapidly improves after the primary psychiatric (or psychological) issue is rectified. Making a diagnosis of pseudodementia at the initial presentation is difficult because neurocognitive tests such as the MMSE often fail to separate depression from true cognitive changes.14 Such a diagnosis typically requires hindsight. Yet, one must also keep in mind that pseudodementia may be part of a NCD prodrome.15
Conversion disorder as well as the dissociative disorders and substance-related disorders are notorious for causing confusion. In Ms. T’s case, pseudodementia stemming from her underlying bipolar disorder and anxiety figured prominently in the differential diagnosis, but she did not have any other overt psychopathology, personality disorder, or signs of malingering to further complicate her picture.
Notebook. I recommend that my patients keep a small notebook to record medical data ranging from blood pressure and glycemic measurements to details about sleep and dietary intake. Such data comprise the necessary metrics to properly assess target conditions and then track changes once treatment is initiated. This exercise not only yields much-needed detail about the patient’s condition for the clinician; the act of journaling also can be therapeutic for the writer through a process known as experimental disclosure, in which writing down one’s thoughts and observations has a positive impact on the writer’s physical health and psychology.16
Diagnosis. The first rule in medicine (perhaps the second, behind primum non nocere) is to determine what you are treating before beginning treatment (decernite quid tractemus, prius cura ministrandi, for Latin buffs). This means trying to fashion the best diagnostic label, even if it is merely a place-holder, while assessment of the confused state continues. DSM-5 has attempted to remove stigma from several neuropsychiatric disorders. On the cognition front, the new name for dementia is “neurocognitive disorder (NCD),” the umbrella term that focuses on the decline from a previous level of cognitive functioning. NCD has been divided into mild or major cognitive impairment headings either “with” or “without behavioral disturbance” subspecifiers.17
Aside from NCD, there are several other diagnoses in the differential for confusion. Delirium remains the most prominent and focuses on disturbances in attention and orientation that develops over a short period of time, with a change seen in an additional cognitive domain, such as memory, but not in the context of a severely reduced level of arousal such as coma. Subjective cognitive impairment (SCI) is when subjective complaints of cognitive impairment are hallmark compared with objective findings—with evidence suggesting that the presence of SCI could predict a 4.5 times higher rate of developing mild cognitive impairment (MCI) over 7 years.18 MCI was originally used to describe the early prodrome of AD, minus functional decline.
Treatment
After even a provisional diagnosis comes the final, all-important challenge: treating the neuropsychiatric symptoms (NPS) of the confused patient. NPS are nearly universal in NCD/delirium throughout the course of illness. There are no FDA-approved treatments for the NPS associated with these conditions. In terms of treating delirium, the best approach is to treat the underlying medical condition. For control of behavior, which can range from agitated to psychotic to hypoactive, nonpharmacotherapeutic interventions are paramount; they include making sure that the patient is at the appropriate level of care, which, for the confused outpatient, could mean hospitalization. Ensuring proper nutrition, hydration, sensory care (hearing aids, glasses, etc.), and stability in ambulation must be done before considering pharmacotherapy.
Antipsychotic use has been the mainstay of drug treatment of behavioral dyscontrol. Haloperidol has been the traditional go-to medication because there is no evidence that low-dose haloperidol (<3 mg/d) has any different efficacy compared with the atypical antipsychotics or has a greater frequency of adverse drug effects. However, high-dose haloperidol (>4.5 mg/d) was associated with a greater incidence of adverse effects, mainly parkinsonism, than atypical antipsychotics.19 Neither the typical nor atypical antipsychotics have shown mortality benefit—the real outcome measure of interest.
In terms of treating major (or minor) NCD, there are only 2 FDA-approved medication classes: cholinesterase inhibitors (donepezil, galantamine, rivastigmine, etc.) and memantine. However, these medication classes—even when combined together—have only shown marginal benefit in terms of improving cognition. Worse, even when given early in the course of illness they do not reduce the rate of NCD. For pseudodementia, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors tend to form the mainstay of treating underlying depression or anxiety leading to cognitive changes. Preliminary data suggest that some SSRIs might improve cognition in terms of processing speed, verbal learning, and memory.20 More studies are needed before definitive conclusions can be drawn.
For the confused patient, a personalized therapeutic program, in which multiple interventions are considered at once (targeting all areas of the patient’s life) is gaining research traction. For example, a novel, comprehensive program involving multiple modalities designed to achieve metabolic enhancement for neurodegeneration (MEND) recently has shown robust benefit for patients with AD, MCI, and SCI.21 Using an individual approach to improve diet, activity, sleep, metabolic status including body mass index, and several other markers that affect neural plasticity, researchers demonstrated symptom improvement in 9 of 10 study patients.
Yet, some of the interventions, such as the use of statins for hyperlipidemia, remain controversial, with some studies suggesting that they help cognition,22,23 and others showing no association.24 The researchers caution that further research is warranted before costly dementia prevention trials with statins are undertaken. It does not appear that there are current MEND-type research projects in delirium but it’s to be hoped that we will see these in the future.
In the case of Ms. T, the cause of delirium vs mild NCD was thought to be multifactorial. Discontinuing Armour Thyroid and metformin—symptoms of hypoglycemia emerged as a leading concern—were simple adjustments that led to resolution of the most concerning elements of her confusion. She continued her other psychotropics, although there might be mild residual cognitive issues that warrant close observation.
Related Resources
• Lin JS, O’Connor E, Rossum RC, et al. Screening for cognitive impairment in older adults: an evidence update for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013.
• Grover S, Kate N. Assessment scales for delirium: a review. World J Psychiatry. 2012;2(4):58-70.
Drug Brand Names
Atorvastatin • Lipitor Lithium • Eskalith, Lithobid
Donepezil • Aricept Lorazepam • Ativan
Escitalopram • Lexapro Memantine • Namenda
Flumazenil • Romazicon Metformin • Glucophage
Galantamine • Razadyne Naloxone • Narcan
Glipizide • Glucotrol Physostigmine • Antilirium
Haloperidol • Haldol Quetiapine • Seroquel
Levothyroxine • Levoxyl, Synthroid Rivastigmine • Exelon
Lithium • Eskalith, Lithobid Valproic acid • Depakene
Disclosure
Dr. Raj is a speaker for Actavis Pharmaceuticals, AstraZeneca, and Merck.
1. Borson S, Scanlan JM, Chen P, et al. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10):1451-1454.
2. Wilber ST, Lofgren SD, Mager TG, et al. An evaluation of two screening tools for cognitive impairment in older emergency department patients. Acad Emerg Med. 2005;12(7):612-616.
3. Tariq SH, Tumosa N, Chibnall JT, et al. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
4. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695- 699.
5. Inouye S, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Int Med. 1990;113(12):941-948.
6. Hillmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167(8):781-787.
7. Raj YP. Psychiatric emergencies. In: Jiang W, Gagliardi JP, Krishnan KR, eds. Clinician’s guide to psychiatric care. New York, NY: Oxford University Press; 2009:33-40.
8. Liptzin B. Clinical diagnosis and management of delirium. In: Stoudemire A, Fogel BS, Greenberg DB, eds. Psychiatric care of the medical patient. 2nd ed. New York, NY: Oxford University Press; 2000:581-596.
9. Raj YP. Subclinical hypothyroidism: merely monitor or time to treat? Current Psychiatry. 2009;8(2):47-48.
10. Poloni N, Vender S, Bolla E, et al. Gluten encephalopathy with psychiatric onset: case report. Clin Pract Epidemiol Ment Health. 2009;5:16.
11. Naughton BJ, Moran M, Ghaly Y, et al. Computed tomography scanning and delirium in elder patients. Acad Emerg Med. 1997;4(12):1107-1110.
12. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666.
13. Zitzmann S, Reimann IR, Schmechel H. Severe hypoglycemia in an elderly patient treated with metformin. Int J Clin Pharmacol Ther. 2002;40(3):108-110.
14. Benson AD, Slavin MJ, Tran TT, et al. Screening for early Alzheimer’s Disease: is there still a role for the Mini-Mental State Examination? Prim Care Companion J Clin Psychiatry. 2005;7(2):62-69.
15. Brown WA. Pseudodementia: issues in diagnosis. Psychiatric Times. http://www.psychiatrictimes.com/ pseudodementia-issues-diagnosis. Published April 9, 2005. Accessed February 2, 2015.
16. Frattaroli J. Experimental disclosure and its moderators: a meta-analysis. Psychol Bull. 2006;132(6):823-865.
17. Stetka BS, Correll CU. A guide to DSM-5: neurocognitive disorder. Medscape. http://www.medscape.com/ viewarticle/803884_13. Published May 21, 2013. Accessed October 30, 2014.
18. Reisberg B, Sulman MD, Torossian C, et al. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6(1):11-24.
19. Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;(2):CD005594.
20. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Intern Clin Psychopharmacol. 2012;27(4):215-223.
21. Bredesen DE. Reversal of cognitive decline: a novel therapeutic program. Aging (Albany NY). 2014;6(9):707-717.
22. Sparks DL, Kryscio RJ, Sabbagh MN, et al. Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr Alzheimer Res. 2008;5(4):416-421.
23. Andrade C, Radhakrishnan R. The prevention and treatment of cognitive decline and dementia: an overview of recent research on experimental treatments. Indian J Psychiatry. 2009;51(1):12-25.
24. Zandi PP, Sparks DL, Khachaturian AS, et al. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry. 2005;62(2):217-224.
1. Borson S, Scanlan JM, Chen P, et al. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10):1451-1454.
2. Wilber ST, Lofgren SD, Mager TG, et al. An evaluation of two screening tools for cognitive impairment in older emergency department patients. Acad Emerg Med. 2005;12(7):612-616.
3. Tariq SH, Tumosa N, Chibnall JT, et al. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
4. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695- 699.
5. Inouye S, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Int Med. 1990;113(12):941-948.
6. Hillmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167(8):781-787.
7. Raj YP. Psychiatric emergencies. In: Jiang W, Gagliardi JP, Krishnan KR, eds. Clinician’s guide to psychiatric care. New York, NY: Oxford University Press; 2009:33-40.
8. Liptzin B. Clinical diagnosis and management of delirium. In: Stoudemire A, Fogel BS, Greenberg DB, eds. Psychiatric care of the medical patient. 2nd ed. New York, NY: Oxford University Press; 2000:581-596.
9. Raj YP. Subclinical hypothyroidism: merely monitor or time to treat? Current Psychiatry. 2009;8(2):47-48.
10. Poloni N, Vender S, Bolla E, et al. Gluten encephalopathy with psychiatric onset: case report. Clin Pract Epidemiol Ment Health. 2009;5:16.
11. Naughton BJ, Moran M, Ghaly Y, et al. Computed tomography scanning and delirium in elder patients. Acad Emerg Med. 1997;4(12):1107-1110.
12. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666.
13. Zitzmann S, Reimann IR, Schmechel H. Severe hypoglycemia in an elderly patient treated with metformin. Int J Clin Pharmacol Ther. 2002;40(3):108-110.
14. Benson AD, Slavin MJ, Tran TT, et al. Screening for early Alzheimer’s Disease: is there still a role for the Mini-Mental State Examination? Prim Care Companion J Clin Psychiatry. 2005;7(2):62-69.
15. Brown WA. Pseudodementia: issues in diagnosis. Psychiatric Times. http://www.psychiatrictimes.com/ pseudodementia-issues-diagnosis. Published April 9, 2005. Accessed February 2, 2015.
16. Frattaroli J. Experimental disclosure and its moderators: a meta-analysis. Psychol Bull. 2006;132(6):823-865.
17. Stetka BS, Correll CU. A guide to DSM-5: neurocognitive disorder. Medscape. http://www.medscape.com/ viewarticle/803884_13. Published May 21, 2013. Accessed October 30, 2014.
18. Reisberg B, Sulman MD, Torossian C, et al. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6(1):11-24.
19. Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;(2):CD005594.
20. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Intern Clin Psychopharmacol. 2012;27(4):215-223.
21. Bredesen DE. Reversal of cognitive decline: a novel therapeutic program. Aging (Albany NY). 2014;6(9):707-717.
22. Sparks DL, Kryscio RJ, Sabbagh MN, et al. Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr Alzheimer Res. 2008;5(4):416-421.
23. Andrade C, Radhakrishnan R. The prevention and treatment of cognitive decline and dementia: an overview of recent research on experimental treatments. Indian J Psychiatry. 2009;51(1):12-25.
24. Zandi PP, Sparks DL, Khachaturian AS, et al. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry. 2005;62(2):217-224.
Treating thyroid disorders and depression: 3 case studies
Discuss this article at www.facebook.com/CurrentPsychiatry
Many endocrine disorders can manifest as depression, including relatively rare disorders such as Cushing’s syndrome (hypercortisolism) or Conn’s syndrome (primary hyperaldosteronism) as well as common ones such as diabetes mellitus. Most clinicians do not routinely screen for adrenal disorders when evaluating depressed patients because the yield is low, but do screen for thyroid disease because these disorders often mimic depression. The following 3 cases from my practice illustrate some nuances of screening and treating depressed patients with suspected thyroid abnormalities.
CASE 1: Feeling ‘like an 80-year-old’
Ms. A, age 25, has a gastrointestinal stromal tumor (GIST) and states that she feels “like an 80-year-old woman.” She is sore all over with facial swelling, abdominal cramping, and fatigue. This feeling has worsened since she started chemotherapy with sunitinib for the GIST. Her Patient Health Questionnaire-9 (PHQ-9) score is 14 out of 27, indicating moderate depression. As part of a workup for her depression, what general laboratory tests would be most helpful?
Because Ms. A is of menstruating age, check hemoglobin/hematocrit levels to evaluate for anemia. Monitoring electrolytes would allow you to assess for hypernatremia/hyponatremia, hyperkalemia/hypokalemia, and impaired renal function, all of which could cause depressive symptoms. Depending on Ms. A’s habitus or risk of metabolic syndrome, a fasting blood glucose or hemoglobin A1C test to screen for diabetes mellitus might be valuable because depression may be associated with diabetes.1 A1C is a preferred primary screening test for diabetes (≥6.5% constitutes a positive screen) based on revised clinical practice recommendations of the American Diabetes Association. A1C is available as an office-based test that requires just a drop of blood from a finger prick and does not require a fasting blood sample or a full laboratory analysis.
A popular test for a workup of depression is serum 25-hydroxyvitamin D [25(OH)D] (vitamin D), particularly for patients who live in areas with limited exposure to ultraviolet B radiation from sunlight.2 In a study of older adults, vitamin D levels were 14% lower in patients with minor depression and 14% lower in patients with major depressive disorder compared with controls. This study suggests that depression severity is associated with decreased serum vitamin D levels,3 but the association between depression and vitamin D insufficiency and deficiency is unknown. Checking sex hormones also may be helpful depending on the patient’s symptoms, because testosterone deficiency in men and dehydroepiandrosterone deficiency in women can have a direct impact on a patient’s libido and overall sense of well-being. If repleted, improved levels of sex hormones can lead to a dramatic improvement in mood as well.
Because more than one-half of the estimated 27 million Americans with hyperthyroidism or hypothyroidism are undiagnosed, the American Thyroid Association recommends universal screening for thyroid dysfunction after age 35, with a recheck every 5 years.4 However, checking serum thyroid-stimulating hormone (TSH) levels this often may not be cost-effective. Typically, I do not follow this recommendation when assessing or treating asymptomatic individuals, but Ms. A has symptoms of hypothyroidism (Table 1) and is taking a medication—sunitinib—thought to be associated with hypothyroidism.5 Her serum TSH was very high (110 mIU/L; range 0.28 to 5.00) and her serum free T4 (FT4) was low (0.5 ng/dL; range 0.7 to 1.8). These values were consistent with overt hypothyroidism, defined as low FT4 and elevated TSH levels. This is in contrast to subclinical hypothyroidism (SH), which is defined as having an elevated serum TSH with normal thyroid hormone (T3 and T4) levels. SH presents in 5% of young patients (age <45) and increasingly is being diagnoses in older patients (age >55), who are most likely to suffer adverse effects in mood or cognition.6
Table 1
Hypothyroidism symptoms
| Psychiatric overlap |
| Fatigue |
| Hypersomnolence |
| Cognitive impairment (forgetfulness) |
| Difficulty concentrating or learning |
| Weight gain or fluid retention |
| Somatic signs and symptoms |
| Dry, itchy skin |
| Brittle hair and nails |
| Constipation |
| Myalgias |
| Heavy and/or irregular menstrual cycle |
| Increased rate of miscarriage |
| Sensitivity to cold |
CASE 1 CONTINUED: A classic case
Ms. A is started on a full levothyroxine replacement dose of 1.6 μg/kg/d. For hypothyroid patients who do not have cardiac symptoms, weight-based replacement is thought to be safe and more convenient than starting with a low dose and titrating up.7 Ms. A responds quickly. At 6-week follow-up—the recommended time interval for repeat thyroid lab testing after initiating thyroid replacement—her depressive symptoms are markedly improved and her PHQ-9 score is 6, indicating mild depression.
CASE 2: Chronic pain, low mood, and fatigue
Ms. B, age 62, has fibromyalgia and chronic back pain. She takes cyclobenzaprine, 5 mg 2 to 3 times daily, and oxycodone, 40 mg/d, and describes mild depressive symptoms when she presents for routine follow-up. Most of her complaints are related to chronic pain, but she has a history of low mood and fatigue. She says she was prescribed levothyroxine, but is unable to remember if she stopped taking it because of financial constraints or laboratory/clinical improvement. Her neurologist recently checked her serum TSH, which was elevated at 8.1 mIU/L. Is it best to restart thyroid replacement or wait 6 weeks and recheck her thyroid panel?
Mild SH typically is defined as TSH between 4.5 and 10 mIU/L. In contrast, TSH between 10 and 20 mIU/L is considered severe SH. Because Ms. B did not have prominent new symptoms, I felt it was reasonable to wait the recommended 6 weeks before rechecking her thyroid function. At follow-up, Ms. B’s TSH was 4.64 mIU/L and her FT4 was normal: 0.7 ng/dL. Thyroid replacement was not indicated because she did not have obvious symptoms and treating SH does not impact overall mood and cognition until TSH is ≥10 mIU/L.8,9
CASE 2 CONTINUED: Prominent symptoms emerge
Ms. B returns several months later. Another clinician prescribed duloxetine, titrated from 30 mg to 60 mg, for worsening fibromyalgia. Her depressive symptoms are more prominent at this visit, and her PHQ-9 score has risen from 7 to 14, indicating moderate depression. She says previously she failed or poorly tolerated several antidepressants—fluoxetine, sertraline, and citalopram—but was hoping for a pharmacologic adjustment. Most evidence-based augmentation algorithms for treating major depression start with adding a second “traditional” antidepressant such as bupropion, then move to lithium, second-generation antipsychotics, or lamotrigine.10 But what about thyroid hormone augmentation?
Thyroid hormone often is on the lower rungs of depression treatment algorithms despite Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial data. The data suggest triiodothyronine’s (T3) lower side effect burden and ease of use may offer an advantage over lithium augmentation for depressed patients who have failed several medication trials.11 Liothyronine sodium (triiodothyronine) is a relatively benign medication with potential for augmentation when started at 25 to 50 mcg/d concurrently with antidepressants such as sertraline.12 Unfortunately, most augmentation trials with T3 have been short-term—generally 4 to 8 weeks. In my practice, T3 has limited application; I use it mainly for patients with treatment-resistant depression who have failed several other treatments.
Lithium, the comparison medication to thyroid hormone in the third augmentation arm of the STAR*D trial, requires an annual check of thyroid function (TSH testing) to properly monitor for potential lithium-related hypothyroidism or thyroiditis. Hypothyroidism, for which thyroid replacement is required, with lithium therapy is common, affecting 8% to 27% of patients.13 Patients who rapidly gain weight at the beginning of lithium treatment seem to have a higher risk of developing hypothyroidism.13 However, the risk of developing lithium-induced hypothyroidism is tied to the length of treatment; the longer a patient has been treated with lithium, the greater the risk of developing lithium-induced hypothyroidism.
CASE 3: Unable to slow down
Mr. C, age 45, has a 20-year history of major depression controlled reasonably well with paroxetine, 40 mg. He presents with escalating anxiety, depression, and irritability. His wife is concerned about his overwhelming thoughts of death, especially because Mr. C’s father committed suicide 30 years ago under similar circumstances. Mr. C has been tremulous for the past month and has not been sleeping well. He feels like he is “in constant motion” and unable to slow down. He screens in the “highly likely” range for bipolar disorder on the Bipolar Spectrum Diagnostic Scale14 and is started on divalproex ER, 500 mg/d.
His thyroid function tests returns with a suppressed TSH of 0.03 mIU/L and an elevated FT4 of 3.26 ng/dL. Divalproex is discontinued and he is started on the beta blocker atenolol, 25 mg/d, to target his anxiety, tachycardia, and akathisia. TSH receptor antibody testing was positive, which, along with an abnormal radioactive iodine uptake scan, confirmed a diagnosis of Graves’ disease. He receives methimazole, 20 mg/d, as a temporizing measure. An endocrinologist completes a radioactive iodine (I-131) ablation procedure on Mr. C, which resolves his mood and anxiety symptoms.
Although hypothyroidism commonly is associated with depressive symptoms, hyperthyroidism also may present as depression. Most cases of overt hyperthyroidism are directly referred to an endocrinologist because when treating disorders such as Graves’ disease—the most common cause of hyperthyroidism, especially among women age 20 to 40—many nuclear medicine teams require the expert guidance of an endocrinologist before considering radioiodine ablation. Hyperthyroidism often is accompanied by psychiatric and somatic symptoms of an “overactive” nature (Table 2). However, older patients (age >65) with hyperthyroidism may develop apathetic hyperthyroidism, a subset that comprises approximately 10% to 15% of all hyperthyroidism cases in older adults.15 Rather than becoming nervous, jittery, and restless, patients with apathetic hyperthyroidism are depressed, lethargic, and weak, and may develop proximal myopathy or cardiomyopathy. It is essential to differentiate apathetic hyperthyroidism from typical hyperthyroidism because accurately diagnosing and treating apathetic hyperthyroidism will improve outcomes.15
Table 2
Hyperthyroidism symptoms
| Psychiatric overlap |
| Decrease or increase in appetite |
| Insomnia |
| Fatigue |
| Mood instability |
| Irritability |
| Anxiety, nervousness |
| Somatic signs and symptoms |
| Frequent bowel movement, eg, diarrhea |
| Heart palpitations |
| Heat intolerance |
| Increased sweating |
| Light or missed menstrual periods, fertility problems |
| Muscle weakness |
| Shortness of breath |
| Sudden paralysis |
| Tremor, shakiness, dizziness |
| Vision changes |
| Weight loss or gain |
| Thinning of hair |
| Itching and hives |
| Possible increase in blood sugar |
Using beta blockers to treat hyperthyroidism can help control tachycardia or palpitations, tremulousness, and anxiety that often are inherent in hyperthyroidism. But can beta blockers induce depressive symptoms? A 1-year prospective Dutch study of patients who had survived a myocardial infarction did not find evidence that beta blockers induced depressive symptoms.16 However, the long-term and high-dosage effects of beta blockers still are in question.16 In Mr. C’s case, beta blockers had only positive effects on his symptoms and did not exacerbate his depressive symptoms.
Related Resources
- National Women’s Health Resource Center, Inc. Thyroid disorders. www.healthywomen.org/condition/thyroid-disorders.
- American Thyroid Association. www.thyroid.org.
- American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. www.aace.com/files/hypo-hyper.pdf.
Drug Brand Names
- Atenolol • Tenormin
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Cyclobenzaprine • Flexeril
- Divalproex ER • Depakote ER
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Levothyroxine • Levoxyl, Synthroid
- Liothyronine sodium • Cytomel, Triostat
- Lithium • Eskalith, Lithobid
- Methimazole • Tapazole
- Oxycodone • OxyContin
- Paroxetine • Paxil
- Sertraline • Zoloft
- Sunitinib • Sutent
Disclosure
Dr. Raj is a speaker for AstraZeneca and Merck.
1. Campayo A, de Jonge P, Roy JF, et al. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry. 2010;167(5):580-588.
2. Gallagher JC, Sai AJ. Vitamin D insufficiency deficiency, and bone health. J Clin Endocrinol Metab. 2010;95(6):2630-2633.
3. Hoogendijk WJ, Lips P, Dik MG, et al. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65(5):508-512.
4. Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med. 2000;160(11):1573-1575.
5. Wolter P, Dumez H, Schöffski P. Sunitinib and hypothyroidism. N Engl J Med. 2007;356(15):1580; author reply 1580-1581.
6. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76-131.
7. Roos A, Linn-Rasker SP, van Domburg RT, et al. The starting dose of levothyroxine in primary hypothyroidism treatment: a prospective, randomized, double-blind trial. Arch Intern Med. 2005;165(15):1714-1720.
8. Raj YP. Subclinical hypothyroidism: merely monitor or time to treat? Current Psychiatry. 2009;8(2):47-48.
9. Samuels MH. Cognitive function in subclinical hypothyroidism. J Clin Endocrinol Metab. 2010;95(8):3611-3613.
10. Mann JJ. The medical management of depression. N Engl J Med. 2005;353(17):1819-1834.
11. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
12. Cooper-Kazaz R, Apter JT, Cohen R, et al. Combined treatment with sertraline and liothyronine in major depression: a randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2007;64(6):679-688.
13. Henry C. Lithium side-effects and predictors of hypothyroidism in patients with bipolar disorder: sex differences. J Psychiatry Neurosci. 2002;27(2):104-107.
14. Ghaemi N, Pies R. The Bipolar Spectrum Diagnostic Scale. http://www.psycheducation.org/depression/BSDS.htm. Published October 2002. Updated June 2003. Accessed October 1 2012.
15. Wu W, Sun Z, Yu J, et al. A clinical retrospective analysis of factors associated with apathetic hyperthyroidism. Pathobiology. 2010;77(1):46-51.
16. van Melle JP, Verbeek DE, van den Berg MP, et al. Beta-blockers and depression after myocardial infarction: a multicenter prospective study. J Am Coll Cardiol. 2006;48(11):2209-2214.
Discuss this article at www.facebook.com/CurrentPsychiatry
Many endocrine disorders can manifest as depression, including relatively rare disorders such as Cushing’s syndrome (hypercortisolism) or Conn’s syndrome (primary hyperaldosteronism) as well as common ones such as diabetes mellitus. Most clinicians do not routinely screen for adrenal disorders when evaluating depressed patients because the yield is low, but do screen for thyroid disease because these disorders often mimic depression. The following 3 cases from my practice illustrate some nuances of screening and treating depressed patients with suspected thyroid abnormalities.
CASE 1: Feeling ‘like an 80-year-old’
Ms. A, age 25, has a gastrointestinal stromal tumor (GIST) and states that she feels “like an 80-year-old woman.” She is sore all over with facial swelling, abdominal cramping, and fatigue. This feeling has worsened since she started chemotherapy with sunitinib for the GIST. Her Patient Health Questionnaire-9 (PHQ-9) score is 14 out of 27, indicating moderate depression. As part of a workup for her depression, what general laboratory tests would be most helpful?
Because Ms. A is of menstruating age, check hemoglobin/hematocrit levels to evaluate for anemia. Monitoring electrolytes would allow you to assess for hypernatremia/hyponatremia, hyperkalemia/hypokalemia, and impaired renal function, all of which could cause depressive symptoms. Depending on Ms. A’s habitus or risk of metabolic syndrome, a fasting blood glucose or hemoglobin A1C test to screen for diabetes mellitus might be valuable because depression may be associated with diabetes.1 A1C is a preferred primary screening test for diabetes (≥6.5% constitutes a positive screen) based on revised clinical practice recommendations of the American Diabetes Association. A1C is available as an office-based test that requires just a drop of blood from a finger prick and does not require a fasting blood sample or a full laboratory analysis.
A popular test for a workup of depression is serum 25-hydroxyvitamin D [25(OH)D] (vitamin D), particularly for patients who live in areas with limited exposure to ultraviolet B radiation from sunlight.2 In a study of older adults, vitamin D levels were 14% lower in patients with minor depression and 14% lower in patients with major depressive disorder compared with controls. This study suggests that depression severity is associated with decreased serum vitamin D levels,3 but the association between depression and vitamin D insufficiency and deficiency is unknown. Checking sex hormones also may be helpful depending on the patient’s symptoms, because testosterone deficiency in men and dehydroepiandrosterone deficiency in women can have a direct impact on a patient’s libido and overall sense of well-being. If repleted, improved levels of sex hormones can lead to a dramatic improvement in mood as well.
Because more than one-half of the estimated 27 million Americans with hyperthyroidism or hypothyroidism are undiagnosed, the American Thyroid Association recommends universal screening for thyroid dysfunction after age 35, with a recheck every 5 years.4 However, checking serum thyroid-stimulating hormone (TSH) levels this often may not be cost-effective. Typically, I do not follow this recommendation when assessing or treating asymptomatic individuals, but Ms. A has symptoms of hypothyroidism (Table 1) and is taking a medication—sunitinib—thought to be associated with hypothyroidism.5 Her serum TSH was very high (110 mIU/L; range 0.28 to 5.00) and her serum free T4 (FT4) was low (0.5 ng/dL; range 0.7 to 1.8). These values were consistent with overt hypothyroidism, defined as low FT4 and elevated TSH levels. This is in contrast to subclinical hypothyroidism (SH), which is defined as having an elevated serum TSH with normal thyroid hormone (T3 and T4) levels. SH presents in 5% of young patients (age <45) and increasingly is being diagnoses in older patients (age >55), who are most likely to suffer adverse effects in mood or cognition.6
Table 1
Hypothyroidism symptoms
| Psychiatric overlap |
| Fatigue |
| Hypersomnolence |
| Cognitive impairment (forgetfulness) |
| Difficulty concentrating or learning |
| Weight gain or fluid retention |
| Somatic signs and symptoms |
| Dry, itchy skin |
| Brittle hair and nails |
| Constipation |
| Myalgias |
| Heavy and/or irregular menstrual cycle |
| Increased rate of miscarriage |
| Sensitivity to cold |
CASE 1 CONTINUED: A classic case
Ms. A is started on a full levothyroxine replacement dose of 1.6 μg/kg/d. For hypothyroid patients who do not have cardiac symptoms, weight-based replacement is thought to be safe and more convenient than starting with a low dose and titrating up.7 Ms. A responds quickly. At 6-week follow-up—the recommended time interval for repeat thyroid lab testing after initiating thyroid replacement—her depressive symptoms are markedly improved and her PHQ-9 score is 6, indicating mild depression.
CASE 2: Chronic pain, low mood, and fatigue
Ms. B, age 62, has fibromyalgia and chronic back pain. She takes cyclobenzaprine, 5 mg 2 to 3 times daily, and oxycodone, 40 mg/d, and describes mild depressive symptoms when she presents for routine follow-up. Most of her complaints are related to chronic pain, but she has a history of low mood and fatigue. She says she was prescribed levothyroxine, but is unable to remember if she stopped taking it because of financial constraints or laboratory/clinical improvement. Her neurologist recently checked her serum TSH, which was elevated at 8.1 mIU/L. Is it best to restart thyroid replacement or wait 6 weeks and recheck her thyroid panel?
Mild SH typically is defined as TSH between 4.5 and 10 mIU/L. In contrast, TSH between 10 and 20 mIU/L is considered severe SH. Because Ms. B did not have prominent new symptoms, I felt it was reasonable to wait the recommended 6 weeks before rechecking her thyroid function. At follow-up, Ms. B’s TSH was 4.64 mIU/L and her FT4 was normal: 0.7 ng/dL. Thyroid replacement was not indicated because she did not have obvious symptoms and treating SH does not impact overall mood and cognition until TSH is ≥10 mIU/L.8,9
CASE 2 CONTINUED: Prominent symptoms emerge
Ms. B returns several months later. Another clinician prescribed duloxetine, titrated from 30 mg to 60 mg, for worsening fibromyalgia. Her depressive symptoms are more prominent at this visit, and her PHQ-9 score has risen from 7 to 14, indicating moderate depression. She says previously she failed or poorly tolerated several antidepressants—fluoxetine, sertraline, and citalopram—but was hoping for a pharmacologic adjustment. Most evidence-based augmentation algorithms for treating major depression start with adding a second “traditional” antidepressant such as bupropion, then move to lithium, second-generation antipsychotics, or lamotrigine.10 But what about thyroid hormone augmentation?
Thyroid hormone often is on the lower rungs of depression treatment algorithms despite Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial data. The data suggest triiodothyronine’s (T3) lower side effect burden and ease of use may offer an advantage over lithium augmentation for depressed patients who have failed several medication trials.11 Liothyronine sodium (triiodothyronine) is a relatively benign medication with potential for augmentation when started at 25 to 50 mcg/d concurrently with antidepressants such as sertraline.12 Unfortunately, most augmentation trials with T3 have been short-term—generally 4 to 8 weeks. In my practice, T3 has limited application; I use it mainly for patients with treatment-resistant depression who have failed several other treatments.
Lithium, the comparison medication to thyroid hormone in the third augmentation arm of the STAR*D trial, requires an annual check of thyroid function (TSH testing) to properly monitor for potential lithium-related hypothyroidism or thyroiditis. Hypothyroidism, for which thyroid replacement is required, with lithium therapy is common, affecting 8% to 27% of patients.13 Patients who rapidly gain weight at the beginning of lithium treatment seem to have a higher risk of developing hypothyroidism.13 However, the risk of developing lithium-induced hypothyroidism is tied to the length of treatment; the longer a patient has been treated with lithium, the greater the risk of developing lithium-induced hypothyroidism.
CASE 3: Unable to slow down
Mr. C, age 45, has a 20-year history of major depression controlled reasonably well with paroxetine, 40 mg. He presents with escalating anxiety, depression, and irritability. His wife is concerned about his overwhelming thoughts of death, especially because Mr. C’s father committed suicide 30 years ago under similar circumstances. Mr. C has been tremulous for the past month and has not been sleeping well. He feels like he is “in constant motion” and unable to slow down. He screens in the “highly likely” range for bipolar disorder on the Bipolar Spectrum Diagnostic Scale14 and is started on divalproex ER, 500 mg/d.
His thyroid function tests returns with a suppressed TSH of 0.03 mIU/L and an elevated FT4 of 3.26 ng/dL. Divalproex is discontinued and he is started on the beta blocker atenolol, 25 mg/d, to target his anxiety, tachycardia, and akathisia. TSH receptor antibody testing was positive, which, along with an abnormal radioactive iodine uptake scan, confirmed a diagnosis of Graves’ disease. He receives methimazole, 20 mg/d, as a temporizing measure. An endocrinologist completes a radioactive iodine (I-131) ablation procedure on Mr. C, which resolves his mood and anxiety symptoms.
Although hypothyroidism commonly is associated with depressive symptoms, hyperthyroidism also may present as depression. Most cases of overt hyperthyroidism are directly referred to an endocrinologist because when treating disorders such as Graves’ disease—the most common cause of hyperthyroidism, especially among women age 20 to 40—many nuclear medicine teams require the expert guidance of an endocrinologist before considering radioiodine ablation. Hyperthyroidism often is accompanied by psychiatric and somatic symptoms of an “overactive” nature (Table 2). However, older patients (age >65) with hyperthyroidism may develop apathetic hyperthyroidism, a subset that comprises approximately 10% to 15% of all hyperthyroidism cases in older adults.15 Rather than becoming nervous, jittery, and restless, patients with apathetic hyperthyroidism are depressed, lethargic, and weak, and may develop proximal myopathy or cardiomyopathy. It is essential to differentiate apathetic hyperthyroidism from typical hyperthyroidism because accurately diagnosing and treating apathetic hyperthyroidism will improve outcomes.15
Table 2
Hyperthyroidism symptoms
| Psychiatric overlap |
| Decrease or increase in appetite |
| Insomnia |
| Fatigue |
| Mood instability |
| Irritability |
| Anxiety, nervousness |
| Somatic signs and symptoms |
| Frequent bowel movement, eg, diarrhea |
| Heart palpitations |
| Heat intolerance |
| Increased sweating |
| Light or missed menstrual periods, fertility problems |
| Muscle weakness |
| Shortness of breath |
| Sudden paralysis |
| Tremor, shakiness, dizziness |
| Vision changes |
| Weight loss or gain |
| Thinning of hair |
| Itching and hives |
| Possible increase in blood sugar |
Using beta blockers to treat hyperthyroidism can help control tachycardia or palpitations, tremulousness, and anxiety that often are inherent in hyperthyroidism. But can beta blockers induce depressive symptoms? A 1-year prospective Dutch study of patients who had survived a myocardial infarction did not find evidence that beta blockers induced depressive symptoms.16 However, the long-term and high-dosage effects of beta blockers still are in question.16 In Mr. C’s case, beta blockers had only positive effects on his symptoms and did not exacerbate his depressive symptoms.
Related Resources
- National Women’s Health Resource Center, Inc. Thyroid disorders. www.healthywomen.org/condition/thyroid-disorders.
- American Thyroid Association. www.thyroid.org.
- American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. www.aace.com/files/hypo-hyper.pdf.
Drug Brand Names
- Atenolol • Tenormin
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Cyclobenzaprine • Flexeril
- Divalproex ER • Depakote ER
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Levothyroxine • Levoxyl, Synthroid
- Liothyronine sodium • Cytomel, Triostat
- Lithium • Eskalith, Lithobid
- Methimazole • Tapazole
- Oxycodone • OxyContin
- Paroxetine • Paxil
- Sertraline • Zoloft
- Sunitinib • Sutent
Disclosure
Dr. Raj is a speaker for AstraZeneca and Merck.
Discuss this article at www.facebook.com/CurrentPsychiatry
Many endocrine disorders can manifest as depression, including relatively rare disorders such as Cushing’s syndrome (hypercortisolism) or Conn’s syndrome (primary hyperaldosteronism) as well as common ones such as diabetes mellitus. Most clinicians do not routinely screen for adrenal disorders when evaluating depressed patients because the yield is low, but do screen for thyroid disease because these disorders often mimic depression. The following 3 cases from my practice illustrate some nuances of screening and treating depressed patients with suspected thyroid abnormalities.
CASE 1: Feeling ‘like an 80-year-old’
Ms. A, age 25, has a gastrointestinal stromal tumor (GIST) and states that she feels “like an 80-year-old woman.” She is sore all over with facial swelling, abdominal cramping, and fatigue. This feeling has worsened since she started chemotherapy with sunitinib for the GIST. Her Patient Health Questionnaire-9 (PHQ-9) score is 14 out of 27, indicating moderate depression. As part of a workup for her depression, what general laboratory tests would be most helpful?
Because Ms. A is of menstruating age, check hemoglobin/hematocrit levels to evaluate for anemia. Monitoring electrolytes would allow you to assess for hypernatremia/hyponatremia, hyperkalemia/hypokalemia, and impaired renal function, all of which could cause depressive symptoms. Depending on Ms. A’s habitus or risk of metabolic syndrome, a fasting blood glucose or hemoglobin A1C test to screen for diabetes mellitus might be valuable because depression may be associated with diabetes.1 A1C is a preferred primary screening test for diabetes (≥6.5% constitutes a positive screen) based on revised clinical practice recommendations of the American Diabetes Association. A1C is available as an office-based test that requires just a drop of blood from a finger prick and does not require a fasting blood sample or a full laboratory analysis.
A popular test for a workup of depression is serum 25-hydroxyvitamin D [25(OH)D] (vitamin D), particularly for patients who live in areas with limited exposure to ultraviolet B radiation from sunlight.2 In a study of older adults, vitamin D levels were 14% lower in patients with minor depression and 14% lower in patients with major depressive disorder compared with controls. This study suggests that depression severity is associated with decreased serum vitamin D levels,3 but the association between depression and vitamin D insufficiency and deficiency is unknown. Checking sex hormones also may be helpful depending on the patient’s symptoms, because testosterone deficiency in men and dehydroepiandrosterone deficiency in women can have a direct impact on a patient’s libido and overall sense of well-being. If repleted, improved levels of sex hormones can lead to a dramatic improvement in mood as well.
Because more than one-half of the estimated 27 million Americans with hyperthyroidism or hypothyroidism are undiagnosed, the American Thyroid Association recommends universal screening for thyroid dysfunction after age 35, with a recheck every 5 years.4 However, checking serum thyroid-stimulating hormone (TSH) levels this often may not be cost-effective. Typically, I do not follow this recommendation when assessing or treating asymptomatic individuals, but Ms. A has symptoms of hypothyroidism (Table 1) and is taking a medication—sunitinib—thought to be associated with hypothyroidism.5 Her serum TSH was very high (110 mIU/L; range 0.28 to 5.00) and her serum free T4 (FT4) was low (0.5 ng/dL; range 0.7 to 1.8). These values were consistent with overt hypothyroidism, defined as low FT4 and elevated TSH levels. This is in contrast to subclinical hypothyroidism (SH), which is defined as having an elevated serum TSH with normal thyroid hormone (T3 and T4) levels. SH presents in 5% of young patients (age <45) and increasingly is being diagnoses in older patients (age >55), who are most likely to suffer adverse effects in mood or cognition.6
Table 1
Hypothyroidism symptoms
| Psychiatric overlap |
| Fatigue |
| Hypersomnolence |
| Cognitive impairment (forgetfulness) |
| Difficulty concentrating or learning |
| Weight gain or fluid retention |
| Somatic signs and symptoms |
| Dry, itchy skin |
| Brittle hair and nails |
| Constipation |
| Myalgias |
| Heavy and/or irregular menstrual cycle |
| Increased rate of miscarriage |
| Sensitivity to cold |
CASE 1 CONTINUED: A classic case
Ms. A is started on a full levothyroxine replacement dose of 1.6 μg/kg/d. For hypothyroid patients who do not have cardiac symptoms, weight-based replacement is thought to be safe and more convenient than starting with a low dose and titrating up.7 Ms. A responds quickly. At 6-week follow-up—the recommended time interval for repeat thyroid lab testing after initiating thyroid replacement—her depressive symptoms are markedly improved and her PHQ-9 score is 6, indicating mild depression.
CASE 2: Chronic pain, low mood, and fatigue
Ms. B, age 62, has fibromyalgia and chronic back pain. She takes cyclobenzaprine, 5 mg 2 to 3 times daily, and oxycodone, 40 mg/d, and describes mild depressive symptoms when she presents for routine follow-up. Most of her complaints are related to chronic pain, but she has a history of low mood and fatigue. She says she was prescribed levothyroxine, but is unable to remember if she stopped taking it because of financial constraints or laboratory/clinical improvement. Her neurologist recently checked her serum TSH, which was elevated at 8.1 mIU/L. Is it best to restart thyroid replacement or wait 6 weeks and recheck her thyroid panel?
Mild SH typically is defined as TSH between 4.5 and 10 mIU/L. In contrast, TSH between 10 and 20 mIU/L is considered severe SH. Because Ms. B did not have prominent new symptoms, I felt it was reasonable to wait the recommended 6 weeks before rechecking her thyroid function. At follow-up, Ms. B’s TSH was 4.64 mIU/L and her FT4 was normal: 0.7 ng/dL. Thyroid replacement was not indicated because she did not have obvious symptoms and treating SH does not impact overall mood and cognition until TSH is ≥10 mIU/L.8,9
CASE 2 CONTINUED: Prominent symptoms emerge
Ms. B returns several months later. Another clinician prescribed duloxetine, titrated from 30 mg to 60 mg, for worsening fibromyalgia. Her depressive symptoms are more prominent at this visit, and her PHQ-9 score has risen from 7 to 14, indicating moderate depression. She says previously she failed or poorly tolerated several antidepressants—fluoxetine, sertraline, and citalopram—but was hoping for a pharmacologic adjustment. Most evidence-based augmentation algorithms for treating major depression start with adding a second “traditional” antidepressant such as bupropion, then move to lithium, second-generation antipsychotics, or lamotrigine.10 But what about thyroid hormone augmentation?
Thyroid hormone often is on the lower rungs of depression treatment algorithms despite Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial data. The data suggest triiodothyronine’s (T3) lower side effect burden and ease of use may offer an advantage over lithium augmentation for depressed patients who have failed several medication trials.11 Liothyronine sodium (triiodothyronine) is a relatively benign medication with potential for augmentation when started at 25 to 50 mcg/d concurrently with antidepressants such as sertraline.12 Unfortunately, most augmentation trials with T3 have been short-term—generally 4 to 8 weeks. In my practice, T3 has limited application; I use it mainly for patients with treatment-resistant depression who have failed several other treatments.
Lithium, the comparison medication to thyroid hormone in the third augmentation arm of the STAR*D trial, requires an annual check of thyroid function (TSH testing) to properly monitor for potential lithium-related hypothyroidism or thyroiditis. Hypothyroidism, for which thyroid replacement is required, with lithium therapy is common, affecting 8% to 27% of patients.13 Patients who rapidly gain weight at the beginning of lithium treatment seem to have a higher risk of developing hypothyroidism.13 However, the risk of developing lithium-induced hypothyroidism is tied to the length of treatment; the longer a patient has been treated with lithium, the greater the risk of developing lithium-induced hypothyroidism.
CASE 3: Unable to slow down
Mr. C, age 45, has a 20-year history of major depression controlled reasonably well with paroxetine, 40 mg. He presents with escalating anxiety, depression, and irritability. His wife is concerned about his overwhelming thoughts of death, especially because Mr. C’s father committed suicide 30 years ago under similar circumstances. Mr. C has been tremulous for the past month and has not been sleeping well. He feels like he is “in constant motion” and unable to slow down. He screens in the “highly likely” range for bipolar disorder on the Bipolar Spectrum Diagnostic Scale14 and is started on divalproex ER, 500 mg/d.
His thyroid function tests returns with a suppressed TSH of 0.03 mIU/L and an elevated FT4 of 3.26 ng/dL. Divalproex is discontinued and he is started on the beta blocker atenolol, 25 mg/d, to target his anxiety, tachycardia, and akathisia. TSH receptor antibody testing was positive, which, along with an abnormal radioactive iodine uptake scan, confirmed a diagnosis of Graves’ disease. He receives methimazole, 20 mg/d, as a temporizing measure. An endocrinologist completes a radioactive iodine (I-131) ablation procedure on Mr. C, which resolves his mood and anxiety symptoms.
Although hypothyroidism commonly is associated with depressive symptoms, hyperthyroidism also may present as depression. Most cases of overt hyperthyroidism are directly referred to an endocrinologist because when treating disorders such as Graves’ disease—the most common cause of hyperthyroidism, especially among women age 20 to 40—many nuclear medicine teams require the expert guidance of an endocrinologist before considering radioiodine ablation. Hyperthyroidism often is accompanied by psychiatric and somatic symptoms of an “overactive” nature (Table 2). However, older patients (age >65) with hyperthyroidism may develop apathetic hyperthyroidism, a subset that comprises approximately 10% to 15% of all hyperthyroidism cases in older adults.15 Rather than becoming nervous, jittery, and restless, patients with apathetic hyperthyroidism are depressed, lethargic, and weak, and may develop proximal myopathy or cardiomyopathy. It is essential to differentiate apathetic hyperthyroidism from typical hyperthyroidism because accurately diagnosing and treating apathetic hyperthyroidism will improve outcomes.15
Table 2
Hyperthyroidism symptoms
| Psychiatric overlap |
| Decrease or increase in appetite |
| Insomnia |
| Fatigue |
| Mood instability |
| Irritability |
| Anxiety, nervousness |
| Somatic signs and symptoms |
| Frequent bowel movement, eg, diarrhea |
| Heart palpitations |
| Heat intolerance |
| Increased sweating |
| Light or missed menstrual periods, fertility problems |
| Muscle weakness |
| Shortness of breath |
| Sudden paralysis |
| Tremor, shakiness, dizziness |
| Vision changes |
| Weight loss or gain |
| Thinning of hair |
| Itching and hives |
| Possible increase in blood sugar |
Using beta blockers to treat hyperthyroidism can help control tachycardia or palpitations, tremulousness, and anxiety that often are inherent in hyperthyroidism. But can beta blockers induce depressive symptoms? A 1-year prospective Dutch study of patients who had survived a myocardial infarction did not find evidence that beta blockers induced depressive symptoms.16 However, the long-term and high-dosage effects of beta blockers still are in question.16 In Mr. C’s case, beta blockers had only positive effects on his symptoms and did not exacerbate his depressive symptoms.
Related Resources
- National Women’s Health Resource Center, Inc. Thyroid disorders. www.healthywomen.org/condition/thyroid-disorders.
- American Thyroid Association. www.thyroid.org.
- American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. www.aace.com/files/hypo-hyper.pdf.
Drug Brand Names
- Atenolol • Tenormin
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Cyclobenzaprine • Flexeril
- Divalproex ER • Depakote ER
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Levothyroxine • Levoxyl, Synthroid
- Liothyronine sodium • Cytomel, Triostat
- Lithium • Eskalith, Lithobid
- Methimazole • Tapazole
- Oxycodone • OxyContin
- Paroxetine • Paxil
- Sertraline • Zoloft
- Sunitinib • Sutent
Disclosure
Dr. Raj is a speaker for AstraZeneca and Merck.
1. Campayo A, de Jonge P, Roy JF, et al. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry. 2010;167(5):580-588.
2. Gallagher JC, Sai AJ. Vitamin D insufficiency deficiency, and bone health. J Clin Endocrinol Metab. 2010;95(6):2630-2633.
3. Hoogendijk WJ, Lips P, Dik MG, et al. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65(5):508-512.
4. Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med. 2000;160(11):1573-1575.
5. Wolter P, Dumez H, Schöffski P. Sunitinib and hypothyroidism. N Engl J Med. 2007;356(15):1580; author reply 1580-1581.
6. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76-131.
7. Roos A, Linn-Rasker SP, van Domburg RT, et al. The starting dose of levothyroxine in primary hypothyroidism treatment: a prospective, randomized, double-blind trial. Arch Intern Med. 2005;165(15):1714-1720.
8. Raj YP. Subclinical hypothyroidism: merely monitor or time to treat? Current Psychiatry. 2009;8(2):47-48.
9. Samuels MH. Cognitive function in subclinical hypothyroidism. J Clin Endocrinol Metab. 2010;95(8):3611-3613.
10. Mann JJ. The medical management of depression. N Engl J Med. 2005;353(17):1819-1834.
11. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
12. Cooper-Kazaz R, Apter JT, Cohen R, et al. Combined treatment with sertraline and liothyronine in major depression: a randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2007;64(6):679-688.
13. Henry C. Lithium side-effects and predictors of hypothyroidism in patients with bipolar disorder: sex differences. J Psychiatry Neurosci. 2002;27(2):104-107.
14. Ghaemi N, Pies R. The Bipolar Spectrum Diagnostic Scale. http://www.psycheducation.org/depression/BSDS.htm. Published October 2002. Updated June 2003. Accessed October 1 2012.
15. Wu W, Sun Z, Yu J, et al. A clinical retrospective analysis of factors associated with apathetic hyperthyroidism. Pathobiology. 2010;77(1):46-51.
16. van Melle JP, Verbeek DE, van den Berg MP, et al. Beta-blockers and depression after myocardial infarction: a multicenter prospective study. J Am Coll Cardiol. 2006;48(11):2209-2214.
1. Campayo A, de Jonge P, Roy JF, et al. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry. 2010;167(5):580-588.
2. Gallagher JC, Sai AJ. Vitamin D insufficiency deficiency, and bone health. J Clin Endocrinol Metab. 2010;95(6):2630-2633.
3. Hoogendijk WJ, Lips P, Dik MG, et al. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65(5):508-512.
4. Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med. 2000;160(11):1573-1575.
5. Wolter P, Dumez H, Schöffski P. Sunitinib and hypothyroidism. N Engl J Med. 2007;356(15):1580; author reply 1580-1581.
6. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76-131.
7. Roos A, Linn-Rasker SP, van Domburg RT, et al. The starting dose of levothyroxine in primary hypothyroidism treatment: a prospective, randomized, double-blind trial. Arch Intern Med. 2005;165(15):1714-1720.
8. Raj YP. Subclinical hypothyroidism: merely monitor or time to treat? Current Psychiatry. 2009;8(2):47-48.
9. Samuels MH. Cognitive function in subclinical hypothyroidism. J Clin Endocrinol Metab. 2010;95(8):3611-3613.
10. Mann JJ. The medical management of depression. N Engl J Med. 2005;353(17):1819-1834.
11. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
12. Cooper-Kazaz R, Apter JT, Cohen R, et al. Combined treatment with sertraline and liothyronine in major depression: a randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2007;64(6):679-688.
13. Henry C. Lithium side-effects and predictors of hypothyroidism in patients with bipolar disorder: sex differences. J Psychiatry Neurosci. 2002;27(2):104-107.
14. Ghaemi N, Pies R. The Bipolar Spectrum Diagnostic Scale. http://www.psycheducation.org/depression/BSDS.htm. Published October 2002. Updated June 2003. Accessed October 1 2012.
15. Wu W, Sun Z, Yu J, et al. A clinical retrospective analysis of factors associated with apathetic hyperthyroidism. Pathobiology. 2010;77(1):46-51.
16. van Melle JP, Verbeek DE, van den Berg MP, et al. Beta-blockers and depression after myocardial infarction: a multicenter prospective study. J Am Coll Cardiol. 2006;48(11):2209-2214.