User login
Cryptococcus neoformans Panniculitis Unmasked: A Paradoxical Reaction to Therapy
To the Editor:
Cryptococcus neoformans is an opportunistic fungus with a predilection for immunocompromised hosts, including solid organ transplant recipients (SOTRs). However, the rapid emergence of diffuse panniculitis only upon the start of therapy for extracutaneous disease is a rare phenomenon. We report the case of a liver transplant recipient who developed a paradoxical inflammatory reaction after initiating liposomal amphotericin B therapy for disseminated C neoformans, which manifested as progressive indurated plaques histologically consistent with cryptococcal panniculitis.
A 44-year-old man who received an orthotopic liver transplant 12 months prior and was on prednisone (20 mg daily) and tacrolimus (7 mg total daily) was admitted for multifocal pneumonia complicated by septic shock. Blood and respiratory cultures grew C neoformans, and lumbar puncture evaluation of cerebrospinal fluid revealed the presence of Cryptococcus antigen in 1:40 titers. Liposomal amphotericin B 5 mg/kg intravenous daily and fluconazole 400 mg intravenous daily were administered starting on the fourth day of admission; maintenance tacrolimus and steroids were stopped. Within 36 hours of treatment initiation, an erythematous papular rash was noted on the extremities, which initially was deemed an infusion reaction. Over the next 6 days, the rash became progressively confluent and hyperpigmented. A dermatologist was consulted on the fifteenth day of admission.
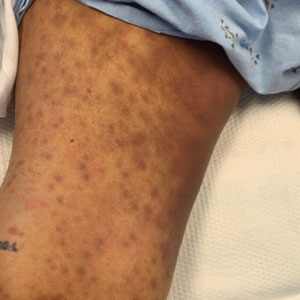
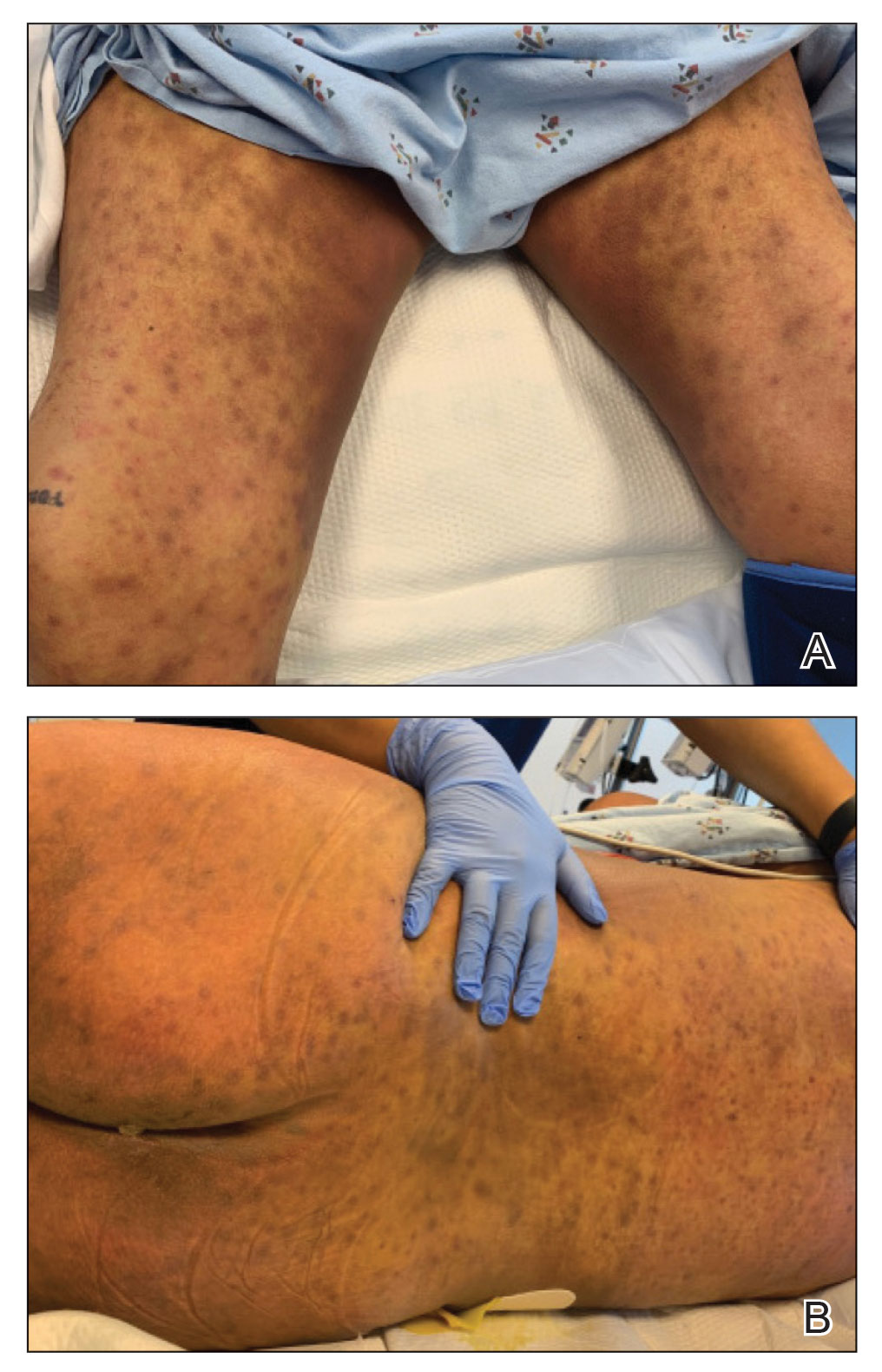
Physical examination by dermatology revealed diffuse, hyperpigmented to erythematous macules on the torso, back, arms, and legs that coalesced into dusky indurated plaques along the thighs, right side of the flank, and right upper arm (Figure 1). Laboratory analysis revealed thrombocytopenia but was otherwise unremarkable. Histoplasma antigen and Coccidioides IgG and IgM enzyme immunoassays were negative, as were cytomegalovirus, HIV, and rapid plasma reagin test results. Blood culture testing was repeated, and the findings were negative.
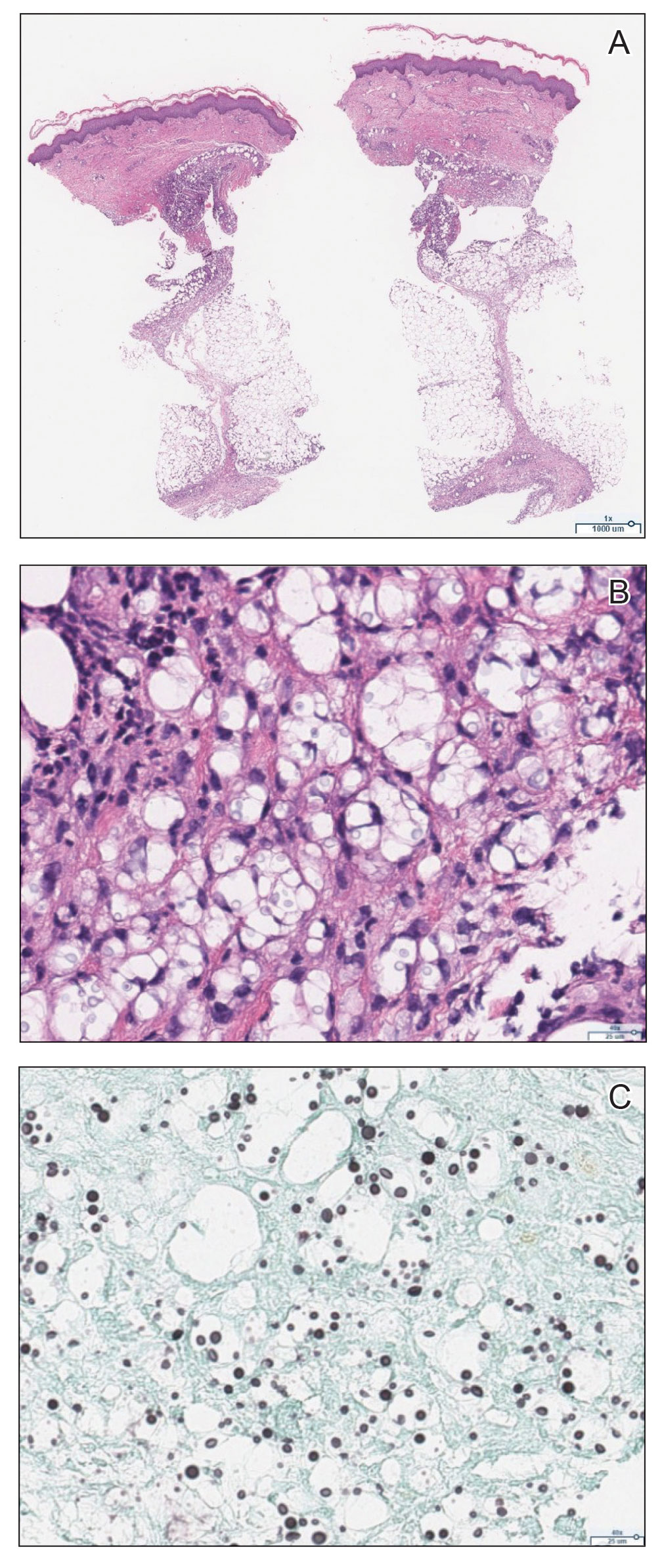
The emergence of the rash after amphotericin initiation prompted concern that the cause was due to a drug reaction rather than cutaneous involvement of cryptococcal infection. Punch biopsies were obtained from the thigh plaque. Hematoxylin and eosin and Grocott-Gomori methenamine-silver stains revealed cryptococcal organisms in the dermis and subcutaneous fat (Figure 2). Bacterial, acid-fast bacillus, and fungal cultures showed no growth.
The patient was diagnosed with cryptococcal panniculitis. Induction therapy with liposomal amphotericin B 5 mg/kg daily and flucytosine 25 mg/kg twice daily was pursued. During the treatment, cutaneous involvement evolved into superficial desquamation. The patient ultimately died from shock secondary to persistent cryptococcal fungemia.
Cryptococcus neoformans is an opportunistic fungal infection that represents a notable hazard to SOTR, inflicting 1.5% to 2.8% of this population and carrying a 19% to 42% mortality rate.1,2 This infection occurs at a median of 1.6 to 2.3 years after transplantation,1,3 though liver transplant recipients and those with immune reconstitution inflammatory syndrome (IRIS)–like complications may present sooner (8.8 and 10.5 months, respectively).4 Cutaneous involvement comprises 17% to 21% of cases and is associated with extensive dissemination, including the central nervous system, lung, and bloodstream (61.5%, 23.1%, and 38.5%, respectively).1-3 When Cryptococcus infects the skin, it classically manifests as multiple nodules, umbilicated papules, ulcers, or cellulitis.3 Involvement of subcutaneous adipose tissue is uncommon and primarily is observed at initial presentation alongside disseminated disease.5-8 Our case is unique because cutaneous involvement was absent until treatment initiation.
Similar patterns of worsened or unmasked disease following treatment initiation have been observed in SOTRs with extracutaneous cryptococcus and were attributed to IRIS-like phenomena that generate a hyperactive inflammatory response to infection.4,9 Common immunosuppressive regimens, particularly tacrolimus, depress helper T cell (TH1) cytokine release and promote a TH2-dominant, anti-inflammatory state.10 In cryptococcosis, the fungus itself may stimulate a comparable cytokine milieu to promote immunologic evasion and dissemination. Cryptococcal IRIS-like responses in SOTRs are precipitated by rapid reduction or withdrawal of calcineurin inhibitors and corticosteroids, in combination with the inherent mitogenicity of the C neoformans polysaccharide capsule and antifungal agents.10 In our patient, cryptococcal yeasts may have invaded subcutaneous tissues when he became fungemic but remained subclinical due to minimal inflammatory recruitment. As treatment began and immunosuppressants diminished, fungal recognition and massive cytokine release resulted in frank panniculitis via precipitous immune dysregulation.
First-line therapy of cryptococcosis entails the use of liposomal amphotericin B and flucytosine for induction, followed by fluconazole for consolidation and maintenance. Use of corticosteroids is atypical to the antifungal regimen; however, a role for them has been suggested in severe IRIS involving individuals who are HIV positive, such as those with lesions demonstrating mass effect.11 Rare case reports have described their utility as adjunctive therapies against cryptococcus in SOTRs when treatment with antifungal agents alone failed.12 Given the paucity of prospective trials to support corticosteroid use in SOTRs as well as the worse global outcomes in cases of cryptococcal meningitis,13 therapeutic corticosteroids were not administered in our patient.
Although our case represents a rare event, cutaneous cryptococcosis and IRIS-like phenomena are clinically relevant complications in immunocompromised patients. In particular, they should be promptly considered in SOTRs receiving maintenance immunosuppressants who demonstrate symptom aggravation despite negative microbial culture results and uninterrupted antifungal therapy.
1. Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7:375-381.
2. Sun HY, Wagener MM, Singh N. Cryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trends. Clin Infect Dis. 2009;48:1566-1576.
3. Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
4. Singh N, Lortholary O, Alexander BD, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40:1756-1761.
5. Reddy BY, Shaigany S, Schulman L, et al. Resident rounds part III: case report: fatal cryptococcal panniculitis in a lung transplant recipient. J Drugs Dermatol. 2015;14:519-252.
6. Bhowmik D, Dinda AK, Xess I, et al. Fungal panniculitis in renal transplant recipients. Transpl Infect Dis. 2008;10:286-289.
7. Gloster HM, Swerlick RA, Solomon AR. Cryptococcal cellulitis in a diabetic, kidney transplant patient. J Am Acad Dermatol. 1994;30:1025-1026.
8. Carlson KC, Mehlmauer M, Evans S, et al. Cryptococcal cellulitis in renal transplant recipients. J Am Acad Dermatol. 1987;17:469-472.
9. French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48:101-107.
10. Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7:395-401.
11. World Health Organization. Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Published March 1, 2018. Accessed September 6, 2020. https://www.who.int/publications/i/item/9789241550277
12. Lanternier F, Chandesris MO, Poirée S, et al. Cellulitis revealing a cryptococcosis-related immune reconstitution inflammatory syndrome in a renal allograft recipient. Am J Transpl. 2007;7:2826-2828.
13. Beardsley J, Wolbers M, Kibengo FM, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374:542-554.
To the Editor:
Cryptococcus neoformans is an opportunistic fungus with a predilection for immunocompromised hosts, including solid organ transplant recipients (SOTRs). However, the rapid emergence of diffuse panniculitis only upon the start of therapy for extracutaneous disease is a rare phenomenon. We report the case of a liver transplant recipient who developed a paradoxical inflammatory reaction after initiating liposomal amphotericin B therapy for disseminated C neoformans, which manifested as progressive indurated plaques histologically consistent with cryptococcal panniculitis.
A 44-year-old man who received an orthotopic liver transplant 12 months prior and was on prednisone (20 mg daily) and tacrolimus (7 mg total daily) was admitted for multifocal pneumonia complicated by septic shock. Blood and respiratory cultures grew C neoformans, and lumbar puncture evaluation of cerebrospinal fluid revealed the presence of Cryptococcus antigen in 1:40 titers. Liposomal amphotericin B 5 mg/kg intravenous daily and fluconazole 400 mg intravenous daily were administered starting on the fourth day of admission; maintenance tacrolimus and steroids were stopped. Within 36 hours of treatment initiation, an erythematous papular rash was noted on the extremities, which initially was deemed an infusion reaction. Over the next 6 days, the rash became progressively confluent and hyperpigmented. A dermatologist was consulted on the fifteenth day of admission.
Physical examination by dermatology revealed diffuse, hyperpigmented to erythematous macules on the torso, back, arms, and legs that coalesced into dusky indurated plaques along the thighs, right side of the flank, and right upper arm (Figure 1). Laboratory analysis revealed thrombocytopenia but was otherwise unremarkable. Histoplasma antigen and Coccidioides IgG and IgM enzyme immunoassays were negative, as were cytomegalovirus, HIV, and rapid plasma reagin test results. Blood culture testing was repeated, and the findings were negative.

The emergence of the rash after amphotericin initiation prompted concern that the cause was due to a drug reaction rather than cutaneous involvement of cryptococcal infection. Punch biopsies were obtained from the thigh plaque. Hematoxylin and eosin and Grocott-Gomori methenamine-silver stains revealed cryptococcal organisms in the dermis and subcutaneous fat (Figure 2). Bacterial, acid-fast bacillus, and fungal cultures showed no growth.

The patient was diagnosed with cryptococcal panniculitis. Induction therapy with liposomal amphotericin B 5 mg/kg daily and flucytosine 25 mg/kg twice daily was pursued. During the treatment, cutaneous involvement evolved into superficial desquamation. The patient ultimately died from shock secondary to persistent cryptococcal fungemia.
Cryptococcus neoformans is an opportunistic fungal infection that represents a notable hazard to SOTR, inflicting 1.5% to 2.8% of this population and carrying a 19% to 42% mortality rate.1,2 This infection occurs at a median of 1.6 to 2.3 years after transplantation,1,3 though liver transplant recipients and those with immune reconstitution inflammatory syndrome (IRIS)–like complications may present sooner (8.8 and 10.5 months, respectively).4 Cutaneous involvement comprises 17% to 21% of cases and is associated with extensive dissemination, including the central nervous system, lung, and bloodstream (61.5%, 23.1%, and 38.5%, respectively).1-3 When Cryptococcus infects the skin, it classically manifests as multiple nodules, umbilicated papules, ulcers, or cellulitis.3 Involvement of subcutaneous adipose tissue is uncommon and primarily is observed at initial presentation alongside disseminated disease.5-8 Our case is unique because cutaneous involvement was absent until treatment initiation.
Similar patterns of worsened or unmasked disease following treatment initiation have been observed in SOTRs with extracutaneous cryptococcus and were attributed to IRIS-like phenomena that generate a hyperactive inflammatory response to infection.4,9 Common immunosuppressive regimens, particularly tacrolimus, depress helper T cell (TH1) cytokine release and promote a TH2-dominant, anti-inflammatory state.10 In cryptococcosis, the fungus itself may stimulate a comparable cytokine milieu to promote immunologic evasion and dissemination. Cryptococcal IRIS-like responses in SOTRs are precipitated by rapid reduction or withdrawal of calcineurin inhibitors and corticosteroids, in combination with the inherent mitogenicity of the C neoformans polysaccharide capsule and antifungal agents.10 In our patient, cryptococcal yeasts may have invaded subcutaneous tissues when he became fungemic but remained subclinical due to minimal inflammatory recruitment. As treatment began and immunosuppressants diminished, fungal recognition and massive cytokine release resulted in frank panniculitis via precipitous immune dysregulation.
First-line therapy of cryptococcosis entails the use of liposomal amphotericin B and flucytosine for induction, followed by fluconazole for consolidation and maintenance. Use of corticosteroids is atypical to the antifungal regimen; however, a role for them has been suggested in severe IRIS involving individuals who are HIV positive, such as those with lesions demonstrating mass effect.11 Rare case reports have described their utility as adjunctive therapies against cryptococcus in SOTRs when treatment with antifungal agents alone failed.12 Given the paucity of prospective trials to support corticosteroid use in SOTRs as well as the worse global outcomes in cases of cryptococcal meningitis,13 therapeutic corticosteroids were not administered in our patient.
Although our case represents a rare event, cutaneous cryptococcosis and IRIS-like phenomena are clinically relevant complications in immunocompromised patients. In particular, they should be promptly considered in SOTRs receiving maintenance immunosuppressants who demonstrate symptom aggravation despite negative microbial culture results and uninterrupted antifungal therapy.
To the Editor:
Cryptococcus neoformans is an opportunistic fungus with a predilection for immunocompromised hosts, including solid organ transplant recipients (SOTRs). However, the rapid emergence of diffuse panniculitis only upon the start of therapy for extracutaneous disease is a rare phenomenon. We report the case of a liver transplant recipient who developed a paradoxical inflammatory reaction after initiating liposomal amphotericin B therapy for disseminated C neoformans, which manifested as progressive indurated plaques histologically consistent with cryptococcal panniculitis.
A 44-year-old man who received an orthotopic liver transplant 12 months prior and was on prednisone (20 mg daily) and tacrolimus (7 mg total daily) was admitted for multifocal pneumonia complicated by septic shock. Blood and respiratory cultures grew C neoformans, and lumbar puncture evaluation of cerebrospinal fluid revealed the presence of Cryptococcus antigen in 1:40 titers. Liposomal amphotericin B 5 mg/kg intravenous daily and fluconazole 400 mg intravenous daily were administered starting on the fourth day of admission; maintenance tacrolimus and steroids were stopped. Within 36 hours of treatment initiation, an erythematous papular rash was noted on the extremities, which initially was deemed an infusion reaction. Over the next 6 days, the rash became progressively confluent and hyperpigmented. A dermatologist was consulted on the fifteenth day of admission.
Physical examination by dermatology revealed diffuse, hyperpigmented to erythematous macules on the torso, back, arms, and legs that coalesced into dusky indurated plaques along the thighs, right side of the flank, and right upper arm (Figure 1). Laboratory analysis revealed thrombocytopenia but was otherwise unremarkable. Histoplasma antigen and Coccidioides IgG and IgM enzyme immunoassays were negative, as were cytomegalovirus, HIV, and rapid plasma reagin test results. Blood culture testing was repeated, and the findings were negative.

The emergence of the rash after amphotericin initiation prompted concern that the cause was due to a drug reaction rather than cutaneous involvement of cryptococcal infection. Punch biopsies were obtained from the thigh plaque. Hematoxylin and eosin and Grocott-Gomori methenamine-silver stains revealed cryptococcal organisms in the dermis and subcutaneous fat (Figure 2). Bacterial, acid-fast bacillus, and fungal cultures showed no growth.

The patient was diagnosed with cryptococcal panniculitis. Induction therapy with liposomal amphotericin B 5 mg/kg daily and flucytosine 25 mg/kg twice daily was pursued. During the treatment, cutaneous involvement evolved into superficial desquamation. The patient ultimately died from shock secondary to persistent cryptococcal fungemia.
Cryptococcus neoformans is an opportunistic fungal infection that represents a notable hazard to SOTR, inflicting 1.5% to 2.8% of this population and carrying a 19% to 42% mortality rate.1,2 This infection occurs at a median of 1.6 to 2.3 years after transplantation,1,3 though liver transplant recipients and those with immune reconstitution inflammatory syndrome (IRIS)–like complications may present sooner (8.8 and 10.5 months, respectively).4 Cutaneous involvement comprises 17% to 21% of cases and is associated with extensive dissemination, including the central nervous system, lung, and bloodstream (61.5%, 23.1%, and 38.5%, respectively).1-3 When Cryptococcus infects the skin, it classically manifests as multiple nodules, umbilicated papules, ulcers, or cellulitis.3 Involvement of subcutaneous adipose tissue is uncommon and primarily is observed at initial presentation alongside disseminated disease.5-8 Our case is unique because cutaneous involvement was absent until treatment initiation.
Similar patterns of worsened or unmasked disease following treatment initiation have been observed in SOTRs with extracutaneous cryptococcus and were attributed to IRIS-like phenomena that generate a hyperactive inflammatory response to infection.4,9 Common immunosuppressive regimens, particularly tacrolimus, depress helper T cell (TH1) cytokine release and promote a TH2-dominant, anti-inflammatory state.10 In cryptococcosis, the fungus itself may stimulate a comparable cytokine milieu to promote immunologic evasion and dissemination. Cryptococcal IRIS-like responses in SOTRs are precipitated by rapid reduction or withdrawal of calcineurin inhibitors and corticosteroids, in combination with the inherent mitogenicity of the C neoformans polysaccharide capsule and antifungal agents.10 In our patient, cryptococcal yeasts may have invaded subcutaneous tissues when he became fungemic but remained subclinical due to minimal inflammatory recruitment. As treatment began and immunosuppressants diminished, fungal recognition and massive cytokine release resulted in frank panniculitis via precipitous immune dysregulation.
First-line therapy of cryptococcosis entails the use of liposomal amphotericin B and flucytosine for induction, followed by fluconazole for consolidation and maintenance. Use of corticosteroids is atypical to the antifungal regimen; however, a role for them has been suggested in severe IRIS involving individuals who are HIV positive, such as those with lesions demonstrating mass effect.11 Rare case reports have described their utility as adjunctive therapies against cryptococcus in SOTRs when treatment with antifungal agents alone failed.12 Given the paucity of prospective trials to support corticosteroid use in SOTRs as well as the worse global outcomes in cases of cryptococcal meningitis,13 therapeutic corticosteroids were not administered in our patient.
Although our case represents a rare event, cutaneous cryptococcosis and IRIS-like phenomena are clinically relevant complications in immunocompromised patients. In particular, they should be promptly considered in SOTRs receiving maintenance immunosuppressants who demonstrate symptom aggravation despite negative microbial culture results and uninterrupted antifungal therapy.
1. Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7:375-381.
2. Sun HY, Wagener MM, Singh N. Cryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trends. Clin Infect Dis. 2009;48:1566-1576.
3. Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
4. Singh N, Lortholary O, Alexander BD, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40:1756-1761.
5. Reddy BY, Shaigany S, Schulman L, et al. Resident rounds part III: case report: fatal cryptococcal panniculitis in a lung transplant recipient. J Drugs Dermatol. 2015;14:519-252.
6. Bhowmik D, Dinda AK, Xess I, et al. Fungal panniculitis in renal transplant recipients. Transpl Infect Dis. 2008;10:286-289.
7. Gloster HM, Swerlick RA, Solomon AR. Cryptococcal cellulitis in a diabetic, kidney transplant patient. J Am Acad Dermatol. 1994;30:1025-1026.
8. Carlson KC, Mehlmauer M, Evans S, et al. Cryptococcal cellulitis in renal transplant recipients. J Am Acad Dermatol. 1987;17:469-472.
9. French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48:101-107.
10. Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7:395-401.
11. World Health Organization. Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Published March 1, 2018. Accessed September 6, 2020. https://www.who.int/publications/i/item/9789241550277
12. Lanternier F, Chandesris MO, Poirée S, et al. Cellulitis revealing a cryptococcosis-related immune reconstitution inflammatory syndrome in a renal allograft recipient. Am J Transpl. 2007;7:2826-2828.
13. Beardsley J, Wolbers M, Kibengo FM, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374:542-554.
1. Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7:375-381.
2. Sun HY, Wagener MM, Singh N. Cryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trends. Clin Infect Dis. 2009;48:1566-1576.
3. Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
4. Singh N, Lortholary O, Alexander BD, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40:1756-1761.
5. Reddy BY, Shaigany S, Schulman L, et al. Resident rounds part III: case report: fatal cryptococcal panniculitis in a lung transplant recipient. J Drugs Dermatol. 2015;14:519-252.
6. Bhowmik D, Dinda AK, Xess I, et al. Fungal panniculitis in renal transplant recipients. Transpl Infect Dis. 2008;10:286-289.
7. Gloster HM, Swerlick RA, Solomon AR. Cryptococcal cellulitis in a diabetic, kidney transplant patient. J Am Acad Dermatol. 1994;30:1025-1026.
8. Carlson KC, Mehlmauer M, Evans S, et al. Cryptococcal cellulitis in renal transplant recipients. J Am Acad Dermatol. 1987;17:469-472.
9. French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48:101-107.
10. Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7:395-401.
11. World Health Organization. Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Published March 1, 2018. Accessed September 6, 2020. https://www.who.int/publications/i/item/9789241550277
12. Lanternier F, Chandesris MO, Poirée S, et al. Cellulitis revealing a cryptococcosis-related immune reconstitution inflammatory syndrome in a renal allograft recipient. Am J Transpl. 2007;7:2826-2828.
13. Beardsley J, Wolbers M, Kibengo FM, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374:542-554.
Practice Points
- Panniculitis caused by Cryptococcus neoformans is a rare complication in solid organ transplant recipients.
- Subclinical panniculitis from C neoformans may be unmasked during paradoxical inflammatory reactions as early as days following immunosuppressant withdrawal and treatment initiation.