User login
In detecting and evaluating pleural effusion, technology has not replaced clinical skills. Yet, despite centuries of lore, data are limited on the role of the physical examination and on its accuracy compared with other noninvasive tests such as conventional chest radiography or ultrasonography.
The following is an overview of the value of the clinical history and physical examination in detecting pleural effusion and a brief review of the available information regarding its accuracy compared with other diagnostic methods.
POTENTIAL CAUSES ARE MANY
The pleurae consist of two membranes that protect the lungs, allow them to move, contribute to their shape, and prevent the alveoli at the pleural surface from becoming overdistended. Between the visceral pleura (covering the lung) and the parietal pleura (covering the diaphragm and the chest wall) is the pleural space.
In healthy adults, the pleural space contains an estimated 5 to 10 mL of pleural fluid (0.1 mg/kg body weight).1 Pleural effusion is an accumulation of an abnormal amount of fluid in the pleural space.
Although the potential causes are many, the most common are congestive heart failure, pneumonia (40% of patients hospitalized with pneumonia have pleural effusion),2,3 cancer, and pulmonary embolism.4
Because many diseases affecting different organs can cause a pleural effusion, we cannot overemphasize the importance of a thorough history and physical examination to uncover clues that will help identify its cause and narrow the diagnostic workup. For example, significant weight loss and cachexia could be due to cancer, and joint, skin, or eye symptoms could be due to a connective tissue disorder.
A thorough review of the patient’s medications is mandatory, since several medications (eg, amiodarone [Cordarone], methotrexate [Rheumatrex, Trexall], and nitrofurantoin [Macrobid]) can be associated with exudative effusions. In addition, the patient’s occupational history must be ascertained, since exposure to asbestos can raise the suspicion of a malignant disease of the pleura such as mesothelioma.
SYMPTOMS ARE NEITHER SENSITIVE NOR SPECIFIC
The symptoms of pleural effusion are neither sensitive nor specific, and many patients have manifestations of the underlying process but not of the effusion itself. The most common symptoms directly related to effusion are cough, dyspnea, and pleuritic chest pain.5
Cough. Many patients with a pleural effusion have a dry, nonproductive cough, a consequence of inflammation of the pleurae or compression of the bronchial walls. Although this symptom is rarely helpful in diagnosing a pleural effusion, if accompanied by purulent sputum it suggests pneumonia, and if complicated by hemoptysis it suggests cancer or pulmonary embolism.
Dyspnea is a consequence of a combination of a restrictive lung defect, a ventilation-perfusion mismatch, and a decrease in cardiac output. Although large pleural effusions reduce lung volume and are generally associated with dyspnea, the symptoms may be out of proportion to the size of the effusion, and patients with small to moderate effusions may also have shortness of breath if their baseline lung function is poor.2
Chest pain accompanying a pleural effusion suggests inflammation of the parietal pleura,6 but could be due to cancer in the chest wall and ribs—or to a benign disease of the thoracic wall such as rib fracture or costo-chondritis.
Pain of pleural origin can remain localized to the adjacent area of the chest, but sometimes it is referred to other areas. If the diaphragmatic pleura is involved, the pain is in many cases referred to the ipsilateral shoulder.5 Pain may also be referred to the abdomen.
Pleuritic chest pain is described as being worse with deep inspiration or when lying down. It is common in patients with pulmonary embolism, parapneumonic effusion, or viral pleurisy, but it can also occur in patients with pneumothorax or pericarditis. A dull, aching chest pain may be due to an underlying pleural malignancy.7
PHYSICAL EXAMINATION: LONG TRADITION, FEW DATA
Our knowledge of the role of physical examination in detecting pleural effusion is still based mostly on expert opinion and on small case series.8,9
Patterson et al11 prospectively compared physical examination (including auscultation, percussion, and tactile fremitus) with bedside ultrasonography and found that physical examination had a lower sensitivity (53% vs 80%, respectively) but a similar specificity (71%).
Bigger effusions are easier to detect
The physical findings are related to the volume of fluid in the pleural effusion and its effects on the chest wall, diaphragm, and lungs. Physical findings are generally normal if less than 300 mL of fluid is present, whereas large effusions (> 1,500 mL) can be associated with significant asymmetry of chest expansion and bulging of intercostal spaces.
Inspection
Although inspection of the chest is not very helpful in detecting a pleural effusion, it can provide other relevant information such as the respiratory rate and the breathing position adopted by the patient (patients with a large pleural effusion may have orthopnea); it can also reveal abnormalities in the shape of the thorax such as the increased anteroposterior diameter (“barrel shape”) seen in patients with chronic obstructive pulmonary disease.18
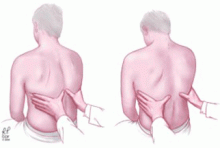
In addition, by inspection we can assess the expansion of the thorax. The utility of inspecting chest expansion to detect lung restriction was first noted by Laennec19 in 1821. A simple method of evaluating chest expansion is to place a measuring tape around the chest at the level of the nipples to compare the circumference at end-inspiration and at end-expiration.20 In the absence of emphysema, the difference should be at least 2 inches. An expansion of 1.5 inches or less is considered abnormal.21 More relevant to pleural effusion than the amount of overall chest expansion is whether the expansion is symmetrical, which we can assess by palpation.
Palpation
Signs of pleural effusion that can be detected by palpation include asymmetric chest expansion and asymmetric tactile fremitus.
Other signs. Palpation of the chest can also help in detecting underlying disease of the thorax sometimes associated with pleurisy or pleural effusions. Chest wall tumors or skin abscesses may be related to underlying empyema, localized tenderness may be associated with rib fractures or costochondritis, and crepitus may be due to subcutaneous emphysema.
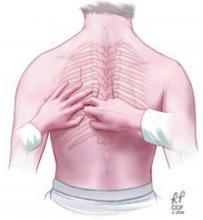
Percussion
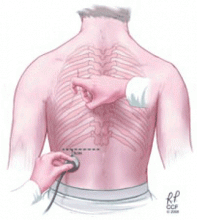
The chest can be percussed directly with the tips of the fingers of one hand or indirectly by placing a third finger against the surface to be percussed. There are two main techniques used to detect pleural effusions: comparative percussion and auscultatory percussion.
Since other conditions such as consolidation of the lung and atelectasis can also be associated with dullness to percussion, some authors advocate percussion in the lateral supine position to detect a shift in the dullness that would indicate movement of fluid in the chest.25
The sensitivity of comparative chest percussion and its accuracy related to the size of the effusion are unknown. Kalantri et al10 found that dullness to percussion had a positive predictive value of only 55% but a negative predictive value of 97%, suggesting that the absence of the sign is very helpful in ruling out an effusion.
According to classic textbook descriptions,26 percussive sounds penetrate a maximum of 6 cm (2 cm of body wall thickness and 4 cm of lung), and at least 500 mL of fluid must be present in order to be able to detect an effusion by physical examination.2,8 Most of these descriptions are based on original studies done in cadavers more than 100 years ago.
The auscultatory percussion technique was first described by Laennec and used to delineate the size of several organs by placing the stethoscope directly above the structure to be outlined, followed by percussion from the periphery towards the organ of interest. The original technique was subsequently modified for the examination of the chest by Guarino.27
Although auscultatory percussion was used initially to try to detect lung lesions, masses and consolidations, Guarino and Guarino12 found this technique to be highly effective in detecting pleural effusion. In a prospective blinded study in 118 patients, this method was highly (95%) sensitive and 100% specific in detecting pleural effusion, even in patients with obesity, pneumonia, or other pleural abnormalities. Of note, their findings suggested that auscultatory percussion can detect as little as 50 mL of pleural fluid.
Bohadana et al13 compared auscultatory and conventional percussion with chest radiographic findings in 281 patients. They found that auscultatory percussion was 100% sensitive for detecting large pleural effusions.
However, when Bourke et al14 compared conventional and auscultatory percussion in 21 patients with abnormal radiographs, both methods had low sensitivity (15.4% vs 19.2%) but high specificity (97.3% vs 85.1%, respectively). It is important to mention that in this series only a few patients had a pleural effusion.
McDermott et al16 compared conventional and auscultatory percussion in detecting pleural effusion in 14 hospitalized patients, using ultrasonography instead of chest radiography as the gold standard for comparison. The findings on auscultatory percussion correlated better with the findings on ultrasonography than did those on conventional percussion. The authors gave no information about sensitivity or specificity.
Kalantri et al10 found that auscultatory percussion had a sensitivity of 58% and a specificity of 85%.
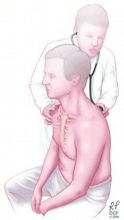
Auscultation
Originally described by Laennec (who invented the stethoscope),19 auscultation is perhaps the physical examination technique most used to detect pleural effusion.
Lichtenstein et al15 performed a study of auscultation in critically ill patients and found it to have a very low sensitivity (42%) but a higher specificity (90%), with an overall diagnostic accuracy of 60%. Of note, compared with chest radiography, auscultatory findings had similar sensitivity but higher accuracy.
Absent or diminished breath sounds strongly suggest an effusion.19
Egophonism. Laennec also described egophonism as a pathognomonic sign associated with a moderate degree of effusion. The word egophony comes from the Greek “ego,” which means goat; it is used to describe the change in the pronounced sound of E to A. The mechanism responsible for finding this sign in massive pleural effusions is probably upward displacement and compression or consolidation of the lung at the top of the effusion.
However, if the effusion is small, the consolidation will not be large enough to produce this sign. Similarly, other lung conditions associated with large consolidations may produce egophonism without a pleural effusion. Little is known about the predictive value of this sign, and significant interob-server variability needs to be taken into account.28
Pleural rub. Pleural effusions that result from any disease that causes direct inflammation of the pleurae can be associated with a pleural rub. This sound, classically described as rubbing of unoiled leather, is pathognomonic of pleural disease but not of pleural effusion. In fact, a pleural rub will disappear once an effusion develops. Little is known about the accuracy of this finding; in the study by Kalantri et al it had a very low sensitivity (5%) but a very high specificity (99%).10 The differential diagnosis includes pleuritis, pneumonia, mesothelioma, and tumors that metastasize to the pleura.
- Noppen M, De Waele M, Li R, et al. Volume and cellular content of normal pleural fluid in humans examined by pleural lavage. Am J Respir Crit Care Med 2000; 162:1023–1026.
- Bouros D. Pleural Disease. New York: Marcel Dekker, 2004.
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006; 3:75–80.
- Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002; 346:1971–1977.
- Light RW. Pleural Diseases, 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2007.
- Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Marel M, Stastny B, Melinova L, Svandova E, Light RW. Diagnosis of pleural effusions. Experience with clinical studies, 1986 to 1990. Chest 1995; 107:1598–1603.
- Leopold SS, Hopkins HU. Leopold’s Principles and Methods of Physical Diagnosis, 3d ed. Philadelphia: W.B. Saunders, 1965.
- Norris GW, Landis HRM, Montgomery CM, Krumbhaar EB. Diseases of the Chest and the Principles of Physical Diagnosis, 4th ed, rev. Philadelphia, W.B. Saunders, 1929.
- Kalantri S, Joshi R, Lokhande T, et al. Accuracy and reliability of physical signs in the diagnosis of pleural effusion. Respir Med 2007; 101:431–438.
- Patterson LA, Costantino TG, Satz WA. Diagnosing pleural effusion: a prospective comparison of physical examination with bedside ultra-sonography [abstract]. Ann Emerg Med 2004; 44:S112.
- Guarino JR, Guarino JC. Auscultatory percussion: a simple method to detect pleural effusion. J Gen Intern Med 1994; 9:71–74.
- Bohadana AB, Coimbra FT, Santiago JR. Detection of lung abnormalities by auscultatory percussion: a comparative study with conventional percussion. Respiration 1986; 50:218–225.
- Bourke S, Nunes D, Stafford F, Hurley G, Graham I. Percussion of the chest re-visited: a comparison of the diagnostic value of auscultatory and conventional chest percussion. Ir J Med Sci 1989; 158:82–84.
- Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004; 100:9–15.
- McDermott TD, McCarthy M, Chestnut T, Schumann L. A comparison of conventional percussion and auscultation percussion in the detection of pleural effusions of hospitalized patients. J Am Acad Nurse Pract 1997; 9:483–486.
- Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest 2003; 123:436–441.
- Pierce JA, Ebert RV. The barrel deformity of the chest, the senile lung and obstructive pulmonary emphysema. Am J Med 1958; 25:13–22.
- Laennec RTH. A Treatise On the Disease[s] of the Chest. New York: Library of the New York Academy of Medicine and Hafner Publishing Company, 1962.
- Bockenhauer SE, Chen H, Julliard KN, Weedon J. Measuring thoracic excursion: reliability of the cloth tape measure technique. J Am Osteopath Assoc 2007; 107:191–196.
- Fries JF. The reactive enthesopathies. Dis Mon 1985; 31 1:1–46.
- McGee SR. Evidence-based physical diagnosis. Philadelphia: W.B. Saunders, 2001.
- Auenbrugger L, Forbes J. On percussion of the chest: being a translation of Auenbrugger’s original treatise entitled “Inventum novum ex percussione thoracis humani, ut signo abstrusos interni pectoris morbos detegendi” (Vienna, 1761). Baltimore: The Johns Hopkins Press, 1936.
- Auenbrugger L, Neuburger M. Inventum Novum. London: Reprinted for Dawsons of Pall Mall, 1966.
- Gilbert VE. Shifting percussion dullness of the chest: a sign of pleural effusion. South Med J 1997; 90:1255–1256.
- Weil A. Handbuch und Atlas der topographischen Percussion nebst einer Darstellung der Lehre vom Percussionsschall. Leipzig: Vogel, 1880.
- Guarino JR. Auscultatory percussion, a new aid in the examination of the chest. J Kansas Med Soc 1974; 75:193–194.
- Sapira JD. About egophony. Chest 1995; 108:865–867.
In detecting and evaluating pleural effusion, technology has not replaced clinical skills. Yet, despite centuries of lore, data are limited on the role of the physical examination and on its accuracy compared with other noninvasive tests such as conventional chest radiography or ultrasonography.
The following is an overview of the value of the clinical history and physical examination in detecting pleural effusion and a brief review of the available information regarding its accuracy compared with other diagnostic methods.
POTENTIAL CAUSES ARE MANY
The pleurae consist of two membranes that protect the lungs, allow them to move, contribute to their shape, and prevent the alveoli at the pleural surface from becoming overdistended. Between the visceral pleura (covering the lung) and the parietal pleura (covering the diaphragm and the chest wall) is the pleural space.
In healthy adults, the pleural space contains an estimated 5 to 10 mL of pleural fluid (0.1 mg/kg body weight).1 Pleural effusion is an accumulation of an abnormal amount of fluid in the pleural space.
Although the potential causes are many, the most common are congestive heart failure, pneumonia (40% of patients hospitalized with pneumonia have pleural effusion),2,3 cancer, and pulmonary embolism.4
Because many diseases affecting different organs can cause a pleural effusion, we cannot overemphasize the importance of a thorough history and physical examination to uncover clues that will help identify its cause and narrow the diagnostic workup. For example, significant weight loss and cachexia could be due to cancer, and joint, skin, or eye symptoms could be due to a connective tissue disorder.
A thorough review of the patient’s medications is mandatory, since several medications (eg, amiodarone [Cordarone], methotrexate [Rheumatrex, Trexall], and nitrofurantoin [Macrobid]) can be associated with exudative effusions. In addition, the patient’s occupational history must be ascertained, since exposure to asbestos can raise the suspicion of a malignant disease of the pleura such as mesothelioma.
SYMPTOMS ARE NEITHER SENSITIVE NOR SPECIFIC
The symptoms of pleural effusion are neither sensitive nor specific, and many patients have manifestations of the underlying process but not of the effusion itself. The most common symptoms directly related to effusion are cough, dyspnea, and pleuritic chest pain.5
Cough. Many patients with a pleural effusion have a dry, nonproductive cough, a consequence of inflammation of the pleurae or compression of the bronchial walls. Although this symptom is rarely helpful in diagnosing a pleural effusion, if accompanied by purulent sputum it suggests pneumonia, and if complicated by hemoptysis it suggests cancer or pulmonary embolism.
Dyspnea is a consequence of a combination of a restrictive lung defect, a ventilation-perfusion mismatch, and a decrease in cardiac output. Although large pleural effusions reduce lung volume and are generally associated with dyspnea, the symptoms may be out of proportion to the size of the effusion, and patients with small to moderate effusions may also have shortness of breath if their baseline lung function is poor.2
Chest pain accompanying a pleural effusion suggests inflammation of the parietal pleura,6 but could be due to cancer in the chest wall and ribs—or to a benign disease of the thoracic wall such as rib fracture or costo-chondritis.
Pain of pleural origin can remain localized to the adjacent area of the chest, but sometimes it is referred to other areas. If the diaphragmatic pleura is involved, the pain is in many cases referred to the ipsilateral shoulder.5 Pain may also be referred to the abdomen.
Pleuritic chest pain is described as being worse with deep inspiration or when lying down. It is common in patients with pulmonary embolism, parapneumonic effusion, or viral pleurisy, but it can also occur in patients with pneumothorax or pericarditis. A dull, aching chest pain may be due to an underlying pleural malignancy.7
PHYSICAL EXAMINATION: LONG TRADITION, FEW DATA
Our knowledge of the role of physical examination in detecting pleural effusion is still based mostly on expert opinion and on small case series.8,9
Patterson et al11 prospectively compared physical examination (including auscultation, percussion, and tactile fremitus) with bedside ultrasonography and found that physical examination had a lower sensitivity (53% vs 80%, respectively) but a similar specificity (71%).
Bigger effusions are easier to detect
The physical findings are related to the volume of fluid in the pleural effusion and its effects on the chest wall, diaphragm, and lungs. Physical findings are generally normal if less than 300 mL of fluid is present, whereas large effusions (> 1,500 mL) can be associated with significant asymmetry of chest expansion and bulging of intercostal spaces.
Inspection
Although inspection of the chest is not very helpful in detecting a pleural effusion, it can provide other relevant information such as the respiratory rate and the breathing position adopted by the patient (patients with a large pleural effusion may have orthopnea); it can also reveal abnormalities in the shape of the thorax such as the increased anteroposterior diameter (“barrel shape”) seen in patients with chronic obstructive pulmonary disease.18
In addition, by inspection we can assess the expansion of the thorax. The utility of inspecting chest expansion to detect lung restriction was first noted by Laennec19 in 1821. A simple method of evaluating chest expansion is to place a measuring tape around the chest at the level of the nipples to compare the circumference at end-inspiration and at end-expiration.20 In the absence of emphysema, the difference should be at least 2 inches. An expansion of 1.5 inches or less is considered abnormal.21 More relevant to pleural effusion than the amount of overall chest expansion is whether the expansion is symmetrical, which we can assess by palpation.
Palpation
Signs of pleural effusion that can be detected by palpation include asymmetric chest expansion and asymmetric tactile fremitus.
Other signs. Palpation of the chest can also help in detecting underlying disease of the thorax sometimes associated with pleurisy or pleural effusions. Chest wall tumors or skin abscesses may be related to underlying empyema, localized tenderness may be associated with rib fractures or costochondritis, and crepitus may be due to subcutaneous emphysema.
Percussion
The chest can be percussed directly with the tips of the fingers of one hand or indirectly by placing a third finger against the surface to be percussed. There are two main techniques used to detect pleural effusions: comparative percussion and auscultatory percussion.
Since other conditions such as consolidation of the lung and atelectasis can also be associated with dullness to percussion, some authors advocate percussion in the lateral supine position to detect a shift in the dullness that would indicate movement of fluid in the chest.25
The sensitivity of comparative chest percussion and its accuracy related to the size of the effusion are unknown. Kalantri et al10 found that dullness to percussion had a positive predictive value of only 55% but a negative predictive value of 97%, suggesting that the absence of the sign is very helpful in ruling out an effusion.
According to classic textbook descriptions,26 percussive sounds penetrate a maximum of 6 cm (2 cm of body wall thickness and 4 cm of lung), and at least 500 mL of fluid must be present in order to be able to detect an effusion by physical examination.2,8 Most of these descriptions are based on original studies done in cadavers more than 100 years ago.
The auscultatory percussion technique was first described by Laennec and used to delineate the size of several organs by placing the stethoscope directly above the structure to be outlined, followed by percussion from the periphery towards the organ of interest. The original technique was subsequently modified for the examination of the chest by Guarino.27
Although auscultatory percussion was used initially to try to detect lung lesions, masses and consolidations, Guarino and Guarino12 found this technique to be highly effective in detecting pleural effusion. In a prospective blinded study in 118 patients, this method was highly (95%) sensitive and 100% specific in detecting pleural effusion, even in patients with obesity, pneumonia, or other pleural abnormalities. Of note, their findings suggested that auscultatory percussion can detect as little as 50 mL of pleural fluid.
Bohadana et al13 compared auscultatory and conventional percussion with chest radiographic findings in 281 patients. They found that auscultatory percussion was 100% sensitive for detecting large pleural effusions.
However, when Bourke et al14 compared conventional and auscultatory percussion in 21 patients with abnormal radiographs, both methods had low sensitivity (15.4% vs 19.2%) but high specificity (97.3% vs 85.1%, respectively). It is important to mention that in this series only a few patients had a pleural effusion.
McDermott et al16 compared conventional and auscultatory percussion in detecting pleural effusion in 14 hospitalized patients, using ultrasonography instead of chest radiography as the gold standard for comparison. The findings on auscultatory percussion correlated better with the findings on ultrasonography than did those on conventional percussion. The authors gave no information about sensitivity or specificity.
Kalantri et al10 found that auscultatory percussion had a sensitivity of 58% and a specificity of 85%.
Auscultation
Originally described by Laennec (who invented the stethoscope),19 auscultation is perhaps the physical examination technique most used to detect pleural effusion.
Lichtenstein et al15 performed a study of auscultation in critically ill patients and found it to have a very low sensitivity (42%) but a higher specificity (90%), with an overall diagnostic accuracy of 60%. Of note, compared with chest radiography, auscultatory findings had similar sensitivity but higher accuracy.
Absent or diminished breath sounds strongly suggest an effusion.19
Egophonism. Laennec also described egophonism as a pathognomonic sign associated with a moderate degree of effusion. The word egophony comes from the Greek “ego,” which means goat; it is used to describe the change in the pronounced sound of E to A. The mechanism responsible for finding this sign in massive pleural effusions is probably upward displacement and compression or consolidation of the lung at the top of the effusion.
However, if the effusion is small, the consolidation will not be large enough to produce this sign. Similarly, other lung conditions associated with large consolidations may produce egophonism without a pleural effusion. Little is known about the predictive value of this sign, and significant interob-server variability needs to be taken into account.28
Pleural rub. Pleural effusions that result from any disease that causes direct inflammation of the pleurae can be associated with a pleural rub. This sound, classically described as rubbing of unoiled leather, is pathognomonic of pleural disease but not of pleural effusion. In fact, a pleural rub will disappear once an effusion develops. Little is known about the accuracy of this finding; in the study by Kalantri et al it had a very low sensitivity (5%) but a very high specificity (99%).10 The differential diagnosis includes pleuritis, pneumonia, mesothelioma, and tumors that metastasize to the pleura.
In detecting and evaluating pleural effusion, technology has not replaced clinical skills. Yet, despite centuries of lore, data are limited on the role of the physical examination and on its accuracy compared with other noninvasive tests such as conventional chest radiography or ultrasonography.
The following is an overview of the value of the clinical history and physical examination in detecting pleural effusion and a brief review of the available information regarding its accuracy compared with other diagnostic methods.
POTENTIAL CAUSES ARE MANY
The pleurae consist of two membranes that protect the lungs, allow them to move, contribute to their shape, and prevent the alveoli at the pleural surface from becoming overdistended. Between the visceral pleura (covering the lung) and the parietal pleura (covering the diaphragm and the chest wall) is the pleural space.
In healthy adults, the pleural space contains an estimated 5 to 10 mL of pleural fluid (0.1 mg/kg body weight).1 Pleural effusion is an accumulation of an abnormal amount of fluid in the pleural space.
Although the potential causes are many, the most common are congestive heart failure, pneumonia (40% of patients hospitalized with pneumonia have pleural effusion),2,3 cancer, and pulmonary embolism.4
Because many diseases affecting different organs can cause a pleural effusion, we cannot overemphasize the importance of a thorough history and physical examination to uncover clues that will help identify its cause and narrow the diagnostic workup. For example, significant weight loss and cachexia could be due to cancer, and joint, skin, or eye symptoms could be due to a connective tissue disorder.
A thorough review of the patient’s medications is mandatory, since several medications (eg, amiodarone [Cordarone], methotrexate [Rheumatrex, Trexall], and nitrofurantoin [Macrobid]) can be associated with exudative effusions. In addition, the patient’s occupational history must be ascertained, since exposure to asbestos can raise the suspicion of a malignant disease of the pleura such as mesothelioma.
SYMPTOMS ARE NEITHER SENSITIVE NOR SPECIFIC
The symptoms of pleural effusion are neither sensitive nor specific, and many patients have manifestations of the underlying process but not of the effusion itself. The most common symptoms directly related to effusion are cough, dyspnea, and pleuritic chest pain.5
Cough. Many patients with a pleural effusion have a dry, nonproductive cough, a consequence of inflammation of the pleurae or compression of the bronchial walls. Although this symptom is rarely helpful in diagnosing a pleural effusion, if accompanied by purulent sputum it suggests pneumonia, and if complicated by hemoptysis it suggests cancer or pulmonary embolism.
Dyspnea is a consequence of a combination of a restrictive lung defect, a ventilation-perfusion mismatch, and a decrease in cardiac output. Although large pleural effusions reduce lung volume and are generally associated with dyspnea, the symptoms may be out of proportion to the size of the effusion, and patients with small to moderate effusions may also have shortness of breath if their baseline lung function is poor.2
Chest pain accompanying a pleural effusion suggests inflammation of the parietal pleura,6 but could be due to cancer in the chest wall and ribs—or to a benign disease of the thoracic wall such as rib fracture or costo-chondritis.
Pain of pleural origin can remain localized to the adjacent area of the chest, but sometimes it is referred to other areas. If the diaphragmatic pleura is involved, the pain is in many cases referred to the ipsilateral shoulder.5 Pain may also be referred to the abdomen.
Pleuritic chest pain is described as being worse with deep inspiration or when lying down. It is common in patients with pulmonary embolism, parapneumonic effusion, or viral pleurisy, but it can also occur in patients with pneumothorax or pericarditis. A dull, aching chest pain may be due to an underlying pleural malignancy.7
PHYSICAL EXAMINATION: LONG TRADITION, FEW DATA
Our knowledge of the role of physical examination in detecting pleural effusion is still based mostly on expert opinion and on small case series.8,9
Patterson et al11 prospectively compared physical examination (including auscultation, percussion, and tactile fremitus) with bedside ultrasonography and found that physical examination had a lower sensitivity (53% vs 80%, respectively) but a similar specificity (71%).
Bigger effusions are easier to detect
The physical findings are related to the volume of fluid in the pleural effusion and its effects on the chest wall, diaphragm, and lungs. Physical findings are generally normal if less than 300 mL of fluid is present, whereas large effusions (> 1,500 mL) can be associated with significant asymmetry of chest expansion and bulging of intercostal spaces.
Inspection
Although inspection of the chest is not very helpful in detecting a pleural effusion, it can provide other relevant information such as the respiratory rate and the breathing position adopted by the patient (patients with a large pleural effusion may have orthopnea); it can also reveal abnormalities in the shape of the thorax such as the increased anteroposterior diameter (“barrel shape”) seen in patients with chronic obstructive pulmonary disease.18
In addition, by inspection we can assess the expansion of the thorax. The utility of inspecting chest expansion to detect lung restriction was first noted by Laennec19 in 1821. A simple method of evaluating chest expansion is to place a measuring tape around the chest at the level of the nipples to compare the circumference at end-inspiration and at end-expiration.20 In the absence of emphysema, the difference should be at least 2 inches. An expansion of 1.5 inches or less is considered abnormal.21 More relevant to pleural effusion than the amount of overall chest expansion is whether the expansion is symmetrical, which we can assess by palpation.
Palpation
Signs of pleural effusion that can be detected by palpation include asymmetric chest expansion and asymmetric tactile fremitus.
Other signs. Palpation of the chest can also help in detecting underlying disease of the thorax sometimes associated with pleurisy or pleural effusions. Chest wall tumors or skin abscesses may be related to underlying empyema, localized tenderness may be associated with rib fractures or costochondritis, and crepitus may be due to subcutaneous emphysema.
Percussion
The chest can be percussed directly with the tips of the fingers of one hand or indirectly by placing a third finger against the surface to be percussed. There are two main techniques used to detect pleural effusions: comparative percussion and auscultatory percussion.
Since other conditions such as consolidation of the lung and atelectasis can also be associated with dullness to percussion, some authors advocate percussion in the lateral supine position to detect a shift in the dullness that would indicate movement of fluid in the chest.25
The sensitivity of comparative chest percussion and its accuracy related to the size of the effusion are unknown. Kalantri et al10 found that dullness to percussion had a positive predictive value of only 55% but a negative predictive value of 97%, suggesting that the absence of the sign is very helpful in ruling out an effusion.
According to classic textbook descriptions,26 percussive sounds penetrate a maximum of 6 cm (2 cm of body wall thickness and 4 cm of lung), and at least 500 mL of fluid must be present in order to be able to detect an effusion by physical examination.2,8 Most of these descriptions are based on original studies done in cadavers more than 100 years ago.
The auscultatory percussion technique was first described by Laennec and used to delineate the size of several organs by placing the stethoscope directly above the structure to be outlined, followed by percussion from the periphery towards the organ of interest. The original technique was subsequently modified for the examination of the chest by Guarino.27
Although auscultatory percussion was used initially to try to detect lung lesions, masses and consolidations, Guarino and Guarino12 found this technique to be highly effective in detecting pleural effusion. In a prospective blinded study in 118 patients, this method was highly (95%) sensitive and 100% specific in detecting pleural effusion, even in patients with obesity, pneumonia, or other pleural abnormalities. Of note, their findings suggested that auscultatory percussion can detect as little as 50 mL of pleural fluid.
Bohadana et al13 compared auscultatory and conventional percussion with chest radiographic findings in 281 patients. They found that auscultatory percussion was 100% sensitive for detecting large pleural effusions.
However, when Bourke et al14 compared conventional and auscultatory percussion in 21 patients with abnormal radiographs, both methods had low sensitivity (15.4% vs 19.2%) but high specificity (97.3% vs 85.1%, respectively). It is important to mention that in this series only a few patients had a pleural effusion.
McDermott et al16 compared conventional and auscultatory percussion in detecting pleural effusion in 14 hospitalized patients, using ultrasonography instead of chest radiography as the gold standard for comparison. The findings on auscultatory percussion correlated better with the findings on ultrasonography than did those on conventional percussion. The authors gave no information about sensitivity or specificity.
Kalantri et al10 found that auscultatory percussion had a sensitivity of 58% and a specificity of 85%.
Auscultation
Originally described by Laennec (who invented the stethoscope),19 auscultation is perhaps the physical examination technique most used to detect pleural effusion.
Lichtenstein et al15 performed a study of auscultation in critically ill patients and found it to have a very low sensitivity (42%) but a higher specificity (90%), with an overall diagnostic accuracy of 60%. Of note, compared with chest radiography, auscultatory findings had similar sensitivity but higher accuracy.
Absent or diminished breath sounds strongly suggest an effusion.19
Egophonism. Laennec also described egophonism as a pathognomonic sign associated with a moderate degree of effusion. The word egophony comes from the Greek “ego,” which means goat; it is used to describe the change in the pronounced sound of E to A. The mechanism responsible for finding this sign in massive pleural effusions is probably upward displacement and compression or consolidation of the lung at the top of the effusion.
However, if the effusion is small, the consolidation will not be large enough to produce this sign. Similarly, other lung conditions associated with large consolidations may produce egophonism without a pleural effusion. Little is known about the predictive value of this sign, and significant interob-server variability needs to be taken into account.28
Pleural rub. Pleural effusions that result from any disease that causes direct inflammation of the pleurae can be associated with a pleural rub. This sound, classically described as rubbing of unoiled leather, is pathognomonic of pleural disease but not of pleural effusion. In fact, a pleural rub will disappear once an effusion develops. Little is known about the accuracy of this finding; in the study by Kalantri et al it had a very low sensitivity (5%) but a very high specificity (99%).10 The differential diagnosis includes pleuritis, pneumonia, mesothelioma, and tumors that metastasize to the pleura.
- Noppen M, De Waele M, Li R, et al. Volume and cellular content of normal pleural fluid in humans examined by pleural lavage. Am J Respir Crit Care Med 2000; 162:1023–1026.
- Bouros D. Pleural Disease. New York: Marcel Dekker, 2004.
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006; 3:75–80.
- Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002; 346:1971–1977.
- Light RW. Pleural Diseases, 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2007.
- Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Marel M, Stastny B, Melinova L, Svandova E, Light RW. Diagnosis of pleural effusions. Experience with clinical studies, 1986 to 1990. Chest 1995; 107:1598–1603.
- Leopold SS, Hopkins HU. Leopold’s Principles and Methods of Physical Diagnosis, 3d ed. Philadelphia: W.B. Saunders, 1965.
- Norris GW, Landis HRM, Montgomery CM, Krumbhaar EB. Diseases of the Chest and the Principles of Physical Diagnosis, 4th ed, rev. Philadelphia, W.B. Saunders, 1929.
- Kalantri S, Joshi R, Lokhande T, et al. Accuracy and reliability of physical signs in the diagnosis of pleural effusion. Respir Med 2007; 101:431–438.
- Patterson LA, Costantino TG, Satz WA. Diagnosing pleural effusion: a prospective comparison of physical examination with bedside ultra-sonography [abstract]. Ann Emerg Med 2004; 44:S112.
- Guarino JR, Guarino JC. Auscultatory percussion: a simple method to detect pleural effusion. J Gen Intern Med 1994; 9:71–74.
- Bohadana AB, Coimbra FT, Santiago JR. Detection of lung abnormalities by auscultatory percussion: a comparative study with conventional percussion. Respiration 1986; 50:218–225.
- Bourke S, Nunes D, Stafford F, Hurley G, Graham I. Percussion of the chest re-visited: a comparison of the diagnostic value of auscultatory and conventional chest percussion. Ir J Med Sci 1989; 158:82–84.
- Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004; 100:9–15.
- McDermott TD, McCarthy M, Chestnut T, Schumann L. A comparison of conventional percussion and auscultation percussion in the detection of pleural effusions of hospitalized patients. J Am Acad Nurse Pract 1997; 9:483–486.
- Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest 2003; 123:436–441.
- Pierce JA, Ebert RV. The barrel deformity of the chest, the senile lung and obstructive pulmonary emphysema. Am J Med 1958; 25:13–22.
- Laennec RTH. A Treatise On the Disease[s] of the Chest. New York: Library of the New York Academy of Medicine and Hafner Publishing Company, 1962.
- Bockenhauer SE, Chen H, Julliard KN, Weedon J. Measuring thoracic excursion: reliability of the cloth tape measure technique. J Am Osteopath Assoc 2007; 107:191–196.
- Fries JF. The reactive enthesopathies. Dis Mon 1985; 31 1:1–46.
- McGee SR. Evidence-based physical diagnosis. Philadelphia: W.B. Saunders, 2001.
- Auenbrugger L, Forbes J. On percussion of the chest: being a translation of Auenbrugger’s original treatise entitled “Inventum novum ex percussione thoracis humani, ut signo abstrusos interni pectoris morbos detegendi” (Vienna, 1761). Baltimore: The Johns Hopkins Press, 1936.
- Auenbrugger L, Neuburger M. Inventum Novum. London: Reprinted for Dawsons of Pall Mall, 1966.
- Gilbert VE. Shifting percussion dullness of the chest: a sign of pleural effusion. South Med J 1997; 90:1255–1256.
- Weil A. Handbuch und Atlas der topographischen Percussion nebst einer Darstellung der Lehre vom Percussionsschall. Leipzig: Vogel, 1880.
- Guarino JR. Auscultatory percussion, a new aid in the examination of the chest. J Kansas Med Soc 1974; 75:193–194.
- Sapira JD. About egophony. Chest 1995; 108:865–867.
- Noppen M, De Waele M, Li R, et al. Volume and cellular content of normal pleural fluid in humans examined by pleural lavage. Am J Respir Crit Care Med 2000; 162:1023–1026.
- Bouros D. Pleural Disease. New York: Marcel Dekker, 2004.
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006; 3:75–80.
- Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002; 346:1971–1977.
- Light RW. Pleural Diseases, 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2007.
- Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Marel M, Stastny B, Melinova L, Svandova E, Light RW. Diagnosis of pleural effusions. Experience with clinical studies, 1986 to 1990. Chest 1995; 107:1598–1603.
- Leopold SS, Hopkins HU. Leopold’s Principles and Methods of Physical Diagnosis, 3d ed. Philadelphia: W.B. Saunders, 1965.
- Norris GW, Landis HRM, Montgomery CM, Krumbhaar EB. Diseases of the Chest and the Principles of Physical Diagnosis, 4th ed, rev. Philadelphia, W.B. Saunders, 1929.
- Kalantri S, Joshi R, Lokhande T, et al. Accuracy and reliability of physical signs in the diagnosis of pleural effusion. Respir Med 2007; 101:431–438.
- Patterson LA, Costantino TG, Satz WA. Diagnosing pleural effusion: a prospective comparison of physical examination with bedside ultra-sonography [abstract]. Ann Emerg Med 2004; 44:S112.
- Guarino JR, Guarino JC. Auscultatory percussion: a simple method to detect pleural effusion. J Gen Intern Med 1994; 9:71–74.
- Bohadana AB, Coimbra FT, Santiago JR. Detection of lung abnormalities by auscultatory percussion: a comparative study with conventional percussion. Respiration 1986; 50:218–225.
- Bourke S, Nunes D, Stafford F, Hurley G, Graham I. Percussion of the chest re-visited: a comparison of the diagnostic value of auscultatory and conventional chest percussion. Ir J Med Sci 1989; 158:82–84.
- Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004; 100:9–15.
- McDermott TD, McCarthy M, Chestnut T, Schumann L. A comparison of conventional percussion and auscultation percussion in the detection of pleural effusions of hospitalized patients. J Am Acad Nurse Pract 1997; 9:483–486.
- Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest 2003; 123:436–441.
- Pierce JA, Ebert RV. The barrel deformity of the chest, the senile lung and obstructive pulmonary emphysema. Am J Med 1958; 25:13–22.
- Laennec RTH. A Treatise On the Disease[s] of the Chest. New York: Library of the New York Academy of Medicine and Hafner Publishing Company, 1962.
- Bockenhauer SE, Chen H, Julliard KN, Weedon J. Measuring thoracic excursion: reliability of the cloth tape measure technique. J Am Osteopath Assoc 2007; 107:191–196.
- Fries JF. The reactive enthesopathies. Dis Mon 1985; 31 1:1–46.
- McGee SR. Evidence-based physical diagnosis. Philadelphia: W.B. Saunders, 2001.
- Auenbrugger L, Forbes J. On percussion of the chest: being a translation of Auenbrugger’s original treatise entitled “Inventum novum ex percussione thoracis humani, ut signo abstrusos interni pectoris morbos detegendi” (Vienna, 1761). Baltimore: The Johns Hopkins Press, 1936.
- Auenbrugger L, Neuburger M. Inventum Novum. London: Reprinted for Dawsons of Pall Mall, 1966.
- Gilbert VE. Shifting percussion dullness of the chest: a sign of pleural effusion. South Med J 1997; 90:1255–1256.
- Weil A. Handbuch und Atlas der topographischen Percussion nebst einer Darstellung der Lehre vom Percussionsschall. Leipzig: Vogel, 1880.
- Guarino JR. Auscultatory percussion, a new aid in the examination of the chest. J Kansas Med Soc 1974; 75:193–194.
- Sapira JD. About egophony. Chest 1995; 108:865–867.
KEY POINTS
- The potential causes of pleural effusion are many and include congestive heart failure, pneumonia, cancer, and pulmonary embolism.
- Cardinal symptoms of pleural effusion are cough, chest pain, and dyspnea, but these are not very sensitive or specific.
- Common signs of pleural effusion are asymmetric chest expansion, asymmetric tactile fremitus, dullness to percussion, absent or diminished breath sounds, and rubs. The larger the effusion, the more sensitive these signs are.
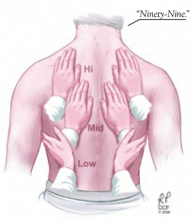
- Some have advocated auscultatory percussion (tapping on the manubrium while listening on the patient’s back) as being more sensitive than conventional percussion for detecting the dullness to percussion of pleural effusion.