User login
Multiple sclerosis (MS) is an autoimmune disorder in which the myelin of the brain and spinal cord is selectively targeted by immune-system cells. As a result, nerve transmission is disrupted, leading to a variety of unpredictable symptoms from weakness and a lack of balance to blindness and paralysis of the body. Clinically, MS can take 4 courses, including relapsing-remitting (RRMS), primary-progressive (PPMS), secondary-progressive, and progressive-relapsing.1 At onset, 85% of diagnosed patients have RRMS, and 10% to 15% have PPMS.2 If untreated, patients with RRMS become secondary-progressive, with progressive disability and indiscrete relapses.3 Hence, disease-modifying therapies are targeted toward decreasing the relapse rate as well as slowing the progression of the disease.4
Annually, about 16,000 veterans with MS receive health care services from the VHA.5 The C.W. Bill Young Bay Pines VA Healthcare System (BPVAHCS) is a level 1 facility that annually serves more than 105,000 veterans. The BPVAMC sees veterans with a wide variety of neurologic illnesses and has 5 full-time neurologists with subspecialty training. The BPVAHCS facility has outpatient clinics and a 200 inpatient bed facility. The Neurology Department sees 125 outpatients per week and consults on about 30 inpatients per week.
Methods
A retrospective review of BPVAHCS patients diagnosed with MS from January 2009 to July 2014 was performed with institutional review board approval. Patient data were collected from ICD-9-CM codes and kept confidential. A list of patients was collected from Neurology Clinic patient visits with “Multiple Sclerosis” on the problem list.
Patient medical records were reviewed to collect the following information: presence of rigorous diagnosis of MS, clinical course of MS in patient, presence or absence of disease-modifying therapy, and disease-modifying agents (DMAs) used.
Determining factors for DMA treatment included increasing tiredness, weakness, visual symptoms, and radiologic evidence (magnetic resonance imaging) of recurrent, active lesions. Each patient was examined on a case-by-case basis to assess whether or not the patient actually had MS and if so, whether they were being treated with DMAs. Only patients with RRMS were included. Patients were excluded from the study if they were deceased, not currently under BPVACHS care, or had symptoms of optic neuritis but were not fully indicative of MS. Patients with clinically isolated syndrome, probable diagnosis of MS, or PPMS also were excluded from the study.
Exclusion from this study was based on 2 additional premises. Patients were excluded if they discontinued an initial ABC (interferon beta-1a, interferon beta-1b, glatiramer acetate) due to DMA treatment relapse or adverse effects (AEs), such as injection site reactions, flulike symptoms, or depression. Additionally, patients who were not willing to take more DMA medications were excluded if they felt they were relatively stable (had infrequent relapses) and believed that additional medication was not worth the risk of potential AEs.
The study patients were seen and followed up by the neurologists. All the data for this study were based on interactions with the neurologists and not primary care providers (PCPs). Because MS treatment is complex, PCPs have little involvement in its management. The percentage of patients not on any DMAs was calculated from the list of BPVAHCS patients with RRMS.
The results were compared with a similar retrospective cohort study conducted using the Commercial Claims database and Medicare Supplemental and Coordination of Benefits database to identify individuals newly diagnosed with MS.6 This study was chosen because it was similar in methodology but investigated a comparable non-VA group. A 2-tailed difference between proportions test was then performed to determine whether the BPVAHCS patients with MS who were not treated with DMAs were significantly different from those from this non-VA population. Additionally, data from VA patients who were receiving DMAs were further examined and presented.
Results
At the BPVAHCS, 262 patients were diagnosed with MS and 43% were not treated with DMAs. Margolis and colleagues found that about 60% of its 11,061 newly diagnosed non-VA patients with MS remained untreated.6 Although the latter proportion is higher, a 2-tailed difference between proportions test indicates that the proportion of patients with MS being treated at the VA was significantly lower (P < .01).
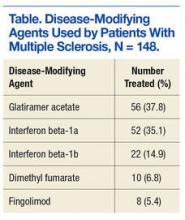
Among the 148 patients who were diagnosed with MS and treated with DMA at BPVAHCS, 5 different DMAs were identified (Table). The most commonly prescribed regimen was glatiramer acetate, which was used by 56 of 148 patients (37.8%). Fifty-two patients (35.1%) used interferon beta-1a. Of the 2 interferon DMAs, beta-1a was twice as popular as beta-1b, which was prescribed to 22 (14.9%) of patients. Dimethyl fumarate (6.8%) and fingolimod (5.4%) were used sparingly, because they were new to the market (cost and availability also were factors). With time, increased efficacy and objective assessment of benefit in the reduction of the T2 lesion load may result in a greater use of these oral DMAs.7–9
Based on this evaluation, 43% of patients who were diagnosed with MS were untreated at BPVAHCS. Concern over treatment AEs, the inconvenience of injectable dosing, and patients who were not 100% service-connected and lost to follow-up because of the cost may have contributed to the poor rate of treatment.
Discussion
Injected-based DMAs, such as interferon beta-1a, interferon beta-1b, and glatiramer acetate, were first introduced in the 1990s, but these proved to be inconvenient and triggered AEs, including injection site reactions. Overall, their efficacy was about 30%, with interferon beta-1a showing a 27% reduction in relapses.10 In 2010, oral DMAs, such as fingolimod, were FDA approved. These oral DMAs were a significant improvement over injectable DMAs but still had AEs. Hence, their use was restricted to neurologists by the BPVAHCS, and rightfully so.
Still, newer and more effective oral DMAs are showing promise, such as dimethyl fumarate, teriflunomide, and alemtuzumab. These new DMAs have significantly impacted the treatment of MS as they are not only easier for patients to adhere to and for neurologists to prescribe, but most significantly, have had a 50% decrease in the rate of relapse.10 Yet, the newer oral DMAs were less commonly prescribed than the older treatments at BPVAHCS.
Since this study did not demonstrate increased use of oral DMAs at the BPVAHCS, more PCP and neurologist-focused educational programs on the use of DMAs may be beneficial. Educational programs should lead to a reevaluation of patients with MS to consider oral DMAs, which offer better efficacy and fewer AEs. The newer oral DMAs have shown a higher reduction of T2 lesions, and the significantly decreased incidence of relapses in many other medical facilities is quite promising for the BPVAHCS.7-9
The data collected at BPVAHCS were part of a quality improvement (QI) study that will be used by the Neurology Department to follow up on the patients with MS in order to implement DMA therapies. A questionnaire was developed for following up with BPVAHCS patients with MS. The primary purpose of the questionnaire is to help neurologists identify the reasons patients avoid DMA therapies and to reduce the number of BPVAHCS patients not on the most efficacious MS DMA treatment.
Conclusion
Multiple sclerosis is a disease without a cure. Current treatment strategies focus on modifying the course of the disease and managing its symptoms. However, even as promising new treatments emerge, the current literature suggests that a significant number of patients diagnosed with MS are not receiving DMAs and may not be receiving optimal treatment.11
Findings from this study indicate that although DMAs are optimal for patients with MS, they may not be prescribed as frequently at BPVAHCS as they are at a non-VA care facility. It is unclear whether this finding is explained by an educational gap, clinical differences between non-VA and VA patients, organizational factors, or a combination of these variables. Further study is warranted to examine the use of DMAs among veterans with MS and factors that facilitate or impede optimal practice. The BPVAHCS will use data from this retrospective cohort study in a QI initiative for patients with MS. Findings from the QI initiative will be reported using the Standards for Quality Improvement Reporting Excellence.12,13
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Bay Pines VA Healthcare System.
1. National Multiple Sclerosis Society. Types of MS. National Multiple Sclerosis Society website. http://www.nationalmssociety.org/What-is-MS/Types-of-MS. Accessed April 7, 2016.
2. McKay KA, Kwan V, Duggan T, Tremlett H. Risk factors associated with the onset of relapsing-remitting and primary progressive multiple sclerosis: a systematic review. Biomed Res Int. 2015;2015:817238.
3. Gold R, Wolinsky JS, Amato MP, Comi G. Evolving expectations around early management of multiple sclerosis. Ther Adv Neurol Disord. 2010;3(6):351-367.
4. Ben-Zacharia A, Lublin FD. Talking About Initiating and Adhering to Treatment With Injectable Disease Modifying Agents. Washington, DC: National Multiple Sclerosis Society; 2009.
5. Cameron MH, Poel AJ, Haselkorn JK, Linke A, Bourdette D. Falls requiring medical attention among veterans with multiple sclerosis: a cohort study. J Rehabil Res Dev. 2011;48(1):13-20.
6. Margolis JM, Fowler R, Johnson BH, Kassed CA, Kahler K. Disease-modifying drug initiation patterns in commercially insured multiple sclerosis patients: a retrospective cohort study. BMC Neurol. 2011;11:122.
7. Johnson KP, Brooks BR, Ford CC, et al. Sustained clinical benefits of glatiramer acetate in relapsing multiple sclerosis patients observed for 6 years. Copolymer 1 Multiple Scleroisis Study Group. Mult Scler. 2000;6(4):255-266.
8. Steinberg SC, Faris RJ, Chang CF, Chan A, Tankersley MA. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig. 2010;30(2):89-100.
9. Agashivala N, Wu N, Abouzaid S, et al. Compliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort study. BMC Neurol. 2013;13:138.
10. Williams UE, Oparah SK, Philip-Ephraim EE. Disease modifying therapy in multiple sclerosis. Int Sch Res Notices. 2014;2014:307064.
11. Lus G, Signoriello E, Maniscalco GT, Bonavita S, Signoriello S, Gallo C. Treatment withdrawal in relapsing-remitting multiple sclerosis: a retrospective cohort study. Eur J Neurol. 2016;23(3):489-493.
12. Davidoff F, Batalden P, Stevens D, Ogrinc G, Mooney S; SQUIRE development group. Publication guidelines for quality improvement studies in health care: evolution of the SQUIRE project. Qual Saf Health Care. 2008;17(suppl 1):i3–i9.
13. Ogrinc G, Mooney SE, Estrada C, et al. The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17(suppl 1):i13-i32.
Multiple sclerosis (MS) is an autoimmune disorder in which the myelin of the brain and spinal cord is selectively targeted by immune-system cells. As a result, nerve transmission is disrupted, leading to a variety of unpredictable symptoms from weakness and a lack of balance to blindness and paralysis of the body. Clinically, MS can take 4 courses, including relapsing-remitting (RRMS), primary-progressive (PPMS), secondary-progressive, and progressive-relapsing.1 At onset, 85% of diagnosed patients have RRMS, and 10% to 15% have PPMS.2 If untreated, patients with RRMS become secondary-progressive, with progressive disability and indiscrete relapses.3 Hence, disease-modifying therapies are targeted toward decreasing the relapse rate as well as slowing the progression of the disease.4
Annually, about 16,000 veterans with MS receive health care services from the VHA.5 The C.W. Bill Young Bay Pines VA Healthcare System (BPVAHCS) is a level 1 facility that annually serves more than 105,000 veterans. The BPVAMC sees veterans with a wide variety of neurologic illnesses and has 5 full-time neurologists with subspecialty training. The BPVAHCS facility has outpatient clinics and a 200 inpatient bed facility. The Neurology Department sees 125 outpatients per week and consults on about 30 inpatients per week.
Methods
A retrospective review of BPVAHCS patients diagnosed with MS from January 2009 to July 2014 was performed with institutional review board approval. Patient data were collected from ICD-9-CM codes and kept confidential. A list of patients was collected from Neurology Clinic patient visits with “Multiple Sclerosis” on the problem list.
Patient medical records were reviewed to collect the following information: presence of rigorous diagnosis of MS, clinical course of MS in patient, presence or absence of disease-modifying therapy, and disease-modifying agents (DMAs) used.
Determining factors for DMA treatment included increasing tiredness, weakness, visual symptoms, and radiologic evidence (magnetic resonance imaging) of recurrent, active lesions. Each patient was examined on a case-by-case basis to assess whether or not the patient actually had MS and if so, whether they were being treated with DMAs. Only patients with RRMS were included. Patients were excluded from the study if they were deceased, not currently under BPVACHS care, or had symptoms of optic neuritis but were not fully indicative of MS. Patients with clinically isolated syndrome, probable diagnosis of MS, or PPMS also were excluded from the study.
Exclusion from this study was based on 2 additional premises. Patients were excluded if they discontinued an initial ABC (interferon beta-1a, interferon beta-1b, glatiramer acetate) due to DMA treatment relapse or adverse effects (AEs), such as injection site reactions, flulike symptoms, or depression. Additionally, patients who were not willing to take more DMA medications were excluded if they felt they were relatively stable (had infrequent relapses) and believed that additional medication was not worth the risk of potential AEs.
The study patients were seen and followed up by the neurologists. All the data for this study were based on interactions with the neurologists and not primary care providers (PCPs). Because MS treatment is complex, PCPs have little involvement in its management. The percentage of patients not on any DMAs was calculated from the list of BPVAHCS patients with RRMS.
The results were compared with a similar retrospective cohort study conducted using the Commercial Claims database and Medicare Supplemental and Coordination of Benefits database to identify individuals newly diagnosed with MS.6 This study was chosen because it was similar in methodology but investigated a comparable non-VA group. A 2-tailed difference between proportions test was then performed to determine whether the BPVAHCS patients with MS who were not treated with DMAs were significantly different from those from this non-VA population. Additionally, data from VA patients who were receiving DMAs were further examined and presented.
Results
At the BPVAHCS, 262 patients were diagnosed with MS and 43% were not treated with DMAs. Margolis and colleagues found that about 60% of its 11,061 newly diagnosed non-VA patients with MS remained untreated.6 Although the latter proportion is higher, a 2-tailed difference between proportions test indicates that the proportion of patients with MS being treated at the VA was significantly lower (P < .01).
Among the 148 patients who were diagnosed with MS and treated with DMA at BPVAHCS, 5 different DMAs were identified (Table). The most commonly prescribed regimen was glatiramer acetate, which was used by 56 of 148 patients (37.8%). Fifty-two patients (35.1%) used interferon beta-1a. Of the 2 interferon DMAs, beta-1a was twice as popular as beta-1b, which was prescribed to 22 (14.9%) of patients. Dimethyl fumarate (6.8%) and fingolimod (5.4%) were used sparingly, because they were new to the market (cost and availability also were factors). With time, increased efficacy and objective assessment of benefit in the reduction of the T2 lesion load may result in a greater use of these oral DMAs.7–9
Based on this evaluation, 43% of patients who were diagnosed with MS were untreated at BPVAHCS. Concern over treatment AEs, the inconvenience of injectable dosing, and patients who were not 100% service-connected and lost to follow-up because of the cost may have contributed to the poor rate of treatment.
Discussion
Injected-based DMAs, such as interferon beta-1a, interferon beta-1b, and glatiramer acetate, were first introduced in the 1990s, but these proved to be inconvenient and triggered AEs, including injection site reactions. Overall, their efficacy was about 30%, with interferon beta-1a showing a 27% reduction in relapses.10 In 2010, oral DMAs, such as fingolimod, were FDA approved. These oral DMAs were a significant improvement over injectable DMAs but still had AEs. Hence, their use was restricted to neurologists by the BPVAHCS, and rightfully so.
Still, newer and more effective oral DMAs are showing promise, such as dimethyl fumarate, teriflunomide, and alemtuzumab. These new DMAs have significantly impacted the treatment of MS as they are not only easier for patients to adhere to and for neurologists to prescribe, but most significantly, have had a 50% decrease in the rate of relapse.10 Yet, the newer oral DMAs were less commonly prescribed than the older treatments at BPVAHCS.
Since this study did not demonstrate increased use of oral DMAs at the BPVAHCS, more PCP and neurologist-focused educational programs on the use of DMAs may be beneficial. Educational programs should lead to a reevaluation of patients with MS to consider oral DMAs, which offer better efficacy and fewer AEs. The newer oral DMAs have shown a higher reduction of T2 lesions, and the significantly decreased incidence of relapses in many other medical facilities is quite promising for the BPVAHCS.7-9
The data collected at BPVAHCS were part of a quality improvement (QI) study that will be used by the Neurology Department to follow up on the patients with MS in order to implement DMA therapies. A questionnaire was developed for following up with BPVAHCS patients with MS. The primary purpose of the questionnaire is to help neurologists identify the reasons patients avoid DMA therapies and to reduce the number of BPVAHCS patients not on the most efficacious MS DMA treatment.
Conclusion
Multiple sclerosis is a disease without a cure. Current treatment strategies focus on modifying the course of the disease and managing its symptoms. However, even as promising new treatments emerge, the current literature suggests that a significant number of patients diagnosed with MS are not receiving DMAs and may not be receiving optimal treatment.11
Findings from this study indicate that although DMAs are optimal for patients with MS, they may not be prescribed as frequently at BPVAHCS as they are at a non-VA care facility. It is unclear whether this finding is explained by an educational gap, clinical differences between non-VA and VA patients, organizational factors, or a combination of these variables. Further study is warranted to examine the use of DMAs among veterans with MS and factors that facilitate or impede optimal practice. The BPVAHCS will use data from this retrospective cohort study in a QI initiative for patients with MS. Findings from the QI initiative will be reported using the Standards for Quality Improvement Reporting Excellence.12,13
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Bay Pines VA Healthcare System.
Multiple sclerosis (MS) is an autoimmune disorder in which the myelin of the brain and spinal cord is selectively targeted by immune-system cells. As a result, nerve transmission is disrupted, leading to a variety of unpredictable symptoms from weakness and a lack of balance to blindness and paralysis of the body. Clinically, MS can take 4 courses, including relapsing-remitting (RRMS), primary-progressive (PPMS), secondary-progressive, and progressive-relapsing.1 At onset, 85% of diagnosed patients have RRMS, and 10% to 15% have PPMS.2 If untreated, patients with RRMS become secondary-progressive, with progressive disability and indiscrete relapses.3 Hence, disease-modifying therapies are targeted toward decreasing the relapse rate as well as slowing the progression of the disease.4
Annually, about 16,000 veterans with MS receive health care services from the VHA.5 The C.W. Bill Young Bay Pines VA Healthcare System (BPVAHCS) is a level 1 facility that annually serves more than 105,000 veterans. The BPVAMC sees veterans with a wide variety of neurologic illnesses and has 5 full-time neurologists with subspecialty training. The BPVAHCS facility has outpatient clinics and a 200 inpatient bed facility. The Neurology Department sees 125 outpatients per week and consults on about 30 inpatients per week.
Methods
A retrospective review of BPVAHCS patients diagnosed with MS from January 2009 to July 2014 was performed with institutional review board approval. Patient data were collected from ICD-9-CM codes and kept confidential. A list of patients was collected from Neurology Clinic patient visits with “Multiple Sclerosis” on the problem list.
Patient medical records were reviewed to collect the following information: presence of rigorous diagnosis of MS, clinical course of MS in patient, presence or absence of disease-modifying therapy, and disease-modifying agents (DMAs) used.
Determining factors for DMA treatment included increasing tiredness, weakness, visual symptoms, and radiologic evidence (magnetic resonance imaging) of recurrent, active lesions. Each patient was examined on a case-by-case basis to assess whether or not the patient actually had MS and if so, whether they were being treated with DMAs. Only patients with RRMS were included. Patients were excluded from the study if they were deceased, not currently under BPVACHS care, or had symptoms of optic neuritis but were not fully indicative of MS. Patients with clinically isolated syndrome, probable diagnosis of MS, or PPMS also were excluded from the study.
Exclusion from this study was based on 2 additional premises. Patients were excluded if they discontinued an initial ABC (interferon beta-1a, interferon beta-1b, glatiramer acetate) due to DMA treatment relapse or adverse effects (AEs), such as injection site reactions, flulike symptoms, or depression. Additionally, patients who were not willing to take more DMA medications were excluded if they felt they were relatively stable (had infrequent relapses) and believed that additional medication was not worth the risk of potential AEs.
The study patients were seen and followed up by the neurologists. All the data for this study were based on interactions with the neurologists and not primary care providers (PCPs). Because MS treatment is complex, PCPs have little involvement in its management. The percentage of patients not on any DMAs was calculated from the list of BPVAHCS patients with RRMS.
The results were compared with a similar retrospective cohort study conducted using the Commercial Claims database and Medicare Supplemental and Coordination of Benefits database to identify individuals newly diagnosed with MS.6 This study was chosen because it was similar in methodology but investigated a comparable non-VA group. A 2-tailed difference between proportions test was then performed to determine whether the BPVAHCS patients with MS who were not treated with DMAs were significantly different from those from this non-VA population. Additionally, data from VA patients who were receiving DMAs were further examined and presented.
Results
At the BPVAHCS, 262 patients were diagnosed with MS and 43% were not treated with DMAs. Margolis and colleagues found that about 60% of its 11,061 newly diagnosed non-VA patients with MS remained untreated.6 Although the latter proportion is higher, a 2-tailed difference between proportions test indicates that the proportion of patients with MS being treated at the VA was significantly lower (P < .01).
Among the 148 patients who were diagnosed with MS and treated with DMA at BPVAHCS, 5 different DMAs were identified (Table). The most commonly prescribed regimen was glatiramer acetate, which was used by 56 of 148 patients (37.8%). Fifty-two patients (35.1%) used interferon beta-1a. Of the 2 interferon DMAs, beta-1a was twice as popular as beta-1b, which was prescribed to 22 (14.9%) of patients. Dimethyl fumarate (6.8%) and fingolimod (5.4%) were used sparingly, because they were new to the market (cost and availability also were factors). With time, increased efficacy and objective assessment of benefit in the reduction of the T2 lesion load may result in a greater use of these oral DMAs.7–9
Based on this evaluation, 43% of patients who were diagnosed with MS were untreated at BPVAHCS. Concern over treatment AEs, the inconvenience of injectable dosing, and patients who were not 100% service-connected and lost to follow-up because of the cost may have contributed to the poor rate of treatment.
Discussion
Injected-based DMAs, such as interferon beta-1a, interferon beta-1b, and glatiramer acetate, were first introduced in the 1990s, but these proved to be inconvenient and triggered AEs, including injection site reactions. Overall, their efficacy was about 30%, with interferon beta-1a showing a 27% reduction in relapses.10 In 2010, oral DMAs, such as fingolimod, were FDA approved. These oral DMAs were a significant improvement over injectable DMAs but still had AEs. Hence, their use was restricted to neurologists by the BPVAHCS, and rightfully so.
Still, newer and more effective oral DMAs are showing promise, such as dimethyl fumarate, teriflunomide, and alemtuzumab. These new DMAs have significantly impacted the treatment of MS as they are not only easier for patients to adhere to and for neurologists to prescribe, but most significantly, have had a 50% decrease in the rate of relapse.10 Yet, the newer oral DMAs were less commonly prescribed than the older treatments at BPVAHCS.
Since this study did not demonstrate increased use of oral DMAs at the BPVAHCS, more PCP and neurologist-focused educational programs on the use of DMAs may be beneficial. Educational programs should lead to a reevaluation of patients with MS to consider oral DMAs, which offer better efficacy and fewer AEs. The newer oral DMAs have shown a higher reduction of T2 lesions, and the significantly decreased incidence of relapses in many other medical facilities is quite promising for the BPVAHCS.7-9
The data collected at BPVAHCS were part of a quality improvement (QI) study that will be used by the Neurology Department to follow up on the patients with MS in order to implement DMA therapies. A questionnaire was developed for following up with BPVAHCS patients with MS. The primary purpose of the questionnaire is to help neurologists identify the reasons patients avoid DMA therapies and to reduce the number of BPVAHCS patients not on the most efficacious MS DMA treatment.
Conclusion
Multiple sclerosis is a disease without a cure. Current treatment strategies focus on modifying the course of the disease and managing its symptoms. However, even as promising new treatments emerge, the current literature suggests that a significant number of patients diagnosed with MS are not receiving DMAs and may not be receiving optimal treatment.11
Findings from this study indicate that although DMAs are optimal for patients with MS, they may not be prescribed as frequently at BPVAHCS as they are at a non-VA care facility. It is unclear whether this finding is explained by an educational gap, clinical differences between non-VA and VA patients, organizational factors, or a combination of these variables. Further study is warranted to examine the use of DMAs among veterans with MS and factors that facilitate or impede optimal practice. The BPVAHCS will use data from this retrospective cohort study in a QI initiative for patients with MS. Findings from the QI initiative will be reported using the Standards for Quality Improvement Reporting Excellence.12,13
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Bay Pines VA Healthcare System.
1. National Multiple Sclerosis Society. Types of MS. National Multiple Sclerosis Society website. http://www.nationalmssociety.org/What-is-MS/Types-of-MS. Accessed April 7, 2016.
2. McKay KA, Kwan V, Duggan T, Tremlett H. Risk factors associated with the onset of relapsing-remitting and primary progressive multiple sclerosis: a systematic review. Biomed Res Int. 2015;2015:817238.
3. Gold R, Wolinsky JS, Amato MP, Comi G. Evolving expectations around early management of multiple sclerosis. Ther Adv Neurol Disord. 2010;3(6):351-367.
4. Ben-Zacharia A, Lublin FD. Talking About Initiating and Adhering to Treatment With Injectable Disease Modifying Agents. Washington, DC: National Multiple Sclerosis Society; 2009.
5. Cameron MH, Poel AJ, Haselkorn JK, Linke A, Bourdette D. Falls requiring medical attention among veterans with multiple sclerosis: a cohort study. J Rehabil Res Dev. 2011;48(1):13-20.
6. Margolis JM, Fowler R, Johnson BH, Kassed CA, Kahler K. Disease-modifying drug initiation patterns in commercially insured multiple sclerosis patients: a retrospective cohort study. BMC Neurol. 2011;11:122.
7. Johnson KP, Brooks BR, Ford CC, et al. Sustained clinical benefits of glatiramer acetate in relapsing multiple sclerosis patients observed for 6 years. Copolymer 1 Multiple Scleroisis Study Group. Mult Scler. 2000;6(4):255-266.
8. Steinberg SC, Faris RJ, Chang CF, Chan A, Tankersley MA. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig. 2010;30(2):89-100.
9. Agashivala N, Wu N, Abouzaid S, et al. Compliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort study. BMC Neurol. 2013;13:138.
10. Williams UE, Oparah SK, Philip-Ephraim EE. Disease modifying therapy in multiple sclerosis. Int Sch Res Notices. 2014;2014:307064.
11. Lus G, Signoriello E, Maniscalco GT, Bonavita S, Signoriello S, Gallo C. Treatment withdrawal in relapsing-remitting multiple sclerosis: a retrospective cohort study. Eur J Neurol. 2016;23(3):489-493.
12. Davidoff F, Batalden P, Stevens D, Ogrinc G, Mooney S; SQUIRE development group. Publication guidelines for quality improvement studies in health care: evolution of the SQUIRE project. Qual Saf Health Care. 2008;17(suppl 1):i3–i9.
13. Ogrinc G, Mooney SE, Estrada C, et al. The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17(suppl 1):i13-i32.
1. National Multiple Sclerosis Society. Types of MS. National Multiple Sclerosis Society website. http://www.nationalmssociety.org/What-is-MS/Types-of-MS. Accessed April 7, 2016.
2. McKay KA, Kwan V, Duggan T, Tremlett H. Risk factors associated with the onset of relapsing-remitting and primary progressive multiple sclerosis: a systematic review. Biomed Res Int. 2015;2015:817238.
3. Gold R, Wolinsky JS, Amato MP, Comi G. Evolving expectations around early management of multiple sclerosis. Ther Adv Neurol Disord. 2010;3(6):351-367.
4. Ben-Zacharia A, Lublin FD. Talking About Initiating and Adhering to Treatment With Injectable Disease Modifying Agents. Washington, DC: National Multiple Sclerosis Society; 2009.
5. Cameron MH, Poel AJ, Haselkorn JK, Linke A, Bourdette D. Falls requiring medical attention among veterans with multiple sclerosis: a cohort study. J Rehabil Res Dev. 2011;48(1):13-20.
6. Margolis JM, Fowler R, Johnson BH, Kassed CA, Kahler K. Disease-modifying drug initiation patterns in commercially insured multiple sclerosis patients: a retrospective cohort study. BMC Neurol. 2011;11:122.
7. Johnson KP, Brooks BR, Ford CC, et al. Sustained clinical benefits of glatiramer acetate in relapsing multiple sclerosis patients observed for 6 years. Copolymer 1 Multiple Scleroisis Study Group. Mult Scler. 2000;6(4):255-266.
8. Steinberg SC, Faris RJ, Chang CF, Chan A, Tankersley MA. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig. 2010;30(2):89-100.
9. Agashivala N, Wu N, Abouzaid S, et al. Compliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort study. BMC Neurol. 2013;13:138.
10. Williams UE, Oparah SK, Philip-Ephraim EE. Disease modifying therapy in multiple sclerosis. Int Sch Res Notices. 2014;2014:307064.
11. Lus G, Signoriello E, Maniscalco GT, Bonavita S, Signoriello S, Gallo C. Treatment withdrawal in relapsing-remitting multiple sclerosis: a retrospective cohort study. Eur J Neurol. 2016;23(3):489-493.
12. Davidoff F, Batalden P, Stevens D, Ogrinc G, Mooney S; SQUIRE development group. Publication guidelines for quality improvement studies in health care: evolution of the SQUIRE project. Qual Saf Health Care. 2008;17(suppl 1):i3–i9.
13. Ogrinc G, Mooney SE, Estrada C, et al. The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17(suppl 1):i13-i32.