User login
The approach to the treatment of common skin disorders and cosmetic concerns in patients with skin of color (SOC) requires the clinician to understand the biological differences, nuances, and special considerations that are unique to patients with darker skin types.1-3 This article addresses 4 common facial concerns in SOC patients—acne, rosacea, facial hyperpigmentation, and cosmetic enhancement—and provides treatment recommendations and management pearls to assist the clinician with optimal outcomes for SOC patients.
Acne in SOC Patients
Acne vulgaris is one of the most common conditions that dermatologists treat and is estimated to affect 40 to 50 million individuals in the United States.1 Many of these acne patients are individuals with SOC.2-4 A study of 2835 females (aged 10–70 years) conducted in 4 different cities—Los Angeles, California; London, United Kingdom; Akita, Japan; and Rome, Italy—demonstrated acne prevalence of 37% in blacks, 32% in Hispanics, 30% in Asians, 24% in whites, and 23% in Continental Indians.5 Blacks, Hispanics, and Continental Indians demonstrated equal prevalence with comedonal and inflammatory acne. Asians displayed more inflammatory acne lesions than comedones. In contrast, whites demonstrated more comedones than inflammatory acne. Dyspigmentation, postinflammatory hyperpigmentation (PIH), and atrophic scars were more common in black and Hispanic females than other ethnicities.5 This study illustrated that acne-induced PIH is a common sequela in SOC patients and is the main reason they seek treatment.6,7
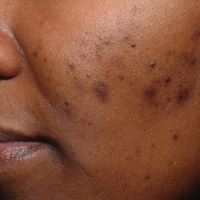
The pathogenesis of acne is the same in all racial and ethnic groups: (1) follicular hyperkeratinization and the formation of a microcomedone caused by abnormal desquamation of the keratinocytes within the sebaceous follicle, (2) production of sebum by circulating androgens, (3) proliferation of Propionibacterium acnes, and (4) inflammation. Subclinical inflammation is present throughout all stages of acne, including normal-appearing skin, inflammatory lesions, comedones, and scarring, and may contribute to PIH in acne patients with SOC (Figure 1).8 A thorough history should be obtained from acne patients, including answers to the following questions7:
- What skin and hair care products do you use?
- Do you use sunscreen daily?
- What cosmetic products or makeup do you use?
- Do you use any ethnic skin care products, including skin lightening creams?
- Do you have a history of keloids?

It is important to ask these questions to assess if the SOC patient has developed pomade acne,9 acne cosmetica,10 or a potential risk of skin irritation from the use of skin care practices. It is best to take total control of the patient’s skin care regimen and discontinue use of toners, astringents, witch hazel, exfoliants, and rubbing alcohol, which may lead to skin dryness and irritation, particularly when combined with topical acne medications.
Treatment
Treatment of acne in SOC patients is similar to generally recommended treatments, with special considerations. Consider the following key points when treating acne in SOC patients:
- Treat acne early and aggressively to prevent or minimize subsequent PIH and acne scarring.
- Balance aggressive treatment with nonirritating topical skin care.
- Most importantly, target PIH in addition to acne and choose a regimen that limits skin irritation that might exacerbate existing PIH.7
Develop a maintenance program to control future breakouts. Topical agents can be used as monotherapy or in fixed combinations and may include benzoyl peroxide, antibiotics, dapsone, azelaic acid (AZA), and retinoids. Similar to white patients, topical retinoids remain a first-line treatment for acne in patients with SOC.11,12
Tolerability must be managed in SOC acne patients. Therapeutic maneuvers that can be instituted should include a discussion on using gentle skin care, initiating therapy with a retinoid applied every other night starting with a low concentration and gradually titrating up, and applying a moisturizer before or after applying acne medication. Oral therapies consist of antibiotics (doxycycline, minocycline), retinoids (isotretinoin), and hormonal modulators (oral contraceptives, spironolactone). Isotretinoin, recommended for patients with nodulocystic acne, may play a possible role in treating acne-induced PIH.13
Two common procedural therapies for acne include comedone extraction and intralesional corticosteroid injection. A 6- to 8-week course of a topical retinoid prior to comedonal extraction may facilitate the procedure and is recommended in SOC patients to help reduce cutaneous trauma and PIH.11 Inflammatory acne lesions can be treated with intralesional injection of triamcinolone acetonide 2.5 or 5.0 mg/mL, which usually reduces inflammation within 2 to 5 days.11
Treatment of acne-induced PIH includes sun protection, topical and oral medications, chemical peels, lasers, and energy devices. Treatment of hypertrophic scarring and keloids involves intralesional injection of triamcinolone acetonide 20, 30, or 40 mg/mL every 4 weeks until the lesion is flat.11
Superficial chemical peels can be used to treat acne and PIH in SOC patients,14 such as salicylic acid (20%–30%), glycolic acid (20%–70%), trichloroacetic acid (15%–30%), and Jessner peels.
Acne Scarring
Surgical approaches to acne scarring in patients with SOC include elliptical excision, punch excision, punch elevation, punch autografting, dermal grafting, dermal planning, subcutaneous incision (subcision), dermabrasion, microneedling, fillers, and laser skin resurfacing. The treatment of choice depends on the size, type, and depth of the scar and the clinician’s preference.
Lasers
Fractional photothermolysis has emerged as a treatment option for acne scars in SOC patients. This procedure produces microscopic columns of thermal injury in the epidermis and dermis, sparing the surrounding tissue and minimizing downtime and adverse events. Because fractional photothermolysis does not target melanin and produces limited epidermal injury, darker Fitzpatrick skin types (IV–VI) can be safely and effectively treated with this procedure.15
Rosacea in SOC Patients
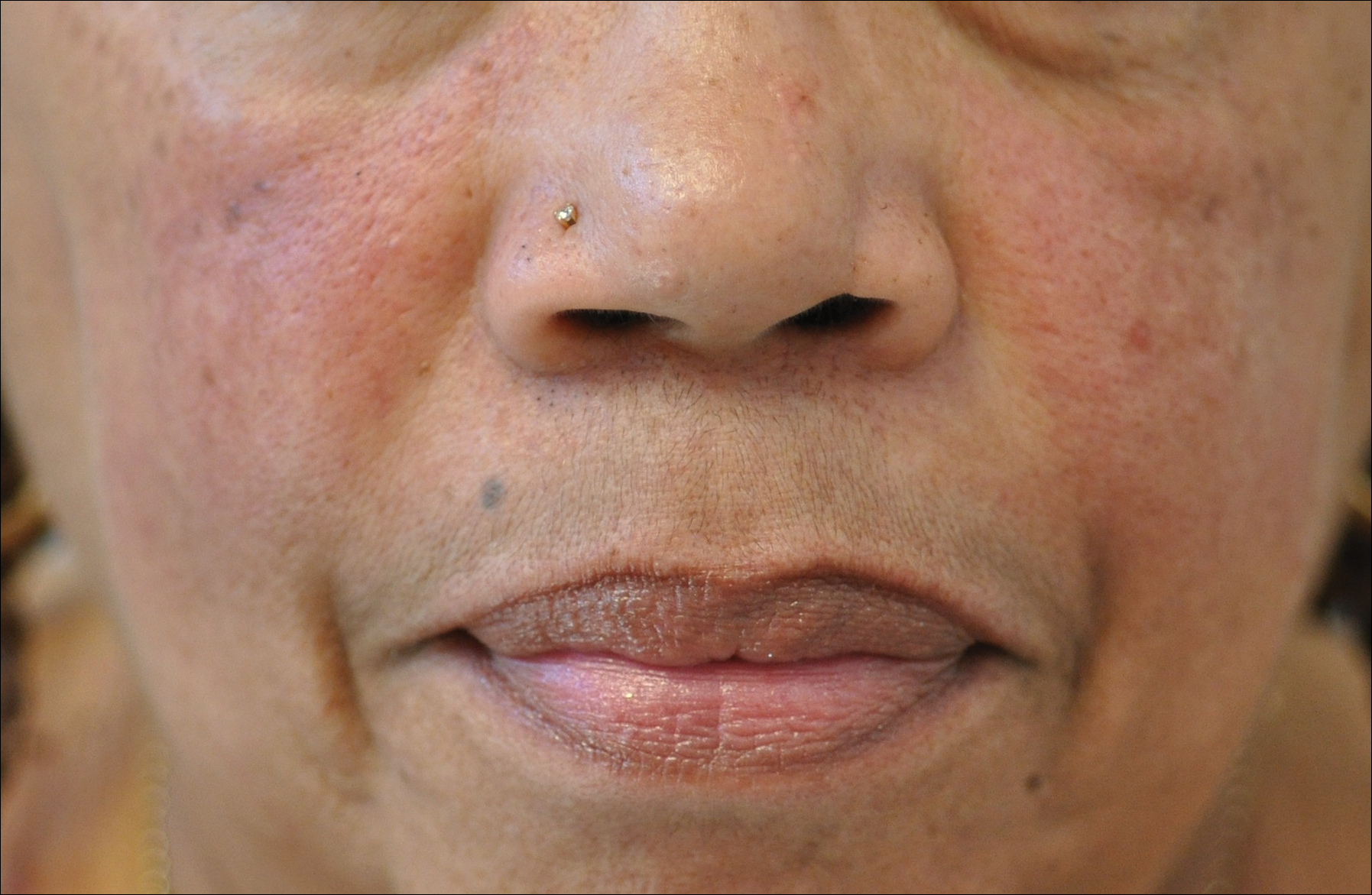
Rosacea is a chronic inflammatory disorder that affects the vasculature and pilosebaceous units of the face. It commonly is seen in Fitzpatrick skin types I and II; however, rosacea can occur in all skin types (Figure 2). Triggers include emotional stress, extreme environmental temperatures, hot and spicy foods, red wine or alcohol, and topical irritants or allergens found in common cosmetic products.16
Data suggest that 4% of rosacea patients in the United States are of African, Latino, or Asian descent.11 National Ambulatory Medical Care Survey data revealed that of 31.5 million rosacea visits, 2% of patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino. In a 5-year longitudinal study of 2587 rosacea patients enrolled in Medicaid in North Carolina who were prescribed at least 1 topical treatment for rosacea, 16.27% were black and 10% were of a race other than white.17
Although the pathogenesis of rosacea is unclear, hypotheses include immune system abnormalities, neurogenic dysregulation, presence of microorganisms (eg, Demodex folliculorum), UV damage, and skin barrier dysfunction.18
The 4 major subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea.16 Interestingly, rosacea in SOC patients may present with hypopigmentation surrounding the borders of the facial erythema. For phymatous rosacea, isotretinoin may reduce incipient rhinophyma but must be carefully monitored and pregnancy must be excluded. Surgical or laser therapy may be indicated to recontour the nose if severe.
There are several skin conditions that can present with facial erythema in patients with SOC, including seborrheic dermatitis, systemic lupus erythematosus, and contact dermatitis. It is important to note that the detection of facial erythema in darker skin types may be difficult; therefore, laboratory evaluation (antinuclear antibodies), patch testing, and skin biopsy should be considered if the clinical diagnosis is unclear.
Treatment
Treatment of rosacea in SOC patients does not differ from other racial groups. Common strategies include gentle skin care, sun protection (sun protection factor 30+), and barrier repair creams. Topical agents include metronidazole, AZA, sodium sulfacetamide/sulfur, ivermectin, and retinoids.16 Oral treatments include antibiotics in the tetracycline family (eg, subantimicrobial dose doxycycline) and isotretinoin.16 Persistent erythema associated with rosacea can be treated with brimonidine19 and oxymetazoline.20 Vascular lasers and intense pulsed light may be used to address the vascular components of rosacea21; however, the latter is not recommended in Fitzpatrick skin types IV through VI.
Facial Hyperpigmentation in SOC Patients
Hyperpigmentation disorders can be divided into conditions that affect Fitzpatrick skin types I through III and IV though VI. Mottled hyperpigmentation (photodamage) and solar lentigines occur in patients with lighter skin types as compared to melasma, PIH, and age-related (UV-induced) hyperpigmentation, which occur more commonly in patients with darker skin types. Facial hyperpigmentation is a common concern in SOC patients. In a survey of cosmetic concerns of 100 women with SOC, hyperpigmentation or dark spots (86%) and blotchy uneven skin (80%) were the top concerns.22 In addition, facial hyperpigmentation has been shown to negatively impact quality of life.23
Postinflammatory hyperpigmentation occurs from a pathophysiological response to inflammation, cutaneous irritation or injury, and subsequent melanocyte lability. Postinflammatory hyperpigmentation is a common presenting concern in patients with SOC and is seen as a result of many inflammatory skin disorders (eg, acne, eczema) and dermatologic procedures (eg, adverse reaction to electrodesiccation, microdermabrasion, chemical peels, laser surgery).24
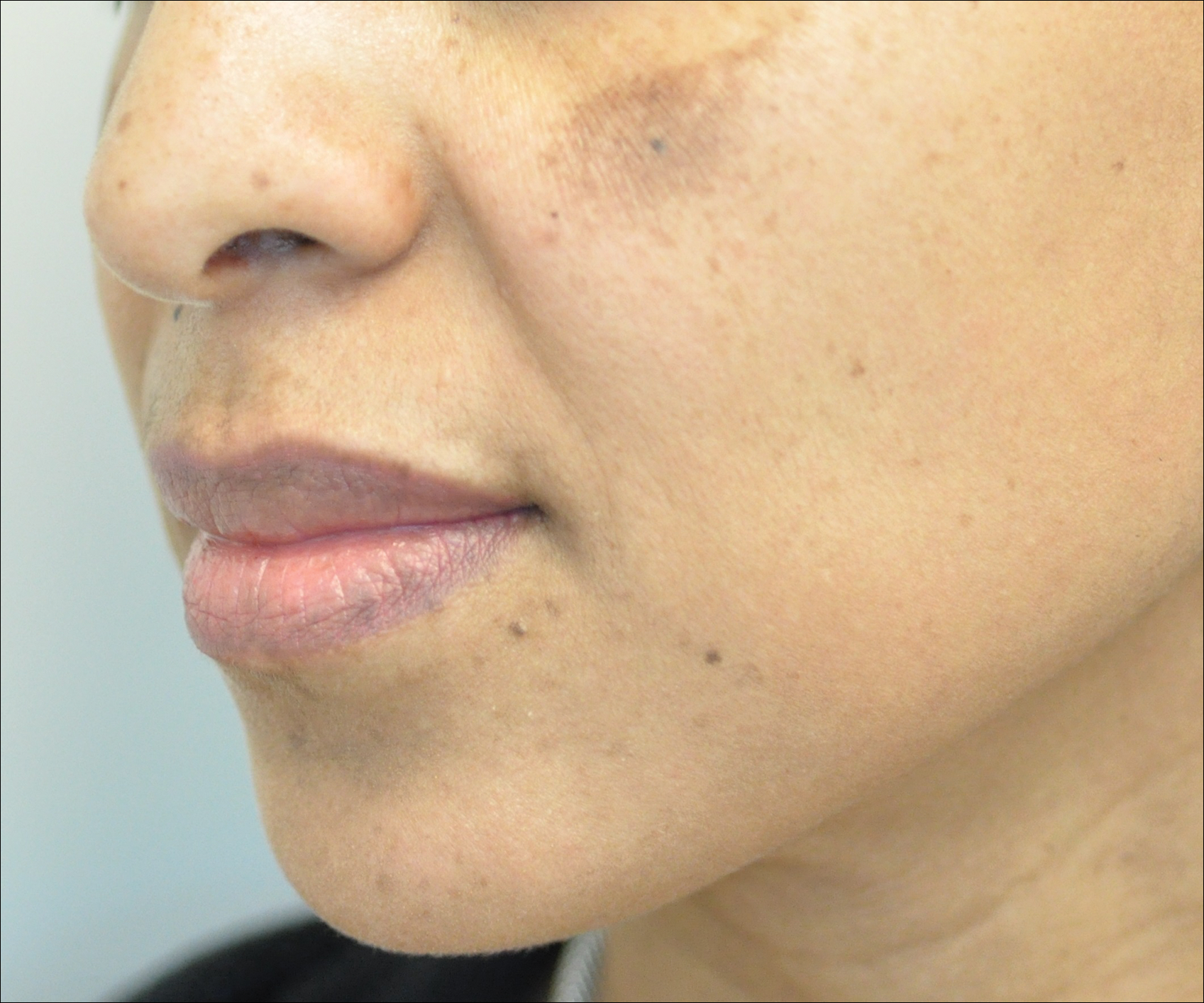
Melasma is an acquired idiopathic disorder of hyperpigmentation and often referred to as the mask of pregnancy (Figure 3). It occurs on sun-exposed areas of skin, mainly in women with Fitzpatrick skin types III through V. Associated factors or triggers include pregnancy, hormonal treatments, exposure to UV radiation, and medications.25 Hereditary factors play a role in more than 40% of cases.26
Other not-so-common facial dyschromias include contact dermatitis, acanthosis nigricans, exogenous ochronosis, lichen planus pigmentosus (associated with frontal fibrosing alopecia),27 drug-induced hyperpigmentation (associated with minocycline or diltiazem),28,29 and UV-induced (age-related) hyperpigmentation.
Treatment
The treatment of hyperpigmentation should provide the following: (1) protection from sun exposure; (2) inhibition of tyrosinase, the enzyme responsible for the conversion of tyrosine to melanin; (3) inhibition of melanosome transfer from the melanocyte to the keratinocyte; (4) removal of melanin from the epidermis through exfoliation; and (5) destruction or disruption of melanin in the dermis.30 Therapies for facial hyperpigmentation are listed in Table 1.

Topical therapies include prescription medications and nonprescription cosmeceuticals. Prescription medications include hydroquinone (HQ), topical retinoids, and AZA. Hydroquinone, a tyrosinase inhibitor, is the gold standard for skin lightening and often is used as a first-line therapy. It is used as a monotherapy (HQ 4%) or as a fixed combination with tretinoin 0.05% and fluocinolone 0.01%.31 Use caution with HQ in high concentrations (6% and higher) and low concentrations (2% [over-the-counter strength]) used long-term due to the potential risk of exogenous ochronosis.
Topical retinoids have been shown to be effective therapeutic agents for melasma and PIH. Tretinoin,32 tazarotene,33 and adapalene34 all have demonstrated efficacy for acne and acne-induced PIH in SOC patients. Patients must be monitored for the development of retinoid dermatitis and worsening of hyperpigmentation.
Azelaic acid is a naturally occurring dicarboxylic acid obtained from cultures of Malassezia furfur. Azelaic acid inhibits tyrosinase activity, DNA synthesis, and mitochondrial enzymes, thus blocking direct cytotoxic effects toward melanocytes. Azelaic acid is approved by the US Food and Drug Administration for acne in a 20% cream formulation and rosacea in 15% gel and foam formulations, and it is used off label for melasma and PIH.35
Oral tranexamic acid is currently used as a hemostatic agent due to its ability to inhibit the plasminogen-plasmin pathway. In melasma, it blocks the interaction between melanocytes and keratinocytes in the epidermis and modulates the vascular component of melasma in the dermis. In an open-label study, 561 Asian melasma patients were treated with oral tranexamic acid 250 mg twice daily for 4 months. Results demonstrated improvement in 90% of patients, and 7.1% reported adverse effects (eg, abdominal bloating and pain, nausea, vomiting, headache, tinnitus, numbness, menstrual irregularities).36 Coagulation screening should be monitored monthly, and any patient with a history of clotting abnormalities should be excluded from off-label treatment with oral tranexamic acid.
Nonprescription cosmeceuticals are available over-the-counter or are office dispensed.37 For optimal results, cosmeceutical agents for skin lightening are used in combination. Most of these combinations are HQ free and have additive benefits such as a multimodal skin lightening agent containing key ingredients that correct and prevent skin pigmentation via several pathways affecting melanogenesis.38 It is an excellent alternative to HQ for mottled and diffuse UV-induced hyperpigmentation and can be used for maintenance therapy in patients with melasma.
Photoprotection is an essential component of therapy for melasma and PIH, but there is a paucity of data on the benefits for SOC patients. Halder et al39 performed a randomized prospective study of 89 black and Hispanic patients who applied sunscreen with a sun protection factor of 30 or 60 daily for 8 weeks. Clinical grading, triplicate L*A*B chromameter, and clinical photography were taken at baseline and weeks 4 and 8. The results demonstrated skin lightening in both black and Hispanic patients and support the use of sunscreen in the prevention and management of dyschromia in SOC patients.39 Visible light also may play a role in melasma development, and thus use of sunscreens or makeup containing iron oxides are recommended.40
Procedural treatments for facial hyperpigmentation include microdermabrasion, chemical peels, lasers, energy-based devices, and microneedling. There are many types and formulations of chemical peeling agents available; however, superficial and medium-depth chemical peels are recommended for SOC patients (Table 2). Deep chemical peels are not recommended for SOC patients due to the potential increased risk for PIH and scarring.

Cosmetic Enhancement in SOC Patients
Cosmetic procedures are gaining popularity in the SOC population and account for more than 20% of cosmetic procedures in the United States.41 Facial cosmetic concerns in SOC include dyschromia, benign growths (dermatosis papulosa nigra), hyperkinetic facial lines, volume loss, and skin laxity.42 Key principles to consider when treating SOC patients are the impact of ethnicity on aging and facial structure, the patient’s desired cosmetic outcome, tissue reaction to anticipated treatments, and the patient’s expectations for recommended therapies.
Aging in SOC Patients
Skin aging can be classified as intrinsic aging or extrinsic aging. Intrinsic aging is genetic and involves subsurface changes such as volume loss, muscle atrophy, and resorption of bony structure. Extrinsic aging (or photoaging) involves surface changes of the epidermis/dermis and manifests as mottled pigmentation, textural changes, and fine wrinkling. Due to the photoprotection of melanin (black skin=SPF 13.4), skin aging in SOC patients is delayed by 10 to 20 years.43 In addition, SOC patients have more reactive collagen and can benefit from noninvasive cosmetic procedures such as fillers and skin-tightening procedures.42
Cosmetic Treatments and Procedures
Dermatosis papulosa nigra (benign growths of skin that have a genetic predisposition)44 occur mainly on the face but can involve the entire body. Treatment modalities include electrodesiccation, cryotherapy, scissor excision, and laser surgery.45
Treatment of hyperkinetic facial lines with botulinum toxin type A is a safe and effective procedure in patients with SOC. Grimes and Shabazz46 performed a 4-month, randomized, double-blind study that evaluated the treatment of glabellar lines in women with Fitzpatrick skin types V and VI. The results demonstrated that the duration of effects was the same in the patients who received either 20 or 30 U of botulinum toxin type A.46 Dynamic rhytides (furrows and frown/scowl lines arising from laughing, frowning, or smiling) can be treated safely in patients with SOC using botulinum toxin type A off label for relaxation of the upper and lower hyperkinetic muscles that result in these unwanted signs of aging. Botulinum toxin type A often is used for etched-in crow’s-feet, which rarely are evident in SOC patients.47 Facial shaping also can be accomplished by injecting botulinum toxin type A in combination with soft-tissue dermal fillers.47
Although black individuals do not experience perioral rhytides at the frequency of white individuals, they experience a variety of other cosmetic issues related to skin sagging and sinking. Currently available hyaluronic acid (HA) fillers have been shown to be safe in patients with Fitzpatrick skin types IV through VI.48 Two studies evaluated fillers in patients with SOC, specifically HA49 and calcium hydroxylapatite,50 focused on treatment of the nasolabial folds and the potential risk for dyspigmentation and keloidal scarring. Taylor et al49 noted that the risk of hyperpigmentation was 6% to 9% for large- and small-particle HA, respectively, and was associated with the serial or multiple puncture injection technique. No hypertrophic or keloidal scarring occurred in both studies.49,50
Facial contouring applications with fillers include glabellar lines, temples, nasal bridge, tear troughs, malar and submalar areas, nasolabial folds, radial lines, lips, marionette lines, mental crease, and chin. Hyaluronic acid fillers also can be used for lip enhancement.47 Although white women are looking to increase the size of their lips, black women are seeking augmentation to restore their lip size to that of their youth. Black individuals do not experience the same frequency of perioral rhytides as white patients, but they experience a variety of other issues related to skin sagging and sinking. Unlike white women, enhancement of the vermilion border rarely is performed in black women due to development of rhytides, predominantly in the body of the lip below the vermilion border in response to volume loss in the upper lip while the lower lip usually maintains its same appearance.47
Facial enhancement utilizing poly-L-lactic acid can be used safely in SOC patients.51 Poly-L-lactic acid microparticles induce collagen formation, leading to dermal thickening over 3 to 6 months; however, multiple sessions are required to achieve optimal aesthetic results.
Patients with more reactive collagen can benefit from noninvasive cosmetic procedures such as skin-tightening procedures.52 Radiofrequency and microfocused ultrasound are cosmetic procedures used to provide skin tightening and facial lifting. They are safe and effective treatments for patients with Fitzpatrick skin types IV to VI.53 Histologically, there is less thinning of collagen bundles and elastic tissue in ethnic skin. Due to stimulation of collagen by these procedures, most SOC patients will experience a more enhanced response, requiring fewer treatment sessions than white individuals.
Conclusion
Medical and aesthetic facial concerns in SOC patients vary and can be a source of emotional and psychological distress that can negatively impact quality of life. The approach to the treatment of SOC patients should be a balance between tolerability and efficacy, considering the potential risk for PIH.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2 pt 3):S34-S37.
- Halder RM, Grimes PE, McLaurin CL, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388, 390.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasians, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Halder RM, Holmes YC, Bridgeman-Shah S, et al. A clinicohistologic study of acne vulgaris in black females (abstract). J Invest Dermatol. 1996;106:888.
- Plewig G, Fulton JE, Kligman AM. Pomade acne. Arch Dermatol. 1970;101:580-584.
- Kligman AM, Mills OH. Acne cosmetica. Arch Dermatol. 1972;106:893-897.
- Halder RM, Brooks HL, Callender VD. Acne in ethnic skin. Dermatol Clin. 2003;21:609-615.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Winhoven SM. Postinflammatory hyperpigmentation in an Asian patient. a dramatic response to oral isotretinoin (13-cis-retinoic acid). Br J Med. 2005;152:368-403.
- Sarkar R, Bansal S, Garg VK. Chemical peels for melasma in dark-skinned patients. J Cutan Aesthet Surg. 2012;5:247-253.
- Alexis AF, Coley MK, Nijhawan RI, et al. Nonablative fractional laser resurfacing for acne scarring in patients with Fitzpatrick skin phototypes IV-VI. Dermatol Surg. 2016;42:392-402.
- Culp B, Scheinfeld N. Rosacea: a review. P T. 2009;34:38-45.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014:20. pii:13030/qt1mv9r0ss.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Jackson JM, Knuckles M, Minni JP, et al. The role of brimonidine tartrate gel in the treatment of rosacea. Clin Cosmet Investig Dermatol. 2015;23:529-538.
- Patel NU, Shukla S, Zaki J, et al. Oxymetazoline hydrochloride cream for facial erythema associated with rosacea. Expert Rev Clin Pharmacol. 2017;10:104954.
- Weinkle AP, Doktor V, Emer J. Update on the management of rosacea. Clin Cosmet Investig Dermatol. 2015;8:159-177.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;19:659-665.
- Taylor A, Pawaskar M, Taylor SL, et al. Prevalence of pigmentary disorders and their impact on quality of life: a prospective cohort study. J Cosmet Dermatol. 2008;7:164-168.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE. Melasma: etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453-1457.
- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Narang T, Sawatkar GU, Kumaran MS, et al. Minocycline for recurrent and/or chronic erythema nodosum leprosum. JAMA Dermatol. 2015;151:1026-1028.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10.
- Pandya AG, Guevara IL. Disorders of hyperpigmentation. Dermatol Clin. 2000;18:91-98.
- Taylor SC, Torok H, Jones T, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72:67-72.
- Bulengo-Ransby SM. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Jacyk WK. Adapalene in the treatment of African patients. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):37-42.
- Kircik LH. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of postinflammatory hyperpigmentation and acne: a 16-week, baseline-controlled study. J Drugs Dermatol. 2011;10:586-590.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma. J Am Acad Dermatol. 2016;75:385-392.
- Kindred C, Okereke U, Callender VD. Skin-lightening agents: an overview of prescription, office-dispensed, and over-the-counter products. Cosmet Dermatol. 2013;26:18-26.
- Makino ET, Kadoya K, Sigler ML, et al. Development and clinical assessment of a comprehensive product for pigmentation control in multiple ethnic populations. J Drugs Dermatol. 2016;15:1562-1570.
- Halder R, Rodney I, Munhutu M, et al. Evaluation and effectiveness of a photoprotection composition (sunscreen) on subjects of skin of color. J Am Acad Dermatol. 2015;72(suppl 1):AB215.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- American Society for Aesthetic Plastic Surgery. 2016 Cosmetic Surgery National Data Bank Statistics. https://www.surgery.org/sites/default/files/ASAPS-Stats2016.pdf. Accessed November 15, 2017.
- Burgess CM. Soft tissue augmentation in skin of color: market growth, available fillers and successful techniques. J Drugs Dermatol. 2007;6:51-55.
- Davis EC, Callender VD. Aesthetic dermatology for aging ethnic skin. Dermatol Surg. 2011;37:901-917.
- Grimes PE, Arora S, Minus HR, et al. Dermatosis papulosa nigra. Cutis. 1983;32:385-386.
- Lupo M. Dermatosis papulosa nigra: treatment options. J Drugs Dermatol. 2007;6:29-30.
- Grimes PE, Shabazz D. A four-month randomized, double-blind evaluation of the efficacy of botulinum toxin type A for the treatment of glabellar lines in women with skin types V and VI. Dermatol Surg. 2009;35:429-435.
- Burgess CM, Awosika O. Ethnic and gender considerations in the use of facial injectables: African-American patients. Plast Reconstr Surg. 2015;136(5 suppl):28S-31S.
- Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill Education; 2016.
- Taylor SC, Burgess CM, Callender VD. Safety of nonanimal stabilized hyaluronic acid dermal fillers in patients with skin of color: a randomized, evaluator-blinded comparative trial. Dermatol Surg. 2009;35(suppl 2):1653-1660.
- Marmur ES, Taylor SC, Grimes PE, et al. Six-month safety results of calcium hydroxylapatite for treatment of nasolabial folds in Fitzpatrick skin types IV to VI. Dermatol Surg. 2009;35(suppl 2):1641-1645.
- Hamilton TK, Burgess CM. Consideration for the use of injectable poly-L-lactic acid in people of color. J Drugs Dermatol. 2010;9:451-456.
- Fabi SG, Goldman MP. Retrospective evaluation of micro-focused ultrasound for lifting and tightening of the face and neck. Dermatol Surg. 2014;40:569-575.
- Harris MO, Sundaram HA. Safety of microfocused ultrasound with visualization in patients with Fitzpatrick skin phototypes III to VI. JAMA Facial Plast Surg. 2015;17:355-357.
The approach to the treatment of common skin disorders and cosmetic concerns in patients with skin of color (SOC) requires the clinician to understand the biological differences, nuances, and special considerations that are unique to patients with darker skin types.1-3 This article addresses 4 common facial concerns in SOC patients—acne, rosacea, facial hyperpigmentation, and cosmetic enhancement—and provides treatment recommendations and management pearls to assist the clinician with optimal outcomes for SOC patients.
Acne in SOC Patients
Acne vulgaris is one of the most common conditions that dermatologists treat and is estimated to affect 40 to 50 million individuals in the United States.1 Many of these acne patients are individuals with SOC.2-4 A study of 2835 females (aged 10–70 years) conducted in 4 different cities—Los Angeles, California; London, United Kingdom; Akita, Japan; and Rome, Italy—demonstrated acne prevalence of 37% in blacks, 32% in Hispanics, 30% in Asians, 24% in whites, and 23% in Continental Indians.5 Blacks, Hispanics, and Continental Indians demonstrated equal prevalence with comedonal and inflammatory acne. Asians displayed more inflammatory acne lesions than comedones. In contrast, whites demonstrated more comedones than inflammatory acne. Dyspigmentation, postinflammatory hyperpigmentation (PIH), and atrophic scars were more common in black and Hispanic females than other ethnicities.5 This study illustrated that acne-induced PIH is a common sequela in SOC patients and is the main reason they seek treatment.6,7
The pathogenesis of acne is the same in all racial and ethnic groups: (1) follicular hyperkeratinization and the formation of a microcomedone caused by abnormal desquamation of the keratinocytes within the sebaceous follicle, (2) production of sebum by circulating androgens, (3) proliferation of Propionibacterium acnes, and (4) inflammation. Subclinical inflammation is present throughout all stages of acne, including normal-appearing skin, inflammatory lesions, comedones, and scarring, and may contribute to PIH in acne patients with SOC (Figure 1).8 A thorough history should be obtained from acne patients, including answers to the following questions7:
- What skin and hair care products do you use?
- Do you use sunscreen daily?
- What cosmetic products or makeup do you use?
- Do you use any ethnic skin care products, including skin lightening creams?
- Do you have a history of keloids?

It is important to ask these questions to assess if the SOC patient has developed pomade acne,9 acne cosmetica,10 or a potential risk of skin irritation from the use of skin care practices. It is best to take total control of the patient’s skin care regimen and discontinue use of toners, astringents, witch hazel, exfoliants, and rubbing alcohol, which may lead to skin dryness and irritation, particularly when combined with topical acne medications.
Treatment
Treatment of acne in SOC patients is similar to generally recommended treatments, with special considerations. Consider the following key points when treating acne in SOC patients:
- Treat acne early and aggressively to prevent or minimize subsequent PIH and acne scarring.
- Balance aggressive treatment with nonirritating topical skin care.
- Most importantly, target PIH in addition to acne and choose a regimen that limits skin irritation that might exacerbate existing PIH.7
Develop a maintenance program to control future breakouts. Topical agents can be used as monotherapy or in fixed combinations and may include benzoyl peroxide, antibiotics, dapsone, azelaic acid (AZA), and retinoids. Similar to white patients, topical retinoids remain a first-line treatment for acne in patients with SOC.11,12
Tolerability must be managed in SOC acne patients. Therapeutic maneuvers that can be instituted should include a discussion on using gentle skin care, initiating therapy with a retinoid applied every other night starting with a low concentration and gradually titrating up, and applying a moisturizer before or after applying acne medication. Oral therapies consist of antibiotics (doxycycline, minocycline), retinoids (isotretinoin), and hormonal modulators (oral contraceptives, spironolactone). Isotretinoin, recommended for patients with nodulocystic acne, may play a possible role in treating acne-induced PIH.13
Two common procedural therapies for acne include comedone extraction and intralesional corticosteroid injection. A 6- to 8-week course of a topical retinoid prior to comedonal extraction may facilitate the procedure and is recommended in SOC patients to help reduce cutaneous trauma and PIH.11 Inflammatory acne lesions can be treated with intralesional injection of triamcinolone acetonide 2.5 or 5.0 mg/mL, which usually reduces inflammation within 2 to 5 days.11
Treatment of acne-induced PIH includes sun protection, topical and oral medications, chemical peels, lasers, and energy devices. Treatment of hypertrophic scarring and keloids involves intralesional injection of triamcinolone acetonide 20, 30, or 40 mg/mL every 4 weeks until the lesion is flat.11
Superficial chemical peels can be used to treat acne and PIH in SOC patients,14 such as salicylic acid (20%–30%), glycolic acid (20%–70%), trichloroacetic acid (15%–30%), and Jessner peels.
Acne Scarring
Surgical approaches to acne scarring in patients with SOC include elliptical excision, punch excision, punch elevation, punch autografting, dermal grafting, dermal planning, subcutaneous incision (subcision), dermabrasion, microneedling, fillers, and laser skin resurfacing. The treatment of choice depends on the size, type, and depth of the scar and the clinician’s preference.
Lasers
Fractional photothermolysis has emerged as a treatment option for acne scars in SOC patients. This procedure produces microscopic columns of thermal injury in the epidermis and dermis, sparing the surrounding tissue and minimizing downtime and adverse events. Because fractional photothermolysis does not target melanin and produces limited epidermal injury, darker Fitzpatrick skin types (IV–VI) can be safely and effectively treated with this procedure.15
Rosacea in SOC Patients
Rosacea is a chronic inflammatory disorder that affects the vasculature and pilosebaceous units of the face. It commonly is seen in Fitzpatrick skin types I and II; however, rosacea can occur in all skin types (Figure 2). Triggers include emotional stress, extreme environmental temperatures, hot and spicy foods, red wine or alcohol, and topical irritants or allergens found in common cosmetic products.16

Data suggest that 4% of rosacea patients in the United States are of African, Latino, or Asian descent.11 National Ambulatory Medical Care Survey data revealed that of 31.5 million rosacea visits, 2% of patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino. In a 5-year longitudinal study of 2587 rosacea patients enrolled in Medicaid in North Carolina who were prescribed at least 1 topical treatment for rosacea, 16.27% were black and 10% were of a race other than white.17
Although the pathogenesis of rosacea is unclear, hypotheses include immune system abnormalities, neurogenic dysregulation, presence of microorganisms (eg, Demodex folliculorum), UV damage, and skin barrier dysfunction.18
The 4 major subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea.16 Interestingly, rosacea in SOC patients may present with hypopigmentation surrounding the borders of the facial erythema. For phymatous rosacea, isotretinoin may reduce incipient rhinophyma but must be carefully monitored and pregnancy must be excluded. Surgical or laser therapy may be indicated to recontour the nose if severe.
There are several skin conditions that can present with facial erythema in patients with SOC, including seborrheic dermatitis, systemic lupus erythematosus, and contact dermatitis. It is important to note that the detection of facial erythema in darker skin types may be difficult; therefore, laboratory evaluation (antinuclear antibodies), patch testing, and skin biopsy should be considered if the clinical diagnosis is unclear.
Treatment
Treatment of rosacea in SOC patients does not differ from other racial groups. Common strategies include gentle skin care, sun protection (sun protection factor 30+), and barrier repair creams. Topical agents include metronidazole, AZA, sodium sulfacetamide/sulfur, ivermectin, and retinoids.16 Oral treatments include antibiotics in the tetracycline family (eg, subantimicrobial dose doxycycline) and isotretinoin.16 Persistent erythema associated with rosacea can be treated with brimonidine19 and oxymetazoline.20 Vascular lasers and intense pulsed light may be used to address the vascular components of rosacea21; however, the latter is not recommended in Fitzpatrick skin types IV through VI.
Facial Hyperpigmentation in SOC Patients
Hyperpigmentation disorders can be divided into conditions that affect Fitzpatrick skin types I through III and IV though VI. Mottled hyperpigmentation (photodamage) and solar lentigines occur in patients with lighter skin types as compared to melasma, PIH, and age-related (UV-induced) hyperpigmentation, which occur more commonly in patients with darker skin types. Facial hyperpigmentation is a common concern in SOC patients. In a survey of cosmetic concerns of 100 women with SOC, hyperpigmentation or dark spots (86%) and blotchy uneven skin (80%) were the top concerns.22 In addition, facial hyperpigmentation has been shown to negatively impact quality of life.23
Postinflammatory hyperpigmentation occurs from a pathophysiological response to inflammation, cutaneous irritation or injury, and subsequent melanocyte lability. Postinflammatory hyperpigmentation is a common presenting concern in patients with SOC and is seen as a result of many inflammatory skin disorders (eg, acne, eczema) and dermatologic procedures (eg, adverse reaction to electrodesiccation, microdermabrasion, chemical peels, laser surgery).24
Melasma is an acquired idiopathic disorder of hyperpigmentation and often referred to as the mask of pregnancy (Figure 3). It occurs on sun-exposed areas of skin, mainly in women with Fitzpatrick skin types III through V. Associated factors or triggers include pregnancy, hormonal treatments, exposure to UV radiation, and medications.25 Hereditary factors play a role in more than 40% of cases.26

Other not-so-common facial dyschromias include contact dermatitis, acanthosis nigricans, exogenous ochronosis, lichen planus pigmentosus (associated with frontal fibrosing alopecia),27 drug-induced hyperpigmentation (associated with minocycline or diltiazem),28,29 and UV-induced (age-related) hyperpigmentation.
Treatment
The treatment of hyperpigmentation should provide the following: (1) protection from sun exposure; (2) inhibition of tyrosinase, the enzyme responsible for the conversion of tyrosine to melanin; (3) inhibition of melanosome transfer from the melanocyte to the keratinocyte; (4) removal of melanin from the epidermis through exfoliation; and (5) destruction or disruption of melanin in the dermis.30 Therapies for facial hyperpigmentation are listed in Table 1.

Topical therapies include prescription medications and nonprescription cosmeceuticals. Prescription medications include hydroquinone (HQ), topical retinoids, and AZA. Hydroquinone, a tyrosinase inhibitor, is the gold standard for skin lightening and often is used as a first-line therapy. It is used as a monotherapy (HQ 4%) or as a fixed combination with tretinoin 0.05% and fluocinolone 0.01%.31 Use caution with HQ in high concentrations (6% and higher) and low concentrations (2% [over-the-counter strength]) used long-term due to the potential risk of exogenous ochronosis.
Topical retinoids have been shown to be effective therapeutic agents for melasma and PIH. Tretinoin,32 tazarotene,33 and adapalene34 all have demonstrated efficacy for acne and acne-induced PIH in SOC patients. Patients must be monitored for the development of retinoid dermatitis and worsening of hyperpigmentation.
Azelaic acid is a naturally occurring dicarboxylic acid obtained from cultures of Malassezia furfur. Azelaic acid inhibits tyrosinase activity, DNA synthesis, and mitochondrial enzymes, thus blocking direct cytotoxic effects toward melanocytes. Azelaic acid is approved by the US Food and Drug Administration for acne in a 20% cream formulation and rosacea in 15% gel and foam formulations, and it is used off label for melasma and PIH.35
Oral tranexamic acid is currently used as a hemostatic agent due to its ability to inhibit the plasminogen-plasmin pathway. In melasma, it blocks the interaction between melanocytes and keratinocytes in the epidermis and modulates the vascular component of melasma in the dermis. In an open-label study, 561 Asian melasma patients were treated with oral tranexamic acid 250 mg twice daily for 4 months. Results demonstrated improvement in 90% of patients, and 7.1% reported adverse effects (eg, abdominal bloating and pain, nausea, vomiting, headache, tinnitus, numbness, menstrual irregularities).36 Coagulation screening should be monitored monthly, and any patient with a history of clotting abnormalities should be excluded from off-label treatment with oral tranexamic acid.
Nonprescription cosmeceuticals are available over-the-counter or are office dispensed.37 For optimal results, cosmeceutical agents for skin lightening are used in combination. Most of these combinations are HQ free and have additive benefits such as a multimodal skin lightening agent containing key ingredients that correct and prevent skin pigmentation via several pathways affecting melanogenesis.38 It is an excellent alternative to HQ for mottled and diffuse UV-induced hyperpigmentation and can be used for maintenance therapy in patients with melasma.
Photoprotection is an essential component of therapy for melasma and PIH, but there is a paucity of data on the benefits for SOC patients. Halder et al39 performed a randomized prospective study of 89 black and Hispanic patients who applied sunscreen with a sun protection factor of 30 or 60 daily for 8 weeks. Clinical grading, triplicate L*A*B chromameter, and clinical photography were taken at baseline and weeks 4 and 8. The results demonstrated skin lightening in both black and Hispanic patients and support the use of sunscreen in the prevention and management of dyschromia in SOC patients.39 Visible light also may play a role in melasma development, and thus use of sunscreens or makeup containing iron oxides are recommended.40
Procedural treatments for facial hyperpigmentation include microdermabrasion, chemical peels, lasers, energy-based devices, and microneedling. There are many types and formulations of chemical peeling agents available; however, superficial and medium-depth chemical peels are recommended for SOC patients (Table 2). Deep chemical peels are not recommended for SOC patients due to the potential increased risk for PIH and scarring.

Cosmetic Enhancement in SOC Patients
Cosmetic procedures are gaining popularity in the SOC population and account for more than 20% of cosmetic procedures in the United States.41 Facial cosmetic concerns in SOC include dyschromia, benign growths (dermatosis papulosa nigra), hyperkinetic facial lines, volume loss, and skin laxity.42 Key principles to consider when treating SOC patients are the impact of ethnicity on aging and facial structure, the patient’s desired cosmetic outcome, tissue reaction to anticipated treatments, and the patient’s expectations for recommended therapies.
Aging in SOC Patients
Skin aging can be classified as intrinsic aging or extrinsic aging. Intrinsic aging is genetic and involves subsurface changes such as volume loss, muscle atrophy, and resorption of bony structure. Extrinsic aging (or photoaging) involves surface changes of the epidermis/dermis and manifests as mottled pigmentation, textural changes, and fine wrinkling. Due to the photoprotection of melanin (black skin=SPF 13.4), skin aging in SOC patients is delayed by 10 to 20 years.43 In addition, SOC patients have more reactive collagen and can benefit from noninvasive cosmetic procedures such as fillers and skin-tightening procedures.42
Cosmetic Treatments and Procedures
Dermatosis papulosa nigra (benign growths of skin that have a genetic predisposition)44 occur mainly on the face but can involve the entire body. Treatment modalities include electrodesiccation, cryotherapy, scissor excision, and laser surgery.45
Treatment of hyperkinetic facial lines with botulinum toxin type A is a safe and effective procedure in patients with SOC. Grimes and Shabazz46 performed a 4-month, randomized, double-blind study that evaluated the treatment of glabellar lines in women with Fitzpatrick skin types V and VI. The results demonstrated that the duration of effects was the same in the patients who received either 20 or 30 U of botulinum toxin type A.46 Dynamic rhytides (furrows and frown/scowl lines arising from laughing, frowning, or smiling) can be treated safely in patients with SOC using botulinum toxin type A off label for relaxation of the upper and lower hyperkinetic muscles that result in these unwanted signs of aging. Botulinum toxin type A often is used for etched-in crow’s-feet, which rarely are evident in SOC patients.47 Facial shaping also can be accomplished by injecting botulinum toxin type A in combination with soft-tissue dermal fillers.47
Although black individuals do not experience perioral rhytides at the frequency of white individuals, they experience a variety of other cosmetic issues related to skin sagging and sinking. Currently available hyaluronic acid (HA) fillers have been shown to be safe in patients with Fitzpatrick skin types IV through VI.48 Two studies evaluated fillers in patients with SOC, specifically HA49 and calcium hydroxylapatite,50 focused on treatment of the nasolabial folds and the potential risk for dyspigmentation and keloidal scarring. Taylor et al49 noted that the risk of hyperpigmentation was 6% to 9% for large- and small-particle HA, respectively, and was associated with the serial or multiple puncture injection technique. No hypertrophic or keloidal scarring occurred in both studies.49,50
Facial contouring applications with fillers include glabellar lines, temples, nasal bridge, tear troughs, malar and submalar areas, nasolabial folds, radial lines, lips, marionette lines, mental crease, and chin. Hyaluronic acid fillers also can be used for lip enhancement.47 Although white women are looking to increase the size of their lips, black women are seeking augmentation to restore their lip size to that of their youth. Black individuals do not experience the same frequency of perioral rhytides as white patients, but they experience a variety of other issues related to skin sagging and sinking. Unlike white women, enhancement of the vermilion border rarely is performed in black women due to development of rhytides, predominantly in the body of the lip below the vermilion border in response to volume loss in the upper lip while the lower lip usually maintains its same appearance.47
Facial enhancement utilizing poly-L-lactic acid can be used safely in SOC patients.51 Poly-L-lactic acid microparticles induce collagen formation, leading to dermal thickening over 3 to 6 months; however, multiple sessions are required to achieve optimal aesthetic results.
Patients with more reactive collagen can benefit from noninvasive cosmetic procedures such as skin-tightening procedures.52 Radiofrequency and microfocused ultrasound are cosmetic procedures used to provide skin tightening and facial lifting. They are safe and effective treatments for patients with Fitzpatrick skin types IV to VI.53 Histologically, there is less thinning of collagen bundles and elastic tissue in ethnic skin. Due to stimulation of collagen by these procedures, most SOC patients will experience a more enhanced response, requiring fewer treatment sessions than white individuals.
Conclusion
Medical and aesthetic facial concerns in SOC patients vary and can be a source of emotional and psychological distress that can negatively impact quality of life. The approach to the treatment of SOC patients should be a balance between tolerability and efficacy, considering the potential risk for PIH.
The approach to the treatment of common skin disorders and cosmetic concerns in patients with skin of color (SOC) requires the clinician to understand the biological differences, nuances, and special considerations that are unique to patients with darker skin types.1-3 This article addresses 4 common facial concerns in SOC patients—acne, rosacea, facial hyperpigmentation, and cosmetic enhancement—and provides treatment recommendations and management pearls to assist the clinician with optimal outcomes for SOC patients.
Acne in SOC Patients
Acne vulgaris is one of the most common conditions that dermatologists treat and is estimated to affect 40 to 50 million individuals in the United States.1 Many of these acne patients are individuals with SOC.2-4 A study of 2835 females (aged 10–70 years) conducted in 4 different cities—Los Angeles, California; London, United Kingdom; Akita, Japan; and Rome, Italy—demonstrated acne prevalence of 37% in blacks, 32% in Hispanics, 30% in Asians, 24% in whites, and 23% in Continental Indians.5 Blacks, Hispanics, and Continental Indians demonstrated equal prevalence with comedonal and inflammatory acne. Asians displayed more inflammatory acne lesions than comedones. In contrast, whites demonstrated more comedones than inflammatory acne. Dyspigmentation, postinflammatory hyperpigmentation (PIH), and atrophic scars were more common in black and Hispanic females than other ethnicities.5 This study illustrated that acne-induced PIH is a common sequela in SOC patients and is the main reason they seek treatment.6,7
The pathogenesis of acne is the same in all racial and ethnic groups: (1) follicular hyperkeratinization and the formation of a microcomedone caused by abnormal desquamation of the keratinocytes within the sebaceous follicle, (2) production of sebum by circulating androgens, (3) proliferation of Propionibacterium acnes, and (4) inflammation. Subclinical inflammation is present throughout all stages of acne, including normal-appearing skin, inflammatory lesions, comedones, and scarring, and may contribute to PIH in acne patients with SOC (Figure 1).8 A thorough history should be obtained from acne patients, including answers to the following questions7:
- What skin and hair care products do you use?
- Do you use sunscreen daily?
- What cosmetic products or makeup do you use?
- Do you use any ethnic skin care products, including skin lightening creams?
- Do you have a history of keloids?

It is important to ask these questions to assess if the SOC patient has developed pomade acne,9 acne cosmetica,10 or a potential risk of skin irritation from the use of skin care practices. It is best to take total control of the patient’s skin care regimen and discontinue use of toners, astringents, witch hazel, exfoliants, and rubbing alcohol, which may lead to skin dryness and irritation, particularly when combined with topical acne medications.
Treatment
Treatment of acne in SOC patients is similar to generally recommended treatments, with special considerations. Consider the following key points when treating acne in SOC patients:
- Treat acne early and aggressively to prevent or minimize subsequent PIH and acne scarring.
- Balance aggressive treatment with nonirritating topical skin care.
- Most importantly, target PIH in addition to acne and choose a regimen that limits skin irritation that might exacerbate existing PIH.7
Develop a maintenance program to control future breakouts. Topical agents can be used as monotherapy or in fixed combinations and may include benzoyl peroxide, antibiotics, dapsone, azelaic acid (AZA), and retinoids. Similar to white patients, topical retinoids remain a first-line treatment for acne in patients with SOC.11,12
Tolerability must be managed in SOC acne patients. Therapeutic maneuvers that can be instituted should include a discussion on using gentle skin care, initiating therapy with a retinoid applied every other night starting with a low concentration and gradually titrating up, and applying a moisturizer before or after applying acne medication. Oral therapies consist of antibiotics (doxycycline, minocycline), retinoids (isotretinoin), and hormonal modulators (oral contraceptives, spironolactone). Isotretinoin, recommended for patients with nodulocystic acne, may play a possible role in treating acne-induced PIH.13
Two common procedural therapies for acne include comedone extraction and intralesional corticosteroid injection. A 6- to 8-week course of a topical retinoid prior to comedonal extraction may facilitate the procedure and is recommended in SOC patients to help reduce cutaneous trauma and PIH.11 Inflammatory acne lesions can be treated with intralesional injection of triamcinolone acetonide 2.5 or 5.0 mg/mL, which usually reduces inflammation within 2 to 5 days.11
Treatment of acne-induced PIH includes sun protection, topical and oral medications, chemical peels, lasers, and energy devices. Treatment of hypertrophic scarring and keloids involves intralesional injection of triamcinolone acetonide 20, 30, or 40 mg/mL every 4 weeks until the lesion is flat.11
Superficial chemical peels can be used to treat acne and PIH in SOC patients,14 such as salicylic acid (20%–30%), glycolic acid (20%–70%), trichloroacetic acid (15%–30%), and Jessner peels.
Acne Scarring
Surgical approaches to acne scarring in patients with SOC include elliptical excision, punch excision, punch elevation, punch autografting, dermal grafting, dermal planning, subcutaneous incision (subcision), dermabrasion, microneedling, fillers, and laser skin resurfacing. The treatment of choice depends on the size, type, and depth of the scar and the clinician’s preference.
Lasers
Fractional photothermolysis has emerged as a treatment option for acne scars in SOC patients. This procedure produces microscopic columns of thermal injury in the epidermis and dermis, sparing the surrounding tissue and minimizing downtime and adverse events. Because fractional photothermolysis does not target melanin and produces limited epidermal injury, darker Fitzpatrick skin types (IV–VI) can be safely and effectively treated with this procedure.15
Rosacea in SOC Patients
Rosacea is a chronic inflammatory disorder that affects the vasculature and pilosebaceous units of the face. It commonly is seen in Fitzpatrick skin types I and II; however, rosacea can occur in all skin types (Figure 2). Triggers include emotional stress, extreme environmental temperatures, hot and spicy foods, red wine or alcohol, and topical irritants or allergens found in common cosmetic products.16

Data suggest that 4% of rosacea patients in the United States are of African, Latino, or Asian descent.11 National Ambulatory Medical Care Survey data revealed that of 31.5 million rosacea visits, 2% of patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino. In a 5-year longitudinal study of 2587 rosacea patients enrolled in Medicaid in North Carolina who were prescribed at least 1 topical treatment for rosacea, 16.27% were black and 10% were of a race other than white.17
Although the pathogenesis of rosacea is unclear, hypotheses include immune system abnormalities, neurogenic dysregulation, presence of microorganisms (eg, Demodex folliculorum), UV damage, and skin barrier dysfunction.18
The 4 major subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea.16 Interestingly, rosacea in SOC patients may present with hypopigmentation surrounding the borders of the facial erythema. For phymatous rosacea, isotretinoin may reduce incipient rhinophyma but must be carefully monitored and pregnancy must be excluded. Surgical or laser therapy may be indicated to recontour the nose if severe.
There are several skin conditions that can present with facial erythema in patients with SOC, including seborrheic dermatitis, systemic lupus erythematosus, and contact dermatitis. It is important to note that the detection of facial erythema in darker skin types may be difficult; therefore, laboratory evaluation (antinuclear antibodies), patch testing, and skin biopsy should be considered if the clinical diagnosis is unclear.
Treatment
Treatment of rosacea in SOC patients does not differ from other racial groups. Common strategies include gentle skin care, sun protection (sun protection factor 30+), and barrier repair creams. Topical agents include metronidazole, AZA, sodium sulfacetamide/sulfur, ivermectin, and retinoids.16 Oral treatments include antibiotics in the tetracycline family (eg, subantimicrobial dose doxycycline) and isotretinoin.16 Persistent erythema associated with rosacea can be treated with brimonidine19 and oxymetazoline.20 Vascular lasers and intense pulsed light may be used to address the vascular components of rosacea21; however, the latter is not recommended in Fitzpatrick skin types IV through VI.
Facial Hyperpigmentation in SOC Patients
Hyperpigmentation disorders can be divided into conditions that affect Fitzpatrick skin types I through III and IV though VI. Mottled hyperpigmentation (photodamage) and solar lentigines occur in patients with lighter skin types as compared to melasma, PIH, and age-related (UV-induced) hyperpigmentation, which occur more commonly in patients with darker skin types. Facial hyperpigmentation is a common concern in SOC patients. In a survey of cosmetic concerns of 100 women with SOC, hyperpigmentation or dark spots (86%) and blotchy uneven skin (80%) were the top concerns.22 In addition, facial hyperpigmentation has been shown to negatively impact quality of life.23
Postinflammatory hyperpigmentation occurs from a pathophysiological response to inflammation, cutaneous irritation or injury, and subsequent melanocyte lability. Postinflammatory hyperpigmentation is a common presenting concern in patients with SOC and is seen as a result of many inflammatory skin disorders (eg, acne, eczema) and dermatologic procedures (eg, adverse reaction to electrodesiccation, microdermabrasion, chemical peels, laser surgery).24
Melasma is an acquired idiopathic disorder of hyperpigmentation and often referred to as the mask of pregnancy (Figure 3). It occurs on sun-exposed areas of skin, mainly in women with Fitzpatrick skin types III through V. Associated factors or triggers include pregnancy, hormonal treatments, exposure to UV radiation, and medications.25 Hereditary factors play a role in more than 40% of cases.26

Other not-so-common facial dyschromias include contact dermatitis, acanthosis nigricans, exogenous ochronosis, lichen planus pigmentosus (associated with frontal fibrosing alopecia),27 drug-induced hyperpigmentation (associated with minocycline or diltiazem),28,29 and UV-induced (age-related) hyperpigmentation.
Treatment
The treatment of hyperpigmentation should provide the following: (1) protection from sun exposure; (2) inhibition of tyrosinase, the enzyme responsible for the conversion of tyrosine to melanin; (3) inhibition of melanosome transfer from the melanocyte to the keratinocyte; (4) removal of melanin from the epidermis through exfoliation; and (5) destruction or disruption of melanin in the dermis.30 Therapies for facial hyperpigmentation are listed in Table 1.

Topical therapies include prescription medications and nonprescription cosmeceuticals. Prescription medications include hydroquinone (HQ), topical retinoids, and AZA. Hydroquinone, a tyrosinase inhibitor, is the gold standard for skin lightening and often is used as a first-line therapy. It is used as a monotherapy (HQ 4%) or as a fixed combination with tretinoin 0.05% and fluocinolone 0.01%.31 Use caution with HQ in high concentrations (6% and higher) and low concentrations (2% [over-the-counter strength]) used long-term due to the potential risk of exogenous ochronosis.
Topical retinoids have been shown to be effective therapeutic agents for melasma and PIH. Tretinoin,32 tazarotene,33 and adapalene34 all have demonstrated efficacy for acne and acne-induced PIH in SOC patients. Patients must be monitored for the development of retinoid dermatitis and worsening of hyperpigmentation.
Azelaic acid is a naturally occurring dicarboxylic acid obtained from cultures of Malassezia furfur. Azelaic acid inhibits tyrosinase activity, DNA synthesis, and mitochondrial enzymes, thus blocking direct cytotoxic effects toward melanocytes. Azelaic acid is approved by the US Food and Drug Administration for acne in a 20% cream formulation and rosacea in 15% gel and foam formulations, and it is used off label for melasma and PIH.35
Oral tranexamic acid is currently used as a hemostatic agent due to its ability to inhibit the plasminogen-plasmin pathway. In melasma, it blocks the interaction between melanocytes and keratinocytes in the epidermis and modulates the vascular component of melasma in the dermis. In an open-label study, 561 Asian melasma patients were treated with oral tranexamic acid 250 mg twice daily for 4 months. Results demonstrated improvement in 90% of patients, and 7.1% reported adverse effects (eg, abdominal bloating and pain, nausea, vomiting, headache, tinnitus, numbness, menstrual irregularities).36 Coagulation screening should be monitored monthly, and any patient with a history of clotting abnormalities should be excluded from off-label treatment with oral tranexamic acid.
Nonprescription cosmeceuticals are available over-the-counter or are office dispensed.37 For optimal results, cosmeceutical agents for skin lightening are used in combination. Most of these combinations are HQ free and have additive benefits such as a multimodal skin lightening agent containing key ingredients that correct and prevent skin pigmentation via several pathways affecting melanogenesis.38 It is an excellent alternative to HQ for mottled and diffuse UV-induced hyperpigmentation and can be used for maintenance therapy in patients with melasma.
Photoprotection is an essential component of therapy for melasma and PIH, but there is a paucity of data on the benefits for SOC patients. Halder et al39 performed a randomized prospective study of 89 black and Hispanic patients who applied sunscreen with a sun protection factor of 30 or 60 daily for 8 weeks. Clinical grading, triplicate L*A*B chromameter, and clinical photography were taken at baseline and weeks 4 and 8. The results demonstrated skin lightening in both black and Hispanic patients and support the use of sunscreen in the prevention and management of dyschromia in SOC patients.39 Visible light also may play a role in melasma development, and thus use of sunscreens or makeup containing iron oxides are recommended.40
Procedural treatments for facial hyperpigmentation include microdermabrasion, chemical peels, lasers, energy-based devices, and microneedling. There are many types and formulations of chemical peeling agents available; however, superficial and medium-depth chemical peels are recommended for SOC patients (Table 2). Deep chemical peels are not recommended for SOC patients due to the potential increased risk for PIH and scarring.

Cosmetic Enhancement in SOC Patients
Cosmetic procedures are gaining popularity in the SOC population and account for more than 20% of cosmetic procedures in the United States.41 Facial cosmetic concerns in SOC include dyschromia, benign growths (dermatosis papulosa nigra), hyperkinetic facial lines, volume loss, and skin laxity.42 Key principles to consider when treating SOC patients are the impact of ethnicity on aging and facial structure, the patient’s desired cosmetic outcome, tissue reaction to anticipated treatments, and the patient’s expectations for recommended therapies.
Aging in SOC Patients
Skin aging can be classified as intrinsic aging or extrinsic aging. Intrinsic aging is genetic and involves subsurface changes such as volume loss, muscle atrophy, and resorption of bony structure. Extrinsic aging (or photoaging) involves surface changes of the epidermis/dermis and manifests as mottled pigmentation, textural changes, and fine wrinkling. Due to the photoprotection of melanin (black skin=SPF 13.4), skin aging in SOC patients is delayed by 10 to 20 years.43 In addition, SOC patients have more reactive collagen and can benefit from noninvasive cosmetic procedures such as fillers and skin-tightening procedures.42
Cosmetic Treatments and Procedures
Dermatosis papulosa nigra (benign growths of skin that have a genetic predisposition)44 occur mainly on the face but can involve the entire body. Treatment modalities include electrodesiccation, cryotherapy, scissor excision, and laser surgery.45
Treatment of hyperkinetic facial lines with botulinum toxin type A is a safe and effective procedure in patients with SOC. Grimes and Shabazz46 performed a 4-month, randomized, double-blind study that evaluated the treatment of glabellar lines in women with Fitzpatrick skin types V and VI. The results demonstrated that the duration of effects was the same in the patients who received either 20 or 30 U of botulinum toxin type A.46 Dynamic rhytides (furrows and frown/scowl lines arising from laughing, frowning, or smiling) can be treated safely in patients with SOC using botulinum toxin type A off label for relaxation of the upper and lower hyperkinetic muscles that result in these unwanted signs of aging. Botulinum toxin type A often is used for etched-in crow’s-feet, which rarely are evident in SOC patients.47 Facial shaping also can be accomplished by injecting botulinum toxin type A in combination with soft-tissue dermal fillers.47
Although black individuals do not experience perioral rhytides at the frequency of white individuals, they experience a variety of other cosmetic issues related to skin sagging and sinking. Currently available hyaluronic acid (HA) fillers have been shown to be safe in patients with Fitzpatrick skin types IV through VI.48 Two studies evaluated fillers in patients with SOC, specifically HA49 and calcium hydroxylapatite,50 focused on treatment of the nasolabial folds and the potential risk for dyspigmentation and keloidal scarring. Taylor et al49 noted that the risk of hyperpigmentation was 6% to 9% for large- and small-particle HA, respectively, and was associated with the serial or multiple puncture injection technique. No hypertrophic or keloidal scarring occurred in both studies.49,50
Facial contouring applications with fillers include glabellar lines, temples, nasal bridge, tear troughs, malar and submalar areas, nasolabial folds, radial lines, lips, marionette lines, mental crease, and chin. Hyaluronic acid fillers also can be used for lip enhancement.47 Although white women are looking to increase the size of their lips, black women are seeking augmentation to restore their lip size to that of their youth. Black individuals do not experience the same frequency of perioral rhytides as white patients, but they experience a variety of other issues related to skin sagging and sinking. Unlike white women, enhancement of the vermilion border rarely is performed in black women due to development of rhytides, predominantly in the body of the lip below the vermilion border in response to volume loss in the upper lip while the lower lip usually maintains its same appearance.47
Facial enhancement utilizing poly-L-lactic acid can be used safely in SOC patients.51 Poly-L-lactic acid microparticles induce collagen formation, leading to dermal thickening over 3 to 6 months; however, multiple sessions are required to achieve optimal aesthetic results.
Patients with more reactive collagen can benefit from noninvasive cosmetic procedures such as skin-tightening procedures.52 Radiofrequency and microfocused ultrasound are cosmetic procedures used to provide skin tightening and facial lifting. They are safe and effective treatments for patients with Fitzpatrick skin types IV to VI.53 Histologically, there is less thinning of collagen bundles and elastic tissue in ethnic skin. Due to stimulation of collagen by these procedures, most SOC patients will experience a more enhanced response, requiring fewer treatment sessions than white individuals.
Conclusion
Medical and aesthetic facial concerns in SOC patients vary and can be a source of emotional and psychological distress that can negatively impact quality of life. The approach to the treatment of SOC patients should be a balance between tolerability and efficacy, considering the potential risk for PIH.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2 pt 3):S34-S37.
- Halder RM, Grimes PE, McLaurin CL, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388, 390.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasians, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Halder RM, Holmes YC, Bridgeman-Shah S, et al. A clinicohistologic study of acne vulgaris in black females (abstract). J Invest Dermatol. 1996;106:888.
- Plewig G, Fulton JE, Kligman AM. Pomade acne. Arch Dermatol. 1970;101:580-584.
- Kligman AM, Mills OH. Acne cosmetica. Arch Dermatol. 1972;106:893-897.
- Halder RM, Brooks HL, Callender VD. Acne in ethnic skin. Dermatol Clin. 2003;21:609-615.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Winhoven SM. Postinflammatory hyperpigmentation in an Asian patient. a dramatic response to oral isotretinoin (13-cis-retinoic acid). Br J Med. 2005;152:368-403.
- Sarkar R, Bansal S, Garg VK. Chemical peels for melasma in dark-skinned patients. J Cutan Aesthet Surg. 2012;5:247-253.
- Alexis AF, Coley MK, Nijhawan RI, et al. Nonablative fractional laser resurfacing for acne scarring in patients with Fitzpatrick skin phototypes IV-VI. Dermatol Surg. 2016;42:392-402.
- Culp B, Scheinfeld N. Rosacea: a review. P T. 2009;34:38-45.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014:20. pii:13030/qt1mv9r0ss.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Jackson JM, Knuckles M, Minni JP, et al. The role of brimonidine tartrate gel in the treatment of rosacea. Clin Cosmet Investig Dermatol. 2015;23:529-538.
- Patel NU, Shukla S, Zaki J, et al. Oxymetazoline hydrochloride cream for facial erythema associated with rosacea. Expert Rev Clin Pharmacol. 2017;10:104954.
- Weinkle AP, Doktor V, Emer J. Update on the management of rosacea. Clin Cosmet Investig Dermatol. 2015;8:159-177.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;19:659-665.
- Taylor A, Pawaskar M, Taylor SL, et al. Prevalence of pigmentary disorders and their impact on quality of life: a prospective cohort study. J Cosmet Dermatol. 2008;7:164-168.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE. Melasma: etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453-1457.
- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Narang T, Sawatkar GU, Kumaran MS, et al. Minocycline for recurrent and/or chronic erythema nodosum leprosum. JAMA Dermatol. 2015;151:1026-1028.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10.
- Pandya AG, Guevara IL. Disorders of hyperpigmentation. Dermatol Clin. 2000;18:91-98.
- Taylor SC, Torok H, Jones T, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72:67-72.
- Bulengo-Ransby SM. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Jacyk WK. Adapalene in the treatment of African patients. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):37-42.
- Kircik LH. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of postinflammatory hyperpigmentation and acne: a 16-week, baseline-controlled study. J Drugs Dermatol. 2011;10:586-590.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma. J Am Acad Dermatol. 2016;75:385-392.
- Kindred C, Okereke U, Callender VD. Skin-lightening agents: an overview of prescription, office-dispensed, and over-the-counter products. Cosmet Dermatol. 2013;26:18-26.
- Makino ET, Kadoya K, Sigler ML, et al. Development and clinical assessment of a comprehensive product for pigmentation control in multiple ethnic populations. J Drugs Dermatol. 2016;15:1562-1570.
- Halder R, Rodney I, Munhutu M, et al. Evaluation and effectiveness of a photoprotection composition (sunscreen) on subjects of skin of color. J Am Acad Dermatol. 2015;72(suppl 1):AB215.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- American Society for Aesthetic Plastic Surgery. 2016 Cosmetic Surgery National Data Bank Statistics. https://www.surgery.org/sites/default/files/ASAPS-Stats2016.pdf. Accessed November 15, 2017.
- Burgess CM. Soft tissue augmentation in skin of color: market growth, available fillers and successful techniques. J Drugs Dermatol. 2007;6:51-55.
- Davis EC, Callender VD. Aesthetic dermatology for aging ethnic skin. Dermatol Surg. 2011;37:901-917.
- Grimes PE, Arora S, Minus HR, et al. Dermatosis papulosa nigra. Cutis. 1983;32:385-386.
- Lupo M. Dermatosis papulosa nigra: treatment options. J Drugs Dermatol. 2007;6:29-30.
- Grimes PE, Shabazz D. A four-month randomized, double-blind evaluation of the efficacy of botulinum toxin type A for the treatment of glabellar lines in women with skin types V and VI. Dermatol Surg. 2009;35:429-435.
- Burgess CM, Awosika O. Ethnic and gender considerations in the use of facial injectables: African-American patients. Plast Reconstr Surg. 2015;136(5 suppl):28S-31S.
- Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill Education; 2016.
- Taylor SC, Burgess CM, Callender VD. Safety of nonanimal stabilized hyaluronic acid dermal fillers in patients with skin of color: a randomized, evaluator-blinded comparative trial. Dermatol Surg. 2009;35(suppl 2):1653-1660.
- Marmur ES, Taylor SC, Grimes PE, et al. Six-month safety results of calcium hydroxylapatite for treatment of nasolabial folds in Fitzpatrick skin types IV to VI. Dermatol Surg. 2009;35(suppl 2):1641-1645.
- Hamilton TK, Burgess CM. Consideration for the use of injectable poly-L-lactic acid in people of color. J Drugs Dermatol. 2010;9:451-456.
- Fabi SG, Goldman MP. Retrospective evaluation of micro-focused ultrasound for lifting and tightening of the face and neck. Dermatol Surg. 2014;40:569-575.
- Harris MO, Sundaram HA. Safety of microfocused ultrasound with visualization in patients with Fitzpatrick skin phototypes III to VI. JAMA Facial Plast Surg. 2015;17:355-357.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2 pt 3):S34-S37.
- Halder RM, Grimes PE, McLaurin CL, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388, 390.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasians, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Halder RM, Holmes YC, Bridgeman-Shah S, et al. A clinicohistologic study of acne vulgaris in black females (abstract). J Invest Dermatol. 1996;106:888.
- Plewig G, Fulton JE, Kligman AM. Pomade acne. Arch Dermatol. 1970;101:580-584.
- Kligman AM, Mills OH. Acne cosmetica. Arch Dermatol. 1972;106:893-897.
- Halder RM, Brooks HL, Callender VD. Acne in ethnic skin. Dermatol Clin. 2003;21:609-615.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Winhoven SM. Postinflammatory hyperpigmentation in an Asian patient. a dramatic response to oral isotretinoin (13-cis-retinoic acid). Br J Med. 2005;152:368-403.
- Sarkar R, Bansal S, Garg VK. Chemical peels for melasma in dark-skinned patients. J Cutan Aesthet Surg. 2012;5:247-253.
- Alexis AF, Coley MK, Nijhawan RI, et al. Nonablative fractional laser resurfacing for acne scarring in patients with Fitzpatrick skin phototypes IV-VI. Dermatol Surg. 2016;42:392-402.
- Culp B, Scheinfeld N. Rosacea: a review. P T. 2009;34:38-45.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014:20. pii:13030/qt1mv9r0ss.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Jackson JM, Knuckles M, Minni JP, et al. The role of brimonidine tartrate gel in the treatment of rosacea. Clin Cosmet Investig Dermatol. 2015;23:529-538.
- Patel NU, Shukla S, Zaki J, et al. Oxymetazoline hydrochloride cream for facial erythema associated with rosacea. Expert Rev Clin Pharmacol. 2017;10:104954.
- Weinkle AP, Doktor V, Emer J. Update on the management of rosacea. Clin Cosmet Investig Dermatol. 2015;8:159-177.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;19:659-665.
- Taylor A, Pawaskar M, Taylor SL, et al. Prevalence of pigmentary disorders and their impact on quality of life: a prospective cohort study. J Cosmet Dermatol. 2008;7:164-168.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE. Melasma: etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453-1457.
- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Narang T, Sawatkar GU, Kumaran MS, et al. Minocycline for recurrent and/or chronic erythema nodosum leprosum. JAMA Dermatol. 2015;151:1026-1028.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10.
- Pandya AG, Guevara IL. Disorders of hyperpigmentation. Dermatol Clin. 2000;18:91-98.
- Taylor SC, Torok H, Jones T, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72:67-72.
- Bulengo-Ransby SM. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Jacyk WK. Adapalene in the treatment of African patients. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):37-42.
- Kircik LH. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of postinflammatory hyperpigmentation and acne: a 16-week, baseline-controlled study. J Drugs Dermatol. 2011;10:586-590.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma. J Am Acad Dermatol. 2016;75:385-392.
- Kindred C, Okereke U, Callender VD. Skin-lightening agents: an overview of prescription, office-dispensed, and over-the-counter products. Cosmet Dermatol. 2013;26:18-26.
- Makino ET, Kadoya K, Sigler ML, et al. Development and clinical assessment of a comprehensive product for pigmentation control in multiple ethnic populations. J Drugs Dermatol. 2016;15:1562-1570.
- Halder R, Rodney I, Munhutu M, et al. Evaluation and effectiveness of a photoprotection composition (sunscreen) on subjects of skin of color. J Am Acad Dermatol. 2015;72(suppl 1):AB215.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- American Society for Aesthetic Plastic Surgery. 2016 Cosmetic Surgery National Data Bank Statistics. https://www.surgery.org/sites/default/files/ASAPS-Stats2016.pdf. Accessed November 15, 2017.
- Burgess CM. Soft tissue augmentation in skin of color: market growth, available fillers and successful techniques. J Drugs Dermatol. 2007;6:51-55.
- Davis EC, Callender VD. Aesthetic dermatology for aging ethnic skin. Dermatol Surg. 2011;37:901-917.
- Grimes PE, Arora S, Minus HR, et al. Dermatosis papulosa nigra. Cutis. 1983;32:385-386.
- Lupo M. Dermatosis papulosa nigra: treatment options. J Drugs Dermatol. 2007;6:29-30.
- Grimes PE, Shabazz D. A four-month randomized, double-blind evaluation of the efficacy of botulinum toxin type A for the treatment of glabellar lines in women with skin types V and VI. Dermatol Surg. 2009;35:429-435.
- Burgess CM, Awosika O. Ethnic and gender considerations in the use of facial injectables: African-American patients. Plast Reconstr Surg. 2015;136(5 suppl):28S-31S.
- Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill Education; 2016.
- Taylor SC, Burgess CM, Callender VD. Safety of nonanimal stabilized hyaluronic acid dermal fillers in patients with skin of color: a randomized, evaluator-blinded comparative trial. Dermatol Surg. 2009;35(suppl 2):1653-1660.
- Marmur ES, Taylor SC, Grimes PE, et al. Six-month safety results of calcium hydroxylapatite for treatment of nasolabial folds in Fitzpatrick skin types IV to VI. Dermatol Surg. 2009;35(suppl 2):1641-1645.
- Hamilton TK, Burgess CM. Consideration for the use of injectable poly-L-lactic acid in people of color. J Drugs Dermatol. 2010;9:451-456.
- Fabi SG, Goldman MP. Retrospective evaluation of micro-focused ultrasound for lifting and tightening of the face and neck. Dermatol Surg. 2014;40:569-575.
- Harris MO, Sundaram HA. Safety of microfocused ultrasound with visualization in patients with Fitzpatrick skin phototypes III to VI. JAMA Facial Plast Surg. 2015;17:355-357.
Practice Points
- Treat acne in skin of color (SOC) patients early and aggressively to prevent or minimize subsequent postinflammatory hyperpigmentation (PIH) and acne scarring.
- Vascular lasers and intense pulsed light may be used to address the vascular components of rosacea; however, the latter is not recommended in Fitzpatrick skin types IV to VI.
- Hydroquinone is the gold standard for skin lightening and is often used as a first-line therapy for melasma and PIH.
- Photoprotection is an essential component of therapy for hyperpigmented skin disorders.
- Cosmetic procedures are gaining popularity in the SOC population. When treating SOC patients, consider the impact of ethnicity on aging and facial structure, the patient's desired cosmetic outcome, tissue reaction to anticipated treatments, and the patient's expectations for recommended therapies.