User login
Metered-dose inhalers with a spacer (MDI/S) are as good as, or better than, nebulizers for children with asthma. This is based on numerous randomized controlled trials that compared outcomes such as hospital admission rates, asthma severity scores, and pulmonary function scores (strength of recommendation: A, based on consistent randomized controlled trials and meta-analysis).
Evidence summary
A Cochrane review of 10 randomized controlled trials comparing nebulizers with MDI/S, both in adults and in children aged >2 years, showed a substantial trend towards improvement in hospital admission rates with MDI/S use. Sample size for each study was small, ranging from 18 to 152 patients, with a total sample size of 880 children and 444 adults.
The relative risk of admission for MDI/S vs nebulizer for children was 0.65 (95% confidence interval, 0.4–1.06). Secondary outcomes were equivalent or slightly improved, including duration in the emergency department, changes in respiratory rate, blood gases, pulse, tremor, symptoms score, lung function, and use of steroids. Patients with life-threatening asthma (for example, those considered for ventilation) or other chronic illnesses were excluded.1
All but 1 of these studies were set in the emergency department and all involved the use of one of a variety of spacers with the MDI, such as the Aerochamber or Inspirease. Whether these efficacy studies can be translated into daily outpatient clinical practice remains unclear. Emergency departments typically have higher staffing levels, and study subjects and their parents may have received more MDI/S training than is practical in many office settings.
While most of the data were for children aged 2 years and older, 1 study published after the Cochrane review did show a lower admission rate in 85 patients who were 2 to 24 months in the MDI/S group.2 Controlling for the initial Pulmonary Index score, children using an MDI and Aerochamber spacer were admitted less often (5% vs 20%, number needed to treat=7; P=.05) than children using nebulizers. Since the results of this single small trial are the only data available for this younger age group, using MDI/S instead of nebulizers should be done with caution for children aged <2 years.
Another randomized controlled trial of 152 patients found no difference in primary outcomes of asthma severity score, oxygen saturation, and percent predicted peak expiratory flow rate (PEFR). Several secondary outcomes slightly favored MDI/S: number of treatments given, whether steroids were used, change in heart rate, side effects, rate of hospital admission, and treatment time in the emergency department.3
A smaller double-blinded randomized controlled trial of 33 children aged 6 to 14 years showed no difference in MDI/S vs nebulizer, as measured by clinical score, respiratory rate, oxygen saturation, and forced expiratory volume at 1 second (FEV1).4 The researchers calculated the study had 90% power to detect a clinically meaningful difference in FEV1 of 12% of the predicted value between the groups.
Other review articles reach the same conclusion. One article reviewed the literature from 1980 to 1996 and examined 17 prospective clinical trials. Outcomes measured included pulmonary function measures and clinical scores. The researchers recommended that MDI/S be used due to clinical benefit, safety, lower cost, personnel time, and speed and ease of administration.5
A review article from the British literature examined 3 randomized controlled trials involving 51 patients and found no superiority of nebulizer vs MDI/S.6 A similar review article examined 14 randomized controlled trials for beta-agonist delivery for patients aged 5 to 15 with stable asthma. They found no obvious benefit of 1 type of device over another, including nebulizer, MDI/S, and dry powder inhalers.7 These last 2 articles claimed to be systematic reviews, although they do not clearly state their search methodology.
Researchers used a wide variety of spacers in all aforementioned studies; accordingly, one cannot be recommended as superior to others. The degree of teaching given to parents and children about MDI/S use was not described in any of the trials.
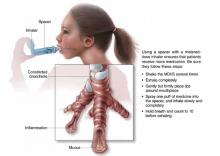
FIGURE
MDI with spacer is beneficial when used properly
Recommendations from others
Guidelines from the Global Health Initiative for Asthma, a collaboration of the National Heart, Lung and Blood Institute and the World Health Organization, recommend MDI/S for children with asthma due to increased efficacy and decreased cost (revised in 2002). Specifically, they recommend a spacer with a face mask for infants and preschool children, a mouthpiece and spacer for children aged 4 to 6 years, and a dry powder inhaler or breath-activated device from age 6 onwards.8 Cincinnati Children’s Hospital’s evidence-based guidelines from 1998 also recommend MDI/S for children aged >1 year with acute asthma exacerbations.9 This guideline suggests using 4 to 8 puffs from a 90 μg albuterol MDI at 1- to 2-minute intervals every 20 minutes for 1 hour, then every 1 to 4 hours subsequently.
Use MDIs with spacers in all but the youngest patients
Grant Hoekzema, MD
Mercy Family Medicine Residency, St. Louis, Mo
Until recently, using a nebulizer for the wheezing child or infant seemed intuitively to be the most effective way to deliver bronchodilators. However, with recent data showing that MDIs with spacers are just as effective, I have been using MDIs with spacers for all but my youngest patients. Parents as well as physicians may need to be convinced that using less technology in this case is better for their child. In some cases, parental acceptance of therapy necessitates using a nebulizer.
1. Cates CJ, Rowe BH, Bara A. Holding chambers versus nebulisers for beta-agonist treatment of acute asthma (Cochrane Review). The Cochrane Library, Issue 2, 2002. Oxford: Update Software, last updated February 21, 2002.
2. Delgado A, Chou KJ, Silver EJ, Crain EF. Nebulizers vs metered dose-inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med 2003;157:76-80.
3. Chou KJ, Cunningham SJ, Crain EF. Metered-dose inhalers with spacers vs nebulizers for pediatric asthma. Arch Pediatr Adolesc Med 1995;149:201-205.
4. Kerem E, Levison H, Schuh S, et al. Efficacy of albuterol administered by nebulizer versus spacer device in children with acute asthma. J Pediatr 1993;123:313-317.
5. Amirav I, Newhouse MT. Metered-dose inhaler accessory devices in acute asthma: efficacy and comparison with nebulizers: a literature review. Arch Pediatr Adolesc Med 1997;151:876-882.
6. Brocklebank D, Ram F, Wright J, et al. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol Assess 2001;5:1-149.
7. Peters J, Stevenson M, Beverley C, Lim JN, Smith S. The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation. Health Technol Assess 2002;6:1-167.
8. National Heart, Lung and Blood Institute (NHLBI), World Health Organization (WHO). Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention. 2002. Available at: http://www.ginasthma.com/workshop.pdf. Accessed on December 3, 2003. Updated from: NHLBI/WHO Workshop Report: Global Strategy for Asthma Management and Prevention, issued January 1995. NIH Publication No. 02-3659.
9. Evidence-Based Clinical Practice Guideline for Managing an Acute Exacerbation of Asthma. Cincinnati, Ohio: Cincinnati Childrens’ Hospital Medical Center; 1998 (revised 2002). Available at: http://www.cincinnatichildrens.org/svc/dept-div/health-policy/ev-based/asthma.htm. Accessed on December 3, 2003.
Metered-dose inhalers with a spacer (MDI/S) are as good as, or better than, nebulizers for children with asthma. This is based on numerous randomized controlled trials that compared outcomes such as hospital admission rates, asthma severity scores, and pulmonary function scores (strength of recommendation: A, based on consistent randomized controlled trials and meta-analysis).
Evidence summary
A Cochrane review of 10 randomized controlled trials comparing nebulizers with MDI/S, both in adults and in children aged >2 years, showed a substantial trend towards improvement in hospital admission rates with MDI/S use. Sample size for each study was small, ranging from 18 to 152 patients, with a total sample size of 880 children and 444 adults.
The relative risk of admission for MDI/S vs nebulizer for children was 0.65 (95% confidence interval, 0.4–1.06). Secondary outcomes were equivalent or slightly improved, including duration in the emergency department, changes in respiratory rate, blood gases, pulse, tremor, symptoms score, lung function, and use of steroids. Patients with life-threatening asthma (for example, those considered for ventilation) or other chronic illnesses were excluded.1
All but 1 of these studies were set in the emergency department and all involved the use of one of a variety of spacers with the MDI, such as the Aerochamber or Inspirease. Whether these efficacy studies can be translated into daily outpatient clinical practice remains unclear. Emergency departments typically have higher staffing levels, and study subjects and their parents may have received more MDI/S training than is practical in many office settings.
While most of the data were for children aged 2 years and older, 1 study published after the Cochrane review did show a lower admission rate in 85 patients who were 2 to 24 months in the MDI/S group.2 Controlling for the initial Pulmonary Index score, children using an MDI and Aerochamber spacer were admitted less often (5% vs 20%, number needed to treat=7; P=.05) than children using nebulizers. Since the results of this single small trial are the only data available for this younger age group, using MDI/S instead of nebulizers should be done with caution for children aged <2 years.
Another randomized controlled trial of 152 patients found no difference in primary outcomes of asthma severity score, oxygen saturation, and percent predicted peak expiratory flow rate (PEFR). Several secondary outcomes slightly favored MDI/S: number of treatments given, whether steroids were used, change in heart rate, side effects, rate of hospital admission, and treatment time in the emergency department.3
A smaller double-blinded randomized controlled trial of 33 children aged 6 to 14 years showed no difference in MDI/S vs nebulizer, as measured by clinical score, respiratory rate, oxygen saturation, and forced expiratory volume at 1 second (FEV1).4 The researchers calculated the study had 90% power to detect a clinically meaningful difference in FEV1 of 12% of the predicted value between the groups.
Other review articles reach the same conclusion. One article reviewed the literature from 1980 to 1996 and examined 17 prospective clinical trials. Outcomes measured included pulmonary function measures and clinical scores. The researchers recommended that MDI/S be used due to clinical benefit, safety, lower cost, personnel time, and speed and ease of administration.5
A review article from the British literature examined 3 randomized controlled trials involving 51 patients and found no superiority of nebulizer vs MDI/S.6 A similar review article examined 14 randomized controlled trials for beta-agonist delivery for patients aged 5 to 15 with stable asthma. They found no obvious benefit of 1 type of device over another, including nebulizer, MDI/S, and dry powder inhalers.7 These last 2 articles claimed to be systematic reviews, although they do not clearly state their search methodology.
Researchers used a wide variety of spacers in all aforementioned studies; accordingly, one cannot be recommended as superior to others. The degree of teaching given to parents and children about MDI/S use was not described in any of the trials.
FIGURE
MDI with spacer is beneficial when used properly
Recommendations from others
Guidelines from the Global Health Initiative for Asthma, a collaboration of the National Heart, Lung and Blood Institute and the World Health Organization, recommend MDI/S for children with asthma due to increased efficacy and decreased cost (revised in 2002). Specifically, they recommend a spacer with a face mask for infants and preschool children, a mouthpiece and spacer for children aged 4 to 6 years, and a dry powder inhaler or breath-activated device from age 6 onwards.8 Cincinnati Children’s Hospital’s evidence-based guidelines from 1998 also recommend MDI/S for children aged >1 year with acute asthma exacerbations.9 This guideline suggests using 4 to 8 puffs from a 90 μg albuterol MDI at 1- to 2-minute intervals every 20 minutes for 1 hour, then every 1 to 4 hours subsequently.
Use MDIs with spacers in all but the youngest patients
Grant Hoekzema, MD
Mercy Family Medicine Residency, St. Louis, Mo
Until recently, using a nebulizer for the wheezing child or infant seemed intuitively to be the most effective way to deliver bronchodilators. However, with recent data showing that MDIs with spacers are just as effective, I have been using MDIs with spacers for all but my youngest patients. Parents as well as physicians may need to be convinced that using less technology in this case is better for their child. In some cases, parental acceptance of therapy necessitates using a nebulizer.
Metered-dose inhalers with a spacer (MDI/S) are as good as, or better than, nebulizers for children with asthma. This is based on numerous randomized controlled trials that compared outcomes such as hospital admission rates, asthma severity scores, and pulmonary function scores (strength of recommendation: A, based on consistent randomized controlled trials and meta-analysis).
Evidence summary
A Cochrane review of 10 randomized controlled trials comparing nebulizers with MDI/S, both in adults and in children aged >2 years, showed a substantial trend towards improvement in hospital admission rates with MDI/S use. Sample size for each study was small, ranging from 18 to 152 patients, with a total sample size of 880 children and 444 adults.
The relative risk of admission for MDI/S vs nebulizer for children was 0.65 (95% confidence interval, 0.4–1.06). Secondary outcomes were equivalent or slightly improved, including duration in the emergency department, changes in respiratory rate, blood gases, pulse, tremor, symptoms score, lung function, and use of steroids. Patients with life-threatening asthma (for example, those considered for ventilation) or other chronic illnesses were excluded.1
All but 1 of these studies were set in the emergency department and all involved the use of one of a variety of spacers with the MDI, such as the Aerochamber or Inspirease. Whether these efficacy studies can be translated into daily outpatient clinical practice remains unclear. Emergency departments typically have higher staffing levels, and study subjects and their parents may have received more MDI/S training than is practical in many office settings.
While most of the data were for children aged 2 years and older, 1 study published after the Cochrane review did show a lower admission rate in 85 patients who were 2 to 24 months in the MDI/S group.2 Controlling for the initial Pulmonary Index score, children using an MDI and Aerochamber spacer were admitted less often (5% vs 20%, number needed to treat=7; P=.05) than children using nebulizers. Since the results of this single small trial are the only data available for this younger age group, using MDI/S instead of nebulizers should be done with caution for children aged <2 years.
Another randomized controlled trial of 152 patients found no difference in primary outcomes of asthma severity score, oxygen saturation, and percent predicted peak expiratory flow rate (PEFR). Several secondary outcomes slightly favored MDI/S: number of treatments given, whether steroids were used, change in heart rate, side effects, rate of hospital admission, and treatment time in the emergency department.3
A smaller double-blinded randomized controlled trial of 33 children aged 6 to 14 years showed no difference in MDI/S vs nebulizer, as measured by clinical score, respiratory rate, oxygen saturation, and forced expiratory volume at 1 second (FEV1).4 The researchers calculated the study had 90% power to detect a clinically meaningful difference in FEV1 of 12% of the predicted value between the groups.
Other review articles reach the same conclusion. One article reviewed the literature from 1980 to 1996 and examined 17 prospective clinical trials. Outcomes measured included pulmonary function measures and clinical scores. The researchers recommended that MDI/S be used due to clinical benefit, safety, lower cost, personnel time, and speed and ease of administration.5
A review article from the British literature examined 3 randomized controlled trials involving 51 patients and found no superiority of nebulizer vs MDI/S.6 A similar review article examined 14 randomized controlled trials for beta-agonist delivery for patients aged 5 to 15 with stable asthma. They found no obvious benefit of 1 type of device over another, including nebulizer, MDI/S, and dry powder inhalers.7 These last 2 articles claimed to be systematic reviews, although they do not clearly state their search methodology.
Researchers used a wide variety of spacers in all aforementioned studies; accordingly, one cannot be recommended as superior to others. The degree of teaching given to parents and children about MDI/S use was not described in any of the trials.
FIGURE
MDI with spacer is beneficial when used properly
Recommendations from others
Guidelines from the Global Health Initiative for Asthma, a collaboration of the National Heart, Lung and Blood Institute and the World Health Organization, recommend MDI/S for children with asthma due to increased efficacy and decreased cost (revised in 2002). Specifically, they recommend a spacer with a face mask for infants and preschool children, a mouthpiece and spacer for children aged 4 to 6 years, and a dry powder inhaler or breath-activated device from age 6 onwards.8 Cincinnati Children’s Hospital’s evidence-based guidelines from 1998 also recommend MDI/S for children aged >1 year with acute asthma exacerbations.9 This guideline suggests using 4 to 8 puffs from a 90 μg albuterol MDI at 1- to 2-minute intervals every 20 minutes for 1 hour, then every 1 to 4 hours subsequently.
Use MDIs with spacers in all but the youngest patients
Grant Hoekzema, MD
Mercy Family Medicine Residency, St. Louis, Mo
Until recently, using a nebulizer for the wheezing child or infant seemed intuitively to be the most effective way to deliver bronchodilators. However, with recent data showing that MDIs with spacers are just as effective, I have been using MDIs with spacers for all but my youngest patients. Parents as well as physicians may need to be convinced that using less technology in this case is better for their child. In some cases, parental acceptance of therapy necessitates using a nebulizer.
1. Cates CJ, Rowe BH, Bara A. Holding chambers versus nebulisers for beta-agonist treatment of acute asthma (Cochrane Review). The Cochrane Library, Issue 2, 2002. Oxford: Update Software, last updated February 21, 2002.
2. Delgado A, Chou KJ, Silver EJ, Crain EF. Nebulizers vs metered dose-inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med 2003;157:76-80.
3. Chou KJ, Cunningham SJ, Crain EF. Metered-dose inhalers with spacers vs nebulizers for pediatric asthma. Arch Pediatr Adolesc Med 1995;149:201-205.
4. Kerem E, Levison H, Schuh S, et al. Efficacy of albuterol administered by nebulizer versus spacer device in children with acute asthma. J Pediatr 1993;123:313-317.
5. Amirav I, Newhouse MT. Metered-dose inhaler accessory devices in acute asthma: efficacy and comparison with nebulizers: a literature review. Arch Pediatr Adolesc Med 1997;151:876-882.
6. Brocklebank D, Ram F, Wright J, et al. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol Assess 2001;5:1-149.
7. Peters J, Stevenson M, Beverley C, Lim JN, Smith S. The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation. Health Technol Assess 2002;6:1-167.
8. National Heart, Lung and Blood Institute (NHLBI), World Health Organization (WHO). Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention. 2002. Available at: http://www.ginasthma.com/workshop.pdf. Accessed on December 3, 2003. Updated from: NHLBI/WHO Workshop Report: Global Strategy for Asthma Management and Prevention, issued January 1995. NIH Publication No. 02-3659.
9. Evidence-Based Clinical Practice Guideline for Managing an Acute Exacerbation of Asthma. Cincinnati, Ohio: Cincinnati Childrens’ Hospital Medical Center; 1998 (revised 2002). Available at: http://www.cincinnatichildrens.org/svc/dept-div/health-policy/ev-based/asthma.htm. Accessed on December 3, 2003.
1. Cates CJ, Rowe BH, Bara A. Holding chambers versus nebulisers for beta-agonist treatment of acute asthma (Cochrane Review). The Cochrane Library, Issue 2, 2002. Oxford: Update Software, last updated February 21, 2002.
2. Delgado A, Chou KJ, Silver EJ, Crain EF. Nebulizers vs metered dose-inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med 2003;157:76-80.
3. Chou KJ, Cunningham SJ, Crain EF. Metered-dose inhalers with spacers vs nebulizers for pediatric asthma. Arch Pediatr Adolesc Med 1995;149:201-205.
4. Kerem E, Levison H, Schuh S, et al. Efficacy of albuterol administered by nebulizer versus spacer device in children with acute asthma. J Pediatr 1993;123:313-317.
5. Amirav I, Newhouse MT. Metered-dose inhaler accessory devices in acute asthma: efficacy and comparison with nebulizers: a literature review. Arch Pediatr Adolesc Med 1997;151:876-882.
6. Brocklebank D, Ram F, Wright J, et al. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol Assess 2001;5:1-149.
7. Peters J, Stevenson M, Beverley C, Lim JN, Smith S. The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation. Health Technol Assess 2002;6:1-167.
8. National Heart, Lung and Blood Institute (NHLBI), World Health Organization (WHO). Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention. 2002. Available at: http://www.ginasthma.com/workshop.pdf. Accessed on December 3, 2003. Updated from: NHLBI/WHO Workshop Report: Global Strategy for Asthma Management and Prevention, issued January 1995. NIH Publication No. 02-3659.
9. Evidence-Based Clinical Practice Guideline for Managing an Acute Exacerbation of Asthma. Cincinnati, Ohio: Cincinnati Childrens’ Hospital Medical Center; 1998 (revised 2002). Available at: http://www.cincinnatichildrens.org/svc/dept-div/health-policy/ev-based/asthma.htm. Accessed on December 3, 2003.
Evidence-based answers from the Family Physicians Inquiries Network