User login
Do antibiotics improve outcomes in chronic rhinosinusitis?
For children, antibiotics do not appear to improve short-term (3-6 weeks) or long-term (3 months) outcomes of chronic rhinosinusitis (strength of recommendation [SOR]: A, randomized controlled trials). No adequate placebo-controlled trials have been performed in adults. Two consensus statements report that 10 to 21 days of antibiotics active against organisms producing beta-lactamase might be beneficial in some cases (SOR: C).
Evidence summary
The American Academy of Otolargynology-Head and Neck Surgery defines chronic rhinosinusitis as the persistence of 2 major or 1 major and 2 minor criteria lasting at least 12 weeks (Table).1 The other categories of rhinosinusitis are acute (symptoms lasting <3 weeks) and subacute (symptoms lasting 3-12 weeks).
Two placebo-controlled trials have evaluated antibiotic treatment of chronic rhinosinusitis in children. In 1 study, 141 children with chronic rhinosinusitis were randomly assigned to 1 of 4 treatment arms: saline nose drops; xylometazoline (Otrivin) drops with oral amoxicillin 3 times daily; surgical drainage; or surgical drainage, amoxicillin 3 times daily and xylometazoline drops.2 Outcomes were resolution of purulent rhinitis, no purulent drainage on exam, and no abnormalities of maxillary sinus on x-ray. The absence of all 3 findings constituted cure. At 6 weeks there was a non-statistically significant higher resolution in the fourth group, but by 26 weeks the groups were indistinguishable. At 6 weeks, 53%, 50%, 55%, and 79% of each group, respectively, were cured. These results increased to 69%, 74%, 69%, and 64% at 26 weeks.
Another study randomized 79 children with chronic sinusitis to treatment with cefaclor vs placebo following antral washout.3 Measured outcomes were similar to those in the prior study. At 6 weeks, 12.3% more patients in the antibiotic group achieved cure than the placebo group (64.8% vs 52.5%), but this difference was not statistically significant (P=.28). At 12 weeks, no differences in improvement were seen between the 2 groups (89% vs 89.5%)
No studies (since 1966) have evaluated antibiotic use compared with placebo in adults. We did not review the numerous studies comparing different antibiotics without placebo.
Recommendations from others
The American Academy of Otolaryngology-Head and Neck Surgery, in conjunction with the American Academy of Rhinology and the American
Academy of Otolaryngic Allergy, state that the use of antibiotics active against beta-lactamase producing organisms might be beneficial in some cases.3 A consensus statement from a panel convened in Belgium in 1996 stated antibiotics should be given for 5 to 7 days with repeat treatments if the child does not respond initially.5
TABLE 2
Diagnostic criteria for rhinosinusitis
| Major criteria |
| Facial pain/pressure* |
| Facial congestion/fullness |
| Nasal obstruction/blockage |
| Nasal discharge/purulence/discolored drainage |
| Hyposmia/anosmia |
| Purulence in nasal cavity on examination |
| Fever (acute only)* |
| Minor criteria |
| Headache |
| Fever (all nonacute) |
| Halitosis |
| Fatigue |
| Dental pain |
| Cough |
| Ear pain/pressure/fullness |
| *Symptom alone does not constitute a major sign in the absence of another major nasal symptom. Adapted from Lanza DC, 1997.1 |
Antibiotics provide only short-term relief, not long-term answers
William A. Hensel, MD
Moses Cone Family Residency Program, Greensboro, NC
For chronic sinusitis, I start by emphasizing nonantibiotic treatments, such as decongestants, nasal steroids, antihistamines, smoking cessation, and avoidance of passive smoke, allergens, and other irritants. With education and experience, patients realize that antibiotics provide only short-term relief, not long-term answers. Having learned this, patients can better participate in antibiotic treatment decisions. Most are able to weigh the short-term, symptomatic benefits against potential medication side effects and the cost. I believe that 2 or 3 courses of antibiotics per year are not excessive, but I try not to exceed that limit.
Finally, I don’t always choose a beta-lactamase-resistant antibiotic. Given that antibiotics do not alter the long-term prognosis, I worry less about resistance and more about minimizing cost and side-effect potential. Therefore, I occasionally treat with amoxicillin or Pen Vee K. Patients seem to appreciate my flexibility and collaborative approach to decision-making.
1. Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg 1997;117(3 Pt 2):S1-S7.
2. Otten FW, Grote JJ. Treatment of chronic maxillary sinusitis in children. Int J Pediatr Otorhinolaryngol 1988;15:269-278.
3. Otten HW, Antvelink JB, Ruyter de Wildt H, Rietema SJ, Siemelink RJ, Hordijk GJ. Is antibiotic treatment of chronic sinusitis effective in children? Clin Otolaryngo 1994;19:215-217.
4. Benninger MS, Anon J, Mabry RL. The medical management of rhinosinusitis. Otolaryngol Head Neck Surg 1997;117(3 Pt 2):S41-S49.
5. Clement PA, Bluestone CD, Gordts F, et al. Management of rhinosinusitis in children: consensus meeting, Brussels, Belgium, September 13, 1996. Arch Otolaryngol Head Neck Surg 1998;124:31-34.
For children, antibiotics do not appear to improve short-term (3-6 weeks) or long-term (3 months) outcomes of chronic rhinosinusitis (strength of recommendation [SOR]: A, randomized controlled trials). No adequate placebo-controlled trials have been performed in adults. Two consensus statements report that 10 to 21 days of antibiotics active against organisms producing beta-lactamase might be beneficial in some cases (SOR: C).
Evidence summary
The American Academy of Otolargynology-Head and Neck Surgery defines chronic rhinosinusitis as the persistence of 2 major or 1 major and 2 minor criteria lasting at least 12 weeks (Table).1 The other categories of rhinosinusitis are acute (symptoms lasting <3 weeks) and subacute (symptoms lasting 3-12 weeks).
Two placebo-controlled trials have evaluated antibiotic treatment of chronic rhinosinusitis in children. In 1 study, 141 children with chronic rhinosinusitis were randomly assigned to 1 of 4 treatment arms: saline nose drops; xylometazoline (Otrivin) drops with oral amoxicillin 3 times daily; surgical drainage; or surgical drainage, amoxicillin 3 times daily and xylometazoline drops.2 Outcomes were resolution of purulent rhinitis, no purulent drainage on exam, and no abnormalities of maxillary sinus on x-ray. The absence of all 3 findings constituted cure. At 6 weeks there was a non-statistically significant higher resolution in the fourth group, but by 26 weeks the groups were indistinguishable. At 6 weeks, 53%, 50%, 55%, and 79% of each group, respectively, were cured. These results increased to 69%, 74%, 69%, and 64% at 26 weeks.
Another study randomized 79 children with chronic sinusitis to treatment with cefaclor vs placebo following antral washout.3 Measured outcomes were similar to those in the prior study. At 6 weeks, 12.3% more patients in the antibiotic group achieved cure than the placebo group (64.8% vs 52.5%), but this difference was not statistically significant (P=.28). At 12 weeks, no differences in improvement were seen between the 2 groups (89% vs 89.5%)
No studies (since 1966) have evaluated antibiotic use compared with placebo in adults. We did not review the numerous studies comparing different antibiotics without placebo.
Recommendations from others
The American Academy of Otolaryngology-Head and Neck Surgery, in conjunction with the American Academy of Rhinology and the American
Academy of Otolaryngic Allergy, state that the use of antibiotics active against beta-lactamase producing organisms might be beneficial in some cases.3 A consensus statement from a panel convened in Belgium in 1996 stated antibiotics should be given for 5 to 7 days with repeat treatments if the child does not respond initially.5
TABLE 2
Diagnostic criteria for rhinosinusitis
| Major criteria |
| Facial pain/pressure* |
| Facial congestion/fullness |
| Nasal obstruction/blockage |
| Nasal discharge/purulence/discolored drainage |
| Hyposmia/anosmia |
| Purulence in nasal cavity on examination |
| Fever (acute only)* |
| Minor criteria |
| Headache |
| Fever (all nonacute) |
| Halitosis |
| Fatigue |
| Dental pain |
| Cough |
| Ear pain/pressure/fullness |
| *Symptom alone does not constitute a major sign in the absence of another major nasal symptom. Adapted from Lanza DC, 1997.1 |
Antibiotics provide only short-term relief, not long-term answers
William A. Hensel, MD
Moses Cone Family Residency Program, Greensboro, NC
For chronic sinusitis, I start by emphasizing nonantibiotic treatments, such as decongestants, nasal steroids, antihistamines, smoking cessation, and avoidance of passive smoke, allergens, and other irritants. With education and experience, patients realize that antibiotics provide only short-term relief, not long-term answers. Having learned this, patients can better participate in antibiotic treatment decisions. Most are able to weigh the short-term, symptomatic benefits against potential medication side effects and the cost. I believe that 2 or 3 courses of antibiotics per year are not excessive, but I try not to exceed that limit.
Finally, I don’t always choose a beta-lactamase-resistant antibiotic. Given that antibiotics do not alter the long-term prognosis, I worry less about resistance and more about minimizing cost and side-effect potential. Therefore, I occasionally treat with amoxicillin or Pen Vee K. Patients seem to appreciate my flexibility and collaborative approach to decision-making.
For children, antibiotics do not appear to improve short-term (3-6 weeks) or long-term (3 months) outcomes of chronic rhinosinusitis (strength of recommendation [SOR]: A, randomized controlled trials). No adequate placebo-controlled trials have been performed in adults. Two consensus statements report that 10 to 21 days of antibiotics active against organisms producing beta-lactamase might be beneficial in some cases (SOR: C).
Evidence summary
The American Academy of Otolargynology-Head and Neck Surgery defines chronic rhinosinusitis as the persistence of 2 major or 1 major and 2 minor criteria lasting at least 12 weeks (Table).1 The other categories of rhinosinusitis are acute (symptoms lasting <3 weeks) and subacute (symptoms lasting 3-12 weeks).
Two placebo-controlled trials have evaluated antibiotic treatment of chronic rhinosinusitis in children. In 1 study, 141 children with chronic rhinosinusitis were randomly assigned to 1 of 4 treatment arms: saline nose drops; xylometazoline (Otrivin) drops with oral amoxicillin 3 times daily; surgical drainage; or surgical drainage, amoxicillin 3 times daily and xylometazoline drops.2 Outcomes were resolution of purulent rhinitis, no purulent drainage on exam, and no abnormalities of maxillary sinus on x-ray. The absence of all 3 findings constituted cure. At 6 weeks there was a non-statistically significant higher resolution in the fourth group, but by 26 weeks the groups were indistinguishable. At 6 weeks, 53%, 50%, 55%, and 79% of each group, respectively, were cured. These results increased to 69%, 74%, 69%, and 64% at 26 weeks.
Another study randomized 79 children with chronic sinusitis to treatment with cefaclor vs placebo following antral washout.3 Measured outcomes were similar to those in the prior study. At 6 weeks, 12.3% more patients in the antibiotic group achieved cure than the placebo group (64.8% vs 52.5%), but this difference was not statistically significant (P=.28). At 12 weeks, no differences in improvement were seen between the 2 groups (89% vs 89.5%)
No studies (since 1966) have evaluated antibiotic use compared with placebo in adults. We did not review the numerous studies comparing different antibiotics without placebo.
Recommendations from others
The American Academy of Otolaryngology-Head and Neck Surgery, in conjunction with the American Academy of Rhinology and the American
Academy of Otolaryngic Allergy, state that the use of antibiotics active against beta-lactamase producing organisms might be beneficial in some cases.3 A consensus statement from a panel convened in Belgium in 1996 stated antibiotics should be given for 5 to 7 days with repeat treatments if the child does not respond initially.5
TABLE 2
Diagnostic criteria for rhinosinusitis
| Major criteria |
| Facial pain/pressure* |
| Facial congestion/fullness |
| Nasal obstruction/blockage |
| Nasal discharge/purulence/discolored drainage |
| Hyposmia/anosmia |
| Purulence in nasal cavity on examination |
| Fever (acute only)* |
| Minor criteria |
| Headache |
| Fever (all nonacute) |
| Halitosis |
| Fatigue |
| Dental pain |
| Cough |
| Ear pain/pressure/fullness |
| *Symptom alone does not constitute a major sign in the absence of another major nasal symptom. Adapted from Lanza DC, 1997.1 |
Antibiotics provide only short-term relief, not long-term answers
William A. Hensel, MD
Moses Cone Family Residency Program, Greensboro, NC
For chronic sinusitis, I start by emphasizing nonantibiotic treatments, such as decongestants, nasal steroids, antihistamines, smoking cessation, and avoidance of passive smoke, allergens, and other irritants. With education and experience, patients realize that antibiotics provide only short-term relief, not long-term answers. Having learned this, patients can better participate in antibiotic treatment decisions. Most are able to weigh the short-term, symptomatic benefits against potential medication side effects and the cost. I believe that 2 or 3 courses of antibiotics per year are not excessive, but I try not to exceed that limit.
Finally, I don’t always choose a beta-lactamase-resistant antibiotic. Given that antibiotics do not alter the long-term prognosis, I worry less about resistance and more about minimizing cost and side-effect potential. Therefore, I occasionally treat with amoxicillin or Pen Vee K. Patients seem to appreciate my flexibility and collaborative approach to decision-making.
1. Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg 1997;117(3 Pt 2):S1-S7.
2. Otten FW, Grote JJ. Treatment of chronic maxillary sinusitis in children. Int J Pediatr Otorhinolaryngol 1988;15:269-278.
3. Otten HW, Antvelink JB, Ruyter de Wildt H, Rietema SJ, Siemelink RJ, Hordijk GJ. Is antibiotic treatment of chronic sinusitis effective in children? Clin Otolaryngo 1994;19:215-217.
4. Benninger MS, Anon J, Mabry RL. The medical management of rhinosinusitis. Otolaryngol Head Neck Surg 1997;117(3 Pt 2):S41-S49.
5. Clement PA, Bluestone CD, Gordts F, et al. Management of rhinosinusitis in children: consensus meeting, Brussels, Belgium, September 13, 1996. Arch Otolaryngol Head Neck Surg 1998;124:31-34.
1. Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg 1997;117(3 Pt 2):S1-S7.
2. Otten FW, Grote JJ. Treatment of chronic maxillary sinusitis in children. Int J Pediatr Otorhinolaryngol 1988;15:269-278.
3. Otten HW, Antvelink JB, Ruyter de Wildt H, Rietema SJ, Siemelink RJ, Hordijk GJ. Is antibiotic treatment of chronic sinusitis effective in children? Clin Otolaryngo 1994;19:215-217.
4. Benninger MS, Anon J, Mabry RL. The medical management of rhinosinusitis. Otolaryngol Head Neck Surg 1997;117(3 Pt 2):S41-S49.
5. Clement PA, Bluestone CD, Gordts F, et al. Management of rhinosinusitis in children: consensus meeting, Brussels, Belgium, September 13, 1996. Arch Otolaryngol Head Neck Surg 1998;124:31-34.
Evidence-based answers from the Family Physicians Inquiries Network
Are inhalers with spacers better than nebulizers for children with asthma?
Metered-dose inhalers with a spacer (MDI/S) are as good as, or better than, nebulizers for children with asthma. This is based on numerous randomized controlled trials that compared outcomes such as hospital admission rates, asthma severity scores, and pulmonary function scores (strength of recommendation: A, based on consistent randomized controlled trials and meta-analysis).
Evidence summary
A Cochrane review of 10 randomized controlled trials comparing nebulizers with MDI/S, both in adults and in children aged >2 years, showed a substantial trend towards improvement in hospital admission rates with MDI/S use. Sample size for each study was small, ranging from 18 to 152 patients, with a total sample size of 880 children and 444 adults.
The relative risk of admission for MDI/S vs nebulizer for children was 0.65 (95% confidence interval, 0.4–1.06). Secondary outcomes were equivalent or slightly improved, including duration in the emergency department, changes in respiratory rate, blood gases, pulse, tremor, symptoms score, lung function, and use of steroids. Patients with life-threatening asthma (for example, those considered for ventilation) or other chronic illnesses were excluded.1
All but 1 of these studies were set in the emergency department and all involved the use of one of a variety of spacers with the MDI, such as the Aerochamber or Inspirease. Whether these efficacy studies can be translated into daily outpatient clinical practice remains unclear. Emergency departments typically have higher staffing levels, and study subjects and their parents may have received more MDI/S training than is practical in many office settings.
While most of the data were for children aged 2 years and older, 1 study published after the Cochrane review did show a lower admission rate in 85 patients who were 2 to 24 months in the MDI/S group.2 Controlling for the initial Pulmonary Index score, children using an MDI and Aerochamber spacer were admitted less often (5% vs 20%, number needed to treat=7; P=.05) than children using nebulizers. Since the results of this single small trial are the only data available for this younger age group, using MDI/S instead of nebulizers should be done with caution for children aged <2 years.
Another randomized controlled trial of 152 patients found no difference in primary outcomes of asthma severity score, oxygen saturation, and percent predicted peak expiratory flow rate (PEFR). Several secondary outcomes slightly favored MDI/S: number of treatments given, whether steroids were used, change in heart rate, side effects, rate of hospital admission, and treatment time in the emergency department.3
A smaller double-blinded randomized controlled trial of 33 children aged 6 to 14 years showed no difference in MDI/S vs nebulizer, as measured by clinical score, respiratory rate, oxygen saturation, and forced expiratory volume at 1 second (FEV1).4 The researchers calculated the study had 90% power to detect a clinically meaningful difference in FEV1 of 12% of the predicted value between the groups.
Other review articles reach the same conclusion. One article reviewed the literature from 1980 to 1996 and examined 17 prospective clinical trials. Outcomes measured included pulmonary function measures and clinical scores. The researchers recommended that MDI/S be used due to clinical benefit, safety, lower cost, personnel time, and speed and ease of administration.5
A review article from the British literature examined 3 randomized controlled trials involving 51 patients and found no superiority of nebulizer vs MDI/S.6 A similar review article examined 14 randomized controlled trials for beta-agonist delivery for patients aged 5 to 15 with stable asthma. They found no obvious benefit of 1 type of device over another, including nebulizer, MDI/S, and dry powder inhalers.7 These last 2 articles claimed to be systematic reviews, although they do not clearly state their search methodology.
Researchers used a wide variety of spacers in all aforementioned studies; accordingly, one cannot be recommended as superior to others. The degree of teaching given to parents and children about MDI/S use was not described in any of the trials.
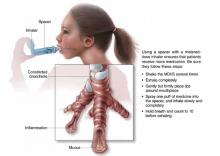
FIGURE
MDI with spacer is beneficial when used properly
Recommendations from others
Guidelines from the Global Health Initiative for Asthma, a collaboration of the National Heart, Lung and Blood Institute and the World Health Organization, recommend MDI/S for children with asthma due to increased efficacy and decreased cost (revised in 2002). Specifically, they recommend a spacer with a face mask for infants and preschool children, a mouthpiece and spacer for children aged 4 to 6 years, and a dry powder inhaler or breath-activated device from age 6 onwards.8 Cincinnati Children’s Hospital’s evidence-based guidelines from 1998 also recommend MDI/S for children aged >1 year with acute asthma exacerbations.9 This guideline suggests using 4 to 8 puffs from a 90 μg albuterol MDI at 1- to 2-minute intervals every 20 minutes for 1 hour, then every 1 to 4 hours subsequently.
Use MDIs with spacers in all but the youngest patients
Grant Hoekzema, MD
Mercy Family Medicine Residency, St. Louis, Mo
Until recently, using a nebulizer for the wheezing child or infant seemed intuitively to be the most effective way to deliver bronchodilators. However, with recent data showing that MDIs with spacers are just as effective, I have been using MDIs with spacers for all but my youngest patients. Parents as well as physicians may need to be convinced that using less technology in this case is better for their child. In some cases, parental acceptance of therapy necessitates using a nebulizer.
1. Cates CJ, Rowe BH, Bara A. Holding chambers versus nebulisers for beta-agonist treatment of acute asthma (Cochrane Review). The Cochrane Library, Issue 2, 2002. Oxford: Update Software, last updated February 21, 2002.
2. Delgado A, Chou KJ, Silver EJ, Crain EF. Nebulizers vs metered dose-inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med 2003;157:76-80.
3. Chou KJ, Cunningham SJ, Crain EF. Metered-dose inhalers with spacers vs nebulizers for pediatric asthma. Arch Pediatr Adolesc Med 1995;149:201-205.
4. Kerem E, Levison H, Schuh S, et al. Efficacy of albuterol administered by nebulizer versus spacer device in children with acute asthma. J Pediatr 1993;123:313-317.
5. Amirav I, Newhouse MT. Metered-dose inhaler accessory devices in acute asthma: efficacy and comparison with nebulizers: a literature review. Arch Pediatr Adolesc Med 1997;151:876-882.
6. Brocklebank D, Ram F, Wright J, et al. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol Assess 2001;5:1-149.
7. Peters J, Stevenson M, Beverley C, Lim JN, Smith S. The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation. Health Technol Assess 2002;6:1-167.
8. National Heart, Lung and Blood Institute (NHLBI), World Health Organization (WHO). Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention. 2002. Available at: http://www.ginasthma.com/workshop.pdf. Accessed on December 3, 2003. Updated from: NHLBI/WHO Workshop Report: Global Strategy for Asthma Management and Prevention, issued January 1995. NIH Publication No. 02-3659.
9. Evidence-Based Clinical Practice Guideline for Managing an Acute Exacerbation of Asthma. Cincinnati, Ohio: Cincinnati Childrens’ Hospital Medical Center; 1998 (revised 2002). Available at: http://www.cincinnatichildrens.org/svc/dept-div/health-policy/ev-based/asthma.htm. Accessed on December 3, 2003.
Metered-dose inhalers with a spacer (MDI/S) are as good as, or better than, nebulizers for children with asthma. This is based on numerous randomized controlled trials that compared outcomes such as hospital admission rates, asthma severity scores, and pulmonary function scores (strength of recommendation: A, based on consistent randomized controlled trials and meta-analysis).
Evidence summary
A Cochrane review of 10 randomized controlled trials comparing nebulizers with MDI/S, both in adults and in children aged >2 years, showed a substantial trend towards improvement in hospital admission rates with MDI/S use. Sample size for each study was small, ranging from 18 to 152 patients, with a total sample size of 880 children and 444 adults.
The relative risk of admission for MDI/S vs nebulizer for children was 0.65 (95% confidence interval, 0.4–1.06). Secondary outcomes were equivalent or slightly improved, including duration in the emergency department, changes in respiratory rate, blood gases, pulse, tremor, symptoms score, lung function, and use of steroids. Patients with life-threatening asthma (for example, those considered for ventilation) or other chronic illnesses were excluded.1
All but 1 of these studies were set in the emergency department and all involved the use of one of a variety of spacers with the MDI, such as the Aerochamber or Inspirease. Whether these efficacy studies can be translated into daily outpatient clinical practice remains unclear. Emergency departments typically have higher staffing levels, and study subjects and their parents may have received more MDI/S training than is practical in many office settings.
While most of the data were for children aged 2 years and older, 1 study published after the Cochrane review did show a lower admission rate in 85 patients who were 2 to 24 months in the MDI/S group.2 Controlling for the initial Pulmonary Index score, children using an MDI and Aerochamber spacer were admitted less often (5% vs 20%, number needed to treat=7; P=.05) than children using nebulizers. Since the results of this single small trial are the only data available for this younger age group, using MDI/S instead of nebulizers should be done with caution for children aged <2 years.
Another randomized controlled trial of 152 patients found no difference in primary outcomes of asthma severity score, oxygen saturation, and percent predicted peak expiratory flow rate (PEFR). Several secondary outcomes slightly favored MDI/S: number of treatments given, whether steroids were used, change in heart rate, side effects, rate of hospital admission, and treatment time in the emergency department.3
A smaller double-blinded randomized controlled trial of 33 children aged 6 to 14 years showed no difference in MDI/S vs nebulizer, as measured by clinical score, respiratory rate, oxygen saturation, and forced expiratory volume at 1 second (FEV1).4 The researchers calculated the study had 90% power to detect a clinically meaningful difference in FEV1 of 12% of the predicted value between the groups.
Other review articles reach the same conclusion. One article reviewed the literature from 1980 to 1996 and examined 17 prospective clinical trials. Outcomes measured included pulmonary function measures and clinical scores. The researchers recommended that MDI/S be used due to clinical benefit, safety, lower cost, personnel time, and speed and ease of administration.5
A review article from the British literature examined 3 randomized controlled trials involving 51 patients and found no superiority of nebulizer vs MDI/S.6 A similar review article examined 14 randomized controlled trials for beta-agonist delivery for patients aged 5 to 15 with stable asthma. They found no obvious benefit of 1 type of device over another, including nebulizer, MDI/S, and dry powder inhalers.7 These last 2 articles claimed to be systematic reviews, although they do not clearly state their search methodology.
Researchers used a wide variety of spacers in all aforementioned studies; accordingly, one cannot be recommended as superior to others. The degree of teaching given to parents and children about MDI/S use was not described in any of the trials.
FIGURE
MDI with spacer is beneficial when used properly
Recommendations from others
Guidelines from the Global Health Initiative for Asthma, a collaboration of the National Heart, Lung and Blood Institute and the World Health Organization, recommend MDI/S for children with asthma due to increased efficacy and decreased cost (revised in 2002). Specifically, they recommend a spacer with a face mask for infants and preschool children, a mouthpiece and spacer for children aged 4 to 6 years, and a dry powder inhaler or breath-activated device from age 6 onwards.8 Cincinnati Children’s Hospital’s evidence-based guidelines from 1998 also recommend MDI/S for children aged >1 year with acute asthma exacerbations.9 This guideline suggests using 4 to 8 puffs from a 90 μg albuterol MDI at 1- to 2-minute intervals every 20 minutes for 1 hour, then every 1 to 4 hours subsequently.
Use MDIs with spacers in all but the youngest patients
Grant Hoekzema, MD
Mercy Family Medicine Residency, St. Louis, Mo
Until recently, using a nebulizer for the wheezing child or infant seemed intuitively to be the most effective way to deliver bronchodilators. However, with recent data showing that MDIs with spacers are just as effective, I have been using MDIs with spacers for all but my youngest patients. Parents as well as physicians may need to be convinced that using less technology in this case is better for their child. In some cases, parental acceptance of therapy necessitates using a nebulizer.
Metered-dose inhalers with a spacer (MDI/S) are as good as, or better than, nebulizers for children with asthma. This is based on numerous randomized controlled trials that compared outcomes such as hospital admission rates, asthma severity scores, and pulmonary function scores (strength of recommendation: A, based on consistent randomized controlled trials and meta-analysis).
Evidence summary
A Cochrane review of 10 randomized controlled trials comparing nebulizers with MDI/S, both in adults and in children aged >2 years, showed a substantial trend towards improvement in hospital admission rates with MDI/S use. Sample size for each study was small, ranging from 18 to 152 patients, with a total sample size of 880 children and 444 adults.
The relative risk of admission for MDI/S vs nebulizer for children was 0.65 (95% confidence interval, 0.4–1.06). Secondary outcomes were equivalent or slightly improved, including duration in the emergency department, changes in respiratory rate, blood gases, pulse, tremor, symptoms score, lung function, and use of steroids. Patients with life-threatening asthma (for example, those considered for ventilation) or other chronic illnesses were excluded.1
All but 1 of these studies were set in the emergency department and all involved the use of one of a variety of spacers with the MDI, such as the Aerochamber or Inspirease. Whether these efficacy studies can be translated into daily outpatient clinical practice remains unclear. Emergency departments typically have higher staffing levels, and study subjects and their parents may have received more MDI/S training than is practical in many office settings.
While most of the data were for children aged 2 years and older, 1 study published after the Cochrane review did show a lower admission rate in 85 patients who were 2 to 24 months in the MDI/S group.2 Controlling for the initial Pulmonary Index score, children using an MDI and Aerochamber spacer were admitted less often (5% vs 20%, number needed to treat=7; P=.05) than children using nebulizers. Since the results of this single small trial are the only data available for this younger age group, using MDI/S instead of nebulizers should be done with caution for children aged <2 years.
Another randomized controlled trial of 152 patients found no difference in primary outcomes of asthma severity score, oxygen saturation, and percent predicted peak expiratory flow rate (PEFR). Several secondary outcomes slightly favored MDI/S: number of treatments given, whether steroids were used, change in heart rate, side effects, rate of hospital admission, and treatment time in the emergency department.3
A smaller double-blinded randomized controlled trial of 33 children aged 6 to 14 years showed no difference in MDI/S vs nebulizer, as measured by clinical score, respiratory rate, oxygen saturation, and forced expiratory volume at 1 second (FEV1).4 The researchers calculated the study had 90% power to detect a clinically meaningful difference in FEV1 of 12% of the predicted value between the groups.
Other review articles reach the same conclusion. One article reviewed the literature from 1980 to 1996 and examined 17 prospective clinical trials. Outcomes measured included pulmonary function measures and clinical scores. The researchers recommended that MDI/S be used due to clinical benefit, safety, lower cost, personnel time, and speed and ease of administration.5
A review article from the British literature examined 3 randomized controlled trials involving 51 patients and found no superiority of nebulizer vs MDI/S.6 A similar review article examined 14 randomized controlled trials for beta-agonist delivery for patients aged 5 to 15 with stable asthma. They found no obvious benefit of 1 type of device over another, including nebulizer, MDI/S, and dry powder inhalers.7 These last 2 articles claimed to be systematic reviews, although they do not clearly state their search methodology.
Researchers used a wide variety of spacers in all aforementioned studies; accordingly, one cannot be recommended as superior to others. The degree of teaching given to parents and children about MDI/S use was not described in any of the trials.
FIGURE
MDI with spacer is beneficial when used properly
Recommendations from others
Guidelines from the Global Health Initiative for Asthma, a collaboration of the National Heart, Lung and Blood Institute and the World Health Organization, recommend MDI/S for children with asthma due to increased efficacy and decreased cost (revised in 2002). Specifically, they recommend a spacer with a face mask for infants and preschool children, a mouthpiece and spacer for children aged 4 to 6 years, and a dry powder inhaler or breath-activated device from age 6 onwards.8 Cincinnati Children’s Hospital’s evidence-based guidelines from 1998 also recommend MDI/S for children aged >1 year with acute asthma exacerbations.9 This guideline suggests using 4 to 8 puffs from a 90 μg albuterol MDI at 1- to 2-minute intervals every 20 minutes for 1 hour, then every 1 to 4 hours subsequently.
Use MDIs with spacers in all but the youngest patients
Grant Hoekzema, MD
Mercy Family Medicine Residency, St. Louis, Mo
Until recently, using a nebulizer for the wheezing child or infant seemed intuitively to be the most effective way to deliver bronchodilators. However, with recent data showing that MDIs with spacers are just as effective, I have been using MDIs with spacers for all but my youngest patients. Parents as well as physicians may need to be convinced that using less technology in this case is better for their child. In some cases, parental acceptance of therapy necessitates using a nebulizer.
1. Cates CJ, Rowe BH, Bara A. Holding chambers versus nebulisers for beta-agonist treatment of acute asthma (Cochrane Review). The Cochrane Library, Issue 2, 2002. Oxford: Update Software, last updated February 21, 2002.
2. Delgado A, Chou KJ, Silver EJ, Crain EF. Nebulizers vs metered dose-inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med 2003;157:76-80.
3. Chou KJ, Cunningham SJ, Crain EF. Metered-dose inhalers with spacers vs nebulizers for pediatric asthma. Arch Pediatr Adolesc Med 1995;149:201-205.
4. Kerem E, Levison H, Schuh S, et al. Efficacy of albuterol administered by nebulizer versus spacer device in children with acute asthma. J Pediatr 1993;123:313-317.
5. Amirav I, Newhouse MT. Metered-dose inhaler accessory devices in acute asthma: efficacy and comparison with nebulizers: a literature review. Arch Pediatr Adolesc Med 1997;151:876-882.
6. Brocklebank D, Ram F, Wright J, et al. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol Assess 2001;5:1-149.
7. Peters J, Stevenson M, Beverley C, Lim JN, Smith S. The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation. Health Technol Assess 2002;6:1-167.
8. National Heart, Lung and Blood Institute (NHLBI), World Health Organization (WHO). Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention. 2002. Available at: http://www.ginasthma.com/workshop.pdf. Accessed on December 3, 2003. Updated from: NHLBI/WHO Workshop Report: Global Strategy for Asthma Management and Prevention, issued January 1995. NIH Publication No. 02-3659.
9. Evidence-Based Clinical Practice Guideline for Managing an Acute Exacerbation of Asthma. Cincinnati, Ohio: Cincinnati Childrens’ Hospital Medical Center; 1998 (revised 2002). Available at: http://www.cincinnatichildrens.org/svc/dept-div/health-policy/ev-based/asthma.htm. Accessed on December 3, 2003.
1. Cates CJ, Rowe BH, Bara A. Holding chambers versus nebulisers for beta-agonist treatment of acute asthma (Cochrane Review). The Cochrane Library, Issue 2, 2002. Oxford: Update Software, last updated February 21, 2002.
2. Delgado A, Chou KJ, Silver EJ, Crain EF. Nebulizers vs metered dose-inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med 2003;157:76-80.
3. Chou KJ, Cunningham SJ, Crain EF. Metered-dose inhalers with spacers vs nebulizers for pediatric asthma. Arch Pediatr Adolesc Med 1995;149:201-205.
4. Kerem E, Levison H, Schuh S, et al. Efficacy of albuterol administered by nebulizer versus spacer device in children with acute asthma. J Pediatr 1993;123:313-317.
5. Amirav I, Newhouse MT. Metered-dose inhaler accessory devices in acute asthma: efficacy and comparison with nebulizers: a literature review. Arch Pediatr Adolesc Med 1997;151:876-882.
6. Brocklebank D, Ram F, Wright J, et al. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol Assess 2001;5:1-149.
7. Peters J, Stevenson M, Beverley C, Lim JN, Smith S. The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation. Health Technol Assess 2002;6:1-167.
8. National Heart, Lung and Blood Institute (NHLBI), World Health Organization (WHO). Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention. 2002. Available at: http://www.ginasthma.com/workshop.pdf. Accessed on December 3, 2003. Updated from: NHLBI/WHO Workshop Report: Global Strategy for Asthma Management and Prevention, issued January 1995. NIH Publication No. 02-3659.
9. Evidence-Based Clinical Practice Guideline for Managing an Acute Exacerbation of Asthma. Cincinnati, Ohio: Cincinnati Childrens’ Hospital Medical Center; 1998 (revised 2002). Available at: http://www.cincinnatichildrens.org/svc/dept-div/health-policy/ev-based/asthma.htm. Accessed on December 3, 2003.
Evidence-based answers from the Family Physicians Inquiries Network
What findings distinguish acute bacterial sinusitis?
No combination of clinical findings can reliably distinguish acute viral rhinosinusitis from acute bacterial rhinosinusitis in primary care. Although unreliable, the best clinical predictor of acute bacterial sinusitis is the combination of unilateral nasal discharge and unilateral pain (positive likelihood ratio [LR+], 4.5; negative likelihood ratio [LR–], 0.25) (strength of recommendation [SOR]: B).1 History of purulent rhinorrhea (LR+, 1.5–1.9), maxillary tooth pain (LR+, 2.1–2.5), and purulent secretions in the nasal cavity (LR+, 2.1–5.5) may increase the likelihood of acute bacterial rhinosinusitis. Illness that starts as the common cold and pain on bending forward were not predictors of acute bacterial rhinosinusitis (SOR: B).2,3,4
Evidence summary
In one series, 87% of patients with the common cold had an abnormal computed tomography (CT) scan of the sinuses 48 to 96 hours after onset. Abnormalities visible on the CT scan persisted in 20% of patients at 2 weeks, yet epidemiological studies have shown that acute bacterial rhinosinusitis develops in only 0.5% to 2% of upper respiratory infections in adults. In primary care, only half of patients with a clinical diagnosis of acute bacterial rhinosinusitis have it proven upon aspiration.5
Two studies compared clinical findings with sinus puncture, the reference standard for acute bacterial rhinosinusitis. Berg found 4 independent predictors of aspirate purulence in Swedish emergency room patients with “paranasal” symptoms lasting <3 months (Table).1 Together, unilateral purulent nasal discharge and predominantly unilateral pain predicated purulence on aspiration (sensitivity 79%, specificity 83%, positive predictive value [PPV], 80%). Clinical exam by an otolaryngologist had a PPV of 72%.
While emergency and primary care patients may differ, this study’s rate of aspiration-proven sinusitis (43%) is closer to that seen in primary care (50%) than in referral practices (70%–80%). This study’s limitations included unclear referral criteria, overlapping clinical predictors, and lack of culture data.
In a study of general practice patients in the United Kingdom with clinically diagnosed acute maxillary sinusitis, no signs or symptoms were independently associated with their illness.6 The authors concluded that the clinical examination was more or less worthless. Only patients with positive findings on CT scan underwent aspiration in this study. Less differentiated, less severe symptoms and a less stringent definition of positive aspiration in this study may account for the different results. Additionally, one third of patients eligible for this study refused participation or withdrew prior to sinus puncture.6
Other primary care studies used less accurate reference tests such as CT2 (sensitivity and specificity unknown),5 x-ray3 (sensitivity 41%–90%, specificity 61%–85%),5 and ultrasound4 (sensitivity 76%, specificity 76%).7
Williams found 5 independent predictors of x-ray findings consistent with sinusitis in 247 male veterans:
- maxillary toothache (LR+, 2.5)
- no improvement with decongestants (LR+, 2.1)
- purulent secretions on exam (LR+, 2.1)
- abnormal transillumination (LR+, 1.6)
- colored nasal discharge (LR+, 1.5).3
In at least 2 of these 4 studies, purulent secretions in the nasal cavity (LR+, 2.1–5.5),2,3 maxillary tooth pain (LR+, 2.1–2.5)3,4 and purulent rhinorrhea (LR+, 1.5–1.9)2,3,4 increased the likelihood of acute bacterial rhinosinusitis.
Finding purulent secretions in the nasal cavity is highly specific for acute bacterial rhinosinusitis (specificity 79%–100%)1,2,3 but is uncommon and difficult to assess, requiring the use of a nasal speculum and possibly topical decongestants. The primary care physician’s overall clinical impression was accurate in Williams’ study but not in others.2,4,6 Illness starting as the common cold and pain on bending forward were not predictors of acute bacterial rhinosinusitis.2,3,4 Headache, bilateral maxillary pain, frontal sinus pain, fever, sinus tenderness on exam, and purulent pharyngeal discharge have not been shown to be useful in acute bacterial rhinosinusitis diagnosis.7
TABLE
Clinical prediction rule for acute bacterial rhinosinusitis
| Symptoms | PPV |
|---|---|
| Local pain, unilateral predominance | 41% |
| Purulent rhinorrhea, unilateral predominance | 48% |
| Purulent rhinorrhea, bilateral | 15% |
| Presence of pus in the nasal cavity | 17% |
| Clinical prediction rule: 3/4 positive: positive likelihood ratio = 6.75, negative likelihood ratio = 0.21 | |
| PPV, positive predictive value | |
Recommendations from others
A recommendation from the Agency for Health Care Policy and Research suggests using symptomatic treatment initially when the prevalence of acute bacterial rhinosinusitis in patients with upper respiratory infection is <25%, and using clinical criteria (see Table) for acute bacterial rhinosinusitis diagnosis when prevalence is higher.5
The Centers for Disease Control and Prevention recommends reserving the diagnosis of acute bacterial rhinosinusitis for patients with symptoms lasting ≥7 days with maxillary pain or tenderness in the face or teeth (especially unilateral) and purulent nasal secretions.8
An otolaryngology guideline recommends considering acute bacterial rhinosinusitis when viral upper respiratory infection persists beyond 10 days or worsens after 5 to 7 days with similar symptoms.9 The 7-to-10-day specification is based on the natural history of rhinovirus infections (SOR: C).
A Canadian Medical Association evidence-based review recommended a score based on Williams’ 5 independent predictor symptoms:
- fewer than 2 symptoms rule out acute bacterial rhinosinusitis (PPV, <40%)
- 4 or more symptoms rule in acute bacterial rhinosinusitis (PPV, 81%) (level of evidence [LOE]: 4)
- 2 or 3 symptoms (PPV, 40%–63%) may benefit from radiography (SOR: C).10
Jacob M. Reider, MD
Department of Family and Community Medicine, Albany Medical College, Albany, NY
This summary emphasizes inconsistencies in the literature and the limited predictive value of clinical findings when diagnosing sinusitis. But there is a way to sidestep this problem. When a patient presents complaining of “sinusitis,” I ask about their expectations for the visit and their understanding of their symptoms’ possible causes, and then I often show the patient a picture of sinus anatomy. By demonstrating that the osteomeatal complex is small, and focusing on obstruction rather than infection, I am able to avoid any confrontation about antibiotics entirely. Then I can recommend irrigation, hydration, and analgesia. For patients whose symptoms persist beyond 10 to 14 days, and for whom these initial interventions have failed, a trial of antibiotics may be indicated.
1. Berg O, Carenfelt C. Analysis of symptoms and clinical signs in the maxillary sinus empyema. Acta Otolaryngol 1988;105:343-349.
2. Lindbaek M, Hjortdahl P, Johnsen UL. Use of symptoms, signs, and blood tests to diagnose acute sinus infections in primary care: comparison with computed tomography. Fam Med 1996;28:183-188.
3. Williams JW, Jr, Simel DL, Roberts L, Samsa GP. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Intern Med 1992;117:705-710.
4. van Duijn NP, Brouwer HJ, Lamberts H. Use of symptoms and signs to diagnose maxillary sinusitis in general practice: comparison with ultrasonography. BMJ 1992;305:684-687.
5. Lau J, Zucker D, Engels EA, et al. Diagnosis and treatment of acute bacterial rhinosinusitis. Evidence Report/Technology Assessment No. 9. Rockville, Md: Agency for Health Care Policy and Research; 1999.
6. Hansen JG, Schmidt H, Rosborg J, Lund E. Predicting acute maxillary sinusitis in a general practice population. BMJ 1995;311:233-236.
7. Lindbaek M, Hjortdahl P. The clinical diagnosis of acute purulent sinusitis in general practice—a review. Br J Gen Pract 2002;52:491-495.
8. Hickner JM, Bartlett JG, Besser RE, Gonzales R, Hoffman JR, Sande MA. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Emerg Med 2001;37:703-710.
9. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Sinus and Allergy Health Partnership. Otolaryngol Head Neck Surg 2000;123(1 Pt 2):5-31.
10. Low DE, Desrosiers M, McSherry J, et al. A practical guide for the diagnosis and treatment of acute sinusitis. CMAJ 1997;156 (Suppl 6):S1-S14.
No combination of clinical findings can reliably distinguish acute viral rhinosinusitis from acute bacterial rhinosinusitis in primary care. Although unreliable, the best clinical predictor of acute bacterial sinusitis is the combination of unilateral nasal discharge and unilateral pain (positive likelihood ratio [LR+], 4.5; negative likelihood ratio [LR–], 0.25) (strength of recommendation [SOR]: B).1 History of purulent rhinorrhea (LR+, 1.5–1.9), maxillary tooth pain (LR+, 2.1–2.5), and purulent secretions in the nasal cavity (LR+, 2.1–5.5) may increase the likelihood of acute bacterial rhinosinusitis. Illness that starts as the common cold and pain on bending forward were not predictors of acute bacterial rhinosinusitis (SOR: B).2,3,4
Evidence summary
In one series, 87% of patients with the common cold had an abnormal computed tomography (CT) scan of the sinuses 48 to 96 hours after onset. Abnormalities visible on the CT scan persisted in 20% of patients at 2 weeks, yet epidemiological studies have shown that acute bacterial rhinosinusitis develops in only 0.5% to 2% of upper respiratory infections in adults. In primary care, only half of patients with a clinical diagnosis of acute bacterial rhinosinusitis have it proven upon aspiration.5
Two studies compared clinical findings with sinus puncture, the reference standard for acute bacterial rhinosinusitis. Berg found 4 independent predictors of aspirate purulence in Swedish emergency room patients with “paranasal” symptoms lasting <3 months (Table).1 Together, unilateral purulent nasal discharge and predominantly unilateral pain predicated purulence on aspiration (sensitivity 79%, specificity 83%, positive predictive value [PPV], 80%). Clinical exam by an otolaryngologist had a PPV of 72%.
While emergency and primary care patients may differ, this study’s rate of aspiration-proven sinusitis (43%) is closer to that seen in primary care (50%) than in referral practices (70%–80%). This study’s limitations included unclear referral criteria, overlapping clinical predictors, and lack of culture data.
In a study of general practice patients in the United Kingdom with clinically diagnosed acute maxillary sinusitis, no signs or symptoms were independently associated with their illness.6 The authors concluded that the clinical examination was more or less worthless. Only patients with positive findings on CT scan underwent aspiration in this study. Less differentiated, less severe symptoms and a less stringent definition of positive aspiration in this study may account for the different results. Additionally, one third of patients eligible for this study refused participation or withdrew prior to sinus puncture.6
Other primary care studies used less accurate reference tests such as CT2 (sensitivity and specificity unknown),5 x-ray3 (sensitivity 41%–90%, specificity 61%–85%),5 and ultrasound4 (sensitivity 76%, specificity 76%).7
Williams found 5 independent predictors of x-ray findings consistent with sinusitis in 247 male veterans:
- maxillary toothache (LR+, 2.5)
- no improvement with decongestants (LR+, 2.1)
- purulent secretions on exam (LR+, 2.1)
- abnormal transillumination (LR+, 1.6)
- colored nasal discharge (LR+, 1.5).3
In at least 2 of these 4 studies, purulent secretions in the nasal cavity (LR+, 2.1–5.5),2,3 maxillary tooth pain (LR+, 2.1–2.5)3,4 and purulent rhinorrhea (LR+, 1.5–1.9)2,3,4 increased the likelihood of acute bacterial rhinosinusitis.
Finding purulent secretions in the nasal cavity is highly specific for acute bacterial rhinosinusitis (specificity 79%–100%)1,2,3 but is uncommon and difficult to assess, requiring the use of a nasal speculum and possibly topical decongestants. The primary care physician’s overall clinical impression was accurate in Williams’ study but not in others.2,4,6 Illness starting as the common cold and pain on bending forward were not predictors of acute bacterial rhinosinusitis.2,3,4 Headache, bilateral maxillary pain, frontal sinus pain, fever, sinus tenderness on exam, and purulent pharyngeal discharge have not been shown to be useful in acute bacterial rhinosinusitis diagnosis.7
TABLE
Clinical prediction rule for acute bacterial rhinosinusitis
| Symptoms | PPV |
|---|---|
| Local pain, unilateral predominance | 41% |
| Purulent rhinorrhea, unilateral predominance | 48% |
| Purulent rhinorrhea, bilateral | 15% |
| Presence of pus in the nasal cavity | 17% |
| Clinical prediction rule: 3/4 positive: positive likelihood ratio = 6.75, negative likelihood ratio = 0.21 | |
| PPV, positive predictive value | |
Recommendations from others
A recommendation from the Agency for Health Care Policy and Research suggests using symptomatic treatment initially when the prevalence of acute bacterial rhinosinusitis in patients with upper respiratory infection is <25%, and using clinical criteria (see Table) for acute bacterial rhinosinusitis diagnosis when prevalence is higher.5
The Centers for Disease Control and Prevention recommends reserving the diagnosis of acute bacterial rhinosinusitis for patients with symptoms lasting ≥7 days with maxillary pain or tenderness in the face or teeth (especially unilateral) and purulent nasal secretions.8
An otolaryngology guideline recommends considering acute bacterial rhinosinusitis when viral upper respiratory infection persists beyond 10 days or worsens after 5 to 7 days with similar symptoms.9 The 7-to-10-day specification is based on the natural history of rhinovirus infections (SOR: C).
A Canadian Medical Association evidence-based review recommended a score based on Williams’ 5 independent predictor symptoms:
- fewer than 2 symptoms rule out acute bacterial rhinosinusitis (PPV, <40%)
- 4 or more symptoms rule in acute bacterial rhinosinusitis (PPV, 81%) (level of evidence [LOE]: 4)
- 2 or 3 symptoms (PPV, 40%–63%) may benefit from radiography (SOR: C).10
Jacob M. Reider, MD
Department of Family and Community Medicine, Albany Medical College, Albany, NY
This summary emphasizes inconsistencies in the literature and the limited predictive value of clinical findings when diagnosing sinusitis. But there is a way to sidestep this problem. When a patient presents complaining of “sinusitis,” I ask about their expectations for the visit and their understanding of their symptoms’ possible causes, and then I often show the patient a picture of sinus anatomy. By demonstrating that the osteomeatal complex is small, and focusing on obstruction rather than infection, I am able to avoid any confrontation about antibiotics entirely. Then I can recommend irrigation, hydration, and analgesia. For patients whose symptoms persist beyond 10 to 14 days, and for whom these initial interventions have failed, a trial of antibiotics may be indicated.
No combination of clinical findings can reliably distinguish acute viral rhinosinusitis from acute bacterial rhinosinusitis in primary care. Although unreliable, the best clinical predictor of acute bacterial sinusitis is the combination of unilateral nasal discharge and unilateral pain (positive likelihood ratio [LR+], 4.5; negative likelihood ratio [LR–], 0.25) (strength of recommendation [SOR]: B).1 History of purulent rhinorrhea (LR+, 1.5–1.9), maxillary tooth pain (LR+, 2.1–2.5), and purulent secretions in the nasal cavity (LR+, 2.1–5.5) may increase the likelihood of acute bacterial rhinosinusitis. Illness that starts as the common cold and pain on bending forward were not predictors of acute bacterial rhinosinusitis (SOR: B).2,3,4
Evidence summary
In one series, 87% of patients with the common cold had an abnormal computed tomography (CT) scan of the sinuses 48 to 96 hours after onset. Abnormalities visible on the CT scan persisted in 20% of patients at 2 weeks, yet epidemiological studies have shown that acute bacterial rhinosinusitis develops in only 0.5% to 2% of upper respiratory infections in adults. In primary care, only half of patients with a clinical diagnosis of acute bacterial rhinosinusitis have it proven upon aspiration.5
Two studies compared clinical findings with sinus puncture, the reference standard for acute bacterial rhinosinusitis. Berg found 4 independent predictors of aspirate purulence in Swedish emergency room patients with “paranasal” symptoms lasting <3 months (Table).1 Together, unilateral purulent nasal discharge and predominantly unilateral pain predicated purulence on aspiration (sensitivity 79%, specificity 83%, positive predictive value [PPV], 80%). Clinical exam by an otolaryngologist had a PPV of 72%.
While emergency and primary care patients may differ, this study’s rate of aspiration-proven sinusitis (43%) is closer to that seen in primary care (50%) than in referral practices (70%–80%). This study’s limitations included unclear referral criteria, overlapping clinical predictors, and lack of culture data.
In a study of general practice patients in the United Kingdom with clinically diagnosed acute maxillary sinusitis, no signs or symptoms were independently associated with their illness.6 The authors concluded that the clinical examination was more or less worthless. Only patients with positive findings on CT scan underwent aspiration in this study. Less differentiated, less severe symptoms and a less stringent definition of positive aspiration in this study may account for the different results. Additionally, one third of patients eligible for this study refused participation or withdrew prior to sinus puncture.6
Other primary care studies used less accurate reference tests such as CT2 (sensitivity and specificity unknown),5 x-ray3 (sensitivity 41%–90%, specificity 61%–85%),5 and ultrasound4 (sensitivity 76%, specificity 76%).7
Williams found 5 independent predictors of x-ray findings consistent with sinusitis in 247 male veterans:
- maxillary toothache (LR+, 2.5)
- no improvement with decongestants (LR+, 2.1)
- purulent secretions on exam (LR+, 2.1)
- abnormal transillumination (LR+, 1.6)
- colored nasal discharge (LR+, 1.5).3
In at least 2 of these 4 studies, purulent secretions in the nasal cavity (LR+, 2.1–5.5),2,3 maxillary tooth pain (LR+, 2.1–2.5)3,4 and purulent rhinorrhea (LR+, 1.5–1.9)2,3,4 increased the likelihood of acute bacterial rhinosinusitis.
Finding purulent secretions in the nasal cavity is highly specific for acute bacterial rhinosinusitis (specificity 79%–100%)1,2,3 but is uncommon and difficult to assess, requiring the use of a nasal speculum and possibly topical decongestants. The primary care physician’s overall clinical impression was accurate in Williams’ study but not in others.2,4,6 Illness starting as the common cold and pain on bending forward were not predictors of acute bacterial rhinosinusitis.2,3,4 Headache, bilateral maxillary pain, frontal sinus pain, fever, sinus tenderness on exam, and purulent pharyngeal discharge have not been shown to be useful in acute bacterial rhinosinusitis diagnosis.7
TABLE
Clinical prediction rule for acute bacterial rhinosinusitis
| Symptoms | PPV |
|---|---|
| Local pain, unilateral predominance | 41% |
| Purulent rhinorrhea, unilateral predominance | 48% |
| Purulent rhinorrhea, bilateral | 15% |
| Presence of pus in the nasal cavity | 17% |
| Clinical prediction rule: 3/4 positive: positive likelihood ratio = 6.75, negative likelihood ratio = 0.21 | |
| PPV, positive predictive value | |
Recommendations from others
A recommendation from the Agency for Health Care Policy and Research suggests using symptomatic treatment initially when the prevalence of acute bacterial rhinosinusitis in patients with upper respiratory infection is <25%, and using clinical criteria (see Table) for acute bacterial rhinosinusitis diagnosis when prevalence is higher.5
The Centers for Disease Control and Prevention recommends reserving the diagnosis of acute bacterial rhinosinusitis for patients with symptoms lasting ≥7 days with maxillary pain or tenderness in the face or teeth (especially unilateral) and purulent nasal secretions.8
An otolaryngology guideline recommends considering acute bacterial rhinosinusitis when viral upper respiratory infection persists beyond 10 days or worsens after 5 to 7 days with similar symptoms.9 The 7-to-10-day specification is based on the natural history of rhinovirus infections (SOR: C).
A Canadian Medical Association evidence-based review recommended a score based on Williams’ 5 independent predictor symptoms:
- fewer than 2 symptoms rule out acute bacterial rhinosinusitis (PPV, <40%)
- 4 or more symptoms rule in acute bacterial rhinosinusitis (PPV, 81%) (level of evidence [LOE]: 4)
- 2 or 3 symptoms (PPV, 40%–63%) may benefit from radiography (SOR: C).10
Jacob M. Reider, MD
Department of Family and Community Medicine, Albany Medical College, Albany, NY
This summary emphasizes inconsistencies in the literature and the limited predictive value of clinical findings when diagnosing sinusitis. But there is a way to sidestep this problem. When a patient presents complaining of “sinusitis,” I ask about their expectations for the visit and their understanding of their symptoms’ possible causes, and then I often show the patient a picture of sinus anatomy. By demonstrating that the osteomeatal complex is small, and focusing on obstruction rather than infection, I am able to avoid any confrontation about antibiotics entirely. Then I can recommend irrigation, hydration, and analgesia. For patients whose symptoms persist beyond 10 to 14 days, and for whom these initial interventions have failed, a trial of antibiotics may be indicated.
1. Berg O, Carenfelt C. Analysis of symptoms and clinical signs in the maxillary sinus empyema. Acta Otolaryngol 1988;105:343-349.
2. Lindbaek M, Hjortdahl P, Johnsen UL. Use of symptoms, signs, and blood tests to diagnose acute sinus infections in primary care: comparison with computed tomography. Fam Med 1996;28:183-188.
3. Williams JW, Jr, Simel DL, Roberts L, Samsa GP. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Intern Med 1992;117:705-710.
4. van Duijn NP, Brouwer HJ, Lamberts H. Use of symptoms and signs to diagnose maxillary sinusitis in general practice: comparison with ultrasonography. BMJ 1992;305:684-687.
5. Lau J, Zucker D, Engels EA, et al. Diagnosis and treatment of acute bacterial rhinosinusitis. Evidence Report/Technology Assessment No. 9. Rockville, Md: Agency for Health Care Policy and Research; 1999.
6. Hansen JG, Schmidt H, Rosborg J, Lund E. Predicting acute maxillary sinusitis in a general practice population. BMJ 1995;311:233-236.
7. Lindbaek M, Hjortdahl P. The clinical diagnosis of acute purulent sinusitis in general practice—a review. Br J Gen Pract 2002;52:491-495.
8. Hickner JM, Bartlett JG, Besser RE, Gonzales R, Hoffman JR, Sande MA. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Emerg Med 2001;37:703-710.
9. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Sinus and Allergy Health Partnership. Otolaryngol Head Neck Surg 2000;123(1 Pt 2):5-31.
10. Low DE, Desrosiers M, McSherry J, et al. A practical guide for the diagnosis and treatment of acute sinusitis. CMAJ 1997;156 (Suppl 6):S1-S14.
1. Berg O, Carenfelt C. Analysis of symptoms and clinical signs in the maxillary sinus empyema. Acta Otolaryngol 1988;105:343-349.
2. Lindbaek M, Hjortdahl P, Johnsen UL. Use of symptoms, signs, and blood tests to diagnose acute sinus infections in primary care: comparison with computed tomography. Fam Med 1996;28:183-188.
3. Williams JW, Jr, Simel DL, Roberts L, Samsa GP. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Intern Med 1992;117:705-710.
4. van Duijn NP, Brouwer HJ, Lamberts H. Use of symptoms and signs to diagnose maxillary sinusitis in general practice: comparison with ultrasonography. BMJ 1992;305:684-687.
5. Lau J, Zucker D, Engels EA, et al. Diagnosis and treatment of acute bacterial rhinosinusitis. Evidence Report/Technology Assessment No. 9. Rockville, Md: Agency for Health Care Policy and Research; 1999.
6. Hansen JG, Schmidt H, Rosborg J, Lund E. Predicting acute maxillary sinusitis in a general practice population. BMJ 1995;311:233-236.
7. Lindbaek M, Hjortdahl P. The clinical diagnosis of acute purulent sinusitis in general practice—a review. Br J Gen Pract 2002;52:491-495.
8. Hickner JM, Bartlett JG, Besser RE, Gonzales R, Hoffman JR, Sande MA. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Emerg Med 2001;37:703-710.
9. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Sinus and Allergy Health Partnership. Otolaryngol Head Neck Surg 2000;123(1 Pt 2):5-31.
10. Low DE, Desrosiers M, McSherry J, et al. A practical guide for the diagnosis and treatment of acute sinusitis. CMAJ 1997;156 (Suppl 6):S1-S14.
Evidence-based answers from the Family Physicians Inquiries Network